1. Introduction
Neurite outgrowth inhibitor B (Nogo-B) receptor
(NgBR), a type I transmembrane protein with multiple functions and
actions at the plasma membrane that is mainly expressed in the
lung, breast, smooth muscle and blood vessels and is widely
distributed in the liver, is able to interact with Nogo-B and
vascular endothelial growth factor (VEGF) or function independently
to exert multiple effects (1–4).
This protein is a key member of the reticulon (RTN) protein family.
The reticulon isoforms of mammalian RTN1, RTN2, RTN3 and RTN4c
proteins have been identified, and these form the largest branch of
the reticulon superfamily (4–6).
NgBR contains a putative residue signalling sequence in the N
terminus, an extracellular domain, a single transmembrane region
and a cytoplasmic domain, which are composed of residues 1–46,
47–119, 120–139 and 140–293, respectively (7). As indicated in the database of the
National Centre for Biotechnology Information, the gene encoding
NgBR in humans, Nus1, dehydrodolichyl diphosphate synthase subunit,
may be located at 6q22.1 (8).
Previous studies have provided an increasing body of evidence
suggesting that NgBR has different cellular localizations and
controls specific membrane transport pathways in numerous human
diseases, including fatty liver, intrauterine pulmonary
hypertension, atherosclerosis, protozoan infections, retinitis
pigmentosa, congenital disorders of glycosylation and relevant
cancer types (3,9).
A previous circular dichromatogram analysis
indicated that the full-length NgBR macromolecule has a high
content of helical structures, and its structure comprises ~38%
α-helices, 15% β-sheets and 47% random coils in the ectodomain and
the cytoplasmic domains (5). The
ectodomain of NgBR, a member of the intrinsically unstructured
protein family, is characterized as intrinsically unstructured
without secondary and tertiary structures (5). The NgBR cytoplasmic domain has
partially secondary structures without tight tertiary packing and
serves as a scaffold that utilizes short motifs for the assembly of
two binding partners, which are isoprenyl lipids and/or prenylated
proteins (5,7).
Activation of the Nogo-B/NgBR signalling axis may
modulate angiogenesis (10), lipid
and cholesterol metabolism (11)
and N-linked glycosylation (12)
and enhance axonal branching (13)
and cellular proliferation and apoptosis (10) under different physiological and
pathological conditions by interacting with multiple downstream
targets, including mammalian target of Niemann-Pick type C2
(11),
cis-prenyltransferase (12)
and endothelial nitric oxide synthase (eNOS (10). More importantly, targeting this
signalling axis has been revealed to be promising for the treatment
of cancer. It has been predominantly reported to have an oncogenic
function and elevated expression in certain cancer types, including
invasive ductal breast carcinoma (IDC) (14), hepatocellular carcinoma (HCC)
(15), malignant melanoma (MM)
(16) and non-small cell lung
carcinomas (NSCLC) (17), which
implies that targeting this signalling axis may have potential for
retarding or ameliorating cancer progression.
In summary, NgBR serves a notable role in a number
of human diseases, particularly cancer, which is a highly studied
and complex subject (3). Once the
first report of its effect on breast cancer was published in 2013
(14), numerous studies on the
function of NgBR in the mechanism of cancer have been reported, and
NgBR has gradually become a novel research hotspot globally
(14–17). However, no systematic review of the
latest research results for NgBR in the cancer research field has
been published, to the best of our knowledge. The present review
assesses and discusses novel advances achieved in previous studies
on NgBR, with a particular emphasis on the expression and
pathophysiological effects of NgBR on relevant human cancer types
(17), including IDC (14), MM (16), HCC (15) and NSCLC (17). The present review additionally
assessed the data implicating NgBR in the regulation of lipid
metabolism (11), N-linked
glycosylation (12) and the
epithelial-mesenchymal transition (EMT) (18). These results identify NgBR as a
novel and potential therapeutic target for mitigating the complex
disease states in relevant human cancer types.
2. Expression of NgBR in cancer
Researchers have demonstrated that NgBR is
recognized as a highly important receptor associated with a number
of cancer types, including IDC (14), HCC (15), MM (16) and NSCLC (17). Furthermore, longitudinal studies
have revealed that the dysregulation of NgBR over time is a risk
factor for the early development of these cancer types (14–17).
NgBR is able to promote the development of IDC (14) and HCC (15) but has an inhibitory effect on MM
(16) and NSCLC (17). Therefore, there is some dispute
regarding the expression and mechanism of NgBR in different types
of malignancies.
Upregulated expression of NgBR in
relevant cancer types
Levels of NgBR are increased in IDC. IDC, a severe
public health problem globally, represents a frequent cause of
cancer-associated morbidity and primarily occurs in women aged 20
to 59 years, resulting in a huge socioeconomic burden (19).
The contribution of NgBR-derived factors to
cancer-induced mammary gland disease is an emerging area of
research. A study conducted by Wang et al (14) revealed that the NgBR levels are
higher in IDC compared with in normal breast tissue and are
strongly associated with estrogen receptor (ER)(+) and human
epidermal growth factor receptor 2 (HER2)(−) breast carcinoma.
Furthermore, these observations suggest a strong association
between altered NgBR protein expression and survivin gene
expression in IDC. Oestradiol is able to increase survivin
expression and proliferation (14). Interestingly, the downregulation of
NgBR is able to decrease the effect of oestradiol on survivin
expression and cellular proliferation (14). An inverse correlation has been
revealed between NgBR expression and the expression of Ki-67
antigen, which is a negative prognostic factor for the initiation,
promotion and progression of breast carcinoma (20). Furthermore, the malignancy grade of
IDC has a close association with the expression of NgBR but no
notable association with the levels of HER2, ER or progesterone
receptor, the size of the primary tumour or lymph node metastasis
(20). Different associations
amongst NgBR, ER(+/−) and HER2(+/−) were revealed by Pula et
al (20) and Wang et al
(14), and the contradictory
results in NgBR expression are likely due to differences in the
socioeconomic status, race/ethnicity, geography, age and sex of the
patients whose breast tumour tissues were tested. Furthermore, the
studies used different antibodies produced by Imgenex (Novus
Biologicals, LLC, Littleton, CO, USA) (20) and Epitomics (Abcam, Cambridge, UK)
(14), which may have resulted in
opposing conclusions. Further clinical discoveries through cell
biology experiments are required. The study conducted by Wang et
al (14) suggested that tissue
sections from human patients with early onset IDC indicate a
pattern of NgBR overexpression.
Levels of NgBR are increased in HCC
HCC is the fifth most common carcinoma in humans and
the second most common cause of cancer-associated mortality
globally (21). The expression of
NgBR in HCC tissues is notably higher compared with that in their
corresponding adjacent liver tissues. Western blot analysis was
used to examine the levels of tumour protein p53 and p21 proteins
in Bel7402 and chemoresistant HCC cells (Bel/5FU) (15). The results indicated that the
protein levels of p53 and downstream p21 are downregulated more in
Bel/5FU compared with in Bel7402 cells. In addition, these
researchers also examined the effects of NgBR knockdown on p53
protein expression (15). A
previous study revealed a notable association between p53
ubiquitination and 5-FU chemoresistance in patients with HCC and
chemoresistant HCC cells (Bel/5FU) (15,22).
However, clinical pathological parameters including sex, hepatitis
history, liver cirrhosis, maximal tumour size, tumour number,
vascular invasion and Tumour Node Metastasis stage are not
specifically associated with NgBR expression (15). However, clinical data analyses
indicated that patients with high NgBR levels have notably lower
survival rates compared with those with low NgBR levels (15).
Downregulated expression of NgBR in
relevant cancer types
Levels of NgBR are decreased in MM. MM is a severe
public health problem globally. The spread of MM cells from a MM
primary tumour to form metastases at distant sites is the most
life-threatening complication and the main cause of mortality in
patients with MM (23). Metastasis
is a complex, multistep and multifactorial process involving
interrelated interactions between tumour and normal cells. Single
MM cells translocate to distant secondary sites, thus these cells
must dissociate and migrate away from the primary tumour (24).
Due to the consistent neuroectodermal origin of MM
and the involvement of the RTN protein family, NgBR may serve an
essential role in the development and progression of MM. Current
research on MM metastasis aims to develop a greater understanding
of the interactions between the depth of invasion of primary MM
tumour cells and the NgBR levels. A study conducted by Calik et
al (16) indicated that NgBR
may serve an important role in reducing the migration and
invasiveness of melanoma cells. Conversely, it has been suggested
that NgBR serves an oncosuppressive role in MM. Future
investigations with larger patient cohorts and in vitro studies may
help to characterize the function of NgBR in MM biology (16).
Levels of NgBR are decreased in
NSCLC
The most common respiratory cancer is lung
carcinoma, which has a prevalence of ~12% among patients with newly
diagnosed cancer. Furthermore, an increased incidence rate has been
observed (19). Current therapy
for NSCLC aims to relieve pain and control symptoms rather than
interfere with the pathophysiology (17,25).
A decrease in the levels of NgBR and Nogo-B have been observed in
various cells, including pneumocytes, bronchial epithelial cells of
non-malignant lung tissue (NMLT) and NSCLC cancer cells (17). In patients with NSCLC, low NgBR
mRNA expression levels result in progressive and generalized
increases in the primary tumour size, lymph node involvement and
advanced disease stages (15),
resulting in exaggerated mortality rates due to an unfavourable
prognosis (17). This indicates
that NgBR may potentially serve a role in tumour growth and lymph
node involvement in patients with NSCLC and NMLT; however, this
hypothesis requires further investigation (17). However, a study by Wu et al
(25) revealed that the levels of
NgBR in NSCLC tissues were higher compared with those in
corresponding tumour cell-positive lymph nodes.
The lung cancer tissues used in the study conducted
by Wu et al (25) were
obtained from patients with different socioeconomic statuses,
races/ethnicities, geographies, ages and sexes, which may have
resulted in contradictory results regarding NgBR expression.
Furthermore, the antibodies used in these previous studies were
purchased from Abcam (25) and
Imgenex (Novus Biologicals, LLC) (17), respectively, and the use of these
different antibodies may result in opposing conclusions. Therefore,
these clinical discoveries obtained from cell biology experiments
require further validation.
3. Potential mechanism of NgBR in malignant
tumour types
Dysregulation of the NgBR mechanism is a
well-recognized aspect of cancer biology, and numerous therapeutic
strategies rely on targeting cancer by altering cellular metabolic
pat hways (15). Until now, the
pathophysiology of NgBR in cancer has remained elusive, and
research has mainly focused on its potential mechanisms in
malignant tumour types (3).
Carcinogenic mechanism of NgBR NgBR
facilitates the phosphatidylinositol-3-kinase/protein kinase B
(PI3K/Akt) pathway
The PI3K/Akt pathway has been implicated in the
carcinogenesis of certain tumour types, and the effects are
attributed to the regulation of cell growth, survival, metabolism
and apoptosis in physiological conditions and are involved in the
development and survival of multiple solid tumour types, including
IDC (26).
Wang et al (14) studied the pathogenesis of breast
cancer, and the results suggested that the expression of NgBR is
essential for oestradiol-stimulated IDC cell growth and survivin
expression. Furthermore, the upregulation of NgBR is associated
with ER(+)/HER2(−) breast carcinoma (14). Zhao et al (27) revealed that the inhibition of NgBR
is able to attenuate the activation of the Akt signalling pathway
and subsequently decrease cellular growth, migration, survival and
proliferation. A follow-up study revealed that the overexpression
of NgBR enhanced the phosphorylation of Akt in MDA-MB-231 cells
(28). Pula et al (20) revealed that NgBR is substantially
increased in IDC at the protein and mRNA levels and concluded that
a low expression of NgBR in IDC is able to downregulate the
PI3K/Akt/mechanistic target of rapamycin (mTOR) signalling pathway
and subsequently reduce cancer cell proliferation, migration,
adhesion, survival and invasiveness. These research results clearly
demonstrate that NgBR may mediate the PI3K/Akt/mTOR signalling
pathway and thereby serves a major role in the pathogenesis of
IDC.
Mouse double minute 2 homolog (MDM2) is a bona
fide ubiquitin ligase for p53 that results in altered
transcription in p53 disorders, indicating that it serves a role in
the stability of p53 (29). p53, a
nuclear phosphorylated protein, inhibits the transformation of
human cancer cells (30). One
previous study has suggested that the phosphorylation and nuclear
translocation of MDM2 and the ubiquitination and destruction of p53
are promoted by the phosphorylation of Akt (31). A gradual and orderly change in the
PI3K/Akt/MDM2 pathway caused by NgBR activation is considered an
important mechanism in the pathogenesis of HCC (15). Researchers have observed that
increased levels of NgBR enhance the phosphorylation of Akt, and
that phosphorylated Akt subsequently upregulates the expression of
phosphorylated MDM2, and phosphorylated MDM2 in turn mediates p53
ubiquitination (15). These
changes in the signalling pathway will mediate the emergence of
chemoresistance in HCC and may induce cancer development and
progression (15). These data
clearly demonstrate that the high levels of NgBR in patients with
HCC bind to phosphorylated Akt and recruit MDM2 to the nucleus,
which is an essential step for the activation of PI3K/Akt/MDM2
signalling and tumourigenesis in HCC cells.
NgBR upregulates the EMT
EMT, a well-recognized physiological phenomenon in
embryonic development that promotes the migration of neural crest
cells out of the neuroectoderm (32), is a critical contributor to the
progression of breast cancer as it not only reinforces that
epithelial cells lose their differentiated characteristics but also
enhances their migratory and invasive abilities by the acquisition
of mesenchymal features, as indicated by the overexpression of
vimentin, a typical marker of mesenchymal cells (33). Importantly, the expression of
epithelial and mesenchymal genes may be regulated by tumour growth
factor-β (TGF-β) activation, which has been proven to be an
upstream regulator of EMT (33).
Furthermore, the Akt signalling pathway is able to facilitate all
these signalling-mediated EMTs (34).
The breast cancer pathogenesis study conducted by
Zhao et al (18) revealed
that the TGF-β-induced expression of zinc finger E-box binding
homeobox 1 and twist family bHLH transcription factor may be
attenuated by inhibiting NgBR, which is necessary for the
progression of EMT. This previous study additionally revealed that
the inhibition of NgBR may attenuate the phosphorylation of Akt
mediated by TGF-β. Furthermore, previous studies have suggested
that the inhibition of Akt may block the TGF-β-mediated progression
of EMT (18,34). It has been suggested that NgBR
functions as a switch between the mesenchymal-to-epithelial
transition and the EMT (18,35,36).
In addition, overexpression of the Akt signalling pathway promotes
the EMT, which has been well-described in the carcinogenesis of IDC
(37). The study of lung cancer
pathogenesis conducted by Wu et al (25) revealed that the overexpression of
NgBR requires the activation and plasma membrane localization of
Ras to increase the levels of Snail family transcriptional
repressor 1 through activation of the mitogen-activated protein
kinase kinase (MEK)/extracellular signal-regulated kinase (ERK)
pathway, which may downregulate the levels of E-cadherin to promote
the EMT in NSCLC cells.
NgBR may promote the epidermal growth
factor (EGF)/Ras/ ERK signalling pathway
The EGF/Ras/Raf/ERK signalling pathway is a MEK
pathway that may be widely activated (38,39).
This signalling pathway is involved in the dysregulation of cell
differentiation, proliferation and apoptosis, and tumorigenesis
likely involves multiple mutations in this pathway (40). Dysregulation of this signalling
pathway may be overactivated by the abnormal expression of numerous
cell factors, including NgBR, the mutational activation of EGF-like
ligands, and the mutation and overexpression of Ras (28).
In the plasma membrane, the combination of guanosine
triphosphate (GTP)-bound H-Ras and K-Ras is reinforced by galectin,
which is a cytosolic protein with hydrophobic farnesyl-binding
pockets (41). Caveolin-1 has a
membrane-docking site in its lipid raft domains that binds to
guanosine diphosphate (GDP)-loaded H-Ras (42). The hydrophobic cytoplasmic domain
of NgBR has a docking site for binding to prenylated Ras, and this
binding promotes the translocation of GDP/GTP-bound H-Ras and K-Ras
to the plasmalemma, a step that has a pivotal role in oncogenic
signalling by these GTPases (28).
A recent cancer pathogenesis study revealed that
NgBR preferentially binds to farnesylated H-Ras, and its carboxyl
terminus composed of hydrophobic residues is essential for
integrating with farnesylated Ras to form a steady complex that
mediates H-Ras signalling (28,43).
Through a cell surface biotinylation assay, Zhao et al
(28) revealed that the
upregulation of NgBR enhances the amounts of membrane-associated
K-Ras and H-Ras without changing their total levels. It has been
reported that NgBR only mediates the accumulation of Ras at the
plasma membrane and does not enhance the levels of Ras (43). In addition, the downregulation of
NgBR decreases the EGF-stimulated phosphorylation of ERK and Akt
(43). These results are
sufficient to suggest that the overexpression of NgBR is able to
enhance the EGF-mediated activation of Ras and its downstream
kinases, including Akt and ERK (28). That NgBR is essential for the
accumulation of Ras in the tumour cell membrane indicates that NgBR
serves a pivotal role in the oncogenic function of Ras in tumour
growth. Furthermore, NgBR may influence the EGF-mediated activation
of K-Ras and H-Ras, as K-Ras and H-Ras must be translocated to the
cell membrane in order to be activated (44) and the upregulation of K-Ras and
H-Ras is thought to mediate downstream kinases throughout the cell
(45).
NgBR facilitates angiogenesis
Angiogenesis, an important step in cancer
progression, involves the development of novel blood vessels
necessary for tumour growth (46).
Furthermore, a recent study of cancer cell invasion indicated that
angiogenesis serves a key role in the proliferation and motility of
cancer cells (47). In the early
phase of the carcinogenic process, proliferating cancer cells
mediate a proangiogenic microenvironment that increases the amount
of supplied nutrients, energy and oxygen (48). VEGF, the primary angiogenic factor
produced and secreted by cancer cells, is strongly involved in this
progression (49). The VEGF/VEGF
receptor (VEGFR) proangiogenic pathway, which is upregulated in
human carcinoma, is the most extensively studied pathway (49). eNOS mediated by VEGFR has a notable
function in carcinogenesis, including the inhibition of DNA repair
systems, the epigenetic regulation of different oncogenic pathways
and the inhibition of apoptosis (50). Furthermore, a recent study by
Ricciuti et al (51)
suggested that angiogenesis promotes the procession of cancer and
is responsible for the invasion and metastasis induced by VEGF,
eNOS, Akt and ERK.
NgBR is able to attenuate defects in angiogenesis by
promoting eNOS and Akt phosphorylation. Furthermore, NgBR may
facilitate tube formation to ameliorate angiogenesis disorders
(52). NgBR is necessary for the
development of cerebral blood vessels. Nogo-B and VEGF may interact
with NgBR, resulting in the phosphorylation of Akt to reinforce
angiogenesis (2). Interestingly,
miR-26a may directly interact with NgBR to inhibit the VEGF-NgBR
pathway and attenuate the phosphorylation of eNOS, which is able to
substantially decrease tube formation, angiogenesis and cellular
migration and proliferation (53).
These results implicate NgBR as necessary for angiogenesis and the
maintenance of migration.
The targeting of endothelial cell metabolism is an
appealing strategy to impact tumour-driven angiogenesis,
particuarly given that proangiogenesis proteins, including NgBR,
VEGF and eNOS, are thought to mediate endothelial cell migration,
proliferation and tube formation; therefore, this NgBR/eNOS/
angiogenesis axis may be a potential therapeutic target in the
switch from neoangiogenesis to carcinogenesis (53).
Anticarcinogenic mechanism of NgBR NgBR
attenuates the Liver X receptor α (LXRα) signalling pathway
Cholesterol, a precursor of bile acids and steroid
hormones, is essential for various biological functions, including
increased tumour angiogenesis, reduced tumour apoptosis and
increased tumour cell proliferation. Lipids, the main cell membrane
component, serve a pivotal role in cell growth and division during
their maintenance of cell integrity (54).
It is thought that lipids are implicated in the
development of a number of carcinomas, including breast cancer,
lung cancer and HCC. A low level of high density
lipoprotein-cholesterol (HDL-C) was revealed to elevate the risk of
breast cancer in 38,823 women in Norway, all between the ages of 17
to 54 years (55). A study on the
pathogenesis of breast cancer conducted by Alikhani et al
(56) suggested that primary
mammary tumour growth and metastasis are substantially accelerated
by dyslipidaemia and that cholesterol may upregulate the metastasis
and growth of breast cancer. These results indicate that
dyslipidaemia serves a key role in the metastasis, recurrence and
growth of breast cancer and that hyperlipidaemia is able to
increase the risk of breast cancer. In a study of the pathogenesis
of liver cancer, Ooi et al (57) revealed that the levels of HDL-C are
lower in metastatic liver cancer compared with in primary HCC. A
study conducted by Rice et al (58) indicates the involvement of
apolipoprotein E in the induction of lung cancer, which may be due
to enhanced cholesterol transport into tumour cells. Altogether,
these data suggest that decreasing the levels of lipids and
cholesterol may suppress cancer development.
LXRα are primarily expressed in the liver and to a
lesser extent in the kidney, small intestine, spleen and adrenal
gland (59). A study conducted by
Hu et al (11) suggests
that NgBR integrates the liver X receptor response element with
LXRα, and this interaction serves an important role in lipid
synthesis. Furthermore, NgBR prevents the translocation of LXR from
the cytoplasm to the nucleus and attenuates the induction of
sterol-regulatory element-binding protein (SREBP-1c), the lipid
regulatory gene of ATP-binding cassette subfamily A member 1 and
fatty acid synthase (FASN), which reduces the lipid levels in
vitro. These results suggest that NgBR is able to mediate LXRα
activation to reduce the levels of lipids.
In summary, these results imply that the NgBR-LXRα
axis may reduce the levels of free fatty acid (FFA) and
triglyceride (TG) in the liver and plasma to suppress the
occurrence and development of cancer types including HCC, breast
cancer and lung cancer.
NgBR facilitates the adenosine
monophosphate-activated protein kinase α (AMPKα) signalling
pathway
AMPK signalling participates in numerous
pathophysiologic processes, particularly lipid metabolism and
tumourigenesis (60,61) During activation, AMPKα enhances the
levels of ATP by regulating the metabolism of fats, proteins and
sugars, thus inducing cells to evade death and engage in metastasis
and the development of drug resistance (61,62).
Furthermore, a previous study suggested that AMPKα is closely
associated with the tumour suppressor gene p53 (63). This protein mediates the expression
of cellular survival-associated signals, including Akt and mTOR. It
has been demonstrated that AMPKα serves an important role in cancer
prevention by inducing cell cycle arrest and blocking tumour growth
(64). Jones et al
(65) previously demonstrated that
AMPKα may directly promote the phosphorylation of p53 on serine 15.
Furthermore, the activation of AMPKα may enhance the levels of p53
in addition to its phosphorylation and ultimately induce cell
arrest at the G1/S phase (66).
Compound C, a cell-permeable pyrazolopyrimidine compound, may
function as a reversible and ATP-competitive inhibitor of AMPK
(67). In addition, Hadad et
al (68) demonstrated that the
activation of AMPKα is notably downregulated in biopsies of breast
cancer compared with normal tissue, which suggests that AMPKα has a
cancer suppressive role. These results suggest that AMPKα has a
carcinostatic action in tumourigenesis.
A previous study by Hu et al (10) revealed that NgBR may not only
enhance the phosphorylation of AMPKα but also upregulate the
expression of phosphorylated acetyl-coenzyme A carboxylase-1
(ACC-1), which is downstream of phosphorylated AMPKα. Ultimately,
the activation of ACC-1 and AMPKα represses the expression of FASN,
SREBP-1c and stearoyl coenzyme A desaturase-1 and enhances the
levels of FFA and TG. Furthermore, the activation of AMPKα may
rescue the translocation of LXRα mediated by a NgBR deficiency to
ensure the maintenance of lipid metabolism (11,69).
These results suggest that NgBR is able to mediate AMPKα in order
to impact lipid homeostasis and LXRα. As mentioned previously,
disorders of lipid homeostasis may serve a role in tumourigenesis.
The AMPKα axis may reduce the levels of lipids and suppress the
translocation of LXRα.
NgBR is able to promote N-glycosylation
to attenuate endoplasmic reticulum stress and the unfolded protein
response (UPR)
NgBR is an extremely conserved subunit of
cis-prenyltransferase, which is essential for the
biosynthesis and phosphorylation of dolichol and ultimately
contributes to the maintenance of protein N-glycosylation (9,70).
Nevertheless, a NgBR deficiency may induce an N-glycosylation
disorder to mediate endoplasmic reticulum stress and thus
activation of the UPR (12). These
results suggest that NgBR is able to modulate this
N-glycosylation/endoplasmic reticulum stress/ UPR signalling
pathway.
One previous study suggested that a glycosylation
disorder is a common occurrence in human cancer, and that these
changes are characteristics of cancer types including breast cancer
and NSCLC (71). Furthermore,
glycosylation serves a key role in the EMT, cell-cell contact,
cancer progression and cancer cell metastasis (72,73).
These results indicate that the inhibitory mechanisms of NgBR on
cancer cells may be mediated by the maintenance of
N-glycosylation.
Recent studies have further investigated whether the
UPR may be involved in intervening in the carcinogenic process and
may be a novel therapeutic target for controlling neoplasia
development or arresting the progression of the malignant phenotype
(77–80). One previous lung cancer study noted
that the upregulation of the UPR is able to induce the expression
of glucose-regulated protein 78 kDa (GRP78) to suppress apoptosis
and resistance to anticancer drugs in lung cancer (74). A previous study indicated that
C/EBP homologous protein and GRP78 may mediate the development and
progression of lung adenocarcinoma (75). Furthermore, the activation of GRP78
is upregulated in lung cancer cells, which are able to induce
endoplasmic reticulum stress tolerance against the pharmacological
action of anticancer drugs (76).
Breast cancer cells may also reduce cell apoptosis and the effects
of antioestrogen therapy by a mechanism similar to the UPR.
Activation of the UPR is induced by tumour hypoxia and endoplasmic
reticulum stress during breast cancer. Subsequently, the
upregulated mRNA of X-box binding protein may bind to oestrogen
receptors and induce agonistic activities, and these agonistic
activities are able to promote the transcription of the mRNA
encoding the B-cell lymphoma 2 protein, which serves a crucial role
in anti-apoptosis and the effects of antioestrogen therapy
(77). A previous study on MM
suggested that the most important oncogenic factor is a B-Raf
proto-oncogene, serine/threonine kinase (BRAF) gene mutation. This
mutation upregulates the Ras-Raf-MEK signalling pathway, which may
produce carcinogenic effects on the human body (78). A recent study indicated that BRAF
mutations also induce the consistent and modest activation of the
UPR with GRP78 upregulation, which is considered a tumour marker of
MM metastasis and recurrence. This mechanism may be considered an
essential factor in MM metastasis (79).
4. Conclusions and future perspectives
The present review summarizes the expression,
characteristics and biological functions of NgBR in numerous cancer
types, which are complicated and novel and had remained unclear
until now. This information is presented in the schematic
representation presented in Fig.
1. Numerous pathophysiologic studies have confirmed that NgBR
serves an important role in carcinogenesis and that its activation
promotes cell proliferation and migration and inhibits apoptosis
via multiple physiopathological mechanisms, including the AMPKα
(11,69), LXRα (11,69),
Akt (20,43) and ERK (28,43)
signalling pathways.
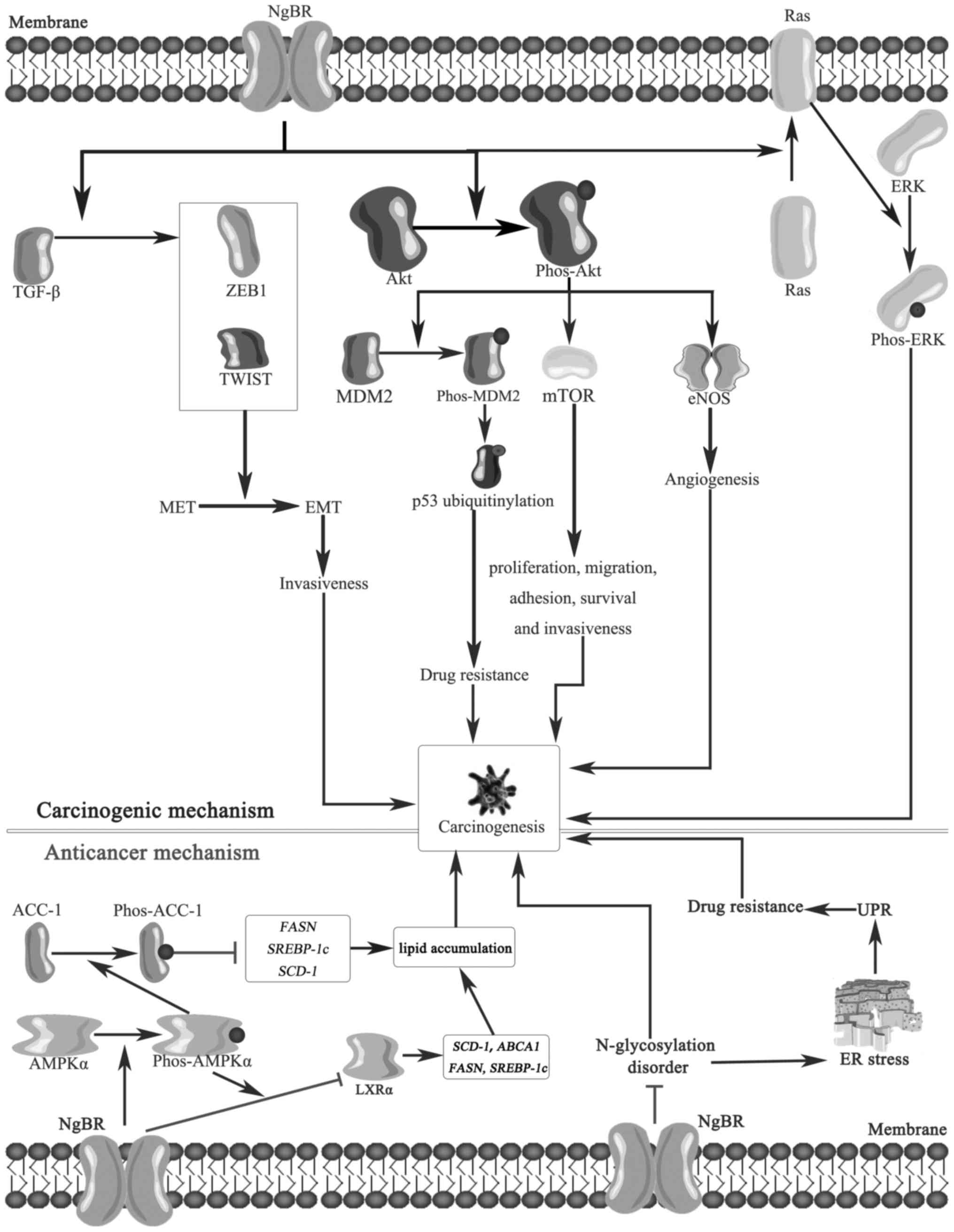 | Figure 1Potential mechanisms underlying the
anticancer and carcinogenetic effects of NgBR. NgBR may promote the
switch from the MET to the EMT via TGF-β, which upregulates the
invasiveness of cancer cells. Furthermore, NgBR enhances the
phosphorylation of Akt, and phos-Akt subsequently activates mTOR,
which promotes cancer cell proliferation, migration, adhesion,
survival and invasiveness. Furthermore, activated Akt enhances the
phosphorylation of MDM2 to promote p53 ubiquitylation, which
induces drug resistance, and increases eNOS to induce angiogenesis.
The phosphorylation of ERK is stimulated by NgBR through the
recruitment of Ras to the plasma membrane. Altogether, these
potential mechanisms all induce carcinogenesis. However, NgBR may
also mediate the phosphorylation of AMPKα and suppress the
activation of LXRα to reduce the level of lipids, which have
anticancer effects. Furthermore, NgBR may maintain normal
N-glycosylation and inhibit ER stress and the UPR to induce a
significant anticancer effect. NgBR, neurite outgrowth inhibitor B
receptor receptor; MET, mesenchymal-to-epithelial transition; EMT,
epithelial-to-mesenchymal transition; TGF-β, tumor growth factor β;
ZEB1, zinc finger E-box binding homeobox 1; TWIST, twist family
bHLH transcription factor 1; Akt, protein kinase B; phos,
phosphorylated; MDM2, mouse double minute 2 homolog; mTOR,
mechanistic target of rapamycin; eNOS, endothelial nitric oxide
synthase; ERK, extracellular signal-regulated kinase; ACC-1,
acetyl-coenzyme A carboxylase-1; AMPKα, adenosine
monophosphate-activated protein kinase α; LXRα, Liver X receptor α;
SCD-1, stearoyl coenzyme A desaturase-1; ABCA1, ATP-binding
cassette subfamily A member 1; FASN, fatty acid synthase; SREBP-1c,
sterol-regulatory element-binding protein; ER, estrogen receptor;
UPR, unfolded protein response. |
NgBR offers novel clinical opportunities, but it is
necessary for researchers to obtain an improved understanding of
the mechanisms underlying the protective biological characteristics
achieved following the elimination of drug resistance, which will
improve the design of systemic therapies for numerous cancer types.
To better understand the chemoresistance mediated by NgBR, the
characteristics of NgBR must be confirmed, including its size,
shape, pathophysiological effect and structure-function
associations (15,43).
Novel studies demonstrated that NgBR activation
serves a key role in reprogramming the metabolism of drug-resistant
cancer cells, including promoting p53 ubiquitylation (15) and the UPR (12). In addition, NgBR has been
demonstrated to be effective in facilitating the EMT, which has
been confirmed to be a root factor affecting cancer
chemosensitivity (18). NgBR
inhibition may be a more powerful and promising tool for reversing
chemoresistance compared with NgBR activation.
Furthermore, the development of highly selective
NgBR inhibitors, including miR-26a, will not only promote further
study on the molecular function of NgBR but also provide a
potential approach for cancer therapy (53). Interestingly, doxycycline is able
to induce the expression of inducible short hairpin RNA
interference targeting NgBR, which is currently being considered
for use in cancer prevention and treatment (29). Doxycycline has been demonstrated to
result in cell transformation and tumour growth arrest in breast
cancer (80).
In contrast, certain molecular mechanisms underlying
the anticancer properties of NgBR may be classified as part of the
LXRα, AMPKα and N-glycosylation/endoplasmic reticulum stress/UPR
pathways, which may aid maintenance of a normal cellular phenotype.
The study of different tumour types and their treatments indicate
that NgBR may be a tumour suppressor protein. Altogether, these
results suggest that NgBR may serve a dual role in carcinogenesis.
In the early stages, NgBR functions as an anticancer molecule, and
its dysregulation may result in the initiation and development of
cancer, whereas in the later stages, the activation of NgBR may
help cancer cells escape from death and develop drug resistance
(11,12).
Therefore, ongoing and future studies are expected
to elaborate on the fundamental structure-function associations of
NgBR that may be useful for the development of novel drugs that
directly target NgBR by providing available projects for the
laboratory validation of its molecular function. Therefore, the
genesis of contraindications postulates that NgBR may induce the
rejuvenation of suppressed vascularity and heighten an opposite
effect conducive to tumour growth. Furthermore, further clinical
research of NgBR is also required to increase its stability in
plasma, the transfer of one of its components to dehydrodolichyl
diphosphate synthase subunit, the dynamics of its metabolism, its
disease specificity, its cell type-specific targeting in cancer
development and responses to conventional therapy.
Funding
The present study was supported by the Hunan
Provincial Natural Sciences Foundation of China (grant nos.
2017JJ2233 and 2018JJ2344), the Key Lab for Clinical Anatomy and
Reproductive Medicine of Hengyang City (grant no. 2017KJ182) and
the Science and Technology Plan Key Projects of Hunan Province
(grant no. 2017SK2183).
Availability of data and materials
Not applicable.
Author contributions
YKL conceptualized the study, performed the
analysis, collected data and participated in writing the paper. YJX
collected data, acquired funding and participated in writing the
paper. DCW participated in the collection of data and in the
writing the paper. SLL performed the analysis and editing and also
participated in the data collection. ST and ZCM reviewed and edited
the paper and jointly designed the structure of the paper. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Miao RQ, Gao Y, Harrison KD, Prendergast
J, Acevedo LM, Yu J, Hu F, Strittmatter SM and Sessa WC:
Identification of a receptor necessary for Nogo-B stimulated
chemotaxis and morphogenesis of endothelial cells. Proc Natl Acad
Sci USA. 103:10997–11002. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rana U, Liu Z, Kumar SN, Zhao B, Hu W,
Bordas M, Cossette S, Szabo S, Foeckler J, Weiler H, et al: Nogo-B
receptor deficiency causes cerebral vasculature defects during
embryonic development in mice. Dev Biol. 410:190–201. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Long SL, Li YK, Xie YJ, Long ZF, Shi JF
and Mo ZC: Neurite outgrowth inhibitor B receptor: A versatile
receptor with multiple functions and actions. DNA Cell Biol.
36:1142–1150. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dodd DA, Niederoest B, Bloechlinger S,
Dupuis L, Loeffler JP and Schwab ME: Nogo-A, -B, and -C are found
on the cell surface and interact together in many different cell
types. J Biol Chem. 280:12494–12502. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li M and Song J: Nogo-B receptor possesses
an intrinsically unstructured ectodomain and a partially folded
cytoplasmic domain. Biochem Biophys Res Commun. 360:128–134. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schwab ME: Nogo and axon regeneration.
Curr Opin Neurobiol. 14:118–124. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Holcomb J, Doughan M, Spellmon N, Lewis B,
Perry E, Zhang Y, Nico L, Wan J, Chakravarthy S, Shang W, et al:
SAXS analysis of a soluble cytosolic NgBR construct including
extracellular and transmembrane domains. PLoS One. 13:e01913712018.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Szafranski P, Von Allmen GK, Graham BH,
Wilfong AA, Kang SH, Ferreira JA, Upton SJ, Moeschler JB, Bi W,
Rosenfeld JA, et al: 6q22.1 microdeletion and susceptibility to
pediatric epilepsy. Eur J Hum Genet. 23:173–179. 2015. View Article : Google Scholar :
|
|
9
|
Grabińska KA, Park EJ and Sessa WC:
cis-prenyltransferase: new insights into protein glycosylation,
rubber synthesis, and human diseases. J Biol Chem. 291:18582–18590.
2016. View Article : Google Scholar
|
|
10
|
Park EJ, Grabińska KA, Guan Z and Sessa
WC: NgBR is essential for endothelial cell glycosylation and
vascular development. EMBO Rep. 17:167–177. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hu W, Zhang W, Chen Y, Rana U, Teng RJ,
Duan Y, Liu Z, Zhao B, Foeckler J, Weiler H, et al: Nogo-B receptor
deficiency increases liver X receptor alpha nuclear translocation
and hepatic lipogenesis through an adenosine
monophosphate-activated protein kinase alpha-dependent pathway.
Hepatology. 64:1559–1576. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Park EJ, Grabińska KA, Guan Z, Stránecký
V, Hartmannová H, Hodaňová K, Barešová V, Sovová J, Jozsef L,
Ondrušková N, et al: Mutation of Nogo-B receptor, a subunit of
cis-prenyltransferase, causes a congenital disorder of
glycosylation. Cell Metab. 20:448–457. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Eckharter C, Junker N, Winter L, Fischer
I, Fogli B, Kistner S, Pfaller K, Zheng B, Wiche G, Klimaschewski
L, et al: Schwann cell expressed Nogo-B modulates axonal branching
of adult sensory neurons through the Nogo-B receptor NgBR. Front
Cell Neurosci. 9:4542015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang B, Zhao B, North P, Kong A, Huang J
and Miao QR: Expression of NgBR is highly associated with estrogen
receptor alpha and survivin in breast cancer. PLoS One.
8:e780832013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dong C, Zhao B, Long F, Liu Y, Liu Z, Li
S, Yang X, Sun D, Wang H, Liu Q, et al: Nogo-B receptor promotes
the chemoresistance of human hepatocellular carcinoma via the
ubiquitination of p53 protein. Oncotarget. 7:8850–8865. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Calik J, Pula B, Piotrowska A, Wojnar A,
Witkiewicz W, Grzegrzolka J, Podhorska-Okolow M and Dziegiel P:
Prognostic significance of NOGO-A/B and NOGO-B receptor expression
in malignant melanoma - A preliminary study. Anticancer Res.
36:3401–3407. 2016.PubMed/NCBI
|
|
17
|
Pula B, Werynska B, Olbromski M,
Muszczynska-Bernhard B, Chabowski M, Janczak D, Zabel M,
Podhorska-Okolow M and Dziegiel P: Expression of Nogo isoforms and
Nogo-B receptor (NgBR) in non-small cell lung carcinomas.
Anticancer Res. 34:4059–4068. 2014.PubMed/NCBI
|
|
18
|
Zhao B, Xu B, Hu W, Song C, Wang F, Liu Z,
Ye M, Zou H and Miao QR: Comprehensive proteome quantification
reveals NgBR as a new regulator for epithelial-mesenchymal
transition of breast tumor cells. J Proteomics. 112:38–52. 2015.
View Article : Google Scholar :
|
|
19
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pula B, Olbromski M, Owczarek T, Ambicka
A, Witkiewicz W, Ugorski M, Rys J, Zabel M, Dziegiel P and
Podhorska-Okolow M: Nogo-B receptor expression correlates
negatively with malignancy grade and ki-67 antigen expression in
invasive ductal breast carcinoma. Anticancer Res. 34:4819–4828.
2014.PubMed/NCBI
|
|
21
|
Maluccio M and Covey A: Recent progress in
understanding, diagnosing, and treating hepatocellular carcinoma.
CA Cancer J Clin. 62:394–399. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kuo TC, Chang PY, Huang SF, Chou CK and
Chao CC: Knockdown of HURP inhibits the proliferation of
hepacellular carcinoma cells via downregulation of gankyrin and
accumulation of p53. Biochem Pharmacol. 83:758–768. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Elder DE: Melanoma screening and
mortality. J Natl Cancer Inst. Mar 29–2018.Epub ahead of print.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee JH, Miele ME, Hicks DJ, Phillips KK,
Trent JM, Weissman BE and Welch DR: KiSS-1, a novel human malignant
melanoma metastasis-suppressor gene. J Natl Cancer Inst.
88:1731–1737. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu D, Zhao B, Qi X, Peng F, Fu H, Chi X,
Miao QR and Shao S: Nogo-B receptor promotes epithelial-mesenchymal
transition in non-small cell lung cancer cells through the
Ras/ERK/Snail1 pathway. Cancer Lett. 418:135–146. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cui J, He W, Yi B, Zhao H, Lu K, Ruan H
and Ma D: mTOR pathway is involved in ADP-evoked astrocyte
activation and ATP release in the spinal dorsal horn in a rat
neuropathic pain model. Neuroscience. 275:395–403. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao B, Chun C, Liu Z, Horswill MA,
Pramanik K, Wilkinson GA, Ramchandran R and Miao RQ: Nogo-B
receptor is essential for angiogenesis in zebrafish via Akt
pathway. Blood. 116:5423–5433. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhao B, Hu W, Kumar S, Gonyo P, Rana U,
Liu Z, Wang B, Duong WQ, Yang Z, Williams CL, et al: The Nogo-B
receptor promotes Ras plasma membrane localization and activation.
Oncogene. 36:3406–3416. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Inoue K and Fry EA: Aberrant splicing of
the DMP1-ARF-MDM2-p53 pathway in cancer. Int J Cancer. 139:33–41.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ryu HW, Shin DH, Lee DH, Won HR and Kwon
SH: A potent hydroxamic acid-based, small-molecule inhibitor A452
preferentially inhibits HDAC6 activity and induces cytotoxicity
toward cancer cells irrespective of p53 status. Carcinogenesis.
39:72–83. 2018. View Article : Google Scholar
|
|
31
|
Abraham AG and O’Neill E:
PI3K/Akt-mediated regulation of p53 in cancer. Biochem Soc Trans.
42:798–803. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jo M, Lester RD, Montel V, Eastman B,
Takimoto S and Gonias SL: Reversibility of epithelial-mesenchymal
transition (EMT) induced in breast cancer cells by activation of
urokinase receptor-dependent cell signaling. J Biol Chem.
284:22825–22833. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Brabletz T: EMT and MET in metastasis:
Where are the cancer stem cells? Cancer Cell. 22:699–701. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Larue L and Bellacosa A:
Epithelial-mesenchymal transition in development and cancer: Role
of phosphatidylinositol 3′ kinase/ AKT pathways. Oncogene.
24:7443–7454. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bakin AV, Tomlinson AK, Bhowmick NA, Moses
HL and Arteaga CL: Phosphatidylinositol 3-kinase function is
required for transforming growth factor beta-mediated epithelial to
mesenchymal transition and cell migration. J Biol Chem.
275:36803–36810. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wendt MK, Smith JA and Schiemann WP:
Transforming growth factor-β-induced epithelial-mesenchymal
transition facilitates epidermal growth factor-dependent breast
cancer progression. Oncogene. 29:6485–6498. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yang L, Han S and Sun Y: An IL6-STAT3 loop
mediates resistance to PI3K inhibitors by inducing
epithelial-mesenchymal transition and cancer stem cell expansion in
human breast cancer cells. Biochem Biophys Res Commun. 453:582–587.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Song Q, Jiang S, Zhang X, Pan C, Lu C,
Peng J and Li Q: Radiosensitivity of human ovarian cancer cells is
enhanced by pseudolaric acid B due to the inhibition of the
Ras/Raf/ERK signaling pathway. Exp Ther Med. 15:685–690.
2018.PubMed/NCBI
|
|
39
|
Sriskanthadevan-Pirahas S, Lee J and
Grewal SS: The EGF/ Ras pathway controls growth in Drosophila via
ribosomal RNA synthesis. Dev Biol. 439:19–29. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Takahashi-Niki K, Kato-Ose I, Murata H,
Maita H, Iguchi-Ariga SM and Ariga H: Epidermal growth
factor-dependent activation of the extracellular signal-regulated
kinase pathway by DJ-1 protein through its direct binding to c-Raf
protein. J Biol Chem. 290:17838–17847. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rotblat B, Belanis L, Liang H, Haklai R,
Elad-Zefadia G, Hancock JF, Kloog Y and Plowman SJ: H-Ras
nanocluster stability regulates the magnitude of MAPK signal
output. PLoS One. 5:e119912010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Roy S, Luetterforst R, Harding A, Apolloni
A, Etheridge M, Stang E, Rolls B, Hancock JF and Parton RG:
Dominant-negative caveolin inhibits H-Ras function by disrupting
cholesterol-rich plasma membrane domains. Nat Cell Biol. 1:98–105.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jin Y, Hu W, Liu T, Rana U,
Aguilera-Barrantes I, Kong A, Kumar SN, Wang B, Gao P, Wang X, et
al: Nogo-B receptor increases the resistance of estrogen receptor
positive breast cancer to paclitaxel. Cancer Lett. 419:233–244.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Buday L and Downward J: Many faces of Ras
activation. Biochim Biophys Acta. 1786:178–187. 2008.PubMed/NCBI
|
|
45
|
Prior IA and Hancock JF: Ras trafficking,
localization and compartmentalized signalling. Semin Cell Dev Biol.
23:145–153. 2012. View Article : Google Scholar :
|
|
46
|
Karadedou CT, Gomes AR, Chen J, Petkovic
M, Ho KK, Zwolinska AK, Feltes A, Wong SY, Chan KY, Cheung YN, et
al: FOXO3a represses VEGF expression through FOXM1-dependent and
-independent mechanisms in breast cancer. Oncogene. 31:1845–1858.
2012. View Article : Google Scholar
|
|
47
|
Sherbet GV: Suppression of angiogenesis
and tumour progression by combretastatin and derivatives. Cancer
Lett. 403:289–295. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Mantovani G, Macciò A, Madeddu C,
Gramignano G, Lusso MR, Serpe R, Massa E, Astara G and Deiana L: A
phase II study with antioxidants, both in the diet and
supplemented, pharmaconutritional support, progestagen, and
anti-cyclooxygenase-2 showing efficacy and safety in patients with
cancer-related anorexia/ cachexia and oxidative stress. Cancer
Epidemiol Biomarkers Prev. 15:1030–1034. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Saharinen P, Eklund L, Pulkki K, Bono P
and Alitalo K: VEGF and angiopoietin signaling in tumor
angiogenesis and metastasis. Trends Mol Med. 17:347–362. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Vasudevan D and Thomas DD: Insights into
the diverse effects of nitric oxide on tumor biology. Vitam Horm.
96:265–298. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ricciuti B, Foglietta J, Bianconi V,
Sahebkar A and Pirro M: Enzymes involved in tumor-driven
angiogenesis: A valuable target for anticancer therapy. Semin
Cancer Biol. Nov 8–2017, (Epub ahead of print).
S1044-579X(17)30043-3. pp. 2017 View Article : Google Scholar
|
|
52
|
Teng RJ, Rana U, Afolayan AJ, Zhao B, Miao
QR and Konduri GG: Nogo-B receptor modulates angiogenesis response
of pulmonary artery endothelial cells through eNOS coupling. Am J
Respir Cell Mol Biol. 51:169–177. 2014.PubMed/NCBI
|
|
53
|
Jo HN, Kang H, Lee A, Choi J, Chang W, Lee
MS and Kim J: Endothelial miR-26a regulates VEGF-Nogo-B
receptor-mediated angiogenesis. BMB Rep. 50:384–389. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Cruz P, Torres C, Ramírez ME, Epuñán MJ,
Valladares LE and Sierralta WD: Proliferation of human mammary
cancer cells exposed to 27-hydroxycholesterol. Exp Ther Med.
1:531–536. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Furberg AS, Veierød MB, Wilsgaard T,
Bernstein L and Thune I: Serum high-density lipoprotein
cholesterol, metabolic profile, and breast cancer risk. J Natl
Cancer Inst. 96:1152–1160. 2004. View Article : Google Scholar
|
|
56
|
Alikhani N, Ferguson RD, Novosyadlyy R,
Gallagher EJ, Scheinman EJ, Yakar S and LeRoith D: Mammary tumor
growth and pulmonary metastasis are enhanced in a hyperlipidemic
mouse model. Oncogene. 32:961–967. 2013. View Article : Google Scholar
|
|
57
|
Ooi K, Shiraki K, Sakurai Y, Morishita Y
and Nobori T: Clinical significance of abnormal lipoprotein
patterns in liver diseases. Int J Mol Med. 15:655–660.
2005.PubMed/NCBI
|
|
58
|
Rice SJ, Liu X, Miller B, Joshi M, Zhu J,
Caruso C, Gilbert C, Toth J, Reed M, Rassaei N, et al: Proteomic
profiling of human plasma identifies apolipoprotein E as being
associated with smoking and a marker for squamous metaplasia of the
lung. Proteomics. 15:3267–3277. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Repa JJ and Mangelsdorf DJ: The role of
orphan nuclear receptors in the regulation of cholesterol
homeostasis. Annu Rev Cell Dev Biol. 16:459–481. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Shackelford DB: Unravelling the connection
between metabolism and tumorigenesis through studies of the liver
kinase B1 tumour suppressor. J Carcinog. 12:162013. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Hardie DG, Schaffer BE and Brunet A: AMPK:
An Energy-Sensing Pathway with Multiple Inputs and Outputs. Trends
Cell Biol. 26:190–201. 2016. View Article : Google Scholar
|
|
62
|
Hardie DG: AMPK: A target for drugs and
natural products with effects on both diabetes and cancer.
Diabetes. 62:2164–2172. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
He G, Zhang YW, Lee JH, Zeng SX, Wang YV,
Luo Z, Dong XC, Viollet B, Wahl GM and Lu H: AMP-activated protein
kinase induces p53 by phosphorylating MDMX and inhibiting its
activity. Mol Cell Biol. 34:148–157. 2014. View Article : Google Scholar :
|
|
64
|
Li W, Saud SM, Young MR, Chen G and Hua B:
Targeting AMPK for cancer prevention and treatment. Oncotarget.
6:7365–7378. 2015.PubMed/NCBI
|
|
65
|
Jones RG, Plas DR, Kubek S, Buzzai M, Mu
J, Xu Y, Birnbaum MJ and Thompson CB: AMP-activated protein kinase
induces a p53-dependent metabolic checkpoint. Mol Cell. 18:283–293.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Vander Heiden MG, Cantley LC and Thompson
CB: Understanding the Warburg effect: The metabolic requirements of
cell proliferation. Science. 324:1029–1033. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Handa N, Takagi T, Saijo S, Kishishita S,
Takaya D, Toyama M, Terada T, Shirouzu M, Suzuki A, Lee S, et al:
Structural basis for compound C inhibition of the human
AMP-activated protein kinase α2 subunit kinase domain. Acta
Crystallogr D Biol Crystallogr. 67:480–487. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Hadad SM, Baker L, Quinlan PR, Robertson
KE, Bray SE, Thomson G, Kellock D, Jordan LB, Purdie CA, Hardie DG,
et al: Histological evaluation of AMPK signalling in primary breast
cancer. BMC Cancer. 9:3072009. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Zhang W, Yang X, Chen Y, Hu W, Liu L,
Zhang X, Liu M, Sun L, Liu Y, Yu M, et al: Activation of hepatic
Nogo-B receptor expression-A new anti-liver steatosis mechanism of
statins. Biochim Biophys Acta. 1863.177–190. 2018.
|
|
70
|
Grabińska KA, Edani BH, Park EJ, Kraehling
JR and Sessa WC: A conserved carboxy-terminal RxG motif in the NgBR
subunit of cis-prenyltransferase is critical for prenyltransferase
activity. J Biol Chem. 292:17351–17361. 2017. View Article : Google Scholar
|
|
71
|
Boersema PJ, Geiger T, Wisniewski JR and
Mann M: Quantification of the N-glycosylated secretome by
super-SILAC during breast cancer progression and in human blood
samples. Mol Cell Proteomics. 12:158–171. 2013. View Article : Google Scholar :
|
|
72
|
Li N, Xu H, Fan K, Liu X, Qi J, Zhao C,
Yin P, Wang L, Li Z and Zha X: Altered β1,6-GlcNAc branched
N-glycans impair TGF-β-mediated epithelial-to-mesenchymal
transition through Smad signalling pathway in human lung cancer. J
Cell Mol Med. 18:1975–1991. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Chen CY, Jan YH, Juan YH, Yang CJ, Huang
MS, Yu CJ, Yang PC, Hsiao M, Hsu TL and Wong CH: Fucosyltransferase
8 as a functional regulator of nonsmall cell lung cancer. Proc Natl
Acad Sci USA. 110:630–635. 2013. View Article : Google Scholar
|
|
74
|
Uramoto H, Sugio K, Oyama T, Nakata S, Ono
K, Yoshimastu T, Morita M and Yasumoto K: Expression of endoplasmic
reticulum molecular chaperone Grp78 in human lung cancer and its
clinical significance. Lung Cancer. 49:55–62. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Kim KM, Yu TK, Chu HH, Park HS, Jang KY,
Moon WS, Kang MJ, Lee DG, Kim MH, Lee JH, et al: Expression of ER
stress and autophagy-related molecules in human non-small cell lung
cancer and premalignant lesions. Int J Cancer. 131:E362–E370. 2012.
View Article : Google Scholar
|
|
76
|
Lin Y, Wang Z, Liu L and Chen L: Akt is
the downstream target of GRP78 in mediating cisplatin resistance in
ER stress-tolerant human lung cancer cells. Lung Cancer.
71:291–297. 2011. View Article : Google Scholar
|
|
77
|
Clarke R, Cook KL, Hu R, Facey CO,
Tavassoly I, Schwartz JL, Baumann WT, Tyson JJ, Xuan J, Wang Y, et
al: Endoplasmic reticulum stress, the unfolded protein response,
autophagy, and the integrated regulation of breast cancer cell
fate. Cancer Res. 72:1321–1331. 2012.PubMed/NCBI
|
|
78
|
Meng XX, Yao M, Zhang XD, Xu HX and Dong
Q: ER stress-induced autophagy in melanoma. Clin Exp Pharmacol
Physiol. 42:811–816. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Martin S, Hill DS, Paton JC, Paton AW,
Birch-Machin MA, Lovat PE and Redfern CP: Targeting GRP78 to
enhance melanoma cell death. Pigment Cell Melanoma Res. 23:675–682.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Matsumoto T, Uchiumi T, Monji K, Yagi M,
Setoyama D, Amamoto R, Matsushima Y, Shiota M, Eto M and Kang D:
Doxycycline induces apoptosis via ER stress selectively to cells
with a cancer stem cell-like properties: Importance of stem cell
plasticity. Oncogenesis. 6:3972017. View Article : Google Scholar : PubMed/NCBI
|















