Introduction
Ovarian cancer is one of the most common types of
cancer among women worldwide, resulting in >14,000 cases of
mortality each year (1).
Epithelial ovarian cancer is the most common type of ovarian
cancer, which has the highest mortality rate of all gynecological
malignancies. This high mortality rate is largely due to the lack
of an effective biomarker; in addition, it is difficult to diagnose
patients at the early stage of the disease and most patients are
diagnosed at advanced stages (2-4).
The Nav1.5 channel protein is a member of the
voltage-gated sodium channel family (5). It is the predominant sodium channel
in cardiomyocytes and permeates sodium currents to initiate the
action potential duration in the heart (6). Previous studies have reported that
Nav1.5 channels are expressed in various types of cancer cells,
including leukemia (7), prostate
cancer (8), breast cancer
(9) and colon cancer (10), in which they serve roles in T
lymphocyte invasiveness, prostate cell pathological
differentiation, breast tumor metastasis and colon cancer
transcriptional activity. Furthermore, growing evidence has
suggested that the Nav1.5 channel is implicated in ovarian cancer
development (11,12), thus suggesting that small
molecule-induced inhibition of this channel may lead to a novel
pharmacological treatment.
Eicosapentaenoic acid (EPA) is a long-chain n-3
poly-unsaturated fatty acid, which has been revealed to reduce
blood lipid levels and lessen inflammation. In addition, EPA has
been reported to balance metabolism, inhibit proliferation and
induce apoptosis (13). EPA may
also inhibit human atrial sodium currents in a
concentration-dependent manner in cardiomyocytes; these effects may
contribute to atrial fibrillation treatment (14). Recently, EPA has been reported to
suppress ovarian cancer cell growth, reduce cancer metastasis and
kill ovarian cancer cells (15);
however, the underlying mechanism by which EPA attenuates ovarian
cancer invasion and cell proliferation remains to be
elucidated.
The present study demonstrated that EPA may inhibit
Nav1.5 sodium currents in the epithelial ovarian cancer cell lines
TOV112D, A2780 and SKOV3, and that EPA alters the gating process of
Nav1.5 and abolishes channel activity in these cells. Furthermore,
the effects of EPA on Nav1.5-mediated ovarian cancer cell migration
and growth were determined.
Materials and methods
Cell culture
TOV112D, A2780 and SKOV3 ovarian cancer cell lines
were purchased from the American Type Culture Collection (Manassas,
VA, USA). TOV112D cells were cultured in a 1:1 mixture of M199 and
MCDB105 media, supplemented with 10% (wt/vol) fetal bovine serum
(FBS) (both from Sigma-Aldrich; Merck KGaA, Darmstadt, Germany).
A2780 and SKOV3 cells were maintained in RPMI-1640 medium (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA), supplemented
with 10% (wt/vol) FBS. In addition, A2780 cells were supplemented
with 50 µg/ml insulin (Sigma-Aldrich; Merck KGaA). All cells
were maintained at 37°C in an incubator containing 5% (vol/vol)
CO2.
Electrophysiological measurements and
analysis
Patch-clamp measurements were performed in
whole-cell configuration using an Axopatch 200B amplifier
controlled by pClamp10 software through an Axon Digidata 1440A
system (all from Molecular Devices, LLC, Sunnyvale, CA, USA)
(16,17). The extracellular solution contained
140 mM NaCl, 4.7 mM KCl, 1.2 mM MgCl2, 2.5 mM
CaCl2, 10 mM HEPES and 11 mM glucose, adjusted to pH 7.4
with NaOH. The intracellular solution contained 140 mM CsCl, 2 mM
MgCl2, 0.1 mM CaCl2, 1.1 mM EGTA and 10 mM
HEPES, adjusted to pH 7.2 with CsOH. The sodium currents were
activated by depolarization to −10 mV for 50 msec from a holding
potential of −110 mV. To test steady-state activation, the sodium
currents were measured from a holding potential of −110 mV to test
potentials ranging between −110 to +70 mV in 10 mV steps, followed
by repolarization to −110 mV. To test steady-state inactivation,
currents were elicited to 0 mV following prepulses ranging between
−140 and 0 mV in 10 mV steps for 200 msec. EPA was purchased from
Sigma-Aldrich; Merck KGaA and dissolved in ethanol to obtain a
stock concentration of 10 mM; butylatedhydroxyanisole was added at
a ratio of 0.005% w/v to the stock solution, which was then stored
at −80°C (18). EPA was added to
the extracel-lular solution at the desired final concentrations
(1-200 µM), and the sodium currents were recorded before and
after the addition of EPA, or after EPA washout. All measurements
were performed at 22±2°C. Pipettes had 2-4 MΩ access resistance
(19,20). Current densities were calculated
according to whole-cell current amplitude and capacitance values
obtained from the amplifier following electronic subtraction of the
capacitive transients.
Dose-responses of channel inhibition were fitted by
the Hill equation, as previously described (21): %i =
%i,max
[B]h/(IC50h
+[B]h), where %i,max is
the maximal percentage of channel inhibition by blocker B,
h is the Hill coefficient and IC50 is the
concentration of an inhibitor required for 50% inhibition.
Steady-state activation G-V were fitted by the Boltzmann equation,
as described previously (22):
G/Gmax =
1/(1+exp(V1/2-V)/K), where
G/Gmax is the relative conductance normalized by
the maximal conductance, V1/2 is the potential of
half activation, V is test pulse, and k is the
Boltzmann coefficient. Steady-state inactivation I-V were fitted by
the Boltzmann equation: I/Imax =
1/(1+exp(V1/2-V)/K).
Western blot analysis
Ovarian cancer cells were rinsed twice with ice-cold
PBS, and were then harvested and lysed with lysis buffer (50 mM
Tris-HCl, 150 mM NaCl, 0.02% NaN3, 1% Nonidet P-40, 0.1%
sodium dodecyl sulfate, 0.5% sodium deoxycholate and 1% protease
inhibitor cocktail; Sigma-Aldrich; Merck KGaA). Protein
concentration was quantified using the bicinchoninic acid assay
(Beyotime Institute of Biotechnology, Haimen, China) and proteins
(25 µg/lane) were separated by 6% SDS-PAGE and transferred
to polyvinylidene fluoride membranes (EMD Millipore, Bedford, MA,
USA). Membranes were blocked at room temperature for 1 h in 5%
non-fat milk dissolved in Tris-buffered saline containing Tween-20
(150 mM NaCl, 50 mM Tris, 0.1% Tween-20; pH 7.5), and were
incubated with rabbit anti-human Nav1.5 (1:1,000, ASC-013; Alomone
Labs, Jerusalem, Israel) and rabbit anti-GAPDH (1:1,000, #5174;
Cell Signaling Technology, Inc., Danvers, MA, USA) primary
antibodies at 4°C overnight. Subsequently, membranes were incubated
with the goat anti-rabbit horse-radish peroxidase-conjugated
secondary antibodies (1:10,000, ab6721; Abcam, Cambridge, UK) for
1.5 h at room temperature. Bands were detected by chemiluminescence
assay (Beyotime Institute of Biotechnology) and were
semi-quantified using ImageJ version 1.4.3 (National Institutes of
Health, Bethesda, MD, USA).
Small interfering (si)RNA
transfection
SKOV3 ovarian cancer cells were transfected with
siRNA directed against sodium voltage-gated channel α subunit 5
(SCN5A) mRNA (Nav1.5-targeting siRNA; forward,
5′-GAACGGCACCUCUGAUGU GTT-3′ and reverse,
5′-CACAUCAGAGGUGCCGUUCTT-3′) or scramble siRNA-A (forward, 5′
GCAACAAUGUGCGUC CUGGTT-3′ and reverse, 5′-CCAGGACGCACAUUGUUG
CTT-3′), which was used as a control (SiCtl). siRNAs were purchased
from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Cells (80%
confluence) were transfected with 20 or 40 nM siRNA using
Lipofectamine® RNAiMAX (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol.
Subsequently, the migration and cell proliferation assays, and the
patch clamp technique, were performed 24 h post-transfection.
Nav1.5 transfection
The pRc/CMV plasmid containing the sequence of the
human Nav1.5 channel was kindly provided by Dr Alfred L. George Jr
(Northwestern University, Chicago, IL, USA). The pRc/CMV-hNav1.5
plasmid contains a 6.1 kb cDNA sequence encoding the human
voltage-gated sodium channel Nav1.5 (SCN5A gene product) inserted
into the NotI (5′) and XbaI (3′) sites of the
mammalian expression vector, pRc/CMV (5.4 kb). Plasmids containing
cDNA encoding human Nav1.5 channels (pRc/CMV-hNav1.5) and green
fluorescent protein (GFP; pRc/CMV-GFP) were co-transfected into
293T cells (cat. no. CRL-3216; American Type Culture Collection),
which do not possess endogenous Nav1.5 channels. Cells (80%
confluence) were transfected with 1 µg/µl plasmids
using Lipofectamine® 3000 reagent (Invitrogen; Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol.
The cells were then cultured for 24 h in Dulbecco's modified
Eagle's medium supplemented with 10% FBS (both from Gibco; Thermo
Fisher Scientific, Inc.). Patch clamp experiments were conducted
24-48 h after 293T cells were reseeded on coverslips in 6-well
plates containing RPMI-1640 medium. A coverslip with 293T cells was
placed in a recording chamber containing bath solution on the stage
of a fluorescence micro-scope (Nikon Corporation, Tokyo, Japan),
and the transfected cells were identified by the fluorescent signal
emitted from GFP (data not shown).
Migration assay
SKOV3 cells were seeded onto 24-well plates (10,000
cells/well). For the wound-healing assay, scratch lesions were
created on confluent SKOV3 cells using a 10 µl pipette tip.
Wounded cultures were incubated at room temperature for 36 h, and
three randomly selected fields at the lesion border were
subsequently examined by inverted phase contrast microscopy (IX73;
Olympus Corporation, Tokyo, Japan) in order to assess cell
migration.
Cell proliferation assay
Cell Counting kit-8 (CCK-8) assay (Dojindo Molecular
Technologies, Inc., Kumamoto, Japan) was used to study SKOV3 cell
proliferation, according to the manufacturer's protocol. SKOV3
cells were seeded at a density of 5×103 cells/well in
96-well plates, after which 10 µl CCK-8 solution was added
to each well. Absorbance was measured at 450 nm using a multiplate
reader (Lambda Bio-20; Beckman Coulter, Inc., Brea, CA, USA). Cell
viability was expressed as a percentage of that of the control
(untreated) cells.
Data analysis
All data are presented as the means ± standard error
of the mean. The n value denotes the number of independent
experiments conducted, unless otherwise stated. Significance
between means was determined using either the two-tailed Student's
paired t-test or one-way analysis of variance with Dunnett's
multiple comparisons test. P<0.05 was considered to indicate a
statistically significant difference.
Results
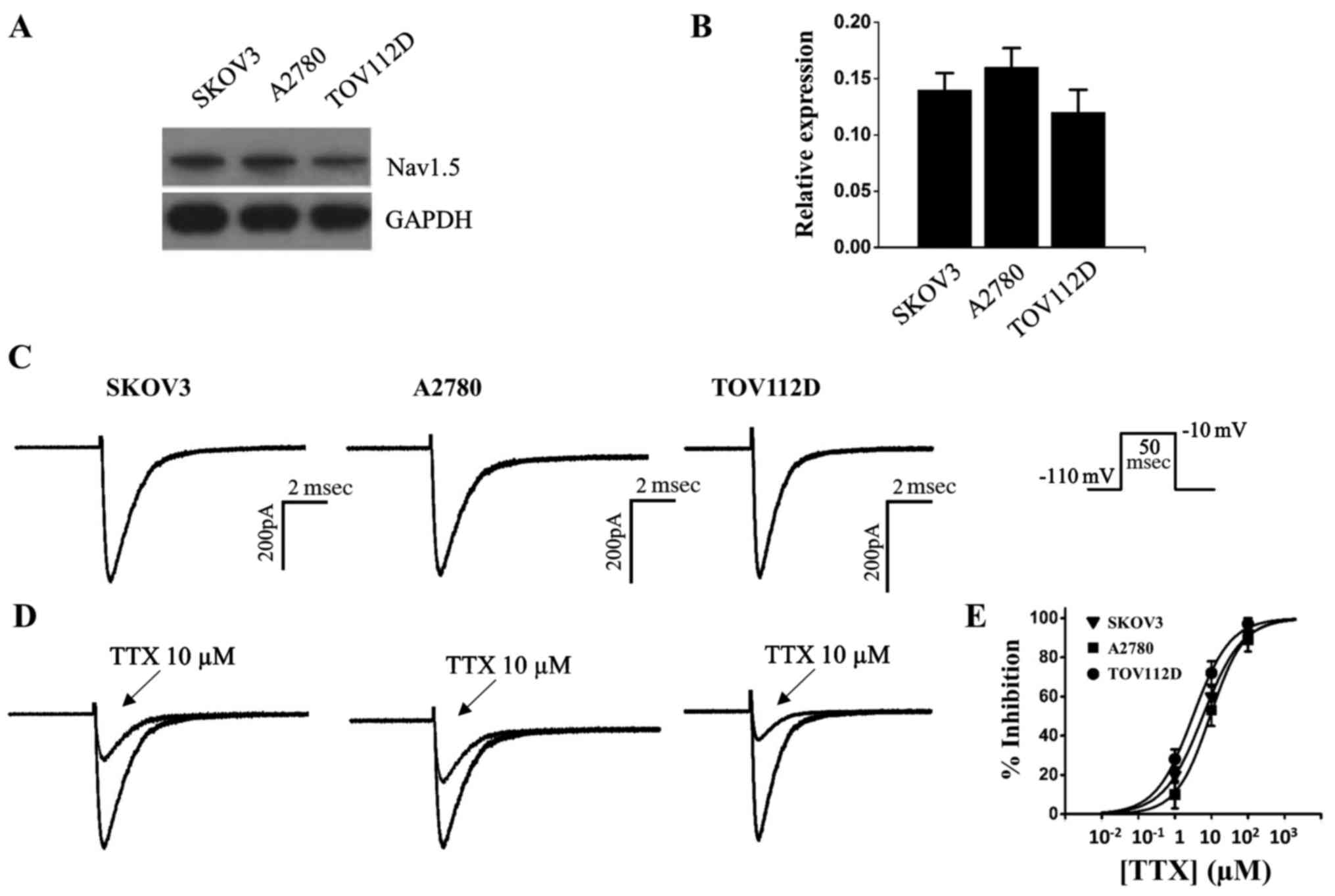
Characterization of voltage-gated sodium
channel Nav1.5 currents in ovarian cancer cells
The voltage-gated sodium channel Nav1.5 has been
implicated in ovarian cancer (12), the present study detected Nav1.5
expression in the epithelial ovarian cancer cell lines TOV112D,
A2780 and SKOV3. The results of a western blot analysis indicated
that Nav1.5 channel protein was expressed in the three ovarian
cancer cells (Fig. 1A and B).
Although the voltage-gated sodium channels have been associated
with ovarian cancer development, to the best of our knowledge, the
functional sodium currents have not been examined in ovarian cancer
cells (11,12). Using whole cell patch-clamp
configuration, the present study observed fast inactivation
currents in ovarian cancer cells (Fig.
1C). The currents elicited by depolarization to −10 mV were
further measured prior to and after the addition of the specific
voltage-gated sodium channel blocker tetrodotoxin (TTX);
Sigma-Aldrich; Merck KGaA) to the bath solution; currents were
partially inhibited by 10 µM TTX (Fig. 1D). TTX had IC50 values
of 5.8, 9.2 and 3.1 µM in SKOV3, A2780, and TOV112D ovarian
cancer cells, respectively (Fig.
1E and Table I). Since the
IC50 values were in the micromolar range, it was
suggested that these cell lines were of the TTX-resistant subtype
(23).
 | Table IIC50 values, as fitted by
the Hill equation, of TTX- or EPA-induced channel inhibition in
ovarian cancer cells. Sodium currents were recorded in independent
cultures. |
Table I
IC50 values, as fitted by
the Hill equation, of TTX- or EPA-induced channel inhibition in
ovarian cancer cells. Sodium currents were recorded in independent
cultures.
| Inhibitor | Cell type | IC50
(µM) | n |
|---|
| TTX | SKOV3 | 5.8±0.4 | 5 |
| TTX | A2780 | 9.2±0.7 | 5 |
| TTX | TOV112D | 3.1±0.3 | 5 |
| EPA | SKOV3 | 11.8±1.0 | 6 |
| EPA | A2780 | 19.6±1.8 | 6 |
| EPA | TOV112D | 18.1±1.2 | 6 |
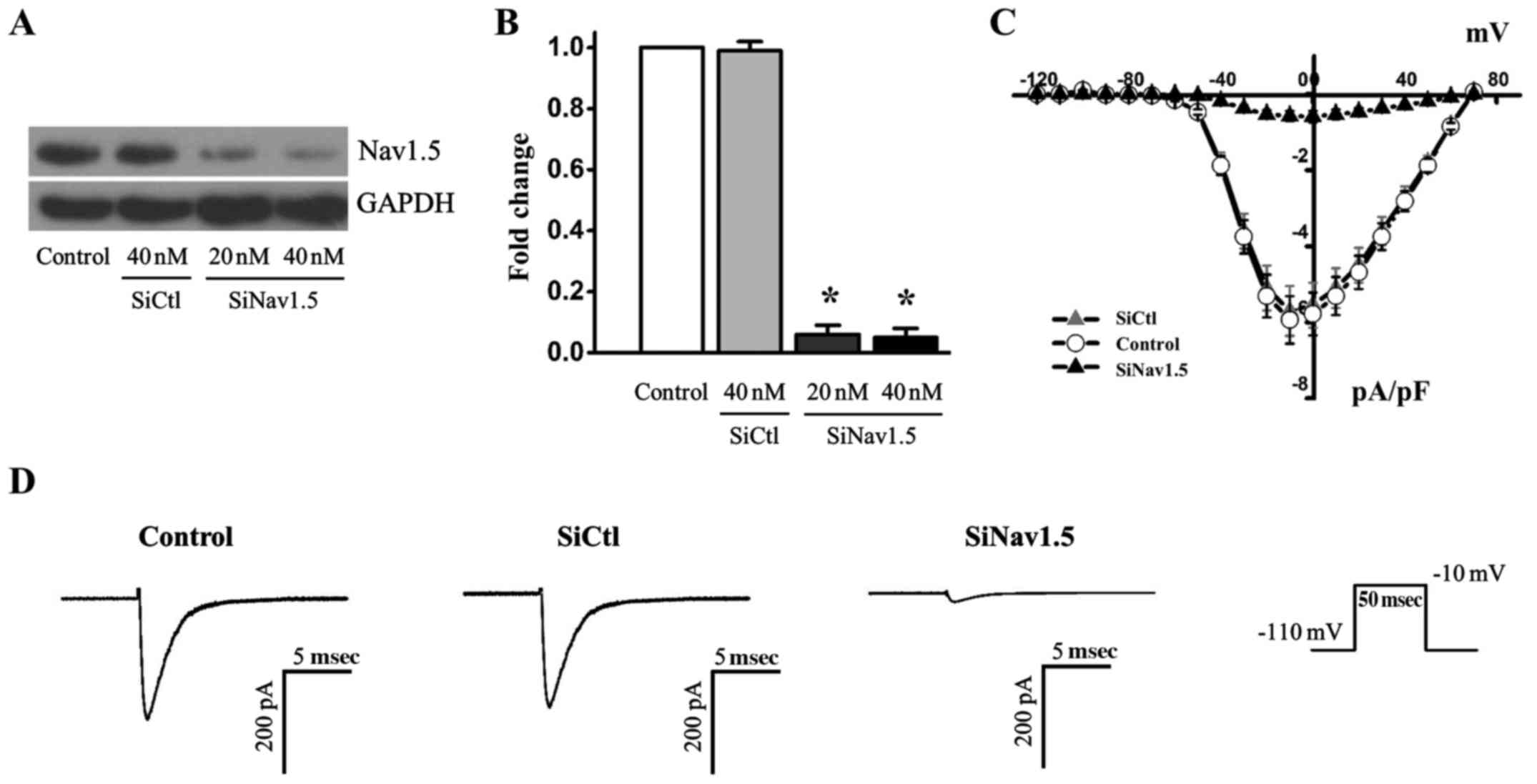
Effects of Nav1.5 knockdown on sodium
currents
To further analyze sodium currents in ovarian cancer
cells, the RNA interference approach was used to knockdown Nav1.5
expression in SKOV3 cancer cells. Transfection with Nav1.5 siRNA
resulted in a significant reduction in the protein expression
levels of Nav1.5, whereas SiCtl did not alter Nav1.5 expression
(Fig. 2A and B). The reduction in
Nav1.5 protein expression was associated with >90% reduction of
the maximal currents recorded when the cells were depolarized to
−10 mV (Fig. 2C and D). These
results indicated that the sodium currents were predominantly
carried by the Nav1.5 channel, and that Nav1.5 channels are
functionally expressed in ovarian cancer cells.
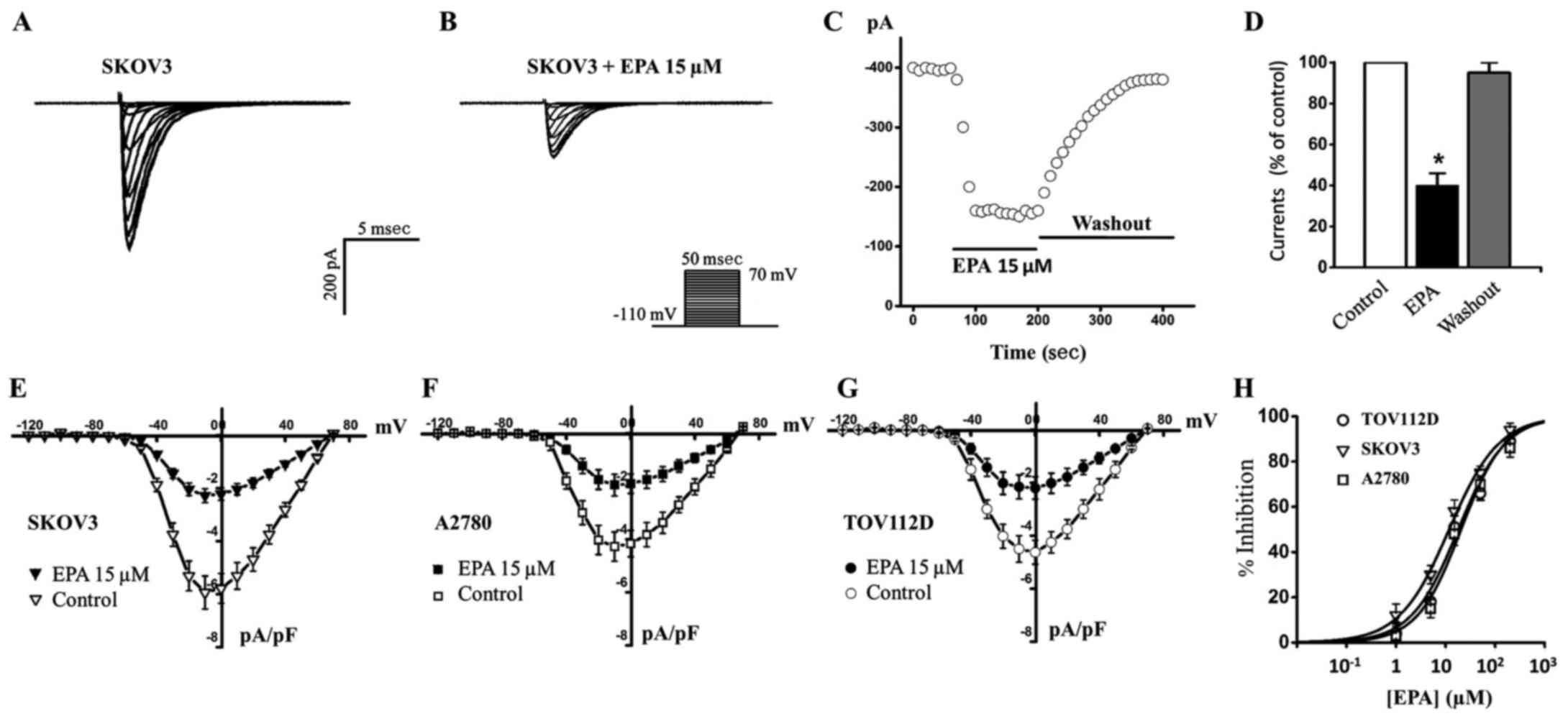
EPA functionally inhibits Nav1.5 sodium
currents in ovarian cancer cells
After examining Nav1.5 channel currents in ovarian
cancer cells, the present study aimed to determine whether EPA
exerted effects on them (Fig. 3).
EPA was previously reported to block the Nav1.5 channel in
cardiomyocytes (14), and to
inhibit ovarian cancer cell growth and reduce cancer metastasis
(15). The present study
hypothesized that EPA may affect the Nav1.5 channel in ovarian
cancer cells; the results indicated that treatment with 15
µM EPA blocked ~60% sodium currents in SKOV3 cancer cells
(Fig. 3A, B and E), and this
inhibition could be completely reversed following EPA washout
(Fig. 3C and D). Furthermore, 15
µM EPA blocked sodium currents to similar extents in A2780
and TOV112D cancer cells (Fig. 3F and
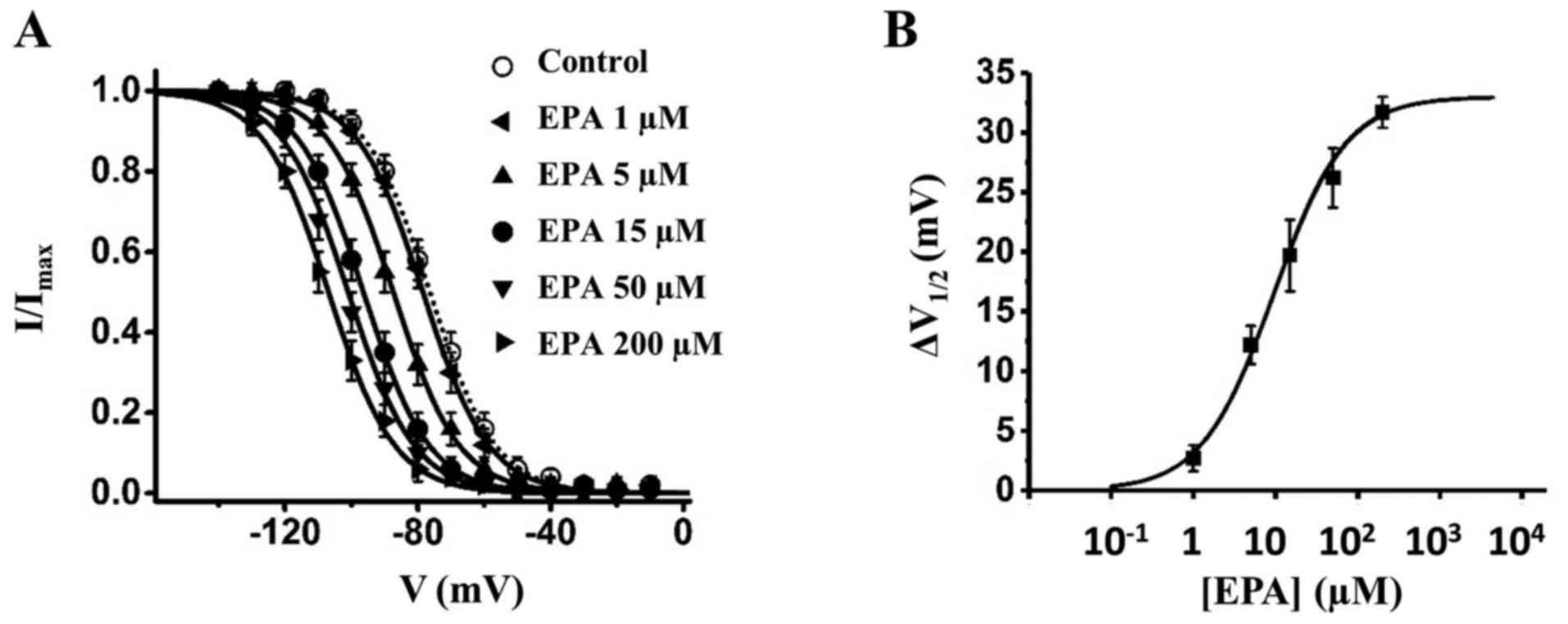
G). Various concentrations of EPA were then used to generate a
dose-response curve; the IC50 values for EPA in SKOV3,
A2780 and TOV112D cells were 11.8, 19.6 and 18.1 µM,
respectively (Fig. 3H and Table I). These results indicated that EPA
may effectively block Nav1.5 sodium currents in ovarian cancer
cells. Subsequently, the present study aimed to explore the
potential mechanism by which EPA inhibits Nav1.5 currents.
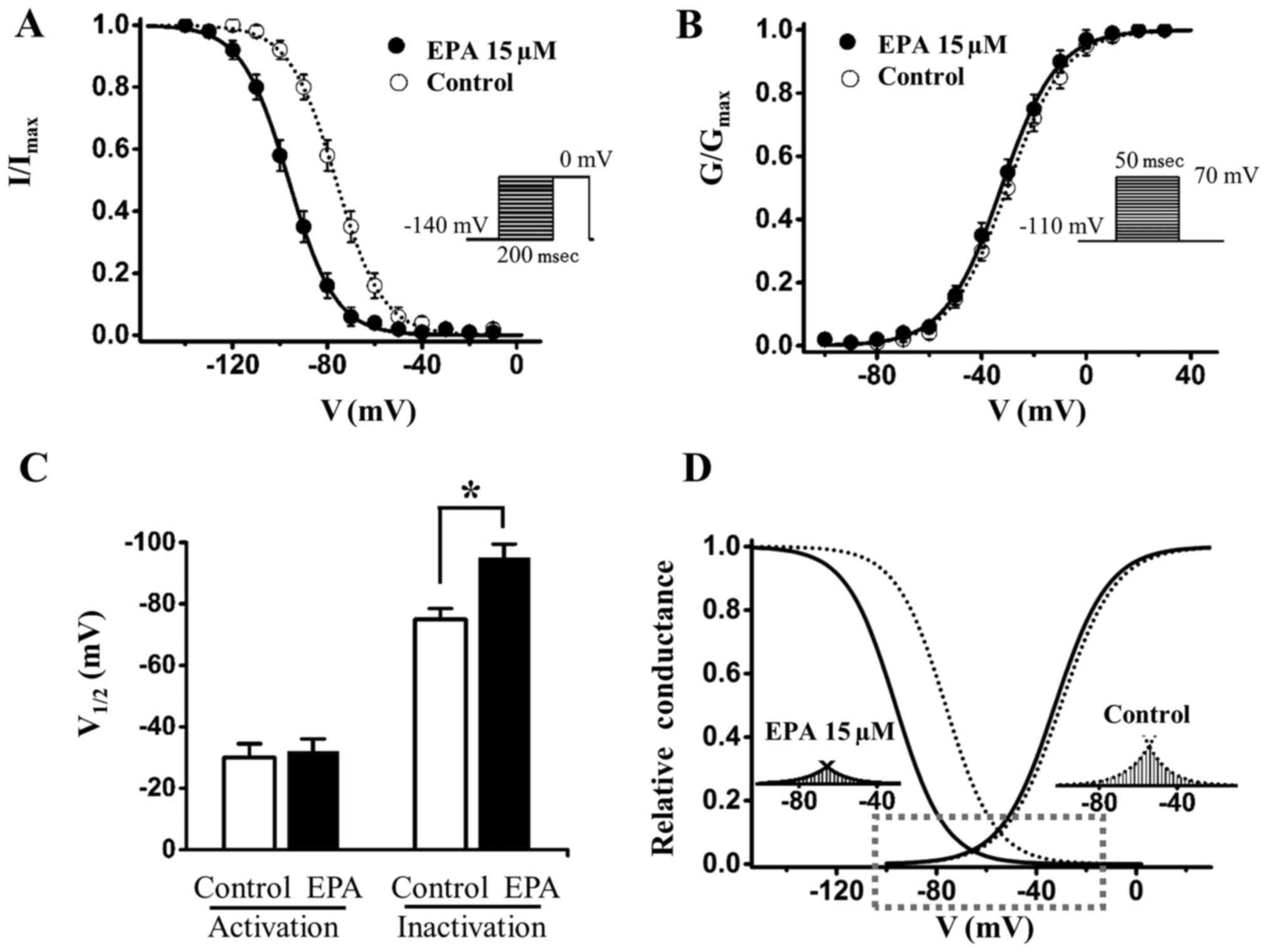
EPA shifts the steady-state inactivation
curve of sodium currents in a hyperpolarizing direction
Previous studies demonstrated that EPA induces
Nav1.5 channels to enter an inactive state and stabilizes the
channel, thus resulting in a reduction of sodium currents (24,25).
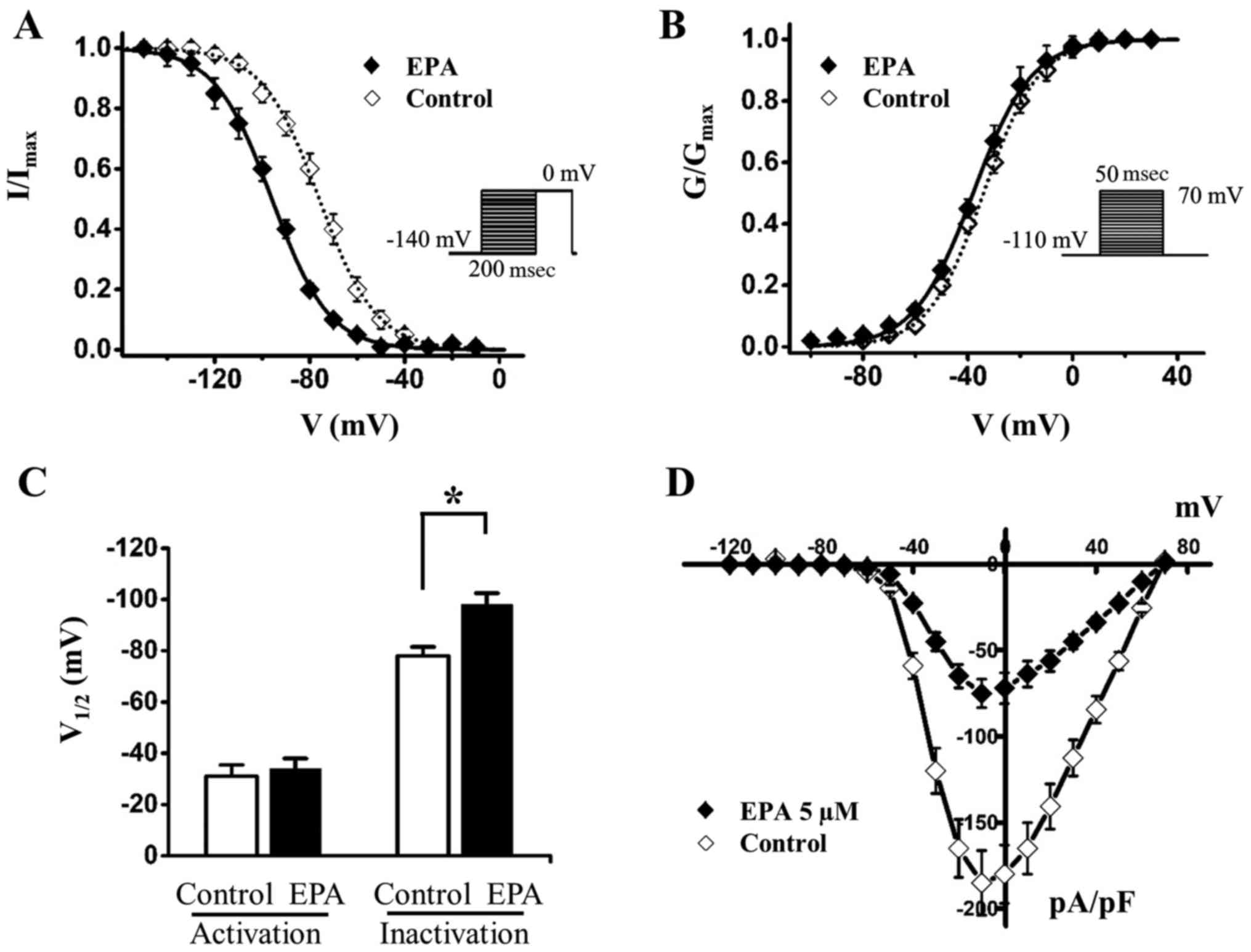
The present study investigated the effects of EPA on SKOV3 cancer
cells (Fig. 4); the results
indicated that 15 µM EPA shifted the steady-state
inactivation of sodium currents by ~20 mV to the hyperpolarizing
direction (Fig. 4A and C and
Table II), whereas it had almost
no effect on channel activation (Fig.
4B and C and Table III). The
window currents were obtained by plotting the area where
steady-state inactivation and activation overlap; EPA significantly
reduced the Nav1.5 sodium window currents (Fig. 4D). In addition, the shifts of
inactivation induced by EPA, along with the rise of EPA
concentration, were gradually enhanced to the hyperpolarizing
direction, indicating that the shift of steady-state inactivation
is EPA dose-dependent (Fig. 5 and
Table II). In the other two
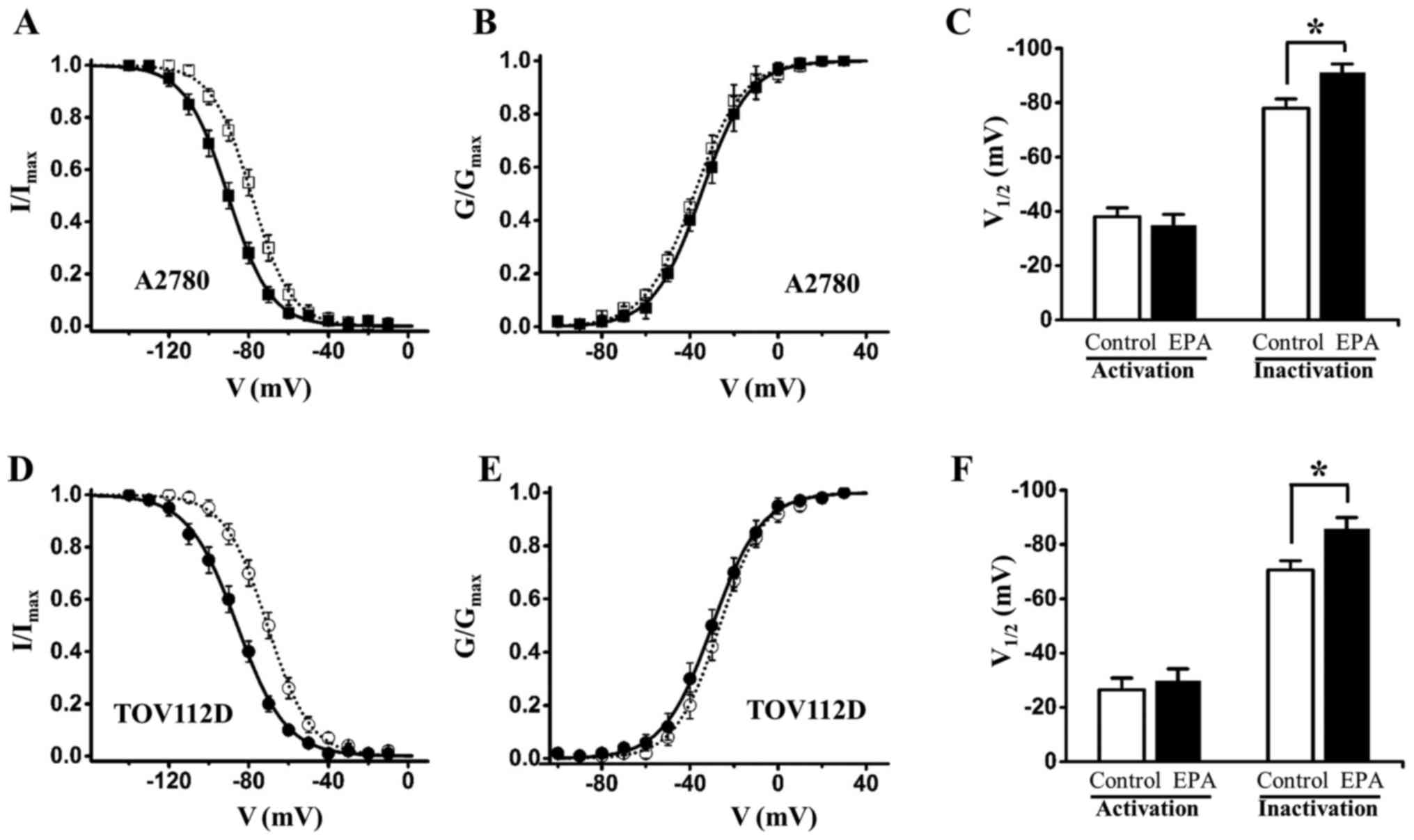
ovarian cancer cells (A2780 and TOV112D cells), 15 µM EPA
also altered steady-state inactivation curves to the
hyperpolarizing direction, and did not significantly alter
steady-state activation curves (Fig.
6 and Tables II and III). Subsequently, the present study
transfected 293T cells with Nav1.5 cDNA; the results revealed that
EPA hyperpolarized the slow inactivation curve of Nav1.5 channels
and reduced Nav1.5 channel availability, which are consistent with
the results in ovarian cancer cells (Fig. 7). In conclusion, these data
suggested that EPA may enhance sodium channel inactivation and
stabilize the inactivation gate of the channel, thus indicating
that EPA-induced reductions in sodium currents may be due to more
channels remaining in the inactivated state post-activation.
 | Table IIV1/2 of voltage
dependence of steady-state inactivation of sodium channels prior to
and after treatment with EPA. Sodium currents were recorded in
independent cultures. |
Table II
V1/2 of voltage
dependence of steady-state inactivation of sodium channels prior to
and after treatment with EPA. Sodium currents were recorded in
independent cultures.
| Group | Cell line |
V1/2 (mV) | n |
|---|
| Control | SKOV3 | −67.3±8.4 | 7 |
| Control | A2780 | −78.0±3.4 | 8 |
| Control | TOV112D | −70.6±3.4 | 6 |
| EPA 15
µM | SKOV3 | −99.0±5.8 | 7 |
| EPA 15
µM | A2780 | −91.1±3.2 | 8 |
| EPA 15
µM | TOV112D | −85.8±4.1 | 6 |
| EPA 1
µM | SKOV3 | −78.1±3.7 | 10 |
| EPA 5
µM | SKOV3 | −87.4±3.5 | 9 |
| EPA 50
µM | SKOV3 | −101.4±7.5 | 9 |
| EPA 200
µM | SKOV3 | −106.9±4.5 | 10 |
 | Table IIIV1/2 values from
Boltzmann fits of voltage dependence of activation of sodium
channels prior to and after treatment with EPA. Sodium currents
were recorded in independent cultures. |
Table III
V1/2 values from
Boltzmann fits of voltage dependence of activation of sodium
channels prior to and after treatment with EPA. Sodium currents
were recorded in independent cultures.
| Group | Cell line |
V1/2 (mV) | n |
|---|
| Control | SKOV3 | −31.1±3.4 | 7 |
| Control | A2780 | −38.0±3.3 | 8 |
| Control | TOV112D | −26.5±4.3 | 6 |
| EPA 15
µM | SKOV3 | −32.6±3.3 | 7 |
| EPA 15
µM | A2780 | −34.9±4.0 | 8 |
| EPA 15
µM | TOV112D | −29.8±4.4 | 6 |
Inhibition of the Nav1.5 channel
attenuates SKOV3 cell migration
The present study also aimed to examine the function
of Nav1.5 during the process of ovarian cancer development. The
Nav1.5 channel has been reported to promote cancer cell invasion
(26); therefore, inhibition of
this channel was expected to reduce cell migration. The present
study used a wound-healing assay to investigate the effects of
either Nav1.5 knockdown or Nav1.5 functional inhibition by EPA on
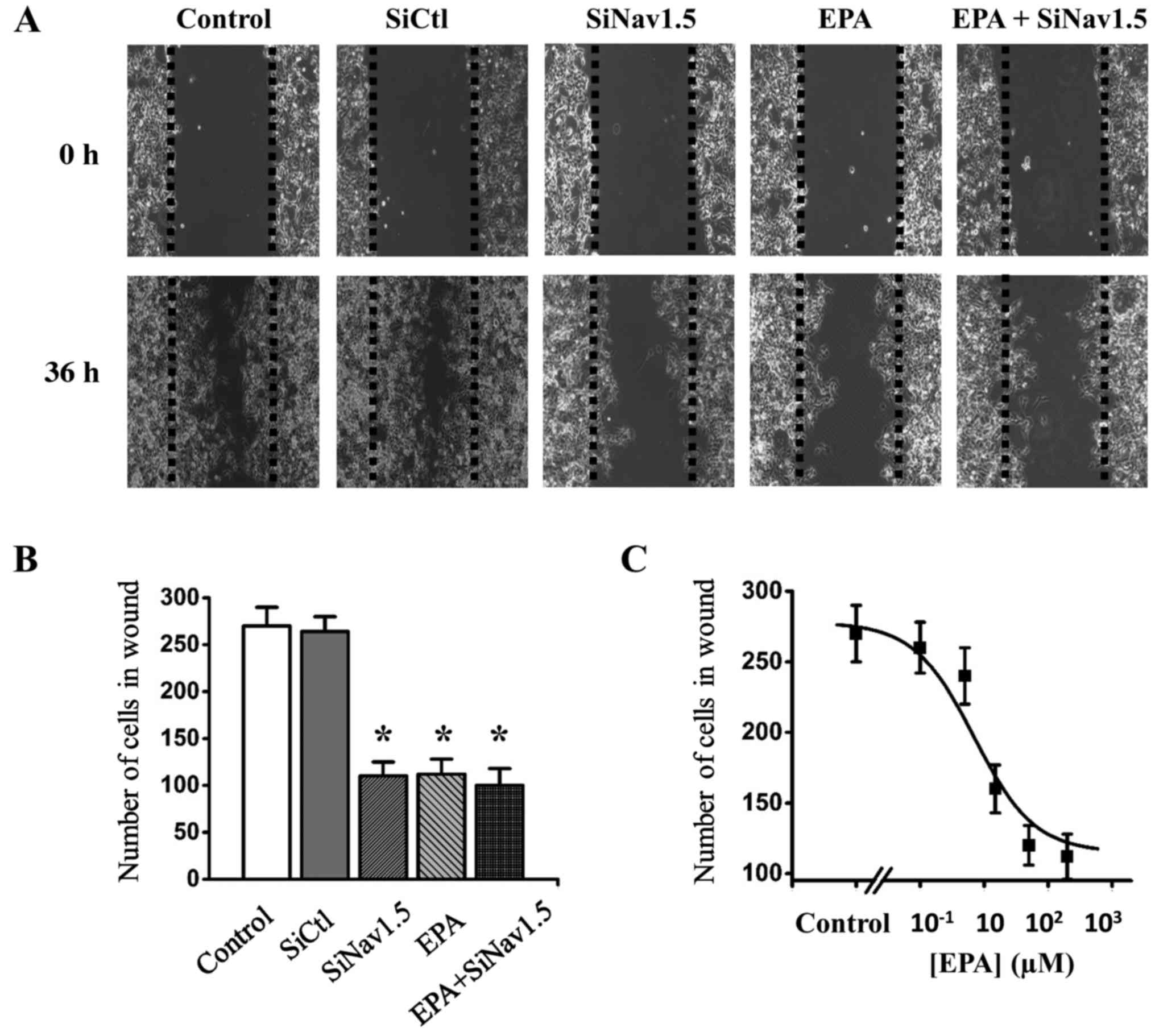
SKOV3 ovarian cancer cell migration. The cells were divided into
five groups: Group 1, control; group 2, cells transfected with
Nav1.5 siRNA; group 3, cells transfected with SiCtl; group 4, cells
treated with 200 µM EPA; group 5, cells transfected with
Nav1.5 siRNA and treated with 200 µM EPA (Fig. 8). The results demonstrated that EPA
was able to inhibit SKOV3 cell migration in a dose-dependent manner
(Fig. 8C), and migration was
significantly reduced in Nav1.5 siRNA-transfected cells compared
with in SiCtl cells (Fig. 8A and
B). Notably, the migration of Nav1.5 siRNA-transfected cells
was not further reduced by 200 µM EPA, thus indicating that
the impaired invasion of these cells was due to the absence of
Nav1.5 activities (Fig. 8B). These
results suggested that the Nav1.5 channel may have a critical role
in ovarian cancer migration, and the effects of EPA on cell
migration were due to the blockade of Nav1.5 sodium channels.
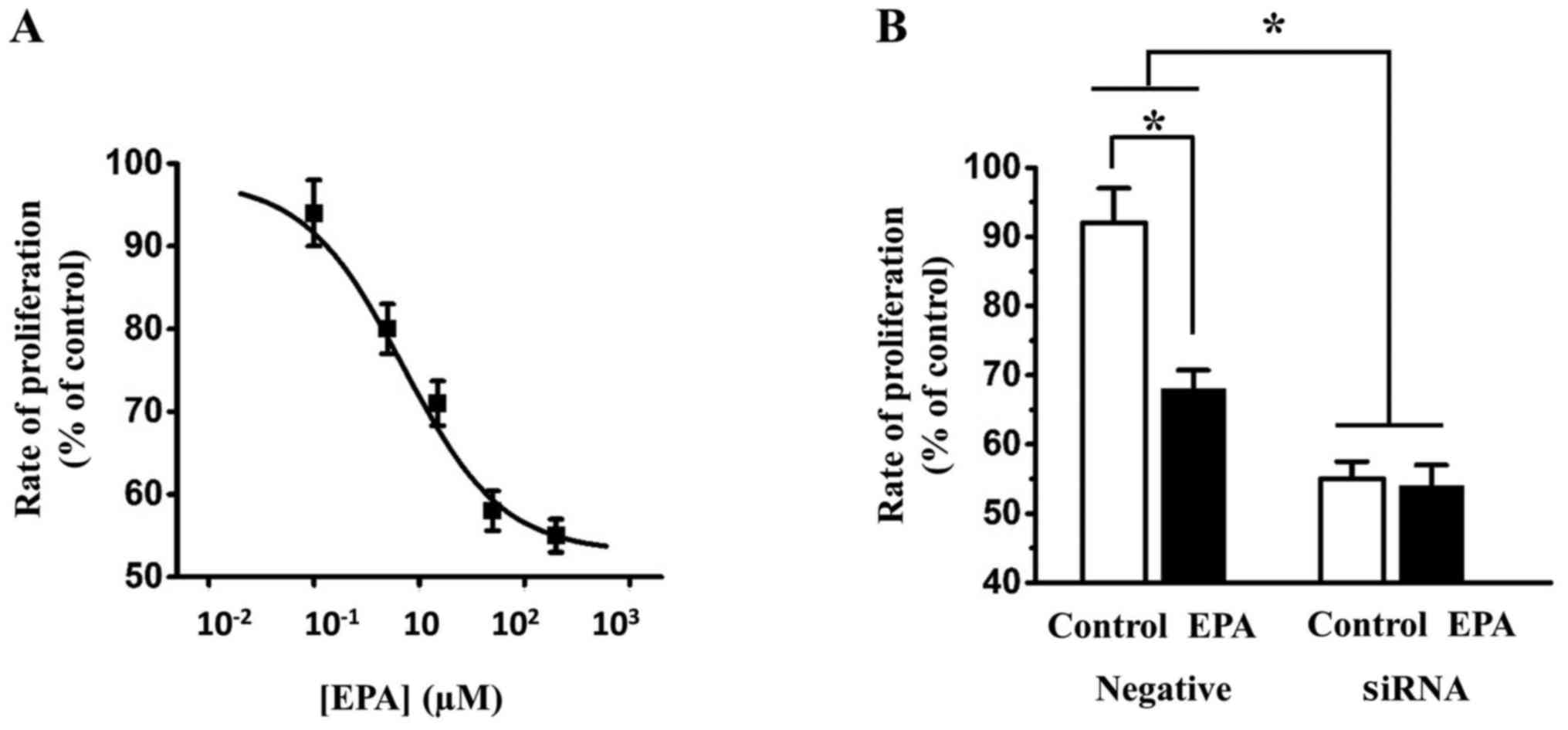
EPA inhibits SKOV3 cell proliferation via
Nav1.5
Using the CCK-8 assay, it was revealed that EPA
inhibited the proliferation of SKOV3 cells in a dose-dependent
manner (Fig. 9A). Knockdown of
Nav1.5 in SKOV3 cells resulted in a marked decrease in the
inhibitory effect of 15 µM EPA on SKOV3 cell proliferation.
In addition, 15 µM EPA alone still inhibited SKOV3 cell
proliferation in SiCtl-transfected cells (Fig. 9B). Collectively, these data
indicated that inhibition of Nav1.5 by EPA may reduce ovarian cell
proliferation.
Discussion
To the best of our knowledge, the present study is
the first to record voltage-gated sodium currents in ovarian cancer
cells; the currents were further characterized to be carried by the
Nav1.5 channel. In addition, EPA effectively inhibited sodium
currents in the ovarian cancer cells, and shifted the steady-state
inactivation of voltage-gated sodium currents to the
hyperpolarizing direction, thus resulting in reduced window
currents and blockade of the channel. The functional inhibition of
Nav1.5 sodium channels by EPA attenuated ovarian cancer cell
migration and proliferation.
EPA is a member of the family of long-chain n-3
poly-unsaturated fatty acids. It is usually used as a nutritional
supplement to treat cancer-associated cachexia (27). Previous studies reported that EPA
exerts a direct effect on cancer development, and induces cancer
apoptosis, suppresses cell migration and affects cancer metastasis
(15,28,29).
EPA has been hypothesized to regulate the apoptotic signaling
pathway, inhibit tumor migration and enhance the effects of
anticancer drugs. EPA was previously reported to block endogenous
Nav1.5 channels in cardiomyocytes; EPA was able to induce Nav1.5
channels to enter an inactive state, thus resulting in a reduction
of sodium currents (14,18). In addition, another study
identified position N406 of the Nav1.5 channel as the key
interacting site with EPA, and an N406 mutation (N406K)
significantly decreases the inhibitory effects of EPA on Nav1.5
sodium currents (24). The present
study revealed that EPA reduced Nav1.5-mediated ovarian cancer cell
migration and growth, and observed that EPA blocked sodium currents
in ovarian cancer cells in a dose-dependent manner. EPA
IC50 values were measured and it was revealed that EPA
was able to inhibit sodium currents in various ovarian cancer cells
within similar concentration ranges; this is consistent with
previous findings regarding the effects of EPA on Nav1.5 channels
in cardiomyocytes (14).
Furthermore, this study indicated that EPA shifted the steady-state
inactivation curve of sodium currents to a hyper-polarizing
direction; however, it had almost no effect on the steady-state
activation curve, thus resulting in a decrease of Nav1.5 channel
window currents.
The window currents are defined as the currents that
flux through voltage-gated sodium channels in a narrow voltage
range, in which steady-state inactivation and activation states
overlap (30). At transmembrane
potentials beyond this window, channels cannot generate any sodium
currents due to the channels being either in a closed state or an
inactivated state. Therefore, the window current serves an
important role in the function of voltage-gated sodium channels
(31). The sodium channel window
currents usually control action potential shape in excitable cells,
such as cardiomyocytes and neurons; however, upregulated window
currents have been reported to trigger arrhythmias or dysfunctional
excitation-contraction coupling (32,33).
The present study demonstrated that EPA effectively inhibited
window currents carried by the Nav1.5 channel in ovarian cancer
cells, and reduced cancer cell migration and proliferation.
It has been reported that a neonatal splice variant
of the Nav1.5 channel (nNav1.5) is predominantly expressed in the
highly metastatic MDA-MB-231 breast cancer cell line; therefore,
nNav1.5 has been proposed as a marker with clinical potential for
the management of metastatic breast cancer (34). Compared with the adult variant
Nav1.5 (aNav1.5), nNav1.5 exhibits a 6-amino acid substitution in
the S3 and S4 loop in domain I, and one amino acid substitution in
S4 of domain I (35). This
structural difference produces unique properties for nNav1.5. For
example, in nNav1.5 voltage-dependence of activation is shifted in
the depolarized direction, and is much slower to open and close.
The V1/2 of activation for nNav1.5 is −19 mV,
whereas for aNav1.5 it is −28 mV (35). The present results indicated that
the V1/2 of activation for Nav1.5 was between −26
and −38 mV in ovarian cancer cells, thus suggesting that being
different from breast cancer cells, aNav1.5 may be the predominant
Nav1.5 in ovarian cancer cells.
It is well known that ion channels have important
roles in the growth and migration of cancer cells (36-41),
and cell proliferation and invasion are crucial hallmarks for
cancer development (42). In
addition, the association between cancer proliferation and resting
potential has been supported by studies in breast cancer cells,
hepatocellular carcinoma cells, fibrosarcoma cells and ovarian
cancer cells (43). It was
revealed that cancer cells have low resting potential and tend to
be more depolarized than normal cells. The resting potential of
ovarian tumor cells was approximately −10 mV, in which most Nav1.5
channels are activated (44). The
low resting potential may activate voltage-dependent Nav1.5
channels in ovarian cancer cells and provide an explanation for the
mechanism underlying Nav1.5-dependent proliferation of cancer
cells. There are several theories regarding how sodium currents
contribute to cancer cell growth and tumor invasiveness. One
explanation is that sodium ions permeate from the sodium channel,
thus leading to the entry of calcium through the sodium-calcium
exchanger (NCX), which induces Ca2+-dependent signaling
to promote cancer cell invasion and metastatic progression
(26). Another theory proposed is
that the local sodium concentration is able to activate the
Na+/H+ exchanger (NHE) and enhance
H+ efflux, consequently leading to increased
intracellular alkalinisation and decreased extra-cellular pH.
Increased glycolytic metabolism in cancer cells gives rise to an
excessive production of intracellular acidity; therefore,
intracellular alkalinisation potentially facilitates cancer
metabolism. In addition, a lower extracellular pH environment
activates pH-dependent cathepsin proteases and invadopodia
formation, which in turn, may enhance cancer cell invasion
(45). In the present study, the
sodium currents that promote ovarian cancer cell migration and
proliferation were suppressed by EPA treatment or by
Nav1.5-targeting siRNA, thus indicating that Nav1.5-mediated sodium
currents have crucial roles during the development of ovarian
cancer. However, whether Nav1.5 induced Ca2+-dependent
signaling by stimulating NCX activity to accelerate ovarian tumor
progression, or activated NHE to increase H+ efflux, in
order to provide a favorable environment for ovarian cancer growth,
remain unclear; further studies are required to address these
uncertainties.
In conclusion, to the best of our knowledge, the
present study was the first to record Nav1.5-mediated sodium
currents in ovarian cancer cells; the results revealed that Nav1.5
currents were effectively inhibited by EPA. The mechanism by which
EPA blocked the Nav1.5 channel was due to increased inactivation of
the Nav1.5 sodium channel, thus resulting in a reduction in window
currents and blockade of the channel. These findings highlight the
importance of Nav1.5 in cancer development, and may provide novel
insights into the development of chemotherapeutics for ovarian
cancer.
Abbreviations:
|
EPA
|
eicosapentaenoic acid
|
|
TTX
|
tetrodotoxin
|
Acknowledgments
The authors would like to thank Dr A.L. George Jr
(Northwestern University, Chicago, IL, USA) for the hNav1.5 channel
cDNA, Dr D. Zhang (Sun Yat-Sen University, Guangzhou, Guangdong,
China) for helpful discussions, and Dr G. Yao (Sun Yat-Sen
University, Guangzhou, Guangdong, China) and other members of the
laboratory for insightful comments on the manuscript.
Funding
The present study was supported by the Young
Scientists Fund of the National Natural Science. Foundation of
China (grant no. 81502226) and the Guangdong Natural Science
Foundation (grant no. 2014A030313038).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
SY and LH designed the project. JL, DL, JJL and CZ
performed research; JL, DL, SY and LH analyzed the data; JL and LH
wrote the paper. All authors read and edited the manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Smith RA, Andrews KS, Brooks D, Fedewa SA,
Manassaram-Baptiste D, Saslow D, Brawley OW and Wender RC: Cancer
screening in the United States, 2017: A review of current American
Cancer Society guidelines and current issues in cancer screening.
CA Cancer J Clin. 67:100–121. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jelovac D and Armstrong DK: Recent
progress in the diagnosis and treatment of ovarian cancer. CA
Cancer J Clin. 61:183–203. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen M, Yao S, Cao Q, Xia M, Liu J and He
M: The prognostic value of Ki67 in ovarian high-grade serous
carcinoma: An 11-year cohort study of Chinese patients. Oncotarget.
8:107877–107885. 2016.
|
|
4
|
Asher V, Khan R, Warren A, Shaw R,
Schalkwyk GV, Bali A and Sowter HM: The Eag potassium channel as a
new prognostic marker in ovarian cancer. Diagn Pathol. 5:782010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yu FH and Catterall WA: Overview of the
voltage-gated sodium channel family. Genome Biol. 4:2072003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu M, Yang KC and Dudley SC Jr: Cardiac
sodium channel mutations: Why so many phenotypes? Nat Rev Cardiol.
11:607–615. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fraser SP, Diss JK, Lloyd LJ, Pani F,
Chioni AM, George AJ and Djamgoz MB: T-lymphocyte invasiveness:
Control by voltage-gated Na+ channel activity. FEBS
Lett. 569:191–194. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shan B, Dong M, Tang H, Wang N, Zhang J,
Yan C, Jiao X, Zhang H and Wang C: Voltage-gated sodium channels
were differentially expressed in human normal prostate, benign
prostatic hyperplasia and prostate cancer cells. Oncol Lett.
8:345–350. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fraser SP, Diss JK, Chioni AM, Mycielska
ME, Pan H, Yamaci RF, Pani F, Siwy Z, Krasowska M, Grzywna Z, et
al: Voltage-gated sodium channel expression and potentiation of
human breast cancer metastasis. Clin Cancer Res. 11:5381–5389.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
House CD, Vaske CJ, Schwartz AM, Obias V,
Frank B, Luu T, Sarvazyan N, Irby R, Strausberg RL, Hales TG, et
al: Voltage-gated Na+ channel SCN5A is a key regulator
of a gene transcriptional network that controls colon cancer
invasion. Cancer Res. 70:6957–6967. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Frede J, Fraser SP, Oskay-Özcelik G, Hong
Y, Ioana Braicu E, Sehouli J, Gabra H and Djamgoz MB: Ovarian
cancer: Ion channel and aquaporin expression as novel targets of
clinical potential. Eur J Cancer. 49:2331–2344. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gao R, Shen Y, Cai J, Lei M and Wang Z:
Expression of voltage-gated sodium channel alpha subunit in human
ovarian cancer. Oncol Rep. 23:1293–1299. 2010.PubMed/NCBI
|
|
13
|
Brinton EA and Mason RP: Prescription
omega-3 fatty acid products containing highly purified
eicosapentaenoic acid (EPA). Lipids Health Dis. 16:232017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li GR, Sun HY, Zhang XH, Cheng LC, Chiu
SW, Tse HF and Lau CP: Omega-3 polyunsaturated fatty acids inhibit
transient outward and ultra-rapid delayed rectifier K+
currents and Na+ current in human atrial myocytes.
Cardiovasc Res. 81:286–293. 2009. View Article : Google Scholar
|
|
15
|
Wan XH, Fu X and Ababaikeli G:
Docosahexaenoic acid induces growth suppression on epithelial
ovarian cancer cells more effectively than eicosapentaenoic acid.
Nutr Cancer. 68:320–327. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pathak MM, Tran T, Hong L, Joós B, Morris
CE and Tombola F: The Hv1 proton channel responds to mechanical
stimuli. J Gen Physiol. 148:405–418. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hong L and Tombola F: Allostery: A lipid
two-step. Nat Chem Biol. 12:202–203. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Leifert WR, McMurchie EJ and Saint DA:
Inhibition of cardiac sodium currents in adult rat myocytes by n-3
polyunsaturated fatty acids. J Physiol. 520:671–679. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Alkhalil A, Hong L, Nguitragool W and
Desai SA: Voltage-dependent inactivation of the plasmodial surface
anion channel via a cleavable cytoplasmic component. Biochim
Biophys Acta. 1818:367–374. 2012. View Article : Google Scholar :
|
|
20
|
Hong L, Wang G and Guan Y: Involvement of
volume-regulated Cl- current in myocardial hypertrophy. Chinese
Pharmacological Bulletin. 23:990–993. 2007.
|
|
21
|
Hong L, Singh V, Wulff H and Tombola F:
Interrogation of the intersubunit interface of the open Hv1 proton
channel with a probe of allosteric coupling. Sci Rep. 5:140772015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim IH, Hevezi P, Varga C, Pathak MM, Hong
L, Ta D, Tran CT, Zlotnik A, Soltesz I and Tombola F: Evidence for
functional diversity between the voltage-gated proton channel Hv1
and its closest related protein HVRP1. PLoS One. 9:e1059262014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zimmer T, Haufe V and Blechschmidt S:
Voltage-gated sodium channels in the mammalian heart. Glob Cardiol
Sci Pract. 2014:449–463. 2014.PubMed/NCBI
|
|
24
|
Xiao YF, Ke Q, Wang SY, Auktor K, Yang Y,
Wang GK, Morgan JP and Leaf A: Single point mutations affect fatty
acid block of human myocardial sodium channel alpha subunit
Na+ channels. Proc Natl Acad Sci USA. 98:3606–3611.
2001. View Article : Google Scholar
|
|
25
|
Xiao YF, Ma L, Wang SY, Josephson ME, Wang
GK, Morgan JP and Leaf A: Potent block of inactivation-deficient
Na+ channels by n-3 polyunsaturated fatty acids. Am J
Physiol Cell Physiol. 290:C362–C370. 2006. View Article : Google Scholar
|
|
26
|
Besson P, Driffort V, Bon É, Gradek F,
Chevalier S and Roger S: How do voltage-gated sodium channels
enhance migration and invasiveness in cancer cells? Biochim Biophys
Acta. 1848:2493–2501. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pappalardo G, Almeida A and Ravasco P:
Eicosapentaenoic acid in cancer improves body composition and
modulates metabolism. Nutrition. 31:549–555. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sharma A, Belna J, Espat J, Rodriguez G,
Cannon VT and Hurteau JA: Effects of omega-3 fatty acids on
components of the transforming growth factor beta-1 pathway:
implication for dietary modification and prevention in ovarian
cancer. Am J Obstet Gynecol. 200:516.e1–6. 2009. View Article : Google Scholar
|
|
29
|
Sharma A, Belna J, Logan J, Espat J and
Hurteau JA: The effects of Omega-3 fatty acids on growth regulation
of epithelial ovarian cancer cell lines. Gynecol Oncol. 99:58–64.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Attwell D, Cohen I, Eisner D, Ohba M and
Ojeda C: The steady state TTX-sensitive ('window') sodium current
in cardiac Purkinje fibres. Pflugers Arch. 379:137–142. 1979.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Frenz CT, Hansen A, Dupuis ND, Shultz N,
Levinson SR, Finger TE and Dionne VE: NaV1.5 sodium channel window
currents contribute to spontaneous firing in olfactory sensory
neurons. J Neurophysiol. 112:1091–1104. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Moreau A, Krahn AD, Gosselin-Badaroudine
P, Klein GJ, Christé G, Vincent Y, Boutjdir M and Chahine M: Sodium
overload due to a persistent current that attenuates the
arrhythmogenic potential of a novel LQT3 mutation. Front Pharmacol.
4:1262013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Janssen LJ: T-type and L-type
Ca2+ currents in canine bronchial smooth muscle:
Characterization and physiological roles. Am J Physiol.
272:C1757–C1765. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Brackenbury WJ, Chioni AM, Diss JK and
Djamgoz MB: The neonatal splice variant of Nav1.5 potentiates in
vitro invasive behaviour of MDA-MB-231 human breast cancer cells.
Breast Cancer Res Treat. 101:149–160. 2007. View Article : Google Scholar
|
|
35
|
Baptista-Hon DT, Robertson FM, Robertson
GB, Owen SJ, Rogers GW, Lydon EL, Lee NH and Hales TG: Potent
inhibition by ropivacaine of metastatic colon cancer SW620 cell
invasion and NaV1.5 channel function. Br J Anaesth. 113(Suppl 1):
i39–i48. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gillet L, Roger S, Besson P, Lecaille F,
Gore J, Bougnoux P, Lalmanach G and Le Guennec JY: Voltage-gated
sodium channel activity promotes cysteine cathepsin-dependent
invasiveness and colony growth of human cancer cells. J Biol Chem.
284:8680–8691. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xing D, Wang J, Ou S, Wang Y, Qiu B, Ding
D, Guo F and Gao Q: Expression of neonatal Nav1.5 in human brain
astrocytoma and its effect on proliferation, invasion and apoptosis
of astrocytoma cells. Oncol Rep. 31:2692–2700. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nelson M, Yang M, Millican-Slater R and
Brackenbury WJ: Nav1.5 regulates breast tumor growth and metastatic
dissemi-nation in vivo. Oncotarget. 6:32914–32929. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ozkucur N, Monsees TK, Perike S, Do HQ and
Funk RH: Local calcium elevation and cell elongation initiate
guided motility in electrically stimulated osteoblast-like cells.
PLoS One. 4:e61312009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Perike S, Özkucur N, Sharma P, Staroske W,
Bläsche R, Barth K and Funk RH: Phospho-NHE3 forms membrane patches
and interacts with beta-actin to sense and maintain constant
direction during cell migration. Exp Cell Res. 324:13–29. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ozkucur N, Perike S, Sharma P and Funk RH:
Persistent directional cell migration requires ion transport
proteins as direction sensors and membrane potential differences in
order to maintain directedness. BMC Cell Biol. 12:42011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Roger S, Gillet L, Le Guennec JY and
Besson P: Voltage-gated sodium channels and cancer: Is excitability
their primary role? Front Pharmacol. 6:1522015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yang M and Brackenbury WJ: Membrane
potential and cancer progression. Front Physiol. 4:1852013.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Redmann K, Müller V, Tanneberger S and
Kalkoff W: The membrane potential of primary ovarian tumor cells in
vitro and its dependence on the cell cycle. Acta Biol Med Ger.
28:853–856. 1972.PubMed/NCBI
|
|
45
|
Brisson L, Driffort V, Benoist L, Poet M,
Counillon L, Antelmi E, Rubino R, Besson P, Labbal F, Chevalier S,
et al: NaV1.5 Na+ channels allosterically regulate the
NHE-1 exchanger and promote the activity of breast cancer cell
invadopodia. J Cell Sci. 126:4835–4842. 2013. View Article : Google Scholar : PubMed/NCBI
|