Introduction
Gliomas, which comprise 80% of malignant primary
brain tumors (1), are recognized
as the leading cause of death from central nervous system tumors
(2). Gliomas are generally
categorized according to the International Classification of
Disease for Oncology (3rd edition) and World Health Organization
grading system (3), classifying
malignant behavior as grades I–IV. The overall incidence of gliomas
is ~6 per 100,000 persons. The grade IV glioma, glioblastoma
(4), accounts for ~45% of all
gliomas and has an overall 5-year survival of <5% (1). Despite significant progress in
developing surgical and adjuvant therapy, the morbidity and
mortality of glioblastoma remain high. In addition to radiotherapy
and surgical resection, temozolomide (TMZ) is used as the
first-line chemotherapy drug for glioblastoma treatment (5). Use of TMZ has improved the overall
survival and progression-free survival, but intrinsic or acquired
chemoresistance is major cause of TMZ treatment failure in patients
with glioblastoma (6). Identifying
novel therapeutic targets is in urgent need for the diagnosis and
treatment for glioblastoma.
WNT1 inducible signaling pathway protein1 (WISP1),
which belongs to the CCN family (connective tissue growth factor,
cysteine-rich protein, and nephroblastoma overexpressed gene), is a
widely recognized downstream target gene of the Wnt/β-catenin
pathway (7). While members of the
CCN family are reported to regulate complex biological processes
during embryogenesis, wound healing, and tissue repair (8), WISP1 was originally identified as
involved in β-catenin-mediated tumorigenesis (9). WISP1 has been implicated in tumor
development in multiple studies. Overexpression of WISP1 is
associated with a poor prognosis in colon cancer by regulating cell
adhesion (10). WISP1 promotes
oral squamous cell carcinoma through angiogenesis (11). In esophageal squamous cell
carcinoma, WISP1 regulates tumor cell radio-sensitivity (12) and is recognized as a marker
suggestive of a poor prognosis (13). These studies strongly suggest that
WISP1 plays an important role in tumor development and might
therefore be a potential target of tumor therapy.
Aberrant WISP1 levels were recently reported in
glioblastoma. Overexpression of WISP1 enhances radiosensitivity of
glioma cells (14). Hypoxia, an
important microenvironment condition for tumor formation, induces
WISP1 expression in U87 human glioblastoma cells (15). These observations indicate that
WISP1 is an important player in glioblastoma. However, the exact
mechanisms by which WISP1 regulates the development of glioblastoma
and its potential for clinical therapeutic targeting remain
elusive. In the present study, we explored the role of WISP1 in
regulating the growth and malignancy of glioblastoma in
vitro and in vivo. We also evaluated the potential
effect of WISP1 in mediating drug resistance against TMZ in
glioblastoma cell lines.
Materials and methods
Tissue microarray and immunohistochemical
staining
A human brain gliocytoma and normal tissue
microarray containing a total of 120 samples (#TC0147; Auragene
Bioscience Co., Changsha, China) was established based on 40 cases,
including 5 normal samples and 35 tumor samples (2 anaplastic
oligodendrogliomas, 24 astrocytomas, and 9 glioblastomas). WISP1
protein levels in the tissue specimens were determined by
immunohistochemical staining. Briefly, tissue sections from the
human brain tissue microarray were deparaffinized in xylene and
rehydrated with decreasing concentrations of ethanol. Endogenous
peroxidase activity was quenched by incubating for 15 min in 3%
hydrogen peroxide. High-temperature antigen retrieval was performed
in sodium citrate buffer (#P019IH; Auragene Bioscience Co.). Tissue
sections were then incubated with primary WISP1 antibody
(#18166-1-AP, 1:100 dilution; Proteintech, Wuhan, China) or PCNA
(AM3547; Abzoom, Dallas, TX, USA) at 4°C overnight, followed by
incubation with horseradish peroxidase (HRP)-conjugated secondary
antibody (#SA009; Auragene Bioscience Co.). Finally, the sections
were colorized with the DAB substrate kit (#P013IH; Auragene
Bioscience Co.). Slides were counterstained with hematoxylin before
dehydration, incubated with xylene, and mounted. The slides were
evaluated by two pathologists blinded to the diagnosis for each
tissue type. Scores ranged from 0 to 4, with 0 indicating no
staining for WISP1 protein, 1 indicating ≤10% staining, 2
indicating 10 to ≤25% staining, 3 indicating 25 to ≤75% staining,
and indicating >75 to 100% staining. In analyzing the
association of WISP1 levels with clinicopathological features, a
score of 0 was categorized as no expression, scores of 1 or 2 as
low expression, and scores of 3 or 4 as high expression.
Clinical specimens
Normal human brain and glioma specimens were
obtained from patients who underwent brain surgery at the Xiangya
Hospital, Central South University from March to June 2016. None of
the patients had undergone radiotherapy, chemotherapy, or other
anticancer treatment before surgery. The histologic features of all
specimens were evaluated by pathologists according to the WHO
criteria. Written informed consent was obtained from all patients.
Protocols in this study were approved by the Medical Ethics
Committee of Xiangya Hospital, Central South University. Analysis
of WISP1 expression levels in normal and glioblastoma tissues by
bioinformatics analysis in GSE4536 from Oncomine datasets.
Statistical analysis of the pathologic stage of glioblastoma
patients in WISP1 expression from tissue microarray. Statistical
analysis of the pathologic stage of glioblastoma patients in WISP1
expression from TCGA datasets.
Reverse transcription and quantitative
real-time polymerase chain reaction
Total RNA of normal brain and glioma tissue samples
were isolated using a TRIzol kit (#R1022; Invitrogen Life
Technologies, Carlsbad, CA, USA). Reverse transcription of RNA into
cDNA was performed with a reverse transcription kit (#K1622;
Fermentans, Vilnius, Lithuania) according to the manufacturer's
instructions. Quantitative real-time polymerase chain reaction
(qRT-PCR) was performed with SYBRGreen qPCR Mix (#P2092; Toyobo,
Shiga, Japan) in real-time PCR system (#ABI 7300; Applied
Biosystems). Primers for qPCR are as follows: WISP1 sense,
5′-ACGCAG GGAAGA AGTGTC-3′; antisense, 5′-GAGAAGCCAAGCCCATCA-3′.
β-actin sense, 5′-AGGGGCCGGACTCGTCATACT-3′; anti-sense,
5′-GGCGGCACCACCATGTACCCT-3′. The β-actin gene was used as an
internal control, and all qRT-PCR reactions were performed in
triplicate. Relative mRNA levels of genes were calculated based on
the 2−∆∆CT method (16).
Cell culture
A normal human astrocyte cell line and human glioma
cell lines including SHG-44, U-251, and U-373 MG, were obtained
from Auragene Bioscience Co. Cells were cultured in RPMI-1640
medium (#SH30809.01; Hyclone, Hudson, NH, USA) supplied with 10%
fetal bovine serum (#900-108; Gemini Bio Products) and 100 U/ml
penicillin-streptomycin. All cells were maintained in humidified
cell incubators with 5% CO2 at 37°C.
shRNA construction and cell
transfection
A human WISP1 shRNA (sh-WISP1) construct was
generated by inserting the following targeting sequence into an
shRNA vector:
5′-CCGGGCTCCTATCAACCCAAGTATTCAAGAGATACTTGGGTTGATAGGAGCTTTTTTG-3′. A
non-specific shRNA sequence was used to construct a control shRNA
plasmid (sh-NC). For shRNA transfection, cells were plated at a
density of 1×105 cells/well in 6-well plates and
cultured for 24 h to reach 80-90% confluence. Culture medium was
replaced with serum-free medium 4 h prior to transfection, and 4
µg/well of plasmid DNA was then transfected using
Lipofectamine 2000 (#12566014; Thermo Fisher Scientific, Waltham,
MA, USA) reagent according to the manufacturer's instructions.
Cells were ready for further experiments after 24 h of culture in
growth medium following transfection.
Western blot analysis
Protein samples from cells with or without
transfection were extracted using RIPA lysis buffer (#P002A;
Auragene Bioscience Co.) containing proteinase inhibitors. Sample
concentrations were measured with a BCA assay. A total of 30
µg of protein from each sample was resolved on 10%
sulfate-polyacrylamide gel and electrophoretically transferred onto
a nitrocellulose membrane. Blots were then blocked with 5% milk and
incubated with a primary antibody against the protein of interest
in 3% BSA overnight at 4°C. After washing, the membranes were
incubated with HRP-labeled secondary antibody, and signals were
detected using an ECL chemiluminescence kit (#P001WB-1; Auragene
Bioscience Co.). Densitometry analysis was evaluated by IPP6.0
(Image-Pro Plus v 6.0, Media Cybernetics, Silver Spring, MD, USA).
The following antibodies were used: anti-WISP1 antibody
(#18166-1-AP, 1:500; Proteintech), anti-Bax antibody (#AM2198,
1:800; Abzoom), anti-Bcl-2 antibody (#YT0496, 1:1,000; ImmunoWay,
USA), anti-caspase 3 polyclonal antibody (#YC0026, 1:1,000;
ImmunoWay), anti-c-myc antibody (#YT0990, 1:1,000; ImmunoWay),
anti-CCND1 antibody (#AM2311, 1:1,000; Abzoom), anti-p53 antibody
(#BM0158, 1:1,000; Abzoom), anti-MMP9 antibody (#BM0616, 1:1,000;
Abzoom), anti-E-cadherin antibody (#BM0530, 1:1,000; Abzoom),
anti-vimentin antibody (#BM0147, 1:1,000; Abzoom), anti-ERK1/2
antibody (#YM0224, 1:1,000; ImmunoWay), anti-phospho-ERK1/2
(Y222/205) antibody (#YP0497, 1:1,000; ImmunoWay), anti-MRK-2
antibody (#YM0435, 1:1,000; ImmunoWay), anti-phospho-MRK-2 (T394)
antibody (#YP0169, 1:1,000; ImmunoWay), goat anti-rabbit IgG-HRP
antibody (#SA009, 1:15,000; Auragene Bioscience Co.), and goat
anti-mouse IgG-HRP antibody (#SA001, 1:15,000; Auragene Bioscience
Co.).
Cell proliferation assay
The effect of WISP1 knockdown on the proliferation
of glioma cell lines was determined by a cell proliferation assay.
Cells transfected with sh-NC or sh-WISP1 were plated in 96-well
plates at a density of 5×103 cells/well with a total
volume of 100 µl/well. When the cells were ready for the
proliferation assay, 10 µl of 3-(4,5-dimethylthiazol-2-yl)
-2,5-diphenyltetrazolium bromide (MTT) solution (#A600799-0005;
Sangon Biotech) was added to each well and the cells were incubated
with 5% CO2 at 37°C for an additional 4 h. Formazan
formed in each well was solubilized by 150 µl DMSO with a
10-min gentle shake incubation. The optical density of the solution
was measured at 570 nm (#MK3; Thermo Fisher Scientific).
Annexin V-FITC/PI staining assay
Human glioma cells with or without WISP1 knockdown
were harvested using trypsin that did not contain EDTA. For
evaluation of apoptosis, cells were washed with PBS and resuspended
in 500 µl binding buffer and incubated sequentially with
Annexin V-FITC and propidium iodide (PI) according to the
manufacturer's instructions using an Annexin V-FITC-PI double
staining kit (#KGA108; KeyGen Biotech, Nangjing, China). Stained
cells were evaluated by flow cytometric analysis (#FACSCanto II; BD
Biosciences, Frankin Lakes, NJ, USA). For cell cycle analysis,
cells were washed with PBS and fixed in 75% precooled ethanol
overnight at 4°C. Fixed cells were washed with PBS again and
treated with RNase A before staining with PI according to the
manufacturer's instructions (#P8080; Solarbio, Beijing, China).
Stained cells were detected by flow cytometric analysis at 488
nm.
Cell migration and invasion assay
The migration and invasion ability of human glioma
cell lines were assessed by a Transwell assay. U373-MG and U251
cell lines were cultured or transfected with either sh-NC or
sh-WISP1. Cells were then harvested and plated at a density of
1×105 cells/well in the upper compartments of Matrigel
Transwell chambers (#354480; Corning) with serum-free medium. For
the invasion assay, the upper chambers were precoated with an ECM
matrix gel layer. The chambers were inserted into 24-well plates
filled with cell growth medium (containing 10% FBS). After
incubation for 24 h, cells that had migrated onto the lower
membrane were stained with crystal violet solution (#C0121;
Beyotime Biotech) and visualized under a microscope. Further
measurement of the optical density at a wavelength of 570 nm was
performed after the cells were treated with glacial acetic
acid.
In vivo tumorigenicity assay
To determine tumor growth in vivo, U251 cell
lines transfected with Lv-sh-NC and Lv-sh-WISP1 were suspended in
culture medium at a density of 1×107 cells/ml. BALB/c
mice were acquired at 4 weeks old (certificate no. 43004700016031;
Hunan SJA Laboratory Animal Co.) and kept in a 10/14-h light-dark
cycle with free access to food and water. Cells were inoculated
subcutaneously (4×106 cells/injection) into the axillary
area of the mice (3 mice/group). The growth of the tumors was
monitored every 3 days, with mouse bodyweight and tumor size
measured and recorded. Any nodule with a diameter >0.4 cm was
considered to be a tumor. The tumor volume was calculated with the
following equation: V = A x B2/2 mm3. At the end of
experiment, the mice were sacrificed and the tumors were isolated
for further measurement.
Statistical analysis
Statistical analysis was performed using SPSS 17.0
statistical software (SPSS, Inc., Chicago, IL, USA). Data are
expressed as mean ± standard error of the mean. P<0.05 was
considered to indicate statistical significance. Correlation
between WISP1 and clinicopathological features of patients were
compared with a χ2 test. Comparisons of WISP1 protein
expression, mRNA levels, cell numbers, and tumor parameters were
analyzed with a 2-tailed t-test. One-way ANOVA was performed to
compare data from more than two groups.
Results
Upregulation of WISP1 in glioblastoma and
its association with clinicopathological features and poor
survival
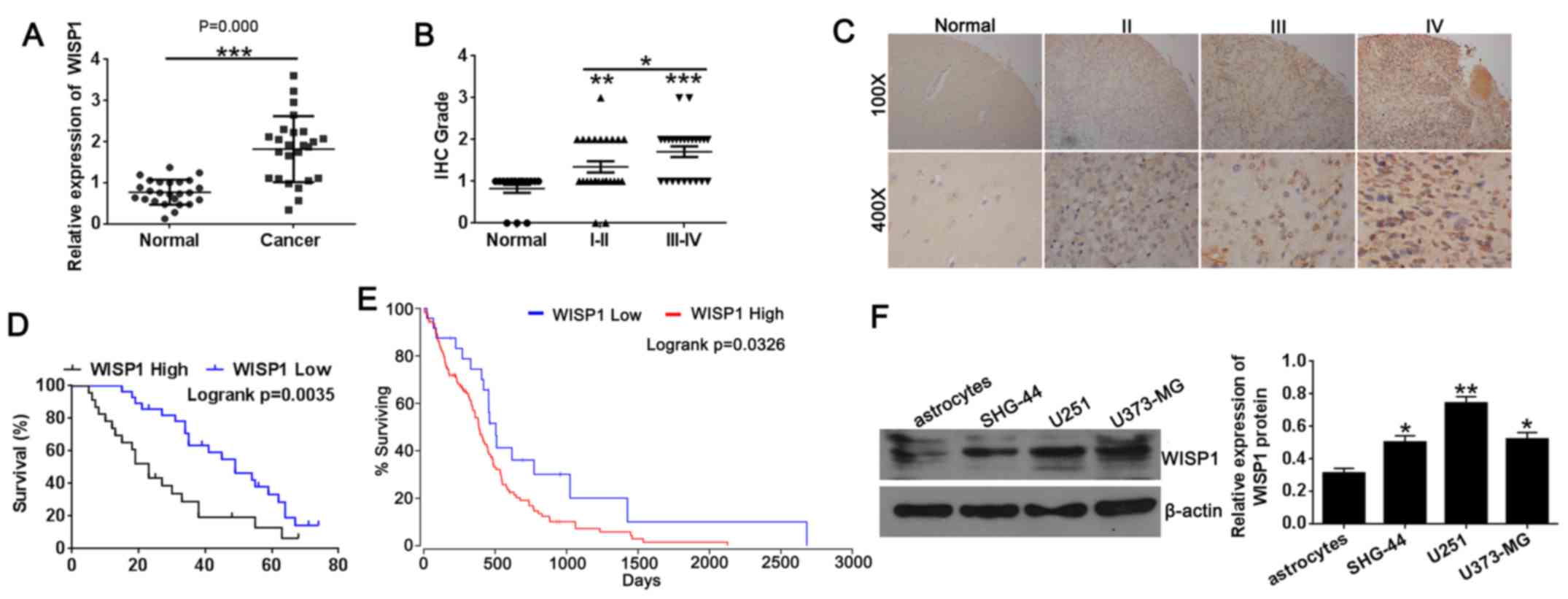
WISP1 expression was dramatically higher in
glioblastoma tissues than in normal tissues from the Oncomine
datasets (GSE4536) (9). We
performed qRT-PCR assay and tissue microarray immunohistochemical
staining to explore the potential involvement of WISP1 in
glioblastoma. The qRT-PCR results and microarray
immunohistochemical results showed that WISP1 levels were
significantly higher in glioblastoma tissues compared with normal
tissues (Fig. 1A and B), similar
to the results of the Oncomine datasets (GSE4536). We further
analyzed the correlation of WISP1 levels with clinicopathological
features of glioblastoma specimens (Table I). The χ2 test indicated
that WISP1 expression was positively associated with the clinical
stage of glioblastoma (P=0.007), but no correlation of WISP1 levels
with age or sex was detected (all P>0.05). As shown in Fig. 1C, the increased expression of WISP1
in normal tissue and in stage II, III, and IV glioblastoma tissues
was specifically distributed in the cytosol. In terms of survival,
patients with glioblastomas expressing lower levels of WISP1
notably lived longer than those with higher levels of WISP1
expression on tissue microarray (Fig.
1D). We also analyzed data of glioblastoma patients in TCGA
datasets, with results similar to those of tissue microarray
(Fig. 1E). Those results indicate
that WISP1 expression was positively associated with the clinical
stage and prognosis in glioblastoma.
 | Table ICorrelation of WISP1 expression and
clinicopathological features of glioblastoma patients. |
Table I
Correlation of WISP1 expression and
clinicopathological features of glioblastoma patients.
| Clinicopathological
features | No. of cases | WISP1 expression
| P-value |
|---|
| Low | High |
|---|
| Age (years) | | | | 0.276 |
| >40 | 22 | 10 | 12 | |
| ≤40 | 30 | 18 | 12 | |
| Sex | | | | 0.177 |
| Female | 25 | 16 | 9 | |
| Male | 27 | 12 | 15 | |
| Grade | | | | 0.028a |
| I–II | 28 | 19 | 9 | |
| III–IV | 24 | 9 | 15 | |
We next examined the levels of WISP1 protein in
normal astrocytes and glioblastoma cell lines (SHG-44, U251 and
U373-MG) by western blotting. Consistent with the tissue results,
the protein expression level of WISP1 (Fig. 1F) was significantly higher in
glioblastoma cell lines compared with normal astrocytes. These
results suggest that higher WISP1 expression was associated with
the development of glioblastoma, which correlated with poor
survival in patients with glioblastoma.
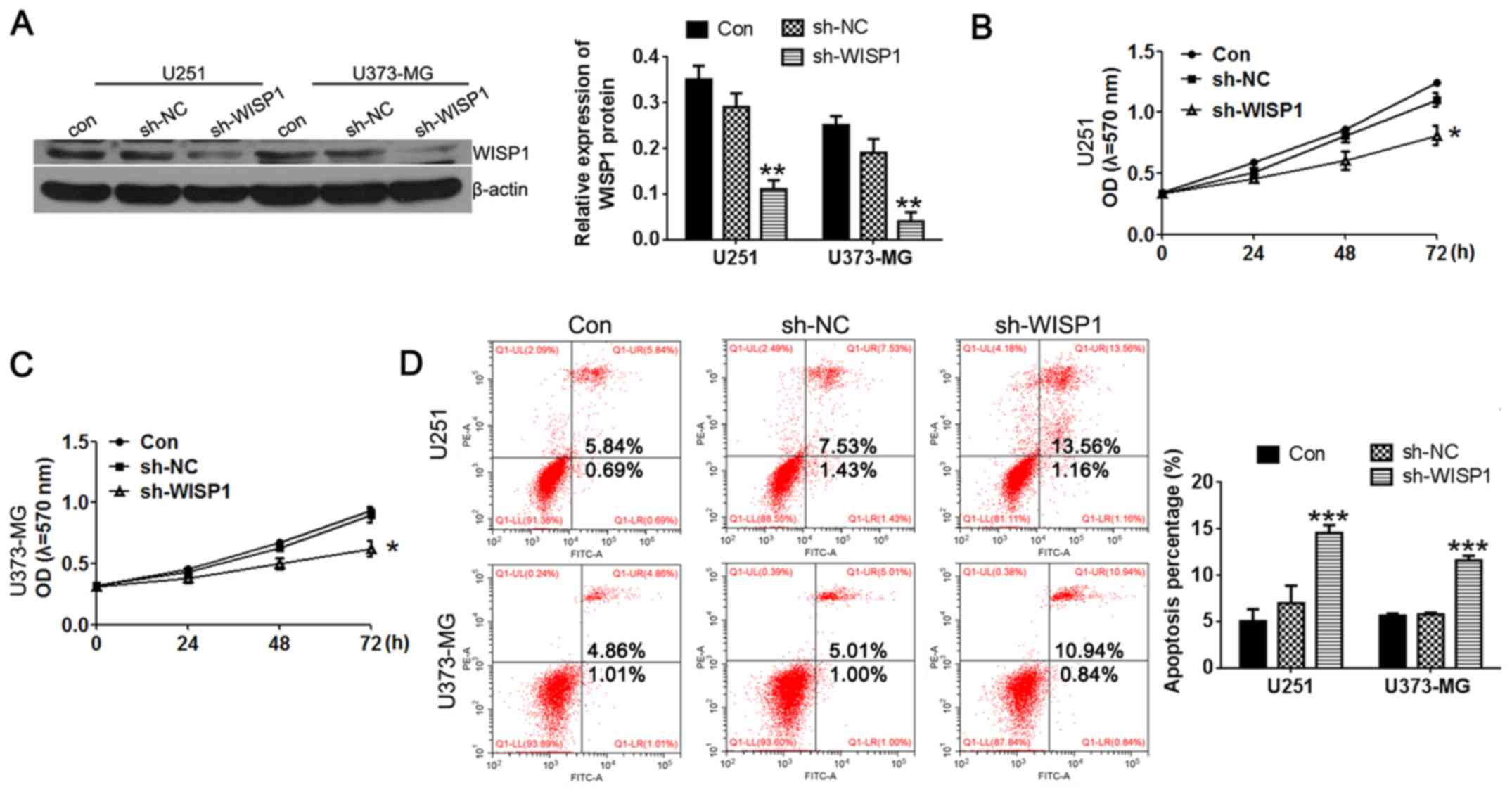
Downregulation of WISP1 suppresses
proliferation and promotes apoptosis and cell cycle arrest of
glioblastoma cell lines
Having confirmed that WISP1 is upregulated in
glioblastoma cell lines, we hypothesized that high levels of WISP1
are important for maintaining the malignant characteristics of
these cells. To test how WISP1 affects glioblastoma cell behavior,
we constructed WISP1 shRNA and transfected it into U251 and U373-MG
cell lines. As shown in Fig. 2A,
western blot results demonstrated efficient knockdown of WISP1 to
30% of its endogenous levels.
To further assess the role of WISP1 on glioblastoma
cell proliferation, MTT assay, and flow cytometry for apoptosis and
cell cycle distribution assays were used. Interestingly, knockdown
of WISP1 dramatically inhibited cell growth (Fig. 2B and C) as measured by MTT assays.
WISP1 deficiency also resulted in increasing cell apoptosis as
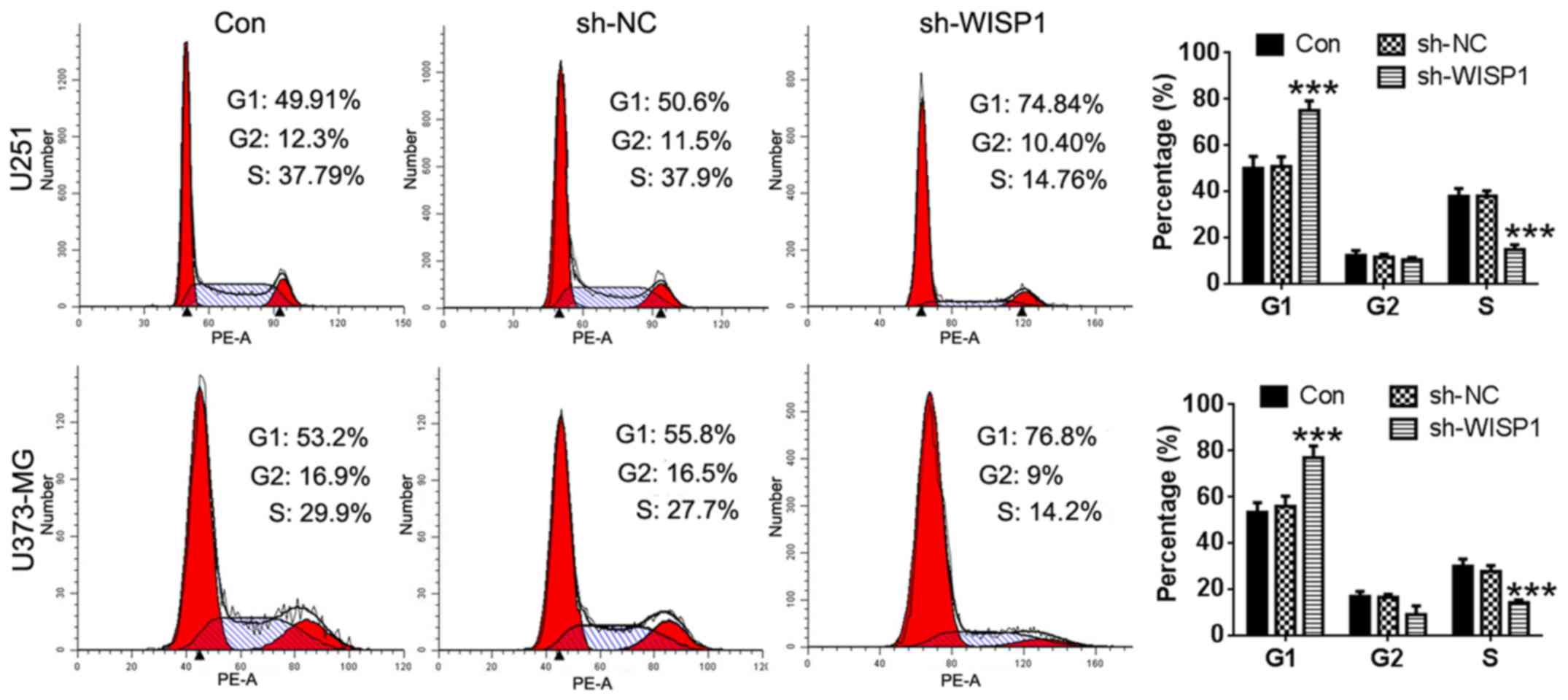
measured with Annexin V-FITC/PI staining (Fig. 2D). As shown in Fig. 3, transfection of the sh-NC plasimid
did not alter cell populations in any phase of the cell cycle
compared with non-transfected cells. Both exhibited normal
distribution of nearly half the cell population in the G1 phase.
However, WISP1 knockdown notably increased cell populations in the
G1 phase (by ~50%), with a concomitant decrease of cell populations
in the S phase. These results suggest that down-regulation of WISP1
inhibits proliferation of glioblastoma cells by arresting cell
cycle progression.
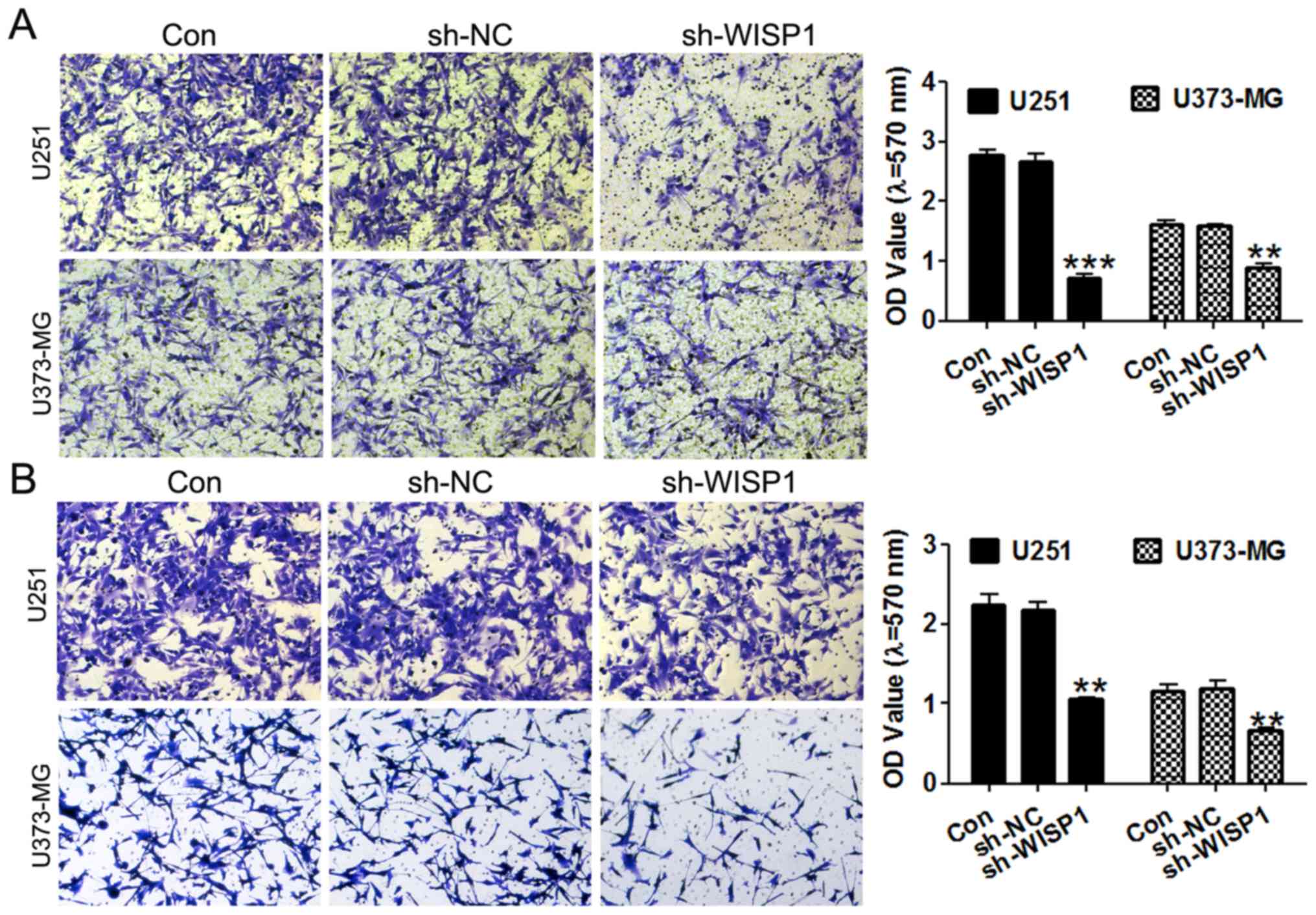
Suppression of WISP1 inhibits migration
and invasion of glioblastoma cells
Invasion of surrounding normal tissues is a critical
process during tumor progression, so the migratory and invasive
capacities of tumor cells are important hallmarks of tumor
malignancy. We performed Transwell assays to evaluate the migration
of glioma cell lines. Importantly, knockdown of WISP1 greatly
inhibited the migratory ability of U251 and U373-MG cells (Fig. 4A), as demonstrated by the 60%
decrease in that cell population that migrated into the lower
chambers compared with control groups. Similarly, WISP1-deficient
cells exhibited a 0.5-fold decrease in invasion through Matrigel to
the lower chambers in cell invasion assays(Fig. 4B).
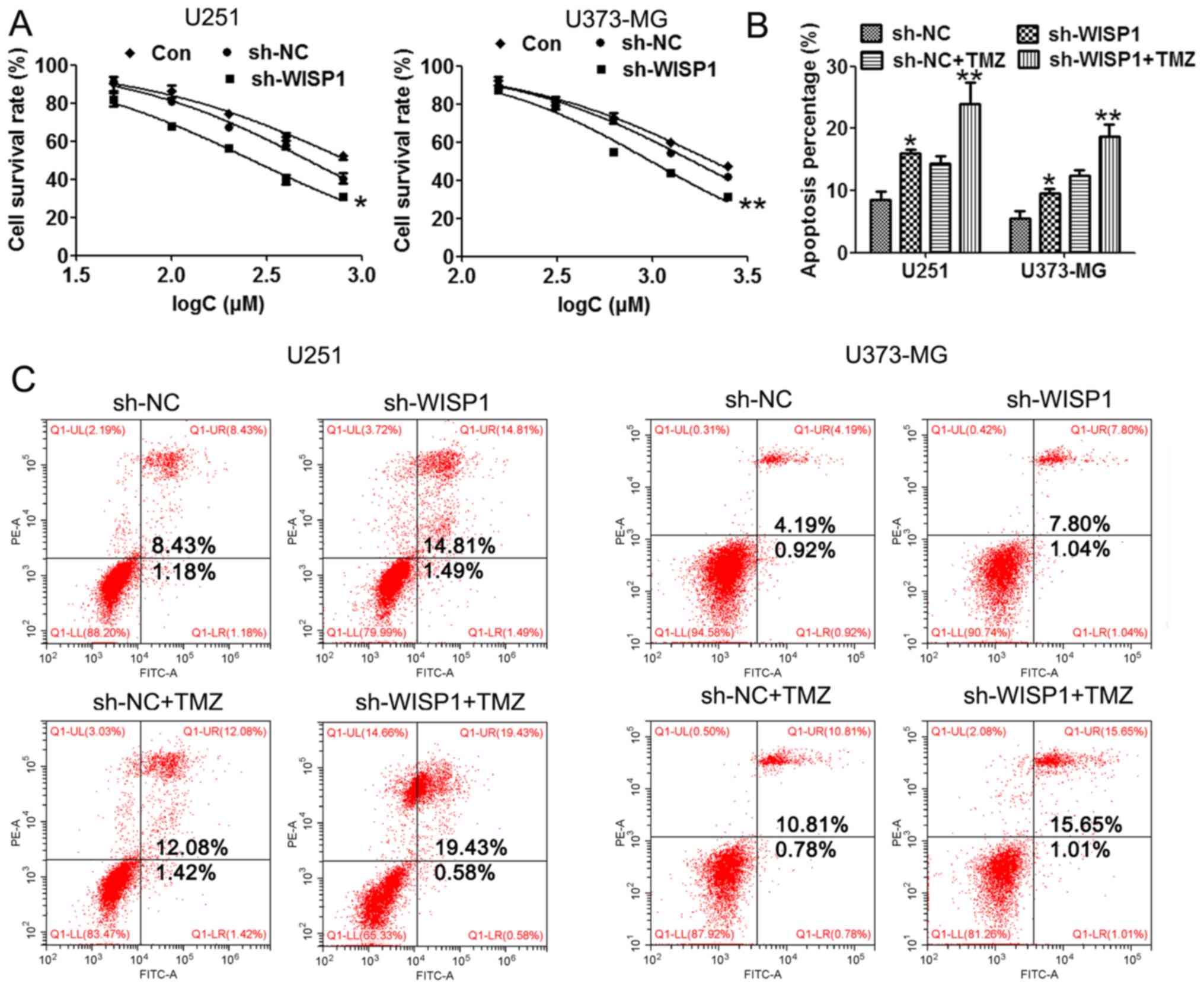
WISP1 downregulation improves drug
sensitivity of glioblastoma cells to temozolomide
TMZ is a recognized and effective drug to improve
the outcome of glioma, but drug resistance often appears to hinder
the effectiveness of TMZ. The mechanisms of TMZ resistance in
glioma cells are not well understood. In order to study the
potential role of WISP1 in regulating glioma drug resistance to
TMZ, the cell survival rate and apoptosis were evaluated by MTT
assay and flow cytometry in WISP1 downregulated cells with or
without TMZ treatment. MTT assay results showed that cells with
downregulation of WISP1 had a significantly lower survival rate
than control groups, indicating enhanced drug sensitivity to TMZ
(Fig. 5A). Flow cytometry results
(Fig. 5B and C) demonstrated that
WISP1 knockdown was associated with dramatically increased cell
apoptosis upon TMZ treatment compared with the control group.
Potential cellular mechanisms of WISP1
function in glioma cells
To further explore the mechanisms by which WISP1
regulates cell proliferation and migration of glioma cells,
associated cellular pathways that are responsible for regulating
cell growth and tumor malignancy were evaluated by western
blotting. Upon WISP1 knockdown, levels of c-myc protein, a key
regulator of cell proliferation, greatly decreased. Cyclin D1, an
important cell cycle regulation factor, as well as profoundly
increased tumor suppressor P53 expression (Fig. 6A). Additionally, epithelial markers
of E-cadherin were greatly increased, while the mesenchymal marker
vimentin and matrix metallopeptidase 9 (MMP9) level was suppressed
(Fig. 6B), indicating that WISP1
may regulate migration of glioma cells by way of
epithelial-mesenchymal transition (EMT). Moreover, the
extracellular signal-regulated kinase (ERK) signaling pathway, a
major regulator of cell proliferation, survival, differentiation,
and motility was suppressed by WISP1 knockdown (Fig. 6C). Collectively, these findings
indicate that WISP1 may be involved in progression of glioma
through regulating classic pathways in cell proliferation,
migration, and apoptosis.
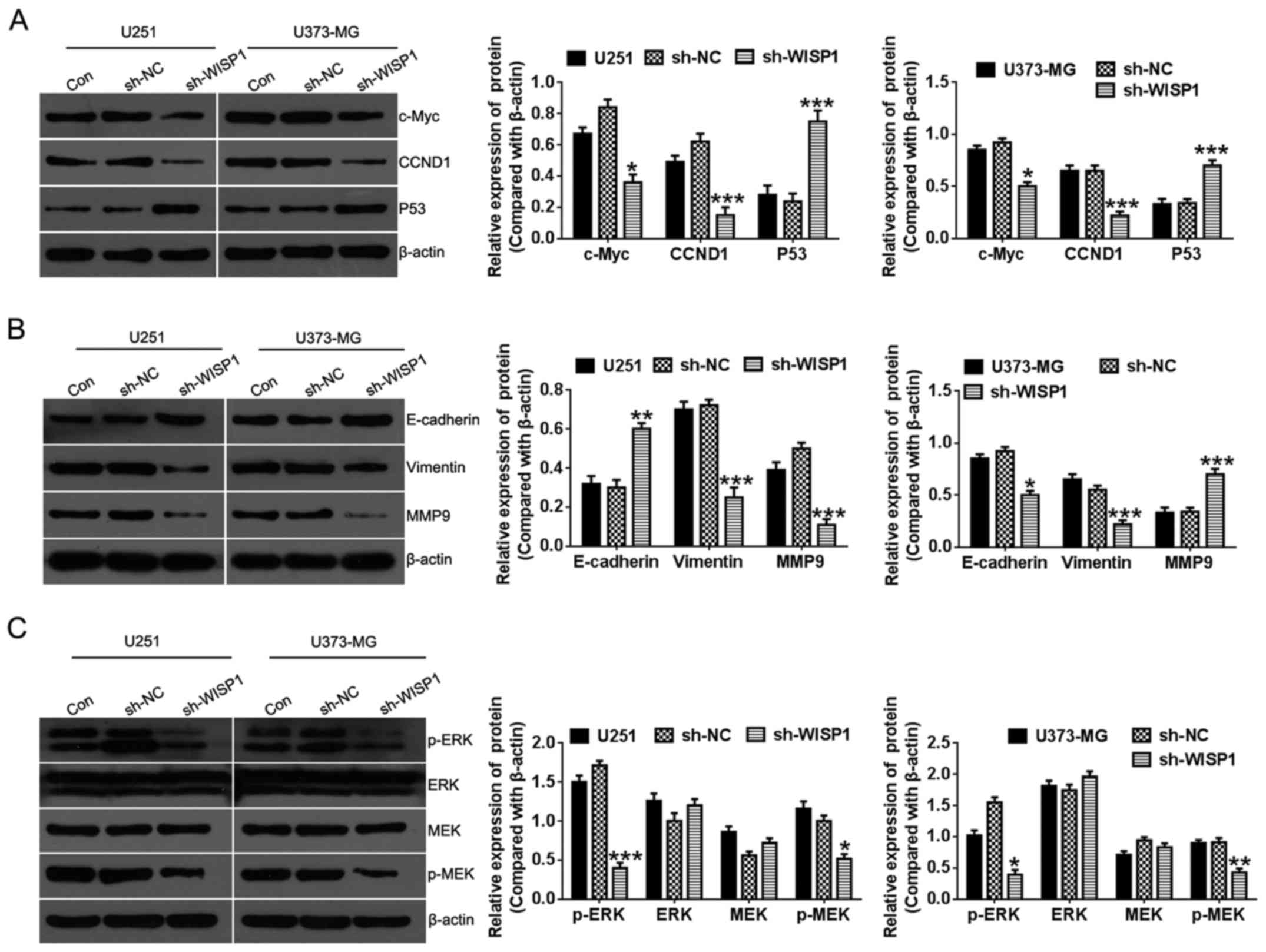 | Figure 6Effect of WISP1 knockdown on
signaling pathways associated with proliferation and migration in
glioma cell lines. After knockdown of WISP1 in U251 and U373-MG
glioma cell lines, protein levels of c-Myc, CCND1, p53 (A),
E-cadherin, Vimentin, MMP9 (B), ERK, MEK and phosphorylated form
(p-ERK, p-MEK) (C) were evaluated with western blotting. Right
panels are quantitative analysis of protein levels that were
normalized to β-actin. *P<0.05,
**P<0.01, ***P<0.001, data represent
mean ± SEM with 5 independent experiments. |
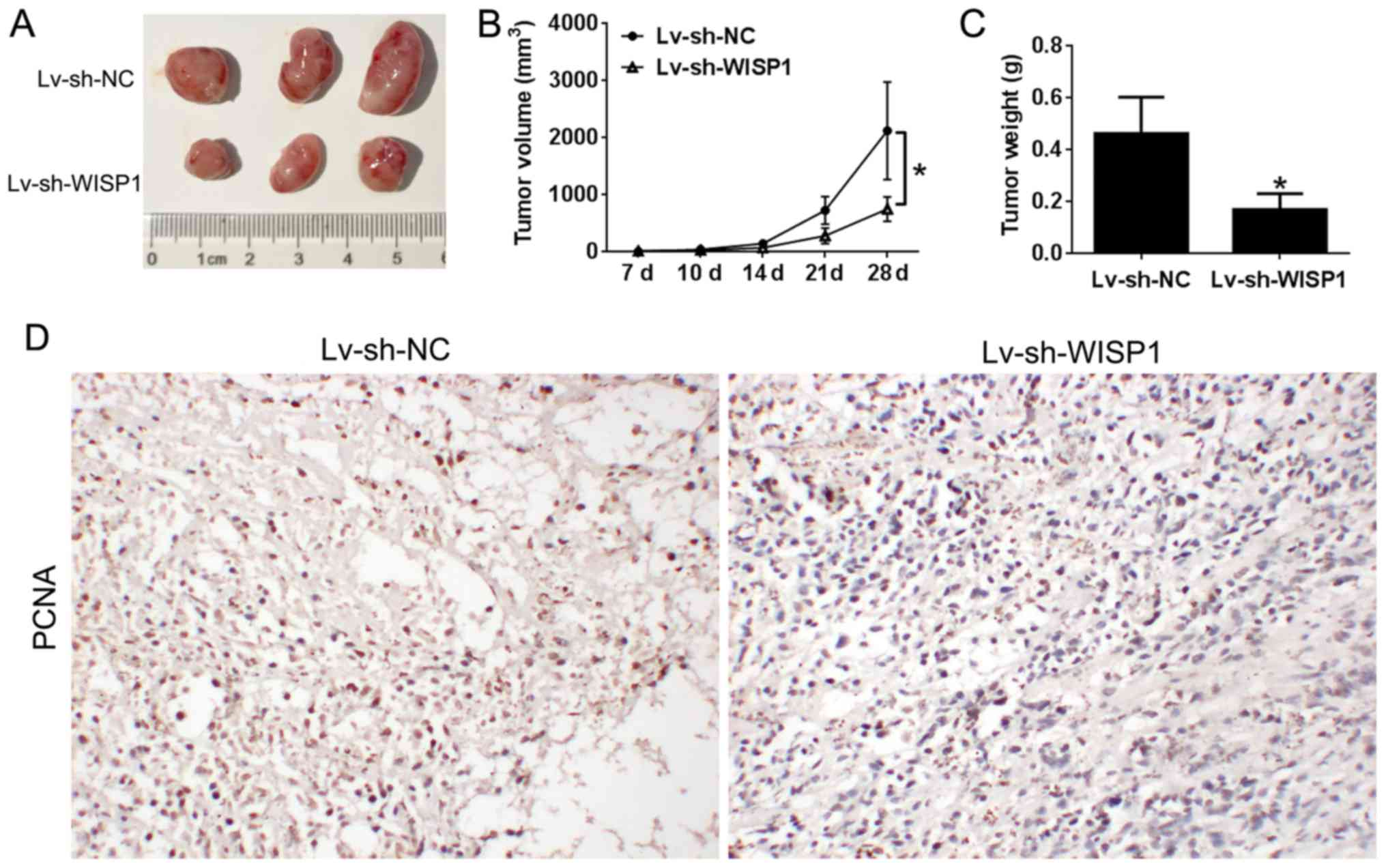
Downregulation of WISP1 suppresses
glioblastoma tumor growth in vivo
In order to examine the involvement of WISP1 in
tumor initiation and progression in vivo, the U251 cell line
transfected with control or Lv-sh-WISP1 was evaluated for
tumorigenesis capacity in BALB/c-nude mice. Interestingly, tumor
volume was significantly smaller in the WISP1 knockdown group
(Fig. 7A and B). Consistently,
tumor weight was considerably lower in mice with WISP1
downregulation (Fig. 7C). As
detected by immunohistochemical assay, the growth marker PCNA was
significantly lower in the WISP1 knockdown group than in the
control group (Fig. 7D). These
results strongly suggest downregulation of WISP1 in glioblastoma
suppresses tumor growth in vivo.
Discussion
In the present study, we explored the biological
functions of WISP1 in glioblastoma. WISP1 levels were markedly
increased in glioblastoma compared with normal tissue.
Consistently, we found that WISP1 expression was significantly
higher in glioblastoma cell lines compared with normal astrocytes.
Analysis of clinical characteristics revealed that high WISP1
levels are positively associated with advanced pathologic stage and
poor prognosis. Therefore, our study suggests a potential oncogenic
role for WISP1 in glioblastoma. This hypothesis is further
supported by our subsequent in vitro studies indicating that
suppression of WISP1 in glioblastoma cells inhibited proliferation,
migration, and invasion and induced apoptosis and cell cycle
arrest. Mechanistically, WISP1 may function through regulation of
pertinent gene signatures and critical proliferation signaling
pathways. Furthermore, WISP1 suppression sensitized glioblastoma
cells to TMZ treatment and significantly inhibited tumor growth
in vivo.
The initiation and progression of human glioma
tumors is a complex, multistep process. Even though a huge number
of growth factors, oncogenes, and tumor suppressors have been
identified as participating in the regulation of cancer cell
survival, proliferation, and invasion, the mechanisms and factors
involved in the development of glioblastoma have not been fully
elucidated. The aberrant expression of WISP1 has been reported in a
number of diseases (17), such as
fibrotic diseases, cancer, and diabetic nephropathy and
retinopathy. Interestingly, WISP1 is differentially regulated in
different types of cancers (colorectal, breast, and esophageal
cancer). High levels of WISP1 are associated with poor prognosis in
breast (18), rectal (19), and esophageal cancers (20), while in melanoma, lower than normal
WISP1 levels were found in patients with a poor outcome (21). Here, we demonstrated for the first
time that WISP1 is upregulated in glioblastoma tissues and cell
lines, and its expression is associated with advanced clinical
stages of glioblastoma and a poor prognosis, supporting the
hypothesis that WISP1 is a candidate biomarker for disease
progression in glioblastoma.
Further supporting information for a link between
WISP1 and glioblastoma progression comes from the in vitro
and in vivo experiments in this study. Downregulation of
WISP1 markedly inhibited cell proliferation, migration, and
invasion, cell cycle progression, and tumorigenesis capacity. These
findings are in line with previous reports of a tumor suppression
effect with inhibition of WISP1 in prostate and colon cancers
(10,22) and provide a plausible explanation
for WISP1 regulation of the progression of glioblastoma.
Resistance to apoptosis is a fundamental part of
carcinogenesis and is critical for chemotherapeutic drug resistance
(23). TMZ is a DNA alkylating
agent known to induce cell cycle arrest and eventually to lead to
apoptosis (24). The main damage
produced by TMZ is through modification of DNA or RNA at the N and
O sites on guanine and the N site on adenine by the addition of
methyl groups. This process produces incorrect pairing of bases,
triggering the intervention of a mismatch repair system and can be
blocked by base excision repair or the activation of DNA
glycosylase and demethylating enzymes (25). In our study, we demonstrated that
downregulation of WISP1 improved the sensitivity of glioblastoma
cell lines to TMZ treatment, suggesting that knockdown of WISP1 may
have affected signaling pathways and gene expression that
facilitate TMZ action. This is supported by decreased c-myc and
increased p53 expression, since both have been suggested to
regulate TMZ sensitivity (5,26).
For example, BACH1 promotes TMZ resistance in glioblastoma by
antagonizing p53 function (27).
It has been shown that EMT and the MAPK signaling
pathway are associated with the progression of several cancers
(28–30). MMPs promote tumor cell invasion by
degrading extracellular matrix proteins and activating signal
transduction cascades that increase lethality (31). EMT is a key factor contributing to
cancer metastasis and chemoresistance (32). Zhang et al proved that
dysregulation of Fra1 expression by Wnt/β-catenin signaling
promotes glioma metastasis through EMT (32). MALAT1 decreased the sensitivity of
resistant glioma cell lines to TMZ by promoting EMT (33). We observed a decrease in MMP9 and
EMT in WISP1 knockdown cells, suggesting that WISP1 may mediate
glioblastoma cell migration and invasion through regulation of cell
adhesion and EMT. In addition, downregulation of WISP1 improved
sensitivity of glioblastoma cells to TMZ, perhaps by suppressing
EMT.
ERK signal pathway has been repoted to contribute to
cancer proliferation and metastasis. Silencing of AQP5 inhibits
cell proliferation, migration, and apoptosis of human glioma cells
through regulation of the EGFR/ERK/p38 MAPK signaling pathway
(34). Hirudin inhibits cell
growth via ERK/MAPK signaling in human gliomas (35). We also detected decreased ERK
signaling pathway activity, indicating that WISP1 may promote
cancer cell proliferation and survival through this pathway.
However, exactly how WISP1 regulates gene expression and signaling
pathways that are critical to glioblastoma cell function is still
not completely understood.
In conclusion, this study showed for the first time
that WISP1 is an important regulator of glioblastoma cell
proliferation, apoptosis, migration, invasion, and TMZ drug
resistance. WISP1 mediates these biological processes through
regulating critical gene expression and pathways that are involved
in cancer progression. Based on the association of WISP1 with
clinicopathological features, downregulation of WISP1 may serve as
a therapeutic strategy for glioblastoma.
Abbreviations:
|
WISP1
|
WNT1 inducible signaling pathway
protein 1
|
|
IHC
|
immunohistochemistry
|
|
SEM
|
standard error of the mean
|
|
FITC
|
fluorescein isothiocyanate
|
|
PI
|
propidium iodide
|
|
Bax
|
Bcl-2 associated X protein
|
|
TMZ
|
temozolomide
|
|
CCND1
|
cyclin D1
|
|
MMP9
|
matrix metallopeptidase 9
|
|
ERK
|
extracellular regulated MAP kinase
|
|
MEK
|
MAP kinase-ERK kinase
|
Acknowledgments
This study was supported by the Scientific Research
Fund of Hunan Provincial Education Department (no. 15B225),
Scientific Research Fund of Xiangnan University (no. 2015XB12) and
Scientific Research Fund of Hunan Provincial Health and Family
Planning Commission (no. C2017014).
References
|
1
|
Ostrom QT, Bauchet L, Davis FG, Deltour I,
Fisher JL, Langer CE, Pekmezci M, Schwartzbaum JA, Turner MC, Walsh
KM, et al: The epidemiology of glioma in adults: A 'state of the
science' review. Neuro-oncol. 16:896–913. 2014. View Article : Google Scholar
|
|
2
|
Rao JS: Molecular mechanisms of glioma
invasiveness: The role of proteases. Nat Rev Cancer. 3:489–501.
2003. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gupta K and Salunke P: Molecular markers
of glioma: An update on recent progress and perspectives. J Cancer
Res Clin Oncol. 138:1971–1981. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Larjavaara S, Mäntylä R, Salminen T,
Haapasalo H, Raitanen J, Jääskeläinen J and Auvinen A: Incidence of
gliomas by anatomic location. Neuro-oncol. 9:319–325. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Martin S, Janouskova H and Dontenwill M:
Integrins and p53 pathways in glioblastoma resistance to
temozolomide. Front Oncol. 2:1572012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pegg AE: Repair of O(6)-alkylguanine by
alkyltransferases. Mutat Res. 462:83–100. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kleer CG: Dual roles of CCN proteins in
breast cancer progression. J Cell Commun Signal. 10:217–222. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Holbourn KP, Acharya KR and Perbal B: The
CCN family of proteins: Structure-function relationships. Trends
Biochem Sci. 33:461–473. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gurbuz I and Chiquet-Ehrismann R:
CCN4/WISP1 (WNT1 inducible signaling pathway protein 1): A focus on
its role in cancer. Int J Biochem Cell Biol. 62:142–146. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu J, Long Z, Cai H, Du C, Liu X, Yu S and
Wang Y: High expression of WISP1 in colon cancer is associated with
apoptosis, invasion and poor prognosis. Oncotarget. 7:49834–49847.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chuang JY, Chen PC, Tsao CW, Chang AC,
Lein MY, Lin CC, Wang SW, Lin CW and Tang CH: WISP-1 a novel
angiogenic regulator of the CCN family promotes oral squamous cell
carcinoma angiogenesis through VEGF-A expression. Oncotarget.
6:4239–4252. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang H, Luo H, Hu Z, Peng J, Jiang Z,
Song T, Wu B, Yue J, Zhou R, Xie R, et al: Targeting WISP1 to
sensitize esophageal squamous cell carcinoma to irradiation.
Oncotarget. 6:6218–6234. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang H, Luo H, Jiang Z, Yue J, Hou Q, Xie
R and Wu S: Fractionated irradiation-induced EMT-like phenotype
conferred radioresistance in esophageal squamous cell carcinoma. J
Radiat Res (Tokyo). 57:370–380. 2016. View Article : Google Scholar
|
|
14
|
Yu W, Song T, Shi L, Wang X, Jiang Z,
Zhang H and Wu S: Targeted inhibition of WISP1 enhanced
radiosensitivity in glioma cells. Zhonghua Yi Xue Za Zhi.
94:1507–1511. 2014.In Chinese. PubMed/NCBI
|
|
15
|
Minchenko OH, Kharkova AP, Minchenko DO
and Karbovskyi LL: Effect of hypoxia on the expression of genes
that encode some IGFBP and CCN proteins in U87 glioma cells depends
on IRE1 signaling. Ukr Biochem J. 87:52–63. 2015. View Article : Google Scholar
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2 (−Delta Delta C (T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
17
|
Jun JI and Lau LF: Taking aim at the
extracellular matrix: CCN proteins as emerging therapeutic targets.
Nat Rev Drug Discov. 10:945–963. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xie D, Nakachi K, Wang H, Elashoff R and
Koeffler HP: Elevated levels of connective tissue growth factor,
WISP-1, and CYR61 in primary breast cancers associated with more
advanced features. Cancer Res. 61:8917–8923. 2001.PubMed/NCBI
|
|
19
|
Tian C, Zhou ZG, Meng WJ, Sun XF, Yu YY,
Li L, Luo HZ, Yang L, Zhou B and Gu J: Overexpression of connective
tissue growth factor WISP-1 in Chinese primary rectal cancer
patients. World J Gastroenterol. 13:3878–3882. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nagai Y, Watanabe M, Ishikawa S, Karashima
R, Kurashige J, Iwagami S, Iwatsuki M, Baba Y, Imamura Y, Hayashi
N, et al: Clinical significance of Wnt-induced secreted protein-1
(WISP-1/CCN4) in esophageal squamous cell carcinoma. Anticancer
Res. 31:991–997. 2011.PubMed/NCBI
|
|
21
|
Shao H, Cai L, Grichnik JM, Livingstone
AS, Velazquez OC and Liu ZJ: Activation of Notch1 signaling in
stromal fibroblasts inhibits melanoma growth by upregulating
WISP-1. Oncogene. 30:4316–4326. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ono M, Inkson CA, Sonn R, Kilts TM, de
Castro LF, Maeda A, Fisher LW, Robey PG, Berendsen AD, Li L, et al:
WISP1/CCN4: A potential target for inhibiting prostate cancer
growth and spread to bone. PLoS One. 8:e717092013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
De Salvo M, Maresca G, D'agnano I,
Marchese R, Stigliano A, Gagliassi R, Brunetti E, Raza GH, De Paula
U and Bucci B: Temozolomide induced c-Myc-mediated apoptosis via
Akt signalling in MGMT expressing glioblastoma cells. Int J Radiat
Biol. 87:518–533. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nagasawa DT, Chow F, Yew A, Kim W, Cremer
N and Yang I: Temozolomide and other potential agents for the
treatment of glioblastoma multiforme. Neurosurg Clin N Am.
23:307–322. ix2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Stavrovskaya AA, Shushanov SS and
Rybalkina EY: Problems of glioblastoma multiforme drug resistance.
Biochemistry (Mosc). 81:91–100. 2016. View Article : Google Scholar
|
|
26
|
Luo H, Chen Z, Wang S, Zhang R, Qiu W,
Zhao L, Peng C, Xu R, Chen W, Wang HW, et al: c-Myc-miR-29c-REV3L
signalling pathway drives the acquisition of temozolomide
resistance in glioblastoma. Brain. 138:3654–3672. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nie E, Jin X, Wu W, Yu T, Zhou X, Zhi T,
Shi Z, Zhang J, Liu N and You Y: BACH1 promotes temozolomide
resistance in glioblastoma through antagonizing the function of
p53. Sci Rep. 6:397432016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li DM and Feng YM: Signaling mechanism of
cell adhesion molecules in breast cancer metastasis: Potential
therapeutic targets. Breast Cancer Res Treat. 128:7–21. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huan J, Wang L, Xing L, Qin X, Feng L, Pan
X and Zhu L: Insights into significant pathways and gene
interaction networks underlying breast cancer cell line MCF-7
treated with 17β-estradiol (E2). Gene. 533:346–355. 2014.
View Article : Google Scholar
|
|
30
|
Burotto M, Chiou VL, Lee JM and Kohn EC:
The MAPK pathway across different malignancies: A new perspective.
Cancer. 120:3446–3456. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Choe G, Park JK, Jouben-Steele L, Kremen
TJ, Liau LM, Vinters HV, Cloughesy TF and Mischel PS: Active matrix
metalloproteinase 9 expression is associated with primary
glioblastoma subtype. Clin Cancer Res. 8:2894–2901. 2002.PubMed/NCBI
|
|
32
|
Zhang L, Liu H, Mu X, Cui J and Peng Z:
Dysregulation of Fra1 expression by Wnt/β-catenin signalling
promotes glioma aggressiveness through epithelial-mesenchymal
transition. Biosci Rep. 37:372017. View Article : Google Scholar
|
|
33
|
Li H, Yuan X, Yan D, Li D, Guan F, Dong Y,
Wang H, Liu X and Yang B: Long non-coding RNA MALAT1 decreases the
sensitivity of resistant glioblastoma cell lines to temozolomide.
Cell Physiol Biochem. 42:1192–1201. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang J, Zhang JN, Chen WL, Wang GS, Mao Q,
Li SQ, Xiong WH, Lin YY, Ge JW, Li XX, et al: Effects of AQP5 gene
silencing on proliferation, migration and apoptosis of human glioma
cells through regulating EGFR/ERK/p38 MAPK signaling pathway.
Oncotarget. 8:38444–38455. 2017.PubMed/NCBI
|
|
35
|
Zhao L: Hirudin inhibits cell growth via
ERK/MAPK signaling in human glioma. Int J Clin Exp Med.
8:20983–20987. 2015.
|