Introduction
Laryngeal carcinoma is the second most malignant
tumor of the upper respiratory tract (after lung cancer),
accounting for 2.4% of all new cases of malignancy worldwide
annually (1,2). According to the regions affected,
laryngeal carcinoma is divided into supraglottis, glottis and
subglottis carcinoma. Based on histology, >95% of laryngeal
carcinomas are squamous cell carcinoma (3). Surgery (with concomitant
chemotherapy) and radiotherapy represent the mainstream treatments
for laryngeal cancer. Function preservation has significant weight
in the selection of therapeutic approaches. Therefore, there is a
pressing need to identify new biological indicators for predicting
prognosis, help selecting treatment strategies, and facilitate the
development of novel therapeutic approaches.
Several studies have shown the downregulation of the
epithelial membrane protein 1 (EMP1), also known as RAP, CL-20 and
B4B, in head and neck carcinomas (4–7).
EMP1 was first cloned from the fetal rat intestinal cDNA library
and is considered to form a gene family with the peripheral myelin
protein and the lens-specific membrane protein 20, due to the
sequence homology of these three proteins (8). The EMP1 gene is located on human
chromosome 12p12.3 (9,10). It encodes a glycoprotein of 157
amino acids that contains four transmembrane domains (11). As indicated by its name, EMP1 is
primarily expressed in squamous epithelium and presents different
levels of expression in a variety of normal tissues, such as the
gastrointestinal tract of the gastric bottom, ileum and colon
(8), liver (12) and prostate (13). EMP1 is associated with a collection
of biological activities, including cell proliferation,
differentiation, and apoptosis (14). It is therefore not surprising that
EMP1 may play a role in cancer development. However, the
significance of EMP1 in cancer seems to vary with different tumor
types: the overexpression of EMP1 was detected in leiomyoma uteri
(15), lung cancer (16), leukemia (17), and brain glioma (18), whereas the downregulation of EMP1
was reported in esophageal carcinoma (19), gastric cancer (20), prostate cancer (21), oral squamous cell carcinoma
(22), breast cancer (23) and nasopharyngeal carcinoma
(24).
No studies have systemically examined the status and
explored the biological activities of EMP1 in laryngeal cancer or
come close to doing this. We tackled this task in this study. By
comparing EMP1 expression in 51 laryngeal carcinoma tissues with
that of matched cancer-free peritumor tissues, we assessed the
expression and clinical correlation of EMP1 in laryngeal carcinoma.
Furthermore, using a gain-of-function approach, we examined the
biological activities of EMP1 in laryngeal cancer cells.
Materials and methods
Human tissue samples and cell lines
This study was approved by the Institutional
Bioethics Committee of China Medical University (Shenyang, China)
and informed consent was obtained from all participating
individuals. A total of 51 pairs of samples, including a tumor
sample (T) and a matched, cancer-free peritumor tissue sample (N),
were examined in this study. All tissues were obtained from
patients diagnosed with laryngeal carcinoma and receiving surgery
(including partial laryngectomy in 29 patients and total
laryngectomy in 22) at the First Hospital of China Medical
University from 2011 to 2012. Immediately after surgical removal
from the patients, 24 pairs of tissue samples were snap-frozen in
liquid nitrogen and then transferred to −80°C until further use
(for western immunoblot); another 27 pairs of tissue samples were
fixed in formalin before being embedded into paraffin and processed
into 4-μm thick sections (for immunohistochemistry).
Human laryngeal carcinoma cell line, Hep-2, was
purchased from the Cell Bank, Chinese Academy of Sciences
(Shanghai, China) and cultured in RPMI-1640 medium (Gibco,
Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS; HyClone,
Logan, UT, USA).
Immunohistochemical (IHC) staining and
scoring
IHC staining was performed using the IHC staining
kit (Maxim, Fuzhou, China), following the manufacturer's
instructions. Briefly, the tissue sections were blocked with
H2O2 (to block endogenous peroxidase)
followed by nonimmune serum (reagent A) for 20 min, and then
incubated with mouse anti-human EMP1 polyclonal antibody (Abnova,
Taiwan, China) at 4°C overnight. After being washed with
phosphate-buffered saline (PBS) 3 times, the sections were
incubated with biotinylated secondary antibody (reagent B) at room
temperature for 15 min, followed by streptavidin-horseradish
peroxidase (reagent C) for 15 min. The signals were detected using
3,3′-diaminobenzidine tetrahydrochloride (DAB; Maxim) and counter
stained with hematoxylin.
The tissue sections were scored by two independent
pathologists blinded to the study, as detailed below. The 'positive
rate' (P) was defined as follows: 0 means <10% of the tissues
showed positive staining; 1, 10–25%; 2, 26–50%; 3, 51–75%; and 4,
76–100%. The 'intensity of staining' (I) was defined as: 0
indicated negative staining; 1, light yellow; 2, brown; and 3, dark
brown. The total staining score (S) was calculated as P × I.
Reverse transcription quantitative
real-time PCR (RT-PCR)
Total RNA was isolated from frozen tissues (~100 mg
each) using TRIzol reagent (Invitrogen, Carlsbad, CA, USA)
following the manufacturer's instructions. Reverse transcription
into cDNA was then performed on total RNA, using the Reverse
Transcription system (A3500; Promega, Madison, WI, USA) per the
manufacturer's instructions. Real-time PCR was performed using the
following primers: EMP1 forward, 5′-AGGGAATACATGGTTTACTCCA-3′ and
reverse, 5′-AGAGAGATTGGCCAGCAAAA-3′; GAPDH (internal control),
forward, 5′-ACGGATTTGGTCGTATTGGG-3′, reverse,
5′-CGCTCCTGGAAGATGGTGAT-3′. The thermal cycling conditions were
95°C for 30 sec, followed by 40 cycles of 95°C for 5 sec and 60°C
for 34 sec.
Western blot analysis
To measure the protein level of EMP1 in tissues, 24
pairs of samples (~200 mg each) were individually packaged in
sterilized tinfoil, frozen in liquid nitrogen for 10 min, and
broken into powder with a pulverizer. The tissue powder was then
homogenized in PBS at 0°C followed by digestion in lysis buffer on
ice for 30 min. After centrifugation at 14,000 × g for 5 min,
protein-containing supernatant was collected and the protein
concentration was measured using the BCA protein assay kit
(Beyotime, Jiangsu, China), according to the manufacturer's
instructions. A total of 80 μg protein from each sample was
separated on SDS-PAGE gel, transferred onto PVDF membrane, blocked
in Tris-HCl and Tween-20 (TBST) buffer containing 5% fat-free milk,
and incubated with either rabbit anti-human EMP1 antibody (1:500;
Abcam, Cambridge, MA, USA) or anti-β-actin antibody (internal
control; Beyotime) at 4°C overnight. After being washed with TBST 3
times (5 min each), the membrane was incubated with a horseradish
peroxidase (HRP)-conjugated goat anti-rabbit-IgG (1:2,000, ZSGB-Bio
Origene, Beijing, China) at 37°C for 90 min. Detection was achieved
using enhanced chemiluminescence (ECL) substrate (Thermo Fisher
Scientific, Waltham, MA, USA) by ECL, and the images were acquired
and quantified by the Gel Imaging system (Chishun, Nanjing,
China).
Transfection
For transient transfection of EMP1 into Hep-2 cells,
the mammalian expression vector pcDNA3.1 expressing no proteins
(control, pcDNA3.1-empty) and that expressing human EMP1
(pcDNA3.1-EMP1; GenScript, Nanjing, China) were transfected into
Hep-2 cells using Lipofectamine 2000 (Invitrogen), according to the
manufacturer's instructions.
MTT assay
Hep-2 cells in log phase were seeded into a 96-well
plate at 5×103/ml, with each condition set up in
quintuplicate. Then cells were transfected with either
pcDNA3.1-empty or pcDNA3.1-EMP1 for 24, 48, 72, 96 or 120 h. Then
MTT (5 mg/ml, 10 μl/well; Sigma, St. Louis, MO, USA) reagent
was added into each well and incubated with cells for a further 4
h, after which, DMSO (150 μl/well) was added to the cells
and the plate was gently shaken for 15 min. Absorbance was measured
at 490 nm.
Colony formation assay
Colony formation assay was performed as previously
described (25). Briefly, at 24 h,
after transfecting Hep-2 cells with either pcDNA3.1-empty or
pcDNA3.1-EMP1, 4,000 cells were seeded into the 6-well tissue
culture plate and cultured at 37°C for two weeks. The colonies were
fixed in 95% ethanol, stained with crystal violet (0.1% w/v), and
counted under a microscope.
Transwell migration assay
For the Transwell migration assay, Hep-2 cells at 24
h after transfection (8×104/ml) were seeded into the
upper chamber of the Transwell insert (pore size 8 μm;
Corning, Lowell, MA, USA). RPMI-1640 medium with 10% FBS was added
into the lower chamber. After 24 h migration at 37°C, the insert
was fixed with 10% methanol and washed 3 times with PBS. The cells
remaining on the upper surface of the insert membrane were gently
removed by wiping with cotton swabs. The insert was stained with
hematoxylin for 40 sec, washed with PBS 3 times, and stained with
eosin for 3 min. After another three washes with PBS, the cells
were re-stained with hematoxylin for 30 sec. Each experimental
condition was set up in quintuplicate. The migrated cells were
imaged under a light microscope and the number of migrated cells
was averaged from five fields (upper, lower, left, right and
center) in each image.
Apoptosis assay
At 24 h and 48 h after transfection of Hep-2 cells
with either pcDNA3.1-empty or pcDNA3.1-EMP1, the cells were
detached with trypsin, washed with PBS 3 times, and stained with 5
μl fluorescein isothiocyanate (FITC)-conjugated Annexin V
and 10 μl propidium iodide (PI) (both from Keygen, Jiangsu,
China) staining solution at room temperature for 15 min. After
staining, the cells were analyzed by a FACScan flow cytometer (BD
Biosciences, Franklin Lakes, NJ, USA).
Statistical analysis
All experiments were independently repeated at least
3 times. Data were presented as mean ± SD. A χ2 test was
used to examine the possible associations between EMP1 expression
and clinicopathological factors. Student's t-test was used to
compare differences between control and treatment cells. SPSS 16.0
for Windows was used for statistical analyses. P<0.05 was
considered statistically significant.
Results
EMP1 is downregulated on both the mRNA
and protein level in laryngeal carcinoma tissues compared to in
peritumor tissues
To assess the status of EMP1 in laryngeal carcinoma,
we first compared its expression on both the steady-state mRNA and
the protein levels in laryngeal carcinoma tissues to that in
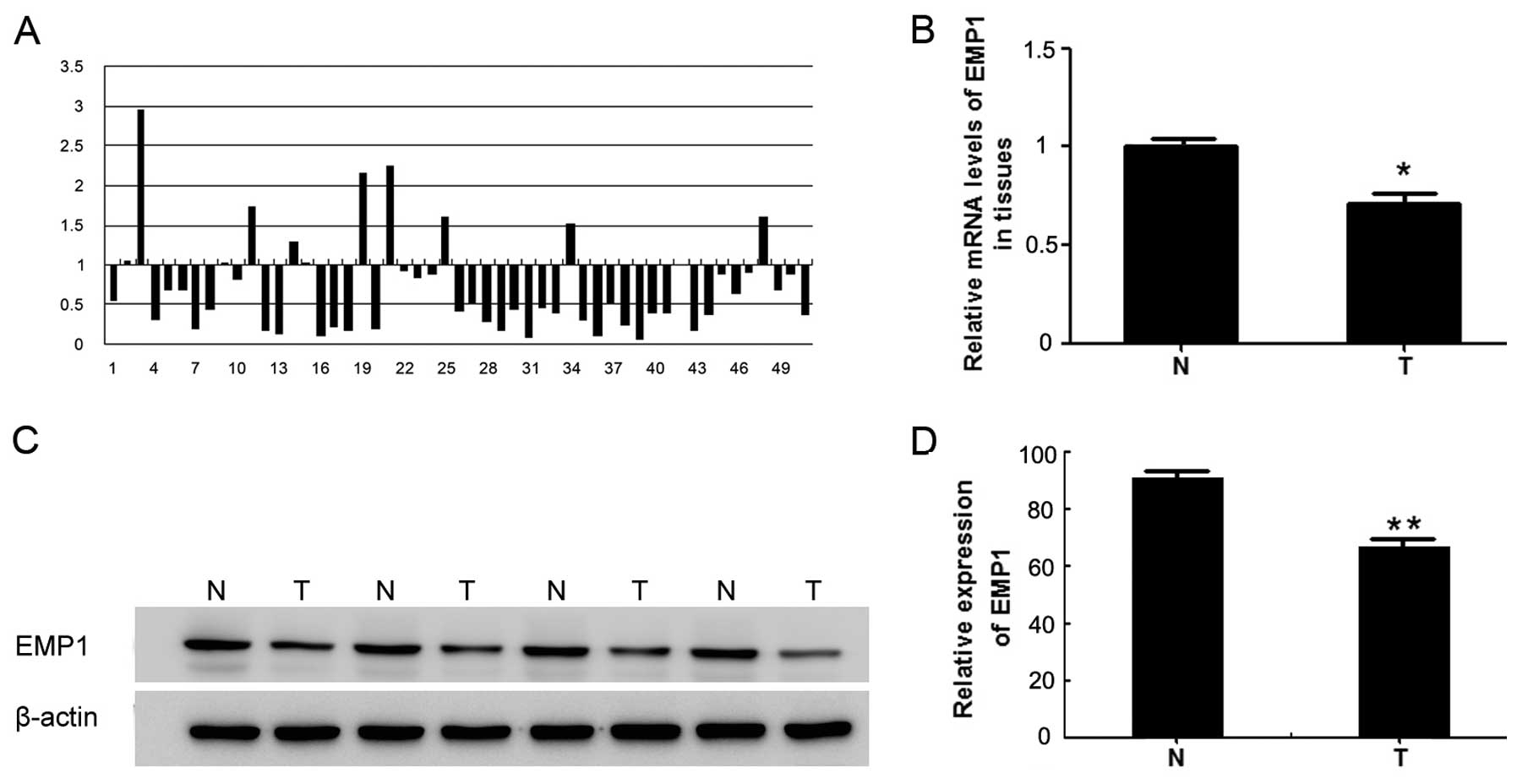
matched cancer-free peritumor tissues. As shown in Fig. 1A, out of the 51 sample pairs
examined, in only 3 pairs was the EMP1 mRNA level more than twice
as high in T than in N tissues; while in 24 pairs, the level was
reduced by >50% in the T sample than in the matched N sample.
The differences between the T and N samples were statistically
significant (P<0.05) (Fig. 1B).
Consistently, the EMP1 protein was significantly lower in the T
samples than in matched N samples (P<0.05) (Fig. 1C and D).
EMP1 expression in laryngeal carcinoma
tissues correlate with histological grading
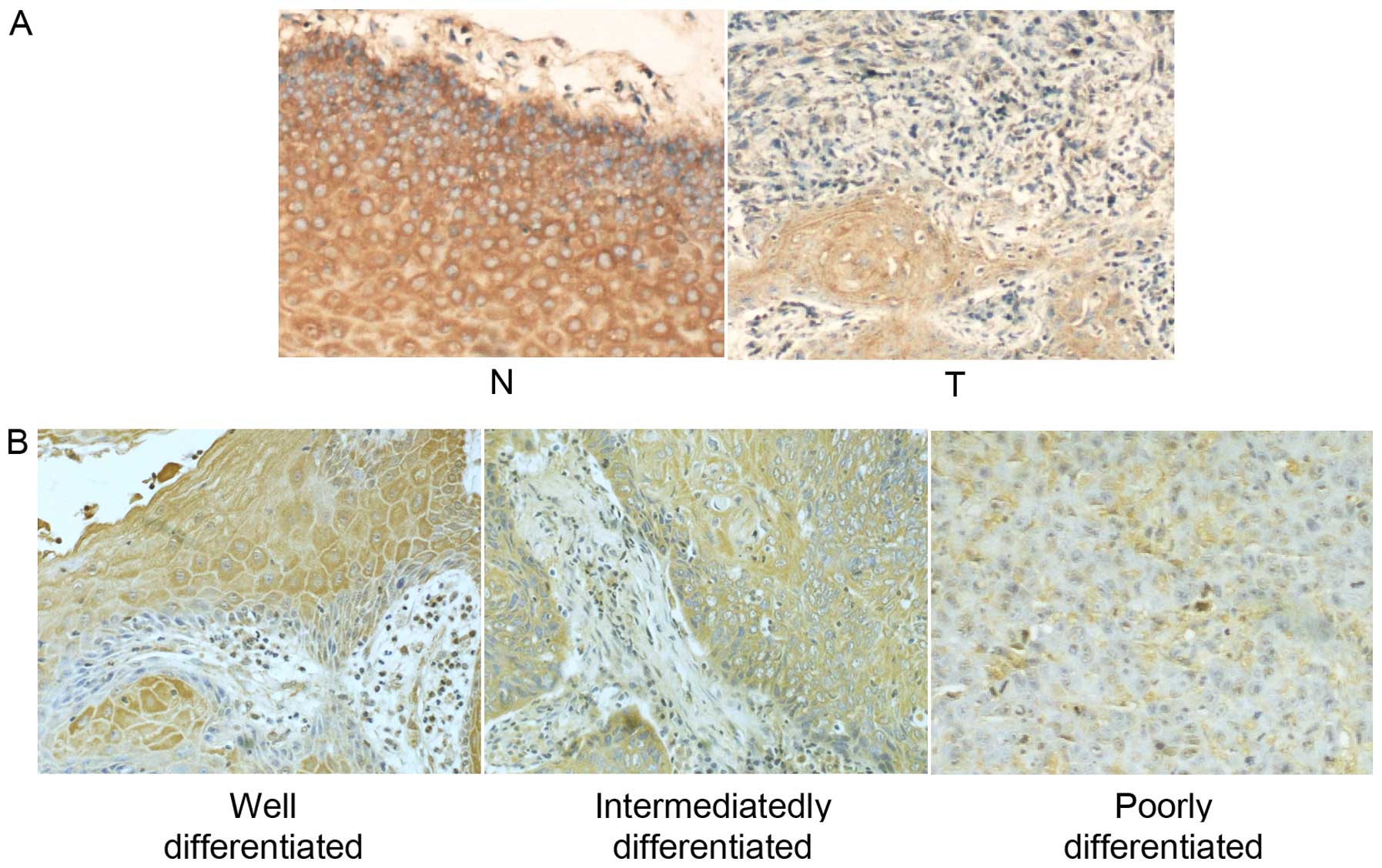
IHC in tissues revealed strong EMP1 expression in
peritumor normal epithelium, predominantly on the cell membrane and
in the cytoplasm (Fig. 2A, left
panel), where the average total staining score (S) was 11.60±1.26.
In contrast, EMP1 staining was markedly reduced in the matched
laryngeal carcinoma tissue (Fig.
2A, right panel), which was associated with a significantly
lower S score (7.55±3.87; P<0.05). In addition, we observed that
staining of EMP1 decreased with the histological grading of the
tumor, with the highest S appearing in well-differentiated tumors,
followed by intermediately differentiated tumors, and the lowest in
poorly differentiated tumors (Fig.
2B). Further correlation analysis confirmed a significant
correlation between the EMP1 S score and pathological grade
(P<0.05) (Table I), but did not
confirm a correlation with other clinicopathological features,
including age, gender, TNM stage or lymph node metastasis
(P>0.05) (Table I).
 | Table ICorrelations between EMP1 total
staining score (S) and different clinicopathological parameters of
patients. |
Table I
Correlations between EMP1 total
staining score (S) and different clinicopathological parameters of
patients.
| Characteristics | No. of patients | EMP1 low
expression | EMP1 high
expression | P-value |
|---|
| Age (years) | | | | |
| <60 | 22 | 9 | 13 | 0.973 |
| ≥60 | 29 | 12 | 17 | |
| Gender | | | | |
| Female | 6 | 4 | 2 | 0.087 |
| Male | 45 | 14 | 31 | |
| TNM stage | | | | |
| I+II | 14 | 7 | 7 | 0.431 |
| III+IV | 37 | 14 | 23 | |
| T stage | | | | |
| 1+2 | 21 | 9 | 12 | 0.227 |
| 3+4 | 30 | 8 | 22 | |
| Lymph node
metastasis | | | | |
| Negative | 36 | 15 | 21 | 0.912 |
| Positive | 15 | 6 | 9 | |
| Pathological
grade | | | | |
| Low | 29 | 16 | 13 | 0.020 |
| High | 22 | 5 | 17 | |
Ectopic EMP1 expression in laryngeal
carcinoma cells reduced cell viability
The significant reduction of EMP1 expression in the
laryngeal carcinoma tissues compared to that in the matching
peritumor laryngeal epithelia and its correlation with the
differentiation status of the tumors suggest the tumor suppressor
activity of EMP1. To characterize the functional significance of
EMP1 in laryngeal carcinoma cells, we applied a gain-of-function
approach and ectopically expressed EMP1 in laryngeal carcinoma
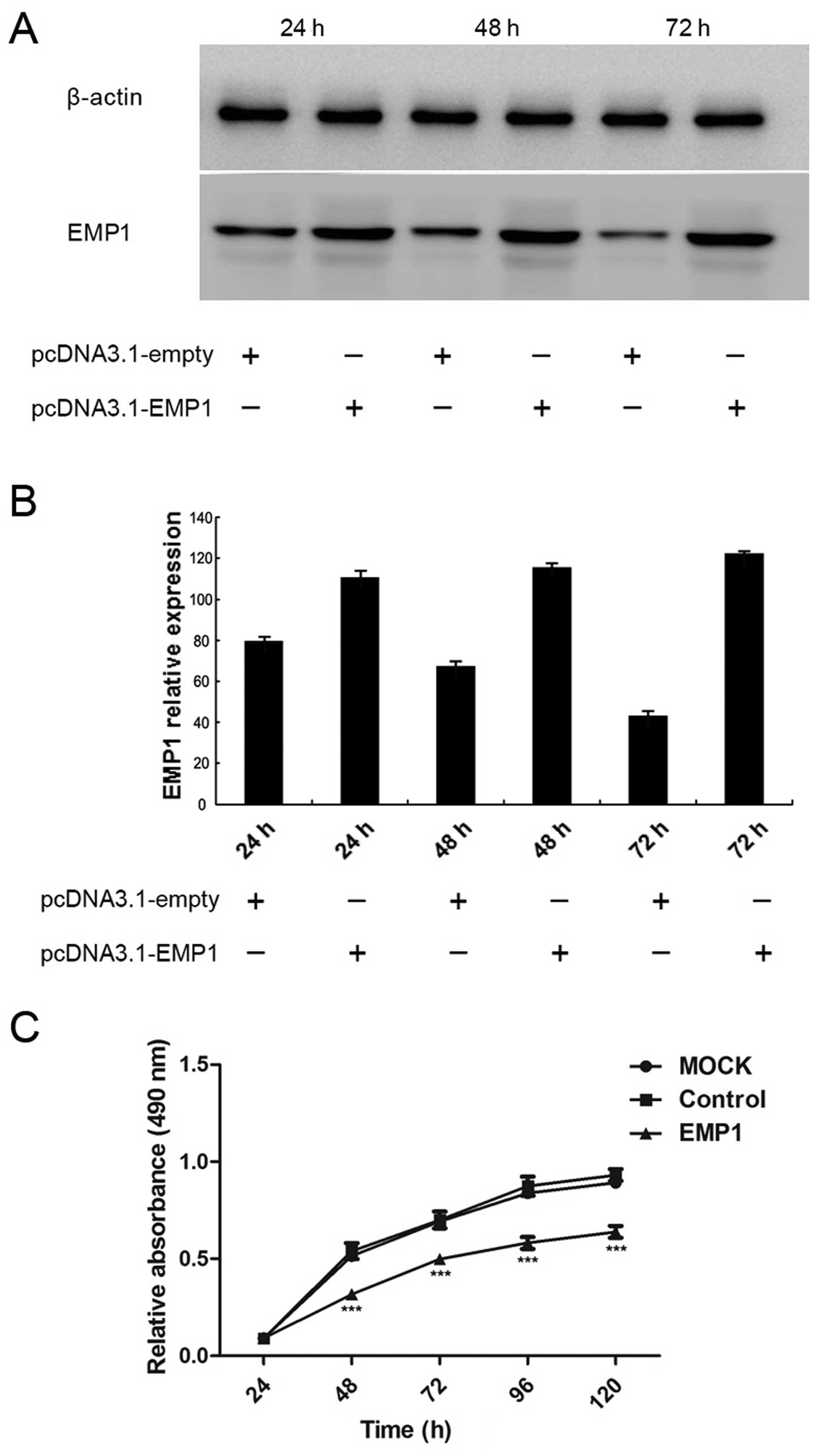
Hep-2 cells. As shown in Fig. 3A,
the transient transfection of Hep-2 cells with pcDNA3.1-EMP1
significantly boosted the expression of EMP1 in the cells, compared
to cells transfected with the control vector pcDNA3.1-empty.
Quantification analysis confirmed significant upregulation of EMP1
expression in pcDNA3.1-EMP1 cells compared to that in
pcDNA3.1-empty cells at 24, 48 and 72 h after transfection
(Fig. 3B).
Upon the overexpression of EMP1 in Hep-2 cells, we
first examined the cell viability by MTT assay. As shown in
Fig. 3C, both the mock-transfected
(MOCK) and pcDNA3.1-empty-transfected (control) cells presented
similar viability over 120 h after the transfection, suggesting
that the control vector did not generate an obviously unfavorable
effect on cell viability. In contrast, starting from 48 h after
transfection, viability in EMP1-expressing cells was significantly
lower than in the other two groups (P<0.05).
EMP1 overexpression promotes the
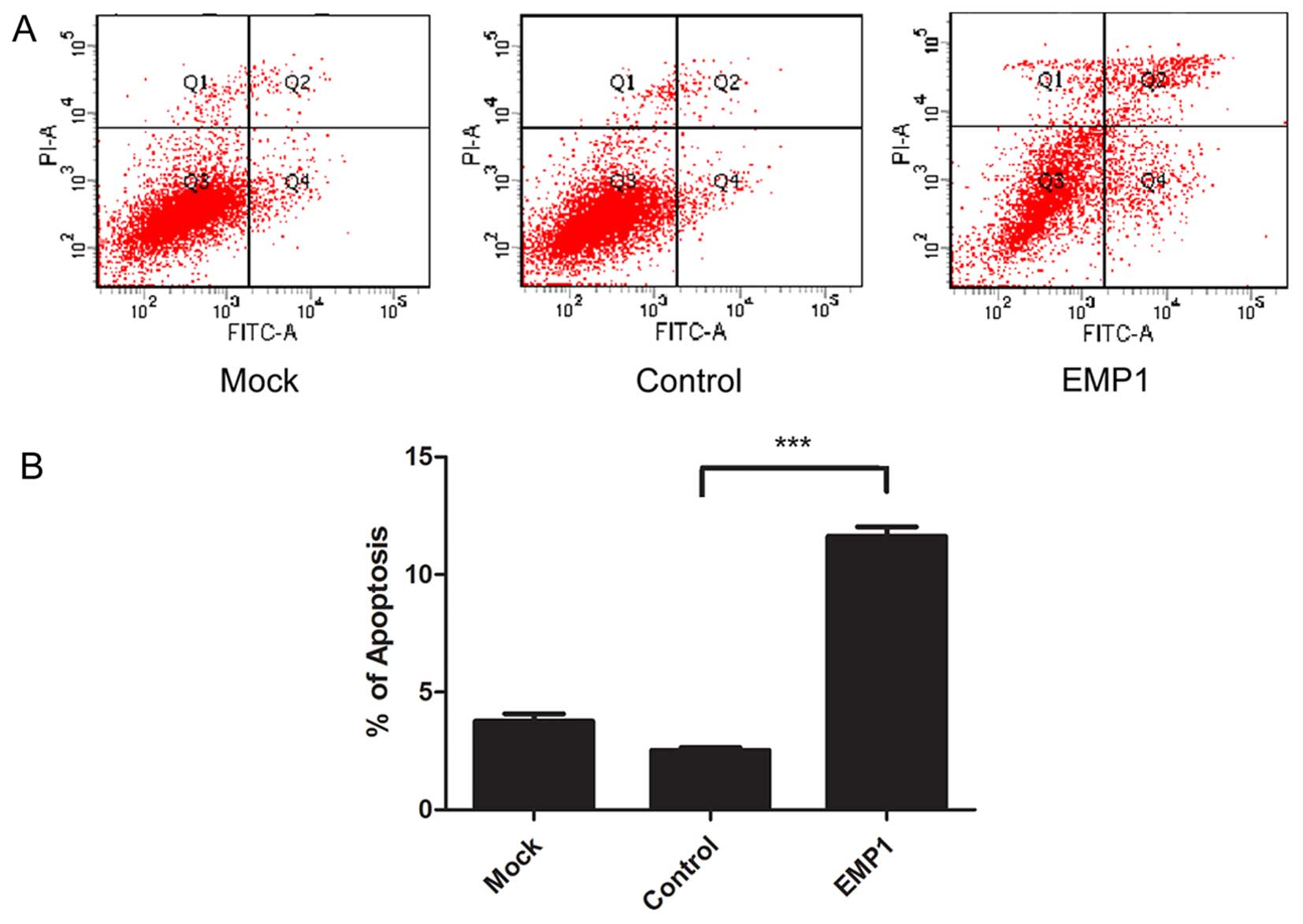
apoptosis of laryngeal carcinoma cells
Cell viability results from a balance between cell
proliferation and apoptosis. The marked decrease in cell viability
following EMP1 ectopic expression prompted us to assess its effect
on cellular apoptosis. By staining cells with Annexin V and PI, we
found that at 48 h after EMP1 overexpression, there was a marked
increase in the percentage of Annexin V+ and
PI+ apoptotic cells (11.63±0.40%), compared to cells
undergoing mock transfection (3.77±0.31%) or transfected with the
control vector (2.53±0.11%) (P<0.05) (Fig. 4).
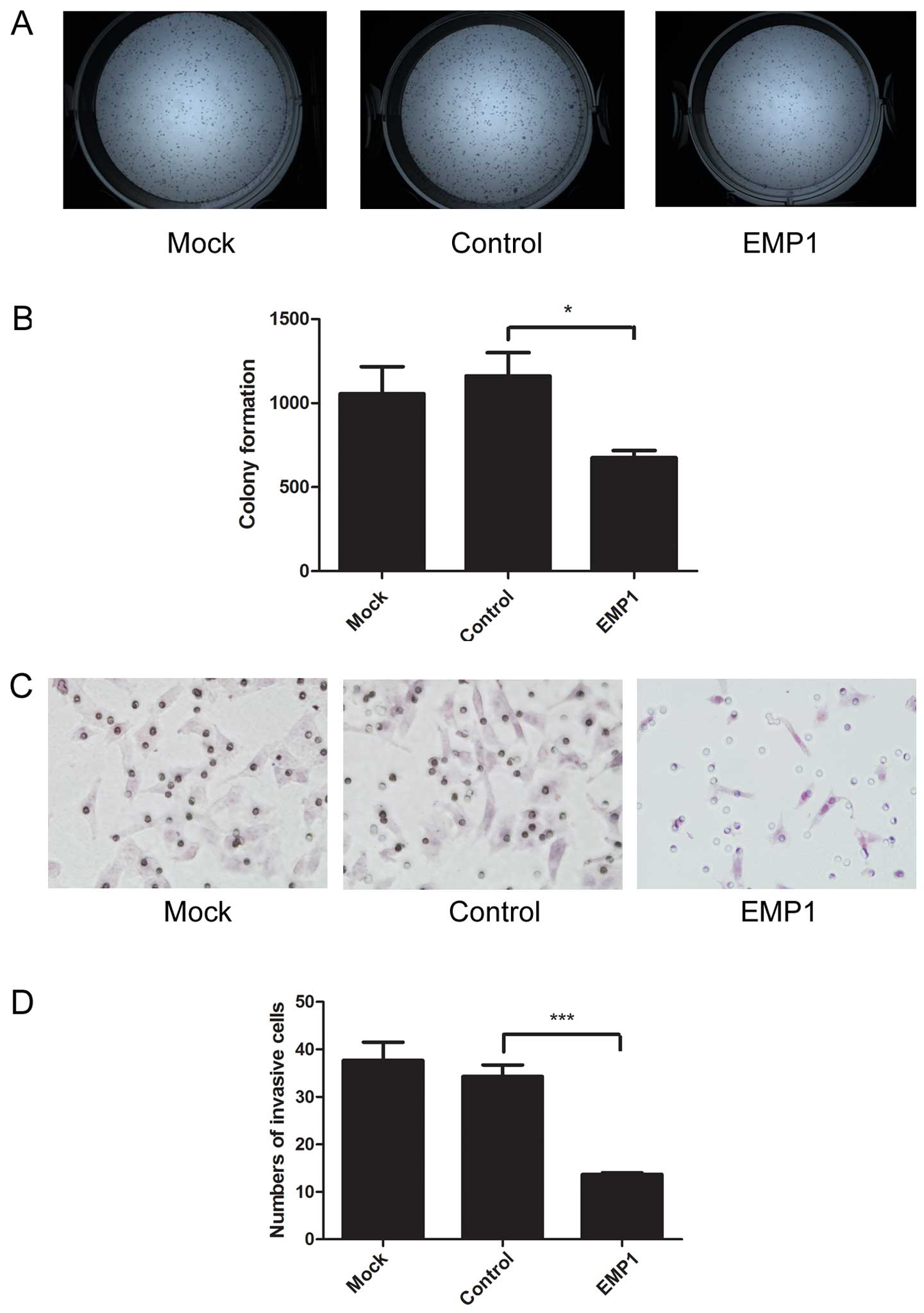
EMP1 overexpression reduces colony
formation and decreases the migratory capacity of laryngeal
carcinoma cells
The capability of tumor cells to generate a large
number of clonogenic progeny (clonogenicity/cancer stemness) is an
important feature of cancer stem cells (26). To measure the effects of EMP1 on
cancer clonogenic capacity, we performed colony formation assay.
EMP1 overexpression in Hep-2 cells significantly reduced the number
of colonies (675.00±43.66) formed, compared to mock-transfected
cells (1055.67±161.55) or cells transfected with the control vector
(1161.67±139.08) (P<0.05) (Fig. 5A
and B).
Migration is another important feature for cancer
cells, closely related to tumor metastasis, the most lethal
phenotype of cancers. Using Transwell migration assay, we revealed
that EMP1 overexpression significantly reduced the number of
migrated cells (13.67±0.33/field), compared to mock-transfected
cells (37.67±3.84/field), or cells transfected with the control
vector (34.33±2.40/field; P<0.05) (Fig. 5C and D).
Discussion
We systematically analyzed the expression of EMP1 on
the mRNA and protein levels from 51 pairs of laryngeal carcinoma
and matched cancer-free peritumor tissues. We corroborated previous
findings that EMP1 is downregulated in laryngeal carcinomas
(5), and its level was
significantly correlated with the differentiation status of the
tumor: the worse the differentiation, the lower EMP1 expression.
Furthermore, we explored the biological activities of EMP1 in
laryngeal carcinoma Hep-2 cells. We showed, for the first time,
that ectopic expression EMP1 in Hep-2 cells significantly reduced
cell viability, which was associated with an increase in cellular
apoptosis. In addition, EMP1 inhibited the colonogenic capacity and
migration of Hep-2 cells. This study supports the notion that EMP1
is a tumor suppressor in laryngeal carcinoma. Upregulating EMP1
initiates multiple anticancer effects and thus provides a promising
therapeutic strategy for laryngeal carcinoma.
In an early study exploring differentially expressed
genes between laryngeal carcinoma cells and matched primary normal
epithelial cells, Liu et al identified 35 significantly
altered genes, and EMP1 was among those downregulated in the cancer
cells, presenting a 20-fold difference between normal and cancer
cells (5). Consistently, EMP1 is
downregulated in other head and neck tumors (4,27)
all supporting tumor suppressive activity of EMP1. However, no
study has systemically looked into the clinical significance of
EMP1 and its biological activities in human laryngeal carcinoma. In
this study, we examined 51 paired samples of laryngeal carcinomas
and matched cancer-free peritumor laryngeal tissues. On the mRNA
level, we showed that 24 pairs of samples presented a >50%
reduction of EMP1 mRNA in the tumor tissues compared to that in the
peritumor tissues; yet three pairs showed a >2-fold increase in
EMP1 mRNA in the former over in the latter. The difference in EMP1
expression on the mRNA level also translates to the protein level,
an ~50% reduction in the average protein level of EMP1 from the
tumor tissues than from the peritumor tissues. In contrast to the
~2-fold difference for EMP1 expression from the present study, Liu
et al reported >20-fold difference in EMP1 mRNA between
normal and cancerous laryngeal cells (5). We noted that the study of Liu et
al focused on EMP1 levels in epithelial cells, while we used
the cancer and peritumor tissues, which contained other cell types
in addition to epithelial cells. The presence of non-epithelial
components in our source materials may dilute EMP1 signals from
epithelial cells, which also suggests that EMP1 is expressed in
non-epithelial components in laryngeal carcinoma. When it comes to
the clinical significance of EMP1 in laryngeal carcinoma, Liu et
al (5) failed to show a
correlation between EMP1 expression and other clinical factors,
while Zhang et al showed that in oral squamous cell
carcinoma, low EMP1 expression correlated with lymphatic metastasis
(7). Our analysis showed that the
EMP1 level, as determined by IHC, only correlates with the
differentiation status of laryngeal carcinoma, but does not
correlate with the other clinicopathological parameters examined,
including age, gender, clinical stages, cancer subtypes or lymph
node metastasis. Although further studies involving a larger number
of clinical samples should be performed on the correlation of EMP1
expression with other clinicopathological factors, including
patient survival, available data suggest that EMP1 may not be a
strong clinical indicator for laryngeal carcinoma. However, the
findings from this study and the study of Liu et al
(5) prompted us to further explore
the potential biological activities of EMP1 in laryngeal carcinoma
cells.
Before this study, several groups have examined the
biological activities of EMP1 in different types of cancer. In
breast cancer MCF-7 cells, nasopharyngeal cancer CNE2 cells,
colorectal cancer SW-480 cells, gastric cancer SGC-7901 cells, and
prostate cancer PC-3 cells, EMP1 transfection reduced cell
survival, increased cellular apoptosis, and inhibited cell
migration and invasion, which was associated with an increased
expression of caspase-9 and a decreased expression of vascular
endothelial growth factor C (20,21,23,24,28).
Consistent with these anticancer biological activities, the
expression of EMP1 was downregulated in these cancers, compared to
normal tissues. In contrast, Lai et al showed that EMP1 was
upregulated in non-small cell lung cancers compared to in benign
lung diseases, and the overexpression of EMP1 in the lung cancer
PC9 cells stimulated xenograft tumor growth in nude mice, which was
associated with the activation of PI3K/AKT pathway (16). These findings suggest that EMP1 may
function either as a tumor suppressor or as an oncoprotein in
different human cancers. Given the relative low level of EMP1
expression in laryngeal carcinoma cells, we applied a
gain-of-function approach, overexpressing EMP1, to study its
effects. EMP1 expression was significantly upregulated in Hep-2
cells through transient transfection. Then we examined the
biological behavior of the cancer cells, including cell viability,
apoptosis, clonogenicity and migration, all closely associated with
the development of laryngeal carcinoma (29). Our findings, that EMP1 reduced cell
viability, increased cellular apoptosis, inhibited colonogenicity,
and decreased cell migration, support tumor suppressor activities
of EMP1 in laryngeal carcinoma.
Several mechanisms have been reported for the
control by EMP1 of cell viability, a phenotype resulting from the
balance of cell proliferation and apoptosis. For cell
proliferation, when overexpressed in esophageal cancer EC9706
cells, EMP1 resulted in cell cycle arrest at S phase (30). In several leukemia cell lines,
however, silencing EMP1 leads to G1 arrest (17). Apoptosis regulated by EMP1 has been
mainly linked to the mitochondrial pathway. Li et al
(31) transfected HEK293 cells
with EMP-1 overexpression vectors and observed a higher activity of
caspase-3 and caspase-9. Furthermore, they increased mitochondrial
membrane potential and found no obvious difference in caspase-8
activity. It is important for future studies to explore the
downstream mechanisms mediating EMP1 regulation on cell viability
and apoptosis in laryngeal carcinoma.
Cancer is composed of a heterogeneous population of
cells, of which small parts present the capability of self-renewal
and differentiation, mimicking normal stem cells, and thus are
termed cancer stem cells. Colony formation assay is a method to
measure the 'stemness' of the cancer cells (25). Cancer stem cells play critical and
significant roles in the initiation, progression, metastasis,
recurrence and drug resistance of cancer. Therefore, targeting
these cells provides a promising strategy for cancer therapy
(32). In this study, we showed
for the first time that overexpressing EMP1 in laryngeal carcinoma
cells reduced colony formation, suggesting that EMP1 may also
regulate cancer stem cells in laryngeal carcinoma, which should be
further evaluated using more comprehensive assays, both in
vitro and in vivo, for cancer stem cells (33).
Migration is critical for local invasion as well as
the distant metastasis of cancer cells. Consistent with multiple
studies showing the effects of EMP1 in modulating cancer cell
migration and/or invasion (20,21,23,24,28)
we showed that EMP1 expression in laryngeal carcinoma cells
inhibited cell migration.
In conclusion, previous studies showed that EMP1 may
function either as a tumor suppressor or an oncoprotein, depending
on the type of cancer. Here we reported tumor suppressor roles of
EMP1 in laryngeal carcinoma. From 51 laryngeal carcinoma tissues,
EMP1 does not seem to be a strong indicator for the progression or
metastasis of laryngeal cancer, which may be limited by the small
sample size and should be examined in future studies involving a
greater number of samples. However, its pleotropic anticancer
activities, specifically the newly identified effect of suppressing
cancer stemness, together with membrane localization (thus allowing
easy access for small molecules or other treatment reagents),
present EMP1 as an ideal therapeutic target for laryngeal
carcinoma. Although the molecular mechanisms underlying EMP1
activities should be further explored, it is expected that
treatments that can stabilize or accumulate membrane EMP1 would
benefit patients with laryngeal carcinoma.
Acknowledgments
We thank Chao Guan (Department of Genetics, Teaching
and Learning Office, the First Affiliated Hospital of China Medical
University) for his kind help with the biopsy screening and
immunohistochemical experiment. We thank Yang Han and Liang Wang
(Department of Pathology, the First Affiliated Hospital of China
Medical University) for the staining score.
References
|
1
|
Wang W, Lin P, Han C, Cai W, Zhao X and
Sun B: Vasculogenic mimicry contributes to lymph node metastasis of
laryngeal squamous cell carcinoma. J Exp Clin Cancer Res.
29:602010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lin HW and Bhattacharyya N: Staging and
survival analysis for nonsquamous cell carcinomas of the larynx.
Laryngoscope. 118:1003–1013. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Marioni G, Marchese-Ragona R, Cartei G,
Marchese F and Staffieri A: Current opinion in diagnosis and
treatment of laryngeal carcinoma. Cancer Treat Rev. 32:504–515.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Choi P and Chen C: Genetic expression
profiles and biologic pathway alterations in head and neck squamous
cell carcinoma. Cancer. 104:1113–1128. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu YH, Tang PZ, Xu ZG, Qi YF, Ding F,
Zhang LY, Wang HT and Liu ZH: Differential expression of the
epithelial membrane protein 1 of laryngeal carcinoma. Zhongguo Yi
Xue Ke Xue Yuan Xue Bao. 25:47–51. 2003.In Chinese. PubMed/NCBI
|
|
6
|
Zhang T, Wang Q, Zhao D, Cui Y, Cao B, Guo
L and Lu SH: The oncogenetic role of microRNA-31 as a potential
biomarker in oesophageal squamous cell carcinoma. Clin Sci (Lond).
121:437–447. 2011. View Article : Google Scholar
|
|
7
|
Zhang J, Cao W, Xu Q and Chen WT: The
expression of EMP1 is downregulated in oral squamous cell carcinoma
and possibly associated with tumour metastasis. J Clin Pathol.
64:25–29. 2011. View Article : Google Scholar
|
|
8
|
Taylor V, Welcher AA, Program AE and Suter
U: Epithelial membrane protein-1, peripheral myelin protein 22, and
lens membrane protein 20 define a novel gene family. J Biol Chem.
270:28824–28833. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liehr T, Kuhlenbäumer G, Wulf P, Taylor V,
Suter U, Van Broeckhoven C, Lupski JR, Claussen U and Rautenstrauss
B: Regional localization of the human epithelial membrane protein
genes 1, 2, and 3 (EMP1, EMP2, EMP3) to 12p12.3, 16p13.2, and
19q13.3. Genomics. 58:106–108. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bredel M, Bredel C, Juric D, Harsh GR,
Vogel H, Recht LD and Sikic BI: High-resolution genome-wide mapping
of genetic alterations in human glial brain tumors. Cancer Res.
65:4088–4096. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ben-Porath I and Benvenisty N:
Characterization of a tumor-associated gene, a member of a novel
family of genes encoding membrane glycoproteins. Gene. 183:69–75.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee HS, Sherley JL, Chen JJ, Chiu CC,
Chiou LL, Liang JD, Yang PC, Huang GT and Sheu JC: EMP-1 is a
junctional protein in a liver stem cell line and in the liver.
Biochem Biophys Res Commun. 334:996–1003. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Taylor V and Suter U: Epithelial membrane
protein-2 and epithelial membrane protein-3: Two novel members of
the peripheral myelin protein 22 gene family. Gene. 175:115–120.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jetten AM and Suter U: The peripheral
myelin protein 22 and epithelial membrane protein family. Prog
Nucleic Acid Res Mol Biol. 64:97–129. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Arslan AA, Gold LI, Mittal K, Suen TC,
Belitskaya-Levy I, Tang MS and Toniolo P: Gene expression studies
provide clues to the pathogenesis of uterine leiomyoma: New
evidence and a systematic review. Hum Reprod. 20:852–863. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lai S, Wang G, Cao X, Li Z, Hu J and Wang
J: EMP-1 promotes tumorigenesis of NSCLC through PI3K/AKT pathway.
J Huazhong Univ Sci Technolog Med Sci. 32:834–838. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ariës IM, Jerchel IS, van den Dungen RE,
van den Berk LC, Boer JM, Horstmann MA, Escherich G, Pieters R and
den Boer ML: EMP1, a novel poor prognostic factor in pediatric
leukemia regulates prednisolone resistance, cell proliferation,
migration and adhesion. Leukemia. 28:1828–1837. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang HT, Lu YC and He J: Expression of
epithelial membrane protein 1 in human gliomas and its clinical
implications. Chin J Cancer Biother. 14:466–470. 2007.
|
|
19
|
Wang HT, Kong JP, Ding F, Wang XQ, Wang
MR, Liu LX, Wu M and Liu ZH: Analysis of gene expression profile
induced by EMP-1 in esophageal cancer cells using cDNA microarray.
World J Gastroenterol. 9:392–398. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun G, Zhao G, Lu Y, Wang Y and Yang C:
Association of EMP1 with gastric carcinoma invasion, survival and
prognosis. Int J Oncol. 45:1091–1098. 2014.PubMed/NCBI
|
|
21
|
Sun GG, Wang YD, Cui DW, Cheng YJ and Hu
WN: EMP1 regulates caspase-9 and VEGFC expression and suppresses
prostate cancer cell proliferation and invasion. Tumour Biol.
35:3455–3462. 2014. View Article : Google Scholar
|
|
22
|
Cheong SC, Chandramouli GV, Saleh A, Zain
RB, Lau SH, Sivakumaren S, Pathmanathan R, Prime SS, Teo SH, Patel
V, et al: Gene expression in human oral squamous cell carcinoma is
influenced by risk factor exposure. Oral Oncol. 45:712–719. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sun GG, Wang YD, Lu YF and Hu WN: EMP1, a
member of a new family of antiproliferative genes in breast
carcinoma. Tumour Biol. 35:3347–3354. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sun GG, Lu YF, Fu ZZ, Cheng YJ and Hu WN:
EMP1 inhibits nasopharyngeal cancer cell growth and metastasis
through induction apoptosis and angiogenesis. Tumour Biol.
35:3185–3193. 2014. View Article : Google Scholar
|
|
25
|
Franken NA, Rodermond HM, Stap J, Haveman
J and van Bree C: Clonogenic assay of cells in vitro. Nat Protoc.
1:2315–2319. 2006. View Article : Google Scholar
|
|
26
|
Baccelli I and Trumpp A: The evolving
concept of cancer and metastasis stem cells. J Cell Biol.
198:281–293. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kuriakose MA, Chen WT, He ZM, Sikora AG,
Zhang P, Zhang ZY, Qiu WL, Hsu DF, McMunn-Coffran C, Brown SM, et
al: Selection and validation of differentially expressed genes in
head and neck cancer. Cell Mol Life Sci. 61:1372–1383. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sun GG, Wang YD, Cui DW, Cheng YJ and Hu
WN: Epithelial membrane protein 1 negatively regulates cell growth
and metastasis in colorectal carcinoma. World J Gastroenterol.
20:4001–4010. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim MM and Califano JA: Molecular
pathology of head-and-neck cancer. Int J Cancer. 112:545–553. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang HT, Liu ZH, Wang XQ and Wu M: Effect
of EMP-1 gene on human esophageal cancer cell line. Ai Zheng.
21:229–232. 2002.PubMed/NCBI
|
|
31
|
Li ZY, Xiong SH and Hu M and Hu M:
Mitochondrial pathway is involved in the EMP1-induced apoptosis.
Chin J Anat. 31:489–514. 2008.
|
|
32
|
Chen K, Huang YH and Chen JL:
Understanding and targeting cancer stem cells: Therapeutic
implications and challenges. Acta Pharmacol Sin. 34:732–740. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Stingl J: Detection and analysis of
mammary gland stem cells. J Pathol. 217:229–241. 2009. View Article : Google Scholar
|