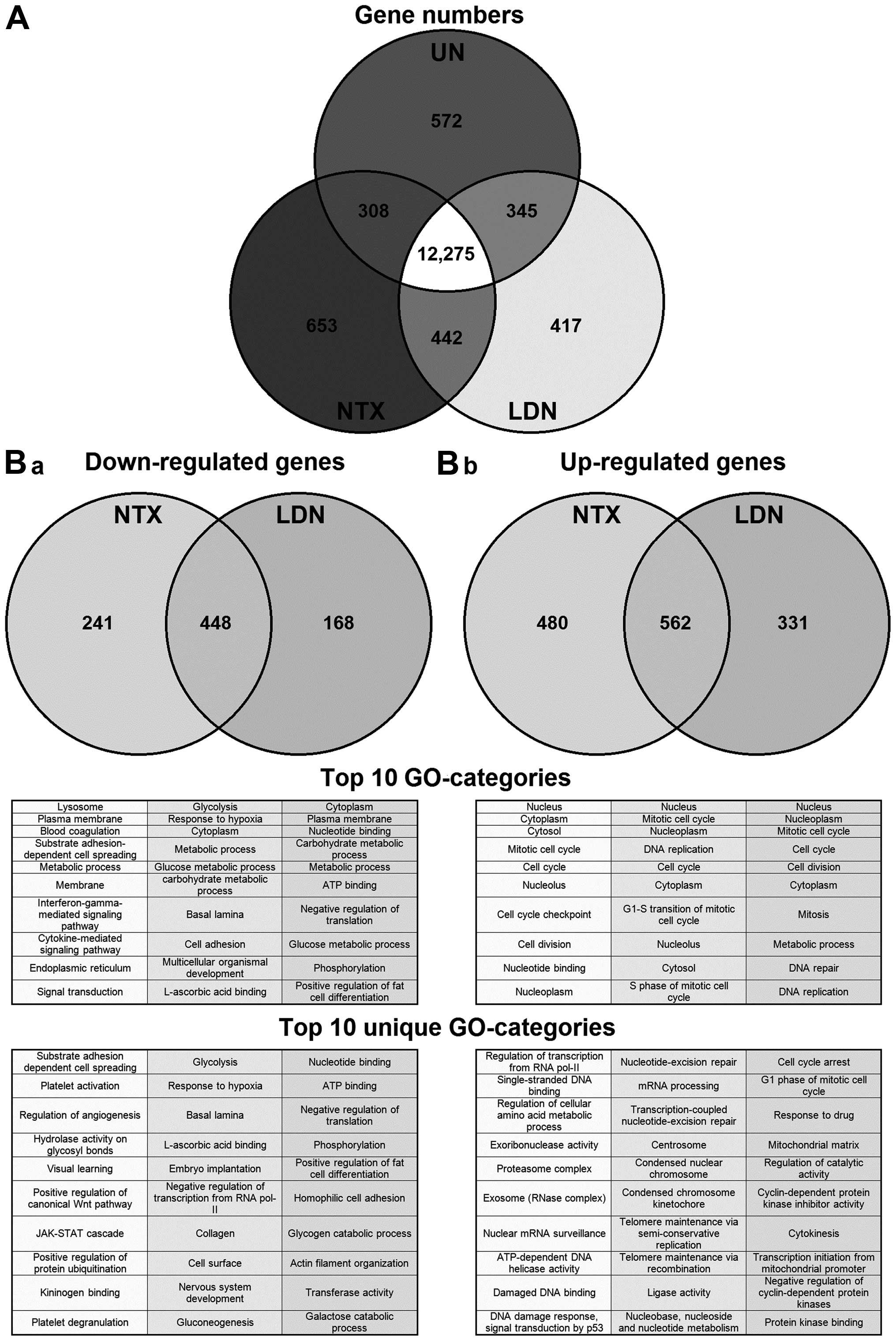
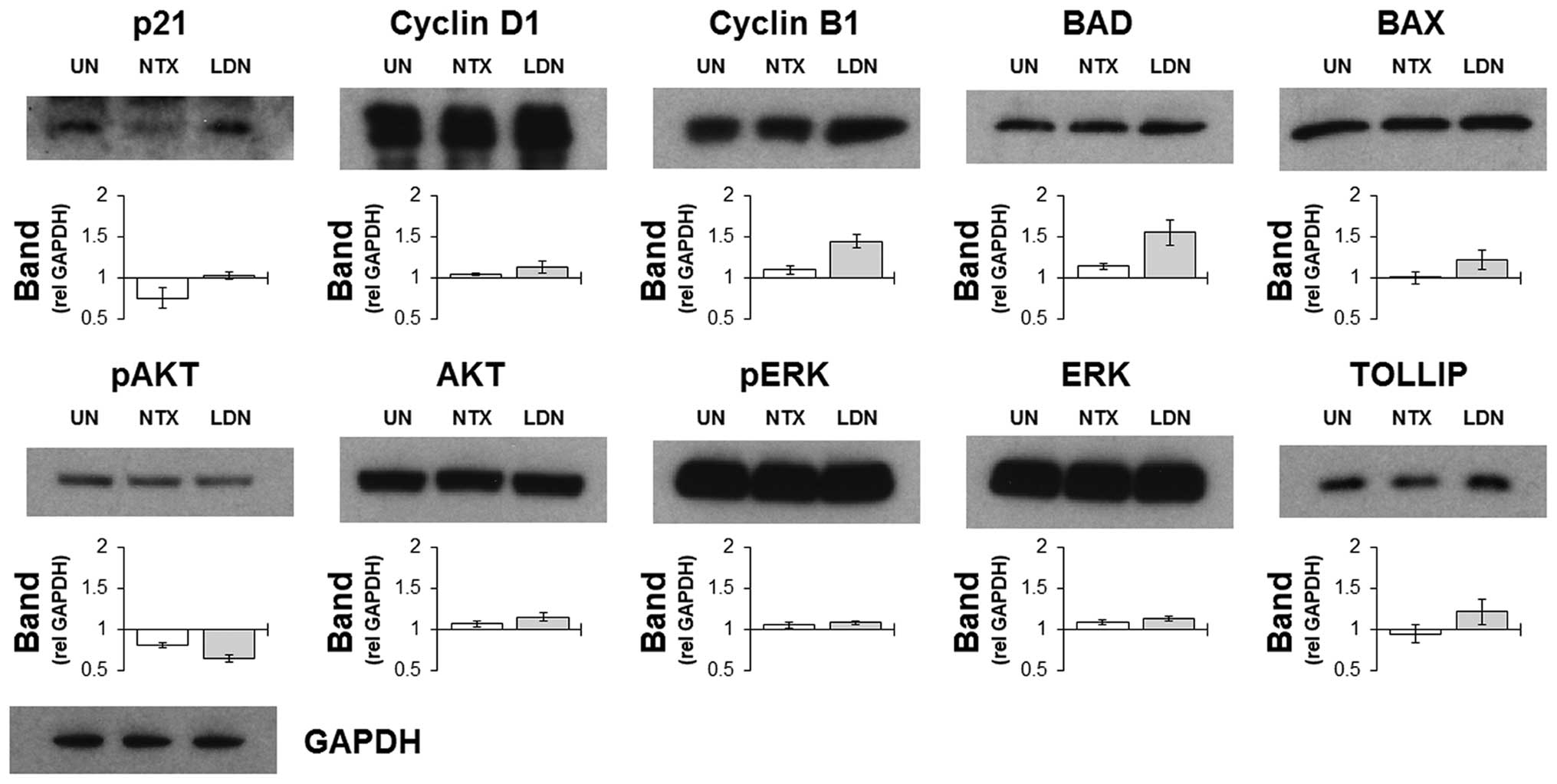
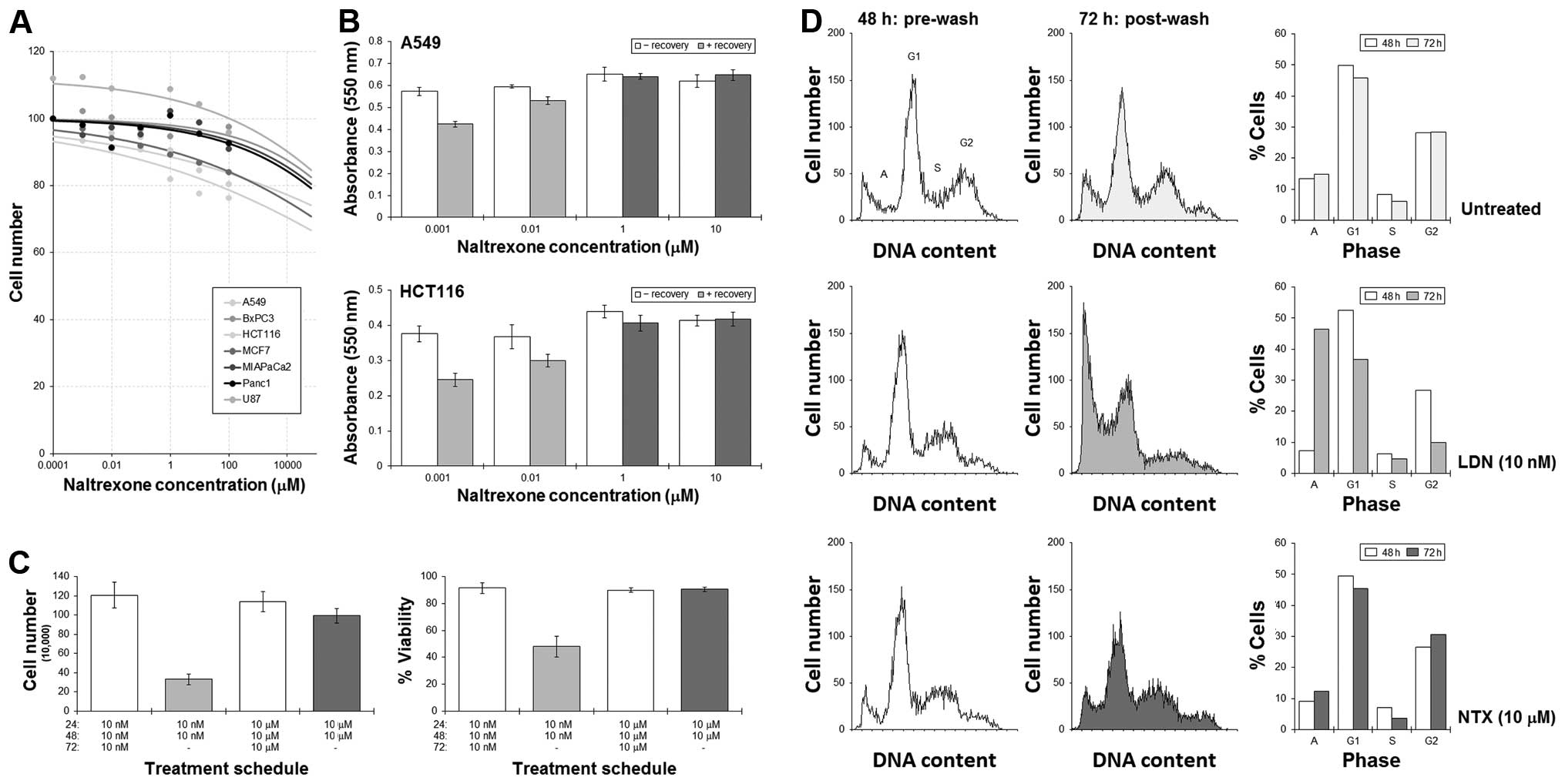
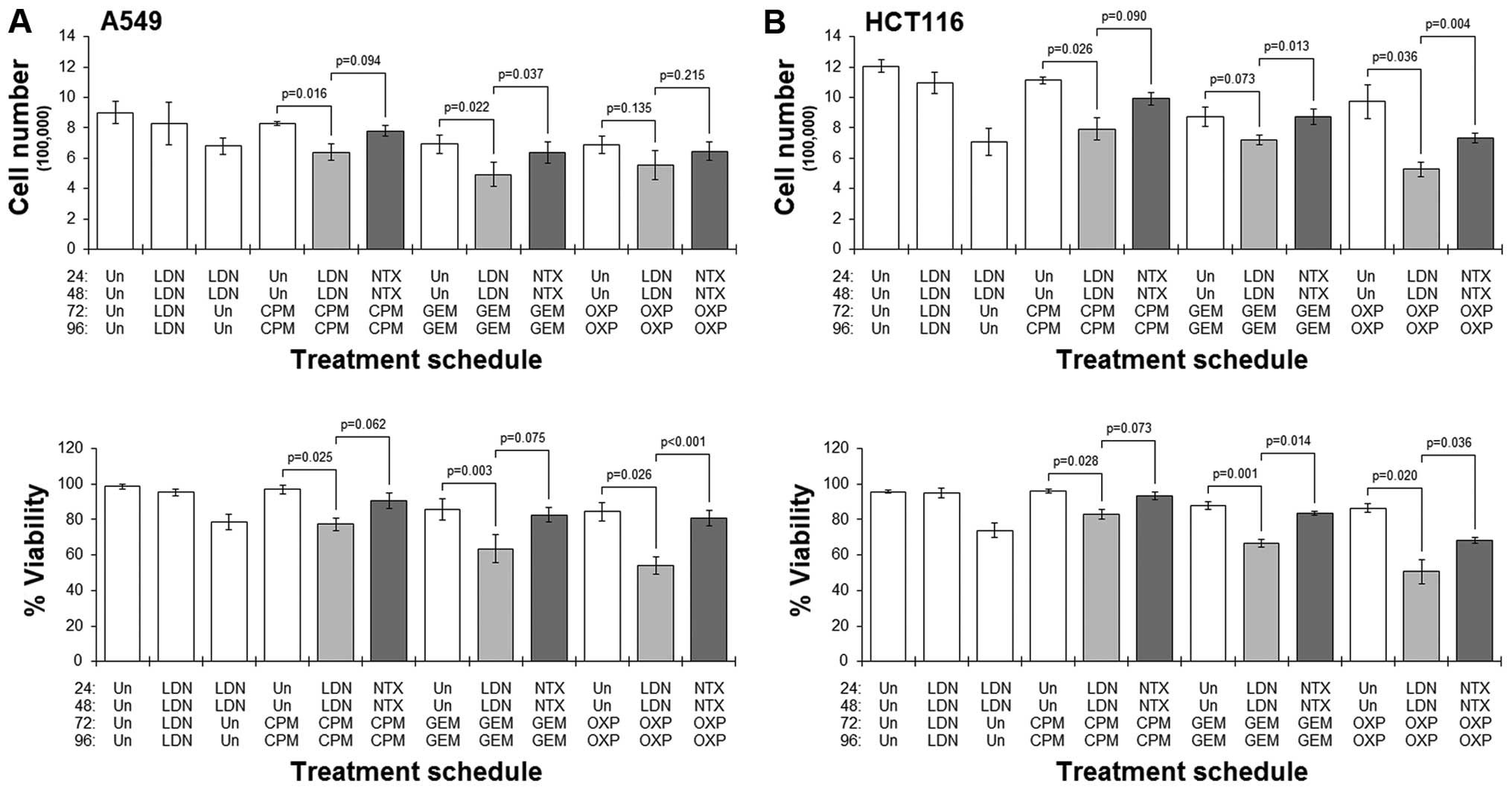
|
1
|
McLaughlin PJ and Zagon IS: Duration of
opioid receptor blockade determines biotherapeutic response.
Biochem Pharmacol. 97:236–246. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tegeder I and Geisslinger G: Opioids as
modulators of cell death and survival - unraveling mechanisms and
revealing new indications. Pharmacol Rev. 56:351–369. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Afsharimani B, Cabot P and Parat MO:
Morphine and tumor growth and metastasis. Cancer Metastasis Rev.
30:225–238. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gach K, Szemraj J, Stasikowska-Kanicka O,
Danilewicz M and Janecka A: Opioid-receptor gene expression and
localization in cancer cells. Cent Eur J Biol. 6:10–15. 2011.
|
|
5
|
Bimonte S, Barbieri A, Palma G and Arra C:
The role of morphine in animal models of human cancer: Does
morphine promote or inhibit the tumor growth? BioMed Res Int.
2013:2581412013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim MS, Cheong YP, So HS, Lee KM, Kim TY,
Oh J, Chung YT, Son Y, Kim BR and Park R: Protective effects of
morphine in peroxynitrite-induced apoptosis of primary rat neonatal
astrocytes: Potential involvement of G protein and
phosphatidylinositol 3-kinase (PI3 kinase). Biochem Pharmacol.
61:779–786. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cui Y, Zhang XQ, Cui Y, Xin WJ, Jing J and
Liu XG: Activation of phosphatidylinositol 3-kinase/Akt-mammalian
target of Rapamycin signaling pathway in the hippocampus is
essential for the acquisition of morphine-induced place preference
in rats. Neuroscience. 171:134–143. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gach K, Wyrębska A, Fichna J and Janecka
A: The role of morphine in regulation of cancer cell growth. Naunyn
Schmiedebergs Arch Pharmacol. 384:221–230. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ishikawa M, Tanno K, Kamo A, Takayanagi Y
and Sasaki K: Enhancement of tumor growth by morphine and its
possible mechanism in mice. Biol Pharm Bull. 16:762–766. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Boland JW, McWilliams K, Ahmedzai SH and
Pockley AG: Effects of opioids on immunologic parameters that are
relevant to anti-tumour immune potential in patients with cancer: A
systematic literature review. Br J Cancer. 111:866–873. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu WM: Enhancing the cytotoxic activity
of novel targeted therapies - is there a role for a combinatorial
approach? Curr Clin Pharmacol. 3:108–117. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu WM, Laux H, Henry JY, Bolton TB,
Dalgleish AG and Galustian C: A microarray study of altered gene
expression in colorectal cancer cells after treatment with
immunomodulatory drugs: Differences in action in vivo and in vitro.
Mol Biol Rep. 37:1801–1814. 2010. View Article : Google Scholar
|
|
13
|
Liu WM, Dennis JL, Fowler DW and Dalgleish
AG: The gene expression profile of unstimulated dendritic cells can
be used as a predictor of function. Int J Cancer. 130:979–990.
2012. View Article : Google Scholar
|
|
14
|
Liu WM, Dennis JL, Gravett AM,
Chanthirakumar C, Kaminska E, Coulton G, Fowler DW, Bodman-Smith M
and Dalgleish AG: Supernatants derived from chemotherapy-treated
cancer cell lines can modify angiogenesis. Br J Cancer.
106:896–903. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Scott KA, Dennis JL, Dalgleish AG and Liu
WM: Inhibiting heat shock proteins can potentiate the cytotoxic
effect of cannabidiol in human glioma cells. Anticancer Res.
35:5827–5837. 2015.PubMed/NCBI
|
|
16
|
Liu WM, Gravett AM and Dalgleish AG: The
antimalarial agent artesunate possesses anticancer properties that
can be enhanced by combination strategies. Int J Cancer.
128:1471–1480. 2011. View Article : Google Scholar
|
|
17
|
Liu WM, Fowler DW, Smith P and Dalgleish
AG: Pre-treatment with chemotherapy can enhance the antigenicity
and immunogenicity of tumours by promoting adaptive immune
responses. Br J Cancer. 102:115–123. 2010. View Article : Google Scholar :
|
|
18
|
Liu WM, Lawrence AJ and Joel SP: The
importance of drug scheduling and recovery phases in determining
drug activity. Improving etoposide efficacy in BCR-ABL-positive CML
cells. Eur J Cancer. 38:842–850. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Scott KA, Shah S, Dalgleish AG and Liu WM:
Enhancing the activity of cannabidiol and other cannabinoids in
vitro through modifications to drug combinations and treatment
schedules. Anticancer Res. 33:4373–4380. 2013.PubMed/NCBI
|
|
20
|
Sobel H and Bonorris G: Effect of morphine
on rats bearing Walker carcinosarcoma 256. Nature. 196:896–897.
1962. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sacerdote P: Opioids and the immune
system. Palliat Med. 20(Suppl 1): S9–S15. 2006.PubMed/NCBI
|
|
22
|
Meriney SD, Gray DB and Pilar G:
Morphine-induced delay of normal cell death in the avian ciliary
ganglion. Science. 228:1451–1453. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu WM and Dalgleish AG: The potential
beneficial effects of drugs on the immune response to vaccination.
Semin Oncol. 39:340–347. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pietenpol JA and Stewart ZA: Cell cycle
checkpoint signaling: Cell cycle arrest versus apoptosis.
Toxicology. 181–182:475–481. 2002. View Article : Google Scholar
|
|
25
|
Shapiro GI and Harper JW: Anticancer drug
targets: Cell cycle and checkpoint control. J Clin Invest.
104:1645–1653. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Waldman T, Zhang Y, Dillehay L, Yu J,
Kinzler K, Vogelstein B and Williams J: Cell-cycle arrest versus
cell death in cancer therapy. Nat Med. 3:1034–1036. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zagon IS and McLaughlin PJ: Naltrexone
modulates tumor response in mice with neuroblastoma. Science.
221:671–673. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Donahue RN, McLaughlin PJ and Zagon IS:
Low-dose naltrexone targets the opioid growth factor-opioid growth
factor receptor pathway to inhibit cell proliferation: Mechanistic
evidence from a tissue culture model. Exp Biol Med (Maywood).
236:1036–1050. 2011. View Article : Google Scholar
|
|
29
|
Tudor G, Aguilera A, Halverson DO, Laing
ND and Sausville EA: Susceptibility to drug-induced apoptosis
correlates with differential modulation of Bad, Bcl-2 and Bcl-xL
protein levels. Cell Death Differ. 7:574–586. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Makin G and Dive C: Recent advances in
understanding apoptosis: New therapeutic opportunities in cancer
chemotherapy. Trends Mol Med. 9:251–255. 2003. View Article : Google Scholar : PubMed/NCBI
|