Introduction
Salinomycin is a potassium ionophore that has been
isolated from Streptomyces albus and is commercially used as an
anti-coccidal agent in veterinary medicine (1). Salinomycin has been shown to reduce
growth in several tumor cell types, including breast cancer,
prostate cancer and chemotherapy-resistant cancer cells (2-5).
Following a high-throughput screen of nearly 16,000 small
molecules, salinomycin was found to selectively inhibit the growth
of breast cancer stem cells with 100-fold higher potency than
paclitaxel (6). Salinomycin has
also been found to induce apoptosis in PC3 prostate cancer cells by
increasing reactive oxygen species (ROS) levels and altering
mitochondrial membrane depolarization through activation of the
intrinsic apoptosis pathway (2).
Recently, amide and ester derivatives of salinomycin have shown
anti-proliferative activity against leukemia and breast cancer
(7). Additionally, the effects of
certain salinomycin derivatives have been shown to surpass those of
cytotoxic drugs such as cisplatin and doxorubicin in LoVo/DX human
colon adenocarcinoma cells that are resistant to doxorubicin
(8).
Despite being relatively uncommon, medullary thyroid
carcinoma (MTC) accounts for the majority of thyroid
cancer-associated mortalities (9).
MTC is characterized as an indolent tumor; however, it is one of
the most aggressive forms of thyroid cancer in its late stages
(10). MTC tumors are derived from
the parafollicular cells of the thyroid gland, which normally
secrete calcitonin (11). The
majority of cases of MTC are sporadic, and only 25% arise as
hereditary multiple endocrine neoplasia (12). Specific mutations are associated
with phenotype and prognosis for both hereditary and sporadic MTC
(9,12). Approximately 15% of patients
present distant metastasis of the tumor into the lung, bone and
liver with a 10-year survival rate of 20% (11). As in other differentiated cancers
of the thyroid gland, thyroidectomy is the treatment of choice
(9,11). Since parafollicular cells lack
iodine transporters and thyroid-stimulating hormone (TSH),
traditional thyroid cancer treatments with radioactive iodine or
TSH suppression are not effective (13). Additionally, MTC develops
resistance to most cytotoxic chemotherapies. Previous studies have
identified few genetic alterations that are involved in the
pathogenesis of MTC (13).
Rearranged during transfection proto-oncogene (RET) appears to be
the primary oncogene in sporadic MTC and other types of endocrine
cancer, such as papillary thyroid carcinoma (PTC) (14). KRAS, HRAS and NRAS are other common
mutations, but are less common than RET (15).
RET is a single-pass transmembrane receptor tyrosine
kinase that binds to glial cell line-derived neurotrophic factor
and dimerizes. Upon RET dimerization, the tyrosine 1062 residue in
the intra-cellular kinase domain is phosphorylated (16). RET plays a crucial role in
mammalian embryonic development of the brain, peripheral nervous
system, calcitonin producing C-cells and thyroid gland (14). The RET-mediated signaling pathway
regulates the molecular mechanisms that are primarily responsible
for biological processes including cell growth, differentiation and
survival. Increase in RET expression levels is associated with
cancer progression and poor survival (17). The constitutive activation of
several growth-factor receptors, including RET, platelet-derived
growth factor receptor, insulin-like growth factor receptor 1 and
epidermal growth factor receptor (EGFR), is generally associated
with the development and progression of different types of cancers
(14). Knocking out RET in mouse
models increases prenatal death rates, demonstrating the importance
of RET signaling in early development (18,19).
Consequently, targeting RET in MTC using small molecule tyrosine
kinase inhibitors may diminish tumor proliferation and induce
apoptosis. The current standard treatment options of cabozantinib
(XL184) and (ZD6474) have shown low response rates and serious side
effects, which include pulmonary embolism and gastrointestinal
bleeding with cabozantinib, and QT prolongation with torsade de
pointes and sudden cardiac death with vandetanib (20-22).
Therefore, the search for safer and more effective small molecules
continues, with the aim of targeting and understanding the role of
the RET proto-oncogene in MTC (14).
Activation of Wnt/β-catenin signaling has been shown
to be associated with several types of cancer, including
colorectal, hepatocellular and breast cancer (23-25).
It is commonly accepted that activation of Wnt signaling is a
late-occurring event in thyroid cancers, particularly in anaplastic
and poorly differentiated thyroid cancers (26). There is evidence to suggest that
Wnt activation serves a pivotal role in the initiation of papillary
thyroid carcinoma; however, the precise nature of that role is not
yet fully understood (26,27).
The present study demonstrated the effects of
salinomycin and its analogs on RET expression, low-density
lipoprotein receptor-related protein 6 (LRP6) expression and
phosphorylation, as well as on their downstream pathways. It also
revealed an association between RET and LRP6 expression in an MTC
model for the first time, to the best of our knowledge, using
salinomycin, salinomycin derivatives, Wnt inhibitors and RET small
interfering RNA (siRNA). This relationship between RET and LRP6 has
previously been observed in early kidney development and cystic
dysplasia in an animal model (19). Finally, the unique capability of
salinomycin to induce cell cycle arrest and apoptosis independently
of ROS generation in MTC was demonstrated.
Materials and methods
Chemicals
Salinomycin and niclosamide (N3510) were purchased
from Sigma-Aldrich (Merck KGaA). The synthesis and characterization
of the 16 salinomycin derivatives were conducted as previously
described (7,28).
Cell culture and media
Human MTC cell lines (TT and MZ-CRC1), an
immortalized normal human thyroid epithelial cell line (Nthy-ori
3-1) and papillary thyroid carcinoma cell line (K1) were obtained
from the American Type Culture Collection. Cell authentication was
completed for K1 using STR profiling, and the results exceeded the
80% match threshold with the K1 reference. K1 is considered as a
subpopulation of Glag-66, both of which are papillary thyroid
carcinoma cell lines (29). TT and
MZ-CRC cells were cultured in Dulbecco's modified Eagle's medium
(DMEM)/F12 (Cellgro; Corning) supplemented with 15% heat
inactivated fetal bovine serum (FBS; Sigma-Aldrich, Merck KGaA).
NThy-ori 3-1 and K1 cell lines were cultured in RPMI-1640 and Ham's
F12 medium (Thermo Fisher Scientific) supplemented with 9% FBS,
respectively. The isogenic cell line 293-RET, which has a
luciferase reporter gene under the control of a wild-type RET
promoter, was generated as described in a previous study conducted
by the present authors (30). The
293-RET cell line was cultured in DMEM supplemented with 9% FBS.
TPC1 cells were kindly provided by Dr Rebecca Schweppe (University
of Colorado Denver) and were cultured in RPMI-1640 medium
supplemented with 9% FBS. All cell lines were maintained in a
humidified atmosphere containing 5% CO2 at 37°C. These
cell lines were also tested for myco-plasma contamination and were
further authenticated using STR profiling.
Cell-based screening using a luciferase
assay
The 293-RET cells were plated in a 96-well plate at
a concentration of 1.5×104 cells/well. The next day, the
cells were treated with various concentrations (0-3.125
µg/ml) of salinomycin and incubated for 24 h. Cells were
then lysed with 25 µl passive lysis buffer (Promega
Corporation), and luciferase expression was determined using the
Steady-Glo Luciferase assay system (Promega Corporation) following
the manufacturer's instructions. Luciferase activity in whole cell
lysates was measured in relative luminescence units and normalized
to the total amount of protein.
Semi-quantitative reverse transcription
(RT)-PCR analysis of RET mRNA synthesis in TT cells
The mRNA expression of TT cells following exposure
to increasing concentrations of salinomycin (0.25-1 µg/ml)
was determined by RT-PCR as described previously (30). Total RNA was extracted from the
cells utilizing the RNeasy Mini QIAcube kit (Qiagen, Inc.) as per
the manufacturer's instructions. The extracted RNA was subjected to
RT using oligo (dT)18 primer with a QuantiTect reverse
transcription kit (Qiagen, Inc.) to obtain cDNA (30). The primers used for RT-PCR were as
follows: RET forward, 5′-GCAGCATTGTTGGGGGACA-3′ and reverse,
5′-CACCGGAAGAGGAGTAGCTG-30; ribosomal protein L9 (RPL9) forward, 5′
CTGAAGGGACGCACAGTTAT-3′ and reverse, 5′- ACGGTAGCCAGTTCCTTTCT-3′.
The PCR step involved initial denaturation at 95°C for 3 min
followed by 33 and 23 cycles for RET and RPL9, respectively, at
95°C for 30 sec, 52°C for 30 sec and 72°C for 30 sec on a
GeneAmp® PCR system 9600 (PerkinElmer, Inc.). PCR
products were analyzed using 1.5% agarose gel electrophoresis. RPL9
gene was used as the reference gene. Densitometric analysis was
performed using ImageJ software (version 1.51; National Institutes
of Health).
Western blotting
After 24 or 48-h treatment with salinomycin or
niclosamide, whole-cell extracts (TT, K1, Nthy-ori3-1 and TPC1)
were prepared as described previously (30). Protein concentration was measured
using Bio-Rad Protein Assay Dye Reagent (Bio-Rad Laboratories,
Inc.) as previously described (31). Proteins were resolved by either
4-12 or 12% (for proteins <40 kDa such as cyclin D, BCL2 and
CDK4) gradient polyacrylamide SDS-PAGE, as described previously
(30,32). For cytosolic protein, protein
fractionation was conducted using NE-PER Nuclear and Cytoplasmic
Extraction Reagents (Pierce; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. The primary antibodies
used were: RET (#3220), mTOR (#2983), phosphorylated (p)-mTOR
(#5536), retinoblastoma (Rb; #9309), p-Rb (#8516S), E2F (#3742S),
p-LRP6 (#2568), LRP6 (#2560), p-Akt (#9271) and Akt (#9272)
(dilution 1:1000) purchased from Cell Signaling Technology, Inc.;
cyclin D (sc-20044), CDK4 (sc-260), BCL-2 (sc-509), P53
(sc-393031), P21 (sc-71811), β-catenin (sc-7963), cyclin B
(sc-166152), Lamin A/C (sc-7293), GAPDH (sc-47724) and β-actin
(sc-47778) (dilution 1:300) purchased from Santa Cruz
Biotechnology, Inc. Mouse or rabbit IgG antibodies tagged with
horseradish peroxidase (#1706516 and #1706515; Bio-Rad
Laboratories, Inc.) were used as secondary antibodies (dilution
1:1,000). An enhanced chemiluminescence substrate kit (#32106;
Thermo Fisher Scientific, Inc.) was used for detection.
Densitometric analysis was performed using ImageJ software (version
1.51). GAPDH and Lamin A/C served as a cytoplasmic and nucleus
markers, respectively (32).
N-acetyl-L-cysteine (NAC)
pretreatment
TT cells were pretreated with 1 mM NAC
(Sigma-Aldrich, Merck KGaA) for 2 h followed by the addition of
salinomycin (1 µg/ml) for 24 and 48 h. Cell extracts were
prepared, and protein expression was analyzed by western
blotting.
Cell viability assay
TT, K1, Nthy-ori 3-1 and TPC1 cells were plated at
7,500 cells/well in a 96-well dish and incubated overnight,
followed by treatment with salinomycin or salinomycin analogs at a
wide range of concentrations for 96 h. Cell viability was
determined using 0.33 mg/ml MTS dye in the presence of 25 µM
phenazine methosulfate (PMS) as described previously (15). The absorbance was measured at 590
nm using a Synergy™ HT Multi-detection Microplate Reader (BioTek
Instruments, Inc.).
Caspase-3 assay
TT cells were treated with different concentrations
of salinomycin for 48 h and caspase-3 activity was measured using
the ApoAlert Caspase Fluorescent assay kit (Clonetech Laboratories,
Inc.) according to the manufacturer's protocol.
Flow cytometry
Cell cycle progression was analyzed in TT cells
treated with increasing concentrations of salinomycin for 48 h, as
described in a previous study conducted by the present authors
(33). In brief, cells were fixed
with ethanol and stained with propidium iodine (PI) before analysis
by fluorescence-activated cell sorting (FACS) using a FACSCanto II
instrument (BD Biosciences). Data were analyzed using BD FACSDiva
v8.0.1 software (BD Biosciences).
RET RNA silencing
An siRNA sequence targeting human RET siRNA
(S11935), Silencer Select Negative Control siRNA (cat. no. 4390843)
and Opti-MEM Reduced Serum Medium were purchased from Thermo Fisher
Scientific, Inc. In 6-well plates, cells (0.25-1×106)
were transfected with 9 µl Lipofectamine RNAiMAX (cat. no.
13778150; Thermo Fisher Scientific, Inc.) and 30 pmol RET siRNA in
reduced serum medium as per the manufacturer's protocol. Control
cells were transfected with the negative control siRNA that has no
significant effect on cell proliferation and viability (data not
shown). After 72 h, cells were harvested for western blot
analyses.
Statistical analysis
All data are presented as the mean ± SEM of at least
three independent experiments. One-way ANOVA with Tukey's post hoc
test or two-way ANOVA with Bonferroni post hoc analysis were used
to test for significant differences among multiple treatment
groups. A Student's t-test was performed where applicable to test
the significant difference between two treatment groups. Difference
between groups were considered to be statistically significant when
P<0.05. GraphPad Prism (version 5.04; GraphPad Software, Inc.)
software was utilized for all data analysis.
Results
Salinomycin reduces endogenous RET
expression and interferes with RET downstream signaling in MTC
cells
The effect of salinomycin on RET gene transcription
was examined by a luciferase reporter assay using 293-RET cells as
described in a previous study by the current authors (30). This reporter cell line was
constructed by stably transfecting the luciferase reporter gene
under the control of the RET wild-type promoter into 293 cells
(30). As shown in Fig. 1A, treatment of 293-RET cells with
various concentrations of salinomycin for 24 h led to a significant
reduction in basal luciferase expression. Next, the inhibitory
effect of salinomycin on endogenous RET expression was verified
using two representative MTC cell lines, TT and MZ-CRC1, which
harbor C634W and M918T RET mutations, respectively (34). As shown in Fig. 1B (top panel), salinomycin decreased
RET protein levels in a time- and concentration-dependent manner in
TT cells. The 24-h treatment of TT cells with 0.25, 0.5 and 1
µg/ml of salinomycin decreased RET protein levels by 70, 80
and 83%, respectively, compared with the vehicle control. The
difference in RET protein expression was significant between the
various treatment groups, with the exception of 0.5 and 1
µg/ml, which did not differ significantly (top panel;
Fig. 1B). The downregulation of
RET protein appeared to be more pronounced in TT cells treated with
salinomycin for 48 h compared with those treated for 24 h. The
difference in RET protein expression was statistically significant
between each treatment group after incubation with salinomycin for
48 h (top panel; Fig. 1B). To
determine if the expression levels of RET protein were consistent
with those of RET mRNA in TT cells treated with salinomycin, the
levels of RET mRNA in TT cells treated with salinomycin were
measured using RT-PCR as previously described (30). As shown in Fig. 1B (lower panel), salinomycin
diminished RET mRNA expression in a time- and
concentration-dependent manner, suggesting that RET protein
expression corresponds with RET mRNA expression in TT cells. The
RPL9 gene was used as a loading control in the present RT-PCR
analysis (35). The lack of
evaluation of the RET mRNA levels by RT-quantitative PCR in the
present study may limit the accuracy of RET mRNA quantification in
the TT cells. The effect of salinomycin on RET protein expression
in MZ-CRC1 cells was also tested, following treatment with various
concentrations of salinomycin for 48 h. As shown in Fig. 1C, the treatment of MZ-CRC1 cells
with salinomycin reduced RET expression compared with that in the
untreated control, although to a lesser extent than in TT
cells.
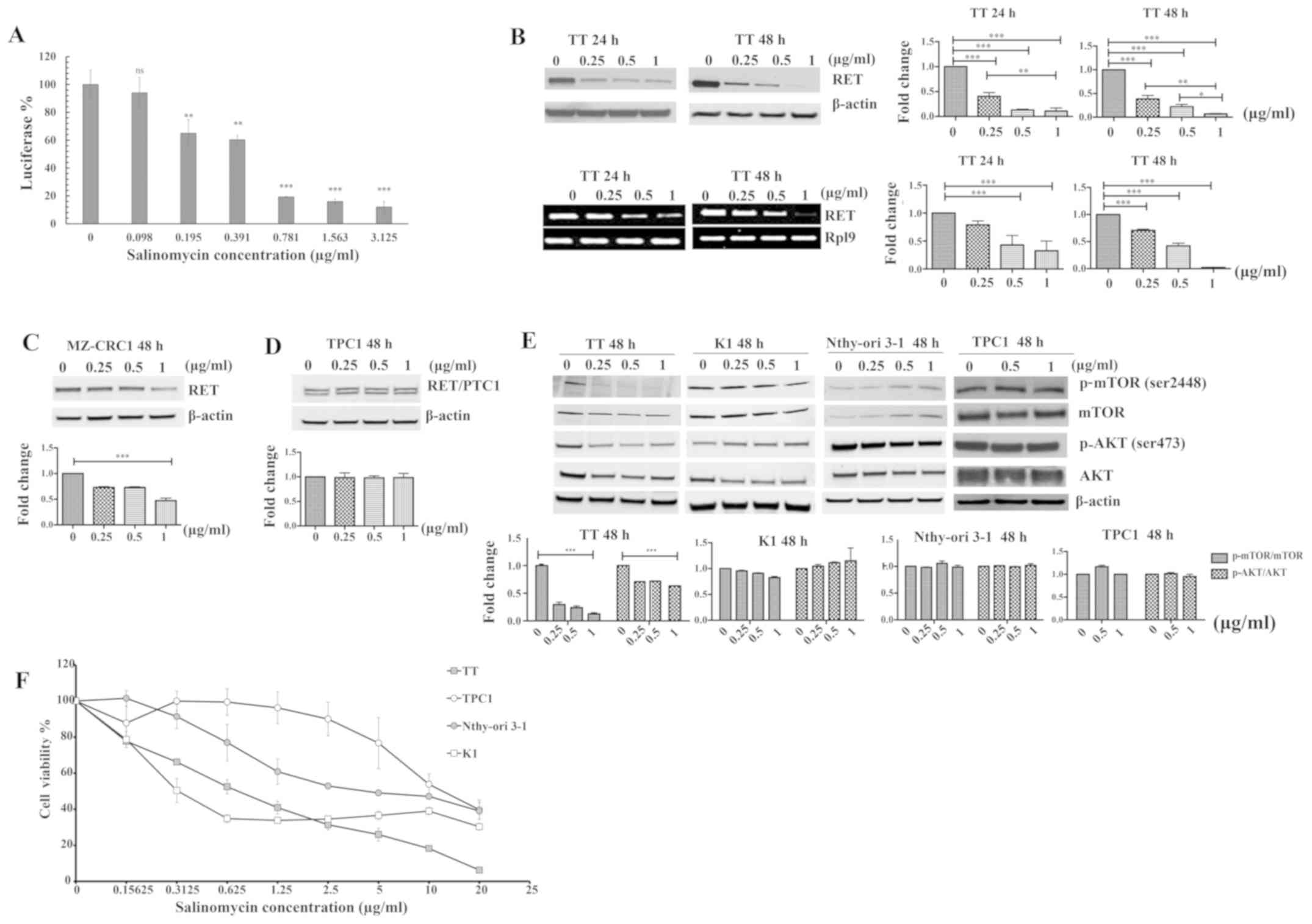 | Figure 1Effect of salinomycin on RET
expression and its downstream pathways. (A) Luciferase expression
in 293-RET cells after treatment with various concentrations of
salinomycin for 24 h. Luciferase activity in whole cell lysates was
measured in relative luminescence units and normalized to total
protein content. The x- and y-axes represent the concentration of
salinomycin and relative luciferase activity in the 293-RET cell
line, respectively. Data were analyzed using one-way ANOVA with
Tukey's post hoc test (n=3; **P<0.01,
***P<0.001). (B) Effect of salinomycin on RET protein
expression (top panel) and mRNA expression (bottom panel) in TT
cells after treatment with various concentrations of salinomycin
for 24 and 48 h (n=3; *P<0.05, **P<0.01
and ***P<0.001). (C and D) Effect of salinomycin on
RET protein expression in (C) MZ-CRC1 and (D) TPC1 cells,
respectively, after treatment with various concentrations of
salinomycin for 48 h. The western blotting results for RET were
determined by densitometric analysis with normalization to basal
expression, and differences among groups were analyzed using
one-way ANOVA with Tukey's post hoc test (n=3;
***P<0.001). (E) Cellular effects mediated by RET
downregulation after treatment with salinomycin. TT, K1, Nthy-ori
3-1 and TPC1 cells were treated with salinomycin for 48-h treatment
at various concentrations, and the levels of phosphorylated and
total mTOR and AKT proteins were measured by western blotting. The
western blotting results for p-mTOR/total mTOR and p-AKT/total AKT
were determined by densitometric analysis with normalization to
basal expression, and differences among groups were analyzed using
one-way ANOVA with Tukey's post hoc test (n=3;
***P<0.001). (F) Effect of salinomycin on the
proliferation of TT, K1, TPC1 and Nthy-ori 3-1 cells. Cell growth
was assessed by MTS assay following treatment with various
concentrations of salinomycin (≤20 µg/ml) for 96 h. Data are
the mean ± SEM of three separate experiments. RET, rearranged
during transfection kinase; PTC, papillary thyroid carcinoma; p,
phosphorylated; ns, not significant. |
The effect of salinomycin on the expression of the
RET fusion proteins (RET/PTC1) was investigated using a PTC cell
line, TPC1, containing a fused RET/PTC1 gene. The fused RET/PTC1
protein in the TPC1 cells is formed by a chromosomal rearrangement
between the RET tyrosine kinase domain coding region and the
5'-terminal region of the coiled-coil domain containing gene 6
(CCD6) at chromosom 10q11.2. This chromosomal inversion causes the
transcription of the RET/PTC1 gene to be regulated by the CCD6 gene
promoter region (36,37). As shown in Fig. 1D, salinomycin exhibited no effect
on fused RET/PTC1 expression in TPC1 cells. This result indicates
that the wild-type RET promoter is required for the repression of
RET transcription in thyroid cancer cell lines.
The PI3K/AKT/mTOR pathway has been reported to play
a critical role in the tumorigenesis induced by RET oncogenes
(38,39). Therefore, the present study
examined the effect of salinomycin on RET downstream signaling
pathway in TT cells by monitoring the changes in the levels of the
phosphorylated forms of AKT and mTOR relative to those of the
respective total proteins. As shown in Fig. 1E, a decrease in the RET protein
level in TT cells was closely associated with a reduction in the
phosphorylation of AKT and mTOR, with only a slight effect on the
amount of total AKT and no effect on the amount of total mTOR.
However, salinomycin showed no effect on the AKT/mTOR pathway in
RET-independent thyroid cells, namely K1, Nthy-ori 3-1 and TPC1,
suggesting that the effect of salinomycin on the AKT/mTOR pathway
is closely associated with its RET downregulatory effect.
Since activating point mutations in the RET
proto-oncogene are well-documented contributors to the progression
of hereditary and sporadic forms of MTC, a cell viability assay of
TT, K1, Nthy-ori 3-1 and TPC1 cells was performed. On the basis of
the cell viability results in Fig.
1F, the half maximal inhibitory concentration (IC50)
values of salinomycin in TT and K1 cells were estimated to be 0.3
and 0.6 µg/ml, respectively. Notably, Nthy-ori 3-1 and TPC1
cells were less sensitive to salinomycin, with IC50
values of 4 and 15 µg//ml, respectively, suggesting that the
inhibitory effect of salinomycin against RET expression could be
closely associated with its anti-proliferative effects on human
thyroid cell lines.
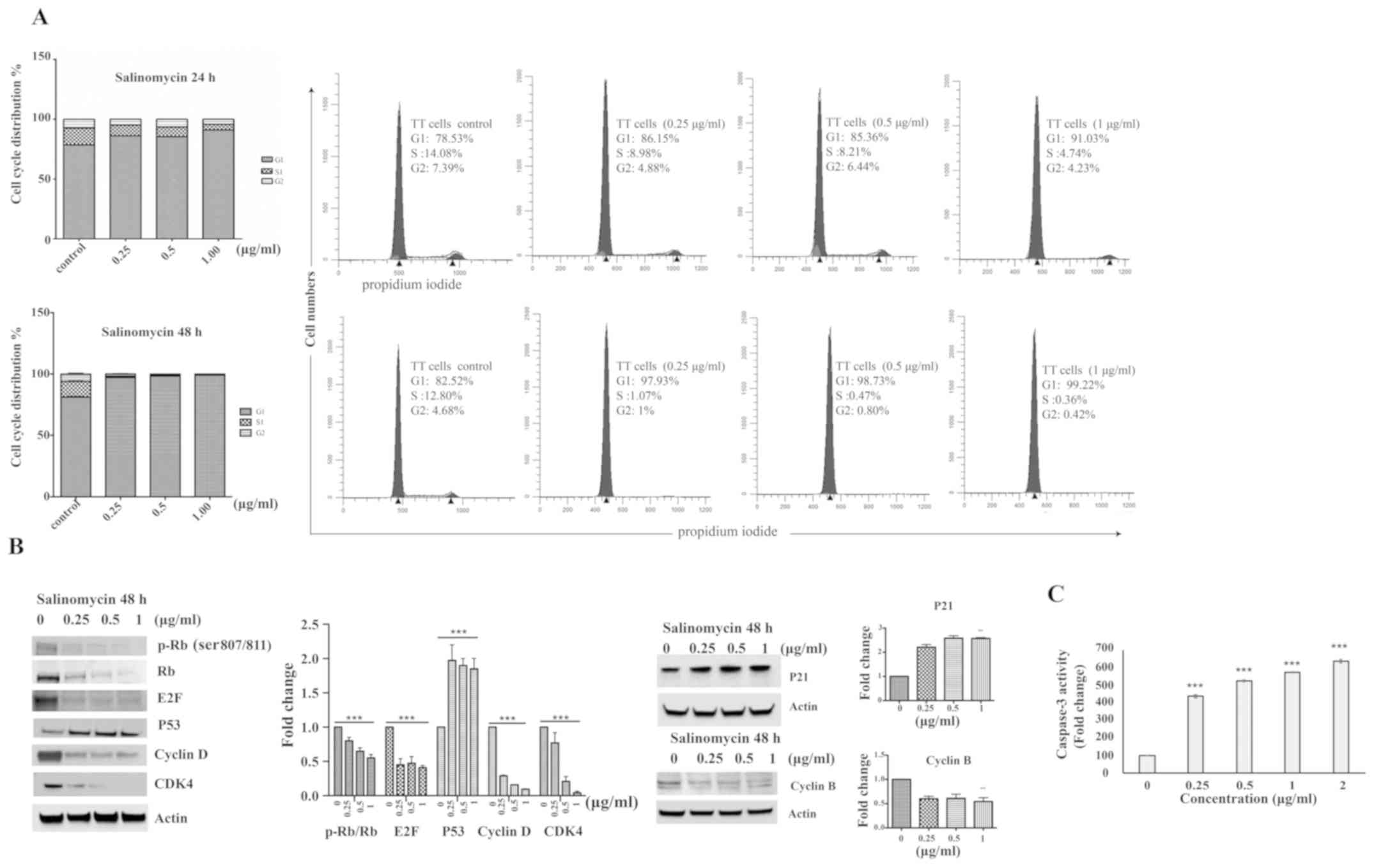
Salinomycin induces G1 cell cycle arrest
and release of caspase-3
The effect of salinomycin on cell cycle progression
was examined by treating TT cells with salinomycin at various
concentrations for 24 and 48 h. As shown in Fig. 2A, at 24 h after treatment with the
highest concentration (1 µg/ml) of salinomycin, there was a
12-15% increase in the proportion of cells in the G1 phase compared
with the control. Treatment with 1 µg/ml salinomycin for 48
h resulted in a greater increase in the proportion of cells in the
G1 phase, by 16-20% compared with the non-treated cells, which was
accompanied by a reduction in the number of cells in the S and G2
phases. To confirm the cell cycle arrest, the expression levels of
core cell cycle regulatory proteins (cyclin D, CDK4, P53, E2F, p-Rb
and Rb) were assessed in TT cells following treatment with
salinomycin for 48 h. As shown in Fig.
2B, western blotting revealed a strong downregulation of the
expression of cyclin D, CDK4 and E2F. Furthermore, salinomycin
decreased p-Rb and total Rb levels compared with the respective
baseline levels, and resulted in a significant increase in P53
expression. P21 and cyclin B proteins were evaluated in TT cells
after treatment with salinomycin for 48 h. P21 was upregulated
while cyclin B was downregulated after the 48-h treatment (Fig. 2B).
Whether salinomycin can induce apoptosis was
determined by measuring the activity of caspase-3, since caspase-3
activation is an apoptotic event that precedes cell death (40). As shown in Fig. 2C, caspase-3 activity in
salinomycin-treated cells was significantly increased in a
concentration-dependent manner compared with the control after 48 h
of treatment, which indicates the apoptosis-inducing effect of
salinomycin on TT cells.
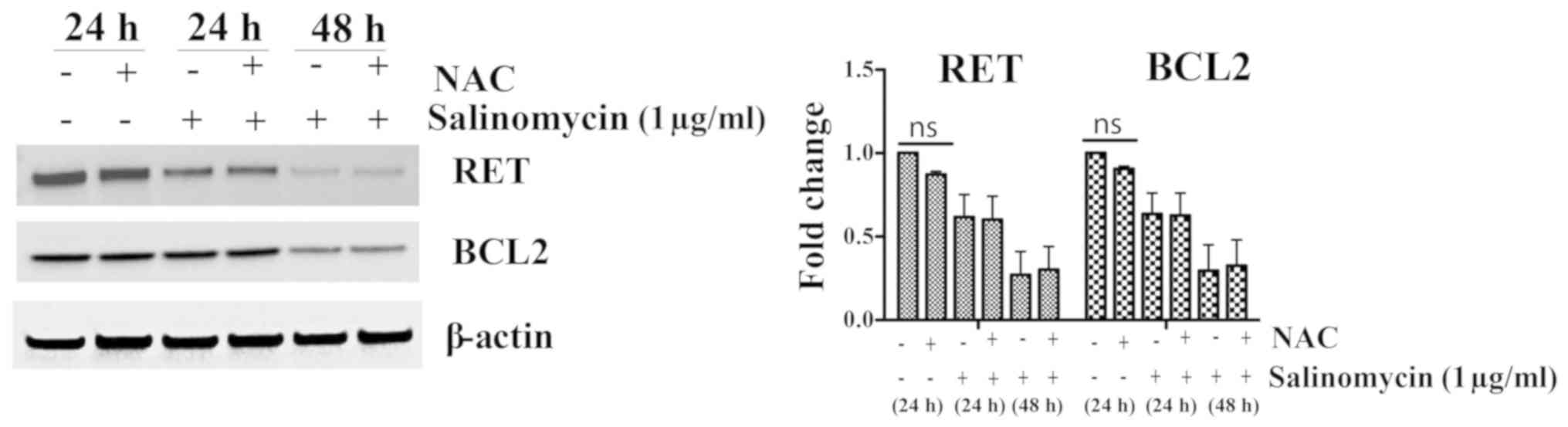
RET downregulation in TT cells after
salinomycin treatment is independent of ROS activation
Oxidative stress can dictate the fate of cells. At
high levels, ROS can induce cytotoxic or cytostatic effects,
whereas at low levels, they can facilitate cell differentiation and
proliferation (41). As has been
documented in previous studies, numerous anticancer drugs induce
programmed cell death, in whole or in part, by generating ROS
(42,43). To assess whether ROS production is
associated with the effect of salinomycin on RET expression or
apoptosis, TT cells were pre-treated with the ROS scavenger
N-acetyl cysteine (NAC). As shown in Fig. 3, after treatment with 1
µg/ml salinomycin for 24 and 48 h, RET and BCL2 protein
expression levels were not changed by NAC pre-treatment, suggesting
that RET suppression by salinomycin is independent of ROS induction
in the MTC model.
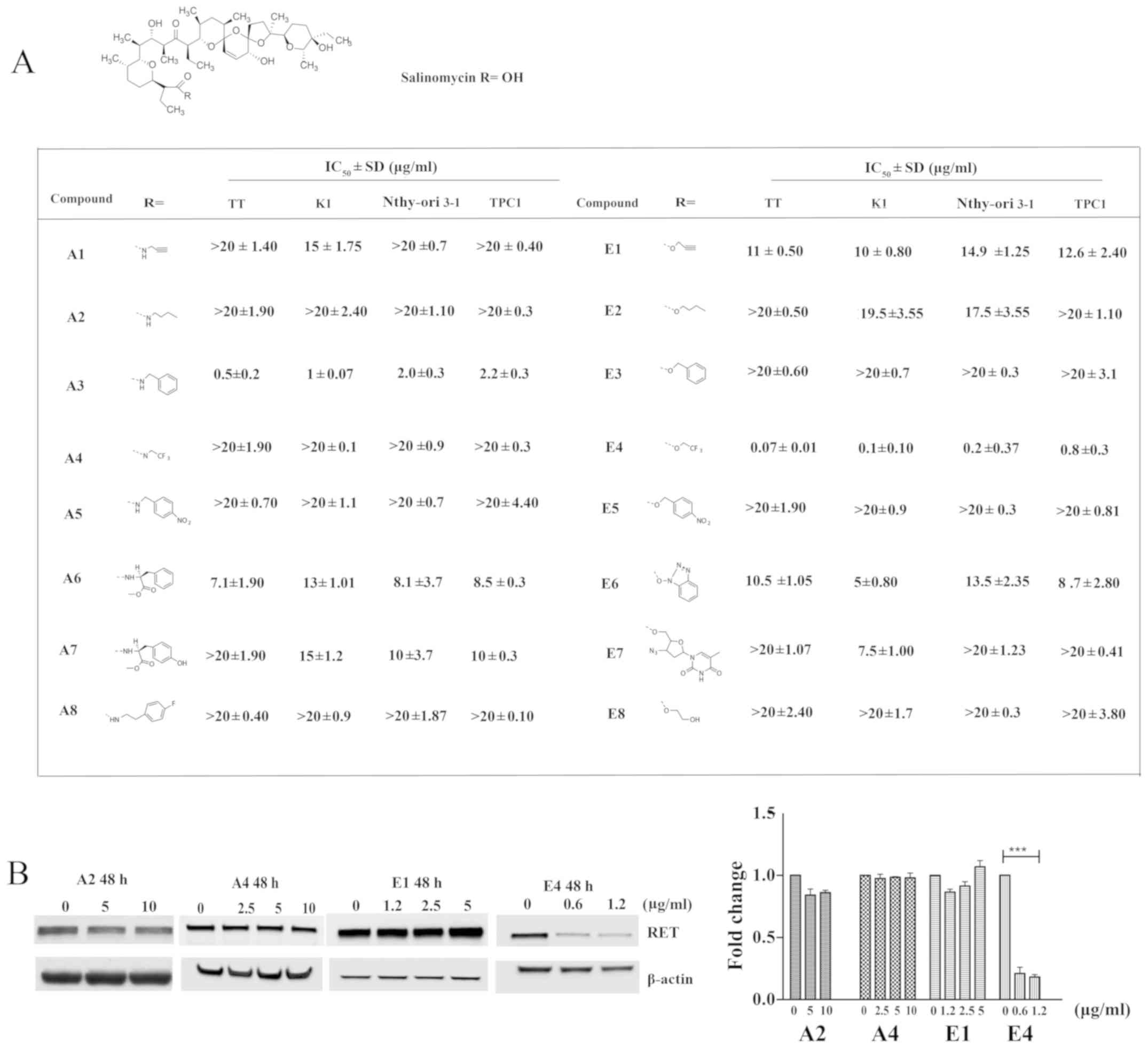
Salinomycin analogs show varying effects
compared with the parent compound on RET expression
To investigate the structure-activity relationship
of salinomycin, analogs of salinomycin generated by chemical
synthesis as described in a previous study, including salinomycin
amide (A1-A8) and ester (E1-E8) derivatives were used (7,8).
Salinomycin analogs have previously been evaluated for cytotoxicity
and selectivity against TT, K1, Nthy-Ori 3-1 and TPC1 cell lines
(Fig. 4A) (28,44).
As shown Fig. 4A, the majority of
the derivatives possessed considerably less potent cytotoxic
effects against normal and cancer thyroid cell lines than did
salinomycin. However, A3 and E4 exhibited more potent cytotoxicity
against these cell lines than the parent compound, with
IC50 values in the ranges of 0.5-2.2 and 0.07-0.8
µg/ml, respectively. It is also worthwhile to note that A3
and E4 exhibited relatively selective cytotoxicity to TT and K1
cells over TPC1 and the normal immortalized thyroid cells, Nthy-ori
3-1. Next, the effects of selected salinomycin analogs on
endogenous RET expression were examined in TT cells to determine if
the structure-related cytotoxic effects of salinomycin analogs are
associated with their potential to downregulate the expression of
RET protein. As shown in Fig. 4B,
nontoxic amide (A2 and A4) and ester (E1) derivatives showed no
effect on RET expression in TT cells after 48-h treatment with ≤10
and 5 µg/ml of the analogs, respectively. However, the most
potent ester derivative, E4, effectively reduced RET expression in
TT after 48 h of treatment at a concentration of 0.6 µg/ml.
The extent of the reduction in RET expression achieved with
salinomycin E4 is comparable to that of salinomycin. These results
suggest that the cytotoxic effects of salinomycin derivatives are
closely associated with their downregulatory effect on RET
expression in TT cells.
RET reduction can lead to LRP6
attenuation in MTC cell lines
Previous studies demonstrated that RET is a novel
transcriptional target of Wnt/β-catenin signaling (19,45).
Since salinomycin is known to interfere with Wnt signaling, whether
the RET downregulatory effect of salinomycin is mediated through
its inhibitory effect on Wnt/β-catenin pathway was examined in TT
cells in the present study (46).
As shown in Fig. 5A, salinomycin
induced a significant reduction in total, cytosolic and nuclear
β-catenin, p-LRP6 and total LRP6 in TT cells after 48 h of
treatment. To further verify the involvement of the Wnt pathway in
the RET-inhibitory effect of salinomycin, the well-characterized
Wnt inhibitor niclosamide, was used as a positive control in the
present study (47,48). Notably, niclosamide was able to
reduce p-LRP6, LRP6 and total β-catenin, as well as RET expression
in TT and MZ-CRC1 cells (Fig. 5B).
These results suggest the existence of a close relationship between
the Wnt/β-catenin and RET pathways in MTC, in agreement with
previous studies (27,45). Whether the activity of the
Wnt/β-catenin pathway is associated with activation of RET pathway
was also investigated in the present study. Therefore, TT cells
were transfected with RET siRNA or negative control siRNA for 72 h.
As shown in Fig. 5C, RET was
successfully knocked down by RET siRNA transfection compared with
the negative control siRNA. Total LRP6 expression was also reduced
by RET siRNA compared with the negative control (Fig. 5C), indicating the likelihood of
crosstalk between RET and the LRP6 (Wnt) pathway in TT cells.
Furthermore, RET and LRP6 levels declined concurrently in TT cells
following treatment with 1 µg/ml salinomycin at different
time points (Fig. 5D). The effects
of various salinomycin analogs on the expression levels of LRP6 and
p-RLP6 in TT cells were also examined. As shown in Fig. 5E, there appears to be a strong
association between the ability of an analog to reduce the
expression of RET and its ability to reduce p-LRP6 and LRP6
expression. Analogs such as A2, A4 and E1, which were not able to
reduce RET expression (Fig. 4B)
were also unable to alter RLP6 expression or phosphorylation
(Fig. 5E), while derivatives that
were able to reduce RET expression, such as E4, were also able to
reduce the expression and phosphorylation of LRP6.
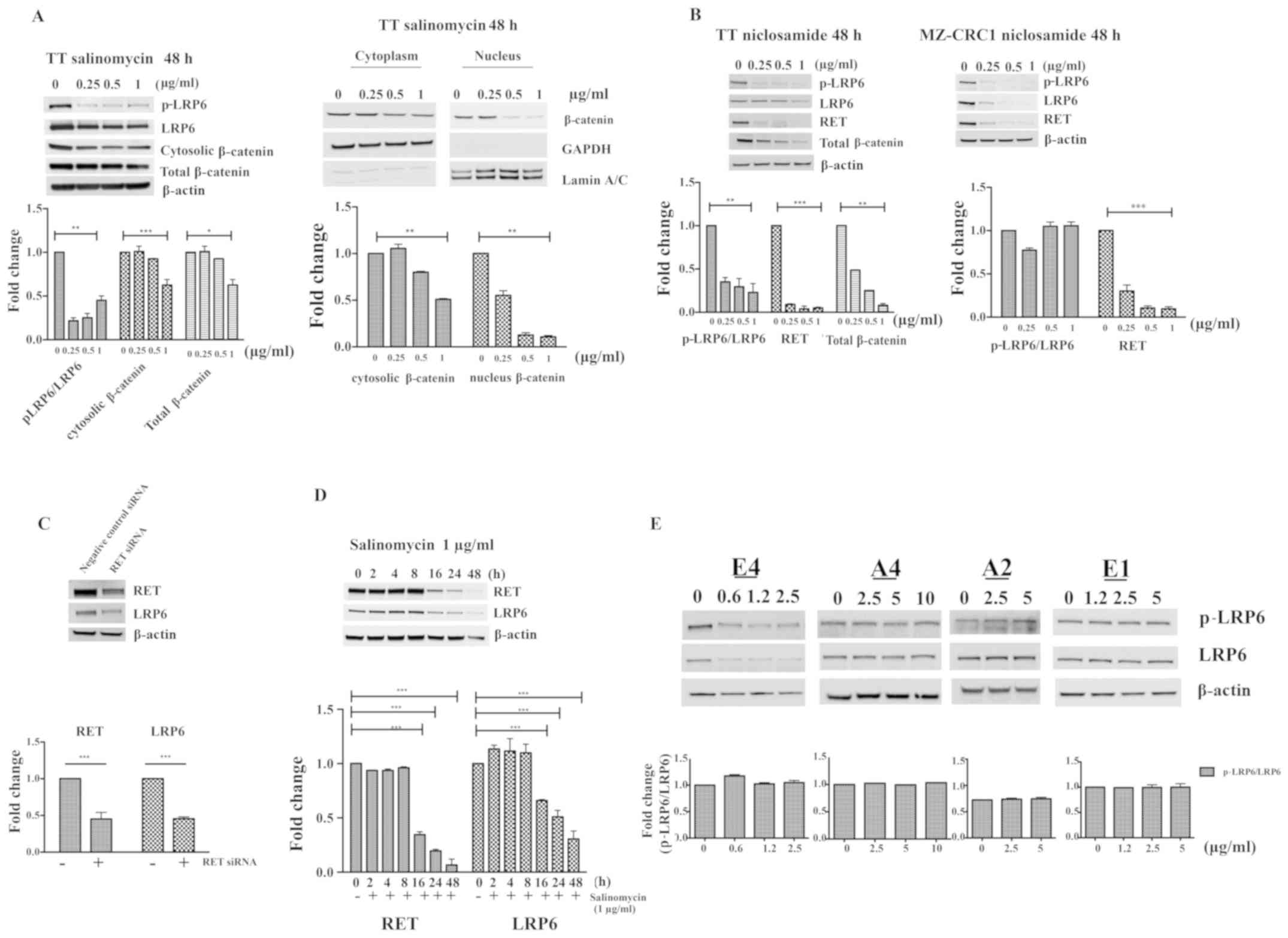 | Figure 5Effect of salinomycin on
Wnt/β-catenin signaling and crosstalk between RET and LRP6. (A)
Expression levels of p-LRP6, LRP6, cytosolic β-catenin and total
β-catenin were determined by western blotting in TT cells after
treatment with salinomycin for 48 h. The expression levels of
cytosolic and nuclear β-catenin were also measured. Proteins
expression were determined by densitometric analysis with
normalization to basal expression, and differences among groups
were analyzed using one-way ANOVA with Tukey's post hoc test (n=3;
*P<0.05, **P<0.01 and
***P<0.001). (B) Effect of niclosamide on the levels
of p-LRP6, LRP6 and RET in TT and MZ-CRC1 cells, and total
β-catenin in TT cells were determined by western blotting. The
western blotting results for the shown proteins were determined by
densitometric analysis with normalization to basal expression, and
differences among groups were analyzed using one-way ANOVA with
Tukey's post hoc test (n=3; **P<0.01 and
***P<0.001). (C) Western blot analysis of the
expression of LRP6 and RET in RET siRNA and silencer negative
control-treated TT cells. Representative images are shown, with
normalized densitometric quantification and analysis of the
difference between groups by Student's t-test (n =3;
***P<0.001). (D) Time course of the expression of RET
and LRP6 protein in TT cells in the presence of 1 μg/ml salinomycin
(n =3; ***P<0.001). (E) Effect of salinomycin analogs
(E4, A4, A2 and E1) on the p-LRP6/LRP6 ratio in TT cells after
treatment for ≤48 h. Western blotting results for all proteins were
determined by densitometric analysis with normalization to basal
expression, and differences among groups were analyzed using
one-way ANOVA with Tukey's post hoc test (n=3). LRP6, low-density
lipoprotein receptor-related protein 6; p, phosphor; RET,
rearranged during transfection kinase; siRNA, small interfering
RNA. |
Discussion
Due to the major contribution of germline mutations
in the RET proto-oncogene to the development of MTC, activated RET
protein is considered to be a weakness of MTC (38,49).
Previous studies have developed several therapeutic strategies
against RET-driven MTC (11,21,50).
These include the use of tyrosine kinase inhibitors, siRNAs that
silence mutant RET expression, and gene therapies that utilize
dominant-negative RET to suppress downstream signaling pathways
(17,39). To date, two multi-target tyrosine
kinase inhibitors (vandetanib and cabozantinib), which target
vascular endothelial growth factor receptor 2, EGFR, MET and RET,
have been approved for the treatment of locally advanced and
metastatic MTC. However, long-term vandetanib or cabozantinib
treatment for advanced MTC has two significant disadvantages:
Efficacy is lost over time as a result of acquired drug resistance,
and prolonged treatment can cause serious side effects (20,21,51,52).
Thus, there is an urgent need to identify and discover new
therapeutic agents that are able to interfere with RET function for
use in the curative therapy of MTC.
The potassium ionophore salinomycin has shown
inhibitory effects on the Wnt/β-catenin pathway in various cancer
models (46). In the present
study, salinomycin was shown to be a potential therapeutic agent
for MTC that can target RET and its downstream signaling. The
present study also demonstrated that the suppression of RET by
salinomycin is closely associated with its anti-proliferative and
apoptotic effects on human MTC cell lines, with a particularly
strong inhibitory effect on cells with RET mutations at C634W, such
as TT cells. Although MZ-CRC1 is an MTC cell line, it appears to be
less sensitive than TT cells to salinomycin (Fig. 1C). It may be speculated that there
are some genes that are highly overexpressed in the RET M918T
mutant but not the C634W mutant that could contribute to
salinomycin sensitivity in MZ-CRC1 (53). Salinomycin demonstrated no clear
effect on the papillary thyroid carcinoma cell line TPC1, in which
its IC50 was >10-fold higher than that for TT cells
(Fig. 1F). Likewise, salinomycin
showed no effect on the expression of the rearranged RET/PTC1
protein in TPC1 cells (Fig. 1D).
RET/PTC1 transcription is regulated by the CCD6 gene promoter
region, leading to the speculation that a wild-type RET promoter
region is essential for mediation of the RET-inhibiting effect of
salinomycin. Furthermore, salinomycin did not exert any detectable
effect on RET downstream proteins such as p-mTOR, mTOR, p-AKT and
AKT in TPC1 cells (Fig. 1E). K1
cells, which are derived from a primary papillary thyroid
carcinoma, have been found to have enhanced Wnt signaling for
growth and survival with wild-type status for RET (54). Thus, the disruption of Wnt
signaling could be the basis of the anti-proliferative activity of
salinomycin against K1 cells. Therefore, at a low concentration,
salinomycin appears to be selective, with a stronger
anti-proliferative effect on TT and K1 cells compared with TPC1 and
Nthy-ori 3-1 cells.
Cell cycle analysis by flow cytometry demonstrated
that the treatment of TT cells with salinomycin for 24 or 48 h
resulted in cell cycle arrest at the G1 phase in a
concentration-dependent manner (Fig.
2A). The cell cycle is highly regulated by different types of
cyclin-dependent kinases and their catalytic units; cyclin D, E2F,
Rb, P53, P21, cyclin B and CDK4 serve important roles in cell cycle
progression through the G1 phase (55,56).
After treatment of TT cells with salinomycin, the aforementioned
regulators were reduced in a concentration-dependent manner
(Fig. 2B). However, P27 was not
evaluated in the current cell cycle analysis. The cell cycle arrest
at the G1 phase was followed by increased caspase-3 activity after
48 h of treatment with salinomycin. Recent reports have shown that
salinomycin induces apoptosis in various human cancer cell lines,
in part due to the accumulation of ROS (57,58);
however, pre-treatment of TT cells with the antioxidant NAC could
not rescue TT cells from apoptosis, nor could it reduce the loss of
BCL2 or RET (Fig. 3). This
indicates that the apoptosis of TT cells after salinomycin
treatment is independent of ROS activation or generation.
In the current study, the potential anticancer
activities of salinomycin amide and ester derivatives are
presented. Although the majority of the salinomycin derivatives
were not capable of either reducing RET expression in TT cells or
inducing selective cytotoxicity, salinomycin E4 produced promising
results. Salinomycin E4 significantly reduced RET expression in TT
cells at submicromolar concentrations and also diminished total and
p-LRP6 levels. The effect on LRP6 demonstrates the importance of
the Wnt/β-catenin pathway in the mediation of the anticancer
activity of salinomycin and its analogs. The Wnt/β-catenin pathway
is essential in embryonic development and thyrocyte proliferation
(23,27). The abnormal activation of
Wnt/β-catenin is associated with multiple types of cancer (23,25).
The Wnt pathway is activated by the binding of Wnt glycoproteins to
Frizzled and its co-receptor LRP6 to form a ternary complex in the
cell membrane. Phosphorylation of LRP6 leads to disruption of the
β-catenin destruction complex, which includes adenomatosis
polyposis coli, glycogen synthase kinase 3, axin and casein kinase
1. This destruction complex typically phosphorylates cytoplasmic
β-catenin, and targets it for ubiquitination and proteolytic
degradation in the absence of Wnt. Upon activation, Wnt/β-catenin
signaling stabilizes cytosolic β-catenin, allowing it to
translocate to the nucleus and form a complex with T-cell
factor/lymphoid enhancer factor. This complex activates target
genes that are vital for cell growth and survival (27,59).
A previous study demonstrated that activation of Wnt/β-catenin by
lithium chloride upregulated RET expression in a
concentration-dependent manner in mIMCD-3 cells, whereas LRP6
knockout downregulated RET expression in mouse embryos (19). In the present study, niclosamide, a
specific Wnt/β-catenin inhibitor, was demonstrated to diminish RET
expression by disrupting Wnt signaling in TT cells. Also,
siRNA-mediated RET knockdown led to the downregulation of LRP6 and
RET expression in TT cells compared with the negative control. The
cytotoxicity of salinomycin derivatives appears to be established
via their ability to reduce the activity and the expression of LRP6
and RET. On the basis of the aforementioned results, it is
hypothesized that LRP6-mediated Wnt/β-catenin signaling either
modulates or closely interacts with RET or RET downstream proteins
in MTC, and that this crosstalk is disrupted by salinomycin,
selected salinomycin analogs, niclosamide or RET siRNA.
In conclusion, the current study demonstrates that
salinomycin, characterized as an inhibitor of the Wnt pathway,
exerts potent antitumor activity against MTC via the reduction of
RET expression. The chemical modification of salinomycin can
provide derivatives with stronger cytotoxity. Niclosamide and other
salinomycin derivatives, which reduce LRP6 expression, highlight
the important role of the Wnt signaling pathway in the regulation
of RET expression in MTC. This preliminary study reveals the
effects of salinomycin and its derivatives on MTC cells in
vitro. Since salinomycin has been reported to have serious side
effects on the cardiovascular system, the evaluation of the
toxicity of salinomycin and salinomycin derivatives as well as
their efficacy in an in vivo MTC model is pivotal (60). However, the findings of the current
study support further preclinical evaluation of salinomycin and its
analogs as novel therapeutic agents for the treatment of MTC.
Funding
The authors thank the American Cancer Society for
their support of this study (grant no. RSGM-12-046-01-CDD).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TA, VMK and DS conceived of and designed the study;
TA and DS analyzed the data, interpreted the results of the
experiments and drafted the manuscript; TA, VMK and DS prepared
figures, and edited and revised the manuscript; TA, AH and VMK
performed the experiments. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
No conflicts of interest, financial or otherwise,
are declared by the authors.
Acknowledgments
Not applicable.
References
|
1
|
Zhou S, Wang F, Wong ET, Fonkem E, Hsieh
TC, Wu JM and Wu E: Salinomycin: A novel anti-cancer agent with
known anti-coccidial activities. Curr Med Chem. 20:4095–4101. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang Y, Liu L, Li F, Wu T, Jiang H, Jiang
X, Du X and Wang Y: Salinomycin exerts anticancer effects on PC-3
cells and PC-3-derived cancer stem cells in vitro and in vivo.
BioMed Res Int. 2017:41016532017.PubMed/NCBI
|
|
3
|
Yu Z, Cheng H, Zhu H, Cao M, Lu C, Bao S,
Pan Y and Li Y: Salinomycin enhances doxorubicin sensitivity
through reversing the epithelial-mesenchymal transition of
cholangiocarcinoma cells by regulating ARK5. Braz J Med Biol Res.
50:e61472017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Verdoodt B, Vogt M, Schmitz I, Liffers ST,
Tannapfel A and Mirmohammadsadegh A: Salinomycin induces autophagy
in colon and breast cancer cells with concomitant generation of
reactive oxygen species. PLoS One. 7:e441322012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
McCarroll JA, Phillips PA, Kumar RK, Park
S, Pirola RC, Wilson JS and Apte MV: Pancreatic stellate cell
migration: Role of the phosphatidylinositol 3-kinase(PI3-kinase)
pathway. Biochem Pharmacol. 67:1215–1225. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gupta PB, Onder TT, Jiang G, Tao K,
Kuperwasser C, Weinberg RA and Lander ES: Identification of
selective inhibitors of cancer stem cells by high-throughput
screening. Cell. 138:645–659. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Antoszczak M, Urbaniak A, Delgado M, Maj
E, Borgström B, Wietrzyk J, Huczyński A, Yuan Y, Chambers TC and
Strand D: Biological activity of doubly modified salinomycin
analogs - Evaluation in vitro and ex vivo. Eur J Med Chem.
156:510–523. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Urbaniak A, Delgado M, Antoszczak M,
Huczyński A and Chambers TC: Salinomycin derivatives exhibit
activity against primary acute lymphoblastic leukemia (ALL) cells
in vitro. Biomed Pharmacother. 99:384–390. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Roy M, Chen H and Sippel RS: Current
understanding and management of medullary thyroid cancer.
Oncologist. 18:1093–1100. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nozhat Z and Hedayati M: Medullary thyroid
carcinoma: A review on ethical considerations in treatment of
children. J Pediatr Endocrinol Metab. 29:633–639. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Priya SR, Dravid CS, Digumarti R and
Dandekar M: Targeted therapy for medullary thyroid cancer: A
review. Front Oncol. 7:2382017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wells SA Jr, Pacini F, Robinson BG and
Santoro M: Multiple endocrine neoplasia type 2 and familial
medullary thyroid carcinoma: An update. J Clin Endocrinol Metab.
98:3149–3164. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nelkin B: Recent advances in the biology
and therapy of medullary thyroid carcinoma. F1000 Res. 6:21842017.
View Article : Google Scholar
|
|
14
|
Roskoski R Jr and Sadeghi-Nejad A: Role of
RET protein-tyrosine kinase inhibitors in the treatment RET-driven
thyroid and lung cancers. Pharmacol Res. 128:1–17. 2018. View Article : Google Scholar
|
|
15
|
Moura MM, Cavaco BM, Pinto AE and Leite V:
High prevalence of RAS mutations in RET-negative sporadic medullary
thyroid carcinomas. J Clin Endocrinol Metab. 96:E863–E868. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Salvatore D, Melillo RM, Monaco C,
Visconti R, Fenzi G, Vecchio G, Fusco A and Santoro M: Increased in
vivo phosphorylation of ret tyrosine 1062 is a potential
pathogenetic mechanism of multiple endocrine neoplasia type 2B.
Cancer Res. 61:1426–1431. 2001.PubMed/NCBI
|
|
17
|
de Groot JWB, Links TP, Plukker JTM, Lips
CJM and Hofstra RMW: RET as a diagnostic and therapeutic target in
sporadic and hereditary endocrine tumors. Endocr Rev. 27:535–560.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kramer ER, Aron L, Ramakers GMJ, Seitz S,
Zhuang X, Beyer K, Smidt MP and Klein R: Absence of Ret signaling
in mice causes progressive and late degeneration of the
nigrostriatal system. PLoS Biol. 5:e392007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Y, Stokes A, Duan Z, Hui J, Xu Y,
Chen Y, Chen HW, Lam K and Zhou CJ: LDL receptor-related protein 6
modulates Ret proto-oncogene signaling in renal development and
cystic dysplasia. J Am Soc Nephrol. 27:417–427. 2016. View Article : Google Scholar
|
|
20
|
Elisei R, Schlumberger MJ, Müller SP,
Schöffski P, Brose MS, Shah MH, Licitra L, Jarzab B, Medvedev V,
Kreissl MC, et al: Cabozantinib in progressive medullary thyroid
cancer. J Clin Oncol. 31:3639–3646. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Grabowski P, Briest F, Baum RP, Zaknun JJ,
Kulkarni HR, Zeitz M and Hörsch D: Vandetanib therapy in medullary
thyroid cancer. Drugs Today (Barc). 48:723–733. 2012. View Article : Google Scholar
|
|
22
|
Wells SA Jr, Robinson BG, Gagel RF, Dralle
H, Fagin JA, Santoro M, Baudin E, Elisei R, Jarzab B, Vasselli JR,
et al: Vandetanib in patients with locally advanced or metastatic
medullary thyroid cancer: A randomized, double-blind phase III
trial. J Clin Oncol. 30:134–141. 2012. View Article : Google Scholar
|
|
23
|
Zhan T, Rindtorff N and Boutros M: Wnt
signaling in cancer. Oncogene. 36:1461–1473. 2017. View Article : Google Scholar :
|
|
24
|
Novellasdemunt L, Antas P and Li VSW:
Targeting Wnt signaling in colorectal cancer. A review in the
theme: Cell signaling: Proteins, pathways and mechanisms. Am J
Physiol Cell Physiol. 309:C511–C521. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Takigawa Y and Brown AMC: Wnt signaling in
liver cancer. Curr Drug Targets. 9:1013–1024. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sastre-Perona A and Santisteban P:
Wnt-independent role of β-catenin in thyroid cell proliferation and
differentiation. Mol Endocrinol. 28:681–695. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sastre-Perona A and Santisteban P: Role of
the wnt pathway in thyroid cancer. Front Endocrinol (Lausanne).
3:312012. View Article : Google Scholar
|
|
28
|
Huczyński A, Antoszczak M, Kleczewska N,
Lewandowska M, Maj E, Stefańska J, Wietrzyk J, Janczak J and
Celewicz L: Synthesis and biological activity of salinomycin
conjugates with floxuridine. Eur J Med Chem. 93:33–41. 2015.
View Article : Google Scholar
|
|
29
|
Meireles AM, Preto A, Rocha AS, Rebocho
AP, Máximo V, Pereira-Castro I, Moreira S, Feijão T, Botelho T,
Marques R, et al: Molecular and genotypic characterization of human
thyroid follicular cell carcinoma-derived cell lines. Thyroid.
17:707–715. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kumarasamy VM and Sun D: Demonstration of
a potent RET transcriptional inhibitor for the treatment of
medullary thyroid carcinoma based on an ellipticine derivative. Int
J Oncol. 51:145–157. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lauf PK, Alqahtani T, Flues K, Meller J
and Adragna NC: Interaction between Na-K-ATPase and Bcl-2 proteins
BclXL and Bak. Am J Physiol Cell Physiol. 308:C51–C60. 2015.
View Article : Google Scholar
|
|
32
|
Ando Y, Tomaru Y, Morinaga A, Burroughs
AM, Kawaji H, Kubosaki A, Kimura R, Tagata M, Ino Y, Hirano H, et
al: Nuclear pore complex protein mediated nuclear localization of
dicer protein in human cells. PLoS One. 6:e233852011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shin YJ, Kumarasamy V, Camacho D and Sun
D: Involvement of G-quadruplex structures in regulation of human
RET gene expression by small molecules in human medullary thyroid
carcinoma TT cells. Oncogene. 34:1292–1299. 2015. View Article : Google Scholar
|
|
34
|
Schweppe RE: Thyroid cancer cell line
misidentification: An update. J Clin Endocrinol Metab. 98:956–957.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
de Jonge HJM, Fehrmann RSN, de Bont ESJM,
Hofstra RM, Gerbens F, Kamps WA, de Vries EG, van der Zee AG, te
Meerman GJ and ter Elst A: Evidence based selection of housekeeping
genes. PLoS One. 2:e8982007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fusco A, Grieco M, Santoro M, Berlingieri
MT, Pilotti S, Pierotti MA, Della Porta G and Vecchio G: A new
oncogene in human thyroid papillary carcinomas and their
lymph-nodal metastases. Nature. 328:170–172. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nikiforov YE: RET/PTC rearrangement in
thyroid tumors. Endocr Pathol. 13:3–16. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nikiforov YE: Thyroid carcinoma: Molecular
pathways and therapeutic targets. Mod Pathol. 21(Suppl 2): S37–S43.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Plaza-Menacho I, Burzynski GM, de Groot
JW, Eggen BJL and Hofstra RMW: Current concepts in RET-related
genetics, signaling and therapeutics. Trends Genet. 22:627–636.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
McIlwain DR, Berger T and Mak TW: Caspase
functions in cell death and disease. Cold Spring Harb Perspect
Biol. 5:a0086562013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fracchiolla NS, Bamonti Catena F,
Novembrino C, Ippolito S, Maisonneuve P and Cortelezzi A: Possible
association between reactive oxygen metabolites and karyotypic
abnormalities in myelodysplastic syndromes. Haematologica.
88:594–597. 2003.PubMed/NCBI
|
|
42
|
Mittler R, Vanderauwera S, Suzuki N,
Miller G, Tognetti VB, Vandepoele K, Gollery M, Shulaev V and Van
Breusegem F: ROS signaling: The new wave? Trends Plant Sci.
16:300–309. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
D'Autréaux B and Toledano MB: ROS as
signalling molecules: Mechanisms that generate specificity in ROS
homeostasis. Nat Rev Mol Cell Biol. 8:813–824. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Antoszczak M, Maj E, Stefańska J, et al:
Synthesis, antiproliferative and antibacterial activity of new
amides of salinomycin. Bioorganic Med Chem Lett. 24:1724–1729
|
|
45
|
Gujral TS, van Veelen W, Richardson DS,
Myers SM, Meens JA, Acton DS, Duñach M, Elliott BE, Höppener JW and
Mulligan LM: A novel RET kinase-β-catenin signaling pathway
contributes to tumorigenesis in thyroid carcinoma. Cancer Res.
68:1338–1346. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lu D, Choi MY, Yu J, Castro JE, Kipps TJ
and Carson DA: Salinomycin inhibits Wnt signaling and selectively
induces apoptosis in chronic lymphocytic leukemia cells. Proc Natl
Acad Sci USA. 108:13253–13257. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lu W, Lin C, Roberts MJ, Waud WR, Piazza
GA and Li Y: Niclosamide suppresses cancer cell growth by inducing
Wnt co-receptor LRP6 degradation and inhibiting the Wnt/β-catenin
pathway. PLoS One. 6:e292902011. View Article : Google Scholar
|
|
48
|
Chen M, Wang J, Lu J, Bond MC, Ren XR,
Lyerly HK, Barak LS and Chen W: The anti-helminthic niclosamide
inhibits Wnt/Frizzled1 signaling. Biochemistry. 48:10267–10274.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hong D, Ye L, Gagel R, Chintala L, El
Naggar AK, Wright J and Kurzrock R: Medullary thyroid cancer:
Targeting the RET kinase pathway with sorafenib/tipifarnib. Mol
Cancer Ther. 7:1001–1006. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Markowitz JN and Fancher KM: Cabozantinib:
A multitargeted oral tyrosine kinase inhibitor. Pharmacotherapy.
38:357–369. 2018. View Article : Google Scholar
|
|
51
|
Cooper MR, Yi SY, Alghamdi W, Shaheen DJ
and Steinberg M: Vandetanib for the treatment of medullary thyroid
carcinoma. Ann Pharmacother. 48:387–394. 2014. View Article : Google Scholar
|
|
52
|
Yakes FM, Chen J, Tan J, Yamaguchi K, Shi
Y, Yu P, Qian F, Chu F, Bentzien F, Cancilla B, et al: Cabozantinib
(XL184), a novel MET and VEGFR2 inhibitor, simultaneously
suppresses metastasis, angiogenesis, and tumor growth. Mol Cancer
Ther. 10:2298–2308. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Maliszewska A, Leandro-Garcia LJ,
Castelblanco E, Macià A, de Cubas A, Goméz-López G, Inglada-Pérez
L, Álvarez-Escolá C, De la Vega L, Letón R, et al: Differential
gene expression of medullary thyroid carcinoma reveals specific
markers associated with genetic conditions. Am J Pathol.
182:350–362. 2013. View Article : Google Scholar
|
|
54
|
Yang D, Wang C, Luo Y, Li X, Song Q, Zhang
J and Xin S: Activated E2F activity induces cell death in papillary
thyroid carcinoma K1 cells with enhanced Wnt signaling. PLoS One.
12:e01789082017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Malumbres M and Barbacid M: Cell cycle,
CDKs and cancer: A changing paradigm. Nat Rev Cancer. 9:153–166.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Bertoli C, Skotheim JM and de Bruin RAM:
Control of cell cycle transcription during G1 and S phases. Nat Rev
Mol Cell Biol. 14:518–528. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kim KY, Park KI, Kim SH, Yu SN, Park SG,
Kim YW, Seo YK, Ma JY and Ahn SC: Inhibition of autophagy promotes
sali-nomycin-induced apoptosis via reactive oxygen species-mediated
PI3K/AKT/mTOR and ERK/p38 MAPK-dependent signaling in human
prostate cancer cells. Int J Mol Sci. 18:E10882017. View Article : Google Scholar
|
|
58
|
Kim SH, Choi YJ, Kim KY, Yu SN, Seo YK,
Chun SS, Noh KT, Suh JT and Ahn SC: Salinomycin simultaneously
induces apoptosis and autophagy through generation of reactive
oxygen species in osteosarcoma U2OS cells. Biochem Biophys Res
Commun. 473:607–613. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Polakis P: Wnt signaling and cancer. Genes
Dev. 14:1837–1851. 2000.PubMed/NCBI
|
|
60
|
Fahim M, del Valle G and Pressman BC:
Comparison of the effects of the ionophore salinomycin and
adrenaline on the haemodynamics and work efficiency of the dog
heart. Cardiovasc Res. 20:145–152. 1986. View Article : Google Scholar : PubMed/NCBI
|