Introduction
The use of charged particles, such as protons or
carbon ions, in radiotherapy offers several advantages compared
with conventional radiotherapy with X-rays. One of these advantages
is the superior dose deposition due to the physical properties of
the particles (1,2). Therefore, the healthy tissue
surrounding the tumour receive a lower radiation dose and,
consequently, fewer side effects are expected. In addition, carbon
ions have increased relative biological effectiveness (RBE), which
indicates that these particles are more effective in inducing DNA
damage, cell cycle arrest and cell death compared with X-rays
(1,3,4). Due
to these advantages, the number of cancer patients receiving carbon
ion therapy, particularly for radioresistant tumours, is increasing
(5).
Breast cancer is the most common cancer in women
worldwide, and the majority of these patients are treated with
surgery and/or radiotherapy (6).
Radiotherapy-related long-term effects include cardiopulmonary
toxicity and secondary malignancies (7). Particle therapy may benefit breast
cancer patients by decreasing the exposure of the heart and lungs,
thereby decreasing cardiotoxicity (8). In Japan, carbon ion therapy has
already been used to treat stage I breast cancer (9). The rationale for using carbon ions in
early-stage breast cancer is the fact that radical conventional
radiotherapy is associated with an inferior cosmetic outcome,
unsatisfactory results and technical difficulties, which are not
observed with carbon ions (9). In
Europe, however, breast cancer has only been treated with proton
therapy and in only few particle therapy centres (10).
Despite the fact that primary breast cancer is
associated with a good local control rate, 25-50% of breast cancer
patients will ultimately develop metastases (11), which is considered to be an
incurable disease state (12). The
migration and invasion of cancer cells is a complicated process
that involves the interplay of different molecular pathways,
including the Hedgehog (Hh) signalling pathway. This is an
important developmental pathway that has also been implicated in
the growth and progression of different tumour types, including
breast cancer (13-15). Several studies have observed
overexpression of the Hh pathway genes sonic Hedgehog (SHH),
glioma-associated oncogene (GLI)1 and patched 1 (PTCH1) in breast
cancer cells (15,16). Moreover, in a study by Cui et
al, upregulation of SHH was observed in early-stage breast
cancer, which may be an indication that Hh pathway activation is an
early event in breast cancer development (17). Several in vitro and in
vivo studies have also reported that active Hh signalling is
associated with increased migration and invasion of different types
of cancer cells (18-23), while inhibition of GLI1/2 has been
found to decrease the migration of ovarian cancer and
medulloblastoma cells (24,25).
In addition, the Hh pathway has also been implicated in resistance
to photon radiation (18,26,27).
Hence, there is an increasing interest in targeting
the Hh pathway for different tumour types. Furthermore, combining
Hh inhibitors with radiation therapy may sensitise cancer cells to
radiation. Several studies have already investigated this treatment
strategy with conventional radiotherapy, mostly using inhibitors
that target the Hh pathway further upstream, such as smoothened
(SMO) inhibitors (28-35). However, resistance to SMO
inhibitors has already been observed (36-38).
Therefore, targeting the Hh pathway further downstream, at the
level of the GLI1/2 transcription factors, appears to be a more
promising approach (29,39,40).
Thus far, the combination of targeted drugs with
particle therapy has not been explored to the same extent as with
X-rays. One example is the combination of poly(ADP-ribose)
polymerase inhibitors with carbon ion radiation, which has
demonstrated an increased sensitivity of cancer cells to particle
radiation (41). However, to the
best of our knowledge, the combination of particle therapy with Hh
inhibitors has not been previously investigated.
The aim of the present study was to compare the
effect of X-rays and carbon ions on cell survival, migration and Hh
pathway gene expression in breast cancer cells, and determine how
this gene expression may be associated with cellular behaviour,
such as migration. In addition, the potential of the Hh inhibitor
GANT61 as an effective modulator of cancer cell radiosensitivity
and migration potential was explored.
Materials and methods
Cell line and drug preparation
Human breast cancer cells (MCF-7) were obtained from
the American Type Culture Collection (ATCC). The MCF-7 cells are
breast cancer cells derived from a pleural effusion (metastatic
site) (42). The MCF-7 cell line
was selected because different components of the Hh signalling
pathway are expressed in these cells (data not shown). The cells
were cultured in Eagle's minimal essential medium (ATCC)
supplemented with 10% foetal bovine serum (FBS), and maintained in
a humidified incubator (37°C and 5% CO2). Mycoplasma
testing was performed periodically.
The GLI1/2 inhibitor GANT61 (Selleck Chemicals) was
selected to inhibit the Hh pathway. Two concentrations of GANT61 (1
and 10 µmol/l) were dissolved in dimethyl sulphoxide (DMSO),
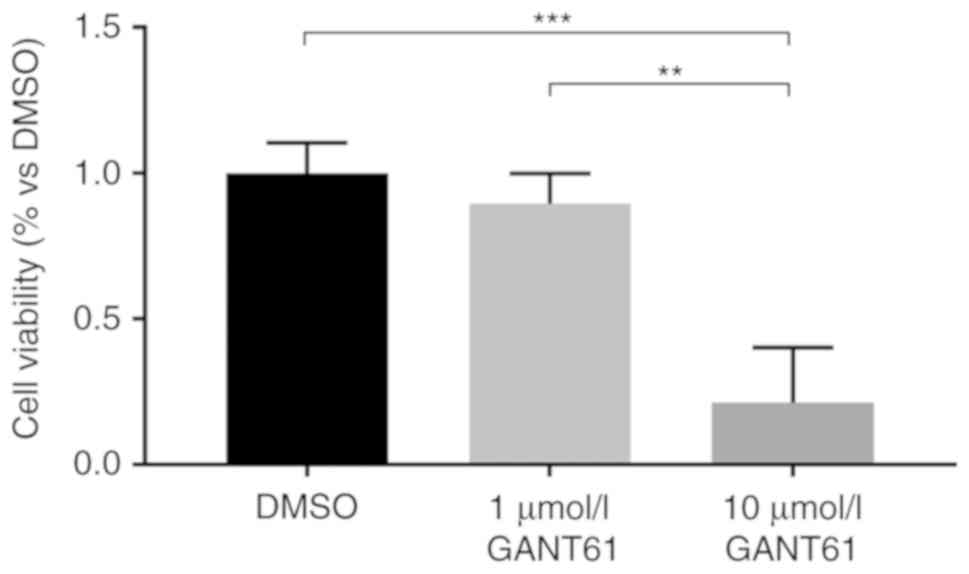
aliquoted and stored at −20°C. Only data relating to 10
µmol/l GANT61 will be discussed, since this was the only
concentration where significant changes were observed compared with
the DMSO-treated control (Fig. 1).
Control cells were treated with the drug solvent, with a final
dilution of DMSO in the cell medium of 1:1,000. The drug was added
72 h prior to irradiation with either X-rays or carbon ions, based
on previous studies using GANT61 (29,39).
The drug was present in the medium during irradiation until the
cells had to be processed for the different assays. After
processing of the cells for the different assays, the drug was
removed.
Cytotoxicity screening
The effect of the Hh inhibitor GANT61 on MCF-7 cell
cytotoxicity was assessed by means of a sulphorhodamine B (SRB)
assay. MCF-7 cells were seeded in 96-well plates (2,500
cells/well). After 72 h of treatment with DMSO (1:1,000), 1
µmol/l GANT61 or 10 µmol/l GANT61 cells were fixed
with trichloric acid and stained with 0.4% SRB (Sigma Aldrich;
Merck KGaA) in 1% acetic acid. The cells were washed five times
with 1% acetic acid, after which time 10 µmol/l Tris-Base
was added. The absorbance was measured at 570 nm with a CLARIOstar
microplate reader (BMG Labtech).
X-irradiation
To irradiate MCF-7 cells with X-rays, the Xstrahl
320 kV generator (250 kV, 12 mA, 3.8 mm Al and 1.4 mm Cu) was used
at the SCK•CEN facility (Mol, Belgium). Cells were irradiated at
room temperature with different X-ray doses (0, 0.25, 0.5, 2, 4 and
6 Gy) at a calculated dose rate of 0.5 Gy/min. The culture medium
was replaced prior to irradiation in a horizontal position
(perpendicular to the vertical X-ray beam). The irradiation of
biological samples was performed with the Xstrahl 320 kV tube,
using the ISO 4037 H-250 beam quality in the following
configuration: Inherent filtration: 0.21 mm Al; additional
filtration: 3.8 mm Al + 1.4 mm Cu + dose-area product meter; tube
voltage: 250 kV; tube current: 12 mA; distance: 50 cm. A Farmer
NE2571 chamber was used for dosimetry measurements. The calibration
in terms of Kair of this chamber was based on the
primary standards of the Physikalisch Technische Bundesanstalt,
Germany.
Carbon ion irradiation
Carbon ion beam time was granted by the iPAC
committee of the Grand Accélérateur National d'Ions Lourds (GANIL).
The cells were transported from Belgium to France by car, inside a
transportable incubator, in completely filled flasks. Upon arrival,
fresh culture medium was added, and the cells were placed in a
humidified incubator overnight. Four days prior to irradiation, the
cells were seeded in 12.5-cm2 flasks (Falcon, VWR).
Immediately prior to irradiation, the cell culture flasks were
completely filled with medium due to the vertical position
(perpendicular to the horizontal carbon ion beam) during
irradiation. Irradiation of the cells was performed at room
temperature, with an initial carbon ion beam energy of 95 and 26-27
MeV/u on target. A poly-methylmethacrylate degrader of 16.9 mm was
used to obtain a linear energy transfer (LET) of 73 keV/µm.
The carbon ion beam had a range in water of 2.5-2.4 mm. The doses
applied were 0, 0.25, 0.5, 1, 2, 3 and 4 Gy, with a calculated dose
rate of ~2 Gy/min. Carbon ion dosimetry was performed as previously
described (43).
Colony survival assay
Immediately after irradiation of the cells in the
culture flasks, the cells were trypsinized and seeded in triplicate
in 6-well plates at low densities (ranging from 800 cells/well when
exposed to the lowest dose to 24.000 cells/well when exposed to the
highest dose). At 14 days after seeding, the cells were fixed with
6% glutaraldehyde and 0.5% crystal violet solution (Sigma-Aldrich;
Merck KGaA). Colonies of >50 cells were counted with a ColCount
colony counter (Oxford Optronix) and used to calculate the
surviving fraction (SF) with the linear quadratic model, as
previously described (44). The
RBE was calculated at 10% survival by dividing the dose of X-rays
at SF10 by the dose of carbon ions at SF10.
Plating efficiency between the DMSO-treated cells and the
GANT61-treated cells differed only slightly (max. 10%
difference).
Gene expression analysis
RNA was isolated at 8 and 24 h after irradiation
using the Allprep DNA/RNA kit (Qiagen) in accordance with the
manufacturer's protocol. The RNA concentration was measured using
DropSense 16 (Trinean), and RNA quality was controlled using the
Agilent 2100 Bioanalyzer (Agilent Technologies, Inc.).
Subsequently, RNA was reverse-transcribed to cDNA using the
GoScript™ Reverse Transcription System (Promega Corporation).
Finally, qPCR reactions were performed by the 7500 Fast Real Time
PCR System (Applied Biosystems; Thermo Fisher Scientific, Inc.)
using MESAGreen qPCR Master Mix Plus for SYBR® Assay Low
ROX (Eurogentec). The following thermocycling conditions were used:
5 min at 95°C followed by 40 cycles of 3 sec at 95°C and 45 sec at
60°C. The primers for the Hh pathway genes [SHH, PTCH1, SMO, GLI1,
GLI2, GLI3 and suppressor of fused homolog (SUFU)] and target genes
of the Hh pathway [cyclin D1 (CCND1), vascular endothelial growth
factor (VEGF)A, B-cell lymphoma (BCL)-2, SNAIL1 and matrix
metallopeptidase (MMP)9] are listed in Table I. Specific target genes of the Hh
pathway were selected based on their involvement in cell cycle
regulation (CCND1), apoptosis (BCL-2), epithelial-to-mesenchymal
transition (EMT; VEGFA and MMP-9) or migration (SNAIL). The gene
expression was normalized to two housekeeping genes (GAPDH and
HPRT1) and calculated using the Pfaffl method (45). The Pfaffl method was selected, as
it takes into account the individual reaction efficiencies of the
housekeeping genes and the target genes (45).
 | Table IPrimer sequences for target
genes. |
Table I
Primer sequences for target
genes.
| Gene | Full gene name | Gene ID
(Entrez) | Forward 5'-3' | Reverse 5′-3′ | Length of
amplicon |
|---|
| GAPDH | Glyceraldehyde
3-phosphate dehydrogenase | 2597 |
CCATCTTCCAGGAGCGAG |
TGAAGACGCCAGTGGAC | 212 |
| HPRT |
Hypoxanthine-guanine
phosphoribosyltransferase | 3251 |
TCAGGCAGTATAATCCAAAGATGGT |
AGTCTGGCTTATATCCAACACTTCG | 83 |
| SHH | Sonic Hedgehog | 6469 |
CCCGACATCATATTTAAGGATGAAGA |
AAGCGTTCAACTTGTCCTTAC | 81 |
| PTCH1 | Patched 1 | 5727 |
AAACAGGTTACATGGATCAGATAATAG |
CCCTTCCCAGAAGCAGT | 80 |
| SMO | Smoothened | 6608 |
ACCTATGCCTGGCACACTTC |
GTGAGGACAAAGGGGAGTGA | 109 |
| GLI1 | Glioma-associated
oncogene 1 | 2735 |
AATGCTGCCATGGATGCTAGA |
GAGTATCAGTAGGTGGGAAGTCCATAT | 105 |
| GLI2 | Glioma-associated
oncogene 2 | 2736 |
GCCCTCACCTCCATCAAT |
TGTTCTGGTTGGTGTCACT | 75 |
| GLI3 | Glioma-associated
oncogene 3 | 2737 |
GTGCTCCACTCGAACAGA |
TCCAGGACTTTCATCCTCATTAGA | 75 |
| SUFU | Suppressor of
fused | 51684 |
CCATGAGTTTACAGGAACAGAT |
GTGCCAAGCCCTGCATTA | 124 |
| CCND1 | Cyclin D1 | 595 |
TGTAGTCACTTTATAAGTCATTG |
CTTCAGCCATGAATAAGG | 80 |
| VEGFA | Vascular
endothelial growth factor | 7422 |
GCTACTGCCATCCAATCGAG |
CTCTCCTATGTGCTGGCCTT | 208 |
| BCL-2 | B-cell lymphoma
2 | 596 |
GGATGCCTTTGTGGAACTGT |
AGCCTGCAGCTTTGTTTCAT | 235 |
| SNAIL | Snail | 6615 |
CCAATCGGAAGCCTAACTAC |
AGAGTCCCAGATGAGCATTG | 151 |
| MMP9 | Matrix
metallopeptidase | 4318 |
GAACCAATCTCACCGACAGG |
GCCACCCGAGTGTAACCATA | 66 |
Boyden chamber assay
The Boyden chamber assay (Neuroprobe) was used to
investigate the migratory potential of cancer cells after
irradiation. Briefly, cells were seeded in serum-free medium in the
upper wells of the Boyden chamber 24 h after irradiation. The lower
wells were filled with medium containing 0.1% FBS. The upper wells
were separated from the lower wells by a polycarbonate membrane
with 8-µm pores (Neuroprobe). The membrane was precoated
with collagen I (Gibco; Thermo Fisher Scientific, Inc.). After the
cells had been allowed to migrate for 16 h, they were fixed with
methanol and stained with 0.5% crystal violet solution. Images were
captured with a Leica MZ12 microscope (Leica Microsystems, Ltd.),
and the migrated cells were counted in five separate fields using
Image J software (National Institutes of Health).
Statistical analysis
The experiments were repeated at least three times
for X-ray experiments and at least twice for carbon ion
experiments. More specifically, for carbon ion irradiation, the
colony survival assay was performed in three different experiments
with two technical repeats per dose. The Boyden chamber assay was
performed twice with three technical repeats per dose. The gene
expression experiments were performed in two independent beam times
with four technical repeats per dose. The results are expressed as
means ± standard error of the mean. Statistical analysis was
performed using GraphPad Prism 7 (GraphPad Software, Inc.). For all
experiments, one-way ANOVA with Tukey's multiple comparison test or
a two-tailed Student's t-test was performed. P-values ≤0.05 were
considered to indicate statistically significant differences.
Results
X-ray and carbon ion radiation decrease
the migration of MCF-7 cells
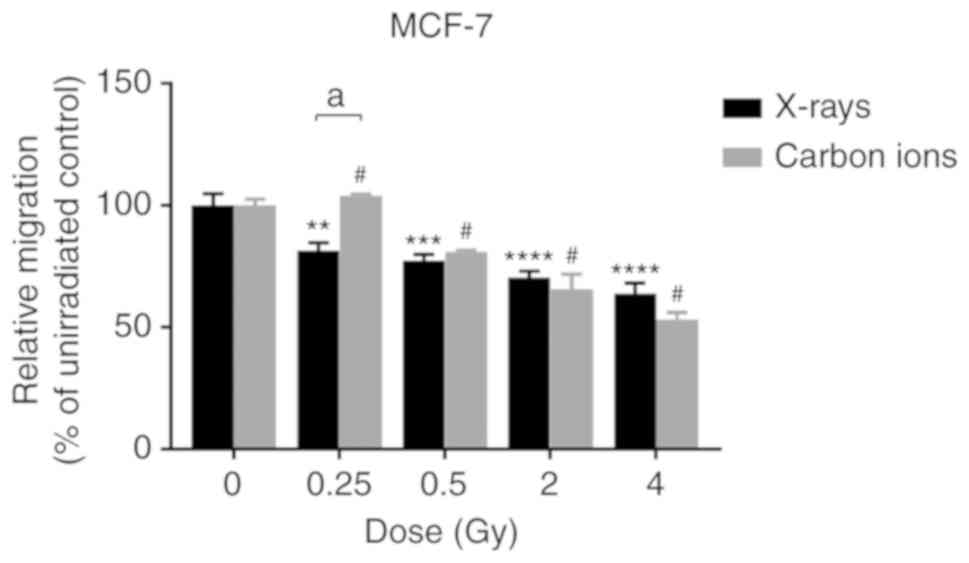
Both X-rays and carbon ions were able to induce a
dose-dependent decrease in the migration of MCF-7 cells (Fig. 2). No significant differences were
observed between the two radiation types, except at 0.25 Gy, where
X-rays decreased cell migration significantly more notably compared
with carbon ions.
Each radiation type induces a specific
gene expression profile of the Hh pathway and its target genes
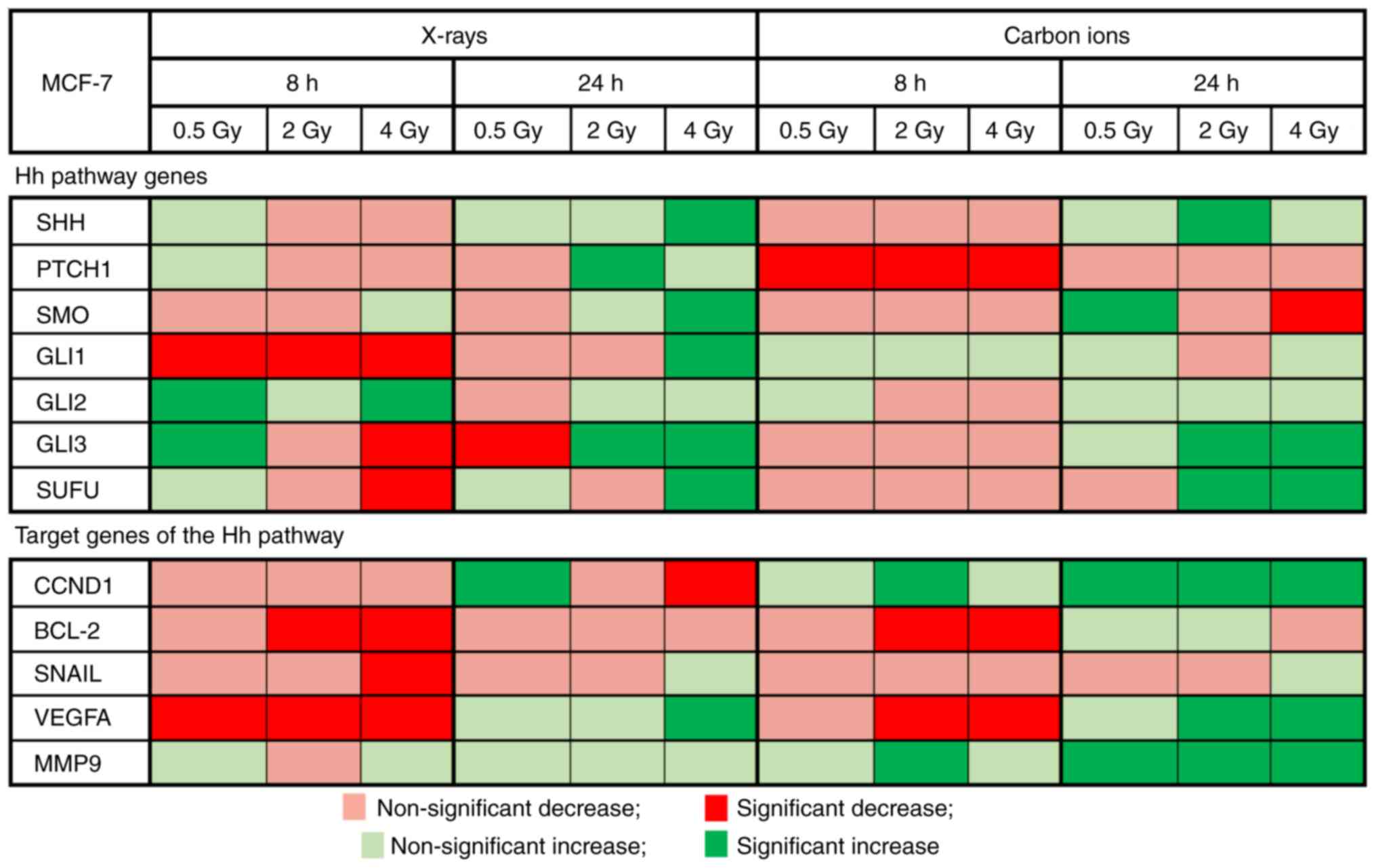
Altered expression of the investigated Hh pathway
genes and certain selected target genes was observed after
irradiation with either X-rays or carbon ions when compared with
non-irradiated controls (Fig. 3).
In addition, both irradiation types induced their own specific
expression profile of the Hh pathway and its target genes.
Significant downregulation of GLI1 was observed at 8 h after X-ray
radiation (P<0.0001). In addition, GLI3 also exhibited a
persistent downregulation (until 24 h after a 4-Gy dose of X-rays).
Carbon ions significantly decreased PTCH1 expression at 8 h after
exposure (0.5 Gy: P=0.0005; 2 Gy: P=0.0024; and 4 Gy: P=0.002).
GLI3 and SUFU, both of which are Hh pathway inhibitors, exhibited a
significant upregulation at 24 h after a 2- and 4-Gy dose of carbon
ions (GLI3: P<0.0001; SUFU 2 Gy: P=0.0003 and 4 Gy: P=0.01).
For the Hh pathway target genes BCL-2 (2 and 4 Gy)
and VEGFA (all doses), X-rays significantly downregu-lated their
expression at 8 h after irradiation (BCL-2 2 Gy: P=0.0019 and 4 Gy:
P<0.0001; VEGFA 0.5 Gy: P=0.03, 2 Gy: P=0.0026 and 4 Gy:
P=0.0028). Carbon ions (2 and 4 Gy) also significantly
downregulated BCL-2 and VEGFA expression at 8 h after exposure
(BCL-2 2 Gy: P=0.001 and 4 Gy: P=0.0007; VEGFA 2 Gy: P=0.02 and 4
Gy: P=0.001). At 24 h after carbon ion irradiation, an upregulation
in the target genes was observed, particularly CCND1, VEGFA and
MMP9, while both up- and downregulation were observed after X-ray
irradiation.
GANT61 does not affect the sensitivity of
MCF-7 cells to X-ray or carbon ion radiation
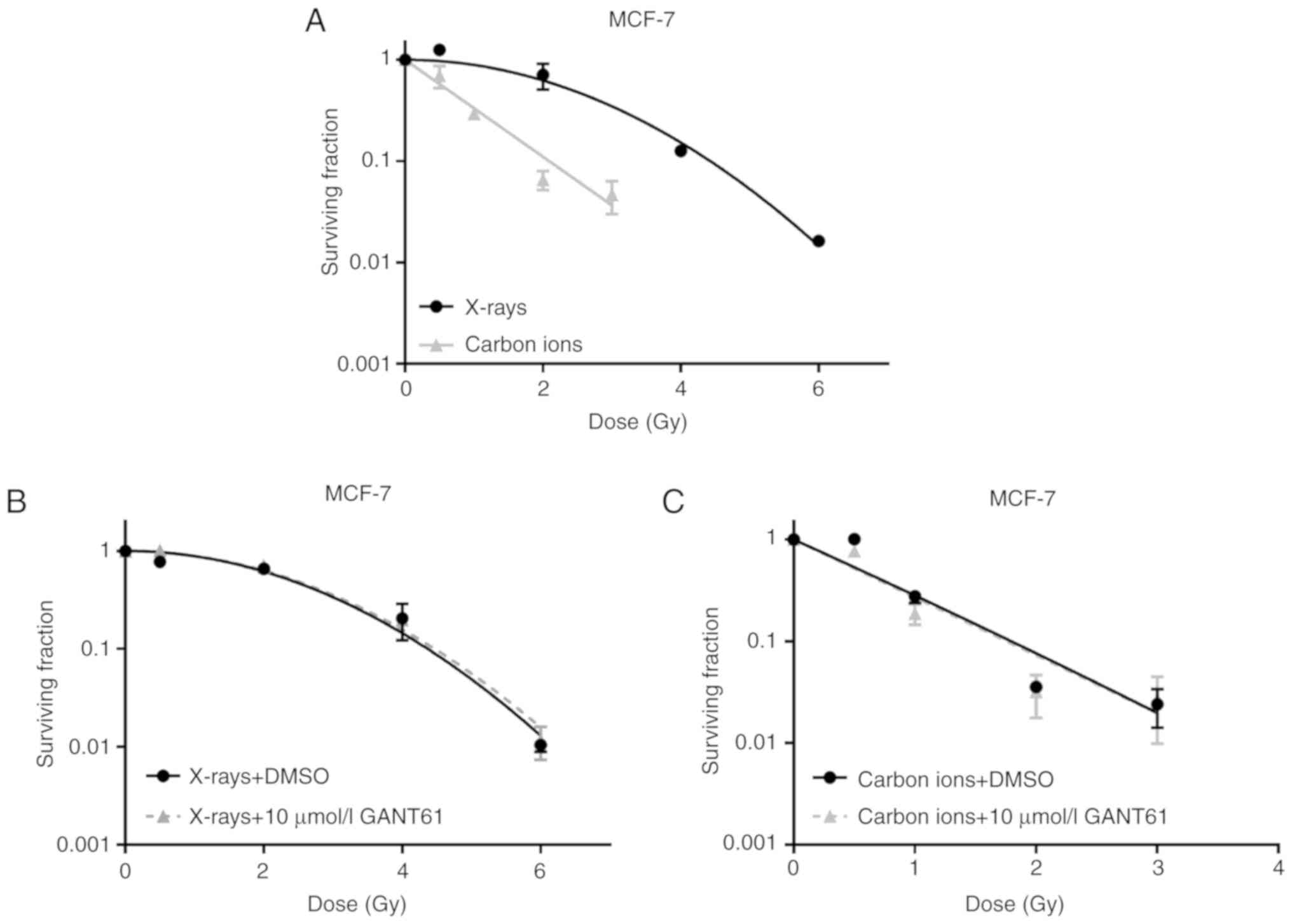
A dose-dependent decrease in MCF-7 cell survival was
observed after irradiation with either X-rays or carbon ions
(Fig. 4A). Carbon ions, however,
were more effective than X-rays in terms of lowering the surviving
fraction, with a calculated RBE of 2.12 for carbon ions at 10%
survival (RBE10).
The combination of the Hh pathway inhibitor GANT61
with different doses of X-rays or carbon ions was not able to exert
a radiosensitising effect on MCF-7 cells (Fig. 4B and C). No significant differences
in the dose enhancement factor at 10% survival (DEF 0.1) were
observed, based on a DEF 0.1 of 0.97 and 1.02 for X-rays and carbon
ions, respectively. The plating efficiencies were calculated and
were found to be 0.71 for the combination of X-rays with 10
µmol/l GANT61 and 0.64 for the combination of carbon ions
with 10 µmol/l GANT61.
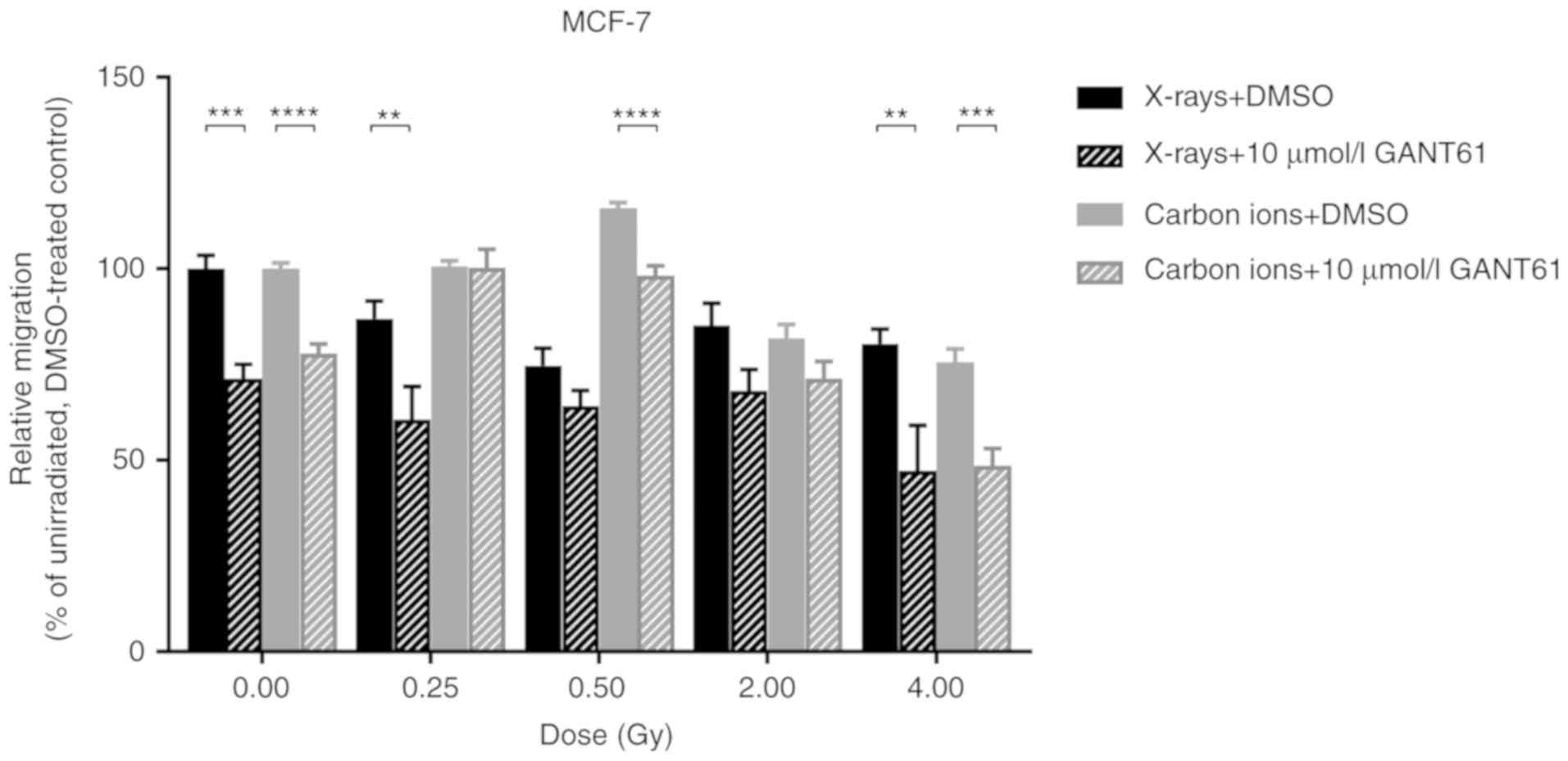
Combining radiation with GANT61 affects
migration differently compared with either radiation type
alone
The combination of GANT61 with X-rays was able to
significantly suppress the migration of MCF-7 cells compared with
X-rays alone, at a dose of 0.5 (P=0.049) and 4 Gy (P=0.0087)
(Fig. 5). However, GANT61 in
combination with carbon ions was only able to significantly
decrease the migration of MCF-7 cells at a dose of 0.5
(P<0.0001) and 4 Gy (P=0.0007) (Fig. 5).
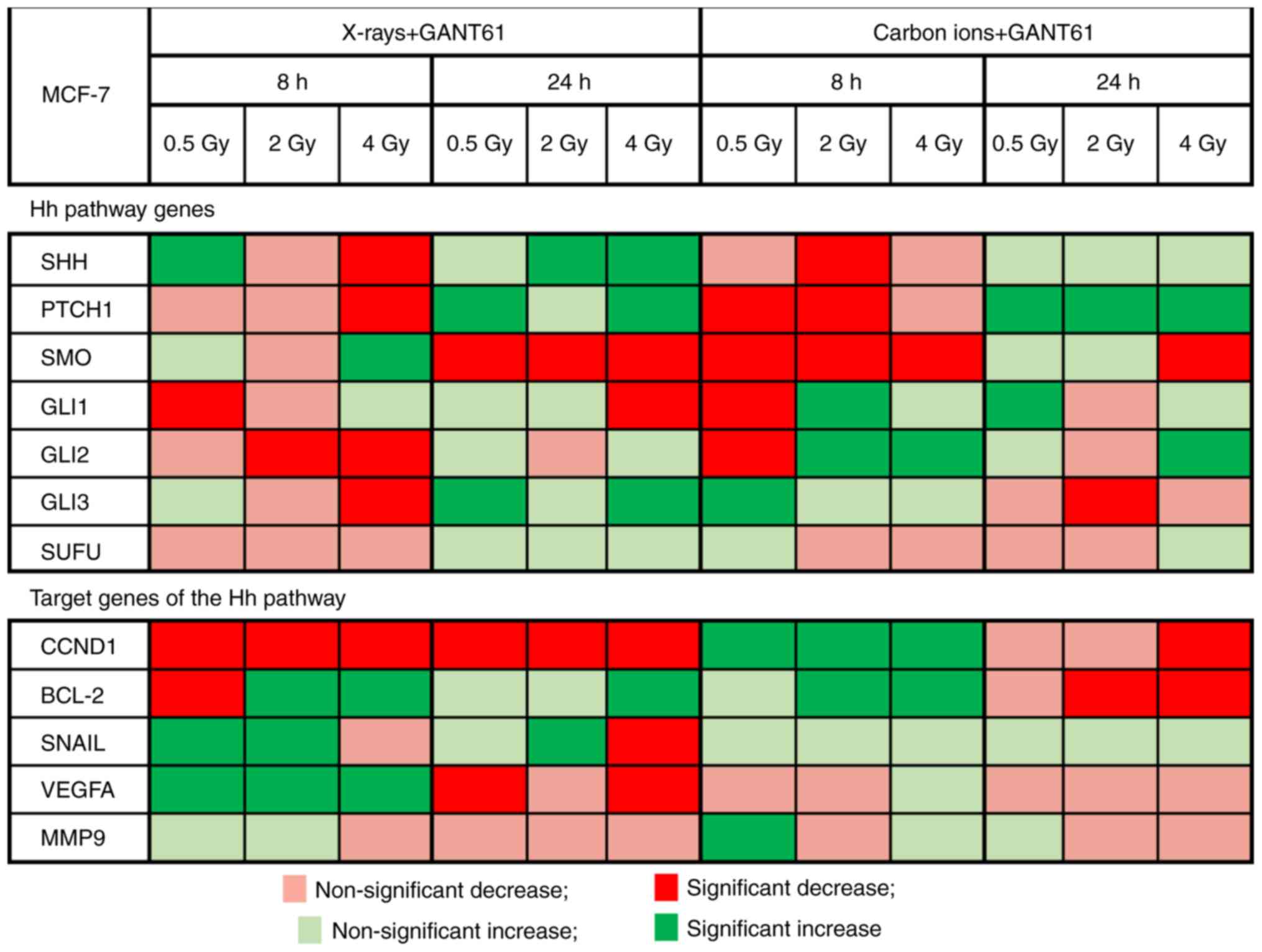
GANT61 induces a differential expression
in Hh pathway and Hh target genes in combination with X-rays or
carbon ions
The combination of GANT61 with X-rays was able to
induce more significant changes in the expression of the Hh target
genes compared with the combination of GANT61 with carbon ions
(Fig. 6; control: DMSO-treated
cells). The majority of the Hh pathway genes exhibited a
significant (SHH: P=0.04, PTCH1: P=0.01, GLI2: P≤0.0001 and GLI3
P=0.0005) downregulation 8 h after 4 Gy of X-rays and GANT61, apart
from SMO, which exhibited a significant upregulation (P=0.002). At
24 h after the combination of X-rays and GANT61, a significant
downregulation was observed only for SMO, whereas the remaining
genes were upregulated. Carbon ions were able to significantly
downregulate SHH at 8 h post-irradiation (2 Gy: P<0.0001), PTCH1
(0.5 Gy: P<0.0001 and 2 Gy: P=0.0002) and SMO (0.5 Gy: P=0.03, 2
Gy: P=0.0002 and 4 Gy: P<0.0001), whereas upregulation of these
genes was observed at 24 h after exposure.
One of the target genes of the Hh pathway, CCND1,
exhibited a significant downregulation, which persisted for up to
24 h after exposure to X-rays and GANT61 (at 8 h 0.5 Gy: P=0.02, 2
Gy: P=0.01 and 4 Gy: P=0.01; at 24 h 0.5 Gy: P=0.04, 2 Gy: P=0.0003
and 4 Gy: P=0.003). By contrast, VEGFA exhibited a significant
upregulation at 8 h after X-ray and GANT61 exposure (all doses
P<0.0001), whereas at 24 h after exposure a significant
downregulation (except at 2 Gy) was observed (0.5 Gy: P=0.002 and 4
Gy: P=0.0007). The combination of carbon ions and GANT61 was able
to significantly upregulate CCND1 (0.5 Gy: P=0.01, 2 Gy: P=0.04 and
4 Gy: P=0.01) and BCL-2 (2 Gy: P=0.0001 and 4 Gy: P<0.0001)
expression (except at 0.5 Gy) 8 h after exposure, whereas a
downregulation was observed at 24 h (except for BCL-2 at 4 Gy).
Discussion
Carbon ions are superior at sparing normal tissues
due to their physical characteristics, and are also known to have a
higher biological effectiveness compared with X-rays (1). As such, carbon ions may be a
promising treatment option for breast cancer patients. Therefore,
the aim of the present study was to investigate whether carbon ions
are superior to X-rays in decreasing breast cancer cell survival
and migration and modulating Hh pathway gene expression.
Furthermore, we aimed to investigate the potential modulatory
effect of the Hh inhibitor GANT61 in combination with different
radiation types for these end points.
The migration of cancer cells after radiotherapy
remains a subject of debate, since contradictory results have been
reported following photon irradiation: Certain studies have
observed an increase in cell migration after radiation, while
others have demonstrated a decrease in migration (46). For particle radiation, more
specifically carbon ions, cell migration has been shown to decrease
(47,48). In the present study, a
dose-dependent decrease in migration was observed for both X-rays
and carbon ions. However, no differences were observed between
carbon ions and X-rays. This is in contrast to a study published by
Matsumoto et al, where a significant difference in melanoma
cell migration was observed between X-ray and carbon ion
irradiation (49). The lack of
difference in migration after X-ray or carbon ion irradiation
observed in the present study may be explained by the similar
expression profile of the migration-related gene VEGFA after both
X-ray and carbon ion irradiation. In addition, the presence of a
cancer stem cell (CSC) subpopulation in the MCF-7 cell line may
account for the lack of difference. Moreover, it is known that CSCs
are characterised by an increased resistance to radiotherapy and
play an important role in treatment failure and recurrence
(metastasis) (50). By contrast, a
study by Zhang et al reported increased migration of MCF-7
cells after X-ray radiation (51).
However, these cells were irradiated with a total dose of 20 Gy
administered in daily fractions of 1 or 2 Gy, whereas a single
irradiation dose was used in the present study. Although different
survival levels are observed after X-ray and carbon ion irradiation
(Fig. 4), migration is not
affected in a similar manner (Fig.
2). This may be attributed to the fact that live cells were
only counted prior to seeding the MCF-7 cells in the Boyden
chamber. Therefore, although more cells may die after carbon ion
irradiation, the difference that may exist between X-rays and
carbon ions is at least partly filtered out. In addition, both
assays were assessed at different time points after 14 days (colony
survival assay) and 24 + 16 h (Boyden chamber assay). Considering
the fact that different responses are observed after 0.5 Gy carbon
ions, as shown in Fig. 2
(decreased migration) and Fig. 5
(increased migration), DMSO may exert an effect on MCF-7 cells,
although we do not consider this to be significant. It has been
previously reported that 0.5% DMSO may exert radioprotective
effects (52,53); however, at the concentration used
herein (1:1,000), this would not be expected to have a major
impact. In addition, both experiments [radiation alone (Fig. 2); radiation and GANT61 (Fig. 5)] were performed separately, which
may account for the differences observed. For the radiation alone
experiments, no DMSO was used or added to the medium, whereas for
the experiments using the combination of radiation and GANT61, DMSO
was added to the medium of the different conditions.
Furthermore, the present study demonstrated a time-
and radiation type-dependent response in the gene expression of the
Hh pathway and selected Hh target genes that are specifically
involved in cell cycle regulation, apoptosis or cell migration.
While the significantly altered genes mostly exhibited a
significantly decreased expression at 8 h after irradiation for
both radiation types, the majority of the genes exhibited a
significantly increased expression at 24 h after irradiation with
either X-rays or carbon ions. In a previous study by Fushimi et
al, a significantly increased CCND1 expression at 24 h was
observed after carbon ions (4 Gy), but not after X-rays (4 Gy), in
oral squamous cell carcinoma cells (54). By contrast, a significant
downregulation in CCND1 was observed after 4 Gy of X-rays (54). In addition, as the combination of
X-rays and GANT61 differentially affected CCND1 expression compared
with the combination of carbon ions and GANT61, it may be of
interest to also investigate the effect of both treatments on the
cell cycle. Overall, radiation induced a variable response in the
gene expression of the Hh pathway genes and its target genes.
Moreover, the observed changes in gene expression after the
different treatments indicate that the investigated genes may not
play a principal role in cell migration. In future experiments, it
may be interesting to focus on the protein expression of the genes
to achieve a better understanding of how the cell may be affected
in functional terms.
Although the role of VEGFA and MMP9 is not
specifically in migration, they do play a role in the entire EMT
process. Together with the expression of SNAIL, these three genes
provide an overview of the different EMT regulators and migration,
possibly indicating in what way the EMT process is directed
(pro/anti-migration). A significantly increased expression of VEGF
and MMP9 was observed in MCF-7 cells 24 h after carbon ion
exposure. This is consistent with a study by Kamlah et al,
in which the gene expression of VEGF was increased in lung cancer
cells 24 h after a 2-Gy dose of carbon ions (55). Gan et al reported an
increased expression of SNAIL1 at 24 h after X-irradiation
(56), whereas in the present
study only non-significant changes in SNAIL expression were
observed 24 h after X-ray exposure. In contrast to these
migration-related gene expression data, MCF-7 cells exhibited a
dose-dependent decrease in migration after carbon ion exposure.
This may, however, be explained by the fact that we only
investigated some of the genes involved in migration and invasion.
For example, a microarray study was performed on PC3 cells, which
also demonstrated that several other genes related to cell
migration were significantly more downregulated after carbon ion
irradiation rather than after X-rays (57). Therefore, it would be of interest
to expand the panel of genes involved in migration and invasion,
including ZEB1 and 2, N-cadherin, E-cadherin, MMP-2, fibronectin 1
and nexilin.
At present, only a few studies have been performed
that compare the effect of X-rays and carbon ions on breast cancer
cell survival (58,59). Our results are consistent with
these studies, showing that carbon ions are more effective compared
with X-rays in decreasing MCF-7 cell survival (RBE ~2 at 10%
survival for our data). Similar RBE values have also been observed
for other cell lines treated with carbon ions with similar LET
values (~70 keV/µm) (60,61).
In addition, PTCH1 and GLI1, two indicators of Hh pathway activity,
did not display significant changes in gene expression up to 24 h
after irradiation with either X-rays or carbon ions. The Hh pathway
is indirectly involved in cell survival, as some of its target
genes are involved in cell survival. However, our data demonstrated
that the Hh pathway, due to its low activity, only plays a minor
role in the survival of MCF-7 cells in response to either X-ray or
carbon ion exposure.
Combining radiation therapy with targeted therapies
may decrease radiation-induced side-effects, as these drugs may
sensitise cancer cells to radiation, thereby making it possible to
use lower radiation doses to achieve the same tumour control rate.
Thus far, only a few targeted therapies have been tested in
combination with particle irradiation in vitro. The
combination of radiation with the Hh inhibitor GANT61 has only been
studied in vitro in combination with photon radiation in a
limited number of cancer types. To the best of our knowledge, this
is the first study to investigate the effect of X-ray and carbon
ion radiation in combination with GANT61 on breast cancer cell
survival. We observed that neither the combination of GANT61 with
X-rays nor with carbon ions was able to sensitise MCF-7 cells. This
is in contrast to previous studies, where GANT61 was able to
sensitise renal cancer and prostate cancer cells to X-irradiation
(29,40). A possible explanation as to why
there was no observed radiosensitising effect of GANT61 may be
because GANT61 at 10 µmol/l induces cytotoxicity in ~80% of
MCF-7 cells (Fig. 1). Therefore,
the 20% of MCF-7 cells that survived GANT61 treatment (resistant
MCF-7 cells) were studied, rather than the entire cell population.
Although the majority of previous studies used a GANT61
concentration of ~10 µmol/l to treat cancer cells, this
concentration may still be too low to reveal changes in cell
radiosensitivity. In addition, the lack of a radiosensitising
effect may be due to the fact that, 24 h after the combination of
X-rays or carbon ions with GANT61, the genes of the Hh pathway were
not significantly downregulated (Fig.
6). This may indicate that the cancer cells would be able to
proliferate in a manner similar to the cells without prior GANT61
treatment and, therefore, no differences were observed between the
two treatments. For future experiments, it may be of interest to
analyse the Hh pathway gene expression at 72 and 72 h + 24 h of the
GANT61 treatment alone.
In conclusion, the present study demonstrated that
carbon ions are more effective in decreasing MCF-7 cell survival
compared with X-rays, while MCF-7 cell migration was similarly
affected by both radiation types. Furthermore, X-rays were able to
induce more significant changes in Hh pathway gene expression
compared with carbon ions. In addition, GANT61 was not able to
sensitise MCF-7 cells to X-ray or carbon ion radiation. However,
the combination of GANT61 with X-ray or carbon ion radiation was
more effective in decreasing cell migration compared with either
radiation type alone. Further studies are required to determine
whether breast cancer cells are a good model for investigating the
combination of Hh inhibition and particle radiation. Moreover,
additional cell lines must be included for further verification of
these results in a wide spectrum of breast cancer types.
Acknowledgments
The authors would like to thank the iPAC committee
of the Grand Accélérateur National d'Ions Lourds (GANIL, Caen,
France) for the carbon ion beam time granted (P1006-H) and the
staff of the LARIA, CIRIL (GANIL) for allowing us access to and use
of their facility. We would also like to thank Bart Marlein, Michel
Doms and Raf Aerts for their continued assistance during the
X-irradiation sessions at SCK•CEN.
Funding
KK is a recipient of a SCK•CEN-KUL PhD grant. KK,
NB, BB and RV received a Horizon 2020 427 travel grant (no. 654002)
for experiments at GANIL. KH is a clinical research fellow of the
Research Foundation Flanders, Belgium.
Availability of materials and data
All the datasets generated and analysed in the
present study are included in this published article.
Authors' contributions
KK performed experiments at SCK•CEN, Belgium and
GANIL, France. MM and SI designed the experimental set-up. NB, BB,
RV and GL helped with experiments performed at GANIL. AJ helped
with qPCR experiments. SB, SI, KH and MM contributed to the design
of the study, as well as with interpretation of obtained data. All
co-authors critically reviewed and approved the final version to be
submitted to this journal.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Durante M and Loeffler JS: Charged
particles in radiation oncology. Nat Rev Clin Oncol. 7:37–43. 2010.
View Article : Google Scholar
|
|
2
|
Suit H, DeLaney T, Goldberg S, Paganetti
H, Clasie B, Gerweck L, Niemierko A, Hall E, Flanz J, Hallman J and
Trofimov A: Proton vs carbon ion beams in the definitive radiation
treatment of cancer patients. Radiother Oncol. 95:3–22. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Weyrather WK and Debus J: Particle beams
for cancer therapy. Clin Oncol (R Coll Radiol). 15:S23–S28. 2003.
View Article : Google Scholar
|
|
4
|
Hamada N: Recent insights into the
biological action of heavy-ion radiation. J Radiat Res. 50:1–9.
2009. View Article : Google Scholar
|
|
5
|
PTCOG Patient Statistics: Particle Therapy
Co-Operative Group (PTCOG), Villigen 2016. https://www.ptcog.ch/index.php/ptcog-patient-statistics.
|
|
6
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Corbin K and Mutter R: Proton therapy for
breast cancer: Progress & pitfalls. Breast Cancer Management.
7:2018. View Article : Google Scholar
|
|
8
|
Menezes KM, Wang H, Hada M and Saganti PB:
Radiation matters of the heart: A mini review. Front Cardiovasc
Med. 5:832018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Akamatsu H, Karasawa K, Omatsu T, Isobe Y,
Ogata R and Koba Y: First experience of carbon-ion radiotherapy for
early breast cancer. Jpn J Radiol. 32:288–295. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Weber DC, Abrunhosa-Branquinho A, Bolsi A,
Kacperek A, Dendale R, Geismar D, Bachtiary B, Hall A, Heufelder J,
Herfarth K, et al: Profile of European proton and carbon ion
therapy centers assessed by the EORTC facility questionnaire.
Radiother Oncol. 124:185–189. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lorusso G and Rüegg C: New insights into
the mechanisms of organ-specific breast cancer metastasis. Semin
Cancer Biol. 22:226–233. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nicolini A, Giardino R, Carpi A, Ferrari
P, Anselmi L, Colosimo S, Conte M, Fini M, Giavaresi G, Berti P and
Miccoli P: Metastatic breast cancer: An updating. Biomed
Pharmacother. 60:548–556. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Karhadkar SS, Bova GS, Abdallah N, Dhara
S, Gardner D, Maitra A, Isaacs JT, Berman DM and Beachy PA:
Hedgehog signalling in prostate regeneration, neoplasia and
metastasis. Nature. 431:707–712. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gonnissen A, Isebaert S and Haustermans K:
Hedgehog signaling in prostate cancer and its therapeutic
implication. Int J Mol Sci. 14:13979–14007. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kubo M, Nakamura M, Tasaki A, Yamanaka N,
Nakashima H, Nomura M, Kuroki S and Katano M: Hedgehog signaling
pathway is a new therapeutic target for patients with breast
cancer. Cancer Res. 64:6071–6074. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
O'Toole SA, Machalek DA, Shearer RF,
Millar EK, Nair R, Schofield P, McLeod D, Cooper CL, McNeil CM,
McFarland A, et al: Hedgehog overexpression is associated with
stromal interactions and predicts for poor outcome in breast
cancer. Cancer Res. 71:4002–4014. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cui W, Wang LH, Wen YY, Song M, Li BL,
Chen XL, Xu M, An SX, Zhao J, Lu YY, et al: Expression and
regulation mechanisms of sonic hedgehog in breast cancer. Cancer
Sci. 101:927–933. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chang L, Zhao D, Liu HB, Wang QS, Zhang P,
Li CL, Du WZ, Wang HJ, Liu X, Zhang ZR and Jiang CL: Activation of
sonic hedgehog signaling enhances cell migration and invasion by
induction of matrix metalloproteinase-2 and -9 via the
phos-phoinositide-3 kinase/AKT signaling pathway in glioblastoma.
Mol Med Rep. 12:6702–6710. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen Q, Gao G and Luo S: Hedgehog
signaling pathway and ovarian cancer. Chin J Cancer Res.
25:346–353. 2013.PubMed/NCBI
|
|
20
|
Yan R, Peng X, Yuan X, Huang D, Chen J, Lu
Q, Lv N and Luo S: Suppression of growth and migration by blocking
the hedgehog signaling pathway in gastric cancer cells. Cell Oncol
(Dordr). 36:421–435. 2013. View Article : Google Scholar
|
|
21
|
Feldmann G, Dhara S, Fendrich V, Bedja D,
Beaty R, Mullendore M, Karikari C, Alvarez H, Iacobuzio-Donahue C,
Jimeno A, et al: Blockade of hedgehog signaling inhibits pancreatic
cancer invasion and metastases: A new paradigm for combination
therapy in solid cancers. Cancer Res. 67:2187–2196. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Isohata N, Aoyagi K, Mabuchi T, Daiko H,
Fukaya M, Ohta H, Ogawa K, Yoshida T and Sasaki H: Hedgehog and
epithelial-mesen-chymal transition signaling in normal and
malignant epithelial cells of the esophagus. Int J Cancer.
125:1212–1221. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yoo YA, Kang MH, Lee HJ, Kim BH, Park JK,
Kim HK, Kim JS and Oh SC: Sonic hedgehog pathway promotes
metastasis and lymphangiogenesis via activation of Akt, EMT, and
MMP-9 pathway in gastric cancer. Cancer Res. 71:7061–7070. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lin Z, Li S, Sheng H, Cai M, Ma LY, Hu L,
Xu S, Yu LS and Zhang N: Suppression of GLI sensitises
medulloblastoma cells to mitochondria-mediated apoptosis. J Cancer
Res Clin Oncol. 142:2469–2478. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen Q, Xu R, Zeng C, Lu Q, Huang D, Shi
C, Zhang W, Deng L, Yan R, Rao H, et al: Down-regulation of Gli
transcription factor leads to the inhibition of migration and
invasion of ovarian cancer cells via integrin β4-mediated FAK
signaling. PLoS One. 9:e883862014. View Article : Google Scholar
|
|
26
|
Magistri P, Battistelli C, Strippoli R,
Petrucciani N, Pellinen T, Rossi L, Mangogna L, Aurello P, D'Angelo
F, Tripodi M, et al: SMO inhibition modulates cellular plasticity
and invasiveness in colorectal cancer. Front Pharmacol. 8:9562018.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yue D, Li H, Che J, Zhang Y, Tseng HH, Jin
JQ, Luh TM, Giroux-Leprieur E, Mo M, Zheng Q, et al: Hedgehog/Gli
promotes epithelial-mesenchymal transition in lung squamous cell
carcinomas. J Exp Clin Cancer Res. 33:342014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zeng J, Aziz K, Chettiar ST, Aftab BT,
Armour M, Gajula R, Gandhi N, Salih T, Herman JM, Wong J, et al:
Hedgehog pathway inhibition radiosensitises non-small cell lung
cancers. Int J Radiat Oncol Biol Phys. 86:143–149. 2013. View Article : Google Scholar
|
|
29
|
Gonnissen A, Isebaert S, McKee CM, Dok R,
Haustermans K and Muschel RJ: The hedgehog inhibitor GANT61
sensitises prostate cancer cells to ionizing radiation both in
vitro and in vivo. Oncotarget. 7:84286–84298. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Teichman J, Dodbiba L, Thai H, Fleet A,
Morey T, Liu L, McGregor M, Cheng D, Chen Z, Darling G, et al:
Hedgehog inhibition mediates radiation sensitivity in mouse
xenograft models of human esophageal adenocarcinoma. PLoS One.
13:e01948092018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tsai CL, Hsu FM, Tzen KY, Liu WL, Cheng AL
and Cheng JC: Sonic Hedgehog inhibition as a strategy to augment
radiosensitivity of hepatocellular carcinoma. J Gastroenterol
Hepatol. 30:1317–1324. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Franco AI, Eastwick G, Farah R, Heyboer M,
Lee M and Aridgides P: Upfront radiotherapy with concurrent and
adjuvant vismodegib is effective and well-tolerated in a patient
with advanced, multifocal basal cell carcinoma. Case Rep Dermatol
Med. 2018:23541462018.PubMed/NCBI
|
|
33
|
Schulze B, Meissner M, Ghanaati S, Burck
I, Rodel C and Balermpas P: Hedgehog pathway inhibitor in
combination with radiation therapy for basal cell carcinomas of the
head and neck: First clinical experience with vismodegib for
locally advanced disease. Strahlenther Onkol. 192:25–31. 2016.
View Article : Google Scholar
|
|
34
|
Pollom EL, Bui TT, Chang AL, Colevas AD
and Hara WY: Concurrent vismodegib and radiotherapy for recurrent,
advanced basal cell carcinoma. JAMA Dermatol. 151:998–1001. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Raleigh DR, Algazi A, Arron ST, Neuhaus IM
and Yom SS: Induction hedgehog pathway inhibition followed by
combined-modality radiotherapy for basal cell carcinoma. Br J
Dermatol. 173:544–546. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Atwood SX, Chang AL and Oro AE: Hedgehog
pathway inhibition and the race against tumor evolution. J Cell
Biol. 199:193–197. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rudin CM, Hann CL, Laterra J, Yauch RL,
Callahan CA, Fu L, Holcomb T, Stinson J, Gould SE, Coleman B, et
al: Treatment of medulloblastoma with hedgehog pathway inhibitor
GDC-0449. N Engl J Med. 361:1173–1178. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chang AL and Oro AE: Initial assessment of
tumor regrowth after vismodegib in advanced Basal cell carcinoma.
Arch Dermatol. 148:1324–1325. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gonnissen A, Isebaert S, McKee CM, Muschel
RJ and Haustermans K: The effect of metformin and GANT61
combinations on the radiosensitivity of prostate cancer cells. Int
J Mol Sci. 18:E3992017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhou J, Wu K, Gao D, Zhu G, Wu D, Wang X,
Chen Y, Du Y, Song W, Ma Z, et al: Reciprocal regulation of
hypoxia-inducible factor 2α and GLI1 expression associated with the
radioresis-tance of renal cell carcinoma. Int J Radiat Oncol Biol
Phys. 90:942–951. 2014. View Article : Google Scholar
|
|
41
|
Lesueur P, Chevalier F, El-Habr EA, Junier
MP, Chneiweiss H, Castera L, Muller E, Stefan D and Saintigny Y:
Radiosensitization effect of talazoparib, a parp inhibitor, on
glioblastoma stem cells exposed to low and high linear energy
transfer radiation. Sci Rep. 8:36642018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Soule HD, Vazguez J, Long A, Albert S and
Brennan M: A human cell line from a pleural effusion derived from a
breast carcinoma. J Natl Cancer Inst. 51:1409–1416. 1973.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Durantel F, Balanzat E, Cassimi A,
Chevalier F, Ngono-Ravache Y, Madi T, Poully JC, Ramillon JM,
Rothard H, Ropars F, et al: Dosimetry for radiobiology experiments
at GANIL. Nuclear instruments and methods in physics research
section A: Accelerators, Spectrometers, Detectors and Associated
Equipment. Elsevier. 816:70–77. 2016.
|
|
44
|
Suetens A, Moreels M, Quintens R, Soors E,
Buset J, Chiriotti S, Tabury K, Gregoire V and Baatout S: Dose- and
time-dependent gene expression alterations in prostate and colon
cancer cells after in vitro exposure to carbon ion and
X-irradiation. J Radiat Res. 56:11–21. 2015. View Article : Google Scholar :
|
|
45
|
Pfaffl MW: A new mathematical model for
relative quantification in real-time RT-PCR. Nucleic Acids Res.
29:e452001. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Moncharmont C, Levy A, Guy JB, Falk AT,
Guilbert M, Trone JC, Alphonse G, Gilormini M, Ardail D, Toillon
RA, et al: Radiation-enhanced cell migration/invasion process: A
review. Crit Rev Oncol Hematol. 92:133–142. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Fujita M, Imadome K, Shoji Y, Isozaki T,
Endo S, Yamada S and Imai T: Carbonion irradiation suppresses
migration and invasiveness of human pancreatic carcinoma cells
MIAPaCa-2 via Rac1 and RhoA degradation. Int J Radiat Oncol Biol
Phys. 93:173–180. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Fujita M, Yamada S and Imai T: Irradiation
induces diverse changes in invasive potential in cancer cell lines.
Semin Cancer Biol. 35:45–52. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Matsumoto Y, Furusawa Y, Uzawa A, Hirayama
R, Koike S, Ando K, Tsuboi K and Sakurai H: Antimetastatic effects
of carbon-ion beams on malignant melanomas. Radiat Res.
190:412–423. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Clarke MF, Dick JE, Dirks PB, Eaves CJ,
Jamieson CH, Jones DL, Visvader J, Weissman IL and Wahl GM: Cancer
stem cells-perspectives on current status and future directions:
AACR Workshop on cancer stem cells. Cancer Res. 66:9339–9344. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhang X, Li X, Zhang N, Yang Q and Moran
MS: Low doses ionizing radiation enhances the invasiveness of
breast cancer cells by inducing epithelial-mesenchymal transition.
Biochem Biophys Res Commun. 412:188–192. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chapman JD, Doern SD, Reuvers AP,
Gillespie CJ, Chatterjee A, Blakely EA, Smith KC and Tobias CA:
Radioprotection by DMSO of mammalian cells exposed to X-rays and to
heavy charged-particle beams. Radiat Environ Biophys. 16:29–41.
1979. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Goddu SM, Narra VR, Harapanhalli RS,
Howell RW and Rao DV: Radioprotection by DMSO against the
biological effects of incorporated radionuclides in vivo-Comparison
with other radioprotectors and evidence for indirect action of
Auger electrons. Acta Oncol. 35:901–907. 1996. View Article : Google Scholar
|
|
54
|
Fushimi K, Uzawa K, Ishigami T, Yamamoto
N, Kawata T, Shibahara T, Ito H, Mizoe JE, Tsujii H and Tanzawa H:
Susceptible genes and molecular pathways related to heavy ion
irradiation in oral squamous cell carcinoma cells. Radiother Oncol.
89:237–244. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kamlah F, Hänze J, Arenz A, Seay U, Hasan
D, Juricko J, Bischoff B, Gottschald OR, Fournier C, Taucher-Scholz
G, et al: Comparison of the effects of carbon ion and photon
irradiation on the angiogenic response in human lung adenocarcinoma
cells. Int J Radiat Oncol Biol Phys. 80:1541–1549. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Gan GN, Eagles J, Keysar SB, Wang G,
Glogowska MJ, Altunbas C, Anderson RT, Le PN, Morton JJ, Frederick
B, et al: Hedgehog signaling drives radioresistance and
stroma-driven tumor repopulation in head and neck squamous cancers.
Cancer Res. 74:7024–7036. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Suetens A, Moreels M, Quintens R,
Chiriotti S, Tabury K, Michaux A, Gregoire V and Baatout S: Carbon
ion irradiation of the human prostate cancer cell line PC3: A whole
genome microarray study. Int J Oncol. 44:1056–1072. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zhou X, Zhang X, Xie Y, Tanaka K, Wang B
and Zhang H: DNA-PKcs inhibition sensitises cancer cells to
carbon-ion irradiation via telomere capping disruption. PLoS One.
8:e726412013. View Article : Google Scholar
|
|
59
|
Sai S, Vares G, Kim EH, Karasawa K, Wang
B, Nenoi M, Horimoto Y and Hayashi M: Carbon ion beam combined with
cisplatin effectively disrupts triple negative breast cancer
stem-like cells in vitro. Mol Cancer. 14:1662015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Suzuki M, Kase Y, Yamaguchi H, Kanai T and
Ando K: Relative biological effectiveness for cell-killing effect
on various human cell lines irradiated with heavy-ion medical
accelerator in Chiba (HIMAC) carbon-ion beams. Int J Radiat Oncol
Biol Phys. 48:241–250. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Wada M, Suzuki M, Liu C, Kaneko Y, Fukuda
S, Ando K and Matsufuji N: Modeling the biological response of
normal human cells, including repair processes, to fractionated
carbon beam irradiation. J Radiat Res. 54:798–807. 2013. View Article : Google Scholar : PubMed/NCBI
|