Introduction
Breast cancer is one of the most prevalent malignant
cancers and the second leading cause of cancer-associated mortality
among women globally (1).
Morbidity and mortality in breast cancer are high in developed
countries and are on the increase in developing countries (2). Susceptibility to breast cancer is
assessed through multiple factors, including environmental factors,
physical factors and genetic factors (1,2). The
phenotype, prognosis and molecular hallmarks of breast cancer are
heterogeneous. Considering the markers of mammary epithelial cell
states, it is possible to classify breast cancer into
stem-cell-like, basal and luminal cancer (3,4).
Triple-negative breast cancer, which lacks estrogen receptors,
progesterone receptors and human epidermal growth factor receptor-2
(HER-2) is the most malignant breast cancer, and effective
treatments remain unavailable (5).
To date, resection followed by loco-regional radiotherapy and
systemic treatments constitutes primary therapy in breast cancer
(6). Surgery has improved outcomes
in primary breast cancer, but resistance to radiotherapy, endocrine
therapy and chemotherapy, along with severe toxicity in locally
advanced and metastatic cancers, limits the efficiency of therapies
and contributes to mortality in breast cancer (1,7).
Therefore, novel therapeutic options for the treatment of breast
cancer are urgently needed.
The center of solid tumors commonly develops a
pathophysiological condition, which is characterized by hypoxia due
to rapid tumor growth and limited oxygen diffusion (8). Intratumoral hypoxic conditions
contribute to tumor angiogenesis, invasion, metastasis and
resistance to therapies; these events are also associated with
aggressive cancer phenotypes (6,9).
When adapting to the hypoxic microenvironment of tumors, cells
activate certain hypoxia-associated proteins and signaling
pathways, including hypoxia inducible factor 1 (HIF-1) (10). HIF-1, a heterodimer consisting of
HIF-1α and HIF-1β, belongs to the family of basic
helix-loop-helix-periodic acid-Schiff domain transcription factors.
It regulates the expression of >70 genes, which serve pivotal
functions in low oxygen metabolism, tumorigenesis, angiogenesis and
metastasis (11). HIF-1α functions
as an oxygen-sensitive factor of HIF-1, which is hydroxylated at
proline resides (pro402 and pro564) of the
oxygen-dependent disintegration domain by prolyl hydroxylase, and
interacts with von Hippel-Lindau protein. This interaction results
in ubiquitination and disintegration of HIF-1α through the
recruitment of the E3 ubiquitin ligase under normoxia; however,
HIF-1α is accumulated under hypoxia (10). In addition to oxygen-dependence,
oxygen-independent mechanisms have also been demonstrated to
regulate HIF-1α in tumor cells under normoxia (11). Excessive accumulation of HIF-1α has
been observed in human tumors compared with para-carcinoma tissues,
such as non-small-cell lung cancer and ovarian cancer, suggesting
that it may be a potential target for neoplastic therapy (8).
The hairy and enhancer of split (HES) family has
seven members, HES1-7, which belong to the basic helix-loop-helix
superfamily of DNA-binding transcription factors and directly
affect cellular activities (12).
Studies have demonstrated that HES1 affects cell proliferation and
differentiation during embryogenesis, maintenance of stem cells,
and the malignancy and maintenance of tumor cells (13). Overexpression of HES1 is associated
with the development of several types of cancer, including lung
cancer, cervical cancer, prostate cancer, oral squamous cell
carcinoma, pancreatic cancer, colon carcinoma, and ovarian cancer
(14–16). The function of HES1 and its
involvement in the regulation in malignant breast cancer remain to
be fully elucidated; therefore, it is necessary to investigate its
function and underlying mechanisms in breast cancer proliferation
and metastasis. Epithelial-mesenchymal transition (EMT) is one of
the crucial mechanisms that induce tumor invasion, metastasis, and
migration, as it facilitates cancer dissemination. EMT is
characterized by the transformation of the cellular phenotype from
the epithelial phenotype (non-motile, polarized and collective) to
the mesenchymal phenotype (individual, non-polarized, motile and
invasive) (17). The EMT process
involves dissolution of epithelial cell-cell adhesion, cytoskeletal
actin remodeling and overexpression of mesenchymal adhesive
molecular markers. Previous studies have suggested that
hypoxia-induced invasion and the EMT processes are associated with
HES1 (10,17).
A number of studies have demonstrated that
phytochemicals have potential for the prevention and treatment of
cancer. Lutein is a natural dihydroxycarotenoid belonging to the
family of non-vitamin A carotenoids, which are the second most
widespread carotenoid in human serum and are present in deep yellow
vegetables and fruits, including lettuce and spinach (18). Lutein has anti-oxidative,
anti-inflammatory, and antitumor properties. Concentrated in the
retina, lutein decreases the risk of age-associated macular
degeneration due to its anti-oxidative properties (19). Previous studies have demonstrated
that lutein exhibits potential antitumor properties in several
cancers, including cervical carcinoma, colon cancer and hepatic
carcinoma (20–22). Lutein is involved in cancer
initiation and progression by regulating cellular redox status and
associated signaling pathways. However, its effects on breast
cancer and underlying mechanisms remain to be fully
investigated.
The aim of the present study was to elaborate the
molecular mechanism underlying the inhibitory effect of lutein in
hypoxia-induced reactive oxygen species (ROS) production, and the
proliferation, invasion and migration of breast cancer cells.
Lutein was demonstrated to inhibit cell vitality in several breast
cancer cell lines in a dose-dependent manner. Lutein downregulated
HES1 expression by inhibiting the generation of ROS and expression
of HIF-1α and NOTCH. Lutein also suppressed EMT, cell invasion, and
metastasis in breast cancer cells. The results of the present study
suggest that the antitumor effects of lutein may enable its
application in the treatment of breast cancer via the inhibition of
HES1.
Materials and methods
Reagents
Lutein (>99% pure) was acquired commercially
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and dissolved in
dimethyl sulfoxide (DMSO). It was then added to RPMI-1640 medium
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) to each
working dose. N-acetylcysteine (NAC) and hydrogen peroxide
(H2O2) were purchased from Sigma-Aldrich;
Merck KGaA. All other reagents were of reagent grade and purchased
from standard commercial sources. All drug solutions were freshly
compounded on the day of the test.
Cell culture and transfection
The human breast cancer cell lines, MDA-MB-157 and
MCF-7, were obtained from the Type Culture Collection of the
Chinese Academy of Sciences (Shanghai, China). Short tandem repeat
profiling analysis was used to examine and authenticate breast cell
lines, and they were used within 6 months of authentication. Cells
were grown in RPMI-1640 medium-supplemented with 10% fetal bovine
serum (Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin,
and 100 μg/ml streptomycin. Cells were cultured under
normoxia in a humidified atmosphere with 5% CO2 at 37°C.
The cells cultured under hypoxia were placed in a hypoxic chamber
(Thermo Fisher Scientific, Inc.) suffused with 1% O2, 5%
CO2, and 94% N2 at 37°C.
The human HES1 expression vector HES1-pCMV6-XL5 was
obtained from OriGene Technologies, Inc. (Rockville, MD, USA). An
empty vector was employed as a negative control. MDA-MB-157 and
MCF-7 cells were transiently transfected with HES1 overexpression
constructs. When these cells reached 60–80% confluence, they were
transfected with the appropriate constructs using Lipofectamine™
2000 transfection reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the Manufacturer’s protocol. After 24 h,
transfection efficiency was checked by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
western blot analysis. Stably transfected cells were used for
subsequent experiments.
Cell proliferation assay
MDA-MB-157 and MCF-7 cells were plated onto 96-well
culture plates with various concentrations (5, 10, 20, 40, 80, and
120 μM) of lutein; 0 μM lutein was used as negative
control. Cells were incubated for 24 h in a humidified incubator
with 5% CO2 at 37°C. At the end of the treatment, cell
viability was examined with the MTT assay. MTT reagent (50
μl, Sigma-Aldrich; Merck KGaA) was added to each well, and
the plate was incubated for another 2 h at 37°C. Formazan, which is
produced from MTT by the action of viable cells, was dissolved in
100 μl DMSO. Absorbance at 570 nm was measured using a
microplate reader (Infinite M200; Tecan Group, Ltd., Mannedorf,
Switzerland). The concentration of lutein required for a 50%
reduction in cell viability (concentration of an inhibitor where
the response/binding is reduced by half) was calculated and used in
further experiments. The half maximal inhibitory concentration
(IC50) values were calculated from the percentage of
cell viability at each concentration of lutein, using probit
analysis.
Cell invasion assay
Invasion assays of breast cancer cells were
performed using Transwell chambers (Corning Incorporated, Corning,
NY, USA) with an 8-micrometer pore insert pre-coated with Matrigel
in a 24-well plate (Corning Incorporated). Cell suspension
(5×104 cells/ml) in serum-free medium was added to the
upper chambers, and 600 μl medium containing 10% fetal
bovine serum was added to the lower chambers. Next, the chamber was
incubated under hypoxia for 24 h or treated with lutein (40
μM) under hypoxia at 37°C for 24 h. A normoxic control was
included to detect the effects of lutein on cell invasion.
Following incubation, the cells in the upper chambers were gently
scraped off using a cotton-tipped swab. Invading cells were fixed
with 5% paraformaldehyde for 30 min and stained with 0.1% crystal
violet for 15 min at room temperature in the lower chambers. Cell
count on the lower side of the membrane was obtained as the average
of the cell counts from five random fields in each well, which was
captured at ×200 magnification under a bright-field microscope.
Wound healing assay
The wound healing assay was performed to detect the
migratory ability of cancer cells. Breast cancer cells were seeded
into 6-well plates to reach a confluent mono-layer. Once the cells
reached 90% confluence, monolayers were scratched with a
200-microliter microtip to produce a wound line among the cells.
Cells were washed with PBS twice and then covered with serum-free
medium either in the absence of stimulus or in the presence of
lutein (40 μM). Next, cells were allowed to migrate for 24 h
at 37°C under normoxia or hypoxia. Cell migration images were
captured at 0 and 24 h under a Leica DMIL inverted microscope (×50
magnification; Leica Microsystems GmbH, Wetzlar, Germany) from
three independent experiments. The edge from 0 to 24 h was assessed
using ImageJ software (National Center for Biotechnology
Information, Bethesda, MD, USA) to calculate the relative distance
travelled.
Cell apoptosis assays
The Annexin V-fluorescein isothio-cyanate
(FITC)/propidium iodide (PI) apoptosis detection kit was obtained
from Beyotime Institute of Biotechnology (Haimen, China). The
content of apoptotic cells was estimated following the
manufacturer’s protocol. Cells were exposed to the indicated
concentrations (20, 40, and 80 μM) of lutein and incubated
for 24 h at 37°C under normoxia or hypoxia. Once they were
harvested from the plates, cells were washed and suspended in PBS.
Next, cells were stained with Annexin V-FITC and PI. The percentage
of apoptotic cells was detected using flow cytometry (BD
Biosciences, San Jose, CA, USA) and the data were analyzed using
CellQuest software (version 5.1; BD Biosciences). A total of
1×104 events were collected for each run, and the assay
was repeated in three independent replicates.
ROS detection
ROS levels were detected using the
2,7-dichlorodihydrofluorescein-diacetate (DCFH-DA) Cellular ROS
Detection assay kit (Beyotime Institute of Biotechnology),
according to the manufacturer’s protocol. In brief, breast cancer
cells were seeded in 6-well plates and exposed to normoxia or
hypoxia or subjected to treatment with lutein (40 μM),
H2O2 (200 μM) or N-acetylcysteine
(NAC; 50 μM) for 24 h at 37°C. Cells were (1×106
cells/ml) trypsinized and suspended in DCFH-DA (10 μM) for
20 min at 37°C then washed with PBS twice. Intracellular ROS levels
were measured in each group according to DCF fluorescence by flow
cytometry (BD Biosciences); data were analyzed using CellQuest 5.1
software (BD Biosciences).
RNA extraction and RT-qPCR
Total RNA was isolated from breast cancer cells
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.).
The concentration of total RNA was measured using a Nanodrop 2000C
spectrophotometer (Thermo Fisher Scientific, Inc.), and reverse
transcription of total RNA was performed using the PrimeScript RT
Reagent kit (Takara Biotechnology Co., Ltd., Dalian, China)
according to the manufacturer’s protocol. qPCR was performed using
the SYBR Green PCR kit (Takara Biotechnology Co., Ltd.) on an
Applied Biosystems 7500 fast Real-Time PCR System (Thermo Fisher
Scientific, Inc.). The following PCR reaction conditions were used:
95°C for 30 sec, followed by 40 cycles consisting of 95°C for 5 sec
and 60°C for 30 sec. Melting curve analysis was performed from 60°C
to 95°C to assess the specificity of PCR products. All RT-qPCR
primer sequences are listed in Table
I. The expression level of each gene was evaluated using the
comparative Cq method (23), and
β-actin mRNA levels were used as a normalization control.
 | Table IPrimer sequences used for reverse
transcription-quantitative polymerase chain reaction analysis. |
Table I
Primer sequences used for reverse
transcription-quantitative polymerase chain reaction analysis.
| Gene name | Forward primer
sequence (5′-3′) | Reverse primer
sequence (5′-3′) |
|---|
| HES1 |
TCAACACGACACCGGATAAA |
CCGCGAGCTATCTTTCTTCA |
| E-cadherin |
CAGCACGTACACAGCCCTAA |
ACCTGAGGCTTTGGATTCCT |
| N-cadherin |
ACAGTGGCCACCTACAAAGG |
CCGAGATGGGGTTGATAATG |
| Vimentin |
GAGAACTTTGCCGTTGAAGC |
TCCAGCAGCTTCCTGTAGGT |
| HIF-1α |
GTCGGACAGCCTCACCAAACAGAGC |
GTTAACTTGATCCAAAGCTCTGAG |
| NOTCH3 |
TCTTGCTGCTGGTCATTCTC |
TGCCTCATCCTCTTCAGTTG |
| JAG1 |
GACTCATCAGCCGTGTCTCA |
TGGGGAACACTCACACTCAA |
| JAG2 |
TCTGCCTTGCTACAATGGTG |
GCGATACCCGTTGATCTCAT |
| DLL1 |
TGCAACCCTGGCTGGAAA |
AATCCATGCTGCTCATCACATC |
| β-actin |
TCCCTGGAGAAGAGCTACGA |
ACCTGAGGCTTTGGATTCCT |
Western blot analysis
Cells were lysed in RIPA lysis buffer (Beyotime
Institute of Biotechnology) supplemented with 1 mM PMSF (Beyotime
Institute of Biotechnology). This lysate was centrifuged at 12,000
× g for 15 min at 4°C and supernatant was harvested. Protein
concentration was measured using BCA reagent (Thermo Fisher
Scientific, Inc.). Protein extracts (50 μg) underwent 10%
SDS-PAGE and transferred to a nitrocellulose membrane (Merck KGaA).
These membranes were soaked in 5% non-fat dry milk for 2 h at 4°C
and incubated with primary antibodies against HIF-1α (1:800,
ab113642; Abcam, Cambridge, UK), HES1 (1:500, ab71559; Abcam),
NOTCH3 (1:800, sc515825; Santa Cruz Biotechnology, Inc., Dallas,
TX, USA), JAGGED1 (JAG1; 1:500, #70109; Cell Signaling Technology,
Inc., Danvers, MA, USA), JAGGED2 (JAG2; 1:500, #2210; Cell
Signaling Technology, Inc.), Delta-like 1 (DLL1; 1:800, MAB1818;
R&D Systems, Inc., Minneapolis, MN, USA), epithelial-cadherin
(E-cadherin; 1:800, ab15148; Abcam), neural-cadherin (N-cadherin;
1:800, ab98952; Abcam), vimentin (1:800, #5741; Cell Signaling
Technology, Inc.), and β-actin (1:1000, ab227387; Abcam) overnight
at 4°C. Next, membranes were incubated with horseradish
peroxidase-conjugated secondary goat anti-mouse (1:2000, HAF007;
R&D Systems, Inc.) or goat anti-rabbit IgG antibodies (1:2000,
HAF008; R&D Systems, Inc.) at 37°C for 2 h. Immunoreactive
protein bands were visualized using a chemiluminescence assay
(Beyotime Institute of Biotechnology).
Immunofluorescence analyses
Following the indicated treatments, breast cancer
cells were rinsed with PBS twice, fixed in fresh 4%
paraformaldehyde for 30 min at 37°C, and permeabilized with 0.2%
Triton X-100 (Sigma-Aldrich; Merck KGaA). Cells were blocked with
5% BSA (Sigma-Aldrich; Merck KGaA) in PBS at 37°C for 30 min and
incubated overnight with primary antibodies against E-cadherin,
N-cadherin and vimentin (all diluted 1:100) at 4°C. It was followed
by incubation with secondary FITC-conjugated goat anti-rabbit IgG
antibody (1:200, AP307F; Chemicon International, Inc., Temecula,
CA, USA) or tetramethylrhodamine-conjugated goat anti-mouse IgG
antibody (1:200, AP500R; Chemicon International, Inc.) for 30 min
at room temperature, and then counterstained with DAPI prior to
observation. The target protein appeared green and the nuclei
appeared blue, as visualized by a fluorescence microscope (Olympus
BX43; Olympus Corporation, Tokyo, Japan) at ×200 magnification.
Image-Pro Plus 6.0 software (Media Cybernetics, Inc., Rockville,
MD, USA) was used to process the images.
Statistical analysis
All data were analyzed using SPSS 21.0 (IBM Corp.,
Armonk, NY, USA) or GraphPad Prism version 5.0 (GraphPad Software,
Inc., La Jolla, CA, USA). Data were obtained from at least three
independent experiments and presented as mean ± standard deviation.
One-way ANOVA and Dunnett’s test was used for the comparison of
means between groups. Unpaired Student’s t-tests were used to
compare between two groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Lutein suppresses the viability and
induces the apoptosis of breast cancer cells under normoxia and
hypoxia
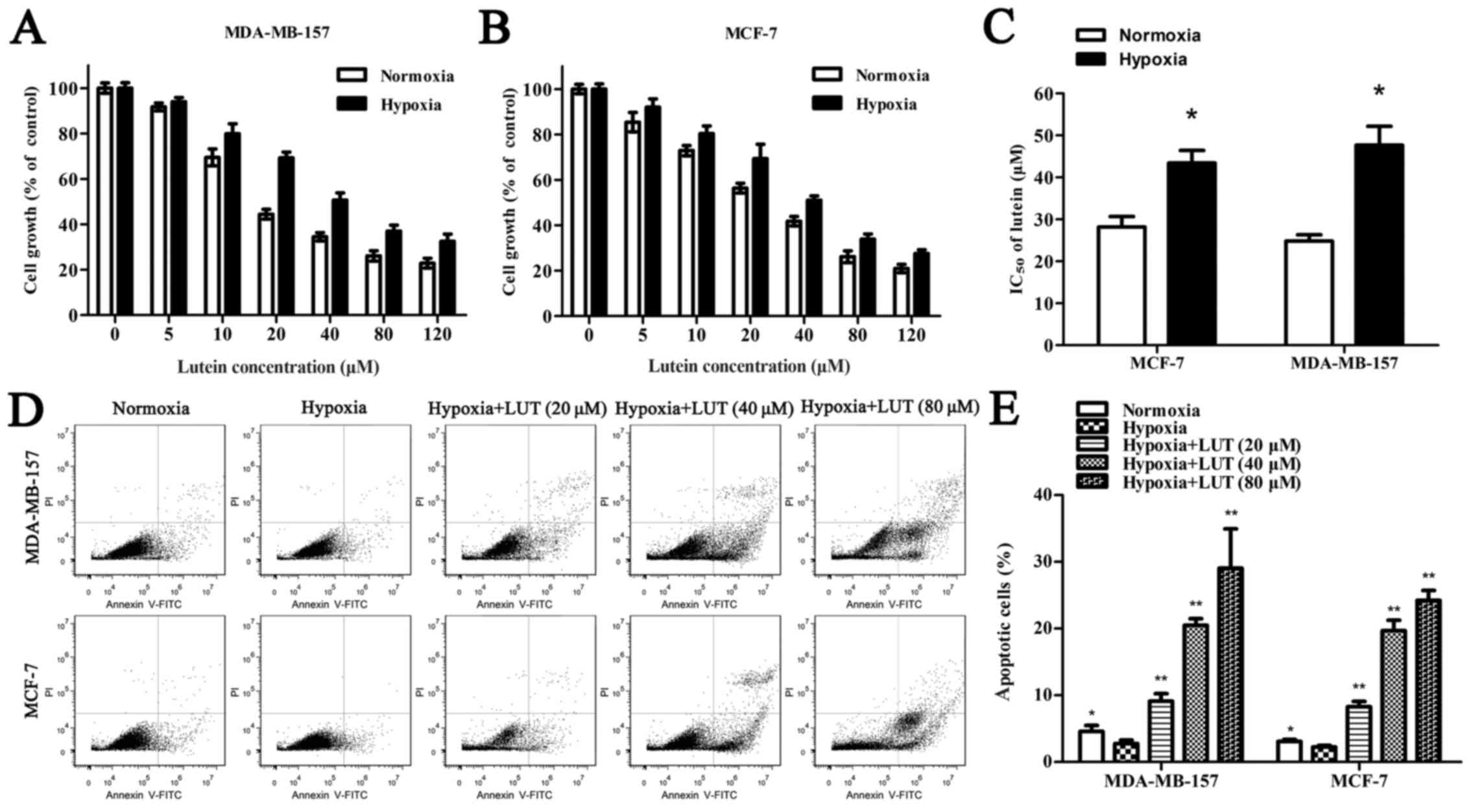
To determine whether lutein affects cell viability,
MDA-MB-157 and MCF-7 human breast cancer cells were treated with
5–120 μM lutein for 24 h under normoxia and hypoxia. As
presented in Fig. 1A and B, lutein
markedly decreased cell viability in a concentration-dependent
manner under normoxia and hypoxia. Hypoxia alone induced a lower
inhibitory rate with lutein intervention than normoxia did in
MDA-MB-157 and MCF-7 cells, and low oxygen exposure improved the
IC50 of lutein in breast cancer cells (Fig. 1C). The Annexin V-PI assay was used
to detect the extent of apoptosis induced by lutein in MDA-MB-157
and MCF-7 cells (Fig. 1D).
Apoptosis was increased in cells treated with lutein compared with
the untreated control. Similarly, hypoxia, compared with normoxia,
inhibited cell apoptosis. These results suggested that lutein
treatment inhibited the viability and promoted the apoptosis of
MDA-MB-157 and MCF-7 cells under normoxia and hypoxia. Furthermore,
hypoxia increased the viability of cancer cells compared with
normoxia.
Antitumor effects of lutein are mediated
through down-regulation of HES1
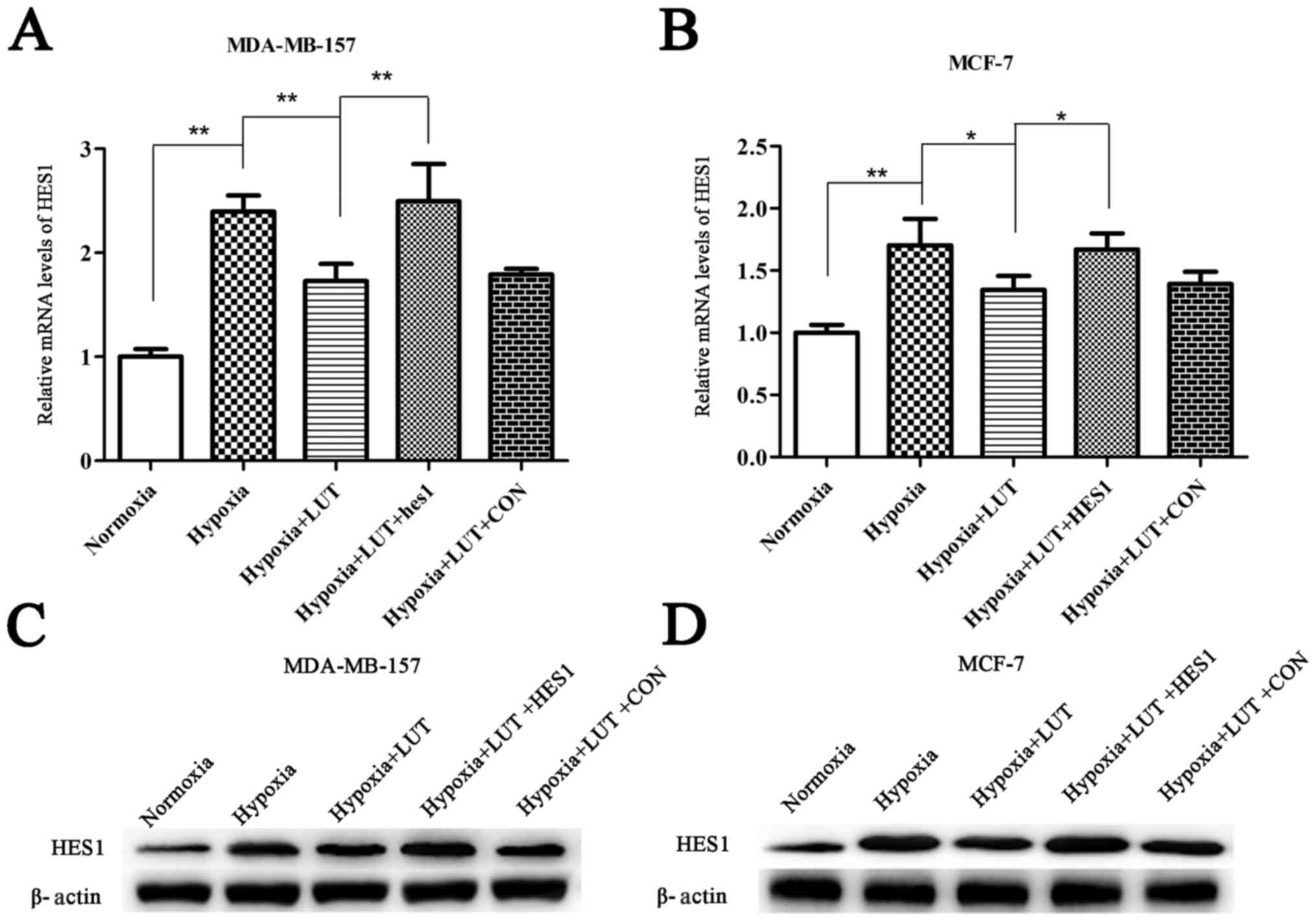
HES1 is involved in the regulation of cell cycle,
growth, differentiation, invasion, and apoptosis in various cancers
(12,13). Therefore, the involvement of HES1
in the effects of lutein on tumor inhibition were investigated. The
results revealed that mRNA and protein levels of HES1 were
significantly upregulated under hypoxia compared with normoxia, and
lutein intervention decreased the expression of HES1 (Fig. 2). To investigate whether
downregulation of HES1 was a mechanism underlying the antitumor
effects of lutein, HES1 mRNA and protein was transiently
overexpressed in lutein-treated MDA-MB-157 and MCF-7 cells using
HES1 overexpression vectors (Fig.
2). These results suggested that the antitumor effects of
lutein were associated with a decrease in HES1 expression, which
was reversed by overexpression of HES1.
Lutein inhibits hypoxia-induced EMT in
breast cancer cells
EMT is considered a key phenomenon that promotes
tumor invasion and metastasis. Expression of HES1 is sufficient to
promote EMT along with the invasive and metastatic potential of
cancer cells (12,17). As presented in Fig 3A, RT-qPCR and western blotting
revealed that treatment with lutein significantly increased the
expression of epithelial markers (E-cadherin) and abrogated the
expression of mesenchymal markers (vimentin and N-cadherin) in
MDA-MB-157 and MCF-7 cells (Fig.
3A–D). The changes in the expression of the EMT phenotype due
to lutein interference in MDA-MB-157 and MCF-7 cell lines were
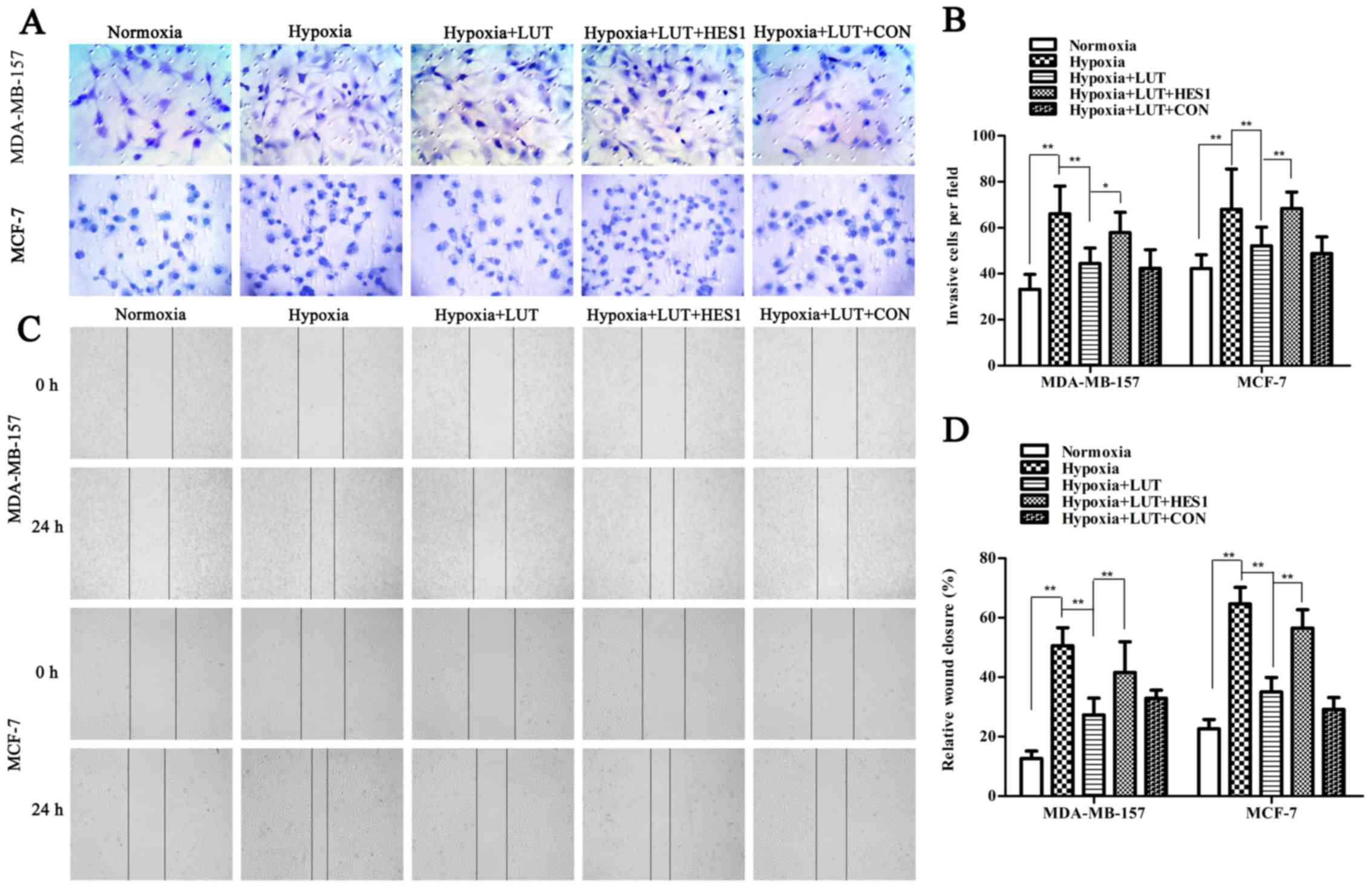
further confirmed using immunocytochemistry (Fig. 3E). Next, the effects of lutein on
cell invasion and motility were also investigated in breast cancer
cells under hypoxia, using Transwell and wound healing assays
(Fig. 4). The Transwell assay
indicated that the invasiveness of MDA-MB-157 and MCF-7 cells under
hypoxia was significantly inhibited by lutein treatment (Fig. 4A and B). The spread of
lutein-treated MDA-MB-157 and MCF-7 cells along the wound edges was
significantly slower compared with control cells (Fig. 4C and D). This indicated that lutein
significantly decreased the invasion and migration abilities of
cells compared with those of control cells under hypoxia.
Lutein-induced inhibition of EMT was significantly reversed by
overexpression of HES1 (Fig.
3A–D). Furthermore, the lutein-induced decrease in invasion and
migration was notably inhibited by overexpression of HES1 (Fig. 4). Taken together, these results
indicated that lutein effectively suppressed the induction of EMT
and prevented the accompanying increase in invasiveness and
metastasis of breast cancer cells by down-regulating HES1.
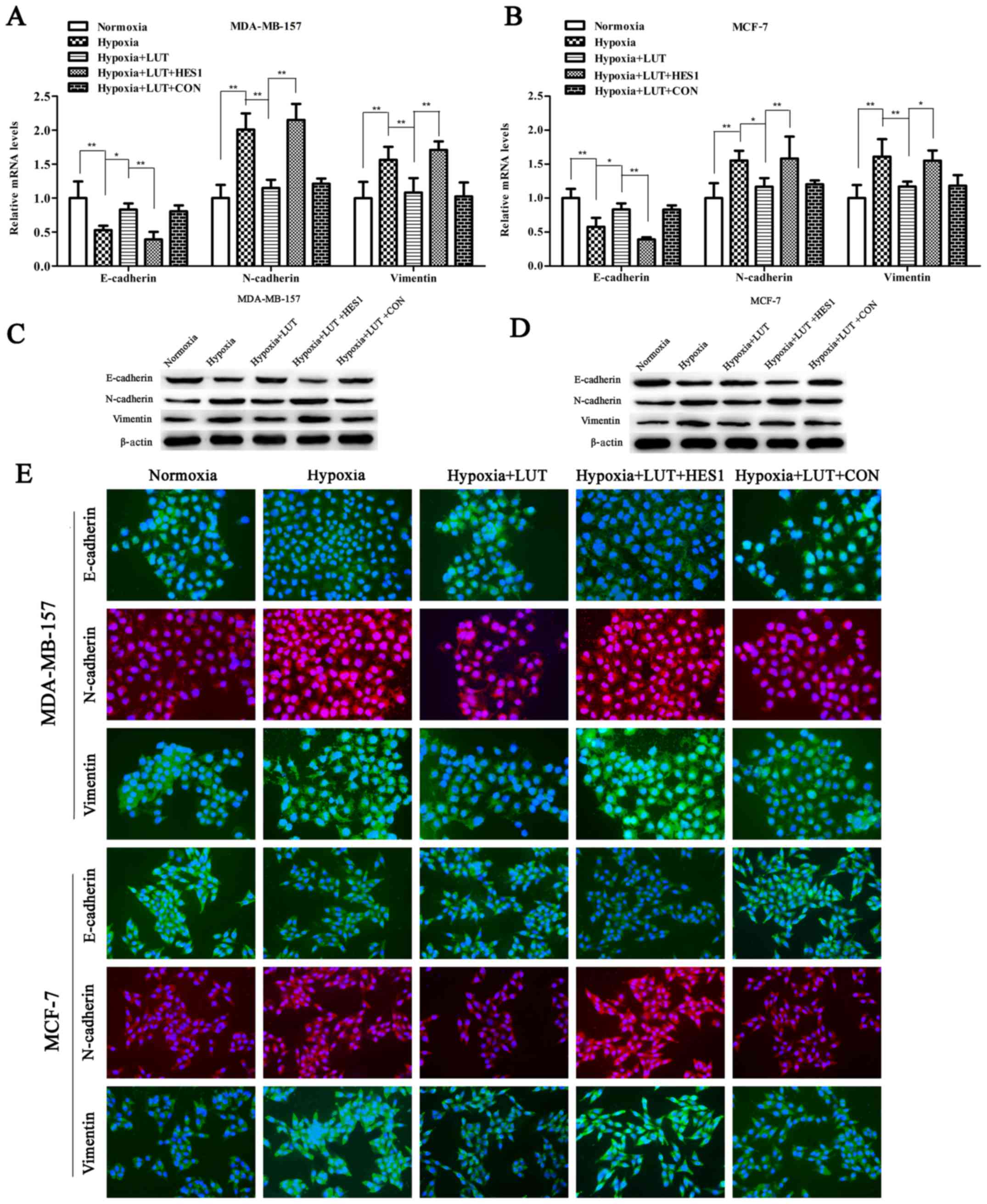 | Figure 3LUT inhibits hypoxia-induced EMT of
breast cancer cells. Reverse transcription-quantitative polymerase
chain reaction analysis of mRNA expression of EMT-associated genes
(E-cadherin, N-cadherin, and vimentin) in (A) MDA-MB-157 or (B)
MCF-7 cells stimulated with LUT (40 μM) and subsequently
transiently transfected with HES1 overexpression vectors or empty
vectors under normoxia or hypoxia. Western blot analysis of
EMT-associated proteins (E-cadherin, N-cadherin, and vimentin) in
(C) MDA-MB-157 or (D) MCF-7 cells stimulated with LUT (40
μM) and subsequently transiently transfected with HES1
overexpression vector or empty vector under normoxia or hypoxia.
(E) MDA-MB-157 and MCF-7 cells were stimulated with LUT (40
μM) and subsequently transiently transfected with HES1
overexpression vectors or empty vectors under normoxia or hypoxia
as indicated. Expression levels of EMT-associated proteins
(E-cadherin, N-cadherin, and vimentin) was visualized by
immunofluorescence (×200 magnification). Green fluorescence
indicates E-cadherin or vimentin expression. Red fluorescence
indicates N-cadherin expression, and blue denotes nuclear DNA
staining by DAPI. The results were presented as the mean ± standard
deviation of triplicate experiments. *P<0.05 and
**P<0.01, with comparisons indicated by lines. LUT,
lutein; EMT, epithelial-mesenchymal transition; HES1, hairy and
enhancer of split 1; E-cadherin, epithelial cadherin; N-cadherin,
neural cadherin. |
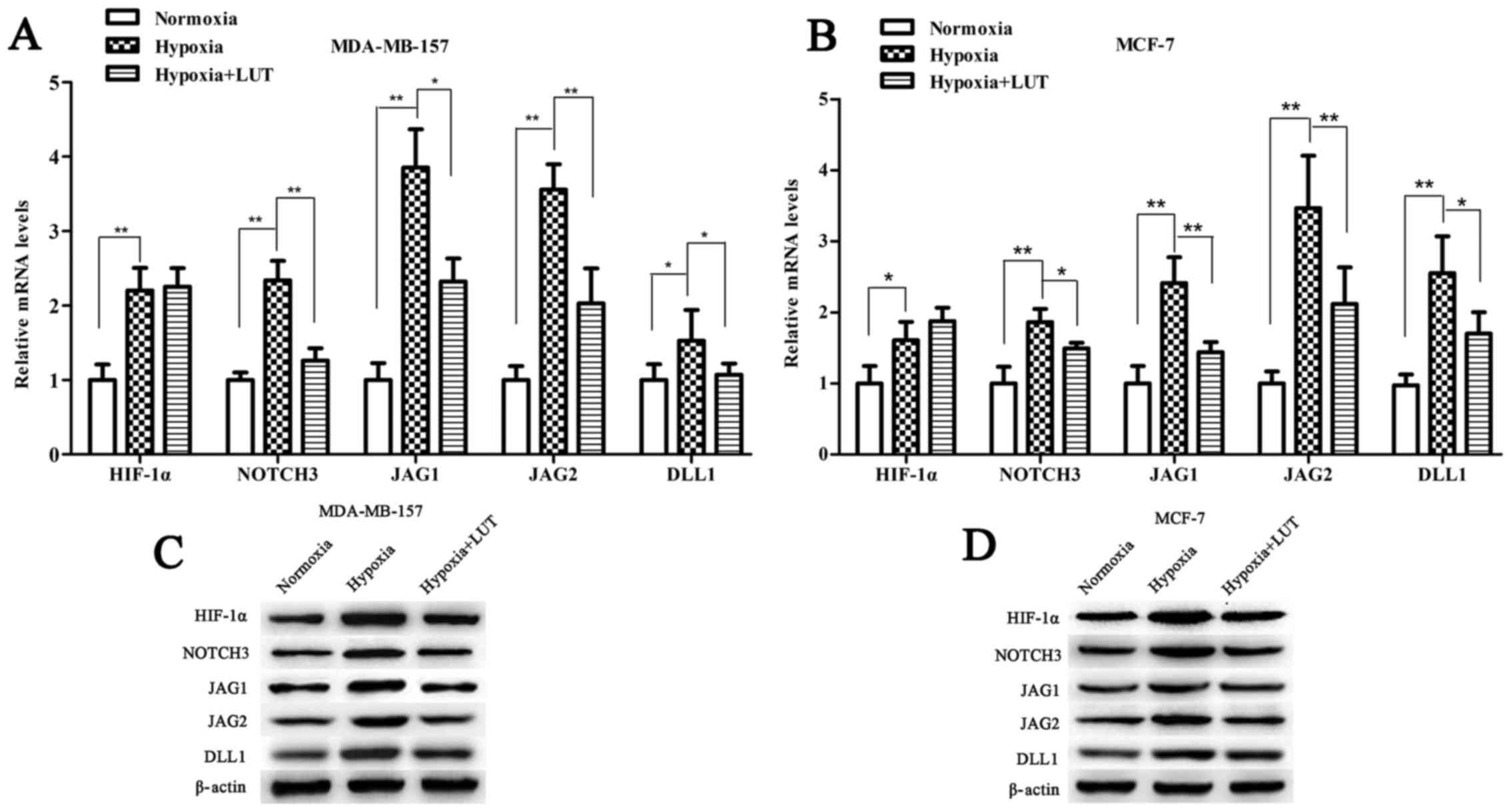
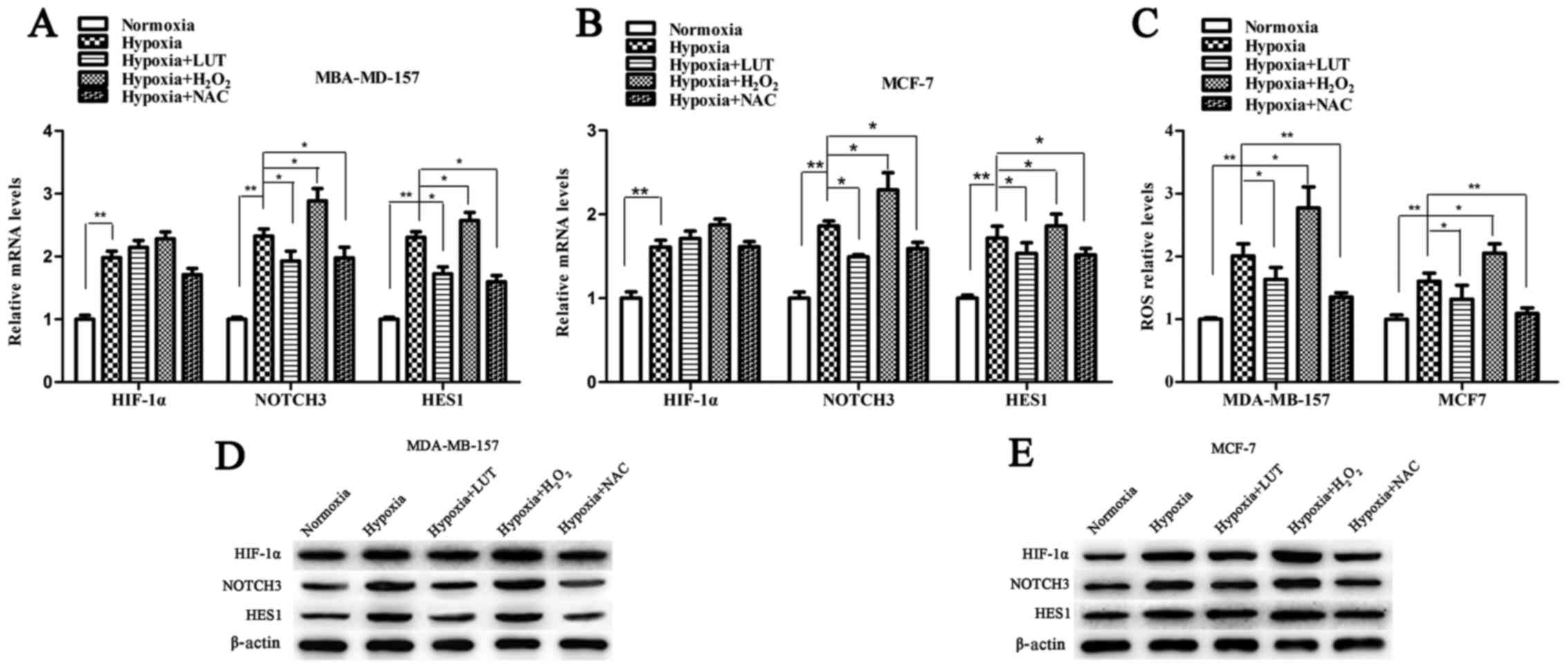
Lutein suppresses the expression of
HIF-1α and downstream NOTCH signaling pathway
Under hypoxia, activation of HIF-1α potentiates
NOTCH signaling by upregulating the expression of NOTCH receptors
and ligands, which in turn induces the expression of the NOTCH
target gene HES1 (24,25). To determine whether the effect of
lutein on HES1 expression was mediated by HIF-1α transcription
factor through the NOTCH signaling pathway, the expression level of
HIF-1α, NOTCH3 and NOTCH ligands (JAG1, JAG2, and DLL1) was
examined. As presented in Fig. 5,
hypoxia increased the expression levels of HIF-1α, NOTCH3, JAG1,
JAG2, and DLL1, which were significantly accumulated in the two
breast cancer cell lines compared with those under normoxia. Lutein
decreased hypoxia-induced HIF-1α protein expression, however, no
significant difference in HIF-1α mRNA expression was detected in
MDA-MB-157 and MCF-7 cells (Fig. 5A
and B). This result indicated that lutein inhibited
hypoxia-induced HIF-1α protein expression in a post-transcriptional
manner, and subsequently suppressed NOTCH receptors and ligands.
These results revealed that lutein downregulated HES1 by
suppressing hypoxia-induced HIF-1α expression at the protein level,
and by suppressing NOTCH signaling expression at the mRNA and
protein levels.
Lutein inhibits the expression of HIF-1α
by decreasing the hypoxia-induced production of ROS in breast
cancer cells
Previous studies have suggested that stabilization
of HIF-1α in low oxygen conditions is associated with an increase
in ROS levels (26). To
investigate whether lutein-induced downregulation of HIF-1α and the
NOTCH pathway in breast cancer cells under hypoxia is associated
with ROS scavenging, the cells were treated under hypoxic
conditions with 5 mM H2O2, 20 mM NAC and 40
mM lutein, respectively. The effect of hypoxia on intracellular ROS
production was detected by flow cytometry. As presented in Fig. 6, cellular ROS levels under hypoxic
conditions exposed to H2O2 were increased
compared with those in the untreated control cells (Fig. 6C). Conversely, treatment with
lutein and NAC (a known ROS scavenger) effectively reduced ROS
levels in MDA-MB-157 and MCF-7 cells under hypoxia. Next, the
association between the ROS accumulation and differential
expression of HIF-1α, NOTCH3, and HES1 was determined using RT-qPCR
and western blot analysis (Fig.
6). The mRNA levels of HIF-1α were not significantly altered in
response to ROS (Fig. 6A and B).
The mRNA level of NOTCH3 and HES1 was significantly increased under
hypoxia and H2O2 interference compared with
the untreated control, but significantly decreased following
treatment with lutein and NAC under hypoxia (Fig. 6A and B). Western blot analysis
revealed that hypoxia and the H2O2-induced
increase in cellular ROS levels significantly upregulated protein
levels of HIF-1α, NOTCH components and HES1 compared with those in
control cells. However, expression of these proteins was markedly
suppressed following lutein and NAC treatment (Fig. 6D and E). Taken together, these
results suggested that the increase of cellular ROS levels
activated HIF-1α, as well as its downstream signaling under
hypoxia, while lutein decreased the expression of HIF-1α via the
suppression of hypoxia-driven ROS in breast cancer cells.
Discussion
Due to improvements in screening, diagnostics,
surgery and chemotherapeutic options, the mortality rate of breast
cancer has abated over the last decade (7). However, metastatic breast cancer is
heterogeneous and has a high mortality rate due to its inherently
aggressive biology. Previous studies have determined that lutein
suppresses multiple types of tumor, and the antitumor effects of
lutein have been associated with a decline in proliferative signals
and growth suppression (21,22,27).
Previous studies have focused on the associations between lutein
and hypoxia. However, the mechanism underlying lutein-induced
interference with the progression of breast cancer under hypoxic
conditions remains unknown. The results of the present study
confirmed that lutein was able to significantly inhibit the
proliferation of human breast cancer cells in a
concentration-dependent manner, by suppressing the proliferation,
invasion, and migration of cells under hypoxia and normoxia.
It has previously been suggested that HES1 is a
crucial transcription factor in cancer cells, and its upregulation
is associated with cancer initiation, cell differentiation, tumor
malignancy and tumorigenicity of cancer stem cells (13). These events contribute to the
malignancy of cancer cells (28).
The present study, consistent with previous studies (14,24),
demonstrated that HES1 expression levels increased in breast cancer
cells under hypoxia compared with normoxia. Furthermore,
hypoxia-induced HES1 accumulation was inhibited following lutein
treatment, and lutein-induced decrement in HES1 expression was
associated with the inhibition of cell proliferation under hypoxia.
To confirm the involvement of HES1 in lutein-mediated antitumor
effects under hypoxia, HES1 was overexpressed by transfecting
breast cancer cells with HES1 overexpression constructs.
Overexpression of HES1 reversed the antitumor effects of
lutein.
Notably, HES1 has also been implicated in
EMT-induced tumor invasion and metastasis, which facilitates the
loss of cell adhesion and increases motility and survival in the
detached condition (17,29). A previous study has suggested that
HES1 facilitates EMT processes by activating the protein kinase
B/phosphatase and tensin homolog axis, which has critical effects
on the regulation of EMT in tumors (17). It is imperative to study the
effects of lutein on EMT. Upregulation of Snail, Zinc finger E-box
binding homeobox and Twist families, which are the direct
transcriptional disruptors of E-cadherin (a marker of epithelial
cells), and upregulation of mesenchymal molecular markers
(including vimentin, N-cadherin and fibronectin) are fundamental
molecular events in EMT progression (30). The results of the present study
indicated that lutein interference notably increased cell-cell
interaction by upregulating E-cadherin, and maintained steady-state
by downregulating N-cadherin and vimentin in breast cancer cells.
Therefore, these results indicated that lutein-induced inhibition
of EMT is mediated by suppression of HES1. In addition,
lutein-induced inhibition of cancer cell invasion and migration was
observed, which was consistent with the EMT-associated features of
cancer cells.
The regulation of HES1 is mediated by NOTCH
signaling, which is a prominent canonical pathway that serves
important functions in proliferation, invasion and apoptosis in
cancer cells (31). Accumulating
evidence indicates that phenotypes in malignant tumors are often
accompanied by activated NOTCH signaling and elevated HES1, which
are blocked by treatment with γ-secretase inhibitors (31). NOTCH intracellular domain (NICD) is
released and translocated within the nucleus, where it facilitates
transcription by binding with ligands of the NOTCH receptor (JAG1,
JAG2 and DLL1, 3 and 4) (12).
Previous studies have suggested a novel intersection between NOTCH
signaling and HIF-1α. HIF-1α induces the upregulation of the NOTCH
ligand DLL4 gene, increases γ-secretase complex activity and
mediates the stabilization of transcriptionally-active NICD
(25). However, the mechanism
underlying lutein-induced inhibition of HES1 remains to be fully
understood. In the present study, HIF-1α was observed to accumulate
in breast cancer cells under hypoxia and to activate NOTCH
signaling, which in turn induces the expression of the NOTCH target
gene HES1 through NOTCH3 and NOTCH ligands in these cells.
Meanwhile, treatment with lutein reversed hypoxia-induced
upregulation of HIF-1α and the NOTCH signaling pathway.
Consequently, NOTCH-induced HES1 expression also decreased.
Increases in the rate of cellular metabolism and
oxygen consumption have been attributed to hypoxic conditions in
the center of breast cancer tissues, and these conditions
exacerbate sprout angiogenesis and increase cancer invasiveness.
ROS, including hydrogen peroxides, superoxide, hydroxyl radical and
singlet oxygen, are chemically reactive molecules arising from
miscellaneous enzymatic processes and the mitochondrial respiratory
chain (32). Malignant cancer
cells show elevated steady-state ROS stress compared with healthy
cells, partly due to its involvement in tumorigenesis and oncogenic
stimulation (11). Numerous
oncogenic factors interact with high levels of ROS and promote the
proliferation, invasion and metastasis of tumor cells. Previous
studies have indicated that ROS are not only produced in hyperoxic
conditions, but also under hypoxia (32). Hypoxia leads to an increase in the
production of ROS from different sources, including mitochondrial
transport of electrons, NADPH oxidase and xanthine oxidase. ROS are
involved in diverse biological and pathological activities. ROS
levels are frequently high in cancer cells and regulate cancer cell
proliferation, invasion, and apoptosis (33,34).
Moderate ROS levels activate a cascade of signals as a secondary
messenger, and regulate responses to various stimulations (33). Previous studies have demonstrated
that ROS serve an important modulatory function in the mediation of
HIF-1α activity under hypoxia in vivo as well as in
vitro (11). ROS production is
required for the direct or indirect regulation of translocation,
activation, and degradation of HIF-1α via PHD family modification
in response to hypoxia (9). To
confirm whether lutein suppresses hypoxia-induced HIF-1α as well as
its downstream pathways, NOTCH signaling and HES1, our group also
evaluated the effect of NAC and H2O2 in
breast cancer cells under hypoxia. The results indicated that
H2O2 exposure was responsible for the
elevated ROS levels and increased HIF-1α expression, which
subsequently increased the expression of NOTCH signaling molecules
and HES1, while the effect of NAC was the opposite. These results
suggested that, compared with NAC, the effect of lutein-induced
HIF-1α expression is attributable to the reduction of ROS under
hypoxia; as this effect was reversible when ROS levels were
elevated by H2O2.
In conclusion, the results of the present study
demonstrated that lutein suppressed hypoxia-induced proliferation,
invasion, and migration of breast cancer cells in vitro by
decreasing HES1 expression. Lutein markedly suppressed
hypoxia-induced ROS levels, which is essential for the stability of
HIF-1α. Subsequently, lutein inhibited HES1 expression through the
NOTCH signaling pathway in breast cancer cells. Furthermore,
lutein-induced HES1 inhibition contributed to inhibition of EMT, a
process in various malignant tumors that is associated with
increased invasion and migration. These results provide insight
into the molecular mechanisms underlying the regulation of HES1 by
lutein through HIF-1α and NOTCH signaling, suggesting that lutein
may be used as a potential agent for the development of novel
therapeutic approaches for the treatment of breast cancer in the
future.
Glossary
Abbreviations
Abbreviations:
|
LUT
|
lutein
|
|
EMT
|
epithelial-mesenchymal transition
|
|
ROS
|
reactive oxygen species
|
|
NAC
|
N-acetylcysteine
|
Acknowledgments
Not applicable.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant no. 81500433)
and the Foundation of Henan Educational Committee (grant no.
16A310003)
Availability of data and materials
The datasets generated during the current study are
available from the corresponding author on reasonable request.
Authors’ contributions
SZ and MW conceived the study and participated in
its design and coordination. YL and XL performed the experiments
and contributed to data collection. YL and YZ analyzed the data and
drafted the manuscript. PW and JY assisted in designing experiments
and provided technical expertise in conducting experiments, and
reviewed the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Harbeck N and Gnant M: Breast cancer.
Lancet. 389:1134–1150. 2017. View Article : Google Scholar
|
|
2
|
Fan L, Strasser-Weippl K, Li JJ, St Louis
J, Finkelstein DM, Yu KD, Chen WQ, Shao ZM and Goss PE: Breast
cancer in China. Lancet Oncol. 15:e279–e289. 2014. View Article : Google Scholar
|
|
3
|
Sariego J: Breast cancer in the young
patient. Am Surg. 76:1397–1400. 2010.
|
|
4
|
Fillmore CM and Kuperwasser C: Human
breast cancer cell lines contain stem-like cells that self-renew,
give rise to phenotypically diverse progeny and survive
chemotherapy. Breast Cancer Res. 10:R252008. View Article : Google Scholar
|
|
5
|
Mayer IA, Abramson VG, Lehmann BD and
Pietenpol JA: New strategies for triple-negative breast cancer -
deciphering the heterogeneity. Clin Cancer Res. 20:782–790. 2014.
View Article : Google Scholar
|
|
6
|
Ward C, Langdon SP, Mullen P, Harris AL,
Harrison DJ, Supuran CT and Kunkler IH: New strategies for
targeting the hypoxic tumour microenvironment in breast cancer.
Cancer Treat Rev. 39:171–179. 2013. View Article : Google Scholar
|
|
7
|
Tang Y, Wang Y, Kiani MF and Wang B:
Classification, treatment strategy, and associated drug resistance
in breast cancer. Clin Breast Cancer. 16:335–343. 2016. View Article : Google Scholar
|
|
8
|
Semenza GL: Targeting HIF-1 for cancer
therapy. Nat Rev Cancer. 3:721–732. 2003. View Article : Google Scholar
|
|
9
|
Zepeda AB, Pessoa A Jr, Castillo RL,
Figueroa CA, Pulgar VM and Farías JG: Cellular and molecular
mechanisms in the hypoxic tissue: Role of HIF-1 and ROS. Cell
Biochem Funct. 31:451–459. 2013. View
Article : Google Scholar
|
|
10
|
Balamurugan K: HIF-1 at the crossroads of
hypoxia, inflammation, and cancer. Int J Cancer. 138:1058–1066.
2016. View Article : Google Scholar
|
|
11
|
Galanis A, Pappa A, Giannakakis A, Lanitis
E, Dangaj D and Sandaltzopoulos R: Reactive oxygen species and
HIF-1 signalling in cancer. Cancer Lett. 266:12–20. 2008.
View Article : Google Scholar
|
|
12
|
Liu ZH, Dai XM and Du B: Hes1: A key role
in stemness, metastasis and multidrug resistance. Cancer Biol Ther.
16:353–359. 2015. View Article : Google Scholar
|
|
13
|
Rani A, Greenlaw R, Smith RA and Galustian
C: HES1 in immunity and cancer. Cytokine Growth Factor Rev.
30:113–117. 2016. View Article : Google Scholar
|
|
14
|
Danza G, Di Serio C, Rosati F, Lonetto G,
Sturli N, Kacer D, Pennella A, Ventimiglia G, Barucci R, Piscazzi
A, et al: Notch signaling modulates hypoxia-induced neuroendocrine
differentiation of human prostate cancer cells. Mol Cancer Res.
10:230–238. 2012. View Article : Google Scholar
|
|
15
|
Liu J, Lu WG, Ye F, Cheng XD, Hong D, Hu
Y, Chen HZ and Xie X: Hes1/Hes5 gene inhibits differentiation via
down-regulating Hash1 and promotes proliferation in cervical
carcinoma cells. Int J Gynecol Cancer. 20:1109–1116. 2010.
View Article : Google Scholar
|
|
16
|
Yuan R, Ke J, Sun L, He Z, Zou Y, He X,
Chen Y, Wu X, Cai Z, Wang L, et al: HES1 promotes metastasis and
predicts poor survival in patients with colorectal cancer. Clin Exp
Metastasis. 32:169–179. 2015. View Article : Google Scholar
|
|
17
|
Wang SC, Lin XL, Wang HY, Qin YJ, Chen L,
Li J, Jia JS, Shen HF, Yang S, Xie RY, et al: Hes1 triggers
epithelial-mesenchymal transition (EMT)-like cellular marker
alterations and promotes invasion and metastasis of nasopharyngeal
carcinoma by activating the PTEN/AKT pathway. Oncotarget.
6:36713–36730. 2015.
|
|
18
|
Johnson EJ: A biological role of lutein.
Food Rev Int. 20:1–16. 2004. View Article : Google Scholar
|
|
19
|
Nwachukwu ID, Udenigwe CC and Aluko RE:
Lutein and zeaxanthin: Production technology, bioavailability,
mechanisms of action, visual function, and health claim status.
Trends Food Sci Technol. 49:74–84. 2016. View Article : Google Scholar
|
|
20
|
Sindhu ER, Firdous AP, Ramnath V and
Kuttan R: Effect of carotenoid lutein on
N-nitrosodiethylamine-induced hepatocellular carcinoma and its
mechanism of action. Eur J Cancer Prev. 22:320–327. 2013.
View Article : Google Scholar
|
|
21
|
Lakshminarayana R, Sathish UV, Dharmesh SM
and Baskaran V: Antioxidant and cytotoxic effect of oxidized lutein
in human cervical carcinoma cells (HeLa). Food Chem Toxicol.
48:1811–1816. 2010. View Article : Google Scholar
|
|
22
|
Rafi MM, Kanakasabai S, Gokarn SV, Krueger
EG and Bright JJ: Dietary lutein modulates growth and survival
genes in prostate cancer cells. J Med Food. 18:173–181. 2015.
View Article : Google Scholar
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
24
|
Chen J, Imanaka N, Chen J and Griffin JD:
Hypoxia potentiates Notch signaling in breast cancer leading to
decreased E-cadherin expression and increased cell migration and
invasion. Br J Cancer. 102:351–360. 2010. View Article : Google Scholar
|
|
25
|
Borggrefe T, Lauth M, Zwijsen A,
Huylebroeck D, Oswald F and Giaimo BD: The Notch intracellular
domain integrates signals from Wnt, Hedgehog, TGFβ/BMP and hypoxia
pathways. Biochim Biophys Acta. 1863:303–313. 2016. View Article : Google Scholar
|
|
26
|
Wang Y, Ma J, Shen H, Wang C, Sun Y,
Howell SB and Lin X: Reactive oxygen species promote ovarian cancer
progression via the HIF-1α/LOX/E-cadherin pathway. Oncol Rep.
32:2150–2158. 2014. View Article : Google Scholar
|
|
27
|
Reynoso-Camacho R, González-Jasso E,
Ferriz-Martínez R, Villalón-Corona B, Loarca-Piña GF, Salgado LM
and Ramos-Gomez M: Dietary supplementation of lutein reduces colon
carcinogenesis in DMH-treated rats by modulating K-ras, PKB, and
β-catenin proteins. Nutr Cancer. 63:39–45. 2011.
|
|
28
|
Sang L, Roberts JM and Coller HA:
Hijacking HES1: How tumors co-opt the anti-differentiation
strategies of quiescent cells. Trends Mol Med. 16:17–26. 2010.
View Article : Google Scholar
|
|
29
|
Gao F, Huang W, Zhang Y, Tang S, Zheng L,
Ma F, Wang Y, Tang H and Li X: Hes1 promotes cell proliferation and
migration by activating Bmi-1 and PTEN/Akt/GSK3β pathway in human
colon cancer. Oncotarget. 6:38667–38680. 2015. View Article : Google Scholar
|
|
30
|
Yilmaz M and Christofori G: EMT, the
cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev.
28:15–33. 2009. View Article : Google Scholar
|
|
31
|
Wong NKY, Fuller M, Sung S, Wong F and
Karsan A: Heterogeneity of breast cancer stem cells as evidenced
with Notch-dependent and Notch-independent populations. Cancer Med.
1:105–113. 2012. View Article : Google Scholar
|
|
32
|
Movafagh S, Crook S and Vo K: Regulation
of hypoxia-inducible factor-1a by reactive oxygen species: New
developments in an old debate. J Cell Biochem. 116:696–703. 2015.
View Article : Google Scholar
|
|
33
|
Zhang C, Cao S, Toole BP and Xu Y: Cancer
may be a pathway to cell survival under persistent hypoxia and
elevated ROS: A model for solid-cancer initiation and early
development. Int J Cancer. 136:2001–2011. 2015. View Article : Google Scholar
|
|
34
|
Cao L, Chen X, Xiao X, Ma Q and Li W:
Resveratrol inhibits hyperglycemia-driven ROS-induced invasion and
migration of pancreatic cancer cells via suppression of the ERK and
p38 MAPK signaling pathways. Int J Oncol. 49:735–743. 2016.
View Article : Google Scholar
|