Introduction
Pancreatic cancer (PC) is the third leading cause of
cancer-associated mortality worldwide (1), with a total of 1,619 cases succumbing
to PC reported by China's Disease Surveillance Point System (DSPS)
between 1991 and 2000 (2). Due to
the high rate of metastasis and lack of effective treatment for
patients with metastatic cancer, the overall 5-year survival rate
of PC is <10% (3). Thus, it is
critical to further elucidate the molecular mechanisms underlying
PC metastasis.
The epithelial-to-mesenchymal transition (EMT) is at
least in part responsible for the metastatic progression of
multiple types of cancer, and this process results in cancer cells
losing epithelial features and acquiring mesenchymal features
(4,5). Among the various signaling pathways
that contribute to the development of PC, the Wnt/β-catenin
signaling pathway plays a prominent role in the EMT and metastasis
in PC (6). The expression and
subcellular localization of β-catenin is closely associated with PC
tumor formation and development. The activation of Wnt signaling
occurs upon nuclear accumulation of β-catenin (7), which activates downstream target
genes. Therefore, investigation of the pivotal upstream regulator
of the Wnt/β-catenin pathway is definitely warranted for a
comprehensive understanding of the molecular mechanism underlying
the metastasis and EMT of PC.
RNA-binding proteins (RBPs), which bind directly to
the RNA of target genes, contribute to tumor biology and
progression by regulating RNA at multiple levels, for example,
through RNA stabilization, translation, localization and
degradation (8-10). Although nearly 2,000 RBPs have been
identified (11), the molecular
mechanisms by which RBPs modulate human cancer progression remain
largely unclear. Negative elongation factor E (NELFE) is one of the
subunits of NELF, which is a multi-subunit that cooperates with DRB
sensitivity-inducing factor (DSIF) and results in the inhibition of
Pol II elongation (12). Recent
research revealed that NELFE could also serve as an RBP oncogene in
hepatocellular carcinoma through the regulation of MYC signaling
(13). However, the expression
patterns and the potential physiological functions of NELFE in
other types of human cancer, including PC, remain largely
unknown.
The present study investigated the role of NELFE in
PC cell proliferation, migration and invasion and assess the
correlation between NELFE expression in clinical PC tissues and
prognosis. The aim of the present study was to determine whether
NELFE is a potential therapeutic target and an effective predictive
biomarker for patients with PC.
Materials and methods
Patients and tissue samples
The Ethics Committee of Clinical Research of Xi'an
Jiaotong University (Xi'an, China) approved the present study, and
written informed consent was obtained from each patient prior to
the study start. The 120 pairs of PC tissues and adjacent non-tumor
clinical samples were collected from patients with PC who underwent
surgical resection at The Second Affiliated Hospital of Xi'an
Jiaotong University between May 2013 and May 2015. The adjacent
tissues were collected ≥5 cm away from the edge of PC cancerous
tissue. Inclusion criteria were as follows: All patients were
determined to have PC via pathological diagnosis and had not
received chemotherapy, radiotherapy or immunotherapy prior to
resection; and the exclusion criteria were as follows: Patients
aged <20 and >70 years. Table
I presents the clinicopathological data of the patients. Every
pair of tissues observed in the present study contained both
cancerous and distant non-cancerous tissues, and were frozen in
liquid nitrogen immediately after surgery, and then stored at
−80°C.
 | Table IAssociation between NELFE expressions
and clinicopathological features in pancreatic cancer. |
Table I
Association between NELFE expressions
and clinicopathological features in pancreatic cancer.
| Variable | Total no. of
patients n=120 | NELFE expression, n
| P-value |
|---|
| High | Low |
|---|
| Age, n (%) | | | | 0.259 |
| <60 years | 48 (40.0) | 36 | 12 | |
| ≥60 years | 72 (60.0) | 47 | 25 | |
| Sex, n (%) | | | | 0.646 |
| Female | 45 (37.5) | 30 | 15 | |
| Male | 75 (62.5) | 53 | 22 | |
| Chronic
pancreatitis, n (%) | | | | 0.546 |
| Yes | 57 (47.5) | 44 | 13 | |
| No | 63 (52.5) | 39 | 24 | |
| Tumor size, n
(%) | | | | 0.04 |
| <2 cm | 42 (35.0) | 34 | 8 | |
| ≥2 cm | 78 (65.0) | 49 | 29 | |
| Differentiation, n
(%) | | | | 0.136 |
| Well-moderate | 69 (57.5) | 44 | 25 | |
| Poor | 51 (42.5) | 39 | 12 | |
| Lymph-node
metastasis, n (%) | | | | 0.001 |
| Yes | 69 (57.5) | 39 | 30 | |
| No | 51 (42.5) | 44 | 7 | |
| TNM stage, n
(%) | | | | 0.003 |
| I-II | 63 (52.5) | 51 | 12 | |
| III-IV | 57 (47.5) | 32 | 25 | |
Immunohistochemistry
The immunohistochemistry assay was performed as
previously described (14). The
anti-NELFE antibody used in the immunohistochemistry assay was
purchased from Beijing Biosynthesis Biotechnology (1:100; cat no.
bs-19198R). The biotinylated goat anti-rabbit antibody (1:200; cat.
no. A0279; Beyotime Institute of Biotechnology) was used as the
secondary antibody. The diaminobenzidine tetrachloride (cat. no.
P0203; Beyotime Institute of Biotechnology) was used for staining
at 37°C for 10 min.
Cell culture and transfection
The normal pancreatic cell line, HPDE6-C7, was
purchased from CELLBIO Cell Center, and the PC cell lines,
including PaCa-2, PANC-1, SW1990, AsPC-1 and BxPC-3, were purchased
from the American Type Culture Collection. All cells were cultured
in Dulbecco's modified Eagle medium (DMEM; Sigma-Aldrich, Merck
KGaA) supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo
Fisher Scientific, Inc.). The specific short hairpin (sh)-RNA
vector (sh-NELFE and sh-NDRG2) to knockdown NELFE or NDRG2
expression in PC and their control vectors were purchased from
Genepharma. PC cells were cultured in 6-well plates until they
reached 40-50% confluence, and the 50 nM vectors were transfected
into PC cells using Lipofectamine® 2000 reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. In order to establish stable clones, PC
cells were expanded by trypsinizing after 48 h, diluted and then
seeded in a 96-well plates with DMEM containing 10% FBS and 500
µg/ml G418 (Gibco; Thermo Fisher Scientific, Inc.). The PC
cells were inspected daily under the fluorescence microscope to
ensure the colonies were derived from a single cell within a well.
After 20-25 days selection, the PC cells were trypsinized and
re-seeded into 24-well plates, and then into 6-well plates in DMEM
containing 10% FBS with 500 µg/ml G418. Finally, the NELFE
or NDRG2 expression levels in the PC cells collected from G418
positive colony were assessed by reverse transcription-quantitative
(RT-q) PCR and western blotting.
RT-qPCR
RT was performed on 0.1 µg total RNA, which
had been extracted from PC cell lines or tissue by
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) to complementary DNA using a RT-PCR kit (ABI; Thermo Fisher
Scientific, Inc.) 45 min at 50°C. qPCR reactions were performed in
triplicate using a SYBR Premix Ex Taq Real-Time PCR kit (Takara
Bio, Inc.) according to the manufacturer's protocol. The
housekeeping genes GAPDH, β-actin and Hydroxymethyl bilane synthase
(HMBS) were selected to serve as the candidate internal controls in
the present study, as they were steadily expressed at a similar
level in the examined PC cell lines. All the three internal
controls were expressed stably, and the RT-qPCR results calculated
using the three internal controls were similar. GAPDH is taken as
the internal control in the figures listed in the paper. The NELFE
and NDRG2 mRNA Expression levels were examined using a relative
quantification approach (2−ΔΔCq method)
compared with the level of GAPDH (15), which is expressed at a similar
level in the examined PC cell lines. The RT-qPCR thermocycling
conditions were as follows: Initial denaturation (90°C, 5 min), 40
cycles of denaturation (90°C, 10 sec), annealing (75°C, 6 sec) and
elongation (75°C, 30 sec), final elongation (75°C, 10 min) and a
final hold (4°C). The primers used in the present study were as
follows: NELFE: Forward, 5′-GCA TAT CCA TAT GCA GGA ATG CCT GGA GAA
GTT CC-3′, reverse, 5′-GCG GAT CCT TAT TCG GCC AGT CGG TAG ATT
AGC-3′; NDRG2: Forward, 5′-CAC TCC AGT GAC AGC ACC TCT-3′; reverse,
5′-GGC TCC AAC ACC AAC TCC AAT T-3′; and GAPDH: Forward, 5′-AAT GGA
CAA CTG GTC GTG GAC-3′; reverse, 5′-CCC TCC AGG GGA TCT GTT
TG-3′.
Western blotting
The protein was collected from PC cell lines via
lysing by RIPA Buffer (Thermo Fisher Scientific, Inc.), and the
protein concentrations were determined by BCA assays (Cell
Signaling Technology, Inc.). Each of the samples were
quantitatively released by the RIPA Buffer at the same
concentration.
Total proteins (25 µg per lane) were
separated via SDS-PAGE (10% gel). The proteins were then
transferred to polyvinylidene fluoride (PVDF) membranes (EMD
Millipore) and blocked in 5% skimed milk PBS with 0.1% Triton X-100
at 37°C for 1 h. The membranes were then incubated with the primary
antibodies at 4°C overnight and the secondary antibodies at room
temperature for 1 h. Finally, the results were visualized using an
ECL blotting analysis system (GE Healthcare Biosciences). The
primary antibodies used in the present study were: Anti-NELFE
(catalog no. ab170104; 1:1,000), anti-GAPDH (catalog no. ab8245;
1:5,000), anti-E-cadherin (catalog no. ab1416; 1:1,000),
anti-N-cadherin (catalog no. ab202030, 1:1,000), anti-Vimentin
(catalog no. ab193555; 1:2,000), anti-β-catenin (catalog no.
ab32572; 1:1,000), anti-NDRG2 (catalog no. ab174850; 1:2,000),
anti-α-tubulin (catalog no. ab210797; 1:2,000) and anti-LaminB1
(catalog no. ab252351; 1:2,000) (all from Abcam). The goat
anti-rabbit secondary antibody (1:10,000; cat. no.
bs-40295G-IRDye8) was purchased from Beijing Bioss Biotechnology.
Finally, the membranes were tested using a bio-imaging system (DNR
Bio-Imaging Systems). ImageJ software (version 1.6.0; National
Institutes of Health) was used to quantify the intensity of protein
bands and normalized by GAPDH.
MTT assays
The PC cells were seeded into the 96-well plates at
a density of 500-700 cells per well. The cells in each well were
cultured in 100 µl DMEM medium with 10% FBS. At the time
points 0, 24, 48, 72 and 96 h, MTT solution (10 µl, 5 mg/ml)
were added into the culture medium. After incubation for 4 h at
room temperature, the medium containing MTT solution was removed,
and dimethyl sulfoxide (DMSO; volume, 200 µl; concentration,
1 mg/ml) was added into each well to dissolve the purple formezan.
Finally, the plate reader (Bio-Rad Laboratories) was used to
observe the absorbance at the 492 nm wavelength.
Colony-forming assays
A total of 100 PC cells per well were seeded into a
6-well cell culture cluster containing 2 ml DMEM supplemented with
10% FBS in each well. Following culture in a humidified atmosphere
for 10 days at 37°C, the colonies were fixed with methanol, and
then stained in 0.1% crystal violet solution at 37°C for 30 min.
The cells were then washed with PBS 2-3 times and then dried
overnight. The colonies forming units (consisting of ≥50 cells)
were assessed under an inverted fluorescence microscope (Nikon,
magnification, ×40).
Cell migration and invasion assays
The 8.0 µm pore Transwell chambers (EMD
Millipore) inserted in 24-well plates were used for the migration
and invasion assays. Migration assays: The PC cells
(2×104) in 200 µl serum-free media were applied
to the upper chamber, while normal culture media (DMEM supplemented
with 10% FBS, 700 µl) was added to the bottom compartment.
After incubation in cell incubator for 36 h at 37°C, the invaded
cells moved into the lower side of the membranes were fixed with 4%
paraformaldehyde at 37°C for 1 h and then stained with 0.1% crystal
violet solution for 30 min at 37°C. Invasion assay: The procedures
was same as those aforementioned in the migration assay except that
the membranes in the upper chamber were pre-coated with 15
µg Matrigel (Becton Dickinson Bioscience). Finally, the
migrated cells were examined in 10 randomly selected fields of view
under a fluorescence microscope (magnification, ×200).
Luciferase reporter assays
The PC cells (1×105/well) in a 24-well
plate were co-transfected with Renilla luciferase (phRL-TK),
sh-NELFE vector or sh-NELFE negative control (NC) empty vector, and
pGL3-NDRG2-3′UTR reporter vectors (or pGL3-TCF promoter)
(GenePharma Co., Ltd.) using Lipofectamine® 3000 (1
µl/well; Invitrogen; Thermo Fisher Scientific, Inc.). After
transfection for 24 h, the luciferase activities were measured
using a Dual-Luciferase Reporter Assay System kit (Promega
Corporation) according to the manufacturer's protocol. The relative
Firefly luciferase activity was measured by normalizing to Renilla
luciferase activity.
mRNA decay assay
The PC cells were seeded into 6-well plates
supplemented with actinomycin D (Sigma Aldrich; Merck KGaA) at a
concentration of 0.8 µg/ml. RT-qPCR assay was performed as
aforementioned in order to detect NDRG2 RNA in PC cells at
scheduled time points following transfection with sh-NELFE or the
control vectors, including 2, 4, 6, 8, 10 and 12 h.
RNA immunoprecipitation (RIP assay)
The RIP assay was performed according to the
instructions of the RIP RNA-binding protein Immunoprecipitation kit
(EMD Millipore) following the manufacturer's protocol. The PC cell
lysates were added with RIP Lysis Buffer and NELFE antibody, normal
mouse IgG (negative control) (1:200; cat. no. PP6421-K; EMD
Millipore) and agarose beads and incubated overnight at 4°C. A
High-Capacity cDNA Reverse Transcription kit (Thermo Fisher
Scientific, Inc.) was used to reverse transcribe the
immunoprecipitated RNAs. Finally, RT-qPCR was performed as
aforementioned in order to examine the targets transcripts.
Statistical analysis
Statistical analyses were performed using SPSS
statistical software (version 19.0; IBM Corp.) and the data are
expressed as the mean ± standard error. Student's t-test was used
to evaluate the difference between two groups. The Kaplan-Meier
method and log-rank test was used to plot the survival curves.
Person correlation was performed in order to assess the correlation
between NELFE and NDRG2 mRNA expression in PC tissues. P<0.05
was considered to indicate a statistically significant result. All
experiments were repeated triplicate.
Results
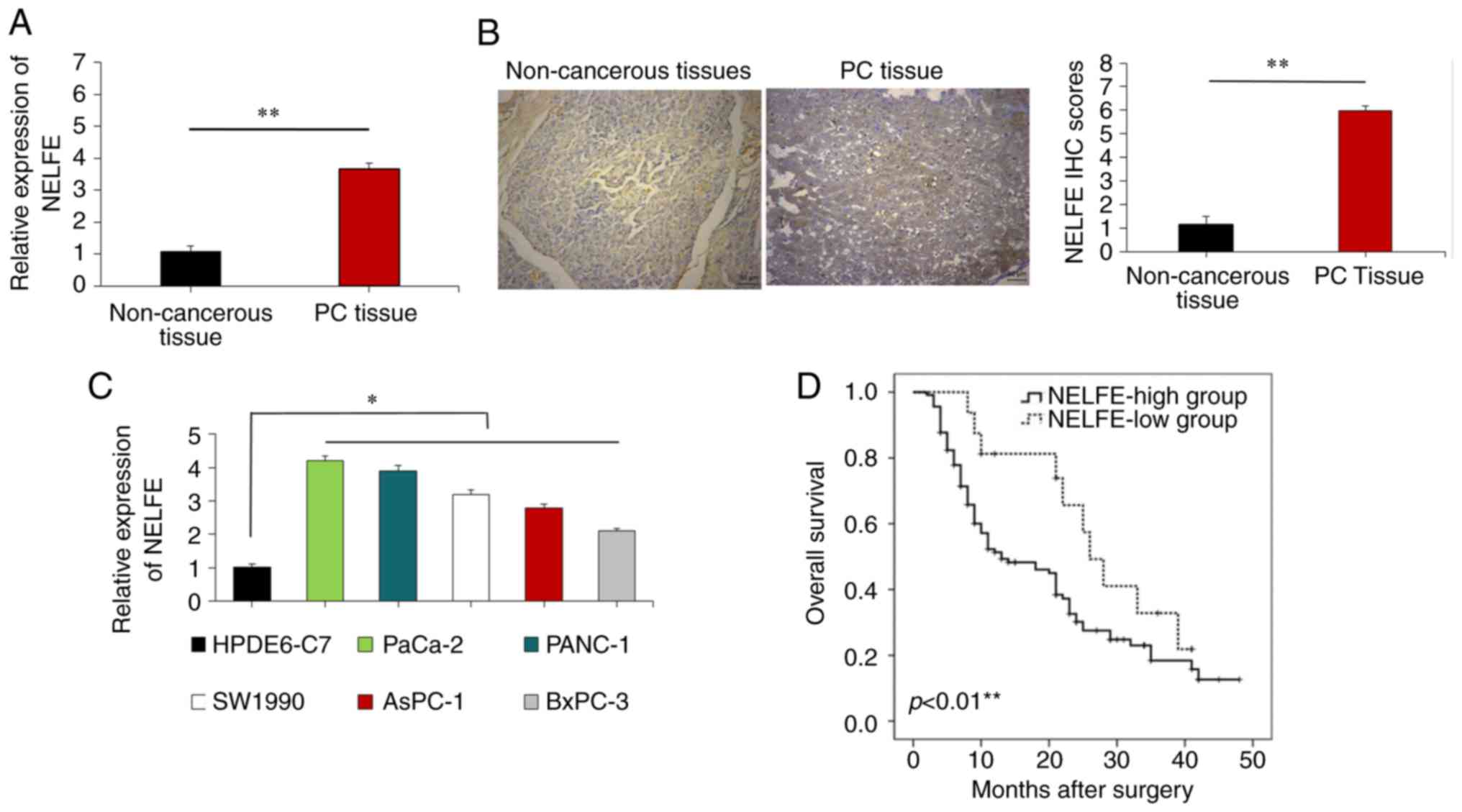
NELFE is increased in PC tissues and cell
lines
In order to determine the expression pattern of
NELFE in clinical PC samples, the present study investigated NELFE
expression in 120 pairs of PC tissues and adjacent non-tumor
clinical samples removed from patients with PC. RT-qPCR assays
suggested that NELFE mRNA expression was primarily higher in the PC
tissues than in the paired non-cancerous tissues (P<0.01;
Fig. 1A). Immunohistochemistry
assays also revealed that NELFE expression was largely upregulated
in PC tissues compared with that in non-cancerous tissues from
patients with PC (P<0.01; Fig.
1B). The present study also analyzed the clinical significance
of NELFE in patients with PC. A high NELFE expression level was
significantly associated with lymph node metastasis (P=0.001),
large tumor size (P=0.04) and advanced Tumor-Node-Metastasis (TNM)
stage PC (16) (P=0.003; Table I). The present study further
measured the expression levels of NELFE in PC cells using RT-qPCR,
and the results revealed that the expression level of NELFE was
higher in PC cell lines than in normal pancreatic cells (HPDE6-C7)
(P<0.05; Fig. 1C). Among the PC
cell lines, the expression of NELFE was highest in the PANC-1 and
PaCa-2 cell lines. In addition, the Kaplan-Meier analysis revealed
that compared with those with higher levels of NELFE, patients with
lower expression levels of NELFE had a shorter survival time
(P<0.01; Fig. 1D).
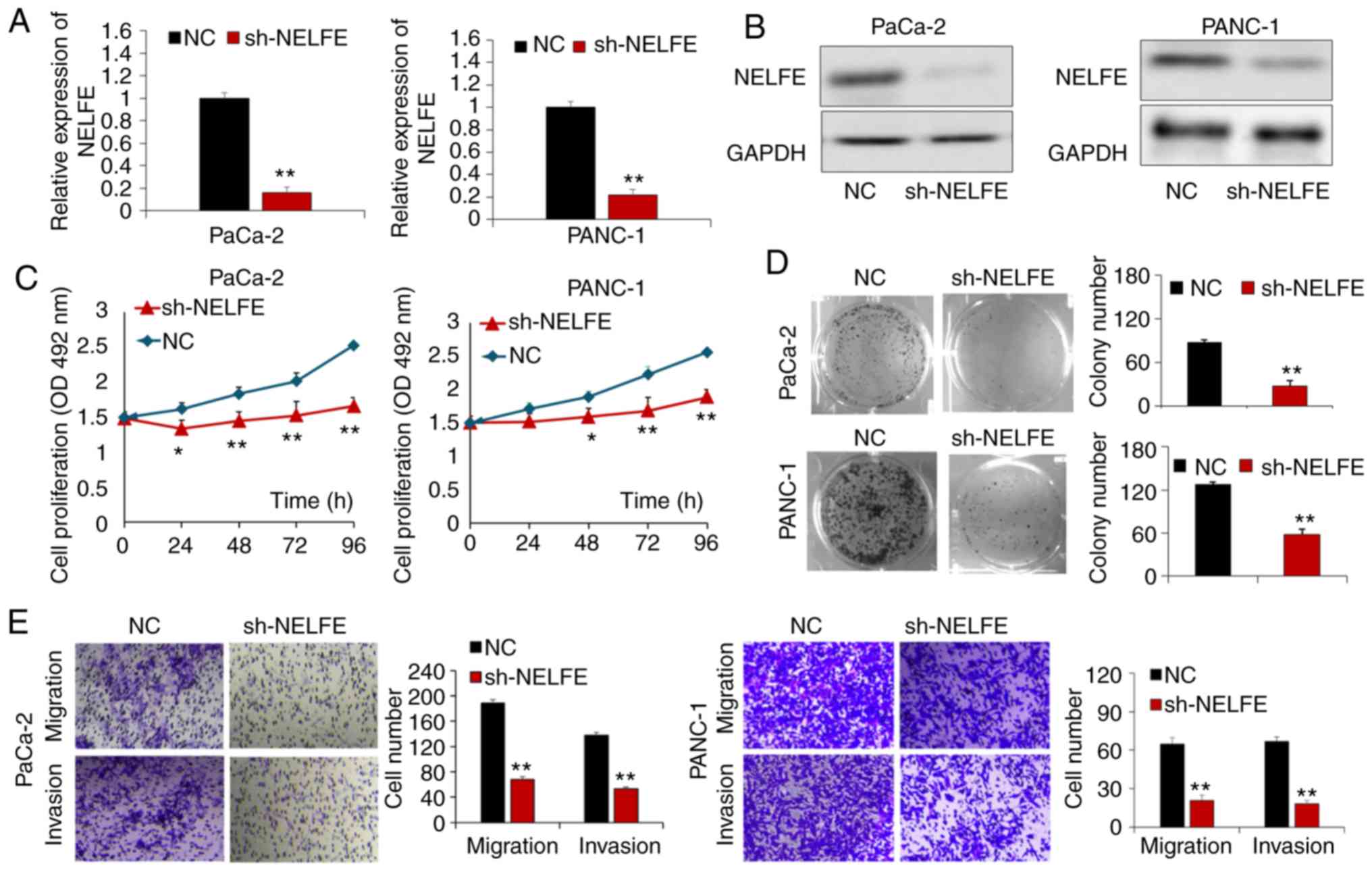
NELFE promotes PC cell proliferation,
invasion and migration
The present study transfected PaCa-2 and PANC-1
cells with a sh-NELFE vector or control vector. RT-qPCR and western
blot assays demonstrated that compared with transfection of the
control vector, transfection of the sh-NELFE vector markedly
decreased the mRNA and protein expression levels of NELFE in PC
cells (P<0.01; Fig. 2A and B).
The MTT assay suggested that the proliferation ability of both
PaCa-2 and PANC-1 cells was significantly inhibited following
knocking-down NELFE expression (P<0.05; Fig. 2C). In addition, the colony
formation results demonstrated that downregulated NELFE expression
inhibited the PC cell colony formation ability (P<0.01; Fig. 2D). The present study also performed
transwell assays in order to assess the function of NELFE in the
migration and invasion ability of PC cells. As presented in
Fig. 2E, decreased NELFE
significantly suppressed the migration and invasion ability of PC
cells (P<0.01). These results further suggested that NELFE
serves as an oncogene in PC cells.
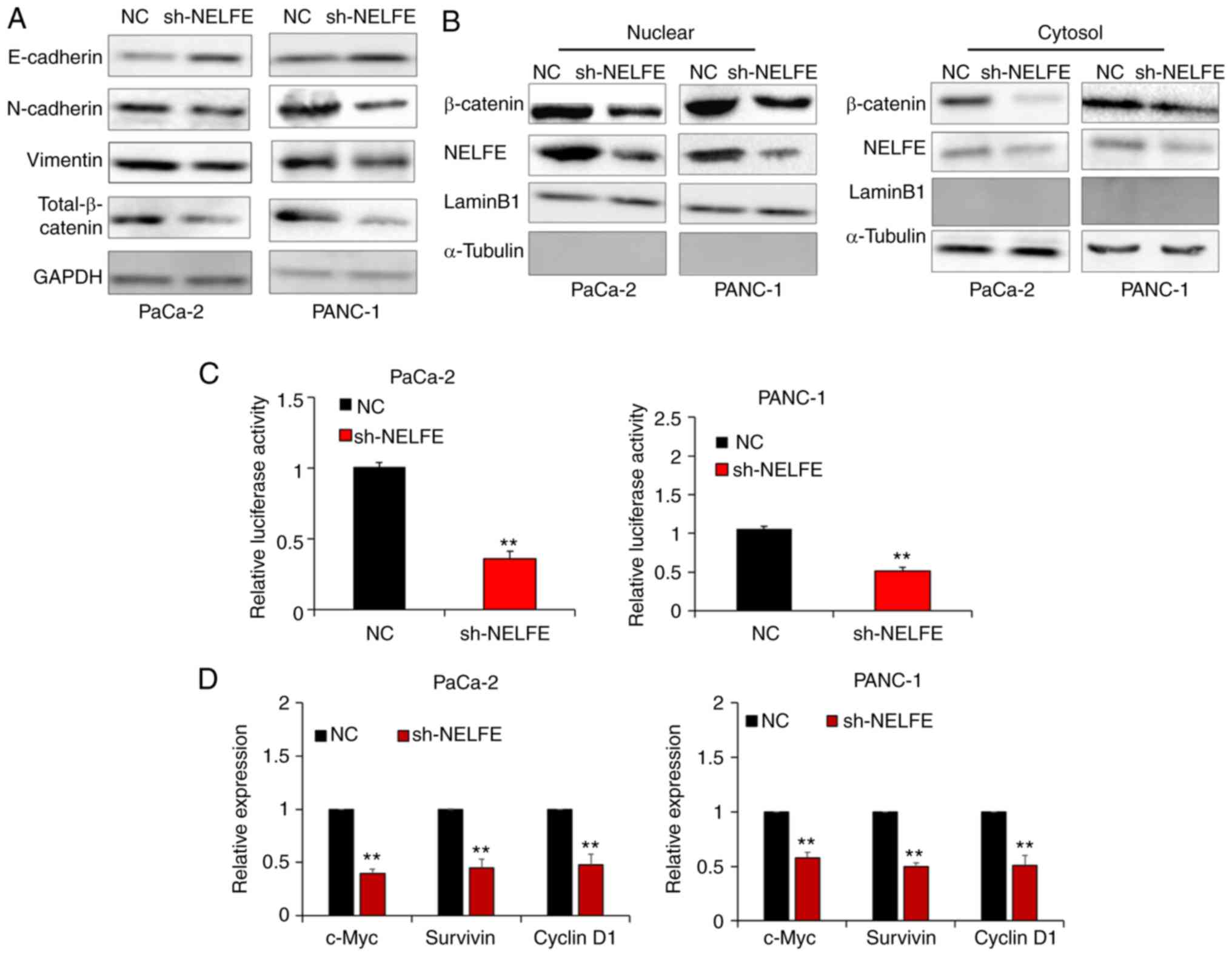
NELFE promotes EMT and Wnt/β-catenin
signaling in PC cells
EMT is well known as the foundation and key
mechanism of the invasion and metastasis of PC cells. Thus, the
present study performed a western blot assay to measure the
expression levels of epithelial markers (E-cadherin) and
mesenchymal markers (N-cadherin and Vimentin) in PC cells
transfected with control vector or sh-NELFE vector, in order to
investigate whether NELFE promoted PC cell migration and invasion
via regulating the EMT. As presented in Fig. 3A, decreased NELFE levels inhibited
the expression of mesenchymal markers and enhanced the expression
of epithelial markers, which indicated that NELFE promoted PC cell
EMT. β-catenin is recognized as a key effector of Wnt/β-catenin
signaling, which is widely implicated in EMT in a number of
different types of cancer, including PC (17). The results from the present study
also revealed that decreased NELFE resulted in the downregulation
of β-catenin expression in PC cells. A decrease in β-catenin
breakdown and its subsequent accumulation in the cytoplasm will
facilitate its nuclear translocation. Then, β-catenin can bind to
TCF transcription factors in the nucleus and successfully
transcriptionally activate a series of genes associated with the
EMT (18). Thus, the present study
performed subcellular fractionation assays in order to evaluate
whether NELFE activated the Wnt/β-catenin signaling pathway in PC
cells. The results revealed that downregulation of NELFE in PC
cells markedly decreased the nuclear accumulation of β-catenin
(Fig. 3B). In addition, a dual
luciferase reporter assay demonstrated that decreased NELFE
significantly inhibited the transactivation of the TCF reporter in
PC cells (Fig. 3C). Furthermore,
the present study measured the expression levels of Wnt/β-catenin
target genes, such as c-Myc, survivin and cyclin D1, via RT-qPCR.
The results revealed that Wnt/β-catenin downstream gene expression
was significantly downregulated following NELFE knockdown in PC
cells (Fig. 3D). Overall, these
results suggested that NELFE promotes EMT via activating the
Wnt/β-catenin signaling pathway in PC.
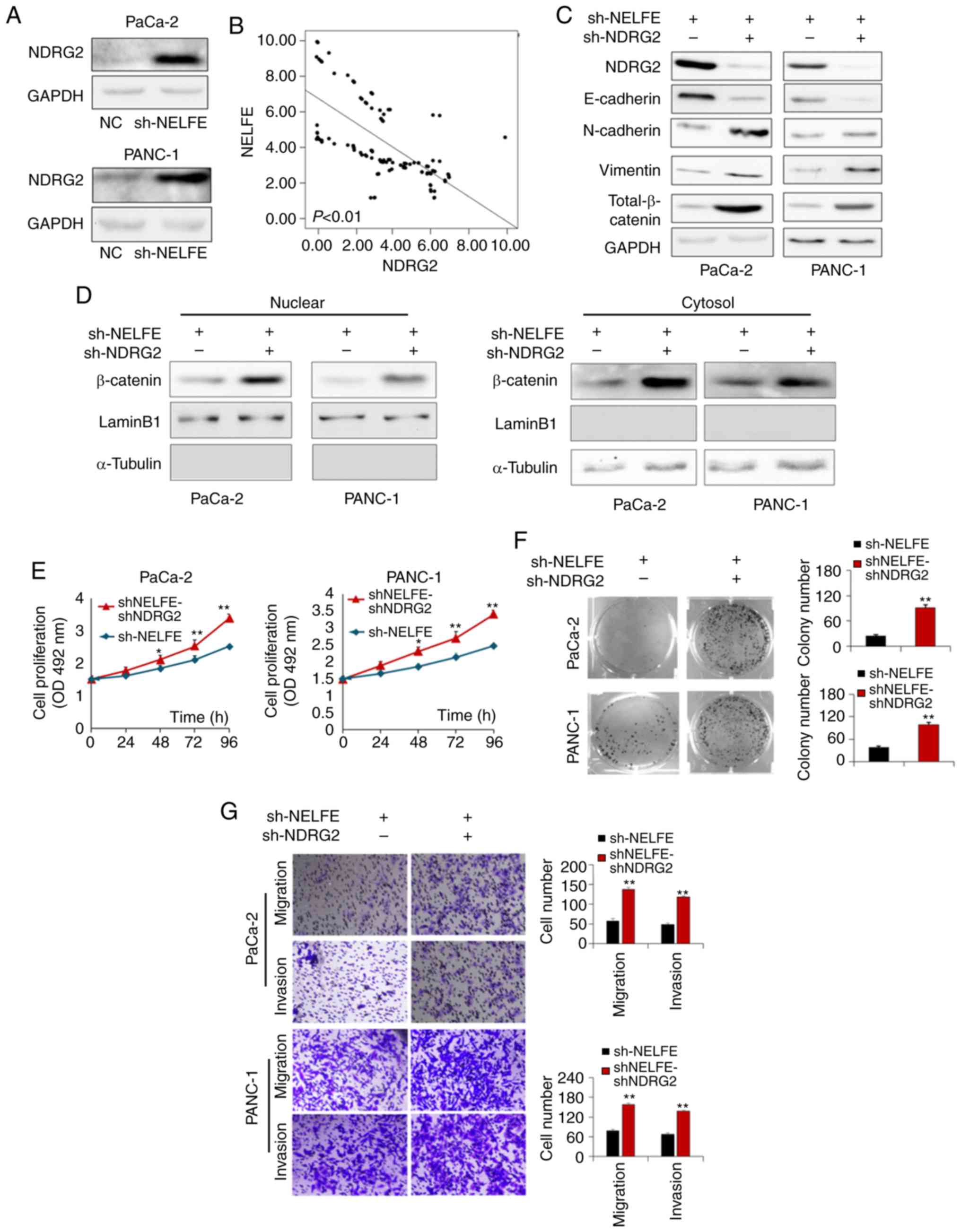
NELFE activates the Wnt/β-catenin
signaling pathway by inhibiting NDRG2 expression in PC
NDRG2, a tumor suppressor belonging to the NDRG
family, has been reported to be decreased in human cancer tissues,
including PC (19). Accumulating
studies have revealed that NDRG2 is involved in the regulation of
EMT via inhibiting β-catenin/c-Myc signaling and then inducing
E-cadherin degradation in human cancers (20-22).
Notably, the present study aimed to determine the potential
molecular mechanisms underlying the regulation of the Wnt/β-catenin
signaling pathway by NELFE, and revealed that NDRG2 expression was
strongly increased following NELFE knockdown (Fig. 4A). In addition, the present study
performed a RT-qPCR to assess whether there is a correlation
between the mRNA expression levels of these two genes in clinical
samples of PC tissues. The pearson correlation analysis of RT-qPCR
results revealed that NELFE mRNA levels were negatively correlated
with NDRG2 mRNA levels (R=−0.776, P<0.01; Fig. 4B). Thus, it was speculated that
NELFE may activate the Wnt/β-catenin signaling pathway by
inhibiting the expression of NDRG2. The present study trans-fected
the sh-NDRG2 vector or control vector into PC cells with NELFE
knockdown. As presented in Fig.
4C, knockdown of NDRG2 in PC cells with decreased NELFE
increased the expression levels of total β-catenin (including
nuclear β-catenin and cytosol β-catenin) and mesenchymal markers
(N-cadherin and Vimentin), and decreased the expression level of an
epithelial marker (E-cadherin) (Fig.
4C). In addition, knockdown of NDRG2 promoted the nuclear
accumulation of β-catenin in PC cells, even though the expression
of NELFE was downregulated (Fig.
4D). Furthermore, sh-NDRG2-mediated downregulation of NDRG2
significantly promoted the proliferation, invasion and migration of
sh-NELFE-transfected PC cells (Fig.
4E-G). Taken together, these results suggested that NELFE
activated the Wnt/β-catenin signaling pathway and promoted PC tumor
progression in a manner at least partly dependent on NDRG2
expression downregulation.
NELFE decreases NDRG2 expression by
directly interacting with its mRNA
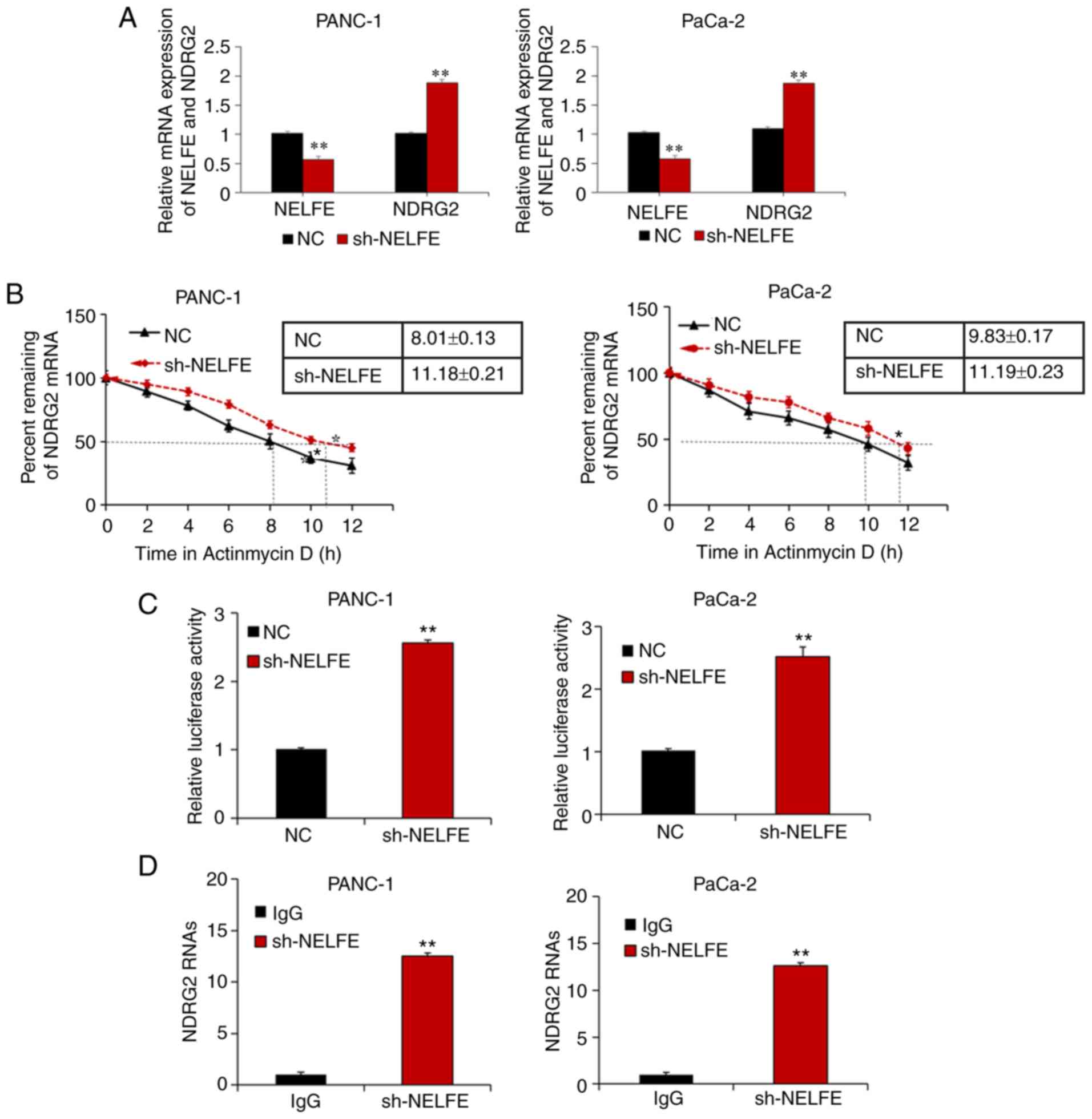
The RT-qPCR results further demonstrated that the
mRNA expression level of NDRG2 was significantly increased
following NELFE knockdown (Fig.
5A), suggesting that NELFE regulated NDRG2 at the
transcriptional level. The effect of NELFE on the stability of
NDRG2 mRNA was also measured, and the results revealed that the
half-life of NDRG2 mRNA was prolonged after NELFE was decreased in
PC cells (Fig. 5B). A luciferase
reporter assay demonstrated that decreased NELFE enhanced the
luciferase activity of the NDRG2 3′ UTR (Fig. 5C). In order to further investigate
whether NELFE binds directly to NDRG2 mRNA, the present study
performed RIP assays, and the results revealed that NDRG2 mRNA was
more enriched in sh-NELFE transfected PC cells than the control
(IgG) (Fig. 5D). Thus, the results
from the present study demonstrated that NELFE inhibited NDRG2
expression by promoting decay of its transcript.
Discussion
To the best of our knowledge, the present study is
the first to demonstrate that NELFE acts as an RBP to promote EMT
and metastasis in PC by activating the Wnt/β-catenin signaling
pathway via post-transcriptional inhibition of NDRG2 expression.
The data from the present study contributes to the current
understanding of the molecular mechanism by which NELFE
overexpression promotes tumorgenesis and progression in PC.
NELFE is an important part of NELF, which is well
known as the pivotal regulatory factor of the Pol II pausing
complex (12,23,24).
NELF contains four multi-functional sub-units, NELFA, NELFB, NELFC
and NELFE, which are all involved in the regulation of MYC
signaling (25-27). In addition, the four sub-units have
been identified to be involved in a variety of different types of
human cancer (23,28,29).
Midorikawa et al (26)
reported that the overexpression of NELFE contributed to the
tumorigenesis of hepatocellular carcinoma (HCC). A recent study
demonstrated that NELFE is upregulated in HCC tissues, and the
overexpression of NELFE promoted the progression of HCC via
directly binding to and enhancing MYC (13). Further research on the role of NELF
will be helpful for understanding the potential molecular mechanism
of PC, and may provide a novel treatment strategy. To the best of
our knowledge, the present study demonstrated for the first time
that NELFE was increased in PC tissues and cell lines. These data
are consistent with other recent results suggesting that NELFE
functions as an oncogene in human cancers (13). The present study further confirmed
that the upregulation of NELFE was associated with poor outcomes in
patients with PC. In addition, the in vitro results
demonstrated that NELFE downregulation weakened the proliferation,
invasion and migration capacities of PC cells, suggesting that
NELFE enhanced the malignant biological behavior of PC cells.
Considering these results have indicated the lower expression of
NELFE in adjacent non-cancerous tissues from PC patients and normal
cells, respectively, compared with that in the PC tissue and PC
cell lines, it was speculated that the abnormally increased
expression of NELFE is associated with the development and
progression of PC. These data strongly suggest that NELFE may work
as a potential therapeutic target for patients with PC.
PC is characterized as one of the most aggressive
types of human tumor, which causes its high probability of
cancer-associated mortality. The poor outcome is in part associated
with the high rate of metastasis for patients with PC. Thus, it is
important to further understand the potential molecular mechanisms
underlying the role of NELFE in promoting PC metastasis. EMT is
recognized as a response to the metastasis of human cancer
(30), and in this process,
epithelial cells lose differentiation characteristics, such as cell
adhesion and cell polarity, and are transformed into mesenchymal
cells and gain the ability to invade and migrate (31). The present study used a western
blot assay to demonstrate that knockdown of NELFE significantly
increased E-cadherin expression and decreased N-cadherin and
vimentin expression in PC cells. These data suggest that NELFE
promotes EMT in PC cells. Previous research has revealed that a
series of signaling factors participate in the regulation of EMT
processes (32), including
Wnt/β-catenin, TGF-β, Notch and HIF-1α (33-35).
Among these signaling pathways, Wnt/β-catenin is well known to
serve as the key mediator of EMT (36). The western blot assay results
revealed that decreased NELFE inhibited the expression and nuclear
accumulation of β-catenin, suggesting that NELFE promoted EMT via
activating the Wnt/β-catenin signaling pathway in PC.
Although increasing research has been focused on the
involvement of RBPs in a range of different types of human cancer
in previous years (37-39), more studies on the details of the
biological effect and underlying mechanisms of RBPs are still
required. To the best of our knowledge, the present study provided
the first evidence that NELFE acts as an RBP to promote PC
tumorigenesis and metastasis via the post-transcriptional
regulation of NDRG2 expression. NELFE has been verified to contain
an RNA recognition motif domain (40), which preferentially binds directly
to RNAs. At first, studies focused on only NELFE revealed that it
binds to certain special RNAs (41,42).
Until recently, the role of NELFE in the regulation of human cancer
progression through binding to certain target genes began to
attract researchers' interest (13,43).
The present study identified NDRG2 as a target gene of NELFE in PC.
The use of the luciferase, mRNA decay and RIP assays demonstrated
that NELFE inhibits NDRG2 expression by binding directly to its
3′UTR. NDRG2 is involved in the regulation of cancer cell
differentiation and proliferation as a tumor suppressor (19). Accumulating studies revealed that
NDRG2 expression was significantly lower in various cancer tissues,
including liver cancer, pancreatic cancer and glioblastoma,
compared with normal tissues, and decreased NDRG2 expression was
closely associated with a shorter overall survival in patients with
tumors (22,44). NDRG2 has been revealed to regulate
the EMT of human cancer in a series of reports. Chen et al
(45) reported that NDRG2
suppressed human cancer upon the metabolic reprogramming. Kim et
al (46) demonstrated that
NDRG2 repressed breast cancer EMT via STAT3/Snail signaling. It has
also been confirmed that NDRG2 could inhibit the prostate cancer
cell invasion and migration through regulating EMT-associated genes
(47) and suppress EMT of
esophageal cancer cells via regulating the AKT/XIAP signaling
pathway (48). Notably, other
studies have confirmed that NDRG2 plays a pivotal role in the
modulation of EMT by inhibiting β-catenin expression (49,50).
Thus, the results of the present study are rational. The present
study also demonstrated that NDRG2 could contribute to rescuing the
function of NELFE in PC cell invasion and migration, further
suggesting that NELFE promoted PC metastasis and EMT by activating
the Wnt/β-catenin signaling pathway via inhibiting NDRG2
expression. These results may help to open novel avenues for
treatment strategies for PC.
In summary, to the best of our knowledge, the
present study revealed the overexpression of NELFE in PC for the
first time. The overexpression of NELFE promoted malignant
phenotypes in PC cells, including proliferation, migration and
invasion. In addition, the results demonstrated that NELFE promoted
the EMT by enhancing the expression and nuclear accumulation of
β-catenin. The present study further revealed that NELFE inhibited
NDRG2 expression via binding with its 3′UTR. The present study also
revealed the significant role of NDRG2 in mediating the function of
NELFE in PC cells. The data also demonstrated for the first time
the significance of the NELFE/Wnt/β-catenin/NDRG2 axis in PC and
may offer a novel therapeutic strategy for PC.
Acknowledgments
Not applicable.
Funding
The present study was funded by the Natural Science
Foundation of Shaanxi Province (grant no. 2019JQ-128) and the China
Postdoctoral Science General Financial Grant (grant no.
2017M623193).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
LH collected the clinical samples and performed most
of the experiments. YZ performed the experiments and the
statistical analysis. CH conceived and designed the study. SZ
assisted with the design of the study and drafting of the
manuscript. All authors have read and approved the final version of
this published manuscript.
Ethics approval and consent for
publication
The present study was approved by the Ethics
Committee of Clinical Research of Xi'an Jiaotong University (Xi'an,
China) and was performed in accordance with the 1964 Declaration of
Helsinki. Written informed consent was obtained from all patients
prior to the study start.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang L, Yang GH, Li H and Lu XH: The
changing pancreatic cancer mortality in China (1991-2000). Zhonghua
Nei Ke Za Zhi. 44:509–513. 2005.In Chinese. PubMed/NCBI
|
|
3
|
Worni M, Guller U, White RR, Castleberry
AW, Pietrobon R, Cerny T, Gloor B and Koeberle D: Modest
improvement in overall survival for patients with metastatic
pancreatic cancer: A trend analysis using the surveillance,
epidemiology, and end results registry from 1988 to 2008. Pancreas.
42:1157–1163. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nieto MA, Huang RY, Jackson RA and Thiery
JP: EMT: 2016. Cell. 166:21–45. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Giannelli G, Koudelkova P, Dituri F and
Mikulits W: Role of epithelial to mesenchymal transition in
hepatocellular carcinoma. J Hepatol. 65:798–808. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gonzalez DM and Medici D: Signaling
mechanisms of the epithelial-mesenchymal transition. Sci Signal.
7:re82014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee JM, Dedhar S, Kalluri R and Thompson
EW: The epithelial-mesenchymal transition: New insights in
signaling, development, and disease. J Cell Biol. 172:973–981.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Scaturrok M, Sala A, Cutrona G, Raimondi
L, Cannino G, Fontana S, Pucci-Minafra I and Di Liegro I:
Purification by affinity chromatography of H1o RNA-binding proteins
from rat brain. Int J Mol Med. 11:509–513. 2003.PubMed/NCBI
|
|
9
|
Roesch A, Becker B, Meyer S, Wild P,
Hafner C, Landthaler M and Vogt T: Retinoblastoma-binding protein
2-homolog 1: A retinoblastoma-binding protein downregulated in
malignant melanomas. Mod Pathol. 18:1249–1257. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hodson DJ, Screen M and Turner M: RNA
binding proteins in hematopoiesis and hematological malignancy.
Blood. 133:2365–2373. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hentze MW, Castello A, Schwarzl T and
Preiss T: A brave new world of RNA-binding proteins. Nat Rev Mol
Cell Biol. 19:327–341. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Narita T, Yung TM, Yamamoto J, Tsuboi Y,
Tanabe H, Tanaka K, Yamaguchi Y and Handa H: NELF interacts with
CBC and participates in 3′ end processing of replication-dependent
histone rnRNAs. Mol Cell. 26:349–365. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dang H, Takai A, Forgues M, Pomyen Y, Mou
H, Xue W, Ray D, Ha KCH, Morris QD, Hughes TR and Wang XW:
Oncogenic activation of the RNA binding protein NELFE and MYC
signaling in hepatocellular carcinoma. Cancer Cell. 32:101–114.e8.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Han LL, Nan HC, Tian T, Guo H, Hu TH, Wang
WJ, Ma JQ, Jiang LL, Guo QQ, Yang CC, et al: Expression and
significance of the novel tumor-suppressor gene SMG-1 in
hepatocellular carcinoma. Oncol Rep. 31:2569–2578. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C (T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
16
|
Liu L, Xu HX, He M, Wang W, Wang WQ, Wu
CT, Wei RQ, Liang Y, Gao HL, Liu C, et al: A novel scoring system
predicts postsurgical survival and adjuvant chemotherapeutic
benefits in patients with pancreatic adenocarcinoma: Implications
for AJCC-TNM staging. Surgery. 163:1280–1294. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhou P, Li Y, Li B, Zhang M, Liu Y, Yao Y
and Li D: NMIIA promotes tumor growth and metastasis by activating
the Wnt/β-catenin signaling pathway and EMT in pancreatic cancer.
Oncogene. 38:5500–5515. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tian X, Liu Z, Niu B, Zhang J, Tan TK, Lee
SR, Zhao Y, Harris DC and Zheng G: Ecadherin/β-catenin complex and
the epithelial barrier. J Biomed Biotechnol. 2011:5673052011.
View Article : Google Scholar
|
|
19
|
Lorentzen A, Vogel LK, Lewinsky RH, Saebø
M, Skjelbred CF, Godiksen S, Hoff G, Tveit KM, Lothe IM, Ikdahl T,
et al: Expression of NDRG2 is down-regulated in high-risk adenomas
and colorectal carcinoma. BMC Cancer. 7:1922007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kang K, Nam S, Kim B, Lim JH, Yang Y, Lee
MS and Lim JS: Inhibition of osteoclast differentiation by
overexpression of NDRG2 in monocytes. Biochem Biophys Res Commun.
468:611–616. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang J, Li F, Liu X, Shen L, Liu J, Su J,
Zhang W, Deng Y, Wang L, Liu N, et al: The repression of human
differentiation-related gene NDRG2 expression by Myc via
Miz-1-dependent interaction with the NDRG2 core promoter. J Biol
Chem. 281:39159–39168. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hu XL, Liu XP, Lin SX, Deng YC, Liu N, Li
X and Yao LB: NDRG2 expression and mutation in human liver and
pancreatic cancers. World J Gastroenterol. 10:3518–3521. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yamaguchi Y, Takagi T, Wada T, Yano K,
Furuya A, Sugimoto S, Hasegawa J and Handa H: NELF, a multisubunit
complex containing RD, cooperates with DSIF to repress RNA
polymerase II elongation. Cell. 97:41–51. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vos SM, Pöllmann D, Caizzi L, Hofmann KB,
Rombaut P, Zimniak T, Herzog F and Cramer P: Architecture and RNA
binding of the human negative elongation factor. Elife.
5:e149812016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Price DH: Regulation of RNA polymerase II
elongation by c-Myc. Cell. 141:399–400. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Midorikawa Y, Tsutsumi S, Taniguchi H,
Ishii M, Kobune Y, Kodama T, Makuuchi M and Aburatani H:
Identification of genes associated with dedifferentiation of
hepatocellular carcinoma with expression profiling analysis. Jpn J
Cancer Res. 93:636–643. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
McChesney PA, Aiyar SE, Lee OJ, Zaika A,
Moskaluk C, Li R and El-Rifai W: Cofactor of BRCA1: A novel
transcription factor regulator in upper gastrointestinal
adenocarcinomas. Cancer Res. 66:1346–1353. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu CH, Lee C, Fan R, Smith MJ, Yamaguchi
Y, Handa H and Gilmour DS: Molecular characterization of Drosophila
NELF. Nucleic Acids Res. 33:1269–1279. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sun J, Watkins G, Blair AL, Moskaluk C,
Ghosh S, Jiang WG and Li R: Deregulation of cofactor of BRCA1
expression in breast cancer cells. J Cell Biochem. 103:1798–1807.
2008. View Article : Google Scholar
|
|
30
|
Thiery JP and Sleeman JP: Complex networks
orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell
Biol. 7:131–142. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cui W, Meng W, Zhao L, Cao H, Chi W and
Wang B: TGF-β-induced long non-coding RNA MIR155HG promotes the
progression and EMT of laryngeal squamous cell carcinoma by
regulating the miR-155-5p/SOX10 axis. Int J Oncol. 54:2005–2018.
2019.PubMed/NCBI
|
|
32
|
Nie J, Jiang HC, Zhou YC, Jiang B, He WJ,
Wang YF and Dong J: MiR-125b regulates the proliferation and
metastasis of triple negative breast cancer cells via the
Wnt/β-catenin pathway and EMT. Biosci Biotechnol Biochem.
83:1062–1071. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang HG, Pan YW, Feng J, Zeng CT, Zhao
XQ, Liang B and Zhang WW: TRIM66 promotes malignant progression of
hepatocellular carcinoma by inhibiting E-cadherin expression
through the EMT pathway. Eur Rev Med Pharmacol Sci. 23:2003–2012.
2019.PubMed/NCBI
|
|
34
|
Nam Y, Weng AP, Aster JC and Blacklow SC:
Structural requirements for assembly of the CSL.intracellular
Notch1. Mastermind-like 1 transcriptional activation complex. J
Biol Chem. 278:21232–21239. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Peng G and Liu Y: Hypoxia-inducible
factors in cancer stem cells and inflammation. Trends Pharmacol
Sci. 36:374–383. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Oh SJ, Shin JH, Kim TH, Lee HS, Yoo JY,
Ahn JY, Broaddus RR, Taketo MM, Lydon JP, Leach RE, et al:
β-Catenin activation contributes to the pathogenesis of adenomyosis
through epithelial-mesenchymal transition. J Pathol. 231:210–222.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kechavarzi B and Janga SC: Dissecting the
expression landscape of RNA-binding proteins in human cancers.
Genome Biol. 15:R142014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gerstberger S, Hafner M and Tuschl T: A
census of human RNA-binding proteins. Nat Rev Genet. 15:829–845.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Galante PA, Sandhu D, de Sousa Abreu R,
Gradassi M, Slager N, Vogel C, de Souza SJ and Penalva LO: A
comprehensive in silico expression analysis of RNA binding proteins
in normal and tumor tissue: Identification of potential players in
tumor formation. RNA Biol. 6:426–433. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Adelman K and Lis JT: Promoter-proximal
pausing of RNA polymerase II: Emerging roles in metazoans. Nat Rev
Genet. 13:720–731. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li J, Liu Y, Rhee HS, Ghosh SK, Bai L,
Pugh BF and Gilmour DS: Kinetic competition between elongation rate
and binding of NELF controls promoter proximal pausing. Mol Cell.
50:711–722. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Pagano JM, Kwak H, Waters CT, Sprouse RO,
White BS, Ozer A, Szeto K, Shalloway D, Craighead HG and Lis JT:
Defining NELF-E RNA binding in HIV-1 and promoter-proximal pause
regions. PLoS Genet. 10:e10040902014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Dang H, Pomyen Y, Martin SP, Dominguez DA,
Yim SY, Lee JS, Budhu A, Shah AP, Bodzin AS and Wang XW:
NELFE-dependent MYC signature identifies a unique cancer subtype in
hepatocellular carcinoma. Sci Rep. 9:33692019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Deng Y, Yao L, Chau L, Ng SS, Peng Y, Liu
X, Au WS, Wang J, Li F, Ji S, et al: N-Myc downstream-regulated
gene 2 (NDRG2) inhibits glioblastoma cell proliferation. Int J
Cancer. 106:342–347. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chen XL, Lei L, Hong LL and Ling ZQ:
Potential role of NDRG2 in reprogramming cancer metabolism and
epithelial-to-mesenchymal transition. Histol Histopathol.
33:655–663. 2018.
|
|
46
|
Kim MJ, Lim J, Yang Y, Lee MS and Lim JS:
N-myc downstream-regulated gene 2 (NDRG2) suppresses the
epithelial-mesenchymal transition (EMT) in breast cancer cells via
STAT3/Snail signaling. Cancer Lett. 354:33–42. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Moradi Monfared M, Alizadeh Zarei M,
Rafiei Dehbidi G, Behzad Behbahani A, Arabsolghar R and Takhshid
MA: NDRG2 regulates the expression of genes involved in epithelial
mesenchymal transition of prostate cancer cells. Iran J Med Sci.
44:118–126. 2019.PubMed/NCBI
|
|
48
|
Yang CL, Zheng XL, Ye K, Ge H, Sun YN, Lu
YF and Fan QX: NDRG2 suppresses proliferation, migration, invasion
and epithelial-mesenchymal transition of esophageal cancer cells
through regulating the AKT/XIAP signaling pathway. Int J Biochem
Cell Biol. 99:43–51. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kim YJ, Yoon SY, Kim JT, Song EY, Lee HG,
Son HJ, Kim SY, Cho D, Choi I, Kim JH and Kim JW: NDRG2 expression
decreases with tumor stages and regulates TCF/beta-catenin
signaling in human colon carcinoma. Carcinogenesis. 30:598–605.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Xu X, Li J, Sun X, Guo Y, Chu D, Wei L, Li
X, Yang G, Liu X, Yao L, et al: Tumor suppressor NDRG2 inhibits
glycolysis and glutaminolysis in colorectal cancer cells by
repressing c-Myc expression. Oncotarget. 6:26161–26176. 2015.
View Article : Google Scholar : PubMed/NCBI
|