Introduction
Excessive chronic alcohol intake is a widely
acknowledged risk factor for breast cancer. In a previous study, we
summarized the epidemiology and known pathology pertaining to
alcohol consumption and breast cancer (1). Briefly, consumption of three or more
drinks per day leads to a 40–50% increase in risk and there are
~50,000 alcohol-attributable breast cancer cases per year,
worldwide (2–4). The risk of breast cancer increases
with the quantity of alcohol consumed, showing a linear
dose-response (5). There is a
greater risk for lobular rather than ductal breast cancer (5–7), and
tumors are more likely to be ER− and HER2+ in
the women with high alcohol consumption (8,9).
One proposed mechanism for the putative alcohol
carcinogenicity involves stimulation of estrogen levels and/or
estrogen responsiveness but other possibilities include effects
unrelated to estrogen (2,3,9–13)
such as inhibition of DNA methylation, interaction with retinoid
metabolism, or oxidative stress. Such changes could operate either
by direct ethanol effects and/or through the first ethanol
metabolite, acetaldehyde.
As summarized previously (1), a few studies in mice and rats have
shown that ethanol consumption promotes mammary tumors via the
estrogen pathway (14). The
estrogen dependence was originally shown and partially explained in
the widely used MCF-7 cell line, a human breast cancer luminal
epithelial cell line which is estrogen and progesterone receptor
positive and lacks ERBB2 gene amplification or Her2/neu protein
overexpression (15–18). It was also shown that ethanol
stimulates the in vitro growth, invasiveness and migration
of these cells (17–24). However, the common denominator of
the previous studies on MCF-7 cells is that the ethanol exposure
was limited to <1 week, concentrations were >50 mM, and the
effects were modest. A similar situation occurred with studies
conducted on other types of more malignant breast cancer cell
lines, such as T47D and erbB2 transformed cells (25–30).
Another potential mechanism of ethanol's
carcinogenicity is through enrichment of a subpopulation of cancer
stem cells, but there are no reports on the effects of ethanol on
this type of stem cells (31–33).
Cancer stem cells are postulated to be involved in the generation
of primary breast tumors and their progression to undifferentiated
tumors and metastasis, and are claimed to be enriched within
mammospheres (34,35). Although ethanol affects the
proliferation and differentiation of normal embryonic and adult
stem cells (36,37), it is not known whether it activates
and/or increases the number of cancer stem cells. The latter
process, as well as the regulation of breast cancer genes in
general, is partially regulated by microRNAs (miR) (34,38–41),
particularly with regard to the epithelial mesenchymal transition
(EMT) (42,43). Ethanol affects the expression of
certain miRs in alcoholic liver injury and other pathologies
(44,45), but no reports link this to breast
cancer. In contrast, there is a substantial recent literature on
miRs in relation to estrogen effects, particularly in MCF-7 cells
(46–48), but none has been directly linked to
ethanol exposure.
In our previous study on the non-malignant
epithelial human breast cell line MCF-12A (1) we found that ethanol, but not
acetaldehyde, induced oncogenic features and EMT, and stimulated
the expression of a collection of mRNAs and miRs, including those
associated with these processes, and also stimulated certain
protein markers for stem-related properties.
In this study, the effects of short- and long-term
exposures to physiologically relevant concentrations of ethanol,
and acetaldehyde up to supraphysiological levels were studied using
MCF-7 monolayers and mammospheres. Stem cell markers, global
transcriptional gene expression signatures including miRs, and
in vitro responses in oncogenic assays were carried out to
better understand the mechanism of action of alcohol on malignant
progression in breast cancer. The aim was to clarify: a) whether
the epidemiological relationship between excessive and long-term
alcohol consumption and the malignant progression of breast cancer
can be elucidated by defining the effects of ethanol on an accepted
epithelial breast cancer cell line such as MCF-7 in vitro;
b) whether ethanol intensifies some of the MCF-7 malignant features
in a dosage- and/or duration of exposure-dependent way; c) the
potential mediation of these effects by stem cell enrichment in
both monolayers and mammospheres; d) the possible role of
acetaldehyde in mediating those changes; and e) the impact of both
alcohol and acetaldehyde on the mRNA and miR global signatures, so
as to define the more affected pathways and to evaluate putative
estrogen mediation.
Materials and methods
Cell culture
The human adherent epithelial adenocarcinoma MCF-7
cell line was obtained from ATCC (Catalog HT-22™ Manassas, VA,
USA), and routinely cultured on monolayers at ≤80% confluence in
MEME (minimum essential medium Eagle's, ATCC), 10% FBS and 0.01
mg/ml bovine insulin (Sigma, St. Louis, MO, USA). For most
experiments, cells were incubated on 6-well plates with 0-25 mM
ethanol (Fisher, molecular grade ethanol) or 0–12.5 mM acetaldehyde
(Sigma, ACS), using freshly prepared solutions. Medium was replaced
2–3 times/week, including addition of ethanol or acetaldehyde, and
cultures were maintained for 1 week (short-term incubations), or
for 4 weeks (long-term incubations). In the latter case, cells were
passaged on average once a week, with a 1:3 splitting.
Mammospheres were generated for 1 week experiments
by seeding 50,000 cells onto Corning Ultra Low Attachment
6-well-plates with 2 ml/well of MEBM medium (Fisher, mammary
epithelial cell growth medium), adding 2% (v/v) of B27 Supplement
(B27 serum-free supplement, Invitrogen, Carlsbad, CA, USA) and 0.01
mg/ml bovine insulin, and then adding ethanol or acetaldehyde. For
4-week incubations, monolayers were cultured for this period with
ethanol or acetaldehyde and used for mammosphere generation that
were maintained in the presence of these agents for an additional
week. Their number and total area were determined by applying
quantitative image analysis (QIA) to digital photographs taken with
a Nikon digital camera of 0.005% crystal violet stained
mammospheres contained in individual wells of a 6-well plate, using
ImagePro-Plus 5.1 software (Media Cybernetics, Silver Spring, MD,
USA). After images were calibrated for background lighting,
integrated optical density (IOD = area × average intensity) was
calculated. Inverted microscopy images were taken under phase
contrast at 40× and 100× using a Nikon Eclipse Ti microscope and a
Leica VCC digital camera.
Western blot analyses
Medium was decanted from wells and cells were washed
twice with PBS at pH 7.4. Boiling buffer (1% SDS, 1 mM sodium
orthovanadate, 10 mM Tris pH 7.4 and protease inhibitors) was added
to each well, cells were scraped from each well and passed several
times through a 26-gauge needle to reduce viscosity, incubated in a
boiling water bath for 5 min, and centrifuged at 16,000 g for 5
min, then 20–40 μg of protein was run on 4–15% gradient
polyacrylamide gels, transferred electrophoretically to
nitrocellulose, and analyzed by immunodetection using antibodies
against: i) Oct-4, (rabbit polyclonal, 1:500, BioVision, Mountain
View, CA, USA); ii) CEACAM-6 (rabbit polyclonal, 1:500, Novus
Biologicals, Littleton, CO, USA); iii) NANOG (rabbit polyclonal,
1:1,000, AVIVA Systems, San Diego, CA, USA) and iv) GAPDH (mouse
monoclonal, 1:3,000, Chemicon). Membranes were incubated with
secondary polyclonal horse anti-mouse or anti-rabbit IgG linked to
horseradish peroxidase (1:2,000; BD Transduction Laboratories,
Franklin Lakes, NJ, USA or 1:5,000; Amersham GE, Pittsburgh, PA,
USA), bands were visualized with luminol (SuperSignal West Pico,
Chemiluminescent, Pierce, Rockford, IL, USA). For the negative
controls the primary antibody was omitted.
Immunocytochemistry
Cultures were grown in 8-well-removable-chamber
slides, subjected to immunofluorescence detection by quenching in
0.3% H2O2, blocking with goat (or
corresponding) serum, and incubated overnight at 4°C with the
primary antibody for Oct-4 or NANOG. This was followed by a
secondary anti-mouse IgG biotinylated antibody (goat, 1:200, Vector
Laboratories) and this complex was detected with streptavidin-Texas
Red. After washing with PBS, the sections were mounted with Prolong
antifade/DAPI (Molecular Probes, Carlsbad, CA, USA). Negative
controls in all cases omitted the first antibodies or were replaced
by IgG isotype.
Flow cytometry
Control and 25 mM ethanol-incubated MCF-7 cells were
grown in GM-20, washed twice with Hanks buffered salt solution,
disaggregated by repeated pipeting in CellStripper (Mediatech,
Manassas VA, USA), pelleted, and resuspended in staining buffer
consisting of PBS, 3% FBS (SB). Cells were incubated in the
presence of antibodies for 30 min on ice, washed twice with SB, and
resuspended in SB for flow cytometry on an LSR II (BD Biosciences).
Controls included samples without any antibody as well as samples
including all combinations of antibodies so as to determine that
the Ceacam6 stained cells and the CD44 stained cells were
accurately identified. Data analysis and plotting were done using
FACSDiva Version 6.1.1 software. Fluorophore-conjugated antibodies
and G12-5841 PE (eBiosciences), performed separately, followed cell
permeabilization with BD CytoFix/CytoPerm kit. BD CompBeads were
used for compensation.
Global DNA microarray transcriptional
profile
RNA was isolated from cells using the RNeasy Plus
Micro kit (Qiagen) with quality determined using the Agilent 2100
Bioanalyzer. Assays were performed by the UCLA DNA microarray core,
applying the Affymetrix Human Gene 1.0 ST array for >30,000
genes. Up- and downregulated genes (by >2-fold) were considered,
except where indicated. DNA microarray results are deposited in the
GEO library under accession no. GSE72013.
RT/PCR
The expression of some of the down- and upregulated
genes identified by DNA microarray analysis was further examined on
triplicate RNA samples. cDNA was synthesized by reverse
transcription using the Superscript III First-Strand Synthesis
SuperMix for qRT-PCr (Life Technologies), and the resulting samples
were amplified using PCR. Primers were designed using the NCBI
Primer Blast program applied to mRNA sequences and synthesized by
Sigma-Aldrich. All primers were designed to include an exon-exon
junction except for GAPDH. Negative controls omitted cDNA. PCR
results were analyzed by electrophoresis through 1% agarose gels in
Tris-acetate EDTA buffer followed by photography under ultraviolet
illumination in a UVP Biodoc transilluminator.
Global miR profiles
RNA was isolated from cells using the mirVana™ miRNA
isolation kit (Ambion), and analysis was carried out by LC Sciences
(Houston, TX, USA) for all miR transcripts listed in the Sanger
miRBase Release 18.0. The miR results are deposited in the GEO
library under accession no. GSE72013.
Anchorage-independence
Cells were trypsinized, suspended in 1 ml/well of
warm (37°C) 0.3–0.5% agar in MEME-10% FBS-bovine serum albumin
(soft agar layer) and 10,000 cells/ml were plated in duplicate or
triplicate above a layer of 1 ml of 1% agar in the same medium that
had previously been allowed to solidify on 6-well plates at 4°C
(hard layer agar). Cultures were allowed to grow for 3–4 weeks and
when foci were visible, they were stained with 0.005% crystal
violet in Hanks solution for 1 h, wells were photographed as
described in 'Cell culture', and colonies were counted, also as
described above.
Cell invasiveness
Matrigel™ basement membrane matrix was diluted 1:5
with serum free culture medium containing 0.5% BSA according to the
manufacturer's instructions. Trypsinized cells were added at 25,000
cells/well to Transwell® Permeable Support 8.0-μm
inserts containing Matrigel and culture medium, and cultured in a
24-well plate using FBS as the chemoattractant for 40 h. Cells were
fixed and stained with 0.5% toluidine blue.
Response to tamoxifen
MCF-7 cells (control vs 4 weeks 25 mM ethanol) were
incubated in estrogen-depletion medium (EDM): phenol red-free
DMEM/F12 (Invitrogen) containing 5% charcoal stripped fetal bovine
serum (Gibco) for 3 days, washed with Hanks, trypsinized,
resuspended in EDM and plated at 1,000 cells/well into a 96-well
plate. 4-OH-tamoxifen was added to a concentration of 0, 1, 2, 5 or
10 μM (6-wells for each concentration). Cells were incubated
at 37°C in 5% CO2 for 4 days, washed once with Hanks,
followed by the addition of 100 μl of EDM. After 30-min
additional incubation, each well received 20 μl of the
metabolic activity indicator CellTiter 96® AQueous One
Solution Cell Proliferation assay (Promega, Madison, WI, USA), and
cells were further incubated (37°C in 5% CO2) for 30
min. The absorbance at 490 nm was measured using an automated plate
reader.
Statistical analyses
Statistical values are expressed as the mean (±
SEM). The normality distribution of the data was established using
the Wilk-Shapiro test. Multiple comparisons were analyzed by a
single factor ANOVA, followed by post hoc comparisons with the
Newman-Keuls test. Differences among groups were considered
statistically significant at p<0.05.
Results
Short-term exposure of MCF-7 monolayers
to low concentrations of ethanol
To investigate the possibility that ethanol exerts
its oncogenic effects through the stimulation of cancer stem cell
proliferation, we carried out experiments to ascertain the effects
on the key stem cell marker proteins Oct4 and Nanog. MCF-7
monolayer cultures were incubated at 30–80% confluence for 7 days
with 1–25 mM ethanol and subjected to western blot analysis for
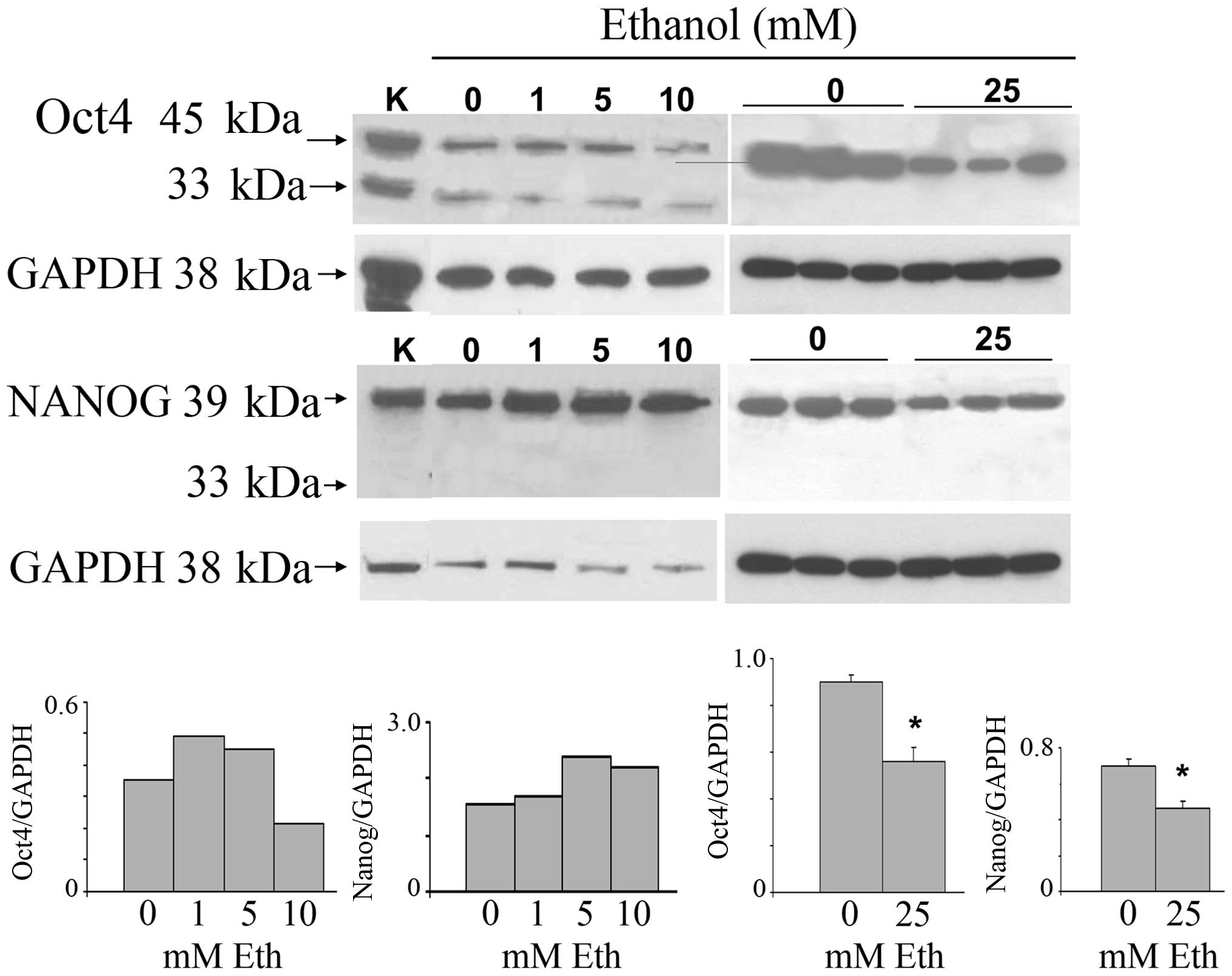
Oct4 and Nanog (Fig. 1). The
highest concentration of ethanol is roughly equivalent to peak
serum levels of alcohol in women within 1 h of consumption of 3–4
glasses of wine. The stemness-related nuclear Oct4a isoform (45
kDa) was increased after exposure to 1 and 5 mM ethanol, and was
reduced at 10 and 25 mM. Triplicate samples of the 25 mM treatment
show downregulation. The cytoplasmic Oct4b (33 kDa), unrelated to
stemness, was expressed at low levels and remained unchanged. The
main 39 kDa Nanog isoform was increased after exposure to 1–10 mM
ethanol, and analogous to the Oct4 result, was also decreased at 25
mM. The nuclear localization of Oct4a, consistent with its known
stemness function, and, in addition, the nuclear localization of
Nanog, were confirmed by double immunofluorescence with Texas red
for the specific antigen and DAPI for nuclei in MCF-7 cells
incubated in the absence of ethanol (data not shown).
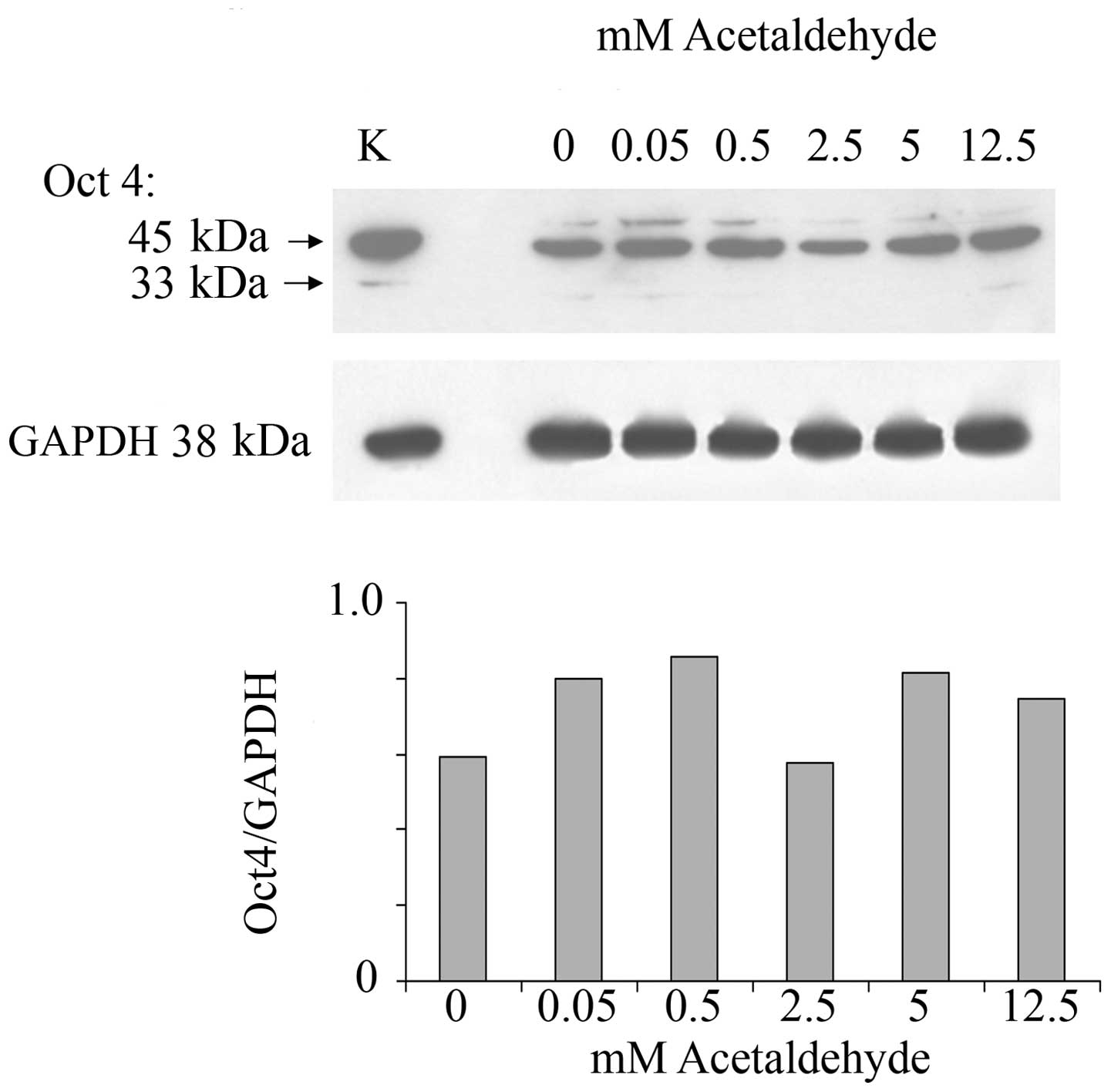
In order to investigate whether ethanol effects are
mediated by its first oxidation product, acetaldehyde, a range of
acetaldehyde concentrations was applied to MCF-7 monolayers and the
effect on Oct4 expression was analyzed. Acetaldehyde over the range
of 0.5–12.5 mM and over the same time of incubation did not affect
the expression of Oct4 (Fig. 2).
These acetaldehyde concentrations, considerably lower than the
respective ethanol concentrations, were chosen based on the fact
that they are marginally to considerably higher (i.e.,
approximately one log value) than the acetaldehyde values that
would be expected in human serum after substantial drinking. These
levels have been measured to be as much as 0.2 mM (49). Even at these levels acetaldehyde
had little or no effect on Oct4 levels.
Ethanol exposure affects gene expression
both in the short-term and long-term
Gene expression analysis of MCF-7 cells grown in
monolayer in the presence or absence of ethanol (25 mM) for 1 and 4
weeks was carried out by subjecting RNA samples to DNA microarray
analysis using the Affymetrix Human Gene 1.0 ST system. The longer
exposure was intended to better model the situation that exists
in vivo in chronic drinkers. In order to determine the
global transcriptional signature that differentiates the malignant
MCF-7 cells from a normal counterpart, we compared the MCF-7 cell
line with the spontaneously immortalized but otherwise benign
breast epithelial line MCF-12A (1). For each gene sequence, the ratio of
MCF-7 expression to MCF-12A expression was determined from
duplicate samples. We refer to the collection of MCF-7/MCF-12A gene
expression ratios shown in Table I
as the MCF-7 oncogenic signature. Some genes related to oncogenic
processes were substantially changed, being up- or downregulated by
a factor ≥2.0. This transcriptional signature was characterized by
15 genes upregulated by a factor ≥2.4, and 3 oncogenesis-related
genes downregulated to a factor of ≤0.27, including some associated
with oncogenic transformation and some associated with
growth-related hormone receptors.
 | Table IThe non-malignant cell line MCF-12A
is compared to the malignant cell line MCF-7 in an oncogenic
signature (column 3).a |
Table I
The non-malignant cell line MCF-12A
is compared to the malignant cell line MCF-7 in an oncogenic
signature (column 3).a
| Gene | Gene
description | Oncogenic
signature
C/C
MCF-7/MCF-12A | 1 week
| 4 weeks
|
|---|
C
MCF-7 | Eth/C
MCF-7 | C
MCF-7 | Eth/C
MCF-7 |
|---|
| CEACAM5 | CEA related cell
adhesion molecule | 42.20 | 181 | 1.03 | 223 | 3.14 |
| PGR | Progesterone
receptor | 12.80 | 137 | 0.98 | 494 | 1.71 |
| ESR1 | Estrogen receptor
1 | 10.10 | 111 | 0.82 | | 0.88 |
| TET2 | Tet oncogene family
member 2 |
9.87 | 392 | 1.08 | 881 | 1.28 |
| BCAS1 | Breast carcinoma
amplified sequence 1 |
8.69 | 161 | 1.38 | 528 | 1.08 |
| CEACAM6 | CEA related cell
adhesion molecule 6 |
8.03 | 121 | 1.15 | 170 | 2.95 |
| ERBB3 | v-erb-b2 erythro
leukemia homolog 3 |
7.95 | 766 | 1.11 | 816 | 1.14 |
| TACSTD1 | Tumor associated Ca
signal transducer 1 |
7.91 | 2,715 | 1.02 | 3,386 | 1.06 |
| BCAS2 | Breast ca amplified
seq 2 |
7.31 | 3,697 | 1.12 | 4,533 | 0.97 |
| MYB | v-myb oncogene
homolog |
6.40 | 474 | 1.33 | 700 | 1.16 |
| TET1 | Tet oncogene 1 |
5.01 | 116 | 1.29 | 147 | 0.78 |
| TOB1 | Transducer of ERBB2
1 |
3.42 | 4,246 | 1.18 | 3,498 | 1.01 |
| BCAS3 | Breast carcinoma
amplified seq 3 |
3.34 | 1,426 | 1.07 | 1,359 | 1.11 |
| ERBB2 | v-erb-b2 oncogene
homolog 1 |
2.53 | 723 | 1.10 | 437 | 1.17 |
| AR | Androgen
receptor |
2.41 | 248 | 1.26 | 71 | 0.98 |
| MTSS1 | Metastasis
suppressor 1 |
0.27 | 285 | 1.03 | 171 | 1.17 |
| CD44 | CD44 molecule
(Indian blood group) |
0.23 | 591 | 0.99 | 492 | 1.23 |
| CTGF | Connective tissue
growth factor |
0.13 | 205 | 0.76 | 86 | 0.96 |
We then investigated whether an oncogenic signature
reflected the effects of ethanol treatment on MCF-7 by itself.
Short-term ethanol incubation for 1 week had little or no effect on
the expression of this group of 18 genes (Table I), whereas 4-week exposure
increased CEACAM5, CEACAM6, and progesterone receptor (PGR) gene
expression (Table I). Neither
1-nor 4-week exposures to ethanol or acetaldehyde affected the
transcriptional expression of Oct-4 or Nanog (Table II).
 | Table IIExpression of stem-related genes
after 1 week of ethanol or acetaldehyde.a |
Table II
Expression of stem-related genes
after 1 week of ethanol or acetaldehyde.a
| Gene | Monolayers with
ethanol
|
|---|
| Control | 25 mM Eth | Eth/cont |
|---|
| OCT-4A | 150 | 144 | 0.96 |
| NANOG | 79 | 70 | 0.89 |
| ALDH2 | 167 | 149 | 0.89 |
| SOX4 | 484 | 475 | 0.98 |
| HEY1 | 109 | 112 | 1.03 |
| JAG1 | 185 | 191 | 1.03 |
| DNER | 124 | 108 | 0.87 |
| DLL1 | 259 | 248 | 0.96 |
|
| Mammospheres with
ethanol
|
| Gene | Control | 25 mM Eth | Eth/cont |
|
| OCT-4A | 116 | 136 | 1.17 |
| NANOG | 75 | 77 | 1.03 |
| ALDH2 | 124 | 147 | 1.19 |
| SOX4 | 450 | 446 | 0.99 |
| HEY1 | 82 | 95 | 1.16 |
| JAG1 | 178 | 176 | 0.99 |
| DNER | 91 | 99 | 1.09 |
| DLL1 | 248 | 268 | 1.08 |
|
| Monolayers with
acetaldehyde
|
| Gene | Control | 25 mM Acet | Acet/cont |
|
| OCT-4A | 76 | 85 | 1.12 |
| NANOG | 38 | 42 | 1.11 |
| ALDH2 | 88 | 105 | 1.19 |
| SOX4 | 544 | 558 | 1.03 |
| HEY1 | 69 | 78 | 1.13 |
| JAG1 | 168 | 202 | 1.20 |
| DNER | 79 | 87 | 1.10 |
| DLL1 | 253 | 267 | 1.06 |
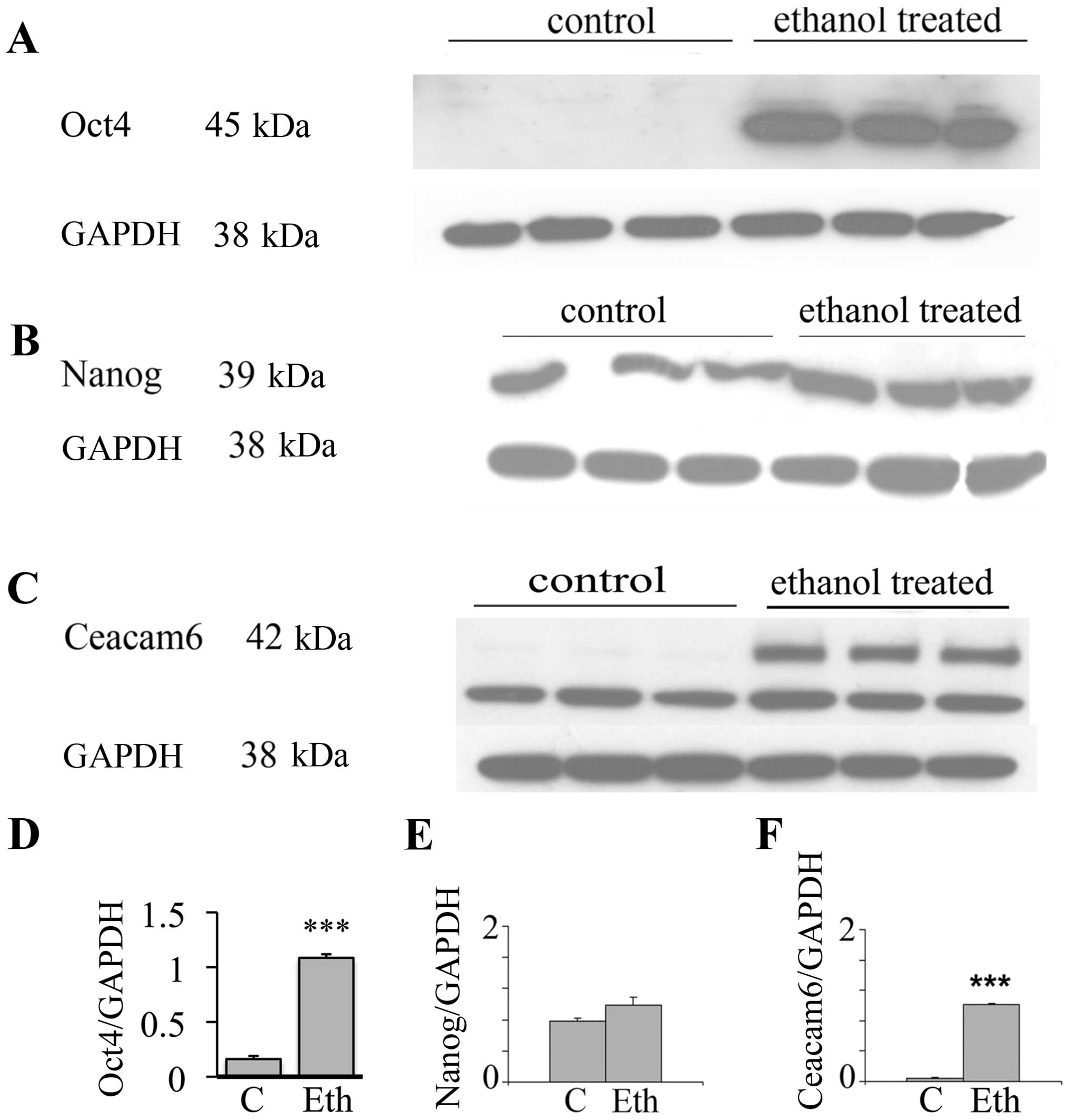
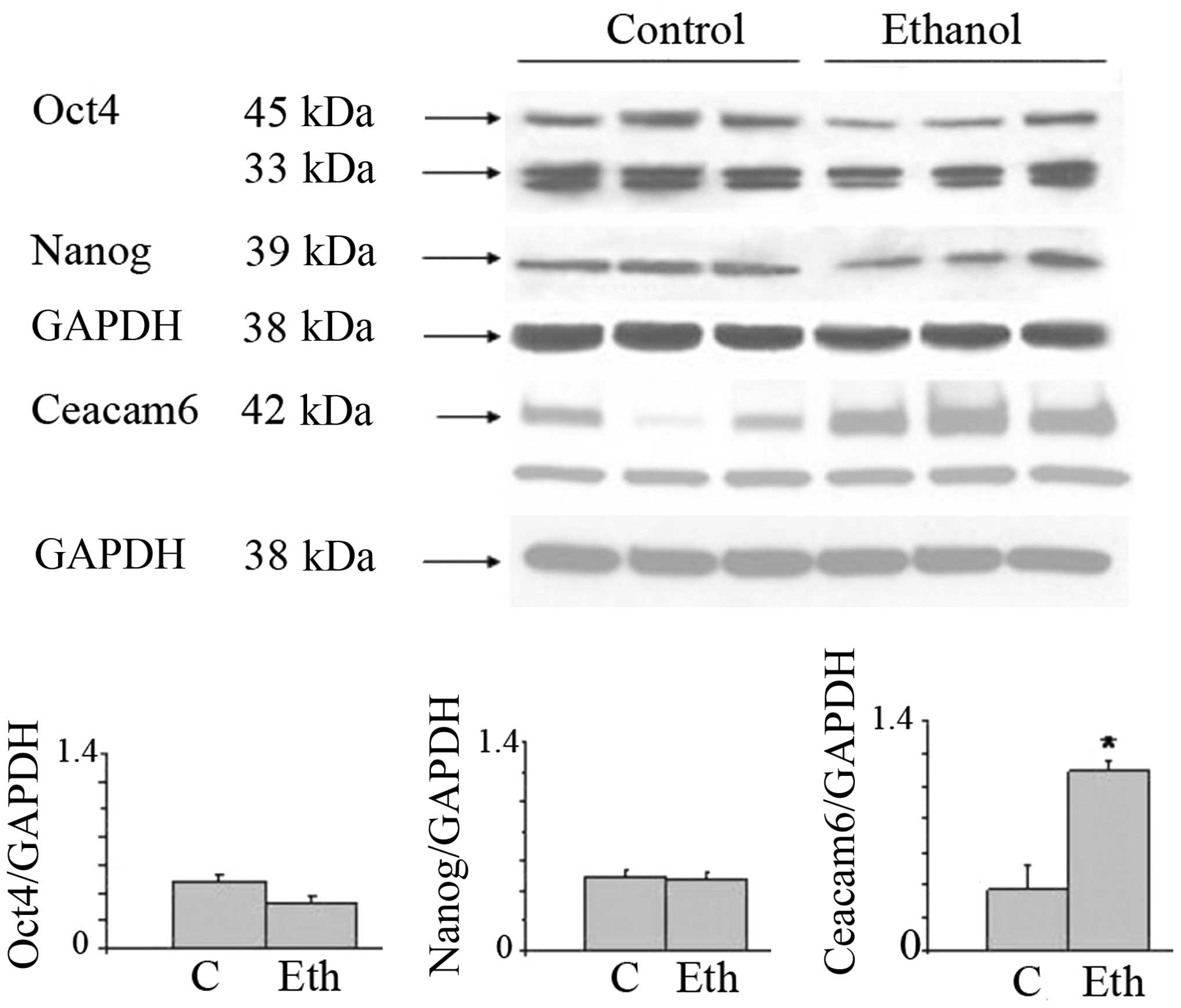
Upregulation of stem-related proteins and
Ceacam6 protein
In view of the interesting results on the
transcriptional expression of the malignancy related CEACAMs, we
evaluated by western blot analysis the effects of long-term ethanol
exposure on Ceacam6, an oncogenic protein associated with breast
cancer (50), along with the Oct4
and Nanog proteins. Fig. 3 shows
that 25 mM ethanol upregulates the expression of these proteins,
with particularly strong upregulation in Oct4 and Ceacam6, and a
visible but non-significant increase of Nanog. In the case of Oct4,
this suggests a post-transcriptional modulation induced by ethanol
(see Discussion). These results potentially may also indicate the
induction of higher stem cell number or the selection of a
subpopulation of cells with some stem-like features.
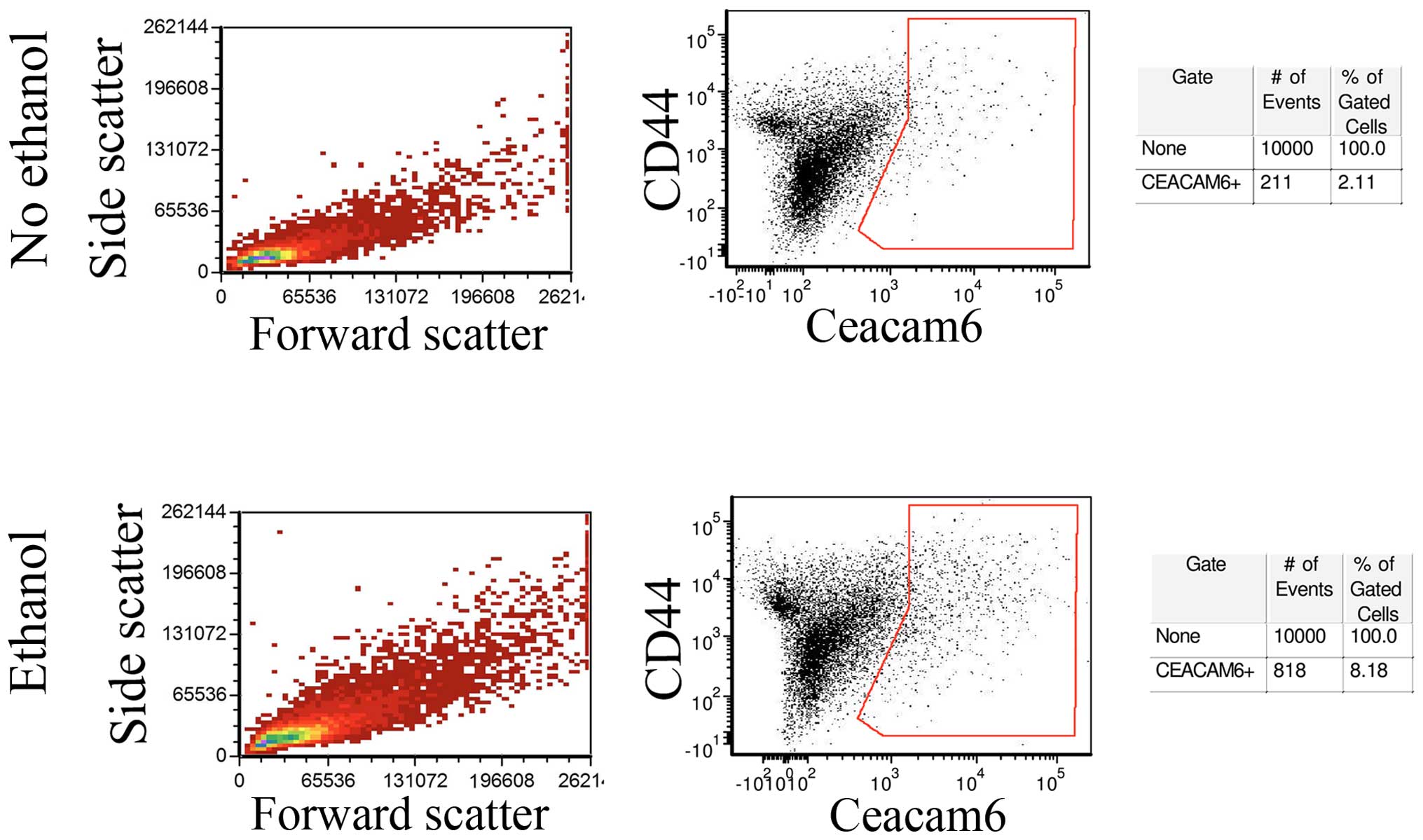
In order to determine whether Ceacam-6 upregulation
represented a global increase or was restricted to a subpopulation
of cells, untreated control MCF-7 monolayers and monolayer cells
exposed to 25 mM ethanol for 4 weeks were analyzed by flow
cytometry. Fig. 4 (center) shows
that a fraction of the cells did show enrichment of Ceacam-6
following ethanol treatment, from 2.11% in controls to 8.18% in
ethanol-treated cells, consistent with levels of upregulation
observed in western blot analyses and PCRs. The control forward-
and side-scattering (Fig. 4, left)
indicate that the ethanol treatment did not appreciably affect cell
size or internal complexity, as shown by the lack of observed
changes.
Long-term ethanol upregulates Ceacam6
protein in mammospheres, but does not induce an increase in Oct4 or
Nanog proteins
In view of the assumption that mammospheres are
enriched in stem cells, we investigated whether ethanol affects
mammosphere formation and composition. MCF-7 cells were maintained
in monolayer culture in the presence or absence of ethanol,
following which mammospheres were prepared from each sample. The
mammospheres derived from control and ethanol treated cultures were
maintained for an additional week in control medium or ethanol
medium respectively. The number of mammospheres and their
morphology did not visually appear to be modified by ethanol
exposure (data not shown).
Despite the observed increase in Oct4a and Nanog
proteins induced by long-term 25 mM ethanol treatment in MCF-7
monolayers, it is of interest that these effects were not
replicated in mammospheres obtained from these cultures and
maintained for an additional week in the presence of ethanol.
Fig. 5 shows that the previously
observed increase in Oct4a and Nanog in monolayer was not observed
in mammospheres where stem cells should be enriched, but Ceacam6
did remain upregulated. More surprisingly, taking into account our
original assumption, when mammospheres were quantitated by
determining both size and number, long-term exposure to ethanol did
not significantly increase their yield, as judged by their relative
area (29,352 control vs. 33,889 ethanol-treated), or by their
numbers either by counting stained mammospheres under the
microscope (1,337 control vs. 1,295 ethanol) or as determined using
quantitative image analysis (1,314 control vs. 1,357
ethanol-treated).
RNA was isolated from the mammospheres obtained
under 1-week exposure to ethanol or acetaldehyde and subjected to
DNA microarray analysis. Table II
shows that mammospheres were not enriched in the expression of a
collection of stem cell genes. Neither ethanol nor acetaldehyde
stimulated the expression of these genes in mammospheres or their
original monolayers, which is noteworthy since some are related to
breast cancer, such as: ALDH2, SOX4, SOX2, KLF4, LIN28, HEY1,
JAG1, DNER or Dll1 (51). No parallel assay was conducted in
the mammospheres exposed long-term to ethanol.
Gene expression in MCF-7 after ethanol
exposure
As stated above, CEACAM6 protein upregulation
induced by long-term ethanol exposure as shown on western blot
analyses was in general agreement with mRNA upregulation, so we
investigated whether other CEACAM mRNAs or those from other gene
families were also upregulated. Table III shows that gene expression of
the CEACAMs, cytokines and HLAs were all stimulated
by 25 mM ethanol, by factors ranging from 1.7 to 8.1.
 | Table IIISome gene families upregulated after
long-term 25 mM ethanol.a |
Table III
Some gene families upregulated after
long-term 25 mM ethanol.a
| Gene ID | Gene
Description | C | Eth/C |
|---|
| Ceacam5 | Carcinoembryonic
antigen-related cell adhesion molecule 5 | 223 | 3.14 |
| Ceacam6 | Carcinoembryonic
antigen-related cell adhesion molecule 6 | 170 | 2.95 |
| Ceacam7 | Carcinoembryonic
antigen-related cell adhesion molecule 7 | 70 | 1.9 |
| Ceacam1 | Carcinoembryonic
antigen-related cell adhesion molecule 1 | 43 | 1.9 |
| IFI6 | Interferon,
α-inducible protein 6 | 333 | 8.1 |
| IFITM1 | Interferon-induced
transmembrane protein 1 | 624 | 2.4 |
| IRF9 | Interferon
regulatory factor 9 | 119 | 1.8 |
| IL24 | Interleukin 24 | 164 | 2.1 |
| HLA-A | Major
histocompatibility complex, Class I,A | 914 | 1.8 |
| HLA-B | Major
histocompatibility complex, Class I,B | 937 | 1.9 |
| HLA-C | Major
histocompatibility complex, Class I,C | 1,237 | 1.8 |
| HLA-G | Major
histocompatibility complex, Class I,G | 518 | 1.7 |
| HLA-H | Major
histocompatibility complex, Class I,H | 561 | 1.8 |
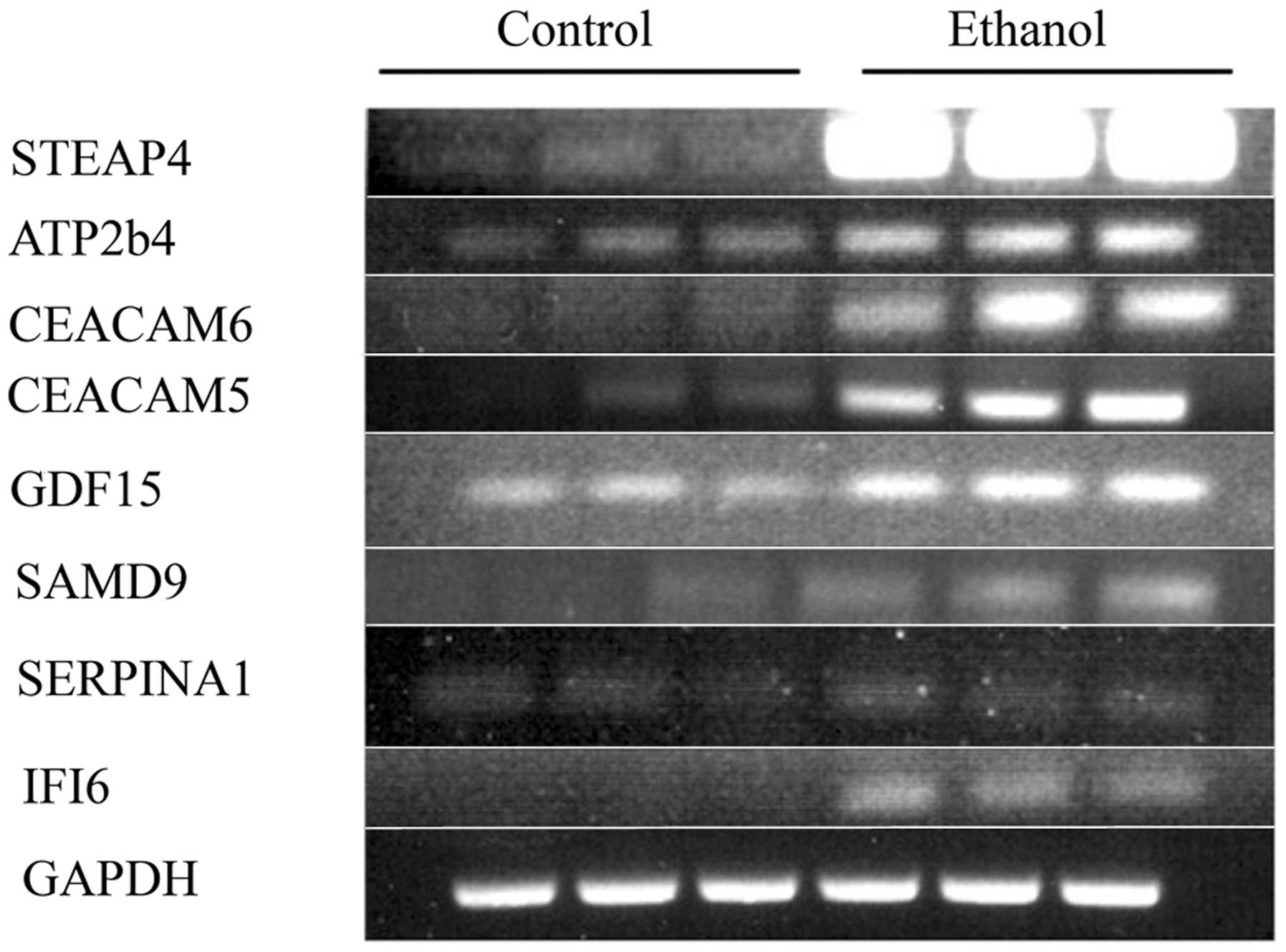
Long-term incubation with ethanol also induces other
changes of oncogenic relevance, as confirmed by the substantial
upregulation of a series of genes related to breast cancer such as
STEAP4, SERPINA3, SAMD9, GDF-15, TP63, PGR, and others, with
the transcriptional expression of 13 genes being increased ≥2.0 in
two experiments (Table IVA). To confirm the DNA microarray results,
selected RNAs from the second of the two experiments were subjected
to RT-PCR for some genes in the families mentioned above (Fig. 6). The correspondence between the
RT-PCR and DNA microarray values was good to excellent (Table IVB),
thus showing that ethanol indeed upregulated these cancer-related
genes, and validating in general the DNA microarray data.
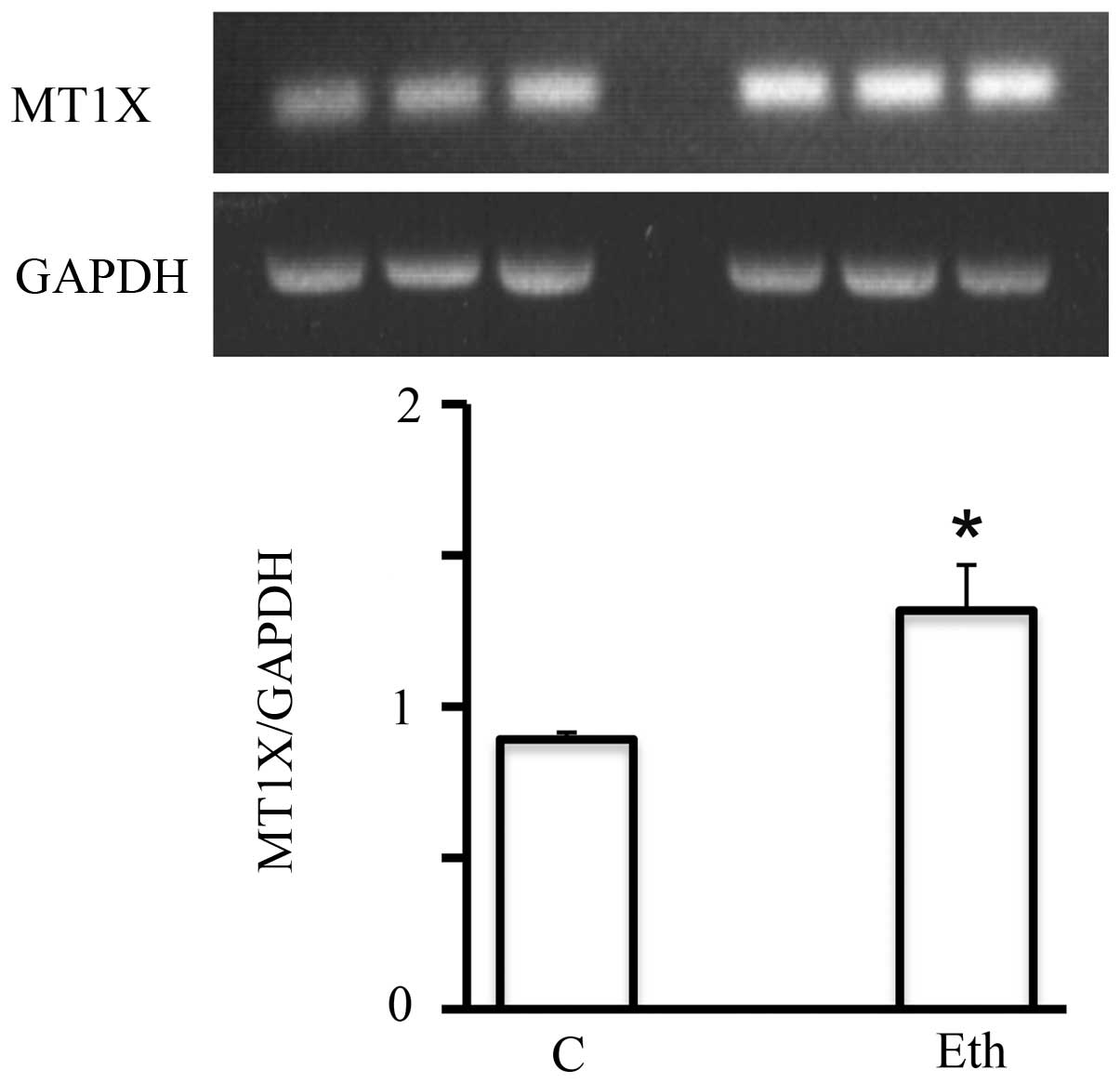
Of particular interest, a family of genes, the
metallothioneins (MTs), is known to be induced by ethanol (52,53).
Short-term 25 mM ethanol upregulated the mRNA expression of
multiple members of this family (MT1F, MT1X, and
MT2A, by a factor of ≥2) (Table
V). This effect was specific to ethanol, as it was not seen in
response to treatment with 2.5 mM acetaldehyde. The upregulation
detected by DNA microarray analysis was also confirmed by RT-PCR,
as for example MT1X (Fig. 7).
However, this significant upregulation seemed to be short-lived,
since after the 4-week exposure, it declined to only a marginal
increase (not shown) suggesting the adaptation of MCF-7 to ethanol
in terms of metallothionein expression.
 | Table VMCF-7 cells treated for 1 week with
25 mM ethanol were analyzed for gene expression using DNA
microarray analysis.a |
Table V
MCF-7 cells treated for 1 week with
25 mM ethanol were analyzed for gene expression using DNA
microarray analysis.a
| Gene ID | cont for ETh | Eth/cont | cont for Acet | Acet/cont |
|---|
| MT1A |
321 | 1.09 |
188 | 1.15 |
| MT1B |
206 | 1.31 | 66 | 0.99 |
| MT1F | 1,749 | 2.27 | 2,014 | 1.03 |
| MT1G |
748 | 1.71 | 4,768 | 0.78 |
| MT1H |
196 | 1.47 |
133 | 1.29 |
| MT1L |
940 | 1.76 |
501 | 1.00 |
| MT1X |
836 | 2.71 |
812 | 0.85 |
| MT2A | 5,225 | 1.95 | 7,099 | 0.81 |
| MT3 |
395 | 0.89 |
275 | 1.11 |
| MT4 |
155 | 1.08 | 90 | 1.19 |
Effects of long-term ethanol exposure on
the expression of miRs controlling the expression of genes related
to stem cells, malignancy, and estrogen effects
MicroRNAs (miRs) function by suppressing the
activities of specific target mRNAs. Although it is possible for
multiple miRs to affect a specific mRNA, sometimes an inverse
relationship is observed between the expression level of a
particular miR and the prevalence of the polypeptide coded by its
target mRNA. Therefore, identifying the global miR transcriptional
signature in response to ethanol exposure is important in
clarifying the mechanism(s) of action of ethanol on breast cancer,
particularly in evaluating its putative estrogen-like effects. We
therefore carried out 4-week incubations of MCF-7 monolayers with
and without 25 mM ethanol in duplicate experiments to investigate
changes in miR prevalence.
Changes in the global expression signature of miRs
induced by ethanol are presented in Table VI, showing that out of 1,904 miRs
analyzed, 18 miRs were consistently upregulated in two separate
assays by a factor of >2.0 and another 24 were downregulated by
at least an equivalent factor (to ≤0.5). Of these, 4 miRs showed
substantial upregulation (by >3.0) and 9 miRs were substantially
downregulated (to ≤0.33). Within the group of 4 upregulated genes,
3 are linked to cancer, namely miR-3170 which is downregulated in
Merkel cell carcinoma (54),
miR-335-5p which is linked to fibrosarcoma (55) and colorectal cancer but was
negative in at least one study in MCF-7 (56), and miR-424-5p which is increased in
breast cancer through an estrogen stimulated pathway (57) but is anti-invasive in another
system (58). Within the group of
9 downregulated miRs, 5 are also related to cancer: miR-2861 is
upregulated in thyroid carcinoma with lymph node metastases
(59) but has been suggested as
one element in a circulating miR screen for cervical cancer (see
Discussion), miR-3185 for chordoma (60), miR-1915 whose downregulation would
predict an antiapoptotic effect (and therefore potential
oncogenicity) mediated through Bcl-2 (61), miR-4492 potentially linked to
breast cancer (62), and miR-1469
downregulation linked to lymphatic metastasis in gastric cancer
(63) but a stimulatory factor for
apoptosis in lung cancer cells (64).
 | Table VILong-term exposure to 25 mM ethanol
and expression of miRs involved in cancer and stem cells.a |
Table VI
Long-term exposure to 25 mM ethanol
and expression of miRs involved in cancer and stem cells.a
| miRs
upregulated | Cont | Eth/Cont | miRs
downregulated | Cont | Eth/Cont |
|---|
| hsa-miR-3170 | 28 | 3.87 | hsa-miR-4507 | 914 | 0.51 |
| hsa-miR-335-5p | 80 | 3.58 |
hsa-miR-4687-3p | 819 | 0.49 |
| hsa-miR-424-5p | 2,046 | 3.18 | hsa-miR-320e | 394 | 0.49 |
|
hsa-miR-3607-3p | 411 | 3.01 | hsa-miR-4734 | 1,819 | 0.48 |
| hsa-miR-20b-5p | 226 | 2.92 | hsa-miR-4516 | 6,902 | 0.48 |
|
hsa-miR-148a-3p | 548 | 2.70 | hsa-miR-4530 | 6,332 | 0.48 |
| hsa-miR-494 | 2,012 | 2.69 | hsa-miR-638 | 11,664 | 0.46 |
| hsa-miR-4284 | 4,767 | 2.53 | hsa-miR-4508 | 4,645 | 0.44 |
| hsa-miR-23c | 85 | 2.38 | hsa-miR-3656 | 6,310 | 0.43 |
| hsa-miR-126-3p | 168 | 2.31 | hsa-miR-3196 | 6,608 | 0.43 |
| hsa-miR-30a-5p | 1,452 | 2.29 |
hsa-miR-5001-5p | 8,936 | 0.42 |
|
hsa-miR-3607-5p | 3,251 | 2.28 | hsa-miR-4505 | 1,873 | 0.40 |
| hsa-miR-141-3p | 1,297 | 2.18 | hsa-miR-663a | 9,642 | 0.39 |
| hsa-miR-454-3p | 255 | 2.16 | hsa-miR-762 | 1,375 | 0.37 |
| hsa-miR-29a-3p | 2,275 | 2.16 |
hsa-miR-3940-5p | 3,288 | 0.34 |
|
hsa-miR-3676-5p | 153 | 2.06 | hsa-miR-1469 | 267 | 0.33 |
| hsa-miR-429 | 369 | 2.04 | hsa-miR-4466 | 5,285 | 0.33 |
|
hsa-miR-106b-5p | 1,869 | 2.02 | hsa-miR-4492 | 1,515 | 0.32 |
|
hsa-miR-106a-5p | 795 | 1.96 |
hsa-miR-4707-5p | 5,583 | 0.30 |
| | |
hsa-miR-1915-3p | 3,695 | 0.29 |
| | | hsa-miR-3185 | 555 | 0.26 |
| | |
hsa-miR-4800-3p | 1,374 | 0.24 |
| | | hsa-miR-2861 | 1,437 | 0.22 |
| | | hsa-miR-654-5p | 5,651 | 0.21 |
In Table VII, we
group other miRs affected by long-term ethanol exposure of MCF-7,
focusing on whether these miRs may be related to two additional
important issues that may underlie the observed intensification of
MCF-7 malignant features: a) the finding of upregulation of Oct4
and Ceacam6 protein expression; and b) the putative mediation of
alcohol effects by estrogen-like mechanisms. In Table VII, miRs whose expression was
affected in general by a factor of >2 are listed based on a
relationship with one or more of these issues. In terms of the Oct4
regulation, Let-7 has been reported to repress Oct4 protein
expression (65) and after ethanol
treatment it is substantially downregulated from high levels seen
in the non-exposed control. Considering the observed lack of OCT4
gene transcriptional upregulation in the presence of ethanol,
concomitant with the observed stimulation of Oct4 protein, this miR
change is very pertinent in this context. In turn, with respect to
possible miRs targeting Ceacam6, only one likely miR was
downregulated (149-3P) (66) which
would be consistent with Ceacam6 upregulation, although three
(15a-5P, 16-5P and 195-5P) were upregulated, but their relationship
with Ceacam6 is less clear.
 | Table VIILong-term exposure to 25 mM ethanol
affects miRs involved in oncogenesis and in estrogen effects. |
Table VII
Long-term exposure to 25 mM ethanol
affects miRs involved in oncogenesis and in estrogen effects.
| miR | C | Eth/C |
|---|
| Let-7a-5p | 6,095 |
0.6 |
| miR-15A-5p |
310 | 2.57 |
| miR-16-5p | 4,286 | 2.01 |
| miR-195-5p |
910 | 2.32 |
| miR-149-3p | 2,067 | 0.15 |
| Let-7b | 2,737 | 0.66 |
| Let-7c | 4,158 | 0.64 |
| miR-424-5pa | 2,046 | 3.18 |
| miR-494a | 2,012 | 2.69 |
| miR-27a | 3,888 | 2.09 |
| miR-27a | 2,254 | 2.43 |
| miR-429a |
369 | 2.04 |
| miR-16 | 4,286 | 2.01 |
| miR-203 | 2,482 | 2.15 |
| miR-342 | 3,339 | 1.83 |
| miR-200a |
854 | 2.44 |
In terms of a possible relationship with estrogen
pathways, Table VII shows
several miRs affected by long-term ethanol exposure that are known
to modulate or be modulated by estrogen-mediated processes or
estrogen responsiveness, specifically 6 that are upregulated (16,
27a, 27b, 200a, 203, and 342) and 3 downregulated (let7b, 7c and
7d) (see Discussion).
Long-term exposure of MCF-7 cells to
ethanol, but not to acetaldehyde, stimulates
anchorage-independence
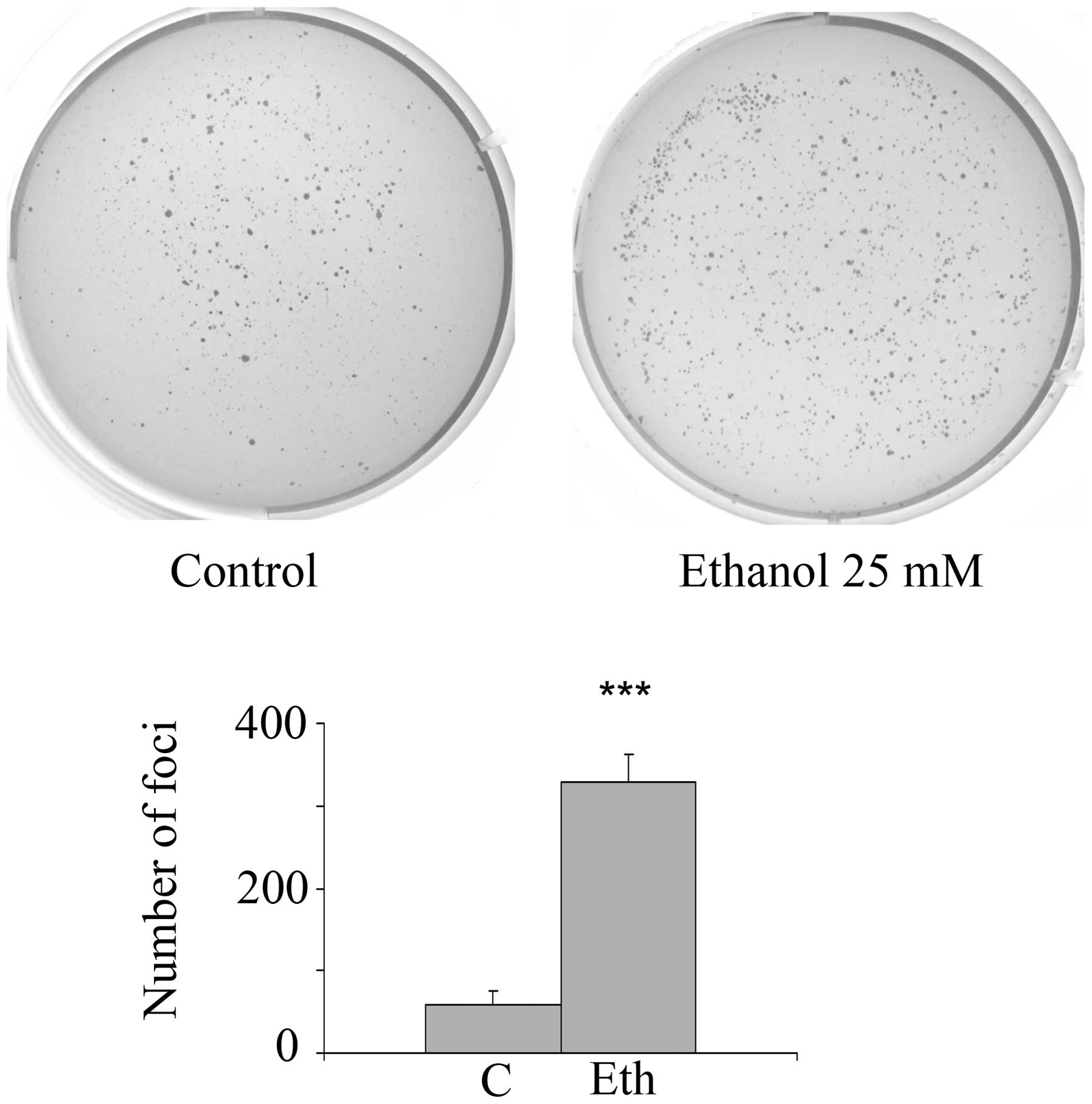
In order to determine whether long-term exposure to
ethanol or acetaldehyde stimulates anchorage-independence, an in
vitro marker of oncogenic transformation, incubations with
2.5–25 mM ethanol or 2.5 mM acetaldehyde were carried out for 4
weeks followed by 3–4 weeks growth in the clonogenic soft agar
assay. Fig. 8 shows that 25 mM
ethanol treatment substantially and significantly increases the
number of anchorage-independent foci by 5.6-fold (4 separate
experiments, each in triplicate), which agrees with the protein
expression and transcriptional signature changes related to stem
cell and oncogenic features. In addition, after exposure to a lower
ethanol concentration (10 mM), there was a substantial and
significant increase in anchorage-independence of 3.85-fold
(p=0.014) for triplicate ethanol wells and duplicate control wells.
However, exposure to further ethanol dilutions, 2.5 or 5 mM, had no
significant effect. As in the case of 1-week incubation, long-term
treatment with 2.5 mM acetaldehyde did not stimulate foci formation
(data not shown).
In contrast to the clonogenic assay, incubation of
25,000 cells with 25 mM ethanol for 4 weeks was not associated with
the stimulation of invasiveness in the Matrigel assay (data not
shown). It should be noted that the MCF-7 cell line is not invasive
per se. There were only trace numbers of positive cells
(mean of 4 samples each: control mean 11.25±2.4 SE; ethanol mean
14.5±2.9 SE) and there was no statistical significance (p=0.68,
two-tailed t-test). Moreover, there was no induction of tamoxifen
resistance after incubation for 4 weeks with 25 mM ethanol as
analyzed by cellular metabolism in the presence of increasing
levels of the drug (data not shown).
Discussion
To our knowledge this is the first report on the
in vitro long-term effects of ethanol on breast cancer cells
at doses comparable to peak exposures in humans, and describing
multiple targets of potential cancer-related impact. These effects
are not exerted by acetaldehyde, and are largely inconsistent with
respect to estrogen-mediated effects. Therefore, our results do not
support the prevalent hypothesis that ethanol action in
cancer-related processes functions mainly through estrogenic or
acetaldehyde mediation, although future, more mechanistic work is
needed to test this assumption, particularly in relation to the
potential estrogen mediation.
In a previous study (1), we investigated the effects of ethanol
exposure on a non-malignant, spontaneously immortalized breast
epithelial cell line, MCF-12A. We found that relatively low doses
of ethanol stimulated markers for epithelial mesenchymal transition
(EMT), as shown by changes in mRNAs and miRs. We also found some
upregulation in the stem-related proteins Oct4 and Nanog, as well
as in the oncogenic marker Ceacam6. The results with respect to
these phenomena are remarkably similar in this study of MCF-7,
although the levels of ethanol necessary to achieve the observed
changes differed. Ethanol incubation induced a gene expression
signature in MCF-12A that includes short-term upregulation of one
gene family in particular, the metallothioneins, similar to that
observed in MCF-7.
In this study, we have shown that: i) an exposure as
short as 1 week to 25 mM ethanol stimulates the transcriptional
expression of the metallothionein gene family in MCF-7, showing
that even in an established cancer cell line, the effect of ethanol
on MT expression occurs; this short-term ethanol treatment does
not, however, induce any substantial change in the global
transcriptional signature related to the expression of stem cell or
oncogenic markers in monolayers or mammospheres; ii) in contrast,
longer (4-week) exposure of MCF-7 monolayers to 5–25 mM ethanol
leads to upregulation of the key stem cell proteins Oct4 and Nanog,
and of a key oncogenic protein, Ceacam6, demonstrating that
phenomena previously observed in MCF-12A are maintained in the
established cancer line MCF-7; iii) in MCF-7 mammospheres incubated
long-term with ethanol, only Ceacam6 remains upregulated, and there
is no effect on mammosphere number or size; iv) changes in Ceacam6
protein expression are reflected in RNA expression, but the same
phenomenon is not observed for Oct4 or Nanog protein expression,
suggesting their regulation at the translational level.
In addition, of even more significant pathological
relevance, we showed that: v) in MCF-7 monolayers, long-term
effects induced by 25 mM ethanol include substantial changes in the
global transcriptional signature, including upregulation of
immune-related genes, HLAs, and in particular, a series of
genes involved in breast cancer; vi) this long-term oncogenic
intensification of the transcriptional signature by ethanol is
associated with the stimulation of anchorage-independence, a marker
of increased malignancy; vii) this putative transformation exerted
by ethanol is not associated with induction of tamoxifen
resistance, potentially related to estrogen effects, or of
invasiveness; viii) ethanol effects on miR expression are largely
inconsistent with the hypothesis that ethanol effects are entirely
or even largely mediated through estrogenic pathways; rather, there
are conflicting effects such that some miR expression follows this
pattern and other miR expression goes in the direction that is
opposite to what would be expected under the estrogenic hypothesis;
ix) most of the alterations we observed following ethanol treatment
do not appear to be mediated by acetaldehyde.
We do not have a conclusive explanation for the
temporal pattern showed by oncogenic effects due to long-term
exposure to ethanol, but speculate as to two possible mechanisms:
i) ethanol effects on gene expression may result from a cascade of
regulatory events that requires multiple cell divisions to
complete; or ii) longer term exposure to ethanol not only affects
gene expression, it selects for a subset of cells within the
original population; thus the partially toxic effects of ethanol
might be reflected in gene expression changes that accumulate over
the course of several cell division cycles.
Before analyzing the mechanistic significance of
these alterations, it is important to stress the limitations of
this study, in that it is essentially an in vitro
proof-of-concept approach, based on the cell line, MCF-7, similar
to what we cautiously stated regarding our previous study on the
normal MCF-12A cell line (1). The
malignant MCF-7 cell line was derived from a metastatic site of a
breast cancer tumor and is widely used as a model for breast cancer
studies. Our results need to be confirmed and extended in
vitro using lower concentrations of ethanol and by examination
of the interconnections between observed gene expression
alterations. It should be noted that in our previous study on
MCF-12A cells, considerably lower levels of ethanol led to
substantial effects on protein, mRNA, and miR prevalence, and that
their long-term exposure to high 25 mM levels arrested their growth
and ultimately was toxic to the cells. In a simplistic comparison,
this would mean that the stimulation of malignant features (MCF-7)
requires a higher concentration of ethanol than their induction
(MCF-12A).
To better extrapolate these MCF-7 results to the
human, in vivo confirmation is required in experimental
animals. Although the in vitro exposure to ethanol
concentrations of 5–25 mM, roughly equivalent in women to peak
human serum concentrations after 0.6–3 glasses of wine taken during
a 2-h period, was maintained continuously, its duration of only 4
weeks is certainly much shorter than the cumulative years of
exposure that a human drinker experiences in a lifetime. There is
no easy calculation of the equivalence between cell culture
incubations and breast tumor tissue exposures, particularly because
MCF-7 cultures may not reflect the complex stromal/epithelial
interactions involved in breast cancer progression. Despite the
above limitations, this study calls attention to the existence of
molecular alterations in breast tumor cells induced by ethanol that
may open up directions to further investigations on breast cancer
in women.
It is of interest that the metallothioneins are
members of a family of genes known to be induced by ethanol
(52) and comprising members such
as MT-I and MT-II that are antiapoptotic,
proliferative, angiogenic, and oncogenic (53). The expression of MT-I and
MT-II is increased in breast cancer and other tumors,
correlating with higher tumor grade/stage, increased recurrence and
poor survival in the highly malignant invasive ductal breast
carcinomas, and predicting poor prognosis in estrogen
receptor-negative patients. Although the stimulation of
metallothionein gene expression is indicative of gene expression
effects beginning as soon as a few days after the administration of
alcohol, the stimulation of metallothionein gene expression did not
persist in our model system, so the long-term effect of these gene
changes with respect to oncogenesis remains uncertain. As noted
above, the temporal course of action in MT genes are similar for
both the malignant MCF-7 cells and the non-malignant MCF-12A
cells.
A consistent pattern of changes induced by long-term
ethanol, but not acetaldehyde, is the correlation between the
upregulation of the key stem cell proteins Oct4a and Nanog
(67) and an important cancer
marker, Ceacam6 (50), in addition
to the induction of anchorage-independence evidenced by soft agar
growth. OCT4 is well known as a key gene responsible for the
stemness network, and the main factor in the generation of induced
pluripotent stem cells (iPS). More recently, OCT4 has
emerged as a key oncogenic factor related to the role of cancer
stem cells (68). For instance,
expression of the Oct4 protein is higher in cancerous tissues than
in adjacent-tumor tissues, is related to histological type, lymph
node status and molecular type of breast cancer, and together with
Her-2, is an independent prognostic factor for breast cancer
(69,70), although it seems to occur later
than SOX2 activation (71,72). In MCF-7 cells, estrogen stimulates
and metformin reduces the size and number of mammospheres and their
expression of Oct4 (73).
However, our current results must be viewed as
speculative as to whether they connect the observed upregulation of
Oct4 to increased malignancy, since in another study, silencing
OCT4 promoted invasion and metastasis in MCF-7 cells by
inducing EMT (74). The very low
expression of Oct4 detected by us in MCF-7 monolayers in the
absence of ethanol is consistent with observations from other
studies (75,76), and the potentially oncogenic
upregulation by ethanol has not been previously reported. As to
NANOG, there is extensive evidence linking it to breast
cancer directly or through its activation by SOX2, alone or
in conjunction with KLF4 (77), and it is known that ethanol induces
Nanog in embryonic stem cells and hepatic carcinogenesis
(78).
The upregulation of CEACAM6 after long-term
25 mM ethanol exposure is consistent with a more aggressive
phenotype of MCF-7 cells in vitro (75). Ceacam6, a membrane-associated cell
adhesion protein, is overexpressed in breast cancer and a variety
of other tumors, and is considered to be a marker of invasiveness
and metastasis, a predictor of breast cancer recurrence, and
specifically of invasive breast cancer in women with atypical
ductal hyperplastic tissues (79–82).
There are no previous reports of ethanol effects on CEACAM6,
or on a link between CEACAM6 and OCT4 expression in
any other context save our previous study on non-malignant cells
(1), and the correlation of this
upregulation to the intensification of anchorage-independence has
not been reported previously except in our previous report
(1), thus showing that this
phenomenon is observed in both malignant and non-malignant cells
derived from breast epithelium. Similarly, the stimulation of MCF-7
growth in soft agar due to exposure to ethanol at a dose as low as
10 mM has not been described before except in the previous report
(1), since previous studies
(20–26) used high concentrations of ethanol
(90–110 mM), short periods of exposure (48 h), and in some cases,
breast cancer cell lines other than MCF-7.
The stimulation by long-term ethanol of the
expression of Ceacam6 protein is in agreement with the observed
transcriptional upregulation of CEACAM6 and CEACAM5
and with other CEACAMs. The observed stimulation is also
paralleled by the increase in mRNA levels for a series of
oncogenesis-related genes, STEAP4 (83,84),
SERPINA3 (84),
SAMD9 (85), GDF-15
(86), KRT15 (87), TP53INPI (88), IF16 or G1P3 (89), and HLA-G (90) as well as other members of the
HLA family, all of which have in common their breast
cancer-related expression and the fact that none has been reported
as being upregulated by ethanol. Some of these genes (SERPINA3,
GDF15, IFI6) are also modulated by estrogen, but only one,
GDF15, was previously studied in MCF-7. Surprisingly, no
reports are available on the relationship of any of these genes
with either OCT4 or CEACAM6.
No evidence of preferential stimulation of stem cell
accumulation following ethanol treatment in either MCF-7 monolayers
or mammospheres could be found by gene expression analysis, as
judged by the lack of meaningful changes in the transcriptional
expression of a series of stem cell genes, including OCT4
and Nanog. This finding is in agreement with the similarity between
the transcriptional signatures of mammospheres and monolayers in
the absence of ethanol. This is puzzling within the framework of
the significant upregulation of Oct4 and Nanog protein expression
induced by long-term ethanol. OCT4 and NANOG are key
stem cell genes in a network of other genes involved in stemness,
but which are expressed transcriptionally in this study at only a
low level, and not changed by ethanol treatment. A possible
explanation could involve translational regulation mediated by miRs
(see below). It is also conceivable that OCT4 and
NANOG act as oncogenic factors per se, unrelated to
stem cell activation, or speculatively may be due to the failure of
stem cells to form mammospheres, but no supporting data exist in
these in respect of other than OCT4 overexpression in breast
cancer and other tumors.
The seeming contradiction between Oct4 protein
upregulation in the absence of detectable Oct4 mRNA increase
suggests a model in which untreated cells exist in a state of
translational repression of Oct4 due to some miR or combination of
miRs. According to this hypothesis, some miRs such as Let-7A-5P
(which is reduced in prevalence by ethanol exposure) allows the
induction, by its partial absence, of overexpression of the Oct4
protein. Other Let-7 family members are also substantially
expressed in control cells and reduced by ethanol. Let-7A has been
shown to function as a tumor suppressor in head and neck cancers
and in their associated tumor initiating cells, where it is
significantly decreased when OCT4 expression was increased
(65). However, it is not clear
that this is only translational repression and NANOG is also
affected, so it is possible that Let-7 might not be responsible by
itself for the selective upregulation of Oct4 protein, and it works
in conjunction with the opposite effects of Lin 28b. Another miR,
miR-145 functions as a protective miRNA in tumor tissues of lung
adenocarcinoma patients and binds to the OCT4
3′-untranslated region (UTR) thus blocking protein expression
correlated with anti-oncogenic action (91–93),
but we did not detect significant changes in this miR. The same
uncertainty applies to the putative interaction between the
downregulation of miR-149-3p by long-term ethanol, that would be
consistent with the observed upregulation of CEACAM6 mRNA
and protein, and the counterintuitive upregulation of miR-15A-5p,
16-5p, and 195-5p that could potentially oppose this effect.
However, these are inferences based purely on sequence analogies in
the miRBase 18.0 (94) and without
mechanistic proof as yet. Therefore, the roles of the changes in
miR levels in relation to the upregulation of Oct4, Nanog, and
Ceacam6 induced by long-term ethanol in MCF-7 require further
study.
The molecular signature of miRs related to estrogen
responsiveness is key to understanding whether ethanol may act via
estrogenic pathways on luminal-like breast tumor cells, such as the
MCF-7, to induce or stimulate their proliferation, survival, and
functional status through the estrogen receptor α. Alcohol is
assumed to increase sex hormone levels in both premenopausal and
postmenopausal women (6) by: a)
increased aromatase activity; b) decreased hepatic catabolism of
androgens; c) modulation of adrenal steroid production; and d) by
increasing the expression or transcriptional activity of
ERα. Alcohol may also preferentially enhance cellular
proliferation and ERα content in ER-positive cell lines
(15–18). Therefore, it might be expected that
long-term ethanol effects on miRs in MCF-7 would mimic those
exerted by estrogen or those related to estrogen responsiveness,
but no reports are available on ethanol effects on any miR profile
except in our recently published study on non-malignant cells
(1).
One of the miRs we found upregulated in MCF-7,
miR-424, was also shown to be induced by 17-β-estradiol in at least
two (95,96) of the multiple studies conducted in
MCF-7. In some cases, ethanol induced upregulation is also seen
after estrogen treatment, such as in miR-27a and b (96). The fact that ethanol affected any
particular miR in this study did not necessarily coincide with
affects on other miRs that would be expected to change
comcomitantly if ethanol action were limited to one specific
pathway such as the estrogenic pathway: thus upregulated miRs such
as miR-107, miR-424, miR-570, miR-618, and miR-760 (95), miR-21 (28), miR-34b (97), miR-98 (98), miR-19A and miR-24 (96), miR-26a and b (99), miR-17 (100,101), miR-7 (102), or miR-190a (103); or downregulated miRs such as
miR-16, miR-143, or miR-203 (104). Interestingly, the latter is the
opposite of what we observed with ethanol.
Although there are 4 miRs where both ethanol and
estradiol induce the same changes in MCF-7, there are at least 18
miRs known to be affected by estrogen that can target a significant
number of transcripts belonging to one or more estrogen-responsive
gene clusters, but were not modified by ethanol in this study. In
turn, 5 of our ethanol responsive changes have not been reported in
global miR transcription studies conducted with estrogen or
estrogenic compounds in MCF-7. The scope of this report does not
cover the elucidation of how these miRs may act in terms of
oncogenic or tumor suppressor effects. However, it is worth
mentioning that out of the miRs whose levels have previously been
shown to be regulated by estrogen and/or to modulate its receptor,
and that were found in our work to be changed by ethanol, only the
miR-27a and miR-27b levels were modulated as predicted by their
role in estrogen responsiveness. It is also known that miR-27a is
oncogenic in the triple-negative MDA-MB-231 breast cancer cell
line, and indirectly regulates E2-responsiveness in MCF-7 cells
through suppression of ZBTB10, thereby enhancing expression
of ERα (47,48). In turn, miR-27a is upregulated in
endometrial adenocarcinoma and precancerous lesions in response to
estrogen overexposure. However, let-7 behaves similarly in the
hyperplastic endometrium (98,105) and most members of this family (b,
c and d) are considered to be pro-estrogenic. In this study they
were uniformly reduced.
The lack of consistency between the ethanol
modulation of miR levels and their relationship with estrogen
responsiveness is confirmed by our results with miR-16 (106), miR-200a (47), miR-203 (47), and miR-342 (107), which, as in the case of let-7a,
b, and c, are opposite to what could be expected from an
estrogen-like effect. However, since miRs exert pleiotropic
effects, a mere association with estrogen-mediated pathways is not
sufficient to conclude that those may not be involved in the
observed ethanol effects on MCF-7 cultures. It should be emphasized
that many of these miRs also affect other processes different from
estrogenic effects, as described in a large and growing literature.
For example, the Let-7 family is highly conserved and is involved
in development, stem cell modulation, oncogenesis, and the
cardiovascular system among other things (108), while the miR 15/107 group
containing miR-15 and miR-16 affects BRCA1 expression (109), and miR-195 and miR-29 have been
linked to aortic aneurism (110).
Regarding the ethanol/estradiol comparison of miR
signatures, we note the limitation that our study was long-term,
whereas the previous estrogen studies varied in duration, but we
propose that estradiol effects do not seem to define more than a
small proportion of the changes in the global miR transcriptional
signature of MCF-7 induced by ethanol. The ethanol induced changes
are specific and at least partially different from estrogen-induced
changes. Thus this study does not provide strong support for the
hypothesis that estrogen mediates ethanol effects. Further studies
are needed, vis-à-vis ethanol and estrogen, and with
anti-estrogenic agents, to clarify the estrogenic mediation of
long-term ethanol effects on MCF-7.
A recent review lists a large number of miRs that
are involved in cancer-related processes ranging from tumor
suppression to various oncogenic features (111). We note that several miRs listed
in that review show ethanol effects in this study, including miRs
15a, 16, the Let-7 family, 27a, 148a, and 149. We should also point
out that ethanol does not necessarily promote oncogenesis in every
case, for example tumor suppressors miR-15a and miR-16 are
upregulated in response to ethanol treatment. However, the same two
miRs were found to be upregulated in a biomarker test
distinguishing sepsis from systemic inflammatory response (112). In this study, we found that
exposure of MCF-7 cells to ethanol results in detectable changes in
the expression of miRs such as miR-29a associated with inflammation
(113). Interestingly, miR-15a
upregulation is found in the serum of humans exposed to particulate
air pollution (114). In another
study, it was found that miR-29a is potentially able to stimulate
the conditions for metastasis by binding to Toll-like receptors
(115).
Some miRs that have been proposed for inclusion as
circulating cancer markers in the clinical setting show changes in
expression levels in response to ethanol. This suggests that such
markers should be considered carefully with regard to the ethanol
intake status of the patient. For example, miR-424 has been
proposed for inclusion in a group of circulating biomarkers for
breast cancer (116), and
miR-2861 is upregulated in thyroid carcinoma with lymph node
metastases (59) but has been
suggested as one element in a circulating miR screen for cervical
cancer, where it has been shown to be decreased (117).
In conclusion, our overall results suggest that
prolonged exposure to ethanol may lead to the intensification of a
series of novel oncogenic features in breast cancer by mechanisms
that are not directly related to acetaldehyde and which do not
strongly indicate estrogen mediation. These features require
further clarification, with the caveat that the in vitro
incubations using a breast cancer cell line are not directly
extrapolatable to alcohol consumption in women. Our results may
suggest some kind of stem cell involvement, but one which so far
shows contradictory transcriptional and translational aspects.
However, in conjunction with our findings on the induction of
oncogenic features in a normal breast epithelial cell line
(1), the current data may
constitute the first comparison of global transcriptional
signatures elicited by long-term ethanol exposure on normal and
cancer breast cells, and the defining of potentially oncogenic
features exacerbated by alcohol. Moreover, miRs in circulation are
now being proposed as a diagnostic tool for both carcinomas and
metatatic carcinomas (118).
Therefore, the observation that ingested ethanol affects miR levels
may have clinical significance, particularly with respect to miR
values measured in heavy drinkers.
References
|
1
|
Gelfand R, Vernet D, Bruhn K, Vadgama J
and Gonzalez-Cadavid NF: Long-term exposure of MCF-12A normal human
breast epithelial cells to ethanol induces epithelial mesenchymal
transition and oncogenic features. Int J Oncol. 48:2399–2414.
2016.PubMed/NCBI
|
|
2
|
Seitz HK, Pelucchi C, Bagnardi V and La
Vecchia C: Epidemiology and pathophysiology of alcohol and breast
cancer: Update 2012. Alcohol Alcohol. 47:204–212. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Coronado GD, Beasley J and Livaudais J:
Alcohol consumption and the risk of breast cancer. Salud Publica
Mex. 53:440–447. 2011.
|
|
4
|
Pelucchi C, Tramacere I, Boffetta P, Negri
E and La Vecchia C: Alcohol consumption and cancer risk. Nutr
Cancer. 63:983–990. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen WY, Rosner B, Hankinson SE, Colditz
GA and Willett WC: Moderate alcohol consumption during adult life,
drinking patterns, and breast cancer risk. JAMA. 306:1884–1890.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Narod SA: Alcohol and risk of breast
cancer. JAMA. 306:1920–1921. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Saxena T, Lee E, Henderson KD, Clarke CA,
West D, Marshall SF, Deapen D, Bernstein L and Ursin G: Menopausal
hormone therapy and subsequent risk of specific invasive breast
cancer subtypes in the California Teachers Study. Cancer Epidemiol
Biomarkers Prev. 19:2366–2378. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Allemani C, Berrino F, Krogh V, Sieri S,
Pupa SM, Tagliabue E, Tagliabue G and Sant M: Do pre-diagnostic
drinking habits influence breast cancer survival? Tumori.
97:142–148. 2011.PubMed/NCBI
|
|
9
|
Jelski W, Chrostek L, Szmitkowski M and
Markiewicz W: The activity of class I, II, III and IV alcohol
dehydrogenase isoenzymes and aldehyde dehydrogenase in breast
cancer. Clin Exp Med. 6:89–93. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Seitz HK and Stickel F: Molecular
mechanisms of alcohol-mediated carcinogenesis. Nat Rev Cancer.
7:599–612. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hirano T: Alcohol consumption and
oxidative DNA damage. Int J Environ Res Public Health. 8:2895–2906.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Balbo S, Meng L, Bliss RL, Jensen JA,
Hatsukami DK and Hecht SS: Time course of DNA adduct formation in
peripheral blood granulocytes and lymphocytes after drinking
alcohol. Mutagenesis. 27:485–490. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Seitz HK and Stickel F: Acetaldehyde as an
underestimated risk factor for cancer development: Role of genetics
in ethanol metabolism. Genes Nutr. 5:121–128. 2010. View Article : Google Scholar :
|
|
14
|
Wong AW, Dunlap SM, Holcomb VB and Nunez
NP: Alcohol promotes mammary tumor development via the estrogen
pathway in estrogen receptor alpha-negative HER2/neu mice. Alcohol
Clin Exp Res. 36:577–587. 2012. View Article : Google Scholar
|
|
15
|
Singletary KW, Frey RS and Yan W: Effect
of ethanol on proliferation and estrogen receptor-alpha expression
in human breast cancer cells. Cancer Lett. 165:131–137. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Etique N, Chardard D, Chesnel A, Merlin
JL, Flament S and Grillier-Vuissoz I: Ethanol stimulates
proliferation, ERalpha and aromatase expression in MCF-7 human
breast cancer cells. Int J Mol Med. 13:149–155. 2004.
|
|
17
|
Etique N, Flament S, Lecomte J and
Grillier-Vuissoz I: Ethanol-induced ligand-independent activation
of ERalpha mediated by cyclic AMP/PKA signaling pathway: An in
vitro study on MCF-7 breast cancer cells. Int J Oncol.
31:1509–1518. 2007.PubMed/NCBI
|
|
18
|
Etique N, Grillier-Vuissoz I, Lecomte J
and Flament S: Crosstalk between adenosine receptor (A2A isoform)
and ERalpha mediates ethanol action in MCF-7 breast cancer cells.
Oncol Rep. 21:977–981. 2009.PubMed/NCBI
|
|
19
|
Przylipiak A, Rabe T, Hafner J, Przylipiak
M and Runnebaum R: Influence of ethanol on in vitro growth of human
mammary carcinoma cell line MCF-7. Arch Gynecol Obstet.
258:137–140. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Meng Q, Gao B, Goldberg ID, Rosen EM and
Fan S: Stimulation of cell invasion and migration by alcohol in
breast cancer cells. Biochem Biophys Res Commun. 273:448–453. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Luo J and Miller MW: Ethanol enhances
erbB-mediated migration of human breast cancer cells in culture.
Breast Cancer Res Treat. 63:61–69. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Izevbigie EB, Ekunwe SI, Jordan J and
Howard CB: Ethanol modulates the growth of human breast cancer
cells in vitro. Exp Biol Med (Maywood). 227:260–265. 2002.
|
|
23
|
Etique N, Chardard D, Chesnel A, Flament S
and Grillier-Vuissoz I: Analysis of the effects of different
alcohols on MCF-7 human breast cancer cells. Ann NY Acad Sci.
1030:78–85. 2004. View Article : Google Scholar
|
|
24
|
Etique N, Grillier-Vuissoz I and Flament
S: Ethanol stimulates the secretion of matrix metalloproteinases 2
and 9 in MCF-7 human breast cancer cells. Oncol Rep. 15:603–608.
2006.PubMed/NCBI
|
|
25
|
Verma M and Davidson EA: MUC1 upregulation
by ethanol. Cancer Biochem Biophys. 17:1–11. 1999.
|
|
26
|
Ma C, Lin H, Leonard SS, Shi X, Ye J and
Luo J: Overexpression of ErbB2 enhances ethanol-stimulated
intracellular signaling and invasion of human mammary epithelial
and breast cancer cells in vitro. Oncogene. 22:5281–5290. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Aye MM, Ma C, Lin H, Bower KA, Wiggins RC
and Luo J: Ethanol-induced in vitro invasion of breast cancer
cells: The contribution of MMP-2 by fibroblasts. Int J Cancer.
112:738–746. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ke Z, Lin H, Fan Z, Cai TQ, Kaplan RA, Ma
C, Bower KA, Shi X and Luo J: MMP-2 mediates ethanol-induced
invasion of mammary epithelial cells over-expressing ErbB2. Int J
Cancer. 119:8–16. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cordes T, Diesing D, Becker S, Diedrich K,
Reichrath J and Friedrich M: Modulation of MAPK ERK1 and ERK2 in
VDR-positive and -negative breast cancer cell lines. Anticancer
Res. 26A:2749–2753. 2006.
|
|
30
|
Xu M, Bower KA, Wang S, Frank JA, Chen G,
Ding M, Wang S, Shi X, Ke Z and Luo J: Cyanidin-3-glucoside
inhibits ethanol-induced invasion of breast cancer cells
overexpressing ErbB2. Mol Cancer. 9:2852010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Raouf A, Sun Y, Chatterjee S and Basak P:
The biology of human breast epithelial progenitors. Semin Cell Dev
Biol. 23:606–612. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bruno RD and Smith GH: Role of epithelial
stem/progenitor cells in mammary cancer. Gene Expr. 15:133–140.
2011. View Article : Google Scholar
|
|
33
|
Korkaya H, Liu S and Wicha MS: Breast
cancer stem cells, cytokine networks, and the tumor
microenvironment. J Clin Invest. 121:3804–3809. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Feifei N, Mingzhi Z, Yanyun Z, Huanle Z,
Fang R, Mingzhu H, Mingzhi C, Yafei S and Fengchun Z: MicroRNA
expression analysis of mammospheres cultured from human breast
cancers. J Cancer Res Clin Oncol. 138:1937–1944. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xie G, Zhan J, Tian Y, Liu Y, Chen Z, Ren
C, Sun Q, Lian J, Chen L, Ruan J, et al: Mammosphere cells from
high-passage MCF7 cell line show variable loss of tumorigenicity
and radioresistance. Cancer Lett. 316:53–61. 2012. View Article : Google Scholar
|
|
36
|
Nash R, Krishnamoorthy M, Jenkins A and
Csete M: Human embryonic stem cell model of ethanol-mediated early
developmental toxicity. Exp Neurol. 234:127–135. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Worley SL, Vaughn BJ, Terry AI, Gardiner
CS and DeKrey GK: Time- and dose-dependent effects of ethanol on
mouse embryonic stem cells. Reprod Toxicol. 57:157–164. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cortez MA, Welsh JW and Calin GA:
Circulating microRNAs as noninvasive biomarkers in breast cancer.
Recent Results Cancer Res. 195:151–161. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Krell J, Frampton AE, Jacob J, Castellano
L and Stebbing J: miRNAs in breast cancer: Ready for real time?
Pharmacogenomics. 13:709–719. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Shore AN, Herschkowitz JI and Rosen JM:
Noncoding RNAs involved in mammary gland development and
tumorigenesis: There's a long way to go. J Mammary Gland Biol
Neoplasia. 17:43–58. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Valastyan S: Roles of microRNAs and other
non-coding RNAs in breast cancer metastasis. J Mammary Gland Biol
Neoplasia. 17:23–32. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Guttilla IK, Adams BD and White BA: ERα,
microRNAs, and the epithelial-mesenchymal transition in breast
cancer. Trends Endocrinol Metab. 23:73–82. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jain P and Alahari SK: Breast cancer stem
cells: A new challenge for breast cancer treatment. Front Biosci
(Landmark Ed). 16:1824–1832. 2011. View
Article : Google Scholar
|
|
44
|
Miranda RC, Pietrzykowski AZ, Tang Y,
Sathyan P, Mayfield D, Keshavarzian A, Sampson W and Hereld D:
MicroRNAs: Master regulators of ethanol abuse and toxicity? Alcohol
Clin Exp Res. 34:575–587. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Meng F, Glaser SS, Francis H, Yang F, Han
Y, Stokes A, Staloch D, McCarra J, Liu J, Venter J, et al:
Epigenetic regulation of miR-34a expression in alcoholic liver
injury. Am J Pathol. 181:804–817. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Klinge CM: miRNAs and estrogen action.
Trends Endocrinol Metab. 23:223–233. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Guttilla IK, Phoenix KN, Hong X, Tirnauer
JS, Claffey KP and White BA: Prolonged mammosphere culture of MCF-7
cells induces an EMT and repression of the estrogen receptor by
microRNAs. Breast Cancer Res Treat. 132:75–85. 2012. View Article : Google Scholar
|
|
48
|
Li X, Mertens-Talcott SU, Zhang S, Kim K,
Ball J and Safe S: MicroRNA-27a indirectly regulates estrogen
receptor {alpha} expression and hormone responsiveness in MCF-7
breast cancer cells. Endocrinology. 151:2462–2473. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Reed TE, Kalant H, Gibbins RJ, Kapur BM
and Rankin JG: Alcohol and acetaldehyde metabolism in Caucasians,
Chinese and Amerinds. Can Med Assoc J. 115:851–855. 1976.PubMed/NCBI
|
|
50
|
Tsang JY, Kwok YK, Chan KW, Ni YB, Chow
WN, Lau KF, Shao MM, Chan SK, Tan PH and Tse GM: Expression and
clinical significance of carcinoembryonic antigen-related cell
adhesion molecule 6 in breast cancers. Breast Cancer Res Treat.
142:311–322. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Pece S, Tosoni D, Confalonieri S, Mazzarol
G, Vecchi M, Ronzoni S, Bernard L, Viale G, Pelicci PG and Di Fiore
PP: Biological and molecular heterogeneity of breast cancers
correlates with their cancer stem cell content. Cell. 140:62–73.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ono S, Ishizaki Y, Tokuda E, Tabata K,
Asami S and Suzuki T: Different patterns in the induction of
metallothionein mRNA synthesis among isoforms after acute ethanol
administration. Biol Trace Elem Res. 115:147–156. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Pedersen MO, Larsen A, Stoltenberg M and
Penkowa M: The role of metallothionein in oncogenesis and cancer
prognosis. Prog Histochem Cytochem. 44:29–64. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ning MS, Kim AS, Prasad N, Levy SE, Zhang
H and Andl T: Characterization of the Merkel Cell Carcinoma
miRNome. J Skin Cancer. 2014:2895482014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Rojas F, Hernandez ME, Silva M, Li L,
Subramanian S, Wilson MJ and Liu P: The oncogenic response to
MiR-335 is associated with cell surface expression of membrane-type
1 matrix metalloproteinase (MT1-MMP) activity. PLoS One.
10:e01320262015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Lu Y, Yang H, Yuan L, Liu G, Zhang C, Hong
M, Liu Y, Zhou M, Chen F and Li X: Overexpression of miR-335
confers cell proliferation and tumour growth to colorectal
carcinoma cells. Mol Cell Biochem. 412:235–245. 2016. View Article : Google Scholar
|
|
57
|
Saumet A, Vetter G, Bouttier M, Antoine E,
Roubert C, Orsetti B, Theillet C and Lecellier CH: Estrogen and
retinoic acid antagonistically regulate several microRNA genes to
control aerobic glycolysis in breast cancer cells. Mol Biosyst.
8:3242–3253. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Ruiz-Llorente L, Ardila-González S, Fanjul
LF, Martínez-Iglesias O and Aranda A: microRNAs 424 and 503 are
mediators of the anti-proliferative and anti-invasive action of the
thyroid hormone receptor beta. Oncotarget. 5:2918–2933. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wang Z, Zhang H, Zhang P, Li J, Shan Z and
Teng W: Upregulation of miR-2861 and miR-451 expression in
papillary thyroid carcinoma with lymph node metastasis. Med Oncol.
30:5772013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Long C, Jiang L, Wei F, Ma C, Zhou H, Yang
S, Liu X and Liu Z: Integrated miRNA-mRNA analysis revealing the
potential roles of miRNAs in chordomas. PLoS One. 8:e666762013.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Nakazawa K, Dashzeveg N and Yoshida K:
Tumor suppressor p53 induces miR-1915 processing to inhibit Bcl-2
in the apoptotic response to DNA damage. FEBS J. 281:2937–2944.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Boo L, Ho WY, Ali NM, Yeap SK, Ky H, Chan
KG, Yin WF, Satharasinghe DA, Liew WC, Tan SW, et al: MiRNA
transcriptome profiling of spheroid-enriched cells with cancer stem
cell properties in human breast MCF-7 cell line. Int J Biol Sci.
12:427–445. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Yang B, Jing C, Wang J, Guo X, Chen Y, Xu
R, Peng L, Liu J and Li L: Identification of microRNAs associated
with lymphangiogenesis in human gastric cancer. Clin Transl Oncol.
16:374–379. 2014. View Article : Google Scholar
|
|
64
|
Xu C, Zhang L, Li H, Liu Z, Duan L and Lu
C: MiRNA-1469 promotes lung cancer cells apoptosis through
targeting STAT5a. Am J Cancer Res. 5:1180–1189. 2015.PubMed/NCBI
|
|
65
|
Yu CC, Chen YW, Chiou GY, Tsai LL, Huang
PI, Chang CY, Tseng LM, Chiou SH, Yen SH, Chou MY, et al: MicroRNA
let-7a represses chemoresistance and tumourigenicity in head and
neck cancer via stem-like properties ablation. Oral Oncol.
47:202–210. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Molina-Pinelo S, Gutiérrez G, Pastor MD,
Hergueta M, Moreno-Bueno G, García-Carbonero R, Nogal A, Suárez R,
Salinas A, Pozo-Rodríguez F, et al: MicroRNA-dependent regulation
of transcription in non-small cell lung cancer. PLoS One.
9:e905242014. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Ogony JW, Malahias E, Vadigepalli R and
Anni H: Ethanol alters the balance of Sox2, Oct4, and Nanog
expression in distinct subpopulations during differentiation of
embryonic stem cells. Stem Cells Dev. 22:2196–2210. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Zeineddine D, Hammoud AA, Mortada M and
Boeuf H: The Oct4 protein: More than a magic stemness marker. Am J
Stem Cells. 3:74–82. 2014.PubMed/NCBI
|
|
69
|
Liu CG, Lu Y, Wang BB, Zhang YJ, Zhang RS,
Lu Y, Chen B, Xu H, Jin F and Lu P: Clinical implications of stem
cell gene Oct-4 expression in breast cancer. Ann Surg.
253:1165–1171. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Liu C, Cao X, Zhang Y, Xu H, Zhang R, Wu
Y, Lu P and Jin F: Co-expression of Oct-4 and Nestin in human
breast cancers. Mol Biol Rep. 39:5875–5881. 2012. View Article : Google Scholar
|
|
71
|
Leis O, Eguiara A, Lopez-Arribillaga E,
Alberdi MJ, Hernandez-Garcia S, Elorriaga K, Pandiella A, Rezola R
and Martin AG: Sox2 expression in breast tumours and activation in
breast cancer stem cells. Oncogene. 31:1354–1365. 2012. View Article : Google Scholar
|
|
72
|
Lengerke C, Fehm T, Kurth R, Neubauer H,
Scheble V, Müller F, Schneider F, Petersen K, Wallwiener D, Kanz L,
et al: Expression of the embryonic stem cell marker SOX2 in
early-stage breast carcinoma. BMC Cancer. 11:422011. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Jung JW, Park SB, Lee SJ, Seo MS, Trosko
JE and Kang KS: Metformin represses self-renewal of the human
breast carcinoma stem cells via inhibition of estrogen
receptor-mediated OCT4 expression. PLoS One. 6:e280682011.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Hu J, Qin K, Zhang Y, Gong J, Li N, Lv D,
Xiang R and Tan X: Downregulation of transcription factor Oct4
induces an epithelial-to-mesenchymal transition via enhancement of
Ca2+ influx in breast cancer cells. Biochem Biophys Res
Commun. 411:786–791. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Trosko JE: From adult stem cells to cancer
stem cells: Oct-4 Gene, cell-cell communication, and hormones
during tumor promotion. Ann NY Acad Sci. 1089:36–58. 2006.
View Article : Google Scholar
|
|
76
|
Wu F, Zhang J, Wang P, Ye X, Jung K, Bone
KM, Pearson JD, Ingham RJ, McMullen TP, Ma Y, et al: Identification
of two novel phenotypically distinct breast cancer cell subsets
based on Sox2 transcription activity. Cell Signal. 24:1989–1998.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Nagata T, Shimada Y, Sekine S, Hori R,
Matsui K, Okumura T, Sawada S, Fukuoka J and Tsukada K: Prognostic
significance of NANOG and KLF4 for breast cancer. Breast Cancer.
21:96–101. 2014. View Article : Google Scholar
|
|
78
|
Machida K, Tsukamoto H, Mkrtchyan H, Duan
L, Dynnyk A, Liu HM, Asahina K, Govindarajan S, Ray R, Ou JH, et
al: Toll-like receptor 4 mediates synergism between alcohol and HCV
in hepatic oncogenesis involving stem cell marker Nanog. Proc Natl
Acad Sci USA. 106:1548–1553. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Lewis-Wambi JS, Cunliffe HE, Kim HR,
Willis AL and Jordan VC: Overexpression of CEACAM6 promotes
migration and invasion of oestrogen-deprived breast cancer cells.
Eur J Cancer. 44:1770–1779. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Ihnen M, Kilic E, Köhler N, Löning T,
Witzel I, Hagel C, Höller S, Kersten JF, Müller V, Jänicke F, et
al: Protein expression analysis of ALCAM and CEACAM6 in breast
cancer metastases reveals significantly increased ALCAM expression
in metastases of the skin. J Clin Pathol. 64:146–152. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Maraqa L, Cummings M, Peter MB, Shaaban
AM, Horgan K, Hanby AM and Speirs V: Carcinoembryonic antigen cell
adhesion molecule 6 predicts breast cancer recurrence following
adjuvant tamoxifen. Clin Cancer Res. 14:405–411. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Blumenthal RD, Leon E, Hansen HJ and
Goldenberg DM: Expression patterns of CEACAM5 and CEACAM6 in
primary and metastatic cancers. BMC Cancer. 7:22007. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Gomes IM, Maia CJ and Santos CR: STEAP
proteins: From structure to applications in cancer therapy. Mol
Cancer Res. 10:573–587. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Sano H, Wada S, Eguchi H, Osaki A, Saeki T
and Nishiyama M: Quantitative prediction of tumor response to
neoadjuvant chemotherapy in breast cancer: Novel marker genes and
prediction model using the expression levels. Breast Cancer.
19:37–45. 2012. View Article : Google Scholar
|
|
85
|
Li CF, MacDonald JR, Wei RY, Ray J, Lau K,
Kandel C, Koffman R, Bell S, Scherer SW and Alman BA: Human sterile
alpha motif domain 9, a novel gene identified as down-regulated in
aggressive fibromatosis, is absent in the mouse. BMC Genomics.
8:922007. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Stone A, Valdés-Mora F, Gee JM, Farrow L,
McClelland RA, Fiegl H, Dutkowski C, McCloy RA, Sutherland RL,
Musgrove EA, et al: Tamoxifen-induced epigenetic silencing of
oestrogen-regulated genes in anti-hormone resistant breast cancer.
PLoS One. 7:e404662012. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Cimino D, Fuso L, Sfiligoi C, Biglia N,
Ponzone R, Maggiorotto F, Russo G, Cicatiello L, Weisz A, Taverna
D, et al: Identification of new genes associated with breast cancer
progression by gene expression analysis of predefined sets of
neoplastic tissues. Int J Cancer. 123:1327–1338. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Ito Y, Motoo Y, Yoshida H, Iovanna JL,
Takamura Y, Miya A, Kuma K and Miyauchi A: Decreased expression of
tumor protein p53-induced nuclear protein 1 (TP53INP1) in breast
carcinoma. Anticancer Res. 26B:4391–4395. 2006.
|
|
89
|
Sorbello V, Fuso L, Sfiligoi C, Scafoglio
C, Ponzone R, Biglia N, Weisz A, Sismondi P and De Bortoli M:
Quantitative real-time RT-PCR analysis of eight novel
estrogen-regulated genes in breast cancer. Int J Biol Markers.
18:123–129. 2003.PubMed/NCBI
|
|
90
|
He X, Dong DD, Yie SM, Yang H, Cao M, Ye
SR, Li K, Liu J and Chen J: HLA-G expression in human breast
cancer: Implications for diagnosis and prognosis, and effect on
allocytotoxic lymphocyte response after hormone treatment in vitro.
Ann Surg Oncol. 17:1459–1469. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Jia Y, Liu H, Zhuang Q, Xu S, Yang Z, Li
J, Lou J and Zhang W: Tumorigenicity of cancer stem-like cells
derived from hepatocarcinoma is regulated by microRNA-145. Oncol
Rep. 27:1865–1872. 2012.PubMed/NCBI
|
|
92
|
Yin R, Zhang S, Wu Y, Fan X, Jiang F,
Zhang Z, Feng D, Guo X and Xu L: MicroRNA-145 suppresses lung
adenocarcinoma-initiating cell proliferation by targeting OCT4.
Oncol Rep. 25:1747–1754. 2011.PubMed/NCBI
|
|
93
|
Xu N, Papagiannakopoulos T, Pan G, Thomson
JA and Kosik KS: MicroRNA-145 regulates OCT4, SOX2, and KLF4 and
represses pluripotency in human embryonic stem cells. Cell.
137:647–658. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Kozomara A and Griffiths-Jones S: miRBase:
Integrating microRNA annotation and deep-sequencing data. Nucleic
Acids Res. 39(Database): D152–D157. 2011. View Article : Google Scholar :
|
|
95
|
Cicatiello L, Mutarelli M, Grober OM,
Paris O, Ferraro L, Ravo M, Tarallo R, Luo S, Schroth GP, Seifert
M, et al: Estrogen receptor alpha controls a gene network in
luminal-like breast cancer cells comprising multiple transcription
factors and microRNAs. Am J Pathol. 176:2113–2130. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Ali HO, Arroyo AB, González-Conejero R,
Stavik B, Iversen N, Sandset PM, Martínez C and Skretting G: The
role of microRNA-27a/b and microRNA-494 in estrogen-mediated
downregulation of tissue factor pathway inhibitor α. J Thromb
Haemost. 14:1226–1237. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Lee YM, Lee JY, Ho CC, Hong QS, Yu SL,
Tzeng CR, Yang PC and Chen HW: miRNA-34b as a tumor suppressor in
estrogen-dependent growth of breast cancer cells. Breast Cancer
Res. 13:R1162011. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Bhat-Nakshatri P, Wang G, Collins NR,
Thomson MJ, Geistlinger TR, Carroll JS, Brown M, Hammond S, Srour
EF, Liu Y, et al: Estradiol-regulated microRNAs control estradiol
response in breast cancer cells. Nucleic Acids Res. 37:4850–4861.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Tan S, Ding K, Li R, Zhang W, Li G, Kong
X, Qian P, Lobie PE and Zhu T: Identification of miR-26 as a key
mediator of estrogen stimulated cell proliferation by targeting
CHD1, GREB1 and KPNA2. Breast Cancer Res. 16:R402014. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Liao XH, Lu DL, Wang N, Liu LY, Wang Y, Li
YQ, Yan TB, Sun XG, Hu P and Zhang TC: Estrogen receptor α mediates
proliferation of breast cancer MCF-7 cells via a
p21/PCNA/E2F1-dependent pathway. FEBS J. 281:927–942. 2014.
View Article : Google Scholar
|
|
101
|
Zhang C, Zhao J and Deng H: 17β-estradiol
up-regulates miR-155 expression and reduces TP53INP1 expression in
MCF-7 breast cancer cells. Mol Cell Biochem. 379:201–211. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Masuda M, Miki Y, Hata S, Takagi K,
Sakurai M, Ono K, Suzuki K, Yang Y, Abe E, Hirakawa H, et al: An
induction of microRNA, miR-7 through estrogen treatment in breast
carcinoma. J Transl Med. 10(Suppl 1): S22012. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Manavalan TT, Teng Y, Litchfield LM,
Muluhngwi P, Al-Rayyan N and Klinge CM: Reduced expression of
miR-200 family members contributes to antiestrogen resistance in
LY2 human breast cancer cells. PLoS One. 8:e623342013. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Yu Y, Xiao CH, Tan LD, Wang QS, Li XQ and
Feng YM: Cancer-associated fibroblasts induce
epithelial-mesenchymal transition of breast cancer cells through
paracrine TGF-β signalling. Br J Cancer. 110:724–732. 2014.
View Article : Google Scholar
|
|
105
|
Shibahara Y, Miki Y, Onodera Y, Hata S,
Chan MS, Yiu CC, Loo TY, Nakamura Y, Akahira J, Ishida T, et al:
Aromatase inhibitor treatment of breast cancer cells increases the
expression of let-7f, a microRNA targeting CYP19A1. J Pathol.
227:357–366. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Yu X, Zhang X, Dhakal IB, Beggs M,
Kadlubar S and Luo D: Induction of cell proliferation and survival
genes by estradiol-repressed microRNAs in breast cancer cells. BMC
Cancer. 12:292012. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Cittelly DM, Das PM, Spoelstra NS,
Edgerton SM, Richer JK, Thor AD and Jones FE: Downregulation of
miR-342 is associated with tamoxifen resistant breast tumors. Mol
Cancer. 9:3172010. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Thornton JE and Gregory RI: How does Lin28
let-7 control development and disease? Trends Cell Biol.
22:474–482. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Quann K, Jing Y and Rigoutsos I:
Post-transcriptional regulation of BRCA1 through its coding
sequence by the miR-15/107 group of miRNAs. Front Genet. 6:2422015.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Fu XM, Zhou YZ, Cheng Z, Liao XB and Zhou
XM: MicroRNAs: Novel players in aortic aneurysm. Biomed Res Int.
2015:8316412015. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Gambari R, Brognara E, Spandidos DA and
Fabbri E: Targeting oncomiRNAs and mimicking tumor suppressor
miRNAs: New trends in the development of miRNA therapeutic
strategies in oncology (Review). Int J Oncol. 49:5–32.
2016.PubMed/NCBI
|
|
112
|
Wang H, Zhang P, Chen W, Feng D, Jia Y and
Xie LX: Evidence for serum miR-15a and miR-16 levels as biomarkers
that distinguish sepsis from systemic inflammatory response
syndrome in human subjects. Clin Chem Lab Med. 50:1423–1428. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
de Gonzalo-Calvo D, Davalos A, Montero A,
Garcia-Gonzalez A, Tyshkovska I, Gonzalez-Medina A, Soares SM,
Martínez-Camblor P, Casas-Agustench P, Rabadán M, et al:
Circulating inflammatory miRNA signature in response to different
doses of aerobic exercise. J Appl Physiol. 119:124–134. 1985.
View Article : Google Scholar
|
|
114
|
Rodosthenous RS, Coull BA, Lu Q, Vokonas
PS, Schwartz JD and Baccarelli AA: Ambient particulate matter and
microRNAs in extracellular vesicles: A pilot study of older
individuals. Part Fibre Toxicol. 13:132016. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Fabbri M, Paone A, Calore F, Galli R,
Gaudio E, Santhanam R, Lovat F, Fadda P, Mao C, Nuovo GJ, et al:
MicroRNAs bind to Toll-like receptors to induce prometastatic
inflammatory response. Proc Natl Acad Sci USA. 109:E2110–E2116.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Zhang L, Xu Y, Jin X, Wang Z, Wu Y, Zhao
D, Chen G, LiD, Wang X, Cao H, et al: A circulating miRNA signature
as a diagnostic biomarker for non-invasive early detection of
breast cancer. Breast Cancer Res Treat. 154:423–434. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Zhang Y, Zhang D, Wang F, Xu D, Guo Y and
Cui W: Serum miRNAs panel (miR-16-2*, miR-195, miR-2861,
miR-497) as novel non-invasive biomarkers for detection of cervical
cancer. Sci Rep. 5:179422015. View Article : Google Scholar
|
|
118
|
Zhao Q, Deng S, Wang G, Liu C, Meng L,
Qiao S, Shen L, Zhang Y, Lü J, Li W, et al: A direct quantification
method for measuring plasma MicroRNAs identified potential
biomarkers for detecting metastatic breast cancer. Oncotarget.
7:21865–21874. 2016.PubMed/NCBI
|