Introduction
Endometrial cancer (EC) is one of the most common
types of malignant tumors worldwide, with an age standardized
incidence rate of 8.4 cases per 100,000 individuals. Each year, an
estimated 382,069 new cases of EC are diagnosed (1). Compared with low-resource countries,
higher-income countries have a higher morbidity rate among patients
with EC, although the former have higher mortality rates. The
estimated cumulative risk of endometrial cancer is 1.6% in
high-income areas and 0.7% in low-income countries up to the age of
75 years (2). High-grade ECs have
a high rate of recurrence, despite the fact that prognosis is
generally good for the initial cancer. The prognosis of patients
with recurrent EC however, is poor. During the treatment of cancer,
it is important to balance treatment efficacy with the toxicity of
the therapy used (3). There are 6
major molecular changes in type I endometrioid carcinoma:
Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit a
(PIK3CA) gene mutations (observed in 26-39% of cases); phosphatase
and tensin homolog deleted on chromosome ten (PTEN) gene mutations
(observed in 30-60% of cases); microsatellite instability (observed
in 25-30% of cases); k-ras gene mutations (observed in 10-30% of
cases); AT-rich interaction domain 1A (ARID1A; observed in 20% of
cases); and catenin beta 1 (CTNNB1) and the accumulation of nuclear
protein mutations (observed in 25-38% of cases). By contrast, the
majority of type II non-endometrioid carcinomas have Her-2/neu
amplification, p53 mutations and multiple chromosomal loss of
heterozygosity. Non-endometrioid carcinomas may also originate from
endometrioid carcinomas, which are unstable due to p53 mutations,
and the resultant instability drives tumor progression (4). The fundamental molecular mechanisms
involving tumor suppression or oncogenic factors remain poorly
understood, even though key mutational events have been
characterized in EC (5).
Therefore, it is of utmost importance to identify novel therapeutic
targets and to develop effective cure strategies for patients with
EC. To achieve this, an improved understanding of the molecular
mechanisms underlying the pathogenesis of EC is required.
Numerous long non-coding RNAs (lncRNAs) have been
shown to be cancer-specific (6-8), and
may thus be used as novel biomarkers for the diagnosis of cancer,
or as therapeutic targets for the treatment of cancer. Certain
lncRNAs regulate gene expression by acting as competing endogenous
RNAs (ceRNAs). This notion has been supported by numerous studies
(9-13). Furthermore, lncRNAs may be more
effective in downregulation of gene expression when acting as
ceRNAs, without the need to interfere with translation (14). However, the roles of
lncRNA-associated ceRNAs in oncogenesis are not yet fully
understood, and the role of lncRNA-microRNA (miRNA/miR) networks in
EC requires further investigation. PTEN has been identified as a
direct target of miR-205-5p in previous studies (15,16),
and the expression of miR-205-5p is significantly increased in EC.
Based on Kaplan-Meier survival analysis, the upregulation of
miR-205-5p is associated with a poor overall survival (17).
A previous study by the authors demonstrated that
miR-205-5p targets the lncRNA LA16c-313D11.11, with one conserved
target site in EC. LA16c-313D11.11 may inhibit the expression and
activity of miR-205-5p in normal and cancer tissues via this
post-transcriptional binding (18). In the present study,
LA16c-313D11.11 was shown to modulate a miR-205-5p-PTEN axis in EC.
These results will improve the understanding of the molecular
mechanisms underlying the development and progression of EC.
Materials and methods
Subjects
In the present study, 60 EC tissues, 20 atypical
hyperplasia endometrium (EAH) tissues and 20 normal endometrial
tissues were obtained from patients who received surgery at the
Obstetrics and Gynecology Hospital of Fudan University between
January, 2013 and February, 2016. Normal endometrial tissues were
obtained from women who had undergone hysterectomy (such as uterine
fibroids or prolapse). The median ages were 55.0 years (range,
26-76 years) in the EC group, 47 years (range, 37-53 years) in the
EAH group and 49 years (range, 49-61 years) in the normal group.
None of the patients recruited in the present study had received
chemotherapy, radiotherapy or hormone therapy prior to surgery.
Cell culture and transfection
HEC-1A and Ishikawa cells were purchased from The
Cell Bank of Type Culture Collection of the Chinese Academy of
Sciences. HEC-1A cells were cultured in McCoy's 5A medium (HyClone;
GE Healthcare) and Ishikawa cells were cultured in Eagle's minimum
essential medium (HyClone; GE Healthcare), both supplemented with
10% FBS. Cells were maintained in a humidified incubator with 5%
CO2 at 37°C. For transfection, all vectors and mimics
were transfected into Ishikawa and HEC-1A using
Lipofectamine® 3000 Transfection Reagent (Invitrogen;
Thermo Fisher Scientific, Inc.). Cells were cultured in plates for
24 h prior to transfection. All vectors and mimics were transfected
into HEC-1A and Ishikawa cells (5x105 cells/well) with
Lipofectamine® 3000 Transfection Reagent, incubated at
37°C for 24 h, and added to the plates. The sequences were as
follows: miR-205-5p mimic (5'-UCCUUCAUUCCACCG GAGUCUG-3';
5'-GACUCCGGUGGAAUGAAGGAUU-3') and mimic NC
(5'-UUCUCCGAACGUGUCACGUTT-3'; 5'-ACGUGACACGUUCGGAGAATT-3'). The
lncRNA overexpression plasmid was pLenti-EF1a-EGFP-F2A-Puro-CMV-
LA16C-313D11.11 and the empty vector was used as a control. The
working concentration of miRNA mimics and NC were 50 nM. The
concentration used for plasmids was 100 nM. The time duration
between transfection subsequent experimentation was approximately
24 h. In order to analyze the dose-response association between
LA16C-313D11.11, miR-205-5p and PTEN, the concentration gradient
(0-5 mg/ml) of LA16C-313D11.11 was set.
Luciferase reporter assay
The wild-type 3'-untranslated region (UTR) sequence
of PTEN (PTEN-WT) were cloned into the luciferase reporter vector
(Obio Technology). A mutant PTEN 3'-UTR vector (PTEN-MuT) was also
constructed, which contained a mutation in the predicted
PTEN-binding sequence. The PTEN-MuT or PTEN-WT, were co-transfected
with either miR-205-5p mimics or negative Control (NC) mimics into
HEC-1A cells using Lipofectamine® 3000. After 48 h of
transfection, luciferase activity was determined using a Luciferase
Reporter Gene kit (Promega Corporation) and normalized to the
Renilla luciferase activity.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA extraction was performed using TRIzol
reagent (Life Technologies; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocols. For the analysis of
miRNA expression, miRNAs were extracted using an miRNeasy Mini kit
(Qiagen, Inc.) and real-time quantification of miRNAs, primer
extension and RNA-tailing were performed as described previously
(19). RT-qPCR was performed using
the SYBR Premix Ex Taq™ (Thermo Fisher Scientific, Inc.) with
primers specific for LA16c-313D1U1, PTEN and miRNA-205-5p. The
primers were designed using a primer designing software package
(LA16c-313D11.11 forward, 5'-T GAAGGAGGTTA TTGACGCA-3' and reverse,
5'-GAGGGGAAACAGTCC AGAGT-3'; miR-205-5p forward, 5'-TCCACCGGAGTCTGT
CTCAT-3' and reverse, 5'-GCTGTCAACGATACGCTACG-3'; PTEN forward,
5'-ACCAACTGAAGTGGCTAAAGAG-3' and reverse, 5'-GGTCCAGAGTCCAGCATA
AAA-3'). GAPDH was used as the internal reference RNA for lncRNA
and mRNA expression analysis, and small nuclear RNA U6 was used as
the internal control for miRNA analysis. Gene expression levels
were calculated based on the comparative quantitative method (the
2-AACq method) (20).
All qPCR reactions were performed using an Applied Biosystems 7500
Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific,
Inc.).
Western blot analysis
Total protein was extracted from the cells using
RIPA lysis buffer (Nanjing KeyGen Biotech Co., Ltd.) supplemented
with 1 nM phenylmethylsulphonyl fluoride. Total protein was
collected, and the concentration was determined using an enhanced
bicinchoninic acid Protein assay kit (Nanjing KeyGen Biotech Co.,
Ltd.). A total of 30 ^g of protein was loaded per lane onto a 10%
SDS-gel, resolved using SDS-PAGE and transferred to PVDF membranes
(EMD Millipore). The membranes were blocked in 5% non-fat milk in
Tris-buffered saline (room temperature, 2 h), and subsequently
incubated overnight at 4°C with one of the following antibodies:
Caspase-3 (1:1,000; Abcam; cat. no. ab32351), PTEN (1:1,000; Abcam;
cat. no. ab32199), phos- phoinositide-dependent kinase-1 (PDK1;
1:1,000; Abcam; cat. no. ab110025), AKT (1:1,000; Cell Signaling
Technology, Inc.; cat. no. #2920), phospho-(p-) Akt (1:1,000; Cell
Signaling Technology, Inc.; cat. no. #4060) and p-actin (1:1,000;
ProteinTech Group, Inc.; cat. no. 66009-1-Ig). The membranes were
subsequently incubated with secondary antibodies (1:5,000;
Immunoway; cat. nos. #RS0001 and #RS0002) for 2 h at room
temperature. Signals were visualized using an enhanced
chemiluminescence kit and densitometric analysis was performed
using ImageJ v1.8.0 (National Institutes of Health).
Cell proliferation analysis
To analyze cell proliferation, a Cell Counting Kit-8
assay was used (CCK-8; Dojindo Molecular Technologies, Inc.). Cells
were plated in 96-well plates (2,000 cells/well) following
transfection. Following 24, 48 and 72 h of incubation, the cells
were incubated with CCK-8 solution at 37°C for 2 h. The viability
of the cells was determined by measuring the absorbance of the
plates at 450 nm using a spectrophotometer (Bio-Tek
Instruments).
Apoptosis assay
HEC-1A and Ishikawa cells (1x106) were
plated in 6-well plates and collected 24 h following transfection.
An Annexin V-FITC Apoptosis Detection kit (BD Biosciences) and flow
cytometry (FACS Calibur; FlowJo10.0BD; Biosciences) was used to
assess apoptosis, and the kit was used according to the
manufacturer's protocol.
Transwell migration assay
Transwell inserts (BD Biosciences) were used to
perform the invasion assays. Transfected Ishikawa and HEC-1A cells
(1x106 cells/well) were added to the upper chamber of
the insert in serum-free medium (200 ^l). In the lower chamber,
medium supplemented with 20% FBS (600 ^l) was added to act as a
chemoattractant. The filter membrane of the invasion assays was
coated with diluted Matrigel (BD Biosciences). Following 24 h of
incubation, the cells which had invaded through the membranes were
fixed in methanol (room temperature, 30 min) and stained (room
temperature, 5 min) with 0.05% crystal violet. The cells which had
invaded were quantified using a microscope (Olympus
Corporation).
Wound-healing assay
HEC-1A and Ishikawa cells (1x106
cells/well) were plated in a 24-well plate; when an 80% confluent
cell monolayer had formed, the monolayer was scratched using a 100
^l pipette tip, and the cells were subsequently cultured in
serum-free medium. Images of the wound were acquired at 0 and 24 h
after scratching to assess cell migration using a light microscope
(scale bar, 100 ^m).
Statistical analysis
Data are presented as the means ± standard deviation
of at least 3 experimental repeats. SPSS version 19.0 (IBM Corp.)
and GraphPad Prism version 7.0 (GraphPad Software, Inc.) were used
for statistical analysis. The results of RT-qPCR and western blot
analysis were compared using ANOVA followed by the post hoc
Student-Newman-Keuls or Tukey's tests. A two-tailed Students'
t-test was used to compare differences between 2 groups.
Qualitative data are expressed as rate and were analyzed using a
x2 test. Spearman's rank correlation analysis was used
for correlation analysis, and R=0 was used as the relevant
standard. P<0.05 was considered to indicate a statistically
significant difference.
Results
LA16c-313D11.11, miRNA-205-5p and PTEN
expression in the endometrium
RT-qPCR was used to assess the expression of
LA16c-313D11.11, PTEN and miRNA-205-5p in 60 EC, 20 EAH and 20
normal endometrium tissues. The relative expression levels of
LA16c-313D11.11 and PTEN in the EC tissues were significantly
decreased compared with those in the EAH and normal endometrial
tissues (LA16c-313D11.11: EC vs. EAH, P<0.0001; EC vs. normal,
P<0.0001; PTEN: EC vs. EAH, P<0.05; EC vs. normal,
P<0.0001; Fig. 1A and B).
However, the relative expression level of miRNA-205-5p in the EC
tissues was significantly higher compared with that in the EAH and
normal endometrial tissues (EC vs. EAH, P<0.05; EC vs. normal,
P<0.0001; Fig. 1C).
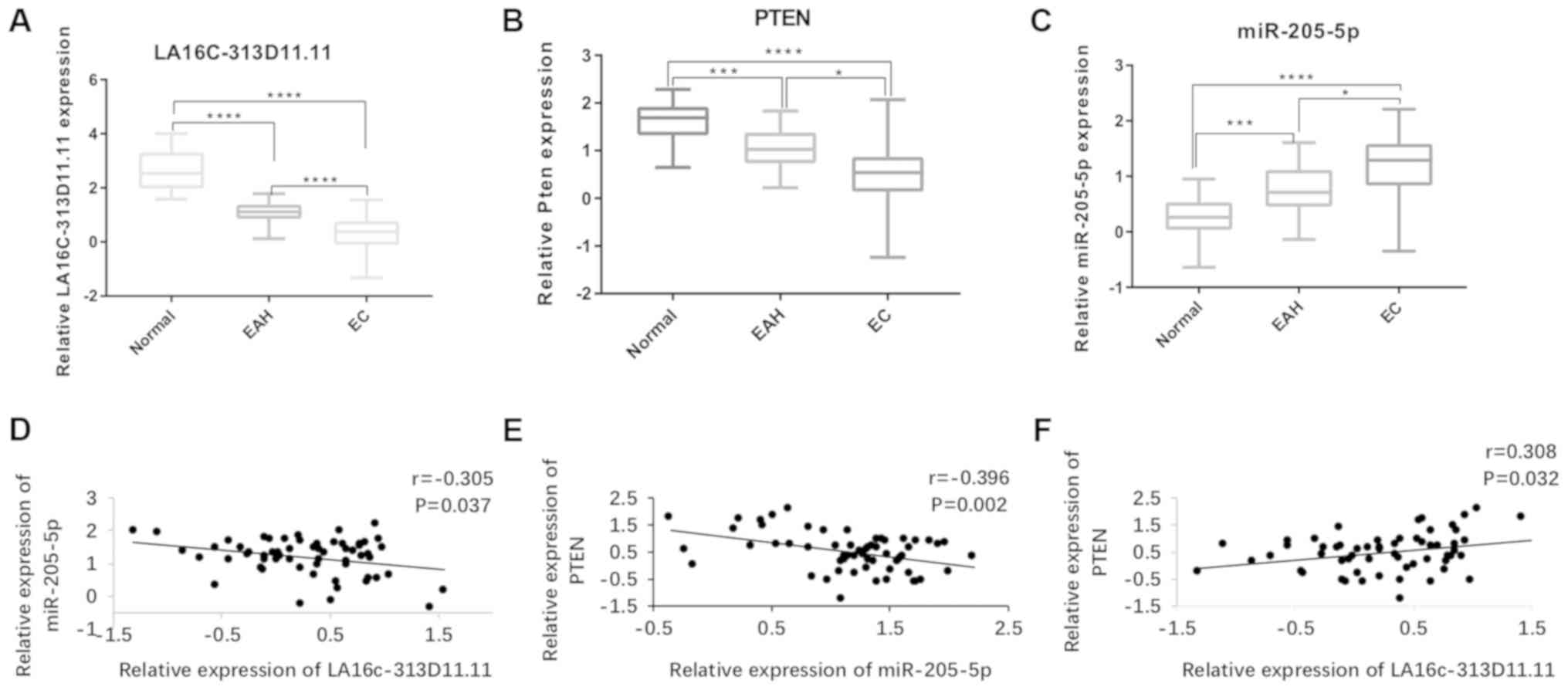 | Figure 1LA16c-313D11.11, PTEN and
microRNA-205-5p are aberrantly expressed in EC, EAH and NE. (A-C)
Expression levels of LA16c-313D11.11, PTEN and microRNA-205-5p in
EC, EAH and NE tissues examined by RT-qPCR. (D-F) Correlation
between LA16c-313D11.11, microRNA-205-5p and PTEN in EC tissues.
*P<0.05, ***P<0.001, *'"P<0.0001. PTEN, phosphatase and
tensin homolog deleted on chromosome ten; EC, endometrial cancer;
EAH, atypical hyperplasia endometrium; NE, normal endometrium. |
Expression and clinical significance of
LA16c-313D11.11 in human EC tissues
As the relative expression level of LA16c-313D11.11
in the human EC tissues was significantly lower compared with that
in the normal endometrial tissues, the clinicopathological
significance of LA16c-313D11.11 was assessed. The mean expression
level of LA16c-313D11.11 was used to stratify patients in to the
high and low expression groups. The low expression of
LA16c-313D11.11 was associated with lymph node metastasis and an
advanced FIGO stage (P<0.05; Table
I). These results suggest that the decreased expression of
LA16c-313D11.11 is associated with an invasive phenotype and an
increased metastatic potential.
 | Table IAssociation of LA16c-313D11.11
expression with the clinicopathological characteristics of patients
with EC. |
Table I
Association of LA16c-313D11.11
expression with the clinicopathological characteristics of patients
with EC.
| Variables | Cases (n) | LA16c-313D11.11
expression
| P-value |
|---|
| Low, n (%) | High, n (%) |
|---|
| Age (years) | | | | 0.639 |
| <50 | 16 | 8 (13.3) | 8 (13.3) | |
| >50 | 44 | 19 (31.7) | 25 (41.7) | |
| Histological
subtype | | | | 0.493 |
| Endometrioid | 45 | 21 (35) | 24 (40) | |
| Serous | 10 | 5 (8.3) | 5 (8.3) | |
| Clear cell | 5 | 1 (1.7) | 4 (6.7) | |
| Menstruation | | | | 0.582 |
| Premenopausal | 20 | 10 (16.7) | 10 (16.7) | |
| Menopausal | 40 | 17 (28.3) | 23 (38.3) | |
| FIGO stage | | | | 0.047a |
| I-II | 47 | 18 (30) | 29 (48.3) | |
| III-IV | 13 | 9 (15) | 4 (6.7) | |
| Histological
grade | | | | 0.962 |
| G1 | 30 | 14 (23.3) | 16 (26.7) | |
| G2 | 8 | 4 (6.7) | 4 (6.7) | |
| G3 | 7 | 3 (5) | 4 (6.7) | |
| Myometrial
invasion | | | | 0.955 |
| <1/2 | 42 | 19 (31.7) | 23 (38.3) | |
| >1/2 | 18 | 8 (13.3) | 10 (16.7) | |
| Lymph node
metastasis | | | | 0.047a |
| Present | 13 | 9 (15) | 4 (6.7) | |
| Absent | 47 | 18 (30) | 29 (48.3) | |
Correlation between LA16c-313D11.11,
miRNA-205-5p and PTEN expression in the endometrium
LA16c-313D11.11 expression negatively correlated
with miRNA-205-5p expression in the EC tissues, suggesting a
possible interaction between LA16c-313D11.11 and microRNA-205-5p in
EC (Fig. 1D). Furthermore, PTEN
expression positively corelated with LA16c-313D11.11 expression,
but negatively correlated with miRNA-205-5p expression in the EC
tissues (Fig. 1E and F).
Interaction between LA16c-313D11.11,
miRNA-205-5p and PTEN in EC cells
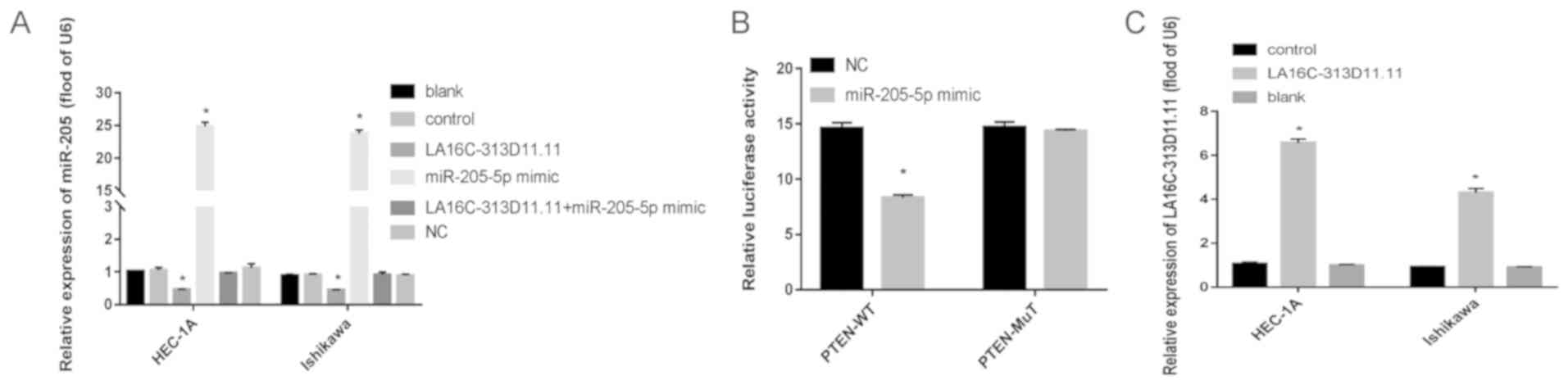
LA16c-313D11.11, miRNA-205-5p and LA16c-313D11.11 +
miRNA-205-5p were overexpressed in the HEC-1A and Ishikawa cells.
LA16c-313D11.11 was successfully overexpressed in the HEC-1A and
Ishikawa cells (Fig. 2C). The
relative expression level of miRNA-205-5p was significantly
decreased in the LA16c-313D11.11-overexpressing cells compared with
the control (P<0.05; Fig. 2A).
However, there was no statistically significant difference compared
with the control when LA16c-313D11.11 and miRNA-205-5p were
co-overexpressed in the HEC-1A and Ishikawa cells (Fig. 2A). Thus, it was demonstrated that
miRNA-205-5p was competitively inhibited by LA16c-313D11.11 in the
HEC-1A and Ishikawa cells. PTEN was found to be a direct target of
miRNA-205-5p by luciferase assay (Fig.
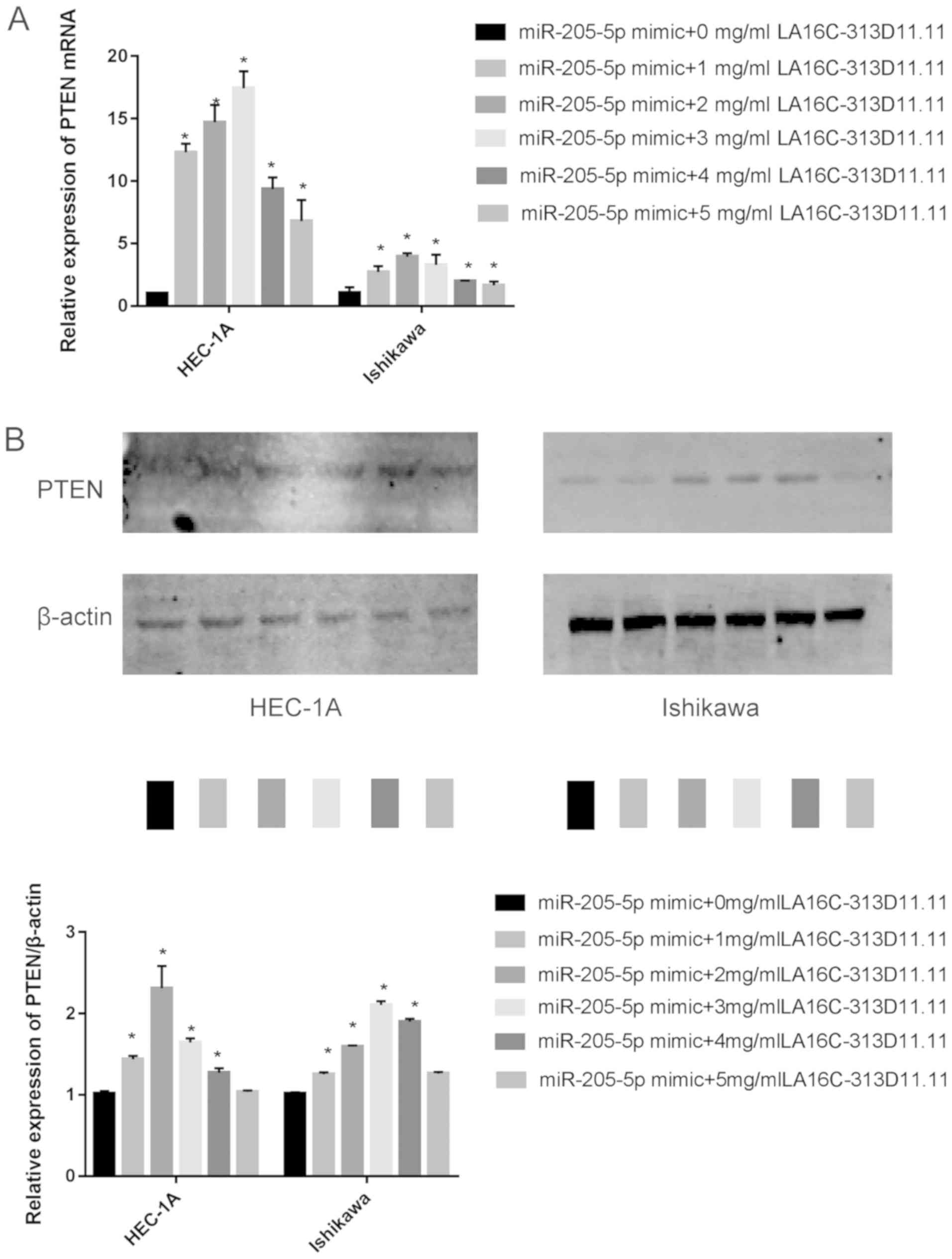
2B). Additionally, the overexpression of LA16c-313D11.11
promoted the expression of PTEN in a concentration-dependent manner
when the LA16c-313D11.11 concentration was up to the 2 mg/ml (in
HEC-1A cells) or 3 mg/ml (in Ishikawa cells) (Fig. 3). At concentrations higher than
these mentioned, the expression of PTEN gradually decreased. Thus,
an LA16c-313D11.11-m iRNA-205-5p-PTEN axis was successfully
identified in the endometrial cancer cells.
LA16c-313D11.11 modulates the expression
of PTEN, the endogenous target of miRNA-205-5p, and thus indirectly
regulates the P13K/Akt signaling pathway in EC cells
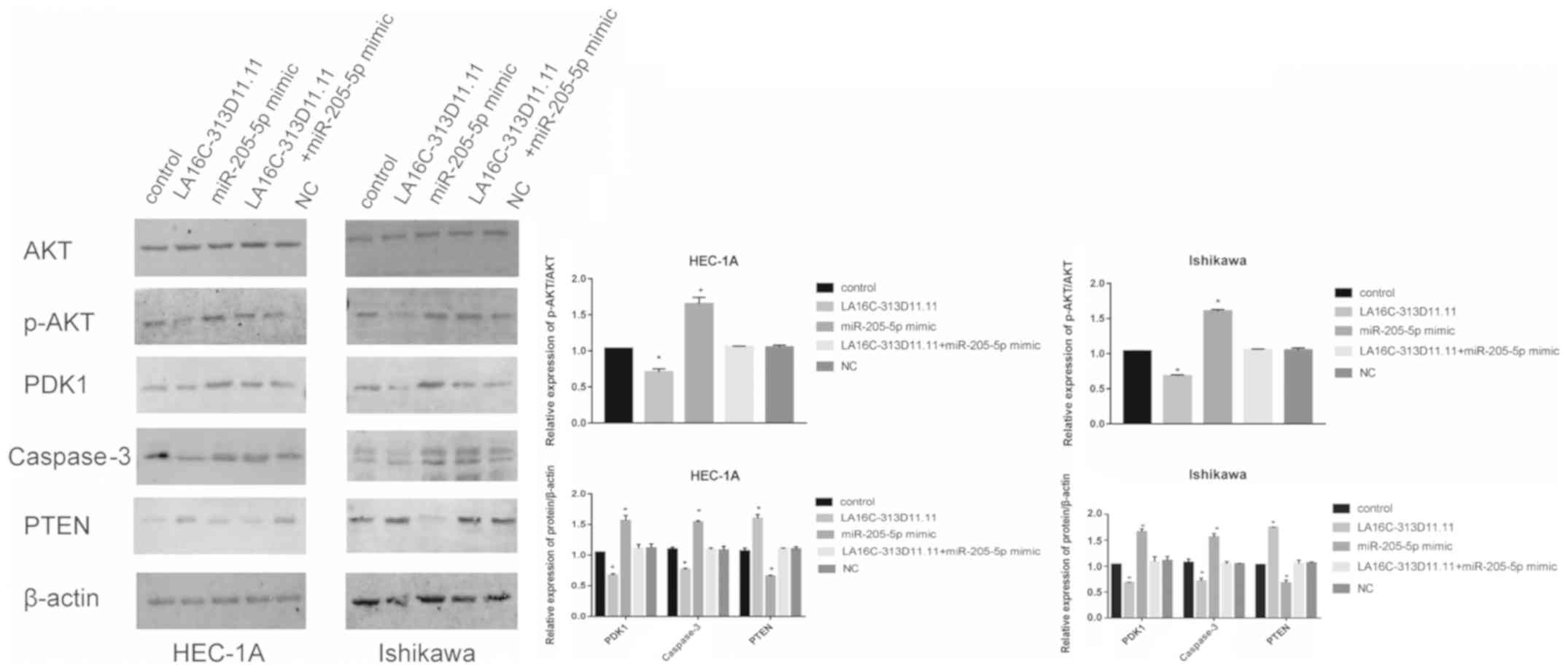
To investigate the function of LA16c-313D11.11 in
endometrial cancer, the expression levels of PTEN and the PI3K/Akt
signaling pathway were analyzed in the HEC-1A and Ishikawa cells.
The expression levels of PTEN were upregulated, while those of
p-AKT/AKT, PDK1 and caspase-3 levels were down- regulated when
LA16c-313D11.11 was overexpressed. The overexpression of
miRNA-205-5p resulted in the opposite effects. However, there was
no significant difference when both LA16c-313D11.11 and miR-205-5p
were both overexpressed (Fig. 4).
Taken together, these results demonstrate that LA16c-313D11.11 may
interfere with the miRNA-205-5p mediated inhibition of PTEN in
EC.
LA16c-313D11.11 decreases the viability,
migration and invasion of EC cells through the inhibition of
miRNA-205-5p
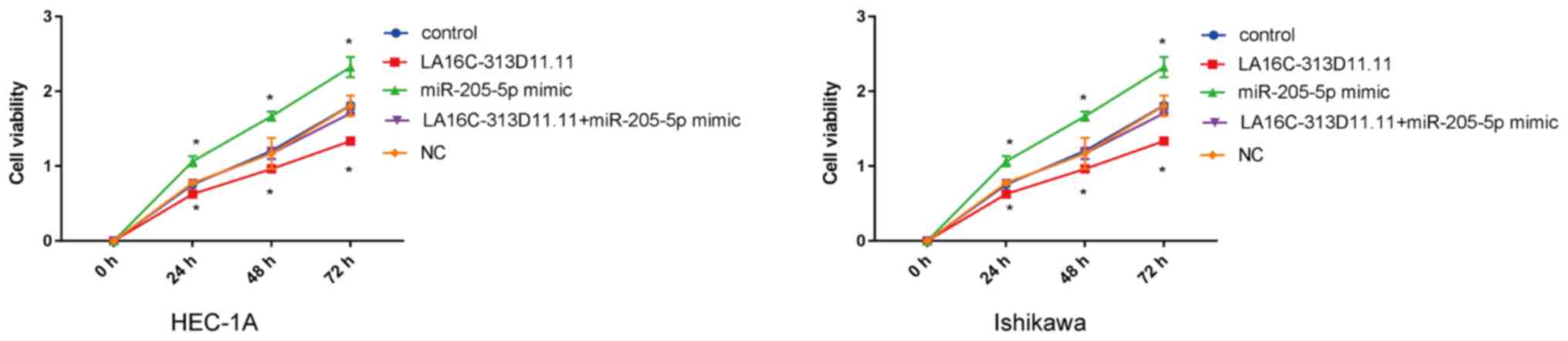
The regulatory effects of the
LA16c-313D11.11-miRNA-205-5p- PTEN axis on the viability, migration
and invasion of endometrial cancer cells were further determined.
CCK-8 assay was performed to detected the proliferation of
transfected Ishikawa and HEC-1A cells at the indicated periods of
time. The results revealed that LA16c-313D11.11 overexpression
significantly inhibited the proliferation of EC cells compared with
the control, whereas miR-205-5p overexpression significantly
increased the proliferation of EC cells. There was no statistically
significant difference in viability compared with the control when
LA16c-313D11.11 and miRNA-205-5p were co-overexpressed in the
HEC-1A and Ishikawa cell lines (Fig.
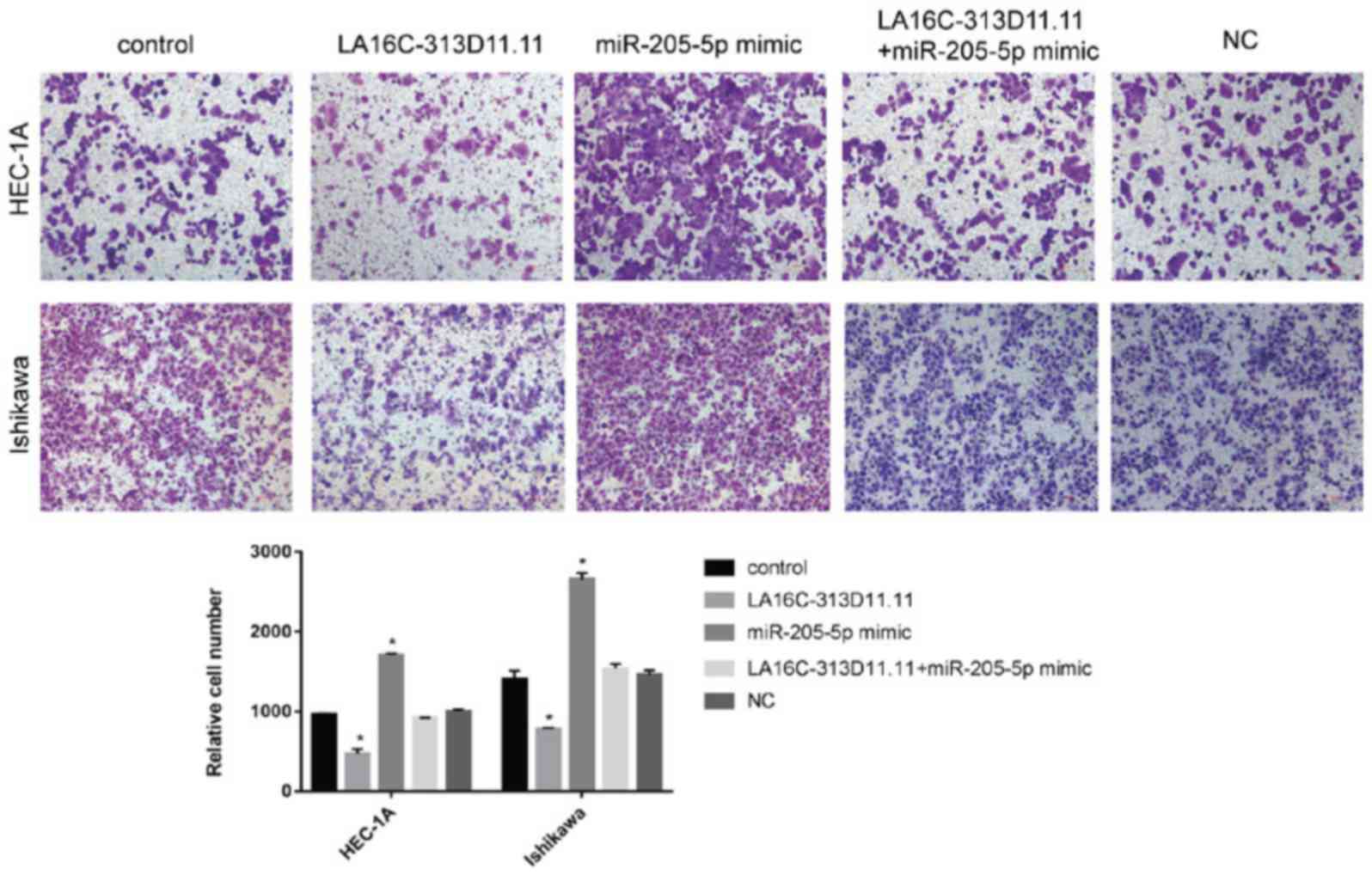
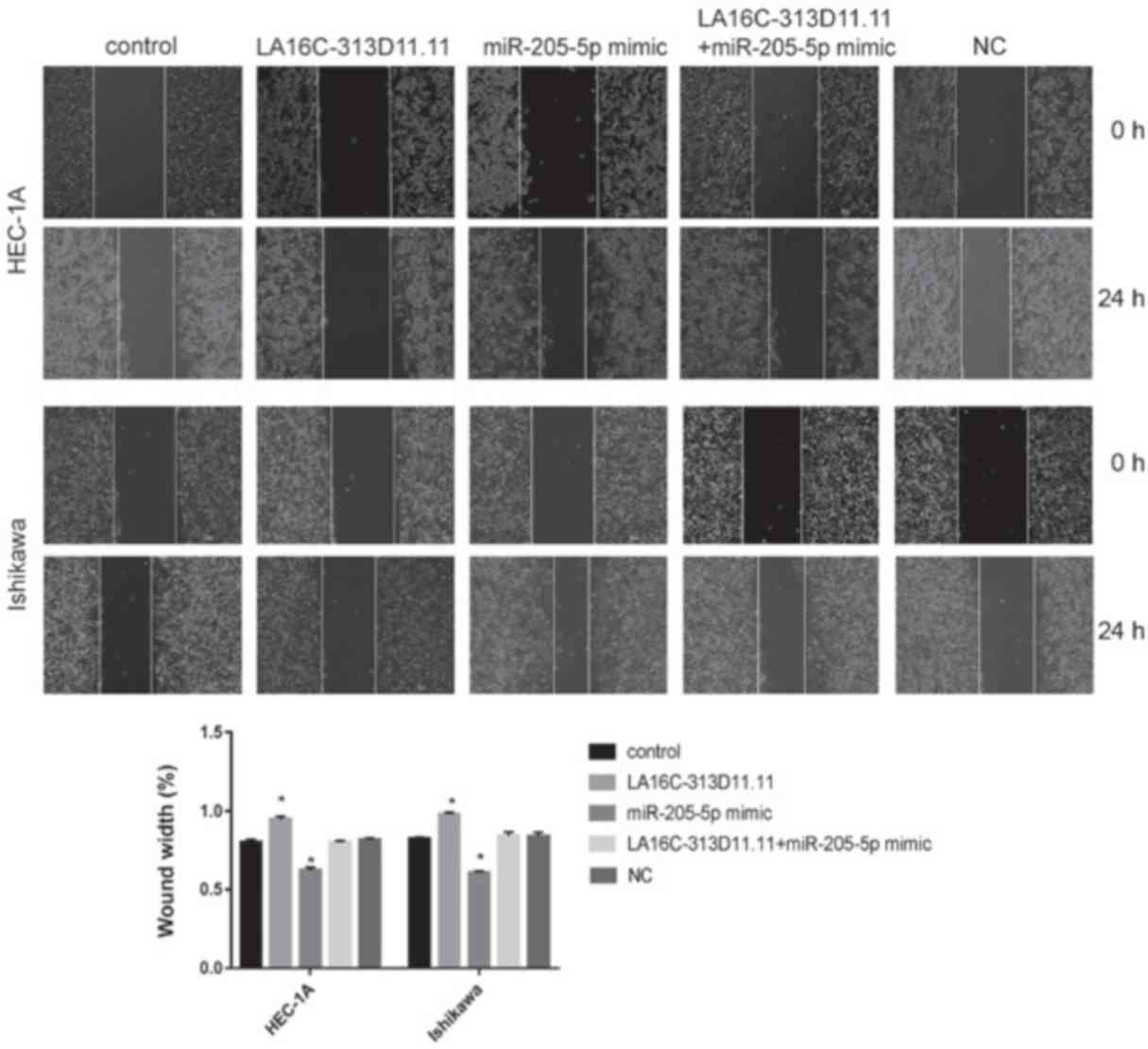
5). Transwell invasion assays were conducted to calculate the
number of invasive cells. Wound-healing assays were used to
evaluate the mobility of EC cells. The overexpression of
LA16c-313D11.11 significantly decreased the invasion and migration
of the HEC-1A and Ishikawa cells compared with the control.
However, the overexpression of miRNA-205-5p significantly increased
the invasion and migration of the HEC-1A and Ishikawa cells
compared with the control. There was no statistically significant
difference in cells overexpressing both LA16c-313D11.11 and
miR-205-5p compared with the control (Figs. 6 and 7). These results suggest that
LA16c-313D11.11 inhibits the effects of miRNA-205-5p on the
viability, invasion and migration of EC cells.
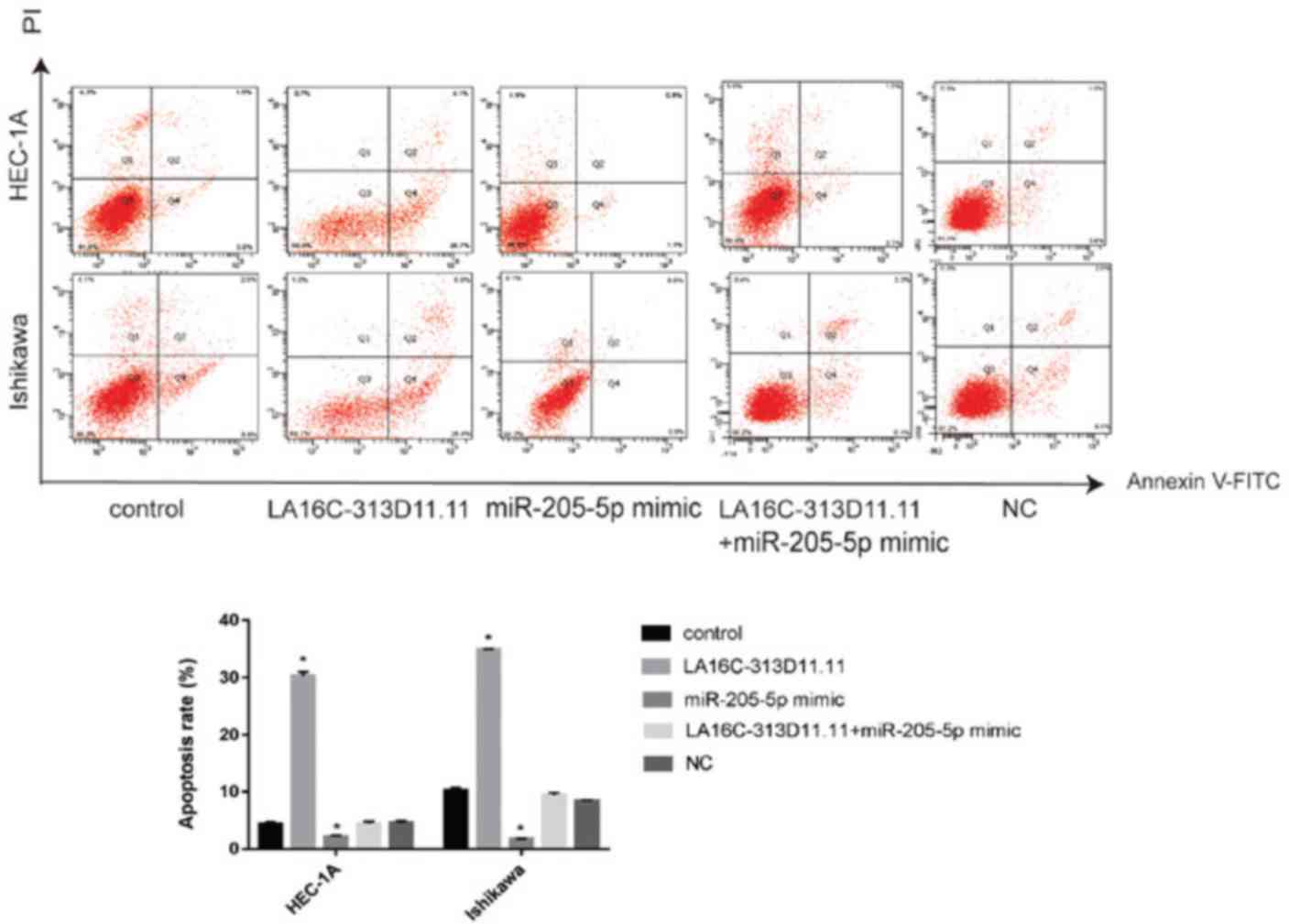
LA16c-313D11.11 induces the apoptosis of
EC cells by inhibiting the binding of miRNA-205-5p to PTEN
As LA16c-313D11.11 decreased the viability, invasion
and migration of endometrial cancer cells, the effects of
LA16c-313D11.11 overexpression on the apoptosis of endometrial
cancer cell lines were also determined. The results revealed that
LA16c-313D11.11 overexpression significantly induced the apoptosis
of EC cells compared with the control, whereas miR-205-5p
overexpression significantly inhibited the apoptosis of EC cells.
There was no statistically significant difference in apoptosis
compared with the control when LA16c-313D11.11 and miRNA-205-5p
were co-overexpressed in HEC-1A and Ishikawa cell lines (Fig. 8). These results suggest that the
dysregulation of LA16c-313D11.11 may contribute to the development
of endometrial cancer and exert a tumor-suppressive effect.
Discussion
The non-coding regions of the genome are considered
more complex compared with the coding regions in higher eukaryotes
(21-23). miRNAs have been demonstrated to be
involved in numerous vital biological processes, including
development, hematopoiesis, organ formation, apoptosis, cell
proliferation and even tumorigenesis (24-26).
Similarly, lncRNAs are receiving increasing interest and are now
considered a novel layer of regulation, adding to the complexity of
mammalian gene regulatory networks (27,28).
Recently, the ceRNA hypothesis has suggested that the regions of
non-coding RNAs may act as molecular sponges of microRNAs, thereby
inhibiting the target miRNAs' function, and thus increasing the
activity of the target of the miRNA, indirectly (14,29-31).
In the present study, it was demonstrated that lncRNA
LA16c-313D11.11 served as an endogenous sponge of miRNA-205-5p, and
abrogated its endogenous inhibition on PTEN, thus affecting
proliferation and migration in endometrial cancer.
A previous study by the authors demonstrated that
LA16c-313D11.11 was associated with the nosogenesis of EC and was
shown to be a non-coding RNA. LA16c-313D11.11 is an effective ceRNA
which is associated with a miRNA-205-5p-PTEN network (18). To further elucidate the molecular
mechanisms through which LA16c-313D11.11 may be involved in
endometrial cancer, in the present study, 60 primary endometrial
cancer tissues, 20 EAH tissues and 20 normal endometrium tissues
were obtained for analysis. Additionally, LA16c-313D11.11 mimic and
miRNA-205-5p mimic were transfected into HEC-1A and Ishikawa
cells.
The expression of miRNA-205-5p was found to be
upregulated in human EC tissues, and was negatively associated with
the PTEN levels in human EC tissues, suggesting a possible
association between miRNA-205-5p and PTEN in the pathogenesis of
EC. It was also demonstrated that the expression levels of
miRNA-205-5p were negatively associated with the overall survival
time of patients with EC (17).
Therefore, it was concluded that miRNA-205-5p may serve as an
effective marker for the diagnosis of EC, and may be used to
predict the clinical prognosis of patients with EC. The expression
of miRNA-205-5p in EC tissues was significantly higher compared
with EAH and normal endometrial tissues.
Furthermore, miRNA-205-5p expression levels were
inversely associated with the viability, migration and invasion of
EC cells in vitro, highlighting its potential role in the
progression of EC.
LA16c-313D11.11 and miRNA-205-5p were shown to
interact in EC cells. The relative expression level of
LA16c-313D11.11 in EC tissues was significantly decreased compared
with EAH and normal endometrial tissues. The clinicopathological
significance of LA16c-313D11.11 indicated that the downregulation
of LA16c-313D11.11 expression was significantly associated with
lymph node metastasis and an advanced FIGO stage. The results
demonstrated that low levels of LA16c-313D11.11 expression were
associated with phenotypically invasive tumors, and in particular,
with an increased metastasis. LA16c-313D11.11 expression negatively
correlated with miRNA-205-5p expression in human EC tissues, and
LA16c-313D11.11 regulated the viability, invasion and migration of
EC cells by competing with miRNA-205-5p, which endogenously
inhibits PTEN function, and PTEN physiologically inhibits the
PI3K/Akt signaling pathway. Therefore, LA16c-313D11.11 indirectly
inhibited the PI3K/Akt signaling pathway.
By acting as ceRNAs, lncRNAs exert their effects by
acting as endogenous inhibitors of miRNAs, thus affecting the
binding of miRNAs to their targets (14). The results of the present study
suggest that LA16c-313D11.11 acts as a ceRNA by sequestering
miRNA-205-5p, thus inhibiting the progression of EC. The
identification of this novel mechanism improves our understanding
of the underlying pathophysiology of EC, and may assist in the
development of novel therapeutics by highlighting potential
therapeutic targets. However, the present study has several
limitations. To address these limitations, in future studies, the
sample size will be increased to verify the association between
LA16c-313D11.11 and the prognosis of patients with EC.
Additionally, whether LA16c-313D11.11 regulates any other potential
mechanisms will be examined, and the role of the miRNA-205-5p-PTEN
axis in vivo will be investigated. Furthermore, the
mechanisms of the three markers will be examined further using
additional in vitro experiments.
In conclusion, LA16c-313D11.11 acts a ceRNA in a
miRNA-205-5p-PTEN axis. To the best of our knowledge, the present
study is the first to demonstrated that LA16c-313D11.11 regulates
the development of EC by inhibiting the binding of miRNA-205-5p to
its target, highlighting a novel lncRNA-miRNA network in EC. The
present study enhances the current knowledge of the molecular
mechanisms underlying the development and progression of EC and may
assist in the development of novel therapeutics for the treatment
of patients with EC, or may improve the diagnosis of patients with
EC.
Abbreviations:
|
EC
|
endometrial cancer
|
|
EAH
|
atypical hyperplasia endometrium
|
|
NE
|
normal endometrium
|
|
PTEN
|
phosphatase and tensin homolog deleted
on chromosome ten
|
|
ceRNA
|
competing endogenous RNA
|
Acknowledgments
Not applicable.
Funding
The present study was subsidized by the Natural
Science Foundation of Shanghai, China (grant no. 16ZR1404100).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article or are available from
the corresponding author on reasonable request.
Authors' contributions
WX, JD, QW and KH designed the study. WX and SZ
performed the experiments. XH, PZ, HY, XG and PL were involved in
the interpretation of the data. WX, SZ, QW and KH analyzed the data
and wrote the manuscript. WX, SZ, XH and HY revised the manuscript.
All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The study' ethical approval was granted by the
Obstetrics and Gynecology Hospital of Fudan University, Shanghai,
China. Written informed consent was provided by the subjects for
the collection of samples and follow-up analysis. The study was
done based on the guidelines and principles stipulated in the
declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bhatla N and Denny L: FIGO Cancer Report
2018. Int J Gynaecol Obstet. 143(Suppl 2): 2–3. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Matias-Guiu X and Prat J: Molecular
pathology of endometrial carcinoma. Histopathology. 62:111–123.
2013. View Article : Google Scholar
|
|
5
|
Kandoth C, Schultz N, Cherniack AD, Akbani
R, Liu Y, Shen H, Robertson AG, Pashtan I, Shen R, Benz CC, et al
Cancer Genome Atlas Research Network: Integrated genomic
characterization of endometrial carcinoma. Nature. 497:67–73. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang H, Chen Z, Wang X, Huang Z, He Z and
Chen Y: Long non-coding RNA: A new player in cancer. J Hematol
Oncol. 6:372013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Seton-Rogers S: Non-coding RNAs: The
cancer X factor. Nat Rev Cancer. 13:224–225. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qi P and Du X: The long non-coding RNAs, a
new cancer diagnostic and therapeutic gold mine. Mod Pathol.
26:155–165. 2013. View Article : Google Scholar
|
|
9
|
Ebert MS, Neilson JR and Sharp PA:
MicroRNA sponges: Competitive inhibitors of small RNAs in mammalian
cells. Nat Methods. 4:721–726. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Poliseno L, Salmena L, Zhang J, Carver B,
Haveman WJ and Pandolfi PP: A coding-independent function of gene
and pseudogene mRNAs regulates tumour biology. Nature.
465:1033–1038. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang J, Liu X, Wu H, Ni P, Gu Z, Qiao Y,
Chen N, Sun F and Fan Q: CREB up-regulates long non-coding RNA,
HULC expression through interaction with microRNA-372 in liver
cancer. Nucleic Acids Res. 38:5366–5383. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee DY, Jeyapalan Z, Fang L, Yang J, Zhang
Y, Yee AY, Li M, Du WW, Shatseva T and Yang BB: Expression of
versican 3'-untranslated region modulates endogenous microRNA
functions. PLoS One. 5:e135992010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jeyapalan Z, Deng Z, Shatseva T, Fang L,
He C and Yang BB: Expression of CD44 3'-untranslated region
regulates endogenous microRNA functions in tumorigenesis and
angiogenesis. Nucleic Acids Res. 39:3026–3041. 2011. View Article : Google Scholar
|
|
14
|
Salmena L, Poliseno L, Tay Y, Kats L and
Pandolfi PP: A ceRNA hypothesis: The Rosetta Stone of a hidden RNA
language? Cell. 146:353–358. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Greene SB, Gunaratne PH, Hammond SM and
Rosen JM: A putative role for microRNA-205 in mammary epithelial
cell progenitors. J Cell Sci. 123:606–618. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Qu C, Liang Z, Huang J, Zhao R, Su C, Wang
S, Wang X, Zhang R, Lee MH and Yang H: MiR-205 determines the
radioresistance of human nasopharyngeal carcinoma by directly
targeting PTEN. Cell Cycle. 11:785–796. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Karaayvaz M, Zhang C, Liang S, Shroyer KR
and Ju J: Prognostic significance of miR-205 in endometrial cancer.
PLoS One. 7:e351582012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xin W, Liu X, Ding J, Zhao J, Zhou Y, Wu Q
and Hua K: Long non-coding RNA derived miR-205-5p modulates human
endometrial cancer by targeting PTEN. Am J Transl Res. 7:2433–2441.
2015.
|
|
19
|
Shi R and Chiang VL: Facile means for
quantifying microRNA expression by real-time PCR. Biotechniques.
39:519–525. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
21
|
Mattick JS: The central role of RNA in
human development and cognition. FEBS Lett. 585:1600–1616. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nagano T and Fraser P: No-nonsense
functions for long noncoding RNAs. Cell. 145:178–181. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cesana M, Cacchiarelli D, Legnini I,
Santini T, Sthandier O, Chinappi M, Tramontano A and Bozzoni I: A
long noncoding RNA controls muscle differentiation by functioning
as a competing endogenous RNA. Cell. 147:358–369. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hatfield S and Ruohola-Baker H: microRNA
and stem cell function. Cell Tissue Res. 331:57–66. 2008.
View Article : Google Scholar
|
|
25
|
Volinia S, Calin GA, Liu CG, Ambs S,
Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, et
al: A microRNA expression signature of human solid tumors defines
cancer gene targets. Proc Natl Acad Sci USA. 103:2257–2261. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yu F, Yao H, Zhu P, Zhang X, Pan Q, Gong
C, Huang Y, Hu X, Su F, Lieberman J, et al: let-7 regulates self
renewal and tumori- genicity of breast cancer cells. Cell.
131:1109–1123. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mercer TR, Dinger ME and Mattick JS: Long
non-coding RNAs: Insights into functions. Nat Rev Genet.
10:155–159. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ponting CP, Oliver PL and Reik W:
Evolution and functions of long noncoding RNAs. Cell. 136:629–641.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang Y, Xu Z, Jiang J, Xu C, Kang J, Xiao
L, Wu M, Xiong J, Guo X and Liu H: Endogenous miRNA sponge
lincRNA-RoR regulates Oct4, Nanog, and Sox2 in human embryonic stem
cell self-renewal. Dev Cell. 25:69–80. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Johnsson P, Ackley A, Vidarsdottir L, Lui
W-O, Corcoran M, Grander D and Morris KV: A pseudogene
long-noncoding-RNA network regulates PTEN transcription and
translation in human cells. Nat Struct Mol Biol. 20:440–446. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang K, Long B, Zhou LY, Liu F, Zhou QY,
Liu CY, Fan YY and Li PF: CARL lncRNA inhibits anoxia-induced
mitochondrial fission and apoptosis in cardiomyocytes by impairing
miR-539-dependent PHB2 downregulation. Nat Commun. 5:35962014.
View Article : Google Scholar : PubMed/NCBI
|