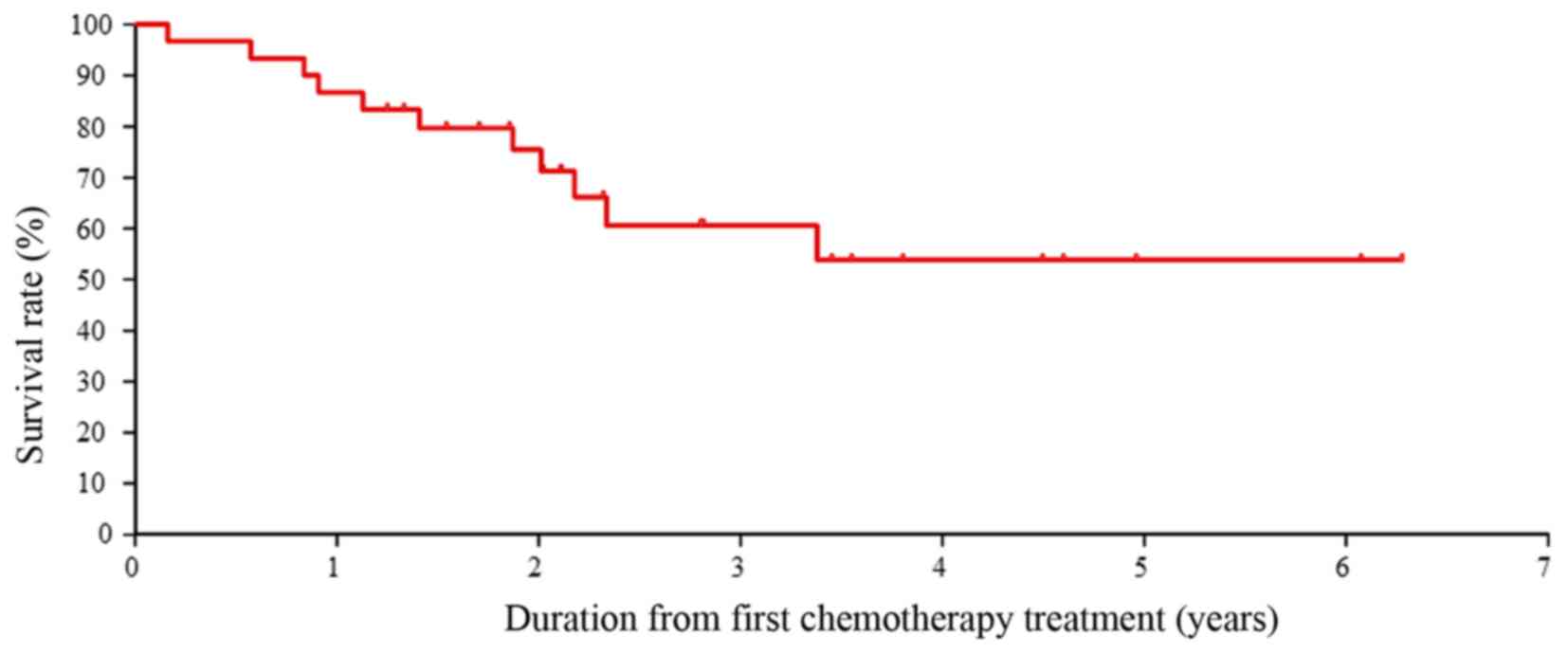
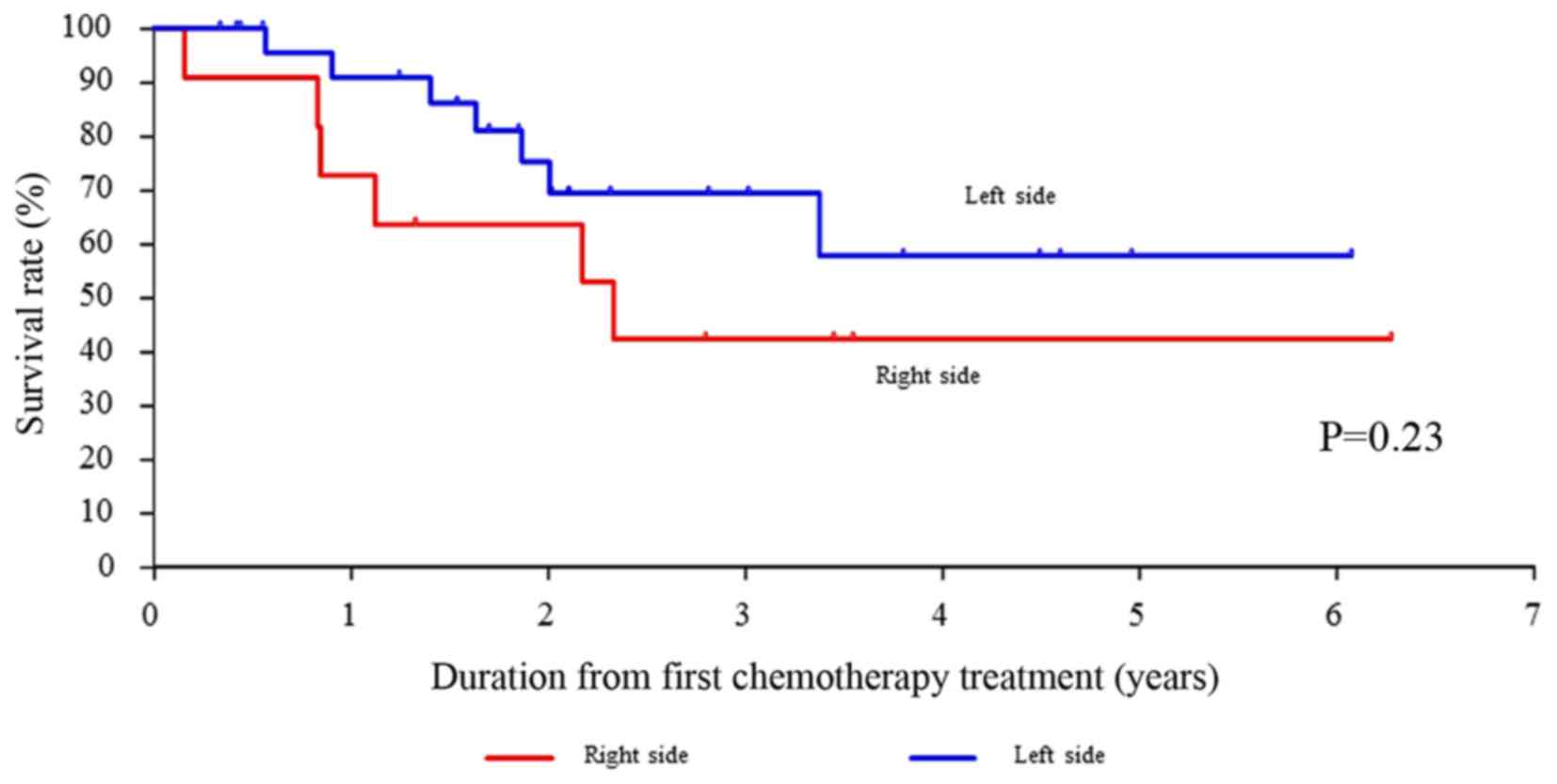
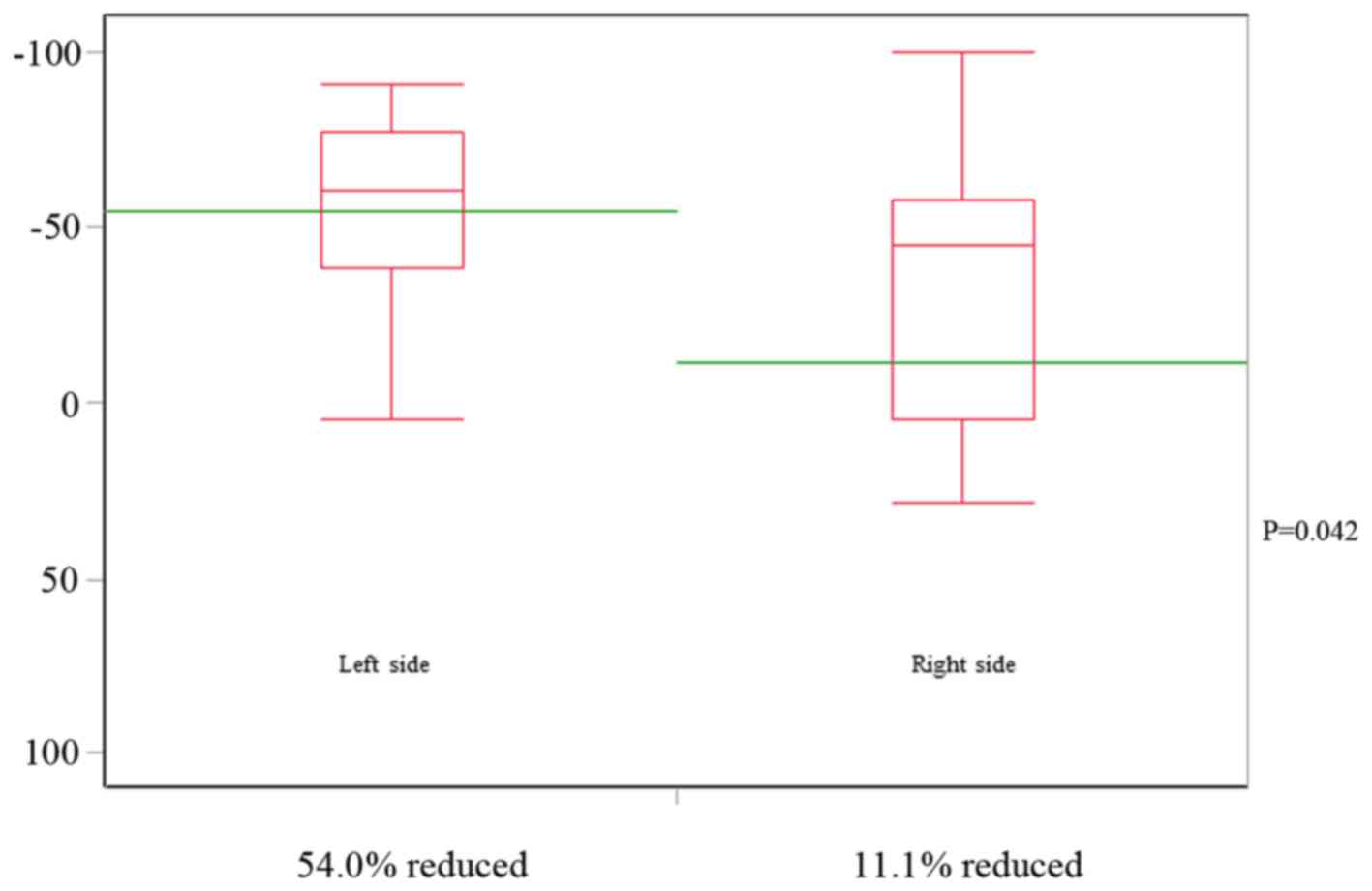
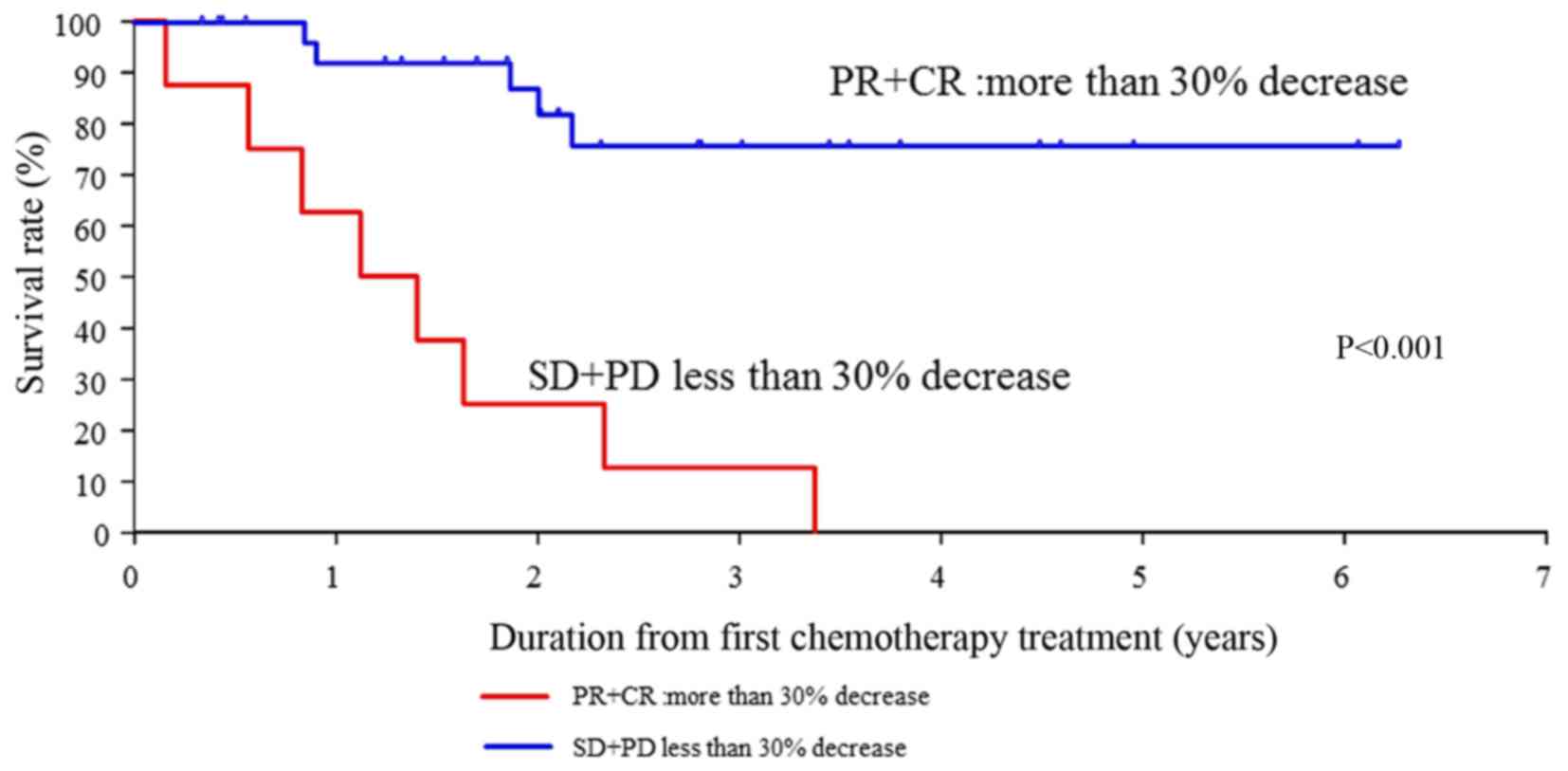
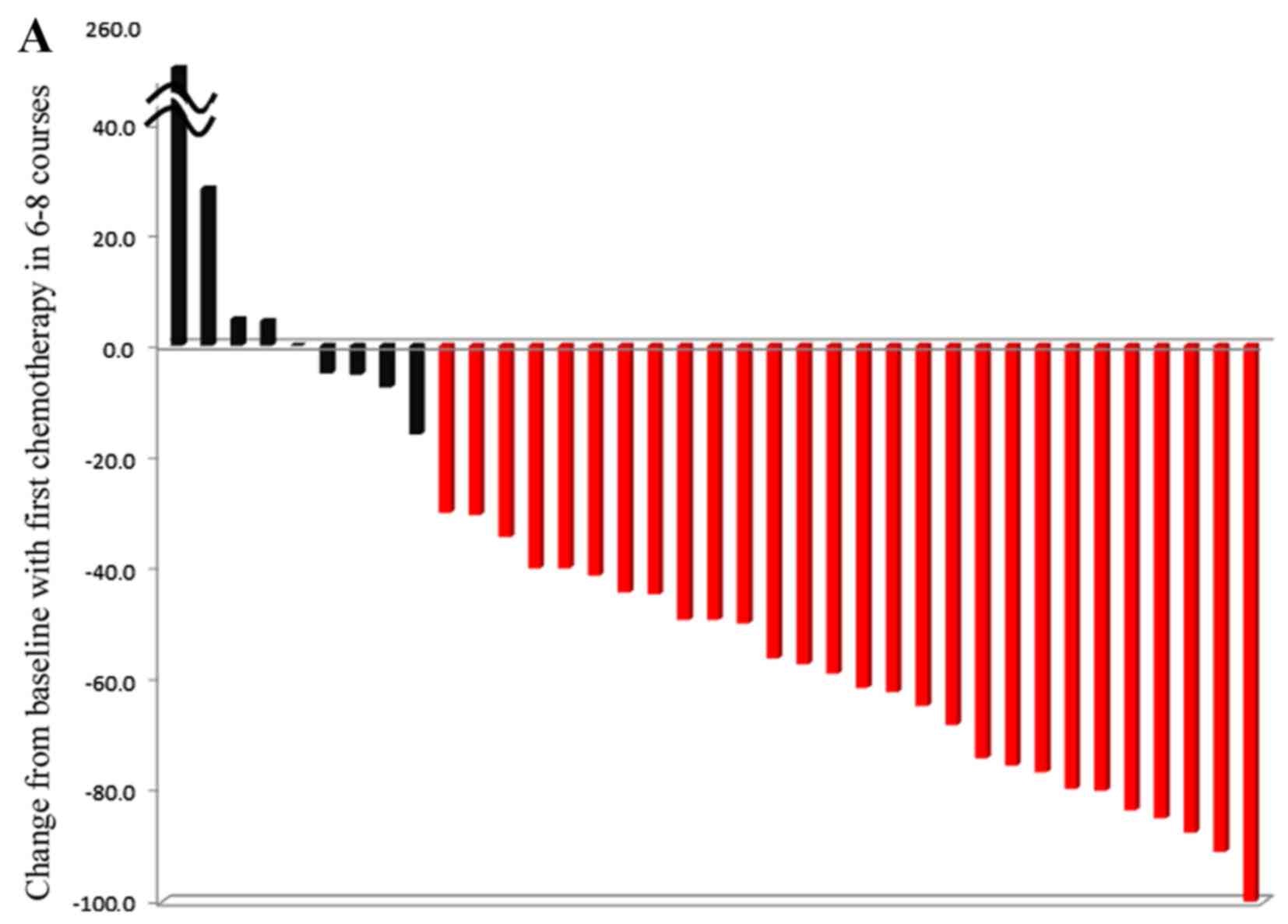
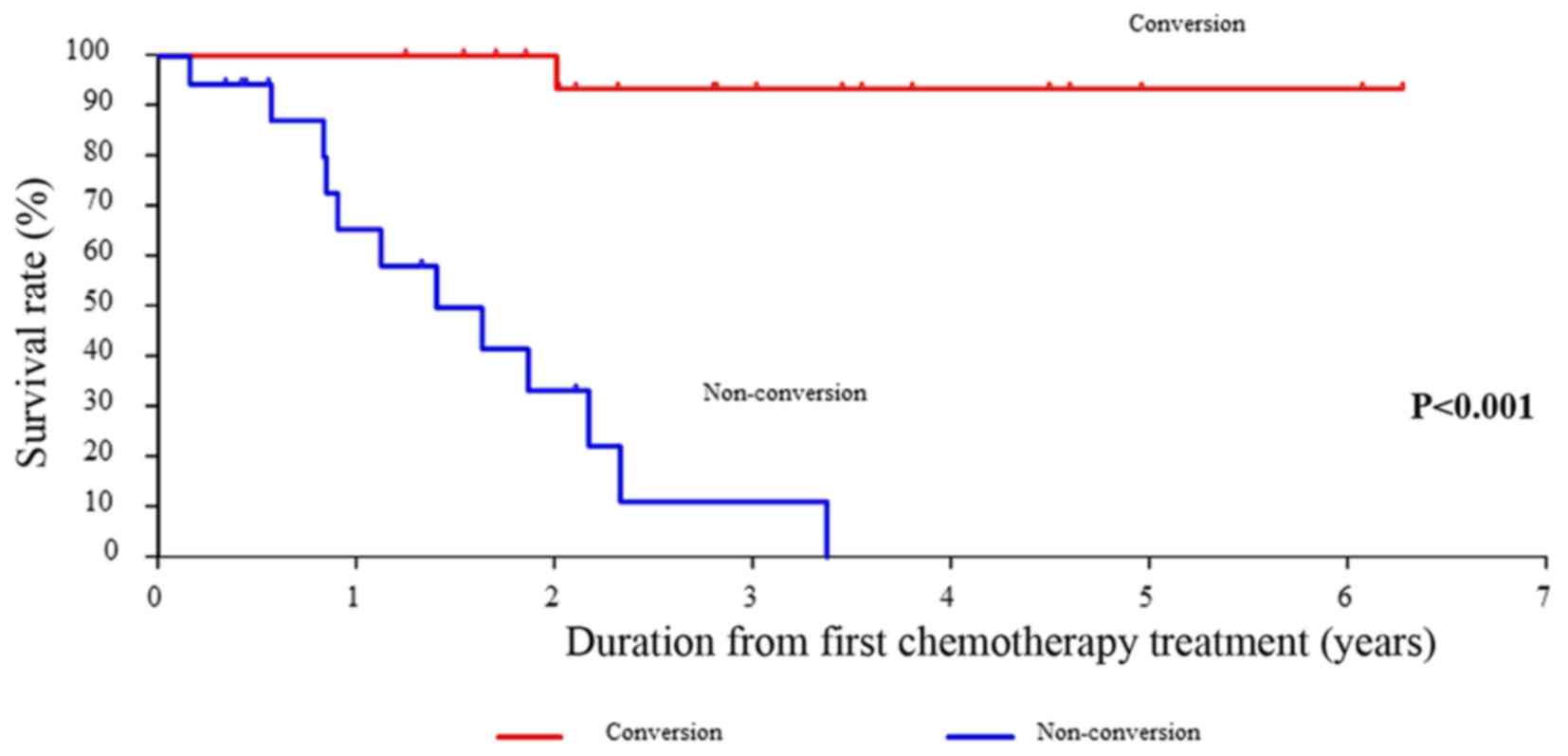
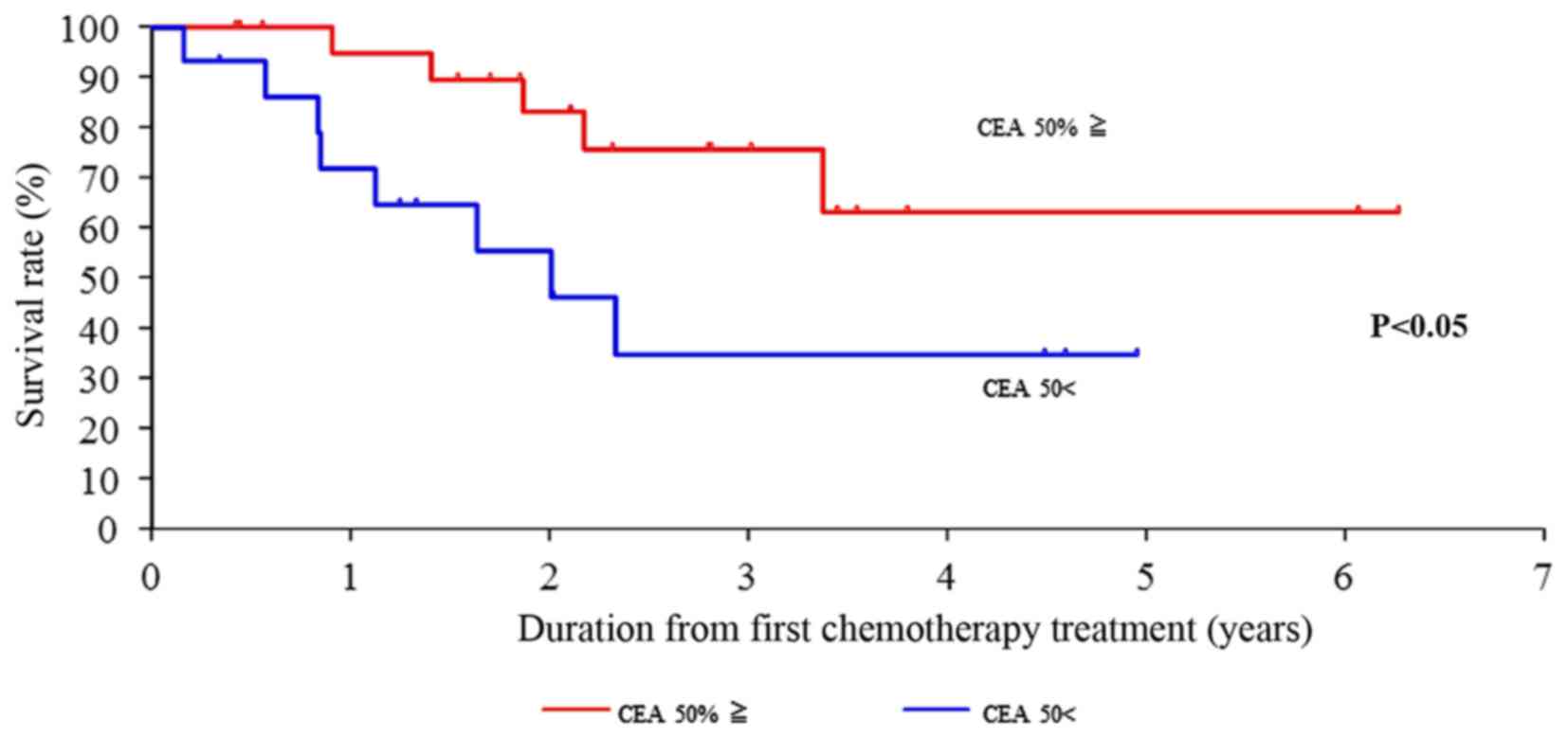
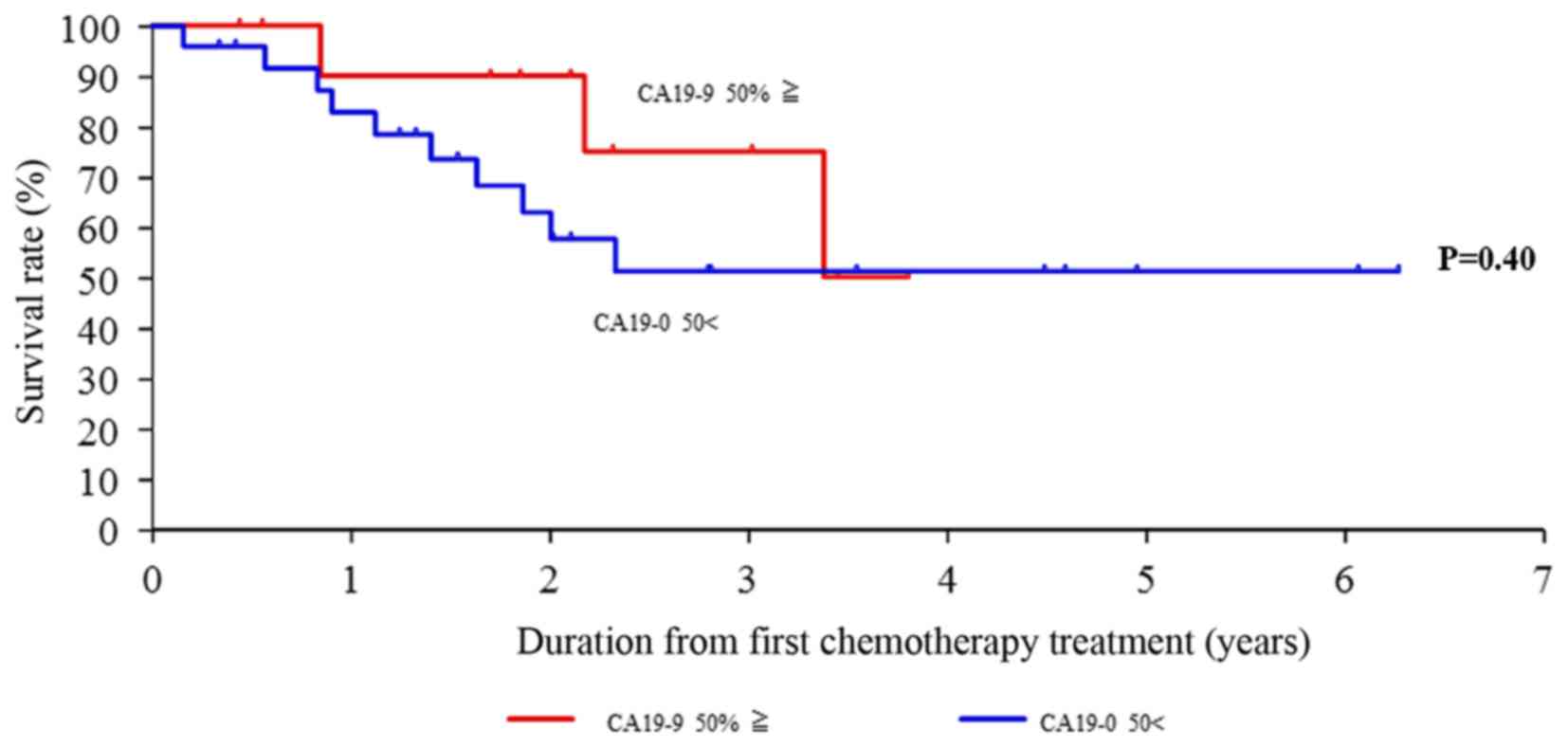
|
1
|
Fernandez FG, Drebin JA, Linehan DC,
Dehdashti F, Siegel BA and Strasberg SM: Five-year survival after
resection of hepatic metastases from colorectal cancer in patients
screened by positron emission tomography with F-18
fluorodeoxyglucose (FDG-PET). Ann Surg. 240:438–450. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
O'Neil BH and Goldberg RM: Innovations in
chemotherapy for metastatic colorectal cancer: An update of recent
clinical trials. Oncologist. 13:1074–1083. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Grothey A, Hedrick EE, Mass RD, Sarkar S,
Suzuki S, Ramanathan RK, Hurwitz HI, Goldberg RM and Sargent DJ:
Response-independent survival benefit in metastatic colorectal
cancer: A comparative analysis of N9741 and AVF2107. J Clin Oncol.
26:183–189. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tyagi P and Grothey A: Commentary on a
phase III trial of bevacizumab plus XELOX or FOLFOX4 for first-line
treatment of metastatic colorectal cancer: The NO16966 trial. Clin
Colorectal Cancer. 6:261–264. 2006. View Article : Google Scholar
|
|
5
|
Arkenau HT, Arnold D, Cassidy J,
Diaz-Rubio E, Douillard JY, Hochster H, Martoni A, Grothey A, Hinke
A, Schmiegel W, et al: Efficacy of oxaliplatin plus capecitabine or
infusional fluorouracil/leucovorin in patients with metastatic
colorectal cancer: A pooled analysis of randomized trials. J Clin
Oncol. 26:5910–5917. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Falcone A, Ricci S, Brunetti I, Pfanner E,
Allegrini G, Barbara C, Crinò L, Benedetti G, Evangelista W,
Fanchini L, et al Gruppo Oncologico Nord Ovest: Phase III trial of
infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan
(FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and
irinotecan (FOLFIRI) as first-line treatment for metastatic
colorectal cancer: The Gruppo Oncologico Nord Ovest. J Clin Oncol.
25:1670–1676. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Van Cutsem E, Köhne CH, Hitre E, Zaluski
J, Chang Chien CR, Makhson A, D'Haens G, Pintér T, Lim R, Bodoky G,
et al: Cetuximab and chemotherapy as initial treatment for
metastatic colorectal cancer. N Engl J Med. 360:1408–1417. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bokemeyer C, Bondarenko I, Makhson A,
Hartmann JT, Aparicio J, de Braud F, Donea S, Ludwig H, Schuch G,
Stroh C, et al: Fluorouracil, leucovorin, and oxaliplatin with and
without cetuximab in the first-line treatment of metastatic
colorectal cancer. J Clin Oncol. 27:663–671. 2009. View Article : Google Scholar
|
|
9
|
Saltz LB, Clarke S, Díaz-Rubio E,
Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS,
Rivera F, et al: Bevacizumab in combination with oxaliplatin-based
chemotherapy as first-line therapy in metastatic colorectal cancer:
A randomized phase III study. J Clin Oncol. 26:2013–2019. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schwartzberg LS, Rivera F, Karthaus M,
Fasola G, Canon JL, Hecht JR, Yu H, Oliner KS and Go WY: PEAK: A
randomized, multicenter phase II study of panitumumab plus modified
fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) or bevacizumab
plus mFOLFOX6 in patients with previously untreated, unresectable,
wild-type KRAS exon 2 metastatic colorectal cancer. J Clin Oncol.
32:2240–2247. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Venook AP, Niedzwiecki D, Lenz HJ,
Innocenti F, Fruth B, Meyerhardt JA, Schrag D, Greene C, O'Neil BH,
Atkins JN, et al: Effect of first-line chemotherapy combined with
cetuximab or bevacizumab on overall survival in patients with KRAS
wild-type advanced or metastatic colorectal cancer: A randomized
clinical trial. JAMA. 317:2392–2401. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Heinemann V, von Weikersthal LF, Decker T,
Kiani A, Vehling-Kaiser U, Al-Batran SE, Heintges T, Lerchenmüller
C, Kahl C, Seipelt G, et al: FOLFIRI plus cetuximab versus FOLFIRI
plus bevacizumab as first-line treatment for patients with
metastatic colorectal cancer (FIRE-3): A randomised, open-label,
phase 3 trial. Lancet Oncol. 15:1065–1075. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Stintzing S, Modest DP, Rossius L, Lerch
MM, von Weikersthal LF, Decker T, Kiani A, Vehling-Kaiser U,
Al-Batran SE, Heintges T, et al FIRE-3 investigators: FOLFIRI plus
cetuximab versus FOLFIRI plus bevacizumab for metastatic colorectal
cancer (FIRE-3): A post-hoc analysis of tumour dynamics in the
final RAS wild-type subgroup of this randomised open-label phase 3
trial. Lancet Oncol. 17:1426–1434. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tejpar S, Stintzing S, Ciardiello F,
Tabernero J, Van Cutsem E, Beier F, Esser R, Lenz HJ and Heinemann
V: Prognostic and predictive relevance of primary tumor location in
patients with RAS wild-type metastatic colorectal cancer:
Retrospective analyses of the CRYSTAL and FIRE-3 trials. JAMA
Oncol. Oct 10–2016.Epub ahead of print. PubMed/NCBI
|
|
15
|
Folprecht G, Gruenberger T, Bechstein WO,
Raab HR, Lordick F, Hartmann JT, Lang H, Frilling A, Stoehlmacher
J, Weitz J, et al: Tumour response and secondary resectability of
colorectal liver metastases following neoadjuvant chemotherapy with
cetuximab: The CELIM randomised phase 2 trial. Lancet Oncol.
11:38–47. 2010. View Article : Google Scholar
|
|
16
|
Van Loon K, Wigler D, Niedzwiecki D,
Venook AP, Fuchs C, Blanke C, Saltz L, Goldberg RM and Meyerhardt
JA: Comparison of dietary and lifestyle habits among stage III and
metastatic colorectal cancer patients: Findings from CALGB 89803
and CALGB 80405. Clin Colorectal Cancer. 12:95–102. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Elez E, Argilés G and Tabernero J:
First-line treatment of metastatic colorectal cancer: Interpreting
FIRE-3, PEAK, and CALGB/SWOG 80405. Curr Treat Options Oncol.
16:522015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wanebo HJ, Rao B, Pinsky CM, Hoffman RG,
Stearns M, Schwartz MK and Oettgen HF: Preoperative
carcinoembryonic antigen level as a prognostic indicator in
colorectal cancer. N Engl J Med. 299:448–451. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Moertel CG, O'Fallon JR, Go VL, O'Connell
MJ and Thynne GS: The preoperative carcinoembryonic antigen test in
the diagnosis, staging, and prognosis of colorectal cancer. Cancer.
58:603–610. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Martin EW Jr, James KK, Hurtubise PE,
Catalano P and Minton JP: The use of CEA as an early indicator for
gastrointestinal tumor recurrence and second-look procedures.
Cancer. 39:440–446. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cooper MJ, Mackie CR, Skinner DB and
Moossa AR: A reappraisal of the value of carcinoembryonic antigen
in the management of patients with various neoplasms. Br J Surg.
66:120–123. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Steele G Jr, Ellenberg S, Ramming K,
O'Connell M, Moertel C, Lessner H, Bruckner H, Horton J, Schein P,
Zamcheck N, et al: CEA monitoring among patients in
multi-institutional adjuvant G.I. therapy protocols. Ann Surg.
196:162–169. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kuusela P, Jalanko H, Roberts P, Sipponen
P, Mecklin JP, Pitkänen R and Mäkelä O: Comparison of CA19-9 and
carcinoembryonic antigen (CEA) levels in the serum of patients with
colorectal diseases. Br J Cancer. 49:135–139. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Filella X, Molina R, Grau JJ, Piqué JM,
Garcia-Valdecasas JC, Astudillo E, Biete A, Bordas JM, Novell A,
Campo E, et al: Prognostic value of CA 19.9 levels in colorectal
cancer. Ann Surg. 216:55–59. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kouri M, Pyrhönen S and Kuusela P:
Elevated CA19-9 as the most significant prognostic factor in
advanced colorectal carcinoma. J Surg Oncol. 49:78–85. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nakayama T, Watanabe M, Teramoto T and
Kitajima M: CA19-9 as a predictor of recurrence in patients with
colorectal cancer. J Surg Oncol. 66:238–243. 1997. View Article : Google Scholar
|
|
27
|
Prager GW, Braemswig KH, Martel A, Unseld
M, Heinze G, Brodowicz T, Scheithauer W, Kornek G and Zielinski CC:
Baseline carcinoembryonic antigen (CEA) serum levels predict
bevacizumab-based treatment response in metastatic colorectal
cancer. Cancer Sci. 105:996–1001. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bramswig KH, Poettler M, Unseld M, Wrba F,
Uhrin P, Zimmermann W, Zielinski CC and Prager GW: Soluble
carcinoembryonic antigen activates endothelial cells and tumor
angiogenesis. Cancer Res. 73:6584–6596. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Carmeliet P: Angiogenesis in life, disease
and medicine. Nature. 438:932–936. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ocvirk J, Brodowicz T, Wrba F, Ciuleanu
TE, Kurteva G, Beslija S, Koza I, Pápai Z, Messinger D, Yilmaz U,
et al: Cetuximab plus FOLFOX6 or FOLFIRI in metastatic colorectal
cancer: CECOG trial. World J Gastroenterol. 16:3133–3143. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Van Cutsem E, Cervantes A, Adam R, Sobrero
A, Van Krieken JH, Aderka D, Aranda Aguilar E, Bardelli A, Benson
A, Bodoky G, et al: ESMO consensus guidelines for the management of
patients with metastatic colorectal cancer. Ann Oncol.
27:1386–1422. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cremolini C, Loupakis F, Antoniotti C,
Lupi C, Sensi E, Lonardi S, Mezi S, Tomasello G, Ronzoni M,
Zaniboni A, et al: FOLFOXIRI plus bevacizumab versus FOLFIRI plus
bevacizumab as first-line treatment of patients with metastatic
colorectal cancer: Updated overall survival and molecular subgroup
analyses of the open-label, phase 3 TRIBE study. Lancet Oncol.
16:1306–1315. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sasaki Y, Osada S, Mori R, Imai H, Tanaka
Y, Matsuhashi N, Okumura N, Nonaka K, Takahashi T and Yoshida K:
Determining timing of hepatectomy for colorectal cancer with
distant metastasis according to imaging-based tumor shrinkage
ratio. Int J Med Sci. 10:1231–1241. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sasaki Y, Osada S, Matsui S, Imai H,
Tanahashi T, Tanaka Y, Matsuhashi N, Okumura N, Yamaguchi K and
Yoshida K: Preoperative chemotherapy can change the surgical
procedure for hepatectomy in patients with liver metastasis of
colorectal cancer. Anticancer Res. 35:5485–5489. 2015.PubMed/NCBI
|
|
35
|
Benson AB III, Venook AP, Cederquist L,
Chan E, Chen YJ, Cooper HS, Deming D, Engstrom PF, Enzinger PC,
Fichera A, et al: Colon Cancer, Version 1.2017, NCCN Clinical
Practice Guidelines in Oncology. J Natl Compr Canc Netw.
15:370–398. 2017. View Article : Google Scholar : PubMed/NCBI
|