1. Structure and function
Akt is a serine/threonine kinase previously known as
protein kinase B (PKB), consisting of three isoforms (Akt1, Akt2
and Akt3), with a crucial role in major cellular functions
including cell size, cell cycle progression, regulation of glucose
metabolism, genome stability, transcription, protein synthesis and
neovascularization. Akt promotes cell survival by mediating the
cellular growth factors and blocking apoptosis by the inactivation
of pro-apoptotic proteins (1-5).
From a structural point of view, Akt/PKB is
characterized by its similarity to protein kinases A (PKA) and C
(PKC), as well as to the retroviral oncoprotein viral akt (v-akt)
(6-9). Structurally, Akt comprises three
domains: An amino-terminal (N-terminal), a central and a
carboxyl-terminal fragment (C-terminal). The N-terminal domain, a
pleckstrin homology (PH) one, consists of 100 amino acids and is
similar to others found in 3-phosphinositide binding molecules,
interacting with membrane lipid products such as
phosphatidylinositol (3,4,5)-trisphosphate (PIP3) and
phosphatidylinositol 4,5-bisphosphate (PIP2). The kinase domain is
highly similar to the AGC protein kinases sharing a regulatory
threonine residue, Thr308. The phosphorylation of this residue
activates Akt. The C-terminal groove consists of 40 amino acids
forming a hydrophobic region, containing a regulatory serine
residue, Ser473 (6).
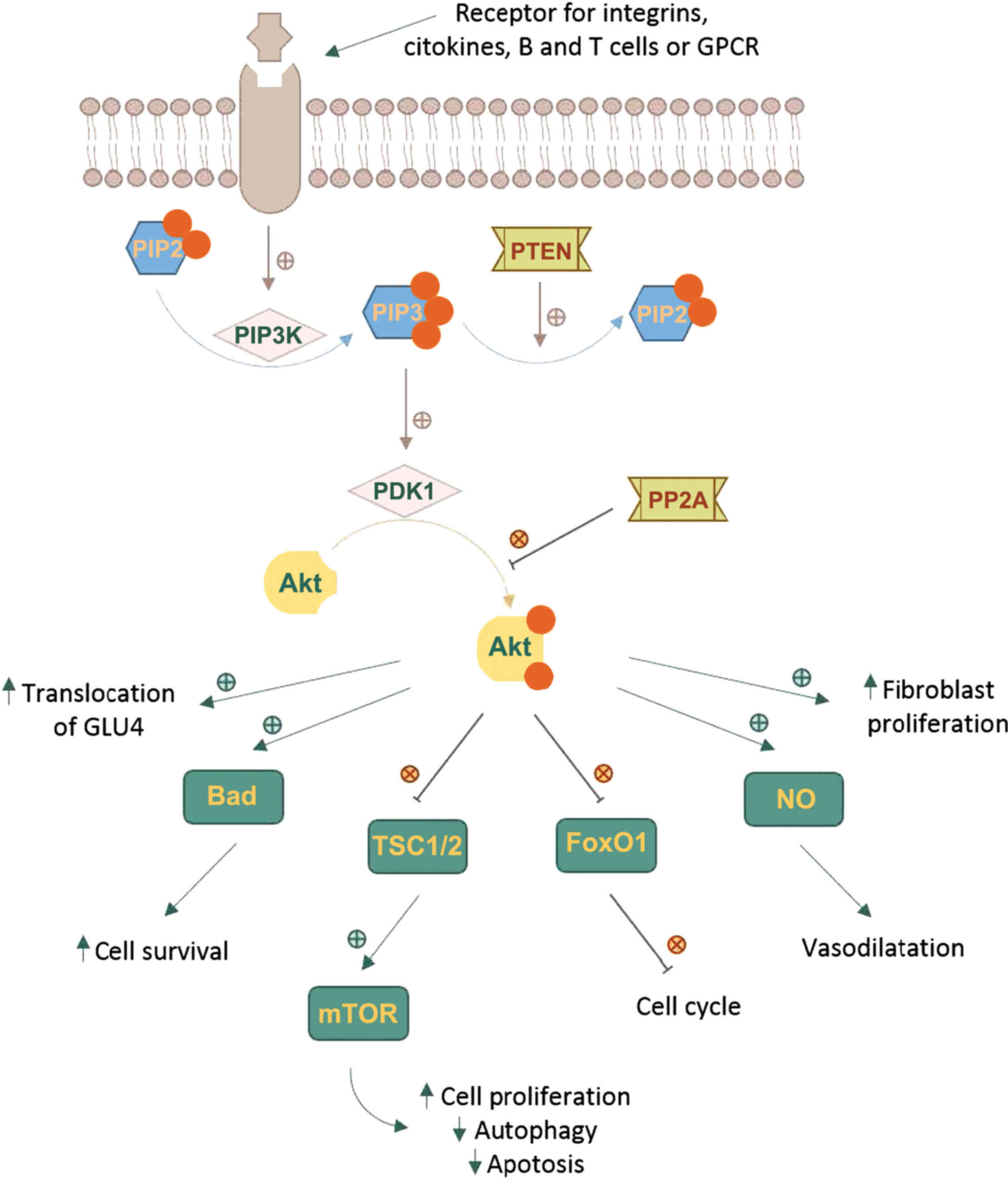
Upstream of molecular signaling pathways of Akt, are
found systems generating PIP3 as an effect of the action of
phosphoinositide 3-kinase (PI3K) (10-12).
Three types of intracellular lipid kinases are described (class I,
II and III) based on their structure and substrate selectivity.
Class I is divided into class IA (PI3Kα, β and δ) and class IB
(PI3Kγ). The PI3Kγ kinases are heterodimers with a catalytic
subunit (p110α, β, γ or δ) and a regulatory subunit (p85α, β or γ).
Class IA PI3Ks (PIK3CA, PIK3CB and PIK3CD), are constituted from a
p110 catalytic unit and a p85 regulatory one, and are the most
important isoforms in cancer studies. PI3Ks are activated by
different agents; PI3Kα, β and δ are activated when the
extracellular ligands bind to one of the transmembrane
glycoproteins with enzymatic activity called receptor tyrosine
kinases (RTKs), while PI3Kγ is activated by G-protein-coupled
receptors (GPCRs) and by the RAS family of GTPases (13-15).
The Akt cascade is activated by various signals
including RTKs, integrins, B and T cell receptors, cytokine
receptors and GPCRs throughPIP3 produced by PI3Ks. Akt is not
directly activated by PIP3, which alters Akt configuration by
binding to its PH region and recruits it to the plasma membrane
allowing phosphoinositide-dependent kinase-1 (PDK1) to
phosphorylate at Thr308 residue in the kinase domain (6,16-18).
The full activation of Akt requires a second phosphorylation at
regulatory Ser473 (Fig. 1).
A number of kinases are known to be capable of
phosphorylating Akt at Ser473. These include PDK-1, integrin-linked
kinase (ILK) or an ILK-associated kinase and Akt itself (19). Binding proteins such as actin,
Erk1/2, Hsp90, Hsp27 or Posh have been found to regulate the
activity of Akt (20). Members of
the PI3K-related kinase (PIKK) family, including DNA-dependent
protein kinase (DNA-PK), can also phosphorylate Akt at Ser473
(2,10,11).
Phosphorylated Akt contributes to the
phosphorylation of different proteins located either in the plasma
membrane, in the nucleus or the cytosol, supporting cell growth and
survival, among other cellular effects. Akt phosphorylates a large
number of targets on RxRxxS/T consensus motifs (21). Such downstream targets of Akt
phosphorylation are PRAS40, a component and regulator of mTOR
complexes, the actin-associated protein palladin, the cell cycle
inhibitors p21 (Cip1) and p27, and vimentin, all enhancing tumor
motility, invasion and metastasis growth (22,23).
One of the key elements of the Akt network is the
serine/threonine protein kinase known as mammalian target of
rapamycin (mTOR). mTOR can form two functionally distinct
multiprotein complexes, mTOR complex 1 (mTORC1) by joining with
regulatory-associated protein of target of rapamycin (RAPTOR) and
mammalian lethal with SEC13 protein 8 (mLST8). mTOR complex 2
(mTORC2) contains the protein RICTOR which is insensitive to
rapamycin, mLST8 and mammalian stress-activated protein kinase
interacting protein (mSIN1) (24,25).
mTOR is a downstream member of Akt and a key regulator of cell
growth and metabolism, but also an activator, mTORC2 directly
phosphorylating Akt's hydrophobic motif Ser473 (26). Ser473 phosphorylation enhances Akt
kinase activity and facilitates the Thr308 phosphorylation by PDK1
(27).
The Akt pathway intercedes cell growth and survival
by influencing the tuberous sclerosis complex (TSC) 1/2 along mTORC
signaling and, respectively, by inhibiting pro-apoptotic proteins
or signals (22,23,28,29).
Activated Akt reduces the formation of TSC1/2 and thus blocks the
activation of Rheb, a RAS family protein that activates mTORC1,
which in turn, induces the phosphorylation of ribosomal protein S6
kinase (S6K) and the eukaryotic translation initiation factor
4E-binding protein 1 (4E-BP1). Phosphorylated 4E-BP1 stimulates the
release of eukaryotic translation initiation factor 4E (eIF4E).
Both S6K and eIF4E promote protein translation and cell
proliferation (30-32). The phosphorylation of TSC2 by Akt
can dissociate the TSC1-TSC2 complex, thereby activating mTORC1.
When mTORC1 is activated, autophagy is inhibited (33).
Akt directly regulates cell survival by inhibiting
pro-apoptotic signals, such as Bad and Forkhead box O (FOXO)
transcription factors. FOXO proteins activate or suppress the
transcription of target genes through a highly conserved winged
helix DNA-binding domain and are regulated by several
posttranslational modifications, such as phosphorylation,
acetylation and ubiquitination (34). FOXOs activate the transcription of
target genes to promote cell cycle arrest, cell death and cellular
oxidative stress to maintain metabolic stability. Akt
phosphorylates and inactivates the FOXO transcription factors,
resulting in their nuclear exclusion and degradation in the
cytoplasm, triggering cell survival. The decreased activity of FOXO
blocks the transcription cyclin-dependent kinase (CDK) inhibitors
p27 and p21, resulting in cell cycle progression (35,36).
It also inhibits the extrinsic apoptotic pathway mediated by the
transcription of proapoptotic factors such as Fas ligand (FasL) and
TNF-related apoptosis-inducing ligand (TRAIL) (16,37).
There are some systems responsible for turning off
Akt. Akt is dephosphorylated by protein phosphatase 2A (PP2A) and
the PH domain leucine-rich repeat-containing protein phosphatases
(PHLPP). PP2A preferentially dephosphorylate Akt at Thr308 residue,
but under certain conditions it can also dephosphorylate the Ser473
residue (38). Although PHLPP1 and
PHLPP2 both dephosphorylate the Ser473 residue, they differentially
stop the signaling by regulating distinct Akt isoforms; PHLPP1
specifically modulates the phosphorylation of HDM2 and glycogen
synthase kinase (GSK)-3α by Akt2, whereas PHLPP2 regulates the
phosphorylation of p27 by Akt3 (39).
Phosphatase and tensin homolog (PTEN) is the most
important negative regulator of Akt function and its metabolic
downstream effects. The phosphatase activity of PTEN functions as
an antagonist of PI3K, dephosphorylating PIP3 in position 3′ to
form PIP2 (40,41). Mutations leading to the
amplification of genes in the receptor-PI3K pathway, and also the
loss of function of PTEN are found frequently in cancer tissues,
resulting in pathologically enhanced PI3K signaling and the loss of
cell growth control by decreased apoptosis (36).
The PI3K/Akt/mTOR pathway is dysregulated in
different diseases, such as solid tumors, immune-mediated disease,
idiopathic pulmonary fibrosis, cardio-vascular disease, metabolic
impairments such as diabetes mellitus, and constitutes a promising
therapeutic target (29,42,43).
2. Akt in malignant disease
The PI3K/Akt pathway has been intensively
investigated in cancer due to its significant role in cell survival
and anti-apoptotic mechanisms. Multiple pathogenic mechanisms,
including the loss of PTEN, mutations that activate the catalytic
subunit of PI3K, the activation of RAS and growth factor receptors,
or tge amplification of the genes encoding PI3K or Akt can lead to
Akt hyperactivation in cancer cells (44).
Studies have proven that the Akt signaling pathway
frequently malfunctions in various types of cancer and, in some
cases, is associated with tumor aggressiveness. Many types of human
cancer are associated with the upregulation of Akt. Anomalies of
Akt genes were described for a variety of human cancers; Akt1 gene
amplifications have been reported in gastric carcinoma,
glioblastomas and gliosarcomas, whereas Akt2 amplification has been
identified in head and neck squamous cell carcinoma, pancreatic,
ovarian and breast cancers (45).
Several studies have reported upregulated Akt3 expression in
androgen resistant prostate cancer cells, estrogen
receptor-deficient breast cancer cells, and in primary ovarian
cancers (46,47).
Several of the proteins involved in the Akt
signaling pathway (eIF4E, periostin, both the p110α and p85α
subunits of PI3K), when overexpressed, can function as
oncoproteins, while the ones involved in quenching this pathway
(PTEN, FOXO and TSC1/2) may constitute tumor suppressors (6,16,48).
PTEN activity can be impaired by various mechanisms, including but
not limited to, somatic mutations, homozygous deletions, epigenic
silencing through gene promoter methylation, or
post-transcriptional modifications (49). PTEN somatic mutations are described
in a large percentage of human cancers, with the highest frequency
in endometrial cancers, glioma, colorectal cancers, melanoma and
prostate cancers (45).
Autophagy is one mechanism through which cells can
move towards programmed cell death. Literature data indicate that
there are multiple links between impaired autophagy and cancer,
autophagy being a mechanism of tumor suppression. The Akt activated
mTOR signaling pathway negatively regulates autophagy; this
constitutes a pharmacological target, as anticancer molecules
blocking the PI3K/Akt/mTOR signal promote autophagy progression,
and also reduce angiogenesis (33,50).
In this context, recent research has proven that anti-inflammatory
molecules (aspirin, celecoxib, meloxicam, or indomethacin) may be
useful tools in dealing with tumors as they have the ability to
interact with these signaling pathways (50-52).
In addition, the overexpression of Akt is linked to
resistance to chemotherapeutic agents such as cisplatin,
methotrexate or paclitaxel (53).
Cisplatin-induced DNA damage causes the phosphorylation of BAD via
Akt, suppressing its apoptotic effect (54). This observation can be capitalized
as a promising strategy for synergic cancer therapy. The Akt
inhibitor, MK-2206, has been shown to improve the effectiveness of
cisplatin in the gastric cancer cell line, AGS (55), and against the ovarian cancer cell
line, SKOV3 (56). MK-2206
demonstrated similar synergic effect in combination with paclitaxel
in SKOV3 cells (56). A low PTEN
expression is a good predictor of poor responses to human epidermal
growth factor receptor (HER2) antagonist therapies and it has been
hypothesized that Akt inhibitors can overcome the development of
resistance (57,58).
The importance of Akt in cancer pathologies renders
it a much pursued target for anticancer therapy, and multiple drug
discovery programs focus on finding selective and potent
inhibitors. One of the mains issues counteracting drug design
efforts consists of achieving selectivity over structurally similar
protein kinase, particularly towards the AGC kinase family. The
existence of three isozymes with distinct function, tissue
distribution and ligand affinity also obstructs the research. The
target-based drug development of Akt-specific and isoform-selective
inhibitors using the catalytic domain has been predicted to be
difficult due to high sequence homology, determining alternative
and novel approaches to identify allo-steric inhibitors (29,44).
In an extensive review, we proposed a new
classification of the Akt inhibitors based on the binding mechanism
related to adenosine triphosphate (ATP) and on their main chemical
scaffold (29). The Akt inhibitors
advanced from ATP-competitive agents to ATP non-competitive
inhibitors binding to allosteric sites in order to solve the high
structural similarity in the catalytic domain between Akt isoforms
and considerable structural analogy to the AGC kinase family
(29,59).
The majority of Akt inhibitors targeting the ATP
binding site are non-selective against the three isoforms, and
poorly selective against structurally similar kinases (60). Akt1-3 share a high ATP-binding site
homology with S6K1 (84%), PKA (81%), PKC (78%), SGK (78%), PRKX
(75%), PKN1 (75%) and Aurora A (72%) (43). The ATP-competitive inhibitors of
Akt can be chemically described as isoquinoline-5-sulfonamides,
azepane derivatives, aminofurazans, heterocyclic 6-5 fused rings,
phenylpyrazoles, thiophene carboxamides and thiazole carboxamides
(29).
The ATP non-competitive Akt inhibitors are
allosteric modulators, the binding mechanism offering important
advantages, as greater specificity, reduced side-effects and lower
toxicity (43). Chemically, they
are categorized as 2,3-diphe-nylquinoxaline and analogs,
alkylphospholipids, derivatives of indole-3-carbinol, sulfonamides,
thiourea deratives and purine derivatives. A special class of ATP
non-competitive are the irreversible inhibitors that interact
covalently with the enzyme (29).
Current pre-clinical and clinical data suggest that
the use of an intermittent high-dose posology is more effective
than a continuous low dose by daily administration. The high doses
are required for induction of apoptosis and pauses can overcome the
toxicological risks. Also, data shows a delay in treatment
resistance mechanisms. The combination with other targeted
therapeutic agents further enhances the antitumor activity of Akt
inhibitors (42,61).
3. Neurological diseases
Early observation associated with increased amounts
of glutamate with excitotoxicity and neural cell death have opened
and facilitated the development of treatments for glutamate-related
disorders, such as Alzheimer's disease (AD), parkinsonism,
epilepsy, or multiple sclerosis (62). The overstimulation of glutamate
receptors (GluRs) and other post-synaptic signaling components
results in excitotoxicity. It is considered that
N-methyl-D-aspartate (NMDA) subtypes play a major role, and
other GluR subtypes such as acid
2-amino-3-(3-hydroxy-5-methylisoxazol-4-yl)propionic (AMPA) or
kainate receptors play a critical role in the excitotoxic neuronal
cell death process (63).
Reversely, the stimulation of metabotropic GluR1 (mGluR1) exerts
neuroprotectives effect by reducing nerve cell death induced by
exposure to NMDA (64,65).
AD is a progressive neurodegenerative disease
characterized by the alteration of memory and cognitive functions,
and is caused by the damage of neurons. Post-mortem
histopathological examinations have revealed extracellular amyloid
β (Aβ) plaques and intracellular neurofibrillary tangles as
hallmark lesions of AD. It has been demonstrated that an elevated
GSK-3β activity is directly linked to increased levels of Aβ
production and deposits, tau hyperphosphorylation and the formation
of neurofibrillary tangles (66,67).
GSK-3β is rendered inactive when it is phosphorylated at Ser9 by
phosphorylated Akt, and therefore an upregulation of Akt may
contribute to a decrease in AD progression. This hypothesis is
associated with the neurotoxic effect of wortmannin, an inhibitor
of PI3K that induces tau hyperphosphorylation similar to that
observed in AD following incubation in hippo-campus slice culture.
Lithium chloride, a GSK-3β inhibitor, prevents the neurotoxic
effects of wortmannin (68).
The treatment of hippocampal and cerebral cortical
cell cultures with toxic doses of NMDA has been shown to cause a
significant decline in phosphorylated Akt levels and phosphorylated
GSK-3β, without caspase-3, caspase-7, or poly(ADP) ribose
polymerase (PARP) cleavage; the total levels of Akt and GSK-3β were
not shown to be affected (69).
Excessive NMDA activity has been demonstrated to cause
excitotoxicity, promoting cell death, a probable mechanism of
neurodegeneration in AD, in agreement with the GSK-3β
hyperactivation hypothesis (70).
The role of Akt hypofunction in AD has been demonstrated by several
studies on PTEN regulation (71,72).
Okadaic acid leads to PTEN activation in SH-SY5Y cells associated
with an increase in tau phosphorylation, an effect that can be
blocked by PTEN knockdown (73).
The elevation of Akt activity has emerged as a
effective strategy with which to prevent progressive neuronal death
in neurological diseases. The 4H-chromene-3-carboxylate
derivative, SC79, was identified as a specific pan-Akt activator.
SC79 specifically binds to the PH domain of Akt, leading to a
conformation favorable for phosphorylation by upstream protein
kinases, without altering total Akt levels. The treatment of
cultured cortical neurons with SC79 has been shown to markedly
enhance Akt phosphorylation and reduce neuronal death elicited by
glutamate excitotoxicity in hippocampal neurons. SC79 effectively
reduced the neocortical lesion size by 35% after a single dose of
0.04 mg/g of body weight in a murine ischemic stroke model
(74).
The inhibition of PTEN function in conditions
associated with neurodegeneration, tissue injury or ischemia may be
a valuable pharmacological intervention with which to activate Akt
function. Bisperoxovanadium compounds are reversible and relatively
specific small PTEN inhibitors, with nanomolar affinity and have
been experimentally explored as a potential therapeutic approach in
AD and other neurological diseases (75).
Parkinson's disease (PD) is a chronic and
progressive neurodegenerative disorder characterized by the
premature death of dopaminergic neurons in the substantia nigra
(76). Oxidative stress is a major
cause of PD pathogenesis, inducing neuronal cell death and
apoptosis by intracellular calcium overload, lipid peroxidation,
DNA damages and excitotoxicity.
The activation of Akt has been shown to efficiently
protect neurons or neuronal cells from oxidative stress and is an
established target of drug design in PA. Pre-treatment of human
dopaminergic neuronal cells with the specific Akt activator, SC79
(10 μM), was previously shown to markedly attenuate hydrogen
peroxide-induced toxicity. The neuroprotective effect of SC79 was
abolished by Akt inhibitors (77).
Sulfuretin, a trihydroxyaurone isolated from the
bark of the lacquer tree (Toxicodendron vernicifluum), was
previously shown to significantly decrease apoptotic cell death
induced by the 1-methyl-4-phenylpyridinium (MPP+) in an
experimental model of PD. Sulfuretin reduced caspase-3 and PARP
activity accompanied by a reduction of intracellular reactive
oxygen species (ROS) production and recovered the normal
mitochondrial membrane potential. The mechanism involved the
augmentation of the phosphorylation of Akt, GSK-3β and Erk pathway,
confirmed by the disappearance of the cytoprotective effects of
sulfuretin following the administration of PI3K/Akt and Erk
inhibitors (78). Vitexin, a
glucoside derivative of apigenin and several other flavonoids had
similar protective effects on cell and mouse models of PD (79).
4. Insulin signaling and diabetes
Akt is involved in the insulin signaling pathway;
the activation of the insulin receptor triggers a phosphorylation
cascade, initiated by receptor autophosphorylation and the
activation of insulin receptor substrate proteins (IRS-1 and
IRS-2), recruiting PI3K that phosphorylates PIP2 to PIP3.
Membrane-anchored PIP3 activates Akt, thus contributing to the
translocation of the glucose transporter 4 (GLUT4) at the membrane
level (80,81). Similarly to insulin, members of the
family of insulin-like growth factors (IGF) activate PI3K to
produce PIP3, which, in turn, recruits two protein kinases to the
plasma membrane via their PH domains Akt and PDK1, leading to Akt
activation (81). Akt2 and its
downstream effectors control the insulin-stimulated translocation
of GLUT4 to the plasma membrane. The most important are AS160, a
Rab GTPase-activating protein, CDP138, a C2 domain-containing
phosphoprotein, and an actin-capping protein, tropomodulin 3
(82,83).
The downstream steps determining the effects of
insulin on target tissues, including glucose uptake in skeletal
muscle and adipose tissue, and hepatic gluconeogenesis are
controlled by Akt2. Glycogen synthesis is regulated by Akt
phosphorylation and the inactivation of GSK-3β, resulting in
glycogen synthase activation (81).
Akt2 is highly expressed in insulin-responsive
tissues and is an important regulator of glucose metabolism. The
deletion of Akt2 in knockout mice was previously shown to result in
insulin resistance, hyperinsulinemia and glucose intolerance,
whereas Akt1 or Akt3 knockout mice have a normal glucose metabolism
(84). Leptin was shown to
increase insulin sensitivity in Akt-deficient mice (85). Consistent with these studies,
defects in the kinase domain of Akt2 are associated with insulin
resistance and diabetes in humans (86). Conversely, an activating mutation
of Akt2 in humans was reported and was shown to be associated with
severe insulin-independent hypoglycemia and asymmetric overgrowth
(87) and a lower risk of diabetes
was observed in individuals with PTEN haploinsufficiency (81).
Insulin is also a potent neurotrophic factor and the
activation of the insulin receptors, highly expressed in sensory
neurons, enhances axon growth through the stimulation of the
PI3K/Akt pathway and counteracts diabetic polyneuropathy symptoms.
Recent research considers that an important strategy for reversing
the neuropathic deficits of diabetic neuropathy may be either the
activation of intrinsic PI3K/Akt signaling or the inhibition of the
PTEN effect (80,81). As in the case of AD, various
vanadium compounds have been proven to reduce blood glucose in
diabetic rats (88,89). Although he systemic long-term PTEN
pharmacological inhibition is likely to promote cancer, it is
possible that a tissue-specific inhibition may be beneficial in
type 2 diabetes treatment (75).
Another strategy which can be used to enhance Akt
signaling to potentially overcome insulin resistance, is the
inhibition of PHLPP. The strategy is enforced by the observation
that the levels of PHLPP1 are highly elevated in the skeletal
muscle of obese humans, and are associated with the increased body
mass index and insulin resistance. Additionally, PHLPP1 mRNA levels
are elevated in muscle cells from diabetic patients (40). Two specific inhibitors of PHLPP2
were identified in a virtual screening coupled with biochemical
assays, a derivative of aminoanthraquinone and a diazenyl
derivative of salicylic acid. Both compounds inhibit the
dephosphorylation of Akt on Ser473 at micromolar doses and have the
potential to improve insulin secretion (90).
Statins are HMG-CoA reductase inhibitors intensively
used to reduce cholesterol levels in a wide range of patient
populations. Several studies have demonstrated that statin use is
associated with an increased risk of developing type 2 diabetes
mellitus by the reduction of insulin secretion and the induction of
insulin resistance. These effects are produced by decreasing GLUT-4
levels and the inhibition of GSK-3β, p38 MAPK and Akt
phosphorylation (91,92). Statin treatment profoundly affects
signaling through Akt in several cell models. Simvastatin and
atorvastatin have been shown to inhibit Akt activation and to be
cytotoxic in mouse myoblast cell line at doses of 10 μM,
while for rosuvastatin the dose is 50 μM (92,93).
These studies highlight the importance of the Akt signaling pathway
in both statin-induced myotoxicity and diabetes, as well as the
anticancer effects of statins.
5. Involvement of Akt in cardiovascular
events
In a number of physiological and pathological
cardiovascular processes, such as vessel remodeling,
atherosclerosis and vascular injury, apoptotic cell death was
identified as a major common factor leading to a close analysis of
the Akt network involvement. Akt activation stimulates downstream
anabolic signaling, favoring cell growth, proliferation and
survival; this is a desired effect for cardiac cells, whose
metabolic improvement following the stimulation of Akt may also
alleviate cardiac cellular damage (94).
Akt1 plays an important role in the regulation of
cardiac hypertrophy and angiogenesis, as the absence of Akt1 leads
to severe atherosclerosis and occlusive coronary arterial disease,
associated with significant reduction in NO production and
endothelial cell viability. It was observed that atherosclerosis
results in the reduction of Akt expression and phosphorylation in
plaque intimal vascular smooth muscle cells and an increased
expression of active FOXO3a. An ectopically activated Akt was used
to demonstrate that Akt activation is sufficient for the survival
of vascular smooth muscle cells in response to oxidative stress.
The PI3K/Akt pathway can stimulate NO production by endothelial
cells and increase the synthesis of prostacyclin, thus leading to
vasodilation and retarding atherogenesis (94-96).
Akt2 is also involved in multiple functions in the
cardiovascular system due to its abundance in insulin-responsive
tissues. Mice lacking Akt2 present with hyperglycemia and
dyslipidemia with high levels of triglycerides and cholesterol,
although the absence of Akt2 does not influence the progression of
atherosclerosis in mice (95).
The PI3K/Akt pathway is involved in cardioprotection
by various mechanisms, such as the heat shock protein (HSP)
90-mediated protection of cardiomyocytes subjected to hypoxia or
the estrogen receptor (97).
Studies using mouse models have shown that a reduced activation of
the PI3K p110α catalytic subunit increases the risk of atrial
fibrillation. Conversely, the augmentation of Akt activity in
murine models of heart failure has been shown to improve cardiac
function (98,99). These findings explain why some
anticancer protein kinase inhibitors, such as ibrutinib and
nilotinib, increase the occurrence of atrial fibrillation and
drug-induced long QT syndrome (98,100).
It is hypothesized that the PI3K/Akt signaling
pathway may provide a solution for addressing proarrhythmia by
regulating cell proliferation and survival through the PIP3
activation of downstream signals; nevertheless, an increased
activity of PI3K/Akt signaling may lead to cancer development
(100).
6. Pulmonary fibrosis
Idiopathic pulmonary fibrosis (IPF) is a chronic,
progressive lung disease, one of the most common types of
interstitial pneumonia associated with high morbidity and
mortality. In normal lung fibroblasts, the interaction with type I
collagen, suppresses the PIP3K/Akt pathway, through the activation
of PTEN, thus inhibiting cell proliferation and promoting
fibroblast apoptosis. Pathologically, in fibroblasts from patients
with IPF, PI3K/Akt activity is enhanced due to the suppression of
PTEN, rendering the cells highly proliferative and resistant to
apoptosis (101-103). It has been demonstrated that the
low activity of PTEN is caused by the decline of the caveolin-1 and
FOXO3a levels (104,105).
Previously, a model of pulmonary fibrosis was used
in rats following the intratracheal administration of bleomycin,
and it was demonstrated that the high levels of phosphorylated Akt
were involved in the progression of pulmonary fibrosis and
contributed to fibrogenesis (106). Tubastatin, a known histone
deacetylase 6 (HDAC6) inhibitor, has been shown to protect mice
against bleomycin-induced fibrosis by decreasing Akt
phosphorylation and increasing Akt association to PHLPP,
independent of its HDAC6 effects (107).
7. Akt in inflammation and autoimmune
diseases
Autoimmune diseases occur when the immune system
mistakenly recognizes self-tissues as foreign and triggers an
immune response. The autoreactivity of the immune system is
multifactorial and may lead to pathogenic autoimmunity and is
related autoimmune diseases (108). Although the exact mechanisms of
the pathogenesis of autoimmune diseases remain unclear, the over
activation and abnormal function of T cells, B cells and myeloid
cells has been extensively studied (109-111). Systemic lupus erythematosus (SLE)
is a systemic autoimmune disease characterized by the high
hyperactivity of T cells and B cells, which result in the
production of antinuclear, anti-double-stranded DNA and
anti-glomerular antibodies, nephritis, renal failure and mortality
(112).
Patients with SLE are characterized by the
activation of mTORC1 and reduced mTORC2 levels, promoting the
expression of pro-inflammatory cytokines, and the deletion of the
regulatory T cell subtype that normally regulates inflammation
(113). To further explore the
underlying mechanisms of SLE, researchers have used mice that are
characterized by the constitutive activation of the PI3K pathway in
T lymphocytes and found that these mice develop SLE-like diseases
(114). The increased activation
of mTORC1 has also been reported in the T cells of patients
suffering from multiple sclerosis (115). In a study on patients with
rheumatoid arthritis, Zhang et al found significantly higher
levels (~4-fold) of p-Akt in fresh syno-vial tissue isolated from
these patients (116). These
findings highlight the central role of the Akt pathway in
autoimmune diseases. It has been suggested that alterations in the
PI3K/Akt axis found in highly active immune cells are mediated
through metabolic changes in these cells (117).
Studies now focus on the therapeutic potency of
molecules that target the PI3K/Akt pathway for the treatment of
autoimmune diseases. Indeed, rapamycin, which inhibits the
antigen-induced proliferation of T cells through the inhibition of
mTOR, has been shown to exert beneficial effects in experimental
models and patients with autoimmune diseases (118,119). Although there are several
challenges that need to be tackled, the further exploration of this
pathway may lead to the identification of disease biomarkers and
therapeutic targets (120).
In Table I the main
pathways that could be modulated by different molecules interfering
with Akt signaling are reviewed.
 | Table IPossible mechanism for Akt modulation
used in clinical and preclinical settings. |
Table I
Possible mechanism for Akt modulation
used in clinical and preclinical settings.
| Pathology models
associated with Akt impairment | Signaling pathway
injury | Molecules involved
in modulating intracellular pathways | (Refs.) |
|---|
| Malignant disease
(carcinomas, glioblastoma, hematological malignancies, gastric
carcinoma, glioblastomas and gliosarcomas, head and neck squamous
cell carcinoma, pancreatic, ovarian, skin, prostate and breast
cancers) | Loss of PTEN
activity, including mutations involving PTEN mutations activating
the catalytic subunit of PI3K, activation of RAS and growth factor
receptors, amplification of the genes encoding PI3K and Akt
impairment of proteins involved in the Akt signaling pathway
impairment of PI3K/Akt/mTOR regulated autophagy |
Isoquinoline-5-sulfonamides, azepane
derivatives, aminofurazans, heterocyclic 6-5 fused rings,
phenylpyrazoles, thiophenecarboxamides and derivatives
2,3-diphenylquinoxaline and analogs, alkylphospholipids,
indole-3-carbinol derivatives, sulfonamides, thiourea deratives,
and purine derivatives anti-inflammatory molecules (aspirin,
celecoxib, meloxicam, or indomethacin) MK-2206 | (30,45,46,
49-51,52-56) |
| Neurological
disorders (Alzheimer's, Parkinson's, and Huntington's disease,
epilepsy and multiple sclerosis) | Elevated GSK-3β
levels (due to Akt inhibition) overactivation of PTEN | Lithium chloride
okadaic acid 4H-chromene-3-carboxylate derivative (SC79),
bisperoxovanadium compounds sulfuretin and vitexin | (63-65,68,69,
70,75,76) |
| Insulin resistance,
hyperinsulinemia, and glucose intolerance, diabetic polyneuropathy
symptoms | Deletion of Akt2
inhibition of PI3K-Akt signaling PTEN activation inhibition of
PHLPP | Vanadium
compounds | (41,85,86,87) |
| Cardiovascular
disease (vessel remodeling, atherosclerosis, etc.) | Inhibition of Akt1
reduction of Akt expression and phosphorylation overexpression of
active FOXO3a | Anticancer protein
kinase inhibitors (ibrutinib and nilotinib) | (91-93,95-97) |
| Idiopathic
pulmonary fibrosis | PTEN suppression
Activation of mTORC1 | Tubastatin | (98-100, 101,103) |
8. Repurposing studies
Drug repositioning is a highly studied alternative
using available drugs for the treatment of various conditions in
order to discover novel therapeutic opportunities. This strategy is
also used on the Akt pathway, capitalizing the lower toxicological
risk of known drugs. Ivermectin is a macrocyclic lactone used as an
antiparasitic drug that has been shown to significantly decrease
Akt phosphorylation at Ser473. This result could allow its clinical
investigation for cancer patients (121). Another antiparasitic drug,
artesunate, may be useful in nasopharyngeal carcinoma by inhibiting
Akt (122). Recent research has
demonstrated the potential of propofol, an intravenous anesthetic
drug, to be repurposed to overcome resistance to imatinib in
chronic myeloid leukemia treatment (123). Cimetidine, a drug used in the
treatment of peptic ulcers, induces the apoptotic death of
cholangiocarcinoma cells by the suppression of Akt phosphorylation
and may be an effective candidate for future treatments (124). A well-known antifungal agent,
itraconazole, has demonstrated anticancer activity in various cell
lines by inhibiting Akt/mTOR signaling (125). Quetiapine, an atypical
antipsychotic, decreases the level of certain pro-inflammatory
cytokines, such as interleukin (IL)-17, IL-6 and IL-1β in mouse
model of arthritis by suppressing the Akt and Erk pathways
(126).
9. Natural products targeting the Akt
pathway
Plants and natural products are used as
complementary or alternative therapies for cancer treatment, as a
large number of patients consider them safer and less toxic
(127,128). Curcumin, the main curcuminoid
found in Curcuma longa, has showed beneficial results
regarding the inhibition of human malignant glioma cells by
inducing autophagic cell death. The mechanisms implicated are the
inhibition of the Akt/mTOR/p70S6K pathway and the activation of
Erk1/2 pathway, and these have been demonstrated in in vitro
and in vivo experiments (129). Lycopene, a carotenoid pigment
found in tomatoes, has been shown to exert antitumorgenic effects
on human colon cancer HT-29 cell lines, partly through the
inhibition of Akt phos-phorylation (130). Lupeol is a triterpenoid compound
found in mango, dandelion and several other species of Acacia
visco and Abronia villosa. Lupeol and its acetyl
derivative have been shown to inhibit the proliferation and induce
the apoptosis of human pancreatic cancer and chemoresistant
prostate cancer cells by decreasing the levels of p-Akt and p-Erk
(131). The chemically similar
tirucallic acids isolated from the oleogum resin of Boswellia
carterii inhibit Akt in prostate cancer cells by bonding within
the PIP3 binding pocket (132).
As mentioned above, several flavonoid compounds,
such as sulfuretin and apigenin, have been proven to target Akt.
Jaceosidin is a flavone from Artemisia princeps, a
traditional medicinal herb, with a marked effect on oral squamous
cell carcinoma by Akt downregulation (133). The antiproliferative and
antiinflammatory effect of luteolin via the inactivation of
PI3K/Akt pathway has also been demonstrated by several studies
(134,135).
Euplotin C is a secondary metabolite of the marine
ciliate Euplotes crassus that has demonstrated marked
cytotoxic effects on human cutaneous melanoma cells via the
inhibition of the Erk and Akt pathways (136). A large diversity of plant
extracts have demonstrated promising anticancer properties in
various cell lines, such as Libidibia ferrea and
Celastrus orbiculatus in colorectal cancer (137,138), Astragalus membranaceus,
Anthriscus sylvestris and Vernonia amygdalina in
breast cancer cells (139-141),
Azadirachta indica in prostate cancer (142), and Fallopia aubertii and
Fallopia convolvulus in cervical cancer cells (143), and these may represent future
sources of leading Akt inhibitors. The usefulness of the natural
sources is not limited to oncology. Bamboo leaf extract
administered for a period of 12 weeks to diabetic rats has been
shown to improve body weight and biochemical markers, and to
alleviated renal injury by increasing p-Akt levels (144,145). Similar properties were observed
for sea buckthorn fruit oil extract and the traditional Chinese
medicine Liuwei Dihaung (146,147).
10. Akt inhibitors in clinical studies
Several compounds have been proven to inhibit Akt in
in vitro and in vivo models, but only a small number
have entered clinical evaluation, and yet no Akt inhibitor has been
approved for anticancer therapy (148). Miltefosine is approved for the
oral treatment of both visceral and cutaneous leishmaniasis, but
not for oncological use (149).
GSK690693 was the first clinically evaluated Akt
inhibitor. In preclinical experiments, GSK690693 was shown to
inhibit all three isoforms at nanomolar concentrations and to
significantly inhibit the growth of various tumors in mouse
xenograft models. Despite the encouraging preclinical data, the
clinical development of the agent was halted due to drug-related
hyperglycemia and peripheral insulin resistance (150,151).
AZD5363 is a pyrrolopyrimidine derivative that
inhibits all Akt isoforms with nanomolar affinity, and inhibits the
proliferation of a large number of solid and hematological tumor
cell lines, with the highest therapeutical potential in breast
cancer cells (152). The safety
and tolerability of AZD5363 as oral monotherapy was evaluated in an
open-label study (NCT01226316) on 90 patients with advanced solid
malignancies. The patients received 320, 480 or 640 mg in a
continuous or intermittent schedule. The most frequently reported
adverse effects in all dosing schedules were diarrhea, nausea and
vomiting. A total of 56 (62%) patients experienced grade 3 adverse
events, such as hyperglycemia (20%), diarrhea (14%) and rash (11%).
An adverse event leading to discontinuation was reported in 23% of
the patients (153).
AZD5363 is under clinical evaluation as a
short-term monotherapy in estrogen receptor-positive breast cancers
(NCT02077569), and in non-small cell lung cancer (NCT02664935). It
has been evaluated in combination with other anticancer drugs in
various clinical trials (153,154).
Ipatasertib belongs to the heterocyclic 6-5 fused
rings class of ATP-competitive inhibitors. In a phase I study
(NCT01090960), the safety and tolerability in patients with
refractory solid tumors was evaluated following orally
administration of ipatasertib at daily doses from 25 to 800 mg.
Ipatasertib was well tolerated, the most frequently reported
adverse events of grade 2 or above were diarrhea (35%), nausea
(27%), asthenia (25%), hyperglycemia (10%), decreased appetite
(6%), rash (6%) and vomiting (6%). The maximum tolerated dose (MTD)
for ipatasertib is 600 mg on a once daily, 21/7 dosing schedule
(155).
The combination of ipatasertib (400 mg daily) and
paclitaxel (80 mg/m2 weekly) was well-tolerated in a
phase II study (NCT02301988) designed to estimate the efficacy in
women with triple-negative breast cancer (42). A double-blind placebo controlled
randomized phase II trial (NCT02162719) demonstrated that the
combination modestly improved the progression-free survival
endpoint, the results being prominent in the patients with
PIK3CA/Akt1/PTEN alterations (156).
Afuresertib (GSK2110183) is a thiophenecarboxamide
derivative, orally bioavailable Akt inhibitor, with higher potency
against Akt1 compared to Akt2 and Akt3 (157). An open-label phase I clinical
study (NCT00881946) evaluated afuresertib in 73 patients with
advanced hematologic malignancies. All patients enrolled in the
study experienced at least one adverse event, the most frequent
being nausea, diarrhea, dyspepsia, fatigue, gastrointestinal reflux
disease and anorexia. Treatment-related adverse events of grade 3
had a frequency under 10% and included neutropenia, rash,
odynophagia, fatigue and asthenia, abnormal liver function test and
thrombocytopenia. No grade 4 or 5 adverse events were observed.
Afuresertib produced a small impact on glycaemia, due to the
selectivity towards PKC (158).
In a phase IIa study (NCT1395004), 17 patients with Langerhans cell
histiocytosis received daily oral doses of 125 mg. The majority of
the reported adverse events were grade 1 or 2 and consisted on
nausea (59%), fatigue (53%), diarrhea (47%) and upper respiratory
infection (47%) (159).
Uprosertib (GSK2141795) is closely related to
afuresertib, the main difference being the replacement of the
thiophene with a bioisostere furan ring (29). The safety, tolerability,
pharmacokinetics, and pharmacodynamics of the drug were evaluated
in a phase I, open-label study in patients with solid tumors.
Uprosertib was safe and well-tolerated, most treatment-related
adverse events were low grade and included diarrhea, fatigue,
vomiting and decreased appetite (160).
MK-2206 is an orally effective, highly potent and
selective allosteric pan-Akt inhibitor (150). Based on a phase I study of
MK-2206 (NCT00670488) the MTD value was established at 60 mg for an
alternate days administration. Most frequent drug-related
toxicities recorded were skin rash (51.5%), nausea (36.4%),
pruritus (24.2%), hyperglycemia (21.2%) and diarrhea (21.2%). The
main dose limiting toxicity is a dose-dependent, generalized
erythematous, nonblistering, maculopapular rash. This rash was
observed also in other clinical trials with MK-2206 (161) and is similar to that produced by
mTOR or PI3K inhibitors, but unlike those caused by epidermal
growth factor receptor inhibitors indicating a mechanism-based
toxicity, and not an off-target effect (162). It was investigated in several
clinical trials. The potential use of MK-2206 goes beyond oncology,
demonstrating a significant reduction of plasma LDL-cholesterol
levels in cultured hepatic cells (163).
Perifosine is an alkylphospholipid derivative that
interferes with the PH domain of Akt rendering it incapable of
phosphorylation and activation. Chemically, it resulted by
replacing the choline moiety of miltefosine with a piperidine
scaffold, leading to higher metabolic stabilityand better
gastrointestinal tract tolerance because perifosine is not able to
generate phosphocholine, responsible for the parasympathomimetic
effects (29,164). Clinical trials using oral
perifosine as a single therapy for various types of cancer have
produced disappointing results. Several combinations of perifosine
are yielded promising results and are undergoing clinical
development (165).
SR13668 was designed as an Akt activation inhibitor
based on the naturally occurring indole-3-carbinol. In preclinical
safety testing, no toxic effects were observed in rats after a
single oral dose of 1,000 mg/kg or multiple doses of 25 up to 600
mg/kg/day for 14 days. In mice, doses up to 500 mg/kg had no
effects on fasting glucose levels and body weight. It has a very
low solubility which may hinder the future development of the drug
(166,167).
Triciribine (API-2, TCN, NSC 154020) is a tricyclic
nucleoside that is metabolically activated inside cells by
adenosine kinase to triciribine phosphate (TCN-P) which binds to
the PH domain near PIP3 binding pocket, preventing PDK1
phosphorylation. TCN is highly selective towards Akt with no
significant effect on PI3K, PKA or PKC (168,169). The antitumor effects of TCN-P
were known before the discovery of the Akt inactivation and were
tested in several clinical trials. The therapeutic development of
TCN-P limited by dose-limiting toxicities, including reversible
hyperglycemia, hepatic toxicity, thrombocytopenia, hypocalcaemia
and high triglyceride levels (170,171). In order to circumvent the
toxicities associated with TCN-P, the clinical development has
focused on tumors that express high p-Akt levels (172).
Miransertib (ARQ 092) binds to the inactive Akt and
inhibits non-competitively all three isoforms at nanomolar
concentrations and has excellent selectivity over other kinases
(173). It is analyzed in several
clinical trials for treatment as single therapy or combinations of
advanced solid tumors and recurrent malignant lymphoma. Positive
results were obtained with miransertib in cells and tissues
harboring Akt1 E17K mutations and is being investigated for
patients with overgrowth diseases and/or vascular anomalies
generated by genetic alterations of the PI3K/AKT pathway (174). Miransertib is under study as a
potential therapy for Proteus syndrome, a disease characterized by
progressive and typically asymmetric overgrowth caused by the Akt1
E17K somatic activating mutation during development (175).
BAY 1125976 is a highly selective, potent
allosteric Akt inhibitor by blocking the PDK1 phosphorylation after
binding in the pocket formed by kinase and PH domain. Chemically it
is a imidazo[1,2-b]pyridazine derivative, closely related to
miransertib. It inhibits cell proliferation in a panel of human
cancer cell lines, especially in hormone dependent breast and
prostate cancer cell lines. It is under investigation is a phase I
study (NCT01915576) in patients with advanced solid tumors
(176).
11. Conclusions
Akt is the core of a complex signaling pathway that
is one of the most intensively investigated cell signaling networks
due to its crucial involvement in cell metabolism, growth,
proliferation, motility, survival and apoptosis. These plethora of
effects render Akt a most valuable target of drug discovery. Even
if cancer treatment is at the center of the research, the potential
applications are numerous other major diseases, such as diabetes,
heart diseases, or neurodegenerative diseases. The position of Akt
at the crossroads between life and death rises multiple challenges,
but increasing understanding of cell biology and accumulating
promising results are raising hope to find personalized
treatments.
Acknowledgments
Not applicable.
Funding
No funding was received.
Availability of data and materials
Data sharing is not applicable to this article, as
no datasets were generated or analyzed during the current
study.
Authors' contributions
GMN, DMdV and AT were involved in the conception of
the study. GMN, MVdV, GN, AU, PJ, QP, OTO, DG, AT, DT, DAS and DM
were involved in the acquisition of the data and study design. GMN,
MVdV, GN, AU, PJ, QP, OTO, DG, AT, DT, DAS and DM were involved in
the writing of the article. GMN, DM, MVdV, PJ and DAS critically
revised the manuscript. All authors have read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Editor-in-Chief for the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this
article.
References
|
1
|
Bellacosa A, de Feo D, Godwin AK, Bell DW,
Cheng JQ, Altomare DA, Wan M, Dubeau L, Scambia G, Masciullo V, et
al: Molecular alterations of the AKT2 oncogene in ovarian and
breast carcinomas. Int J Cancer. 64:280–285. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bellacosa A, Kumar CC, Di Cristofano A and
Testa JR: Activation of AKT kinases in cancer: Implications for
therapeutic targeting. Adv Cancer Res. 94:29–86. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fresno Vara JA, Casado E, de Castro J,
Cejas P, Belda-Iniesta C and González-Barón M: PI3K/Akt signalling
pathway and cancer. Cancer Treat Rev. 30:193–204. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhao GX, Pan H, Ouyang DY and He XH: The
critical molecular interconnections in regulating apoptosis and
autophagy. Ann Med. 47:305–315. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Duronio V: The life of a cell: Apoptosis
regulation by the PI3K/PKB pathway. Biochem J. 415:333–344. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Song G, Ouyang G and Bao S: The activation
of Akt/PKB signaling pathway and cell survival. J Cell Mol Med.
9:59–71. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Coffer PJ and Woodgett JR: Molecular
cloning and characterisation of a novel putative protein-serine
kinase related to the cAMP-dependent and protein kinase C families.
Eur J Biochem. 201:475–481. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jones PF, Jakubowicz T, Pitossi FJ, Maurer
F and Hemmings BA: Molecular cloning and identification of a
serine/threonine protein kinase of the second-messenger subfamily.
Proc Natl Acad Sci USA. 88:4171–4175. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bellacosa A, Testa J, Staal SP and
Tsichlis PN: A retroviral oncogene, akt, encoding a
serine-threonine kinase containing an SH2-like region. Science.
254:274–277. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Scheid MP and Woodgett JR: Unravelling the
activation mechanisms of protein kinase B/Akt. FEBS Lett.
546:108–112. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brazil DP, Yang ZZ and Hemmings BA:
Advances in protein kinase B signalling: AKTion on multiple fronts.
Trends Biochem Sci. 29:233–242. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rodgers SJ, Ferguson DT, Mitchell CA and
Ooms LM: Regulation of PI3K effector signalling in cancer by the
phosphoinositide phosphatases. Biosci Rep. 37:372017. View Article : Google Scholar
|
|
13
|
Dobbin ZC and Landen CN: The importance of
the PI3K/AKT/MTOR pathway in the progression of ovarian cancer. Int
J Mol Sci. 14:8213–8227. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ahmad A, Biersack B, Li Y, Kong D, Bao B,
Schobert R, Padhye SB and Sarkar FH: Targeted regulation of
PI3K/Akt/mTOR/NF-κB signaling by indole compounds and their
derivatives: Mechanistic details and biological implications for
cancer therapy. Anticancer Agents Med Chem. 13:1002–1013. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Montaño A, Forero-Castro M,
Marchena-Mendoza D, Benito R and Hernández-Rivas JM: New Challenges
in Targeting Signaling Pathways in Acute Lymphoblastic Leukemia by
NGS Approaches: An Update. Cancers (Basel). 10. pp. 102018,
View Article : Google Scholar
|
|
16
|
Altomare DA and Testa JR: Perturbations of
the AKT signaling pathway in human cancer. Oncogene. 24:7455–7464.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Conus NM, Hannan KM, Cristiano BE,
Hemmings BA and Pearson RB: Direct identification of tyrosine 474
as a regulatory phosphorylation site for the Akt protein kinase. J
Biol Chem. 277:38021–38028. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mora A, Komander D, van Aalten DMF and
Alessi DR: PDK1, the master regulator of AGC kinase signal
transduction. Semin Cell Dev Biol. 15:161–170. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Memmott RM and Dennis PA: Akt-dependent
and -independent mechanisms of mTOR regulation in cancer. Cell
Signal. 21:656–664. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jhaveri K and Modi S: Ganetespib: Research
and clinical development. Onco Targets Ther. 8:1849–1858.
2015.PubMed/NCBI
|
|
21
|
Georgescu MM: PTEN tumor suppressor
network in PI3K-Akt pathway control. Genes Cancer. 1:1170–1177.
2010. View Article : Google Scholar
|
|
22
|
Hers I, Vincent EE and Tavaré JM: Akt
signalling in health and disease. Cell Signal. 23:1515–1527. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu P, Cheng H, Roberts TM and Zhao JJ:
Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev
Drug Discov. 8:627–644. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hindupur SK, González A and Hall MN: The
opposing actions of target of rapamycin and AMP-activated protein
kinase in cell growth control. Cold Spring Harb Perspect Biol.
7:a0191412015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Soliman GA: The role of mechanistic target
of rapamycin (mTOR) complexes signaling in the immune responses.
Nutrients. 5:2231–2257. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Laplante M and Sabatini DM: mTOR signaling
in growth control and disease. Cell. 149:274–293. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sarbassov DD, Guertin DA, Ali SM and
Sabatini DM: Phosphorylation and regulation of Akt/PKB by the
rictor-mTOR complex. Science. 307:1098–1101. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bozulic L and Hemmings BA: PIKKing on PKB:
Regulation of PKB activity by phosphorylation. Curr Opin Cell Biol.
21:256–261. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nitulescu GM, Margina D, Juzenas P, Peng
Q, Olaru OT, Saloustros E, Fenga C, Spandidos DA, Libra M and
Tsatsakis AM: Akt inhibitors in cancer treatment: The long journey
from drug discovery to clinical use (Review). Int J Oncol.
48:869–885. 2016. View Article : Google Scholar :
|
|
30
|
Inoki K, Li Y, Zhu T, Wu J and Guan KL:
TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR
signalling. Nat Cell Biol. 4:648–657. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hay N and Sonenberg N: Upstream and
downstream of mTOR. Genes Dev. 18:1926–1945. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dowling RJ, Topisirovic I, Alain T,
Bidinosti M, Fonseca BD, Petroulakis E, Wang X, Larsson O, Selvaraj
A, Liu Y, et al: mTORC1-mediated cell proliferation, but not cell
growth, controlled by the 4E-BPs. Science. 328:1172–1176. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yu C, Li WB, Liu JB, Lu JW and Feng JF:
Autophagy: Novel applications of nonsteroidal anti-inflammatory
drugs for primary cancer. Cancer Med. 7:471–484. 2018. View Article : Google Scholar
|
|
34
|
Zhang X, Tang N, Hadden TJ and Rishi AK:
Akt, FoxO and regulation of apoptosis. Biochim Biophys Acta.
1813:1978–1986. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jin J, Jin L, Lim SW and Yang CW: Klotho
deficiency aggravates tacrolimus-induced renal injury via the
phosphatidylinositol 3-kinase-Akt-Forkhead box protein O pathway.
Am J Nephrol. 43:357–365. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ouyang ZH, Wang WJ, Yan YG, Wang B and Lv
GH: The PI3K/Akt pathway: A critical player in intervertebral disc
degeneration. Oncotarget. 8:57870–57881. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pommier Y, Sordet O, Antony S, Hayward RL
and Kohn KW: Apoptosis defects and chemotherapy resistance:
Molecular interaction maps and networks. Oncogene. 23:2934–2949.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liao Y and Hung MC: Physiological
regulation of Akt activity and stability. Am J Transl Res. 2:19–42.
2010.PubMed/NCBI
|
|
39
|
Brognard J, Sierecki E, Gao T and Newton
AC: PHLPP and a second isoform, PHLPP2, differentially attenuate
the amplitude of Akt signaling by regulating distinct Akt isoforms.
Mol Cell. 25:917–931. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Newton AC and Trotman LC: Turning off AKT:
PHLPP as a drug target. Annu Rev Pharmacol Toxicol. 54:537–558.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Haddadi N, Lin Y, Travis G, Simpson AM,
Nassif NT and McGowan EM: PTEN/PTENP1: 'Regulating the regulator of
RTK-dependent PI3K/Akt signalling', new targets for cancer therapy.
Mol Cancer. 17:372018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Brown JS and Banerji U: Maximising the
potential of AKT inhibitors as anti-cancer treatments. Pharmacol
Ther. 172:101–115. 2017. View Article : Google Scholar
|
|
43
|
Huck BR and Mochalkin I: Recent progress
towards clinically relevant ATP-competitive Akt inhibitors. Bioorg
Med Chem Lett. 27:2838–2848. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang Q, Chen X and Hay N: Akt as a target
for cancer therapy: More is not always better (lessons from studies
in mice). Br J Cancer. 117:159–163. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chalhoub N and Baker SJ: PTEN and the
PI3-kinase pathway in cancer. Annu Rev Pathol. 4:127–150. 2009.
View Article : Google Scholar :
|
|
46
|
Nakatani K, Thompson DA, Barthel A, Sakaue
H, Liu W, Weigel RJ and Roth RA: Up-regulation of Akt3 in estrogen
receptor-deficient breast cancers and androgen-independent prostate
cancer lines. J Biol Chem. 274:21528–21532. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Cristiano BE, Chan JC, Hannan KM, Lundie
NA, Marmy-Conus NJ, Campbell IG, Phillips WA, Robbie M, Hannan RD
and Pearson RB: A specific role for AKT3 in the genesis of ovarian
cancer through modulation of G(2)-M phase transition. Cancer Res.
66:11718–11725. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Xia P and Xu XY: PI3K/Akt/mTOR signaling
pathway in cancer stem cells: From basic research to clinical
application. Am J Cancer Res. 5:1602–1609. 2015.PubMed/NCBI
|
|
49
|
Milella M, Falcone I, Conciatori F, Cesta
Incani U, Del Curatolo A, Inzerilli N, Nuzzo CM, Vaccaro V, Vari S,
Cognetti F, et al: PTEN: Multiple functions in human malignant
tumors. Front Oncol. 5:242015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Sato K, Tsuchihara K, Fujii S, Sugiyama M,
Goya T, Atomi Y, Ueno T, Ochiai A, Esumi H, et al: Autophagy is
activated in colorectal cancer cells and contributes to the
tolerance to nutrient deprivation. Cancer Res. 67:9677–9684. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Huang KH, Kuo KL, Ho IL, Chang HC, Chuang
YT, Lin WC, Lee PY, Chang SC, Chiang CK, Pu YS, et al:
Celecoxib-induced cytotoxic effect is potentiated by inhibition of
autophagy in human urothelial carcinoma cells. PLoS One.
8:e820342013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Bibbins-Domingo K: US Preventive Services
Task Force: Aspirin use for the primary prevention of
cardiovascular disease and colorectal cancer: US preventive
services task force recommendation statement. Ann Intern Med.
164:836–845. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zheng HC: The molecular mechanisms of
chemoresistance in cancers. Oncotarget. 8:59950–59964.
2017.PubMed/NCBI
|
|
54
|
Dasari S and Tchounwou PB: Cisplatin in
cancer therapy: Molecular mechanisms of action. Eur J Pharmacol.
740:364–378. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Tao K, Yin Y, Shen Q, Chen Y, Li R, Chang
W, Bai J, Liu W, Shi L and Zhang P: Akt inhibitor MK-2206 enhances
the effect of cisplatin in gastric cancer cells. Biomed Rep.
4:365–368. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Lin YH, Chen BYH, Lai WT, Wu SF, Guh JH,
Cheng AL and Hsu LC: The Akt inhibitor MK-2206 enhances the
cytotoxicity of paclitaxel (Taxol) and cisplatin in ovarian cancer
cells. Naunyn Schmiedebergs Arch Pharmacol. 388:19–31. 2015.
View Article : Google Scholar
|
|
57
|
Nagata Y, Lan KH, Zhou X, Tan M, Esteva
FJ, Sahin AA, Klos KS, Li P, Monia BP, Nguyen NT, et al: PTEN
activation contributes to tumor inhibition by trastuzumab, and loss
of PTEN predicts trastuzumab resistance in patients. Cancer Cell.
6:117–127. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Nahta R, Yu D, Hung MC, Hortobagyi GN and
Esteva FJ: Mechanisms of disease: Understanding resistance to
HER2-targeted therapy in human breast cancer. Nat Clin Pract Oncol.
3:269–280. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Crowell JA, Steele VE and Fay JR:
Targeting the AKT protein kinase for cancer chemoprevention. Mol
Cancer Ther. 6:2139–2148. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Mayer IA and Arteaga CL: The PI3K/AKT
pathway as a target for cancer treatment. Annu Rev Med. 67:11–28.
2016. View Article : Google Scholar
|
|
61
|
Geuna E, Roda D, Rafii S, Jimenez B,
Capelan M, Rihawi K, Montemurro F, Yap TA, Kaye SB, De Bono JS, et
al: Complications of hyperglycaemia with PI3K-AKT-mTOR inhibitors
in patients with advanced solid tumours on Phase I clinical trials.
Br J Cancer. 113:1541–1547. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Ribeiro FM, Paquet M, Cregan SP and
Ferguson SS: Group I metabotropic glutamate receptor signalling and
its implication in neurological disease. CNS Neurol Disord Drug
Targets. 9:574–595. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Sattler R and Tymianski M: Molecular
mechanisms of glutamate receptor-mediated excitotoxic neuronal cell
death. Mol Neurobiol. 24:107–129. 2001. View Article : Google Scholar
|
|
64
|
Baskys A, Bayazitov I, Fang L, Blaabjerg
M, Poulsen FR and Zimmer J: Group I metabotropic glutamate
receptors reduce excitotoxic injury and may facilitate
neurogenesis. Neuropharmacology. 49(Suppl 1): 146–156. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Costa C, Tsatsakis A, Mamoulakis C,
Teodoro M, Briguglio G, Caruso E, Tsoukalas D, Margina D, Dardiotis
E, Kouretas D, et al: Current evidence on the effect of dietary
polyphenols intake on chronic diseases. Food Chem Toxicol.
110:286–299. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Reddy PH: Amyloid beta-induced glycogen
synthase kinase 3β phosphorylated VDAC1 in Alzheimer's disease:
implications for synaptic dysfunction and neuronal damage. Biochim
Biophys Acta. 1832:1913–1921. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Hernandez F, Lucas JJ and Avila J: GSK3
and tau: Two convergence points in Alzheimer's disease. J
Alzheimers Dis. 3(Suppl 1): 141–144. 2012. View Article : Google Scholar
|
|
68
|
Li X, Lu F, Tian Q, Yang Y, Wang Q and
Wang JZ: Activation of glycogen synthase kinase-3 induces
Alzheimer-like tau hyper-phosphorylation in rat hippocampus slices
in culture. J Neural Transm (Vienna). 113:93–102. 2006. View Article : Google Scholar
|
|
69
|
Luo HR, Hattori H, Hossain MA, Hester L,
Huang Y, Lee-Kwon W, Donowitz M, Nagata E and Snyder SH: Akt as a
mediator of cell death. Proc Natl Acad Sci USA. 100:11712–11717.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Wang R and Reddy PH: Role of glutamate and
NMDA receptors in Alzheimer's disease. J Alzheimers Dis.
57:1041–1048. 2017. View Article : Google Scholar
|
|
71
|
Ferrarelli LK: PTEN contributes to
Alzheimer's disease. Sci Signal. 9:ec452016. View Article : Google Scholar
|
|
72
|
Frere S and Slutsky I: Targeting PTEN
interactions for Alzheimer's disease. Nat Neurosci. 19:416–418.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Chen Z, Chen B, Xu WF, Liu RF, Yang J and
Yu CX: Effects of PTEN inhibition on regulation of tau
phosphorylation in an okadaic acid-induced neurodegeneration model.
Int J Dev Neurosci. 30:411–419. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Jo H, Mondal S, Tan D, Nagata E, Takizawa
S, Sharma AK, Hou Q, Shanmugasundaram K, Prasad A, Tung JK, et al:
Small molecule-induced cytosolic activation of protein kinase Akt
rescues ischemia-elicited neuronal death. Proc Natl Acad Sci USA.
109:10581–10586. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Pulido R: PTEN inhibition in human disease
therapy. Molecules. 23:E2852018. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Mhyre TR, Boyd JT, Hamill RW and
Maguire-Zeiss KA: Parkinson's disease. Subcell Biochem. 65:389–455.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Xu Y, Gao YW and Yang Y: SC79 protects
dopaminergic neurons from oxidative stress. Oncotarget.
9:12639–12648. 2017.
|
|
78
|
Pariyar R, Lamichhane R, Jung HJ, Kim SY
and Seo J: Sulfuretin attenuates MPP+-induced
neurotoxicity through Akt/GSK3β and ERK signaling pathways. Int J
Mol Sci. 18:E27532017. View Article : Google Scholar
|
|
79
|
Hu M, Li F and Wang W: Vitexin protects
dopaminergic neurons in MPTP-induced Parkinson's disease through
PI3K/Akt signaling pathway. Drug Des Devel Ther. 12:565–573. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Masaki K and Douglas Z: Diabetic
neuropathy and the sensory neuron: New aspects of pathogenesis and
their treatment implications. J Diabetes Investig. Mar 13–2018.Epub
ahead of print. View Article : Google Scholar
|
|
81
|
Boucher J, Kleinridders A and Kahn CR:
Insulin receptor signaling in normal and insulin-resistant states.
Cold Spring Harb Perspect Biol. 6:a0091912014. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Zaid H, Antonescu CN, Randhawa VK and Klip
A: Insulin action on glucose transporters through molecular
switches, tracks and tethers. Biochem J. 413:201–215. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Lim CY, Bi X, Wu D, Kim JB, Gunning PW,
Hong W and Han W: Tropomodulin3 is a novel Akt2 effector regulating
insulin-stimulated GLUT4 exocytosis through cortical actin
remodeling. Nat Commun. 6:59512015. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Cho H, Mu J, Kim JK, Thorvaldsen JL, Chu
Q, Crenshaw EB III, Kaestner KH, Bartolomei MS, Shulman GI and
Birnbaum MJ: Insulin resistance and a diabetes mellitus-like
syndrome in mice lacking the protein kinase Akt2 (PKB beta).
Science. 292:1728–1731. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Gradinaru D, Khaddour H, Margina D,
Ungurianu A, Borsa C, Ionescu C, Prada GI, Usher J and Elshimali Y:
Insulin-leptin axis, cardiometabolic risk and oxidative stress in
elderly with metabolic syndrome. Exp Clin Endocrinol Diabetes.
126:445–452. 2018. View Article : Google Scholar
|
|
86
|
Koren S, DiPilato LM, Emmett MJ, Shearin
AL, Chu Q, Monks B and Birnbaum MJ: The role of mouse Akt2 in
insulin-dependent suppression of adipocyte lipolysis in vivo.
Diabetologia. 58:1063–1070. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Hussain K, Challis B, Rocha N, Payne F,
Minic M, Thompson A, Daly A, Scott C, Harris J, Smillie BJ, et al:
An activating mutation of AKT2 and human hypoglycemia. Science.
334:4742011. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Badea M, Olar R, Uivarosi V, Marinescu D,
Aldea V, Barbuceanu SF and Nitulescu GM: Thermal behavior of some
vanadyl complexes with flavone derivatives as potential insulin-
mimetic agents. J Therm Anal Calorim. 105:1052011. View Article : Google Scholar
|
|
89
|
Domingo JL and Gómez M: Vanadium compounds
for the treatment of human diabetes mellitus: A scientific
curiosity? A review of thirty years of research. Food Chem Toxicol.
95:137–141. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Sierecki E, Sinko W, McCammon JA and
Newton AC: Discovery of small molecule inhibitors of the PH domain
leucine-rich repeat protein phosphatase (PHLPP) by chemical and
virtual screening. J Med Chem. 53:6899–6911. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Zhao W and Zhao SP: Different effects of
statins on induction of diabetes mellitus: An experimental study.
Drug Des Devel Ther. 9:6211–6223. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Bonifacio A, Sanvee GM, Bouitbir J and
Krähenbühl S: The AKT/mTOR signaling pathway plays a key role in
statin-induced myotoxicity. Biochim Biophys Acta. 1853:1841–1849.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Beckwitt CH, Shiraha K and Wells A:
Lipophilic statins limit cancer cell growth and survival, via
involvement of Akt signaling. PLoS One. 13:e01974222018. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Yu H, Littlewood T and Bennett M: Akt
isoforms in vascular disease. Vascul Pharmacol. 71:57–64. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Rotllan N, Chamorro-Jorganes A, Araldi E,
Wanschel AC, Aryal B, Aranda JF, Goedeke L, Salerno AG, Ramírez CM,
Sessa WC, et al: Hematopoietic Akt2 deficiency attenuates the
progression of atherosclerosis. FASEB J. 29:597–610. 2015.
View Article : Google Scholar :
|
|
96
|
Yao S, Fan LY and Lam EW: The FOXO3 FOXM1
axis: A key cancer drug target and a modulator of cancer drug
resistance. Semin Cancer Biol. 50:77–89. 2018. View Article : Google Scholar
|
|
97
|
Dhalla NS and Müller AL: Protein kinases
as drug development targets for heart disease therapy.
Pharmaceuticals (Basel). 3:2111–2145. 2010. View Article : Google Scholar
|
|
98
|
McMullen JR, Boey EJ, Ooi JY, Seymour JF,
Keating MJ and Tam CS: Ibrutinib increases the risk of atrial
fibrillation, potentially through inhibition of cardiac PI3K-Akt
signaling. Blood. 124:3829–3830. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Pretorius L, Du XJ, Woodcock EA, Kiriazis
H, Lin RC, Marasco S, Medcalf RL, Ming Z, Head GA, Tan JW, et al:
Reduced phosphoinositide 3-kinase (p110α) activation increases the
susceptibility to atrial fibrillation. Am J Pathol. 175:998–1009.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Ezeani M and Elom S: Necessity to evaluate
PI3K/Akt signalling pathway in proarrhythmia. Open Hear.
4:e0005962017. View Article : Google Scholar
|
|
101
|
Tamura M, Gu J, Danen EHJ, Takino T,
Miyamoto S and Yamada KM: PTEN interactions with focal adhesion
kinase and suppression of the extracellular matrix-dependent
phosphatidylinositol 3-kinase/Akt cell survival pathway. J Biol
Chem. 274:20693–20703. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Nho RS, Hergert P, Kahm J, Jessurun J and
Henke C: Pathological alteration of FoxO3a activity promotes
idiopathic pulmonary fibrosis fibroblast proliferation on type i
collagen matrix. Am J Pathol. 179:2420–2430. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Lawrence J and Nho R: The Role of the
Mammalian Target of Rapamycin (mTOR) in Pulmonary Fibrosis. Int J
Mol Sci. 19:7782018. View Article : Google Scholar :
|
|
104
|
Xia H, Khalil W, Kahm J, Jessurun J,
Kleidon J and Henke CA: Pathologic caveolin-1 regulation of PTEN in
idiopathic pulmonary fibrosis. Am J Pathol. 176:2626–2637. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Nho RS, Peterson M, Hergert P and Henke
CA: FoxO3a (Forkhead Box O3a) deficiency protects Idiopathic
Pulmonary Fibrosis (IPF) fibroblasts from type I polymerized
collagen matrix-induced apoptosis via caveolin-1 (cav-1) and Fas.
PLoS One. 8:e610172013. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Lu Y, Azad N, Wang L, Iyer AKV, Castranova
V, Jiang BH and Rojanasakul Y: Phosphatidylinositol-3-kinase/akt
regulates bleomycin-induced fibroblast proliferation and collagen
production. Am J Respir Cell Mol Biol. 42:432–441. 2010. View Article : Google Scholar :
|
|
107
|
Saito S, Zhuang Y, Shan B, Danchuk S, Luo
F, Korfei M, Guenther A and Lasky JA: Tubastatin ameliorates
pulmonary fibrosis by targeting the TGFβ-PI3K-Akt pathway. PLoS
One. 12:e01866152017. View Article : Google Scholar
|
|
108
|
Theofilopoulos AN, Kono DH and Baccala R:
The multiple pathways to autoimmunity. Nat Immunol. 18:716–724.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Goodnow CC, Crosbie J, Adelstein S, Lavoie
TB, Smith-Gill SJ, Brink RA, Pritchard-Briscoe H, Wotherspoon JS,
Loblay RH, Raphael K, et al: Altered immunoglobulin expression and
functional silencing of self-reactive B lymphocytes in transgenic
mice. Nature. 334:676–682. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Sakaguchi S: Naturally arising
Foxp3-expressing CD25+CD4+ regulatory T cells
in immunological tolerance to self and non-self. Nat Immunol.
6:345–352. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
111
|
Matthews R: Autoimmune diseases. The B
cell slayer. Science. 318:1232–1233. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Waldman M and Madaio MP: Pathogenic
autoantibodies in lupus nephritis. Lupus. 14:19–24. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Lai ZW, Borsuk R, Shadakshari A, Yu J,
Dawood M, Garcia R, Francis L, Tily H, Bartos A, Faraone SV, et al:
Mechanistic target of rapamycin activation triggers IL-4 production
and necrotic death of double-negative T cells in patients with
systemic lupus erythematosus. J Immunol. 191:2236–2246. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Di Cristofano A, Kotsi P, Peng YF,
Cordon-Cardo C, Elkon KB and Pandolfi PP: Impaired Fas response and
autoimmunity in Pten+/− mice. Science. 285:2122–2125.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Carbone F, De Rosa V, Carrieri PB,
Montella S, Bruzzese D, Porcellini A, Procaccini C, La Cava A and
Matarese G: Regulatory T cell proliferative potential is impaired
in human autoimmune disease. Nat Med. 20:69–74. 2014. View Article : Google Scholar
|
|
116
|
Zhang HG, Wang Y, Xie JF, Liang X, Liu D,
Yang P, Hsu HC, Ray RB and Mountz JD: Regulation of tumor necrosis
factor α-mediated apoptosis of rheumatoid arthritis synovial
fibroblasts by the protein kinase Akt. Arthritis Rheum.
44:1555–1567. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Chimenti MS, Triggianese P, Conigliaro P,
Candi E, Melino G and Perricone R: The interplay between
inflammation and metabolism in rheumatoid arthritis. Cell Death
Dis. 6:e18872015. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Warner LM, Adams LM and Sehgal SN:
Rapamycin prolongs survival and arrests pathophysiologic changes in
murine systemic lupus erythematosus. Arthritis Rheum. 37:289–297.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Fernandez D, Bonilla E, Mirza N, Niland B
and Perl A: Rapamycin reduces disease activity and normalizes T
cell activation-induced calcium fluxing in patients with systemic
lupus erythematosus. Arthritis Rheum. 54:2983–2988. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Wu T and Mohan C: The AKT axis as a
therapeutic target in autoimmune diseases. Endocr Metab Immune
Disord Drug Targets. 9:145–150. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Juarez M, Schcolnik-Cabrera A and
Dueñas-Gonzalez A: The multitargeted drug ivermectin: From an
antiparasitic agent to a repositioned cancer drug. Am J Cancer Res.
8:317–331. 2018.PubMed/NCBI
|
|
122
|
Li Q, Ni W, Deng Z, Liu M, She L and Xie
Q: Targeting nasopharyngeal carcinoma by artesunate through
inhibiting Akt/mTOR and inducing oxidative stress. Fundam Clin
Pharmacol. 31:301–310. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Tan Z, Peng A, Xu J and Ouyang M: Propofol
enhances BCR-ABL TKIs' inhibitory effects in chronic myeloid
leukemia through Akt/mTOR suppression. BMC Anesthesiol. 17:1322017.
View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Dana P, Vaeteewoottacharn K, Kariya R,
Matsuda K, Wongkham S and Okada S: Repurposing cimetidine for
cholangiocarcinoma: Antitumor effects in vitro and in vivo. Oncol
Lett. 13:1432–1436. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Tsubamoto H, Inoue K, Sakata K, Ueda T,
Takeyama R, Shibahara H and Sonoda T: Itraconazole inhibits
AKT/mTOR signaling and proliferation in endometrial cancer cells.
Anticancer Res. 37:515–519. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Pan YJ, Wang WH, Huang TY, Weng WH, Fang
CK, Chen YC and Hwang JJ: Quetiapine ameliorates collagen-induced
arthritis in mice via the suppression of the AKT and ERK signaling
pathways. Inflamm Res. 67:847–861. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Margină D, Ilie M, Grădinaru D,
Androutsopoulos VP, Kouretas D and Tsatsakis AM: Natural
products-friends or foes? Toxicol Lett. 236:154–167. 2015.
View Article : Google Scholar
|
|
128
|
Seca AML and Pinto DCGA: Plant secondary
metabolites as anticancer agents: Successes in clinical trials and
therapeutic application. Int J Mol Sci. 19:2632018. View Article : Google Scholar :
|
|
129
|
Aoki H, Takada Y, Kondo S, Sawaya R,
Aggarwal BB and Kondo Y: Evidence that curcumin suppresses the
growth of malignant gliomas in vitro and in vivo through induction
of autophagy: Role of Akt and extracellular signal-regulated kinase
signaling pathways. Mol Pharmacol. 72:29–39. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Tang FY, Shih CJ, Cheng LH, Ho HJ and Chen
HJ: Lycopene inhibits growth of human colon cancer cells via
suppression of the Akt signaling pathway. Mol Nutr Food Res.
52:646–654. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Schmidt C, Loos C, Jin L, Schmiech M,
Schmidt CQ, Gaafary ME, Syrovets T and Simmet T: Acetyl-lupeolic
acid inhibits Akt signaling and induces apoptosis in chemoresistant
prostate cancer cells in vitro and in vivo. Oncotarget.
8:55147–55161. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Estrada AC, Syrovets T, Pitterle K, Lunov
O, Büchele B, Schimana-Pfeifer J, Schmidt T, Morad SA and Simmet T:
Tirucallic acids are novel pleckstrin homology domain-dependent Akt
inhibitors inducing apoptosis in prostate cancer cells. Mol
Pharmacol. 77:378–387. 2010. View Article : Google Scholar
|
|
133
|
Han HY, Kim HJ, Jeong SH, Kim J, Jeong SH,
Kim GC, Hwang DS, Kim UK and Ryu MH: The flavonoid Jaceosidin from
Artemisia princeps induces apoptotic cell death and inhibits the
Akt pathway in oral cancer cells. Evid Based Complement Alternat
Med. 2018:57650472018. View Article : Google Scholar :
|
|
134
|
Lin CH, Chang CY, Lee KR, Lin HJ, Chen TH
and Wan L: Flavones inhibit breast cancer proliferation through the
Akt/FOXO3a signaling pathway. BMC Cancer. 15:9582015. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Lim W, Yang C, Bazer FW and Song G:
Luteolin inhibits proliferation and induces apoptosis of human
placental choriocarcinoma cells by blocking the PI3K/AKT pathway
and regulating sterol regulatory element binding protein activity.
Biol Reprod. 95:822016. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Carpi S, Polini B, Poli G, Alcantara
Barata G, Fogli S, Romanini A, Tuccinardi T, Guella G, Frontini FP,
Nieri P, et al: Anticancer activity of euplotin C, isolated from
the marine ciliate Euplotes crassus, against human melanoma cells.
Mar Drugs. 16:E1662018. View Article : Google Scholar
|
|
137
|
Guerra AC, Soares LA, Ferreira MR, Araújo
AA, Rocha HA, Medeiros JS, Cavalcante RD and Júnior RF: Libidibia
ferrea presents antiproliferative, apoptotic and antioxidant
effects in a colorectal cancer cell line. Biomed Pharmacother.
92:696–706. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Yang L, Liu Y, Wang M, Qian Y, Dai X, Zhu
Y, Chen J, Guo S and Hisamitsu T: Celastrus orbiculatus extract
triggers apoptosis and autophagy via PI3K/Akt/mTOR inhibition in
human colorectal cancer cells. Oncol Lett. 12:3771–3778. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Zhou R, Chen H, Chen J, Chen X, Wen Y and
Xu L: Extract from Astragalus membranaceus inhibit breast cancer
cells proliferation via PI3K/AKT/mTOR signaling pathway. BMC
Complement Altern Med. 18:832018. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Wong FC, Woo CC, Hsu A and Tan BKH: The
anti-cancer activities of Vernonia amygdalina extract in human
breast cancer cell lines are mediated through caspase-dependent and
p53-independent pathways. PLoS One. 8:e780212013. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Olaru OT, Niţulescu GM, Ortan A and
Dinu-Pîrvu CE: Ethnomedicinal, phytochemical and pharmacological
profile of anthriscus sylvestris as an alternative source for
anticancer lignans. Molecules. 20:15003–15022. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Gunadharini DN, Elumalai P, Arunkumar R,
Senthilkumar K and Arunakaran J: Induction of apoptosis and
inhibition of PI3K/Akt pathway in PC-3 and LNCaP prostate cancer
cells by ethanolic neem leaf extract. J Ethnopharmacol.
134:644–650. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Olaru OT and Venables L: Anticancer
potential of selected Fallopia Adans species. Oncol Lett.
10:1323–1332. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Ying C, Mao Y, Chen L, Wang S, Ling H, Li
W and Zhou X: Bamboo leaf extract ameliorates diabetic nephropathy
through activating the AKT signaling pathway in rats. Int J Biol
Macromol. 105:1587–1594. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Lu Q, Zuo WZ, Ji XJ, Zhou YX, Liu YQ, Yao
XQ, Zhou XY, Liu YW, Zhang F and Yin XX: Ethanolic Ginkgo biloba
leaf extract prevents renal fibrosis through Akt/mTOR signaling in
diabetic nephropathy. Phytomedicine. 22:1071–1078. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Dai B, Wu Q, Zeng C, Zhang J, Cao L, Xiao
Z and Yang M: The effect of Liuwei Dihuang decoction on PI3K/Akt
signaling pathway in liver of type 2 diabetes mellitus (T2DM) rats
with insulin resistance. J Ethnopharmacol. 192:382–389. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Gao S, Guo Q, Qin C, Shang R and Zhang Z:
Sea buckthorn fruit oil extract alleviates insulin resistance
through the PI3K/Akt signaling pathway in type 2 diabetes mellitus
cells and rats. J Agric Food Chem. 65:1328–1336. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Mundi PS, Sachdev J, McCourt C and
Kalinsky K: AKT in cancer: New molecular insights and advances in
drug development. Br J Clin Pharmacol. 82:943–956. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Dorlo TP, Balasegaram M, Beijnen JH and de
Vries PJ: Beijnen JH and de vries PJ: Miltefosine: A review of its
pharmacology and therapeutic efficacy in the treatment of
leishmaniasis. J Antimicrob Chemother. 67:2576–2597. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Pal SK, Reckamp K, Yu H and Figlin RA: Akt
inhibitors in clinical development for the treatment of cancer.
Expert Opin Investig Drugs. 19:1355–1366. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Mattmann ME, Stoops SL and Lindsley CW:
Inhibition of Akt with small molecules and biologics: Historical
perspective and current status of the patent landscape. Expert Opin
Ther Pat. 21:1309–1338. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Davies BR, Greenwood H, Dudley P, Crafter
C, Yu DH, Zhang J, Li J, Gao B, Ji Q, Maynard J, et al: Preclinical
pharmacology of AZD5363, an inhibitor of AKT: Pharmacodynamics,
antitumor activity, and correlation of monotherapy activity with
genetic background. Mol Cancer Ther. 11:873–887. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Banerji U, Dean EJ, Pérez-Fidalgo JA,
Batist G, Bedard PL, You B, Westin SN, Kabos P, Garrett MD, Tall M,
et al: A phase I open-label study to identify a dosing regimen of
the Pan-AKT inhibitor AZD5363 for evaluation in solid tumors and in
PIK3CA-mutated breast and gynecologic cancers. Clin Cancer Res.
24:2050–2059. 2018. View Article : Google Scholar
|
|
154
|
Sundar R, Chénard-Poirier M, Collins DC
and Yap TA: Imprecision in the era of precision medicine in
non-small cell lung cancer. Front Med (Lausanne). 4:392017.
|
|
155
|
Saura C, Roda D, Roselló S, Oliveira M,
Macarulla T, Pérez-Fidalgo JA, Morales-Barrera R, Sanchis-García
JM, Musib L, Budha N, et al: A first-in-human phase I study of the
ATP-competitive AKT inhibitor ipatasertib demonstrates robust and
safe targeting of AKT in patients with solid tumors. Cancer Discov.
7:102–113. 2017. View Article : Google Scholar :
|
|
156
|
Dent RA, Kim SB, Im SA, Espie M, Blau S,
Tan AR, Isakoff S, Oliveira M, Saura C, Wongchenko M, et al: LOTUS
(NCT02162719): A double-blind placebo (PBO)-controlled randomized
phase II trial of first-line ipatasertib (IPAT) + paclitaxel (P)
for metastatic triple-negative breast cancer (TNBC). J Clin Oncol.
35(Suppl 15): 10092017. View Article : Google Scholar
|
|
157
|
Dumble M, Crouthamel M-C, Zhang S-Y,
Schaber M, Levy D, Robell K, Liu Q, Figueroa DJ, Minthorn EA,
Seefeld MA, et al: Discovery of novel AKT inhibitors with enhanced
anti-tumor effects in combination with the MEK inhibitor. PLoS One.
9:e1008802014. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Spencer A, Yoon SS, Harrison SJ, Morris
SR, Smith DA, Brigandi RA, Gauvin J, Kumar R, Opalinska JB and Chen
C: The novel AKT inhibitor afuresertib shows favorable safety,
pharmacokinetics, and clinical activity in multiple myeloma. Blood.
124:2190–2195. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Arceci RJ, Allen CE, Dunkel IJ, Jacobsen
E, Whitlock J, Vassallo R, Morris SR, Portnoy A, Reedy BA, Smith
DA, et al: A phase IIa study of afuresertib, an oral pan-AKT
inhibitor, in patients with Langerhans cell histiocytosis. Pediatr
Blood Cancer. 64:642017. View Article : Google Scholar
|
|
160
|
Aghajanian C, Bell-McGuinn KM, Burris HA
III, Siu LL, Stayner LA, Wheler JJ, Hong DS, Kurkjian C, Pant S,
Santiago-Walker A, et al: A phase I, open-label, two-stage study to
investigate the safety, tolerability, pharmacokinetics, and
pharmacodynamics of the oral AKT inhibitor GSK2141795 in patients
with solid tumors. Invest New Drugs. Apr 3–2018.Epub ahead of
print. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Ma BB, Goh BC, Lim WT, Hui EP, Tan EH,
Lopes GL, Lo KW, Li L, Loong H, Foster NR, et al: Multicenter phase
II study of the AKT inhibitor MK-2206 in recurrent or metastatic
nasopharyngeal carcinoma from patients in the mayo phase II
consortium and the cancer therapeutics research group (MC1079).
Invest New Drugs. 33:985–991. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Yap TA, Yan L, Patnaik A, Fearen I, Olmos
D, Papadopoulos K, Baird RD, Delgado L, Taylor A, Lupinacci L, et
al: First-in-man clinical trial of the oral pan-AKT inhibitor
MK-2206 in patients with advanced solid tumors. J Clin Oncol.
29:4688–4695. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Bjune K, Sundvold H, Leren TP and Naderi
S: MK-2206, an allosteric inhibitor of AKT, stimulates LDLR
expression and LDL uptake: A potential hypocholesterolemic agent.
Atherosclerosis. 276:28–38. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Richardson PG, Eng C, Kolesar J, Hideshima
T and Anderson KC: Perifosine, an oral, anti-cancer agent and
inhibitor of the Akt pathway: Mechanistic actions,
pharmacodynamics, pharmacokinetics, and clinical activity. Expert
Opin Drug Metab Toxicol. 8:623–633. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Ríos-Marco P, Marco C, Gálvez X,
Jiménez-López JM and Carrasco MP: Alkylphospholipids: An update on
molecular mechanisms and clinical relevance. Biochim Biophys Acta -
Biomembr. 1859:1657–1667. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Reid JM, Walden CA, Qin R, Ziegler KL,
Haslam JL, Rajewski RA, Warndahl R, Fitting CL, Boring D, Szabo E,
et al: Cancer Prevention Network: Phase 0 clinical chemoprevention
trial of the Akt inhibitor SR13668. Cancer Prev Res (Phila).
4:347–353. 2011. View Article : Google Scholar
|
|
167
|
Kapetanovic IM, Muzzio M, Hu SC, Crowell
JA, Rajewski RA, Haslam JL, Jong L and McCormick DL:
Pharmacokinetics and enhanced bioavailability of candidate cancer
preventative agent, SR13668 in dogs and monkeys. Cancer Chemother
Pharmacol. 65:1109–1116. 2010. View Article : Google Scholar
|
|
168
|
Yang L, Dan HC, Sun M, Liu Q, Sun XM,
Feldman RI, Hamilton AD, Polokoff M, Nicosia SV, Herlyn M, et al:
Akt/protein kinase B signaling inhibitor-2, a selective small
molecule inhibitor of Akt signaling with antitumor activity in
cancer cells overexpressing Akt. Cancer Res. 64:4394–4399. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Berndt N, Yang H, Trinczek B, Betzi S,
Zhang Z, Wu B, Lawrence NJ, Pellecchia M, Schönbrunn E, Cheng JQ,
et al: The Akt activation inhibitor TCN-P inhibits Akt
phosphorylation by binding to the PH domain of Akt and blocking its
recruitment to the plasma membrane. Cell Death Differ.
17:1795–1804. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Hoffman K, Holmes FA, Fraschini G, Esparza
L, Frye D, Raber MN, Newman RA and Hortobagyi GN: Phase I–II study:
Triciribine (tricyclic nucleoside phosphate) for metastatic breast
cancer. Cancer Chemother Pharmacol. 37:254–258. 1996. View Article : Google Scholar
|
|
171
|
Feun LG, Savaraj N, Bodey GP, Lu K, Yap
BS, Ajani JA, Burgess MA, Benjamin RS, McKelvey E and Krakoff I:
Phase I study of tricyclic nucleoside phosphate using a five-day
continuous infusion schedule. Cancer Res. 44:3608–3612.
1984.PubMed/NCBI
|
|
172
|
Sampath D, Malik A, Plunkett W, Nowak B,
Williams B, Burton M, Verstovsek S, Faderl S, Garcia-Manero G, List
AF, et al: Phase I clinical, pharmacokinetic, and pharmacodynamic
study of the Akt-inhibitor triciribine phosphate monohydrate in
patients with advanced hematologic malignancies. Leuk Res.
37:1461–1467. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Kim K, Li J, Barazia A, Tseng A, Youn SW,
Abbadessa G, Yu Y, Schwartz B, Andrews RK, Gordeuk VR, et al: ARQ
092, an orally-available, selective AKT inhibitor, attenuates
neutrophil-platelet interactions in sickle cell disease.
Haematologica. 102:246–259. 2017. View Article : Google Scholar :
|
|
174
|
Ranieri C, Di Tommaso S, Loconte DC,
Grossi V, Sanese P, Bagnulo R, Susca FC, Forte G, Peserico A, De
Luisi A, et al: In vitro efficacy of ARQ 092, an allosteric AKT
inhibitor, on primary fibroblast cells derived from patients with
PIK3CA-related overgrowth spectrum (PROS). Neurogenetics. 19:77–91.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Lindhurst MJ, Yourick MR, Yu Y, Savage RE,
Ferrari D and Biesecker LG: Repression of AKT signaling by ARQ 092
in cells and tissues from patients with Proteus syndrome. Sci Rep.
5:171622015. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Politz O, Siegel F, Bärfacker L, Bömer U,
Hägebarth A, Scott WJ, Michels M, Ince S, Neuhaus R, Meyer K, et
al: BAY 1125976, a selective allosteric AKT1/2 inhibitor, exhibits
high efficacy on AKT signaling-dependent tumor growth in mouse
models. Int J Cancer. 140:449–459. 2017. View Article : Google Scholar
|