Introduction
Pancreatic adenocarcinoma is one of the most common
types of cancers (1). As the
symptoms of pancreatic adenocarcinoma are generally non-specific,
early diagnostic rates are extremely low; as such, pancreatic
adenocarcinoma is often detected at an advanced stage with
extensive metastasis, and has a poor prognosis (2,3). The
median survival time of pancreatic adenocarcinoma is 8–12 months
for patients with locally advanced disease, and 3–6 months for
patients with metastases (4).
Pancreatic ductal adenocarcinoma (PDAC) is the most common type of
pancreatic cancer and is the fourth leading cause of cancer-related
mortality, with a 5-year overall survival (OS) rate for patients
with metastatic PDAC at 8%, which is the lowest OS rate among all
types of cancer (5,6). Although new therapies have been
introduced, there has not been a notable improvement in OS rates
for patients with PDAC (7). Thus,
there is an urgent need to elucidate the underlying mechanisms of
pancreatic cancer metastasis.
Asparaginyl endopeptidase (AEP; also known as
legumain) is a member of the C13 family of cysteine proteases; it
specifically hydrolyzes carboxy-terminally to asparagine (8). AEP occurs in acidic endosomes and
lysosomes, and participates in intracellular protein degradation
under physiological conditions (9). AEP was reported to function in kidney
physiology (10), immunity
(11) and osteoclast formation
(12). High AEP expression levels
have been identified in certain solid tumors, including colorectal
cancer and breast cancer, and high AEP expression was previously
reported to correlate with a more metastatic phenotype, which was
partially due to the activation of cathepsin proteases and
pro-protein matrix metalloproteinase 2 (13–16).
A previous study reported that AEP exhibited a vesicular staining
pattern, and the expression of AEP was significantly related to
advanced tumor stage, high Gleason score, perineural invasion and
larger tumor size in patients with prostate cancer (17). However, whether AEP participates in
pancreatic cancer metastasis remains unknown.
Exosomes are nanosized membrane vesicles, with a
diameter between 30 and 100 nm, which are generated from endosomal
compartment invaginations (18–20).
As reported previously, tumor cell-derived exosomes serve important
roles in regulating certain functions, such as cell proliferation,
invasion and angiogenesis, by effectively delivering microRNAs,
mRNAs and proteins to other cells (21–23).
However, the functions and underlying mechanisms of exosomes
secreted by pancreatic cancer cells remains unknown.
Pancreatic cancer cell survival is often due to
survival-promoting signals, including increased expression of
apoptosis regulator BCL-2 and activation of phosphoinositide
3-kinase (PI3K)/RAC-α serine/threonine-protein kinase (AKT)
signaling (24–26), both of which have been associated
with pancreatic adenocarcinoma progression in human tissues and in
animal models. Activation of the PI3K/AKT pathway due to gene
amplification, activating mutations or loss of suppressors has been
reported in several types of human cancer, such as colorectal,
lung, cervical, gastric and pancreatic cancer (27–29).
Materials and methods
Patients and tissue samples
The present study was approved by the Ethics
Committee of Huzhou Central Hospital, Zhejiang University (Huzhou,
China). Written informed consent was obtained from patients, or
from the guardians on behalf of the minors, prior to enrollment in
the present study. Patient diagnoses were independently reviewed by
two pathologists and classified according to the WHO criteria. A
total of 63 patients (age range, 43–85 years) with histologically
confirmed PDAC that were treated at Huzhou Central Hospital of
Zhejiang University were recruited for this study between May 2009
and December 2014. Of the 63 patient samples collected, 6 were
paired fresh PDAC tissues and adjacent normal tissues. Follow-up
data were available for all 63 patients. Sera were also collected
from three patients that suffered pancreatitis and three patients
with PDAC.
Cell lines
The human PDAC cell lines PANC-1 (catalog no.
TCHu98), BxPC3 (catalog no. TCHu12) and ASPC-1 (catalog no. TCHu8)
were purchased from the Cell Bank of Type Culture Collection of the
Chinese Academy of Sciences (Shanghai, China). Capan-1 was
purchased from the American Type Culture Collection (catalog no.
HTB-79; Manassas, VA, USA). Cells were maintained in Dulbecco’s
modified Eagle’s medium supplemented with 10% heat-inactivated
fetal bovine serum (FBS; Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA), penicillin (100 U/ml) and streptomycin
(100 μg/ml) at 37°C in a humidified atmosphere of 5%
CO2. All cells were free of mycoplasma
contamination.
Plasmids and reagents
Lentiviral vectors for AEP knockdown (KD) or
overexpression (OE) were constructed by Shanghai Hanyin
Biotechnology Co., Ltd. (Shanghai, China). Empty vector was used as
negative control (NC) for AEP-KD and -OE experiments; AEP-targeted
KD sequences and AEP-OE sequences were used as previously described
(30). AEP-targeted KD sequences
were: KD1, 5′-GATGGTGTTCTACATTGAA-3′, and KD2, 5′-GGGGACTGGTACAGCG
TCA-3′. The lentiviral particles were packaged using psPAX2 and
pMD2G plasmids (Shanghai Hanyin Biotechnology Co., Ltd.). To obtain
stable cells with reduced or overexpressed AEP,
lentivirus-containing supernatants (Shanghai Hanyin Biotechnology
Co., Ltd.) were added to the PDAC cells, followed by selection with
1 μg/ml puromycin (Shanghai Hanyin Biotechnology Co., Ltd.)
for 2 weeks to select stably expressing AEP-KD1, AEP-KD2 or AEP-OE
cells (31).
Primary antibodies used in the present study
included: Goat anti-human AEP (catalog no. AF2199; R&D Systems;
Bio-Techne, Abingdon, UK), rabbit anti-human AKT (catalog no. 4685;
Cell Signaling Technology, Inc., Danvers, MA, USA), rabbit
anti-human phosphorylated (p)-AKT (catalog no. 4060; Cell Signaling
Technology, Inc.), rabbit anti-CD63 (catalog no. ab68418; Abcam,
Cambridge, UK), rabbit anti-human PI3K (catalog no. 3811; Cell
Signaling Technology, Inc.), and rabbit anti-β-actin (catalog no.
ab8227; Abcam); and the horseradish peroxidase (HRP)-conjugated
donkey anti-goat immunoglobulin G (IgG; catalog no. 705-036-147;
Jackson ImmunoResearch, Inc.) the horseradish peroxidase
(HRP)-conjugated goat anti-rabbit IgG (catalog no. 7074; Cell
Signaling Technology, Inc.) secondary antibody was also used in the
present study.
Immunohistochemical analysis
Tissues were fixed in 4% paraformaldehyde overnight
at 4°C, embedded in paraffin and sectioned (6 μm).
Immunohistochemical analyses were performed as previously described
(32). Goat anti-human AEP
antibody (catalog no. AF2199; R&D Systems; Bio-Techne,
Abingdon, UK; diluted 1:200 in blocking buffer) was used as primary
antibody. Biotin-conjugated donkey anti-goat IgG (catalog no.
705-066-147; Jackson ImmunoResearch, Inc.) was used as secondary
antibody. Normal goat IgG (catalog no. AB-108-C; R&D Systems;
Bio-Techne) was included as negative control. The proportion of
positive protein expressions were evaluated as follows: A score of
0 was indicated if 0% of the tumor cells showed positive staining;
1 if 0–10% of cells were stained; 2, 11–50% stained; 3, 51–75%
stained; and 4 if 75–100% stained. The intensity of staining was
rated on a scale of 0 to 3: 0, negative; 1, weak; 2, moderate; and
3, strong. The proportion and intensity scores were combined to
obtain a total score (range 0–6) and designated 0–3.5 as low
expression and 4–6 as high expression.
Western blot analysis
Total protein was extracted from cells, exosomes and
tissue samples using RIPA lysis and extraction buffer (Thermo
Fisher Scientific, Inc.). Protein concentration was determined
using the bicinchoninic acid protein assay method. Lysates (50
μg per lane) were separated by 8% SDS-PAGE and transferred
to nitrocellulose membranes. Membranes were blocked with 5% non-fat
milk for 30 min at 25°C, followed by overnight incubation with
primary antibodies (1:500) at 4°C. Subsequently, membranes were
incubated with HRP-conjugated secondary antibodies (1:3,000) for 60
min at 25°C. Immunoreactive proteins were visualized with the
Immobilon Western Chemiluminescent HRP Substrate (cat. no.
WBKLS0500; EMD Millipore, Billerica, MA, USA). Quantity One
analysis software version 4.6.9 (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) was used to quantify the relative band
intensities from western blotting images; actin or CD63 was used
for loading controls and for normalization. The assays were
conducted in triplicate.
Total RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
Total RNA was extracted from PDAC cells
(2×106) using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer’s instructions.
cDNA was reverse transcribed from 1 μg total RNA using the
Promega Reverse Transcription System (cat no. A3500; Promega
Corporation, Madison, WI, USA). qPCR was performed with the SYBR
Premix Ex Taq kit (Takara Biotechnology Co., Ltd., Dalian, China).
Primers were purchased from Sangon Biotech Co., Ltd. (Shanghai,
China), and the sequences are as follows: AEP, forward 5′-TCA
GGGTATGAAACGCAAAGC-3′, reverse 5′-GAGACGATCT TACGCACTGAC-3′; GAPDH,
forward 5′-CATGGCCTTCCGTGTTCCTA-3′, reverse
5′-GCGGCACGTCAGATCCA-3′; GAPDH was used as a loading control.
Thermocycling conditions comprised initial denaturation at 95°C (5
min), followed by 36 cycles of denaturation at 95°C (10 sec) and
annealing/elongation at 60°C (30 sec). Relative mRNA expression
levels were calculated using the 2−ΔΔCq method using the
housekeeping gene GAPDH for normalization (33). The assays were conducted in
triplicate.
Exosome isolation and culture method
To isolate exosomes, PDAC cells were cultured for 48
h at 37°C and the supernatants of these cells were collected and
centrifuged twice (1,000 × g for 10 min at 4°C, and 3,000 × g for
30 min at 4°C) to remove cells and fragments. Subsequently, the
exosome isolation reagent from the Total Exosome Isolation kit
(Invitrogen; Thermo Fisher Scientific, Inc.) was added to the cell
media sample and incubated overnight at 4°C. The precipitated
exosomes were recovered by centrifugation at 10,000 × g for 1 h at
4°C. For exosome isolation in sera, the ExoQuick Exosome
Precipitation Solution (catalog no. EXOQ5A-1; System Biosciences,
Palo Alto, CA, USA) was used to isolate exosomes from serum
samples, according to the manufacturer’s instructions. Exosomes
were re-suspended in PBS and stored at −80°C. The concentration of
exosomes was determined by BCA protein assay. Exosomes (50 ng/rl)
were added to 1×105 cells in culture medium for 24 h at
37°C, as previously described (34). The assays were conducted in
triplicate.
Transmission electron microscopy
The exosome suspension was added to an equal volume
of 4% paraformaldehyde at 4°C for 30 min and applied to a
Formvar/Carbon film-coated transmission electron microscope grid
(Alliance Biosystems, Inc., Osaka, Japan). Subsequently, the sample
was fixed by incubation with 1% glutaraldehyde for 5 min at 25°C,
washed with PBS and contrasted with 1% uranyl acetate for 5 min at
25°C. Samples were embedded in epoxy resin and polymerized at 35°C
for 12 h, 45°C for 12 h and 60°C for 24 h. Exosomes were
subsequently observed under a Hitachi H-7650 transmission electron
microscope (Hitachi, Ltd., Tokyo, Japan).
Cell invasion assay
Cells (1×105) were seeded into the upper
chambers of Matrigel-coated Transwell chambers (pore size, 8
μm) in serum-free DMEM. DMEM containing 10% FBS was added to
the lower chamber as a chemoattractant. Following incubation for 24
h at 37°C, the upper surfaces of the inserts were gently wiped with
a cotton swab and cells that had invaded the lower chambers were
fixed with 4% paraformaldehyde and stained with 0.1% crystal violet
at 37°C for 30 min. The number of invading cells was counted under
an Olympus CKX41 inverted microscope (Olympus Corporation, Tokyo,
Japan); five random microscopic fields were analyzed for each
insert. The assays were conducted in triplicate.
Statistical analysis
OS rates were calculated from the date of surgery to
the date of death or last follow-up; survival curves were plotted
using the Kaplan-Meier method and compared using the log-rank test.
Median survival times and hazard ratios (HRs) were shown with 95%
confidence intervals (CIs). Data are presented as the mean ±
standard deviation. To assess the differences between groups,
categorical variables were compared by means of χ2
analysis. Analysis of variance tests were followed by two-tailed
Dunn’s post-hoc analysis or Tukey’s multiple comparisons test to
identify statistically significant differences. Statistical
analyses were performed using SPSS software version 15.0 (Chicago,
IL, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
AEP expression in human PDAC tissues
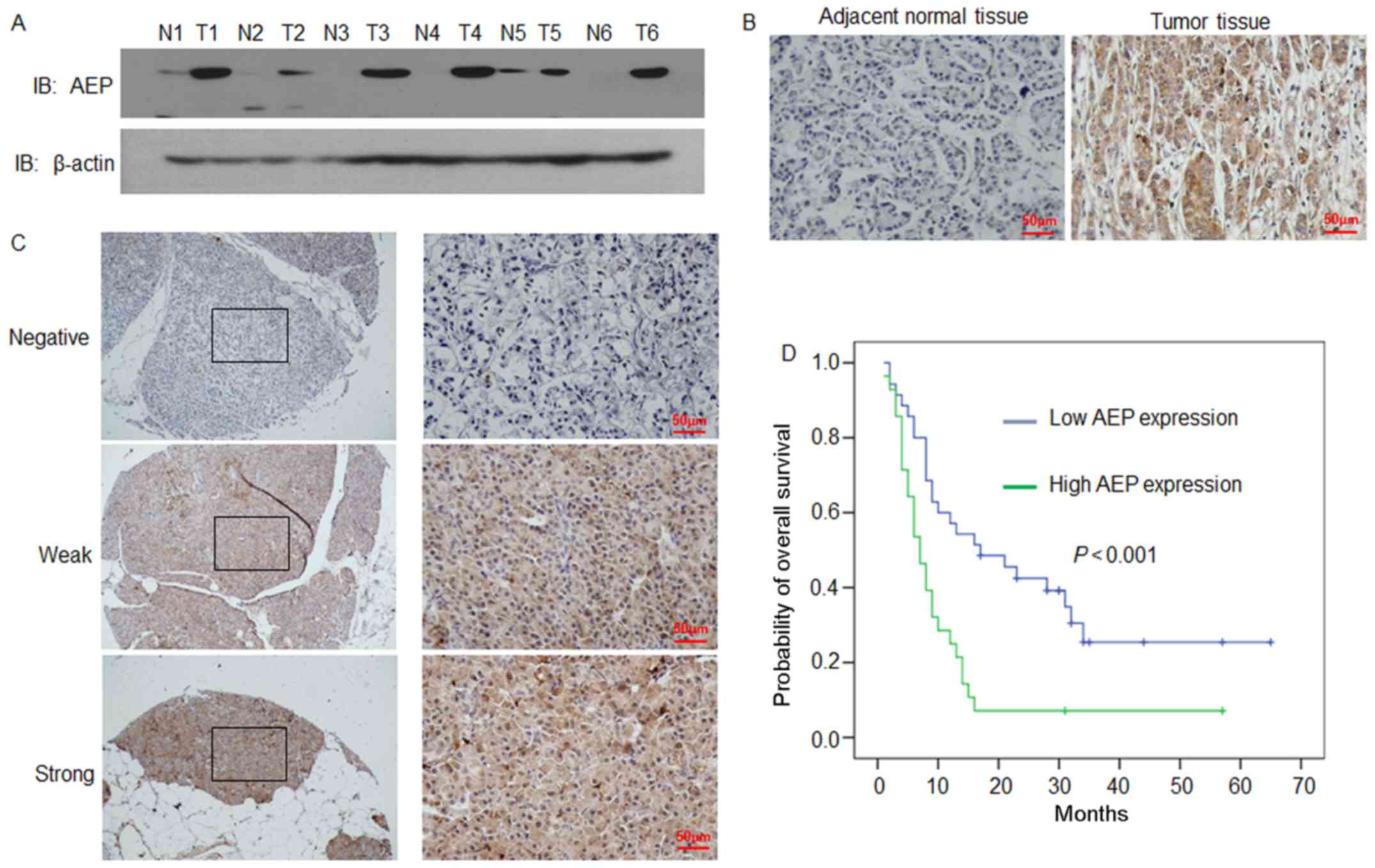
AEP protein expression levels were analyzed in
freshly collected human PDAC tissues (n=6) and adjacent normal
tissues (n=6) by western blotting (Fig. 1A). AEP expression levels were
notably higher in PDAC tissues compared with expression levels in
adjacent normal tissues (Fig. 1A).
AEP protein expression levels were also examined by
immunohistochemical analysis in the 6 matched tissues as well as
the remaining 57 tumoral tissues (Fig.
1B and C, respectively). Consistent with the western blotting
results, AEP staining was stronger in tumoral tissues compared with
expression levels in the adjacent normal tissues (Fig. 1B). The staining of AEP was revealed
to be mainly localized in the cytoplasm in the PDAC tissues
(Fig. 1B and C).
Relationship between AEP expression and
the clinicopathological features of patients with PDAC
According to the expression level score of AEP
protein in PDAC samples, all cases were distributed into two
groups: A low AEP expression group (n=35), and a high AEP
expression group (n=28; Fig. 1C;
Table I). Following evaluation of
the immunohistochemical staining results, AEP staining levels in
the American Joint Committee on Cancer (AJCC) stage II cases were
significantly higher compared with staining level in the AJCC stage
I cases (P=0.009; Table I). The
expression of AEP in PDAC tissues exhibited a strong association
with AJCC stage, although no associations were found between AEP
expression and other clinicopathological features (Table I).
 | Table IAssociation of AEP expression with
clinicopathological variables in pancreatic ductal
adenocarcinoma. |
Table I
Association of AEP expression with
clinicopathological variables in pancreatic ductal
adenocarcinoma.
| Clinicopathological
characteristic | n (%) | AEP staining (n; %)
| P-value |
|---|
| Low | High |
|---|
| Age (year) | | | | 0.599 |
| <60 | 22 (34.92) | 11 (50.00) | 11 (50.00) | |
| ≥60 | 41 (60.08) | 24 (58.54) | 17 (41.46) | |
| Sex | | | | 0.798 |
| Male | 27 (42.86) | 16 (59.26) | 11 (40.74) | |
| Female | 36 (57.14) | 19 (52.78) | 17 (47.22) | |
| AJCC stage | | | | 0.009 |
| I | 7 (11.11) | 6 (85.71) | 1 (14.29) | |
| II | 56 (88.89) | 29 (51.79) | 27 (48.21) | |
| Tumor location | | | | 0.209 |
| Head | 37 (58.73) | 18 (48.65) | 19 (51.35) | |
| Body/tail | 26 (41.27) | 17 (65.38) | 9 (34.62) | |
AEP expression and patient prognosis
To assess the relationship between the level of AEP
expression with patient prognosis the Kaplan-Meier and log-rank
tests were used to evaluate the effects of AEP expression on
patient OS. Patients with a high level of AEP expression in tumoral
tissues had significantly shorter OS times compared with patients
with low AEP expression (n=63; P=0.005; Fig. 1D and Table II). The mean OS time of patients
with low AEP expression was 20.29 months (n=35; 95% CI,
14.95–25.63; Table II), whereas
the OS time of patients with high AEP expression was 10.11 months
(n=28; 95% CI, 5.84–14.37). The log-rank test (univariate analysis)
revealed that the patients with low AEP expression had a longer OS
time (χ2 = 2.536; P=0.005; Table II). Furthermore, multivariate Cox
regression analysis was also performed, which indicated that AEP
expression was an independent prognostic factor (HR = 2.415; 95%
CI, 1.345–4.334; P=0.003; Table
III), whereas AJCC stage was not (HR = 1.475; 95% CI,
0.574–3.787; P=0.419).
 | Table IIMedian for survival time with 95% CI
and the log-rank test. |
Table II
Median for survival time with 95% CI
and the log-rank test.
| AEP expression | n | Mean (months) | 95% CI | χ2
(log-rank) | P-value |
|---|
| Low | 35 | 20.29 | 14.95-25.63 | | |
| High | 28 | 10.11 | 5.84-14.37 | 2.536 | 0.005 |
| Overall | 63 | 15.76 | 12.10-19.42 | | |
 | Table IIIUnivariate and multivariate analysis
of overall survival for patients with pancreatic ductal
adenocarcinoma. |
Table III
Univariate and multivariate analysis
of overall survival for patients with pancreatic ductal
adenocarcinoma.
| Clinicopathological
characteristic | Overall survival
|
|---|
Univariate analysis
| Multivariate
analysis
|
|---|
| Mean ± SEM | P-valuea | HR | P-valuea |
|---|
| AEP expression in
tumor tissues | | | | |
| Low | 20.29±2.63 | 0.005 | – | – |
| High | 10.11±2.08 | | 2.415 | 0.003 |
| Age (year) | | | | |
| <60 | 16.91±2.86 | 0.65 | – | – |
| ≥60 | 15.15±2.38 | | – | – |
| Sex | | | | |
| Male | 16.96±2.66 | 0.574 | – | – |
| Female | 14.86±2.53 | | – | – |
| AJCC stage | | | | |
| I | 29.86±8.45 | 0.006 | – | – |
| II | 15.76±1.83 | | 1.475 | 0.419 |
| Tumor location | | | | |
| Head | 12.81±1.65 | 0.054 | – | – |
| Body/tail | 19.76±1.83 | | – | – |
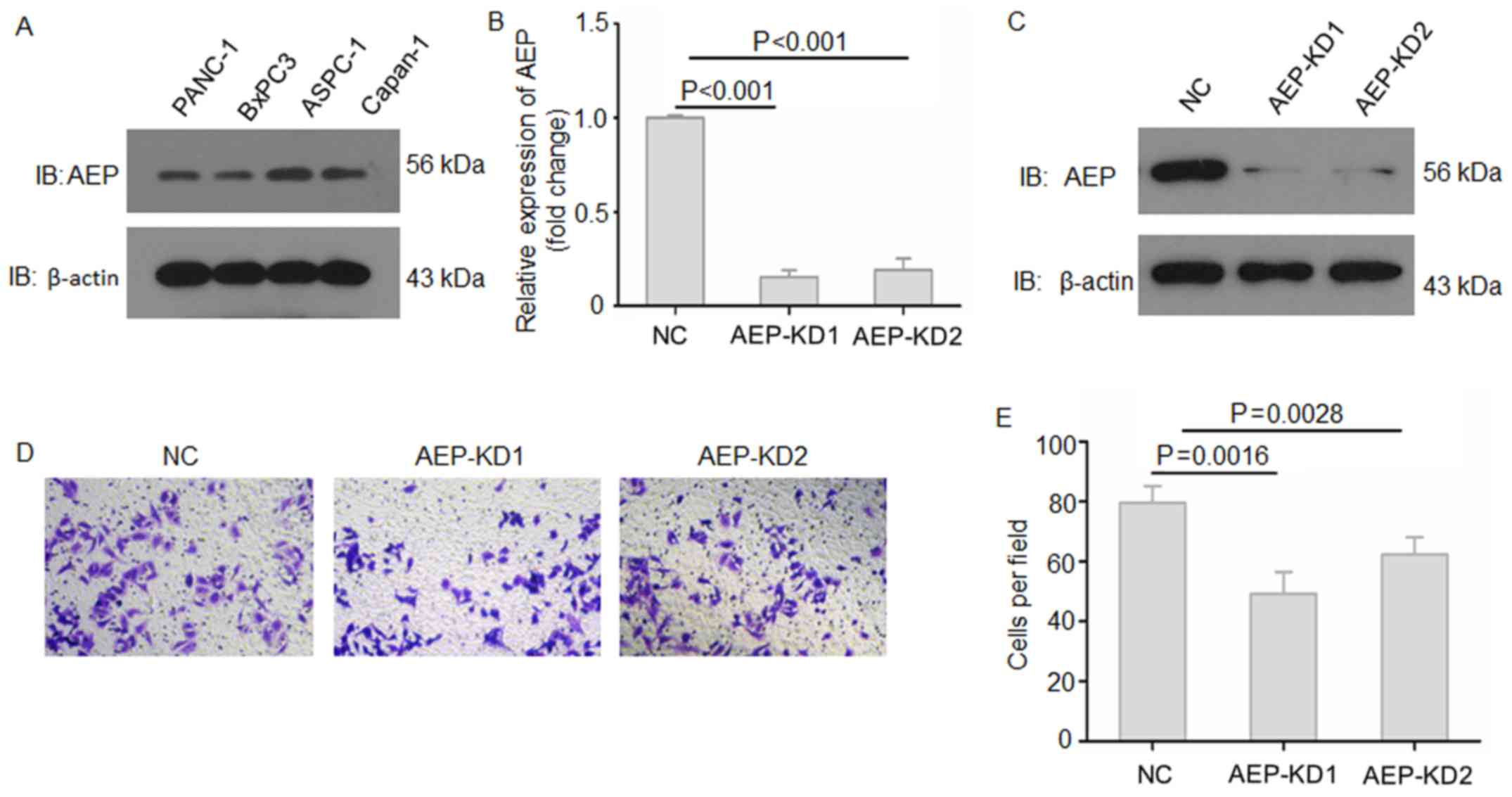
AEP enhances PDAC cell invasive
ability
To examine the functions of AEP in pancreatic
adenocarcinoma progression, the expression levels of AEP protein
were first examined in several PDAC cell lines by western blotting
(Fig. 2A). The results showed that
AEP was expressed in all PDAC cell lines. Subsequently, two AEP-KD
lentiviral vectors were constructed and used to knock down AEP
expression in ASPC-1 cells (Fig. 2B
and C). The RT-qPCR and western blotting results demonstrated
that AEP was effectively knocked down upon treatment with AEP-KD1
and -KD2 compared with NC-treated cells. The effects of AEP on the
invasive ability of PDAC cells were assessed by Matrigel-Transwell
invasion assay, which indicated that suppression of AEP expression
resulted in reduced invasive ability of ASPC-1 cells compared with
NC-treated cells (Fig. 2D and E).
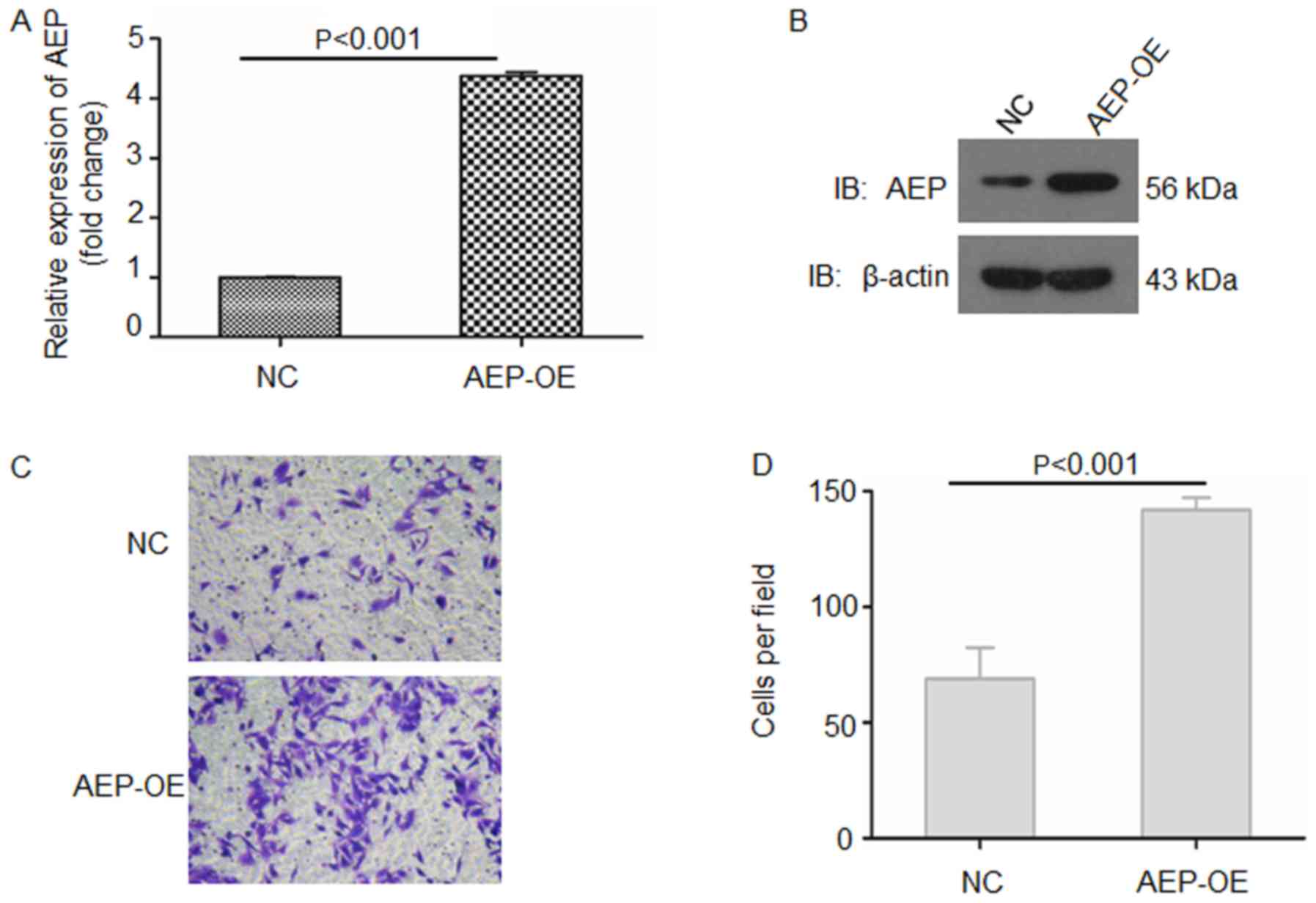
AEP-OE BxPC-3 cells were also constructed and verified by RT-qPCR
and western blotting (Fig. 3A and
B); overexpression of AEP in BxPC3 cells significantly
increased the invasive ability of these cells compared with
NC-treated cells (Fig. 3C and D).
These data suggested that AEP may be crucial for the invasive
phenotype of PDAC cells.
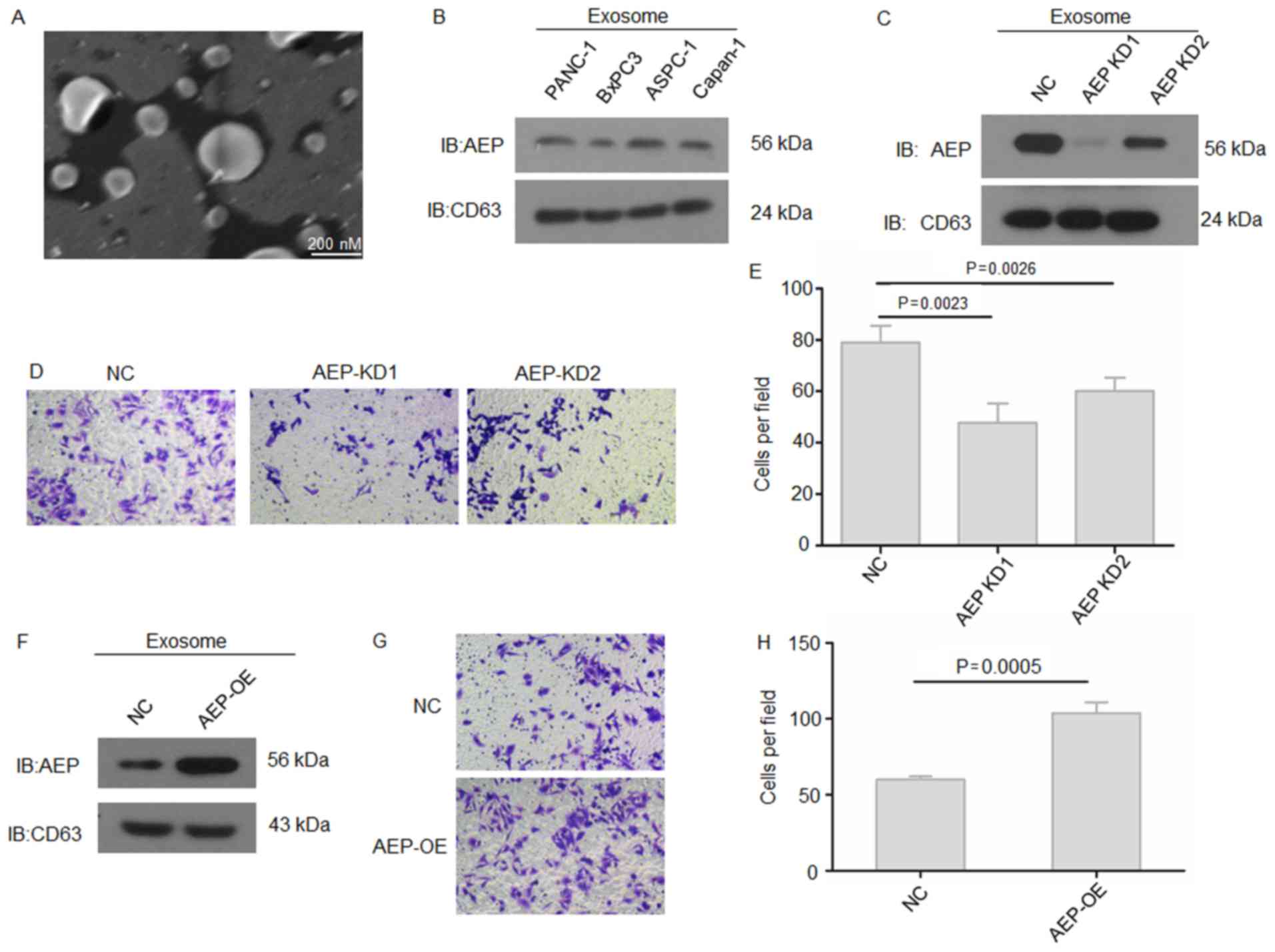
Secreted exosomal AEP regulates the
invasive ability of PDAC cells
AEP was previously reported to be a secreted protein
(27), and exosomes are key
mediators and modulators of cell-cell communications to promoter
tumor metastasis. Therefore, the exosomes secreted by PDAC cells
were collected and analyzed. The morphology of exosomes was
observed under transmission electron microscopy; exosomes are round
in appearance and ~100 nm in diameter (Fig. 4A). Western blotting results
demonstrated that the exosomes from each PDAC cell line expressed
AEP protein (Fig. 4B). When AEP
expression was knocked down in ASPC-1 cells, exosomal AEP protein
expression was notably reduced in AEP-KD1-treatd cells compared
with AEP-KD2- and NC-treated cells (Fig. 4C). To further determine the
putative functions of pancreatic cancer cell-derived exosomal AEP
on PDAC metastasis, ASPC-1 cells were cultured with the exosomes
isolated from either untreated cells or cells treated with AEP-KD1
or -KD2 and the invasive ability was examined. Results from the
Matrigel-Transwell invasion assay indicated that exosomes with a
low content of AEP (that is, exosomes isolated from cells treated
with either AEP-KD1 or -KD2) exhibited a significantly reduced
ability to promote the invasion of PDAC cells compared with cells
treated with NC-exosomes (Fig. 4D and
E). Conversely, exosomes derived from AEP-overexpressing BxPC3
cells significantly increased the invasive ability of treated PDAC
cells compared with cells treated with NC-exosomes (Fig. 4F–H). These results suggested that
exosomal AEP may be crucial for the invasive phenotype of PDAC
cells.
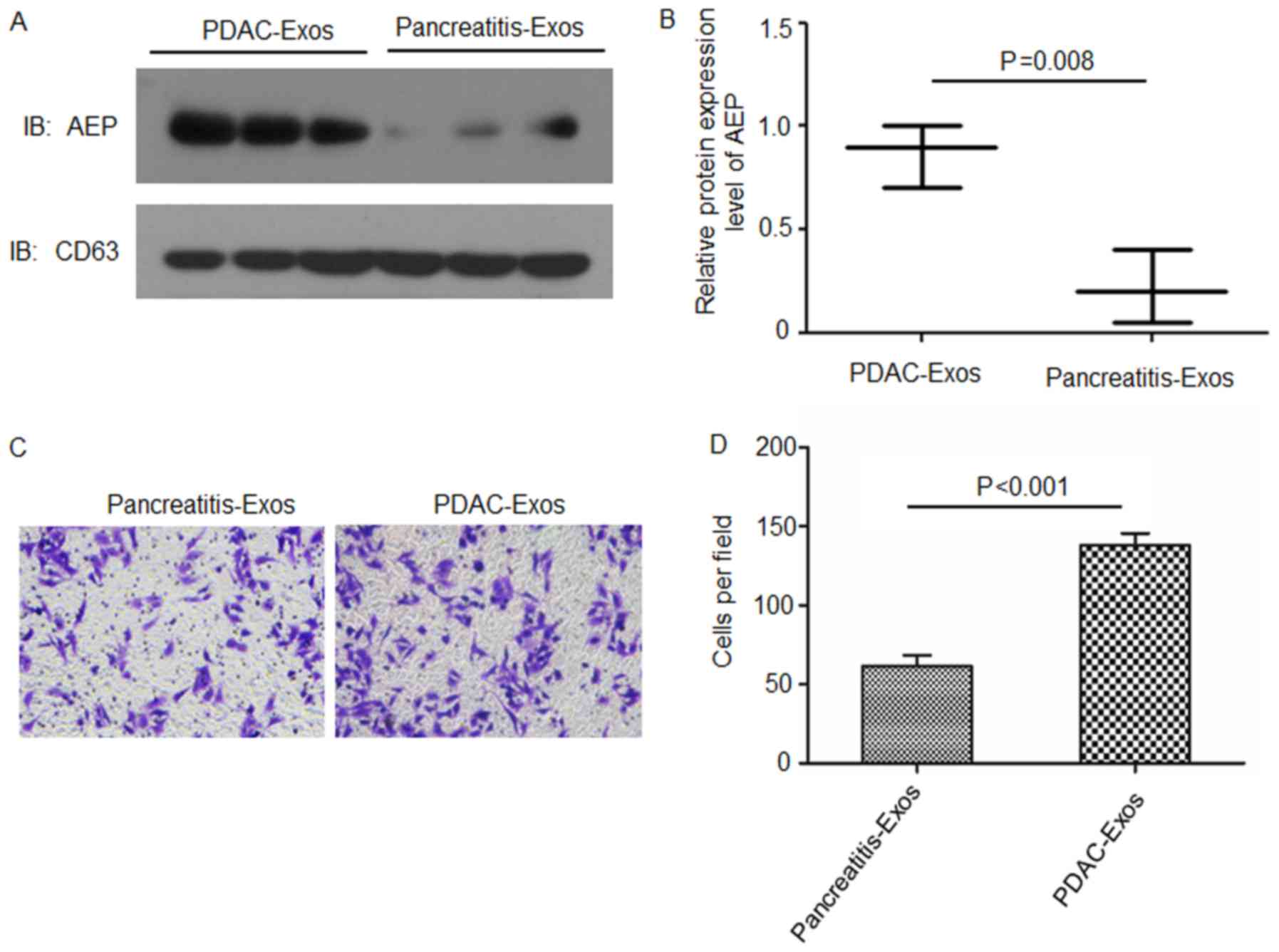
Exosomal AEP proteins are enriched in the
serum of patients with PDAC
Exosomes were isolated from the sera of patients
with either PDAC or pancreatitis. Western blotting results revealed
that AEP was enriched in the exosomes isolated from the sera of
patients with PDAC compared with expression levels in patients with
pancreatitis (Fig. 5A and B).
BxPC3 cells were co-cultured with these isolated exosomes and the
invasive ability was examined. Results from the Matrigel-Transwell
invasion assays indicated that BxPC3 cells treated with exosomes
collected from patients with PDAC (with a high content of AEP)
exhibited a significantly higher invasive ability compared with
cells treated with exosomes derived from patients with pancreatitis
(Fig. 5C and D).
AEP regulates the activation of PI3K/AKT
signaling in PDAC cells
PI3K/AKT signaling is an important survival pathway
that is involved in carcinogenesis and malignant cell progression
(22–24). Therefore, whether AEP was able to
regulate the PI3K/AKT pathway in PDAC cells was investigated.
Reduced AEP expression in BxPC3 cells led to decreased expression
levels of p-PI3K and p-AKT, but not total PI3K or AKT expression,
compared with the respective expression levels in NC-treated cells
(Fig. 6A). Furthermore, AEP
overexpression in BxPC3 cells resulted in significantly elevated
p-PI3K and p-AKT expression levels (Fig. 6B). Cells cultured with exosomes
expressing reduced levels of AEP also exhibited significantly
reduced p-PI3K and p-AKT expression compared with NC-treated cells
(Fig. 6C), whereas cells treated
with AEP-OE exosomes exhibited increased expression levels of
p-PI3K and p-AKT (Fig. 6D). These
results indicated that AEP may be an important element in
pancreatic cancer cell invasion and survival by regulating the
PI3K/AKT pathway.
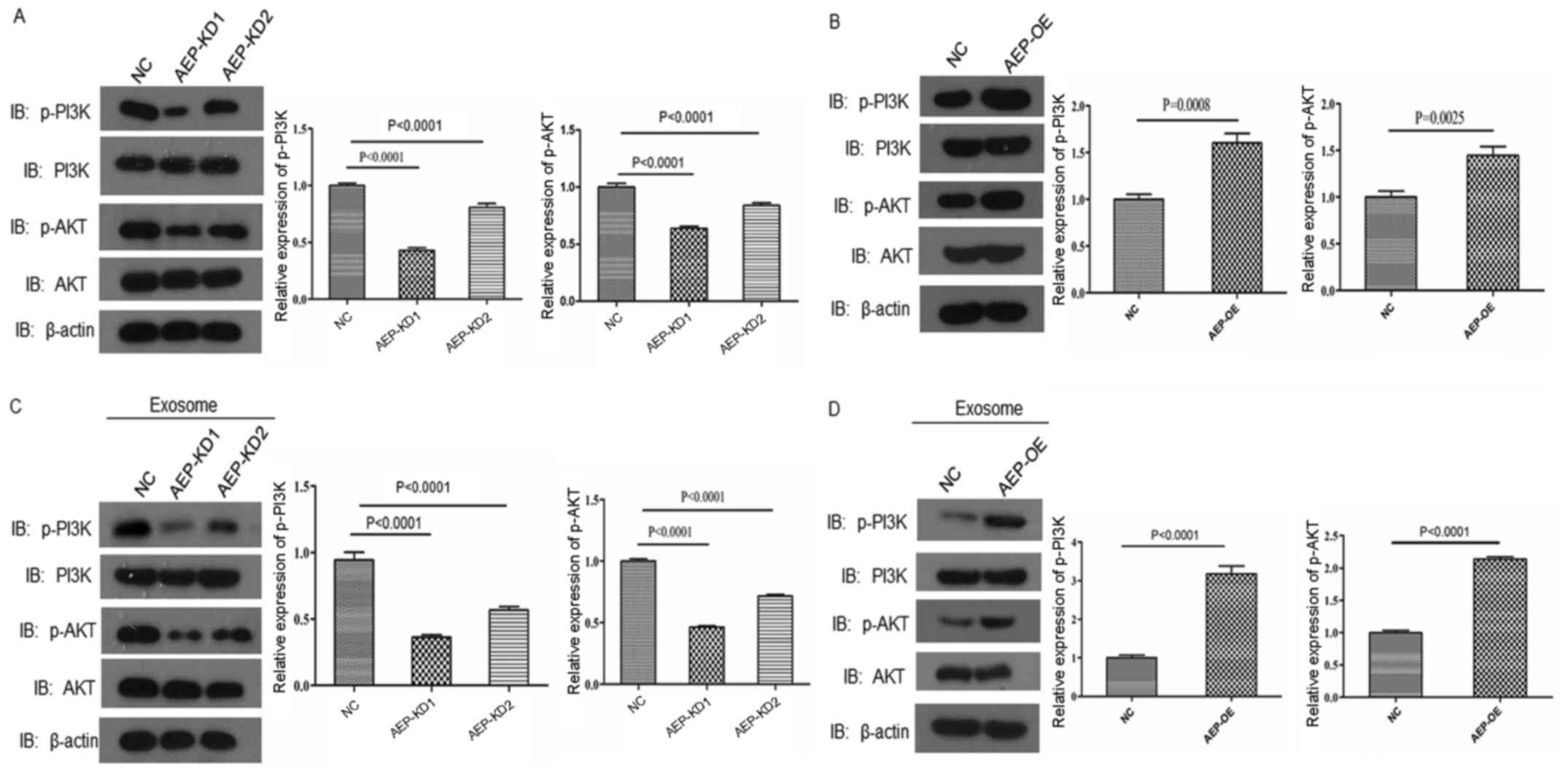 | Figure 6AEP regulates PI3K-AKT pathway
activation. Western blotting results for p-PI3K, PI3K, p-AKT and
AKT protein expression in (A) AEP-KD1 or -KD2-treated BxPC3 cells,
(B) AEP-OE-treated BxPC3 cells, (C) BxPC3 cells cultured with
exosomes derived from AEP-KD1 or -KD2-treated BxPC3 cells, or (D)
BxPC3 cells cultured with exosomes derived from AEP-OE-treated
BxPC3 cells. AEP, asparaginyl endopeptidase; AKT, RAC-α
serine/threonine-protein kinase; KD, knockdown; NC, negative
control; OE, overexpression; p, phosphorylated; PI3K,
phosphoinositide 3-kinase. |
Discussion
Pancreatic cancer is a common malignancy worldwide
and has a high rate of mortality (35). Therefore, the discovery of
potential biomarkers and therapeutic targets is important for the
improvement of clinical strategies for pancreatic adenocarcinoma.
AEP is highly specific to an asparagine residue at the P1 site of
its substrates (36). A study by
Liu et al reported that numerous solid tumors expressed AEP,
including breast cancer, colon cancer and central nervous system
neoplasms (32). In addition, AEP
expression was positively associated with certain
clinicopathological features in patients with ovarian cancer
(37) and breast cancer (14) such as stage and ascetic cytology.
Although the possible involvement of AEP in several solid tumors
has been reported, the present study is the first to examine the
expression and function of AEP in PDAC. In the present study, AEP
was demonstrated to be expressed in PDAC cell lines, and AEP was
highly expressed in pancreatic cancer tissues compared with
adjacent normal tissues. In addition, high AEP expression was
determined to be associated with poor prognosis. Taken together,
the present study results indicated that high expression of AEP was
associated with pancreatic carcinoma progression and that AEP
expression may independently indicate poor prognosis in patients;
therefore, AEP may be a novel prognostic biomarker or therapeutic
target in pancreatic carcinoma.
To date, little is known about the biological
processes in which AEP may be involved in cancer progression. In
the present study, gain- and loss-of-function experiments revealed
that knockdown of AEP expression levels significantly reduced the
invasive ability of PDAC cells, whereas overexpression of AEP
increased the invasive ability. Furthermore, AEP was detected in
exosomes derived from PDAC cells as well as in serum from patients
with PDAC. The Matrigel-Transwell invasion assay revealed that
exosomes enriched with AEP enhanced the invasive ability of PDAC
cells, whereas exosomes lacking AEP decreased the invasive ability.
Thus, AEP may be important for pancreatic carcinoma progression in
an exosome-dependent manner. A previous study reported that
AEP-containing vesicles may be found at the invasive front of a
tumor, and AEP overexpression can increase cell migration and
invasion (32). The present study
demonstrated that the extracellular AEP-containing exosomes
promoted pancreatic carcinoma cell invasive ability.
Although AEP has been reported to be an important
regulator of cancer invasion and metastasis (14), the biological functions of AEP in
cancer progression have not been fully investigated. Biochemical
analyses revealed that AEP may be involved in pancreatic
adenocarcinoma cell invasion and survival through the regulation of
the PI3K-AKT pathway. A previous study reported that AEP forms a
complex with integrin αvβ3, an upstream regulator of AKT signaling,
which indicated that AEP may regulate AKT signaling through
integrins (32). The PI3K/AKT
pathway is frequently activated during tumor progression and may be
involved in inducing EMT and subsequent tumor metastasis (38,39).
Consistent with these reports, the present study demonstrated that
the suppression of AEP expression significantly reduced AKT and
PI3K phosphorylation. Further investigations are required to
determine whether AEP may be a potential target for pancreatic
adenocarcinoma treatment.
In conclusion, the present study identified the
tumor-promoting functions of AEP in pancreatic adenocarcinoma and
suggested that AEP may be a new target for the treatment of
pancreatic adenocarcinoma; the discovery of novel therapeutic
targets is important to improve the efficacy of pancreatic
adenocarcinoma treatment.
Acknowledgments
Not applicable.
Notes
[1]
Funding
The present study was supported by The Zhejiang
Science and Technology Plan Project (grant no. 2017C33189).
[2] Availability
of data and materials
All data generated or analyzed during this study are
included in this published article.
[3] Authors’
contributions
QY designed the study, acquired, analyzed and
interpreted the data, and wrote the manuscript. WY, XS, MZ, FC, SZ,
WW and YX collected and analyzed the data. YX, LT and ZM revised
the manuscript.
[4] Ethics
approval and consent to participate
The present study was approved by the Ethics
Committee of Huzhou Central Hospital, Zhejiang University (Huzhou,
China). Written informed consent was obtained from patients, or
from the guardians on behalf of the minors, prior to enrollment in
the present study.
[5] Consent for
publication
Not applicable.
[6] Conflicts of
interest
The authors declare that they have no competing
interests.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wood NJ: Pancreatic cancer: Pancreatic
tumour formation and recurrence after radiotherapy are blocked by
targeting CD44. Nat Rev Gastroenterol Hepatol. 11:732014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu P, Zhu Y and Liu L: Elevated
pretreatment plasma D-dimer levels and platelet counts predict poor
prognosis in pancreatic adenocarcinoma. Onco Targets Ther.
8:1335–1340. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Colbert LE, Hall WA, Nickleach D,
Switchenko J, Kooby DA, Liu Y, Gillespie T, Lipscomb J, Kauh J and
Landry JC: Chemoradiation therapy sequencing for resected
pancreatic adenocarcinoma in the National Cancer Data Base. Cancer.
120:499–506. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jagadeeshan S, Krishnamoorthy YR, Singhal
M, Subramanian A, Mavuluri J, Lakshmi A, Roshini A, Baskar G, Ravi
M, Joseph LD, et al: Transcriptional regulation of fibronectin by
p21-activated kinase-1 modulates pancreatic tumorigenesis.
Oncogene. 34:455–464. 2015. View Article : Google Scholar
|
|
6
|
Ibrahim AM and Wang YH: Viro-immune
therapy: A new strategy for treatment of pancreatic cancer. World J
Gastroenterol. 22:748–763. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Al Haddad AH and Adrian TE: Challenges and
future directions in therapeutics for pancreatic ductal
adenocarcinoma. Expert Opin Investig Drugs. 23:1499–1515. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Haugen MH, Johansen HT, Pettersen SJ,
Solberg R, Brix K, Flatmark K and Maelandsmo GM: Nuclear legumain
activity in colorectal cancer. PLoS One. 8:e529802013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Herskowitz JH, Gozal YM, Duong DM, Dammer
EB, Gearing M, Ye K, Lah JJ, Peng J, Levey AI and Seyfried NT:
Asparaginyl endopeptidase cleaves TDP-43 in brain. Proteomics.
12:2455–2463. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Miller G, Matthews SP, Reinheckel T,
Fleming S and Watts C: Asparagine endopeptidase is required for
normal kidney physiology and homeostasis. FASEB J. 25:1606–1617.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
van Endert P: Toll-like receptor 9: AEP
takes control. Immunity. 31:696–698. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Choi SJ, Reddy SV, Devlin RD, Menaa C,
Chung H, Boyce BF and Roodman GD: Identification of human
asparaginyl endopeptidase (legumain) as an inhibitor of osteoclast
formation and bone resorption. J Biol Chem. 274:27747–27753. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhen Y, Chunlei G, Wenzhi S, Shuangtao Z,
Na L, Rongrong W, Xiaohe L, Haiying N, Dehong L, Shan J, et al:
Clinicopathologic significance of legumain overexpression in
cancer: A systematic review and meta-analysis. Sci Rep.
5:165992015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
D’Costa ZC, Higgins C, Ong CW, Irwin GW,
Boyle D, McArt DG, McCloskey K, Buckley NE, Crawford NT,
Thiagarajan L, et al: TBX2 represses CST6 resulting in uncontrolled
legumain activity to sustain breast cancer proliferation: A novel
cancer-selective target pathway with therapeutic opportunities.
Oncotarget. 5:1609–1620. 2014.
|
|
15
|
Sevenich L and Joyce JA: Pericellular
proteolysis in cancer. Genes Dev. 28:2331–2347. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Edgington-Mitchell LE, Rautela J,
Duivenvoorden HM, Jayatilleke KM, van der Linden WA, Verdoes M,
Bogyo M and Parker BS: Cysteine cathepsin activity suppresses
osteoclastogenesis of myeloid-derived suppressor cells in breast
cancer. Oncotarget. 6:27008–27022. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhu W, Shao Y, Yang M, Jia M and Peng Y:
Asparaginyl endopeptidase promotes proliferation and invasiveness
of prostate cancer cells via PI3K/AKT signaling pathway. Gene.
594:176–182. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hingorani SR: Intercepting Cancer
Communiques: Exosomes as heralds of malignancy. Cancer Cell.
28:151–153. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jørgensen M, Bæk R, Pedersen S,
Søndergaard EK, Kristensen SR and Varming K: Extracellular Vesicle
(EV) Array: Microarray capturing of exosomes and other
extracellular vesicles for multiplexed phenotyping. J Extracell
Vesicles. 2:22013. View Article : Google Scholar
|
|
20
|
Hoshino A, Costa-Silva B, Shen TL,
Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di
Giannatale A, Ceder S, et al: Tumour exosome integrins determine
organotropic metastasis. Nature. 527:329–335. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jeong D, Jo W, Yoon J, Kim J, Gianchandani
S, Gho YS and Park J: Nanovesicles engineered from ES cells for
enhanced cell proliferation. Biomaterials. 35:9302–9310. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gao Q, Zhao YJ, Wang XY, Guo WJ, Gao S,
Wei L, Shi JY, Shi GM, Wang ZC, Zhang YN, et al: Activating
mutations in PTPN3 promote cholangiocarcinoma cell proliferation
and migration and are associated with tumor recurrence in patients.
Gastroenterology. 146:1397–1407. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kalluri R: The biology and function of
exosomes in cancer. J Clin Invest. 126:1208–1215. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vitale G, Zappavigna S, Marra M, Dicitore
A, Meschini S, Condello M, Arancia G, Castiglioni S, Maroni P,
Bendinelli P, et al: The PPAR-γ agonist troglitazone antagonizes
survival pathways induced by STAT-3 in recombinant interferon-β
treated pancreatic cancer cells. Biotechnol Adv. 30:169–184. 2012.
View Article : Google Scholar
|
|
25
|
Missiaglia E, Dalai I, Barbi S, Beghelli
S, Falconi M, della Peruta M, Piemonti L, Capurso G, Di Florio A,
delle Fave G, et al: Pancreatic endocrine tumors: Expression
profiling evidences a role for AKT-mTOR pathway. J Clin Oncol.
28:245–255. 2010. View Article : Google Scholar
|
|
26
|
Stoll V, Calleja V, Vassaux G, Downward J
and Lemoine NR: Dominant negative inhibitors of signalling through
the phosphoinositol 3-kinase pathway for gene therapy of pancreatic
cancer. Gut. 54:109–116. 2005. View Article : Google Scholar
|
|
27
|
Martini M, De Santis MC, Braccini L,
Gulluni F and Hirsch E: PI3K/AKT signaling pathway and cancer: An
updated review. Ann Med. 46:372–383. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Polivka J Jr and Janku F: Molecular
targets for cancer therapy in the PI3K/AKT/mTOR pathway. Pharmacol
Ther. 142:164–175. 2014. View Article : Google Scholar
|
|
29
|
Toren P and Zoubeidi A: Targeting the
PI3K/Akt pathway in prostate cancer: Challenges and opportunities
(review). Int J Oncol. 45:1793–1801. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lin Y, Qiu Y, Xu C, Liu Q, Peng B,
Kaufmann GF, Chen X, Lan B, Wei C, Lu D, et al: Functional role of
asparaginyl endopeptidase ubiquitination by TRAF6 in tumor invasion
and metastasis. J Natl Cancer Inst. 106:dju0122014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Qiu JJ, Lin YY, Ye LC, Ding JX, Feng WW,
Jin HY, Zhang Y, Li Q and Hua KQ: Overexpression of long non-coding
RNA HOTAIR predicts poor patient prognosis and promotes tumor
metastasis in epithelial ovarian cancer. Gynecol Oncol.
134:121–128. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu C, Sun C, Huang H, Janda K and
Edgington T: Overexpression of legumain in tumors is significant
for invasion/metastasis and a candidate enzymatic target for
prodrug therapy. Cancer Res. 63:2957–2964. 2003.PubMed/NCBI
|
|
33
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Δ Δ C(T)) Method. Methods. 25:402–408. 2001. View Article : Google Scholar
|
|
34
|
Kogure T, Lin WL, Yan IK, Braconi C and
Patel T: Intercellular nanovesicle-mediated microRNA transfer: A
mechanism of environmental modulation of hepatocellular cancer cell
growth. Hepatology. 54:1237–1248. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kamisawa T, Wood LD, Itoi T and Takaori K:
Pancreatic cancer. Lancet. 388:73–85. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen JM, Dando PM, Rawlings ND, Brown MA,
Young NE, Stevens RA, Hewitt E, Watts C and Barrett AJ: Cloning,
isolation, and characterization of mammalian legumain, an
asparaginyl endopeptidase. J Biol Chem. 272:8090–8098. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang L, Chen S, Zhang M, Li N, Chen Y, Su
W, Liu Y, Lu D, Li S, Yang Y, et al: Legumain: A biomarker for
diagnosis and prognosis of human ovarian cancer. J Cell Biochem.
113:2679–2686. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mulholland DJ, Kobayashi N, Ruscetti M,
Zhi A, Tran LM, Huang J, Gleave M and Wu H: Pten loss and RAS/MAPK
activation cooperate to promote EMT and metastasis initiated from
prostate cancer stem/progenitor cells. Cancer Res. 72:1878–1889.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bartolomé RA, García-Palmero I, Torres S,
Lopez-Lucendo M, Balyasnikova IV and Casal JI: IL13 Receptor α2
signaling requires a scaffold protein, FAM120A, to activate the FAK
and PI3K pathways in colon cancer metastasis. Cancer Res.
75:2434–2444. 2015. View Article : Google Scholar
|