Introduction
Prostate cancer (PC) is a major health problem
causing high morbidity and mortality worldwide (1). PC is a heterogeneous cancer
phenotypically and biologically, particularly with regard to its
hormone sensitivity. In order to reproduce this context in
vitro, DU145 and PC-3 androgen-insensitive PC cells (AIPC) may
be used. These cells differ in terms of tumor suppressor proteins,
cell adhesion molecules and aggressiveness (2–5).
Although the synthesis of androgens and androgen
receptors (AR) constitutes a therapeutic target (6), there is currently no definitive
treatment for PC beyond surgery or radiotherapy in the early stages
of the disease. When recurrence occurs, androgen ablation therapy
(ADT) is a standard systemic therapy. Although the initial response
to treatment is high, after ~18 months there is a state of
resistance to androgens, termed castration-resistant PC (CRPC)
(7), which is associated in up to
40% of cases with metastasis and heterogeneity in the intra patient
intermetastasis therapeutic response. The expected survival rate
for patients with PC is ~18%, but when recurrences appear it
decreases to 5–10% (8). Treatment
options for CRPC and metastatic CRPC (mCRPC) include docetaxel
(DOCE)-based regimens (9) alone or
in association with, for example, ADT (10–13)
or anti-angiogenic (bevacizumab), anti-proliferative and
anti-migratory (tyrosine kinase inhibitors) drugs (14,15).
DOCE has been extensively used and is likely to continue to be used
in PC, improving tumor response rate, time to progression and
overall survival time (7).
Although DOCE is a first-line reference drug in CRPC, side effects
(15) and DOCE resistance
(16) limit its clinical efficacy.
Additionally, the widespread use of novel drugs is limited by the
high cost (17). Therefore, there
is a requirement to develop novel therapeutic strategies to improve
efficacy and reduce adverse effects.
Phytochemicals are a heterogeneous set of bioactive
compounds that are found naturally in vegetables, fruits, grains
and other plant products. The compounds are often responsible for
certain unique plant characteristics, including smell and
pigmentation, and a number are vital for the protection of the host
against parasites, viruses and other externally damaging agents.
Moreover, phytochemicals could be compounds of interest in cancer
chemoprevention due to their lower toxic effects compared with
conventional chemotherapeutic drugs, their action on molecular
targets involved in carcinogenesis (18) or their resensitizing effects on
anti-androgen-resistant cells (19). An inverse correlation between
consumption of cruciferous vegetables and cancer risk has been
observed in PC and breast, colon, lung and gastric cancer (20–22).
This effect has been attributed to a group of plant compounds, the
isothiocyanates (ITCs), which are present in substantial
concentrations in all brassica vegetables. ITCs are formed by the
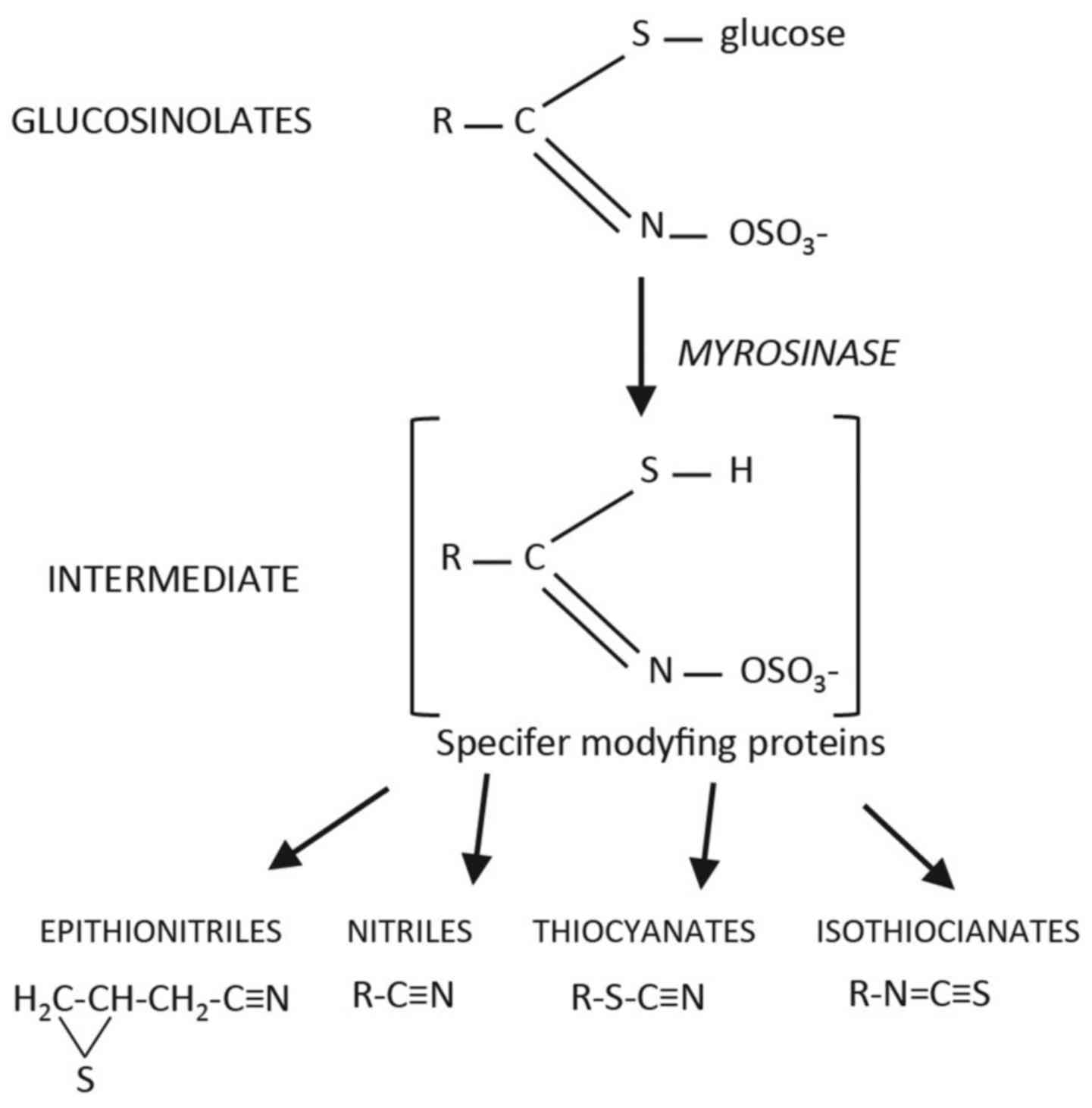
hydrolysis of their precursor parent compounds, the glucosinolates.
The glucosinolates are a large group of sulphur-containing
compounds that are present in all Brassica crops; a β-D-thioglucose
group, a sulphonated oxime moiety and a variable side-chain derived
from methionine, tryptophan or phenylalanine forms their common
structure. Upon damage to the plant tissues during consumption, the
endogenous enzyme myrosinase (thioglucoside glycohydrolase EC
3:2:3:1) and other gut enzymes hydrolize the glucosino-lates to
release a range of breakdown products, including the biologically
active ITCs (23) (Fig. 1). ITCs have inhibitory effects on
the metabolic activation of carcinogenic dietary or tobacco
components (24), along with the
inhibition of mutagenesis (25)
and anti-carcinogenic effects in vitro (26–30)
and in vivo (31,32). In PC, ITCs modulate epigenetic
changes, induce the arrest of the cell cycle, activate apoptosis
pathways and increase chemotherapeutic agent sensitivity; leading
to the inhibition of cell proliferation, progression and
invasion-metastasis (33).
ITCs are compounds with the structure R-N=C=S, where
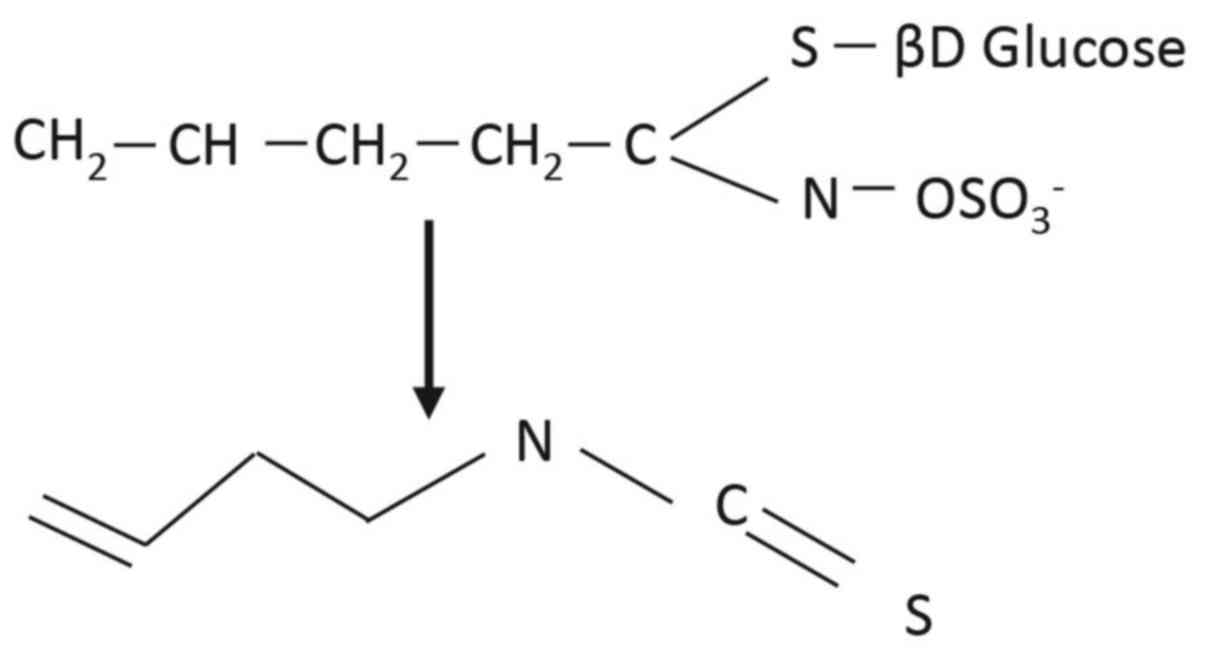
R is an alkyl or aryl group. One such ITC is 3-Butenyl ITC (3-BI),
found particularly in brassica crops from Brassica rapa
species, including pak choi, Chinese cabbage, turnips, turnip
greens and turnip tops (34),
where it is present as the glucosinolate gluconapin (Fig. 2). 3-BI is known to have multiple
effects, including anti-proliferative and anti-apoptotic effects,
on colon (26,35), prostate, bone, cervical, liver,
breast and neuroblastoma tumor cells (30). To date, there are no data on the
effects of combination therapy of 3-BI and DOCE in PC. In order to
elucidate this interaction, the present study examined the in
vitro anticancer effects of 3-BI, with and without DOCE, using
primary cultures of healthy human prostate epithelial cells (PECs)
and the AIPC PC-3 and DU145 cell lines. Comparing the effects of
3-BI with or without DOCE on normal versus cancer cell lines will
provide insight into their role in the treatment of PC.
Materials and methods
Cell culture
Tumorigenic cell lines, DU145 (HTB-81) and PC-3
(cat. no. CRL-1435), and primary PECs (PCS-440-010; used to
determine non-toxic nature of 3-BI only) were obtained from the
American Type Culture Collection (ATCC; Manassas, VA, USA) and
cultured according to company protocols with F-12K Medium (cat. no.
30-2004) and a Prostate Epithelial Cell Growth kit (cat. no.
PCS-440-040) (both from ATCC), supplemented with fetal bovine serum
(FBS; cat. no. 10270; Life Technologies; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) and 100 U/ml penicillin and 100 mg/ml
streptomycin (Penicillin-Streptomycin Solution 30-2300; LGC
Standards SL, Barcelona, Spain). Stock solutions of 3-BI (cat. no.
I0443; TCI, Paris, France) and DOCE (cat. no. 01885) were prepared
in dimethyl sulfoxide (DMSO; cat. no. D2650) (both from
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and diluted with
complete medium (medium supplemented with FBS, penicillin and
streptomycin). An equal volume of DMSO (final concentration
<0.05%) was added to the controls.
Cell viability assay
The effect of 3-BI and/or DOCE treatments on cell
viability was determined by trypan blue dye exclusion assay as
described previously (36).
Briefly, 5×103 cells in 1 ml complete medium were plated
in 6-well plates and allowed to attach overnight. The medium was
replaced with fresh complete medium containing different
concentrations of 3-BI (0, 5, 10, 30 and 50 μM) and/or DOCE
(0, 1 and 2 nM), and the plates were incubated for 24, 48 or 72 h
at 37°C. At the end of the incubation period, floating and adherent
cells were collected and suspended in 25 ml phosphate-buffered
saline (PBS; cat. no. D8537; Sigma-Aldrich; Merck KGaA). The cells
were then mixed with 5 ml 0.4% trypan blue solution (cat. no.
T6146; Sigma-Aldrich; Merck KGaA), and live and dead cells were
counted under an Olympus inverted microscope (Olympus, Tokyo,
Japan).
Determination of apoptosis
Apoptosis induction by 3-BI and/or DOCE treatments,
at the same concentrations that were used to evaluate cellular
viability, was assessed by fluorescence microscopy following
staining with 4′,6-diamidino-2-phe-nylindole (DAPI), caspase-3
activation assay and cleaved poly(ADP-ribose) polymerase (PARP)
assay.
Fluorescence microscopy
Cells (2×104) were plated on cover-slips,
allowed to attach overnight, and exposed to DMSO or 3-BI and/or
DOCE at the same concentrations that were used to evaluate cellular
viability, for 72 h. The cells were washed with PBS and fixed with
3% paraformaldehyde for 1 h at room temperature. The cells were
washed three times with PBS, permeabilized with 1% Triton X-100
(cat. no. 142314.1611142314.1611; Panreac Quimica SLU, Barcelona,
Spain) for 4 min, washed again with PBS and stained with 1
μg/ml DAPI (cat. no. D9542; Sigma-Aldrich; Merck KGaA) for 5
min. The cells with condensed and fragmented DNA (apoptotic cells)
were counted under a fluorescence microscope at ×40 magnification
(37).
Caspase-3 activity and cleaved PARP
assay
The effect of 3-BI and/or DOCE treatments on
caspase-3 activity and cleaved PARP level in PC-3 cells was
determined using commercially available ELISA kits. Active
caspase-3 and cleaved PARP levels (ng/mg total protein of cell
lysate) were determined, and the results were expressed relative to
the control. Briefly, 1×104 cells were plated in 24-well
plates and allowed to attach overnight. Next, the cells were
treated with DMSO, 50 μM 3-BI, 2 nM DOCE or 50 μM
3-BI + 2 nM DOCE for 72 h at 37°C. The cells were collected and
lysed in cell extraction buffer (cat. no. FNN0011; Thermo Fisher
Scientific, Inc.) mixed with protease inhibitor cocktail (cat. no.
P-2714) and 1 mM phenyl methane sulfonyl fluoride (cat. no.
10837091001) (both from Sigma-Aldrich; Merck KGaA). ELISA assays
for caspase-3 activity (cat. no. KHO1091) and cleaved PARP level
(cat. no. 10650704) (both from Thermo Fisher Scientific, Inc.) were
performed according to the manufacturer's protocols.
In vitro migration assay
The effect of 3-BI (0, 5, 10, 30 and 50 μM)
on PC-3 and DU145 cell migration was assessed using 24-well
Transwell cell culture chambers (6.5-mm diameter, 8.0-μm
pore size, polycarbonate membrane) (cat. no. C6932; Sigma-Aldrich;
Merck KGaA) with a Millicell Cell Culture Insert (cat. no.
PI8P01250; Merck KGaA) (38).
Briefly, 1×105 cells were added into the upper chamber
with 100 μl serum-free medium. The lower chamber was filled
with 600 μl complete medium containing 10% fetal bovine
serum (Life Technologies; Thermo Fisher Scientific, Inc.). After 24
h of incubation at 37°C in a 5% CO2 incubator, the cells
in the upper chamber were carefully removed with a cotton swab and
the cells that had migrated through the membrane and had stuck to
the lower surface of the membrane were fixed with 4%
paraformaldehyde (20 min at room temperature) and stained with
crystal violet (cat. no. C6158; Sigma-Aldrich; Merck KGaA) (15 min
at room temperature). For quantification, the stained cells were
counted by capturing images of the membrane under a microscope in
five randomly selected fields using an Olympus microscope with a
digital camera (Olympus, Tokyo, Japan). At least three chambers
from three different experiments were analyzed.
Confocal microscopy
As 3-BI exhibited noticeable effects on PC-3 cells
but not in DU 145 cells (see Results section), PC-3 cells were
seeded in glass coverslips into incubation chambers for 24 h at
37°C and with 5% CO2 for full adherence. Next, the
medium was replaced with fresh complete medium without adding any
treatment (control) or containing 3-BI 50 μM and/or DOCE (1
and 2 nM), and the cells were incubated for 72 h at 37°C.
Subsequently, the PC-3 cells were fluorescently stained for F-actin
(Phalloidin-ATTO 647N; 1:500; cat. no. 65906; Sigma-Aldrich; Merck
KGaA), nuclei (DAPI) and cytoplasm [Carboxyfluorescein diacetate
succinimidyl ester (CFDA-SE); cat. no. 1351201EDU; Bio-Rad
Laboratories, Inc., Hercules, CA, USA] according to the following
procedure. The culture medium was replaced by PBS solution
containing fluorescent dye CFDA-SE at 1 mM and the cells were
incubated for 15 min at room temperature. Following incubation,
CFDA-SE solution was removed and the cells were washed with PBS,
fixed with 70% ethanol for 5 min, washed again with PBS and stained
for with Phalloidin-ATTO for 1 h at room temperature. Next, the
cells were washed with 0.9% sodium chloride (cat. no. S7653;
Sigma-Aldrich; Merck KGaA) and stained with DAPI at 1 μg/ml
for 10 min at room temperature. Thereafter, slides were embedded in
Vecta Shield antifade mounting medium (cat. no. H-1000; Vector
Laboratories, Inc., Burlingame, CA, USA). A Leica confocal
microscope (Leica TCS SP5 X microscope; Leica Microsystems, Inc.,
Buffalo Grove, IL, USA) was used for the analysis of the cells
(39).
Statistical analysis
The results represent the mean of at least three
independent experiments (mean ± standard deviation). The
significance of difference in measured variables between control
and treated groups was studied by two-way analysis of variance.
Significance between multiple experimental groups was determined
using Tukey's post hoc analysis. P≤0.05 was considered to indicate
a statistically significant difference.
Results
Inhibition of PC cell proliferation by
3-BI and/or DOCE
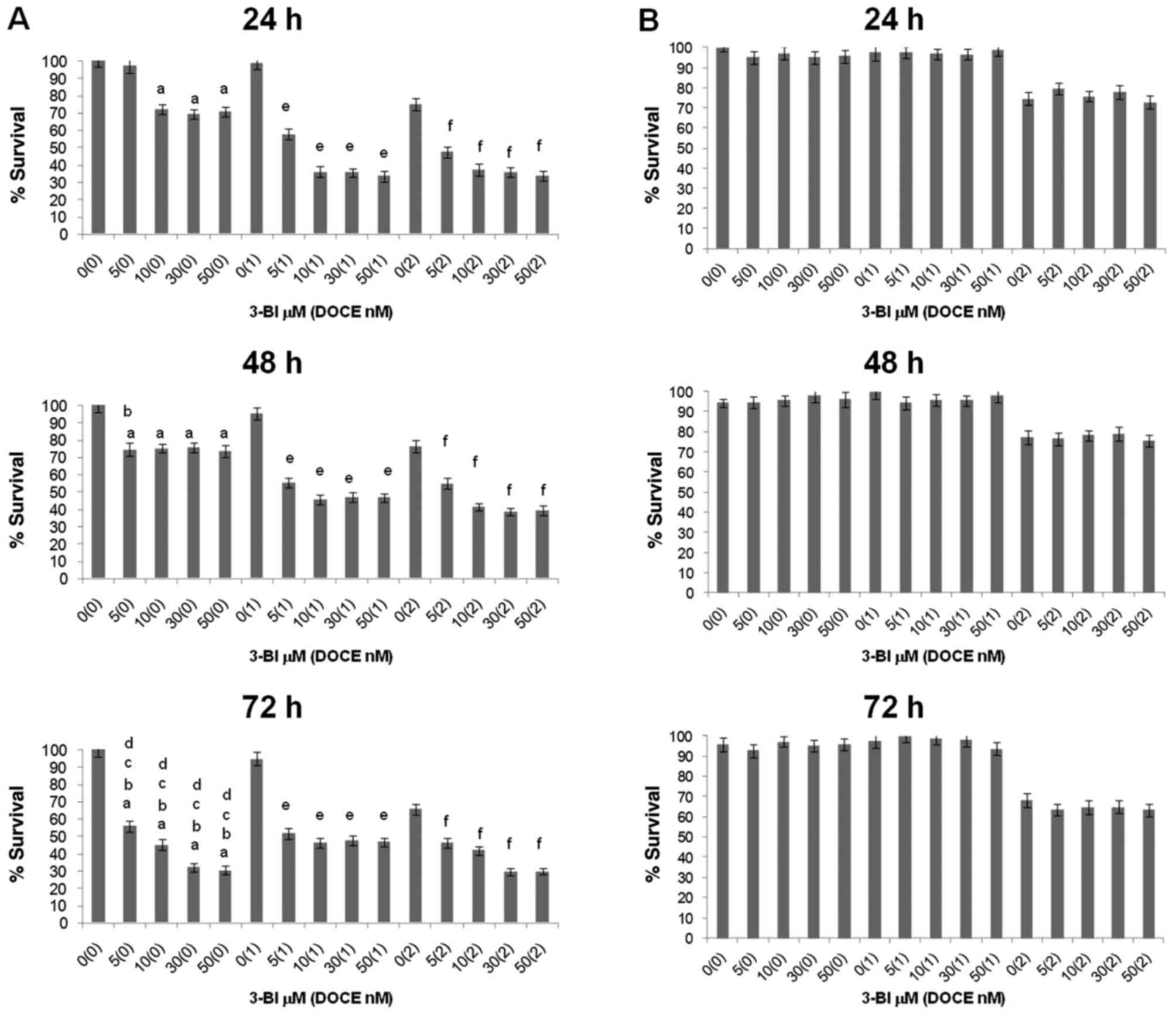
The effect of 3-BI treatment on the survival of PC-3
and DU145 cells was assessed by trypan blue dye exclusion assay
(Fig. 3). The viability of the
DU145 cells was not affected by 3-BI (Fig. 3B), whereas the compound
significantly inhibited the survival of PC-3 cells in a
concentration- and time-dependent manner (Fig. 3A). The inhibitory effect of 3-BI
was most pronounced at doses of 50 μM (30% at 24 h).
Otherwise, survival of PECs was minimally affected (P>0.05) by
3-BI. Thus, ~96% of PECs were viable following a 72-h exposure to
50 μM 3-BI (Table I),
whereas only ~30% of PC-3 survived under similar conditions to 3-BI
treatment.
 | Table IEffects of the treatment with 3-BI
(72 h) on proliferation of PEC cells, as determined by trypan blue
dye exclusion assay. |
Table I
Effects of the treatment with 3-BI
(72 h) on proliferation of PEC cells, as determined by trypan blue
dye exclusion assay.
| DOCE, nM | 3-BI,
μM | Cell proliferation
|
|---|
| Mean (%) | SD |
|---|
| 0 | 0 | 98.04 | 0.52 |
| 0 | 5 | 97.55 | 1.37 |
| 0 | 10 | 98.52 | 1.26 |
| 0 | 30 | 96.52 | 0.57 |
| 0 | 50 | 95.64 | 1.32 |
| 1 | 0 | 93.43 | 0.97 |
| 1 | 5 | 96.75 | 1.01 |
| 1 | 10 | 96.40 | 1.34 |
| 1 | 30 | 95.32 | 0.98 |
| 1 | 50 | 95.35 | 1.19 |
| 2 | 0 | 96.48 | 1.08 |
| 2 | 5 | 96.37 | 1.33 |
| 2 | 10 | 95.51 | 0.95 |
| 2 | 30 | 96.48 | 0.77 |
| 2 | 50 | 96.04 | 0.90 |
3-BI was more effective in PC-3 cells than DOCE only
at 72 h; 50 μM 3-BI reduced PC-3 cell viability by ~70%,
whereas 2 nM DOCE alone inhibited the growth of these cells by
~35%.
Sensitization of PC cells to growth
suppression by DOCE
Growth inhibition of PC-3 cells (but not of DU145
cells) by DOCE was increased by 3-BI. Fig. 3 shows the viability of PC cells
following 24–72 h of exposure to 3-BI and/or DOCE, as assessed by
trypan blue dye exclusion assay.
The 3-BI and DOCE combination was significantly more
efficacious against the viability of PC-3 cells compared with 3-BI
or DOCE treatment alone. This effect was not observed for DU145
cells, so the 3-BI-mediated sensitization to growth suppression by
DOCE was a cell line-specific response. Analyzing the results using
a previously described method (40), it was observed that 3-BI
synergistically enhanced the anti-proliferative effects of DOCE on
PC-3 cells at 24 and 48 h when the lowest dose of the chemotherapy
drug was administered (Table II).
For example, the viability of the PC-3 cells was slightly affected
in the presence of 1 nM DOCE alone for 48 h (95%), while exposure
to 50 μM 3-BI resulted in a survival rate of 73% compared
with that in the DMSO-treated control group. The viability of PC-3
cells was reduced by ~53% by a 48-h co-treatment with 50 μM
3-BI and 1 nM DOCE in comparison with that of the vehicle-treated
control cells, with an observed combination index of 1.47,
indicating synergy between 3-BI and DOCE (Table II).
 | Table IIAnalysis of synergy between 3-BI and
DOCE calculated by survival rate for PC-3 cells. |
Table II
Analysis of synergy between 3-BI and
DOCE calculated by survival rate for PC-3 cells.
| Time, h | DOCE
| 3-BI
| Combination
treatment
| Indexe |
|---|
| Dose, nM | MSRa | P-valueb | Dose, nM | MSR | P-value | Expectedc | Observedd | P-value |
|---|
| 24 | 1 | 0.99 | >0.05 | 5 | 0.97 | >0.05 | 0.96 | 0.58 | <0.05 | 1.66 |
| 24 | 1 | 0.99 | >0.05 | 10 | 0.72 | <0.05 | 0.71 | 0.36 | <0.05 | 1.97 |
| 24 | 1 | 0.99 | >0.05 | 30 | 0.69 | <0.05 | 0.68 | 0.36 | <0.05 | 1.89 |
| 24 | 1 | 0.99 | >0.05 | 50 | 0.71 | <0.05 | 0.70 | 0.33 | <0.05 | 2.12 |
| 24 | 2 | 0.37 | <0.05 | 5 | 0.97 | >0.05 | 0.36 | 0.48 | <0.05 | 0.75 |
| 24 | 2 | 0.37 | <0.05 | 10 | 0.72 | <0.05 | 0.27 | 0.37 | <0.05 | 0.73 |
| 24 | 2 | 0.37 | <0.05 | 30 | 0.69 | <0.05 | 0.26 | 0.36 | <0.05 | 0.72 |
| 24 | 2 | 0.37 | <0.05 | 50 | 0.71 | <0.05 | 0.26 | 0.34 | <0.05 | 0.76 |
| 48 | 1 | 0.95 | >0.05 | 5 | 0.75 | <0.05 | 0.71 | 0.55 | <0.05 | 1.29 |
| 48 | 1 | 0.95 | >0.05 | 10 | 0.75 | <0.05 | 0.71 | 0.46 | <0.05 | 1.54 |
| 48 | 1 | 0.95 | >0.05 | 30 | 0.76 | <0.05 | 0.72 | 0.47 | <0.05 | 1.53 |
| 48 | 1 | 0.95 | >0.05 | 50 | 0.73 | <0.05 | 0.69 | 0.47 | <0.05 | 1.47 |
| 48 | 2 | 0.38 | <0.05 | 5 | 0.75 | <0.05 | 0.29 | 0.55 | <0.05 | 0.53 |
| 48 | 2 | 0.38 | <0.05 | 10 | 0.75 | <0.05 | 0.29 | 0.41 | <0.05 | 0.71 |
| 48 | 2 | 0.38 | <0.05 | 30 | 0.76 | <0.05 | 0.29 | 0.39 | <0.05 | 0.74 |
| 48 | 2 | 0.38 | <0.05 | 50 | 0.73 | <0.05 | 0.28 | 0.39 | <0.05 | 0.72 |
| 72 | 1 | 0.95 | >0.05 | 5 | 0.56 | <0.05 | 0.53 | 0.54 | >0 .05 | 0.98 |
| 72 | 1 | 0.95 | >0.05 | 10 | 0.45 | <0.05 | 0.43 | 0.46 | >0 .05 | 0.93 |
| 72 | 1 | 0.95 | >0.05 | 30 | 0.32 | <0.05 | 0.30 | 0.48 | >0 .05 | 0.63 |
| 72 | 1 | 0.95 | >0.05 | 50 | 0.30 | <0.05 | 0.29 | 0.47 | <0.05 | 0.62 |
| 72 | 2 | 0.33 | <0.05 | 5 | 0.56 | <0.05 | 0.18 | 0.46 | <0.05 | 0.39 |
| 72 | 2 | 0.33 | <0.05 | 10 | 0.45 | <0.05 | 0.15 | 0.42 | <0.05 | 0.36 |
| 72 | 2 | 0.33 | <0.05 | 30 | 0.32 | <0.05 | 0.11 | 0.30 | <0.05 | 0.37 |
| 72 | 2 | 0.33 | <0.05 | 50 | 0.30 | <0.05 | 0.10 | 0.30 | <0.05 | 0.33 |
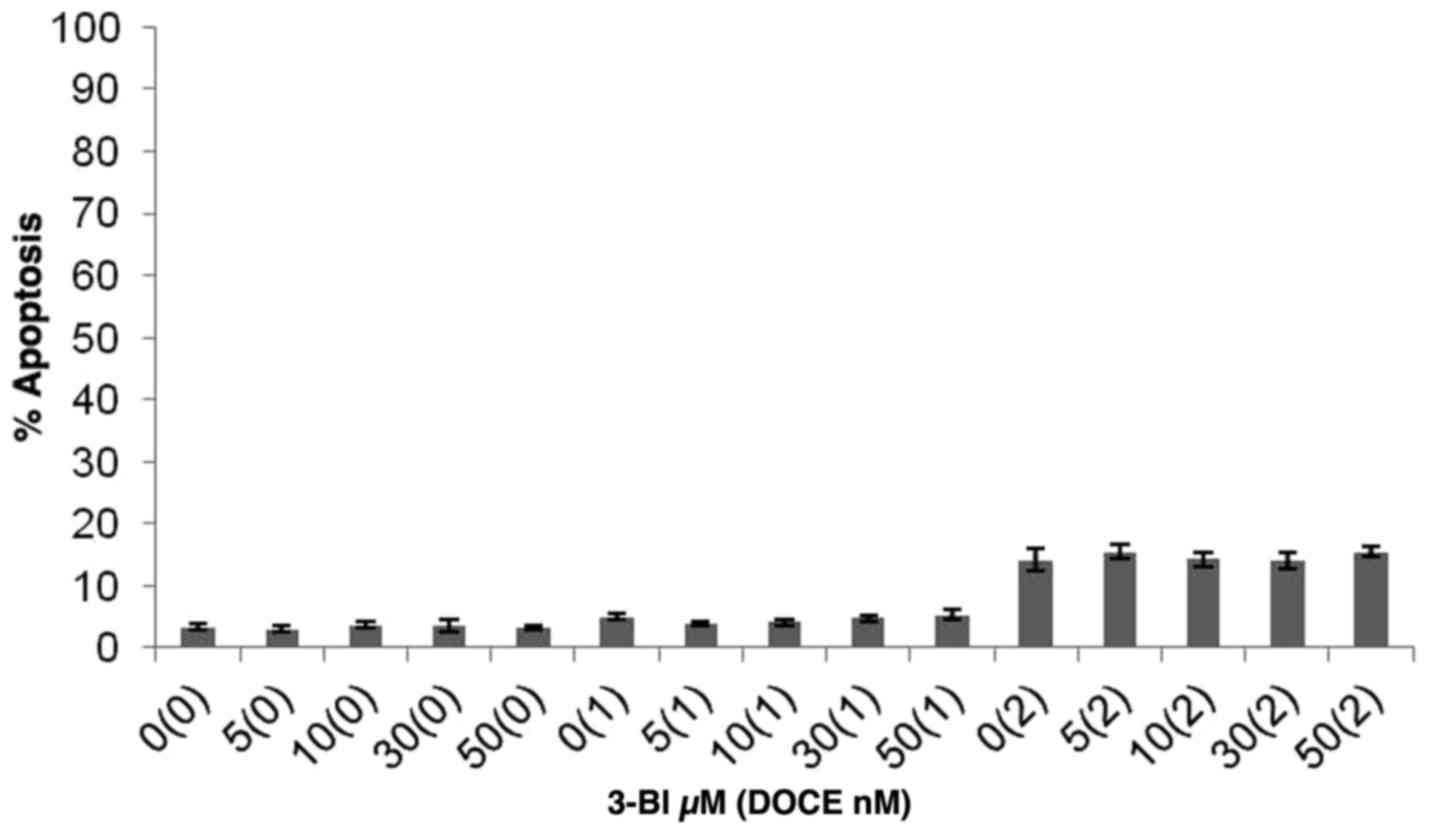
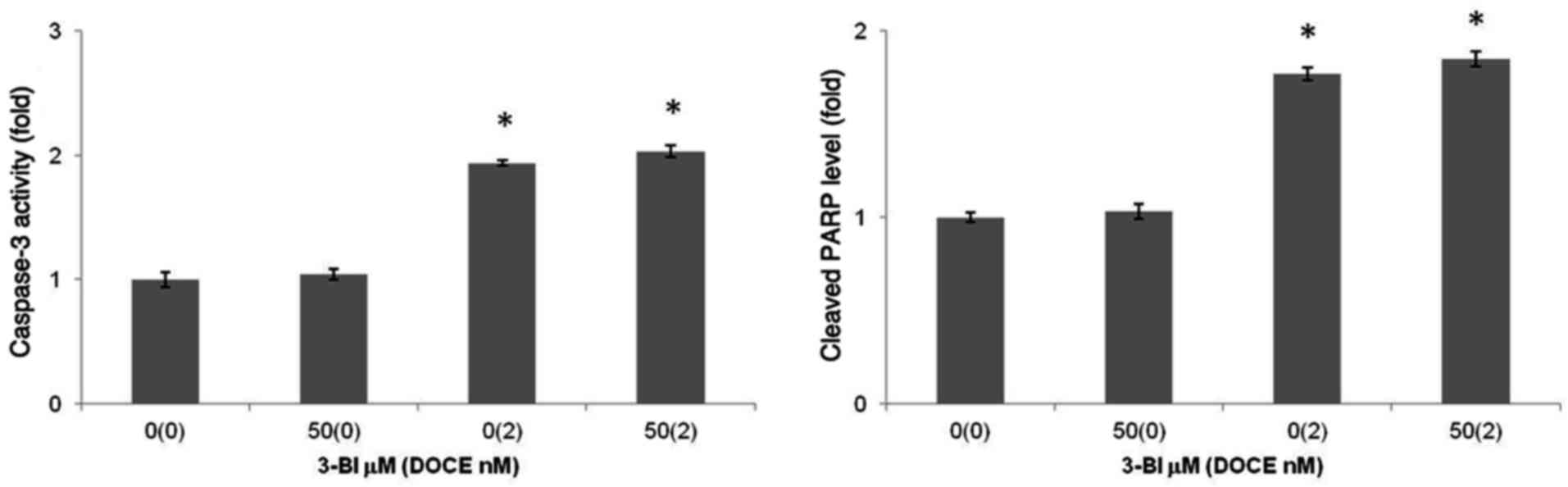
Apoptosis
Apoptotic induction by 3-BI was assessed by
fluorescence microscopy subsequent to staining with DAPI (Fig. 4). Furthermore, taking into account
that characteristic events of apoptosis include proteolytic
activation of caspase-3, and proteolytic cleavage of PARP-1, PARP
and caspase-3 cleavage measurements were performed. It was
concluded that the 3-BI-mediated death of androgen-independent PC
cells induced by 3-BI cannot be explained by apoptotic mechanisms,
as apoptotic cells were rarely observed in the cultures of PC-3
cells following a 72-h treatment with 3-BI (Fig. 5).
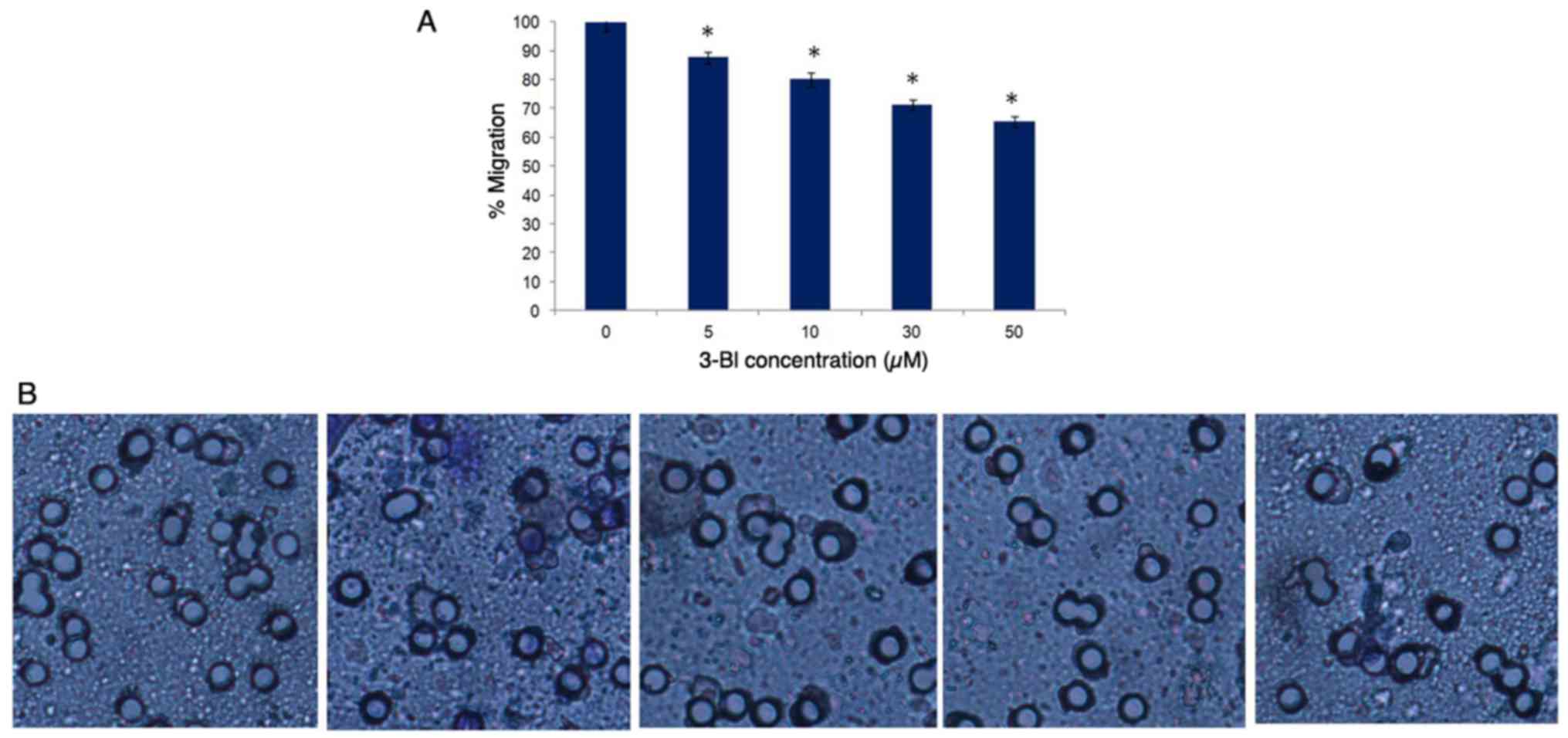
Migration
Transwell chamber assays were used to assess the
effects of 3-BI on PC-3 and DU145 cell migration. In contrast to
that in DU145 cells, following 3-BI treatment for 72 h, the
migration ability of the PC-3 cells was decreased, with its maximum
effect at 50 μM (Fig.
6).
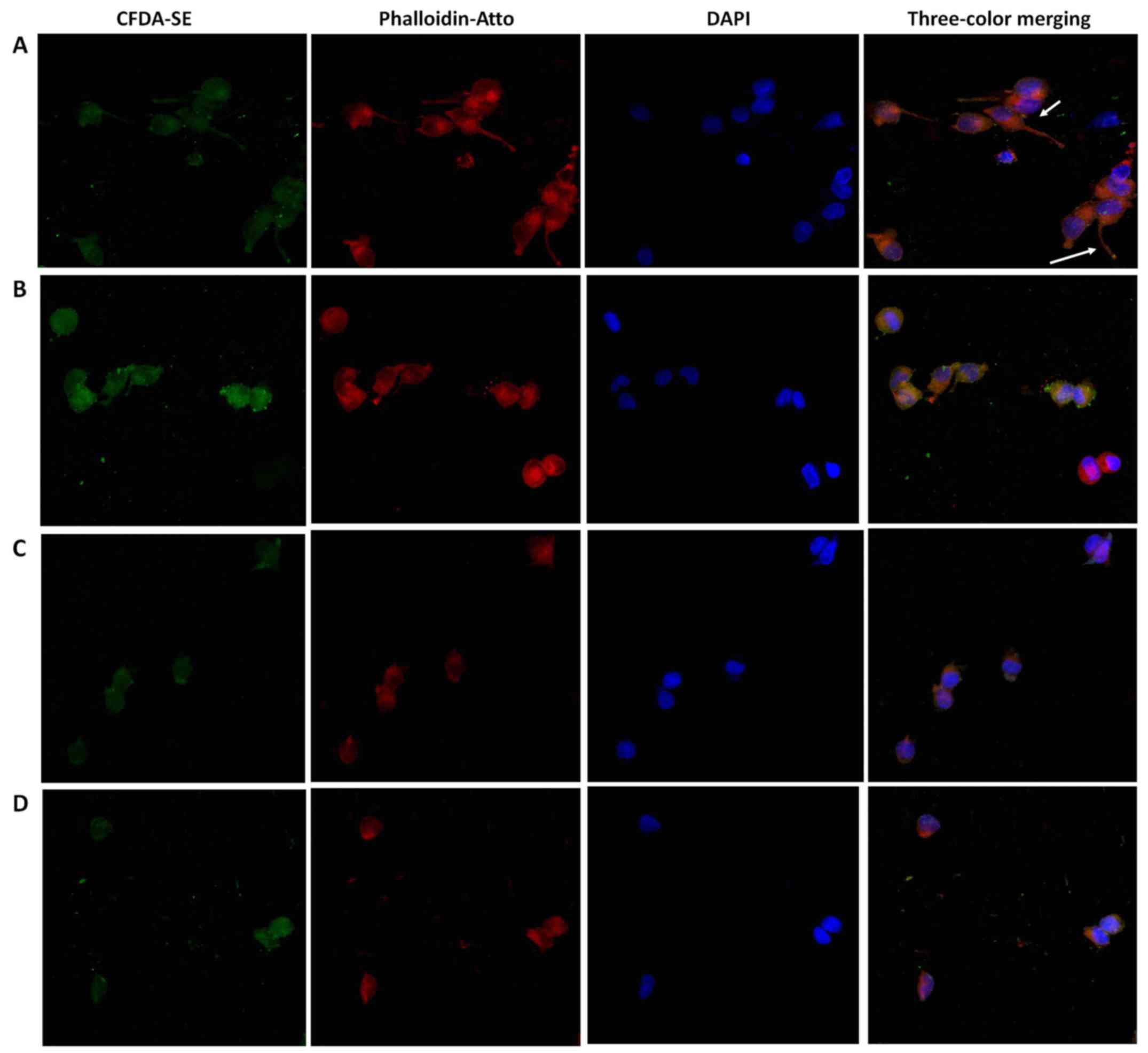
Confocal microscopy
To investigate the role of 3-BI in cell damage in
PC-3, cytopathic changes were examined (Fig. 7). As the maximum effect had
previously been observed at 50 μM 3-BI, this dose was used
as the treatment concentration in this experiment. In the control
group, PC-3 cells showed elongated morphology, cell appendages
(arrows), ellipsoid nuclei with euchromatin and heterochromatin,
actin filaments localized mainly beneath the plasma membrane and
the cytoplasm stained homogenously with CFDA-SE. In the treatment
groups (50 μM 3-BI, 50 μM 3-BI and 1 nM DOCE, and 50
μM 3-BI and 2 nM DOCE) the cells exhibited a rounded shape
as the predominant phenotype and actin skeleton reorganization with
actin dislocation into the perinuclear zone.
Discussion
The present study found that the PC-3 cell line, but
not the DU145 cell line, was sensitive to survival inhibition
induced by 3-BI. This was a time- and dose-dependent effect. At the
highest doses and more prolonged times of exposure, PC-3 cell
survival was ~30%. By contrast, survival of PECs was ~96%. These
findings may be noteworthy since the ability of a chemopreventive
agent to cause specific cytotoxicity in cancer cells and not in
normal cells is an important determinant of its clinical relevance
and safety.
There are a few studies on the effects of 3-BI on
cancer cells under in vitro conditions (26,30,35).
Other 3-butenyl ITCs, including 4-methylthio-3-BI-ITC (raphasatin)
or 4-methylsulfinyl-3-BI-ITC (sulforaphene), have been found not to
cause toxicity in T lymphocytes, despite being cytotoxic in Jurkat
T-leukemia (41), colon cancer
(LoVo, CaCo-2, HCT-116 and HT-29) (42,43)
and breast cancer (MDA-MB-231 and MCF-7) (43) cells.
Unlike other ITCs, the molecular mechanisms by which
3-BI produces an inhibitory effect on the survival and
proliferation of cancer cells are not fully known. The
anti-proliferative effect of ITCs is usually associated with cell
cycle arrest and the activation of apoptosis (33,44,45).
Regarding cell cycle arrest, previous studies have found that
sulforaphene inhibits proliferative activity by increasing the
expression of p21 (cyclin-dependent kinase inhibitor), which in
turn promotes G1 cell cycle phase arrest (esophageal carcinogenesis
in vivo). By contrast, sulforaphene induces apoptosis
through increased expression of p21 (32), downregulation of the B-cell
lymphoma-2 (Bcl-2)/apoptosis regulator BAX (Bax) ratio (in
vivo) and activation of caspase-3 and -9 (in vitro, A549
lung cancer cells) (46). In the
present study, few apoptotic cells were observed following 3-BI
treatment of PC-3 cells, suggesting that the effect of 3-BI could
be mediated through other pathways, which will be investigated in
future studies. These controversial results may be due to the
different cell and ITC characteristics and/or ITC treatment
concentrations.
In an attempt to overcome DOCE resistance, the
effective cytotoxic, apoptotic and anti-migratory concentrations of
the microtubule-targeting drug DOCE and 3-BI, and the synergistic
effects in DU145 and PC-3 CRPC lines were studied. DU145 cells are
p53-mutant, Bax-negative, signal transducer and activator of
transcription 3 (STAT3)-positive and E-cadherin-positive, while
PC-3 cells are p53-null, STAT3-negative, and phosphatase and tensin
homologue (PTEN)-negative, with negative or low E-cadherin
expression and high matrix metalloproteinase (MMP)1 and 3
expression; therefore making PC-3 cells more aggressive (2–5,47).
Furthermore, PC-3 cells show features that are characteristic of
prostatic small cell neuroendocrine PC (48).
The present results demonstrated that 3-BI
synergizes with DOCE against cultured human PC-3 AIPC cells. To the
best of our knowledge, the present study is the first published
report to document this synergistic anti-proliferative effect. This
effect could be explained by interaction with a DOCE molecular
mechanism (some of which are already under investigation by our
group): i) DOCE is a well-known microtubule-target agent (49). Certain ITCs, including allyl-ITC
(45), benzyl-ITC, phenethyl-ITC
and 4-methyl-sulphynilbutyl-ITC (SFN) (50–52),
interact with microtubules causing inhibition of tubulin
polymerization, tubulin depolymerization, histone deacetylase
inhibition or tubulin acetylation (Fig. 8). ii) DOCE induces Bcl-2
phosphorylation, which in turn causes cellular apoptosis through
different routes (53). Bcl-2 is
associated with the development of AIPC, being overexpressed in
advanced stages of the disease. Bcl-2 proteins, independent of
their known anti-apoptotic effects, are also implicated in cancer
cell migration and invasion. Certain ITCs can upregulate Bcl-2
(33). iii) DOCE inhibits
androgen-dependent and -independent activation of AR. The
association between AR and tubulin, and the preferential binding of
micro-tubules to AR can recruit AR to determine apoptotic-signaling
promotion and tumor growth inhibition (54). SFN can decrease the expression of
AR in a dose- and time-dependent manner in PC cells (55). The chemical structure of this ITC
determines this effect. Thus, while propyl-, butyl- and pentyl-thio
analogues have similar effectiveness, sulfonyl derivatives of SFN
are either inactive or less effective than the thio- or
sulfinyl-derivatives (56).
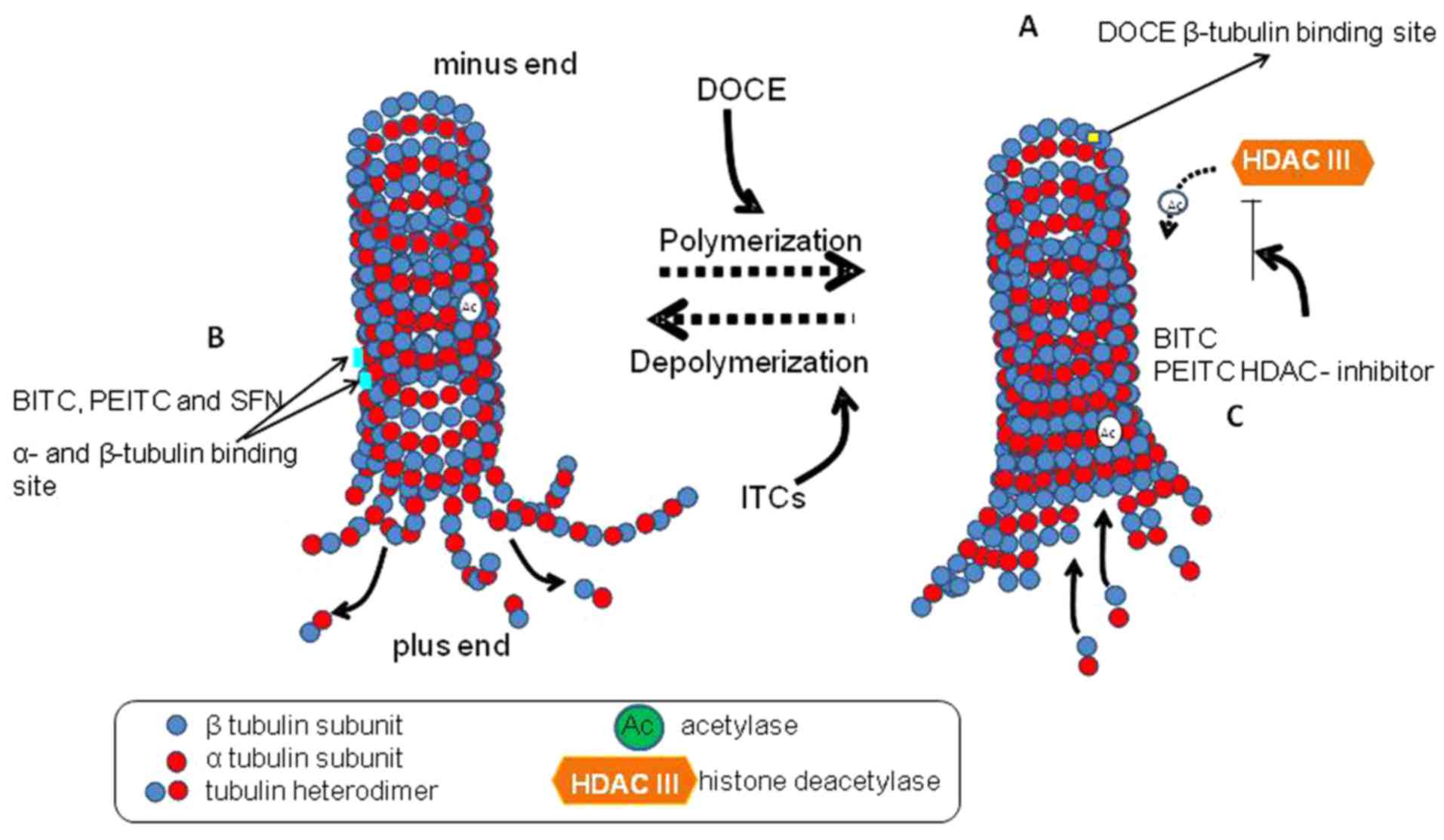 | Figure 8Possible mechanisms of 3-butenyl
isothiocyanate microtubule-targeting effects. Tubulin-containing
microtubules serve a pivotal role in different cellular processes,
including cell division, motility and intracellular trafficking.
Microtubules are dynamic polymers, formed by α and β tubulin
heterodimers, which undergo periods of polymerization and
depolymerization. The dynamic process of polymerization and
depolymerization is required to form mitotic spindles that are
necessary to segregate the replicated chromosomes of the daughter
cells. (A) DOCE binds to β-tubulin and stabilizes it. Subsequent to
the union, the microtubules cannot be disassembled, so the static
polymerization arrests the normal mitotic process in the G2M phase
and finally induces cell death (49). (B) ITCs can bind to α and β
tubulin, inhibiting microtubule polymerization in vitro
(human lung cancer A549 cells, human cervix cancer HeLa cells and
mouse mammary carcinoma F3II cells) (50,51,67),
causing depolymerization (51,67)
or (C) inhibiting HDAC with increasing microtubule acetylation
(51). The degree of binding
affinity and microtubule polymerization inhibition is conditioned
by the ITC structure (51,52). DOCE, docetaxel; HDAC, histone
deacetylase; ITC, isothiocyanate; BITC, benzyl ITC; PEITC,
phenethyl ITC; SFN, sulphynilbutyl-ITC. |
On the other hand, the present study observed that
3-BI-mediated DOCE sensitization to growth suppression was
significantly higher in the PC-3 cell line as opposed to that in
the DU145 cell line. Although the precise mechanism underlying this
divergence is not known, it could likely be attributed to
differential cells characteristics, particularly with regard to
p53, STAT3 and PTEN. The tumor suppressor p53 is expressed in
response to different stresses (57), orchestrating biological outputs
such as cell proliferation, apoptosis, cell-cycle arrest or
autophagy (58). In advanced PC, a
complete loss of p53 is found (59) and a lack or mutation of p53 is
associated with chemotherapeutic resistance (60–62).
As the PC-3 and DU145 cell lines lack functional p53, similarities
could be expected; however, according to Muller and Vousden
(63), mutant p53 proteins could
acquire oncogenic properties that are different to those resulting
from loss of wild-type tumor-suppressing p53 function and that
could promote proliferation, survival, migration, invasion and
metastasis. STAT3 is an oncogenic transcription factor implicated
not only in development, but also in PC progression, and is
constitutively active in the DU145 cell line, but not in the PC-3
cell line. PTEN, a tumor suppressor gene, is mutated or lost in
50–80% of primary PC cases, with the complete loss associated with
aggressiveness and metastasis. Likewise, Akt is constitutively
active in the PC-3 cell line, but not in the DU145 cell line, due
to the lack and the robust expression of PTEN, respectively
(5). Nevertheless, the PTEN
loss-induced Akt pathway promotes cell survival, proliferation and
metastasis in DU145 and PC-3 cells (64).
With regard to the ability of PC to metastasize, the
present study showed that the migration of PC-3 cells is reduced
significantly by 3-BI treatment. Migration involves changes in the
motility and adhesion of PC cells that are associated with
metastasis (65). Inhibition of
E-cadherin expression is considered as one of the main indicators
of the epithelial/mesenchymal phenotype transition of PC,
diminishing cell-cell adhesion and facilitating metastasis
(66). Certain ITCs can modulate
the expression of a variety of metastasis target genes. The
regulation of migration is quite complex and is mediated by Bcl-2,
p53, Akt, PTEN, E-cadherin and MMP, among others (33). Further studies are required to
solve the differential 3-BI-induced response in PC-3 and DU145
cells. This could be of note, as anti-migratory drugs such as
tyrosine kinase inhibitors are commonly used in CRPC (14,15).
In addition, visualization of cytopathic changes by
confocal microscopy could be a sensitive indicator for malignant
progression and for the anti-invasive and cytotoxic drug effects.
In particular, the reduction of cell size and the loss of cell
appendages are considered as indicators of growth inhibitory and
anti-invasive effects of anticancer compounds in PC-3 cells
(67). In the present study, it
was found that 3-BI with or without DOCE exhibits cytopathic
effects in PC-3 cells, manifested by cell morphology changes and
actin skeleton reorganization. Härmä et al (67) showed that cell migration can be
more rapid in elongated bipolar PC-3 cells in comparison to that in
rounded cells. The changes in cell morphology and motility could be
driven by organization of the actin cytoskeleton, likely mediated
by putative motility modifiers (68,69).
A detailed study of these mechanisms requires further study and
future research.
In conclusion, DOCE-based combination therapy was
shown to increase the number and/or the duration of the therapeutic
response in the present study. 3-BI demonstrated anti-proliferative
and anti-migratory effects. Furthermore, 3-BI showed a synergic
effect with DOCE without toxic effects on prostatic normal cells.
According to these findings, 3-BI could be clinically attractive as
an adjuvant to conventional chemotherapy with DOCE. The present
results are promising, but further studies are required to
determine the molecular mechanisms involved in 3-BI-induced
cytotoxicity DOCE synergism.
Funding
This study was supported by the Feder-Innterconecta
project (ITC-20151009), the Spanish National Plan for Research and
Development (grant no. AGL2015-66256-C2-1-R), and financed by the
European Regional Development Funds.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
CG contributed substantially to the conception,
design, drafting, critical revision and supervision of the
submitted manuscript. PS contributed substantially to the analysis
and interpretation of data, drafting, and technical and
administrative support for the submitted manuscript. MEC and PV
contributed substantially to the acquisition of data, drafting and
administrative support for the submitted manuscript. SN, MJNI and
MFG contributed substantially to the analysis and interpretation of
data, design, drafting, critical revision and supervision of the
submitted manuscript. All authors approved the final version to be
published and agree to be accountable for all aspects of the work
in ensuring that questions related to the accuracy or integrity of
any part of the work are appropriately investigated and
resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
Authors declare that they have no competing
interests.
Acknowledgments
This abstract was presented at the 7th Symposium on
Brassica Crops, May 2017, in Pontevedra, Spain.
References
|
1
|
Zhou CK, Check DP, Lortet-Tieulent J,
Laversanne M, Jemal A, Ferlay J, Bray F, Cook MB and Devesa SS:
Prostate cancer incidence in 43 populations worldwide: An analysis
of time trends overall and by age group. Int J Cancer.
138:1388–1400. 2016. View Article : Google Scholar :
|
|
2
|
Mitchell S, Abel P, Ware M, Stamp G and
Lalani E: Phenotypic and genotypic characterization of commonly
used human prostatic cell lines. BJU Int. 85:932–944. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Spiotto MT and Chung TD: STAT3 mediates
IL-6-induced neuro-endocrine differentiation in prostate cancer
cells. Prostate. 42:186–195. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mori K, Le Goff B, Charrier C, Battaglia
S, Heymann D and Rédini F: DU145 human prostate cancer cells
express functional receptor activator of NFkappaB: New insights in
the prostate cancer bone metastasis process. Bone. 40:981–990.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
van Duijn PW and Trapman J: PI3K/Akt
signaling regulates p27(kip1) expression via Skp2 in PC3 and DU145
prostate cancer cells, but is not a major factor in p27(kip1)
regulation in LNCaP and PC346 cells. Prostate. 66:749–760. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Proverbs-Singh T, Feldman JL, Morris MJ,
Autio KA and Traina TA: Targeting the androgen receptor in prostate
and breast cancer: Several new agents in development. Endocr Relat
Cancer. 22:R87–R106. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Quinn DI, Tangen CM, Hussain M, Lara PN
Jr, Goldkorn A, Moinpour CM, Garzotto MG, Mack PCA, Carducci MA,
Monk JP, et al: Docetaxel and atrasentan versus docetaxel and
placebo for men with advanced castration-resistant prostate cancer
(SWOG S0421): A randomised phase 3 trial. Lancet Oncol. 14:893–900.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Morin F, Beauregard JM, Bergeron M, Nguile
Makao M, Lacombe L, Fradet V, Fradet Y and Pouliot F: Metabolic
Imaging of Prostate Cancer Reveals Intrapatient Intermetastasis
Response Heterogeneity to Systemic Therapy. Eur Urol Focus.
3:639–642. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Katzenwadel A and Wolf P: Androgen
deprivation of prostate cancer: Leading to a therapeutic dead end.
Cancer Lett. 367:12–17. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gravis G, Fizazi K, Joly F, Oudard S,
Priou F, Esterni B, Latorzeff I, Delva R, Krakowski I, Laguerre B,
et al: Androgen-deprivation therapy alone or with docetaxel in
non-castrate metastatic prostate cancer (GETUG-AFU 15): A
randomised, open-label, phase 3 trial. Lancet Oncol. 14:149–158.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gillessen S, Omlin A, Attard G, de Bono
JS, Efstathiou E, Fizazi K, Halabi S, Nelson PS, Sartor O, Smith
MR, et al: Management of patients with advanced prostate cancer:
Recommendations of the St Gallen Advanced Prostate Cancer Consensus
Conference (APCCC) 2015. Ann Oncol. 26:1589–1604. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ohlmann CH, Goebell PJ, Grimm MO, Klier J,
König F, Machtens S, Schostak M, Schrader AJ and Albers P:
Metastatic prostate cancer: Update: position paper for the use of
chemotherapy. Urologe A. 56:1597–1602. 2017.In German. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Oudard S, Fizazi K, Sengeløv L, Daugaard
G, Saad F, Hansen S, Hjälm-Eriksson M, Jassem J, Thiery-Vuillemin
A, Caffo O, et al: Cabazitaxel versus docetaxel as first-line
therapy for patients with metastatic castration-resistant prostate
cancer: A randomized phase III trial-FIRSTANA. J Clin Oncol.
35:3189–3197. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Galsky MD and Vogelzang NJ:
Docetaxel-based combination therapy for castration-resistant
prostate cancer. Ann Oncol. 21:2135–2144. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pitcher B, Khoja L, Hamilton RJ, Abdallah
K, Pintilie M and Joshua AM: Assessment of a prognostic model, PSA
metrics and toxicities in metastatic castrate resistant prostate
cancer using data from Project Data Sphere (PDS). PLoS One.
12:e01705442017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hwang C: Overcoming docetaxel resistance
in prostate cancer: A perspective review. Ther Adv Med Oncol.
4:329–340. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Attard G, Parker C, Eeles RA, Schröder F,
Tomlins SA, Tannock I, Drake CG and de Bono JS: Prostate cancer.
Lancet. 387:70–82. 2016. View Article : Google Scholar
|
|
18
|
Mouhid L, Corzo-Martínez M, Torres C,
Vázquez L, Reglero G, Fornari T and Ramírez de Molina A: Improving
in vivo efficacy of bioactive molecules: An overview of potentially
antitumor phytochemicals and currently available lipid-based
delivery systems. J Oncol. 2017:73519762017. View Article : Google Scholar :
|
|
19
|
Tummala R, Lou W, Gao AC and Nadiminty N:
Quercetin targets hnRNPA1 to overcome enzalutamide resistance in
prostate cancer cells. Mol Cancer Ther. 16:2770–2779. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang Y and Talalay P: Anticarcinogenic
activities of organic isothiocyanates: Chemistry and mechanisms.
Cancer Res. 54 (Suppl 7): 1976s–1981s. 1994.PubMed/NCBI
|
|
21
|
Zhao B, Seow A, Lee EJ, Poh WT, Teh M, Eng
P, Wang YT, Tan WC, Yu MC and Lee HP: Dietary isothiocyanates,
glutathione S-transferase -M1, -T1 polymorphisms and lung cancer
risk among Chinese women in Singapore. Cancer Epidemiol Biomarkers
Prev. 10:1063–1067. 2001.
|
|
22
|
Ambrosone CB, McCann SE, Freudenheim JL,
Marshall JR, Zhang Y and Shields PG: Breast cancer risk in
premenopausal women is inversely associated with consumption of
broccoli, a source of isothiocyanates, but is not modified by GST
genotype. J Nutr. 134:1134–1138. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Halkier BA and Gershenzon J: Biology and
biochemistry of glucosinolates. Annu Rev Plant Biol. 57:303–333.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guo Z, Smith TJ, Wang E, Eklind KI, Chung
FL and Yang CS: Structure-activity relationships of arylalkyl
isothiocyanates for the inhibition of
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone metabolism and the
modulation of xenobiotic-metabolizing enzymes in rats and mice.
Carcinogenesis. 14:1167–1173. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rampal G, Thind TS, Arora R, Vig AP and
Arora S: Synergistic antimutagenic effect of isothiocyanates
against varied mutagens. Food Chem Toxicol. 109:879–887. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Papi A, Orlandi M, Bartolini G, Barillari
J, Iori R, Paolini M, Ferroni F, Grazia Fumo M, Pedulli GF and
Valgimigli L: Cytotoxic and antioxidant activity of
4-methylthio-3-butenyl isothiocyanate from Raphanus sativus L.
(Kaiware Daikon) sprouts. J Agric Food Chem. 56:875–883. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cheung KL and Kong AN: Molecular targets
of dietary phenethyl isothiocyanate and sulforaphane for cancer
chemoprevention. AAPS J. 12:87–97. 2010. View Article : Google Scholar :
|
|
28
|
Wu CL, Huang AC, Yang JS, Liao CL, Lu HF,
Chou ST, Ma CY, Hsia TC, Ko YC and Chung JG: Benzyl isothiocyanate
(BITC) and phenethyl isothiocyanate (PEITC)-mediated generation of
reactive oxygen species causes cell cycle arrest and induces
apoptosis via activation of caspase-3, mitochondria dysfunction and
nitric oxide (NO) in human osteogenic sarcoma U-2 OS cells. J
Orthop Res. 29:1199–1209. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kadir NH, David R, Rossiter JT and
Gooderham NJ: The selective cytotoxicity of the alkenyl
glucosinolate hydrolysis products and their presence in Brassica
vegetables. Toxicology. 334:59–71. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Arora R, Kumar R, Mahajan J, Vig AP, Singh
B, Singh B and Arora S: 3-Butenyl isothiocyanate: A hydrolytic
product of glucosinolate as a potential cytotoxic agent against
human cancer cell lines. J Food Sci Technol. 53:3437–3445. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Okamura T, Umemura T, Inoue T, Tasaki M,
Ishii Y, Nakamura Y, Park EY, Sato K, Matsuo T, Okamoto S, et al:
Chemopreventive effects of 4-methylthio-3-butenyl Isothiocyanate
(Raphasatin) but not curcumin against pancreatic carcinogenesis in
hamsters. J Agric Food Chem. 61:2103–2108. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Suzuki I, Cho YM, Hirata T, Toyoda T,
Akagi JI, Nakamura Y, Sasaki A, Nakamura T, Okamoto S, Shirota K,
et al: Toxic effects of 4-methylthio-3-butenyl isothiocyanate
(Raphasatin) in the rat urinary bladder without genotoxicity. J
Appl Toxicol. 37:485–494. 2017. View Article : Google Scholar
|
|
33
|
Novío S, Cartea ME, Soengas P,
Freire-Garabal M and Núñez-Iglesias MJ: Effects of Brassicaceae
isothiocyanates on prostate cancer. Molecules. 21:E6262016.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Padilla G, Cartea ME, Velasco P, de Haro A
and Ordás A: Variation of glucosinolates in vegetable crops of
Brassica rapa. Phytochemistry. 68:536–545. 2007. View Article : Google Scholar
|
|
35
|
Smith TK, Lund EK, Clarke RG, Bennett RN
and Johnson IT: Effects of Brussels sprout juice on the cell cycle
and adhesion of human colorectal carcinoma cells (HT29) in vitro. J
Agric Food Chem. 53:3895–3901. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xiao D, Choi S, Johnson DE, Vogel VG,
Johnson CS, Trump DL, Lee YJ and Singh SV: Diallyl
trisulfide-induced apoptosis in human prostate cancer cells
involves c-Jun N-terminal kinase and extracellular-signal regulated
kinase-mediated phosphorylation of Bcl-2. Oncogene. 23:5594–5606.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xiao D, Vogel V and Singh SV: Benzyl
isothiocyanate-induced apoptosis in human breast cancer cells is
initiated by reactive oxygen species and regulated by Bax and Bak.
Mol Cancer Ther. 5:2931–2945. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mao L, Yang C, Wang J, Li W, Wen R, Chen J
and Zheng J: SATB1 is overexpressed in metastatic prostate cancer
and promotes prostate cancer cell growth and invasion. J Transl
Med. 11:1112013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
da Silva Ferreira R, Zhou D, Gasperazzo J,
Cabral MC, Silva-Lucca RA, Mentele R, Paredes-Gamero EJ, Bertolin
TC, dos Santos MT, Guedes PM, et al: Crystal structure of Crataeva
tapia bark protein (CrataBL) and its effect in human prostate
cancer cell lines. PLoS One. 8:e644262013. View Article : Google Scholar
|
|
40
|
Lee HY, Oh SH, Suh YA, Baek JH,
Papadimitrakopoulou V, Huang S and Hong WK: Response of non-small
cell lung cancer cells to the inhibitors of phosphatidylinositol
3-kinase/ Akt- and MAPK kinase 4/c-Jun NH2-terminal kinase
pathways: An effective therapeutic strategy for lung cancer. Clin
Cancer Res. 11:6065–6074. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fimognari C, Nüsse M, Iori R,
Cantelli-Forti G and Hrelia P: The new isothiocyanate
4-(methylthio)butylisothiocyanate selectively affects cell-cycle
progression and apoptosis induction of human leukemia cells. Invest
New Drugs. 22:119–129. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Barillari J, Iori R, Papi A, Orlandi M,
Bartolini G, Gabbanini S, Pedulli GF and Valgimigli L: Kaiware
Daikon (Raphanus sativus L.) extract: A naturally multipotent
chemopreventive agent. J Agric Food Chem. 56:7823–7830. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Papi A, Farabegoli F, Iori R, Orlandi M,
De Nicola GR, Bagatta M, Angelino D, Gennari L and Ninfali P:
Vitexin-2-O-xyloside, raphasatin and (-)-epigallocatechin-3-gallate
synergistically affect cell growth and apoptosis of colon cancer
cells. Food Chem. 138:1521–1530. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang Y, Yao S and Li J: Vegetable-derived
isothiocyanates: Anti-proliferative activity and mechanism of
action. Proc Nutr Soc. 65:68–75. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang Y: The molecular basis that unifies
the metabolism, cellular uptake and chemopreventive activities of
dietary isothiocyanates. Carcinogenesis. 33:2–9. 2012. View Article : Google Scholar :
|
|
46
|
Wang N, Wang W, Huo P, Liu CQ, Jin JC and
Shen LQ: Mitochondria-mediated apoptosis in human lung cancer A549
cells by 4-methylsulfinyl-3-butenyl isothiocyanate from radish
seeds. Asian Pac J Cancer Prev. 15:2133–2139. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Daja MM, Niu X, Zhao Z, Brown JM and
Russell PJ: Characterization of expression of matrix
metalloproteinases and tissue inhibitors of metalloproteinases in
prostate cancer cell lines. Prostate Cancer Prostatic Dis. 6:15–26.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Tai S, Sun Y, Squires JM, Zhang H, Oh WK,
Liang CZ and Huang J: PC3 is a cell line characteristic of
prostatic small cell carcinoma. Prostate. 71:1668–1679. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Stanton RA, Gernert KM, Nettles JH and
Aneja R: Drugs that target dynamic microtubules: A new molecular
perspective. Med Res Rev. 31:443–481. 2011. View Article : Google Scholar :
|
|
50
|
Mi L, Xiao Z, Hood BL, Dakshanamurthy S,
Wang X, Govind S, Conrads TP, Veenstra TD and Chung FL: Covalent
binding to tubulin by isothiocyanates. A mechanism of cell growth
arrest and apoptosis. J Biol Chem. 283:22136–22146. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Mi L, Gan N, Cheema A, Dakshanamurthy S,
Wang X, Yang DC and Chung FL: Cancer preventive isothiocyanates
induce selective degradation of cellular alpha- and beta-tubulins
by proteasomes. J Biol Chem. 284:17039–17051. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Xiao Z, Mi L, Chung FL and Veenstra TD:
Proteomic analysis of covalent modifications of tubulins by
isothiocyanates. J Nutr. 142:1377S–1381S. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Magadoux L, Isambert N, Plenchette S,
Jeannin JF and Laurens V: Emerging targets to monitor and overcome
docetaxel resistance in castration resistant prostate cancer
(review). Int J Oncol. 45:919–928. 2014.review. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zhu ML, Horbinski CM, Garzotto M, Qian DZ,
Beer TM and Kyprianou N: Tubulin-targeting chemotherapy impairs
androgen receptor activity in prostate cancer. Cancer Res.
70:7992–8002. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Khurana N, Talwar S, Chandra PK, Sharma P,
Abdel-Mageed AB, Mondal D and Sikka SC: Sulforaphane increases the
efficacy of anti-androgens by rapidly decreasing androgen receptor
levels in prostate cancer cells. Int J Oncol. 49:1609–1619. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kim SH and Singh SV: D, L-Sulforaphane
causes transcriptional repression of androgen receptor in human
prostate cancer cells. Mol Cancer Ther. 8:1946–1954. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Lane DP: Cancer p53, guardian of the
genome. Nature. 358:15–16. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zhou M, Gu L, Li F, Zhu Y, Woods WG and
Findley HW: DNA damage induces a novel p53-survivin signaling
pathway regulating cell cycle and apoptosis in acute lymphoblastic
leukemia cells. J Pharmacol Exp Ther. 303:124–131. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Qian J, Hirasawa K, Bostwick DG,
Bergstralh EJ, Slezak JM, Anderl KL, Borell TJ, Lieber MM and
Jenkins RB: Loss of p53 and c-myc overrepresentation in stage
T(2-3)N(1-3)M(0) prostate cancer are potential markers for cancer
progression. Mod Pathol. 15:35–44. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Strano S, Dell'Orso S, Di Agostino S,
Fontemaggi G, Sacchi A and Blandino G: Mutant p53: An oncogenic
transcription factor. Oncogene. 26:2212–2219. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Gan L, Wang J, Xu H and Yang X: Resistance
to docetaxel-induced apoptosis in prostate cancer cells by
p38/p53/p21 signaling. Prostate. 71:1158–1166. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Liu C, Zhu Y, Lou W, Nadiminty N, Chen X,
Zhou Q, Shi XB, deVere White RW and Gao AC: Functional p53
determines docetaxel sensitivity in prostate cancer cells.
Prostate. 73:418–427. 2013. View Article : Google Scholar
|
|
63
|
Muller PA and Vousden KH: p53 mutations in
cancer. Nat Cell Biol. 15:2–8. 2013. View Article : Google Scholar
|
|
64
|
Conley-LaComb MK, Saliganan A, Kandagatla
P, Chen YQ, Cher ML and Chinni SR: PTEN loss mediated Akt
activation promotes prostate tumor growth and metastasis via
CXCL12/ CXCR4 signaling. Mol Cancer. 12:852013. View Article : Google Scholar
|
|
65
|
Guan X: Cancer metastases: Challenges and
opportunities. Acta Pharm Sin B. 5:402–418. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Wells A, Yates C and Shepard CR:
E-cadherin as an indicator of mesenchymal to epithelial reverting
transitions during the metastatic seeding of disseminated
carcinomas. Clin Exp Metastasis. 25:621–628. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Härmä V, Haavikko R, Virtanen J, Ahonen I,
Schukov HP, Alakurtti S, Purev E, Rischer H, Yli-Kauhaluoma J,
Moreira VM, et al: Optimization of Invasion-Specific Effects of
Betulin Derivatives on Prostate Cancer Cells through Lead
Development. PLoS One. 10:e01261112015. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Bai SW, Herrera-Abreu MT, Rohn JL, Racine
V, Tajadura V, Suryavanshi N, Bechtel S, Wiemann S, Baum B and
Ridley AJ: Identification and characterization of a set of
conserved and new regulators of cytoskeletal organization, cell
morphology and migration. BMC Biol. 9:542011. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Jackson SJ and Singletary KW:
Sulforaphane: A naturally occurring mammary carcinoma mitotic
inhibitor, which disrupts tubulin polymerization. Carcinogenesis.
25:219–227. 2004. View Article : Google Scholar
|