Introduction
Gastric cancer (GC) is considered to be one of the
most common grave alignancies with high mortality rate worldwide,
and particularly in China (1).
This is due to its high drug resistance along with the
unavailability of proficient diagnostic tools (2). In the past few decades, great efforts
were made to explore cogent therapeutic approaches for GC including
chemotherapy (3) as well as
immunotherapy (4). Natural drugs
for example, hispolon, lentinan, and grifolin derived from
different mushrooms showcased a highly therapeutic effect to curb
GC. Of great interest is that these natural compounds do not
possess any side effects on bone marrow, blood haemoglobin, and
immune system in respect of traditional therapeutic methods
(5–7).
Cordycepin an adenosine analogue, extracted from
Cordyceps militaris mushroom has gained attention with its
anti-inflammatory, antioxidant, and antibacterial features
(8,9). Currently, tumor genesis of cordycepin
has been presented in many types of cancers. For example,
cordycepin integrates inhibition of the proliferation of lung and
renal cancer cells through mediating their cell death due to
apoptosis (10,11). Apoptosis (programmed cell death) is
a stern mechanism of physiological cell death, that keeps up cell
proliferation and homoeostasis in tissue (12). Furthermore, the processes through
which cordycepin mediated apoptosis was presented to be by
mitochondrial extrinsic pathways (13). The mitochondrial extrinsic
apoptosis is monitored by death receptors like DR3 that, once
activated, trigger activation of caspase-8 that proceeds with
mediating the activation of caspase-3 as well as cleaved PARP
(14). Death receptors have a
direct apoptotic pathway, transfer apoptosis signals (from death
receptor) to death ligands, and consequently play an important role
in instructive apoptosis (15).
These receptors can activate death caspases within seconds of
ligand binding, causing an apoptotic demise of the cell within
hours (15). Generally, DR3
regulate inflammation and autoimmune diseases: experimental
autoimmune uveoretinitis, allergic lung inflammation and
inflammatory arthritis (16,17).
Cordycepin was used to treated gastric cancer EBV-positive (SNU-791
cell line), without affecting their cell proliferation or cell
cycle arrest (18). Interestingly,
the combination of cordycepin with doxorubicin enhanced gastric
cancer (AGS cell line) EBV-positive cell proliferation notably by
p38 MAPK signalig pathways (19).
Moreover, cordycepin also mediated apoptosis with the help of
mitochondrial intrinsic pathways by inhibiting PI3K/Akt signaling
pathway and generating ROS (20,21).
PI3K/Akt plays a potential role in several cancer
cells by regulating cell proliferation, survival and metabolism. It
is reported that phosphorylarylation of PI3K/Akt leads to
inhibition of gastric cancer cell proliferation (22). In the same manner, activation of
Akt results into inhibition of MDM2 and generation of p53,
incorporating gastric cancer cell proliferation arrest (23). p53 also induces upregulation of the
Bax and downregulation of Bcl-2 during p53-related apoptosis
(24). In addition, the inhibition
of Akt is also linked to the production of ROS in SGC-7901 cells
(25). ROS is a product of oxygen
metabolism following cellular stress. In accordance with its
generation, ROS can either possess positive or negative influence
on cells. Many cancer cells generate a moderate level of ROS to
sustain their proliferation, migration and proliferation.
Furthermore, most of the gastric cancer cells pose resistance to
chemotherapy drugs quite clearly due to their moderate production
of ROS. As a result, extreme levels of ROS bring forth adverse
environment thereby promoting cells death by apoptosis (26,27).
Although, previous studies underline the mechanism
by which cordycepin regulates many cancers, its mechanism in
gastric cancer is still unclear. We performeded an in vitro
study to observe the anti-proliferative result of cordycepin,
Cordyceps militaris, mushroom, on the gastric cancer
SGC-7901 cells. SGC-7901 cells (EBU_) are frequently investigated
representative gastric cancer cell line. Aim of this study was to
demonstrate the effect of cordycepin on cancer cells and the
molecular pathway by which it induces apoptosis and prevent cell
survival signal in human gastric cancer SGC-7901 cells. We also
verified the role of cordycepin as ROS inducer promoting selective
killing of cancer cells.
Materials and methods
Chemicals and reagents
Cordycepin was purchased from Chengdu Pufei De
Biotech Co., Ltd. (China). Fetal bovine serums was purchased from
Hangzhou Sijiqing Biological Engineering Materials Co. (China).
Dulbecco's modified Eagle's medium (DMEM) were purchased from
(Gibco, China), 3–4,5-dimethylthiazol-2-yl-2,5-diphenyltetrazolium
bromide (MTT), and dimethyl sulfoxide (DMSO) were from Sigma
Chemical Co. (St. Louis, MO, USA). Propidium iodide (PI), Annexin
V-FITC Apoptosis Detection kit, rhodamine 123 and reactive oxygen
species kit were from Beyotime Institute of Biotechnology
(Shanghai, China). Rabbit polyclonal anti-human Bcl-2, anti-human
Bax, P-PI3K, Akt, P-Akt, caspase-8, antibodies were purchased from
Cell Signaling Technology (Beverly, MA, USA). Mouse, anti-rabbit,
caspase-3 p-JAK2 (catalog no. bs-2485R), p-Stat3 (catalog no.
bs-1658R) were purchased from Bioss (Beijing Biosynthesis
Biotechnology), p53, MDM2, survivin, Cdk2, Cdk1, and cyclin E
antibodies were purchased from Santa Cruz Biotechnology, DR3,
A3AR antibodies were purchased from Kanghexin Biotech
Co. (Suzhou, China).
Ethics/guidelines
We followed the ethical classification standards and
our laboratory guidelines; SGC-7901 (gastric cancer) cell line was
purchased from ATCC (Shanghai, China).
Cell culture
SGC-7901 cells were seeded and cultured in 10-cm
dish with DMEM medium contained 10% FBS (Gibco), then incubated in
an incubator (humidified 37°C, the atmosphere of 5%
CO2). Cells were allowed to grow to 70–80% before
used.
Determination of cell cytotoxicity by
MTT
SGC-7901 cells were seed and cultured in a 96-well
plate to a final concentration of 5×103 with DMEM medium
with 1% FBS then incubated at 37°C for 18–24 h. After all, cells
were treated with the appropriated concentration of cordycepin for
24 h. Afterward, the cell medium was discarded, and fresh medium
was added to each well, with 20 µl MTT solutions (5 mg/ml),
and then incubated at 37°C for 4 h. Finally, 150 µl of DMSO
was added to each well, incubated in the dark for 10 min, then read
at the wavelength of 570 nm using Varioskan Flash Multimode Reader
(Thermo Scientific, USA). The absorbance was easured at 570 nm, and
results were expressed as a percentage, relative to solvent-control
incubations, and the IC50 were determined using
non-linear regression analysis (percentage survival versus
concentration).
Annexin V/PI assays for apoptosis
For Annexin V/PI assays, SCG-7901 cells were stained
with Annexin V-FITC and PI, and evaluated for apoptosis by flow
cytometry according to the manufacturer's protocol (Beyotime,
China). After treatment with 0, 20, 40 and 80 µM of
cordycepin, and incubation at 37°C for 24 h, SGC-7901 cells were
collect and washed twice with PBS, then stained with 5 µl of
Annexin V-FITC and 10 µl of PI in 500 µl binding
buffer for 15 min at room temperature in the dark. The apoptotic
cells were determined using flow cytometry, and the data were
analysed using Cellquest analysis software.
DAPI (4′,6-diamidino-2-phenylindole)
staining
SGC-7901 cells were cultured in 12-well plates.
After 24 h, cells were treated with cordycepin for 24 h, and then
fixed in 4% cold paraformaldehyde (PFA) for 30 min. Subsequently,
SGC-7901 cells were incubated with high DAPI (1 mg/ml) for 30 min
in the dark; then, the cells were washed with PBS. Apoptotic nuclei
characterized as intensively stained were detected using
fluorescent microscopy (model IX71; Olympus, Tokyo, Japan).
Cell cycle analysis
The cell cycle distribution in different phases
after exposure of cordycepin were analysed by flow cytometry. In
brief, SGC-7901 cells were harvested and washed with PBS after
exposure to 0, 20, 40 and 80 µM of cordycepin for 24 h.
Subsequently, the cells were fixed with 70% of ethanol at −20°C for
2 h and then stained with PI solution consisting of 1 mg/ml PI and
RNase A. The fluorescence-activated cells were analysed by the flow
cytometry, and the data were analyzed using Cellquest analysis
software.
Determination of intracellular reactive
oxygen species (ROS) production
The ROS generation was measured after staining the
cells with DCFH-DA and flow cytometry. Briefly, after exposure of
different concentrations of cordycepin, SGC-7901 stained by 1 ml of
PBS containing 10 µM DCFH-DA, and incubated for 30 min at
37°C. The fluorescence emission from DCF was analysed via Cellquest
analysis software flow cytometry (Cytomics FC 500; Beckman Coulter
Inc., Miami, FL, USA), with excitation and emission spectra set at
480 and 530 nm, respectively.
Determination of mitochondrial
trans-membrane potential (MMP)
The MMP was measured using a flow cytometry
(Cytomics FC 500; Beckman Coulter Inc.), and the fluorescent dye
rhodamine 123. In brief SGC-7901 cells were cultured in 12-well
plates, after treatment with cordycepin cells were harvested, and
washed with PBS. Then stained with Rh123 (100 µg/l) for 30
min at 37°C. The cells were collected by pipetting and washed twice
with PBS and analysed by flow cytometry.
Western blot analysis
SGC-7901 cells were treated with 0, 20, 40 and 80
µM cordycepin in DMEM medium with 1% FBS incubated for 24 h.
The cells were harvested and collected on ice-cold PBS; further
RIPA containing proteinase inhibitor cocktail was added in cells,
incubated on ice for 30 min. Afterward, insoluble protein lysate
was removed by centrifugation at 1,350 rpm for 15 min at 4°C. The
protein concentrations were measured by using NanoDrop 1000 (Thermo
Scientific) spectrophotometer, 70 µg of proteins were
resolved on 10–12% SDS-PAGE and transferred to PVDF membranes.
After blocking with 5% (w/v) non-fat milk and washing with a
Tris-buffered saline-Tween solution (TBST), membranes were
incubated with respective primary antibodies at 4°C overnight and
washed three times with TBST. The blots were then incubated with
anti-rabbit or anti-mouse horseradish peroxidase conjugated
secondary antibodies for 1 h at room temperature. Finally membrane
was washed again with TBST three times; signals were detected using
ECL plus chemiluminescence kit on X-ray film (Millipore Corp.,
Billerica, USA) (28).
Statistical analysis
Statistical analysis was performed using Origin lab
8. Each experiment was repeated at least three times. All data are
presented as the mean ± standard deviation (SD). Statistical
significance was evaluated using one-way analysis of variance
(ANOVA). Differences were considered to be statistically
significant at P<0.05.
Results
Cytotoxicity of cordycepin
MTT assay was used to assess the cell viability of
SGC-7901 cells in the presence of several concentrations of
cordycepin (Fig. 1A) ranging from
0 to 100 µM. The growth inhibition improved consistently
with time over the period of the incubation time. Specifically, the
estimated half-maximal inhibitory concentration (IC50)
values were 40, 32 and 7 µM after 24, 48 and 72 h,
correspondingly (Fig. 1B). The
cytotoxicity of cordycepin was further observed after the SGC-7901
cells were treated with 0, 20, 40 and 80 µM cordycepin. As
seen in Fig. 1C cordycepin
inhibited SGC-7901 cell proliferation and cells death rate
increased with incensement of cordycepin concentration.
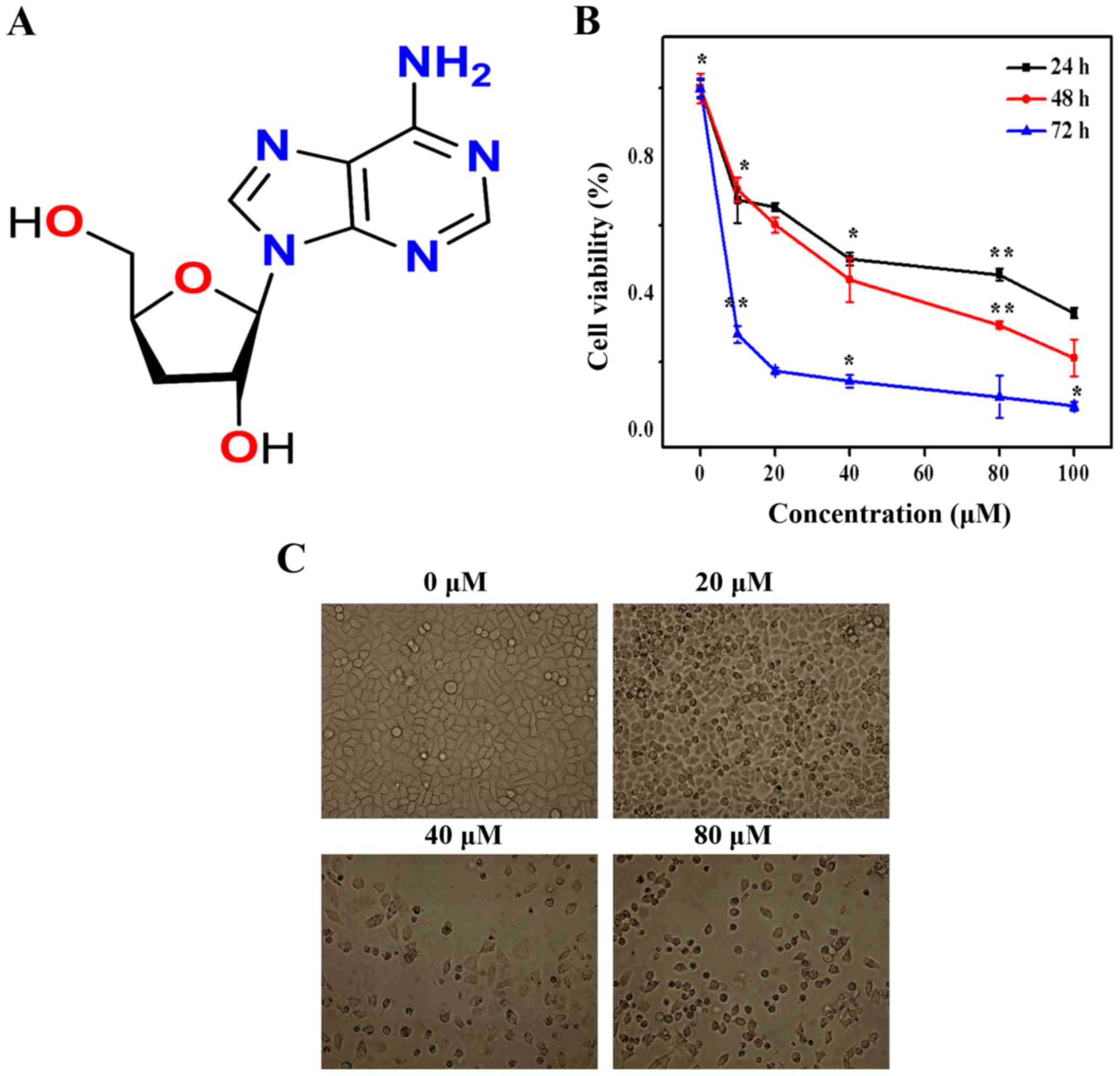 | Figure 1Effect of cordycepin on SGC-7901 cell
morphology and viability. (A) Structure of cordycepin. (B) SGC-7901
cells were treated with 0, 10, 20, 40, 80 and 100 µM,
Proliferation was assessed after 24, 48, and 72 h, as described in
Materials and methods. Each bar represents the mean ± standard
deviation of three experiments. (C) Morphological changes of
SGC-7901 cells were observed by phase-contrast microscopy, control
(without cordycepin treatment) and with 20, 40 and 80 µM of
cordycepin for 24 h. |
Cordycepin induces apoptosis in SGC-7901
cells
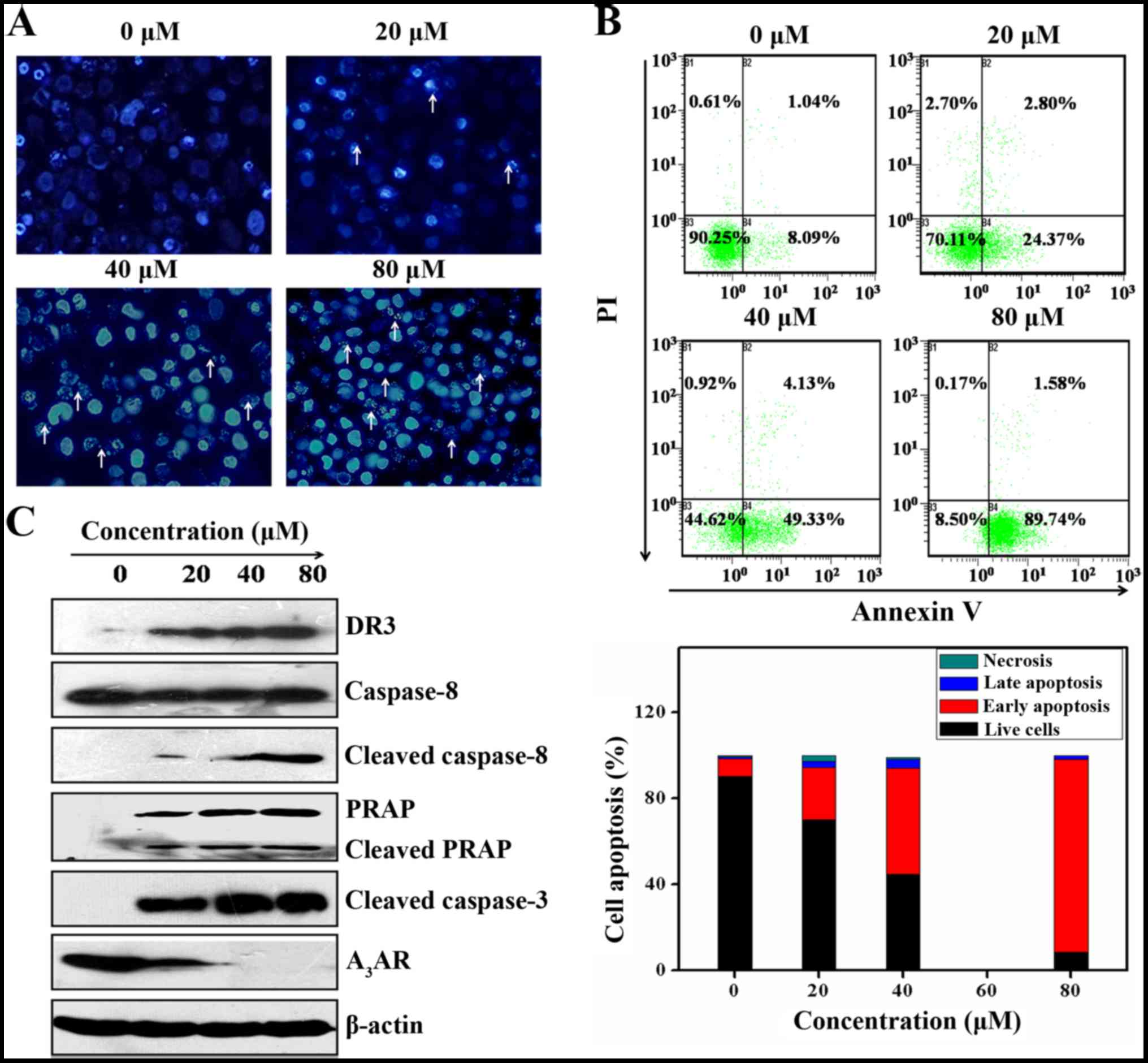
Loss of membrane plasma and DNA fragmentation are
the key cha racteristics possessed by apoptotic cell death. The
impact of cordycepin on SGC-7901 cells death was evaluated by DNA
fragmentation with the help of DAPI staining and fluorescent
microscopy. As seen in Fig. 2A
cordycepin incorporates the shape of the SGC-7901 cells modified to
a considerable extent via increasing dose-dependently.
Particularly, cordycepin broke the cell membranes leading to
inducing the nuclear condensation by apoptotic in comparison with
the control cells. Induction of apoptosis was further validated by
Annexin V/PI assay. This is based on the probe of the initial
apoptosis (B4), late apoptosis (B2), and necrosis (B1) of SGC-7901
cells. The results indicated that the B4 values increased
8.091±1.435, 24.37±1.829, 49.33±1,492 and 89.74±2.714% by utilizing
0, 20, 40 and 80 µM of cordycepin, correspondingly (Fig. 2B).
Additionally, to evaluate whether cordycepin-induced
apoptosis was dependent upon mitochondrial pathways, western blot
analysis was carried out to monitor protein expression of
mitochondrial extrinsic apoptosis. As shown in Fig. 2C, treatment of SGC-7901 cells by
cordycepin, induces activation of death receptor DR3. Indeed, in
the absence of cordycepin DR3 protein expression is null while
increased together with the cordycepin concentration. This
activation of DR3 encouraging activation of caspase-8, that
functions as an initiator of caspase-3, further enhancing cleavage
of PARP consequently inducing SGC-7901 cell death by apoptosis
(14).
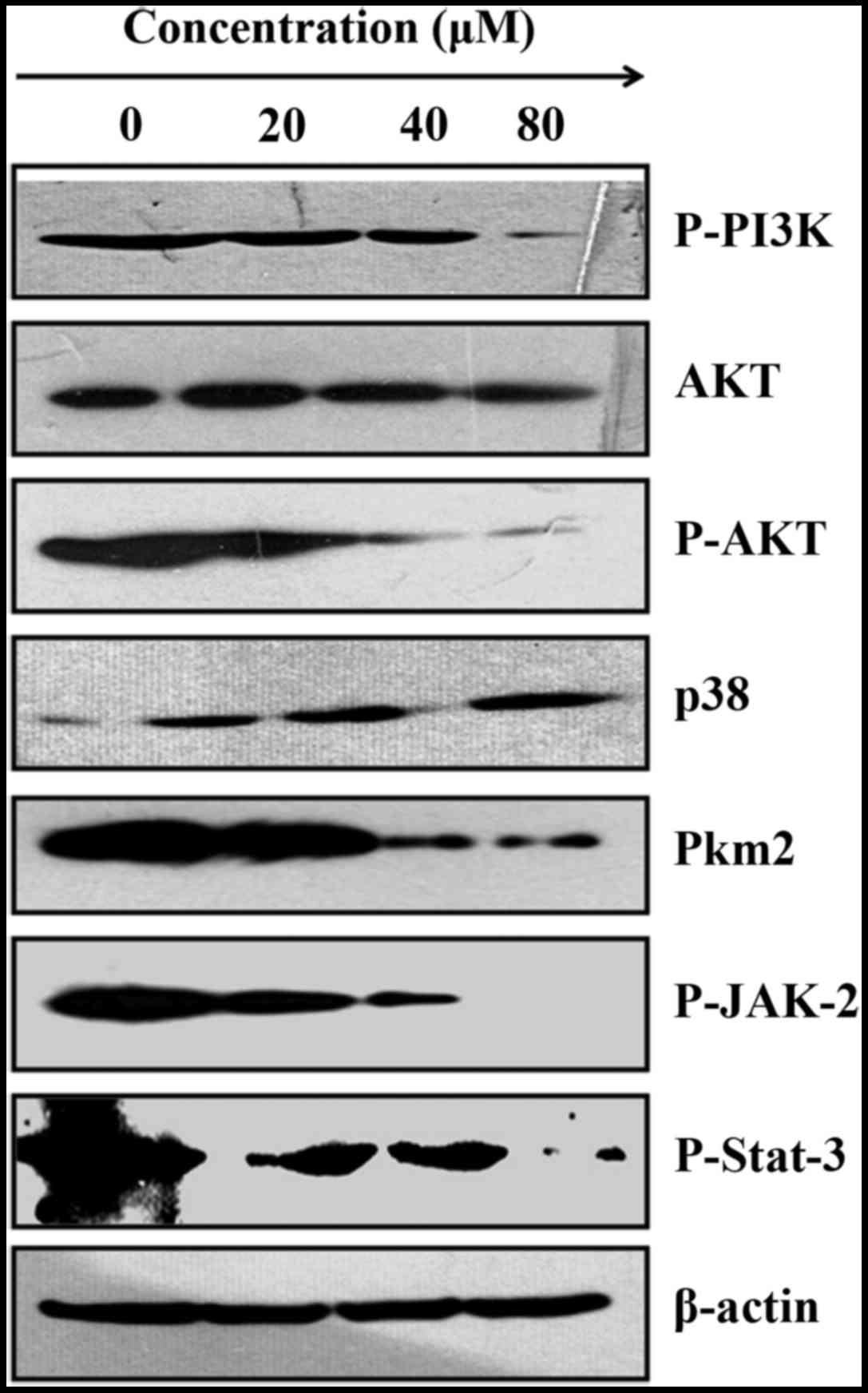
Cordycepin induces SGC-7901 cell
apoptosis by inhibiting PI3K/AKT Pkm2, and increases, p38 in
SGC-7901 cells
The phosphatidylinositol 3-kinase/Akt pathways are
engaged in the SGC-7901 tumor expansion and its metastasis.
Especially, the inhibition of Akt results in disturbance of the
biological activities of SGC-7901 cells by mediating their cell
cycle arrest (29). A previous
report, supported that the activation of DR3 in colon cancer cells,
leads to activation of PI3K inducing cell apoptosis (30). We therefore, evaluated whether the
activation of DR3 in SGC-7901 cells was associated with activation
of PI3K/Akt signaling. As presented in Fig. 3, cordycepin inhibits the expression
level of PI3K, considerably decreasing P-Akt protein expression. At
the same time, cordycepin boosts the expression of p38 whereas Akt
remains almost the same. These findings show that cordycepin is
likely to induce SGC-7901 death by mediating their cells cycle
arrest, and this through inhibition of AKT and increased p38.
PKM2 is a key enzyme that regulates aerobic
glycolysis in tumor cells and particularly in gastric cancer. Its
inhibition was report to affect SGC-7901 cells growth (31). We therefore, investigated the
expression of PKM2 in SGC-7901 cells. As shown in Fig. 5, cordycepin suppressed PKM2
expression. We can therefore suggest that this inhibition of PKM2
by cordycepin contributed to the induction apoptosis in SGC-7901
cells.
Cordycepin induces SGC-7901 cell
apoptosis by inhibition of A3AR
A3AR is a normal purine metabolite
extensively expressed in most cancer cells. Its inhibition is
involved in inhibiting the proliferation and induction of cell
death by apoptosis (32). To gauge
whether the apoptosis mediated by cordycepin possesses the capacity
to influence A3AR expression in SGC-7901 cells western
blot analysis was carried out and results showed that cordycepin
inhibited drastically the protein expression of A3AR
expression (Fig. 2C). Based on
this we suggest that inhibition of A3AR is likely to
play a role in the induction of apoptosis mediated by cordycepin on
SGC-7901 cells.
Cordycepin exerts anti-inflammatory
activity in SGC-7910 cells by phosphorylated STAT-3/JAK2
The Jak-Stat cascade proteins are essential for
inflammatory as well as immune responses of anticancer agents
(33). The impacts of cordycepin
on the Jak-Stat protein expression was probed with the help of
western blot analysis. The findings brought to light that
cordycepin improved the phosphorylation of Jak2 and Stat3 proteins
in SGC-7901 cells (Fig. 3). This
is clearly due to the potential of cordycepin to translocate Stat3
and Jak2 from the cytoplasm into the nucleus leading to the
initiation of the gene expression of pro-inflammatory response.
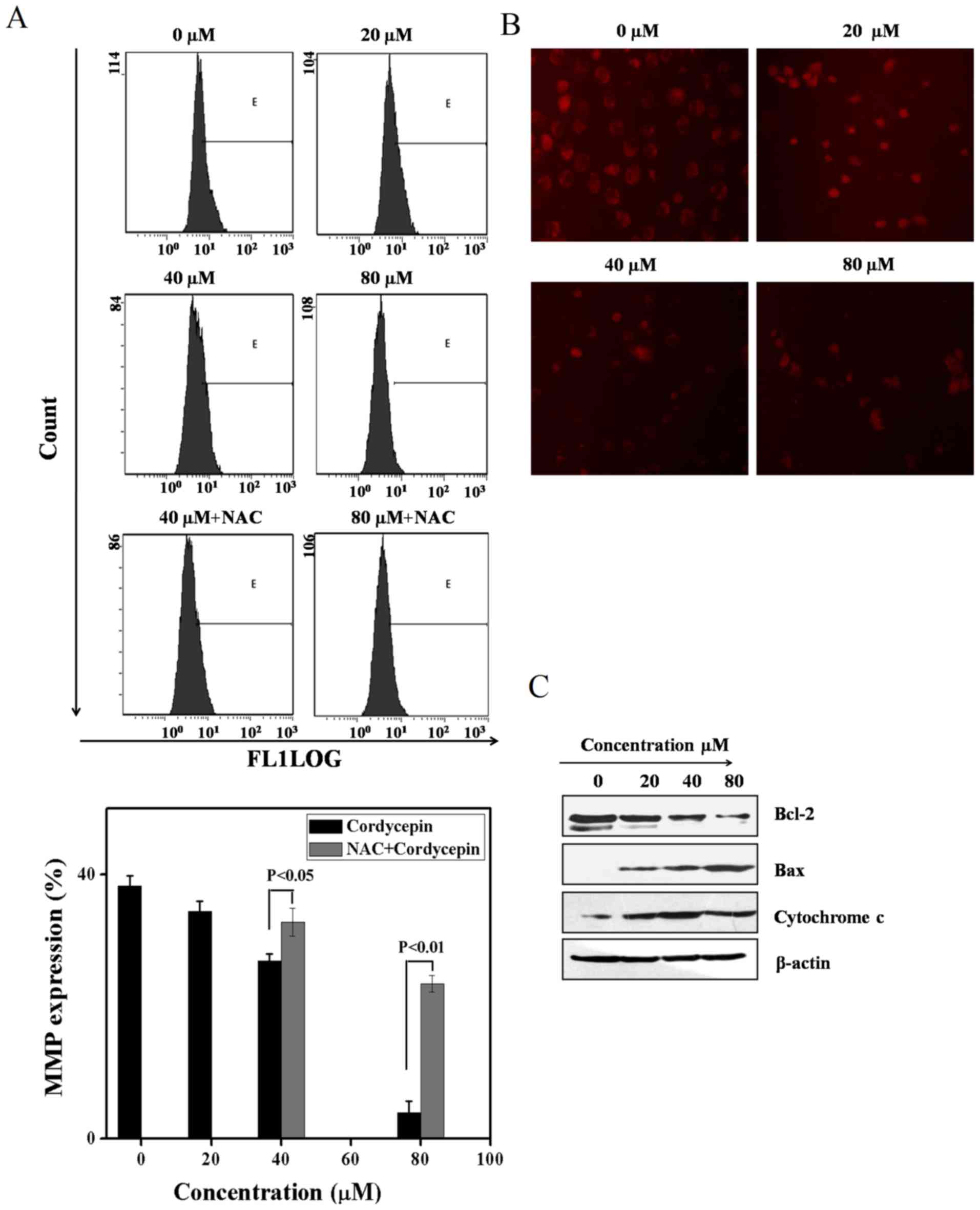
Cordycepin induces apoptosis in SGC-7901
cells by collapse of mitochondrial membrane potential and
generation of reactive oxygen species
To validate whether mitochondrial events were
involved in induction of apoptosis, flow cytometry of rhodamine 123
staining and western blot analysis was carried out. As depicted in
Fig. 4A, during the unavailability
of NAC, the mitochondrial membrane potential expression is
minimized to a significant extent. On the other hand, the
mitochondrial membrane potential expression was restored in the
availability of NAC upon utilization of cordycepin (0, 20, 40 and
80 µM) for 24 h, recommending that mitochondrial were taking
part in cordycepin induced SGC-7901 cell apoptosis. To confirm our
findings immunoflorescence of Rhodamine were further processed. As
seen in Fig. 4B, cordycepin induce
SC-7901 cells morphology changes due to the lost of mitochondrial
membrane metabolite resulting in their collapse.
Moreover, to develop more understanding of the
process by which cordycepin decreased mitochondrial membrane
potential, western blot analysis is carried out for the purpose of
validating the level of cytochrome c, Bax, and Bcl-2 to
obtain more insight into cell apoptosis. The findings suggest that
cordycepin resulted in release of cytochrome c from the
mitochondrial membrane which is the major factor in the development
of apoptosomes, which trigger the activation of Bax and deactivates
Bcl-2 (Fig. 4C).
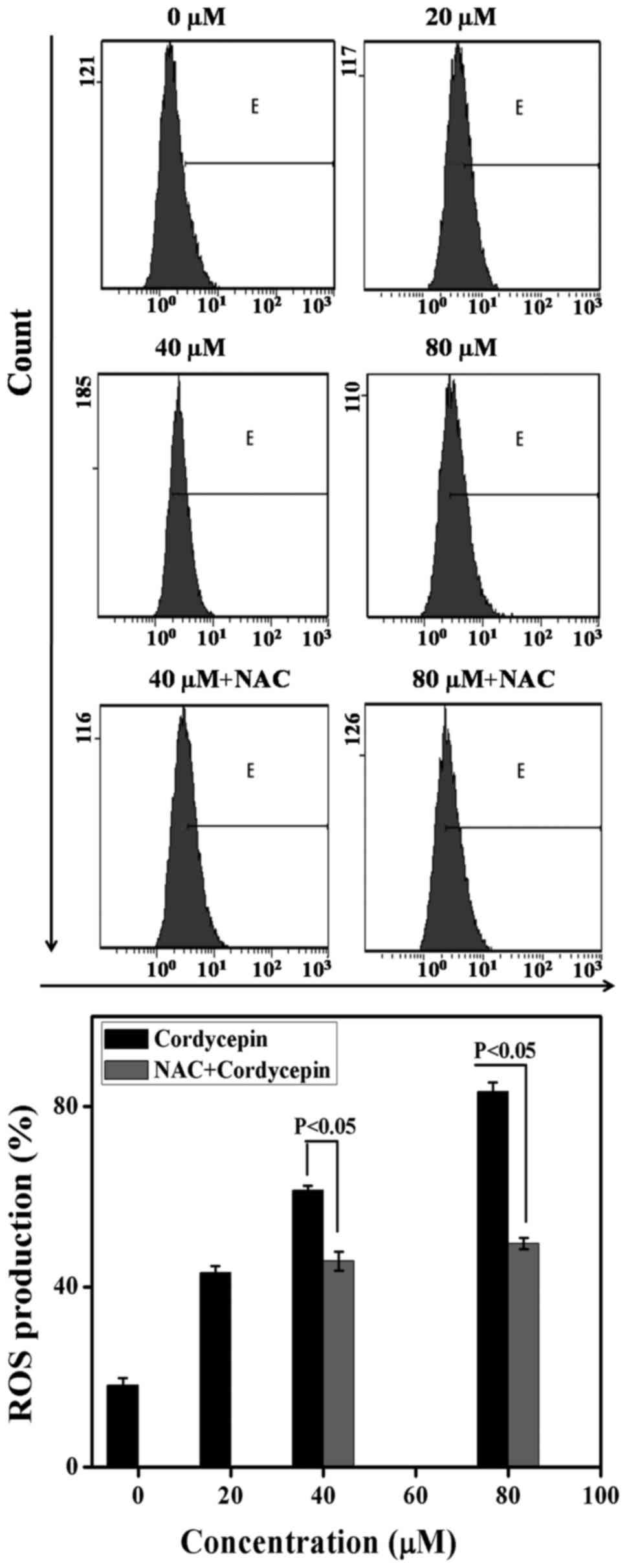
Previous study on cordycepin shows that ROS is
linked to a collapse of mitochondrial membrane potential (34). We carried out an analysis of the
production of intracellular ROS level with the help of flow
cytometry to evaluate whether apoptosis was caused by cordycepin.
Specifically, the cells treated with cordycepin were loaded with
the fluorescent probes DCF-DA to gauge the
H2O2 in the availability as well as
unavailability of NAC and incubated for 24 h. The findings show
that, in the absence of NAC, the ROS values are 18.22±1.52,
43.14±1.52, 61.39±1.73 and 83.23±2.075 upon 0, 20, 40 and 80
µM of cordycepin, correspondingly. On the other hand, in the
presence of NAC, the generation of ROS expression are 45.72±2.08
and 49.57±1.25 via utilization of 40, and 80 µM cordycepin
correspondingly denoting the role of NAC in minimizing
H2O2 (Fig.
5). These results validate the role of cordycepin in the
generation of oxidative stress in SGC-7901 cells.
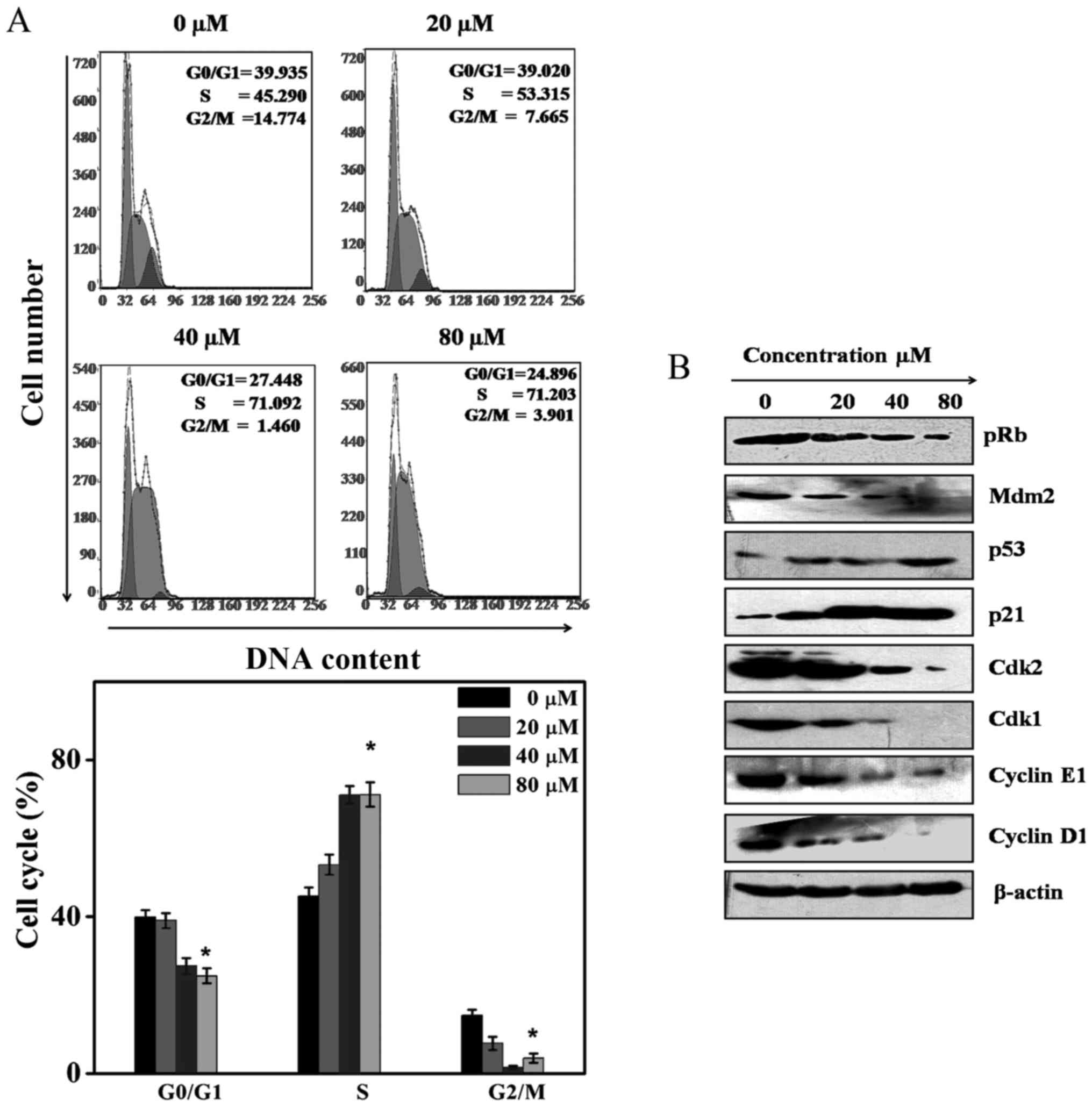
Cordycepin induces cell cycle arrest at S
phase
To validate whether the inhibition of Akt can
influence SGC-7901 cell cycle arrest, flow cytometry was carried
out. In the four cell cycle phases, the S phase, M phase, G1, and
G2 phase, the treatment of SGC-7901 cell line with 0, 20, 40, and
80 µM for 24 h induce an increase in the S phase percentages
to 45.29±2.13, 53.315±2.54, 71.092±2.24 and 71.203±3.1. These
concentrations also resulted in the decrease of the G0/G1 and G2/M
phase percentages. Based on the above evidence, we confirmed that
cordycepin induces SGC-7901 cell cycle arrest at the S phase
(Fig. 6A). To further delineate
and validate the process of the SGC-7901 cell cycle arrest by
cordycepin, the protein expression of cyclin E, CDK1 and CDK2,
cyclin D1, p53, p21 MDM2, and pRb proteins were explored by western
blot analysis. Fig. 6B suggests
that cordycepin suppressed significantly the expression of cyclin
E, CDK1, CDK2, MDM2, cyclin D1, as well as phosphorylated pRb. At
the same time, cordycepin augmented the p53 and p21 expression.
These findings indicated that cordycepin induces SGC-7901 cell
progression at S phase (Fig.
6A).
Discussion
It has been reported that there are two types of
gastric carcinoma cells EBV-positive and EBV-negative. Recently, it
has been suggested that EBV-positive gastric carcinomas have
distinct molecular characteristics in comparison with EBV-negative
gastric carcinomas (35).
Regarding this we evaluated the cytotoxicity of cordycepin against
SGC-7901 (EBV-negative) to compare with a previous study (18).
To shed light on the cytotoxicity mechanism trigged
by cordycepin, we attempted to identify the molecular mechanisms
involved in cordycepin apoptosis and cell cycle in SGC-7901. To our
knowledge, this is the first report showing the mechanism behind
with cordycepin induced gastric cancer cell cytotoxicity.
Cordycepin not only induced SGC-7901 cells growth changes but also
triggers their morphology changes in line with initial reports
(36,37). This is due to the unique
resemblance of cordycepin to disturb the cell membranes leading to
a DNA damage influencing cell death by apoptosis. This is
additionally validated through the evaluation of the expression of
pro- and anti-apoptotic Bcl-2 proteins during SGC-7901 cell
apoptosis (38). The imbalance
between these proteins resulted in the loss in the mitochondrial
trans-membrane potential (Δψm) leading to cell death by apoptosis
(38). Apoptosis can take place
due to death receptors such as DR3. When activated by external
stimuli DR3 can eliminate the activation of caspase-8 resulting in
activating the downstream caspase-9/3. Cordycepin activates the DR3
and eliminates SGC-7901 celldeath by apoptosis. In the same manner,
cordycepin releases cytochrome c a major factor in the
development of apoptosomes that activates caspase-3, which drives
cleavage of the PARP and that is why it induces cell death by
apoptosis. These findings are similar to previous reports (14). Moreover, it has been reported that
cordycepin induces inhibition of thyroid carcinoma cells through
suppressing the expression of A3AR (39). Herein, treatment of SGC-7901 cell
line induced inhibition of A3AR protein expression thus
we suggest that cordycepin induces SGC-7901 cell apoptosis mainly
by suppression of A3AR.
Activation of Akt affects cell growth and
progression. Earlier, cordycepin was observed to assert anticancer
impact by minimizing PI3K/Akt pathway (21). Herein our findings brought to light
that cordycepin downregulated PI3K/Akt that was linked to SGC-7901
cell apoptosis. Furthermore, our current study indicates that the
anti-apoptotic impacts of Akt on gastric cancer cell death is
linked to the generation of ROS. These findings are similar to a
previous study and confirmed the elimination of PI3K/Akt signaling
by cordycepin (20). Moreover,
pyruvate protein kinase isoform M2 (PKM2) imparts a leading role in
the nucleus phosphorylation of Akt and PI3K (31). In this way, inhibition of PKM2
expression by cordycepin triggers the phosphorylation of PI3K/Akt
that is ascribed to the efficacy of cordycepin to downregulate
PI3K/Akt which is presented to be involved in the secretion of PKM2
in SGC-7910 cells. Furthermore, cordycepin phosphorylated STAT-3
and JAK-2 protein result from their translocation from the
cytoplasm to the nucleus which assists in expression of
pro-inflammatory genes (40).
The molecular mechanism of cordycepin is further
validated by the benchmarking the SGC-7901 cell cycle arrest. The
molecular process of the cancer cell cycle regulation is interfered
by the modifications in the major checkpoint of genes (41). Cordycepin drive SGC-7901 cell cycle
arrested at the S phase. This is due to the potential of cordycepin
to downregulate the protein expression of the complex CDK2/cyclin E
in addition to upregulation of the p21 and phosphorylated pRb
proteins that are playing a crucial role in promoting the cell
progression with the help of S phase (42). Furthermore, the DNA damage in
gastric cancer results in the inhibition of MDM2 and improvement of
p53 (43). This is where we can
presume that the inhibition of MDM2 protein expression and increase
of p53 might play their parts in the cell cycle arrest induced by
cordycepin in SGC-7901 cells.
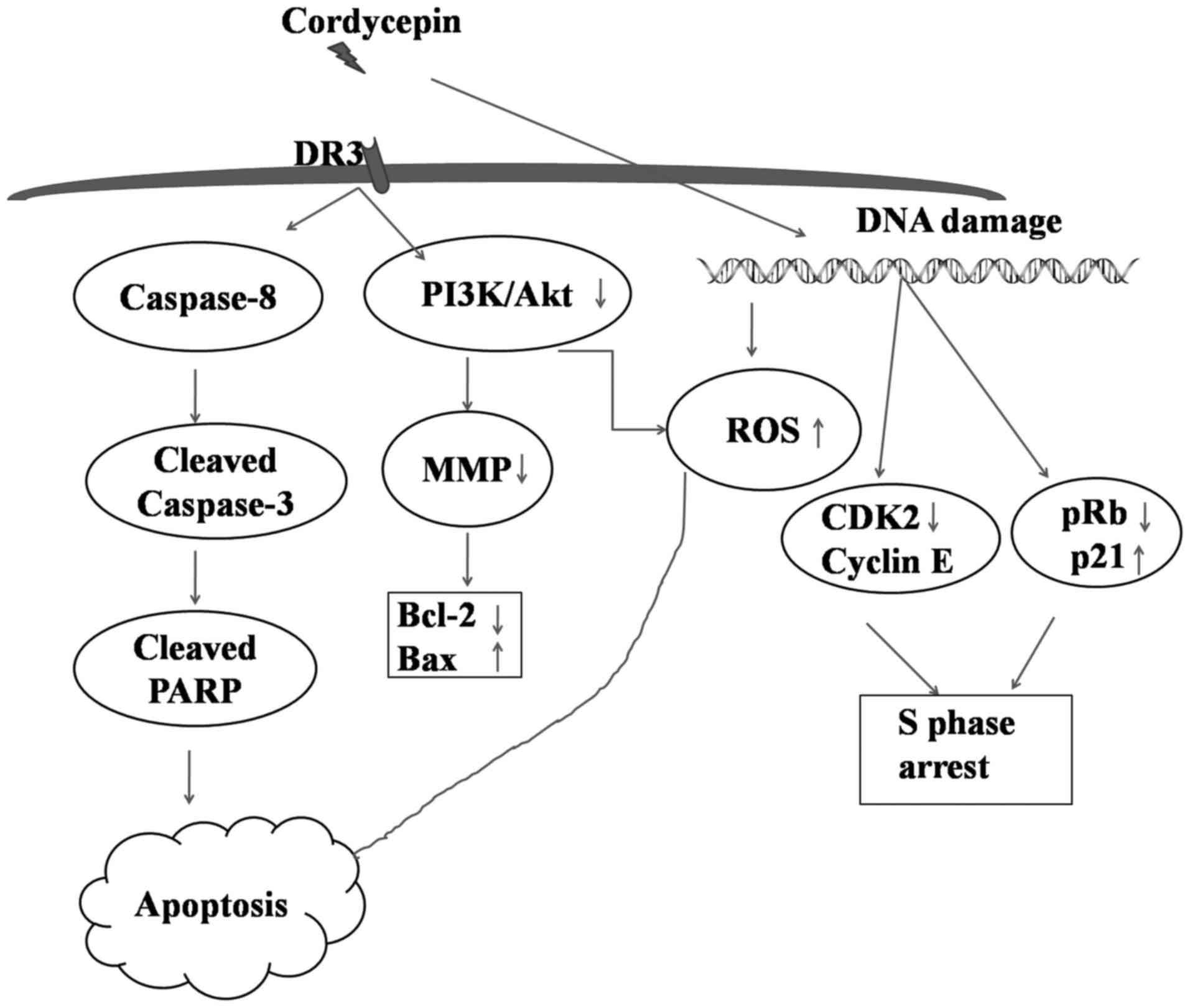
This study, revealed the process by which cordycepin
functions as an effectual anti-proliferating agent to deal with
SGC-7901 gastric cancer cell line. Apoptosis induced by cordycepin
was linked to the mitochondrial extrinsic pathways mainly by
activation of DR3, inhibition of A3AR and the collapse
of mitochondrial membrane potential. In addition, cordycepin
promotes phosphorylation of PI3K/Akt that results into the
generation of ROS in addition to cell cycle arrest at S phase
(Fig. 7). Considered collectively,
these findings suggest cordycepin as an efficient drug against
gastric cancer.
However, cordycepin being an adenosine analogue must
affect the cells with adenosine receptors, more studies are
required to investigate the effect and its molecular mechanism on
cordycepin in other cell lines.
Acknowledgments
This study was supported by Ministry of Science and
Technology (no. 2016YFE0128500), Jilin Provincial Science and
Technology Department (20130521010JH, 20150101187JC, and
20150414007GH), Jilin Province Education Department (2015–526,
2015–551); the Fundamental Research Funds for the Central
Universities (2412015ZH005, 2412016KJ037, 130017507, 130028633).
University S&T Innovation Platform of Jilin Province for
Economic Fungi (no. 2014B-1). National Natural Science Foundation
of China (nos. 30871301 and 30700827), and the Program for
Introducing Talents to Universities (no. B07017).
References
|
1
|
Zhao EH, Ling TL and Cao H: Current status
of surgical treatment of gastric cancer in the era of minimally
invasive surgery in China: Opportunity and challenge. Int J Surg.
28:45–50. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Oba K, Paoletti X, Bang YJ, Bleiberg H,
Burzykowski T, Fuse N, Michiels S, Morita S, Ohashi Y, Pignon JP,
et al GASTRIC (Global Advanced/Adjuvant Stomach Tumor Research
International Collaboration) Group: Role of chemotherapy for
advanced/recurrent gastric cancer: An individual-patient-data
meta-analysis. Eur J Cancer. 49:1565–1577. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim HS, Kim JH, Kim JW and Kim BC:
Chemotherapy in elderly patients with gastric cancer. J Cancer.
7:88–94. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Moehler M, Delic M, Goepfert K, Aust D,
Grabsch HI, Halama N, Heinrich B, Julie C, Lordick F, Lutz MP, et
al: Immunotherapy in gastrointestinal cancer: Recent results,
current studies and future perspectives. Eur J Cancer. 59:160–170.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen W, Zhao Z, Li L, Wu B, Chen SF, Zhou
H, Wang Y and Li YQ: Hispolon induces apoptosis in human gastric
cancer cells through a ROS-mediated mitochondrial pathway. Free
Radic Biol Med. 45:60–72. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nishiyama M, Yoshino S, Matsui H, Sakamoto
K, Suzuki N, Tamesa T, Takeda S, Ueno T, Hazama S and Oka M: A case
of metachronous liver metastasis from gastric cancer successfully
treated with hepatectomy. Gan To Kagaku Ryoho. 41:2352–2354.
2014.In Japanese.
|
|
7
|
Cui F, Zan X, Li Y, Sun W, Yang Y and Ping
L: Grifola frondosa glycoprotein GFG-3a arrests S phase, alters
proteome, and induces apoptosis in human gastric cancer cells. Nutr
Cancer. 68:267–279. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang F, Yin P, Lu Y, Zhou Z, Jiang C, Liu
Y and Yu X: Cordycepin prevents oxidative stress-induced inhibition
of osteogenesis. Oncotarget. 6:35496–35508. 2015.PubMed/NCBI
|
|
9
|
Tianzhu Z, Shihai Y and Juan D: The
effects of cordycepin on ovalbumin-induced allergic inflammation by
strengthening Treg response and suppressing Th17 responses in
ovalbumin-sensitized mice. Inflammation. 38:1036–1043. 2015.
View Article : Google Scholar
|
|
10
|
Hwang JH, Joo JC, Kim DJ, Jo E, Yoo HS,
Lee KB, Park SJ and Jang IS: Cordycepin promotes apoptosis by
modulating the ERK-JNK signaling pathway via DUSP5 in renal cancer
cells. Am J Cancer Res. 6:1758–1771. 2016.PubMed/NCBI
|
|
11
|
Shao LW, Huang LH, Yan S, Jin JD and Ren
SY: Cordycepin induces apoptosis in human liver cancer HepG2 cells
through extrinsic and intrinsic signaling pathways. Oncol Lett.
12:995–1000. 2016.PubMed/NCBI
|
|
12
|
Baig S, Seevasant I, Mohamad J, Mukheem A,
Huri HZ and Kamarul T: Potential of apoptotic pathway-targeted
cancer therapeutic research: Where do we stand? Cell Death Dis.
7:e20582016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tian X, Li Y, Shen Y, Li Q, Wang Q and
Feng L: Apoptosis and inhibition of proliferation of cancer cells
induced by cordycepin. Oncol Lett. 10:595–599. 2015.PubMed/NCBI
|
|
14
|
Lee SY, Debnath T, Kim SK and Lim BO:
Anti-cancer effect and apoptosis induction of cordycepin through
DR3 pathway in the human colonic cancer cell HT-29. Food Chemical
Toxicol. 60:439–447. 2013. View Article : Google Scholar
|
|
15
|
Ashkenazi A and Dixit VM: Death receptors:
Signaling and modulation. Science. 281:1305–1308. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Williams JO, Wang EC, Lang D and Williams
AS: Characterization of death receptor 3-dependent aortic changes
during inflammatory arthritis. Pharmacol Res Perspect.
4:e002402016. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Calder CJ and Wang EC: An essential role
for death receptor 3 in experimental autoimmune uveoretinitis. Ocul
Immunol Inflamm. 20:212–214. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ryu E, Son M, Lee M, Lee K, Cho JY, Cho S,
Lee SK, Lee YM, Cho H, Sung GH, et al: Cordycepin is a novel
chemical suppressor of Epstein-Barr virus replication. Oncoscience.
1:866–881. 2014. View Article : Google Scholar
|
|
19
|
Du Y, Yu J, Du L, Tang J and Feng WH:
Cordycepin enhances Epstein-Barr virus lytic infection and
Epstein-Barr virus-positive tumor treatment efficacy by
doxorubicin. Cancer Lett. 376:240–248. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pan BS, Wang YK, Lai MS, Mu YF and Huang
BM: Cordycepin induced MA-10 mouse Leydig tumor cell apoptosis by
regulating p38 MAPKs and PI3K/AKT signaling pathways. Sci Rep.
5:133722015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jeong JW, Jin CY, Park C, Han MH, Kim GY,
Moon SK, Kim CG, Jeong YK, Kim WJ, Lee JD, et al: Inhibition of
migration and invasion of LNCaP human prostate carcinoma cells by
cordycepin through inactivation of Akt. Int J Oncol. 40:1697–1704.
2012.PubMed/NCBI
|
|
22
|
Ye Y, Ge YM, Xiao MM, Guo LM, Li Q, Hao
JQ, Da J, Hu WL, Zhang XD, Xu J, et al: Suppression of SHIP2
contributes to tumorigenesis and proliferation of gastric cancer
cells via activation of Akt. J Gastroenterol. 51:230–240. 2016.
View Article : Google Scholar
|
|
23
|
Zheng T, Meng X, Wang J, Chen X, Yin D,
Liang Y, Song X, Pan S, Jiang H and Liu L: PTEN- and p53-mediated
apoptosis and cell cycle arrest by FTY720 in gastric cancer cells
and nude mice. J Cell Biochem. 111:218–228. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Martin DA and Elkon KB: Mechanisms of
apoptosis. Rheum Dis Clin North Am. 30:vii 441–vii454. 2004.
View Article : Google Scholar
|
|
25
|
Zhao Z, Han F, Yang S, Wu J and Zhan W:
Oxamate-mediated inhibition of lactate dehydrogenase induces
protective autophagy in gastric cancer cells: Involvement of the
Akt-mTOR signaling pathway. Cancer Lett. 358:17–26. 2015.
View Article : Google Scholar
|
|
26
|
Panieri E and Santoro MM: ROS homeostasis
and metabolism: A dangerous liason in cancer cells. Cell Death Dis.
7:e22532016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen Y, Zhang H, Zhou HJ, Ji W and Min W:
Mitochondrial redox signaling and tumor progression. Cancers
(Basel). 8:82016. View Article : Google Scholar
|
|
28
|
Khan M, Rasul A, Yi F, Zhong L and Ma T:
Jaceosidin induces p53-dependent G2/M phase arrest in U87
glioblastoma cells. Asian Pac J Cancer Prev. 12:3235–3238.
2011.PubMed/NCBI
|
|
29
|
Singh SS, Yap WN, Arfuso F, Kar S, Wang C,
Cai W, Dharmarajan AM, Sethi G and Kumar AP: Targeting the PI3K/Akt
signaling pathway in gastric carcinoma: A reality for personalized
medicine? World J Gastroenterol. 21:12261–12273. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Porquet N, Poirier A, Houle F, Pin AL,
Gout S, Tremblay PL, Paquet ER, Klinck R, Auger FA and Huot J:
Survival advantages conferred to colon cancer cells by
E-selectin-induced activation of the PI3K-NFκB survival axis
downstream of death receptor-3. BMC Cancer. 11:2852011. View Article : Google Scholar
|
|
31
|
Chen G, Feng W, Zhang S, Bian K, Yang Y,
Fang C, Chen M, Yang J and Zou X: Metformin inhibits gastric cancer
via the inhibition of HIF1α/PKM2 signaling. Am J Cancer Res.
5:1423–1434. 2015.PubMed/NCBI
|
|
32
|
Fishman P, Cohen S and Bar-Yehuda S:
Targeting the A3 adenosine receptor for glaucoma treatment
(Review). Mol Med Rep. 7:1723–1725. 2013.PubMed/NCBI
|
|
33
|
Khanna P, Chua PJ, Bay BH and Baeg GH: The
JAK/STAT signaling cascade in gastric carcinoma (Review). Int J
Oncol. 47:1617–1626. 2015.PubMed/NCBI
|
|
34
|
Lee HH, Park C, Jeong JW, Kim MJ, Seo MJ,
Kang BW, Park JU, Kim GY, Choi BT, Choi YH, et al: Apoptosis
induction of human prostate carcinoma cells by cordycepin through
reactive oxygen species-mediated mitochondrial death pathway. Int J
Oncol. 42:1036–1044. 2013.PubMed/NCBI
|
|
35
|
He D, Zhang YW, Zhang NN, Zhou L, Chen JN,
Jiang Y and Shao CK: Aberrant gene promoter methylation of p16,
FHIT, CRBP1, WWOX, and DLC-1 in Epstein-Barr virus-associated
gastric carcinomas. Med Oncol. 32:922015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tao X, Ning Y, Zhao X and Pan T: The
effects of cordycepin on the cell proliferation, migration and
apoptosis in human lung cancer cell lines A549 and NCI-H460. J
Pharm Pharmacol. 68:901–911. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li Y, Li R, Zhu S, Zhou R, Wang L, Du J,
Wang Y, Zhou B and Mai L: Cordycepin induces apoptosis and
autophagy in human neuroblastoma SK-N-SH and BE (2) -M17 cells.
Oncol Lett. 9:2541–2547. 2015.PubMed/NCBI
|
|
38
|
Qiao L and Wong BC: Targeting apoptosis as
an approach for gastrointestinal cancer therapy. Drug Resist Updat.
12:55–64. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen Y, Chen YC, Lin YT, Huang SH and Wang
SM: Cordycepin induces apoptosis of CGTH W-2 thyroid carcinoma
cells through the calcium-calpain-caspase 7-PARP pathway. J Agric
Food Chem. 58:11645–11652. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zearfoss NR, Alarcon JM, Trifilieff P,
Kandel E and Richter JD: A molecular circuit composed of CPEB-1 and
c-Jun controls growth hormone-mediated synaptic plasticity in the
mouse hippocampus. J Neurosci. 28:8502–8509. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Viallard JF, Lacombe F, Belloc F,
Pellegrin JL and Reiffers J: Molecular mechanisms controlling the
cell cycle: Fundamental aspects and implications for oncology.
Cancer Radiother. 5:109–129. 2001.In French. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liao Y, Ling J, Zhang G, Liu F, Tao S, Han
Z, Chen S, Chen Z and Le H: Cordycepin induces cell cycle arrest
and apoptosis by inducing DNA damage and up-regulation of p53 in
leukemia cells. Cell Cycle. 14:761–771. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Choi HS, Seo HS, Kim JH, Um JY, Shin YC
and Ko SG: Ethanol extract of paeonia suffruticosa Andrews (PSE)
induced AGS human gastric cancer cell apoptosis via fas-dependent
apoptosis and MDM2-p53 pathways. J Biomed Sci. 19:822012.
View Article : Google Scholar : PubMed/NCBI
|