1. Introduction
In mammals, thymus-derived T lymphocytes and
bone-marrow-derived B lymphocytes can generate wide repertoires of
antigen receptors [T-cell receptors (TCR) and B-cell receptors
(BCR), respectively]. These receptors enable the recognition of a
myriad of antigens in mammals through primary participation in
cell-mediated immunity (T cells) and humoral immunity (B cells). T
cells are divided into the following two subsets: αβ and γδ T
cells, according to the heterodimeric, disulphide bond-linked TCR
structure (1). The domain
structures of TCRs are strikingly similar to the structure of
immunoglobulin Fab fragments, therefore, TCRs are also classified
as members of the immunoglobulin superfamily. However, unlike
immunoglobulins, which recognize intact protein antigens, the
complementarity-determining region (CDR) loops of TCRs only
recognize processed fragments of antigens that are presented by
major histocompatibility complex (MHC) molecules on
antigen-presenting cells (APCs), such as B cells, dendritic cells
and macrophages (2,3). These antigens that are recognized by
TCR family members include peptides, glycoproteins, lipids and
small molecule metabolites. Thus, the resulting TCR-peptide-MHC
complex is of great importance in cellular immunity.
αβ TCR are mainly expressed on the surfaces of αβ T
and NKT cells, where they play a significant role in adaptive
immune response by interacting with foreign antigens that are
presented by MHC class I/II molecules on APCs. However, recent
studies have described unconventional subsets of αβ T cells bearing
TCRs that can recognize ligand antigens that are presented by
molecules of the MHC-like CD1 family or vitamin B based metabolites
that are bound to MHC-related protein 1 (MR1). These subsets
include: i) mucosal-associated invariant T cells which exhibited
restricted diversity and are involved in antibacterial immunity
(4,5); and ii) invariant natural killer T
(NKT) cells and germ-line-encoded mycolyl-reactive (GEM) T cells,
which recognize glycolipids with respect to CD1d and CD1b,
respectively (6,7). Other less well-characterized
molecules are also known to recognize lipids that are presented by
CD1a and CD1c molecules. In contrast, γδ TCRs are mainly expressed
on the surfaces of γδ T cells, which comprise minority of the T
cell pool but may represent more than half of the total T cells in
tissues such as the gut and skin. In contrast to αβ TCRs, γδ TCRs
recognize lipid-based antigens that are presented by CD1
molecules.
Regarding the functions of these two T-cell subsets,
αβ T cells play an important role in protective immunity against
various antigens, whereas γδ T cells were previously considered to
be a necessary component of innate immunity, the term ‘T cell’
generally refers to αβ T cells. However, in recent years, they have
been considered to share important characteristics of both the
innate and adaptive immune responses and have garnered increasing
attention in the field of immunotherapy because of their prominent
functions and direct or indirect participation in disease recovery
(8).
Abundant TCR diversity is a prerequisite for broad
immunological recognition, and antigen-specific TCRs are an
important step in TCR gene transduction-based immunotherapy.
Therefore, a clear understanding of TCR structure and diversity
generation represents an elementary step in determining the best
incorporation of αβ TCRs in disease treatment strategies. Moreover,
knowledge regarding TCR bias in different diseases could promote a
good prognosis in patients treated via gene transfer. Accordingly,
in the present review, we briefly introduce background information
regarding TCR structure and diversity. In addition, we summarize
recent data concerning TCR selection bias in some diseases, which
might serve as a theoretical basis for clinical applications, and
thus, further illuminate potential functions in disease
treatment.
2. TCR diversity and antigen
recognition
The highly variable TCR comprises a number of
segments that are encoded by genes at discrete chromosomal
locations: the α and γ glycoprotein chains, which comprise V
(variable), J (joining) and C (constant) regions and the β and δ
chains, which feature an additional D (diversity) region (9). The re-arrangement of V (D) and J
through recombination signal sequences (RSS) and the formation of a
functional antigen receptor via the activity of the
lymphoid-specific protein recombination activating gene (RAG) 1 and
RAG 2 exclusively occurs during thymocyte development (Fig. 1). This process involves the random
re-arrangement of various V and J genes at the TCR-α locus and V, D
and J genes at the TCR-β locus during T-cell development,
additional diversity is produced by the random insertion or
deletion of >20 non-germline nucleotides at region junctions
(V-(N)-J, V-(N)-D and/or D-(N)-J; N represents nucleotide), thus,
yielding a TCR recognition spectrum with an estimated theoretical
diversity of ~1018 in human (10) and 1015 in mouse
(2) although most of those
specificities will never be used during an individual's life, as
peripheral repertoire in human are composed of 25×106
clonotypes (11) and
2×106 in mouse (1).
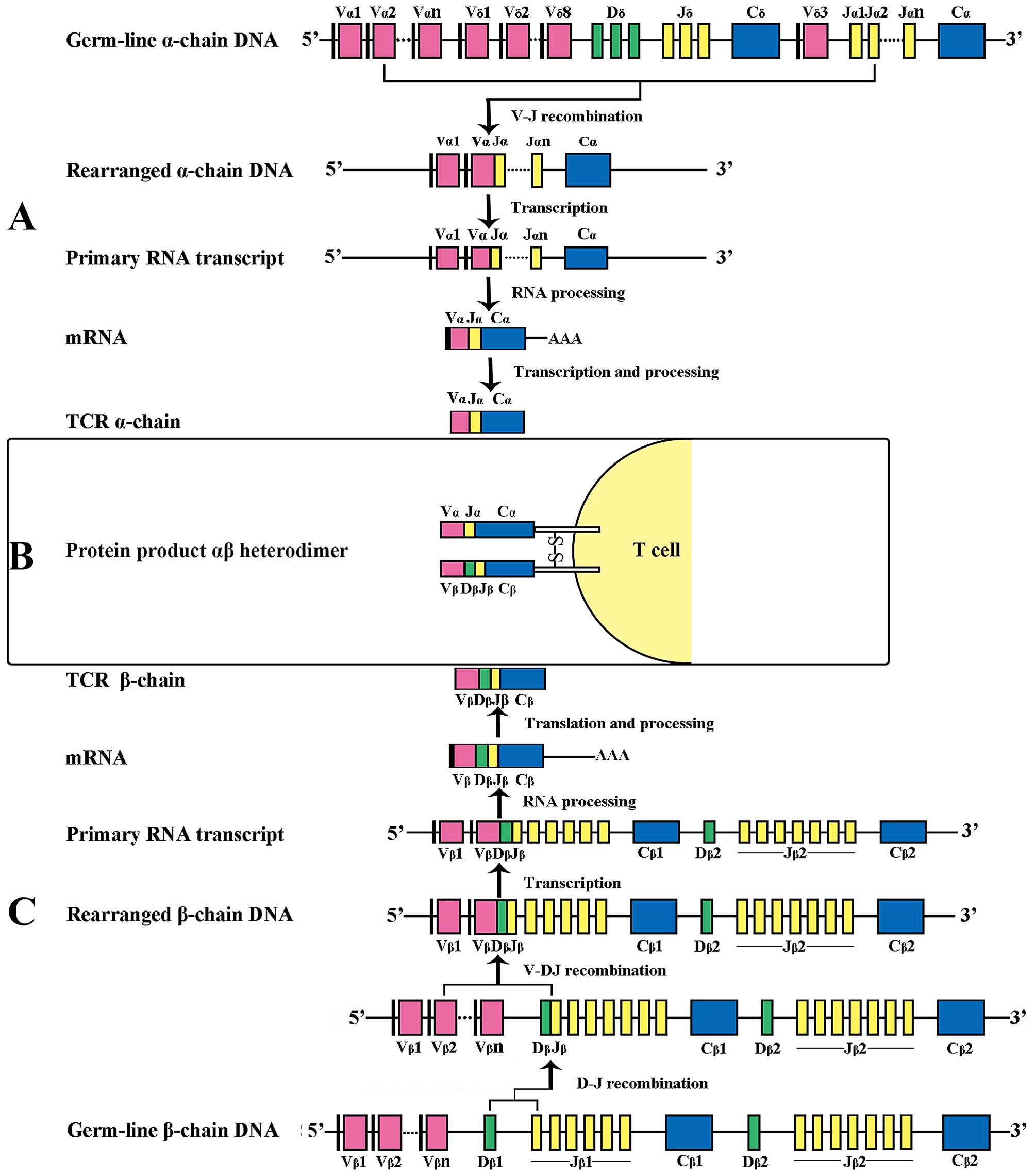 | Figure 1Examples of human TCR gene
rearrangement, forming the functional gene encoding the αβ
heterodimer on the surface of T cells. (A) V-J recombination of the
TCR-α chain DNA. The α-chain DNA (in which the TCR-δ locus is also
embedded), similar to the light-chain (L-chain) DNA of
immunoglobulin, undergoes V-J recombination, brings together one of
46 TRAV segments and one of 51 TRAJ segments. The TCR-α transcript
produced where V, J and C segments connect directly after the
intron sequences are spliced out. (B) Heterodimer structure of
αβ-TCR on the surface of T lymphocytes. (C) V-D-J recombination of
the TCR-β chain DNA. The β-chain DNA is analogous to the heavy
chain (H-chain) DNA of immunoglobulin, undergoes two-step
recombination: first Dβ to Jβ and then Vβ to Dβ-Jβ rearrangement.
The intervening sequences are then cut off, generating the TCR-β
chain transcript with V, D, J and C region adjacent. The leader
sequence is removed from the nascent peptide chain. TCR, T cell
receptor. |
Three defined TCR hypervariable regions (CDR1, CDR2
and CDR3) combine to form the TCR antigen-binding site.
Crystallographic analysis has revealed that CDR1 and CDR2 interact
with a particular MHC molecule, whereas CDR3 interacts with the
antigenic peptide bound to the MHC molecule (12,13).
Most variation in each chain lies within CDR3, which is responsible
for the specific recognition of and interaction with peptide
antigens that are presented by MHC molecules. As this region
determines the antigen specificity of a T cell, each CDR3
represents a single T-cell clone (14). CDR1α, CDR1β, CDR2α and CDR2β are
entirely encoded by the V region in germline DNA segments, whereas
CDR3 loops are encoded by the V (D) J junction and the N additions
and deletions during the recombination process; relative to CDR1
and CDR2 loops, CDR3 loops are significantly more diverse, and
thus, mediate contact between TCR and the antigenic peptide-MHC
complex. In addition, the D segment insertion occurs in CDR3 of the
TRB/TRD locus, thus, yielding a broader TRB repertoire for antigen
recognition compared with TRA and TRG. Whereas the CDR3 loop size
distributions of the IgH and L chains and TCR γ and δ chains are
relatively broad and dissimilar, in contrast, the CDR3 loop size
distribution of the α and β chain are narrow and closely matched,
suggesting that a pairing of TCR α and β chains with similar CDR3
loop sizes might be generally required to generate a functional αβ
TCR repertoire (14,15). Furthermore, the greater diversity
of the CDR3β loop (relative to the CDR3α loop) might indicate that
the TCR Vβ chain is the main factor in determining TCR usage bias
(16).
As each CDR3 sequence represents one T cell clone,
the polyclonal or oligoclonal expansion of T cells can be
determined through the detection of CDR3 spectratype by various
methods such as PCR DNA blotting, PCR GeneScan sequence analysis,
and high throughput TCR sequencing (TCR-seq) (17). The biased TCR AV and BV gene
families are considered to be antigen-specific and can be used in
immunotherapy.
3. TCR bias in disease
TCR diversity largely remains constant throughout
the life span, except in infants and the elderly, because most
specificity is generated in the thymus. However, under conditions
of antigen exposure, the expansion of specific clonotypes is a
common feature of immunity (Table
I). Antigen-specific TCRs will demonstrate a preferential usage
of particular TCR variable region gene segments, resulting in a TCR
spectrum with a ‘skewed’, ‘immunodominant’, ‘restricted’ or
‘limited’ distribution, known as a ‘biased’ repertoire (16). TCR bias can be mainly classified
into three types. Type 1 bias is characterised by selecting a
single TCR gene family but with obvious diversity in the CDR3
sequences of different individual clones. Type 2 bias refers to the
selection of the same amino acid motif in the CDR3 region of an
antigen-specific TCR. Type 3 bias indicates a similar spectrum
sequence, with differences resulting from different α and β chain
pairings. Several factors can influence TCR bias, including thymic
selection, the initial immune response, persistent infection and
selection of a ‘private’ versus ‘public’ TCR (16).
 | Table IExamples of TCR bias in disease. |
Table I
Examples of TCR bias in disease.
| Diseases | Antigen/antigen
peptide | MHC
restriction | Source of T
cell | Priority of TCR
genes | Ref. |
|---|
|
|---|
| TRAV | TRBV |
|---|
| Tumor | | | | | | |
| B-CLL | | | CD4 | | 2, 3, 5, 6 | (19) |
| | | CD8 | | 6, 19 | (19) |
| | | PB | | 5 | (25) |
| Melanoma | | | PB | | 6 | (21) |
| DLBCL | | | CD4 | 6, 23 | 3, 13 | (28) |
| | | PB, TIL | | 13, 23 | (29) |
| CRC | | | CD4 | | 12, 16, | (31) |
| | | CD8 | | 19, 21 | (31) |
| | | PB, TIL | | 12, 16, 19, 21 | (30) |
| Infectious
disease | | | | | | |
| EBV | EBNA3(339–347) | HLA-B*0801 | | 26-2 | 7 | (35,43) |
| EBNA1(407–417) | HLA-B*3508 | | 20, 29 | 9 | (44) |
| | HLA-B*3501 | | | | |
| BZLF1(52–64) | HLA-B*3508 | | 1 | 10 | (45) |
| Influenza
virus | Matrix
protein(58–66) | HLA-A2 | | | 19 | (48) |
| HIV | Gag p17(77–85) | HLA-A2 | PB, CD8 | | 5–6 | (47) |
| Env | | | | 23-1 | (36) |
| p24 capsid | HLA-B*5703 | | 5 | 19 | (39) |
| SIV | Tat(28–35) | Mamu-A*01 | PB, CD8 | 22 | 6-5, 14 | (37) |
| Gag(181–189) | Mamu-A*01 | | | 13 | (37) |
| Tuberculosis | | | | 11/3 | 8 | (54) |
| Malaria | | | | | 8.1, 8.2 | (56) |
| HCMV | pp65(495–503) | HLA-A2 | PB, CD8 | 18 | 6-5, 12-4 | (38) |
| Autoimmune
disease | | | | | | |
| SLE | | | PBMC | | 1, 13-1, 15,
16 | (60,62) |
| | | Kidney | | 8, 20 | (58) |
| | | Skin | | 8, 13 | (59) |
| | | Th lines | 8 | | (61) |
| T1D | | | Pancreatic
islets | | 1, 7, 11, 17,
22 | (64) |
| | | Spleen, PB | | 22 | (63) |
Tumor antigens
T lymphocytes play an effective role in antitumor
immune responses (18). Various
tumor-associated antigens (TAAs) that are used to discriminate
between healthy and malignant tissue have been identified. In
addition, antitumor cytotoxic T cells (CTLs), which exist within
tumor-infiltrating lymphocyte (TIL) populations and the peripheral
blood and generally comprise αβ T cells, have been detected in
patients with cancer (19). The
overexpression of a restricted TCR gene is a common characteristic
of TILs. These proliferating monoclonal T cells, which play a basic
role in tumor-specific cellular immune therapy, can be detected and
separated using a gene re-arrangement analysis technology.
In some patients with melanoma, the number of T-cell
clones of the same TRBV subfamily accounted for >65% of TILs
(20,21), and TRBV14 was overexpressed in all
patients who were human leukocyte antigen (HLA)-A2 positive but not
in patients who were HLA-A2 negative (22,23).
In 1994, Puisieux et al (24) observed that clonally proliferating
TCR Vα and TCR Vβ tumor-specific CTLs could kill autologous tumor
cells and subsequently transform into specific T-cell clones
following isolation and in vitro cultivation. Monoclonal and
oligoclonal expansions have been reported in patients with B-cell
malignancies (19), and in B-cell
chronic lymphocytic leukaemia (B-CLL), an oligoclonally expanded
BV19+CD8+ T-cell subset has been demonstrated
to specifically react with autologous leukemic B cells (25). TRBV overexpression is more
frequently observed in CD4+ T cells than in
CD8+ T cells from patients with B-CLL (23), suggesting that leukemic B cells
present MHC class II-restricted peptides to CD4+ T
cells.
Specific T cells from some patients with myeloma can
recognize tumor-derived idiotypic immunoglobulin, and patients with
these idiotype-reactive T cells are likely to have a better
prognosis (26). No change in TRBV
usage was detected between T cells that were cultured in a culture
medium alone and with phytohemagglutinin stimulation, suggesting
that the tumor-associated antigen-mediated selection of monoclonal
and oligoclonal TCR families from stimulated T cells were indeed
tumor specific (27). In addition,
the clonal expansion of TRBV13 and TRBV23 in CD4+ T
cells was identified in patients with diffuse large B-cell lymphoma
(DLBCL) (28). Tan et al
(29) conducted RT-PCR and
GeneScan analysis of the CDR3 repertoires of 29 TRAV and 24 TRBV
gene families in PBMCs from patients with DLBCL and identified TRAV
and TRBV gene bias.
Using a DNA melting curve method, Zhou et al
(30) analysed the CDR3
spectratypes of peripheral blood and TIL from several patients with
colorectal cancer (CRC). With respect to the response to CRC
antigens, the prominent usage of TRBV16 was observed in peripheral
blood T cells, and limited expression of the BV12, 19 and 21 gene
families was detected; TILs from patients with CRC exhibited the
same phenomena. Although no significant difference was observed
between PBMCs and TILs, they found that the ratio of skewed TRBV
gene families was higher among TILs than among PBMCs. Although
dominant TCR repertoire usage varied among individuals, there is no
lack of the same motif, which might imply that different patients
can react to the same tumor antigen. Of greater interest is the
dissimilarity in CDR3 gene sequences of PBMCs and TILs from the
same patients (31). It remains
unknown whether the same CDR3 motifs signify PBMCs and TILs that
have proliferated in response to the same peptide antigen and
whether proliferated PBMCs and/or TILs play a primary role in
patients with CRC (30).
Pathogenic infections
Viral infection
Preferential selection of T-cell clones can
contribute to the narrowing of an antigen-selected TCR repertoire
(32), and such limited TCR
repertoires have been observed in some CD8+ T-cell
subpopulations (33,34). An increasing number of examples
demonstrate the existence of TCR bias in antiviral immunity, and
those restricted Vα and Vβ families expression and conserved CDR3
motifs are considered to be immune responders to definitive
antigens. Such TCR bias has been observed in response to persistent
infections with entities, such as the Epstein-Barr virus (EBV)
(35), human immunodeficiency
virus (HIV) (36), SIV (37), cytomegalovirus (CMV) (38) and influenza (39). However, this effect of selection is
less apparent in the context of non-persistent antigens (16,34).
Studies of EBV and CMV infection have demonstrated that TCR bias
can result from the prior selection of T cells with high-affinity
TCRs (38,40); virus-specific memory
CD8+ T cells have been detected in vitro
(41).
EBV is a persistent entity that has infected >90%
of the population; in this context, biased TCR usage is mainly
directed against several EBV antigens (42), and most restricted TCR family
members exhibit sequence conservation according to specific antigen
epitopes. EBNA1, EBNA3 and BZLF1 are three antigens for which
structural data exist (43–45).
Furthermore, in SIV-infected rhesus macaques, specific clonotypes
are associated with protection, and a number of common clonotypes
are closely associated with the viral load. Turner et al
(16) considered three factors
that might determine the constraint of a TCR repertoire during
consistent infection: recognition of the peptide-MHC complex,
antigen load (higher antigen load = narrower TCR repertoire) and
TCR diversity in the naïve pool. In patients with HIV, three
so-called ‘protective MHC alleles’ have been identified: HLA-B*57,
HLA-B*27 and HLA-B*58. These specific clonotypes are associated
with protection and can predict disease prognosis (46,47).
CD8+ T cells are the main population of
immune cells that directly act against the pp65 protein (ALV/A2,
495–503) of HCLV, an immunodominant HLA-A2-restricted epitope.
ALV/A2-specific T cells exhibited limited clonal diversity and
restricted usage of the Vβ regions. The expansion of
ALV/A2-specific T cells were observed in response to HCMV
activation and/or chronic inflammation, resulting in the selection
of a single clonotype with similar public TCR features. High
antigen avidity was also observed with ALV/A2-specific clonotypes,
further proving that TCR avidity/affinity is the main concern with
regard to persistent antigenic stimulation.
During the acute phase of SIV infection,
CD8+ T cells respond to the TL8 (TTPESANL; Tat, residues
28–35) and CM9 (CTPYDINQM; Gag, residues 181–189) epitopes that are
bound to codominant Mamu-A*01. Although TL8 and CM9 exhibit the
same MHC class I restriction, they are associated with different
patterns of conserved TRBV gene usage. Whereas TRBV13 usage was
restricted in a majority of CM9-specific CD8+ T cells,
TRBV14 usage was predominantly observed in TL8-specific
CD8+ T cells. A highly conserved CDR3 motif
(CASSXXRXSNQPQY) and preferential TRBJ1.5 usage were prevalent
among TL8-specific clonotypes. In contrast, no such consensus was
evident among CM9-specific CD8+ T cells. Furthermore,
TL8-specific subtypes preferred a specific TRAV22 (37).
Conserved usage of the Vα and Vβ sequences were also
observed in T cells that were specific for the influenza matrix
peptide (58–66) (47,48).
In addition, the KF11 peptide (KAFSPEVIPMF) is among the
immunodominant epitopes that are derived from the p24 capsid
peptide of HIV (39,42). A biased TCR repertoire displaying
TRAV5 and TRBV19 and sharing common CDR3α and CDR3β sequences was
selected.
Tuberculosis
Tuberculosis (TB), which is caused by
Mycobacterium tuberculosis, is a well-known common
infectious disease (49). As M.
tuberculosis is an intracellular bacterium, cell-mediated
immunity acts as the driving force during bacterial elimination; in
other words, CD4+ and CD8+ T cells play
important prophylactic roles against TB (50). The clonal expansion of
CD8+ T cells in granuloma lesions and PBMCs has been
observed (51,52). In the infected lung,
CD4+ T cells have been observed to secrete interferon
gamma (IFN-γ) and tumor necrosis factor alpha (TNF-α), both of
which recruit and activate infected macrophages, thereby killing
intracellular TB (53). Similarly,
CD8+ T cells also produce IFN-γ and TNF-α and mediate
the cytolysis of infected macrophages; in addition, CD8+
T cells can kill M. tuberculosis within macrophages via
granule-dependent mechanisms (54). As the CD4+ and
CD8+ T cells of patients with TB exhibit decreased
levels of functionality, promising therapeutic methods involve
promoting an increase in T-cell functionality. Luo et al
(54) screened TCR Vα11 and Vβ8
expression in CD4+ T cells and TCR Vα3 and Vβ8
expression in CD8+ T cells because these are specific
for a 38-kDa antigen of M. tuberculosis; following
transduction into primary CD4+ and CD8+ T
cells, these specific genes induced anti-M. tuberculosis
activity. Furthermore, the CDR3 spectratypes of two T cell
subpopulations were also analysed in 86 patients with different
degrees of TB (55), and patients
with TB were found to possess a restricted TCR repertoire when
compared with healthy controls. A sequence analysis of CDR3 in
clonally expanded T cells revealed a highly conserved amino acid
motif in both the TCR α and β chains. Severely infected patients
exhibited less TCR diversity than did patients with mild disease,
suggesting that TCR abundance negatively correlates with disease
severity. Furthermore, CDR3 sequence conservation was found to be
unrelated to the HLA phenotype; in other words, despite differences
in individual HLA phenotypes, common TCR structural features may be
observed in associated T cells.
Malaria
Among mice infected with cerebral malaria (CM),
those lacking either the αβ or γδ T cells (TCRαβ−/− or
TCRγδ−/−) differently manifested the disease.
TCRγδ−/− mice were susceptible to CM, whereas
TCRαβ−/− mice were resistant. Flow cytometric analysis
of peripheral blood lymphocytes revealed that a subset of
CD8+ T cells bearing the Vβ8.1 and 2 segments was
associated with disease severity (56).
Autoimmune disease
Autoimmune diseases are characterised by tissue
damage resulting from an abnormal immune response toward
autoantigens. During normal development, positive and negative
thymic selection exclude T cells with a low or high affinity for
self-MHC; under normal circumstances, the remaining TCRs with
moderate self-affinity will not lead to autoimmune disease because
of the inhibitory effects of regulatory T cells (Tregs) (57). However, under conditions of genetic
vulnerability, many environmental factors can lead to the
production of inflammatory cytokines and autoantibodies, which in
turn can facilitate disease episodes. Abnormalities in humoral and
cellular immunity are likely to be involved in autoimmune disease.
Although autoantibodies, which are produced by the humoral immune
system, target autoantigens, more recent reports demonstrate the
contribution of abnormal T-cell proliferation to the secretion of
these aberrant antibodies. As a result, studies concerning abnormal
T cell activation address not only the cellular immune component of
autoimmunity but also the humoral component.
In patients with SLE, limited expansion of T cells
of the TCRβ gene family has been observed in the kidney (58), damaged areas of the skin (59) and peripheral blood (60–62).
A conserved CDR3 amino acid motif (GGX) has also been detected in
PBMCs from 20 patients with SLE. In human patients with type 1
diabetes (T1D), T cells that infiltrate the pancreatic islets
mainly express TCRs of the BV gene family, with monoclonal
expansion of T cells expressing Vβ1, Vβ7, Vβ11, Vβ17 and Vβ22. A
monoclonal expansion of cells expressing Vβ22 with a conserved CDR3
sequence was also observed in the peripheral blood, indicating the
homing of circulating cells (63).
Zhou et al (64) also
analysed αβ TCR distributions in the peripheral blood of patients
with T1D, which may be relevant to the onset.
Furthermore, TCR plays a key role in T cell-mediated
transplant rejection. Kim et al (65) analysed the TCRβ chain variable gene
usage bias in the graft-infiltrating CD8+ T lymphocytes
in a human transplanted hand over 178 days, they found the TCR
variable gene usage and size distribution revealed the
oligoclonality expansion of some TCR gene families. These
restricted TCR BV gene are likely to be associated with graft
rejection. Aberrant TCR activity has been found to be associated
with drug-linked hypersensitivity. Changes in TCR repertoire during
treatment correspond with the patients' levels of morbidity
(66), thus, a diverse TCR
repertoire indicates a healthy condition or good disease
outcome.
The tracking of antigen-specific TCRs related to
tumors and various pathogenic infections will facilitate an
understanding of disease progression. For example, in patients
expressing HLA-A2, the CDR3 regions of T cells expressing Vβ17
share the same unique sequence; in contrast, this sequence is
difficult to detect in healthy individuals. Another example is
TCR-β oligoclonality with respect to autologous stem cell
transplantation. Given the differences in TCR repertoire
distribution between a healthy and diseased state, oligoclonality
can be used as a marker of the degree of tumor cell elimination and
the extent of immune reconstitution, and thus, may facilitate the
evaluation of treatment efficiency (49). In patients with colorectal cancer,
chemotherapy gradually increases the TCR diversity, suggesting
favourable prognosis (66).
Treating metastatic CRC with bevacizumab combined with modified
irinotecan, fluorouracil and leucovorin (mIFL) results in a more
normal TCR repertoire than does treatment with mIFL alone (67). All of these changes in repertoire
prior to disease onset and after treatment may reflect a
relationship between TCR repertoire normalization and relief from
disease, and therefore, might indicate the potential diagnostic
value of this parameter in a clinical setting (66).
4. Antigen-specific TCR gene
transduction
In addition to the various disease treatments that
have already been applied in the clinic, including tyrosine
inhibitors and monoclonal antibody targeting of tumor-associated
surface antigens, and potential suppression of antitumor immune
responses, cytokines represent another form of immunotherapy that
can modify the outcomes of innate or adaptive immunity, antitumor
vaccines and adaptive gene therapies (68–78).
However, the increased toxicity resulting from cytokine therapy can
induce lymphocyte degeneration, reduce TCR repertoire diversity and
increase susceptibility to opportunistic pathogens (79). To date, there are three kind of
therapies: tumor-infiltrating lymphocytes (CILs), chimeric antigen
receptors (CARs) and T cell receptor engineered T cells (80). This kind of transfer of lymphocytes
to mediate an effector function is known as adaptive cell transfer
(ACT). TILs have been developed with slow but continuing progress
(80). Antigen-associated
monoclonal TCR Vα and Vβ gene families can be defined according to
the length and sequence of the CDR3 region. In vitro
experiments have confirmed that following efficient separation and
amplification, these specific TCR genes can be incorporated into
antitumor or antiviral disease therapy. However, it is difficult to
gain a sufficient number of CTLs from the patients' T cell
populations, thus, presenting a challenge to the application of
this technique. In addition to the limited number of CTLs,
immunosuppressive factors might help to downregulate effector
T-cell functions. Accordingly, it would be meaningful to study the
features and amplification methods that are associated with
tumor-specific CTLs because these will play pivotal roles in
antitumor adoptive immunotherapy.
All of these factors increase the attractiveness of
T cell transduction-based immunotherapy, particularly with regard
to long-term survival. The endowment of T cells with high-affinity
antigen specificity via TCR transduction has highlighted the
growing importance of this effective method with respect to the
control and elimination of malignant cells and infectious
agents.
Mainstream research has focused on the transduction
of tumor or viral antigen-related TCR genes into normal T cells,
including mandatory expression of the idiotypic TCR; as a result,
the recognition patterns of endogenous TCRs are changed, leading to
an increase in cytokine secretion and lethality after a large
amplification, an immune response and the generation of the
adequate numbers of specific CTLs during treatment. During this
process, the TCR repertoires are dynamically monitored before, and
for several cycles after therapy, by analysing the CDR3 length
distributions within CD4+ and CD8+ T cell
subsets. Recently, an in vitro expansion of tumor-specific
CTLs was applied with clinical results. These TCR gene-engineered T
cells were first used in the clinical treatment of melanoma. The
introduction of a MART-1 antigen specific TCR gene into the
peripheral blood lymphocytes of patients with melanoma may restore
the capacity to resist positive tumor-surface antigens and might
completely alter the disease prognosis.
Two methods can facilitate the transfer of exogenous
genes into cells: viral vectors or chemical/physical methods
(81). Viral vectors like gamma
retrovirus and lentivirus enable efficient gene transfer with the
least amount of damage; however, viral vectors are expensive, and
the process represents a waste of time and resources. In addition,
viral vectors cannot deliver large DNA fragments because of package
capacity limitations. Furthermore, the potential for widespread
viral contamination limits the application of this technique. In
comparison, although physical methods, such as electroporation, can
induce more cell death, these methods are faster, easier and safer
methods of gene transfection, which explains their prominence. In
recent years, a more advanced electroporation-based gene transfer
method involving nucleofection has been introduced; this method
transfers the gene of interest into the nuclei of non-dividing
cells, with an high transfection efficiency.
In 1999, CD8+ T cells that were
genetically engineered to express the melanoma MART-1-restricted
TCRα/β gene exhibited cytotoxicity against HLA-A2-restricted
melanoma cells (82). In 2006, the
first clinical trial was conducted (83); condition of 2 patients was
completely restrained in 15 patients, win 18 months life time
without this disease. Many examples of immunotherapy have been
reported. The transduction of an HIV-specific TCR gene into
patients with HIV-1+ can enhance immunity. In addition,
Tang et al (84) recently
reviewed the mechanism by which the tumor microenvironment (TME)
affects the efficiency of immunotherapy and how to best use TME as
a contributor to immunotherapy. However, this kind of introduction
of exogenous TCR gene may lead to mispairing of the endogenous TCR
subunits with introduced TCR chain, which may alter the TCR
specificity of antigen recognition, not only lead to the loss of
antitumor, but also the formation of autoreactive T cells (85–87).
Hu et al (91) have shown
the humanized mouse (hu-mouse) model which transplantation of human
fetal thymus tissue and CD34+ fetal liver cells into
immunodeficient mice, leading to the development of human
lymphohematopoietic cells and the formation of secondary lymphoid
organs (88–90). They used this hu-mouse model to
generate melanoma antigen (MART-1) specific human T cells for
studies of human cancer immunotherapy. They found that MART-1
specific T cells can be generated after transduction of
lentiviruses containing MART-1 specific TCR gene, implying that it
was possible to produce large quantity of MART-1 specific T cells
with antitumor activity.
The third and a powerful new immunotherapeutic
approach intended to target TAAs comprises chimeric antigen
receptors (CARs), which comprise an extracellular region - the
antigen-binding domain of a monoclonal antibody (containing the
variable domains of the light and heavy chains), a stem-like
region, a transmembrane region and the intracellular signalling
portion of a T-cell receptor, can combine the specificity and
effectiveness of a monoclonal antibody with the cytotoxic effects
and long-term persistence of CTLs (92), thus, it can make it possible to
endow immune system with reactivity and add benefits which can be
seen with cytotoxic chemotherapy or targeted therapy of the rapid
onset of action. This method has been proven safe and effective for
the induction of tumor remission in patients with neuroblastoma
(93) and hematological
malignancies (94). To date, the
most successful CARs were those specific for CD19 on B cell
malignances (92). Several
research centres have confirmed the compelling efficacy of CARs
against acute and chronic B-cell malignancies (95).
Compared with TCRs, there are several different
parameters. First of all, these two format receptors target
different antigens, TCRs react to natural ligands with MHC
restriction, and it is necessary to enhance the affinity of the TCR
with specific peptide-MHC in order to mediate the activities of T
cells against the peptide-MHC antigen during the process of
adoptive T cell therapy. In contrast, CARs are not MHC-dependent,
and can function both in CD4+ and CD8+ T
cells. Furthermore, CARs can induce activity even against
low-density antigens. The CAR density, antigen-binding domain
affinity still need to be optimized to match the antigen density of
tumor and tissue (92). However,
risk also exits because both on-target and off-target recognition
of normal tissue occur in engineered T cells. For instance, in
patients treated with carcino-embryonic antigen specific TCR
modified T cells showed on-target toxicity, resulting in
inflammatory colitis in normal colon (96). Apart from toxicity, both TCR and
CARs will give rise to autoimmunity and inflammation from the
infusion of ex vivo activated autologous lymphocytes.
However, the incidence can be calculated to be less than one in
1,000 patients, which is lower than cytotoxic chemotherapy
(97). A common challenge to all
therapies, including antigen specific TCR gene based therapy, is to
develop cost-effective, and efficient manufacturing and delivery
capabilities (80).
5. Conclusions
Gene re-arrangement during the thymic development of
T cells introduces sufficient TCR diversity to meet the varied
requirements for antigen recognition. Over the last two decades,
considerable research has demonstrated a close relationship between
TCR diversity and immune responses against various antigens. Under
natural conditions, the TCR repertoire is diverse and exhibits a
Gaussian distribution, and the CDR3 of each gene family is
represented by at least eight different lengths. As each CDR3
sequence represents a specific T-cell clone, CDR3 region
spectratyping can reflect TCR clonality, and antigen-specific TCRs
can be selected for immunotherapeutic use. A basic understanding of
the T-cell receptor structure and recombination mechanisms can help
us to understand the aetiology of TCR diversity and thus identify
antigens.
An analysis of TCR bias in tumor, pathogenic
infection, autoimmune disease and other disease settings is a basic
prerequisite to a deep understanding of the immune mechanisms
underlying antigen-specific responses and specific immunotherapy.
Many studies have described how TCR abundance might be affected by
certain infections, malignancies and immune dysfunctions. A broader
specific peptide-MHC complex repertoire can help to reduce the
emergence of a persistent viral infection. Three factors, thymic
selection, initial immune response and persistent infection, are
all known to influence specific TCR selection. In addition, some
pathologies can induce the appearance of specific TCR clonotypes,
which might be useful as immunological markers in clinical
settings.
An understanding of T-cell responses against
specific antigens is particularly important with respect to
adaptive immunity. The immunotherapeutic role of αβ T cells has
been strongly focused on their function in recovery from disease.
In contrast, how would one capture the ideal TCR? It is necessary
to monitor CDR3 spectratypes and conserved amino acid motifs. In
addition to several above mentioned common methods, nascent
high-throughput sequencing (TCR-seq) using next-generation
sequencing also provides new insights to the sequence analysis of
TCR repertoires under conditions of health and disease. The
transduction of specific TCR genes with conserved CDR3 motifs into
normal T cells can alter the original identification model and form
antigen-specific T cells; this approach to disease treatment has
yielded results that could further enhance the likelihood of
survival. However, new strategies and methods still need be
developed to enhance specific recognition between TCRs and
antigens, with the aim of improving immunotherapeutic approaches to
persistent infection and cancer.
Acknowledgements
The authors appreciate the support of the National
Natural Science Funds (nos. 31372423 and 31302072) from the
National Natural Science Foundation of China.
References
|
1
|
Casrouge A, Beaudoing E, Dalle S,
Pannetier C, Kanellopoulos J and Kourilsky P: Size estimate of the
alpha beta TCR repertoire of naive mouse splenocytes. J Immunol.
164:5782–5787. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Davis MM and Bjorkman PJ: T-cell antigen
receptor genes and T-cell recognition. Nature. 334:395–402. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jorgensen JL, Reay PA, Ehrich EW and Davis
MM: Molecular components of T-cell recognition. Annu Rev Immunol.
10:835–873. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Treiner E, Duban L, Bahram S,
Radosavljevic M, Wanner V, Tilloy F, Affaticati P, Gilfillan S and
Lantz O: Selection of evolutionarily conserved mucosal-associated
invariant T cells by MR1. Nature. 422:164–169. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Le Bourhis L, Martin E, Péguillet I,
Guihot A, Froux N, Coré M, Lévy E, Dusseaux M, Meyssonnier V,
Premel V, et al: Antimicrobial activity of mucosal-associated
invariant T cells. Nat Immunol. 11:701–708. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Van Rhijn I, Kasmar A, de Jong A, Gras S,
Bhati M, Doorenspleet ME, de Vries N, Godfrey DI, Altman JD, de
Jager W, et al: A conserved human T cell population targets
mycobacterial antigens presented by CD1b. Nat Immunol. 14:706–713.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Beckman EM, Porcelli SA, Morita CT, Behar
SM, Furlong ST and Brenner MB: Recognition of a lipid antigen by
CD1-restricted αβ+ T cells. Nature. 372:691–694. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Latha TS, Reddy MC, Durbaka PV, Rachamallu
A, Pallu R and Lomada D: γδ T Cell-mediated immune responses in
disease and therapy. Front Immunol. 5:5712014. View Article : Google Scholar
|
|
9
|
Caccia N, Bruns GA, Kirsch IR, Hollis GF,
Bertness V and Mak TW: T cell receptor alpha chain genes are
located on chromosome 14 at 14q11-14q12 in humans. J Exp Med.
161:1255–1260. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sewell AK: Why must T cells be
cross-reactive? Nat Rev Immunol. 12:669–677. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Arstila TP, Casrouge A, Baron V, Even J,
Kanellopoulos J and Kourilsky P: A direct estimate of the human
alphabeta T cell receptor diversity. Science. 286:958–961. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bentley GA, Boulot G, Karjalainen K and
Mariuzza RA: Crystal structure of the beta chain of a T cell
antigen receptor. Science. 267:1984–1987. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fields BA, Ober B, Malchiodi EL, Lebedeva
MI, Braden BC, Ysern X, Kim JK, Shao X, Ward ES and Mariuzza RA:
Crystal structure of the V α domain of a T cell antigen receptor.
Science. 270:1821–1824. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pannetier C, Cochet M, Darche S, Casrouge
A, Zöller M and Kourilsky P: The sizes of the CDR3 hypervariable
regions of the murine T-cell receptor beta chains vary as a
function of the recombined germ-line segments. Proc Natl Acad Sci
USA. 90:4319–4323. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rock EP, Sibbald PR, Davis MM and Chien
YH: CDR3 length in antigen-specific immune receptors. J Exp Med.
179:323–328. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Turner SJ, Doherty PC, McCluskey J and
Rossjohn J: Structural determinants of T-cell receptor bias in
immunity. Nat Rev Immunol. 6:883–894. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Woodsworth DJ, Castellarin M and Holt RA:
Sequence analysis of T-cell repertoires in health and disease.
Genome Med. 5:982013. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Imai N, Ikeda H, Tawara I and Shiku H:
Tumor progression inhibits the induction of multifunctionality in
adoptively transferred tumor-specific CD8+ T cells. Eur
J Immunol. 39:241–253. 2009. View Article : Google Scholar
|
|
19
|
Rezvany MR, Jeddi-Tehrani M, Osterborg A,
Kimby E, Wigzell H and Mellstedt H: Oligoclonal TCRBV gene usage in
B-cell chronic lymphocytic leukemia: Major perturbations are
preferentially seen within the CD4 T-cell subset. Blood.
94:1063–1069. 1999.PubMed/NCBI
|
|
20
|
Lake DF, Salgaller ML, van der Bruggen P,
Bernstein RM and Marchalonis JJ: Construction and binding analysis
of recombinant single-chain TCR derived from tumor-infiltrating
lymphocytes and a cytotoxic T lymphocyte clone directed against
MAGE-1. Int Immunol. 11:745–751. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Farina C, van der Bruggen P, Boël P,
Parmiani G, Sensi M and Moretta L: Conserved TCR usage by HLA-Cw*
1601-restricted T cell clones recognizing melanoma antigens. Int
Immunol. 8:1463–1466. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Boon T, Gajewski TF and Coulie PG: From
defined human tumor antigens to effective immunization? Immunol
Today. 16:334–336. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Salvi S, Segalla F, Rao S, Arienti F,
Sartori M, Bratina G, Caronni E, Anichini A, Clemente C, Parmiani
G, et al: Overexpression of the T-cell receptor beta-chain variable
region TCRBV14 in HLA-A2-matched primary human melanomas. Cancer
Res. 55:3374–3379. 1995.PubMed/NCBI
|
|
24
|
Puisieux I, Even J, Pannetier C, Jotereau
F, Favrot M and Kourilsky P: Oligoclonality of tumor-infiltrating
lymphocytes from human melanomas. J Immunol. 153:2807–2818.
1994.PubMed/NCBI
|
|
25
|
Farace F, Orlanducci F, Dietrich PY,
Gaudin C, Angevin E, Courtier MH, Bayle C, Hercend T and Triebel F:
T cell repertoire in patients with B chronic lymphocytic leukemia.
Evidence for multiple in vivo T cell clonal expansions. J Immunol.
153:4281–4290. 1994.PubMed/NCBI
|
|
26
|
Brown RD, Yuen E, Nelson M, Gibson J and
Joshua D: The prognostic significance of T cell receptor beta gene
rearrangements and idiotype-reactive T cells in multiple myeloma.
Leukemia. 11:1312–1317. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rezvany MR, Jeddi-Tehrani M, Wigzell H,
Österborg A and Mellstedt H: Leukemia-associated monoclonal and
oligoclonal TCR-BV use in patients with B-cell chronic lymphocytic
leukemia. Blood. 101:1063–1070. 2003. View Article : Google Scholar
|
|
28
|
Li H, Ma X, Moskovits T, Inghirami G and
Tsiagbe VK: Identification of oligoclonal CD4 T cells in diffuse
large B cell lymphomas. Clin Immunol. 107:160–169. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tan H, Ye J, Luo X, Chen S, Yin Q, Yang L
and Li Y: Clonal expanded TRA and TRB subfamily T cells in
peripheral blood from patients with diffuse large B-cell lymphoma.
Hematology. 15:81–87. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhou J, Ma R, Luo R, Sun Y, He X, Sun W,
Tang W and Yao X: Primary exploration of CDR3 spectratyping and
molecular features of TCR β chain in the peripheral blood and
tissue of patients with colorectal carcinoma. Cancer Epidemiol.
34:733–740. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Baier PK, Wimmenauer S, Hirsch T, von
Specht BU, von Kleist S, Keller H and Farthmann EH: Analysis of the
T cell receptor variability of tumor-infiltrating lymphocytes in
colorectal carcinomas. Tumor Biol. 19:205–212. 1998. View Article : Google Scholar
|
|
32
|
McHeyzer-Williams LJ, Panus JF, Mikszta JA
and McHeyzer-Williams MG: Evolution of antigen-specific T cell
receptors in vivo: Preimmune and antigen-driven selection of
preferred complementarity-determining region 3 (CDR3) motifs. J Exp
Med. 189:1823–1838. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Busch DH and Pamer EG: T cell affinity
maturation by selective expansion during infection. J Exp Med.
189:701–710. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhong W and Reinherz EL: In vivo selection
of a TCR Vbeta repertoire directed against an immunodominant
influenza virus CTL epitope. Int Immunol. 16:1549–1559. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Argaet VP, Schmidt CW, Burrows SR, Silins
SL, Kurilla MG, Doolan DL, Suhrbier A, Moss DJ, Kieff E, Sculley
TB, et al: Dominant selection of an invariant T cell antigen
receptor in response to persistent infection by Epstein-Barr virus.
J Exp Med. 180:2335–2340. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pantaleo G, Demarest JF, Soudeyns H,
Graziosi C, Denis F, Adelsberger JW, Borrow P, Saag MS, Shaw GM,
Sekaly RP, et al: Major expansion of CD8+ T cells with a
predominant V β usage during the primary immune response to HIV.
Nature. 370:463–467. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Price DA, West SM, Betts MR, Ruff LE,
Brenchley JM, Ambrozak DR, Edghill-Smith Y, Kuroda MJ, Bogdan D,
Kunstman K, et al: T cell receptor recognition motifs govern immune
escape patterns in acute SIV infection. Immunity. 21:793–803. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Trautmann L, Rimbert M, Echasserieau K,
Saulquin X, Neveu B, Dechanet J, Cerundolo V and Bonneville M:
Selection of T cell clones expressing high-affinity public TCRs
within Human cytomegalovirus-specific CD8 T cell responses. J
Immunol. 175:6123–6132. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gillespie GMA, Stewart-Jones G, Rengasamy
J, Beattie T, Bwayo JJ, Plummer FA, Kaul R, McMichael AJ,
Easterbrook P, Dong T, et al: Strong TCR conservation and altered T
cell cross-reactivity characterize a B*57-restricted immune
response in HIV-1 infection. J Immunol. 177:3893–3902. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Price DA, Brenchley JM, Ruff LE, Betts MR,
Hill BJ, Roederer M, Koup RA, Migueles SA, Gostick E, Wooldridge L,
et al: Avidity for antigen shapes clonal dominance in
CD8+ T cell populations specific for persistent DNA
viruses. J Exp Med. 202:1349–1361. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Miconnet I, Marrau A, Farina A, Taffé P,
Vigano S, Harari A and Pantaleo G: Large TCR diversity of
virus-specific CD8 T cells provides the mechanistic basis for
massive TCR renewal after antigen exposure. J Immunol.
186:7039–7049. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gras S, Kjer-Nielsen L, Burrows SR,
McCluskey J and Rossjohn J: T-cell receptor bias and immunity. Curr
Opin Immunol. 20:119–125. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Callan MF, Annels N, Steven N, Tan L,
Wilson J, McMichael AJ and Rickinson AB: T cell selection during
the evolution of CD8+ T cell memory in vivo. Eur J
Immunol. 28:4382–4390. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Miles JJ, Borg NA, Brennan RM, Tynan FE,
Kjer-Nielsen L, Silins SL, Bell MJ, Burrows JM, McCluskey J,
Rossjohn J, et al: TCR α genes direct MHC restriction in the potent
human T cell response to a class I-bound viral epitope. J Immunol.
177:6804–6814. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Tynan FE, Burrows SR, Buckle AM, Clements
CS, Borg NA, Miles JJ, Beddoe T, Whisstock JC, Wilce MC, Silins SL,
et al: T cell receptor recognition of a ‘super-bulged’ major
histocompatibility complex class I-bound peptide. Nat Immunol.
6:1114–1122. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
46
|
Dolton G, Tungatt K, Lloyd A, Bianchi V,
Theaker SM, Trimby A, Holland CJ, Donia M, Godkin AJ, Cole DK, et
al: More tricks with tetramers: A practical guide to staining T
cells with peptide-MHC multimers. Immunology. 146:11–22. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wilson JD, Ogg GS, Allen RL, Goulder PJ,
Kelleher A, Sewell AK, O'Callaghan CA, Rowland-Jones SL, Callan MF
and McMichael AJ: Oligoclonal expansions of CD8+ T cells
in chronic HIV infection are antigen specific. J Exp Med.
188:785–790. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Moss PA, Moots RJ, Rosenberg WM,
Rowland-Jones SJ, Bodmer HC, McMichael AJ and Bell JI: Extensive
conservation of alpha and beta chains of the human T-cell antigen
receptor recognizing HLA-A2 and influenza A matrix peptide. Proc
Natl Acad Sci USA. 88:8987–8990. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Boehme CC, Nicol MP, Nabeta P, Michael JS,
Gotuzzo E, Tahirli R, Gler MT, Blakemore R, Worodria W, Gray C, et
al: Feasibility, diagnostic accuracy, and effectiveness of
decentralised use of the Xpert MTB/RIF test for diagnosis of
tuberculosis and multidrug resistance: A multicentre implementation
study. Lancet. 377:1495–1505. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Geiger R, Duhen T, Lanzavecchia A and
Sallusto F: Human naive and memory CD4+ T cell
repertoires specific for naturally processed antigens analyzed
using libraries of amplified T cells. J Exp Med. 206:1525–1534.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Tully G, Kortsik C, Höhn H, Zehbe I,
Hitzler WE, Neukirch C, Freitag K, Kayser K and Maeurer MJ: Highly
focused T cell responses in latent human pulmonary Mycobacterium
tuberculosis infection. J Immunol. 174:2174–2184. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Jacobsen M, Detjen AK, Mueller H,
Gutschmidt A, Leitner S, Wahn U, Magdorf K and Kaufmann SHE: Clonal
expansion of CD8+ effector T cells in childhood
tuberculosis. J Immunol. 179:1331–1339. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Flynn JL and Chan J: Immunology of
tuberculosis. Annu Rev Immunol. 19:93–129. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Luo W, Zhang XB, Huang YT, Hao PP, Jiang
ZM, Wen Q, Zhou MQ, Jin Q and Ma L: Development of genetically
engineered CD4+ and CD8+ T cells expressing
TCRs specific for a M. tuberculosis 38-kDa antigen. J Mol Med Berl.
89:903–913. 2011. View Article : Google Scholar
|
|
55
|
Luo W, Su J, Zhang XB, Yang Z, Zhou MQ,
Jiang ZM, Hao PP, Liu SD, Wen Q, Jin Q, et al: Limited T cell
receptor repertoire diversity in tuberculosis patients correlates
with clinical severity. PLoS One. 7:e481172012. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Boubou MI, Collette A, Voegtlé D, Mazier
D, Cazenave PA and Pied S: T cell response in malaria pathogenesis:
Selective increase in T cells carrying the TCR Vβ8 during
experimental cerebral malaria. Int Immunol. 11:1553–1562. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Li Y: Research and application of T-cell
receptor. People's Medical Publishing House; Beijing: pp. 1322009,
(in Chinese).
|
|
58
|
Murata H, Matsumura R, Koyama A, Sugiyama
T, Sueishi M, Shibuya K, Tsutsumi A and Sumida T: T cell receptor
repertoire of T cells in the kidneys of patients with lupus
nephritis. Arthritis Rheum. 46:2141–2147. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Furukawa F, Tokura Y, Matsushita K,
Iwasaki-Inuzuka K, Onagi-Suzuki K, Yagi H, Wakita H and Takigawa M:
Selective expansions of T cells expressing Vβ8 and Vβ13 in skin
lesions of patients with chronic cutaneous lupus erythematosus. J
Dermatol. 23:670–676. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Mato T, Masuko K, Misaki Y, Hirose N, Ito
K, Takemoto Y, Izawa K, Yamamori S, Kato T, Nishioka K, et al:
Correlation of clonal T cell expansion with disease activity in
systemic lupus erythematosus. Int Immunol. 9:547–554. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Desai-Mehta A, Mao C, Rajagopalan S,
Robinson T and Datta SK: Structure and specificity of T cell
receptors expressed by potentially pathogenic anti-DNA
autoantibody-inducing T cells in human lupus. J Clin Invest.
95:531–541. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Luo W, Ma L, Wen Q, Wang N, Zhou MQ and
Wang XN: Analysis of the interindividual conservation of T cell
receptor α- and β-chain variable regions gene in the peripheral
blood of patients with systemic lupus erythematosus. Clin Exp
Immunol. 154:316–324. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Codina-Busqueta E, Scholz E, Muñoz-Torres
PM, Roura-Mir C, Costa M, Xufré C, Planas R, Vives-Pi M,
Jaraquemada D and Martí M: TCR bias of in vivo expanded T cells in
pancreatic islets and spleen at the onset in human type 1 diabetes.
J Immunol. 186:3787–3797. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Zhou J, Kong C, Jia Y, Wang L, Jin C and
Wang X: The skewness of alpha beta T cell receptors in peripheral
blood of the patients with type 1 diabetes. Exp Clin Endocrinol
Diabetes. 124:1–4. 2016.
|
|
65
|
Kim JY, Balamurugan A, Azari K, Hofmann C,
Ng HL, Reed EF, McDiarmid S and Yang OO: Clonal CD8+ T
cell persistence and variable gene usage bias in a human
transplanted hand. PLoS One. 10:e01362352015. View Article : Google Scholar
|
|
66
|
Luo W, Liao WJ, Huang YT, Shi M, Zhang Y,
Wen Q, Zhou MQ and Ma L: Normalization of T cell receptor
repertoire diversity in patients with advanced colorectal cancer
who responded to chemotherapy. Cancer Sci. 102:706–712. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Luo W, Liao WJ, Ma L, Huang YT, Shi M, Wen
Q and Wang XN: Dynamic monitoring the TCR CDR3 spectratypes in
patients with metastatic CRC treated with a combination of
bevacizumab, irinotecan, fluorouracil, and leucovorin. Cancer
Immunol Immunother. 59:247–256. 2010. View Article : Google Scholar
|
|
68
|
Yu AL, Gilman AL, Ozkaynak MF, London WB,
Kreissman SG, Chen HX, Smith M, Anderson B, Villablanca JG, Matthay
KK, et al; Children's Oncology Group. Anti-GD2 antibody with
GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J
Med. 363:1324–1334. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Hank JA, Robinson RR, Surfus J, Mueller
BM, Reisfeld RA, Cheung NK and Sondel PM: Augmentation of antibody
dependent cell mediated cytotoxicity following in vivo therapy with
recombinant interleukin 2. Cancer Res. 50:5234–5239.
1990.PubMed/NCBI
|
|
70
|
Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ,
Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al:
Safety and activity of anti-PD-L1 antibody in patients with
advanced cancer. N Engl J Med. 366:2455–2465. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Topalian SL, Hodi FS, Brahmer JR,
Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD,
Sosman JA, Atkins MB, et al: Safety, activity, and immune
correlates of anti-PD-1 antibody in cancer. N Engl J Med.
366:2443–2454. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Wolchok JD, Kluger H, Callahan MK, Postow
MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K,
et al: Nivolumab plus ipilimumab in advanced melanoma. N Engl J
Med. 369:122–133. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Bowman L, Grossmann M, Rill D, Brown M,
Zhong WY, Alexander B, Leimig T, Coustan-Smith E, Campana D,
Jenkins J, et al: IL-2 adenovector-transduced autologous tumor
cells induce antitumor immune responses in patients with
neuroblastoma. Blood. 92:1941–1949. 1998.PubMed/NCBI
|
|
74
|
Brenner MK, Heslop H, Krance R, Horowitz
M, Strother D, Nuchtern J, Grilley B, Martingano E and Cooper K:
Phase I study of chemokine and cytokine gene-modified autologous
neuroblastoma cells for treatment of relapsed/refractory
neuroblastoma using an adenoviral vector. Hum Gene Ther.
11:1477–1488. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Pule MA, Savoldo B, Myers GD, Rossig C,
Russell HV, Dotti G, Huls MH, Liu E, Gee AP, Mei Z, et al:
Virus-specific T cells engineered to coexpress tumor-specific
receptors: Persistence and antitumor activity in individuals with
neuroblastoma. Nat Med. 14:1264–1270. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Louis CU, Savoldo B, Dotti G, Pule M, Yvon
E, Myers GD, Rossig C, Russell HV, Diouf O, Liu E, et al: Antitumor
activity and long-term fate of chimeric antigen receptor-positive T
cells in patients with neuroblastoma. Blood. 118:6050–6056. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Park JR, Digiusto DL, Slovak M, Wright C,
Naranjo A, Wagner J, Meechoovet HB, Bautista C, Chang WC, Ostberg
JR, et al: Adoptive transfer of chimeric antigen receptor
re-directed cytolytic T lymphocyte clones in patients with
neuroblastoma. Mol Ther. 15:825–833. 2007.PubMed/NCBI
|
|
78
|
Cheung NKV, Cheung IY, Kushner BH,
Ostrovnaya I, Chamberlain E, Kramer K and Modak S: Murine anti-GD2
monoclonal antibody 3F8 combined with granulocyte-macrophage
colony-stimulating factor and 13-cis-retinoic acid in high-risk
patients with stage 4 neuroblastoma in first remission. J Clin
Oncol. 30:3264–3270. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Mackall CL, Fleisher TA, Brown MR, Magrath
IT, Shad AT, Horowitz ME, Wexler LH, Adde MA, McClure LL and Gress
RE: Lymphocyte depletion during treatment with intensive
chemotherapy for cancer. Blood. 84:2221–2228. 1994.PubMed/NCBI
|
|
80
|
June CH, Riddell SR and Schumacher TN:
Adoptive cellular therapy: A race to the finish line. Sci Transl
Med. 7:280ps72015. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Zhang M, Ma Z, Selliah N, Weiss G, Genin
A, Finkel TH and Cron RQ: The impact of Nucleofection®
on the activation state of primary human CD4 T cells. J Immunol
Methods. 408:123–131. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Clay TM, Custer MC, Sachs J, Hwu P,
Rosenberg SA and Nishimura MI: Efficient transfer of a tumor
antigen-reactive TCR to human peripheral blood lymphocytes confers
anti-tumor reactivity. J Immunol. 163:507–513. 1999.PubMed/NCBI
|
|
83
|
Duval L, Schmidt H, Kaltoft K, Fode K,
Jensen JJ, Sorensen SM, Nishimura MI and von der Maase H: Adoptive
transfer of allogeneic cytotoxic T lymphocytes equipped with a
HLA-A2 restricted MART-1 T-cell receptor: a phase I trial in
metastatic melanoma. Clin Cancer Res. 12:1229–1236. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Tang H, Qiao J and Fu YX: Immunotherapy
and tumor microenvironment. Cancer Lett. 370:85–90. 2016.
View Article : Google Scholar
|
|
85
|
Cohen CJ, Zhao Y, Zheng Z, Rosenberg SA
and Morgan RA: Enhanced antitumor activity of murine-human hybrid
T-cell receptor (TCR) in human lymphocytes is associated with
improved pairing and TCR/CD3 stability. Cancer Res. 66:8878–8886.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Kuball J, Dossett ML, Wolfl M, Ho WY, Voss
RH, Fowler C and Greenberg PD: Facilitating matched pairing and
expression of TCR chains introduced into human T cells. Blood.
109:2331–2338. 2007. View Article : Google Scholar
|
|
87
|
Bendle GM, Linnemann C, Hooijkaas AI, Bies
L, de Witte MA, Jorritsma A, Kaiser ADM, Pouw N, Debets R, Kieback
E, et al: Lethal graft-versus-host disease in mouse models of T
cell receptor gene therapy. Nat Med. 16:565–570. 1p5702010.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Lan P, Wang L, Diouf B, Eguchi H, Su H,
Bronson R, Sachs DH, Sykes M and Yang YG: Induction of human T-cell
tolerance to porcine xenoantigens through mixed hematopoietic
chimerism. Blood. 103:3964–3969. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Lan P, Tonomura N, Shimizu A, Wang S and
Yang YG: Reconstitution of a functional human immune system in
immunodeficient mice through combined human fetal thymus/liver and
CD34+ cell transplantation. Blood. 108:487–492. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Tonomura N, Habiro K, Shimizu A, Sykes M
and Yang YG: Antigen-specific human T-cell responses and T
cell-dependent production of human antibodies in a humanized mouse
model. Blood. 111:4293–4296. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Hu Z, Xia J, Fan W, Wargo J and Yang YG:
Human melanoma immunotherapy using tumor antigen-specific T cells
generated in humanized mice. Oncotarget. Jan 27–2016.(Epub ahead of
print).
|
|
92
|
Harris DT, Kranz DM and Adoptive T: Cell
therapies: A comparison of T cell receptors and chimeric antigen
receptors. Trends Pharmacol Sci. 37:220–230. 2015. View Article : Google Scholar
|
|
93
|
Heczey A and Louis CU: Advances in
chimeric antigen receptor immunotherapy for neuroblastoma. Discov
Med. 16:287–294. 2013.PubMed/NCBI
|
|
94
|
Maus MV, Grupp SA, Porter DL and June CH:
Antibody-modified T cells: CARs take the front seat for hematologic
malignancies. Blood. 123:2625–2635. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Alrifai D, Sarker D and Maher J: Prospects
for adoptive immunotherapy of pancreatic cancer using chimeric
antigen receptor-engineered T-cells. Immunopharm Immunot. 38:50–60.
2016. View Article : Google Scholar
|
|
96
|
Parkhurst MR, Yang JC, Langan RC, Dudley
ME, Nathan DAN, Feldman SA, Davis JL, Morgan RA, Merino MJ, Sherry
RM, et al: T cells targeting carcinoembryonic antigen can mediate
regression of metastatic colorectal cancer but induce severe
transient colitis. Mol Ther. 19:620–626. 2011. View Article : Google Scholar :
|
|
97
|
Kaldor JM, Day NE, Pettersson F, Clarke
EA, Pedersen D, Mehnert W, Bell J, Høst H, Prior P, Karjalainen S,
et al: Leukemia following chemotherapy for ovarian cancer. N Engl J
Med. 322:1–6. 1990. View Article : Google Scholar : PubMed/NCBI
|















