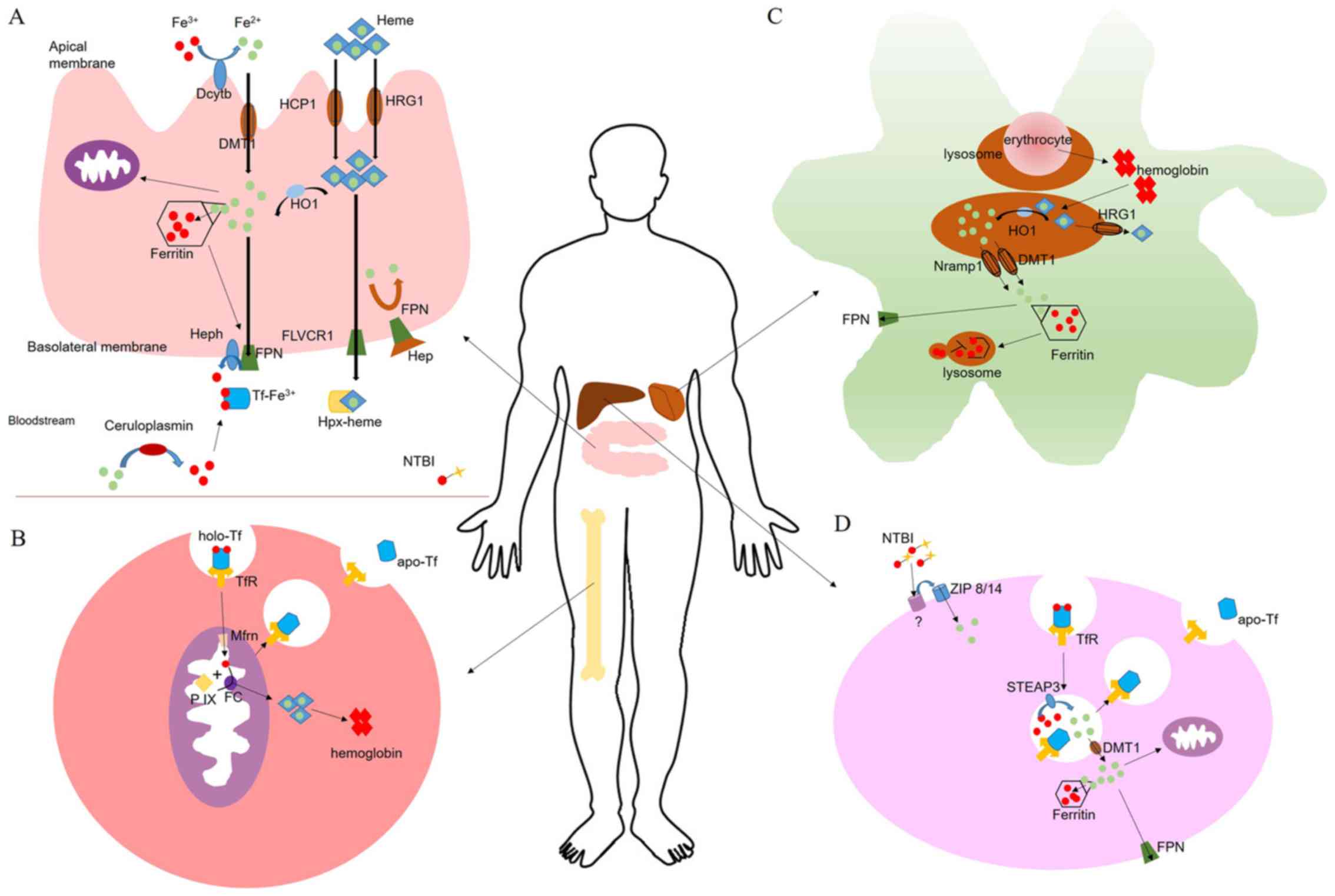
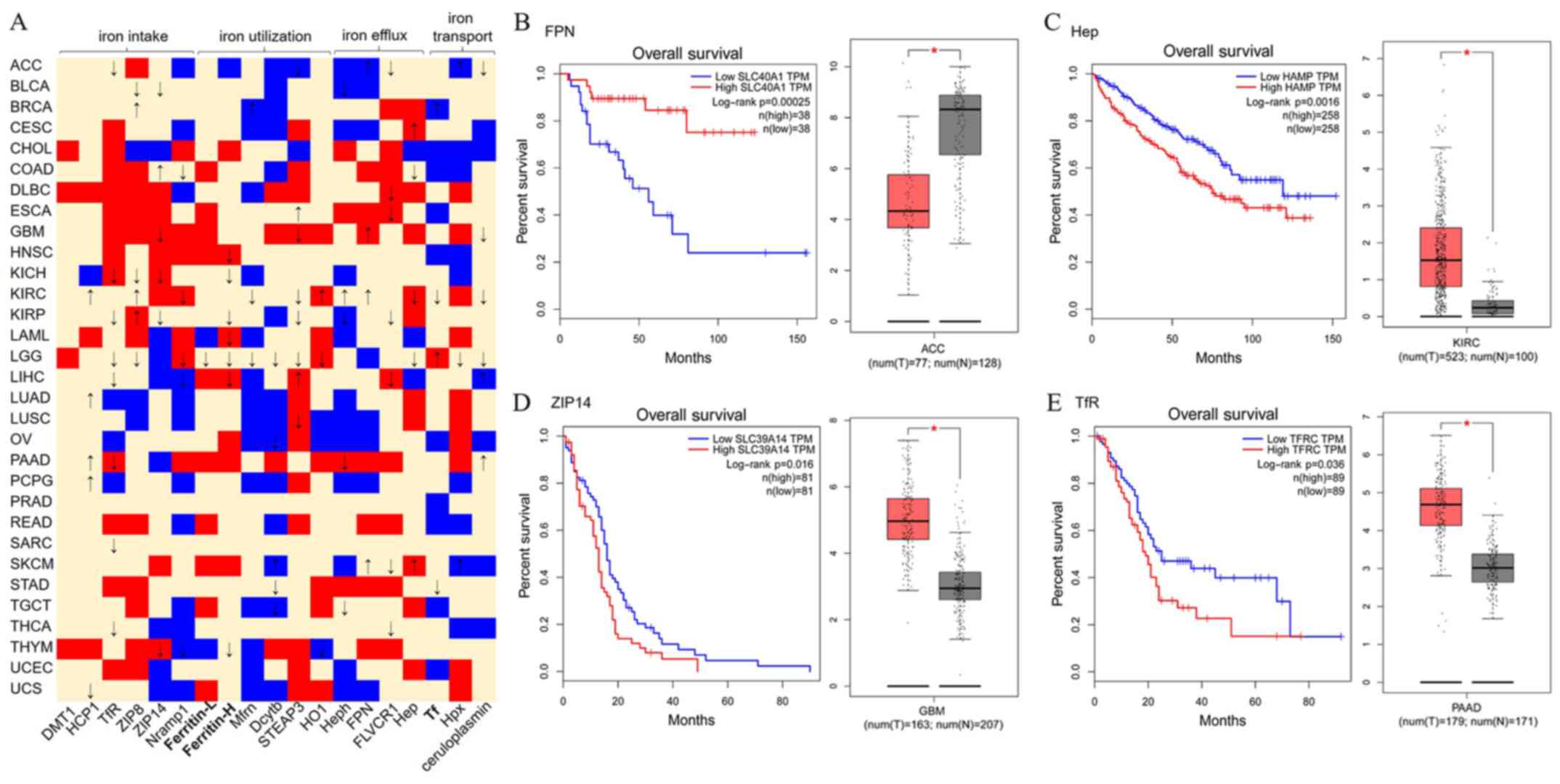
|
1
|
Kerins MJ and Ooi A: The roles of NRF2 in
modulating cellular iron homeostasis. Antioxid Redox Signal.
29:1756–1773. 2018. View Article : Google Scholar :
|
|
2
|
Andrews NC: Forging a field: The golden
age of iron biology. Blood. 112:219–230. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dielschneider RF, Henson ES and Gibson SB:
Lysosomes as oxidative targets for cancer therapy. Oxid Med Cell
Longev. 2017:37491572017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ali MK, Kim RY, Karim R, Mayall JR, Martin
KL, Shahandeh A, Abbasian F, Starkey MR, Loustaud-Ratti V,
Johnstone D, et al: Role of iron in the pathogenesis of respiratory
disease. Int J Biochem Cell Biol. 88:181–195. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Manz DH, Blanchette NL, Paul BT, Torti FM
and Torti SV: Iron and cancer: Recent insights. Ann NY Acad Sci.
1368:149–161. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fonseca-Nunes A, Jakszyn P and Agudo A:
Iron and cancer risk - a systematic review and meta-analysis of the
epidemiological evidence. Cancer Epidemiol Biomarkers Prev.
23:12–31. 2014. View Article : Google Scholar
|
|
7
|
Torti SV and Torti FM: Iron and cancer:
More ore to be mined. Nat Rev Cancer. 13:342–355. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Leftin A, Ben-Chetrit N, Klemm F, Joyce JA
and Koutcher JA: Iron imaging reveals tumor and metastasis
macrophage hemosiderin deposits in breast cancer. PLoS One.
12:e01847652017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rouault TA and Maio N: Biogenesis and
functions of mammalian iron-sulfur proteins in the regulation of
iron homeostasis and pivotal metabolic pathways. J Biol Chem.
292:12744–12753. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fuss JO, Tsai CL, Ishida JP and Tainer JA:
Emerging critical roles of Fe-S clusters in DNA replication and
repair. Biochim Biophys Acta. 1853:1253–1271. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Imlay JA and Linn S: DNA damage and oxygen
radical toxicity. Science. 240:1302–1309. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Brandt KE, Falls KC, Schoenfeld JD, Rodman
SN, Gu Z, Zhan F, Cullen JJ, Wagner BA, Buettner GR, Allen BG, et
al: Augmentation of intracellular iron using iron sucrose enhances
the toxicity of pharmacological ascorbate in colon cancer cells.
Redox Biol. 14:82–87. 2018. View Article : Google Scholar
|
|
13
|
Callens C, Coulon S, Naudin J,
Radford-Weiss I, Boissel N, Raffoux E, Wang PH, Agarwal S, Tamouza
H, Paubelle E, et al: Targeting iron homeostasis induces cellular
differentiation and synergizes with differentiating agents in acute
myeloid leukemia. J Exp Med. 207:731–750. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Heath JL, Weiss JM, Lavau CP and Wechsler
DS: Iron deprivation in cancer - potential therapeutic
implications. Nutrients. 5:2836–2859. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
González A, Gálvez N, Martín J, Reyes F,
Pérez-Victoria I and Dominguez-Vera JM: Identification of the key
excreted molecule by Lactobacillus fermentum related to host iron
absorption. Food Chem. 228:374–380. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Staroń R, Lipiński P, Lenartowicz M,
Bednarz A, Gajowiak A, Smuda E, Krzeptowski W, Pieszka M, Korolonek
T, Hamza I, et al: Dietary hemoglobin rescues young piglets from
severe iron defi-ciency anemia: Duodenal expression profile of
genes involved in heme iron absorption. PLoS One. 12:e01811172017.
View Article : Google Scholar
|
|
17
|
Li Y, Jiang H and Huang G: Protein
hydrolysates as promoters of non-haem iron absorption. Nutrients.
9:92017. View Article : Google Scholar
|
|
18
|
Martínez-Torres C and Layrisse M: Iron
absorption from veal muscle. Am J Clin Nutr. 24:531–540. 1971.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ascenzi P, Leboffe L and Polticelli F:
Cyanide binding to human plasma heme-hemopexin: A comparative
study. Biochem Biophys Res Commun. 428:239–244. 2012. View Article : Google Scholar : PubMed/NCBI
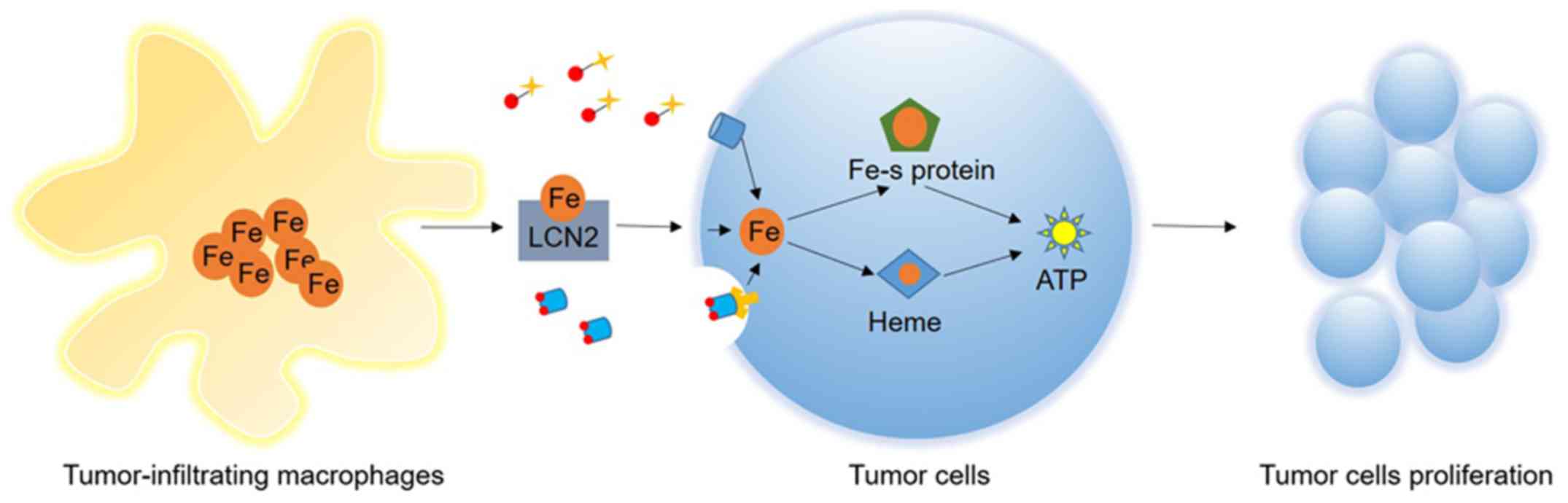
|
|
20
|
Colins A, Gerdtzen ZP, Nuñez MT and
Salgado JC: Mathematical modeling of intestinal iron absorption
using genetic programming. PLoS One. 12:e01696012017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Donovan A, Lima CA, Pinkus JL, Pinkus GS,
Zon LI, Robine S and Andrews NC: The iron exporter
ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab.
1:191–200. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sokolov AV, Voynova IV, Kostevich VA,
Vlasenko AY, Zakharova ET and Vasilyev VB: Comparison of
interaction between ceruloplasmin and lactoferrin/transferrin: To
bind or not to bind. Biochemistry (Mosc). 82:1073–1078. 2017.
View Article : Google Scholar
|
|
23
|
Nemeth E, Tuttle MS, Powelson J, Vaughn
MB, Donovan A, Ward DM, Ganz T and Kaplan J: Hepcidin regulates
cellular iron efflux by binding to ferroportin and inducing its
internalization. Science. 306:2090–2093. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lane DJ, Bae DH, Merlot AM, Sahni S and
Richardson DR: Duodenal cytochrome b (DCYTB) in iron metabolism: An
update on function and regulation. Nutrients. 7:2274–2296. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Skjørringe T, Burkhart A, Johnsen KB and
Moos T: Divalent metal transporter 1 (DMT1) in the brain:
Implications for a role in iron transport at the blood-brain
barrier, and neuronal and glial pathology. Front Mol Neurosci.
8:192015.PubMed/NCBI
|
|
26
|
Harrison PM and Arosio P: The ferritins:
Molecular properties, iron storage function and cellular
regulation. Biochim Biophys Acta. 1275:161–203. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
McKie AT and Barlow DJ: The SLC40
basolateral iron transporter family (IREG1/ferroportin/MTP1).
Pflugers Arch. 447:801–806. 2004. View Article : Google Scholar
|
|
28
|
Park CH, Valore EV, Waring AJ and Ganz T:
Hepcidin, a urinary antimicrobial peptide synthesized in the liver.
J Biol Chem. 276:7806–7810. 2001. View Article : Google Scholar
|
|
29
|
Liuzzi JP, Aydemir F, Nam H, Knutson MD
and Cousins RJ: Zip14 (Slc39a14) mediates non-transferrin-bound
iron uptake into cells. Proc Natl Acad Sci USA. 103:13612–13617.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lin W, Vann DR, Doulias P-T, Wang T,
Landesberg G, Li X, Ricciotti E, Scalia R, He M, Hand NJ, et al:
Hepatic metal ion transporter ZIP8 regulates manganese homeostasis
and manganese-dependent enzyme activity. J Clin Invest.
127:2407–2417. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mwanjewe J and Grover AK: Role of
transient receptor potential canonical 6 (TRPC6) in
non-transferrin-bound iron uptake in neuronal phenotype PC12 cells.
Biochem J. 378:975–982. 2004. View Article : Google Scholar
|
|
32
|
Knutson MD: Non-transferrin-bound iron
transporters. Free Radic Biol Med. 133:101–111. 2019. View Article : Google Scholar
|
|
33
|
Yoshizaki T, Uematsu M, Obata JE, Nakamura
T, Fujioka D, Watanabe K, Nakamura K and Kugiyama K: Angiotensin II
receptor blockers suppress the release of stromal cell-derived
factor-1alpha from infarcted myocardium in patients with acute
myocardial infarction. J Cardiol. 71:367–374. 2018. View Article : Google Scholar
|
|
34
|
Recuenco MC, Rahman MM, Takeuchi F,
Kobayashi K and Tsubaki M: Electron transfer reactions of candidate
tumor suppressor 101F6 protein, a cytochrome b561 homologue, with
ascorbate and monodehydroascorbate radical. Biochemistry.
52:3660–3668. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lane DJ and Lawen A: Ascorbate and plasma
membrane electron transport - enzymes vs efflux. Free Radic Biol
Med. 47:485–495. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Anderson SA, Nizzi CP, Chang YI, Deck KM,
Schmidt PJ, Galy B, Damnernsawad A, Broman AT, Kendziorski C,
Hentze MW, et al: The IRP1-HIF-2α axis coordinates iron and oxygen
sensing with erythropoiesis and iron absorption. Cell Metab.
17:282–290. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Coates TD: Physiology and pathophysiology
of iron in hemoglobin-associated diseases. Free Radic Biol Med.
72:23–40. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gunshin H, Allerson CR, Polycarpou-Schwarz
M, Rofts A, Rogers JT, Kishi F, Hentze MW, Rouault TA, Andrews NC
and Hediger MA: Iron-dependent regulation of the divalent metal ion
transporter. FEBS Lett. 509:309–316. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Anderson CP, Shen M, Eisenstein RS and
Leibold EA: Mammalian iron metabolism and its control by iron
regulatory proteins. Biochim Biophys Acta. 1823:1468–1483. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ford GC, Harrison PM, Rice DW, Smith JM,
Treffry A, White JL and Yariv J: Ferritin: Design and formation of
an iron-storage molecule. Philos Trans R Soc Lond B Biol Sci.
304:551–565. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Carmona U, Li L, Zhang L and Knez M:
Ferritin light-chain subunits: Key elements for the electron
transfer across the protein cage. Chem Commun (Camb).
50:15358–15361. 2014. View Article : Google Scholar
|
|
42
|
Kukulj S, Jaganjac M, Boranic M, Krizanac
S, Santic Z and Poljak-Blazi M: Altered iron metabolism,
inflammation, trans-ferrin receptors, and ferritin expression in
non-small-cell lung cancer. Med Oncol. 27:268–277. 2010. View Article : Google Scholar
|
|
43
|
Ganz T and Nemeth E: Iron metabolism:
Interactions with normal and disordered erythropoiesis. Cold Spring
Harb Perspect Med. 2:a0116682012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cianetti L, Gabbianelli M and Sposi NM:
Ferroportin and erythroid cells: an update. Adv Hematol.
2010:4041732010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wallace DF, McDonald CJ, Ostini L, Iser D,
Tuckfield A and Subramaniam VN: The dynamics of
hepcidin-ferroportin internalization and consequences of a novel
ferroportin disease mutation. Am J Hematol. 92:1052–1061. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
El Hage Chahine JM, Hémadi M and Ha-Duong
NT: Uptake and release of metal ions by transferrin and interaction
with receptor 1. Biochim Biophys Acta. 1820:334–347. 2012.
View Article : Google Scholar
|
|
47
|
Frazer DM and Anderson GJ: The regulation
of iron transport. Biofactors. 40:206–214. 2014. View Article : Google Scholar
|
|
48
|
Addo L, Ikuta K, Tanaka H, Toki Y,
Hatayama M, Yamamoto M, Ito S, Shindo M, Sasaki Y, Shimonaka Y, et
al: The three isoforms of hepcidin in human serum and their
processing determined by liquid chromatography-tandem mass
spectrometry (LC-tandem MS). Int J Hematol. 103:34–43. 2016.
View Article : Google Scholar
|
|
49
|
Qiao B, Sugianto P, Fung E,
Del-Castillo-Rueda A, Moran-Jimenez MJ, Ganz T and Nemeth E:
Hepcidin-induced endocytosis of ferroportin is dependent on
ferroportin ubiquitination. Cell Metab. 15:918–924. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ramos E, Kautz L, Rodriguez R, Hansen M,
Gabayan V, Ginzburg Y, Roth MP, Nemeth E and Ganz T: Evidence for
distinct pathways of hepcidin regulation by acute and chronic iron
loading in mice. Hepatology. 53:1333–1341. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Coffey R and Ganz T: Iron homeostasis: An
anthropocentric perspective. J Biol Chem. 292:12727–12734. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Pietrangelo A, Dierssen U, Valli L, Garuti
C, Rump A, Corradini E, Ernst M, Klein C and Trautwein C: STAT3 is
required for IL-6-gp130-dependent activation of hepcidin in vivo.
Gastroenterology. 132:294–300. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Palis J: Primitive and definitive
erythropoiesis in mammals. Front Physiol. 5:32014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Papanikolaou G and Pantopoulos K: Systemic
iron homeostasis and erythropoiesis. IUBMB Life. 69:399–413. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Shaw GC, Cope JJ, Li L, Corson K, Hersey
C, Ackermann GE, Gwynn B, Lambert AJ, Wingert RA, Traver D, et al:
Mitoferrin is essential for erythroid iron assimilation. Nature.
440:96–100. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Beaumont C and Canonne-Hergaux F:
Erythrophagocytosis and recycling of heme iron in normal and
pathological conditions; regulation by hepcidin. Transfus Clin
Biol. 12:123–130. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Theurl I, Hilgendorf I, Nairz M, Tymoszuk
P, Haschka D, Asshoff M, He S, Gerhardt LM, Holderried TA, Seifert
M, et al: On-demand erythrocyte disposal and iron recycling
requires transient macrophages in the liver. Nat Med. 22:945–951.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Soe-Lin S, Apte SS, Mikhael MR, Kayembe
LK, Nie G and Ponka P: Both Nramp1 and DMT1 are necessary for
efficient macrophage iron recycling. Exp Hematol. 38:609–617. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Poss KD and Tonegawa S: Heme oxygenase 1
is required for mammalian iron reutilization. Proc Natl Acad Sci
USA. 94:10919–10924. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Qian ZM and Tang PL: Mechanisms of iron
uptake by mammalian cells. Biochim Biophys Acta. 1269:205–214.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Morgan EH: Chelator-mediated iron efflux
from reticulocytes. Biochim Biophys Acta. 733:39–50. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Wang CY, Jenkitkasemwong S, Duarte S,
Sparkman BK, Shawki A, Mackenzie B and Knutson MD: ZIP8 is an iron
and zinc transporter whose cell-surface expression is up-regulated
by cellular iron loading. J Biol Chem. 287:34032–34043. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Pinilla-Tenas JJ, Sparkman BK, Shawki A,
Illing AC, Mitchell CJ, Zhao N, Liuzzi JP, Cousins RJ, Knutson MD
and Mackenzie B: Zip14 is a complex broad-scope metal-ion
transporter whose functional properties support roles in the
cellular uptake of zinc and nontransferrin-bound iron. Am J Physiol
Cell Physiol. 301:C862–C871. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Tsushima RG, Wickenden AD, Bouchard RA,
Oudit GY, Liu PP and Backx PH: Modulation of iron uptake in heart
by L-type Ca2+ channel modifiers: Possible implications
in iron overload. Circ Res. 84:1302–1309. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Brittenham GM, Andersson M, Egli I, Foman
JT, Zeder C, Westerman ME and Hurrell RF: Circulating
non-trans-ferrin-bound iron after oral administration of
supplemental and fortification doses of iron to healthy women: A
randomized study. Am J Clin Nutr. 100:813–820. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Pinto JP, Arezes J, Dias V, Oliveira S,
Vieira I, Costa M, Vos M, Carlsson A, Rikers Y, Rangel M, et al:
Physiological implications of NTBI uptake by T lymphocytes. Front
Pharmacol. 5:242014. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Ramey G, Deschemin JC, Durel B,
Canonne-Hergaux F, Nicolas G and Vaulont S: Hepcidin targets
ferroportin for degradation in hepatocytes. Haematologica.
95:501–504. 2010. View Article : Google Scholar :
|
|
68
|
Iancu TC, Ward RJ and Peters TJ:
Ultrastructural changes in the pancreas of carbonyl iron-fed rats.
J Pediatr Gastroenterol Nutr. 10:95–101. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Paragas N, Qiu A, Hollmen M, Nickolas TL,
Devarajan P and Barasch J: NGAL-Siderocalin in kidney disease.
Biochim Biophys Acta. 1823:1451–1458. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Martines AM, Masereeuw R, Tjalsma H,
Hoenderop JG, Wetzels JF and Swinkels DW: Iron metabolism in the
pathogenesis of iron-induced kidney injury. Nat Rev Nephrol.
9:385–398. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Lakhal-Littleton S, Wolna M, Carr CA,
Miller JJ, Christian HC, Ball V, Santos A, Diaz R, Biggs D,
Stillion R, et al: Cardiac ferro-portin regulates cellular iron
homeostasis and is important for cardiac function. Proc Natl Acad
Sci USA. 112:3164–3169. 2015. View Article : Google Scholar
|
|
72
|
Richmond HG: Induction of sarcoma in the
rat by iron-dextran complex. BMJ. 1:947–949. 1959. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Xue X and Shah YM: Intestinal iron
homeostasis and colon tumorigenesis. Nutrients. 5:2333–2351. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Kew MC: Hepatic iron overload and
hepatocellular carcinoma. Liver Cancer. 3:31–40. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Stevens RG, Cologne JB, Nakachi K, Grant
EJ and Neriishi K: Body iron stores and breast cancer risk in
female atomic bomb survivors. Cancer Sci. 102:2236–2240. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Huang X: Iron overload and its association
with cancer risk in humans: Evidence for iron as a carcinogenic
metal. Mutat Res. 533:153–171. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Tang Z, Li C, Kang B, Gao G, Li C and
Zhang Z: GEPIA: A web server for cancer and normal gene expression
profiling and interactive analyses. Nucleic Acids Res. 45:W98–W102.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar
|
|
79
|
Haggar FA and Boushey RP: Colorectal
cancer epidemiology: Incidence, mortality, survival, and risk
factors. Clin Colon Rectal Surg. 22:191–197. 2009. View Article : Google Scholar :
|
|
80
|
Chua ACG, Klopcic B, Lawrance IC, Olynyk
JK and Trinder D: Iron: An emerging factor in colorectal
carcinogenesis. World J Gastroenterol. 16:663–672. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Kato I, Dnistrian AM, Schwartz M, Toniolo
P, Koenig K, Shore RE, Zeleniuch-Jacquotte A, Akhmedkhanov A and
Riboli E: Iron intake, body iron stores and colorectal cancer risk
in women: A nested case-control study. Int J Cancer. 80:693–698.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Wilson MJ, Dekker JWT, Harlaar JJ, Jeekel
J, Schipperus M and Zwaginga JJ: The role of preoperative iron
deficiency in colorectal cancer patients: Prevalence and treatment.
Int J Colorectal Dis. 32:1617–1624. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
de Juan D, Reta A, Castiella A, Pozueta J,
Prada A and Cuadrado E: HFE gene mutations analysis in Basque
hereditary haemochromatosis patients and controls. Eur J Hum Genet.
9:961–964. 2001. View Article : Google Scholar
|
|
84
|
Castiella A, Múgica F, Zapata E, Zubiaurre
L, Iribarren A, de Juan MD, Alzate L, Gil I, Urdapilleta G, Otazua
P, et al: Gender and plasma iron biomarkers, but not HFE gene
mutations, increase the risk of colorectal cancer and polyps.
Tumour Biol. 36:6959–6963. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Asberg A, Thorstensen K, Irgens WO,
Romundstad PR and Hveem K: Cancer risk in HFE C282Y homozygotes:
Results from the HUNT 2 study. Scand J Gastroenterol. 48:189–195.
2013. View Article : Google Scholar
|
|
86
|
Ludwig H, Müldür E, Endler G and Hübl W:
Prevalence of iron deficiency across different tumors and its
association with poor performance status, disease status and
anemia. Ann Oncol. 24:1886–1892. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Nelson RL: Dietary iron and colorectal
cancer risk. Free Radic Biol Med. 12:161–168. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Wilson MJ, Harlaar JJ, Jeekel J,
Schipperus M and Zwaginga JJ: Iron therapy as treatment of anemia:
A potentially detrimental and hazardous strategy in colorectal
cancer patients. Med Hypotheses. 110:110–113. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Joosten E, Meeuwissen J, Vandewinckele H
and Hiele M: Iron status and colorectal cancer in symptomatic
elderly patients. Am J Med. 121:1072–1077. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Wilson MJ, Dekker JW, Bruns E, Borstlap W,
Jeekel J, Zwaginga JJ and Schipperus M: Short-term effect of
preoperative intravenous iron therapy in colorectal cancer patients
with anemia: Results of a cohort study. Transfusion. 58:795–803.
2018. View Article : Google Scholar
|
|
91
|
Laso-Morales M, Jericó C, Gómez-Ramírez S,
Castellví J, Viso L, Roig-Martínez I, Pontes C and Muñoz M:
Preoperative management of colorectal cancer-induced iron
deficiency anemia in clinical practice: Data from a large
observational cohort. Transfusion. 57:3040–3048. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Baecker A, Liu X, La Vecchia C and Zhang
ZF: Worldwide incidence of hepatocellular carcinoma cases
attributable to major risk factors. Eur J Cancer Prev. 27:205–212.
2018.PubMed/NCBI
|
|
93
|
Sun B and Karin M: Obesity, inflammation,
and liver cancer. J Hepatol. 56:704–713. 2012. View Article : Google Scholar
|
|
94
|
Bardou-Jacquet E, Morcet J, Manet G, Lainé
F, Perrin M, Jouanolle AM, Guyader D, Moirand R, Viel JF and
Deugnier Y: Decreased cardiovascular and extrahepatic
cancer-related mortality in treated patients with mild HFE
hemochromatosis. J Hepatol. 62:682–689. 2015. View Article : Google Scholar
|
|
95
|
Grosse SD, Rogowski WH, Ross LF, Cornel
MC, Dondorp WJ and Khoury MJ: Population screening for genetic
disorders in the 21st century: Evidence, economics, and ethics.
Public Health Genomics. 13:106–115. 2010. View Article : Google Scholar
|
|
96
|
Da Costa GG, Gomig TH, Kaviski R, Santos
Sousa K, Kukolj C, De Lima RS, De Andrade Urban C, Cavalli IJ and
Ribeiro EM: Comparative proteomics of tumor and paired normal
breast tissue highlights potential biomarkers in breast cancer.
Cancer Genomics Proteomics. 12:251–261. 2015.PubMed/NCBI
|
|
97
|
Nunes-Xavier CE, Martín-Pérez J, Elson A
and Pulido R: Protein tyrosine phosphatases as novel targets in
breast cancer therapy. Biochim Biophys Acta. 1836:211–226.
2013.PubMed/NCBI
|
|
98
|
Tonks NK: Protein tyrosine phosphatases:
From genes, to function, to disease. Nat Rev Mol Cell Biol.
7:833–846. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Kuban-Jankowska A, Sahu KK,
Gorska-Ponikowska M, Tuszynski JA and Wozniak M: Inhibitory
activity of iron chelators ATA and DFO on MCF-7 breast cancer cells
and phos-phatases PTP1B and SHP2. Anticancer Res. 37:4799–4806.
2017.PubMed/NCBI
|
|
100
|
Wang YF, Zhang J, Su Y, Shen YY, Jiang DX,
Hou YY, Geng MY, Ding J and Chen Y: G9a regulates breast cancer
growth by modulating iron homeostasis through the repression of
ferroxidase hephaestin. Nat Commun. 8:2742017. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Lemler DJ, Lynch ML, Tesfay L, Deng Z,
Paul BT, Wang X, Hegde P, Manz DH, Torti SV and Torti FM: DCYTB is
a predictor of outcome in breast cancer that functions via
iron-independent mechanisms. Breast Cancer Res. 19:252017.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Zheng J, Ren W, Chen T, Yinhua J, Li A,
Yan K, Wu Y and Wu A: Recent advances in superparamagnetic iron
oxide based nano-probes as multifunctional theranostic agents for
breast cancer imaging and therapy. Curr Med Chem. 25:3001–3016.
2018. View Article : Google Scholar
|
|
103
|
Ridge CA, McErlean AM and Ginsberg MS:
Epidemiology of lung cancer. Semin Intervent Radiol. 30:93–98.
2013. View Article : Google Scholar :
|
|
104
|
Wild P, Bourgkard E and Paris C: Lung
cancer and exposure to metals: The epidemiological evidence.
Methods Mol Biol. 472:139–167. 2009. View Article : Google Scholar
|
|
105
|
Brookes MJ, Boult J, Roberts K, Cooper BT,
Hotchin NA, Matthews G, Iqbal T and Tselepis C: A role for iron in
Wnt signalling. Oncogene. 27:966–975. 2008. View Article : Google Scholar
|
|
106
|
Wu KJ, Polack A and Dalla-Favera R:
Coordinated regulation of iron-controlling genes, H-ferritin and
IRP2, by c-MYC. Science. 283:676–679. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Chanvorachote P and Luanpitpong S: Iron
induces cancer stem cells and aggressive phenotypes in human lung
cancer cells. Am J Physiol Cell Physiol. 310:C728–C739. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Lee BJ, Kim B and Lee K: Air pollution
exposure and cardiovascular disease. Toxicol Res. 30:71–75. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Lovera-Leroux M, Crobeddu B, Kassis N,
Petit PX, Janel N, Baeza-Squiban A and Andreau K: The iron
component of particulate matter is antiapoptotic: A clue to the
development of lung cancer after exposure to atmospheric
pollutants? Biochimie. 118:195–206. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Bidoli E, Barbone F, Collarile P, Valent
F, Zanier L, Daris F, Gini A, Birri S and Serraino D: Residence in
proximity of an iron foundry and risk of lung cancer in the
municipality of trieste, Italy, 1995-2009. Int J Environ Res Public
Health. 12:9025–9035. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Song MK, Chung JS, Seol YM, Shin HJ, Choi
YJ and Cho GJ: Elevation of serum ferritin is associated with the
outcome of patients with newly diagnosed multiple myeloma. Korean
Korean J Intern Med. 24:368–373. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Strasser-Weippl K and Ludwig H: Ferritin
as prognostic marker in multiple myeloma patients undergoing
autologous transplantation. Leuk Lymphoma. 55:2520–2524. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Gu Z, Wang H, Xia J, Yang Y, Jin Z, Xu H,
Shi J, De Domenico I, Tricot G and Zhan F: Decreased ferroportin
promotes myeloma cell growth and osteoclast differentiation. Cancer
Res. 75:2211–2221. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Kim JL, Lee D-H, Na YJ, Kim BR, Jeong YA,
Lee SI, Kang S, Joung SY, Lee S-Y, Oh SC, et al: Iron
chelator-induced apoptosis via the ER stress pathway in gastric
cancer cells. Tumour Biol. 37:9709–9719. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Timofeeva OA, Palechor-Ceron N, Li G, Yuan
H, Krawczyk E, Zhong X, Liu G, Upadhyay G, Dakic A, Yu S, et al:
Conditionally reprogrammed normal and primary tumor prostate
epithelial cells: A novel patient-derived cell model for studies of
human prostate cancer. Oncotarget. 8:22741–22758. 2017. View Article : Google Scholar :
|
|
116
|
Wachowius F, Attwater J and Holliger P:
Nucleic acids: Function and potential for abiogenesis. Q Rev
Biophys. 50:e42017. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Puig S, Ramos-Alonso L, Romero AM and
Martínez-Pastor MT: The elemental role of iron in DNA synthesis and
repair. Metallomics. 9:1483–1500. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Friedman JR and Nunnari J: Mitochondrial
form and function. Nature. 505:335–343. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Ren JG, Seth P, Ye H, Guo K, Hanai JI,
Husain Z and Sukhatme VP: Citrate suppresses tumor growth in
multiple models through inhibition of glycolysis, the tricarboxylic
acid cycle and the IGF-1R pathway. Sci Rep. 7:45372017. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Dutkiewicz R and Nowak M: Molecular
chaperones involved in mitochondrial iron-sulfur protein
biogenesis. J Biol Inorg Chem. 23:569–579. 2018. View Article : Google Scholar
|
|
121
|
Miller LD, Coffman LG, Chou JW, Black MA,
Bergh J, D'Agostino R Jr, Torti SV and Torti FM: An iron regulatory
gene signature predicts outcome in breast cancer. Cancer Res.
71:6728–6737. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Mettert EL and Kiley PJ: Fe-S proteins
that regulate gene expression. Biochim Biophys Acta.
1853:1284–1293. 2015. View Article : Google Scholar :
|
|
123
|
Zhang L, Reyes A and Wang X: The role of
DNA repair in maintaining mitochondrial DNA stability. Adv Exp Med
Biol. 1038:85–105. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Chen YR and Zweier JL: Cardiac
mitochondria and reactive oxygen species generation. Circ Res.
114:524–537. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Urra FA, Muñoz F, Lovy A and Cárdenas C:
The mitochondrial complex(I)ty of cancer. Front Oncol. 7:1182017.
View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Bastian A, Matsuzaki S, Humphries KM,
Pharaoh GA, Doshi A, Zaware N, Gangjee A and Ihnat MA: AG311, a
small molecule inhibitor of complex I and hypoxia-induced HIF-1α
stabilization. Cancer Lett. 388:149–157. 2017. View Article : Google Scholar
|
|
127
|
Bridges HR, Jones AJ, Pollak MN and Hirst
J: Effects of metformin and other biguanides on oxidative
phosphorylation in mitochondria. Biochem J. 462:475–487. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Esser L, Zhou F, Zhou Y, Xiao Y, Tang WK,
Yu CA, Qin Z and Xia D: Hydrogen bonding to the substrate is not
required for rieskeiron-sulfur protein docking to the quinol
oxidation site of complex III. J Biol Chem. 291:25019–25031. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Wang F, Zhang R, Xia T, Hsu E, Cai Y, Gu Z
and Hankinson O: Inhibitory effects of nitric oxide on invasion of
human cancer cells. Cancer Lett. 257:274–282. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Fiorillo M, Lamb R, Tanowitz HB, Mutti L,
Krstic-Demonacos M, Cappello AR, Martinez-Outschoorn UE, Sotgia F
and Lisanti MP: Repurposing atovaquone: Targeting mitochondrial
complex III and OXPHOS to eradicate cancer stem cells. Oncotarget.
7:34084–34099. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Oyedotun KS and Lemire BD: The quaternary
structure of the Saccharomyces cerevisiae succinate dehydrogenase.
Homology modeling, cofactor docking, and molecular dynamics
simulation studies. J Biol Chem. 279:9424–9431. 2004. View Article : Google Scholar
|
|
132
|
Sun F, Huo X, Zhai Y, Wang A, Xu J, Su D,
Bartlam M and Rao Z: Crystal structure of mitochondrial respiratory
membrane protein complex II. Cell. 121:1043–1057. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Guo L, Shestov AA, Worth AJ, Nath K,
Nelson DS, Leeper DB, Glickson JD and Blair IA: Inhibition of
mitochondrial complex II by the anticancer agent lonidamine. J Biol
Chem. 291:42–57. 2016. View Article : Google Scholar :
|
|
134
|
Kluckova K, Bezawork-Geleta A, Rohlena J,
Dong L and Neuzil J: Mitochondrial complex II, a novel target for
anti-cancer agents. Biochim Biophys Acta. 1827:552–564. 2013.
View Article : Google Scholar
|
|
135
|
Quail DF and Joyce JA: Microenvironmental
regulation of tumor progression and metastasis. Nat Med.
19:1423–1437. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Shree T, Olson OC, Elie BT, Kester JC,
Garfall AL, Simpson K, Bell-McGuinn KM, Zabor EC, Brogi E and Joyce
JA: Macrophages and cathepsin proteases blunt chemotherapeutic
response in breast cancer. Genes Dev. 25:2465–2479. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Gocheva V, Wang HW, Gadea BB, Shree T,
Hunter KE, Garfall AL, Berman T and Joyce JA: IL-4 induces
cathepsin protease activity in tumor-associated macrophages to
promote cancer growth and invasion. Genes Dev. 24:241–255. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Yeung OW, Lo CM, Ling CC, Qi X, Geng W, Li
CX, Ng KT, Forbes SJ, Guan XY, Poon RT, et al: Alternatively
activated (M2) macrophages promote tumour growth and invasiveness
in hepatocellular carcinoma. J Hepatol. 62:607–616. 2015.
View Article : Google Scholar
|
|
139
|
Wu L, Zhang X, Zhang B, Shi H, Yuan X, Sun
Y, Pan Z, Qian H and Xu W: Exosomes derived from gastric cancer
cells activate NF-κB pathway in macrophages to promote cancer
progression. Tumour Biol. 37:12169–12180. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Torti SV and Torti FM: Cellular iron
metabolism in prognosis and therapy of breast cancer. Crit Rev
Oncog. 18:435–448. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Duan X, He K, Li J, Cheng M, Song H, Liu J
and Liu P: Tumor associated macrophages deliver iron to tumor cells
via Lcn2. Int J Physiol Pathophysiol Pharmacol. 10:105–114.
2018.PubMed/NCBI
|
|
142
|
Mertens C, Mora J, Ören B, Grein S,
Winslow S, Scholich K, Weigert A, Malmström P, Forsare C, Fernö M,
et al: Macrophage-derived lipocalin-2 transports iron in the tumor
microenvironment. OncoImmunology. 7:e14087512017. View Article : Google Scholar
|
|
143
|
Flower DR: The lipocalin protein family: A
role in cell regulation. FEBS Lett. 354:7–11. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Laskar A, Eilertsen J, Li W and Yuan XM:
SPION primes THP1 derived M2 macrophages towards M1-like
macrophages. Biochem Biophys Res Commun. 441:737–742. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Fearnhead HO, Vandenabeele P and Vanden
Berghe T: How do we fit ferroptosis in the family of regulated cell
death? Cell Death Differ. 24:1991–1998. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta
R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS,
et al: Ferroptosis: An iron-dependent form of nonapoptotic cell
death. Cell. 149:1060–1072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Sheng X, Shan C, Liu J, Yang J, Sun B and
Chen D: Theoretical insights into the mechanism of ferroptosis
suppression via inactivation of a lipid peroxide radical by
liproxstatin-1. Phys Chem Chem Phys. 19:13153–13159. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Fanzani A and Poli M: Iron, oxidative
damage and ferroptosis in rhabdomyosarcoma. Int J Mol Sci.
18:182017.
|
|
149
|
Alvarez SW, Sviderskiy VO, Terzi EM,
Papagiannakopoulos T, Moreira AL, Adams S, Sabatini DM, Birsoy K
and Possemato R: NFS1 undergoes positive selection in lung tumours
and protects cells from ferroptosis. Nature. 551:639–643.
2017.PubMed/NCBI
|
|
150
|
Sun X, Ou Z, Xie M, Kang R, Fan Y, Niu X,
Wang H, Cao L and Tang D: HSPB1 as a novel regulator of ferroptotic
cancer cell death. Oncogene. 34:5617–5625. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Doll S, Proneth B, Tyurina YY, Panzilius
E, Kobayashi S, Ingold I, Irmler M, Beckers J, Aichler M, Walch A,
et al: ACSL4 dictates ferroptosis sensitivity by shaping cellular
lipid composition. Nat Chem Biol. 13:91–98. 2017. View Article : Google Scholar :
|
|
152
|
Friedmann Angeli JP, Schneider M, Proneth
B, Tyurina YY, Tyurin VA, Hammond VJ, Herbach N, Aichler M, Walch
A, Eggenhofer E, et al: Inactivation of the ferroptosis regulator
Gpx4 triggers acute renal failure in mice. Nat Cell Biol.
16:1180–1191. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Cao JY and Dixon SJ: Mechanisms of
ferroptosis. Cell Mol Life Sci. 73:2195–2209. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Stockwell BR, Friedmann Angeli JP, Bayir
H, Bush AI, Conrad M, Dixon SJ, Fulda S, Gascón S, Hatzios SK,
Kagan VE, et al: Ferroptosis: A regulated cell death nexus linking
metabolism, redox biology, and disease. Cell. 171:273–285. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Ishii T, Sugita Y and Bannai S: Regulation
of glutathione levels in mouse spleen lymphocytes by transport of
cysteine. J Cell Physiol. 133:330–336. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Lou L, Kang J, Pang H, Li Q, Du X, Wu W,
Chen J and Lv J: Sulfur protects Pakchoi (Brassica chinensis L.)
seedlings against cadmium stress by regulating
ascorbate-glutathione metabolism. Int J Mol Sci. 18:182017.
View Article : Google Scholar
|
|
157
|
Dolma S, Lessnick SL, Hahn WC and
Stockwell BR: Identification of genotype-selective antitumor agents
using synthetic lethal chemical screening in engineered human tumor
cells. Cancer Cell. 3:285–296. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Seiler A, Schneider M, Förster H, Roth S,
Wirth EK, Culmsee C, Plesnila N, Kremmer E, Rådmark O, Wurst W, et
al: Glutathione peroxidase 4 senses and translates oxidative stress
into 12/15-lipoxygenase dependent- and AIF-mediated cell death.
Cell Metab. 8:237–248. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Ursini F and Bindoli A: The role of
selenium peroxidases in the protection against oxidative damage of
membranes. Chem Phys Lipids. 44:255–276. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Chu FF: The human glutathione peroxidase
genes GPX2, GPX3, and GPX4 map to chromosomes 14, 5, and 19,
respectively. Cytogenet Cell Genet. 66:96–98. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Yang WS, SriRamaratnam R, Welsch ME,
Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji
AF, Clish CB, et al: Regulation of ferroptotic cancer cell death by
GPX4. Cell. 156:317–331. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Shen Z, Liu T, Li Y, Lau J, Yang Z, Fan W,
Zhou Z, Shi C, Ke C, Bregadze VI, et al:
Fenton-reaction-acceleratable magnetic nanoparticles for
ferroptosis therapy of orthotopic brain tumors. ACS Nano.
12:11355–11365. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Shen J, Sheng X, Chang Z, Wu Q, Wang S,
Xuan Z, Li D, Wu Y, Shang Y, Kong X, et al: Iron metabolism
regulates p53 signaling through direct heme-p53 interaction and
modulation of p53 localization, stability, and function. Cell Rep.
7:180–193. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Tarangelo A, Magtanong L, Bieging-Rolett
KT, Li Y, Ye J, Attardi LD and Dixon SJ: p53 suppresses metabolic
stress-induced ferroptosis in cancer cells. Cell Rep. 22:569–575.
2018. View Article : Google Scholar : PubMed/NCBI
|