Introduction
Breast cancer is one of the most common types of
cancer worldwide, and is a major cause of mortality (1,2).
Several types of breast cancer can be differentiated based on
receptor expression; ~20% of breast cancer cases express epidermal
growth factor receptor (EGFR) and are classified as triple-negative
breast cancer (TNBC) (3-5). Notably, EGFR-targeted therapies have
been reported to reduce EGFR activity and decrease cancer cell
proliferation (6).
All types of breast cancer cells express signal
transducer and activator of transcription (STAT) proteins, which
serve key roles in cell death, differentiation and proliferation
(7,8). STAT3 and STAT5b have important roles
in the initiation, progression and metastasis of malignant breast
tumors. In particular, STAT3 is strongly associated with cell
migration, invasion and metastasis, and its activation is mediated
through ligand-associated tyrosine kinases, Janus kinase 2 (Jak2)
and EGFR. Phosphorylation of certain STAT3 residues results in the
formation of a homodimer or heterodimer that is subsequently
translocated to the nucleus. The activated STAT3 molecule acts as a
transcription factor in the nucleus, and binds to specific
activation elements of the target gene promoter region (9). Previous studies have reported that
EGFR, Jak2 and STAT3 signaling has a role in cancer cell
proliferation, differentiation, apoptosis and metastasis (8,10).
Rhodiola rosea is widely distributed in high
altitude areas, predominantly in Northeast Asia and Eastern Europe,
and has long been used as a medicinal material in China. Among the
isolated components of R. rosea, salidroside
(p-hydroxyphenethyl-β-d-glucoside) is one of the most potent, and
is found in all Rhodiola species. Numerous studies have
investigated the biological functions of salidroside, high-lighting
its antioxidant and anticancer effects (11-16).
Various anticancer investigations have examined the
effects of salidroside on the cell cycle and apoptosis; however, to
the best of our knowledge, no studies have specifically analyzed
the effects of salidroside on breast cancer. The present study
investigated how salidroside affected major factors involved in
breast cancer, including STAT3, Jak2 and EGFR, in order to
elucidate its ability to inhibit metastasis and invasion. The
results demonstrated that salidroside treatment downregulated the
STAT3 signaling pathway through matrix metalloproteinases (MMPs),
thus resulting in the inhibition of cell viability, metastasis,
migration and invasion in breast cancer cells.
Materials and methods
Antibodies and cell culture reagents
Dulbecco's modified Eagle's medium (DMEM) was
purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
Penicillin-streptomycin solution and fetal bovine serum (FBS) were
purchased from HyClone (GE Healthcare Life Sciences, Logan, UT,
USA). Trypsin-EDTA (0.05%) was obtained from Gibco (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Antibodies specific for
phosphorylated (p)-STAT3 (Tyr705; sc-7993), STAT1 (sc-464), STAT3
(sc-8019), STAT5b (sc-1656), MMP3 (sc-6839), MMP9 (sc-21733),
vascular endothelial growth factor (VEGF; sc-507), VEGF-R2
(sc-6251) and β-actin (sc-47778), together with secondary
antibodies [goat anti-mouse (sc-2005), anti-rabbit (sc-2004) and
donkey anti-goat (sc-2020) immunoglobulin G-horseradish peroxidase
(HRP)], were obtained from Santa Cruz Biotechnology, Inc. (Dallas,
TX, USA). Anti-TBP (TATA-binding protein; ab818) was purchased from
Abcam (Cambridge, UK). An antibody against MMP2 (E90317) was
purchased from EnoGene Biotech Co., Ltd. (New York, NY, USA) and an
anti-Jak2 (06-1310) antibody was obtained from EMD Millipore
(Billerica, MA, USA). Anti-p-Jak2 (#3776s), p-EGFR (#2235), EGFR
(#4267), p-STAT1 (#9167s), p-STAT5 (#9314) and p-VEGF-R2 (#2478)
antibodies were purchased from Cell Signaling Technology, Inc.
(Beverly, MA, USA). Salidroside was purchased from Sigma-Aldrich
[Merck KGaA; diluted in dimethyl sulfoxide (DMSO)].
Cell culture and treatment
MDA-MB 231 (no. 30026, Korean Cell Line Bank, Seoul,
Korea) human breast cancer cells were cultured in DMEM supplemented
with 10% FBS, 2 mM glutamine, and 100 U/ml penicillin and
streptomycin. MCF-10A immortalized normal human breast epithelial
cells [CRL-10317; American Type Culture Collection (ATCC),
Manassas, VA, USA] were grown to confluence in phenol red-free
DMEM/F12 medium (11320033; Gibco; Thermo Fisher Scientific, Inc.)
supplemented with cholera toxin (1 mg/ml; C8052, Sigma-Aldrich;
Merck KGaA), insulin (10 mg/ml; I0516; Sigma-Aldrich; Merck KGaA),
EGF (5 mg/ml; E9644; Sigma-Aldrich; Merck KGaA), 1%
penicillin/streptomycin and 5% FBS. Human umbilical vein
endothelial cells (HUVECs; PCS-100-010; ATCC) were maintained in
EBM-2 (CC-3156; Lonza, Walkersville, MD USA) endothelial growth
basal media. Cells were cultured at 37°C in an incubator containing
5% CO2. For each experiment, cells were resuspended in
medium at a density of 2.5×105 cells/ml. Unless
otherwise specified, cells were treated with 40 µM
salidroside for 24 h at 37°C.
Cell viability assay
Cell viability was assessed by MTT assay. Briefly,
cells were resuspended in DMEM 1 day prior to drug treatment, at a
density of 3×103 cells/well in 96-well culture plates.
Culture medium was replaced with fresh medium containing DMSO as a
vehicle control. Cells were incubated with increasing
concentrations of salidroside (5-80 µM) for 24 h at 37°C.
Following drug treatment, MTT (5 mg/ml) was added, and the culture
dishes were incubated at 37°C for 4 h. The resulting formazan
product was dissolved in DMSO and absorbance was measured at 550 nm
on an Ultra Multifunctional Microplate Reader (Tecan US, Inc.,
Morrisville, NC, USA). All measurements were performed in
triplicate, with experiments repeated at least three times.
Western blotting
Whole cell lysates from untreated or
salidroside-treated MDA-MB 231 cells and HUVECs were prepared on
ice using radioimmunoprecipitation lysis buffer (20-188; EMD
Millipore) containing phosphatase and protease inhibitors. Cells
were disrupted by aspiration through a 23-gauge needle and
centrifuged at 18,300 × g for 10 min at 4°C to remove cellular
debris. Protein concentrations were measured using the Bradford
method (Thermo Fisher Scientific, Inc.). Equal amounts of protein
(100 µg/lane) were resolved by 10% SDS-PAGE and were then
transferred onto nitrocellulose membranes. The blots were blocked
for 1 h with 5% skimmed milk in TBS-T buffer [20 mM Tris-HCl (pH
7.6), 137 mM NaCl, 0.1X Tween-20]. Membranes were then probed
overnight at 4°C with the relevant primary antibodies (MMP2, MMP3
and MMP9, 1:500 dilutions; STAT1, STAT3, STAT5b, p-STAT1, p-STAT3,
p-STAT5, VEGF, VEGF-R2, p-Jak2, Jak2, p-EGFR, EGFR, p-VEGF-R2, TBP
and β-actin, 1:1,000 dilutions) diluted in 5% bovine serum albumin
(BSA; EMD Millipore) or skim milk (Difco™ Skim Milk; BD
Biosciences, Franklin Lakes, NJ, USA), followed by washing with
TBS-T and incubation for 1 h at room temperature with
HRP-conjugated secondary antibodies (1:2,000 dilution in 5% BSA or
skim milk). Detection was performed using the Enhanced
Chemiluminescence Plus detection kit (Amersham; GE Healthcare,
Chicago, IL, USA) and an LAS-4000 imaging device (Fujifilm, Tokyo,
Japan). Blots were stripped using Restore Western Blot Stripping
Buffer (Thermo Fisher Scientific, Inc.).
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was extracted using the RNeasy Mini kit
(Qiagen GmbH, Hilden, Germany) according to the manufacturer's
protocol. RNA was quantified spectrophotometrically at 260 nm.
Subsequently, RT-PCR analyses were performed to detect MMP2, MMP3,
MMP9 and 18s RNA expression. cDNA was synthesized from total RNA at
42°C for 1 h and 95°C for 5 min using first-strand cDNA synthesis
kits (K-2041; Bioneer Corporation, Daejeon, Korea) and oligo d(T)
primers. The RT-PCR Premix kit (K-2016; Bioneer Corporation) was
used for MMP2, MMP3 MMP9 and 18s amplification with primers
synthesized by Bioneer Corporation. PCR amplification to generate a
472-bp MMP2 fragment was conducted with the following primers:
MMP2, sense 5′-GGCCCTGTCAC TCCTGAGAT-3′ and antisense 5′-GGC
ATCCAGGTTATCGG GGA-3′. The PCR conditions were as follows: 94°C for
5 min, followed by 32 cycles at 94°C for 30 sec, 58°C for 30 sec
and 72°C for 45 sec, followed by 72°C for 7 min. PCR amplification
to generate a 432-bp MMP3 fragment was conducted with the following
primers and PCR conditions: MMP3, sense 5′-CCT GCTTTGTCCTTTGATGC-3′
and antisense 5′-TGAGTCAA TCCCTGGAAAGT-3′; 95°C for 5 min, followed
by 32 cycles at 95°C for 45 sec, 60°C for 45 sec and 72°C for 60
sec, followed by 72°C for 10 min. PCR amplification to generate a
455-bp MMP9 fragment was conducted with the following primers and
PCR conditions: MMP9, sense 5′-CCTGCCAGTTTCC ATTCATC-3′ and
antisense 5′-GCCATTCACGTCGTCCT TAT-3′; 94°C for 5 min, followed by
30 cycles at 94°C for 40 sec, 60°C for 40 sec and 68°C for 50 sec,
followed by 72°C for 7 min. Finally, a 489-bp amplified 18s mRNA
fragment was generated using the following primers and PCR
conditions: 18s, sense 5′-CGGCTACCACATCCAAGGAA-3′ and antisense
5′-CCGGCGTCCCTCTTAATC-3′; 95°C for 5 min, followed by 30 cycles at
95°C for 60 sec, 58°C for 60 sec and 72°C for 60 sec, followed by
72°C for 10 min. PCR products were resolved by electrophoresis on a
2% agarose gel and were visualized by ethidium bromide (E7637;
Sigma-Aldrich; Merck KGaA) staining.
Electrophoretic mobility shift assay
(EMSA)
STAT3 DNA-binding activity was detected using
LightShift Chemiluminescent EMSA kit (20148; Thermo Fisher
Scientific, Inc.). Oligonucleotide probes (STAT3) and reporter
lysis buffer were purchased from Promega Corporation (Madison, WI,
USA). MDA-MB 231 cells were grown to ~90% confluence with nuclear
protein extracts prepared using the Nuclear Extract kit
(Affymetrix; Thermo Fisher Scientific, Inc.). EMSA was performed
using the EMSA kit (20148; Thermo Fisher Scientific, Inc.),
according to the manu-facturer's protocol. Briefly, nuclear protein
was subjected to hybridization to a double-stranded, biotin-labeled
oligonucleotide probe, containing the consensus-binding site for
STAT3 (sense strand, 5′-GATCCTTCTGGGAATTCCTAGATC-3′). Proteins were
resolved on a non-denaturing 6% PAGE gel (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). Proteins were transferred from the gel to
a nylon membrane and detected using streptavidin-HRP and a
chemiluminescent substrate (Affymetrix; Thermo Fisher Scientific,
Inc.).
Small interfering RNA (siRNA)
analyses
MDA-MB 231 cells (1×105) were cultured in 6-well
plates, and grown to 60% confluence. Cells were then transfected
with the ON-TARGETplus SMARTpool siRNA targeting STAT3 (siSTAT3),
or ON-TARGETplus non-target siRNA (GE Healthcare Dharmacon, Inc.,
Lafayette, CO, USA), as a control. Transfection was conducted using
the DharmaFECT transfection reagent (GE Healthcare Dharmacon,
Inc.), according to the manufacturer's protocol. Following siRNA
transfection (1 µg/µl) for 24 h at 37°C, salidroside
treatment was applied for an additional 24 h. The expression levels
of STAT3, p-STAT3, MMP2 and β-actin were detected by western
blotting.
Wound healing assay
MDA-MB 231 cells were cultured in 6-well plates at a
concentration of 1×105 cells/well in DMEM containing 10%
FBS and were then incubated for 24 h in a humidified chamber. After
forming a confluent monolayer, the cell layer was scratched with a
pipette tip and washed with PBS to remove cell debris. Cells were
untreated (controls) or exposed to 10, 20 and 40 µM
salidroside. Images of the scratch sites (wounds) were captured at
0 and 24 h using a fluorescence microscope (IX71; Olympus
Corporation, Center Valley, PA, USA), and the average area of the
wound was calculated using DP Controller software (Version 3.2;
Olympus Corporation).
Matrigel invasion assay
A Transwell invasion assay was performed using
Matrigel pre-coated, ready-to-use invasion chambers (BD BioCoat; BD
Biosciences). MDA-MB 231 cells in DMEM, suspended at a
concentration of 5×104 were added to the inserts.
Drug-containing (10, 20 and 40 µM) media were added to the
receiver plate, and the inserts containing cells were placed onto
it. After 24-h incubation in a humidified chamber at 37°C, the
cells that had invaded the apical surface of the inserts were
identified using crystal violet. The plates were then incubated in
ambient conditions for 24 h, after which they were washed and fixed
with 3.7% formaldehyde for 5 min at room temperature. The cells on
the upper surface were removed using a cotton swab and the invaded
cells were quantified under a fluorescence microscope. Cells were
counted in four fields of view.
In vitro angiogenesis assay
ECMatrix (In Vitro Angiogenesis Assay kit;
ECM625; EMD Millipore) was thawed at 4°C over-night, after which
the wells of pre-chilled 96-well plates were coated with 50
µl diluted ECMatrix and incubated at 37°C for 1 h. A total
of 150 µl HUVECs (1×104; American Type Culture
Collection; CRL-1730) with or without salidroside was added to the
solidified matrix and incubated at 37°C for 12 h. Endothelial cell
formation was observed under a fluores-cence microscope. Focus was
placed on distinct areas and the tubes formed were counted.
Transfection and STAT3 overexpression
analysis
MDA-MB 231 cells (1×105) were cultured in
6-well plates and grown to 60% confluence. The cells were then
transfected with STAT3-pMX vector (provided by Dr M. Shong,
Chungnam National University, Daejeon, Korea) or empty pMX vector
(control, 0.5 µg/µl) using the DharmaFECT
transfection reagent (GE Healthcare Dharmacon, Inc.) for 24 h at
37°C. Transfected cells were washed with ice-cold PBS, and treated
for an additional 24 h with media that contained 40 µM
salidroside. Proteins were isolated and analyzed by western
blotting to determine p-STAT3, STAT3, MMP2 and β-actin expression
levels.
Molecular docking
The binding of salidroside to EGFR was determined by
molecular docking using the AutoDock Vina program in PyRx software
version 0.8 (17). The 3D
structure of salidroside was obtained from PubChem (ID: 159278;
https://pubchem.ncbi.nlm.nih.gov/) and
that of EGFR was obtained from Protein Data Bank (PDB ID: 2GS2;
https://www.rcsb.org/). The obtained binding was
analyzed by PyMol software version 0.99 (https://pymol.en.uptodown.com/).
Statistical analyses
All experiments were performed at least three times
with results expressed as means ± standard error of the mean.
Statistical analyses were conducted using one-way analysis of
variance (ANOVA) or Student's t-test. One-way ANOVA was performed
with Duncan's multiple range test as a post hoc test. Analyses were
performed using the SAS 9.3 program (SAS Institute, Inc., Cary, NC,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
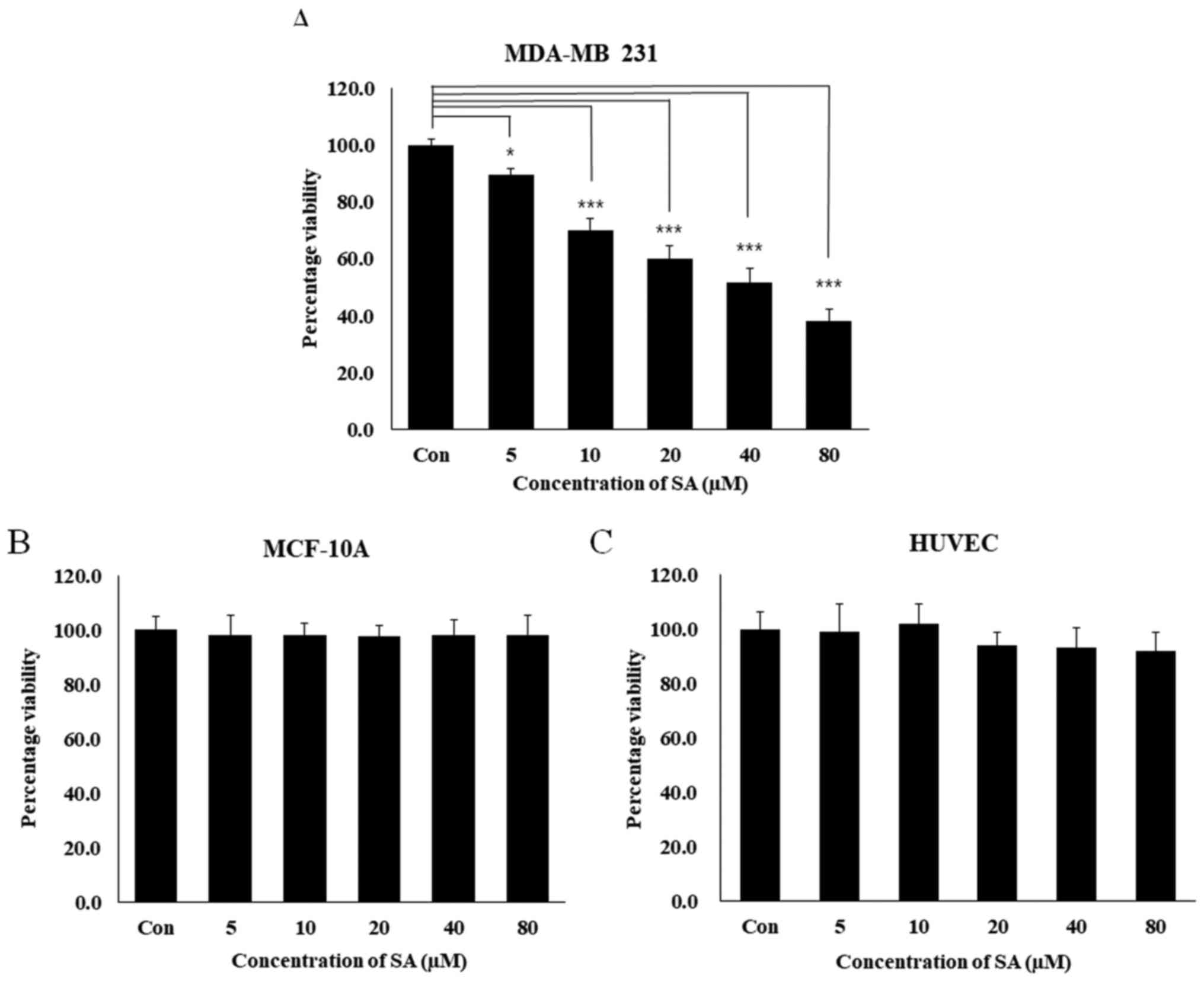
Salidroside inhibits MDA-MB 231 cell
viability
MTT assay was performed to examine how salidroside
affected the viability of the human breast cancer cell lines MDA-MB
231 and MCF-10A. For 24 h, MDA-MB 231, MCF-10A and HUVECs were
exposed to increasing concentrations of salidroside (5, 10, 20, 40
and 80 µM). The numbers of salidroside-treated cells
compared with control cells were then compared during the
logarithmic growth phase. MDA-MB 231 cell growth was inhibited by
49% following treatment with 40 µM salidroside, and by 62%
following treatment with 80 µM salidroside (Fig. 1A, P<0.001). Conversely,
salidroside did not inhibit the viability of normal cell lines,
MCF-10A and HUVECs (Fig. 1B and
C). Salidroside treatment markedly decreased the viability of
MDA-MB 231 cells in a dose-dependent manner, with 40 µM
salidroside determined to be the half maximal inhibitory
concentration; this concentration was used in subsequent
experiments.
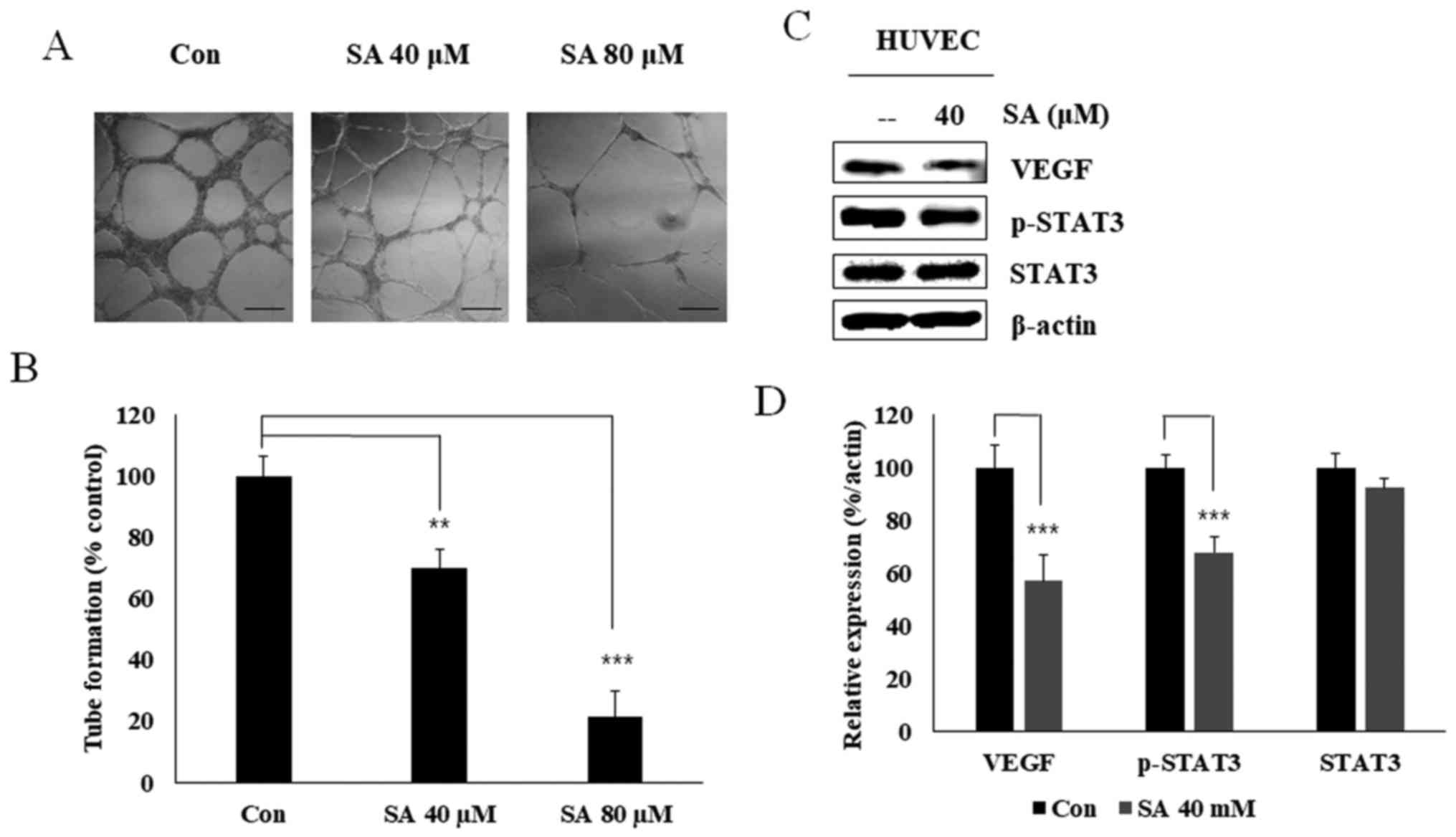
Salidroside inhibits angiogenesis through
VEGF and STAT3 in HUVEC cells
To confirm that salidroside exerted inhibitory
effects on angiogenesis, an in vitro angiogenesis assay was
conducted using 40 and 80 µM salidroside (Fig. 2A). Salidroside significantly
inhibited tube formation in the extracellular matrix (Fig. 2B). VEGF serves an important role in
angiogenesis. Western blot analysis confirmed that treatment with
40 µM salidroside inhibited the expression levels of VEGF
and p-STAT3 in HUVECs without altering the levels of total STAT3
(Fig. 2C and D). These results
suggested that salidroside may inhibit VEGF-dependent angiogenesis
through STAT3.
Salidroside suppresses EGFR
phosphorylation and Jak2/STAT3 signaling in MDA-MB 231 cells
The EGF-initiated Jak and STAT signaling cascade has
been implicated in cell survival responses; Jak phosphorylates STAT
proteins localized at the plasma membrane (18,19).
The present study evaluated the binding ability of salidroside with
EGFR using molecular docking with the AutoDock Vina platform.
Salidroside docked with the ATP-binding site of EGFR, thus
confirming direct binding of salidroside with EGFR (Fig. 3A). Western blot analysis revealed
that salidroside treatment led to down-regulation of p-EGFR. These
results suggested that salidroside treatment may inhibit EGFR
binding, and thus downregulate the expression of its downstream
molecules p-Jak2 and p-STAT3. Compared with in the control group,
treatment with 40 µM salidroside resulted in a reduction in
the expression of these proteins (Fig.
3B). These data suggested that salidroside may inhibit EGFR
phosphorylation, which resulted in reduced phosphorylation of Jak2
and STAT3. Furthermore, inhibition of the angiogenesis markers VEGF
and p-VEGF-R2 confirmed that salidroside inhibited
angiogenesis.
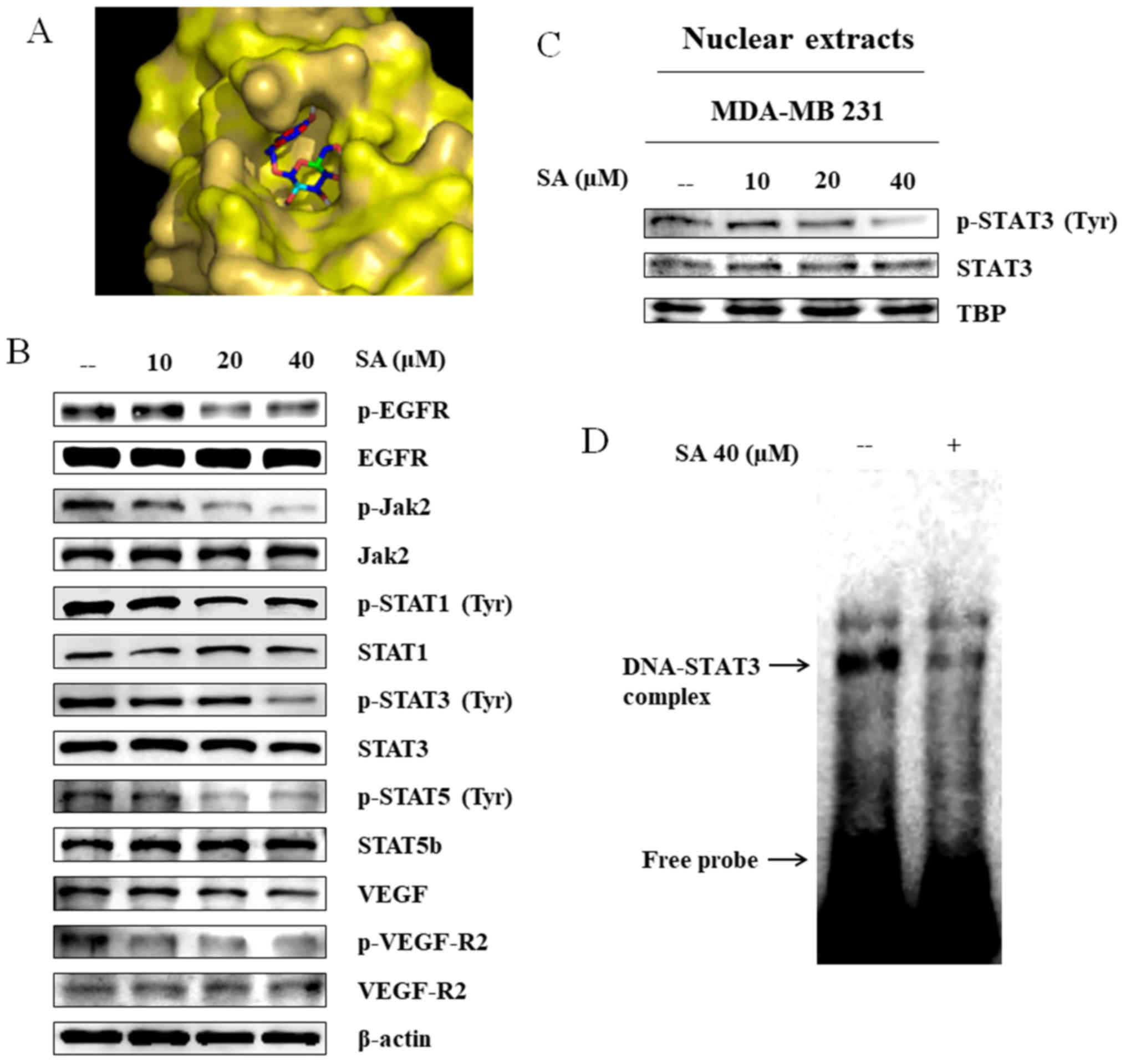 | Figure 3SA inhibits EGFR/Jak2/STAT signaling
activity and nuclear translocation, as well as the DNA-binding
activity of STAT3. (A) Binding of SA (PubChem ID: 159278) to the
ATP-binding domain of EGFR (Protein Data Bank ID: 2GS2), as
determined by molecular docking using AutoDock Vina. (B) Western
blot analysis revealed inhibition of EGFR activity and STAT3
signaling in MDA-MB 231 cells following treatment with increasing
concentrations of SA for 24 h. (C) MDA-MB 231 cells were exposed to
10-40 µM salidroside for 24 h, and nuclear proteins were
analyzed by western blotting, with TBP as a loading control. SA
inhibited nuclear translocation of STAT3. (D) Gel shift analysis of
nuclear extracts revealed that salidroside treatment inhibited the
DNA-binding activity of STAT3 to the γ-interferon activation site
element. EGFR, epidermal growth factor receptor; Jak2, Janus kinase
2; p, phosphorylated; SA, salidroside; STAT, signal transducer and
activator of transcription; TBP, TATA-binding protein; VEGF,
vascular endothelial growth factor; VEGF-R2, VEGF receptor 2. |
Salidroside inhibits nuclear
translocation and DNA-binding activity of STAT3
A recent study reported that STAT3 activation
regulates MMP2 expression (20).
MMP2 transcription requires translocation of p-STAT3 to the
nucleus, where it binds to the MMP2 gene promoter. To analyze
nuclear translocation, nuclear extracts were isolated from
untreated and salidroside-treated MDA-MB 231 cells. Western blot
analysis of these nuclear extracts revealed that the expression
levels of p-STAT3 were reduced in salidroside-treated cells
(Fig. 3C). In our previous study,
the MMP2 gene promoter was analyzed, in order to detect a
γ-interferon activation site (GAS) element (20), which is the DNA-binding sequence
for the STAT family of transcription factors. Upon locating this
element, an EMSA was conducted to analyze the DNA-binding activity
of STAT3 to the GAS element. The results indicated that the
DNA/STAT3 complex was down-regulated in salidroside-treated cells
compared with in the untreated control cells (Fig. 3D). These findings revealed that
salidroside may inhibit expression of the STAT3/MMP2 complex, thus
indicating that salidroside acts through STAT3 signaling.
Salidroside suppresses MMP2 expression
via STAT3 regu- lation
Upon confirming the downregulation of MMP2
expression, and the downregulation of STAT3 expression,
phosphorylation and DNA-binding activity, the present study
investigated the association between STAT3 and MMP2. To test the
hypothesis that STAT3 may be a positive regulator of MMP2, STAT3
was knocked down using specific siSTAT3, and MMP2 expression was
analyzed by western blotting. The results verified the role of
STAT3 in MMP2 transcription. Among salidroside-treated cells, MMP2
expression was reduced in cells targeted with siSTAT3 compared with
in cells transfected with non-targeting siRNA (Fig. 4A). Analyzing the protein expression
levels relative to β-actin clearly elucidated the impact of
salidroside on STAT3-related MMP2 expression (Fig. 4B).
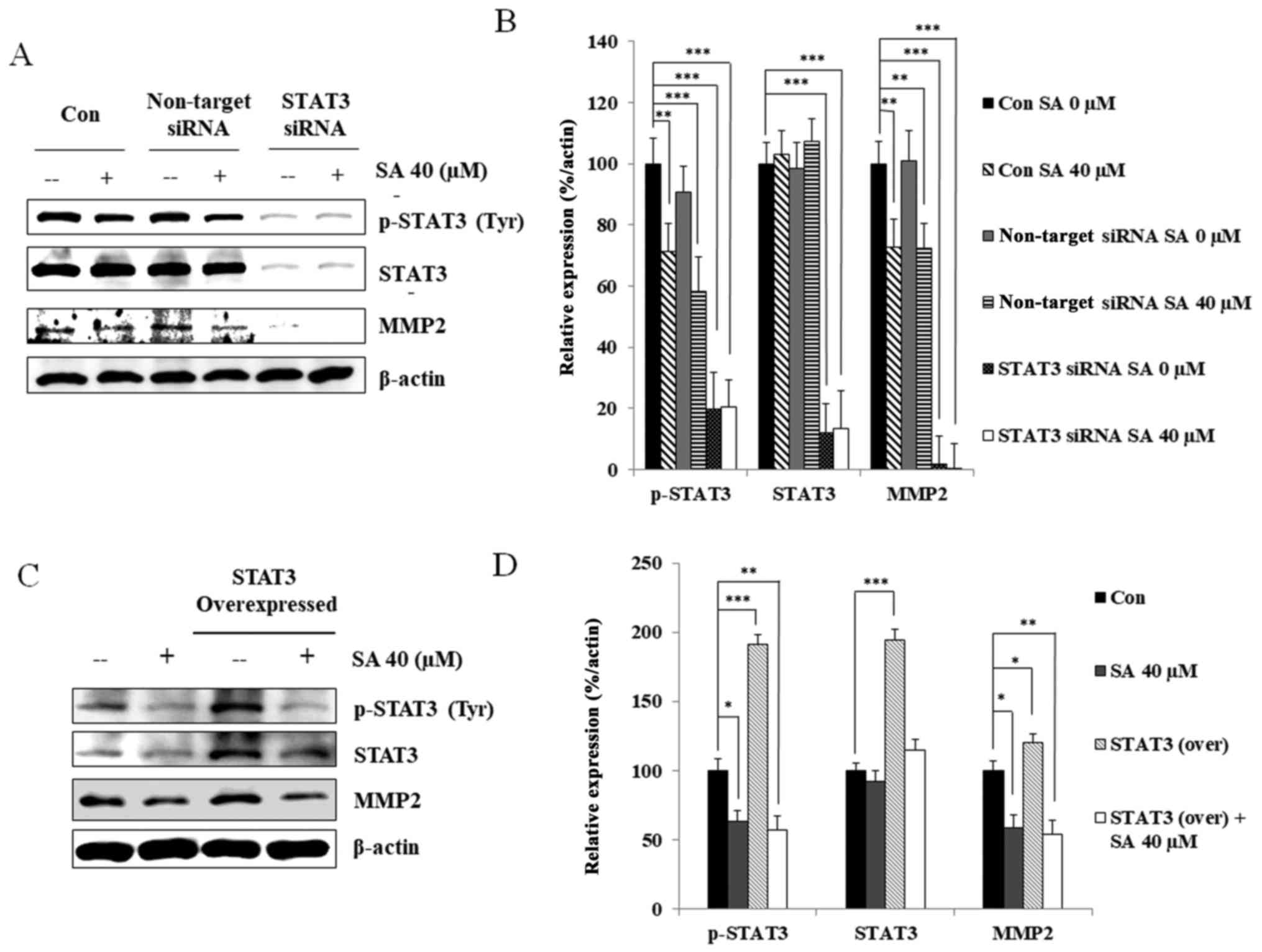 | Figure 4STAT3 regulates MMP2 expression in
MDA-MB 231 cells upon SA treatment. (A) Targeted STAT3 silencing
decreased MMP2 expression in MDA-MB 231 cells. (B) Relative protein
expression levels of p-STAT3, STAT3 and MMP2 were determined using
densitometry, and were normalized to β-actin. Data are
representative of three independent experiments. Statistical
analyses were performed using one-way analysis of variance.
**P<0.01, ***P<0.001 compared with the
control. (C) STAT3-overexpressing MDA-MB 231 cells exhibited
decreased p-STAT3 and MMP2 protein expression following treatment
with 40 µM SA. (D) Relative levels of p-STAT3, STAT3, and
MMP2 proteins were determined using densitometry, and normalized
β-actin. Statistical analyses were performed using one-way analysis
of variance. *P<0.05, **P<0.01,
***P<0.001. Data are representative of three
independent experiments. MMP2, matrix metalloproteinase 2; p,
phosphorylated; SA, salidroside; siRNA, small interfering RNA;
STAT3, signal transducer and activator of transcription 3. |
STAT3 protein was subsequently overexpressed in
MDA-MB 231 cells, and STAT3 and MMP2 expression were analyzed using
western blotting (Fig. 4C).
Salidroside treatment led to ~40% inhibition of p-STAT3 and MMP2
expression in MDA-MB 231 cells compared with in the untreated
control cells. In STAT3-overexpressing cells, salidroside treatment
led to ~55% inhibition of MMP2 compared with untreated
STAT3-overexpressing MDA-MB 231 cells (Fig. 4D). Analysis of cell lysates
revealed similar expression patterns of p-STAT3 and MMP2 following
salidroside treatment, indicating that salidroside suppressed STAT3
and MMP2 expression. Based on these results, it may be concluded
that STAT3 serves an essential role in MMP2 activation.
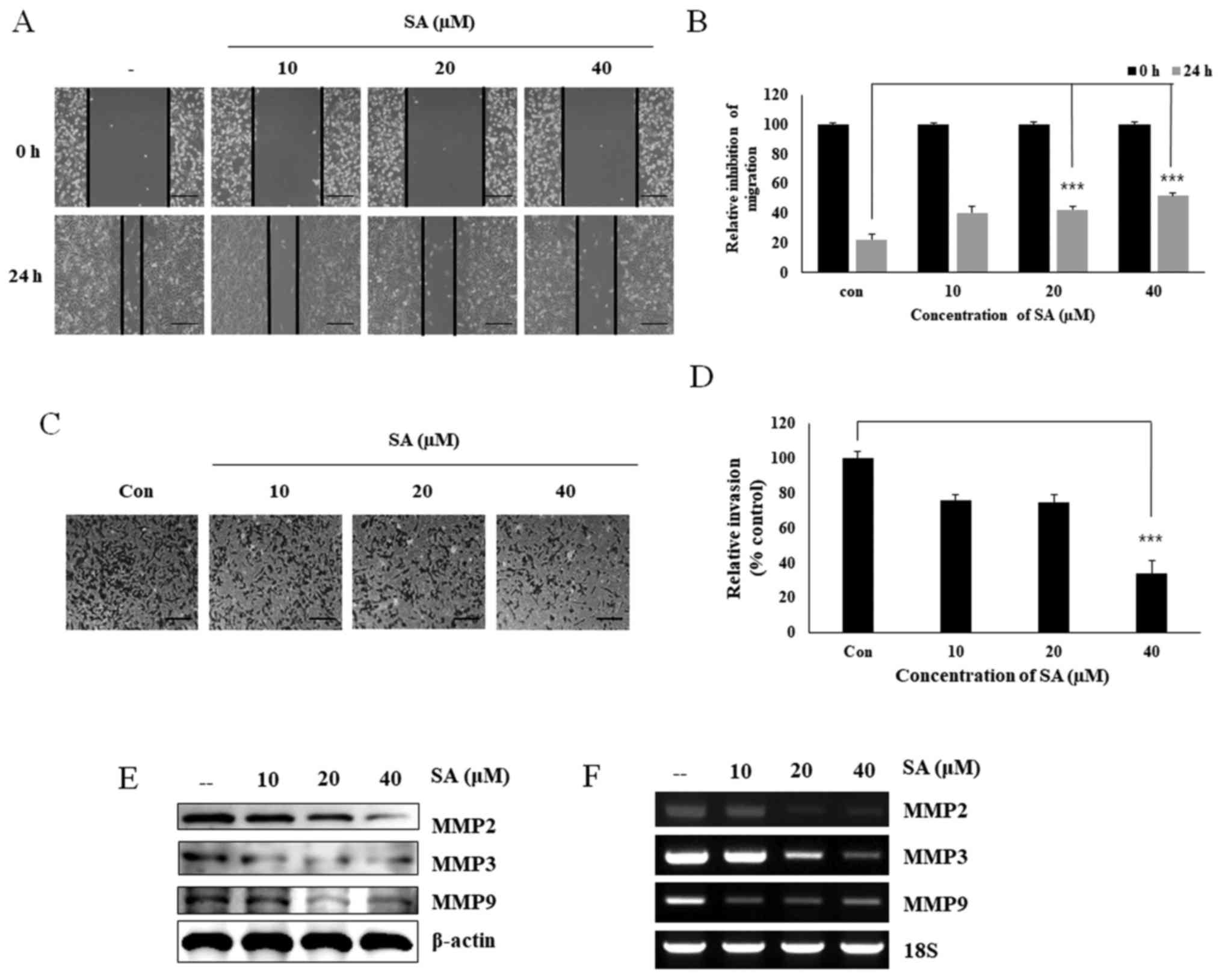
Salidroside suppresses cell migration and
cellular invasion
Migration and invasion are considered key regulatory
processes in tumor progression, angiogenesis and metastasis.
Therefore, the present study analyzed whether salidroside inhibited
cell migration and invasion via its effects on MMPs and STAT3. A
wound-healing assay was performed in MDA-MB 231 cells following
treatment with salidroside for 24 h (Fig. 5A). The results suggested that
salidroside dose-dependently inhibited the migratory ability of
MDA-MB 231 cells (Fig. 5B). To
assess whether salidroside could inhibit cell invasion, a Matrigel
invasion assay (Fig. 5C) was
conducted, which demonstrated that salidroside treatment decreased
the relative cell invasion (Fig.
5D). Invasion was largely dependent on the release of MMPs, and
salidroside downregulated MMP2, MMP3 and MMP9 expression at both
the translational (Fig. 5E) and
transcriptional levels (Fig. 5F).
Overall, these results confirmed that salidroside inhibited cell
migration and invasion by inhibiting STAT3 phosphorylation and MMP2
expression.
Discussion
Previous studies have extensively investigated the
effects of salidroside on cancer cells, and a vast body of research
exists on breast cancer, including studies regarding cell cycle
inhibition and mammalian target of rapamycin molecular pathway
analysis (14-16,21,22).
However, despite work in these areas, only scarce data are
available regarding the specific roles of salidroside with regards
to breast cancer growth, progression and metastasis. The present
study aimed to elucidate the mechanism underlying the ability of
salidroside to inhibit tumor progression and metastasis by
targeting EGFR signaling and its downstream molecule STAT3 as a
specific target.
The results provided evidence to suggest that
salidroside, extracted from R. rosea, inhibited the growth
of breast cancer cells in vitro. Specifically, 40 µM
salidroside killed 49% of MDA-MB 231 TNBC cells. The observed
cytotoxic effect was concentration-dependent. Conversely,
salidroside exhibited no cytotoxic effect on MCF-10A and HUVECs,
which indicated that salidroside may have no side effects on normal
cells. These results suggested that salidroside does not induce
cell death in normal cell lines, whereas it may induce cancer cell
death, and therefore may be considered a good candidate as an
anticancer drug. These in vitro data strongly suggested that
salidroside is a candidate chemotherapeutic agent for breast cancer
treatment.
A recent report demonstrated that salidroside
significantly and dose-dependently suppresses MMP2 and MMP9
activity, and decreases protein expression in MCF-7 cells (23). This is consistent with the present
findings, which indicated that salidroside treatment reduced MMP2
and MMP9 expression at the mRNA and protein levels in MDA-MB 231
cells. It has also been demonstrated that MMP2 and MMP9 are
associated with the Jak2/STAT3 pathway (20,24,25).
STAT3 phosphorylation provokes homo- or heterodimerization, which
is followed by nuclear translocation and binding to gene-specific
response elements in target gene promoters (26). Together with these data, the
present experimental results suggested that treatment with 40
µM salidroside resulted in a reduction of binding site
activity. Examination of the MMP2 gene promoter sequence revealed a
GAS element in the transcription start site, and gel shift analyses
confirmed that STAT3 possessed DNA-binding activity to the MMP2
gene promoter, which was inhibited by salidroside. Furthermore,
STAT3 knockdown effectively eliminated MMP2 expression, whereas
STAT3 overexpression led to the same pattern of STAT3 and MMP2
reduction following salidroside treatment. Finally, it was
demonstrated that salidroside treatment markedly inhibited cell
migration and invasion in MDA-MB 231 cells. However, the results of
a wound-healing assay indicated that the inhibitory effect of
salidroside on migration may be due to cell toxicity. In order to
confirm this effect was not due to cell death, a Matrigel invasion
assay was performed; therefore, the contact between cells and
salidroside occurred through Matrigel, clearly indicating that cell
death had no role in the invasion process. These findings confirmed
that salidroside induced inhibition of tumor migration and
invasion, which was not caused by cell toxicity, thus indicating
the ability of salidroside to inhibit metastasis.
The present experimental results are consistent with
the previously reported roles of Jak2 and STAT3. The Jak/STAT
pathway has important signaling roles in cancer growth and
progression, and has been implicated in numerous cancer types,
including human colon cancer (12), breast cancer (23), melanoma (27), osteosarcoma (28), gastric cancer (21), and head and neck cancer (29). Jak proteins are implicated in cell
proliferation, migration and apoptosis; they bind with and promote
the phosphorylation of downstream molecules, including STAT3. Upon
phosphorylation, STAT3 is activated and regulates a broad range of
downstream target genes, mostly associated with malignant
transformation and metastasis (30-32).
The preesnt study also observed that salidroside
exerted an inhibitory effect on angiogenesis in HUVECs. Therefore,
the protein expression levels of VEGF in HUVECs and MDA-MB 231
cells were detected. VEGF is considered a key endothelial
cell-specific signaling factor required for tumor angiogenesis
(33). In the present study, VEGF
expression was reduced in HUVECs following treatment with
salidroside. Previously, Ariyanti et al (34) and Zhang et al (35) reported an elevation in the
expression of VEGF in salidroside-treated skeletal muscle cells
compared with in non-treated cells, which indicated that
angiogenesis activity is dependent on the upregulation of VEGF-A
and platelet-derived growth factor-BB, thus suggesting that it is
independent of VEGF downregulation. However, in the present study,
salidroside inhibited angiogenesis by inhibiting VEGF activity,
thus suggesting that salidroside inhibited VEGF-dependent
angiogenesis. Since angiogenesis can foster tumor progression and
metastasis, it is an important consideration in developing
anti-cancer therapy (36). The
present study revealed that salidroside treatment inhibited in
vitro angiogenesis in endothelial cells, and led to reduced
VEGF protein expression in breast cancer cells, thus suggesting the
anti-angiogenic ability of salidroside. Further experimentation in
a breast cancer xenograft model is required to confirm the roles of
these signaling molecules in salidroside-induced inhibition of
migration, invasion and angiogenesis.
Overall, it appears that salidroside treatment may
inhibit STAT3 phosphorylation (in a manner involving EGFR and
Jak2), which in turn inhibited STAT3 signaling by blocking STAT3
nuclear translocation and DNA-binding. This may lead to inhibition
of the gene-specific transcriptional activation of MMP2, ultimately
resulting in inhibition of viability, migration, invasion and
angiogenesis of breast cancer cells.
In conclusion, the present data revealed that
salidroside inhibited TNBC tumor migration and invasion by
regulating EGFR/Jak2/STAT3 signaling in a manner that inhibited
MMP2 transcription. Additionally, it was demonstrated that
salidroside inhibited VEGF-dependent tumor angiogenesis via a
mechanism that also involves STAT3.
Acknowledgments
Not applicable.
Funding
The present study received funding from Konkuk
University in 2017.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
DYK conceived and designed the experiments,
performed the experiments and wrote the paper. YMY contributed in
designing the experiments and data analysis. YHJ, NSP, DHK, HGL and
YMP analyzed experiments and data along with YMY. All authors
contributed to revise the manuscript and approved the final version
for publication.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cleator S, Heller W and Coombes RC:
Triple-negative breast cancer: Therapeutic options. Lancet Oncol.
8:235–244. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Anders CK and Carey LA: Biology,
metastatic patterns, and treatment of patients with triple-negative
breast cancer. Clin Breast Cancer. 9(Suppl 2): S73–S81. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Carey LA, Perou CM, Livasy CA, Dressler
LG, Cowan D, Conway K, Karaca G, Troester MA, Tse CK, Edmiston S,
et al: Race, breast cancer subtypes, and survival in the Carolina
Breast Cancer Study. JAMA. 295:2492–2502. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Potemski P, Kusinska R, Watala C,
Pluciennik E, Bednarek AK and Kordek R: Prognostic relevance of
basal cytokeratin expression in operable breast cancer. Oncology.
69:478–485. 2005. View Article : Google Scholar
|
|
5
|
Dent R, Trudeau M, Pritchard KI, Hanna WM,
Kahn HK, Sawka CA, Lickley LA, Rawlinson E, Sun P and Narod SA:
Triple-negative breast cancer: Clinical features and patterns of
recurrence. Clin Cancer Res. 13:4429–4434. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Masuda H, Zhang D, Bartholomeusz C,
Doihara H, Hortobagyi GN and Ueno NT: Role of epidermal growth
factor receptor in breast cancer. Breast Cancer Res Treat.
136:331–345. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Furth PA: STAT signaling in different
breast cancer sub-types. Mol Cell Endocrinol. 382:612–615. 2014.
View Article : Google Scholar
|
|
8
|
Bowman T, Garcia R, Turkson J and Jove R:
STATs in oncogenesis. Oncogene. 19:2474–2488. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Darnell JE Jr: STATs and gene regulation.
Science. 277:1630–1635. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Thomas SJ, Snowden JA, Zeidler MP and
Danson SJ: The role of JAK/STAT signalling in the pathogenesis,
prognosis and treatment of solid tumours. Br J Cancer. 113:365–371.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang J, Li JZ, Lu AX, Zhang KF and Li BJ:
Anticancer effect of salidroside on A549 lung cancer cells through
inhibition of oxidative stress and phospho-p38 expression. Oncol
Lett. 7:1159–1164. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sun KX, Xia HW and Xia RL: Anticancer
effect of salidroside on colon cancer through inhibiting JAK2/STAT3
signaling pathway. Int J Clin Exp Pathol. 8:615–621.
2015.PubMed/NCBI
|
|
13
|
Zhang Y, Yao Y, Wang H, Guo Y, Zhang H and
Chen L: Effects of salidroside on glioma formation and growth
inhibition together with improvement of tumor microenvironment.
Chin J Cancer Res. 25:520–526. 2013.PubMed/NCBI
|
|
14
|
Hu X, Zhang X, Qiu S, Yu D and Lin S:
Salidroside induces cell-cycle arrest and apoptosis in human breast
cancer cells. Biochem Biophys Res Commun. 398:62–67. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hu X, Lin S, Yu D, Qiu S, Zhang X and Mei
R: A preliminary study: The anti-proliferation effect of
salidroside on different human cancer cell lines. Cell Biol
Toxicol. 26:499–507. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu Z, Li X, Simoneau AR, Jafari M and Zi
X: Rhodiola rosea extracts and salidroside decrease the growth of
bladder cancer cell lines via inhibition of the mTOR pathway and
induction of autophagy. Mol Carcinog. 51:257–267. 2012. View Article : Google Scholar
|
|
17
|
Trott O and Olson AJ: AutoDock Vina:
Improving the speed and accuracy of docking with a new scoring
function, efficient optimization, and multithreading. J Comput
Chem. 31:455–461. 2010.
|
|
18
|
Hofmann HD and Kirsch M: JAK2-STAT3
signaling: A novel function and a novel mechanism. JAK-STAT.
1:191–193. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Looyenga BD, Hutchings D, Cherni I,
Kingsley C, Weiss GJ and Mackeigan JP: STAT3 is activated by JAK2
independent of key oncogenic driver mutations in non-small cell
lung carcinoma. PLoS One. 7:e308202012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Byun HJ, Darvin P, Kang DY, Sp N, Joung
YH, Park JH, Kim SJ and Yang YM: Silibinin downregulates MMP2
expression via Jak2/STAT3 pathway and inhibits the migration and
invasive potential in MDA-MB-231 cells. Oncol Rep. 37:3270–3278.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kisseleva T, Bhattacharya S, Braunstein J
and Schindler CW: Signaling through the JAK/STAT pathway, recent
advances and future challenges. Gene. 285:1–24. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang J, Liu A, Hou R, Zhang J, Jia X,
Jiang W and Chen J: Salidroside protects cardiomyocyte against
hypoxia-induced death: A HIF-1α-activated and VEGF-mediated
pathway. Eur J Pharmacol. 607:6–14. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao G, Shi A, Fan Z and Du Y: Salidroside
inhibits the growth of human breast cancer in vitro and in vivo.
Oncol Rep. 33:2553–2560. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Björklund M and Koivunen E:
Gelatinase-mediated migration and invasion of cancer cells. Biochim
Biophys Acta. 1755:37–69. 2005.PubMed/NCBI
|
|
25
|
Xie TX, Wei D, Liu M, Gao AC, Ali-Osman F,
Sawaya R and Huang S: Stat3 activation regulates the expression of
matrix metalloproteinase-2 and tumor invasion and metastasis.
Oncogene. 23:3550–3560. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Darvin P, Baeg SJ, Joung YH, Sp N, Kang
DY, Byun HJ, Park JU and Yang YM: Tannic acid inhibits the
Jak2/STAT3 pathway and induces G1/S arrest and mitochondrial
apoptosis in YD-38 gingival cancer cells. Int J Oncol.
47:1111–1120. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nam S, Xie J, Perkins A, Ma Y, Yang F, Wu
J, Wang Y, Xu RZ, Huang W, Horne DA, et al: Novel synthetic
derivatives of the natural product berbamine inhibit Jak2/Stat3
signaling and induce apoptosis of human melanoma cells. Mol Oncol.
6:484–493. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu Y, Wang L, Wu Y, Lv C, Li X, Cao X,
Yang M, Feng D and Luo Z: Pterostilbene exerts antitumor activity
against human osteosarcoma cells by inhibiting the JAK2/STAT3
signaling pathway. Toxicology. 304:120–131. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Henson ES and Gibson SB: Surviving cell
death through epidermal growth factor (EGF) signal transduction
pathways: Implications for cancer therapy. Cell Signal.
18:2089–2097. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Siveen KS, Sikka S, Surana R, Dai X, Zhang
J, Kumar AP, Tan BK, Sethi G and Bishayee A: Targeting the STAT3
signaling pathway in cancer: Role of synthetic and natural
inhibitors. Biochim Biophys Acta. 1845:136–154. 2014.PubMed/NCBI
|
|
31
|
Yu H, Lee H, Herrmann A, Buettner R and
Jove R: Revisiting STAT3 signalling in cancer: New and unexpected
biological functions. Nat Rev Cancer. 14:736–746. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Darvin P, Joung YH, S P N, Kang DY, Byun
HJ, Hwang DY, Cho KH, Park KD, Lee HK and Yang YM, SP N, Kang DY,
Byun HJ, Hwang DY, Cho KH, Park KD, Lee HK and Yang YM: Sorghum
polyphenol suppresses the growth as well as metastasis of colon
cancer xenografts through co-targeting jak2/STAT3 and PI3K/Akt/mTOR
pathways. J Funct Foods. 15:193–206. 2015. View Article : Google Scholar
|
|
33
|
McMahon G: VEGF receptor signaling in
tumor angiogenesis. Oncologist. 5(Suppl 1): 3–10. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ariyanti AD, Sisjayawan J, Zhang J, Zhang
JQ, Wang GX, Miyagishi M, Wu SR and Kasim V: Elevating VEGF-A and
PDGF-BB secretion by salidroside enhances neoangiogenesis in
diabetic hind-limb ischemia. Oncotarget. 8:97187–97205. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang J, Liu A, Hou R, Zhang J, Jia X,
Jiang W and Chen J: Salidroside protects cardiomyocyte against
hypoxia-induced death: a HIF-1alpha-activated and VEGF-mediated
pathway. Eur J Pharmacol. 607:6–14. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Weis SM and Cheresh DA: Tumor
angiogenesis: Molecular pathways and therapeutic targets. Nat Med.
17:1359–1370. 2011. View Article : Google Scholar : PubMed/NCBI
|