Introduction
Hepatocellular carcinoma (HCC) is a common
malignancy with an increasing global incidence rate, and was
reported to be the second leading cause of cancer-associated
mortality worldwide in 2012 (1).
Surgical intervention, including resection and liver
transplantation, is the most effective strategy for improving
5-year survival rates of patients with HCC (2). Unfortunately, the proportion of
patients with HCC who receive surgery for treatment remains at ~22%
(3). This is primarily because
numerous patients with HCC are not candidates for resection, due to
insufficient liver function at the time of diagnosis. Infection
with the hepatitis B virus (HBV) is not only responsible for almost
half of all new HCC cases around the world each year (4), but also leads to serious liver
cirrhosis in patients with underlying HCC. In patients with
unresectable HCC, chemotherapy is the main strategy used to prevent
tumor progression at present.
Doxorubicin (DOX) is a cytotoxic anthracycline
antibiotic with susbtantial treatment potential in a broad variety
of solid neoplasms (5). The major
mechanism underlying the tumor-inhibiting activity of DOX is
intercalation into primary double-stranded DNA, which blocks the
interaction between DNA and RNA polymerases and results in impaired
protein synthesis (6). In 1975,
Olweny et al (7) first
reported that 11 of 14 treated patients responded, with three
patients showing complete tumor regression following two, three and
five courses of DOX, respectively (7). Although DOX is considered a standard
cytostatic agent for the treatment of HCC, response rates are
typically low (8). Furthermore,
DOX treatment may cause multidirectional cytotoxic effects,
including cardiotoxicity, fever, urticarial and vomiting. Recently,
several clinical studies have presented favorable safety profiles
for transarterial chemoembolization (TACE) using DOX-eluting beads,
compared with conventional TACE or oral chemotherapy. In 2002,
Llovet et al (9) revealed
that TACE combined with DOX improved the survival of certain
patients with HCC, while decreasing the rate of cardiotoxic effects
(9). TACE combined with
DOX-eluting beads has become a common treatment method in patients
with primary and metastatic liver cancer. The advantage of
DOX-eluting beads is the limited toxicity to the normal liver
parenchyma and other organs. Indeed, Forner et al (10) reported that the objective tumor
response rate in patients with HCC following TACE with DOX-eluting
beads was >80%. Furthermore, the 1, 3, 4, and 5-year survival
rates were 89.7%, 67.8%, 50.8%, and 33.9% in patients with
Barcelona clinic liver cancer (BCLC) stage A (11), respectively (and 88.2%, 64.4%,
47.3%, and 39.4% patients with BCLC stage B, respectively)
following treatment with DOX-eluting beads (11).
However, DOX resistance still is a major challenge
faced during the treatment of HCC. Therefore, the identification of
novel molecular target is essential to improve the efficacy of Dox
chemotherapy. Epithelial-mesenchymal transition (EMT) is known to
be a primary mechanism underlying resistance to chemotherapy in
HCC. During EMT, expression of the desmosome protein desmoplakin,
the cell-adhesion molecule epithelial (E-)cadherin and the tight
junction protein claudin-1 is inhibited in HCC cells, while
neural-cadherin, several matrix metalloproteinases (MMPs), the
intermediate filament protein vimentin and a number of
transcription factors, including snail family transcription
repressor 1, snail family transcription repressor 2, zinc finger
E-box binding homeobox (ZEB)1, ZEB2 and Twist are overexpressed
(12,13). Furthermore, EMT triggers the
activation of the transforming growth factor (TGF)-β/SMAD,
Wnt/β-catenin, mitogen-activated protein kinase/extracellular
signal-regulated kinase (ERK), phosphoinositide 3-kinase
(PI3K)/protein kinase B (Akt) and Notch signaling pathways
(14). These EMT-induced
characteristics significantly increase the resistance of HCC cells
to apoptosis and chemotherapy.
Fascin-1 (FSCN-1) is an actin bundling protein that
serves key functions in cell-cell interactions, adhesion and
motility via regulating the function of filopodial protrusions and
microfilaments (15). Aberrant
FSCN-1 expression has been observed in various types of cancer, and
has been demonstrated to be associated with a more aggressive
clinical course. Yoder et al (16) revealed that FSCN-1 is primarily
overexpressed in estrogen receptor-negative breast cancer tissues,
and that positive FSCN-1 expression is associated with decreased
mean tumor-free survival and overall survival time (16). Likewise, in colorectal
adenocarcinomas, strong and diffuse FSCN-1 expression is associated
with disease progression and reduced survival (17). Furthermore, similar prognostic
significance of FSCN-1 expression has been confirmed in renal cell
carcinoma (18), gastric
adenocarcinoma (19), laryngeal
squamous cell carcinoma (20) and
ovarian carcinoma (21). There is
also evidence suggesting that FSCN-1 may be involved in regulating
the EMT process. During vertebrate development, FSCN-1 is
principally expressed throughout the embryonic and mesenchymal
tissues, as well as in the developing nervous system (22). Suppression of microRNA (miR)-145 in
breast cancer regulates cell migration by targeting FSCN-1 and
inhibiting EMT (23). Furthermore,
FSCN-1 expression was associated with suppressed E-cadherin
expression and increased invasiveness, thus serving as a promoter
of cancer aggressiveness in HCC (24). Therefore, investigation of FSCN-1
expression patterns and its association with EMT in HCC is
necessary.
Furthermore, previous investigations have revealed
that FSCN-1 promotes cancer metastasis and recurrence primarily
through increasing cell motility (25). Furthermore, several studies have
demonstrated that FSCN-1 knockdown suppresses cellular
proliferation and cloning efficiency (17,26).
FSCN-1 expression has also been implicated in the production of
MMP9, which is induced by activation of the tumor necrosis factor-α
signaling pathway in cholangiocarcinoma (27). However, up to now, no studies have
investigated the associations between FSCN-1 and tumor drug
resistance in HCC cells.
In the present study, our group investigated FSCN-1
expression patterns and the association between FSCN-1 and DOX
resistance in HCC cell lines. HCC cells with increased expression
of FSCN-1 were more resistant to DOX. Knockdown of FSCN-1 by small
interfering (si)RNA reduced the resistance of HCC to DOX. In
addition, FSCN-1 promoted EMT in HCC cells. FSCN-1 siRNA reversed
hypoxia-induced EMT and DOX resistance, while suppressing EMT using
Twist siRNA blocked the effect of FSCN-1 siRNA. In conclusion, the
results of the present study demonstrated that FSCN-1 conferred
doxorubicin resistance in HCC cells through the promotion of
EMT.
Materials and methods
Cell lines and reagents
Five human HCC cell lines (SNU387, Huh7, Hep3B, and
SNU449) were purchased from the Type Culture Collection of the
Chinese Academy of Sciences (Shanghai, China). Huh7 cells were
grown in Dulbecco's modified Eagle's medium (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), Hep3B cells were cultured in
minimum essential medium, and SNU387, SNU449 cells were incubated
in RPMI-1640 complete medium (Gibco; Thermo Fisher Scientific,
Inc.). All cell media were supplemented with 10% fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc.) and 100 U/ml streptomycin
and penicillin. All cell lines were incubated at 37°C in 5%
CO2. DOX and DAPI were purchased from Sigma-Aldrich;
Merck KGaA (Darmstadt, Germany). The following antibodies were
used: FSCN-1 (1:1,000, ab126772), while antibodies against β-actin
(1:1,000, ab8227), Twist (1:1,000, ab50581), E-cadherin (1:1,000,
ab40772), Vimentin (1:1,000, ab8978) and horseradish peroxidase
(HRP)-conjugated goat anti-rabbit secondary antibodies (1:2,000,
ab6721), all of which were purchased from Abcam (Cambridge, MA,
USA), whereas HRP-conjugated goat anti-mouse secondary antibodies
(1:2,000, 7076) were purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA).
siRNA transfection
Untreated SNU387, Huh7, Hep3B and SNU449 cells were
plated at a density of 1×105/well in 2 ml of their corresponding
media. Once the cells reached 20-30% confluence, 50 nM siRNA
(targeting FSCN-1 or Twist) or a negative control siRNA (Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) combined with 50 μl
Lipofectamine 2000 transfection reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) were added into each well, following the
manufacturer's protocol. The siRNA sequences were as follows:
FSCN-1, sense 5′-GCU GCUACUUUGACAUCGATT-3′, antisense 5′-UCGAUGUC
AAAGUAGCAGCTT-3′; Twist1, sense 5′-GGUACAUCGACUUCCUCUATT-3′,
antisense 5′-UAGAGGAAGUCGAUGUACCTT-3′; negative control siRNA,
sense 5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense
5′-ACGUGACACGUUCGGAGAATT-3′. The transfection medium (Opti-MEM;
Gibco; Thermo Fisher Scientific, Inc.) was replaced with complete
medium 6 h following transfection, and the cells were incubated for
2 h. All treatments were initiated 24 h after transfection.
Cell viability assay
Following siRNA transfection, SNU387, Huh7, Hep3B
and SNU449 cells were added to 96-well plates at a density of
3×103/well and incubated for 24 h. Following
administration of DOX [half maximal inhibitory concentration
(IC50) value] or the same amount of phosphate-buffered
saline (PBS), cell viability was measured using Cell Counting Kit-8
(CCK-8; Dojindo Molecular Technologies, Inc., Kumamoto, Japan)
according to the manufacturer's protocol at an optical density of
450 nm using a microplate reader (Elx800; BioTek Instruments, Inc.,
Winooski, VT, USA) following incubation for 24, 48, and 72 h at
37°C. To induce hypoxia, the HCC cells were incubated in an
atmosphere of 5% CO2, 1% O2 and 94%
N2 at 37°C for 12 h.
Protein extraction and western
blotting
The protein from HCC cells was harvested using cell
lysis buffer (10X) (Cell Signaling Technology, Inc.) at 4°C
following the manufacturer's protocol. Western blot analysis was
conducted according to a standard protocol. Briefly, a BCA Protein
assay kit (Pierce; Thermo Fisher Scientific, Inc.) was utilized to
detect protein concentrations using a Bradford assay (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). A total of 40 μg/10
μl protein was added per lane and separated by 10% SDS-PAGE
at 80-120 V. Subsequently, the protein was transferred to 0.45
μm polyvinylidene fluoride membranes (EMD Millipore,
Billerica, MA, USA) at 350 mA for 90 min. Following incubating with
5% non-fat milk prepared with Tris-buffered saline containing 0.05%
Tween-20 (TBST) for 1 h at room temperature, the membrane was
incubated with the primary antibodies overnight at 4°C. Following
washing three times with TBST, the membrane was further incubated
with the secondary antibodies for 1 h at room temperature. Finally,
the protein concentrations on the membrane were determined using
SuperSignal West Pico Chemiluminescent Substrate (Pierce; Thermo
Fisher Scientific, Inc.).
EDU assay
A total of 1×105 cells were plated into
24-well plates and incubated for 24 h. Following treatment as
aforementioned, EdU (50 μM; Invitrogen; Thermo Fisher
Scientific, Inc.) was added into the cultures. Following 2-h
incubation at 37°C, the cells were fixed with 4% methanol-free
formaldehyde in PBS for 20 min at 4°C, followed by treatment with
0.1% Triton X-100 in PBS for 20 min.
Immunofluorescence
HCC cells were seeded into 48-well plates at a
density of 5×103 cells/well. Cells were fixed with 4%
formaldehyde for 15 min at 37°C, washed with PBS, blocked with 5%
bovine serum albumin (Sangon Biotech Co., Ltd., Shanghai, China)
for 30 min at room temperature, and incubated with anti-E-cadherin
(1:200, 3195) or anti-human vimentin (1:200, 5741) primary
antibodies (Cell Signaling Technology, Inc.) at 4°C overnight. The
cells were then incubated with foat anti-rabbit immunoglobulin G
H&L (Alexa Fluor® 488) antibodies (Abcam; 1:1000,
ab150077) at 4°C for 2 h. Nuclear staining was performed with DAPI
(Sigma-Aldrich; Merck KGaA) at room temperature for 2 min.
Following washing with PBS three times, cells were observed using
an inverted fluorescence microscope (Olympus Corporation, Tokyo,
Japan).
Statistical analysis
Experimental data is presented as the mean with
standard deviation (SD) or frequency. Two groups were compared
using an unpaired Student's t-tests and multiple groups were
performed using one-way analysis of variance followed by Tukey's
post hoc test. All statistical analysis was performed with the SPSS
software (version 19.0; IBM Corp., Armonk, NY, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
FSCN-1 expression is positively
associated with DOX resistance in HCC cell lines
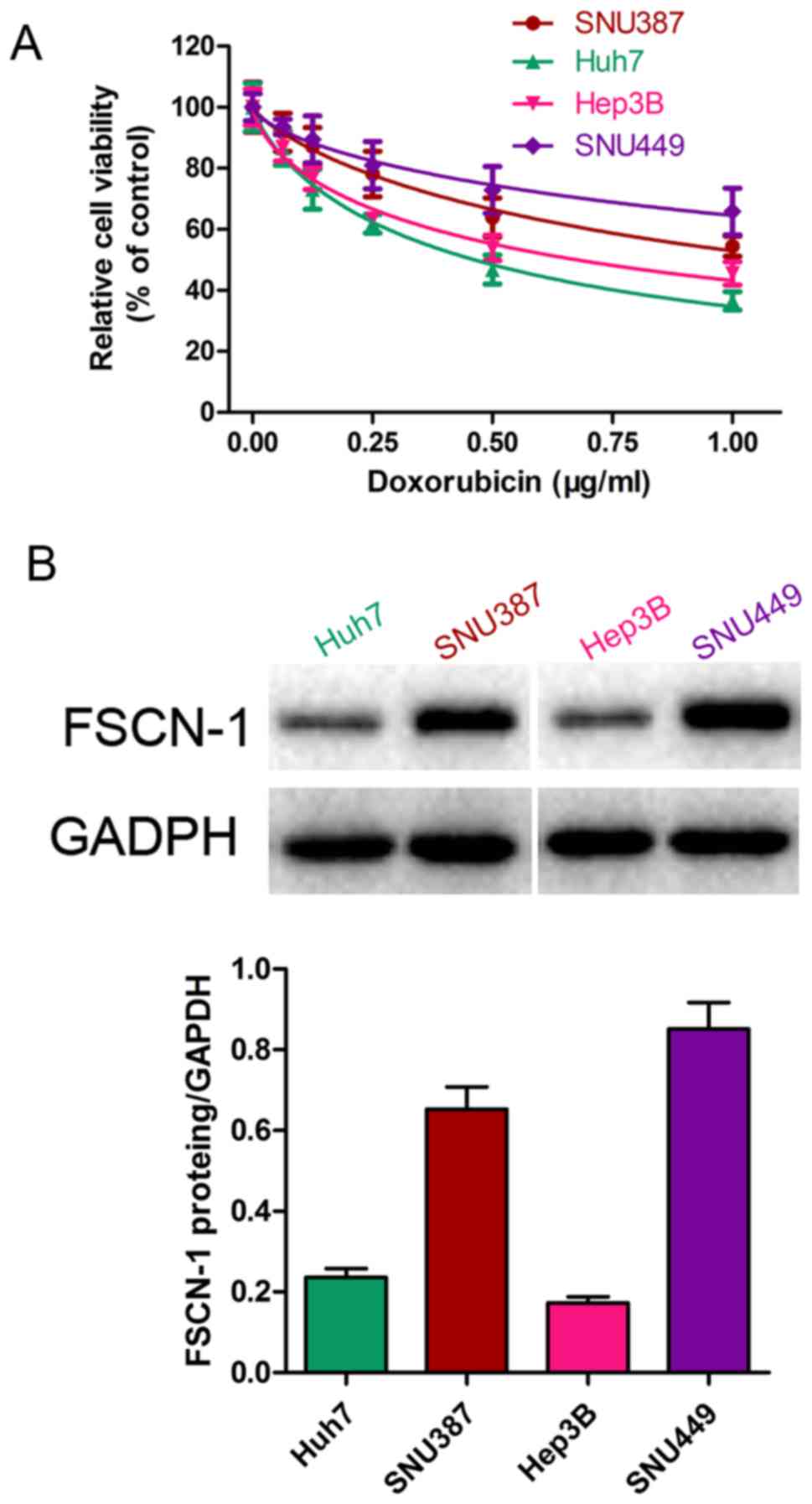
The sensitivity of five HCC cell lines (SNU387,
Huh7, Hep3B and SNU449) to DOX was estimated by the change in cell
viability following drug administration for 48 h, using a CCK-8
assay. The IC50 of DOX from high to low (thus, from
least sensitive to most sensitive) was SNU449, SNU387, Hep3B and
Huh7 (Fig. 1A). Western blot
analysis revealed that the basal level of FSCN-1 expression
presented a similar trend to the IC50 of DOX (Fig. 1B).
To further investigate the relationship between
FSCN-1 expression and DOX resistance, the expression of FSCN-1 was
knocked down with siRNA (Fig. 2A).
The results of the cell viability (CCK-8) assays and EDU staining
assays revealed that HCC cells with decreased FSCN-1 expression had
lower cell viability (Fig. 2B–E)
and inhibitory DNA copies compared with the control groups
(negative siRNA; Fig. 2F–I). These
results suggested that FSCN-1 expression is positively associated
with DOX resistance in HCC cell lines.
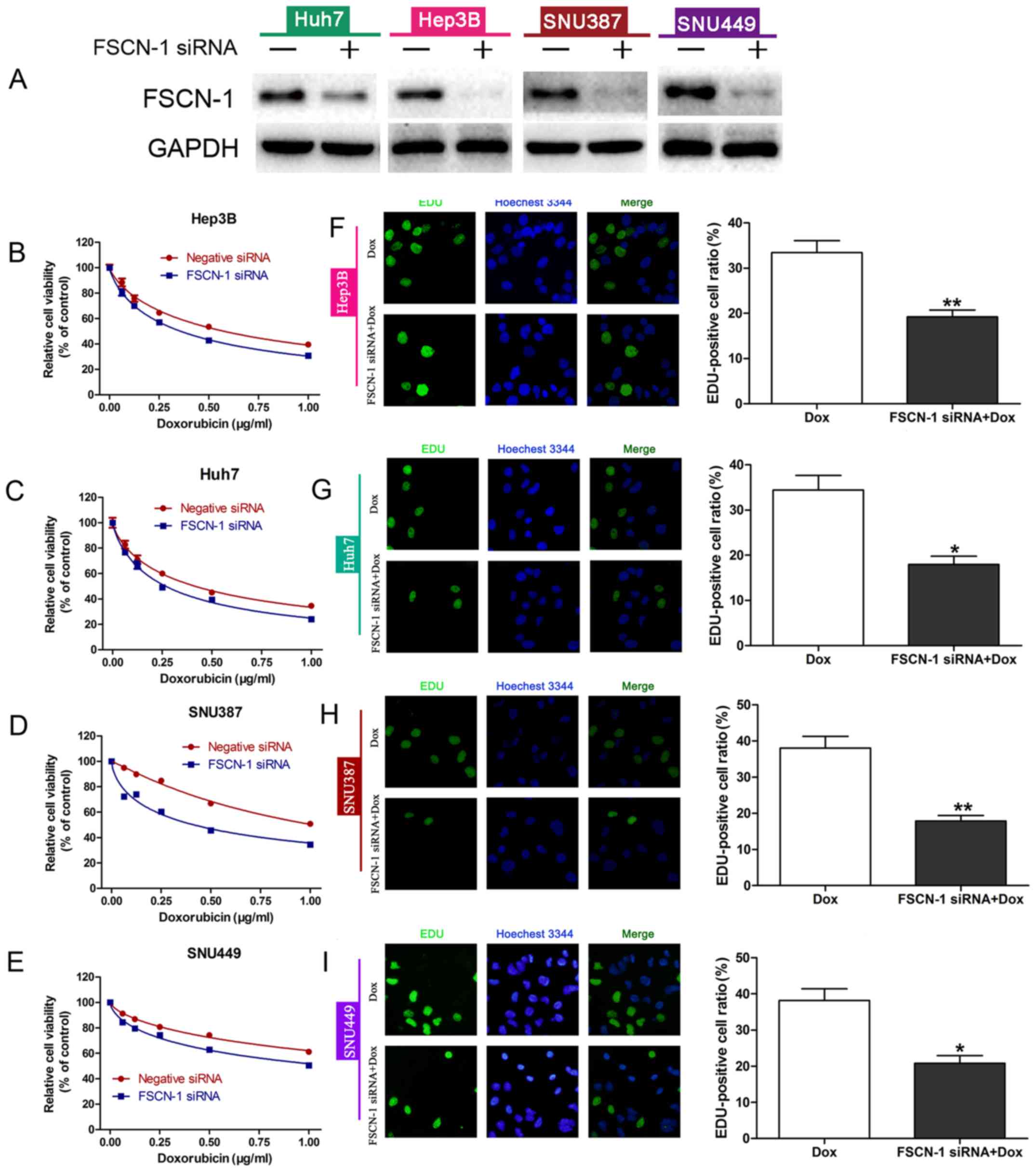 | Figure 2FSCN-1 expression is associated with
DOX resistance in HCC cell lines. (A) Western blot analysis of
FSCN-1 expression in HCC cell lines following siRNA transfection.
Cell viability following FSCN-1 knockdown or transfection with
negative siRNA in (B) Hep3B, (C) Huh7, (D) SNU387 and (E) SNU449
cells in the presence of different concentrations of DOX was
assessed using Cell Counting Kit-8. EDU staining assays (DNA copy
number) following DOX treatment and FSCN-1 knockdown in (F) Hep3B,
(G) Huh7, (H) SNU387 and (I) SNU449 cells compared with the control
(magnification, ×200). DOX concentrations (μg/ml) were as
follows: Hep3B, 0.6751; Huh7, 0.4620; SNU387, 1.154; SNU449, 2.402.
*P<0.05 and **P<0.01 vs. DOX. FSCN-1,
Fascin-1; DOX, doxorubicin; HCC, hepatocellular carcinoma; siRNA,
small interfering RNA. |
FSCN-1 promotes EMT of HCC cells
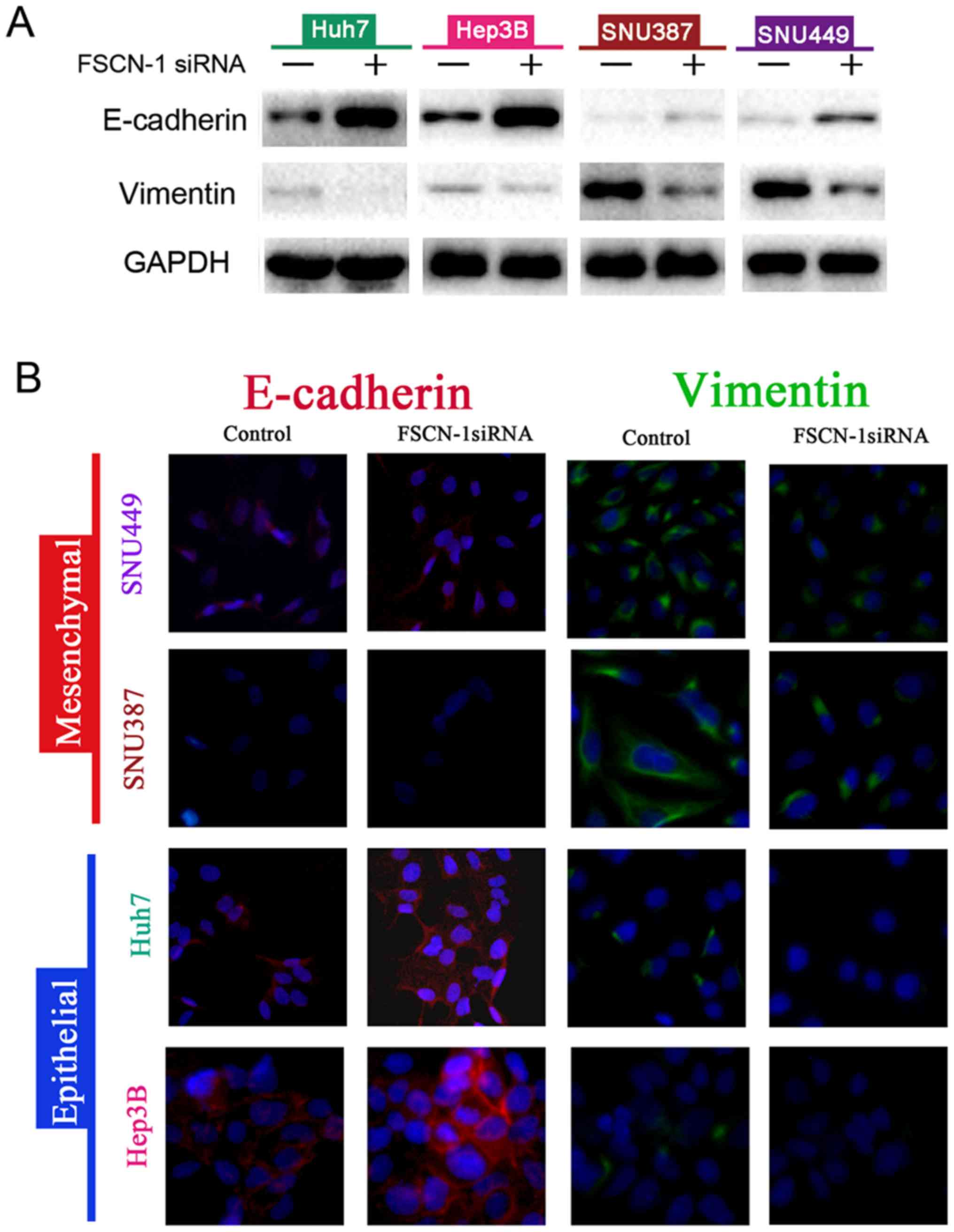
Next, the expression of EMT markers in siRNA-FSCN-1
transfected HCC cell lines was detected. Following inhibition of
FSCN-1 expression, vimentin expression was significantly suppressed
while E-cadherin expression was increased (Fig. 3A). These results were confirmed by
the immunofluorescence experiments (Fig. 3B). Notably, E-cadherin expression
was higher in Huh7 and Hep3B cells, which were sensitive to DOX,
compared with SNU387 and SNU449 cells with DOX resistance, while
the opposite pattern was observed for vimentin expression (Fig. 3A). These data indicated that EMT is
associated with DOX resistance in HCC cells.
FSCN-1 increases HCC resistance to DOX by
promoting EMT
The change in expression of EMT markers was further
investigated in HCC cells following DOX treatment. While vimentin
expression was increased, E-cadherin expression was suppressed
(Fig. 4A). Following FSCN-1
knockdown, DOX-induced EMT of HCC cells was inhibited (Fig. 4A). These results were further
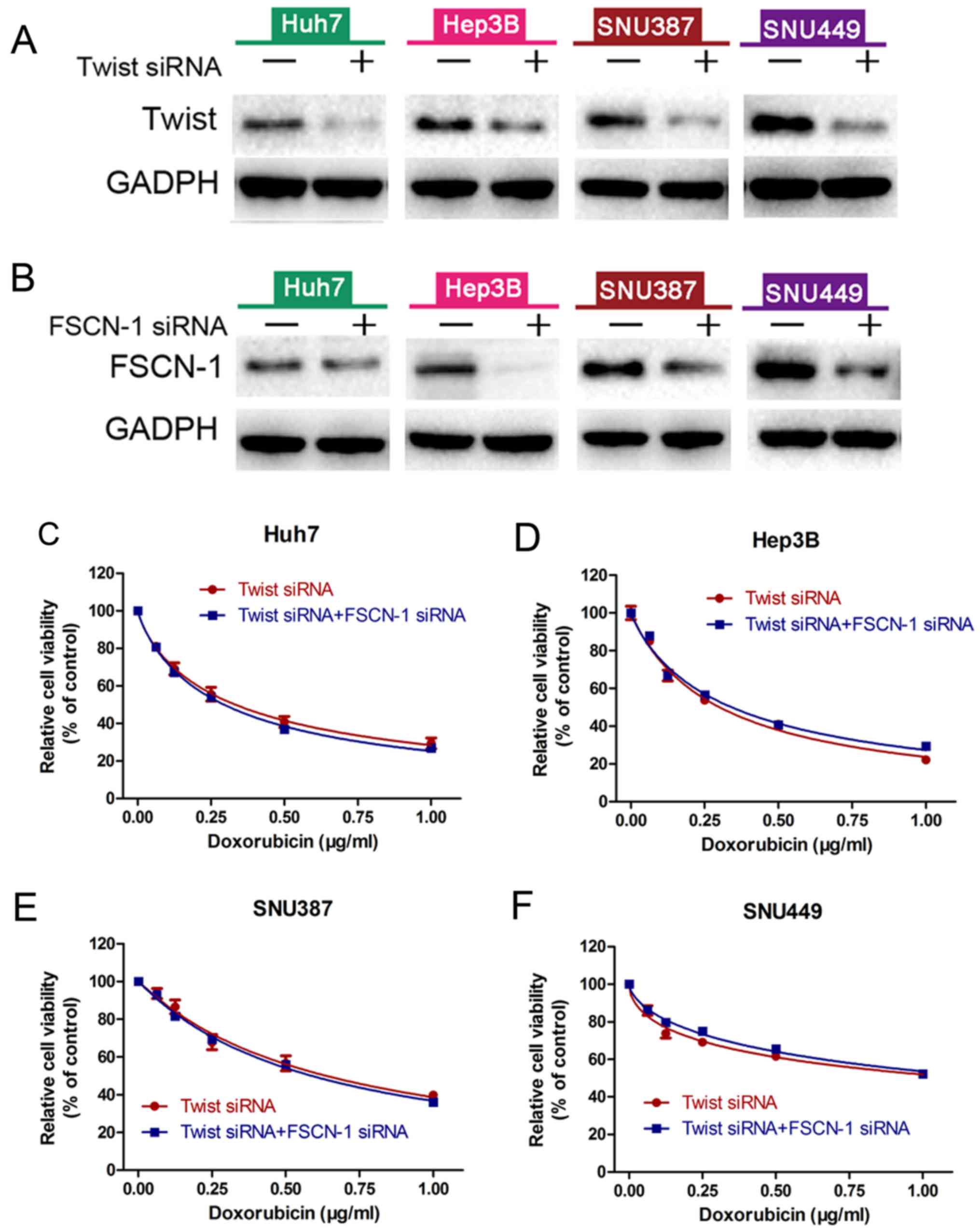
verified through immunofluorescence confocal experiments (Fig. 4B). Twist has been reported to play
an vital role in adriamycin (DOX) induced EMT (28). HCC cells were transfected with
Twist siRNA alone or combined with FSCN-1 siRNA, and then treated
with DOX. There was no difference in cell viability between these
two groups (Fig. 5C-F). FSCN-1
protein expression was detected by western blotting in order to
verify knockdown efficiency (Fig. 5A
and B). These data indicated that FSCN-1 enhances DOX
resistance via promotion of the EMT process in HCC cell lines.
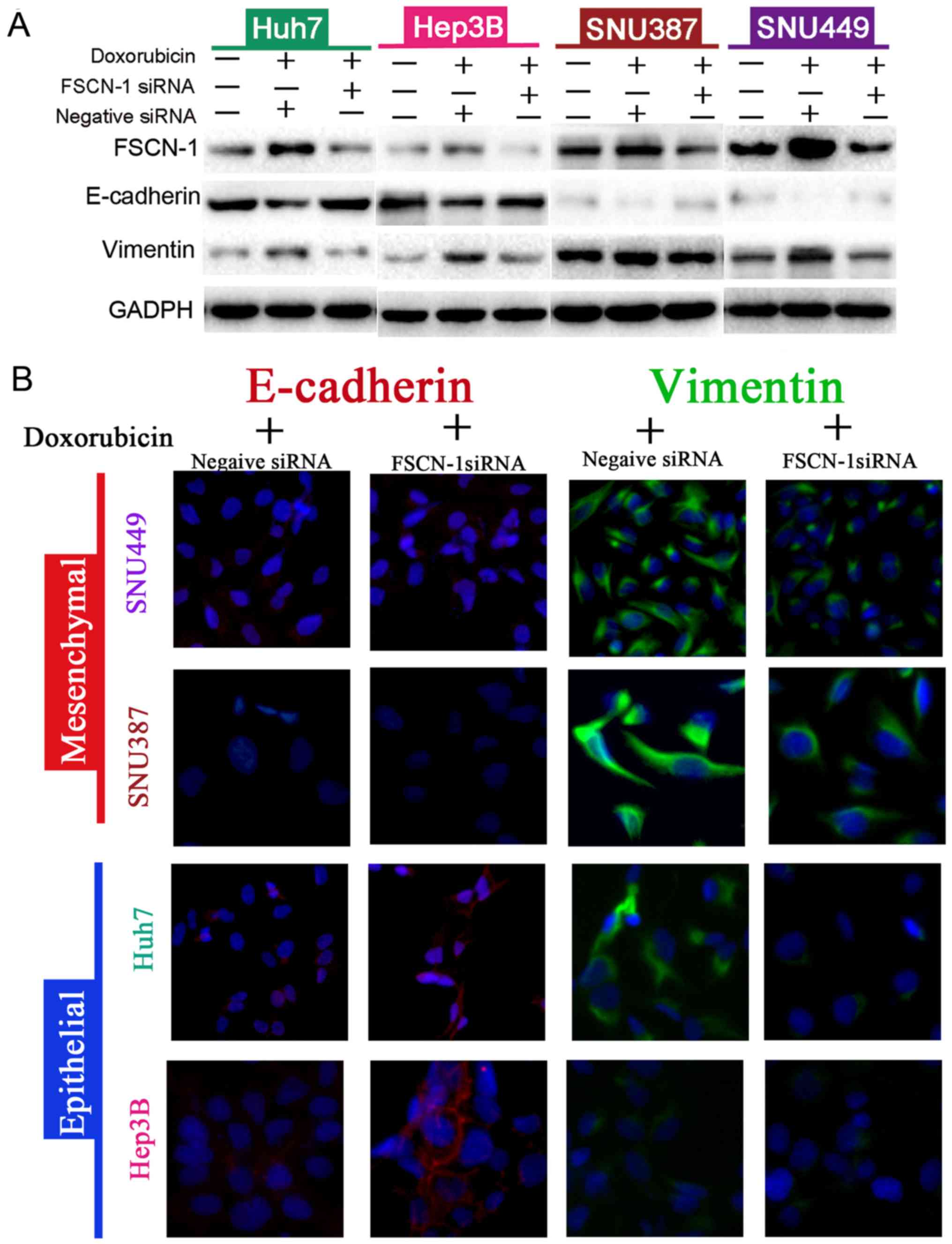 | Figure 4Knockdown of FSCN-1-reverses
doxorubicin-induced EMT in different HCC cell lines. (A) Western
blot analysis of the expression of EMT markers (vimentin and
E-cadherin) in the various HCC cell lines, with or without FSCN-1
knockdown, and in the presence or absence of DOX. (B) Confocal
immunofluorescence images showing the expression of EMT markers
(magnification, ×200). DOX concentrations (μg/ml) were as
follows: Hep3B, 0.6751; Huh7, 0.4620; SNU387, 1.154; SNU449, 2.402.
FSCN-1, Fascin-1; EMT, epithelial-mesenchymal transition; HCC,
hepatocellular carcinoma; E-cadherin, epithelial-cadherin; DOX,
doxorubicin; siRNA, small interfering RNA. |
Knockdown of FSCN-1 reverses EMT and
resistance to DOX of HCC under hypoxic conditions
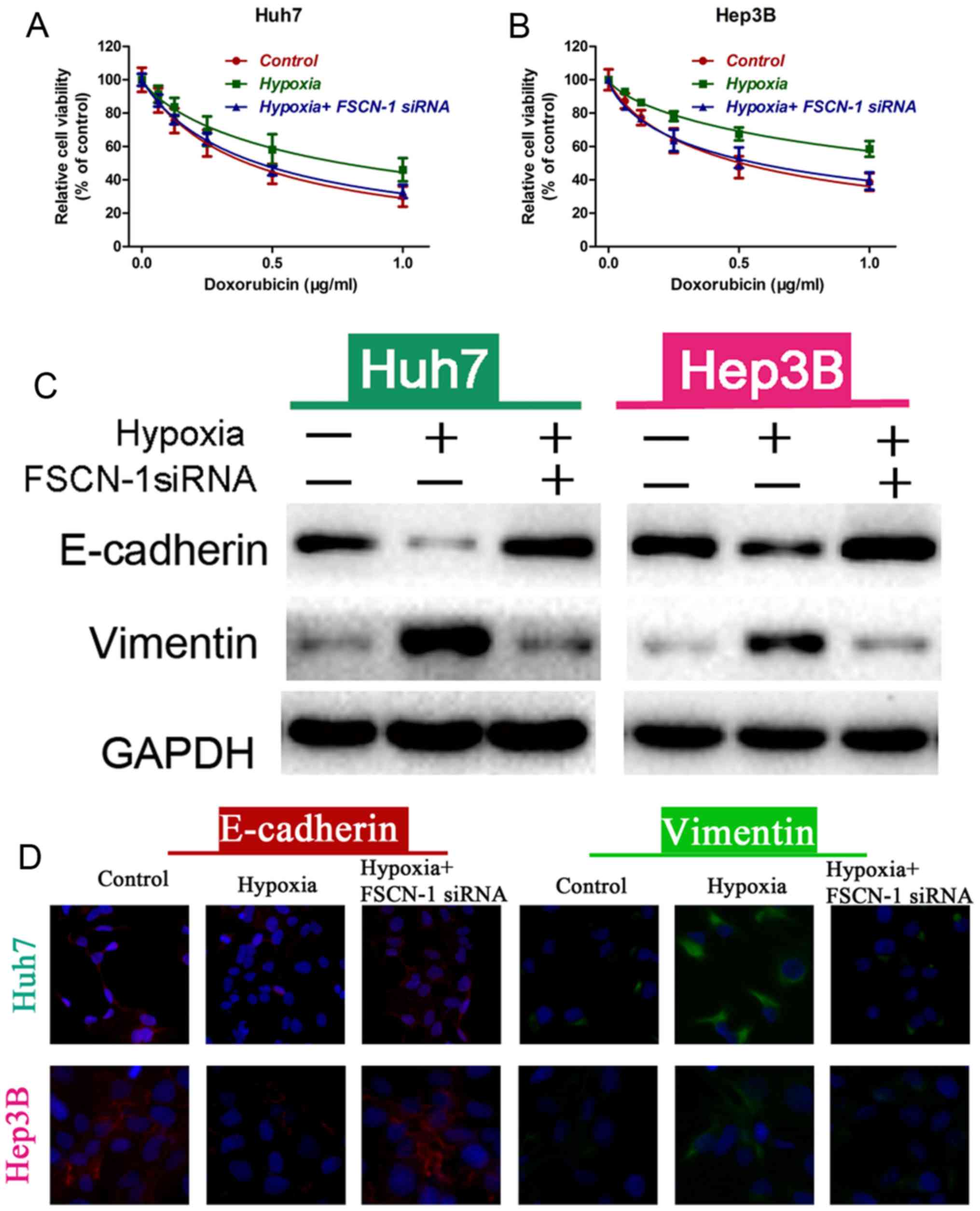
Hypoxia induces drug resistance and EMT in
vitro and in vivo. Indeed, cells cultured under hypoxia
were more resistant to DOX compared with normoxia (control group;
Fig. 6A and B). Western blot
analysis and immunofluorescence confocal assay results revealed
that hypoxia decreased E-cadherin expression and upregulated
vimentin expression, indicating that hypoxia induced the EMT
process in HCC cells (Fig. 6C and
D). However, HCC cells under hypoxia were more sensitive to DOX
treatment following FSCN-1 knockdown by siRNA compared to the
hypoxia group, similar to the control group (Fig. 6A and B). The expression of
E-cadherin was increased and vimentin was downregulated, while the
cells were transfected with FSCN-1 siRNA (Fig. 6C and D). These results indicated
that FACN-1 reversed hypoxia-induced drug resistance and EMT.
Discussion
As the prevalence of unresectable cases of HCC
increases, so too does the demand for nonsurgical and minimally
invasive alternatives to surgery to limit disease progression. In
the present study, our group investigated whether the mechanism
behind the resistance to the chemotherapy agent DOX was associated
with FSCN-1 expression, a key protein involved in cell-cell
interaction and motility. FSCN-1 expression was relatively
increased in HCC cells with DOX resistance compared with DOX
sensitive cells. FSCN-1 knockdown further suppressed the viability
of HCC cells in the presence of DOX compared with control cells.
Furthermore, investigation of the underlying mechanisms revealed
that FSCN-1 promoted the EMT process and increased HCC cell
resistance to DOX even under hypoxia. These results suggest that
FSCN-1 overexpression is primarily responsible for DOX resistance
in HCC.
Typically, FSCN-1 expression is low or absent in
normal epithelia, but high in mesenchymal tissues. Previous studies
have demonstrated that FSCN-1 is overexpressed in response to
TGF-β1-mediated activation of the JNK and ERK signaling pathways
(29). In addition, Zhao et
al (23) demonstrated that
inhibition of miR-145, a main regulatory miRNA for FSCN-1
expression, significantly increased EMT in breast cancer cells.
These previous data suggested that upregulation of FSCN-1
expression increased HCC resistance to DOX, potentially through the
induction of EMT. As expected, FSCN-1 suppression significantly
suppressed vimentin expression and increased E-cadherin
expression.
EMT in tumor cells allows them to gain metastatic
features via the induction of cell-cell disconnection, cell
depolarization and transitioning to an elongated, fibroblast-like
morphology. However, certain controversies concerning the
involvement of EMT in the promotion of tumor distant metastasis
have occurred. In breast cancer and pancreatic ductal
adenocarcinoma, EMT was revealed to be unessential for tumor
distant dissemination or metastasis (30,31).
Nonetheless, these two studies demonstrated that EMT is closely
associated with chemotherapy resistance and immune escape. In the
present study, our group observed that HCC cells that survived
following treatment with DOX had increased expression of EMT
markers. Meanwhile, Twist knockdown reduced the viability of HCC
cells in the presence of DOX. These data confirmed that EMT was
contributing to increased resistance of HCC cells to DOX.
Ischemia is a common phenomenon in HCC tissues
during TACE, and is associated with metastatic capacity, resistance
to chemotherapy and tumor progression, as well as poor patient
prognosis (32). Sridharan et
al (33) developed a Boolean
network model using targeted drug intervention to mimic persistent
hypoxia in a cell, and revealed that hypoxia regulated the
p53/mouse double minute 2 homolog, PI3K/Akt/mechanistic target of
rapamycin and the glycolysis/tricarboxylic acid cycle pathways to
influence cell energy production, apoptosis and survival.
Furthermore, the hypoxic microenvironment of tumors is known to
enhance stemness features and EMT (34). Therefore, our group detected the
effect of hypoxia on the viability of HCC cells following DOX
administration. Hypoxia increased the survival of HCC cells
following DOX treatment via the promotion of EMT. The effect of
hypoxia on DOX resistance was inhibited in HCC cells following
knockdown of FSCN-1. Tumor ischemia may also stimulate angiogenesis
to support HCC development (35).
Sorafenib, a tyrosine kinase inhibitor, is considered to be
standard treatment for patients with advanced HCC. This drug not
only directly suppresses HCC cell proliferation, but also
significantly inhibits angiogenesis. In a large, randomized and
double blind clinical trial, concurrent treatment of DOX-eluting
beads and sorafenib presented a manageable safety and tolerability
(36).
Finally, hypoxia-inducible factor (HIF)-1α has
previously been demonstrated to promote autophagy (37). Upregulation of autophagy is a
crucial mechanism of resistance to multiple antitumor drugs,
including DOX (38). In addition,
Zhao et al (39) revealed
that the mechanisms underlying HIF-1α-mediated increased invasion
and metastasis of pancreatic ductal adenocarcinoma was dependent on
the upregulation of FSCN-1 expression. Therefore, the relationship
between FSCN-1 and autophagy is worthy of further
investigation.
In conclusion, the present study demonstrated that
that FSCN-1 serves a critical role in doxorubicin resistance in HCC
under normoxic and hypoxic conditions through the promotion of EMT.
Therefore, FSCN-1 is potentially a novel target to overcome HCC
resistance to DOX. Further studies, including animal experiments
and clinical specimen analysis, should be performed in the future
to verify the results of the present study.
Acknowledgments
Not applicable.
Notes
[1]
Funding
The present study was funded by the National Health
and Family Planning Research Fund-Major Technology Plan of Medical
and Health Science and Technology Project (grant no. WKJ-ZJ-1602),
the Medical and Health Science and Technology Project of Zhejiang
Province (grant no. 2016KYB025) and the Science and Technology
Department Public Welfare Project of Zhejiang Province (grant no.
GF18H160058).
[2] Availability
of data and materials
All data generated or analyzed during this study are
included in this published article.
[3] Authors'
contributions
WY, WC and WW designed the study; YuaZ, JS, YL and
CZ performed the experiments; YC, DH and YuhZ analyzed the data; WY
wrote the manuscript. All authors have read and approved the final
version of the manuscript.
[4] Ethics
approval and consent to participate
Not applicable.
[5] Consent for
publication
Not applicable.
[6] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
El-Serag HB: Hepatocellular carcinoma:
Recent trends in the United States. Gastroenterology. 127(Suppl 1):
S27–S34. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Njei B, Rotman Y, Ditah I and Lim JK:
Emerging trends in hepatocellular carcinoma incidence and
mortality. Hepatology. 61:191–199. 2015. View Article : Google Scholar
|
|
4
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Piska K, Koczurkiewicz P, Bucki A,
Wójcik-Pszczoła K, Kołaczkowski M and Pękala E: Metabolic carbonyl
reduction of anthracyclines - role in cardiotoxicity and cancer
resistance. Reducing enzymes as putative targets for novel
cardioprotective and chemosensitizing agents. Invest New Drugs.
35:375–385. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Minotti G, Menna P, Salvatorelli E, Cairo
G and Gianni L: Anthracyclines: Molecular advances and
pharmacologic developments in antitumor activity and
cardiotoxicity. Pharmacol Rev. 56:185–229. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Olweny CL, Toya T, Katongole-Mbidde E,
Mugerwa J, Kyalwazi SK and Cohen H: Treatment of hepatocellular
carcinoma with adriamycin. Preliminary communication. Cancer.
36:1250–1257. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Asghar U and Meyer T: Are there
opportunities for chemotherapy in the treatment of hepatocellular
cancer? J Hepatol. 56:686–695. 2012. View Article : Google Scholar
|
|
9
|
Llovet JM, Real MI, Montaña X, Planas R,
Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R, et al
Barcelona Liver Cancer Group: Arterial embolisation or
chemoembolisation versus symptomatic treatment in patients with
unresectable hepatocellular carcinoma: A randomised controlled
trial. Lancet. 359:1734–1739. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Forner A, Ayuso C, Varela M, Rimola J,
Hessheimer AJ, de Lope CR, Reig M, Bianchi L, Llovet JM and Bruix
J: Evaluation of tumor response after locoregional therapies in
hepatocellular carcinoma: Are response evaluation criteria in solid
tumors reliable? Cancer. 115:616–623. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Burrel M, Reig M, Forner A, Barrufet M, de
Lope CR, Tremosini S, Ayuso C, Llovet JM, Real MI and Bruix J:
Survival of patients with hepatocellular carcinoma treated by
transarterial chemoembolisation (TACE) using Drug Eluting Beads.
Implications for clinical practice and trial design. J Hepatol.
56:1330–1335. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Batlle E, Sancho E, Francí C, Domínguez D,
Monfar M, Baulida J, García De and Herreros A: The transcription
factor snail is a repressor of E-cadherin gene expression in
epithelial tumour cells. Nat Cell Biol. 2:84–89. 2000. View Article : Google Scholar
|
|
14
|
Lee JM, Dedhar S, Kalluri R and Thompson
EW: The epithelial-mesenchymal transition: New insights in
signaling, development, and disease. J Cell Biol. 172:973–981.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vignjevic D, Kojima S, Aratyn Y, Danciu O,
Svitkina T and Borisy GG: Role of fascin in filopodial protrusion.
J Cell Biol. 174:863–875. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yoder BJ, Tso E, Skacel M, Pettay J, Tarr
S, Budd T, Tubbs RR, Adams JC and Hicks DG: The expression of
fascin, an actin-bundling motility protein, correlates with hormone
receptor-negative breast cancer and a more aggressive clinical
course. Clin Cancer Res. 11:186–192. 2005.PubMed/NCBI
|
|
17
|
Hashimoto Y, Skacel M, Lavery IC,
Mukherjee AL, Casey G and Adams JC: Prognostic significance of
fascin expression in advanced colorectal cancer: An
immunohistochemical study of colorectal adenomas and
adenocarcinomas. BMC Cancer. 6:2412006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jin JS, Yu CP, Sun GH, Lin YF, Chiang H,
Chao TK, Tsai WC and Sheu LF: Increasing expression of fascin in
renal cell carcinoma associated with clinicopathological parameters
of aggressiveness. Histol Histopathol. 21:1287–1293.
2006.PubMed/NCBI
|
|
19
|
Tsai WC, Jin JS, Chang WK, Chan DC, Yeh
MK, Cherng SC, Lin LF, Sheu LF and Chao YC: Association of
cortactin and fascin-1 expression in gastric adenocarcinoma:
Correlation with clinicopathological parameters. J Histochem
Cytochem. 55:955–962. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zou J, Yang H, Chen F, Zhao H, Lin P,
Zhang J, Ye H, Wang L and Liu S: Prognostic significance of
fascin-1 and E-cadherin expression in laryngeal squamous cell
carcinoma. Eur J Cancer Prev. 19:11–17. 2010. View Article : Google Scholar
|
|
21
|
Lin CK, Su HY, Tsai WC, Sheu LF and Jin
JS: Association of cortactin, fascin-1 and epidermal growth factor
receptor (EGFR) expression in ovarian carcinomas: Correlation with
clinicopathological parameters. Dis Markers. 25:17–26. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
De Arcangelis A, Georges-Labouesse E and
Adams JC: Expression of fascin-1, the gene encoding the
actin-bundling protein fascin-1, during mouse embryogenesis. Gene
Expr Patterns. 4:637–643. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao H, Kang X, Xia X, Wo L, Gu X, Hu Y,
Xie X, Chang H, Lou L and Shen X: miR-145 suppresses breast cancer
cell migration by targeting FSCN-1 and inhibiting
epithelial-mesenchymal transition. Am J Transl Res. 8:3106–3114.
2016.PubMed/NCBI
|
|
24
|
Hayashi Y, Osanai M and Lee GH: Fascin-1
expression correlates with repression of E-cadherin expression in
hepatocellular carcinoma cells and augments their invasiveness in
combination with matrix metalloproteinases. Cancer Sci.
102:1228–1235. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim SJ, Choi IJ, Cheong TC, Lee SJ, Lotan
R, Park SH and Chun KH: Galectin-3 increases gastric cancer cell
motility by up-regulating fascin-1 expression. Gastroenterology.
138:1035–1045. e1031–1032. 2010. View Article : Google Scholar
|
|
26
|
Fu H, Wen JF, Hu ZL, Luo GQ and Ren HZ:
Knockdown of fascin1 expression suppresses the proliferation and
metastasis of gastric cancer cells. Pathology. 41:655–660. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Onodera M, Zen Y, Harada K, Sato Y, Ikeda
H, Itatsu K, Sato H, Ohta T, Asaka M and Nakanuma Y: Fascin is
involved in tumor necrosis factor-alpha-dependent production of
MMP9 in cholangiocarcinoma. Lab Invest. 89:1261–1274. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li QQ, Xu JD, Wang WJ, Cao XX, Chen Q,
Tang F, Chen ZQ, Liu XP and Xu ZD: Twist1-mediated
adriamycin-induced epithelial-mesenchymal transition relates to
multidrug resistance and invasive potential in breast cancer cells.
Clin Cancer Res. 15:2657–2665. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fu H, Hu Z, Wen J, Wang K and Liu Y:
TGF-beta promotes invasion and metastasis of gastric cancer cells
by increasing fascin1 expression via ERK and JNK signal pathways.
Acta Biochim Biophys Sin (Shanghai). 41:648–656. 2009. View Article : Google Scholar
|
|
30
|
Fischer KR, Durrans A, Lee S, Sheng J, Li
F, Wong ST, Choi H, El Rayes T, Ryu S, Troeger J, et al:
Epithelial-to-mesenchymal transition is not required for lung
metastasis but contributes to chemoresistance. Nature. 527:472–476.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zheng X, Carstens JL, Kim J, Scheible M,
Kaye J, Sugimoto H, Wu CC, LeBleu VS and Kalluri R:
Epithelial-to-mesenchymal transition is dispensable for metastasis
but induces chemoresistance in pancreatic cancer. Nature.
527:525–530. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sergio A, Cristofori C, Cardin R, Pivetta
G, Ragazzi R, Baldan A, Girardi L, Cillo U, Burra P, Giacomin A, et
al: Transcatheter arterial chemoembolization (TACE) in
hepatocellular carcinoma (HCC): The role of angiogenesis and
invasiveness. Am J Gastroenterol. 103:914–921. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sridharan S, Varghese R, Venkatraj V and
Datta A: Hypoxia stress response pathways: Modeling and targeted
therapy. IEEE J Biomed Health Inform. 21:875–885. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Prasad P, Mittal SA, Chongtham J, Mohanty
S and Srivastava T: Hypoxia-mediated epigenetic regulation of
stemness in brain tumor cells. Stem Cells. 35:1468–1478. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Myung SJ and Yoon JH: Hypoxia in
hepatocellular carcinoma. Korean J Hepatol. 13:9–19. 2007.In
Korean. PubMed/NCBI
|
|
36
|
Lencioni R, Llovet JM, Han G, Tak WY, Yang
J, Guglielmi A, Paik SW, Reig M, Kim DY, Chau GY, et al: Sorafenib
or placebo plus TACE with doxorubicin-eluting beads for
intermediate stage HCC: The SPACE trial. J Hepatol. 64:1090–1098.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang P, Long M, Zhang S, Cheng Z, Zhao X,
He F, Liu H and Ming L: Hypoxia inducible factor-1α regulates
autophagy via the p27-E2F1 signaling pathway. Mol Med Rep.
16:2107–2112. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tan Q, Joshua AM, Wang M, Bristow RG,
Wouters BG, Allen CJ and Tannock IF: Up-regulation of autophagy is
a mechanism of resistance to chemotherapy and can be inhibited by
pantoprazole to increase drug sensitivity. Cancer Chemother
Pharmacol. 79:959–969. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhao X, Gao S, Ren H, Sun W, Zhang H, Sun
J, Yang S and Hao J: Hypoxia-inducible factor-1 promotes pancreatic
ductal adenocarcinoma invasion and metastasis by activating
transcription of the actin-bundling protein fascin. Cancer Res.
74:2455–2464. 2014. View Article : Google Scholar : PubMed/NCBI
|