Introduction
Currently, prostate cancer is the most commonly
diagnosed cancer in male and is a major cause of death in men aged
40–70 years (1,2). The gland of prostate surrounds the
base of the male bladder and is likely prone to tumorigenesis in
aged men (3,4). The burden of human prostate cancer in
the world is increasing annually (5). Thus, focusing on therapeutic
strategies for prostate cancer is urgently necessary.
Naturally occurring compounds are considered as the
most interesting agents to test for cancer prevention and therapy,
due to their anticipated multimodal actions and limited toxicity,
affecting the signaling pathways within the cells, including
regulation of cell proliferation, activation of apoptosis and
modulation of cell cycle arrest (6,7).
Chrysophanol (1,8-dihydroxy-3-methylanthraquinone), extracted from
plants of Rheum genus, is one of the anthraquinone
compounds, which has been suggested to induce cell death in
different types of cancer cells (8,9). The
effects of chrysophanol on human prostate cancer cell death have
not been studied. However, the naturally derived compounds have
limitations of preservation, bioavailability and low water
solubility. Thus, delivering the compound requires product
formulations to maintain the active molecular form until
consumption, as well as to preserve stability, bioactivity, and
bio-availability, which is the central goal of developing a
nanoparticle (NP)-based system. Nanoparticulate drug delivery
system for drug intranasal administration needed less amounts of
administrations to induce the required pharmacological reaction due
to its ability to locate on the target region and supply controlled
drug delivery for prolonged time periods (10,11).
Accordingly, the concentrations of polyphenols, which appear to be
effective in vitro, are usually an order of magnitude higher
than the levels tested in vivo (12,13).
Thus, delivering these natural compounds needs product formulations
to keep the active form of the molecule until consumption, and to
maintain stability, bioavailability, and bioactivity, an essential
point to explore a nanoparticle-based system. Surface
functionalization of gold nanoparticles (AuNPs) is important for
biomedical applications, which target them to specific disease
areas and selectively allow them to interact with biomolecules or
cells. Surface conjugation is usually achieved by adsorption of the
ligand to the surface of gold. Thus, they have been widely
investigated for cancer because of their unique size and intrinsic
optical properties, including localized surface plasmon resonance
(14,15). Additionally, long-term circulating
NPs are desirable in systemic applications, including passive
targeting of tumors and inflammatory sites. Poly (ethylene glycol)
(PEG)/poly (lactic-co-glycolic acid (PLGA)-modified NPs have a
long-term circulating property, as they can evade
macrophage-mediated uptake and removal from systemic circulation
(16,17).
Inhibiting cancer cell cycle and proliferation rates
relies on various parameters, including DNA structural alterations
and suppressing the activities or expression of histone
deacetylases (HDACs) (18). These
anti-proliferation promoting activities can make drugs more
specific for various cancers (19,20).
As previously indicated, HDACs was highly expressed during the
cellular oncogenesis (21). HDAC1
was the first identified mammalian HDAC and is considered the
prototype of the HDAC family (22). Overexpression of HDAC1 is
significantly associated with higher lymphatic metastases and
decreased the survival rates in patients with gastric cancer
(23). Recently studies showed
that elevated levels of HDAC3 expression and activity caused
epigenetic alterations associated with malignancies (24). HDAC6 is involved in protein
trafficking and degradation, cell shape and migration. Deregulation
of HDAC6 activity is associated with a variety of diseases
including cancer leading to a growing interest for developing HDAC6
inhibitors (25,26). Increased HDAC6 expression and/or
activity have been demonstrated to promote cell migration and
tissue invasiveness. HDAC6 has also been shown to be required for
oncogenic transformation and tumor formation. Upregulated HDAC6 has
been observed in a number of different cancers and recently,
specific HDAC6 inhibitors have been found to inhibit cell growth
and prevent tumor formation in mouse models (27–29).
Also, the use of HDACs inhibitors could suppress cancer cells both
in vivo and in vitro through regulating gene
expression, and protein levels to prevent tumor progression
(30). We explored the effects of
formulated chrysophanol nanoparticle on human prostate cancer cell
lines in vitro and confirmed the possible molecular
mechanisms involved in apoptosis induction in prostate cancer
cells. We found that chrysophanol nanoparticle could reduce
prostate cancer cell viability by the induction of apoptosis
through ROS, which was associated with p53 expression. Chrysophanol
nanoparticle also decreased the expression of HDACs, indicating its
role in suppressing human prostate cancer cell proliferation. Also,
in vivo, the mouse models revealed that chrysophanol
nanoparticle showed high bioavailability compared to the free
chrysophanol. The significantly reduced tumor volume and size was
observed.
Materials and methods
The preparation of chrysophanol
nanoparticle
High performance liquid chromatography-grade
chrysophanol was purchased from SeeBio (Shanghai, China) in an
anhydrous powder formation. AuNPs were synthesized by
downregulating gold chloride (1 mM) with a freshly prepared
chrysophanol solution in absolute alcohol. The pale-yellow solution
changed to the deep red as chrysophanol nanoparticle (0, 10, 30,
50, 70, 90, 110 and 130 μM) were formed. PLGA (50 mg) was
added into an AuNPs aqueous dispersion, which were added to an
aqueous solution containing a stabilizer. The mixture was then
stirred at 400 rpm, 4°C, until the organic solution had completely
evaporated. The redundant stabilizer was diminished by washing and
centrifugation at 25,000 × g, 4°C, for 30 min. Then, the pellet was
re-suspended in Milli-Q water. Chrysophanol nanoparticles were
stored at 4°C for the following study. Furthermore, the
concentrations of chrysophanol nanoparticles used in our study were
dependent on the doses of chrysophanol attached to nanoparticles.
Thus, the mount of chrysophanol attached to nanomaterial was not
higher than free chrysophanol used in each group. The mean size and
the size distribution of the chrysophanol nanoparticle were
determined by dynamic light scattering (DLS) with a Zetasizer Nano
ZS90 by Malvern Instruments (UK). The surface morphology of the
chrysophanol nanoparticle was determined by SEM (Jeol NeoScope
JCM-5000 Benchtop, WA, USA).
Cells and culture
Human prostate cancer cell lines, DU145, LNCap and
PC3, were purchased from American Type Tissue Collection (ATCC,
USA). Human prostate normal cell line, RWPE-1, and human normal
liver cell line, L02, were obtained from KeyGene Biotech (Nanjing,
China). DU145, LNCap and PC3 cells were cultured in RPMI-1640
(Gibco, USA) supplemented with 10% fetal bovine serum (Gibco).
RWPE-1 and L02 were cultured in DMEM (Gibco) supplemented with 10%
fetal bovine serum (Gibco). All cells were incubated at 37°C and
maintained at 5% CO2. The cells were cultivated in the
absence or presence of various concentrations of chrysophanol
nanoparticles for 24 h, which were harvested for the following
research. All cancer cells were transfected with (100 nM) nonsense
control and siRNA against p53 (#1:5′-GUA AGG AGA AUG GGU AUG GCG U;
#2:5′-GUA UGG GUG AUG AGC AGG GAG AU) for 48 h using Lipofectamine
2000 (Invitrogen, USA) according to the manufacturer's protocol.
The ROS scavenger NAC was purchased from Sigma-Aldrich (USA), which
was treated in the culture medium at a final concentration of 1 mM
for 1 h before chrysophanol nanoparticles administration. P53
inhibitor, pifithrin-α (PIF-α), was obtained from TargetMol (USA)
and was administered to the culture medium at a final concentration
of 30 μM in the absence or presence of chrysophanol
nanoparticles (90 μM) for 24 h. Also, caspase-3 inhibitor,
Z-VAD-FMK (Sigma-Aldrich), at dose of 10 μM, was
administered to cells according to the manufacturer's
instructions.
Survival rate analysis
Human prostate cancer cells (1×104 cells
per well) were planted in triplicate on a 96-well plate and
incubated overnight before various treatment for different time as
indicated. After incubation,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
dye was added, and the mixture was then incubated for 4 h. The dye
was solubilized with 100 μl of DMSO (KeyGen Biotech). The
plates were read at 570 nm on an automated microtiter plate reader.
A blank well that contained only media and drug was used as a
control for all the experiments.
Flow cytometric analysis
The cells were seeded at 0.75×105
cells/ml. Cell cycle assays were carried out in LNCap cells.
Chrysophanol nanoparticle (90 μM) was applied on LNCap cells
for 24 h. Then, all cells were harvested, washed with PBS, fixed
and subsequently incubated with RNaseA (KeyGen Biotech). Next, the
cells were stained with 50 μg/ml propidium iodide (PI,
Sigma-Aldrich) and the readings were obtained in flow cytometer (BD
Biosciences, USA).
For apoptosis analysis, the cells were harvested
after treatment with chrysophanol nanoparticle (90 μM) or
PIF-α (30 μM) for 24 h and stained with the Annexin V/PI
Cell Apoptosis Detection Kit (KeyGen Biotech) according to the
manufacturer's instructions. The cells in early stages of apoptosis
were Annexin V-positive and PI-negative, and the cells in the late
stages of apoptosis were both Annexin V and PI-positive. The
acquisition of results and analysis were carried out with a
Becton-Dickinson FACSCalibur flow cytometer using Cell Quest
software.
The number of apoptotic cells was calculated using
the Fluorescein Active Caspase-3 Staining kit (Abcam, UK) and In
Situ BrdU DNA Fragmentation assay kit (Abnova, USA) according to
the manufacturer's instructions. The proportion of caspase-3 in
active form and TUNEL-positive cells was analyzed by using a flow
cytometer (BD Biosciences).
ROS generation was analyzed by flow cytometry. The
cells treated under various conditions as described, and then were
harvested and fixed with the ice-cold methanol (70%). Fixed cancer
cells were incubated with H2DCFDA (Sigma-Aldrich) for 20
min in the dark according to the manufacturer's instructions.
Fluorescence intensity was determined by the use of FL1H filter of
the flow cytometer.
The cellular uptake and binding
capability with DNA in cells
The cellular uptake of chrysophanol nanoparticle in
LNCap cells was analyzed using fluorescence method. The cancer
cells were washed with PBS twice. The cells after washing were
re-suspended in medium and subsequently incubated with chrysophanol
nanoparticle for different time intervals (0, 60, 90, 120, 180, and
480 min). Cells were then observed under a fluorescence microscope
(Olympus, Japan), and the representative images were captured. For
assessment of the interaction of chrysophanol nanoparticle with
nuclear DNA, LNCap cells were treated with chrysophanol
nanoparticle for different times as indicated. After incubation,
the nuclear DNA of LNCap cells were extracted and purified using
DNA and RNA Purification:Genomic DNA (Sigma-Aldrich). The collected
DNA was applied to analyze the circular dichroism spectra using a
spectroscope.
Hoechst analysis
The prostate cancer cells, after chrysophanol
nanoparticle administration (0, 70, 90 and 110 μM) for 24 h
on 12-well plates were stained with 10 μg/ml of Hoechst
33342 (Life Technologies Corp., USA) in 1 ml PBS for 30 min.
Hoechst 33342 was applied for staining of nuclei of cells. After
staining, samples were rinsed with PBS once and 1ml of PBS was
added for observation using a fluorescent microscope.
Western blot analysis
The prostate cancer cells were harvested after
various treatments and were homogenized into 10% (wt/vol) hypotonic
buffer (pH 8.0, 1 mM EDTA, 5 μg/ml leupeptin, 25 mM
Tris-HCl, 1 mM Pefabloc SC, 5 μg/ml soybean trypsin
inhibitor, 50 μg/ml aprotinin, 4 mM benzamidine) to yield a
homogenate. Also, then the final supernatants were obtained by
centrifugation at 15,000 rpm for 15 min. Protein concentration was
determined by BCA protein assay kit (Thermo Fisher Scientific, USA)
with bovine serum albumin as a standard. The total protein extract
was used for western blot analysis. Equal amounts (40 μg) of
total protein were subjected to 10% SDS-PAGE followed by
immunoblotting with the following primary polyclonal antibodies:
rabbit anti-p53 (Abcam, USA), rabbit anti-Bax (1:1,000, Abcam),
rabbit anti-caspase-3 (1:1,000, Abcam), rabbit anti-AMPK (1:1,000,
Cell Signaling Technology), rabbit anti-p-AMPK (1:1,000, Cell
Signaling Technology), rabbit anti-AKT (1:1,000, Cell Signaling
Technology), rabbit anti-p-AKT (1:1,000, Cell Signaling
Technology), rabbit anti-HDAC1 (1:1,000, Abcam), rabbit anti-HDAC3
(1:1,000, Abcam), rabbit anti-HDAC6 (1:1,000, Abcam), rabbit
anti-Apaf-1 (1:1,000, Abcam), mouse anti-Bcl-2 (1:1,000, Cell
Signaling Technology), rabbit anti-PARP (1:1,000, Cell Signaling
Technology), rabbit anti-p27 (1:1,000, Cell Signaling Technology),
mouse anti-Bcl-xl (1:1,000, Abcam), rabbit anti-Ac-p53 (K382)
(1:1,000, Abcam), rabbit anti-Ac-p53 (K373) (1:1,000, Abcam), mouse
anti-Ac-Histone H3 (AH3-120) (1:500, Santa Cruz Biotechnology,
USA), rabbit anti-Cyto-c (1:1,000, Abcam) and anti-GAPDH (1:500,
Abcam). Immunoreactive bands were visualized by ECL Immunoblot
Detection system (Pierce Biotechnology, Inc., Rockford, IL, USA)
and exposed to Kodak (Eastman Kodak Co., USA) X-ray film. Each
protein expression level was defined as grey value (Version 1.4.2b,
ImageJ, National Institutes of Health, USA) and standardized to
housekeeping gene of GAPDH and expressed as a fold of control. All
experiments were performed in triplicate and done three times
independently.
Chrysophanol pharmacokinetics in
mice
Eighty, 8-week-old C57BL/6J male mice (18–22 g),
were purchased from Shanghai Slac Laboratory Animal Co. Ltd.
(Shanghai, China). The mice were raised in air-conditioned
pathogen-free rooms under controlled lighting (12 h light/day) and
fed with water and standard laboratory food. The animal study was
carried out according to the regulations of The Sixth Affliated
Hospital of Sun Yat-Sen University (Guangzhou, China). The
chrysophanol dose was 100 mg/kg for the free fisetin (n=35) and 50
mg/kg of its nanoemulsion (n=35). Mice were sacrificed at 0, 2, 4,
6, 8, 10, 12, 14, 16, 18, 20, 22 and 24 h. The blood was obtained
by cardiac puncture, centrifuged at 10,000 × g for 10 min, and the
harvested plasma was kept frozen at −20°C until HPLC analysis.
Finally, liver, renal and lung tissue samples were isolated from
mice, and then stored in 4% paraformaldehyde and embedded in
paraffin. Paraffin sections (3-μm thick) were stained with
hematoxylin-eosin (H&E) and observed by light microscopy.
Mouse therapeutic trial
Four to six-week-old, female, BALB/c athymic nude
mice (nu/nu) were purchased from Shanghai Slac Laboratory Animal
Co. Ltd. Mouse care and usage were performed in accordance to local
ethics guidelines. The mice were raised in air-conditioned
pathogen-free rooms under controlled lighting (12 h light/day) and
fed with water and standard laboratory food. The animal study was
carried out according to the regulations of The Sixth Affliated
Hospital of Sun Yat-Sen University (Guangzhou, China). Single-tumor
cell suspensions (LNCap, 2×105) were injected into the
left flank of each mouse subcutaneously to obtain prostate cancer
xenografts. The mice were divided into three groups (15 mice/group)
5 days after cell implantation. Mice in the experimental groups
were intraperitoneally injected with chrysophanol nanoparticle at a
dose of 25 and 50 mg/kg body weight per day. Mice in the control
group received an equal volume of normal saline. The tumor volume
was calculated every two days by two cross-sectional measurements,
and the tumor size was measured as follows: tumor volume =
width2 × length × 0.4. Mice were sacrificed after 28
days, and the tumors were weighed and then fixed in 10% formalin
for the following experiments.
Immunofluorescent assays
All cells were seeded onto glass coverslips for the
experiment. After various treatments with chrysophanol nanoparticle
for 24 h, the cells were harvested and washed with PBS, fixed with
4% formaldehyde for 10 min, permeabilized with 0.1% Triton X-100
for 5 min, blocked with 5% goat serum for 1 h, incubated with
primary antibody overnight and then with secondary antibody (Alexa
Fluor 488 labeled anti-rabbit; Sigma-Aldrich). The antibody used in
the immunofluorescence staining was Cyto-c (1:200; Abcam, UK).
Localization of Cyto-c was visualized using a confocal
microscopy.
Immunohistochemical analysis
Paraffin-embedded tumor sections were used for the
blinded assessment of apoptosis levels. Mouse tumors were sectioned
at 3 μM thickness, and terminal deoxynucleotidyl transferase
(TdT) dUTP nick-end labeling (TUNEL) assay was carried out using
light and electron microscope-based kits (R&D Systems, USA) for
detecting DNA fragments. The tumor samples isolated from mice were
collected, stored in 4% paraformaldehyde and embedded in paraffin.
Paraffin sections (3-μm thick) were stained with
hematoxylin-eosin (H&E) and observed by light microscopy.
Statistical analysis
Data are expressed as the means ± SEM. The treated
cells, tissues and corresponding controls were compared using
GraphPad PRI SM (version 6.0; GraphPad Software, USA) by a one-way
ANOVA with Dunn's least significant difference tests or Student's
t-tests. Differences between groups were considered significant at
P<0.05.
Results
Gold-chrysophanol nanoparticle
characterization and its cytotoxicity to prostate cancer cells
The surface morphology of chrysophanol nanoparticle
was investigated under SEM. The images exhibited the spherically
shaped chrysophanol nanoparticles with a smooth surface without
cracks or pinholes (Fig. 1A). The
DLS results indicated that mean chrysophanol nanoparticle diameter
was 107.5 nm (Fig. 1B), and the
zeta potential of chrysophanol nanoparticle was −18.8 mV (Fig. 1C). In this study, we attempted to
explore if chrysophanol nanoparticle could be useful to prevent
human prostate cancer development. Three prostate cancer cell lines
were chosen to evaluate whether chrysophanol nanoparticle was
effective and specific. Therefore, a dose-dependent (0–130
μM) study using human prostate cancer cell lines, DU145,
LNCap, and PC3, were performed. DU145 cell viability was
downregulated by chrysophanol nanoparticle from 100 to 48.5±1.8%,
LNCap cells were reduced from 100 to 36.8±2.2%, and PC3 cells were
decreased from 100 to 46.9±2.8% (Fig.
1D). As chrysophanol nanoparticle was more toxic to LNCap
cells, all subsequent studies and experiments were conducted using
LNCap cells. Prostate normal cells, RWPE-1, and human L02 normal
liver cells were treated with chrysophanol nanoparticle to
calculate the toxicity to normal cells. As shown in Fig. 1D, cell viability of the two cell
types was decreased but showed a much lesser degree than that in
the prostate cancer cell lines (Fig.
1D). As shown in Fig. 1E–I,
DU145, LNCap, PC3, RWPE-1, and L02 cells were not given any
treatment (Con) or only the nanoparticles (Con-NP) for different
times (0, 2, 4, 6, 8, 12, 14, 16, 18, 20, 22 and 24 h). Then, the
cell viability was evaluated using MTT analysis. The results
indicated that there was no significant difference among various
groups of cells treated under Con and Con-NP for different times,
indicating insignificant difference in results between the control
and the drug untreated groups (only with nanoparticles). Thus, the
media as control was used for comparing the experimental data for
the sake of clarity and brevity.
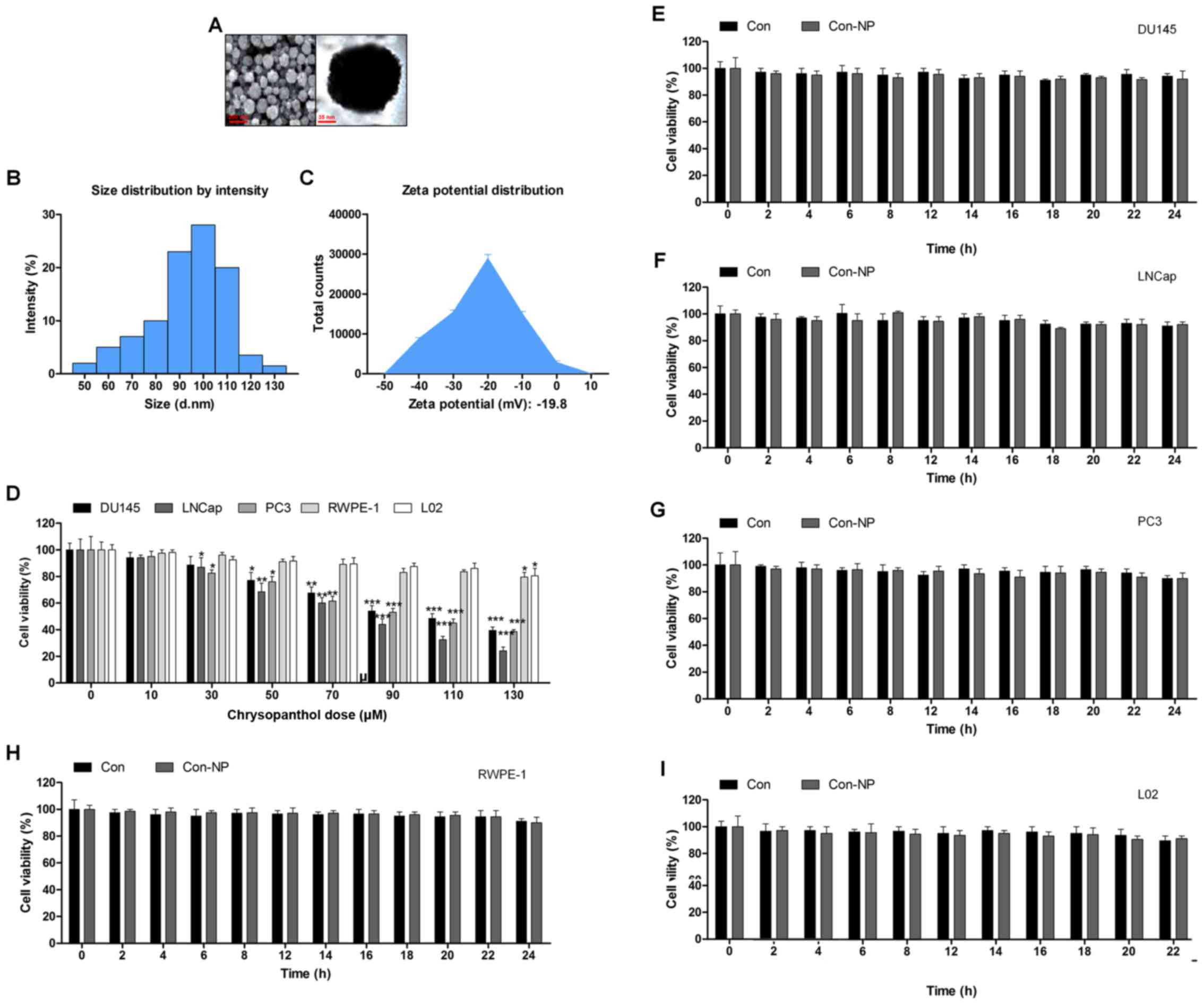 | Figure 1Gold-chrysophanol nanoparticle
characterization and its cytotoxicity to prostate cancer cells. (A)
The scanning electron microscopy (SEM) images of chrysophanol
nanoparticles. (B) Dynamic light scattering (DLS) image of cerium
oxide nanoparticles. (C) Zeta potential of PLGA encapsulated
chrysophanol nanoparticle. (D) Chrysophanol nanoparticle (0–130
μM) was added to human prostate cancer cell line cultures
and human prostate normal epithelia cells, as well as normal liver
cell culture for 24 h. The cell viability was calculated by MTT
assay, and the graph exhibits a gradual downregulation in prostate
cancer cell viability. (E) DU145, (F) LNCap, (G) PC3, (H) RWPE-1,
and (I) L02 cells were not given any treatment (Con) or only the
nanoparticles (Con-NP) for different times as indicated (0, 2, 4,
6, 8, 12, 14, 16, 18, 20, 22 and 24 h). Then, the cell viability
was evaluated using MTT analysis. Data are shown as mean ± SEM.
*P<0.05, **P<0.01 and
***P<0.001 versus the control group in the absence of
any treatment. |
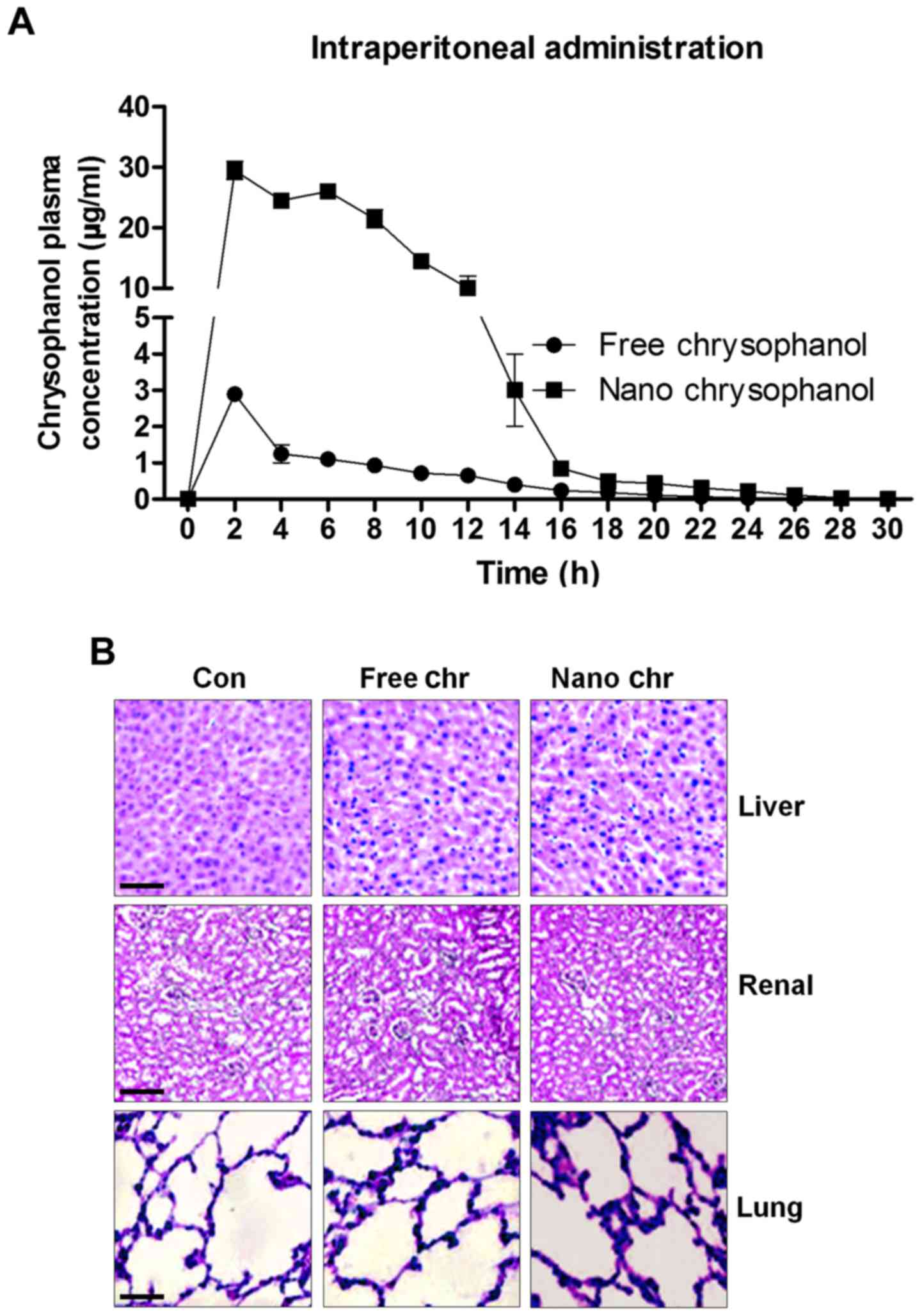
The release of chrysophanol in mice after
intraperitoneal administration and its toxicity to mice
According to the results above, chrysophanol
nanoparticle was effective to suppress the growth of human prostate
cancer cells, especially LNCap. For the chrysophanol nanoemulsion,
a comparison of its pharmacokinetics after intraperitoneal (i.p.)
administration with the free chrysophanol injected by the same
route is shown in Fig. 2A.
Compared to the i.p. administration of free chrysophanol, the
results indicated that injection of chrysophanol nanoparticle
resulted in a significant upregulation of chrysophanol
concentrations in plasma, even at the dose of 50 mg/kg half that of
the free chrysophanol dose (100 mg/kg), indicating the
effectiveness of chrysophanol nanoparticle in the organisms.
Furthermore, H&E staining of liver, renal and lung were
performed to investigate if chrysophanol nanoparticle showed any
toxicity to animals. Compared to the Con groups, no significant
histology was observed in different groups of liver, renal and lung
tissue samples (Fig. 2B). The data
above indicated that chrysophanol nanoparticle might be more
effective than the free chrysophanol for human prostate cancer
prevention with little toxicity to animals.
The effects of chrysophanol nanoparticle
on prostate cancer cell proliferation and cell arrest
A proliferation assay was carried out to further
determine the anti-proliferative action of chrysophanol
nanoparticle and free chrysophanol. The results revealed that the
growth rates of prostate cancer cells treated with chrysophanol
nanoparticle decreased compared to that of the control, and similar
results were observed in LNCap cells with free chrysophanol
treatment. Also, consistently, no significant difference was
observed between the Con and Con-NP group (Fig. 3A). Of note, chrysophanol
nanoparticle exhibited higher anti-proliferative action, which was
comparable to the free chrysophanol group. Chrysophanol
nanoparticle increased the sub G-phase population and reduced the
S-phase cell population, indicating induction of apoptosis in
prostate cancer cells (Fig. 3B).
Additionally, expression of CHK1 and p27, cell cycle-related
proteins, increased, while CDK1 and cyclin D1 were decreased,
indicating that these proteins were related to induction of cell
cycle arrest in chrysophanol nanoparticle-treated cancer cells
(Fig. 3C). We confirmed p-AMPK and
p-AKT expression through western blotting. The results indicated
that expression of the activated form of AMPK (p-AMPK) was
upregulated in a time-dependent manner after chrysophanol
nanoparticle treatment (Fig. 3D).
In contrast, p-AKT was downregulated by chrysophanol nanoparticle
administration. The result suggested that chrysophanol nanoparticle
affected AMPK and AKT phosphorylation, reducing the survivability
and proliferative potential of LNCap cells. Histone deacetylation
activity has been reported to be involved in cell proliferation
(14–16,18).
Here, western blot analysis indicated that HDAC1, HDAC3 and HDAC6
were significantly reduced by chrysophanol nanoparticle treatment
to cells in a time-dependent manner (Fig. 3E). We found that the global level
acetylated Histone 3 (Ac-H3) was highly expressed with the increase
of chrysophanol nanoparticle treatment (Fig. 3F). In addition, chrysophanol
nanoparticle-induced Ac-H3 expression was in a time-dependent
manner, which was contrary to the expression of HDACs in cancer
cells treated under the same conditions. HDACs inhibitor of TSA was
exposed to cells. MTT analysis indicated that the cell viability
was reduced by TSA time-dependently, which was similar to
chrysophanol nanoparticle administration, indicating that
chrysophanol nanoparticle inhibited HDAC activity (Fig. 3F). The data above suggested that
chrysophanol nanoparticle is a suppressor of transcription and
proliferation.
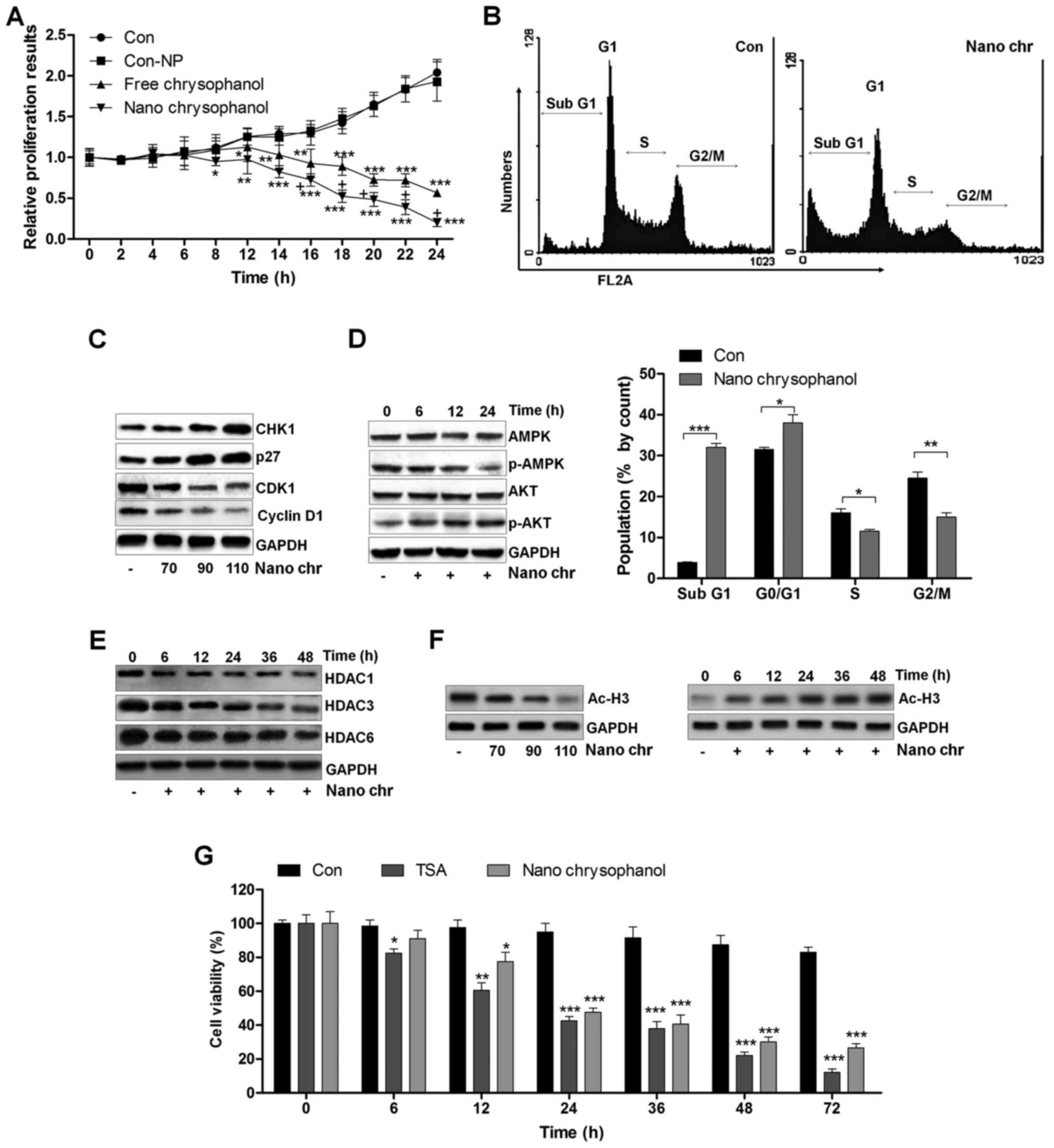 | Figure 3The effects of chrysophanol
nanoparticles on prostate cancer cell proliferation and cell
arrest. (A) LNCap cells were exposed to free chrysophanol,
chrysophanol nanoparticle or only the nanoparticles in the absence
of drug conjugation at 90 μM for 0, 2, 4, 6, 8, 12, 14, 16,
18, 20, 22 or 24 h. Then, the cell viability was evaluated using
MTT analysis. *P<0.05, **P<0.01 and
***P<0.001 versus the control group in the absence of
any treatments. (B) Upper, chrysophanol nanoparticle (90 μM)
was added to LNCap cells for 24 h, and then cell cycle analysis was
performed. Lower, the quantification of cells in different phases
of cell cycle is exhibited. *P<0.05,
**P<0.01 and ***P<0.001. LNCap cells
were treated with 90 μM chrysophanol nanoparticle for 24 h.
(C) Then, western blot analysis of CHK1, p27, CDK1 and cyclin D1
was carried out. (D) AMPK and AKT phosphorylation was calculated
using western blot analysis. (E) LNCap cells were treated with 90
μM chrysophanol nanoparticle for different times, ranging
from 0 to 48 h, followed by HDAC1, HDAC3 and HDAC6 measurement by
western blot assays. (F) LNCap cells were treated as indicated.
Then, western blot analysis was used to calculate the expression
levels of Ac-H3. (G) LNCap cells were administered with HDACs
inhibitor, TSA (5 μM), and chrysophanol nanoparticle (90
μM) for the described time, followed by MTT analysis.
*P<0.05, **P<0.01 and
***P<0.001 versus the control group in the absence of
any treatments. Data are shown as mean ± SEM. |
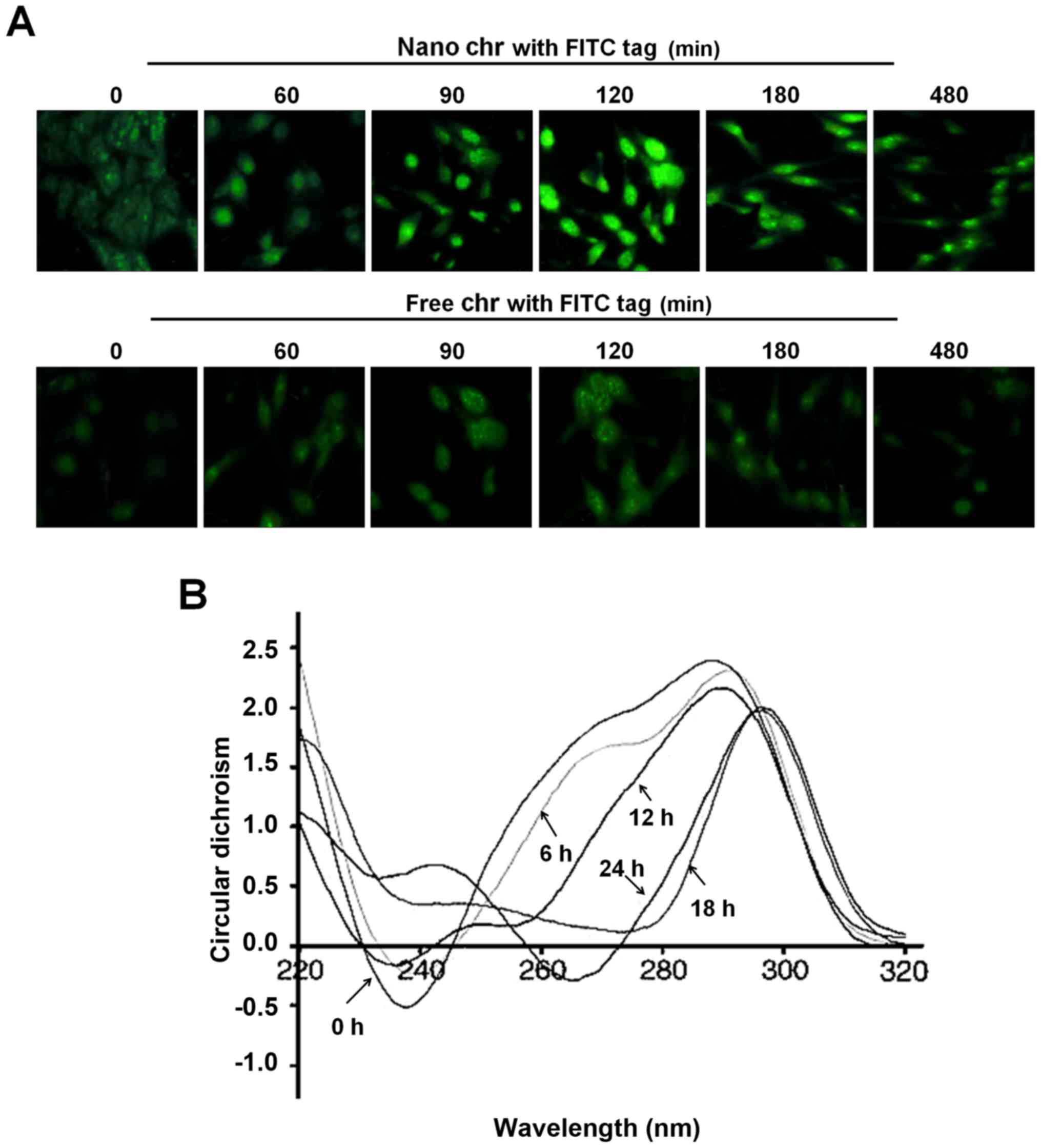
The interaction of chrysophanol
nanoparticle with DNA of prostate cancer cells
A drug entering into cells and its binding capacity
with DNA are important ways to produce cytotoxicity. The
immunofluorescent analysis suggested that chrysophanol nanoparticle
reached a maximum level inside the cells after incubation for 120
min (Fig. 4A). However, in the
cells treated with free chrysophanol exhibited weaker fluorescence
compared to the group of cells with chrysophanol nanoparticles,
indicating the more effective uptake of chrysophanol nanoparticles
to cancer cells. In addition, the circular dichroism spectroscopic
results indicated that chrysophanol nanoparticle injured the native
B-confirmation of DNA in LNCap cells, with a gradually reduced
negative signal and positive hypochromism signals with a peak
shift. These alterations might to be the result of structural
changes in the double helical DNA structure caused by chrysophanol
nanoparticle (Fig. 4B).
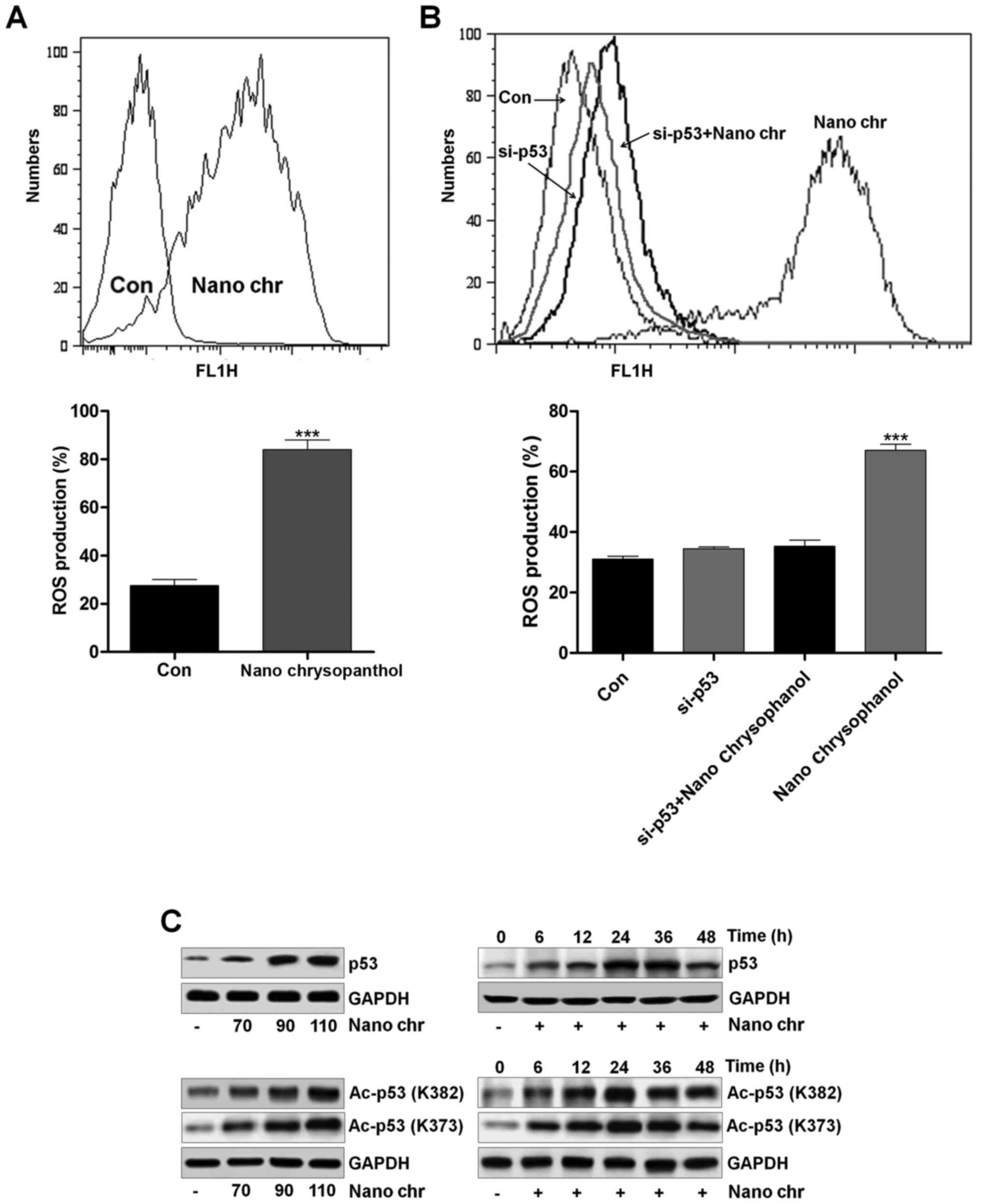
Chrysophanol nanoparticle increases ROS
generation, p53 expression and apoptosis
To reveal the molecular mechanism behind the cell
death, ROS activity in chrysophanol nanoparticle-treated cells was
evaluated using flow cytometry. The results indicated that ROS
levels were produced in LNCap cells after chrysophanol nanoparticle
treatment (Fig. 5A). P53 knockdown
reduced ROS generation (Fig. 5B).
This result confirmed that ROS production following chrysophanol
nanoparticle treatment was an important event to regulate the cell
death and production of ROS might be also controlled by p53
(Fig. 5B). p53 expression was
determined using western blot analysis. As shown in Fig. 5C, p53 expression in LNCap cells was
increased dose-dependently. P53 expression increased early in
chrysophanol nanoparticle treatment and then returned to the lower
level. In addition, we found that Ac-p53 both at K382 and K373
sites was highly induced by chrysophanol nanoparticles in a
dose-dependent manner. Also, administration of chrysophanol
nanoparticles at 110 μM, we found that both Ac-p53 (K382)
and Ac-p53 (K373) were highly induced, especially when treated for
24 h. The results indicated that acetylation of p53 was also
involved in chrysophanol nanoparticle-suppressed prostate cancer
cells. According to previous studies, HDAC inhibitors are also
reported to induce acetylation in non-histone proteins (31). For example, the HDAC1 inhibitor TSA
leads to acetylation of p53 on K373 primarily under conditions in
which cells are subjected to ionizing radiation, acting as a
transcription factor regulating multiple genes important for the
regulation of cell cycle arrest, senescence and apoptosis (32).
LNCap cells were treated with the ROS scavenger NAC
and p53 inhibitor PIF-α, as indicated in Fig. 6A to determine the relationship of
p53 and ROS production. The data indicated that NAC treatment
reduced p53 expression, which was enhanced by chrysophanol
nanoparticle administration. Furthermore, the results showed that
the NAC-treated cells combined with chrysophanol nanoparticle had a
higher level of p53 expression compared to that of NAC-treated
cells, while reduced p53 expression in comparison to the
chrysophanol nanoparticle-treated cells. Also, in PIF-α-treated
cancer cells, p53 was highly reduced, while its combination with
chrysophanol nanoparticle upregulated p53 expression. Additionally,
similar results were observed in p27 expression levels. Then, the
cell viability was measured using MTT analysis. As shown in
Fig. 6B and C, NAC and PIF-α
treatment to cells showed upregulated cell viability, which were
significantly reduced by treatment of chrysophanol nanoparticles.
Also, NAC or PIF-α co-treated with chrysophanol nanoparticle
increased the viability of LNCap cells. The data above indicated
that chrysophanol nanoparticle-reduced cell proliferation was
associated with ROS and p53 expression. Next, p53 expression was
inhibited by PIF-α. Also, apoptosis of cancer cells was
investigated using flow cytometry. The results indicated that PIF-α
treatment decreased the number of apoptotic cells induced by
chrysophanol nanoparticle (Fig.
6D). Thus, chrysophanol nanoparticle-regulated p53 activity was
involved in apoptotic response in prostate cancer cells.
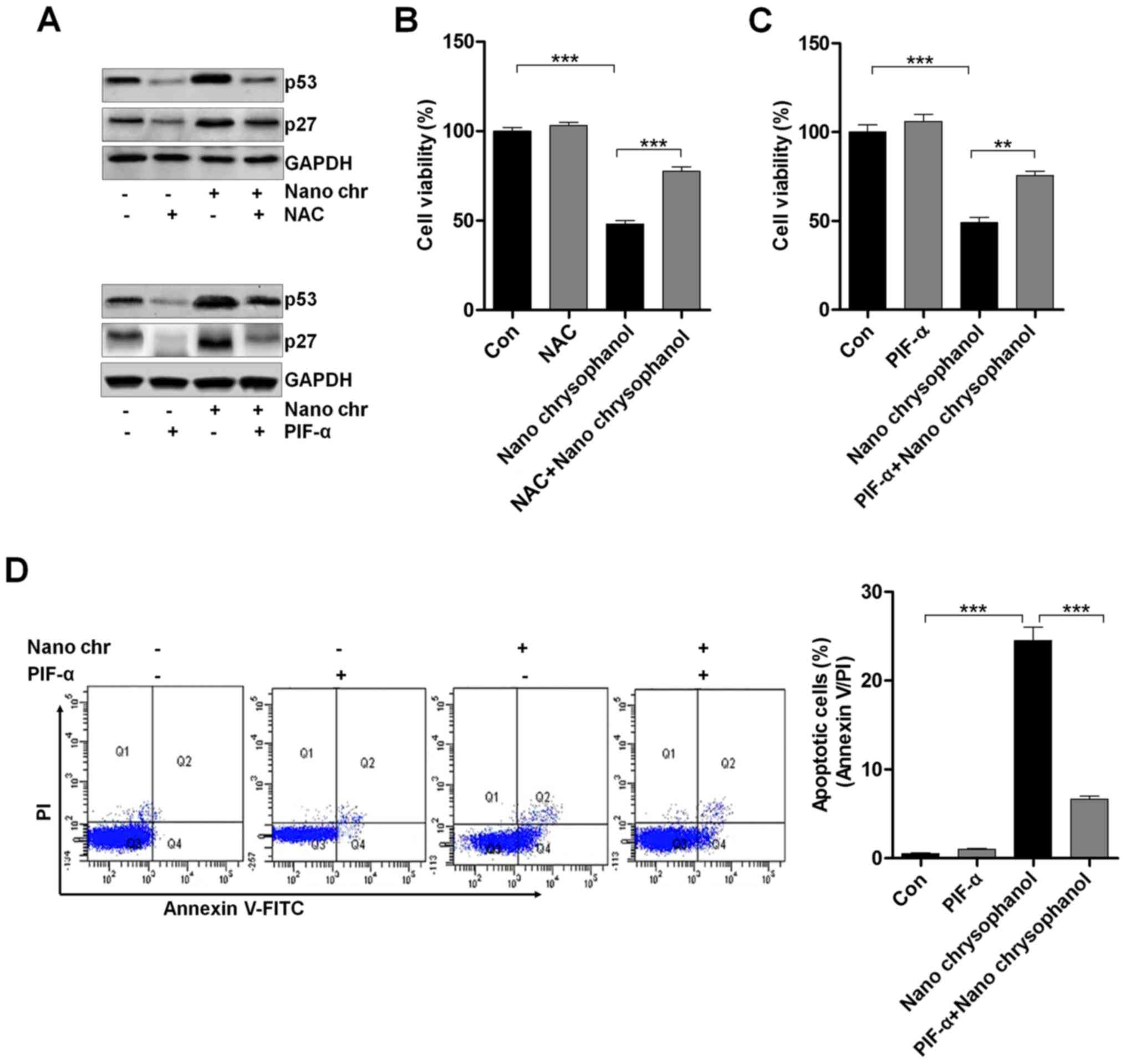 | Figure 6The effects of chrysophanol
nanoparticle on ROS, p53 and apoptosis. (A) Upper, LNCap cells were
treated with 90 μM chrysophanol nanoparticle for 24 h with
or without ROS scavenger of NAC (1 mM) pre-treatment for 1 h. Then,
p53 and p27 protein levels were assessed using western blot
analysis. Lower, LNCap cells were treated with 90 μM
chrysophanol nanoparticle in the presence or absence of p53
inhibitor, PIF-α (30 μM), for 24 h. Then, all cells were
harvested for p53 and p27 assessment through western blot analysis.
Viability assay of LNCap cells using (B) NAC (1 mM) and (C)
p53-inhibitor (PIF-α, 30 μM) with 90 μM chrysophanol
nanoparticle was conducted via MTT analysis. (D) LNCap cells were
exposed to 90 μM chrysophanol nanoparticle and 30 μM
PIF-α in single or double treatments for 24 h. Then, flow cytometry
analysis was carried out to evaluate apoptosis. Data are shown as
mean ± SEM. *P<0.05, **P<0.01 and
***P<0.001. |
Chrysophanol nanoparticle promotes the
release of Cyto-c into cytoplasm
Cyto-c expression has been reported to be linked
with apoptosis induction in cancer cells, which is also related to
ROS generation (33,34). Thus, here we attempted to explore
its role in chrysophanol nanoparticle-induced apoptosis in LNCap
cells. As shown in Fig. 7A,
chrysophanol nanoparticle treatment significantly enhanced the
fluorescent intensity, indicating the Cyto-c expression levels.
Also, NAC treatment reduced chrysophanol nanoparticle-caused high
expression of Cyto-c through immunofluorescent assays (Fig. 7B). Western blot analysis further
confirmed that expression of Apaf-1 and Cyto-c in LNCap cells was
upregulated by chrysophanol nanoparticle dose- and time-dependently
(Fig. 7C and D). Together, the
results above suggested that chrysophanol nanoparticle-reduced
LNCap proliferation was related to intrinsic pathway through
modulating Cyto-c expression.
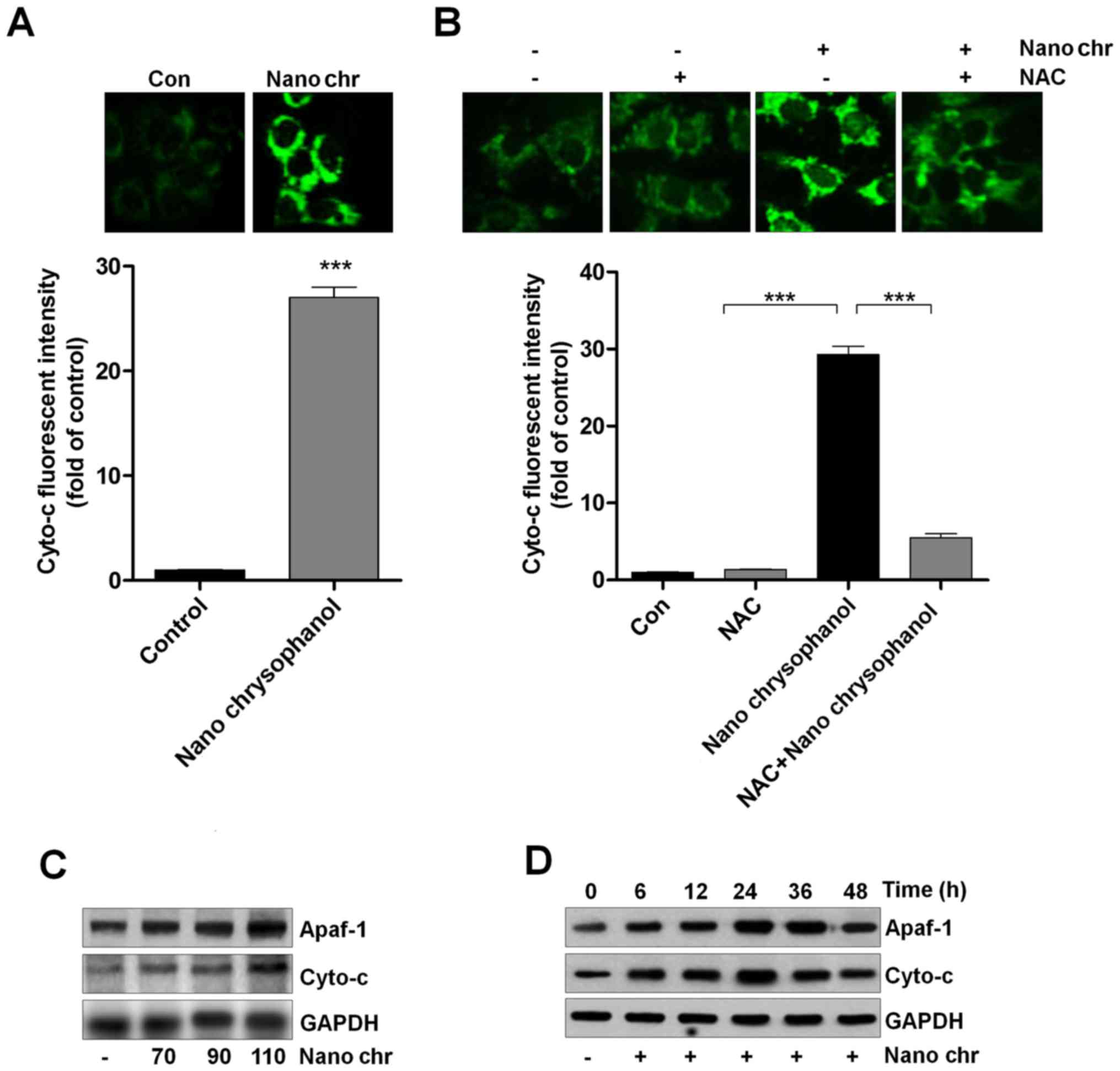 | Figure 7Chrysophanol nanoparticle promotes
the release of Cyto-c into the cytoplasm. (A) LNCap cells were
treated with 90 μM chrysophanol nanoparticle for 24 h, and
then immunofluorescent analysis was used to evaluate Cyto-c
expression levels. (B) LNCap cells were treated with 90 μM
chrysophanol nanoparticle for 24 h with or without NAC (1 mM)
pre-treatment for 1 h, followed by fluorescent intensity analysis
of Cyto-c. (C) Chrysophanol nanoparticle (70, 90 and 110 μM)
was administered to LNCap cells for 24 h. Then, Apaf-1 and Cyto-c
expression levels were evaluated using western blot analysis. (D)
LNCap cells were treated with 90 μM chrysophanol
nanoparticle for 0, 6, 12, 24, 36 and 48 h, followed by Apaf-1 and
Cyto-c measurement using western blot assays. Data are shown as
mean ± SEM. *P<0.05, **P<0.01 and
***P<0.001. |
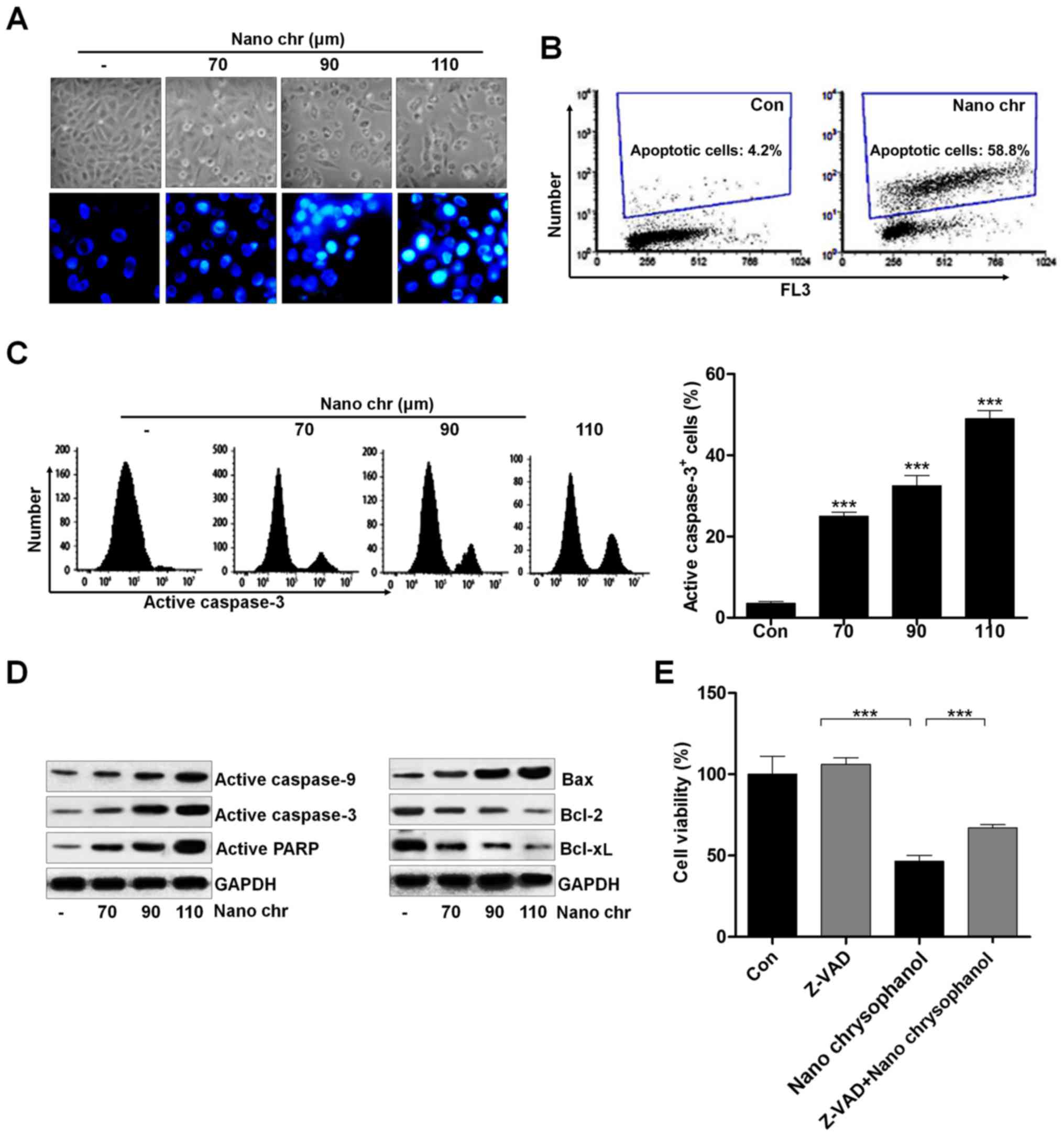
Chrysophanol nanoparticle induces
apoptosis in human prostate cancer cells through activating
caspase-3 signaling pathway
Activating apoptosis needs to inhibit anti-apoptotic
proteins and enhance pro-apoptotic proteins, activating caspase-3
and PARP to induce apoptosis. We investigated the cellular
morphological changes and DNA damage induced by NQ. NQ induced the
formation of blebs and rounding off of cellular structures. The
morphology and Hoechst 33342 staining of cancer cells indicated
that chrysophanol nanoparticle dose-dependently induced cell death
(Fig. 8A). Also, TUNEL analysis
using flow cytometry revealed that apoptosis was induced by
chrysophanol nanoparticle in LNCap cells (Fig. 8B). Caspase-3 activation is well
reported to be essential for inducing apoptosis. Flow cytometry
analysis indicated that active caspase-3 was dose-dependently
induced by chrysophanol nanoparticle in prostate cancer cells
(Fig. 8C). The immunoblotting was
used here to calculate the role of different proteins associated
with apoptosis. The overall results indicated the increased
expression of caspase-9, and caspase-3, PARP, and Bax, and
decreased expression of Bcl-2, and Bcl-xL when LNCap cells were
incubated with chrysophanol nanoparticle for 24 h (Fig. 8D). Treatment with ZDVD-FMK
(caspase-3 inhibitor) increased the cell viability, and
chrysophanol nanoparticle-reduced cell survival rate was enhanced
after caspase-3 inhibition (Fig.
8E). Together, these results above indicated that chrysophanol
nanoparticle accelerates DNA damage and induces caspase-3-regulated
apoptosis in LNCap cells.
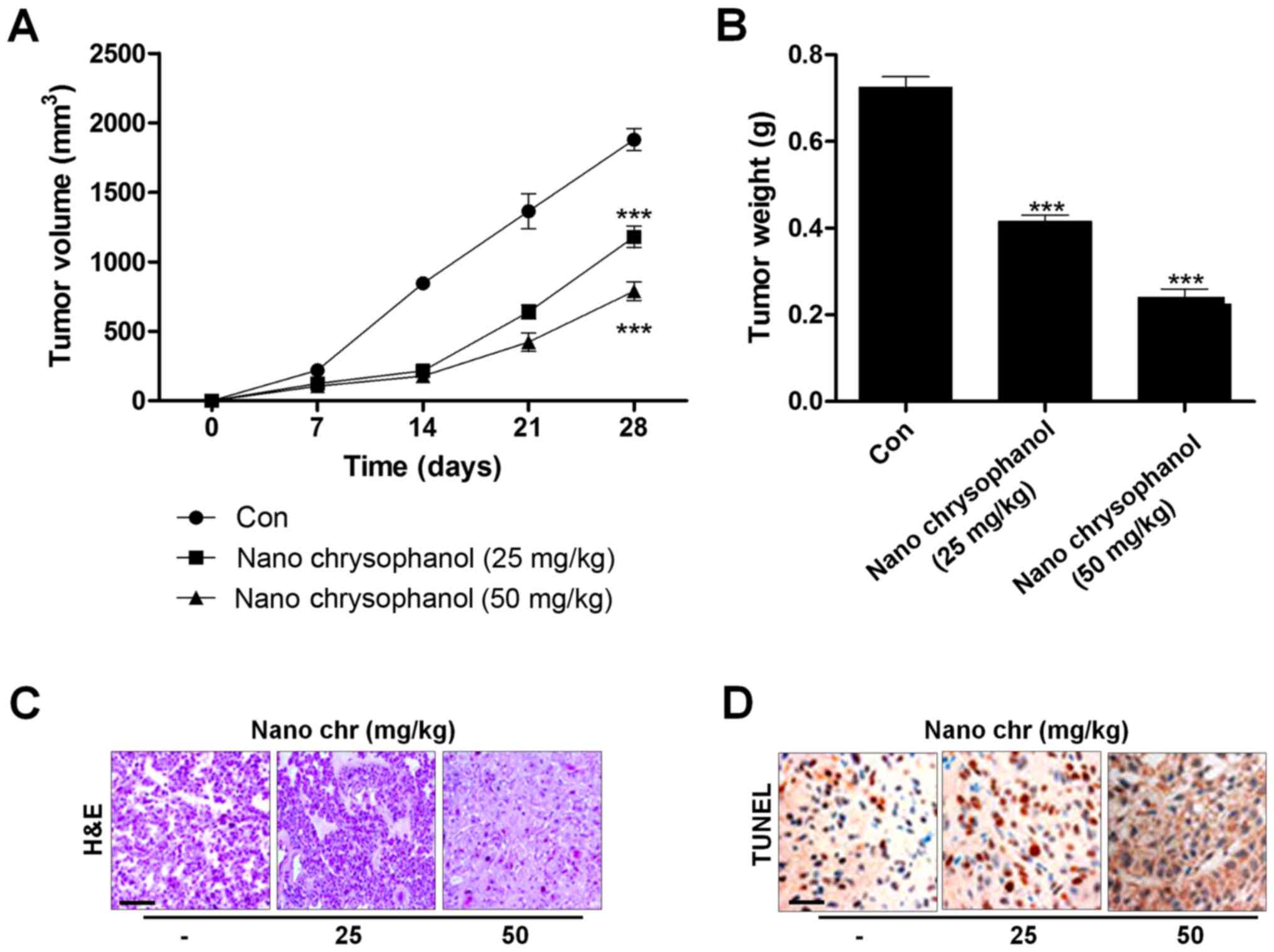
Chrysophanol nanoparticle reduces tumor
growth in vivo
Next, xenograft tumor models were established in
nude mice using LNCap cancer cells to further investigate the
effects of chrysophanol nanoparticle in vivo. The
tumorgenicity of the cells was calculated. LNCap cells at
2×105 were inoculated subcutaneously into nude mice.
When tumors were obvious (tumor size 50 mm3), mice were
randomly grouped to receive 25 and 50 mg/kg chrysophanol
nanoparticle for 28 days. Then, all mice were sacrificed for tumor
weight, and IHC assays. The tumor volumes were tested every 7 days.
After 28 days, the tumor size and weight were significantly reduced
by chrysophanol nanoparticle in mice (Fig. 9A and B). Subsequently, IHC analysis
was performed to evaluate TUNEL levels in tumor tissue sections
isolated from mice treated with different concentrations of
chrysophanol nanoparticle. As shown in Fig. 9C, H&E staining indicated that
the suppressed progression of tumor after chrysophanol nanoparticle
administration. Furthermore, TUNEL-positive cells were found to be
upregulated in tumor sections with chrysophanol nanoparticle
treatments (Fig. 9D). Together,
the data above indicated that chrysophanol nanoparticle inhibited
tumor growth in vivo.
Discussion
Prostate cancer has one of the highest incidence
rates amongst all diagnosed cancers in males worldwide (35,36).
Finding effective therapeutic strategies is urgently necessary to
prevent or treat human prostate cancer progression. According to
the role of chrysophanol in suppressing lung cancer, leukaemia and
breast cancer, it was applied in our study to investigate if it
could be a novel candidate for prostate cancer treatment (37–39).
Also, the molecular mechanism revealing preventing prostate cancer
by chrysophanol remains poorly understood.
Application of nanoparticles as carriers or delivery
systems for chemotherapeutic drugs is attracting attention due to
the specificity of nanoparticle to cancer cells, which improve drug
efficiency and reduce systemic toxicity (40,41).
AuNPs have some advantages, such as a bio-compatible core, making
them an ideal initiating point for a nanocarrier system (42). Furthermore, AuNPs experience
functionalization of multiple surfaces, highly rendering them
multi-use for targeting. However, some studies have pointed out
high toxicity of AuNPs (43,44).
Hence, decreasing the undesirable toxicity and enhancing sustained
releasing ability and bioavailability of drugs are essential
considerations. In the present study, reduced AuNPs with
chrysophanol and PLGA were encapsulated for the first time. PEG
tagging or PLGA encapsulation could protect the compound inside
from being attacked by immune cells (11). Following the results of our study,
we found that nano-chrysophanol showed a significant enhancement of
chrysophanol concentrations in serum, even at the half dose of the
free chrysophanol, demonstrating the effectiveness of chrysophanol
nanoparticle in organisms to prevent prostate cancer. In addition,
chrysophanol nanoparticles exhibited more suppressive role in the
cell proliferation compared to the free chrysophanol treatment.
Thus, we supposed that chrysophanol nanoparticles have more
advantages than its free form, which might be associated with the
enhanced bioavailability, preservation, and water solubility. We
attempted to calculate the underlying molecular mechanism of the
tumor-killing role of the new drug in prostate cancer. We found
that chrysophanol nanoparticles showed remarkable anticancer
effects and toxicity towards human prostate cancer cells, and more
effectively towards LNCap cells than other two prostate cancer
cells. Furthermore, no significant difference was observed in human
normal prostate cells and normal liver cells, indicating its low
toxicity. We revealed that the i.p. administration of chrysophanol
nanoparticles led to a significant improvement of bioavailability
in comparison to the i.p. administration of free chrysophanol
administered through the same route.
The therapy of targeting DNA was developed recently.
DNA functions as a large receptor for a variety numbers of small
molecules and thus becomes an important target for exploring
anticancer agents (45,46). Circular dichroism spectroscopic
data suggested that chrysophanol nanoparticles have the activity to
interact with DNA. Therefore, chrysophanol nanoparticle
cytotoxicity might be due to its inhibition of DNA synthetic
process at least partly. The blocking capacity of chrysophanol
nanoparticles made it effective to induce apoptosis in LNCap cells,
and enhancing chrysophanol nanoparticles into nuclear DNA also
caused nuclear damage. The circular dichroism spectroscopic data
indicated that the interaction between chrysophanol nanoparticles
and the cellular DNA in LNCap cells was more pronounced 120 min
after treatment of chrysophanol nanoparticles. The interaction of
chrysophanol nanoparticles with DNA promoted the ability of the
drug to suppress the cell proliferation and to induce cell cycle
arrest, which was accompanied with reduced cyclin D1 and CDK1,
essential signals to promote cell proliferation through affecting
G1 progression (47,48). In contrast, elevated p27 levels,
regulating cell differentiation and cell cycle arrest, were found
to be upregulated by chrysophanol nanoparticles (49).
Histone deacetylase inhibitors have been
investigated as therapeutic strategy for various cancers (19,20).
The exact molecular mechanisms by which the inhibitors work
remained unclear. Histone deacetylase inhibitors induced p27 and
p21 expression, associated with p53 activity to inhibit tumor
progression via triggering cell differentiation, growth arrest, or
apoptosis-related death in a variety of cultured cells, such as
leukemia cells, neuroblastoma, and melanoma, and cells from
prostate, ovary, liver, lung, and gastric cancers (50–52).
Histone deacetylase inhibitors induce apoptosis, which could be
assayed by DNA fragmentation, and the number of sub-G1 population
was assessed using flow cytometry. Overexpression of HDACs,
including HDAC1, HDAC3 and HDAC6, which are reported as the key
histone deacetylation regulators, is associated with cancer
development through silencing p53, p27 and p21 (53,54).
In our study, we found that chrysophanol nanoparticles, similar to
inhibitors of HDACs, could reduce HDAC1, HDAC3 and HDAC6
expression, a possible molecular mechanism by which chrysophanol
nanoparticles performed their role in preventing human prostate
cancer progression. The treatment of chrysophanol nanoparticles
further resulted in the concomitant enrichment of acetyl histones
such as Ac-H3.
Chrysophanol nanoparticle has a similar ability to
trigger ROS generation. Chrysophanol has efficacy to induce
apoptosis in p53-expressing cancer cells by promoting ROS. The
molecular mechanism behind the enhancement in ROS is increased p53
activity (55). P53 expression and
ROS generation are correlated, and p53 could modulate proliferative
proteins, including cyclin D1 (56). Thus, elevated p53 activity and ROS
production occurred in prostate cancer cells. ROS-dependent
apoptosis involving p53 relies on damage to mitochondrial
generally. The dramatic alterations in mitochondrial morphology
during the early stages of apoptotic cell death, involve the
network fragmentation and the remodeling of cristae.
Activation-inactivation of p53 is also dependent on acetylation.
P53 acetylation was found to be indispensable for its activation
(57,58). In this study, we also found that
expression levels of Ac-p53 were induced by chrysophanol
nanoparticle, which suggested that p53 activation through its
acetylation might be included in chrysophanol
nanoparticle-prevention of the progression of prostate cancer.
Translocation of Bax onto mitochondrial membrane produces pores,
depolarizing the potential of mitochondrial membrane. Subsequently,
cytochrome c (Cyto-c) moves into the cytoplasm and
initiates the formation of apoptosomes along with adopter molecule
Apaf-1, as well as other pro-caspase molecules, including caspase-9
and caspase-3 (59,60). Caspase-3 is regarded as the
terminal mediator of cell apoptosis in caspase family. Caspase-3
activation has been suggested to activate PARP and induce
apoptosis, which is also related to ROS production (61,62).
In our study, we found that chrysophanol nanoparticles enhanced
Cyto-c expression in cells and elevated caspase-9, caspase-3 and
PARP activity. Accordingly, the anti-apoptotic signals, Bcl-2 and
Bcl-xl, were reduced by chrysophanol nanoparticles in prostate
cancer cells (63).
AMPK is a pleiotropic enzyme, playing a role in the
regulation of numerous functions both peripherally and centrally
(64). AMPK has now been
considered as a potential therapeutic target for preventing cancer
as a consequence of being a substrate (65). Furthermore, AKT signaling pathways
are linked to the inhibition of proliferation, apoptosis, and the
metastasis of cells, angiogenesis and various other processes
(66,67). AKT is involved in the proliferation
of cells through its activation of cyclin D1, and inactivates
caspases (68,69). Our results indicated that
chrysophanol nanoparticles upregulated AMPK activation, while
downregulated AKT phosphorylation, contributing to the suppression
of prostate cancer cells.
In conclusion, chrysophanol nanoparticles reduced
the expression of HDACs, CDK1, cyclin D1 and p-AKT, which might be
related to the cell cycle arrest. The upregulation of p27, CHK1 and
p-AMPK may have led to the arrest of LNCap cell proliferation. Flow
cytometry analysis indicated that chrysophanol nanoparticles
arrested the LNCap cell cycle in sub-G phase. Additionally,
treatment of chrysophanol nanoparticles resulted in ROS production,
along with the release of Cyto-c and Apaf-1 expression, inducing
apoptosis in LNCap cells through activating caspase-3. Hence,
inhibition of proliferation and induction of ROS and apoptosis by
chrysophanol nanoparticles may provide a potential therapeutic
strategy to prevent human prostate cancer development.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (no. 81402111).
References
|
1
|
Øverbye A, Skotland T, Koehler CJ, Thiede
B, Seierstad T, Berge V, Sandvig K and Llorente A: Identification
of prostate cancer biomarkers in urinary exosomes. Oncotarget.
6:30357–30376. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tanno T, Rabel A, Alleyne M, Lee YT, Dahut
WL, Gulley JL and Miller JL: Hepcidin, anaemia, and prostate
cancer. BJU Int. 107:678–679. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu T, Wu LY, Kazak M and Berkman CE:
Cell-Surface labeling and internalization by a fluorescent
inhibitor of prostate-specific membrane antigen. Prostate.
68:955–964. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tagawa ST, Beltran H, Vallabhajosula S,
Goldsmith SJ, Osborne J, Matulich D, Petrillo K, Parmar S, Nanus DM
and Bander NH: Anti-prostate-specific membrane antigen-based
radioimmunotherapy for prostate cancer. Cancer. 116(Suppl):
1075–1083. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cho SY, Gage KL, Mease RC,
Senthamizhchelvan S, Holt DP, Jeffrey-Kwanisai A, Endres CJ,
Dannals RF, Sgouros G, Lodge M, et al: Biodistribution, tumor
detection, and radiation dosimetry of 18F-DCFBC, a
low-molecular-weight inhibitor of prostate-specific membrane
antigen, in patients with metastatic prostate cancer. J Nucl Med.
53:1883–1891. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
López-Lázaro M: Distribution and
biological activities of the flavonoid luteolin. Mini Rev Med Chem.
9:31–59. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Webb KM and DiRuggiero J: Role of
Mn2+ and compatible solutes in the radiation resistance
of thermophilic bacteria and archaea. Archaea. 2012:8457562012.
View Article : Google Scholar
|
|
8
|
Lu CC, Yang JS, Huang AC, Hsia TC, Chou
ST, Kuo CL, Lu HF, Lee TH, Wood WG and Chung JG: Chrysophanol
induces necrosis through the production of ROS and alteration of
ATP levels in J5 human liver cancer cells. Mol Nutr Food Res.
54:967–976. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Darzynkiewicz Z, Carter SP, Kapuscinski J
and Watanabe KA: Effect of derivatives of chrysophanol, a new type
of potential antitumor agents of anthraquinone family, on growth
and cell cycle of L1210 leukemic cells. Cancer Lett. 46:181–187.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zeng X, Tao W, Mei L, Huang L, Tan C and
Feng SS: Cholic acid-functionalized nanoparticles of star-shaped
PLGA-vitamin E TPGS copolymer for docetaxel delivery to cervical
cancer. Biomaterials. 34:6058–6067. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Thamake SI, Raut SL, Gryczynski Z, Ranjan
AP and Vishwanatha JK: Alendronate coated poly-lactic-co-glycolic
acid (PLGA) nanoparticles for active targeting of metastatic breast
cancer. Biomaterials. 33:7164–7173. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hrkach J, Von Hoff D, Mukkaram Ali M,
Andrianova E, Auer J, Campbell T, De Witt D, Figa M, Figueiredo M,
Horhota A, et al: Preclinical development and clinical translation
of a PSMA-targeted docetaxel nanoparticle with a differentiated
pharmacological profile. Sci Transl Med. 4:128ra392012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ganju A, Yallapu MM, Khan S, Behrman SW,
Chauhan SC and Jaggi M: Nanoways to overcome docetaxel resistance
in prostate cancer. Drug Resist Updat. 17:13–23. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang X: Gold nanoparticles: Recent
advances in the biomedical applications. Cell Biochem Biophys.
72:771–775. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang CC, Wu SM, Li HW and Chang HT:
Biomedical applications of DNA-conjugated gold nanoparticles.
ChemBioChem. 17:1052–1062. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Simon LC, Stout RW and Sabliov C:
Bioavailability of orally delivered alpha-tocopherol by poly
(lactic-co-glycolic) acid (PLGA) nanoparticles and chitosan covered
PLGA nanoparticles in F344 rats. Nanobiomedicine. 3:82016.
View Article : Google Scholar
|
|
17
|
Lin TsT, Gao DY, Liu YC, Sung YC, Wan D,
Liu JY, Chiang T, Wang L and Chen Y: Development and
characterization of sorafenib-loaded PLGA nanoparticles for the
systemic treatment of liver fibrosis. J Control Release. 221:62–70.
2016. View Article : Google Scholar
|
|
18
|
Gu W and Roeder RG: Activation of p53
sequence-specific DNA binding by acetylation of the p53 C-terminal
domain. Cell. 90:595–606. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Horikoshi M: Histone acetylation: From
code to web and router via intrinsically disordered regions. Curr
Pharm Des. 19:5019–5042. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sachweh MC, Drummond CJ, Higgins M,
Campbell J and Laín S: Incompatible effects of p53 and HDAC
inhibition on p21 expression and cell cycle progression. Cell Death
Dis. 4:e5332013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu T, Kuljaca S, Tee A and Marshall GM:
Histone deacetylase inhibitors: Multifunctional anticancer agents.
Cancer Treat Rev. 32:157–165. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang XJ and Seto E: The Rpd3/Hda1 family
of lysine deacetylases: From bacteria and yeast to mice and men.
Nat Rev Mol Cell Biol. 9:206–218. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Weichert W, Röske A, Gekeler V, Beckers T,
Ebert MP, Pross M, Dietel M, Denkert C and Röcken C: Association of
patterns of class I histone deacetylase expression with patient
prognosis in gastric cancer: A retrospective analysis. Lancet
Oncol. 9:139–148. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
West AC and Johnstone RW: New and emerging
HDAC inhibitors for cancer treatment. J Clin Invest. 124:30–39.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hubbert C, Guardiola A, Shao R, Kawaguchi
Y, Ito A, Nixon A, Yoshida M, Wang XF and Yao TP: HDAC6 is a
microtubule-associated deacetylase. Nature. 417:455–458. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zuo Q, Wu W, Li X, Zhao L and Chen W:
HDAC6 and SIRT2 promote bladder cancer cell migration and invasion
by targeting cortactin. Oncol Rep. 27:819–824. 2012.
|
|
27
|
Park SY, Jun JA, Jeong KJ, Heo HJ, Sohn
JS, Lee HY, Park CG and Kang J: Histone deacetylases 1, 6 and 8 are
critical for invasion in breast cancer. Oncol Rep. 25:1677–1681.
2011.PubMed/NCBI
|
|
28
|
Aldana-Masangkay GI and Sakamoto KM: The
role of HDAC6 in cancer. J Biomed Biotechnol. 2011:8758242011.
View Article : Google Scholar
|
|
29
|
Haggarty SJ, Koeller KM, Wong JC,
Grozinger CM and Schreiber SL: Domain-selective small-molecule
inhibitor of histone deacetylase 6 (HDAC6)-mediated tubulin
deacetylation. Proc Natl Acad Sci USA. 100:4389–4394. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Henderson C, Mizzau M, Paroni G, Maestro
R, Schneider C and Brancolini C: Role of caspases, Bid, and p53 in
the apoptotic response triggered by histone deacetylase inhibitors
trichostatin-A (TSA) and suberoylanilide hydroxamic acid (SAHA). J
Biol Chem. 278:12579–12589. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Choudhary C, Kumar C, Gnad F, Nielsen ML,
Rehman M, Walther TC, Olsen JV and Mann M: Lysine acetylation
targets protein complexes and co-regulates major cellular
functions. Science. 325:834–840. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kloster MM, Naderi EH, Haaland I, Gjertsen
BT, Blomhoff HK and Naderi S: cAMP signalling inhibits p53
acetylation and apoptosis via HDAC and SIRT deacetylases. Int J
Oncol. 42:1815–1821. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jiang X and Wang X: Cytochrome c-mediated
apoptosis. Annu Rev Biochem. 73:87–106. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Garrido C, Galluzzi L, Brunet M, Puig PE,
Didelot C and Kroemer G: Mechanisms of cytochrome c release from
mitochondria. Cell Death Differ. 13:1423–1433. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Prensner JR, Zhao S, Erho N, Schipper M,
Iyer MK, Dhanasekaran SM, Magi-Galluzzi C, Mehra R, Sahu A,
Siddiqui J, et al: RNA biomarkers associated with metastatic
progression in prostate cancer: A multi-institutional
high-throughput analysis of SChLAP1. Lancet Oncol. 15:1469–1480.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Severi G, Morris HA, MacInnis RJ, English
DR, Tilley W, Hopper JL, Boyle P and Giles GG: Circulating steroid
hormones and the risk of prostate cancer. Cancer Epidemiol
Biomarkers Prev. 15:86–91. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ni CH, Yu CS, Lu HF, Yang JS, Huang HY,
Chen PY, Wu SH, Ip SW, Chiang SY, Lin JG, et al:
Chrysophanol-induced cell death (necrosis) in human lung cancer
A549 cells is mediated through increasing reactive oxygen species
and decreasing the level of mitochondrial membrane potential.
Environ Toxicol. 29:740–749. 2014. View Article : Google Scholar
|
|
38
|
Hong JY, Chung HJ, Bae SY, Trung TN, Bae K
and Lee SK: Induction of cell cycle arrest and apoptosis by
physcion, an anthraquinone isolated from rhubarb (rhizomes of Rheum
tanguticum), in MDA-MB-231 human breast cancer cells. J Cancer
Prev. 19:273–278. 2014. View Article : Google Scholar
|
|
39
|
Ozenver N, Saeed M, Guvenalp Z, et al:
Chrysophanol-and nepodin-8-O-β-D-glucopyranoside from Rumex
acetosella, the cytotoxicity towards drug sensitive and multi-drug
resistant T leukaemia cancer cells. Planta Med. 81:3882016.
|
|
40
|
Gradishar WJ, Tjulandin S, Davidson N,
Shaw H, Desai N, Bhar P, Hawkins M and O'Shaughnessy J: Phase III
trial of nanoparticle albumin-bound paclitaxel compared with
polyethylated castor oil-based paclitaxel in women with breast
cancer. J Clin Oncol. 23:7794–7803. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li Y, Tan B and Wu Y: Mesoporous
Co3O4 nanowire arrays for lithium ion
batteries with high capacity and rate capability. Nano Lett.
8:265–270. 2008. View Article : Google Scholar
|
|
42
|
Park SY, Chae SY, Park JO, Lee KJ and Park
G: Gold-conjugated resveratrol nanoparticles attenuate the invasion
and MMP-9 and COX-2 expression in breast cancer cells. Oncol Rep.
35:3248–3256. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Saha K, Agasti SS, Kim C, Li X and Rotello
VM: Gold nanoparticles in chemical and biological sensing. Chem
Rev. 112:2739–2779. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Shi J, Chan C, Pang Y, Ye W, Tian F, Lyu
J, Zhang Y and Yang M: A fluorescence resonance energy transfer
(FRET) biosensor based on graphene quantum dots (GQDs) and gold
nanoparticles (AuNPs) for the detection of mecA gene sequence of
Staphylococcus aureus. Biosens Bioelectron. 67:595–600. 2015.
View Article : Google Scholar
|
|
45
|
Zeng L, Wu GZ, Goh KJ, Lee YM, Ng CC, You
AB, Wang J, Jia D, Hao A, Yu Q, et al: Saturated fatty acids
modulate cell response to DNA damage: Implication for their role in
tumorigenesis. PLoS One. 3:e23292008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Fahrer J and Kaina B: O6-methylguanine-DNA
methyltransferase in the defense against N-nitroso compounds and
colorectal cancer. Carcinogenesis. 34:2435–2442. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Casagrande F and Darbon JM: Effects of
structurally related flavonoids on cell cycle progression of human
melanoma cells: Regulation of cyclin-dependent kinases CDK2 and
CDK1. Biochem Pharmacol. 61:1205–1215. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hansel DE, Dhara S, Huang RC, Ashfaq R,
Deasel M, Shimada Y, Bernstein HS, Harmon J, Brock M, Forastiere A,
et al: CDC2/CDK1 expression in esophageal adenocarcinoma and
precursor lesions serves as a diagnostic and cancer progression
marker and potential novel drug target. Am J Surg Pathol.
29:390–399. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lu Z and Hunter T: Ubiquitylation and
proteasomal degradation of the p21(Cip1), p27(Kip1) and p57(Kip2)
CDK inhibitors. Cell Cycle. 9:2342–2352. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Pandey M, Kaur P, Shukla S, Abbas A, Fu P
and Gupta S: Plant flavone apigenin inhibits HDAC and remodels
chromatin to induce growth arrest and apoptosis in human prostate
cancer cells: In vitro and in vivo study. Mol Carcinog. 51:952–962.
2012. View Article : Google Scholar
|
|
51
|
Schäfer C, Göder A, Beyer M, Kiweler N,
Mahendrarajah N, Rauch A, Nikolova T, Stojanovic N, Wieczorek M,
Reich TR, et al: Class I histone deacetylases regulate p53/NF-κB
crosstalk in cancer cells. Cell Signal. 29:218–225. 2017.
View Article : Google Scholar
|
|
52
|
Ververis K, Hiong A, Karagiannis TC and
Licciardi PV: Histone deacetylase inhibitors (HDACIs):
Multitargeted anticancer agents. Biologics. 7:47–60.
2013.PubMed/NCBI
|
|
53
|
Varricchio L, Dell'Aversana C, Nebbioso A,
Migliaccio G, Altucci L, Mai A, Grazzini G, Bieker JJ and
Migliaccio AR: Identification of NuRSERY, a new functional HDAC
complex composed by HDAC5, GATA1, EKLF and pERK present in human
erythroid cells. Int J Biochem Cell Biol. 50:112–122. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yuan H, Li AJ, Ma SL, Cui LJ, Wu B, Yin L
and Wu MC: Inhibition of autophagy significantly enhances
combination therapy with sorafenib and HDAC inhibitors for human
hepatoma cells. World J Gastroenterol. 20:4953–4962. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhao Y, Chaiswing L, Velez JM,
Batinic-Haberle I, Colburn NH, Oberley TD and St Clair DK : p53
translocation to mitochondria precedes its nuclear translocation
and targets mitochondrial oxidative defense protein-manganese
superoxide dismutase. Cancer Res. 65:3745–3750. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Erster S, Mihara M, Kim RH, Petrenko O and
Moll UM: In vivo mitochondrial p53 translocation triggers a rapid
first wave of cell death in response to DNA damage that can precede
p53 target gene activation. Mol Cell Biol. 24:6728–6741. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Proietti S, Cucina A, Dobrowolny G,
D'Anselmi F, Dinicola S, Masiello MG, Pasqualato A, Palombo A,
Morini V, Reiter RJ, et al: Melatonin down-regulates MDM2 gene
expression and enhances p53 acetylation in MCF-7 cells. J Pineal
Res. 57:120–129. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Ono W, Hayashi Y, Yokoyama W, Kuroda T,
Kishimoto H, Ito I, Kimura K, Akaogi K, Waku T and Yanagisawa J:
The nucleolar protein Myb-binding protein 1A (MYBBP1A) enhances p53
tetramerization and acetylation in response to nucleolar
disruption. J Biol Chem. 289:4928–4940. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Estaquier J, Vallette F, Vayssiere JL and
Mignotte B: The mitochondrial pathways of apoptosis. Adv Exp Med
Biol. 942:157–183. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Pradelli LA, Bénéteau M and Ricci JE:
Mitochondrial control of caspase-dependent and -independent cell
death. Cell Mol Life Sci. 67:1589–1597. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Shi Y: caspase activation: Revisiting the
induced proximity model. Cell. 117:855–858. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Isabelle M, Moreel X, Gagné JP, Rouleau M,
Ethier C, Gagné P, Hendzel MJ and Poirier GG: Investigation of
PARP-1, PARP-2, and PARG interactomes by affinity-purification mass
spectrometry. Proteome Sci. 8:222010. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Czabotar PE, Lessene G, Strasser A and
Adams JM: Control of apoptosis by the BCL-2 protein family:
Implications for physiology and therapy. Nat Rev Mol Cell Biol.
15:49–63. 2014. View Article : Google Scholar
|
|
64
|
Li W, Saud SM, Young MR, Chen G and Hua B:
Targeting AMPK for cancer prevention and treatment. Oncotarget.
6:7365–7378. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Oakhill JS, Scott JW and Kemp BE: AMPK
functions as an adenylate charge-regulated protein kinase. Trends
Endocrinol Metab. 23:125–132. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Vivanco I and Sawyers CL: The
phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev
Cancer. 2:489–501. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
67
|
Benbrook DM and Masamha CP: The
pro-survival function of Akt kinase can be overridden or altered to
contribute to induction of apoptosis. Curr Cancer Drug Targets.
11:586–599. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Unger C, Popescu R, Giessrigl B, Rarova L,
Herbacek I, Seelinger M, Diaz R, Wallnöfer B, Fritzer-Szekeres M,
Szekeres T, et al: An apolar extract of Critonia morifolia inhibits
c-Myc, cyclin D1, Cdc25A, Cdc25B, Cdc25C and Akt and induces
apoptosis. Int J Oncol. 40:2131–2139. 2012.PubMed/NCBI
|
|
69
|
Polivka J Jr and Janku F: Molecular
targets for cancer therapy in the I3K/AKT/mTOR pathway. Pharmacol
Ther. 142:164–175. 2014. View Article : Google Scholar
|