Introduction
Esophageal cancer has a high incidence in East Asia,
with annual morbidity and mortality rates that rank third and
fourth among all cancers, retrospectively, based on analysis of
2012 East Asian cancer statistics (1). The major histological type in East
Asia is squamous cell carcinoma. Despite ongoing research regarding
treatments for esophageal squamous cell carcinoma (ESCC), the
long-term survival rates remain poor (2). As the majority of patients are
diagnosed with locally advanced disease and are not suitable for
curative surgery, definitive chemo-radiotherapy or radiotherapy
have became the main treatment modalities (3). However, the local tumor recurrence
rate is as high as 30-40% following radiotherapy (4), which is one of the main causes of
treatment failure. Therefore, re-irradiation combined with systemic
chemotherapy is often the only choice; however, re-irradiation is
difficult to perform. In radical radiotherapy, local normal tissue
can receive high radiation doses, therefore a radical irradiation
dose cannot be administered to the recurred tumor (5). Notably, the impact of irradiation on
the tumor microenvironment, which predominately consists of damage
to tumor microvascules, may increase the hypoxia fraction in
recurred tumors, which leads to radiotherapy resistance (6,7).
Therefore, an efficacious therapy with minimal toxicity that
improves the efficacy of radiotherapy is urgently needed.
A recurrent tumor xenograft model can be established
to simulate the clinical situation of the tumor. This widely
accepted approach involves administering a certain dose of
radiation to the transplantation site prior to tumor cell
transplantation. In a variety of animal experiments with different
recurrence tumor models, it has been found that the level of
hypoxia in recurrent tumors is significantly higher compared with
in normal tumors (8,9). In addition, epidermal growth factor
receptor (EGFR) overexpression is observed in hypoxic regions
(10,11). Additionally, EGFR overexpression in
esophageal cancer has been identified to be inversely associated
with tumor radiocurability (12,13).
In a number or studies, EGFR overexpression has been demonstrated
to be associated with lower tumor control rates following
irradiation (14,15). EGFR overexpression is also
correlated with poor disease-free and overall survival rates in
patients with esophageal adenocarcinoma (16). Previous studies have reported that
nimotuzumab can improve the radiosensitivity of lung cancer and
brain tumors with high expression of EGFR (17,18).
Therefore, EGFR-targeted therapy combined with radiotherapy is
expected to become an effective method to control recurrent tumors.
Nimotuzumab, an important EGFR-targeted antibody, is currently used
clinically in combination with radiotherapy and is approved for
treating esophageal cancer (19,20).
However, to the best of our knowledge, there has been no
preclinical study of the radiosensitivity of nimotuzumab in
recurrent esophageal cancer. Therefore, the present study
investigated the efficacy of the anti-EGFR therapy on recurrent
ESCC radiotherapy and the potential underlying mechanisms in
vitro and in vivo.
Materials and methods
Drug and chemicals
Nimotuzumab was provided by Baitai Bio
Pharmaceutical Co., Ltd. Nimotuzumab was dissolved in saline (0.9%
sodium chloride) to the required concentration and stored in the
dark at 4°C for follow-up experiments.
Cell culture and antibodies
The human esophageal cancer cell lines EC-109
(Chinese Academy of Sciences) and TE-1 (Shanghai Institute of Cell
Bank) were cultured in RPMI-1640 medium (Gibco; Thermo Fisher
Scientific, Inc.), supplemented with 10% fetal bovine serum (Gibco;
Thermo Fisher Scientific, Inc.), penicillin (100 U/ml) and
streptomycin (100 µg/ml). The cells were cultured under
normoxic (20% O2) or hypoxic conditions (0.2%
O2). The temperature of culture was 37°C. PBS with 1%
BSA was used as the primary antibody diluent. Hypoxia-inducible
factor 1-α (HIF-1α; cat. no. ABE279; EMD Millipore; western
blotting, 1:1,000; immunohistochemistry, 1:100) and carbonic
anhydrase 9 (CA9; cat. no. D120346; Sangon Biotech, Co. Ltd;
western blotting, 1:1,000; immunohistochemistry, 1:100) antibodies
were used for western blotting and staining. The cleaved caspase-3
rabbit mAb (cat. no. 9664; western blotting, 1:1,000), EGFR rabbit
mAb (cat. no. 2085; western blotting, 1:1,000;
immunohistochemistry, 1:100; immunofluorescence, 1:250),
anti-phosphorylated (p)-EGFR rabbit mAb (cat. no. 3777; western
blotting, 1:1,000; immunohistochemistry, 1:800; immunofluorescence,
1:400), Bcl-2 rabbit mAb (cat. no. 2870; western blotting, 1:1,000)
and caspase-3 rabbit mAb (cat. no. 9665; western blotting, 1:1,000)
were purchased from Cell Signaling Technology, Inc. CDK1 rabbit mAb
(cat. no. ab133327; western blotting, 1:10,000), Cdc25c rabbit mAb
(cat. no. ab32444; western blotting, 1:2,000), Bax rabbit mAb (cat.
no. ab32503; western blotting, 1:2,000), cyclin B1 rabbit mAb (cat.
no. ab32053; western blotting, 1:5,000), cyclin A2 rabbit mAb (cat.
no. ab181591; western blotting, 1:2,000) and cyclin D1 rabbit mAb
(cat. no. ab134175; western blotting, 1:10,000) were purchased from
Abcam.
Western blot analysis
The esophageal cancer cells were lysed on ice for 5
min with RIPA lysis buffer (Beyotime Institute of Biotechnology)
and then centrifuged for 10 min at 4°C and 10,800 × g to separate
the supernatant as the protein extract. The protein concentration
was measured using the BCA assay kit (Beyotime Institute of
Biotechnology). Total protein (20 µg) from each sample was
dissolved on a 12% Bis-Tris gel with MOPS running buffer and
transferred to nitrocellulose membranes. The membrane was blocked
with 5% milk-TBST (10 nm Tris-HCl, pH 7.5; 150 mM, 0.05% Tween-20)
and incubated overnight at room temperature with antibodies against
HIF-1α, CA9, cleaved caspase-3, caspase-3, EGFR, p-EGFR, Bcl-2,
CDK1, Cdc25c, Bax, cyclin B1, cyclin A2 and cyclin D1. The membrane
was then washed with TBST and incubated for 1 h with a goat
anti-rabbit secondary antibody (1:3,000; Cell Signaling Technology,
Inc.). The protein bands were detected using G:BOX (Syngene) with
Chemiluminescent horseradish peroxidase substrate (EMD Millipore).
The data were analyzed using ImageJ software (version 1.8.0;
National Institutes of Health) and GraphPad Prism software (version
7.04; GraphPad Software, Inc.).
CCK-8 assay
For proliferative curve measurement, EC109 and TE-1
cells were divided into the following groups: i) Control
(untreated); ii) Nitmotuzumab treatment group (25 µg/ml, 24
h); iii) radiotherapy group (5 Gy); and iv) drug combined
radiotherapy group (5 Gy 12 h post-nimotuzumab treatment), and
treated at 37°C. Esophageal cancer cells (2,000/100 µl/well)
were seeded in 96-well plates with the supplemented RPMI-1640
medium (control) or the supplemented RPMI-1640 medium containing
nitmotuzumab (25 µg/ml) for the treatment and combined
groups. The cells were then incubated for 2 h with CCK-8 reagent
(10 µl; Dojindo Molecular Technologies). The optical density
value was measured using a microplate reader at a wavelength of 450
nm.
Colony formation assay
Cells (2,000/100 µl/well) were seeded in
6-well dishes and divided into the four aforementioned groups. The
cells were cultured in drug-free supplemented RPMI-1640 medium for
10-14 days, and then fixed in 4% para-formaldehyde for 15 min at
room temperature and stained with crystal violet for 20 min at room
temperature. The stained cells were then counted after photographs
were obtained using a light microscope (magnification, ×100).
Cell cycle and apoptosis assays
EC109 and TE-1 esophageal cancer cells were divided
into the four aforementioned groups. Subsequently, the cells were
fixed with 70% ethanol (2 ml) for 48-72 h at 4°C. Apoptosis was
assessed by Annexin V and propidium iodide (PI) staining using the
Apoptosis Detection kit (cat. no. 88-8102-74; Invitrogen; Thermo
Fisher Scientific, Inc.). At room temperature, aliquots of
1×105 cells were incubated with Annexin V/PI for 15 min.
Cells were then analyzed by flow cytometry with a two-color
fluorescence-activated cell sorting flow cytometer.
Animal xenograft models
A total of 150 female nude mice (18-22 g; 4-6 weeks
old) were purchased from Huafukang Biotechnology Co., Ltd. Animal
experimental protocols were approved by the Institutional Animal
Experimentation Committee of Shandong Cancer Hospital (Shandong,
China). To generate tumor xenografts, the right hind legs of the
mice were subcutaneously injected with 4×106 EC109 cells
or 1×107 TE-1 cells. The tumors were measured with a
digital caliper in three orthogonal dimensions (a, b and c), and
the volume of tumor was calculated as πabc/6 (21). When the volume of tumors reached
~150 mm3, the tumor growth delay assay was performed.
Three different tumor models were established: i) common xenograft
model, Pre-IR 0 Gy; ii) recurrent tumor model 1, tumors were
allowed to continue grow after 20 Gy irradiation, Pre-IR 20 Gy; and
iii) recurrent tumor model 2: the right hind leg was irradiated
with 10 Gy X-ray 24 h before tumor implantation, Pre-IR 10 Gy,
according to previously published studies (22,23).
Then mice were randomly divided into the following groups: i)
Untreated control; ii) irradiation alone; iii) nimotuzumab alone;
iv) and irradiation+nimotuzumab (n=6 per group). The different
mouse models are presented in Fig.
S1.
Tumor irradiation and growth delay
assays
The tumor irradiation equipment, a X-ray irradiator
(X-rad225Cx; National Instruments Corporation), was provided by the
laboratory of Shandong Cancer Hospital (Shandong, China). The mice
under anesthesia (Ketamine, 80 mg/kg BW; Xylazine 10 mg/kg BW, IP)
were given a total of 30 Gy in six fractions (5 Gy every other day)
over the course of 3 weeks. Tumor-bearing mice in the experimental
group were injected intravenously with nimotuzumab at a single dose
of 1.0 mg (24). Tumor-bearing
mice in the control and radiation alone groups (used as controls)
were injected with 0.9% sodium chloride. Radiation therapy began 6
h after drug treatment. As described in our previous study
(21), the relative tumor volume
(RTV) was calculated as follows: RTV = Vt / V0, where Vt
is the volume of tumor at any given time and V0 is the
initial volume before treatment. The RTV values were recorded and
the tumor growth delay curve was analyzed.
Immunohistochemistry and
immunofluorescence staining
Immunohistochemistry was performed as described
previously (21). The mice were
sacrificed by pentobarbital (100 mg/kg) followed by cervical
dislocation, then half of the tumors were removed quickly, embedded
in optimal cutting medium (OCT 4583; Sakura Finetek) and frozen
until use in immunofluorescence experiments. The other half of the
tumors were fixed overnight (within 12 h) at 25°C in 10% formalin
and embedded in paraffin blocks, from which 4-µm thick
sections were prepared for immunohistochemical staining. For
immunohistochemistry, the paraffin-embedded tumors were sectioned
and dewaxed. After antigen retrieval was performed using 10 mmol/l
citrate buffer, sections were incubated with 3%
H2O2 and blocked with 5% BSA at 37°C for 30
min. The sections were then incubated with HIF-1α, CA9, EGFR and
p-EGFR primary antibodies at 4°C overnight. After rewarming at room
temperature the next day, the sections were incubated with
secondary antibody (1:200; cat. no. A-21442; Thermo Fisher
Scientific, Inc.) using the two-step polymer HRP (cat. no. PV-9005;
OriGene Technologies, Inc.) detection system at room temperature
for 1 h. The samples were visualized using
3,3-diaminobenzidine.
For immunofluorescence, 8-10 µm sections were
fixed with 4% paraformaldehyde for at 4°C for 20 min and blocked
with 5% BSA at 37°C for 30 min. Then the sections were incubated
with EGFR and p-EGFR primary antibodies at 4°C overnight. After
being rewarmed for 1 h, samples were washed and incubated with
specific secondary antibody (1:200; cat. no. A-21442; Thermo Fisher
Scientific, Inc.) using the two-step polymer HRP (cat. no. PV-9005;
OriGene Technologies, Inc.) for 1 h at 37°C. After washing three
times, nuclei were stained with DAPI for 5 min at room temperature.
Fluorescence images were captured used a Nikon H600L ECLIPSE 90i
fluorescence microscope (Nikon Corporation; magnification, ×400)
and immunohistochemistry images were acquired using a light
microscope (Olympus Corporation; magnification, ×400). EGFR and
p-EGFR were imaged using red filters.
Statistical analysis
SPSS (version 16.0; SPSS, Inc.) was used for data
analyses. Quantitative data are expressed as the mean ± standard
error. All experiments were performed a minimum of three times.
One-way ANOVA followed by Bonferroni's post hoc test was used to
compare multiple groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Nimotuzumab enhances the radiation
response of ESCC cells with high expression of EGFR in vitro
To investigate the effect of nimotuzumab and
radiotherapy on ESCC proliferation in vitro, EC109 and TE-1
cells were treated with nimotuzumab alone or in combination with
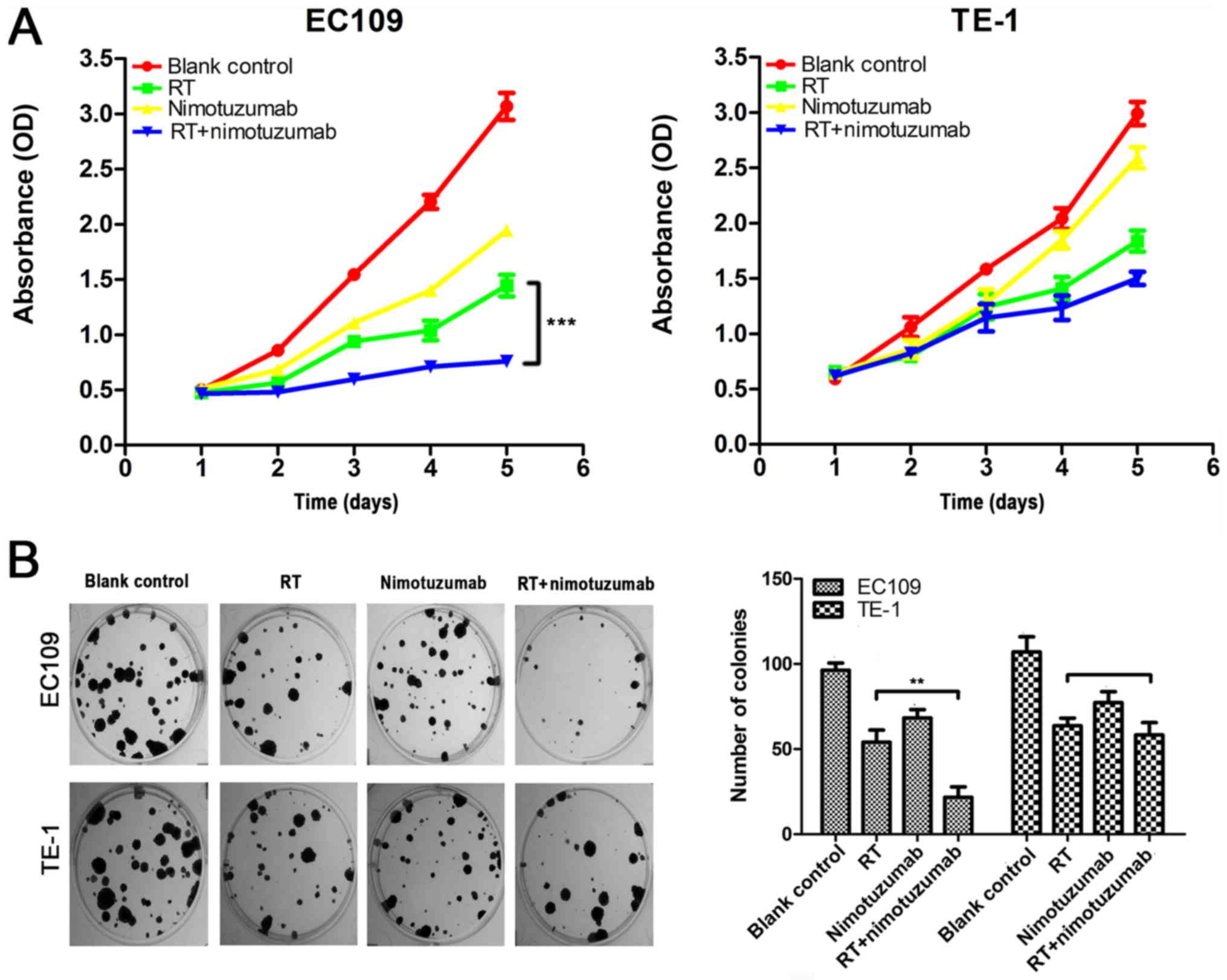
radiotherapy, and then analyzed by CCK-8 assay. The results
(Fig. 1A) demonstrated that the
viability of EC109 cells with radiotherapy and nimotuzumab was
markedly decreased compared with the radiotherapy alone group
(P<0.001), whereas the viability of TE-1 cells [the cell line
with low levels of EGFR (20)]
treated with radiotherapy and nimotuzumab was not significantly
different compared with the radiotherapy alone group (P=0.215).
Additionally, a colony formation assay was used to assess whether
radiotherapy and nimotuzumab affected the ability of the two cell
lines to form colonies. As presented in Fig. 1B, compared with the radiotherapy
alone group, EC109 cells with radiotherapy and nimotuzumab
exhibited a significant decrease in the number of colonies
(P<0.01), and colonies were smaller. Whereas, in TE-1 cells,
compared with the radiotherapy alone group, combined radiotherapy
and nimotuzumab did not decrease the number of colonies (P=0.85).
These data demonstrated that nimotuzumab could enhance the
sensitivity of ESCC cells with high expression of EGFR to
radiotherapy in vitro.
Nimotuzumab accelerates ESCC cell
apoptosis
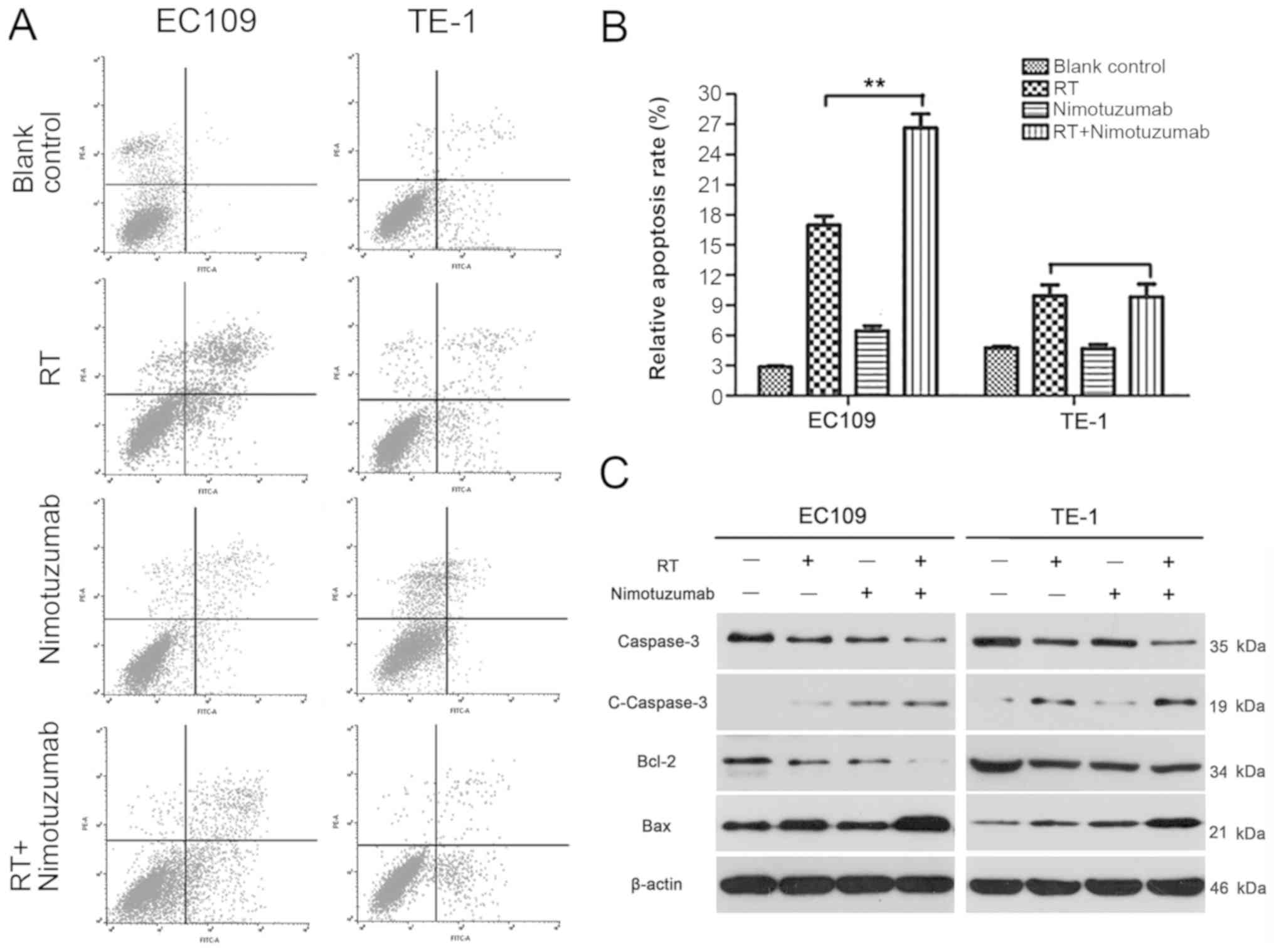
The present study further examined whether
nimotuzumab alone or combined with radiotherapy could influence the
apoptosis of ESCC cells. In EC109 cells, it was observed that the
apoptosis rate was significantly increased in the combination
treatment group compared with the radiotherapy alone group
(P<0.01). However, no similar result was observed in TE-1 cells
(P>0.05; Fig. 2A and B).
Additionally, to investigate the mechanism of nimotuzumab alone or
combination with radiotherapy-induced apoptosis, the present study
examined apoptosis-related caspase-3, anti-apoptotic protein Bcl-2
and pro-apoptotic protein Bax in ESCC cells. As presented in
Fig. 2C, the protein expression of
caspase-3 was decreased in the RT+nimotuzumab group compared with
the RT group in EC109 cells, and cleaved caspase-3 was increased.
In addition, Bcl-2 was markedly decreased and Bax was increased in
the RT+nimotuzumab group compared with the RT group, particularly
in EC109 cells. Analysis of the relative expression of apoptosis
proteins is presented in Fig. S2;
for RT vs. combined groups, significant differences have been shown
in TE-1 cells and EC109 cells). These data indicated that
nitmotuzumab combined with radiotherapy could accelerate apoptosis
in EC109 cells.
Nimotuzumab arrests the ESCC cell cycle
at the G2 phase
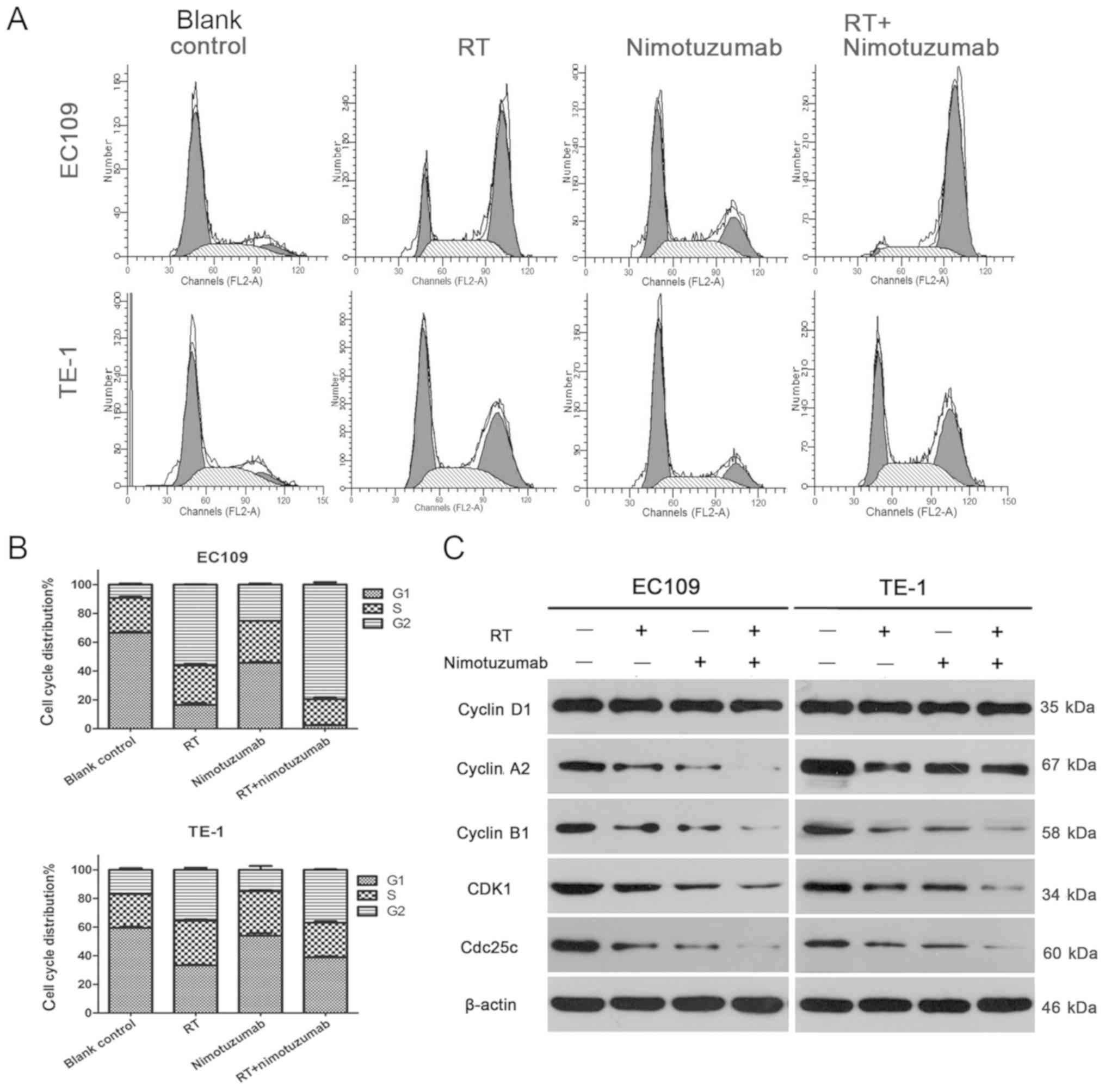
As presented in Fig. 3A
and B, the percentage of EC109 cells in the G2 phase was
79.56±2.86% for the radiotherapy+drug group, 56.22±0.37% for the
radiotherapy group, 9.25±1.26% for the blank control and
25.14±1.19% for the drug group (combined vs. RT group, P<0.001).
In addition, the percentage of TE-1 cells in the G2 phase was
37.01±0.66% for radiotherapy+drug group, 35.57±2.24% for the
radiotherapy group, 18.71±4.04% for the blank control and
15.03±4.49% for the drug group (combined vs. RT group, P>0.05).
Furthermore, the percentages of ESCC cells in the G1 and S phases
correspondingly decreased. In addition, the expression levels of
several cell cycle kinases were examined in ESCC cells, including
cyclin A2, cyclin D1, cyclin B1, CDK1 and Cdc25c. As shown in
Fig. 3C, in EC109 cells, cyclin
A2, cyclin B1, CDK1 and Cdc25c were decreased in the
radiotherapy+drug group compared with the blank control, drug alone
and radiotherapy alone groups. In TE-1 cells, cyclin B1, CDK1 and
Cdc25c were decreased. However, no notable change was observed for
cyclin D1, which is a regulator that drives cells from the G1 phase
to S phase (25). All these data
suggest that nimotuzumab induces cell cycle arrest at the G2 phase
to inhibit cell proliferation.
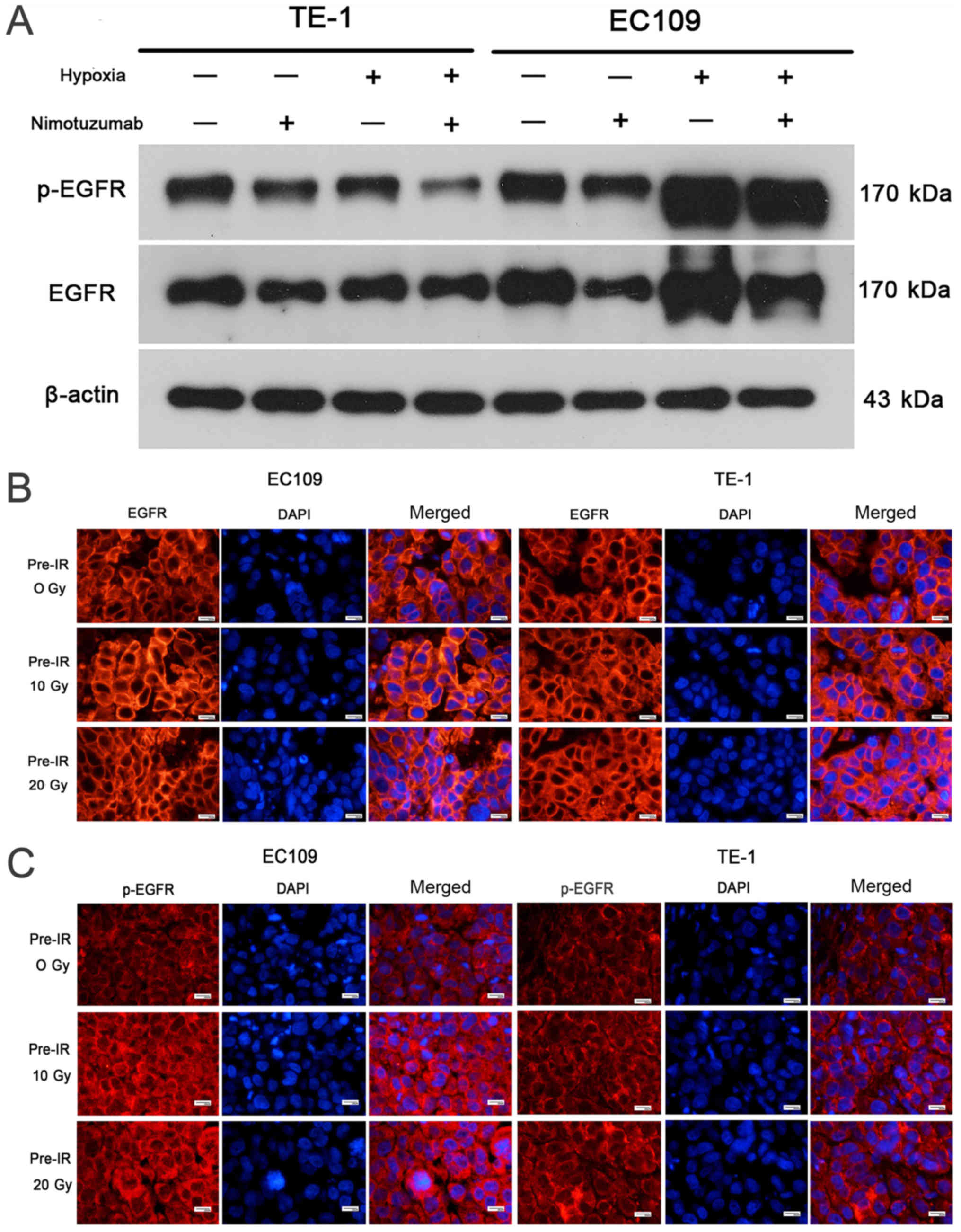
Level of hypoxia increases in the
recurrent esophageal tumor models
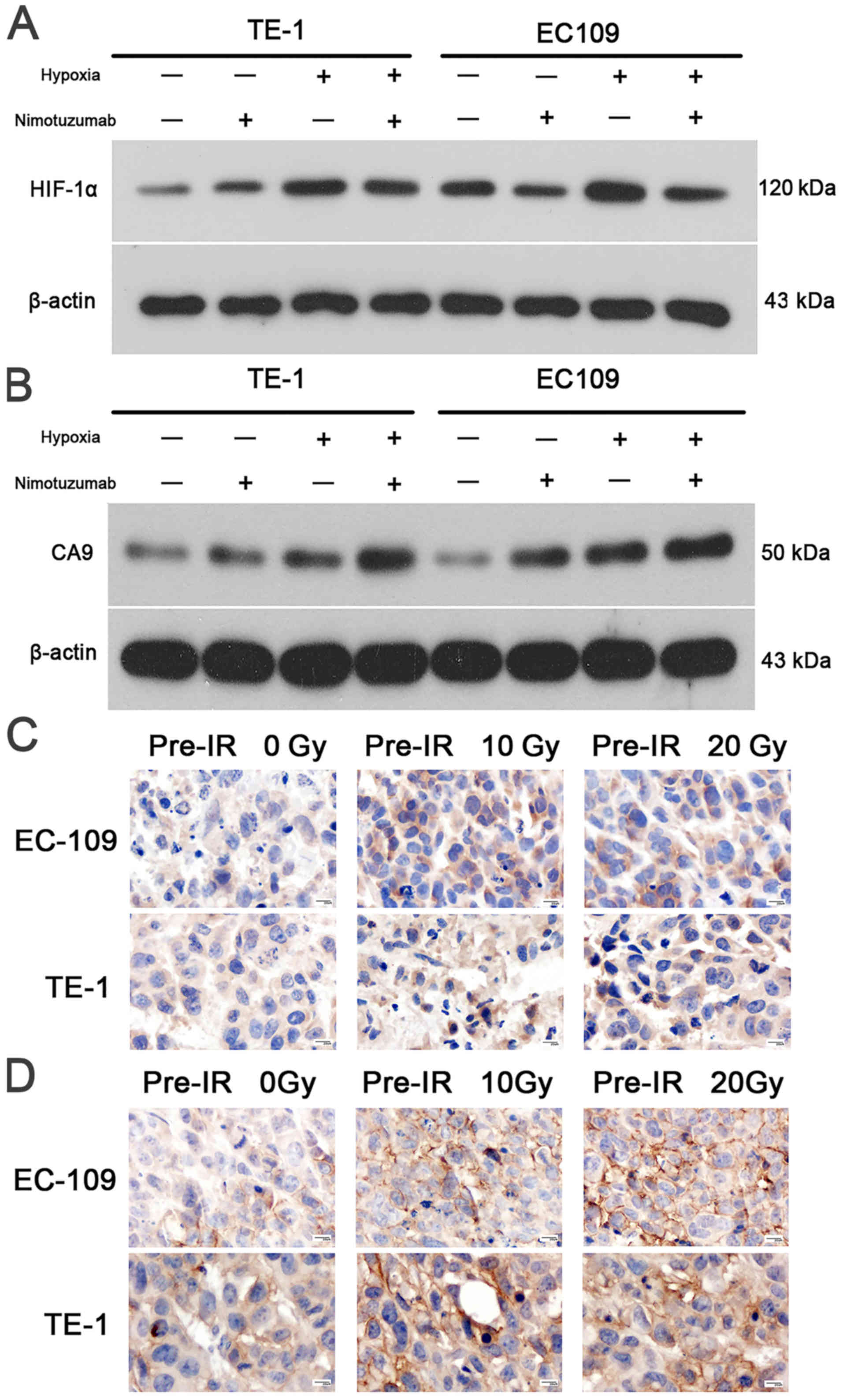
Western blot experiments revealed that HIF-1α and
CA9 levels were increased in hypoxic cells compared with normoxic
EC109 and TE1 cells (Fig. 4A and
B). The relative expression levels of HIF-1α and CA9 are
presented in Figs. S3 and
S4. It was demonstrated that
nimotuzumab may downregulate the expression of Hif-1α in EC109
cells during hypoxia, but no effect was observed for CA9, which
needs to be further verified. As presented in Fig. 4C and D, the HIF-1α and CA9
expression levels were also examined using immunohistochemical
staining in the different xenograft models. In the nuclei and
cytoplasm of 0 Gy pre-irradiated tumors, the content of HIF-1α and
CA9 protein was low; however, in the 10 Gy and 20 Gy pre-irradiated
xenograft models, abundant levels of HIF-1α (especially in the
nucleus) and CA9 (especially in the cytoplasm) protein were
observed in EC109 and TE1 tumors (Fig.
4C and D). These results suggested that the recurrent tumor
models had been successfully constructed, and the degree of hypoxia
had increased in recurrent tumors.
Tumor hypoxia is positively associated
with EGFR overexpression
As shown in Fig.
5A, EGFR expression was markedly increased in hypoxic cells
compared with normoxic cells, particularly in EC109 cells. The
relative expression levels of p-EGFR and EGFR are presented in
Figs. S5 and S6, and the ratio of p-EGFR/EGFR is shown
in Fig. S7. As presented in
Fig. S7, hypoxia increased the
p-EGFR/EGFR ratio in EC109 cells. However, this result needs to be
further verified. In addition, the immunofluorescence studies
demonstrated that EGFR and p-EGFR expression levels were markedly
increased in the cell membrane in EC109 10 Gy and 20 Gy
pre-irradiated models compared with the 0 Gy pre-irradiated tumor
model. However, this effect was not detected in TE-1 cells
(Fig. 5B and C). These results
indicate that the level of tumor hypoxia was positively associated
with EGFR overexpression in ESCC.
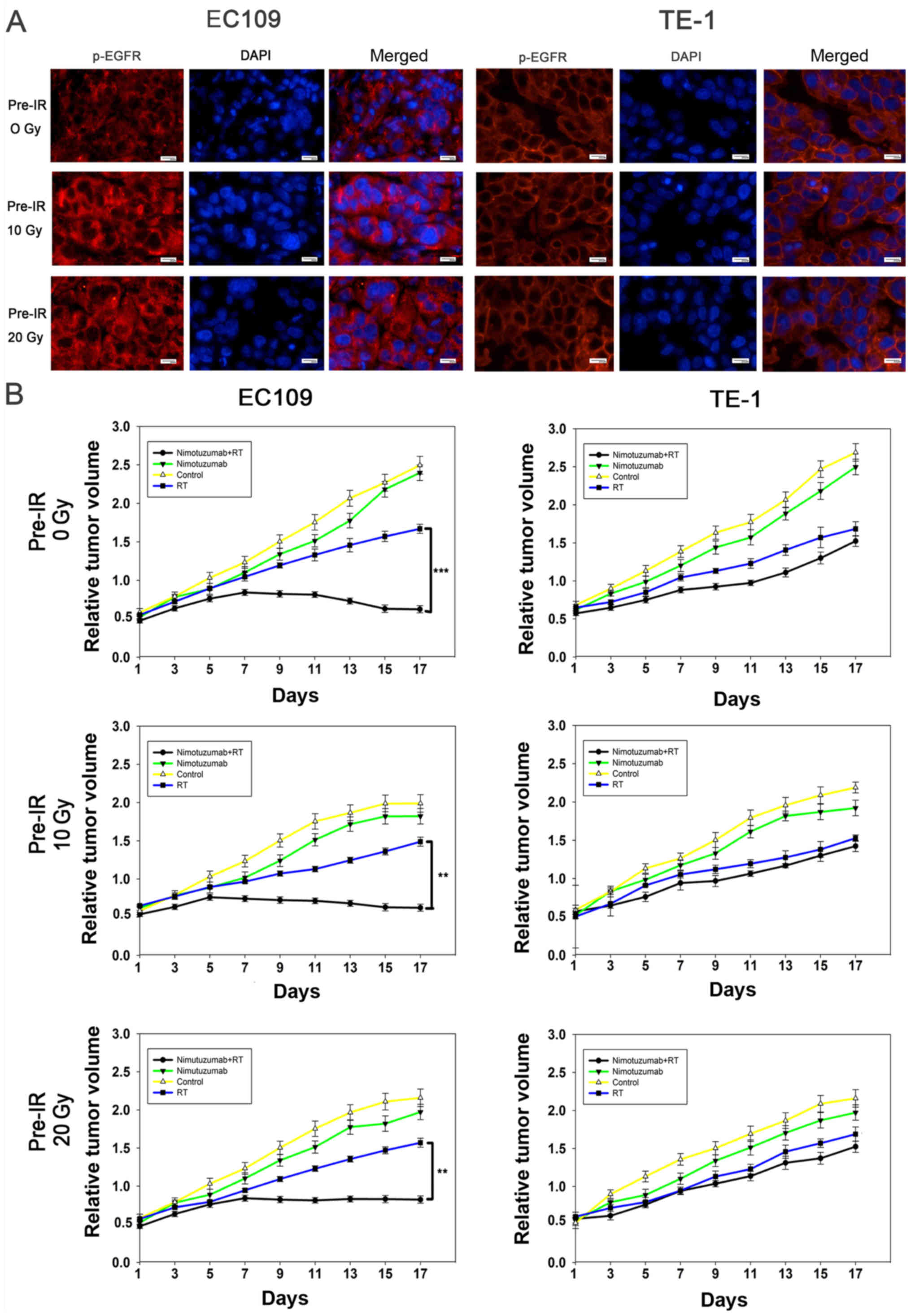
Nimotuzumab inhibits EGFR phosphorylation
and increases radiosensitivity of recurrent ESCC cells with EGFR
overexpression in vivo
The present study further investigated the
mechanisms of nimotuzumab in the promotion of ESCC cell
radiosensitivity. As presented in Figs. 5A, S5 and S6, nimotuzumab significantly
downregulated the level of p-EGFR and EGFR in EC109 cells and
hypoxic EC109 cells, indicating that the anti-EGFR effect of
nimotuzumab is predominantly via a downregulated level of p-EGFR.
However, this result was not obvious in TE-1 cells due to a low
expression of EGFR. To confirm whether nimotuzumab could increase
the radio-sensitivity of ESCC cells in vivo, routine and
recurrent ESCC xenograft models were established. As shown in
Fig. 6A, expression levels of
p-EGFR in tumors slides of ESCC cell xenografts following
radiotherapy with nimotuzumab treatment were examined using
immunofluorescence analysis. Similar to the in vitro
results, following treatment with nimotuzumab and radiotherapy, the
level of p-EGFR was inhibited in the three xenograft EC109 tumor
models (0 Gy, 10 Gy and 20 Gy pre-irradiated); however, this effect
was not detected in TE-1 cells.
The tumor growth delay assay demonstrated that in
EC109 xenografts, tumor growth was significantly inhibited in the
groups treated with a combination of nimotuzumab and radiotherapy
compared with those treated with radiation alone (P<0.001 for 0
Gy pre-irradiation, P=0.005 for 20 Gy pre-irradiation, P=0.005 for
10 Gy pre-irradiation; Fig. 6B).
However, in the TE-1 xenografts models, it was observed that tumor
growth was inhibited by radiation, but there were no significant
differences between the irradiation groups and groups also treated
with nimotuzumab (P=0.071 for 0 Gy pre-irradiation, P=0.096 for 20
Gy pre-irradiation, P=0.091 for 10 Gy pre-irradiation). The present
results indicated that nimotuzumab could downregulate the levels of
p-EGFR and increase radiosensitivity in both primary and recurrent
EGFR-overexpressing ESCC cells.
Discussion
Local recurrence is one of the main causes for the
failure of esophageal cancer treatment following radiotherapy.
Secondary radiotherapy remains the most important therapeutic
method for recurrent tumors (3).
As few preclinical research studies and clinical trials have
reported a treatment for recurrent esophageal cancer, this was
investigated in the present study. The current study measured the
effect of nimotuzumab on radiation response of recurrent esophageal
cancer and investigated the underling mechanisms in vitro
and in vivo. The results demonstrated that nimotuzumab could
enhance the radiation response of EC109 cells, which exhibit a high
expression of EGFR, both in primary and recurrent cells. However,
similar results were not found in TE-1 cells, which exhibit low
EGFR expression. Previous studies have demonstrated that the
antitumor effect of EGFR inhibitors is due to restrained ligand
binding to EGFR, which inhibits EGFR activation (26,27).
To investigate the potential mechanisms of nimotuzumab in the
improvement of radiosensitivity, the expression levels of EGFR and
p-EGFR were detected in ESCC cells. The results revealed that
expression of EGFR was increased in the hypoxic (recurrent) cells
compared with in primary EC109 and TE-1 cells. Nimotuzumab
treatment decreased the p-EGFR level in EC109, but not in TE-1
cells. These data demonstrated that nimotuzumab inhibited the
activation of EGFR in EGFR-overexpressing ESCC cells, and hypoxia
may be involved in the regulation of EGFR over-expression and its
ligands. The findings of the present study are valuable for further
clinical translational studies for the following reasons. Firstly,
nimotuzumab enhanced the radiation response of EGFR-overexpressing
ESCC cells. Secondly, in future clinical situations, it is
necessary to assess EGFR expression in patients with recurrent
esophageal tumors.
Retrospective trials have shown that EGFR inhibitor
can improve the radiotherapy response and local control of certain
cancer types, including rectal, lung and brain cancer, providing a
new treatment strategy for anticancer therapy (14,28).
However, the SCOPE1 (29) and RTOG
0436 (30) trials demonstrated
that adding cetuximab to definitive chemoradiotherapy in patients
with localized esophageal cancer failed to improve the patients'
survival. These phase III trials demonstrated little benefit to
current EGFR-targeted agents in the patients with no detectable
EGFR expression, and highlighted the need for predictive biomarkers
in the treatment of esophageal cancer. Another EGFR inhibitor is
nimotuzumab, a humanized IgG1 isotype monoclonal antibody of EGFR,
which adheres to the cell surface to inhibit tumor growth by
binding both antibody arms to two targets. Two phase II clinical
trials (31,32) and the NICE trial (33) evaluated the efficacy of nimotuzumab
with definitive chemoradiotherapy in patients with advanced
esophageal cancer, which revealed that a combination with
nimotuzumab improved the patients local control rate, endoscopic
complete response rate, pathological complete response rate and
overall survival rate. Nimotuzumab is currently used in phase III
clinical trials of concurrent chemoradiotherapy for esophageal
cancer; which indicate that a combination with nimotuzumab is more
effective (unpublished data).
Baumann et al (15) observed that the antitumor
capabilities of nimotuzumab depend on EGFR expression levels. Zhao
et al (24) demonstrated
that nimotuzumab enhances the radiation response in KYSE30 cells
with high EGFR expression; however, the effect is not observed in
TE-1 cells with low EGFR expression. Akashi et al (18) observed that nimotuzumab enhances
the radiosensitivity of NSCLC cell lines with high levels of EGFR
expression. Compared with these previous studies, the present study
also used two cell lines, in addition recurrent tumor models were
constructed. Additionally, the present study focused on the effect
of hypoxia on EGFR expression.
The present study demonstrated that the level of
tumor hypoxia was positively associated with EGFR overexpression.
The regulatory mechanism has been reported in previous studies
(34-36), which is that hypoxia induces
expression of EGFR and its ligands. In return, EGFR may enhance the
response of cells to hypoxia by increasing the expression of
HIF-1α, thereby acting as a survival factor for hypoxic cancer
cells. EGFR signaling activates the PI3K/AKT pathway, which
increases the level of HIF-1α. HIF-1α then activates survivin gene
transcription by direct binding to the survivin promoter (37). Several studies (10,24)
have shown that under normoxic or hypoxic conditions, the abundance
of HIF-1α protein increases with the activation of EGFR signaling,
which is also achieved via the PI3K/AKT pathway. As described in
our previous study (38), the
present study succeeded in establishing a recurrent esophageal
xenograft tumor model, which was characterized by a hypoxic
microenvironment. The extent of hypoxia increase was assessed in
the recurrent esophageal tumor models. Chen et al (23) reported similar results; the level
of hypoxia increases in recurrent prostate tumor models, which is
due to a lower microvascular density in pre-irradiated tissues.
Compared with the study by Zhao et al (24), the present study verified that the
anti-apoptotic protein Bcl-2 and caspase-3 were decreased, while
pro-apoptotic Bax was increased when ESCC cells were treated with a
combination of drug and radiation, demonstrating that treatment
with nimotuzumab in combination with radiation promotes cell
apoptosis, particularly in EC109 cells. Furthermore, in the present
study, cyclin A2, cyclin B1, CDK1 and Cdc25c expression levels were
markedly decreased following combined drug and radiotherapy
treatment. By contrast, cyclin D1, an essential factor in the
regulation of the cell cycle transition from G1 to S phase, was not
affected; demonstrating that treatment with nimotuzumab affected
the cell cycle at the G2 phase.
There are a number of limitations of the present
study. It is understood that in the USA and other Western
countries, the incidence of esophageal adenocarcinoma is equal to
or exceeds the incidence of ESCC. Therefore, one limitation of the
current study is that no esophageal adenocarcinoma cell lines were
investigated. In addition, the lack of investigation of HIF-1α
translocation is another limitation.
In conclusion, the present study demonstrated that
nimotuzumab enhanced the radiation response both in primary and
recurrent ESCC cells with high expression of EGFR. Additionally,
nimotuzumab accelerated ESCC cell apoptosis and arrested the ESCC
cell cycle at the G2 phase. It was also identified that the degree
of hypoxia was increased in recurrent tumors. Furthermore, the
potential mechanisms of how nimotuzumab improves radiosensitivity
were investigated, which revealed that nimotuzumab inhibits the
activation of EGFR in ESCC cells with a high EGFR expression. In
addition, it was found that the level of tumor hypoxia was
positively associated with EGFR expression in ESCC and hypoxia may
be involved in the regulation of EGFR and its ligands. However,
further studies are required to clarify the precise mechanism and
therapeutic significance of the hypoxic response and EGFR signaling
pathway in cancer.
Supplementary Data
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant no. 81572970),
Shandong Provincial Natural Science Foundation (grant nos.
ZR2015HZ004 and ZR2014YL033), the Subject Assignment of China
National Key Research and Development Program (grant no.
2018YFC1313201), and the Jinan Scientific and Technology
Development Project (grant no. 201805005).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XS designed the study and revised the draft. YY
drafted the manuscript and completed the main experiments. HG
created the figures and performed statistical analyses. XL
performed the histological examination of the tumors. LX assisted
in designing the study. LJ analyzed the data and revised the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The animal experiments were approved by the Animal
Ethics Committee at the Shandong Cancer Hospital Affiliated to
Shandong University (Jinan, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Malhotra GK, Yanala U, Ravipati A, Follet
M, Vijayakumar M and Are C: Global trends in esophageal cancer. J
Surg Oncol. 115:564–579. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jha N, Harris J, Seikaly H, Jacobs JR,
McEwan AJ and Robbins KT: A phase II study of submandibular gland
transfer prior to radiation for prevention of radiation-induced
xerostomia in head-and-neck cancer (RTOG 0244). Int J Radiat Oncol
Biol Phys. 84:437–442. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brown JM: Tumor hypoxia in cancer therapy.
Methods Enzymol. 435:297–321. 2007.PubMed/NCBI
|
|
5
|
Hengstler JG, Bockamp EO, Hermes M,
Brulport M, Bauer A, Schormann W, Schiffer IB, Hausherr C, Eshkind
L, Antunes C, et al: Oncogene-blocking therapies: New insights from
conditional mouse tumor models. Curr Cancer Drug Targets.
6:603–612. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee AWM, Ng WT, Chan JYW, Corry J, Mäkitie
A, Mendenhall WM, Rinaldo A, Rodrigo JP, Saba NF, Strojan P, et al:
Management of locally recurrent nasopharyngeal carcinoma. Cancer
Treat Rev. 79:1018902019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Moeller BJ, Cao Y, Li CY and Dewhirst MW:
Radiation activates HIF-1 to regulate vascular radiosensitivity in
tumors: Role of reoxygenation, free radicals, and stress granules.
Cancer Cell. 5:429–441. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Harada H, Itasaka S, Zhu Y, Zeng L, Xie X,
Morinibu A, Shinomiya K and Hiraoka M: Treatment regimen determines
whether an HIF-1 inhibitor enhances or inhibits the effect of
radiation therapy. Br J Cancer. 100:747–757. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kwong LN, Zou L, Chagani S, Pedamallu CS,
Liu M, Jiang S, Protopopov A, Zhang J, Getz G and Chin L: Modeling
Genomic Instability and Selection Pressure in a Mouse Model of
Melanoma. Cell Rep. 19:1304–1312. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bussink J, van der Kogel AJ and Kaanders
JH: Activation of the PI3-K/AKT pathway and implications for
radioresistance mechanisms in head and neck cancer. Lancet Oncol.
9:288–296. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nijkamp MM, Hoogsteen IJ, Span PN, Takes
RP, Lok J, Rijken PF, van der Kogel AJ, Bussink J and Kaanders JH:
Spatial relationship of phosphorylated epidermal growth factor
receptor and activated AKT in head and neck squamous cell
carcinoma. Radiother Oncol. 101:165–170. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Harari PM and Huang SM: Combining EGFR
inhibitors with radiation or chemotherapy: Will preclinical studies
predict clinical results? Int J Radiat Oncol Biol Phys. 58:976–983.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Milas L, Fan Z, Andratschke NH and Ang KK:
Epidermal growth factor receptor and tumor response to radiation:
In vivo preclinical studies. Int J Radiat Oncol Biol Phys.
58:966–971. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Giralt J, de las Heras M, Cerezo L, Eraso
A, Hermosilla E, Velez D, Lujan J, Espin E, Rosello J, Majó J, et
al Grupo Español de Investigacion Clinica en Oncologia
Radioterápica (GICOR): The expression of epidermal growth factor
receptor results in a worse prognosis for patients with rectal
cancer treated with preoperative radiotherapy: A multicenter,
retrospective analysis. Radiother Oncol. 74:101–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Baumann M, Krause M, Dikomey E, Dittmann
K, Dörr W, Kasten-Pisula U and Rodemann HP: EGFR-targeted
anti-cancer drugs in radiotherapy: Preclinical evaluation of
mechanisms. Radiother Oncol. 83:238–248. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang KL, Wu TT, Choi IS, Wang H, Resetkova
E and Correa AM: Expression of epidermal growth factor receptor in
esophageal and esophagogastric junction adenocarcinomas:
association with poor outcome. Cancer. 109:658–667. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Diaz Miqueli A, Rolff J, Lemm M, Fichtner
I, Perez R and Montero E: Radiosensitisation of U87MG brain tumours
by anti-epidermal growth factor receptor monoclonal antibodies. Br
J Cancer. 100:950–958. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Akashi Y, Okamoto I, Iwasa T, Yoshida T,
Suzuki M, Hatashita E, Yamada Y, Satoh T, Fukuoka M, Ono K, et al:
Enhancement of the antitumor activity of ionising radiation by
nimotuzumab, a humanised monoclonal antibody to the epidermal
growth factor receptor, in non-small cell lung cancer cell lines of
differing epidermal growth factor receptor status. Br J Cancer.
98:749–755. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kawaguchi Y, Kono K, Mimura K, Sugai H,
Akaike H and Fujii H: Cetuximab induce antibody-dependent cellular
cytotoxicity against EGFR-expressing esophageal squamous cell
carcinoma. Int J Cancer. 120:781–787. 2007. View Article : Google Scholar
|
|
20
|
Zhao L, Li QQ, Zhang R, Xi M, Liao YJ,
Qian D, He LR, Zeng YX, Xie D and Liu MZ: The overexpression of
IGFBP-3 is involved in the chemosensitivity of esophageal squamous
cell carcinoma cells to nimotuzumab combined with cisplatin. Tumour
Biol. 33:1115–1123. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu Y, Li X, Xu H, Liu J, Dong M, Yang J,
Sun L, Sun X and Xing L: Correlation of hypoxia status with
radiosensitizing effects of sodium glycididazole: A preclinical
study. Oncol Lett. 15:6481–6488. 2018.PubMed/NCBI
|
|
22
|
Zips D, Eicheler W, Brüchner K, Jackisch
T, Geyer P, Petersen C, van der Kogel AJ and Baumann M: Impact of
the tumour bed effect on microenvironment, radiobiological hypoxia
and the outcome of fractionated radiotherapy of human FaDu
squamous-cell carcinoma growing in the nude mouse. Int J Radiat
Biol. 77:1185–1193. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen FH, Chiang CS, Wang CC, Fu SY, Tsai
CS, Jung SM, Wen CJ, Lee CC and Hong JH: Vasculatures in tumors
growing from preirradiated tissues: Formed by vasculogenesis and
resistant to radiation and antiangiogenic therapy. Int J Radiat
Oncol Biol Phys. 80:1512–1521. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhao L, He LR, Xi M, Cai MY, Shen JX, Li
QQ, Liao YJ, Qian D, Feng ZZ, Zeng YX, et al: Nimotuzumab promotes
radiosensitivity of EGFR-overexpression esophageal squamous cell
carcinoma cells by upregulating IGFBP-3. J Transl Med. 10:2492012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Baraban E, Sadigh S, Rosenbaum J, Van
Arnam J, Bogusz AM, Mehr C and Bagg A: Cyclin D1 expression and
novel mutational findings in Rosai-Dorfman disease. Br J Haematol.
186:837–844. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Okamoto I: Epidermal growth factor
receptor in relation to tumor development: EGFR-targeted anticancer
therapy. FEBS J. 277:309–315. 2010. View Article : Google Scholar
|
|
27
|
Li S, Kussie P and Ferguson KM: Structural
basis for EGF receptor inhibition by the therapeutic antibody
IMC-11F8. Structure. 16:216–227. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chakravarti A, Winter K, Wu CL, Kaufman D,
Hammond E, Parliament M, Tester W, Hagan M, Grignon D, Heney N, et
al: Expression of the epidermal growth factor receptor and Her-2
are predictors of favorable outcome and reduced complete response
rates, respectively, in patients with muscle-invading bladder
cancers treated by concurrent radiation and cisplatin-based
chemotherapy: A report from the Radiation Therapy Oncology Group.
Int J Radiat Oncol Biol Phys. 62:309–317. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Crosby T, Hurt CN, Falk S, Gollins S,
Mukherjee S, Staffurth J, Ray R, Bashir N, Bridgewater JA, Geh JI,
et al: Chemoradiotherapy with or without cetuximab in patients with
oesophageal cancer (SCOPE1): A multicentre, phase 2/3 randomised
trial. Lancet Oncol. 14:627–637. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Suntharalingam M, Winter K, Ilson D,
Dicker AP, Kachnic L, Konski A, Chakravarthy AB, Anker CJ, Thakrar
H, Horiba N, et al: Effect of the Addition of Cetuximab to
Paclitaxel, Cisplatin, and Radiation Therapy for Patients With
Esophageal Cancer: The NRG Oncology RTOG 0436 Phase 3 Randomized
Clinical Trial. JAMA Oncol. 3:1520–1528. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lu M, Wang X, Shen L, Jia J, Gong J, Li J,
Li J, Li Y, Zhang X, Lu Z, et al: Nimotuzumab plus paclitaxel and
cisplatin as the first line treatment for advanced esophageal
squamous cell cancer: A single centre prospective phase II trial.
Cancer Sci. 107:486–490. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ramos-Suzarte M, Lorenzo-Luaces P, Lazo
NG, Perez ML, Soriano JL, Gonzalez CE, Hernadez IM, Albuerne YÁ,
Moreno BP, Alvarez ES, et al: Treatment of malignant,
non-resectable, epithelial origin esophageal tumours with the
humanized anti-epidermal growth factor antibody nimotuzumab
combined with radiation therapy and chemotherapy. Cancer Biol Ther.
13:600–605. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
de Castro Junior G, Segalla JG, de Azevedo
SJ, Andrade CJ, Grabarz D, de Araújo Lima França B, Del Giglio A,
Lazaretti NS, Álvares MN, Pedrini JL, et al: A randomised phase II
study of chemoradiotherapy with or without nimotuzumab in locally
advanced oesophageal cancer: NICE trial. Eur J Cancer. 88:21–30.
2018. View Article : Google Scholar
|
|
34
|
Cheng JC, Klausen C and Leung PC:
Hypoxia-inducible factor 1 alpha mediates epidermal growth
factor-induced down-regulation of E-cadherin expression and cell
invasion in human ovarian cancer cells. Cancer Lett. 329:197–206.
2013. View Article : Google Scholar
|
|
35
|
Lin P, Wang W, Cai WJ, Han CR, Sun Y, Li M
and Sun BC: Relationship between the expression of
hypoxia-inducible factor-1alpha and epidermal growth factor
receptor and micro vessel density and their clinical significance.
Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 44:480–485. 2009.In
Chinese. PubMed/NCBI
|
|
36
|
Wouters A, Boeckx C, Vermorken JB, Van den
Weyngaert D, Peeters M and Lardon F: The intriguing interplay
between therapies targeting the epidermal growth factor receptor,
the hypoxic microenvironment and hypoxia-inducible factors. Curr
Pharm Des. 19:907–917. 2013. View Article : Google Scholar
|
|
37
|
Peng XH, Karna P, Cao Z, Jiang BH, Zhou M
and Yang L: Cross-talk between epidermal growth factor receptor and
hypoxia-inducible factor-1alpha signal pathways increases
resistance to apoptosis by up-regulating survivin gene expression.
J Biol Chem. 281:25903–25914. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wu P, Liu J, Sun X, Li X, Xing L and Yu J:
Enhanced radio-sensitizing by sodium glycididazole in a recurrent
esophageal carcinoma tumor model. Oncotarget. 8:63871–63880.
2017.PubMed/NCBI
|