Introduction
Glioma is the most common type of primary malignant
tumor of the central nervous system (1). Despite the current standard of care
for glioma patients, which involves surgery, radiotherapy and
chemotherapy, the prognosis of glioma patients remains poor. The
median overall survival for World Health Organization (WHO) grade
IV glioblastoma patients is only 14.6 months (2). Therefore, it is essential to improve
the treatments for glioma patients.
A previous report shows that the prognosis and
malignant degree of gliomas are correlated with the presence of
glioma stem-like cells (GSLCs) (3). Glioma stem-like cells are a small
cellular subpopulation that exhibits the capacity for self-renewal
and differentiation. Glioma stem-like cells are also thought to
contribute to glioma resistance to conventional radiotherapy and
chemotherapy. The expression of cancer stem cell-related markers in
gliomas is often associated with the survival of glioma stem cells
(4). Therefore, enriching and
identifying glioma stem-like cells is essential for the development
of novel therapeutic approaches targeting glioma stem cells.
β-catenin is a crucial factor involved in cancer
stem cell self-renewal. After entering the nucleus and binding with
the TCF/LEF family transcription factors, the activation of
β-catenin induces the expression of target genes to maintain cancer
stem cell survival (5). The
expression of β-catenin affects the mRNA stability of the cancer
stem growth factor IL6 (6). In
Wnt/β-catenin signaling, GSK3β forms a multimeric complex with
β-catenin, AXIN1 and APC. The destruction of the inactivated
complex results in the cytoplasmic accumulation and nuclear
translocation of β-catenin, where it associates with TCF/LEF and
alters the transcriptional state of the cell. Recent studies have
shown that the activation of Wnt/β-catenin signaling helps maintain
the glioma stem-like phenotype (7,8).
Wnt/β-catenin signaling also plays an important role in regulating
colorectal cancer stem cells (9).
Thus, the inhibition of Wnt/β-catenin signaling in GSLCs could be a
promising strategy for glioma therapy.
Tetrandrine (Tet) is a compound extracted from the
dried root of Stephania tetrandra S. Moore. Tet has been
widely used due to its anti-inflammatory, immunosuppressive and
anti-hypertensive effects (10).
Recent studies have shown that Tet has antitumor activity for
several cancer cells, including neuroblastoma, breast, lung, colon
and prostate cancer cells, and the anticancer effects of Tet could
be mediated in part by the inactivation of Wnt/β-catenin signal
transduction (11–15). However, neither the effects of Tet
in GSLCs nor its mechanism of action have been evaluated. In this
study, we identified and enriched GSLCs from the U87 and U251 cell
lines. Furthermore, we evaluated the effects of Tet on GSLCs, and
we found that Tet inhibits GSLCs by repressing the expression of
β-catenin and preventing its nuclear translocation.
Materials and methods
Patients and tissue collection
Eighty-eight patients with gliomas were included in
the present study. All patients underwent surgery at the Second
People's Hospital of Shenzhen (Shenzhen, China) between October
2004 and August 2010. This study cohort consisted of 51 males and
37 females, with a median age of 36 years (range, 3–72 years). All
patients underwent magnetic resonance imaging (MRI) a few days
before surgery and within 72 h after surgery. The extent of tumor
resection was determined using postoperative MRI scans. Surgical
resection was defined as macroscopic total resection, partial
resection, or biopsy, as appropriate. To confirm the diagnosis, two
neuropathologists independently evaluated the tumor samples
according to the WHO criteria: 47 patients presented with stage
I–II disease and 41 patients presented with stage III–IV
disease.
Cell lines and cultures
Human glioblastoma cell lines U87 and U251 were
obtained from the Shanghai Institute of Cell Biology, Chinese
Academy of Sciences (Shanghai, China). All cells were cultured in
Dulbecco's modified Eagle's medium (DMEM; Gibco-Life Technologies,
Paisley, UK) supplemented with 10% fetal bovine serum (FBS), 100
IU/ml penicillin and 100 mg/ml streptomycin. Cells were maintained
in an atmosphere of 5% CO2 at 37°C.
Neurosphere culture
Glioblastoma cells were plated in a 60-mm dish
(5,000 cells/ml) in neurobasal medium containing B27 (Invitrogen,
San Diego, CA, USA), 20 ng/ml human basic fibroblast growth factor
(Sigma-Aldrich, Taufkirchen, Germany) and 20 ng/ml epidermal growth
factor (Invitrogen, Carlsbad, CA, USA) for 7 days. Spheroids were
collected after 7 days and were dissociated with Accutase (a
mixture of enzymes with proteolytic, collagenolytic and DNase
activity; Invitrogen). The cells obtained from dissociation were
filtered through a 40-µm cell strainer. Then, the
dissociated cells were plated in a 60-mm dish and were maintained
in neurobasal medium to enrich for GSLCs. Subsequently, the protein
expression levels of stem cell markers were evaluated by western
blotting and immunofluorescence staining.
Cytotoxicity assays with Cell Counting
kit-8 (CCK-8)
The cytotoxic effect of Tet (Sigma-Aldrich, St.
Louis, MO, USA) was measured using the CCK-8 assay (Dojindo
Laboratories, Kumamoto, Japan). GSLCs were dissociated into single
cells, which were seeded into 96-well plates at a density of
5×103 cells/well. Various doses of Tet were added to
each well 24 h after seeding. Spheroids in each well were
photographed after treatment by microscopy. The optical density at
450 nm was measured using a microplate reader (BioTek Instruments,
Inc., Winooski, VT, USA). The IC50 value, determined by
the relative absorbance of CCK-8, was assessed using probit
regression analysis in the SPSS 13.0 statistical software package.
The half maximal inhibitory concentration (IC50) was
calculated using SPSS 13.0 statistical software.
Tumor spheroid formation assay
To assess the sphere-forming capability of GSLCs
in vitro, spheroid cells were dissociated into single cells
and then were plated in 96-well plates at a density of
1×103 cells/ml in an atmosphere of 5% CO2 at
37°C. Various doses of Tet were added to each well 24 h after
seeding. Spheroids >0.2 mm in diameter in each well were
photographed under a microscope after 7 days of incubation.
Transwell migration assay
For the Transwell migration assays, GSLCs were
dissociated into single cells, after which 5×103
cells/ml were plated in the top Transwell chamber (6.5 mm diameter,
8.0 µm pore size polycarbonate filters; Corning
Incorporated, Corning, NY, USA) and were allowed to migrate toward
600 µl of serum-containing medium in the lower Transwell
chamber. After 24 h, the cells were fixed with methanol and stained
with 0.1% crystal violet (2 mg/ml). The number of cells that
migrated through the membrane was counted in five randomly selected
fields under a light microscope.
FACS analysis
Apoptosis was measured using an Annexin V-FITC/PI
apoptosis detection kit (BD Biosciences, San Jose, CA, USA). GSLCs
were dissociated into single cells, after which the cells were
treated for the indicated amount of time and then harvested and
washed twice with cold phosphate-buffered saline (PBS). Then, cells
were re-suspended in 100 µl of binding buffer
(1×105 cells), after which the cells were stained with 5
µl of Annexin V-FITC and 10 µl of PI for 30 min at
room temperature in the dark. The percentage of apoptotic cells was
determined by flow cytometry (Beckman Coulter, Brea, CA, USA).
Western blotting
Protein expression levels were determined by western
blotting. In brief, the cells were lysed for 30 min on ice in 300
µl of radioimmunoprecipitation assay (RIPA) lysis buffer.
The lysates were subjected to sodium dodecyl sulfate
(SDS)-polyacrylamide gel electrophoresis (PAGE) separation, after
which the proteins were transferred onto polyvinylidene fluoride
(PVDF) membranes (Roche Diagnostics GmbH, Penzberg, Germany). The
membranes were blocked with 5% fat-free milk and were incubated
overnight at 4°C with primary antibodies against ALDH1 (Abcam,
Cambridge, UK), GAPDH, β-catenin, GSK3β, Bax, Bcl-2 and cleaved
PARP, as indicated (Cell Signaling Technology, Danvers, MA, USA).
After five washes of 10 min each in TBST, the membranes were
incubated with horseradish peroxidase (HRP)-conjugated secondary
antibodies (1:5,000; Cell Signaling Technology) for 1 h.
Immunoreactive bands were visualized using an enhanced
chemiluminescence assay (Pierce, Rockford, IL, USA).
Immunofluorescence staining
GSLCs were plated on poly-L-lysine-coated coverslips
(Sigma-Aldrich) and incubated overnight at 37°C, after which the
cells were treated with Tet, BIO (Selleck Chemicals, Houston TX,
USA) or ICG-001 (Selleck Chemicals). After treatment, the cells
were rinsed with PBS and fixed in 3.7% paraformaldehyde for 15 min.
Cells were then washed in PBS three times for 5 min, and 0.2%
Triton X-100 was added to the cells. Cells were blocked in 5%
bovine serum albumin for 60 min after three washes in PBS, and then
the cells were incubated with anti-β-catenin antibody (1:100),
anti-rabbit-FITC secondary antibody (1:1,000; Cell Signaling
Technology), TUNEL reaction mixture containing a nucleotide mixture
and terminal deoxynucleotidyl transferase (TdT) (In Situ
Cell Death Detection kit; Roche Diagnostics GmbH), and
4′,6-diamidino-2-phenylindole (DAPI; Cell Signaling Technology).
Finally, the slides were mounted and examined by laser scanning
confocal microscopy (LSM).
Immunohistochemical analysis
We prepared slides using 2-mm-thick sections of
paraffin-embedded specimens. The slides were baked at 60°C for 2 h,
deparaffinized in xylene, rehydrated in decreasing concentrations
of ethanol and rinsed in PBS. The slides were then microwaved with
a 10 mmol/l citrate buffer (pH 6.0; Maixin Bio, Fuzhou, China) on
the 'high' setting for 5 min and on the 'mid-high' setting for 10
min. The Ultra-Sensitive™ SP kit (Maixin Bio) was used to incubate
the slides with hydrogen peroxide and normal serum for 10 min each.
Next, the slides were incubated overnight with rabbit anti-human
monoclonal antibodies to β-catenin (diluted 1:100; Cell Signaling
Technology) at 4°C. The slides were then processed with the
Ultra-Sensitive™ SP kit for 30 min at room temperature, followed by
development with diaminobenzidine (DAB) for visualization. Negative
controls were included by substituting the primary antibodies with
non-immune serum.
Statistical analysis
The data are expressed as the means and standard
deviations (SD). The χ2 test was used to analyze the
correlations between the clinicopathological features and the
β-catenin expression. Statistical analysis of the remaining data
was conducted using a one-way analysis of variance (ANOVA) in the
statistical package SPSS 13.0 (SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered statistically significant.
Results
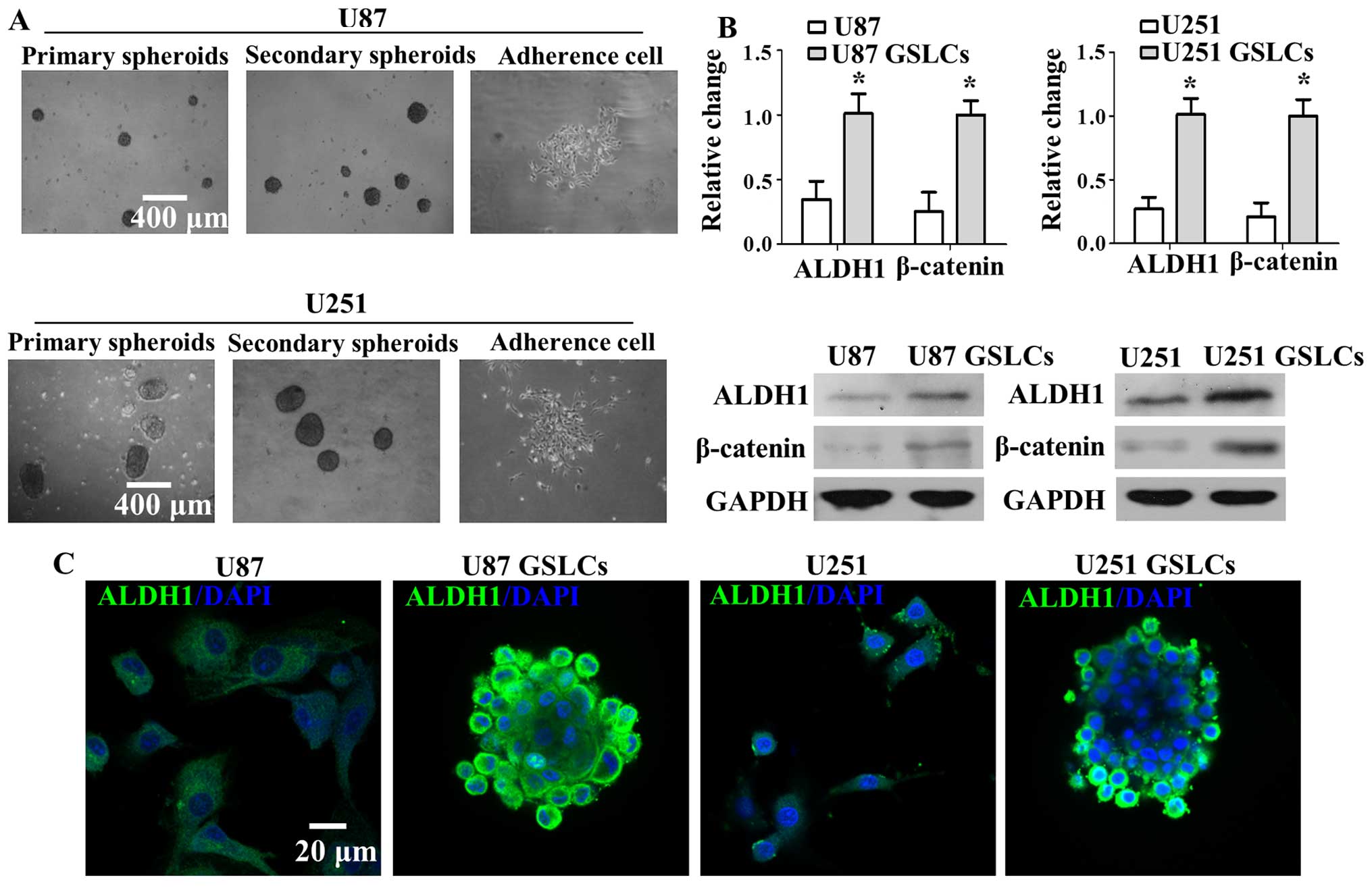
Generation of GSLCs from U87 and U251
sphere cultures induces neural stem cell properties with the
elevated expression of ALDH1 and β-catenin
We enriched for primary neurospheres from the human
glioma cell lines U87 and U251 by incubating the cells in
neurobasal medium containing B27, bFGF and EGF for 7 days. Then,
primary neurospheres were dissociated with Accutase into single
cell suspensions. Primary neurospheres were incubated in neurobasal
medium again to enrich for GSLCs (secondary neurospheres). GSLCs
attached to the plate and began proliferating for 3 days after the
serum-free medium was replaced with medium supplemented with serum
(Fig. 1A). Our results suggest
that GSLCs have the capacity for self-renewal and differentiation.
As shown in Fig. 1B and C, the
protein expression level of β-catenin and CSC marker ALDH1 was
significantly increased in U87 GSLCs and U251 GSLCs compared with
U87 and U251 cells, respectively (Fig.
1B). A similar result was observed with immunofluorescence
staining. ALDH1 (green fluorescence), which is located in the cell
membrane, is upregulated in U87 GSLCs and U251 GSLCs compared with
U87 and U251 cells, respectively (Fig.
1C). Blue fluorescence represents DAPI signal, which is located
in the cell nucleus. These results show that U87 GSLCs and U251
GSLCs possess CSC characteristics relative to the parental cells,
which includes elevated expression of ALDH1 and β-catenin.
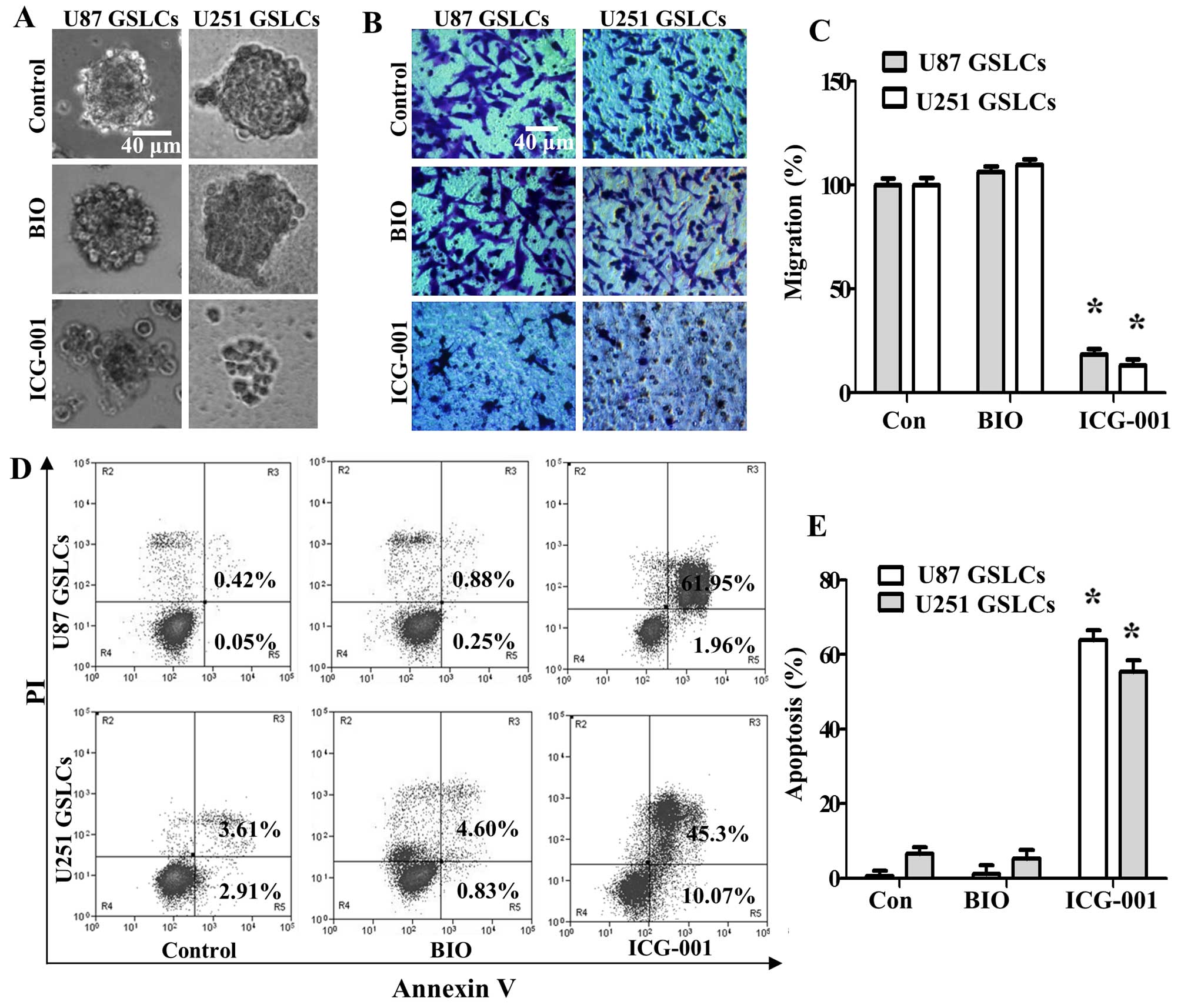
The regulation of β-catenin activity is
vital to maintain the neural stem cell traits of U87 GSLCs and U251
GSLCs
To determine whether the regulation of β-catenin
activity is vital to maintain CSC properties, we activated
β-catenin by treating U87 GSLCs and U251 GSLCs cells with the GSK3β
inhibitor BIO (2 µM) or we inactivated β-catenin/TCF
transcription by treating U87 GSLCs and U251 GSLCs cells with
ICG-001 (10 µM). Our results showed that BIO treatment
maintained sphere formation of U87 GSLCs and U251 GSLCs, whereas
ICG-001 significantly decreased sphere formation of U87 GSLCs and
U251 GSLCs (Fig. 2A). The
Transwell migration assay was used to assess the effects of BIO and
ICG-001 on the migration of GSLCs in vitro. Our results show
that compared with controls, the migration capability of GSLCs was
significantly reduced following ICG-001 treatment, but the
migration capability of GSLCs was conserved following BIO treatment
(Fig. 2B and C). We next analyzed
apoptosis of GSLCs upon treatment with BIO or ICG-001 by flow
cytometry. The percentage of apoptotic GSLCs increased
significantly after treatment with ICG-001, whereas GSLC apoptosis
did not increase following BIO treatment (Fig. 2D and E). Our results suggest that
the stem-like characteristics of U87 GSLCs and U251 GSLCs can be
effectively inhibited by inactivating β-catenin signaling, and that
activating β-catenin could promote the maintenance of CSC
properties.
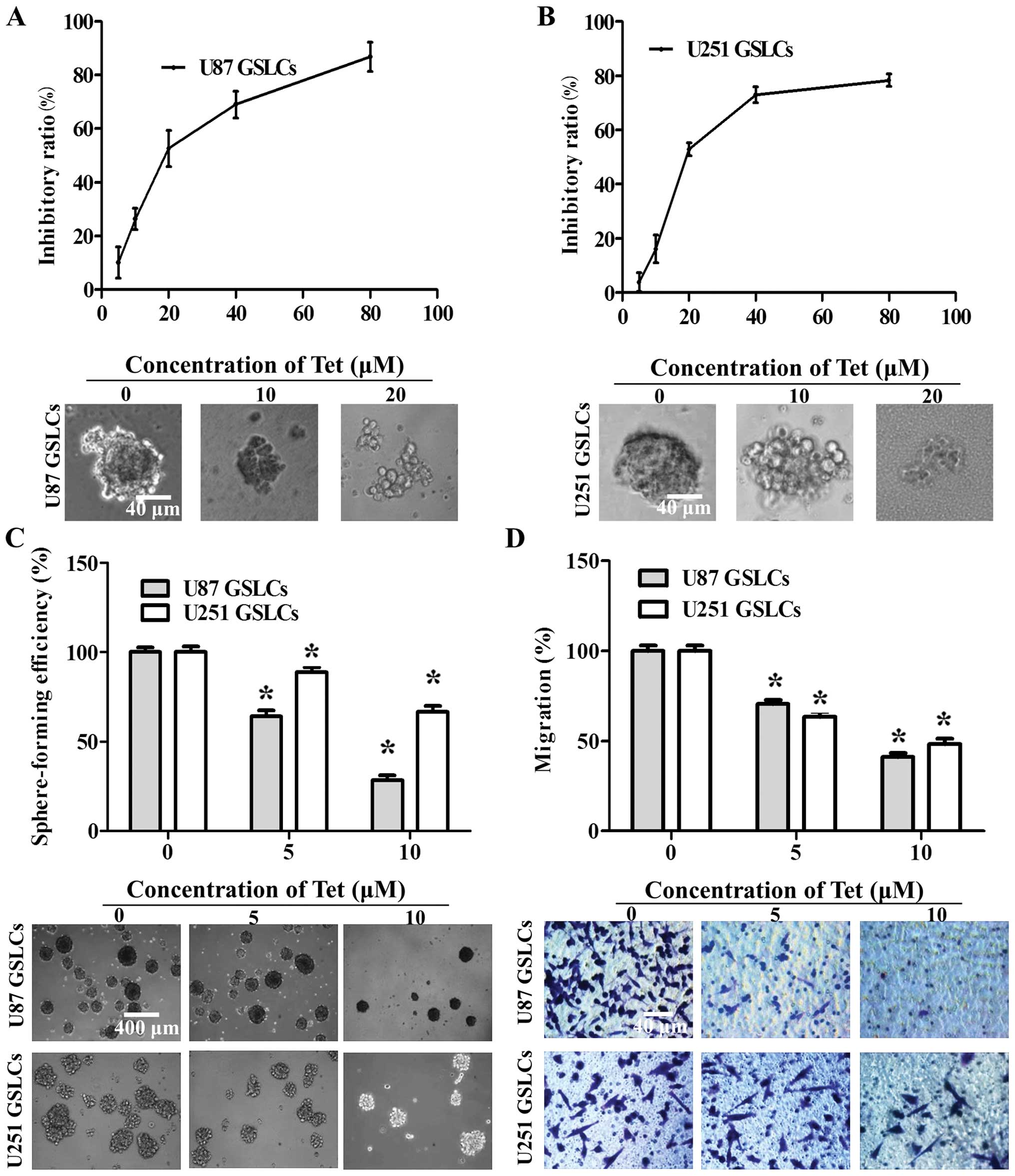
Tet inhibits cell viability, neurosphere
formation, and the migration of U87 GSLCs and U251 GSLCs in
vitro
To investigate whether Tet inhibits cell growth in
GSLCs, we analyzed cell viability following Tet treatment using a
CCK-8 assay. U87 GSLCs and U251 GSLCs were grown in neurobasal
medium containing B27, bFGF and EGF in the presence of various
doses of Tet (0–80 µM). The spheroids in each well were
photographed at the end of the incubation period. Our results show
that Tet treatment decreases the viability of GSLCs in a
dose-dependent manner (Fig. 3A and
B). Tet IC50 values in U87 GSLCs and U251 GSLCs were
30.41 and 27.5 µM, respectively. When GSLCs were
dissociated, suspended, and treated with Tet (0–10 µM) for 7
days, we observed changes in cell morphology. Tet treatment
decreased sphere formation in GSLCs in a dose-dependent manner
(Fig. 3C), suggesting that Tet may
inhibit the self-renewal capacity of GSLCs. Additionally, a
Transwell migration assay was used to investigate the effects of
Tet on the migration of GSLCs in vitro. Tet treatment
reduced the migration efficiency of GSLCs in a dose-dependent
manner (Fig. 3D).
Tet induces the apoptosis of GSLCs as
indicated by the upregulation of Bax, the cleavage of PARP, and the
downregulation of Bcl-2
To understand the mechanisms underlying the effects
of Tet on U87 GSLCs and U251 GSLCs, we analyzed the rate of
apoptosis in GSLCs treated with Tet by flow cytometry. Our results
show that the proportion of apoptotic GSLCs increases in a
dose-dependent manner in response to Tet treatment (Fig. 4A and B). We next assessed whether
GSLC treatment with Tet has an effect on the expression of
apoptosis-related proteins, including Bax, Bcl-2 and cleaved PARP.
We found that Tet treatment results in significant downregulation
of the expression of the anti-apoptotic protein Bcl-2, upregulation
of the expression of the apoptosis-promoting protein Bax, and an
increase in the cleavage of PARP in U87 GSLCs and U251 GSLCs
(Fig. 4C).
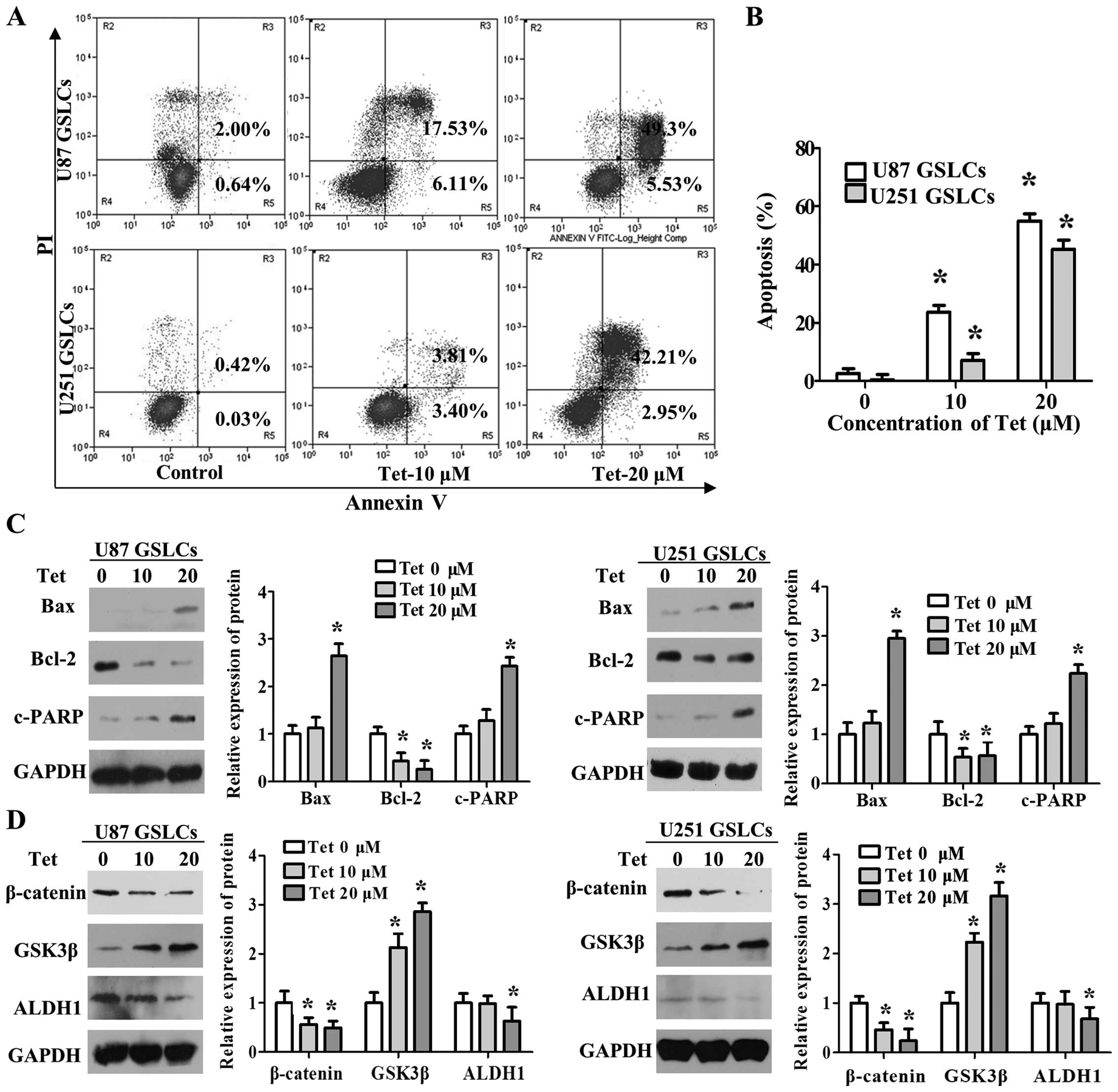 | Figure 4Tet induces GSLC apoptosis,
upregulates expression of GSK3β, Bax and cleaves PARP, and
downregulates expression of β-catenin, Bcl-2 and ALDH1. (A and B)
GSLCs were dissociated, seeded in suspension and treated with Tet
for 48 h. The cells were then stained with Annexin V-FITC and
propidium iodide (PI) and analyzed by flow cytometry (left). The
Annexin V/PI-positive cells were considered apoptotic cells.
Fold-changes were calculated (right). (C and D) GSLCs were treated
with Tet (0–20 µM) for 48 h. The expression levels of Bcl-2,
Bax, c-PARP, β-catenin, GSK3β, ALDH1 and GAPDH were determined by
western blot analysis (left). Fold-changes were calculated (right).
Data represent the means ± SD. *P<0.05 different from
the respective controls. |
Tet inhibits neural stem cell properties
of GSLCs with the upregulation of GSK3β and the upregulation of
β-catenin
We next analyzed whether Tet has an effect on the
Wnt/β-catenin pathway as well as the stemness marker ALDH1. Upon
dissociation, suspension and treatment of GSLCs with Tet for 48 h,
we found that Tet significantly reduced the protein expression
levels of β-catenin and increased the expression of GSK3β.
Moreover, the stem cell marker ALDH1 was significantly
downregulated in response to treatment with 20 µM Tet
(Fig. 4D). These data indicate
that Tet has inhibitory effects on GSLCs, which are partly
associated with the repression of the Wnt/β-catenin pathway.
Tet inhibits GSLCs by repressing the
nuclear translocation and expression of β-catenin
To determine whether the inhibitory effects of Tet
are related to the downregulation and inactivation of β-catenin, we
assessed additional Wnt pathway-related proteins (β-catenin and
GSK3β), the CSC marker ALDH1, and apoptosis-related proteins
(Bcl-2, Bax and c-PARP) upon treatment of GSLCs with 2 µM
BIO, 10 µM ICG-001, or 20 µM Tet treatment (Fig. 5A–D). The expression levels of
GSK3β, Bax and cleaved PARP significantly increased, whereas the
expression levels of β-catenin, ALDH1 and Bcl-2 significantly
decreased following ICG-001 and Tet treatment. Compared with Tet
treatment, BIO treatment downregulated the expression of GSK3β and
upregulated the expression of β-catenin and Bcl-2. We further
analyzed the proportion of apoptotic cells and the expression and
localization of β-catenin by TUNEL assay and confocal
immunofluorescence staining, respectively, following treatment with
BIO, ICG-001 and Tet (Fig. 5E and
F). Our results show that β-catenin becomes concentrated in the
cytoplasm and nucleus, and TUNEL staining is negative in response
to BIO treatment. Upon ICG-001 or Tet treatment, the expression of
β-catenin decreased and TUNEL staining increased. These results
suggest that the nuclear translocation and expression of β-catenin
are vital for cell survival and the maintenance of CSC properties
in U87 GSLCs and U251 GSLCs.
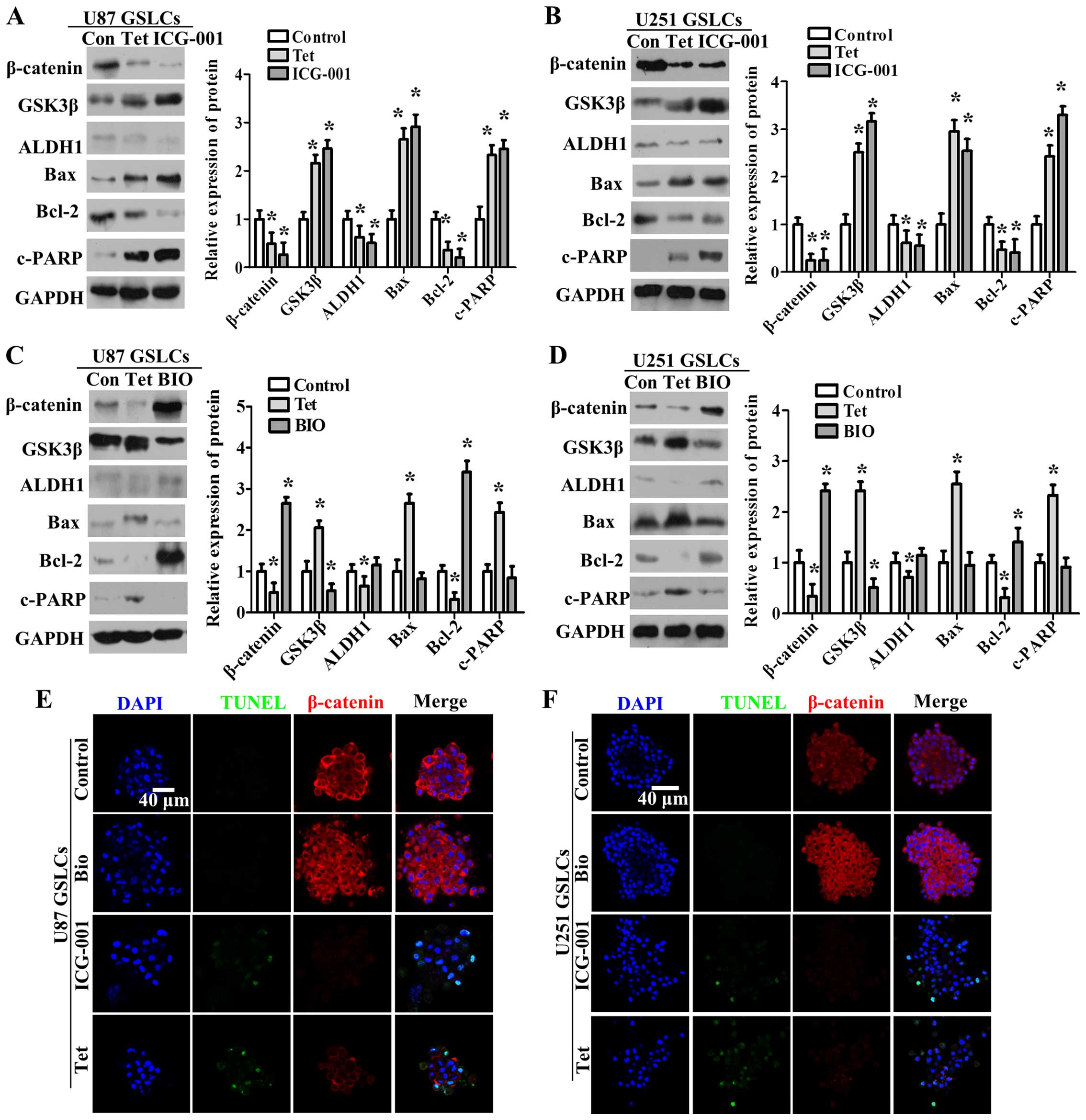 | Figure 5Tet inhibits β-catenin expression and
nuclear translocation to modulate the Wnt signaling pathway and
stimulates apoptosis in U87 GSLCs and U251 GSLCs. (A–D) GSLCs were
dissociated and seeded in suspension culture, after which they were
treated with Tet or BIO (A and B) or Tet or ICG-001 (C and D). The
expression levels of β-catenin, GSK3β, ALDH1, Bcl-2, Bax, c-PARP
and GAPDH were determined by western blot analysis (left panel).
Fold-changes were calculated (right panel). The data represent the
means ± SD. *P<0.05 different from the respective
controls. (E and F) Confocal immunofluorescence staining. U87 GSLCs
and U251 GSLCs were seeded in suspension and treated with control,
Tet, BIO or ICG-001 for 48 h. Blue fluorescence represents DAPI,
red fluorescence represents β-catenin, and green fluorescence
represents TUNEL. Scale bars, 40 µm. |
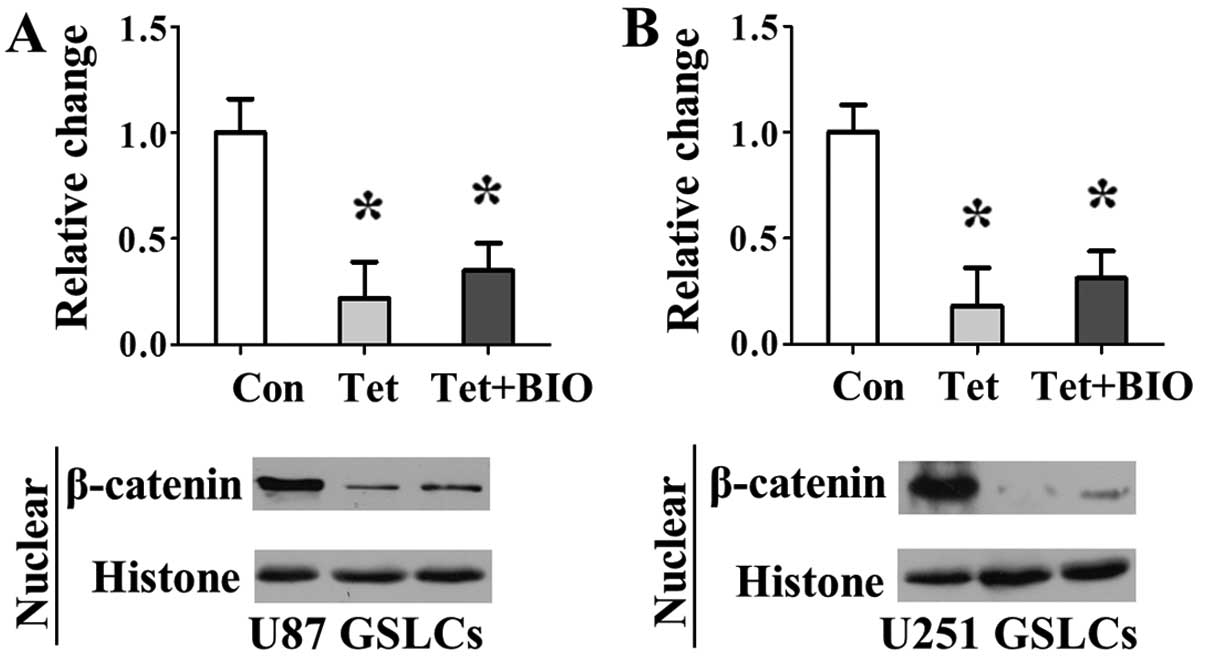
Tet inhibits the β-catenin activation
induced by BIO
To determine whether Tet inhibited the β-catenin
signaling activation induced by BIO, we assessed nuclear β-catenin
expression upon treatment of GSLCs with 20 µM Tet or 20
µM Tet + 2 µM BIO treatment. The expression levels of
nuclear β-catenin significantly decreased following Tet and Tet +
BIO treatment. Compared with the control treatment group, the Tet +
BIO treatment group downregulated the expression of nuclear
β-catenin significantly (Fig.
6).
Association between β-catenin nuclear
localization and clinicopathological features
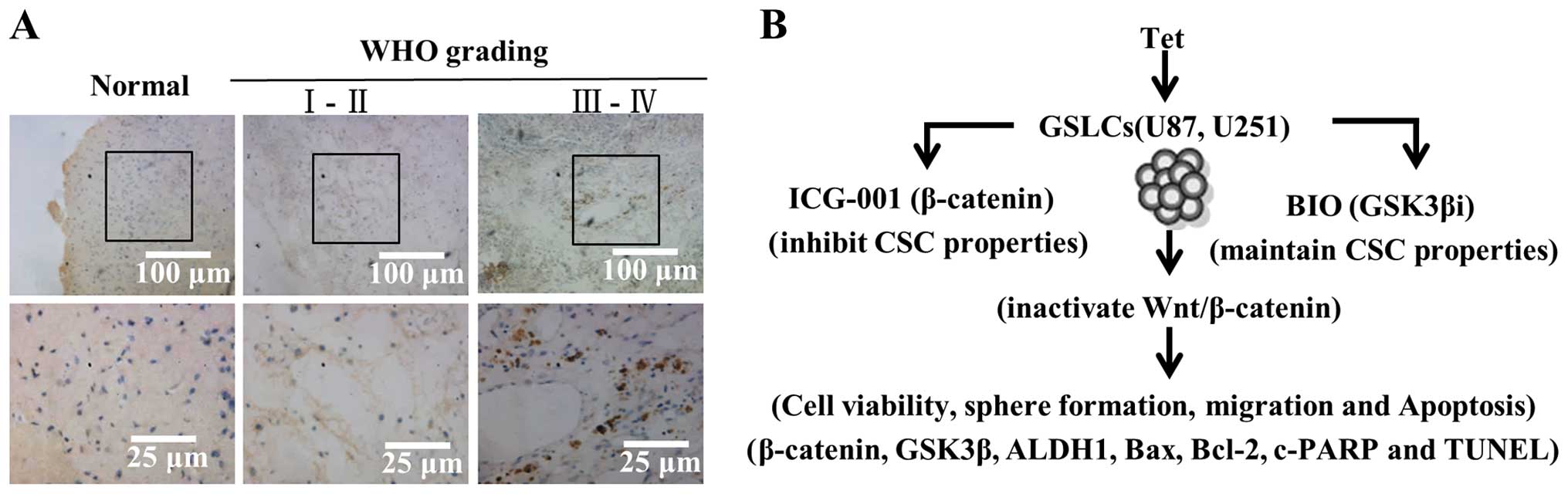
Immunohistochemical detection of β-catenin was
analyzed in 88 gliomas for which sufficient tissue was available.
WHO grading for the samples was distributed as follows: I + II, 47
cases; III + IV, 41 cases. According to our IHC staining results,
in tumor cells, β-catenin signal was observed in the cytoplasm,
nucleus, or both, and the β-catenin levels were heterogeneous. In
contrast, β-catenin staining in normal brain tissue was constantly
negative (Fig. 7A). As shown in
Table I, β-catenin
immunoreactivity in the nucleus was low in grade I–II tumors,
whereas β-catenin signal increased significantly in grade III–IV
tumors. Nuclear β-catenin immunoreactivity significantly correlated
with WHO grading based on analysis using the chi-square test
(χ2=43.59, P=0.001). No significant differences between
gender and age were observed. These results suggest that the
nuclear expression of β-catenin is related to the degree of
malignancy in gliomas.
 | Table ICorrelation between the expression of
β-catenin and clinicopathological parameters in glioma patients
(χ2 test). |
Table I
Correlation between the expression of
β-catenin and clinicopathological parameters in glioma patients
(χ2 test).
| Variables | n | β-catenin
expression
| χ2 | P-value |
|---|
|
Cytoplasm/member | Nuclear |
|---|
| Gender | | | | 2.503 | 0.114 |
| Male | 51 | 23 | 28 | | |
| Female | 37 | 23 | 14 | | |
| Age (years) | | | | 0.059 | 0.808 |
| <36 | 41 | 22 | 19 | | |
| ≥36 | 47 | 24 | 23 | | |
| WHO grading | | | | 43.591 | 0.001 |
| I–II | 47 | 40 | 7 | | |
| III–IV | 41 | 6 | 35 | | |
Discussion
Recent studies have shown that cancer stem cells in
malignant glioma closely correlate with poor prognosis. Glioma
patients often relapse after treatment because of the persistence
of glioma stem cells. In the present study, we enriched cells with
CSC properties to obtain U87 GSLCs and U251 GSLCs based on high
levels of ALDH1 expression. Tet cytotoxicity was demonstrated in
U87 GSLCs and U251 GSLCs. Importantly, as shown in Fig. 7B, we found for the first time that
Tet induces GSLC apoptosis by repressing the expression of
β-catenin and preventing β-catenin nuclear translocation.
A small fraction of cancer stem cells (CSC) are
thought to initiate cancer and drive the tumorigenesis of gliomas.
Studies have shown that ALDH1 activity can be used as a marker to
identify stem-like cells in glioma, breast, liver, hepatic cancer
and embryonal rhabdomyosarcoma (16–20).
We enriched for GSLCs from glioma cell lines U87 and U251 using a
neurosphere culture technique, and we resuspended the GSLCs for
subculture using Accutase. The data in Fig. 1B and C show that ALDH1 is highly
expressed in U87 GSLCs and U251 GSLCs compared with their parental
cell lines. This suggests that we enriched U87 GSLCs and U251 GSLCs
based on increased expression of the cancer stem cell marker
ALDH1.
The Wnt/β-catenin pathway is one of the key
signaling pathways in cancer stem cells. A recent study also showed
that the suppression of GSK3β activity enhances β-catenin signaling
and increases the level of nuclear β-catenin, which promotes the
stemness of colorectal cancer cells (21). The inactivation of Wnt/β-catenin
signaling results in decreased tumor growth and increased invasive
ability of glioma stem cells (8,22).
The activation of Wnt/β-catenin signaling contributes to the
maintenance of glioma stem cells and the stem cell phenotype in
glioma (7,23,24).
Our results show that the pharmacologic inhibition of GSK3β by BIO
treatment in U87 GSLCs and U251 GSLCs results in increased
β-catenin activity as well as no change in sphere formation,
migration capability, or apoptosis compared with control cells. In
contrast, the pharmacologic inhibition of β-catenin in U87 GSLCs
and U251 GSLCs by ICG-001, which decreases the expression of
nuclear β-catenin and β-catenin transcription, resulted in reduced
sphere formation and migration capability and an increase in
apoptosis compared with control cells (Fig. 2). In addition, the
apoptosis-related protein Bcl-2 was upregulated and c-PARP was
decreased in GSLCs in response to BIO treatment, whereas Bcl-2 was
downregulated, and Bax and c-PARP were upregulated in GSLCs in
response to ICG-001 treatment (Fig.
4).
Recent studies have reported that Tet is a strong
anticancer agent based on in vitro and in vivo
studies (25). Tet also showed a
time- and concentration-dependent cytotoxic effect on neuroblastoma
cells (11). In this study, we
found for the first time that Tet reduces the viability,
neurosphere formation capacity, and migration capacity in GSLCs in
a dose-dependent manner (Fig. 3).
Tet treatment also induced apoptosis in GSLCs by reducing the
expression levels of β-catenin, increasing the protein expression
of GSK3β, inactivating the anti-apoptotic protein Bcl-2,
upregulating the apoptosis-promoting protein Bax, and promoting the
cleavage of PARP (Fig. 4). The
Wnt/β-catenin signaling pathway is associated with the anticancer
activity of Tet in chronic myeloid leukemia and human colorectal
cancer, which is consistent with our results (26,27).
Moreover, our results are in agreement with the finding that Tet
treatment reduces Bcl-2 levels, increases Bax levels, and promotes
the cleavage of PARP in other cancer cells (28–30).
These data suggest that Tet may induce GSLC apoptosis partly
through the inhibition of the Wnt/β-catenin signaling pathway.
Wnt/β-catenin signaling plays a critical role in
cancer cell proliferation and invasion. β-catenin protein is
persistently upregulated in a variety of human cancers, including
glioma (31). The nuclear
translocation and expression of β-catenin is critical for the
survival, invasion and tumorigenesis of glioma (32–34).
Previous studies have reported that aberrant Wnt/β-catenin
signaling is essential for the maintenance of cancer stem cells
(CSCs) of various origins, including the bladder, blood, breast and
colon (35). Our results suggest
that the inhibition of Wnt/β-catenin signaling in CSCs could be an
effective treatment for cancer, which has been suggested by other
studies (36). The Wnt/β-catenin
pathway critically regulates the self-renewal and differentiation
of neural stem/progenitor cells (37–39).
A previous study showed that the inhibition of nuclear β-catenin
expression decreases the in vitro proliferation and sphere
formation capability of glioma stem cells (40). These results suggest that
Wnt/β-catenin signaling activation is vital for the survival of
glioma stem cells. GSCs are also closely related to the degree of
malignancy in gliomas. In this study, we found that the nuclear
expression of β-catenin in glioma tissues significantly correlated
with WHO grading (Fig. 6A). Tet
treatment inhibits GSLCs by repressing the nuclear translocation
and expression of β-catenin (Fig.
5A–E). Tet treatment (10 mg/kg body weight) also inhibits tumor
metastasis in the mouse breast in vivo (12). Additional studies in other cell
lines and animal models will be necessary to demonstrate the
inhibitory effect of Tet on GSLCs through the repression of
β-catenin signaling.
In conclusion, we observed for the first time that
Tet inhibits cell viability, neurosphere formation, and migration
in U87 GSLCs and U251 GSLCs in vitro. The WHO grading of
glioma tissues was significantly correlated with the nuclear
expression of β-catenin. Importantly, Tet treatment repressed the
nuclear translocation and expression of β-catenin and induced
apoptosis in GSLCs through the upregulation of Bax, the cleavage of
PARP, and the downregulation of Bcl-2. Our results provide a basis
for Tet development and provide novel insight into GSC-based
anti-glioma treatments.
Abbreviations:
|
CSCs
|
cancer stem cells
|
|
GSLCs
|
glioma stem-like cells
|
|
Tet
|
tetrandrine
|
|
TdT
|
terminal deoxynucleotidyl
transferase
|
Acknowledgments
The present study was supported by the Science and
Technology Project of Guangzhou (no. 2014J4100103), the China
Postdoctoral Science Foundation (no. 2015M572414), the Guangdong
Natural Science Foundation (no. 2016A030310096) and the Shenzhen
Science and Technology Project (no. JCYJ20150324141711568).
References
|
1
|
Parsons DW, Jones S, Zhang X, Lin JC,
Leary RJ, Angenendt P, Mankoo P, Carter H, Siu IM, Gallia GL, et
al: An integrated genomic analysis of human glioblastoma
multiforme. Science. 321:1807–1812. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
You G, Sha ZY, Yan W, Zhang W, Wang YZ, Li
SW, Sang L, Wang Z, Li GL, Li SW, et al: Seizure characteristics
and outcomes in 508 Chinese adult patients undergoing primary
resection of low-grade gliomas: A clinicopathological study. Neuro
Oncol. 14:230–241. 2012. View Article : Google Scholar :
|
|
3
|
Dahlrot RH: The prognostic value of
clinical factors and cancer stem cell-related markers in gliomas.
Dan Med J. 61:B49442014.PubMed/NCBI
|
|
4
|
Tabatabai G and Weller M: Glioblastoma
stem cells. Cell Tissue Res. 343:459–465. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
D'Uva G, Bertoni S, Lauriola M, De Carolis
S, Pacilli A, D'Anello L, Santini D, Taffurelli M, Ceccarelli C,
Yarden Y, et al: Beta-catenin/HuR post-transcriptional machinery
governs cancer stem cell features in response to hypoxia. PLoS One.
8:e807422013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Korkaya H, Kim GI, Davis A, Malik F, Henry
NL, Ithimakin S, Quraishi AA, Tawakkol N, D'Angelo R, Paulson AK,
et al: Activation of an IL6 inflammatory loop mediates trastuzumab
resistance in HER2+ breast cancer by expanding the
cancer stem cell population. Mol Cell. 47:570–584. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen X, Hu W, Xie B, Gao H, Xu C and Chen
J: Downregulation of SCAI enhances glioma cell invasion and stem
cell like phenotype by activating Wnt/β-catenin signaling. Biochem
Biophys Res Commun. 448:206–211. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rathod SS, Rani SB, Khan M, Muzumdar D and
Shiras A: Tumor suppressive miRNA-34a suppresses cell proliferation
and tumor growth of glioma stem cells by targeting Akt and Wnt
signaling pathways. FEBS Open Bio. 4:485–495. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brabletz S, Schmalhofer O and Brabletz T:
Gastrointestinal stem cells in development and cancer. J Pathol.
217:307–317. 2009. View Article : Google Scholar
|
|
10
|
Wang H and Chen X: Tetrandrine ameliorates
cirrhosis and portal hypertension by inhibiting nitric oxide in
cirrhotic rats. J Huazhong Univ Sci Technolog Med Sci. 24:385–388.
3952004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen Y, Chen JC and Tseng SH: Effects of
tetrandrine plus radiation on neuroblastoma cells. Anticancer Res.
29:3163–3171. 2009.PubMed/NCBI
|
|
12
|
Gao JL, Ji X, He TC, Zhang Q, He K, Zhao
Y, Chen SH and Lv GY: Tetrandrine suppresses cancer angiogenesis
and metastasis in 4T1 tumor bearing mice. Evid Based Complement
Alternat Med. 2013:2650612013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cho HS, Chang SH, Chung YS, Shin JY, Park
SJ, Lee ES, Hwang SK, Kwon JT, Tehrani AM, Woo M, et al:
Synergistic effect of ERK inhibition on tetrandrine-induced
apoptosis in A549 human lung carcinoma cells. J Vet Sci. 10:23–28.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu K, Zhou M, Wu QX, Yuan SX, Wang DX, Jin
JL, Huang J, Yang JQ, Sun WJ, Wan LH, et al: The role of IGFBP-5 in
mediating the anti-proliferation effect of tetrandrine in human
colon cancer cells. Int J Oncol. 46:1205–1213. 2015.
|
|
15
|
Kou B, Liu W, He W, Zhang Y, Zheng J, Yan
Y, Zhang Y, Xu S and Wang H: Tetrandrine suppresses metastatic
phenotype of prostate cancer cells by regulating Akt/mTOR/MMP-9
signaling pathway. Oncol Rep. 35:2880–2886. 2016.PubMed/NCBI
|
|
16
|
Kida K, Ishikawa T, Yamada A, Shimada K,
Narui K, Sugae S, Shimizu D, Tanabe M, Sasaki T, Ichikawa Y, et al:
Effect of ALDH1 on prognosis and chemoresistance by breast cancer
subtype. Breast Cancer Res Treat. 156:261–269. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gehlot P, Shukla V, Gupta S and Makidon
PE: Detection of ALDH1 activity in rabbit hepatic VX2 tumors and
isolation of ALDH1 positive cancer stem cells. J Transl Med.
14:492016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang Y, Wang SX, Ma JW, Li HY, Ye JC, Xie
SM, Du B and Zhong XY: EGCG inhibits properties of glioma stem-like
cells and synergizes with temozolomide through downregulation of
P-glycoprotein inhibition. J Neurooncol. 121:41–52. 2015.
View Article : Google Scholar
|
|
19
|
Nakahata K, Uehara S, Nishikawa S, Kawatsu
M, Zenitani M, Oue T and Okuyama H: Aldehyde dehydrogenase 1
(ALDH1) is a potential marker for cancer stem cells in embryonal
rhabdomyosarcoma. PLoS One. 10:e01254542015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu M, Inoue K, Leng T, Guo S and Xiong
ZG: TRPM7 channels regulate glioma stem cell through STAT3 and
Notch signaling pathways. Cell Signal. 26:2773–2781. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Venugopal A, Subramaniam D, Balmaceda J,
Roy B, Dixon DA, Umar S, Weir SJ and Anant S: RNA binding protein
RBM3 increases beta-catenin signaling to increase stem cell
characteristics in colorectal cancer cells. Mol Carcinog.
55:1503–1516. 2015. View
Article : Google Scholar
|
|
22
|
Gong A and Huang S: FoxM1 and
Wnt/β-catenin signaling in glioma stem cells. Cancer Res.
72:5658–5662. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim KH, Seol HJ, Kim EH, Rheey J, Jin HJ,
Lee Y, Joo KM, Lee J and Nam DH: Wnt/β-catenin signaling is a key
downstream mediator of MET signaling in glioblastoma stem cells.
Neuro Oncol. 15:161–171. 2013. View Article : Google Scholar
|
|
24
|
Shi L, Fei X, Wang Z and You Y: PI3K
inhibitor combined with miR-125b inhibitor sensitize TMZ-induced
anti-glioma stem cancer effects through inactivation of
Wnt/beta-catenin signaling pathway. In Vitro Cell Dev Biol Anim.
51:1047–1055. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bhagya N and Chandrashekar KR: Tetrandrine
- A molecule of wide bioactivity. Phytochemistry. 125:5–13. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xu XH, Gan YC, Xu GB, Chen T, Zhou H, Tang
JF, Gu Y, Xu F, Xie YY, Zhao XY, et al: Tetrandrine citrate
eliminates imatinib-resistant chronic myeloid leukemia cells in
vitro and in vivo by inhibiting Bcr-Abl/β-catenin axis. J Zhejiang
Univ Sci B. 13:867–874. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
He BC, Gao JL, Zhang BQ, Luo Q, Shi Q, Kim
SH, Huang E, Gao Y, Yang K, Wagner ER, et al: Tetrandrine inhibits
Wnt/β-catenin signaling and suppresses tumor growth of human
colorectal cancer. Mol Pharmacol. 79:211–219. 2011. View Article : Google Scholar :
|
|
28
|
Zhang YX, Liu XM, Wang J, Li J, Liu Y,
Zhang H, Yu XW and Wei N: Inhibition of AKT/FoxO3a signaling
induced PUMA expression in response to p53-independent cytotoxic
effects of H1: A derivative of tetrandrine. Cancer Biol Ther.
16:965–975. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhu R, Liu T, Tan Z, Wu X, Li M, Jiang L,
Bao R, Shu Y, Lu A and Liu Y: Tetrandrine induces apoptosis in
gallbladder carcinoma in vitro. Int J Clin Pharmacol Ther.
52:900–905. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yu VW and Ho WS: Tetrandrine inhibits
hepatocellular carcinoma cell growth through the caspase pathway
and G2/M phase. Oncol Rep. 29:2205–2210. 2013.PubMed/NCBI
|
|
31
|
Zheng H, Ying H, Wiedemeyer R, Yan H,
Quayle SN, Ivanova EV, Paik JH, Zhang H, Xiao Y, Perry SR, et al:
PLAGL2 regulates Wnt signaling to impede differentiation in neural
stem cells and gliomas. Cancer Cell. 17:497–509. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Náger M, Santacana M, Bhardwaj D, Valls J,
Ferrer I, Nogués P, Cantí C and Herreros J: Nuclear phosphorylated
Y142 β-catenin accumulates in astrocytomas and glioblastomas and
regulates cell invasion. Cell Cycle. 14:3644–3655. 2015. View Article : Google Scholar
|
|
33
|
Kahlert UD, Suwala AK, Koch K, Natsumeda
M, Orr BA, Hayashi M, Maciaczyk J and Eberhart CG: Pharmacologic
Wnt inhibition reduces proliferation, survival, and clonogenicity
of glioblastoma cells. J Neuropathol Exp Neurol. 74:889–900. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang N, Wei P, Gong A, Chiu WT, Lee HT,
Colman H, Huang H, Xue J, Liu M, Wang Y, et al: FoxM1 promotes
β-catenin nuclear localization and controls Wnt target-gene
expression and glioma tumorigenesis. Cancer Cell. 20:427–442. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Holland JD, Klaus A, Garratt AN and
Birchmeier W: Wnt signaling in stem and cancer stem cells. Curr
Opin Cell Biol. 25:254–264. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Takebe N, Harris PJ, Warren RQ and Ivy SP:
Targeting cancer stem cells by inhibiting Wnt, Notch, and Hedgehog
pathways. Nat Rev Clin Oncol. 8:97–106. 2011. View Article : Google Scholar
|
|
37
|
Chenn A and Walsh CA: Regulation of
cerebral cortical size by control of cell cycle exit in neural
precursors. Science. 297:365–369. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Clevers H, Loh KM and Nusse R: Stem cell
signaling. An integral program for tissue renewal and regeneration:
Wnt signaling and stem cell control. Science. 346:12480122014.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nusse R: Wnt signaling and stem cell
control. Cell Res. 18:523–527. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kierulf-Vieira KS, Sandberg CJ, Grieg Z,
Günther CC, Langmoen IA and Vik-Mo EO: Wnt inhibition is
dysregulated in gliomas and its re-establishment inhibits
proliferation and tumor sphere formation. Exp Cell Res. 340:53–61.
2016. View Article : Google Scholar
|