Introduction
Esophageal carcinoma is a commonly occurring tumor,
with no effective therapy, which threatens human health, causing
>400,000 mortalities worldwide annually in 2016 (1,2). The
most prevalent type of esophageal carcinoma is esophageal squamous
cell carcinoma (ESCC), which mainly occurs in Eastern Asia, as well
as Eastern and Southern Africa (3). In China, ~477,900 patients were
diagnosed with esophageal cancer and ~375,00 patients succumbed to
the disease in 2015 (4). The
prognosis of ESCC is very poor, with a 5-year survival rate
following surgery, chemotherapy or radiotherapy treatment of only
15-25%. As metastases are often present prior to diagnosis
(5), it is crucial to understand
the molecular mechanisms of metastasis in ESCC.
Tumor metastasis is the movement of tumor cells from
a primary site to colonize distant organs progressively (6). The process of tumor metastasis is
complicated and is associated with tumor microenvironment (7), changes in the cytoskeleton (8), activation or inactivation of
signaling pathways (9),
angiogenesis (10),
epithelial-mesenchymal transition (EMT) process (11), cell proliferation, as well as the
mobilization of oncogenes and the restrain of tumor suppressors
(12). In the course of tumor
progression, epithelial cells can exhibit plasticity and transition
into a mesenchymal state by activating the EMT process, while cells
in the mesenchymal state have a greater ability to migrate and
invade (11). The Wnt/β-catenin
signaling pathway plays an important role in the process of cell
proliferation and differentiation, while abnormal activation of
this pathway can lead to tumor (13). A previous study reported that EMT
could be regulated by Wnt/β-catenin signal pathway (14), and revealed a new mechanism of
tumor metastasis.
DJ-1 is an oncogene that is highly expressed in
numerous cancer types compared with corresponding normal tissue,
such as in lung cancer, breast cancer and endometrial cancer
(15-17). The effects of DJ-1 in cancer are
possibly through its ability to transform normal cells, prevent
oxidative damage, and inhibit apoptosis and promote metastasis
(18). DJ-1 has been reported to
be an independent prognostic indicator of poor survival in ESCC
(19). However, the mechanisms of
DJ-1 that promote ESCC metastasis remain unclear. The present study
aimed to investigate the role of DJ-1 in ESCC tumor cell
proliferation and invasion, and its underlying mechanisms.
Materials and methods
Patient information and tissue
specimens
The samples for the present study were collected
from 84 patients diagnosed with ESCC who underwent surgical
treatment at the Department of Thoracic Surgery, The Affiliated
Hospital of Yangzhou University (Yangzhou, China) between January
2015 to December 2016. The age range was 49-81 years (median, 64.46
years), and 58 male and 26 females were included. None of the
patients received radiotherapy or chemotherapy prior to surgery.
The ESCC specimens obtained from thora-coscopic surgery were stored
at -80˚C, fixed in 10% formalin at room temperature for 24 h and
embedded in paraffin. The study was reviewed and approved by the
Institutional Ethics Committee of the Affiliated Hospital of
Yangzhou University (Yangzhou, China). All patients signed informed
consent forms.
Reagents and antibodies
UltraVision Quanto Detection system HRP DAB was
obtained from Thermo Fisher Scientific, Inc. Roswell Park Memorial
Institute-1640 (RPMI-1640) and fetal bovine serum (FBS) were
purchased from Gibco; Thermo Fisher Scientific, Inc. Recombinant
transforming growth factor-β1 (TGF-β1) was acquired from R&D
Systems. Matrigel was obtained from BD Biosciences. Anti-DJ-1
antibody was obtained from Santa Cruz Biotechnology, Inc (catalog
no. sc-55572; 1:1,000 for western blotting; 1:200 for
immunohistochemistry). Anti-E-Cadherin antibody was acquired from
Abcam (catalog no. ab231303; 1:1,000 for western blotting; 1:1,000
for immunohistochemistry). Antibodies against vimentin (catalog no.
5741; 1:1,000 for western blotting; 1:200 for
immunohistochemistry), N-Cadherin (catalog no. 13116S; 1:1,000 for
western blotting), β-actin (catalog no. 4970S; 1:1,000 for western
blotting), low density lipoprotein receptor-related protein 6
(LRP6) (catalog no. 2560S; 1:1,000 for western blotting),
phosphorylation-LRP6 (p-LRP6) (catalog no. 2568S; 1:1,000 for
western blotting), axin1 (catalog no. 2087S; 1:1,000 for western
blotting) and β-catenin (catalog no. 8480S; 1:1,000 for western
blotting; 1:100 for immunohistochemistry) were purchased from Cell
Signaling Technology, Inc. HRP-conjugated goat anti mouse IgG
(catalog no. G1006-1; 1:2,000 for western blotting) and
HRP-conjugated goat anti-rabbit IgG (catalog no. HA1001; 1:2,000
for western blotting) were from Hangzhou Hua-an Medical &
Health Instruments Co., Ltd. Other chemicals of analytical grade
were obtained from commercial sources.
Immunohistochemistry
Immunohistochemical staining of human
paraffin-embedded ESCC was performed using UltraVision Quanto
Detection system HRP DAB, according to the manufacturer's protocol.
Briefly, slides were cut to a 5-µm thickness. Following
deparaffinization and rehydration with gradient alcohol ranging
between 100 and 70%, the tissues were incubated in digestive enzyme
for pretreatment. Sections were washed with PBS at room
temperature, and then incubated in Hydrogen Peroxide Block for 10
min and Ultra V Block for 5 min at 37˚C to reduce non-specific
background staining. Following washing with PBS, the sections were
incubated with primary antibodies against E-cadherin, vimentin,
DJ-1 and b-catenin for 20 min at 37˚C. Following washing, the
sections were then incubated with Applied HRP Polymer Quanto for 10
min at 37˚C. DAB Quanto Chromogen (30 µl) was added to 1 ml DAB
Quanto Substrate, mixed by swirling and applied to tissue for 5
min, followed by washing with distilled water at room temperature.
Finally, the sections were counterstained with hematoxylin for 1
min at room temperature followed by dehydration and coverslip
mounting. Negative controls were treated with PBS in place of
primary antibodies. The stained slides were observed with a light
microscope (magnification, x100 and x200) and evaluated using Image
Pro Plus (version 6.0; Media Cybernetics) as previously described
(20).
Cell lines and cell culture
The human ESCC cell line ECA-109 was purchased from
the Type Culture Collection of the Chinese Academy of Sciences and
cultured in RPMI-1640 medium containing 10% FBS and 1%
penicillin-streptomycin mixture. Cells were maintained at 37˚C in a
humidified atmosphere containing 5% CO2. Prior to
experiments, ECA-109 cells in the log phase of growth were cultured
in six-well plates in media containing only 1% FBS for 24 h.
EMT model
ECA-109 cells (1x105) were plated in
six-well plates 12 h before the experiment, and RPMI-1640
containing 10 µg/l TGF-β1 was subsequently added to each well for
24 h under normal culture conditions (21). The cell morphology and distribution
were captured under a light microscope (magnification, x200;
Olympus Corporation). Subsequently, the protein expression levels
of EMT-associated proteins E-cadherin, N-cadherin, vimentin and
DJ-1 were detected by western blotting.
Lentivirus-mediated DJ-1 overexpression
and knockdown
A lentiviral vector carrying green fluorescent
protein (GFP) for DJ-1 overexpression (LV-DJ-1) was applied to
mediate DJ-1 overexpression and an empty lentiviral vector was
applied as the negative control (LV-con). A lentiviral vector
carrying GFP and encoding short interfering RNA (siRNA) targeting
DJ-1 was applied to mediate DJ-1 knockdown (LV-siRNA-DJ-1) and an
empty lentiviral vector was used as the negative control
(LV-siRNA-con). All lentivirus vectors were constructed by Shanghai
Genechem Co., Ltd. GV358 lentiviral vector was used to upregulate
the expression of DJ-1 and GV248 lentiviral vector was used to
knockdown the expression of DJ-1. The negative controls were also
generated using GV248 and GV358, respectively. The primer sequence
of DJ-1 was as follow: Forward, 5'-GAG GAT CCC CGG GTA CCG GTC GCC
ACC ATG GCT TCC AAA AGA GCT CTG G-3' and reverse, 5'-TCC TTG TAG
TCC ATA CCG TCT TTA AGA ACA AGT GGA G-3'. The siRNA sequence was
5'-TTA GAG AAA CAG GCC GTT A-3'. ECA-109 cells (1.0x106)
were seeded in six-well plates, and lentivirus (MOI, 10) with
viral-plus transduction enhancer and polybrene (Shanghai Genechem
Co., Ltd.) at a final concentration of 8 µg/ml were added and
incubated at 37˚C for 48 h. Subsequently, the cells were treated
with 10 g/ml puromycin to eliminate the uninfected cells for 72 h.
The GFP positive cells were evaluated using a fluorescence
microscope (Olympus Corporation) and transfection was further
confirmed by western blotting.
Western blot analyses
Total proteins of each cell group were prepared
using RIPA buffer containing 1 mmol/l PMSF and a protease inhibitor
cocktail (Beyotime Institute of Biotechnology). Proteins were
quantified using the BCA assay kit (Beyotime Institute of
Biotechnology). Total proteins (30 µg/lane) from each lysate were
separated by 10% SDS-PAGE and then transferred onto PVDF membranes.
Membranes were blocked in blocking buffer (5% non-fat dry milk and
1% Tween-20 in PBS) for 2 h at room temperature. The membranes were
incubated with the primary antibodies against DJ-1, E-cadherin,
vimentin, N-cadherin, β-actin, LRP6, p-LRP6, Axin1 and β-catenin
overnight at 4˚C, and then incubated with HRP-conjugated rabbit or
anti-mouse antibody at room temperature for 2 h. Immunoreactive
signals were visualized using ECL detection reagent (Thermo Fisher
Scientific, Inc.) with the Molecular Imager Chemi Doc XRS system
(Bio-Rad Laboratories, Inc.). The bands were quantified using
Quantity One analysis software version 4.62 (Bio-Rad Laboratories,
Inc.). The value of each protein was normalized to the β-actin
level in the same sample.
Cell proliferation assay
Thymidine analog 5-ethynyl-2'-de-oxyuridine (EdU)
(Invitrogen; Thermo Fisher Scientific, Inc.) was used to detect
cell proliferation. According to the manufacturer's protocol,
2.0x104 cells/well were seeded into 96-well plates and
incubated at 37˚C in a 5% CO2 incubator for 24 h. Then,
25 µM EdU was added for 2 h at 37˚C. Following washing with PBS,
cells were fixed with 4% para-formaldehyde for 30 min at room
temperature and terminated with 2 mg/ml glycine. The Apollo
staining reaction liquid (catalog no. C10310; Guangzhou RiboBio
Co., Ltd.) was then added to the wells and incubated in dark at
room temperature for 30 min. Subsequently, 100 µl 0.5% TritonX-100
was applied to reduce the dye background. DAPI was diluted and used
to dye the nucleus in dark at room temperature for 30 min. The
results were observed and photographed using a fluorescence
inversion microscope system (magnification, x100; Olympus
Corporation).
Cell clone assay
To examine clonogenic ability, 500 cells were seeded
in six-well plates and cultured with complete medium for 2 weeks in
a 5% CO2 incubator at 37˚C (22). Then, the cells were fixed with 99%
methanol for 10 min at room temperature, and stained with 0.1%
crystal violet (Sigma-Aldrich; Merck KGaA) for 15 min at room
temperature. Clones with ≥50 cells were scored under a light
microscope (Olympus Corporation) and five random views were
evaluated (magnification, x100).
Cell adhesion assay
The 96-well plates were coated with 0.04 µg/µl
Matrigel (BD Biosciences) prior to the experiments. Cells
(5x104) were seeded in the plates for 2 h and then
washed with PBS to remove non-adherent cells. MTT (5 mg/ml;
Sigma-Aldrich; Merck KGaA) was added to each well in the dark for 4
h in a 5% CO2 incubator at 37˚C. The optical density
(OD) value was measured at 490 nm. The adhesion rate was calculated
as: OD of objective cells/OD of negative control cells x100%.
Transwell assay
A Corning Matrigel invasion chamber (Corning, Inc.)
was used for the Transwell assay. In the invasion assay, Matrigel
gel diluted with serum-free RPMI-1640 (1:8) was added to the upper
chamber. A single cell suspension in serum-free RPMI-1640 was added
to the upper chamber at a density of 2x104 cells/well
and incubated for 24 h (23). The
non-invading cells on the upper side of the chamber were removed
using cotton swabs. The membrane containing the invaded cells was
fixed with 99% methanol for 10 min at room temperature and stained
with 0.1% crystal violet for 10 min at room temperature. Migration
assays were performed using the same procedure, except that the
polycarbonate membrane was not coated with Matrigel. Each
experiment was repeated three times. The number of invading and
migrating cells was detected by light microscopy (magnification,
x200). The invasion and migration capacity of cells was calculated
as follows: Cell number of the treatment group/cell number of the
control group x100%.
Inhibitor treatment
To evaluate the exact mechanism, the present study
investigated the effect of β-catenin inhibition on the role of DJ-1
in ESCC malignant behaviors using XAV939 (MedChemExpress), a small
molecule inhibitor of Wnt/β-catenin. LV-DJ-1 cells were cultured in
6-well plates in advance, then 10 mM XAV939 was added for 24 h in a
5% CO2 incubator at 37˚C and the same concentration of
DMSO was used as a negative control. The treated cells were then
subjected to a cell proliferation assay, cell clone assay, cell
adhesion assay, Transwell assay and western blotting.
Nude mice intraperitoneal xenograft
model
A total of 20 4-week-old female athymic nude BALB/c
mice were obtained from the Comparative Medicine Laboratory Animal
Center of Yangzhou University (Jiangsu, China). All mice were
raised in a specific pathogen-free atmosphere environment at 25˚C
with a 12 h light/dark cycle and allowed free access to food and
water. All animal experiments were approved by the Institutional
Animal Care and Use Committee of Yangzhou University and performed
in accordance with internationally accepted guidelines on the use
of laboratory animals. Nude mice were randomly assigned to four
groups; each group contained 5 mice. ECA-109/LV-con,
ECA-109/LV-DJ-1, ECA-109/LV-siRNA-DJ-1 and ECA-109/LV-siRNA-con
cells (1x106) were separately inoculated into the
peritoneal cavity of nude mice (24). Because the intraperitoneal nodules
could not be measured directly for tumor progression, we referred
to relevant literature and chose 28 days as the total time of the
experiment (24). In the 28 days,
tumor progression was assessed by abdominal palpation and in
vivo imaging of the small animals using IVIS® Lumina
Series III (PerkinElmer, Inc.) every 2 days. Isoflurane (3%) was
used for induction and 1.5% isoflurane was used for maintenance of
anesthesia in the process of imaging small animals in vivo.
However, due to the poor imaging effect of GFP in cells and the
high background fluorescence in the picture, tumor progress was
roughly evaluated according to the intensity of the strongest
fluorescence. During the process, all the mice were alive, and
almost all of them showed wasting, but they were able to eat and
drink normally; therefore, the experiment was continued until the
end of the experiment period. A total of 28 days following
injection, all the mice were anesthetized with 3% isoflurane gas
inhalation and sacrificed by cervical dislocation, then a
laparotomy was performed. Peritoneal dissemination and organ
metastasis were examined. The peritoneal nodules and liver were
fixed in 10% formalin and embedded in paraffin for pathological
analysis.
Statistical analyses
All the experiments were repeated three times.
Protein expression levels and clinicopathological features were
compared using the χ2 test. Correlations between DJ-1,
E-cadherin and vimentin in clinical samples were analyzed using
Pearson's correlation analysis. Other data are presented as the
mean ± standard deviation, and groups were compared using Student's
t-test. One-way ANOVA followed by Fisher's least significant
difference post hoc test was used for multiple comparisons. All
statistical analysis was performed using SPSS version 16.0 software
(SPSS, Inc.). P<0.05 was considered to indicate a statistically
significant difference.
Results
DJ-1 is associated with metastasis and
EMT in clinical human esophageal squamous cancer patients
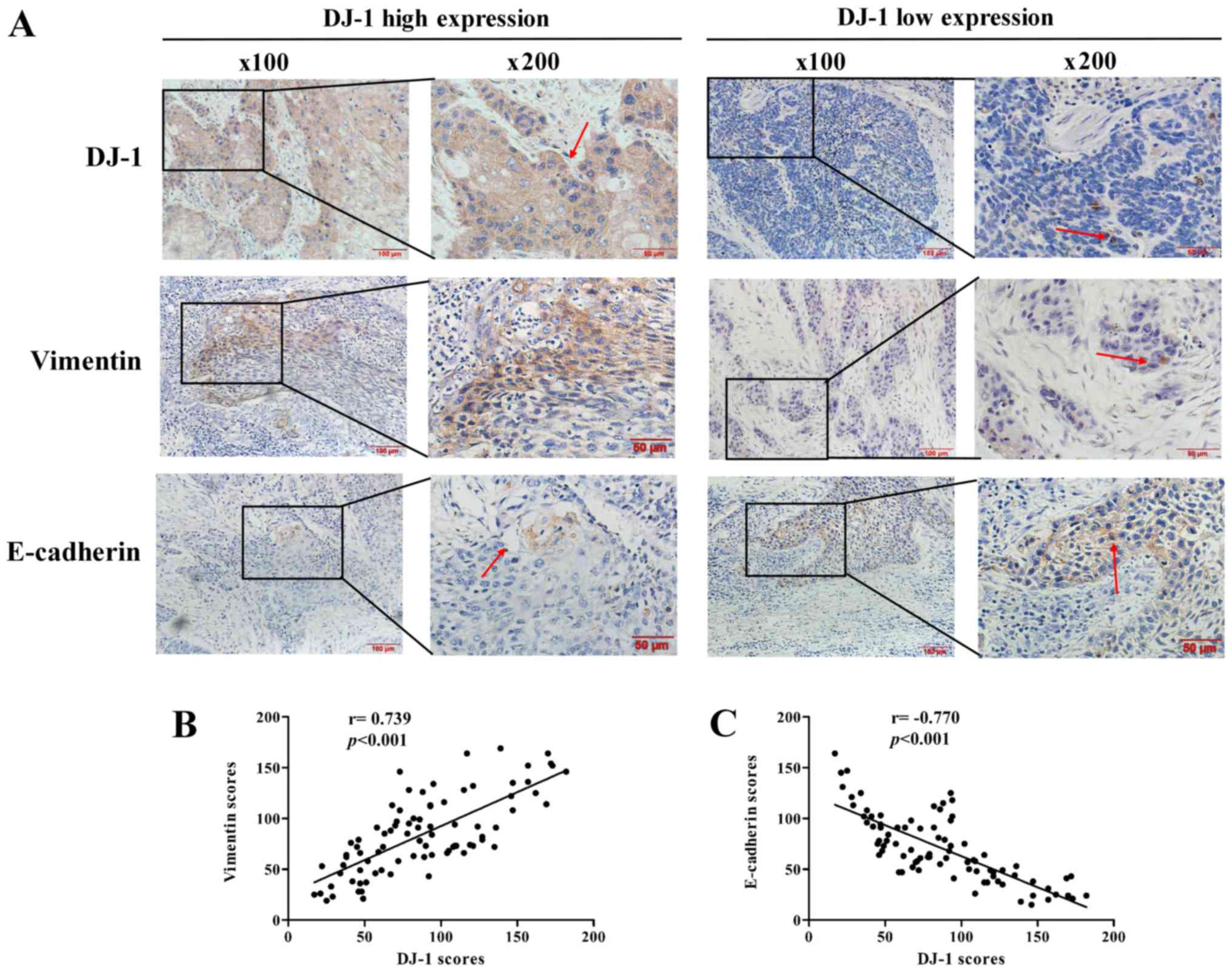
In order to elucidate the association between DJ-1
and EMT in ESCC, immunohistochemistry analyses were first performed
to detect the expression levels of DJ-1, vimentin and E-cadherin in
84 human ESCC tissue specimens (Fig.
1A). The associations between the clinical pathological
features of patients with ESCC and the DJ-1, E-cadherin ad vimentin
immunohis-tochemical staining scores are summarized in Table I. The data demonstrated that high
expression of DJ-1 was significantly associated with the lymph node
metastasis and distant metastasis. In addition, high expression of
vimentin and low expression of E-cadherin were significantly
associated with the tumor cell differentiation, pT status, lymph
node metastasis and distant metastasis (P<0.05).
 | Table IAssociations between DJ-1, E-cadherin
and vimentin expression and the clinicopathological features of 84
patients with esophageal squamous cell carcinoma. |
Table I
Associations between DJ-1, E-cadherin
and vimentin expression and the clinicopathological features of 84
patients with esophageal squamous cell carcinoma.
| Parameter | DJ-1 expression
| Vimentin expression
| E-cadherin
expression
|
|---|
| n | High | Low | χ2 | P-value | High | Low | χ2 | P-value | High | Low | χ2 | P-value |
|---|
| Age, years | | | | 0.350 | 0.554 | | | 0.239 | 0.625 | | | 0.075 | 0.784 |
| ≤60 | 15 | 7 | 8 | | | 8 | 7 | | | 6 | 9 | | |
| >60 | 69 | 38 | 31 | | | 32 | 37 | | | 25 | 44 | | |
| Sex | | | | 0.257 | 0.612 | | | 0.032 | 0.857 | | | 0.472 | 0.492 |
| Male | 58 | 30 | 28 | | | 28 | 30 | | | 20 | 38 | | |
| Female | 26 | 15 | 11 | | | 12 | 14 | | | 11 | 15 | | |
| Tumor size, cm | | | | 1.440 | 0.230 | | | 1.925 | 0.165 | | | 0.614 | 0.433 |
| ≤5 | 48 | 23 | 25 | | | 26 | 22 | | | 16 | 32 | | |
| >5 | 36 | 22 | 14 | | | 14 | 22 | | | 15 | 21 | | |
| Location | | | | 3.127 | 0.209 | | | 1.359 | 0.507 | | | 0.470 | 0.791 |
| Upper | 14 | 8 | 6 | | | 7 | 7 | | | 6 | 8 | | |
| Middle | 39 | 17 | 22 | | | 16 | 23 | | | 13 | 26 | | |
| Lower | 31 | 20 | 11 | | | 17 | 14 | | | 12 | 19 | | |
|
Differentiation | | | | 4.503 | 0.105 | | | 7.489 | 0.024 | | | 8.531 | 0.014 |
| Well | 7 | 8 | | | | 5 | 10 | | | 10 | 5 | | |
| Moderate | 25 | 27 | | | | 22 | 30 | | | 18 | 34 | | |
| Poor | 13 | 4 | | | | 13 | 4 | | | 3 | 14 | | |
| pT status | | | | 3.489 | 0.062 | | | 4.587 | 0.032 | | | 4.741 | 0.029 |
| Tl-2 | 24 | 9 | 15 | | | 7 | 17 | | | 13 | 11 | | |
| T3-T4 | 60 | 36 | 24 | | | 33 | 27 | | | 18 | 42 | | |
| Lymph node
metastasis | | | | 6.489 | 0.011 | | | 7.126 | 0.008 | | | 6.347 | 0.012 |
| No | 29 | 10 | 19 | | | 8 | 21 | | | 16 | 13 | | |
| Yes | 55 | 35 | 20 | | | 32 | 23 | | | 15 | 40 | | |
| Metastasis | | | | 8.526 | 0.004 | | | 9.185 | 0.002 | | | 10.181 | 0.001 |
| No | 46 | 18 | 28 | | | 15 | 31 | | | 24 | 22 | | |
| Yes | 38 | 27 | 11 | | | 25 | 13 | | | 7 | 29 | | |
Furthermore, the expression levels of EMT biomarkers
E-cadherin and vimentin were significantly correlated with DJ-1. As
presented in Fig. 1B and C, DJ-1
expression was positively correlated with vimentin expression
(r=0.739, P<0.001) and negatively correlated with E-cadherin
(r=-0.770, P<0.001). These results collectively suggest that
DJ-1 may be closely associated with metastasis and the EMT in
ESCC.
DJ-1 is highly expressed during EMT in a
ESCC cell model
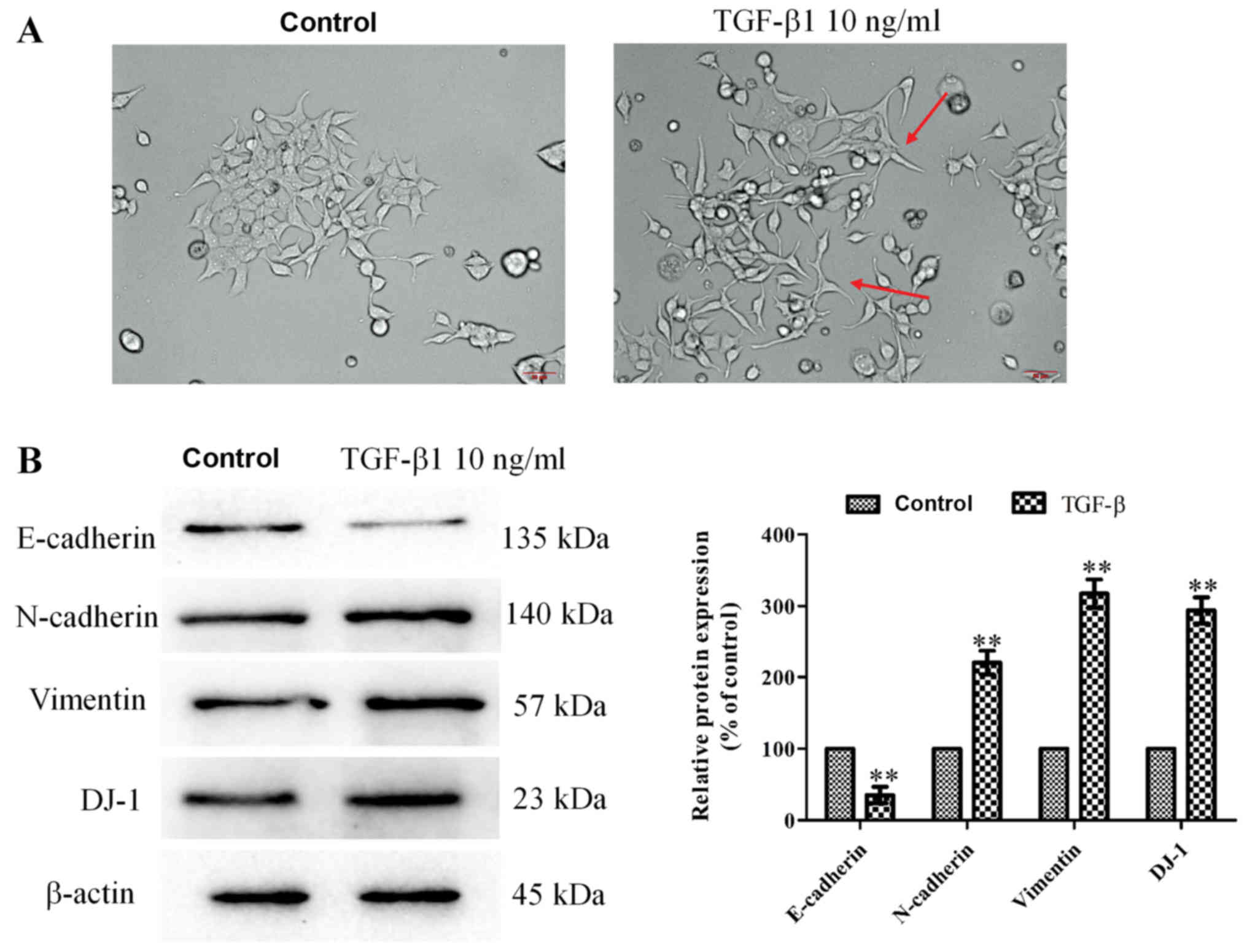
To study the DJ-1 expression in EMT progress, EMT
was induced in ECA-109 cells using 10 ng/ml TGF-β1. Following 24 h,
cells were photographed under a light microscope and examined by
western blotting. As presented in Fig.
2A, the cell morphology of the TGF-β1 group exhibited
spindle-like shapes. The distribution of cells in the TGF-β1 group
was more extensive and changes in migration patterns were observed
compared with the control group. Furthermore, the expression levels
of EMT biomarkers were significantly different following TGF-β1
treatment; E-cadherin was significantly decreased, and N-cadherin
and vimentin were significantly increased (P<0.01). In addition,
DJ-1 expression was significantly increased following TGF-β1
treatment (P<0.01; Fig. 2B).
Thus, DJ-1 was highly expressed during EMT in ECA-109 cells.
DJ-1 overexpression promotes cell growth
of ESCC cells
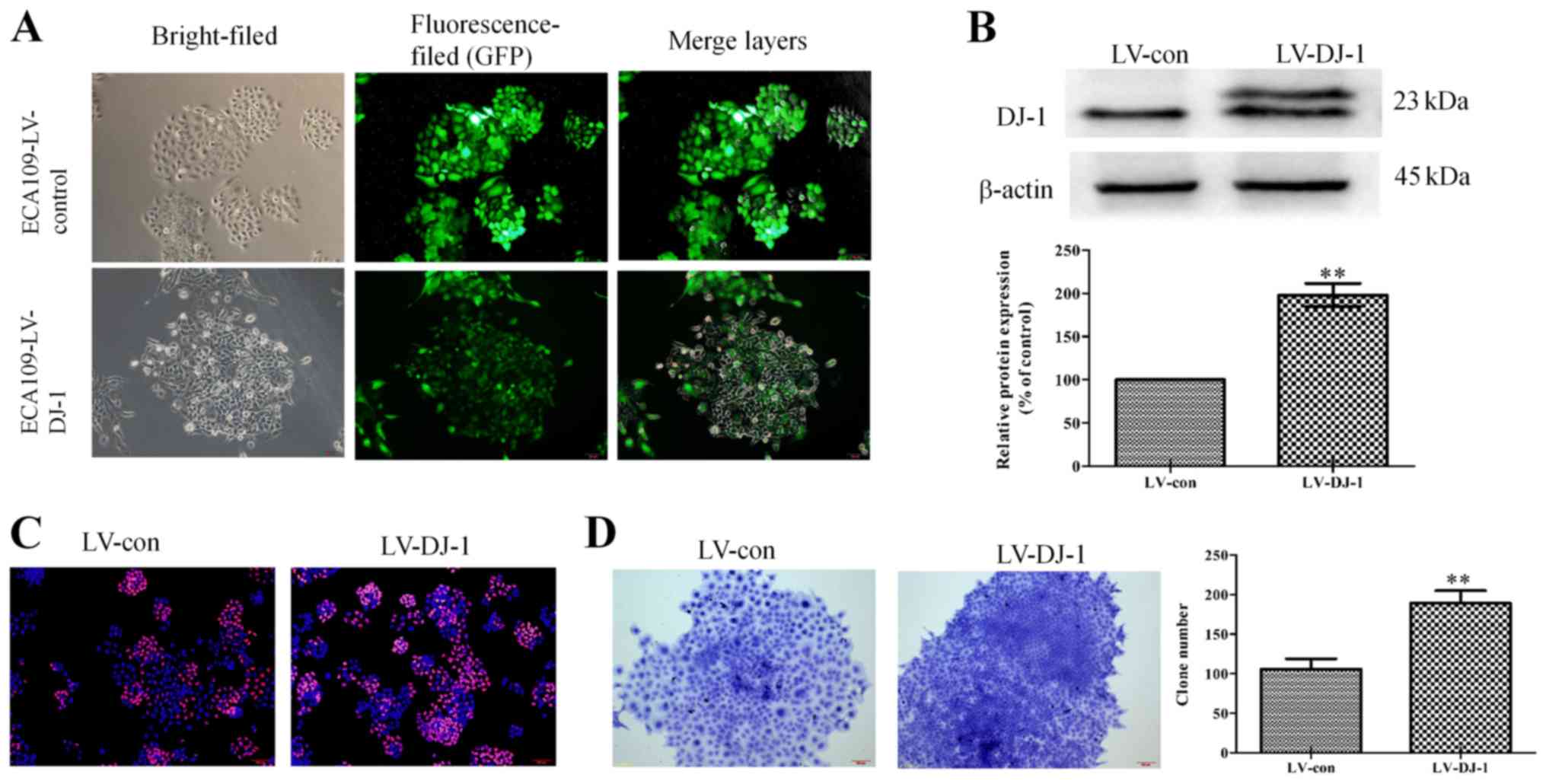
To validate the role of DJ-1 in tumor metastasis and
EMT, lentivirus vectors were used to overexpress DJ-1 in Eca-109
cells. Successful overexpression of DJ-1 of was verified by
fluorescence observation of the GFP signal (Fig. 3A). In addition, western blotting
demonstrated that DJ-1 expression was significantly increased in
LV-DJ-1 cells compared with control cells (P<0.01; Fig. 3B). As presented in Fig. 3C, cell proliferation was higher
following DJ-1 overexpression compared with the control group, as
the Edu staining was stronger. In order to further observe the
change of cell proliferation ability, a cell clone assay was
performed. This revealed that LV-DJ-1 cells had a larger colony
size and significantly more colonies (189.33±15.50) compared with
the control cells (106±12.77) (P<0.01; Fig. 3D).
DJ-1 overexpression promotes migration,
invasion, adhesion and EMT via the Wnt/b-catenin signaling pathway
of ESCC cells
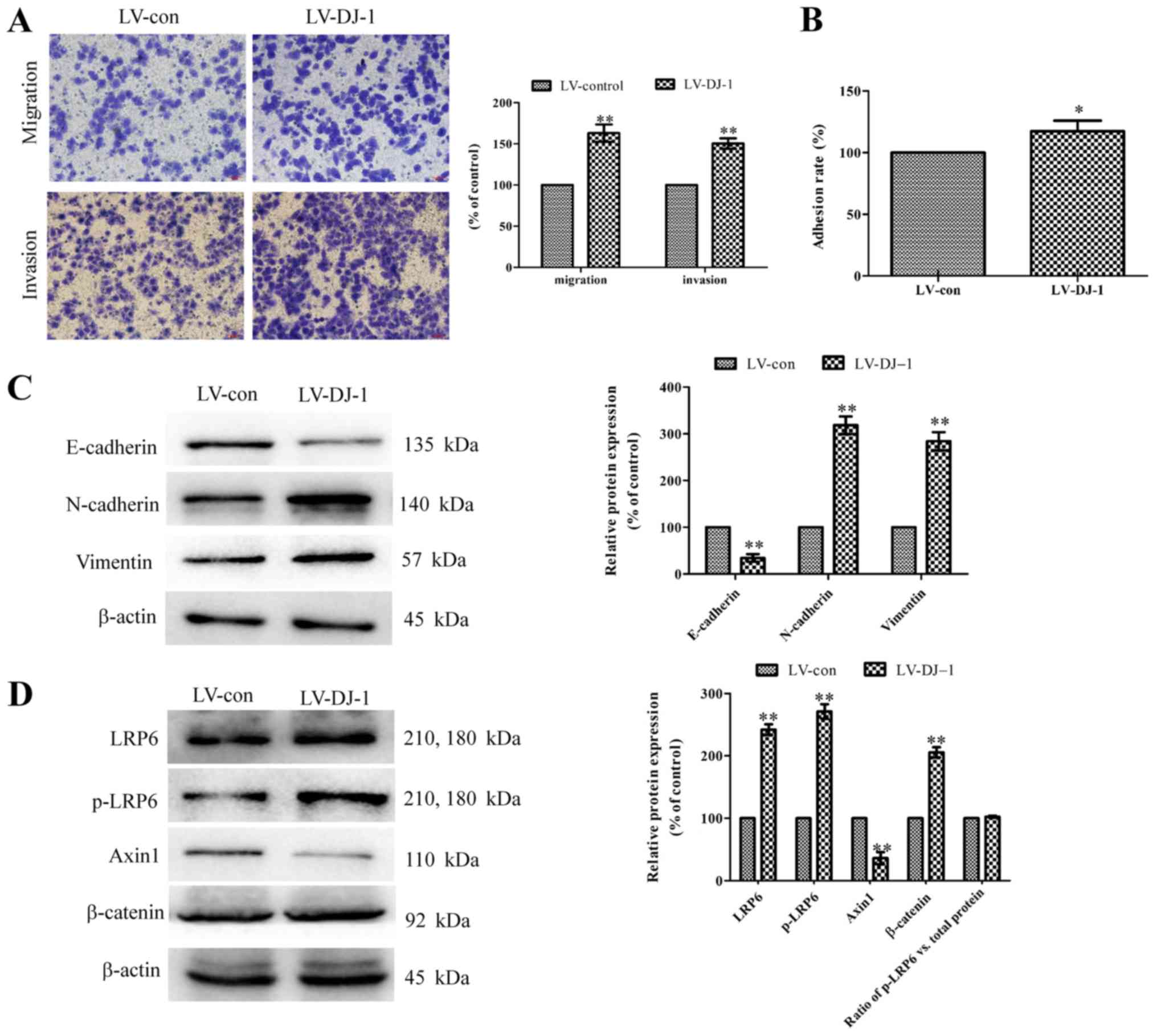
A Transwell assay was performed to detect the
migration and invasion ability of DJ-1 overexpressed cells. As
presented in Fig. 4A, LV-DJ-1
cells had a significantly higher migration and invasion ability
compared with the LV-con group (P<0.01). Similarly, the adhesion
rate was significantly higher in LV-DJ-1 cells compared with the
LV-con cells (P<0.05; Fig. 4B).
Western blotting results demonstrated that DJ-1 overexpression
could significantly reduce the E-cadherin expression, and
significantly increase the vimentin and N-cadherin expression
(P<0.01; Fig. 4C), which
indicates DJ-1 promotes the EMT process. LRP6, p-LRP6, axin1 and
β-catenin are key proteins of the canonical Wnt/β-catenin signal
pathway (13). It was identified
that in LV-DJ-1 cells, LRP6, p-LRP6 and β-catenin expression levels
were significantly upregulated, while axin1 expression was
significantly downregulated compared with control cells (P<0.01;
Fig. 4D). However, the ratio of
p-LRP6 to total LRP6 protein had no significant change between the
control group and DJ-1 overexpressed group (P>0.05; Fig. 4D). Therefore, this suggests that a
high expression of DJ-1 does not effect the phosphorylation of
LPR6.
DJ-1 knockdown suppresses cell growth of
ESCC cells
To further confirm the effects of DJ-1 in tumor
metastasis and EMT of ESCC, lentivirus vectors encoding siRNAs
targeting DJ-1 were used to knockdown DJ-1 expression in ECA-109
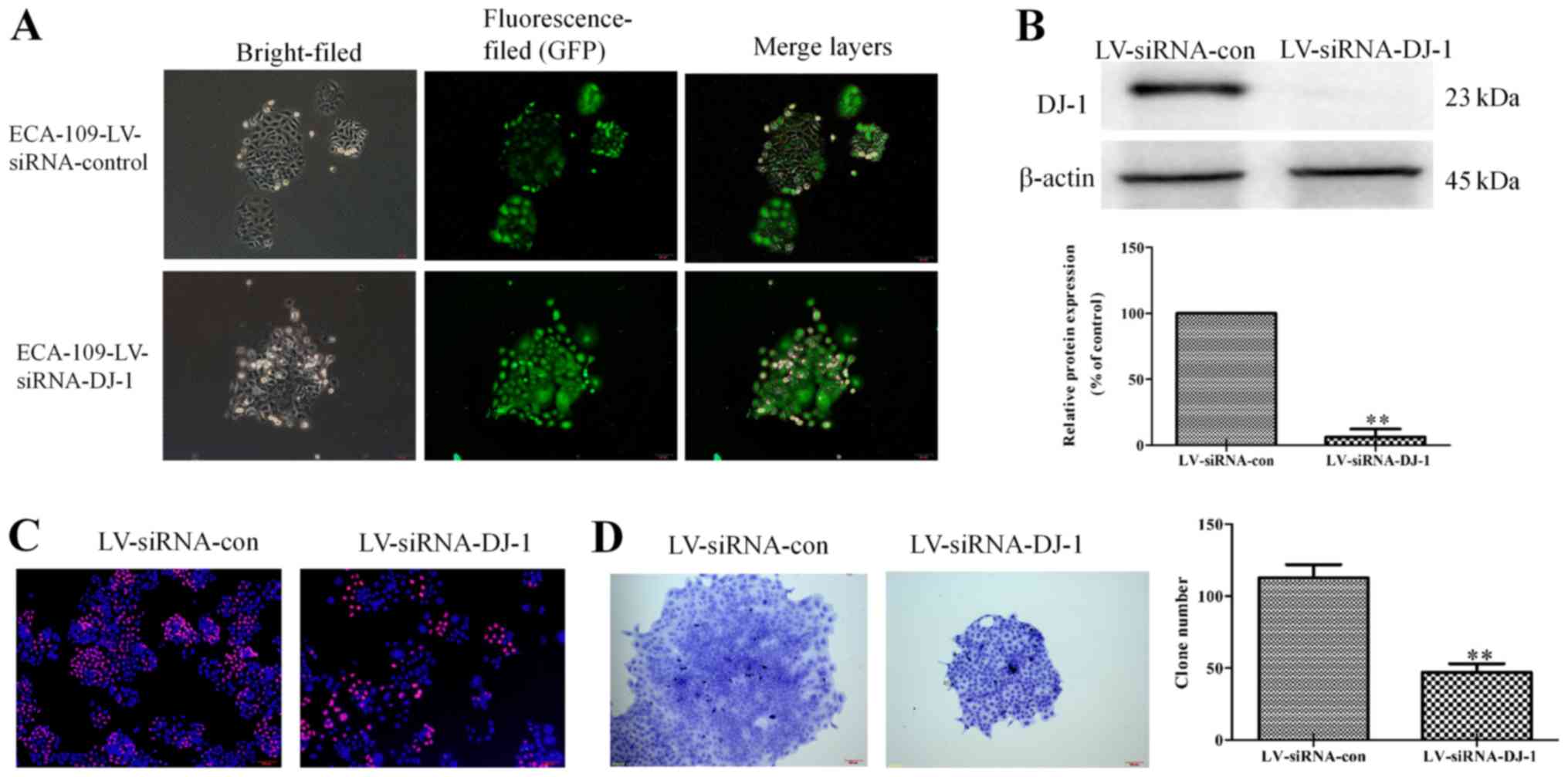
cells. As presented in Fig. 5A,
DJ-1 was knocked-down, according to the extent of green
fluorescence. Additionally, western blot assay revealed that the
protein expression of DJ-1 was significantly reduced in
LV-siRNA-con cells (P<0.01; Fig.
5B). These results suggest that the DJ-1 low-expression cell
model was successfully constructed. Edu staining assay demonstrated
that LV-siRNA-DJ-1 cells had a low proliferative capacity compared
with control cells, as the red fluorescence was enhanced. (Fig. 5C). Furthermore, the ability of
cells in the LV-siRNA-DJ-1 group to proliferate and form clones was
significantly lower compared with the control group (P<0.01;
Fig. 5D).
DJ-1 knockdown suppresses migration,
invasion, adhesion and EMT via the Wnt/β-catenin signaling pathway
in ESCC cells
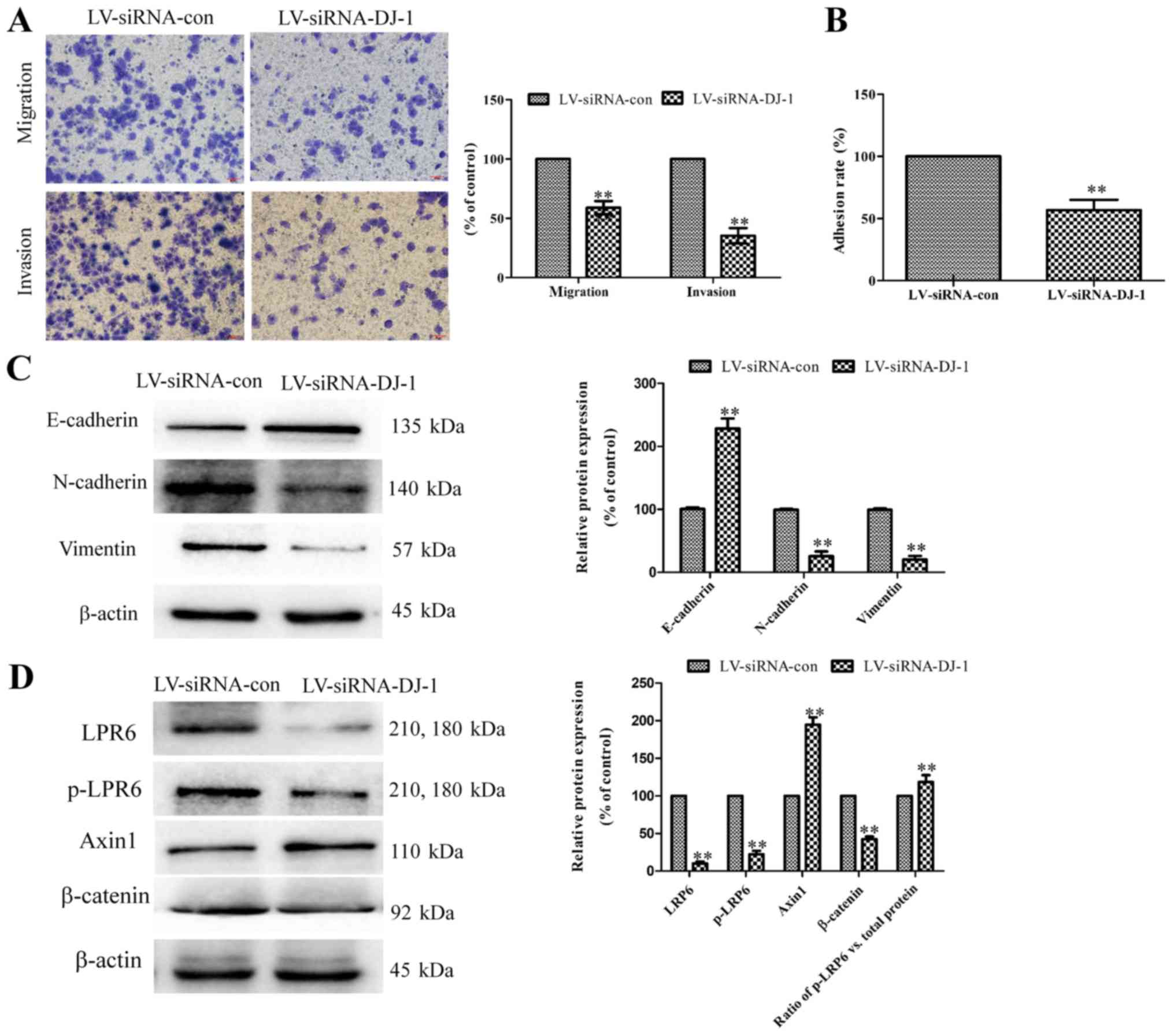
In contrast to DJ-1 overexpressed cells, the
LV-siRNA-DJ-1 cells had significantly lower migration, invasion and
adhesion abilities compared with LV-siRNA-control cells (P<0.01;
Fig. 6A and B). The E-cadherin
expression was significantly higher, while N-cadherin and Vimentin
expression were significantly lower in LV-siRNA-DJ-1 cells compared
with control cells (P<0.01; Fig.
6C). Furthermore, LRP6, p-LRP6 and b-catenin expression were
significantly downregulated, while Axin1 expression was
significantly upregulated in LV-siRNA-DJ-1 cells (P<0.01;
Fig. 6D). Finally, the ratio of
phosphorylated LRP6 to total LRP6 protein was significantly
increased in LV-siRNA-DJ-1 cells compared with control cells.
Inhibition of Wnt/β-catenin reduces tumor
malignant behaviors induced by DJ-1
In order to investigate the exact mechanism of the
Wnt/β-catenin signaling pathway in the regulation of DJ-1 in tumor
malignant behaviors, the β-catenin inhibitor XAV939 was used in
in vitro studies. LV-DJ-1 cells were treated with XAV939; as
presented in Fig. 7A, the
proliferation of LV-DJ-1 cells treated with XAV939 was decreased
compared with LV-DJ-1 cells. In the colony formation assay, cells
treated with XAV939 had a significantly smaller number of colonies
compared with the control LV-DJ-1 cells (P<0.01; Fig. 7B). In the Transwell assay, LV-DJ-1
cells treated with XAV939 demonstrated significantly reduced
abilities to migrate and invade compared with LV-con group
(P<0.01; Fig. 7C). Similarly,
the adhesion ability was significantly decreased in the XAV939
group compared with the LV-con group (P<0.01; Fig. 7D). Western blotting results
demonstrated that XAV939 treatment could signifi-cantly increase
the E-cadherin, while it significantly reduced the vimentin and
N-cadherin expression levels compared with the untreated LV-DJ-1
cells (P<0.01; Fig. 7E), which
indicates XAV939 inhibited the EMT process promoted by DJ-1.
Additionally, LRP6, p-LRP6 and b-catenin expression levels were
significantly downregulated, while Axin1 expression was
significantly upregulated compared with control cells, and the
ratio of phosphorylated LRP6 to total LRP6 protein was
significantly increased in XAV939-treated cells compared with the
control untreated LV-DJ-1 cells (P<0.05; Fig. 7F), These data suggest XAV939 could
reverse tumor malignant behavior induced by overexpression of
DJ-1.
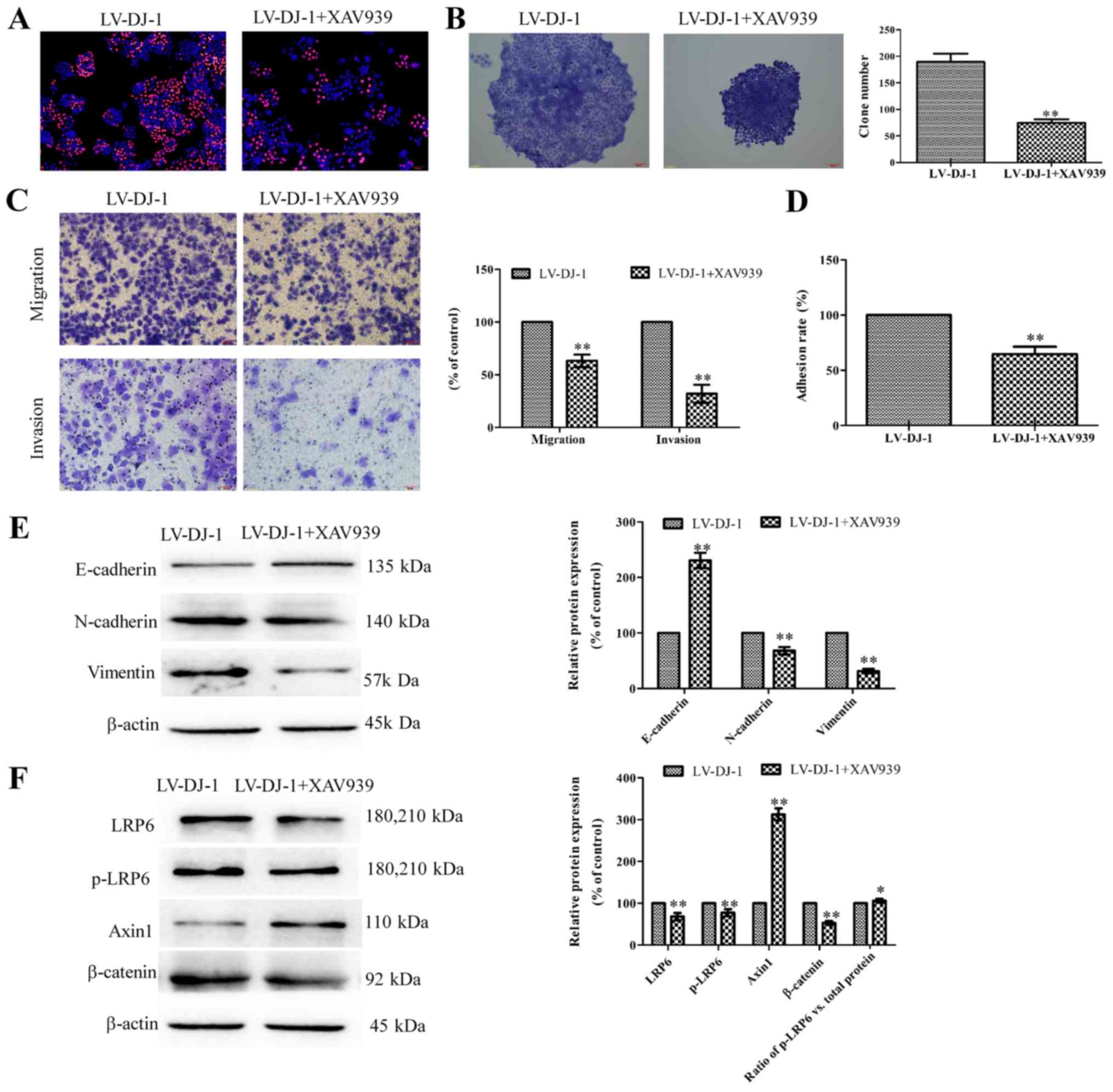 | Figure 7Inhibition of Wnt/β-catenin reduces
tumor malignant behaviors caused by DJ-1. (A) The proliferation of
LV-DJ-1 cells treated with XAV939 was significantly decreased
compared with LV-DJ-1 cells (magnification, x100). (B) In the
colony formation assay, cells treated with XAV939 had fewer
colonies compared with the control LV-DJ-1 cells (magnification,
x100). (C) In the Transwell assay, cells of the LV-DJ-1 + XAV939
group demonstrated a weaker ability to migrate and invade compared
with the LV-DJ-1 group (magnification, x200). (D) The adhesion
ability was decreased in the LV-DJ-1 + XAV939 group compared with
the LV-DJ-1 group. (E) Western blotting results demonstrated XAV939
treatment could increase the E-cadherin expression, while it
reduced the vimentin and N-cadherin expression levels. (F) LRP6,
p-LRP6 and β-catenin expression were downregulated, while Axin1
expression was upregulated in XAV939-treated compared with control
cells. The ratio of p-LRP6 to total LRP6 protein was significantly
increased in XAV939-treated cells compared with the control
untreated LV-DJ-1 cells. *P<0.05,
**P<0.01 vs. LV-DJ-1 group. LV-DJ-1, lentivirus
overexpressing DJ-1; LRP-6, lipoprotein receptor-related protein 6;
p-. phosphorylated. |
Manipulation on DJ-1 expression
influences ESCC xeno- graft metastasis and EMT via the
Wnt/β-catenin signaling pathway
Since the important roles of DJ-1 in metastasis and
EMT have been demonstrated by clinical analysis and in vitro
experiments, its biological effect on ESCC metastasis and EMT was
further examined in vivo using a nude mice abdominal
transplantation model. In vivo imaging of the small animals
was also used to observe tumor growth dynamically. However, due to
the poor imaging effect of green fluorescent protein in cells and
the high background fluorescence in the picture, tumor progress was
roughly estimated according to the intensity of the strongest
fluorescence. In the late stage of tumor growth, differences were
observed between each group. At day 28, the LV-DJ-1 group had a
larger area and stronger fluorescence compared with the control,
while the LV-siRNA-DJ-1 group had a smaller area of fluorescence
(Fig S1). The numbers of
peritoneal dissemination nodules were examined and the liver
metastases were removed and analyzed. As presented in Fig. 8A and B, the number of peritoneal
dissemination nodules in LV-DJ-1 group was significantly increased
compared with the LV-con group, while in the LV-siRNA-DJ-1 group,
the number of peritoneal dissemination nodules was significantly
reduced compared with the LV-siRNA-con group (P<0.01). Notably,
liver metastases in the LV-DJ-1 group exhibited a larger volume and
contained a larger number of nodules (Fig. 8C). By contrast, in the
LV-siRNA-DJ-1 group, liver metastases had a smaller volume and
fewer nodules (Fig. 8D).
Immunohistochemistry results further explained the mechanism of
DJ-1 promoting tumor metastasis. Immunohistochemical staining
(Fig. 8E) revealed increased DJ-1,
vimentin and β-catenin levels, and decreased E-cadherin levels in
the LV-DJ-1 group compared with the control group. In addition,
staining demonstrated decreased DJ-1, vimentin and β-catenin
levels, and increased E-cadherin levels in the LV-siRNA-DJ-1 group
compared with the control group.
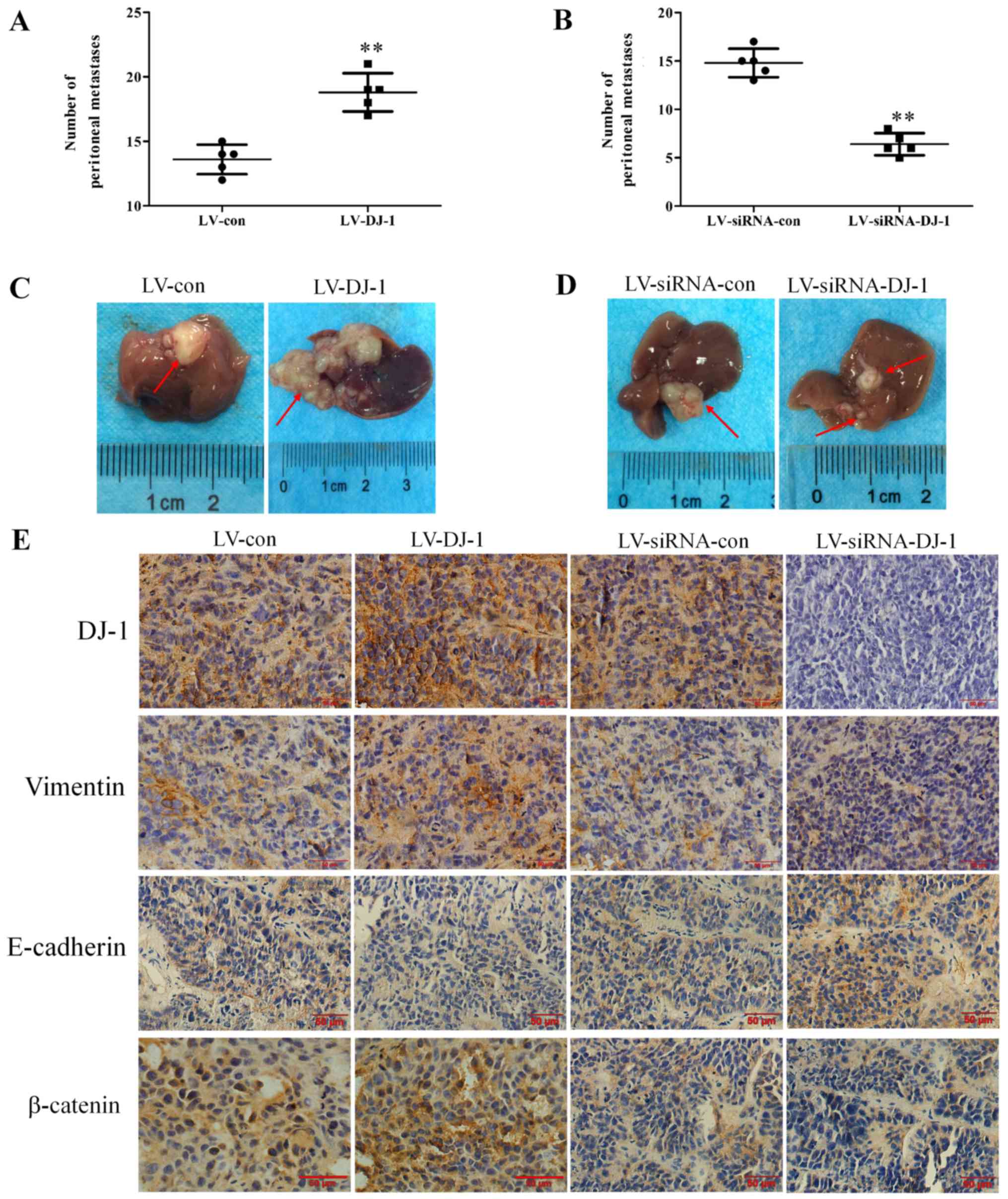 | Figure 8Manipulation of DJ-1 expression
influences esophageal squamous cell carcinoma xenograft metastasis
and EMT via the Wnt/β-catenin signal pathway. (A) The number of
peritoneal dissemination nodules in the LV-DJ-1 group was
significantly higher compared with the LV-con group.
**P<0.01 vs. LV-con. (B) In LV-siRNA-DJ-1 group, the
number of peritoneal dissemination nodules was significantly lower
compared with the LV-siRNA-con group. **P<0.01 vs.
LV-siRNA-DJ-1. (C) Liver metastases in the LV-DJ-1 group exhibited
a larger volume and more nodules. (D) In the LV-siRNA-DJ-1 group,
liver metastases exhibited a smaller volume and fewer nodules. (E)
Immunohistochemistry results revealed increased DJ-1, vimentin and
β-catenin, and decreased E-cadherin levels in the LV-DJ-1 group,
and decreased DJ-1, vimentin and β-catenin, and increased
E-cadherin levels in the LV-siRNA-DJ-1 group (magnification, x200).
LV-DJ-1, lentivirus overexpressing DJ-1; LV-con, lentivirus
control; LV-siRNA-DJ-1, lentivirus encoding DJ-1 small interfering
RNA; LV-siRNA-con, lentivirus encoding control small interfering
RNA. |
Discussion
The role of DJ-1 has been extensively investigated
in numerous human cancer types. In the process of tumor
progression, DJ-1 activates the AKT/mTOR and MAPK signaling pathway
by suppressing the PTEN gene, and promotes proliferation, survival,
anti-apoptosis, invasion and metastasis of tumor cells (25,26).
In addition, DJ-1 serves as an endogenous antioxidant to protect
cells from oxidative stress via oxidizing itself and/or stabilizing
the antioxidant transcriptional responses regulator Nrf2 (27). A previous study reported that
cytoplasmic DJ-1 expression is significantly higher in ESCC and
ESCC lymph node metastases compared with in non-neoplastic
esophageal epithelium. ESCC specimens with high distant metastatic
potential also had a significantly higher level of nuclear DJ-1
expression (19). However, the
mechanisms of DJ-1 promoting esophageal squamous carcinoma
metastasis remain unclear.
The present study investigated the role of DJ-1 in
regulation of the EMT process and metastasis, and clarified its
potential mechanisms in ESCC. To understand the association between
DJ-1 and EMT, the current study investigated the expression levels
of DJ-1, the mesenchymal cell marker vimentin and the epithelial
cell marker E-cadherin in 84 paraffin-embedded ESCC tissue samples
by immunohistochemistry. It was identified that DJ-1 was
significantly associated with local lymph node metastasis and
distant metastasis, while the vimentin and E-cadherin were
significantly associated with tumor cell differentiation, depth of
tumor invasion, lymph node metastasis and distant metastases.
Furthermore, DJ-1 was positively correlated with vimentin and a
negatively correlated with E-cadherin (P<0.01). Furthermore,
DJ-1 was highly expressed in the TGF-β1-induced EMT model of
ECA-109 cells. Altogether, the present data demonstrated that DJ-1
was closely associated with tumor metastasis and EMT. To further
investigate the effects of DJ-1 on metastasis and EMT, DJ-1 was
upregulated and knocked-down in ECA-109 cells using lentivirus
vectors. Cell proliferation is an important process for successful
tumor transformation (28). Cells
with DJ-1 overexpression had a greater proliferation rate and
colony formation ability compared with the control cells, while
DJ-1 knockdown resulted in a slower proliferation rate and colony
formation compared with the control. Tumor cells gradually migrate
to the vicinity of blood vessels and invade blood vessels, travel
with the blood for adhesion, and then proliferate to form new
metastases (29). The present
results demonstrated that overex-pression of DJ-1 could increase
the adhesion, migration and invasion. By contrast, the adhesion,
migration and invasion were significantly decreased in the DJ-1
low-expression group. Notably, DJ-1 promoted EMT by increasing
N-cadherin and vimentin expression levels, and reducing E-cadherin
expression.
The Wnt/β-catenin signaling pathway serves a
fundamental role in the cell fate specification during early
embryonic development, proliferation, body axis patterning,
survival, apoptosis, and in tissue homeostasis in adults (30). A mutated Wnt pathway leads to
multiple growth-related pathologies and cancer (31). Zhou et al (32) found that DJ-1 can promote CRC
metastasis by activating the PLAGL2-Wnt-BMP4 axis (32). This result suggested that DJ-1
could regulate the Wnt/β-catenin signaling pathway in cancer.
However, it remains unclear whether DJ-1 can inhibit the invasion
and metastasis in ESCC by regulating the Wnt/β-catenin signaling
pathway. Therefore, the present study further investigated the role
and mechanism of DJ-1 and the Wnt/β-catenin signaling pathway in
the invasion and metastasis of ESCC. Canonical Wnt/β-catenin
signaling begins with secreted Wnt ligands binding to the Frizzled
family receptors, and then the LRP5/LRP6 co-receptor triggers the
β-catenin signaling cascade (33).
Du et al (34) demonstrated
that silencing LRP6 leads to less metastasis and angiogenesis in
HCC cells. Tung et al (35)
identified that regulation of LRP6 could promote
hepato-carcinogenesis and enhance cell invasion. LRP6
phosphorylation is accompanied by receptor internalization in
caveolin-containing vesicles and endocytosis, which is essential
for Wnt/β-catenin signaling (36).
Tang et al (37) reported
that β-catenin expression has higher in ESCC tissues compared with
corresponding normal mucosa tissues, and exhibited a correlation
with histological grade and invasion depth. Axin1 protein
negatively regulates the Wnt signaling pathway based on its role as
a scaffolding protein and its binding to cytoplasmic β-catenin
(38).
The present study detected the expression levels of
LRP6, p-LRP6, Axin1 and β-catenin in LV-DJ-1, LV-con, siRNA-DJ-1
and siRNA-con cells. The western blotting results demonstrated DJ-1
upregulation could increase LRP6, p-LRP6 and β-catenin levels, and
decrease Axin1. Whereas, DJ-1-knockdown decreased the LRP6, p-LRP6
and β-catenin levels, and increased Axin1. These data revealed that
the Wnt/β-catenin signaling pathway participates in the process of
DJ-1-induced regulation of tumor metastasis and EMT. These results
were consistent with the data in literature that DJ-1 can promote
CRC metastasis by activating the Wnt axis (32). The present study identified that
the ratio of p-LRP6 to total LRP6 protein was not significantly
different between the control group and DJ-1-overexpressing group.
These results suggest that a high expression of DJ-1 did not result
in changes in the phosphorylation of LPR6.
β-catenin protein is a core molecule and important
regulatory site of the Wnt signaling pathway, and the level of
β-catenin has a decisive influence on this pathway (39). It has been reported that β-catenin
is one of the differentially expressed proteins between
high-metastatic human ovarian cancer and non-metastatic ovarian
cancer, and subsequent in vitro and in vivo studies
have confirmed that overexpression of β-catenin can promote ovarian
cancer metastasis, while knockdown of β-catenin expression can
significantly weaken the cell metastasis ability (39). Therefore, the present study
speculated that β-catenin plays an important role in the process of
DJ-1 inhibiting Wnt/β-catenin signal pathway. The β-catenin
inhibitor XAV939 was used to further clarify the role of the
Wnt/β-catenin signaling pathway in DJ-1 promoting tumor metastasis.
XAV939 is a novel small molecule inhibitor of the Wnt signaling
pathway, which may restrain the abnormal activation of
Wnt/β-catenin and does not affect CRE, NF-κB or TGF-β (40). Guo et al (41) found that XAV939 could suppress the
viability of small cell lung cancer NCI-H446 cells and induce
apoptosis. The present results demonstrated that XAV939 treatment
could reduce the reverse tumor malignant behavior caused by
overexpression of DJ-1. It has been reported that DJ-1-induced
β-catenin nuclear translocation stimulates T-cell factor
transcription activity, which promotes BMP4 expression for CRC cell
migration and invasion, and elevates CCND1 expression for CRC cell
proliferation (32). This report
was consistent with the present results, which identified that DJ-1
inhibited ESCC cell invasion through the Wnt/β-catenin pathway by
inhibiting β-catenin. However, the current experiment focused on
the relationship between DJ-1 and the invasion and metastasis of
ESCC, and the mechanism of DJ-1 inhibiting β-catenin was not
further investigated. The present results suggested that when the
Wnt/β-catenin signaling pathway was blocked, DJ-1 could not promote
tumor metastasis as before. Furthermore, in vivo experiments
were conducted using a nude mouse abdominal xenograft model. Data
revealed that DJ-1 promoted a stronger metastasis ability and the
EMT, and this result may be achieved by DJ-1 regulating the
Wnt/β-catenin signaling pathway.
In conclusion, the present study demonstrated that
DJ-1 promoted tumor metastasis and EMT via the Wnt/β-catenin
signaling pathway in ESCC. These results indicate that DJ-1 may be
a candidate therapeutic target for new ESCC drug development.
Supplementary Data
Funding
This work was financially supported by the National
Natural Science Foundation of China (grant nos. 81573656 and
81773944), the Natural Science Foundation of Jiangsu Province of
China (grant no. BK20171290) and the Natural Science Foundation of
Jiangsu Province for Youths (grant no. BK20170516).
Authors' contributions
FJ and HW designed the experimental procedures. FJ,
DL, CF and WL jointly performed the experiments. QS, YD, ZD and FW
helped in clinical specimen collection and performed statistical
analysis. FJ, XD and MS analyzed the data. FJ, LT and YQ helped in
designing the in vivo experiments and revised the manuscript
critically for important intellectual content. YL contributed to
the conception or design of the work and gave final approval of the
version to be published. All authors reviewed the article and
approved the final manuscript for publication.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Ethics approval and consent to
participate
The animal experiments protocol was reviewed and
approved by ethics committee of Medical College, Yangzhou
University (Yangzhou, China). The human tissues study protocol was
approved by the Institutional Ethics Committee of the Affiliated
Hospital of Yangzhou University (Yangzhou, China), and informed
consent was obtained from each patient.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgements
The authors would like to thank Dr Jun Feng (Gaoyou
Traditional Chinese Medicine Hospital, Yangzhou, China) for
providing assistance with the cell culture, and Mr. Yerong Yan
(Comparative Medicine Laboratory Animal Center, Yangzhou
University, Yangzhou, China) for taking care of the animals used in
the study.
References
|
1
|
Lin DC, Wang MR and Koeffler HP: Genomic
and epigenomic aberrations in esophageal squamous cell carcinoma
and implications for patients. Gastroenterology. 154:374–389. 2018.
View Article : Google Scholar :
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar
|
|
4
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Song Y, Li L, Ou Y, Gao Z, Li E, Li X,
Zhang W, Wang J, Xu L, Zhou Y, et al: Identification of genomic
alterations in oesopha-geal squamous cell cancer. Nature.
509:91–95. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Steeg PS: Targeting metastasis. Nat Rev
Cancer. 16:201–218. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Joyce JA and Pollard JW:
Micro-environmental regulation of metastasis. Nat Rev Cancer.
9:239–252. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fife CM, McCarroll JA and Kavallaris M:
Movers and shakers: Cell cytoskeleton in cancer metastasis. Br J
Pharmacol. 171:5507–5523. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
McCubrey JA, Abrams SL, Fitzgerald TL,
Cocco L, Martelli AM, Montalto G, Cervello M, Scalisi A, Candido S,
Libra M and Steelman LS: Roles of signaling pathways in drug
resistance, cancer initiating cells and cancer progression and
metastasis. Adv Biol Regul. 57:75–101. 2015. View Article : Google Scholar
|
|
10
|
Bonapace L, Coissieux MM, Wyckoff J, Mertz
KD, Varga Z, Junt T and Bentires-Alj M: Cessation of CCL2
inhibition accelerates breast cancer metastasis by promoting
angiogenesis. Nature. 515:130–133. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tsai JH and Yang J: Epithelial-mesenchymal
plasticity in carcinoma metastasis. Genes Dev. 27:2192–2206. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Turajlic S and Swanton C: Metastasis as an
evolutionary process. Science. 352:169–175. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
White BD, Chien AJ and Dawson DW:
Dysregulation of Wnt/β-catenin signaling in gastrointestinal
cancers. Gastroenterology. 142:219–232. 2012. View Article : Google Scholar
|
|
14
|
Gu Y, Wang Q, Guo K, Qin W, Liao W, Wang
S, Ding Y and Lin J: TUSC3 promotes colorectal cancer progression
and epithelial-mesenchymal transition (EMT) through WNT/β-catenin
and MAPK signalling. J Pathol. 239:60–71. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Han B, Wang J, Gao J, Feng S, Zhu Y, Li X,
Xiao T, Qi J and Cui W: DJ-1 as a potential biomarker for the early
diagnosis in lung cancer patients. Tumour Biol.
39:10104283177146252017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kawate T, Iwaya K, Koshikawa K, Moriya T,
Yamasaki T, Hasegawa S, Kaise H, Fujita T, Matsuo H, Nakamura T, et
al: High levels of DJ-1 protein and isoelectric point 63 isoform in
sera of breast cancer patients. Cancer Sci. 106:938–943. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Benati M, Montagnana M, Danese E, Paviati
E, Giudici S, Ruzzenente O, Franchi M and Lippi G: The clinical
significance of DJ-1 and HE4 in patients with endometrial cancer. J
Clin Lab Anal. 32:2018. View Article : Google Scholar
|
|
18
|
Chan JY and Chan SH: Activation of
endogenous antioxidants as a common therapeutic strategy against
cancer, neurodegeneration and cardiovascular diseases: A lesson
learnt from DJ-1. Pharmacol Ther. 156:69–74. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yuen HF, Chan YP, Law S, Srivastava G,
El-Tanani M, Mak TW and Chan KW: DJ-1 could predict worse prognosis
in esophageal squamous cell carcinoma. Cancer Epidemiol Biomarkers
Prev. 17:3593–3602. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jue C, Lin C, Zhisheng Z, Yayun Q, Feng J,
Min Z, Haibo W, Youyang S, Hisamitsu T, Shintaro I, et al: Notch1
promotes vasculogenic mimicry in hepatocellular carcinoma by
inducing EMT signaling. Oncotarget. 8:2501–2513. 2017. View Article : Google Scholar :
|
|
21
|
Da C, Liu Y, Zhan Y, Liu K and Wang R:
Nobiletin inhibits epithelial-mesenchymal transition of human
non-small cell lung cancer cells by antagonizing the TGF-β1/Smad3
signaling pathway. Oncol Rep. 35:2767–2774. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Geng XF, Fang M, Liu SP and Li Y: Quantum
dot-based molecular imaging of cancer cell growth using a clone
formation assay. Mol Med Rep. 14:3007–3012. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xiao J, Yang W, Xu B, Zhu H, Zou J, Su C,
Rong J, Wang T and Chen Z: Expression of fibronectin in esophageal
squamous cell carcinoma and its role in migration. BMC Cancer.
18:9762018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhu Y, Liu Y, Qian Y, Dai X, Yang L, Chen
J, Guo S and Hisamitsu T: Research on the efficacy of Celastrus
Orbiculatus in suppressing TGF-β1-induced epithelial-mesenchymal
transition by inhibiting HSP27 and TNF-α-induced NF-κB/Snail
signaling pathway in human gastric adenocarcinoma. BMC Complement
Altern Med. 14:4332014. View Article : Google Scholar
|
|
25
|
Aleyasin H, Rousseaux MW, Marcogliese PC,
Hewitt SJ, Irrcher I, Joselin AP, Parsanejad M, Kim RH, Rizzu P,
Callaghan SM, et al: DJ-1 protects the nigrostriatal axis from the
neurotoxin MPTP by modulation of the AKT pathway. Proc Natl Acad
Sci USA. 107:3186–3191. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim RH, Peters M, Jang Y, Shi W, Pintilie
M, Fletcher GC, DeLuca C, Liepa J, Zhou L, Snow B, et al: DJ-1, a
novel regulator of the tumor suppressor PTEN. Cancer Cell.
7:263–273. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Clements CM, McNally RS, Conti BJ, Mak TW
and Ting JP: DJ-1, a cancer- and Pa2rkinson's disease-associated
protein, stabilizes the antioxidant transcriptional master
regulator Nrf2. Proc Natl Acad Sci USA. 103:15091–15096. 2006.
View Article : Google Scholar
|
|
28
|
Hurst DR and Welch DR: Metastasis
suppressor genes at the interface between the environment and tumor
cell growth. Int Rev Cell Mol Biol. 286:107–180. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Valastyan S and Weinberg RA: Tumor
metastasis: Molecular insights and evolving paradigms. Cell.
147:275–292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Clevers H: Wnt/beta-catenin signaling in
development and disease. Cell. 127:469–480. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nusse R and Clevers H: Wnt/β-catenin
signaling, disease, and emerging therapeutic modalities. Cell.
169:985–999. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhou J, Liu H, Zhang L, Liu X, Zhang C,
Wang Y, He Q, Zhang Y, Li Y, Chen Q, et al: DJ-1 promotes
colorectal cancer progression through activating PLAGL2/Wnt/BMP4
axis. Cell Death Dis. 9:8652018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
MacDonald BT, Tamai K and He X:
Wnt/beta-catenin signaling: Components, mechanisms, and diseases.
Dev Cell. 17:9–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Du C, Lv Z, Cao L, Ding C, Gyabaah OA, Xie
H, Zhou L, Wu J and Zheng S: MiR-126-3p suppresses tumor metastasis
and angiogenesis of hepatocellular carcinoma by targeting LRP6 and
PIK3R2. J Transl Med. 12:2592014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tung EK, Wong BY, Yau TO and Ng IO:
Upregulation of the Wnt Co-receptor LRP6 promotes
hepatocarcinogenesis and enhances cell invasion. PLoS One.
7:e365652012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yamamoto H, Komekado H and Kikuchi A:
Caveolin is necessary for Wnt-3a-dependent internalization of LRP6
and accumulation of beta-catenin. Dev Cell. 11:213–223. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tang X, Fan Z, Wang Y, Ji G, Wang M, Lin J
and Huang S: Expression of klotho and β-catenin in esophageal
squamous cell carcinoma, and their clinicopathological and
prognostic signifi-cance. Dis Esophagus. 29:207–214. 2016.
View Article : Google Scholar
|
|
38
|
Chiurillo MA: Role of the Wnt/β-catenin
pathway in gastric cancer: An in-depth literature review. World J
Exp Med. 5:84–102. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
To SKY, Mak ASC, Eva Fung YM, Che CM, Li
SS, Deng W, Ru B, Zhang J and Wong AST: β-catenin downregulates
Dicer to promote ovarian cancer metastasis. Oncogene. 36:5927–5938.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wu X, Luo F, Li J, Zhong X and Liu K:
Tankyrase 1 inhibitior XAV939 increases chemosensitivity in colon
cancer cell lines via inhibition of the Wnt signaling pathway. Int
J Oncol. 48:1333–1340. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Guo W, Shen F, Xiao W, Chen J and Pan F:
Wnt inhibitor XAV939 suppresses the viability of small cell lung
cancer NCI-H446 cells and induces apoptosis. Oncol Lett.
14:6585–6591. 2017.
|