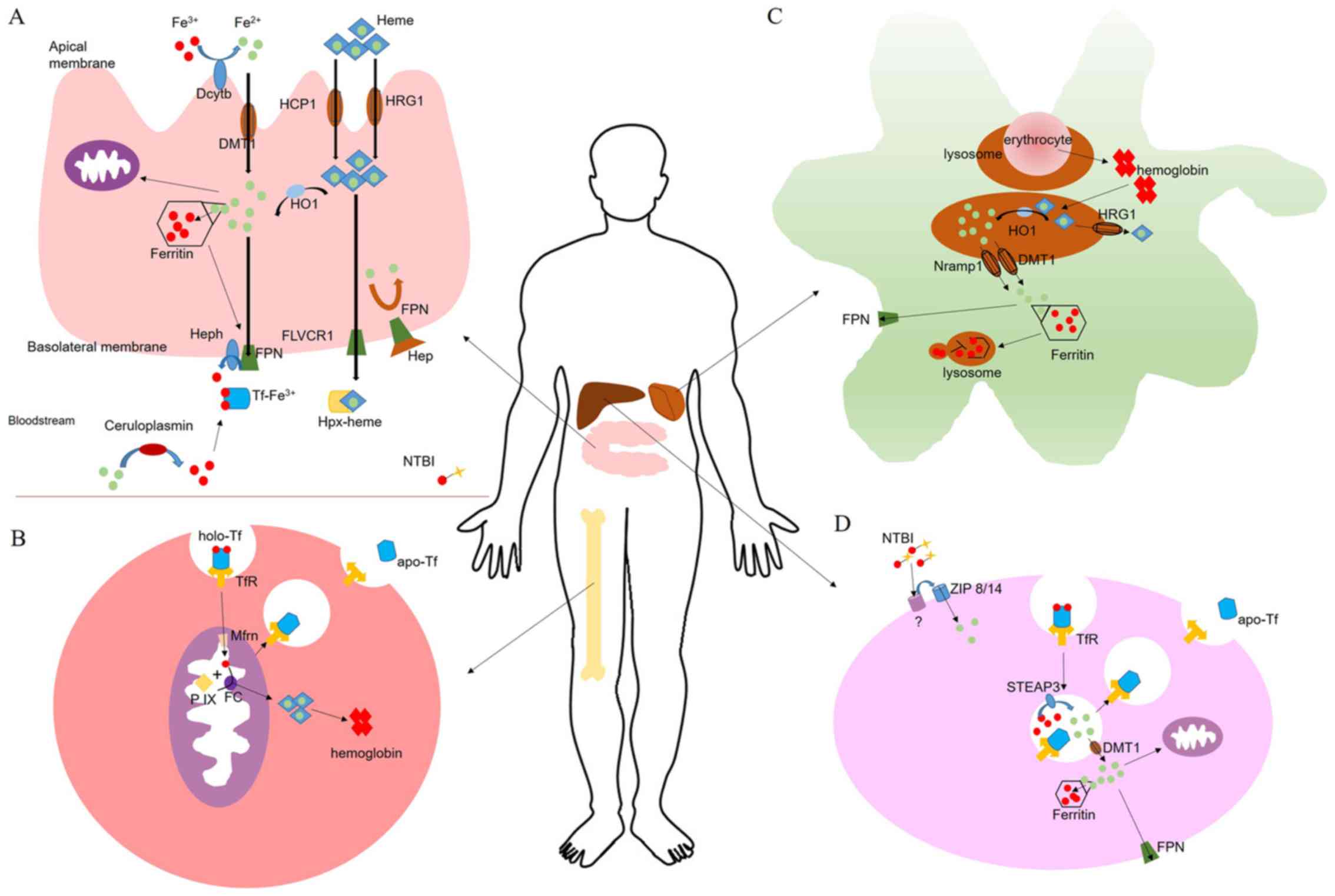
As a fundamental inorganic nutrient in the human
body, iron serves an important role in numerous biological
processes, including DNA and RNA synthesis, oxygen transport,
cellular respiration, the activity of numerous enzymes, heme
synthesis, detoxification processes, and immune function and
metabolism (1). Iron homeostasis
is tightly regulated in healthy cells by balancing absorption,
systemic transportation, and cellular uptake and storage (2). However, dysregulation of this balance
may increase the risk of cancer and has been associated with
carcinogenesis (3,4). Numerous studies have investigated
iron regulation pathways and examined the association between
increases in iron concentration and enhanced tumor growth (5-7). For
instance, high-iron clusters were observed in macrophage deposits
in mammary tumors, lung metastases and brain metastases with the
accumulation of hemosiderin (8).
Iron is an indispensable element for the synthesis
of iron sulfur clusters, which are versatile and used by enzymes
for vital cellular processes in normal and cancer cells (9). However, a high concentration of
oxygen makes iron sulfur clusters susceptible to oxidation and
Fenton reactions resulting in DNA damage (10,11).
Increasing intracellular labile iron pools, using iron sucrose, may
be applied to enhance the toxicity of pharmacological ascorbate in
human colon cancer cells by increasing the generation of
H2O2 (12).
Additionally, iron excess in tumor cells, due to an excessive
dietary intake and/or genetic factors, makes iron deprivation a
principal strategy of chemotherapy for multiple types of human
cancer (13,14). However, despite great progress, the
association between iron metabolism and cancer has yet to be fully
elucidated. The present review summarizes recent studies on novel
processes and mechanisms of iron transport into cells, which could
promote cancer cellular proliferation. The concepts discussed in
the present study may provide a novel approach to cancer prognosis
and therapy.
Iron is not synthesized during physiological
processes. Therefore, iron concentrations must be maintained
through nutrition. Iron from food is primarily absorbed by duodenum
enterocytes (90%). The stomach does not contribute much in the
assimilation of iron, as its absorption is ≤2% of the total intake
(15). There are two types of
dietary iron that may be absorbed: Heme iron, from the breakdown of
hemoglobin and myoglobin in red meat, or non-heme inorganic iron,
which is predominantly released from foods, including vegetables
and cereals (16,17).
In the cytosol, iron is neutralized by ferritin (a
multimeric iron-storage protein) (Table I) and weakly bound together to form
a pool of iron termed the cytoplasmic labile iron pool (cLIP)
(20). The cLIP supplies iron to a
number of cytoplasmic enzymes, in addition to mitochondria for heme
and iron sulfur cluster synthesis. Ferroportin (FPN) (Table I), the iron transporter at the
basolateral membrane of intestinal enterocytes, is the principal
cellular iron exporter and transfers duodenal iron out of cells
into circulation; this is the principal way for iron to enter
systemic circulation (21). The
released iron is reduced to ferrous ions by membrane-bound
hephaestin or caeruloplasmin in plasma. These enzymes oxidize
Fe2+ to Fe3+ and load Fe3+ onto
transferrin (Tf) in the blood (22). Subsequently, Tf combines with the
transferrin receptor (TfR) on cytomembranes. Hepcidin (Hep), a
protein secreted by hepatocytes, binds to FPN, ceasing cellular
iron export (Table I) (23-32).
Ferric ions are reduced by Dcytb and subsequently
enter enterocyte epithelial cells via DMT1. Some of the iron is
stored in ferritin and some passes through FPN into the plasma. In
specific cases, Hep may prevent FPN from releasing the iron. Tf
takes the released iron to TfR on the cell surface, the ion is then
used by the cells. Other specific proteins are involved in iron
intake, including zinc transporter (ZIP)14, ZIP8, transient
receptor potential cation channel 6 (TRPC6), L-type calcium
channels (LTCCs) and T-type calcium channels (TTCCs; Fig. 1).
As a member of the cytochrome b561 family, Dcytb
serves a vital role in the ascorbate-dependent reduction of
inorganic iron in duodenal enterocytes. Besides Dcytb, there are
additional members of the cytochrome b561 family, including stromal
cell-derived receptor 2, cytochrome b ascorbate-dependent protein 3
and cytochrome b561 domain-containing protein 2 (33,34).
Dcytb reduces extracellular ferric iron to ferrous ion (24,35).
The expression of Dcytb may be modulated by the iron regulatory
protein 1-hypoxia-inducible factor 2α axis (24,36).
DMT1 is well known for its involvement in ferric
iron influx into the duodenum cytoplasm. In erythroid precursors,
hepatocytes and macrophages, DMT1 is the principal contributor in
iron transport, transporting iron out of the endosome and into the
cytosol (37). DMT1 is also
expressed in other tissues, including the kidneys, liver, brain and
heart (25). When animals exhibit
iron deficiency, the expression of DMT1 is increased in the
duodenum to elevate iron absorption (38). This process is regulated by an iron
responsive element (IRE) at the 3'-untranslated region of DMT1
(37,39).
Ferritin forms a hollow shell that may bind ≤4,500
atoms of iron. Ferritin consists of 24 subunits, and has a
combination of a heavy (H) chain and light (L) chain ferritin types
(40). H-type ferritin ferroxidase
quickly oxidizes iron to Fe3+ following Fe2+
incorporation into ferritin, whereas, L-type ferritin may be
responsible for the electron transfer across the globular protein
cage (41). Since free iron is
toxic inside the cells, ferritin stores excess iron and is ligated
in labile cellular iron to protect cells from iron toxicity.
Ferritin stores are subsequently exported as ferrous ions to the
plasma via FPN, or utilized when cells are subjected to iron
deficiency. Ferritin is not only detected intracellularly, it is
also located extracellularly in the serum, cerebrospinal fluid and
synovial fluid. Serum ferritin is associated with inflammation and
body iron load in patient populations (42). The expression of ferritin may be
modulated by the iron regulatory protein 1 (IRP)-IRE system at the
post-transcriptional level.
FPN, encoded by SLC40A1 (additionally termed
SLC11A3, MTP1 or IREG1), is the only known cellular iron exporter
in mammals and is highly expressed in spleen macrophages, the
liver, and the basolateral membranes of enterocytes and erythroid
precursors (43,44). Macrophages, liver cells and
enterocytes export Fe2+ to the blood via FPN.
Subsequently, hephaestin or ceruloplasmin oxidizes Fe2+
to Fe3+ in order to bind Tf. FPN may be inhibited by Hep
binding to cell surface-localized FPN (Fig. 1A), which leads to FPN
internalization and degradation, and consequently to the loss of
iron export capacity (45).
Tf is the primary extracellular iron transport
protein in blood. Tf possesses a single chain bilobal protein. Each
lobe has a high affinity for Fe3+ (45). The combination of
Tf-Fe3+ is reversible, thus making it a convenient
method to deliver iron to cells. Tf has two conformations: Apo Tf
(the iron-free form of Tf) and holo Tf (the iron-saturated form),
of which the latter buries iron deeply within each lobe (46). Tf is secreted by the liver and
under normal circumstances 20-30% of Tf is bound to iron. If
circulating iron levels exceed the binding capacity of Tf, toxic
non-Tf-bound iron may accumulate, leading to various diseases
(47). TfR mediates cellular iron
uptake by binding and internalizing Tf. TfR expression is tightly
regulated by iron levels in the cLIP inside cells.
Hep (also termed liver-expressed antimicrobial
peptide 1) is a peptide comprising 25 amino acids encoded by
HAMP, and is predominantly expressed in the liver, and to a
lower extent, in the heart. Additionally, Hep may be secreted by
macrophages, lymphocytes, adipocytes, pancreatic β-cells,
neutrophils and renal cells (48).
Hep regulates cellular iron export and regulates the iron
concentration of plasma by binding to FPN, thus triggering the
internalization and degradation of FPN (49). The storage of systemic iron is
closely associated with the synthesis of Hep. When plasma iron is
deficient, Hep transcription is decreased in the liver. As the
plasma iron concentration increases, the Hep expression level
increases. However, a high concentration of Hep in the blood
negatively regulates iron absorption, and consequently, the release
of iron into the plasma is decreased (50).
Iron is transported in a cycle of Tf-bound and
non-Tf-bound iron (NTBI) forms. In steady state conditions, iron is
loaded onto Tf. Iron distribution is achieved using primary
pathways: Iron utilization in erythropoiesis, erythrocyte recycling
by macrophages and iron uptake into tissue (Fig. 1B-D). In total, ~24 mg of iron is
utilized at erythropoiesis (the process that generates
erythrocytes) per day. Macrophages phagocytose aged erythrocytes to
recycle iron (51). For humans,
there is no biological mechanism for the excretion of iron. Healthy
adults lose ~5% of plasma iron through gastro-intestinal tract
lining desquamation, skin or blood loss (37,52).
Erythropoiesis is characterized by three progressive
stages from progenitor cells to precursor cells and, ultimately, to
mature RBCs in bone marrow (53).
Erythroid progenitors (burst forming unit-erythroids and colony
forming unit-erythroids) have the capacity to differentiate to
proerythroblasts, basophilic erythroblasts, polychromatophilic
erythroblasts, orthochromatic erythroblasts, reticulocytes and
mature erythrocytes (53).
Maturation and proliferation of early erythroid progenitor cells
depends on erythropoietin, which is a cytokine produced by the
kidneys (54).
The body produces ~200 billion erythrocytes every
day. Erythropoiesis is the largest consumer of iron in the body
(>80% of plasma iron). Macrophages phagocytose senescent
erythrocytes and release hemoglobin-derived iron into the blood.
Hemoglobin-derived iron is the primary source of RBC regeneration.
Erythrocytes contain hemoglobin, which are functionally responsible
for transporting oxygen. Iron is the active site of hemoglobin
binding oxygen. When heme synthesis and Fe-S cluster biogenesis
occur, the iron demand increases. To meet this demand, transferred
iron is delivered to the mitochondrial matrix by mitoferrin and the
insertion of Fe2+ into protoporphyrin IX is catalyzes to
make heme by ferrochelatase, which is exported to the cytoplasm
(Fig. 1B) (55).
Iron homeostasis is regulated by circulating
erythrocytes. Macrophages remove senescent erythrocytes through
erythrophagocytosis (56). The
majority of aged or damaged erythrocytes are removed to release
iron for the production of novel RBCs. Red pulp splenic macrophages
and the Kupffer cells (specialized macrophages) in liver sinusoids
are primarily responsible for erythrophagocytosis. When the tissue
microenvironment is triggered by iron instability, monocyte-derived
macrophages are recruited to the liver, forming a liver-specific
phenomenon (57).
Heme degradation is catalyzed by HO-1 in macrophage
lysosomes. Prior to degradation, a part of heme is exported from
the lysosome to the cytosol by HRG1. Fe2+ is exported
from the lysosome to the cytoplasm by DMT1 and natural
resistance-associated macrophage protein 1 (58). Subsequently, the iron is released
into blood or accumulated by ferritin (Fig. 1C) (59).
Iron is transported in the Tf cycle normally;
however, iron additionally exists in the state of NTBI in iron
loading conditions. TfR is expressed ubiquitously on the surface of
the majority of cells and combines with holo Tf to form a complex,
which is swallowed by the cells as an endosome. When the complex
enters a cell, ferric ions are dissociated from Tf by
acidification. At that time, apo-Tf remains bound to TfR; apo-Tf is
released once the complex is transferred to the cell surface
(60,61). The ferrireductase protein,
six-transmembrane epithelial antigen of prostate 3 (STEAP3),
subsequently reduces Fe3+ to Fe2+ within the
endosome. Fe2+ enters the cytoplasm of cells via
DMT1.
In 1959, an animal study first demonstrated that
repeated intramuscular injections of iron dextran were able to
induce malignant tumors (72).
Over time, accumulating research demonstrated that the injection of
iron preparations caused serious side effects, such as sarcomas,
and exacerbated diseases (73,74).
Supersaturation ferric ion or iron exposure may increase cancer
risk (75). Iron-status biomarkers
are Tf saturation, the total iron binding capacity of Tf and the
serum iron concentration, which are regulated by dietary iron
intake, gene status and iron overload disease (76). Accumulating evidence suggests that
iron excess is closely associated with tumorigenesis in multiple
types of human cancer.
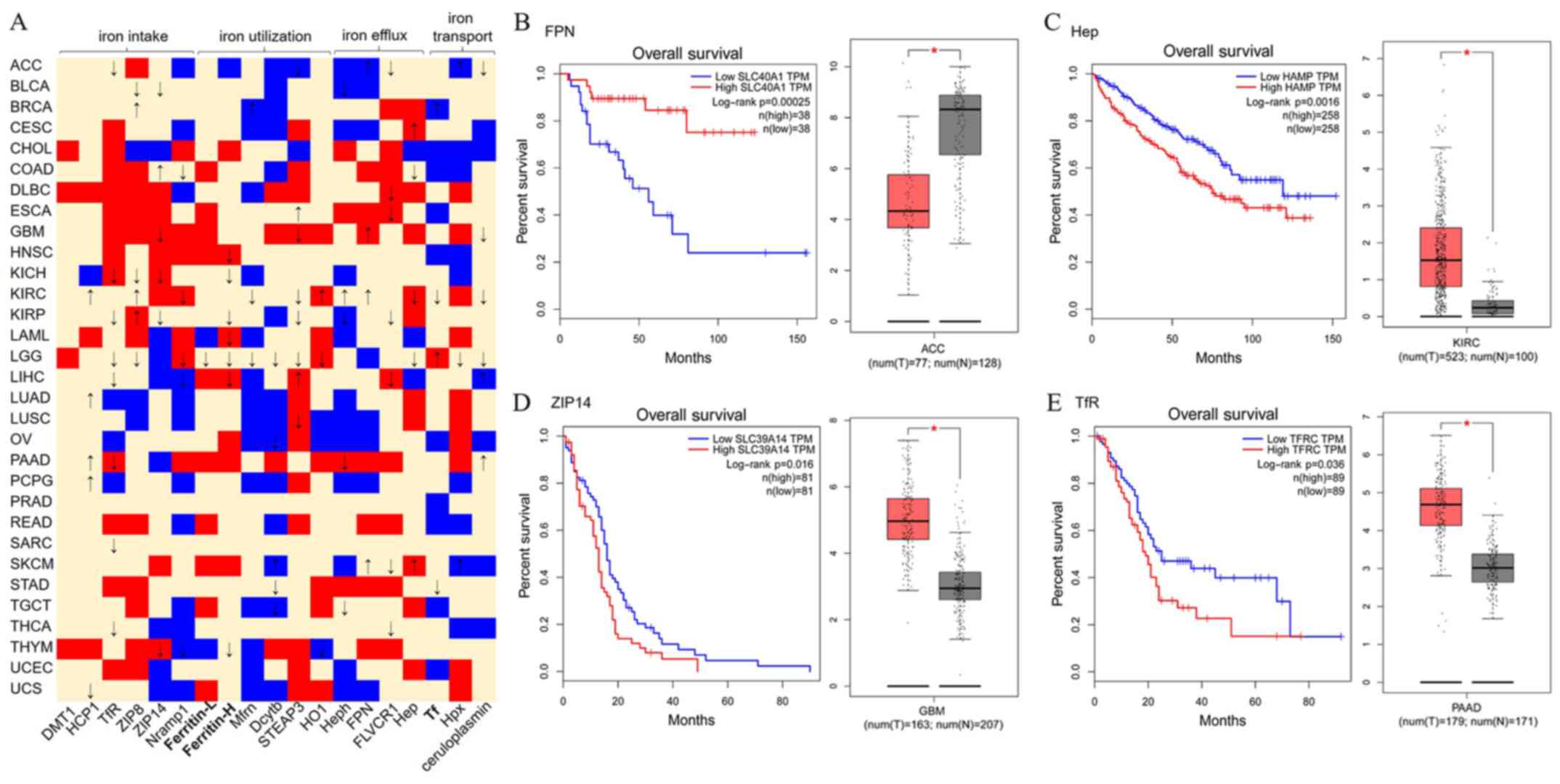
To evaluate the contribution of iron metabolism to
tumorigenesis, the expression of iron metabolism-associated genes
and clinical datasets were retrieved from The Cancer Genome Atlas
projects and mined using GEPIA online tools (version 2017;
http://gepia.cancer-pku.cn), which
processed high-throughput transcriptomic data using standard
pipeline (77). As presented in
Fig. 2A, for the 31 cancer types,
19 genes involved in iron intake, utilization, efflux or transport
were identified, demonstrating either upregulated or downregulated
expression in tumor samples compared with normal control samples.
To test the hypothesis, the overall survival (OS) data from these
patients were analyzed using Log-rank test (also known as the
Mantel-Cox test). Altered gene expression data of these patients
were compared with controls using one-way analysis of variance
(|log2fold change| Cutoff: 1; P-value Cutoff: 0.01).
Considering the patients' OS percentage, it was also observed that
specific cancer types exhibit frequent poor or good survival rates,
particularly for kidney chromophobe (KICH), kidney renal clear cell
carcinoma (KIRC), kidney renal papillary cell carcinoma (KIRP),
brain lower grade glioma (LGG) and liver hepatocellular carcinoma
(LIHC). The majority of proteins expressed at high levels
expression predicted a poor OS for these patients. However, high
HCP1, ZIP8, HO-1, Heph and FPN expression demonstrated a good OS in
patients with KIRC. Additionally, high Zip8 expression, high Tf
expression, and high STEAP3 and ceruloplasmin expression
demonstrated a good OS in patients with KIRP, LGG and LIHC,
respectively. The survival rates associated with these cancers may
be partially due to interrupted iron metabolism in liver and kidney
tissues. As presented in Fig.
2B-E, in patients with adrenocortical carcinoma, low FPN
expression demonstrated a lower survival rate (Fig. 2B), whereas, high Hep expression
predicted a poor OS in patients with KIRC (Fig. 2C). In addition, the high expression
of ZIP14 in patients with glioblastoma multiforme or TfR expression
in patients with pancreatic adenocarcinoma predicted a poor OS
(Fig. 2D and E). Notably, FPN had
been demonstrated to transport iron between the inside and outside
of the cell, whereas, Hep stops the transit (49). ZIP14 and TfR transfer iron from
outside to inside of the cell, which increases the intracellular
iron concentration. Supporting the hypothesis that iron-excess
contributes to tumorigenesis (29,60).
Colorectal cancer is the third most common cancer in
men and second in women worldwide (78). With the exception of hereditary
factors, lifestyle factors, including physical activity, obesity,
smoking and alcohol consumption are closely associated with
colorectal cancer (79). The
correlation between excess iron and colorectal cancer risk has been
examined in numerous previous studies (80-82),
and iron overload associated with the H63D mutation and C282Y in
HFE may increase the risk for developing colorectal cancer
(83-85).
Preoperative anemia is a common phenomenon in
patients with colorectal cancer and iron supplementation is the
most common therapy (86).
However, an analysis of previous studies investigating ingested
iron as treatment of anemia identified it as potentially
detrimental and hazardous for human colorectal cancer risk
(87-89). Recent studies demonstrated that
compared with oral iron, intravenous iron therapy is more effective
in anemic patients with colorectal cancer with higher Tf and lower
ferritin levels (90,91).
Liver cancer is one of the most common malignancies
in numerous countries worldwide and hepatocellular carcinoma is the
most frequent type of global cancer mortality rates (92). A number of factors, including
hepatitis B virus, hepatitis C virus, alcohol, tobacco, aflatoxin
and chronic inflammation are associated with hepatic carcinogenesis
(93). Iron overload is a
significant risk for hepatocellular carcinoma as the liver is the
main organ for iron storage.
Excessive iron accumulation may cause hepatocellular
injury. Oversaturated ferritin subunits result in ionic iron
releasing into hepatocyte cytoplasm when iron is overloaded
(74). If iron stores are
excessively overloaded in the liver, the lobules may develop
fibrosis. Hereditary haemochromatosis (HH), an inherited iron
metabolism disorder, and excess dietary iron are associated with
hepatic iron overload. HH induces hepatic fibrosis and cirrhosis
when treatment is not timely and appropriate (94). Patients with HH possess C282Y
mutations on HFE (95).
C282Y homozygosity has been correlated with an increased risk of
hepatocellular cancer in men (85).
Breast cancer is the most commonly diagnosed cancer
in women, and the number of cases of breast cancer is still
increasing (96). It was
identified that the development of breast cancer is associated with
protein tyrosine phosphorylation (97). Tyrosine phosphorylation is
regulated by a careful balance of activity of tyrosine kinases and
tyrosine phosphatases, which may activate or inactivate oncogenic
pathways in human breast cancer cells (98). Iron chelator aurintricarboxylic
acid may inhibit tyrosine phosphatases (99).
A number of genes serve important roles in breast
cancer progression through increased iron content in cells.
Histone-lysine N-methyltransferase EHMT2 regulates breast cancer
growth by modulating iron homeostasis through the repression of
ferroxidase hephaestin (100).
Dcytb is an important predictor of outcome and is associated with
the response to therapy in patients with breast cancer (101).
A mammogram is a test performed to check whether
women have breast cancer using x-rays, which subjects the body to
radiation. At present, with advances in technology, iron imaging
may identify macrophage hemosiderin deposits in metastatic breast
cancer (8). Recently,
superparamagnetic iron oxide based nanoprobes as multifunctional
theranostic agents were applied to breast cancer imaging and
therapy (102).
Lung cancer is the most common leading cause of
mortality in cancer during the past several decades (103). There are two types of lung
cancer: Non-small cell lung cancer (NSCLC) and small cell lung
cancer. Accumulating evidence suggests that iron overload is
associated with lung cancer (104). WNT, MYC and hypoxia-inducible
factor signaling pathways may be activated by iron (105,106). Subtoxic concentrations of iron
induce cellular hydroxyl radicals, affecting cancer stem cell-like
subpopulations of human NSCLC cells, which is important for
aggressive cancer behaviors and metastasis via transcription factor
SOX9 upregulation (107).
Air pollution, particularly particulate matter (PM),
increases the risk of respiratory morbidity and mortality, and even
lung cancer (108). In PM, iron
components have anti-apoptotic effects, which activate nuclear
factor erythroid 2-related factor 2-dependent antioxidant processes
(109). This previous study
provided insight for the development of lung cancer caused by PM
pollution; it was hypothesized that a nearby iron foundry may
influence the physical condition of local residents. Another study
demonstrated that men (aged <75 years), but not women, residing
within 800 m of the iron foundry coke oven had a high lung cancer
risk (110).
In multiple myeloma, iron metabolism remains
unclear. Serum ferritin may serve as a negative prognostic
indicator (111,112). Decreased FPN leads to an
intracellular iron overload and promotes myeloma cell growth
(113). In gastric cancer, iron
chelators induce gastric cancer cell apoptosis, involving
endoplasmic reticulum stress formed by reactive oxygen species
(ROS) and c-Jun N-terminal kinase activation (114).
Cancer cells have a strong ability to proliferate
and metastasize, requiring higher levels of environmental nutrients
compared with their healthy counterparts (115). As proliferation is closely
associated with the vast biosynthesis of nucleic acids and
proteins, the acquisition of energy is particularly vital.
Mitochondria are essential organelles for cells, which generate
energy and contain diverse enzymes involved in the synthesis
(116). Iron is a crucial element
of these enzymes and has an important function in the synthesis of
these enzymes (117) (Fig. 3).
Mitochondria are one of the principal ancient
endomembrane systems and have a circular genome of ~16 kb. The
numbers of mitochondria are associated with the vitality of cell
types in different tissues (118). Recently, the association between
mitochondria and iron was identified. Apart from classic pathways,
including β-oxidation of fatty acids and the tricarboxylic acid
cycle, mitochondria are the principal organelles for the metabolism
of iron (119). There are three
metabolic pathways of mitochondrial iron: Iron-sulfur cluster
biogenesis, heme synthesis and iron storage (120). Macrophages store large amounts of
iron and serve an important role in tumor progression.
Iron-sulfur cluster biogenesis and mitochondrial
iron transport are complex, and includes 16 genes, including
SFXN1 and SFXN5 (121). There are numerous biological
processes that require Fe-S proteins, including the mitochondrial
respiratory chain, DNA replication and repair, and RNA modification
(122). As a source of energy,
the mitochondrial respiratory chain depends on specific Fe-S
cluster-containing enzymes, including NADH-ubiquinone
oxidoreductase [additionally termed Complex I (CI)], Rieske
iron-sulfur protein (RISP) and subunits of succinate dehydrogenase
(SDH, additionally termed succinate-coenzyme Q reductase or
respiratory Complex II) (123). A
high concentration of iron and these enzymes promote cellular
growth significantly in tumors.
CI is one of largest membrane-bound enzymes in the
cell and is the largest complex of the mitochondrial respiratory
chain. The primary function of CI is ATP production, which drives
protons across the inner membrane by reducing the potential of
NADH. In total, CI is responsible for ~40% of ATP synthesis. There
are three subcomplexes of CI: An iron sulfur protein fraction, a
flavoprotein fraction and a hydrophobic fraction. The electron
transfer centers of CI are associated with redox reactions, which
includes eight iron-sulfur clusters, a flavin mononucleotide and
ubiquinone (124). CI is
indispensable in healthy cells. However, in cancer cells, CI serves
an important role in proliferation (125). Small molecular inhibitors serving
through CI have been identified as anticancer agents. For example,
rotenoids, polyphenols AG311, metformin, BAY 87-2243, fenofibrate,
canagliflozin and kalkitoxin offer potential anticancer treatment
(126,127).
RISP is an essential subunit of mitochondrial
complex III. The progression of the electron transfer reaction
requires RISP to dock to the quinol oxidation site; the electron
transfer reaction serves a crucial role in the synthesis of ATP
(128). A previous study
demonstrated that knocking down RISP of mitochondrial complex III
in human cancer cells decreased their invasive potential (129). Atovaquone, as a oxidative
phosphorylation inhibitor, has been observed to target
mitochondrial complex III to eliminate cancer stem cells (130).
SDH is composed of four subunits: A flavoprotein
(SDHA), an iron-sulfur protein (SDHB), a cytochrome b560 subunit
(SDHC) and cytochrome b small subunit (SDHD). There are three
iron-sulfur clusters of SDHB: [2Fe-2S], [4Fe-4S] and [3Fe-4S]. SDH
is a key respiratory enzyme complex in the citric acid cycle that
converts succinate to fumarate and is also involved in the
mitochondrial electron transport chain (131). SDH transfers electrons from the
[Fe-S] clusters to ubiquinone (132). SDH has been proposed as a target
for cancer therapy. As an inhibitor of SDH, lonidamine provides
novel approaches for the treatment of cancer (133). Vitamin E analogues,
3-Bromopyruvate, Malonate, 3-nitropropionic acid,
Thenoyltrifluoroacetone and Troglitazone were identified as
anticancer agents targeting SDH (134).
The traditional role of macrophages for tumor
clearance has been challenged, as it was demonstrated that
macrophages have pro-tumor properties. Over the course of cancer
progression, tumor cells secrete various mediators to construct
their own niche in order to adapt to the surrounding environment
(135). It was even observed that
tumor-infiltrating macrophages helped tumor cells. Tumor-associated
macrophages are principal suppliers of cytokines, proteases and
growth factors, such as cysteine cathepsin proteases, which enhance
tumor progression and the therapeutic resistance of different
cancer types (136,137). For example, in hepatocellular
carcinoma and gastric cancer, tumor-associated macrophages promote
tumor growth and invasiveness via C-C motif chemokine 22-induced
epithelial-mesenchymal transition and the activated nuclear
factor-κB signaling pathway (138,139). Furthermore, growing cancer cells
demand plenty of iron. Therefore, how tumor cells obtain iron from
their microenvironment requires further study.
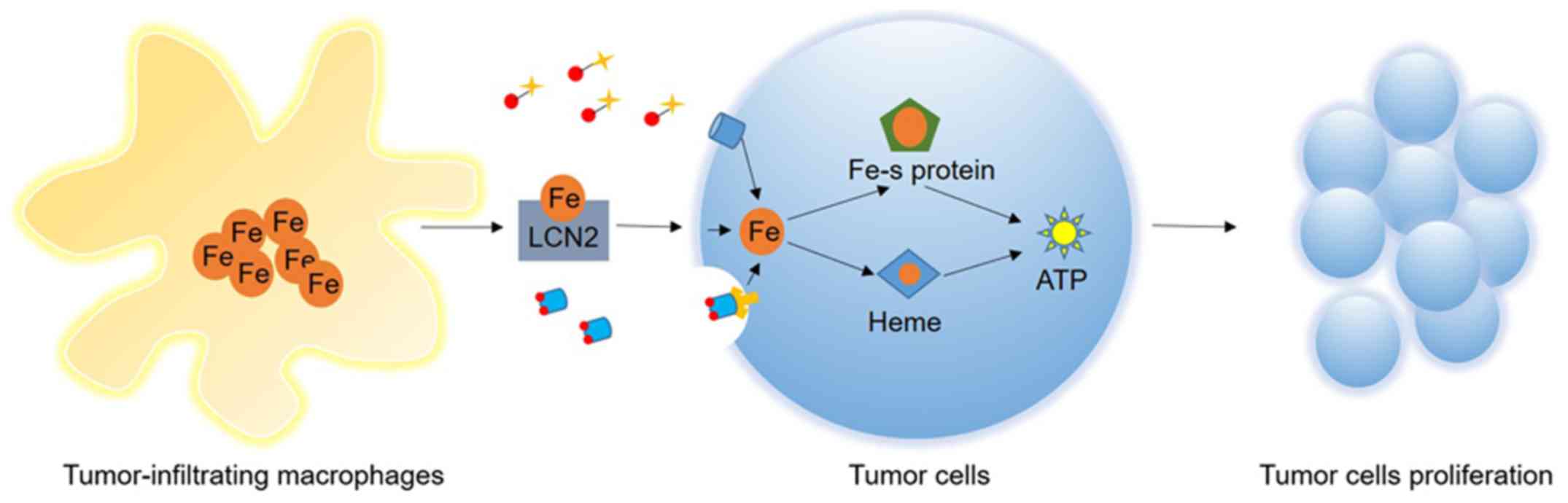
For the tumor itself, iron may be increased by
upregulated expression of iron import and storage proteins,
including TfR and ferritin, and downregulated expression of iron
export proteins, including FPN (140). In the tumor microenvironment,
macrophages secrete lipocalin2 (LCN2), which increases tumor
intracellular iron concentration (141,142). LCN2 is a member of the lipocalin
superfamily and functions as a carrier protein whose structural
feature is a β-barrel (143).
LCN2 is able to bind to iron-loaded siderophores. As a result,
tumor-infiltrating macrophages release unlimited iron for tumor
cells. A previous study observed that iron in super paramagnetic
iron-oxide nanoparticles may target tumor-associated macrophages,
which may be used for cancer therapy (144).
Ferroptosis, as a novel form of iron-dependent
programmed cell death characterized by the accumulation of lipid
peroxides, is genetically and biochemically different from
pyroptosis, apoptosis and necroptosis (145). Ferroptosis may be triggered by
erastin, sorafenib and sulfasalazine (5,146).
The accumulation of iron, through the Fenton reaction, generates
lipid peroxides and ROS, leading to the occurrence of
erastin-induced ferroptosis (147). A number of cancer types exhibit
sensitivity to ferroptosis inducers, including large B-cell
lymphoma, cervical carcinoma, renal cell carcinoma, osteosarcoma,
prostate adenocarcinoma, liver cancer, ovarian carcinoma,
pancreatic carcinoma and carcinoma of the lungs (148-151). Notably, ferroptosis inhibitors,
such as Liproxstatin-1, may reduce ischemia/reperfusion-induced
hepatic damage in Gpx4−/− mice (152). Other molecular mechanisms of
ferroptosis have been identified (153,154).
Intracellular iron may regulate the sensitivity of
cells to ferroptosis. Enhanced intracellular iron promotes
erastin-induced ferroptosis; conversely, reduced intracellular iron
diminishes ROS, thus suppressing ferroptosis (146). Although ROS induction and iron
deprivation therapy may be examined as a possible therapeutic
intervention in a variety of cancer types (13,14),
the optimal therapeutic strategies have yet to be identified.
Therefore, novel therapies, termed ferroptosis therapy, have
emerged. Fenton-reaction-acceleratable magnetic nanoparticles
(FeGd-HN@Pt@LF/RGD2) significantly inhibit tumor growth by
delivering Fenton reaction reactants to the tumor site (162). Furthermore, two studies examined
tumor suppression mediated by a direct interaction between heme and
p53 in iron deprivation (163,164). p53 downregulating metabolic
stress-induced ferroptosis in tumors (164) demonstrated that this well-known
tumor-suppressor gene is associated with iron metabolism, in
addition to cancer cell proliferation and death. In summary, there
is great potential for the development of ferroptosis-based
effective treatments for iron metabolism-associated diseases and
cancer.
Iron is essential for numerous vital metabolic
processes in mammalian systems. The present review focused on the
importance of iron regulation, and the association between iron
homeostasis and carcinogenic mechanisms, summarizing the
progression of research on tumors. Altering iron homeostasis in
various cancers may influence patient outcome. Ferroptosis, a
unique form of regulated cell death, may serve as a
tumor-suppressor for cancer therapy. Taken together, future studies
are required to develop novel methods to disturb iron-induced
activation, and the resulting signal transduction cascades leading
to carcinogenesis and progression.
This work was supported by National Natural Science
Foundation of China to YY and CG (grant nos. 81670200, 81600177 and
81770220), the 2016 outstanding youth fund of Jiangsu Province to
YY (grant no. BK20160048), and the Natural Science Foundation of
Jiangsu Province to CG (grant no. BK20161041).
Not applicable.
YY, CG and ZF were involved in the conception of
the review. CG and YC were involved in the writing of the article.
All authors have read and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
The authors thank Dr Michael Pisano (Department of
Pathology, School of Medicine, University of Iowa, Iowa City, IA,
USA) for polishing the language in the current manuscript.
|
1
|
Kerins MJ and Ooi A: The roles of NRF2 in
modulating cellular iron homeostasis. Antioxid Redox Signal.
29:1756–1773. 2018. View Article : Google Scholar :
|
|
2
|
Andrews NC: Forging a field: The golden
age of iron biology. Blood. 112:219–230. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dielschneider RF, Henson ES and Gibson SB:
Lysosomes as oxidative targets for cancer therapy. Oxid Med Cell
Longev. 2017:37491572017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ali MK, Kim RY, Karim R, Mayall JR, Martin
KL, Shahandeh A, Abbasian F, Starkey MR, Loustaud-Ratti V,
Johnstone D, et al: Role of iron in the pathogenesis of respiratory
disease. Int J Biochem Cell Biol. 88:181–195. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Manz DH, Blanchette NL, Paul BT, Torti FM
and Torti SV: Iron and cancer: Recent insights. Ann NY Acad Sci.
1368:149–161. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fonseca-Nunes A, Jakszyn P and Agudo A:
Iron and cancer risk - a systematic review and meta-analysis of the
epidemiological evidence. Cancer Epidemiol Biomarkers Prev.
23:12–31. 2014. View Article : Google Scholar
|
|
7
|
Torti SV and Torti FM: Iron and cancer:
More ore to be mined. Nat Rev Cancer. 13:342–355. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Leftin A, Ben-Chetrit N, Klemm F, Joyce JA
and Koutcher JA: Iron imaging reveals tumor and metastasis
macrophage hemosiderin deposits in breast cancer. PLoS One.
12:e01847652017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rouault TA and Maio N: Biogenesis and
functions of mammalian iron-sulfur proteins in the regulation of
iron homeostasis and pivotal metabolic pathways. J Biol Chem.
292:12744–12753. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fuss JO, Tsai CL, Ishida JP and Tainer JA:
Emerging critical roles of Fe-S clusters in DNA replication and
repair. Biochim Biophys Acta. 1853:1253–1271. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Imlay JA and Linn S: DNA damage and oxygen
radical toxicity. Science. 240:1302–1309. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Brandt KE, Falls KC, Schoenfeld JD, Rodman
SN, Gu Z, Zhan F, Cullen JJ, Wagner BA, Buettner GR, Allen BG, et
al: Augmentation of intracellular iron using iron sucrose enhances
the toxicity of pharmacological ascorbate in colon cancer cells.
Redox Biol. 14:82–87. 2018. View Article : Google Scholar
|
|
13
|
Callens C, Coulon S, Naudin J,
Radford-Weiss I, Boissel N, Raffoux E, Wang PH, Agarwal S, Tamouza
H, Paubelle E, et al: Targeting iron homeostasis induces cellular
differentiation and synergizes with differentiating agents in acute
myeloid leukemia. J Exp Med. 207:731–750. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Heath JL, Weiss JM, Lavau CP and Wechsler
DS: Iron deprivation in cancer - potential therapeutic
implications. Nutrients. 5:2836–2859. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
González A, Gálvez N, Martín J, Reyes F,
Pérez-Victoria I and Dominguez-Vera JM: Identification of the key
excreted molecule by Lactobacillus fermentum related to host iron
absorption. Food Chem. 228:374–380. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Staroń R, Lipiński P, Lenartowicz M,
Bednarz A, Gajowiak A, Smuda E, Krzeptowski W, Pieszka M, Korolonek
T, Hamza I, et al: Dietary hemoglobin rescues young piglets from
severe iron defi-ciency anemia: Duodenal expression profile of
genes involved in heme iron absorption. PLoS One. 12:e01811172017.
View Article : Google Scholar
|
|
17
|
Li Y, Jiang H and Huang G: Protein
hydrolysates as promoters of non-haem iron absorption. Nutrients.
9:92017. View Article : Google Scholar
|
|
18
|
Martínez-Torres C and Layrisse M: Iron
absorption from veal muscle. Am J Clin Nutr. 24:531–540. 1971.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ascenzi P, Leboffe L and Polticelli F:
Cyanide binding to human plasma heme-hemopexin: A comparative
study. Biochem Biophys Res Commun. 428:239–244. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Colins A, Gerdtzen ZP, Nuñez MT and
Salgado JC: Mathematical modeling of intestinal iron absorption
using genetic programming. PLoS One. 12:e01696012017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Donovan A, Lima CA, Pinkus JL, Pinkus GS,
Zon LI, Robine S and Andrews NC: The iron exporter
ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab.
1:191–200. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sokolov AV, Voynova IV, Kostevich VA,
Vlasenko AY, Zakharova ET and Vasilyev VB: Comparison of
interaction between ceruloplasmin and lactoferrin/transferrin: To
bind or not to bind. Biochemistry (Mosc). 82:1073–1078. 2017.
View Article : Google Scholar
|
|
23
|
Nemeth E, Tuttle MS, Powelson J, Vaughn
MB, Donovan A, Ward DM, Ganz T and Kaplan J: Hepcidin regulates
cellular iron efflux by binding to ferroportin and inducing its
internalization. Science. 306:2090–2093. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lane DJ, Bae DH, Merlot AM, Sahni S and
Richardson DR: Duodenal cytochrome b (DCYTB) in iron metabolism: An
update on function and regulation. Nutrients. 7:2274–2296. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Skjørringe T, Burkhart A, Johnsen KB and
Moos T: Divalent metal transporter 1 (DMT1) in the brain:
Implications for a role in iron transport at the blood-brain
barrier, and neuronal and glial pathology. Front Mol Neurosci.
8:192015.PubMed/NCBI
|
|
26
|
Harrison PM and Arosio P: The ferritins:
Molecular properties, iron storage function and cellular
regulation. Biochim Biophys Acta. 1275:161–203. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
McKie AT and Barlow DJ: The SLC40
basolateral iron transporter family (IREG1/ferroportin/MTP1).
Pflugers Arch. 447:801–806. 2004. View Article : Google Scholar
|
|
28
|
Park CH, Valore EV, Waring AJ and Ganz T:
Hepcidin, a urinary antimicrobial peptide synthesized in the liver.
J Biol Chem. 276:7806–7810. 2001. View Article : Google Scholar
|
|
29
|
Liuzzi JP, Aydemir F, Nam H, Knutson MD
and Cousins RJ: Zip14 (Slc39a14) mediates non-transferrin-bound
iron uptake into cells. Proc Natl Acad Sci USA. 103:13612–13617.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lin W, Vann DR, Doulias P-T, Wang T,
Landesberg G, Li X, Ricciotti E, Scalia R, He M, Hand NJ, et al:
Hepatic metal ion transporter ZIP8 regulates manganese homeostasis
and manganese-dependent enzyme activity. J Clin Invest.
127:2407–2417. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mwanjewe J and Grover AK: Role of
transient receptor potential canonical 6 (TRPC6) in
non-transferrin-bound iron uptake in neuronal phenotype PC12 cells.
Biochem J. 378:975–982. 2004. View Article : Google Scholar
|
|
32
|
Knutson MD: Non-transferrin-bound iron
transporters. Free Radic Biol Med. 133:101–111. 2019. View Article : Google Scholar
|
|
33
|
Yoshizaki T, Uematsu M, Obata JE, Nakamura
T, Fujioka D, Watanabe K, Nakamura K and Kugiyama K: Angiotensin II
receptor blockers suppress the release of stromal cell-derived
factor-1alpha from infarcted myocardium in patients with acute
myocardial infarction. J Cardiol. 71:367–374. 2018. View Article : Google Scholar
|
|
34
|
Recuenco MC, Rahman MM, Takeuchi F,
Kobayashi K and Tsubaki M: Electron transfer reactions of candidate
tumor suppressor 101F6 protein, a cytochrome b561 homologue, with
ascorbate and monodehydroascorbate radical. Biochemistry.
52:3660–3668. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lane DJ and Lawen A: Ascorbate and plasma
membrane electron transport - enzymes vs efflux. Free Radic Biol
Med. 47:485–495. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Anderson SA, Nizzi CP, Chang YI, Deck KM,
Schmidt PJ, Galy B, Damnernsawad A, Broman AT, Kendziorski C,
Hentze MW, et al: The IRP1-HIF-2α axis coordinates iron and oxygen
sensing with erythropoiesis and iron absorption. Cell Metab.
17:282–290. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Coates TD: Physiology and pathophysiology
of iron in hemoglobin-associated diseases. Free Radic Biol Med.
72:23–40. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gunshin H, Allerson CR, Polycarpou-Schwarz
M, Rofts A, Rogers JT, Kishi F, Hentze MW, Rouault TA, Andrews NC
and Hediger MA: Iron-dependent regulation of the divalent metal ion
transporter. FEBS Lett. 509:309–316. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Anderson CP, Shen M, Eisenstein RS and
Leibold EA: Mammalian iron metabolism and its control by iron
regulatory proteins. Biochim Biophys Acta. 1823:1468–1483. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ford GC, Harrison PM, Rice DW, Smith JM,
Treffry A, White JL and Yariv J: Ferritin: Design and formation of
an iron-storage molecule. Philos Trans R Soc Lond B Biol Sci.
304:551–565. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Carmona U, Li L, Zhang L and Knez M:
Ferritin light-chain subunits: Key elements for the electron
transfer across the protein cage. Chem Commun (Camb).
50:15358–15361. 2014. View Article : Google Scholar
|
|
42
|
Kukulj S, Jaganjac M, Boranic M, Krizanac
S, Santic Z and Poljak-Blazi M: Altered iron metabolism,
inflammation, trans-ferrin receptors, and ferritin expression in
non-small-cell lung cancer. Med Oncol. 27:268–277. 2010. View Article : Google Scholar
|
|
43
|
Ganz T and Nemeth E: Iron metabolism:
Interactions with normal and disordered erythropoiesis. Cold Spring
Harb Perspect Med. 2:a0116682012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cianetti L, Gabbianelli M and Sposi NM:
Ferroportin and erythroid cells: an update. Adv Hematol.
2010:4041732010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wallace DF, McDonald CJ, Ostini L, Iser D,
Tuckfield A and Subramaniam VN: The dynamics of
hepcidin-ferroportin internalization and consequences of a novel
ferroportin disease mutation. Am J Hematol. 92:1052–1061. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
El Hage Chahine JM, Hémadi M and Ha-Duong
NT: Uptake and release of metal ions by transferrin and interaction
with receptor 1. Biochim Biophys Acta. 1820:334–347. 2012.
View Article : Google Scholar
|
|
47
|
Frazer DM and Anderson GJ: The regulation
of iron transport. Biofactors. 40:206–214. 2014. View Article : Google Scholar
|
|
48
|
Addo L, Ikuta K, Tanaka H, Toki Y,
Hatayama M, Yamamoto M, Ito S, Shindo M, Sasaki Y, Shimonaka Y, et
al: The three isoforms of hepcidin in human serum and their
processing determined by liquid chromatography-tandem mass
spectrometry (LC-tandem MS). Int J Hematol. 103:34–43. 2016.
View Article : Google Scholar
|
|
49
|
Qiao B, Sugianto P, Fung E,
Del-Castillo-Rueda A, Moran-Jimenez MJ, Ganz T and Nemeth E:
Hepcidin-induced endocytosis of ferroportin is dependent on
ferroportin ubiquitination. Cell Metab. 15:918–924. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ramos E, Kautz L, Rodriguez R, Hansen M,
Gabayan V, Ginzburg Y, Roth MP, Nemeth E and Ganz T: Evidence for
distinct pathways of hepcidin regulation by acute and chronic iron
loading in mice. Hepatology. 53:1333–1341. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Coffey R and Ganz T: Iron homeostasis: An
anthropocentric perspective. J Biol Chem. 292:12727–12734. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Pietrangelo A, Dierssen U, Valli L, Garuti
C, Rump A, Corradini E, Ernst M, Klein C and Trautwein C: STAT3 is
required for IL-6-gp130-dependent activation of hepcidin in vivo.
Gastroenterology. 132:294–300. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Palis J: Primitive and definitive
erythropoiesis in mammals. Front Physiol. 5:32014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Papanikolaou G and Pantopoulos K: Systemic
iron homeostasis and erythropoiesis. IUBMB Life. 69:399–413. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Shaw GC, Cope JJ, Li L, Corson K, Hersey
C, Ackermann GE, Gwynn B, Lambert AJ, Wingert RA, Traver D, et al:
Mitoferrin is essential for erythroid iron assimilation. Nature.
440:96–100. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Beaumont C and Canonne-Hergaux F:
Erythrophagocytosis and recycling of heme iron in normal and
pathological conditions; regulation by hepcidin. Transfus Clin
Biol. 12:123–130. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Theurl I, Hilgendorf I, Nairz M, Tymoszuk
P, Haschka D, Asshoff M, He S, Gerhardt LM, Holderried TA, Seifert
M, et al: On-demand erythrocyte disposal and iron recycling
requires transient macrophages in the liver. Nat Med. 22:945–951.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Soe-Lin S, Apte SS, Mikhael MR, Kayembe
LK, Nie G and Ponka P: Both Nramp1 and DMT1 are necessary for
efficient macrophage iron recycling. Exp Hematol. 38:609–617. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Poss KD and Tonegawa S: Heme oxygenase 1
is required for mammalian iron reutilization. Proc Natl Acad Sci
USA. 94:10919–10924. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Qian ZM and Tang PL: Mechanisms of iron
uptake by mammalian cells. Biochim Biophys Acta. 1269:205–214.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Morgan EH: Chelator-mediated iron efflux
from reticulocytes. Biochim Biophys Acta. 733:39–50. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Wang CY, Jenkitkasemwong S, Duarte S,
Sparkman BK, Shawki A, Mackenzie B and Knutson MD: ZIP8 is an iron
and zinc transporter whose cell-surface expression is up-regulated
by cellular iron loading. J Biol Chem. 287:34032–34043. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Pinilla-Tenas JJ, Sparkman BK, Shawki A,
Illing AC, Mitchell CJ, Zhao N, Liuzzi JP, Cousins RJ, Knutson MD
and Mackenzie B: Zip14 is a complex broad-scope metal-ion
transporter whose functional properties support roles in the
cellular uptake of zinc and nontransferrin-bound iron. Am J Physiol
Cell Physiol. 301:C862–C871. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Tsushima RG, Wickenden AD, Bouchard RA,
Oudit GY, Liu PP and Backx PH: Modulation of iron uptake in heart
by L-type Ca2+ channel modifiers: Possible implications
in iron overload. Circ Res. 84:1302–1309. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Brittenham GM, Andersson M, Egli I, Foman
JT, Zeder C, Westerman ME and Hurrell RF: Circulating
non-trans-ferrin-bound iron after oral administration of
supplemental and fortification doses of iron to healthy women: A
randomized study. Am J Clin Nutr. 100:813–820. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Pinto JP, Arezes J, Dias V, Oliveira S,
Vieira I, Costa M, Vos M, Carlsson A, Rikers Y, Rangel M, et al:
Physiological implications of NTBI uptake by T lymphocytes. Front
Pharmacol. 5:242014. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Ramey G, Deschemin JC, Durel B,
Canonne-Hergaux F, Nicolas G and Vaulont S: Hepcidin targets
ferroportin for degradation in hepatocytes. Haematologica.
95:501–504. 2010. View Article : Google Scholar :
|
|
68
|
Iancu TC, Ward RJ and Peters TJ:
Ultrastructural changes in the pancreas of carbonyl iron-fed rats.
J Pediatr Gastroenterol Nutr. 10:95–101. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Paragas N, Qiu A, Hollmen M, Nickolas TL,
Devarajan P and Barasch J: NGAL-Siderocalin in kidney disease.
Biochim Biophys Acta. 1823:1451–1458. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Martines AM, Masereeuw R, Tjalsma H,
Hoenderop JG, Wetzels JF and Swinkels DW: Iron metabolism in the
pathogenesis of iron-induced kidney injury. Nat Rev Nephrol.
9:385–398. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Lakhal-Littleton S, Wolna M, Carr CA,
Miller JJ, Christian HC, Ball V, Santos A, Diaz R, Biggs D,
Stillion R, et al: Cardiac ferro-portin regulates cellular iron
homeostasis and is important for cardiac function. Proc Natl Acad
Sci USA. 112:3164–3169. 2015. View Article : Google Scholar
|
|
72
|
Richmond HG: Induction of sarcoma in the
rat by iron-dextran complex. BMJ. 1:947–949. 1959. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Xue X and Shah YM: Intestinal iron
homeostasis and colon tumorigenesis. Nutrients. 5:2333–2351. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Kew MC: Hepatic iron overload and
hepatocellular carcinoma. Liver Cancer. 3:31–40. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Stevens RG, Cologne JB, Nakachi K, Grant
EJ and Neriishi K: Body iron stores and breast cancer risk in
female atomic bomb survivors. Cancer Sci. 102:2236–2240. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Huang X: Iron overload and its association
with cancer risk in humans: Evidence for iron as a carcinogenic
metal. Mutat Res. 533:153–171. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Tang Z, Li C, Kang B, Gao G, Li C and
Zhang Z: GEPIA: A web server for cancer and normal gene expression
profiling and interactive analyses. Nucleic Acids Res. 45:W98–W102.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar
|
|
79
|
Haggar FA and Boushey RP: Colorectal
cancer epidemiology: Incidence, mortality, survival, and risk
factors. Clin Colon Rectal Surg. 22:191–197. 2009. View Article : Google Scholar :
|
|
80
|
Chua ACG, Klopcic B, Lawrance IC, Olynyk
JK and Trinder D: Iron: An emerging factor in colorectal
carcinogenesis. World J Gastroenterol. 16:663–672. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Kato I, Dnistrian AM, Schwartz M, Toniolo
P, Koenig K, Shore RE, Zeleniuch-Jacquotte A, Akhmedkhanov A and
Riboli E: Iron intake, body iron stores and colorectal cancer risk
in women: A nested case-control study. Int J Cancer. 80:693–698.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Wilson MJ, Dekker JWT, Harlaar JJ, Jeekel
J, Schipperus M and Zwaginga JJ: The role of preoperative iron
deficiency in colorectal cancer patients: Prevalence and treatment.
Int J Colorectal Dis. 32:1617–1624. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
de Juan D, Reta A, Castiella A, Pozueta J,
Prada A and Cuadrado E: HFE gene mutations analysis in Basque
hereditary haemochromatosis patients and controls. Eur J Hum Genet.
9:961–964. 2001. View Article : Google Scholar
|
|
84
|
Castiella A, Múgica F, Zapata E, Zubiaurre
L, Iribarren A, de Juan MD, Alzate L, Gil I, Urdapilleta G, Otazua
P, et al: Gender and plasma iron biomarkers, but not HFE gene
mutations, increase the risk of colorectal cancer and polyps.
Tumour Biol. 36:6959–6963. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Asberg A, Thorstensen K, Irgens WO,
Romundstad PR and Hveem K: Cancer risk in HFE C282Y homozygotes:
Results from the HUNT 2 study. Scand J Gastroenterol. 48:189–195.
2013. View Article : Google Scholar
|
|
86
|
Ludwig H, Müldür E, Endler G and Hübl W:
Prevalence of iron deficiency across different tumors and its
association with poor performance status, disease status and
anemia. Ann Oncol. 24:1886–1892. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Nelson RL: Dietary iron and colorectal
cancer risk. Free Radic Biol Med. 12:161–168. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Wilson MJ, Harlaar JJ, Jeekel J,
Schipperus M and Zwaginga JJ: Iron therapy as treatment of anemia:
A potentially detrimental and hazardous strategy in colorectal
cancer patients. Med Hypotheses. 110:110–113. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Joosten E, Meeuwissen J, Vandewinckele H
and Hiele M: Iron status and colorectal cancer in symptomatic
elderly patients. Am J Med. 121:1072–1077. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Wilson MJ, Dekker JW, Bruns E, Borstlap W,
Jeekel J, Zwaginga JJ and Schipperus M: Short-term effect of
preoperative intravenous iron therapy in colorectal cancer patients
with anemia: Results of a cohort study. Transfusion. 58:795–803.
2018. View Article : Google Scholar
|
|
91
|
Laso-Morales M, Jericó C, Gómez-Ramírez S,
Castellví J, Viso L, Roig-Martínez I, Pontes C and Muñoz M:
Preoperative management of colorectal cancer-induced iron
deficiency anemia in clinical practice: Data from a large
observational cohort. Transfusion. 57:3040–3048. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Baecker A, Liu X, La Vecchia C and Zhang
ZF: Worldwide incidence of hepatocellular carcinoma cases
attributable to major risk factors. Eur J Cancer Prev. 27:205–212.
2018.PubMed/NCBI
|
|
93
|
Sun B and Karin M: Obesity, inflammation,
and liver cancer. J Hepatol. 56:704–713. 2012. View Article : Google Scholar
|
|
94
|
Bardou-Jacquet E, Morcet J, Manet G, Lainé
F, Perrin M, Jouanolle AM, Guyader D, Moirand R, Viel JF and
Deugnier Y: Decreased cardiovascular and extrahepatic
cancer-related mortality in treated patients with mild HFE
hemochromatosis. J Hepatol. 62:682–689. 2015. View Article : Google Scholar
|
|
95
|
Grosse SD, Rogowski WH, Ross LF, Cornel
MC, Dondorp WJ and Khoury MJ: Population screening for genetic
disorders in the 21st century: Evidence, economics, and ethics.
Public Health Genomics. 13:106–115. 2010. View Article : Google Scholar
|
|
96
|
Da Costa GG, Gomig TH, Kaviski R, Santos
Sousa K, Kukolj C, De Lima RS, De Andrade Urban C, Cavalli IJ and
Ribeiro EM: Comparative proteomics of tumor and paired normal
breast tissue highlights potential biomarkers in breast cancer.
Cancer Genomics Proteomics. 12:251–261. 2015.PubMed/NCBI
|
|
97
|
Nunes-Xavier CE, Martín-Pérez J, Elson A
and Pulido R: Protein tyrosine phosphatases as novel targets in
breast cancer therapy. Biochim Biophys Acta. 1836:211–226.
2013.PubMed/NCBI
|
|
98
|
Tonks NK: Protein tyrosine phosphatases:
From genes, to function, to disease. Nat Rev Mol Cell Biol.
7:833–846. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Kuban-Jankowska A, Sahu KK,
Gorska-Ponikowska M, Tuszynski JA and Wozniak M: Inhibitory
activity of iron chelators ATA and DFO on MCF-7 breast cancer cells
and phos-phatases PTP1B and SHP2. Anticancer Res. 37:4799–4806.
2017.PubMed/NCBI
|
|
100
|
Wang YF, Zhang J, Su Y, Shen YY, Jiang DX,
Hou YY, Geng MY, Ding J and Chen Y: G9a regulates breast cancer
growth by modulating iron homeostasis through the repression of
ferroxidase hephaestin. Nat Commun. 8:2742017. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Lemler DJ, Lynch ML, Tesfay L, Deng Z,
Paul BT, Wang X, Hegde P, Manz DH, Torti SV and Torti FM: DCYTB is
a predictor of outcome in breast cancer that functions via
iron-independent mechanisms. Breast Cancer Res. 19:252017.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Zheng J, Ren W, Chen T, Yinhua J, Li A,
Yan K, Wu Y and Wu A: Recent advances in superparamagnetic iron
oxide based nano-probes as multifunctional theranostic agents for
breast cancer imaging and therapy. Curr Med Chem. 25:3001–3016.
2018. View Article : Google Scholar
|
|
103
|
Ridge CA, McErlean AM and Ginsberg MS:
Epidemiology of lung cancer. Semin Intervent Radiol. 30:93–98.
2013. View Article : Google Scholar :
|
|
104
|
Wild P, Bourgkard E and Paris C: Lung
cancer and exposure to metals: The epidemiological evidence.
Methods Mol Biol. 472:139–167. 2009. View Article : Google Scholar
|
|
105
|
Brookes MJ, Boult J, Roberts K, Cooper BT,
Hotchin NA, Matthews G, Iqbal T and Tselepis C: A role for iron in
Wnt signalling. Oncogene. 27:966–975. 2008. View Article : Google Scholar
|
|
106
|
Wu KJ, Polack A and Dalla-Favera R:
Coordinated regulation of iron-controlling genes, H-ferritin and
IRP2, by c-MYC. Science. 283:676–679. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Chanvorachote P and Luanpitpong S: Iron
induces cancer stem cells and aggressive phenotypes in human lung
cancer cells. Am J Physiol Cell Physiol. 310:C728–C739. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Lee BJ, Kim B and Lee K: Air pollution
exposure and cardiovascular disease. Toxicol Res. 30:71–75. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Lovera-Leroux M, Crobeddu B, Kassis N,
Petit PX, Janel N, Baeza-Squiban A and Andreau K: The iron
component of particulate matter is antiapoptotic: A clue to the
development of lung cancer after exposure to atmospheric
pollutants? Biochimie. 118:195–206. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Bidoli E, Barbone F, Collarile P, Valent
F, Zanier L, Daris F, Gini A, Birri S and Serraino D: Residence in
proximity of an iron foundry and risk of lung cancer in the
municipality of trieste, Italy, 1995-2009. Int J Environ Res Public
Health. 12:9025–9035. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Song MK, Chung JS, Seol YM, Shin HJ, Choi
YJ and Cho GJ: Elevation of serum ferritin is associated with the
outcome of patients with newly diagnosed multiple myeloma. Korean
Korean J Intern Med. 24:368–373. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Strasser-Weippl K and Ludwig H: Ferritin
as prognostic marker in multiple myeloma patients undergoing
autologous transplantation. Leuk Lymphoma. 55:2520–2524. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Gu Z, Wang H, Xia J, Yang Y, Jin Z, Xu H,
Shi J, De Domenico I, Tricot G and Zhan F: Decreased ferroportin
promotes myeloma cell growth and osteoclast differentiation. Cancer
Res. 75:2211–2221. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Kim JL, Lee D-H, Na YJ, Kim BR, Jeong YA,
Lee SI, Kang S, Joung SY, Lee S-Y, Oh SC, et al: Iron
chelator-induced apoptosis via the ER stress pathway in gastric
cancer cells. Tumour Biol. 37:9709–9719. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Timofeeva OA, Palechor-Ceron N, Li G, Yuan
H, Krawczyk E, Zhong X, Liu G, Upadhyay G, Dakic A, Yu S, et al:
Conditionally reprogrammed normal and primary tumor prostate
epithelial cells: A novel patient-derived cell model for studies of
human prostate cancer. Oncotarget. 8:22741–22758. 2017. View Article : Google Scholar :
|
|
116
|
Wachowius F, Attwater J and Holliger P:
Nucleic acids: Function and potential for abiogenesis. Q Rev
Biophys. 50:e42017. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Puig S, Ramos-Alonso L, Romero AM and
Martínez-Pastor MT: The elemental role of iron in DNA synthesis and
repair. Metallomics. 9:1483–1500. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Friedman JR and Nunnari J: Mitochondrial
form and function. Nature. 505:335–343. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Ren JG, Seth P, Ye H, Guo K, Hanai JI,
Husain Z and Sukhatme VP: Citrate suppresses tumor growth in
multiple models through inhibition of glycolysis, the tricarboxylic
acid cycle and the IGF-1R pathway. Sci Rep. 7:45372017. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Dutkiewicz R and Nowak M: Molecular
chaperones involved in mitochondrial iron-sulfur protein
biogenesis. J Biol Inorg Chem. 23:569–579. 2018. View Article : Google Scholar
|
|
121
|
Miller LD, Coffman LG, Chou JW, Black MA,
Bergh J, D'Agostino R Jr, Torti SV and Torti FM: An iron regulatory
gene signature predicts outcome in breast cancer. Cancer Res.
71:6728–6737. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Mettert EL and Kiley PJ: Fe-S proteins
that regulate gene expression. Biochim Biophys Acta.
1853:1284–1293. 2015. View Article : Google Scholar :
|
|
123
|
Zhang L, Reyes A and Wang X: The role of
DNA repair in maintaining mitochondrial DNA stability. Adv Exp Med
Biol. 1038:85–105. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Chen YR and Zweier JL: Cardiac
mitochondria and reactive oxygen species generation. Circ Res.
114:524–537. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Urra FA, Muñoz F, Lovy A and Cárdenas C:
The mitochondrial complex(I)ty of cancer. Front Oncol. 7:1182017.
View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Bastian A, Matsuzaki S, Humphries KM,
Pharaoh GA, Doshi A, Zaware N, Gangjee A and Ihnat MA: AG311, a
small molecule inhibitor of complex I and hypoxia-induced HIF-1α
stabilization. Cancer Lett. 388:149–157. 2017. View Article : Google Scholar
|
|
127
|
Bridges HR, Jones AJ, Pollak MN and Hirst
J: Effects of metformin and other biguanides on oxidative
phosphorylation in mitochondria. Biochem J. 462:475–487. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Esser L, Zhou F, Zhou Y, Xiao Y, Tang WK,
Yu CA, Qin Z and Xia D: Hydrogen bonding to the substrate is not
required for rieskeiron-sulfur protein docking to the quinol
oxidation site of complex III. J Biol Chem. 291:25019–25031. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Wang F, Zhang R, Xia T, Hsu E, Cai Y, Gu Z
and Hankinson O: Inhibitory effects of nitric oxide on invasion of
human cancer cells. Cancer Lett. 257:274–282. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Fiorillo M, Lamb R, Tanowitz HB, Mutti L,
Krstic-Demonacos M, Cappello AR, Martinez-Outschoorn UE, Sotgia F
and Lisanti MP: Repurposing atovaquone: Targeting mitochondrial
complex III and OXPHOS to eradicate cancer stem cells. Oncotarget.
7:34084–34099. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Oyedotun KS and Lemire BD: The quaternary
structure of the Saccharomyces cerevisiae succinate dehydrogenase.
Homology modeling, cofactor docking, and molecular dynamics
simulation studies. J Biol Chem. 279:9424–9431. 2004. View Article : Google Scholar
|
|
132
|
Sun F, Huo X, Zhai Y, Wang A, Xu J, Su D,
Bartlam M and Rao Z: Crystal structure of mitochondrial respiratory
membrane protein complex II. Cell. 121:1043–1057. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Guo L, Shestov AA, Worth AJ, Nath K,
Nelson DS, Leeper DB, Glickson JD and Blair IA: Inhibition of
mitochondrial complex II by the anticancer agent lonidamine. J Biol
Chem. 291:42–57. 2016. View Article : Google Scholar :
|
|
134
|
Kluckova K, Bezawork-Geleta A, Rohlena J,
Dong L and Neuzil J: Mitochondrial complex II, a novel target for
anti-cancer agents. Biochim Biophys Acta. 1827:552–564. 2013.
View Article : Google Scholar
|
|
135
|
Quail DF and Joyce JA: Microenvironmental
regulation of tumor progression and metastasis. Nat Med.
19:1423–1437. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Shree T, Olson OC, Elie BT, Kester JC,
Garfall AL, Simpson K, Bell-McGuinn KM, Zabor EC, Brogi E and Joyce
JA: Macrophages and cathepsin proteases blunt chemotherapeutic
response in breast cancer. Genes Dev. 25:2465–2479. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Gocheva V, Wang HW, Gadea BB, Shree T,
Hunter KE, Garfall AL, Berman T and Joyce JA: IL-4 induces
cathepsin protease activity in tumor-associated macrophages to
promote cancer growth and invasion. Genes Dev. 24:241–255. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Yeung OW, Lo CM, Ling CC, Qi X, Geng W, Li
CX, Ng KT, Forbes SJ, Guan XY, Poon RT, et al: Alternatively
activated (M2) macrophages promote tumour growth and invasiveness
in hepatocellular carcinoma. J Hepatol. 62:607–616. 2015.
View Article : Google Scholar
|
|
139
|
Wu L, Zhang X, Zhang B, Shi H, Yuan X, Sun
Y, Pan Z, Qian H and Xu W: Exosomes derived from gastric cancer
cells activate NF-κB pathway in macrophages to promote cancer
progression. Tumour Biol. 37:12169–12180. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Torti SV and Torti FM: Cellular iron
metabolism in prognosis and therapy of breast cancer. Crit Rev
Oncog. 18:435–448. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Duan X, He K, Li J, Cheng M, Song H, Liu J
and Liu P: Tumor associated macrophages deliver iron to tumor cells
via Lcn2. Int J Physiol Pathophysiol Pharmacol. 10:105–114.
2018.PubMed/NCBI
|
|
142
|
Mertens C, Mora J, Ören B, Grein S,
Winslow S, Scholich K, Weigert A, Malmström P, Forsare C, Fernö M,
et al: Macrophage-derived lipocalin-2 transports iron in the tumor
microenvironment. OncoImmunology. 7:e14087512017. View Article : Google Scholar
|
|
143
|
Flower DR: The lipocalin protein family: A
role in cell regulation. FEBS Lett. 354:7–11. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Laskar A, Eilertsen J, Li W and Yuan XM:
SPION primes THP1 derived M2 macrophages towards M1-like
macrophages. Biochem Biophys Res Commun. 441:737–742. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Fearnhead HO, Vandenabeele P and Vanden
Berghe T: How do we fit ferroptosis in the family of regulated cell
death? Cell Death Differ. 24:1991–1998. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta
R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS,
et al: Ferroptosis: An iron-dependent form of nonapoptotic cell
death. Cell. 149:1060–1072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Sheng X, Shan C, Liu J, Yang J, Sun B and
Chen D: Theoretical insights into the mechanism of ferroptosis
suppression via inactivation of a lipid peroxide radical by
liproxstatin-1. Phys Chem Chem Phys. 19:13153–13159. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Fanzani A and Poli M: Iron, oxidative
damage and ferroptosis in rhabdomyosarcoma. Int J Mol Sci.
18:182017.
|
|
149
|
Alvarez SW, Sviderskiy VO, Terzi EM,
Papagiannakopoulos T, Moreira AL, Adams S, Sabatini DM, Birsoy K
and Possemato R: NFS1 undergoes positive selection in lung tumours
and protects cells from ferroptosis. Nature. 551:639–643.
2017.PubMed/NCBI
|
|
150
|
Sun X, Ou Z, Xie M, Kang R, Fan Y, Niu X,
Wang H, Cao L and Tang D: HSPB1 as a novel regulator of ferroptotic
cancer cell death. Oncogene. 34:5617–5625. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Doll S, Proneth B, Tyurina YY, Panzilius
E, Kobayashi S, Ingold I, Irmler M, Beckers J, Aichler M, Walch A,
et al: ACSL4 dictates ferroptosis sensitivity by shaping cellular
lipid composition. Nat Chem Biol. 13:91–98. 2017. View Article : Google Scholar :
|
|
152
|
Friedmann Angeli JP, Schneider M, Proneth
B, Tyurina YY, Tyurin VA, Hammond VJ, Herbach N, Aichler M, Walch
A, Eggenhofer E, et al: Inactivation of the ferroptosis regulator
Gpx4 triggers acute renal failure in mice. Nat Cell Biol.
16:1180–1191. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Cao JY and Dixon SJ: Mechanisms of
ferroptosis. Cell Mol Life Sci. 73:2195–2209. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Stockwell BR, Friedmann Angeli JP, Bayir
H, Bush AI, Conrad M, Dixon SJ, Fulda S, Gascón S, Hatzios SK,
Kagan VE, et al: Ferroptosis: A regulated cell death nexus linking
metabolism, redox biology, and disease. Cell. 171:273–285. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Ishii T, Sugita Y and Bannai S: Regulation
of glutathione levels in mouse spleen lymphocytes by transport of
cysteine. J Cell Physiol. 133:330–336. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Lou L, Kang J, Pang H, Li Q, Du X, Wu W,
Chen J and Lv J: Sulfur protects Pakchoi (Brassica chinensis L.)
seedlings against cadmium stress by regulating
ascorbate-glutathione metabolism. Int J Mol Sci. 18:182017.
View Article : Google Scholar
|
|
157
|
Dolma S, Lessnick SL, Hahn WC and
Stockwell BR: Identification of genotype-selective antitumor agents
using synthetic lethal chemical screening in engineered human tumor
cells. Cancer Cell. 3:285–296. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Seiler A, Schneider M, Förster H, Roth S,
Wirth EK, Culmsee C, Plesnila N, Kremmer E, Rådmark O, Wurst W, et
al: Glutathione peroxidase 4 senses and translates oxidative stress
into 12/15-lipoxygenase dependent- and AIF-mediated cell death.
Cell Metab. 8:237–248. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Ursini F and Bindoli A: The role of
selenium peroxidases in the protection against oxidative damage of
membranes. Chem Phys Lipids. 44:255–276. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Chu FF: The human glutathione peroxidase
genes GPX2, GPX3, and GPX4 map to chromosomes 14, 5, and 19,
respectively. Cytogenet Cell Genet. 66:96–98. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Yang WS, SriRamaratnam R, Welsch ME,
Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji
AF, Clish CB, et al: Regulation of ferroptotic cancer cell death by
GPX4. Cell. 156:317–331. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Shen Z, Liu T, Li Y, Lau J, Yang Z, Fan W,
Zhou Z, Shi C, Ke C, Bregadze VI, et al:
Fenton-reaction-acceleratable magnetic nanoparticles for
ferroptosis therapy of orthotopic brain tumors. ACS Nano.
12:11355–11365. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Shen J, Sheng X, Chang Z, Wu Q, Wang S,
Xuan Z, Li D, Wu Y, Shang Y, Kong X, et al: Iron metabolism
regulates p53 signaling through direct heme-p53 interaction and
modulation of p53 localization, stability, and function. Cell Rep.
7:180–193. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Tarangelo A, Magtanong L, Bieging-Rolett
KT, Li Y, Ye J, Attardi LD and Dixon SJ: p53 suppresses metabolic
stress-induced ferroptosis in cancer cells. Cell Rep. 22:569–575.
2018. View Article : Google Scholar : PubMed/NCBI
|