Introduction
Lung cancer (LC) is the leading cause of cancer
associated mortality and represents the most common malignancy
world wide (1). The outcome of LC
remains unsatisfactory, with low survival rates, presumably due to
a late diagnosis made in the advanced stages (2). Therefore, it is important to identify
novel biomarkers that can contribute to a better understanding of
LC biology and serve as new therapeutic targets.
Non small cell LC (NSCLC) is the major type of LC,
which accounts for ~85% of all LC cases. NSCLC comprises
adenocarcinoma (ADC), squamous cell carcinoma (SCC), large cell
carcinoma (LCC) and other rare subtypes (3).
Although smoking remains the main risk factor for LC
and the lung is not classically thought to be a target tissue for
sex steroid hormones, numerous studies have revealed sex
differences in LC pathogenesis (4,5).
These observations have shed light on the potential role of
oestrogens in the development of LC. Among never-smokers, women are
more likely to develop LC, with ADC being the predominant subtype
(6). Considering the same amount
of smoked cigarettes, women are more frequently diagnosed with
NSCLC than men are (7). These data
raise the question of whether there is a link between carcinogens
from cigarette smoke and oestrogens. Notably, several studies have
revealed that women are more vulnerable to the hazardous effects of
smoking (7-9). Smoking has been reported to enhance
the expression and activity of cytochrome P450 family 1 subfamily B
member 1 (CYP1B1) in lung tissue (10), which may consequently lead to the
formation of 2 and 4 catechol oestrogens. Subsequently, the
conversion of these oestrogens to toxic metabolites mediates DNA
damage (11,12). These reports support the role
played by oestrogens during LC development.
In addition, LC in young women demonstrates
aggressive biology with rapid growth, whereas postmenopausal women
with advanced NSCLC have survival advantages over men and younger
counterparts (13,14). Furthermore, high levels of serum 17
β oestradiol (E2) have been associated with shorter survival of men
with advanced NSCLC (15), and
high plasma concentrations of dihydrotestosterone and testosterone
(T) have been correlated with an increased LC incidence in older
men (16).
Numerous studies have confirmed the presence of
classical oestrogen receptors (ERs), ER α and ER β, as well as a
membrane G protein coupled ER (GPER) in NSCLC tumours, regardless
of sex (17 23). A growing body of evidence has indicated that
oestrogen signalling mediated by these recep tors may promote the
growth, survival and aggressiveness of human NSCLC cells,
contributing to cancer development and progression (18,24 27).
Although the ovaries are the main source of
oestrogens in women before menopause and the testicles are mostly
responsible for the synthesis of T in men, it is well known that
active sex steroids can be synthesized locally, in peripheral
tissues, from inactive precursors of adrenal origin (28,29).
Studies by Luu-The (28) and
Labrie (29) revealed that the
majority of peripheral tissues possess different sets of
steroidogenic enzymes involved in the local interconversion of
inactive precursors into their active forms. Synthesized sex
steroids act in an intracrine manner without being released into
the circulation (28,29). It was previously indicated that
oestrogens and androgens are metabolized within the lung and that
NSCLC cells are able to produce their own sex steroid hormones
(22,30). Niikawa et al (31) detected higher concentrations of E2
in LC tissues than in corresponding non neoplastic tissues from
patients with NSCLC, regard less of sex, suggesting that oestrogens
may be synthesized locally during LC development. Therefore, an
altered expression of enzymes responsible for the generation,
activation or inactivation of sex steroids may lead to a local
imbalance in androgen/oestrogen concentrations and trigger
carcinogenesis.
Earlier studies associated an elevated level of E2
in LC tissues with an increased expression of aromatase, which
converts 4 androstenedione into oestrone (E1) and T into E2
(31,32). However, the research carried out by
Verma et al (33), and the
results of our previous studies (34,35),
pinpointed another important pathway for E2 synthesis in NSCLC. The
presence of 17 β hydroxysteroid dehydrogenase type 1 (HSD17B1),
which catalyses the reduction of weak E1 into highly potent E2, was
detected in the investigated NSCLC cells. Our previous study
identified the ability of HSD17B1 to convert E1 into E2 in
vitro, and demonstrated an elevated expression of
HSD17B1 in NSCLC tissues compared with matched,
histopathologically unchanged specimens (34,35).
Encouraged by these results, we decided to continue our research
related to enzymes belonging to the HSD17B family.
The present study focused on 17 β hydroxysteroid
dehydrogenase type 2 (HSD17B2), an enzyme that catalyses the
oxidation of active steroids into their corresponding 17-keto
forms, efficiently inactivating E2, T and dihydrotestosterone in
various tissues (36,37). Therefore, HSD17B2 may regulate the
amount of active sex steroids within the lung, thus protecting
cells from their excess.
The aim of this study was to evaluate the expression
levels of HSD17B2 in LC and corresponding histopathologically
unchanged tissues from patients with NSCLC at the mRNA and protein
levels, and to determine the association between HSD17B2 and
clinicopathological features. Three different methods, which
complement each other, were conducted: Reverse transcription
quantitative PCR (RT-qPCR), western blotting and
immunohistochemistry. In addition, a retrospective analysis was
performed to investigate whether HSD17B2 mRNA or protein expression
may have prognostic significance in the survival outcome of
patients with NSCLC. To date, to the best of our knowledge, only
one study investigated the amount of HSD17B2 protein in NSCLC
clinical specimens. However, it was performed exclusively by
immunohistochemistry, and only in cancer tissues, without
comparison with adjacent normal tissues (33). To the best of our knowledge, this
study is the first to compare HSD17B2 mRNA and protein expression
between LC and adjacent histopathologically unchanged tissues, and
to evaluate their prognostic significance in NSCLC.
Materials and methods
Antibodies and reagents
The following antibodies (Abs) were used for western
blotting: Rabbit polyclonal anti HSD17B2 Ab (cat. no. ab103161;
Abcam), rabbit polyclonal anti-GAPDH Ab (FL-335; cat. no. sc-25778;
Santa Cruz Biotechnology, Inc.) and goat anti rabbit horseradish
peroxidase (HRP)-conjugated Ab (cat. no. 7074S; Cell Signaling
Technology, Inc.). For immunohistochemistry, rabbit polyclonal anti
HSD17B2 Ab (cat. no. 10978-1-AP) was purchased from ProteinTech
Group, Inc. TRI Reagent® for RNA isolation and RIPA
lysis buffer for protein isolation were provided by Sigma-Aldrich
(Merck KGaA).
Patient samples
Primary LC tissues and histopathologically unchanged
lung tissues, the latter located at a distance of 10 20 cm from the
cancerous lesions were obtained from 161 patients diagnosed with
NSCLC between March 2012 and May 2016. All patients underwent
surgical resection at the Department of Thoracic Surgery, Poznan
University of Medical Sciences (Poznan, Poland). None of the
patients received any preoperative chemotherapy or radiation
therapy. All patients provided written informed consent for the use
of clinical specimens. The procedures of the study were approved by
the Local Ethical Committee of Poznan University of Medical
Sciences, and all procedures were in accordance with the 1964
Declaration of Helsinki and its later amendments. After surgical
intervention, tissue samples were divided into two sets. One set
was immediately snap-frozen in liquid nitrogen for further
processing (homogenization, RNA and protein isolation). The other
set was used for histopathological examination, which was performed
by an experienced pathologist according to the 7th edition of the
tumour-node-metastasis (TNM) staging system (38). The residual tumour status after
surgical resection was also examined and classified in accordance
with the TNM staging system.
Measurement of overall survival (OS)
For the measurement of OS, patients were observed
from the moment of surgery (the first patient underwent surgery on
March 6, 2012) until death or December 31, 2017. In the OS
analysis, patients who died of non cancer related causes were
excluded. Follow-up data concerning OS were available for 147
patients. None of the patients received any preoperative
chemotherapy or radiation therapy.
Kaplan-Meier Plotter database and
survival analysis
To analyse the prognostic value of HSD17B2
expression in a wider group of patients, the online tool
Kaplan-Meier Plotter (http://kmplot.com/analysis/) was used. This database
contains gene expression data from multiple microarrays [from the
Gene Expression Omnibus (Affymetrix microarrays only), European
Genome Phenome Archive and The Cancer Genome Atlas databases] and
survival information for 1,926 patients with NSCLC (39). This study focused on OS,
post-progression survival (PPS) and first progression (FP)
survival.
The Affymetrix probe set ID for HSD17B2 was
204818_at. This study used 'JetSet best probe set', 'Auto select
best cutoff' options, and an array quality control set at 'exclude
biased arrays' during analysis. Patients were divided into high and
low HSD17B2 expression groups, and the Kaplan Meier survival
plots with P-values from the log-rank test, hazard ratio (HR) with
95% confidence intervals (CI) and numbers-at-risk were obtained. In
addition, plot data were exported as text and P values were
calculated using the Gehan Breslow Wilcoxon test when the log-rank
test was not applicable (when survival curves were not parallel at
late stages of analysis). P<0.05 was considered to indicate a
statistically significant difference.
RT-qPCR analysis
All investigated tissues (cancerous and
histopathologically unchanged tissues obtained from 161 patients)
were homogenized into a powder in liquid nitrogen, and total RNA
was isolated using TRI Reagent® (Sigma-Aldrich; Merck
KGaA). Subsequently, RNA samples were treated with recombinant
DNase I using a DNA free™ DNA Removal kit (Ambion; Thermo Fisher
Scientific, Inc.) to eliminate any residual DNA. RNA quality and
concentration were determined by denaturing agarose gel (1.2%)
electro phoresis and by absorbance measurements using a NanoDrop
One spectrophotometer (NanoDrop; Thermo Fisher Scientific, Inc.).
Quantified samples were then reverse-transcribed into cDNA using
M-MLV reverse transcriptase (Invitrogen; Thermo Fisher Scientific,
Inc.), according to the manufactur er's protocol. For cDNA
synthesis, a mixture of oligo(dT)23 and random
hexaprimers was used, with 1 μg isolated total RNA as a
template. RT-qPCR was conducted using a Light Cycler®
480 Real Time PCR system (Roche Diagnostics GmbH) with SYBR Green I
used as the fluorophore. Target cDNA was quantified according to
the relative quantification method using a calibrator. For the
calibrator, 1 μl cDNA from each tissue sample was mixed
together. Consecutive dilutions of the calibrator served for the
generation of standard curves for all investigated genes, as
provided in the Relative Quantification Manual (Roche Diagnostics
GmbH) (40). The quantity of
HSD17B2 transcript in each sample was standardized by the
geometric mean of porphobilinogen deaminase, human mitochondrial
ribosomal protein L19 and RNA polymerase II subunit A cDNA levels.
The PCR amplification efficiency for target and reference genes was
92, 100, 92 and 98%, respec tively. For amplification, 1 μl
total (20 μl) cDNA solution was added to 9 μl 1X
concentrated Light Cycler® 480 SYBR Green I Master mix
(Roche Diagnostics GmbH), containing 2.5 mM MgCl2, and
0.5 μM or 1 μM of primers, and subjected to 40 PCR
cycles preceded by 10 min of activation at 95°C. Primer sequences
and specific amplification conditions are presented in Table I. A sample of non reverse
transcribed RNA and a no template control were included in each
batch of samples as negative controls. Melting curve analysis and
agarose gel (1.5%) electrophoresis were applied to confirm the
specificity of the amplified products. All cDNA samples were
applied in triplicate for each gene of interest. HSD17B2
transcript levels in the investigated tissues were expressed as the
decimal logarithm of multiplicity of cDNA concentrations in the
calibrator.
 | Table IPrimer sequences used for reverse
transcription quantitative PCR analysis. |
Table I
Primer sequences used for reverse
transcription quantitative PCR analysis.
| Gene | Sequence
(5′-3′) | ENST/ENSG number
(www.ensembl.org/) | Product size
(bp) | Final primer
concentration | RT-qPCR cycling
conditions (denaturation; annealing; extension) |
|---|
| HSD17B2 | F:
CAATGCTGCAGGACAGAGGA
R: GTTCACGGCCATGCATTGTT |
ENST00000199936 | 116 | 0.5 μM | 95°C/6 sec; 60°C/6
sec; 72°C/6 sec |
| PBGD | F:
GCCAAGGACCAGGACATC
R: TCAGGTACAGTTGCCCATC |
ENST00000278715 | 160 | 0.5 μM | 95°C/6 sec; 61°C/6
sec; 72°C/6 sec |
| hMRPL19 | F:
ACTTTATAATCCTCGGGTC
R: ACTTTCAGCTCATTAACAG |
ENST00000393909 | 171 | 1 μM | 95°C/10 sec;
56°C/10 sec; 72°C/10 sec |
| POLR2A | F:
AAGTGGTGGAGGGAGTCAAG
R: CGCAGGTGGATGTTGAAGAG |
ENST00000617998 | 115 | 0.5 μM | 95°C/6 sec; 58°C/6
sec; 72°C/6 sec |
Sodium dodecyl sulphate-polyacrylamide
gel electrophoresis (SDS-PAGE) and western blotting
Proteins for western blotting were isolated as
previously described (41).
Briefly, all tissue specimens obtained from 161 patients were
homogenized in liquid nitrogen and treated with RIPA lysis buffer
(Sigma-Aldrich; Merck KGaA) supplemented with protease inhibitor
cocktail (Roche Diagnostics GmbH). Samples were incubated on ice
for 30 min and were then centrifuged at 10,000 × g for 10 min at
4°C. Supernatants were collected for western blotting.
Subsequently, 2 μl isolated proteins from each sample were
denatured in sample loading buffer and separated by standard
SDS-PAGE on 10% Tris-glycine gels. Gel proteins were
electrotransferred to a nitrocellulose membrane, which was then
blocked in 5% non-fat dry milk in 1X concentrated Tris buffered
saline/Tween-20 (0.1%) for 1 h at room temperature. After blocking,
each membrane was incubated overnight at 4°C with rabbit
anti-HSD17B2 Ab (1:800), followed by 2 h incubation with goat anti
rabbit HRP conjugated Ab (1:2,500) at room temperature. Bands were
visualized using SuperSignal West Femto Chemiluminescent Substrate
(Thermo Fisher Scientific, Inc.). To ensure equal protein loading,
membranes were stripped and incubated with rabbit polyclonal anti
GAPDH Ab (1:3,300) for 2 h, followed by incubation with goat anti
rabbit HRP conjugated Ab (1:2,500) for 1 h at room temperature.
Bands were revealed using Clarity Western ECL Substrate (Bio Rad
Laboratories, Inc.). For signal visualization, the Biospectrum
Imaging system 500 (UVP, LLC) was used. The time of exposure and
camera settings were identical for all membranes. The amount of
HSD17B2 protein is presented as the decimal logarithm of the
HSD17B2 to GAPDH band optical density ratio, which was measured
using ImageJ2x program (https://imagej.net/ImageJ2). Samples were run in
technical duplicates to confirm the reproducibility of the
results.
Immunohistochemistry
Cancer tissues (n=161) were fixed in 10% formalin
for 24 h at room temperature, embedded in paraffin and cut into
4-μm sections. Samples were then mounted on
SuperFrost® Plus adhesion microscope slides (Menzel;
Thermo Fisher Scientific, Inc.) and stained according to the
presented optimized procedure. Briefly, slides were deparaffinized
(overnight at 60°C and immersed in xylene for 30 min) and
rehydrated (two washes in 100, 96, 75 and 50% ethanol and distilled
water; 5 min/wash). Subsequently, heat induced epitope retrieval
was carried out by heating slides in low pH EnVision FLEX Target
Retrieval Solution (Dako; Agilent Technologies, Inc.) for 50 min at
97°C in a water bath. The sections were then incubated for 10 min
at room temperature in Novocastra Peroxidase Block reagent (Leica
Microsystems, Ltd.) to neutralize endogenous peroxidase activity
and were treated with Novocastra Protein Block (Leica Microsystems,
Ltd.) for 10 min at room temperature to reduce non-specific binding
of Ab and polymer. Sections were then incubated over night with
rabbit polyclonal anti HSD17B2 Ab at a dilution of 1:120 in a
humidified chamber at 4°C. The primary Ab was diluted in EnVision
FLEX Antibody Diluent (Dako; Agilent Technologies, Inc.).
Immunodetection was achieved using the Novolink Polymer Detection
system (Leica Microsystems, Ltd.), which is a two step streptavidin
biotin HRP method. Each slide was incubated for 30 min at room
temperature, and between each step, slides were washed in EnVision
FLEX Wash Buffer (Dako; Agilent Technologies, Inc.). Finally, the
reaction product was visualized using 3,3′ diaminobenzidine
tetrahydrochloride (DAB) prepared from Novolink Polymer DAB
Chromogen and Novolink DAB Substrate Buffer (two incubations for 5
min at room temperature). Sections were counterstained with Mayer's
haematoxylin for 5 min at room temperature, dehydrated, cleared and
mounted in DPX. Sections from formalin-fixed and paraffin-embedded
normal human liver were used as a positive control for anti HSD17B2
Ab. Normal liver tissue was obtained from a patient with squamous
lung cancer with liver metastasis, and written informed consent was
provided for the use of tissue samples. As a negative control,
sections were incubated with an omission of the primary Ab.
Evaluation of immunohistochemical staining was
scored independently by two experienced pathologists and was
repeated twice for each sample. Both pathologists were blinded to
the clinical characteristics of the patients and scores were
accepted if investigators agreed with each other. Otherwise,
specimens were re-evaluated until a consensus was reached.
Immunohistochemical reactivity was assessed using a semi
quantitative method based on staining intensity, with a score of 0,
negative staining; 1, weak staining; and 2, strong staining, in
>60% of tumour cells. Moderate staining was omitted, as it is
highly subjective. Furthermore, in each group, subgroups were
extracted based on the percentage of positively stained tumour
cells (2/1, strong staining in >60% and weak staining in 30-40%
of tumour cells; 1/2, weak staining in >60% and strong staining
in 30-40% of tumour cells; 1/0, weak staining in >60% and
negative staining in 30-40% of tumour cells; and 0/1, negative
staining in >60% and weak staining in 30-40% of tumour cells).
Based on this evaluation, cases were considered to have high (2 and
2/1) and low (1/2; 1/0; 0/1; and 0) expression of HSD17B2 protein.
In order to perform univariate survival analysis based on the
immunohis tochemical results, LC specimens were subdivided into
three groups: High (score 2 and 2/1), intermediate (score 1/2) and
low (score 1/0, 0/1 and 1) HSD17B2 protein levels.
Statistical analysis
Statistical analyses were performed using STATISTICA
12 software (StatSoft, Inc.) and GraphPad Prism 8.3.0 version
(GraphPad Software, Inc.). The Shapiro-Wilk test was used to assess
the normality of the observed patient data distribution. Because
HSD17B2 mRNA and protein expression levels were not normally
distributed, the Wilcoxon matched-pairs signed rank test was used
to consider statistically significant differences in HSD17B2 mRNA
and protein levels between LC and histopathologically unchanged
tissues (P<0.05). χ2 and Fisher's exact tests were
used to determine whether there was a significant association
between HSD17B2 expression and various clinicopathological
parameters in LC tissues. U Mann Whitney (for comparisons between
two groups) and Kruskal-Wallis tests (for comparisons between three
or more groups) were performed to evaluate the relationship between
HSD17B2 expression and clinicopathological parameters in
histopathologically unchanged lung tissues. Survival curves were
plotted using the Kaplan Meier method, and differences were
estimated by log-rank test or Gehan Breslow Wilcoxon test.
Univariate and multivariate Cox proportional hazard models were
used to estimate HR. P<0.05 was considered to indicate a
statistically significant difference.
Results
Patient characteristics
In total, 161 patients diagnosed with NSCLC were
included in the present study. The median age of patients at the
moment of resection was 65 years (range, 29-80). Among the 63 women
enrolled, all patients were postmenopausal. Only four women
received hormone replacement therapy (E2 transdermal patches). The
majority of patients were smokers (n=146). LC grading was evaluated
for 153 patients, excluding eight patients with carcinoid tumours.
Detailed clinicopathological characteristics of the patients are
presented in Table II.
 | Table IIDifferences in HSD17B2 transcript and
protein levels between lung cancer and corresponding
histopathologically unchanged tissues from patients with non small
cell lung cancer, including clinicopathological
characteristics. |
Table II
Differences in HSD17B2 transcript and
protein levels between lung cancer and corresponding
histopathologically unchanged tissues from patients with non small
cell lung cancer, including clinicopathological
characteristics.
| | HSD17B2
transcript level
| HSD17B2 protein
level
|
|---|
| | Cancerous tissues
| Histopathologically
unchanged tissues
| | Cancerous tissues
| Histopathologically
unchanged tissues
| |
|---|
| Variables | Number of
cases | Median (range) | Median (range) | P value | Median (range) | Median (range) | P value |
|---|
| No. of
patients | 161 | 2.65
(0.13-4.39) | 3.43
(0.07-5.61) |
<10−6 | 2.54
(0.07-4.39) | 3.60
(1.03-6.81) |
<10−6 |
| Sex | | | | | | | |
| Male | 98 | 2.60
(0.18-4.06) | 3.31
(0.07-5.61) |
<10−6 | 2.50 (0.38
4.05) | 3.40 (1.03
6.81) |
<10−6 |
| Female | 63 | 2.70
(0.13-4.39) | 3.63
(2.46-5.28) |
<10−6 | 2.58
(0.07-4.39) | 4.06
(2.36-6.30) |
<10−6 |
| Patient age
(years) | | | | | | | |
| ≤60
(males:females) | 45 (29;16) | 2.61
(0.13-3.81) | 3.41
(1.53-5.45) |
<10−6 | 2.50
(0.07-3.97) | 3.56
(2.06-6.53) |
<10−6 |
| >60
(males:females) | 116 (69:47) | 2.66
(0.13-4.39) | 3.45
(0.07-5.61) |
<10−6 | 2.55
(0.43-4.39) | 3.63
(1.03-6.81) |
<10−6 |
| Histological
type | | | | | | | |
| Adenocarcinoma
(males:females) | 70 (37:33) | 2.49
(0.13-.39) | 3.53
(0.07-5.49) |
<10−6 | 2.34
(0.07-4.39) | 3.65
(1.03-6.30) |
<10−6 |
| Squamous cell
carcinoma (males:females) | 71 (50:21) | 2.76
(0.58-3.89) | 3.35
(1.53-5.61) |
<10−6 | 2.66 (0.38
4.04) | 3.48 (1.80
6.81) |
<10−6 |
| Large cell
carcinoma (males:females) | 12 (9:3) | 2.55
(0.43-3.21) | 3.50
(2.83-4.54) | 0.0029 | 2.66
(0.43-3.26) | 3.54
(2.81-5.25) | 0.0022 |
| Carcinoid
(males:females) | 8 (2:6) | 2.09
(0.13-3.67) | 3.35
(2.70-4.64) | 0.012 | 2.47
(1.12-3.77) | 3.36
(2.67-5.19) | 0.012 |
| Lung cancer
stage | | | | | | | |
| 0
(males:females) | 6 (3:3) | 2.02
(0.13-3.67) | 3.07
(2.70-4.01) | 0.028 | 1.88
(1.12-3.77) | 3.18
(2.67-4.95) | 0.028 |
| IA-IB
(males:females) | 63 (29:34) | 2.84
(0.13-4.15) | 3.50
(2.46-5.61) |
<10−6 | 2.77 (0.07
4.21) | 3.84 (2.36
6.81) |
<10−6 |
| IIA-IIB
(males:females) | 53 (35:18) | 2.41
(0.15-4.39) | 3.60
(0.07-5.49) |
<10−5 | 2.30
(0.10-4.39) | 3.64
(1.03-6.53) |
<10−6 |
| IIIA-IV
(males:females) | 39 (31:8) | 2.60
(1.20-3.81) | 3.24
(2.42-5.11) |
<10−5 | 2.41
(1.04-3.97) | 3.34
(2.38-6.11) |
<10−6 |
| Lung cancer
gradea | | | | | | | |
| G1
(males:females) | 12 (7:5) | 2.94
(1.64-4.39) | 3.31
(2.76-5.21) | 0.21 | 2.89
(1.44-4.39) | 3.36
(2.72-6.30) | 0.019 |
| G2
(males:females) | 68 (44:24) | 2.71
(0.62-4.15) | 3.29
(2.34-5.49) |
<10−6 | 2.59
(0.42-4.21) | 3.39
(1.80-6.53) |
<10−6 |
| G3
(males:females) | 73 (47:26) | 2.58
(0.13-3.89) | 3.59
(0.07-5.61) |
<10−6 | 2.46 (0.07
4.05) | 3.84 (1.03
6.81) |
<10−6 |
| Tumour size | | | | | | | |
| Tis
(males:females) | 6 (3:3) | 2.02
(0.13-3.67) | 3.07
(2.70-4.01) | 0.028 | 1.88
(1.12-3.77) | 3.18
(2.67-4.95) | 0.028 |
| T1
(males:females) | 23 (13:10) | 3.11
(2.04-3.89) | 3.71
(2.42-5.25) | 0.00092 | 3.04
(1.92-4.04) | 4.19
(2.85-6.29) | 0.00013 |
| T2
(males:females) | 99 (55:44) | 2.59
(0.13-4.39) | 3.48
(1.53-5.61) |
<10−6 | 2.53 (0.07
4.35) | 3.64 (1.803
6.81) |
<10−6 |
| T3-T4
(males:females) | 33 (27:6) | 2.51
(0.58-4.35) | 3.23
(0.07-4.91) | 0.0003 | 2.39
(0.38-4.39) | 3.31
(1.03-5.63) |
<10−4 |
| Lymph node
metastasis | | | | | | | |
| N0
(males:females) | 95 (49:46) | 2.73
(0.13-4.35) | 3.46
(0.07-5.61) |
<10−6 | 2.61
(0.07-4.39) | 3.63
(1.03-6.81) |
<10−6 |
| N1
(males:females) | 44 (34:10) | 2.37
(0.15-4.39) | 3.34
(1.53-5.50) |
<10−6 | 2.27 (0.10
4.35) | 3.45 (1.80
6.53) |
<10−6 |
| N2
(males:females) | 22 (15:7) | 2.66
(1.73-3.81) | 3.42
(2.42-5.11) | 0.00098 | 2.47
(1.63-3.97) | 3.50
(2.53-6.11) |
<10−4 |
| Distant
metastasis | | | | | | | |
| M0
(males:females) | 158 (95:63) | 2.60
(0.13-4.38) | 3.45
(0.07-5.61) |
<10−6 | 2.53
(0.07-4.39) | 3.62
(1.03-6.81) |
<10−6 |
| M1
(males:females) | 3 (3:0) | 2.78 (2.65
3.05) | 3.33 (2.82
3.34) | | 2.73 (2.55
2.81) | 3.34 (2.72
3.37) | |
| Smoking | | | | | | | |
| Yes
(males:females) | 146 (92:54) | 2.66
(0.13-4.39) | 3.34
(0.07-5.61) |
<10−6 | 2.55 (0.07
4.35) | 3.58 (1.03
6.81) |
<10−6 |
| No
(males:females) | 15 (6:9) | 2.41
(0.18-4.35) | 3.41
(2.70-5.21) | 0.0045 | 2.29
(1.13-4.39) | 3.63
(2.67-6.30) | 0.0015 |
| Residual tumour
status | | | | | | | |
| R0
(males:females) | 144 (84:60) | 2.60
(0.13-4.39) | 3.42
(0.07-5.61) |
<10−6 | 2.53
(0.07-4.39) | 3.56
(1.03-6.81) |
<10−6 |
| R1
(males:females) | 16 (13:3) | 2.94
(1.87-3.81) | 3.43
(2.84-5.44) | 0.0072 | 2.85
(1.75-3.77) | 3.78
(2.85-6.53) | 0.0013 |
| R2
(males:females) | 1 (1:0) | – | - | - | - | - | - |
Differences in HSD17B2 transcript and
protein levels between LC tissues and adjacent histopathologically
unchanged tissues from patients with NSCLC
RT-qPCR and western blotting were performed to
compare the expression status of HSD17B2 in LC and
histopathologically unchanged tissues. HSD17B2 mRNA (P<10
6) and protein (P<10 6) expression levels
were significantly lower in cancer tissues compared with in
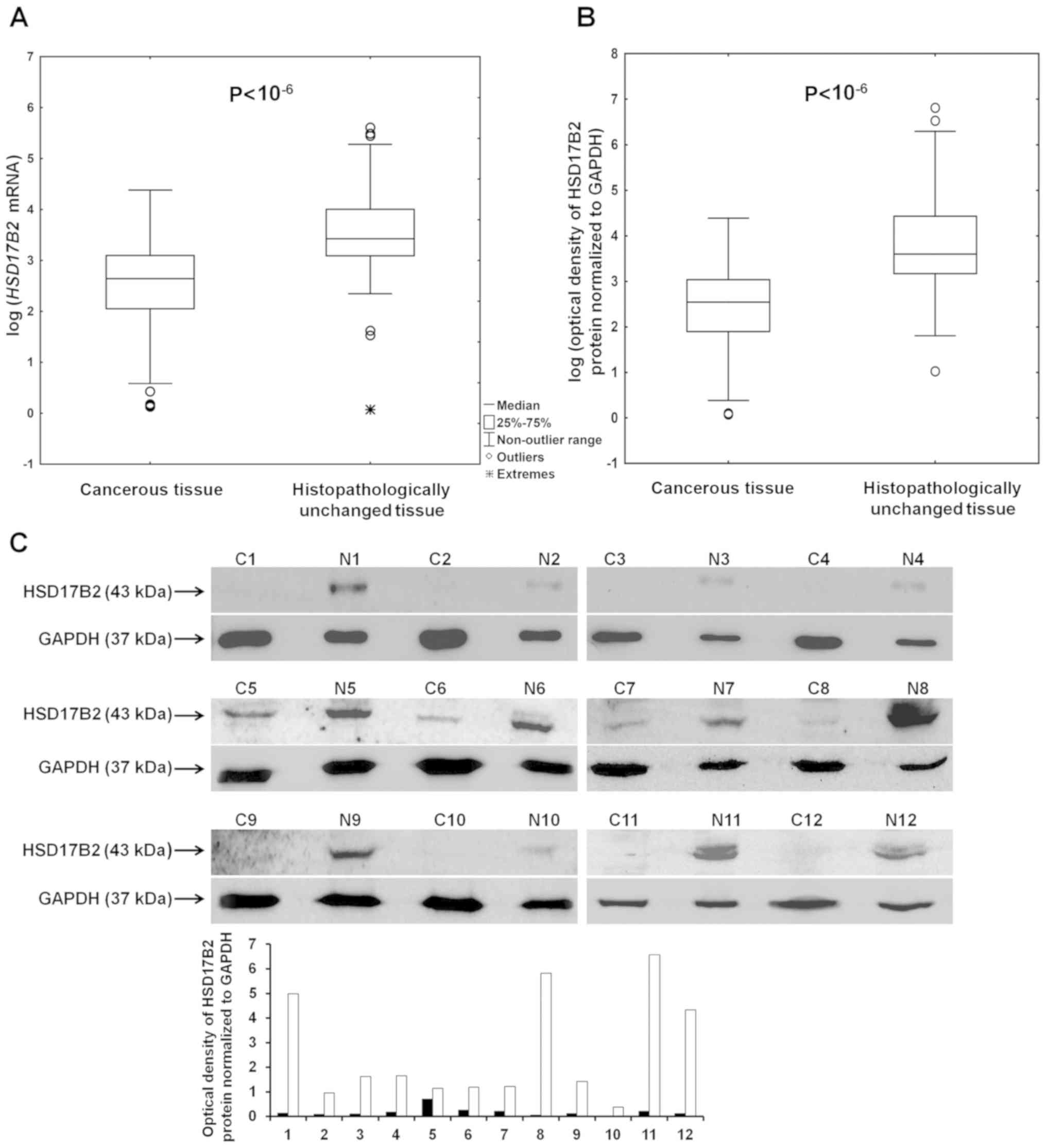
adjacent normal tissues (Fig. 1A and
B; Table II). A
representative image of western blotting results with a bar graph
including optical density measurements of bands is presented in
Fig. 1C. The clinicopathological
characteristics of patients whose samples were used to generate the
image presented in Fig. 1C are
summarized in Table SI.
In addition, this study aimed to determine whether
the differences in HSD17B2 mRNA and protein levels among the
investigated tissues were associated with various
clinicopathological features (Table
II). HSD17B2 mRNA and protein expression levels were
significantly lower in cancer tissues regardless of sex or age
(P<10 6). With regards to NSCLC histological
subtypes, a substantial decrease in HSD17B2 mRNA and protein
expression was detected in ADC and SCC specimens (P<10
6). Although only 12 patients included in this study
presented with LCC, HSD17B2 mRNA and protein expression was also
significantly diminished in cancer tissues compared with
histopathologically unchanged specimens (P=0.0029 and P=0.0022,
respectively). Furthermore, the expression of HSD17B2 was detected
in tissues from eight patients with carcinoid tumours; notably,
reduced mRNA and protein expression levels of HSD17B2 were detected
in tumour tissues compared with matched normal specimens (P=0.012
and P=0.012, respectively). Statistically significant differences
in HSD17B2 mRNA and protein levels between investigated tissues
were also detected in all LC stages and all tumour sizes (Table II). Notably, only six patients
were diagnosed with stage 0 cancer and carcinoma in situ;
therefore, this is not a representative group to consider the
statistical significance of HSD17B2 expression. HSD17B2 mRNA and
protein expression levels were decreased in LC tissues compared
with their matched normal counterparts in all grades of lymph node
metastasis (Table II), and were
associated with G2 and G3 LC histological grades (P<10
6; Table II). In the
group of patients with low grade tumours (G1), the difference in
HSD17B2 protein levels was statistically significant, whereas a
tendency towards lower HSD17B2 mRNA levels in cancer tissues
was observed; however, this was not significant. Furthermore,
HSD17B2 mRNA and protein levels were significantly decreased in LC
tissues regardless of smoking status and residual tumour status
(Table II). The majority of
patients included in this study (n=158) presented no distant
metastasis (M0); therefore, no conclusions can be made concerning
this parameter. A strong positive association between HSD17B2 mRNA
expression and protein expression was detected in all investigated
tissues (data not shown).
HSD17B2 immunoreactivity in clinical
tissue specimens
Although LC tissues used for RT-qPCR and western
blotting were obtained from the centre of the tumour, it is
possible that these specimens contained some non tumoural cells and
stromal cells. Therefore, to verify the present results and to
reveal the location of HSD17B2 protein, immunohistochemical
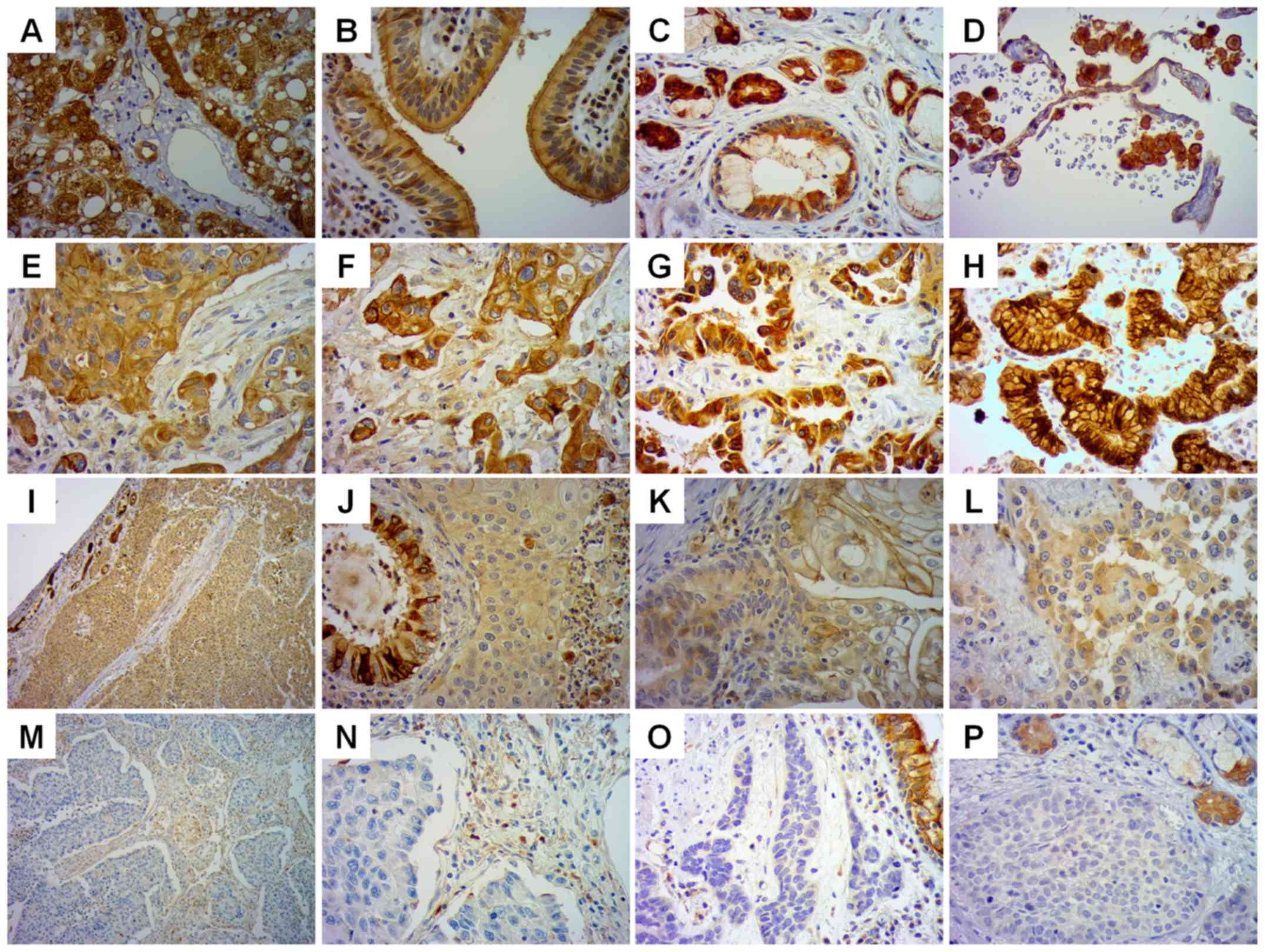
staining was performed with anti-HSD17B2 Ab (Fig. 2).
Sections of normal human liver were used as a
positive control for anti HSD17B2 Ab (Fig. 2A). A positive reaction, with strong
cytoplasmic staining, was detected in normal respiratory
epithelium, histopathologically unchanged glands, and macrophages
(Fig. 2B-D). In addition, weak
staining was detected in lung endothelial cells (Fig. 2D). Among all NSCLC specimens, only
58 (36%) demonstrated high cytoplasmic staining of HSD17B2 in
cancer cells (Fig. 2E-H). A total
of 72 specimens (45%) exhibited weak HSD17B2 immunoreactivity in
cancer cells (Fig. 2I-L), whereas
31 (19%) were negative (Fig.
2M-P). A weak positive reaction was revealed in tumour stromal
cells. The presence of HSD17B2 protein was also confirmed in normal
submucosal glands (Fig. 2I and P)
and in normal bronchial epithelial cells (Fig. 2J and O), located in the tumour
field. The clinicopathological characteristics of the patients
whose tissues were used to generate the staining images presented
in Fig. 2 are detailed in Table SII.
Association between HSD17B2 mRNA and
protein levels in LC tissues and various clinicopathological
parameters
To evaluate the clinical significance of HSD17B2
expression in NSCLC, this study investigated whether there was an
association between HSD17B2 mRNA and/or protein expression and
clinicopathological features in tumour specimens. The results from
RT-qPCR analysis were divided into two groups according to the
median value of the detected HSD17B2 expression in cancer
tissues. Tissues that displayed values higher than the median were
classified as having high expression, and those that displayed
values equal to or lower than the median were classified as having
low expression. With regards to HSD17B2 protein levels,
immunohistochemistry results were used. LC specimens were
classified into two groups: Those with high and low levels of
HSD17B2 protein in cancer cells, according to immunohistochemistry
staining classification.
In cancer tissues, HSD17B2 mRNA and protein
expression levels were significantly associated with LC stages
(P=0.017; P=0.0006), tumour size (P=0.0061; P=0.046), and lymph
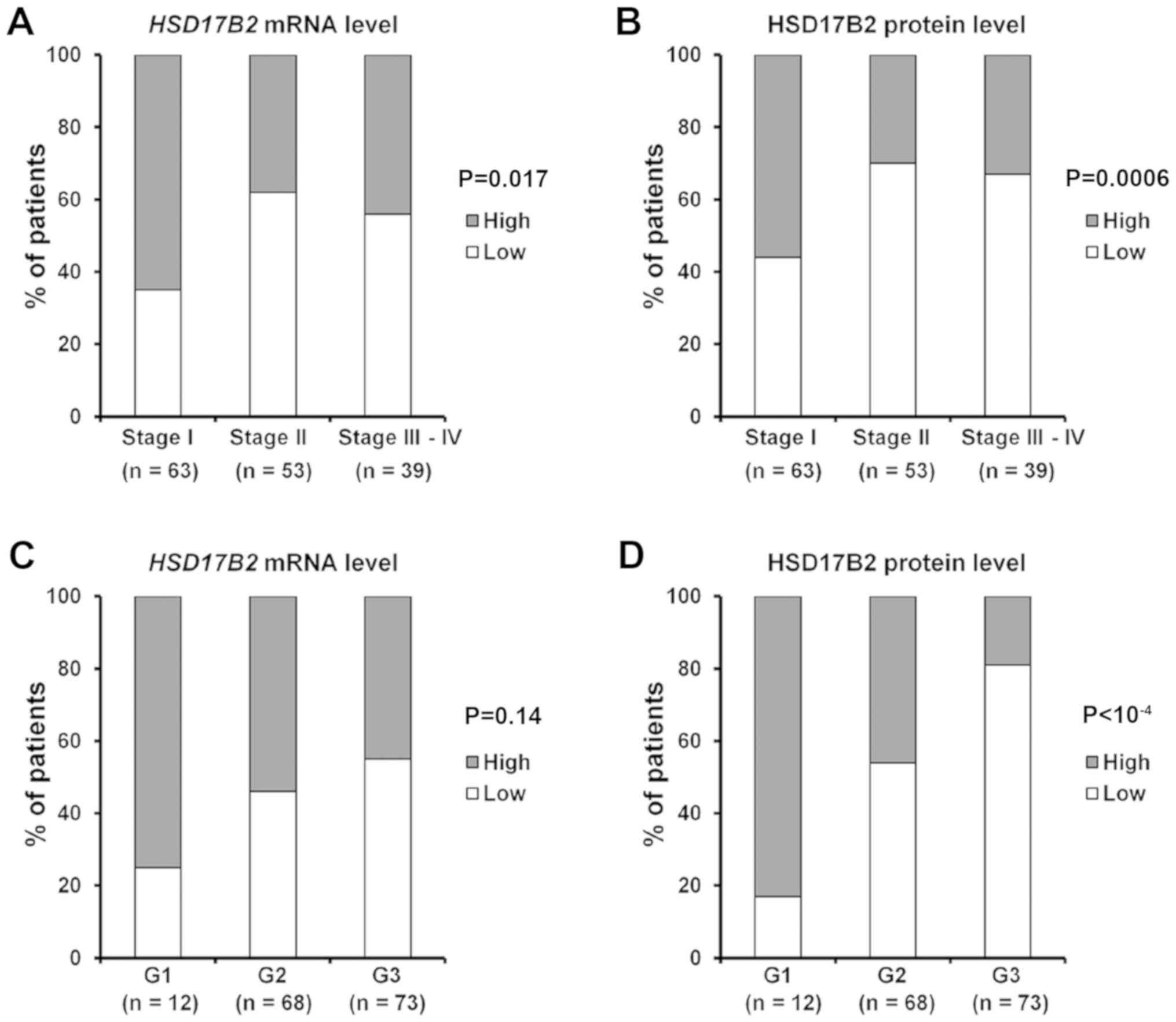
node metastasis (P=0.0055; P=0.028; Table III). Notably, 65% of patients
with stage I disease presented a high level of HSD17B2
transcripts, and this feature was diminished to 38% of patients
with stage II disease and 44% of patients with stage III disease
(Fig. 3A; Table III). The difference was even more
substantial for HSD17B2 protein expression. Only 44% of patients
with stage I disease had low HSD17B2 levels in cancer cells,
whereas as much as 77 and 74% of patients with stage II and III IV
disease showed decreased amounts of HSD17B2 protein in cancer
specimens, respectively (Fig. 3B;
Table III). Similarly, the same
tendency was maintained regarding tumour size. Furthermore,
HSD17B2 expression was strongly associated with LC grading.
Although the difference in transcript levels among patients with
various LC grades appeared to be not statistically significant, the
tendency towards lower mRNA expression in higher grade LC
(moderately or poorly differentiated tumours, G2 and G3) was
revealed (Fig. 3C; Table III). Notably, a great majority of
patients with G3 LC (81%) exhibited weak or negative staining for
HSD17B2 protein, whereas in the G1 group (well differentiated, low
grade tumours), 83% of patients had high amounts of HSD17B2 protein
(P<10 4; Fig. 3D;
Table III). Furthermore, lower
levels of HSD17B2 mRNA and protein were observed in LC tissues from
patients with N1 lymph node metastasis.
 | Table IIIAssociation between HSD17B2 mRNA and
protein levels and clinicopathological parameters in lung cancer
tissues from patients with non small cell lung cancer. |
Table III
Association between HSD17B2 mRNA and
protein levels and clinicopathological parameters in lung cancer
tissues from patients with non small cell lung cancer.
| | HSD17B2 mRNA
expression
| | HSD17B2 protein
expression
| |
|---|
| Variable | Number of
cases | High | Low | P value | High | Low | P value |
|---|
| Sex | | | | 0.13 | | | 0.07 |
| Male | 98 | 44 (45%) | 54 (55%) | | 30 (31%) | 68 (69%) | |
| Female | 63 | 36 (57%) | 27 (43%) | | 28 (44%) | 35 (56%) | |
| Patient age
(years) | | | | 0.63 | | | 0.51 |
| ≤60 | 45 | 21 (47%) | 24 (53%) | | 18 (40%) | 27 (60%) | |
| >60 | 116 | 59 (51%) | 57 (49%) | | 40 (34%) | 76 (66%) | |
| Histological
type | | | | 0.11 | | | 0.089 |
|
Adenocarcinoma | 70 | 29 (41%) | 41 (59%) | | 30 (43%) | 40 (57%) | |
| Squamous cell
carcinoma | 71 | 43 (61%) | 28 (39%) | | 23 (32%) | 48 (68%) | |
| Large cell
carcinoma | 12 | 5 (42%) | 7 (58%) | | 1 (8%) | 11 (92%) | |
| Carcinoid | 8 | 3 (37%) | 5 (63%) | | 4 (50%) | 4 (50%) | |
| Lung cancer
stage | | | | 0.017 | | | 0.0006 |
| 0 | 6 | 2 (33%) | 4 (67%) | | 1 (17%) | 5 (83%) | |
| IA-IB | 63 | 41 (65%) | 22 (35%) | | 35 (56%) | 28 (44%) | |
| IIA-IIB | 53 | 20 (38%) | 33 (62%) | | 12 (23%) | 41 (77%) | |
| IIIA-IV | 39 | 17 (44%) | 22 (56%) | | 10 (26%) | 29 (74%) | |
| Lung cancer
gradea | | | | 0.14 | | |
<10−4− |
| G1 | 12 | 9 (75%) | 3 (25%) | | 10 (83%) | 2 (17%) | |
| G2 | 68 | 37 (54%) | 31 (46%) | | 31 (46%) | 37 (54%) | |
| G3 | 73 | 33 (45%) | 40 (55%) | | 14 (19%) | 59 (81%) | |
| Tumour size | | | | 0.0061 | | | 0.046 |
| Tis | 6 | 2 (33%) | 4 (67%) | | 1 (17%) | 5 (83%) | |
| T1 | 23 | 19 (83%) | 4 (17%) | | 14 (61%) | 9 (39%) | |
| T2 | 99 | 46 (46%) | 53 (54%) | | 33 (33%) | 66 (67%) | |
| T3-T4 | 33 | 13 (39%) | 20 (61%) | | 10 (30%) | 23 (70%) | |
| Lymph node
metastasis | | | | 0.0055 | | | 0.028 |
| N0 | 95 | 56 (59%) | 39 (41%) | | 38 (40%) | 57 (60%) | |
| N1 | 44 | 13 (30%) | 31 (70%) | | 9 (20%) | 35 (80%) | |
| N2 | 22 | 11 (50%) | 11 (50%) | | 11 (50%) | 11 (50%) | |
| Distant
metastasis | | | | 0.55 | | | 0.19 |
| M0 | 158 | 78 (49%) | 80 (51%) | | 58 (37%) | 100 (63%) | |
| M1 | 3 | 2 (67%) | 1 (33%) | | 0 | 3 (100%) | |
| Smoking | | | | 0.81 | | | 0.82 |
| Yes | 146 | 73 (50%) | 73 (50%) | | 53 (36%) | 93 (64%) | |
| No | 15 | 7 (47%) | 8 (53%) | | 5 (33%) | 10 (67%) | |
| Residual tumour
status | | | | 0.29 | | | 0.51 |
| R0 | 144 | 70 (49%) | 74 (51%) | | 51 (35%) | 93 (65%) | |
| R1 | 16 | 10 (62%) | 6 (38%) | | 7 (44%) | 9 (56%) | |
Association between HSD17B2 mRNA and
protein levels in histopathologically unchanged lung tissues and
various clinicopathological parameters
In addition, the relationship between the
clinicopathological features of patients with NSCLC and HSD17B2
expression status in histopathologically unchanged lung tissues was
assessed (Table IV). RT-qPCR and
western blotting results were used, because tumour adjacent,
histopathologically unchanged lung specimens were not available for
immunohistochemical staining. Notably, women had significantly
higher levels of HSD17B2 mRNA and protein in tumour matched,
macroscopically unchanged tissue specimens than men (P=0.0012;
P=0.0022). The expression levels of HSD17B2 mRNA and protein were
also associated with tumour size (P=0.030; P=0.021). The expression
levels of HSD17B2 tended to decrease in histopathologically
unchanged tissues obtained from patients diagnosed with larger
tumour dimensions. This tendency was also maintained for advanced
LC stages. No significant associations were detected between
HSD17B2 expression and the other clinicopathological variables in
the investigated macroscopically unchanged tissues.
 | Table IVAssociation between HSD17B2 mRNA and
protein levels and clinicopathological parameters in lung
histopathologically unchanged tissues from patients with non small
cell lung cancer. |
Table IV
Association between HSD17B2 mRNA and
protein levels and clinicopathological parameters in lung
histopathologically unchanged tissues from patients with non small
cell lung cancer.
| | HSD17B2 mRNA
| HSD17B2 proteinb
|
|---|
| Variable | Number of
cases | Median (range) | P value | Median (range) | P value |
|---|
| Sex | | | 0.0012c | | 0.0022c |
| Male | 98 | 3.31
(0.07-5.61) | | 3.40
(1.03-6.81) | |
| Female | 63 | 3.63 (2.46
5.28) | | 4.06 (2.36
6.30) | |
| Patient age
(years) | | | 0.69c | | 0.75c |
| ≤60 | 45 | 3.41
(1.53-5.45) | | 3.56
(2.06-6.53) | |
| >60 | 116 | 3.45 (0.07
5.61) | | 3.63 (1.03
6.81) | |
| Histological
type | | | 0.67d | | 0.68d |
|
Adenocarcinoma | 70 | 3.53
(0.07-5.49) | | 3.65
(1.03-6.30) | |
| Squamous cell
carcinoma | 71 | 3.35 (1.53
5.61) | | 3.48 (1.80
6.81) | |
| Large cell
carcinoma | 12 | 3.50 (2.83
4.54) | | 3.54 (2.81
5.25) | |
| Carcinoid | 8 | 3.35
(2.70-4.64) | | 3.36
(2.67-5.19) | |
| Lung cancer
stages | | | 0.10d | | 0.14d |
| 0 | 6 | 3.07
(2.70-4.01) | | 3.18
(2.67-4.95) | |
| IA IB | 63 | 3.50 (2.46
5.61) | | 3.84 (2.36
6.81) | |
| IIA-IIB | 53 | 3.60
(0.07-5.49) | | 3.64
(1.03-6.53) | |
| IIIA-IV | 39 | 3.24
(2.42-5.11) | | 3.34
(2.38-6.11) | |
| Lung cancer
gradesa | | | 0.17d | | 0.051d |
| G1 | 12 | 3.31 (2.76
5.21) | | 3.36 (2.72
6.30) | |
| G2 | 68 | 3.29
(2.34-5.49) | | 3.39
(1.80-6.53) | |
| G3 | 73 | 3.59
(0.07-5.61) | | 3.84
(1.03-6.81) | |
| Tumour size | | | 0.030d | | 0.021d |
| Tis | 6 | 3.07
(2.70-4.01) | | 3.18
(2.67-4.95) | |
| T1 | 23 | 3.71
(2.42-5.25) | | 4.19
(2.85-6.29) | |
| T2 | 99 | 3.48
(1.53-5.61) | | 3.64
(1.80-6.81) | |
| T3-T4 | 33 | 3.23
(0.07-4.91) | | 3.31
(1.03-5.63) | |
| Lymph node
metastasis | | | 0.8d | | 0.87d |
| N0 | 95 | 3.46
(0.07-5.61) | | 3.63
(1.03-6.81) | |
| N1 | 44 | 3.34 (1.53
5.50) | | 3.45 (1.80
6.53) | |
| N2 | 22 | 3.42 (2.42
5.11) | | 3.50 (2.53
6.11) | |
| Distant
metastasis | | | 0.31c | | 0.22c |
| M0 | 158 | 3.45 (0.07
5.61) | | 3.62 (1.03
6.81) | |
| M1 | 3 | 3.33 (2.82
3.34) | | 3.34 (2.72
3.37) | |
| Smoking | | | 0.66c | | 0.52c |
| Yes | 146 | 3.34 (0.07
5.61) | | 3.58 (1.03
6.81) | |
| No | 15 | 3.41 (2.70
5.21) | | 3.63 (2.67
6.30) | |
| Residual tumour
status | | | 0.85c | | 0.62c |
| R0 | 144 | 3.42 (0.07
5.61) | | 3.56 (1.03
6.81) | |
| R1 | 16 | 3.43 (2.84
5.44) | | 3.78 (2.85
6.53) | |
Association of HSD17B2 mRNA and protein
levels in cancer tissues with clinical outcome in patients with
NSCLC
A retrospective analysis was performed to
investigate whether HSD17B2 mRNA or protein expression may have
prognostic significance in outcomes for patients with NSCLC. The
median OS among 147 patients enrolled in this analysis was 30.7
months (range, 1.3 80 months). The results from RT-qPCR and western
blotting in cancer tissues were divided into two groups, high and
low mRNA and protein levels, according to the median value of
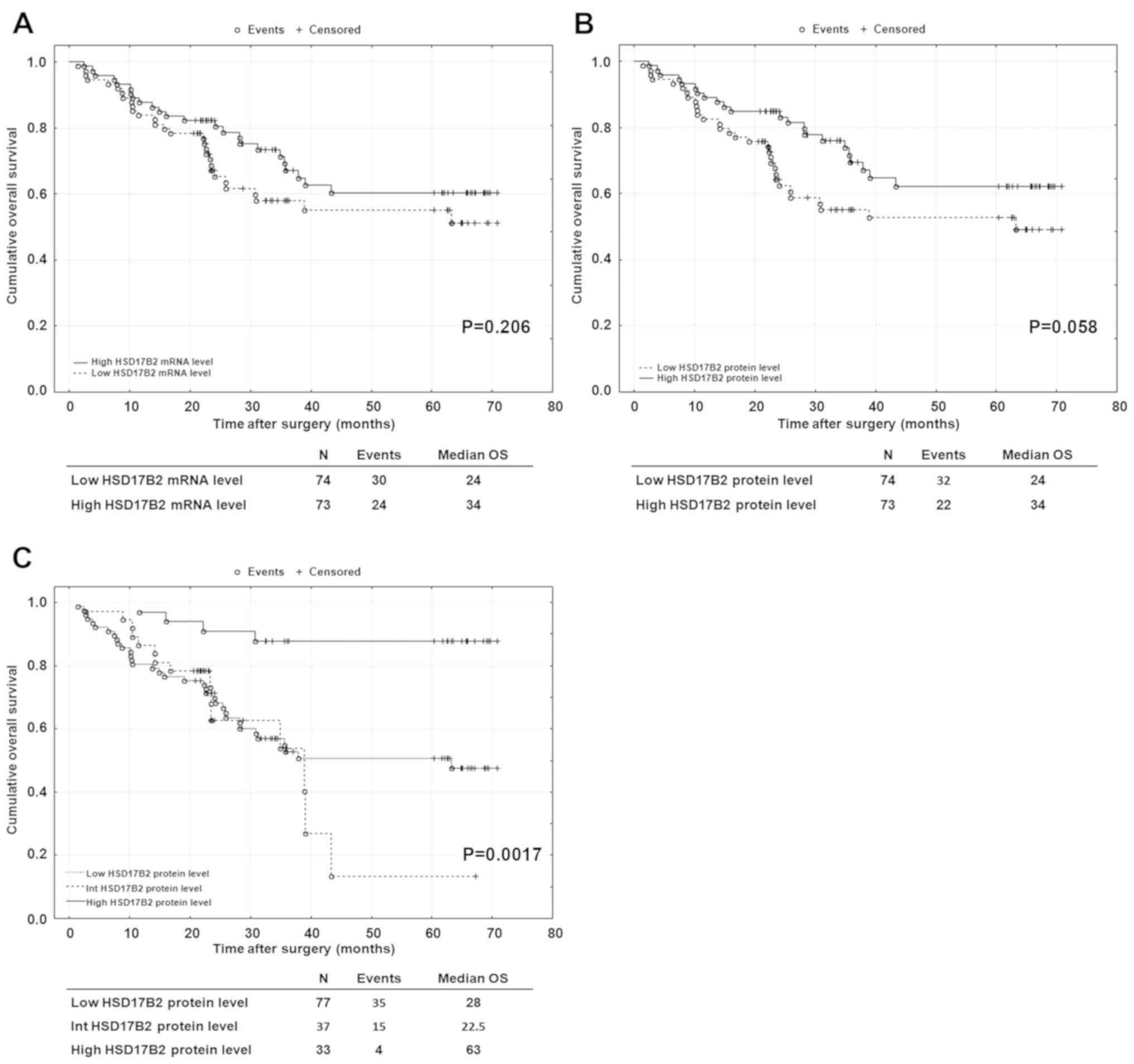
measurements. Although univariate analysis revealed results that
did not reach statistical significance, median survival was longer
for patients with higher HSD17B2 mRNA and protein expression
(Fig. 4A and B). In particular, a
certain trend towards significance was observed for HSD17B2 protein
content (P=0.058; Fig. 4B). As
aforementioned, specimens used for western blotting may be burdened
with the presence of non tumoural cells. Therefore, univariate
analysis was performed based on the immunohistochemical results. LC
specimens were subdivided into three groups: High (score 2 and
2/1), intermediate (score 1/2) and low (1/0; 0/1 and 1) HSD17B2
protein levels. Notably, using the log-rank test, patients with
high levels of HSD17B2 protein presented a significant increase in
OS (P=0.0017; Fig. 4C). The median
value of OS for those patients was 63 months vs. 28 and 22.5 months
for patients with low and intermediate HSD17B2 protein levels,
respectively. Univariate Cox regression analysis revealed that high
HSD17B2 protein content was associated with a better prognosis in
patients with NSCLC (HR=0.18; 95% CI=0.06-0.51; P=0.0012; Table V). Furthermore, a large tumour size
and LCC subtype were poor predictors in this study (Table V). Subsequently, variables with a P
value <0.15 in the univariate analysis (HSD17B2 protein level,
LC histological type, tumour size and residual tumour status) were
included in the multivariate analysis during stepwise selection to
determine independent predictors of outcome in patients with
NSCLC.
 | Table VUnivariate and multivariate Cox
regression analyses for overall survival in patients with non small
cell lung cancer. |
Table V
Univariate and multivariate Cox
regression analyses for overall survival in patients with non small
cell lung cancer.
| Univariate analysis
| Multivariate
analysis
|
|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| HSD17B2 protein
level | | | | |
| Low | 1 (Reference) | | 1 (Reference) | |
| Intermediate | 1.17 (0.63
2.17) | 0.62 | 1.25 (0.67
2.34) | 0.48 |
| High | 0.18 (0.06
0.51) | 0.0012 | 0.21 (0.07
0.63) | 0.0055 |
| Sex | | | | |
| Male | 1 (Reference) | | | |
| Female | 0.75 (0.43
1.31) | 0.31 | | |
| Patient age
(years) | | | | |
| ≤60 | 1 (Reference) | | | |
| >60 | 1.08
(0.59-1.96) | 0.81 | | |
| Histological
type | | | | |
|
Adenocarcinoma | 1 (Reference) | | 1 (Reference) | |
| Squamous cell
carcinoma | 1.23
(0.69-2.21) | 0.48 | 0.76
(0.41-1.4) | 0.38 |
| Large cell
carcinoma | 3.64
(1.58-8.39) | 0.0025 | 2.49
(1.06-5.84) | 0.036 |
| Tumour size | | | | |
| Tis T1 | 1 (Reference) | | 1 (Reference) | |
| T2 | 2.56
(0.99-6.59) | 0.051 | 1.31
(0.49-3.52) | 0.59 |
| T3-T4 | 5.42
(1.98-14.83) | 0.001 | 2.95
(1.03-8.49) | 0.044 |
| Lung cancer
stage | | | | |
| 0 I | 1 (Reference) | | | |
| II | 1.15 (0.62
2.15) | 0.65 | | |
| III IV | 1.31 (0.66
2.58) | 0.44 | | |
| Lymph node
metastasis | | | | |
| N0 | 1 (Reference) | | | |
| N1 | 1.07
(0.60-1.91) | 0.82 | | |
| N2 | 0.68 (0.26
1.74) | 0.42 | | |
| Lung cancer
grade | | | | |
| G1 | 1 (Reference) | | | |
| G2 | 1.09
(0.38-3.18) | 0.87 | | |
| G3 | 1.07
(0.38-3.05) | 0.9 | | |
| Smoking | | | | |
| No | 1 (Reference) | | | |
| Yes | 1.15 (0.38
3.05) | 0.75 | | |
| Residual tumour
status | | | | |
| R0 | 1 (Reference) | | 1 (Reference) | |
| R1 | 1.94
(0.91-4.11) | 0.085 | 2.47
(1.12-5.46) | 0.025 |
Results from multivariate analysis revealed that
HSD17B2 protein levels in cancer tissues could serve as an
independent prognostic factor for OS (HR=0.21; 95% CI=0.07-0.63;
P=0.0055). The rate of mortality for patients with low HSD17B2
protein expression within cancer tissues was 4.75 times higher than
that for patients with high HSD17B2 expression. As well as HSD17B2
protein content, a large tumour size (T3 T4), LCC subtype and R1
residual tumour status were also found to predict a poorer OS in
patients with NSCLC in an independent manner (Table V).
Prognostic significance of HSD17B2
expression in the NSCLC patient cohort from the Kaplan-Meier
Plotter database
To investigate whether downregulation of
HSD17B2 expression was associated with unfavourable survival
in a larger group of patients with NSCLC, Kaplan Meier Plotter was
used to generate survival curves for all available patient cohorts.
Since some clinical characteristics of patients are available in
the Kaplan-Meier Plotter database, a stratified analysis of
HSD17B2 expression in different subgroups of patients with
NSCLC was also performed. This approach allows us to distinguish
groups of patients in which HSD17B2 expression may have
prognostic significance. The Kaplan-Meier Plotter database also
offers an opportunity to perform multivariate analysis. However, it
is important to note that some patients do not have complete
clinical information and therefore are not included in such
analyses. The total number of cases enrolled in each univariate or
multivariate analysis is presented in Table VI. When the group had <50
patients, analysis was not performed. For all survival analyses,
patients were divided into two groups, with low and high
HSD17B2 mRNA expression classified according to the 'auto
select best cutoff' value.
 | Table VIStratified univariate analysis of
prognostic significance of HSD17B2 expression for OS, FP
survival and PPS in non- small cell lung cancer patient cohorts
from the Kaplan Meier Plotter database. |
Table VI
Stratified univariate analysis of
prognostic significance of HSD17B2 expression for OS, FP
survival and PPS in non- small cell lung cancer patient cohorts
from the Kaplan Meier Plotter database.
| OS
| FP
| PPS
|
|---|
| Variable | No. of cases | HR (95% CI) | P-value | No. of cases | HR (95% CI) | P-value | No. of cases | HR (95% CI) | P-value |
|---|
| Total no. of
patients | 1,926 | 0.85
(0.74-0.97) | 0.008a | 982 | 0.88
(0.72-1.07) | 0.062a | 344 | 0.65
(0.5-0.84) |
9.9×10−4b |
| Histological
type | | | | | | | | | |
|
Adenocarcinoma | 720 | 0.63
(0.5-0.79) |
9.2×10−5b | 461 | 0.76
(0.55-.04) | 0.088b | 125 | 0.5 (0.3-0.82) | 0.0056b |
| Squamous cell
carcinoma | 524 | 0.93
(0.73-1.19) | 0.57b | 141 | 0.76
(0.44-1.29) | 0.3b | 20 | - | - |
| Sex | | | | | | | | | |
| Male | 1,100 | 1.06
(0.91-1.25) | 0.38a | 514 | 1.28
(0.96-1.7) | 0.098b | 179 | 0.74(0.5-1.08) | 0.078a |
| Female | 715 | 0.65
(0.51-0.83) | <0.0001a | 468 | 0.81 (0.6-1.1) | 0.17b | 165 |
0.46(0.31-0.69) |
9.1×10−5b |
| Tumour size | | | | | | | | | |
| T1 | 437 |
0.87(0.65-1.17) | 0.17a | 177 | 0.63
(0.34-1.17) | 0.14b | 61 | 0.32
(0.16-0.61) |
3.1×10−4b |
| T2 | 589 | 1.09
(0.86-1.39) | 0.48b | 351 |
1.57(1.16-2.13) | 0.0032b | 169 | 0.63
(0.44-0.9) | 0.0095b |
| T3 | 81 | 0.63
(0.37-1.08) | 0.09b | 21 | - | - | 17 | - | - |
| T4 | 46 | 0.52 (0.25-
1.05) | 0.065b | 7 | - | - | 5 | - | - |
| Lung cancer
stage | | | | | | | | | |
| I | 577 | 0.65
(0.49-0.87) | 0.0033b | 325 | 0.55
(0.35-0.87) | 0.0097b | 78 | 0.41
(0.21-0.79) | 0.0059b |
| II | 244 |
0.74(0.51-1.09) | 0.17a | 130 | 0.71
(0.42-1.19) | 0.19b | 58 | 0.47
(0.24-0.95) | 0.03b |
| III | 70 |
0.74(0.43-1.28) | 0.32a | 19 | - | - 10 | - | - | |
| Lymph node
metastasis | | | | | | | | | |
| N0 | 781 | 1.14
(0.92-1.41) | 0.23a | 374 | 1.72
(1.22-2.43) | 0.0019b | 146 | 0.5
(0.34-0.75) |
5.4×10−4b |
| N1 | 252 | 0.87
(0.63-1.2) | 0.17a | 130 | 0.78
(0.49-1.24) | 0.26a | 71 | 0.57
(0.33-0.99) | 0.042b |
| N2 | 111 | 0.66 (0.44 -
0.99) | 0.05b | 51 | 0.81
(0.38-1.72) | 0.85a | 35 | - | - |
| Lung cancer
grade | | | | | | | | | |
| G1 | 201 | 0.83
(0.56-.21) | 0.33b | 140 | 1.41
(0.91-2.18) | 0.12b | 79 | 0.65
(0.37-1.15) | 0.13b |
| G2 | 310 | 1.15
(0.81-1.63) | 0.44b | 165 | 1.29
(0.81-2.04) | 0.29a | 89 | 0.67(0.41-1.1) | 0.11b |
| G3 | 77 | 0.52
(0.24-1.08) | 0.22a | 51 | 0.58
(0.24-1.4) | 0.22b | 24 | - | - |
The results of univariate analysis revealed that
high HSD17B2 expression was significantly associated with a
favourable prognosis (HR=0.85; 95% CI=0.74-0.97; P=0.008) in an
independent verification cohort of 1,926 patients with NSCLC
(Fig. 5A; Table VI). The median OS in the group of
patients with high HSD17B2 expression was 76 months, in comparison
with 62.3 months in the low expression group. Next, the
relationship between HSD17B2 mRNA levels and clinical
outcomes in various subgroups of patients with NSCLC was
investigated. The results indicated that high HSD17B2
expression was significantly associated with a better OS in
patients with ADC (HR=0.63; 95% CI=0.5-0.79; P=9.2x10
5), whereas it was not associated with OS in patients
with SCC (HR=0.93; 95% CI=0.73-1.19; P=0.57) (Fig. 5B and C; Table VI). Furthermore, during the
preliminary analysis, it was revealed that high HSD17B2
expression significantly improved the OS rates in female patients,
but not in male patients (Fig. 5D and
E; Table VI). Subsequently,
sex-stratified analyses were performed in the ADC and SCC patient
subgroups; it was observed that high mRNA expression levels of
HSD17B2 predicted better OS in women and men with ADC
(Fig. 5F and G), but it had no
impact on OS in either women or men with SCC (Fig. S1A and B). Furthermore, high
HSD17B2 expression significantly improved OS rates in
patients with stage I LC (Fig.
S1C; Table VI). In addition,
multivariate Cox regression analysis was performed, which revealed
that HSD17B2 mRNA expression, LC histological type, stage
and sex were associated with OS. Notably, high HSD17B2
expression exerted a protective effect, as it was associated with
an improved OS in an independent manner (HR=0.73; 95% CI=0.59-0.9;
P=0.0031; Table VII).
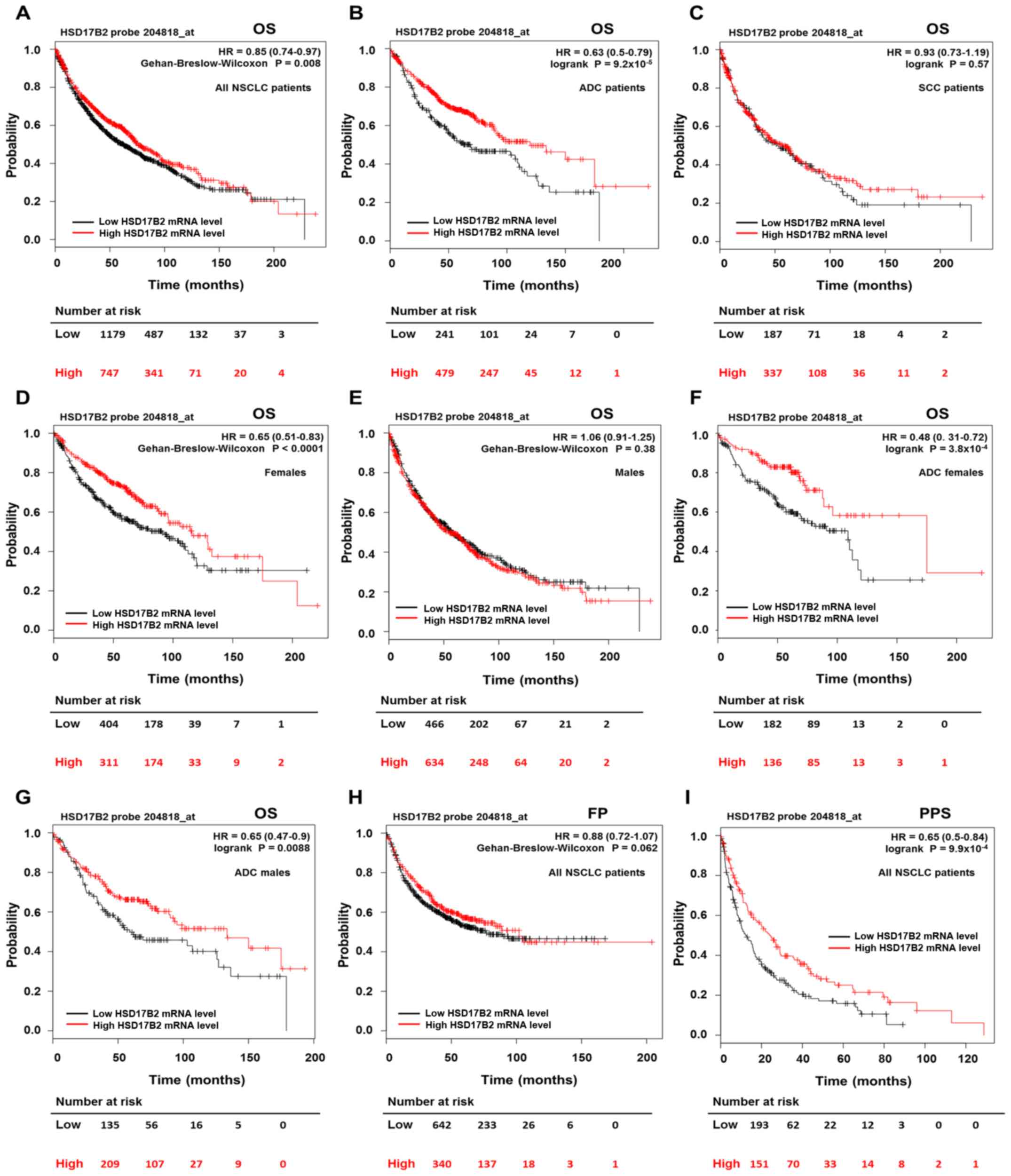 | Figure 5Prognostic value of HSD17B2
expression in the NSCLC patient cohort from the Kaplan Meier
Plotter online database. OS, FP survival and PPS survival curves
demonstrating survival rates of patients with NSCLC and high (red
line) or low (black line) HSD17B2 expression levels.
HSD17B2 expression was categorized into high and low
according to the 'Auto select best cutoff' value. The number of
patients at risk at specific time (in months) is presented in
tables below each graph. (A G) Kaplan Meier survival curves showing
the OS of patients with NSCLC. (A) Survival curves were plotted for
all patients with NSCLC (n=1,926). (B) Survival curves were plotted
only for patients with ADC (n=720). (C) Survival curves were
plotted only for patients with SCC (n=524). (D) Survival curves
were plotted only for female patients (n=715). (E) Survival curves
were plotted only for male patients (n=1,100). (F) Survival curves
were plotted only for female patients with ADC (n=318). (G)
Survival curves were plotted only for male patients with ADC
(n=344). (H) Kaplan Meier survival curves showing FP survival for
all patients with NSCLC (n=982). (I) Kaplan-Meier survival curves
showing PPS for all patients with NSCLC (n=344). P<0.05 was
considered to indicate a statistically significant difference. ADC,
adenocarcinoma; FP, first progression; HSD17B2, 17-β hydroxysteroid
dehydrogenase type 2; n NSCLC, non-small cell lung cancer; OS,
overall survival; PPS, post-progression survival; SCC, squamous
cell carcinoma. |
 | Table VIIStratified multivariate analysis of
prognostic significance of HSD17B2 expression for OS, FP
survival and PPS in non small cell lung cancer patient cohorts from
the Kaplan Meier Plotter database. |
Table VII
Stratified multivariate analysis of
prognostic significance of HSD17B2 expression for OS, FP
survival and PPS in non small cell lung cancer patient cohorts from
the Kaplan Meier Plotter database.
| OSa
| FPb
| PPSc
|
|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| HSD17B2 mRNA
level | 0.73
(0.59-0.9) | 0.0031 | 0.72
(0.49-1.04) | 0.082 | 0.6
(0.39-0.92) | 0.019 |
| Histological
type | 1.45
(1.23-1.7) | <0.0001 | 0.9
(0.61-1.33) | 0.6 | 1.94
(1.17-3.23) | 0.011 |
| Sex | 1.34
(1.09-1.66) | 0.0059 | 1.26
(0.91-1.75) | 0.17 | 1.24
(0.79-1.94) | 0.35 |
| Lung cancer
stage | 1.52
(1.33-1.74) | <0.0001 | 2.25
(1.74-2.91) | <0.0001 | 1.32
(0.95-1.82) | 0.098 |
No association between HSD17B2 mRNA
expression and FP survival was detected in the entire cohort
(Fig. 5H; Table VI). However, when patients were
divided according to clinicopathological variables, HSD17B2
expression influenced FP survival in particular subgroups. Patients
diagnosed with stage I LC, presenting high HSD17B2
transcript levels, had better FP survival times than patients with
lower expression (Fig. S1D;
Table VI). Unexpectedly, higher
HSD17B2 expression was negatively associated with FP
survival in patients with T2 tumour size and no regional lymph node
metastases (Fig. S1E and F;
Table VI). The multivariate
analysis revealed that HSD17B2 expression had no prognostic
impact on FP survival (HR=0.72; 95% CI=0.49-1.04; P=0.082; Table VII).
This study revealed that HSD17B2 mRNA levels
were strongly associated with improved PPS in the whole cohort of
patients (HR=0.65; 95% CI=0.5-0.84; P=9.9×10 4) as well
as in various cohort subsets (Fig.
5I; Table VI). The median PPS
in the group of patients with high HSD17B2 expression was
24.5 months, in comparison with 12.3 months in the low expression
group. According to the univariate analysis, low expression of
HSD17B2 was a significant unfavourable prognostic factor in
terms of PPS in patients with ADC, in women, in patients diagnosed
with early stages of LC (I and II), with T1 and T2 tumour size, and
with N0 and N1 lymph node metastasis (Fig. S2A-H; Table VI). Because of the lack of data,
we were unable to perform analysis regarding PPS for patients with
SCC, advanced LC stages and larger tumour sizes. The prognostic
effect of HSD17B2 expression on PPS remained significant and
independent from other risk factors, as determined by multivariate
analysis (HR=0.6; 95% CI=0.39-0.92; P=0.019; Table VII).
Discussion
At present, numerous studies have demonstrated that
oestrogens may exert an impact on LC development (22,42).
It has been shown that NSCLC cells express classical ERs as well as
the membrane GPER, and the administration of oestrogens has been
reported to activate genomic and non genomic signalling pathways in
these cells (17 21,25,43). Many studies have confirmed that the
activation of ERs and GPER by their agonists and oestrogens was
associated with enhanced proliferation and migration of human NSCLC
cells in vitro and in vivo, whereas their inhibition
or knockdown significantly reduced these events (18,26,27,44).
For example, an administration of E2 initiated rapid activation of
the p42/p44 MAPK signalling cascade and promoted the
phosphorylation of nuclear (steroid) receptor coactivator SRC 3,
which may enhance ER transcriptional activity in NSCLC cells,
whereas downregulation of ERs by small interfering RNA diminished
cancer cell proliferation (18).
Furthermore, E2 treatment in lung adenocarcinoma mouse models with
expression of oncogenic K-ras and concurrent deletion of
Tp53 significantly increased the number of tumours and their
volume in male and ovariectomized female mice (44). Fan et al (26) indicated that E2 and ERβ agonists
increased the protein levels of ERβ and matrix metalloproteinase 2,
leading to increased proliferation, migration and invasion of NSCLC
cells. Using an experimental lung metastatic mouse model, it was
confirmed that oestrogens enhanced NSCLC aggressiveness, which
resulted in an increased number of lung metastatic lesions in the
group of mice treated with E2 (26). Previous studies have indicated that
both oestrogens and androgens are metabolized within the lung, and
can be synthesized locally in lung tumours by various steroidogenic
enzymes (30 35). However, all of the mechanisms and pathways that
may lead to an exaggerated accumulation of active sex steroids in
LC tissue are currently not known.
The present study demonstrated, using three
different techniques, that the expression of HSD17B2 was signifi
cantly reduced in NSCLC tissues compared with adjacent
histopathologically unchanged tissue specimens. Because HSD17B2
inactivates biologically potent steroid hormones and regulates
their balance in various tissues, these results may indicate the
protective role of this enzyme within the lung. This study
complemented and expanded the results of a previous study performed
by Verma et al (33). This
previous study evaluated HSD17B2 protein levels in NSCLC specimens
for the first time (33); however,
the evaluation was made exclusively using immunohistochemical
staining, and the authors did not compare the differences in
HSD17B2 mRNA and protein levels between NSCLC tissues and
histopathologically unchanged tissue specimens. The present study
revealed that downregulation of HSD17B2 expression may be a
frequent feature in LC tissues. Similar to Verma et al
(33), this study detected that
the immunoreactivity of HSD17B2 was mostly located in the cytoplasm
of cells. In their previous study, higher HSD17B2 immunoreactivity
was associated with SCC and adenosquamous cell carcinoma subtypes
(33). The present study also
observed that among all histological subtypes of NSCLC, the highest
amount of HSD17B2 mRNA was detected in SCC. However, the
expression of HSD17B2 was substantially decreased in all LC
subtypes in comparison with adjacent histopathologically unchanged
counterparts, and no significant association among subtypes alone
was identified.
The present study demonstrated that normal
respiratory epithelium and normal glands within the lung were
positively stained for HSD17B2 protein. Low HSD17B2 mRNA and
protein expression levels in cancer tissues were associated with LC
stage, tumour size, lymph node metastasis and LC grading. Notably,
in higher grade, poorly differentiated tumours, most often
characterized by a poor prognosis, and in advanced NSCLC, the
amount of HSD17B2 expression was significantly diminished. Lower
expression was also detected in LC tissues from patients with N1
lymph node metastasis; however, in patients with N2, HSD17B2 mRNA
and protein expression was higher than that in N1 and comparable
with that in N0. Notably, during the evaluation of
immunohistochemical staining, it was revealed that the amount of
HSD17B2 protein was elevated in apoptotic regions of tumour
specimens (data not shown). A recent study by Hilborn et al
(45) revealed that E2 may
regulate HSD17B2 in breast cancer cells; the long-term
exposure to E2 (7 days) resulted in increased HSD17B2 mRNA
levels in the MCF7 cell line. Therefore, it may be possible that
decreased expression of HSD17B2 is crucial during the first
steps of NSCLC development, as it could provide a high level of sex
steroids, which may support cancer progression. Furthermore, it has
been reported that oestrogens stimulated proliferation of
preneoplastic parenchymal cells in the lung, suggesting that those
hormones may be a driver of LC at early stages of the disease
(44). On the other hand, the
reactivation of HSD17B2 expression in NSCLC apoptotic cells
after prolonged exposure to elevated levels of active sex steroids
may exert a protec tive role. This process may eliminate an
excessive amount of potent E2 and therefore diminish its pro
apoptotic properties, as reported previously (46).
This study also carried out a retrospective analysis
to estimate the prognostic significance of HSD17B2
expression in cancer tissues in patients with NSCLC. To date, to
the best of our knowledge, only one study has raised this issue.
Verma et al (33) reported
that patients with a negative HSD17B2 protein status in NSCLC cells
had poorer OS than HSD17B2 positive patients. The results based on
immunohistochemical analysis revealed that only negative cases
possessed prognostic significance because patients with high
HSD17B2 protein levels presented a steeper Kaplan Meier curve than
patients with a low amount of HSD17B2 protein (33). In the aforementioned study,
multivariate analysis demonstrated that HSD17B2 protein content
could not be considered an independent prognostic factor in the
investigated group of patients the NSCLC. The present study used a
log-rank test to assess the impact of HSD17B2 mRNA and protein
levels on the OS of patients. Even though univariate analysis
revealed no statistically significant benefits of high HSD17B2 mRNA
and protein levels (from western blotting), a clear trend towards
longer survival rates in these groups of patients was detected.
Because specimens used for western blotting usually represent a
mixture of various cells, it was decided that this study would
focus on immunohistochemical results considering only cancer cells.
The specimens were subdivided into three groups (high, intermediate
and low HSD17B2 protein levels); high expression levels of HSD17B2
protein in cancer cells were significantly associated with better
OS of patients with NSCLC. The median OS was clearly longer for
those patients than for patients with low or intermediate HSD17B2
immunoreactivity. This result indicated the potential value of
HSD17B2 as a predictor of survival in patients with NSCLC.
Subsequently, multivariate Cox regression analysis was performed,
which revealed that a high level of HSD17B2 protein in NSCLC cells
was associated with prolonged patient survival. The current study
indicated that the HSD17B2 protein expression in cancer cells could
serve as a prognostic factor in NSCLC. In addition, stratified
survival analysis was performed in an independent cohort of
patients with NSCLC from the Kaplan Meier Plotter database. The
analysis clearly demonstrated that patients with higher expression
levels of HSD17B2 presented better OS and PPS. This
favourable prognosis was particularly observed in women, patients
with ADC of both sexes, and patients with early stages of LC. This
online analysis confirmed that HSD17B2 expression may possess a
prognostic value concerning OS and PPS, at least in some groups of
patients with NSCLC. Unfortunately, no online database that
contained data concerning HSD17B2 protein status in patients with
NSCLC was found, which could verify the preliminary results. The
present study revealed that high protein expression of HSD17B2 in
LC tissues of patients was an independent factor associated with
favourable OS. However, because of limited follow up data, we were
not able to perform stratified survival analysis concerning HSD17B2
protein significance in various subgroups of patients, or to assess
its association with FP survival or PPS. Thus, the present study is
still preliminary, and it postulates that HSD17B2 could be a
promising prognostic factor for NSCLC, but further studies are
required to confirm its value in this disease.
The disturbed expression of HSD17B2 and its
prognostic significance have also been demonstrated for other types
of cancer. This fact emphasizes the role of this enzyme during
carcinogenesis. HSD17B2 appears to be an important player during
breast cancer development; while an oxidative pathway is preferred
in normal breast epithelium, where HSD17B2 activity may protect
cells from an excess of E2 (47),
in malignant breast tumours, the reductive pathway becomes
dominant, with considerably elevated expression of HSD17B1,
resulting in an elevated E2/E1 ratio (48 50). Gunnarsson et
al (51) reported that
HSD17B2 expression was lost in breast cancer tissues,
particularly in ER positive tumours, whereas it was detectable in
normal mammary gland specimens. Diminished HSD17B2
transcript levels were correlated with a higher risk of later
recurrence in the group of investigated patients. Furthermore, in a
later study, the same team revealed that the expression of
HSD17B2 can be a valuable predictor for the prognosis of
patients with breast cancer, as its low or absent transcript levels
were associated with decreased survival (52). Immunohistochemical staining of
HSD17B2 in breast cancer revealed a positive reaction in only 20%
of cancer specimens, while 83% of adjacent non-malignant tissues
exhibited immunoreactivity (53).
In the present work, only 36% of NSCLC specimens demonstrated high
HSD17B2 protein content.
Additionally, reduced expression of HSD17B2
has been associated with advanced stages of urothelial carcinoma
and was postulated to be an unfavourable prognostic factor
(54). Lower levels of HSD17B2
mRNA and protein were also detected in gastric tumour tissues
(55). Conversely, HSD17B2
expression was significantly upregulated in non-responding patients
with colorectal cancer treated with preoperative chemoradiotherapy
(56). Its overexpression was
associated with a poor prognosis and with an aggressive phenotype
of cancer. It is thought that in this type of cancer, E2 may serve
a protective role (57).
Therefore, in different types of cancer, HSD17B2 expression
is regulated in a different manner, presumably to sustain the best
environmental conditions for cancer development.
Notably, the disturbed expression of HSD17B2,
with the concomitant induction of the expression of reductive
HSD17B genes, led to an elevated E2/E1 ratio in breast
cancer cells and was associated with a poorer outcome in patients
(49,50,52).
Recently, a relationship between HSD17B1 and HSD17B2
expression levels, and an outcome in patients with endometrial
cancer has also been established. Concerning HSD17B1 and
HSD17B2 mRNA levels analysed in combination, patients with
tumours exhibiting high HSD17B1 and low HSD17B2 transcript levels
had the worst prognosis (58).
Previous studies have also revealed that the expression of
HSD17B1 was significantly increased in NSCLC tissues
(33,35). In the present work, a substantial
decrease in HSD17B2 mRNA and protein levels was detected in NSCLC
tissue specimens. Therefore, another important pathway that may
contribute to an elevated E2 concentration in the lung tumour
milieu has emerged. Therefore, it is important to clarify the exact
role played by the HSD17B2 enzyme in the process of sex steroid
inactivation in normal lung and LC tissues.
As HSD17B2 catalyses the conversion of T into 4
androstenedione, androstenediol into dehydroepiandrosterone, and
dihydrotestosterone into androstanedione (29,36,37),
it is inarguably involved in androgen inactivation within the lung.
An androgen receptor and the formation of active androgens were
detected in NSCLC cells and led to a significant growth response
(30,59,60).
High plasma levels of T and dihydrotestosterone have also been
associated with an increased incidence of LC in men (16). Androgen deprivation therapy,
applied after, or before and after, the diagnosis of LC in men,
contributed to a greater survival rate among patients (61). Collectively, these reports
suggested that androgens are also implicated in LC pathogenesis.
The present results indicated an alteration in NSCLC tissues that
may maintain a relatively high concentration of not only oestrogens
but also androgens, enhancing cancer development.
Smoking remains the most important risk factor for
LC, and an increasing number of studies have aimed to investigate
the interaction between tobacco exposure and oestrogen signalling
in lung tissue. It has been reported that polycyclic aromatic
hydrocarbons found in cigarette smoke stimulated the expression of
CYP1B1 in lung tissue (10). As this enzyme possesses an affinity
not only for tobacco carcinogens but also for the most potent
oestrogen, E2, the reaction of hydroxylation may result in the
generation of 2 and 4 catechol oestrogens with their subsequent
conversion to quinones. These compounds are able to damage DNA by
inducing single-strand breaks, 8 hydroxylation of guanine bases,
and may enhance formation of free radicals and DNA adducts
(11,12). Huuskonen et al (62) revealed that among the HSD17B enzyme
family, the expression of HSD17B2 was repressed in the human
placenta of smokers (62).
However, this study did not find any important relationship between
the smoking status of patients and the differences in
HSD17B2 expression in LC specimens. In smokers and
non-smokers, the expression of HSD17B2 mRNA and protein was
significantly diminished in LC tissues compared with adjacent
histopathologically unchanged tissues. However, in the present
study, a great majority of patients were smokers, so further
investigations are required to determine whether smoking may have
an influence on HSD17B2 expression in NSCLC.
Very little is known about the mechanisms
responsible for the regulation of HSD17B2 gene expression.
It has been reported that retinoic acid (RA) increased the
transcriptional activity of the HSD17B2 gene in breast
cancer, endometrial cancer cells and in human placental endothelial
cells (63 65). RA exerts its effect through RA receptors, which are
thought to be tumour suppressors in several types of cancer. RA
receptor β was reported to be severely hypermethylated in NSCLC
tissues, particularly in smokers (66). In addition, recent evidence
suggests that the lack of nuclear RA receptors may serve a critical
role during lung carcinogenesis (67). Therefore, alterations in the RA
signalling pathway in LC tissues may contribute to the disturbed
expression of HSD17B2. Furthermore, the HSD17B2 gene
is located at chromosome 16q24.1, and an allelic loss at this
region was reported as a frequent event in prostate and breast
cancer (68,69). This allelic deletion was also
detected as a common feature in LC (70). Collectively, these mechanisms may
be responsible for inactivation of HSD17B2 expression.
Additionally, previous studies showed that hormone
replacement therapy or oral contraceptives may influence the
expression level of steroidogenic enzymes (48). Hilborn et al (45) investigated the impact of active sex
steroids on HSD17B2 expression in breast cancer cell lines.
It was reported that E2 stimulation significantly altered the
expression status of HSD17B2, but the final effect was
dependent on the cell type and time of exposure (45). In the present study, there was a
small group of patients who were treated by hormone replacement
therapy (n=4), and all women were postmenopausal. Therefore, this
study cannot verify how and whether postmenopausal oestrogen
therapy may affect HSD17B2 expression in LC or
histopathologically unchanged tissues. Because of the lack of
premenopausal women in the patient group, this study was not able
to compare HSD17B2 expression levels in LC as well as in
histopathologically unchanged tissues between pre and
postmenopausal women. Further investigations are required to
clarify this issue.
In conclusion, this study demonstrated that HSD17B2
transcript and protein levels were significantly reduced in LC
tissues compared with in matched, normal specimens. However, this
study investigated tissues obtained only from patients suffering
from NSCLC and a lack of healthy control patients may be a
limitation. It would be helpful and interesting to compare the
expression levels of HSD17B2 in lung tissues from healthy
individuals with expression levels in histopathologically unchanged
tissues and LC tissues from patients with NSCLC. Further
investigations are required to obtain a comprehensive view on this
point. The present study also revealed that normal respiratory
epithelium and histopathologically unchanged glandular cells
exhibited strong cytoplasmic staining for HSD17B2. A strong
association between reduced HSD17B2 mRNA and protein expression,
and NSCLC stages, tumour size, lymph node metastasis and LC grading
was detected in the group of cancer tissues. The majority of the
investigated cancer tissue specimens exhibited weak or negative
staining with increasing tumour grade. The results from the
log-rank test and a multivariate Cox regression model revealed a
beneficial effect of high HSD17B2 protein levels in cancer tissues
on OS, and indicated that HSD17B2 protein status may be a
prognostic factor of OS in patients with NSCLC. Furthermore, online
survival analysis using an independent verification cohort revealed
statistically significant differences in OS and PPS according to
HSD17B2 mRNA level. The survival time of patients with high
HSD17B2 expression was significantly longer than that of
patients with low expression levels. This beneficial effect was
particularly evident in patients with ADC of both sexes, and in
patients with early stages of LC. The results from multivariate
analysis pinpointed HSD17B2 as an independent prognostic
factor.
Collectively, decreased expression of HSD17B2 is
frequently observed in NSCLC; however, the mechanisms underlying
the disturbed expression of HSD17B2 in NSCLC remains unexplained.
The restoration of HSD17B2 expression in NSCLC tissues may lead to
patient benefits and may be a target for future therapy.
Supplementary Data
Abbreviations:
|
Ab
|
antibody
|
|
ADC
|
adenocarcinoma
|
|
CI
|
confidence interval
|
|
E1
|
oestrone
|
|
E2
|
17-β-oestradiol
|
|
ERs
|
oestrogen receptors
|
|
FP
|
first progression
|
|
GPER
|
G protein-coupled oestrogen
receptor
|
|
hMRPL19
|
human mitochondrial ribosomal protein
L19
|
|
HR
|
hazard ratio
|
|
HRP
|
horseradish peroxidase
|
|
HSD17B1
|
17 β-hydroxysteroid dehydrogenase
type 1
|
|
HSD17B2
|
17 β-hydroxysteroid dehydrogenase
type 2
|
|
LC
|
lung cancer
|
|
LCC
|
large cell carcinoma
|
|
NSCLC
|
non-small cell lung cancer
|
|
OS
|
overall survival
|
|
PBGD
|
porphobilinogen deaminase
|
|
POLR2A
|
RNA polymerase II subunit A
|
|
PPS
|
post-progression survival
|
|
RA
|
retinoic acid
|
|
RT-qPCR
|
reverse transcription-quantitative
PCR
|
|
SDS-PAGE
|
sodium dodecyl
sulphate-polyacrylamide gel electrophoresis
|
|
SCC
|
squamous cell carcinoma
|
|
T
|
testosterone
|
|
TNM
|
tumour node metastasis
|
Acknowledgments
The authors would like to thank Mr. Bartosz
Słowikowski M.Sc. (Department of Biochemistry and Molecular
Biology, Poznan University of Medical Sciences) for his technical
assistance and Mrs. Małgorzata Janicka-Jedyńska PhD., MD.
(Department of Clinical Pathomorphology, Poznan University of
Medical Sciences) for independent immunohistochemistry
evaluation.
Funding
This work was supported by the National Science
Centre, Poland (grant no. UMO 2013/11/B/NZ5/00039) and by Poznan
University of Medical Sciences (grant no.
502-14-01124182-09698).
Availability of data and materials
All data generated or analyzed during this study
are included in this published article.
Authors' contributions
HD developed the concept of the project, designed
and performed the experiments, analysed the results and wrote the
manuscript. DJJ was responsible for the histopathological
classification and evaluation of HSD17B2 staining in the
investigated specimens. AK performed immunohistochemical staining.
BG informed patients about the aim of the research study, collected
and analysed clinical specimens, and collected all clinical data.
WD and PPJ participated in design and coordination of the study,
were involved in statistical analysis of the obtained results and
were responsible for revision of the manuscript for substantive and
methodological correctness. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Local Ethical
Committee of Poznan University of Medical Sciences. All procedures
performed in this study were in accordance with the 1964
Declaration of Helsinki and its later amendments. Written informed
consent was obtained from all patients included in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dholaria B, Hammond W, Shreders A and Lou
Y: Emerging therapeutic agents for lung cancer. J Hematol Oncol.
9:1382016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Alì G, Donati V, Loggini B, Servadio A,
Dell'Omodarme M, Prati MC, Camacci T, Lucchi M, Melfi F, Mussi A,
et al: Different estrogen receptor beta expression in distinct
histologic subtypes of lung adenocarcinoma. Hum Pathol.
39:1465–1473. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gasperino J: Gender is a risk factor for
lung cancer. Med Hypotheses. 76:328–331. 2011. View Article : Google Scholar
|
|
5
|
Schwartz AG, Prysak GM, Murphy V, Lonardo
F, Pass H, Schwartz J and Brooks S: Nuclear estrogen receptor beta
in lung cancer: Expression and survival differences by sex. Clin
Cancer Res. 11:7280–7287. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wakelee HA, Chang ET, Gomez SL, Keegan TH,
Feskanich D, Clarke CA, Holmberg L, Yong LC, Kolonel LN, Gould MK,
et al: Lung cancer incidence in never smokers. J Clin Oncol.
25:472–478. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Papadopoulos A, Guida F, Leffondré K,
Cénée S, Cyr D, Schmaus A, Radoï L, Paget Bailly S, Carton M,
Menvielle G, et al: Heavy smoking and lung cancer: Are women at
higher risk? Result of the ICARE study. Br J Cancer. 110:1385–1391.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ryu JS, Jeon SH, Kim JS, Lee JH, Kim SH,
Hong JT, Jeong JH, Jeong JJ, Lee MD, Min SJ, et al: Gender
differences in susceptibility to smoking among patients with lung
cancer. Korean J Intern Med (Korean Assoc Intern Med). 26:427–431.
2011.
|
|
9
|
Guo H, Huang K, Zhang X, Zhang W, Guan L,
Kuang D, Deng Q, Deng H, Zhang X, He M, et al: Women are more
susceptible than men to oxidative stress and chromosome damage
caused by polycyclic aromatic hydrocarbons exposure. Environ Mol
Mutagen. 55:472–481. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Spivack SD, Hurteau GJ, Reilly AA, Aldous
KM, Ding X and Kaminsky LS: CYP1B1 expression in human lung. Drug
Metab Dispos. 29:916–922. 2001.PubMed/NCBI
|
|
11
|
Cavalieri E, Chakravarti D, Guttenplan J,
et al: Catechol estrogen quinones as initiators of breast and other
human cancers: Implications for biomarkers of susceptibility and
cancer prevention. Biochim Biophys Acta. 1766:63–78.
2006.PubMed/NCBI
|
|
12
|
Belous AR, Hachey DL, Dawling S, Roodi N
and Parl FF: Cytochrome P450 1B1 mediated estrogen metabolism
results in estrogen deoxyribonucleoside adduct formation. Cancer
Res. 67:812–817. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Siegfried JM, Hershberger PA and Stabile
LP: Estrogen receptor signaling in lung cancer. Semin Oncol.
36:524–531. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lienert T, Serke M, Schönfeld N and
Loddenkemper R: Lung cancer in young females. Eur Respir J.
16:986–990. 2000. View Article : Google Scholar
|
|
15
|
Ross H, Oldham FB, Bandstra B, Sandalic L,
Bianco J, Bonomi P and Singer JW: Serum free estradiol (E2) levels
are prognostic in men with chemotherapy naive advanced non small
cell lung cancer (NSCLC) and performance status (PS) 2. J Clin
Oncol. (Suppl 18)25:76832007. View Article : Google Scholar
|
|
16
|
Chan YX, Alfonso H, Chubb SAP, Handelsman
DJ, Fegan PG, Hankey GJ, Golledge J, Flicker L and Yeap BB: Higher
Dihydrotestosterone Is Associated with the Incidence of Lung Cancer
in Older Men. Horm Cancer. 8:119–126. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stabile LP, Davis AL, Gubish CT, Hopkins
TM, Luketich JD, Christie N, Finkelstein S and Siegfried JM: Human
non-small cell lung tumors and cells derived from normal lung
express both estrogen receptor alpha and beta and show biological
responses to estrogen. Cancer Res. 62:2141–2150. 2002.PubMed/NCBI
|
|
18
|
Márquez-Garbán DC, Chen HW, Fishbein MC,
Goodglick L and Pietras RJ: Estrogen receptor signaling pathways in
human non small cell lung cancer. Steroids. 72:135–143. 2007.
View Article : Google Scholar
|
|
19
|
Fasco MJ, Hurteau GJ and Spivack SD:
Gender-dependent expression of alpha and beta estrogen receptors in
human nontumor and tumor lung tissue. Mol Cell Endocrinol.
188:125–140. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jala VR, Radde BN, Haribabu B and Klinge
CM: Enhanced expression of G protein coupled estrogen receptor
(GPER/GPR30) in lung cancer. BMC Cancer. 12:6242012. View Article : Google Scholar
|
|
21
|
Liu C, Liao Y, Fan S, Tang H, Jiang Z,
Zhou B, Xiong J, Zhou S, Zou M and Wang J: G protein coupled
estrogen receptor (GPER) mediates NSCLC progression induced by 17β
estradiol (E2) and selective agonist G1. Med Oncol. 32:1042015.
View Article : Google Scholar
|
|
22
|
Słowikowski BK, Lianeri M and Jagodziński
PP: Exploring estrogenic activity in lung cancer. Mol Biol Rep.
44:35–50. 2017. View Article : Google Scholar
|
|
23
|
Hsu LH, Chu NM and Kao SH: Estrogen,
Estrogen Receptor and Lung Cancer. Int J Mol Sci. 18:17132017.
View Article : Google Scholar :
|
|
24
|
Zhang G, Liu X, Farkas AM, Parwani AV,
Lathrop KL, Lenzner D, Land SR and Srinivas H: Estrogen receptor
beta functions through nongenomic mechanisms in lung cancer cells.
Mol Endocrinol. 23:146–156. 2009. View Article : Google Scholar
|
|
25
|
Hershberger PA, Stabile LP, Kanterewicz B,
Rothstein ME, Gubish CT, Land S, Shuai Y, Siegfried JM and Nichols
M: Estrogen receptor beta (ERbeta) subtype specific ligands
increase transcription, p44/p42 mitogen activated protein kinase
(MAPK) activation and growth in human non small cell lung cancer
cells. J Steroid Biochem Mol Biol. 116:102–109. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fan S, Liao Y, Liu C, Huang Q, Liang H, Ai
B, Fu S and Zhou S: Estrogen promotes tumor metastasis via estrogen
receptor beta mediated regulation of matrix metalloproteinase 2 in
non-small cell lung cancer. Oncotarget. 8:56443–56459.
2017.PubMed/NCBI
|
|
27
|
Liu C, Liao Y, Fan S, Fu X, Xiong J, Zhou
S, Zou M and Wang J: G Protein Coupled Estrogen Receptor Antagonist
G15 Decreases Estrogen Induced Development of Non Small Cell Lung
Cancer. Oncol Res. 27:283–292. 2019. View Article : Google Scholar
|
|
28
|
Luu The V: Assessment of steroidogenesis
and steroidogenic enzyme functions. J Steroid Biochem Mol Biol.
137:176–182. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Labrie F: All sex steroids are made
intracellularly in peripheral tissues by the mechanisms of
intracrinology after menopause. J Steroid Biochem Mol Biol.
145:133–138. 2015. View Article : Google Scholar
|
|
30
|
Provost PR, Blomquist CH, Drolet R,
Flamand N and Tremblay Y: Androgen inactivation in human lung
fibroblasts: Variations in levels of 17 beta hydroxysteroid
dehydrogenase type 2 and 5 alpha reductase activity compatible with
androgen inactivation. J Clin Endocrinol Metab. 87:3883–3892.
2002.PubMed/NCBI
|
|
31
|
Niikawa H, Suzuki T, Miki Y, Suzuki S,
Nagasaki S, Akahira J, Honma S, Evans DB, Hayashi S, Kondo T, et
al: Intratumoral estrogens and estrogen receptors in human non
small cell lung carcinoma. Clin Cancer Res. 14:4417–4426. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Verma MK, Miki Y and Sasano H: Aromatase
in human lung carcinoma. Steroids. 76:759–764. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Verma MK, Miki Y, Abe K, Suzuki T, Niikawa
H, Suzuki S, Kondo T and Sasano H: Intratumoral localization and
activity of 17β hydroxysteroid dehydrogenase type 1 in non small
cell lung cancer: A potent prognostic factor. J Transl Med.
11:1672013. View Article : Google Scholar
|
|
34
|
Drzewiecka H and Jagodzinski PP:
Conversion of estrone to 17 beta estradiol in human non small cell
lung cancer cells in vitro. Biomed Pharmacother. 66:530–534. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Drzewiecka H, Gałęcki B,
Jarmołowska-Jurczyszyn D, Kluk A, Dyszkiewicz W and Jagodziński PP:
Increased expression of 17 beta hydroxysteroid dehydrogenase type 1
in non small cell lung cancer. Lung Cancer. 87:107–116. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Miettinen MM, Mustonen MV, Poutanen MH,
Isomaa VV and Vihko RK: Human 17 beta-hydroxysteroid dehydrogenase
type 1 and type 2 isoenzymes have opposite activities in cultured
cells and characteristic cell and tissue specific expression.
Biochem J. 314(Pt 3): 839–845. 1996. View Article : Google Scholar
|
|
37
|
Vihko P, Isomaa V and Ghosh D: Structure
and function of 17beta hydroxysteroid dehydrogenase type 1 and type
2. Mol Cell Endocrinol. 171:71–76. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Goldstraw P, Crowley J, Chansky K, Giroux
DJ, Groome PA, Rami-Porta R, Postmus PE, Rusch V and Sobin L;
International Association for the Study of Lung Cancer
International Staging Committee Participating Institutions: The
IASLC Lung Cancer Staging Project: Proposals for the revision of
the TNM stage groupings in the forthcoming (seventh) edition of the
TNM Classification of malignant tumours. J Thorac Oncol. 2:706–714.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Győrffy B, Surowiak P, Budczies J and
Lánczky A: Online survival analysis software to assess the
prognostic value of biomarkers using transcriptomic data in
non-small-cell lung cancer. PLoS One. 8:e822412013. View Article : Google Scholar
|
|
40
|
Tellmann G: The E Method: A highly
accurate technique for gene expression analysis. Nat Methods.
3:i–ii. 2006. View Article : Google Scholar
|
|
41
|
Drzewiecka H, Gałęcki B,
Jarmołowska-Jurczyszyn D, Kluk A, Dyszkiewicz W and Jagodziński
PPPP: Decreased expression of connective tissue growth factor in
non small cell lung cancer is associated with clinicopathological
variables and can be restored by epigenetic modifiers. J Cancer Res
Clin Oncol. 142:1927–1946. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Burns TF and Stabile LP: Targeting the
estrogen pathway for the treatment and prevention of lung cancer.
Lung Cancer Manag. 3:43–52. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ivanova MM, Mazhawidza W, Dougherty SM and
Klinge CM: Sex differences in estrogen receptor subcellular
location and activity in lung adenocarcinoma cells. Am J Respir
Cell Mol Biol. 42:320–330. 2010. View Article : Google Scholar :
|
|
44
|
Hammoud Z, Tan B, Badve S and Bigsby RM:
Estrogen promotes tumor progression in a genetically defined mouse
model of lung adenocarcinoma. Endocr Relat Cancer. 15:475–483.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hilborn E, Stål O, Alexeyenko A and
Jansson A: The regulation of hydroxysteroid 17β dehydrogenase type
1 and 2 gene expression in breast cancer cell lines by estradiol,
dihydrotestosterone, microRNAs, and genes related to breast cancer.
Oncotarget. 8:62183–62194. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lewis Wambi JS and Jordan VC: Estrogen
regulation of apoptosis: How can one hormone stimulate and inhibit?
Breast Cancer Res. 11:2062009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Speirs V, Green AR, Walton DS, Kerin MJ,
Fox JN, Carleton PJ, Desai SB and Atkin SL: Short-term primary
culture of epithelial cells derived from human breast tumours. Br J
Cancer. 78:1421–1429. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hilborn E, Stål O and Jansson A: Estrogen
and androgen converting enzymes 17β hydroxysteroid dehydrogenase
and their involvement in cancer: With a special focus on 17β
hydroxysteroid dehydrogenase type 1, 2, and breast cancer.
Oncotarget. 8:30552–30562. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Miyoshi Y, Ando A, Shiba E, Taguchi T,
Tamaki Y and Noguchi S: Involvement of up regulation of 17beta
hydroxys teroid dehydrogenase type 1 in maintenance of intratumoral
high estradiol levels in postmenopausal breast cancers. Int J
Cancer. 94:685–689. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhang CYY, Chen J, Yin DCC and Lin SXX:
The contri bution of 17beta hydroxysteroid dehydrogenase type 1 to
the estradiol estrone ratio in estrogen sensitive breast cancer
cells. PLoS One. 7:e298352012. View Article : Google Scholar
|
|
51
|
Gunnarsson C, Olsson BM and Stål O;
Southeast Sweden Breast Cancer Group: Abnormal expression of 17beta
hydroxysteroid dehydrogenases in breast cancer predicts late
recurrence. Cancer Res. 61:8448–8451. 2001.PubMed/NCBI
|
|
52
|
Gunnarsson C, Hellqvist E and Stål O:
17beta Hydroxysteroid dehydrogenases involved in local oestrogen
synthesis have prog nostic significance in breast cancer. Br J
Cancer. 92:547–552. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Han B, Li S, Song D, Poisson Paré D, Liu
G, Luu The V, Ouellet J, Li S, Labrie F and Pelletier G: Expression
of 17β hydroxysteroid dehydrogenase type 2 and type 5 in breast
cancer and adjacent non malignant tissue: A correlation to
clinicopathological parameters. J Steroid Biochem Mol Biol.
112:194–200. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wang CT, Li CFCC, Wu WJ, Huang CN, Li CC,
Li WM, Chan TC, Liang PI, Hsing CH and Liao KM: High Expression of
17β hydroxysteroid Dehydrogenase Type 2 is Associated with a Better
Prognosis in Urothelial Carcinoma of the Urinary Tract. J Cancer.
7:2221–2230. 2016. View Article : Google Scholar :
|
|
55
|
Frycz BA, Murawa D, Borejsza-Wysocki M,
Marciniak R, Murawa P, Drews M and Jagodziński PP: Expression of
17β hydroxysteroid dehydrogenase type 2 is associated with some
clinicopathological features in gastric cancer. Biomed
Pharmacother. 70:24–27. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Lee YE, He HL, Shiue YL, Lee SW, Lin LC,
Wu TF, Chang IW, Lee HH and Li CF: The prognostic impact of lipid
biosyn thesis-associated markers, HSD17B2 and HMGCS2, in rectal
cancer treated with neoadjuvant concurrent chemoradiotherapy.
Tumour Biol. 36:7675–7683. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Marino M: Xenoestrogens challenge 17β
estradiol protective effects in colon cancer. World J Gastrointest
Oncol. 6:67–73. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Cornel KMC, Krakstad C, Delvoux B,
Xanthoulea S, Jori B, Bongers MY, Konings GF, Kooreman LF,
Kruitwagen RF, Salvesen HB, et al ENITEC: High mRNA levels of 17β
hydroxysteroid dehydrogenase type 1 correlate with poor prognosis
in endometrial cancer. Mol Cell Endocrinol. 442:51–57. 2017.
View Article : Google Scholar
|
|
59
|
Jeong Y, Xie Y, Lee W, Bookout AL, Girard
L, Raso G, Behrens C, Wistuba II, Gadzar AF, Minna JD, et al:
Research resource: Diagnostic and therapeutic potential of nuclear
receptor expression in lung cancer. Mol Endocrinol. 26:1443–1454.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Recchia AG, Musti AM, Lanzino M, Panno ML,
Turano E, Zumpano R, Belfiore A, Andò S and Maggiolini M: A
cross-talk between the androgen receptor and the epidermal growth
factor receptor leads to p38MAPK dependent activation of mTOR and
cyclinD1 expression in prostate and lung cancer cells. Int J
Biochem Cell Biol. 41:603–614. 2009. View Article : Google Scholar
|
|
61
|
Harlos C, Musto G, Lambert P, Ahmed R and
Pitz MW: Androgen pathway manipulation and survival in patients
with lung cancer. Horm Cancer. 6:120–127. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Huuskonen P, Amezaga MR, Bellingham M,
Jones LH, Storvik M, Häkkinen M, Keski-Nisula L, Heinonen S,
O'Shaughnessy PJ, Fowler PA, et al: The human placental proteome is
affected by maternal smoking. Reprod Toxicol. 63:22–31. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Ito K, Suzuki T, Moriya T, Utsunomiya H,
Sugawara A, Konno R, Sato S and Sasano H: Retinoid receptors in the
human endometrium and its disorders: A possible modulator of 17 β
hydroxysteroid dehydrogenase. J Clin Endocrinol Metab.
86:2721–2727. 2001.PubMed/NCBI
|
|
64
|
Reed MJ, Rea D, Duncan LJ and Parker MG:
Regulation of estradiol 17 beta hydroxysteroid dehydrogenase
expression and activity by retinoic acid in T47D breast cancer
cells. Endocrinology. 135:4–9. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Su EJ, Cheng YH, Chatterton RT, Lin ZH,
Yin P, Reierstad S, Innes J and Bulun SE: Regulation of 17 beta
hydroxysteroid dehydrogenase type 2 in human placental endothelial
cells. Biol Reprod. 77:517–525. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Li Y, Lu DG, Ma YM and Liu H: Association
between Retinoic acid receptor β hypermethylation and NSCLC risk: A
meta analysis and literature review. Oncotarget. 8:5814–5822.
2017.
|
|
67
|
Muñiz Hernández S, Huerta Yepez S,
Hernández Pedro N, Ramírez Tirado LA, Aviles Salas A, Maldonado A,
Hernández Cueto D, Baay Guzmán G and Arrieta O: Association between
nuclear expression of retinoic acid receptor alpha and beta and
clinicopathological features and prognosis of advanced non small
cell lung cancer. Int J Clin Oncol. 21:1051–1061. 2016. View Article : Google Scholar
|
|
68
|
Elo JP, Härkönen P, Kyllönen AP,
Lukkarinen O and Vihko P: Three independently deleted regions at
chromosome arm 16q in human prostate cancer: Allelic loss at
16q24.1 q24.2 is associated with aggressive behaviour of the
disease, recurrent growth, poor differentiation of the tumour and
poor prognosis for the patient. Br J Cancer. 79:156–160. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Jansson A: 17Beta hydroxysteroid
dehydrogenase enzymes and breast cancer. J Steroid Biochem Mol
Biol. 114:64–67. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Sato M, Mori Y, Sakurada A, Fukushige S,
Ishikawa Y, Tsuchiya E, Saito Y, Nukiwa T, Fujimura S and Horii A:
Identification of a 910-kb region of common allelic loss in
chromosome bands 16q24.1 q24.2 in human lung cancer. Genes
Chromosomes Cancer. 22:1–8. 1998. View Article : Google Scholar : PubMed/NCBI
|