Introduction
Breast cancer is the most commonly diagnosed
neoplasm and the leading cause of cancer mortality among females
worldwide (1,2). Clinically, it is divided into three
main subtypes based on hormone receptor expression (3-6). One
of the main characteristics of aggressive breast cancers is
epithelial-mesenchymal transition (EMT), via which cells transition
from an epithelial to a mesenchymal phenotype, a phenomenon that
has been closely linked to metastasis, drug resistance and cancer
recurrence, and reported to result in poor prognosis (7). Hallmarks of EMT include the
downregulation of E-cadherin, decreased expression of claudins and
occludins at tight junctions, and repression of genes encoding
desmoplakin and plakophilin desmosomes (8). Reductions in the levels of these
epithelial proteins disrupt cell junction complexes and
cytoskeletal connections (9);
occurring in parallel with activation of genes such as N-cadherin,
vimentin, fibronectin, α-smooth muscle actin and matrix
metalloproteinases (MMPs), this gives cells a mesenchymal-like
phenotype (10,11). EMT increases cancer cell motility
and facilitates invasive behavior by enabling cells to penetrate
through the extracellular matrix, and invade lymphatic and vascular
elements (7).
In the process of studying therapeutic resistance to
endocrine therapy, several cell lines have been established in our
laboratory via short hairpin RNA (shRNA)-mediated knockdown of the
estrogen receptor (ER) in parental endocrine-sensitive MCF-7 breast
cancer cells, resulting in cells resistant to both tamoxifen and
estrogen, and associated with EMT, leading to increased motility
and invasion (8,11,12).
One of the main characteristics of these endocrine-resistant cells,
but not of endocrine-sensitive breast cancer cells or normal breast
epithelial cells, is the induction of a remarkable alteration in
their morphology, including cell rounding and the formation of
dynamic actin-rich blebs along the outer membrane upon brief
exposure to alkaline but not acidic pH conditions (13,14).
Under such conditions, ER-silenced pII cells exhibit enhanced
motility and migration, in part through increased MMP activity
(12). These blebs can be observed
at the leading edge of cells moving towards a chemoattractant
source in an amoeboid-like manner (13-15).
Bleb formation can be reversed or prevented by inhibitors of
cytoplasmic streaming or drugs known to inhibit certain ion
channels; the most effective of these is ouabain, a potent
inhibitor of Na+/K+ ATPase (13,14).
The reversibility of bleb formation - which disappear upon
returning the cells to pH 7.4 - suggests that this may be a
physiological process to promote cellular motility in order to
enable cells to move away from an essentially hostile environment.
The process of cytoplasmic streaming required for bleb formation
must involve considerable water movement and thus is likely to be
subject to osmotic changes; therefore, the expression of membrane
channel proteins responsible for water transport, such as
aquaporins (AQPs), is of major research interest.
AQPs are small, hydrophobic, integral transmembrane
proteins whose major function is to facilitate the transport of
water molecules (16,17). To date, ~300 distinct AQPs have
been discovered in diverse organisms, with 13 mammalian isoforms
having been identified (AQP0-AQP12). Based on their functional and
structural properties, AQPs are classified into subfamilies: The
classical AQPs, including AQP0, AQP1, AQP2, AQP4, AQP5, AQP6 and
AQP8, are selective for water transport, whereas the
aquaglyceroporins and the super-aquaporins, which include AQP3,
AQP7, AQP9 and AQP10, also transport small solutes such as ammonia,
urea and glycerol. AQP3 is selective for water and glycerol
(16,17).
In addition to the various physiological roles of
AQPs, they have also been linked to cancer pathogenesis in various
organs including the lung (18),
colon (19), brain (22) and liver (21). Shi et al (16) reported the expression of several
AQPs in breast cancer, including AQPs 1, 3, 4 and 5; all were
upregulated in cancer tissue apart from AQP4, which was more
intensely expressed in normal tissue. AQP1 expression was detected
in basal-like (22) and luminal
cancer subtypes (23), and
associated with poor prognosis. AQP1 overexpression was shown to
enhance breast cancer proliferation and invasion in vitro
(23), while small interfering RNA
(siRNA)-mediated knockdown of AQP1 decreased tumor mass and volume
in vivo (24). Enhanced
AQP3 expression in patients with early breast cancer was associated
with poor prognosis (25).
Treating ER-positive (ER+) breast cancer cells with
estrogen upregulated expression of AQP3; this is likely via
activation of estrogen response elements (EREs) in its gene
promoter (26). Additionally,
siRNA knockdown of AQP3 significantly reduced cell migration and
invasion (26-28). AQP4 has been reported to be
elevated in breast cancer tissues (16), and its siRNA-mediated
downregulation in ER+ breast cancer cells enhanced the
expression of E-cadherin, and decreased cell proliferation,
motility and invasion (29).
Enhanced expression of AQP5 in tumors has been associated with poor
clinical prognosis and lymph node metastasis (30), and shRNA-knockdown of AQP5 in MCF-7
cells significantly decreased cell proliferation and migration
(31).
In the present study, it was determined that
alkaline pH induces bleb formation in endocrine-resistant pII
breast cancer cells, regulated in part by water movement in
response to osmotic changes in the extracellular environment. AQP3
(and to a certain extent, AQP1) was translocated to newly formed
blebs. In addition, siRNA-mediated knockdown of AQP3 in pII cells
significantly decreased bleb formation, and cell motility and
invasion. These data suggested that AQP3 may play a role in bleb
formation/stabilization, and thereby help promote invasion and
metastasis.
Materials and methods
Cell lines
The ER-negative (ER−) MDA-MB-231 human
breast carcinoma cell line was originally obtained from the
American Type Culture Collection (ATCC). MCF10A normal breast
epithelial cells were obtained from Dr Elizabeth Saunderson through
Dr Jenny Gomm (St Bartholomew's Hospital, London). pII
(ER−) cells were generated via shRNA-mediated knockdown
of ER in MCF7 cells (which were also originally obtained from the
ATCC); YS1.2 was derived from similarly transfected MCF7 cells, in
which ER was not downregulated; therefore, this cell line is used
as ER+ control for pII (8,11).
For routine culture, all cell lines were maintained as monolayers
at 37°C in an incubator with an atmosphere of 5% CO2 at
95% humidity, in advanced DMEM containing phenol red as a pH
indicator and supplemented with 5% FBS, 600 µg/ml
L-glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin and
6 ml/500 ml 100X non-essential amino acids (all from Invitrogen;
Thermo Fisher Scientific, Inc.).
Morphology of pII cells exposed to
alkaline pH and osmotic changes
Cells were removed from the CO2 incubator
and exposed to standard atmospheric conditions, which caused the
medium pH to rise to 8.3 (13)
within 10-30 min (depending on whether grown in culture plates or
flasks). The morphology of cells under control (pH 7.4) and
alkaline (pH 8.3) conditions was monitored via phase contrast light
microscopy. Images were captured for the same field at 0 and 30 min
(4 fields for each condition; magnification, ×40). The number of
blebbing cells was counted manually to compare the percentage of
cells blebbing. Live cell microscopy with time-lapse photography
was used to continuously monitor the effect of osmotic changes on
the bleb morphology of pII cells. For this, cells were grown in a
10-mm culture dish containing 2 ml DMEM and exposed to standard
atmospheric conditions for 30 min to alkalinize the medium. The
cells were then placed inside an imaging chamber (Cell Observer HS;
Carl Zeiss AG) heated with an airstream to 37°C with normal
atmosphere and monitored for 120 min. Cells were photographed at
×40 magnification and images were captured at 5-min intervals. This
experiment was repeated by first inducing alkaline conditions for
30 min, followed by the removal of the original media, and
substitution with media diluted with water (1:1 and 1:4) or sucrose
solution (1-100 mM) for 30 min to produce acute hypotonic or
hyper-tonic conditions, respectively.
Reverse transcription-quantitative PCR
(RT-qPCR)
RNA was extracted from cell pellets using an RNeasy
kit from Qiagen, Inc., and 2 µg (in 20 µl reaction
mix) was converted to cDNA using a High Capacity cDNA Reverse
Transcriptase kit from Applied Biosystems (Thermo Fisher
Scientific, Inc.). qPCR was performed on 1 µl of the cDNA
reaction mix using a standard multiplexed TaqMan PCR kit protocol
(Applied Biosystems; Thermo Fisher Scientific, Inc.) to determine
the expression of AQPs. Control gene probes (human β-actin; cat.
no. 4310881E; Thermo Fisher Scientific, Inc.) were labeled with
VIC™, and target gene probes for AQP1 (cat. no. Hs01028916_m1),
AQP3 (cat. no. Hs00185020_m1), AQP4 (cat. no. Hs00242342_m1) and
AQP5 (cat. no. Hs00387048_m1; all Applied Biosystems; Thermo Fisher
Scientific, Inc.) were labeled with fluorescein amidite. The
amplifications were performed on an Applied Biosystems 7500 HT Fast
thermocycler under the following conditions: 95°C for 10 min,
followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min. The
raw quantification cycle (Cq) values were converted via the ∆∆Cq
method to determine normalized expression ratios of the target
genes relative to β-actin (32).
Western blotting
Cells were cultured in 6-well plates with complete
DMEM to 80-90% confluence. Medium was subsequently aspirated off,
and cell monolayers were harvested by scraping and resuspended into
300 µl of lysis buffer containing 50 mM HEPES, 50 mM NaCl, 5
mM EDTA 1% Triton X, 100 µg/ml PMSF, 10 µg/ml
aprotinin and 10 µg/ml leupeptin. Protein concentration was
determined via the Bradford assay using BSA (Sigma-Aldrich; Merck
KGaA) as standard, and 8 µg protein lysate was mixed with an
equal volume of 2X SDS and heated at 90°C for 10 min. Samples were
loaded onto a 10% SDS-polyacrylamide gel and electrophoresed at 150
V for 1 h. Proteins were transferred to a PVDF membrane and blocked
with 2% BSA for 1 h at 4°C before being incubated overnight at 4°C
with AQP3 antibody (1:500; cat. no. ab125219; Abcam) prepared in 2%
BSA. The membrane was washed and incubated with anti-HRP-conjugated
secondary antibody (1:500; cat. no. 7074; Cell Signaling
Technology, Inc.) for 1 h at 4°C, developed with Super Signal ECL
(Thermo Fisher Scientific, Inc.) and visualized with Kodak X-ray
film. Blots were also probed with β-actin antibody (1:1,000; cat.
no. 8457; Cell Signaling Technology, Inc.) as a loading
control.
Immunofluorescence analysis
Cells were seeded at ~5,000 cells/well in an 8-well
chambered slide containing 300 µl/well DMEM, and left to
grow under standard culture conditions for 2 days. pII cells were
also cultured under standard conditions for 2 days before
subsequent exposure to alkaline pH conditions for 30 min. Media
were removed, and cells were immediately fixed by addition of 3.7%
formaldehyde (200 µl/well) for 10 min with gentle agitation
at room temperature. Alternatively, cells were fixed by incubation
with ice-cold 100% methanol for 15 min at -20°C. Removal of the
fixative was followed by three washing steps with ice-cold PBS,
each for 5 min. Non-specific binding sites were blocked by addition
of 1% BSA in PBS (100 µl), and cells were incubated at room
temperature for 1 h. The BSA was then aspirated, and 200
µl/well of primary antibody solution was added and left to
incubate overnight at 4°C, with anti-AQP1 (cat. no. ab11025),
anti-AQP3 (cat. no. ab125219), anti-AQP4 (cat. no. ab46182) or
anti-AQP5 (cat. no. ab15119) antibodies added (all 1:100; all
Abcam). Following removal of the primary antibodies, cells were
washed as aforementioned and the secondary antibody (Alexa
Fluor® 555-conjugated anti-rabbit IgG; 1:500; cat. no.
4413; Cell Signaling Technology, Inc.) was added for a further 2-h
incubation at room temperature in the dark. This was followed by a
repeat of the previously described washing steps after removal of
the antibody solution. Then, 200 µl/well of diluted
phalloidin (6 µl in 1 ml PBS; Thermo Fisher Scientific,
Inc.) was added and incubated for 10 min at room temperature in the
dark. After removing the phalloidin and washing with PBS three
times, the chambers and borders of the 8-well chambered slides were
removed, and a drop of DAPI was added onto the slide immediately
prior to mounting. Staining was visualized and photographed using
an LSM 510 Meta confocal microscope (Carl Zeiss AG) at an
excitation wavelength of 450 nm.
For comparison, experiments were also performed
using AQP antibodies sourced from Biorbyt Ltd. [AQP1 (cat. no.
orb10122), AQP3 (cat. no. orb47955), AQP4 (cat. no. orb10125) and
AQP5 (cat. no. orb395791); all 1:100) and Santa Cruz Biotechnology,
Inc. [AQP1 (cat. no. sc-25287), AQP3 (cat. no. sc-518001), AQP4
(cat. no. sc-32739) and AQP5 (cat. no. sc-514022); all 1:100; data
not shown].
AQP3 siRNA transfection
Cells were plated in 12-well plates in complete
DMEM, incubated for 24 h at 37°C with 5% CO2, and
allowed to grow until reaching 70-90% confluence. Transfection was
then performed using control scramble siRNA (cat. no. sc-37007) or
AQP3 siRNA (cat. no. sc-29713; both Santa Cruz Biotechnology,
Inc.). Solution A was prepared by adding 6 µl
Lipofectamine® RNAiMAX transfection reagent (cat. no.
56532; Invitrogen; Thermo Fisher Scientific, Inc.) to 100 µl
of Opti-MEM reduced serum medium (Invitrogen; Thermo Fisher
Scientific, Inc.), and solution B was prepared by diluting 2
µl of AQP3 siRNA into 100 µl of Opti-MEM reduced
serum medium (0.2 µM final concentration). Solutions A and B
were mixed, incubated for 15 min and added dropwise to the cells.
Cells were then incubated at 37°C with 5% CO2. Following
48-72-h incubation, cells were harvested and RNA was extracted for
the determination of AQP3 expression via RT-qPCR. Other transfected
cells were harvested at 72 h for AQP3 protein determination via
western blotting, or after 48 h for motility and invasion assays,
as well as determining the effects of alkaline pH on cell
morphology via immunofluorescence confocal microscopy.
Motility assay
Cells (control and following AQP3 siRNA transfection
for 48 h) were cultured in 12-well plates with complete DMEM
containing 5% FBS to 80-90% confluence. A scratch was created in
the cell monolayer using a sterile P1000 pipette tip, and a
photograph of the scratched area was captured immediately (0 h).
After overnight incubation, another photograph (both magnification,
×10) was captured of the same scratched area. The width of the
scratch at 24 h was calculated as a percentage of the width at 0 h;
a minimum of three areas along the scratch were measured.
Cultrex basement membrane extract (BME)
cell invasion assay
Cell invasion was assessed using a Cultrex 24-well
BME cell invasion assay purchased from Trevigen, Inc. (cat. no.
3455-024-K) according to the manufacturer's instructions. For this,
the invasion chamber was coated with 100 µl of 1X BME
solution and incubated overnight at 37°C. After 24 h of
transfection, cells were serum-starved overnight at 37°C with 5%
CO2. On the following day (after 48 h of transfection),
pII cells were harvested, counted and diluted to 1×106
cells/ml in serum-free medium. After that, 100 µl of cells
were added to the top chamber of the Cultrex dish. The lower
chamber was loaded with 500 µl of DMEM supplemented with 30%
FBS (used as a chemoattractant). Cells were incubated at 37°C with
5% CO2 and allowed to invade to the bottom chamber.
After 24 h, the top and the bottom chambers were aspirated and
washed with 1X cell wash buffer. Calcein-AM/cell dissociation
solution complex was added to the bottom chamber and left for 1 h
at 37°C with 5% CO2. Cells internalize
calcein-acetomethylester (AM), and intracellular esterases cleave
the AM moiety, generating fluorescencefree calcein. Invading cells
were determined by recording the fluorescence emission using a
microplate reader, with an excitation/emission filter set of
485/535 nm.
Statistical analysis
Data are presented as the mean ± SEM of three
independent experiments, and were analyzed using GraphPad Prism 5
software (GraphPad Software, Inc.). Student's two-tailed unpaired
t-test or one-way ANOVA followed by Bonferroni post hoc test were
used to compare means of individual groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Effects of alkaline pH and
hypotonic/hypertonic conditions in the extracellular environment on
the morphology and bleb formation of pII cells
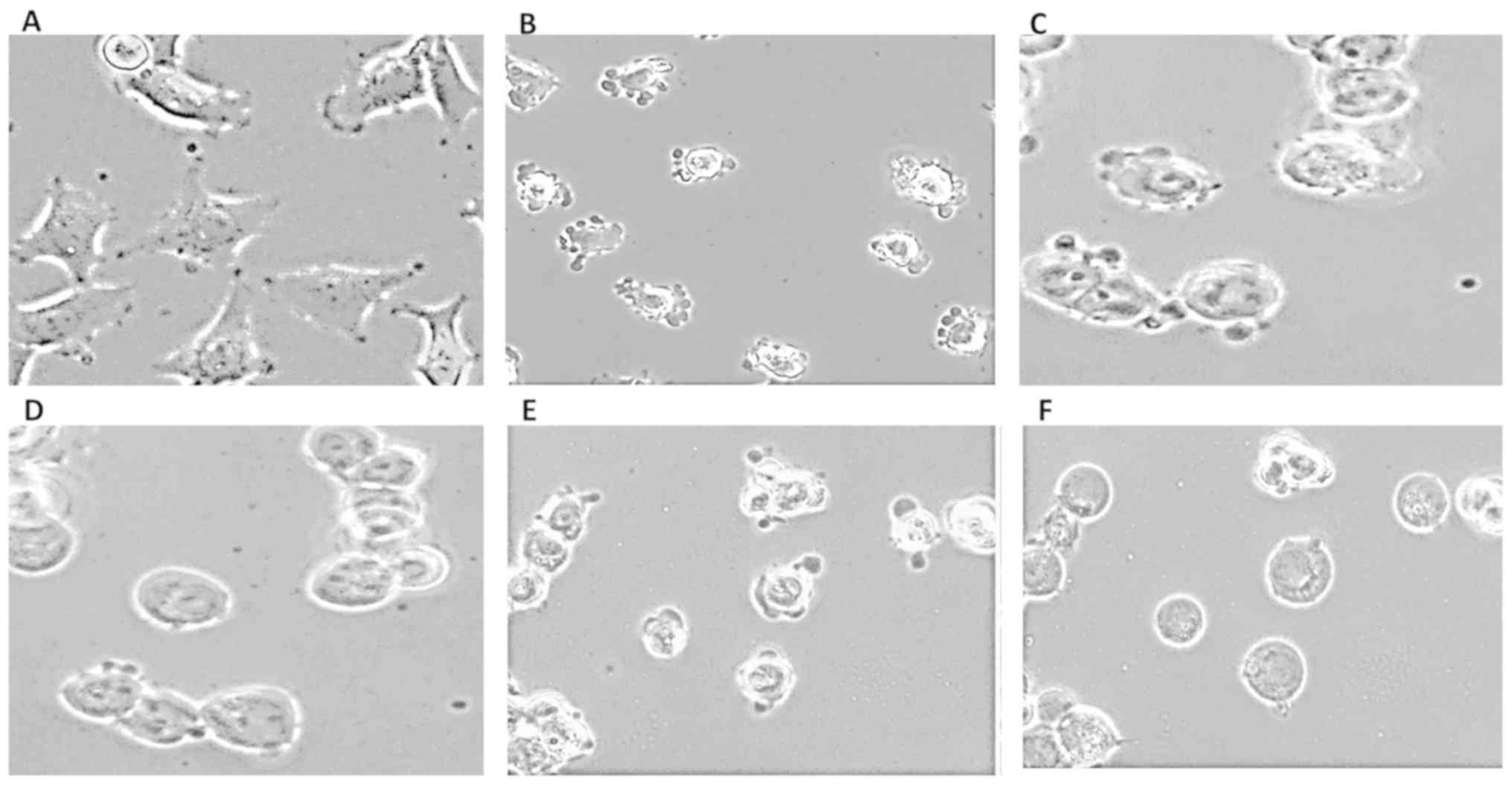
Changes in the morphology of pII cells were recorded
by photographing immediately after removal from the incubator at pH
7.4 (control; Fig. 1A), and then
again after 30 min exposure to atmospheric conditions (pH 8.3;
Fig. 1B). From their typical
mesenchymal-like spindle morphology, the cells became rounded and
exhibited extensive blebbing on the plasma membrane. To determine
the effect of osmotic changes on pH-induced bleb formation, pII
cells were first exposed to alkaline conditions for 30 min (to form
the blebs) followed by the removal of the original media and
substitution with media diluted with water to produce acute
hypotonic conditions. As shown in Fig.
1C-F, substitution with DMEM diluted 1:1 or 1:4 with water for
30-60 min caused the cells to become more rounded and enlarged in
size, and after 60 min (Fig. 1D and
F), the blebs had almost entirely disappeared. Notably, the
cells were able to withstand severe hypotonic conditions without
lysing even after exposure for 120 min (data not shown). Prior
hypotonic conditions caused the cells to swell; this prevented bleb
formation when the medium pH was allowed to alkalinize (data not
shown).
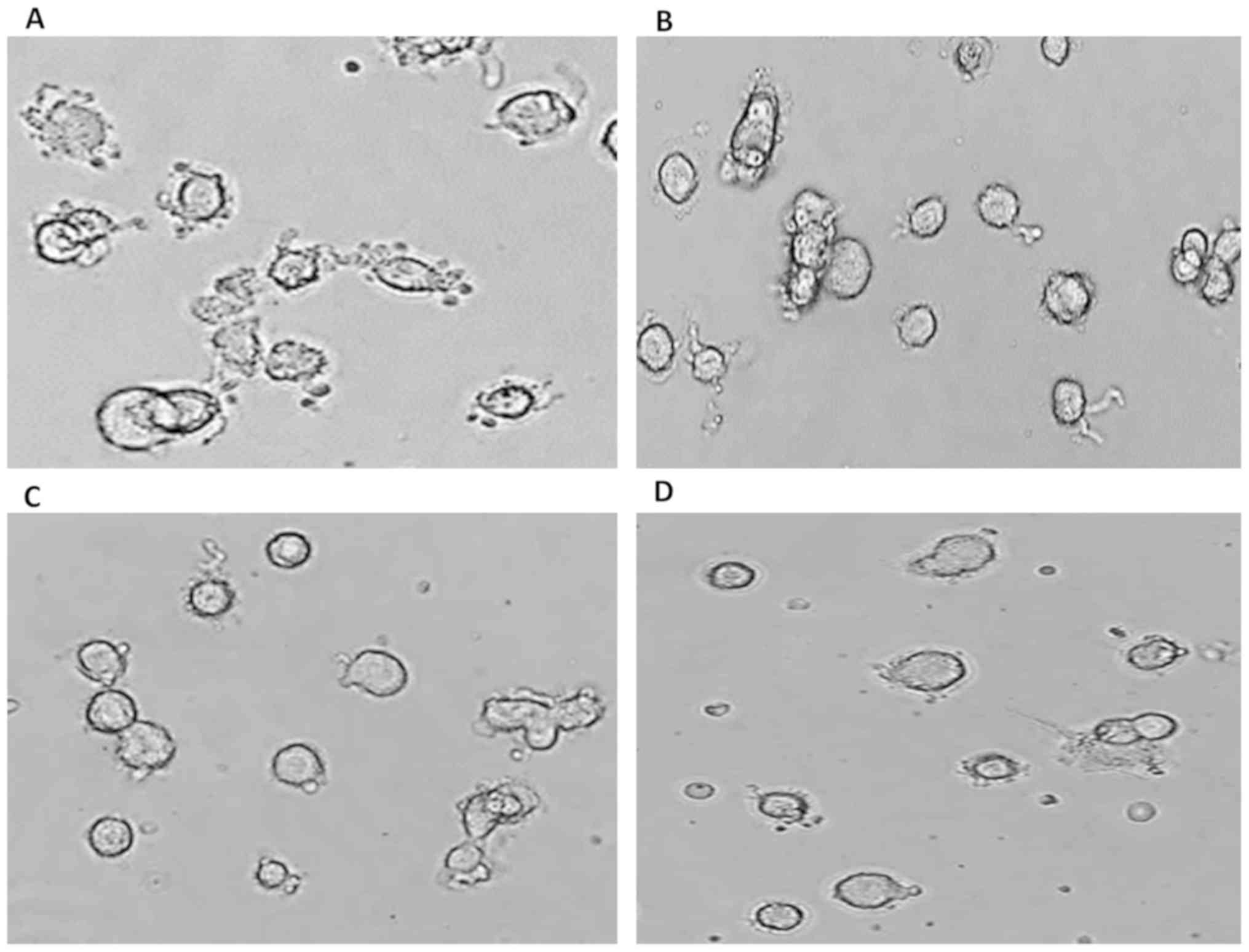
In another experimental setup, pII cells were first
exposed to alkaline pH conditions (Fig. 2A) followed by the addition of
sucrose solution to the media at various concentrations (1, 10 and
100 mM) for 30 min (Fig. 2B-D,
respectively) to induce acute hypertonic conditions. The cells
became rounded and the blebs almost entirely disappeared. These
data suggested that water movement across the plasma membrane is
essential for alkaline pH-induced bleb formation in pII cells.
Expression profile of various AQPs in
breast epithelial and cancer cell lines
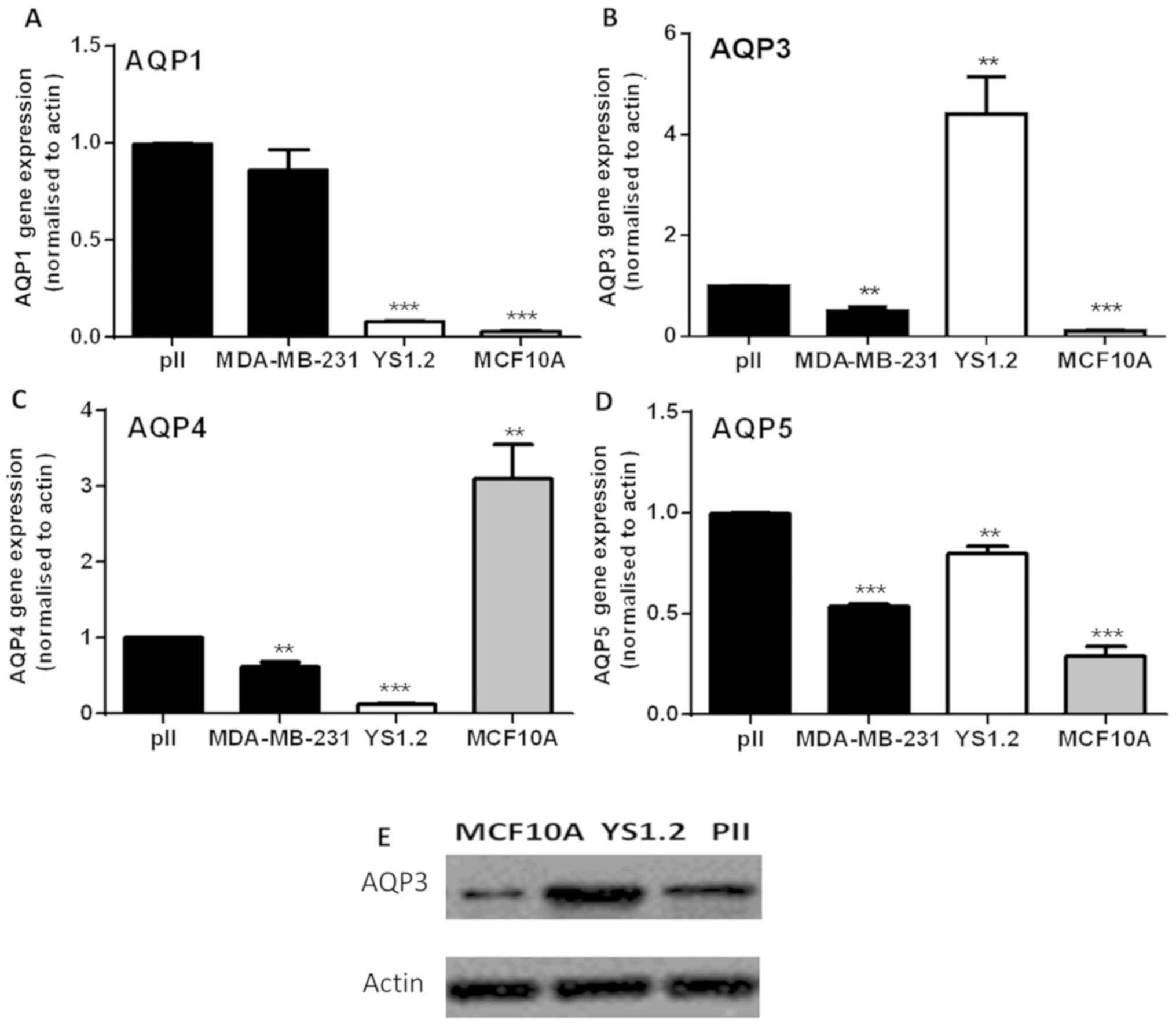
RT-qPCR analysis showed that AQP1 was expressed at
very low levels in MCF10A and non-invasive YS1.2 cells (12), but its levels were significantly
higher in the ER− breast cancer cells (Fig. 3A). AQP3 was highly expressed in
YS1.2, followed by pII and MDA-MB-231, with the lowest expression
in MCF10A cells (Fig. 3B). AQP4
was mainly expressed in MCF10A, with very low expression in breast
cancer cells (Fig. 3C). Similar to
AQP1 and AQP3, AQP5 was highly expressed in breast cancer cells
compared with MCF10A (Fig. 3D).
Western blotting was used to confirm the expression profile of AQP3
at the protein level in MCF10A, YS1.2 and pII cells (Fig. 3E). These results are consistent
with the gene expression profile, which shows differing expression
between ER− and ER+ breast cancer cell lines.
In general, AQP3 expression was higher in cancerous compared to
non-cancerous breast cells. The gene expression profile of the
tested AQPs was not modified in pII cells upon exposure to alkaline
pH for 30 min (data not shown).
Localization pattern of various AQPs at
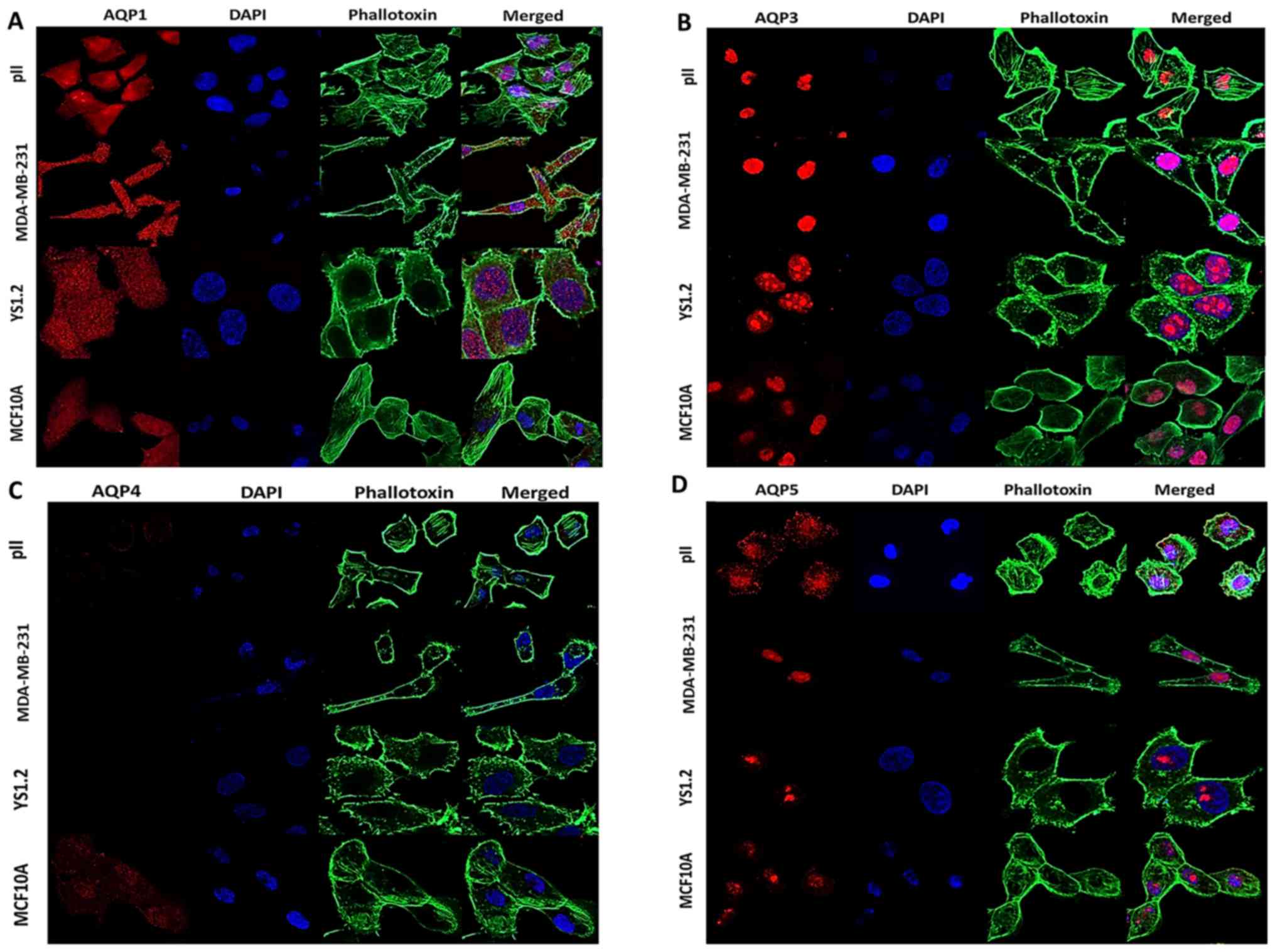
normal pH and upon exposure to alkaline pH
The localization patterns of AQPs 1, 3, 4 and 5 in
the tested cell lines cultured in pH 7.4 were determined via
immunofluorescence. Due to differences in microscope settings and
digital enhancement of certain images (necessary to visualise low
expression), fluorescence intensities presented in the figures do
not accurately reflect relative quantitative differences. AQP1
showed diffuse cytoplasmic staining in all the cell lines examined,
as well as expression over the nuclear surface; immunoreactivity
was weakest in MCF10A cells, confirming comparatively low levels of
expression of this protein in normal breast epithelial cells
(Fig. 4A). Staining with an
anti-AQP3 antibody from Abcam, expression was detected only in the
nuclear region, with no clear staining in the cytoplasm (Fig. 4B); this pattern was observed in all
the cell lines. In view of this somewhat unexpected distribution of
AQP3 compared with other literature reports (28,33),
these experiments were repeated in pII cells with antibodies
purchased from other sources, which showed similar nuclear
localization (data not shown). Additionally, an alternative
fixation method using methanol instead of formaldehyde was used to
determine whether this affected the interaction of the antibody,
and similar distribution patterns were also observed (data not
shown). Weak diffuse staining of AQP4 was observed only in MCF10A
(Fig. 4C). AQP5 appeared to be
mainly nuclear in all the cell lines, with some additional
cytoplasmic immunoreactivity detected in pII cells (Fig. 4D).
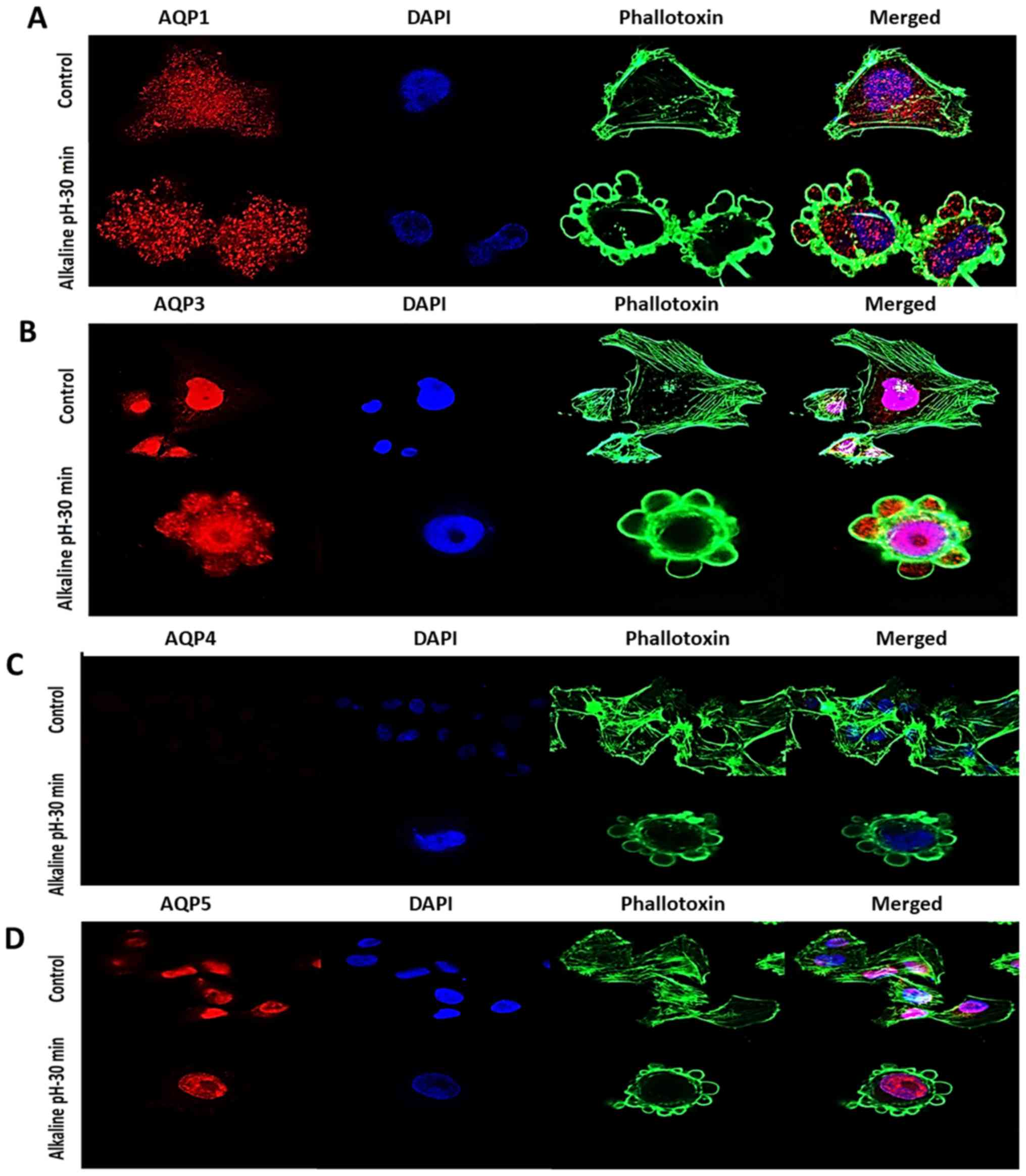
The locations of AQPs 1, 3, 4 and 5 were also
determined in pII cells following exposure to alkaline pH for 30
min. As presented in Fig. 5A, AQP1
exhibited a similar diffuse distribution in alkaline pH, but was
now also detected inside the blebs. AQP3 remained predominantly
nuclear but had also markedly migrated into the blebs, in a far
more pronounced manner than AQP1 (Fig.
5B). AQP4 was not detectable at either pH (Fig. 5C). AQP5 maintained its nuclear
localization, and was not observed in either the extranuclear space
or inside the blebs (Fig. 5D).
These data suggested that AQP3 may play an important role in pII
cell migration and invasion due to its substantial translocation
into the newly formed blebs.
Effects of siRNA knockdown of AQP3 on pII
cell blebbing, motility and invasion
AQP3 gene expression was significantly reduced in
pII cells at 48 and 72 h post-transfection with AQP3-targeting
siRNA (Fig. 6A). This was
paralleled by a reduction in AQP3 protein detected via western
blotting at 72 h (Fig. 6B).
Cultures assayed at 48 h post-transfection exhibited significant
reduction in cell motility (Fig. 6C
and D) and invasion towards serum components (Fig. 6E). It should be noted that the
motility assays were performed in media supplemented with 5% FBS
over 24 h. This is expected to result in doubling of the cell
number; however, the contribution of cell proliferation to total
wound closure is expected to be relatively minor. This can be
clearly demonstrated by the difference between migratory and
non-migratory cells cultured in growth media. Assessment of the
proportion of blebbing cells in siRNA-transfected cultures exposed
to alkaline pH also showed a significant reduction (Fig. 6F). The immunofluorescence images
presented in Fig. 6 G show
examples of the heterogeneity of response in individual cells; the
left panel shows AQP3 expression in control (scrambled
siRNA)-transfected blebbing cells, whereas the right panels show
AQP3 siRNA-transfected cells that either failed to bleb, or blebbed
despite the absence of AQP3.
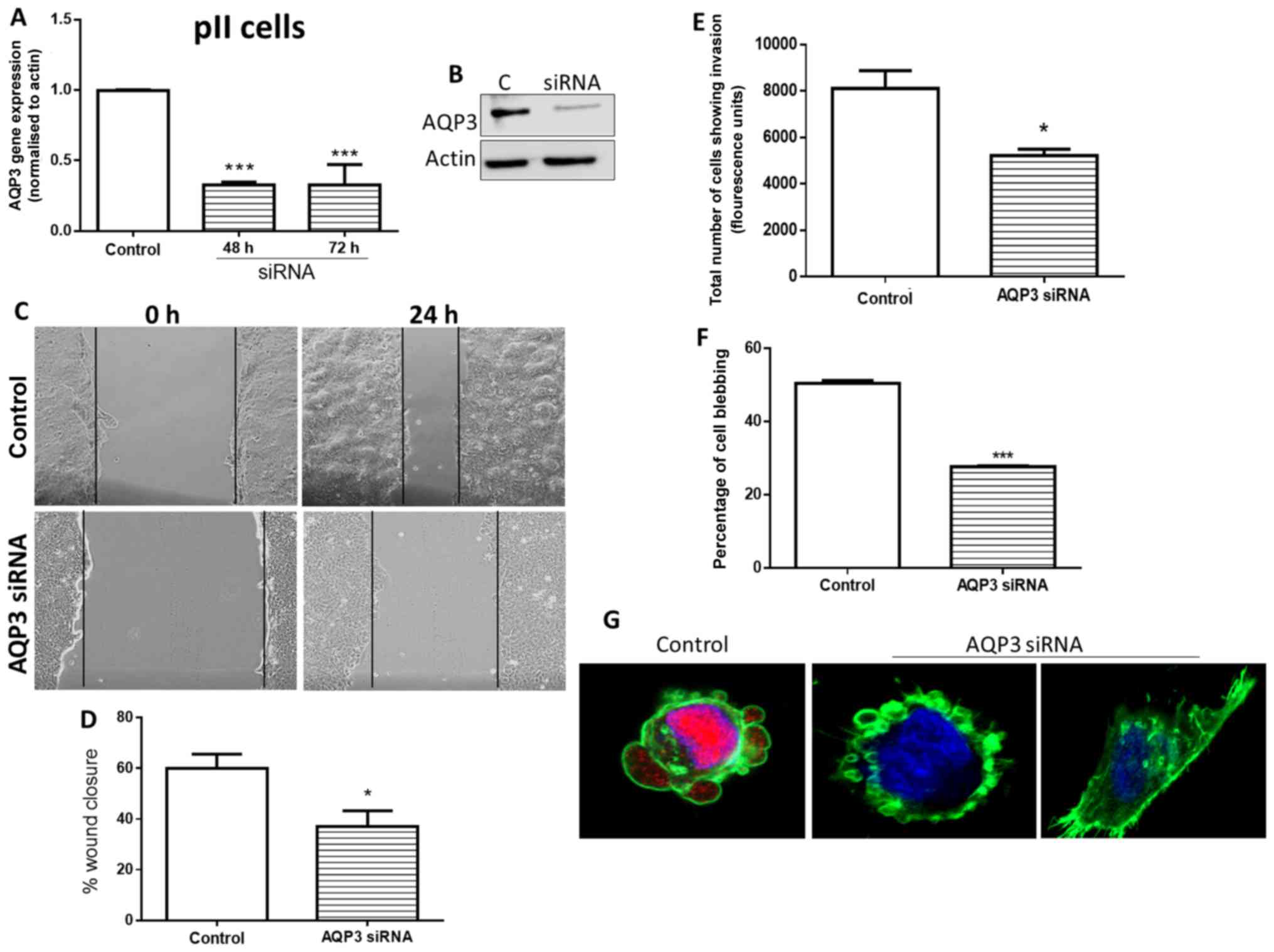 | Figure 6Effects of siRNA-mediated knockdown
of AQP3 in pII cells on cell motility, invasion and alkaline
pH-induced bleb formation. (A) Reverse transcription-quantitative
PCR analysis of AQP3 mRNA expression in control siRNA-transfected
cells, and AQP3 siRNA-transfected cells at 48 and 72 h. (B) AQP3
protein expression was determined in control siRNA- and AQP3
siRNA-transfected cells at 72 h via western blot analysis. (C) At
48 h following transfection, cells were grown to confluency, and a
scratch was made through the center of the cell monolayer using a
1000 µl Eppendorf pipette. The scratch width was measured
under a microscope at 0 and 24 h. (D) Percentage of wound closure
was quantified. (E) Effect of AQP3 siRNA knockdown on invasion of
pII cells through basement membrane extract towards serum
components. The y-axis represents arbitrary fluorescence units
showing uptake of calcein into the invading cells in the bottom
chamber of the Cultrex dish. (F) At 48 h after transfection with
AQP3 siRNA, cells were removed from the incubator and exposed to
atmospheric conditions. Cells were photographed immediately (pH
7.4, control) and after 30 min (pH 8.3). The number of blebbing
cells was counted manually to compare the percentage of cells
blebbing between transfected and untreated pII cells. (G) Confocal
images of AQP3/phalloidin/DAPI staining of individual cells in
control siRNA- and AQP3 siRNA-transfected cultures exposed to
alkaline pH (magnification, ×60). Data are presented as the mean ±
SEM of 3 independent experiments. *P<0.05,
***P<0.001 vs. control. AQP, aquaporin; siRNA, small
interfering RNA. |
Discussion
The present study revealed distinct expression
profiles for AQPs 1, 3, 4 and 5 in normal epithelial breast cells
and breast cancer cells. mRNA expression levels of AQPs 1, 3 and 5
were higher in breast cancer cells compared with MCF10A.
Conversely, AQP4 expression was higher in MCF10A than in the cancer
cells. These results are in agreement with a number of reports
which demonstrated enhanced AQP 1, 3 and 5 expression in breast
cancer tissues (16,22,31),
particularly in those with invasive ductal carcinoma and lymph node
invasion (for AQP5) (31). AQP1
expression was found to be correlated with high tumor grade and
triple negativity, and exhibited a negative relationship with ER
status (22). This inverse
correlation is in agreement with the present findings into AQP1
mRNA expression. In contrast, the expression of AQP3 was elevated
in ER+ breast cancer tissues obtained from premenopausal
women in comparison to those from postmenopausal women (33). Additionally, treating
ER+ (T47D and MCF7) cells and ER−
(MDA-MB-231) cells with estrogen enhanced AQP3 mRNA levels only in
the ER+ cells (26),
which was attributed to the presence of an ERE in the promoter
region of the AQP3 gene in the ER+ breast cancer cells.
This observation was also in agreement with the present findings
regarding AQP3 expression.
Immunocytochemical analysis showed that AQPs 1, 3, 4
and 5 exhibited distinct cellular localization. AQP1 was evenly
distributed along the cytoplasm and surrounding the nucleus. AQP3
and AQP5 were localized predominantly in the nuclear region of all
cell lines, except in pII cells, which showed some cytoplasmic
staining for AQP5. In contrast, AQP4 was most strongly expressed in
MCF10A cells, with low expression in breast cancer cell lines. The
observed cellular distribution contrasts with published reports
that have generally shown the AQPs to be located in the cytoplasm
or cell membrane. For example, a study conducted by Luo et
al (34) demonstrated
immunohistochemical localization of AQP1 in the cell membrane of
breast cancer tissues, and at lower levels in the corresponding
normal tissues. In their clinical study, they showed strong
cytoplasmic AQP1 staining in the cells of invasive ductal carcinoma
tissues and membranous staining in the cells of benign breast
lesions (23). They further
verified the cytoplasmic expression of AQP1 via in vitro
analysis of primary breast cancer cells and MDA-MB-231 cells
overexpressing AQP1. Shi et al (16) also detected the expression of AQPs
1, 4 and 5 in breast cancer and normal breast tissues, and found
that AQPs 1 and 5 were located in the cellular membranes, whereas
AQP4 exhibited membranous and cytoplasmic localization. Huang et
al (26) showed that AQP3
exhibited cytoplasmic and membranous expression in the tumor cells
of ER+ breast cancer tissues. A recent study also
investigated the cellular localization of AQPs 3 and 5 in triple
negative breast cancer tissue, and found that both were expressed
in the membrane and the cytoplasm (35). In view of these reports, the
immunolocalization experiments were repeated to detect AQP3 in pII
cells with antibodies from several commercial sources, including
those used in the abovementioned studies, as well as using
different fixation methods to determine whether this may account
for the disparity of these observations. The results obtained with
antibodies from 3 different companies all consistently confirmed
the nuclear localization of AQP3 in pII cells. AQP1 was the only
AQP to exhibit consistent cytoplasmic localization; AQP4 and AQP5
showed some cytoplasmic and membranous distribution in certain cell
lines. Western blot analysis using the same anti-AQP3 antibody
identified a band of the expected molecular weight of 32 kDa, which
supports the specificity of these antibodies.
Only the ER-silenced pII cells are affected by
alkaline pH and exhibit blebbing. Neither normal breast cells nor
ER+ cells show this phenomenon. ER−
MDA-MB-231 cells do undergo some blebbing, but not at comparable
levels to pII cells (13,14,36).
Therefore, the pH experiments were only conducted using pII cells.
At present, no other stimuli that cause blebbing without inducing
apoptosis have been identified. Notably, exposure to acidic pH does
not induce this response (14). In
the current study, the alterations in the morphology of pII cells
exposed to alkalinity were further verified. As the formation of
the blebs involves dynamic cytoplasmic streaming, this would
presumably require substantial water flux, which is generally
hypothesized to be controlled by the AQPs to maintain osmolality.
Thus, the effects of changing the medium of pII cells that had
previously been exposed to alkaline pH on bleb formation were
examined, with the medium replaced with culture medium diluted with
various ratios of water or sucrose. As would be expected the cells
enlarged with increasing hypotonicity of the medium, and the blebs
disappeared. Notably, the cells did not burst even after 90-120 min
of observation. The redistribution of some AQP subtypes into the
cellular blebs may aid to maintain the integrity of the cell and
support the cytoskeleton. Under hypertonic conditions, there was
also a reduction in the blebbing. As these were only exploratory
observations, it will be interesting to examine the expression and
distribution of AQPs during this process in further experiments.
Indeed, Monzani et al (37)
demonstrated that downregulation of AQP1 in a human melanoma cell
line and a human microvascular cell line altered the organization
of F-actin. In addition, AQP1 was found to co-immunoprecipitate
with Lin-7 in these cell lines; a model was proposed that AQP1 acts
as a scaffolding protein of the plasma membrane that is involved in
the organization of the cytoskeleton by interacting with the
Lin-7/β-catenin pathway (37). In
the present study, only AQP1 and AQP3 were observed to be
translocated into the formed blebs. The redistribution of these
proteins into the blebs suggests their involvement in the process
of bleb formation and/or stabilization and, by implication, in cell
migration and invasion. The process of cell migration has been
associated with membrane protrusions such as lamel-lipodia and
ruffling at the leading edge of migrating cells (38). Hu and Verkman (39) reported that AQP1 showed polarized
distribution at the leading edge of migrating mouse melanoma B16F10
cells and was associated with increased migration. In the present
study, siRNA-mediated knockdown of AQP3 in pII cells resulted in a
significant reduction in cell migration and invasion through BME;
the percentage of blebbing cells was also significantly reduced
upon subsequent exposure to alkaline pH. It should be noted that a
proportion of the transfected cells still blebbed despite loss of
AQP3. This is not unexpected, as our previous studies showed that
several other molecules and signaling pathways are involved in the
complex process of blebbing in pII cells (13,14,40);
these would be unaffected by AQP3 knockdown.
A number of studies have suggested that AQP3 is
associated with cancer progression and metastasis in esophageal,
colorectal, hepatocellular and gastric cancers (21,41-43).
Hara-Chikuma and Verkman (33)
demonstrated that AQP3 contributes to breast cancer cell migration
by facilitating the transport of hydrogen peroxide. Another study
showed that lentiviral shRNA-mediated knockdown of AQP3 in
MDA-MB-231 and Bcap-37 breast cancer cell lines significantly
reduced fibroblast growth factor (FGF)-2-induced cell migration
(28). In addition, FGF receptor
(FGFR) kinase, PI3K and mitogen-activated protein kinase kinase 1/2
inhibitors reduced FGF-2-dependent increases in AQP3 expression and
cell migration, indicating the involvement of the FGFR-PI3K and
FGFR-ERK signaling pathways. Another study reported that
siRNA-mediated knockdown of AQP3 in the ER+ breast
cancer cell lines T47D and MCF7 led to a prominent reduction in
cell migration; upregulation of this protein in T47D cells markedly
increased both processes (26). It
was found that AQP3 regulates estradiol-induced breast cancer cell
migration and invasion by inducing reorganization of the actin
cytoskeleton (including formation of filopodia) and affecting the
expression of EMT inducer molecules, favoring the metastasis of
breast cancer (33). Similarly,
transfection of AQP3 shRNA into MDA-MB-231 breast cancer cells
reduced cell proliferation, migration and invasion (44). However, downregulation of AQP3 was
also associated with a marked decrease in the viability of cells
exposed to 100 µM 5-fluorouracil, a fluoropyrimidine
pro-drug administered to patients with breast or colorectal tumors
(44).
It should be considered that the process of cancer
cells attaining motility and the capacity for invasion is a complex
process involving numerous molecular participants, and evaluating
only one of these molecules at a time ignores the contributory
effect of others; this has been demonstrated in our previous
studies by the wide range of agents that inhibit blebbing (13,14,36).
Interpretation of data should take this into consideration. A
shortcoming of siRNA knockdown is the incomplete silencing of the
gene of interest, which may be more effectively achieved using gene
editing techniques such as CRISPR. Further direct evidence is also
required to directly connect the AQPs with the osmotic effects on
blebbing.
In conclusion, the expression of AQPs 1, 3, 4 and 5
is modulated in breast cancer; all of them were upregulated in
breast cancer cells compared with normal breast cells, except for
AQP4, which was more intensely expressed in normal breast cells.
AQP3 serves a significant role in endocrine-resistant breast cancer
cell motility and invasion, and may participate in the processes
leading to the blebbing of endocrine-resistant cells. Therefore,
AQP3 (and potentially other AQPs) may represent additional
therapeutic targets in the prevention of metastatic spread of
breast cancers.
Funding
This work was supported by Kuwait University College
of Graduate Studies and the Research Sector (grant no. YP02/17), a
Distinguished Scholarship from Kuwait University, and a Kuwait
University grant (grant no. SRUL02/13) that funds the Research Unit
for Genomics, Proteomics and Cellomics Studies facilities used
during the study.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MAK and YAL conceived the study and designed the
experiments. AEA with some assistance from SK performed the
experiments. AEA, MAK and YAL analyzed the data. YAL and MAK
contributed reagents, materials and analytical tools. MAK, AEA and
YAL drafted the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar
|
|
2
|
Servick K: Breast cancer: Breast cancer: A
world of differences. Science. 343:1452–1453. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Carey LA, Perou CM, Livasy CA, Dressler
LG, Cowan D, Conway K, Karaca G, Troester MA, Tse CK, Edmiston S,
et al: Race, breast cancer subtypes, and survival in the Carolina
Breast Cancer Study. JAMA. 295:2492–2502. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Higgins MJ and Baselga J: Targeted
therapies for breast cancer. J Clin Invest. 121:3797–3803. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Perou CM, Sørlie T, Eisen MB, van de Rijn
M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA,
et al: Molecular portraits of human breast tumours. Nature.
406:747–752. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sørlie T, Perou CM, Tibshirani R, Aas T,
Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey
SS, et al: Gene expression patterns of breast carcinomas
distinguish tumor subclasses with clinical implications. Proc Natl
Acad Sci USA. 98:10869–10874. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang Y and Zhou BP: Epithelial-mesenchymal
Transition - A Hallmark of Breast Cancer Metastasis. Cancer Hallm.
1:38–49. 2013. View Article : Google Scholar
|
|
8
|
Al Saleh S, Al Mulla F and Luqmani YA:
Estrogen receptor silencing induces epithelial to mesenchymal
transition in human breast cancer cells. PLoS One. 6:e206102011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Al Saleh S, Sharaf LH and Luqmani YA:
Signalling pathways involved in endocrine resistance in breast
cancer and associations with epithelial to mesenchymal transition
(Review). Int J Oncol. 38:1197–1217. 2011.PubMed/NCBI
|
|
11
|
Luqmani YA, Al Azmi A, Al Bader M, Abraham
G and El Zawahri M: Modification of gene expression induced by
siRNA targeting of estrogen receptor alpha in MCF7 human breast
cancer cells. Int J Oncol. 34:231–242. 2009.
|
|
12
|
Khajah MA, Al Saleh S, Mathew PM and
Luqmani YA: Differential effect of growth factors on invasion and
proliferation of endocrine resistant breast cancer cells. PLoS One.
7:e418472012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Khajah MA, Almohri I, Mathew PM and
Luqmani YA: Extracellular alkaline pH leads to increased metastatic
potential of estrogen receptor silenced endocrine resistant breast
cancer cells. PLoS One. 8:e763272013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Khajah MA, Mathew PM, Alam-Eldin NS and
Luqmani YA: Bleb formation is induced by alkaline but not acidic pH
in estrogen receptor silenced breast cancer cells. Int J Oncol.
46:1685–1698. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Khajah MA and Luqmani YA: Involvement of
Membrane Blebbing in Immunological Disorders and Cancer. Medical
principles and practice: International journal of the Kuwait
University. Health Sci Cent. 25(Suppl 2): 18–27. 2016.
|
|
16
|
Shi Z, Zhang T, Luo L, Zhao H, Cheng J,
Xiang J and Zhao C: Aquaporins in human breast cancer:
Identification and involvement in carcinogenesis of breast cancer.
J Surg Oncol. 106:267–272. 2012. View Article : Google Scholar
|
|
17
|
Kasa P, Farran B, Prasad GLV and Nagaraju
GP: Aquaporins in female specific cancers. Gene. 700:60–64. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
López-Campos JL, Sánchez Silva R, Gómez
Izquierdo L, Márquez E, Ortega Ruiz F, Cejudo P, Barrot Cortés E,
Toledo Aral JJ and Echevarría M: Overexpression of Aquaporin-1 in
lung adenocarcinomas and pleural mesotheliomas. Histol Histopathol.
26:451–459. 2011.PubMed/NCBI
|
|
19
|
Yoshida T, Hojo S, Sekine S, Sawada S,
Okumura T, Nagata T, Shimada Y and Tsukada K: Expression of
aquaporin-1 is a poor prognostic factor for stage II and III colon
cancer. Mol Clin Oncol. 1:953–958. 2013. View Article : Google Scholar
|
|
20
|
El Hindy N, Bankfalvi A, Herring A,
Adamzik M, Lambertz N, Zhu Y, Siffert W, Sure U and Sandalcioglu
IE: Correlation of aquaporin-1 water channel protein expression
with tumor angiogenesis in human astrocytoma. Anticancer Res.
33:609–613. 2013.PubMed/NCBI
|
|
21
|
Guo X, Sun T, Yang M, Li Z, Li Z and Gao
Y: Prognostic value of combined aquaporin 3 and aquaporin 5
overexpression in hepatocellular carcinoma. BioMed Res Int.
2013:2065252013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Otterbach F, Callies R, Adamzik M, Kimmig
R, Siffert W, Schmid KW and Bankfalvi A: Aquaporin 1 (AQP1)
expression is a novel characteristic feature of a particularly
aggressive subgroup of basal-like breast carcinomas. Breast Cancer
Res Treat. 120:67–76. 2010. View Article : Google Scholar
|
|
23
|
Qin F, Zhang H, Shao Y, Liu X, Yang L,
Huang Y, Fu L, Gu F and Ma Y: Expression of aquaporin1, a water
channel protein, in cytoplasm is negatively correlated with
prognosis of breast cancer patients. Oncotarget. 7:8143–8154. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Esteva-Font C, Jin BJ and Verkman AS:
Aquaporin-1 gene deletion reduces breast tumor growth and lung
metastasis in tumor-producing MMTV-PyVT mice. FASEB J.
28:1446–1453. 2014. View Article : Google Scholar :
|
|
25
|
Kang S, Chae YS, Lee SJ, Kang BW, Kim JG,
Kim WW, Jung JH, Park HY, Jeong JH, Jeong JY, et al: Aquaporin 3
Expression Predicts Survival in Patients with HER2-positive Early
Breast Cancer. Anticancer Res. 35:2775–2782. 2015.PubMed/NCBI
|
|
26
|
Huang YT, Zhou J, Shi S, Xu HY, Qu F,
Zhang D, Chen YD, Yang J, Huang HF and Sheng JZ: Identification of
Estrogen Response Element in Aquaporin-3 Gene that Mediates
Estrogen-induced Cell Migration and Invasion in Estrogen
Receptor-positive Breast Cancer. Sci Rep. 5:124842015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cao C, Sun Y, Healey S, Bi Z, Hu G, Wan S,
Kouttab N, Chu W and Wan Y: EGFR-mediated expression of aquaporin-3
is involved in human skin fibroblast migration. Biochem J.
400:225–234. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cao XC, Zhang WR, Cao WF, Liu BW, Zhang F,
Zhao HM, Meng R, Zhang L, Niu RF, Hao XS, et al: Aquaporin3 is
required for FGF-2-induced migration of human breast cancers. PLoS
One. 8:e567352013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li YB, Sun SR and Han XH: Down-regulation
of AQP4 Inhibits Proliferation, Migration and Invasion of Human
Breast Cancer Cells. Folia Biol (Praha). 62:131–137. 2016.
|
|
30
|
Lee SJ, Chae YS, Kim JG, Kim WW, Jung JH,
Park HY, Jeong JY, Park JY, Jung HJ and Kwon TH: AQP5 expression
predicts survival in patients with early breast cancer. Ann Surg
Oncol. 21:375–383. 2014. View Article : Google Scholar
|
|
31
|
Jung HJ, Park JY, Jeon HS and Kwon TH:
Aquaporin-5: A marker protein for proliferation and migration of
human breast cancer cells. PLoS One. 6:e284922011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
33
|
Hara-Chikuma M and Verkman AS: Aquaporin-3
facilitates epidermal cell migration and proliferation during wound
healing. J Mol Med (Berl). 86:221–231. 2008. View Article : Google Scholar
|
|
34
|
Luo L, Yang R, Zhao S, Chen Y, Hong S,
Wang K, Wang T, Cheng J, Zhang T and Chen D: Decreased miR-320
expression is associated with breast cancer progression, cell
migration, and invasiveness via targeting Aquaporin 1. Acta Biochim
Biophys Sin (Shanghai). 50:473–480. 2018. View Article : Google Scholar
|
|
35
|
Zhu Z, Jiao L, Li T, Wang H, Wei W and
Qian H: Expression of AQP3 and AQP5 as a prognostic marker in
triple-negative breast cancer. Oncol Lett. 16:2661–2667.
2018.PubMed/NCBI
|
|
36
|
Khajah MA and Luqmani YA: Involvement of
Membrane Blebbing in Immunological Disorders and Cancer. Med Princ
Pract. 25(Suppl 2): 18–27. 2016. View Article : Google Scholar
|
|
37
|
Monzani E, Bazzotti R, Perego C and La
Porta CA: AQP1 is not only a water channel: It contributes to cell
migration through Lin7/beta-catenin. PLoS One. 4:e61672009.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Schwab A: Function and spatial
distribution of ion channels and transporters in cell migration. Am
J Physiol Renal Physiol. 280:F739–F747. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hu J and Verkman AS: Increased migration
and metastatic potential of tumor cells expressing aquaporin water
channels. FASEB J. 20:1892–1894. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Khajah MA, Mathew PM and Luqmani YA:
Na+/K+ ATPase activity promotes invasion of
endocrine resistant breast cancer cells. PLoS One. 13:e01937792018.
View Article : Google Scholar
|
|
41
|
Chen J, Wang T, Zhou YC, Gao F, Zhang ZH,
Xu H, Wang SL and Shen LZ: Aquaporin 3 promotes
epithelial-mesenchymal transition in gastric cancer. J Exp Clin
Cancer Res. 33:382014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li A, Lu D, Zhang Y, Li J, Fang Y, Li F
and Sun J: Critical role of aquaporin-3 in epidermal growth
factor-induced migration of colorectal carcinoma cells and its
clinical significance. Oncol Rep. 29:535–540. 2013. View Article : Google Scholar
|
|
43
|
Liu S, Zhang S, Jiang H, Yang Y and Jiang
Y: Co-expression of AQP3 and AQP5 in esophageal squamous cell
carcinoma correlates with aggressive tumor progression and poor
prognosis. Med Oncol. 30:6362013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Arif M, Kitchen P, Conner MT, Hill EJ,
Nagel D, Bill RM, Dunmore SJ, Armesilla AL, Gross S, Carmichael AR,
et al: Downregulation of aquaporin 3 inhibits cellular
proliferation, migration and invasion in the MDA-MB-231 breast
cancer cell line. Oncol Lett. 16:713–720. 2018.PubMed/NCBI
|