Cancer drug therapy was developed from chemotherapy
and radiotherapy to molecular targeting therapy combined with a
'guiding missile', for cancer-targeted delivery to avoid healthy
tissue damage (1). For example, in
genome targeted therapy, DNAs and RNAs can interfere with the
normal host genome, and genetic modification is difficult as the
modified genes may mutate the original genome or the off-target
(2). Furthermore, immunotherapy
with antibodies against cancer cell surface antigens can provide
specific delivery, but some healthy cells can express the same
targeted antigens, resulting in limited effectiveness (3). Small molecules can also exert
antitumor effects on cancer cells, such as C188-9, a STAT3
inhibitor, in head and neck squamous cell carcinoma, and GNS561, a
lysosomotropic molecule, in intrahepatic cholangiocarcinoma
(4,5). Moreover, these small molecules can be
used in drug delivery systems (6);
however, they are difficult to synthesize. Therefore, peptides
against cancer cells are an alternative therapeutic method in
anticancer drug development.
ACPs, as small peptides containing amino acid
sequences, are selective and toxic to cancer cells (7). ACPs are a superior choice of
therapeutics compared with antibodies and small molecules due to
their high selectivity, high penetration and easy modifications
(8-10). Ideally, anticancer therapy should
destroy a range of cancer types, but not all healthy cells.
A different property between cancerous and healthy
cells is the cell membrane. Numerous anticancer peptides destroy
cancer cells via apoptosis and necrosis by membrane lysis or pore
formation (11-13). The eukaryotic cell membrane
contains cholesterol to protect lytic action by modifying membrane
fluidity (14). Moreover, a high
level of membrane cholesterol can inhibit lytic activity. It has
been shown that membrane fluidity of cancer cells is higher
compared with healthy cells (15).
Cancer cells also contain more abundant microvilli compared with
healthy cells, which increases the cell surface area (16). Furthermore, healthy cells have
electrical neutrality, whereas cancer cells contain a negatively
charge component on their surface (17), leading to membrane destabilization,
cytotoxicity and cancer cell lysis when interacting with small
molecules, such as ACPs (18,19).
In addition, the primary driving force for the interactions between
peptides and the healthy cell membrane is the hydrophobic
interactions, while that between peptides and the cancer cell
membrane is the electrostatic interactions (20).
Anticancer medicines contain molecularly targeted
drugs with or without 'guiding missiles' to interact with specific
molecular targets on cancer cells (21). Besides molecularly targeted drugs,
drug-delivery to the cancer cell surface was developed using the
most important properties, including high specificity, high
selectivity and the binding capability to various targeted drugs,
as well as being easy to synthesize and produce (21). Peptide properties can be used both
in molecularly targeted drugs and 'guiding missiles' to inhibit
cell proliferation or eradicate cancer cells completely, depending
on the amino acid residue composition, sequence length, isoelectric
point, molecular weight, net charge, hydrophobicity,
amphiphilicity, secondary structure and structural orientation
(22). These ideal anticancer
peptide characteristics are summarized in Fig. 1. Membrane characteristics promote
or inhibit drug penetration, drug conformation and/or location
within the membrane and sequentially affect therapeutic targets
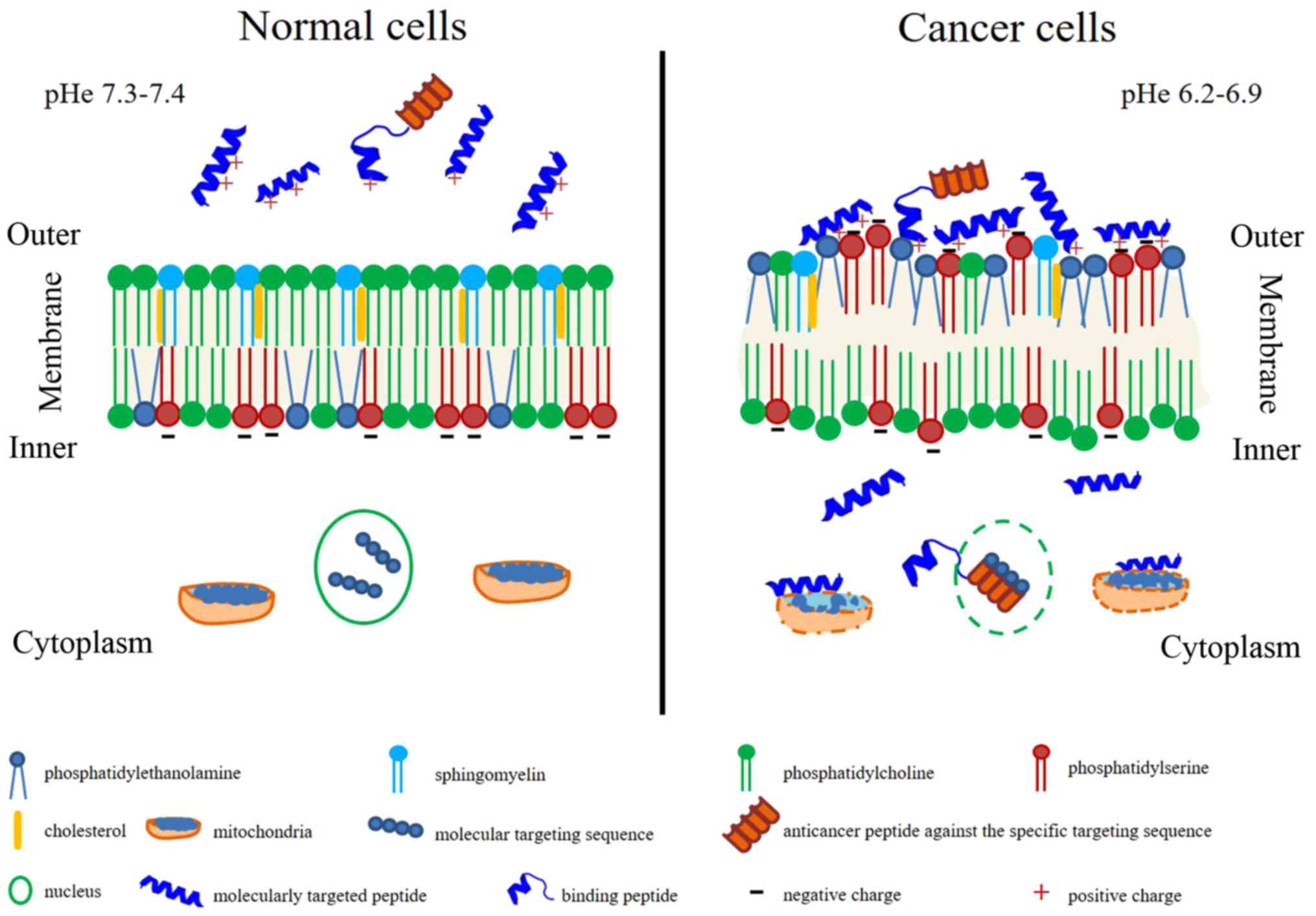
(23). Healthy cell membranes have
zwitterion phosphatidylcholine and sphingomyelin in an outer
leaflet and anionic phosphatidylserine and the
phosphatidylethanonlamine in the inner leaflet with the asymmetric
distribution (24). The inner
leaflet with the asymmetric distribution is primarily maintained by
flippases (phosphatidylserine and phosphatidylethanonlamine from
outer to inner membrane), floppases (phosphatidylcholine and
cholesterol from inner to outer membrane) and scramblases
(facilitated the flip-flop of lipids) (24,25).
In contrast, the cancer cell membrane loses this asymmetric
distribution and alterations in membrane fluidity, resulting in
exposure of negative charge of phosphatidylserine on the surface of
the membrane, as well as the locating of phosphatidylethanonlamine
on the outer leaflet (26-28). Furthermore, sphingomyelin is
decreased in the cancer cell membrane and is associated with
tumorigenesis (29). Different
lipid composition affects membrane fluidity, influencing drug
penetration and biological action (30,31).
Extracellular acidity with or without exosome release affects the
pH, changing from 7.4 to 6.5 (typical pH of cancer), forming the
malignant tumor phenotype (32).
The surrounding environment in the acidic extracellular pH (pHe)
can promote cancer invasiveness (33). Specific interaction between
anticancer peptides and cell membrane components are mostly bound
by electrostatic interactions (34).
Anticancer peptides act as either molecularly
targeted peptides, which can penetrate and directly bind to the
specific cancer cell or organelle membranes, or binding peptides
linking to the anticancer drugs (35-37).
In cancer cells, anticancer peptides, as molecular targeting
peptides, particularly in the α-helical form, penetrate the plasma
membrane, the nuclear membrane and/or the mitochondrial membrane
exerting pharmacological activity via different mechanisms (such as
the inhibition of DNA synthesis or cell division), thus promoting
cancer cell apoptosis (38-41).
However, binding peptides, also referred to as cancer-targeting
peptides or cell-penetrating peptides, that have no anticancer
property, can recognize and penetrate the cancer cell membrane
(42). Binding peptides can also
be used for drug delivery by binding to the anticancer drugs, such
as those that are non-penetrable (43).
Internal prolines in peptides are crucial for
membrane interaction and conformational flexibility, which is the
same as glycine residues (53). It
has been reported that serine and glycine-free diets can slow tumor
growth and enhance antiproliferative effects (54). Methionine, a moderately hydrophobic
amino acid, does not serve a major role in ACPs, but its elevated
levels can be consumed by cancer cells. Furthermore, a
methionine-deficient diet causes a metabolic defect in cancer cells
by arresting cancer cell proliferation (55). Phenylalanine, a strongly
hydrophobic residue, is highly present in primary tumors and acts
as a protective amino acid (56).
Phenylalanine-containing peptides can also enhance the affinity for
targeting the cancer cell membrane (57). Tyrosine and tryptophan are weakly
hydrophobic amino acids; tyrosine does not serve a role in toxicity
of ACPs, whereas tryptophan may exert a role in the toxicity of
some ACPs against cancer cells such as indolicidin and
trans-activator of transportation (TAT)-Ras GTPase-activating
protein-326 peptides (19,58,59).
However, synthesized peptides containing tryptophan and histidine
may decrease cytotoxicity, while those containing tyrosine,
phenylalanine or proline may be able to increase cytotoxic activity
(60). The tryptophan position on
the cell-penetrating peptides serves an important role in entering
cancer cells, which subsequently involves an endocytic pathway and
binding at the major groove of nuclear DNA (61). The role of amino acid residues on
ACPs and on cancer cells is summarized in Table I. Collectively, these findings
suggested ACPs should contain cationic and hydrophobic amino acid
residues to further form secondary structures that affect cancer
cells.
The association between ACPs and SAR has been
investigated and analyzed using machine learning, and it has been
demonstrated that the majority of ACPs contained 21-30 amino acids
and were predominately composed of glycine, lysine and leucine
(47). In addition, amino acid
residues on a peptide influences its anticancer activity depending
on the cationic, hydrophobic and amphiphilic properties associated
with forming helical structure (62-64).
Anticancer activity is primarily determined by the IC50
value associated with cancer cell membrane disruption (62). It has been reported that peptides
with a higher hydrophobicity can penetrate into the hydrophobic
core of the cancer cell membrane, resulting in cancer cell
disruption via necrosis (62).
Several studies have aimed to substitute low hydrophobic and
neutral or acidic amino acid residues with positively charged amino
acid residues, such as lysine and leucine, on the polar and
non-polar faces of α-helical peptides (63,65).
As a result, high cationic peptides with moderate hydrophobicity
can enhance the cytotoxicity of cancer cells (63). Peptides in free-form do not fold in
solution, but arrange in an α-helix or β-sheet via electrostatic
interaction on the membrane surface of the cells (11).
As well as the physicochemical properties, the
secondary structure of the peptides is important in cell surface
interaction, such as peptide structural orientation (57). The orientation of peptides can
enhance the surface-activity for targeted interaction with the
cancer cell membrane (66). The
angle of the interaction leads to destabilized lipid packing on the
cancer cell membrane, thus resulting in membrane penetration
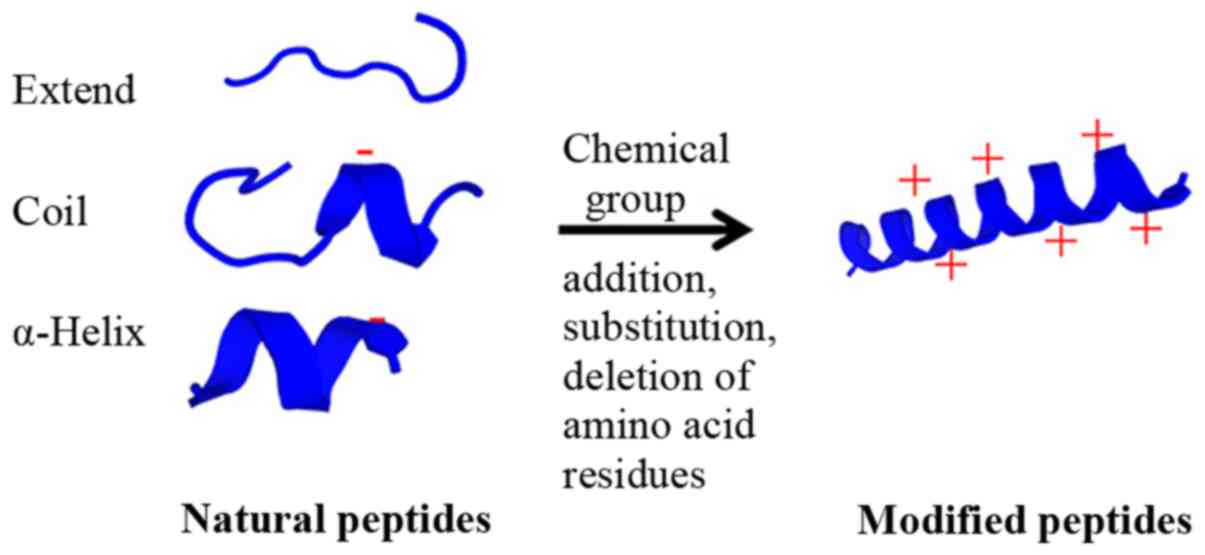
(67). Furthermore, modifying
peptides by adding chemical groups, including methylation,
acetylation or phosphorylation (particularly phosphorylation at
tyrosine), can inhibit STAT3 phosphorylation, leading to cancer
cell death (68). The potential
modification of natural peptides is presented in Fig. 2. Therefore, the results indicated
that the secondary structure of ACPs serves a crucial role in
peptide-cancer cell membrane interaction, leading to cancer cell
disruption and cell death.
Anticancer peptide creation should consider the
peptide structure, mode of action, selectivity and efficacy to
specific cancer cells (69,70).
In the present review, active peptides were classified into three
types depending on their actions, including: i) Molecularly
targeted peptides, which directly act on cancer cells via
cytotoxic, anti-proliferative and apoptotic activities; ii)
'guiding missile' peptides or binding peptides, which are drug
binding peptides used for transporting drugs into the cancer cell
targets; and iii) cell-stimulating peptides that indirectly effect
other stimulating cells to kill cancer cells, such as via
immunomodulatory activities and hormone receptors (71-73).
Molecularly targeted peptides, which are specific to
the cancer cell targets, can penetrate, bind and then inhibit or
kill cancer cells that are in an important stage of carcinogenesis
or proliferation (74). The
peptides concerning target cells can be classified into two major
groups, including: i) Peptides against only cancer cells, and not
against healthy cells (75,76)
and, ii) peptides against both cancerous and healthy cells
(77). Numerous peptides have
selectivity for cancer cells but not healthy cells, such as
peptides derived from defensins, lactoferricin B, cecropins,
magainin-2 and chrysophsin-1 (22).
The majority of ACPs are collected using the
CancerPPD resource for predicting peptide structure and identifying
the best ACP for further study (7). In addition, ACPs are identified via
computational methods that consider amino acid composition, binary
profiles and sequence-based methods (78-80).
Membranolytic ACPs are generated de novo using automated
designs based on α-helical cationic amphipathic peptide sequences
against the cancer cells (81).
Anionic molecules in the malignant cells conferring a net negative
charge are different from the normal mammalian cell membrane, which
have a neutral net charge (17).
High cholesterol contents in healthy cells can obstruct the
cationic peptide entry via cell fluidity; healthy cells are less
fluid compared with cancer cells (15,82).
Furthermore, peptides can permeate into the cells, causing
mitochondrial swelling with cytochrome c release, followed by
apoptosis (83). For example,
Mastoparan I, a peptide with a α-helical structure, can act on the
negative charge of prostate and liver cancer cell surfaces causing
cell injury, cell swelling, cell bursting and then necrosis
(84). Moreover, SVS-1
(KVKVKVKVDPLPTKVKVKVK-NH2), as a
β-sheet structure, disrupts cell membranes via pore formation in
lung-, epidermal- and breast-cancer cells (85,86).
Peptides extracted from marine organisms, such as sponges,
mollusks, tunicates, bryozoans, algae, fish, soft corals and sea
slugs, can act against human cancer cells via, for example,
anti-proliferative, cytotoxicity and anti-tubulin activities, as
well as suppressing microtubule depolymerization (87).
Amino acid composition of the peptides can act
directly against various cancer cell types. For example, highly
cationic peptides can enhance cancer cell specificity, while an
increase in hydrophobic peptides can decrease the degree of
specificity (63). Moreover,
polycationic peptides have selectivity against human acute T-cell
leukemia via a higher membrane potential compared with healthy
cells (88). Lysine and
argi-nine-rich peptides with an intact amphipathic helical
interface can also enhance cell lysis via membrane lysis mechanisms
by penetrating and inducing caspase-3-dependent apoptotic cell
death (89). The methods of
peptide designing, such as cyclization, hybridization,
fragmentation and modification, have potential advantages in
increasing drug half-life time in plasma, enhancing stability and
activity and decreasing toxicity of ACPS, for improving their
therapeutic efficacy (90).
Therapeutic peptides are classified into three
classes based on the mechanism of peptide entry into cancer cells,
including: i) Pore-forming peptides, which bind to negatively
charged molecules on the cancer cell membrane for inducing
apoptosis or necrosis; ii) cell-penetrating peptides, which
translocate across the plasma membrane and transporting small
molecules to oligonucleotides or proteins, known as
internalization; and iii) tumor-targeting peptides, which bind to
receptors on the cancer cell surface for cell internalization
(91). Based on the mechanism of
entry, therapeutic peptides are also classified into three groups
based on their biological targets, including: i) Signal
transduction pathways; ii) cell cycle regulation; and iii) cell
death pathways (92,93). For instance, a tumor-penetrating
peptide, KLA, exerts pro-apoptotic activity, which disrupts the
mitochondrial membrane, leading to programmed cell death in tumors
(40). In a tumor suppressor
mechanism, kisspeptin-1 metastasis suppressor, a precursor for
several shorter peptides, which regularly exhibits decreased
expression in metastatic tumors, can suppress colonization of
disseminated cancer cells in distant organs and is involved in
mechanisms of tumor angiogenesis, autophagy and apoptosis
regulation in breast cancer (94).
Furthermore, the tubulysin analogue KEMTUB10 can inhibit tubulin
polymerization during mammalian cancer cell proliferation, block
the G2/M phase of the cell cycle and stimulate apoptosis
or cell death via p53, Bcl-2-interacting mediator of cell death and
Bcl-2 (95). Although ACPs can
induce cancer cell death and specify an expressed molecule to
cellular targets, such as a cationic anticancer peptide,
temporin-1CEa and melanoma cell surface-expressed
phosphatidylserine (96), ACPs
have limitations, including drug binding peptide delivery to cancer
cell targets (97). Thus, ACPs
could be developed for their high penetration into the tumor tissue
and tumor cells, as well as high antitumor activity (40). While ACPs can progress from binding
to killing cancer cells, in terms of molecular targeting peptides,
ACPs cannot be specific or penetrated all cancer cell types,
leading to the need for an addition of a binding cancer cell
target, such as 'guiding missile' peptides or binding peptides.
Optimizing anticancer drug delivery requires the
safety of healthy cells, as well as cancer cell elimination
(98). 'Guiding missile' peptides
or binding peptides, used as delivery carriers, should hold the
poorly stable, non-soluble drugs and control drug-release inside
the tumor environments (99).
Furthermore, these peptides require specificity, affinity and dose
effectiveness (98). Anticancer
drug concentration is continually diluted during transport until
reaching the target areas. However, drug binding adjuvant and
nanoparticles can retain drug concentration during transport to
target areas and induce the slow-release of the drug at these
target areas (100,101).
Drug concentration and cell and tissue barriers are
an obstacle for therapeutic efficacy. Medical application for drug
delivery requires biologically active conjugates (cargoes) and/or
binding peptides ('guiding missile') to reach specific
intracellular targets (102-104). Minimal amino acid sequences of
various cell-penetrating peptides, typically comprising 5-30 amino
acid residues, especially cationic residues, can pass through
tissue and cell membranes using energy-dependent or -independent
mechanisms without the interaction of specific receptors (36). Binding peptides can bind to the
cargoes with either covalent (mainly disulfide and thioester bonds)
or non-covalent bonds (electrostatic and/or hydrophobic
interactions between negatively charged cargoes and positively
charged peptides) to protect the cargoes from enzymatic degradation
(105).
The physical and chemical properties of binding
peptides can be categorized into three main classes: Cationic,
amphipathic and hydrophobic peptides (42). Firstly, cationic peptides contain
highly positive net charges comprising lysines and arginines.
Arginine contains a guanidine head group, which is used to form
bidentate hydrogen bonds with the negatively charged carboxylic,
sulfate and phosphate groups on the cell membrane, resulting in
binding peptide internalization into the cells; however, lysine
does not contain the guani-dine head group, leading to lower
penetration into the cell membrane (106). Secondly, amphipathic peptides,
which contain both hydrophilic and hydrophobic amino acids, are
classified into primary (covalent binding hydrophobic domain
targeting to cell membrane and nuclear localization signal),
secondary (α-helical structure with hydrophilic and hydrophobic
residues on different sides of the helix or β-sheet for cellular
internalization) and proline-rich (pyrolidine ring without hydrogen
bonds on α-amino group able to allow cell permeability) peptides
(107,108). Hydrophobic peptides contain
non-polar amino acids with a low net charge and have a high
affinity for the hydrophobic domain of the cell membrane, leading
to cellular internalization and translocation across the membrane
via energy-independent mechanisms (107,109).
Binding peptides enter target cells via cell
penetration (pore formation and membrane destabilization) and
endocytosis (macropinocytosis, clathrin or caveolin-mediated
endocytosis, and clathrin/caveolin-independent endocytosis with
enhanced endosomal escape from a lysosome) depending on
physico-chemical properties, size and concentration of the peptides
(110). There are various
mechanistic studies examining binding peptides depending on their
targets. For example, D-form octa-arginines stimulates the
intestinal epithelial transport of drugs, such as insulin, via
energy-independent unsaturable internalization (111). Furthermore, a specific peptide
derived from nuclear localization signal (NLS) and epidermal growth
factor receptor pathway substrate 8 (EPS8), called CP-EPS8-NLS, can
cross the cellular membrane and interfere with the nuclear
translocation of EPS8, leading to inhibited cell viability and
proliferation in acute myeloid leukemia (AML) (112). It has also been revealed that
cell-penetrating peptide TAT-conjugated gambogic acid promotes
tumor apoptosis via reactive oxygen species (ROS)-mediated
apoptosis by increasing the ROS level in bladder cancer cells
(113).
Development of cell-permeable therapeutic peptides
with polar side chains has used advantage of adding methyl groups,
asparagine residues and D-amino acids (45). Similarly, another drug delivery
system, known as nanoparticles, can carry ACPs to tumor sites
without enzymatic degradation and can then enter inaccessible tumor
sites (114). However, anticancer
drug-carrying nanoparticles should be optimized for synergistic
effect, drug release control, circulating stability and drug
combination (115). Moreover,
binding peptides could be modified to protect enzymatic digestion,
penetrate cancer cell or organelle membranes, specifically bind to
cancer targets and stimulate biological cells around tumor
environments (116).
Host defense mechanisms against pathogens or
transformed cells, such as the cancer cells, is a novel therapeutic
approach that involves recruiting the immune cells into the tumors
(117). Antigenic peptide-human
leukocyte antigen class I complex respond to cytotoxic
CD8+ T-cells against malignant diseases and brain tumors
(118). However, ACP-produced
vaccines exhibit poor immunogenicity, and thus require adjuvants to
increase specific immune responses (119). For example, E75 peptide breast
cancer vaccine (Her2 p369-p377) containing polyactin A can increase
CD4+ and CD8+ T lymphocytes, enhance
proliferation of splenocytes and increase levels of interferon-γ in
splenocytes (120). Furthermore,
a melittin-RADA32 hybrid peptide hydrogel-linked
doxorubicin can recruit activated natural killer cells in the
primary melanoma tumor, resulting in growth retardation, as well as
activation of dendritic cells of draining lymph nodes and
production of cytotoxic T-cells against the remaining tumors
(121). Tyrosinase-related
protein 2 melanoma antigen peptide nanovaccine combined with CpG
adjuvant could slowly result in growth of the melanoma tumor
(122). Moreover, the 5-mer
peptide, A-P-D-T-R, is a potential target for immunotherapy against
breast cancer due to its highly immunogenic property that exists
within the variable number of tandem repeats found in all mucins,
particularly mucin1, which is increased by 10-fold in
adenocarcinomas (123). Some
peptide vaccines have been studied in phase I/II clinical trials
(124-126). For instance, an adjuvant
multi-peptide vaccine (UroRCC) was administered in patients with
metastatic renal cell carcinoma following metastasectomy (127). Furthermore, a multipeptide
vaccine (IMA950) containing 11 tumor-associated peptides, which
targets IMA950 antigens, has been used as a tumor-targeting vaccine
involving the T-cell response in grade II and III glioma (128). In metastatic hormone-naïve
prostate cancer, the novel human telomerase reverse transcrip-tase
(hTERT) peptide vaccine UV1 can induce an immune response,
affecting the prostate-specific antigen level (129). A vaccine containing peptides can
also be an adjuvant for activating the immune system. For instance,
Hp91 peptide has formed the adjuvant for a protein vaccine against
human papillomavirus to control cervical cancer (130). Therefore, immune system
stimulating peptides are an alternative cancer therapy to control
metastasis and eradicate cancer cells by activating host immunity
with the specific tumor antigens.
The therapeutic peptides can inhibit cancer cell
proliferation by controlling hormone release via their receptors
(131). Cancer cells can produce
hormones, such as growth hormone-releasing hormone (GHRH), to
stimulate the pituitary gland and then the release of growth
hormone (132,133). In a previous study, a GHRH
antagonist was synthesized to inhibit proliferation in AML cell
lines, including K562, THP-1 and KG-1a cells (134). Follicle stimulating hormone
(FSH), for which the circulating level is increased by leptin,
serves an important role in the initiation and the proliferation of
the ovarian cancer cells (135).
Moreover, the obese OB3 peptide, a derivative of leptin, may
prevent leptin-induced ovarian cancer cells by disrupting
leptin-induced ovarian cancer cell proliferation signal via
stimulation of STAT3 phosphorylation and estrogen receptor
α-activation (135). Furthermore,
nanoparticle drug vehicles containing 21-amino acid peptides
[YTRDLVYGDPARPGIQGTGTF (D-FP21)] conjugated to polyethylenimine and
methoxy polyethylene glycol target the FSH receptor, leading to
anti-proliferative effects on ovarian cancer (136). For chemotherapeutic improvement
of metastatic hormone-refractory prostate cancer, it was found that
the AlkB homolog 2 proliferating cell nuclear antigen (PCNA)
interacting motif peptide targeting PCNA, an essential scaffold
protein, in combination with docetaxel could decrease prostate
volume and inhibit cancer cell regrowth in vivo (137).
The issues with conventional therapeutic agents
associated with the majority of cancer drugs, include poor water
solubility, lack of target specificity and capability, non-specific
distribution, system cytotoxicity and low therapeutic index, can be
solved by creating a water-soluble form, targeting the delivery of
ACPs, non-systemic side effects and specific treatment efficacy
(138). Numerous natural peptides
derived from natural products, such as bioactive peptides, are
applied in cancer therapy (139).
Although naturally bioactive peptides exhibited beneficial
biocompatibility and low cytotoxicity, a number of bioactive
peptides cannot provide the active targeting, cell uptake, cancer
cell cytotoxicity and targeted delivery (140). The natural active peptides can be
modified to novel peptides with special properties, including
specificity, higher cell penetration, cancer cell cytotoxicity and
therapeutic efficacy with no side effect. The present review
focused on the therapeutic peptide development from natural
peptides to modified peptides and targeting peptides for increasing
the specific cancer cell targets.
Anticancer peptides have been discovered and
modified from antimicrobial peptides, and these resources produce
natural peptides from various organisms, such as marine, plant,
yeast, fungi, bacteria and bovine (141,142). Antimicrobial and anticancer
peptides, especially cationic peptides, can kill both bacteria and
cancer cells due to the similar negative net charge on their
membranes (143). Proteins from
nutrients can release bioactive peptides via enzymatic hydrolysis,
gastrointestinal digestion or during fermentation (144). Bioactive peptides discovered from
natural peptides have an electrostatic interaction between the
peptides and cell membrane, leading to cancer cell or mitochondrial
membrane disruption and then necrosis or apoptosis (145). For example, bioactive
milk-derived peptides released during digestion have a vital role
in cancer prevention (146).
Moreover, germinated soybean protein-derived peptides from
enzymatic hydrolysis exert antiproliferative activity against human
colorectal cancer cells (147).
It has also been shown that the extracted peptides from Lentinus
squarrosulus mushrooms can mediate human lung cancer cells via
apoptosis (148). Cyclic peptides
isolated from marine cyanobacteria, such as Urumamide,
exhibited low proliferative inhibitory activity on human cancer
cells (149). Additional examples
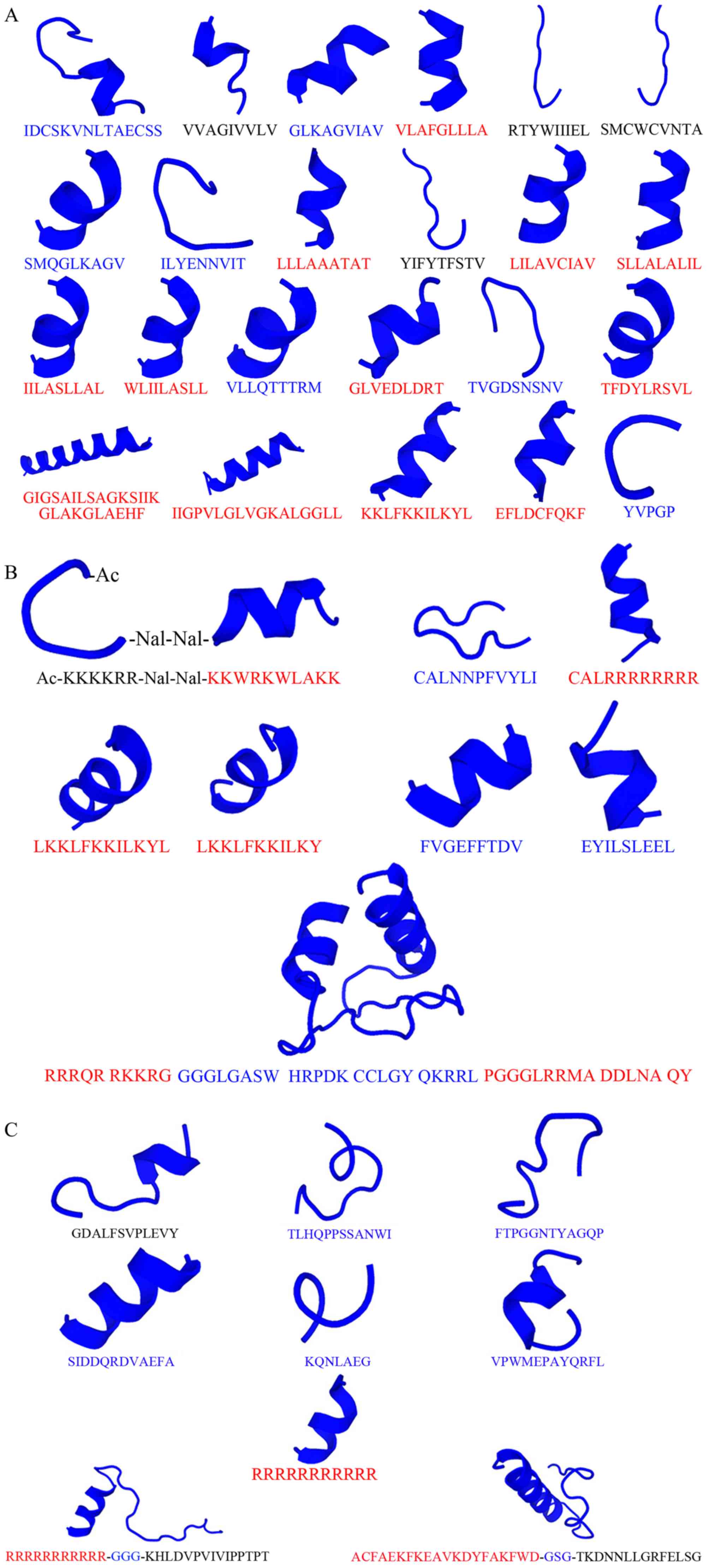
of natural peptides that have anticancer properties are presented
in Table II and Fig. 3A. The majority of natural peptides
that exert effects against cancer cell survival are α-helical
folding peptides that have cationic properties (150,151). However, a minority of peptides,
including other folding with neutral or anionic peptides, are able
to disrupt cancer cell survival (152). Recently, a number of anionic
antimicrobial peptides that originate from amphibians, including
frogs, toads, newts and salamanders across Africa, South America
and China, demonstrated anticancer activity (153). Thus, natural ACPs can exhibit
both cationic and anionic or neutral properties; also, the majority
of cationic peptides are found to have a significant cytotoxic
effect against cancer cells compared with anionic or neutral
peptides. In the future, these natural ACPs can be modified to
further ACP development.
Highly cationic and amphipathic peptide properties
can be synthesized and designed via in silico creations. For
example, some chemical groups, such as acetylation or amidation,
are added into the natural peptides to increase the cationic
properties and target cell specificity (162). Replacement of D-amino acids in an
amphipathic peptide, KLALKLALKALKAAKLA-NH2, and a
hydrophobic interaction can increase the membrane-disrupting effect
on high negative surface charge bilayers, which then promotes
peptide penetration into the inner membrane regions (163). Moreover, the folding and
formation of peptides, such as the α-helix or cyclization, results
in an increase in anticancer properties and stability (164,165). It has also been revealed that
fewer helical peptides can decrease the bilayer disruption activity
(163), and that cyclic peptides
can act on cell permeability (45). Furthermore, substitution, deletion
or addition of positively charged or polar and non-polar amino
acids on natural peptides could modify their properties to improve
therapeutic application (164,165). Some modified peptides are
displayed in Table III and
Fig. 3B.
Besides the aforementioned modifications, ACPs have
been constructed via genetic engineering, including anticancer
fusion peptides; for example, the structure of bovine lactoferricin
and hexapeptide derived from bovine milk protein for ovarian cancer
treatment (166). NT4 peptides
bound to GAG chains of heparan sulfate proteoglycans have a
modulatory effect on the cancer cell migration and invasion ability
(167). A recombinant protein
consisting of iRGD (CRGDKGPDC)-conjugated KLA peptide
(KLAKLAKKLAKLAK) exerts a pro-apoptotic activity and high
penetration to tumor tissue and cells for gastric cancer treatment
(40). Collectively, modified
peptides can be developed to improve anticancer properties and the
effect on the cancer targets directly.
The discovery of cancer cell targets can promote
cell target specificity to avoid healthy cell damage (172). Targeting peptides on various
cancer cell types should bind to cancer cell targets and eliminate
cancer cells at the same time (173). Molecular targets in cancer cells
are important for clinical therapy, including vascular endothelial
growth factor, RAS/mitogen-activated protein kinase pathway
inhibitors, aurora kinase inhibitors or endothelin receptor
antagonists (174,175). Some molecular targets can induce
an immune response, such as cytokines, while others directly bind
to specific cancer cell biomarkers (175,176). Upregulation of specific cancer
proteins or peptides has been used as cancer targets (139). For instance, high expression
levels of MDM2 proto-oncogene (MDM2) and MDM4 regulator of p53
(MDMX), as negative regulators of tumor suppressor protein p53, and
upregulated expression of the cell surface receptor CD33 have been
targeted for AML therapy (177).
Lanthanide oxyfluoride nanoparticle (LONp) bound dual-specific
peptide antagonists of MDM2 and MDMX (PMI) and antiCD33-LONp-PMI
can activate the p53 pathway, thus inducing AML cell apoptosis
(177). Furthermore, upregulated
urokinase plasminogen activator receptors (uPAR) on cancer cells
are targeted to uptake a specific peptide, 68Ga-labeled AE105
peptide, as uPAR PET-probes, into U87MG tumor cells (178). Examples of the targeting peptides
are presented in Table IV and
Fig. 3C. Besides disturbing cancer
cell survival, targeting peptides for cancer cell labeling was an
advantage for cancer cell detection and diagnosis. For example,
99mTc-(tricine)-HYNIC-Lys-FROP peptides were taken up by breast
cancer cells for tumor targeting and molecular imaging (179). Therefore, targeting peptides can
specifically and directly bind and destroy cancer cells, but not
healthy cells. However, their targets are difficult to discover and
develop for specific cancer cell therapy.
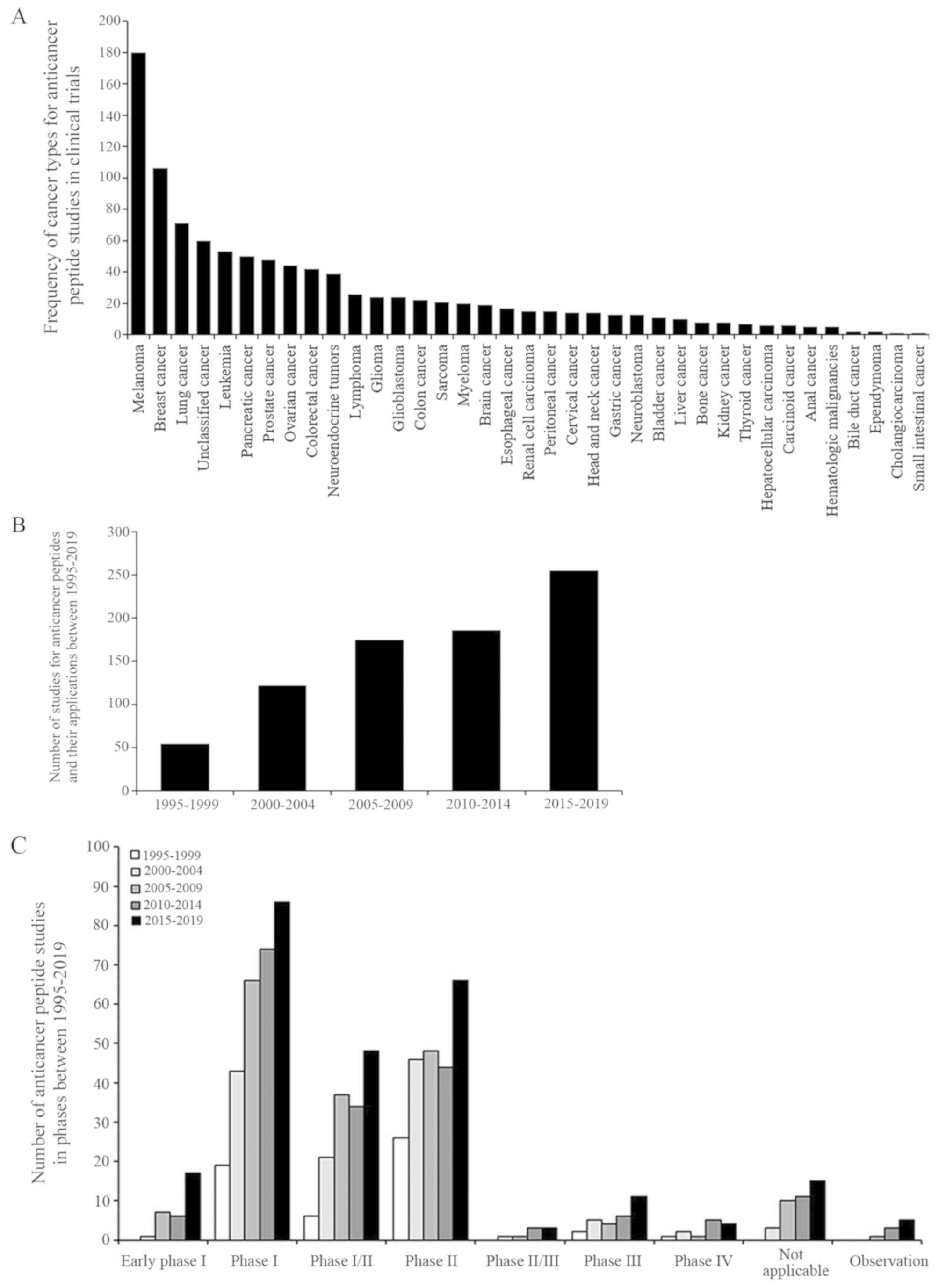
Several synthetic peptide-based drugs and vaccines
are currently undergoing clinical trials. The National Library of
Medicine (NLM) at the National Institutes of Health (NIH) provides
and updates clinical trial information on the ClinicalTrials.gov website. A total of 792 studies
between 1995-2019 were identified and searched for 'cancer',
'peptide', 'drug' or 'biological' key words, excluding
non-anti-cancer peptide interventions such as behavior and surgery.
The search result is presented in Fig.
4.
For example, CIGB-300, an amidated disulfide cyclic
undecapeptide fused to the TAT cell-penetrating peptide via a
β-alanine spacer, inhibits CK-2-mediated phosphorylation leading to
cancer cell apoptosis in patients with cervical and non-small cell
lung cancer (185-187). Wilms' tumor 1 (WT1) peptide-based
vaccination combined with the adjuvant drug OK-432 administered to
pediatric patients with a solid tumor has been demonstrated to be
safe for these children (188).
Furthermore, WT1-pulsed dendritic cell vaccine has been used to
treat patients with surgically resected pancreatic cancer under a
phase I study (189). A modified
9-mer WT1 peptide vaccine was also used in patients with
gynecological cancer for inducing myeloid dendritic cells, and was
demonstrated to be associated with cytotoxic T-cell activation
(190). Subsequently, WT1 peptide
vaccine therapy was evaluated in patients with gynecological cancer
in a phase II clinical trial (191). A target of esophageal squamous
cell carcinoma and lung cancer types is lymphocyte antigen 6
complex locus K (LY6K), which is expressed in gastric cancer
(192). LY6K-177 peptide vaccine
emulsified with Montanide ISA 51 was evaluated in patients with
gastric cancer as a phase I clinical trial, and was found to be
tolerated by patients with advanced gastric cancer (50% of patients
with gastric cancer had stable disease and 16% patients had a tumor
contraction effect) (192).
B-cell lymphocytic leukemia and pancreatic cancer
have demonstrated a high level of telomerase activity (193). GV1001, a peptide based-cancer
vaccine derived from the hTERT (hTERT 616-626; EARPALLTSRLRFIPK),
was administrated in patients with non-resectable pancreatic cancer
undergoing a dose-escalating phase I/II study (194); GV1001 was capable of inducing
CD4+ and CD8+ T-cells, interacting with
professional antigen-presenting cells and then engulfing dead tumor
tissue or cells (194). Moreover,
GV1001 may be a candidate vaccine in patients with B-cell chronic
lymphocytic leukemia that exhibit telomerase-specific leukemic
cells (195).
A combination of the ACPs and other drugs have also
been evaluated in phase I trials, such as cyclodepsipeptide
plitidepsin and bevacizumab in refractory solid tumors (196). For the binding peptide strategy,
a carrier peptide, as a luteinizing hormone-releasing hormone
(LHRH) agonist, is linked to the cytotoxic analogs of LHRH for
cancer expressing receptors for LHRH (197). The LHRH agonist under phase II
clinical trial exhibits anticancer activity in LHRH
receptor-positive cancer types, such as human endometrial, ovarian
and prostate cancer (197).
Previously, a personalized peptide vaccination (PPV) has been
developed as a novel approach for a cancer vaccine to boost the
immune response using specific peptides for each patient (198). The peptides for PPV treatment
under a randomized phase II trial in patients with bladder cancer
were selected from the candidate peptides, according to human
leukocyte antigen types and peptide-reactive IgG titers, to observe
progression-free survival, overall survival, immune response and
toxicity (198). Similarly, 19
mixed peptides were selected from 31 PPVs according to the
anti-tumor immunological effect, and the safety profiles for
patients with metastatic breast cancer were also assessed in a
phase II clinical trial (199).
While some peptides, such as gp100:209-217 (210M)/Montanide™
ISA-51/Imiquimod for high risk melanoma and E39 peptide/GM-CSF
vaccine plus E39 booster for ovarian cancer, have been approved by
the Food and Drug Administration (FDA), these have been improved in
clinical therapy, such as peptide boronate bortezomib (200-202). The peptide boronate bortezomib is
a reversible 26S proteasome inhibitor, degenerating several
intracellular proteins, with antitumor and antiproliferative
activities and can be used in multiple myeloma therapy (202). Due to adverse effects, such as
hematotoxicity and peripheral neuropathy, poor penetration into
solid tumors and low clinical stability and bioavailability,
bortezomib was developed for delivery using nanoparticles, and
treatment for bortezomib resistant multiple myeloma was improved
using target chemical modification during synthetic processes
(203,204). Additional ACP examples are
presented in Table V. As
aforementioned, various cancer vaccines have been produced using
ACPs and ACPs combined with adjuvants or drugs, and the effects of
carrier peptides on targeting cancer cell directly and/or by
activating immune response have been tested in clinical trials for
safety, side effects and effectiveness.
Although ACPs have a number of disadvantages, such
as biological instability, low bioavailability, short half-life,
protease sensitivity, poor pharmacokinetics and first-pass
metabolism, their most notable advantage is the protein-protein
interaction with a target, thus overcoming limitations via
designing peptide modifications and conjugation to improve
affinity, stability and selectivity (205,206). For example, the peptide
BBN7-14 (Gln-Trp-Ala-Val-Gly-His-Leu-Met-NH2)
composed of natural amino acids has a higher binding affinity with
the CFPAC-1 cell line compared with the modified peptide GB-6
(Gln-5-Htp-β-Ala-Nva-Gln-His-NH2) that consists of
unnatural amino acids (in vitro). However, in vivo,
BBN7-14 has a reduced tumor-targeting ability compared
with GB-6, which is stable against protease-mediate degradation and
has a slightly lower uptake and slow metabolism (207). Currently, ACPs have been modified
to improve specific cancer cell targets and enhance cancer cell
elimination. Some anticancer peptides as drugs and vaccinations
have been tested in phase I/II clinical trials (175). For example, dTCApFs, a natural
hormone peptide for the treatment of advanced or metastatic solid
tumors, enters the cells via the Toll/interleukin-1 receptor
superfamily, suppresses angiogenic factors and induces anticancer
cytokine production and ER stress, leading to cancer cell apoptosis
(208). dTCApFs anticancer
activity in humans was firstly studied in a phase I clinical trial
by investigating the safety and efficacy with regards to both
pharmacokinetics and pharmacodynamics, with intravenous dTCApFs
(6-96 mg/m2; 3 times/week; in consecutive 28-day cycles)
(209). The intravenous dTCApFs
is decreased at lower limit of detection in serum after 24-h
administration and its concentration in serum is present in
dose-dependent manner (209).
Furthermore, ACPs have been combined with immunogens for clinical
therapeutic improvement (210).
Upregulation of molecular cancer targets, such as Ras protein that
has been discovered in various cancer cell types (lung, colon and
pancreatic), could also be direct targets for ACP development
(211). The aim of ACP therapy
should promote cancer cell death and intermit tumor regression,
without contributing to tumorigenesis and resistance in cancer cell
treatment (212). The first ACP
approved by the FDA was the peptide boronate bortezomib
(Velcade®) for multiple myeloma treatment in 2003 and
mantle cell lymphoma in 2006 (213). In the near future, combination
therapy with a drug or vaccine containing i) the specific targeting
peptides, ii) the ACPs and iii) the cell-penetrating peptides
and/or the conjugated delivery materials (such as liposome,
nanoparticles or adjuvants) may facilitate the development of
cancer therapy with cancer cell specificity, stability, safety and
efficacy, without healthy cell eradication (214). ACP construction for specific
cancer cell targets, and predictive, preventive and personalized
medicine may be beneficial to the cancer research field due to the
different complexity of the whole-body system in each individual
(215). Besides the
aforementioned therapeutic peptides, peptides with specific cancer
cell targets are applied to bind the cancer cell targets for cancer
detection and therapy (216,217). For example, a sodium pump
Na+/K+ ATPase α1-targeted peptide for
positron emission tomography imaging of breast cancer, as
peptide-based platform on dual-targeted molecular imaging, is able
to more obviously visualize the disease state of a patient, leading
to improved informed treatment decisions (218,219).
ACP therapy affects molecular targets, binds the
anticancer drugs and stimulates biological systems involving cancer
and healthy cell environments. Notably, natural and synthetic
peptides have been developed as novel strategies against cancer
types. Natural anticancer peptides can be modified to enable high
penetration, specific cancer cell targets, increase efficacy and
reduce side effects. A number of ACPs have been demonstrated to be
anti-proliferative, apoptotic and proliferation inhibitors in
various cancer cell types, both in vitro and in vivo,
leading to clinical trials for the evaluation of cancer treatment.
The development of drug or vaccine technology could further ACPs in
design, synthesis and delivery to eliminate cancer cells directly
or by affecting the anticancer immune responses (220). Collectively, it was suggested
ACPs may promote cancer drugs or vaccine development to decrease
emerging cases and mortality rates in the future.
The authors would like to thank Dr Thitinee
Vanichapol, Division of Hematology and Oncology, Department of
Pediatrics, Faculty of Medicine, Ramathibodi Hospital, Mahidol
University for revising the manuscript and providing kind
suggestions.
This study was financially supported by BRAND'S
Health Research Award 2017 (Cerebos Award 2017), Children Cancer
Fund under the Patronage of HRH Princess Soamsawali and Faculty
Staff Development Program of Faculty of Medicine Ramathibodi
Hospital, Mahidol University, Thailand (grant no. BHR2017).
The datasets used and/or analyzed during the
present study are available from the corresponding author on
reasonable request.
WC wrote and edited the manuscript and was involved
in the creation of the figures and data analysis. All authors were
involved in the drafting and revising of the manuscript. All
authors read the manuscript and approved the final version.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Wang SH and Yu J: Structure-based design
for binding peptides in anti-cancer therapy. Biomaterials.
156:1–15. 2018. View Article : Google Scholar
|
|
2
|
Wang X, Zhang L, Ding N, Yang X, Zhang J,
He J, Li Z and Sun LQ: Identification and characterization of
DNAzymes targeting DNA methyltransferase I for suppressing bladder
cancer proliferation. Biochem Biophys Res Commun. 461:329–333.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ingram JR, Blomberg OS, Rashidian M, Ali
L, Garforth S, Fedorov E, Fedorov AA, Bonanno JB, Le Gall C,
Crowley S, et al: Anti-CTLA-4 therapy requires an Fc domain for
efficacy. Proc Natl Acad Sci USA. 115:3912–3917. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Di JX and Zhang HY: C188-9 a
small-molecule STAT3 inhibitor, exerts an antitumor effect on head
and neck squamous cell carcinoma. Anticancer Drugs. 30:846–853.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brun S, Bassissi F, Serdjebi C, Novello M,
Tracz J, Autelitano F, Guillemot M, Fabre P, Courcambeck J, Ansaldi
C, et al: GNS561, a new lysosomotropic small molecule, for the
treatment of intrahe-patic cholangiocarcinoma. Invest New Drugs.
37:1135–1145. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jahangirian H, Kalantari K, Izadiyan Z,
Rafiee-Moghaddam R, Shameli K and Webster TJ: A review of small
molecules and drug delivery applications using gold and iron
nanoparticles. Int J Nanomedicine. 14:1633–1657. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tyagi A, Tuknait A, Anand P, Gupta S,
Sharma M, Mathur D, Joshi A, Singh S, Gautam A and Raghava GP:
CancerPPD: A database of anticancer peptides and proteins. Nucleic
Acids Res. 43(Database Issue): D837–D843. 2015. View Article : Google Scholar :
|
|
8
|
Thundimadathil J: Cancer treatment using
peptides: Current therapies and future prospects. J Amino Acids.
2012:9673472012. View Article : Google Scholar
|
|
9
|
Vlieghe P, Lisowski V, Martinez J and
Khrestchatisky M: Synthetic therapeutic peptides: Science and
market. Drug Discov Today. 15:40–56. 2010. View Article : Google Scholar
|
|
10
|
Otvos L Jr: Peptide-based drug design:
Here and now. Methods Mol Biol. 494:1–8. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hoskin DW and Ramamoorthy A: Studies on
anticancer activities of antimicrobial peptides. Biochim Biophys
Acta. 1778:357–375. 2008. View Article : Google Scholar
|
|
12
|
Rodrigues EG, Dobroff AS, Taborda CP and
Travassos LR: Antifungal and antitumor models of bioactive
protective peptides. An Acad Bras Cienc. 81:503–520. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Droin N, Hendra JB, Ducoroy P and Solary
E: Human defensins as cancer biomarkers and antitumour molecules. J
Proteomics. 72:918–927. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Simons K and Ikonen E: How cells handle
cholesterol. Science. 290:1721–1726. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sok M, Sentjurc M and Schara M: Membrane
fluidity characteristics of human lung cancer. Cancer Lett.
139:215–220. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zwaal RF and Schroit AJ: Pathophysiologic
implications of membrane phospholipid asymmetry in blood cells.
Blood. 89:1121–1132. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schweizer F: Cationic amphiphilic peptides
with cancer-selective toxicity. Eur J Pharmacol. 625:190–194. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Utsugi T, Schroit AJ, Connor J, Bucana CD
and Fidler IJ: Elevated expression of phosphatidylserine in the
outer membrane leaflet of human tumor cells and recognition by
activated human blood monocytes. Cancer Res. 51:3062–3066.
1991.PubMed/NCBI
|
|
19
|
Harris F, Dennison SR, Singh J and Phoenix
DA: On the selectivity and efficacy of defense peptides with
respect to cancer cells. Med Res Rev. 33:190–234. 2013. View Article : Google Scholar
|
|
20
|
Li G, Huang Y, Feng Q and Chen Y:
Tryptophan as a probe to study the anticancer mechanism of action
and specificity of alpha-helical anticancer peptides. Molecules.
19:12224–12241. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Marqus S, Pirogova E and Piva TJ:
Evaluation of the use of therapeutic peptides for cancer treatment.
J Biomed Sci. 24:212017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Roudi R, Syn NL and Roudbary M:
Antimicrobial peptides as biologic and immunotherapeutic agents
against Cancer: A comprehensive overview. Front Immunol.
8:13202017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Alves AC, Ribeiro D, Nunes C and Reis S:
Biophysics in cancer: The relevance of drug-membrane interaction
studies. Biochim Biophys Acta. 1858:2231–2244. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yamaji-Hasegawa A and Tsujimoto M:
Asymmetric distribution of phospholipids in biomembranes. Biol
Pharm Bull. 29:1547–1553. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Clark MR: Flippin' lipids. Nat Immunol.
12:373–375. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Deliconstantinos G: Physiological aspects
of membrane lipid fluidity in malignancy. Anticancer Res.
7:1011–1021. 1987.PubMed/NCBI
|
|
27
|
Ran S, Downes A and Thorpe PE: Increased
exposure of anionic phospholipids on the surface of tumor blood
vessels. Cancer Res. 62:6132–6140. 2002.PubMed/NCBI
|
|
28
|
Stafford JH and Thorpe PE: Increased
exposure of phosphati-dylethanolamine on the surface of tumor
vascular endothelium. Neoplasia. 13:299–308. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Barcelo-Coblijn G, Martin ML, de Almeida
RF, Noguera-Salva MA, Marcilla-Etxenike A, Guardiola-Serrano F,
Lüth A, Kleuser B, Halver JE and Escribá PV: Sphingomyelin and
sphin-gomyelin synthase (SMS) in the malignant transformation of
glioma cells and in 2-hydroxyoleic acid therapy. Proc Natl Acad Sci
USA. 108:19569–19574. 2011. View Article : Google Scholar
|
|
30
|
Preetha A, Huilgol N and Banerjee R:
Comparison of paclitaxel penetration in normal and cancerous
cervical model monolayer membranes. Colloids Surf B Biointerfaces.
53:179–186. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhao L, Feng SS and Go ML: Investigation
of molecular interactions between paclitaxel and DPPC by Langmuir
film balance and differential scanning calorimetry. J Pharm Sci.
93:86–98. 2004. View Article : Google Scholar
|
|
32
|
Logozzi M, Spugnini E, Mizzoni D, Di Raimo
R and Fais S: Extracellular acidity and increased exosome release
as key phenotypes of malignant tumors. Cancer Metastasis Rev.
38:93–101. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cardone RA, Casavola V and Reshkin SJ: The
role of disturbed pH dynamics and the Na+/H+ exchanger in
metastasis. Nat Rev Cancer. 5:786–795. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jobin ML and Alves ID: On the importance
of electrostatic interactions between cell penetrating peptides and
membranes: A pathway toward tumor cell selectivity? Biochimie.
107:154–159. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Peyressatre M, Prevel C, Pellerano M and
Morris MC: Targeting cyclin-dependent kinases in human cancers:
From small molecules to Peptide inhibitors. Cancers (Basel).
7:179–237. 2015. View Article : Google Scholar
|
|
36
|
Raucher D and Ryu JS: Cell-penetrating
peptides: Strategies for anticancer treatment. Trends Mol Med.
21:560–570. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li J, Tan S, Chen X, Zhang CY and Zhang Y:
Peptide aptamers with biological and therapeutic applications. Curr
Med Chem. 18:4215–4222. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fuertes MA, Castilla J, Alonso C and Perez
JM: Cisplatin biochemical mechanism of action: from cytotoxicity to
induction of cell death through interconnections between apoptotic
and necrotic pathways. Curr Med Chem. 10:257–266. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Horwitz SB: Taxol (paclitaxel): Mechanisms
of action. Ann Oncol. 5(Suppl 6): S3–S6. 1994.PubMed/NCBI
|
|
40
|
Huang Y, Li X, Sha H, Zhang L, Bian X, Han
X and Liu B: Tumor-penetrating peptide fused to a pro-apoptotic
peptide facilitates effective gastric cancer therapy. Oncol Rep.
37:2063–2070. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang B, Shi W, Li J, Liao C, Yang L,
Huang W and Qian H: Synthesis and biological evaluation of novel
peptides based on antimicrobial peptides as potential agents with
antitumor and multidrug resistance-reversing activities. Chem Biol
Drug Des. 90:972–980. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ramsey JD and Flynn NH: Cell-penetrating
peptides transport therapeutics into cells. Pharmacol Ther.
154:78–86. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kapoor P, Singh H, Gautam A, Chaudhary K,
Kumar R and Raghava GP: TumorHoPe: A database of tumor homing
peptides. PLoS One. 7:e351872012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ghasemy S, Garcia-Pindado J, Aboutalebi F,
Dormiani K, Teixido M and Malakoutikhah M: Fine-tuning the
physicochemical properties of peptide-based blood-brain barrier
shuttles. Bioorg Med Chem. 26:2099–2106. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Buckton LK and McAlpine SR: Improving the
cell permeability of polar cyclic peptides by replacing residues
with alkylated amino acids, asparagines, and d-Amino Acids. Org
Lett. 20:506–509. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Perry SR, Hill TA, de Araujo AD, Hoang HN
and Fairlie DP: Contiguous hydrophobic and charged surface patches
in short helix-constrained peptides drive cell permeability. Org
Biomol Chem. 16:367–371. 2018. View Article : Google Scholar
|
|
47
|
Shoombuatong W, Schaduangrat N and
Nantasenamat C: Unraveling the bioactivity of anticancer peptides
as deduced from machine learning. EXCLI J. 17:734–752.
2018.PubMed/NCBI
|
|
48
|
Dai YX, Cai XG, Shi W, Bi XZ, Su X, Pan
MB, Li HL, Lin HY, Huang WL and Qian H: Pro-apoptotic cationic host
defense peptides rich in lysine or arginine to reverse drug
resistance by disrupting tumor cell membrane. Amino Acids.
49:1601–1610. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Navarro S, Aleu J, Jimenez M, Boix E,
Cuchillo CM and Nogues MV: The cytotoxicity of eosinophil cationic
protein/ribonuclease 3 on eukaryotic cell lines takes place through
its aggregation on the cell membrane. Cell Mol Life Sci.
65:324–337. 2008. View Article : Google Scholar
|
|
50
|
Midoux P, Kichler A, Boutin V, Maurizot JC
and Monsigny M: Membrane permeabilization and efficient gene
transfer by a peptide containing several histidines. Bioconjug
Chem. 9:260–267. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yamaguchi Y, Yamamoto K, Sato Y, Inoue S,
Morinaga T and Hirano E: Combination of aspartic acid and glutamic
acid inhibits tumor cell proliferation. Biomed Res. 37:153–159.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Oancea E, Teruel MN, Quest AF and Meyer T:
Green fluorescent protein (GFP)-tagged cysteine-rich domains from
protein kinase C as fluorescent indicators for diacylglycerol
signaling in living cells. J Cell Biol. 140:485–498. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Shamova O, Orlov D, Stegemann C, Czihal P,
Hoffmann R, Brogden K, Kolodkin N, Sakuta G, Tossi A, Sahl HG, et
al: ChBac3.4: A Novel proline-rich antimicrobial peptide from goat
leukocytes. Int J Pept Res Ther. 15:107–119. 2009. View Article : Google Scholar
|
|
54
|
Maddocks ODK, Athineos D, Cheung EC, Lee
P, Zhang T, van den Broek NJF, Mackay GM, Labuschagne CF, Gay D,
Kruiswijk F, et al: Modulating the therapeutic response of tumours
to dietary serine and glycine starvation. Nature. 544:372–376.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kawaguchi K, Han Q, Li S, Tan Y, Igarashi
K, Kiyuna T, Miyake K, Miyake M, Chmielowski B, Nelson SD, et al:
Targeting methionine with oral recombinant methioninase (o-rMETase)
arrests a patient-derived orthotopic xenograft (PDOX) model of
BRAF-V600E mutant melanoma: Implications for chronic clinical
cancer therapy and prevention. Cell Cycle. 17:356–361. 2018.
View Article : Google Scholar :
|
|
56
|
Gueron G, Anselmino N, Chiarella P, Ortiz
EG, Lage Vickers S, Paez AV, Giudice J, Contin MD, Leonardi D,
Jaworski F, et al: Game-changing restraint of Ros-damaged
phenylalanine, upon tumor metastasis. Cell Death Dis. 9:1402018.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Dennison SR, Whittaker M, Harris F and
Phoenix DA: Anticancer alpha-helical peptides and
structure/function relationships underpinning their interactions
with tumour cell membranes. Curr Protein Pept Sci. 7:487–499. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Marchand C, Krajewski K, Lee HF, Antony S,
Johnson AA, Amin R, Roller P, Kvaratskhelia M and Pommier Y:
Covalent binding of the natural antimicrobial peptide indolicidin
to DNA abasic sites. Nucleic Acids Res. 34:5157–5165. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Barras D, Chevalier N, Zoete V, Dempsey R,
Lapouge K, Olayioye MA, Michielin O and Widmann C: A WXW motif is
required for the anticancer activity of the TAT-RasGAP317-326
peptide. J Biol Chem. 289:23701–23711. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ahmaditaba MA, Shahosseini S, Daraei B,
Zarghi A and Houshdar Tehrani MH: Design, synthesis, and biological
evaluation of new peptide analogues as selective cox-2 inhibitors.
Arch Pharm (Weinheim). 350:e17001582017. View Article : Google Scholar
|
|
61
|
Bhunia D, Mondal P, Das G, Saha A,
Sengupta P, Jana J, Mohapatra S, Chatterjee S and Ghosh S: Spatial
position regulates power of tryptophan: Discovery of a
major-groove-specific nuclear-localizing, cell-penetrating
tetrapeptide. J Am Chem Soc. 140:1697–1714. 2018. View Article : Google Scholar
|
|
62
|
Huang YB, Wang XF, Wang HY, Liu Y and Chen
Y: Studies on mechanism of action of anticancer peptides by
modulation of hydrophobicity within a defined structural framework.
Mol Cancer Ther. 10:416–426. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Yang QZ, Wang C, Lang L, Zhou Y, Wang H
and Shang DJ: Design of potent, non-toxic anticancer peptides based
on the structure of the antimicrobial peptide, temporin-1CEa. Arch
Pharm Res. 36:1302–1310. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Dennison SR, Harris F, Bhatt T, Singh J
and Phoenix DA: A theoretical analysis of secondary structural
characteristics of anticancer peptides. Mol Cell Biochem.
333:129–135. 2010. View Article : Google Scholar
|
|
65
|
Wu JM, Jan PS, Yu HC, Haung HY, Fang HJ,
Chang YI, Cheng JW and Chen HM: Structure and function of a custom
anticancer peptide, CB1a. Peptides. 30:839–848. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Lins L and Brasseur R: Tilted peptides: A
structural motif involved in protein membrane insertion? J Pept
Sci. 14:416–422. 2008. View Article : Google Scholar
|
|
67
|
Lins L, Decaffmeyer M, Thomas A and
Brasseur R: Relationships between the orientation and the
structural properties of peptides and their membrane interactions.
Biochim Biophys Acta. 1778:1537–1544. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Mandal PK, Gao F, Lu Z, Ren Z, Ramesh R,
Birtwistle JS, Kaluarachchi KK, Chen X, Bast RC Jr, Liao WS, et al:
Potent and selective phosphopeptide mimetic prodrugs targeted to
the Src homology 2 (SH2) domain of signal transducer and activator
of transcription 3. J Med Chem. 54:3549–3563. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Gabernet G, Gautschi D, Muller AT, Neuhaus
CS, Armbrecht L, Dittrich PS, Hiss JA and Schneider G: In silico
design and optimization of selective membranolytic anticancer
peptides. Sci Rep. 9:112822019. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Singh M, Kumar V, Sikka K, Thakur R,
Harioudh MK, Mishra DP, Ghosh JK and Siddiqi MI: Computational
design of biologically active anticancer peptides and their
interactions with heterogeneous POPC/POPS Lipid membranes. J Chem
Inf Model. 60:332–341. 2020. View Article : Google Scholar
|
|
71
|
Ray T, Kar D and Pal A, Mukherjee S, Das C
and Pal A: Molecular targeting of breast and colon cancer cells by
PAR1 mediated apoptosis through a novel pro-apoptotic peptide.
Apoptosis. 23:679–694. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Bohmova E, Machova D, Pechar M, Pola R,
Venclikova K, Janouskova O and Etrych T: Cell-penetrating peptides:
A useful tool for the delivery of various cargoes into cells.
Physiol Res. 67(Suppl 2): S267–S279. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Levely ME, Mitchell MA and Nicholas JA:
Synthetic immunogens constructed from T-cell and B-cell stimulating
peptides (T:B chimeras): Preferential stimulation of unique T- and
B-cell specificities is influenced by immunogen configuration. Cell
Immunol. 125:65–78. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Asao T, Takahashi F and Takahashi K:
Resistance to molecu-larly targeted therapy in non-small-cell lung
cancer. Respir Investig. 57:20–26. 2019. View Article : Google Scholar
|
|
75
|
Zhang H, Han D, Lv T, Liu K, Yang Y, Xu X
and Chen Y: Novel peptide myristoly-CM4 induces selective
cytotoxicity in leukemia K562/MDR and Jurkat cells by necrosis
and/or apop-tosis pathway. Drug Des Devel Ther. 13:2153–2167. 2019.
View Article : Google Scholar :
|
|
76
|
Chen YQ, Min C, Sang M, Han YY, Ma X, Xue
XQ and Zhang SQ: A cationic amphiphilic peptide ABP-CM4 exhibits
selective cytotoxicity against leukemia cells. Peptides.
31:1504–1510. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Jiang R, Du X and Lonnerdal B: Comparison
of bioactivities of talactoferrin and lactoferrins from human and
bovine milk. J Pediatr Gastroenterol Nutr. 59:642–652. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Tyagi A, Kapoor P, Kumar R, Chaudhary K,
Gautam A and Raghava GP: In silico models for designing and
discovering novel anticancer peptides. Sci Rep. 3:29842013.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Hajisharifi Z, Piryaiee M, Mohammad Beigi
M, Behbahani M and Mohabatkar H: Predicting anticancer peptides
with Chou's pseudo amino acid composition and investigating their
muta-genicity via Ames test. J Theor Biol. 341:34–40. 2014.
View Article : Google Scholar
|
|
80
|
Chen W, Feng PM, Lin H and Chou KC:
iRSpot-PseDNC: Identify recombination spots with pseudo
dinucleotide composition. Nucleic Acids Res. 41:e682013. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Grisoni F, Neuhaus C, Gabernet G, Muller
A, Hiss J and Schneider G: Designing anticancer peptides by
constructive machine learning. ChemMedChem. 13:1300–1302. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Kozlowska K, Nowak J, Kwiatkowski B and
Cichorek M: ESR study of plasmatic membrane of the transplantable
melanoma cells in relation to their biological properties. Exp
Toxicol Pathol. 51:89–92. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Mai JC, Mi Z, Kim SH, Ng B and Robbins PD:
A proapoptotic peptide for the treatment of solid tumors. Cancer
Res. 61:7709–7712. 2001.PubMed/NCBI
|
|
84
|
Zhang W, Li J, Liu LW, Wang KR, Song JJ,
Yan JX, Li ZY, Zhang BZ and Wang R: A novel analog of antimicrobial
peptide Polybia-MPI, with thioamide bond substitution, exhibits
increased therapeutic efficacy against cancer and diminished
toxicity in mice. Peptides. 31:1832–1838. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Sinthuvanich C, Veiga AS, Gupta K, Gaspar
D, Blumenthal R and Schneider JP: Anticancer β-hairpin peptides:
Membrane-induced folding triggers activity. J Am Chem Soc.
134:6210–6217. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Gaspar D, Veiga AS, Sinthuvanich C,
Schneider JP and Castanho MA: Anticancer peptide SVS-1: Efficacy
precedes membrane neutralization. Biochemistry. 51:6263–6265. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Negi B, Kumar D and Rawat DS: Marine
peptides as anticancer agents: A remedy to mankind by nature. Curr
Protein Pept Sci. 18:885–904. 2017. View Article : Google Scholar
|
|
88
|
Lemeshko VV: Electrical potentiation of
the membrane permeabilization by new peptides with anticancer
properties. Biochim Biophys Acta. 1828:1047–1056. 2013. View Article : Google Scholar
|
|
89
|
Liu X, Cao R, Wang S, Jia J and Fei H:
Amphipathicity determines different cytotoxic mechanisms of lysine-
or arginine-rich cationic hydrophobic peptides in cancer cells. J
Med Chem. 59:5238–5247. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Hu C, Chen X, Zhao W, Chen Y and Huang Y:
Design and modification of anticancer peptides. Drug Des.
5:1000138–1000147. 2016. View Article : Google Scholar
|
|
91
|
Boohaker RJ, Lee MW, Vishnubhotla P, Perez
JM and Khaled AR: The use of therapeutic peptides to target and to
kill cancer cells. Curr Med Chem. 19:3794–3804. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Bidwell GL III and Raucher D: Therapeutic
peptides for cancer therapy. Part I-peptide inhibitors of signal
transduction cascades. Expert Opin Drug Deliv. 6:1033–1047. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Raucher D, Moktan S, Massodi I and Bidwell
GL III: Therapeutic peptides for cancer therapy. Part II-cell cycle
inhibitory peptides and apoptosis-inducing peptides. Expert Opin
Drug Deliv. 6:1049–1064. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Ulasov IV, Borovjagin AV, Timashev P,
Cristofanili M and Welch DR: KISS1 in breast cancer progression and
autophagy. Cancer Metastasis Rev. 38:493–506. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Lamidi OF, Sani M, Lazzari P, Zanda M and
Fleming IN: The tubulysin analogue KEMTUB10 induces apoptosis in
breast cancer cells via p53, Bim and Bcl-2. J Cancer Res Clin
Oncol. 141:1575–1583. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Wang C, Chen YW, Zhang L, Gong XG, Zhou Y
and Shang DJ: Melanoma cell surface-expressed phosphatidylserine as
a therapeutic target for cationic anticancer peptide,
temporin-1CEa. J Drug Target. 24:548–556. 2016. View Article : Google Scholar
|
|
97
|
Cao XW, Yang XZ, Du X, Fu LY, Zhang TZ,
Shan HW, Zhao J and Wang FJ: Structure optimisation to improve the
delivery efficiency and cell selectivity of a tumour-targeting
cell-penetrating peptide. J Drug Target. 26:777–792. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Shull AY, Hu CA and Teng Y: Zebrafish as a
model to evaluate peptide-related cancer therapies. Amino Acids.
49:1907–1913. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Leite ML, da Cunha NB and Costa FF:
Antimicrobial peptides, nanotechnology, and natural metabolites as
novel approaches for cancer treatment. Pharmacol Ther. 183:160–176.
2018. View Article : Google Scholar
|
|
100
|
Sun T, Zhang YS, Pang B, Hyun DC, Yang M
and Xia Y: Engineered nanoparticles for drug delivery in cancer
therapy. Angew Chem Int Ed Engl. 53:12320–12364. 2014.PubMed/NCBI
|
|
101
|
Dossa F, Acuna SA, Rickles AS, Berho M,
Wexner SD, Quereshy FA, Baxter NN and Chadi SA: Association between
adjuvant chemotherapy and overall survival in patients with rectal
cancer and pathological complete response after neoadjuvant
chemotherapy and resection. JAMA Oncol. 4:930–937. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Xu J, Khan AR, Fu M, Wang R, Ji J and Zhai
G: Cell-penetrating peptide: A means of breaking through the
physiological barriers of different tissues and organs. J Control
Release. 309:106–124. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Duarte D, Fraga AG, Pedrosa J, Martel F
and Vale N: Increasing the potential of cell-penetrating peptides
for cancer therapy using a new pentagonal scaffold. Eur J
Pharmacol. 860:1725542019. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Park SE, Sajid MI, Parang K and Tiwari RK:
Cyclic cell-penetrating peptides as efficient intracellular drug
delivery tools. Mol Pharm. 16:3727–3743. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Copolovici DM, Langel K, Eriste E and
Langel U: Cell-penetrating peptides:Design, synthesis, and
applications. ACS Nano. 8:1972–1994. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Rothbard JB, Jessop TC, Lewis RS, Murray
BA and Wender PA: Role of membrane potential and hydrogen bonding
in the mechanism of translocation of guanidinium-rich peptides into
cells. J Am Chem Soc. 126:9506–9507. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Guidotti G, Brambilla L and Rossi D:
Cell-penetrating peptides: From basic research to clinics. Trends
Pharmacol Sci. 38:406–424. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Pujals S and Giralt E: Proline-rich,
amphipathic cell-penetrating peptides. Adv Drug Deliv Rev.
60:473–484. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Marks JR, Placone J, Hristova K and Wimley
WC: Spontaneous membrane-translocating peptides by orthogonal
high-throughput screening. J Am Chem Soc. 133:8995–9004. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Koren E and Torchilin VP: Cell-penetrating
peptides: Breaking through to the other side. Trends Mol Med.
18:385–393. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Kamei N, Onuki Y, Takayama K and
Takeda-Morishita M: Mechanistic study of the uptake/permeation of
cell-penetrating peptides across a caco-2 monolayer and their
stimulatory effect on epithelial insulin transport. J Pharm Sci.
102:3998–4008. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Chen Y, Xie X, Wu A, Wang L, Hu Y, Zhang H
and Li Y: A synthetic cell-penetrating peptide derived from nuclear
localization signal of EPS8 exerts anticancer activity against
acute myeloid leukemia. J Exp Clin Cancer Res. 37:122018.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Lyu L, Huang LQ, Huang T, Xiang W, Yuan JD
and Zhang CH: Cell-penetrating peptide conjugates of gambogic acid
enhance the antitumor effect on human bladder cancer EJ cells
through ROS-mediated apoptosis. Drug Des Devel Ther. 12:743–756.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Benergossi J, Calixto G, Fonseca-Santos B,
Aida KL, de Cassia Negrini T, Duque C, Gremiao MP and Chorilli M:
Highlights in peptide nanoparticle carriers intended to oral
diseases. Curr Top Med Chem. 15:345–355. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Meng F, Han N and Yeo Y: Organic
nanoparticle systems for spatiotemporal control of multimodal
chemotherapy. Expert Opin Drug Deliv. 14:427–446. 2017. View Article : Google Scholar :
|
|
116
|
Conibear AC, Schmid A, Kamalov M, Becker
CFW and Bello C: Recent advances in peptide-based approaches for
cancer treatment. Curr Med Chem. 27:1174–1205. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Asadzadeh Z, Mohammadi H, Safarzadeh E,
Hemmatzadeh M, Mahdian-Shakib A, Jadidi-Niaragh F, Azizi G and
Baradaran B: The paradox of Th17 cell functions in tumor immunity.
Cell Immunol. 322:15–25. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Darabi A, Thuring C and Paulsson KM: HLA-I
antigen presentation and tapasin influence immune responses against
malignant brain tumors-considerations for successful immunotherapy.
Anticancer Agents Med Chem. 14:1094–1100. 2014. View Article : Google Scholar
|
|
119
|
Li M, Shi HS, Zhang HL, Luo ZC, Wan Y, Lu
L, Luo ST and Yang L: bFGF peptide combined with the pVAX-8CpG
plasmid as adjuvant is a novel anticancer vaccine inducing
effective immune responses against Lewis lung carcinoma. Mol Med
Rep. 5:625–630. 2012. View Article : Google Scholar
|
|
120
|
Wang W, Li Y, Wang Y, Ren S, Li Y and Wang
B: Polyactin A is a novel and potent immunological adjuvant for
peptide-based cancer vaccine. Int Immunopharmacol. 54:95–102. 2018.
View Article : Google Scholar
|
|
121
|
Jin H, Wan C, Zou Z, Zhao G, Zhang L, Geng
Y, Chen T, Huang A, Jiang F, Feng JP, et al: Tumor ablation and
therapeutic immunity induction by an injectable peptide hydrogel.
ACS Nano. 12:3295–3310. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Kakwere H, Ingham ES, Allen R, Mahakian
LM, Tam SM, Zhang H, Silvestrini MT, Lewis JS and Ferrara KW:
Toward personalized peptide-based cancer nanovaccines: A facile and
versatile synthetic approach. Bioconjug Chem. 28:2756–2771. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Apostolopoulos V and McKenzie IF: Cellular
mucins: Targets for immunotherapy. Crit Rev Immunol. 14:293–309.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Obara W, Eto M, Mimata H, Kohri K,
Mitsuhata N, Miura I, Shuin T, Miki T, Koie T, Fujimoto H, et al: A
phase I/II study of cancer peptide vaccine S-288310 in patients
with advanced urothelial carcinoma of the bladder. Ann Oncol.
28:798–803. 2017. View Article : Google Scholar
|
|
125
|
Antonilli M, Rahimi H, Visconti V,
Napoletano C, Ruscito I, Zizzari IG, Caponnetto S, Barchiesi G,
Iadarola R, Pierelli L, et al: Triple peptide vaccination as
consolidation treatment in women affected by ovarian and breast
cancer: Clinical and immunological data of a phase I/II clinical
trial. Int J Oncol. 48:1369–1378. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Asahara S, Takeda K, Yamao K, Maguchi H
and Yamaue H: Phase I/II clinical trial using HLA-A24-restricted
peptide vaccine derived from KIF20A for patients with advanced
pancreatic cancer. J Transl Med. 11:2912013. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Rausch S, Gouttefangeas C, Hennenlotter J,
Laske K, Walter K, Feyerabend S, Chandran PA, Kruck S, Singh-Jasuja
H, Frick A, et al: Results of a Phase 1/2 Study in Metastatic Renal
Cell Carcinoma Patients Treated with a Patient-specific Adjuvant
Multi-peptide Vaccine after Resection of Metastases. Eur Urol
Focus. 5:604–607. 2019. View Article : Google Scholar
|
|
128
|
Dutoit V, Migliorini D, Ranzanici G,
Marinari E, Widmer V, Lobrinus JA, Momjian S, Costello J, Walker
PR, Okada H, et al: Antigenic expression and spontaneous immune
responses support the use of a selected peptide set from the IMA950
glio-blastoma vaccine for immunotherapy of grade II and III glioma.
Oncoimmunology. 7:e13919722018. View Article : Google Scholar
|
|
129
|
Lilleby W, Gaudernack G, Brunsvig PF,
Vlatkovic L, Schulz M, Mills K, Hole KH and Inderberg EM: Phase
I/IIa clinical trial of a novel hTERT peptide vaccine in men with
metastatic hormone-naive prostate cancer. Cancer Immunol
Immunother. 66:891–901. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Talebi S, Bolhassani A, Azad TM, Arashkia
A and Modaresi MH: Immuno-stimulating peptide derived from HMGB1 is
more effective than the N-terminal domain of Gp96 as an endogenous
adjuvant for improvement of protein vaccines. Protein Pept Lett.
24:190–196. 2017. View Article : Google Scholar
|
|
131
|
Glover S, Delaney M, Dematte C, Kornberg
L, Frasco M, Tran-Son-Tay R and Benya RV: Phosphorylation of focal
adhesion kinase tyrosine 397 critically mediates gastrin-releasing
peptide's morphogenic properties. J Cell Physiol. 199:77–88. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Schally AV, Zhang X, Cai R, Hare JM,
Granata R and Bartoli M: Actions and potential therapeutic
applications of growth hormone-releasing hormone agonists.
Endocrinology. 160:1600–1612. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Munoz-Moreno L, Bajo AM, Prieto JC and
Carmena MJ: Growth hormone-releasing hormone (GHRH) promotes
metastatic phenotypes through EGFR/HER2 transactivation in prostate
cancer cells. Mol Cell Endocrinol. 446:59–69. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Jimenez JJ, DelCanto GM, Popovics P, Perez
A, Vila Granda A, Vidaurre I, Cai RZ, Rick FG, Swords RT and
Schally AV: A new approach to the treatment of acute myeloid
leukaemia targeting the receptor for growth hormone-releasing
hormone. Br J Haematol. 181:476–485. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Chin YT, Wang LM, Hsieh MT, Shih YJ, Nana
AW, Changou CA, Yang YSH, Chiu HC, Fu E, Davis PJ, et al: Leptin
OB3 peptide suppresses leptin-induced signaling and progression in
ovarian cancer cells. J Biomed Sci. 24:512017. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Zhang M, Zhang M, Wang J, Cai Q, Zhao R,
Yu Y, Tai H, Zhang X and Xu C: Retro-inverso follicle-stimulating
hormone peptide-mediated polyethylenimine complexes for targeted
ovarian cancer gene therapy. Drug Deliv. 25:995–1003. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Sogaard CK, Moestue SA, Rye MB, Kim J,
Nepal A, Liabakk NB, Bachke S, Bathen TF, Otterlei M and Hill DK:
APIM-peptide targeting PCNA improves the efficacy of docetaxel
treatment in the TRAMP mouse model of prostate cancer. Oncotarget.
9:11752–11766. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Farokhzad OC and Langer R: Impact of
nanotechnology on drug delivery. ACS Nano. 3:16–20. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Liu X, Peng J, He J, Li Q, Zhou J, Liang X
and Tang S: Selection and identification of novel peptides
specifically targeting human cervical cancer. Amino Acids.
50:577–592. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Serrill JD, Wan X, Hau AM, Jang HS,
Coleman DJ, Indra AK, Alani AW, McPhail KL and Ishmael JE:
Coibamide A, a natural lariat depsipeptide, inhibits VEGFA/VEGFR2
expression and suppresses tumor growth in glioblastoma xenografts.
Invest New Drugs. 34:24–40. 2016. View Article : Google Scholar
|
|
141
|
Chakrabarti S, Guha S and Majumder K:
Food-derived bioac-tive peptides in human health: Challenges and
opportunities. Nutrients. 10:17382018. View Article : Google Scholar
|
|
142
|
Sable R, Parajuli P and Jois S: Peptides,
Peptidomimetics, and polypeptides from marine sources: A wealth of
natural sources for pharmaceutical applications. Mar Drugs.
15:1242017. View Article : Google Scholar :
|
|
143
|
O'Brien-Simpson NM, Hoffmann R, Chia CSB
and Wade JD: Editorial: Antimicrobial and Anticancer Peptides.
Front Chem. 6:132018. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Sultan S, Huma N, Butt MS, Aleem M and
Abbas M: Therapeutic potential of dairy bioactive peptides: A
contemporary perspective. Crit Rev Food Sci Nutr. 58:105–115. 2018.
View Article : Google Scholar
|
|
145
|
Felicio MR, Silva ON, Goncalves S, Santos
NC and Franco OL: Peptides with dual antimicrobial and anticancer
activities. Front Chem. 5:52017. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Mohanty DP, Mohapatra S, Misra S and Sahu
PS: Milk derived bioactive peptides and their impact on human
health-A review. Saudi J Biol Sci. 23:577–583. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Gonzalez-Montoya M, Hernandez-Ledesma B,
Silvan JM, Mora-Escobedo R and Martinez-Villaluenga C: Peptides
derived from in vitro gastrointestinal digestion of germinated
soybean proteins inhibit human colon cancer cells proliferation and
inflammation. Food Chem. 242:75–82. 2018. View Article : Google Scholar
|
|
148
|
Prateep A, Sumkhemthong S, Suksomtip M,
Chanvorachote P and Chaotham C: Peptides extracted from edible
mushroom: Lentinus squarrosulus induces apoptosis in human lung
cancer cells. Pharm Biol. 55:1792–1799. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Newman DJ and Cragg GM: Natural products
as sources of new drugs over the last 25 years. J Nat Prod.
70:461–477. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Fahs S, Patil-Sen Y and Snape TJ:
Foldamers as anticancer therapeutics: Targeting protein-protein
interactions and the cell membrane. Chembiochem. 16:1840–1853.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Dhar A, Mallick S, Ghosh P, Maiti A, Ahmed
I, Bhattacharya S, Mandal T, Manna A, Roy K, Singh S, et al:
Simultaneous inhibition of key growth pathways in melanoma cells
and tumor regression by a designed bidentate constrained helical
peptide. Biopolymers. 102:344–358. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Tanishiki N, Yano Y and Matsuzaki K:
Endowment of pH responsivity to anticancer peptides by introducing
2,3-diami-nopropionic acid residues. Chembiochem. 20:2109–2117.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Dennison SR, Harris F, Mura M and Phoenix
DA: An atlas of anionic antimicrobial peptides from amphibians.
Curr Protein Pept Sci. 19:823–838. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Khamessi O, Ben Mabrouk H, ElFessi-Magouri
R and Kharrat R: RK1, the first very short peptide from Buthus
occi-tanus tunetanus inhibits tumor cell migration, proliferation
and angiogenesis. Biochem Biophys Res Commun. 499:1–7. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Choi YJ, Park SJ, Park YS, Park HS, Yang
KM and Heo K: EpCAM peptide-primed dendritic cell vaccination
confers significant anti-tumor immunity in hepatocellular carcinoma
cells. PLoS One. 13:e01906382018. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Matsueda S, Itoh K and Shichijo S:
Antitumor activity of antibody against cytotoxic T lymphocyte
epitope peptide of lymphocyte-specific protein tyrosine kinase.
Cancer Sci. 109:611–617. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Peng X, Zhou C, Hou X, Liu Y, Wang Z, Peng
X, Zhang Z, Wang R and Kong D: Molecular characterization and
bioactivity evaluation of two novel bombinin peptides from the skin
secretion of Oriental fire-bellied toad, Bombina orientalis. Amino
Acids. 50:241–253. 2018. View Article : Google Scholar
|
|
158
|
Xie X, Zhou W, Hu Y, Chen Y, Zhang H and
Li Y: A dual-function epidermal growth factor receptor pathway
substrate 8 (Eps8)-derived peptide exhibits a potent cytotoxic T
lymphocyte-activating effect and a specific inhibitory activity.
Cell Death Dis. 9:3792018. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Wu ZZ, Ding GF, Huang FF, Yang ZS, Yu FM,
Tang YP, Jia YL, Zheng YY and Chen R: Anticancer activity of
anthopleura anjunae oligopeptides in prostate cancer DU-145 cells.
Mar Drugs. 16:pii: E125. 2018. View Article : Google Scholar
|
|
160
|
Shen Y, Maupetit J, Derreumaux P and
Tuffery P: Improved PEP-FOLD approach for peptide and miniprotein
structure prediction. J Chem Theory Comput. 10:4745–4758. 2014.
View Article : Google Scholar
|
|
161
|
Thevenet P, Shen Y, Maupetit J, Guyon F,
Derreumaux P and Tuffery P: PEP-FOLD: An updated de novo structure
prediction server for both linear and disulfide bonded cyclic
peptides. Nucleic Acids Res. 40:W288–W293. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Hao Y, Yang N, Teng D, Wang X, Mao R and
Wang J: A review of the design and modification of lactoferricins
and their derivatives. Biometals. 31:331–341. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Dathe M, Schumann M, Wieprecht T, Winkler
A, Beyermann M, Krause E, Matsuzaki K, Murase O and Bienert M:
Peptide helicity and membrane surface charge modulate the balance
of electrostatic and hydrophobic interactions with lipid bilayers
and biological membranes. Biochemistry. 35:12612–12622. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Sun S, Zhao G, Huang Y, Cai M, Yan Q, Wang
H and Chen Y: Enantiomeric effect of d-Amino acid substitution on
the mechanism of action of α-helical membrane-active peptides. Int
J Mol Sci. 19:672017. View Article : Google Scholar
|
|
165
|
Hicks RP: Antibacterial and anticancer
activity of a series of novel peptides incorporating cyclic
tetra-substituted C(α) amino acids. Bioorg Med Chem. 24:4056–4065.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Zhou J, Yang X, Zhang W, Wang J, Wei C, Gu
F, Lei T and Qin Y: Construction of an Anticancer Fusion Peptide
(ACFP) derived from milk proteins and an assay of anti-ovarian
cancer cells in vitro. Anticancer Agents Med Chem. 17:635–643.
2017. View Article : Google Scholar
|
|
167
|
Bracci L, Mandarini E, Brunetti J, Depau
L, Pini A, Terzuoli L, Scali S and Falciani C: The GAG-specific
branched peptide NT4 reduces angiogenesis and invasiveness of tumor
cells. PLoS One. 13:e01947442018. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Chu HL, Yip BS, Chen KH, Yu HY, Chih YH,
Cheng HT, Chou YT and Cheng JW: Novel antimicrobial peptides with
high anticancer activity and selectivity. PLoS One.
10:e01263902015. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
He B, Yang D, Qin M, Zhang Y, He B, Dai W,
Wang X, Zhang Q, Zhang H and Yin C: Increased cellular uptake of
peptide-modified PEGylated gold nanoparticles. Biochem Biophys Res
Commun. 494:339–345. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Tsuchiya N, Hosono A, Yoshikawa T, Shoda
K, Nosaka K, Shimomura M, Hara J, Nitani C, Manabe A, Yoshihara H,
et al: Phase I study of glypican-3-derived peptide vaccine therapy
for patients with refractory pediatric solid tumors.
Oncoimmunology. 7:e13778722017. View Article : Google Scholar
|
|
171
|
Liang AL, Qian HL, Zhang TT, Zhou N, Wang
HJ, Men XT, Qi W, Zhang PP, Fu M, Liang X, et al: Bifunctional
fused polypeptide inhibits the growth and metastasis of breast
cancer. Drug Des Devel Ther. 9:5671–5686. 2015.PubMed/NCBI
|
|
172
|
Herbert KJ, Ashton TM, Prevo R, Pirovano G
and Higgins GS: T-LAK cell-originated protein kinase (TOPK): An
emerging target for cancer-specific therapeutics. Cell Death Dis.
9:10892018. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Wu D, Gao Y, Qi Y, Chen L, Ma Y and Li Y:
Peptide-based cancer therapy: Opportunity and challenge. Cancer
Lett. 351:13–22. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Zachos I, Konstantinopoulos PA, Tzortzis
V, Gravas S, Karatzas A, Karamouzis MV, Melekos M and Papavassiliou
AG: Systemic therapy of metastatic bladder cancer in the molecular
era: Current status and future promise. Expert Opin Investig Drugs.
19:875–887. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Amir E, Hughes S, Blackhall F, Thatcher N,
Ostoros G, Timar J, Tovari J, Kovacs G and Dome B: Targeting blood
vessels for the treatment of non-small cell lung cancer. Curr
Cancer Drug Targets. 8:392–403. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Kim-Schulze S, Taback B and Kaufman HL:
Cytokine therapy for cancer. Surg Oncol Clin N Am. 16:793–818.
viii2007. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Niu F, Yan J, Ma B, Li S, Shao Y, He P,
Zhang W, He W, Ma PX and Lu W: Lanthanide-doped nanoparticles
conjugated with an anti-CD33 antibody and a p53-activating peptide
for acute myeloid leukemia therapy. Biomaterials. 167:132–142.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Vats K, Sharma R, Sarma HD, Satpati D and
Dash A: 68Ga-labeled HBED-CC variant of uPAR targeting
peptide AE105 compared with 68Ga-NODAGA-AE105. Anticancer Agents
Med Chem. 18:1289–1294. 2018. View Article : Google Scholar
|
|
179
|
Ahmadpour S, Noaparast Z, Abedi SM and
Hosseinimehr SJ: 99mTc-(tricine)-HYNIC-Lys-FROP peptide for breast
tumor targeting. Anticancer Agents Med Chem. 18:1295–1302. 2018.
View Article : Google Scholar
|
|
180
|
Zhang J, Spring H and Schwab M:
Neuroblastoma tumor cell-binding peptides identified through random
peptide phage display. Cancer Lett. 171:153–164. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Soudy R, Etayash H, Bahadorani K,
Lavasanifar A and Kaur K: Breast cancer targeting peptide binds
keratin 1: A new molecular marker for targeted drug delivery to
breast cancer. Mol Pharm. 14:593–604. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Du Y, Wang L, Wang W, Guo T, Zhang M,
Zhang P, Zhang Y, Wu K, Li A, Wang X, et al: Novel application of
cell penetrating R11 peptide for diagnosis of bladder cancer. J
Biomed Nanotechnol. 14:161–167. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Sato S, Nakamura T, Katagiri T, Tsuchikawa
T, Kushibiki T, Hontani K, Takahashi M, Inoko K, Takano H, Abe H,
et al: Molecular targeting of cell-permeable peptide inhibits
pancreatic ductal adeno-carcinoma cell proliferation. Oncotarget.
8:113662–113672. 2017. View Article : Google Scholar
|
|
184
|
Kang T, Huang Y, Zhu Q, Cheng H, Pei Y,
Feng J, Xu M, Jiang G, Song Q, Jiang T, et al: Necroptotic cancer
cells-mimicry nanovaccine boosts anti-tumor immunity with tailored
immune-stimulatory modality. Biomaterials. 164:80–97. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Garay H, Espinosa LA, Perera Y, Sanchez A,
Diago D, Perea SE, Besada V, Reyes O and Gonzalez LJ:
Characterization of low-abundance species in the active
pharmaceutical ingredient of CIGB-300: A clinical-grade anticancer
synthetic peptide. J Pept Sci. 24:e30812018. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Perea SE, Reyes O, Baladron I, Perera Y,
Farina H, Gil J, Rodriguez A, Bacardi D, Marcelo JL, Cosme K, et
al: CIGB-300, a novel proapoptotic peptide that impairs the CK2
phosphorylation and exhibits anticancer properties both in vitro
and in vivo. Mol Cell Biochem. 316:163–167. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Rodriguez-Ulloa A, Ramos Y, Gil J, Perera
Y, Castellanos-Serra L, Garcia Y, Betancourt L, Besada V, Gonzalez
LJ, Fernandez-de-Cossio J, et al: Proteomic profile regulated by
the anticancer peptide CIGB-300 in non-small cell lung cancer
(NSCLC) cells. J Proteome Res. 9:5473–5483. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Hirabayashi K, Yanagisawa R, Saito S,
Higuchi Y, Koya T, Sano K, Koido S, Okamoto M, Sugiyama H, Nakazawa
Y, et al: Feasibility and immune response of WT1 peptide
vaccination in combination with OK-432 for paediatric solid tumors.
Anticancer Res. 38:2227–2234. 2018.PubMed/NCBI
|
|
189
|
Yanagisawa R, Koizumi T, Koya T, Sano K,
Koido S, Nagai K, Kobayashi M, Okamoto M, Sugiyama H and Shimodaira
S: WT1-pulsed dendritic cell vaccine combined with chemotherapy for
resected pancreatic cancer in a phase I study. Anticancer Res.
38:2217–2225. 2018.PubMed/NCBI
|
|
190
|
Ohno S, Takano F, Ohta Y, Kyo S, Myojo S,
Dohi S, Sugiyama H, Ohta T and Inoue M: Frequency of myeloid
dendritic cells can predict the efficacy of Wilms' tumor 1 peptide
vaccination. Anticancer Res. 31:2447–2452. 2011.PubMed/NCBI
|
|
191
|
Ohno S, Kyo S, Myojo S, Dohi S, Ishizaki
J, Miyamoto K, Morita S, Sakamoto J, Enomoto T, Kimura T, et al:
Wilms' tumor 1 (WT1) peptide immunotherapy for gynecological
malignancy. Anticancer Res. 29:4779–4784. 2009.PubMed/NCBI
|
|
192
|
Ishikawa H, Imano M, Shiraishi O, Yasuda
A, Peng YF, Shinkai M, Yasuda T, Imamoto H and Shiozaki H: Phase I
clinical trial of vaccination with LY6K-derived peptide in patients
with advanced gastric cancer. Gastric Cancer. 17:173–180. 2014.
View Article : Google Scholar
|
|
193
|
Vasef MA, Ross JS and Cohen MB: Telomerase
activity in human solid tumors. Diagnostic utility and clinical
applications. Am J Clin Pathol. 112(1 Suppl): S68–S75.
1999.PubMed/NCBI
|
|
194
|
Bernhardt SL, Gjertsen MK, Trachsel S,
Moller M, Eriksen JA, Meo M, Buanes T and Gaudernack G: Telomerase
peptide vaccination of patients with non-resectable pancreatic
cancer: A dose escalating phase I/II study. Br J Cancer.
95:1474–1482. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Kokhaei P, Palma M, Hansson L, Osterborg
A, Mellstedt H and Choudhury A: Telomerase (hTERT 611-626) serves
as a tumor antigen in B-cell chronic lymphocytic leukemia and
generates spontaneously antileukemic, cytotoxic T cells. Exp
Hematol. 35:297–304. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Aspeslagh S, Awada A, Matos-Pita A,
Aftimos P, Bahleda R, Varga A and Soria JC: Phase I dose-escalation
study of plitidepsin in combination with bevacizumab in patients
with refractory solid tumors. Anticancer Drugs. 27:1021–1027. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Engel JB, Tinneberg HR, Rick FG, Berkes E
and Schally AV: Targeting of peptide cytotoxins to LHRH receptors
for treatment of cancer. Curr Drug Targets. 17:488–494. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Noguchi M, Matsumoto K, Uemura H, Arai G,
Eto M, Naito S, Ohyama C, Nasu Y, Tanaka M, Moriya F, et al: An
open-label, randomized phase II trial of personalized peptide
vaccination in patients with bladder cancer that progressed after
platinum-based chemotherapy. Clin Cancer Res. 22:54–60. 2016.
View Article : Google Scholar
|
|
199
|
Toh U, Saku S, Okabe M, Iwakuma N,
Kimitsuki Y, Akashi M, Ogo E, Yamada A, Shichijo S, Itoh K, et al:
Development of peptide vaccines for triple-negative breast cancer
treatment. Gan To Kagaku Ryoho. 43:1249–1251. 2016.In Japanese.
PubMed/NCBI
|
|
200
|
Brown TA, Byrd K, Vreeland TJ, Clifton GT,
Jackson DO, Hale DF, Herbert GS, Myers JW, Greene JM, Berry JS, et
al: Final analysis of a phase I/IIa trial of the folate-binding
protein-derived E39 peptide vaccine to prevent recurrence in
ovarian and endometrial cancer patients. Cancer Med. 8:4678–4687.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Schwartzentruber DJ, Lawson DH, Richards
JM, Conry RM, Miller DM, Treisman J, Gailani F, Riley L, Conlon K,
Pockaj B, et al: gp100 peptide vaccine and interleukin-2 in
patients with advanced melanoma. N Engl J Med. 364:2119–2127. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Mikecin AM, Walker LR, Kuna M and Raucher
D: Thermally targeted p21 peptide enhances bortezomib cytotoxicity
in androgen-independent prostate cancer cell lines. Anticancer
Drugs. 25:189–199. 2014. View Article : Google Scholar
|
|
203
|
Korani M, Korani S, Zendehdel E, Nikpoor
AR, Jaafari MR, Orafai HM, Johnston TP and Sahebkar A: Enhancing
the therapeutic efficacy of bortezomib in cancer therapy using
polymeric nanostructures. Curr Pharm Des. 25:4883–4892. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Zhou Y, Liu X, Xue J, Liu L, Liang T, Li
W, Yang X, Hou X and Fang H: Discovery of peptide boronate
derivatives as histone deacetylase and proteasome dual inhibitors
for overcoming bortezomib resistance of multiple myeloma. J Med
Chem. 63:4701–4715. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Garofalo M, Grazioso G, Cavalli A and
Sgrignani J: How computational chemistry and drug delivery
techniques can support the development of new anticancer drugs.
Molecules. 25:17562020. View Article : Google Scholar :
|
|
206
|
Adlakha S, Sharma A, Vaghasiya K, Ray E
and Verma RK: Inhalation delivery of host defence peptides (HDP)
using nano-formulation strategies: A pragmatic approach for therapy
of pulmonary ailments. Curr Protein Pept Sci. 21:369–378. 2020.
View Article : Google Scholar
|
|
207
|
Tu Y, Tao J, Wang F, Liu P, Han Z, Li Z,
Ma Y and Gu Y: A novel peptide targeting gastrin releasing peptide
receptor for pancreatic neoplasm detection. Biomater Sci.
8:2682–2693. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Ohana J, Sandler U, Kass G, Stemmer SM and
Devary Y: dTCApFs, a derivative of a novel human hormone peptide,
induces apoptosis in cancer cells through a mechanism involving
loss of Golgi function. Mol Clin Oncol. 7:991–999. 2017.PubMed/NCBI
|
|
209
|
Stemmer SM, Benjaminov O, Silverman MH,
Sandler U, Purim O, Sender N, Meir C, Oren-Apoteker P, Ohana J and
Devary Y: A phase I clinical trial of dTCApFs, a derivative of a
novel human hormone peptide, for the treatment of
advanced/metastatic solid tumors. Mol Clin Oncol. 8:22–29.
2018.PubMed/NCBI
|
|
210
|
Murali R and Kieber-Emmons T: Cancer
immunotherapeutics: Evolution of monoclonal antibodies to peptide
immunogens. Monoclon Antib Immunodiagn Immunother. 33:179–182.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Tan Z and Zhang S: Past, present, and
future of targeting ras for cancer therapies. Mini Rev Med Chem.
16:345–357. 2016. View Article : Google Scholar
|
|
212
|
Li QX, Yu DH, Liu G, Ke N, McKelvy J and
Wong-Staal F: Selective anticancer strategies via intervention of
the death pathways relevant to cell transformation. Cell Death
Differ. 15:1197–1210. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
213
|
Micale N, Scarbaci K, Troiano V, Ettari R,
Grasso S and Zappala M: Peptide-based proteasome inhibitors in
anticancer drug design. Med Res Rev. 34:1001–1069. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
214
|
Wang X, Chen X, Yang X, Gao W, He B, Dai
W, Zhang H, Wang X, Wang J, Zhang X, et al: A nanomedicine based
combination therapy based on QLPVM peptide functionalized liposomal
tamoxifen and doxorubicin against Luminal A breast cancer.
Nanomedicine. 12:387–397. 2016. View Article : Google Scholar
|
|
215
|
Cheng T and Zhan X: Pattern recognition
for predictive, preventive, and personalized medicine in cancer.
EPMA J. 8:51–60. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
216
|
Vadevoo SMP, Gurung S, Khan F, Haque ME,
Gunassekaran GR, Chi L, Permpoon U and Lee B: Peptide-based
targeted therapeutics and apoptosis imaging probes for cancer
therapy. Arch Pharm Res. 42:150–158. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
217
|
Sun X, Li Y, Liu T, Li Z, Zhang X and Chen
X: Peptide-based imaging agents for cancer detection. Adv Drug
Deliv Rev. 110-111:38–51. 2017. View Article : Google Scholar :
|
|
218
|
Ehlerding EB, Sun L, Lan X, Zeng D and Cai
W: Dual-targeted molecular imaging of cancer. J Nucl Med.
59:390–395. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
219
|
Wang Q, Li SB, Zhao YY, Dai DN, Du H, Lin
YZ, Ye JC, Zhao J, Xiao W, Mei Y, et al: Identification of a sodium
pump Na(+)/K(+) ATPase alpha1-targeted peptide for PET imaging of
breast cancer. J Control Release. 281:178–188. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
220
|
Perez SA, von Hofe E, Kallinteris NL,
Gritzapis AD, Peoples GE, Papamichail M and Baxevanis CN: A new era
in anticancer peptide vaccines. Cancer. 116:2071–2080.
2010.PubMed/NCBI
|