Introduction
In all gynecologic cancers, ovarian cancer (OvCa) is
one of the most lethal, mostly diagnosed at advanced stages for
lack of the effective prior-diagnostic methods (1). It ranked the fifth most common cause
of cancer-related death among women in the United States (2). After tumor cytoreductive surgery or
administration of platinum-based chemotherapy, almost all the
patients developed recurrent and disseminated malignancies with
multiple drug resistance (3,4).
Approximately 30% of epithelial ovarian cancer patients died in
less than five years even with the progress in therapeutic methods
(5). Therefore, it is urgent and
essential to develop a novel non-toxic drug for improving the
existing therapy.
Bile is composed by large amounts of bile acids such
as chenodeoxycholic acid (CDCA), ursodeoxycholic acid (UDCA),
cholic acid (CA), and deoxycholic acid (DCA) (6). Snake bile has been found to possess
anti-inflammatory, anti-convulsion and analgesic physiological
functions (7). In a previous
study, we found that extracts from Crocodylus siamensis bile
could induce apoptosis effectively in human cholangiocarcinoma
cells lines (QBC939, Sk-ChA-1 and MZ-ChA-1) and liver cancer cell
(SMMC-7721) (8). After purifying
from the extracts, we obtained a more effective inducer ESC-3
(9) and studied the localization
of prohibitin during apoptosis of human cholangiocarcinoma Mz-ChA-1
cells (10). However, it is still
unclear whether ESC-3 could suppress the growth of ovarian tumor
and the xenograft tumorigenesis in vivo.
In this study, we firstly demonstrated the antitumor
effects of novel inducer ESC-3 on human ovarian carcinomas cell
lines (A2780 cells, SKOV-3 cells and OVCAR-3 cells) in vitro
and elucidated its inducing apoptosis mechanism. We also employed
A2780 xenograft models to confirm the effectiveness and the
potential as a candidate for ovarian cancer therapy.
Materials and methods
Cell preparation
SKOV-3 and IOSE-80 cells were cultured in Roswell
Park Memorial Institute (RPMI)-1640 medium supplemented with 10%
FBS and penicillin (100 U/ml)/streptomycin (100 µg/ml). The
human OvCa A2780 cells were cultured in DMEM, supplemented with 10%
FBS, penicillin (100 U/ml) and streptomycin (100 µg/ml).
OVCAR-3 cells were cultured in complete medium supplemented with 10
µg/ml insulin. Cells were incubated at 37°C in a humidified
atmosphere of 95% air and 5% CO2.
Cell proliferation assay
Cell viability was determined using the CCK-8 assay.
A2780 cells were treated with ESC-3 at different concentrations (0,
5, 10, 20, 40 and 80 µg/ml) for 24, 48 and 72 h,
respectively. Cell viability was determined using CCK-8 according
to the manufacturer's instructions. Briefly, 4×103 cells
per well were seeded in a 96-well plate and incubated at 37°C for
24 h. Subsequently, cells were treated with different
concentrations of ESC-3 for 24, 48 and 72 h respectively. Then 10
µl WST-8 dye was added to each well, cells were incubated at
37°C for 1 h and the absorbance was finally determined at 450 nm
using a microplate reader.
Morphological changes
The cells from control group and the group treated
with ESC-3 (40 µg/ml) for 48 h were seeded onto coverslips
and grown for 24 h. After washing with PBS three times, the cells
were stained with Giemsa staining solution/Hoechst 33258/AO&EB
and observed under standard inverted phase-contrast microscope or a
fluorescence microscope.
Colony-forming assay
Cells were plated into a 6-well culture plate (1,200
cells/well) and allowed to adhere for 12 h before treatment. The
next day, cells were treated with ESC-3 and equal volumes of DMSO.
After 48 h, ESC-3-containing media was removed, and cells were
allowed to form colonies in serum-free media for 14 days, and then
the colonies were fixed with a solution of acetic acid and methanol
(1:3) for 15 min, stained with Giemsa for 15 min and counted
manually.
Flow cytometry assay
Cells were treated with ESC-3 (5, 10, 20, 40 and 80
µg/ml) for 48 h and then collected and washed twice with
PBS. After fixing in ice-cold 70% ethanol for 12 h, the samples
were washed twice with PBS and then incubated with 10 mg/ml RNase
and 1 mg/ml PI (propidium iodide) for 30 min in the dark. Finally,
the samples were evaluated by Flow Cytometry 500, and the data were
analyzed using Cell Fit software. The Annexin V-fluorescein
isothiocyanate (V-FITC)/PI double staining assay was conducted to
quantify cell apoptotic proportion according to the manufacturer's
instructions. Briefly, after exposure to 40 µg/ml ESC-3 the
A2780 cells were harvested and stained with Annexin V-FITC and PI
for 20 min at room temperature. Following washing with PBS, we used
Flow Cytometry 500 to detect the fluorescence of the cells.
Western blot analysis
To explore the mechanism of apoptosis induced by
ESC-3, proteins were extracted with RIPA buffer (10 mM Tris, 150 mM
NaCl, 0.5% NP-40, 0.1% SDS, 0.1% deoxycholate, 1 mM PMSF, 2 mM
sodium fluoride, and 1 mM sodium orthovanadate), then centrifuged
at 13,000 rpm for 30 min at 4°C. Briefly, equivalent amounts of
proteins were analyzed by 10–15% SDS-PAGE, then transferred onto
polyvinylidene difluoride (PVDF) membranes (Millipore, Bedford, MA,
USA), which were then incubated with specific primary antibodies.
Finally, proteins were visualized with peroxidase-coupled secondary
antibody, using the ECL system (Pierce Co., USA) for detection.
Xenograft models
Female Balb/c nude mice (16±2 g) were purchased from
SLRC Laboratory Animal Co., Ltd. Shanghai, China. The animals were
kept on 12-h-light/12-h-dark cycle under the condition of a
constant temperature of 21–22°C and 60–65% humidity. Additionally,
they were maintained on standard pellet diet and water ad
libitum throughout the experiments. The experimental procedures
were performed in accordance with the guidelines for the humane
treatment of animals set by the Laboratory Animal Center. Briefly,
~5×106 A2780 cells were subcutaneously injected into
nude mice to establish human ovarian cancer xenograft. When the
tumor reached a volume of 100 mm3, the mice were
randomized to control and treatment groups, then the control groups
received corn oil (every three days, i.g. administration) and the
other groups 50 mg/kg ESC-3 (every other three days, i.g.
administration) for 30 days. Tumor volume (V) was calculated as V =
(length × width2)/2. The tumor volume at day n was
expressed as relative tumor volume (RTV) according to the following
formula: RTV = TVn/TV0, where TVn
is the tumor volume at day n and TV0 is the tumor volume
at day 0. Therapeutic effects of treatment were expressed in terms
of T/C (%) using the calculation formula T/C (%) = mean RTV of the
treated group/mean RTV of the control group × 100%. Tumors and
internal organs (heart, liver, spleen, lung and liver) were fixed
in formalin and processed for hematoxylin-eosin staining. The
samples were processed by the following published standard methods.
In brief, the sections (4–5-µm) mounted on glass slides were
deparaffinized, rehydrated through grated alcohols to distilled
water, stained with hematoxylin and eosin, and then observed under
a light microscope (Olympus BH-2).
Results
ESC-3 inhibits cell proliferation and
colony-forming ability in human OvCa cell lines
To evaluate effects of ESC-3 on proliferation of
A2780 cells using CCK-8 assays, A2780 cells were treated with ESC-3
at different concentrations (5, 10, 20, 40 and 80 µg/ml) for
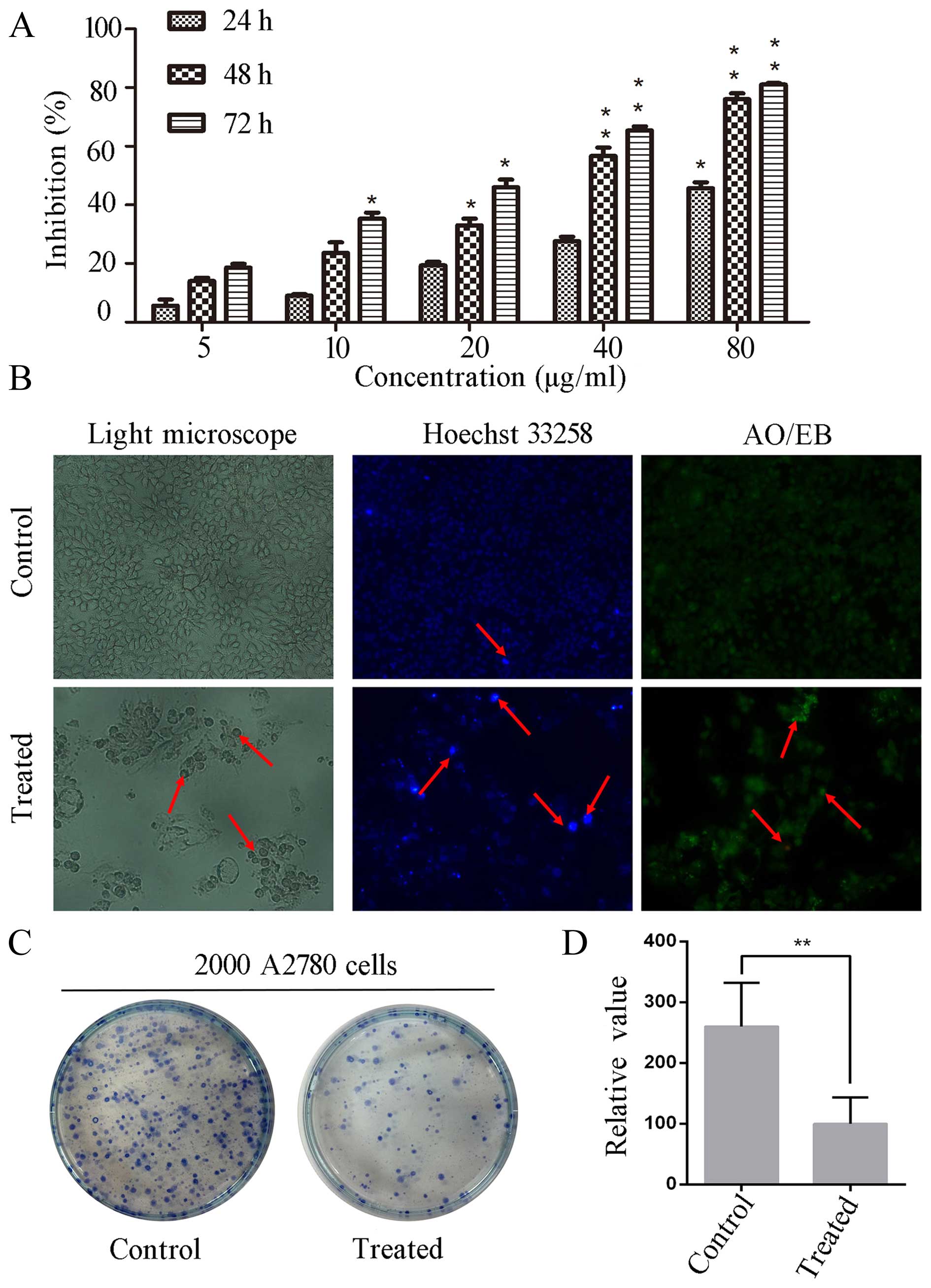
24, 48 and 72 h, respectively. As shown in Fig. 1A, after treated with different
concentration of ESC-3, cell proliferation became slower compared
to that of untreated cells (P<0.01). With ESC-3 concentrations
of 5, 10, 20, 40 and 80 µg/ml for 48 h, the inhibition rates
were 18, 22, 33, 57 and 76%, respectively. Besides, ESC-3
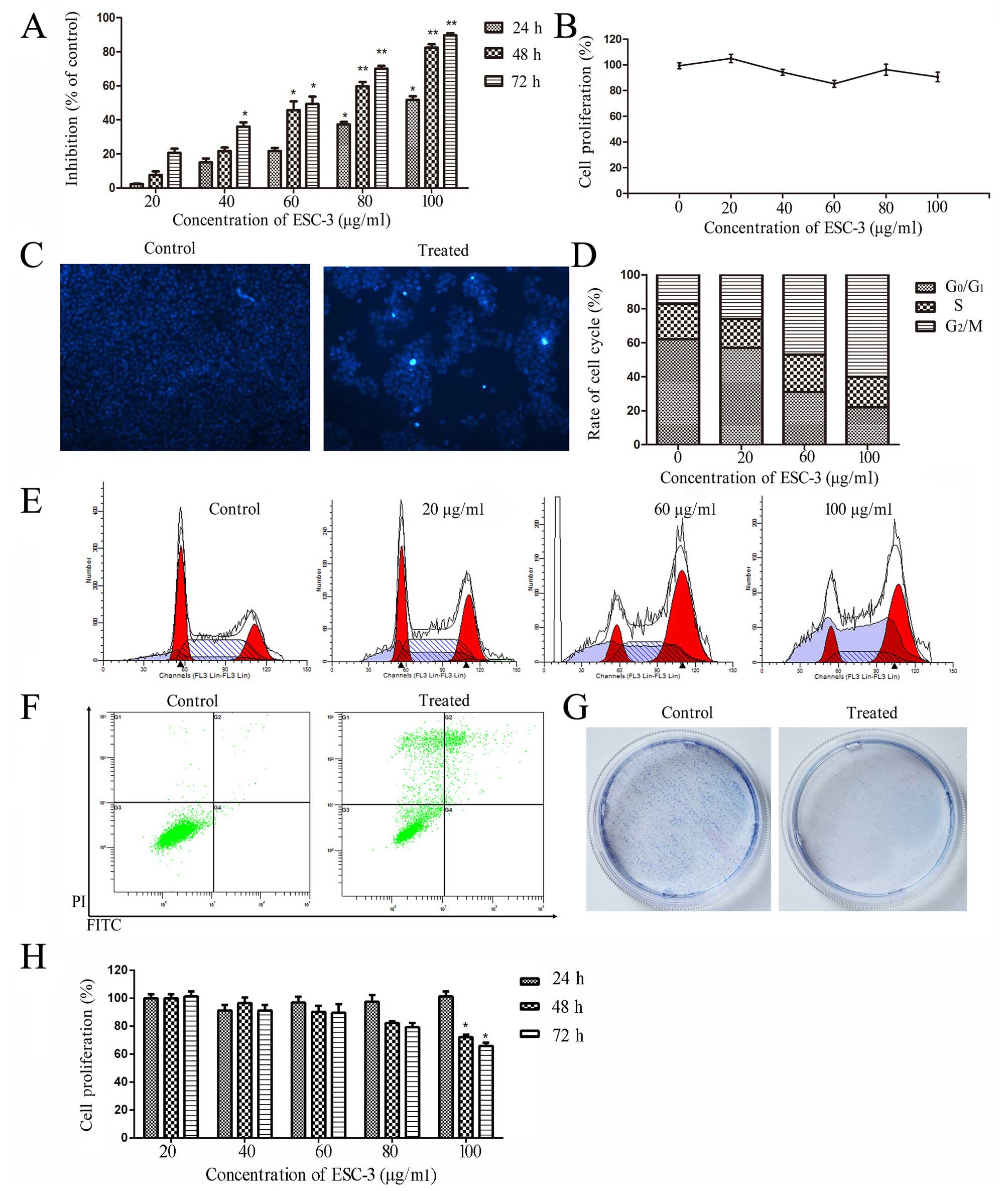
significantly suppressed the proliferation of SKOV-3 cells and
inhibited colony-formation ability as shown in Fig. 3A and G. After exposure to 60
µg/ml ESC-3, the changes in cell morphology occurred with
typical trait of apoptosis: cell shrinkage, chromatin condensation,
apoptotic body formation, dense nuclei (Fig. 3C). However, ESC-3 did not induce
apoptosis in human ovarian carcinomas OVCAR-3 effectively (Fig. 3H). Our data indicated that ESC-3
could inhibit the proliferation of A2780 cells and SKOV-3 cells in
a dose- and time-dependent manner.
To investigate whether morphological changes
happened after ESC-3-treatment, we used an optical inverted
microscope to visualized morphological features. As shown in
Fig. 1B, after treated with 40
µg/ml ESC-3, A2780 cells were smaller in size and close to
rotundity compared to the control group. With Hoechst 33258
staining, the treated cells emitted a higher fluorescence intensity
and were smaller than those of the control group in size. After
AO/EB staining, the treated cells displayed orange and red
fluorescence, while the untreated cells emitted a low green
fluorescence in a homogeneous manner. To determine the ability of
colony-forming, 2,000 A2780 cells were seeded into and treated with
40 µg/ml ESC-3. As shown in Fig. 1C and D, the ESC-3-treated cells
showed a significant decrease in colony number compared to the
untreated A2780 cells. There results suggested that ESC-3-treated
A2780 cells displayed typical morphological features of apoptosis
and a significant reduction in the colony-forming ability
(P<0.01).
ESC-3 causes cell cycle arrest and
induces apoptotic cell death in A2780 and SKOV3 cell lines
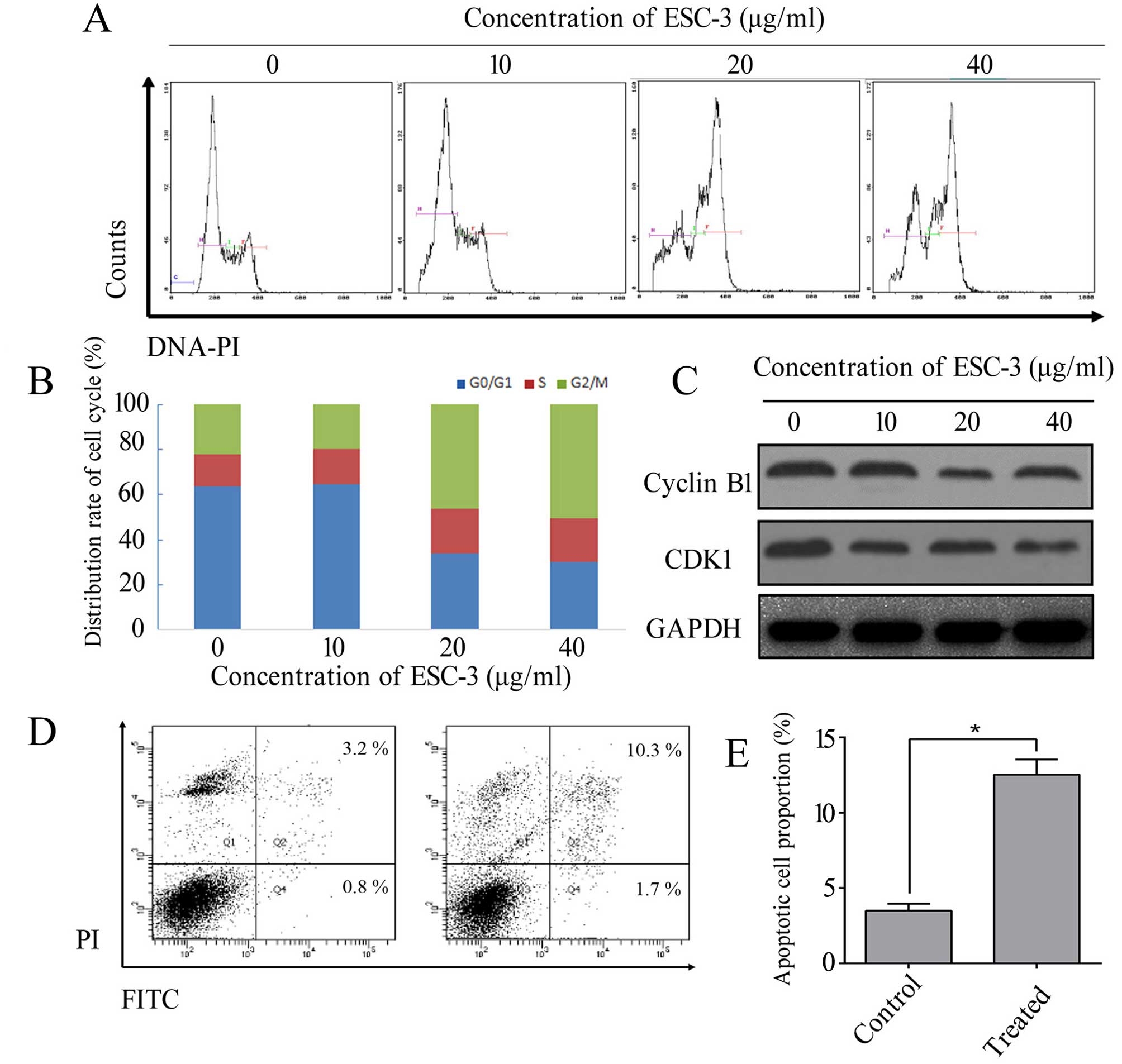
To confirm whether the inhibition of cellular
proliferation was associated with the cell cycle distribution, we
performed a cell cycle analysis after exposure to different
concentration of ESC-3 (5, 10, 20, 40 and 80 µg/ml). As
shown in Fig. 2A and B, after
treated with ESC-3 for 48 h, the cell cycle distribution of A2780
cells was altered in a dose-dependent manner. The proportion of
cells at the G2/M increased from 22.1 to 55.3%
(P<0.01), while the percentage of cells in
G0/G1 phase was 63.8% in the control group
and decreased to 30.3% after treatment with 40 µg/ml ESC-3
(P<0.01). On the other hand, the proportion of ESC-3-treated at
S phase displayed non-significance compared to untreated cells.
ESC-3 caused cell cycle arrest and induced apoptotic cell death in
SKOV-3, which could confirm the consistency of the in vitro
study in A2780 cells as shown in Fig.
3E. Our data indicated that ESC-3 arrested A2780 cells and
SKOV-3 cells at G2/M phase and suppressed cell
proliferation. The protein level of CDK1 and cyclin B1 were
decreased after exposure to ESC-3 compared with the untreated group
in A2780 cells (Fig. 2C).
We preformed flow cytometric analysis using dual
staining with Annexin V and propidium iodide to distinguish between
early apoptotic and late apoptotic cells. As shown in Fig. 2D and E, the apoptotic proportion of
cells with 40 µg/ml was 13.5% compared to untreated cells
with 2.5% apoptotic proportion (P<0.05). Therefore, we
demonstrated that apoptosis could be induced by ESC-3.
ESC-3 induces A2780 apoptotic cell death
through Wnt/β-catenin and Notch pathway
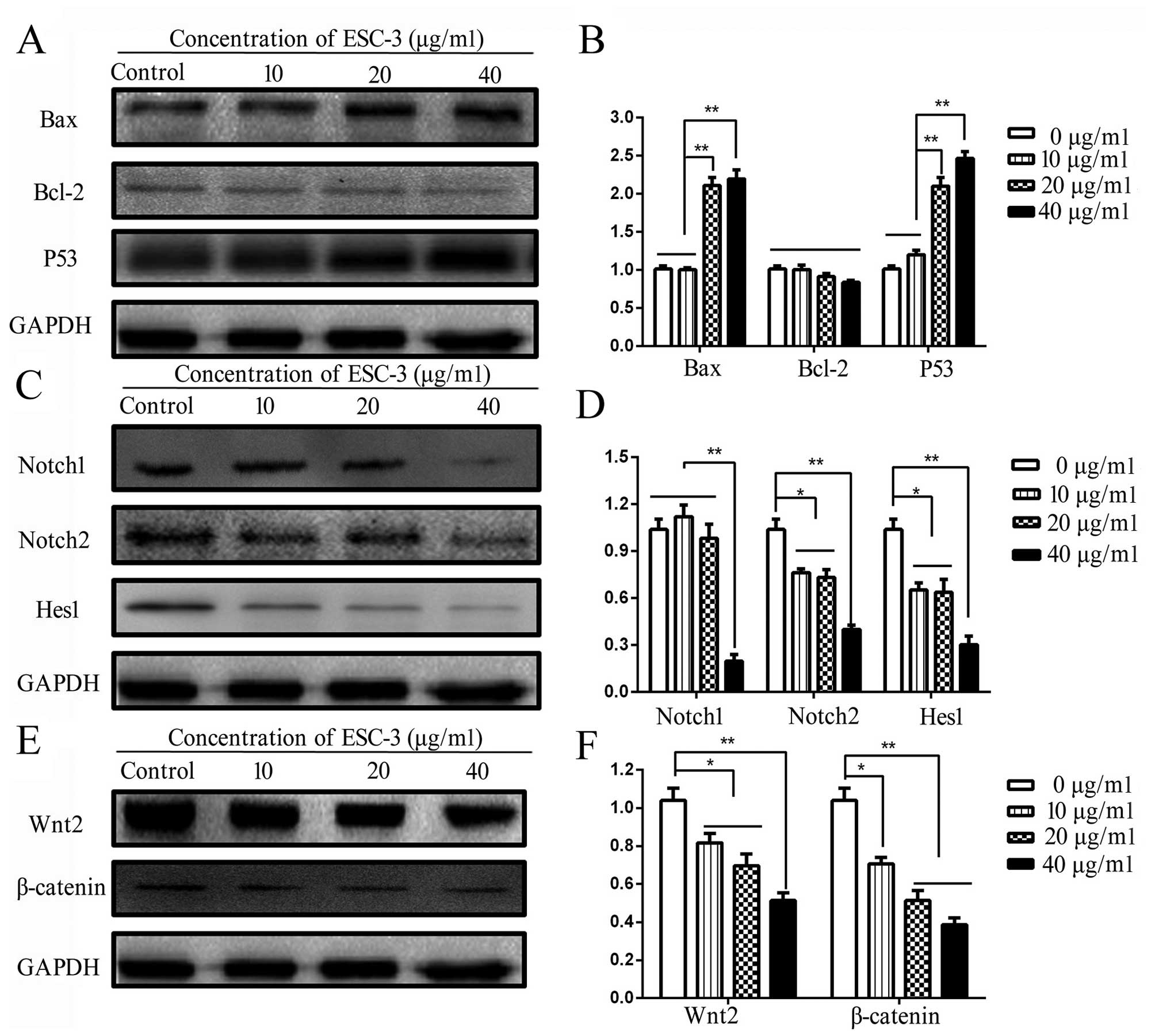
To investigate the apoptosis mechanism induced by
ESC-3, the expression of apoptosis-related (Bax, Bcl-2 and P53) and
pathway-related (Wnt2, β-catenin, Notch1, Notch2 and Hes1) proteins
were measured by western blotting and quantified using ImageJ
software. As shown in Fig. 4A and
B, the proteins level of Bax were significantly increased
(P<0.01) after exposure to ESC-3 for 24 h, while the change in
expression of Bcl-2 proteins remained non-significant; therefore,
the ratio of Bax to Bcl-2 increased (P<0.01) significantly
compared to the untreated group. Moreover, the protein levels of
P53 were remarkably increased in a dose-dependent manner. As shown
in Fig. 4E and F, the expression
of Wnt2 proteins were decreased to 65% protein levels of the
untreated group, and the expression of β-catenin at the protein
levels decreased (P<0.01) significantly compared the proteins
obtained from the untreated cells. Besides, the expression of
Notch1 and Notch2 proteins, the receptor located at cell membrane
initiating the Notch pathway, decreased significantly (P<0.01)
in a dose-dependent manner; the expression levels of the Hes1
proteins, the downstream proteins of Notch pathway, decreased
(P<0.01) obviously (Fig. 3C and
D). Our data suggested that the Wnt/β-catenin and Notch pathway
might play a significant role in induction of cell apoptosis by
ESC-3.
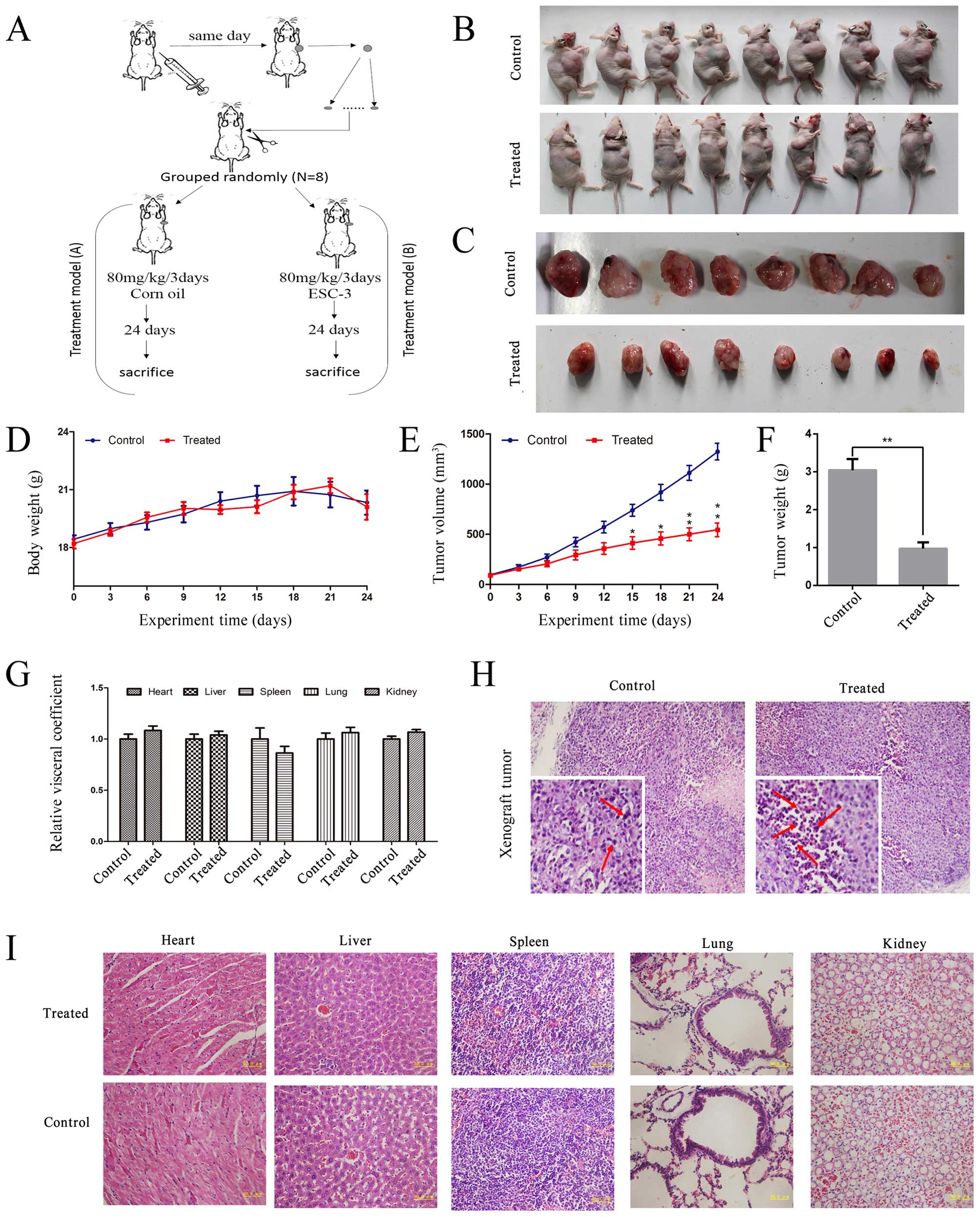
ESC-3 inhibits the growth of A2780
xenograft tumor in Balb/c nude mice without noticeable
toxicity
To determine the antitumor effects of ESC-3,
1×106 A2780 cells were injected subcutaneously into the
right flank of Balb/c nude mice to build the tumor xenograft models
as shown in Fig. 5A. During the
study, the body weight and tumor volume of nude mice was tracked
every three days to detect the non-toxic and effectivity of ESC-3
in vivo. As shown in Fig. 5D
and E, the body weight of nude mice treated with ESC-3
displayed non-significant changes compared to the control group,
while the volume demonstrated apparent difference between the
treated and the control group (at the 15th day P<0.05 and at the
21st day P<0.01). After administration (i.g) with ESC-3 for 24
days, the mice were sacrificed (Fig.
5B) and the tumors was excised (Fig. 5C) and weighed (Fig. 5F), the tumors from ESC-3-treated
mice were smaller and lighter than those of the control group
(P<0.01). As shown in Fig. 5G,
hematoxylin-eosin staining of ESC-3-treated pathological paraffin
sections displayed typical apoptotic features: condensed chromatin
and pyknotic nuclei. To confirm the non-toxicity of ESC-3 further,
the viscus of Balb/c nude mice were excised, weight and stained
with hematoxylin-eosin, it showed that the relative visceral
coefficient have no remarkable difference between the control group
and ESC-3-treated group (Fig. 5H),
and hematoxylin-eosin staining of ESC-3-treated viscus pathological
paraffin sections displayed no significant changes in
organizational structure (Fig.
5I). Our data demonstrated that ESC-3 effectively suppressed
the growth of A2780 xenograft tumors (T/C=42%) without affecting
the weight and viscus of nude mice as shown in Tables I and II. Therefore, the ESC-3 is efficient and
non-toxic to ovarian cancer.
 | Table IEvaluation system of ESC-3 on ovarian
xenograft models. |
Table I
Evaluation system of ESC-3 on ovarian
xenograft models.
| Mean
volume
(t=0) | Mean
volume
(t=24) | RTV | T/C |
|---|
| Control group | 95.53
mm3 | 1,323.32
mm3 | 13.85 | |
| Treated group | 91.61
mm3 | 545.04
mm3 | 5.95 | 42% |
 | Table IIWeight of nude mouse body, tumor and
viscera at the 24th day. |
Table II
Weight of nude mouse body, tumor and
viscera at the 24th day.
| No. | Heart | Liver | Spleen | Lung | Kidney | Body | Tumor |
|---|
| 1 | 0.0914 | 1.5033 | 0.2346 | 0.1227 | 0.3212 | 21.5 | 4.1511 |
| 2 | 0.1115 | 1.3387 | 0.1555 | 0.1273 | 0.3929 | 22.7 | 2.2685 |
| 3 | 0.1158 | 1.4238 | 0.1171 | 0.1310 | 0.3031 | 17.2 | 1.6610 |
| 4 | 0.0983 | 1.3953 | 0.1316 | 0.1445 | 0.3381 | 19.5 | 3.0444 |
| 5 | 0.1156 | 1.3570 | 0.2425 | 0.1993 | 0.3298 | 21.8 | 2.8495 |
| 6 | 0.1055 | 1.1678 | 0.1108 | 0.1370 | 0.3011 | 21.1 | 3.8889 |
| 7 | 0.1061 | 1.3355 | 0.1233 | 0.1321 | 0.3055 | 19.5 | 2.9054 |
| 8 | 0.0965 | 1.1336 | 0.1064 | 0.1497 | 0.2968 | 19.3 | 3.6030 |
| Mean | 0.1051 | 1.3319 | 0.1527 | 0.1430 | 0.3236 | 20.3 | 3.0465 |
| 9 | 0.1246 | 1.3500 | 0.1402 | 0.1650 | 0.3457 | 22.3 | 0.4304 |
| 10 | 0.1090 | 1.4654 | 0.1072 | 0.1266 | 0.3446 | 21.1 | 1.3244 |
| 11 | 0.1125 | 1.3435 | 0.1322 | 0.1499 | 0.3332 | 20.9 | 0.6967 |
| 12 | 0.1203 | 1.5170 | 0.1361 | 0.1841 | 0.3503 | 21.8 | 1.2925 |
| 13 | 0.0913 | 1.2272 | 0.1514 | 0.1161 | 0.3252 | 18.1 | 0.4968 |
| 14 | 0.1128 | 1.4028 | 0.1710 | 0.1651 | 0.3587 | 20.3 | 1.6308 |
| 15 | 0.1186 | 1.4050 | 0.1023 | 0.1520 | 0.3124 | 16.8 | 1.3545 |
| 16 | 0.1147 | 1.2606 | 0.0899 | 0.1454 | 0.3535 | 19.5 | 0.5738 |
| Mean | 0.1130 | 1.3734 | 0.1288 | 0.1505 | 0.3405 | 20.1 |
0.9750b |
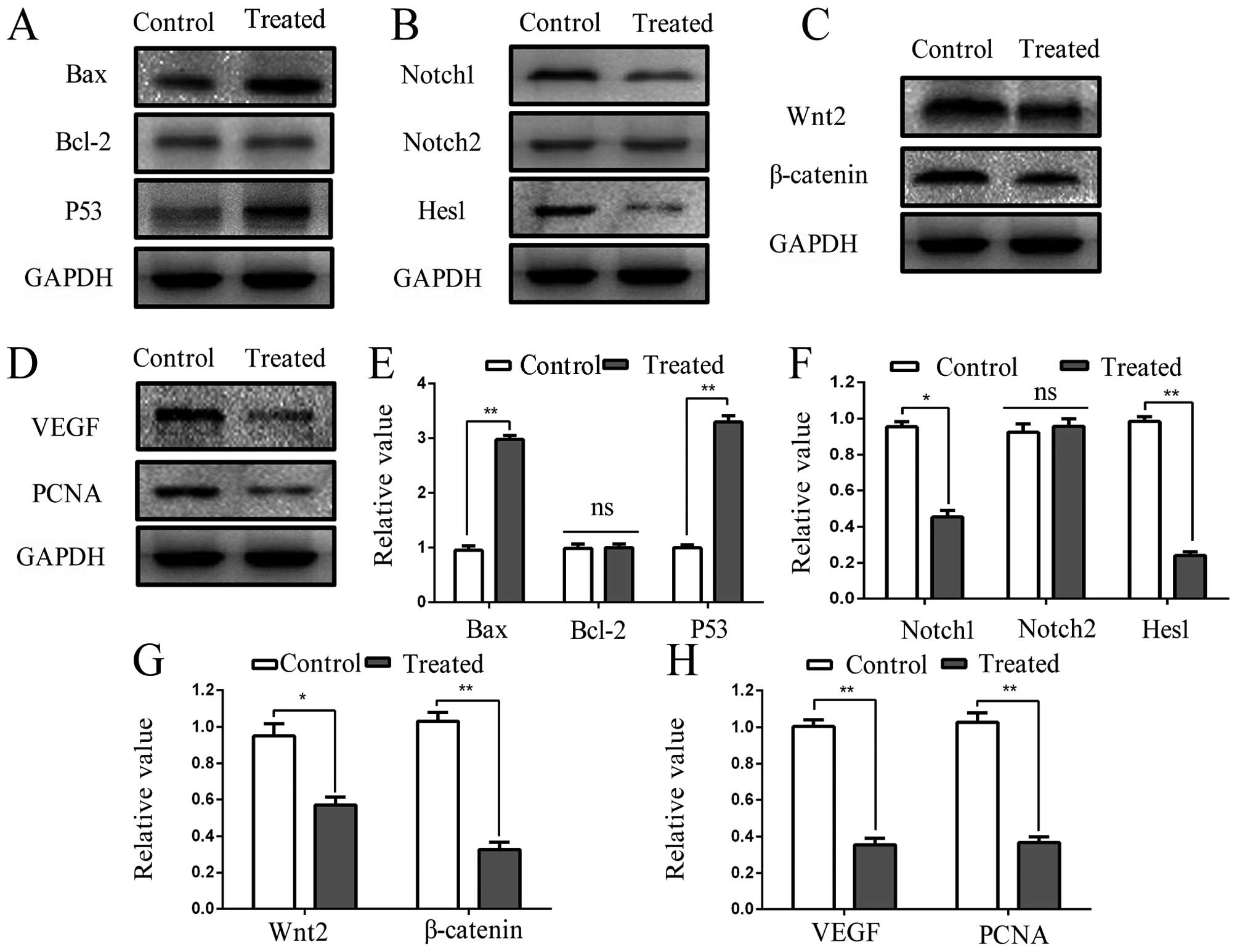
Tumor inhibition is induced by ESC-3
through Wnt/β-catenin and Notch pathway
After tumors were treated (i.g) with 80 mg/kg ESC-3
every three days for 24 days, the expression of proteins obtained
from the tumors were measured by western blot analyses to determine
the consistency of the results in vitro and in vivo.
We examined the expression levels of apoptosis-related and
pathway-related proteins, including Bax, Bcl-2, P53, Wnt2,
β-catenin, Notch1, Notch2, Hes1, VEGF and PCNA. As shown in
Fig. 6A and E, the result of
western blot analyses revealed that the expression of Bax was
significantly increased ~2.9-fold (P<0.01) after administration
(i.g) with ESC-3, while proteins of Bcl-2 remained about the same
compared to the control group; therefore, the ratio of Bax to Bcl-2
also increased (P<0.01). Moreover, we observed that the levels
of P53 proteins obtained from ESC-3-treated increased ~3.3-fold
(P<0.01), which displayed the consistency also observed in
vitro and in vivo. Furthermore, we determined the
proteins in Wnt/β-catenin and Notch pathway, as shown in Fig. 6B and F, the expression of Notch1
and Notch2 proteins decreased ~1.21-fold (P<0.05) and 0.06-fold
respectively, the proteins level of Hes1, the downstream effector
proteins of Notch pathway, were decreased (P<0.01) significantly
after administration (i.g) of ESC-3 for 24 days. ESC-3 has a
significant effect on the expression levels of Wnt2 and β-catenin
proteins based on the ESC-3-treated tumors (Fig. 6C and G). Furthermore, the
expression of VEGF and PCNA were significantly decreased at the
protein level compared with the control group (Fig. 6D and H).
Discussion
Apoptosis, programmed cell death under physiological
or pathological conditions, plays a critical role in the tissue
homeostasis of eukaryotes (11).
It is desirable to prevent the occurrence and metastasis of cancer
through inducing apoptosis (12).
The cancer cells have the ability to sustain chronic proliferation
through the cell cycle, which is the most fundamental feature
(13). Our data were suggested
that ESC-3 significantly suppressed the proliferation of A2780
cells and inhibited colony-formation ability. After exposure to
different concentrations of ESC-3, the A2780 cells were arrested at
G2/M phase through downregulation of CDK1 and cyclin B1,
two critical G2/M transition regulators (14). The primary indicators of apoptosis
under physiological and pathological conditions, are the
morphological changes (11,15,16).
After exposure to 40 µg/ml ESC-3, the changes in cell
morphology occurred with typical trait of apoptosis: cell
shrinkage, chromatin condensation, apoptotic body formation, and
dense nuclei. Our results were in agreement with the study by
Horowitz et al (17),
concluding that chenodeoxycholic acid (CDCA) and deoxycholic acid
(DCA) possess the ability to induce apoptotic phenomenon in ovarian
cancers. Then, SKOV-3 and OVCAR-3 cell lines were analyzed in
vitro. The results suggested that ESC-3-treated SKOV-3 cells
displayed typical morphological features of apoptosis and a
significant reduction in the colony-forming ability. Besides, ESC-3
caused cell cycle arrest and induced apoptotic cell death in
SKOV-3, which confirmed the consistency with the in vitro
study in A2780 cells. However, ESC-3 did not induce apoptosis in
human ovarian carcinomas OVCAR-3 effectively, this difference in
suppression of ESC-3 on A2780, SKOV-3 and OVCAr-3 cell
proliferation confirmed the different phenotypes of these three
ovarian cancer cell lines, which emphasizes the need to recognize
the heterogeneity of cancer cell populations (18–21).
Furthermore, we used normal human ovarian epithelial cells
(IOSE-80) to perform the CCK-8 assay. We found that, compared to
the control, ESC-3 dose-dependently inhibited A2780 cells and
SKOV-3 cells, but not IOSE-80 in cell proliferation as shown in
Figs. 1A and 3A and B.
The Bcl-2 family plays a vital role in regulating of
apoptosis, including Bax and Bcl-2, of which the former induces
apoptosis and the latter prevents apoptosis (22). In the present study, our data
demonstrated that ESC-3 could significantly upregulate the
expression of Bax proteins while the protein levels of Bcl-2
remained steady, resulting in the elevation of Bax/Bcl-2 ratio
which usually induces apoptosis (23). In the previous study, bile extract
from crocodile could induce apoptosis in human cholangiocarcinoma
through the mitochondrial pathway (24). Furthermore, ESC-3 isolated from
Crocodylus siamensis bile through a Sephadex LH-20 column
(Pharmacia, Sweden) and an RP-18 reversed-phase column (25×0.3 cm),
induced apoptosis in Mz-ChA-1 cells in a dose-dependent manner via
the mitochondria-dependent pathway (9). In this study, we demonstrated the
mechanism of apoptosis induced by ESC-3 in human OvCa carcinomas.
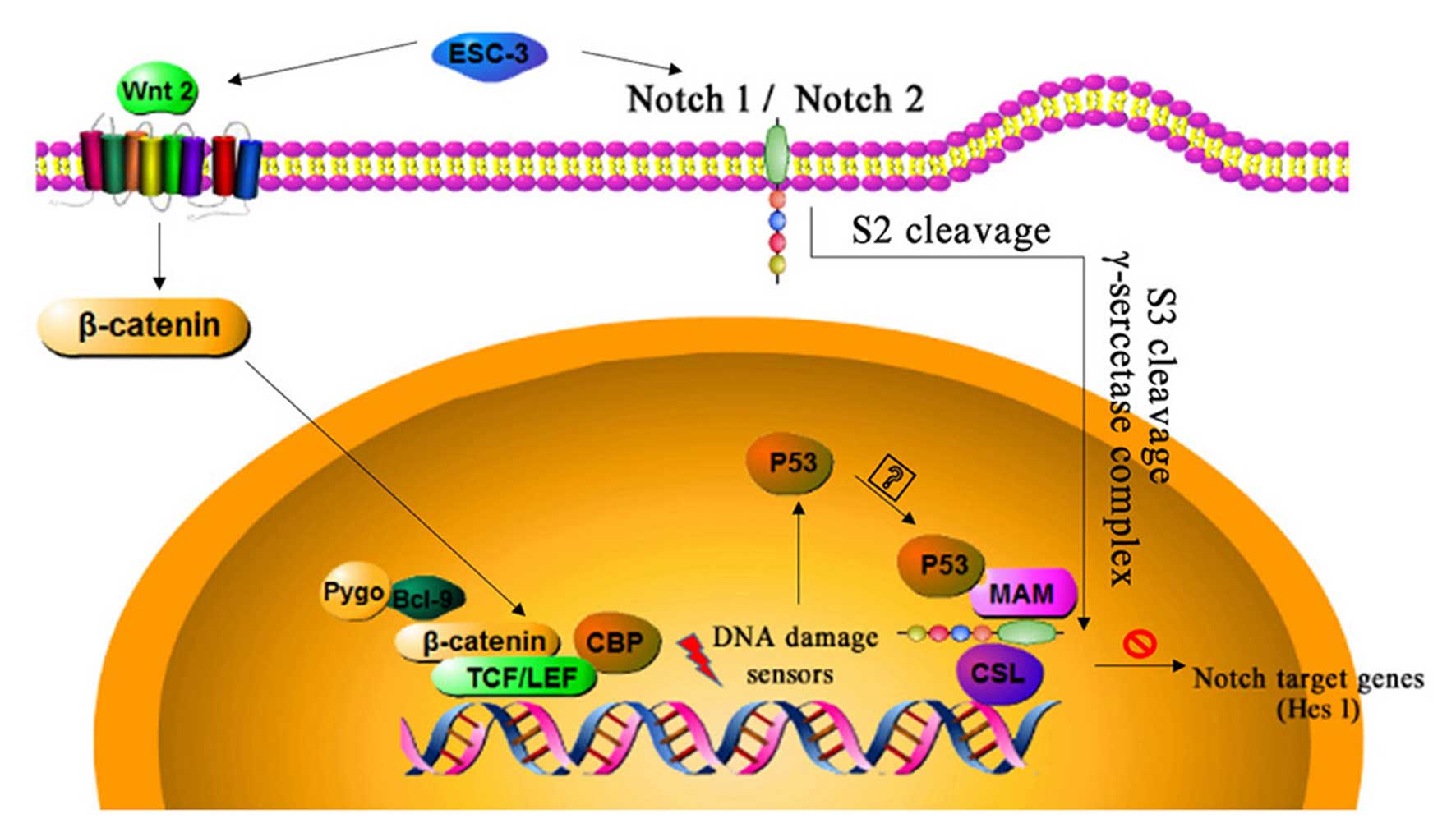
The development and progression of several malignancies have
association with the Notch signal pathway (25,26).
There are four Notch receptors the Notch1, 2, 3 and 4 and five
ligands (Delta-like1, 3 and 4) in Notch signaling mediating via
cell-to-cell contact (27). A
basic platform consisting of the ternary complex (Notch-CSL-MAM)
could recruit coactivators including p300 to increasing the
expression levels of Notch pathway downstream proteins (28–30),
however, this process could be blocked with the abnormal elevation
of P53, a tumor suppressor in human cancers (31). Our results suggested that ESC-3
could significantly (P<0.01) upregulate the expression of P53,
while hes1 remarkably decreased at the proteins levels. As previous
research reported that Hes1, the downstream protein of Notch
pathway, could be suppressed by the abnormal elevation of P53
through the combination with Notch-CSL-MAM complex, which might
disturb the tendency of dose-response at the protein level
(31). Our data do not indicate
whether the association between p53 and the NTCs is mediated by
direct association with MAM or through additional proteins.
However, it was reported that P53 can be combination with MAM
directly to block the recruitment of a coactivator. The
associations between Wnt signaling and ovarian cancer confirmed
that Wnt signaling played a critical role in the embryonic
development of ovary and homeostasis including proliferation,
differentiation, and migration (32,33).
Our data suggested that ESC-3 could downregulate the expression of
Wnt2 at the protein levels, whereas, the protein levels of
β-catenin, the key effector in Wnt signaling, were decreased
(P<0.01) significantly. In conclusion, ESC-3 induced apoptosis
of human ovarian carcinomas through Wnt/β-catenin and Notch
signaling as shown in Fig. 7.
The xenograft models were employed to confirm the
consistency with the in vitro assays and to determine the
non-toxicity and effectiveness of ESC-3. Our data in vivo
demonstrated that 80 mg/kg dose of ESC-3 every three days was
highly effective and did not have toxic side effects. ESC-3 also
effectively inhibited the expression of the proliferation marker
PCNA (34), indicating its
important role in the growth of ovarian tumors. Furthermore, the
expression of vascular endothelial growth factor (VEGF), playing a
critical role in angiogenesis (proliferation, migration and
survival of endothelial cells) in cancer (35,36),
was significantly decreased measured by western blotting. The
molecular mechanism demonstrated the consistency between result
in vitro and in vivo after treated with ESC-3.
Therefore, our data suggested that ESC-3 was a safe, natural and
effective compound in ovarian cancer therapy.
In conclusion, ESC-3 is a novel active compound that
could arrest the A2780 cells and SKOV-3 cells at the
G2/M phase and cyclin B1 proteins and induce apoptosis
in a dose-dependent manner via the Wnt/β-catenin and Notch pathway,
moreover, xenograft models displayed the consistency as showed in
the results in vitro. Therefore, ESC-3 could be a potential
therapeutic in ovarian carcinomas.
Acknowledgments
This study was supported by the Natural Science
Foundation of China (grant nos. 81571418 and 81402309), the
National Science Foundation for Fostering Talents in Basic Research
of the National Natural Science Foundation of China (grant no.
J1310027) and by the Natural Sciences Foundation of Fujian
Province, China (2016J05105).
Abbreviations:
|
SCB
|
siamese crocodile bile
|
|
OvCa
|
ovarian cancer
|
|
FBS
|
fetal bovine serum
|
|
DMEM
|
Dulbecco's modified Eagle's essential
medium
|
|
CCK-8
|
cell counting kit-8
|
|
DMSO
|
dimethyl sulfoxide
|
|
FITC
|
fluorescein isothiocyanate
|
|
PI
|
propidium iodide
|
|
PVDF
|
polyvinylidene difluoride
|
|
PMSF
|
phenylmethanesulfonyl fluoride
|
|
VEGF
|
vascular endothelial growth factor
|
References
|
1
|
Seidman JD, Horkayne-Szakaly I, Haiba M,
Boice CR, Kurman RJ and Ronnett BM: The histologic type and stage
distribution of ovarian carcinomas of surface epithelial origin.
Int J Gynecol Pathol. 23:41–44. 2004. View Article : Google Scholar
|
|
2
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vaughan S, Coward JI, Bast RC JR, Berchuck
A, Berek JS, Brenton JD, Coukos G, Crum CC, Drapkin R,
Etemadmoghadam D, et al: Rethinking ovarian cancer: Recommendations
for improving outcomes. Nat Rev Cancer. 11:719–725. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Matsuo K, Lin YG, Roman LD and Sood AK:
Overcoming platinum resistance in ovarian carcinoma. Expert Opin
Investig Drugs. 19:1339–1354. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang Y and Morrow JS: Identification and
characterization of human SLP-2, a novel homologue of stomatin
(band 7.2b) present in erythrocytes and other tissues. J Biol Chem.
275:8062–8071. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tint GS, Dayal B, Batta AK, Shefer S,
Joanen T, McNease L and Salen G: Biliary bile acids, bile alcohols,
and sterols of Alligator mississippiensis. J Lipid Res. 21:110–117.
1980.PubMed/NCBI
|
|
7
|
Yeh YH, Wang DY, Liau MY, Wu ML, Deng JF,
Noguchi T and Hwang DF: Bile acid composition in snake bile juice
and toxicity of snake bile acids to rats. Comp Biochem Physiol C
Toxicol Pharmacol. 136:277–284. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Song W, Li SS, Qiu PP, Shen DY, Tian L,
Zhang QY, Liao LX and Chen QX: Apoptosis induced by aqueous
extracts of crocodile bile in human heptacarcinoma SMMC-7721. Appl
Biochem Biotechnol. 170:15–24. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Song W, Shen DY, Kang JH, Li SS, Zhan HW,
Shi Y, Xiong YX, Liang G and Chen QX: Apoptosis of human
cholangiocarcinoma cells induced by ESC-3 from Crocodylus siamensis
bile. World J Gastroenterol. 18:704–711. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Song W, Tian L, Li SS, Shen DY and Chen
QX: The aberrant expression and localization of prohibitin during
apoptosis of human cholangiocarcinoma Mz-ChA-1 cells. FEBS Lett.
588:422–428. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qin F, Song Y, Li Z, Zhao L, Zhang Y and
Geng L: S100A8/A9 induces apoptosis and inhibits metastasis of
CasKi human cervical cancer cells. Pathol Oncol Res. 16:353–360.
2010. View Article : Google Scholar
|
|
12
|
Reed JC and Pellecchia M: Apoptosis-based
therapies for hematologic malignancies. Blood. 106:408–418. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Stark GR and Taylor WR: Analyzing the G2/M
checkpoint. Methods Mol Biol. 280:51–82. 2004.PubMed/NCBI
|
|
15
|
Danial NN and Korsmeyer SJ: Cell death:
Critical control points. Cell. 116:205–219. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hunot S and Flavell RA: Apoptosis. Death
of a monopoly? Science. 292:865–866. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Horowitz NS, Hua J, Powell MA, Gibb RK,
Mutch DG and Herzog TJ: Novel cytotoxic agents from an unexpected
source: Bile acids and ovarian tumor apoptosis. Gynecol Oncol.
107:344–349. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yap TA, Carden CP and Kaye SB: Beyond
chemotherapy: Targeted therapies in ovarian cancer. Nat Rev Cancer.
9:167–181. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Geurts van Kessel A: The cancer genome:
From structure to function. Cell Oncol (Dordr). 37:155–165. 2014.
View Article : Google Scholar
|
|
20
|
Li Y, Wang K, Jiang Y-Z, Chang X-W, Dai
C-F and Zheng J: 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)
inhibits human ovarian cancer cell proliferation. Cell Oncol.
37:429–437. 2014. View Article : Google Scholar
|
|
21
|
Wang K, Li Y, Jiang YZ, Dai CF, Patankar
MS, Song JS and Zheng J: An endogenous aryl hydrocarbon receptor
ligand inhibits proliferation and migration of human ovarian cancer
cells. Cancer Lett. 340:63–71. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang TT and Phang JM: Effects of estrogen
on apoptotic pathways in human breast cancer cell line MCF-7.
Cancer Res. 55:2487–2489. 1995.PubMed/NCBI
|
|
23
|
Earnshaw WC, Martins LM and Kaufmann SH:
Mammalian caspases: Structure, activation, substrates, and
functions during apoptosis. Annu Rev Biochem. 68:383–424. 1999.
View Article : Google Scholar
|
|
24
|
Kang JH, Zhang WQ, Song W, Shen DY, Li SS,
Tian L, Shi Y, Liang G, Xiong YX and Chen QX: Apoptosis mechanism
of human cholangiocarcinoma cells induced by bile extract from
crocodile. Appl Biochem Biotechnol. 166:942–951. 2012. View Article : Google Scholar
|
|
25
|
Espinoza I and Miele L: Notch inhibitors
for cancer treatment. Pharmacol Ther. 139:95–110. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pannuti A, Foreman K, Rizzo P, Osipo C,
Golde T, Osborne B and Miele L: Targeting Notch to target cancer
stem cells. Clin Cancer Res. 16:3141–3152. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Koch U and Radtke F: Notch and cancer: A
double-edged sword. Cell Mol Life Sci. 64:2746–2762. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nam Y, Sliz P, Pear WS, Aster JC and
Blacklow SC: Cooperative assembly of higher-order Notch complexes
functions as a switch to induce transcription. Proc Natl Acad Sci
USA. 104:2103–2108. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nam Y, Sliz P, Song L, Aster JC and
Blacklow SC: Structural basis for cooperativity in recruitment of
MAML coactivators to Notch transcription complexes. Cell.
124:973–983. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Borggrefe T and Oswald F: The Notch
signaling pathway: Transcriptional regulation at Notch target
genes. Cell Mol Life Sci. 66:1631–1646. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yun J, Espinoza I, Pannuti A, Romero D,
Martinez L, Caskey M, Stanculescu A, Bocchetta M, Rizzo P, Band V,
et al: p53 modulates Notch signaling in MCF-7 breast cancer cells
by associating with the Notch transcriptional complex via MAML1. J
Cell Physiol. 230:3115–3127. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jeays-Ward K, Hoyle C, Brennan J,
Dandonneau M, Alldus G, Capel B and Swain A: Endothelial and
steroidogenic cell migration are regulated by WNT4 in the
developing mammalian gonad. Development. 130:3663–3670. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yao HH, Matzuk MM, Jorgez CJ, Menke DB,
Page DC, Swain A and Capel B: Follistatin operates downstream of
Wnt4 in mammalian ovary organogenesis. Dev Dyn. 230:210–215. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Batheja N, Suriawinata A, Saxena R,
Ionescu G, Schwartz M and Thung SN: Expression of p53 and PCNA in
cholangiocarcinoma and primary sclerosing cholangitis. Mod Pathol.
13:1265–1268. 2000. View Article : Google Scholar
|
|
35
|
Ferrara N: Role of vascular endothelial
growth factor in regulation of physiological angiogenesis. Am J
Physiol Cell Physiol. 280:C1358–C1366. 2001.PubMed/NCBI
|
|
36
|
Redmer DA, Doraiswamy V, Bortnem BJ,
Fisher K, Jablonka-Shariff A, Grazul-Bilska AT and Reynolds LP:
Evidence for a role of capillary pericytes in vascular growth of
the developing ovine corpus luteum. Biol Reprod. 65:879–889. 2001.
View Article : Google Scholar : PubMed/NCBI
|