Introduction
Pancreatic cancer (PC) accounts for the majority of
pancreatic malignancies, and is a devastating disease characterized
by a late diagnosis, poor prognosis and resistance to chemotherapy
(1,2). Many patients have advanced
unresectable tumors at the time of diagnosis. The effectiveness of
current chemotherapeutic regimens and radiotherapy is not
sufficient. Therefore, additional treatment options for patients
with advanced PC are urgently required.
Heme oxygenase (HO)-1 is the inducible isoform of
the three heme oxygenases (HO-1, HO-2 and HO-3) that catalyze the
degradation of heme into biliverdin, carbon monoxide (CO) and free
iron (3). HO-1 and its metabolites
affect inflammatory and apoptotic properties. Furthermore, HO-1
regulates cancer cell proliferation and angiogenesis (4). In a recent studies, HO-1 expression
and activity was found in various tumors, such as prostate cancer
(5), lung cancer (6) and colon cancer (7). Data from previous studies have also
shown that HO-1 inhibits the proliferation of pancreatic stellate
cells (8), and that HO-1 affects
the responsiveness of PC to anticancer treatment (9).
The sonic hedgehog (SHH) signaling pathway plays a
fundamental role in organ development and differentiation. SHH,
which is a secreted protein, binds to Patched (PTCH) allows
smoothened (SMO) to activate downstream factors, such as Gli1 or
Gli2, and regulate target gene expression. The SHH signaling
pathway, a major regulator of cancer cell proliferation and
differentiation, contributes to metastasis (10). In PC cells, the SHH signaling
pathway has been shown to be involved in pain associated with PC
(11), and in the development of
pancreatic fibrosis (12).
Gemcitabine (Gem) is one of the most important
chemotherapeutic agents used in the treatment of patients with PC.
Although this drug is effective, its cytotoxic effects and drug
resistance limit its application and curative effects, leading to
poor patient outcomes. An increasing amount of research has been
carried out with an aim to determine the mechanisms responsible for
sensitivity and resistance to Gem (9,13,14).
Furthermore, the SHH signaling pathway is partly involved in the
resistance of PC cells to Gem (15,16).
The above-mentioned findings suggest that HO-1 and
SHH signaling may play a crucial role in PC progression and
sensitivity to chemotherapy. In this study, we examined the
potential effects of HO-1 on PC cell proliferation and sensitivity
to Gem. Furthermore, the role of the SHH signaling pathway in the
mechanisms of HO-1-induced PC cell proliferation were
investigated.
Materials and methods
Cell culture and reagents
The established human PC cell lines, BxPc-3, MIA
PaCa-2, PANC-1 and SW1990, were obtained from the American Type
Culture Collection (ATCC, Manassas, VA, USA) and cultured in DMEM
supplemented with 10% fetal bovine serum (FBS) and 1%
antibiotic/antimycotic (Life Technologies, Carlsbad, CA, USA). The
cells were maintained at 37°C in a humidified 5% CO2
atmosphere. Antibodies against SHH (ab53281), SMO (ab5694), PTCH
(ab53715), Gli1 (ab49314), Gli2 (ab26056), GAPDH (ab8245) and HO-1
(ab13248) were purchased from Abcam (Cambridge, MA, USA).
Recombinant SHH, and zinc proto-porphyrin IX (ZnPPIX) were obtained
from R&D Systems (Minneapolis, MN, USA).
Real-time PCR
Total RNA was extracted from the cells using TRIzol
reagent (Invitrogen, Carlsbad, CA, USA), and cDNA was synthesized
using a Prime Script RT reagent kit (Takara, Dalian, China). The
real-time PCR experiments were conducted on an iQ5 Multicolor
Real-Time PCR Detection System (Bio-Rad Laboratories Inc.,
Hercules, CA, USA) using SYBR-Green Real-time PCR Master Mix
(Takara). Amplification was carried out as follows: denaturation at
94°C for 3 min, 35 cycles of 94°C for 30 sec, 58°C for 30 sec, and
72°C for 35 sec. The sequences of the primers used were as follows:
HO-1 forward, 5′-CAG GCA GAG GGT GAT AGA AGA GG-3′ and reverse,
5′-CTG GGA GCG GGT GTT GAG TG-3′; and GAPDH forward,
5′-ACCACAGTCCATGCCATCAC-3′ and reverse, 5′-TCCACCACCCTGTTGCTGTA-3′.
The expression of the target gene was calculated using the
2−ΔΔCq method (17).
All experiments were repeated independently 3 times.
Western blot analysis
Protein was extracted from the cells using lysis
buffer [50 mM Tris (pH 7.5), 150 mM NaCl, 1% NP-40, 0.5% sodium
deoxycholate, 1 mM EDTA, and 0.1% SDS] containing a protease
inhibitor cocktail (Sigma-Aldrich, St. Louis, MO, USA), and protein
concentrations were measured by DC Protein Assay (Bio-Rad
Laboratories Inc.). Following separation on 7.5% SDS-polyacrylamide
gels, the proteins (20 μl) were transferred onto
nitrocellulose membranes (Millipore, Billerica, MA, USA), which
were then incubated with the primary antibodies (1:1,000) at 4°C
overnight. After washing 3 times with TBST, the membranes were
incubated with horseradish peroxidase-conjugated secondary
antibodies (rabbit, 1:1,000, ab191866; mouse, 1:1000, ab193651;
Abcam) for 1 h. Immunoreactive bands were visualized using an
enhanced chemiluminescence kit (Millipore). Quantitative analysis
was performed using Image-Pro Plus 6.0 software (Media Cybernetics,
Inc., Rockville, MD, USA). The relative protein expression levels
were normalized to GAPDH. All experiments were repeated
independently 3 times.
MTT assay
Cell proliferation rates were measured by MTT
assays. Briefly, the cells were seeded in 96-well plates at a
density of 1×104 cells per well and incubated overnight
in medium containing 10% FBS. The DMSO concentration was adjusted
to 0.4%. The cells incubated in serum-free medium were used as the
control group. Following incubation for 24, 48 and 72 h at 37°C, 20
μl of MTT solution [5 mg/ml in phosphate-buffered saline
(PBS)] were added to each well, and the cells were incubated for an
additional 4 h at 37°C. Subsequently, 100 μl DMSO were added
to each well at 37°C. The optical density (OD) value was determined
using a spectrophotometer (Bio-Rad Laboratories Inc.) at 490 nm.
The proliferation rate was defined as OD (cell plate)/OD (blank
plate). All experiments were repeated independently 3 times.
Immunofluorescence staining
HO-1 localization in PC cells was examined by
immunofluorescence. The prepared cells were washed 3 times with PBS
and then fixed with 100 ml 4% paraformaldehyde in PBS. The cells
were permeabilized in blocking buffer (0.1% Triton X-100 or
0.1–0.5% saponin, 10% NGS, 100 mM PBS, pH 7.4) for 1 h at room
temperature and then incubated with primary antibody to HO-1
overnight at 4°C. The following day, the cells were washed and
incubated with FITC-conjugated goat anti-mouse IgG (green
fluorescence; 1:500, anti-mouse, #115165003; Jackson
ImmunoResearch, West Grove, PA, USA) for 1 h at room temperature.
The cell nuclei were stained with 4′,6-diamidino-2-phenylindole
(DAPI; #0100-20, SouthernBiotech, Birmingham, AL, USA) for 10 min.
After washing with PBS 3 times, cells were blocked for 5 min. As a
negative control, the primary antibody was substituted with
antibody diluent. All experiments were repeated independently 3
times.
Transfection with HO-1 small interfering
RNA (siRNA) and plasmid construction
HO-1 expression was specifically suppressed by the
introduction of 21-nucleotide duplex siRNA. The sequences of the
ribonucleotides used were as follows:
5′-rGACUGCGUUCCUGCUCAACdTdT-3′ and 5′-rGUUGA GCAGGAACGCAGUCdTdT-3′
(Ambion, Inc., Austin, TX, USA). The cells seeded into small dishes
were transfected with 100 nM siRNA using Lipofectamine 2000
(Invitrogen) according to the manufacturer’s instructions. Negative
control siRNA (Ambion Inc.) was used as a negative control. In
addition, plasmids for the overexpression of HO-1 were constructed
according to manufacturer’s instructions (Roche, Penzberg,
Germany). The untransfected cells used in the experiment were
cultured under normal conditions. All experiments were repeated
independently 3 times.
Drug treatments
Recombinant SHH (#CYT597; ProSpec-Tany TechnoGene
Ltd., Ness-Ziona, Israel) was applied to the cultured PC cells at 5
mg/ml. In addition, cyclopamine (Cyc) (#4449518; ApexBio, Houston,
TX, USA), a SHH pathway inhibitor, was diluted to 18 μg/ml
in PC cell medium. The compound, ZnPPIX, an inhibitor of HO-1, was
used at 50 μM (18). Gem
was applied according to the PC cell line-specific EC50 (15
μg/ml for the MIA PaCa-2 cells and 100 μg/ml for the
PANC-1 cells (9,19).
Statistical analysis
The analyses of the results was carried out using
the SPSS statistical software package (version 13.0). The
significance of the data was determined using a Student’s t-test or
one-way analysis of variance (ANOVA), followed by the Bonferroni
post hoc test, where applicable. A value of P<0.05 was
considered to indicate a statistically significant difference. All
results are expressed as the means ± SD. All the experiments were
repeated 3 times.
Results
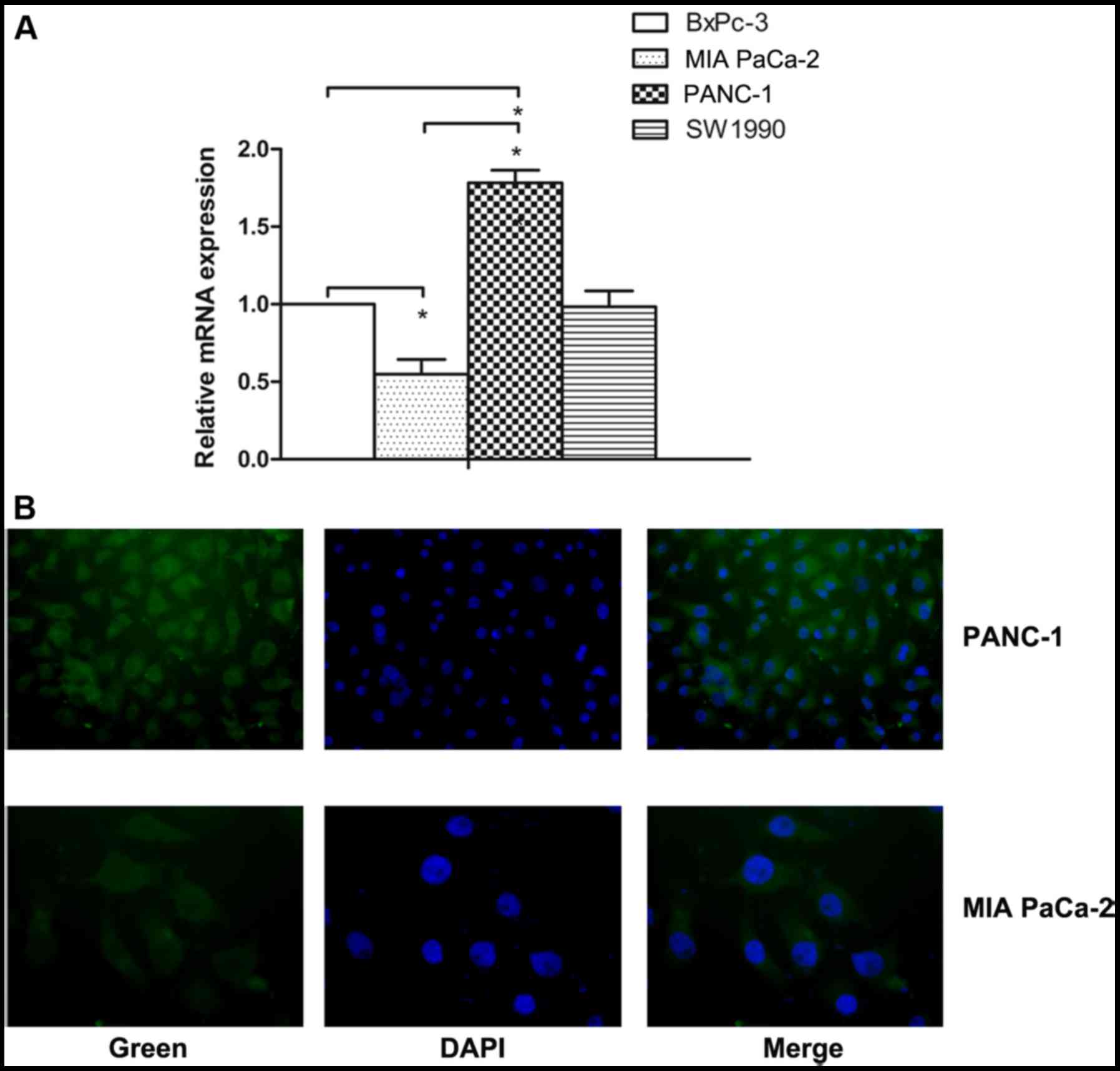
HO-1 is overexpressed in PC cells
To determine whether HO-1 plays an important role in
the progression of PC, the mRNA expression of HO-1 was identified
by real-time PCR in the BxPc-3, MIA PaCa-2, PANC-1 and SW1990 cells
human PC cells (Fig. 1A). The
results revealed that HO-1 mRNA expression differed significantly
in the different PC cells. On average, a 3-fold upregulation of
HO-1 mRNA expression was observed in the PANC-1 cells compared with
the MIA PaCa-2 cells (P<0.05).
Thus, we selected the MIA PaCa-2 and PANC-1 cell
lines for use in the following experiments due to the fact that
they expressed low and high levels of HO-1, respectively. The
protein expression of HO-1 was examined by the immunofluorescence
staining of the MIA PaCa-2 and PANC-1 cell lines (Fig. 1B). As a negative control, the
primary antibody was substituted with antibody diluent (data not
shown). The expression of HO-1 in the PC cells provided the basis
for our subsequent research.
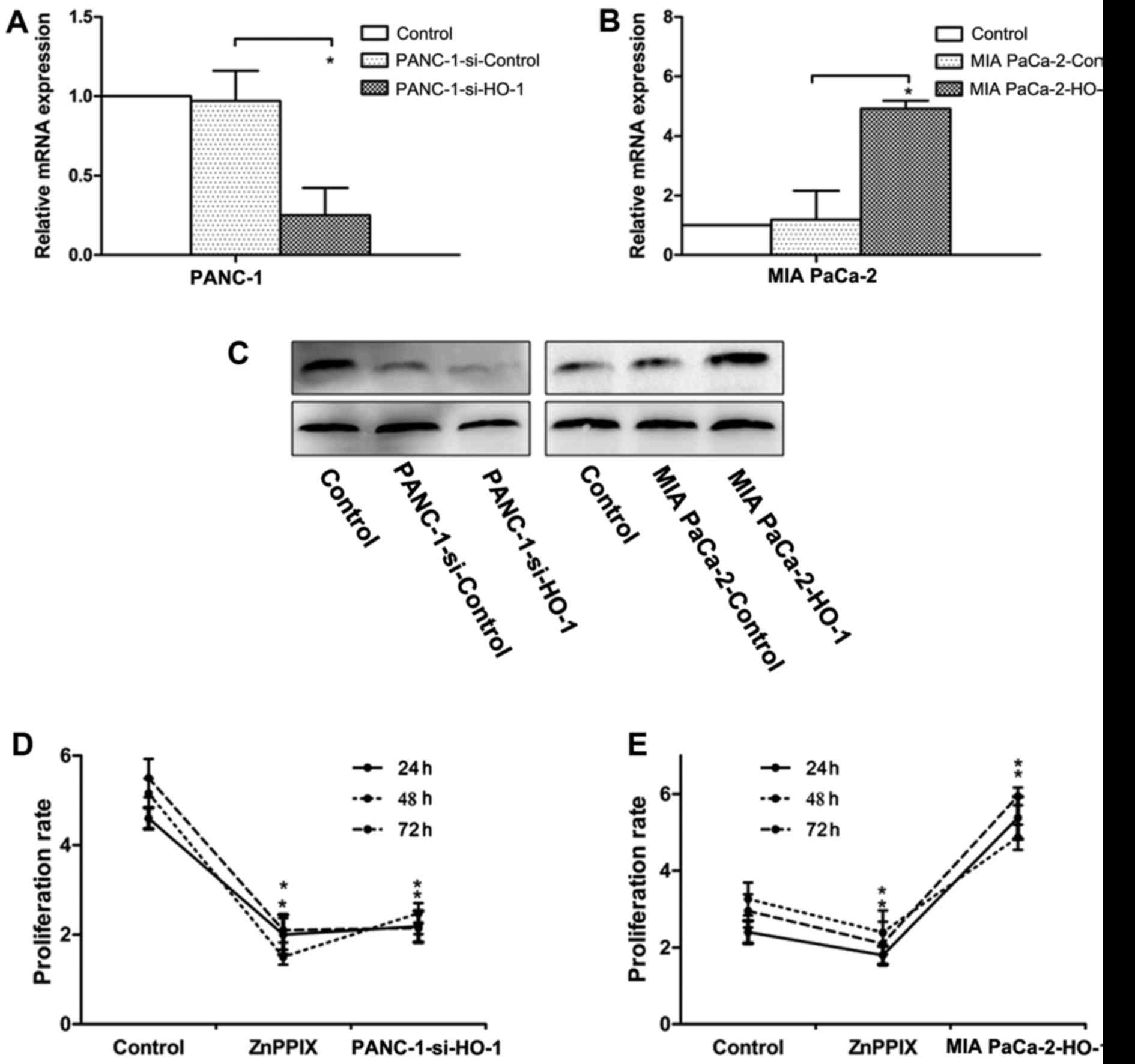
Expression of HO-1 induces PC cell
proliferation and inhibition of HO-1 reduces the proliferation
ability
To examine the effects of HO-1 on PC cell growth,
the PANC-1 (high HO-1 expression) and MIA PaCa-2 cells (low HO-1
expression) were used for transfection. The PANC-1 cells were
transfected with HO-1 siRNA, and the MIA PaCa-2cells were
transfected with HO-1 expression plasmids. At 12 h after
transfection, the transfection efficiencies were verified by
real-time PCR (Fig. 2A and B) and
western blot analysis (Fig. 2C).
Cell proliferation in response to HO-1 stimulation was measured by
MTT assays at 24, 48 and 72 h following transfection or treatment
with ZnPPIX. Our results revealed that the PANC-1 cells transfected
with siRNA (PANC-1-si-HO-1) exhibited a decreased rate of
proliferation compared with the control group; ZnPPIX was used as a
positive control and treatment with ZnPPIX also decreased cell
proliferation (P<0.05) (Fig.
2D). On the contrary, the MIA PaCa-2 cells transfected with the
HO-1 expression plasmid exhibited an increased proliferation rate
(P<0.05) (Fig. 2E).
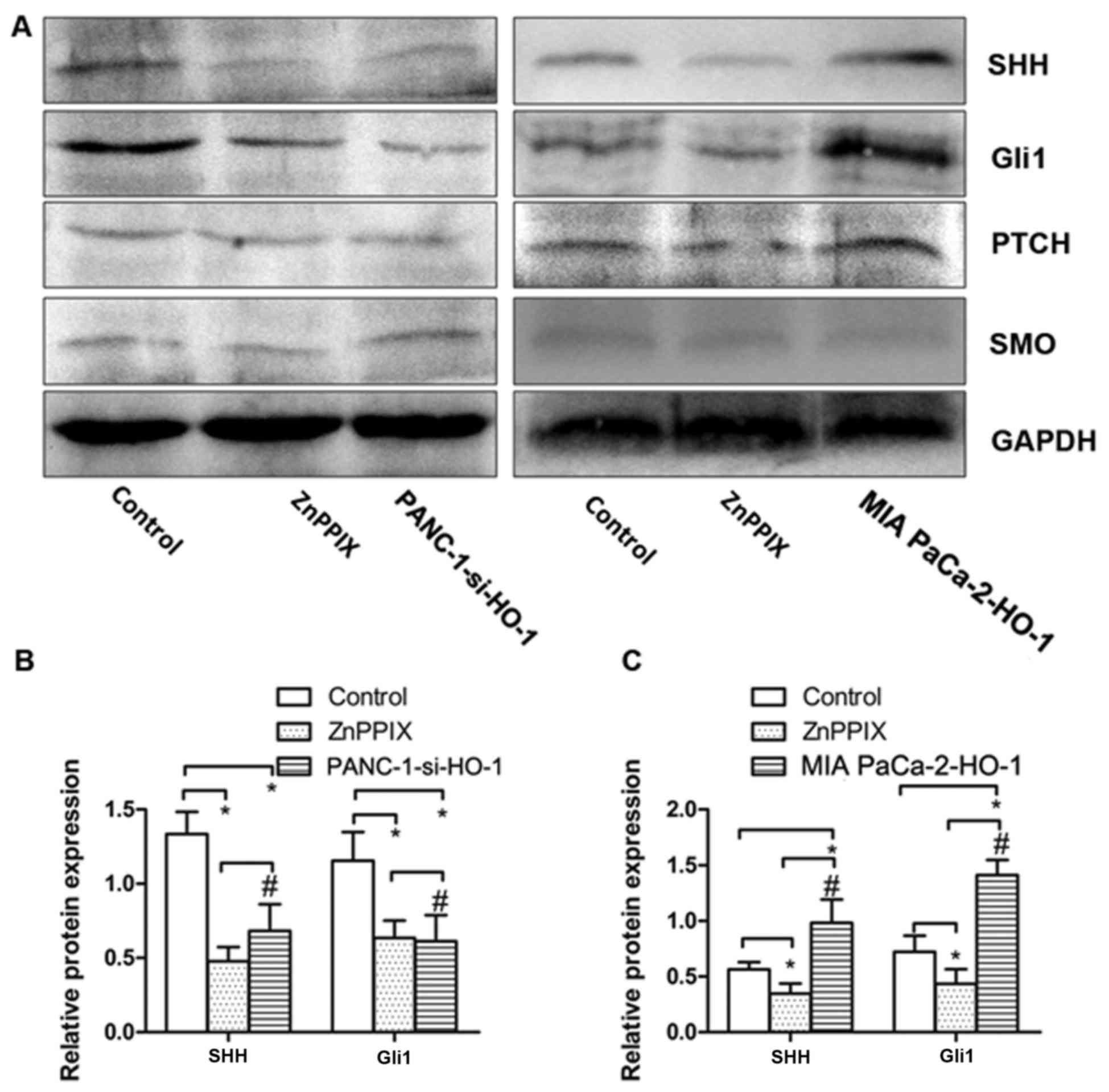
HO-1 induces SHH signaling pathway
activation in PC cells
To examine the effects of HO-1 on the SHH signaling
pathway in the PC cells, we examined the expression of molecules
associated with the SHH signaling pathway in the PC cells under
various treatment conditions by western blot analysis (Fig. 3A). Based on this analysis, the
PANC-1 cells transfected with siRNA against HO-1 (PANC-1-si-HO-1)
exhibited a decreased expression of SHH and Gli1 compared with the
untransfected PANC-1 cells; similar results were obtained with the
PANC-1 cells treated with the positive control, ZnPPIX (P<0.050
(Fig. 3B). We also found that the
MIA PaCa-2 cells transfected with the HO-1 expression plasmid (MIA
PaCa-2-HO-1) exhibited an increased expression of SHH and Gli1
(P<0.050 (Fig. 3B). However, no
marked differences were observed in the expression of PTCH and SMO
the cell groups (P>0.05). Thus, these results indicate that HO-1
activates the SHH signaling pathway in PC cells.
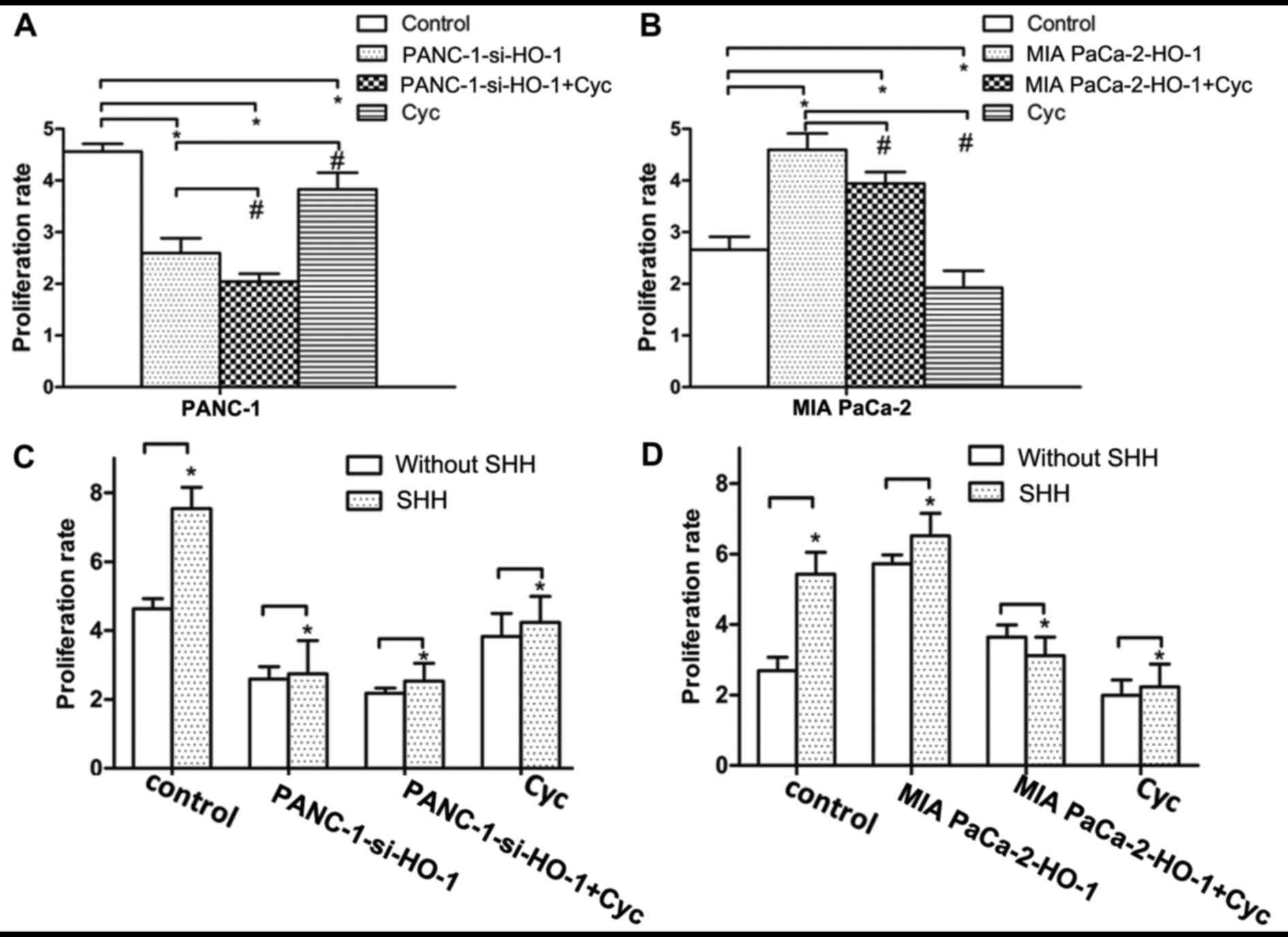
SHH signaling pathway plays role in
HO-1-induced PC cell proliferation
To determine whether the SHH pathway is involved in
the high proliferation rate induced by HO-1, as shown in Fig. 4, the PANC-1 and MIA PaCa-2 cells
were treated with Cyc, which is a SHH signaling pathway inhibitor.
The results revealed the dose-dependent effects of Cyc on cell
proliferation (data not shown). In addition, treatment of the
PANC-1 cells with Cyc in combination with transfection with the
HO-1 overexpression vector significantly decreased the PANC-1 cell
proliferative ability in comparison with the controls or the other
intervention groups (P<0.05) (Fig.
4A). Additionally, as regards the MIA PaCa-2 cells, the results
revealed that treatment of these cells with Cyc inhibited the
proliferation, compared with the control group and the MIA PaCa-2
cells transfected with the expression vector and not treated with
Cyc (P<0.05) (Fig. 4B). Thus,
HO-1 was found to activate the SHH signaling pathway in PC cells;
thus, this suggests that the activation of the SHH signaling
pathway is involved in HO-1-induced PC cell proliferation. However,
the mechanisms through which the SHH signaling pathway is activated
by HO-1 remain unknown. Whether the activation of the SHH signaling
pathway occurs through an autocrine manner or through other
mechanisms, is worthy of investigation. To clarify this, we used
exogenous SHH to treat the PC cells. The results revealed that
differences were only found between the cells not treated with SHH
and those that were treated with SHH in the control group; no
significant changes in the cell proliferative ability were observed
in the other 3 intervention groups in both cell lines. (P>0.05)
(Fig. 4C and D).
Inhibition of HO-1 enhances the
responsiveness of PC cells to Gem
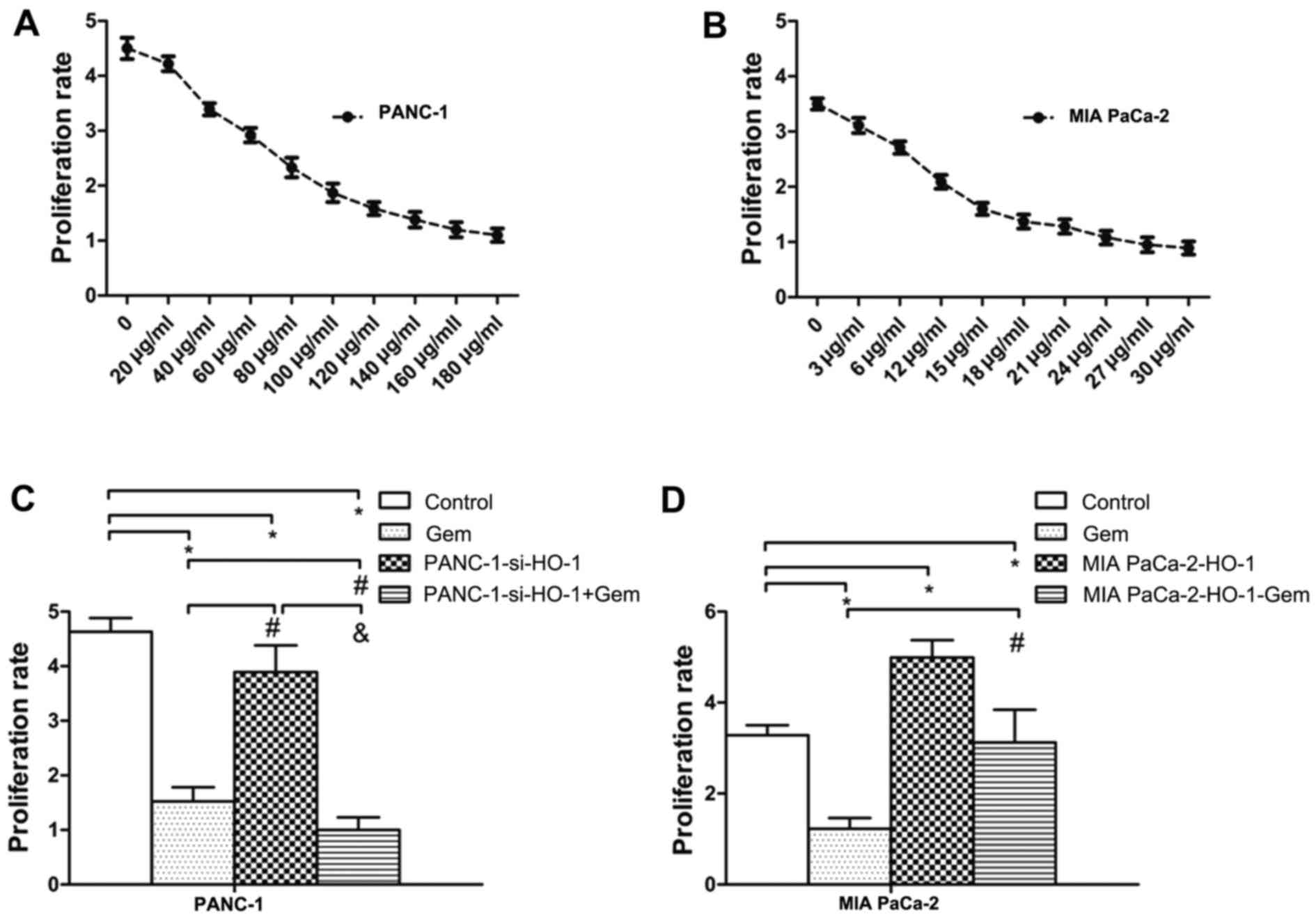
To determine whether the chemoresistance of PC cells
is due to HO-1, we examined PC cell proliferation when the MIA
PaCa-2 and PANC-1 cells were transfected with either HO-1 siRNA or
the HO-1 expression plasmid and treated with Gem (EC50 dose). The
results of the dose-dependent experiments of Gem treatment are
shown in Fig. 5A and B). We found
that the PANC-1 cells transfected with HO-1 siRNA (PANC-1-si-HO-1)
and treated with Gem exhibited a decreased proliferative ability
(P<0.05) (Fig. 5C). By
contrast, MIA PaCa-2 cells transfected with the HO-1 expression
vector and treated with Gem exhibited a greater proliferative
ability than the MIA PaCa-2 cells treated with Gem alone
(P<0.05) (Fig. 5D). Thus, a
decreased proliferative ability was observed after HO-1 inhibition
and treatment with Gem.
HO-1 and SHH pathway activation can be
induced by Gem in PC cells chemosensitivity
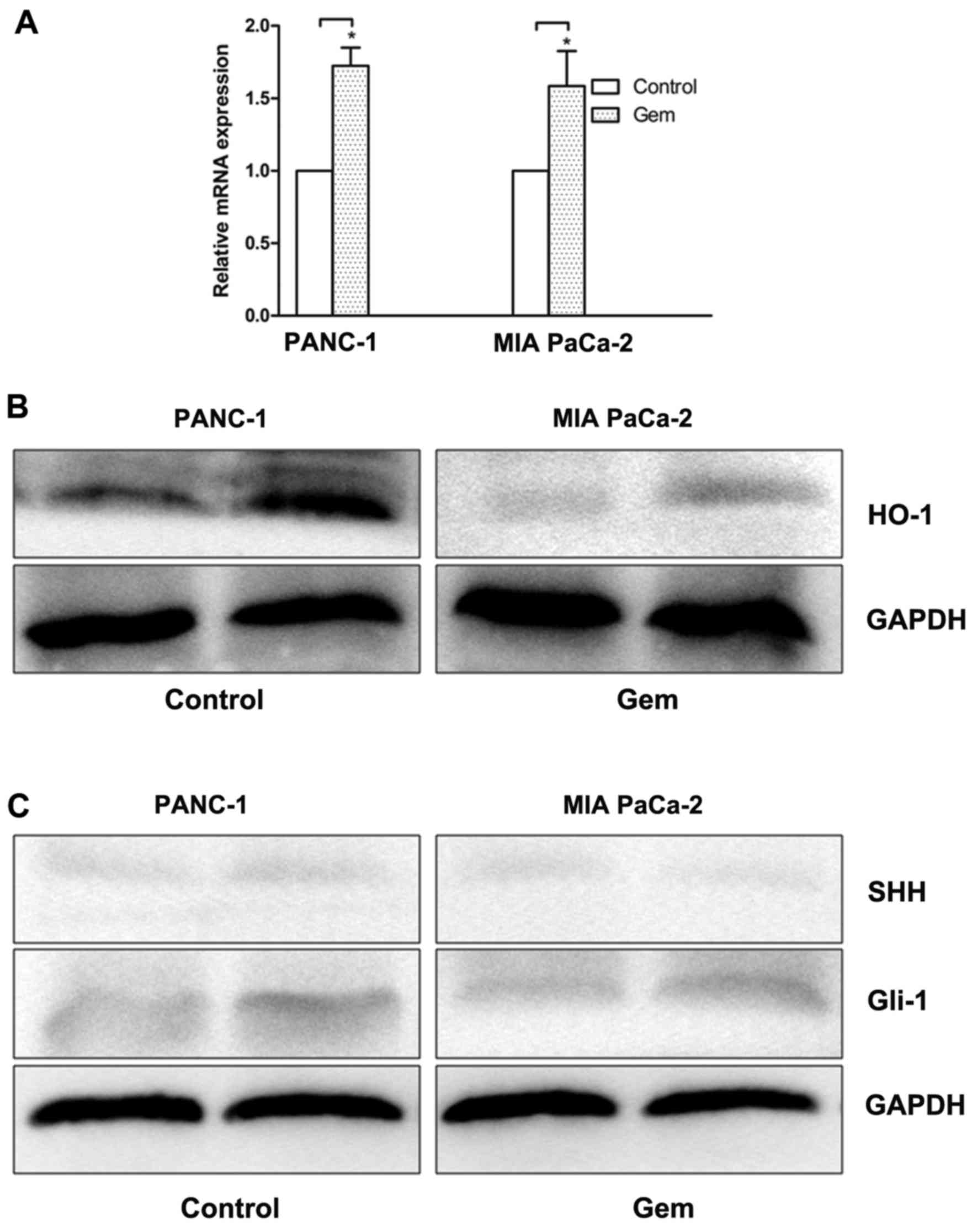
To determine whether HO-1 affects the
chemosensitivity of PC cells, we treated the PC cells with an EC50
dose of Gem for 24 h. The quantification data from real-time PCR
revealed that Gem increased the mRNA expression of HO-1 in the
PANC-1 and MIA PaCa-2 cells compared with the untreated controls
(P<0.05) (Fig. 6A).
Furthermore, the cells treated with Gem exhibited an increased
protein expression of HO-1 in both cell lines (P<0.05) (Fig. 6B). To determine whether Gem is
responsible for the activation of the SHH pathway, we examined the
expression of SHH and Gli1 in the PC cells treated with Gem.
Treatment with Gem resulted in an increase in the expression of
Gli1 in the PANC-1 and MIA PaCa-2 cells (P<0.05) (Fig. 6C). However, no signifi-cant
induction of SHH expression observed in both cell lines
(P>0.05).
Discussion
PC is one of the most lethal types of cancer. While
the mortality rates are improving as with other types of cancer,
the clinical outcomes of patients with PC remains abysmally poor
(1). HO-1 is the rate-limiting
enzyme involed in the degradation of heme into carbon monoxide,
iron and biliverdin. HO-1 regulates cell proliferation (4) and accelerates PC angiogenesis
(20). The proliferation of
pancreatic stellate cells, which were the key process in PC
development has been shown to be inhibited by HO-1 via the
extracellular signal-regulated kinase (ERK)1/2 pathway (8). Furthermore, HO-1 functions as a
mediator of the therapeutic effects (21). The present study focused on the
association between HO-1 and SHH in PC. As reported previously, SHH
regulates pancreatic fibrosis and cancer cell proliferation and
differentiation (22,23). Gem is one of the most important
chemotherapeutic agents used in the treatment of the majority of
patients with PC (13,24). Over the past several years,
first-line therapy with Gem has boosted the median overall survival
(OS) of patients with PC (25,26).
However, the effectiveness of Gem is limited due to drug resistance
in patients with PC. Thus far, the mechanisms of resistance have
been tested for mutations in several genes; however, the mechanisms
involved are not yet very clear (27,28).
Some studies have reported the correlation between resistance to
Gem and SHH signaling (15,29).
The main results of this study indicate that the
upregulation of HO-1 expression promotes PC cell proliferation, and
that the inhibition of HO-1 enhances the responsiveness of PC cells
to Gem. In addition, our data indicate that the SHH signaling
pathway plays an important role in this process.
This study demonstrated that the expression levels
of HO-1 differed among the PC cell lines. Furthermore, the MIA
PaCa-2 and PANC-1 cell lines were selected for use in the following
experiments due to the fact that they expressed low and high levels
of HO-1, respectively. We suppressed HO-1 expression by siRNA
transfection and evaluated the growth behavior of the PANC-1 cells.
The analysis revealed a significant inhibitory effect on the
proliferation of the cells expressing low levels of HO-1; similar
results were obtained with cells treated with the HO-1 activity
inhibitor, ZnPPIX. Moreover, we found a significantly increased
proliferation rate in the MIA PaCa-2 cells expressing high HO-1
expression levels by transfection with an HO-1 expression plasmid
in comparison to the control group. Thus, the expression of HO-1
induced PC cell proliferation and the inhibition of HO-1 suppresses
PC cell proliferation.
More importantly, we examined the potential
mechanisms responsible for HO-1-induced PC cell proliferation. We
examined the effect of HO-1 on the SHH signaling pathway in PC
cells. The results revealed that the PANC-1 cells transfected with
siRNA against HO-1 exhibited a decreased expression of SHH and Gli1
compared with the untransfected PANC-1 cells. However, the MIA
PaCa-2 cells expressing high levels of HO-1 by transfection with an
HO-1 expression plasmid exhibited an increased expression of SHH
and Gli1. No marked differences were observed in the levels of PTCH
and SMO. In our study, it was evident that HO-1 activated the SHH
signaling pathway in PC cells. Our results supported by evidence
from a previous study on the effects of HO-1 upregulation on the
SHH signaling pathway (30).
In following experiments, treatment with Cyc
significantly decreased the proliferative ability when the
expression of HO-1 was decreased by siRNA. Thus, it was proven that
the SHH signaling pathway also acts as a downstream target of HO-1
in PC cells. Moreover, PC cells were treated with exogenous SHH.
However, no significant differences in proliferation were observed
among the different intervention groups. Therefore, the activation
of the SHH signaling pathway did not occur in an autocrine manner
(31). Thus, the activation of SHH
signaling pathway by HO-1 occurs through other mechanisms, as for
example, via transcriptional activation or alternative pathways
(32,33). Unfortunately, in this study, the
detailed mechanisms of SHH signaling pathway activation and their
role in HO-1-induced PC cell proliferation were not investigated,
and thus further research is required on this matter.
Our data demonstrated that treatment with Gem led to
the pronounced growth inhibition of the PC cells with the
downregulation of HO-1. On the contrary, the upregulation of HO-1
suppressed the inhibitory effect of Gem on PC cell proliferation.
Our in vitro results are similar to those of other studies
on HO-1 or its metabolites and PC growth in vivo (4) and on HO-1 and the responsiveness of
PC to anticancer treatment (9).
Furthermore, our results provide further information and add to the
findings of these other studies, and the SHH pathway may be one of
the mechanisms involved. Therefore, the specific inhibition of HO-1
expression may be used as a sensitizer to chemotherapy (9,34).
In addition, we explored the potential mechanisms of
HO-1-induced PC cell proliferation and the response of PC cells to
Gem. Treatment of the PC cells with Gem strongly induced HO-1
expression. Moreover, Gem enhanced Gli1 expression, although no
difference was observed in SHH expression. Thus, Gem is responsible
for activation of the SHH pathway through Gli1, but not through
autocrine SHH. The results revealed the combined antitumor effects
of Gem and the SHH signaling pathway on PC cells (16). Although the relatively integrated
analysis of the HO-1 mechanisms was carried out in this study, the
actual cause and effect were not expounded completely, and thus
further studies are warranted. However, the mechanisms of
HO-1-induced cell proliferation are complex, and the results were
shown only in vitro in this study. Thus, further studies
performing in vivo experiments are also required.
In conclusion, the data from this study demonstrate
that high HO-1 levels in PC cells are responsible for tumor cell
proliferation and resistance to anticancer therapy. Furthermore,
the SHH signaling pathway, which is initiated by HO-1, may act as
an endogenous mechanism in this process. This study enhanced our
understanding of the exact molecular processes of HO-1 and SHH in
PC cells, may aid in the development of novel therapeutic targets
for the treatment of patients with PC.
Acknowledgments
Not applicable.
Funding
This study was supported by the National Natural
Science Foundation of China (grant no. 81502074).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors’ contributions
The author contributions were as follows: Design of
the experiments, ZWa and LH; performing the experiments, LH and JJ;
data analysis, ZWu and QM; contribution of
reagents/materials/analysis tools, ZWa; writing of the article, LH
and JJ. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar
|
|
2
|
Liu Q, Liao Q and Zhao Y: Chemotherapy and
tumor microenvi-ronment of pancreatic cancer. Cancer Cell Int.
17:682017. View Article : Google Scholar
|
|
3
|
Otterbein LE and Choi AM: Heme oxygenase:
Colors of defense against cellular stress. Am J Physiol Lung Cell
Mol Physiol. 279:L1029–L1037. 2000. View Article : Google Scholar
|
|
4
|
Nuhn P, Künzli BM, Hennig R, Mitkus T,
Ramanauskas T, Nobiling R, Meuer SC, Friess H and Berberat PO: Heme
oxygenase-1 and its metabolites affect pancreatic tumor growth in
vivo. Mol Cancer. 8:372009. View Article : Google Scholar
|
|
5
|
Heeba GH, Hamza AA and Hassanin SO:
Induction of heme oxygenase-1 with hemin alleviates
cisplatin-induced reproductive toxicity in male rats and enhances
its cytotoxicity in prostate cancer cell line. Toxicol Lett.
264:38–50. 2016. View Article : Google Scholar
|
|
6
|
Jo EJ, Park SJ and Kim BC: Propyl gallate
sensitizes human lung cancer cells to cisplatin-induced apoptosis
by targeting heme oxygenase-1 for TRC8-mediated degradation. Eur J
Pharmacol. 788:321–327. 2016. View Article : Google Scholar
|
|
7
|
Jang HJ, Hong EM, Kim M, Kim JH, Jang J,
Park SW, Byun HW, Koh DH, Choi MH, Kae SH, et al: Simvastatin
induces heme oxygenase-1 via NF-E2-related factor 2 (Nrf2)
activation through ERK and PI3K/Akt pathway in colon cancer.
Oncotarget. 7:46219–46229. 2016. View Article : Google Scholar
|
|
8
|
Schwer CI, Guerrero AM, Humar M, Roesslein
M, Goebel U, Stoll P, Geiger KK, Pannen BH, Hoetzel A and Schmidt
R: Heme oxygenase-1 inhibits the proliferation of pancreatic
stellate cells by repression of the extracellular signal-regulated
kinase1/2 pathway. J Pharmacol Exp Ther. 327:863–871. 2008.
View Article : Google Scholar
|
|
9
|
Berberat PO, Dambrauskas Z, Gulbinas A,
Giese T, Giese N, Künzli B, Autschbach F, Meuer S, Büchler MW and
Friess H: Inhibition of heme oxygenase-1 increases responsiveness
of pancreatic cancer cells to anticancer treatment. Clin Cancer
Res. 11:3790–3798. 2005. View Article : Google Scholar
|
|
10
|
Yoo YA, Kang MH, Lee HJ, Kim BH, Park JK,
Kim HK, Kim JS and Oh SC: Sonic hedgehog pathway promotes
metastasis and lymphangiogenesis via activation of Akt, EMT, and
MMP-9 pathway in gastric cancer. Cancer Res. 71:7061–7070. 2011.
View Article : Google Scholar
|
|
11
|
Han L, Ma J, Duan W, Zhang L, Yu S, Xu Q,
Lei J, Li X, Wang Z, Wu Z, et al: Pancreatic stellate cells
contribute pancreatic cancer pain via activation of sHH signaling
pathway. Oncotarget. 7:18146–18158. 2016.
|
|
12
|
Jung IH, Jung DE, Park YN, Song SY and
Park SW: Aberrant Hedgehog ligands induce progressive pancreatic
fibrosis by paracrine activation of myofibroblasts and ductular
cells in transgenic zebrafish. PLoS One. 6:e279412011. View Article : Google Scholar
|
|
13
|
Shukla SK, Purohit V, Mehla K, Gunda V,
Chaika NV, Vernucci E, King RJ, Abrego J, Goode GD, Dasgupta A, et
al: MUC1 and HIF-1alpha signaling crosstalk induces anabolic
glucose metabolism to impart gemcitabine resistance to pancreatic
cancer. Cancer Cell. 32:3922017. View Article : Google Scholar
|
|
14
|
Jin X, Pan Y, Wang L, Ma T, Zhang L, Tang
AH, Billadeau DD, Wu H and Huang H: Fructose-1,6-bisphosphatase
inhibits ERK activation and bypasses gemcitabine resistance in
pancreatic cancer by blocking IQGAP1-MAPK interaction. Cancer Res.
77:4328–4341. 2017. View Article : Google Scholar
|
|
15
|
Wang H, Ning Z, Li Y, Zhu X and Meng Z:
Bufalin suppresses cancer stem-like cells in gemcitabine-resistant
pancreatic cancer cells via Hedgehog signaling. Mol Med Rep.
14:1907–1914. 2016. View Article : Google Scholar
|
|
16
|
Kim EJ, Sahai V, Abel EV, Griffith KA,
Greenson JK, Takebe N, Khan GN, Blau JL, Craig R, Balis UG, et al:
Pilot clinical trial of hedgehog pathway inhibitor GDC-0449
(vismodegib) in combination with gemcitabine in patients with
metastatic pancreatic adenocarcinoma. Clin Cancer Res.
20:5937–5945. 2014. View Article : Google Scholar
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
18
|
Cao Z, Geng B, Xu S, Xuan W, Nie L, Shen
W, Liang Y and Guan R: BnHO1, a haem oxygenase-1 gene from Brassica
napus, is required for salinity and osmotic stress-induced lateral
root formation. J Exp Bot. 62:4675–4689. 2011. View Article : Google Scholar
|
|
19
|
Chen R, Lai LA, Sullivan Y, Wong M, Wang
L, Riddell J, Jung L, Pillarisetty VG, Brentnall TA and Pan S:
Disrupting glutamine metabolic pathways to sensitize
gemcitabine-resistant pancreatic cancer. Sci Rep. 7:79502017.
View Article : Google Scholar
|
|
20
|
Sunamura M, Duda DG, Ghattas MH, Lozonschi
L, Motoi F, Yamauchi J, Matsuno S, Shibahara S and Abraham NG: Heme
oxygenase-1 accelerates tumor angiogenesis of human pancreatic
cancer. Angiogenesis. 6:15–24. 2003. View Article : Google Scholar
|
|
21
|
Jakstaite A, Maziukiene A, Silkuniene G,
Kmieliute K, Gulbinas A and Dambrauskas Z: HuR mediated
post-transcriptional regulation as a new potential adjuvant
therapeutic target in chemotherapy for pancreatic cancer. World J
Gastroenterol. 21:13004–13019. 2015. View Article : Google Scholar
|
|
22
|
Bai Y, Bai Y, Dong J, Li Q, Jin Y, Chen B
and Zhou M: Hedgehog signaling in pancreatic fibrosis and cancer.
Medicine (Baltimore). 95:e29962016. View Article : Google Scholar
|
|
23
|
Lee RT, Zhao Z and Ingham PW: Hedgehog
signalling. Development. 143:367–372. 2016. View Article : Google Scholar
|
|
24
|
Tadros S, Shukla SK, King RJ, Gunda V,
Vernucci E, Abrego J, Chaika NV, Yu F, Lazenby AJ, Berim L, et al:
De novo lipid synthesis facilitates gemcitabine resistance through
endoplasmic reticulum stress in pancreatic cancer. Cancer Res.
77:5503–5517. 2017. View Article : Google Scholar
|
|
25
|
Pishvaian MJ and Brody JR: Therapeutic
implications of molecular subtyping for pancreatic cancer. Oncology
(Williston Park). 31:159–166. 1682017.
|
|
26
|
Adamska A, Domenichini A and Falasca M:
Pancreatic ductal adenocarcinoma: Current and evolving therapies.
Int J Mol Sci. 18:182017. View Article : Google Scholar
|
|
27
|
Borowa-Mazgaj B: Pancreatic
cancer-mechanisms of chemoresistance. Postepy Hig Med Dosw.
70:169–179. 2016.In Polish. View Article : Google Scholar
|
|
28
|
Güngör C, Hofmann BT, Wolters-Eisfeld G
and Bockhorn M: Pancreatic cancer. Br J Pharmacol. 171:849–858.
2014. View Article : Google Scholar
|
|
29
|
Catenacci DV, Junttila MR, Karrison T,
Bahary N, Horiba MN, Nattam SR, Marsh R, Wallace J, Kozloff M,
Rajdev L, et al: Randomized phase Ib/II study of gemcitabine plus
placebo or vismodegib, a hedgehog pathway inhibitor, in patients
with metastatic pancreatic cancer. J Clin Oncol. 33:4284–4292.
2015. View Article : Google Scholar
|
|
30
|
Cao J, Peterson SJ, Sodhi K, Vanella L,
Barbagallo I, Rodella LF, Schwartzman ML, Abraham NG and Kappas A:
Heme oxygenase gene targeting to adipocytes attenuates adiposity
and vascular dysfunction in mice fed a high-fat diet. Hypertension.
60:467–475. 2012. View Article : Google Scholar
|
|
31
|
Felley-Bosco E, Opitz I and Meerang M:
Hedgehog signaling in malignant pleural mesothelioma. Genes
(Basel). 6:500–511. 2015. View Article : Google Scholar
|
|
32
|
Wendling-Keim DS, Wanie L, von Schweinitz
D, Grantzow R and Kappler R: Transcriptional activation of Hedgehog
pathway components in aggressive haemangioma. Exp Dermatol.
26:934–939. 2017. View Article : Google Scholar
|
|
33
|
Huang H, Cotton JL, Wang Y, Rajurkar M,
Zhu LJ, Lewis BC and Mao J: Specific requirement of Gli
transcription factors in Hedgehog-mediated intestinal development.
J Biol Chem. 288:17589–17596. 2013. View Article : Google Scholar
|
|
34
|
Frank J, Lornejad-Schäfer MR, Schöffl H,
Flaccus A, Lambert C and Biesalski HK: Inhibition of heme
oxygenase-1 increases responsiveness of melanoma cells to ALA-based
photodynamic therapy. Int J Oncol. 31:1539–1545. 2007.
|