Introduction
In recent years, the health-promoting and disease
prevention properties of the Mediterranean diet (MD) have been
highlighted (1). The Mediterranean
populations, which follow this pattern of eating for cultural and
natural reasons, present a reduced incidence of chronic
inflammation-derived diseases. The view that the MD is associated
with a reduced incidence of inflammatory diseases is supported by a
large number of studies (2).
Extra-virgin olive oil (EVOO) is a common component of MD, which
for some time has been examined in studies that have highlighted
its health benefits (3–6). In EVOO a number of phenolic compounds
have been identified, including tyrosol (7), hydroxytyrosol (8), oleuropein (9), and many others, as well as
Oleocanthal (OC) [(−)-deacetoxylig-stroside aglycone]. In 2005,
Beauchamp et al identified in OC the pungent component of
EVOO that induces a strong prickling sensation in the throat,
similar to that caused by the non-steroidal anti-inflammatory drug
(NSAID) ibuprofen (10). The
authors showed that OC exhibits anti-inflammatory activity, as it
is an inhibitor of cyclooxygenases (COXs), COX1 and COX2, two
enzymes involved in the synthesis of prostaglandins and
thromboxanes from arachidonic acid (10).
Recently, various studies have shown that OC
exhibits anticancer activities by inhibiting cell proliferation,
migration, and invasion in different human cancer cell types
(11–16). In the breast cancer model OC
suppresses cell proliferation, invasiveness and tumor growth by
inhibiting the HGF-induced phosphorylation of c-Met and suppressing
the Brk/paxillin/Rac1 signaling pathway, via inhibition of Brk
phosphorylation (11). In
vivo studies in mice have shown that OC treatment suppresses
tumor cell growth (11). Moreover,
further studies have demonstrated that OC inhibits the growth of
several breast cancer cell lines by inhibiting the enzymatic
activity of mTOR, a serine/threonine kinase which is involved in
cell survival and proliferation in cancer cells (17).
Hepatocellular carcinoma (HCC), is an
inflammation-related cancer that arises in the context of hepatic
damage and inflammation. HCC is the fifth most common cancer
worldwide, characterized by an increasing incidence and a poor
prognosis (18–22). It is largely asymptomatic until it
is in the advanced state, when the treatments available are often
unsuccessful, the standard treatments being surgical resection and
liver transplantation. Other treatments, such as chemoembolization
and ultrasound ablation techniques also rarely lead to a complete
recovery (22). Standard cancer
drugs such as doxorubicin, cisplatin, and 5-fluorouracil have a
very limited efficacy (22).
Moreover, the latest new targeted therapy approved for the
treatment of patients with advanced HCC, e.g. sorafenib, has a poor
efficacy (23).
In the inflammation process an important role is
attributed to the COX enzymes, although the role of the
cyclooxygenases in hepatocellular carcinogenesis is still unclear.
Some studies have shown an increased expression of COX-2 in
patients with different types of liver disease, suggesting its
possible role in hepatocarcinogenesis, especially in the early
stages (24–26). Results from our laboratory have
confirmed the antitumor and pro-apoptotic effects of COX inhibitors
used alone or in combination with other targeted specific drugs
(27–32). However, our results and those of
other authors suggest that often the anticancer activities of COX
inhibitors might be due to COX-independent effects (32).
Colorectal cancer (CRC) is one of most common
cancers worldwide, with a number of different etiologies. However,
the largest proportion of CRC cases has been linked to
environmental causes, such as chronic intestinal inflammation
(33). Elevated COX-2 expression
has been found in most CRC cancer tissues and it is associated with
poor prognosis (34–37). Large epidemiological studies have
demonstrated that NSAIDs reduce the risk of CRC cancer in humans
and, recently, also an antitumoral activity of NSAIDs has been
described in CRC (38–40).
Although OC has already been shown to inhibit growth
and metastasis as well as tumorigenicity in different tumor cell
types, the underlying molecular mechanism of action in HCC and CRC
is not yet fully understood.
In this study, we investigated the anticancer
effects of OC in HCC and CRC cell lines. Interestingly, OC caused a
loss of cell viability and induced apoptosis in both liver and
colon cancer cells, without affecting the cell viability of healthy
primary hepatocytes, through ROS generation and independently of
COX-2 expression.
Materials and methods
Cell lines, cell culture and
reagents
The human hepatocarcinoma cell lines HepG2, Hep3B,
Huh7, PLC/PRF/5 and the colon carcinoma cell lines SW480 and HT29
used in this study were maintained in RPMI medium (Sigma-Aldrich,
St. Louis, MO, USA), containing 10% (v/v) Fetal Bovine Serum (FBS)
(Gibco, Life Technologies, Monza, Italy).
The HCC cell lines have different characteristics of
differentiation, biological behavior, and genetic defects (31). HepG2 and Hep3B cells were obtained
from the American Type Culture Collection (ATCC, Rockville, MD,
USA). The PLC/PRF/5 cells used in this study were a gift from
Professor O. Bussolati (University of Parma, Parma, Italy). The
other cell lines used were gifts from various sources: the Huh-7
cells from Professor M. Levrero (Department of Internal Medicine,
Sapienza University, Rome, Italy); SW480 cells from Dr J.L. Iovanna
(Inserm, Marseille, France); and HT29 cells from Professor S.
Travali (University of Catania, Catania, Italy). All cell lines
were authenticated by short tandem repeat (STR) profiling (BMR
Genomics, Padua, Italy), and used within 6 months of receipt.
OC was synthesized as previously described (41). Nimesulide, SC560 and ibuprofen were
purchased from Cayman Chemical (Ann Arbor, MI, USA). All the
reagents were dissolved in dimethyl sulfoxide (DMSO;
Sigma-Aldrich)
Cell viability assays
Cells (5×103/well) were distributed into
each well of 96-well microtiter plates and then incubated
overnight. At time 0, the medium was replaced with fresh complete
medium plus 1% (v/v) FBS, and different doses of OC, nimesulide,
SC560 and ibuprofen were added. Cells were cultured for 24, 48 and
72 h. At the end of treatment, MTS assays were performed using the
CellTiter Aqueous OneSolution kit (Promega Corp., Madison, WI, USA)
according to the manufacturer's instructions. Cell viability was
expressed as a percentage of the absorbance measured in the control
cells. Values were expressed as means ± SD of three separate
experiments, each performed in triplicate. In some experiments,
cells were treated with OC plus the antioxidant N-acetyl-L-cysteine
(NAC) (Sigma-Aldrich).
Colony formation assays
The effects of different inhibitor concentrations on
cell growth were also assessed using a clonogenic assay. For this
analysis, 1.0–1.5×103 cells were plated in 6-well plates
in growth medium, and after overnight attachment cells were exposed
either to OC or vehicle for 48 h in fresh complete medium with 1%
(v/v) FBS. The cells were then washed and allowed to grow for 14
days in drug-free complete medium with 10% (v/v) FBS, after which
the cell colonies were fixed with 70% ethanol at 4°C for 20 min.
and stained with crystal violet (0.1% in H2O) for 5 min.
The plates were rinsed with water, air-dried, photographed and
evaluated for colony formation. Colonies containing more than 50
cells were counted.
Data are expressed as a percentage of colonies in
untreated cells and are the means ± SD of three separate
experiments, each of which was performed in duplicate.
Caspase activity assays
Cells (5×103/well) were treated with 25
µM OC, and after 24 h the activity of caspases 3 and 7 were
measured by the Caspase-Glo® 3/7 (Promega Corp.) Assay
according to the manufacturer's instructions. Results were
expressed as arbitrary units (AU). Values were the mean ± SD of
three separate experiments, each performed in duplicate.
Western blot analysis
Cell/well (35×104) were plated in 6-well
plates. After 24 h of treatment whole cellular lysates from cells
were obtained using RIPA buffer (Cell Signaling Technologies Inc.,
Beverly, MA, USA) and western blots were performed using the
methodology for the Odyssey® infrared imaging system
(LI-COR Biosciences, Lincoln, NE, USA), as previously described
(42). Membranes were scanned and
analyzed with an Odyssey infrared imaging system (LI-COR
Biosciences) using Odyssey 3.0 imaging software. Antibody signals
were analyzed as integrated intensities of regions defined around
the bands of interest in either channel, with primary antibodies
raised against β-actin (Sigma-Aldrich), phospho-p38, p38, PARP and
γH2AX (Cell Signaling Technologies Inc.).
Flow cytometry analysis
After 24 h of OC treatment, 0.5×106 cells
were collected and stained with FITC-conjugated Annexin V antibody,
and propidium iodide (Apoptosis detection kit; Dojindo, Munich,
Germany). The number of viable, apoptotic and necrotic cells were
determined using the FACSCalibur flow cytometer (Becton Dickinson,
San Jose, CA, USA). Results are presented as percentage. Values
represent the mean ± SD of two separate experiments.
Measurement of reactive oxygen species
(ROS)
The intracellular accumulation of ROS was determined
using the fluorescent probe 2′,7′-difluorodihydrofluorescein
diacetate (H2DCFDA) and MitoSOX™ Red mitochondrial
superoxide indicator (Invitrogen Corp., Camarillo, CA, USA). Cells
(2×104) were treated with 25–50 µM OC for 24 h
and then incubated with the probe in the dark at 37°C in 5%
CO2 incubator according to the manufacturer's
instructions. Cells were observed with fluorescence microscopy
(Axioskop; Zeiss, Oberkochen, Germany) and photographed.
TUNEL assays
Cells were cultured in 8-well chamber slides
overnight. After treatment for 24 h with 25–50 µM OC, cells
were washed twice with PBS and fixed in 4% paraformaldehyde
solution for 25 min at room temperature. Apoptotic cells were
detected by terminal deoxynucleotidyl transferase-mediated dUTP
nick end-labeling (TUNEL) assay using the DeadEnd™ Colorimetric
TUNEL System kit from Promega Corp., following the manufacturer's
instructions. Cells were visualized with an Axioskop microscope
(Zeiss).
Measurement of mitochondrial membrane
potential
For ΔΨm measurement, cells (2 × 104
cells/well) in chamber slides were treated with 25–50 µM OC
for 24 h. JC-1 staining solution (5 µg/ml, Thermo Fisher
Scientific Inc., Waltham, MA, USA) was added to both treated and
untreated cells at 37°C for 15 min, according to the manufacturer's
instructions. After washing twice with PBS, mitochondrial membrane
potential was monitored by determining the relative amounts of dual
emission with a multiple fluorescence reader (Victor; Perkin Elmer,
Waltham, MA, USA) and cells were visualized with a fluorescence
microscope.
Purification of normal human
hepatocytes
All human tissues were collected with informed
consent following ethical and institutional guidelines. Liver
tissue dissociation and subsequent hepatocyte isolation procedures
were performed as previously described (43). Cell viability was assessed by
Trypan Blue (Sigma-Aldrich) exclusion method, and plating
efficiency assessed as previously described (43).
After 24–48–72 h of exposure to OC, hepatocyte
status (ATP content) was assessed using the
CellTiter-Glo® Luminescent Cell Viability Assay (Promega
Corp.). Fresh medium with drugs was changed daily.
Double-stranded DNA (dsDNA) quantification was
performed with a Quant-iT™ PicoGreen® dsDNA
ultrasensitive fluorescent nucleic acid staining kit (Molecular
Probes; Invitrogen Corp.), as previously described (44). Briefly, after CellTiter-Glo
measurement, each well was incubated with 80 µl Quant-iT
PicoGreen in Tris-Ethylenediaminetetraacetic acid (EDTA) buffer,
and the fluorescence intensity was read on a fluorescent
spectrometer (Synergy HT; BioTek Instruments, Inc., Winooski, VT,
USA) at an excitation wavelength of 488/15 nm and an emission
wavelength of 528/20 nm. dsDNA concentration was quantified by
interpolating the A528 values for the unknowns from a standard
curve of λ DNA using the equation (dsDNA (mg/ml) = 0.1057 × A528 −
61.322; R2=0.9941). The luminescence produced was normalized to the
amount of DNA in each well, and normalized to control cells (0.1%
DMSO). Three separate experiments using different batches of
primary isolated human hepatocytes were used, each experiment
performed in triplicate.
Statistical analysis
Statistical analysis was performed using Student's
two-tailed t-test. Statistical significance was assessed at
p<0.05.
Results
Oleocanthal inhibits cell viability and
colony formation capacity of HCC and CRC cells
To investigate the potential anticancer effects of
OC in hepatocellular carcinoma (HCC), we exposed 3 human HCC cell
lines, characterized by different properties such as:
differentiation status, biological behavior and genetic defects
(27). In particular, as regards
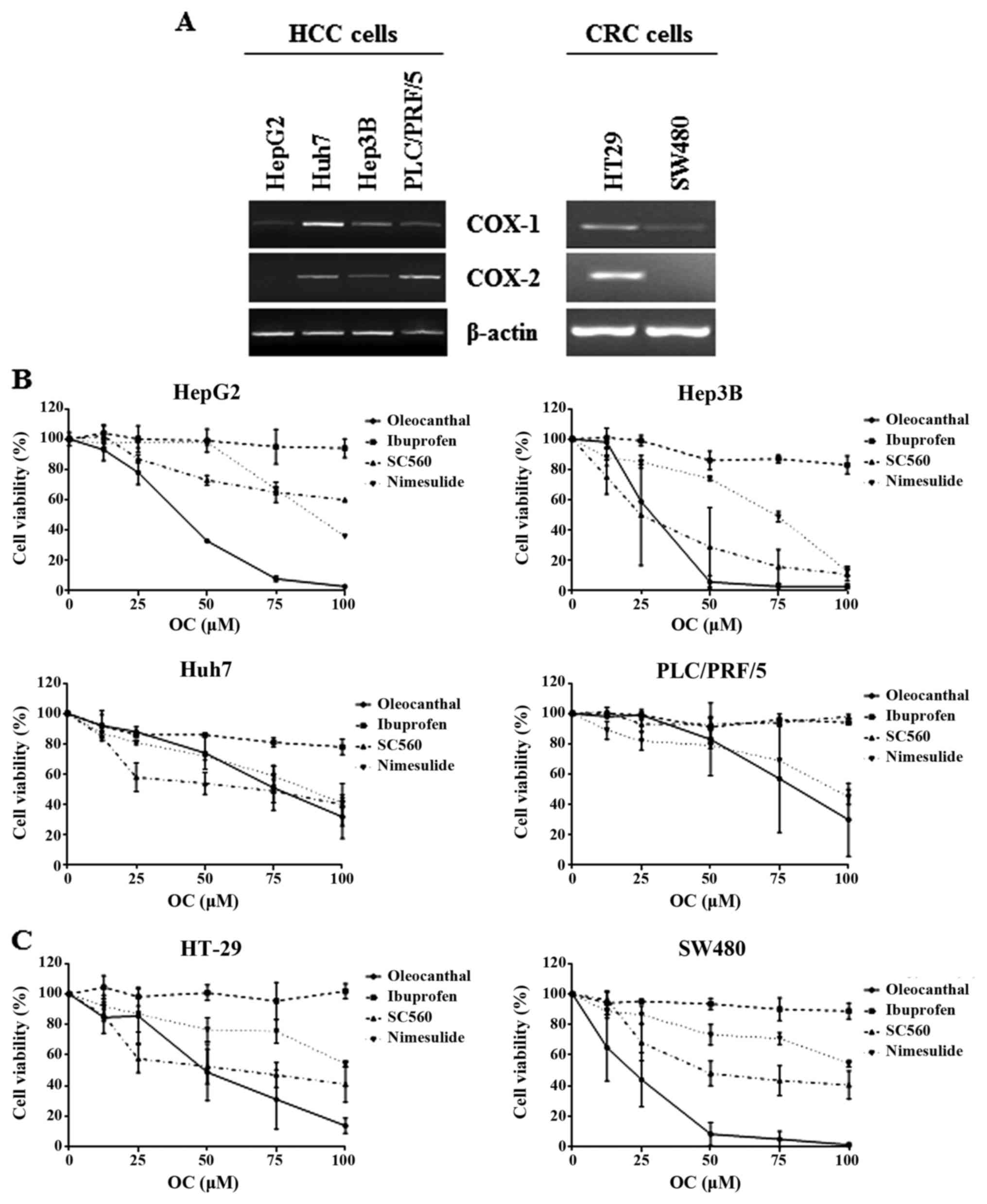
COX-1 and COX-2 mRNA expression, all cell lines expressed COX-1
mRNA, whereas each type of the cell lines showed different
expression levels of COX-2 mRNA (Fig.
1A); PLC/PRF/5 cells expressed the highest COX-2 mRNA levels
while in HepG2 cells COX-2 mRNA was undetectable (Fig. 1A).
To determine whether the effects of OC were
cell-type specific we also used a different cancer model, i.e.
human colorectal carcinoma (CRC). For this purpose, we used the
HT29 and SW480 cells, which are known to be COX-2-positive and
COX-2-negative, respectively (45)
(Fig. 1A).
Since OC displays anti-inflammatory properties
similar to the nonsteroidal anti-inflammatory drug (NSAID)
ibuprofen (10), a dual inhibitor
of cyclooxygenase (COX) enzymes COX-1 and COX-2, we compared the
effects on cell viability and cell survival of OC with that of
ibuprofen, as well as, the COX-2 inhibitor nimesulide and the COX-1
inhibitor SC560.
Cell viability assays were performed in HCC and CRC
cells using different concentrations of OC and other
anti-inflammatory compounds (Fig. 1B
and C). Of note, after treatment for 72 h, OC was more
effective than ibuprofen in inhibiting proliferation in all the
cell lines analyzed. In Hep3B cells, treatment with OC showed the
strongest inhibition of cell viability with an IC50
value of 26.6 µM, followed by HepG2 cells
(IC50=41.9 µM), whereas in Huh7 and PLC/PRF/5
cells, OC displayed an effect comparable to that of nimesulide and
SC560 (Fig. 1B).
In addition, as shown in Fig. 1C, OC inhibited cell viability in a
dose-dependent manner in both CRC cell lines. OC was more effective
than the NSAIDs nimesulide and ibuprofen, and also SC560, as
already observed in HCC cells (Fig.
1B). Time course analyses of cell viability assays did not show
any time-dependent effects of OC (data not shown).
Several lines of evidence have pointed out that the
effect of NSAIDs does not always depend on their effect on the
inhibition of COX enzyme activities, i.e. the mechanism of action
of NSAIDs is also COX-2-independent (32,45–47).
Therefore, we continued all our studies using the HCC cell line
HepG2 cells and the CRC SW480 cells, as models for COX-2 negative
cells, and Hep3B cells and HT-29, respectively, as models for COX-2
positive cells.
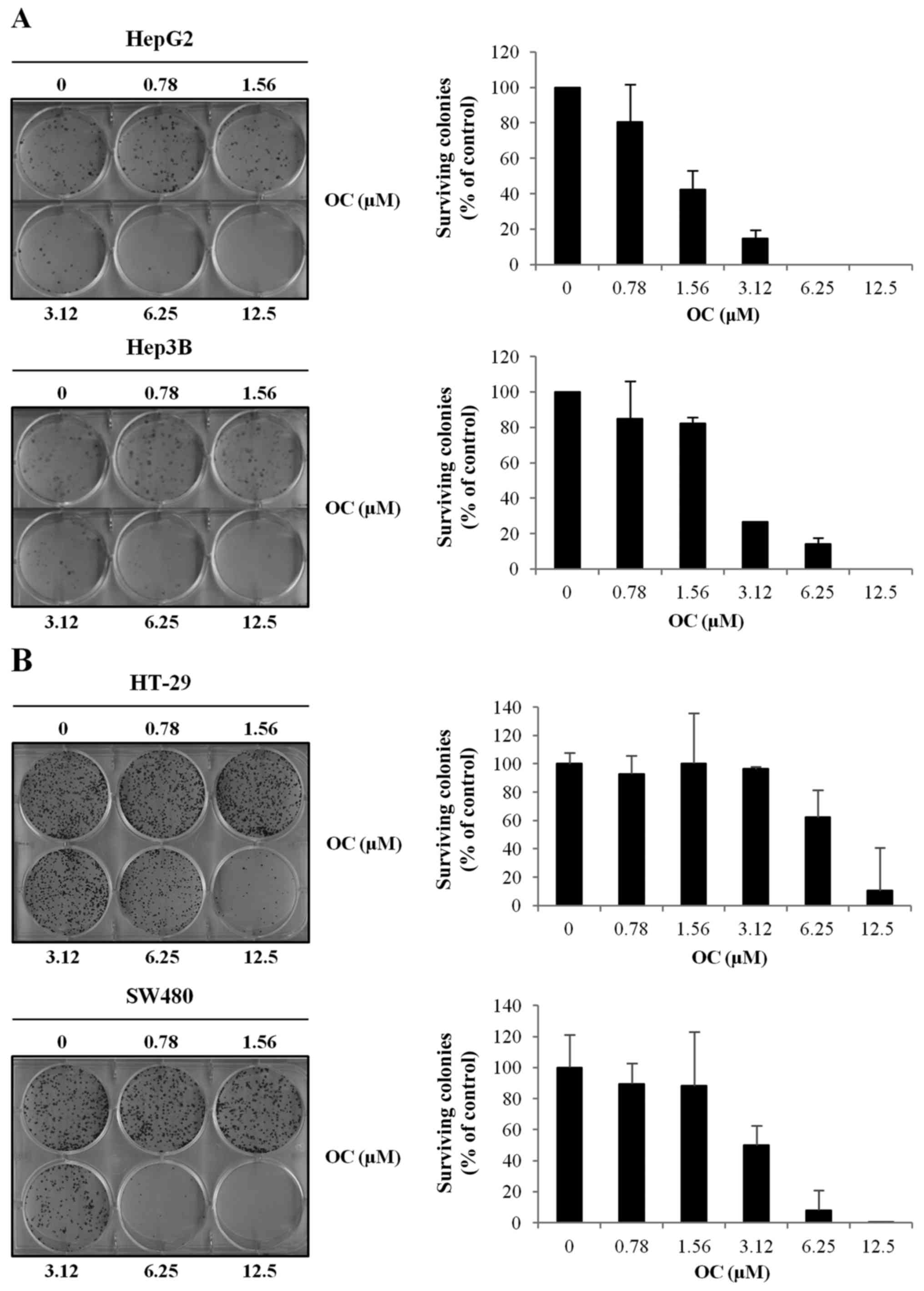
We then investigated the effect of OC on colony
formation in HCC and CRC cells. In both cancer types, OC displayed
a strong dose-dependent inhibition of colony-forming capacity that
was independent of COX-2 expression (Fig. 2A and B).
Oleocanthal induces apoptosis in HCC and
CRC cells
To further explore the mechanism of loss of cell
viability observed after OC treatment, we analyzed the activation
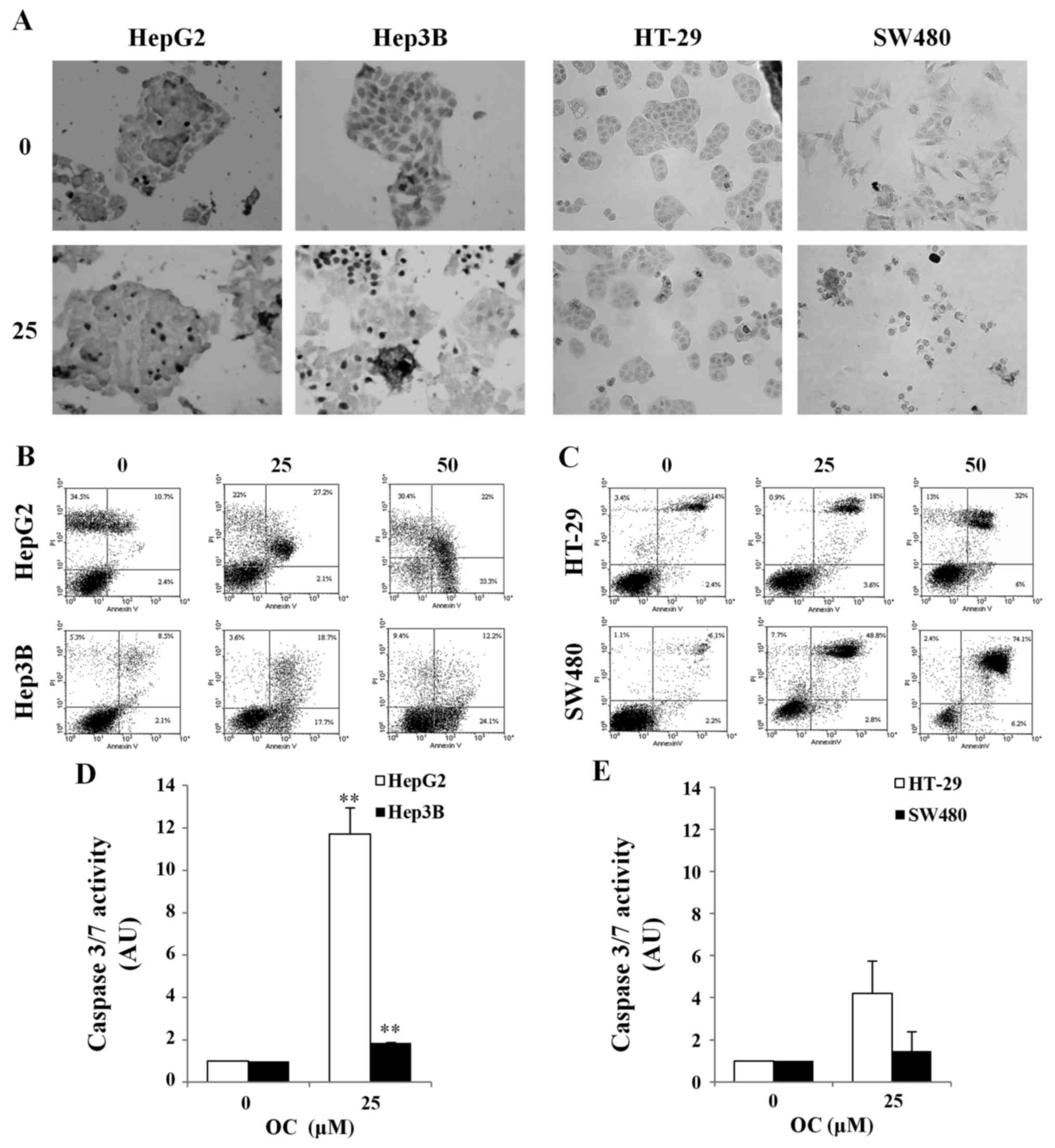
of apoptotic response in HCC and CRC cell lines. The results in
Fig. 3A show that inhibition of
cell viability in HCC and CRC cells after OC treatment is related
to the induction of apoptosis as confirmed by the increased number
of apoptotic cells (brown/dark nuclei). We also quantified
apoptosis after 24 h of treatment with OC by flow cytometry
analysis after staining cells with Annexin V/PI (Fig. 3B and C). The percentage of
apoptotic cells increased after OC treatment from 10% in untreated
Hep3B cells to 30% in Hep3B cells treated with 50 µM OC, and
from 10 to 56% in HepG2 cells (Fig.
3B).
In CRC cells, the percentage of apoptotic cells
increased from 14% to 29% in HT29 and from 17 to 65% in SW480 after
treatment with 50 µM OC (Fig.
3C). All experiments were performed in 1% FCS, a growth
condition that could explain the presence of some dead cells among
the untreated cells (control).
Apoptosis induction was also investigated by
analyzing caspase activation. The caspase assay displayed an
activation of caspases 3/7 after 24 h of treatment with 25
µM OC (Fig. 3D and E) in
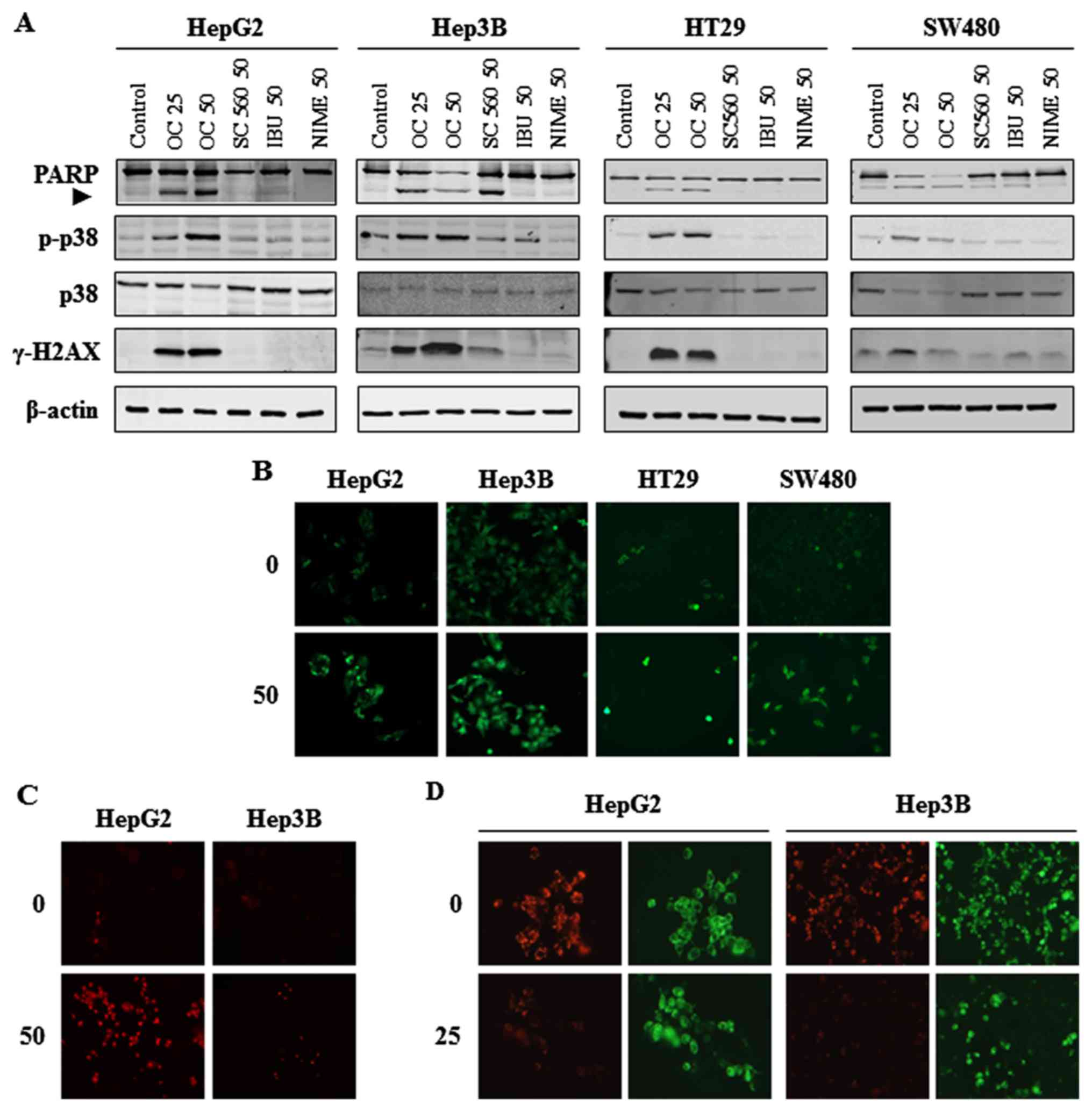
all cell lines. In addition, western blot analyses of HCC and CRC
cell lysates showed cleavage of Poly (ADP-ribose) polymerase (PARP)
after treatment with OC in HepG2, HT29 and SW480 cells, and also
after treatment with OC and SC560 in Hep3B cells. In contrast, a
dose of either 50 µM ibuprofen or nimesulide did not induce
the cleavage of PARP in any of the cell lines (Fig. 4A). Western blot analyses after OC
treatment also indicated an increase in phosphorylated levels of
the stress kinase p38, known to be involved in death signaling
(Fig. 4A). Taken together these
data confirm that OC induced apoptosis in HCC and CRC cells.
Oleocanthal increases reactive oxygen
species (ROS) generation in HCC and CRC cells
A number of studies have shown that phenolic
compounds may have pro-oxidant activities via the production of
reactive oxygen species (ROS), and that ROS generation in cells is
an effective apoptotic inducer (48–50).
To better understand the mechanism of apoptosis
induced by OC, we evaluated ROS production, using the
cell-permeable fluorescent probe H2DCFDA. OC treatment
induced intracellular ROS production in both HCC and CRC cells
(Fig. 4B). Moreover, to identify
the type of ROS produced on OC treatment, we evaluated the presence
of superoxide anions with the MitoSOX-red fluorescent probe. As
shown in Fig. 4C, the production
of superoxide anions increased after treatment with OC in HCC cell
lines.
Since high levels of ROS are known to produce DNA
damage and impair mitochondrial integrity, we next evaluated the
effect of OC treatment on phospho-H2AX (γ-H2AX) expression levels
and on mitochondrial membrane potential. Western blot analysis
showed a strong increase in γ-H2AX histone levels on OC treatment
in all cell lines (Fig. 4A). These
results indicate a strong induction of ROS production which caused
DNA damage after treatment with OC.
Δψm were assessed by staining OC-treated HCC cells
with the membrane permeable dye JC-1, a widely-used probe for
determining changes of ∆ψm (Fig.
4D). JC-1 specifically shows potential-dependent accumulation
in depolarized mitochondria, displaying a red to green fluorescence
shift. The intensity of JC-1 red fluorescence was lower in the
OC-treated cells than in the control, indicating a depolarization
of the mitochondrial membrane (Fig.
4D).
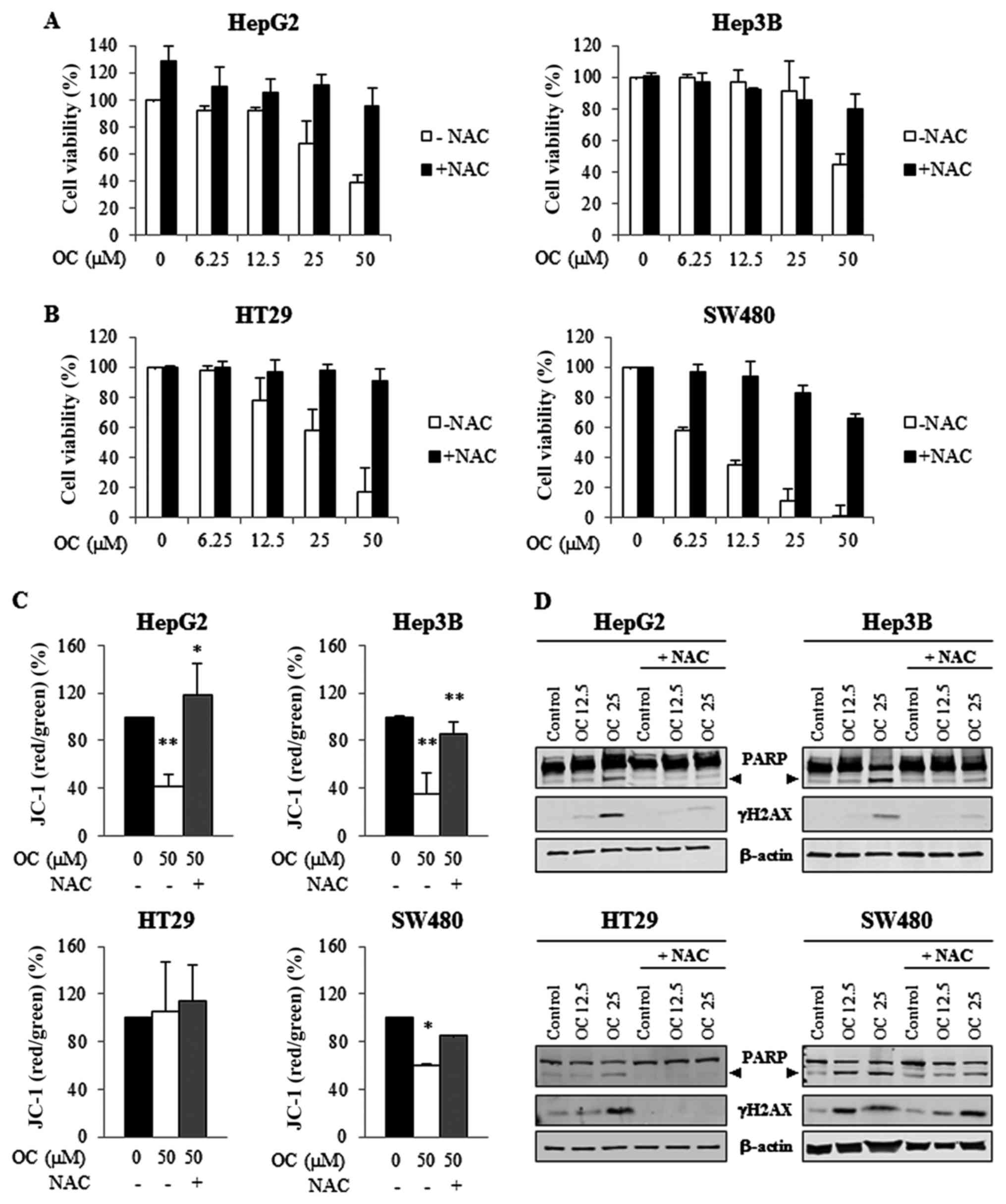
Treatment with N-acetyl-L-cysteine (NAC)
reverses the cytotoxic effects of oleocanthal in HCC and CRC
cells
In the previous experiments, we observed an
induction of ROS generation due to treatment with OC in HCC and CRC
cells. To determine whether OC induces cytotoxic effects via ROS
generation, we tested the effects of the ROS scavenger
N-acetyl-L-cysteine (NAC) on cell viability, on apoptosis
activation and on DNA damage. For these purposes, cells were
pre-treated with NAC (1 mM) for 2 h and subsequently treated with
different concentrations of OC for an additional 24 h in the
presence of NAC.
The cell morphology of HCC and CRC cell lines after
24 h of OC treatment was examined by light microscopy with or
without the presence of NAC (not shown). In samples treated with
both OC and NAC there was a reduction in the number of floating
cells, cells remained spread as non-treated cells, suggesting a
recovery of cell vitality, otherwise in samples treated with OC
alone cells were shrunken, detached, and fragmented into
membrane-bound apoptotic bodies. The recovery in cell viability was
confirmed by MTS assays. In all cell lines, NAC significantly
decreased the OC-induced inhibition of cell viability (Fig. 5A and B). In addition, treatment
with NAC abrogated OC-induced mitochondrial membrane depolarization
and the ratio of red/green JC-1 fluorescence was restored to normal
levels (Fig. 5C).
Furthermore, as shown by western blot analysis
(Fig. 5D), treatment with NAC also
prevented OC-induced PARP cleavage and γ-H2AX activation.
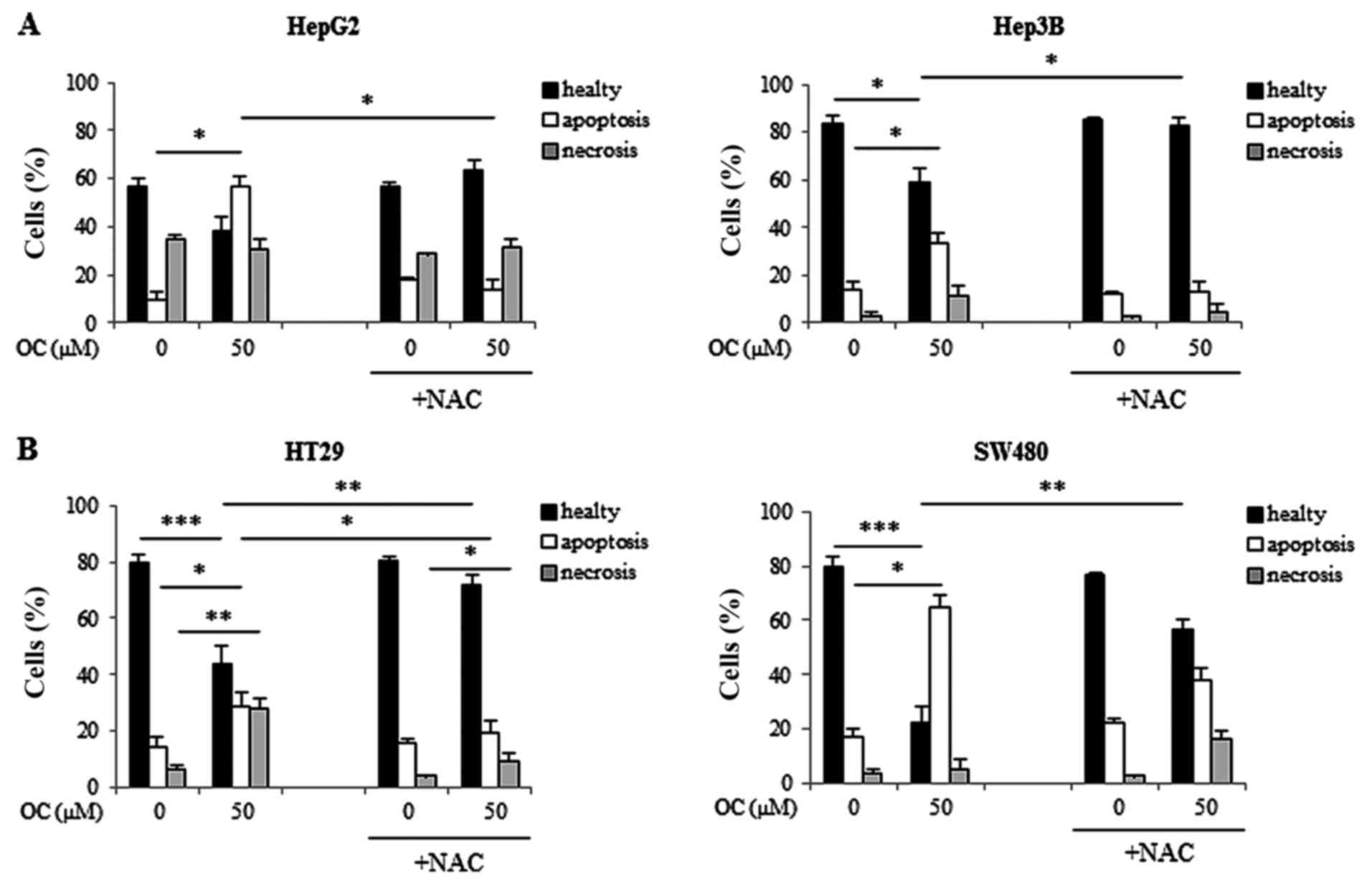
Finally, apoptosis inhibition by NAC treatment was
also confirmed by flow cytometry analysis. Flow cytometry analysis
of cells treated with 50 µM OC in the presence or absence of
NAC confirmed that NAC prevented OC-induced apoptosis, leading to
the recovery of viable cells (Fig. 6A
and B). In Hep3B cells, the percentage of apoptotic cells
declined from 33±8% after treatment with OC to 13±1% after
treatment with OC plus NAC. In HepG2 cells the percentage of
apoptotic cells decreased from 56±6% after treatment with OC to
13±9% after treatment with OC plus NAC (Fig. 6A). In SW480 cells, the percentage
of apoptotic cells diminished from 65±18% after treatment with OC
to 38±2% after treatment with OC plus NAC. In HT29 cells, the
percentage of apoptotic cells declined from 29±4% after treatment
with OC to 19±4% after treatment with OC plus NAC (Fig. 6B).
Taken together, these results indicated that the
oxidative stress induced by OC treatment reduced the cell viability
of HCC and CRC cells, and that apoptosis activation, mitochondrial
and DNA damage are downstream of the oxidative stress.
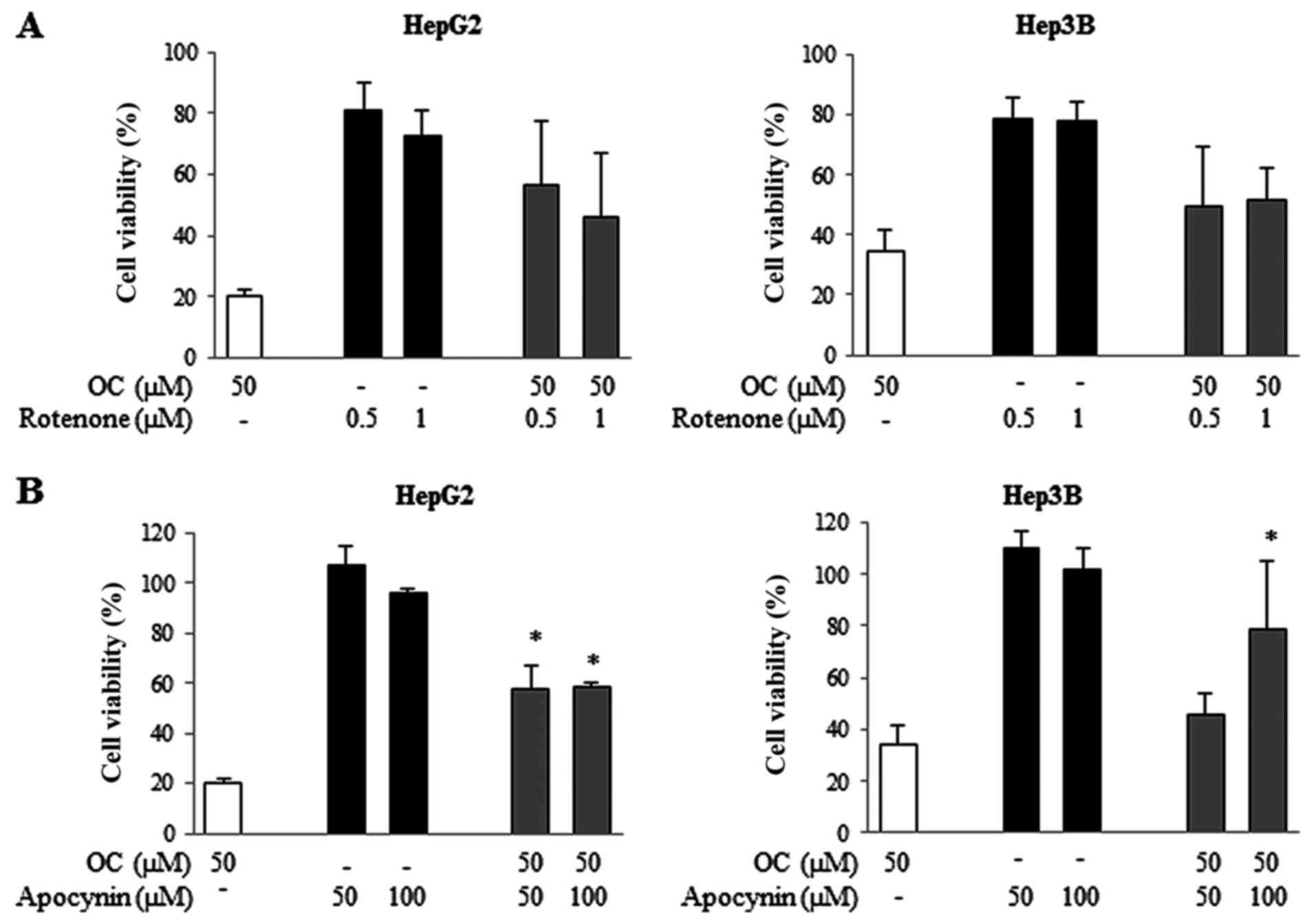
NADPH oxidase enzyme is the main source
of ROS in HCC cells treated with OC
We then investigated the source(s) of ROS in cells
treated with OC. The major recognized sources of ROS in cells are
the mitochondrial respiratory chain (MRC) complexes (51) and the NADPH oxidase (NOX) enzyme
(52,53). We analyzed the effects of specific
inhibitors of each component of MRC and of NADPH oxidase in cells
treated with OC. The MRC complex I inhibitor rote-none, complex II
inhibitor thenoyltrifluoroacetone (TTFA), complex III inhibitor
antimycin, complex IV inhibitor sodium azide and complex V
inhibitor oligomycin were used. HCC cells were treated with OC
alone or in combination with each single MRC inhibitor at different
concentrations for 24 h, after which cell viability assays were
performed. All of them failed to revert cell growth inhibition
induced by OC (data not shown), with the exception of rotenone,
which reversed the effect of OC, however, the inhibition did not
reach statistical significance (Fig.
7A).
Next, the effects of apocynin, an inhibitor of NADPH
oxidase, were examined. The results shown in Fig. 7B demonstrate that in HCC cell lines
apocynin significantly prevented OC-induced cell growth inhibition.
In Hep3B cells cultured in the presence of apocynin, cell viability
increased from 36% to 78.8% in samples treated with OC, whereas in
HepG2 cells it increased from 20 to 58%.
These results suggest that both MRC complex I and
NADPH oxidase are the main sources of ROS in HCC cells treated with
OC.
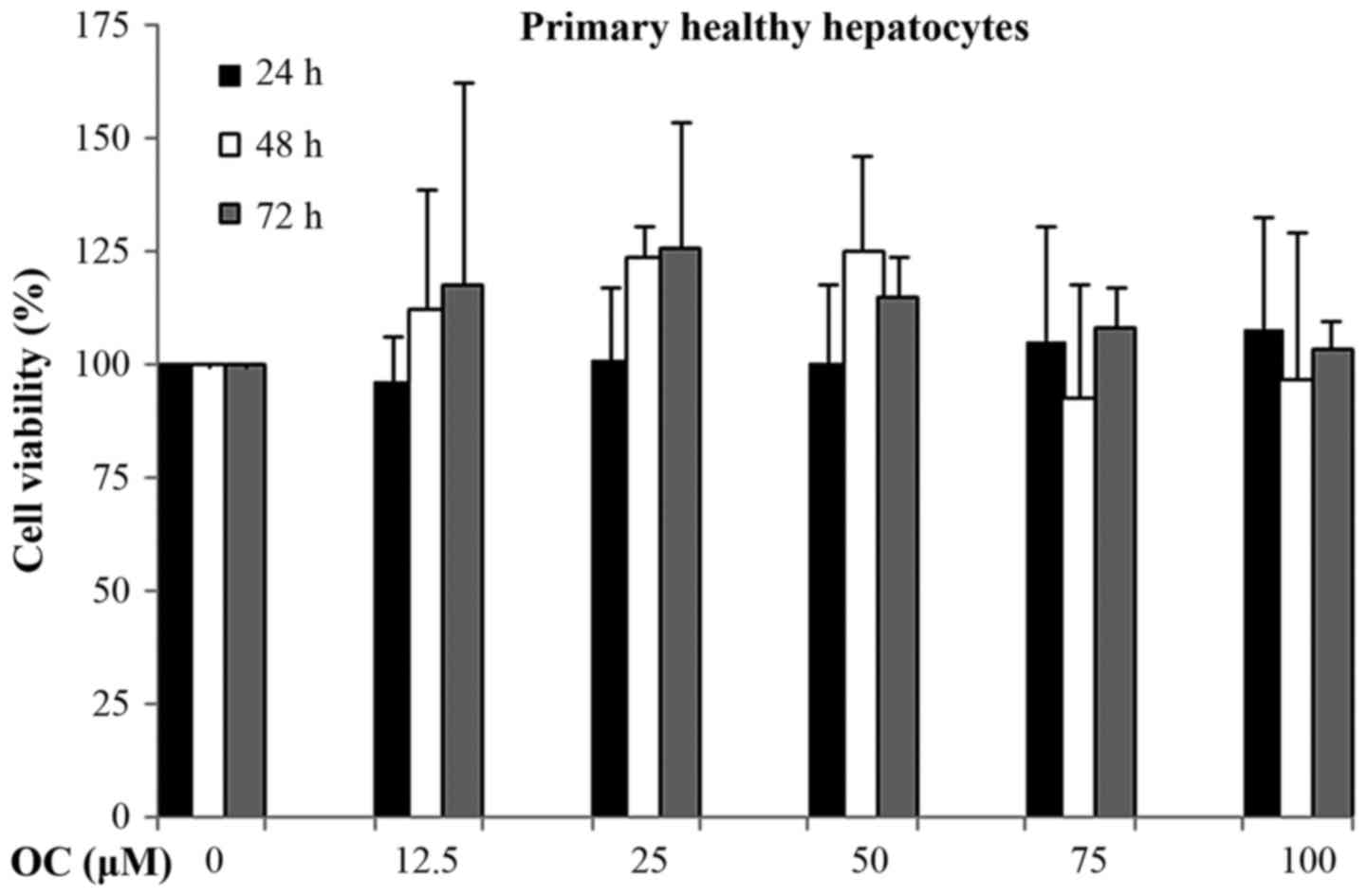
OC is not cytotoxic in primary normal
human hepatocytes
Finally, we tested the effects of OC on primary
normal human hepatocytes, measuring the levels of cytotoxicity by
cell viability assays. Surprisingly, prolonged treatment with OC
(72 h) did not result in reduced hepatocyte viability (Fig. 8). The percentage of hepatocyte
viability was unaffected even at higher OC doses (100 µM).
These results suggest that OC, a natural compound, is cytotoxic for
cancer cells but does not have any effect on the cell viability of
normal healthy hepatocytes.
Discussion
Oleocanthal (OC) was first described as
anti-inflammatory ibuprofen-like compound (10). We and other research groups have
demonstrated that ibuprofen and other NSAID molecules can act as
anticancer drugs (27,28,45–47).
In the current study, we compared the effects on cell viability of
OC in comparison to other NSAIDs, such as the COX-1 inhibitor
SC560, the dual COX-1/COX-2 inhibitor ibuprofen and the COX-2
inhibitor nimesulide. Moreover, to better investigate the role of
COX-2 we used HCC and CRC cell lines which expressed different
levels of COX-2, i.e. Hep3B and HT29 as COX-2 positive cells, and
HepG2 and SW480 as COX-2 negative cells. We demonstrated that OC
exerted antitumor activities in HCC and CRC cells. Our results
demonstrated that the OC inhibitory effect on cell viability was
more effective than the effects of NSAIDs in all the cell lines
tested, and, moreover, its effects were COX-2-independent.
Interestingly at the same dose, OC had no effect on normal human
hepatocytes, suggesting that it inhibits cancer cell viability
while sparing normal cells.
OC inhibited the capacity of HCC and CRC cells to
form colonies and induced apoptosis as demonstrated by the
induction of PARP cleavage as well as the activation of caspases
3/7. Moreover, OC treatment induced intracellular ROS production in
HCC and CRC cells. In particular, in HCC cells we observed a
specific induction in superoxide anions.
It is well known that high levels of ROS cause
damage in all cellular compartments. For example, they can impair
mitochondrial integrity. The γ-H2AX histone is a well-known and
established marker of DNA damage, recruited in DNA double strand
break (DSBs) foci to allow the assembling of repair machinery.
Mitochondrial membrane potential (∆ψm) represents an important
parameter of mitochondrial function and integrity. After OC
treatment, the stability of mitochondrial membrane potential was
significantly impaired in all cell lines.
ROS also causes DNA damage, and we observed a strong
induction of γ-H2AX levels, a marker of DNA damage, in all cell
lines treated with OC. Consequently, the ROS scavenger NAC rescued
cell viability, reduced the number of apoptotic cells and prevented
PARP cleavage after OC treatment in all cell lines. Furthermore,
the increase in γ-H2AX expression, observed after treatment with
OC, was reversed by treatment with NAC, and mitochondrial function
was also restored after co-treatment with NAC.
We identified NADPH oxidase as a main source of ROS
induced by OC treatment, as demonstrated by the improvement in cell
viability of cells co-treated with OC and the NADPH oxidase
inhibitor apocynin. However, our results suggest that also the MRC
complex I might be a source of ROS, particularly in the form of
superoxide anions. In fact, cells co-treated with OC and the MRC
complex I inhibitor rotenone showed a tendency to recover cell
viability in HepG2 and in Hep3B cells.
Polyphenols were first indicated as antioxidant
components of the diet (54) and
initial evidence supported a role of polyphenols in the prevention
of cardiovascular and neurodegenerative disorders, cancers and
other diseases due to their antioxidant activities. Despite this,
the results of the present study demonstrate that oleocanthal, a
phenolic compound of EVOO, exerts a pro-oxidant action on HCC and
CRC cells. In agreement with this finding, numerous studies in
recent years have suggested that polyphenols might also exhibit
pro-oxidant effects by generating ROS (49,50,55).
Therefore, ROS could be said to have a Janus face nature, as it
might contribute on the one hand to carcinogenesis, but, on the
other, might induce cancer cell growth arrest, apoptosis, or
necrosis (56). The precise
mechanism(s) of this behavior is, however, as yet unclear. It has
been speculated that malignant cells, living under an increased
level of oxidative stress, could be more vulnerable to further ROS
increases (57). Studies indicate
that normal cells show a lower steady-state level of ROS than
cancer cells and a constant level of reducing equivalents (58). The different redox status of normal
and cancer cells would allow the development of new promising
therapeutic strategies based on drugs that might alter redox
equilibrium.
In our present study, OC proved to be an excellent
anticancer drug for its ability to kill cancer cells without
affecting normal cells. Overall, our results highlight the
potential of OC in the treatment of HCC and CRC, and provide a
basis for future investigation into its use in humans.
Acknowledgments
This work was supported by PON DI.ME.SA. (Programma
Operativo Nazionale Ricerca e Competitività 2007/2013 -Progetto
'DI.ME.SA'. PON02_00451_3361785. Valorisation of typical products
of the Mediterranean diet and their nutraceutical use to improve
health) granted to G.M. and M.C. Authors thank Dr Amos B. Smith III
and Dr Gary Beauchamp, The Monell Chemical Senses Center,
Philadelphia, PA, USA, for a generous sample of OC.
References
|
1
|
Colomer R and Menéndez JA: Mediterranean
diet, olive oil and cancer. Clin Transl Oncol. 8:15–21. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tripoli E, Giammanco M, Tabacchi G, Di
Majo D, Giammanco S and La Guardia M: The phenolic compounds of
olive oil: Structure, biological activity and beneficial effects on
human health. Nutr Res Rev. 18:98–112. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cicerale S, Lucas LJ and Keast RS:
Antimicrobial, antioxidant and anti-inflammatory phenolic
activities in extra virgin olive oil. Curr Opin Biotechnol.
23:129–135. 2012. View Article : Google Scholar
|
|
4
|
Cicerale S, Conlan XA, Sinclair AJ and
Keast RS: Chemistry and health of olive oil phenolics. Crit Rev
Food Sci Nutr. 49:218–236. 2009. View Article : Google Scholar
|
|
5
|
Cicerale S, Lucas L and Keast R:
Biological activities of phenolic compounds present in virgin olive
oil. Int J Mol Sci. 11:458–479. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Caramia G, Gori A, Valli E and Cerretani
L: Virgin olive oil in preventive medicine: From legend to
epigenetics. Eur J Lipid Sci Technol. 114:375–388. 2012. View Article : Google Scholar
|
|
7
|
Rafehi H, Ververis K and Karagiannis TC:
Mechanisms of action of phenolic compounds in olive. J Diet
(Suppl). 9:96–109. 2012. View Article : Google Scholar
|
|
8
|
Visioli F and Bernardini E: Extra virgin
olive oil's polyphenols: Biological activities. Curr Pharm Des.
17:786–804. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Omar SH: Oleuropein in olive and its
pharmacological effects. Sci Pharm. 78:133–154. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Beauchamp GK, Keast RSJ, Morel D, Lin J,
Pika J, Han Q, Lee C-H, Smith AB and Breslin PAS: Phytochemistry:
Ibuprofen-like activity in extra-virgin olive oil. Nature.
437:45–46. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Akl MR, Ayoub NM, Mohyeldin MM, Busnena
BA, Foudah AI, Liu Y-Y and Sayed KA: Olive phenolics as c-Met
inhibitors: (−)-Oleocanthal attenuates cell proliferation,
invasiveness, and tumor growth in breast cancer models. PLoS One.
9:e976222014. View Article : Google Scholar
|
|
12
|
Elnagar AY, Sylvester PW and El Sayed KA:
(−)-Oleocanthal as a c-Met inhibitor for the control of metastatic
breast and prostate cancers. Planta Med. 77:1013–1019. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Scotece M, Gómez R, Conde J, Lopez V,
Gómez-Reino JJ, Lago F, Smith AB III and Gualillo O: Oleocanthal
inhibits proliferation and MIP-1α expression in human multiple
myeloma cells. Curr Med Chem. 20:2467–2475. 2013. View Article : Google Scholar
|
|
14
|
Fogli S, Arena C, Carpi S, Polini B,
Bertini S, Digiacomo M, Gado F, Saba A, Saccomanni G, Breschi MC,
et al: Cytotoxic activity of oleocanthal isolated from virgin olive
oil on human melanoma cells. Nutr Cancer. 68:873–877. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pei T, Meng Q, Han J, Sun H, Li L, Song R,
Sun B, Pan S, Liang D and Liu L: (−)-Oleocanthal inhibits growth
and metastasis by blocking activation of STAT3 in human
hepatocellular carcinoma. Oncotarget. 7:43475–43491.
2016.PubMed/NCBI
|
|
16
|
Khanal P, Oh W-K, Yun HJ, Namgoong GM, Ahn
S-G, Kwon S-M, Choi H-K and Choi HS: p-HPEA-EDA, a phenolic
compound of virgin olive oil, activates AMP-activated protein
kinase to inhibit carcinogenesis. Carcinogenesis. 32:545–553. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Khanfar MA, Bardaweel SK, Akl MR and El
Sayed KA: Olive oil-derived oleocanthal as potent inhibitor of
mammalian target of rapamycin: Biological evaluation and molecular
modeling studies. Phytother Res. 29:1776–1782. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dhanasekaran R, Limaye A and Cabrera R:
Hepatocellular carcinoma: Current trends in worldwide epidemiology,
risk factors, diagnosis, and therapeutics. Hepat Med. 4:19–37.
2012.PubMed/NCBI
|
|
19
|
El-Serag HB: Epidemiology of viral
hepatitis and hepatocellular carcinoma. Gastroenterology.
142:1264–1273.e1. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ferlay J, Shin H-R, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar
|
|
21
|
Schütte K, Bornschein J and Malfertheiner
P: Hepatocellular carcinoma - epidemiological trends and risk
factors. Dig Dis. 27:80–92. 2009. View Article : Google Scholar
|
|
22
|
Thomas MB and Zhu AX: Hepatocellular
carcinoma: The need for progress. J Clin Oncol. 23:2892–2899. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc J-F, de Oliveira AC, Santoro A, Raoul J-L, Forner
A, et al SHARP Investigators Study Group: Sorafenib in advanced
hepatocellular carcinoma. N Engl J Med. 359:378–390. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cervello M and Montalto G: Cyclooxygenases
in hepatocellular carcinoma. World J Gastroenterol. 12:5113–5121.
2006.PubMed/NCBI
|
|
25
|
Koga H, Sakisaka S, Ohishi M, Kawaguchi T,
Taniguchi E, Sasatomi K, Harada M, Kusaba T, Tanaka M, Kimura R, et
al: Expression of cyclooxygenase-2 in human hepatocellular
carcinoma: Relevance to tumor dedifferentiation. Hepatology.
29:688–696. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Leng J, Han C, Demetris AJ, Michalopoulos
GK and Wu T: Cyclooxygenase-2 promotes hepatocellular carcinoma
cell growth through Akt activation: Evidence for Akt inhibition in
celecoxib-induced apoptosis. Hepatology. 38:756–768. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Foderà D, D'Alessandro N, Cusimano A, Poma
P, Notarbartolo M, Lampiasi N, Montalto G and Cervello M: Induction
of apoptosis and inhibition of cell growth in human hepatocellular
carcinoma cells by COX-2 inhibitors. Ann NY Acad Sci. 1028:440–449.
2004. View Article : Google Scholar
|
|
28
|
Lampiasi N, Foderà D, D'Alessandro N,
Cusimano A, Azzolina A, Tripodo C, Florena AM, Minervini MI,
Notarbartolo M, Montalto G, et al: The selective cyclooxygenase-1
inhibitor SC-560 suppresses cell proliferation and induces
apoptosis in human hepatocellular carcinoma cells. Int J Mol Med.
17:245–252. 2006.PubMed/NCBI
|
|
29
|
Cusimano A, Foderà D, D'Alessandro N,
Lampiasi N, Azzolina A, Montalto G and Cervello M: Potentiation of
the antitumor effects of both selective cyclooxygenase-1 and
cyclooxygenase-2 inhibitors in human hepatic cancer cells by
inhibition of the MEK/ERK pathway. Cancer Biol Ther. 6:1461–1468.
2007. View Article : Google Scholar
|
|
30
|
Cusimano A, Foderà D, Lampiasi N, Azzolina
A, Notarbartolo M, Giannitrapani L, D'Alessandro N, Montalto G and
Cervello M: Prostaglandin E2 receptors and COX enzymes in human
hepatocellular carcinoma: Role in the regulation of cell growth.
Ann NY Acad Sci. 1155:300–308. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cusimano A, Azzolina A, Iovanna JL,
Bachvarov D, McCubrey JA, D'Alessandro N, Montalto G and Cervello
M: Novel combination of celecoxib and proteasome inhibitor MG132
provides synergistic antiproliferative and proapoptotic effects in
human liver tumor cells. Cell Cycle. 9:1399–1410. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cervello M, Bachvarov D, Cusimano A,
Sardina F, Azzolina A, Lampiasi N, Giannitrapani L, McCubrey JA and
Montalto G: COX-2-dependent and COX-2-independent mode of action of
celecoxib in human liver cancer cells. OMICS. 15:383–392. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Terzić J, Grivennikov S, Karin E and Karin
M: Inflammation and colon cancer. Gastroenterology.
138:2101–2114.e5. 2010. View Article : Google Scholar
|
|
34
|
Eberhart CE, Coffey RJ, Radhika A,
Giardiello FM, Ferrenbach S and DuBois RN: Up-regulation of
cyclooxygenase 2 gene expression in human colorectal adenomas and
adenocarcinomas. Gastroenterology. 107:1183–1188. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gupta RA and Dubois RN: Colorectal cancer
prevention and treatment by inhibition of cyclooxygenase-2. Nat Rev
Cancer. 1:11–21. 2001. View Article : Google Scholar
|
|
36
|
Marnett LJ and DuBois RN: COX-2: A target
for colon cancer prevention. Annu Rev Pharmacol Toxicol. 42:55–80.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ogino S, Kirkner GJ, Nosho K, Irahara N,
Kure S, Shima K, Hazra A, Chan AT, Dehari R, Giovannucci EL, et al:
Cyclooxygenase-2 expression is an independent predictor of poor
prognosis in colon cancer. Clin Cancer Res. 14:8221–8227. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Piazuelo E and Lanas A: NSAIDS and
gastrointestinal cancer. Prostaglandins Other Lipid Mediat.
120:91–96. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ghanghas P, Jain S, Rana C and Sanyal SN:
Chemopreventive action of non-steroidal anti-inflammatory drugs on
the inflammatory pathways in colon cancer. Biomed Pharmacother.
78:239–247. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang D and DuBois RN: The role of COX-2 in
intestinal inflammation and colorectal cancer. Oncogene.
29:781–788. 2010. View Article : Google Scholar
|
|
41
|
Smith AB III, Han Q, Breslin PA and
Beauchamp GK: Synthesis and assignment of absolute configuration of
(−)-oleocanthal: A potent, naturally occurring non-steroidal
anti-inflammatory and anti-oxidant agent derived from extra virgin
olive oils. Org Lett. 7:5075–5078. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cusimano A, Puleio R, D'Alessandro N,
Loria GR, McCubrey JA, Montalto G and Cervello M: Cytotoxic
activity of the novel small molecule AKT inhibitor SC66 in
hepatocellular carcinoma cells. Oncotarget. 6:1707–1722. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gramignoli R, Green ML, Tahan V, Dorko K,
Skvorak KJ, Marongiu F, Zao W, Venkataramanan R, Ellis ECS, Geller
D, et al: Development and application of purified tissue
dissociation enzyme mixtures for human hepatocyte isolation. Cell
Transplant. 21:1245–1260. 2012. View Article : Google Scholar
|
|
44
|
Gramignoli R, Tahan V, Dorko K,
Venkataramanan R, Fox IJ, Ellis ECS, Vosough M and Strom SC: Rapid
and sensitive assessment of human hepatocyte functions. Cell
Transplant. 23:1545–1556. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Smith ML, Hawcroft G and Hull MA: The
effect of non-steroidal anti-inflammatory drugs on human colorectal
cancer cells: Evidence of different mechanisms of action. Eur J
Cancer. 36:664–674. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang Y-J, Bao Y-J, Dai Q, Yang W-Y, Cheng
P, Zhu L-M, Wang B-J and Jiang F-H: mTOR signaling is involved in
indo-methacin and nimesulide suppression of colorectal cancer cell
growth via a COX-2 independent pathway. Ann Surg Oncol. 18:580–588.
2011. View Article : Google Scholar
|
|
47
|
Janssen A, Maier TJ, Schiffmann S, Coste
O, Seegel M, Geisslinger G and Grösch S: Evidence of COX-2
independent induction of apoptosis and cell cycle block in human
colon carcinoma cells after S- or R-ibuprofen treatment. Eur J
Pharmacol. 540:24–33. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Khan HY, Zubair H, Ullah MF, Ahmad A and
Hadi SM: A prooxidant mechanism for the anticancer and
chemopreventive properties of plant polyphenols. Curr Drug Targets.
13:1738–1749. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lecci RM, Logrieco A and Leone A:
Pro-oxidative action of polyphenols as action mechanism for their
pro-apoptotic activity. Anticancer Agents Med Chem. 14:1363–1375.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
León-González AJ, Auger C and Schini-Kerth
VB: Pro-oxidant activity of polyphenols and its implication on
cancer chemoprevention and chemotherapy. Biochem Pharmacol.
98:371–380. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Richter C, Ghafourifar P, Schweizer M and
Laffranchi R: Nitric oxide and mitochondrial Ca2+.
Biochem Soc Trans. 25:914–918. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Trudel S, Pâquet MR and Grinstein S:
Mechanism of vanadate-induced activation of tyrosine
phosphorylation and of the respiratory burst in HL60 cells. Role of
reduced oxygen metabolites. Biochem J. 276:611–619. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Bedard K and Krause K-H: The NOX family of
ROS-generating NADPH oxidases: Physiology and pathophysiology.
Physiol Rev. 87:245–313. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Scalbert A, Johnson IT and Saltmarsh M:
Polyphenols: antioxidants and beyond. Am J Clin Nutr. 81(Suppl):
215S–217S. 2005.PubMed/NCBI
|
|
55
|
Ferroni F, Maccaglia A, Pietraforte D,
Turco L and Minetti M: Phenolic antioxidants and the protection of
low density lipoprotein from peroxynitrite-mediated oxidations at
physiologic CO2. J Agric Food Chem. 52:2866–2874. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Gorrini C, Harris IS and Mak TW:
Modulation of oxidative stress as an anticancer strategy. Nat Rev
Drug Discov. 12:931–947. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Trachootham D, Lu W, Ogasawara MA, Nilsa
RD and Huang P: Redox regulation of cell survival. Antioxid Redox
Signal. 10:1343–1374. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Ivanova D, Bakalova R, Lazarova D, Gadjeva
V and Zhelev Z: The impact of reactive oxygen species on anticancer
therapeutic strategies. Adv Clin Exp Med. 22:899–908. 2013.
|