Introduction
Osteosarcoma is the most common primary malignant
tumor that arises from osteoid tissues in young adults and
adolescents (1,2). Most patients with osteosarcoma are
diagnosed at a late stage with strong requirement of surgical
radiotherapy or adjuvant chemotherapy, however, usually it is not
effective (3,4). Since platinum-based drugs such as
cisplatin are the standard first-line agents used alone or in
combination with other drugs for osteosarcoma treatment,
osteosarcoma cells may acquire resistance to cisplatin with tumor
recurrence through the expansion of cisplatin-resistance cell
population (5,6). Unfortunately, a major challenge
exists in bone cancer fields due to the limited efficiency of
current therapeutic strategies. Therefore, there is an urgent need
to develop novel early molecular markers of therapeutic targets for
osteosarcoma with cisplatin-resistant scenario (7,8).
Lysophosphatidic acid acyltransferase β (LPAATβ,
1-acylglycerol-3-phosphate O-acyltransferase 2, Agpat2), one of
transmembrane proteins, are involved in regulating osteosarcoma
cell proliferation (9,10). During this process, LPAATβ could
activate the acyltransferase activity and convert lysophosphotidic
acid (LPA) into phosphatidic acid (PA), such as the mTOR and Raf-1
signaling pathways, which is considered as an important secondary
messenger for cell survival and proliferation (11–13).
However, the comprehensive underlying molecular mechanisms
responsible for LPAATβ as a cell viability regulator in
osteosarcoma disease remain unclear. Recently LPAATβ has become a
pharmacological research target of osteosarcoma. It was reported
that overexpression of miR-24 inhibited osteosarcoma cell
proliferation both in vitro and in vivo through
binding to 3′UTR of LPAATβ (14).
Other research revealed that exogenous expression of LPAATβ
effectively promoted osteosarcoma tumor growth, while knockdown of
which inhibited tumor growth in vitro (15). However, the effect of LPAATβ
expression alteration on human osteosarcoma with
cisplatin-resistant environment is not well known.
We examined the LPAATβ expression in tumor samples
from 40 osteosarcoma patients and then analyzed the relationship
between LPAATβ expression and clinical behavior of osteosarcoma. We
further generated cisplatin-resistant sublines using
lentivirus-mediated RNAi and fianlly mechanistically investigated
the functional role of LPAATβ and PI3K/Akt/mTOR signaling pathway
involvement in cisplatin-resistant environment of osteosarcoma
in vivo and in vitro.
Materials and methods
Clinical specimens and samples
collection
Cancer tissue specimens were obtained from 40
osteosarcoma patients aged from 13 to 46 years who had undergone
resection at the Orthopaedics Department of First Affliated
Hospital, Third Military Medical University between 2014 and 2016.
Clinical information of all the patients was collected and shown in
Table I. This study was approved
by the ethics committee of First Affliated Hospital, Third Military
Medical University, and all patients provided informed consent.
 | Table IClinicopathological features of
osteosarcoma in all study subjects. |
Table I
Clinicopathological features of
osteosarcoma in all study subjects.
| Clinicopathological
features | No. of cases
(n=40) |
|---|
| Age (years) |
| ≤25 | 24 |
| >25 | 16 |
| Gender |
| Male | 29 |
| Female | 11 |
| Tumor site |
| Femur | 18 |
| Tibia | 14 |
| Humeral bone | 7 |
| Other | 1 |
| Pathological
fracture |
| Absent | 15 |
| Present | 25 |
| Recurrence |
| Absent | 27 |
| Present | 13 |
| Response to
preoperative chemotherapy |
| Good | 10 |
| Poor | 28 |
| N/A | 2 |
Ethical approval
All procedures performed in studies involving human
participants were in accordance with the ethical standards of the
institutional and/or national research committee and with the 1964
Helsinki Declaration and its later amendments or comparable ethical
standards. All animal experiments were carried out strictly in
accordance with international ethical guidelines and the National
Institutes of Health Guide concerning the Care and Use of
Laboratory Animals. The experiments were approved by Medical Ethics
Committee of the Southwest Hospital and the First Affiliated
Hospital of the Third Military Medical University.
Cell culture
Two human osteosarcoma cell lines MG-63 and SaOS-2
[American Type Culture Collection (ATCC), Manassas, VA, USA] were
maintained in DMEM high glucose media (PAA Laboratories, Pasching,
Austria) supplemented with 10% (v/v) fetal bovine serum (PAA
Laboratories, Pasching, Austria) at 37°C and 5% CO2.
Culture medium was changed twice a week, and splitting of the cell
culture was done every ten days at confluency of 70–80%. Cells were
kept in a 5% CO2 atmosphere at 37°C before
analyzing.
Acute cytotoxicity assay
The acute cytotoxic effects of cisplatin (1.5, 3,
4.5, 6, 8 and 4 µM, 8, 12 and 16 µM) on cell
viability were measured in confluent monolayers in 96-well plates,
using the CCK8 kit (Sigma-Aldrich) according to the standard
method. Briefly, cells were allowed to grow in a 75-cm2
cell culture flask (TPP Techno Plastic Products, Trasadingen,
Switzerland) until 95% confluent. The cells were then seeded into
each well and incubated for 24 h at 37°C in an atmosphere of 7.5%
CO2 in air. In the last hour of incubation, 100
µl CCK8 solution (Dojindo, Japan) was added to each well for
1 h, and the absorbance was read at 450 nm on the BioTek
FL600® fluorescence/absorbance plate reader (BioTek
Instruments Inc., Winooski, VT, USA). Control groups consisted of
cells in media (minus chemical) which were processed identically
and incubated simultaneously as treated groups. Parallel sets of
wells without cells were incubated as process blanks. Calculation
of inhibitory concentration 50 (IC50) value was
determined from the absorbance versus concentration curve for each
chemical based on a minimum of 3 experiments performed at separate
times.
Establishment of cisplatin-resistant
subline clonogenic assay
A monoclonal strain was separated by dilution
culture method. MG-63 and SaOS-2 were derived by incubation with
stepwise increasing cisplatin concentrations (from 1.5 to 16
µM). Cells were seeded in a 24-well dish at 100 cells/well
and allowed to adhere for 24 h in an incubator after which the
cisplatin (Sigma, USA) was added. Cells were replaced with fresh
DMEM media after 24-h incubation. After each treatment, the
survival cells re-expanded and were conventionally propagated for
four generations in cisplatin-free media. Each individual
experiment was performed in triplicate and repeated three
times.
Quantitative real-time PCR
Total RNA (2 µg) was extracted using an
RNeasy Mini kit (Qiagen, Germany). RNA purity and concentration
were estimated with an ND-1000 spectrophotometer (NanoDrop
Technologies, Thermo Scientific, UK). Total messenger RNA was
reverse-transcribed into cDNA using the Verso™ cDNA synthesis kit
(Thermo Fisher Scientific) as described by the manufacturer. Each
forward and reverse validated primer (5 µM) (Sigma-Aldrich,
UK) is listed in Table II. Each
reaction contained 50 ng cDNA and was carried out using
SYBR® green RT-PCR master mix (Life Technologies). PCR
reactions with 50 ng/µl of the cDNA samples per 10 µl
final reaction volume, were performed using standard cycling
parameters (stage 1, 50°C for 2 min, stage 2, 95°C for 10 min then
40 cycles of 95°C for 15 sec and 60°C for 1 min) on an ABI 7900HT
sequence detection system. Normalization was performed using GAPDH
as the internal control, and relative gene expression was
calculated by the comparative 2−ΔΔCt method using SDS
2.2 software (Applied Biosystems).
 | Table IISequences of the gene-specific
primers used for real-time PCR detection in vitro. |
Table II
Sequences of the gene-specific
primers used for real-time PCR detection in vitro.
| Gene | Primer sequence
(5′-3′) |
|---|
| LPAATβ | F:
CGGACCGATGTTCGCCGCCATGAACGG |
| R:
AATCTTCCATCATCGCACTTGTAGTTGC |
| P-gp | F:
AGTTCGTAGGGCTAGCTAATCGATCGAT |
| R:
CGCACGTGATCGATCCGTCCCGATCGAT |
| MRP1 | F:
AGGCCCCTAGCTAGTAGCTAGCCATCG |
| R:
GTAGCTAAAACGTAGCTGTAGCCCTA |
| GST | F:
ATAGATATCAGTCCCCCTAGTAGCTAG |
| R:
CTCGAAAATCGATCGTAGTCGATGCC |
| bcl-2 | F:
CGCGCTAGCTAGACGCGCGATGATCC |
| R:
CGCGCTAGCTAGTAAGCTAGCTCTAGC |
| PIK3CA | F:
CGGGATCGATCCCCGATCGTATAGCT |
| R:
AGATCGATCGTATATCGATAGCTAAC |
| Akt1 | F:
CGCGATCTATAGCTCGCCTAGCTAGC |
| R:
GTAGTCTAGCGCGCCCCTAGCTAGAT |
| Akt2 | F:
CGCGATGATCGTAGTAGCTAGCTAAC |
| R:
GCTAGATAGCGCTAGCTGACCGATCGC |
| mTOR | F:
CGCATACGTAGCTAGCTAGCAACGAC |
| R:
GTAGCTAGCCCTAGCTAGACTGCGCG |
| GAPDH | F:
CGGAGTCAACGGATTTGGTCGTAT |
| R:
AGCCTTCTCCATGGTGGTGAAGAC |
Western blot analysis
Before employing a set of phospho-specific
antibodies, lysis buffer (12.5 ml Tris-HCl, 2 g SDS, 10 ml
glycerol, 67.5 ml distilled water) was used to harvest whole-cell
lysates, followed by sonication. The concentration of protein in
the cell lysates was estimated by using a bicinchoninic acid (BCA)
assay. Novex® 4–20% Tris-glycine 12-well polyacrylamide
gradient gels (Invitrogen, UK) were used to separate proteins.
Subsequently, proteins were transferred onto a nitrocellulose
membrane (GE Healthcare, Little Chalfont, UK) via the Protean Mini
Cell system (Bio-Rad, München, Germany). After blocking in 5%
non-fat milk in TBS/0.1% Tween-20 (Merck, Darmstadt, Germany) (2 h,
RT), the membrane was incubated with the corresponding primary
antibody (overnight, 4°C). After washing with TBS/0.1% Tween-20 the
secondary (peroxidase-conjugated) antibody was added (1:10,000, 2
h, RT). For visualization of the bound antibodies the Fusion FX7
imaging system (PeqLab, Erlangen, Germany) was used. All antibodies
were diluted in 5% milk/1X TBS-Tween (w/v). Enhanced
chemiluminescence (GE Life Sciences) and X-ray film (Fujifilm) were
finally used to visualize the proteins. Sections were incubated
with the primary antibody, anti-human LPAATβ polyclonal antibody
(1:10,000; Santa Cruz), anti-human P-gp polyclonal antibody
(1:10,000; Abcam), anti-human MRP1 polyclonal antibody (1:4,000;
Abcam), anti-human GST polyclonal antibody (1:4,000; Abcam),
anti-human Bcl-2 polyclonal antibody (1:4,000; Santa Cruz);
anti-human Akt1/2 polyclonal antibody (1:4,000; Santa Cruz),
anti-human Akt1/2 polyclonal antibody (1:4,000; Santa Cruz),
anti-human mTOR polyclonal antibody (1:5,000; Santa Cruz) and
anti-human PIK3CA polyclonal antibody (1:10,000; Santa Cruz),
respectively, at 4°C overnight.
Immunohistochemistry analysis
We detected the expression of LPAATβ in tissue
samples and phospho-mTOR in tumor samples by immunohistochemisty in
nude mice. Samples were carefully digested and fixed overnight in
4% paraformaldehyde with a 0.1 M phosphate buffer solution (pH
7.4). Then cells were embedded in paraffin and sliced into
5-µm-thick serial sections using a Microtome (Leica
Microsystems Inc., Buffalo Grove, IL, USA). The sections were
deparaffinized and fixed after hydration. Sections were incubated
with the primary antibody, anti-human LPAATβ polyclonal antibody
(1:100; Abcam), anti-mouse phospho-mTOR polyclonal antibody (1:50;
Abcam), respectively. at 4°C overnight. The photographs of the
identified adjacent areas were taken under high magnification
(×400).
Lentivirus-mediated gene silencing
To further prove the importance of LPAATβ in the
process of cisplatin resistance, small intering RNA (siRNA
GCCGGACGGUGGAGAACAUGA) transfection was performed using three
sequences designed to target LPAATβ which were designed by the
Invitrogen RNAi design tool (http://www.invitrogen.com) and synthesized by
Invitrogen, Ltd. Non-targeting negative control of siRNA (control
TTCTCCGAACGTGTCACGTTTC) was also synthesized. The oligonucleotides
were annealed and inserted into the pLKO.1 siRNA expression vector.
The LPAATβ-interference cell lines MG-63 and SaOS-2 were
established using lentivirus transfection and puromycin selection
with the second sequence. For lentivirus-mediated RNAi, the 293T
cells were selected for transfection with two kinds of auxiliary
packaging vector plasmids (LPAATβ-siRNA-pLKO.1 and pLKO.1)
respectively with polyethylenimine (PEI). The supernatant was
collected, and MG-63, MG-63-CR, SaOS-2 and SaOS-2-CR cells were
infected for 5 h with polybrene. To obtain the stable cell line,
puromycin selection was performed. The efficiency of LPAATβ
inhibition was evaluated by RT-PCR.
Immunofluorescence staining
Cells were plated onto coverslips in MEM medium with
10% FBS for 24 h before being transfected with lentivirus-mediated
siRNA or negative control. At 48 h after transfection, the cells
were fixed with 4% paraformaldehyde for 20 min, incubated in 0.3%
Triton X-100-PBS for 10 min at room temperature, followed by
blocking with 5% goat serum at 37°C for 30 min. The cells were then
incubated with the primary antibody at 4°C overnight; mouse
anti-phospho-mTOR IgG (1:200, Santa Cruz). The samples were soaked
in goat anti-human IgG conjugated to Cy3 (1:400; Jackson
ImmunoResearch) at 37°C for 1 h. Subsequently, the nuclei were
counter stained with DAPI (1:1,000; Sigma-Aldrich, Inc., MO, USA).
Images were obtained using an inverted fluorescence microscope
(Olympus). The primary antibody was replaced with PBS as the
negative control.
Animal experiments
Divided into 4 groups (n=32), right flank of SCID
mice were inoculated subcutaneously with cisplatin-resistant
MG-63-CR-LV3-siRNA (1×107 cells) and SaOS-2-CR-LV3-siRNA
(1×107 cells), respectively, at day 0, followed by
intraperitoneal injection of cisplatin (4 mg/kg) when the tumor
volume reached 100 mm3. In addition, MG-63-CR-LV3
(1×107 cells) and SaOS-2-CR-LV3 (1×107 cells)
were employed for in vivo experiments as control vector
groups. All mice were monitored for tumor growth for 40 days before
sacrifice. The tumor size in these tumor-bearing mice was measured
and tumor volumes were calculated as: length × width2 ×
0.45.
Histopathology assays
Tumor samples from nude mice were collected at the
indicated time-points and fixed in 10% formalin solution for 48 h,
and then embedded in paraffin for the 5-µm-thick sections.
Serial sections of the embedded specimens were stained with
hematoxylin and eosin (H&E) using standard pathology procedures
and evaluated by a pathologist.
Statistical analysis
The differences between each group are expressed as
the mean ± SD. Statistical significance was assessed by Student's
t-test and one-way ANOVA followed by a Tukey post hoc test.
Differences were considered statistically significant at P-value of
<0.05.
Results
Differentially expressed LPAATβ with
strong correlation with cisplatin-resistant scenario in vivo and in
vitro
Immunohistochemical detection was performed in
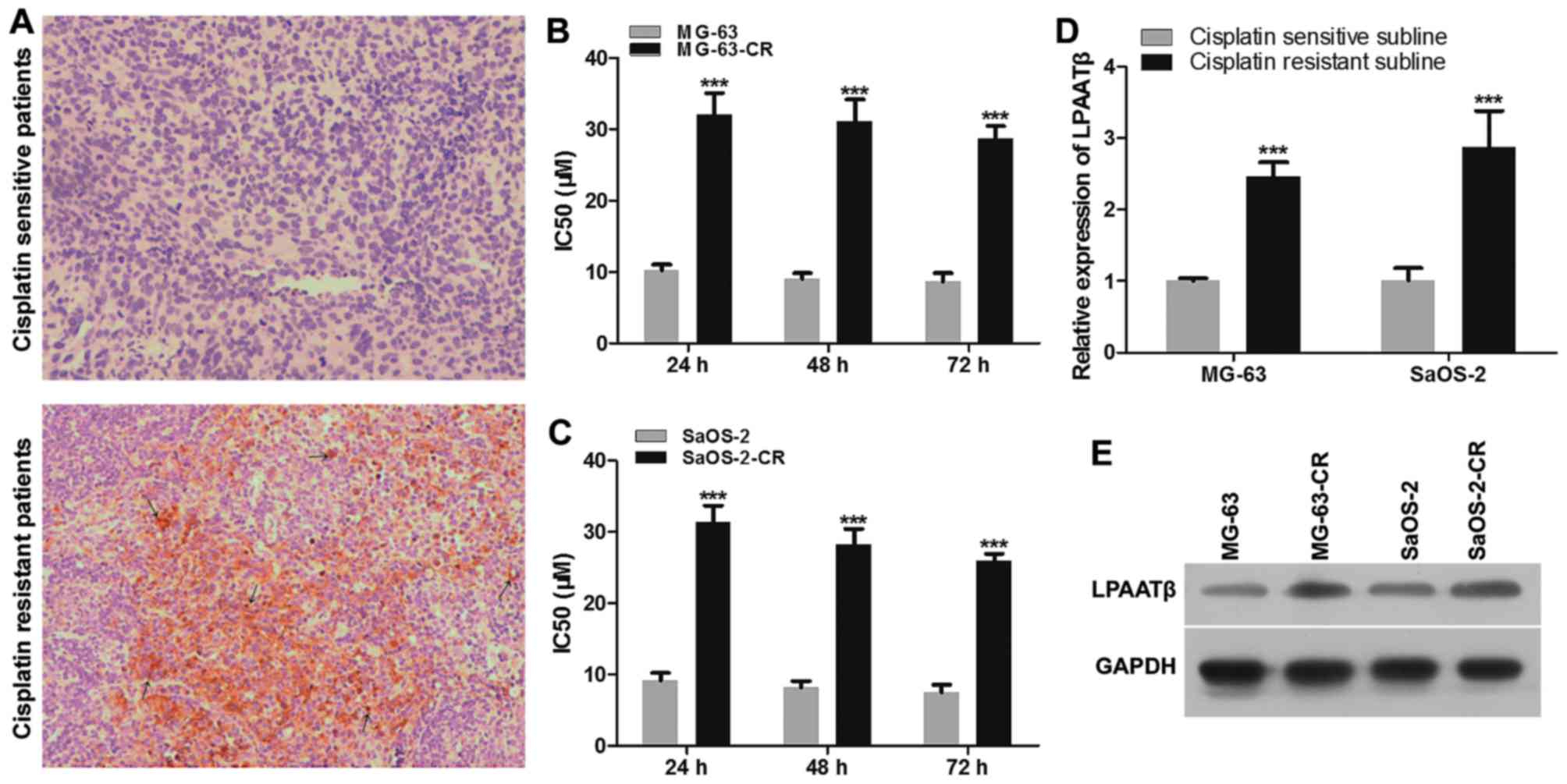
tissue samples of both cohorts. As Fig. 1A shown, LPAATβ in
cisplatin-resistant osteosarcoma patients was stained as brown in
cell membrane and nuclear while in osteosarcoma patients was very
weak, similar to the control group (Fig. 1A). The cell membrane and nuclear
LPAATβ protein expression of mature and new born tumor cells was
observed in the nuclear of nascent tumor cells at various
differentiation states, compared to control group.
Two human osteosarcoma cell lines were subjected to
treatment with 7 different concentrations of cisplatin, ranging
from 0.05 to 2 µM for 24, 48 and 72 h. Cytotoxicity of
cisplatin in control and drug-resistant pairs (MG-63 and MG-63-CR;
SaOS-2 and SaOS-2-CR) were measured by CCK8 assays. Examples of
resulting volumes are shown in Fig. 1B
and C. As shown in Fig. 1B,
MG-63-CR cells displayed enhanced cisplatin IC50 value
after cisplatin treatment, compared with those of parental cells.
The IC50 value at 24 h was the highest compared to 48
and 72 h. The same result is shown as Fig. 1C, SaOS-2-CR cells displayed the
highest cisplatin IC50 value after cisplatin treatment
for 24 h, compared with those of parental cells. In order to extend
our findings, we measured LPAATβ level in these cell lines. LPAATβ
mRNA level and protein level were significantly increased in
drug-resistant cell lines, compared with those of control parental
cells, which were positively correlated with cisplatin
IC50 (Fig. 1D and
E).
Effect of cisplatin-resistance on
expression of relevant important transporters
In order to explore the effect of cisplatin in
osteosarcoma, we harvested MG-63-CR and SaOS-2-CR after cisplatin
treatment for 72 h, and then detected P-gp, MRP1, GST, Bcl-2
expression in mRNA level and protein level as Fig. 2 shows. The mRNA levels of P-gp,
MRP1, GST, Bcl-2 in MG-63-CR and SaOS-2-CR were upregulated
(Fig. 2A–D), compared to those of
parental cells. Similar with results of mRNA level detection,
protein levels of P-gp, MRP1, GST, Bcl-2 were increased
significantly (Fig. 2E).
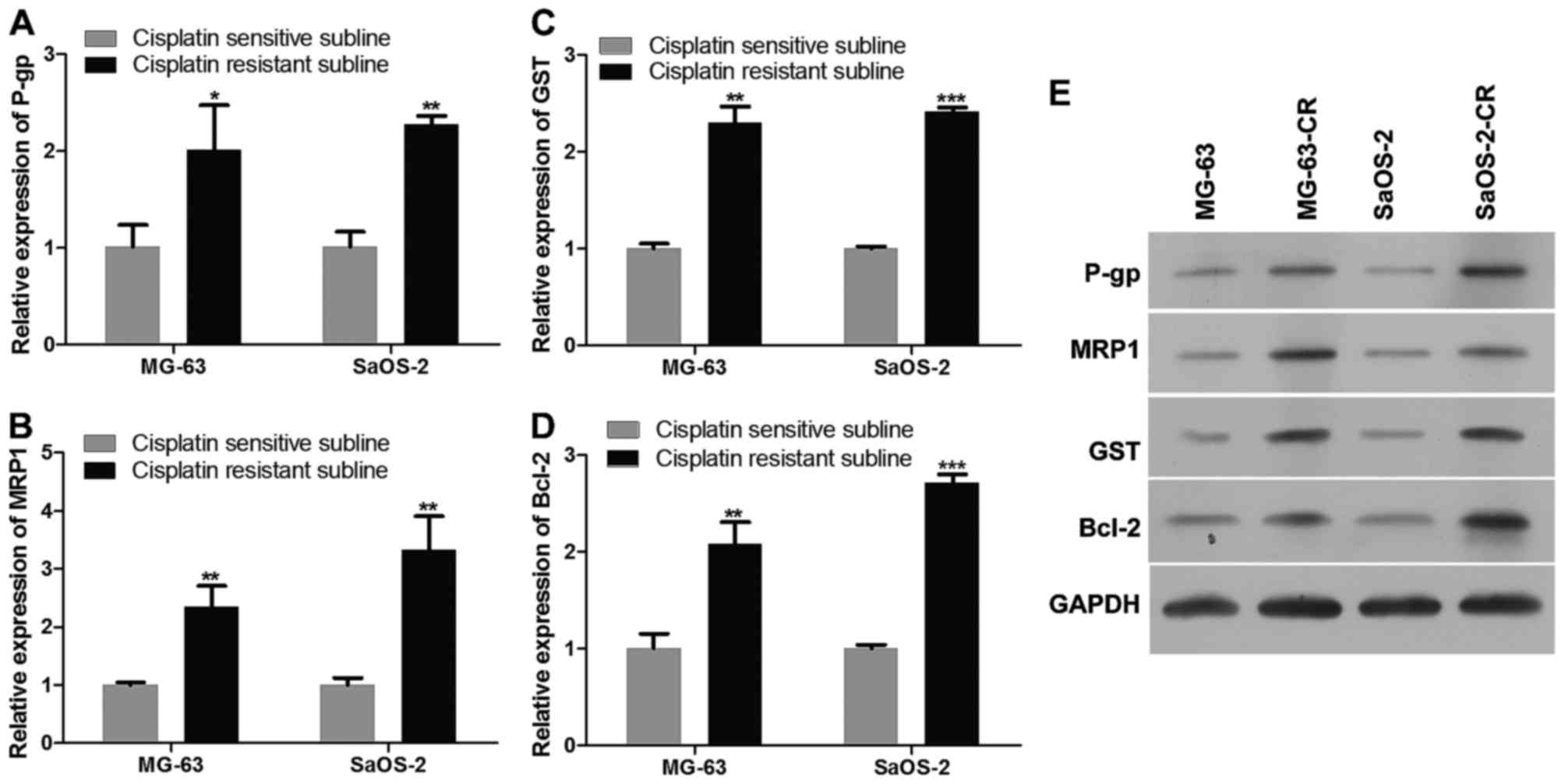 | Figure 2Expression of P-gp, MRP1, GST, Bcl-2
in both cisplatin-resistant and cisplatin-sensitive cells. (A–D)
The mRNA levels of P-gp, MRP1, GST, bcl-2 in cisplatin-resistant
MG-63-CR and SaOS-2-CR, as detected by real-time PCR. (E) Protein
levels of P-gp, MRP1, GST, bcl-2 in cisplatin-resistant MG-63-CR
and SaOS-2-CR, as detected by western blotting. All detections were
repeated independently three times. *P<0.05,
**P<0.01 and ***P<0.001 versus the
parental cells (n=3 experiments). |
Effect of silencing LPAATβ on
cisplatin-induced cytotoxicity in MG-63 and SaOS-2 cell lines
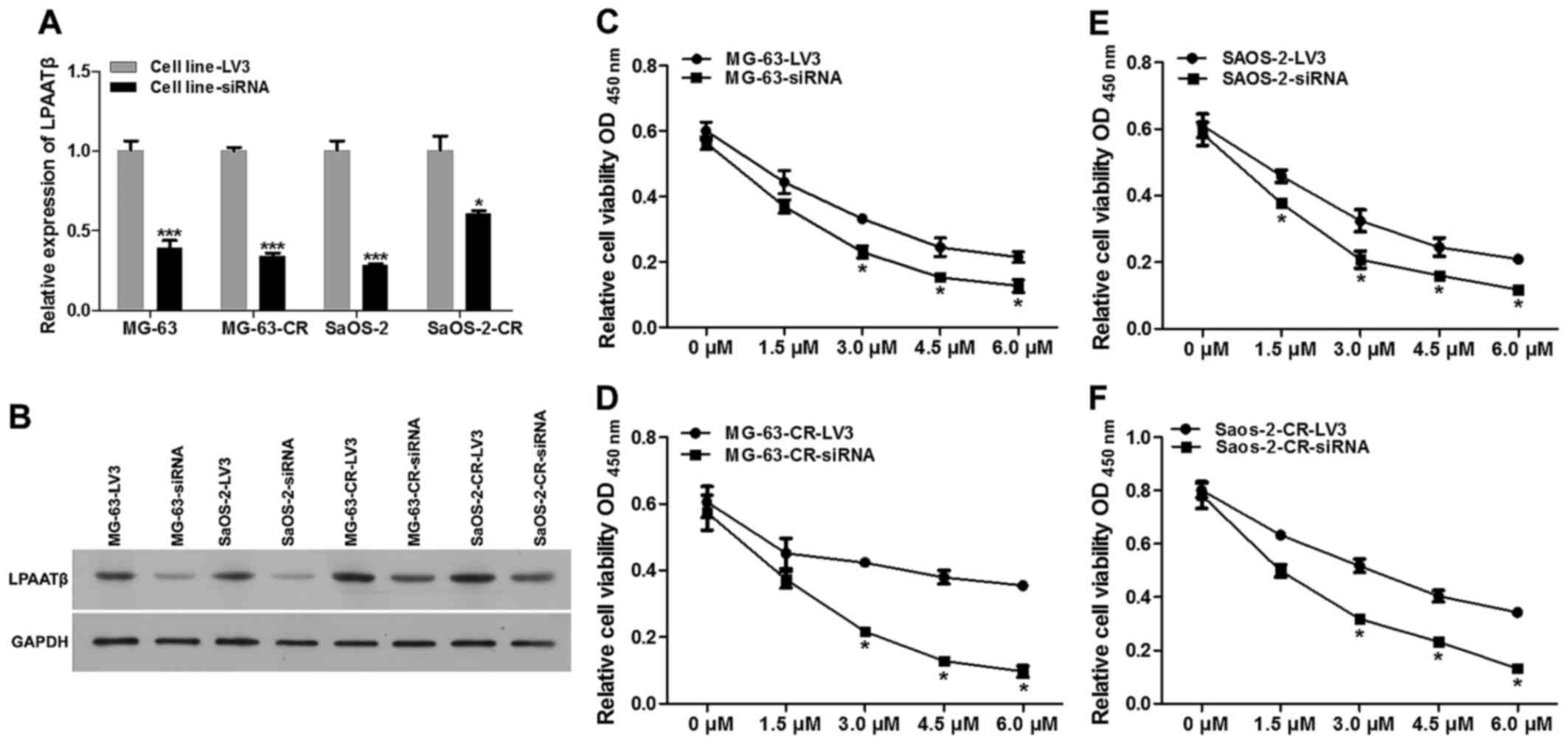
First we validated mRNA and protein levels of LPAATβ
after effective siRNA mediated lentivirus transfection. As shown in
Fig. 3A and B, mRNA level
(Fig. 3A) and protein level
(Fig. 3B) were both significantly
inhibited after siRNA interference, compared to blank vector
group.
To characterize the role of LPAATβ in cisplatin
resistance, two pairs of LPAATβ knockdown cell lines from MG-63 and
SaOS-2 were established, stably transfected with blank vector LV3
or effective LPAATβ siRNA. Cytotoxicity of cisplatin in control and
LPAATβ knockdown pairs (MG-63-LV3 and MG-63-siRNA; MG-63-CR-LV3 and
MG-63-CR-siRNA; SaOS-2-LV3 and SaOS-2-siRNA; SaOS-2-CR-LV3 and
SaOS-2-CR-siRNA) were measured by CCK8 assays. We examined whether
depletion of LPAATβ can re-sensitize cisplatin resistant cells, as
shown in Fig. 3C–F, the
drug-resistant cells depleted of LPAATβ displayed markedly reduced
cisplatin IC50, compared with blank vector trasnfection
groups, which was lower than those of parental cells.
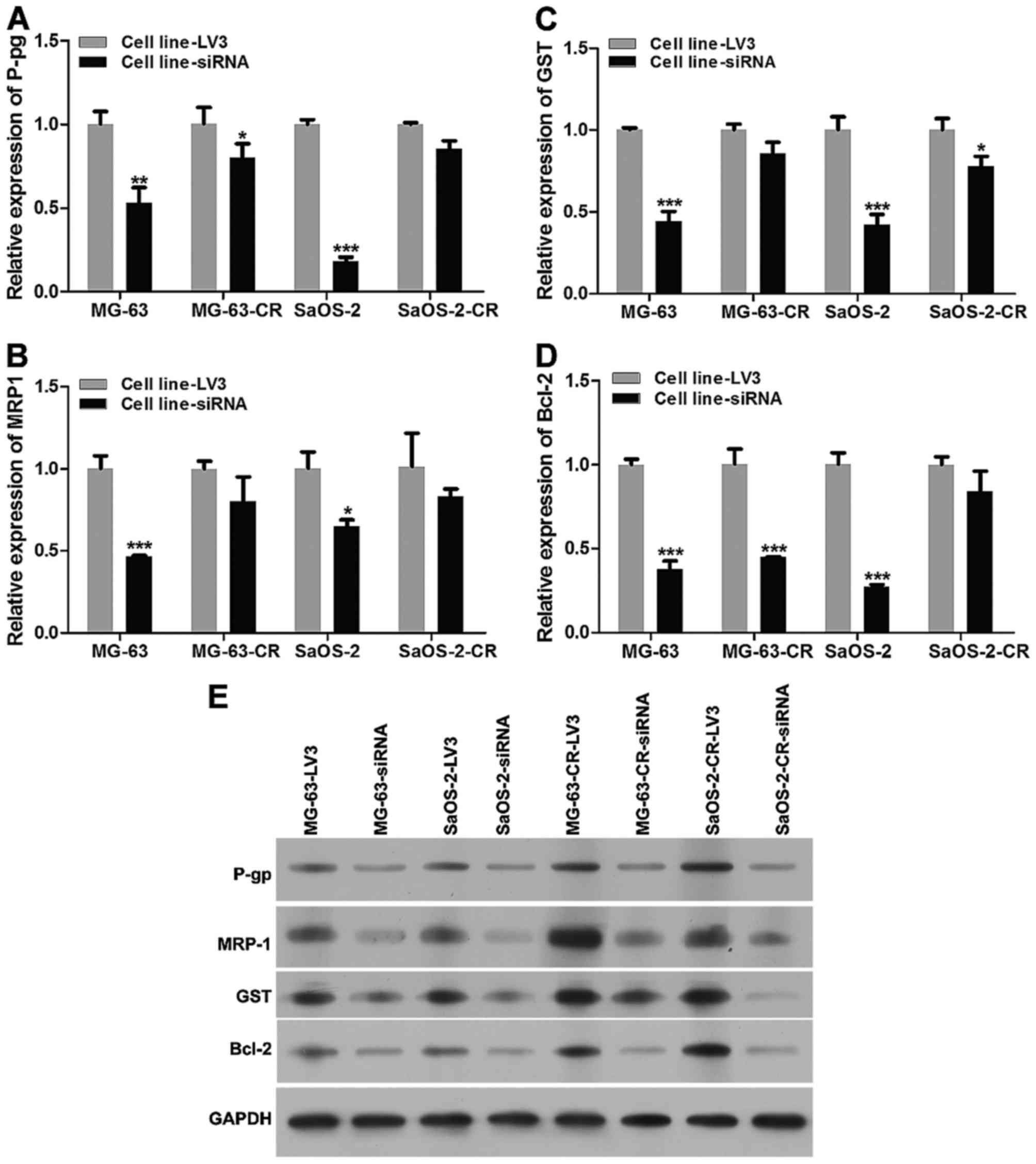
Effect of silencing LPAATβ on expression
of relevant important transporters in cisplatin-resistant cell
lines
We monitored the change of P-gp, MRP1, GST, Bcl-2
expression at mRNA level and protein level after LPAATβ was
silenced (Fig. 4), mRNA levels of
P-gp, MRP1, GST and Bcl-2 in parental and drug-resistant cells were
downregulated (Fig. 4A–D), which
is similar with result of protein level detection (Fig. 4E), compared to blank vector
transfection groups.
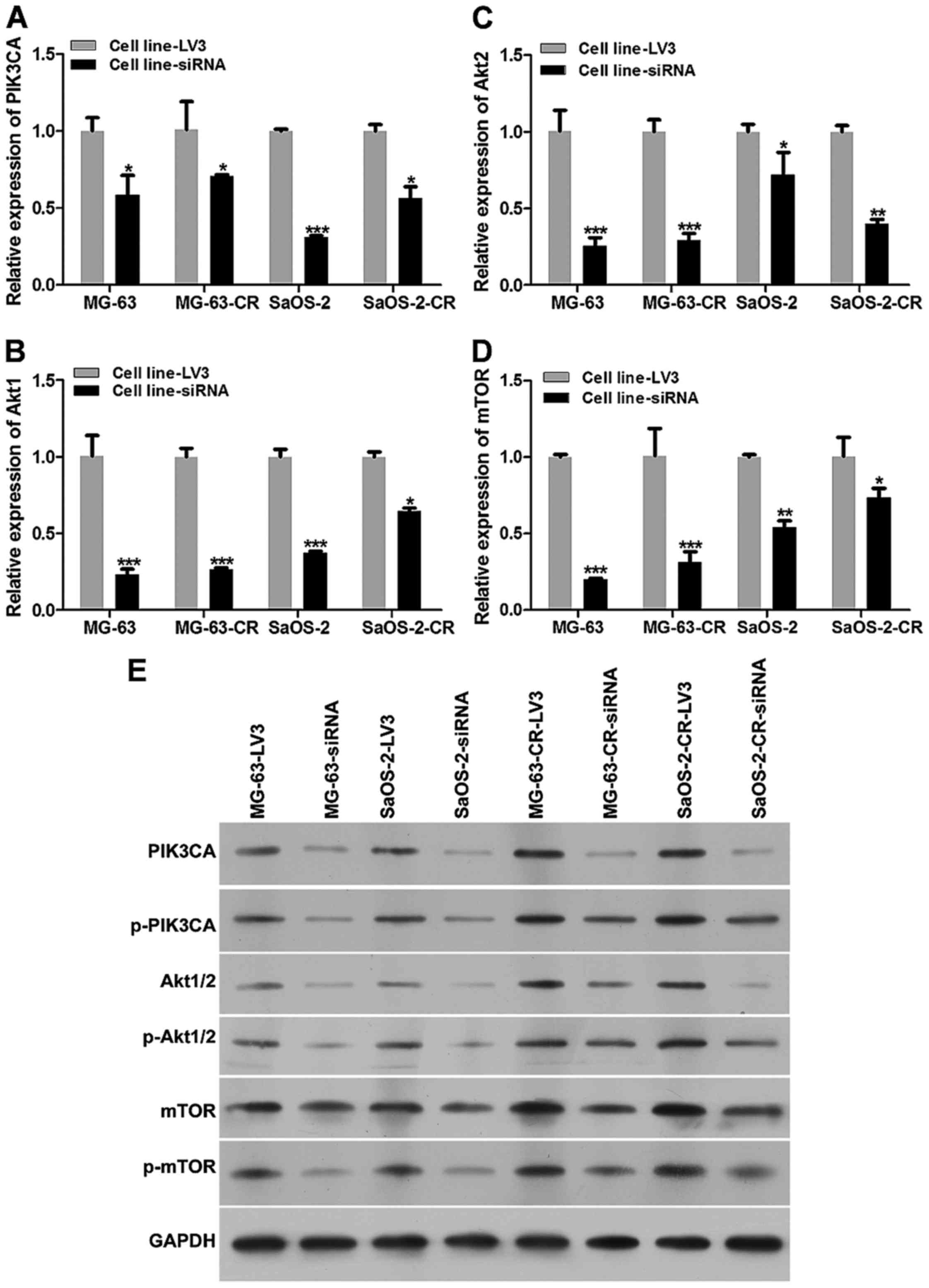
Silencing LPAATβ changed cellular
characteristics through activating PI3K/Akt/mTOR signaling
pathway
We also harvested cells after lentivirus treatment,
and then detected expression of PI3K/Akt/mTOR signaling pathway
relevant proteins (Fig. 5). The
mRNA levels of PIK3CA, Akt1/2 and mTOR in MG-63-CR-siRNA and
SaOS-2-CR-siRNA were downregulated (Fig. 5A–D), compared to those of NC group.
Similar with results of mRNA level detection, protein levels of
PIK3CA, p-PIK3CA, Akt1/2, p-Akt1/2, mTOR and p-mTOR were decreased
significantly (Fig. 5E).
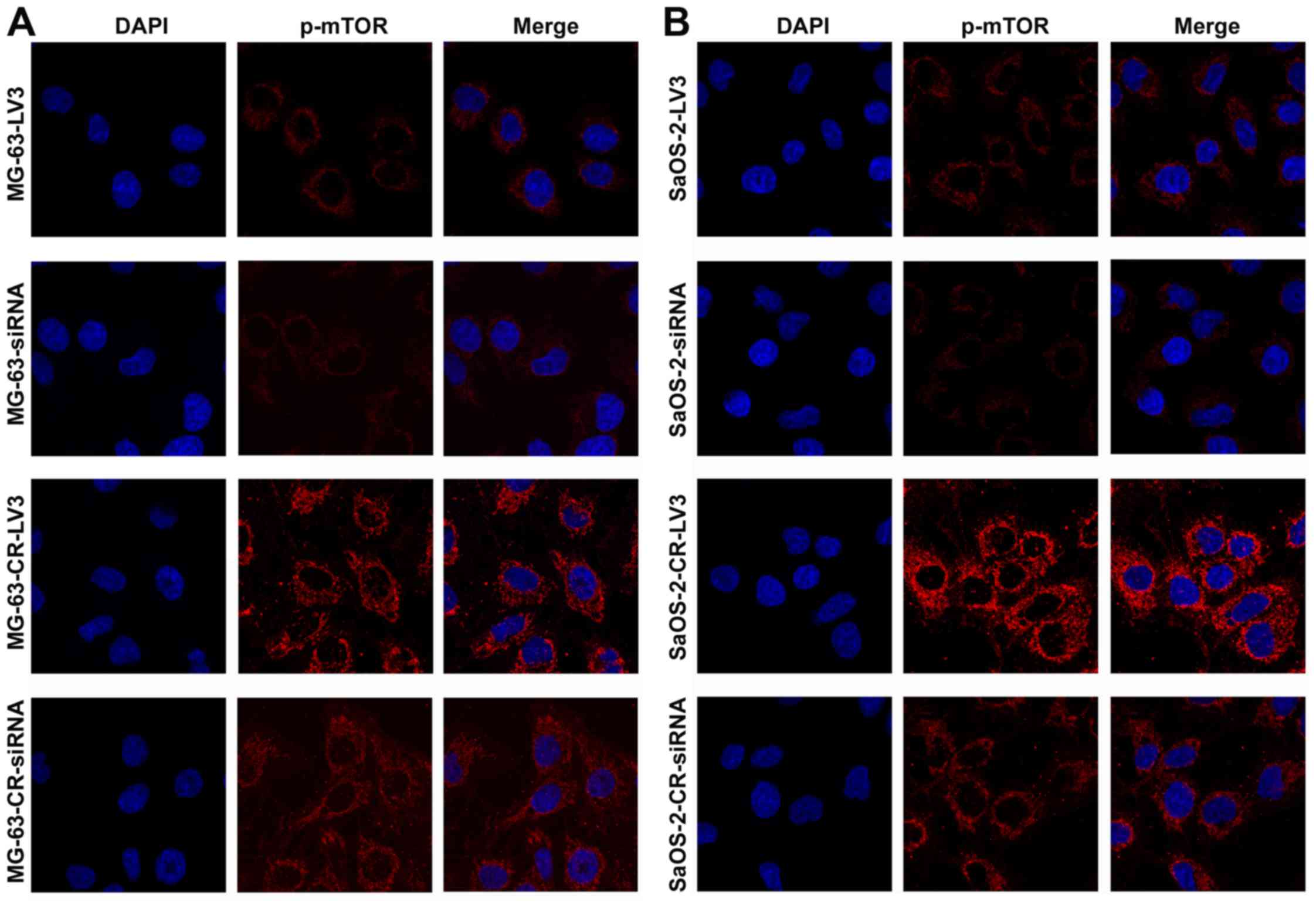
Immunofluorescence results showed that phospho-mTOR
expression at 48 h was increased significantly in MG-63-CR cells
(Fig. 6A) and SaOS-2-CR cells
(Fig. 6B) compared to their
parental cell lines, which were downregulated in MG-63-CR cells
(Fig. 6A) and SaOS-2-CR cells
(Fig. 6B) transfected with siRNA
mediated by lentivirus compared with negative control.
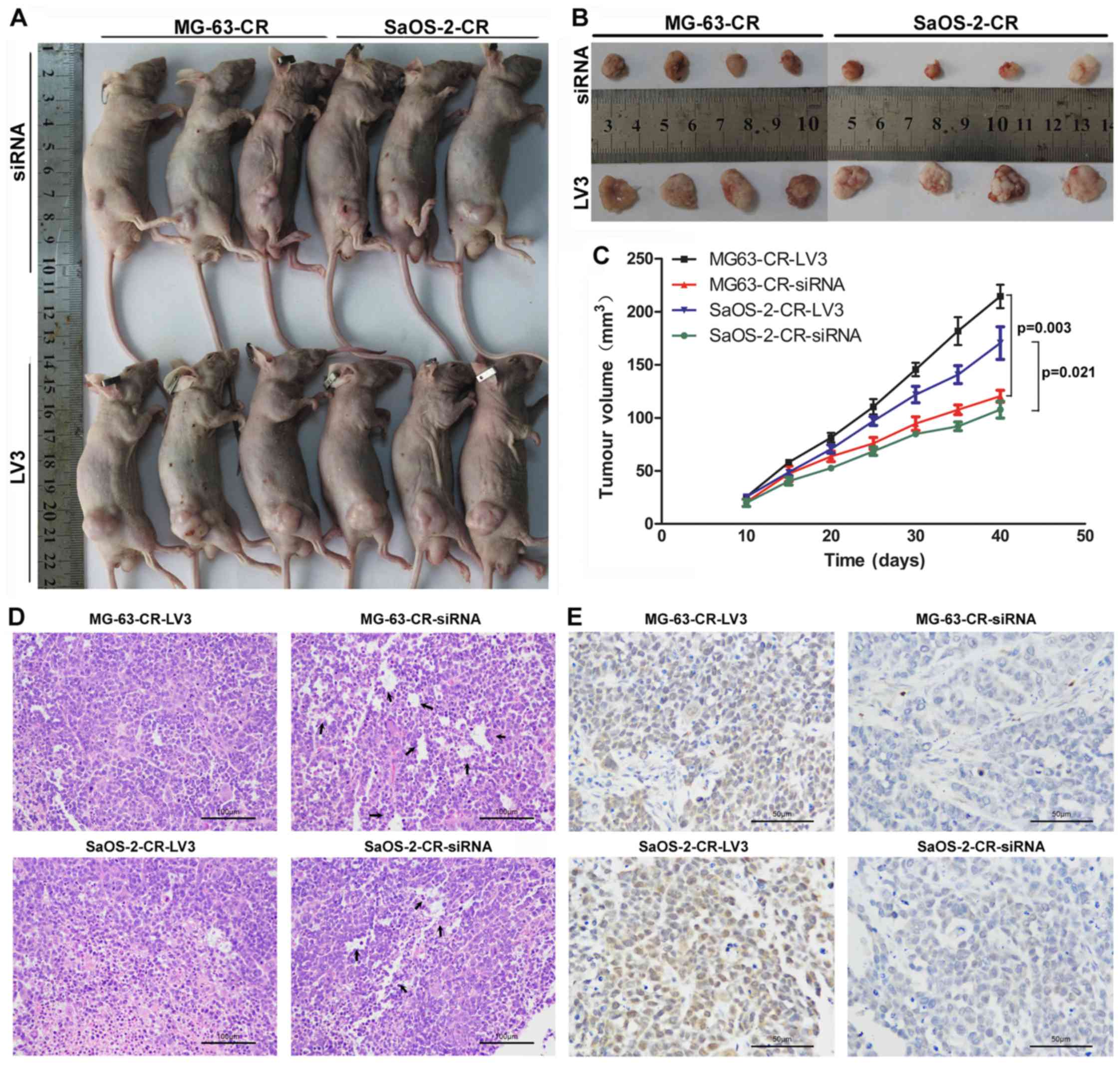
In vivo LPAATβ gene silencing using
lentivirus as a potential strategy for cisplatin resistance
Using a nude mouse model, it was found that mice
with subcutaneous LPAATβ-depleted cell line derived tumors had
smaller tumor burden, with consequent enhanced survival benefit
(Fig. 7A and B). Interestingly, we
monitored the tumor volumn after cells implantation for 40 days and
found that tumor volume in MG-63/SaOS-2-siRNA group was
significantly inhibited with respect of MG-63-LV3/SaOS-2-LV3 group,
based on pair comparison at every time-point (Fig. 7C). Moreover, extensive tubular
necrosis was observed in the nude mice with implantation of drug
re-sensitive cancer cells by lentivirus-mediated siRNA insertion
using histopathological examinations (Fig. 7D). Immunohistochemical detection
displayed phospho-mTOR expression alteration in both
cisplatin-resistant cells and cisplatin re-sensitive cells. As
Fig. 7E shows, phospho-mTOR in
cisplatin-resistant osteosarcoma cells (MG-63-CR-LV3 group and
SaOS-2-CR-LV3 group) was stained brown in cell membrane and nuclear
while in cisplatin re-sensitive cells was very weak (MG-63-CR-siRNA
group and SaOS-2-CR-RNA group).
Discussion
As the most common primary malignancy of bone,
osteosarcoma has complicated occurrence and development which have
not been clarified yet. Although development of surgery combined
with neoadjuvant chemotherapy has significantly improved, the
survival rate of osteosarcoma in the last few decades has
plateaued, which may be heavily influenced by resistance to
chemotherapy drugs (16,17). Therefore, discovery of effective
ways that can increase the sensitivity to chemotherapy will be
important in antitumor effect. In this study, we investigated the
role of LPAATβ in osteosarcoma with cisplatin-resistance scenario.
The results show that LPAATβ had high expression in osteosarcoma
patients who received cisplatin treatment and cisplatin-resistant
MG-63-CR/SaOS-2-CR cells and the IC50 was higher than in
the control groups. Expression of some important relevant proteins
including P-gp, MRP1, GST, Bcl-2 were downregulated after
downregulating LPAATβ expression mediated by lentivirus. Also
silencing LPAATβ triggered the PI3K/Akt/mTOR signaling pathway in
cisplatin-resistant MG-63-CR/SaOS-2-CR cells with lower
IC50 compared to control group. Using the nude mouse
model bearing osteosarcoma tumor xenografts of cisplantin-resistant
osteosarcoma cells, we demonstrated that silencing LPAATβ
effectively inhibited osteosarcoma tumor growth. These results
suggested that LPAATβ may play an important role of lowering the
sensitivity to chemotherapy in regulating cisplatin-resistant
osteosarcoma cell proliferation through activating PI3K/Akt/mTOR
signaling pathway. Thus, our results confirm the role of LPAATβ in
osteosarcoma growth.
It has been shown that inhibition of LPAATβ may play
an important role in regulating osteosarcoma cell proliferation and
tumor growth through catalysing antitumor activity, which usually
results in an arrest of cell signaling pathways and apoptosis
(15,18). Previous studies have indicated that
inhibition of LPAATβ expression via siRNA interfering suppresses
basal Erk phosphorylation, prevents the translocation of Raf to the
plasma Erk phosphorylation and inhibits the activation of proteins
in the phosphoinositide-3-kinase/Akt pathway, including Akt, mTOR,
and S6 kinase (12,19). Therefore, LPAATβ may be considered
and exploited as a novel therapeutic target for osteosarcoma
clinical management, as indicated consistently in this study.
Previous research revealed that aberrant activation
of PI3K/Akt/mTOR signaling pathway usually plays a pivotal role in
malignant transformation and chemoresistance for cancer cells
through regulating multidrug resistance gene 1/P-glycoprotein
(MDR1/P-gp) (20–22). PI3K stimulated by multiple growth
factors contributes to chronic activation of Akt in cancer cells,
downstream of which both activate the mTOR kinase (23,24).
It was reported that VEGF expression in different tumors for
angiogenesis can be regulated by activating PI3K/Akt/mTOR signal
pathway (25). Through negative
regulation of P53, the activated Akt protein induces cisplatin
resistance in ovarian cancer, which could be reversed by PI3K
inhibitor to increase the mitochondrial Bax translocation and cytC
release (26,27). Other studies also demonstrated that
higher mTOR phosphorylation is involved in cisplatin resistance
with strong sensitivity to mTOR inhibitor in vitro, which
was validated in our results (28). Also the activation of the
PI3K/Akt/mTOR signaling pathway was indicated to inhibit
cisplatin-induced apoptosis and improve cisplatin resistance in
cancer cells (28–30).
Our results in vitro indicate that LPAATβ may
increase cisplatin-resistant osteosarcoma cell proliferation and
viability which may be reversed by silencing LPAATβ, with
significant variation of relevant proteins and activation of
PI3K/Akt/mTOR signally pathway. Therefore, we chose a nude mouse
model to further investigate the effect of LPAATβ on tumor growth
and found silencing LPAATβ significantly inhibited the osteosarcoma
tumor growth in vivo after cisplatin-resistant osteosarcoma
cell transplantation. Subsequently, targeting LPAATβ may be
exploited as a novel therapeutic strategy for osteosarcoma clinical
treatment, which is especially attractive given the availability of
selective pharmacological inhibitors.
Acknowledgments
This study was supported by grants from the National
Natural Science Foundation of China (no. 81302347) and the National
Natural Science Foundation of China (no. 81400418).
References
|
1
|
Alcantara MB, Nemazannikova N, Elahy M and
Dass CR: Pigment epithelium-derived factor upregulates collagen I
and downregulates matrix metalloproteinase 2 in osteosarcoma cells,
and colocalises to collagen I and heat shock protein 47 in fetal
and adult bone. J Pharm Pharmacol. 66:1586–1592. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fortunati D, Reppe S, Fjeldheim AK,
Nielsen M, Gautvik VT and Gautvik KM: Periostin is a collagen
associated bone matrix protein regulated by parathyroid hormone.
Matrix Biol. 29:594–601. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ferrari D, Codecà C, Battisti N, Broggio
F, Crepaldi F, Violati M, Bertuzzi C, Dottorini L, Caldiera S,
Luciani A, et al: Multimodality treatment of osteosarcoma of the
jaw: A single institution experience. Med Oncol. 31:1712014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yu W, Tang L, Lin F, Li D, Wang J, Yang Y
and Shen Z: Stereotactic radiosurgery, a potential alternative
treatment for pulmonary metastases from osteosarcoma. Int J Oncol.
44:1091–1098. 2014.PubMed/NCBI
|
|
5
|
Li Z, Zhao L and Wang Q: Overexpression of
long non-coding RNA HOTTIP increases chemoresistance of
osteosarcoma cell by activating the Wnt/β-catenin pathway. Am J
Transl Res. 8:2385–2393. 2016.PubMed/NCBI
|
|
6
|
Yu L, Fan Z, Fang S, Yang J, Gao T, Simões
BM, Eyre R, Guo W and Clarke RB: Cisplatin selects for stem-like
cells in osteosarcoma by activating Notch signaling. Oncotarget.
7:33055–33068. 2016.PubMed/NCBI
|
|
7
|
Dell'Amore A, Asadi N, Caroli G, Dolci G,
Bini A and Stella F: Recurrent primary cardiac osteosarcoma: A case
report and literature review. Gen Thorac Cardiovasc Surg.
62:175–180. 2014. View Article : Google Scholar
|
|
8
|
Hawkins DS and Arndt CA: Pattern of
disease recurrence and prognostic factors in patients with
osteosarcoma treated with contemporary chemotherapy. Cancer.
98:2447–2456. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pagel JM, Laugen C, Bonham L, Hackman RC,
Hockenbery DM, Bhatt R, Hollenback D, Carew H, Singer JW and Press
OW: Induction of apoptosis using inhibitors of lysophosphatidic
acid acyltransferase-beta and anti-CD20 monoclonal antibodies for
treatment of human non-Hodgkin's lymphomas. Clin Cancer Res.
11:4857–4866. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bonham L, Leung DW, White T, Hollenback D,
Klein P, Tulinsky J, Coon M, de Vries P and Singer JW:
Lysophosphatidic acid acyltransferase-beta: A novel target for
induction of tumour cell apoptosis. Expert Opin Ther Targets.
7:643–661. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schmid B, Finnen MJ, Harwood JL and
Jackson SK: Acylation of lysophosphatidylcholine plays a key role
in the response of monocytes to lipopolysaccharide. Eur J Biochem.
270:2782–2788. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Blaskovich MA, Yendluri V, Lawrence HR,
Lawrence NJ, Sebti SM and Springett GM: Lysophosphatidic acid
acyltransferase beta regulates mTOR signaling. PLoS One.
8:e786322013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Douvas MG, Hogan KN, Ji Y, Hollenback D,
Bonham L, Singer JW and Mitchell BS: Effect of lysophosphatidic
acid acyltransferase-beta inhibition in acute leukemia. Leuk Res.
30:1027–1036. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Song L, Yang J, Duan P, Xu J, Luo X, Luo
F, Zhang Z, Hou T, Liu B and Zhou Q: MicroRNA-24 inhibits
osteosarcoma cell proliferation both in vitro and in vivo by
targeting LPAATβ. Arch Biochem Biophys. 535:128–135. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rastegar F, Gao JL, Shenaq D, Luo Q, Shi
Q, Kim SH, Jiang W, Wagner ER, Huang E, Gao Y, et al:
Lysophosphatidic acid acyltransferase β (LPAATβ) promotes the tumor
growth of human osteosarcoma. PLoS One. 5:e141822010. View Article : Google Scholar
|
|
16
|
Huang Z, Huang Y, He H and Ni J:
Podocalyxin promotes cisplatin chemoresistance in osteosarcoma
cells through phosphatidylinositide 3-kinase signaling. Mol Med
Rep. 12:3916–3922. 2015.PubMed/NCBI
|
|
17
|
Ma Y, Ren Y, Han EQ, Li H, Chen D, Jacobs
JJ, Gitelis S, O'Keefe RJ, Konttinen YT, Yin G, et al: Inhibition
of the Wnt-β-catenin and Notch signaling pathways sensitizes
osteosarcoma cells to chemotherapy. Biochem Biophys Res Commun.
431:274–279. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Körbes AP, Kulcheski FR, Margis R,
Margis-Pinheiro M and Turchetto-Zolet AC: Molecular evolution of
the lysophosphatidic acid acyltransferase (LPAAT) gene family. Mol
Phylogenet Evol. 96:55–69. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Coon M, Ball A, Pound J, Ap S, Hollenback
D, White T, Tulinsky J, Bonham L, Morrison DK, Finney R, et al:
Inhibition of lysophosphatidic acid acyltransferase beta disrupts
proliferative and survival signals in normal cells and induces
apoptosis of tumor cells. Mol Cancer Ther. 2:1067–1078.
2003.PubMed/NCBI
|
|
20
|
Ma Q, Chang Z, Wang W and Wang B:
Rapamycin-mediated mTOR inhibition reverses drug resistance to
adriamycin in colon cancer cells. Hepatogastroenterology.
62:880–886. 2015.
|
|
21
|
Marklein D, Graab U, Naumann I, Yan T,
Ridzewski R, Nitzki F, Rosenberger A, Dittmann K, Wienands J,
Wojnowski L, et al: PI3K inhibition enhances doxorubicin-induced
apoptosis in sarcoma cells. PLoS One. 7:e528982012. View Article : Google Scholar
|
|
22
|
Randle RA, Raguz S, Higgins CF and Yagüe
E: Role of the highly structured 5′-end region of MDR1 mRNA in
P-glycoprotein expression. Biochem J. 406:445–455. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang SF, Chou YC, Mazumder N, Kao FJ, Nagy
LD, Guengerich FP, Huang C, Lee HC, Lai PS and Ueng YF:
7-Ketocholesterol induces P-glycoprotein through PI3K/mTOR
signaling in hepatoma cells. Biochem Pharmacol. 86:548–560. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Markova B, Albers C, Breitenbuecher F,
Melo JV, Brümmendorf TH, Heidel F, Lipka D, Duyster J, Huber C and
Fischer T: Novel pathway in Bcr-Abl signal transduction involves
Akt-independent, PLC-gamma1-driven activation of mTOR/p70S6-kinase
pathway. Oncogene. 29:739–751. 2010. View Article : Google Scholar
|
|
25
|
Tsai JL, Lee YM, Pan CY and Lee AY: The
novel VEGF121-VEGF165 fusion attenuates angiogenesis and drug
resistance via targeting VEGFR2-HIF-1α-VEGF165/Lon signaling
through PI3K-AKT-mTOR pathway. Curr Cancer Drug Targets.
16:275–286. 2016. View Article : Google Scholar
|
|
26
|
Zhao Z, Wang J, Tang J, Liu X, Zhong Q,
Wang F, Hu W, Yuan Z, Nie C and Wei Y: JNK-and Akt-mediated Puma
expression in the apoptosis of cisplatin-resistant ovarian cancer
cells. Biochem J. 444:291–301. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fraser M, Leung BM, Yan X, Dan HC, Cheng
JQ and Tsang BK: p53 is a determinant of X-linked inhibitor of
apoptosis protein/Akt-mediated chemoresistance in human ovarian
cancer cells. Cancer Res. 63:7081–7088. 2003.PubMed/NCBI
|
|
28
|
Yuge K, Kikuchi E, Hagiwara M, Yasumizu Y,
Tanaka N, Kosaka T, Miyajima A and Oya M: Nicotine induces tumor
growth and chemoresistance through activation of the PI3K/Akt/mTOR
pathway in bladder cancer. Mol Cancer Ther. 14:2112–2120. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gan Y, Wang Y, Tan Z, Zhou J, Kitazawa R,
Jiang X, Tang Y and Yang J: TDRG1 regulates chemosensitivity of
seminoma TCam-2 cells to cisplatin via PI3K/Akt/mTOR signaling
pathway and mitochondria-mediated apoptotic pathway. Cancer Biol
Ther. 17:741–750. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cai Y, Tan X, Liu J, Shen Y, Wu D, Ren M,
Huang P and Yu D: Inhibition of PI3K/Akt/mTOR signaling pathway
enhances the sensitivity of the SKOV3/DDP ovarian cancer cell line
to cisplatin in vitro. Chin J Cancer Res. 26:564–572.
2014.PubMed/NCBI
|