Introduction
Cervical cancer is the fourth most common type of
cancer among women worldwide, with 527,600 estimated new cases in
2012 (1). Infection with the human
papillomavirus (HPV) has been established to be a necessary cause
of invasive cervical cancer (2–4). The
lifetime risk of acquiring an HPV infection is ~80% among sexually
active women, but most women clear the virus uneventfully (5). When infection with HPV persists, this
can lead to malignant cellular changes ranging from cervical
intraepithelial neoplasia (CIN)1, CIN2 and CIN3 to invasive
cervical cancer. Persistent HPV infections have been associated
with chronic inflammation, with large numbers of inflammatory cells
often surrounding cervical cancer cells (6). Macrophages are a major component of
cancer-related inflammation, and they are roughly divided into two
main subgroups: the classically activated type 1 macrophages (M1),
which are pro-inflammatory (enhanced expression and release of
TNF-α, IL-6, IL-12), and the alternative activated type 2
macrophages (M2), which show anti-inflammatory properties (enhanced
expression and release of TGF-β1, IL-10, prostaglandin E2)
(6–11). Tumors mediate the accumulation and
differentiation of macrophages to sustain tumor growth, and
increased numbers of tumor-associated macrophages (TAM) are
associated with disease progression and poor prognosis in cervical
cancer (12–14).
In cervical cancer, high expression of the epidermal
growth factor receptor (EGFR) is associated with tumor development,
as EGFR expression increases with increasing malignant stage and
subsequent EGFR activation leads to cell growth, differentiation,
resistance to apoptosis, cell cycle progression and angiogenesis.
EGFR-based therapy has established efficacy in selected patients
with head and neck squamous cell carcinoma, colorectal carcinoma
and non-small cell lung carcinoma (15–17).
It is currently being explored as a therapeutic target in cervical
cancer, as EGFR expression is overexpressed in 70–90% of the cases
and has been associated with poor prognosis (17–22).
Cell line studies and mouse models show that TAM aid
tumor progression through a paracrine loop with EGFR. EGFR
activation regulates the expression of granulocyte-macrophage
colony-stimulating factor (GM-CSF/CFS2) by tumor cells, thus
attracting macrophages to EGFR-expressing tumors where these
macrophages express and release EGFR ligands, leading to EGFR
activation and subsequent tumor cell proliferation (23–29).
Although ~70% of cervical cancers show increased EGFR expression,
and high EGFR expression is associated with decreased
disease-specific survival, this paracrine loop has never been
investigated in human cervical cancer (18,30–32).
We hypothesized that macrophages are the main source
of EGFR ligands and that a paracrine loop between tumor cells and
macrophages is responsible for ligand expression. First, we
investigated whether macrophages are the predominant source of EGFR
ligands in human cervical cancer. Therefore, we first assessed
which of the known EGFR ligands was present in cervical cancer
specimens and which ligand was associated with EGFR expression.
Subsequently, we tested whether this ligand was predominantly
expressed by TAM. Finally we determined whether an association
could be found between EGFR expression by cervical cancer cells and
the number of cells expressing the EGFR ligand.
Materials and methods
Tissue samples
All tissue samples were collected from the archives
of the Department of Pathology, Leiden University Medical Center,
Leiden, The Netherlands. Only material from cervical cancer
patients who underwent radical hysterectomy with lymphadenectomy
between 1985 and 1995, had not received radiotherapy or
chemotherapy prior to surgery, had a histopathological diagnosis of
squamous cell carcinoma, adenosquamous carcinoma or adenocarcinoma
and had enough primary tumor material available was used. For mRNA
expression analysis of amphiregulin (AREG), β-cellulin, epidermal
growth factor (EGF), epiregulin, heparin-binding EGF-like growth
factor (HB-EGF), transforming growth factor a (TGFα) and EGFR, 32
frozen cervical cancer tissue samples were collected. For
immunohistochemistry and mRNA in situ hybridization, 36
formalin-fixed, paraffin-embedded cervical cancer tissue samples
were collected. The group of 32 frozen cervical cancer specimens
did not overlap with the group of 36 formalin-fixed,
paraffin-embedded cervical cancer specimens. Tissue samples were
used according to the guidelines of the Ethical Committee of the
Leiden University Medical Center.
mRNA expression analysis
mRNA extraction and analysis of microarray
expression data were performed as previously described (33). In short, mRNA was obtained from 32
frozen cervical squamous cell carcinoma tissue samples, using
TRIzol (Life Technologies, Carlsbad, CA, USA). mRNA was
subsequently purified, after which cDNA was synthesized and
transcribed into cRNA using the Illumina Totalprep RNA
amplification kit following the manufacturer's instructions
(Ambion, Austin, Tx, USA). Labeled cRNA was hybridized to Illumina
Sentrix-human 6 expression bead-chips. Gene expression levels were
quantified using beadstudio gene expression module 2.1, and data
were subsequently normalized using the VSN method (34).
Immunohistochemistry
Four-micrometre tissue sections were deparaffinized,
rehydrated, and endogenous peroxidase was blocked with 0.3%
hydrogen peroxide (H2O2) for 20 min. For
HB-EGF, AREG and TGFα, antigen retrieval was performed in 0.01 M
citrate buffer (pH 6.0, 12 min). Subsequently, slides were
incubated overnight with polyclonal goat anti-human HB-EGF (1:200
at 4°C, af-259-na, R&D Systems Europe Ltd., Abingdon, UK),
polyclonal goat anti-human AREG (1:100, af-262, R&D Systems)
and mouse monoclonal anti-human TGFα (1:50, clone P/T1, Abcam,
Cambridge, UK) diluted in phosphate-buffered saline (PBS)
containing 1% bovine serum albumin (BSA). After washing with PBS,
tissue sections were incubated with a goat probe and anti-goat
horseradish peroxidase (HRP) according to the manufacturer's
instructions (Goat HRP-polymer kit, GHP516, Biocare Medical,
Concord, CA, USA) for AREG and HB-EGF. Tissue sections were
incubated with anti-TGFα with BrightVision-Poly/HRP (Immunologic,
Duiven, The Netherlands). Immunoreactions were visualized using a
3.3′-diaminobenzidine-tetrahydrochloryde (DAB)+
chromogen (Dako, Heverlee, Belgium), and counterstained with
hematoxylin. The results were scored for tumor compartment staining
intensity [negative (0), weak (1),
moderate (2) or strong (3)] and for the presence of ligand
positive cells in the stroma (positive or negative).
EGFR protein expression was determined as previously
described (18). The intensity of
EGFR membrane staining was scored as negative (0), weak (1), moderate (2) or strong (3). Strong immunoreactivity was defined as
complete membrane staining, producing a thick outline of the cell.
Presence of TAM was determined though immunohistochemical staining
of CD68 as previously described (25). The total number of TAM in the tumor
and stromal compartment was quantified by counting the number of
positive cells per six, randomly selected, high-power fields of
view.
For immunohistochemical double staining for CD68 and
HB-EGF, four representative tissue slides were deparaffinized,
rehydrated, and endogenous peroxidase was blocked with 0.3%
H2O2 for 20 min. Antigen retrieval was
performed with 0.01 M citrate buffer, and slides were incubated
overnight with anti-HB-EGF and mouse monoclonal anti-human CD68
(1:50, 514H12, AbD Serotec, Oxford, Uk) diluted in
Tris(hydroxymethyl)aminomethane buffered saline (TBS) containing 1%
BSA. After washing with TBS, tissue sections were first incubated
with a goat probe, subsequent anti-goat HRP and DAB+ to
visualize HB-EGF. Then, to visualize CD68, tissue slides were
incubated with an alkaline phosphatase (AP)-labeled secondary
antibody (1:200, rabbit anti-mouse IgG2a-AP, Southern Biotech,
Uithoorn, The Netherlands) and subsequent PermaBlue (Diagnostic
Biosystems, Uithoorn, The Netherlands) according to the
manufacturer's instructions. No counterstaining was applied. For
corresponding single staining of CD68 and HB-EGF the same protocol
was used, but only one of the two primary antibodies was
applied.
Analysis of immunohistochemically stained
slides
The number of CD68 and HB-EGF double positive cells
was assessed using spectral imaging (35). In short, five representative
spectral images, containing both CD68 and HB-EGF-positive cells,
were taken for each tissue specimen with a Leica DM4000 B
microscope, equipped with a Nuance FX Multispectral Imaging System.
The spectral libraries were created from PermaBlue and DAB single
staining, after which spectral images from double stained tissue
slides were acquired at the same wavelengths. The obtained spectral
images were analyzed using Nuance software version 2.10, allowing
unmixing into monochrome PermaBlue and DAB images. Subsequently, a
simulated fluorescence composite image was created, after which the
appropriate threshold for analysis was determined and the
percentage of pixel-based co-localization was calculated for each
image.
To measure the HB-EGF expression in the epithelial
and stromal compartment, the single stained tissue slides were
scanned, using the Pannoramic 250 Flash digital slide scanner
(3DHistech), after which five representative images, containing
both tumor and HB-EGF-positive stromal cells, were taken from each
digitalized slide. Color deconvolution was applied to assess the
staining intensity of HB-EGF (DAB) only and not the counterstaining
(hematoxylin). The average staining intensity [gray scale range: 0
(black) – 255 (white)] was assessed in a standardized area size
(42×42 μm) in three representative parts of the tumor. All
measured staining intensities were subsequently inverted as follows
for all measurements: 255 - measured staining intensity. Since
HB-EGF expression was homogeneous in the tumor compartment (all
tumor cells expressed HB-EGF equally), an average of three measured
areas was used as a standardized measure to determine the relative
HB-EGF amount in the tumor compartment. In the stromal compartment,
single cells were positive for HB-EGF. Therefore, first, the
staining intensity was determined for three separate DAB-positive
cells in the stroma, after which the average of these three
measurements was calculated. Then, the percentage of positive cells
in the stroma was determined for each image, using the same
threshold settings for all analyses. The average signal intensity
was multiplied by the percentage of positive cells, to obtain a
standardized measure for the relative HB-EGF amount in the stroma.
One image was analyzed per slide, as the measurements proved to be
consistent. The analyses were performed using ImageJ (version
1.45s, National Institutes of Health, MD, USA, freely available on
http://imagej.nih.gov/ij).
mRNA in situ hybridization
Expression of the chemokines GM-CSF and CCL2 was
determined by mRNA in situ hybridization as previously
described and scored for staining intensity [negative (0), weak
(1), moderate (2) and strong (3)] as well as percentage of tumor cells
positive for chemokine expression [1–5% (1), 6–25% (2), 26–50% (3), 51–75% (4) and 76–100% (5)]. The sum of the staining intensity
score and the percentage of positive cells resulted in an overall
score for mRNA expression by tumor cells of either 0 or 2–8, which
was then dichotomized into low (0–5) and high expression (6–8)
(24,25).
Statistical analysis
Statistical analyses were performed using the SPSS
program (Version 17.0 for Windows; SPSS Inc. Chicago, Ill, USA).
Significance tests were two-sided and statistical significance was
assumed when P<0.05, corresponding to 95% confidence intervals
(CI). For correlation analysis, the Spearman's correlation
coefficient (ϱ, rho) was determined using ungrouped mRNA or protein
expression scores. For crosstab analyses, odds ratios (OR) were
calculated and the Fisher's exact test was used for calculation of
P-values. In case of empty cells, 0.5 was added to each cell for OR
calculation.
Results
Expression of HB-EGF in cervical
cancer
To assess which EGFR ligands are expressed in
cervical cancer specimens, a genome-wide mRNA expression array of
32 cervical cancer specimens was analyzed for mRNA expression of
EGFR and EGFR ligands. Median normalized mRNA expression was found
to be substantial for AREG, HB-EGF, TGFα and EGFR,
while expression of EGF, β-cellulin and epiregulin
was low or absent (Table I). To
assess protein expression of HB-EGF, AREG and TGFα in cervical
cancer and to determine the primary site of ligand expression,
immunohistochemistry was performed on 36 specimens. Expression of
all three ligands was observed in both the tumor and stromal
compartment (Table II).
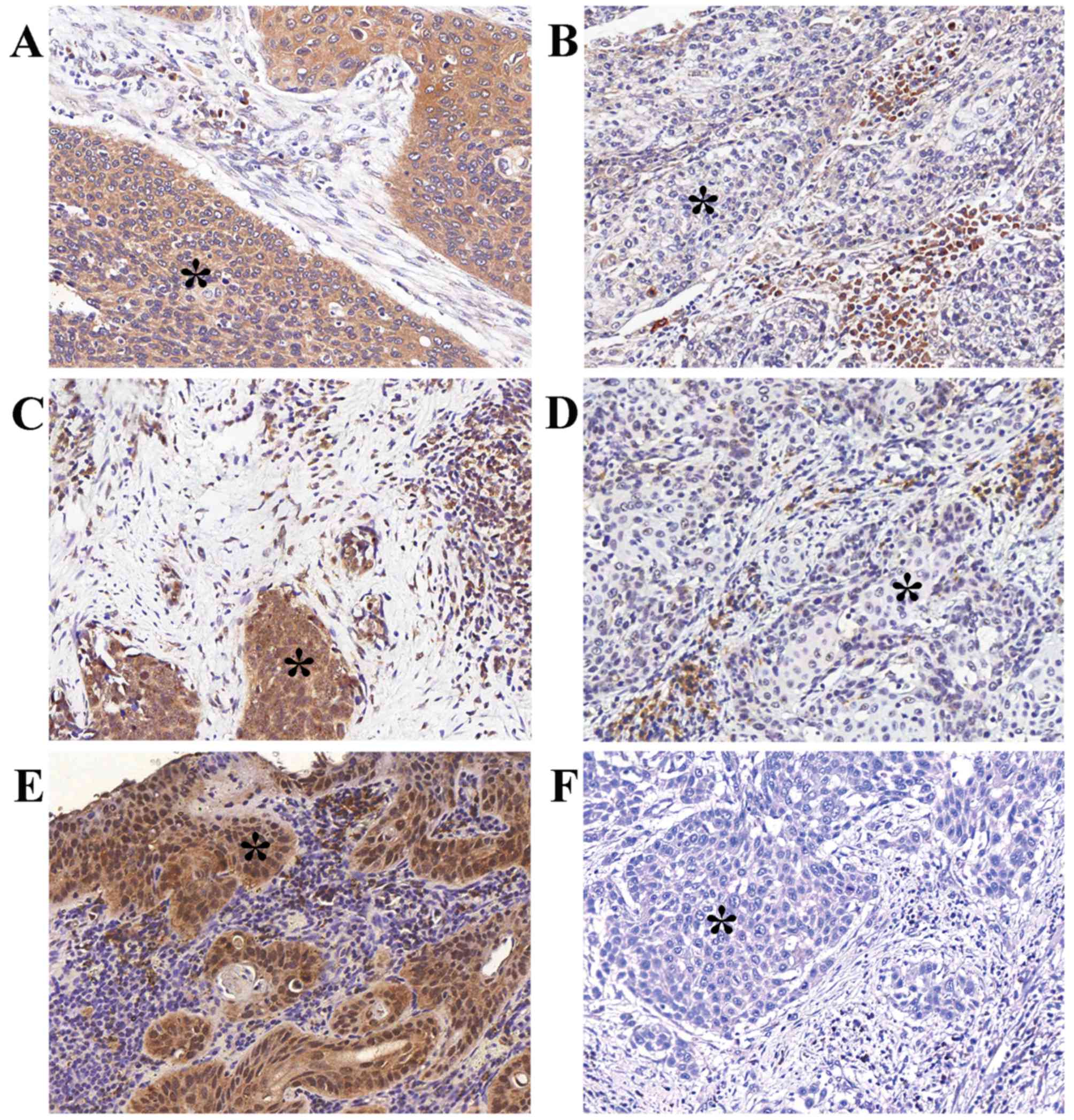
Representative examples of positive tumor compartment staining are
shown in Fig. 1. The correlation
between EGFR ligand expression and EGFR expression was determined,
to assess which ligand is most likely the primary EGFR ligand in
cervical cancer and whether this ligand was primarily expressed in
the epithelial or stromal compartment (Table III). Both stromal compartment
(ϱ=0.58, P<0.001) and tumor compartment HB-EGF expression
(ϱ=0.33, P=0.049) were correlated to EGFR expression. No
correlations were observed between EGFR and AREG or TGFα.
 | Table IEpidermal growth factor receptor
(EGFR) ligand and EGFR mRNA expression in cervical cancer. |
Table I
Epidermal growth factor receptor
(EGFR) ligand and EGFR mRNA expression in cervical cancer.
| mRNA
expressiona
|
|---|
| Median | (25th–75th
percentile) |
|---|
| AREG | 355 | 149–870 |
| BTC | 15 | 0–43 |
| EGF | −6 | −14–3 |
| EREG | 15 | 7–27 |
| HB-EGF | 233 | 145–461 |
| TGFα | 145 | 99–196 |
| EGFR | 63 | 37–94 |
 | Table IITumor and stromal protein expression
of HB-EGF, AREG and TGFα in cervical cancer patients. |
Table II
Tumor and stromal protein expression
of HB-EGF, AREG and TGFα in cervical cancer patients.
| Na (%) |
|---|
| AREG | |
| Tumor | |
| Negative | – |
| Weak | 13 (36) |
| Moderate | 11 (31) |
| Strong | 12 (33) |
| Stroma | |
| Negative | 9 (25) |
| Positive | 27 (75) |
| HB-EGF | |
| Tumor | |
| Negative | – |
| Weak | 8 (22) |
| Moderate | 18 (50) |
| Strong | 10 (28) |
| Stroma | |
| Negative | 12 (33) |
| Positive | 24 (67) |
| TGFα | |
| Tumor | |
| Negative | – |
| Weak | 19 (53) |
| Moderate | 10 (28) |
| Strong | 7 (19) |
| Stroma | |
| Negative | 10 (28) |
| Positive | 26 (72)\ |
 | Table IIICorrelations between protein
expression of epidermal growth factor receptor (EGFR), EGFR ligands
and tumor associated macrophages. |
Table III
Correlations between protein
expression of epidermal growth factor receptor (EGFR), EGFR ligands
and tumor associated macrophages.
| EGFR expression
|
|---|
| ϱ | P-value |
|---|
| AREG | | |
| Tumor | 0.11 | 0.521 |
| Stromal | 0.15 | 0.392 |
| HB-EGF | | |
| Tumor | 0.33 | 0.049 |
| Stromal | 0.58 | <0.001 |
| TGFα | | |
| Tumor | −0.06 | 0.751 |
| Stromal | −0.02 | 0.927 |
| TAM | | |
| Tumor | 0.43 | 0.009 |
| Stromal | 0.56 | <0.001 |
Association between intensity of EGFR
expression, number of TAM and expression of chemotactic factors by
tumor cells
Next, we assessed the correlation between EGFR
expression and the number of CD68-positive TAM (cluster of
differentiation 68, membrane glycoprotein that binds low density
lipoprotein, a marker predominantly expressed by
monocytes/macrophages). High EGFR expression was correlated to a
high number of stromal TAM (ϱ=0.56, P<0.001) and a high number
of TAM in the tumor compartment (ϱ=0.43, P=0.009, Table III). To assess whether EGFR
expression was associated with macrophage recruitment, the
association between CD68+ TAM, EGFR, M-CSF,
GM-CSF and CCL2 expression by cervical cancer cells was
determined. First, the Spearman's correlation between mRNA
expression of CD68 and M-CSF, GM-CSF, CCL2 and their
receptors M-CSFR, GM-CFSR and CCR was determined.
CD68 was correlated with M-CSFR (P=0.003) and CCR2
(P=0.018). However, EGFR was not associated with M-CSF,
GM-CSF, CCL2, M-CSFR, GM-CFSR or CCR. Next,
GM-CSF and CCL2 mRNA expression, assessed through
mRNA in situ hybridization was divided into low expression
(combined intensity and percentage scores 0–5) and high expression
(combined intensity and percentage scores 6–8). EGFR expression was
divided into low (0–1) and high (2–3)
intensity scores. High EGFR expression was associated with high
GM-CSF and CCL2 expression (OR, 11; P=0.039 and OR,
16; P=0.006, respectively).
Expression of HB-EGF in tumor-associated
macrophages
We further investigated whether TAM could be an
important source of HB-EGF in cervical cancer. Therefore, the
correlation between HB-EGF expression and the number of TAM was
determined. Stromal HB-EGF expression was positively correlated to
the number of stromal TAM (ϱ=0.34, P=0.044), but no correlation was
observed between tumor HB-EGF expression and the number of TAM in
the tumor compartment (ϱ=0.24, P=0.163). To investigate whether the
observed HB-EGF-positive cells in the stroma were indeed
macrophages, immunohistochemical double staining was performed for
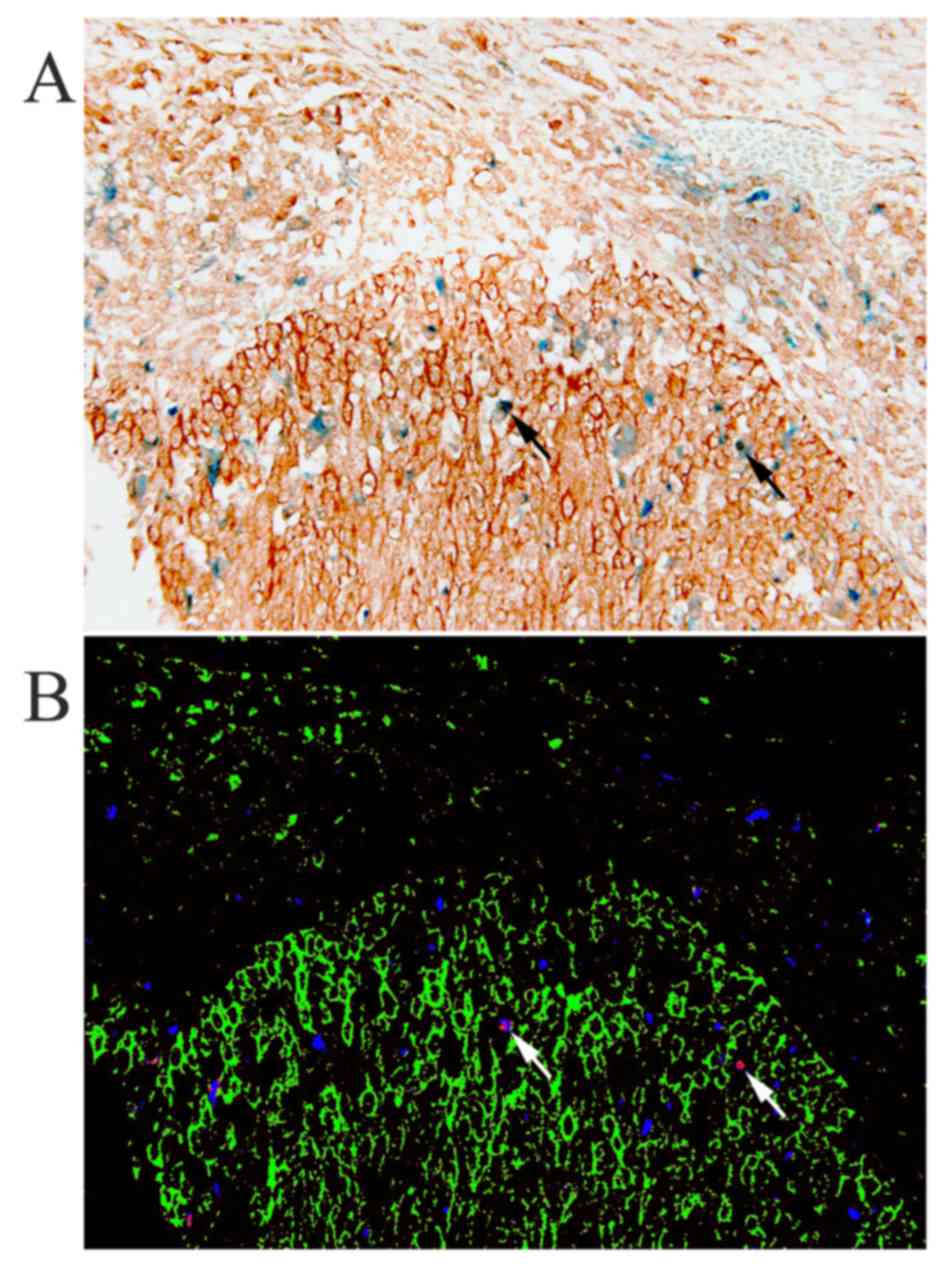
CD68 and HB-EGF. CD68 and HB-EGF-double-positive cells were
observed, i.e., the cervical cancer specimens showed macrophages
expressing HB-EGF (Fig. 2), but
analysis using spectral imaging showed that a subset of 4–14% of
all observed macrophages expressed HB-EGF, with a mean of 9%.
Furthermore, the majority of HB-EGF-positive cells in the stroma
were CD68-negative, most likely fibroblasts or B-cells, suggesting
that macrophages are not the predominant stromal source of HB-EGF
in cervical cancer.
HB-EGF is expressed on tumor cells
Immunohistochemical staining showed high HB-EGF
expression in 78% of the cervical tumor specimens, suggesting that
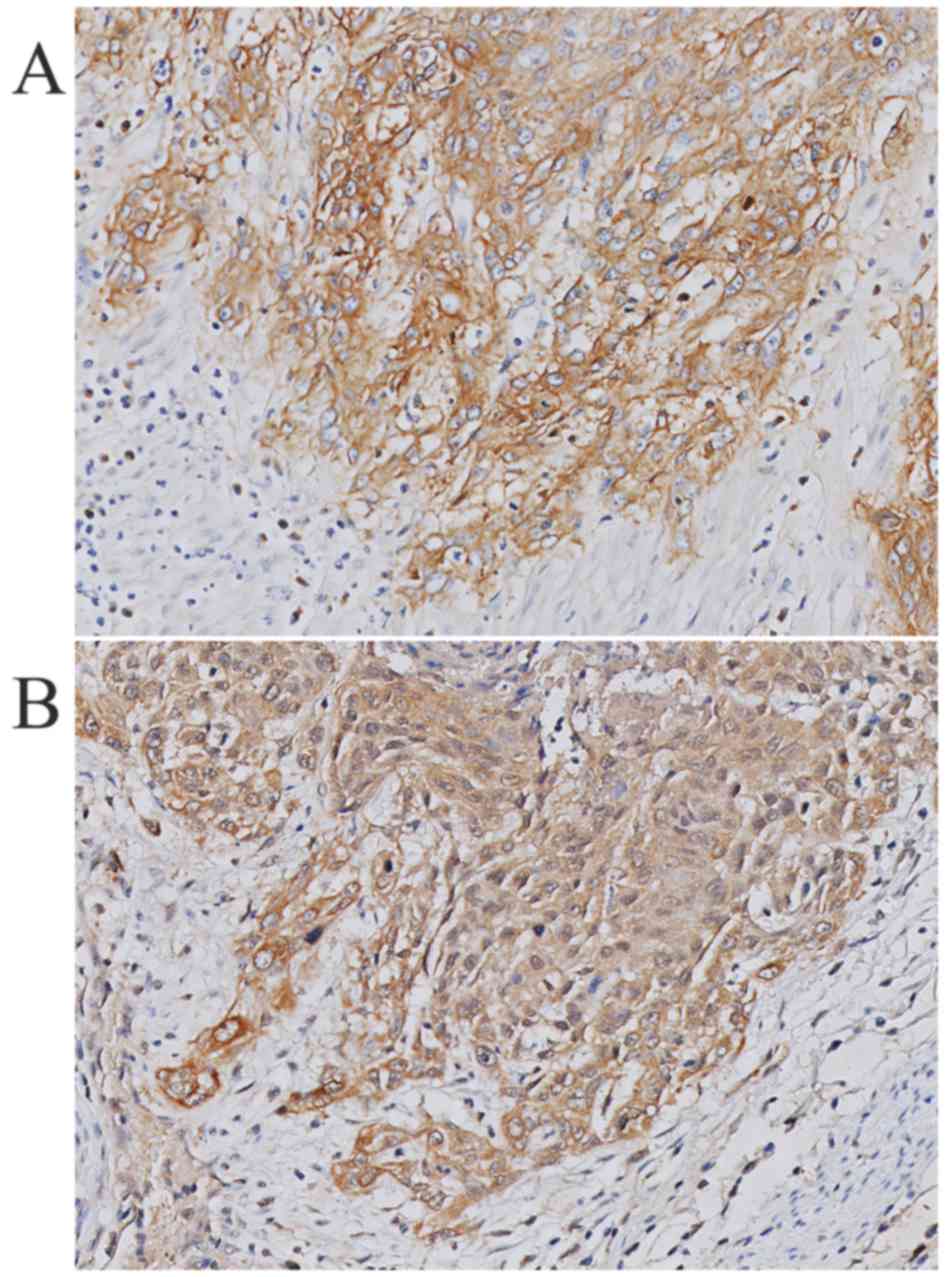
HB-EGF is utilized in an autocrine manner. Fig. 3 shows a representative example of
HB-EGF staining and EGFR staining within the same tumor specimen.
To estimate the amount of HB-EGF in the tumor and stromal
compartment, we measured the HB-EGF staining intensity in the
cervical cancer samples, and adjusted the HB-EGF staining intensity
for the number of HB-EGF-positive cells in the stroma and the
epithelial compartment (relative HB-EGF amount). The average
staining intensities as determined using ImageJ corresponded to the
previously appointed immunohistochemical (weak, moderate or strong)
intensity scores (data not shown). Subsequently, the relative
HB-EGF expression in the tumor and stromal compartment was
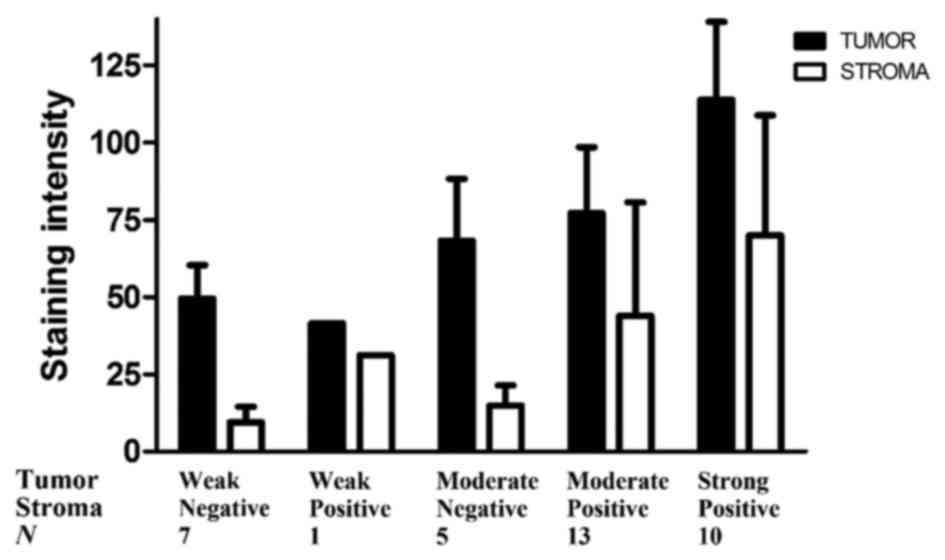
determined in each tissue slide. All individual relative HB-EGF
amount ratios (epithelial cancer cells/stromal cells) were >1,
except for 2 cases (N=36), suggesting that even in cases with weak
HB-EGF expression in the tumor compartment, the tumor compartment
showed higher expression of HB-EGF than the stromal compartment
(Fig. 4).
Discussion
In this study, we investigated which EGFR ligands
are expressed in cervical cancer and whether macrophages are the
predominant source of these EGFR ligands. The results obtained
suggest HB-EGF as the predominant EGFR ligand. Although EGFR
expression is associated with macrophage counts and the expression
of CCL2 and GM-CSF, and macrophage numbers are
associated with the expression of M-CSFR and CCR2, macrophages do
not appear to be the predominant source of HB-EGF since the
relative amount of HB-EGF measured in the epithelial compartment
was on average four times higher than the relative amount of HB-EGF
in the stromal compartment. These results suggest that, in cervical
cancer, the tumor cells are the major source of HB-EGF.
mRNA expression analysis showed that three EGFR
ligands were expressed in cervical cancer tissue, namely HB-EGF,
AREG and TGFα. In previous studies in pancreatic cancer,
where mRNA transcription of several EGFR ligands was observed, only
HB-EGF was shown to be of significance at protein level (36). To substantiate the findings on mRNA
level in our study, we performed immunohistochemical staining on an
independent group of cervical cancer patients, to determine protein
expression of HB-EGF, AREG and TGFα. All three ligands were
strongly expressed both in tumor stroma and in the epithelial
compartment. However, only HB-EGF expression was associated with
EGFR expression on the tumor cell membrane, indicating HB-EGF as
the primary ligand for EGFR in cervical cancer.
Pollard (32) and
Vlaicu et al (37), have
shown through cell line research and mouse models, that in breast
cancer, macrophage production of EGF induces EGFR activation on
tumor cells, which in turn leads to M-CSF (CSF-1) production,
consequently attracting more macrophages. M-CSF and its receptor
have been shown to be upregulated in cervical cancer both on the
mRNA and protein level, when compared to normal cervical tissue
(38). In turn, the inhibition of
M-CSF prevents tumor progression in a cervical cancer mouse model
(39). In cervical cancer HeLa
cells and macrophages, an analogous paracrine loop has been
described with GM-CSF and HB-EGF expression (26,27).
In addition to cervical cancer cell lines, HB-EGF, AREG and TGFα
expression and EGFR activation have previously been shown to induce
GM-CSF release in several human cell lines, such as airway
epithelial cells, keratinocytes and colon adenocarcinoma cells
(DLD-1) (27,28,40).
The present study analyzed whether this interdependent paracrine
signaling between tumor cells and macrophages through HB-EGF and
GM-CSF was present in human cervical cancer. We observed that EGFR
expression was associated with high numbers of TAM in the tumor and
stromal compartment, and with high GM-CSF and CCL2
expression. Previously, increased EGFR expression has been shown to
downregulate CCL2, while EGFR inhibition has been shown to
upregulate CCL2 expression in vitro in head and neck
squamous cell carcinoma and primary cultures of normal human
keratinocytes (41,42). Only in osteoblasts and vascular
smooth muscle cells has EGFR activation been shown to increase CCL2
expression (43,44). Our results are concordant with the
findings that EGFR expression is associated with GM-CSF and CCL2
expression by tumor cells, thus attracting macrophages to the tumor
site. However, although EGFR expression was associated with
macrophage recruitment, double staining for CD68 and HB-EGF showed
that only a limited number (9%) of the macrophages were
HB-EGF-positive. Furthermore, only a small proportion of
HB-EGF-positive cells in the stroma was CD68-positive, as
fibroblasts and B-cells also appeared to be HB-EGF-positive,
suggesting that macrophages are not the predominant source of
HB-EGF in cervical cancer stroma.
It has been suggested that HB-EGF is expressed by
cervical cancer-associated stromal fibroblasts, thus promoting
cancer cell proliferation in a paracrine manner (45). However, although our study showed
that tumor stroma was indeed positive for HB-EGF, the largest
relative amount of HB-EGF was detected in the cytoplasm of cervical
cancer cells, as the relative amount of HB-EGF in the epithelial
compartment exceeded the relative amount of HB-EGF in the stromal
compartment by a factor of four. Thus, we conclude that HB-EGF is
expressed in an autocrine manner in cervical cancer. In
vitro experiments with head and neck squamous cell carcinoma
cells show that this HB-EGF autocrine loop is associated with
invasive processes through the EGFR-Src-cortactin cascade (46).
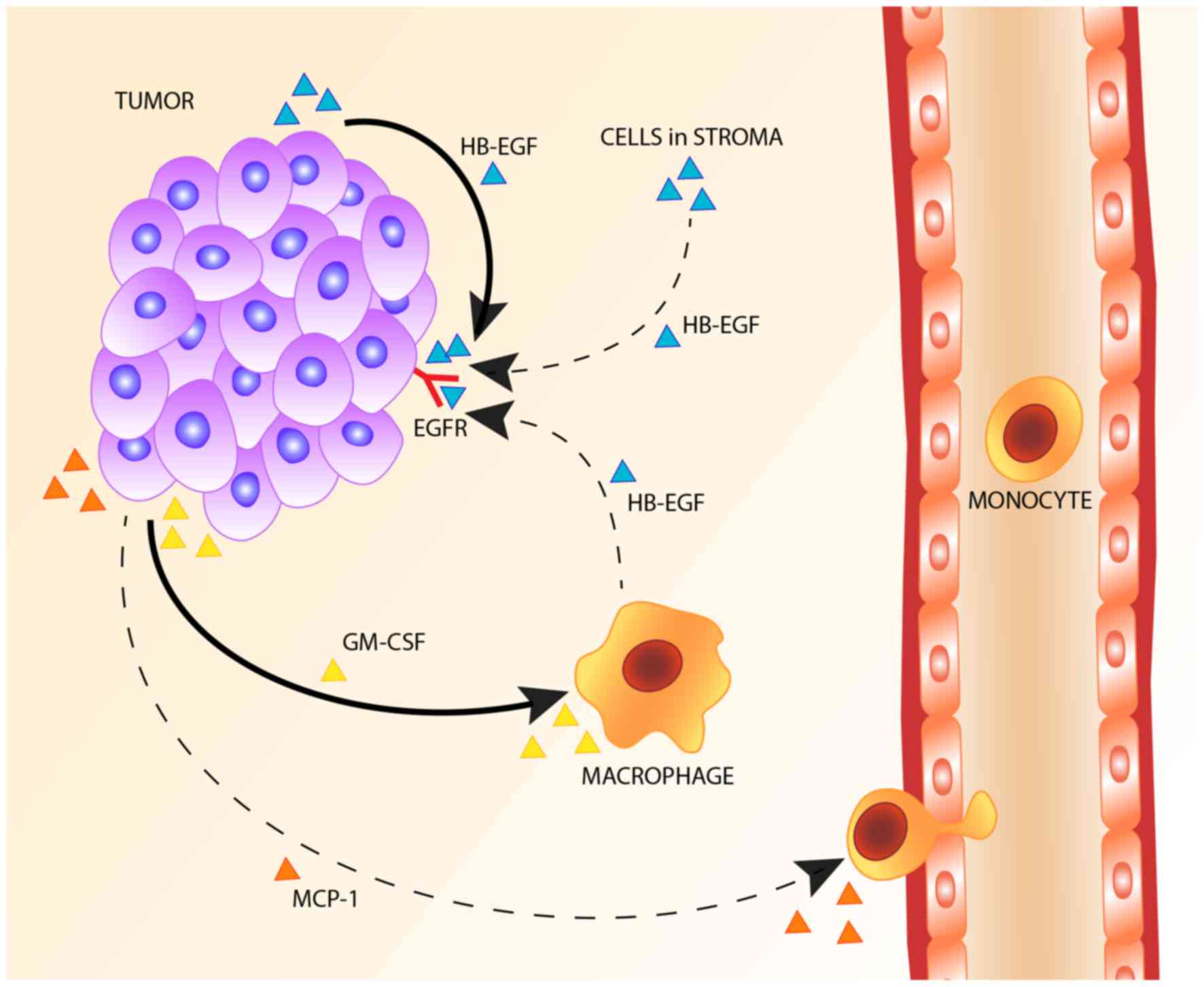
Based on our results, we propose an autocrine EGFR
stimulation model in cervical carcinoma, with cervical cancer cells
being the predominant source of HB-EGF. Cervical cancer cells are
shown to express GM-CSF to attract macrophages to the tumor
environment. Although macrophages and other stromal cells express
the primary EGFR ligand HB-EGF, they do not appear to be the major
source of HB-EGF, as the relative amount of HB-EGF measured in the
tumor cell compartment was on average four times higher than the
relative amount of HB-EGF in the stromal compartment. The proposed
autocrine mechanism of HB-EGF expression is shown in Fig. 5.
Abbreviations:
|
AREG
|
amphiregulin
|
|
CCL2
|
C-C motif ligand 2
|
|
EGF
|
epidermal growth factor
|
|
EGFR
|
epidermal growth factor receptor
|
|
GM-CSF
|
granulocyte-macrophage
colony-stimulating factor
|
|
HB-EGF
|
heparin-binding EGF-like growth
factor
|
|
HPV
|
human papillomavirus
|
|
TAM
|
tumor-associated macrophages
|
|
TGFα
|
transforming growth factor α
|
Acknowledgments
We gratefully acknowledge Natalja T. ter Haar for
her technical support and Patty M. Jansen for her helpful
suggestions concerning the HB-EGF-positive cells in the stroma.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Walboomers JM, Jacobs MV, Manos MM, Bosch
FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ and Muñoz N:
Human papillomavirus is a necessary cause of invasive cervical
cancer worldwide. J Pathol. 189:12–19. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
de Martel C, Ferlay J, Franceschi S,
Vignat J, Bray F, Forman D and Plummer M: Global burden of cancers
attributable to infections in 2008: A review and synthetic
analysis. Lancet Oncol. 13:607–615. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Joura EA, Ault KA, Bosch FX, Brown D,
Cuzick J, Ferris D, Garland SM, Giuliano AR, Hernandez-Avila M, Huh
W, et al: Attribution of 12 high-risk human papillomavirus
genotypes to infection and cervical disease. Cancer Epidemiol
Biomarkers Prev. 23:1997–2008. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Koutsky L: Epidemiology of genital human
papillomavirus infection. Am J Med. 102A:3–8. 1997. View Article : Google Scholar
|
|
6
|
Utrera-Barillas D, Castro-Manrreza M,
Castellanos E, Gutiérrez-Rodríguez M, Arciniega-Ruíz de Esparza O,
García-Cebada J, Velazquez JR, Flores-Reséndiz D,
Hernández-Hernández D and Benítez-Bribiesca L: The role of
macrophages and mast cells in lymphangiogenesis and angiogenesis in
cervical carcinogenesis. Exp Mol Pathol. 89:190–196. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Boccardo E, Lepique AP and Villa LL: The
role of inflammation in HPV carcinogenesis. Carcinogenesis.
31:1905–1912. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lepique AP, Daghastanli KR, Cuccovia IM
and Villa LL: HPV 16 tumor associated macrophages suppress
antitumor T cell responses. Clin Cancer Res. 15:4391–4400. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mantovani A, Germano G, Marchesi F,
Locatelli M and Biswas SK: Cancer-promoting tumor-associated
macrophages: New vistas and open questions. Eur J Immunol.
41:2522–2525. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mantovani A and Sica A: Macrophages,
innate immunity and cancer: Balance, tolerance, and diversity. Curr
Opin Immunol. 22:231–237. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
de Vos van Steenwijk PJ, Ramwadhdoebe TH,
Goedemans R, Doorduijn EM, van Ham JJ, Gorter A, van Hall T,
Kuijjer ML, van Poelgeest MI, van der Burg SH, et al:
Tumor-infiltrating CD14-positive myeloid cells and CD8-positive
T-cells prolong survival in patients with cervical carcinoma. Int J
Cancer. 133:2884–2894. 2013.PubMed/NCBI
|
|
12
|
Fujimoto J, Sakaguchi H, Aoki I and Tamaya
T: Clinical implications of expression of interleukin 8 related to
angiogenesis in uterine cervical cancers. Cancer Res. 60:2632–2635.
2000.PubMed/NCBI
|
|
13
|
Pollard JW: Tumour-educated macrophages
promote tumour progression and metastasis. Nat Rev Cancer. 4:71–78.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Petrillo M, Zannoni GF, Martinelli E,
Pedone Anchora L, Ferrandina G, Tropeano G, Fagotti A and Scambia
G: Polarisation of tumor-associated macrophages toward M2 phenotype
correlates with poor response to chemoradiation and reduced
Survival in patients with locally advanced cervical cancer. PLoS
One. 10:e01366542015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gong W, Xiao Y, Wei Z, Yuan Y, Qiu M, Sun
C, Zeng X, Liang X, Feng M and Chen Q: Toward the use of precision
medicine for the treatment of head and neck squamous cell
carcinoma. Oncotarget. 8:2141–2152. 2017.
|
|
16
|
Heinemann V, von Weikersthal LF, Decker T,
Kiani A, Vehling-Kaiser U, Al-Batran SE, Heintges T, Lerchenmüller
C, Kahl C, Seipelt G, et al: FOLFIRI plus cetuximab versus FOLFIRI
plus bevacizumab as first-line treatment for patients with
metastatic colorectal cancer (FIRE-3): A randomised, open-label,
phase 3 trial. Lancet Oncol. 15:1065–1075. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Greenhalgh J, Dwan K, Boland A, Bates V,
Vecchio F, Dundar Y, Jain P and Green JA: First-line treatment of
advanced epidermal growth factor receptor (EGFR) mutation positive
non-squamous non-small cell lung cancer. Cochrane Database Syst
Rev. 5:CD0103832016.
|
|
18
|
Schrevel M, Gorter A, Kolkman-Uljee SM,
Trimbos JB, Fleuren GJ and Jordanova ES: Molecular mechanisms of
epidermal growth factor receptor overexpression in patients with
cervical cancer. Mod Pathol. 24:720–728. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Narayanan R, Kim HN, Narayanan NK, Nargi D
and Narayanan B: Epidermal growth factor-stimulated human cervical
cancer cell growth is associated with EGFR and cyclin D1
activation, independent of COX-2 expression levels. Int J Oncol.
40:13–20. 2012.
|
|
20
|
Reyes HD, Thiel KW, Carlson MJ, Meng X,
Yang S, Stephan JM and Leslie KK: Comprehensive profiling of
EGFR/HER receptors for personalized treatment of gynecologic
cancers. Mol Diagn Ther. 18:137–151. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kassouf E, Tabchi S and Tehfe M: Anti-EGFR
therapy for metastatic colorectal cancer in the era of extended RAS
gene mutational analysis. BioDrugs. 30:95–104. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sacco AG and Worden FP: Molecularly
targeted therapy for the treatment of head and neck cancer: A
review of the ErbB family inhibitors. Onco Targets Ther.
9:1927–1943. 2016.PubMed/NCBI
|
|
23
|
Leek RD, Hunt NC, Landers RJ, Lewis CE,
Royds JA and Harris AL: Macrophage infiltration is associated with
VEGF and EGFR expression in breast cancer. J Pathol. 190:430–436.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zijlmans HJ, Fleuren GJ, Baelde HJ, Eilers
PH, Kenter GG and Gorter A: The absence of CCL2 expression in
cervical carcinoma is associated with increased survival and loss
of heterozygosity at 17q11.2. J Pathol. 208:507–517. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zijlmans HJ, Fleuren GJ, Baelde HJ, Eilers
PH, Kenter GG and Gorter A: Role of tumor-derived proinflammatory
cytokines GM-CSF, TNF-alpha, and IL-12 in the migration and
differentiation of antigen-presenting cells in cervical carcinoma.
Cancer. 109:556–565. 2007. View Article : Google Scholar
|
|
26
|
Edwards JP, Zhang X and Mosser DM: The
expression of heparin-binding epidermal growth factor-like growth
factor by regulatory macrophages. J Immunol. 182:1929–1939. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rigo A, Gottardi M, Zamo A, Mauri P,
Bonifacio M, Krampera M, Damiani E, Pizzolo G and Vinante F:
Macrophages may promote cancer growth via a GM-CSF/HB-EGF paracrine
loop that is enhanced by CXCL12. Mol Cancer. 9:2732010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mascia F, Cataisson C, Lee TC, Threadgill
D, Mariani V, Amerio P, Chandrasekhara C, Souto Adeva G, Girolomoni
G, Yuspa SH, et al: EGFR regulates the expression of
keratinocyte-derived granulocyte/macrophage colony-stimulating
factor in vitro and in vivo. J Invest Dermatol. 130:682–693. 2010.
View Article : Google Scholar
|
|
29
|
Fu YY, Nergard JC, Barnette NK, Wang YL,
Chai KX and Chen LM: Proteasome inhibition augments cigarette
smoke-induced GM-CSF expression in trophoblast cells via the
epidermal growth factor receptor. PLoS One. 7:e430422012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wyckoff J, Wang W, Lin EY, Wang Y, Pixley
F, Stanley ER, Graf T, Pollard JW, Segall J and Condeelis J: A
paracrine loop between tumor cells and macrophages is required for
tumor cell migration in mammary tumors. Cancer Res. 64:7022–7029.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Goswami S, Sahai E, Wyckoff JB, Cammer M,
Cox D, Pixley FJ, Stanley ER, Segall JE and Condeelis JS:
Macrophages promote the invasion of breast carcinoma cells via a
colony-stimulating factor-1/epidermal growth factor paracrine loop.
Cancer Res. 65:5278–5283. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pollard JW: Macrophages define the
invasive microenvironment in breast cancer. J Leukoc Biol.
84:623–630. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kloth JN, Gorter A, Fleuren GJ, Oosting J,
Uljee S, ter Haar N, Dreef EJ, Kenter GG and Jordanova ES: Elevated
expression of SerpinA1 and SerpinA3 in HLA-positive cervical
carcinoma. J Pathol. 215:222–230. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Huber W and Gentleman R: matchprobes: A
Bioconductor package for the sequence-matching of microarray probe
elements. Bioinformatics. 20:1651–1652. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lehr HA, van der Loos CM, Teeling P and
Gown AM: Complete chromogen separation and analysis in double
immunohistochemical stains using Photoshop-based image analysis. J
Histochem Cytochem. 47:119–126. 1999. View Article : Google Scholar
|
|
36
|
Sloss CM, Wang F, Palladino MA and Cusack
JC Jr: Activation of EGFR by proteasome inhibition requires HB-EGF
in pancreatic cancer cells. Oncogene. 29:3146–3152. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Vlaicu P, Mertins P, Mayr T, Widschwendter
P, Ataseven B, Hogel B, Eiermann W, Knyazev P and Ullrich A:
Monocytes/macrophages support mammary tumor invasivity by
co-secreting lineage-specific EGFR ligands and a STAT3 activator.
BMC Cancer. 13:1972013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kirma N, Hammes LS, Liu YG, Nair HB,
Valente PT, Kumar S, Flowers LC and Tekmal RR: Elevated expression
of the oncogene c-fms and its ligand, the macrophage
colony-stimulating factor-1, in cervical cancer and the role of
transforming growth factor-beta1 in inducing c-fms expression.
Cancer Res. 67:1918–1926. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Strachan DC, Ruffell B, Oei Y, Bissell MJ,
Coussens LM, Pryer N and Daniel D: CSF1R inhibition delays cervical
and mammary tumor growth in murine models by attenuating the
turnover of tumor-associated macrophages and enhancing infiltration
by CD8(+) T cells. OncoImmunology. 2:e269682013. View Article : Google Scholar
|
|
40
|
Rumelhard M, Ramgolam K, Hamel R, Marano F
and Baeza-Squiban A: Expression and role of EGFR ligands induced in
airway cells by M2.5 and its components. Eur Respir J.
30:1064–1073. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pastore S, Mascia F, Mariotti F, Dattilo
C, Mariani V and Girolomoni G: ERK1/2 regulates epidermal chemokine
expression and skin inflammation. J Immunol. 174:5047–5056. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hoffmann TK, Schirlau K, Sonkoly E,
Brandau S, Lang S, Pivarcsi A, Balz V, Müller A, Homey B, Boelke E,
et al: A novel mechanism for anti-EGFR antibody action involves
chemokine-mediated leukocyte infiltration. Int J Cancer.
124:2589–2596. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhu J, Jia X, Xiao G, Kang Y, Partridge NC
and Qin L: EGF-like ligands stimulate osteoclastogenesis by
regulating expression of osteoclast regulatory factors by
osteoblasts: Implications for osteolytic bone metastases. J Biol
Chem. 282:26656–26664. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Singh NK, Wang D, Kundumani-Sridharan V,
Van Quyen D, Niu J and Rao GN: 15-Lipoxygenase-1-enhanced Src-Janus
kinase 2-signal transducer and activator of transcription 3
stimulation and monocyte chemoattractant protein-1 expression
require redox-sensitive activation of epidermal growth factor
receptor in vascular wall remodeling. J Biol Chem. 286:22478–22488.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Murata T, Mizushima H, Chinen I, Moribe H,
Yagi S, Hoffman RM, Kimura T, Yoshino K, Ueda Y, Enomoto T, et al:
HB-EGF and PDGF mediate reciprocal interactions of carcinoma cells
with cancer-associated fibroblasts to support progression of
uterine cervical cancers. Cancer Res. 71:6633–6642. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hayes KE, Walk EL, Ammer AG, Kelley LC,
Martin KH and Weed SA: Ableson kinases negatively regulate
invadopodia function and invasion in head and neck squamous cell
carcinoma by inhibiting an HB-EGF autocrine loop. Oncogene.
32:4766–4777. 2013. View Article : Google Scholar
|