Deoxynucleotide triphosphates (dNTPs) are the raw
materials for DNA replication and repair, rendering them
indispensable components for transmitting genetic information in
cells and maintaining genomic stability (1,2).
Sterile alpha motif and histidine/aspartic acid domain-containing
protein 1 (SAMHD1), the only dNTP hydrolase in eukaryotes, is
involved in several pathological processes. SAMHD1 is well known
for its vital role in the resistance to virus transcription and
replication by limiting the volume of the dNTP pool, thus resulting
in the protection of the host cellular genome integrity. It has
been reported that SAMHD1 acetylation enhances its dNTP hydrolase
(dNTPase) activity and regulates cancer cell proliferation
(3). Moreobver, the dNTPase
activity of SAMHD1 is dependent on the stability of the catalytic
core tetramer, which can be inhibited by cyclin-dependent kinase
phosphorylation on threonine 592 (T592) (4-8). In
addition, viral protein kinases can also phosphorylate SAMHD1,
thereby inhibiting its dNTPase activity (9,10).
The transcriptional repression of Samhd1 is mediated by
methylation of its promoter (11-13).
It has been also reported that viral protein X (Vpx) interacts with
SAMHD1, resulting in the proteasomal degradation of SAMHD1 and an
increase in dNTP levels (14-17).
Therefore, it is necessary to systematically summarize the
modifications of SAMHD1 and reveal its related downstream
functions.
In the present review, the current knowledge of the
role of SAMHD1 in the dynamic regulation of dNTP cellular
homeostasis and genomic stability is summarized. In addition, the
potential role of SAMHD1 as a housekeeping protein in the
maintenance of dNTP homeostasis and the prevention of tumorigenesis
is discussed.
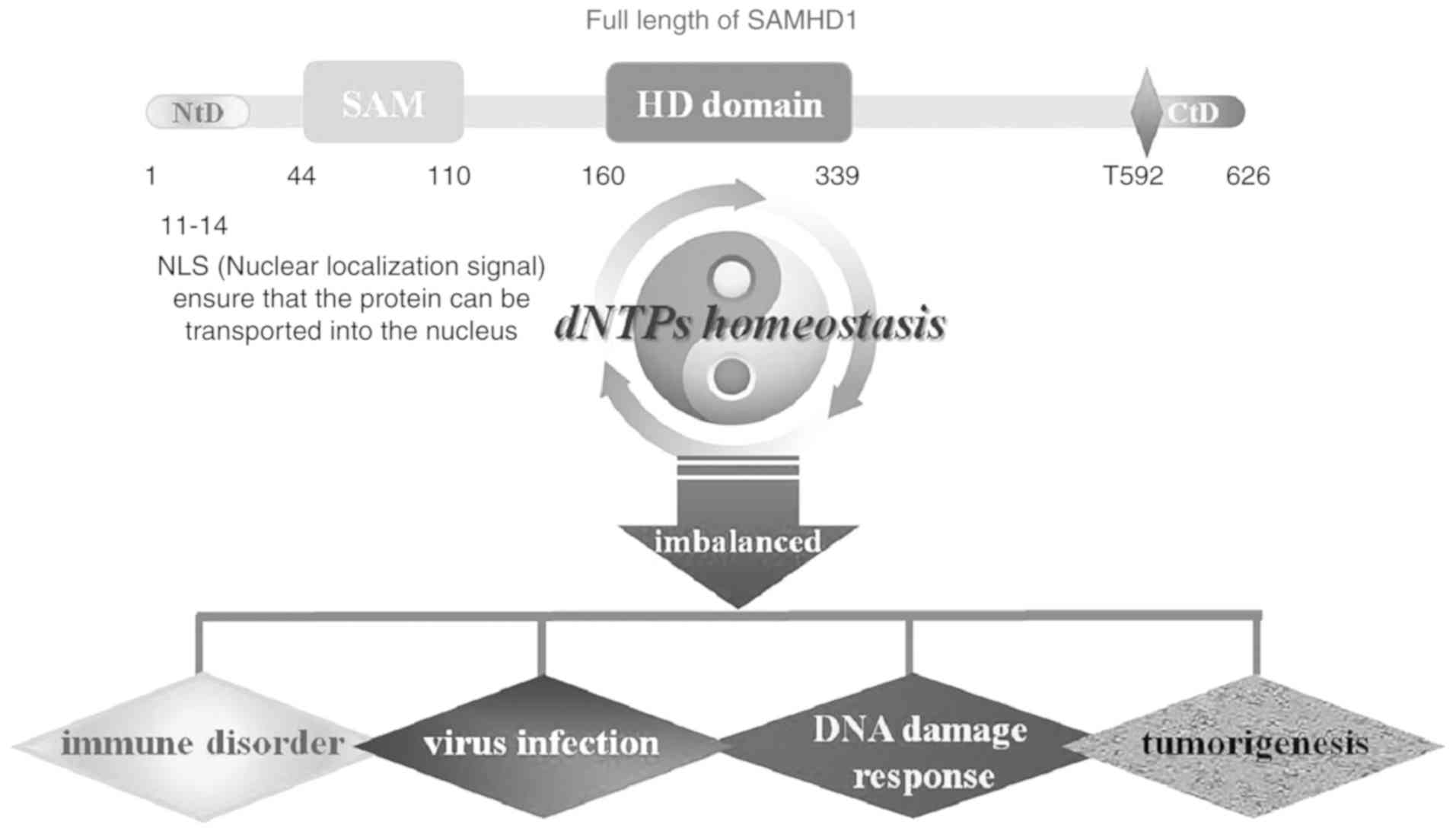
Human SAMHD1 is 626 amino acids (aa) in length and
contains an N-terminal nuclear localization domain
11KRPR14 followed by a conserved sterile
alpha motif (SAM) and a histidine/aspartic acid (HD) domain
(25,26). These domains are connected by a
short linker and flanked by unstructured regions. The SAM domain
(44-110 aa) is involved in protein-protein and protein-DNA/RNA
interactions, whereas the HD domain is a conserved sequence
containing 160-339 aa comprising an arrangement of alternating
histidine/aspartic acid amino acids (27-29)
(Fig. 1). HD is the main
functional domain of SAMHD1 with antiviral activity, which is
involved in nucleotide metabolism and exhibits dNTPase and
ribonuclease (RNase) activity. However, the RNase activity of
SAMHD1 is controversial. Ryoo et al (31) suggested that SAMHD1 restricted
HIV-1 infection by cleaving the viral RNA genome via its RNase
activity. In addition, the SAMHD1 phosphorylation at T592
negatively regulates its RNase activity in vivo and impedes
HIV-1 restriction. By contrast, Antonucci et al (30) reported that SAMHD1 did not exhibit
broad nuclease activity; however, they did not rule out a specific
nucleolytic interaction between SAMHD1 and incoming HIV-1 genomic
RNA (gRNA). Furthermore, Antonucci et al (30) demonstrated that both SAMHD1D137N
(RNase-positive and dNTPase-negative) and SAMHD1Q548A
(RNase-negative and dNTPase-positive) mutants were expressed at
comparable levels with wild-type SAMHD1 and each efficiently
restricted HIV-1 infection (30,31).
Several studies have demonstrated that the C-terminus of SAMHD1
(600-626 aa) is included in the crystal structure of the
GTP/dNTP-bound tetramer and forms a short alpha-helical structure
with an extended loop (32-34).
The C-terminus of SAMHD1 is required for the efficient depletion of
dNTP pools and the inhibition of HIV-1 infection in monocytes
(35). Although the C-terminal
region contains conserved amino acid sequences, it extends
interspersed with more divergent ones among vertebrate species
(17). A recent study demonstrated
that SAMHD1 catalytic activity is regulated by redox signaling.
SAMHD1 is inactivated in a dose-dependent, yet reversible manner
when treated with the oxidizing agent, H2O2
(36).
The oxidation of SAMHD1 has been demonstrated to
inhibit tetramerization, and has been emphasized as a central
regulatory mechanism for the regulation of SAMHD1 activity in
vivo (37). Recent research
has highlighted that rapid protein degradation is not mediated by
SAMHD1 phosphorylation at T592. In addition, it has been documented
that the dNTPase activity of SAMHD1 is not only retained during the
G1 and G0 phases, but throughout the entire cell cycle, independent
of phosphorylation at T592 (38).
Other researchers have indicated that constructed mutant SAMHD1
fragments generated by deleting the HD domain and C-terminal
segment inhibit the ability to restrict HIV-1 infection (39). In the absence of the dGTP
co-factor, SAMHD1 exists as an inactive monomer or dimer in which
the substrate-binding pocket is unable to bind dNTP, thus losing
its dNTPase activity (27,35). Upon dGTP-Mg2+-dGTP
binding at the allosteric sites, the catalytically inactive SAMHD1
dimers tetramerize, thereby inducing a large conformational change
at the tetramer interface and the recovery of its catalytic
activity. Therefore, the dNTPase activity of SAMHD1 is mainly
dependent on its active tetramer structure (35,40).
Taken together, the aforementioned features of SAMHD1 verify its
ability to properly regulate dNTP levels, which are indispensable
for the transcription and replication of viruses, such as herpes
simplex virus (HSV) type 1 (41,42)
and hepatitis B virus (HBV) (43,44),
and the inhibition of HIV-1 reverse transcription. The structure of
SAMHD1 forms the basis of its biological functions and may thus
provide novel insight into the elucidatation of the internal
regulatory mechanisms of immune disorders, viral infections, DNA
damage responses and tumorigenesis (45).
SAMHD1 is subjected to a vast array of
post-translational modifications, including phosphorylation,
acetylation and methylation. It has been suggested that
cyclin-dependent kinase 1 (CDK1)/cyclin A2 phosphorylates SAMHD1 at
T592 only in proliferating cells and completely abolish its ability
to resist viral infections (46).
In addition, SAMHD1 has been shown to be phosphorylated at T592 in
proliferating leukocytes in the G1/S and G2/M phase of the cell
cycle by the key S-phase kinase complex, CDK2-cyclin A (38). The study by Pauls et al
(47) suggested that the
CDK6-dependent CDK2 phosphorylation of SAMHD1 inhibited its
restriction activity against HIV-1 replication in primary cells.
Therefore, the synergistic effect of CDK2 and CDK6 during cell
cycle progression is essential for determining the susceptibility
to HIV-1 infection by modulating viral dNTP access through SAMHD1.
Notably, SAMHD1 is also phosphorylated at T592 from the G0 to G1
phase of the cell cycle through the activation of CDK2 and cyclin E
expression, resulting in increased dNTP pools (47).
Furthermore, the levels of SAMHD1 phosphorylation at
T592 may be reduced following treatment with type I IFN,
reinforcing the link between the phosphorylation of SAMHD1 and its
antiviral activity (46).
Recently, several studies have suggested that the expression of
p21Waf1/Cip1 (referred to as p21), a CDK inhibitor, may
lead to reduced phosphorylation at T592 residue by CDKs. Thus,
SAMHD1 antiviral activity is regulated by CDK1 phosphorylation at
amino acid T592, and type I IFN renders Vpx unable to induce SAMHD1
degradation (48-50). Type II IFN can stimulate the
transcription of SAMHD1 to degrade dNTP and to restrict viral
infection positively (51,52) and type III IFN exhibits modest to
undetectable activity (53).
However, further research is required in this field to explore the
underlying molecular biological mechanisms.
The folding of the SAMHD1 region is disrupted around
T592E due to negative charge repulsion generated by a
phosphomimetic mutation. Subsequently, this disruption leads to the
substantial destabilization of the active tetrameric form of SAMHD1
and an approximately 3-fold decrease in its dNTPase activity.
However, the T592V variant does not perturb the crystal structure
of SAMHD1; thus, the available active SAMHD1 tetramers are not
significantly decreased (54). In
addition, the importance of SAMHD1 dephosphorylation has also been
investigated. Thus, phosphatase PP2A-B55α is responsible for
rendering the antiviral activity of SAMHD1. These results suggest
that phosphorylation and dephosphorylation at T592, the key
regulatory site of SAMHD1 protein, is responsible for the diverse
physiological functions of SAMHD1 (55).
Although alanine substitution at T592 exerts only a
minimal effect on the viral restriction ability of SAMHD1 in
differentiated U937 cells, phosphomimetic substitution by aspartate
and glutamate completely eliminates its antiviral effect. In
addition, introducing a T592A alanine mutation does not rescue
SAMHD1 restriction in cycling U937 cells, suggesting that the
inhibition of phosphorylation is not sufficient to restore SAMHD1
in proliferating cells (5,39). However, the antiviral activity of
SAMHD1 is limited to non-cycling cells. As previously mentioned,
SAMHD1 is phosphorylated on residue T592 in cycling cells; however,
the phosphorylation dissipates when cells are in a non-cycling
state, thus modulating the ability of SAMHD1 to block retroviral
infection without affecting its dNTPase activity (6).
Moreover, it has been reported that SAMHD1 is
acetylated on K405 by the acetyltransferase arrest defective
protein 1 (ARD1) and enhances its dNTPase activity in vitro.
However, the non-acetylated arginine substitution mutant (K405R)
does not exert a similar effect. Compared with cells expressing
wild-type SAMHD1, cancer cells expressing K405R mutant exhibit an
attenuated G1/S cell cycle transition and a decreased cell
proliferation. SAMHD1 acetylation levels are increased during the
G1 phase of the cell cycle. Collectively, these findings suggest
that SAMHD1 acetylation enhances its ability to hydrolyze dNTPs and
promote cancer cell proliferation. Therefore, SAMHD1 may be a
potent effective target for cancer treatment (3).
Finally, it has been documented that promoter
hypermethylation suppresses the transcriptional regulation of
SAMHD1, thereby downregulating its protein expression and
its tumori-genesis-related functions (11-13).
SAMHD1 activity demonstrates a significant
association between dNTP homeostasis and disease progression. Thus,
further research on the post-translational modifications of SAMHD1
is urgently required in order for its additional benefits to be
fully elucidated.
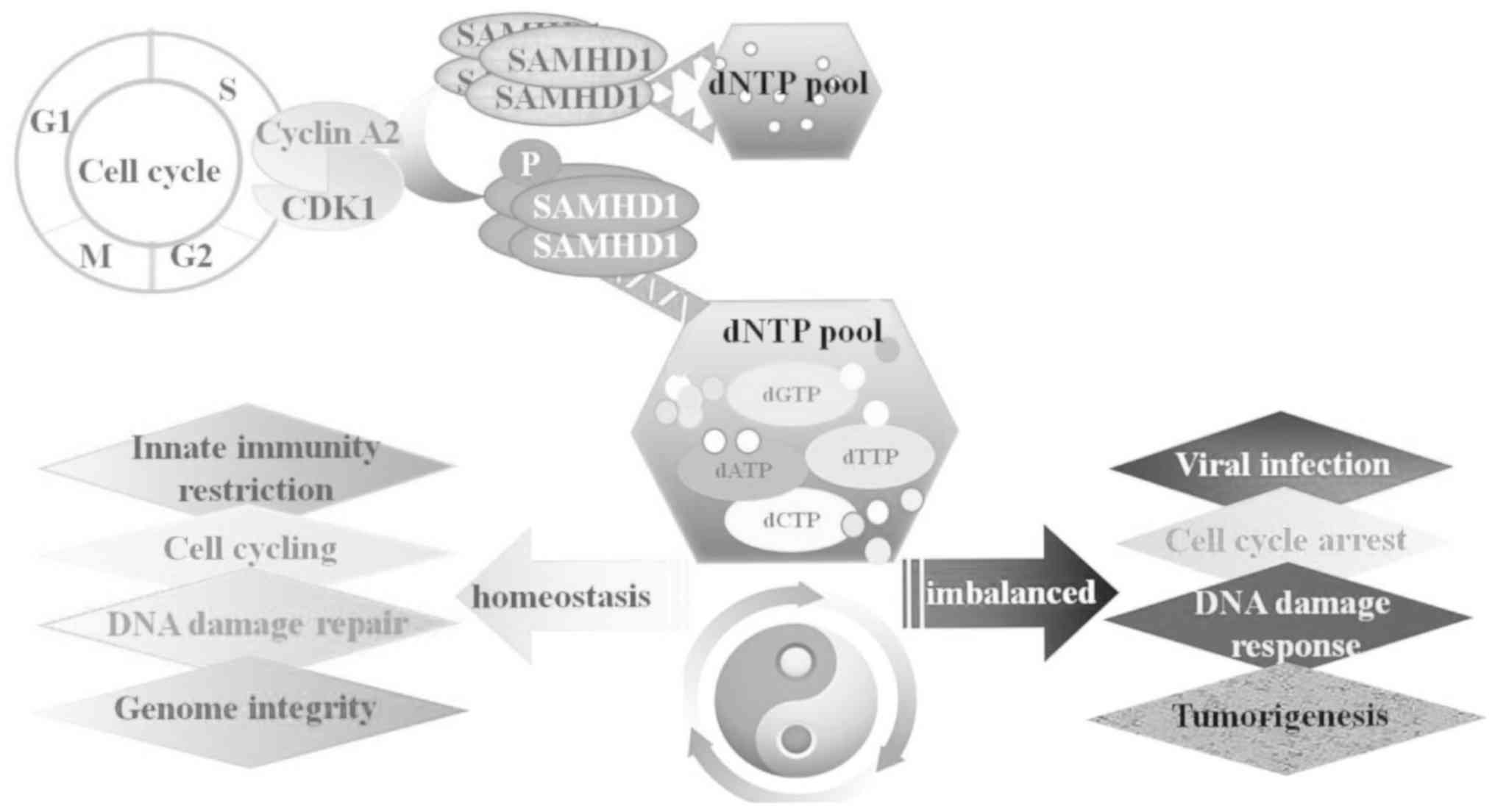
SAMHD1, as a dNTP hydrolytic enzyme, plays a key
role in the maintenance of homeostasis of cellular dNTP pools
(20,56,57)
and it is essential for preserving genome integrity. It has been
reported that dNTP pool imbalance caused by SAMHD1 deficiency may
lead to DNA damage, accompanied by the activation of IFN signaling
(57). In addition, the surplus of
dNTPs induces mismatches and increases the mutation rate during
cellular DNA replication (58),
which is an important molecular mechanism of tumorigenesis
(1). There is increasing evidence
to suggest that imbalanced dNTP levels are associated with the rate
of replication fork formation under DNA replication stress, leading
to gene mutations, genomic instability and cancer development
(59,60). Therefore, SAMHD1 is considered a
key regulator involved in the maintenance of the dNTP pool and
genome homeostasis. The role of SAMHD1 is illustrated in Fig. 2.
DNA damage in cells, mainly single-strand breaks,
arises frequently (approximately 10,000 lesions per cell per day)
by a variety of endogenous and exogenous stimuli (61,62).
It has been well established that the DNA damage response (DDR)
pathway detects lesions in DNA strands and activates the repair
system (63). Subsequently, cell
cycle checkpoints are activated, providing sufficient time to allow
lesions to be repaired. However, an unrepaired or improperly
repaired DNA response leads to cell death or abnormal cell mitosis,
which may induce malignant transformation and proliferation
(64,65). Additionally, inherited defects in
DNA damage repair mechanisms are associated with cancer
predisposition (66),
immunodeficiency (67),
neurodegenerative disorders (68),
infertility (69) and premature
aging, highlighting the critical role of DDR in human health.
Several studies have demonstrated that SAMHD1
participates in the DDR process. Thus, SAMHD1 promotes
dNTPase-independent DNA end resection to facilitate DNA
double-strand breaks (DSBs) repair by homologous recombination (HR)
(70). In addition, SAMHD1
exhibits a hydrolase-independent function though its C-terminal
recruitment of interacting proteins (CTIP) to DSB sites. These
observations suggest that SAMHD1 may contribute to anticancer
therapy (71). Clifford et
al (72) investigated the
expression of SAMHD1 in patients with chronic lymphocytic leukemia
(CLL) in the UK and revealed that SAMHD1 affected cell
proliferation and survival following DNA damage induction. More
specifically, the overexpression of wild-type SAMHD1 inhibited
proliferation and increased cell death following DSB treatment.
Furthermore, SAMHD1 was co-localized with p53-binding protein 1
(53BP1) at the DNA DSB site in the nucleus, which further indicated
that SAMHD1 is involved in the DDR process and related diseases
(72). By contrast, SAMHD1
downregulation may cause excess dNTPs and a subsequent imbalance of
dNTP pools, resulting in base mismatches and mutations during
replication, eventually leading to the activation of the intrinsic
interferon signal (57). These
findings indicate a novel association between SAMHD1 and DDR
process in the pathogenesis of several diseases.
SAMHD1 is widely expressed in the majority of
tissues and cells, and its restrictive function in the innate
immunity has been extensively reviewed since it was first
discovered. The SAMHD1 gene mutation was detected in
autoimmune AGS (22,73-75),
which was first described by Jean Aicardi and Francoise Goutières
in 1984 (76). The common clinical
features of AGS overlap with the autoimmune disease systemic lupus
erythematosus (SLE), including brain atrophy and severe sequelae
(75,77,78).
It has been reported that SAMHD1 mutations at residues 123, 143,
145, 201, 209, 254, 369 and 385 result in impaired endogenous
SAMHD1 protein function and induce nucleotide metabolism disorders
in myeloid cells (22). Abnormally
increased dNTP pools in fibroblasts derived from patients with AGS
are caused by the loss of functional SAMHD1. Subsequently, dNTP
accumulation may induce the immune system to secrete excessive
amount of antibodies, as it has been previously described (57). These results suggest that SAMHD1 is
a key regulator of the immune system by maintaining nucleotide pool
homeostasis.
Reverse transcription is a unique DNA synthesis
process through which retroviruses and retrotransposons convert
single stranded RNA genomes into double stranded DNA. This process
is catalyzed by reverse transcriptase, which is a virally encoded
DNA polymerase (79,80). Retroviruses consume cellular dNTPs
regulated by SAMHD1 to convert their RNA genomes into proviral DNA
through reverse transcription (81). DNTPs differ by only a single atom
from ribonucleotide triphosphates (NTPs), yet are maintained at
10-1,000-fold lower concentrations (82). Ryoo et al also found that
SAMHD1 restricted HIV-1 infection through its RNase activity by
cleaving the viral RNA genome, and SAMHD1 associated with HIV-1 RNA
and degraded it during the early phases of cell infection (31). The poor dNTP availability in
macrophages infected with HIV infection mainly promotes viral
mutagenesis induced by frequent rNMP and non-canonical dUMP
incorporation (83,84). Finally, SAMHD1 may be a primitive
cellular defense tool that was developed to effectively control the
replication of dNTP-utilizing pathogens (81).
Human SAMHD1 is a key restriction factor against
HIV-1 infection and is highly expressed in non-circulating cells,
such as resting CD4+ T cells and terminally
differentiated macrophages. SAMHD1 limits HIV-1 infection in
non-dividing cells by reducing the levels of intracellular dNTPs
during viral reverse transcription, which is indispensable for
viral storage and incubation (85). Thus, the overexpression of
wild-type SAMHD1 inhibits HIV-1 long terminal repeat (LTR)-driven
gene expression at the transcriptional level. In addition, it has
been well documented that non-phosphorylated (T592A) and dNTPase
inactive [H206D R207N (HD/RN)] mutants of SAMHD1 fail to
efficiently inhibit HIV-1 LTR-driven gene expression or the latent
virus reactivation (85). SAMHD1
has been reported to be a potent inhibitor of LINE-1
retrotrans-position. SAMHD1 is a potent regulator of LINE-1 and
LINE-1-mediated Alu/SVA reverse transcriptional transposon. It has
also been found that the mutant of SAMHD1 has a defect in LINE-1
inhibition. At the same time, the ability of SAMHD1 to inhibit
ORF2p-mediated LINE-1 RNP reverse transcription has been shown to
be associated with SAMHD1-mediated LINE-1 inhibition (86). Furthermore, SAMHD1 attenuates IFN-
and T-cell-mediated responses by suppressing the induction of
virus-specific cytotoxic T-cells in vivo (87). Of note, HIV-2 and simian
immunodeficiency viruses (SIVs) with Vpx or viral protein R (Vpr)
can induce SAMHD1 degradation, by inhibiting SAMHD1 downregulation
during viral infection (25,88-91).
Additionally, it has been reported that SAMHD1 blocks feline
immunodeficiency virus (FIV), bovine immunodeficiency virus (BIV),
equine infectious anemia virus (EIAV), N-tropic murine leukemia
virus (N-MLV) and B-tropic murine leukemia virus (B-MLV) infections
(92). These findings indicate
that SAMHD1 exerts inhibitory effects on infectious diseases.
Several studies have demonstrated that SAMHD1 is
associated with the development of multiple types of cancer, such
as lung and colon cancer. Thus, in lung adenocarcinoma, SAMHD1 mRNA
and protein levels have been shown to be downregulated compared
with those noted in adjacent normal tissues. In addition, it has
been suggested that the SAMHD1 promoter is highly methylated
in lung adenocarcinoma, resulting in a suppressed SAMHD1 expression
(13). Similarly, frequent
mutations in SAMHD1 in colon cancer cells induce SAMHD1
downregulation (94). The
aforementioned results suggest that SAMHD1 is closely associated
with an increased risk of both lung and colon cancer, and
presumably with other types of cancer.
Recently, it was demonstrated that a low level of
exogenous SAMHD1 expression can significantly reduce the growth,
proliferation and colony formation of HuT78 cells by increasing
apoptosis; thus, it may play a potential anticancer role in
cutaneous T cell lymphoma (CTCL) (95). In view of the role of SAMHD1 in
maintaining genomic stability, it may play an additional role in
cells as a cancer suppressor enzyme.
Exogenous SAMHD1 expression in HuT78 cells has also
been shown to result in increased spontaneous and Fas ligand
(Fas-L)-induced apoptosis levels via the activation of the
extrinsic pathway, including caspase-8, -3 and -7. Mechanistically,
SAMHD1 expression in HuT78 cells leads to a significant reduction
in the expression of cFLIPS, a key anti-apoptotic regulator that is
commonly overexpressed in patients with CTCL (95-97).
The catalogue of somatic mutations in cancer
(COSMOS) records 164 unique mutations in SAMHD1 found in samples
from various cancer tissues (98).
Widely expressed in several tissues, SAMHD1 mutations have
also been detected in breast cancer, myeloma, pancreatic cancer and
others. The mutation and modification sites of SAMHD1 in different
types of cancer are presented in Tables ITable II (99-104) and II, respectively.
The importance of SAMHD1 in dNTP metabolism and
genome integrity has been well established; thus, strategies
targeting SAMHD1 gene replication, post-translational
modifications and protein expression have been evaluated for the
treatment of cancer and autoimmune diseases (105). SAMHD1 acetylation enhances its
dNTPase activity, and thereby, cancer cell arrest at the G1 phase
to aid G1/S phase transition and promote cell cycle progression.
This observation suggests that the acetylation level of SAMHD1 may
be a potential therapeutic target for cancer treatment. In
addition, this finding also unveils a potential method for
therapeutically targeting SAMHD1 activity in cells through the use
of small molecule inhibitors of acetyltransferases (3). Furthermore, SAMHD1 protects cancer
cells from several antinucleoside metabolite treatments, such as
cytarabine (Ara-C) which is mainly used in the treatment of acute
myeloid leukemia (AML) (106-109). Combination therapy with an
anthracycline (commonly doxorubicin or daunorubicin) and Ara-C is
the standard treatment for AML (110). Ara-C is converted by the
canonical dNTP synthesis pathway to Ara-CTP, the active
triphosphate of Ara-C, which serves as a substrate of SAMHD1
(107). Herold et al
(106) demonstrated that
wild-type SAMHD1 reduced Ara-C treatment efficacy in vivo in
an AML mouse model. In addition, THP-1 cells lacking a functional
SAMHD1 gene have been shown to exhibit an increased
sensitivity to antimetabolites, including fludarabine, decitabine,
vidarabine and clofarabine (106). SAMHD1 downregulation or the
inhibition of its post-translational modifications may be promising
strategies with which overcome tumor resistance. Therefore, SAMHD1
is considered a potential biomarker for the stratification of
patients with AML and a target for the treatment of
Ara-C-refractory AML (109). The
aforementioned findings suggest that the invention of a potent
SAMHD1 inhibitor that enhances the efficiency of nucleotide
analogues should perhaps be a top priority for researchers. Thus,
high-throughput assays have already been established from several
research groups (111,112). Such approaches seem to be
particularly promising for future developments in this field.
Studies on the unique, natural viral restriction and
dNTPase properties of SAMHD1 have demonstrated its involvement in
the pathogenesis of several diseases and have provided guidance for
progress in the development of clinical applications. More
specifically, studies on the underlying mechanisms of antiviral
agents to fight infection have revealed that SAMHD1 inhibits HIV-1
infection in non-dividing cells by restricting viral reverse
transcription, resulting in decreased virus activity and storage
(14,15,113,114). In addition, SAMHD1 inhibits SIV
activity containing Vpx or Vpr (116-118).
The dNTPase activity of SAMHD1 maintains balanced
cellular dNTP pools, thus preventing genomic instability and
tumorigenesis. SAMHD1 loss-of-function mutations are
associated with abnormal dNTP accumulation, which induces rapid
cancer cell proliferation (37,105,119,120) and immune system disfunctions. On
the other hand, SAMHD1 protects cancer cells from DNA replication
inhibitors, such as pyrimidine antimetabolite antitumor agents
(104,105).
Therefore, future studies on SAMHD1 may provide
further insight into the clinical treatment of cancer and other
severe diseases. Finally, strategies targeting SAMHD1 are expected
to provide more effective health-related interventions.
This study was supported by the National Key R&D
Program of China (2016YFC1302400), the Ministry of Education
Innovation Team Development plan to LC(IRT_17R107), and the
National Science Foundation of China to XS (31300963, LFWK201725,
2018225083) and QG (81502400).
Not applicable.
XS and LC designed and conceived the general idea
and context of this review article. XS, LC and ZZ conceived and
wrote the Abstract. ZZ and YY conceived and wrote the Introduction
and 'Overview of SAMHD1' sections. LZ and JW contributed to the
'Modifications of SAMHD1' and the 'Role of SAMHD1 in dNTP
homeostasis' sections. FY, YX and QG conducted the writing of the
'Role of SAMHD1 in DNA damage response' and 'Role of SAMHD1 in
immune disorders and viral infections' sections. XW and SC
completed the 'Role of SAMHD1 in tumorigenesis and cancer
treatment' sections. ZZ integrated all sections and relevant
references of this manuscript. XS, LC and ZZ revised the
manuscript. All authors read and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
|
1
|
Kunz BA, Kohalmi SE, Kunkel TA, Mathews
CK, McIntosh EM and Reidy JA: International commission for
protection against environmental mutagens and carcinogens.
Deoxyribonucleoside triphosphate levels: A critical factor in the
maintenance of genetic stability. Mutat Res. 318:1–64. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Reichard P: Interactions between
deoxyribonucleotide and DNA synthesis. Annu Rev Biochem.
57:349–374. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee EJ, Seo JH, Park JH, Vo TTL, An S, Bae
SJ, Le H, Lee HS, Wee HJ, Lee D, et al: SAMHD1 acetylation enhances
its deoxy-nucleotide triphosphohydrolase activity and promotes
cancer cell proliferation. Oncotarget. 8:68517–68529.
2017.PubMed/NCBI
|
|
4
|
Koharudin LM, Wu Y, DeLucia M, Mehrens J,
Gronenborn AM and Ahn J: Structural basis of allosteric activation
of sterile α motif and histidine-aspartate domain-containing
protein 1 (SAMHD1) by nucleoside triphosphates. J Biol Chem.
289:32617–32627. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Welbourn S, Dutta SM, Semmes OJ and
Strebel K: Restriction of virus infection but not catalytic dNTPase
activity is regulated by phosphorylation of SAMHD1. J Virol.
87:11516–11524. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
White TE, Brandariz-Nunez A, Valle-Casuso
JC, Amie S, Nguyen LA, Kim B, Tuzova M and Diaz-Griffero F: The
retroviral restriction ability of SAMHD1, but not its
deoxynucleotide triphosphohydrolase activity, is regulated by
phosphorylation. Cell Host Microbe. 13:441–451. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
St Gelais C, de Silva S, Hach JC, White
TE, Diaz-Griffero F, Yount JS and Wu L: Identification of cellular
proteins interacting with the retroviral restriction factor SAMHD1.
J Virol. 88:5834–5844. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ji X, Tang C, Zhao Q, Wang W and Xiong Y:
Structural basis of cellular dNTP regulation by SAMHD1. Proc Natl
Acad Sci USA. 111:E4305–E4314. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang K, Lv DW and Li R: Conserved
herpesvirus protein kinases target SAMHD1 to facilitate virus
replication. Cell Rep. 28:449–459 e445. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim ET, Roche KL, Kulej K, Spruce LA,
Seeholzer SH, Coen DM, Diaz-Griffero F, Murphy EA and Weitzman MD:
SAMHD1 modulates early steps during human cytomegalovirus infection
by limiting NF-kB activation. Cell Rep. 28:434–448 e436. 2019.
View Article : Google Scholar
|
|
11
|
de Silva S, Hoy H, Hake TS, Wong HK, Porcu
P and Wu L: Promoter methylation regulates SAMHD1 gene expression
in human CD4+ T cells. J Biol Chem. 288:9284–9292. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
de Silva S, Wang F, Hake TS, Porcu P, Wong
HK and Wu L: Downregulation of SAMHD1 expression correlates with
promoter DNA methylation in Sezary syndrome patients. J Invest
Dermatol. 134:562–565. 2014. View Article : Google Scholar
|
|
13
|
Wang JL, Lu FZ, Shen XY, Wu Y and Zhao LT:
SAMHD1 is down regulated in lung cancer by methylation and inhibits
tumor cell proliferation. Biochem Biophys Res Commun. 455:229–233.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Laguette N, Sobhian B, Casartelli N,
Ringeard M, Chable-Bessia C, Ségéral E, Yatim A, Emiliani S,
Schwartz O and Benkirane M: SAMHD1 is the dendritic- and
myeloid-cell-specific HIV-1 restriction factor counteracted by Vpx.
Nature. 474:654–657. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hrecka K, Hao C, Gierszewska M, Swanson
SK, Kesik-Brodacka M, Srivastava S, Florens L, Washburn MP and
Skowronski J: Vpx relieves inhibition of HIV-1 infection of
macrophages mediated by the SAMHD1 protein. Nature. 474:658–661.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Berger A, Sommer AF, Zwarg J, Hamdorf M,
Welzel K, Esly N, Panitz S, Reuter A, Ramos I, Jatiani A, et al:
SAMHD1-deficient CD14+ cells from individuals with aicardigoutieres
syndrome are highly susceptible to HIV-1 infection. PLoS Pathog.
7:e10024252011. View Article : Google Scholar
|
|
17
|
Ahn J, Hao C, Yan J, DeLucia M, Mehrens J,
Wang C, Gronenborn AM and Skowronski J: HIV/simian immunodeficiency
virus (SIV) accessory virulence factor Vpx loads the host cell
restriction factor SAMHD1 onto the E3 ubiquitin ligase complex
CRL4DCAF1. J Biol Chem. 287:12550–12558. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li N, Zhang W and Cao X: Identification of
human homologue of mouse IFN-gamma induced protein from human
dendritic cells. Immunol Lett. 74:221–224. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kueck T, Cassella E, Holler J, Kim B and
Bieniasz PD: The aryl hydrocarbon receptor and interferon gamma
generate antiviral states via transcriptional repression. Elife.
7:e388672018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Goldstone DC, Ennis-Adeniran V, Hedden JJ,
Groom HC, Rice GI, Christodoulou E, Walker PA, Kelly G, Haire LF,
Yap MW, et al: HIV-1 restriction factor SAMHD1 is a deoxynucleoside
triphosphate triphosphohydrolase. Nature. 480:379–382. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Leshinsky-Silver E, Malinger G, Ben-Sira
L, Kidron D, Cohen S, Inbar S, Bezaleli T, Levine A, Vinkler C, Lev
D and Lerman-Sagie T: A large homozygous deletion in the SAMHD1
gene causes atypical aicardi-goutieres syndrome associated with
mtDNA deletions. Eur J Hum Genet. 19:287–292. 2011. View Article : Google Scholar
|
|
22
|
Rice GI, Bond J, Asipu A, Brunette RL,
Manfield IW, Carr IM, Fuller JC, Jackson RM, Lamb T, Briggs TA, et
al: Mutations involved in aicardi-goutieres syndrome implicate
SAMHD1 as regulator of the innate immune response. Nat Genet.
41:829–832. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Thiele H, du Moulin M, Barczyk K, George
C, Schwindt W, Nürnberg G, Frosch M, Kurlemann G, Roth J, Nürnberg
P and Rutsch F: Cerebral arterial stenoses and stroke: Novel
features of Aicardi-Goutieres syndrome caused by the arg164X
mutation in SAMHD1 are associated with altered cytokine expression.
Hum Mutat. 31:E1836–E1850. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dale RC, Gornall H, Singh-Grewal D,
Alcausin M, Rice GI and Crow YJ: Familial aicardi-goutieres
syndrome due to SAMHD1 mutations is associated with chronic
arthropathy and contractures. Am J Med Genet A. 152A:938–942. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Brandariz-Nunez A, Valle-Casuso JC, White
TE, Laguette N, Benkirane M, Brojatsch J and Diaz-Griffero F: Role
of SAMHD1 nuclear localization in restriction of HIV-1 and SIVmac.
Retrovirology. 9:492012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hofmann H, Logue EC, Bloch N, Daddacha W,
Polsky SB, Schultz ML, Kim B and Landau NR: The Vpx lentiviral
accessory protein targets SAMHD1 for degradation in the nucleus. J
Virol. 86:12552–12560. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
DeLucia M, Mehrens J, Wu Y and Ahn J:
HIV-2 and SIVmac accessory virulence factor Vpx down-regulates
SAMHD1 enzyme catalysis prior to proteasome-dependent degradation.
J Biol Chem. 288:19116–19126. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim CA and Bowie JU: SAM domains: Uniform
structure, diversity of function. Trends Biochem Sci. 28:625–628.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Laguette N and Benkirane M: How SAMHD1
changes our view of viral restriction. Trends Immunol. 33:26–33.
2012. View Article : Google Scholar
|
|
30
|
Antonucci JM, St Gelais C, de Silva S,
Yount JS, Tang C, Ji X, Shepard C, Xiong Y, Kim B and Wu L:
SAMHD1-mediated HIV-1 restriction in cells does not involve
ribonuclease activity. Nat Med. 22:1072–1074. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ryoo J, Choi J, Oh C, Kim S, Seo M, Kim
SY, Seo D, Kim J, White TE, Brandariz-Nuñez A, et al: The
ribonuclease activity of SAMHD1 is required for HIV-1 restriction.
Nat Med. 20:936–941. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhu CF, Wei W, Peng X, Dong YH, Gong Y and
Yu XF: The mechanism of substrate-controlled allosteric regulation
of SAMHD1 activated by GTP. Acta Crystallogr D Biol Crystallogr.
71:516–524. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li Y, Kong J, Peng X, Hou W, Qin X and Yu
XF: Structural insights into the high-efficiency catalytic
mechanism of the sterile α-motif/histidine-aspartate
domain-containing protein. J Biol Chem. 290:29428–29437. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Patra KK, Bhattacharya A and Bhattacharya
S: Allosteric signal transduction in HIV-1 restriction factor
SAMHD1 proceeds via reciprocal handshake across monomers. J Chem
Inf Model. 57:2523–2538. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yan J, Kaur S, DeLucia M, Hao C, Mehrens
J, Wang C, Golczak M, Palczewski K, Gronenborn AM, Ahn J and
Skowronski J: Tetramerization of SAMHD1 is required for biological
activity and inhibition of HIV infection. J Biol Chem.
288:10406–10417. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mauney CH, Rogers LC, Harris RS, Daniel
LW, Devarie-Baez NO, Wu H, Furdui CM, Poole LB, Perrino FW and
Hollis T: The SAMHD1 dNTP triphosphohydrolase is controlled by a
redox switch. Antioxid Redox Signal. 27:1317–1331. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mauney CH and Hollis T: SAMHD1: Recurring
roles in cell cycle, viral restriction, cancer, and innate
immunity. Autoimmunity. 51:96–110. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tramentozzi E, Ferraro P, Hossain M,
Stillman B, Bianchi V and Pontarin G: The dNTP triphosphohydrolase
activity of SAMHD1 persists during S-phase when the enzyme is
phosphorylated at T592. Cell Cycle. 17:1102–1114. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Arnold LH, Groom HC, Kunzelmann S,
Schwefel D, Caswell SJ, Ordonez P, Mann MC, Rueschenbaum S,
Goldstone DC, Pennell S, et al: Phospho-dependent regulation of
SAMHD1 oligomerisation couples catalysis and restriction. PLoS
Pathog. 11:e10051942015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ji X, Wu Y, Yan J, Mehrens J, Yang H,
DeLucia M, Hao C, Gronenborn AM, Skowronski J, Ahn J and Xiong Y:
Mechanism of allosteric activation of SAMHD1 by dGTP. Nat Struct
Mol Biol. 20:1304–1309. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Badia R, Angulo G, Riveira-Munoz E,
Pujantell M, Puig T, Ramirez C, Torres-Torronteras J, Martí R,
Pauls E, Clotet B, et al: Inhibition of herpes simplex virus type 1
by the CDK6 inhibitor PD-0332991 (palbociclib) through the control
of SAMHD1. J Antimicrob Chemother. 71:387–394. 2016. View Article : Google Scholar :
|
|
42
|
Kim ET, White TE, Brandariz-Nunez A,
Diaz-Griffero F and Weitzman MD: SAMHD1 restricts herpes simplex
virus 1 in macrophages by limiting DNA replication. J Virol.
87:12949–12956. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hu J, Qiao M, Chen Y, Tang H, Zhang W,
Tang D, Pi S, Dai J, Tang N, Huang A and Hu Y: Cyclin E2-CDK2
mediates SAMHD1 phosphorylation to abrogate its restriction of HBV
replication in hepatoma cells. FEBS Lett. 592:1893–1904. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sommer AF, Riviere L, Qu B, Schott K,
Riess M, Ni Y, Shepard C, Schnellbächer E, Finkernagel M,
Himmelsbach K, et al: Restrictive influence of SAMHD1 on Hepatitis
B Virus life cycle. Sci Rep. 6:266162016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Li M, Zhang D, Zhu M, Shen Y, Wei W, Ying
S, Korner H and Li J: Roles of SAMHD1 in antiviral defense,
autoimmunity and cancer. Rev Med Virol. 27:2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Cribier A, Descours B, Valadao AL,
Laguette N and Benkirane M: Phosphorylation of SAMHD1 by Cyclin
A2/CDK1 regulates its restriction activity toward HIV-1. Cell Rep.
3:1036–1043. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Pauls E, Ruiz A, Badia R, Permanyer M,
Gubern A, Riveira-Muñoz E, Torres-Torronteras J, Alvarez M, Mothe
B, Brander C, et al: Cell cycle control and HIV-1 susceptibility
are linked by CDK6-dependent CDK2 phosphorylation of SAMHD1 in
myeloid and lymphoid cells. J Immunol. 193:1988–1997. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Valle-Casuso JC, Allouch A, David A, Lenzi
GM, Studdard L, Barré-Sinoussi F, Müller-Trutwin M, Kim B, Pancino
G and Sáez-Cirión A: p21 Restricts HIV-1 in monocyte-derived
dendritic cells through the reduction of deoxynucleoside
triphos-phate biosynthesis and regulation of SAMHD1 antiviral
activity. J Virol. 91:e01324–e01317. 2017. View Article : Google Scholar :
|
|
49
|
Bloch N, O'Brien M, Norton TD, Polsky SB,
Bhardwaj N and Landau NR: HIV type 1 infection of plasmacytoid and
myeloid dendritic cells is restricted by high levels of SAMHD1 and
cannot be counteracted by Vpx. AIDS Res Hum Retroviruses.
30:195–203. 2014. View Article : Google Scholar :
|
|
50
|
Dragin L, Nguyen LA, Lahouassa H, Sourisce
A, Kim B, Ramirez BC and Margottin-Goguet F: Interferon block to
HIV-1 transduction in macrophages despite SAMHD1 degradation and
high deoxynucleoside triphosphates supply. Retrovirology.
10:302013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lafuse WP, Brown D, Castle L and Zwilling
BS: Cloning and characterization of a novel cDNA that is
IFN-gamma-induced in mouse peritoneal macrophages and encodes a
putative GTP-binding protein. J Leukoc Biol. 57:477–483. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Taylor GA, Jeffers M, Largaespada DA,
Jenkins NA, Copeland NG and Vande Woude GF: Identification of a
novel GTPase, the inducibly expressed GTPase, that accumulates in
response to interferon gamma. J Biol Chem. 271:20399–20405. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Szaniawski MA, Spivak AM, Cox JE, Catrow
JL, Hanley T, Williams ESCP, Tremblay MJ, Bosque A and Planelles V:
SAMHD1 phosphorylation coordinates the Anti-HIV-1 response by
diverse interferons and tyrosine kinase inhibition. Mbio.
9:e00819–e00818. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Tang C, Ji X, Wu L and Xiong Y: Impaired
dNTPase activity of SAMHD1 by phosphomimetic mutation of Thr-592. J
Biol Chem. 290:26352–26359. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Schott K, Fuchs NV, Derua R, Mahboubi B,
Schnellbächer E, Seifr ied J, Tondera C, Schm itz H, Shepa rd C,
Brandariz-Nuñez A, et al: Dephosphorylation of the HIV-1
restriction factor SAMHD1 is mediated by PP2A-B55 α holoenzymes
during mitotic exit. Nat Commun. 9:22272018. View Article : Google Scholar
|
|
56
|
Franzolin E, Pontarin G, Rampazzo C,
Miazzi C, Ferraro P, Palumbo E, Reichard P and Bianchi V: The
deoxynucleotide triphosphohydrolase SAMHD1 is a major regulator of
DNA precursor pools in mammalian cells. Proc Natl Acad Sci USA.
110:14272–14277. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kretschmer S, Wolf C, Konig N, Staroske W,
Guck J, Häusler M, Luksch H, Nguyen LA, Kim B, Alexopoulou D, et
al: SAMHD1 prevents autoimmunity by maintaining genome stability.
Ann Rheum Dis. 74:e172015. View Article : Google Scholar :
|
|
58
|
Mathews CK: DNA precursor metabolism and
genomic stability. FASEB J. 20:1300–1314. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Poli J, Tsaponina O, Crabbe L, Keszthelyi
A, Pantesco V, Chabes A, Lengronne A and Pasero P: dNTP pools
determine fork progression and origin usage under replication
stress. EMBO J. 31:883–894. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Coquel F, Silva MJ, Techer H, Zadorozhny
K, Sharma S, Nieminuszczy J, Mettling C, Dardillac E, Barthe A,
Schmitz AL, et al: SAMHD1 acts at stalled replication forks to
prevent interferon induction. Nature. 557:57–61. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Seo YR, Sweeney C and Smith ML:
Selenomethionine induction of DNA repair response in human
fibroblasts. Oncogene. 21:3663–3669. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Lin Y, Ha A and Yan S: Methods for
studying DNA single-strand break repair and signaling in xenopus
laevis egg extracts. Methods Mol Biol. 1999:161–172. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Hanawalt PC: Historical perspective on the
DNA damage response. DNA Repair (Amst). 36:2–7. 2015. View Article : Google Scholar
|
|
64
|
Chu G: Double strand break repair. J Biol
Chem. 272:24097–24100. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Rooney S, Chaudhuri J and Alt FW: The role
of the non-homologous end-joining pathway in lymphocyte
development. Immunol Rev. 200:115–131. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Figueroa-Gonzalez G and Perez-Plasencia C:
Strategies for the evaluation of DNA damage and repair mechanisms
in cancer. Oncol Lett. 13:3982–3988. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Morio T: Recent advances in the study of
immunodeficiency and DNA damage response. Int J Hematol.
106:357–365. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Barzilai A: DNA damage, neuronal and glial
cell death and neurodegeneration. Apoptosis. 15:1371–1381. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Brown JS and Jackson SP: Ubiquitylation,
neddylation and the DNA damage response. Open Biol. 5:1500182015.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Medeiros AC, Soares CS, Coelho PO, Vieira
NA, Baqui MMA, Teixeira FR and Gomes MD: DNA damage response
signaling does not trigger redistribution of SAMHD1 to nuclear
foci. Biochem Biophys Res Commun. 499:790–796. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Cabello-Lobato MJ, Wang S and Schmidt CK:
SAMHD1 sheds moonlight on DNA double-strand break repair. Trends
Genet. 33:895–897. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Clifford R, Louis T, Robbe P, Ackroyd S,
Burns A, Timbs AT, Wright Colopy G, Dreau H, Sigaux F, Judde JG, et
al: SAMHD1 is mutated recurrently in chronic lymphocytic leukemia
and is involved in response to DNA damage. Blood. 123:1021–1031.
2014. View Article : Google Scholar :
|
|
73
|
Oh C, Ryoo J, Park K, Kim B, Daly MB, Cho
D and Ahn K: A central role for PI3K-AKT signaling pathway in
linking SAMHD1-deficiency to the type I interferon signature. Sci
Rep. 8:842018. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Martinez-Lopez A, Martin-Fernandez M, Buta
S, Kim B, Bogunovic D and Diaz-Griffero F: SAMHD1 deficient human
monocytes autonomously trigger type I interferon. Mol Immunol.
101:450–460. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Ramantani G, Kohlhase J, Hertzberg C,
Innes AM, Engel K, Hunger S, Borozdin W, Mah JK, Ungerath K,
Walkenhorst H, et al: Expanding the phenotypic spectrum of lupus
erythematosus in aicardi-goutieres syndrome. Arthritis Rheum.
62:1469–1477. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Aicardi J and Goutieres F: A progressive
familial encephalopathy in infancy with calcifications of the basal
ganglia and chronic cerebrospinal fluid lymphocytosis. Ann Neurol.
15:49–54. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Pendergraft WF III and Means TK: AGS, SLE,
and RNASEH2 mutations: Translating insights into therapeutic
advances. J Clin Invest. 125:102–104. 2015. View Article : Google Scholar :
|
|
78
|
Ramantani G, Hausler M, Niggemann P,
Wessling B, Guttmann H, Mull M, Tenbrock K and Lee-Kirsch MA:
Aicardi-Goutieres syndrome and systemic lupus erythematosus (SLE)
in a 12-year-old boy with SAMHD1 mutations. J Child Neurol.
26:1425–1428. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Hu WS and Hughes SH: HIV-1 reverse
transcription. Cold Spring Harb Perspect Med. 2:a0068822012.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Sarafianos SG, Marchand B, Das K, Himmel
DM, Parniak MA, Hughes SH and Arnold E: Structure and function of
HIV-1 reverse transcriptase: Molecular mechanisms of polymerization
and inhibition. J Mol Biol. 385:693–713. 2009. View Article : Google Scholar
|
|
81
|
Amie SM, Noble E and Kim B: Intracellular
nucleotide levels and the control of retroviral infections.
Virology. 436:247–254. 2013. View Article : Google Scholar :
|
|
82
|
Traut TW: Physiological concentrations of
purines and pyrimidines. Mol Cell Biochem. 140:1–22. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Kennedy EM, Amie SM, Bambara RA and Kim B:
Frequent incorporation of ribonucleotides during HIV-1 reverse
transcription and their attenuated repair in macrophages. J Biol
Chem. 287:14280–14288. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Kennedy EM, Gavegnano C, Nguyen L, Slater
R, Lucas A, Fromentin E, Schinazi RF and Kim B: Ribonucleoside
triphosphates as substrate of human immunodeficiency virus type 1
reverse transcriptase in human macrophages. J Biol Chem.
285:39380–39391. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Antonucci JM, Kim SH, St Gelais C,
Bonifati S, Li TW, Buzovetsky O, Knecht KM, Duchon AA, Xiong Y,
Musier-Forsyth K and Wu L: SAMHD1 impairs HIV-1 gene expression and
negatively modulates reactivation of viral latency in CD4(+) T
cells. J Virol. 92:e00292–e00218. 2018. View Article : Google Scholar :
|
|
86
|
Gao W, Li G, Bian X, Rui Y, Zhai C, Liu P,
Su J, Wang H, Zhu C, Du Y, et al: Defective modulation of LINE-1
retrotransposition by cancer-associated SAMHD1 mutants. Biochem
Biophys Res Commun. 519:213–219. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Maelfait J, Bridgeman A, Benlahrech A,
Cursi C and Rehwinkel J: Restriction by SAMHD1 limits
cGAS/STING-dependent innate and adaptive immune responses to HIV-1.
Cell Rep. 16:1492–1501. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Baldauf HM, Stegmann L, Schwarz SM, Ambiel
I, Trotard M, Martin M, Burggraf M, Lenzi GM, Lejk H, Pan X, et al:
Vpx overcomes a SAMHD1-independent block to HIV reverse
transcription that is specific to resting CD4 T cells. Proc Natl
Acad Sci USA. 114:2729–2734. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Miyakawa K, Matsunaga S, Yokoyama M,
Nomaguchi M, Kimura Y, Nishi M, Kimura H, Sato H, Hirano H, Tamura
T, et al: PIM kinases facilitate lentiviral evasion from SAMHD1
restriction via Vpx phosphorylation. Nat Commun. 10:18442019.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Yurkovetskiy L, Guney MH, Kim K, Goh SL,
McCauley S, Dauphin A, Diehl WE and Luban J: Primate
immunodeficiency virus proteins Vpx and Vpr counteract
transcriptional repression of proviruses by the HUSH complex. Nat
Microbiol. 3:1354–1361. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Reinhard C, Bottinelli D, Kim B and Luban
J: Vpx rescue of HIV-1 from the antiviral state in mature dendritic
cells is independent of the intracellular deoxynucleotide
concentration. Retrovirology. 11:122014. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
White TE, Brandariz-Nunez A, Valle-Casuso
JC, Amie S, Nguyen L, Kim B, Brojatsch J and Diaz-Griffero F:
Contribution of SAM and HD domains to retroviral restriction
mediated by human SAMHD1. Virology. 436:81–90. 2013. View Article : Google Scholar :
|
|
93
|
Rossi D: SAMHD1: A new gene for CLL.
Blood. 123:951–952. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Rentoft M, Lindell K, Tran P, Chabes AL,
Buckland RJ, Watt DL, Marjavaara L, Nilsson AK, Melin B, Trygg J,
et al: Heterozygous colon cancer-associated mutations of SAMHD1
have functional significance. Proc Natl Acad Sci USA.
113:4723–4728. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Kodigepalli KM, Li MH, Liu SL and Wu L:
Exogenous expression of SAMHD1 inhibits proliferation and induces
apoptosis in cutaneous T-cell lymphoma-derived HuT78 cells. Cell
Cycle. 16:179–188. 2017. View Article : Google Scholar :
|
|
96
|
Contassot E, Kerl K, Roques S, Shane R,
Gaide O, Dupuis M, Rook AH and French LE: Resistance to FasL and
tumor necrosis factor-related apoptosis-inducing ligand-mediated
apoptosis in Sezary syndrome T-cells associated with impaired death
receptor and FLICE-inhibitory protein expression. Blood.
111:4780–4787. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Zhang CL, Kamarashev J, Qin JZ, Burg G,
Dummer R and Dobbeling U: Expression of apoptosis regulators in
cutaneous T-cell lymphoma (CTCL) cells. J Pathol. 200:249–254.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Forbes SA, Beare D, Boutselakis H, Bamford
S, Bindal N, Tate J, Cole CG, Ward S, Dawson E, Ponting L, et al:
COSMIC: Somatic cancer genetics at high-resolution. Nucleic Acids
Res. 45:D777–D783. 2017. View Article : Google Scholar :
|
|
99
|
Sjoblom T, Jones S, Wood LD, Parsons DW,
Lin J, Barber TD, Mandelker D, Leary RJ, Ptak J, Silliman N, et al:
The consensus coding sequences of human breast and colorectal
cancers. Science. 314:268–274. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Kohnken R, Kodigepalli KM, Mishra A, Porcu
P and Wu L: MicroRNA-181 contributes to downregulation of SAMHD1
expression in CD4+T-cells derived from Sezary syndrome patients.
Leuk Res. 52:58–66. 2017. View Article : Google Scholar
|
|
101
|
Liu J, Lee W, Jiang Z, Chen Z,
Jhunjhunwala S, Haverty PM, Gnad F, Guan Y, Gilbert HN, Stinson J,
et al: Genome and transcriptome sequencing of lung cancers reveal
diverse mutational and splicing events. Genome Res. 22:2315–2327.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Shang Z, Qian L, Liu S, Niu X, Qiao Z, Sun
Y, Zhang Y, Fan LY, Guan X, Cao CX and Xiao H: Graphene
oxide-facilitated comprehensive analysis of cellular nucleic acid
binding proteins for lung cancer. Acs Appl Mater Interfaces.
10:17756–17770. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Yang CA, Huang HY, Chang YS, Lin CL, Lai
IL and Chang JG: DNA-sensing and nuclease gene expressions as
markers for colorectal cancer progression. Oncology. 92:115–124.
2017. View Article : Google Scholar
|
|
104
|
Herrmann A, Wittmann S, Thomas D, Shepard
CN, Kim B, Ferreirós N and Gramberg T: The SAMHD1-mediated block of
LINE-1 retroelements is regulated by phosphorylation. Mob DNA.
9:112018. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Kohnken R, Kodigepalli KM and Wu L:
Regulation of deoxy-nucleotide metabolism in cancer: Novel
mechanisms and therapeutic implications. Mol Cancer. 14:1762015.
View Article : Google Scholar
|
|
106
|
Herold N, Rudd SG, Sanjiv K, Kutzner J,
Bladh J, Paulin CBJ, Helleday T, Henter JI and Schaller T: SAMHD1
protects cancer cells from various nucleoside-based
antimetabolites. Cell Cycle. 16:1029–1038. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Rudd SG, Schaller T and Herold N: SAMHD1
is a barrier to antimetabolite-based cancer therapies. Mol Cell
Oncol. 4:e12875542017. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Zhu KW, Chen P, Zhang DY, Yan H, Liu H,
Cen LN, Liu YL, Cao S, Zhou G, Zeng H, et al: Association of
genetic polymorphisms in genes involved in Ara-C and dNTP
metabolism pathway with chemosensitivity and prognosis of adult
acute myeloid leukemia (AML). J Transl Med. 16:902018. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Schneider C, Oellerich T, Baldauf HM,
Schwarz SM, Thomas D, Flick R, Bohnenberger H, Kaderali L, Stegmann
L, Cremer A, et al: SAMHD1 is a biomarker for cytarabine response
and a therapeutic target in acute myeloid leukemia. Nat Med.
23:250–255. 2017. View Article : Google Scholar
|
|
110
|
Ossenkoppele G and Lowenberg B: How I
treat the older patient with acute myeloid leukemia. Blood.
125:767–774. 2015. View Article : Google Scholar
|
|
111
|
Arnold LH, Kunzelmann S, Webb MR and
Taylor IA: A continuous enzyme-coupled assay for
triphosphohydrolase activity of HIV-1 restriction factor SAMHD1.
Antimicrob Agents Chemother. 59:186–192. 2015. View Article : Google Scholar :
|
|
112
|
Seamon KJ and Stivers JT: A
high-throughput enzyme-coupled assay for SAMHD1 dNTPase. J Biomol
Screen. 20:801–809. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Baldauf HM, Pan X, Erikson E, Schmidt S,
Daddacha W, Burggraf M, Schenkova K, Ambiel I, Wabnitz G, Gramberg
T, et al: SAMHD1 restricts HIV-1 infection in resting CD4(+) T
cells. Nat Med. 18:1682–1687. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Descours B, Cribier A, Chable-Bessia C,
Ayinde D, Rice G, Crow Y, Yatim A, Schwartz O, Laguette N and
Benkirane M: SAMHD1 restricts HIV-1 reverse transcription in
quiescent CD4(+) T-cells. Retrovirology. 9:872012. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Lahouassa H, Daddacha W, Hofmann H, Ayinde
D, Logue EC, Dragin L, Bloch N, Maudet C, Bertrand M, Gramberg T,
et al: SAMHD1 restricts the replication of human immunodeficiency
virus type 1 by depleting the intracellular pool of
deoxynucleo-side triphosphates. Nat Immunol. 13:223–228. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Sakai Y, Doi N, Miyazaki Y, Adachi A and
Nomaguchi M: Phylogenetic insights into the functional relationship
between primate lentiviral reverse transcriptase and accessory
proteins vpx/vpr. Front Microbiol. 7:16552016. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Plitnik T, Sharkey ME, Mahboubi B, Kim B
and Stevenson M: Incomplete suppression of hiv-1 by samhd1 permits
efficient macrophage infection. Pathog Immun. 3:197–223. 2018.
View Article : Google Scholar
|
|
118
|
Mereby SA, Maehigashi T, Holler JM, Kim
DH, Schinazi RF and Kim B: Interplay of ancestral non-primate
lentiviruses with the virus-restricting SAMHD1 proteins of their
hosts. J Biol Chem. 293:16402–16412. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Wang Z, Bhattacharya A, Villacorta J,
Diaz-Griffero F and Ivanov DN: Allosteric activation of SAMHD1
protein by deoxynucleotide triphosphate (dNTP)-dependent
tetramerization requires dNTP concentrations that are similar to
dNTP concentrations observed in cycling T cells. J Biol Chem.
291:21407–21413. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Bonifati S, Daly MB, St Gelais C, Kim SH,
Hollenbaugh JA, Shepard C, Kennedy EM, Kim DH, Schinazi RF, Kim B
and Wu L: SAMHD1 controls cell cycle status, apoptosis and HIV-1
infection in monocytic THP-1 cells. Virology. 495:92–100. 2016.
View Article : Google Scholar : PubMed/NCBI
|