Introduction
Breast cancer is the most common type of cancer and
the leading cause of cancer-associated mortality and morbidity in
women worldwide (1,2). The mortality rate for this disease
has decreased due to early diagnosis, but treatment outcomes of
patients with advanced breast cancer remain poor (3). There are currently several options
for the treatment of breast cancer such as surgery, radiotherapy
and chemotherapy, but their use is limited by adverse effects, drug
resistance and rapid cancer cell metastasis (4). Therefore, an effective treatment for
certain aggressive forms of breast cancer, such as inflammatory
breast cancer and triple-negative breast cancer (TNBC), is still
required. TNBC cannot be treated with hormonal therapy, as TNBC
cells do not express receptors for estrogen, progesterone and
epidermal growth factor (5).
Despite some considerable limitations, the leading cancer
treatments have generally improved over the years; though the
financial cost remains high (6).
Hence, cancer research should move towards developing and applying
herbal treatment strategies with lower costs and fewer side effects
to patients.
Bridelia ovata and Croton
oblongifolius, from the Euphorbiaceae family, are traditional
herbs found in Thailand; Thai traditional medicine uses the dried
leaf of B. ovata as an expectorant and a laxative, whereas
the bark is used as a medicinal astringent (7). Few previous phytochemical studies
have demonstrated the presence of triterpenes and phytosterols in
B. ovata (8,9); its crude ethanolic extract has been
shown to inhibit the invasive and migratory abilities of HepG2
cells (10), thus the present
study focused on the identification of these compounds in B.
ovata.
C. oblongifolius has been used as a medicinal
plant for the treatment of several diseases and ailments; for
example, the leaf is used as a blood tonic for general weakness and
poor appetite, and the flower is used to treat flatworm infections.
Furthermore, the fruit is used to alleviate dysmenorrhea, the seed
acts as a purgative (11) and the
root is used in the treatment of dysentery (12). In addition, C. oblongifolius
bark has been used for the treatment of dyspepsia, chronic liver
enlargement and remittent fever (13). Moreover, in Thai traditional
medicine, C. oblongifolius is utilized in combination with
C. sublyratus to treat gastric ulcers and gastric cancers
(14,15). Several types of phytochemical have
been isolated from C. oblongifolius, including megastigmane
glycosides (16), labdane
diterpenoids (11,17), clerodanes (18-21),
halimanes (22), cembranes
(21,23,24),
pimarane-types (acantoic acid) (25) and (-)-ent-kaur-16-en-19-oic
acid (12). A study investigating
clerodanes from C. oblongifolius showed that this
phytochemical has cytotoxic effect against human cancer cell lines,
including HepG2, SW620, CHAGO, KATO3 and BT474 (17). However, there are currently no
reports of the activity of B. ovata and C.
oblongifolius ethyl acetate extracts against TNBC cells, and to
the best of our knowledge, the underlying mechanisms mediating the
induction of cancer cell death remain unknown.
In the present study, apoptosis (programmed cell
death) was investigated. Apoptosis is an important process for
normal tissue homeostasis (26),
and any disruption of the underlying mechanisms may cause the onset
of a variety of diseases, including autoimmunity, neurodegenerative
diseases and cancer (27).
Therefore, therapeutic strategies targeting apoptosis have been
applied to treat these diseases (28). The two primarily routes of
apoptosis are the extrinsic and intrinsic pathways; the extrinsic,
or death receptor pathway is induced by the binding of death
ligands (such as FasL, TRAIL and TNF) to specific receptors (such
as FAS, TRAILR1-2 and TNFR1) at the cell membrane (29). Subsequently, the intracellular
death domain of the receptor recruits the adaptor FAS-associated
death domain protein and the initiator caspase 8. These form the
death-inducing signaling complex (DISC). DISC proteolytically
auto-activates caspase 8, which then cleaves and activates the
effector caspase 3 to induce apoptosis (30,31).
The intrinsic, or mitochondrial pathway, is
triggered by diverse stimuli, including DNA damage,
chemotherapeutic drugs and oxidative stress. These stimuli induce
the loss of mitochondrial transmembrane potential, permitting
cytochrome c release from the mitochondria intermembranous
space into the cytosol, resulting in apoptosome formation. The
apoptosome is a protein complex comprising cytochrome c,
apoptosis-activating factor 1, adenosine triphosphate (ATP), and
procaspase-9 (32). The apoptosome
autoactivates caspase 9, which in turn cleaves and activates
caspase 3 (33), resulting in
apoptosis. Mitochondrial apoptosis is controlled by the Bcl-2
protein family. This comprises anti-apoptotic proteins, including
Bcl-2, Bcl-xL and Mcl-1, and two other groups of pro-apoptotic
proteins, including the multi-domain proteins BAX and Bcl-2
homologous antagonist/killer (BAK), and BH3-only proteins
Bcl-2-like protein 11 (BIM), and
phorbol-12-myristate-13-acetate-induced protein 1 (NOXA). During
apoptosis, the expression of anti-apoptotic molecules is
downregulated, and regulatory BH3-only proteins induce the
formation of transport channels on the outer mitochondrial
membrane, comprised of dimers of BAX and/or BAK (34). These channels promote cytochrome
c release and the sequential activation of caspase 9 and 3
(35). Therefore, the aim of the
present study was to investigate the pro-apoptotic potential of BEA
and CEA against MDA-MB-231 triple-negative breast cancer cells.
This cell line has the highest potential for invasiveness (36) and is commonly used as a model for
late-stage breast cancer (37).
Materials and methods
Plant materials
The trunks of B. ovata and C.
oblongifolius were collected from northeastern Thailand by the
plant genetic conservation project under the Royal Initiative of
Her Royal Highness Princess Maha Chakri Sirindhorn (RSPG). The
plants were extracted using ethyl acetate as a solvent, and
authenticated by Professor Dr. Bungorn Sripanidkulchai (Center for
Research and Development of Herbal Health Products, Department of
Pharmaceutical Chemistry, Faculty of Pharmaceutical Sciences, Khon
Kaen University, Thailand); the voucher numbers of B. ovata
and C. oblongifolius are TT-OC-SK-1253 and TT-OC-SK-1215,
respectively. The collected plants were washed, cut and dried, and
then crushed into powders. The extracts were obtained using ethyl
acetate at a plant-to-solvent ratio of 1:5 at room temperature for
72 h, filtered using Whatman filter paper no. 1 and then
centrifuged at 500 × g for 10 min to obtain the supernatant. The
extracts were then concentrated under a rotary evaporator and the
final products were weighed prior to determination of the
percentage yield. For the cell treatment, the crude ethyl acetate
extracts were dissolved in dimethyl sulfoxide (DMSO) to obtain an
appropriate concentration of the extract stock solutions (to avoid
DMSO-associated cellular toxicity). The final concentrations of
DMSO under the treatment conditions were less than 0.2%, at which
they were not cytotoxic (38).
Reagents and assay kits
The chemicals and solvents used were of analytical
grade, which included chloroform, ethyl acetate, ferric chloride,
hydrochloric acid, sulfuric acid, acetic acid anhydride, sodium
hydroxide, copper acetate, potassium iodide and mercuric chloride
(all Merck KGaA). Dulbecco's modified Eagle's medium (DMEM),
DMEM/Ham's F-12, fetal bovine serum (FBS), horse serum (HS),
phosphate-buffered saline (PBS), trypsin-EDTA solution, penicillin
and streptomycin were purchased from Gibco; Thermo Fisher
Scientific, Inc.
3-(4,5-Dimethythiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT),
the Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide
(PI) kit, 3,3′-dihexyloxacarbocyanine iodide (DiOC6),
dichlorodihydrofluorescein diacetate (DCFH-DA), dihydroethidium
(DHE), DMSO, epidermal growth factor (EGF), cholera toxin,
hydrocortisone and insulin were purchased from Sigma-Aldrich; Merck
KGaA. Caspase 9 (LEHD-paranitroaniline; LEHD-p-NA), caspase
8 (IETD-p-NA) and caspase 3 (DEVD-p-NA) activity
colorimetric assay kits were obtained from Invitrogen; Thermo
Fisher Scientific, Inc. The primers and RevertAid First Strand cDNA
Synthesis kit were obtained from Thermo Fisher Scientific, Inc. The
RNA Prep Pure kit was purchased from Tiangen Biotech Co., Ltd and
the SensiFAST SYBR Lo-ROX kit was purchased from Bioline; Meridian
Biosciences. The mitochondria/cytosol fractionation kit, primary
antibodies against NOXA (ab13654), BAX (ab32503), Bcl-xL (ab2568),
cytochrome c (ab6400), β-actin (ab8227), COX-4 (abl6056) and
Rip3 (ab56164), rabbit HRP-conjugated secondary antibody (ab97051),
and mouse HRP-conjugated secondary antibody (ab97046) were
purchased from Abcam. The ECL Prime Western Blotting System was
purchased from GE Healthcare.
Phytochemical screening
The ethyl acetate crude extracts were screened for
the presence of bioactive chemical constituents, including
alkaloids, flavonoids, phytosterols, terpenoids, saponins, phenols
and tannins using the standard methods outlined below (39-41).
The results were reported as -, absent; +, present; ++
abundant.
Detection of alkaloids
Dry extracts (50 mg each) were dissolved in 1 ml of
2% hydrochloric acid and filtered using Whatman filter paper no. 1.
The filtrates were treated with 100 μ1 potassium mercuric
iodide, and a yellow-colored precipitate indicated the presence of
alkaloids.
Detection of flavonoids
Dry extracts (100 mg each) were dissolved in 4 ml
ethyl acetate and filtered using Whatman filter paper no. 1. The
filtrates were treated with 200 μl sodium hydroxide solution
followed by 1% sulfuric acid. The formation of an intense yellow
color became colorless when 1% sulfuric acid was added, which
indicated the presence of flavonoids.
Detection of phytosterols
Dry extracts (50 mg each) were dissolved in 1 ml
chloroform and filtered using Whatman filter paper no. 1. The
filtrates were treated with 100 μl acetic anhydride, boiled
at 90-100°C and then subsequently cooled at room temperature. Then,
1 ml 95% sulfuric acid was added and the formation of a brown ring
at the junction indicated the presence of phytosterols.
Detection of triterpenoids
Dry extracts (50 mg each) were dissolved in 1 ml
chloroform and filtered using Whatman filter paper no. 1. The
filtrates were treated with 100 μ1 95% sulfuric acid, gently
shaken and then allowed to stand for 5 min. Formation of a
golden-yellow color indicated the presence of triterpenoids.
Detection of diterpenes
Dry extracts (50 mg each) were dissolved in 1 ml
distilled water and treated with 200 μ1 copper acetate
solution. The Appearance of an emerald green color indicated the
presence of diterpenes.
Detection of saponins
Dry extracts (50 mg each) were dissolved in 20 ml
distilled water and shaken in a graduated cylinder for 15 min. The
formation of a 1-cm layer of foam indicated the presence of
saponins.
Detection of phenols
Dry extracts (50 mg each) were dissolved in 1 ml
distilled water and treated with 100 μl 2% ferric chloride.
The formation of a bluish-green or black coloration confirmed the
presence of phenols.
Detection of tannins
Dry extracts (50 mg each) were boiled at 90-100°C in
1 ml distilled water for 5 min and then filtered using Whatman
filter paper no. 1; 100 μ1 0.1% ferric chloride was added to
the filtrates, and the appearance of a brownish-green or a
blue-black color indicated the presence of tannins.
Gas chromatography-mass spectrometry
(GC-MS) analysis
BEA and CEA were subjected to GC-MS detection, which
was performed using a GC 7890A attached to a MSD 5975C (Agilent
Technologies, Inc.). The chromatograph was fitted with a HP5-MS
capillary column (30 m × 0.25 mm, 0.25-μm film thickness)
using a temperature program initially set to 100-150°C, at a rate
of 3°C/min for 16.67 min. Then, the temperature was raised to 300°C
at a rate of 5°C/min, for 30 min, and then held at 300°C for 10
min; the total run time was 56.67 min. The injector was set at
280°C and the injection volume was at 1.0 μ1 in the split
mode (2:1). The flow speed of helium, the carrier gas, was 1
ml/min. The mass spectra were scanned from m/z 50-550, and the
electron impact ionization energy was set at 70 eV. Identification
of the components was based on the comparison of their mass spectra
and retention times with those of the standard authentic compounds.
This was accomplished using Agilent ChemStation Integrator (Agilent
Technologies, Inc.) software computer matching using the National
Institute Standard and Technology Library (nist.gov/nist-research-library).
Cell culture
The human MDA-MB-231 breast cancer cell line was
obtained from the American Type Culture Collection (ATCC). The
cells were cultured in DMEM and supplemented with 10% FBS, 100 U/ml
penicillin and 100 μg/ml streptomycin. The human MCF10A
mammary epithelial cell line was obtained from the ATCC and
cultured in DMEM/Ham's F-12 supplemented with 5% HS, 20 ng/ml EGF,
0.5 mg/ml hydrocortisone, 100 ng/ml cholera toxin, 10 μg/ml
insulin, 50 U/ml penicillin and 50 μg/ml streptomycin. Both
cell lines were cultured at 37°C in a 5% CO2
atmosphere.
Cytotoxicity determination by MTT
assay
MDA-MB-231 and MCF10A cells were seeded into 96-well
plates at 5×104 cells/ml and treated with BEA, CEA or
Doxorubicin at various concentrations. The cells were then
incubated for 24 h at 37°C before detecting cell viability using
the MTT method, as previously described (42). Briefly, MTT reagent was added to
the cells, which were then incubated for 4 h at 37°C. The formazan
crystals were dissolved using DMSO and the optical density was
measured at 540 nm (with a reference wavelength of 630 nm). The
results were expressed as a percentage of cell viability and were
compared with untreated control cells. The inhibitory concentration
(IC)10, IC20 and IC50 values were
calculated in the presence of each of the extracts.
Apoptosis investigation using annexin
V-FITC and PI staining
MDA-MB-231 cells were treated with BEA and CEA at
IC10, IC20 and IC50 for 24 h, at
37°C, and then stained with annexin V-FITC and PI for 15 min. The
percentages of apoptotic cells were quantified by annexin V-FITC
positive staining (43), detected
using the BD FACSAria™ III Cell Sorter flow cytometer (BD
Biosciences) and then analyzed with BD FACSDiva software version
7.0 (BD Biosciences). The treated cell results were compared with
untreated control cells.
Assessment of mitochondrial transmembrane
depolarization
To detect changes in mitochondrial transmembrane
potential (A1 I'm), MDA-MB-231 cells were treated with
BEA and CEA for 24 h at 37°C. BEA and CEA-treated and untreated
control cells were stained using DiOC6 at a final
concentration of 40 nM for 15 min at 37°C. Then, the cells were
washed and analyzed using a FACSCAtia™ III Cell Sorter flow
cytometer (BD Biosciences) (44).
Measurement of reactive oxygen species
(ROS) generation
The levels of intracellular ROS were measured, and
included those of superoxide anion, hydrogen peroxide and peroxide
radicals; two intracellular redox-sensitive fluorescent dyes, DHE
and DCFH-DA, were used according to previously described methods
(45). Briefly, after the
MDA-MB-231 cells were treated with BEA and CEA at IC10,
IC20 and IC50 for 12 h, the cells were
stained with DHE and DCFH-DA at a final concentration of 2 for 30
min at 37°C. Fluorescence intensity was measured using a
fluorescence microplate reader (BioTek Instruments, Inc.) at 535 nm
excitation and 635 nm emission wavelengths for DHE, and 485 nm
excitation and 525 nm emission wavelengths for DCFH-DA (46,47).
The results were compared between treated and untreated cells. 3%
H2O2 was used as the positive control for
DCFH-DA staining, whereas FeSO4 was used as the positive
control for DHE staining.
Determination of caspase activities
Caspase 3, 8 and 9 activities were measured in
cellular protein extracts using colorimetric assay kits. Following
treatment with BEA and CEA at IC10, IC20 and
IC50, the MDA-MB-231 cells were lysed and the total
protein was quantified using a Bradford protein assay. Caspase
activity was detected using the specific substrates
DEVD-p-NA, IETD-p-NA and LEHD-p-NA for caspase
3, 8 and 9, respectively. The absorbance was then determined at 405
nm using a microplate reader (BioTek Instruments, Inc.) (44). The results were compared between
treated and untreated cells.
Analysis of gene expression levels by
reverse transcription-quantitative (RT-qPCR)
After treatment of the MDA-MB-231 cells with BEA and
CEA at IC10, IC20 and IC50 (for 24
h at 37°C), the total RNA was extracted using the Tiangen RNA Prep
Pure kit, and then reverse transcribed into cDNA using the
RevertAid First Strand cDNA Synthesis kit. mRNA expression levels
of NOXA, BAX and Bcl-xL were quantified using the SensiFAST SYBR
Lo-ROX kit (Bioline; Meridian Biosciences) and the 7500 Fast
Real-time PCR system (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The relative gene expression levels were analyzed using the
2-ΔΔCq method (48)
with GAPDH as the housekeeping gene. The primers for NOXA, BAX,
Bcl-xL and GAPDH were as follows: NOXA, forward 5′GCT GGA AGT
CGAGTG TGC TA3′ and reverse 5′CCTGAGCAGAAGAGTTTG GA3′; BAX, forward
5′AGC AGATCATGAAGACAGGGG3′ and reverse 5′ACACTCGCT CAGCTTCTTGG3′;
Bcl-xL, forward 5′GCTGGGACACTTT TGT GGAT3′ and reverse 5′TGT CTG
GTCACT TCC GAC TG3′; and GAPDH, forward 5′TGCACCACCAACTGCTTAGC3′
and reverse 5′GGCATGGACTGTGGTCATGAG3′. The primers were designed
and selected using the Primer-BLAST tool (ncbi.nlm.nih.gov/tools/primer-blast/) (48,49).
Western blotting
To determine the protein expression levels in
MDA-MB-231 cells, the cells were seeded into culture dishes and
treated with BEA or CEA for 24 h at IC10,
IC20 and IC50; untreated MDA-MB-231 cells
were used as the control. Following treatment, the cells were
harvested and lysed using RIPA lysis buffer supplemented with a
protease inhibitor. The mitochondria/cytosol fractionation kit was
used to extract cytosolic and mitochondrial proteins per the
manufacturer's protocol. Protein concentrations were measured using
a Bradford protein assay (50),
and protein expression levels were assessed using western blot
analysis (51). A total of 20
μg (for NOXA, BAX, Bcl-xL, Rip3, COX-IV and p-actin) and 80
μg (for cytochrome c) total protein per lane was
loaded onto a 12% gel, resolved using SDS-PAGE and subsequently
transferred onto nitrocellulose membranes. The membranes were
blocked with 5% skim milk at room temperature for 1 h, and then
incubated with primary antibodies against NOXA, BAX, Bcl-xL,
cytochrome c, Rip3 and COX-IV (1:1,000) at 4°C overnight
(B-actin was used at 1:20,000). Next, the membranes were washed and
incubated with HRP-conjugated secondary antibodies (1:5,000) for 2
h at room temperature. For visualization, an ECL Prime Western
Blotting System was used and antibody-specific chemiluminescent
bands were detected using an Omega Lum W Imager (Gel Company,
Inc.). Quantification of the band density was conducted using
ImageJ 1.52p (National Institutes of Health). B-actin and COX-IV
were used as the loading controls for whole cell/cytosolic and
mitochondrial proteins, respectively.
Statistical analysis
The data are presented as the mean ± standard
deviation (SD), calculated from three independent experiments
(unless otherwise stated). The statistical differences between
multiple-group comparisons were determined using the Kruskal-Wallis
test and Dunn's multiple comparisons post-hoc test, and P<0.05
was considered to indicate a statistically significant difference.
All statistical analyses were performed using SPSS version 22.0.
(IBM Corp.).
Results
Percentage yield and phytochemical
screening of BEA and CEA
The percentage yields of BEA and CEA were 0.31 and
0.95%, respectively. For phytochemical screening of the extracts,
standard methods were applied (41). BEA contained alkaloids, flavonoids,
phytosterols, triterpenoids, saponins, phenols and tannins with an
absence of diterpenoids. The CEA results revealed the presence of
alkaloids, flavonoids, phytosterols, triterpenoids, diterpenoids
and tannins with an absence of saponins and phenols. The results of
these qualitative phytochemical analyses are presented in Table I.
 | Table IPhytochemical screening test of BEA
and CEA. |
Table I
Phytochemical screening test of BEA
and CEA.
|
Phytoconstituent | BEA | CEA |
|---|
| Alkaloids | + | + |
| Flavonoids | ++ | ++ |
| Phytosterols | ++ | + |
| Triterpenoids | ++ | + |
| Diterpenoids | | ++ |
| Saponins | + | − |
| Phenols | ++ | − |
| Tannins | ++ | + |
Identification of BEA and CEA compounds
using GC-MS
The characterization of BEA and CEA extracts are
presented in Table II. A total of
24 BEA compounds and 12 CEA compounds were identified. Their
respective retention times and percentages of the total are stated.
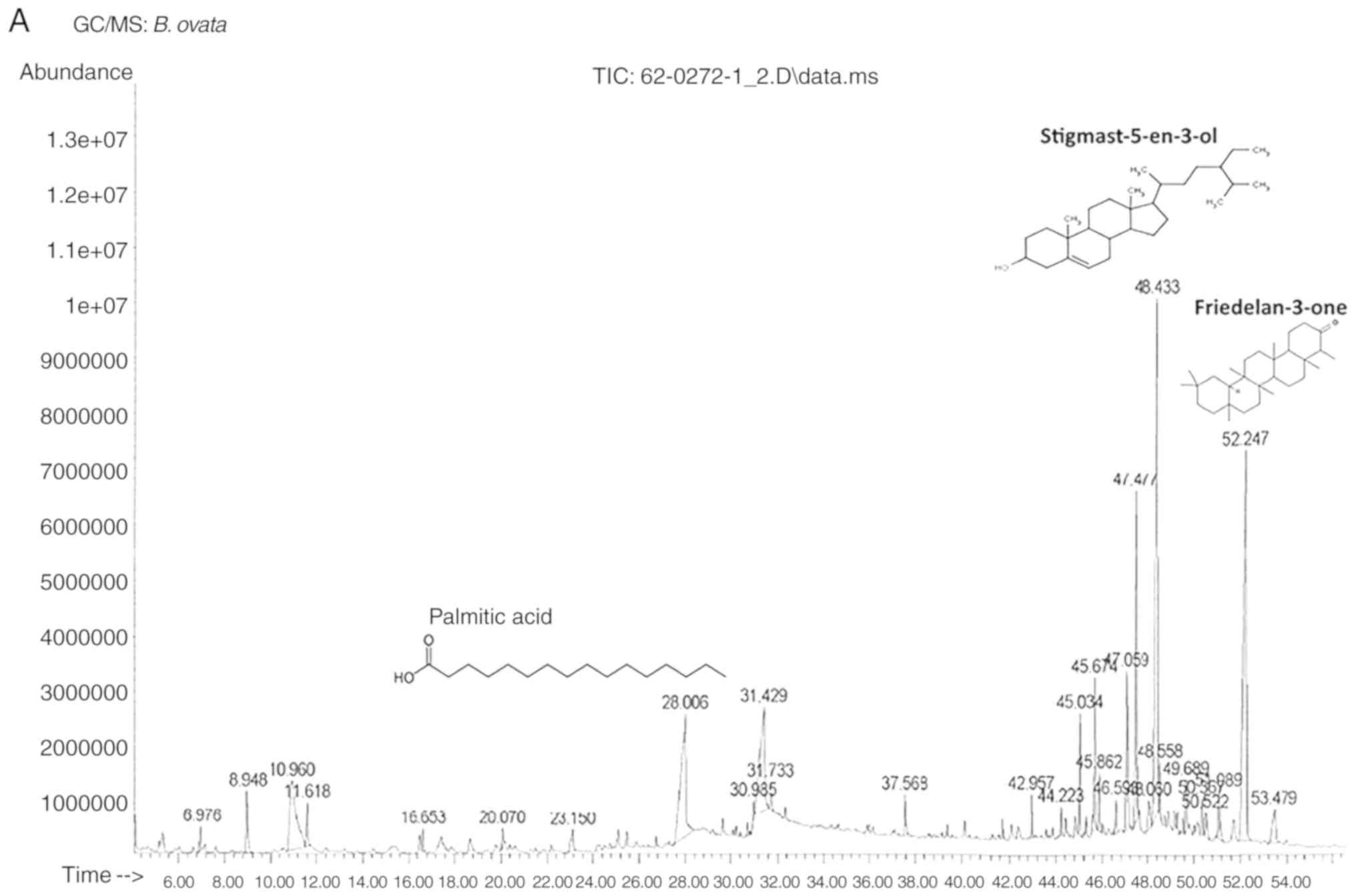
The GC-MS chromatogram and structures of the 3 major identified
compounds of each extract are presented in Fig. 1. The GC-MS results show that the
primary phytoconstituent of BEA is friedelan-3-one (20.1%) and that
the primary active compound of CEA is kaur-16-en-18-oic acid
(36.1%).
 | Table IIPhytochemical analyses and identified
compound profiles with retention time, name and percentage of total
in BEA and CEA treatment. |
Table II
Phytochemical analyses and identified
compound profiles with retention time, name and percentage of total
in BEA and CEA treatment.
A, BEA
|
|---|
| Retention time
(min) | Compounds | % of total |
|---|
| 8.948 |
2-methoxy-4-vinylphenol | 1.62 |
| 10.960 |
1,2,3-benzenetriol | 7.70 |
| 11.618 | Benzaldehyde,
4-hydroxy-3-methoxy- | 1.15 |
| 16.653 | Benzene,
1,2,3-trimethoxy-5 (2-propenyl) | 0.43 |
| 20.070 | Benzaldehyde,
4-hydroxy-3,5-dimethoxy- | 0.43 |
| 23.150 | Tetradecanoic
acid | 0.88 |
| 28.006 | Palmitic acid | 10.28 |
| 30.986 | Linoleic acid | 0.25 |
| 31.429 | Oleic acid | 7.92 |
| 31.733 | Stearic acid | 0.63 |
| 37.568 |
Di-n-octylphthalate | 0.72 |
| 42.957 | 1-Nonadecene | 0.69 |
| 45.674 | 1-Hexacosanol | 2.59 |
| 45.862 | α-Tocopherol | 1.03 |
| 46.593 | Cirsilineol | 0.83 |
| 47.059 | Campesterol | 4.18 |
| 47.477 | Stigmasterol | 7.74 |
| 48.060 |
24-n-propylidenecholesterol | 1.06 |
| 48.433 |
Stigmast-5-en-3-ol | 19.55 |
| 48.558 | Fucosterol | 1.09 |
| 49.689 |
Stigmasta-3,5-dien-7-one | 1.18 |
| 50.367 |
Stigmast-4-en-3-one | 0.92 |
| 50.522 |
9,19-Cyclolanostan-3-ol, 24-methylene-,
(3.beta.)- | 0.80 |
| 52.247 |
Friedelan-3-one | 20.09 |
|
B, CEA
|
| Retention time
(min) | Compounds | % of total |
| 5.589 | Benzoic acid | 4.54 |
| 27.688 | Palmitic acid | 2.38 |
| 29.082 | Biformen | 1.15 |
| 29.210 |
(-)-Kaur-16-ene | 2.00 |
| 33.331 |
4-Hydroxy-3,5,4′-trimethoxystilbene | 3.25 |
| 36.634 | Kaur-16-en-18-oic
acid | 36.06 |
| 36.794 |
9-Isopropylidene-5,6,7,8-tetramethyl-1,4-dihydro-1,4-methano-naphthalene | 2.66 |
| 43.416 | Aflatoxin G1 | 2.54 |
| 47.055 | Campesterol | 1.25 |
| 47.413 | Stigmasterol | 2.52 |
| 48.286 |
Stigmast-5-en-3-ol | 4.70 |
| 49.673 |
Stigmasta-3,5-dien-7-one | 3.06 |
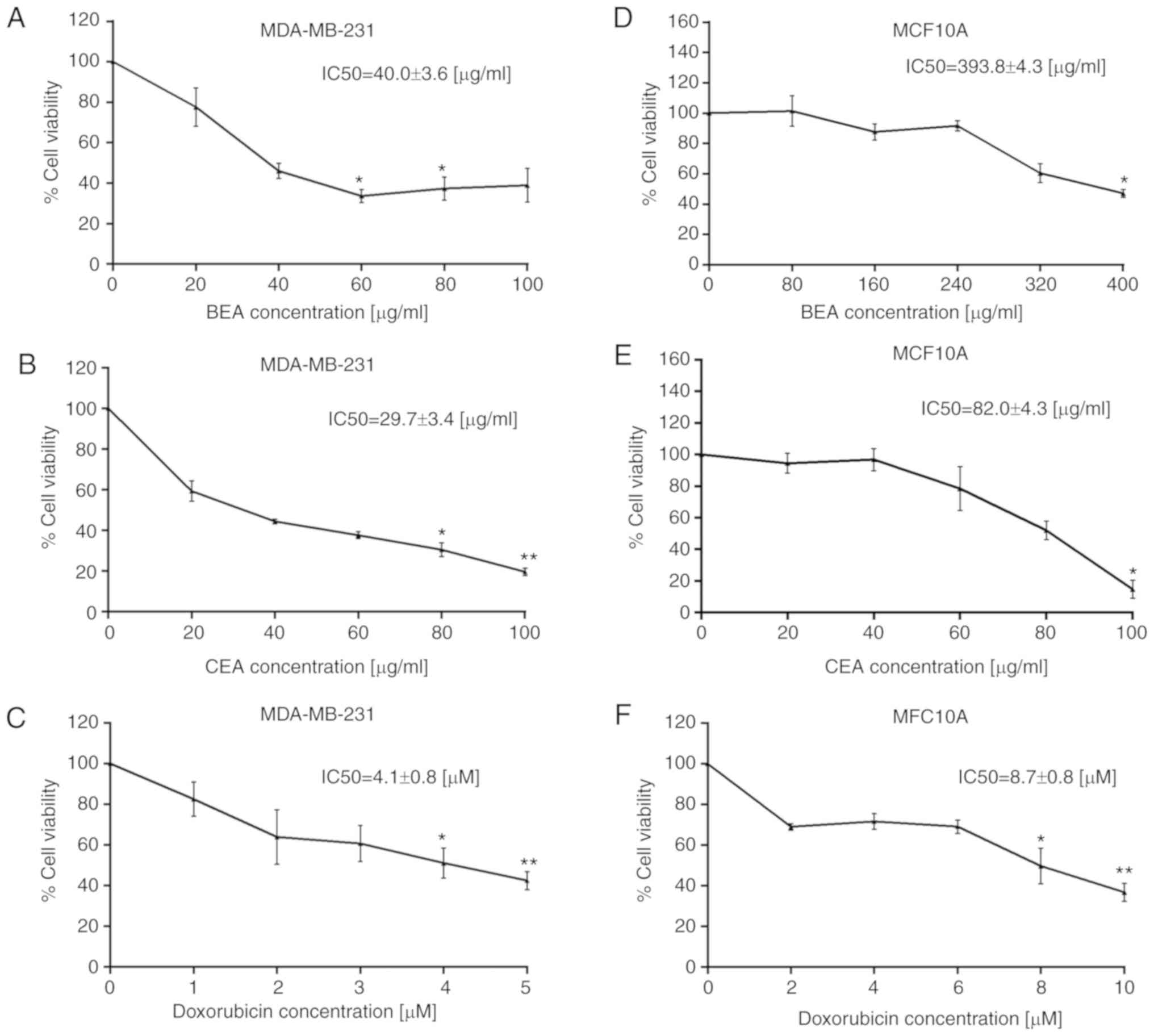
Cytotoxicity of BEA and CEA in MDA-MB-231
and MCF10A cells
BEA and CEA exhibited a cytotoxic effect on
MDA-MB-231 and MCF10A cells. The percentage of cell viability was
calculated compared with the untreated control cells (100% cell
viability). The IC10, IC20, IC50
and selectivity index (SI) of BEA, CEA and doxorubicin for both
cell types were calculated (Table
III). BEA and CEA significantly decreased MDA-MB-231 cell
viability in a dose-dependent manner (Fig. 2A and B). The IC50 of BEA
and CEA were 40.0±3.6 and 29.7±3.4 μg/ml, respectively.
However, BEA and CEA also decreased the viability of MCF10A cells
(Fig. 2D and E) but at higher
concentrations compared with their effect on MDA-MB-231 cells. The
results are in accordance with the selectivity index criteria:
SI>3=high selectivity and SI<3=less selectivity (52,53).
Therefore, BEA possessed a high SI (9.84), whereas CEA had less
selectivity (2.76). Doxorubicin, the conventional chemotherapeutic
drug used for breast cancer (54),
was used to treat both cell types (Fig. 2C and F). The IC50 of
doxorubicin in MCF10A cells was higher than that in MDA-MB-231
cells and the SI of both BEA and CEA were higher than doxorubicin
(2.13). This suggests that these two extracts have more selectivity
towards breast cancer cells than noncancerous cells, which may
relate to a reduction in adverse effects.
 | Table IIIIC10, IC20 and
IC50 values in MDA MB 231 cells and IC50 in
MCF10A cells treated with BEA, CEA and doxorubicin. |
Table III
IC10, IC20 and
IC50 values in MDA MB 231 cells and IC50 in
MCF10A cells treated with BEA, CEA and doxorubicin.
A, MDA MA 231
|
|---|
| Concentration | BEA ± SD
(µg/ml) | CEA ± SD
(µg/ml) | Doxorubicin ± SD
(µM) |
|---|
|
IC10 | 7.6±1.9 | 3.9±0.7 | - |
|
IC20 | 14.4±2.4 | 8.5±1.3 | - |
|
IC50 | 40.0±3.6 | 29.7±3.4 | 4.1±0.8 |
|
| B, MCF10A | | | |
|
| Concentration | BEA ± SD,
µg/ml | CEA ± SD,
µg/ml | Doxorubicin ± SD
(µM) |
|
|
IC50 | 393.8±4.3 | 82.0±4.3 | 8.7±0.8 |
| SIa | 9.8 | 2.8 | 2.1 |
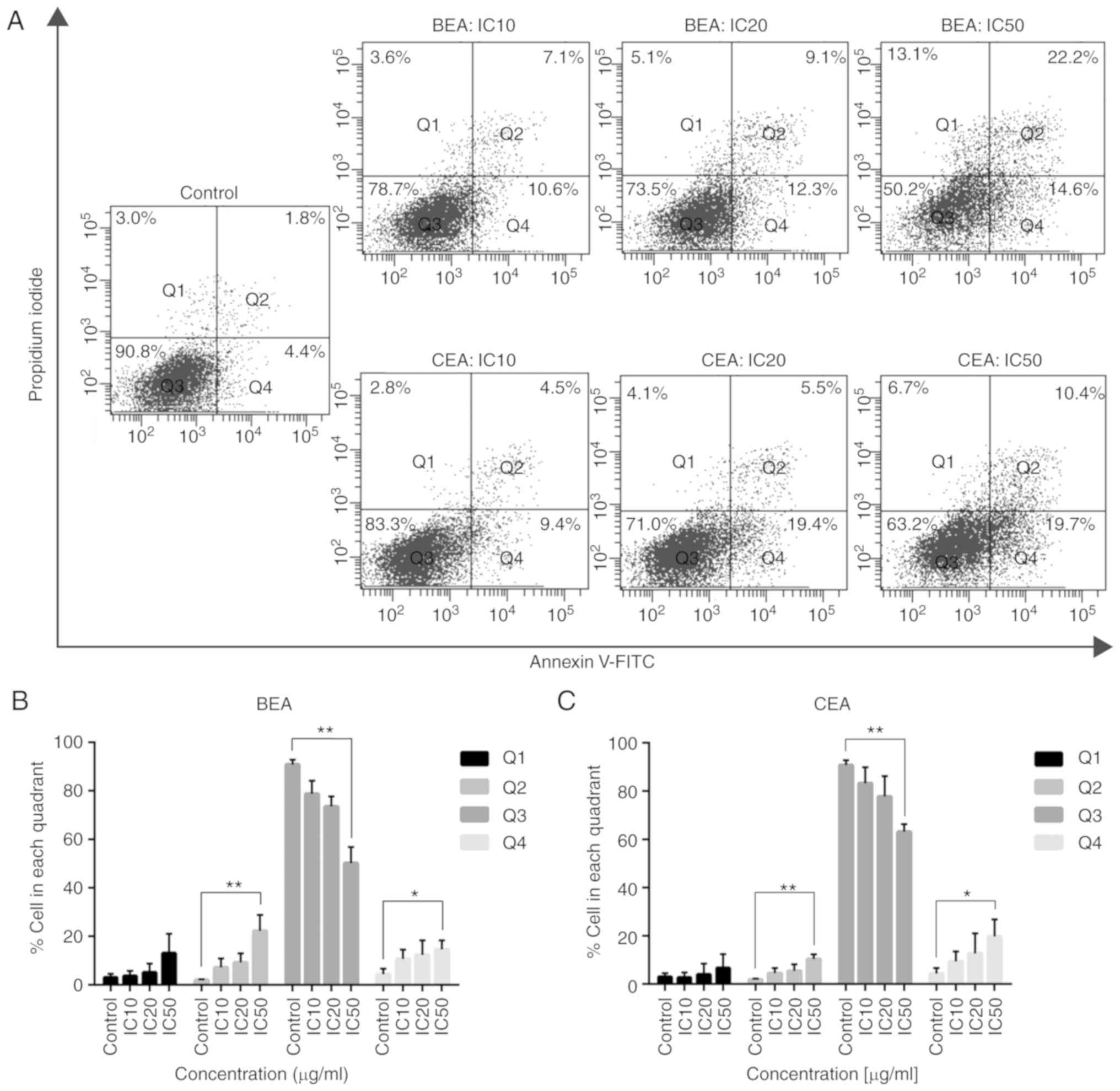
Mode of cell death induced by BEA and CEA
in MDA-MB-231 cells
Annexin V-FITC/PI staining was employed to determine
the mode of cell death in MDA-MB-231 cells after treatment with
both extracts. Dot plot analyses show the percentage of cells in
each quadrant (Fig. 3A). The
percentages of cells in early (Q4) and late apoptosis (Q2) were
significantly increased compared with the control, whereas the
percentage of living cells (Q3) was significantly decreased after
BEA treatment at IC50 concentration compared with the
control; similar results were observed following CEA treatment
(Fig. 3B and C). However, the
number of necrotic cells in Q1 was increased, but not significantly
compared with the control cells; supplementary data demonstrates
that necroptosis marker protein RIP3 did not change the expression
levels following BEA and BEA treatment (Fig. S1). Therefore, BEA and CEA induced
MDA-MB-231 cell death via the apoptosis pathway.
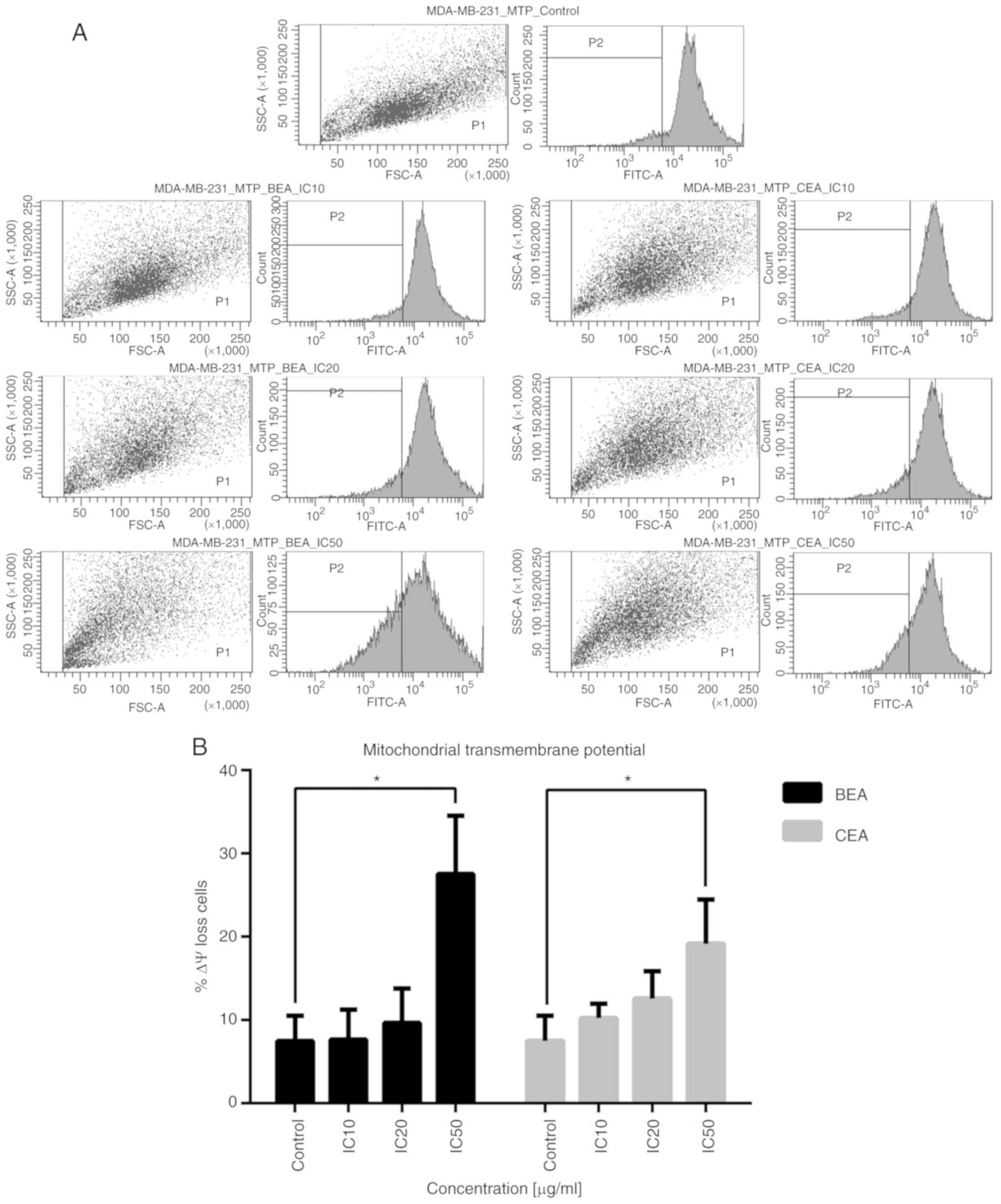
Reduction of Δψm and intracellular ROS
generation
Mitochondrial dysfunction is a key event in the
intrinsic apoptosis pathway, and depolarization of Δψm, which
occurs during apoptosis (55), was
measured to determine the level of mitochondrial disruption induced
by BEA and CEA. The percentage of MDA-MB-231 cells with Δψm loss
was significantly increased after treatment with BEA and CEA at
IC50 (Fig. 4B). The
reduction of Δψm indicates mitochondrial dysfunction. The
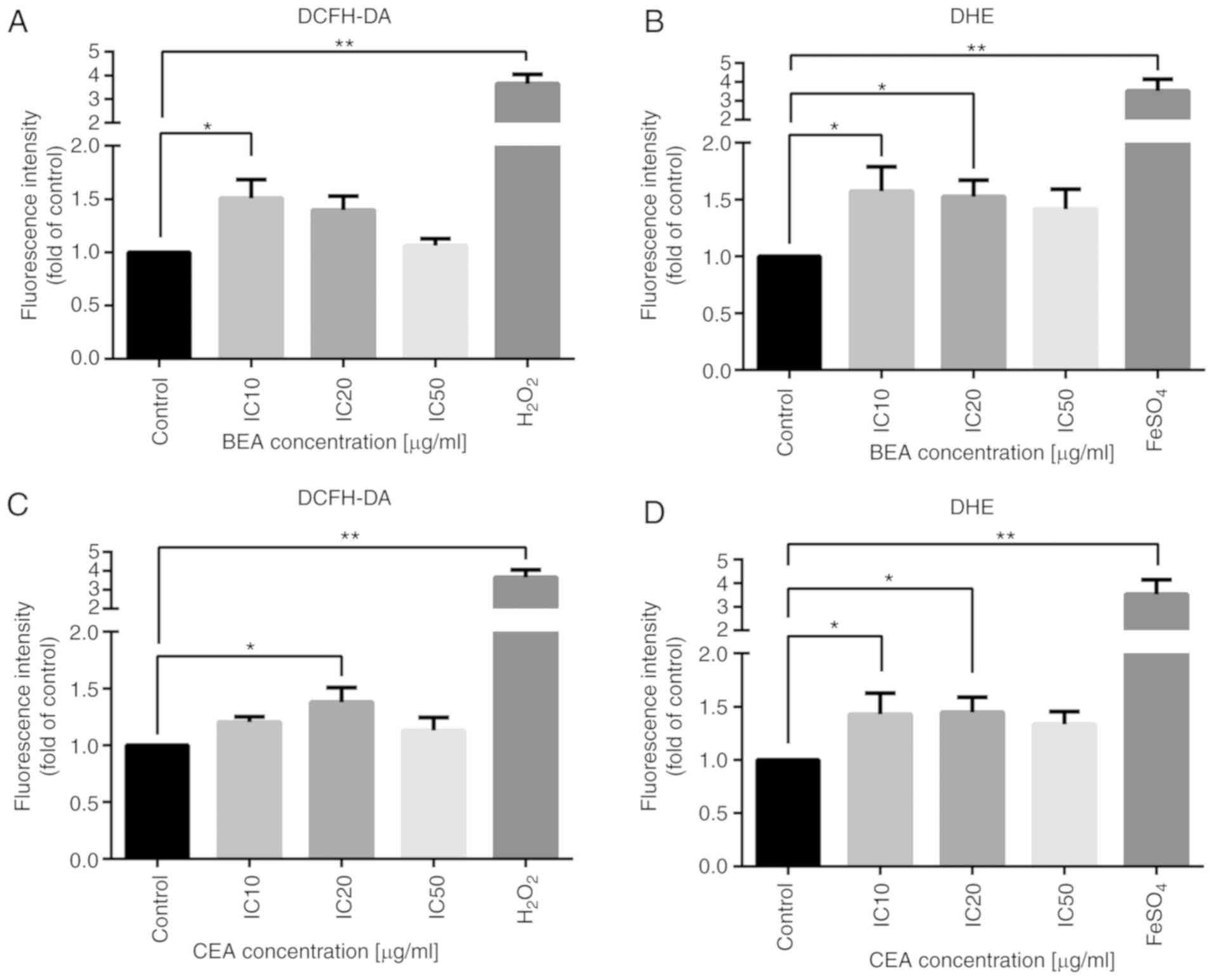
intracellular ROS were measured using two fluorescent dyes. DCFH-DA
is widely used probe for detecting H2O2 and
oxidative stress and DHE is another used probe for detecting
intracellular O•-2 (56). The fluorescent intensity of both
DCFH-DA and DHE probes were increased following BEA and CEA
treatment, compared with the untreated control (Fig. 5), thus H2O2
and O•-2 were generated. However, the level of ROS
generation did not increase in a dose-dependent manner (though they
were significantly increased). This indicates that BEA and CEA
induced oxidative stress and mitochondrial dysfunction during
MDA-MB-231 cell apoptosis.
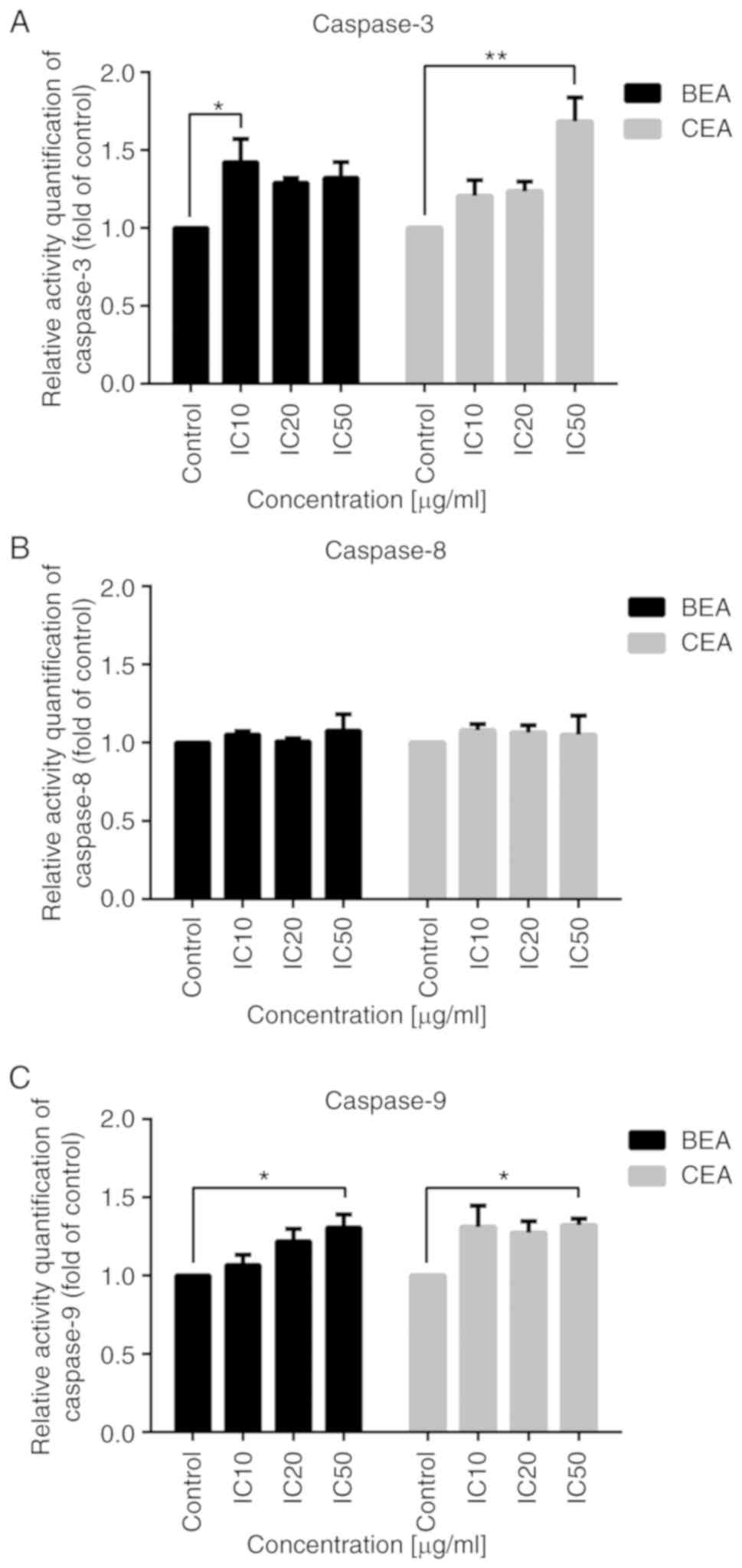
Effect of BEA and CEA on caspase
activity
In MDA-MB-231 cells, caspase 3 and 9 activities were
significantly increased following treatment with BEA and CEA
(Fig. 6A and C), while caspase 8
activity was not significantly altered, compared with untreated
control cells (Fig. 6B). These
results indicate that BEA and CEA induce MDA-MB-231 apoptosis
through the intrinsic pathway.
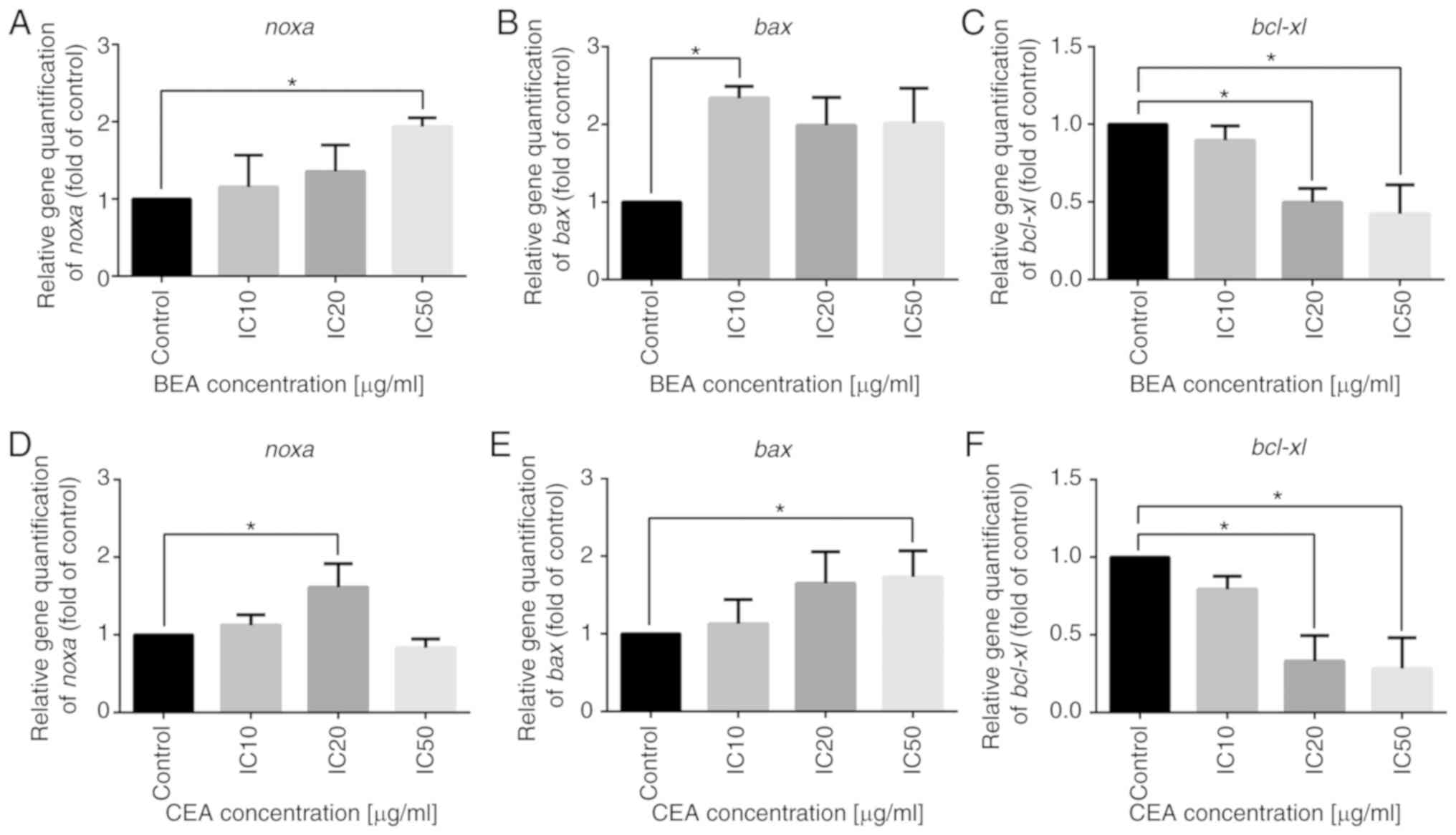
Effects of BEA and CEA treatment on Bcl-2
family mRNA expression levels
Bcl-2 family proteins play an important role in the
mitochondrial pathway (57).
Expression levels of NOXA and BAX were significantly increased in
cells treated with BEA and CEA, whereas Bcl-xL mRNA expression
levels were significantly attenuated compared with those of the
control cells (Fig. 7). These
results indicate that BEA and CEA induce NOXA expression, which
subsequently downregulates Bcl-xL and upregulates BAX expression,
inducing apoptosis.
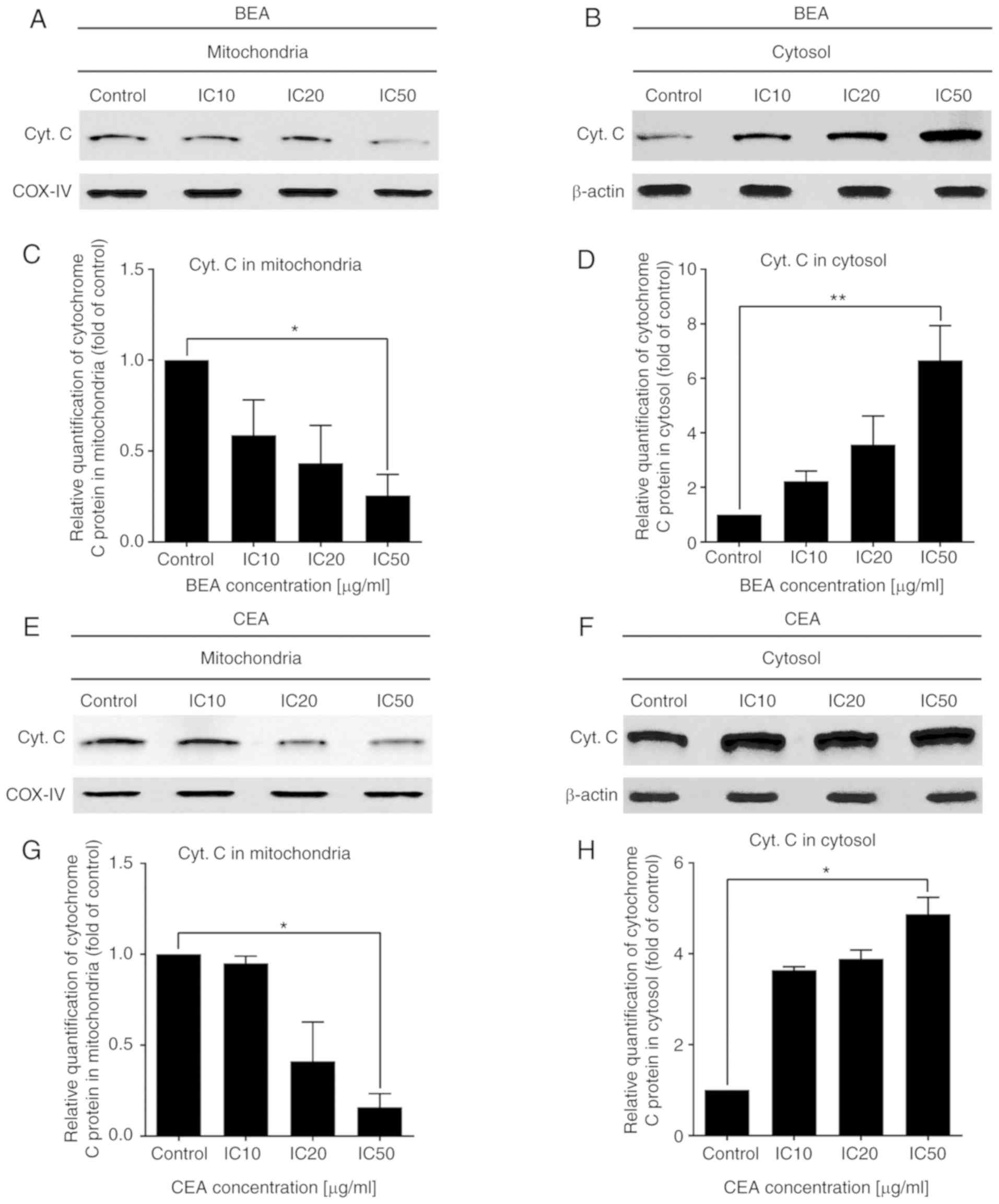
Effects of BEA and CEA treatment on
apoptotic-related protein expression levels
The expression levels of NOXA, BAX, Bcl-xL and
cytochrome c proteins were investigated in MDA-MB-231 cells
following BEA and CEA treatment. Protein expression levels of NOXA
and BAX were significantly increased, whereas Bcl-xL expression
levels were significantly decreased following BEA and CEA treatment
(Fig. 8). In addition, cytochrome
c expression levels were measured in the mitochondria and
cytosol. Following treatment with BEA and CEA, cytochrome c
expression levels in the mitochondria decreased in a dose-dependent
manner, while those in the cytosol were increased (Fig. 9). These results suggest that BEA
and CEA induce the mitochondrial apoptosis pathway in MDA-MB-231
cells.
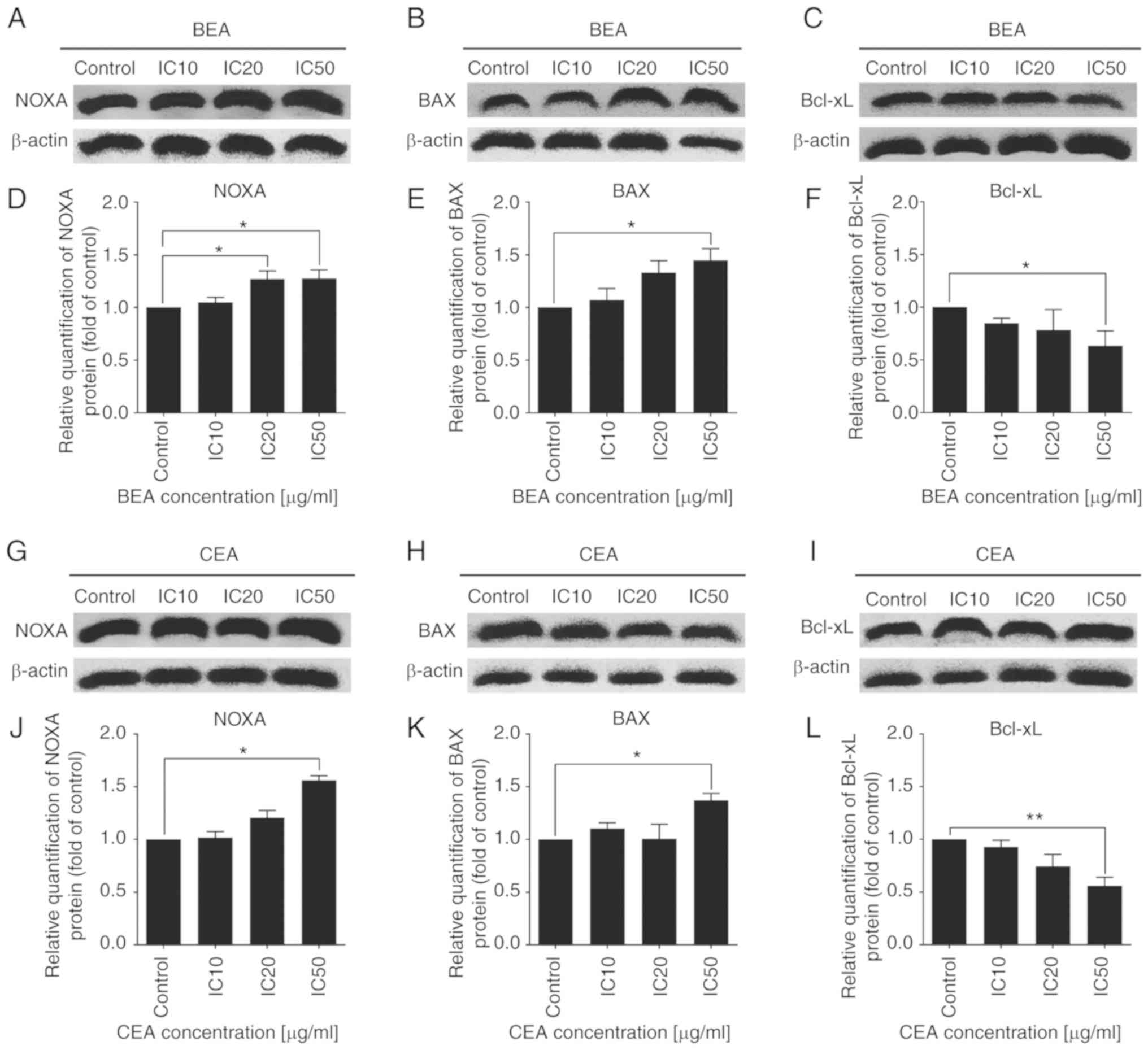 | Figure 8The effect of BEA and CEA on Bcl-2
family protein expression levels in MDA-MB-231 cells. BEA treatment
increased the protein expression levels of (A) NOXA and (B) BAX,
and deceased the protein expression levels of (C) Bcl-xL.
Quantification of the protein band density for (D) NOXA, (E) BAX
and (F) Bcl-xL after BEA treatment. CEA treatment increased the
protein expression levels of (G) NOXA and (H) BAX, and decreased
that of (I) Bcl-xL. Quantification of the protein band density for
(J) NOXA, (K) BAX and (L) Bcl-xL after CEA treatment.
*P<0.05 and **P<0.01 vs. non-treated
control MDA-MB-231 cells. BEA, Bridelia ovata; CEA,
Croton oblongifolius; NOXA,
phorbol-12-myristate-13-acetate-induced protein 1; BAX,
Bcl2-associated X protein; Bcl-xL, B-cell lymphoma-extra large; IC,
inhibitory concentration. |
Discussion
B. ovata and C. oblongifolius are
medicinal plants used in Thai traditional medicine. These herbs are
used in the treatment of several diseases and symptoms, including
as an expectorant (7), and to
treat flatworm infections and dysmenorrhea (11). However, previous studies have also
reported the cytotoxicity of these herbs against some cancer cell
lines (10,17). However, there are no reports
investigating the underlying mechanisms of action of these two
herbs for potential use in breast cancer treatment. In the present
study, BEA and CEA were screened for the presence of
phytoconstituent groups using standard methods. The phytochemical
screening results showed several groups of phytochemical compounds,
such as alkaloids, flavonoids, phytosterols and tannins. However,
previous phytochemical reports of B. ovata showed the
presence of triterpenoids and phytosterols, whereas those of C.
oblongifolius revealed diterpenoids and megastigmane
glycosides. Therefore, GC-MS analysis in the present study
primarily aimed to identify triterpenoids, phytosterols and
diterpenoids in BEA and CEA. To the best of our knowledge, this is
first report of the phytoconstituents of crude ethyl acetate
extracts of BEA and CEA by GC-MS analysis.
Friedelan-3-one is a friedelane-type triterpenoid,
which was a primary phytoconstituent of BEA in the present study. A
previous study reported the cytotoxic effect of friedelan-3-one
against mouse 4T1 breast cancer cells (58). Friedelan-3-one also inhibits
VEGF-induced Kaposi's sarcoma cell proliferation and induces
apoptotic cell death (59). These
findings indicate the cytotoxic effect and apoptotic induction of
BEA in MDA-MB-231 cells, which may be associated with friedelan-3
-one.
Kaur-16-en-18-oic acid (or kaurenoic acid) is a
kaurane-type diterpenoid, which is present in some medicinal plants
that possess potential anti-cancer properties (60). Kaurane diterpenes have been
considered for the development of novel effective anti-cancer
chemotherapeutic agents (61).
Kaurenoic acid is also reported to possess apoptosis-inducing
properties against various cell lines, including the HL-60 human
leukemia cell line (62), the PC-3
human prostate cancer cell line and the A-549 human lung cancer
cell line (63). Furthermore, the
genotoxicity of kaurenoic acid has been demonstrated in gastric
cancer cell lines, including ACP02, ACP03, AGP01 and PG100
(64), and cervical cancer cell
lines such as HeLa, CaSki and C33A (65). Moreover, the epimer of kaurenoic
acid isolated from Croton antisyphiliticus exhibits moderate
cytotoxic activity against HeLa cervical cancer cells and B-16
murine melanoma cells, by inducing apoptosis (66).
In the present study, the second most abundant
compound in both BEA and CEA was the phytosterol
stigmast-5-en-3-ol. The anti-proliferative and pro-apoptotic
effects of stigmast-5-en-3-ol (from Dendronephthya
gigantean) have been demonstrated in HL-60 and MCF-7 cells
(67). Diterpenes, triterpenes and
phytosterols have shown anti-tumor activity in several models
(68-71), hence they may have value as novel
anti-tumor agents. In the present study, high contents of
friedelan-3-one and stigmast-5-en-3-ol in BEA, and
kaur-16-en-18-oic acid in CEA, were observed. Therefore, these two
herbs show potential as anti-breast cancer agents.
However, in the present study, crude ethyl acetate
extracts of B. ovata and C. oblongifolius were
investigated for their anti-cancer activity as an alternative to
purified forms of the constituents identified. The present study
aimed to investigate all of the constituents in these herbal
extracts on the basis of possible synergy required to generate the
anti-cancer effects. A previous study demonstrated that the
IC50 values of crude ethanolic extracts of B.
ovata and C. oblongifolius in the HepG2 cell line
(72) were higher than the crude
ethyl acetate extracts in the MDA-MB-231 cell line in the present
study. These differences suggest that these two herbs have more
potential as ethyl acetate extracts against breast cancer cells
than ethanolic extracts against liver cancer; however, further
study of these extracts against liver cancer is required. Comparing
the IC50 and SI values of BEA and CEA suggests that CEA
exerts greater toxic against MDA-MB-213 cells than BEA, whereas BEA
acts more selectively towards breast cancer cells than CEA.
Annexin V-FITC and PI staining showed that
MDA-MB-231 cells had undergone apoptosis and necrosis. However, the
number of necrotic cells was not significantly different compared
with that of the control cells, and Rip3 protein expression levels
did not change following BEA and CEA treatment. The percentage of
apoptotic cells was significantly increased following treatment
with both extracts in a dose-dependent manner, compared with that
of the control cells.
The present study further investigated the mechanism
of apoptosis, which revealed that the percentage of cells with
mitochondrial disruption increased following treatment with both
extracts. The mitochondria are the primary source of intracellular
ROS production. High generation of ROS induces mitochondrial
dysfunction, cell damage and apoptosis (73). This Δψm loss was associated with
ROS generation, as the high energy source equates to the
mitochondrial electron transport chain (74). Intracellular ROS induce cellular
stress and consequently induce apoptosis (75). During apoptosis, the loss of
mitochondrial membrane potential leads to the inhibition of ATP
synthase, subsequently resulting in ROS production (76). However, a number of drugs and
natural compounds can directly induce ROS generation; this includes
doxorubicin, which also induces mitochondrial dysfunction and
apoptosis (77,78). In the present study, the level of
ROS increased ~1.5-fold in MDA-MB-213 cells treated with BEA and
CEA, compared with the control group, demonstrating that BEA and
CEA induce oxidative stress, and subsequent apoptotic cell death.
ROS level increased in a dose-independent manner, which may be due
to the presence of antioxidants in the cell or the cellular
microenvironment (79).
Alternatively, this may be due to the oxidant content of the
microenvironment, which contains superoxide dismutase (80), reduced glutathione (81), catalase, bilirubin, NADPH oxidase
and lipoxygenase and toxins (82,83).
Cellular stress can lead to DNA damage that
activates the p53 tumor suppressor gene; this can induce apoptotic
cell death and cell cycle arrest. However, the downstream effects
of p53 activation depend on cell type, differentiation state and
the local microenvironment (84).
It has been reported that p53-mediated apoptosis is favorably
selected in cells expressing oncoproteins (85); for example, mouse embryonic
fibroblasts (MEFs) normally undergo p53-mediated cell cycle arrest,
whereas MEFs expressing E1A oncoprotein undergo p53-mediated
apoptosis (86). p53-mediated
apoptosis involves the transcriptional targets PUMA and NOXA, and
the expression of other BH-3 only proteins (85,87).
Several studies have reported that NOXA functions as an integral
mediator of p53-dependent apoptosis (88). Moreover, PUMA and NOXA indirectly
induce mitochondrial outer membrane permeabilization (MOMP) by
direct binding to members of the anti-apoptotic Bcl-2 family, such
as Bcl-2 and Bcl-xL, which interferes with pro-apoptotic BAX and
BAK and anti-apoptotic interactions. Consequently, BAX serves a
role in MOMP by homo- or heterodimerizing with BAK, forming pores
in the mitochondrial outer membrane and permitting the release of
cytochrome c from the mitochondrial intermembranous space
into the cytosol (89). This
subsequently leads to the activation of initiator caspase 9,
resulting in downstream activation of executioner caspase 3, which
proteolyzes key proteins, including those of cytoskeletal proteins.
Also, caspase 3 inactivates other enzymes such as poly(ADP) ribose
polymerase (PARP), a DNA repair enzyme (90). Finally, the cells undergo apoptotic
cell death and exhibit unique morphological changes, including DNA
fragmentation and membrane blebbing (91).
The present study investigated the effects of BEA
and CEA on MDA-MB-231 human breast cancer cells, showing that
treatment with these herbs increased the mRNA and protein
expression levels of the pro-apoptotic proteins NOXA and BAX. By
contrast, BEA and CEA treatment led to the downregulation of the
anti-apoptotic protein Bcl-xL. Additionally, cytochrome c,
which is essential for apoptosome formation and the progression of
apoptosis (92), was released from
the mitochondria into the cytosol. These data show that BEA and CEA
induced breast cancer cell death via the mitochondria-mediated
pathway. Caspases are a group of cysteine proteases which play a
pivotal role in the apoptosis pathway (93). The mitochondrial dysfunction
observed following BEA and CEA treatment demonstrated that only the
intrinsic apoptotic pathway was activated by these herbs, and that
there was no evidence of a significant increase in caspase 8
activity, which would indicate the activation of the extrinsic
pathway. By contrast, significantly enhanced caspase 9 activity was
observed. Caspase 3 activity was activated by active caspase 9, and
this resulted in MDA-MB-231 cell apoptosis. Therefore, BEA and CEA
induced apoptotic cell death via the mitochondrial apoptosis
pathway.
To conclude, crude BEA and CEA extracts induced
MDA-MB-231 human breast cancer cell apoptosis via oxidative stress
and mitochondria-mediated pathways. The altered expression levels
of NOXA, BAX and Bcl-xL resulted in disruption of the mitochondrial
membrane potential and the release of cytochrome c into the
cytosol. This led to caspase 9 and 3 activation and induced
apoptotic cell death. The pathway by which BEA and CEA induce
MDA-MB-231 cell apoptosis is illustrated in Fig. 10. The lack of in vivo
experiments using animal models and patient studies are the primary
limitations of the present study. Therefore, further investigation
into the associated active compounds in these extracts is required,
and in vivo experiments need to be performed before these
herbs can be developed as therapeutic agents for human breast
cancer treatment.
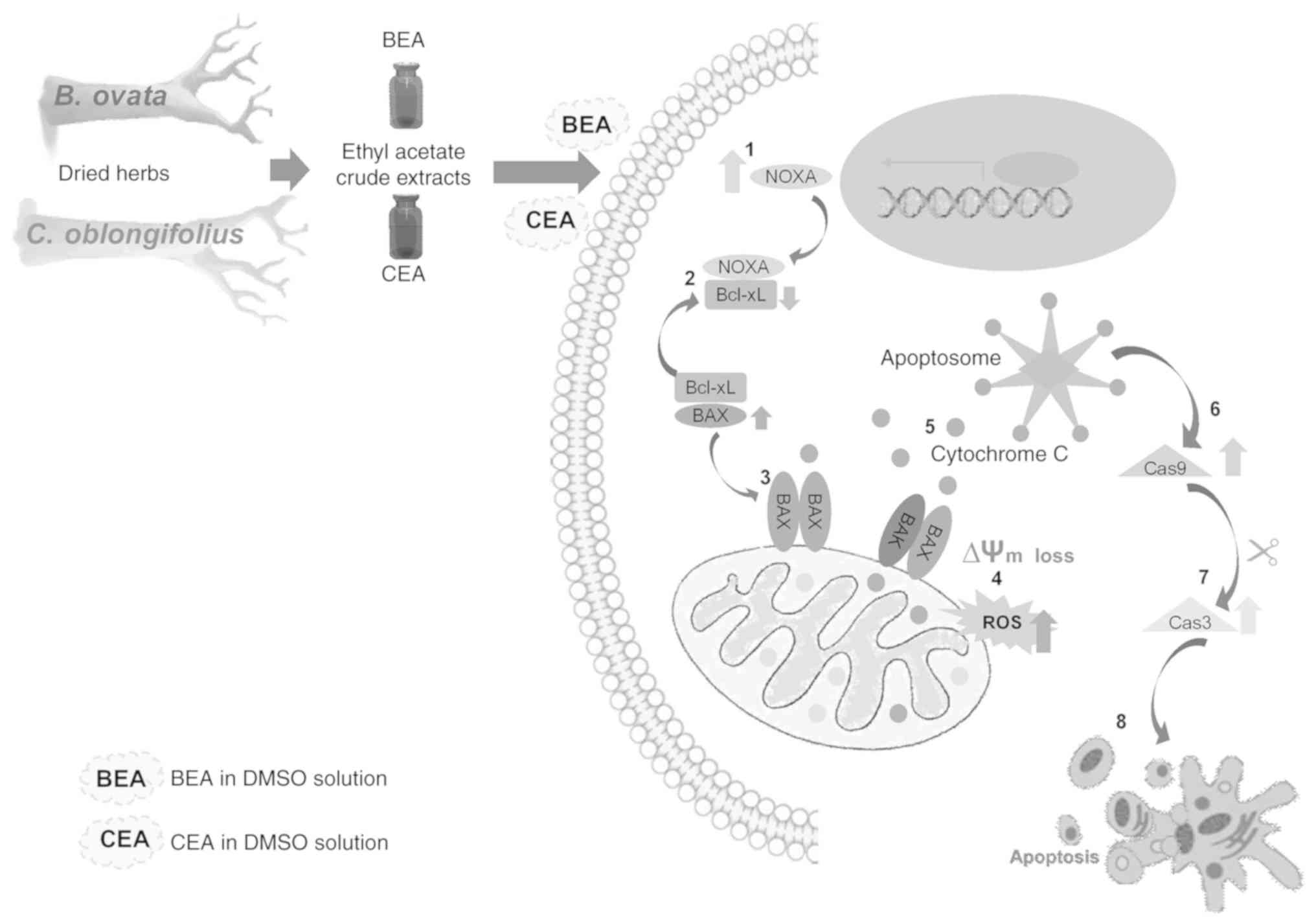 | Figure 10The apoptosis pathway induced by BEA
and CEA in MDA-MB-231 cells. BEA and CEA induce gene and protein
expression of NOXA and BAX pro-apoptotic proteins and reduced those
of anti-apoptotic Bcl-xL, leading to the loss of mitochondrial
membrane potential and ROS production. Cytochrome c is then
released from the mitochondria into the cytosol, consequently
inducing activation of the caspase 9 and 3 cascades, and ultimately
resulting in apoptosis. BEA, Bridelia ovata; CEA, Croton
oblongifolius; ROS, reactive oxygen species; Cas9, caspase 9;
Cas3, caspase 3; Alm, mitochondrial transmembrane potential; NOXA,
phorbol-12-myristate-13-acetate-induced protein 1; BAX,
Bcl2-associated X protein; Bcl-xL, B-cell lymphoma-extra large;
DMSO, dimethyl sulfoxide. |
Supplementary Data
Funding
The present study was supported by The Research and
Researchers for Industries (grant no. PHD58I0008), and the Research
Fund for Graduate Student of Faculty of Medicine, Chiang Mai
University (grant no. 038/2018).
Availability of data and materials
The datasets used and/or analyzed during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
JP and RB designed the study. BS collected the
herbs and took responsibility for the extraction. JP performed the
biochemical experiments and wrote the manuscript. All authors read
and approved the final manuscript.
Ethical approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Abbreviations:
|
BEA
|
Bridelia ovata ethyl acetate
extract
|
|
CEA
|
Croton oblongifolius ethyl
acetate extract
|
|
ΔΨm
|
mitochondrial transmembrane
potential
|
|
NOXA
|
phorbol-12-myristate-13-acetate-induced protein 1 or PMAIP1
|
|
BAX
|
Bcl2-associated X protein
|
|
Bcl-xL
|
B-cell lymphoma-extra large
|
Acknowledgments
The group would like to thank The Medical Science
Research Equipment Center, Faculty of Medicine, Chiang Mai
University.
References
|
1
|
DeSantis CE, Ma J, Goding Sauer A, Newman
LA and Jemal A: Breast cancer statistics, 2017, racial disparity in
mortality by state. CA Cancer J Clin. 67:439–448. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sledge GW, Mamounas EP, Hortobagyi GN,
Burstein HJ, Goodwin PJ and Wolff AC: Past, present, and future
challenges in breast cancer treatment. J Clin Oncol. 32:1979–1986.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Moiseenko F, Volkov N, Bogdanov A, Dubina
M and Moiseyenko V: Resistance mechanisms to drug therapy in breast
cancer and other solid tumors: An opinion. F1000Res. 6:2882017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu W, Fu X, Yang Z, Li S, Cao Y, Li Q and
Luan J: Moderate intermittent negative pressure increases
invasiveness of MDA-MB-231 triple negative breast cancer cells.
Breast. 38:14–21. 2018. View Article : Google Scholar
|
|
6
|
Sun L, Legood R, Dos-Santos-Silva I, Gaiha
SM and Sadique Z: Global treatment costs of breast cancer by stage:
A systematic review. PLoS One. 13:e02079932018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chotchoungchatchai S, Saralamp P,
Jenjittikul T, Pornsiripongse S and Prathanturarug S: Medicinal
plants used with thai traditional medicine in modern healthcare
services: A case study in Kabchoeng Hospital, Surin Province,
Thailand. J Ethnopharmacol. 141:193–205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Boonyaratavej S, Tantayanontha S,
Kitchanachai P, Chaichantipyuth C, Chittawong V and Miles DH:
Trans-triacontyl-4-hydroxy-3-methoxycinnamate, a new compound from
the thai plant bridelia ovata. J Nat Prod. 55:1761–1763. 1992.
View Article : Google Scholar
|
|
9
|
Thongkorn N: Chemical constituents of the
leaves of bridelia ovata decne (unpublished PhD thesis).
Chulalongkorn University; 1995
|
|
10
|
Baig H, Diskul-Na-Ayudthaya P, Weeraphan
C, Paricharttanakul M, Svasti J and Srisomsap C: Inhibitory effect
of bridelia ovata decne extract on HepG2 cell migration and
invasion stimulated by fibroblast-conditioned media. Naresuan
Phayao J. 1:6–10. 2015.
|
|
11
|
Sommit D, Petsom A, Ishikawa T and
Roengsumran S: Cytotoxic activity of natural labdanes and their
semi-synthetic modified derivatives from Croton oblongifolius.
Planta Med. 69:167–170. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ngamrojnavanich N, Sirimongkon S,
Roengsumran S, Petsom A and Kamimura H: Inhibition of Na+,K+-ATPase
activity by (-)-ent-Kaur-16-en-19-oic acid and its derivatives.
Planta Med. 69:555–556. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ahmed B, Alam T, Varshney M and Khan SA:
Hepatoprotective activity of two plants belonging to the Apiaceae
and the euphor-biaceae family. J Ethnopharmacol. 79:313–316. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Salatino A, Salatino MLF and Negri G:
Traditional uses, chemistry and pharmacology of Croton species
(Euphorbiaceae). J Braz Chem Soc. 18:11–33. 2007. View Article : Google Scholar
|
|
15
|
Singh M, Pal M and Sharma RP: Biological
activity of the labdane diterpenes. Planta Med. 65:2–8. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Takeshige Y, Kawakami S, Matsunami K,
Otsuka H, Lhieochaiphant D and Lhieochaiphant S: Oblongionosides
A-F, megastigmane glycosides from the leaves of Croton
oblongifolius Roxburgh. Phytochemistry. 80:132–136. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Roengsumran S, Petsom A, Kuptiyanuwat N,
Vilaivan T, Ngamrojnavanich N, Chaichantipyuth C and Phuthong S:
Cytotoxic labdane diterpenoids from Croton oblongifolius.
Phytochemistry. 56:103–107. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pudhom K and Sommit D: Clerodane
diterpenoids and a trisubstituted furan from Croton oblongifolius.
Phytochem Lett. 4:147–150. 2011. View Article : Google Scholar
|
|
19
|
Roengsumran S, Musikul K, Petsom A,
Vilaivan T, Sangvanich P, Pornpakakul S, Puthong S, Chaichantipyuth
C, Jaiboon N and Chaichit N: Croblongifolin, a new anticancer
clerodane from Croton oblongifolius. Planta Med. 68:274–277. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Youngsa-ad W, Ngamrojanavanich N, Mahidol
C, Ruchirawat S, Prawat H and Kittakoop P: Diterpenoids from the
roots of Croton oblongifolius. Planta Med. 73:1491–1494. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pudhom K, Vilaivan T, Ngamrojanavanich N,
Dechangvipart S, Sommit D, Petsom A and Roengsumran S:
Furanocembranoids from the stem bark of Croton oblongifolius. J Nat
Prod. 70:659–661. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Roengsumran S, Pornpakakul S, Muangsin N,
Sangvanich P, Nhujak T, Singtothong P, Chaichit N, Puthong S and
Petsom A: New halimane diterpenoids from Croton oblongifolius.
Planta Med. 70:87–89. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Roengsumran S, Achayindee S, Petsom A,
Pudhom K, Singtothong P, Surachetapan C and Vilaivan T: Two new
cembranoids from Croton oblongifolius. J Nat Prod. 61:652–654.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Roengsumran S, Singtothong P, Pudhom K,
Ngamrochanavanich N, Petsom A and Chaichantipyuth C:
Neocrotocembranal from Croton oblongifolius. J Nat Prod.
62:1163–1164. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Suwancharoen S, Tommeurd W, Phurat C,
Muangsin N and Pornpakakul S: Acanthoic acid. Acta Crystallogr Sect
E Struct Rep Online. 66:o15312010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Renehan AG, Booth C and Potten CS: What is
apoptosis, and why is it important? BMJ. 322:1536–1538. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Favaloro B, Allocati N, Graziano V, Di
Ilio C and De Laurenzi V: Role of apoptosis in disease. Aging
(Albany N Y). 4:330–349. 2012.
|
|
28
|
Fathi N, Rashidi G, Khodadadi A, Shahi S
and Sharifi S: STAT3 and apoptosis challenges in cancer. Int J Biol
Macromol. 117:993–1001. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kumar R, Herbert PE and Warrens AN: An
introduction to death receptors in apoptosis. Int J Surg.
3:268–277. 2005. View Article : Google Scholar
|
|
30
|
O'Brien MA and Kirby R: Apoptosis: A
review of pro-apoptotic and anti-apoptotic pathways and
dysregulation in disease. J Veterinary Emergency Crit Care.
18:572–585. 2008. View Article : Google Scholar
|
|
31
|
Schneider P and Tschopp J: Apoptosis
induced by death receptors. Pharm Acta Helv. 74:281–286. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chinnaiyan AM: The apoptosome: Heart and
soul of the cell death machine. Neoplasia. 1:5–15. 1999. View Article : Google Scholar
|
|
33
|
Tait SW and Green DR: Mitochondria and
cell death: Outer membrane permeabilization and beyond. Nat Rev Mol
Cell Biol. 11:621–632. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cao K and Tait SWG: Apoptosis and cancer:
Force awakens, phantom menace, or both? Int Rev Cell Mol Biol.
337:135–152. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dong N, Liu X, Zhao T, Wang L, Li H, Zhang
S, Li X, Bai X, Zhang Y and Yang B: Apoptosis-inducing effects and
growth inhibitory of a novel chalcone, in human hepatic cancer
cells and lung cancer cells. Biomed Pharmacother. 105:195–203.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Neve RM, Chin K, Fridlyand J, Yeh J,
Baehner FL, Fevr T, Clark L, Bayani N, Coppe JP, Tong F, et al: A
collection of breast cancer cell lines for the study of
functionally distinct cancer subtypes. Cancer Cell. 10:515–527.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dai X, Cheng H, Bai Z and Li J: Breast
cancer cell line classification and its relevance with breast tumor
subtyping. J Cancer. 8:3131–3141. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen X and Thibeault S: Effect of DMSO
concentration, cell density and needle gauge on the viability of
cryopreserved cells in three dimensional hyaluronan hydrogel. Conf
Proc IEEE Eng Med Biol Soc. 2013:6228–6231. 2013.PubMed/NCBI
|
|
39
|
Arega ED: Phytochemical studies of the
ethyl acetate extract of the fruit of piper capense. J Pharm Nat
Products. 4:148–152. 2018.
|
|
40
|
Obasi NL, Egbuonu ACC, Ukoha PO and
Ejikeme PM: Comparative phytochemical and antimicrobial screening
of some solvent extracts of Samanea saman (fabaceae or mimosaceae)
pods. Afr J Pure Appl Chem. 4:206–212. 2010.
|
|
41
|
Tiwari P, Kumar B, Kaur M, Kaur G and Kaur
H: Phytochemical screening and extraction: A review. Int Pharm Sci.
1:98–106. 2011.
|
|
42
|
Banjerdpongchai R, Yingyurn S and
Kongtawelert P: Sesamin induces human leukemic cell apoptosis via
mitochondrial and endoplasmic reticulum stress pathways. World J
Oncol. 1:78–86. 2010.PubMed/NCBI
|
|
43
|
Wudtiwai B, Sripanidkulchai B,
Kongtawelert P and Banjerdpongchai R: Methoxyflavone derivatives
modulate the effect of TRAIL-induced apoptosis in human leukemic
cell lines. J Hematol Oncol. 4:522011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Khaw-on P and Banjerdpongchai R: Induction
of intrinsic and extrinsic apoptosis pathways in the human leukemic
MOLT-4 cell line by terpinen-4-ol. Asian Pac J Cancer Prev.
13:3073–3076. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Khaw-On P, Pompimon W and Banjerdpongchai
R: Goniothalamin induces necroptosis and anoikis in human invasive
breast cancer MDA-MB-231 cells. Int J Mol Sci. 20:E39532019.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Dikalov S, Griendling KK and Harrison DG:
Measurement of reactive oxygen species in cardiovascular studies.
Hypertension. 49:717–727. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhao H, Kalivendi S, Zhang H, Joseph J,
Nithipatikom K, Vasquez-Vivar J and Kalyanaraman B: Superoxide
reacts with hydroethidine but forms a fluorescent product that is
distinctly different from ethidium: Potential implications in
intracellular fluorescence detection of superoxide. Free Radic Biol
Med. 34:1359–1368. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
49
|
Mitas M, Mikhitarian K, Walters C, Baron
PL, Elliott BM, Brothers TE, Robison JG, Metcalf JS, Palesch YY,
Zhang Z, et al: Quantitative real-time RT-PCR detection of breast
cancer micrometastasis using a multigene marker panel. Int J
Cancer. 93:162–171. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hanf A, Oelze M, Manea A, Li H, Munzel T
and Daiber A: The anti-cancer drug doxorubicin induces substantial
epigenetic changes in cultured cardiomyocytes. Chem Biol Interact.
313:1088342019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wudtiwai B, Pitchakarn P and
Banjerdpongchai R: Alpha-mangostin, an active compound in Garcinia
mangostana, abrogates anoikis-resistance in human hepatocellular
carcinoma cells. Toxicol In Vitro. 53:222–232. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Badisa RB, Darling-Reed SF, Joseph P,
Cooperwood JS, Latinwo LM and Goodman CB: Selective cytotoxic
activities of two novel synthetic drugs on human breast carcinoma
MCF-7 cells. Anticancer Res. 29:2993–2996. 2009.PubMed/NCBI
|
|
53
|
Prayong P, Barusrux S and Weerapreeyakul
N: Cytotoxic activity screening of some indigenous Thai plants.
Fitoterapia. 79:598–601. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Rivankar S: An overview of doxorubicin
formulations in cancer therapy. J Cancer Res Ther. 10:853–858.
2014. View Article : Google Scholar
|
|
55
|
Kalogeris T, Bao Y and Korthuis RJ:
Mitochondrial reactive oxygen species: A double edged sword in
ischemia/reperfusion vs. preconditioning Redox Biol. 2:702–714.
2014. View Article : Google Scholar
|
|
56
|
Kalyanaraman B, Darley-Usmar V, Davies KJ,
Dennery PA, Forman HJ, Grisham MB, Mann GE, Moore K, Roberts LJ II
and Ischiropoulos H: Measuring reactive oxygen and nitrogen species
with fluorescent probes: Challenges and limitations. Free Radic
Biol Med. 52:1–6. 2012. View Article : Google Scholar
|
|
57
|
Tsujimoto Y: Role of Bcl-2 family proteins
in apoptosis: Apoptosomes or mitochondria? Genes Cells. 3:697–707.
1998. View Article : Google Scholar
|
|
58
|
Sousa GFd, Soares DCF, Mussel WdN, Pompeu
NFE, Silva GDdF, Vieira Filho SA and Duarte LP: Pentacyclic
triterpenes from branches of Maytenus robusta and in vitro
cytotoxic property against. J Braz Chem Soc. 25:1338–1345.
2014.
|
|
59
|
Martucciello S, Balestrieri ML, Felice F,
Estevam Cdos S, Sant'Ana AE, Pizza C and Piacente S: Effects of
triterpene derivatives from Maytenus rigida on VEGF-induced
Kaposi's sarcoma cell proliferation. Chem Biol Interact.
183:450–454. 2010. View Article : Google Scholar
|
|
60
|
Balbinot RB, de Oliveira JAM, Bernardi DI,
Melo UZ, Zanqueta EB, Endo EH, Ribeiro FM, Volpato H, Figueiredo
MC, Back DF, et al: Structural characterization and biological
evaluation of 18-Nor-ent-labdane diterpenoids from grazielia
gaudichaudeana. Chem Biodivers. 16:e18006442019. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Cavalcanti BC, Ferreira JR, Moura DJ, Rosa
RM, Furtado GV, Burbano RR, Silveira ER, Lima MA, Camara CA, Saffi
J, et al: Structure-mutagenicity relationship of kaurenoic acid
from Xylopia sericeae (Annonaceae). Mutat Res. 701:153–163. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Cavalcanti BC, Bezerra Dp, Magalhaes HI,
Moraes MO, Lima MA, Silveira ER, Camara CA, Rao VS, Pessoa C and
Costa-Lotufo LV: Kauren-19-oic acid induces DNA damage followed by
apoptosis in human leukemia cells. J Appl Toxicol. 29:560–568.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Cuca LE, Coy ED, Alarcon MA, Fernandez A
and Aristizabal FA: Cytotoxic effect of some natural compounds
isolated from Lauraceae plants and synthetic derivatives.
Biomedica. 31:335–343. 2011. View Article : Google Scholar
|
|
64
|
Cardoso PCDS, Rocha CAMD, Leal MF, Bahia
MO, Alcantara DDFA, Santos RAD, Gongalves NDS, Ambrosio SR,
Cavalcanti BC, Moreira-Nunes CA, et al: Effect of diterpenoid
kaurenoic acid on genotoxicity and cell cycle progression in
gastric cancer cell lines. Biomed Pharmacother. 89:772–780. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Rocha SMMD, Cardoso PCDS, Bahia MO, Pessoa
CDO, Soares PC, Rocha SMD, Burbano RMR and Rocha CAMD: Effect of
the kaurenoic acid on genotoxicity and cell cycle progression in
cervical cancer cells lines. Toxicol In Vitro. 57:126–131. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Fernandes VC, Pereira SI, Coppede J,
Martins JS, Rizo WF, Beleboni RO, Marins M, Pereira PS, Pereira AM
and Fachin AL: The epimer of kaurenoic acid from Croton
antisyphiliticus is cytotoxic toward B-16 and HeLa tumor cells
through apoptosis induction. Genet Mol Res. 12:1005–1011. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Fernando IPS, Sanjeewa KKA, Ann YS, Ko CI,
Lee SH, Lee WW and Jeon YJ: Apoptotic and antiproliferative effects
of Stigmast-5-en-3-ol from Dendronephthya gigantea on human
leukemia HL-60 and human breast cancer MCF-7 cells. Toxicol In
Vitro. 52:297–305. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Jian B, Zhang H, Han C and Liu J:
Anti-cancer activities of diterpenoids derived from Euphorbia
fischeriana steud. Molecules. 23:E3872018. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Petiwala SM and Johnson JJ: Diterpenes
from rosemary (Rosmarinus officinalis): Defining their potential
for anti-cancer activity. Cancer Lett. 367:93–102. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Suttiarporn P, Chumpolsri W,
Mahatheeranont S, Luangkamin S, Teepsawang S and Leardkamolkarn V:
Structures of phytosterols and triterpenoids with potential
anti-cancer activity in bran of black non-glutinous rice.
Nutrients. 7:1672–1687. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Saleem M: Lupeol, a novel
anti-inflammatory and anti-cancer dietary triterpene. Cancer Lett.
285:109–115. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Weerapreeyakul N, Nonpunya A, Barusrux S,
Thitimetharoch T and Sripanidkulchai B: Evaluation of the
anticancer potential of six herbs against a hepatoma cell line.
Chin Med. 7:152012. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Sinha K, Das J, Pal PB and Sil PC:
Oxidative stress: The mitochondria-dependent and
mitochondria-independent pathways of apoptosis. Arch Toxicol.
87:1157–1180. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Cairns RA, Harris IS and Mak TW:
Regulation of cancer cell metabolism. Nat Rev Cancer. 11:85–95.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Circu ML and Aw TY: Reactive oxygen
species, cellular redox systems, and apoptosis. Free Radic Biol
Med. 48:749–762. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Redza-Dutordoir M and Averill-Bates DA:
Activation of apoptosis signalling pathways by reactive oxygen
species. Biochim Biophys Acta. 1863:2977–2992. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
He H, Zang LH, Feng YS, Chen LX, Kang N,
Tashiro S, Onodera S, Qiu F and Ikejima T: Physalin A induces
apoptosis via p53-Noxa-mediated ROS generation, and autophagy plays
a protective role against apoptosis through p38-NF-kB survival
pathway in A375-S2 cells. J Ethnopharmacol. 148:544–555. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Yu CC, Ko FY, Yu CS, Lin CC, Huang YP,
Yang JS, Lin JP and Chung JG: Norcantharidin triggers cell death
and DNA damage through S-phase arrest and ROS-modulated apoptotic
pathways in TSGH 8301 human urinary bladder carcinoma cells. Int J
Oncol. 41:1050–1060. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Weinberg F, Ramnath N and Nagrath D:
Reactive oxygen species in the tumor microenvironment: An overview.
Cancers (Basel). 11:E11912019. View Article : Google Scholar
|
|
80
|
Crompton M: The mitochondrial permeability
transition pore and its role in cell death. Biochem J. 341:233–249.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Griffith OW: Biologic and pharmacologic
regulation of mammalian glutathione synthesis. Free Radic Biol Med.
27:922–935. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Komonrit P and Banjerdpongchai R: Effect
of Pseuderanthemum palatiferum (Nees) radlk fresh leaf ethanolic
extract on human breast cancer MDA-MB-231 regulated cell death.
Tumour Biol. 40:10104283188001822018. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Kumari S, Badana AK, GMM GS and Malla R:
Reactive oxygen species: A key constituent in cancer survival.
Biomark Insights. 13:11772719187553912018. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Kastenhuber ER and Lowe SW: Putting p53 in
context. Cell. 170:1062–1078. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Vousden KH and Lu X: Live or let die: The
cell's response to p53. Nat Rev Cancer. 2:594–604. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Lowe SW, Ruley HE, Jacks T and Housman DE:
p53-dependent apoptosis modulates the cytotoxicity of anticancer
agents. Cell. 74:957–967. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Shibue T, Suzuki S, Okamoto H, Yoshida H,
Ohba Y, Takaoka A and Taniguchi T: Differential contribution of
Puma and Noxa in dual regulation of p53-mediated apoptotic
pathways. EMBO J. 25:4952–4962. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Shibue T, Takeda K, Oda E, Tanaka H,
Murasawa H, Takaoka A, Morishita Y, Akira S, Taniguchi T and Tanaka
N: Integral role of Noxa in p53-mediated apoptotic response. Genes
Dev. 17:2233–2238. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Zhang LN, Li JY and Xu W: A review of the
role of Puma, Noxa and Bim in the tumorigenesis, therapy and drug
resistance of chronic lymphocytic leukemia. Cancer Gene Ther.
20:1–7. 2013. View Article : Google Scholar
|
|
90
|
McIlwain DR, Berger T and Mak TW: Caspase
functions in cell death and disease. Cold Spring Harb Perspect
Biol. 5:a0086562013. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Saraste A and Pulkki K: Morphologic and
biochemical hallmarks of apoptosis. Cardiovasc Res. 45:528–537.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Huttemann M, Pecina P, Rainbolt M,
Sanderson TH, Kagan VE, Samavati L, Doan JW and Lee I: The multiple
functions of cytochrome c and their regulation in life and death
decisions of the mammalian cell: From respiration to apoptosis.
Mitochondrion. 11:369–381. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Salvesen GS: Caspases and apoptosis.
Essays Biochem. 38:9–19. 2002. View Article : Google Scholar : PubMed/NCBI
|