Introduction
Epidermal growth factor (EGF)-mediated activation of
its receptor (EGFR) is a principal regulatory event in breast
cancer growth and progression (1).
Cell migration and invasion can be enhanced by numerous growth
factors. High expression of EGFR is a major contributing factor to
the development and progression of cancer, and is associated with a
poor prognosis (2). Basal or
triple-negative (ER-negative, PR-negative, and Her2/neu-negative)
breast cancer cells, like MD Anderson-mammary breast cancer cell
line-231 (MDA-MB-231), often express high levels of EGFR, which is
associated with a loss of sensitivity to hormonal therapies and/or
high probability of metastasis (3). The metastasis and invasion of cancer
cells have been considered important therapeutic targets, because
the inability to control metastasis and invasion remains the most
formidable obstacle to successful treatment (4).
Cancer metastasis occurs by a complex series of
events, which include detachment of cells from the primary tumor,
migration, invasion of the cells into either blood or lymphatic
vessels with the help of matrix metalloproteinases (MMPs), and
finally, interaction with target organs such as the lungs, liver,
and bones (5,6). Among the MMPs, MMP-9 is known to be
involved in the degradation of type IV collagen, an important
component of the extracellular matrix (ECM) (7). Focal adhesion kinase (FAK) is also a
critical factor for cell motility and metastasis, and it is found
in the cell membrane where the cytoskeleton interacts with the
proteins of the ECM (8). Cancer
cell invasion is regulated by growth factors that can rapidly
activate cell surface receptors to induce actin polymerization and
reorganization into actin-based extensions (9). Recent reports have suggested that
MMP-9 and FAK are associated with the EGFR activation via EGF
induction, and that they promote tumor cell motility and invasion
(10). It has been shown that
EGF-induced phosphorylation of mitogen-activated protein kinase
(MAPK) and phosphatidylinositol 3-kinase/protein kinase B
(PI3K/Akt), mammalian target of rapamycin (mTOR) pathway, leads to
MMP-9 expression and FAK phosphorylation (5). Furthermore, the EGFR/PI3K/Akt
signaling pathways regulate mTOR. The inhibition of the mTOR
signaling pathway suppresses filamentous-actin (F-actin)
reorganization, MMP expression, and FAK phosphorylation, which is a
functional indicator of cell migration (11,12).
Triterpenoids (friedeline, glut-5-en-ol, pomolic
acid and methylotundate) were isolated from Euscaphis
japonica (Tunb.) Kantiz (Staphyleaceae) found in China,
Japan, and Korea (13). Among
them, pomolic acid (PA) was shown to inhibit the growth of leukemia
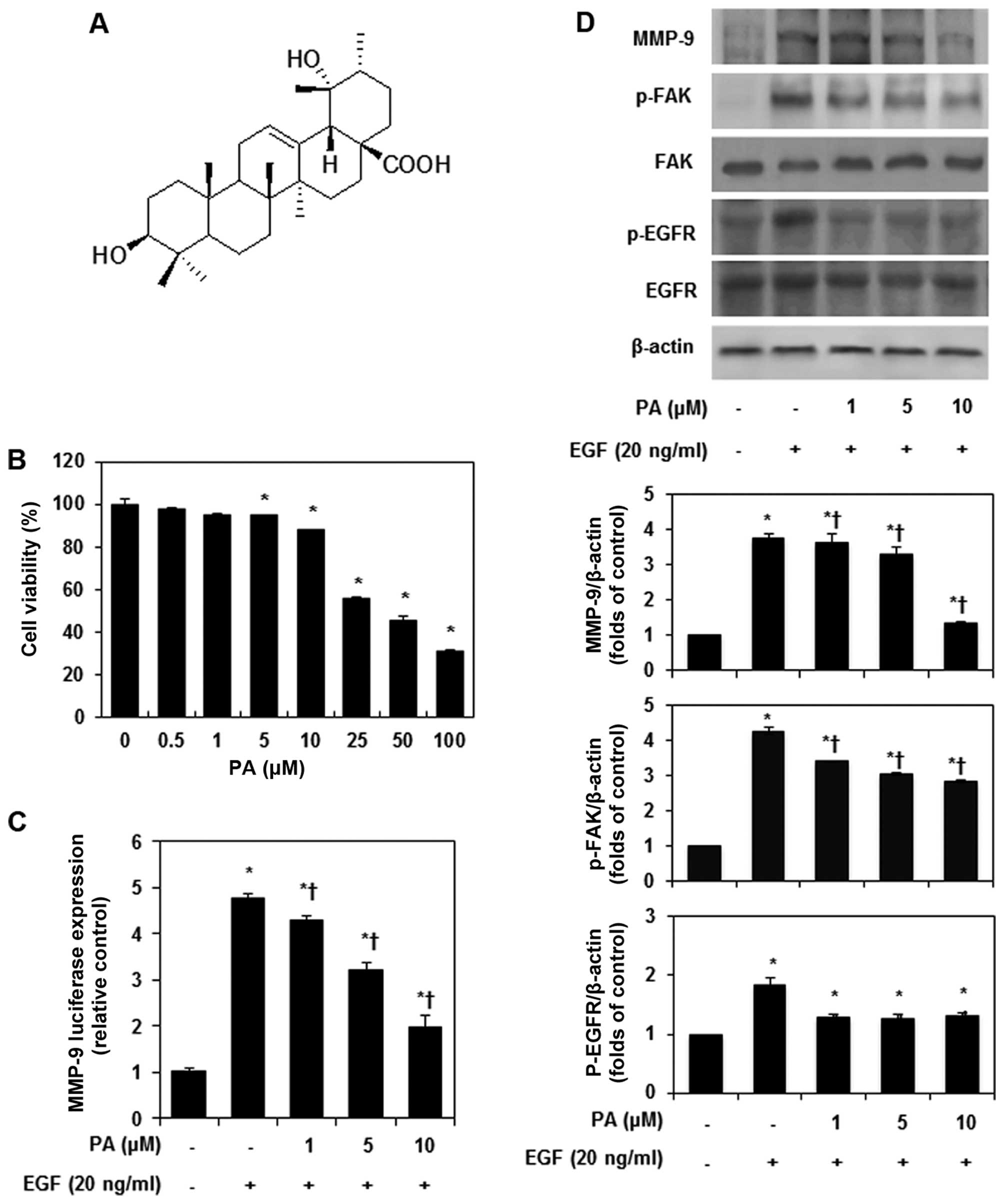
cell lines with high efficacy (Fig.
1A) (14,15), and other investigations have
demonstrated that PA mediates the apoptosis of human ovarian cancer
cells through the mitochondrial-mediated intrinsic and death
receptor-induced extrinsic pathways (16). PA has demonstrated
anti-proliferative activity against human gastric adenocarcinoma,
human uterine carcinoma, and murine melanoma (17). We previously demonstrated that PA
inhibits invasion of breast cancer cells through the suppression of
CXC chemokine receptor type 4 (CXCR4) expression (18). However, the molecular mechanisms of
the anti-metastatic potential of PA have not been fully elucidated
in breast cancer cells. Therefore, we investigated the
anti-metastasis mechanism by examining the effect of PA on
EGF-induced breast cancer cells.
Materials and methods
Cell cultures and reagents
MDA-MB-231 cells were obtained from the America
Tissue Culture Collection (Rockville, MD, USA). Cells were cultured
in Dulbecco’s modified Eagle’s medium (DMEM) or RPMI-1640
supplemented with 10% fetal bovine serum (FBS) and antibiotics (100
U/ml penicillin and 100 μg/ml streptomycin) at 37°C in a humidified
incubator 5% CO2 and 95% air. DMEM, RPMI-1940, FBS,
antibiotics, and trypsin-EDTA were obtained from Gibco BRL (Grand
Island, NY, USA). Pomolic acid (PA) was purified and received from
Dr Ki Yong Lee, professor of the College of Pharmacy, Korea
University (13). PA was dissolved
in dimethylsulfoxide (DMSO) as a 10-mM stock solution and stored at
4°C. Further dilution was done in cell culture medium. Recombinant
human EGF was purchased from R&D System Inc. (Minneapolis, MN,
USA). Signal inhibitors were obtained from Calbiochem (Cambridge,
MA, USA).
Wound-healing and invasion assays
Wound healing assay was performed in 6-well plates.
When cells reached 80% confluence, synchronized cells were
pretreated with PA (10 μM) for 1 h, followed by stimulated with EGF
(20 ng/ml) for 24 h. A single wound was created in the center of
the cell monolayers by gentle removal of the attached cells with a
sterile plastic pipette tip. After 24 h of incubation, the cells
that migrated into the wounded area or protruded from the border of
the wound were visualized and photographed with a microscope (Carl
Zeiss, Sartrouville, France). In vitro invasion assay was
done using Bio-Coat Matrigel invasion assay system (BD Biosciences,
Lexington, KY, USA) according to the manufacturer’s instructions
(19). MDA-MB-231 cells were
suspended in medium and seeded into the Matrigel-precoated
Transwell chambers with polycarbonate membranes of 8 μm pore size.
The lower chambers were filled with DMEM supplemented with EGF (20
ng/ml) in the absence or presence of PA (10 μM). The chamber was
incubated at 37°C for 24 h. The cells from the upper surface of the
filters were carefully removed while the cells on the lower surface
of the filters were fixed with 4% paraformaldehyde and stained with
0.1% crystal violet. Migrant cells were quantified by blind
counting under a microscope (Carl Zeiss). The number of
transmigrated cells were counted and averaged in five random
areas.
Actin filament staining
Serum-starved cells grown in 6-well-plates were
pretreated with or without PA for 1 h, followed by stimulation with
or without EGF (20 ng/ml) for 24 h. Cells were then fixed with 4%
ice cold formaldehyde in PBS for 20 min at 4°C and washed with 0.2%
Triton X-100 in PBS for 5 min. Cells were stained with FITC
conjugated phalloidin (1 μg/ml, Sigma, MO, USA) for 30 min. Slides
were mounted using ProLong® Gold antifade reagent
(Molecular Probes® by Life TechnologiesTM,
CA, USA). Actin filaments (F-actin) were examined using a confocal
microscope (Nikon, Tokyo, Japan).
Protein isolation and immunoblot
analysis
Cytosolic and nuclei protein fractions were obtained
as described (20). The protein
concentration was determined with a Bio-Rad Bradford kit (Bio-Rad
Laboratories, CA, USA). The samples were boiled for 5 min and equal
volumes were loaded on a sodium dodecylsulfate-polyacrylamide gel
electrophoresis (SDS-PAGE). After separation, the proteins were
transferred to a nitrocellulose membrane for 1 h at 4°C and blocked
overnight with PBS-T [0.1% (v/v) Tween-20, 5% (w/v) powdered milk
in 137 nm NaCl, 2.7 mM KCl, 1.5 mM KH2PO4, pH
7.4] at 4°C. Immune complexes were detected with a horseradish
peroxidase (HRP)-conjugated secondary antibody and were visualized
by an enhanced chemiluminescence (ECL) detection system (Bio-Rad
Laboratories). The primary antibodies used in this study were:
anti-MMP-9, anti-p-FAK, anti-FAK, anti-p-EGFR, anti-EGFR,
anti-p-IKKα/β, anti-IKKα/β, anti-p-IκBα, anti-IκBα, anti-p-NF-κB
p65, anti-NF-κB, anti-p-ERK1/2, anti-ERK1/2, anti-p-PI3K,
anti-PI3K, anti-p-Akt, anti-Akt, anti-p-mTOR, anti-mTOR,
anti-p-70S6K, anti-70S6K, anti-p-4E-BP1, anti-4E-BP1, anti-lamin B
and anti-β-actin (all from Cell Signaling Technology, MA, USA).
Lamin B and β-actin were used as a loading control. The luminescent
signals were analyzed using an ImageQuant LAS 4000 Scanner of GE
Healthcare (Piscataway, NJ, USA).
Construction of human MMP-9 promoter,
transient transfec-tion and luciferase gene assays
A 700-bp fragment at the 5′-flanking region of the
human MMP-9 gene was amplified by PCR from human genomic DNA
(6). Specific primers were
designed to contain the appropriate restriction enzyme site: sense
5′-CGG GGT ACC TGC TAC TGT CCC CTT TAC TG-3′ (KpnI) and
antisense 5′-CCC AGA TCT GTG AGG GCA GAG GTG TCT-3′ (BglII).
The amplified promoter DNA was digested with KpnI and
BglII, and then cloned upstream of the luciferase gene in
pGL3 plasmid. The DNA sequence of the MMP-9 promoter was confirmed,
and the resultant reporter plasmid was named pGL3-wtMMP-9. The
AP-1, NF-κB, and SP-1 mutants from pGL-3-wtMMP-9 were generated
using the QuickChange Site-Directed Mutagenesis kit (Stratagene,
CA, USA); all the mutants were confirmed by DNA sequencing.
MMP-9 wild-type (pGL3-wtMMP-9), AP-1 site-mutated
(pGL3-MMP-9-mtAP-1), NF-κB site-mutated (pGL3-MMP-9-mtNF-κB), and
SP-1 site-mutated MMP-9 luciferase promoter constructs
(pGL3-MMP-9-mtSP-1) were used in transient transfection assays.
MDA-MB-231 cells were co-transfected with 1 μg of MMP-9
promoter-luciferase reporter constructs and 0.2 μg of the
Renilla reporter plasmid for 6 h using Lipofectamine reagent
(Invitrogen, CA, USA) according to the manufacturer’s protocol.
After transfection, MDA-MB-231 cells were pretreated with PA for 1
h and then stimulated with EGF for 24 h. Luciferase and
Renilla activities were determined by following the
manufacturer’s protocol (Dual-Luciferase Reporter Assay system,
Promega, WI, USA).
Electrophoretic mobility shift analysis
(EMSA) and super shift assay
According to our previous studies, DIG Gel Shift kit
(Roche, Mannheim, Germany) was performed to detect NF-κB p65, AP-1
and SP-1 DNA-binding activity, with the manufacturer’s instructions
(20,21). The binding activity of NF-κB p65,
AP-1 and SP-1 in the EGF-induced MDA-MB-231 cell nuclear extract
was confirmed by EMSA or super shift assay with a DIG-labeled
oligonucleotide (NF-κB, 5′-AGT TGA GGG GAC TTT CCC AGG C-3′; AP-1,
5′-CGC TTG ATG AGT CAG CCG GAA-3′; SP-1, 5′-CTT GAA CCC CGC CCC TGT
CTT-3′) and NF-κB p65 antibody (Abcam, Cambridge, UK). EMSA was
performed by incubating 10 μg of the MDA-MB-231 cell nuclear
extract in a 9-μl binding reaction mixture containing 10 mM
Tris-HCl (pH 7.5), 50 mM NaCl, 0.5 mM EDTA, 0.5 mM DTT, 3 mM
MgCl2, 0.05 mg/ml poly (dI-dC) and 10% (v/v) glycerol at
37°C for 10 min. The binding reaction mixture for the super shift
assay containing 1 μl of the non-diluted antibody of NF-κB p65 was
added to 1 μl of DIG-labeled double-stranded oligonucleotide and
was incubated at 37°C for 20 min, followed by the addition of 1 μl
of the gel loading 10X buffer (250 mM Tris-HCl, pH 7.5, 40%
glycerol) at room temperature. This mixture was then loaded on a
pre-run 4% polyacrylamide gel and run at 10°C in 0.5X TBE buffer at
350 V until the bromophenol blue dye was three-fourth of the way
down the gel. After electrophoresis, the DIG-labeled DNA was
electroblotted on the positively-charged nylon membrane (Roche).
The blotted nylon membrane was fixed by baking at 120°C for 15 min
and the chemiluminescent detection was performed according to the
manufacturer’s instructions.
MAPK inhibitor treatment and transient
transfection with small interfering RNA
MDA-MB-231 cells were pre-treated with
mitogen-activated protein kinase (MAPK) inhibitors such as
ERK-specific inhibitor: PD98059 (20 μM), JNK-specific inhibitor:
SP600125 (40 μM), p38-specific inhibitor: SB203580 (20 μM),
PKC-specific inhibitor: GO6978 (10 μM), PI3K/Akt-specific
inhibitor: LY294002 (50 μM), mTOR-specific inhibitor: rapamycin (20
nM), and EGFR-specific inhibitor: AG1478 (2 μM). After 30 min, the
cells were treated and co-cultured with EGF for 24 h. Cell were
transfected with negative control small interfering RNA (siRNA) or
mTOR specific siRNA (Cell Signaling Technology) using RNAiMAX
transfection reagent (Invitrogen) according to the manufacturer’s
instructions.
Statistical analysis
Statistical analysis was performed by Student’s
t-test and ANOVA using SPSS 12.0 software (SPSS Ins., Chicago, IL,
USA) to study the relationship between the different variables.
Values of p<0.05 were considered to indicate statistical
significance.
Results
Effects of PA on the EGF-induced MMP-9
expression and FAK phosphorylation
To investigate the cytotoxic effect of PA on the
proliferation of MDA-MB-231 cells, we treated them with PA for 24 h
and measured cell viability using the MTT assay. As shown in
Fig. 1B, PA treatment for 24 h at
concentration ranging from 0.5 to 10 μM had no significant
cytotoxic effect on MDA-MB-231 cells. Thus, we used PA at a
concentration of 1, 5 and 10 μM for subsequent experiments. We
investigated whether the PA treatment inhibited the MMP-9
transcriptional activity induced by EGF. To this end, MDA-MB-231
cells were transiently transfected with MMP-9 promoter reported
construct. As shown Fig. 1C, PA
inhibited EGF-stimulated MMP-9 transcriptional activity in a
dose-dependent manner. Previous studies have shown that EGF induces
significant MMP-9 expression and phosphorylation of FAK in
MDA-MB-231 cells (7). Therefore,
the inhibitory effects of PA on the activation of MMPs and FAK were
analyzed in EGF-stimulated MDA-MB-231 cells. PA strongly inhibited
EGF-induced MMP-9 expression and FAK phosphorylation in a
dose-dependent manner (Fig. 1D).
Also, PA significantly suppressed EGF-induced EGF receptor (EGFR)
phosphorylation.
Effects of PA on the EGF-induced
migration and invasion
The regulatory proteins of ECM, such as MMPs and
FAK, are important pharmaceutical targets for the prevention of
tumor invasion and metastasis (22). Since the most important
characteristic of metastasis is the migratory and invasive ability
of tumor cells, we next examined the effect of PA on the
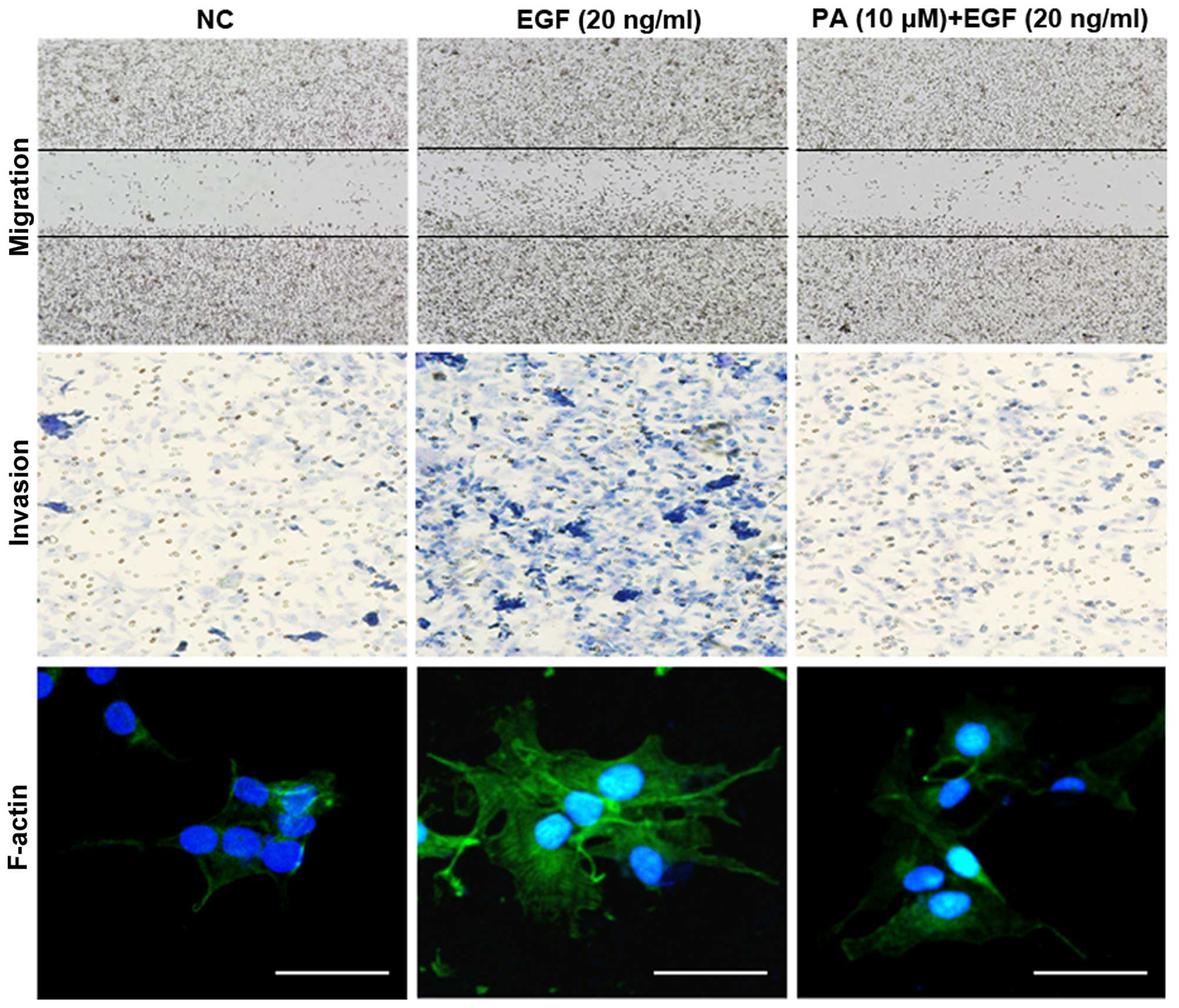
EGF-induced migration and invasion of the MDA-MB-231 cells using
wound-healing and Transwell invasion assays. As shown in Fig. 2, 10 μM of PA significantly
inhibited EGF-induced cell migration and invasion. Tumor metastasis
is associated with the polymerization of F-actin (23). Thus, to investigate the inhibitory
effect of PA on EGF-induced cell motility, F-actin staining was
performed. F-actin was randomly distributed across cells in the
absence of EGF. After stimulation with EGF for 24 h, however,
F-actin was condensed at the leading edge within structures
resembling fans, an effect not seen in non-treated cells. PA
markedly reduced EGF-stimulated F-actin reorganization at the
leading edge. These results indicate that PA has an inhibitory
effect on cell motility through the prevention of F-actin
reorganization in MDA-MB-231 cells. Taken together, these results
suggest that PA has a strong inhibitory effect on EGF-induced
metastasis, and that F-actin reorganization can be associated with
inhibition of MMP-9 expression and FAK phosphorylation in
MDA-MB-231 cells.
PA suppresses the EGF-induced MMP-9
expression via inhibition of NF-κB and AP-1
The promoter of MMP-9 contains cis-acting regulatory
elements for transcription factors including an NF-κB site (located
at −600 bp), an SP-1 site (located at −558 bp), and two AP-1 sites
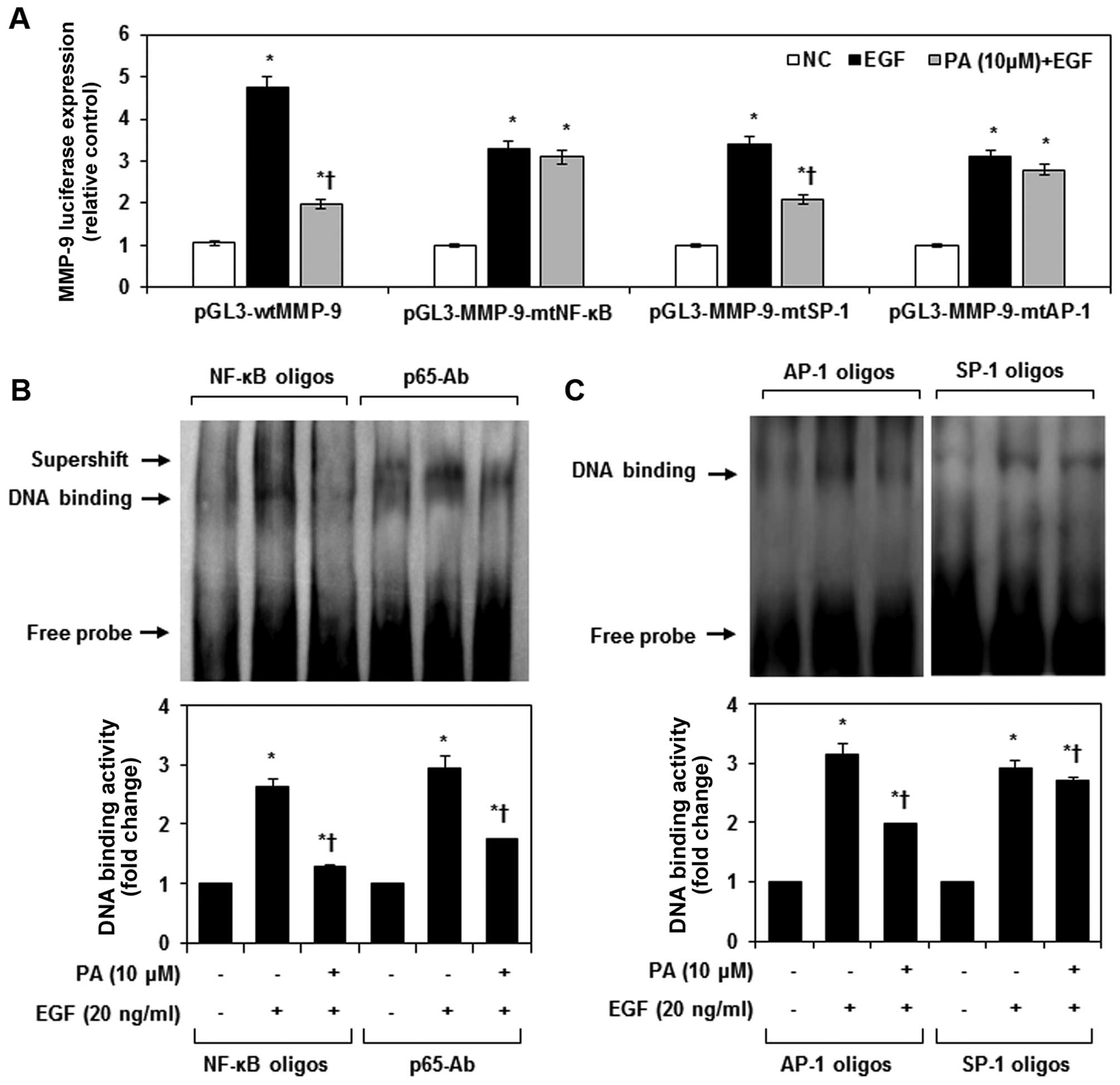
(located at −79 and −533 bp) (24). To test which of these transcription
factors may regulate the MMP-9 gene in MDA-MB-231 cells, cells were
transiently transfected with a reporter gene, either the wild-type
(wt) MMP-9 promoter or the promoter with mutations in the NF-κB
site, the AP-1 sites and the SP-1 site. As shown in Fig. 3A, wtMMP-9 luciferase activity was
activated ≤5-fold in cells treated with EGF. PA inhibited the
EGF-induced wtMMP-9 luciferase activity, suggesting that PA may
inhibit MMP-9 expression at the transcriptional level. Transfection
of MDA-MB-231 cells with MMP-9-mtAP-1 promoter and MMP-9-mtAP-1
promoter, which have mutated NF-κB and AP-1 binding sites,
neutralized the ability of PA to prevent EGF-induced MMP-9
activation. We further evaluated the effects of PA on NF-κB DNA
binding activity when cells were treated with EGF. NF-κB-DNA and
NF-κB-Ab complexes were strongly upregulated in EGF-stimulated
MDA-MB-231 cells, an effect that was significantly suppressed by
treatment with PA (Fig. 3B). Also,
PA significantly inhibited EGF-induced AP-1 DNA binding activity in
MDA-MB-231 cells, but had no effect on SP-1 DNA binding activity
(Fig. 3C).
Effects of PA on EGF-induced NF-κB signal
pathway
NF-κB regulates many responses of cancer cells
involved in proliferation, apoptosis, metastasis, and angiogenesis
(25). EGF-induced cellular
migration corresponds to an increase in MMP-9 expression, which
targets NF-κB activation and is correlated with NF-κB binding
activity. Therefore, we examined the effects of PA on the NF-κB
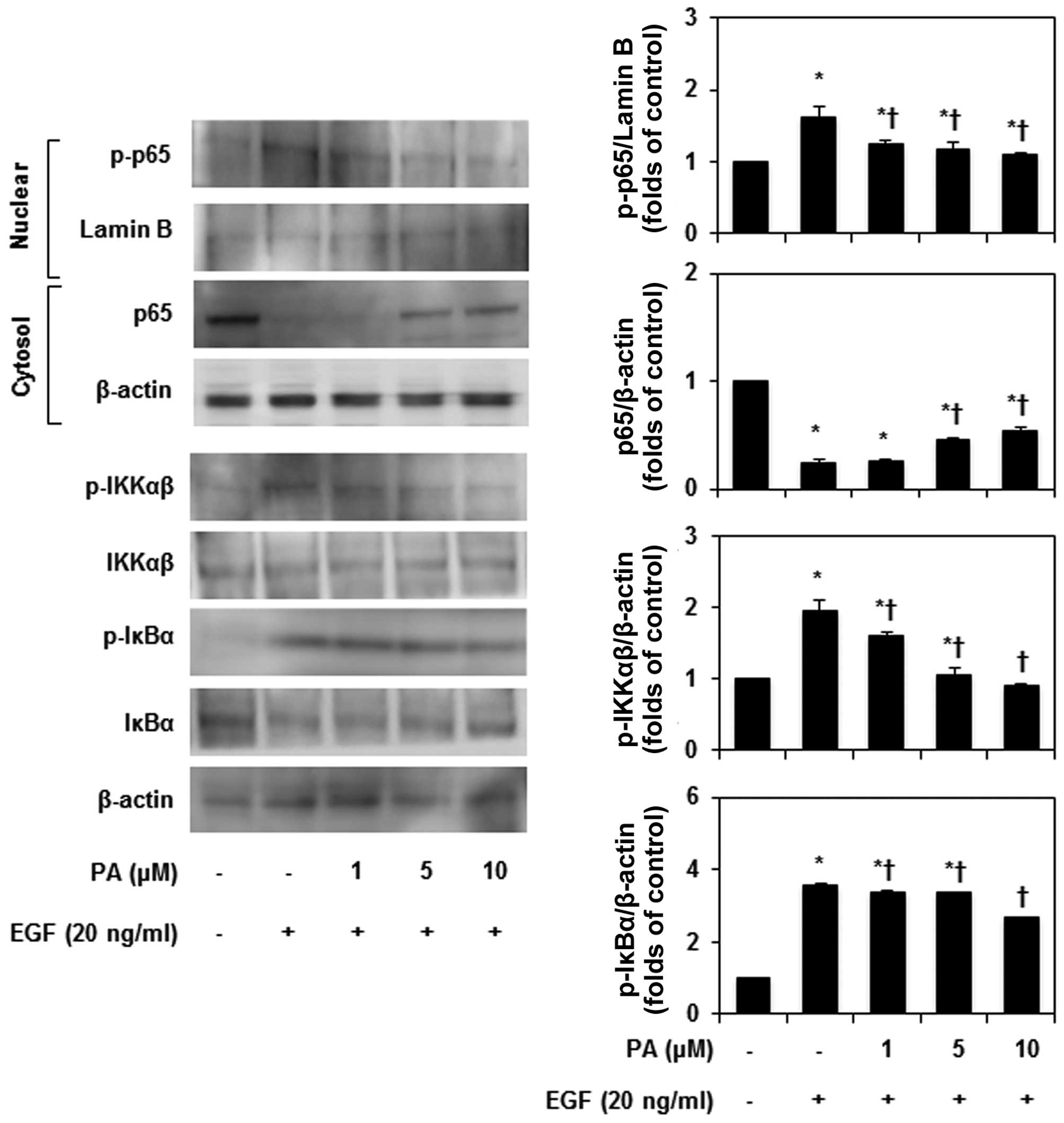
singling pathway in MDA-MB-231 cells treated with EGF. As shown in
Fig. 4, following EGF
administration, expression of nuclear NF-κB and cytosolic
phosphorylated IKKα/β and IκBα increased when MDA-MB-231 cells were
treated with EGF. In addition, PA significantly reduced IKKα/β and
IκBα phosphorylation in EGF-treated MDA-MB-231 cells. EGF
stimulated nuclear translocation of p65, and PA inhibited the p65
translocation in a dose-dependent manner. These results confirmed
that expression of the NF-κB DNA binding activity, decreased as a
result of PA. It is likely that PA inhibited MMP-9 expression by
decreasing the MMP-9 gene promoter binding activity of the NF-κB
and AP-1 transcription factor. These results indicate that
treatment with PA abrogated the effect of EGF on the expression
levels of genes, relevant to breast cancer metastasis through NF-κB
signaling.
Effects of PA on EGF-induced MAPKs and
PI3K/Akt/mTOR signal pathways
MAPKs and PI3K/Akt/mTOR pathways play important
roles in the migration and invasion of cancer cells through the
regulation of MMP-9 and FAK (26,27).
We therefore aimed to identify the precise MAPKs and PI3K/Akt/mTOR
pathway involved in PA inhibition of EGF-induced MMP-9 expression
and FAK phosphorylation, the MDA-MB-231 cells were exposed to
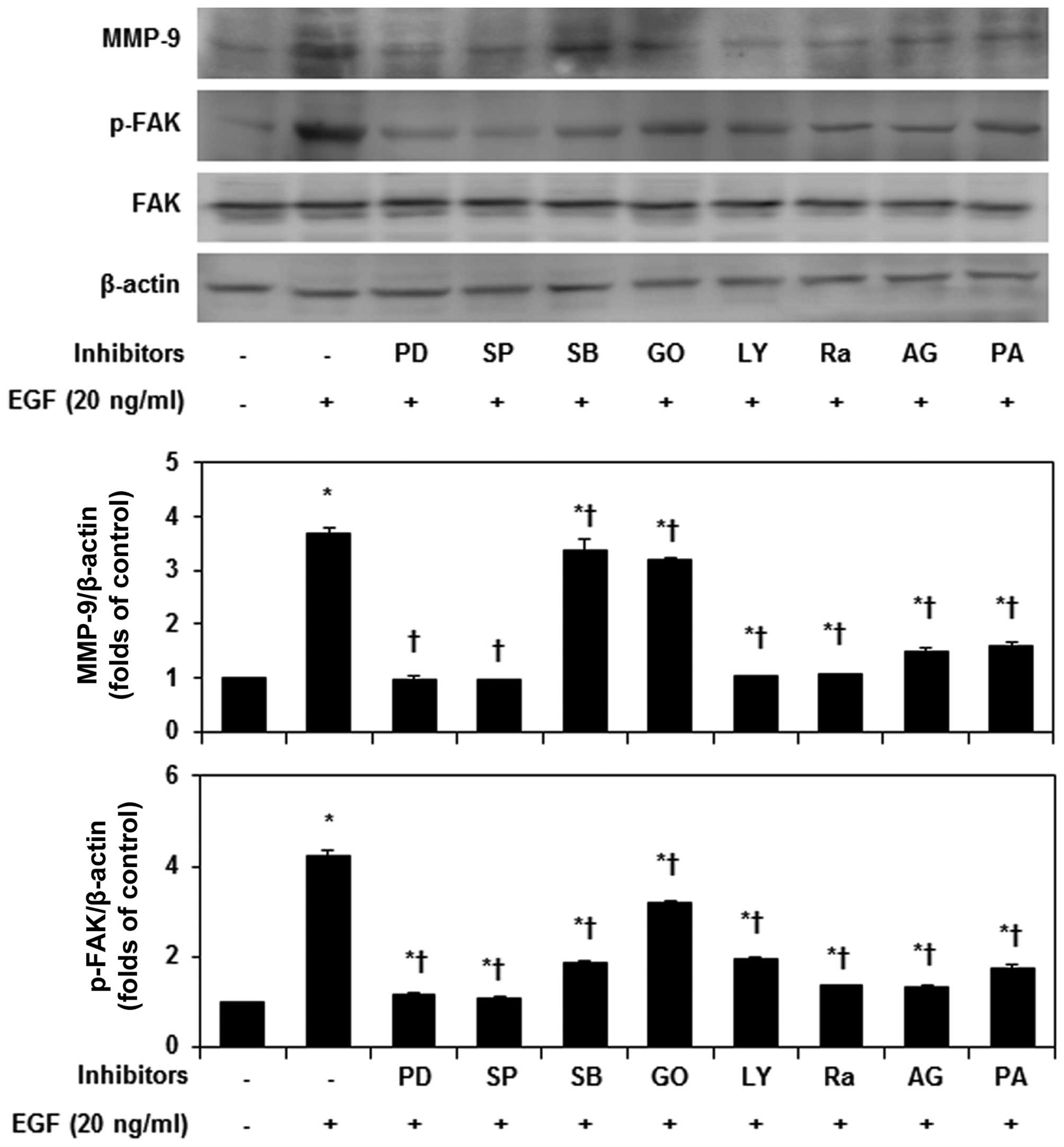
various kinase inhibitors. PD98059, SP600125, LY294002, rapamycin,
AG1478 and PA most strongly inhibited MMP-9 expression and FAK
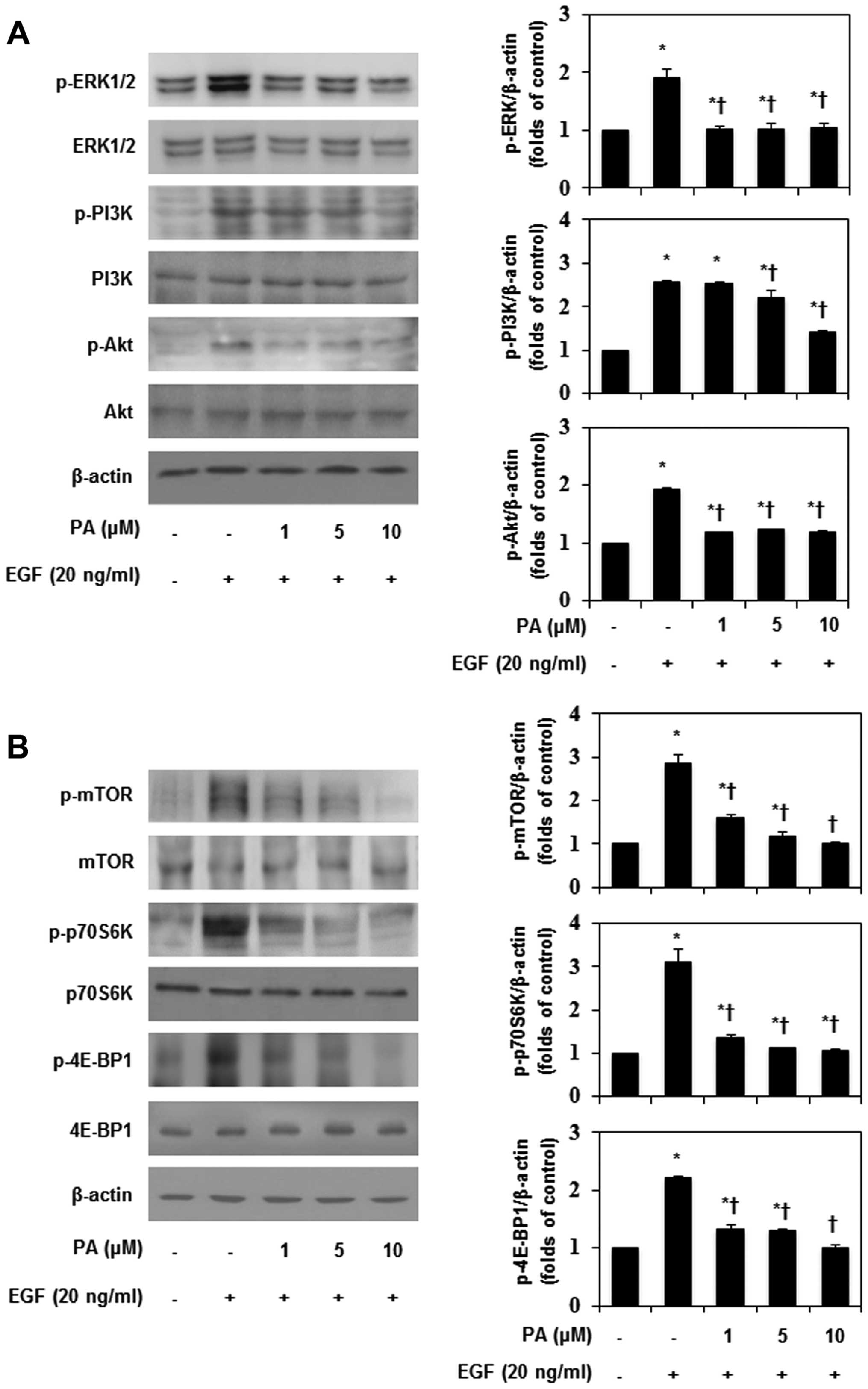
phosphorylation, in EGF-stimulated MDA-MB-231 cells (Fig. 5). As shown in Fig. 6A, PA inhibited the EGF-induced
phosphorylation of ERK, but had no effect on JNK and p38
phosphorylation (data not shown). In addition, PA significantly
suppressed EGF-induced PI3K/Akt phosphorylation. These results
indicate that inhibition of ERK and PI3K/Akt phosphorylation would
be an important way to block the invasion of MDA-MB-231 cells.
Additionally, EGF-induced MMP-9 expression and FAK phosphorylation
seem to be involved in the PI3K and NF-κB signaling pathways in
MDA-MB-231 cells. Akt-dependent regulation of NF-κB is controlled
by the mTOR signaling pathway (28). Therefore, the effects of PA on
EGF-induced mTOR phosphorylation were investigated in this study.
PA significantly inhibited EGF-induced mTOR phosphorylation in
MDA-MB-231 cells (Fig. 6B), and
inhibited the phosphorylation of p70S6K and 4E-BP1, which are
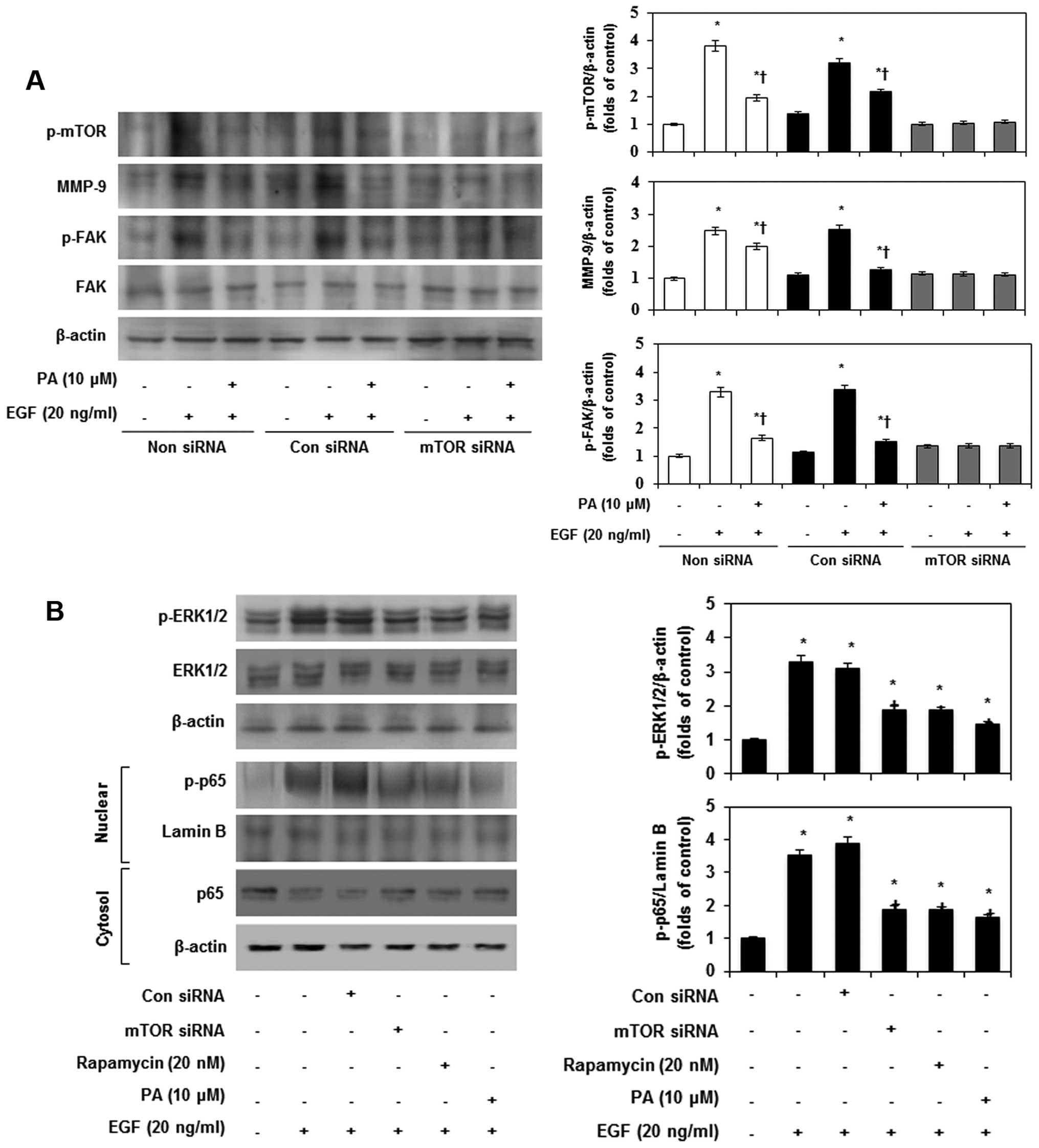
downstream effector molecules of mTOR. Subsequently, to confirm the
ERK-MAPK and mTOR-dependent MMP-9 expression and FAK
phosphorylation, MDA-MB-231 cells were pretreated with mTOR siRNA.
After transfection with mTOR siRNA for 24 h, the expression of mTOR
declined in EGF-stimulated MDA-MB-231 cells (Fig. 7A). However, non-siRNA and
transfection with control siRNA did not have any effects on mTOR
accumulation. Furthermore, PA and mTOR siRNA most strongly
suppressed MMP-9 expression and FAK phosphorylation in
EGF-stimulated MDA-MB-231 cells. To further investigate the role of
mTOR in EGF-induced phosphorylation of ERK-MAPK and translocation
of p65, we examined the effects of mTOR siRNA and rapamycin on
EGF-induced ERK-MAPK phosphorylation and p65 translocation by
immunoblotting. As shown in Fig.
7B, EGF-induced phosphorylation of ERK-MAPK and p65 were
inhibited by mTOR siRNA, rapamycin and PA. These results indicated
that mTOR partially contributes to EGF-induced MMP-9 expression,
phosphorylation of FAK by ERK-MAPK, and p65 activation in
MDA-MB-231 cells.
Discussion
Cancer invasion and metastasis are multistep and
complex processes that involve changes in cell adhesion, migration,
and invasion through the ECM (29). Aberrations in EGFR expression and
downstream signaling pathways contribute to the progression,
invasion, and maintenance of the malignant phenotype of many human
cancers, including breast cancer (2). In recent years, considerable
enthusiasm was generated for EGFR inhibitors as targeted agents for
cancer treatment. Evidence supporting the important role of MMP-9
in the invasive potential of malignancy in vitro and in
vivo has been reported (27).
A downstream intracellular signaling effector, FAK is mainly
located at the focal adhesion sites of migrating cells, and upon
phosphorylation, FAK promotes cell adhesion (30). EGF stimulates the upregulation of
MMP-9 and FAK signaling pathways in breast cancer cells (31).
Pomolic acid (PA), isolated from Euscaphis
japonica, has been shown to have anticancer activities such as
inhibiting the growth of leukemia cell lines and mediating the
apoptosis of human ovarian cancer cells. PA has also been reported
to demonstrate anti-proliferative activity against various cancer
cell lines (17). However, the
molecular mechanisms of the anti-metastatic potential of PA are not
fully understood in breast cancer cells.
In this study, PA effectively suppressed EGF-induced
MDA-MB-231 cell migration, invasion and cell motility through
inhibition of the EGFR-mediated ERK/PI3K/Akt/mTOR signaling
pathway, abating the expression of NF-κB and AP-1 and reducing the
expression of MMP-9 and the phosphorylation of FAK resulting an
anti-metastatic effect.
Lamellipodia and invadopodia formation are caused by
the polymerization of F-actin through the regulation of
cytoskeletal reorganization (23),
a process that is stimulated by EGF at the leading edge of cell. We
also found that PA inhibited EGF-induced cell motility and invasion
in MDA-MB-231 cells.
EGF controls MMP-9 expression and FAK
phosphorylation using various transcription factors such as AP-1,
SP-1, and NF-κB through MAPK, PI3K/Akt, and mTOR signaling pathways
(4,7,32).
It has been reported that NF-κB, AP-1, and SP-1 regulate cancer
proliferation and metastasis, and that they are also important
transcriptional elements of MMP-9 (32). In agreement with these reports, our
results showed that PA markedly suppresses the EGF-induced nuclear
translocation of NF-κB and AP-1 DNA binding activity, whereas it
did not affect the EGF-induced binding activity of SP-1.
The mitogenic effect of EGF is initiated by its
interaction with overexpressed EGFR and is transmitted by the
activation of the MAPKs, PI3K/Akt, and mTOR pathways (11). As several studies also indicated,
the MAPKs and PI3K/Akt pathways play crucial roles in regulating
the MMP-9 expression and FAK phosphorylation, and the suppression
of MAPKs has the potential to prevent invasion and metastasis in
various tumors (5). Our results
showed that PA significantly inhibited EGF-induced ERK-MAPK and
PI3K/Akt phosphorylation in MDA-MB-231 cells. The mitogenic effect
of EGF is mediated via activation of PI3K/Akt and IKK-dependent
activation of NF-κB (3). In this
study, PA inhibited the activation of PI3K and Akt which led to
inhibition of the downstream effector, NF-κB.
mTOR is regulated by the ERK-MAPK and PI3K/Akt
pathways in various cancer cells. The inhibition of mTOR suppressed
cell motility, which is independent of cell type and stimuli
(33). Also, mTOR regulates the
phosphorylation of 70S6K and 4E-BP1, which inhibit cell
proliferation and growth by blocking the translation of mRNA. In
addition, activated EGFR can regulate MMP-9 expression and cell
proliferation through EGF binding. It has been reported that MMP-9
expression is needed to regulate the EGF-mediated activation of the
PI3K/Akt/mTOR and MAPK pathways by phosphorylating protein
translational regulators, including 70S6K and 4E-BP1 (34). Additionally, previous studies have
demonstrated that the mTOR inhibitor also inhibited EGF-induced
actin reorganization and cell migration (35). In accordance with these results,
our results showed that PA suppresses the EGF-induced
phosphorylation of mTOR/p70S6K/4E-BP1 in MDA-MB-231 cells. Also, we
found that PD98059, SP600125, LY294002, rapamycin, AG1478, and PA
specifically inhibited expression of MMP-9 and phosphorylation of
FAK. Furthermore, mTOR siRNA transfection inhibited EGF-induced
MMP-9 expression and FAK phosphorylation, and the suppressive
effect of PA on the expression of MMP-9 and phosphorylation of FAK
also decreased in mTOR-knockdown cells. These results suggest that
PA can inhibit EGF-induced expression of MMP-9 and phosphorylation
of FAK in MDA-MB-231 cells by inhibiting PI3K/Akt/mTOR signaling
pathways, leadings to activation of NF-κB and ERK/MAPK signaling
pathways. Our findings demonstrated for the first time that PA
inhibits the migration, invasion, and cell motility of highly
metastatic MDA-MB-231 breast cancer cells by downregulating MMP-9
expression and FAK phosphorylation via the inhibition of
EGFR-mediated NF-κB/ERK/mTOR signaling pathways. Based on these
results, we suggest that PA is a potent candidate for a therapeutic
anticancer agent and may be used for the prevention and treatment
of breast cancer.
Acknowledgements
This study was supported by Basic Science Research
Program through the National Research Foundation of Korea (NRF)
funded by the Ministry of Education (NRF-2016R1A6A1A03011325).
Abbreviations:
|
AP-1
|
activator protein-1
|
|
4E-BP1
|
eukaryotic translation initiation
factor 4E-binding protein 1
|
|
ECM
|
extracellular matrix
|
|
EGF
|
epidermal growth factor
|
|
EGFR
|
epidermal growth factor receptor
|
|
ERK
|
extracellular signal-regulated
kinase
|
|
F-actin
|
filamentous actin
|
|
FAK
|
focal adhesion kinase
|
|
JNK
|
c-Jun N-terminal kinase
|
|
MAPK
|
mitogen-activated protein kinase
|
|
MDA-MB-231
|
MD Anderson-mammary breast cancer cell
line-231
|
|
MMPs
|
matrix metalloproteinases
|
|
NF-κB
|
nuclear factor-κB
|
|
mTOR
|
mammalian target of rapamycin
|
|
p70S6K
|
70 kDa ribosomal protein S6 kinase
|
|
PI3K/Akt
|
phosphatidylinositol 3-kinase/protein
kinase B
|
References
|
1
|
Hu Z, Xu R, Liu J, Zhang Y, Du J, Li W,
Zhang W, Li Y, Zhu Y and Gu L: GEP100 regulates epidermal growth
factor-induced MDA-MB-231 breast cancer cell invasion through the
activation of Arf6/ERK/uPAR signaling pathway. Exp Cell Res.
319:1932–1941. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee YC, Lee LM, Yang CH, Lin AM, Huang YC,
Hsu CC, Chen MS, Chi CW, Yin PH, Kuo CD, et al: Norcantharidin
suppresses cell growth and migration with enhanced anticancer
activity of gefitinib and cisplatin in human non-small cell lung
cancer cells. Oncol Rep. 29:237–243. 2013.
|
|
3
|
Saxena R, Chandra V, Manohar M, Hajela K,
Debnath U, Prabhakar YS, Saini KS, Konwar R, Kumar S, Megu K, et
al: Chemotherapeutic potential of
2-[piperidinoethoxyphenyl]-3-phenyl-2H-benzo(b)pyran in estrogen
receptor-negative breast cancer cells: Action via prevention of
EGFR activation and combined inhibition of PI-3-K/Akt/FOXO and
MEK/Erk/AP-1 pathways. PLoS One. 8:e662462013. View Article : Google Scholar
|
|
4
|
Jeong YJ, Choi Y, Shin JM, Cho HJ, Kang
JH, Park KK, Choe JY, Bae YS, Han SM, Kim CH, et al: Melittin
suppresses EGF-induced cell motility and invasion by inhibiting
PI3K/Akt/mTOR signaling pathway in breast cancer cells. Food Chem
Toxicol. 68:218–225. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chun J and Kim YS: Platycodin D inhibits
migration, invasion, and growth of MDA-MB-231 human breast cancer
cells via suppression of EGFR-mediated Akt and MAPK pathways. Chem
Biol Interact. 205:212–221. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cho HJ, Kang JH, Kwak JY, Lee TS, Lee IS,
Park NG, Nakajima H, Magae J and Chang YC: Ascofuranone suppresses
PMA-mediated matrix metalloproteinase-9 gene activation through the
Ras/Raf/MEK/ERK- and Ap1-dependent mechanisms. Carcinogenesis.
28:1104–1110. 2007. View Article : Google Scholar
|
|
7
|
Wang L, Ling Y, Chen Y, Li CL, Feng F, You
QD, Lu N and Guo QL: Flavonoid baicalein suppresses adhesion,
migration and invasion of MDA-MB-231 human breast cancer cells.
Cancer Lett. 297:42–48. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
McLean GW, Carragher NO, Avizienyte E,
Evans J, Brunton VG and Frame MC: The role of focal-adhesion kinase
in cancer - a new therapeutic opportunity. Nat Rev Cancer.
5:505–515. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee WJ, Chen WK, Wang CJ, Lin WL and Tseng
TH: Apigenin inhibits HGF-promoted invasive growth and metastasis
involving blocking PI3K/Akt pathway and beta 4 integrin function in
MDA-MB-231 breast cancer cells. Toxicol Appl Pharmacol.
226:178–191. 2008. View Article : Google Scholar
|
|
10
|
Hwang YP, Yun HJ, Choi JH, Han EH, Kim HG,
Song GY, Kwon KI, Jeong TC and Jeong HG: Suppression of EGF-induced
tumor cell migration and matrix metalloproteinase-9 expression by
capsaicin via the inhibition of EGFR-mediated FAK/Akt, PKC/Raf/ERK,
p38 MAPK, and AP-1 signaling. Mol Nutr Food Res. 55:594–605. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shin JM, Jeong YJ, Cho HJ, Park KK, Chung
IK, Lee IK, Kwak JY, Chang HW, Kim CH, Moon SK, et al: Melittin
suppresses HIF-1α/VEGF expression through inhibition of ERK and
mTOR/p70S6K pathway in human cervical carcinoma cells. PLoS One.
8:e693802013. View Article : Google Scholar
|
|
12
|
Jeong YJ, Cho HJ, Magae J, Lee IK, Park KG
and Chang YC: Ascofuranone suppresses EGF-induced HIF-1α protein
synthesis by inhibition of the Akt/mTOR/p70S6K pathway in
MDA-MB-231 breast cancer cells. Toxicol Appl Pharmacol.
273:542–550. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee MK, Lee KY, Jeon HY, Sung SH and Kim
YC: Antifibrotic activity of triterpenoids from the aerial parts of
Euscaphis japonica on hepatic stellate cells. J Enzyme Inhib Med
Chem. 24:1276–1279. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee HH, Lin CT and Yang LL:
Neuroprotection and free radical scavenging effects of Osmanthus
fragrans. J Biomed Sci. 14:819–827. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fernandes J, Weinlich R, Castilho RO,
Amarante-Mendes GP and Gattass CR: Pomolic acid may overcome
multidrug resistance mediated by overexpression of anti-apoptotic
Bcl-2 proteins. Cancer Lett. 245:315–320. 2007. View Article : Google Scholar
|
|
16
|
Yoo KH, Park JH, Lee DK, Fu YY, Baek NI
and Chung IS: Pomolic acid induces apoptosis in SK-OV-3 human
ovarian adenocarcinoma cells through the mitochondrial-mediated
intrinsic and death receptor-induced extrinsic pathways. Oncol
Lett. 5:386–390. 2013.
|
|
17
|
Yoshida M, Fuchigami M, Nagao T, Okabe H,
Matsunaga K, Takata J, Karube Y, Tsuchihashi R, Kinjo J, Mihashi K,
et al: Antiproliferative constituents from Umbelliferae plants VII.
Active triterpenes and rosmarinic acid from Centella asiatica. Biol
Pharm Bull. 28:173–175. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim B, Kim JH and Park B: Pomolic acid
inhibits invasion of breast cancer cells through the suppression of
CXC chemokine receptor type 4 expression. J Cell Biochem.
117:1296–1307. 2016. View Article : Google Scholar
|
|
19
|
Kim B and Park B: Baohuoside I suppresses
invasion of cervical and breast cancer cells through the
downregulation of CXCR4 chemokine receptor expression.
Biochemistry. 53:7562–7569. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Park JH, Lee KY, Park B and Yoon J:
Suppression of Th2 chemokines by crocin via blocking of
ERK-MAPK/NF-κB/STAT1 signalling pathways in TNF-α/IFN-γ-stimulated
human epidermal keratinocytes. Exp Dermatol. 24:634–636. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Park JH and Yoon J: Schizandrin inhibits
fibrosis and epithelial-mesenchymal transition in transforming
growth factor-β1-stimulated AML12 cells. Int Immunopharmacol.
25:276–284. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nabeshima K, Inoue T, Shimao Y and
Sameshima T: Matrix metalloproteinases in tumor invasion: Role for
cell migration. Pathol Int. 52:255–264. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Marchesin V, Montagnac G and Chavrier P:
ARF6 promotes the formation of Rac1 and WAVE-dependent ventral
F-actin rosettes in breast cancer cells in response to epidermal
growth factor. PLoS One. 10:e01217472015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cho HJ, Jeong YJ, Park KK, Park YY, Chung
IK, Lee KG, Yeo JH, Han SM, Bae YS and Chang YC: Bee venom
suppresses PMA-mediated MMP-9 gene activation via JNK/p38 and
NF-kappaB-dependent mechanisms. J Ethnopharmacol. 127:662–668.
2010. View Article : Google Scholar
|
|
25
|
Sandur SK, Deorukhkar A, Pandey MK, Pabón
AM, Shentu S, Guha S, Aggarwal BB and Krishnan S: Curcumin
modulates the radiosensitivity of colorectal cancer cells by
suppressing constitutive and inducible NF-kappaB activity. Int J
Radiat Oncol Biol Phys. 75:534–542. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rothhut B, Ghoneim C, Antonicelli F and
Soula-Rothhut M: Epidermal growth factor stimulates matrix
metalloproteinase-9 expression and invasion in human follicular
thyroid carcinoma cells through Focal adhesion kinase. Biochimie.
89:613–624. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hsieh CY, Tsai PC, Tseng CH, Chen YL,
Chang LS and Lin SR: Inhibition of EGF/EGFR activation with
naphtho[1,2-b]furan-4,5-dione blocks migration and invasion of
MDA-MB-231 cells. Toxicol In Vitro. 27:1–10. 2013. View Article : Google Scholar
|
|
28
|
Dan HC, Cooper MJ, Cogswell PC, Duncan JA,
Ting JP and Baldwin AS: Akt-dependent regulation of NF-{kappa}B is
controlled by mTOR and Raptor in association with IKK. Genes Dev.
22:1490–1500. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Friedl P, Locker J, Sahai E and Segall JE:
Classifying collective cancer cell invasion. Nat Cell Biol.
14:777–783. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gialeli Ch, Theocharis AD, Kletsas D,
Tzanakakis GN and Karamanos NK: Expression of matrix macromolecules
and functional properties of EGF-responsive colon cancer cells are
inhibited by panitumumab. Invest New Drugs. 31:516–524. 2013.
View Article : Google Scholar
|
|
31
|
Sen T and Chatterjee A:
Epigallocatechin-3-gallate (EGCG) downregulates EGF-induced MMP-9
in breast cancer cells: Involvement of integrin receptor α5β1 in
the process. Eur J Nutr. 50:465–478. 2011. View Article : Google Scholar
|
|
32
|
Han H, Du B, Pan X, Liu J, Zhao Q, Lian X,
Qian M and Liu M: CADPE inhibits PMA-stimulated gastric carcinoma
cell invasion and matrix metalloproteinase-9 expression by
FAK/MEK/ERK-mediated AP-1 activation. Mol Cancer Res. 8:1477–1488.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen JS, Wang Q, Fu XH, Huang XH, Chen XL,
Cao LQ, Chen LZ, Tan HX, Li W, Bi J, et al: Involvement of
PI3K/PTEN/AKT/mTOR pathway in invasion and metastasis in
hepatocellular carcinoma: Association with MMP-9. Hepatol Res.
39:177–186. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu L, Li F, Cardelli JA, Martin KA,
Blenis J and Huang S: Rapamycin inhibits cell motility by
suppression of mTOR-mediated S6K1 and 4E-BP1 pathways. Oncogene.
25:7029–7040. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Berven LA, Willard FS and Crouch MF: Role
of the p70(S6K) pathway in regulating the actin cytoskeleton and
cell migration. Exp Cell Res. 296:183–195. 2004. View Article : Google Scholar : PubMed/NCBI
|