Introduction
Breast cancer (BC) is the leading cause of
cancer-associated mortality in women worldwide, with >1,000,000
diagnosed cases annually (1).
Although the diagnosis and treatment of BC has improved, the
overall survival rate for patients with BC, particularly for those
with advanced stage, remains poor (2). Several molecules have been reported
to serve essential roles in BC development and progression
(3,4). However, the precise regulatory
mechanisms of these molecules remain poorly understood (5). Determining the molecular mechanisms
that are associated with BC progression and metastasis is therefore
imperative.
Long non-coding RNAs (lncRNAs), which are
transcripts >200 nucleotides without a protein-coding capacity,
have been demonstrated to be involved in the initiation,
progression and metastasis of numerous types of cancer (6). lncRNA cancer susceptibility candidate
2 (CASC2) is a novel lncRNA located at chromosome 10q26, which has
been identified as a tumor suppressor in multiple human
malignancies (7,8). CASC2 was reported to inhibit gastric
cancer and hepatocellular carcinoma cell proliferation, migration
and invasion through the suppression of the mitogen activated
protein kinase signaling pathway, which is involved in the
pathogenesis of various cancer types (9,10).
CASC2 was also revealed to suppress glioma cell and bladder cancer
cell proliferation and metastasis through the inactivation of the
Wnt/â-catenin pathway, which is a conserved molecular mechanism
with an important role in multiple human malignancies (11,12).
In addition, CASC2 has been demonstrated to function as a competing
endogenous RNA for microRNA and in turn modulates the expression of
microRNA (miR) target genes (13).
In colorectal cancer, CASC2 acts as a sponge of miR-18a to regulate
the expression of protein inhibitor of activated STAT3 thereby
inhibiting cancer growth in vitro and in vivo, and
represents a biomarker for the diagnosis and therapeutic of
colorectal cancer (14). CASC2
inhibits tumorigenesis in esophageal carcinoma by targeting
miR-18a-5p, whereas it increases the sensitivity of prostate cancer
cells to docetaxel by sponging miR-183 (15,16).
Zhang et al (17) reported
that lncRNA CASC2 suppresses BC cell proliferation and metastasis
through inactivation of the tumor growth factor-β signaling
pathway.
A number of studies have demonstrated that miR-96-5p
is implicated in the regulation of proliferation, apoptosis,
migration and invasion of several types of cancer (18-20).
Serum concentrations of miR-96-5p are significantly upregulated in
BC and are associated with a reduced survival rate of patients with
BC (21). Furthermore, miR-96-5p
expression is significantly elevated in BC tissues and cell lines
compared with adjacent normal tissues and non-malignant breast
epithelial cells, respectively (22,23).
The overexpression of miR-96-5p promotes the proliferation and
migration of BC cells, while the inhibition of miR-96-5p leads to a
decrease in cell viability and an increase in cell death (24,25).
miR-96-5p serves an oncogenic role in BC progression, but the
regulatory mechanism requires further elucidation.
In the present study, lncRNA CASC2 expression was
determined in BC. The results demonstrated that the lncRNA CASC2
level was significantly decreased in BC tissues and cells compared
with adjacent normal tissues and mammary epithelial cells,
respecitively. Furthermore, the overexpression of CASC2 was
identified to induce apoptosis and suppress the migration of BC
cells. The upregulation of lncRNA CASC2 resulted in decreased
miR-96-5p expression and increased synoviolin (SYVN1) expression.
In combination, these results demonstrated that lncRNA CASC2
inhibited BC cell growth and metastasis through the regulation of
the miR-96-5p/SYVN1 signaling pathway.
Methods and materials
Patients and tissue samples
A total of 35 paired tissue samples of breast cancer
and adjacent normal tissues were obtained from the Affiliated
Hospital of Integrated Traditional Chinese and Western Medicine
(Nanjing, China) between January 2016 and February 2017 from female
patients with a median age of 52 years (age range, 31-69 years). A
total of eight patients had stage I, 17 had stage II and 10 had
stage III breast cancer at the time of the surgery. In addition, 19
(54.3%) patients were negative and 16 (45.7%) patients were
positive for lymph node-metastasis, and 22 (62.9%) patients were
estrogen receptor-positive, 18 (51.4%) patients were
progesterone-positive, and 12 (34.3%) patients were human epidermal
growth factor receptor 2-positive. The pathological features and
tumor stage were reviewed by two different experienced pathologists
according to the World Health Organization Classification. Clinical
information was obtained from patient charts and pathological
reports. No patient had received radiotherapy or chemotherapy prior
to surgical resection. Patients were excluded from the present
study if they exhibited bilateral disease or pregnancy with the
diagnosis of breast cancer. All the tissue samples were immediately
frozen in liquid nitrogen and stored at −80°C until total RNA and
protein were extracted. The study on BC samples was approved and
supervised by the Research Ethics Committee of Nanjing Medical
University (Nanjing, China). Written informed consent was obtained
from all patients.
Cell lines and cell culture
Human BC (MCF-7, MDA-MB-231) and mammary epithelial
(MCF10A) cell lines were maintained at Nanjing Medical University
(Nanjing, China). MCF10A cells were cultured in Dulbecco’s modified
Eagle’s medium (DMEM)/F12 (3:1) supplemented with 10% fetal bovine
serum (FBS) (both from Thermo Fisher Scientific, Inc., Waltham, MA,
USA), 20 ng/ml of epidermal growth factor, 0.5 µg/ml
hydrocortisone, 10 µg/ml insulin, 50 U/ml penicillin and 50
µg/ml streptomycin. MCF7 and MDA-MB-231 cells were cultured
in DMEM + GlutaMAX™ (Thermo Fisher Scientific, Inc., Waltham, MA,
USA) containing 10% FBS, 50 U/ml penicillin and 50 µg/ml
streptomycin. All cells were cultured at 37°C in a humidified
atmosphere containing 5% CO2 (26).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from tissues and cells using
TRIzol reagent (Thermo Fisher Scientific, Inc.) according to the
manufacturer’ s protocol. For each sample, 1 µg of the total
RNA was converted to cDNA using the High-Capacity cDNA Reverse
Transcription kit (Thermo Fisher Scientific, Inc.) and performed at
45°C for 60 min followed by 70°C for 10 min. The expression of
CASC2, miR-96-5p, miR-183-5p, miR-182-5p, miR-155, miR-21, miR-31,
miR-221 and miR-27a was measured by qPCR using a LightCycler480 II
Sequence Detection system (Roche Diagnostics, Basel, Switzerland).
The following primers were used: CASC2 forward,
5′-GCTGATCAGAGCACATTGGA-3′ and reverse, 5′-ATAAAGGTGGCCACAACTGC-3′;
SYVN1 forward, 5′-AACCCCTGGGACAACAAGG-3′ and reverse,
5′-GCGAGACATGATGGCATCTG-3′; GAPDH forward, 5′-GGGAGCCAAAAGGGTCAT-3′
and reverse, 5′-GAGTCC TTCCACGATACCAA-3′; miR-96-5p forward,
5′-TTTGGC ACTAGCACAT-3′ and reverse, 5′-GAGCAGGCTGGAGAA-3′;
miR-183-5p forward, 5′-CGCGGTATGGCACTGGTAGA-3′ and reverse,
5′-AGTGCAGGGTCCGAGGTATTC-3′; miR-182-5p forward,
5′-TGCGGTTTGGCAATGGTAGAAC-3′ and reverse,
5′-CCAGTGCAGGGTCCGAGGT-3′; miR-155 forward, 5′-GCG
GTTAATGCTAATCGTGAT-3′ and reverse, 5′-GTGCAGGGT CCGAGGT-3′; miR-21
forward, 5′-UAGCUUAUCAGACUGA UGUUGA-3′ and reverse,
5′-CGAGGAAGAAGACGGAAG AAT-3′; miR-31 forward,
5′-GCGGCGGAGGCAAGATGCT GGC-3′ and reverse,
5′-AGGCAAGATGCTGGCATAGCT-3′; miR-221 forward,
5′-CGAGCTACATTGTCTGCTGGGT-3′ and reverse, 5′-GTGCAGGGTCCGAGGT-3′;
miR-27a forward, 5′-TGTATTTTAGTCGTGGCGATA-3′ and reverse, 5′-ATAACG
ACTCACGCCTATAATC-3′; U6 forward, 5′-GTGCGTGTCGT G GAGTCG-3′ and
reverse, 5′-AACGCTTCACGAATTTGCGT-3′. U6 and GAPDH were used as
internal standards. The qPCR analysis was performed using the SYBR
premix Ex Taq II kit (Takara Biotechnology, Co., Ltd., Dalian,
China). The thermocycling conditions were as follows: 95°C for 30
sec; followed by 40 cycles at 95°C for 15 sec, 57°C for 30 sec and
72°C for 34 sec; and a final extension step at 72°C for 5 min.
Relative expression levels were analyzed using the
2−∆∆Cq method as previously described (27).
Western blot analysis
Total protein from BC cells were extracted in lysis
buffer were lysed with ice-cold lysis buffer containing: 50 mmol/l
Tris-HCl, pH 7.4; 1% NP-40; 150 mmol/l NaCl; 1 mmol/l EDTA; 1
mmol/l phenylmethylsulfonyl fluoride; and complete proteinase
inhibitor mixture (one tablet/10 ml; Roche Molecular Biochemicals,
Pleaston, CA, USA). The protein concentration was measured using a
Bradford protein assay. Equal quantities of protein (30 µg)
were subjected to 10% SDS-PAGE and transferred onto polyvinylidene
difluoride membranes. The membranes were blocked with 5% bovine
serum albumin (Beyotime Institute of Biotechnology, Haimen, China)
for 2 h at room temperature and incubated overnight at 4°C with
primary antibodies. Western blot analysis was performed as
previously described (26).
Individual immunoblots were probed with a rabbit anti-SYVN1
(1:1,000; cat. no. AV43360; Merck KGaA, Darmstadt, Germany) and a
mouse anti-β-actin antibody (1:3,000; cat. no. sc-517582; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA). The blots were
incubated with horseradish peroxidase-conjuagated goat anti-rabbit
IgG (cat. no. A0208) and goat anti-mouse IgG (cat. no. A0216) (both
1:1,000; Beyotime Institute of Biotechnology) secondary antibodies
for 1 h at room temperature. The relative protein expression was
determined using ImageJ V1.8.0 (National Institutes of Health,
Bethesda, MD, USA) with β-actin used as the internal reference.
Plasmid construction
CASC2 cDNA coding sequence was amplified according
to the full-length CASC2 sequence using the following primer pair:
Forward, 5′-TGCATCAGACAG GAGTAGATG-3′ and reverse,
5′-GCTATGCGCCAAGTTAA CAG-3′. PCR products were subcloned into a
pcDNA3.1 vector (Thermo Fisher Scientific, Inc.). The plasmid was
sequenced and confirmed to be correct. An empty pcDNA3.1 vector
served as the negative control.
Cell viability assay
Cell viability was quantified using the Cell
Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan). Briefly, MCF-7 and MDA-MB-231 cells were seeded
into a 48-well plate (6×103 cells/well) and were
transfected with pcDNA3.1 or pcDNA3.1-CASC2 (0.5 µg/well).
Following transfection for 24, 48 and 72 h, the CCK-8 reagent was
added to the culture wells, which were then incubated at 37°C for
an additional 2 h. The absorbance was determined at 450 nm using a
microtiter plate reader. Experiments were performed in
triplicate.
Cell apoptosis assay
Apoptosis was analyzed by flow cytometric analysis.
The pcDNA3.1 or pcDNA3.1-CASC2-transfected MCF-7 and MDA-MB-231
cells were cultured in 6-well plates for 48 h. The cells were
harvested by trypsinization. Following double staining with
FITC-Annexin V and propidium iodide (BD Biosciences, San Jose, CA,
USA) for 15 min at room temperature in the dark, the cells were
analyzed using flow cytometry (BD FACScan™ system; BD Biosciences).
Flow cytometry data were analyzed using Kaluza analysis software
version 2.0 (Beckman Coulter, Inc., Brea, CA, USA). The assay was
repeated in triplicate.
Invasion and migration assay
For the migration assays, 1×105
MDA-MB-231 cells in serum-free medium were placed into the upper
chamber of a Transwell insert (8-µm pore size;
Sigma-Aldrich; Merck KGaA). For the invasion assays, MDA-MB-231
cells in serum-free medium were placed into the upper chamber of an
insert coated with Matrigel (Sigma-Aldrich; Merck KGaA). DMEM
containing 10% FBS was added to the lower chamber. Following
incubation for 12 h, the cells remaining on the upper membrane were
removed using cotton wool. BC cells that had migrated or invaded
through the membrane were fixed in 10% methanol for 15 min at room
temperature, and then stained with crystal violet dye (0.04% in
H2O; 100 µl) for 20 min at room temperature,
counted using an inverted microscope and imaged (magnification,
×200).
Pull-down assay with biotinylated
lncRNA-CASC2 DNA probe
CASC2 and its antisense RNA were in vitro
transcribed and biotin-labeled with the Biotin RNA Labeling mix and
T7/SP6 RNA polymerase (both from Roche Diagnostics), and purified
using a RNeasy Mini kit (Qiagen, Inc., Valencia, CA, USA) according
to the manufacturer’s protocol. The biotinylated lncRNA-CASC2 DNA
probe was dissolved in binding and washing buffer, and incubated
with Dynabeads M-280 streptavidin (Invitrogen; Thermo Fisher
Scientific, Inc.) at room temperature for 10 min to generate
probe-coated beads according to the manufacturer’s protocol.
Subsequently, MDA-MB-231 cell lysates were incubated with the
probe-coated beads, and the RNA complexes bound to these beads were
eluted and extracted for RT-qPCR as aforementioned.
Pull-down assay with biotinylated
miR-96-5p
MDA-MB-231 cells were transiently transfected with
biotinylated miR-96-5p, miR-96-5p-mutant (Mut) and negative control
(Guangzhou RiboBio Co., Ltd., Guangzhou, China), then harvested and
lysed 48 h after transfection. Subsequently, 50 µl of the
samples were aliquoted for input. The remaining lysates were
incubated with Dynabeads M-280 streptavidin according to the
manufacturer’ s protocol. In brief, the washed beads were treated
with RNase-free solutions and incubated with an equal volume of
biotinylated miR-96-5p for 10 min at room temperature in binding
and washing buffer on a rotator. Next, the beads with the
immobilized miR-96-5p fragment were incubated with 10 mM EDTA (pH
8.2) with 95% formamide at 65°C for 5 min. The bound RNAs were
purified using TRIzol for RT-qPCR as aforementioned.
miRNA target prediction
Prediction of the miR-96-5p targets was performed
using two publicly available algorithms: TargetScan6.2 (http://www.targetscan.org/) and miRanda (http://www.microrna.org/).
Luciferase reporter assays
To construct the reporter vector, one fragment of
SYVN1 3′-untranslated region (3′-UTR) [wild-type (WT) or mutant
(MUT), respectively] and the fragment of CASC2 containing predicted
miR-96-5p binding site, or the fragment of miR-96-5p, containing
predicted CASC2 binding site were separately amplified, and fused
to a modified pcDNA3.1 vector containing a luciferase gene, which
was cloned into upstream of cloning sites. The luciferase assay was
performed using the Dual-Luciferase® Reporter assay
system (Promega Corporation, Madison, WI, USA). Briefly, BC cells
were co-transfected with 100 pmol miR-96-5p mimics or 100 pmol
pre-negative control (NC) and 0.5 µg pMIR-reporter
luciferase vector containing a specific sequence of WT or MUT CASC2
or SYVN1 fragment, using Lipofectamine 2000 (Thermo Fisher
Scientific, Inc.). The miR-96-5p mimics,
5′-UUUGGCACUAGCACAUUUUUGCU-3′, and NC, 5′-UUC UCCGAACGUGUCACGUTT-3′
were obtained from Shanghai GenePharma Co., Ltd. (Shanghai, China).
The luciferase activity was measured 48 h post-transfection. The
relative luciferase activity was normalized to the Renilla
luciferase activity.
Animal tumor model
Female athymic 6-weeks-old nude mice (mean weight,
10-12 g; n=7 mice/group) were purchased from the Shanghai
Laboratory Animal Centre (Chinese Academy of Sciences, Shanghai,
China) and maintained in cage housing under specific pathogen-free
conditions with free access to food and water. Cultured MDA-MB-231
cells transfected with enhanced green fluorescent protein (EGFP)
(Len-GFP, 10-fold multiplicity of infection virus particle
concentration) or EGFP-tagged CASC2 lentivirus (Len-CASC2, 10-fold
multiplicity of infection virus particle concentration) in the
presence of 4 µg/ml polybrene (Sigma-Aldrich; Merck KGaA)
were harvested from 6-well plates and resuspended in 0.2 ml PBS at
5×107 cells/ml (1×106 cells/mouse). Len-GFP
and Len-CASC2 were obtained from Shanghai GenePharma Co., Ltd.
Cells were injected into the right or the left flank region of the
mice to generate the orthotopic model. Tumor volumes
(mm3) in mice were measured with a slide caliper every 4
days according to the formula: 1/2 × width2 × length.
Animals were treated humanely, using approved procedures in
accordance with the guidelines of the Institutional Animal Care and
Use Committee at Nanjing Medical University. The study was approved
by the Experimental Animal Ethics Committee of Nanjing Medical
University.
Statistical analysis
Statistical analysis was performed using SPSS 18.0
statistical analysis software (SPSS, Inc., Chicago, IL, USA).
Comparisons between two groups were performed using the Student’s
t-test and comparisons among multiple groups using one-way analysis
of variance with Tukey’s post hoc test. The correlation between
CASC2 and miR-96-5p expression was analyzed using the Pearson
correlation analysis. Data are expressed as the mean ± standard
deviation. P<0.05 was considered to indicate a statistically
significant difference. All experiments were repeated
triplicate.
Results
lncRNA CASC2 expression is downregulated
in BC tissues and cell lines
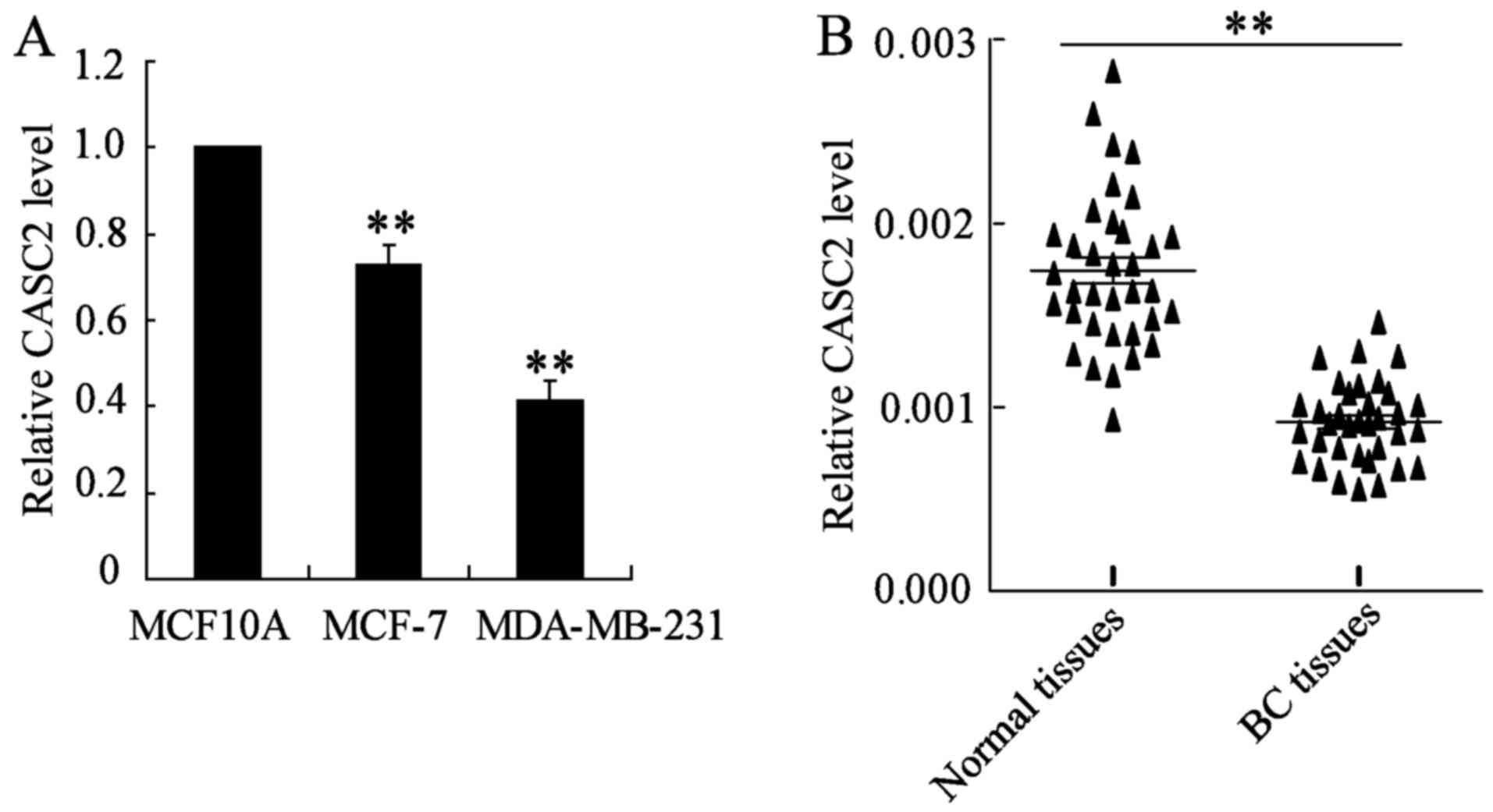
CASC2 expression was determined in MCF10A, MCF-7 and
MDA-MB-231 cells using RT-qPCR. The results demonstrated that the
CASC2 expression level was significantly decreased in BC cells
compared with the mammary epithelial MCF10A cells (Fig. 1A). In addition, the expression of
lncRNA CASC2 in BC and matched adjacent normal tissue samples was
measured. As shown in Fig. 1B, the
CASC2 expression level was significantly downregulated in BC
tissues, as compared with adjacent normal tissues.
CASC2 upregulation inhibits BC cell
viability, migration and invasion
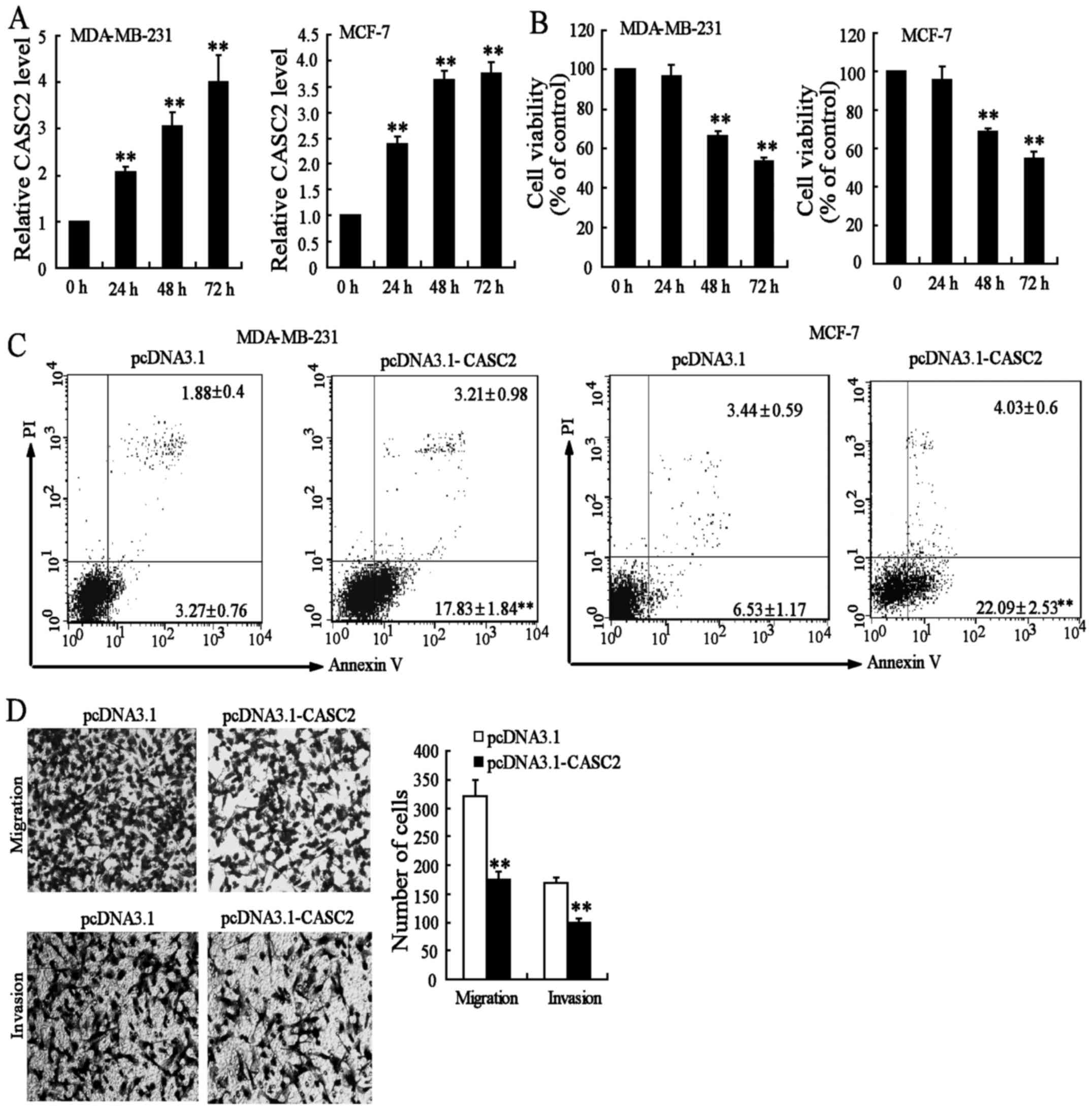
To explore the role of CASC2 in BC cells, CASC2
expression was increased through the transfection of pcDNA3.1-CASC2
in MDA-MB-231 and MCF-7 cells. The results revealed that
pcDNA3.1-CASC2 significantly increased the expression of CASC2
(Fig. 2A). Next, the effect of
pcDNA3.1-CASC2 on the growth of MDA-MB-231 and MCF-7 cells was
investigated. The overexpression of CASC2 led to a decrease of cell
growth in a time-dependent manner (Fig. 2B). In addition, the effects of
CASC2 on the apoptosis of BC cells were measured by flow cytometry.
As shown in Fig. 2C, the rate of
apoptotic cells in pcDNA3.1- and pcDNA3.1-CASC2-transfected
MDA-MB-231 cells was 5.1 and 21%, respectively. Similar results
were observed in MCF-7 cells. However, pcDNA3.1-CASC2 had no
significant effect on cell cycle distribution (data not shown).
These data indicated that the decrease in the number of BC cells
upon pcDNA3.1-CASC2 transfection was caused by apoptosis.
Next, migration and invasion assays were performed
in MDA-MB-231 cells transfected with pcDNA3.1-CASC2. The results
demonstrated that the overexpression of CASC2 significantly
decreased migration and invasion in MDA-MB-231 cells, when compared
with the controls (Fig. 2D). These
results indicated that CASC2 may act as a tumor suppressor through
the promotion of cell apoptosis and the suppression of cell
migration and invasion in BC.
CASC2 overexpression inhibits BC growth
in vivo
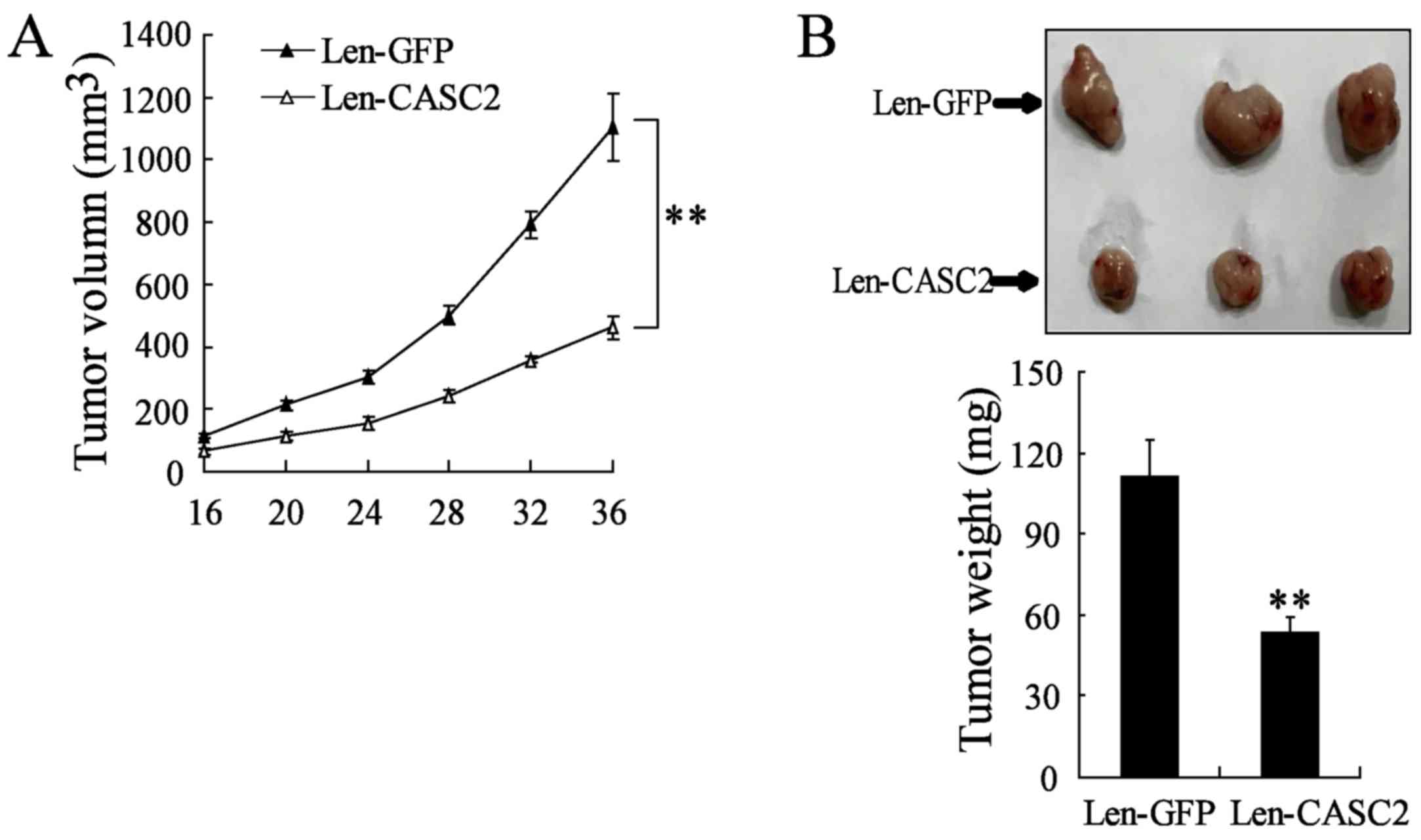
To further explore the effect of CASC2
overexpression on BC growth in vivo an animal tumor model
was established using MDA-MB-231 cells transfected with Len-GFP or
Len-CASC2. The growth of the BC xenograft was significantly
inhibited in mice treated with Len-CASC2, as compared with mice
treated with Len-GFP (Fig. 3A).
The mean tumor weight in Len-CASC2-treated BC xenografts was
significantly reduced compared with that in the Len-GFP group
(53.33±5.5 vs. 113.33±13.41 mg; P<0.01; Fig. 3B). Therefore, the present data
demonstrated that CASC2 overexpression inhibited BC development
in vivo.
CASC2 functions as a miR-96-5p sponge in
BC cells
lncRNA CASC2 may function as a competing endogenous
RNA for microRNA due to sequence complementarity and in turn may
regulate the expression of microRNA target genes (13-15).
In the present study, the association between CASC2 and miR-96-5p
expression in 35 BC tissues was explored, with the results revealed
a significantly negative correlation between CASC2 and miR-96-5p
expression levels (r=−0.825; P<0.001; Fig. 4A). Furthermore, overexpression of
CASC2 was demonstrated to significantly inhibit miR-96-5p
expression in MDA-MB-231 cells (Fig.
4B). A total of seven other miRNAs (miR-183-5p, miR-182-5p,
miR-155, miR-21, miR-31, miR-221 and miR-27a) were also measured,
which act as oncogenes in BC (28), and were predicted to be likely
downstream targets of CASC2 from the database (LncBase Predicted
V.2, http://www.microrna.gr/LncBase). The
results revealed that the inhibitory effect of CASC2 on the
expression of these seven miRNAs was lower, compared with that on
miR-96-5p (Fig. 4B).
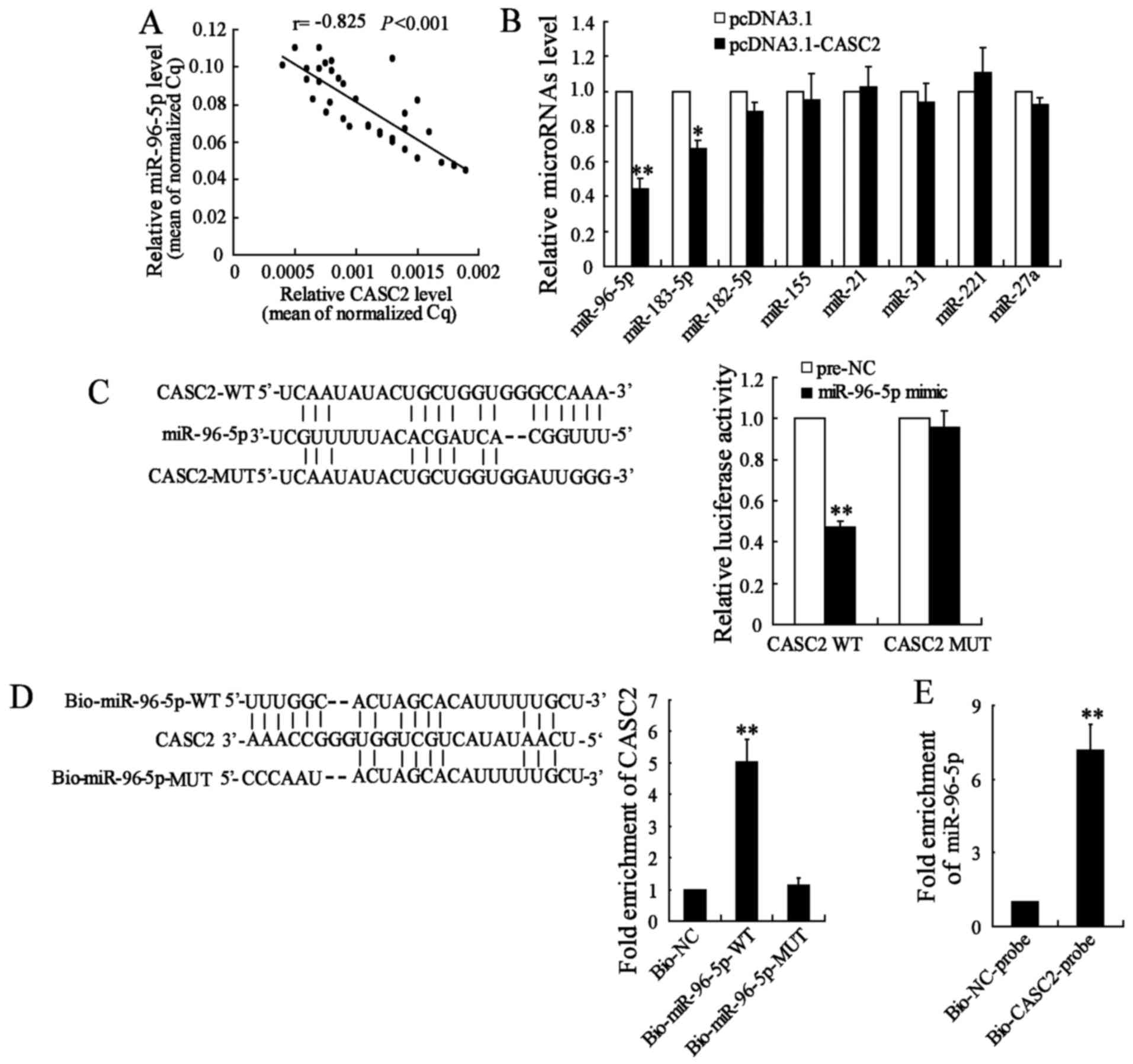 | Figure 4CASC2 functions as a miR-96-5p sponge
in BC cells. (A) Correlation between the CASC2 and miR-96-5p
expression in 35 BC tissues. (B) MDA-MB-231 cells were transfected
with pcDNA3.1 or pcDNA3.1-CASC2 for 48 h and the expression of
miR-96-5p, miR-183, miR-182, miR-155, miR-21, miR-205, miR-221 and
miR-27a was determined. (C) Sequence alignment of miR-96-5p with
the putative binding sites within the WT regions of CASC2.
MDA-MB-231 cells were co-transfected with a miR-96-5p mimic and
CASC2-WT vector or CASC2-MUT vector for 48 h and the luciferase
activity was measured. (D) The WT and mutated forms of the
miR-96-5p sequence are shown. Detection of CASC2 using RT-qPCR in
the sample pulled down by biotinylated miR-96-5p. (E) Detection of
miR-96-5p using RT-qPCR in the sample pulled down by biotinylated
CASC2 probe. *P<0.05 and **P<0.01,
compared with pcDNA3.1, Pre-NC or Bio-NC. CASC2, long non-coding
RNAs cancer susceptibility candidate 2; BC, breast cancer; WT, wild
type; MUT, mutant; NC, negative control; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; UTR,
untranslated region; miR, microRNA. |
The database revealed that there were binding sites
between CASC2 and miR-96-5p. The luciferase reporter assay
demonstrated that the overexpression of miR-96-5p significantly
decreased CASC2-WT activity, while it had no significant effect on
CASC2-MUT (Fig. 4C). In addition,
CASC2 was pulled down by miR-96-5p, but the mutations resulted in
the inability of miR-96-5p to pull down CASC2 (Fig. 4D), which suggested that the
recognition of miR-96-5p to CASC2 was in a sequence-specific
manner. It was also observed that CASC2 pulled down miR-96-5p
(Fig. 4E). These results revealed
that CASC2 functioned as a miR-96-5p sponge in BC cells.
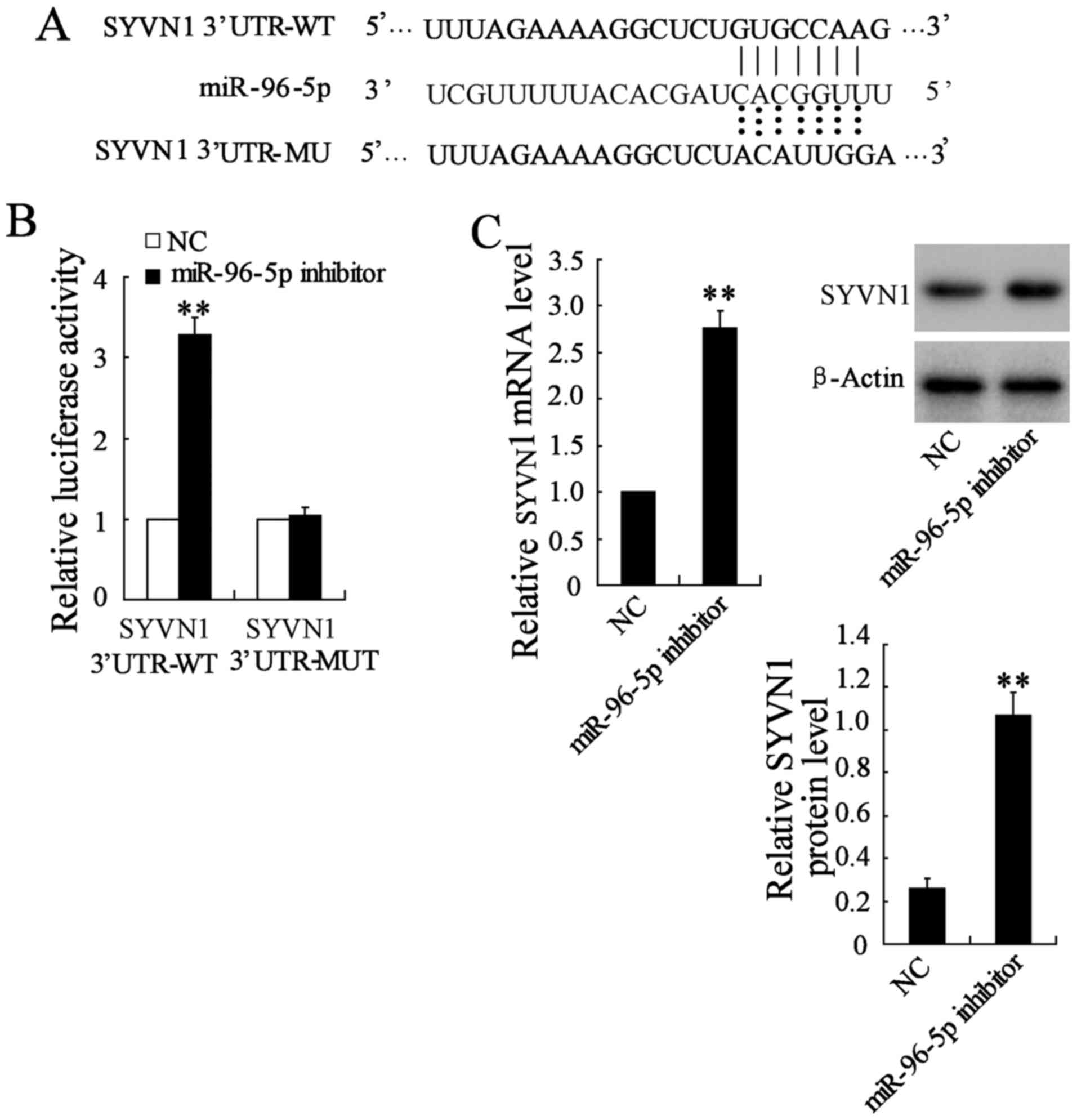
miR-96-5p directly targets SYVN1 in BC
cells
It was demonstrated that miR-96-5p served an
oncogenic role in BC. Our previous study reported that the
overexpression of SYVN1 inhibited the growth, migration and
invasion of BC cells in vitro and in vivo (26). TargetScan and miRanda revealed that
the 3′-UTR of SYVN1 contained the complementary site for the seed
region of miR-96-5p (Fig. 5A).
Further examination demonstrated that miR-96-5p inhibition
significantly increased the SYVN1 3′-UTR activity, which was not
observed for the mutant SYVN1 3′-UTR activity (Fig. 5B). In addition, miR-96-5p
inhibition significantly elevated the mRNA and protein expression
of SYVN1 in MDA-MB-231 cells compared with the negative control
(Fig. 5C). These data indicated
that miR-96-5p targeted human SYVN1 by directly binding to the
predicted sites in 3′-UTR of SYVN1 mRNA.
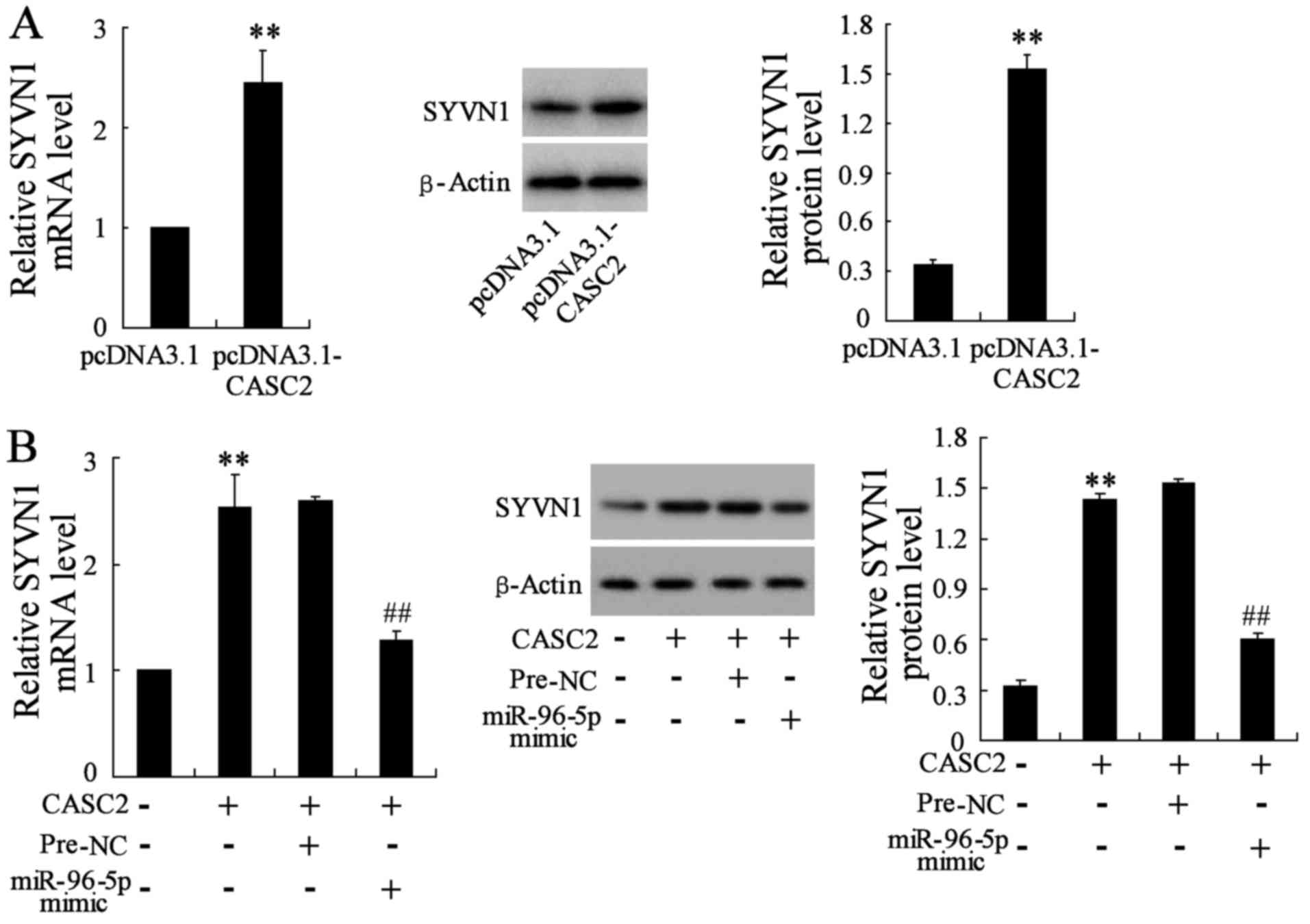
CASC2 regulates SYVN1 expression through
modulating miR-96-5p
As CASC2 share regulatory miR-96-5p with SYVN1 mRNA,
the possibility of CASC2 regulating SYVN1 in BC cells was explored.
As shown in Fig. 6A, the
overexpression of CASC2 significantly increased SYVN1 mRNA and
protein levels in MDA-MB-231 cells compared with the control cells.
In addition, the upregulation of miR-96-5p upon pcDNA3.1-CASC2
transfection abrogated this increase (Fig. 6B). All these data suggested an
important role of CASC2 in regulating SYVN1 by competitively
binding miR-96-5p.
CASC2 overexpression suppresses BC cell
viability, migration and invasion via the miR-96-5p/SYVN1 axis
Whether the upregulation of CASC2 inhibited BC cell
viability, migration and invasion through the miR-96-5p/SYVN1 axis
was investigated. Notably, miR-96-5p overexpression inhibited
apoptosis, and promoted migration and invasion of
CASC2-overexpressing MDA-MB-231 cells compared with cells with
CASC2 overexpression only (Fig. 7A and
B). Furthermore, the restoration of SYVN1 abrogated the
promoting effects of miR-96-5p on viability, migration and invasion
of MDA-MB-231 cells (Fig. 7C and
D). Thus, it was demonstrated that CASC2 suppressed BC cell
viability, migration and invasion via the miR-96-5p/SYVN1 axis.
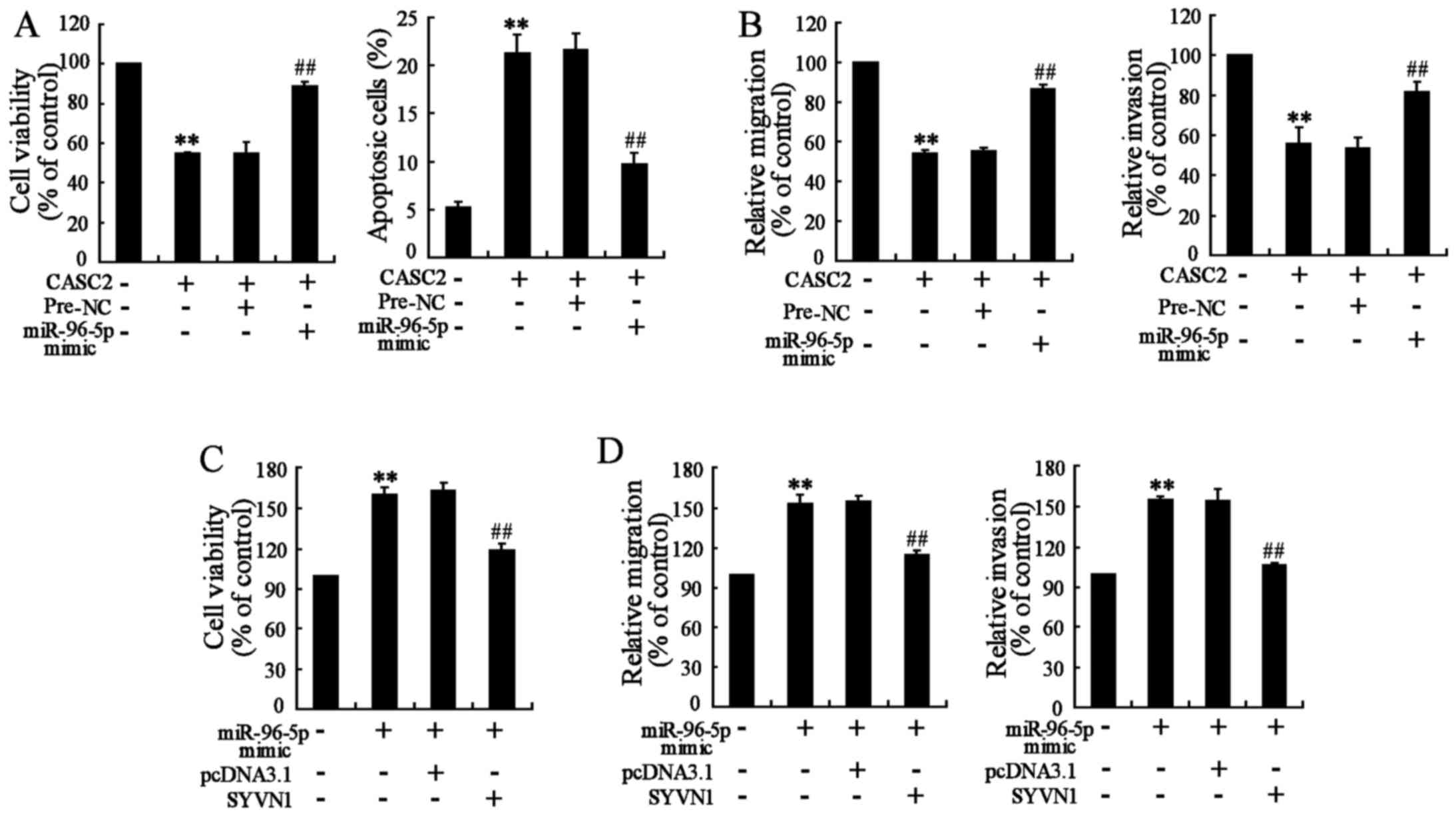 | Figure 7The CASC2 overexpression suppresses
BC cell viability, migration and invasion through the
miR-96-5p/SYVN1 axis. MDA-MB-231 cells were transfected with
pcDNA3.1-CASC2 (CASC2) and miR-96-5p mimic for 48 h, and then the
(A) cell viability and cell apoptosis, and (B) cell migration and
invasion were determined. MDA-MB-231 cells were transfected with an
miR-96-5p mimic and pcDNA3.1-SYVN1 (SYVN1) for 48 h, and the (C)
cell viability, and (D) cell migration and cell invasion was
determined. **P<0.01, compared with pcDNA3.1 or
Pre-NC; ##P<0.01, compared with pcDNA3.1-SYVN1 +
Pre-NC or miR-96-5p mimic + pcDNA3.1. CASC2, long non-coding RNAs
cancer susceptibility candidate 2; NC, negative control; miR,
microRNA; SYVN1, synoviolin. |
Discussion
Several studies have reported that a number of
lncRNAs serve an important role in the occurrence and development
of tumors in humans (29). lncRNA
CASC2 has been revealed to be involved in the proliferation and
metastasis of various types of cancer, including gastric, bladder
and colorectal cancer, hepatocellular carcinoma, and glioma
(6). However, the role and
mechanism of lncRNA CASC2 in BC remain unclear. The findings of the
present study suggested that downregulation of lncRNA CASC2 in BC
tissues and cell lines may act as a tumor suppressor. The mechanism
revealed that dysregulation of the CASC2/miR-96-5p/SYVN1 axis
contributed to BC cell proliferation, migration and invasion.
Low expression of CASC2 has been reported in several
human malignancies (9-12). In the present study, CASC2
expression was significantly inhibited in BC tissues and cell lines
compared with adjacent normal tissues and nonmalignant breast
epithelial cells, respectively. Furthermore, low expression of
CASC2 was demonstrated to be significantly associated with the TNM
stage, differentiation grade and lymph node metastasis of BC. In
addition, CASC2 overexpression significantly inhibited BC growth
in vitro and in vivo, and induced apoptosis in BC
cells. CASC2 overexpression also inhibited the migration and
invasion of BC cells. These findings demonstrated that CASC2 acted
as a tumor suppressor in BC tissues and cells, which was in
accordance with the function of CASC2 identified in other cancer
tissues (30).
lncRNA CASC2 may function as a competing endogenous
RNA by sponging miR-18a, miR-193a-5p or miR-183 and subsequently
regulating the expression of the target genes of these microRNA
(14-16,31).
In the present study, a significantly negative association was
identified between the CASC2 and miR-96-5p expression levels in BC
tissues. Furthermore, the CASC2 overexpression significantly
decreased the expression level of miR-96-5p, but had a minimal
effect on the expression of miR-183-5p, miR-182-5p, miR-155,
miR-21, miR-31, miR-221 and miR-27a in BC cells. Since lncRNAs
contain microRNA responsive elements and act as microRNA sponges to
downregulate microRNA expression, it was observed that CASC2 may
directly interact with miR-96-5p and downregulate its expression in
BC cells. In addition, the present data indicated that miR-96-5p
overexpression may reverse the anticancer effects of CASC2
overexpression on BC cell growth, migration and invasion.
Therefore, lncRNA CASC2 acted as a tumor suppressor in BC tissues
and cell lines, at least partly, through the inhibition of
miR-96-5p.
In BC, miR-96-5p is a positive regulator of the
proliferation and metastasis processes, in which the miR-183-5p
expression is increased (22-25).
In our previous study, SYVN1 was revealed to be a tumor suppressor
in BC (26). Bioinformatics
analysis revealed that SYVN1 may be a downstream target of
miR-96-5p. Further investigations confirmed that miR-96-5p targeted
human SYVN1 by directly binding to the predicted sites in the
3′-UTR of SYVN1 mRNA. Subsequently, it was observes that CASC2
positively regulated SYVN1 expression via targeting miR-96-5p in BC
cells. The restoration of SYVN1 abrogated the promoter effects of
miR-96-5p on the migration, invasion and viability of BC cells.
In conclusion, CASC2 may inhibit cell migration,
invasion and viability via the CASC2/miR-96-5p/SYVN1 axis in BC.
Therefore, the suppressive effect of CASC2 on BC development
indicated that lncRNA-MIAT may be a potential therapeutic target in
BC.
Funding
The present study was supported by the National Key
Research and Development Program of China (grant no.
2016YFC0905900), The ‘333’ Talent Project of Jiangsu Province
[grant no. 4(2016)], The National Key Clinical Specialist
Construction Programs of China [grant no. 544 (2013)], The Natural
Science Foundation of Jiangsu Province (grant no. BK20151579) and
The National Natural Science Foundation of China (grant nos.
81570779 and HG13-06).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors’ contributions
DS and JT designed the study and wrote the
manuscript. ZG, ML, HW, HL, JW and WZ performed the experiments. XL
analyzed the data. All authors reviewed and approved the final
manuscript.
Ethics approval and consent to
participate
The study on BC samples was approved and supervised
by the Research Ethics Committee of Nanjing Medical University
(Nanjing, China). Written informed consent was obtained from all
patients. Animals were treated humanely, using approved procedures
in accordance with the guidelines of the Institutional Animal Care
and Use Committee at Nanjing Medical University. The study was
approved by the Experimental Animal Ethics Committee of Nanjing
Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Zduriencikova M, Gronesova P, Cholujova D
and Sedlak J: Potential biomarkers of exosomal cargo in endocrine
signaling. Endocr Regul. 49:141–150. 2015. View Article : Google Scholar
|
|
2
|
Paracha N, Thuresson PO, Moreno SG and
MacGilchrist KS: Health state utility values in locally advanced
and metastatic breast cancer by treatment line: A systematic
review. Expert Rev Pharmacoecon Outcomes Res. 16:549–559. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Osborne C, Wilson P and Tripathy D:
Oncogenes and tumor suppressor genes in breast cancer: Potential
diagnostic and therapeutic applications. Oncologist. 9:361–377.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Logan GJ, Dabbs DJ, Lucas PC, Jankowitz
RC, Brown DD, Clark BZ, Oesterreich S and McAuliffe PF: Molecular
drivers of lobular carcinoma in situ. Breast Cancer Res. 17:762015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Panno ML, Naimo GD, Spina E, Andò S and
Mauro L: Different molecular signaling sustaining adiponectin
action in breast cancer. Curr Opin Pharmacol. 31:1–7. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sun W, Yang Y, Xu C and Guo J: Regulatory
mechanisms of long noncoding RNAs on gene expression in cancers.
Cancer Genet. 216-217:105–110. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Baldinu P, Cossu A, Manca A, Satta MP,
Sini MC, Palomba G, Dessole S, Cherchi P, Mara L, Tanda F, et al:
CASC2a gene is down-regulated in endometrial cancer. Anticancer
Res. 27:235–243. 2007.PubMed/NCBI
|
|
8
|
Palmieri G, Paliogiannis P, Sini MC, Manca
A, Palomba G, Doneddu V, Tanda F, Pascale MR and Cossu A: Long
non-coding RNA CASC2 in human cancer. Crit Rev Oncol Hematol.
111:31–38. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li P, Xue WJ, Feng Y and Mao QS: Long
non-coding RNA CASC2 suppresses the proliferation of gastric cancer
cells by regulating the MAPK signaling pathway. Am J Transl Res.
8:3522–3529. 2016.PubMed/NCBI
|
|
10
|
Gan Y, Han N, He X, Yu J, Zhang M, Zhou Y,
Liang H, Deng J, Zheng Y, Ge W, et al: Long non-coding RNA CASC2
regulates cell biological behaviour through the MAPK signalling
pathway in hepatocellular carcinoma. Tumour Biol.
39:10104283177062292017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pei Z, Du X, Song Y, Fan L, Li F, Gao Y,
Wu R, Chen Y, Li W, Zhou H, et al: Down-regulation of lncRNA CASC2
promotes cell proliferation and metastasis of bladder cancer by
activation of the Wnt/β-catenin signaling pathway. Oncotarget.
8:18145–18153. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang R, Li Y, Zhu G, Tian B, Zeng W, Yang
Y and Li Z: Long noncoding RNA CASC2 predicts the prognosis of
glioma patients and functions as a suppressor for gliomas by
suppressing Wnt/β-catenin signaling pathway. Neuropsychiatr Dis
Treat. 13:1805–1813. 2017. View Article : Google Scholar :
|
|
13
|
Liao Y, Shen L, Zhao H, Liu Q, Fu J, Guo
Y, Peng R and Cheng L: lncRNA CASC2 interacts with miR-181a to
modulate glioma growth and resistance to TMZ through PTEN pathway.
J Cell Biochem. 118:1889–1899. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang G, Wu X, Li S, Xu X, Zhu H and Chen
X: The long noncoding RNA CASC2 functions as a competing endogenous
RNA by sponging miR-18a in colorectal cancer. Sci Rep. 6:265242016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang W, He W, Gao J, Wang Y, Zang W, Dong
Z and Zhao G: RETRACTED: The long noncoding RNA CASC2 inhibits
tumorigenesis through modulating the expression of PTEN by
targeting miR-18a-5p in esophageal carcinoma. Exp Cell Res.
361:30–38. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gao W, Lin S, Cheng C, Zhu A, Hu Y, Shi Z,
Zhang X and Hong Z: Long non-coding RNA CASC2 regulates Sprouty2
via functioning as a competing endogenous RNA for miR-183 to
modulate the sensitivity of prostate cancer cells to docetaxel.
Arch Biochem Biophys. Jan 23–2018, Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang Y, Zhu M, Sun Y, Li W, Wang Y and Yu
W: Up-regulation of lncRNA CASC2 suppresses cell proliferation and
metastasis of breast cancer via inactivating of the TGF-β signaling
pathway. Oncol Res. Mar 9–2018, Epub ahead of print. View Article : Google Scholar
|
|
18
|
Li C, Du X, Tai S, Zhong X, Wang Z, Hu Z,
Zhang L, Kang P, Ji D, Jiang X, et al: GPC1 regulated by miR-96-5p,
rather than miR-182-5p, in inhibition of pancreatic carcinoma cell
proliferation. Int J Mol Sci. 15:6314–6327. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ress AL, Stiegelbauer V, Winter E,
Schwarzenbacher D, Kiesslich T, Lax S, Jahn S, Deutsch A,
Bauernhofer T, Ling H, et al: MiR-96-5p influences cellular growth
and is associated with poor survival in colorectal cancer patients.
Mol Carcinog. 54:1442–1450. 2015. View
Article : Google Scholar
|
|
20
|
Assal RA, El Tayebi HM, Hosny KA, Esmat G
and Abdelaziz AI: A pleiotropic effect of the single clustered
hepatic metastamiRs miR-96-5p and miR-182-5p on insulin-like growth
factor II, insulin-like growth factor-1 receptor and insulin-like
growth factor-binding protein-3 in hepatocellular carcinoma. Mol
Med Rep. 12:645–650. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang K, Wang YW, Wang YY, Song Y, Zhu J,
Si PC and Ma R: Identification of microRNA biomarkers in the blood
of breast cancer patients based on microRNA profiling. Gene.
619:10–20. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang J, Kong X, Li J, Luo Q, Li X, Shen
L, Chen L and Fang L: miR-96 promotes tumor proliferation and
invasion by targeting RECK in breast cancer. Oncol Rep.
31:1357–1363. 2014. View Article : Google Scholar
|
|
23
|
Hong Y, Liang H, Uzair-Ur-Rehman, Wang Y,
Zhang W, Zhou Y, Chen S, Yu M, Cui S, Liu M, et al: miR-96 promotes
cell proliferation, migration and invasion by targeting PTPN9 in
breast cancer. Sci Rep. 6:374212016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shi Y, Zhao Y, Shao N, Ye R, Lin Y, Zhang
N, Li W, Zhang Y and Wang S: Overexpression of microRNA-96-5p
inhibits autophagy and apoptosis and enhances the proliferation,
migration and invasiveness of human breast cancer cells. Oncol
Lett. 13:4402–4412. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xie W, Sun F, Chen L and Cao X: miR-96
promotes breast cancer metastasis by suppressing MTSS1. Oncol Lett.
15:3464–3471. 2018.PubMed/NCBI
|
|
26
|
Xu YM, Wang HJ, Chen F, Guo WH, Wang YY,
Li HY, Tang JH, Ding Y, Shen YC, Li M, et al: HRD1 suppresses the
growth and metastasis of breast cancer cells by promoting IGF-1R
degradation. Oncotarget. 6:42854–42867. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
28
|
Lo PK, Wolfson B, Zhou X, Duru N,
Gernapudi R and Zhou Q: Noncoding RNAs in breast cancer. Brief
Funct Genomics. 15:200–221. 2016. View Article : Google Scholar :
|
|
29
|
Kondo Y, Shinjo K and Katsushima K: Long
non-coding RNAs as an epigenetic regulator in human cancers. Cancer
Sci. 108:1927–1933. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lu L, Dai Z, Luo Q and Lv G: The long
noncoding RNA cancer susceptibility candidate 2 inhibits tumor
progression in osteosarcoma. Mol Med Rep. 17:1947–1953. 2018.
|
|
31
|
Jiang C, Shen F, Du J, Fang X, Li X, Su J,
Wang X, Huang X and Liu Z: Upregulation of CASC2 sensitized glioma
to temozolomide cytotoxicity through autophagy inhibition by
sponging miR-193a-5p and regulating mTOR expression. Biomed
Pharmacother. 97:844–850. 2018. View Article : Google Scholar
|