Introduction
Hepatocellular carcinoma (HCC) is a primary liver
cancer with a high mortality rate (1). In recent years, the incidence of
liver cancer has not only increased, but also exhibits a tendency
to occur in young individuals (2).
Due to the strong compensatory function of the liver and the
structural features of blood supply, liver tumors grow rapidly and
are prone to metastasis (3).
Therefore, these tumors are clinically diagnosed mostly during the
mid and late stages of the disease, when the opportunity for
surgical resection has been missed and the prognosis is poor.
Surgical resection and liver transplantation are the main treatment
methods for HCC (4), but
intrahepatic metastasis and recurrence after surgery adversely
affect the prognosis of HCC patients. Liver transplantation is
often limited by a shortage of liver donors. Therefore, it is
crucial to explore new methods to treat HCC based on traditional
surgery, chemotherapy, radiotherapy, and other treatment
strategies.
In recent years, there have been several reports on
the development of new delivery systems for traditional
chemotherapeutic drugs, such as cytotoxic drugs, new drugs for gene
and molecular targets, and their various combinations (5,6).
However, due to the resistance of a small proportion of HCC stem
cells against chemotherapeutic drugs, almost all currently
investigated drugs have failed. Glucocorticoids are a type of
anti-inflammatory drug that is widely used in the clinical setting
to relieve symptoms in patients with advanced liver cancer, and
have demonstrated certain therapeutic effects (7-9).
However, to date, the therapeutic effect and synergistic mechanism
of action of glucocorticoids combined with chemotherapeutic drugs
for the treatment of patients with HCC remain unclear.
Accumulating studies have demonstrated that
hypoxia-inducible factor (HIF)-1α increases the tolerance of tumor
stem cells to a hypoxic microenvironment by upregulating the
expression of vascular endothelial growth factor, which is
considered to be one of the major mechanisms of chemotherapy
resistance in cancer stem cells (10-12).
The persistent and stable presence of HIF-1α in the cytoplasm under
hypoxic conditions depends on its degree of conjugate binding to
small ubiquitin-like modifier (SUMO) (13,14).
Similarly, the function of Oct4, a crucial stemness maintenance
protein, is also dependent on SUMO modification (15,16).
SUMO4 is hardly expressed in hepatocytes (17) and the SUMO modification caused by
SUMO2 and SUMO3 mainly occurs when cells respond to acute stress
(18). Therefore, in the present
study, only SUMO1 was assessed in order to analyze the effects of
glucocorticoids on the stemness maintenance potential of HCC stem
cells and the mechanism of chemotherapy resistance from the
perspective of SUMOylation of these two important proteins, and to
provide new targeted treatment options for HCC stem cells.
Materials and methods
HCC stem cell sorting
The human-derived HCC cell line Hep3B was purchased
from the American Type Culture Collection. The culture medium was
DMEM supplemented with 10% FBS, 100 U/ml penicillin and 100
µg/ml streptomycin (all from Gibco; Thermo Fisher
Scientific, Inc.). The cells were cultured at 37°C with 5%
CO2. To collect HCC stem cells, Hep3B cells were
routinely digested and resuspended in staining buffer (BioLegend,
Inc.) at 1×107 cells/ml, followed by incubation with
phycoerythrin-CD133 (cat. no. 130-080-801; 1:11; Miltenyi Biotec
GmbH) and fluorescein isothiocya-nate-CD44 (cat. no.11-0441-82;
1:1,000; eBioscience; Thermo Fisher Scientific, Inc.) antibodies at
4°C for 30 min. The stained tumor cells were collected by a flow
cytometer (BD Immunocytometry Systems) and cultured in DMEM/F12
containing 1X B27 (Invitrogen; Thermo Fisher Scientific, Inc.), 20
ng/ml basic fibroblast growth factor (Miltenyi Biotec GmbH) and 20
ng/ml epidermal growth factor (Provitro Biosciences LLC) at 37°C
and 5% CO2.
HCC stem cell identification
After the collected cells had been cultured for 48
h, HCC stem cell markers [sex-determining region Y-box2 (SOX2) and
the octamer-binding transcription factor 4 (Oct4)] were detected by
immunofluorescence. Briefly, cell-laden coverslips were soaked in
4% paraformaldehyde at 4°C for 30 min. After the cells were
permeabilized with 0.5% Triton X-100 and blocked in 5% BSA
(Sigma-Aldrich; Merck KGaA) for 1 h, they were incubated with
antibodies against SOX2 (1:200, ab137385; Abcam) or Oct4 (1:200,
ab18976; Abcam) overnight at 4°C. On the following day, the cells
were washed and incubated with Alexa Fluor 488-conjugated
anti-rabbit IgG H&L (1:500, ab150080; Abcam) for 1 h at room
temperature. Finally, the nuclei were counterstained with DAPI, and
the fluorescence signal was captured under a fluorescence
microscope (XF-73; Olympus Corporation) at a magnification of
×200.
Sphere formation assay
HCC stem cells (1×103 cells/ml) were
seeded in a 24-well culture plate containing a poly-lysine-coated
coverslip and cultured for 7-10 days in DMEM/F12 medium containing
1X B27, 20 ng/ml basic fibroblast growth factor, and 20 ng/ml
epidermal growth factor with low-dose (1×10−7 M),
high-dose (1×10−5 M), or no dexamethasone (Dex) (D4902;
Sigma-Aldrich; Merck KGaA). Suspended cloned spheres with diameters
>50 µm were counted under an inverted microscope (cell
Sens Entry 1.16; Olympus Corporation) at magnifications of ×40 and
×400.
Cytotoxicity assay
To assess the side effects of various concentrations
of Dex, mouse-derived bone marrow mesenchymal stem cells (BMSCs;
Central Laboratory of The Fifth Central Hospital of Tianjin) were
used. BMSCs were incubated in DMEM with 10% FBS, 100 U/ml
penicillin and 100 µg/ml streptomycin with low-dose
(1×10−7 M), high-dose (1×10−5 M), or no Dex
for 72 h. Then, cell morphology was observed and lactate
dehydrogenase (LDH) content in the culture supernatant was measured
by an LDH Activity Assay Kit (Yuanmu Biotechnology Co., Ltd.),
according to the manufacturer's instructions.
Western blotting
Total protein was extracted from cells by incubation
in RIPA buffer (Beijing Solarbio Science & Technology Co.,
Ltd.) supplemented with 1 mM phenylmethanesulfonyl fluoride and 20
mM N-ethylmaleimide, as previously reported (19). Then, 100 µg proteins were
separated by 10% SDS-PAGE and then transferred onto a PVDF membrane
(EMD Millipore, Inc.). Then, the membrane was blocked in 5% BSA
(Sigma-Aldrich; Merck KGaA) at room temperature for 1 h and
incubated with the specific antibodies against SOX2 (ab137385;
Abcam; 1:1,000), Oct4 ab18976; 1:1,000), SUMO1(ab11672; Abcam;
1:1,000), HIF-1α (ab216842; Abcam; 1:500), vimentin (5741; Cell
Signaling Technology, Inc.; 1:1,000), E-cadherin (610404; BD
Biosciences; 1:500) and β-actin as an internal control (ab8227;
Abcam; 1:1,000). The gray value of the bands was quantified by
ImageJ image analysis software, version 1.48 (National Institutes
of Health).
Wound healing assay
HCC stem cells were seeded into a 6-well plate at of
0.25×106 cells per well and cultured in DMEM/F12 at 37°C
with 5% CO2, as previously reported (20). When the confluence of the cell
monolayer cells had reached ~95%, a scratch ~1 mm wide was created
using a 200-µl pipette tip. Then, the cells were treated
with or without 1×10−7 M Dex. Images were captured at 0
and 24 h, an image was captured under an inverted microscope
(cellSens Entry 1.16; Olympus Corporation) at a magnification of
×200 and the distance between the edges of the scratch was measured
by Image-Pro Plus 6.0 software (Media Cybernetics, Inc.).
Invasion assay
Cell invasion was assessed using a 24-well Transwell
culture chamber with a filter pore size of 8 µm. Briefly,
HCC stem cells (1×104 in 100 µl medium with or
without 1×10−7 M Dex) were seeded into the upper chamber
precoated by Matrigel (1:3; BD Biosciences) at 37°C overnight, and
DMEM containing 10% FBS was placed in the lower chamber. After
incubation at 37°C for 48 h, the cells invading to the lower
surface of the membrane were stained with 0.2% crystal violet
solution at room temperature for 10 min. Then, the number of
migrated cells was counted under an inverted microscope (cellSens
Entry 1.16) at a magnification of ×200.
Angiogenesis assay
The bottom of a 96-well plate was precoated with a
2-mm layer of semi-solid Matrigel (1:3; BD Biosciences) at 37°C
overnight. Then, HCC stem cells (0.25×106/well) were
seeded on the surface of the gel in 0.1 ml medium with or without
1×10−7 M Dex. After incubation at 37°C for 96 h,
formation of blood vessels was observed under an inverted
microscope (cellSens Entry 1.16) at a magnification of ×200.
Apoptosis assay
Hep3B cells with stable herpes simplex virus
thymidine kinase (HSVtk) gene transduction were provided by the
Central Laboratory, The Fifth Central Hospital of Tianjin (Tianjin,
China). The cells were seeded in 24-well plates with DMEM
containing 1 mg/ml ganciclovir (GCV; cat. no. Y0001129;
Sigma-Aldrich; Merck KGaA). After 24 h, the cells were harvested
and apoptosis was analyzed by flow cytometry using an Annexin
V-FITC/PI Apoptosis Detection kit (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions.
Briefly, the cells were washed twice with cold PBS and then
resuspended in binding buffer. Subsequently, 100 µl of the
solution was supplemented by 5 µl FITC Annexin V and 5
µl PI. The cells were incubated for 15 min at 25°C in the
dark. The experimental data were analyzed by flow cytometry within
1 h.
Immunofluorescence
HCC stem cells were cultured on glass coverslips
with or without 1×10−7 M Dex at 37°C for 48 h. The
expression of target proteins was detected by immunofluorescence
with anti-SUMO1 (1:300, ab11672; Abcam), anti-HIF-1α (1:100,
ab216842; Abcam), or anti-Oct4 (1:100, ab18976; Abcam)
antibodies.
Gene transduction
The transfer vector pWPXLD-GFP-HA-SUMO1 or
pWPXLD-GFP or overexpressing vector for SUMO1/sentrin-specific
peptidase 1 (pWPXLD-His-SENP1) or pWPXLD-His (Biogot Technology,
Co., Ltd.), the packaging plasmid psPAX2 (Addgene, Inc.), and the
envelope plasmid pMD2.G (Addgene, Inc.) were transfected into 293T
cells at the ratio of 4:3:1 for production of lentiviral particles.
Lipofectamine 2000™ (Thermo Fisher Scientific, Inc.) was used as
the transfection reagent, and the ratio of transfection reagent to
plasmid was 1:2.5. The supernatant was filtered through a
0.45-µm filter and was concentrated by passing through an
ultrafiltration tube (EMD Millipore). The concentrated virus was
used to infect Hep3B cells with 20-30% confluence (5-105
cells) in a 60-mm dish with 8 mg/ml polybrene. After 48 h, western
blotting was used to verify the gene transduction efficiency. The
extent of cellular phenotypic changes was detected by the methods
mentioned above.
Xenograft tumor assay
All animal experiments in the present study met the
ethical requirements of the Animal Ethics Committee of Tianjin
Fifth Central Hospital (Tianjin, China). The mice were kept at the
Experimental Animal Center of The Fifth Central Hospital of Tianjin
that conforms to international certification standards. A total of
50 BALB/c female athymic mice (aged 6 weeks and weighing 14-16 g)
were injected subcutaneously with 5×106 HSVtk/Hep3B stem
cells. When the subcutaneous tumor volume approached 100
mm3, the mice were randomly assigned to five
experimental groups (n=10) that received vehicle, SUMO1 plasmid (10
mg/kg), Dex (10 mg/kg), or combined SUMOl plasmid and Dex, while
GCV (15 mg/kg) was injected intraperitoneally every other day up to
28 days. The longest and shortest diameters of the tumor were
measured every 3 days using vernier calipers, and the tumor volume
was calculated using the formula: V = L × W2 × 0.5 (L, length; W,
width). On the 28th day, the mice were euthanized with
CO2 gas in sealed chambers at a flow rate of 25%
volume/min. The tumors were resected and apoptosis was detected
using an in situ cell death kit (Roche Diagnostics), according to
the manufacturer's protocol.
Statistical analysis
All experiments were repeated at least three times.
The data were analyzed using GraphPad Prism 6 (GraphPad Software,
Inc.). Measurement data are presented as means ± SD. Comparisons
between two groups were analyzed using Student's t-test.
Differences among multiple groups was analyzed by one-way ANOVA
followed by Tukey's post hoc test. P<0.05 was considered to
indicate statistically significant differences.
Results
Successful isolation and identification
of HCC stem cells
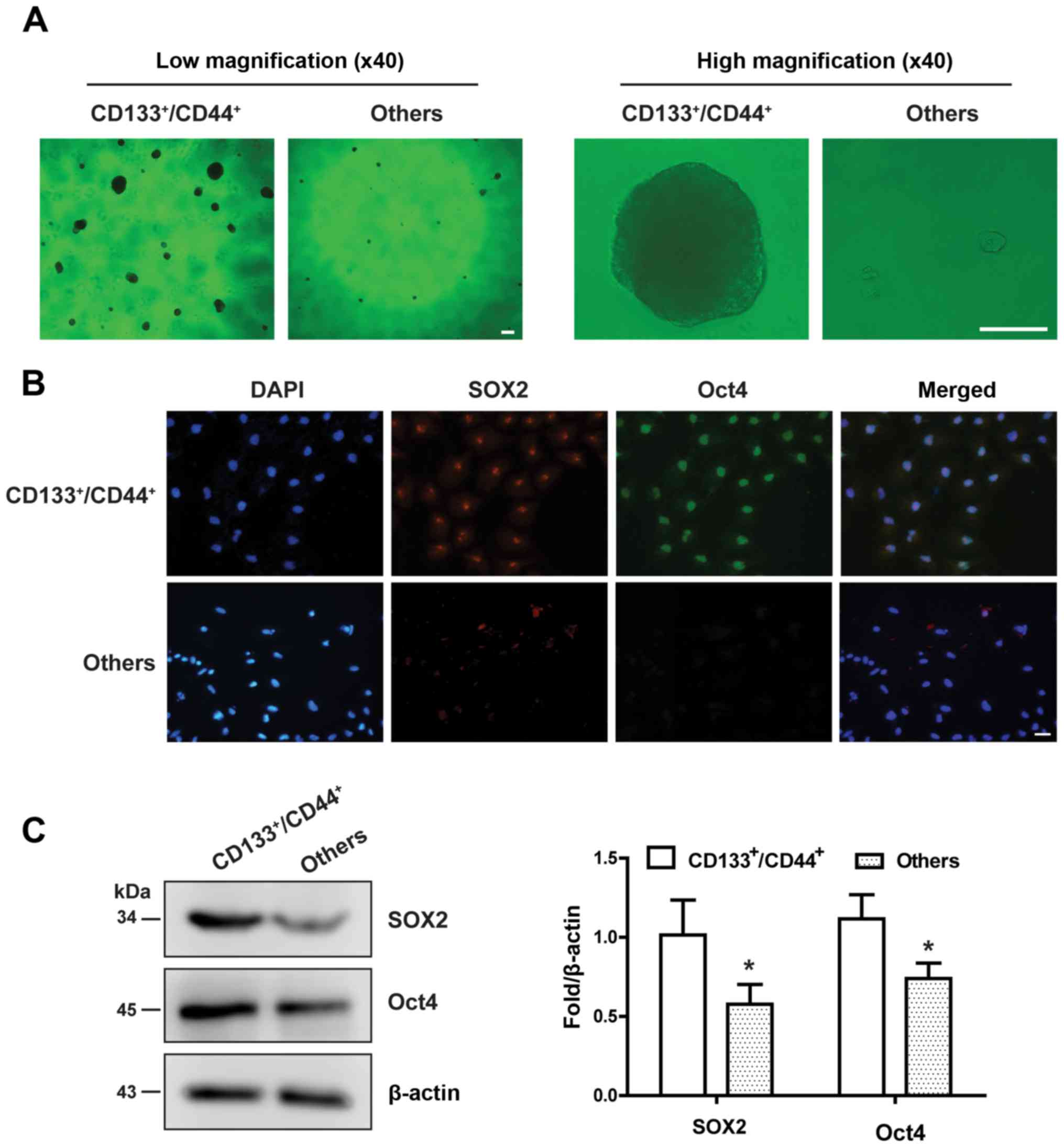
CD133+/CD44+ cells sorted by
flow cytometry were capable of forming a large number of suspended
cloned spheres. Conversely, other cells (including
CD133−/CD44−,
CD133+/CD44− and
CD133−/CD44+ cells) were almost unable to
form cloned spheres (Fig. 1A).
Immunofluorescence and western blot analysis demonstrated that the
CD133+/CD44+ cells expressed both SOX2 and
Oct4, whereas the other cells hardly expressed the two stem cell
markers (Fig. 1B and C). These
results confirmed that the collected
CD133+/CD44+ cells were HCC stem cells.
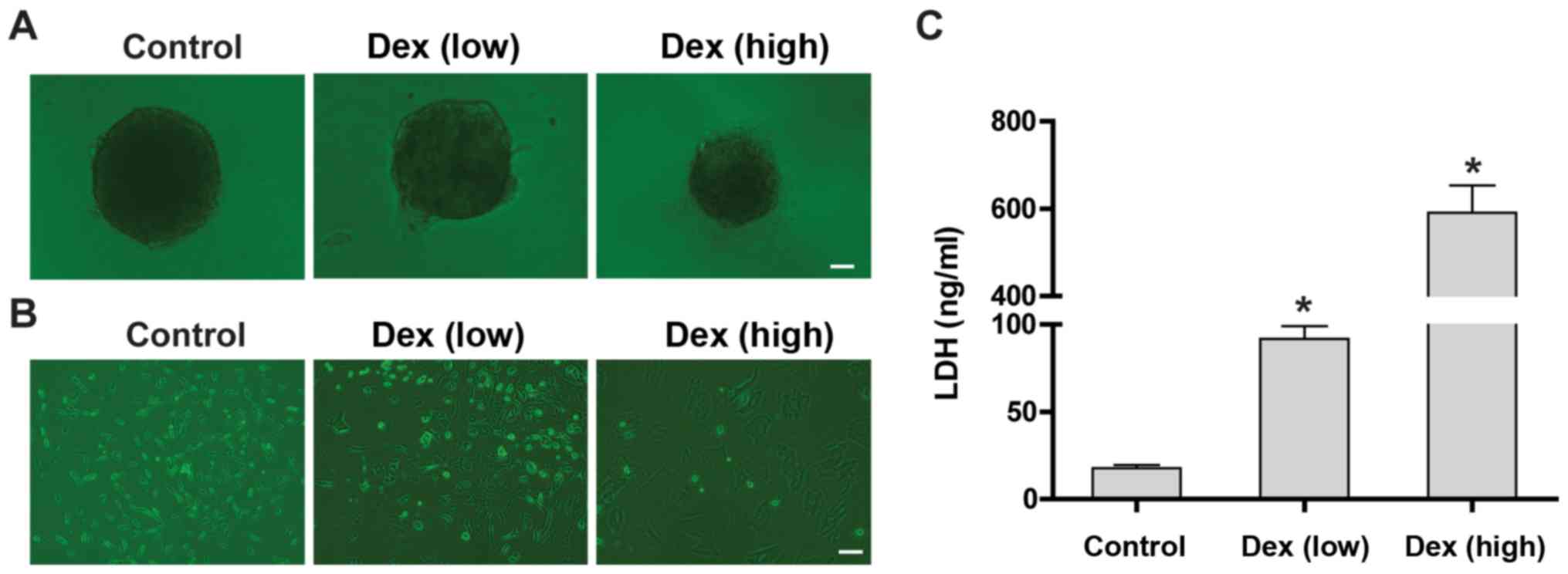
High-dose Dex damages mesenchymal stem
cells and produces strong side effects
High doses of Dex are often used with caution due to
the severe clinical side effects (21). According to the shape of the HCC
stem cell cloned sphere, although low doses of Dex increased the
adhesion of the cloned spheres to the bottom of the culture dish,
they did not appear to significantly induce differentiation or
aging of these cells. However, high doses of Dex significantly
increased adhesion and aging of HCC stem cells (Fig. 2A). Next, the toxicity of various
doses of Dex against BMSCs was examined. The results demonstrated
that low-dose Dex changed the morphology of BMSCs, mainly from
spindled to polygonal, whereas high-dose Dex caused significant
aging (Fig. 2B). The results of
cytotoxicity assays demonstrated that low doses of Dex slightly
increased the LDH content in the culture supernatant of BMSCs,
whereas high doses of Dex significantly increased the LDH levels
(Fig. 2C).
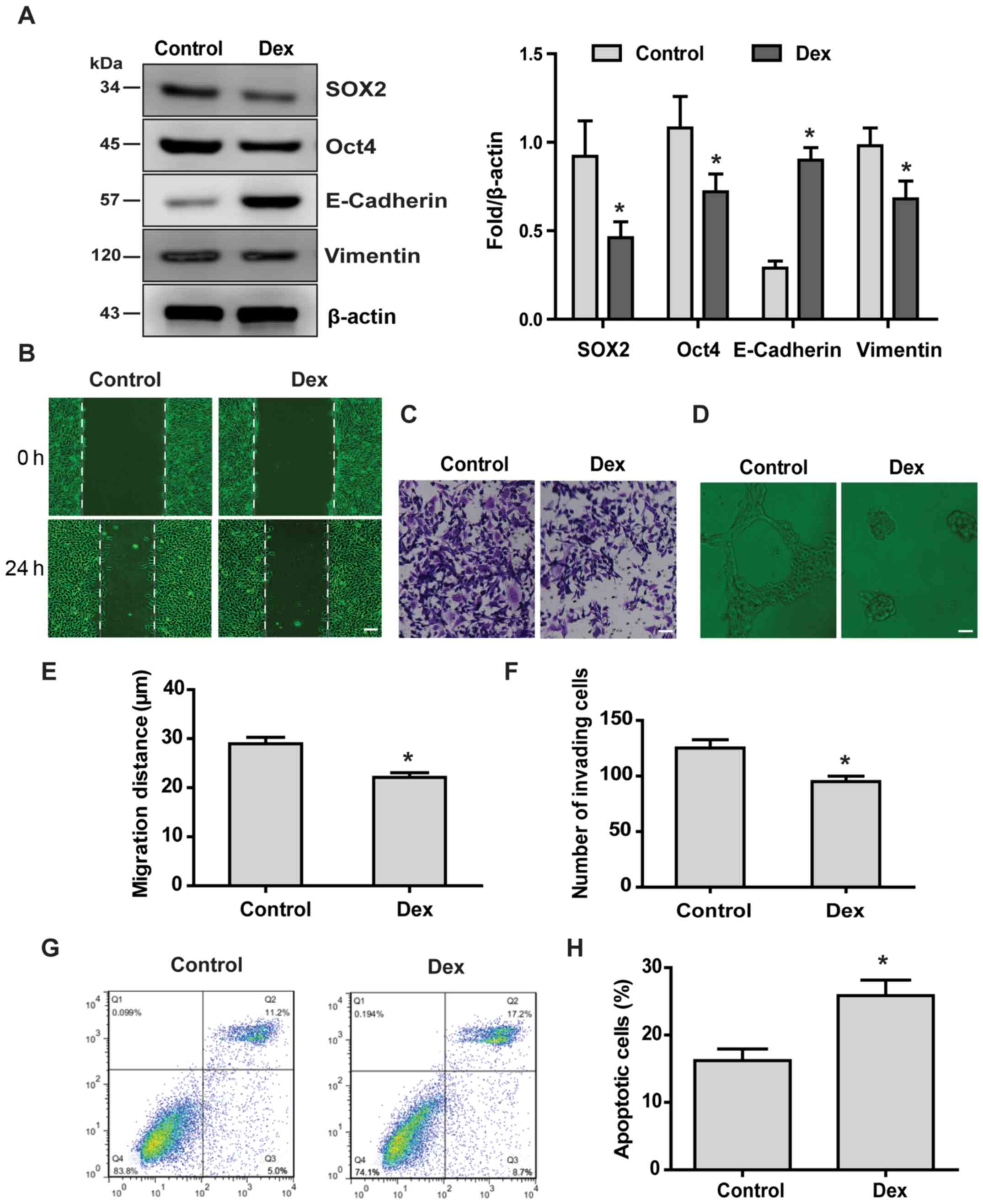
Low doses of Dex suppress the malignant
behavior of HCC stem cells and increase chemosensitivity
SOX2 and Oct4 are involved in stemness maintenance
of HCC stem cells (22). The
results of the present study demonstrated that Dex reduced the
protein levels of SOX2 and Oct4, suggesting that low-dose Dex
reduced the stemness maintenance potential of HCC stem cells
(Fig. 3A). Elevated vimentin
protein levels and decreased E-cadherin protein levels are
important markers of epithelial-to-mesenchymal transition (EMT)
(23). Our results demonstrated
that Dex reduced vimentin levels, while it increased E-cadherin
levels, suggesting that low-dose Dex inhibited EMT of HCC stem
cells (Fig. 3A). Next, the effect
of low-dose Dex on the malignant behavior of HCC stem cells was
examined. Low-dose Dex suppressed the malignant behavior of HCC
stem cells, including self-repair (Fig. 3B and E), invasion (Fig. 3C and F), neovascularization
(Fig. 3D) ability, and increased
the sensitivity of HCC stem cells to HSVtk/GCV treatment (Fig. 3G and H).
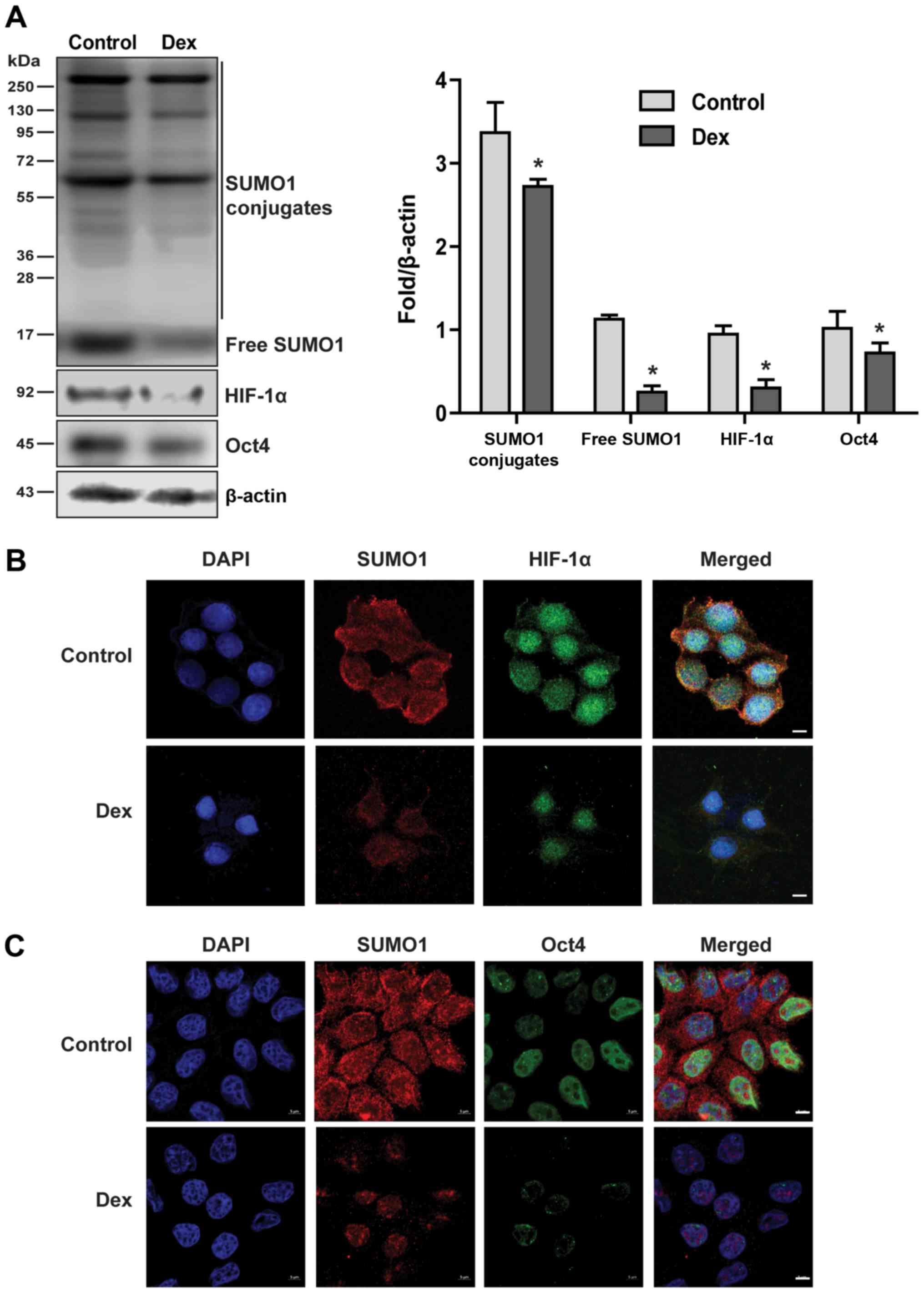
Low doses of Dex reduce SUMOylation of
target proteins and inhibit nuclear translocation of HIF-1α and
Oct4
SUMO modification usually acts by stabilizing the
target protein structure and inhibiting degradation and the shift
from the cytoplasm to the nucleus by antagonizing ubiquitination
modification (24-26). In the present study, how Dex
affects the SUMOylation of target proteins was investigated. The
results demonstrated that low doses of Dex significantly reduced
the covalent modification of target proteins (Fig. 4A). HIF-1α and Oct4 are two
confirmed SUMO1 target proteins (27,28).
Therefore, the effects of Dex on HIF-1α and Oct4 protein expression
were examined. The results demonstrated that low-dose Dex
significantly reduced the expression of HIF-1α and Oct4 proteins
(Fig. 4A), while it inhibited
their translocation from the cytoplasm to the nucleus (Fig. 4B and C).
Overexpression of SUMO1 slightly affects
the malignant phenotype of HCC stem cells
The effects of SUMO1 overexpression on the malignant
phenotype of HCC stem cells were next examined. The results
demonstrated that overexpression of SUMO1 only slightly affected
the malignant phenotype of HCC stem cells, including stemness
maintenance and EMT (Fig. 5A and
B), wound healing (Fig. 5C and
G), migration (Fig. 5D and H)
and angiogenesis (Fig. 5E). In
addition, overexpression of SUMO1 did not significantly affect the
sensitivity of HCC stem cells to HSVtk/GCV (Fig. 5F and I).
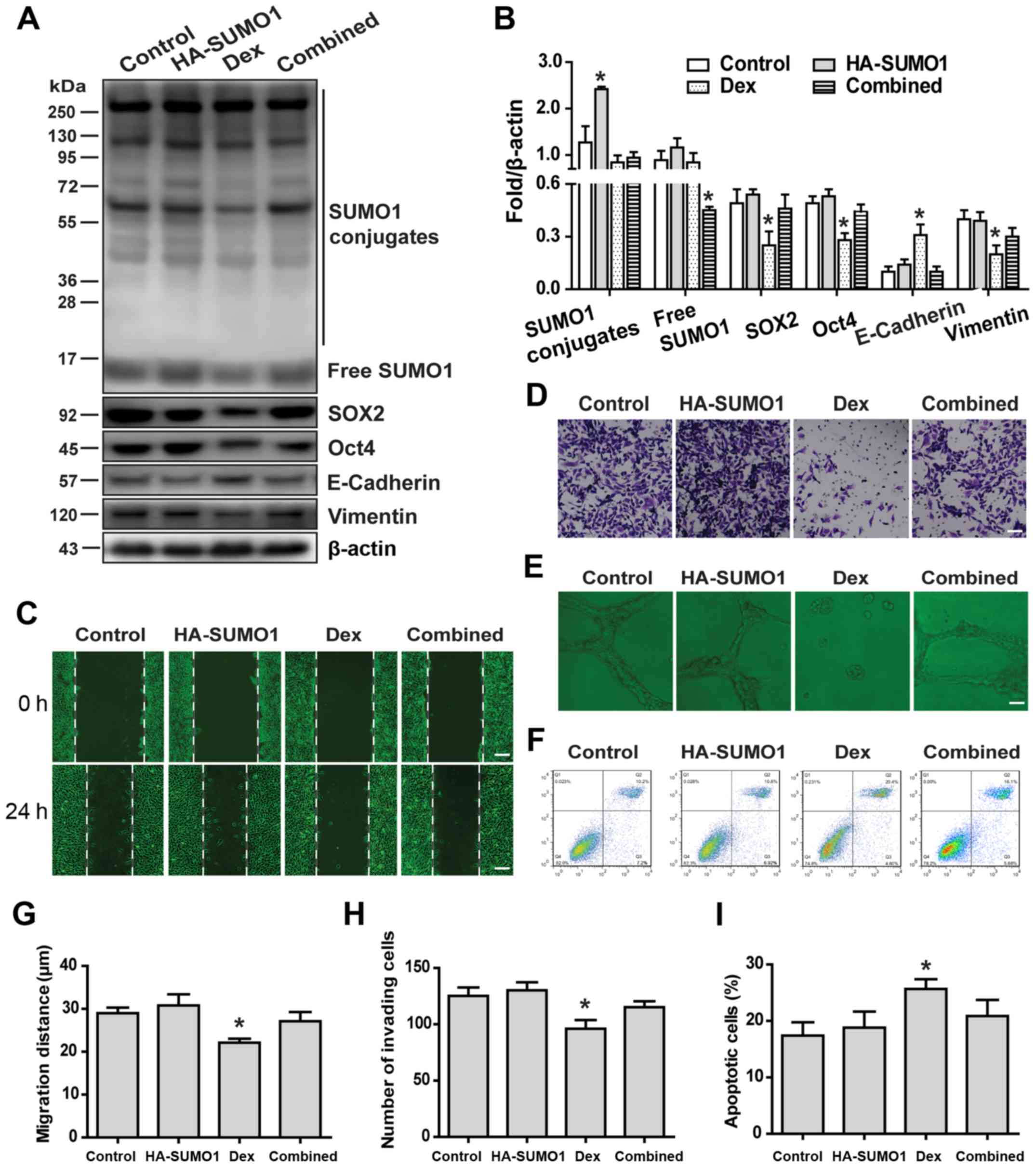 | Figure 5Overexpression of SUMO1 slightly
affects the malignant phenotype of HCC stem cells. (A and B) The
effects of SUMO1 overexpression and Dex or their combination on
stemness maintenance and EMT-related protein expression of HCC stem
cells were examined by western blotting. The malignant phenotypes
caused by SUMO1 overexpression and Dex or their combination were
examined by (C and G) wound healing (scale bar, 50 µm), (D
and H) invasion (scale bar, 50 µm), (E) angiogenesis (scale
bar, 20 µm), and (F and I) apoptosis assays. Data are shown
as means ± SD (n=3). *P<0.05 compared with the
control group (Student's t-test). SUMO, small ubiquitin-like
modifier; HCC, hepatocellular carcinoma; EMT,
epithelial-to-mesenchymal transition; Dex, dexamethasone. |
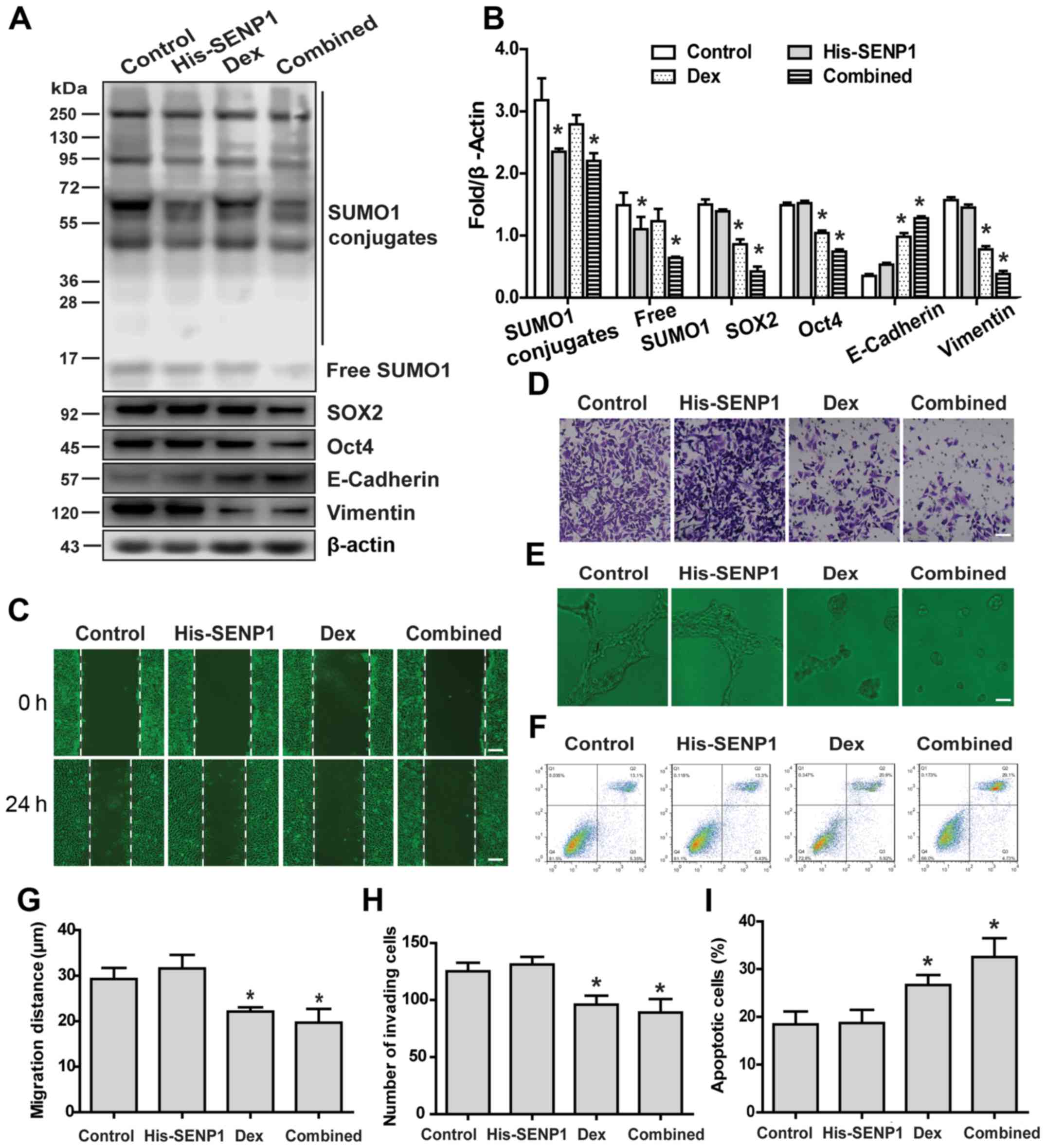
Overexpression of SENP1 partially
antagonizes the effect of Dex on the malignant phenotype of HCC
stem cells
Next, the effects of SENP1 overexpression on
malignant phenotypic changes of HCC stem cells induced by Dex were
examined. Compared with normal HCC stem cells, HCC stem cells
overexpressing SENP1 exhibited a stronger stemness maintenance
ability and partially inhibited EMT (Fig. 6A and B), enhanced wound healing
(Fig. 6C and G), increased
migration (Fig. 6D and H) and
angiogenesis (Fig. 6E) under Dex
treatment. More importantly, overexpression of SENP1 decreased the
sensitivity of HCC stem cells to HSVtk/GCV when combined with Dex
treatment (Fig. 6F and I).
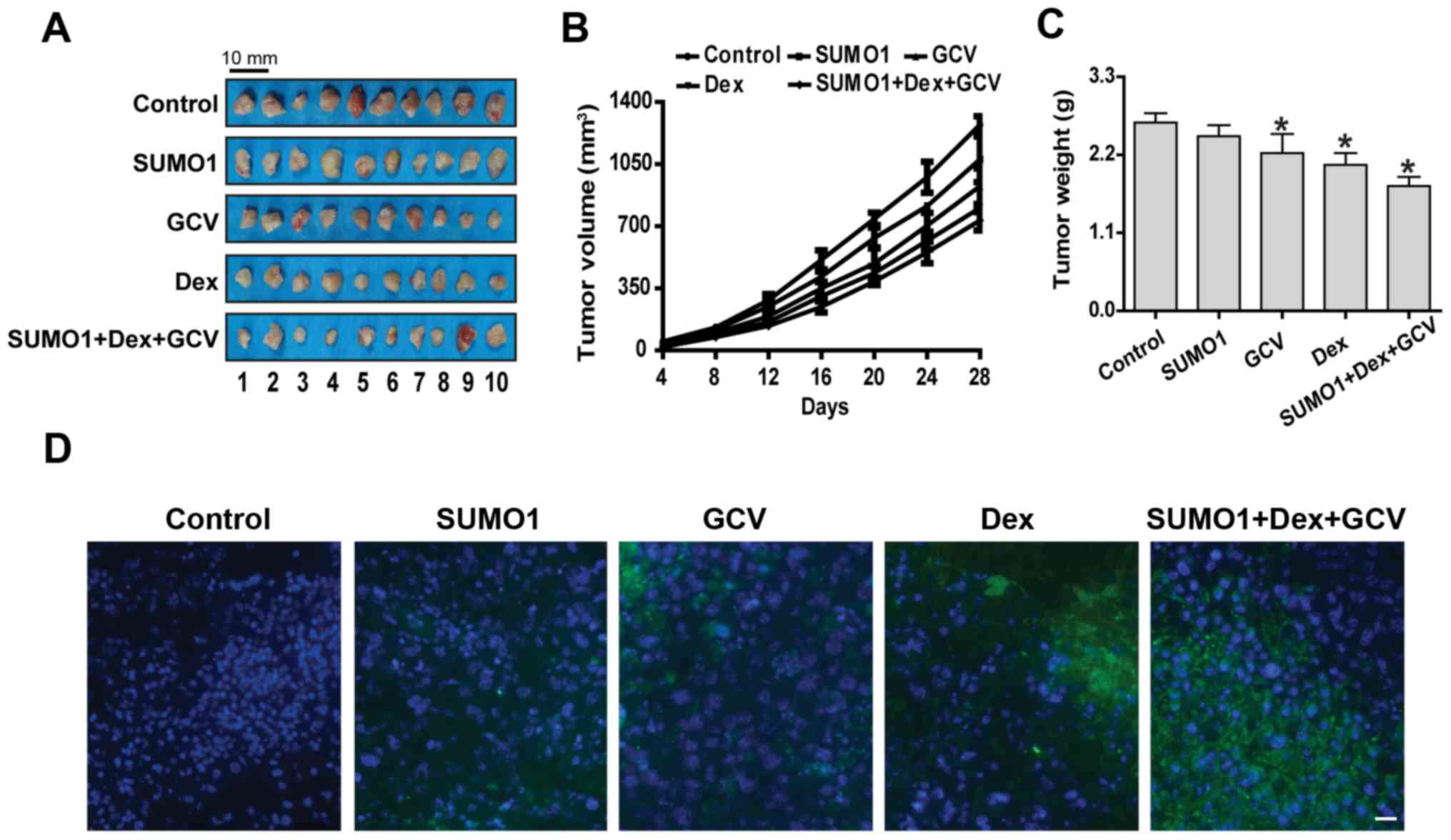
Overexpression of SUMO1 decreases the
sensitivity of HCC stem cells to HSVtk/GCV when combined with Dex
in vivo
Finally, the effects of SUMO1 overexpression
combined with Dex on the sensitivity of HCC stem cells to HSVtk/GCV
were observed in a tumor-bearing mouse model. Although
administration of HSVtk/GCV or Dex alone inhibited the growth of
subcutaneous HCC tumors and caused tumor cell apoptosis, the tumor
continued to grow until the tumor-bearing mice were sacrificed
(Fig. 7A-D). However, upon
combined application of SUMO1 and Dex, the sensitivity of HCC stem
cells to HSVtk/GCV was reduced, which was characterized by
accelerated tumor growth and lower rate of tumor cell apoptosis
(Fig. 7A-D).
Discussion
HCC is the main histological subtype of liver
cancer, accounting for 90% of primary liver cancers (1). In recent years, the incidence rate of
HCC in China has gradually increased, which has been attracting
increasing attention (29). Due to
the structural features of the rich liver blood transport and the
powerful compensatory function, liver cancer grows rapidly and is
prone to metastasis. Therefore, the clinical diagnosis mostly
occurs in the mid- and late stages of the disease, when the
opportunity for surgical resection has been missed and the
prognosis is poor (30). Surgical
resection and liver transplantation are the main methods for
treating liver cancer, but intrahepatic metastasis and
postoperative recurrence adversely affect the prognosis (31,32).
Liver transplantation is often abandoned due to the shortage of
liver donors. Therefore, it is crucial to explore new methods for
treating liver cancer based on traditional surgery, chemotherapy,
radiotherapy, as well as other treatment strategies.
HSVtk/GCV systems have been highly anticipated for
the treatment of multiple solid tumors, including breast cancer,
gastric cancer, brain tumors and HCC (33-35).
Theoretically, HSVtk catalyzes GCV phosphorylation, and then
phosphorylated GCV binds to DNA polymerase, interrupting the DNA
replication process, hindering cell division and, therefore killing
tumor cells. However, the discovery of cancer stem cells has
uncovered a weakness of the HSVtk/GCV system, as it cannot kill
cells in the G0 phase. Improving the therapeutic
sensitivity of HSVtk/GCV in cancer stem cells has come to represent
a new challenge.
Dex has been widely used in clinical practice for
several years. In recent years, it has been reported that this drug
affects glucose metabolism in HCC cells and inhibits tumor growth
(36-38). However, whether Dex can be combined
with other chemotherapeutic drugs to treat tumors has been a
subject of debate among clinicians. Therefore, Dex was combined
with HSVtk/GCV to assess its therapeutic effect on HCC stem cells
in the present study.
The survival of tumor stem cells under hypoxic
conditions is dependent on stable expression of the HIF-1α protein,
and their stemness maintenance is dependent on stable Oct4
expression in the nucleus (39,40).
The stable presence of HIF-1α and Oct4 in the nucleus is known to
depend on a dynamic equilibrium state mediated by ubiquitin and
SUMO modification (41).
Typically, ubiquitin modification mediates degradation of target
proteins, whereas SUMO modification antagonizes this process
(42). However, whether Dex can
change the stable forms of HIF-1α and Oct4 proteins in cells by
affecting this dynamic balance remains unknown.
In the present study, HCC stem cells and BMSCs were
first treated with low- and high-dose Dex to assess the effects.
Although high-dose Dex induced aging of HCC stem cells, it also
induced severe aging of BMSCs, suggesting that high-dose Dex causes
abnormalities in mesenchymal stem cells, leading to serious side
effects. Therefore, subsequent experiments used low-dose Dex to
treat HCC stem cells to avoid adverse reactions. Next, the effect
of low-dose Dex on the malignant phenotype of HCC stem cells were
observed. The results demonstrated that low-dose Dex inhibited the
malignant phenotype of HCC stem cells, particularly by increasing
their sensitivity to HSVtk/GCV. We then examined the effect of Dex
on the expression of HIF-1α and Oct4 proteins. The results revealed
that low-dose Dex reduced the degree of SUMOylation of both HIF-1α
and Oct4, increased their degradation, and inhibited their
translocation from the cytoplasm to the nucleus, thereby reducing
their accumulation and stable presence in the nucleus and
ultimately reducing hypoxia tolerance and stemness maintenance
potential.
To investigate the effect of low-dose Dex on the
sensitivity of HCC stem cells to HSVtk/GCV, gene transduction was
used to obtain HCC stem cells overexpressing SUMO1. First, it was
observed that overexpression of SUMO1 only mildly affected the
malignant phenotype of HCC stem cells, but increased their
therapeutic sensitivity to HSVtk/GCV in vitro and in
vivo. More importantly, overexpression of SUMO1 partially
antagonized the effect of Dex on the malignant phenotype of HCC
stem cells and further increased their sensitivity to HSVtk/GCV.
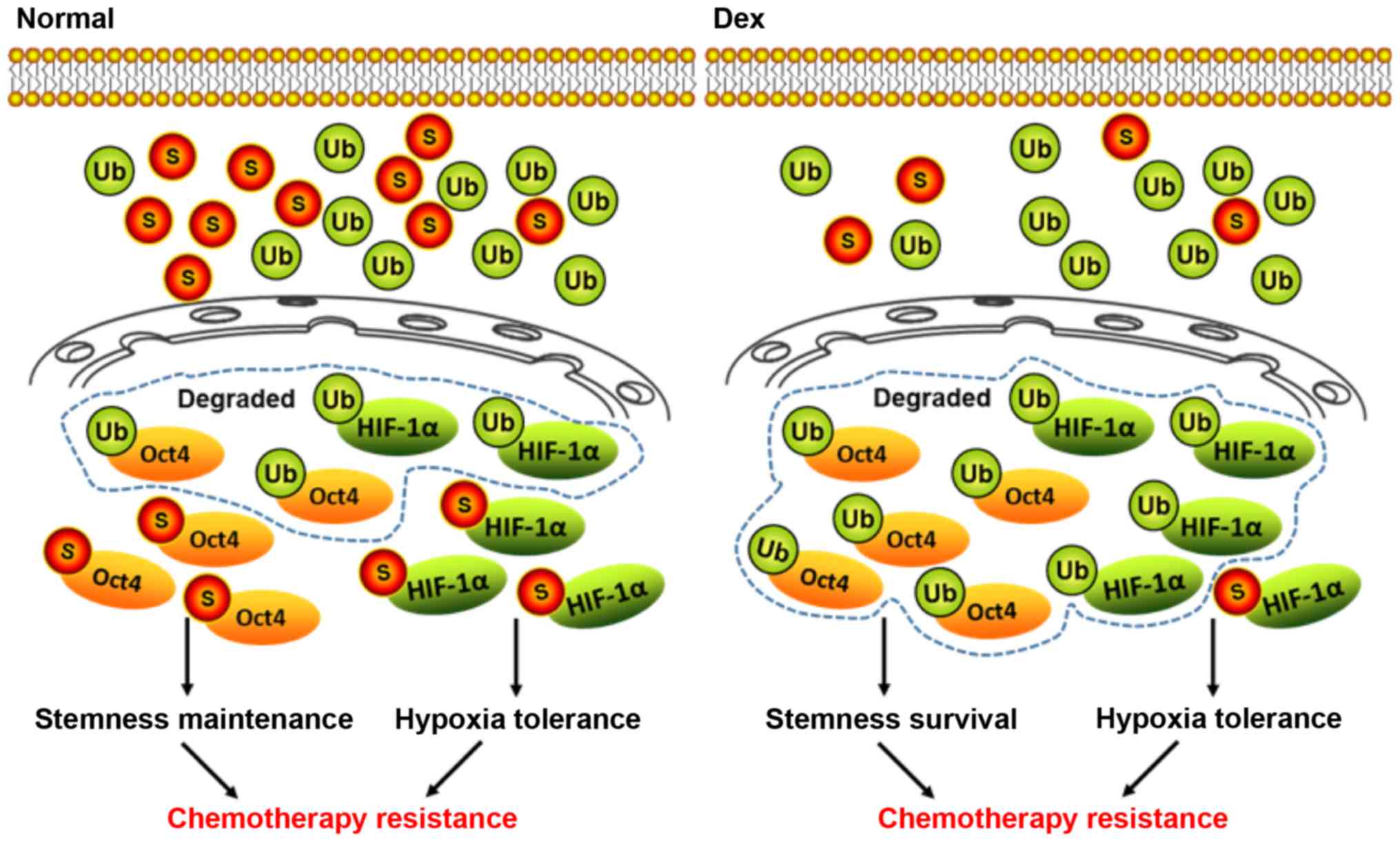
Therefore, Dex inhibited stemness maintenance and enhanced
chemosensitivity of HCC stem cells by inducing deSUMOylation of
HIF-1α and Oct4 (Fig. 8).
These results may enable a better understanding of
the mechanism through which Dex enhances the therapeutic
sensitivity of HCC stem cells to HSVtk/GCV from the perspective of
protein ubiquitination and SUMO modification equilibrium. However,
Dex increases the sensitivity to chemotherapy drugs, while
partially restoring the malignant phenotype of HCC stem cells. In
conclusion, the combined application of Dex and other
chemotherapeutic drugs requires further extensive research in order
to improve its strengths and limit or avoid adverse effects.
Acknowledgments
Not applicable.
Funding
The present study was supported by the National Nat
u ra l Science Fou nd at ion of Ch i na (g ra nt nos. 81901526 and
81900407), the Tianjin Natural Science Foundation of China (grant
nos. 18JCQNJC12800, 19JCZDJC35200, 19JCQNJC12100 and
19JCQNJC11900), the Tianjin Special Project of New Generation
Artificial Intelligence Technology (grant no. 18ZXZNSY00260) and
the Binhai Health and Family Planning Commission Science and
Technology Projects (grant nos. 2019BWKQ030 and 2019BWKQ029).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
WHW and PZ designed the experiments. ZMJ, CYZ, XFM,
RG and YJS performed the experiments and collected data. ZMJ, CYZ,
XZL, XYB and XXX analyzed and interpreted the data. ZMJ, CYZ and
XZL drafted the manuscript. ZMJ, WHW and PZ revised the paper
critically for important intellectual content. WHW and PZ agree to
be accountable for all aspects of the work in ensuring that
questions related to the accuracy or integrity of any part of the
work are appropriately investigated and resolved. All the authors
have read and approved the final version of the manuscript for
publication.
Ethics approval
The animal experimental protocols were reviewed and
approved by the Ethics Committee of the Fifth Central Hospital of
Tianjin.
Patient consent for publication
Not applicable.
Competing interests
All the authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
El-Karaksy HM, Mogahed E, El-Sayed R,
El-Raziky M, Sheba M, Besheer M, Elkiki H and Ghita H: Focal
hepatic lesions in Egyptian infants and children: The pediatric
hepatologist perspective. Minerva Pediatr. 70:35–45. 2018.
|
|
3
|
Hartke J, Johnson M and Ghabril M: The
diagnosis and treatment of hepatocellular carcinoma. Semin Diagn
Pathol. 34:153–159. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li Y, Ruan DY, Jia CC, Zhao H, Wang GY,
Yang Y and Jiang N: Surgical resection versus liver transplantation
for hepatocellular carcinoma within the Hangzhou criteria: A
preoperative nomogram-guided treatment strategy. Hepatobiliary
Pancreat Dis Int. 16:480–486. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gnutzmann D, Kortes N, Sumkauskaite M,
Schmitz A, Weiss KH and Radeleff B: Transvascular therapy of
hepatocellular carcinoma (HCC), status and developments. Minim
Invasive Ther Allied Technol. 27:69–80. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
da Motta Girardi D, Correa TS, Crosara
Teixeira M and Dos Santos Fernandes G: Hepatocellular carcinoma:
Review of targeted and immune therapies. J Gastrointest Cancer.
49:227–236. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lui WY, P'eng FK, Chang YF, Chang TJ, Tsai
TF, Hsu ML, Su TS, Tsay SH, Wu CW, Liu TY, et al: Analysis of
glucocorticoid receptors in human hepatocellular carcinoma and
HepG2 cells. Hepatology. 18:1167–1174. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ray K: Hepatocellular carcinoma: restoring
gluconeogenesis: steroids could treat liver cancer. Nat Rev
Gastroenterol Hepatol. 10:6932013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Woods CP, Hazlehurst JM and Tomlinson JW:
Glucocorticoids and non-alcoholic fatty liver disease. J Steroid
Biochem Mol Biol. 154:94–103. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee DH, Cheul Oh S, Giles AJ, Jung J,
Gilbert MR and Park DM: Cardiac glycosides suppress the maintenance
of stemness and malignancy via inhibiting HIF-1α in human glioma
stem cells. Oncotarget. 8:40233–40245. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xiang L, Gilkes DM, Chaturvedi P, Luo W,
Hu H, Takano N, Liang H and Semenza GL: Ganetespib blocks HIF-1
activity and inhibits tumor growth, vascularization, stem cell
maintenance, invasion, and metastasis in orthotopic mouse models of
triple-negative breast cancer. J Mol Med (Berl). 92:151–164. 2014.
View Article : Google Scholar
|
|
12
|
Yoon C, Chang KK, Lee JH, Tap WD, Hart CP,
Simon MC and Yoon SS: Multimodal targeting of tumor vasculature and
cancer stem like cells in sarcomas with VEGF A inhibition, HIF 1α
inhibition, and hypoxia activated chemotherapy. Oncotarget.
7:42844–42858. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cui CP, Wong CC, Kai AK, Ho DW, Lau EY,
Tsui YM, Chan LK, Cheung TT, Chok KS, Chan ACY, et al: SENP1
promotes hypoxia-induced cancer stemness by HIF-1α deSUMOylation
and SENP1/HIF-1α positive feedback loop. Gut. 66:2149–2159. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Han X, Wang XL, Li Q, Dong XX, Zhang JS
and Yan QC: HIF-1α SUMOylation affects the stability and
transcriptional activity of HIF-1α in human lens epithelial cells.
Graefes Arch Clin Exp Ophthalmol. 253:1279–1290. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tahmasebi S, Ghorbani M, Savage P,
Gocevski G and Yang XJ: The SUMO conjugating enzyme Ubc9 is
required for inducing and maintaining stem cell pluripotency. Stem
Cells. 32:1012–1020. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu Y, Guo Z, Wu H, Wang X, Yang L, Shi X,
Du J, Tang B, Li W, Yang L, et al: SUMOylation represses Nanog
expression via modulating transcription factors Oct4 and Sox2. PLoS
One. 7:e396062012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Han ZJ, Feng YH, Gu BH, Li YM and Chen H:
The post-translational modification, SUMOylation, and cancer
(Review). Int J Oncol. 52:1081–1094. 2018.Review. PubMed/NCBI
|
|
18
|
Gupta KJ, Kolbert Z, Durner J, Lindermayr
C, Corpas FJ, Brouquisse R, Barroso JB, Umbreen S, Palma JM,
Hancock JT, et al: Regulating the regulator: Nitric oxide control
of post-translational modifications. New Phytol. Apr 27–2020.Epub
ahead of print. View Article : Google Scholar
|
|
19
|
Li R, Wei J, Jiang C, Liu D, Deng L, Zhang
K and Wang P: Akt SUMOylation regulates cell proliferation and
tumorigenesis. Cancer Res. 73:5742–5753. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ni P, Xu H, Chen C, Wang J, Liu X, Hu Y,
Fan Q, Hou Z and Lu Y: Serum starvation induces DRAM expression in
liver cancer cells via histone modifications within its promoter
locus. PLoS One. 7:e505022012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Polderman JA, Farhang-Razi V, Van Dieren
S, Kranke P, DeVries JH, Hollmann MW, Preckel B and Hermanides J:
Adverse side effects of dexamethasone in surgical patients.
Cochrane Database Syst Rev. 8:CD0119402018.PubMed/NCBI
|
|
22
|
Tang H, Jin Y, Jin S, Tan Z, Peng Z and
Kuang Y: Arsenite inhibits the function of CD133+
CD13+ liver cancer stem cells by reducing PML and Oct4
protein expression. Tumour Biol. 37:14103–14115. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Luo T, Wang L, Wu P, Gong W, Chen W, Zhao
H and Zheng Z: Downregulated vimentin and upregulated E-cadherin in
T1 stage non-small-cell lung cancer: Does it suggest a
mesenchymal-epithelial transition? Neoplasma. 64:693–699. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zilio N, Eifler-Olivi K and Ulrich HD:
Functions of SUMO in the Maintenance of Genome Stability. Adv Exp
Med Biol. 963:51–87. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao X: SUMO-mediated regulation of
nuclear functions and signaling processes. Mol Cell. 71:409–418.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pichler A, Fatouros C, Lee H and
Eisenhardt N: SUMO conjugation - a mechanistic view. Biomol
Concepts. 8:13–36. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang L, Zhang T, Fang M, Shen N, Wang D,
Teng J, Fu B, Xie H, Hong Q and Lin H: Podocytes protect glomerular
endothelial cells from hypoxic injury via deSUMOylation of HIF-1α
signaling. Int J Biochem Cell Biol. 58:17–27. 2015. View Article : Google Scholar
|
|
28
|
Gupta P, Ho PC, Huq MM, Ha SG, Park SW,
Khan AA, Tsai NP and Wei LN: Retinoic acid-stimulated sequential
phosphorylation, PML recruitment, and SUMOylation of nuclear
receptor TR2 to suppress Oct4 expression. Proc Natl Acad Sci USA.
105:11424–11429. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Parikh ND, Fu S, Rao H, Yang M, Li Y,
Powell C, Wu E, Lin A, Xing B, Wei L, et al: Risk assessment of
hepatocellular carcinoma in patients with hepatitis C in China and
the USA. Dig Dis Sci. 62:3243–3253. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mak LY, Cruz-Ramón V, Chinchilla-López P,
Torres HA, LoConte NK, Rice JP, Foxhall LE, Sturgis EM, Merrill JK,
Bailey HH, et al: Global epidemiology, prevention, and management
of hepatocellular carcinoma. Am Soc Clin Oncol Educ Book.
38:262–279. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pinna AD, Yang T, Mazzaferro V, De Carlis
L, Zhou J, Roayaie S, Shen F, Sposito C, Cescon M, Di Sandro S, et
al: Liver transplantation and hepatic resection can achieve cure
for hepatocellular carcinoma. Ann Surg. 268:868–875. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Akoad ME and Pomfret EA: Surgical
resection and liver transplantation for hepatocellular carcinoma.
Clin Liver Dis. 19:381–399. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fillat C, Carrió M, Cascante A and Sangro
B: Suicide gene therapy mediated by the Herpes Simplex virus
thymidine kinase gene/Ganciclovir system: Fifteen years of
application. Curr Gene Ther. 3:13–26. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang JH, Wan MX, Pan BR and Yu B:
Cytotoxicity of HSVtk and hrTNF-alpha fusion genes with IRES in
treatment of gastric cancer. Cancer Biol Ther. 3:1075–1080. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Parada C, Hernández Losa J, Guinea J,
Sánchez-Arévalo V, Fernández Soria V, Alvarez-Vallina L,
Sánchez-Prieto R and Ramón y Cajal S: Adenovirus E1a protein
enhances the cytotoxic effects of the herpes thymidine
kinase-ganciclovir system. Cancer Gene Ther. 10:152–160. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shang F, Liu M, Li B, Zhang X, Sheng Y,
Liu S, Han J, Li H and Xiu R: The anti-angiogenic effect of
dexamethasone in a murine hepatocellular carcinoma model by
augmentation of gluconeogenesis pathway in malignant cells. Cancer
Chemother Pharmacol. 77:1087–1096. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ogasawara S, Chiba T, Ooka Y, Kanogawa N,
Motoyama T, Suzuki E, Tawada A, Nagai K, Nakagawa T, Sugawara T, et
al: A randomized placebo-controlled trial of prophylactic
dexa-methasone for transcatheter arterial chemoembolization.
Hepatology. 67:575–585. 2018. View Article : Google Scholar
|
|
38
|
Yang H, Seon J, Sung PS, Oh JS, Lee HL,
Jang B, Chun HJ, Jang JW, Bae SH, Choi JY and Yoon SK:
Dexamethasone Prophylaxis to Alleviate Postembolization Syndrome
after Transarterial Chemoembolization for Hepatocellular Carcinoma:
A Randomized, Double Blinded, Placebo Controlled Study. J Vasc
Interv Radiol. 28:1503–1511.e2. 2017. View Article : Google Scholar
|
|
39
|
Tang YA, Chen YF, Bao Y, Mahara S, Yatim
SMJM, Oguz G, Lee PL, Feng M, Cai Y, Tan EY, et al: Hypoxic tumor
microenvironment activates GLI2 via HIF-1α and TGF-β2 to promote
chemoresistance in colorectal cancer. Proc Natl Acad Sci USA.
115:E5990–E5999. 2018. View Article : Google Scholar
|
|
40
|
Covello KL, Kehler J, Yu H, Gordan JD,
Arsham AM, Hu CJ, Labosky PA, Simon MC and Keith B: HIF-2alpha
regulates Oct-4: Effects of hypoxia on stem cell function,
embryonic development, and tumor growth. Genes Dev. 20:557–570.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yuan L, Jiang ZM, Chen XH, Bian XY, Li YX,
Ma XF and Liu XZ: Hypoxia inducible factor-1α deSUMOylation reduces
the stemness maintenance ability of endometrial cancer stem cell
and increases its chemosensitivity. Zhonghua Yi Xue Za Zhi.
97:3579–3582. 2017.In Chinese. PubMed/NCBI
|
|
42
|
Liebelt F and Vertegaal AC:
Ubiquitin-dependent and independent roles of SUMO in proteostasis.
Am J Physiol Cell Physiol. 311:C284–C296. 2016. View Article : Google Scholar : PubMed/NCBI
|