Introduction
Of female malignant tumour types, breast cancer is
one of the most common and has the highest incidence, which
seriously endangers women's health (1,2).
Triple-negative breast cancer (TNBC) refers to a subtype of breast
cancer that is negative for oestrogen receptor, progesterone
receptor and human epidermal growth factor receptor 2 expression,
accounting for 10-20% of all breast cancer cases (3). Compared with other types of breast
cancer, TNBC has a poor prognosis (4). Of the pathological types, most are
invasive ductal carcinoma, which is characterized by a strong
invasiveness, high recurrence rate, high metastasis rate, short
survival time and high mortality (5). Investigating the pathogenesis of TNBC
can provide a theoretical basis for improving its treatment.
However, the molecular regulatory mechanisms of TNBC development
remain unclear.
B-cell lymphoma/leukaemia 11A (BCL11A) was first
found in B cell chronic lymphocytic leukaemia. Its coding region
encodes a transcription factor activating protein with a Kruppel
zinc finger structure (6,7). A previous study by our group has
shown that BCL11A promotes TNBC stemness and tumorigenesis
(8). However, as a transcription
factor cofactor, the molecular mechanism by which it interacts with
transcription factors to promote TNBC metastasis remains to be
studied. A recent study has reported that in lung cancer, BCL11A is
involved in the transcriptional upregulation of pro-cancer factors
by directly binding to SRY (sex determining region Y)-box 2 (SOX2)
(9). SOX2 is a factor associated
with a variety of processes, such as cancer cell stemness and
epithelial-mesenchymal transition (EMT) (10-12).
However, whether this regulation is also present in TNBC remains to
be studied.
The SKI like proto-oncogene (SKIL; also termed SnoN
or SnoA) is a gene that was discovered in the 1980s, which belongs
to the SKI family of intranuclear oncoproteins (13). Studies have shown that SKIL gene
expression is increased in numerous tumour types, such as
oesophageal cancer, ovarian cancer, melanoma, lung cancer and
breast cancer (14-19). SKIL serves a dual role in the
development of breast cancer. Zhu et al (20) found that inhibition of SKIL
expression promoted the metastasis of breast cancer to the bone and
lung, while Band and Laiho (21)
showed that high expression of SKIL promoted the invasion and
metastasis of breast cancer. A recent study has demonstrated that
SKIL is regulated by transcription factor SOX2, and SKIL can
promote breast cancer proliferation, migration and EMT by
regulating the Hippo pathway (22). Therefore, the mechanism of SKIL in
breast cancer deserves further study.
Transcriptional co-activator with PDZ-binding motif
(TAZ), a classical effector protein of the Hippo pathway, mediates
different tissue growth. As an oncogene, unrestrained TAZ activity
is reported to counteract classical tumor suppressor checkpoints
(23). TAZ is usually highly
expressed in many cancer types, including breast cancer cells, and
as the final effector of the Hippo pathway, TAZ is an attractive
target in tumor treatment (24).
Connective tissue growth factor (CTGF), a well-known oncogene
involved in EMT and tumour metastasis, is a direct target gene of
TAZ (22). Zhu et al
(22) found that SKIL could
enhance the transcription and onco-genic activity of TAZ and that
SKIL-knockdown could lead to the downregulation of TAZ and its
target CTGF.
MicroRNAs (miRNAs) are a class of endogenous
non-coding small RNAs that were discovered in 1993; they are ~22
nucleotides in length and are encoded by endogenous genes (25,26).
miRNAs can be involved in numerous biological pathways, such as
embryonic development, cell proliferation, cell differentiation,
growth and apoptosis (27-29). miR-574 is a tumour suppressor
miRNA, and it has been reported that the long non-coding RNA
linc-ZNF469-3 can promote breast cancer by inhibiting miR-574
(30). However, there are still
few reports on the mechanism of miR-574 in breast cancer, and its
regulation of EMT remains unclear. Bioinformatics analysis
performed in the present study revealed that miR-574 can
simultaneously target BCL11A and SOX2. Therefore, the present study
hypothesized that miR-574-5p may inhibit the expression of SKIL by
targeting BCL11A and SOX2, thereby inhibiting the proliferation,
migration and EMT of TNBC.
In summary, the present study investigated the role
of miR-574-5p in TNBC by studying the regulatory relationship and
mechanism of miR-574-5p on the BCL11A/SOX2 axis. The current study
provides novel ideas to further the understanding of TNBC.
Materials and methods
Clinical samples and cell lines
A total of 45 pairs of BC and respective adjacent
normal tissues were obtained from 45 female patients (mean age,
55.4 years; age range, 24-76 years) who underwent surgery at the
Department of Breast Surgery, Xiangya Hospital, Central South
University (Changsha, China) between January 2017 and February
2018. Adjacent tissue samples were obtained within 3 cm of the edge
of the cancer tissue. The 45 paired BC samples were divided into
TNBC (n=16) and non-TNBC (n=29) based on immunohistochemistry
staining of estrogen receptor (ER), progesterone receptor (PR) and
human epidermal growth factor receptor (HER-2), and according to
the Chinese Society of Clinical Oncology Breast Cancer Diagnosis
and Treatment Guidelines 2018 (31).
No preoperative radiotherapy, chemotherapy or other
tumour treatments were performed prior to tumour resection.
Informed consent was signed by all patients, and this research was
approved by the Ethics Committee of Xiangya Hospital Affiliated to
Central South University (approval no. 201803377). MCF10A, MCF7,
T47D, MDA-MB-436, MDA-MB-231, MDA-MB-468, SK-BR-3 cell lines were
purchased from American Type Culture Collection and identified by
STR verification. The MDA‑MB‑436, MDA‑MB‑231 and MDA-MB-468 cells
were TNBC cell lines. The cells were cultured in DMEM (Invitrogen;
Thermo Fisher Scientific, Inc.) supplemented with 10% foetal bovine
serum (FBS; Invitrogen; Thermo Fisher Scientific, Inc.), 1%
streptomycin (Invitrogen; Thermo Fisher Scientific, Inc.) and 1%
penicillin (Invitrogen; Thermo Fisher Scientific, Inc.) in a 5%
CO2 atmosphere at 37˚C.
Immunohistochemistry
Tumor tissue samples were fixed with 10% formalin
for 24 h at 4˚C, embedded in paraffin and sectioned to a thickness
on 2 µm. The sections were then depa-raffinized and blocked with
10% goat serum (Gibco; Thermo Fisher Scientific, Inc.) in PBS for 1
h at 4˚C. The slides were incubated with anti-ER (1:100; Abcam;
cat. no. ab108398), anti-PR (1:100; Abcam; cat. no. ab32085) and
anti-HER-2 (1:100; Abcam; cat. no. ab32085) for 12 h at 4˚C.
Following washing with PBS, the sections were incubated with a goat
anti-rabbit HRP-conjugated secondary antibody (1:10,000; cat. no.
4414; Cell Signalling Technology, Inc.) for an additional 30 min at
25˚C. Following staining with hematoxylin for 5 min at 25˚C, the
slices were blocked with neutral gum at 25˚C for 24 h and images
were obtained using a light microscope (magnification, ×100).
Non‑specific primary antibody staining was assessed by substituting
the primary antibody with PBS, and at least 10 fields were randomly
selected from each section for evaluation. For scoring, ER negative
and PR negative were defined as <1% of cells with positive ER
and positive PR staining based on five independent fields.
HER‑2- or HER-2+ were considered to be
negative for HER-2. HER-2- was when the pathologist
observed no staining or membrane staining in <10% of the tumour
cells. HER-2+ was when faint or barely perceptible
membrane staining was detected in >10% of tumour cells. A sample
was considered HER-2++ when the pathologist observed a
weak-to-moderate complete membrane staining in >10% of the
tumour cells. HER-2++ samples were applied for
fluorescence in situ hybridization, and the cases with
amplification were defined as HER2-positive. The samples negative
for all three markers were defined as TNBC, whereas all other cases
were considered non-TNBC.
Fluorescence in situ hybridization
HER-2++ samples were applied for
fluorescence in situ hybridization. The
ZytoLight® SPEC ERBB2/CEN 17 Dual Color Probe (Zytomed,
Systems GmbH), which employs two DNA probes, was used to perform
the assay, according to the manufacturer's protocol. The following
probes were used: HER‑2(ERBB2) gene specific probe, ZyGreen
(excitation 503 nm/emission 528 nm)-labelled polynucleotides (~10.0
ng/µl), which target sequences mapping in 17q12-q21.1*
(chr17:37,572,531-38,181,308) harbouring the ERBB2 gene region; and
an α satellite probe targeting the centromere region of chromosome
17 (CEP17): ZyOrange (excitation 547 nm/emission 572 nm)-labelled
polynucleotides (~1.5 ng/µl), which target sequences mapping in
17p11.1-q11.1 specific for the alpha satellite centromeric region
D17Z1 of chromosome 17. In brief, specimens were incubated with
pre‑treatment solution (99˚C for 20 min) and then digested with
protease (37˚C for 10 min). The DNA probe was applied and
hybridized to each section overnight at 37˚C. The slides were then
washed, counterstained with DAPI, and observed by fluorescence
microscopy. In each of the specimens of papillary carcinoma, the
total numbers of HER2 and CEP17 signals were counted in 20 tumour
cell nuclei, and the ratios of HER2 signals to CEP17 signals were
calculated. Polysomy 17 was defined as the occurrence of a
centromere copy number of three or more for chromosome 17 per
cell.
Prediction targeting sequence
Possible miR-574-5p targets were predicted by
bioinformatics analysis with TargetScan (version 7.2; http://www.targetscan.org/vert_72/).
Plasmid construction and
transfection
Short hairpin RNA (shRNA)-negative control (NC),
shRNA-BCL11A (shBCL11A), NC mimic and miR-574-5p mimic (for
miR-574-5p overexpression) were purchased from Shanghai GenePharma
Co., Ltd. The target sequences of the shRNA are presented in
Table I. The miRNA mimics (2 µg,
100 nM) or shRNA (1 µg, 100 nM) were transfected into MDA-MB-468 or
MDA-MB-231 cells using Lipofectamine® 3000 (Invitrogen;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocol. For SOX2 and BCL11A overexpression, the pcDNA3.1 vector
(Shanghai GenePharma Co., Ltd.) was used to construct the
recombinant plasmids pcDNA3.1-SOX2 and pcDNA3.1-BCL11A,
respectively. The recombinant vectors (1 µg, 100 nM) were
transfected into MDA-MB-468 or MDA-MB-231 cells using
Lipofectamine® 3000, according to the manufacturer's
protocol. A total of 48 h after transfection, cells were used for
subsequent experiments.
 | Table IshRNA target and mimics
sequences. |
Table I
shRNA target and mimics
sequences.
| shRNA or mimic | Target sequence
(5′-3′) |
|---|
| shNC |
GTTCTCCGAACGTGTCACGT |
| BCL11A shRNA#1 |
GCCTCTTGAAGCCATTCTTAC |
| BCL11A shRNA#2 |
GGAACACATAGCAGATAAACT |
| BCL11A shRNA#3 |
GGATTTCTCTAGGAGACTTAG |
| miR-574 mimic
sense |
UGAGUGUGUGUGUGUGAGUGUGU |
| miR-574 mimic
antisense |
ACACUCACACACACACACUCAUU |
| Mimic NC sense |
UUCUCCGAACGUGUCACGUTT |
| Mimic NC
antisense |
ACGUGACACGUUCGGAGAATT |
MTT assay
The MTT experiment was applied to analyse cell
viability. MDA-MB-468 and MDA-MB-231 cells were seeded in 96-well
plates (5x104 cells/µl). Subsequently, 0.5% MTT
(Sigma-Aldrich; Merck KGaA) was added to each well and incubated
for 4 h. Then, DMSO (150 µl) was added to each well and incubated
for 10 min. The absorbance at 490 nm was detected using the Thermo
Fisher Multiskan FC plate reader (Thermo Fischer Scientific,
Inc.).
Colony formation assay
MDA-MB-468 and MDA-MB-231 cells in the logarithmic
growth phase were serially diluted and then inoculated separately
in culture dishes and cultured at 37˚C for 2 weeks. The cells were
fixed with a mixture of acetic acid and formic acid (at a ratio of
1:3) for 15 min at 25˚C, stained with Giemsa solution for 15 min at
25˚C and then rinsed slowly with running water. Colonies were
counted using a light microscope (magnification, x40). Five fields
were randomly selected and counted for each well.
Transwell assay
Cells were seeded into the upper chamber
(1.0x106 cells/chamber). RPMI-1640 medium (Gibco; Thermo
Fisher Scientific, Inc.) with 10% FBS was added to the lower
chamber. Cells were incubated in the chambers at 37˚C for 48 h. The
lower chamber was stained with 0.1% crystal violet 30 min at 25˚C,
and the cells were counted under a light microscope (magnification,
x100). Five fields were randomly selected and counted for each
well. For the cell migration assay, 24-well plates (8-µm pores;
Corning, Inc.) without coating were used. For the invasion assay,
the upper chambers were coated with Matrigel (1:8 dilution; BD
Biosciences).
Total RNA extraction and reverse
transcription‑quantitative PCR (RT‑qPCR)
Total RNA was extracted using TRIzol (Thermo Fisher
Scientific, Inc.). The PrimeScript RT Reagent kit with gDNA Eraser
(Takara Bio, Inc.) and the SYBR Green qPCR Master mix kit (Takara
Bio, Inc.) were used for RT (45 min at 50˚C) and qPCR,
respectively. The thermocycling conditions were as follows: 96˚C
for 6 min, followed by 40 cycles of 96˚C for 15 sec, 57˚C for 30
sec and 72˚C for 30 sec. GADPH was used as an internal control for
SOX2 and BCL11A. U6 was used as an internal control for
miRNA-574-5p. The 2-ΔΔCq method (32) was used to calculate the relative
expression level. The primer sequences are listed in Table II.
 | Table IIPrimers used for reverse
transcription-quantitative PCR. |
Table II
Primers used for reverse
transcription-quantitative PCR.
| Gene | Forward primer
(5′-3′) | Reverse primer
(5′-3′) |
|---|
| miR-574-5p |
GCCTGAGTGTGTGTGTGTGA |
GTGCAGGGTCCGAGGT |
| BCL11A |
ATGCGAGCTGTGCAACTATG |
CAACACTCGATCACTGTGCC |
| SOX2 |
CATGTCCCAGCACTACCAGA |
TTTGAGCGTACCGGGTTTTC |
| U6 |
CTCGCTTCGGCAGCACA |
AACGCTTCACGAATTTGCGT |
| GAPDH |
ACAAGATGGGCTTCATCCAC |
CTCCATGCTGTCCTGTCTGA |
Western blot analysis
Total protein of MDA-MB-468 or MDA-MB-231 cells was
extracted using RIPA buffer (Invitrogen; Thermo Fisher Scientific,
Inc.). The protein concentration was measured using a BCA Protein
assay kit (Takara Bio, Inc.). Protein (30 µg) was separated by 10%
SDS-PAGE at 40 V for 4-5 h and then transferred to a PVDF membrane
(Sigma-Aldrich; Merck KGaA). The PVDF membrane was stained with 1x
Ponceau S solution (Beijing Solarbio Science & Technology, Co.,
Ltd.) for 5 min. The membranes were then blocked with 5% non-fat
milk at room temperature for 1 h and incubated with the following
primary antibodies: Anti-SOX2 (1:1,000; cat. no. 3579), anti-BCL11A
(1:1,000; cat. no. 75432), anti-Slug (1:1,000; cat. no. 9585),
anti-N-cadherin (1:1,000; cat. no. 13116), anti-vimentin (1:1,000;
cat. no. 5741), anti-E-cadherin (1:1,000; cat. no. 14472),
anti-SKIL (1:1,000; cat. no. 4973), anti-TAZ (1:1,000; cat. no.
83669), anti-CTGF (1:1,000; cat. no. 86641) and anti‑GAPDH
(1:10,000; cat. no. 5174) at 4˚C overnight. All primary antibodies
were from Cell Signaling Technology, Inc. Then, the membranes were
washed with TBST buffer and incubated with goat anti-rabbit IgG
antibody tagged with horseradish peroxidase (1:10,000; cat. no.
4414; Cell Signaling Technology, Inc.) at room temperature for 2 h.
The membranes were scanned using an Odyssey Imaging system and
analysed with the Odyssey v2.0 software (LI-COR Biosciences).
Luciferase reporter assay
The binding elements of miRNA-574-5p in BCL11A and
SOX2 were predicted by Starbase (http://starbase.sysu.edu.cn). The wild-type (WT)
BCL11A, mutant (Mut) BCL11A, WT SOX2 and Mut SOX2 were synthesized
and inserted into the pGL3 vector (YouBio) to construct
pGL3-BCL11A-WT, pGL3-BCL11A-Mut, pGL3-SOX2-WT and pGL3-SOX2-Mut,
respectively, which were obtained from Shanghai GenePharma Co.,
Ltd. Then, the recombinant plasmids were transfected into cells
with NC mimic or miR-574-5p mimics using Lipofectamine 3000
(Invitrogen; Thermo Fisher Scientific, Inc.). A Dual-Luciferase™
Reporter (DLR™) assay system (Promega Corporation) was applied for
luciferase activity analysis. The activity of normalized reporter
was expressed as the firefly luciferase value divided by the
renilla luciferase value, and normalized to the control
vector activity.
Animal experiments
This research was approved by the Ethics Committee
of Xiangya Hospital affiliated to Central South University
(approval no. 201803245). In total, 20 male BALB/c nude mice (4-6
weeks old) were obtained from SLAC Laboratory Animal Co. Ltd,
(Hunan, China). Mice were raised in pathogen‑free conditions at
20‑25˚C, 40‑70% humidity, 15 times/h ventilation and a 12 h
light/12 h dark cycle, with access to food and water ad
libitum. For the xenograft assay, the mice were transplanted
subcutaneously with MDA-MB-231 cells (transfected with NC mimic or
miR-574-5p mimics) into the right flank and then housed until the
tumour volume reached ~200 mm3. Tumour size was measured
every 5 days using the following formula: Length x
width2 x 0.5. On the 30th day, the mice were sacrificed
and the tumour tissue was photographed. For the in vivo
metastasis assay, 5x105 MDA-MB-231 cells (transfected
with NC mimic or miR-574-5p mimics) were injected into the tail
veins of nine mice. On the 30th day, the mice were sacrificed and
the lung tissues were photographed. The number of pulmonary nodules
was counted.
Haematoxylin-eosin staining was used for pulmonary
migration analysis. Tissues were fixed by 4% formalin at 25˚C for
24 h, embedded in paraffin and cut into 5‑µm slices. The slices
were stained with hematoxylin (Beyotime Institute of Biotechnology)
for 5 min at 25˚C and eosin (Beyotime Institute of Biotechnology)
for 3 min at 25˚C. The slices were then blocked with neutral gum at
25˚C for 24 h and imaged using a light microscope (magnification,
x100).
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 6.0 software (GraphPad Software, Inc.). Three independent
experiments were performed, and data are expressed as the mean ±
standard deviation. Student's t-test was performed for comparisons
between two groups, while one-way ANOVA followed by Tukey's
post-hoc test was performed for multiple groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Expression of miR‑574‑5p in BC tissues
and cells
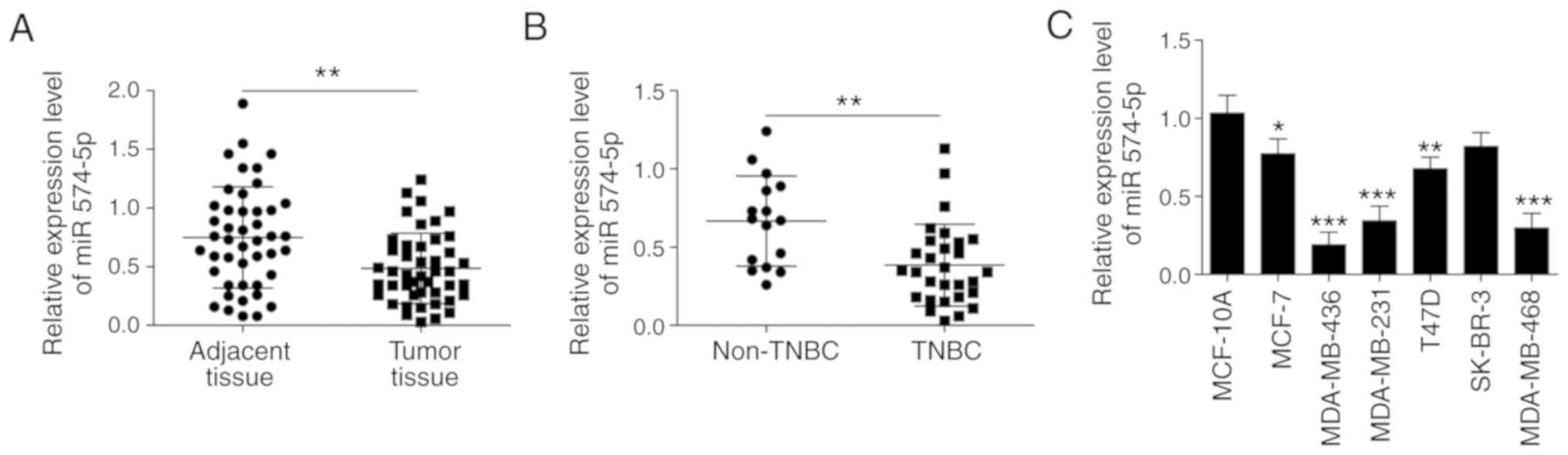
The RT-qPCR results revealed that the expression
level of miR-574-5p was significantly lower in tumour tissue
compared with in the adjacent tissue of 45 patients (Fig. 1A). Subsequently, miR-574-5p
expression levels in TNBC (n=29) and non-TNBC (n=16) were compared.
As presented in Fig. 1B,
miR‑574‑5p expression level in TNBC was significantly lower
compared with that in non-TNBC. Furthermore, the expression of
miR-574-5p was lower in both TNBC and non-TNBC cell lines
(MDA-MB-436, MDA-MB-231, MDA-MB-468) compared with that in the
normal breast epithelial cell line MCF10A (Fig. 1C). In summary, the expression of
miR-574-5p was shown to be lower in both BC tissues and cells,
particularly in TNBC tissue.
miR‑574‑5p inhibits proliferation,
migration and EMT in TNBC cells
To explore the effects of miR-574-5p on TNBC
progression, miR-574-5p was overexpressed in TNBC cells via
transfection with miR-574-5p mimics or NC mimic. Compared with the
NC mimic group, miR-574-5p expression was significantly increased
in the miR‑575‑5p mimic‑transfected group (Fig. 2A). The MTT assay revealed that cell
viability was significantly suppressed in cells that overexpressed
miR-574-5p (Fig. 2B and C). A
colony formation assay was performed to detect the effect of
miR-574-5p overexpression on colony formation. The results
demonstrated that the colony formation ability of
miR-574-5p-overexpressing cells was significantly inhibited
compared with that of cells transfected with NC mimic (Fig. 2D). In addition, Transwell assays
showed that miR‑574‑5p overexpression significantly inhibited cell
migration and invasion (Fig. 2E and
F). The effects of miR-574-5p on the expression of EMT-related
proteins, E-cadherin, vimentin, Slug and N-cadherin, were analysed
by western blotting. Overexpression of miR‑574‑5p significantly
inhibited the expression of Slug, N-cadherin and vimentin, and
significantly increased the expression of E‑cadherin (Fig. 2G). Taken together, these findings
suggested that miR-574-5p inhibits proliferation, migration and EMT
in TNBC cells.
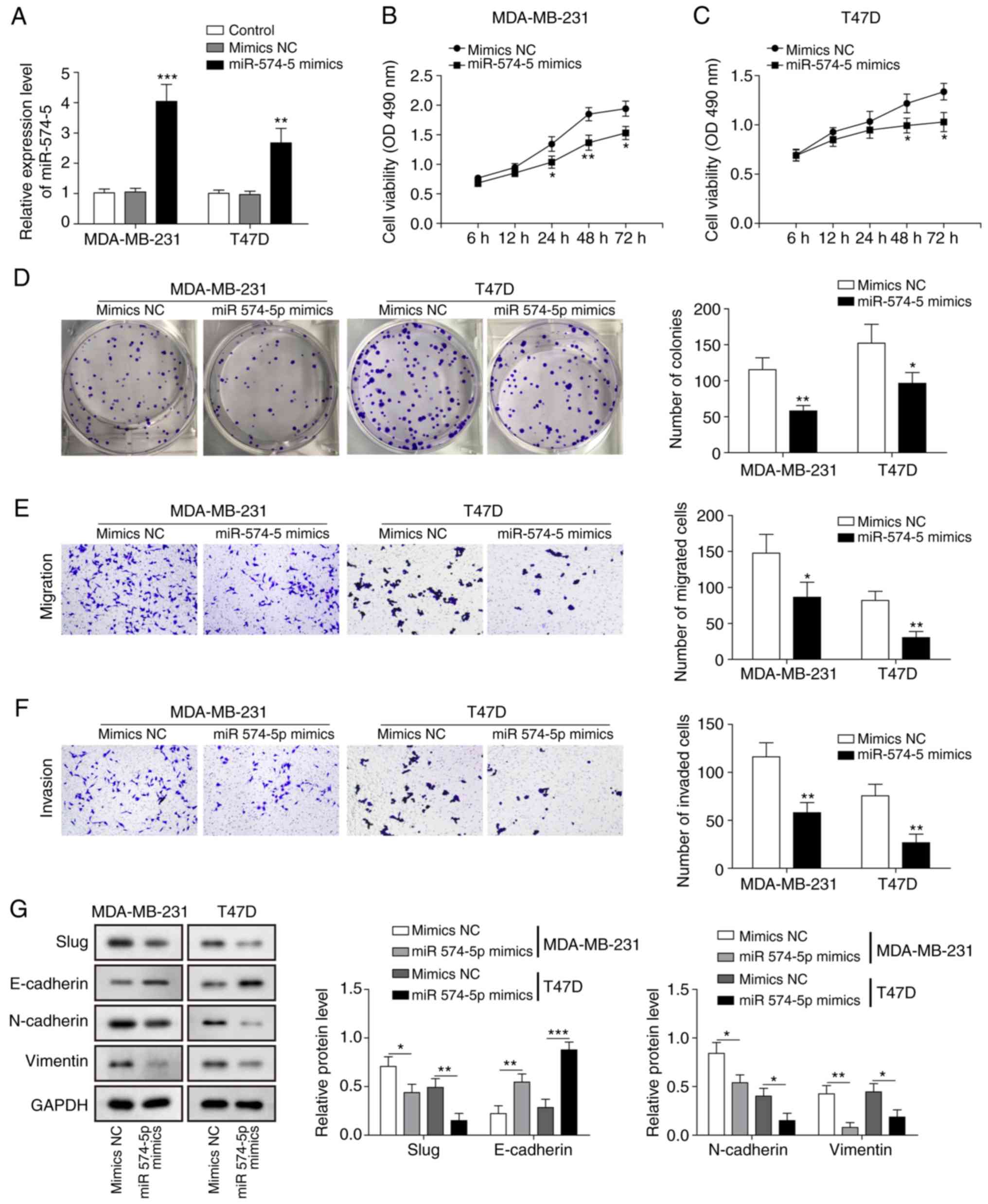 | Figure 2miR-574-5p inhibits the
proliferation, migration and EMT of TNBC cells. (A) Reverse
transcription-quantitative PCR was used to assess the relative
miR-574-5p expression in MDA-MB-231 and MDA-MB-468 cells
transfected with miR-574-5p mimics or NC mimic. MTT assay was
performed to measure cell viability in (B) MDA-MB-231 and (C)
MDA-MB-468 cells transfected with miR-574-5p mimics or NC mimic at
6, 12, 24, 48 and 72 h. (D) Cell colony formation ability in
MDA-MB-231 and MDA-MB-468 cells transfected with miR-574-5p mimics
or NC mimic was analysed by a colony formation assay. (E) Cell
migration and (F) invasion ability in MDA-MB-231 and MDA-MB-468
cells transfected with miR-574-5p mimics or NC mimic was detected
by Transwell experiments. Magnification, x100. (G) The effects of
miR‑574‑5p on EMT‑related proteins, E‑cadherin, vimentin, Slug and
N‑cadherin, in MDA-MB-231 and MDA-MB-468 cells transfected with
miR-574-5p mimics or NC mimic were analysed by western blotting.
Data are presented as the mean ± standard deviation from at least
three independent experiments. *P<0.05,
**P<0.01, ***P<0.001 vs. mimics NC.
miR-574-5p, microRNA-574-5p; TNBC, triple-negative breast cancer;
NC, negative control; EMT, epithelial-mesenchymal transition; OD,
optical density. |
miR‑574‑5p targets BCL11A and SOX2 to
inhibit the SKIL/TAZ/CTGF axis
To explore the mechanism by which miR-574 regulates
the malignant phenotype, the present study examined the proteins
involved in the SKIL pathway. The results of the western blot
analysis revealed that overexpression of miR‑574‑5p significantly
decreased the expression of SKIL, TAZ and CTGF (Fig. 3A). Next, how miR-574 regulates SKIL
was examined. The effect of miR-574-5p overexpression on BCL11A and
SOX2 expression levels was investigated, which have been reported
to regulate SKIL transcription (22). The data demonstrated that the
expression levels of BCL11A and SOX2 in cells transfected with
miR-574-5p mimics were significantly lower compared that in control
cells (Fig. 3B). Additionally, the
relative mRNA expression levels of BCL11A and SOX2 in cells
transfected with miR-574-5p mimics were significantly lower
compared with in the NC mimic group (Fig. 3C and D). Bioinformatics prediction
revealed that miR-574-5p targets the 3′-untranslated regions
(3′-UTRs) of BCL11A and SOX2 (Fig.
3E). Subsequently, a luciferase assay was used to verify that
miR-574-5p binds directly to the 3′-UTRs of BCL11A and SOX2. In the
WT group, miR-574-5p overexpression significantly inhibited the
luciferase activity of the reporter gene containing the WT BCL11A
and SOX2 3′-UTR fragments, whereas for the Mut group, there was no
significantly difference in luciferase activity between the NC
mimic and miR-574-5p mimic groups, demonstrating that miR-574-5p
binds directly to the predicted sequences of BCL11A and SOX2
(Fig. 3F and G). These combined
data suggested that miR-574-5p targets BCL11A and SOX2, which may
lead to miR-574-5p-mediated inhibition of the SKIL/TAZ/CTGF
axis.
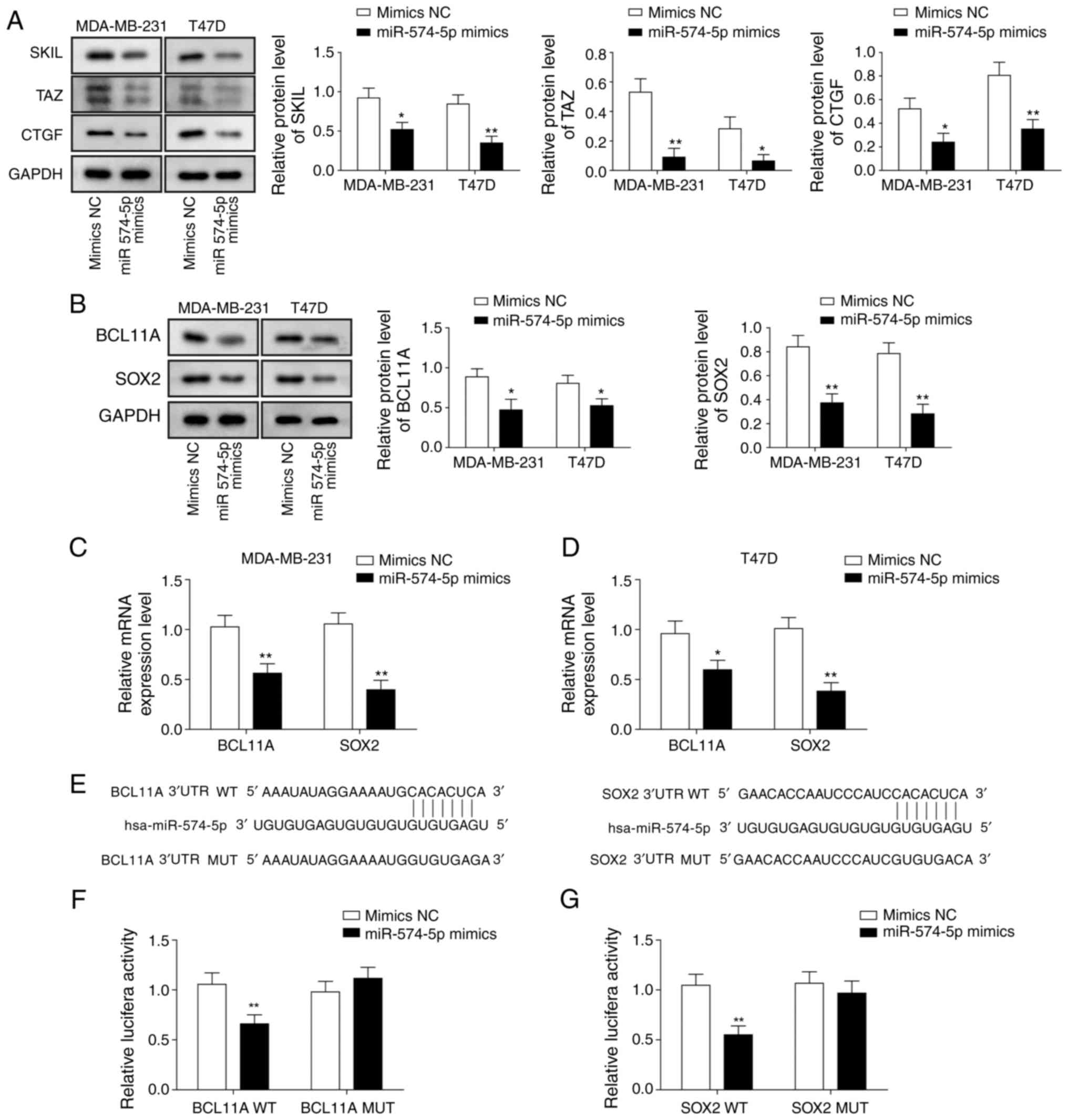 | Figure 3miR-574-5p targets BCL11A and SOX2 to
inhibit the SKIL/TAZ/CTGF axis. Western blotting was used to assess
the levels of (A) SKIL, TAZ, CTGF, (B) BCL11A and SOX2 in
MDA-MB-231 and MDA-MB-468 cells transfected with miR-574-5p mimics
or NC mimic. Reverse transcription-quantitative PCR was applied to
evaluate the relative mRNA expression of BCL11A and SOX2 in (C)
MDA-MB-231 and (D) MDA-MB-468 cells transfected with miR-574-5p
mimics or NC mimic. (E) Binding sites of WT BCL11A and SOX2 3′-UTR
to miR-574-5p and the mutation sites of BCL11A and SOX2. A
luciferase assay was used to demonstrate that miR-574-5p directly
targets the 3′-UTRs of (F) BCL11A and (G) SOX2. The luciferase
activity of NC mimic-transfected cells was set to 1. Data are
presented as the mean ± standard deviation from at least three
independent experiments. *P<0.05,
**P<0.01 vs. mimics NC. miR-574-5p, microRNA-574-5p;
SOX2, SRY (sex determining region Y)-box 2; BCL11A, B-cell
lymphoma/leukaemia 11A; SKIL, SKI like proto-oncogene; TAZ,
transcriptional co-activator with PDZ-binding motif; CTGF,
connective tissue growth factor; NC, negative control; WT,
wild-type; 3′-UTR, 3′-untranslated region; MUT, mutated. |
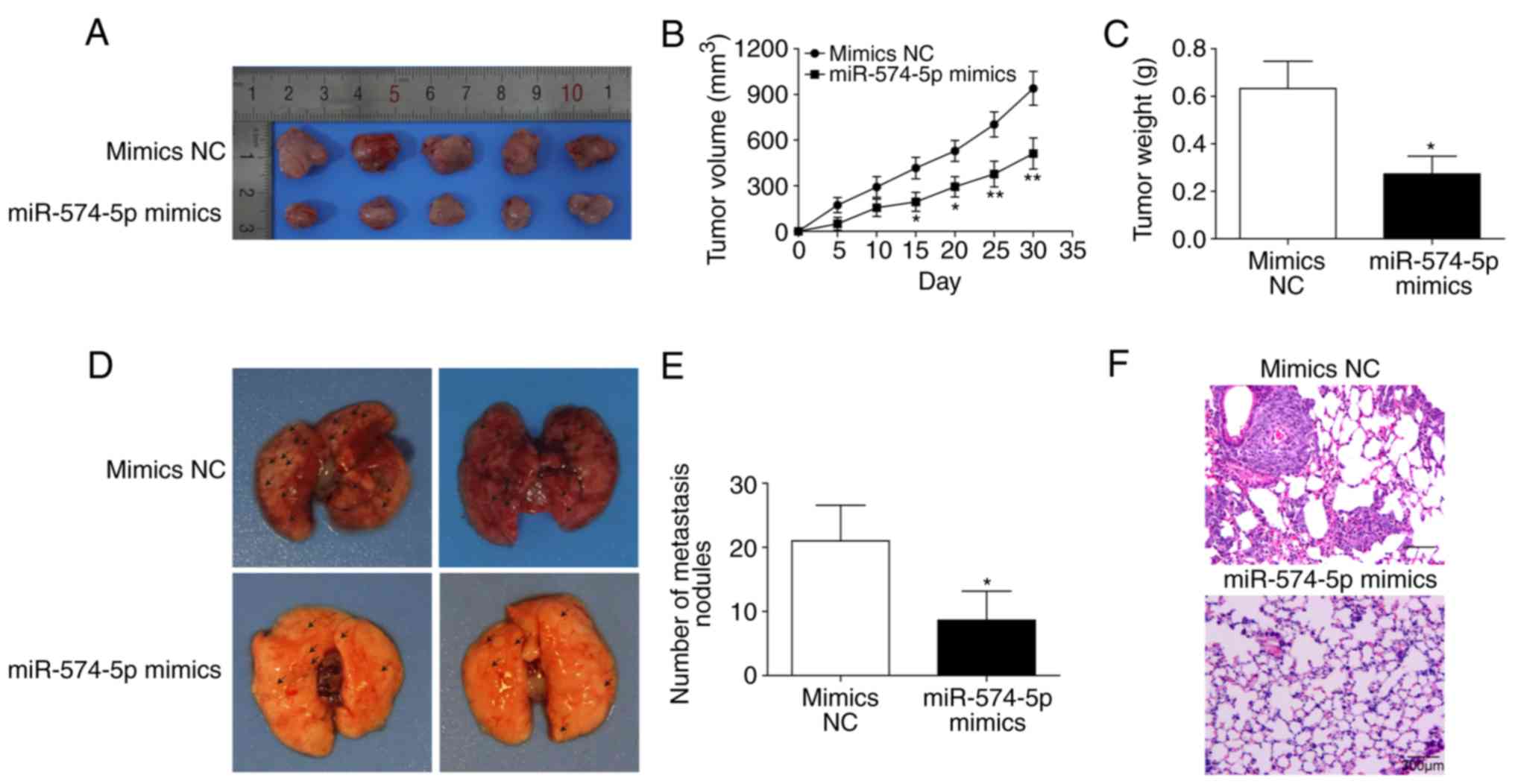
miR‑574‑5p inhibits tumorigenesis and
lung metastasis in vivo
As presented in Fig.
4A-C, miR-574-5p overexpres-sion significantly inhibited tumour
size and weight in vivo. Additionally, miR-574-5p
overexpression inhibited pulmonary migration and significantly
reduced the number of metastatic nodules (Fig. 4D-F). Hence, it can been suggested
that miR-574-5p may inhibit tumorigenesis and lung metastasis in
vivo.
Inhibition of proliferation, migration
and EMT by miR‑574‑5p in TNBC cells is at least partly dependent on
targeting SOX2 and BCL11A
Co-overexpression of miR-574-5p and SOX2 or BCL11A
was performed by co-transfection with miR-574-5p mimics and
pcDNA3.1-SOX2 or pcDNA3.1-BCL11A in MDA-MB-231 and MDA-MB-468
cells. Transfection efficiency is presented in Fig. S1A and B. Western blot analysis
revealed that co-overexpression of miR-574-5p and SOX2 or BCL11A
significantly reversed the inhibition of SKIL by miR-574-5p
(Fig. 5A and B). Overexpression of
SOX2 restored both BCL11A and SOX2 expression (Fig. 5A and B). However, BCL11A
overexpression only restored its own expression and had no effect
on the expression of SOX2 (Fig. 5A and
B). Co-overexpression of miR-574-5p and SOX2 or BCL11A restored
the inhibition of cell viability by miR-574-5p (Fig. 5C and D). miR-574-5p overexpression
inhibited cell migration ability, while overexpression of
miR-574-5p and SOX2 or BCL11A restored migration (Fig. 5E). Furthermore, western blot
analysis demonstrated that co-overexpression of miR-574-5p and SOX2
or BCL11A reversed the inhibition of vimentin, Slug, and N-cadherin
expression and the promotion of E-cadherin expression by miR-574-5p
(Fig. 5F and G). In summary, the
inhibition of proliferation, migration and EMT by miR-574-5p in
TNBC cells was at least partly dependent on targeting SOX2 and
BCL11A.
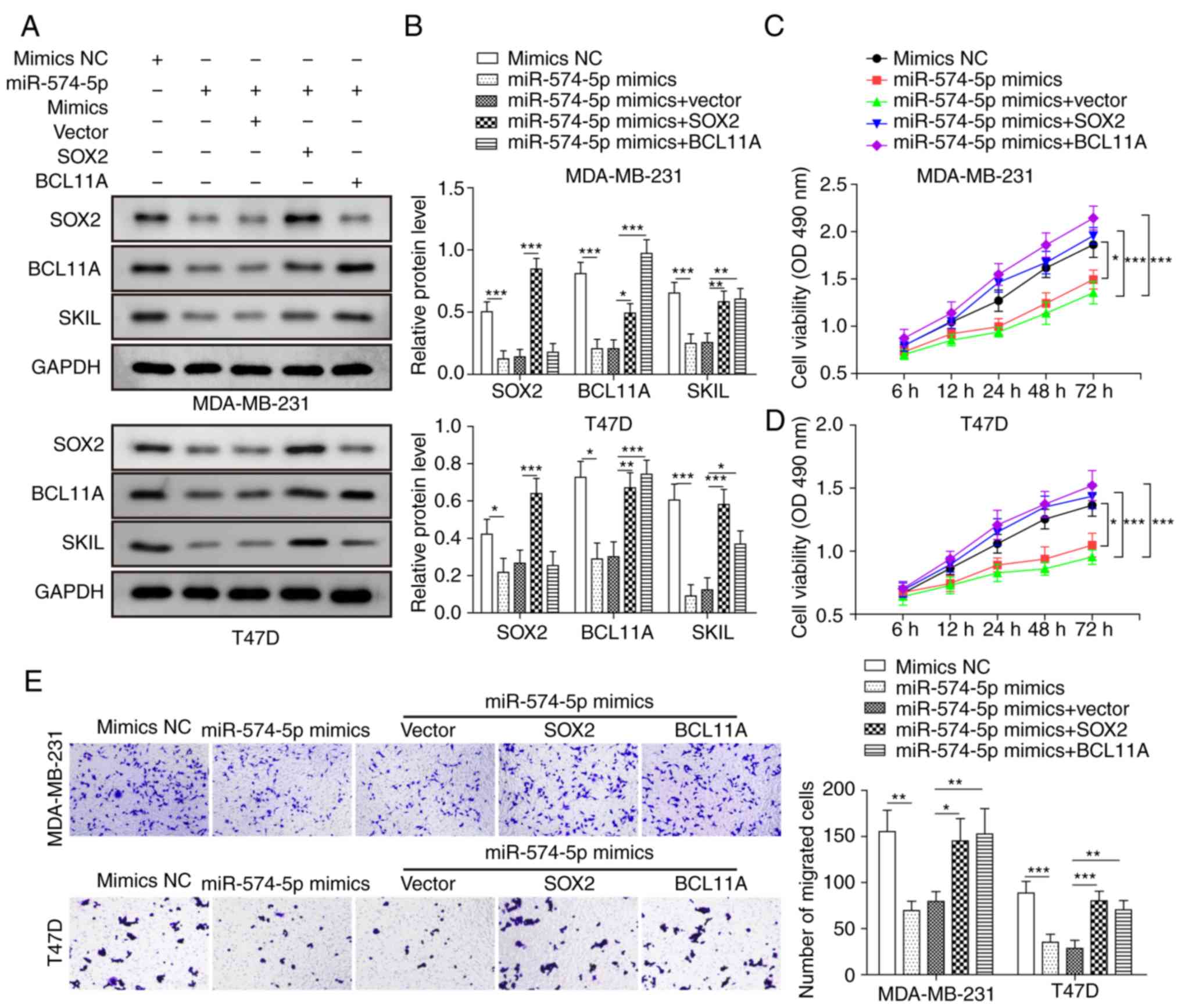 | Figure 5Inhibition of proliferation,
migration and EMT by miR-574-5p in TNBC cells is dependent on SOX2
and BCL11A. MDA-MB-231 and MDA-MB-468 cells were divided into five
groups: NC mimic, miR‑574‑5p mimic, miR‑574‑5p mimic + vector,
miR‑574‑5p mimic + SOX2 and miR‑574‑5p mimic + BCL11A. (A and B)
Western blotting was used to measure the expression of SOX2, BCL11A
and SKIL. (C and D) The MTT assay was performed to assess cell
viability at 6, 12, 24, 48 and 72 h. (E) Transwell assay was used
to analyse migration ability. Magnification, x100. (F and G)
Western blotting was applied to measure the expression levels of
E-cadherin, vimentin, Slug and N-cadherin. Data are presented as
the mean ± SD for three independent experiments.
*P<0.05, **P<0.01,
***P<0.001. miR-574-5p, microRNA-574-5p; EMT,
epithelial-mesenchymal transition; OD, optical density; SOX2, SRY
(sex determining region Y)-box 2; BCL11A, B-cell lymphoma/leukaemia
11A; SKIL, SKI like proto-oncogene; NC, negative control. |
SOX2‑mediated regulation of downstream
oncogenes depends on BCL11A
As presented in Figs.
6A and S1C and D, the
knockdown efficiency of BCL11A shRNAs was examined and shRNA#3 was
selected for subsequent experiments as it demonstrated the best
knockdown efficiency in MDA‑MB‑231 cells. To further investigate
the underlying interactive regulation of SOX2 and BCL11A,
pcDNA3.1-SOX2 and shBCL11A were co-transfected into MDA-MB-231 and
MDA-MB-468 cells. Western blot assays indicated that transfection
with pcDNA3.1‑SOX2 significantly upregulated the expression of
SOX2, SKIL and BCL11A. BCL11A knockdown reversed this effect but
had no effect on SOX2 expression (Fig.
6B and C). SOX2 overexpression significantly promoted cell
viability and migration, while silencing BCL11A reversed this
effect (Fig. 6D‑F). Moreover,
overexpression of SOX2 significantly increased the expression of
Slug, vimentin and N-cadherin, and decreased the expression of
E-cadherin, while BCL11A knockdown reversed this effect (Fig. 6G and H). Collectively, these
results demonstrated that the oncogenic effect of SOX2 is partly
dependent on BCL11A.
 | Figure 6SOX2-mediated regulation of
downstream oncogenes depends on BCL11A. MDA-MB-231 and MDA-MB-468
cells were divided into four groups: Vector, pcDNA3.1-SOX2,
pcDNA3.1-SOX2 + shNC and pcDNA3.1-SOX2 + shBCL11A. (A)
BCL11A-knockdown was performed with different shRNAs. (B and C)
Western blotting was used to detect SOX2, SKIL and BCL11A levels.
(D and E) The MTT assay was applied to analyse cell viability at 6,
12, 24, 48 and 72 h. (F) Cell migration ability was analysed by
Transwell assay. Magnification, x100. (G and H) Slug, vimentin,
N‑cadherin and E‑cadherin levels were measured by western blotting.
Data are presented as the mean ± standard deviation for three
independent experiments. *P<0.05,
**P<0.01, ***P<0.001. SOX2, SRY (sex
determining region Y)-box 2; BCL11A, B-cell lymphoma/leukaemia 11A;
SKIL, SKI like proto-oncogene; NC, negative control; sh, short
hairpin; OD, optical density. |
Discussion
TNBC is a subtype of breast cancer with poor
prognosis that lacks specific markers for effective therapeutic
targets (5). miRNAs are a class of
short-chain non-coding RNAs that normally bind to the 3′-UTR of a
target gene mRNA in a complementary manner to downregulate the
expression of the target gene (33). Previous studies have confirmed the
abnormal expression of multiple miRNAs in TNBC that are closely
associated with the occurrence and development of TNBC (34,35).
Different miRNAs and their regulated target genes play different
roles in the development and progression of TNBC, but the specific
molecular mechanisms still require further study. The present study
found that miR-574-5p was lower in TNBC tissues and cells.
miR-574-5p inhibited the proliferation, migration and EMT of TNBC
cells, and this was at least partly dependent on targeting SOX2 and
BCL11A, which may involve the SKIL/TAZ/CTGF axis.
A number of studies have shown that miR-574-5p is
involved in a variety of biological processes and has a wide range
of effects (36-38). Wang et al (30) demonstrated that the long non-coding
RNA linc-ZNF469-3 can promote breast cancer by inhibiting miR-574.
However, to the best of our knowledge, the more detailed mechanism
by which miR-574-5p regulates breast cancer progression remains
unclear. The present study revealed a further mechanism of
miR-574-5p in TNBC, which was mediated by inhibition of the
SOX2-induced SKIL/TAZ/CTGF axis. On the other hand, it was
identified that miR‑574‑5p exerted a suppressive effect on TNBC by
simultaneously targeting both SOX2 and BCL11A, suggesting that
miRNAs can maximize their function by regulating multiple targets
in the same pathway. Future work needs to focus on whether
miR-574-5p targets other genes in this pathway.
BCL11A is a member of the B cell lymphoma/leukaemia
family and is reported as an oncogene in TNBC (39,40).
Khaled et al (39) reported
that high expression of BCL11A can promote the colony formation of
mammary epithelial cells and stimulate the formation of tumours.
The present study found that BCL11A mediated regulation of SOX2,
which transcriptionally regulates the SKIL gene, thereby
facilitating a malignant phenotype. SKIL is a cancer-promoting
regulator mediated by SOX2 (9).
SKIL can upregulate CTGF through the TAZ pathway, and CTGF is an
important molecule that regulates cell migration and EMT (22). The present study identified that
miR‑574‑5p inhibits the SKIL/TAZ/CTGF axis by inhibiting SOX2 and
BCL11A and finally inhibits the proliferation, migration and EMT of
TNBC. In addition, the present study also found that SOX2
overexpression could upregulate the expression of BCL11A, while the
overexpression of BCL11A had no such effect on SOX2. This is
consistent with the findings of Lazarus et al (9), which the present study has confirmed
again in TNBC. SOX2 is an important oncogene for maintaining the
embryonic development and multipotential differentiation of stem
cells, and its expression level is related to the stem cell
characteristics of various malignant cells (11,41).
The present study found that miR-574-5p targeted the expression of
SOX2. Therefore, it can be hypothesized that miR-574-5p may also be
able to regulate tumour cell stemness by targeting SOX2; however,
this requires further experimental validation. In addition, it was
recognized that SOX2 may promote TNBC independent of SKIL; hence, a
definite conclusion that the miR-574-5p/SOX2/BCL11A axis regulates
the malignant phenotype of TNBC via SKIL/TAZ/CTGF cannot be
made.
In conclusion, the present study identified the
novel miR-574-5p/SOX2/BCL11A signalling axis in TNBC. It was
demonstrated that miR‐574‑5p inhibited the proliferation, migration
and EMT of TNBC, and this was at least partly dependent on directly
targeting BCL11A and SOX2, which may therefore regulate the
downstream SKIL/TAZ/CTGF axis. To the best of our knowledge, this
is the first study to report the underlying mechanism between the
miR-574-5p/BCL11A/SOX2 axis and the tumorigenesis of TNBC, which
provides new insight into the progression of TNBC.
Supplementary Data
Funding
This study was supported by the Science and
Technology Project of Hunan Province (grant no. 2015JC3016) and the
Scientific Research Project of Hunan Provincial Health and Family
Planning Commission (grant no. B2016106).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KJZ designed the study, performed the literature
research, performed experimental studies, acquired the data,
analysed the data, and prepared, edited and reviewed the
manuscript. LG designed the study, performed the statistical
analysis, reviewed the manuscript and is responsible for the
integrity of the study. JQY performed the clinical and experimental
studies. YH and XL performed the statistical analysis. NL edited
the manuscript and performed the statistical analysis. FYC acquired
the data of the clinical study and performed the statistical
analysis of clinical study. All authors read and approved the
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Xiangya Hospital Affiliated to Central South
University (approval no. 201803377). Written informed consent was
obtained from the participants. The animal study was approved by
the Ethics Committee of Xiangya Hospital Affiliated to Central
South University (approval no. 201803245).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wu Y, Zhang Z, Cenciarini ME, Proietti CJ,
Amasino M, Hong T, Yang M, Liao Y, Chiang HC, Kaklamani VG, et al:
Tamoxifen resistance in breast cancer is regulated by the
EZH2-ERα-GREB1 transcriptional axis. Cancer Res. 78:671–684. 2018.
View Article : Google Scholar
|
|
3
|
Kumar P and Aggarwal R: An overview of
triple-negative breast cancer. Arch Gynecol Obstet. 293:247–269.
2016. View Article : Google Scholar
|
|
4
|
Liu F, Zhuang L, Wu R and Li D: miR-365
inhibits cell invasion and migration of triple negative breast
cancer through ADAM10. J BUON. 24:1905–1912. 2019.PubMed/NCBI
|
|
5
|
Denkert C, Liedtke C, Tutt A and von
Minckwitz G: Molecular alterations in triple-negative breast
cancer-the road to new treatment strategies. Lancet. 389:2430–2442.
2017. View Article : Google Scholar
|
|
6
|
Yu Y, Wang J, Khaled W, Burke S, Li P,
Chen X, Yang W, Jenkins NA, Copeland NG, Zhang S and Liu P: Bcl11a
is essential for lymphoid development and negatively regulates p53.
J Exp Med. 209:2467–2483. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nakamura T, Yamazaki Y, Saiki Y, Moriyama
M, Largaespada DA, Jenkins NA and Copeland NG: Evi9 encodes a novel
zinc finger protein that physically interacts with BCL6, a known
human B-cell proto-oncogene product. Mol Cell Biol. 20:3178–3186.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen F, Luo N, Hu Y, Li X and Zhang K:
MiR-137 suppresses triple-negative breast cancer stemness and
tumorigenesis by perturbing BCL11A-DNMT1 interaction. Cell Physiol
Biochem. 47:2147–2158. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lazarus KA, Hadi F, Zambon E, Bach K,
Santolla MF, Watson JK, Correia LL, Das M, Ugur R, Pensa S, et al:
BCL11A interacts with SOX2 to control the expression of epigenetic
regulators in lung squamous carcinoma. Nat Commun. 9:33272018.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Boumahdi S, Driessens G, Lapouge G, Rorive
S, Nassar D, Le Mercier M, Delatte B, Caauwe A, Lenglez S, Nkusi E,
et al: SOX2 controls tumour initiation and cancer stem-cell
functions in squamous-cell carcinoma. Nature. 511:246–250. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mamun MA, Mannoor K, Cao J, Qadri F and
Song X: SOX2 in cancer stemness: Tumor malignancy and therapeutic
potentials. J Mol Cell Biol. Dec 5–2018.Epub ahead of print.
PubMed/NCBI
|
|
12
|
Tong B, Zeng J, Wu Y and Xiong W: Enhanced
SOX2 expression in retinoblastoma tissues and peripheral blood is
associated with the clinicopathological characteristics of the
disease. Oncol Lett. 9:1244–1248. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nomura N, Sasamoto S, Ishii S, Date T,
Matsui M and Ishizaki R: Isolation of human cDNA clones of ski and
the ski-related gene, sno. Nucleic Acids Res. 17:5489–5500. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jahchan NS and Luo K: SnoN in mammalian
development, function and diseases. Curr Opin Pharmacol.
10:670–675. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shinozuka E, Miyashita M, Mizuguchi Y,
Akagi I, Kikuchi K, Makino H, Matsutani T, Hagiwara N, Nomura T,
Uchida E and Takizawa T: SnoN/SKIL modulates proliferation through
control of hsa-miR-720 transcription in esophageal cancer cells.
Biochem Biophys Res Commun. 430:101–106. 2013. View Article : Google Scholar
|
|
16
|
Kodigepalli KM, Anur P, Spellman P, Sims
PJ and Nanjundan M: Phospholipid Scramblase 1, an
interferon-regulated gene located at 3q23, is regulated by
SnoN/SkiL in ovarian cancer cells. Mol Cancer. 12:322013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Javelaud D, van Kempen L, Alexaki VI, Le
Scolan E, Luo K and Mauviel A: Efficient TGF‑β/SMAD signaling in
human melanoma cells associated with high c-SKI/SnoN expression.
Mol Cancer. 10:22011. View Article : Google Scholar
|
|
18
|
Makino Y, Yoon JH, Bae E, Kato M, Miyazawa
K, Ohira T, Ikeda N, Kuroda M and Mamura M: Repression of Smad3 by
Stat3 and c‑Ski/SnoN induces gefitinib resistance in lung
adeno-carcinoma. Biochem Biophys Res Commun. 484:269–277. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sengupta S, Jana S, Biswas S, Mandal PK
and Bhattacharyya A: Cooperative involvement of NFAT and SnoN
mediates transforming growth factor-β (TGF-β) induced EMT in
metastatic breast cancer (MDA-MB 231) cells. Clin Exp Metastasis.
30:1019–1031. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhu Q, Krakowski AR, Dunham EE, Wang L,
Bandyopadhyay A, Berdeaux R, Martin GS, Sun L and Luo K: Dual role
of SnoN in mammalian tumorigenesis. Mol Cell Biol. 27:324–339.
2007. View Article : Google Scholar :
|
|
21
|
Band AM and Laiho M: SnoN oncoprotein
enhances estrogen receptor-α transcriptional activity. Cell Signal.
24:922–930. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhu Q, Le Scolan E, Jahchan N, Ji X, Xu A
and Luo K: SnoN antagonizes the hippo kinase complex to promote TAZ
signaling during breast carcinogenesis. Dev Cell. 37:399–412. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Janse van Rensburg HJ, Azad T, Ling M, Hao
Y, Snetsinger B, Khanal P, Minassian LM, Graham CH, Rauh MJ and
Yang X: The hippo pathway component TAZ promotes immune evasion in
human cancer through PD-L1. Cancer Res. 78:1457–1470. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Harvey KF, Zhang X and Thomas DM: The
Hippo pathway and human cancer. Nat Rev Cancer. 13:246–257. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Krol J, Loedige I and Filipowicz W: The
widespread regulation of microRNA biogenesis, function and decay.
Nat Rev Genet. 11:597–610. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tian Y, Fu X, Li Q, Wang Y, Fan D, Zhou Q,
Kuang W and Shen L: MicroRNA181 serves an oncogenic role in breast
cancer via the inhibition of SPRY4. Mol Med Rep. 18:5603–5613.
2018.PubMed/NCBI
|
|
27
|
Krutzfeldt J: Strategies to use microRNAs
as therapeutic targets. Best Pract Res Clin Endocrinol Metab.
30:551–561. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Peng G, Liao Y and Shen C: miRNA-429
inhibits astrocytoma proliferation and invasion by targeting BMI1.
Pathol Oncol Res. 23:369–376. 2017. View Article : Google Scholar
|
|
29
|
Deng Z, Wang Y, Fang X, Yan F, Pan H, Gu
L, Xie C, Li Y, Hu Y, Cao Y and Tang Z: Research on miRNA-195 and
target gene CDK6 in oral verrucous carcinoma. Cancer Gene Ther.
24:282–288. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang PS, Chou CH, Lin CH, Yao YC, Cheng
HC, Li HY, Chuang YC, Yang CN, Ger LP, Chen YC, et al: A novel long
non-coding RNA linc-ZNF469-3 promotes lung metastasis through
miR-574-5p-ZEB1 axis in triple negative breast cancer. Oncogene.
37:4662–4678. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huang X and Yin YM: Updates of chinese
society of clinical oncology (CSCO) guideline for breast cancer in
2018. Zhonghua Yi Xue Za Zhi. 98:1213–1217. 2018.In Chinese.
PubMed/NCBI
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
33
|
Shah MY and Calin GA: MicroRNAs as
therapeutic targets in human cancers. Wiley Interdiscip Rev RNA.
5:537–548. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
D'Ippolito E and Iorio MV: MicroRNAs and
triple negative breast cancer. Int J Mol Sci. 14:22202–22220. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chang YY, Kuo WH, Hung JH, Lee CY, Lee YH,
Chang YC, Lin WC, Shen CY, Huang CS, Hsieh FJ, et al: Deregulated
microRNAs in triple-negative breast cancer revealed by deep
sequencing. Mol Cancer. 14:362015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang X, Lu X, Geng Z, Yang G and Shi Y:
LncRNA PTCSC3/miR-574-5p governs cell proliferation and migration
of papillary thyroid carcinoma via wnt/β-catenin signaling. J Cell
Biochem. 118:4745–4752. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lai Z, Lin P, Weng X, Su J, Chen Y, He Y,
Wu G, Wang J, Yu Y and Zhang L: MicroRNA-574-5p promotes cell
growth of vascular smooth muscle cells in the progression of
coronary artery disease. Biomed Pharmacother. 97:162–167. 2018.
View Article : Google Scholar
|
|
38
|
Ku T, Li B, Gao R, Zhang Y, Yan W, Ji X,
Li G and Sang N: NF-κB-regulated microRNA-574-5p underlies synaptic
and cognitive impairment in response to atmospheric PM2.5
aspiration. Part Fibre Toxicol. 14:342017. View Article : Google Scholar
|
|
39
|
Khaled WT, Choon Lee S, Stingl J, Chen X,
Raza Ali H, Rueda OM, Hadi F, Wang J, Yu Y, Chin SF, et al: BCL11A
is a triple-negative breast cancer gene with critical functions in
stem and progenitor cells. Nat Commun. 6:59872015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Errico A: Genetics: BCL11A-targeting
triple-negative breast cancer? Nat Rev Clin Oncol. 12:1272015.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liu DW, Zhang JH, Liu FX, Wang XT, Pan SK,
Jiang DK, Zhao ZH and Liu ZS: Silencing of long noncoding RNA PVT1
inhibits podocyte damage and apoptosis in diabetic nephropathy by
upregulating FOXA1. Exp Mol Med. 51:882019. View Article : Google Scholar :
|