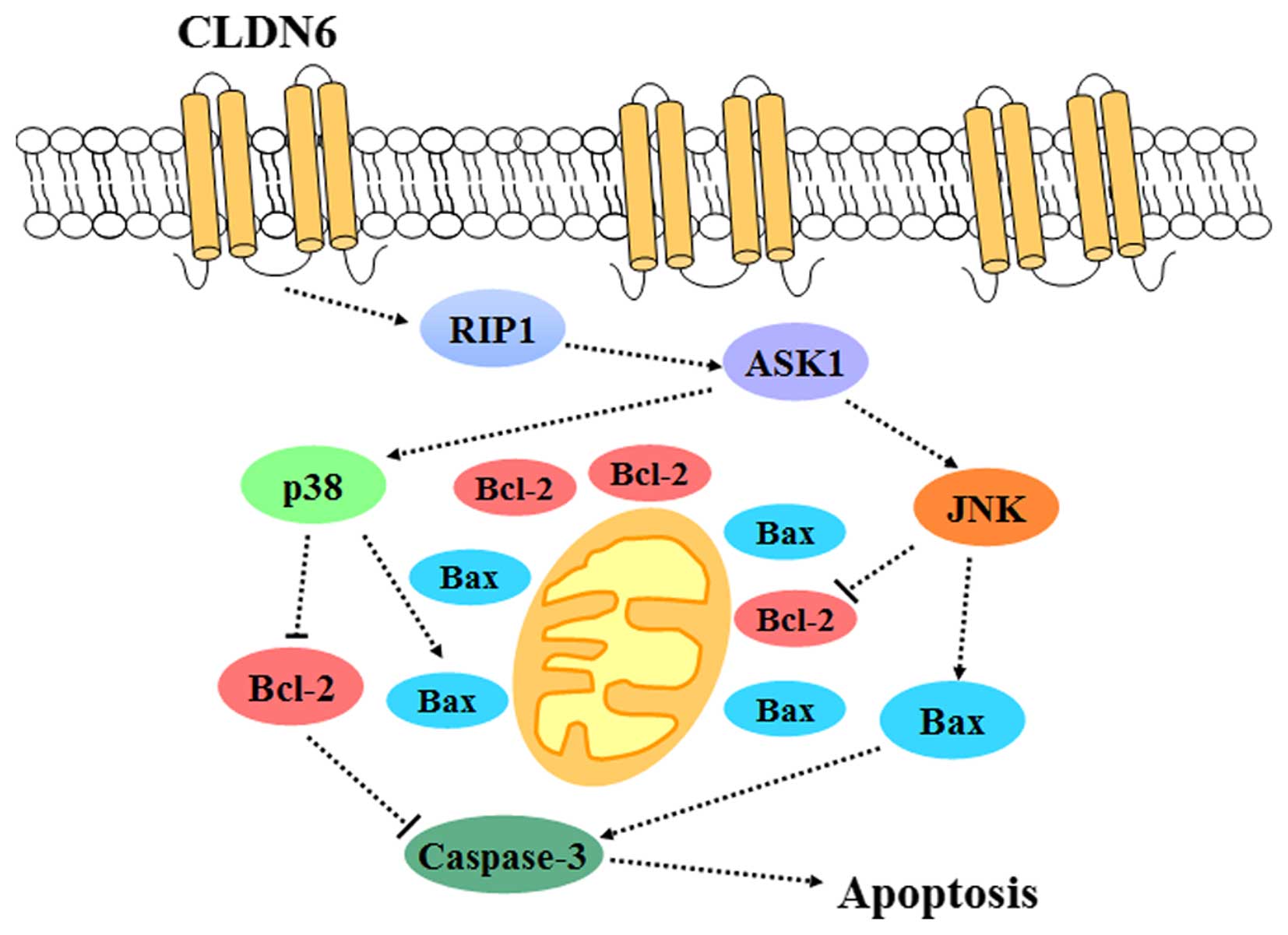
Introduction
Claudins (CLDNs) are scaffolding proteins of tight
junction strands in epithelium and endothelium cells (1). Claudin 6 (CLDN6) is one of the 27
CLDN family members (2). Numerous
studies have suggested that abnormal expression of CLDN6 could
benefit the occurrence and development of tumor (3). We cloned and identified CLDN6 gene in
Copenhagen rat mammary epithelial cells, which are almost
completely resistant to mammary cancer, indicating that CLDN6 may
be a breast cancer suppressor gene, we found that CLDN6 inhibits
proliferation and induces apoptosis in MCF-7 breast cancer cells
through p38 signaling (4–6). However, the precise molecular
mechanisms by which CLDN6 promotes apoptosis remain largely
elusive. We also previously found that CLDN6 expression levels were
correlated with apoptosis signal-regulating kinase 1 (ASK1)
expression levels using cDNA array and bioinformatics analysis
(unpublished data). Another study showed that CLDN6 overexpression
led to increased expression of ASK1 protein in breast cancer
tissues (7). Together these data
suggest a potential link between CLDN6 and ASK1 signaling.
ASK1 is a member of the mitogen-activated protein
kinase kinase kinase (MAP3K) family that induces cells apoptosis,
including in breast cancer cells, in response to various stresses
(8). The phosphorylation state of
serine/threonine kinase (serine/threonine kinase) as an essential
component of the MAPK pathway plays an important role in the
induction of cellular signaling. A previous study showed that
phosphorylation of the Thr-838 site of ASK1 leads to ASK1
activation, while ASK1 is inactivated by Ser83, Ser1034 and Ser967
phosphorylation (9). Interactions
between ASK1 and various binding proteins also regulate ASK1 active
and inactive status. These mechanisms may play a major part in
regulating the activation of ASK1 in cancer cells (10). ASK1 induces apoptosis via
activation of downstream MAPKs, c-Jun N-terminal kinases (JNKs) and
p38 MAPKs (11). The mechanisms of
MAPK-induced apoptosis via Bcl-2 family proteins, and caspase
family proteins in cancer cells have been documented (12–16).
Accumulating evidence suggests that CLDNs induce
tumor apoptosis through activating MAPK signal pathway, such as
CLDN6 induces tumor apoptosis through activating the ERK1/2
signaling pathway (17), and CLDN7
promotes apoptosis through the activation of p38 protein kinase in
MKN28 gastric epithelial cells (18). The present study dissects the
molecular mechanisms by which CLDN6 induced apoptosis in MCF-7
breast cancer cells. We hypothesize that CLDN6 induces MCF-7 cells
apoptosis by early activation of ASK1 followed by the subsequent
activation of p38/JNK pro-apoptotic signaling. We examined the
potential relationship between CLDN6 and ASK1 signaling, and
specifically through p-p38 and p-JNK downstream targets.
Materials and methods
Antibodies and chemicals
Antibodies agaist CLDN6, Bcl-2, Bax and β-actin were
purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). Antibodies against p-ASK1ser976, ASK1, p-JNK and
JNK were from Bioworld (Dublin, OH, USA). Anti-human p-p38 and p38
antibodies were from Cell Signaling Technology, Inc. (Beverly, MA,
USA). Antibody against RIP1, caspase-3 and cleaved caspase-3 were
purchased from BD Biosciences (San Diego, CA, USA). TRX1 was
purchased from Sigma-Aldrich (St. Louis, MO, USA).
Cells and culture conditions
MCF-7/pcDNA3.1(+) (MCF-7/vector) or
MCF-7/pcDNA3.1(+)-CLDN6 (MCF-7/CLDN6) stable transfection cell
clones were established and obtained as previously described
(19). All cells were cultured in
H-DMEM medium (Gibco, Carlsbad, CA, USA) containing 10% fetal
bovine serum (FBS; HyClone Laboratories, Inc., Logan, UT USA) and
100 U/ml penicillin and 100 U/ml streptomycin in a 5%
CO2 humidified incubator at 37°C. G418 (Sigma-Aldrich)
was added to the medium of transfected cells at a concentration of
400 μg/ml.
TRX1 treatment
MCF-7/CLDN6 stably transfected cells were incubated
with defined concentrations of Thioredoxin-1 (TRX1; Sigma-Aldrich)
(a physiological inhibitor of ASK1) 0, 1, 10 and 100 ng/ml for 24 h
and with 10 ng/ml of TRX1 for 0, 24, 48 and 72 h.
p-ASK1ser967 and ASK1 expression levels were
subsequently measured using western blot analysis (described below)
to determine the optimal treatment concentration and time. Then
these cells with TRX1 (10 ng/ml) treatment for 48 h were used to a
follow-up study.
Western blot analysis
Cells were lysed with RIPA and PMSF (Roche Applied
Science) buffer, and protein concentrations were quantitated based
on the BCA (Protein assay kit; Beyotime Institute of Biotechnology,
Haimen, China) method according to the manufacturer's protocol.
Equal amounts of cell lysates were resolved on SDS-PAGE by
electrophoresis, and proteins were transferred to PVDF membranes
(Millipore, Billerica, MA, USA) and membranes were blocked with
PBST buffered saline containing 5% (w/v) non-fat dried milk
(20). The membranes were blotted
with the appropriate primary antibodies and dilutions (Table I), followed by an HRP-conjugated
secondary antibody. The detection of immunoreactive bands was
visualized by chemiluminiscence using ECL-plus reagent (Beyotime
Institute of Biotechnology) via the western blot system (GeneSnap,
Frederick, MD, USA).
 | Table IPrimary antibodies used by western
blot analysis. |
Table I
Primary antibodies used by western
blot analysis.
| Antibody | Company | Dilution | Species |
|---|
| CLND6 | Santa Cruz
Biotechnology | 1:1000 | Goat |
| β-actin | Santa Cruz,
Biotechnology | 1:1000 | Mouse |
| p-ASK1 | Bioworld | 1:500 | Rabbit |
| ASK1 | Bioworld | 1:1000 | Rabbit |
| p-JNK | Abcam | 1:500 | Rabbit |
| JNK | Abcam | 1:500 | Rabbit |
| p-p38 | Abcam | 1:500 | Rabbit |
| p38 | Abcam | 1:500 | Rabbit |
Cell death assay
MCF-7/CLDN6 stably transfected cell death treated
with TRX1 was measured by trypan blue (Beijing Donglinchangsheng
Biotechnology, Co., Ltd., Beijing, China) exclusion assay (21). The cell relative viability
percentage was recorded using the formula below: Survival ratio (%)
= (number of viable cells/number of total cells) × 100%.
Colony formation experiment
MCF-7/CLDN6 stably transfected cells in the
logarithmic growth phase were seeded in 6-well plate with the cell
density of 3×102 cells in each dish, and treated with
TRX1. The visible clones appeared after 3 weeks, and were fixed
with methanol for 20 min, stained with Giemsa solution for 5 min.
Cell clones with diameter >0.5 mm were counted under an optical
microscope and the cloned formation efficiency was calculated with
the following formula: The cloning efficiency (CE) (%) = (number of
clones formed/number of cells inoculated) × 100% (22).
Analysis of mitochondrial transmembrane
potential using rhodamine 123 staining
The disruption of MCF-7/CLDN6 stably transfected
cell mitochondrial membrane potential ΔΨm was analyzed by staining
with rhodamine (Rho) 123 (Beijing Donglinchangsheng Biotechnology)
according to manufacturer's protocol. Loss of ΔΨm resulting in
strong yellow-green fluorescence was observed by fluorescence
microscopy (Olympus, Tokyo, Japan) and measured by Image-Pro Plus
software (Media Cybernetics, Inc., Rockville, MD, USA). Results are
given in percentage of strong fluorescence cells with low ΔΨm
compared to untreated control.
Evaluation of apoptotic cells using TUNEL
staining
MCF-7/CLDN6 stably transfected cells were grown with
TRX1 for 24 h and fixed in 1% paraformaldehyde in PBS,
permeabilized in 70% ethanol. TUNEL staining procedure was carried
out according to In Situ Cell Death Detection kit (Roche Products,
Ltd., Basel, Switzerland), as per the manufacturer's protocol. The
stained cells were analyzed and mounted under light microscope. The
eosinophils count was done at ×40 magnification and the apoptosis
index was calculated by dividing the sum of eosinophils in
apoptosis by the global sum of counted eosinophils (23).
DNA fragmentation assay
MCF-7/CLDN6 stably transfected cells were harvested
after 48-h incubation with 10 ng/ml TRX1 by centrifugation (300 × g
for 5 min) and lysed in lysis buffer as previously described
(24). The integrity of DNA was
assessed by agarose gel electrophoresis.
Electron microscopy
MCF-7/vector and MCF-7/CLDN6 stably transfected
cells were fixed with 2% paraformaldehyde/2% glutaraldehyde in 0.1
M, pH 7.4 phosphate buffer, followed by 1% OsO4. After
dehydration, thin sections were stained with uranyl acetate and
lead citrate for observation under a FEI Tecnai Spirit electron
microscope.
Statistical analysis
All data are presented as mean ± standard deviation
(SD). The differences between groups were statistically analyzed
using one-way analysis of variance (ANOVA) followed by the
Dunnett's test or LSD-t test for multiple comparisons. P<0.05
was considered as significant to evaluate the presence of a
significant statistical difference.
Results
CLDN6 decreases p-ASK1ser967
levels and increases RIP1, p-p38 and p-JNK proteins in MCF-7
cells
To investigate the mechanism underlying CLDN6
induction of apoptosis, the levels of Ser967 phosphorylation of
ASK1 in MCF-7 cells overexpressing CLDN6 and control cells were
assessed by western blot analysis. The results showed that the
relative expression level of p-ASK1ser967 was
significantly decreased in MCF-7/CLDN6 stably transfected cells
(Fig. 1A and B), suggesting that
CLDN6-induced apoptosis may involve activation of ASK1. The
expression levels of RIP1 (Fig. 1C and
D), p-JNK and p-p38 (Fig.
1E–G) were significantly increased in MCF-7/CLDN6 stably
transfected cells. We consider ASK1 is associated with upstream
activators of p38 and JNK signaling in response to restoration of
CLDN6 expression in MCF-7 cells. Taken together, these data suggest
that CLDN6-induced apoptosis is associated with activation of ASK1
and upstream of RIP1 and downstream of JNK, and p38.
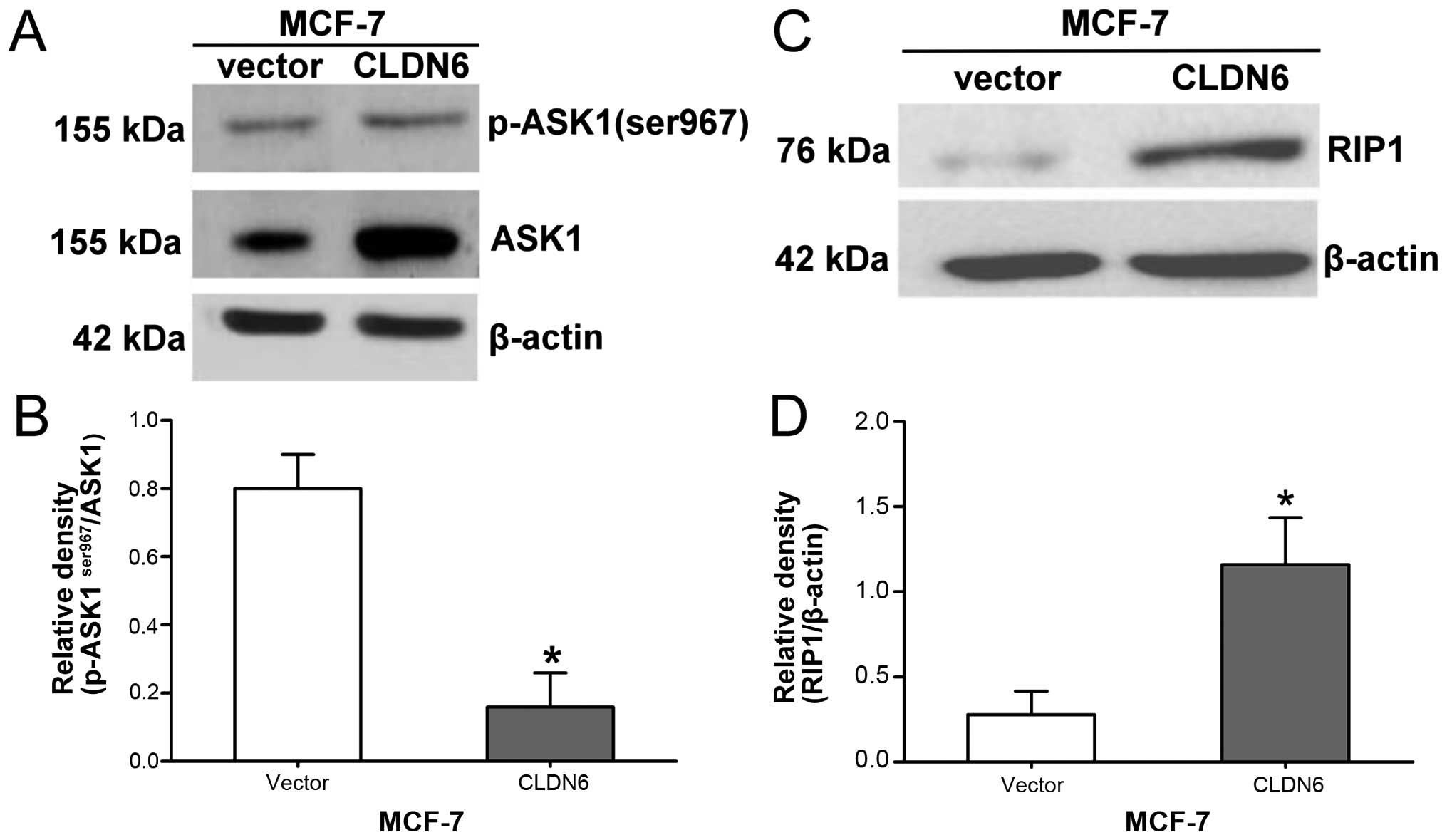 | Figure 1CLDN6 decreased the expression of
ASK1 at Ser967 site, increased the expression of RIP1, and
activated phosphorylation of p38, JNK proteins in human MCF-7
cells. (A and B) The expression level of p-ASK1ser967
and ASK1 proteins were detected by western blot analysis.
Quantification of the protein levels are shown in the histograms,
compared with the vector, *P=0.037<0.05; (C and D)
RIP1 protein expression level was detected by western blot
analysis. Compared with the vector, relative density of
RIP1/β-actin was increased in MCF-7/CLDN6 stably transfected cells,
*P=0.014<0.05. (E–G) p-JNK and p-p38 protein
expression level were detected by western blot analysis. Compared
with the vector, relative density of p-JNK/JNK and p-p38/p38 was
increased in MCF-7/CLDN6 cells, *P=0.035<0.05,
*P=0.026<0.05. Data are mean ± SD of vertical
bars. |
ASK1 plays a crucial role in
CLDN6-activation of p38/JNK signaling molecules
We next assess the role of ASK1 in CLDN6-induced
activation of p38/JNK signaling. MCF-7/CLDN6 stably transfected
cells were treated with TRX1, a physiological inhibitor of ASK1
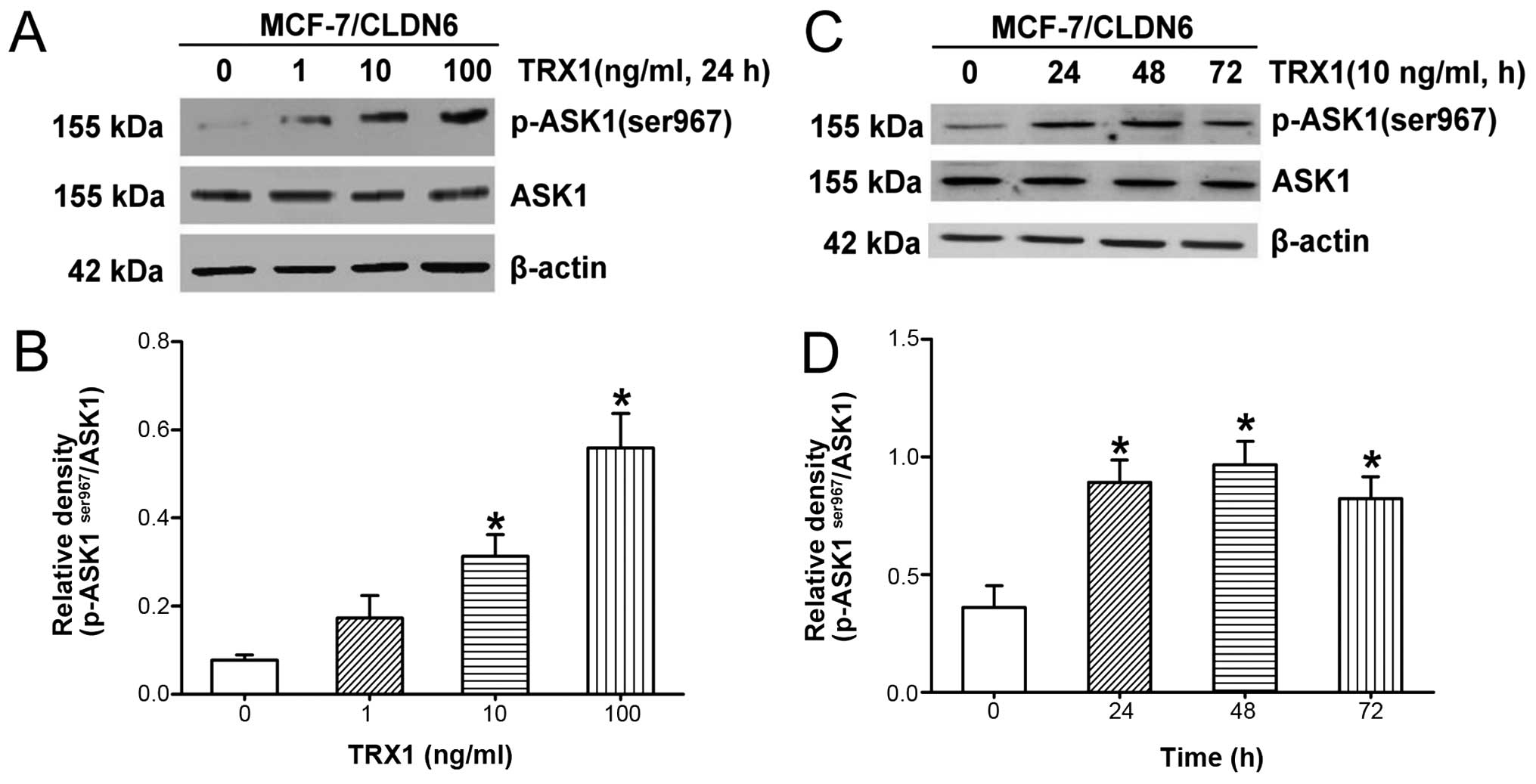
expression, for various exposure times and various doses (Fig. 2A–D). We confirmed increased
p-ASK1ser967 in response to TRX1 in a dose-dependent and
time-dependent manner. Our results showed increased
p-ASK1ser967 in response to TRX1 at 10 ng/ml for 48 h,
suggesting that TRX1 effectively inhibited ASK1 activation under
these conditions, therefore, we selected this concentration for
further experiments. We next tested the impact of TRX1 on the
phosphorylation of ASK1 downstream targets JNK and p38 in
MCF-7/CLDN6 stably transfected cells. TRX1 significantly
downregulated p-JNK and p-p38 levels (Fig. 2G–I). These results provide further
evidence that ASK1 activation is required for CLDN6-promoted
activation of JNK and p38.
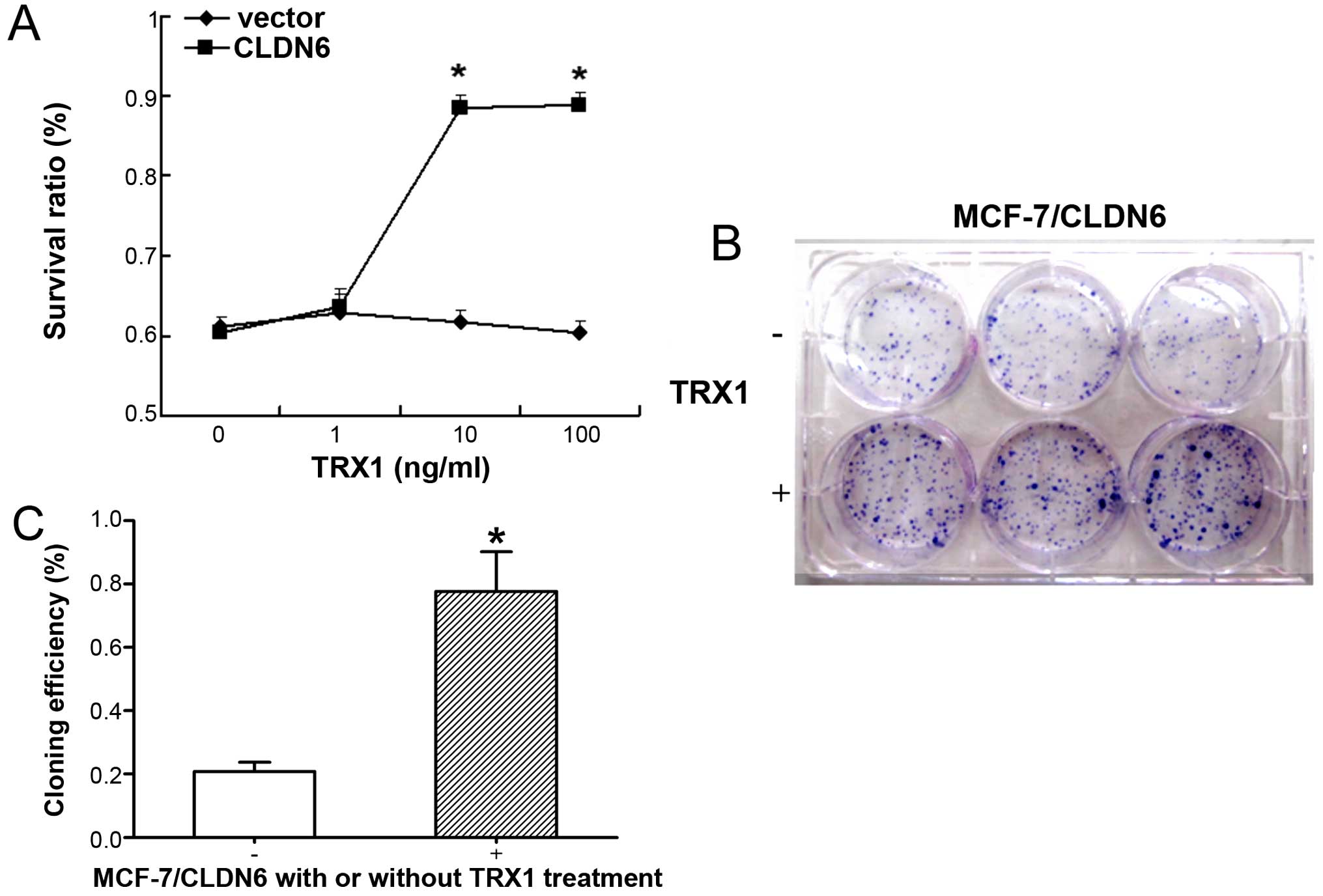
TRX1 suppresses the apoptosis of
MCF-7/CLDN6 stably transfected cells by inhibiting the cell
viability and enhancing the clone formation ability
A previous study demonstrated that CLDN6 induced
apoptosis of MCF-7 breast cancer cells (19). We analyzed cellular apoptosis using
various assays including trypan blue staining, colony formation,
TUNEL staining and DNA ladder analysis. MCF-7/CLDN6 stably
transfected cells showed detectable levels of apoptosis in all
assays (Fig. 3). Notably,
treatment of MCF-7/CLDN6 stably transfected cells with TRX1
significantly decreased these apoptotic changes. Together these
results suggest that ASK1 plays a major role in CLDN6-induced MCF-7
cell apoptosis.
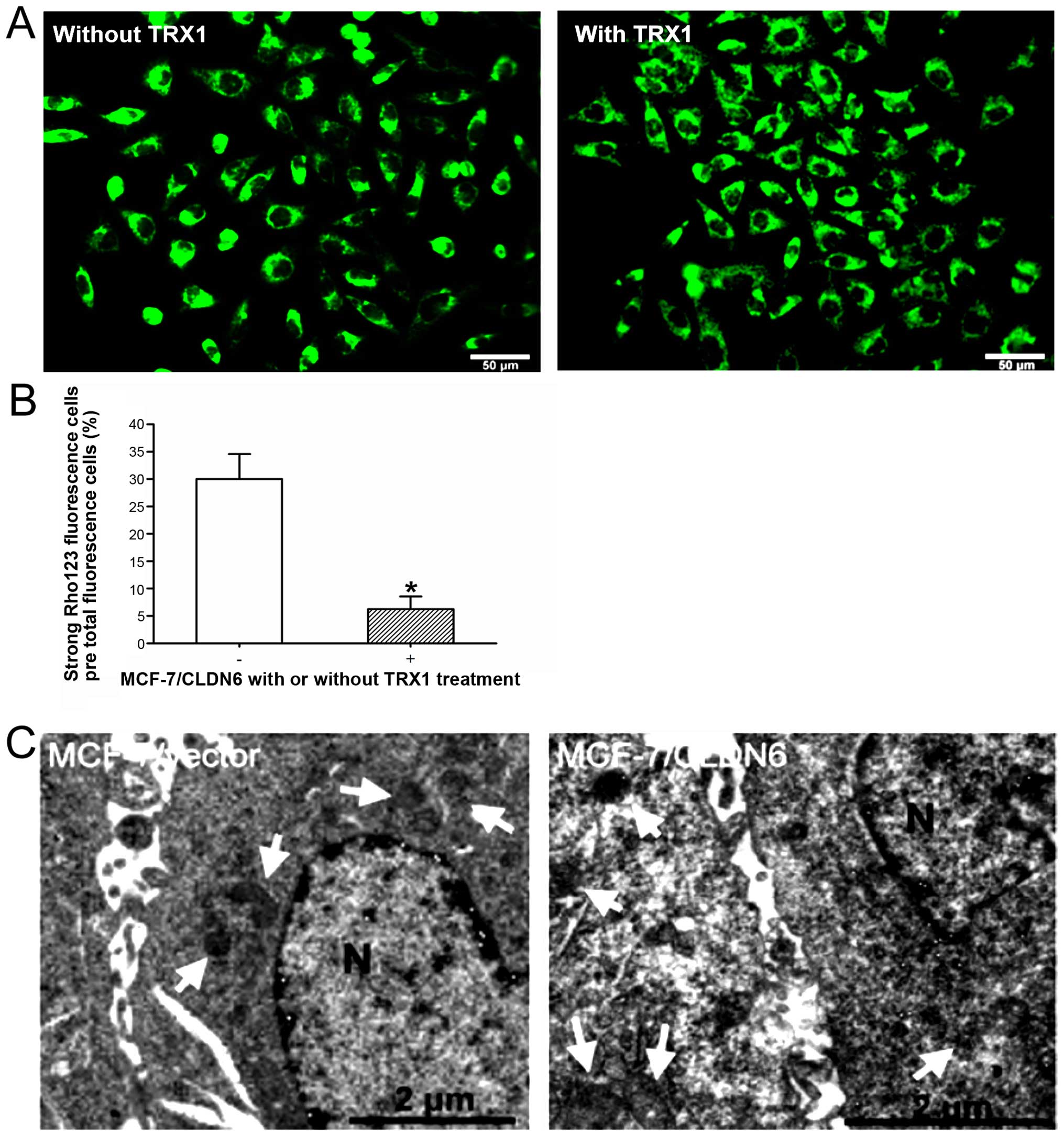
TRX1 inhibits apoptosis by disruption of
the mitochondrial structure in MCF-7/CLDN6 stably transfected
cells
To analyze if ASK1 induces apoptosis via the
mitochondrial pathway, we measured the disruption of the cell
transmembrane mitochondrial potential (ΔΨm) in MCF-7/CLDN6 stably
transfected cells with TRX1 treatment. Using Rho-123 staining
strong fluorescence was observed in MCF-7/CLDN6 stably transfected
cells (Fig. 4A). TRX1 treatment of
MCF-7/CLDN6 stably transfected cells induced a strong reduction of
ΔΨm after 48 h (Fig. 4A and B).
Analysis of mitochondrial structure further confirmed disruption of
mitochondrial structure in MCF-7/CLDN6 stably transfected cells and
these changes were observed less frequently upon TRX1 treatment
(Fig. 4C). These results indicate
that CLDN6 induces the activation of mitochondrial apoptotic
pathway in MCF-7 cells.
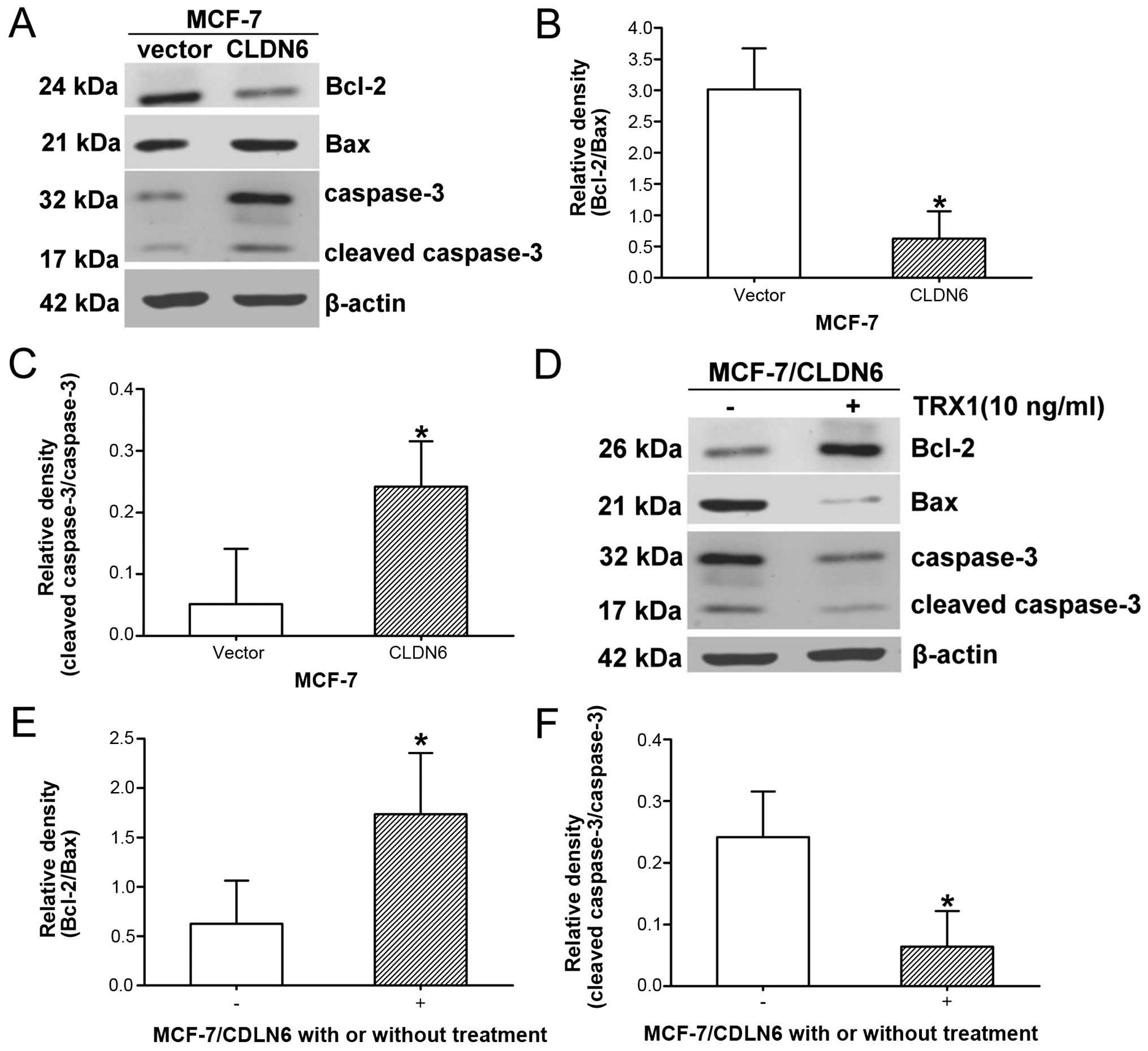
Changes of Bcl-2/Bax ratio and caspase-3
activation are involved in CLDN6-modulated pro-apoptotic
effect
We continued investigating the molecular mechanisms
of apoptosis induction by CLDN6 in MCF-7 cells. We evaluated
expression levels of Bcl-2 and Bax proteins, which are involved in
the mitochondrial apoptosis pathway, and the expression level of
caspase-3 before and after TRX1 treatment. Bcl-2 levels were
significantly lower, and Bax protein expression levels
significantly increased in MCF-7/CLDN6 stably transfected cells
compared with control cells, resulting in a decrease in Bcl-2/Bax
ratio (Fig. 5A and B). However,
TRX1 treatment caused an increase in Bcl-2 and decrease in Bax,
resulting in significant increase in the Bcl-2/Bax ratio (Fig. 5D and E). Caspase-3 was
significantly activated in MCF-7/CLDN6 stably transfected cells
compared with control (Fig. 5C)
and significantly inhibited in MCF-7/CLDN6 stably transfected cells
after TRX1 treatment (Fig. 5F).
Taken together, the constitutive deregulation of the balance of
Bcl-2 family proteins and activation of caspase-3 are involved in
the mitochondrial apoptotic pathways.
Discussion
Previous studies have demonstrated that CLDN6
functions as a cancer suppressor in MCF-7 breast cancer cells and
CLDN6 could be attributed to inhibition of cell proliferation and
induction of apoptosis (5).
Immunohistochemical analysis in breast invasive ductal carcinomas
tissues showed that ASK1 expression level is significantly related
with CLDN6 (7). The purpose of the
present study was to examine the involvement of the ASK1 molecular
signaling pathway in the anti-apoptotic effects of CLDN6 in MCF-7
cells.
ASK1 is a member of the mitogen-activated protein
kinase kinase kinase family, in response to various stimuli such as
oxidative stress, endoplasmic reticulum stress, infection and
calcium influx, it is also activated by its upstream RIP1 and
activates downstream JNK and p38 apoptotic signaling (25–28).
Using cDNA microarray approaches, we previously analyed genes
differentially expressed in the CLDN6 transfected cells
(unpublished observations). The result showed ASK1 mRNA is
increased, suggesting ASK1 may be a target of CLDN6. MCF-7/CLDN6
stable transfection cell clone treated with TRX1, an ASK1
inhibitor, showed p-ASK1ser967 increased, suggesting
that TRX1 effectively inhibited ASK1 activation. Some related genes
of oxidative stress, endoplasmic reticulum stress, infection, and
calcium influx were increased in transfection cells, they may play
an important role in ASK1 activation and will be further
investigated in our future work. Here ASK1 was activated, RIP1 was
upregulated, p-p38 and p-JNK were increased by CLDN6 overexpression
in MCF-7 cells. We considered that CLDN6 activated p-JNK and p-p38
through RIP1-ASK1 apoptotic signaling pathway.
TRX1, a physiological inhibitor of ASK1, is a 12-kDa
ubiquitous protein with a redox-active disulfide/dithiol within the
conserved active site sequence (-Cys-Gly-Pro-Cys-) (29). Oxidized TRX1 with a disulfide on
its active site is reduced by NADPH-dependent thioredoxin
reductase1 (TR1) to restore its functions (30,31).
TRX1 combined with ASK1 to form a TRX1-ASK1 complex which in return
inactivated the ASK1 protein expression (27). To test the role of ASK1 during the
effect of CLDN6 on JNK/p38 activation in MCF-7/CLDN6 stably
transfected cells, these cells were incubated with 10 ng/ml of TRX1
for 48 h. The results showed that TRX1 treatment significantly
downregulated p-JNK and p-p38 expression. These data further
confirmed that ASK1 is required for CLDN6-promoted activation of
JNK and p38. ASK1 serves as a general mediator of cell death such
as apoptosis in cancer (32–34).
Stress induced apoptosis worked through activation of the ASK1-p38
MAPK pathway (35–37). Several studied have shown that
CLDNs induce apoptosis of cancer cells such as cervical carcinoma
and breast cancer cells (17,38–40).
Our data showed that CLDN6 significantly inhibited MCF-7 cell
apoptosis with TRX1 treatment. Taken together, ASK1 by CLDN6
overexpression adjusted MAPK activity, increased JNK and p38
phosphorylation, and cell apoptosis reaction may be cascaded.
The dissociation between TRX1 and ASK1 induced by
ROS leads to the activation of the ASK1/JNK signaling pathway and
subsequent increase of apoptosis (41,42).
The mechanism for the inhibition of apoptosis by ASK1 has been
suggested such as Bcl-2 protein, caspase protein family members and
mitochondria, whereby TRX1 could bind to and inhibit ASK1 further
regulating the JNK/p38 signaling pathway in response to
environmental stresses (26,27,43).
Bcl-2 family proteins, including Bax, Bak, Bik and Bid, promote
apoptosis by translocating to and disrupting the mitochondrial
membrane. The caspase protein family also plays a critical role in
mediating apoptosis. Increasing studies have shown that caspase-1
and caspase-3 are associated with mitochondrial transmembrane
potential and induce thymocyte apoptosis through the Fas/APO-1
(44). During apoptosis,
mitochondria releasing caspase activation factors such as
cytochrome c, undergo loss of electric transfer function and
reduce generation of cellular energy.
We used Rho 123 staining on MCF-7 cells to evaluate
the mitochondrial transmembrane potential. Changes in the cell
membrane potential was detected in MCF-7/CLDN6 stably transfected
cells, indicating a serious collapse of mitochondrial membrane
potential and apoptosis rate. Our results further showed that TRX1
inhibited the ASK1 signaling pathway, reduced the degree of the
mitochondrial transmembrane potential collapse and reduced the rate
of apoptosis. These data suggest that CLDN6 regulates ASK1-JNK/p38
signal in apoptosis process and activates the mitochondrial
apoptotic pathway. A previous study also found that
JNK/p38-activated the inhibition of the growth of cancer cells
through the mitochondrial apoptotic signaling pathway (45).
Among TJ proteins, multiple claudin isoforms are
expressed in a homophilic and heterophilic manner and in varied
patterns of expression that are tissue specific to regulate
junctional permeability, and the selectivity and strength of the
TJs are conferred by these proteins in most cell types (46). CLDN7 has a similar function and
signaling transduction in the induction of tumor cell apoptosis.
For example, CLDN7 expression shows a significant correlation with
grading, locoregional and distant metastases, nodal involvement and
cellular cohesion in invasive carcinomas of the breast (47,48).
Oshima et al (18) found
that CLDN7 expression is increased in gastric cancer, and as a
first report, showed that aspirin induces gastric epithelial
barrier dysfunction by activating p38 MAPK via CLDN7. To precisely
define whether CLDN7 is involved in the same events as CLDN6, more
studies are still needed.
In conclusion, the present study shows that CLDN6 is
a tumor suppressor that inhibits MCF-7 cell survival and induces
cell apoptosis. The breast cancer suppressive role of CLDN6 may be
initiated by downregulation of ASK1 phosphorylation and subsequent
decrease in JNK and p38 phosphorylation, along with decreasing the
Bcl-2/Bax ratio and increasing caspase-3 activation (Fig. 6). This study highlighted the
central role of CLDN6 in breast cancer cell survival and suggests
that the CLDN6 gene is an ideal target for developing agents for
breast cancer therapy.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (Code: 81172499) and the Jilin
Province Science and Technology Development Projects
(20140414036GH).
Abbreviations:
|
CLDN6
|
claudin 6
|
|
ASK1
|
apoptosis signal-regulating kinase
1
|
|
JNK
|
c-jun N-terminal kinase
|
|
MAP3K
|
mitogen-activated protein kinase
kinase kinase
|
|
Rho 123
|
rhodamine 123
|
|
RIP1
|
receptor interacting prtein 1
|
References
|
1
|
Chiba H, Osanai M, Murata M, Kojima T and
Sawada N: Transmembrane proteins of tight junctions. Biochim
Biophys Acta. 1778:588–600. 2008. View Article : Google Scholar
|
|
2
|
Van Itallie CM and Anderson JM: Claudins
and epithelial para-cellular transport. Annu Rev Physiol.
68:403–429. 2006. View Article : Google Scholar
|
|
3
|
Sawada N: Tight junction-related human
diseases. Pathol Int. 63:1–12. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Quan C, Wang HL and Lu SJ: Resistance to
mammary carcinogenesis in Copenhagen rats: Potential roles of
vascular endothelial growth factor and mast cells. Cancer Lett.
186:165–175. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu Q, Liu YF, Ren Y, Xu XM, Yu LN, Li YL
and Quan CS: Effects of stable up-regulation of tight junction
protein claudin-6 upon biological phenotypes of breast cancer cell
MCF-7. Zhonghua Yi Xue Za Zhi. 90:407–412. 2010.(In Chinese).
PubMed/NCBI
|
|
6
|
Ren Y, Wu Q, Liu Y, Xu X and Quan C: Gene
silencing of claudin-6 enhances cell proliferation and migration
accompanied with increased MMP-2 activity via p38 MAPK signaling
pathway in human breast epithelium cell line HBL-100. Mol Med Rep.
8:1505–1510. 2013.PubMed/NCBI
|
|
7
|
Guo Y, Xu X, Liu Z, Zhang T, Zhang X, Wang
L, Wang M, Liu Y, Lu Y, Liu Y, et al: Apoptosis signal-regulating
kinase 1 is associated with the effect of claudin-6 in breast
cancer. Diagn Pathol. 7:1112012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shen K, Xie J, Wang H, Zhang H, Yu M, Lu
F, Tan H and Xu H: Cambogin induces caspase-independent apoptosis
through the ROS/JNK pathway and epigenetic regulation in breast
cancer cells. Mol Cancer Ther. 14:1738–1749. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Singh O, Shillings A, Craggs P, Wall I,
Rowland P, Skarzynski T, Hobbs CI, Hardwick P, Tanner R, Blunt M,
et al: Crystal structures of ASK1-inhibtor complexes provide a
platform for structure-based drug design. Protein Sci.
22:1071–1077. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vauzour D, Pinto JT, Cooper AJ and Spencer
JP: The neurotoxicity of 5-S-cysteinyldopamine is mediated by the
early activation of ERK1/2 followed by the subsequent activation of
ASK1/JNK1/2 pro-apoptotic signalling. Biochem J. 463:41–52. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen Z, Seimiya H, Naito M, Mashima T,
Kizaki A, Dan S, Imaizumi M, Ichijo H, Miyazono K and Tsuruo T:
ASK1 mediates apoptotic cell death induced by genotoxic stress.
Oncogene. 18:173–180. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li G, Xiao Y and Zhang L: Cocaine induces
apoptosis in fetal rat myocardial cells through the p38
mitogen-activated protein kinase and mitochondrial/cytochrome c
pathways. J Pharmacol Exp Ther. 312:112–119. 2005. View Article : Google Scholar
|
|
13
|
Ye Q, Zhang N, Chen K, Zhu J and Jiang H:
Effects of portulacerebroside a on apoptosis of human leukemia HL60
cells and p38/JNK signaling pathway. Int J Clin Exp Pathol.
8:13968–13977. 2015.
|
|
14
|
Niso-Santano M, Bravo-San Pedro JM,
Gómez-Sánchez R, Climent V, Soler G, Fuentes JM and González-Polo
RA: ASK1 overexpression accelerates paraquat-induced autophagy via
endoplasmic reticulum stress. Toxicol Sci. 119:156–168. 2011.
View Article : Google Scholar
|
|
15
|
Jiang Q, Yuan Y, Zhou J, Wu Y, Zhou Q, Gui
S and Wang Y: Apoptotic events induced by high glucose in human
hepatoma HepG2 cells involve endoplasmic reticulum stress and
MAPK's activation. Mol Cell Biochem. 399:113–122. 2015. View Article : Google Scholar
|
|
16
|
Zhang ZY, Sun BL, Liu JK, Yang MF, Li DW,
Fang J, Zhang S, Yuan QL and Huang SL: Activation of mGluR5
attenuates microglial activation and neuronal apoptosis in early
brain injury after experimental Subarachnoid Hemorrhage in rats.
Neurochem Res. 40:1121–1132. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang X, Ruan Y, Li Y, Lin D and Quan C:
Tight junction protein claudin-6 inhibits growth and induces the
apoptosis of cervical carcinoma cells in vitro and in vivo. Med
Oncol. 32:148–157. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Oshima T, Miwa H and Joh T: Aspirin
induces gastric epithelial barrier dysfunction by activating p38
MAPK via claudin-7. Am J Physiol Cell Physiol. 295:C800–C806. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu Q, Liu Y, Ren Y, Xu X, Yu L, Li Y and
Quan C: Tight junction protein, claudin-6, downregulates the
malignant phenotype of breast carcinoma. Eur J Cancer Prev.
19:186–194. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Griffiths GS, Grundl M, Leychenko A,
Reiter S, Young-Robbins SS, Sulzmaier FJ, Caliva MJ, Ramos JW and
Matter ML: Bit-1 mediates integrin-dependent cell survival through
activation of the NFkappaB pathway. J Biol Chem. 286:14713–14723.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Beppu K, Jaboine J, Merchant MS, Mackall
CL and Thiele CJ: Effect of imatinib mesylate on neuroblastoma
tumorigenesis and vascular endothelial growth factor expression. J
Natl Cancer Inst. 96:46–55. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun D, Yang K, Zheng G, Li Z and Cao Y:
Study on effect of peptide-conjugated near-infrared fluorescent
quantum dots on the clone formation, proliferation, apoptosis, and
tumorigenicity ability of human buccal squamous cell carcinoma cell
line BcaCD885. Int J Nanomed. 5:401–405. 2010. View Article : Google Scholar
|
|
23
|
Anjos CP, Vasconcelos AC, Crosara PF,
Anjos GC, Becker CG and Guimarães RE: Apoptosis in eosinophilic
nasal polyps treated in vitro with mitomycin C. Braz J
Otorhinolaryngol. 78:32–37. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jiang CP, Ding H, Shi DH, Wang YR, Li EG
and Wu JH: Pro-apoptotic effects of tectorigenin on human
hepatocellular carcinoma HepG2 cells. World J Gastroenterol.
18:1753–1764. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ichijo H, Nishida E, Irie K, ten Dijke P,
Saitoh M, Moriguchi T, Takagi M, Matsumoto K, Miyazono K and Gotoh
Y: Induction of apoptosis by ASK1, a mammalian MAPKKK that
activates SAPK/JNK and p38 signaling pathways. Science. 275:90–94.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tobiume K, Matsuzawa A, Takahashi T,
Nishitoh H, Morita K, Takeda K, Minowa O, Miyazono K, Noda T and
Ichijo H: ASK1 is required for sustained activations of JNK/p38 MAP
kinases and apoptosis. EMBO Rep. 2:222–228. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shiizaki S, Naguro I and Ichijo H:
Activation mechanisms of ASK1 in response to various stresses and
its significance in intracellular signaling. Adv Biol Regul.
53:135–144. 2013. View Article : Google Scholar
|
|
28
|
Zhang H, Zhang H, Lin Y, Li J, Pober JS
and Min W: RIP1-mediated AIP1 phosphorylation at a 14-3-3-binding
site is critical for tumor necrosis factor-induced ASK1-JNK/p38
activation. J Biol Chem. 282:14788–14796. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kosek D, Kylarova S, Psenakova K,
Rezabkova L, Herman P, Vecer J, Obsilova V and Obsil T: Biophysical
and structural characterization of the thioredoxin-binding domain
of protein kinase ASK1 and its interaction with reduced
thioredoxin. J Biol Chem. 289:24463–24474. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nakamura H, Nakamura K and Yodoi J: Redox
regulation of cellular activation. Annu Rev Immunol. 15:351–369.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rodrigues J, Branco V, Lu J, Holmgren A
and Carvalho C: Toxicological effects of thiomersal and
ethylmercury: Inhibition of the thioredoxin system and
NADP+-dependent dehydrogenases of the pentose phosphate
pathway. Toxicol Appl Pharmacol. 286:216–223. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sanmartín C, Plano D, Sharma AK and Palop
JA: Selenium compounds, apoptosis and other types of cell death: An
overview for cancer therapy. Int J Mol Sci. 13:9649–9672. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Evan GI and Vousden KH: Proliferation,
cell cycle and apoptosis in cancer. Nature. 411:342–348. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Du J, Cai SH, Shi Z and Nagase F: Binding
activity of H-Ras is necessary for in vivo inhibition of ASK1
activity. Cell Res. 14:148–154. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Michalak M and Gye MC: Endoplasmic
reticulum stress in peri-implantation embryos. Clin Exp Reprod Med.
42:1–7. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Noro T, Namekata K, Kimura A, Guo X,
Azuchi Y, Harada C, Nakano T, Tsuneoka H and Harada T: Spermidine
promotes retinal ganglion cell survival and optic nerve
regeneration in adult mice following optic nerve injury. Cell Death
Dis. 6:e17202015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cheng X, Holenya P, Can S, Alborzinia H,
Rubbiani R, Ott I and Wölfl S: A TrxR inhibiting gold(I) NHC
complex induces apoptosis through ASK1-p38-MAPK signaling in
pancreatic cancer cells. Mol Cancer. 13:221–236. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shiozaki A, Shimizu H, Ichikawa D, Konishi
H, Komatsu S, Kubota T, Fujiwara H, Okamoto K, Iitaka D, Nakashima
S, et al: Claudin 1 mediates tumor necrosis factor alpha-induced
cell migration in human gastric cancer cells. World J
Gastroenterol. 20:17863–17876. 2014.PubMed/NCBI
|
|
39
|
Soini Y, Eskelinen M, Juvonen P, Kärjä V,
Haapasaari KM, Saarela A and Karihtala P: Strong claudin 5
expression is a poor prognostic sign in pancreatic adenocarcinoma.
Tumour Biol. 35:3803–3808. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Achari C, Winslow S and Larsson C: Down
regulation of CLDND1 induces apoptosis in breast cancer cells. PLoS
One. 10:e01303002015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wei L, Zhu S, Wang J, Zhang C, Quan R, Yan
X and Liu J: Regulatory role of ASK1 in porcine circovirus type
2-induced apoptosis. Virology. 447:285–291. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li X, Zhang R, Luo D, Park SJ, Wang Q, Kim
Y and Min W: Tumor necrosis factor alpha-induced desumoylation and
cytoplasmic translocation of homeodomain-interacting protein kinase
1 are critical for apoptosis signal-regulating kinase 1-JNK/p38
activation. J Biol Chem. 280:15061–15070. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Matsuura H, Nishitoh H, Takeda K,
Matsuzawa A, Amagasa T, Ito M, Yoshioka K and Ichijo H:
Phosphorylation-dependent scaffolding role of JSAP1/JIP3 in the
ASK1-JNK signaling pathway. A new mode of regulation of the MAP
kinase cascade. J Biol Chem. 277:40703–40709. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Marsden VS, O'Connor L, O'Reilly LA, Silke
J, Metcalf D, Ekert PG, Huang DC, Cecconi F, Kuida K, Tomaselli KJ,
et al: Apoptosis initiated by Bcl-2-regulated caspase activation
independently of the cytochrome c/Apaf-1/caspase-9 apoptosome.
Nature. 419:634–637. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Grubb RL, Deng J, Pinto PA, Mohler JL,
Chinnaiyan A, Rubin M, Linehan WM, Liotta LA, Petricoin EF and
Wulfkuhle JD: Pathway biomarker profiling of localized and
metastatic human prostate cancer reveal metastatic and prognostic
signatures. J Proteome Res. 8:3044–3054. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hewitt KJ, Agarwal R and Morin PJ: The
claudin gene family: Expression in normal and neoplastic tissues.
BMC Cancer. 6:186–194. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kominsky SL, Argani P, Korz D, Evron E,
Raman V, Garrett E, Rein A, Sauter G, Kallioniemi OP and Sukumar S:
Loss of the tight junction protein claudin-7 correlates with
histological grade in both ductal carcinoma in situ and invasive
ductal carcinoma of the breast. Oncogene. 22:2021–2033. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sauer T, Pedersen MK, Ebeltoft K and Naess
O: Reduced expression of Claudin-7 in fine needle aspirates from
breast carcinomas correlate with grading and metastatic disease.
Cytopathology. 16:193–198. 2005. View Article : Google Scholar : PubMed/NCBI
|