Introduction
The American Cancer Society estimated 45,780 new
cases of oral cavity and pharyngeal cancer, and 8,650 deaths from
these tumors, in 2015 in the United States (1). By advances in surgery and radiation
therapy, the 5-year survival rate for oropharyngeal cancer has
increased to 66%, but the rate is still unsatisfactory in
comparison with some other site cancers including prostate, thyroid
and breast; the rates of these types of cancer are >90%
(1). A primary cause for the
unfavorable prognosis is patient death from the cancer metastasized
at regional and distant sites (2–5).
Squamous cell carcinoma (SCC) accounts for approximately 90% of
oral and oropharyngeal malignancies in the United States and tongue
is a common site of the malignant diseases (6,7). The
rate of nodal metastasis is higher in tongue cancer patients than
oral cavity cancer patients whose rate is 30% on their initial
evaluation (8,9). Several studies have shown a high rate
of occult nodal metastasis (20–40%) in tongue SCC patients with no
evidence of regional spread on clinical or radiographic evaluation
(8,10–15).
There is a tendency that tongue cancer increases in young females
in recent 2–3 decades (16–18).
Thus, metastasis suppression is a main and urgent subject in the
treatments of patients with tongue SCC.
The process of metastasis is complex and involves
tumor growth, the extra-cellular matrix breakdown, invasion to the
vessels, escape from immune surveillance, transport to other sites
with adhesion to the vessel, subsequent invasion into an organ
where tumor cells proliferate, grow and spread. Various molecules
participate in the process but their roles remain incompletely
understood. Tumor necrosis factor (TNF)-α is one of major
regulators of inflammation, playing in the cytokine network. TNF-α
is synthesized primarily by immune cells as a 34-kDa type II
transmembrane protein (19) and a
soluble form of the C-terminal 17 kDa portion is released from
cells through cleavage by TNF-α-converting enzyme (20). TNF-α forms a homotrimer that
activates cells via two distinct receptors (21). TNFR1 (p55, CD120a) binds the
soluble ligand and is expressed on most cells, and TNFR2 (p75,
CD120b) primarily binds the transmembrane form and is found on
hematopoietic cells (22). TNF-α
required high doses or injection to the tumor tissue for induction
of hemorrhagic tumor necrosis (19). In contrast, TNF-α at low doses
exerted angiogenic activity in both the rabbit cornea and chick
chorioallantoic membrane models (23,24).
By treating tumor cells or mice TNF-α augmented the metastatic
activity of transplanted tumor cells (25,26).
These reports suggest TNF-α activity for cancer progression. Thus,
whether TNF-α is an anticancer molecule or therapeutic target is an
important issue in the treatment of cancer including tongue
SCC.
To study a relationship between TNF-α and tongue
cancer metastasis, we made highly metastatic oral squamous cell
carcinoma (OSCC) cells by repeated implantation of lymph
node-metastasized OSCC cells into a nude mouse tongue (27) and investigated secretion of TNF-α
and MMPs, and expression of TNF-α receptors and molecules involved
in TNF-α signaling pathway. Moreover, mRNA expression in highly
metastatic OSCC cells after lung metastasis was measured by DNA
micro-array method, and migration activity by wound healing
assay.
Materials and methods
Preparation of highly metastatic cell
lines from a GFP-expressing OSCC cell line
The human OSCC cell line SAS cells were transfected
with the pAcGFP1-C1 vector (Clontech Laboratories, Mountain View,
CA, USA) using FuGENE 6 (Roche Diagnostics, Indianapolis, IN, USA),
then the cells with strong GFP fluorescence were isolated by flow
cytometry. The cells were selected in Dulbecco’s modified Eagle’s
medium (DMEM) supplemented with 1 mg/ml G418 (Life Technologies,
Grand Island, NY, USA). The cells with bright GFP were designated
GSAS and used as the parental cells. Highly metastatic cell lines
were prepared according the method previously described (27). Briefly, GSAS cells
(5.0×105 in 50 μl serum-free DMEM medium were injected
into the tongue of a nude mouse and after 21 days were harvested
from metastasized cervical lymph nodes. These GSAS cells were
cultured in DMEM medium supplemented with 10% FBS and transplanted
into the tongue of another nude mouse. This procedure was repeated
and GSAS cells after 5 passages were referred to as GSAS/N5. To
obtain a lung metastatic cell line, GSAS/N5 was injected into the
tongue of a nude mouse. The cells that metastasized to the lungs
(GSAS/L) were cultured for use.
TNF-α assay
TNF-α in the GSAS culture medium was assessed
by enzyme-linked immunosorbent assay (ELISA) using Human TNF-α
Quatikaine ELISA kit (R&D Systems, Inc., Minneapolis, MN, USA).
Cells (2.0×106) were cultured in serum-free DMEM medium
for 24 h and 25 μl of the supernatant was incubated in an
anti-TNF-α antibody-adsorbed well for 4 h at room temperature,
followed by washing. Biotin-labeled anti-TNF-α antibody was poured
into the well, and after washing streptavidin-labeled peroxidese
was incubated for 10 min in the well. After washing, 100 μl of TMB
solution was added and incubated for 10 min, followed by 50 μl of 2
M HCl. The absorbance of the solution was measured at 450 nm.
Total RNA extraction and reverse
transcription-PCR
The cells were incubated in serum-free DMEM in the
presence or absence of 100 μM NBD peptide (Imgenex Corp., San
Diego, CA, USA). After 24 h, total RNA was isolated using the
RNeasy Mini kit (Qiagen, Valencia, CA, USA). The RNA quantity,
purity, and integrity were evaluated using a NanoDrop
spectrophotometer (Thermo Fisher Scientific, Inc., Waltham, MA,
USA). Complementary DNA was synthesized from total RNA using the
PrimeScript RT reagent kit (Takara Bio Inc., Shiga, Japan).
Gene-specific primer sets were designed using the Custom Primers
software program (Invitrogen, Carlsbad, CA, USA). The primer
sequences and the amplification conditions are shown in Table I. The expression of β-actin was
used as an internal control.
 | Table IRT-PCR conditions. |
Table I
RT-PCR conditions.
| Gene | Primer sequences
(5′-3′) | Annealing
temperature (°C) | Cycles |
|---|
| TNF-α | Forward:
CGGGACGTCGAGCTGGCCGAGGAG
Reverse: TTGCAGTGTGTTATCCGTGCTGTC | 50 | 35 |
| TNFR-1 | Forward:
TACATTGCAGCCTCTGCCTC
Reverse: AGAGCTTGGACTTCCACCGT | 50 | 35 |
| TNFR-2 | Forward:
ACATCAGACGTGGTGTGCAA
Reverse: CCAACTGGAAGAGCGAAGTC | 50 | 35 |
| Angptl4 | Forward:
CCTCAGGGGTCTCCGCCATTTT
Reverse: GGGCCGGTTGAAGTCCACTGA | 60 | 30 |
| β-actin | Forward:
CCAAGGCCAACCGCGAGAAGATTGAC
Reverse: AGGGTACATGGTGGTGCCGCCAGAC | 57 | 27 |
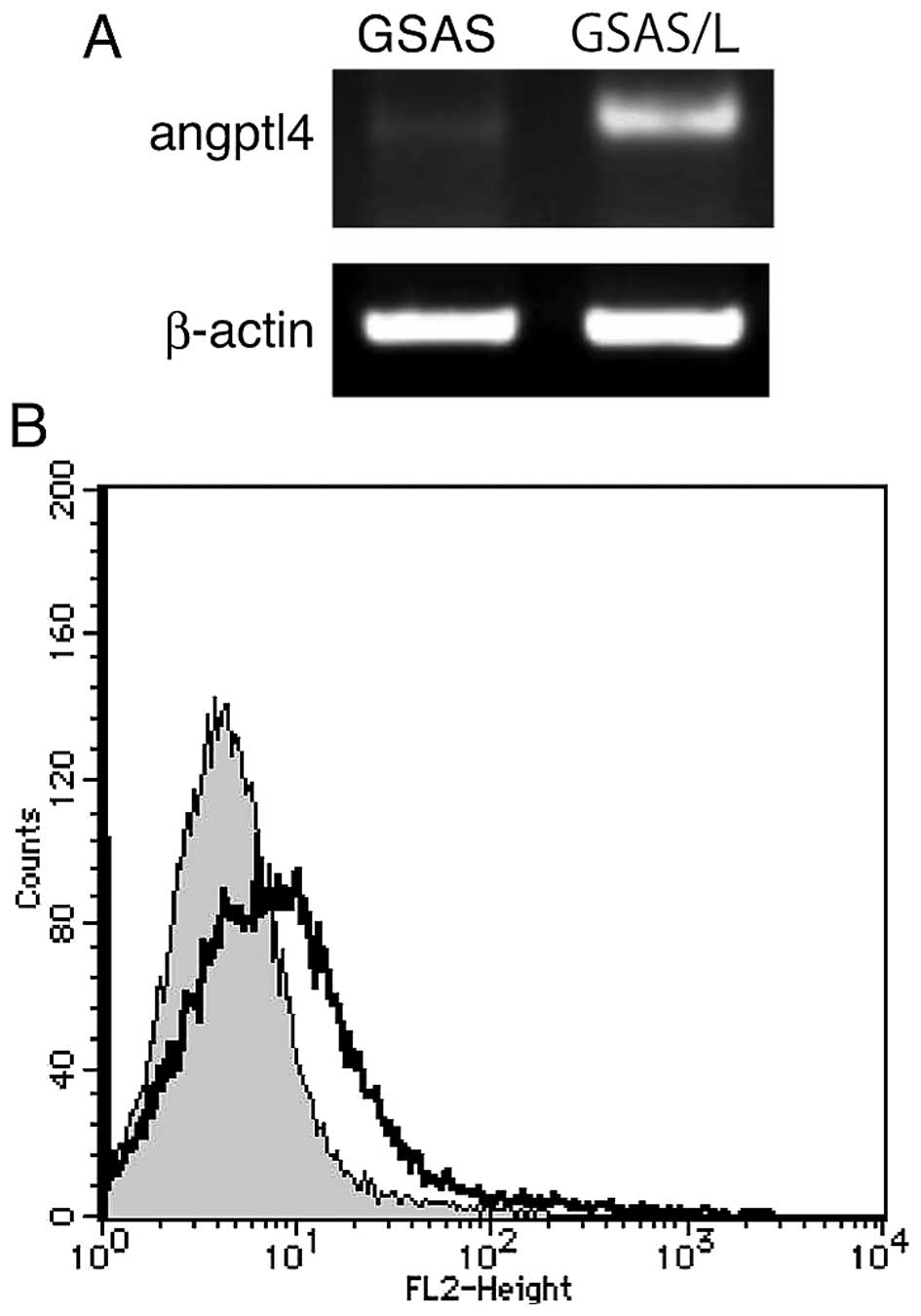
Flow cytometry
GSA/L cells were treated for 30 min with a rabbit
anti-Angptl4 antibody (Abgent, Inc., San Diego, CA, USA) or a
control rabbit IgG (Dako Corp., Carpinteria, CA, USA), followed by
washing with PBS twice and incubation with phycoerythrin-conjugated
anti-rabbit IgG (Santa Cruz Biotechnology). Angptl4 antigen was
quantified by FACScan (BD Biosciences, San Jose, CA, USA).
Western blotting
GSAS, GSAS/N3 and GSAS/N5 cells (2.0×106)
were cultured in serum-free DMEM medium for 24 h. Cell extracts
were analyzed in an SDS-polyacrylamide gel and transferred onto a
nitrocellulose membrane (Bio-Rad Laboratories, Hercules, CA, USA).
After blocking with 5% skim milk, the membrane was incubated
overnight with the first antibody against Akt (1:1,000; Cell
Signaling Technology, Boston, MA, USA), phosho-Akt (1:1,000; Santa
Cruz Biotechnology, Dallas, TX, USA), phospho-IKKα/β (1:1,000; Cell
Signaling Technology), or β-actin (1:5,000; Sigma-Aldrich, St.
Louis, MO, USA). After washing, the membrane was treated for 1 h
with HRP-labeled second antibody (EnVision+, Dako Corp.). Bands
were visualized using ECL (GE Healthcare, Buckinghamshire, UK).
Electrophoretic mobility shift assay
(EMSA)
GSAS, GSAS/N3 and GSAS/N5 cells (2.0×106)
were cultured in serum-free DMEM medium for 24 h. Nuclear extracts
were prepared using the NE-PER™ Nuclear and Cytoplasmic Extraction
reagents (Pierce Chemical Co., Dallas, TX, USA). The DNA binding
reaction was performed with a biotin end-labeled NF-κB
oligonucleotide (5′-AGCTTGGGGACTTTCCGAG-3′) using a LightShift™
Chemiluminescent EMSA kit (Pierce Chemical). A total of 20 μl of
the extracts was electrophoresed in 6% polyacrylamide gels and
transferred onto a nitrocellulose membrane (Bio-Rad Laboratories).
Biotin-labeled DNA was detected with the Chemiluminescent Nucleic
Acid Detection Module (Pierce Chemical). For supershift reactions,
extracts were preincubated with an anti-p65 antibody (Santa Cruz
Biotechnology) for 20 min. All of the above procedures were done
according to the manufacturer’s protocols.
Gelatin zymography
Cells were seeded in a well of a 24-well plate to
form a subconfluent sheet, then cultured in serum-free DMEM medium
containing 0.5% BSA for 24 h, followed by a 24-h incubation in 10
ng/ml TNF-α in the presence or absence of 100 μM NBD peptide. The
supernatant was obtained by centrifugation. To adjust the sample
conditions, cells in a well were treated with 50 mM Tris-HCl, pH
6.8, 2% SDS, 10% glycerol and protein amount was measured using DC
protein assay (Bio-Rad Laboratories). Supernatant samples were
diluted with 50 mM Tris-HCl, pH 6.8, 10% SDS, 50% glycerol to
adjust to a same concentration per the protein amount of the cells
in a well and 40 μl of the sample was electrophoresed in a 10% SDS
polyacrylamide gel containing 1 mg/ml gelatin. After washing twice
in 10 mM Tris-HCl, pH 8.0, 2.5% Triton X-100, the gel was incubated
in 50 mM Tris-HCl, pH 8.0, 0.5 mM CaCl2, 1 μM
ZnCl2 at 37°C for 16 h. Then, the gel was stained in
Coomassie brilliant blue solution. To determine the MMPs, standard
pro-MMP-2, active MMP-2 and pro-MMP-9 (Cosmo Bio Co., Ltd., Tokyo,
Japan) were electrophoresed in the same gel together with the
samples.
Invasion assay
CytoSelect™ 24-well Cell Invasion Assay (Cell
Biolabs, Inc., San Diego, CA, USA) was used to measure cancer cell
invasion activity according to the manufacturer’s instructions.
Cells (2.0×105) were suspended in serum-free DMEM, and
then seeded into the upper chamber in the presence or absence of
100 μM NBD peptide. Serum-free DMEM was placed in the lower
chamber. After incubation for 24 h, cells that invaded through
Matrigel (BD Biosciences) layer to the lower surface of the 8
μm-pored membrane partitioning the chambers were fixed in 100%
methanol, stained with 1% toluidine blue and counted in 4
microscopic fields (×400). The average cell number per a field in
triplicate assay is shown.
Gene expression microarrays
Total RNA of GSAS, GSAS/N3, GSAS/N5 or GSAS/L cells
was isolated using the RNeasy Mini kit (Qiagen). The RNA quantity,
purity, and integrity were evaluated using a NanoDrop
spectrophotometer (Thermo Fisher Scientific). The cRNA was
amplified, labelled, and hybridised by an Agilent Human GE 4×44K v2
Microarray (Agilent Technologies, Santa Clara, CA, USA) according
to the manufacturer’s instructions. Signals of probes in all
hybridised microarrays were scanned by an Agilent scanner and
calculated using Feature Extraction Software version 9.5.1.1
(Agilent Technologies).
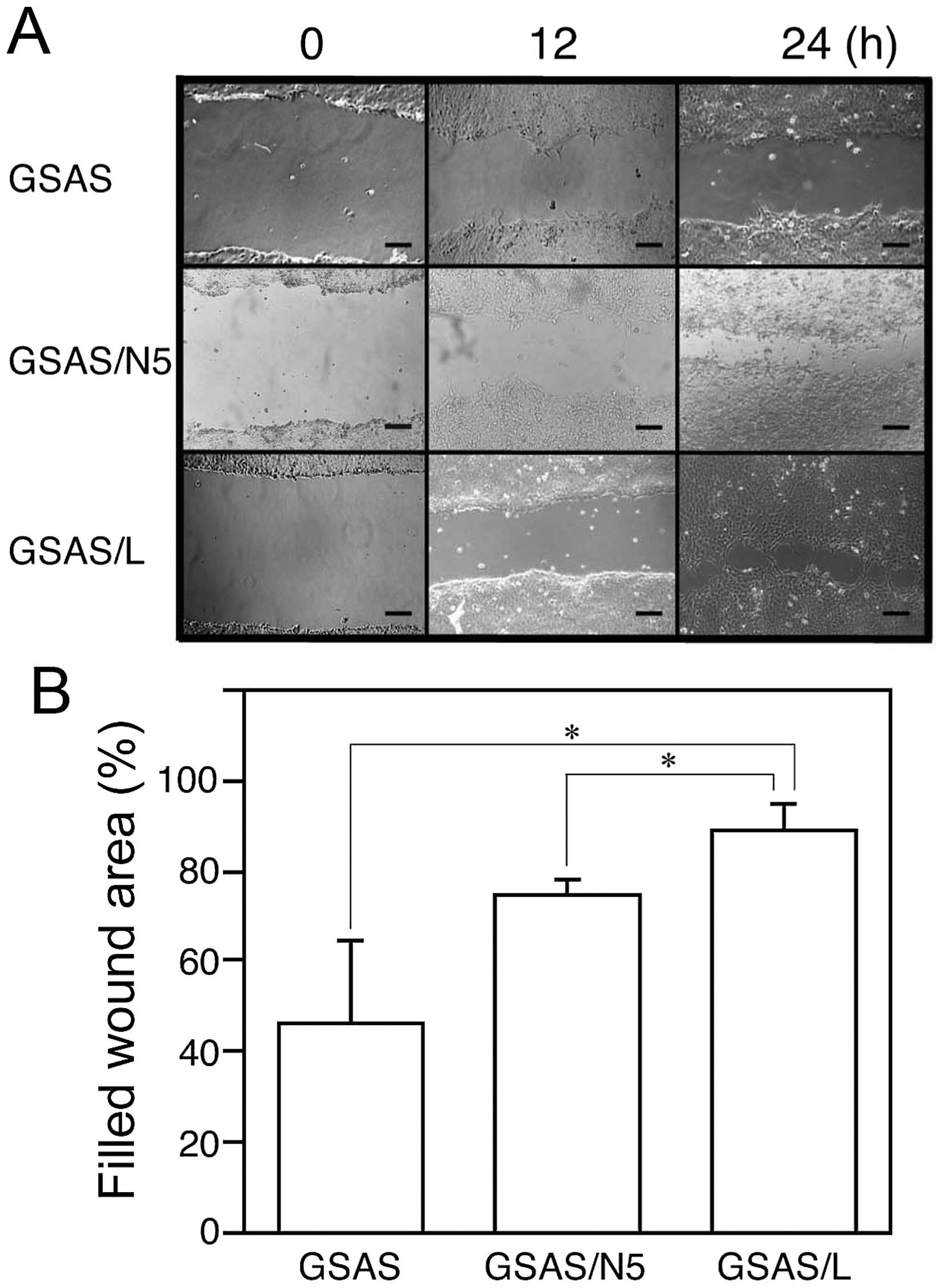
Wound healing assay
GSAS, GSAS/N3 and GSAS/N5 cells (5.0×105)
were seeded in 6-well plates and were cultured in DMEM containing
10% of FBS for 24 h. A line was scratched with a yellow pipette tip
in the 70–80% confluent monolayer, and the cellular debris were
removed by washing with serum-free DMEM. Then, the cell sheets were
incubated in serum-free DMEM and the cell migration into the wound
area was photographed with a phase-contrast microscope for 24 h.
The distance between the cell migration fronts of both sides was
measured vertically against the initial scratch border at three
points at random after 24 h from scratching and the percentage of
the average distance vs. the distance between the initial scratch
borders was calculated. All the assays were performed as a set of
three independent experiments.
Statistical analysis
Statistical analysis was performed using the
unpaired Student’s t-test. Values are expressed as means ± SD.
Results
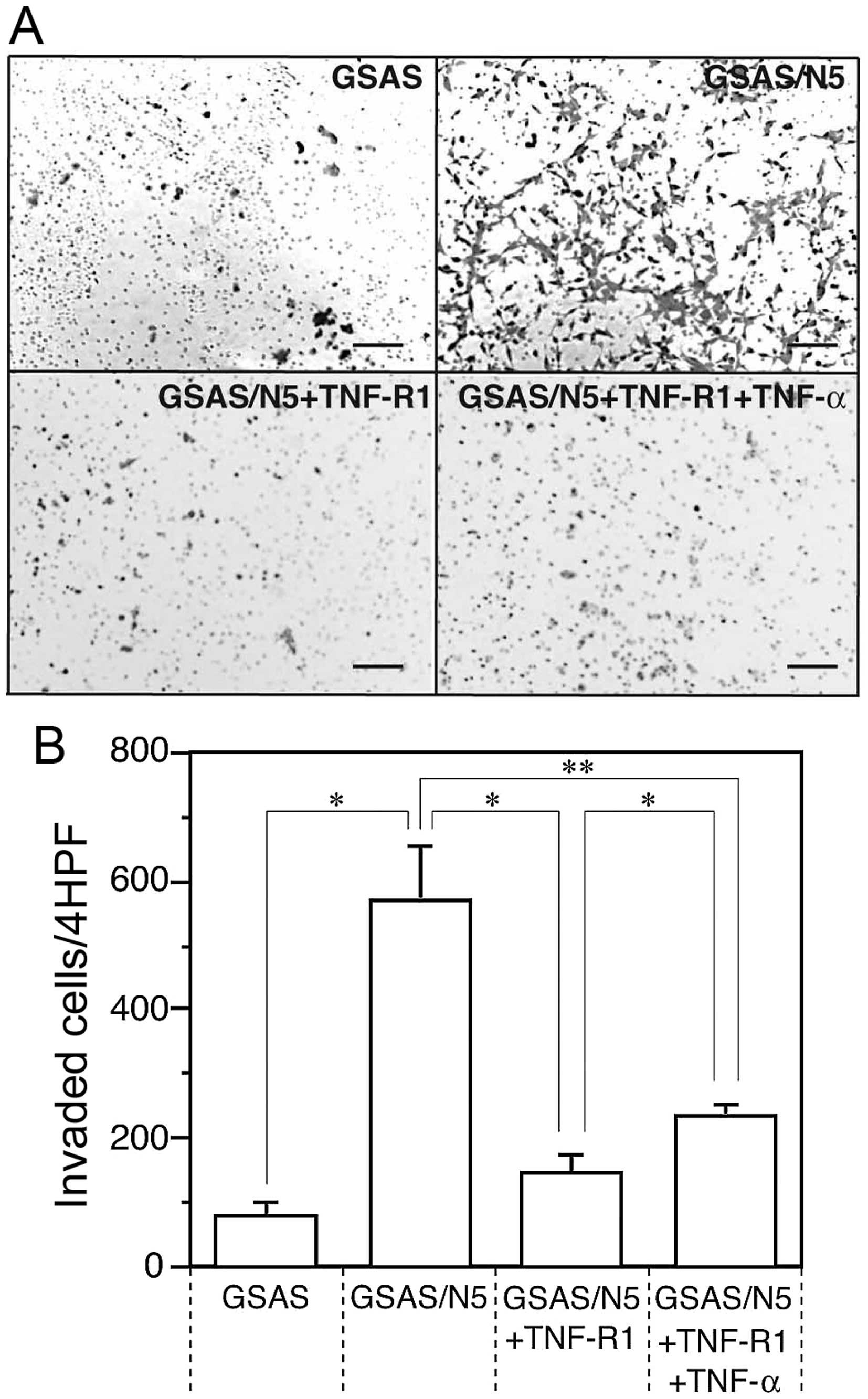
Enhancement of invasive activity of
GSAS/N5 cells
High invasiveness facilitates spread and metastasis
of cancer cells, thus, invasive activity was measured for GSAS
cells and GSAS/N5 cells and compared between them. Invasive
activity of GSAS/N5 cells was >7-fold higher than that of GSAS
cells (Fig. 1). The invasive
activity of GSAS/N5 cells was mostly inhibited in the presence of
soluble TNF-R1 and the inhibitory effect of the receptor was
decreased by TNF-α addition (Fig.
1B). The result indicated that invasive activity was enhanced
in GSAS/N5 cells and the enhanced invasive activity of the highly
metastatic cancer cells was mainly dependent on TNF-R1.
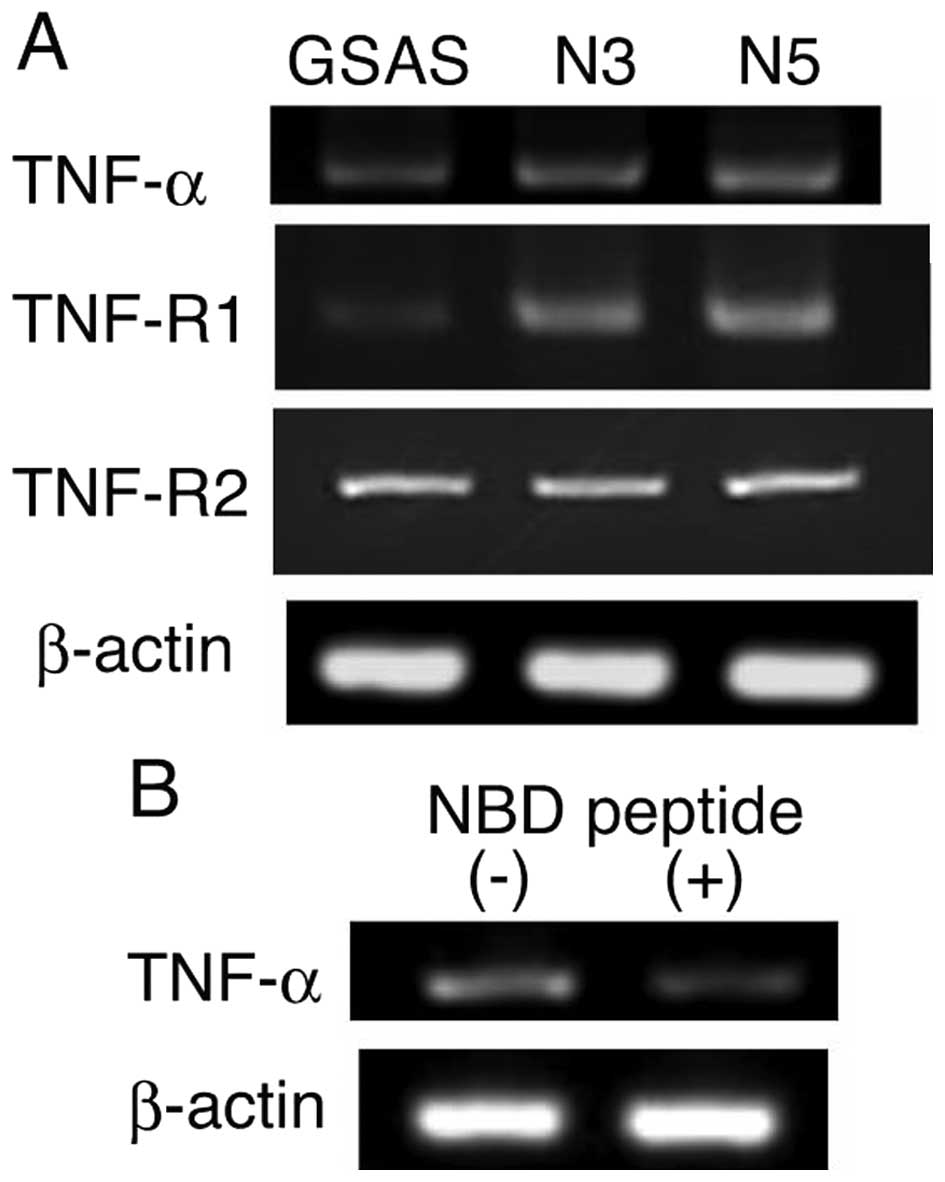
Elevation of TNF-R1-NF-κB pathway and
TNF-α secretion in GSAS/N cells
TNFR1-dependent enhancement of GSAS/N5 cell invasive
activity suggested involvement of TNF-α-TNF-R1 system and elevation
of the TNF-R1-initiated signaling pathway in the highly metastatic
cancer cells. Hence, this system of the cancer cells was
investigated. TNF-α and the receptor TNF-R1 mRNA expression
increased in GSAS/N3 and GSAS/N5 cells in comparison with GSAS
cells, but TNF-R2 mRNA expression did not change among the three
GSAS cells (Fig. 2A). Suppression
of TNF-α mRNA expression by NBD peptide (Fig. 2B) that inhibits NF-κB activation
(28) indicated that increase of
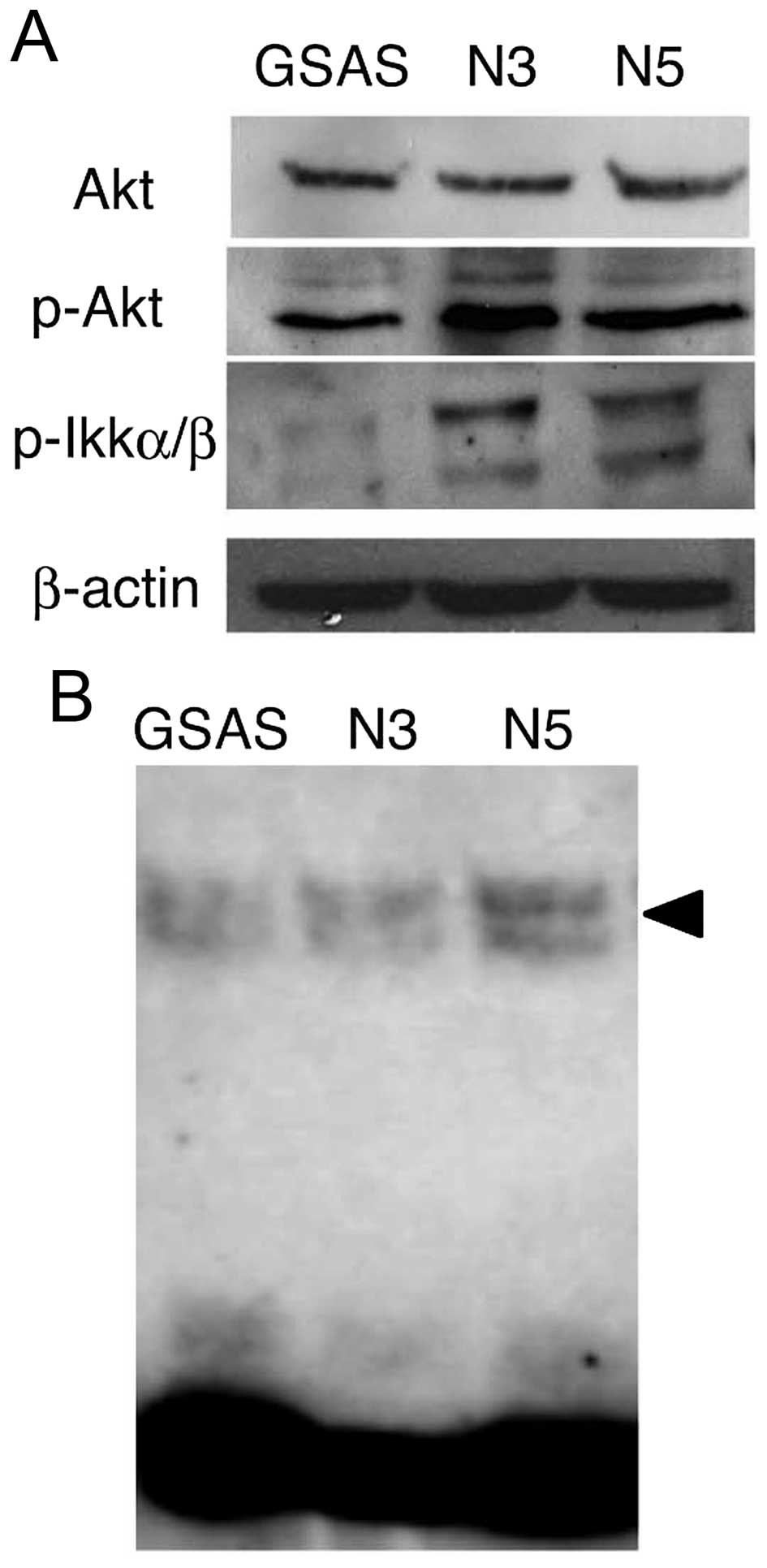
TNF-α mRNA expression was caused by NF-κB activation. The signaling
pathway triggered by binding of TNF-α to TNFR1 mediates NF-κB
activation in human head and neck squamous cell carcinoma (29). Thus, molecules participating in the
signaling pathway from TNFR1 to NF-κB (30) were examined. Phospho-Akt protein
increased in GSAS/N3 and GSAS/N5 cells, but Akt expression did not
change (Fig. 3A). Phospho-IKKα/β
was significantly increased in the highly metastatic cells, whereas
it was negligible in GSAS cells (Fig.
3A). Expression of p50/p65, components of NF-κB (29), was enhanced in GSAS/N5 cells
(Fig. 3B). These results indicated
elevation of the signaling pathway to NF-κB and resultant increase
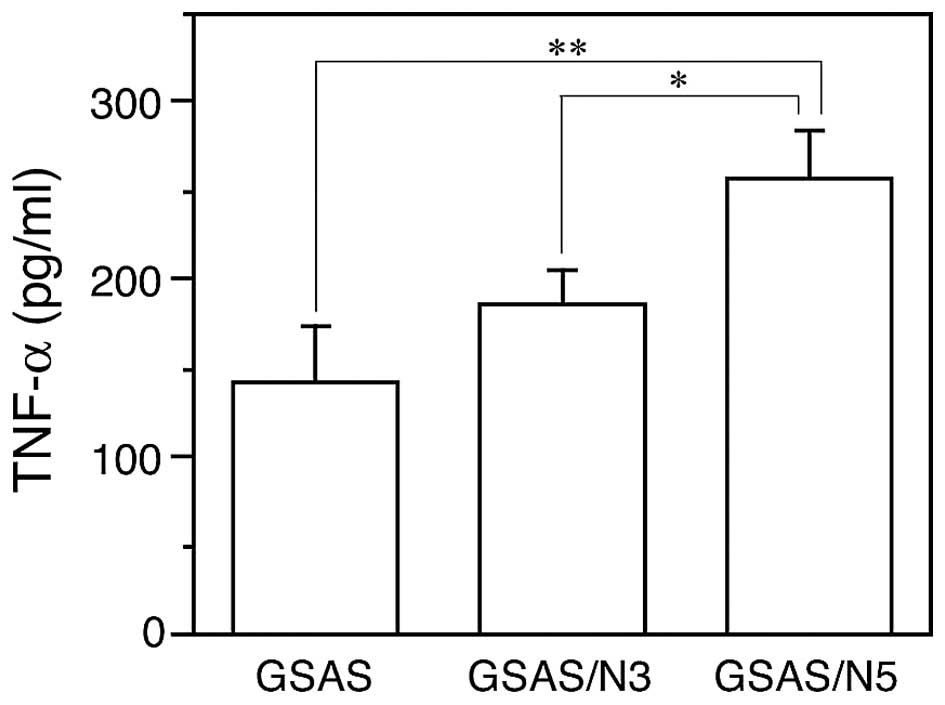
of NF-κB activation in the highly metastatic oral SCC cells. NF-κB
activation leads to TNF-α production in cancer cells (31,32),
thus, TNF-α secretion from the cancer cells was measured. In
agreement with increased TNF-α mRNA expression (Fig. 2A) GSAS/N5 secreted significantly
more TNF-α into the culture medium than GSAS and GSAS/N3 (Fig. 4).
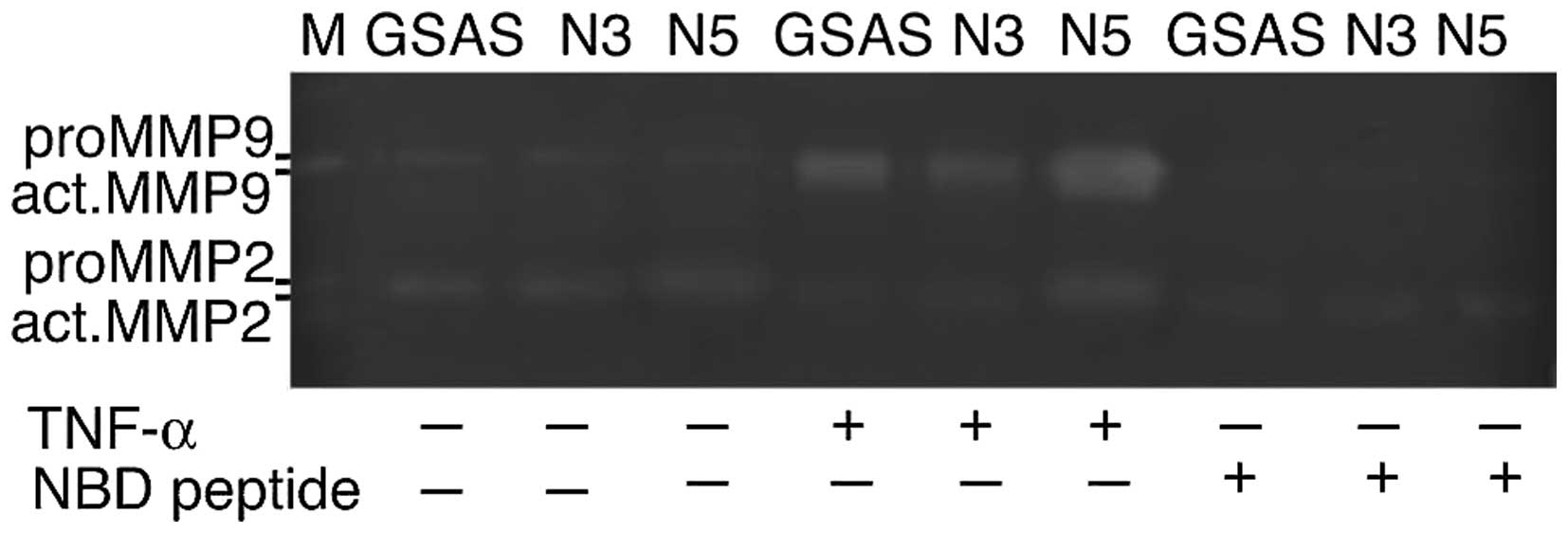
Enhancement of active MMP release from
GSAS/N5 cells by TNF-α and its inhibition by NBD peptide
Elevation of TNF-R1-NF-κB pathway in GSAS/N5 cells
suggested that TNF-α stimulation was associated with enhanced
invasive activity of the cells. We previously showed that elevated
mRNA expression of MMP-2 and MMP-9 in GSAS/N5 cells (27). We examined whether the cancer cells
secreted active forms of these MMPs by TNF-α. By stimulation with
TNF-α, cancer cells secreted active MMPs that were converted from
latent forms; GSAS/N5 cells released more active MMPs than other
cells (Fig. 5). NBD peptide
induced pronounced inhibition of TNF-α-elicited active MMP-2 and
MMP-9 secretion from the cancer cells (Fig. 5), which indicated a close
association of TNF-α-triggered NF-κB activation to upregulation of
active MMP release from the cancer cells.
Elevated angiopoietin-like 4 (Angptl4)
expression and mobility of GSAS/L cells
To study tongue cancer metastasis further, we made
another highly metastatic GSAS cell line (GSAS/L), which were
GSAS/N5 cells that metastasized to the lung by implantation into a
nude mouse tongue. GSAS, GSAS/N5 and GSAS/L cells were examined for
exhaustive mRNA expression by the microarray assay and mRNAs that
increased in comparison with GSAS cell mRNAs were sought. Table II shows the ten highest increase
ratio mRNAs of GSAS/L cell and corresponding mRNAs of GSAS/N5
cells. This assay revealed mRNA elevation of several genes in the
highly metastatic cells and Angptl4 increased most among the mRNAs.
Angptl4 mRNA expression of GSAS/L increased 141-fold for GSAS and
25-fold for GSAS/N5. Indeed, high expression of Angptl4 mRNA and
the protein were confirmed in GSAS/L cells but GSAS cell Angptl4
mRNA expression was faint (Fig.
6). To study GSAS/L cells further, migration activity of the
cells was investigated. In the scratch assay GSAS/L cells migrated
more rapidly than GSAS/N5 and GSAS cells (Fig. 7). These results may suggest that
the lung metastatic activity of GSAS/L is associated with
enhancement of Angptl4 expression and cell mobility.
 | Table IIGene expression microarrays. |
Table II
Gene expression microarrays.
| Signal | GSAS/N5 vs.
GSAS | GSAS/L vs.
GSAS |
|---|
|
|
|
|
|---|
| Gene | GSAS | GSAS/N5 | GSAS/L | Ratio | Z-score | Ratio | Z-score |
|---|
| Angptl4 | 120.180 | 677.890 | 17002.502 | 5.64 | 3.6 | 141.48 | 14.8 |
| HSPA1A | 1692.248 | 16530.238 | 67702.449 | 9.77 | 6.2 | 40.01 | 13.3 |
| VNN1 | 10.544 | 46.645 | 388.448 | 4.42 | 2.1 | 36.84 | 4.8 |
| GJB4 | 77.626 | 301.386 | 2711.984 | 3.88 | 2.8 | 34.94 | 8.9 |
| HSPA1B | 1255.094 | 5619.184 | 20255.974 | 4.48 | 4.1 | 16.14 | 8.3 |
| NLRP3 | 23.420 | 123.228 | 291.084 | 5.26 | 2.3 | 12.43 | 5.6 |
| TNFAIP3 | 104.419 | 305.699 | 1274.084 | 2.93 | 2.2 | 12.20 | 6.3 |
| ZBED2 | 16.635 | 187.705 | 183.027 | 11.28 | 3.3 | 11.00 | 3.2 |
| GRHL3 | 49.419 | 190.636 | 485.379 | 3.86 | 2.8 | 9.82 | 5.1 |
| ZMAT4 | 3.177 | 49.333 | 31.066 | 15.53 | 2.5 | 9.78 | 2.1 |
Discussion
We revealed enhancement of invasive activity in the
highly metastatic OSCC cells (Fig.
1) accompanying elevated TNF-α signaling via TNFR1 (Fig. 2A), leading to NF-κB activation
through phospholylation of Akt and IKKα/β (Fig. 3). The increase of TNF-α mRNA
expression (Fig. 2A) and protein
secretion (Fig. 4) in relation to
elevated NF-κB activation (Fig.
3B) agrees with that NF-κB activation leading to TNF-α
production in tumor cells (31,32).
NF-κB is a key regulator of MMP-2 and MMP-9 expression (33,34),
even in SAS cells (35) used in
the present study, which suggests that the high NF-κB level of
GSAS/N5 cells implicated secretion enhancement of these proteases
from the cells (Fig. 5).
Upregulation of MMP-2 and MMP-9 expression is associated with tumor
invasion and metastasis (36,37).
Accordingly, elevated secretion of active forms of these proteases
in the GSAS/N5 cells (Fig. 5)
likely accounts for high invasive (Fig. 1) and metastatic (27) activities of the cells.
We have shown that NF-κB level was elevated as GSAS
cell metastasis passage advanced (Fig.
3B). The highly metastatic GSAS cells, particularly GSAS/N5,
secrete more TNF-α (Fig. 4)
together with increased expression of TNFR1 (Fig. 2A), the receptor for soluble TNF-α
(22). The increase of TNF-α
release brings about further NF-κB activation via TNFR1 signaling
pathway in the cells and resultant elevation of MMPs secretion. As
an autocrine TNF-α from tumor cells stimulated by this cytokine was
reported (38), TNF-α release from
the highly metastatic cells is possibly amplified in an autocrine
mannaer. These findings suggest an auto-activation mechanism for
TNF-α-triggered NF-κB activation in these cells. This may be an
underlying mechanism by which GSAS/N3 and GSAS/N5 cells exert high
invasive and metastatic activities. Such a positive feedback
between NF-κB and TNF-α was reported in leukemia-initiating cells
and contributes to leukemia progression (32). The present study suggests that this
mechanism is present in the oral cancer cells and associated with
enhanced secretion of active MMPs, augmenting invasion and probably
metastasis of the malignant cells. In addition, the stimulative
effect of TNF-α on the epithelial-mesenchymal transition (38) may also be augmented through the
auto-activation mechanism, participating in the progression of the
highly metastatic cancer cells.
Angptl4 is a member of proteins which are
structurally similar to the angiopoietins but they do not bind to
the angiopoietin receptors, namely the tyrosine kinase with
immunoglobulin-like and EGF-like domain 1 (Tie1) and the
endothelial-specific receptor tyrosine kinase (TEK or Tie2)
(39). Angptl4 is a critical
mediator in the transmigration process (40,41)
and promotes transendothelial migration of cancer cells through the
upregulation of vascular endothelial adhesion molecule 1 (VCAM-1)
expression on endothelial cells (42). The increase of VCAM-1 on
endothelial cells facilitates the attachment of cancer cells in the
circulation to the vessels, allowing subsequent extravasation and
tumor establishment. Clinical studies showed correlation of Angptl4
expression with venous and lymphatic invasion in human squamous
cell carcinoma (43). Moreover,
Angptl4 was reported as a lung metastasis gene in breast cancer
(44). Considering these Angptl4
activities to support cancer progression, a high expression of the
molecule (Table II and Fig. 6) in GSAS/L cells seems to be
associated with metastasis of the OSCC cells, particularly to
lungs, in addition to the enhanced invasiveness (Fig. 7).
In the highly metastatic OSCC cells, the elevated
TNFR1 signaling pathway enhanced resultant NF-κB activation that
was amplified through the auto-activation mechanism, leading to
increase of active MMP-2 and MMP-9 secretion and invasive activity.
Angptl4 mRNA was multiplied 25-fold in GSAS/L cells in comparison
with GSAS/N5 cells, suggesting an involvement of this molecule in
lung metastasis. The high expression level of Angptl4 was
predictive of poor prognosis and prognostic of poor survival in
patients with tongue cancer (45).
These results may indicate that TNF-α, TNFR1 and Angptl4 were
therapeutic targets of OSCC, including tongue cancers. Thus,
blocking the TNF-α-TNFR1 system and Angptl4 with antibodies and
receptor antagonists is possibly effective on the treatment of
OSCC, inhibiting progression of the cancer.
Acknowledgements
The present study was supported in part by the
Japanese Science Progress Society KAKENHI Grant (26463049) to
T.T.
Abbreviations:
|
OSCC
|
oral squamous cell carcinoma
|
|
TNF-α
|
tumor necrosis factor-α
|
|
GSAS
|
GFP expressing SAS cell
|
|
GSAS/N3
|
lymph node metastasized GSAS after
three passages
|
|
GSAS/N5
|
lymph node metastasized GSAS after
five passages
|
|
GSAS/L
|
lung metastasized GSAS/N5
|
|
NBD
|
NEMO-binding domain
|
|
TNFR1
|
tumor necrosis factor-α-receptor 1
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kalnins IK, Leonard AG, Sako K, Razack MS
and Shedd DP: Correlation between prognosis and degree of lymph
node involvement in carcinoma of the oral cavity. Am J Surg.
134:450–454. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schuller DE, McGuirt WF, McCabe BF and
Young D: The prognostic significance of metastatic cervical lymph
nodes. Laryngoscope. 90:557–570. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Snow GB, Annyas AA, van Slooten EA,
Bartelink H and Hart AA: Prognostic factors of neck node
metastasis. Clin Otolaryngol Allied Sci. 7:185–192. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Grandi C, Alloisio M, Moglia D, Podrecca
S, Sala L, Salvatori P and Molinari R: Prognostic significance of
lymphatic spread in head and neck carcinomas: Therapeutic
implications. Head Neck Surg. 8:67–73. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chi AC, Day TA and Neville BW: Oral cavity
and oropharyngeal squamous cell carcinoma - an update. CA Cancer J
Clin. 65:401–421. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sano D and Myers JN: Metastasis of
squamous cell carcinoma of the oral tongue. Cancer Metastasis Rev.
26:645–662. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ho CM, Lam KH, Wei WI, Lau SK and Lam LK:
Occult lymph node metastasis in small oral tongue cancers. Head
Neck. 14:359–363. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Myers EN and Simental AA Jr: Cancer of the
oral cavity. Cancer of the head and neck. 4th edition. Myers EN,
Suen JY, Myers JN and Hanna EY: Elsevier; pp. 279–319. 2004
|
|
10
|
Teichgraeber JF and Clairmont AA: The
incidence of occult metastases for cancer of the oral tongue and
floor of the mouth: Treatment rationale. Head Neck Surg. 7:15–21.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cunningham MJ, Johnson JT, Myers EN,
Schramm VL Jr and Thearle PB: Cervical lymph node metastasis after
local excision of early squamous cell carcinoma of the oral cavity.
Am J Surg. 152:361–366. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fakih AR, Rao RS and Patel AR:
Prophylactic neck dissection in squamous cell carcinoma of oral
tongue: A prospective randomized study. Semin Surg Oncol.
5:327–330. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lydiatt DD, Robbins KT, Byers RM and Wolf
PF: Treatment of stage I and II oral tongue cancer. Head Neck.
15:308–312. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yuen AP, Wei WI, Wong YM and Tang KC:
Elective neck dissection versus observation in the treatment of
early oral tongue carcinoma. Head Neck. 19:583–588. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yuen AP, Lam KY, Chan AC, Wei WI, Lam LK,
Ho WK and Ho CM: Clinicopathological analysis of elective neck
dissection for N0 neck of early oral tongue carcinoma. Am J Surg.
177:90–92. 1999. View Article : Google Scholar
|
|
16
|
Müller S, Pan Y, Li R and Chi AC: The
Emory University Experience: Changing trends in oral squamous cell
carcinoma with particular reference to young patients: 1971–2006.
The Emory University experience. Head Neck Pathol. 2:60–66. 2008.
View Article : Google Scholar
|
|
17
|
Patel SC, Carpenter WR, Tyree S, Couch ME,
Weissler M, Hackman T, Hayes DN, Shores C and Chera BS: Increasing
incidence of oral tongue squamous cell carcinoma in young white
women, age 18 to 44 years. J Clin Oncol. 29:1488–1494. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Toporcov TN, Znaor A, Zhang ZF, Yu GP,
Winn DM, Wei Q, Vilensky M, Vaughan T, Thomson P, Talamini R, et
al: Risk factors for head and neck cancer in young adults: A pooled
analysis in the INHANCE consortium. Int J Epidemiol. 44:169–185.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pennica D, Nedwin GE, Hayflick JS, Seeburg
PH, Derynck R, Palladino MA, Kohr WJ, Aggarwal BB and Goeddel DV:
Human tumour necrosis factor: Precursor structure, expression and
homology to lymphotoxin. Nature. 312:724–729. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Black RA, Rauch CT, Kozlosky CJ, Peschon
JJ, Slack JL, Wolfson MF, Castner BJ, Stocking KL, Reddy P,
Srinivasan S, et al: A metalloproteinase disintegrin that releases
tumour-necrosis factor-alpha from cells. Nature. 385:729–733. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Locksley RM, Killeen N and Lenardo MJ: The
TNF and TNF receptor superfamilies: Integrating mammalian biology.
Cell. 104:487–501. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Balkwill F: Tumour necrosis factor and
cancer. Nat Rev Cancer. 9:361–371. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fràter-Schröder M, Risau W, Hallmann R,
Gautschi P and Böhlen P: Tumor necrosis factor type α, a potent
inhibitor of endothelial cell growth in vitro, is angiogenic in
vivo. Proc Natl Acad Sci USA. 84:5277–5281. 1987. View Article : Google Scholar
|
|
24
|
Leibovich SJ, Polverini PJ, Shepard HM,
Wiseman DM, Shively V and Nuseir N: Macrophage-induced angiogenesis
is mediated by tumour necrosis factor-alpha. Nature. 329:630–632.
1987. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Malik STA, Naylor MS, East N, Oliff A and
Balkwill FR: Cells secreting tumour necrosis factor show enhanced
metastasis in nude mice. Eur J Cancer. 26:1031–1034. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Orosz P, Echtenacher B, Falk W, Rüschoff
J, Weber D and Männel DN: Enhancement of experimental metastasis by
tumor necrosis factor. J Exp Med. 177:1391–1398. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tanaka T, Nakayama H, Yoshitake Y, Irie A,
Nagata M, Kawahara K, Takamune Y, Yoshida R, Nakagawa Y, Ogi H, et
al: Selective inhibition of nuclear factor-κB by nuclear factor-κB
essential modulator-binding domain peptide suppresses the
metastasis of highly metastatic oral squamous cell carcinoma.
Cancer Sci. 103:455–463. 2012. View Article : Google Scholar
|
|
28
|
May MJ, D’Acquisto F, Madge LA, Glöckner
J, Pober JS and Ghosh S: Selective inhibition of NF-kappaB
activation by a peptide that blocks the interaction of NEMO with
the IkappaB kinase complex. Science. 289:1550–1554. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jackson-Bernitsas DG, Ichikawa H, Takada
Y, Myers JN, Lin XL, Darnay BG, Chaturvedi MM and Aggarwal BB:
Evidence that TNF-TNFR1-TRADD-TRAF2-RIP-TAK1-IKK pathway mediates
constitutive NF-kappaB activation and proliferation in human head
and neck squamous cell carcinoma. Oncogene. 26:1385–1397. 2007.
View Article : Google Scholar
|
|
30
|
Rehman AO and Wang C-Y: SDF-1α promotes
invasion of head and neck squamous cell carcinoma by activating
NF-kappaB. J Biol Chem. 283:19888–19894. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Stathopoulos GT, Kollintza A, Moschos C,
Psallidas I, Sherrill TP, Pitsinos EN, Vassiliou S, Karatza M,
Papiris SA, Graf D, et al: Tumor necrosis factor-alpha promotes
malignant pleural effusion. Cancer Res. 67:9825–9834. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kagoya Y, Yoshimi A, Kataoka K, Nakagawa
M, Kumano K, Arai S, Kobayashi H, Saito T, Iwakura Y and Kurokawa
M: Positive feedback between NF-κB and TNF-α promotes
leukemia-initiating cell capacity. J Clin Invest. 124:528–542.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Epanchintsev A, Shyamsunder P, Verma RS
and Lyakhovich A: IL-6, IL-8, MMP-2, MMP-9 are overexpressed in
Fanconi anemia cells through a NF-κB/TNF-α dependent mechanism. Mol
Carcinog. 54:1686–1699. 2015. View Article : Google Scholar
|
|
34
|
Shi M, Cao M, Song J, Liu Q, Li H, Meng F,
Pan Z, Bai J and Zheng J: PinX1 inhibits the invasion and
metastasis of human breast cancer via suppressing NF-κB/MMP-9
signaling pathway. Mol Cancer. 14:662015. View Article : Google Scholar
|
|
35
|
Lai WW, Hsu SC, Chueh FS, Chen YY, Yang
JS, Lin JP, Lien JC, Tsai CH and Chung JG: Quercetin inhibits
migration and invasion of SAS human oral cancer cells through
inhibition of NF-κB and matrix metalloproteinase-2/-9 signaling
pathways. Anticancer Res. 33:1941–1950. 2013.PubMed/NCBI
|
|
36
|
Egeblad M and Werb Z: New functions for
the matrix metal-loproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Littlepage LE, Sternlicht MD, Rougier N,
Phillips J, Gallo E, Yu Y, Williams K, Brenot A, Gordon JI and Werb
Z: Matrix metalloproteinases contribute distinct roles in
neuroendocrine prostate carcinogenesis, metastasis, and
angiogenesis progression. Cancer Res. 70:2224–2234. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bates RC and Mercurio AM: Tumor necrosis
factor-α stimulates the epithelial-to-mesenchymal transition of
human colonic organoids. Mol Biol Cell. 14:1790–1800. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Santulli G: Angiopoietin-like proteins: A
comprehensive look. Front Endocrinol (Lausanne). 5:42014.
|
|
40
|
Zhu P, Tan MJ, Huang RL, Tan CK, Chong HC,
Pal M, Lam CR, Boukamp P, Pan JY, Tan SH, et al: Angiopoietin-like
4 protein elevates the prosurvival intracellular
O2−:H2O2 ratio and
confers anoikis resistance to tumors. Cancer Cell. 19:401–415.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Huang RL, Teo Z, Chong HC, Zhu P, Tan MJ,
Tan CK, Lam CRI, Sng MK, Leong DTW, Tan SM, et al: ANGPTL4
modulates vascular junction integrity by integrin signaling and
disruption of intercellular VE-cadherin and claudin-5 clusters.
Blood. 118:3990–4002. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li H, Ge C, Zhao F, Yan M, Hu C, Jia D,
Tian H, Zhu M, Chen T, Jiang G, et al: Hypoxia-inducible factor 1
alpha-activated angiopoietin-like protein 4 contributes to tumor
metastasis via vascular cell adhesion molecule-1/integrin β1
signaling in human hepatocellular carcinoma. Hepatology.
54:910–919. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shibata K, Nakayama T, Hirakawa H, Hidaka
S and Nagayasu T: Clinicopathological significance of
angiopoietin-like protein 4 expression in oesophageal squamous cell
carcinoma. J Clin Pathol. 63:1054–1058. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Minn AJ, Gupta GP, Padua D, Bos P, Nguyen
DX, Nuyten D, Kreike B, Zhang Y, Wang Y, Ishwaran H, et al: Lung
metastasis genes couple breast tumor size and metastatic spread.
Proc Natl Acad Sci USA. 104:6740–6745. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang Z, Han B, Zhang Z, Pan J and Xia H:
Expression of angiopoietin-like 4 and tenascin C but not cathepsin
C mRNA predicts prognosis of oral tongue squamous cell carcinoma.
Biomarkers. 15:39–46. 2010. View Article : Google Scholar
|