The pituitary gland is an organ that physiologically
connects the hypothalamus with the peripheral organs. The pituitary
gland is an important regulator of body homeostasis during
development, stress, and other physiological processes. This small
organ is localized in a tiny cavity called sella turcica. The
pituitary fossa is a depression in the bone structure at the base
of the brain.
The pituitary is functionally and anatomically
connected to the hypothalamus by the median eminence. The gland
receives blood through the hypophyseal portal circulation, which
carries the hypothalamic hormones to the specialized
adenohypophyseal cells.
The pituitary gland is composed by adenohypophysis
(or anterior pituitary) and neurohypophysis (or posterior
pituitary) that are two different lobes. The adenohypophysis
contains three regions: the pars tuberalis (pars infundibularis),
the pars intermedia (intermediate lobe) and pars distalis (also
known as the anterior lobe). The pars intermedia is placed in the
marginal area between the anterior pituitary and the posterior
pituitary. The pars distalis represents the largest part of the
adenohypophysis while the intermediate lobe is a small portion of
the gland. The different cells of pituitary gland secrete many
molecules: endocrine hormones, cytokines and growth factors.
The neurohypophysis, or posterior pituitary, is
characterized by axonal terminals of the hypothalamic supraoptic
and paraventricular (PVN) nuclei. The neurohypophysis contains the
pituicytes: fusiform cells related with microglia. The pituicytes
surround the axons influencing the secretion of neurohypophyseal
hormones (1) as oxytocin and
vasopressin.
Hypothalamic stimulatory and inhibitory factors and
other molecules interact with the auto- and paracrine factors to
induce transcriptional regulation, translation and secretion of the
pituitary hormones. Hormones released by the anterior pituitary are
produced by specialized cells. Growth hormone (GH) is produced by
somatotroph, prolactin (PRL) by lactotrophs, adrenocorticotropic
hormone (ACTH) by corticotrophs, thyroid-stimulating hormone (TSH)
by thyreotrophs, luteinizing hormone (LH) and follicle-stimulating
hormone (FSH) by gonadotrophs cells. The function of the
specialized cells is influenced by the interaction between cells
via systemic signals.
The lactotrophs are the cells located in the
anterior pituitary and they interact with the gonadotrophs to
improve the paracrine secretion. Gonadotrophs are in the pars
distalis and tuberalis, while thyrotrophs, that represent a small
section of the total pituitary cells, are found in the
anterior-medial part of the pituitary gland and they secrete the
subunits of PRL, LH, and FSH. In the central mucoid wedge of the
pituitary gland, we can observe the presence of corticotrophs that
constitute 15–20% of the adenophypophyseal cells (2). Folliculostellate cells (FS) represent
a small rate of total population of pituitary cells. Some
immunohistochemical studies described the presence of molecular
connections between the FS cells in anterior gland (3,4). FS
cells in the normal pituitary tissue secrete large amounts of VEGF
(5,6) that improve blood vessel system of the
gland (7).
The hypophysis may be affected by different
pathologies causing endocrine and neurological disorders. Among
these pathologies there are craniopharyngioma, pituicytoma and
granular cell tumor, chordoma and the pituitary adenomas that are
associated with endocrine dysfunctions such as Cushing's disease or
acromegaly. Some pituitary neoplasms are simulated by pathological
conditions as lymphocytic or granulomatous hypophysitis.
Craniopharyngiomas are relatively uncommon tumors
and arise in the suprasellar region. They are believed to be
congenital. Craniopharyngiomas involve the pituitary gland and they
can modify the endocrine functions and damage optical nerves and
chiasm, causing vision problems (8).
The pituicytoma is a rare neoplasm in the posterior
pituitary gland that arises in the infundibulum or the
neurohypophyseal cells. A possible development of this tumor was
also noticed from the FS cells. In a recent study, a positivity for
vascular endothelial growth factor (VEGF) was detected in patients
with pituicytoma (9).
Granular cell tumors (GCTs) can arise in different
regions of the body: skin, head and neck. GCTs occur rarely in the
central nervous system developing in the posterior hypophysis and
cerebral hemispheres (10,11).
Intracranial chordomas are characterized by soft and
gelatinous lesions usually developing at the dorsum sellae. The
cells are large and vacuolated. Chordomas may produce compression
of the pituitary and destruction of the pituitary fossa. These
lesions slowly reach neurovascular bundles and often cannot be
treated surgically (12).
Pituitary adenomas constitute a common group of
benign tumors arising in adenohypophyseal cells of the anterior
lobe of the pituitary gland (13,14).
Some adenomas are similar to malignant tumors, invading the
cavernous sinus, sphenoid sinus and hypothalamus (15). The invasive adenomas are
characterized by large size, rapid growth and scarce response to
treatment. Pituitary adenomas may be subdivided to functional and
non-functional, depending on their hormonal activity in vivo
(14,16,17).
Tumors may hypersecrete pituitary hormones which can
determine endocrine disturbances of physiological mechanisms that
are controlled by the gland. The pituitary adenomas can produce
great doses of GH causing gigantism or acromegaly, ACTH leading to
Cushing's disease and PRL, which negatively influences
reproduction. Some very rare pituitary adenomas secrete FSH and LH
(which cause reproductive dysfunction) or TSH that leads to
hyperthyroidism. However, some pituitary tumors do not produce
hormones, but their growth and expansion may produce a reduced
function of the gland (hypopituitarism). Non-functioning pituitary
adenomas represent ~30% of all pituitary tumors (18). Non-functioning pituitary adenomas
may be characterized by the feature of invasive macroadenomas that
determine the onset of neurological symptoms (19).
Regarding the different therapeutic approaches,
which include trans-sphenoidal resection, pharmacotherapy, and
radiation therapy, the results remain inadequate in a significant
number of patients (20,21). The invasiveness of pituitary
adenomas seems to be an important determinant for the success rate
of surgical treatment. Generally, this tumor has no capsule that
could separate it from the adjacent tissue and, consequently, the
growing adenoma can reach and invade the adjacent structures
(22). Such adenomas may produce
some symptoms through two mechanisms: i) hypersecretion or
hyposecretion of hormones and i) compression exerted on the
neighbouring structures (23).
Functioning pituitary adenomas become symptomatic because they lead
to hormone secretion whereas the non-functioning variety may grow
slowly and compress the optic chiasm situated directly above the
pituitary gland, producing progressive visual loss (24).
Actually, the causes responsible for the pathogenic
processes of initiation, expansion and invasion of pituitary tumors
are not clear, but different mechanisms are involved in the
pituitary tumorigenesis. A small percentage of tumors is hereditary
and so these tumors may be due to genetic mutations (20). Recent studies suggest that
neurotrophins and other growth factors play a significant role in
pituitary adenoma development (25–29)
(Table I). In this review we
summarize and discuss the data regarding the trophic and
neurotrophic factors which seem to influence the proliferation and
growth of pituitary adenomas.
The CNTF is a neurotrophin of the IL-6 family and
has important neuroprotective effects on neurons. CNTF acts by
binding to several receptors: CNTF receptor (CNTFR), gp130 and the
leukemia inhibitory factor receptor (LIFR). The resultant
CNTF-CNTFRα complex induces the formation of the LIFRα-gp130
heterodimer. When CNTF interacts with the receptors it activates
the Janus kinases/signal transducer and activator of transcription
(JAK/STAT), mitogen-activated protein kinase (MAPK), and
phosphatidyl inositol 3-kinase/protein kinase B (PI3K/Akt)
signalling pathways (30). Some
studies found that the deletion of the CNTF gene in mice determines
the degeneration of motor neurons and the increasing of an
inflammatory demyelinating disease (31,32).
The CNTF have been demonstrated to be important for autocrine and
paracrine mechanisms that act in the pituitary gland (33). CNTF is expressed in
folliculostellate cells and in lactosomatotropic cells and its
secretion stimulates the production of GH and PRL (34,35).
Some authors observed that folliculostellate and
lactosomatotrophic cells express the mRNA of CNTF (34) and the mRNA of the α-chain specific
for the CNTFR. This mRNA for the CNTFR was detected in tumors
secreting PRL, GH and in non-functioning tumors (35). CNTFR are expressed on lactotropic,
somatotropic and non-functioning pituitary adenomas demonstrating
that these receptors are also present on human pituitary cells.
CNTFR was seen to be involved in pituitary pathophysiology
(35). In particular, CNTF did not
influence the secretion of either GH or PRL and on GH mRNA in
monolayer cell cultures obtained from normal rat anterior
pituitary. However, CTNF significantly stimulated both PRL and GH
secretion when the cells were amassed in cultures, restoring the
three-dimensional structure of the cells. These results underline
that the three-dimensional structure of the pituitary cells
represents a key role for the regulatory action of CTNF in anterior
pituitary cells (35). The
three-dimensional organization of the cells constitutes their
physiological conformation that can also result in a better
expression of receptors. It was also observed that the interaction
of hormone-secreting cells with the extracellular matrix is
determining the role of CNTF in regulation of GH and PRL in
producing the pituitary cells.
TGF-β is a suppressor or a promoter of tumor
development in relation to the tumor stage and type (25,36).
TGF-β signalling starts through the binding of some ligands
(TGF-β1, TGF-β2 and TGF-β3) with type II TGF-β receptors (TGF-β
RII): subsequently the recruitment of type I TGF-β receptor (TGF-β
RI) forms a complex (37–39). Moreover, TGF-β RII phosphorylates
TGF-β RI to activate it (37–39).
TGF-β signal transducer proteins are Smads and the activated TGF-β
receptor complexes can phosphorylate Smad2 and Smad3. These
proteins bind Smad4 to form a complex that regulates the
transcriptional activity. On the other hand, Smad7 is an inhibitory
protein that suppresses the phosphorylation of Smad2 and Smad3
(40–42). Some authors reported that Smad3 and
phospho-Smad3 are potential markers of invasive non-functioning
pituitary adenomas. In particular, it was observed that the
invasion of non-functioning pituitary adenoma is associated with
low level of expression of Smad3 and phospho-Smad3 and that
proliferative activity was higher in invasive non-functioning
pituitary adenomas when compared to non-invasive non-functioning
pituitary adenomas (43).
The clinical importance of TGF-β ligands and
downstream signalling mediators has been analyzed in some studies
performed in different types of tumors although the results
obtained are discordant (37–39).
In non-functioning pituitary adenomas, the expression of mRNA
TGF-β1 levels was significantly lower than in invasive
non-functioning pituitary adenomas and in non-invasive
non-functioning pituitary adenomas, in comparison to normal
anterior pituitaries (43). It has
been suggested that the invasiveness of pituitary adenomas could be
predicted by TGF-β1 blood serum concentration (44). Some studies widely evaluated the
role of the TGF-β1 gene in some tumors. The expression of this gene
was studied in subjects with breast cancer and a relationship was
found between TGF-β1 gene and a poorer patient clinical outcome
(45). The evaluation of a tumor
in lung cell lines, established that this gene plays an important
role in cell proliferation (46).
TGF-β1 is commonly evaluated in tumors: in fact, the in
vitro studies were performed on TGF-β1 function in cancer cell
lines other than lung cancer and pituitary adenoma cells (47).
A study described the development of prolactinomas
in transgenic female mice with pituitary TGF-α transgene expression
(48). The levels of pituitary
TGF-α mRNA become high before initiation of lactotroph hyperplasia.
TGF effects are increased in vivo conditions by estrogen,
and TGFs do not seem to improve other pituitary cell type
cancers.
GDNF is a component of a large family of
neurotrophic factors that include GDNF, Neurturin, Artemin, and
Persephin (49,50). GDNF plays a crucial role in the
development and survival of various neuronal populations (51,52).
However, the intracellular trafficking mechanisms of GDNF are not
fully understood. Some studies demonstrated that although GDNF is
not essential for the development of dopaminergic neurons (49,50),
the presence of GDNF is important for the maintenance of these
cells in A9 dopaminergic neurons of tyrosine kinase receptor (RET)
knockout mice (51). In fact,
degeneration of A9 neurons is evident in Parkinson's disease (PD)
and GDNF was retained to be a possible therapeutic drug for PD. The
signalling of GDNF is mediated through a system including GFRa1
which binds to GDNF. Subsequently, this complex binds and triggers
the RET (52,53). Recently, GDNF and RET gene
expression have been found in anterior pituitary glands from male
rats (54). In an interesting
study, a positive immunostaining for GDNF was observed in all of
the GH-secreting pituitary adenomas and in 10% of the
corticotropinomas (55). Some
experimental evaluations showed that both RET and GDNF are normally
secreted in the human pituitary gland. These results were confirmed
through other experimental techniques, demonstrating that the
pituitary gland produces GDNF and that the gland itself is also a
target tissue of neurotrophins (55). GDNF is mostly present in
somatotrophs and, to a lesser extent, in corticotrophs, but it is
not present in gonadotrophs of the human pituitary gland (55). In particular, a positive
immunostaining for GDNF was observed in all of the GH secreting
adenomas and in 10% of the corticotropinomas, but it was negative
in all other pituitary tumors.
NGF is a growth factor expressed by peripheral
tissues that are innervated by sensory and sympathetic neuronal
projections and it belongs to the nerve growth factor family of
neurotrophins. NGF controls neuronal survival, differentiation and
growth binding to two receptors: the p75 neurotrophin receptor
(p75NTR) and tropomyosin-related kinase A (TrkA) (56–58).
Some studies have demonstrated that NGF is involved in tumor
progression, increasing cancer cell survival and proliferation
(59–61). NGF is not only described in the
nervous system, but also in some normal and neoplastic human
tissues (62,63). In the submandibular gland of the
mouse NGF is composed of 2α subunits, 1β subunit, 2γ subunits
(α2βγ2) and also one or two zinc ions (64). The β subunit of NGF represents a
biologically active region, the 2γ subunits of NGF have proteolytic
activity and the 2α subunits do not possess enzymatic activity
(65–67).
NGF acts as a regulator of neuronal survival,
proliferation, and differentiation in the peripheral and central
nervous systems by binding to its receptors: TrkA and p75NTR. The
binding between p75NTR and NGF controls the activation of c-Jun
N-terminal kinase (JNK) signalling pathways to promote apoptosis,
and the activation of nuclear factor κB (NF-κB) pathways to promote
cell survival. Moreover, NGF increases cell proliferation and
metastasis binding to TrkA (68,69).
Some researchers have also found that p75NTR may help to improve
the binding of NGF with TrkA, TrkA activation and the number of
binding sites (70). TrkA
regulates growth and differentiation of neurons in peripheral and
central nervous systems (71). NGF
facilitates the development of perivascular nerves to regulate the
blood flow in tumors. NGF may promote angiogenesis by interacting
with α9β1 integrin (72). In one
study it was observed that p75NTR in tumor cells may negatively
regulate cell growth and proliferation (73). An increased apoptosis
(pro-apoptotic effect) was demonstrated in cells with a high
expression of p75NTR from patients with medulloblastoma (74). In prolactinoma cells, NGF binds
p75NTR and activates NF-κB in a TrkA-independent way (75). It was observed that TrkA triggers
proliferation in some tumor cells (76,77),
but inhibits cell growth in other tumors (78,79).
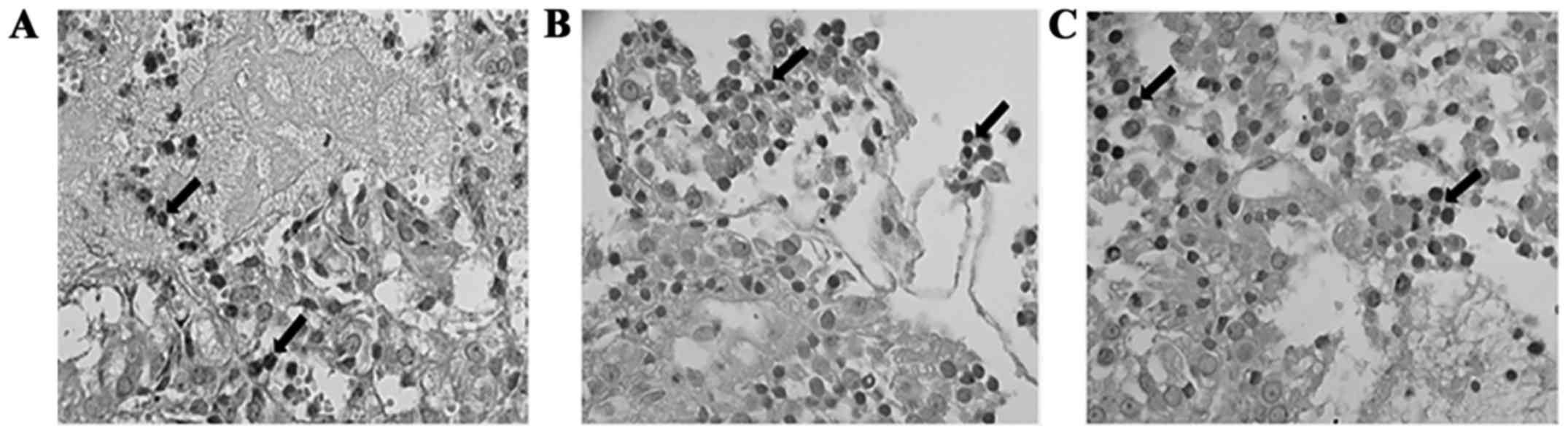
In some patients with pituitary adenomas, a moderate expression for
NGF, and its relative receptor TrKA and p75NTR were observed on
epithelial glands (29) (Fig. 1). It has also been found that
p75NTR suppresses some tumors (80,81),
but has a mitogenic effect in others (82). The cell cycle controls the cell
proliferation and the G1 phase of the cycle is regulated by the
protein p53 that is a tumor suppressor (83). Inactivation of this pathway
influences tumorigenesis. Some mutations that inactivate the p53
gene are observed in more than 50% of all human cancers (84). However, p53 is rarely mutated in
human pituitary adenomas (85).
Mammosomatotroph pituitary cells express NGF and its receptors.
Mice, in which transgenic NGF is driven by the prolactin promoter,
develop lactotroph hyperplasia without adenomas, despite having
markedly enlarged pituitary glands (86).
VEGF is a central regulatory protein of angiogenesis
with a hydrophobic leader sequence. It has a homodimeric structure
with three intramolecular and two intermolecular disulfide (S-S)
bonds. It is a member of a family that exerts important functions
in vasculogenesis, angiogenesis and lymphangiogenesis. It was found
that VEGF is a tumor-secreted protein that can increase
microvascular permeability to plasma proteins. In particular, it
improves vascular permeability to plasma and plasma proteins, a
typical characteristic of the tumor microvasculature and a critical
early step in cancer stroma generation. VEGF is overexpressed in
the cells of several human vascular tumors of the brain, colon,
gastrointestinal tract, ovary, and breast (87). Some studies found that VEGF may not
directly participate to tumoral invasion, but it may regulate
pathways that increase tumor volume or invasiveness (88–91).
Results obtained in a study, described that VEGF operates as a
neurotrophic factor and plays an important role during the
regeneration of peripheral nerves (92).
VEGF binds and activates the type 1 and 2 vascular
endothelial growth factor receptors (VEGFR1 and VEGFR2) on the
vascular endothelium (93). In the
pituitary VEGF and VEGFR2 are expressed (94,95).
Various experimental publications reported that VEGF expression is
not necessarily related to endothelium and vessels, but it is also
found in adenoma cells (28,96).
Distribution and location of VEGF receptors in pituitary adenomas
has also been studied. A relevant expression of fetal liver
kinase-1, a type of VEGF receptor that controls mitogenesis and
influences endothelial cell characteristics, may have a potential
role in the pituitary tumorigenesis (97). According to this observation fetal
liver kinase-1 expression was very marked in non-functioning
adenomas in comparison to functioning tumors (97). Other reports concerning the
expression and the distribution of VEGF and its receptors in
pituitary adenomas are not conclusive. In another study, VEGF
expression was recognized as different in the subtypes, thus
suggesting possible different modalities of VEGF expression and/or
effects (28). The tyrosine kinase
receptors of VEGF are mainly expressed on vascular endothelium, and
it behaves as a selective mitogen for vascular endothelium.
VEGF contributes to the formation of the vascular
network of a new pituitary tumor (98,99)
and is also involved in the proliferative action of estrogen on
lactotrophs (100), VEGF might
contribute to adequate temporal vascular supply. It was observed
that VEGF is expressed in all cell types of the pituitary, but
mainly in somatotrophic and follicle-stellate cells. In the normal
human pituitary gland, VEGF expression was higher than adenomas
(28). One study found no
differences in VEGF expression among tumors of different histotypes
(101). Also, comparing VEGF in
different types, the highest expression was observed in
non-functioning adenomas and GH producing adenomas (97). Elevated serum VEGF levels have been
determined in patients with pituitary tumors and VEGF secretion was
measured in human pituitary tumors cultured in vitro. In a
group of patients with pituitary adenomas it was observed that VEGF
protein expression was higher in dopamine agonist resistant
prolactinomas compared to non-functioning GH and ACTH secreting
adenomas (102). Different VEGF
levels in ACTH-secreting adenomas may be produced by
glucocorticoids which are efficient inhibitors of VEGF secretion
(103). VEGF targeting in
pituitary adenomas may be useful as shown in a study performed on
mice (104).
The VEGF is characterized from several variants of
the growth factor that can bind one of the three VEGF-specific
receptor tyrosine kinases to influence angiogenesis or related
processes (105). The VEGF
secretion is increased by hypoxia of the tumor. The deficiency of
oxygen increases the expression of hypoxia-inducible factor 1
(HIF1), which enhances the VEGF expression.
VEGI is a protein member of the TNF superfamily that
can inhibit the proliferation of endothelial cells and exerts an
anti-angiogenic effect on the endothelial cells (106). VEGI is mainly produced by vessel
endothelial cells and also be expressed on antigen-presenting cells
and lymphocytes such as T cells and dendritic cells. VEGI always
acts as a co-stimulator to induce T cell proliferation and cytokine
secretion (107,108). VEGI acts by interacting with two
receptors: death receptor-3 (DR-3) and the decoy receptor-3
(DcR-3). DR-3 is also known as TNFRSF25: this receptor is a protein
of approximate 47 kDa in size and is a member of the TNF receptor
superfamily. So far DR3 is the only known functional receptor for
VEGI and its role is to induce apoptosis after activation.
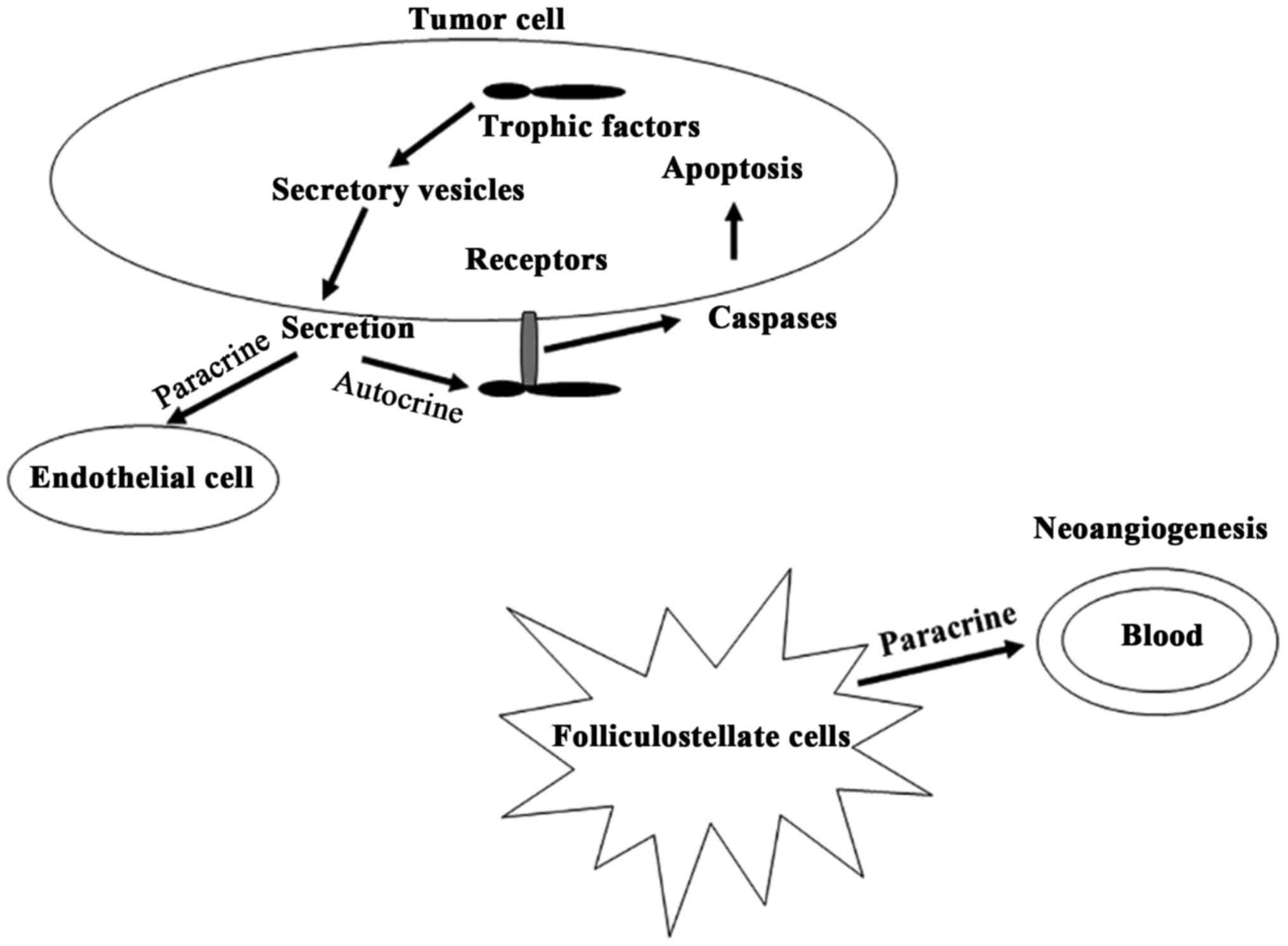
Many studies have shown that VEGI is related to
various diseases including bowel diseases (109), lung cancer (110), prostate cancer (111), breast cancer (112) and pituitary adenomas (27). The authors of these studies
described how VEGI expression is decreased in late stage tumors and
is associated with survival of patients. VEGI and other factors may
influence the development of diseases through the involvement of
some pathways (Fig. 2). It was
recently found that VEGI has a direct effect on both epithelial and
cancer cells, also by exerting an inhibitory effect on the
migration and growth of neoplastic cells. Some results indicated
that VEGI plays an essential role in activating the transcription
factor κB and caspase-3 (113).
Other studies suggested that VEGI may also be involved in the
immune response by inducing the secretion of granulocyte-macrofage
colony-stimulating factor (GM-CSF) and interferon γ IFN-γ (114). VEGI gene expression also
decreases inflammation and angiogenesis in cancers or wounds
(114). Studies that involved the
cell cycle suggested that VEGI maintained early G1 arrest in the
G0/G1 cells and induced programmed death in the endothelial cell
cycle (115).
Pituitary tumors with cystic lesions tended to
express low levels of VEGI and, in particular, pituitary tumors
that invade the floor of the sella turcica showed reduced levels of
VEGI. VEGI has an anti-angiogenesis function and some studies
demonstrated that it acts as a powerful angiogenesis inhibitor.
Lack of VEGI was negatively correlated with angiogenesis in solid
tumors, due to the removal of the inhibitory mechanisms in the
tissues (116,117). In pituitary adenomas, VEGI
inhibits the growth and the migration of tumor cells through DR3
(113). In particular, high
levels of VEGI and DR3 were found to be associated with
intratumoral haemorrhage. It is thus argued that, in a similar
pattern, the lack of VEGI in pituitary tumors may trigger an
increase in angiogenesis within the tumor tissues.
FGFs are a family of molecules that control the
differentiation, migration, and angiogenesis of the cells (118,119). FGFs includes 23 ligands and FGF2
is the basic FGF, which regulates the production of pituitary
hormones and the proliferation and differentiation of parenchyma
and vessels (120). FGF2 isoform
of 18 kDa is highly secreted in the normal human pituitary gland,
whereas the 24 kDa isoform is largely secreted by pituitary
adenomas (121). It was observed
that high levels of FGF2 were expressed in patients with pituitary
neoplasms and the secretion decreased after surgical adenomectomy
(122). In a recent study, it was
found that FGF2 is secreted in different types of pituitary tumors,
including GH secreting adenomas (123). FGF2 expression was significantly
higher in patients without postoperative remission observed at the
third month and later, if compared to subjects with remission
(123). Also, the levels of FGF2
were higher in patients who presented a sphenoid bone invasion if
compared to patients without bony lesions (123).
The interaction between FGF2 and some transmembrane
receptors (FGFRs) with tyrosine kinase activity, determines the
biological effects of FGF2 (124). The FGFRs are encoded by different
genes to obtain multiple isoforms, also the FGFRs are expressed in
the membrane surfaces of different type of cells, including
endothelial cells in which FGF2 influences the angiogenic
development. Each FGFR is characterized by three Ig-like
extracellular domains, a transmembrane domain, a tyrosine kinase
cytoplasmic domain and a -COOH domain that comprehends a group of
tyrosines phosphorylated by the binding with the ligand (125,126). A great percentage of FGFs shows a
specific affinity for the receptor isoforms. It was observed that
FGFRs are expressed in different types of tumors, including
pituitary adenomas (127–132). In particular, FGFR1 was highly
expressed in pituitary adenomas compared to healthy gland (132) and the cytoplasmic
immunoreactivity of the receptor was inversely correlated with
pituitary lesion size (133).
A study has demonstrated the lack of FGFR2 in the
pituitary adenomas that causes the upregulation of the
melanoma-associated antigen 3 (MAGEA3) (134). The presence of arginine at codon
388 of the FGFR4 gene, which encodes the receptor, was associated
with the phosphorylation process of mitochondrial STAT3 serine that
improves the GH secretion in pituitary cells promoting
tumorigenesis development (135).
Also, this arginine allele was associated with other forms of
tumors resistant to the pharmacological treatment (136–139). The expression of FGFR isoforms
was really modified in pituitary adenomas (140). It was observed that the modified
FGFR4 expression in pituitary tumors is caused by the pituitary
tumor-derived FGFR4 (ptd-FGFR4) (141). This is an isoform with a deletion
at the N-terminal produced by substitute transcription initiation
(141,142). The FGFR4 isoforms have a
different capability to link the cell adhesion molecules and, in
particular, ptd-FGFR4 blocks cell adhesion interacting with
N-cadherin signaling (143). The
complex constituted by FGFR4 and N-cadherin may be considered an
important therapeutic target to reduce the growth and the
invasiveness of tumor cells (143).
EGF is a small protein of 6 kDa containing 53 amino
acids which comprises three disulfide bridges (144). EGF displays a homology of
function and sequence with human type-α transforming growth factor
(hTGF-α), which can link the EGF receptor sites. EGF is
characterized by mitogenic activity and so it is involved in the
process of cell growth and tumorigenesis. In fact, EGF is a protein
contained in a network of growth factors and receptors that
controls the growth and the division of cells. EGF is produced by
pituitary cells and it acts as a growth factor, stimulating
prolactin synthesis (145). In
human pituitary gland EGF is localized mainly in thyrotrophic and
gonadotrophic cells (145). An
immunohistochemical study described the EGF expression in
functional and non-functional pituitary adenomas (145).
It was observed that EGF can promote the
transphosphorylation of the related oncogene neu through the
binding with epidermal growth factor receptor (EGF-R) (146). EGF-R is a 170-kDa transmembrane
glycoprotein constituted by: a tyrosine kinase domain, a
transmembrane domain and an extracellular domain of binding. EFG-R
is a component of a family which includes four known members and it
is considered the homolog of the v-erbB oncogene protein (147). Some studies evidenced EGF-R
overexpression in different tumors: lung (148), breast (149), ovarian (150) and gastric (151) cancers. Different studies observed
a higher expression in non-functional pituitary tumors (152–154). Interestingly, Onguru et al
described a moderate or a strong positivity of EGFR in more than
50% of adenomas (154). In each
group of adenomas the percentage of EGFR overexpression was
variable and it was higher in non-functional compared to functional
adenomas.
Also, in ACTH secreting adenomas the lowest number
of tumors expressing EGFR was detected (154). However, high expression of EGFR
is frequently found in ACTH secreting adenomas, in which EGF
signaling is deleted and consequently there is inhibition of ACTH
secretion (155,156). The binding between intracellular
EGFR domain and its specific antibody showed an immunoreactivity in
corticotroph adenomas and many cells of ACTH secreting tumors were
EGFR immunoreactive (157). Also,
a low or absent immunoreactivity was observed for p27 protein that
blocks the cell cycle (157).
Probably, EGFR promotes the signaling activation that induces the
downregulation of p27, which promotes tumorigenesis by the
stimulation of ACTH secretion (158).
Pituitary adenomas are common tumors that are
classified on the basis of certain characteristics: size, invasion
of adjacent structures, sporadic or familial cases, biochemical
activity, clinical manifestations, morphological characteristics,
response to treatment and recurrence. Although they are considered
benign tumors, some of them are difficult to treat because they can
recur after standardized treatment. Some studies indicate that
trophic and neurotrophic factors play a significant role in
neuroendocrine systems and in the biological effects of molecules
involved in the development and maintenance of the nervous system.
There is some evidence suggesting that trophic and neurotrophic
factors may be involved in pituitary endocrine cell function,
suggesting a possible role of trophic and neurotrophic factors in
the normal development of the pituitary gland and in the
progression of some pituitary adenomas.
The pituitary cells are regulated by endocrine and
paracrine systems through the action of some growth factors and
their receptors. A different expression and action of these factors
and their receptors may determine the development and progression
of pituitary tumors. The vascularization in the normal pituitary
regulates the growth of pituitary cells and the hormone secretion
by some molecules: hormones, hypothalamic and pituitary growth
factors. However, the high secretion of EGF, FGF and VEGF in
pituitary is involved in gland tumorigenesis (159). Generally, the pituitary adenomas
have a low grade of carcinogenicity. In fact, these tumors are
rarely aggressive and the angiogenesis is not predominantly
involved in the improvement of the release of nutrients to the
tumor. Basing upon these reasons, it may be interesting to study
the possible role of VEGF involved in pituitary angiogenesis. The
development of the vascularization in pituitary tumors is less
present than in the normal anterior pituitary tissue (160,161).
The biological role of these factors in the
development and progression of this type of tumor should be further
investigated to ameliorate the knowledge of the pathogenesis of
pituitary adenomas.
This study was supported by Nobile S.p.A. Grant from
REGIONE LAZIO Prot. FILAS-RU-2014-1020 is gratefully acknowledged
(EA).
|
1
|
Rosso L and Mienville JM: Pituicyte
modulation of neurohormone output. Glia. 57:235–243. 2009.
View Article : Google Scholar
|
|
2
|
Doniach I: Histopathology of the
pituitary. Clin Endocrinol Metab. 14:765–789. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chauvet N, El-Yandouzi T, Mathieu MN,
Schlernitzauer A, Galibert E, Lafont C, Le Tissier P, Robinson IC,
Mollard P and Coutry N: Characterization of adherens junction
protein expression and localization in pituitary cell networks. J
Endocrinol. 202:375–387. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stojilkovic SS: A novel view of the
function of pituitary folliculostellate cell network. Trends
Endocrinol Metab. 12:378–380. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cristina C, Díaz-Torga G, Baldi A, Góngora
A, Rubinstein M, Low MJ and Becú-Villalobos D: Increased pituitary
vascular endothelial growth factor-a in dopaminergic D2 receptor
knockout female mice. Endocrinology. 146:2952–2962. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Alfer J, Neulen J and Gaumann A:
Lactotrophs: The new and major source for VEGF secretion and the
influence of ECM on rat pituitary function in vitro. Oncol Rep.
33:2129–2134. 2015.PubMed/NCBI
|
|
7
|
Chauvet N, Romanò N, Lafont C, Guillou A,
Galibert E, Bonnefont X, Le Tissier P, Fedele M, Fusco A, Mollard
P, et al: Complementary actions of dopamine D2 receptor agonist and
anti-vegf therapy on tumoral vessel normalization in a transgenic
mouse model. Int J Cancer. 140:2150–2161. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hoffmann A, Boekhoff S, Gebhardt U,
Sterkenburg AS, Daubenbüchel AM, Eveslage M and Müller HL: History
before diagnosis in childhood craniopharyngioma: Associations with
initial presentation and long-term prognosis. Eur J Endocrinol.
173:853–862. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mende KC, Matschke J, Burkhardt T, Saeger
W, Buslei R, Buchfelder M, Fahlbusch R, Westphal M and Flitsch J:
Pituicytoma-An outlook on possible targeted therapies. CNS Neurosci
Ther. 23:620–626. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li P, Yang Z, Wang Z, Zhou Q, Li S, Wang
X, Wang B, Zhao F and Liu P: Granular cell tumors in the central
nervous system: A report on eight cases and a literature review. Br
J Neurosurg. 30:611–618. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mumert ML, Walsh MT, Chin SS and Couldwell
WT: Cystic granular cell tumor mimicking Rathke cleft cyst. J
Neurosurg. 114:325–328. 2011. View Article : Google Scholar
|
|
12
|
Larkin S and Ansorge O: Pathology and
pathogenesis of pituitary adenomas and other sellar lesions
Endotext [Internet]. De Groot LJ, Chrousos G, Dungan K, Feingold
KR, Grossman A, Hershman JM, Koch C, Korbonits M, McLachlan R, New
M, Purnell J, Rebar R, Singer F and Vinik A: MDText.com, Inc. 2000;
South Dartmouth, MA: 2017
|
|
13
|
Ezzat S, Asa SL, Couldwell WT, Barr CE,
Dodge WE, Vance ML and McCutcheon IE: The prevalence of pituitary
adenomas: A systematic review. Cancer. 101:613–619. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Asa SL: Tumors of the pituitary gland.
Atlas of Tumor Pathology. Rosai J: (3rd series Fascicle 22). Armed
Forces Institute of Pathology (AFIP); Washington DC: pp. 1–214.
1998
|
|
15
|
Blevins LS Jr, Verity DK and Allen G:
Aggressive pituitary tumors. Oncology (Williston Park).
12:1307–1312. 1315discussion 1315–1318. 1998.
|
|
16
|
Asa SL and Ezzat S: The pathogenesis of
pituitary tumours. Nat Rev Cancer. 2:836–849. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nammour GM, Ybarra J, Naheedy MH, Romeo JH
and Aron DC: Incidental pituitary macroadenoma: A population-based
study. Am J Med Sci. 314:287–291. 1997.PubMed/NCBI
|
|
18
|
Katznelson L, Alexander JM and Klibanski
A: Clinical review 45: Clinically nonfunctioning pituitary
adenomas. J Clin Endocrinol Metab. 76:1089–1094. 1993.PubMed/NCBI
|
|
19
|
Colao A, Di Somma C, Pivonello R, Faggiano
A, Lombardi G and Savastano S: Medical therapy for clinically
non-functioning pituitary adenomas. Endocr Relat Cancer.
15:905–915. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Daly AF, Tichomirowa MA and Beckers A: The
epidemiology and genetics of pituitary adenomas (Review). Best
Pract Res Clin Endocrinol Metab. 23:543–554. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Galland F, Lacroix L, Saulnier P, Dessen
P, Meduri G, Bernier M, Gaillard S, Guibourdenche J, Fournier T,
Evain-Brion D, et al: Differential gene expression profiles of
invasive and non-invasive non-functioning pituitary adenomas based
on microarray analysis. Endocr Relat Cancer. 17:361–371. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Syro LV, Rotondo F, Ramirez A, Di Ieva A,
Sav MA, Restrepo LM, Serna CA and Kovacs K: Progress in the
diagnosis and classification of pituitary adenomas. Front
Endocrinol (Lausanne). 6:972015.
|
|
23
|
Kovacs K, Scheithauer BW, Horvath E and
Lloyd RV: The World Health Organization classification of
adenohypophysial neoplasms. A proposed five-tier scheme. Cancer.
78:502–510. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jagannathan J, Dumont AS, Prevedello DM,
Lopes B, Oskouian RJ, Jane JA Jr and Laws ER Jr: Genetics of
pituitary adenomas: Current theories and future implications.
Neurosurg Focus. 19:E42005.
|
|
25
|
Wakefield LM and Roberts AB: TGF-beta
signaling: Positive and negative effects on tumorigenesis. Curr
Opin Genet Dev. 12:22–29. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Massagué J: TGFbeta in cancer (Review).
Cell. 134:215–230. 2008. View Article : Google Scholar
|
|
27
|
Jia W, Sander AJ, Jia G, Ni M, Liu X, Lu R
and Jiang WG: Vascular endothelial growth inhibitor (VEGI) is an
independent indicator for invasion in human pituitary adenomas.
Anticancer Res. 33:3815–3822. 2013.PubMed/NCBI
|
|
28
|
Lloyd RV, Scheithauer BW, Kuroki T, Vidal
S, Kovacs K and Stefaneanu L: Vascular endothelial growth factor
(VEGF) expression in human pituitary adenomas and carcinomas.
Endocr Pathol. 10:229–235. 1999. View Article : Google Scholar
|
|
29
|
Artico M, Bianchi E, Magliulo G, De
Vincentiis M, De Santis E, Orlandi A, Santoro A, Pastore FS,
Giangaspero F, Caruso R, et al: Neurotrophins, their receptors and
KI-67 in human GH-secreting pituitary adenomas: An
immunohistochemical analysis. Int J Immunopathol Pharmacol.
25:117–125. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ernst M and Jenkins BJ: Acquiring
signalling specificity from the cytokine receptor gp130. Trends
Genet. 20:23–32. 2004. View Article : Google Scholar
|
|
31
|
Masu Y, Wolf E, Holtmann B, Sendtner M,
Brem G and Thoenen H: Disruption of the CNTF gene results in motor
neuron degeneration. Nature. 365:27–32. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Linker RA, Mäurer M, Gaupp S, Martini R,
Holtmann B, Giess R, Rieckmann P, Lassmann H, Toyka KV, Sendtner M,
et al: CNTF is a major protective factor in demyelinating CNS
disease: A neurotrophic cytokine as modulator in neuroinflammation.
Nat Med. 8:620–624. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ray D and Melmed S: Pituitary cytokine and
growth factor expression and action. Endocr Rev. 18:206–228. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Perez Castro C, Nagashima AC, Pereda MP,
Goldberg V, Chervin A, Largen P, Renner U, Stalla GK and Arzt E:
The gp130 cytokines interleukin-11 and ciliary neurotropic factor
regulate through specific receptors the function and growth of
lactosomatotropic and folliculostellate pituitary cell lines.
Endocrinology. 141:1746–1753. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Perez Castro C, Carbia Nagashima A, Páez
Pereda M, Goldberg V, Chervin A, Carrizo G, Molina H, Renner U,
Stalla GK and Arzt E: Effects of the gp130 cytokines ciliary
neurotropic factor (CNTF) and interleukin-11 on pituitary cells:
CNTF receptors on human pituitary adenomas and stimulation of
prolactin and GH secretion in normal rat anterior pituitary
aggregate cultures. J Endocrinol. 169:539–547. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yang L, Pang Y and Moses HL: TGF-beta and
immune cells: An important regulatory axis in the tumor
microenvironment and progression. Trends Immunol. 31:220–227. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Johnson MD, Shaw AK, O'Connell MJ, Sim FJ
and Moses HL: Analysis of transforming growth factor β receptor
expression and signaling in higher grade meningiomas. J Neurooncol.
103:277–285. 2011. View Article : Google Scholar
|
|
38
|
Bruna A, Darken RS, Rojo F, Ocaña A,
Peñuelas S, Arias A, Paris R, Tortosa A, Mora J, Baselga J, et al:
High TGFbeta-Smad activity confers poor prognosis in glioma
patients and promotes cell proliferation depending on the
methylation of the PDGF-B gene. Cancer Cell. 11:147–160. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wu Y, Li Q, Zhou X, Yu J, Mu Y, Munker S,
Xu C, Shen Z, Müllenbach R, Liu Y, et al: Decreased levels of
active SMAD2 correlate with poor prognosis in gastric cancer. PLoS
One. 7:e356842012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Massagué J: TGFβ signalling in context.
Nat Rev Mol Cell Biol. 13:616–630. 2012. View Article : Google Scholar
|
|
41
|
Heldin CH, Miyazono K and ten Dijke P:
TGF-beta signalling from cell membrane to nucleus through SMAD
proteins. Nature. 390:465–471. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
42
|
Nakao A, Afrakhte M, Morén A, Nakayama T,
Christian JL, Heuchel R, Itoh S, Kawabata M, Heldin NE, Heldin CH,
et al: Identification of Smad7, a TGFbeta-inducible antagonist of
TGF-beta signalling. Nature. 389:631–635. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu C, Li Z, Wu D, Li C and Zhang Y: Smad3
and phospho-Smad3 are potential markers of invasive nonfunctioning
pituitary adenomas. Onco Targets Ther. 9:2265–2271. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Elenkova A, Atanassova I, Kirilov G,
Vasilev V, Kalinov K and Zacharieva S: Transforming growth factor
β1 is not a reliable biomarker for valvular fibrosis but could be a
potential serum marker for invasiveness of prolactinomas (pilot
study). Eur J Endocrinol. 169:299–306. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chen C, Zhao KN, Masci PP, Lakhani SR,
Antonsson A, Simpson PT and Vitetta L: TGFβ isoforms and receptors
mRNA expression in breast tumours: Prognostic value and clinical
implications. BMC Cancer. 15:10102015. View Article : Google Scholar
|
|
46
|
Liu ZY, Zhang GL, Wang MM, Xiong YN and
Cui HQ: MicroRNA-663 targets TGFB1 and regulates lung cancer
proliferation. Asian Pac J Cancer Prev. 12:2819–2823.
2011.PubMed/NCBI
|
|
47
|
Wang Y, Jiang M, Li Z, Wang J, Du C,
Yanyang L, Yu Y, Wang X, Zhang N, Zhao M, et al: Hypoxia and TGF-β1
lead to endostatin resistance by cooperatively increasing cancer
stem cells in A549 transplantation tumors. Cell Biosci. 5:722015.
View Article : Google Scholar
|
|
48
|
McAndrew J, Paterson AJ, Asa SL, McCarthy
KJ and Kudlow JE: Targeting of transforming growth factor-alpha
expression to pituitary lactotrophs in transgenic mice results in
selective lactotroph proliferation and adenomas. Endocrinology.
136:4479–4488. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Airaksinen MS, Titievsky A and Saarma M:
GDNF family neurotrophic factor signaling: Four masters, one
servant? Mol Cell Neurosci. 13:313–325. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Airaksinen MS and Saarma M: The GDNF
family: Signalling, biological functions and therapeutic value. Nat
Rev Neurosci. 3:383–394. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kramer ER, Aron L, Ramakers GM, Seitz S,
Zhuang X, Beyer K, Smidt MP and Klein R: Absence of Ret signaling
in mice causes progressive and late degeneration of the
nigrostriatal system. PLoS Biol. 5:e392007. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Treanor JJ, Goodman L, de Sauvage F, Stone
DM, Poulsen KT, Beck CD, Gray C, Armanini MP, Pollock RA, Hefti F,
et al: Characterization of a multicomponent receptor for GDNF.
Nature. 382:80–83. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Robertson K and Mason I: The GDNF-RET
signalling partnership. Trends Genet. 13:1–3. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Urbano AG, Suárez-Peñaranda JM, Diéguez C
and Alvarez CV: GDNF and RET-gene expression in anterior
pituitary-cell types. Endocrinology. 141:1893–1896. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Japón MA, Urbano AG, Sáez C, Segura DI,
Cerro AL, Diéguez C and Alvarez CV: Glial-derived neurotropic
factor and RET gene expression in normal human anterior pituitary
cell types and in pituitary tumors. J Clin Endocrinol Metab.
87:1879–1884. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Lykissas MG, Batistatou AK,
Charalabopoulos KA and Beris AE: The role of neurotrophins in
axonal growth, guidance, and regeneration. Curr Neurovasc Res.
4:143–151. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Cui X, Chen L, Ren Y, Ji Y, Liu W, Liu J,
Yan Q, Cheng L and Sun YE: Genetic modification of mesenchymal stem
cells in spinal cord injury repair strategies. Biosci Trends.
7:202–208. 2013.PubMed/NCBI
|
|
58
|
Wiesmann C and de Vos AM: Nerve growth
factor: Structure and function. Cell Mol Life Sci. 58:748–759.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Mancino M, Ametller E, Gascón P and
Almendro V: The neuronal influence on tumor progression. Biochim
Biophys Acta. 1816:105–118. 2011.PubMed/NCBI
|
|
60
|
Krüttgen A, Schneider I and Weis J: The
dark side of the NGF family: Neurotrophins in neoplasias. Brain
Pathol. 16:304–310. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Molloy NH, Read DE and Gorman AM: Nerve
growth factor in cancer cell death and survival. Cancers (Basel).
3:510–530. 2011. View Article : Google Scholar
|
|
62
|
MacGrogan D, Saint-André JP and Dicou E:
Expression of nerve growth factor and nerve growth factor receptor
genes in human tissues and in prostatic adenocarcinoma cell lines.
J Neurochem. 59:1381–1391. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Vanhecke E, Adriaenssens E, Verbeke S,
Meignan S, Germain E, Berteaux N, Nurcombe V, Le Bourhis X and
Hondermarck H: Brain-derived neurotrophic factor and
neurotrophin-4/5 are expressed in breast cancer and can be targeted
to inhibit tumor cell survival. Clin Cancer Res. 17:1741–1752.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Varon S, Nomura J and Shooter EM: The
isolation of the mouse nerve growth factor protein in a high
molecular weight form. Biochemistry. 6:2202–2209. 1967. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Thoenen H and Barde YA: Physiology of
nerve growth factor. Physiol Rev. 60:1284–1335. 1980.PubMed/NCBI
|
|
66
|
Fahnestock M, Yu G, Michalski B, Mathew S,
Colquhoun A, Ross GM and Coughlin MD: The nerve growth factor
precursor proNGF exhibits neurotrophic activity but is less active
than mature nerve growth factor. J Neurochem. 89:581–592. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Seidel MF, Herguijuela M, Forkert R and
Otten U: Nerve growth factor in rheumatic diseases. Semin Arthritis
Rheum. 40:109–126. 2010. View Article : Google Scholar
|
|
68
|
Masoudi R, Ioannou MS, Coughlin MD,
Pagadala P, Neet KE, Clewes O, Allen SJ, Dawbarn D and Fahnestock
M: Biological activity of nerve growth factor precursor is
dependent upon relative levels of its receptors. J Biol Chem.
284:18424–18433. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Haase G, Pettmann B, Raoul C and Henderson
CE: Signaling by death receptors in the nervous system. Curr Opin
Neurobiol. 18:284–291. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Barker PA: High affinity not in the
vicinity? Neuron. 53:1–4. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Nakagawara A: Trk receptor tyrosine
kinases: A bridge between cancer and neural development. Cancer
Lett. 169:107–114. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Walsh EM, Kim R, Del Valle L, Weaver M,
Sheffield J, Lazarovici P and Marcinkiewicz C: Importance of
interaction between nerve growth factor and α9β1 integrin in glial
tumor angiogenesis. Neurooncol. 14:890–901. 2012.
|
|
73
|
Reis-Filho JS, Steele D, Di Palma S, Jones
RL, Savage K, James M, Milanezi F, Schmitt FC and Ashworth A:
Distribution and significance of nerve growth factor receptor
(NGFR/p75NTR) in normal, benign and malignant breast tissue. Mod
Pathol. 19:307–319. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Küchler J, Hartmann W, Waha A, Koch A,
Endl E, Wurst P, Kindler D, Mikeska T, Waha A, Goodyer CG, et al:
p75(NTR) induces apoptosis in medulloblastoma cells. Int J Cancer.
128:1804–1812. 2011. View Article : Google Scholar
|
|
75
|
Fiorentini C, Guerra N, Facchetti M,
Finardi A, Tiberio L, Schiaffonati L, Spano P and Missale C: Nerve
growth factor regulates dopamine D(2) receptor expression in
prolactinoma cell lines via p75(NGFR)-mediated activation of
nuclear factor-kappaB. Mol Endocrinol. 16:353–366. 2002.PubMed/NCBI
|
|
76
|
Descamps S, Toillon RA, Adriaenssens E,
Pawlowski V, Cool SM, Nurcombe V, Le Bourhis X, Boilly B, Peyrat JP
and Hondermarck H: Nerve growth factor stimulates proliferation and
survival of human breast cancer cells through two distinct
signaling pathways. J Biol Chem. 276:17864–17870. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Sortino MA, Condorelli F, Vancheri C,
Chiarenza A, Bernardini R, Consoli U and Canonico PL: Mitogenic
effect of nerve growth factor (NGF) in LNCaP prostate
adenocarcinoma cells: Role of the high- and low-affinity NGF
receptors. Mol Endocrinol. 14:124–136. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Hughes AL, Gollapudi L, Sladek TL and Neet
KE: Mediation of nerve growth factor-driven cell cycle arrest in
PC12 cells by p53. Simultaneous differentiation and proliferation
subsequent to p53 functional inactivation. J Biol Chem.
275:37829–37837. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Decker SJ: Nerve growth factor-induced
growth arrest and induction of p21Cip1/WAF1 in NIH-3T3 cells
expressing TrkA. J Biol Chem. 270:30841–30844. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Krygier S and Djakiew D: Neurotrophin
receptor p75(NTR) suppresses growth and nerve growth
factor-mediated metastasis of human prostate cancer cells. Int J
Cancer. 98:1–7. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Khwaja F and Djakiew D: Inhibition of
cell-cycle effectors of proliferation in bladder tumor epithelial
cells by the p75NTR tumor suppressor. Mol Carcinog. 36:153–160.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Weis C, Wiesenhofer B and Humpel C: Nerve
growth factor plays a divergent role in mediating growth of rat C6
glioma cells via binding to the p75 neurotrophin receptor. J
Neurooncol. 56:59–67. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Zilfou JT and Lowe SW: Tumor suppressive
functions of p53. Cold Spring Harb Perspect Biol. 1:a0018832009.
View Article : Google Scholar :
|
|
84
|
Rivlin N, Brosh R, Oren M and Rotter V:
Mutations in the p53 tumor suppressor gene: Important milestones at
the various Steps of tumorigenesis. Genes Cancer. 2:466–474. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Tanizaki Y, Jin L, Scheithauer BW, Kovacs
K, Roncaroli F and Lloyd RV: P53 gene mutations in pituitary
carcinomas. Endocr Pathol. 18:217–222. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Borrelli E, Sawchenko PE and Evans RM:
Pituitary hyperplasia induced by ectopic expression of nerve growth
factor. Proc Natl Acad Sci USA. 89:2764–2768. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Ferrara N and Davis-Smyth T: The biology
of vascular endothelial growth factor (Review). Endocr Rev.
18:4–25. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Fukui S, Nawashiro H, Otani N, Ooigawa H,
Yano A, Nomura N, Tokumaru AM, Miyazawa T, Ohnuki A, Tsuzuki N, et
al: Vascular endothelial growth factor expression in pituitary
adenomas. Acta Neurochir (Suppl). 86:519–521. 2003.
|
|
89
|
Niveiro M, Aranda FI, Peiró G, Alenda C
and Picó A: Immunohistochemical analysis of tumor angiogenic
factors in human pituitary adenomas. Hum Pathol. 36:1090–1095.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Pan LX, Chen ZP, Liu YS and Zhao JH:
Magnetic resonance imaging and biological markers in pituitary
adenomas with invasion of the cavernous sinus space. J Neurooncol.
74:71–76. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Arita K, Kurisu K, Tominaga A, Sugiyama K,
Eguchi K, Hama S, Yoshioka H, Yamasaki F and Kanou Y: Relationship
between intratumoral hemorrhage and overexpression of vascular
endothelial growth factor (VEGF) in pituitary adenoma. Hiroshima J
Med Sci. 53:23–27. 2004.PubMed/NCBI
|
|
92
|
Sondell M, Sundler F and Kanje M: Vascular
endothelial growth factor is a neurotrophic factor which stimulates
axonal outgrowth through the flk-1 receptor. Eur J Neurosci.
12:4243–4254. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Maeda K, Chung YS, Takatsuka S, Ogawa Y,
Sawada T, Yamashita Y, Onoda N, Kato Y, Nitta A and Arimoto Y:
Tumor angiogenesis as a predictor of recurrence in gastric
carcinoma. J Clin Oncol. 13:477–481. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Ochoa AL, Mitchner NA, Paynter CD, Morris
RE and Ben-Jonathan N: Vascular endothelial growth factor in the
rat pituitary: Differential distribution and regulation by
estrogen. J Endocrinol. 165:483–492. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Vidal S, Lloyd RV, Moya L, Scheithauer BW
and Kovacs K: Expression and distribution of vascular endothelial
growth factor receptor Flk-1 in the rat pituitary. J Histochem
Cytochem. 50:533–540. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Yamada S and Takada K: Angiogenesis in
pituitary adenomas. Microsc Res Tech. 60:236–243. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
McCabe CJ, Boelaert K, Tannahill LA,
Heaney AP, Stratford AL, Khaira JS, Hussain S, Sheppard MC,
Franklyn JA and Gittoes NJ: Vascular endothelial growth factor, its
receptor KDR/Flk-1, and pituitary tumor transforming gene in
pituitary tumors. J Clin Endocrinol Metab. 87:4238–4244. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Banerjee SK, Zoubine MN, Tran TM, Weston
AP and Campbell DR: Overexpression of vascular endothelial growth
factor164 and its co-receptor neuropilin-1 in estrogen-induced rat
pituitary tumors and GH3 rat pituitary tumor cells. Int J Oncol.
16:253–260. 2000.PubMed/NCBI
|
|
99
|
Kim K, Yoshida D and Teramoto A:
Expression of hypoxia-inducible factor 1alpha and vascular
endothelial growth factor in pituitary adenomas. Endocr Pathol.
16:115–121. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Onofri C, Carbia Nagashima A, Schaaf L,
Feirer M, Lohrer P, Stummer W, Berner S, Chervin A, Goldberg V,
Stalla GK, et al: Estradiol stimulates vascular endothelial growth
factor and interleukin-6 in human lactotroph and lactosomatotroph
pituitary adenomas. Exp Clin Endocrinol Diabetes. 112:18–23. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Viacava P, Gasperi M, Acerbi G, Manetti L,
Cecconi E, Bonadio AG, Naccarato AG, Acerbi F, Parenti G, Lupi I,
et al: Microvascular density and vascular endothelial growth factor
expression in normal pituitary tissue and pituitary adenomas. J
Endocrinol Invest. 26:23–28. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Cristina C, Perez-Millan MI, Luque G,
Dulce RA, Sevlever G, Berner SI and Becu-Villalobos D: VEGF and
CD31 association in pituitary adenomas. Endocr Pathol. 21:154–160.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Lohrer P, Gloddek J, Hopfner U, Losa M,
Uhl E, Pagotto U, Stalla GK and Renner U: Vascular endothelial
growth factor production and regulation in rodent and human
pituitary tumor cells in vitro. Neuroendocrinology. 74:95–105.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Korsisaari N, Ross J, Wu X, Kowanetz M,
Pal N, Hall L, Eastham-Anderson J, Forrest WF, Van Bruggen N, Peale
FV, et al: Blocking vascular endothelial growth factor-A inhibits
the growth of pituitary adenomas and lowers serum prolactin level
in a mouse model of multiple endocrine neoplasia type 1. Clin
Cancer Res. 14:249–258. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Fowkes RC and Vlotides G: Hypoxia-induced
VEGF production 'RSUMEs' in pituitary adenomas. Endocr Relat
Cancer. 19:C1–C5. 2012. View Article : Google Scholar
|
|
106
|
Zhai Y, Ni J, Jiang GW, Lu J, Xing L,
Lincoln C, Carter KC, Janat F, Kozak D, Xu S, et al: VEGI, a novel
cytokine of the tumor necrosis factor family, is an angiogenesis
inhibitor that suppresses the growth of colon carcinomas in vivo.
FASEB J. 13:181–189. 1999.PubMed/NCBI
|
|
107
|
Prehn JL, Thomas LS, Landers CJ, Yu QT,
Michelsen KS and Targan SR: The T cell costimulator TL1A is induced
by FcgammaR signaling in human monocytes and dendritic cells. J
Immunol. 178:4033–4038. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Migone TS, Zhang J, Luo X, Zhuang L, Chen
C, Hu B, Hong JS, Perry JW, Chen SF, Zhou JX, et al: TL1A is a
TNF-like ligand for DR3 and TR6/DcR3 and functions as a T cell
costimulator. Immunity. 16:479–492. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Bamias G, Martin C III, Marini M, Hoang S,
Mishina M, Ross WG, Sachedina MA, Friel CM, Mize J, Bickston SJ, et
al: Expression, localization, and functional activity of TL1A, a
novel Th1-polarizing cytokine in inflammatory bowel disease. J
Immunol. 171:4868–4874. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Liang PH, Tian F, Lu Y, Duan B, Stolz DB
and Li LY: Vascular endothelial growth inhibitor (VEGI; TNFSF15)
inhibits bone marrow-derived endothelial progenitor cell
incorporation into Lewis lung carcinoma tumors. Angiogenesis.
14:61–68. 2011. View Article : Google Scholar :
|
|
111
|
Zhang N, Sanders AJ, Ye L, Kynaston HG and
Jiang WG: Vascular endothelial growth inhibitor, expression in
human prostate cancer tissue and the impact on adhesion and
migration of prostate cancer cells in vitro. Int J Oncol.
35:1473–1480. 2009.PubMed/NCBI
|
|
112
|
Parr C, Gan CH, Watkins G and Jiang WG:
Reduced vascular endothelial growth inhibitor (VEGI) expression is
associated with poor prognosis in breast cancer patients.
Angiogenesis. 9:73–81. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Haridas V, Shrivastava A, Su J, Yu GL, Ni
J, Liu D, Chen SF, Ni Y, Ruben SM, Gentz R, et al: VEGI, a new
member of the TNF family activates nuclear factor-kappa B and c-Jun
N-terminal kinase and modulates cell growth. Oncogene.
18:6496–6504. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Lu Y, Gu X, Chen L, Yao Z, Song J, Niu X,
Xiang R, Cheng T, Qin Z, Deng W, et al: Interferon-γ produced by
tumor-infiltrating NK cells and CD4+ T cells
downregulates TNFSF15 expression in vascular endothelial cells.
Angiogenesis. 17:529–540. 2014. View Article : Google Scholar
|
|
115
|
Yu J, Tian S, Metheny-Barlow L, Chew LJ,
Hayes AJ, Pan H, Yu GL and Li LY: Modulation of endothelial cell
growth arrest and apoptosis by vascular endothelial growth
inhibitor. Circ Res. 89:1161–1167. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Kaptein A, Jansen M, Dilaver G, Kitson J,
Dash L, Wang E, Owen MJ, Bodmer JL, Tschopp J and Farrow SN:
Studies on the interaction between TWEAK and the death receptor
WSL-1/TRAMP (DR3). FEBS Lett. 485:135–141. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Locksley RM, Killeen N and Lenardo MJ: The
TNF and TNF receptor superfamilies: Integrating mammalian biology.
Cell. 104:487–501. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Gospodarowicz D, Jones KL and Sato G:
Purification of a growth factor for ovarian cells from bovine
pituitary glands. Proc Natl Acad Sci USA. 71:2295–2299. 1974.
View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Ezzat S, Zheng L and Asa SL: Pituitary
tumor-derived fibroblast growth factor receptor 4 isoform disrupts
neural cell-adhesion molecule/N-cadherin signaling to diminish cell
adhesiveness: A mechanism underlying pituitary neoplasia. Mol
Endocrinol. 18:2543–2552. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Gospodarowicz D, Ferrara N, Schweigerer L
and Neufeld G: Structural characterization and biological functions
of fibroblast growth factor. Endocr Rev. 8:95–114. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Li Y, Koga M, Kasayama S, Matsumoto K,
Arita N, Hayakawa T and Sato B: Identification and characterization
of high molecular weight forms of basic fibroblast growth factor in
human pituitary adenomas. J Clin Endocrinol Metab. 75:1436–1441.
1992.PubMed/NCBI
|
|
122
|
Zimering MB, Katsumata N, Sato Y, Brandi
ML, Aurbach GD, Marx SJ and Friesen HG: Increased basic fibroblast
growth factor in plasma from multiple endocrine neoplasia type 1:
Relation to pituitary tumor. J Clin Endocrinol Metab. 76:1182–1187.
1993.PubMed/NCBI
|
|
123
|
Ozkaya HM, Comunoglu N, Keskin FE, Oz B,
Haliloglu OA, Tanriover N, Gazioglu N and Kadioglu P: Locally
produced estrogen through aromatization might enhance tissue
expression of pituitary tumor transforming gene and fibroblast
growth factor 2 in growth hormone-secreting adenomas. Endocrine.
52:632–640. 2016. View Article : Google Scholar
|
|
124
|
Moscatelli D: High and low affinity
binding sites for basic fibroblast growth factor on cultured cells:
Absence of a role for low affinity binding in the stimulation of
plasminogen activator production by bovine capillary endothelial
cells. J Cell Physiol. 131:123–130. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Givol D and Yayon A: Complexity of FGF
receptors: Genetic basis for structural diversity and functional
specificity. FASEB J. 6:3362–3369. 1992.PubMed/NCBI
|
|
126
|
Qian ZR, Sano T, Asa SL, Yamada S,
Horiguchi H, Tashiro T, Li CC, Hirokawa M, Kovacs K and Ezzat S:
Cytoplasmic expression of fibroblast growth factor receptor-4 in
human pituitary adenomas: Relation to tumor type, size,
proliferation, and invasiveness. J Clin Endocrinol Metab.
89:1904–1911. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Jaakkola S, Salmikangas P, Nylund S,
Partanen J, Armstrong E, Pyrhönen S, Lehtovirta P and Nevanlinna H:
Amplification of fgfr4 gene in human breast and gynecological
cancers. Int J Cancer. 54:378–382. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Ohta T, Yamamoto M, Numata M, Iseki S,
Tsukioka Y, Miyashita T, Kayahara M, Nagakawa T, Miyazaki I,
Nishikawa K, et al: Expression of basic fibroblast growth factor
and its receptor in human pancreatic carcinomas. Br J Cancer.
72:824–831. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Ahmed NU, Ueda M, Ito A, Ohashi A,
Funasaka Y and Ichihashi M: Expression of fibroblast growth factor
receptors in naevus-cell naevus and malignant melanoma. Melanoma
Res. 7:299–305. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Giri D, Ropiquet F and Ittmann M:
Alterations in expression of basic fibroblast growth factor (FGF) 2
and its receptor FGFR-1 in human prostate cancer. Clin Cancer Res.
5:1063–1071. 1999.PubMed/NCBI
|
|
131
|
Henriksson ML, Edin S, Dahlin AM,
Oldenborg PA, Öberg Å, Van Guelpen B, Rutegård J, Stenling R and
Palmqvist R: Colorectal cancer cells activate adjacent fibroblasts
resulting in FGF1/FGFR3 signaling and increased invasion. Am J
Pathol. 178:1387–1394. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
McCabe CJ, Khaira JS, Boelaert K, Heaney
AP, Tannahill LA, Hussain S, Mitchell R, Olliff J, Sheppard MC,
Franklyn JA, et al: Expression of pituitary tumour transforming
gene (PTTG) and fibroblast growth factor-2 (FGF-2) in human
pituitary adenomas: Relationships to clinical tumour behaviour.
Clin Endocrinol (Oxf). 58:141–150. 2003. View Article : Google Scholar
|
|
133
|
Fukui S, Otani N, Nawashiro H, Yano A,
Nomura N, Miyazawa T, Ohnuki A, Tsuzuki N, Katoh H, Ishihara S, et
al: Subcellular localization of basic fibroblast growth factor and
fibroblast growth factor receptor 1 in pituitary adenomas. Brain
Tumor Pathol. 19:23–29. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Zhu X, Asa SL and Ezzat S: Fibroblast
growth factor 2 and estrogen control the balance of histone 3
modifications targeting MAGE-A3 in pituitary neoplasia. Clin Cancer
Res. 14:1984–1996. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Tateno T, Asa SL, Zheng L, Mayr T, Ullrich
A and Ezzat S: The FGFR4-G388R polymorphism promotes mitochondrial
STAT3 serine phosphorylation to facilitate pituitary growth hormone
cell tumorigenesis. PLoS Genet. 7:e10024002011. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
da Costa Andrade VC, Parise O Jr, Hors CP,
de Melo Martins PC, Silva AP and Garicochea B: The fibroblast
growth factor receptor 4 (FGFR4) Arg388 allele correlates with
survival in head and neck squamous cell carcinoma. Exp Mol Pathol.
82:53–57. 2007. View Article : Google Scholar
|
|
137
|
Frullanti E, Berking C, Harbeck N,
Jézéquel P, Haugen A, Mawrin C, Parise O Jr, Sasaki H, Tsuchiya N
and Dragani TA: Meta and pooled analyses of FGFR4 Gly388Arg
polymorphism as a cancer prognostic factor. Eur J Cancer Prev.
20:340–347. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Serra S, Zheng L, Hassan M, Phan AT,
Woodhouse LJ, Yao JC, Ezzat S and Asa SL: The FGFR4-G388R
single-nucleotide polymorphism alters pancreatic neuroendocrine
tumor progression and response to mTOR inhibition therapy. Cancer
Res. 72:5683–5691. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Marmé F, Werft W, Benner A, Burwinkel B,
Sinn P, Sohn C, Lichter P, Hahn M and Schneeweiss A: FGFR4 Arg388
genotype is associated with pathological complete response to
neoadjuvant chemotherapy for primary breast cancer. Ann Oncol.
21:1636–1642. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Abbass SA, Asa SL and Ezzat S: Altered
expression of fibroblast growth factor receptors in human pituitary
adenomas. J Clin Endocrinol Metab. 82:1160–1166. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Ezzat S, Zheng L, Zhu XF, Wu GE and Asa
SL: Targeted expression of a human pituitary tumor-derived isoform
of FGF receptor-4 recapitulates pituitary tumorigenesis. J Clin
Invest. 109:69–78. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Ezzat S, Yu S and Asa SL: Ikaros isoforms
in human pituitary tumors: Distinct localization, histone
acetylation, and activation of the 5′ fibroblast growth factor
receptor-4 promoter. Am J Pathol. 163:1177–1184. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Ezzat S, Zheng L, Winer D and Asa SL:
Targeting N-cadherin through fibroblast growth factor receptor-4:
Distinct pathogenetic and therapeutic implications. Mol Endocrinol.
20:2965–2975. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Fisher DA and Lakshmanan J: Metabolism and
effects of epidermal growth factor and related growth factors in
mammals (Review). Endocr Rev. 11:418–442. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Murdoch GH, Potter E, Nicolaisen AK, Evans
RM and Rosenfeld MG: Epidermal growth factor rapidly stimulates
prolactin gene transcription. Nature. 300:192–194. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Qian X, LeVea CM, Freeman JK, Dougall WC
and Greene MI: Heterodimerization of epidermal growth factor
receptor and wild-type or kinase-deficient Neu: A mechanism of
interreceptor kinase activation and transphosphorylation. Proc Natl
Acad Sci USA. 91:1500–1504. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Downward J, Yarden Y, Mayes E, Scrace G,
Totty N, Stockwell P, Ullrich A, Schlessinger J and Waterfield MD:
Close similarity of epidermal growth factor receptor and v-erb-B
oncogene protein sequences. Nature. 307:521–527. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Bethune G, Bethune D, Ridgway N and Xu Z:
Epidermal growth factor receptor (EGFR) in lung cancer: An overview
and update. J Thorac Dis. 2:48–51. 2010.PubMed/NCBI
|
|
149
|
Nicholson S, Richard J, Sainsbury C,
Halcrow P, Kelly P, Angus B, Wright C, Henry J, Farndon JR and
Harris AL: Epidermal growth factor receptor (EGFr); results of a 6
year follow-up study in operable breast cancer with emphasis on the
node negative subgroup. Br J Cancer. 63:146–150. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Hudson LG, Zeineldin R, Silberberg M and
Stack MS: Activated epidermal growth factor receptor in ovarian
cancer. Cancer Treat Res. 149:203–226. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Takehana T, Kunitomo K, Suzuki S, Kono K,
Fujii H, Matsumoto Y and Ooi A: Expression of epidermal growth
factor receptor in gastric carcinomas. Clin Gastroenterol Hepatol.
1:438–445. 2003. View Article : Google Scholar
|
|
152
|
LeRiche VK, Asa SL and Ezzat S: Epidermal
growth factor and its receptor (EGF-R) in human pituitary adenomas:
EGF-R correlates with tumor aggressiveness. J Clin Endocrinol
Metab. 81:656–662. 1996.PubMed/NCBI
|
|
153
|
Chaidarun SS, Eggo MC, Sheppard MC and
Stewart PM: Expression of epidermal growth factor (EGF), its
receptor, and related oncoprotein (erbB-2) in human pituitary
tumors and response to EGF in vitro. Endocrinology. 135:2012–2021.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Onguru O, Scheithauer BW, Kovacs K, Vidal
S, Jin L, Zhang S, Ruebel KH and Lloyd RV: Analysis of epidermal
growth factor receptor and activated epidermal growth factor
receptor expression in pituitary adenomas and carcinomas. Mod
Pathol. 17:772–780. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Fukuoka H, Cooper O, Ben-Shlomo A, Mamelak
A, Ren SG, Bruyette D and Melmed S: EGFR as a therapeutic target
for human, canine, and mouse ACTH-secreting pituitary adenomas. J
Clin Invest. 121:4712–4721. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Vallar L, Spada A and Giannattasio G:
Altered Gs and adenylate cyclase activity in human GH-secreting
pituitary adenomas. Nature. 330:566–568. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Theodoropoulou M, Arzberger T, Gruebler Y,
Jaffrain-Rea ML, Schlegel J, Schaaf L, Petrangeli E, Losa M, Stalla
GK and Pagotto U: Expression of epidermal growth factor receptor in
neoplastic pituitary cells: Evidence for a role in corticotropinoma
cells. J Endocrinol. 183:385–394. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Theodoropoulou M, Reincke M, Fassnacht M
and Komada M: Decoding the genetic basis of Cushing's disease: USP8
in the spotlight. Eur J Endocrinol. 173:M73–M83. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Yu R and Melmed S: Pathogenesis of
pituitary tumors. Prog Brain Res. 182:207–227. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Onofri C, Theodoropoulou M, Losa M, Uhl E,
Lange M, Arzt E, Stalla GK and Renner U: Localization of vascular
endothelial growth factor (VEGF) receptors in normal and
adenomatous pituitaries: Detection of a non-endothelial function of
VEGF in pituitary tumours. J Endocrinol. 191:249–261. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Turner HE, Nagy Z, Gatter KC, Esiri MM,
Harris AL and Wass JA: Angiogenesis in pituitary adenomas and the
normal pituitary gland. J Clin Endocrinol Metab. 85:1159–1162.
2000. View Article : Google Scholar : PubMed/NCBI
|