Introduction
Colorectal cancer (CRC), the third most common
malignant tumor in humans, is a major cause of cancer-associated
mortality in the West. Metastatic tumors are present in 40–50% of
patients newly diagnosed with metastatic CRC (mCRC) (1), and their prognosis remains poor. The
standard first-line chemotherapy for mCRC comprises a combination
of fluorouracil (5-FU) and folinic acid with either oxaliplatin
(FOLFOX-4 and FOLFOX-6 regimens) or irinotecan (FOLFIRI and AIO
regimens) (2). The first-line
treatment of the anti-vascular endothelial growth factor (VEGF)
mono-clonal antibody bevacizumab for mCRC has also been widely used
with good effect, as shown in the pivotal AVF2107g and NO16966
trials (3–5). In addition, the combinations of
irinotecan- or oxaliplatin-based chemotherapy with epidermal growth
factor receptor (EGFR) inhibitors (cetuximab and panitumumab) are
other standard first-line treatments for mCRC. A similar efficacy
of FOLFIRI and FOLFOX has been shown in patients treated in this
manner (6–9). In particular, these drugs are being
investigated in terms of early tumor shrinkage (ETS) and deepness
of response in unresectable-type and borderline resectable-type
cancer with liver metastasis treated with anti-EGFR monoclonal
antibodies (10), as the
CALGB/SWOG 80405 trial showed no superiority in overall survival
(OS) between anti-VEGF monoclonal antibodies and anti-EGFR
monoclonal antibodies (11).
Currently, the superiority of treatment with anti-EGFR monoclonal
antibodies over that of anti-VEGF monoclonal antibodies remains
confusing. The present study evaluated the possibility of proposing
a novel treatment strategy for personalized medicine in which
anti-EGFR monoclonal antibodies are used positively as a first-line
treatment.
Patients and methods
The present study included 37 patients with mCRC
that was considered unresectable or borderline resectable who were
confirmed to have the wild-type RAS gene. The patients underwent
administration of first-line treatment with anti-EGFR monoclonal
antibodies in the Department of Surgical Oncology of Gifu
University School of Medicine (Gifu, Japan) between January 2010
and March 2017. Written informed consent was obtained from all
patients enrolled in the present study. The study protocol
conformed to the ethical guidelines of the 1975 Declaration of
Helsinki and the guidelines of the regional Ethics Committees of
Zurich and Basel, Switzerland, following approval by the
Institutional Review Board of the Gifu University Graduate School
of Medicine.
The demographics and disease characteristics of the
patients were recorded (Table I).
Tumor shrinkage was evaluated according to the Response Evaluation
Criteria In Solid Tumors (RECIST) version 1.1 classifications for
these 37 patients who underwent first-line treatment consisting of
6–8 courses of FOLFOX plus anti-EGFR monoclonal antibody
therapy.
 | Table ICharacteristics of 37 patients with
metastatic colorectal cancer who received anti-epidermal growth
factor receptor monoclonal antibodies as first-line treatment. |
Table I
Characteristics of 37 patients with
metastatic colorectal cancer who received anti-epidermal growth
factor receptor monoclonal antibodies as first-line treatment.
| Characteristics | Value |
|---|
| Sex, n (%) | |
| Female | 10 (27.0) |
| Male | 27 (73.0) |
| Age, years | |
| Mean ± SD
(range) | 62.1±9.4 (39–77) |
| Primary tumor, n
(%) | |
| C | 2 (5.4) |
| A | 5 (13.5) |
| T | 4 (10.8) |
| D | 2 (5.4) |
| S | 11 (29.7) |
| Rs | 5 (13.5) |
| Ra-b | 8 (21.6) |
| Pathology, n
(%) | |
| pap | 1 (2.7) |
| tub1 | 6 (16.2) |
| tub2 | 27 (73.0) |
| por | 2 (5.4) |
| muc | 1 (2.7) |
| Metastatic site, n
(%) | |
| Liver | 29 (78.4) |
| Lung | 7 (18.9) |
| Spleen | 1 (2.7) |
| Lymph node | 9 (24.3) |
| Peritoneal | 6 (16.2) |
| Bone | 2 (5.4) |
| Metastases, n
(%) | |
| 1 | 24 (64.9) |
| 2 | 8 (21.6) |
| 3 | 4 (10.8) |
| 4 | 1 (2.7) |
RECIST guideline (version 1.1)
Currently, computed tomography (CT) is the best and
most reproducible method to measure lesions when assessing
response. The RECIST guideline defines measurability of lesions on
CT scan based on a CT slice thickness of 5 mm or less. When the CT
slice thickness is >5 mm, the minimum size of a measurable
lesion must be at least twice the slice thickness.
Evaluation of target lesions
The following definitions were applied for the
evaluation of the target lesions. Complete response (CR): The
disappearance of all target lesions. Any pathological lymph nodes
(whether target or non-target) must have a reduction in the short
axis to <10 mm. Partial response (PR): At least a 30% decrease
in the sum of the diameters of the target lesions as compared with
the baseline sum diameters. Progressive disease (PD): At least a
20% increase in the sum of the diameters of the target lesions as
compared with the smallest sum in the study (this includes the
baseline sum if that is the smallest in the study). In addition to
the relative increase of 20%, the sum must also show an absolute
increase of at least 5 mm. The appearance of one or more new
lesions is also considered progression. Stable disease (SD):
Neither sufficient shrinkage to qualify for PR nor sufficient
increase to qualify for PD as compared with the smallest sum
diameters in the study.
The present study also investigated the association
between the predictive value of pre-chemotherapy changes in the
levels of carcinoembryonic antigen (CEA) and carbohydrate antigen
19-9 (CA19-9), by determining whether there was a 50% drop in their
values after the 6–8 courses of therapy, and the clinical outcome
following the anti-EGFR monoclonal antibody-based treatment
regimen.
Statistical analysis
All data are presented as the mean ± standard
deviation. Student's t-test, Wilcoxon's signed-rank test, the
Kaplan-Meier method, the log-rank test and Pearson's product-moment
correlation coefficient were used to evaluate the data to determine
statistical significances. A two-sided P-value of <0.05 was
considered to indicate a statistically significant difference. All
statistical analyses were performed with SPSS 11.5J software (SPSS
Japan, Inc., Tokyo, Japan).
Results
The study consisted of 37 patients with mCRC (27 men
and 10 women; mean age, 62.1±9.4 years). Primary tumor locations in
the 37 patients were as follows: The cecum in 2 (5.4%) patients,
the ascending colon in 5 (13.5%) patients, the transverse colon in
4 (10.8%) patients, the descending colon in 2 (5.4%) patients, the
sigmoid colon in 11 (29.7%) patients, the rectosigmoid rectum in 5
(13.5%) patients and the rectum above-below the peritoneal
reflection in 8 (21.6%) patients. Pathological types in the 37
patients were categorized as follows: 1 (2.7%) patient with
papillary adenocarcinoma, 6 (16.2%) patients with
well-differentiated adenocarcinoma, 27 (73.0%) patients with
moderately differentiated adenocarcinoma, 2 (5.4%) patients with
poorly differentiated adenocarcinoma and 1 (2.7%) patient with
mucinous adenocarcinoma. Metastatic sites of the 37 patients
included the liver in 29 (78.4%) patients, the lungs in 7 (18.9%)
patients, the spleen in 1 (2.7%) patient, the lymph nodes in 9
(24.3%) patients and the bones in 2 (5.4%) patients. The number of
metastatic sites included 1 site in 24 (64.9%) patients, 2 sites in
8 (21.6%) patients, 3 sites in 4 (10.8%) patients and 4 sites in 1
(2.7%) patient (Table I).
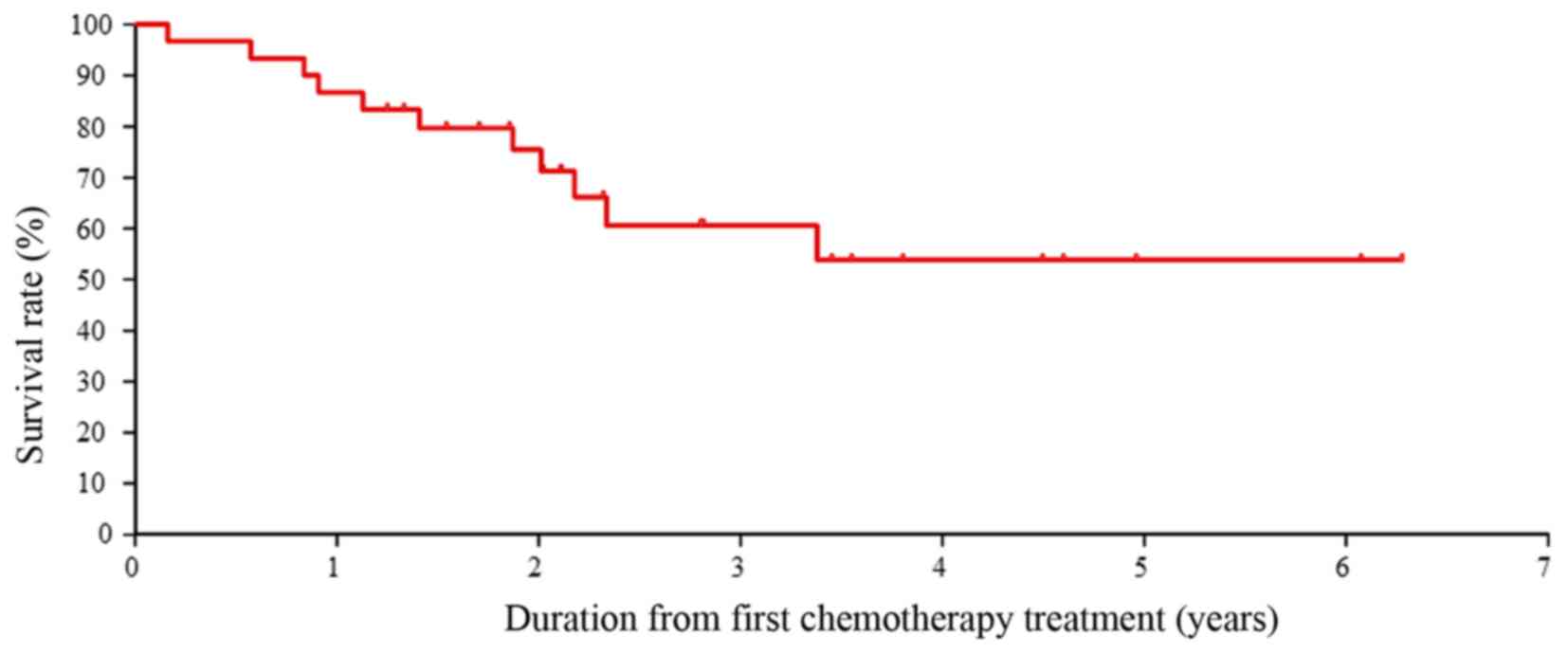
Curves showing cumulative survival are presented in
Fig. 1. The 3-year OS rate was
>60.0% in all patients (n=37). Patients receiving the anti-EGFR
monoclonal antibodies as first-line treatment did not reach the
median survival time. These 3-year OS data are meaningful as the OS
time in a recent study reached only slightly more than 30 months
(11).
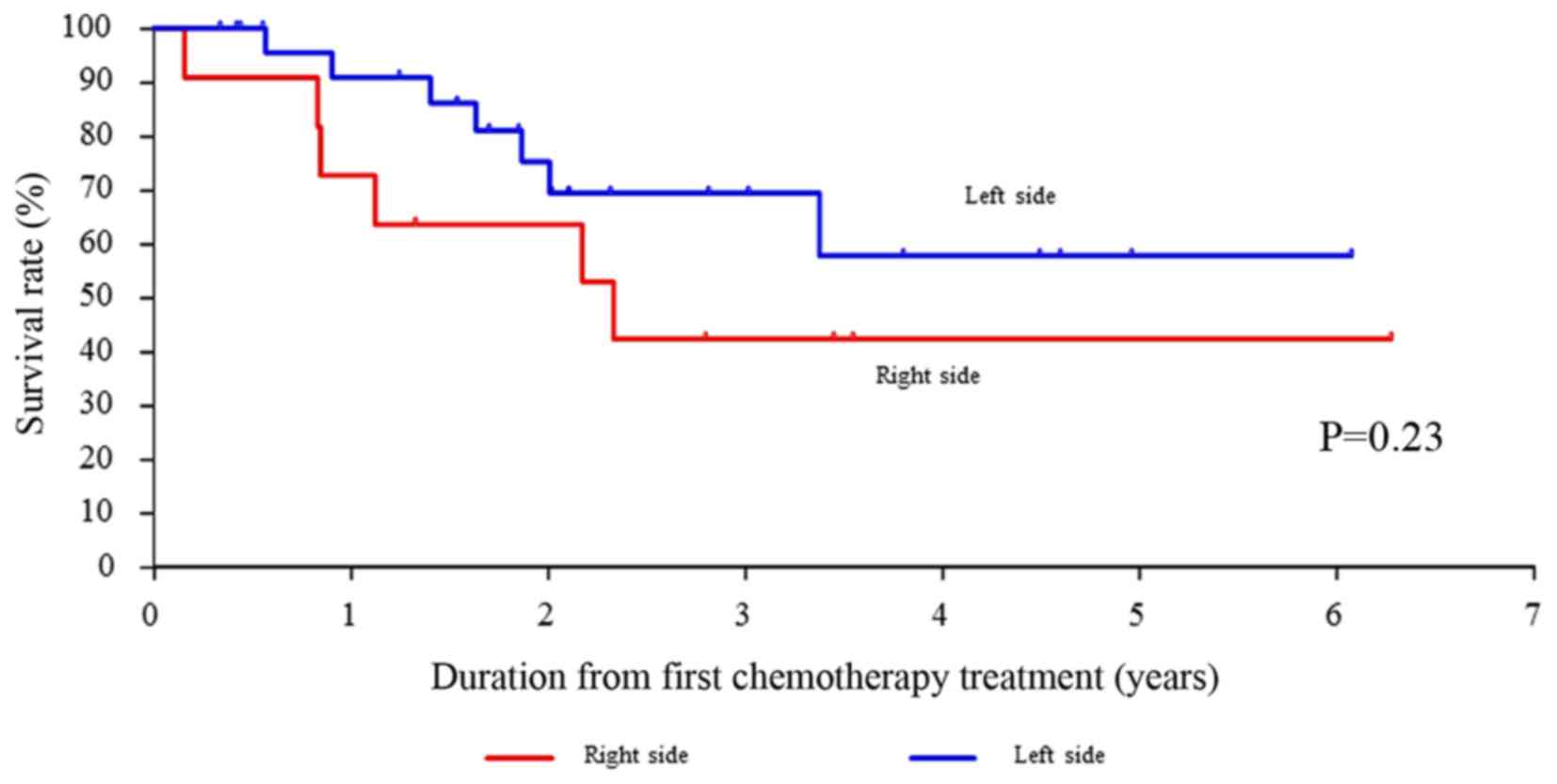
The OS rates for right-sided CRC compared with
left-sided CRC were not significantly different (P=0.235). However,
the OS rates for left-sided CRC treated with the anti-EGFR
monoclonal antibodies as first-line treatment tended to be better
than those for right-sided CRC (Fig.
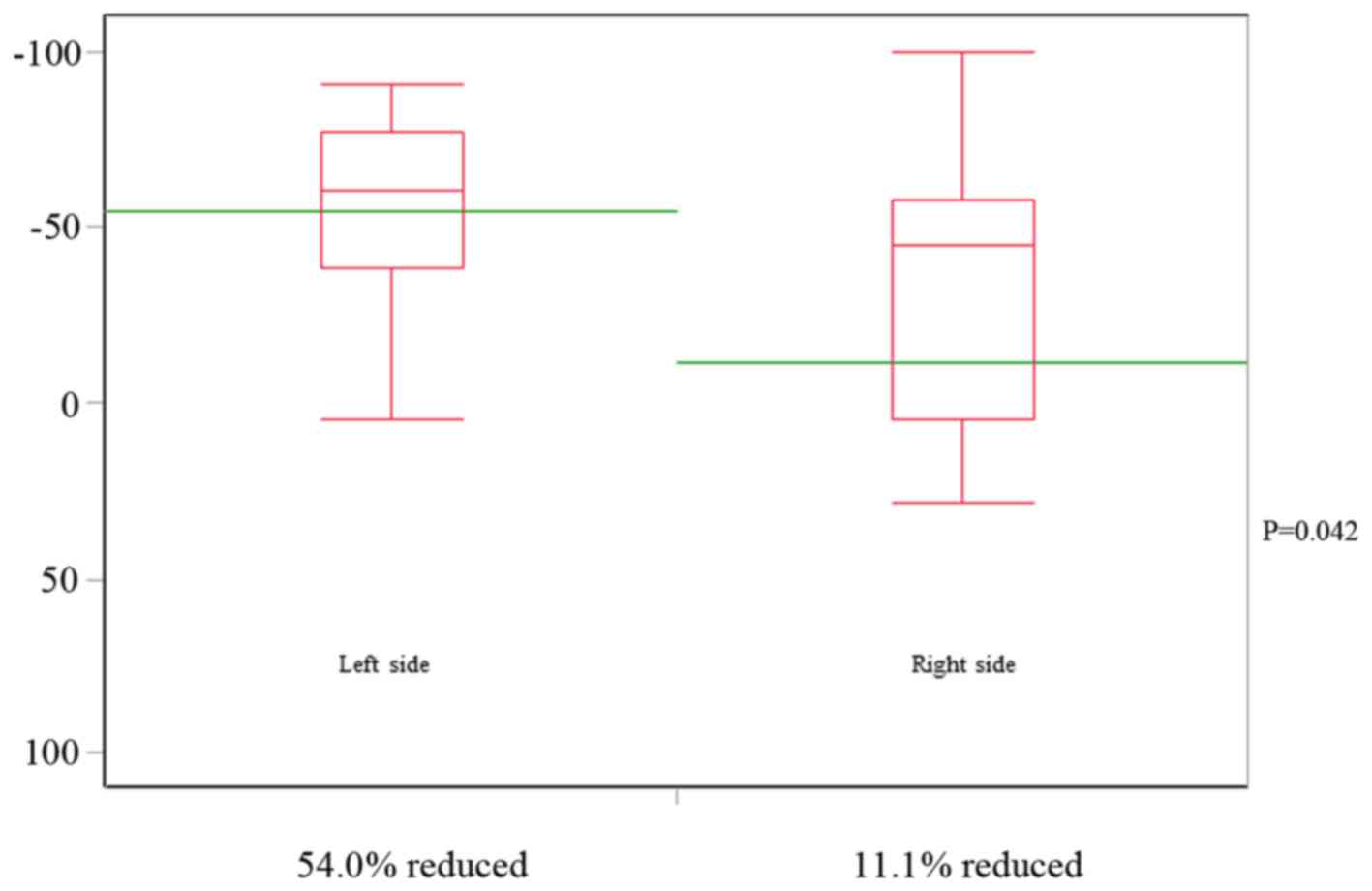
2). The mean tumor shrinkage rate in right-sided CRC according
to the classification of the RECIST guidelines (version 1.1) was
−11.1%, whereas that for the left-sided CRC was significantly
different at −54.0% (P=0.042) (Fig.
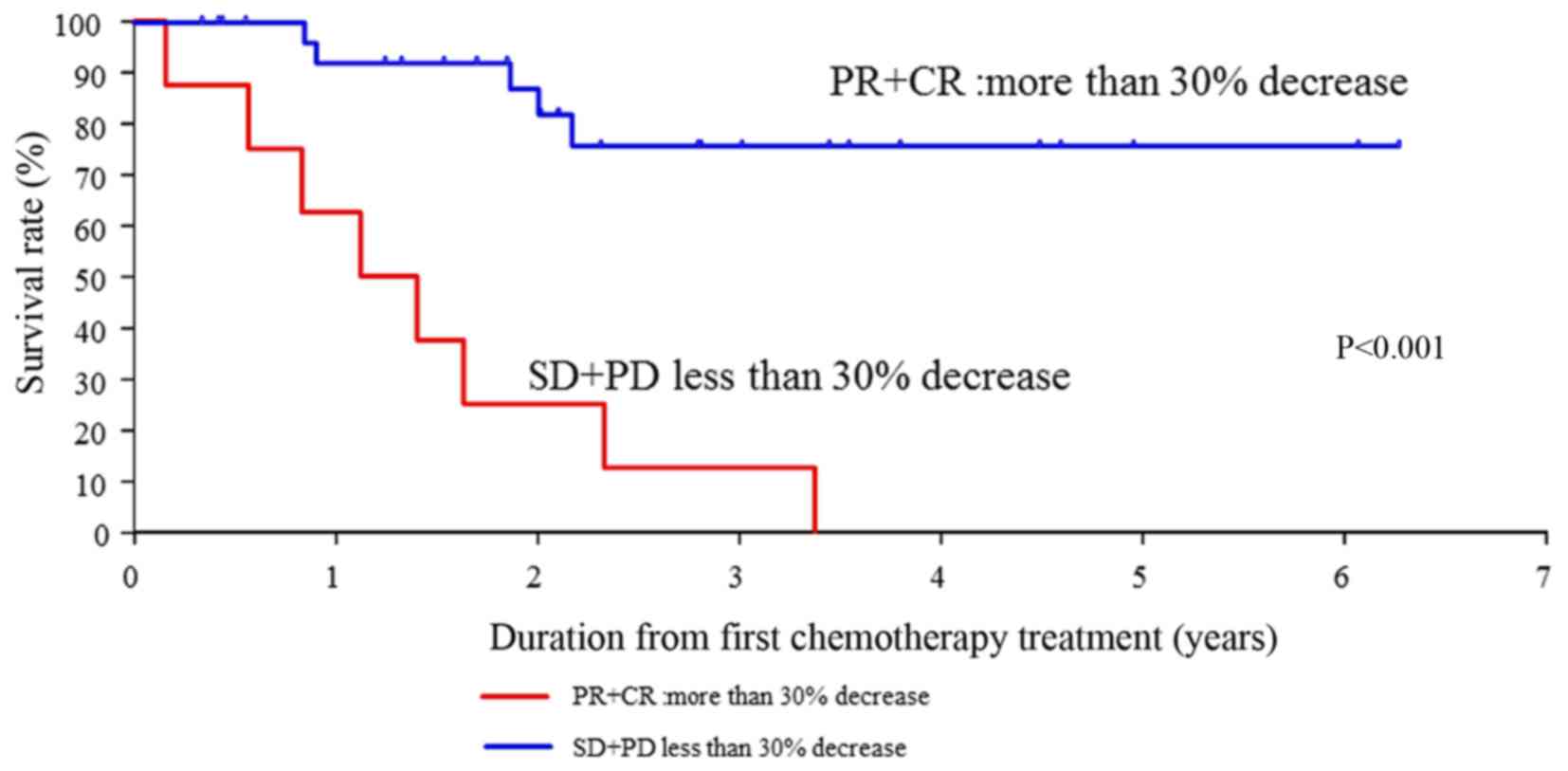
3). In addition, the OS rates for SD+PD compared with PR+CR
were significantly different (P<0.001) (Fig. 4).
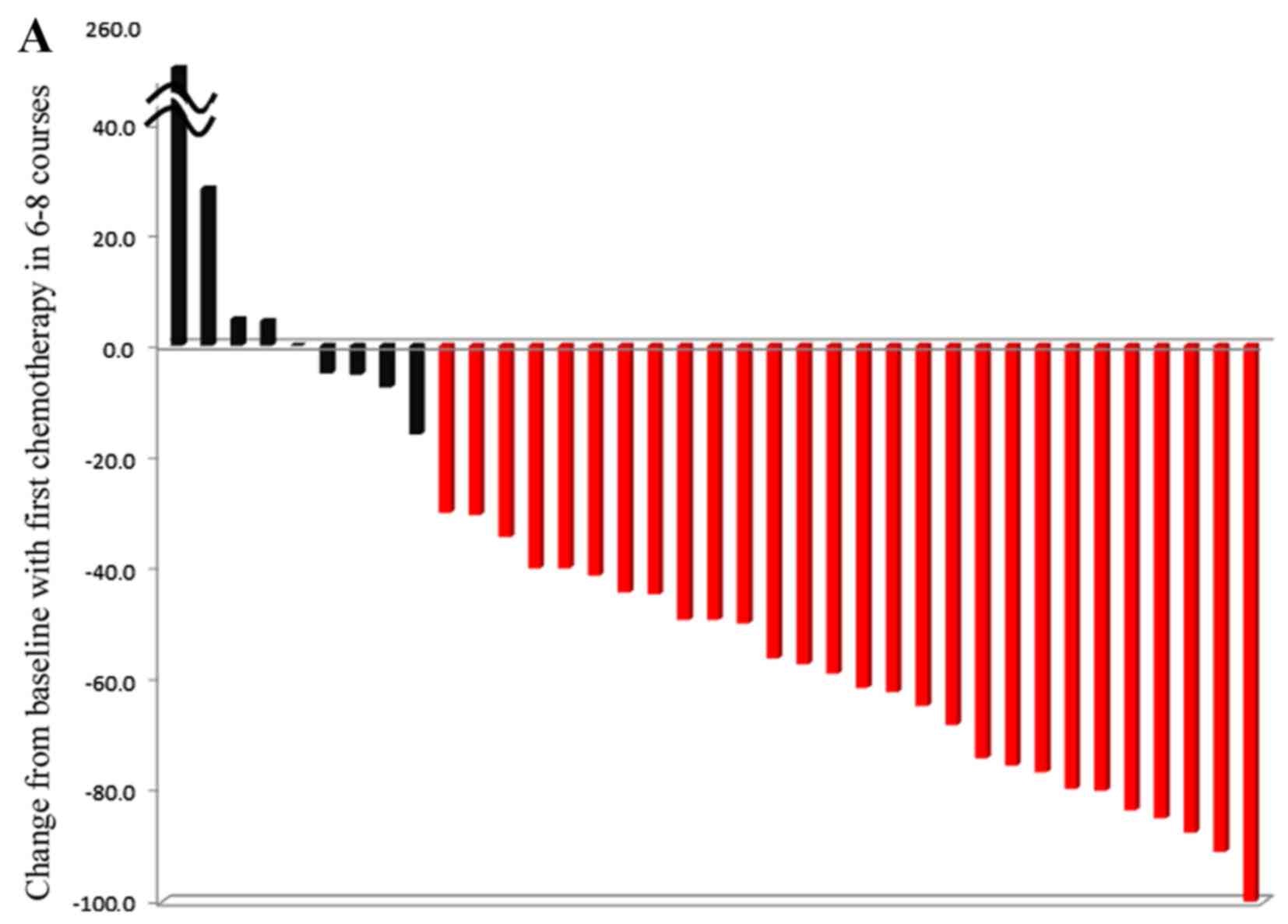
The overall median tumor shrinkage rate of all
patients according to the RECIST guideline (version 1.1)
classification was −49.6%. The estimated median shrinkage rate in
the 29 patients with CR+PR was −60.3%. In addition, the 8 patients
with SD+PD did not show apparent shrinkage, with an estimated
median shrinkage rate of −0.3%. Waterfall plots dividing PR+CR and
SD+PD and left-sided and right-sided CRC location are shown in
Fig. 5. Characteristics of patient
who received first-line treatment with FOLFOX plus anti-EGFR
monoclonal antibodies are presented by RECIST (version 1.1)
response to chemotherapy according to changes in CEA and CA19-9 in
Table II.
 | Table IIRECIST response to chemotherapy
according to changes in CEA and CA19-9. |
Table II
RECIST response to chemotherapy
according to changes in CEA and CA19-9.
| CEA change, % | |
| <50 | 15 (40.5) |
| ≥50 | 22 (59.5) |
| CA 19-9 change,
% | |
| <50 | 25 (67.6) |
| ≥50 | 12 (32.4) |
| Disease
statusa | |
| CR | 1 (2.7) |
| PR | 28 (75.7) |
| SD | 6 (16.2) |
| PD | 2 (5.4) |
| Conversion
therapy | |
| Yes | 19 (51.4) |
| No | 18 (48.6) |
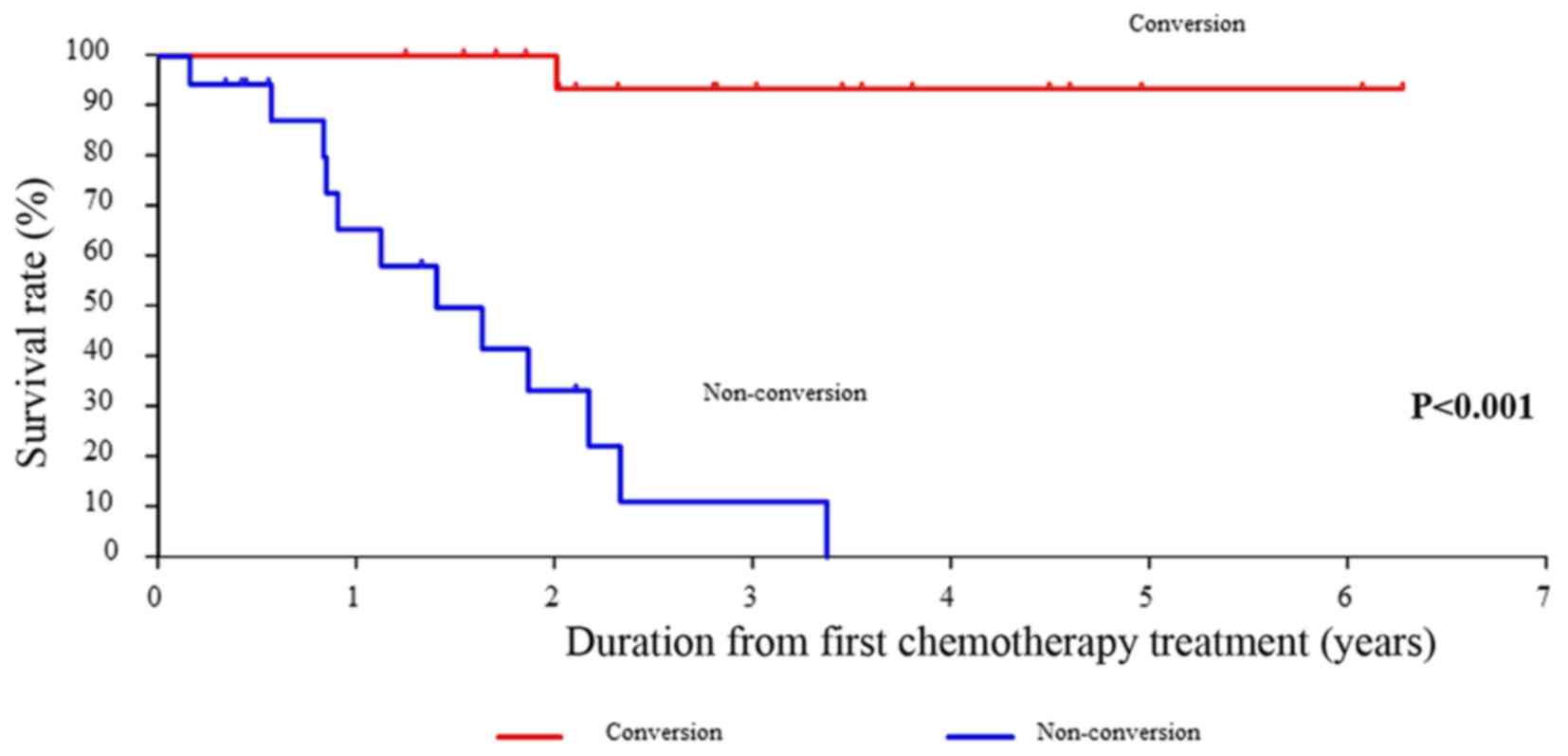
The OS rates for patients treated with conversion
therapy, which was defined as surgical therapy with R0 or R1
margins in unresectable and marginally resectable metastases,
compared with patients treated with non-conversion therapy were
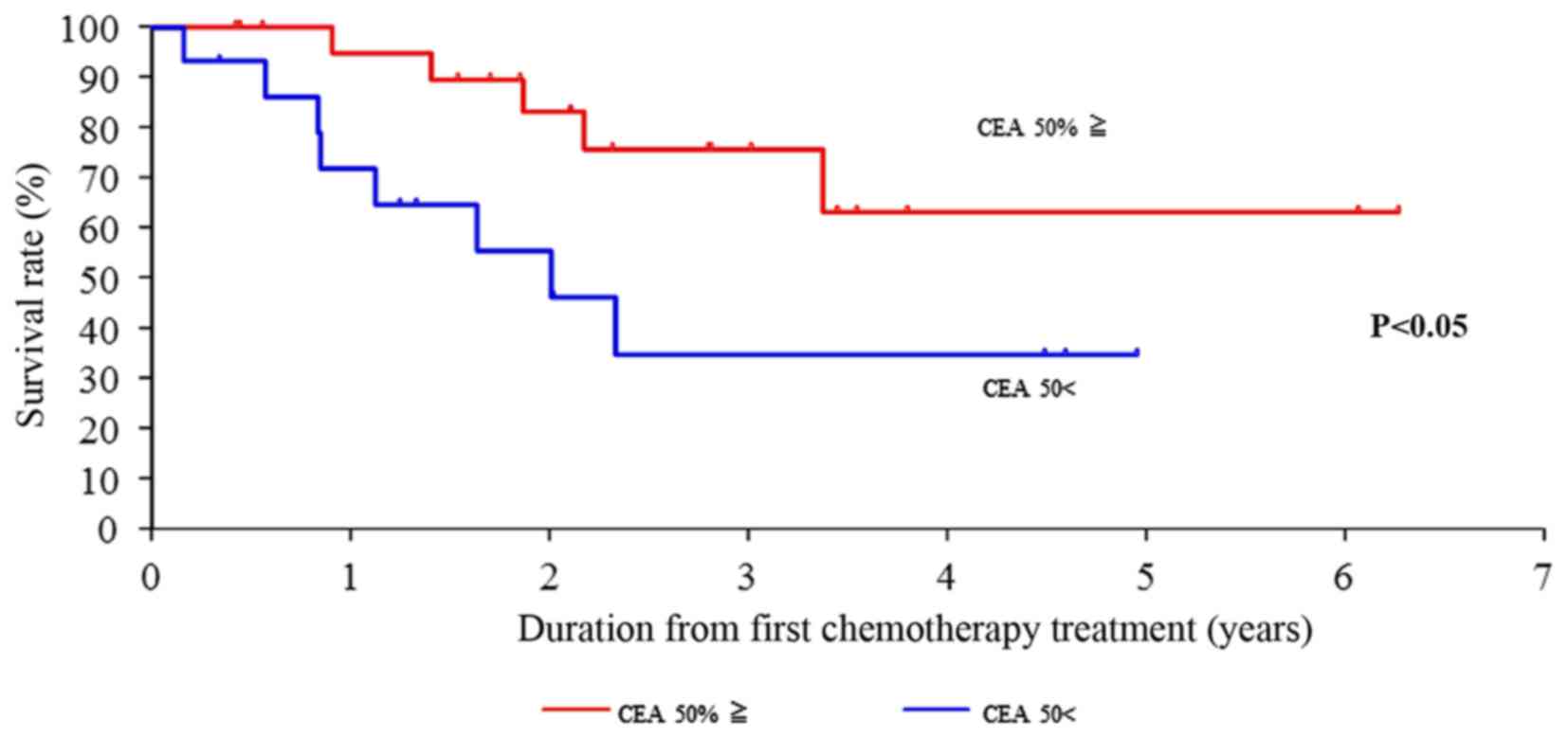
significantly different (P<0.001) (Fig. 6). In addition, CEA was suggested as
a possible predictive factor for survival due to the significant
50% drop in its value (P<0.05) following the 6–8 courses of
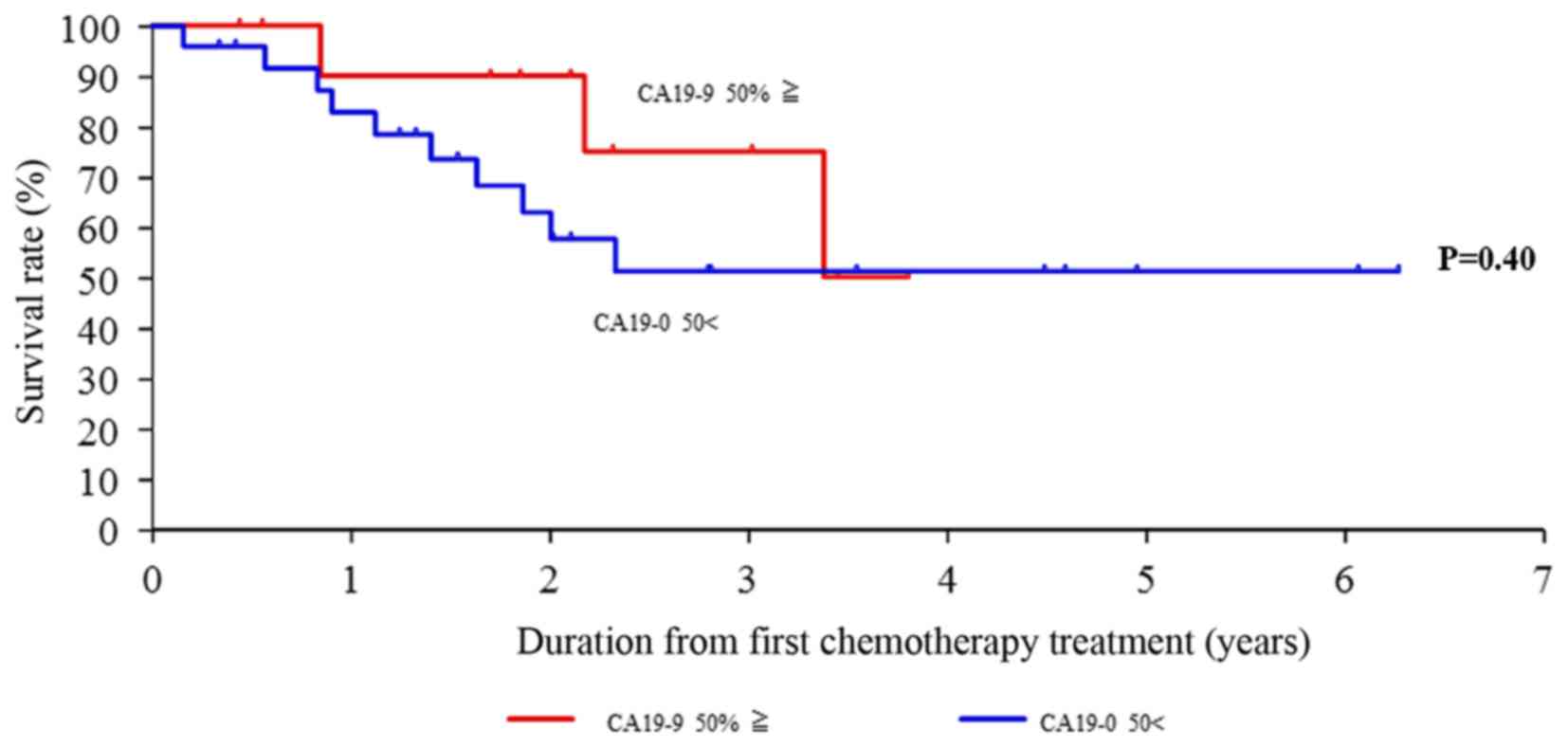
therapy (Fig. 7). However, the
level of CA19-9 did not decrease by 50% following the 6–8 courses
of therapy, suggesting that it was not likely to be a predictive
factor of survival (Fig. 8).
Discussion
The combinations of irinotecan- or oxaliplatin-based
chemotherapy with EGFR inhibitors (cetuximab and panitumumab) are
standard first-line treatments for mCRC. A similar efficacy of
FOLFIRI and FOLFOX has been shown in these patients (6–9).
The randomized phase III FIRE-3 trial, first
reported by Heinemann et al (12) in 2014, aimed to compare the
efficacy of FOLFIRI plus cetuximab with FOLFIRI plus bevacizumab in
592 patients with KRAS-wild-type mCRC. A significant OS advantage
was found in the patients treated with first-line FOLFIRI plus
cetuximab, as their median OS time increased by almost 4 months
[28.8 vs. 25.0 months; hazard ratio (HR), 0.77; P=0.0164]. Results
from a sub-analysis that excluded patients with other activating
mutations in the RAS family genes (all-RAS wild-type population)
showed an increase in median OS time of 7.5-months: 33.1 months
with FOLFIRI plus cetuximab vs. 25.6 months with FOLFIRI plus
bevacizumab (HR, 0.70; P=0.011) (13,14).
In the PEAK trial, 278 patients with previously
untreated wild-type KRAS exon 2 (codons 12 and 13) tumors received
either panitumumab plus modified 5-FU, leucovorin and oxaliplatin
(mFOLFOX6) or bevacizumab plus mFOLFOX6. A pre-planned analysis of
extended all-RAS genes (including exons 2, 3 and 4 of KRAS and
NRAS) was also included. Despite the similar rates of
progression-free survival (PFS) (HR, 0.87; P=0.353), FOLFOX plus
panitumumab showed a significant advantage in in terms of OS, with
a 12-month survival gain in the all-RAS wild-type population
(median OS, 41.3 vs. 28.9 months; P=0.058). The present analysis is
the first to evaluate the concepts of ETS and deepness of response,
which were previously assessed in first-line trials that included
anti-EGFR monoclonal antibodies (10)
Achieving tumor shrinkage may be clinically relevant
when treating mCRC. Not only may it permit the secondary resection
of metastatic lesions, which can provide a possible cure even in a
subgroup of patients with metastasis, it may also improve or delay
the occurrence of symptoms in patients with aggressive diseases and
high tumor load (15). In the
present study, the rates of OS for conversion therapy compared with
those for non-conversion therapy were significantly different
(P<0.001). This result suggests that if it is performed
concomitantly with surgery, conversion therapy will prolong the OS
rate.
On the basis of these results, >1,100 KRAS
wild-type patients were enrolled in the CALGB 80405 trial, and
among them, 526 all-RAS wild-type patients underwent a separate
sub-analysis to assess study endpoints. No advantage for cetuximab
in terms of either OS or PFS could be confirmed. In particular, the
median PFS time in the all-RAS wild-type population was ~11 months
for the two arms (HR, 1.1; P=0.31), and the median OS time was
31–32 months (HR, 0.9; P=0.40) (16).
The predictive performance of ETS is at least as
good as the standard RECIST response with respect to PFS, OS and
post-progression survival, and it allows for earlier assessment.
Therefore, it would be extremely appealing to use this endpoint in
clinical trials to expedite drug development and to potentially
guide therapeutic decisions. As the surrogacy of ETS has yet to be
shown at trial level (17), the
present study evaluated whether ETS could be a significant
prognosticator of OS.
The mean tumor shrinkage rate of all patients
according to the RECIST guideline (version 1.1) classification was
−49.6%. The rate in the 29 patients with CR+PR was estimated to be
−60.3%, whereas that in the 8 patients with SD+PD was estimated to
be −0.3%, indicating no shrinkage.
CEA and CA19-9 are used as surrogate markers of
predictive factors in mCRC. Although CEA is a glycoprotein present
in normal mucosal cells, its elevation is observed in a number of
different malignancies (18–22).
CA19-9 was first identified as a mucin-like product in a human CRC
cell line (23). The importance of
an elevated serum level of CA19-9 as a useful marker for diagnosing
adenocarcinoma of the upper gastrointestinal tract and for
monitoring colon tumors has been suggested by a number of studies
(24–26). CEA and CA19-9 are considered to be
useful as tumor markers in the assessment of prognosis, in the
detection of recurrence and in the monitoring of the treatment of
patients with CRC.
Prager et al (27) first reported that the canonical
biomarker CEA, a monitor of adenocarcinoma growth and treatment
efficacy, may also be a predictive marker of anti-VEGF-based
combination therapies in addition to its biological role (28,29).
The described effects of CEA on treatment outcome were clearly
limited to bevacizumab-based treatment. In addition, Ocvirk et
al (30) evaluated CEA and
treatment efficacy in a cetuximab-based cohort in comparison to a
bevacizumab cohort. Negative results in the control cohort
indicated that baseline CEA levels were predictive only for
bevacizumab-based treatment, suggesting that for anti-VEGF
monoclonal antibody treatment in mCRC, CEA may be a specific marker
of response and PFS. In the present study, CEA was suggested to be
a predictor of convalescence, as its level dropped by 50% following
the 6–8 courses of therapy. However, CA19-9 was not suggested to be
a predictive factor, as its level did not decrease by 50% following
the 6–8 courses. These results were similar to those of numerous
other studies (27–30).
It was suggested that patients with right-sided CRC
had a worse rate of tumor reduction than patients with left-sided
CRC. Recently, the location of the primary tumor, whether of right-
or left-sided origin, has been investigated for its role in
assisting in the prediction of outcomes. Median OS time with
anti-EGFR monoclonal antibody treatment in CALGB/SWOG80405 trial
(11) was significantly different
between patients with CRC of right- and left-sided origins.
Specifically, OS with anti-EGFR monoclonal antibody treatment was
significantly worse for patients with CRC of right-sided origin.
The present study also suggested that the shrinkage rate was worse
in the patients with a right-sided versus left-sided origin
(14). Anti-EGFR monoclonal
antibodies have an effect on ETS and may improve OS in patients
with mCRC. If anti-EGFR monoclonal antibodies are used for 6–8
courses, this may be useful as a predictor of convalescence. If SD
is determined with treatment using anti-EGFR monoclonal antibodies,
the switch to anti-VEGF monoclonal antibodies should be considered
at the time of the 6–8 course, as recommended by the RECIST
guideline classification (version 1.1). Anti-EGFR monoclonal
antibodies are not therefore not expected to be effective as
first-line treatment, but their use as third-line treatment should
be considered. This concept means the re-introduction of treatment
using anti-EGFR monoclonal antibodies.
Identifying prognostic molecules that can predict
the effectiveness of aggressive chemotherapy to prevent tumor
relapse represents a challenge for clinical practice. The 2016
European Society for Medical Oncology (ESMO) guidelines (31) recommend that the best
benefit-to-risk ratio can be obtained with a cytotoxic doublet plus
an anti-EGFR antibody for patients with RAS wild-type disease;
however, the combination of FOLFOXIRI with bevacizumab can also be
considered (32). The TRIBE study
reported that FOLFOXIRI plus bevacizumab, compared with FOLFIRI
plus bevacizumab, resulted in significantly higher response rates
(65 vs. 53%) (32). On this basis,
cytoreduction of the disease may prolong OS and PFS times. We
recently reported that the overall rate of tumor shrinkage was
highest during the first 50 days after the start of therapy and
gradually decreased over the next 210 days subsequent to plateauing
at 105 days (33,34). The present results indicated that
the effect of anti-EGFR monoclonal antibodies plus FOLFOX on tumor
size is achieved at around 3 months (6–8 courses).
Taking into consideration the present results and
the evidence in the literature, we believe that patients with RAS
wild-type disease should still undergo 6–8 courses of therapy with
anti-EGFR monoclonal antibodies plus FOLFOX. If tumor shrinkage
shows a PR, these patients should continue on this same therapy.
Also, it is possible to perform conversion therapy (surgery) in
patients with unresectable or borderline resectable liver and lung
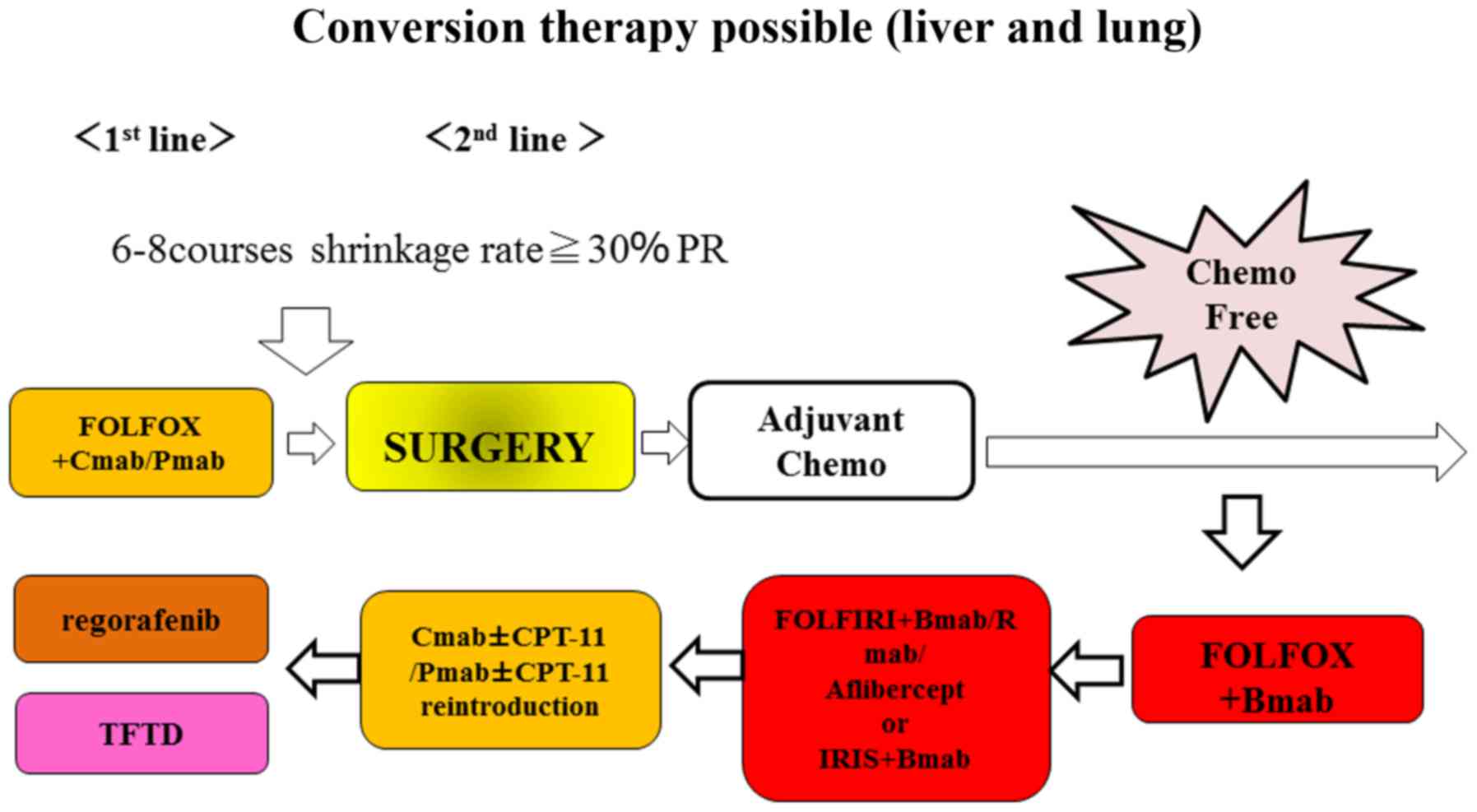
mCRC (Fig. 9).
We thus propose a novel treatment strategy for
personalized medicine in patients in whom anti-EGFR monoclonal
antibodies are used as first-line treatment. If the rate of tumor
shrinkage indicates PD, patients with RAS wild-type disease (BRAF
mutant-type) may switch to the combination of FOLFOXIRI plus
bevacizumab to provide a stronger therapeutic agent. If the rate of
tumor shrinkage indicates SD, these patients may be switched to
therapy with anti-VEGF monoclonal antibodies plus FOLFOX to provide
a maintenance dose of the therapeutic agent (Fig. 10).
Recent National Comprehensive Cancer Network
(35) and ESMO guidelines
(31) have recommended that
anti-PDL-1 antibody treatment should be used in patients with high
micro-satellite instability. Therefore, physicians should consider
not only RAS-type disease, but also diseases with other individual
genomic types.
In conclusion, at present in RAS wild-type patients,
it is important to evaluate the rate of tumor shrinkage from the
beginning of the first-line treatment until 6–8 courses of
anti-EGFR monoclonal antibody administration have been completed,
and it is important to determine whether conversion therapy is
possible. In addition, it is important to determine whether
anti-EGFR monoclonal antibodies are effective. In the case of
localized liver metastasis, anti-VEGF monoclonal antibodies plus
FOLFOXIRI may be used after 6–8 courses of anti-EGFR monoclonal
antibodies plus FOLFOX in order to make the treatment more
effective and obtain stable disease. In this case, we expect that
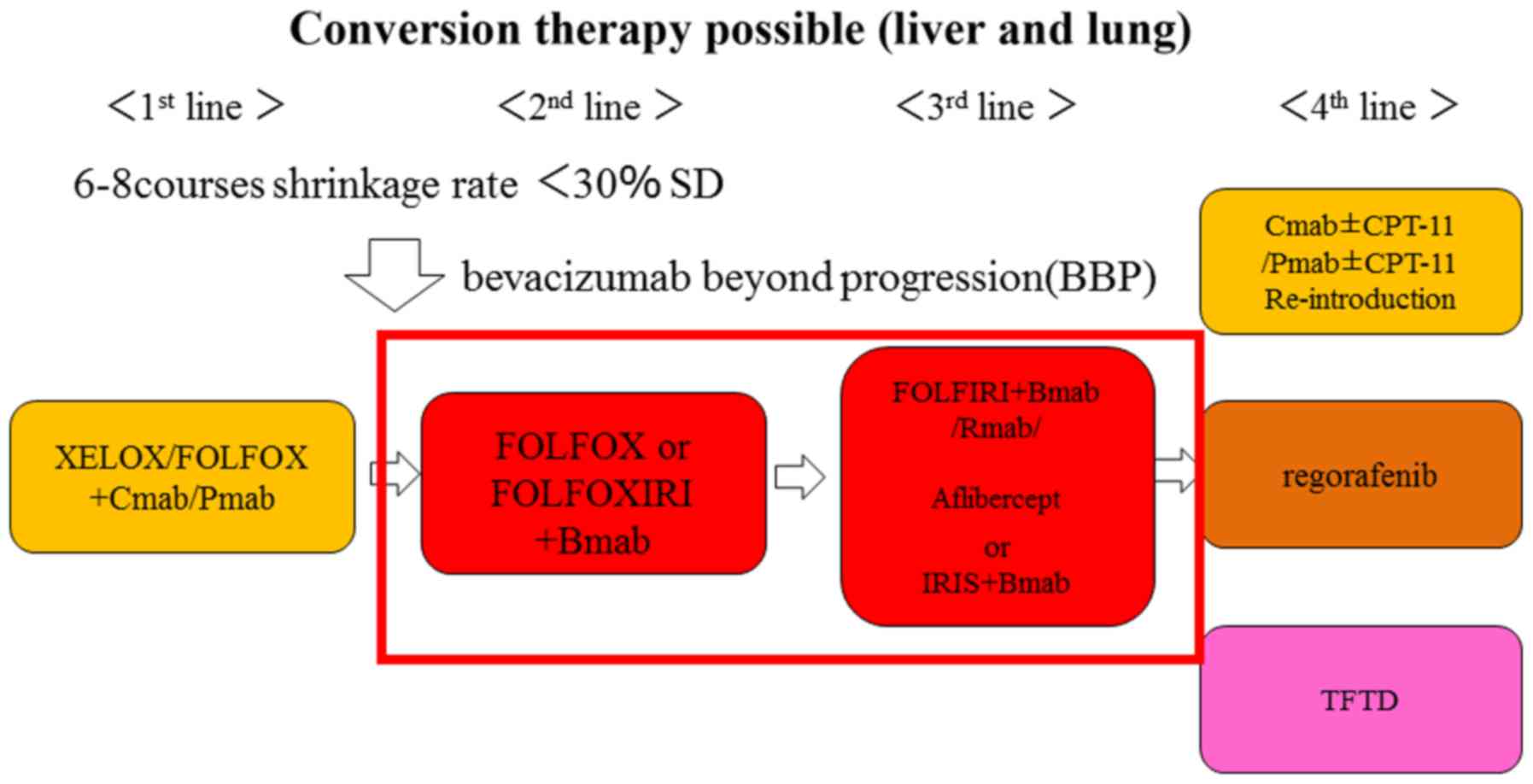
the use of this novel strategy of anti-VEGF monoclonal antibodies
plus FOLFOXIRI or bevacizumab beyond progression will be possible,
and furthermore, that anti-VEGF monoclonal antibodies of the new
generation, such as ramucirumab and aflibercept, will also be used.
In addition, we expect that strategic treatments, including
re-challenge and re-introduction with anti-EGFR monoclonal
antibodies, will further prolong the OS and PFS times of these
patients with mCRC.
Acknowledgments
Not applicable.
Notes
[1]
Funding
No funding was received.
[2] Competing
interests
KYo has received honoraria for lectures from Chugai
Pharmaceutical Co., Ltd., Taiho Pharmaceutical Co., Ltd., Takeda
Pharmaceutical Co., Ltd., Eli Lilly and Company, Daiichi Sankyo
Co., Ltd., Ono Pharmaceutical Co., Ltd., Merck Serono Co., Ltd.,
Novartis Pharma K.K. and Sanofi K.K., and research funding from
Ajinomoto Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co.,
Ltd., Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd.,
Taiho Pharmaceutical Co., Ono Pharmaceutical Co., and Yakult Honsha
Co., Ltd., outside the submitted work. TaT has received honoraria
for lectures from Takeda Pharmaceutical Co., Ltd. All remaining
authors declare that they have no conflicts of interest.
[3] Availability
of data and materials
All data generated or analyzed during this study are
included in this published article.
[4] Authors'
contributions
NM and TaT conceived the study and its design. NM,
TaT, SM, ToT, HI, YT, KYa and KYo acquired the data. NM analyzed
and interpreted the data and drafted the manuscript. NM, TaT, and
KYo performed critical revision of the manuscript. KYo supervised
the study. All authors have read and approved the final
manuscript.
[5] Ethics
approval and consent to participate
All procedures performed in studies involving human
participants were in accordance with the ethical standards of the
institutional and/or national research committee and with the 1975
Declaration of Helsinki and its later amendments or comparable
ethical standards. Informed consent was obtained from all
individual participants included in the study.
[6] Consent for
publication
Not applicable.
References
|
1
|
Fernandez FG, Drebin JA, Linehan DC,
Dehdashti F, Siegel BA and Strasberg SM: Five-year survival after
resection of hepatic metastases from colorectal cancer in patients
screened by positron emission tomography with F-18
fluorodeoxyglucose (FDG-PET). Ann Surg. 240:438–450. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
O'Neil BH and Goldberg RM: Innovations in
chemotherapy for metastatic colorectal cancer: An update of recent
clinical trials. Oncologist. 13:1074–1083. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Grothey A, Hedrick EE, Mass RD, Sarkar S,
Suzuki S, Ramanathan RK, Hurwitz HI, Goldberg RM and Sargent DJ:
Response-independent survival benefit in metastatic colorectal
cancer: A comparative analysis of N9741 and AVF2107. J Clin Oncol.
26:183–189. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tyagi P and Grothey A: Commentary on a
phase III trial of bevacizumab plus XELOX or FOLFOX4 for first-line
treatment of metastatic colorectal cancer: The NO16966 trial. Clin
Colorectal Cancer. 6:261–264. 2006. View Article : Google Scholar
|
|
5
|
Arkenau HT, Arnold D, Cassidy J,
Diaz-Rubio E, Douillard JY, Hochster H, Martoni A, Grothey A, Hinke
A, Schmiegel W, et al: Efficacy of oxaliplatin plus capecitabine or
infusional fluorouracil/leucovorin in patients with metastatic
colorectal cancer: A pooled analysis of randomized trials. J Clin
Oncol. 26:5910–5917. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Falcone A, Ricci S, Brunetti I, Pfanner E,
Allegrini G, Barbara C, Crinò L, Benedetti G, Evangelista W,
Fanchini L, et al Gruppo Oncologico Nord Ovest: Phase III trial of
infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan
(FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and
irinotecan (FOLFIRI) as first-line treatment for metastatic
colorectal cancer: The Gruppo Oncologico Nord Ovest. J Clin Oncol.
25:1670–1676. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Van Cutsem E, Köhne CH, Hitre E, Zaluski
J, Chang Chien CR, Makhson A, D'Haens G, Pintér T, Lim R, Bodoky G,
et al: Cetuximab and chemotherapy as initial treatment for
metastatic colorectal cancer. N Engl J Med. 360:1408–1417. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bokemeyer C, Bondarenko I, Makhson A,
Hartmann JT, Aparicio J, de Braud F, Donea S, Ludwig H, Schuch G,
Stroh C, et al: Fluorouracil, leucovorin, and oxaliplatin with and
without cetuximab in the first-line treatment of metastatic
colorectal cancer. J Clin Oncol. 27:663–671. 2009. View Article : Google Scholar
|
|
9
|
Saltz LB, Clarke S, Díaz-Rubio E,
Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS,
Rivera F, et al: Bevacizumab in combination with oxaliplatin-based
chemotherapy as first-line therapy in metastatic colorectal cancer:
A randomized phase III study. J Clin Oncol. 26:2013–2019. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schwartzberg LS, Rivera F, Karthaus M,
Fasola G, Canon JL, Hecht JR, Yu H, Oliner KS and Go WY: PEAK: A
randomized, multicenter phase II study of panitumumab plus modified
fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) or bevacizumab
plus mFOLFOX6 in patients with previously untreated, unresectable,
wild-type KRAS exon 2 metastatic colorectal cancer. J Clin Oncol.
32:2240–2247. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Venook AP, Niedzwiecki D, Lenz HJ,
Innocenti F, Fruth B, Meyerhardt JA, Schrag D, Greene C, O'Neil BH,
Atkins JN, et al: Effect of first-line chemotherapy combined with
cetuximab or bevacizumab on overall survival in patients with KRAS
wild-type advanced or metastatic colorectal cancer: A randomized
clinical trial. JAMA. 317:2392–2401. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Heinemann V, von Weikersthal LF, Decker T,
Kiani A, Vehling-Kaiser U, Al-Batran SE, Heintges T, Lerchenmüller
C, Kahl C, Seipelt G, et al: FOLFIRI plus cetuximab versus FOLFIRI
plus bevacizumab as first-line treatment for patients with
metastatic colorectal cancer (FIRE-3): A randomised, open-label,
phase 3 trial. Lancet Oncol. 15:1065–1075. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Stintzing S, Modest DP, Rossius L, Lerch
MM, von Weikersthal LF, Decker T, Kiani A, Vehling-Kaiser U,
Al-Batran SE, Heintges T, et al FIRE-3 investigators: FOLFIRI plus
cetuximab versus FOLFIRI plus bevacizumab for metastatic colorectal
cancer (FIRE-3): A post-hoc analysis of tumour dynamics in the
final RAS wild-type subgroup of this randomised open-label phase 3
trial. Lancet Oncol. 17:1426–1434. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tejpar S, Stintzing S, Ciardiello F,
Tabernero J, Van Cutsem E, Beier F, Esser R, Lenz HJ and Heinemann
V: Prognostic and predictive relevance of primary tumor location in
patients with RAS wild-type metastatic colorectal cancer:
Retrospective analyses of the CRYSTAL and FIRE-3 trials. JAMA
Oncol. Oct 10–2016.Epub ahead of print. PubMed/NCBI
|
|
15
|
Folprecht G, Gruenberger T, Bechstein WO,
Raab HR, Lordick F, Hartmann JT, Lang H, Frilling A, Stoehlmacher
J, Weitz J, et al: Tumour response and secondary resectability of
colorectal liver metastases following neoadjuvant chemotherapy with
cetuximab: The CELIM randomised phase 2 trial. Lancet Oncol.
11:38–47. 2010. View Article : Google Scholar
|
|
16
|
Van Loon K, Wigler D, Niedzwiecki D,
Venook AP, Fuchs C, Blanke C, Saltz L, Goldberg RM and Meyerhardt
JA: Comparison of dietary and lifestyle habits among stage III and
metastatic colorectal cancer patients: Findings from CALGB 89803
and CALGB 80405. Clin Colorectal Cancer. 12:95–102. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Elez E, Argilés G and Tabernero J:
First-line treatment of metastatic colorectal cancer: Interpreting
FIRE-3, PEAK, and CALGB/SWOG 80405. Curr Treat Options Oncol.
16:522015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wanebo HJ, Rao B, Pinsky CM, Hoffman RG,
Stearns M, Schwartz MK and Oettgen HF: Preoperative
carcinoembryonic antigen level as a prognostic indicator in
colorectal cancer. N Engl J Med. 299:448–451. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Moertel CG, O'Fallon JR, Go VL, O'Connell
MJ and Thynne GS: The preoperative carcinoembryonic antigen test in
the diagnosis, staging, and prognosis of colorectal cancer. Cancer.
58:603–610. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Martin EW Jr, James KK, Hurtubise PE,
Catalano P and Minton JP: The use of CEA as an early indicator for
gastrointestinal tumor recurrence and second-look procedures.
Cancer. 39:440–446. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cooper MJ, Mackie CR, Skinner DB and
Moossa AR: A reappraisal of the value of carcinoembryonic antigen
in the management of patients with various neoplasms. Br J Surg.
66:120–123. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Steele G Jr, Ellenberg S, Ramming K,
O'Connell M, Moertel C, Lessner H, Bruckner H, Horton J, Schein P,
Zamcheck N, et al: CEA monitoring among patients in
multi-institutional adjuvant G.I. therapy protocols. Ann Surg.
196:162–169. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kuusela P, Jalanko H, Roberts P, Sipponen
P, Mecklin JP, Pitkänen R and Mäkelä O: Comparison of CA19-9 and
carcinoembryonic antigen (CEA) levels in the serum of patients with
colorectal diseases. Br J Cancer. 49:135–139. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Filella X, Molina R, Grau JJ, Piqué JM,
Garcia-Valdecasas JC, Astudillo E, Biete A, Bordas JM, Novell A,
Campo E, et al: Prognostic value of CA 19.9 levels in colorectal
cancer. Ann Surg. 216:55–59. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kouri M, Pyrhönen S and Kuusela P:
Elevated CA19-9 as the most significant prognostic factor in
advanced colorectal carcinoma. J Surg Oncol. 49:78–85. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nakayama T, Watanabe M, Teramoto T and
Kitajima M: CA19-9 as a predictor of recurrence in patients with
colorectal cancer. J Surg Oncol. 66:238–243. 1997. View Article : Google Scholar
|
|
27
|
Prager GW, Braemswig KH, Martel A, Unseld
M, Heinze G, Brodowicz T, Scheithauer W, Kornek G and Zielinski CC:
Baseline carcinoembryonic antigen (CEA) serum levels predict
bevacizumab-based treatment response in metastatic colorectal
cancer. Cancer Sci. 105:996–1001. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bramswig KH, Poettler M, Unseld M, Wrba F,
Uhrin P, Zimmermann W, Zielinski CC and Prager GW: Soluble
carcinoembryonic antigen activates endothelial cells and tumor
angiogenesis. Cancer Res. 73:6584–6596. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Carmeliet P: Angiogenesis in life, disease
and medicine. Nature. 438:932–936. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ocvirk J, Brodowicz T, Wrba F, Ciuleanu
TE, Kurteva G, Beslija S, Koza I, Pápai Z, Messinger D, Yilmaz U,
et al: Cetuximab plus FOLFOX6 or FOLFIRI in metastatic colorectal
cancer: CECOG trial. World J Gastroenterol. 16:3133–3143. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Van Cutsem E, Cervantes A, Adam R, Sobrero
A, Van Krieken JH, Aderka D, Aranda Aguilar E, Bardelli A, Benson
A, Bodoky G, et al: ESMO consensus guidelines for the management of
patients with metastatic colorectal cancer. Ann Oncol.
27:1386–1422. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cremolini C, Loupakis F, Antoniotti C,
Lupi C, Sensi E, Lonardi S, Mezi S, Tomasello G, Ronzoni M,
Zaniboni A, et al: FOLFOXIRI plus bevacizumab versus FOLFIRI plus
bevacizumab as first-line treatment of patients with metastatic
colorectal cancer: Updated overall survival and molecular subgroup
analyses of the open-label, phase 3 TRIBE study. Lancet Oncol.
16:1306–1315. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sasaki Y, Osada S, Mori R, Imai H, Tanaka
Y, Matsuhashi N, Okumura N, Nonaka K, Takahashi T and Yoshida K:
Determining timing of hepatectomy for colorectal cancer with
distant metastasis according to imaging-based tumor shrinkage
ratio. Int J Med Sci. 10:1231–1241. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sasaki Y, Osada S, Matsui S, Imai H,
Tanahashi T, Tanaka Y, Matsuhashi N, Okumura N, Yamaguchi K and
Yoshida K: Preoperative chemotherapy can change the surgical
procedure for hepatectomy in patients with liver metastasis of
colorectal cancer. Anticancer Res. 35:5485–5489. 2015.PubMed/NCBI
|
|
35
|
Benson AB III, Venook AP, Cederquist L,
Chan E, Chen YJ, Cooper HS, Deming D, Engstrom PF, Enzinger PC,
Fichera A, et al: Colon Cancer, Version 1.2017, NCCN Clinical
Practice Guidelines in Oncology. J Natl Compr Canc Netw.
15:370–398. 2017. View Article : Google Scholar : PubMed/NCBI
|