Introduction
Breast cancer is becoming an increasingly malignant
cancer type with the highest morbidity rate globally, of which the
onset age is decreasing (1). Each
year, ~1.2 million females are diagnosed with breast cancer
globally, amongst which the majority succumb at 40-45 years old,
however the onset age of the majority of patients is becoming
younger (2). Over one-third of
patients with breast cancer succumb to mortality from the disease
(3). Radiation therapy may have a
transformative impact on the treatment of breast cancer, as it
allows women with an early stage of the disease to maintain the
integrity of their body and those with advanced disease to have
relief from suffering (4). Fisher
et al (5) proved that
breast-conserving surgery with radiation has the same therapeutic
effect as a mastectomy through a 20-year randomized trial in 1,851
women with invasive breast tumor types. Although radiation therapy
is a powerful anticancer modality, radiation-induced stress
responses and gene expression alongside adaptive resistance may
severely compromise the effectiveness of radiation (6). Mechanisms resulting in radiation
therapy resistance are diverse and still poorly defined. One
previous study has indicated that breast cancer stem cells develop
resistance to radiation due to intrinsic and extrinsic mechanisms,
genetic mutations and epigenetic modifications (7).
Traditional Chinese Medicine has had an impact on
the tumor cell death pathway, which may guide tumor treatment
decisions and clinical management (8). Nowadays, compounds derived from
natural medicines with unique and diverse chemical entities still
constitute a considerable resource for developing novel
medicaments. Rotundic acid (RA) belongs to the pentacyclic
triterpenoid family and is mainly identified in Ilex rotunda
(I. rotunda), Ilex purpurea, Ilex integra and
other Aquifoliaceae plants which are widely distributed in
China (9). RA was also isolated
from Mussaenda Pubescens and Guettarda platypoda of
the Rubiaceae family (10,11).
Olea europaea and Planchonella duclitan, which are
part of the Oleaceae and Sapotaceae families,
respectively, also contain RA (12,13).
Xu et al (14) demonstrated
that RA, as one of a number of isolated compounds, demonstrated
anticancer activity. Li et al (13) also reported that RA exerted
cytotoxicity, with half maximal inhibitory concentration
(IC50) values of 9.5 µM, when it was applied on
the MCF-7 cell line, but the exact mechanism underlying this effect
remain unclear. Although RA exerted potential antitumor activity,
its free carboxylic acid may result in problems when administered
in vivo (15).
Radiation therapy is a standard treatment for local
breast cancer. Adjuvant radiotherapy following breast conserving
surgery may reduce the 10-year risk of first recurrence from 35.0
to 19.3% and 15-year risk from 25.2 to 21.4% (16). However, high-dose and large-field
radiotherapy may result in side effects including radiation
dermatitis, lymphedema, lung toxicity, long-term cardiac toxicity
and thyroid toxicity (17,18). Chemotherapy and radiotherapy have
effects on normal and tumor cells, which means patient have to
suffer concurrent side effects, and the toxicity is dose-dependent
(19-23). Therefore, identifying methods to
reduce the dose of chemotherapy and radiotherapy, without affecting
their therapeutic efficiency, is particularly important to tumor
therapy. p53 serves a key role in the process of radiation
response, controlling the activation of DNA repair and cell
apoptosis pathways following acute radiation injury (24,25).
Therefore, novel therapeutic methods or novel antitumor methods for
breast cancer may be identified by studying the p53 pathway
(26). The results of combined
effect of chemotherapy and radiotherapy will be beneficial for
cancer therapy by reducing the side effects. As an important
sensor, p53 may be a useful checkpoint for toxicity and radiation
response. The present study was to investigate whether RA combined
with radiotherapy exerts an effect on MCF-7 cells, which are a p53
gene wild-type cell line and are suitable for p53-dependent
mechanism study. It may present as a potential antitumor drug
combined with radiotherapy for the treatment of the breast cancer
disease by reducing side effects.
Materials and methods
Reagents
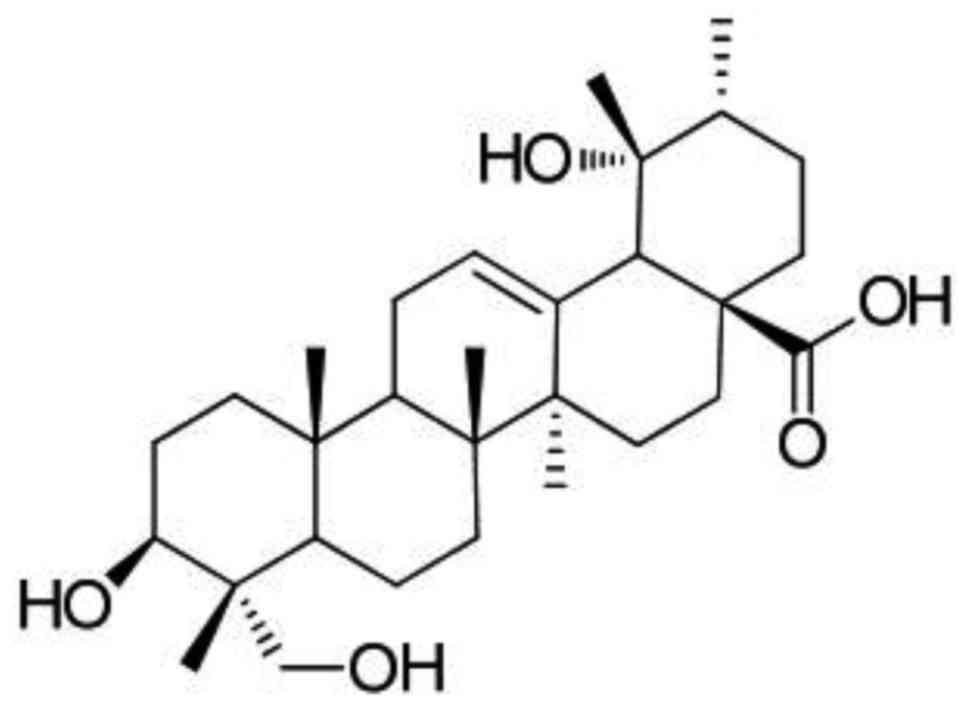
RA (Fig. 1) was
isolated and purified from I. rotunda, as reported in a
previous study (27). Its purity
was determined to be ≥98% using a high-performance liquid
chromatography (HPLC) assay and the extraction yield of RA was up
to 100 mg/g. The HPLC system consisted of a Beckman 114 M solvent
delivery module (Beckman Coulter, Inc., Brea, CA, USA), a Rheodyne
syringe loading injector (model 9725) with a 100 µl PEEK
injection loop (Dionex Corporation, Sunnyvale, CA, USA), a Shimadzu
ultraviolet spectrophotometric detector (UV VIS SPD-20A; Man-Tech
Associates Inc., Guelph, ON, Canada), and a Hewlett-Packard HP3395
Integrator (Agilent Technologies, Inc., Santa Clara, CA, USA).
Chromatographic separation was achieved on a 3 mm 110 A 150×3.0 mm
i.d. C18 reversed phase analytical column oven at 35°C
(Gemini®-NX; Phenomenex, Torrance, CA, USA) coupled with
a 5 µm 4.0×3.0 mm i.d. C18 reversed phase guard column
(Security® Guard Cartridges; Phenomenex) using a mobile
phase consisting of a mixture of 0.005 M potassium phosphate buffer
at pH 7.2 (KH2PO4):acetonitrile:methanol
(23:7:70). A total of 5 µl sample was injected into the
column every time. The flow rate used was 0.3 ml/min.
MTT kit and RNase were purchased from Sigma-Aldrich
(Merck KGaA, Darmstadt, Germany). The propidium iodide (PI) and
Annexin V-fluorescein isothiocyanate (FITC) Apoptosis Detection kit
was purchased from BD Biosciences (Franklin Lakes, NJ, USA).
Dulbecco’s modified Eagle’s medium (DMEM), trypsin, fetal bovine
serum (FBS), PBS, penicillin and streptomycin were obtained from
Gibco (Thermo Fisher Scientific, Inc., Waltham, MA, USA). RA was
dissolved in PBS (pH 7.2) to prepare a stock solution at a
concentration of 1.0 mM which was stored at −20°C. DMEM complete
medium was added to dilute the RA to the 2.0, 5.0 and 12.5
µM prior to use.
Cell cultivation and treatments
Human breast cancer cell line MCF-7 was purchased
from American Type Culture Collection (Manassas, VA, USA). Cells
were maintained in DMEM supplemented with 10% FBS and 1%
antibiotics. (penicillin-streptomycin). All cells were cultured at
37°C in a humidified incubator with a constant air flow of 5%
CO2. In order to inhibit the function of
ataxia-telangiectasia mutated (ATM), 5 mM caffeine (Sigma-Aldrich;
Merck KGaA) dissolved in distilled water were added to the cell
culture media 2 and 24 h prior to irradiation. Media containing
this inhibitor were replaced with fresh medium immediately
following irradiation.
Irradiation strategy
Monolayer cells were ionizing irradiated at the dose
rate of 500 mGy/min using the X-RAD 320 X-Ray system (Precision
X-Ray, North Branford, CT, USA). The total doses used were 0.5, 2.0
and 10.0 Gy. Subsequently, the cell culture media was replaced and
cells were harvested immediately or continually cultured until the
next step experiment was performed. Control groups were treated
similarly but without irradiation.
Cell viability assay
The inhibitory effect of RA, irradiation or the two
combined on the viability of MCF-7 cells were detected via MTT
assays. All experiment steps were performed using cells seeded on
96-well plates at a density of 5×104/ml, and were
performed according to the manufacturer’s protocol (Sigma-Aldrich;
Merck KGaA). Briefly, the cells were seeded at a density of
5×104/ml at a volume of 200 µl per well. All
groups without or with RA (0.0, 2.0, 5.0 and 12.5 µM) were
incubated at 37°C for 24 h. Irradiation treatment was completed 2 h
prior RA administration. MTT reagent (1.0 mg/ml) was added to each
well, and the cells were incubated at 37°C for 4 h. The MTT
solution was then aspirated, and 100 µl dimethyl sulfoxide
was then added. The 96-well plates were read using a microplate
spectrophotometer (Synergy H1; BioTek Instruments, Inc., Winooski,
VT, USA) at 540 nm. The inhibition percentage was calculated as
(1-the value of absorbance in the experimental group/the value of
absorbance in the control group) ×100%.
Flow cytometry for cell apoptosis
Annexin V-FITC and PI double staining flow cytometry
analyses were employed to assess cell apoptosis. MCF-7 cells were
plated in 96-well plates containing 200 µl DMEM at a density
of 5×104 cells/well. The induction of apoptosis in the
MCF-7 cells were examined following treatment. The cell apoptosis
was analysed using a flow cytometer (FACScan; BD Biosciences) with
Flowjo 7.6 FACS analysis software (FlowJo LLC, Ashland, OR, USA).
Briefly, the MCF-7 cells were washed with cold PBS then resuspended
in 1× binding buffer [cat. no. 51-66121E; BD Biosciences; 0.1 M
HEPES/NaOH (pH 7.4), 1.4 M NaCl and 25 mM CaCl2] at a
density of 1×106 cells/ml. A total of 100 µl
solution (1×105 cells) was then transferred to a 5 ml
culture tube. Then, 5 µl Annexin V-FITC (cat. no. 51-65874X;
BD Biosciences) and 5 µl PI (cat. no. 51-66211E; BD
Biosciences) was added. The cells were gently vortexed and incubate
for 15 min at room temperature (25°C) in the dark. A total of 400
µl 1× binding buffer was then added to each tube, and the
cells were analysed using a flow cytometer within 1 h. The cells in
the different sections represented the different cell states as
follows: Late-apoptotic cells were present in the upper right
section, viable cells were present in the lower left section, and
the early apoptotic were cells present in the lower right
section.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RT-qPCR was performed using ABI Plus one (Applied
Biosystems; Thermo Fisher Scientific, Inc.) and the FastStart
Universal SYBR Green Master (Roche Diagnostics GmbH, Mannheim,
Germany). Total RNA was isolated from the MCF-7 cells using a
Qiagen RNAeasy kit (Qiagen, Inc., Valencia, CA, USA). cDNA was
synthesized with Superscript II reverse transcriptase using random
hexamer primers (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer’s protocol. The primers for p53, ATM
and GAPDH were designed and synthesized by Sangon Biotech Co., Ltd.
(Shanghai, China). An Applied Biosystems StepOne plus Fast
Real-Time PCR system was used to determine the mRNA levels of p53,
ATM and GAPDH (used as the internal control). The primer sequences
for p53 were as follows: Sense, 5ʹ-TTCCCACTGAGGAGTCCAAC-3ʹ and
antisense, 5ʹ-TTGT TCCCGAAACGCTGAG-3ʹ. The primer sequences for ATM
were as follows: Sense, 5ʹ-TTGCCTTGTATCTACTTTTG GGG-3ʹ and
antisense, 5ʹ-TCAACACTGTTATGTTTGTG GGT-3ʹ. The GAPDH primers were
as follows: Sense, 5ʹ-CCA GGTGGTCTCCTCTGACTT-3ʹ and antisense,
5ʹ-GTTGCTG TAGCCAAATTCGTTGT-3ʹ. Amplification was performed for 40
cycles with a denaturation temperature of 94°C (5 sec), annealing
temperature of 58°C (15 sec) and extension temperature of 74°C (10
sec) for p53, ATM and GAPDH in a thermal cycler (Veriti; Applied
Biosystems; Thermo Fisher Scientific, Inc.). The PCR products were
200 bp in length. Analysis of relative gene expression data was
performed using the 2−ΔΔCq method (28).
Western blotting
Cell total protein was extracted using
radioimmunoprecipitation buffer (Beyotime Institute of
Biotechnology, Shanghai, China) supplemented with a cocktail
protease inhibitor (Roche Molecular Diagnostics, Pleasanton, CA,
USA), and the protein concentration was determined using a BCA
protein assay kit (Beyotime Institute of Biotechnology) according
to the manufacturer’s protocol. A total of 5-40 µg cell
total protein was separated by 10% SDS-PAGE and then were
electrophoretically transferred to polyvinylidene fluoride
membranes (0.45 µm; EMD Millipore, Billerica, MA, USA) and
blocked at 37°C for 1 h with 5% skim milk in Tris-buffered saline
(TBS) with Tween-20 (0.1%). Subsequently, membranes were incubated
with monoclonal antibodies against p53 (cat. no. 2254; 1:1,000),
ATM (cat. no. 92356; 1:1,000), B-cell lymphoma 2 (Bcl-2; cat. no.
4223; 1:1,000), Bcl-2-associated X protein (Bax; cat. no. 2774;
1:1,000) and β-actin (cat. no. 3700; 1:1,000) at 4°C overnight. The
primary antibodies used were all obtained from Cell Signaling
Technology, Inc. (Danvers, MA, USA). Subsequent to washing 3 times
for 5 min each with TBS, membranes were incubated with horseradish
peroxidase-conjugated goat anti-mouse (cat. no. TA130001) or goat
anti-rabbit (cat. no. TA130015) second antibodies (1:2,000; OriGene
Technologies, Inc., Beijing, China) at 37°C for 1 h. Subsequent to
washing another 3 times, the immunocomplexes were detected using an
enhanced chemiluminescence system (Thermo Fisher Scientific, Inc.)
and X-ray film (Kodak, Rochester, NY, USA). Protein expression
levels were determined semi-quantitatively by densitometric
analysis with the Quantity One software (V4.62, Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
All data and results were calculated from at least
three replicate measurements and presented as the mean ± standard
deviation. The differences between experimental groups and the
control group were determined using a two-way analysis of variance
followed by a Dunnett’s t-test using SPSS version 20.0 (IBM Corp.,
Armonk, NY, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
RA and irradiation inhibit MCF-7 cell
viability
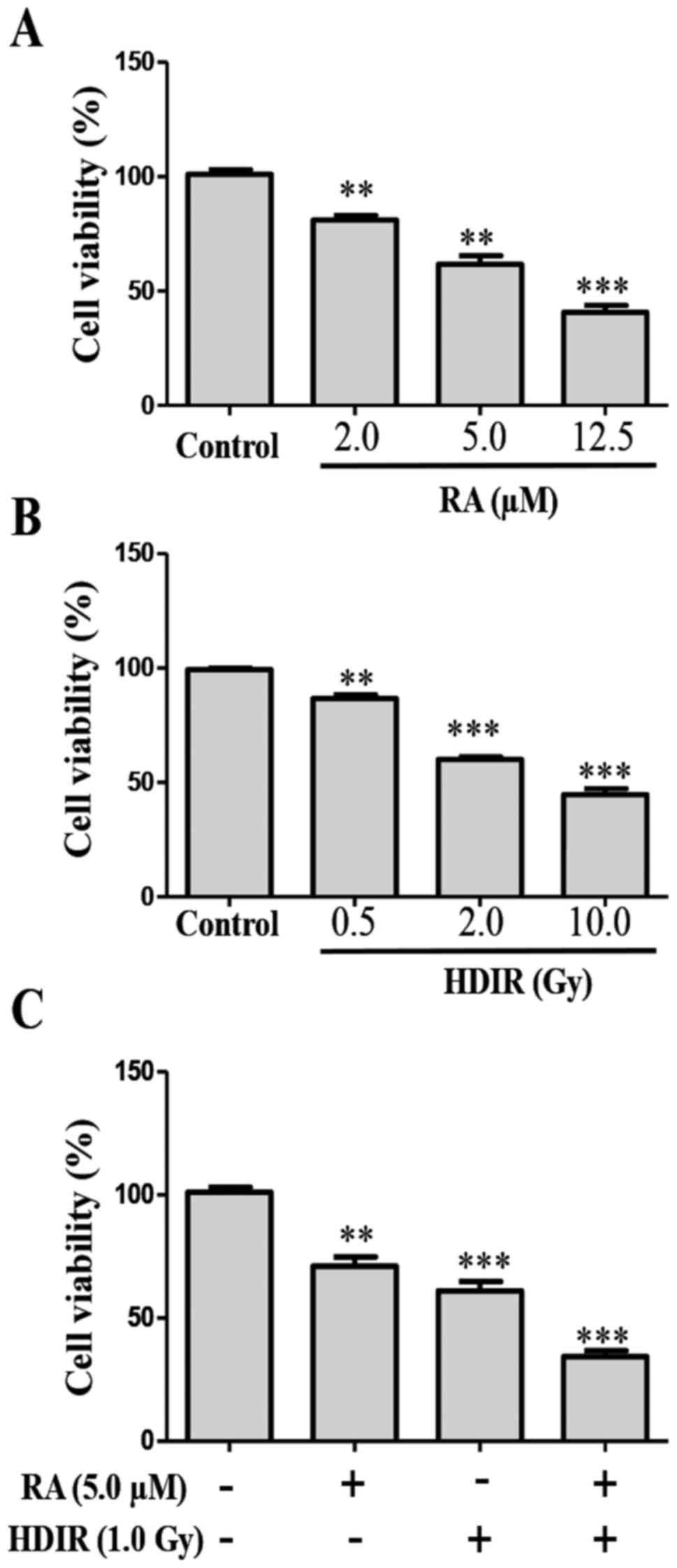
The inhibitory effect of RA and irradiation on the
viability of MCF-7 cells were detected via an MTT assay. The
structure of RA is presented in Fig.
1. MTT detection revealed that RA significantly inhibited the
viability of MCF-7 cells compared with the control cells
(P<0.01). The inhibitory effects of RA on MCF-7 cells were
dose-dependent within the ranges of 2.0-12.5 µmol/l.
Treatment with RA at 12.5 µmol/l elicited the greatest
inhibitory effect, with a cell viability of 41.3% compared with
that of the blank control group (Fig.
2A; P<0.001). The irradiation experiment revealed that 0.5,
2.0 and 10.0 Gy doses of high dose irradiation (HDIR) significantly
inhibited the growth of MCF-7 cells compared with the
sham-irradiated group (Fig. 2B;
P<0.01). Among them, the 10.0 Gy irradiation had the most
significant effect (P<0.001). In order to test whether RA
exerted a combined effect with radiation on MCF-7 cells, low doses
of RA (5 µM) and radiation (1 Gy) doses were used in
combination. Suprisingly, RA combined with irradiation exerted a
significantly greater inhibitory effect on MCF-7 cell proliferation
compared with no RA or irradiation (Fig 2C; P<0.001).
RA and irradiation induce apoptosis in
MCF-7 cells
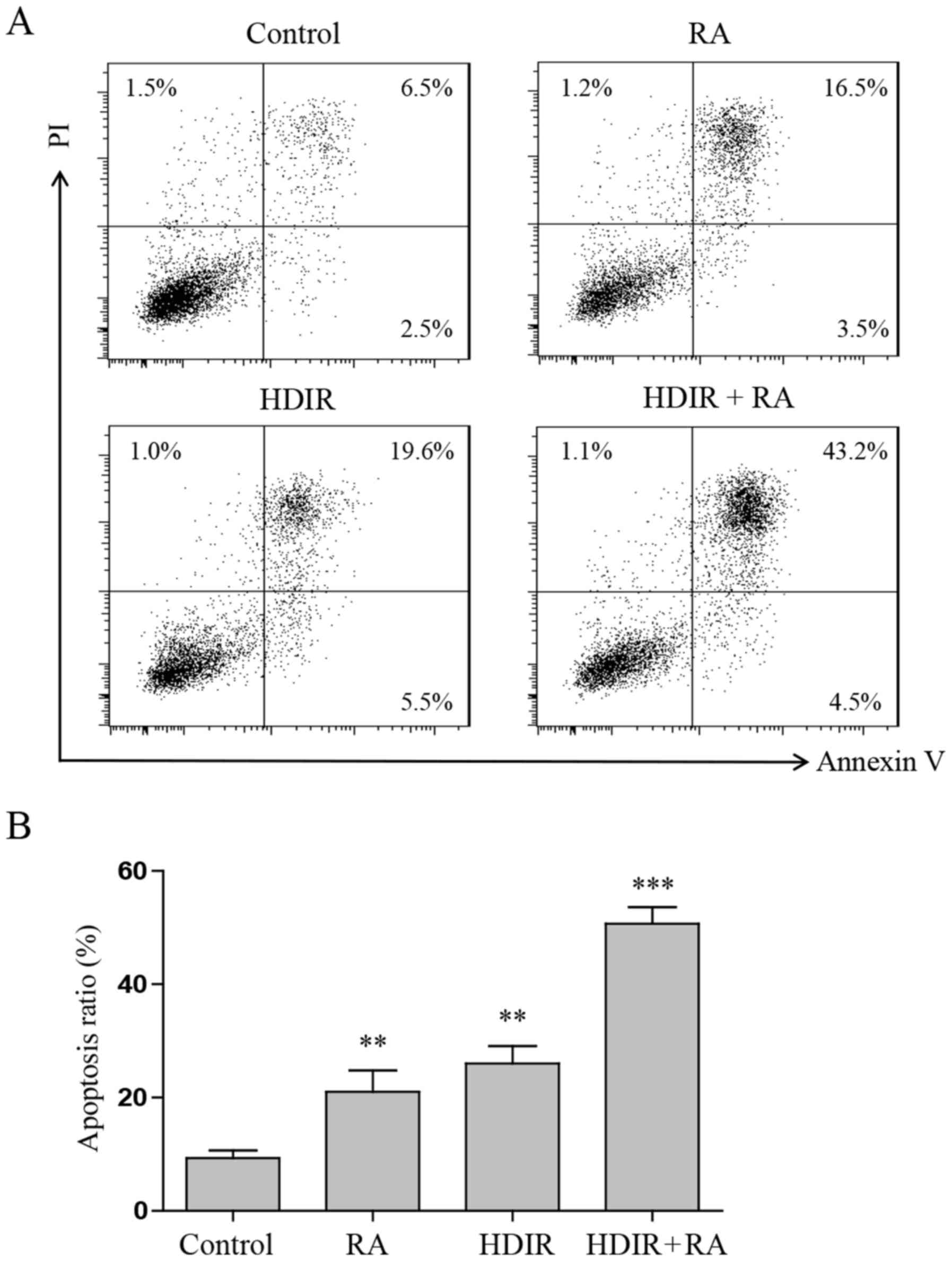
To investigate whether RA and irradiation also
induced MCF-7 cell apoptosis when combined, an Annexin V-FITC and
PI double staining assay was performed. MCF-7 cells were treated
with irradiation, RA or the two combined, and then analysed using
flow cytometry. Fig. 3A and B
indicate that compared with either treatment alone, the numbers of
early and late apoptotic cells increased significantly in the RA
and irradiation combined treatment groups (P<0.001). Treatment
with RA at 5 µM elicited a low apoptotic function
(21.3±2.6%; P<0.01), and treatment with irradiation at 1 Gy
elicited a greater apoptosis function (27.2±3.8%; P<0.01).
However, RA combined with irradiation groups elicited the greatest
apoptotic capacity, which reached 51.2±4.5%.
ATM/p53 pathway is involved in the effect
of RA and irradiation combined in MCF-7 cell apoptosis
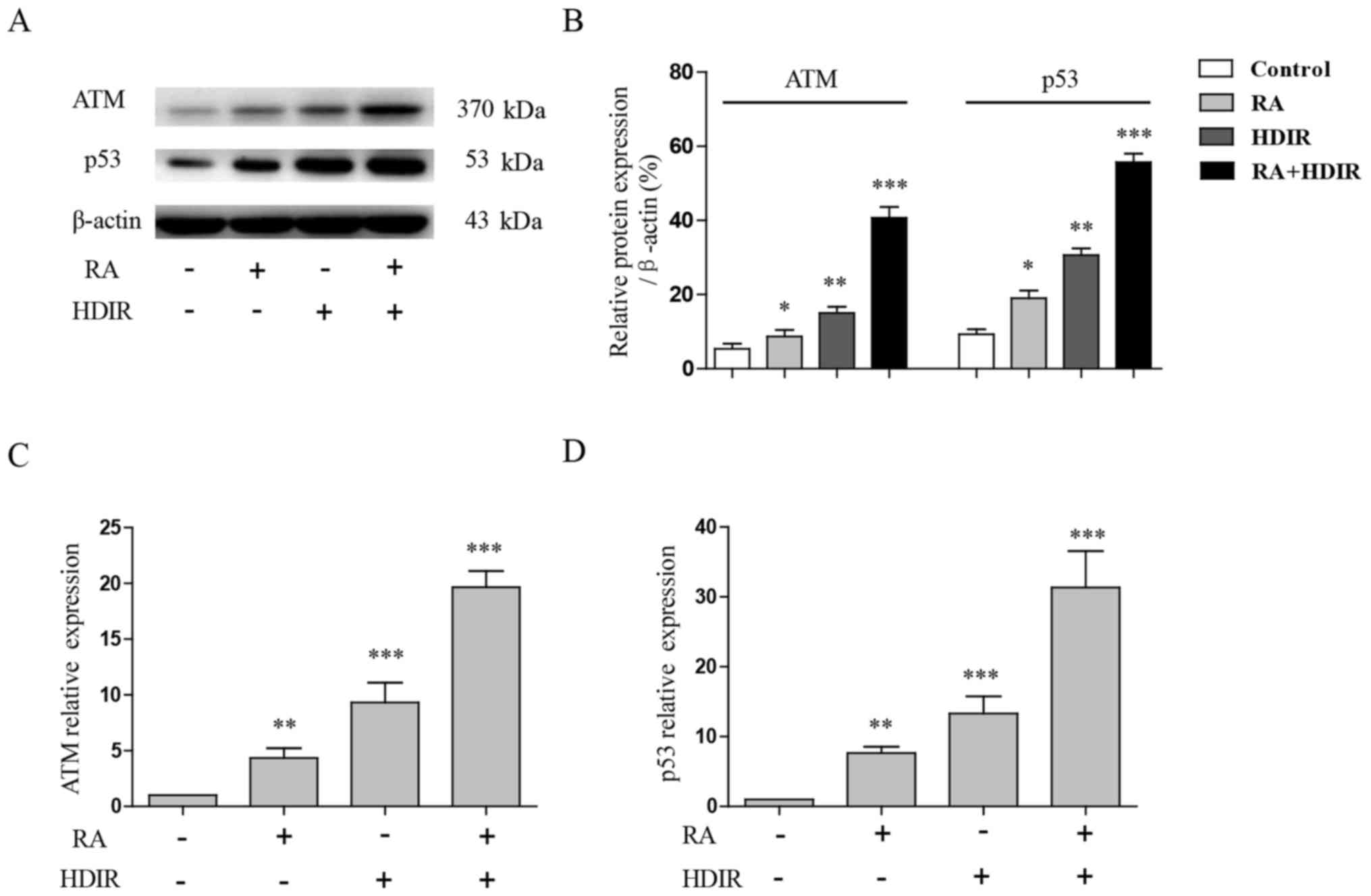
The present study further examined the expression of
ATM and p53, which are also well known as critical DNA damage and
radiation response sensors in mammalian cells (29). At 4 h post-irradiation, RA or the
two treatments combined, the whole cell proteins of MCF-7 cells
were extracted and separated by 4-8% SDS-PAGE, and the expression
of ATM and p53 were detected by western blotting. The expression of
ATM and p53 in MCF-7 cells were revealed to be significantly
upregulated by irradiation or RA treatment compared with the
control (P<0.05), but the combined use of irradiation and RA
exerted the most significant effect (P<0.001; Fig. 4A and B). A RT-qPCR experiment
confirmed that the gene expression of ATM and p53 were also
significantly induced by RA and irradiation in Fig. 4C and D (P<0.01). Altogether, the
results revealed that the ATM/p53 pathway serves a critical role in
the effect of RA and irradiation combined on the apoptosis of MCF-7
cells.
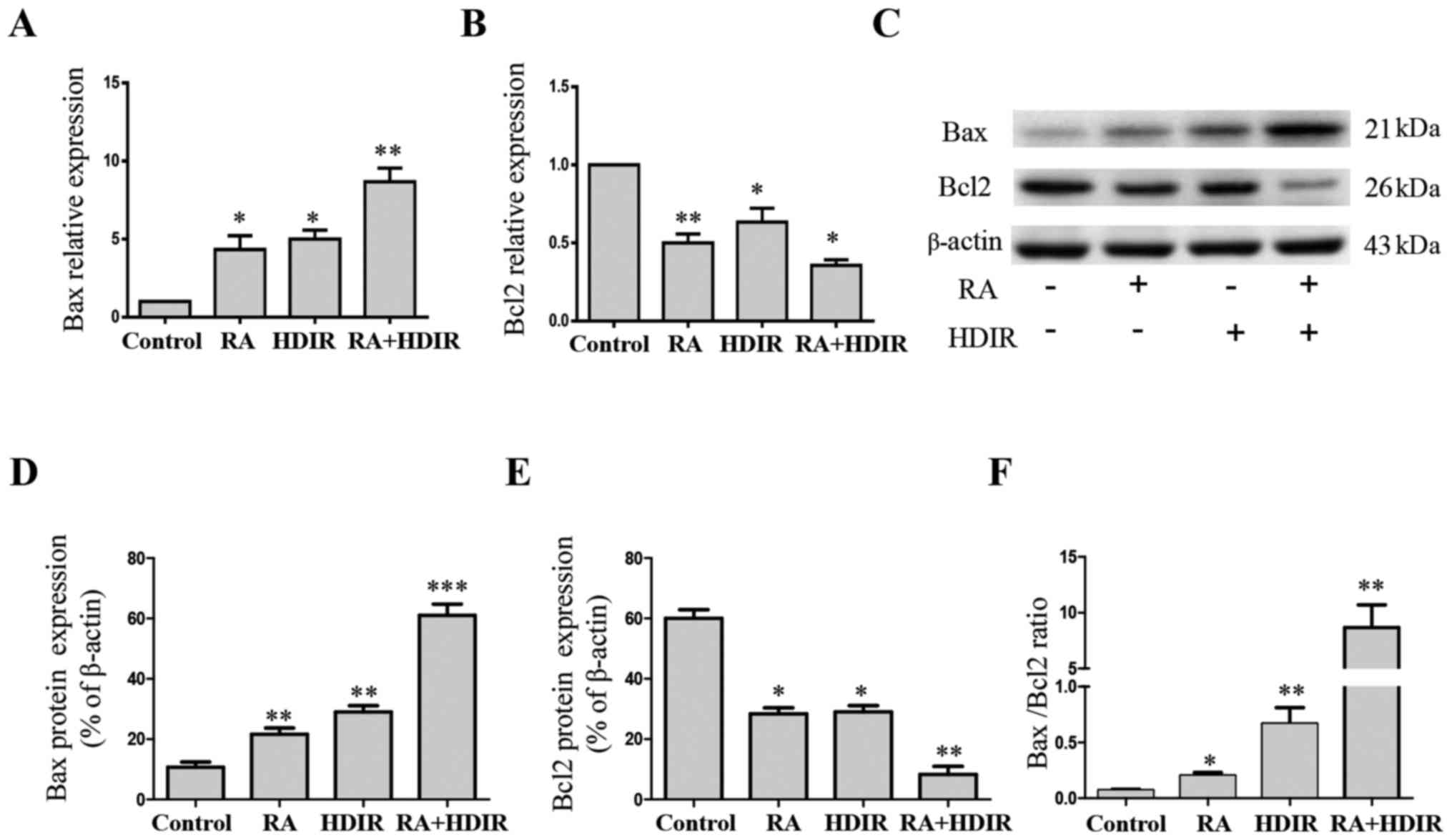
RA and irradiation activate the
Bax/mitochondria cell apoptosis pathway in MCF-7 cells
Previously, antitumor mechanisms have mainly focused
on their ability to trigger apoptosis. Apoptosis, known as
programmed cell death, is closely associated with numerous
anticancer reagents. When patients with cancer undergo
chemotherapy, proapoptotic Bax may be upregulated in response to
sensing DNA damage; and Bax in turn stimulates mitochondria to
release cytochrome c. Cytochrome c is the major
inducer of mitochondria apoptosis pathway (30). The protein expression of Bax and
Bcl-2 were detected by western blotting. As presented in Fig. 5, RA and irradiation alone were able
to significantly increase the gene (Fig. 5A; P<0.05) and protein (Fig. 5C and D; P<0.01) expression of
Bax and significantly decrease the gene (Fig. 5B; P<0.05) and protein (Fig. 5C and E; P<0.05) expression of
Bcl-2 compared with the control. The combined use of RA and
irradiation amplified the effect further (P<0.05). The ratio of
Bax to Bcl-2 has been used in numerous studies (31,32)
in order to evaluate the level of apoptosis. In the present study,
RA and irradiation combined significantly increased this ratio by
elevating Bax expression and decreasing Bcl-2 expression in
Fig. 5F (P<0.01).
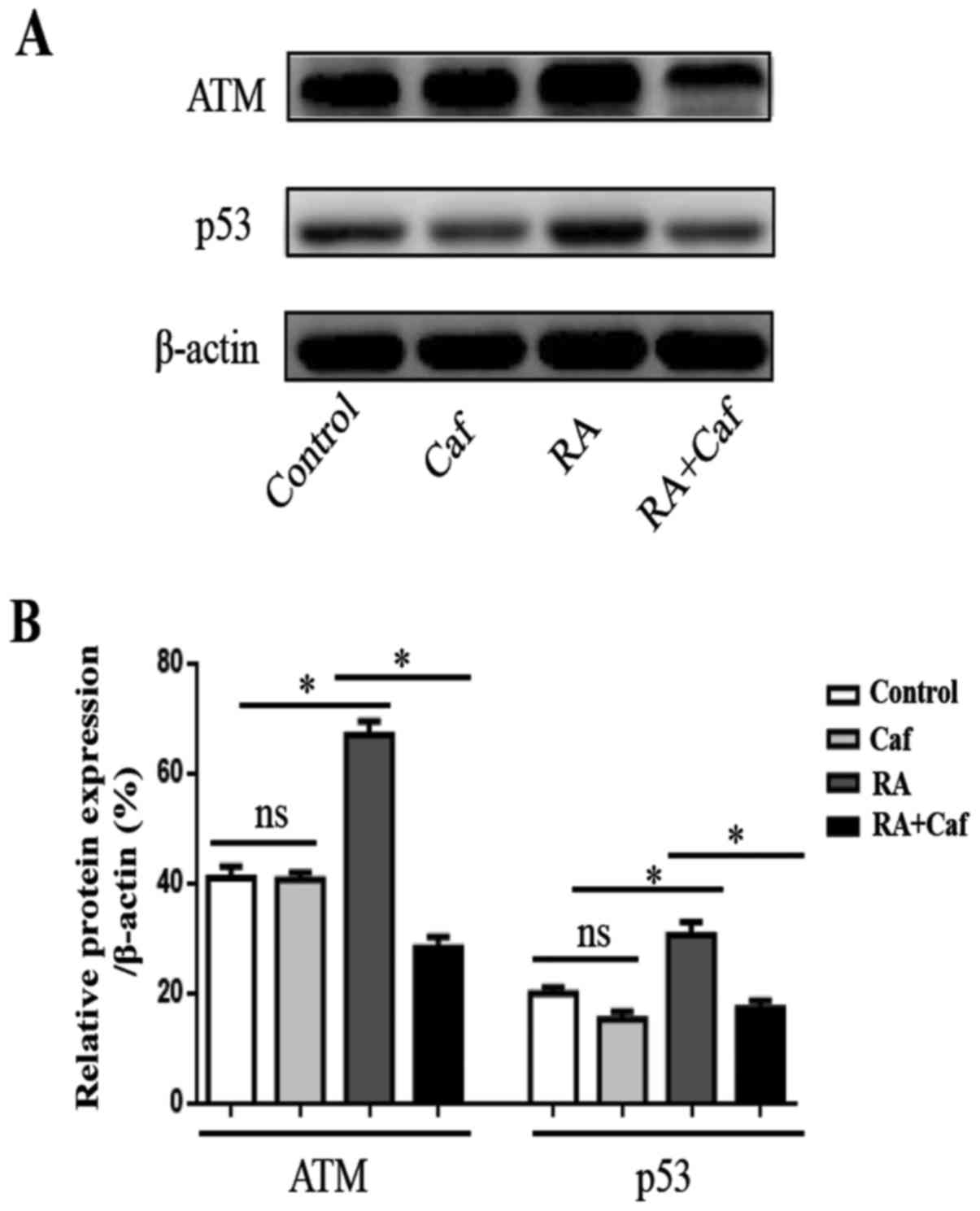
ATM inhibitor restores RA and
irradiation-induced apoptosis in MCF-7 cells
To further address the function of the ATM pathway
in RA and irradiation combined apoptosis induction in MCF-7 cells,
caffeine was used to block the function of ATM. MCF-7 cells were
treated without RA, caffeine or RA alone and RA and caffeine
combined. The results revealed that caffeine does not affect ATM
expression, but significantly inhibits p53 expression in MCF-7
cells compared with untreated cells (P<0.05; Fig. 6A and B). Subsequent to the blocking
of ATM with caffeine, the expression of ATM, p53, Bax and Bcl-2
were measured by western blotting. It was revealed that the
expression of ATM, p53 and Bax was decreased significantly but the
Bcl-2 expression was increased significantly in cells treated with
RA and irradiation combined in addition to caffeine compared with
cells treated without caffeine (Fig.
7A and B; P<0.001). These results revealed that ATM is
involved in mitochondrial apoptotic activity. Further apoptosis
detection revealed that following treatment with caffeine, the
viability inhibition in MCF-7 cells caused by RA and irradiation
was abolished (Fig. 7C and D;
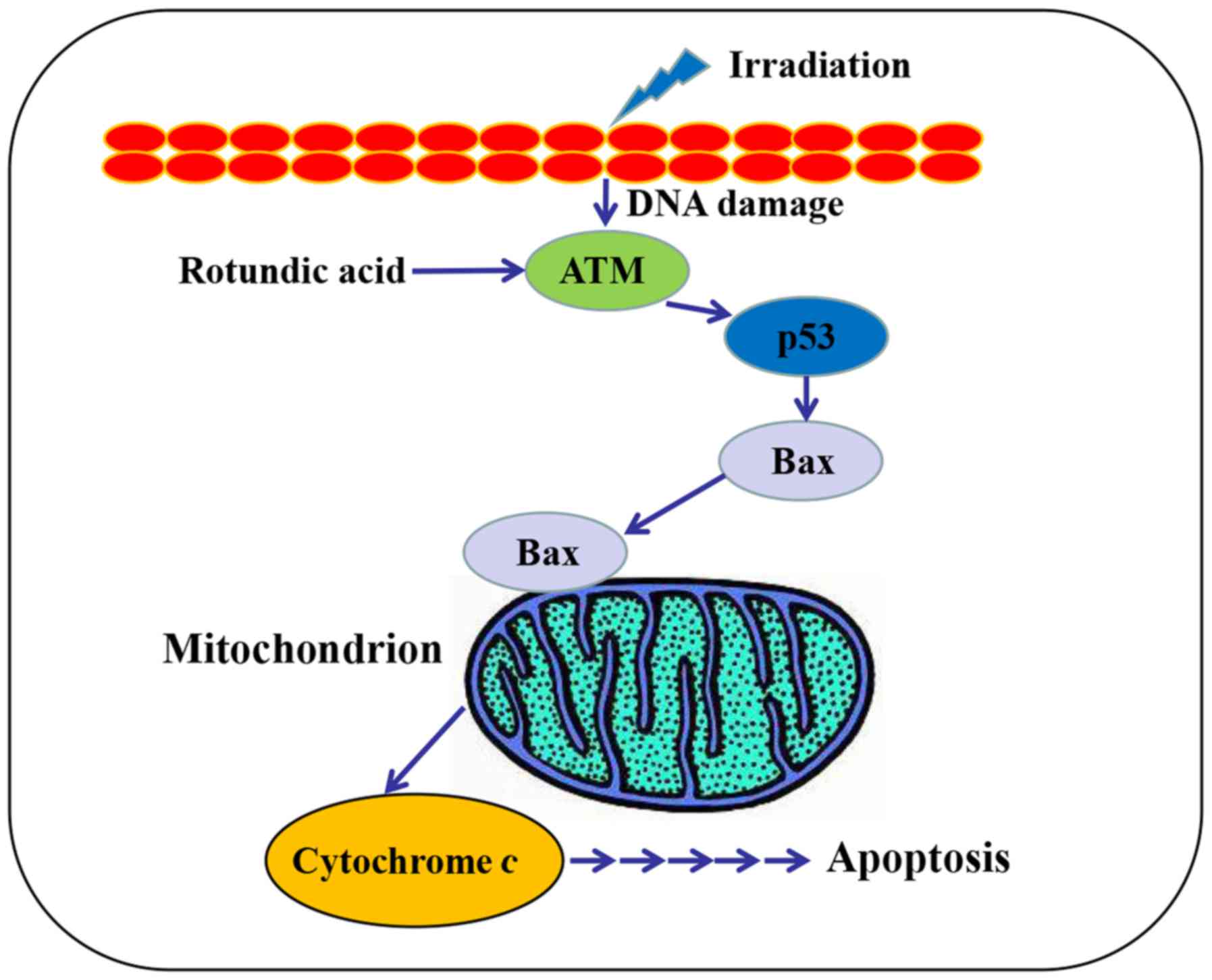
P<0.001). Together, these data suggest the involvement of the
ATM/p53 pathway in the RA and irradiation combined-induced
apoptosis of MCF-7 cells (Fig.
8).
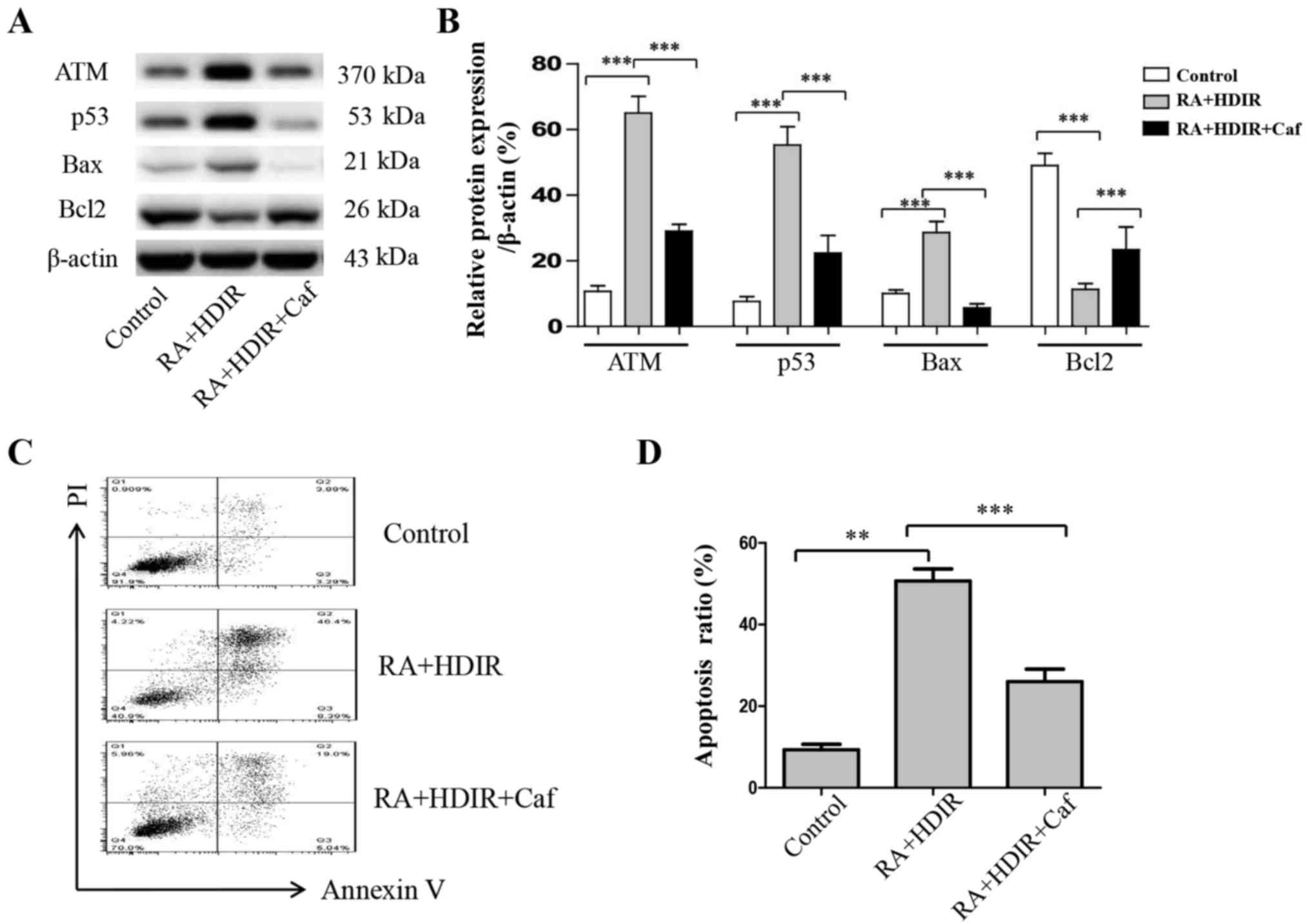 | Figure 7ATM inhibitor counteracts the RA and
irradiation synergistical apoptosis induction in MCF-7 cells. MCF-7
cells were treated with RA (1.0 µM) and HDIR (1 Gy) for 24 h
subsequent to blocking with caffeine (an ATM inhibitor). (A) The
protein expression of ATM, p53, Bax and Bcl-2 were measured by
western blotting. (B) Quantitation of ATM, p53, Bax and Bcl-2
protein expression levels. (C) Flow cytometric analysis of
apoptosis of MCF-7 cells. (D) Percentage of apoptotic cells. The
data are expressed as the mean ± the standard deviation of three
experiments. **P<0.01 and ***P<0.001
vs. control. ATM, ataxia-telangiectasia mutated; Caf, caffeine; RA,
rotundic acid; HDIR, high dose irradiation; Bcl-2, B-cell lymphoma
2; Bax, Bcl-2-associated X protein; PI, propidium iodide. |
Discussion
Breast cancer is a common malignant cancer globally
and has threatened the health of women over the past few decades
(33). There are a number of
dangerous internal and external factors affecting the biology of
tumor cells during the occurrence and progression of breast cancer
(34). Oncogene disorder in the
body has emerged as an increasingly important factor in the
development of cancer. The metastasis and prognosis of breast
cancer are associated with the mutation and abnormal expression of
oncogenes (35). p53 is an
important tumor suppressor gene and is present in the majority of
normal cells, but is often mutated in cancer cells. One previous
study revealed that the p53 gene mutation is associated with the
resistance of breast cancer (36).
Li et al (13) also
reported that RA exerted cytotoxicity, with IC50 values
of 9.5 µM when it was applied to MCF-7 cells, but the exact
underlying mechanisms remains unknown.
Although there are sufficient sources for the
extraction of RA in China, as aforementioned, there remain few
reports on its bioactivity due to little interest from
pharmacological researchers. In an open patent, a substantial
quantity of RA was isolated and purified from I. rotunda
(37). Since RA may be a potential
native anticancer drug with sufficient sources, a previous study
has investigated and applied for a series of patents regarding RA
and its derivatives during the past few years to investigate and
make use of this compound (38).
Radiation therapy is a standard treatment for local breast cancer.
Adjuvant radiotherapy following breast conservation surgery may
reduce the 10-year risk of first recurrence. However, high-dose and
large-field radiotherapy may result in side effects. Chemotherapy
and radiotherapy have effects on normal and tumor cells, which
means patient have to suffer concurrent side effects, and the
toxicity is always dose-dependent (39). Therefore, identifying methods to
reduce the dose of chemotherapy and radiotherapy, without affecting
their therapeutic efficiency, is particularly important to tumor
therapy (40). The present study
aimed to investigate the combined effect of RA and radiotherapy,
which may be beneficial for cancer therapy by reducing the side
effects. Initially, the inhibitory effect of RA and irradiation on
the viability of MCF-7 cells were detected via an MTT assay. MTT
detection and flow cytometry revealed that RA significantly
inhibited the viability of MCF-7 cells. The inhibitory effects of
RA on MCF-7 cells were dose-dependent within the ranges of 2.0-12.5
µmol/l. An irradiation experiment revealed that 0.5, 2.0 and
10.0 Gy doses of HDIR significantly inhibited the growth of MCF-7
cells compared with the sham-irradiated group. In order to assess
whether RA exerts a combined function with radiation on MCF-7
cells, low doses of RA (5 µM) and radiation (1 Gy) were
used. Surprisingly, RA combined with the irradiation demonstrated
the greatest inhibitory effect on MCF-7 cell proliferation.
ATM is a crucial factor involved in the processing
of DNA damage, maintenance of genome stability and control of cell
cycle progression. According to Suzuki et al (41) phosphorylated ATM foci were detected
immediately once normal human diploid cells were radiated. As a
radiation sensor, the activation of ATM has been proved to be an
early event of activated molecules and will interact with a number
of cell signaling pathways, including the AKT and mitogen-activated
protein kinase kinase/extracellular signal-regulated kinase
pathways (42). p53 is a key
factor in the process of radiation response, controlling the
activation of DNA repair and cell apoptosis pathways subsequent to
acute radiation injury (25). The
present study further examined the expression of ATM and p53, which
are also well known as critical DNA damage and radiation response
sensors in mammalian cells. It was revealed that the expression of
ATM and p53 in MCF-7 cells were upregulated by irradiation or RA
treatment alone, but the combined use of irradiation and RA
demonstrated substantially higher levels of ATM and p53. The
ATM/p53 pathway serves a critical role in RA and
irradiation-induced MCF-7 cell apoptosis. When a patient with
cancer undergoes chemotherapy, proapoptotic Bax may be upregulated
in response to sensing DNA damage. Bax in turn stimulates
mitochondria to release cytochrome c. Cytochrome c is
the major inducer of mitochondria (30). The ratio of Bax to Bcl-2 has been
used in numerous studies in order to evaluate the level of
apoptosis (43,44). The protein expression of Bax and
Bcl-2 were detected in the present study. RA and irradiation alone
may increase the expression of Bax and decrease Bcl-2 expression.
The combined use of RA and irradiation amplifies this effect.
To further address the function of the ATM/p53
pathway in RA and irradiation-induced apoptosis in MCF-7 cells,
caffeine was used to block the function of ATM. Subsequent to the
blocking of ATM with caffeine, the expression of ATM was not
decreased, but the expression of p53 and Bax decreased, and Bcl-2
expression was increased. These results demonstrated that ATM was
involved in mitochondrial apoptotic activity.
Cancer stem cells are a hot topic in current cancer
research. Breast cancer stem cells display different proliferation
and inhibition mechanisms to breast cancer cell lines. Future
studies will examine the effects of RA and irradiation alone and
combined on breast cancer stem cells, which will be more effective
for understanding and curing breast cancer. In summary, the present
study revealed that the ATM/p53 pathway directly participates in
the radiation and RA combined induction of MCF-7 cell apoptosis. RA
has potential for development as a novel drug for the treatment of
human breast cancer combined with radiation therapy, thereby
reducing the concurrent side effects.
Funding
The present study was supported by the Special
Financial Grant from the China Postdoctoral Science Foundation
(grant no. 2018T110251), the Jilin Provincial Natural Science
Foundation of China (grant no. 20140520014JH to Dr Hai-Jun Li; and
grant no. 20180101135JC to Dr Hong-Mei Xu), the Interdisciplinary
Chemistry and Medicine Foundation of Jilin University (grant no.
JDYYJCHX004) and the National Natural Science Foundation of China
(grant no. 31470418 to Y.H.).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors’ contributions
ZW and WS planed and performed the experiments,
analyzed the data and wrote the manuscript. DY, YZ, HX and YH
performed the experiments. HL designed, interpreted and funded the
study, and wrote the manuscript.
Ethics approval and consent to
participate
The present study was approved by the medical ethics
committee of The First Hospital of Jilin University (Changchun,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Metzger-Filho O, de Azambuja E, Bradbury
I, Saini KS, Bines J, Simon SD, Dooren VV, Aktan G, Pritchard KI,
Wolff AC, et al: Analysis of regional timelines to set up a global
phase III clinical trial in breast cancer: The adjuvant lapatinib
and/or trastuzumab treatment optimization experience. Oncologist.
18:134–140. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bower JE, Greendale G, Crosswell AD, Garet
D, Sternlieb B, Ganz PA, Irwin MR, Olmstead R, Arevalo J and Cole
SW: Yoga reduces inflammatory signaling in fatigued breast cancer
survivors: A randomized controlled trial. Psychoneuroendocrinology.
43:20–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pu Z, Zhang X, Chen Q, Yuan X and Xie H:
Establishment of an expression platform of OATP1B1 388GG and 521CC
genetic polymorphism and the therapeutic effect of tamoxifen in
MCF-7 cells. Oncol Rep. 33:2420–2428. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rodin D, Knaul FM, Lui TY and
Gospodarowicz M: Radiotherapy for breast cancer: The predictable
consequences of an unmet need. Breast. 29:120–122. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fisher B, Anderson S, Bryant J, Margolese
RG, Deutsch M, Fisher ER, Jeong JH and Wolmark N: Twenty-year
follow-up of a randomized trial comparing total mastectomy,
lumpectomy, and lumpectomy plus irradiation for the treatment of
invasive breast cancer. N Engl J Med. 347:1233–1241. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim JJ and Tannock IF: Repopulation of
cancer cells during therapy: An important cause of treatment
failure. Nat Rev Cancer. 5:516–525. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Peitzsch C, Kurth I, Kunz-Schughart L,
Baumann M and Dubrovska A: Discovery of the cancer stem cell
related determinants of radioresistance. Radiother Oncol.
108:378–387. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gerber DE: Targeted therapies: A new
generation of cancer treatments. Am Fam Physician. 77:311–319.
2008.PubMed/NCBI
|
|
9
|
Haraguchi H, Kataoka S, Okamoto S, Hanafi
M and Shibata K: Antimicrobial triterpenes from Ilex integra and
the mechanism of antifungal action. Phytother Res. 13:151–156.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao WM, Wolfender JL, Hostettmann K,
Cheng KF, Xu RS and Qin GW: Triterpenes and triterpenoid saponins
from Mussaenda pubescens. Phytochemistry. 45:1073–1078. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bhattacharyya J and de Almeida MZ:
Isolation of the constituents of the root-bark of Guettarda
platypoda. J Nat Prod. 48:148–149. 1985. View Article : Google Scholar
|
|
12
|
Saimaru H, Orihara Y, Tansakul P, Kang YH,
Shibuya M and Ebizuka Y: Production of triterpene acids by cell
suspension cultures of Olea europaea. Chem Pharm Bull (Tokyo).
55:784–788. 2007. View Article : Google Scholar
|
|
13
|
Lee TH, Juang SH, Hsu FL and Wu CY:
Triterpene acids from the leaves of Planchonella duclitan (Blanco)
Bakhuizan. J Chin Chem Soc (Taipei). 52:1275–1280. 2005. View Article : Google Scholar
|
|
14
|
Kim MH, Park KH, Oh MH, Kim HH, Choe KI,
Park SH and Lee MW: Two new hemiterpene glycosides from the leaves
of Ilex otunda. Thunb Arch Pharm Res. 35:1779–84. 2012. View Article : Google Scholar
|
|
15
|
Lin FP, Shao JW, Du HD, Dai YC and Wang T:
Synthesis, characterization and anti-tumor activity of ursolic acid
derivatives. Chin J Appl Chem. 27:893–898. 2010.Chinese.
|
|
16
|
Darby S, McGale P, Correa C, Taylor C,
Arriagada R, Clarke M, Cutter D, Davies C, Ewertz M, Godwin J, et
al Early Breast Cancer Trialists’ Collaborative Group (EBCTCG):
Effect of radiotherapy after breast-conserving surgery on 10-year
recurrence and 15-year breast cancer death: Meta-analysis of
individual patient data for 10,801 women in 17 randomised trials.
Lancet. 378:1707–1716. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Darby SC, Ewertz M, McGale P, Bennet AM,
Blom-Goldman U, Brønnum D, Correa C, Cutter D, Gagliardi G, Gigante
B, et al: Risk of ischemic heart disease in women after
radiotherapy for breast cancer. N Engl J Med. 368:987–998. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hayes SB, Freedman GM, Li T, Anderson PR
and Ross E: Does axillary boost increase lymphedema compared with
supraclavicular radiation alone after breast conservation? Int J
Radiat Oncol Biol Phys. 72:1449–1455. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sandoo A, Kitas GD and Carmichael AR:
Breast cancer therapy and cardiovascular risk: Focus on
trastuzumab. Vasc Health Risk Manag. 11:223–228. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lian L, Li W, Li ZY, Mao YX, Zhang YT,
Zhao YM, Chen K, Duan WM and Tao M: Inhibition of MCF-7 breast
cancer cell-induced platelet aggregation using a combination of
antiplatelet drugs. Oncol Lett. 5:675–680. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li W, Liang RR, Zhou C, Wu MY, Lian L,
Yuan GF, Wang MY, Xie X, Shou LM, Gong FR, et al: The association
between expressions of Ras and CD68 in the angiogenesis of breast
cancers. Cancer Cell Int. 15:172015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shou LM, Zhang QY, Li W, Xie X, Chen K,
Lian L, Li ZY, Gong FR, Dai KS, Mao YX, et al: Cantharidin and
norcantharidin inhibit the ability of MCF-7 cells to adhere to
platelets via protein kinase C pathway-dependent downregulation of
α2 integrin. Oncol Rep. 30:1059–1066. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Majeed W, Aslam B, Javed I, Khaliq T,
Muhammad F, Ali A and Raza A: Breast cancer: Major risk factors and
recent developments in treatment. Asian Pac J Cancer Prev.
15:3353–3358. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee CL, Blum JM and Kirsch DG: Role of p53
in regulating tissue response to radiation by mechanisms
independent of apoptosis. Transl Cancer Res. 2:412–421. 2013.
|
|
25
|
Menon V and Povirk L: Involvement of p53
in the repair of DNA double strand breaks: Multifaceted roles of
p53 in homologous recombination repair (HRR) and non-homologous end
joining (NHEJ). Subcell Biochem. 85:321–336. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hu D, Su C, Jiang M, Shen Y, Shi A, Zhao
F, Chen R, Shen Z, Bao J and Tang W: Fenofibrate inhibited
pancreatic cancer cells proliferation via activation of p53
mediated by upregulation of LncRNA MEG3. Biochem Biophys Res
Commun. 471:290–295. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
He YF, Nan ML, Sun JM, Meng ZJ, Yue FG,
Zhao QC, Yang XH and Wang H: Synthesis, characterization and
cytotoxicity of new rotundic acid derivatives. Molecules.
17:1278–1291. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
29
|
Kim GD, Choi YH, Dimtchev A, Jeong SJ,
Dritschilo A and Jung M: Sensing of ionizing radiation-induced DNA
damage by ATM through interaction with histone deacetylase. J Biol
Chem. 274:31127–31130. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu XY, Zhang FR, Shang JY, Liu YY, Lv XF,
Yuan JN, Zhang TT, Li K, Lin XC, Liu X, et al: Renal inhibition of
miR-181a ameliorates 5-fluorouracil-induced mesangial cell
apoptosis and nephrotoxicity. Cell Death Dis. 9:6102018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zaki I, Abdelhameid MK, El-Deen IM, Abdel
Wahab AHA, Ashmawy AM and Mohamed KO: Design, synthesis and
screening of 1, 2, 4-triazinone derivatives as potential antitumor
agents with apoptosis inducing activity on MCF-7 breast cancer cell
line. Eur J Med Chem. 156:563–579. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Singh PK, Weber A and Häcker G: The
established and the predicted roles of dynein light chain in the
regulation of mitochondrial apoptosis. Cell Cycle. 18:1–11.
2018.
|
|
33
|
O’Brien KM, Sandler DP, Xu Z, Kinyamu HK,
Taylor JA and Weinberg CR: Vitamin D, DNA methylation, and breast
cancer. Breast Cancer Res. 20:702018. View Article : Google Scholar
|
|
34
|
Mrózek E, Layman R, Ramaswamy B, Lustberg
M, Vecchione A, Knopp MV and Shapiro CL: Phase II trial of
neoadjuvant weekly nanoparticle albumin-bound paclitaxel,
carboplatin, and biweekly bevacizumab therapy in women with
clinical stage II or III HER2-negative breast cancer. Clin Breast
Cancer. 14:228–234. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zuo S, Liu C, Wang J, Wang F, Xu W, Cui S,
Yuan L, Chen X, Fan W, Cui M, et al: IGFBP-rP1 induces p21
expression through a p53-independent pathway, leading to cellular
senescence of MCF-7 breast cancer cells. J Cancer Res Clin Oncol.
138:1045–1055. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shokouh TZ, Ezatollah A and Barand P:
Interrelationships Between Ki67, HER2/neu, p53, ER, and PR status
and their associations with tumor grade and lymph node involvement
in breast carcinoma subtypes: Retrospective-observational
analytical study. Medicine (Baltimore). 94:e13592015. View Article : Google Scholar
|
|
37
|
Zhao QC, Nan ML, He YF and Chen SW:
Application of rotundic Aacid in the cardiovascular disease
prevention. CHN Patent 201010204596.9. 2010, Chinese.
|
|
38
|
Zhao QC, Nan ML, He YF and Chen SW:
Application of rotundic acid in the preparation of lipid-lowering
drugs. CHN. 201010204607:32010.Chinese.
|
|
39
|
Cao W, Gu Y, Meineck M and Xu H: The
combination of chemotherapy and radiotherapy towards more efficient
drug delivery. Chem Asian J. 9:48–57. 2014. View Article : Google Scholar
|
|
40
|
Li SJ, Liang XY, Li HJ, Yang GZ, Li W, Li
Z, Zhou L, Wen X, Yu DH and Cui JW: Low-dose irradiation inhibits
proliferation of the p53null type human prostate cancer cells
through the ATM/p21 pathway. Int J Mol Med. 41:548–554. 2018.
|
|
41
|
Suzuki K, Okada H, Yamauchi M, Oka Y,
Kodama S and Watanabe M: Qualitative and quantitative analysis of
phosphorylated ATM foci induced by low-dose ionizing radiation.
Radiat Res. 165:499–504. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Viniegra JG, Martínez N, Modirassari P,
Hernández Losa J, Parada Cobo C, Sánchez-Arévalo Lobo VJ, Aceves
Luquero CI, Alvarez-Vallina L, Ramón y Cajal S, Rojas JM, et al:
Full activation of PKB/Akt in response to insulin or ionizing
radiation is mediated through ATM. J Biol Chem. 280:4029–4036.
2005. View Article : Google Scholar
|
|
43
|
Yin J, Wang F, Kong Y, Wu R, Zhang G, Wang
N, Wang L, Lu Z and Liang M: Antithrombin III prevents progression
of chronic kidney disease following experimental
ischaemic-reperfusion injury. J Cell Mol Med. 21:3506–3514. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lu Z, Cheng D, Yin J, Wu R, Zhang G, Zhao
Q, Wang N, Wang F and Liang M: Antithrombin III protects against
contrast-induced nephropathy. EBioMedicine. 17:101–107. 2017.
View Article : Google Scholar : PubMed/NCBI
|