Introduction
Cervical cancer is the fourth most common cancer in
women and the fourth leading cause of cancer-associated mortality
in women (with incidence and mortality rates of 6.6 and 7.5%
considering the 10 most common cancers in women in 2018,
respectively), with an estimated 570,000 cases and 311,000 deaths
worldwide in 2018 (1). Moreover,
in regions of the world with low (e.g. Niger, Chad, Sierra Leone,
Gambia and Nigeria) and medium (Paraguay, Egypt, Vietnam, El
Salvador, Nicaragua, Zambia and Pakistan) human development index,
cervical cancer has the second highest incidence (18.2%) and
mortality (12%) rates, behind breast cancer (1). In the USA 13,800 new cases and 4,290
cervical cancer-associated deaths have been estimated for 2020, and
the probability, from birth to death, of developing invasive
cervical cancer from 2014 to 2016 was of 0.6% (2). Since the mid-1970s cancer survival
has improved in the USA for all of the most common cancers (e.g.
lung and bronchus, colon and rectum, breast, prostate, oral cavity
and pharynx, esophagus, stomach, pancreas, liver, kidney, urinary
bladder, melanoma, ovary and thyroid), except those of the uterine
cervix and uterine corpus (3). In
cervical cancer the 5-year relative survival rate for all races,
according to the stage at diagnosis, was 92%, 56% and 17%, for
localized, regional and distant lesions, respectively (2). Moreover, cervical cancer remains the
second leading cause of cancer-associated death in women aged 20 to
39 years in the USA (2).
Infection with a subtype of the human
papillomavirus, known as high-risk (HR), is the most common cause
of cervical cancer (4). However,
not all women infected with HR-HPV will develop cervical cancer, as
this disease develops over 2 decades following exposure to these
viruses and might require additional contributing factors,
including tobacco smoke (5),
parity (6,7), estrogens (8), oral contraceptives (9), immune deficiencies, such as human
immunodeficiency virus-seropositive women (10), genetic polymorphisms (11), epigenetic regulation of
costimulatory factors Tim-3 and galectin-9 (12) and methylation sensitivity of the
enhancer from HPV16 (13). Dietary
factors also appear to play a role, as a diet rich in plant-based
nutrients, which increases dietary fiber, vitamins -C, -E, -A, α-
and β-carotenes and lutein, has been found to reduce the risk of
cervical cancer (14,15).
HPVs are DNA viruses containing two genes, which are
expressed early named E6 and E7, which in the high-risk viral types
encode proteins that promote cervical carcinogenesis (16). E6 and E7 oncoproteins associate
with and inactivate the tumor suppressor proteins p53 (17) and retinoblastoma (Rb) (18), respectively. Employing chemical
carcinogens in the K14E7 transgenic mouse, E7 was found to promote
the formation of benign tumors in the skin, while in the transgenic
mice K14E6 treated with these carcinogens, E6 accelerate the
progression of the benign tumors to the malignant state (16). However, in the cervix, E7 increased
cell proliferation and generated micro-invasive cervical cancers,
while E6 did not produce neoplasia or cancer even following
17β-estradiol (E2) treatment, which is a contributing
factor for cervical cancer, and was delivered in continuous release
pellets implanted in the dorsal skin (8,19).
The co-expression of E6 and E7 in double transgenic mice
(K14-E6:E7) revealed that E6 modulated the malignant phenotype
produced by E7, causing micro-invasive cervical cancers to be
dispersed over a larger area of cervical stromal tissue (19). E2 treatment is required
not only for the genesis of cervical cancer (20,21)
but also for its persistence and continued development (22).
The disassembly of the TJ, which is a cell-cell
adhesion structure characteristic of epithelial cells, constitutes
one of the earliest steps in cell transformation (23,24).
However, its role in the development of cervical cancer has not
been elucidated, therefore further investigation was performed in
the present study. TJs are comprised of a complex group of
molecules, including the peripheral proteins, cingulin, ZO-1, -2
and -3 and the integral proteins, claudins, occludin and JAMs.
While numerous TJ proteins are downregulated during cell
transformation (e.g. ZO-1, -2, and -3, occludin, JAM-A and MUPP1),
others are overexpressed (e.g. claudins -2, -3 and -4), even though
they are no longer present at the cell borders (23). With respect to claudins, there are
differences in expression levels of the protein in different
tissues and different types of cancer. For example, in lung
squamous cell carcinomas claudins -1, -2, -4 and -7 are expressed,
while claudins -2, -3, -4, -5 and -7 are expressed in lung
adenocarcinomas, and claudin-4 was found to be expressed in lung
large cell carcinomas express. Claudin-1 is expressed in esophageal
squamous cell carcinomas, while in the adenocarcinoma form of the
disease claudins -2, -3, -4, and -7 are expressed. Claudins -1, -4,
-5 and -7 are expressed in the squamous cell carcinomas found in
the oral cavity, while claudin-1 is expressed in the low
mucoepidermoid carcinoma, and claudin-3 is expressed in the high
mucoepidermoid carcinoma (23).
Therefore the expression of claudins is a useful molecular tool for
distinguishing between different types of tumors and for the
prediction of patient survival rates. For example, claudin-6 is the
most distinctive molecular marker of atypical teratoid/rhabdoid
tumors, which are highly aggressive malignant central nervous
system tumors of children, in comparison to other brain tumors,
such as choroid plexus papilloma, ependymoma, large-cell
medulloblastoma, classic medulloblastoma and pediatric glioblastoma
(25).
In our previous study claudin-4 expression was found
to increase in the cervix of K14E7 transgenic mice compared with
that in non-transgenic mice, particularly in the presence of
E2 (26). In the
present study the impact of the E7 onco-protein on the expression
levels of claudins -1 and -10 in the murine cervix was
investigated, as the expression of claudin-1 has been found to
decrease in breast carcinomas, stage II colon cancer, lymph node
metastasis in colon carcinomas, colon mucinous carcinomas, lung
adenocarcinomas, prostate adenocarcinomas, pancreatic endocrine
tumors, gastric cancer, type I endometrial carcinomas,
hepatocellular carcinomas, kidney clear cell carcinomas, papillary
urothelial neoplasms of low malignant potential and low grade
urothelial cell carcinomas, thyroid undifferentiated carcinomas and
thyroid medullary carcinomas, while it is overexpressed in
colorectal cancer, in the early phase of carcinogenesis in cervical
intraepithelial lesions, esophageal squamous cell carcinoma,
squamous cell carcinoma of the tongue, low grade mucoepidermoid
carcinoma of minor salivary glands, and in melanoma (23). With regards to claudin-10, its
expression was reduced in the biliary tract, breast carcinomas and
in lung adenocarcinomas of the invasive type (23,24);
however, it was increased in chicken ovarian cancer (27), hepatocellular carcinoma (28) and papillary thyroid cancer
(29). To further confirm the
results, the impact of the stable expression of the E7 oncoprotein
in Madin-Darby Canine Kidney (MDCK) cells was also investigated,
including monolayer morphology, transepithelial electrical
resistance (TER), claudin expression, invasive properties and cell
stiffness. The results indicate that E7 perturbs the expression
pattern of claudins and the degree of sealing of TJs in the cervix,
and in MDCK cells, and also alters the cytoarchitecture of the
cervix and MDCK monolayer, induces migration of MDCK cells in
three-dimensional (3D) cultures and triggers the stiffening of
their apical membrane.
Materials and methods
Mouse model and hormone treatment
The K14E7 trans-genic mice were a gift of Dr Paul F.
Lambert (Department of Oncology, McArdle Laboratory for Cancer
Research, University of Wisconsin, Madison, WI, USA) and were
backcrossed in the FvB background, maintained and used as
heterozygotes in further experiments. The FvB mice, used as the
control group, were obtained from the animal facility of the Center
for Research and Advanced Studies, Mexico. FvB and K14E7 transgenic
female mice were housed in cages with double air filtration (HEPA
level 99% of particles) and maintained at 20-22°C, under a 12 h
dark-light cycles with food and water ad libitum. Animals
were sacrificed by cervical dislocation at 2-, 4- and 7-months of
age. Hormone treatment was performed with continuous release
pellets delivering 0.05 mg of E2 over 60 days (cat. no.
SE-121; Innovative Research of America). Pellets were implanted in
the dorsal skin of 1-month-old virgin female transgenic and
non-transgenic mice. Mice sacrificed at 4-months of age received
two E2 pellets: one at the first month of age and the
other at the 3rd month of age; whereas mice sacrificed at 7-months
of age received an additional third pellet at the 5th month of age.
A total of 42 mice were used in the study: 5 FvB mice, and 3 K14E7
at each age (2-, 4- and 7-months of age) were analyzed and 3 FvB
and K14E7 mice treated with E2 were also used for each
age group (2-, 4- and 7-months of age). The weight of the FvB and
K14E7 mice was similar to what had been previously reported (20, 26
and 30, and 18, 23 and 26 g for 2-, 4- and 7-month-old FvB and
K14E7 mice, respectively) (30).
Cell culture
Epithelial MDCK cells were obtained from the
American Type Culture Collection (cat. no. CCL-34). Cells between
the 60th and 90th passage were grown at 36.5°C in disposable
plastic bottles in a humidified atmosphere with 5% CO2
in DMEM (cat. no. 12800-082; Gibco; Thermo Fisher Scientific, Inc.)
with 100 U/ml penicillin-streptomycin (cat. no. A-01; In Vitro,
S.A; www.invitro.com.mx) and 10% fetal bovine
serum (cat. no. S1650-500; Biowest). Cells were harvested with
trypsin-EDTA (cat. no. EN-005; In Vitro). Mycoplasma testing of
parental and MDCK-E7 cells was performed using a mycoplasma
detection kit for conventional PCR (cat. no. 11-8005;
Vector® Gem OneStep; Minerva Biolabs GmbH) according to
the manufacturer’s instructions.
Immunofluorescence
Reproductive female tracts containing the vagina,
cervix and both uterine horns were removed from the FvB and K14E7
mice treated with or without E2. The tissue was then
immersed in tissue freezing mounting media (cat. no. 14020108926,
Tissue Tek; Leica Microsystems GmbH) and incubated at -70°C for 24
h. Next, 5-7-µm sections were cut using a Leica MC1510
cryostat (Leica Microsystems GmbH) and mounted on pre-cooled
(-20°C) gelatin-coated slides. The slides were then incubated at
-70°C for 24 h. Subsequently, the sections were fixed with 100%
ethanol at -20°C for 20 min and the remaining protocol was
performed as previously described (31). Using a confocal microscope (Leica
TG5 SP8; Leica Microsystems GmbH) with a ×63 objective, images were
captured of the squamous multilayered epithelium of the exocervix
only, where the basal epithelial cells are the site of infection of
HPV in women (32).
Immunofluorescence of claudins in parental and MDCK-E7 cells at the
TER peak (18 h) following the Ca-switch, was performed following a
previously described protocol (31).
The following primary antibodies were used for the
immunofluorescence experiments in both mouse cervix and MDCK cells:
rabbit antibodies against claudin-1 (cat. no. 51-9000, dilution
1:100) and claudin-10 (cat. no. 38-8400; dilution 1:100) (both
Invitrogen; Thermo Fisher Scientific, Inc.). In addition, in MDCK
cells the following mouse monoclonal primary anti-bodies were used:
against E7 protein of HPV16 (cat. no. ab30731; dilution 1:100;
Abcam), claudin-2 (cat. no. sc-293233; dilution 1:100; Santa Cruz
Biotechnology Inc.) and claudin-4 (cat. no. 32-9400; dilution
1:100; Invitrogen; Thermo Fisher Scientific, Inc.). The following
secondary antibodies were used: donkey anti-rabbit IgG coupled to
Alexa-Fluor 488 (cat. no. A21206; dilution 1:500), donkey
anti-mouse coupled to Alexa-Fluor 594 (cat. no. A21203; dilution
1:1,000), or donkey anti-rabbit coupled to Alexa-Fluor 594 (cat.
no. A21207; dilution 1:1,000) (all Invitrogen; Thermo Fisher
Scientific, Inc.). In parental MDCK and MDCK-E7 cells actin was
detected using rhodaminated phalloidin (cat. no. P1951; dilution
1:50; Sigma-Aldrich; Merck KGaA). Slides were mounted with
Vectashield with DAPI (cat. no. H1200; Vector Laboratories, Inc;
Maravai Life Science).
All the antibodies used reacted with mouse and dog
tissues according to the manufacturer's instructions. The two
exceptions are the monoclonal antibody against claudin-2 and the
anti-claudin-10 polyclonal antibody, in which no details were
provided regarding the reactivity with dog tissue. However, the
monoclonal antibody against claudin-2, was raised against amino
acids 29-80 of the human claudin-2 protein, and a blast sequence
analysis revealed a 96% identity and 98% similarity between human
and dog claudin-2 (data not shown). The poly-clonal antibody
against claudin-10 was successfully used in MDCK cells in a
previous study (33).
Relative mean fluorescence intensity
measurements
The relative mean fluorescence intensity
measurements of claudins -1 and -10 in the cervix of the FvB and
K14E7 mice treated with or without E2, and of claudins
-1, -2, -4 and -10 in MDCK cells were obtained using ImageJ
software (v1.52n, National Institutes of Health) using the freehand
function. The integrated density feature of ImageJ was used to
record pixel intensities per area. Data were derived from three
images of the cervix for all the experimental groups: FvB and K14E7
mice treated with or without E2 at 2-, 4- and 7-months
of age.
Transmission electron microscopy
(TEM)
The reproductive female tracts containing the
vagina, cervix and both uterine horns were removed from 2- and
7-month-old female FvB and transgenic K14E7 mice treated with or
without E2. Subsequently 1-mm width discs from the
middle of the exocervix were excised. Samples were fixed at room
temperature with 2.5% (v/v) glutaraldehyde in 0.1 M sodium
cacodylate buffer, pH 7.2, for 60 min. Samples were then treated at
room temperature for 60 min with a solution of 1% (w/v) osmium
tetroxide in 0.1 M sodium cacodylate buffer, containing 0.5 mg/ml
ruthenium red. Following dehydration with increasing concentrations
of ethanol and propylene oxide, samples were embedded in Polybed
epoxy resins and polymerized at 60°C for 24 h. Thin sections
(60-nm) were stained at room temperature for 20 min with uranyl
acetate and subsequently for 2 min with lead citrate prior to
examination at a magnification of ×20,000, using a Jeol JEM-1011
transmission electron microscope.
The permeability of the TJ present in the
superficial cells of the multi-stratified epithelium of the cervix
of mice was evaluated using the paracellular pathway marker
ruthenium red, as previously reported (34,35).
Thus, if the ruthenium red stain only highlighted the apical
surface of the superficial cells in the cervix, this indicated that
passage through the paracellular pathway was blocked due to TJ
sealing. Instead, if the staining was present along the
paracellular pathway, below the TJ region, in the superficial cells
of cervix, the TJ was opened.
Stable transfection of MDCK cells with E7
oncoprotein
MDCK cells at 70% confluence were transfected with 3
µg of pcDNA3E7 plasmid as previously described (36) using Lipofectamine® 2000
(cat. no. 11668019; Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. To generate the
stable MDCK-E7 clones, the cells were harvested using
trypsinization and plated at a density of 3×104
cells/cm2 on 100-mm culture dishes with DMEM containing
800 µg/ml geneticin (G418; cat. no. 11811-031; Gibco; Thermo
Fisher Scientific, Inc.), 48 h following transfection. Resistant
clones were selected and re-cloned manually using the cloning ring
technique, after 2-3 weeks (37).
Stable colonies were maintained in the presence of 200 µg/ml
G418.
PCR amplification of E7
Total RNA was extracted from parental and E7 MDCK
cells using TRIzol® reagent (cat. no. 15596026;
Invitrogen; Thermo Fisher Scientific, Inc.) following the
manufacturer's instructions. RNA purity and concentration were
measured using a Nanodrop™ ND-8000 spectrophotometer (cat. no.
ND-8000-GL; Thermo Fisher Scientific, Inc.) and its integrity was
determined using electrophoresis in 1% agarose gels, by resolving
the 28S and 18S ribosomal RNA bands. cDNA was synthesized from RNA
using SuperScript One-Step reverse transcription (RT)-PCR with
platinum Taq DNA polymerase (cat. no. 10928042; Invitrogen; Thermo
Fisher Scientific, Inc.), following the manufacturer's instructions
and a Biometra GmbH Professional Basic Gradient thermocycler (cat.
no. 070-601; Analytik, Jena US LLC). The PCR for HPV16 E7 was
performed in a final 25 µl volume containing: 1 µg
template RNA, 0.2 µM forward and reverse primers for HPV16
E7, 1U RT/Platinum Taq mix, 1.2 mM MgCl2 and 200
µM dNTPs. The primers were designed according to E7 gene
sequence obtained from GenBank (accession no. AF125673; www.ncbi.nlm.nih.gov/nuccore/4927719);
and had the following sequence: Forward,
5′-CTCAGAGGAGGAGGATGAAATAG-3′; and reverse,
5′-CTGAGAACAGATGGGGCACAC-3′. cDNA synthesis was performed at 50°C
for 30 min and at 94°C for 2 min. The following thermocycling
conditions were used: Initial denaturation at 94°C for 15 sec
followed by 25 cycles of 94°C for 1 min, 60°C for 30 sec and 72°C
for 1 min. The PCR products were separated on 1.5% agarose gels and
images were obtained following ethidium bromide staining. The
expected amplicon size was 196 bp.
Western blot analysis
Western blots of lysates from the cervix of FvB and
K14E7 mice treated with or without E2, of 2-, 4- and
7-months of age and of parental and MDCK-E7 cells at the TER peak
(18 h) following the Ca-switch, was performed following standard
procedures, as previously described (31). The following primary antibodies
were used: rabbit polyclonal anti-claudin-1 (cat. no. 51-9000;
dilution 1:500; Invitrogen; Thermo Fisher Scientific, Inc.),
claudin-10 (cat. no. 38-8400; dilution, 1:500; Invitrogen, Thermo
Fisher Scientific, Inc.) and GAPDH (cat. no. RPCA-GAPDH; dilution
1:40,000; Encor Biotechnology Inc.). In addition, for MDCK cells
the following primary antibodies were used: rabbit polyclonal
anti-claudin-2 (cat. no. 51-6100, dilution 1:1,000; Invitrogen;
Thermo Fisher Scientific, Inc.), and claudin-4 (cat. no. 36-4800;
dilution 1:500; Invitrogen; Thermo Fisher Scientific, Inc.), and a
mouse monoclonal against actin (generated and provided by Dr Manuel
Hernández, Department of Cell Biology, Center for Research and
Advanced Studies, Mexico city, Mexico). The following secondary
antibodies were used: goat anti-rabbit IgG conjugated to
horseradish peroxidase (HRP; cat. no. 6111620; dilution 1:20,000;
Invitrogen; Thermo Fisher Scientific, Inc.), or goat anti-mouse IgG
conjugated to HRP (cat. no. 626520; dilution 1:10,000; Invitrogen;
Thermo Fisher Scientific, Inc.). The proteins were visualized using
a Immobilon chemiluminescence detection (cat. no. WVKLS 0500; EMD
Millipore).
All the antibodies used react with mouse and dog
tissues according the manufacturer's instructions. The only
exception was the polyclonal anti-claudin-10 antibody, in which
there was no information regarding its reactivity on dog tissue.
However, this antibody was successfully used in MDCK cells in a
previous study (33).
TER
Parental and MDCK-E7 cells were cultured at a
density of 1.2×105 cells/cm2 on 1.12
cm2 Transwell poly-ester membrane clear inserts (cat.
no. 3460; pore size 0.4 µm; Corning Inc.). Confluent
monolayers of control and MDCK-E7 cells were transferred from
normal calcium (NC; 1.8 mM Ca2+; cat. no. 12800-082;
Gibco; Thermo Fisher Scientific Inc.) containing media to low
calcium (LC; 1-5 µM Ca2+; cat. no. D9800-10.10;
United States Biological) media for 22 h. Subsequently, the
monolayers with no TER (time 0) were changed to NC media
(Ca-switch) to trigger TJ formation and TER development. TER was
measured continuously and without interruptions for 63 h from each
insert using an automated cell monitoring system (cellZscope
version CZS0101909; nanoAnalytics GmbH). TER values were obtained
using the CellZscope software (version 2.2.0.16827; nanoAnalytics
GmbH). Statistical analysis using the Student's t-test was used to
investigate the TER observed in parental and MDCK-E7 cells at the
peak of resistance obtained at 18 h.
Cell migration assay in a 3D matrix
MDCK and MDCK-E7 cells were plated at a density of
2.4×105 cells/cm2 on 12-well inserts
containing Alvetex® 3-dimentional polystyrene scaffold
(cat. no. AVP005-12; Reinnervate Ltd.) precoated at room
temperature for 2 h with basement membrane matrix BD Matrigel™
(cat. no. 356230, BD Biosciences) at a concentration of 0.8 mg/ml,
according to the manufacturer's instructions. Every second day DMEM
media with 10% fetal calf serum was changed from the upper and
lower chambers. The migration of MDCK and MDCK-E7 cells was
evaluated using confocal immunofluorescence in 10-day-old cultures
where the cells were stained with rhodaminated phalloidin (cat. no.
P1951; Sigma-Aldrich; Merck KGaA) for 2 h at room temperature, and
mounted with Vectashield (cat. no. H-1200; Vector Laboratories;
Maravai LifeSciences) antifade mounting medium containing DAPI,
which fluoresces blue when bound to DNA. Images were captured using
a confocal microscope (Leica TG5 SP8) with a ×63 objective in the z
plane using LAS AF X software (version 3.0; Leica Microsystems
GmbH). A depth coded z series image was generated from red to blue,
where warmer colors (red) correspond to cells that have not
migrated to the bottom of the scaffold and are still located on the
surface, whereas colder colors (blue) represent the cells that have
migrated deeper in the scaffold.
Atomic force microscopy
The nanomechanical properties of the apical surface
of parental and MDCK-E7 cells present as either isolated cells or
as islands of cells was analyzed using Atomic force microscopy. The
cells were plated at a density of 1.5×104
cells/cm2 on ultra flat silicon wafers placed in 12-well
plates and incubated at 36.5°C in a humidified incubator with 5%
CO2 with DMEM (cat. no. 12800-082; Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% fetal bovine serum (cat.
no. S1650-500; Biowest). The cells were fixed for 10 min with 4%
paraformaldehyde at room temperature, after 8 h. All measurements
were performed using a Solver Next Atomic Force Microscope from
NT-MDT Spectrum Instruments. Elastic Modulus were measured based on
the deviation force lateral (DFL) curves (displacement of
cantilever vs. distance). A cantilever contact type CSG10, was used
with a curvature radius of 10 nm. Scanning conditions for the cells
were as follows: A ramp size set to 80 µm for islets and 50
µm for isolated cells, with a gain of 0.18 and a rate of 0.3
Hz with 256 points. Experimental data were analyzed using the Image
Analysis Software (v3.5; NT-MDT Spectrum Instruments) to determine
Young’s modulus in kPa.
Statistical analysis
The statistical analysis between multiple groups in
different experimental conditions (Figs. 2B and C, and 4B and C) was performed using three-way
ANOVA followed by Tukey's post hoc test. The statistical analysis
between 2 groups (Figs. 3B,
5B, 8A, C and D, and 9B) was performed with Student's t-test.
Statistical analysis was performed using Prism GraphPad v6
(GraphPad Software Inc.). Data are presented as mean ± standard
deviation. Number of repeats are indicated in the legend of each
figure.
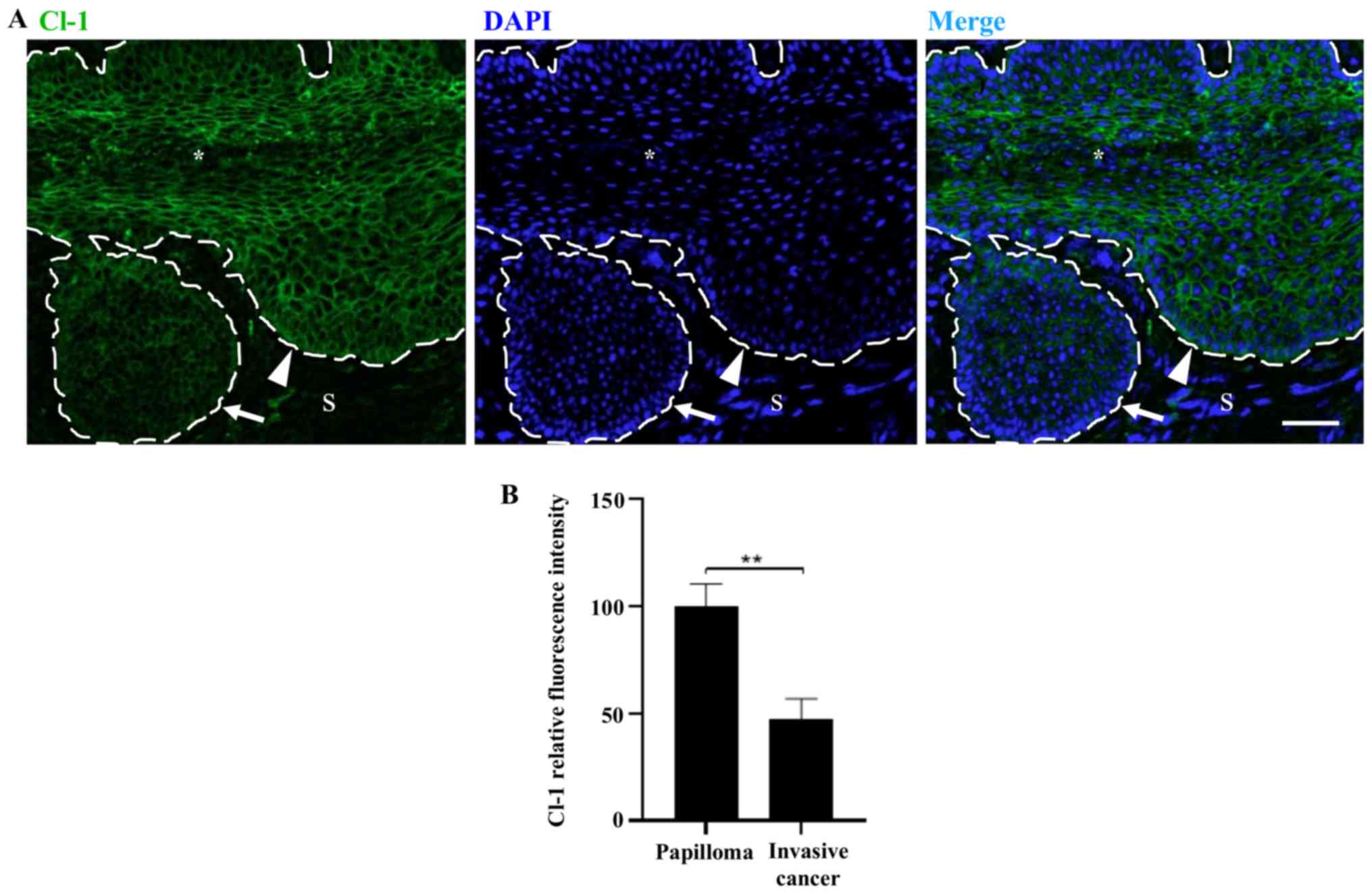
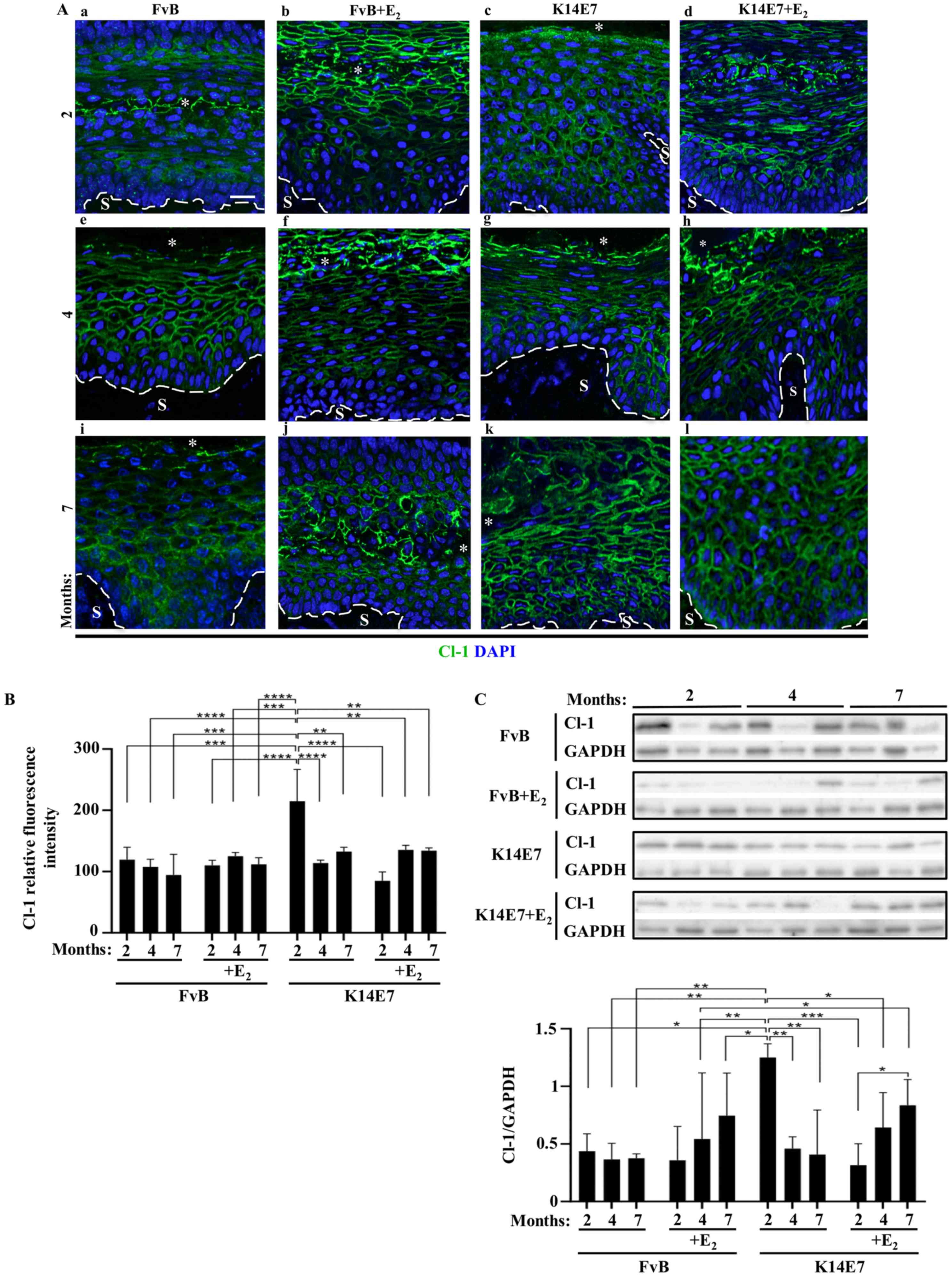 | Figure 2Oncogenic protein E7 augments the
expression of Cl-1 in the cervix of 2-month-old mice, while
treatment with E2 abolishes this effect. Expression of
Cl-1 from frozen sections of the cervix obtained from (A-a-d) 2-,
(A-e-h) 4- or (Ai-l) 7-month-old (A-a, -e and -i) control FvB
without E2 or (A-b, -f and -j) with E2 and
transgenic K14E7 mice, treated (A-d, -h and -l) with E2
or (A-c, -g and -k) without E2, using
immunofluorescence. Nuclei were stained with DAPI. Dashed white
line indicates the border between the epithelial basal cell layer
and the S. *Cervical lumen. Scale bar, 20 µm. (B)
Quantitative analysis of the mean fluorescence intensity of Cl-1
observed in frozen sections of the cervix obtained from 2-, 4- or
7-month-old control FvB and transgenic K14E7 mice, treated with or
without E2. Results obtained from three independent
images from each experimental group. Statistical analysis performed
using three-way ANOVA followed by Tukey's post hoc test. Data are
presented as mean ± standard deviation. **P<0.01,
***P<0.001, ****P<0.001. (C)
Representative western blot of Cl-1 in cervix lysates obtained from
2-, 4- or 7-month-old control FvB and transgenic K14E7 mice,
treated with or without E2 (upper panel). Densitometry
analysis from at least three independent experiments (lower panel).
Statistical analysis was performed using three-way ANOVA followed
by Tukey's post hoc test. Data are presented as mean ± standard
deviation. *P<0.05, **P<0.01,
***P<0.001. S, Stroma; Cl, claudin; E2,
17β-estradiol. |
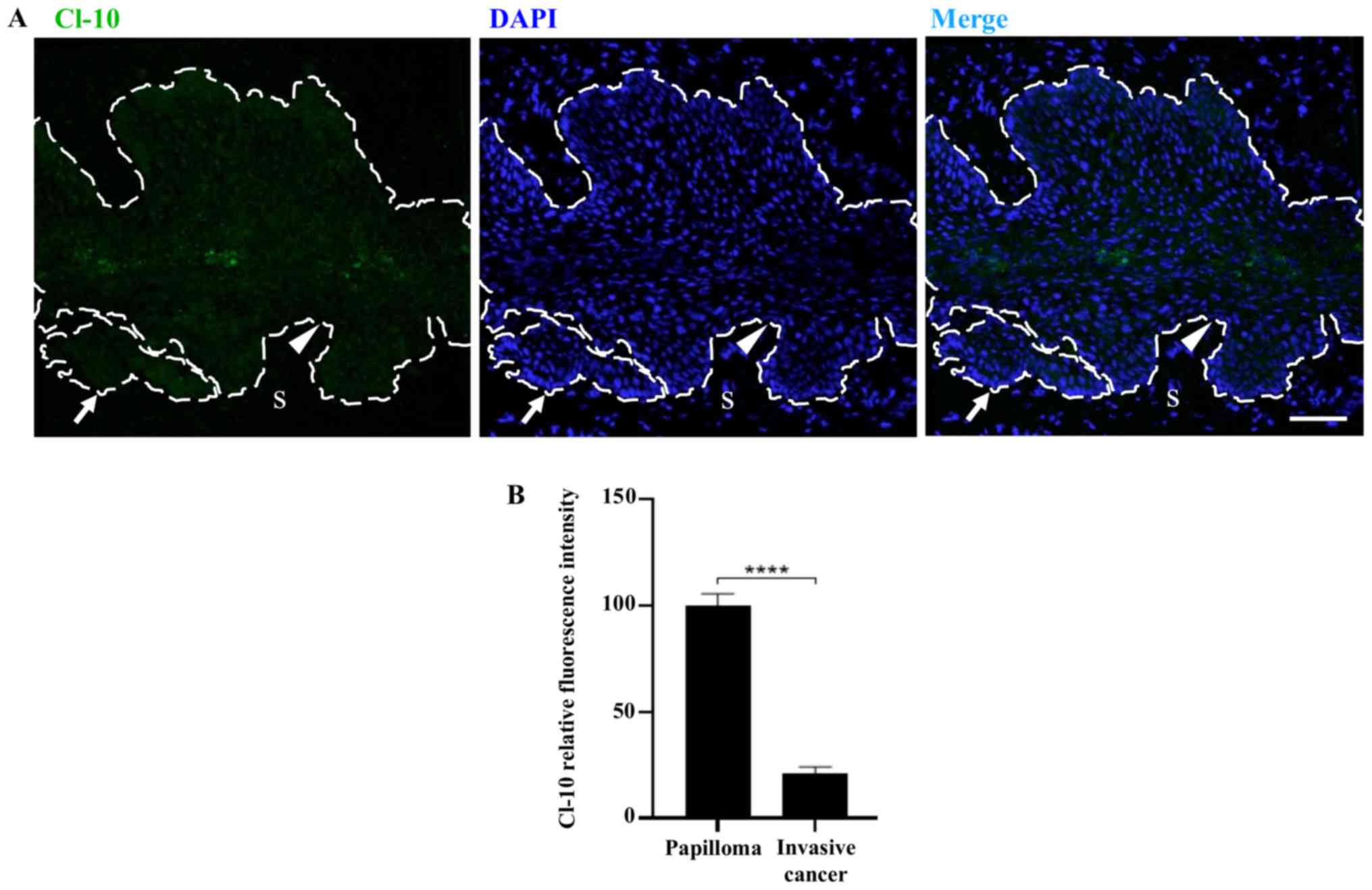
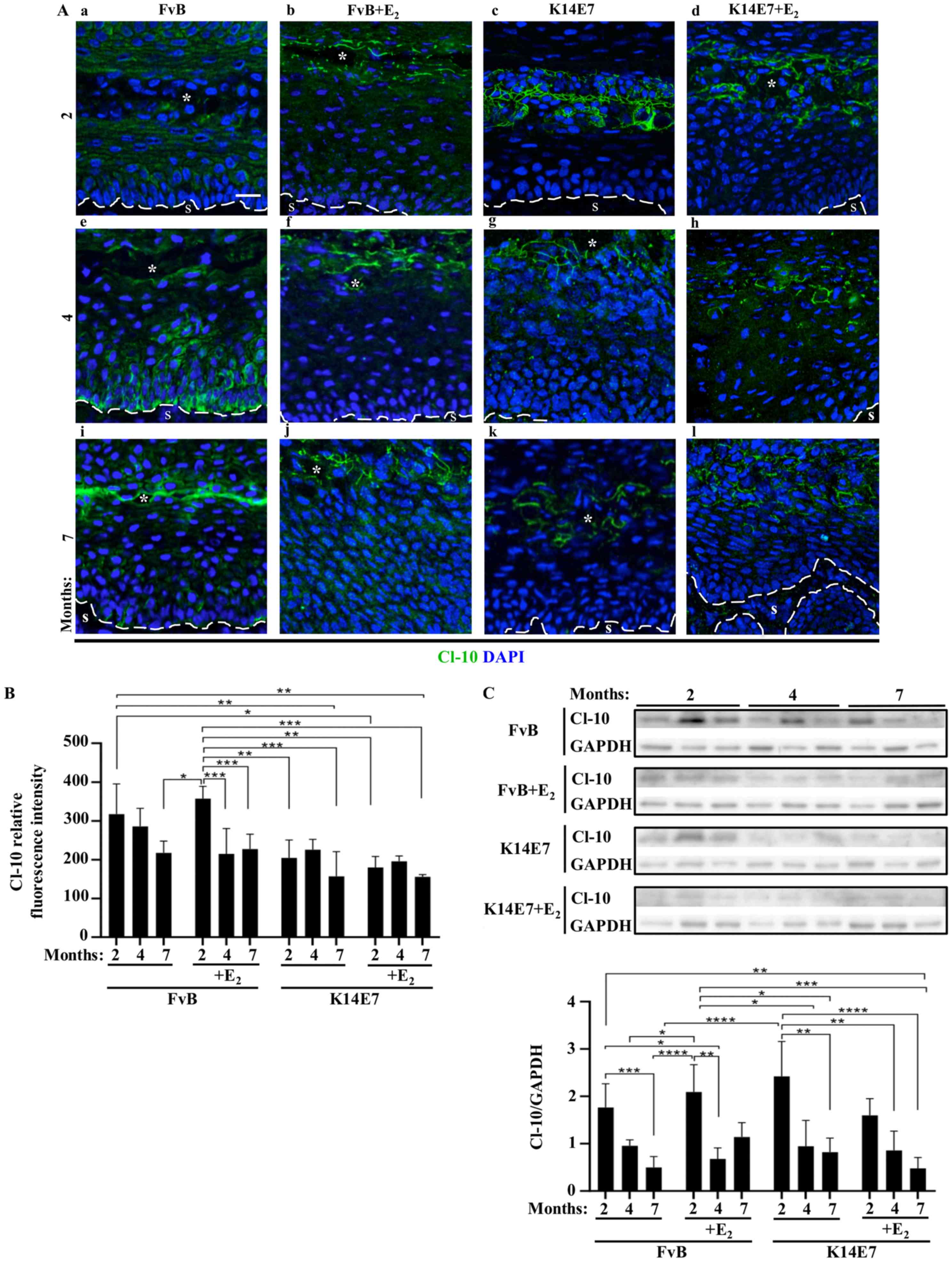 | Figure 4Treatment of FvB and K14E7 mice with
and without E2 increases the expression of Cl-10 at the
border of cells in the superficial layer of the cervix. Expression
of Cl-10 in frozen sections of the cervix obtained from (A-a-d) 2-,
(A-e-h) 4- or (Ai-l) 7-month-old control FvB (A-a, -e and -i)
without E2 or (A-b, -f, and -j) with E2 and
transgenic K14E7 mice, treated (A-d, -h and -l) with E2
or (A-c, -g and -k) without E2, using
immunofluorescence. Nuclei were stained with DAPI. Scale bar, 20
µm. The dashed white line indicates the border between the
epithelial basal cell layer and the S. *Cervical lumen.
(B) Quantitative analysis of the mean fluorescence intensity of
Cl-10 from frozen sections of the cervix obtained from 2-, 4- or
7-month-old control FvB and transgenic K14E7 mice, treated with or
without E2. Results obtained from three independent
images from each experimental group. Statistical analysis was
performed using three-way ANOVA followed by Tukey's post hoc test.
Data are presented as the mean ± standard deviation.
*P<0.05, **P<0.01,
***P<0.001. (C) Representative western blot of Cl-10
in cervix lysates obtained from 2-, 4- or 7-month-old control FvB
and transgenic K14E7 mice, treated with or without E2
(upper panel). Densitometry analysis from at least three
independent experiments (lower panel). Statistical analysis was
performed using three-way ANOVA followed by Tukey's post hoc test.
Data are presented as the mean ± standard deviation.
*P<0.05, **P<0.01,
***P<0.001, ****P<0.0001.
E2, 17β-estradiol; S, stroma; Cl, claudin. |
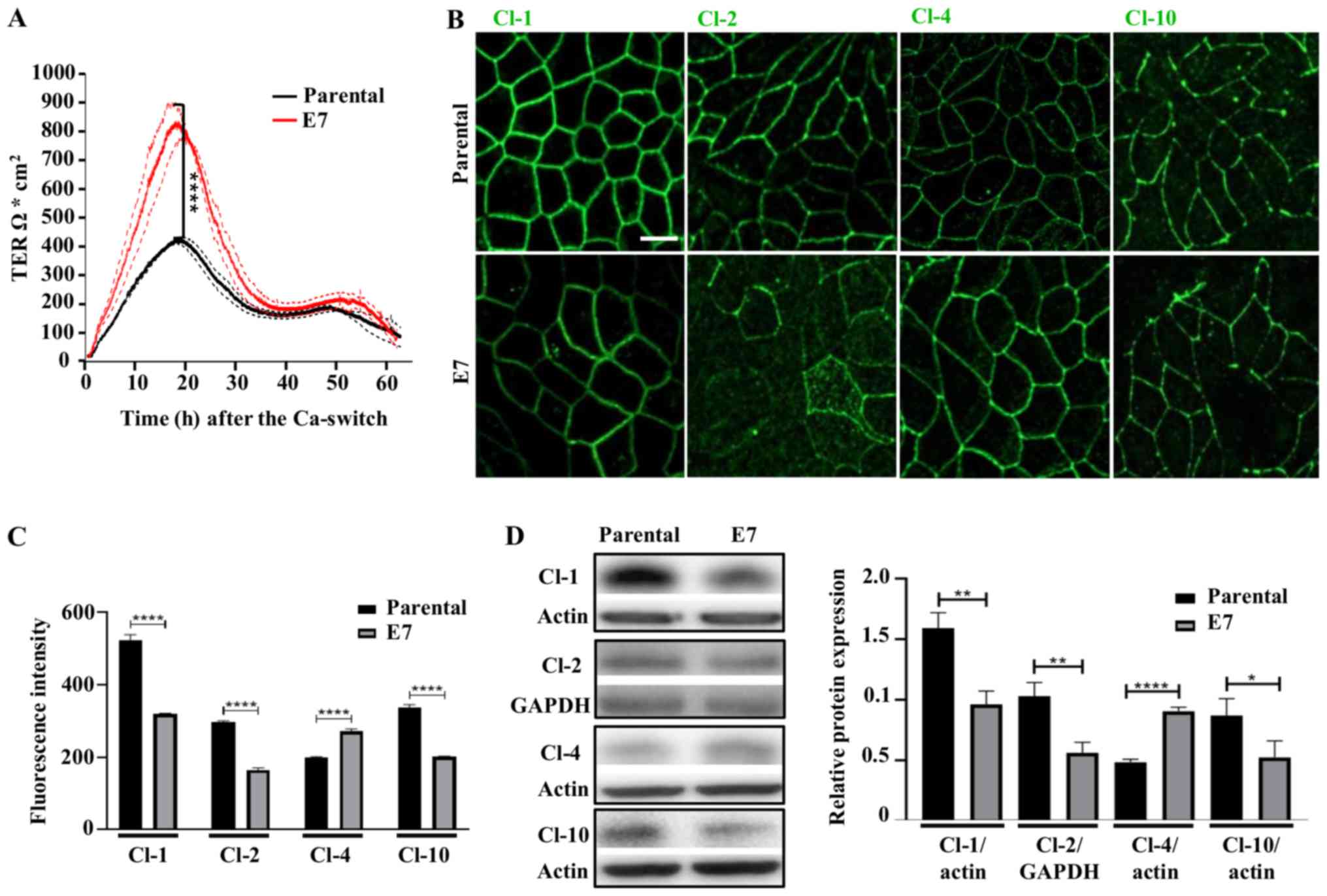 | Figure 8Stable expression of E7 in MDCK
monolayers induces the development of a higher peak of TER and
changes the expression of claudins. (A) Parental MDCK and MDCK-E7
monolayers plated in Transwell inserts were subjected to a
Ca-switch and the development of TER was measured using the
automated cell monitoring system, cellZscope. Data are presented
the mean ± SD from three independent monolayers per condition
plated on Transwell inserts. Statistical analysis was performed at
the 18 h with Student's t-test. ****P<0.0001. (B)
Immunofluorescence detection of Cl-1, -2, -4 and -10 at the peak of
TER (18 h) in parental and MDCK-E7 monolayers. Representative
images from three independent experiments. Scale bar, 10 µm.
(C) Mean fluorescence intensity measurements of Cl-1, -2, -4 and
-10 performed at the peak of TER (18 h) in parental and MDCK-E7
monolayers. Data derived from three independent images from each
condition and from three independent experiments. Statistical
analysis was performed using Student's t-test. Data are presented
as the mean ± standard deviation. ****P<0.0001. (D)
Western blot analysis of Cl-1, -2, -4 and -10 at the peak of TER
(18 h) in parental and MDCK-E7 monolayers. Representative western
blots of at least three independent experiments (left panel) and
the densitometry analysis (right panel). Statistical analysis was
performed using Student's t-test. Data are presented as the mean ±
standard deviation. *P<0.05, **P<0.01,
****P<0.0001. Ca, calcium; Cl, claudin; TER,
transepithelial electrical resistance; MDCK, Madin-Darby Canine
Kidney; MDCK-E7, MDCK cells transfected with E7 from HPV; HPV,
human papillomavirus. |
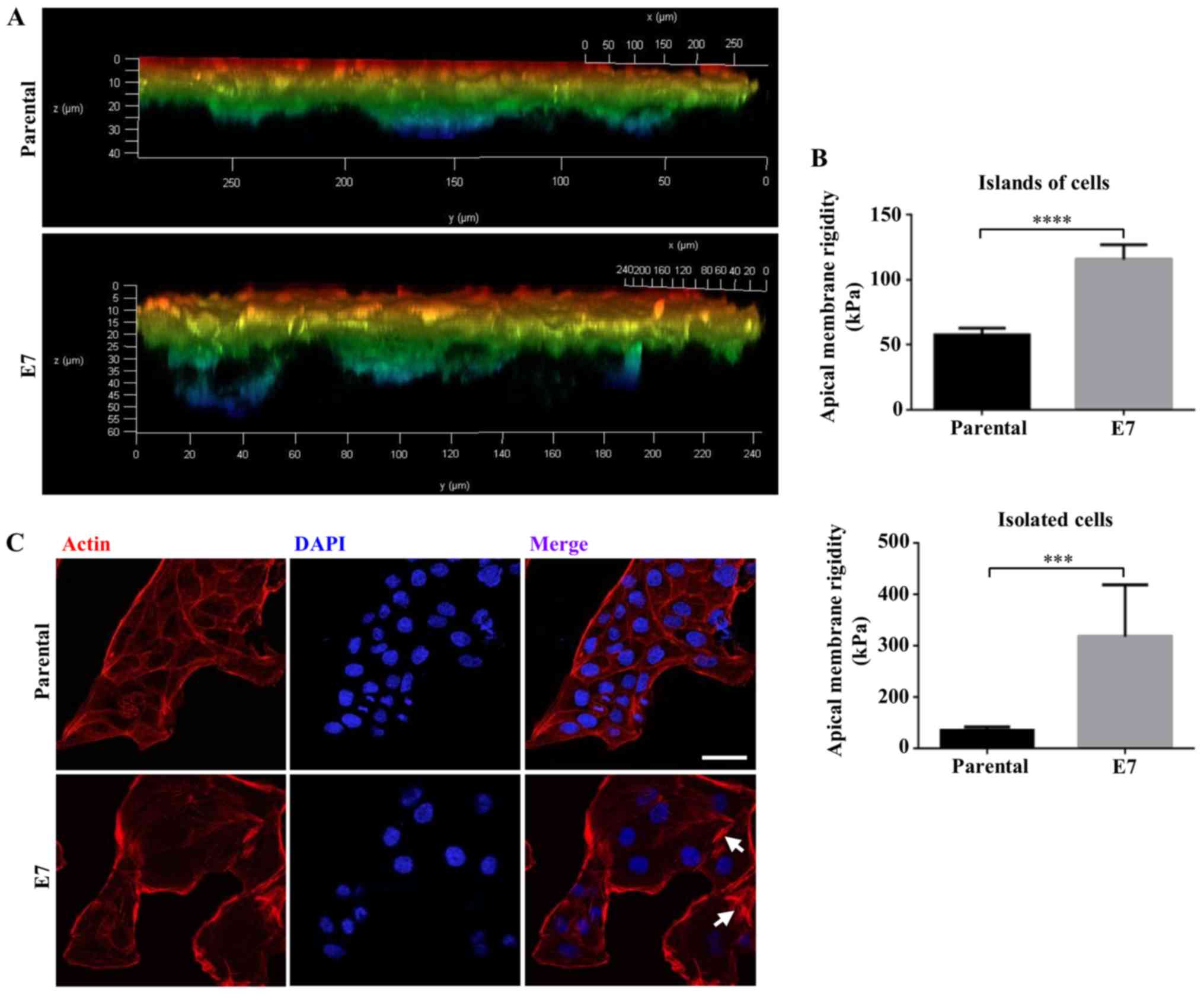 | Figure 9MDCK-E7 cells migrate further through
a 3D scaffold, have a stiffer apical surface and display more
stress fibers compared with that in control MDCK cells. (A) MDCK
and MDCK-E7 cells were seeded on top of 12-well insert containing
Alvetex® Scaffold precoated with Matrigel™. Cells were
stained with rhodaminated phalloidin following 10 days of culture.
Images were captured using a confocal microscope and data analyzed
using the Leica LAS AF X software (v3.0), in which a z series image
was generated, with a depth color code from red to blue where
warmer colors correspond to cells closest to the surface of the
scaffold and colder colors to cells that have migrated towards the
bottom of the scaffold. Results derived from two independent
experiments. (B) Apical membrane rigidity measures were performed
using Atomic Force Microscopy in parental and MDCK-E7 cells present
as isolated cells or in islets. Statistical analysis was performed
using Student's t-test. Data are presented as the mean ± standard
deviation. ***P<0.001, ****P<0.0001.
(C) The expression of stress fibers (arrows) was higher in MDCK-E7
compared with that in control MDCK cells. MDCK and MDCK-E7 cells
were treated with rhodaminated-phalloidin and DAPI, to stain the
stress fibers and nuclei, respectively. Scale bar, 30 µm.
MDCK, Madin-Darby Canine Kidney; MDCK-E7, MDCK cells transfected
with E7 from HPV; HPV, human papillomavirus. |
Results
Transgenic K14E7 mice treated with
E2 develop invasive cancer with a reduced expression of
claudin-1
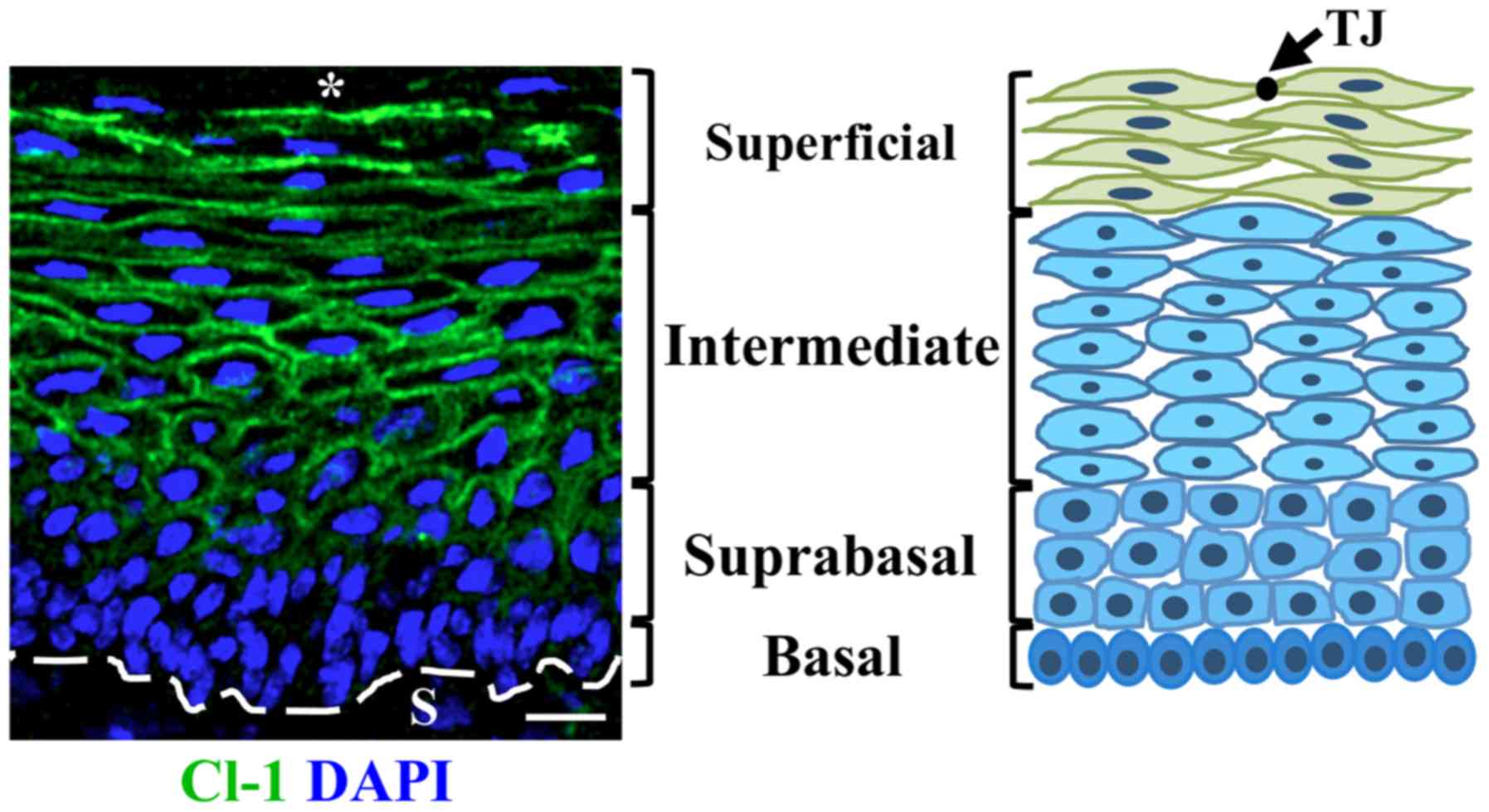
The expression of claudin-1 in the multi-stratified
epithelium of the cervix of control FvB and transgenic K14E7 mice
treated with or without E2 was investigated. In control
FvB mice, at 2 months of age, claudin-1 is present at the border of
cells from the suprabasal region up to the most superficial cell
layer facing the cervix lumen where it is expressed with higher
intensity, while no claudin-1 expression is present in the basal
cell layer (Fig. 1). In mice at 4-
and 7-months of age the expression of claudin-1 was present at the
basal cell layer augments and neither, treatment of FvB mice with
E2 nor the expression of E7 in the transgenic K14E7 mice
changed this pattern of expression (Figs. 2A and S1-4). Quantitative analysis revealed no
change in claudin-1 immunofluorescence intensity in the cervix
between FvB mice at 2-, 4-, and 7-months of age with or without
E2 treatment (Fig. 2B).
In 2-month-old K14E7 mice without E2 treatment, there
was an increase in claudin-1 expression compared with that in FvB
mice, and FvB and K14E7 mice treated with E2 (Fig. 2B). Western blot analysis of
claudin-1 in the cervix of the aforementioned mice confirmed the
increase in claudin-1 protein expression in 2-month-old K14E7 mice
without treatment with E2 compared with that in 4- and
7-month-old K14E7 mice without E2 treatment, and in
2-month-old FvB mice without E2 and K14E7 mice with
E2 (Fig. 2C).
Treatment of transgenic K14E7 mice with
E2 induces the appearance of papillomas which develop
progressively with age. In carcinomas that project into the
cervical stroma of 7-month-old mice, the expression of claudin-1
was reduced compared with that in the rest of the cervix (Fig. 3A and B).
Claudin-10 localizes at an earlier age in
the upper layer of the cervix in FvB mice treated with
E2 and K14E7 mice treated with or without
E2
Subsequently, the expression of claudin-10 in the
cervix of control FvB and transgenic K14E7 mice treated with or
without E2 was investigated (Figs. S5-8). Claudin-10 was expressed in
a diffuse cytoplasmic pattern, from the basal to the uppermost
layers of the cervix in FvB mice without E2 treatment at
2- and 4-months of age (Fig. 4A-a and
-e); however, it was found to be expressed at the border of
cells in the superficial cell layers in 7-month-old mice (Fig. 4A-i). The expression of claudin-10
in FvB mice treated with E2 and in K14E7 mice treated
with and without E2 was located in the border of cells
in the in the upper cell layers from the second month of life
(Fig. 4A--b-d, -f-h, and -j-l).
Quantitative analysis revealed a significant higher fluorescence
intensity of claudin-10 in the cervix of FvB mice treated with
E2 at 2-months of age compared with that in mice at 4-
and 7-months of age, and these values were similar to those in
K14E7 mice treated with or without E2 at 2-, 4- and
7-months of age (Fig. 4B). In
addition, the quantitative fluorescence intensity of claudin-10 in
2-month-old FvB mice was higher compared with that observed in
7-month-old K14E7 mice without E2 treatment and in 2-
and 7-month-old K14E7 mice treated with E2 (Fig. 4B). The western blot analysis of
claudin-10 protein expression in the cervix of the aforementioned
mice decreased in a time-dependent manner in both FvB and K14E7
mice treated with or without E2, whereas in K14E7 mice
treated with E2 the level of claudin-10 at 4- and
7-month of age was lower compared with that in K14E7 mice without
E2 treatment, which were 2-months old (Fig. 4C).
Furthermore, in 7-month-old transgenic K14E7 mice
treated with E2, the papillomas which can invade the
stroma showed a low expression levels of claudin-10, similar to
that observed in the lower layers of the rest of the cervix
(Fig. 5A and B).
Opening of TJs at the cervix superficial
cell layer is induced by E2 or E7
To determine if the changes observed in the
expression of claudins -1 and -10 were accompanied with an increase
in the paracellular permeability of the cervix, further analysis
was performed. Fig. 6A shows a
semi-thin section of the cervix in a 2-month-old FvB mouse without
E2 treatment, where the ruthenium red stain was found to
be located in the lumen, which was in contact with the apical
surface of the superficial layer of cells in the multistratified
epithelium. Subsequently, using transmission electron microscopy,
the thin sections found in the cervix of 2-month-old FvB mice,
without E2 treatment, were impermeable to the
paracellular electrodense marker, ruthenium red, which was found to
be maintained in FvB mice at 7-months-old (Fig. 6B-a and -e). In contrast, in FvB
mice at 2- and 7-months-old treated with E2, 29 and 36%
of TJ were permeable to ruthenium red, respectively (Fig. 6B-b and -f). There percentage
permeability in K14E7 mice treated without E2 was 67 and
55%, at 2- and 7-months of age, respectively (Fig. 6B-c and -g), which was similar to
that found in K14E7 mice treated with E2 (Fig. 6B-d and -h).
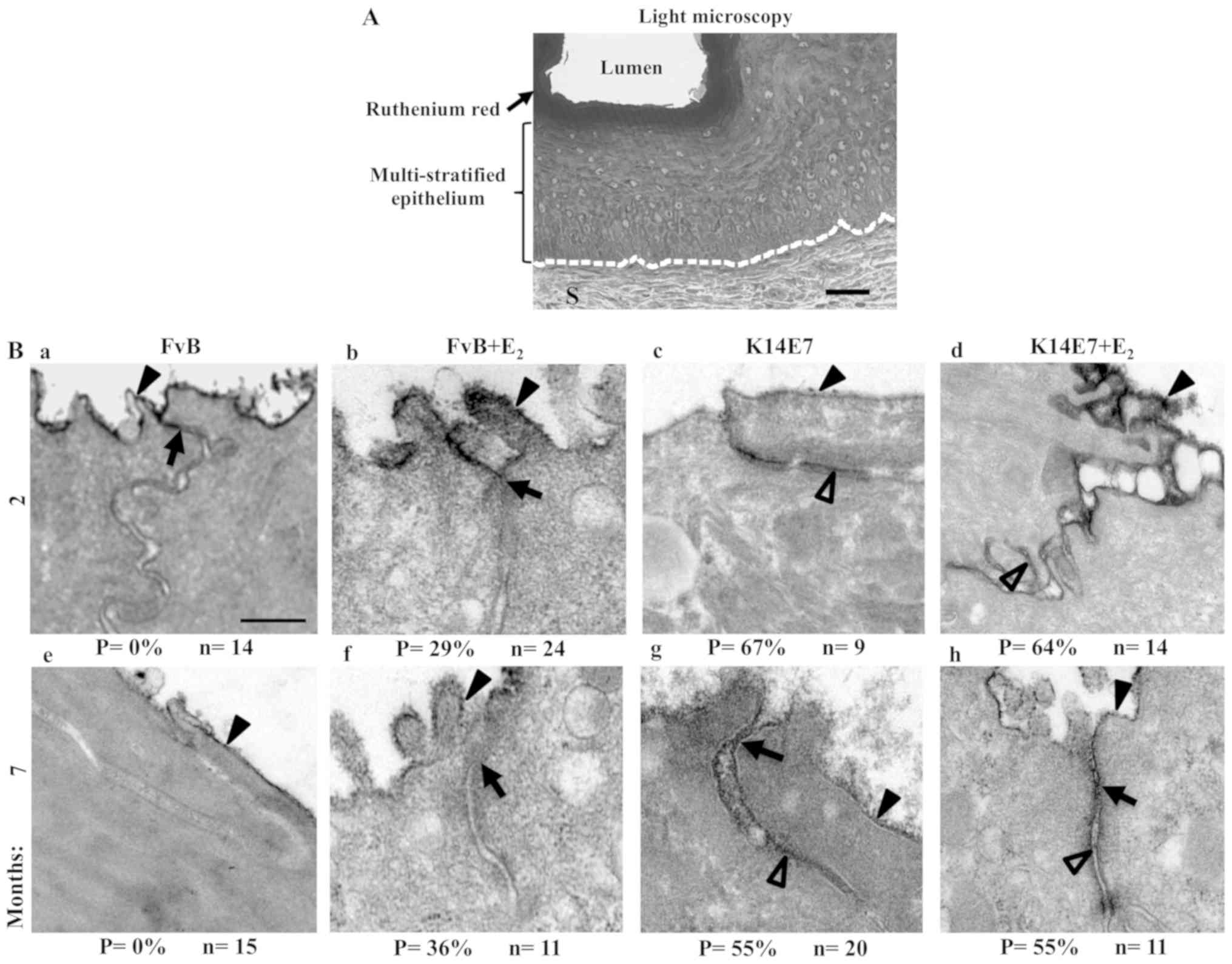 | Figure 6Treatment with E2 induces
opening of TJs in the cervix of transgenic K14E7 mice. (A)
Semi-thin section of the cervix from a 2-month-old FvB mouse
stained with ruthenium red. The dashed white line indicates the
border between the epithelial basal cell layer and the S. Scale
bar, 25 µm. Transmission electron microscopy of thin
sections from ruthenium red stained cervix from (B-a-d) 2- and
(B-e-h) 7-month-old FvB mice (B-b and -f) with E2 or
(B-a and -e) without E2, and transgenic K14E7 mice,
treated (B-d and -h) with E2 or (B-c and -g) without
E2. In 2- and 7-month-old FvB mice the ruthenium red
stain highlighted the apical surface of the superficial cells in
the multi-stratified epithelium of the cervix, while negative
staining was found in the paracellular pathway, below the TJ
region, in this layer of cells. In FvB mice treated with
E2 and in K14E7 mice treated with or without
E2, the ruthenium red stain highlighted a percentage of
the cells along the paracellular route of the superficial layer of
cells in the cervix. Scale bar, 250 nm. Black arrowheads indicate
ruthenium red staining in the apical surface. The arrows indicate
TJs and the empty arrowheads indicate ruthenium red staining in the
paracellular pathway between adjacent cells. P, permeable TJs; n,
number of observations; TJ, tight junctions; E2,
17β-estradiol. |
Therefore, it was concluded that the increase in TJ
perme-ability in the cervix of FvB mice treated with E2
occurs at the same age (2-months old) when a change in the
expression pattern of claudin-10 was observed.
The stable expression of E7 alters the
monolayer architecture of epithelial MDCK cells
To improve the understanding of E7 on epithelial
cell transformation, a stable transfection of HPV16 E7 oncoprotein
in epithelial MDCK cells was created, which is a cell line
characterized for exhibiting well developed TJs (38), and has frequently been used to
investigate the effect of viruses and viral proteins on TJs
(39-43). Fig.
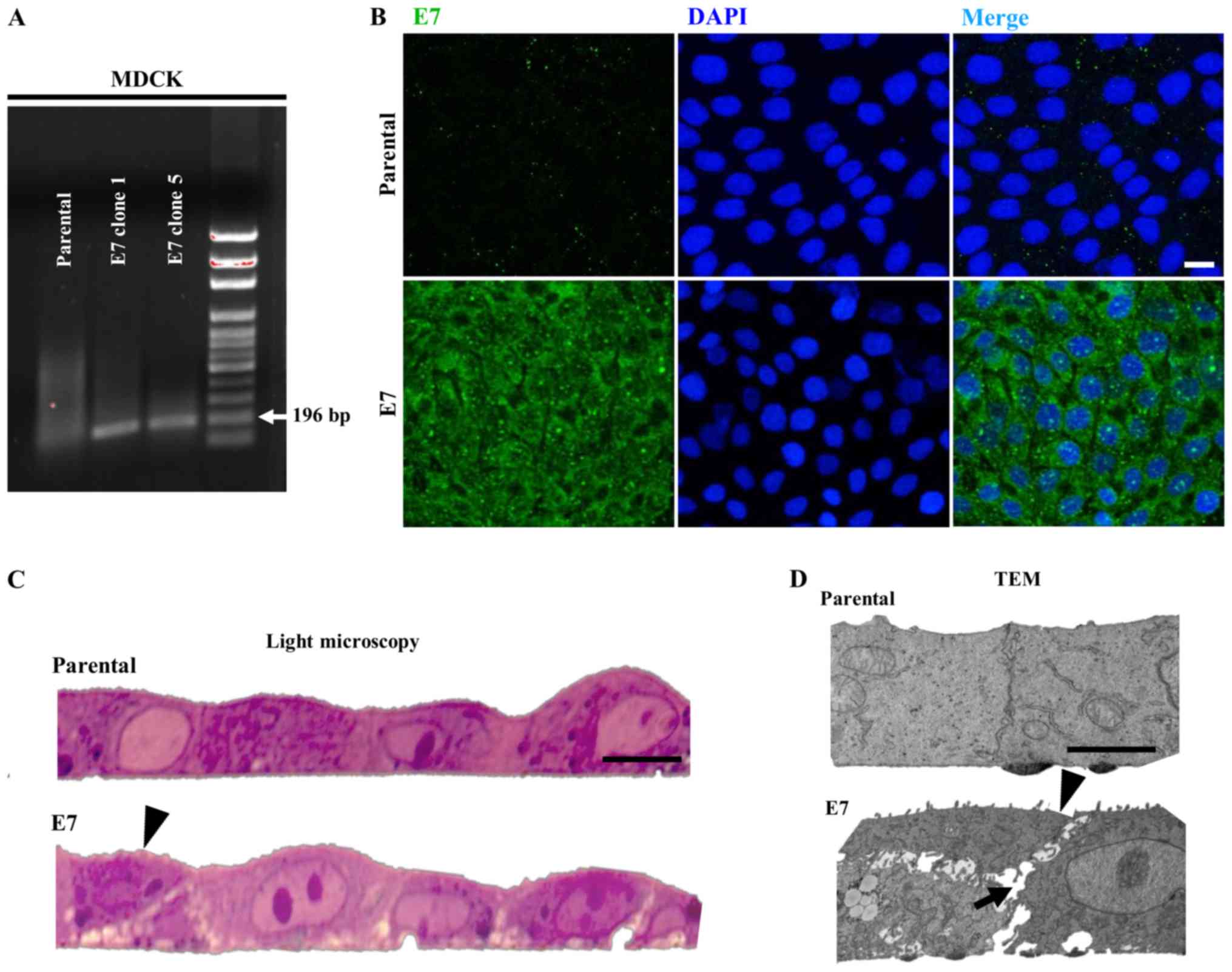
7A revealed that E7 was expressed in clones 1 and 5 of MDCK-E7
cells but not in parental MDCK cells using RT-PCR. As the
expression of E7 was the same in all clones, clone 5 was selected
to be used in further experimentation. Fig. 7B shows that the E7 protein is
expressed in a diffuse manner in MDCK-E7 cells, while there is no
expression in parental MDCK cells, using immunofluorescence. Light
microscopy images of semi-thin sections reveal that MDCK-E7 cells
grow in an abnormal manner characterized by the presence of some
cells growing on top of one another and by the widening of
intercellular spaces (Fig. 7C).
These observations were further confirmed by TEM (Fig. 7D).
E7 induces the development of an acute
peak of TER in MDCK monolayers and changes the expression pattern
of claudins-1 and -10
Subsequently, if the development of TER was altered
by the expression of E7 in MDCK cells was investigated and the
Ca-switch procedure to trigger TJ formation and TER development was
used. Fig. 8A shows that MDCK-E7
cells reached a much higher peak of TER compared with that in the
control cells. However, at 40 h following the Ca-switch, when the
TER stabilizes in both cell lines, both experimental groups display
similar TER values. Next, the expression of claudins-1 and -10 at
the TER peak (18 h) following the Ca-switch was investigated. In
addition, the expression levels of claudins-2 and -4 were also
investigated, as the former forms paracellular cationic pores
(44,45) which increases TJ permeability
(46) and decreases TER (47), while the latter exerts the opposite
effect, functioning as a cationic barrier (48) or an anionic pore (49). Using immunofluorescence, the
expression of claudins-1, and -10 was found to be reduced, while
that of claudin-2 was reduced at the cell borders and appeared in a
diffuse pattern in the cytoplasm at the peak of TER in MDCK-E7
cells compared with that in parental cells. On the other hand,
claudin-4 expression was increased (Fig. 8B and C). These results were further
confirmed using western blot analysis (Fig. 8D).
Taken together, the results suggest that the higher
peak of TER found in MDCK-E7 cells compared with that in parental
monolayers, was due to an altered expression of claudins.
E7 enhances the migrate ability of MDCK
cells through a 3D matrix and induces cell stiffening and stress
fiber formation
Next, if the presence of E7 induced the migration of
MDCK cells was investigated, as this characteristic is important
for cancerous cells to invade the underlying stroma and
metastasize. A 3D in vitro model was used, as it replicates
the tissue organization compared with that in 2D models (50). MDCK and MDCK-E7 cells were plated
on top of a Matrigel® coated
Alvetex®Scaffold, which is a porous and inert
polystyrene platform with large voids that create 3D spaces where
cells can grow. Fig. 9A shows that
MDCK cells migrated through the scaffold between 20 and 30
µm, whereas MDCK-E7 cells migrated to a distance of 50
µm, thus revealing that E7 enhanced the ability of MDCK
cells to migrate.
It has been previously shown that cells undergo a
stiffening stage prior to acquiring malignant features (51). Therefore, the apical surface
elastic Young's module in parental and MDCK-E7 cells was measured
using standard nanoindentation force microscopy. The experimental
data are supported by their theoretical analysis, which is based on
the description of the tip-sample interactions using the
Euler-Bernoulli equation (www.efunda.com/formulae/solid_mechanics/beams/theory.cfm)
and the asymptotic solutions of the oscillatory tip behavior during
its interaction with the sample. Fig.
9B shows the rigidity of the apical surface of MDCK-E7
increases compared with that in parental cells by 5.64- and
1.99-fold in isolated and in cell islands, respectively. As
stiffening is characterized by the accumulation of stress fibers
(51), if E7 promoted the
expression of stress fibers in MDCK cells was investigated.
Fig. 9C shows a proliferation of
stress fibers in MDCK-E7 compared with that in control MDCK
cells.
Taken together, these results indicate that the E7
oncoprotein promotes cell stiffening and invasion.
Discussion
In the present study a previously characterized
transgenic mouse containing the hK14HPV16E7 transgene, where the
expression of E7 oncoprotein from HR-HPV16 is regulated by the
promoter for human keratin 14 (hK14) (52) was used. The activity of this
promoter is restricted to the basal cell layer of multi-stratified
epithelia, to guarantee that the expression of E7 is directed to
the cell types where HPV infection is targeted. K14E7 transgenic
mice display several characteristic phenotypes, including wrinkled
skin, thickened ears and loss of hair in adults, and develop skin
tumors, which begins at 9-months of age (52). In addition, animals exhibit stunted
growth and a high mortality rate during the first 2 weeks of life
caused by the incapacity to feed due to esophagus hyperplasia. This
hyperplasia is characterized by an expansion of the keratin
10-positive basal layer of cells and is found in several additional
sites, including the skin, palate, forestomach and exocervix
(52).
The expression of claudin-1 in the cervix of FvB
mice, without E2 treatment, was higher at the most
superficial cell layer facing the lumen, while the presence at the
basal cell layers augments, as the age of the mice increases from
2- to 7-months of age, in the present study. This same pattern of
expression was found in FvB mice treated with E2, and in
K14E7 mice treated with or without E2. However, in
2-month-old K14E7 mice treated without E2 the amount of
claudin-1 found in the cervix was higher compared with that in the
other experimental groups. On the other hand, the expression of
claudin-1 was reduced compared with that in the rest of the cervix
in the invasive papillomas that developed in 7-month-old K14E7 mice
treated with E2. These results are consistent with
previous research which shows that claudin-1 is markedly expressed
in grades I, II and III of human intraepithelial neoplasias, and in
situ carcinomas (53-55), but decreases with progression to
the invasive phenotype (56).
In addition, the expression of claudin-1 was found
to be low in aggressive colorectal carcinomas (57,58)
and in gastric cancer (59,60).
In the latter (61), as well as in
oral squamous cell carcinomas (62) and thyroid cancer (63), the absence of claudin-1 is
associated with poorly differentiated tumors. Both papillary
urothelial neoplasms of low malignant potential (PUNLMP) and
low-grade urothelial cell carcinoma have a decreased expression
level of claudin-1 compared with that in inverted urothelial
neoplasms, which almost always exhibit a benign behavior (64). The low expression level of
claudin-1 was found to be a predictor of recurrence and poor
patient survival in colon cancer (65-67),
hepatocellular carcinoma (68,69),
thyroid (63) and prostate
(70) cancer, lung adenocarcinoma
(71), non-small cell lung cancer
(72) and PUNLMPs (64). With respect to breast cancer, the
expression of claudin-1 was initially associated with the
basal-like type, which has a very poor prognosis (73,74).
However, a previous study has found that a claudin-1 negative
phenotype predicts a high risk of recurrence and mortality in
triple-negative breast cancers (75). In addition, a reduction or the
total loss of the expression levels of expression claudin-1 was
also observed in the group of patients with recurrent breast cancer
in comparison to the non-recurrent group, as well as in the lymph
node metastasis-positive group (76,77).
Taken together, the findings from previous research studies are
consistent with the results of the present study, that there is a
reduced expression level of claudin-1 in the invasive papillomas in
7-month-old K14E7 mice treated with E2.
Nevertheless, claudin-1 has also been reported to
be overexpressed in several cancerous tissues, including high-grade
cervical intraepithelial neoplasias (78) and invasive cervical cancer
(79), where it has been
considered as a biomarker of the disease (80), with a prognosis potential (81). Claudin-1 was also found to be
overexpressed in colorectal tumors (82-84),
mucoepidermoid carcinomas of the salivary gland (85), thyroid papillary carcinomas,
papillary microcarcinomas primary tumors and lymph node metastases
(86), and esophageal (87,88),
hypopharyngeal (89,90), tongue (91) and oral (92) squamous cell carcinomas, where
claudin-1 was found to be associated with cell invasion and disease
recurrence (93). Claudin-1
expression in melanoma cells promotes the secretion and activation
of metalloproteinase 2, which enhances cell motility (94,95)
and mediates tumor necrosis factor α-induced cell migration in
gastric cancer cells (96).
Claudin-1 was also found to be a prognostic marker and shorter
patient survival in kidney clear cell carcinomas (97), intestinal-type gastric cancer
(98), lung adenocarcinoma
(99) and stage N2 non-small cell
lung cancer (100).
The localization of claudin-1 is also altered in
cancerous cells. For example, in colorectal cancer, the membrane
staining intensity of claudin-1 was reduced compared with that in
adjacent non-neoplastic tissue, while a significant increase in
claudin-1 cytoplasmic staining was found in colorectal cancer
tissue (101). Similarly, in
follicular thyroid carcinoma there was negative claudin-1 staining
in the membrane and an increase in the nucleus, resulting in
increased cell migration and invasion (102). This expression is abnormal as
benign thyroid tissue and peritumoral non-malignant thyroid tissue
are negative for claudin-1 staining (103,104) and in normal epithelia, in which
claudin-1 is expressed [such as the skin (105), kidney (106) and intestine (107,108)], the expression is negative in the
nucleus and concentrates at the TJ in the lateral membrane. Thus,
evidence is emerging showing that in human cancer, the expression
and localization of claudin-1 is altered when compared with that in
normal tissue, which was consistent with the results in the murine
cervical cancer model in the present study.
It was found in the current study that the
expression of claudin-10 in the cervix of FvB mice, without
E2 treatment, was reduced after 2-months of age and was
found to be increased in the border of cells in the upper cell
layers from the 2-month of age in FvB mice treated with
E2, and in K14E7 mice with and without E2
treatment. The quantitative analysis of the fluorescence revealed
lower values of claudin-10 in K14E7 mice treated with or without
E2 in the second month of life compared with that in FvB
mice treated with E2; however, this effect was not
confirmed using western blot analysis. The reason for this
discrepancy remains unknown, although it could be due to the
greater statistical variance of the western blots results, which
makes them not statistically significant.
Furthermore, in 7-month-old transgenic K14E7 mice
treated with E2, the expression level of claudin-10 in
the papillomas, which invades the stroma, was not detectable. This
was consistent with previous research which found that the
expression of claudin-10 diminishes in breast (109) and biliary tract carcinomas
compared with that in normal tissues (110). Moreover, the expression of
claudin-10 was associated with the overall survival of patients
with lung adenocarcinoma, as it was found to be lower in invasive
lepidic adenocarcinoma compared with that in the in situ lung
adenocarcinomas (111).
In contrast, the mRNA expression level of
claudin-10 was found to be associated with the recurrence of
primary hepatocellular carcinoma following curative hepatectomy
(112). In addition, type III
hepatic tumors with a high malignant potential, display cytoplasmic
staining of claudin-10 around the nucleus (113). In the thyroid, claudin-10 was
found to be negatively expressed in follicular thyroid carcinomas,
and overexpressed in papillary thyroid carcinomas (114), where it serves as a biomarker to
distinguish tumors from benign thyroid lesions (115).
With respect to the paracellular permeability of
the cervix, treatment of FvB mice with E2 increased the
permeability of TJ to ruthenium red. However, the effect was
markedly higher in K14E7 mice treated with or without
E2, which suggest that both E2 and E7 induce
the development of weaker TJs. In this respect, we have previously
found that in the skin of ovariectomized FvB mice, E2
treatment diminishes the expression of TJ proteins occludin and
ZO-2 (39). Notably, in mice the
blockade of ruthenium red paracellular passage can be established
at the most superficial layer of cells in the cervix, while in the
human cervix the epithelial junctions that restrict the diffusion
of paracellular markers are localized in the cells located three or
four layers below the lumen (116).
Following the investigation of the cervix in the
trans-genic mice, further analysis was performed to determine if
the expression of E7 altered the TJs and transformed the
characteristics of the normal epithelia, which required
transfection of E7 into an epithelial cell line. A non-cancerous
cell line of cervix from a mouse or other mammal was not used as to
the best of our knowledge these are not currently commercially
available. In addition, from the seminal study of TJs in MDCK cells
performed by Cereijido et al (117) in 1978, the cell line has been
widely used to investigate the electrical properties of TJs, the
permeability of the paracellular pathway (118-120), the changes in the ultrastructure
of TJs visualized in freeze-fracture replicas (38,121), the molecular composition of TJs
(46,122-126), as well as the response of TJs to
a wide variety of factors, including temperature (127), ions (128-130), signaling cascades (131,132), toxins (133) and growth factors (134,135). Moreover, the role of claudins, in
particular claudins -1 (136,137), -2 (46,125,138), -4 (46,125,126) and -10 (33,139) has been extensively studied in
MDCK cells. Furthermore, the effect of numerous viruses and viral
proteins on TJs has also been investigated in MDCK cells (39-43,140-160). Therefore, the MDCK cell line was
selected as it is an ideal in vitro model system to
investigate factors that regulate or have a harmful effect on
TJs.
A stable MDCK cell line was created which expressed
E7, and it was found that the monolayers had widened intercellular
spaces and had areas in which some cells were growing on top of one
another. A similar phenotype was observed in MDCK monolayers where
the expression of the TJ protein ZO-2 was knocked down (161,162), which suggests that the E7
oncoprotein exerts a harmful effect on TJs. However, when the
development of TER in MDCK-E7 monolayers was analyzed it was found
that they achieved a higher peak of TER compared with that in
parental cells. This unexpected result led to the investigation
into the expression pattern of claudins at the time where TER
reaches its highest values. It was found that the protein
expression level of claudins -1, -2 and -10 decreased, while that
of claudin-4 increased in MDCK-E7 monolayers. The alteration of a
single type of claudin can modify, in a significant manner, the
permeability and transepithelial electrical resistance of a tissue
(163). The increased protein
expression level of claudin-4 in MDCK-E7 cells was found to be
important, as transfection of this protein can function as a cation
barrier in MDCK cells and induce a significant decrease in
permeability and an increase in TER (46,48).
The decreased expression level of claudin-2 in MDCK-E7 monolayers
was expected to have a significant effect on TER, as this claudin,
which is highly expressed in leaky epithelia, such as the proximal
tubule of the kidney (164) and
the intestinal crypts (165),
functions as a high conductance cation-permeable pore (44,47).
Claudin-1 was found to be ubiquitously expressed claudin and in
vitro overexpression studies reveal that it acts as a barrier,
which increases TER (136,137)
therefore, decreased expression would not be expected to contribute
to the increased TER observed in MDCK-E7 cells. Claudin-10 has been
found to be expressed in numerous tissues, including breast
(109), biliary tract (110), lung (111), kidney (33) and liver (113). The function of the two major
claudin-10 isoforms revealed that while claudin-10a acts as an
anion pore in MDCK II cells (166), claudin-10b has no strong ion
selectivity in MDCK II cells which exhibit a high expression of
claudin-2 that forms cation pores, but instead claudin-10b acts as
a strong cation-permeating pore in high resistance MDCK-C7 cells
(33). Claudin-10a expression was
found to be restricted to the kidney (33), therefore the remaining tissues,
including the cervix and those where the mRNA for claudin-10b has
been detected (heart, brain, spleen, lung, liver, skeletal muscle,
testis, placenta, eye, lymph node, smooth muscle, prostate, thymus,
stomach and uterus) are expected to express claudin-10b. However,
as none of the available antibodies, including the one used in the
present study, discriminate between claudin-10a and -10b, this was
not confirmed in the murine cervix or in MDCK cells.
In MDCK cells it was found that the stable
expression of E7 also increases cell migration through a 3D
scaffold, stiffens the apical cell membrane, induces the appearance
of stress fibers, decreases the expression level of claudins -1, -2
and -10, and augments the expression of claudin-4. All these
changes suggest that the cell phenotype was transformed. With
respect to increased cell migration and altered claudin expression,
previous research has found that migration augments upon
overexpression of claudin-2 in lung cancerous cells (167), claudin-4 in ovarian tumor cells
(168), claudin-7 in ovarian
cancer (169), claudin-8 in
prostate cancer cells (170),
claudin-10 in papillary thyroid cancer cells (29) and in hepatocellular carcinomas
cells (171), and claudin-17 in
hepatic cells (172); or upon
silencing of claudin-3 (173) and
claudin-4 (173) in ovarian cells
OV2008, of claudin-6 in human breast epithelium cell line HBL-100
(174), of claudin-7 in clear
cell renal cell carcinoma (175),
and of claudin-11 in nasopharyngeal carcinoma (176). In MDCK cells migration augments
following claudin-2 silencing (156), and in normal mammary epithelial
cells as well as in breast and ovarian tumor cells, blocking the
second extracellullar loop in claudin-4, with a peptide, which
mimics a conserved sequence in this claudin loop, inhibited cell
mobility (167,177,178).
With respect to cell stiffening, tumors have long
been characterized for being harder compared with that in normal
tissue, and while stiffening of the stroma is a crucial factor that
favors tumorigenesis (179,180), changes in the tension of the cell
have also been observed during cell transformation (51). MDCK cells also acquire a more rigid
apical membrane when the TJ proteins ZO-1 and ZO-2 are both knocked
out (181). Moreover, cell
stiffening, accompanied by the appearance of stress fibers, has
been found to be essential to drive breast tumor growth during
premalignant stages (51).
In summary, the results from the present study
indicate that the oncogenic protein E7 derived from HPV16 induces
epithelial cells changes, including in the expression of claudins
-1, -2, -4 and -10 and TJ sealing, which are accompanied by changes
in cell stiffness, motility and cytoarchitecture, which could be
important in the development of tumorigenesis.
Supplementary Data
Acknowledgments
This study was part of the doctoral dissertation by
Perla Yaceli Uc, a student from the Posgrado en Ciencias
Biomédicas, Universidad Nacional Autónoma de México, in which she
also received a doctoral fellowship from Consejo Nacional de
Ciencia y Tecnología (no. 362696).
Funding
This study was supported by grants from the Miguel
Alemán Valdés Foundation 2018, and Secretaría de Educación
Pública-Center for Research and Advanced Studies, México (grant no.
FIDSC2018/33).
Availability of data and materials
All data generated or analyzed during this study
are included in this published article.
Authors' contributions
LGM and PG contributed with the conception and
design of the study, and analyzed and interpreted the data. PYU,
JM, ARS, LA, BCM, MLR. and GR performed the experiments and
analyzed and interpretation the data. ROD maintained and treated
the FvB and K14E7 transgenic mice. EMCM obtained cervical samples
from the aforementioned mice. LS was involved in the design of the
experiment for the development of MDCK-E7 cells. RA designed the
procedure for the measurements of the nanomechanical properties of
the cells. LGM and PYU wrote the manuscript. PG and ROD revised the
manuscript critically. All authors read and revised the manuscript,
and approved the final version.
Ethics approval and consent to
participate
All animal procedures were performed according to
inter-national laws and the Mexican Official Norm (approval no.
NOM-062-ZOO-1999) and with the approval of the Center of Research
and Advanced Studies Institutional Animal Care and Use Committee
(approval no. 0193-16).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2020. CA Cancer J Clin. 70:7–30. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jemal A, Ward EM, Johnson CJ, Cronin KA,
Ma J, Ryerson B, Mariotto A, Lake AJ, Wilson R, Sherman RL, et al:
Annual Report to the Nation on the Status of Cancer, 1975-2014
Featuring Survival. J Natl Cancer Inst. 109:1092017. View Article : Google Scholar
|
|
4
|
Paavonen J, Naud P, Salmerón J, Wheeler
CM, Chow SN, Apter D, Kitchener H, Castellsague X, Teixeira JC,
Skinner SR, et al HPV PATRICIA Study Group: Efficacy of human
papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical
infection and precancer caused by oncogenic HPV types (PATRICIA):
Final analysis of a double-blind, randomised study in young women.
Lancet. 374:301–314. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Muñoz JP, Carrillo-Beltrán D,
Aedo-Aguilera V, Calaf GM, León O, Maldonado E, Tapia JC, Boccardo
E, Ozbun MA and Aguayo F: Tobacco Exposure Enhances Human
Papillomavirus 16 Oncogene Expression via EGFR/PI3K/Akt/c-Jun
Signaling Pathway in Cervical Cancer Cells. Front Microbiol.
9:30222018. View Article : Google Scholar
|
|
6
|
Muñoz N, Franceschi S, Bosetti C, Moreno
V, Herrero R, Smith JS, Shah KV, Meijer CJ and Bosch FX;
International Agency for Research on Cancer: Multicentric Cervical
Cancer Study Group: Role of parity and human papillomavirus in
cervical cancer: The IARC multicentric case-control study. Lancet.
359:1093–1101. 2002. View Article : Google Scholar
|
|
7
|
Berraho M, Amarti-Riffi A, El-Mzibri M,
Bezad R, Benjaafar N, Benideer A, Matar N, Qmichou Z, Abda N,
Attaleb M, et al: HPV and cofactors for invasive cervical cancer in
Morocco: A multicentre case-control study. BMC Cancer. 17:4352017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chung SH, Franceschi S and Lambert PF:
Estrogen and ERalpha: Culprits in cervical cancer? Trends
Endocrinol Metab. 21:504–511. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Asthana S, Busa V and Labani S: Oral
contraceptives use and risk of cervical cancer-A systematic review
& meta-analysis. Eur J Obstet Gynecol Reprod Biol. 247:163–175.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mapanga W, Singh E, Feresu SA and
Girdler-Brown B: Treatment of pre- and confirmed cervical cancer in
HIV-seropositive women from developing countries: A systematic
review. Syst Rev. 9:792020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Du GH, Wang JK, Richards JR and Wang JJ:
Genetic poly-morphisms in tumor necrosis factor alpha and
interleukin-10 are associated with an increased risk of cervical
cancer. Int Immunopharmacol. 66:154–161. 2019. View Article : Google Scholar
|
|
12
|
Zhang L, Tian S, Pei M, Zhao M, Wang L,
Jiang Y, Yang T, Zhao J, Song L and Yang X: Crosstalk between
histone modification and DNA methylation orchestrates the
epigenetic regulation of the costimulatory factors, Tim-3 and
galectin-9, in cervical cancer. Oncol Rep. 42:2655–2669.
2019.PubMed/NCBI
|
|
13
|
List HJ, Patzel V, Zeidler U, Schopen A,
Rühl G, Stollwerk J and Klock G: Methylation sensitivity of the
enhancer from the human papillomavirus type 16. J Biol Chem.
269:11902–11911. 1994.PubMed/NCBI
|
|
14
|
Chih HJ, Lee AH, Colville L, Binns CW and
Xu D: A review of dietary prevention of human
papillomavirus-related infection of the cervix and cervical
intraepithelial neoplasia. Nutr Cancer. 65:317–328. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ghosh C, Baker JA, Moysich KB, Rivera R,
Brasure JR and McCann SE: Dietary intakes of selected nutrients and
food groups and risk of cervical cancer. Nutr Cancer. 60:331–341.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Song S, Liem A, Miller JA and Lambert PF:
Human papillomavirus types 16 E6 and E7 contribute differently to
carcinogenesis. Virology. 267:141–150. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Werness BA, Levine AJ and Howley PM:
Association of human papillomavirus types 16 and 18 E6 proteins
with p53. Science. 248:76–79. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dyson N, Howley PM, Münger K and Harlow E:
The human papilloma virus-16 E7 oncoprotein is able to bind to the
retinoblastoma gene product. Science. 243:934–937. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Riley RR, Duensing S, Brake T, Münger K,
Lambert PF and Arbeit JM: Dissection of human papillomavirus E6 and
E7 function in transgenic mouse models of cervical carcinogenesis.
Cancer Res. 63:4862–4871. 2003.PubMed/NCBI
|
|
20
|
Brake T, Connor JP, Petereit DG and
Lambert PF: Comparative analysis of cervical cancer in women and in
a human papillomavirus-transgenic mouse model: Identification of
mini-chromosome maintenance protein 7 as an informative biomarker
for human cervical cancer. Cancer Res. 63:8173–8180.
2003.PubMed/NCBI
|
|
21
|
Arbeit JM, Howley PM and Hanahan D:
Chronic estrogen-induced cervical and vaginal squamous
carcinogenesis in human papillomavirus type 16 transgenic mice.
Proc Natl Acad Sci USA. 93:2930–2935. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Brake T and Lambert PF: Estrogen
contributes to the onset, persistence, and malignant progression of
cervical cancer in a human papillomavirus-transgenic mouse model.
Proc Natl Acad Sci USA. 102:2490–2495. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
González-Mariscal L, Díaz-Coránguez M and
Quirós M: Regulation of tight junctions for therapeutic advanges.
Cancer Metastasis-Biology and Treatment. Martin TA and Jiang WG:
Springer; Dondrecht: pp. 197–246. 2013, View Article : Google Scholar
|
|
24
|
González-Mariscal L, Lechuga S and Garay
E: Role of tight junctions in cell proliferation and cancer. Prog
Histochem Cytochem. 42:1–57. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Birks DK, Kleinschmidt-DeMasters BK,
Donson AM, Barton VN, McNatt SA, Foreman NK and Handler MH: Claudin
6 is a positive marker for atypical teratoid/rhabdoid tumors. Brain
Pathol. 20:140–150. 2010. View Article : Google Scholar
|
|
26
|
Cortés-Malagón EM, Bonilla-Delgado J,
Díaz-Chávez J, Hidalgo-Miranda A, Romero-Cordoba S, Uren A, Celik
H, McCormick M, Munguía-Moreno JA, Ibarra-Sierra E, et al: Gene
expression profile regulated by the HPV16 E7 oncoprotein and
estradiol in cervical tissue. Virology. 447:155–165. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Seo HW, Rengaraj D, Choi JW, Ahn SE, Song
YS, Song G and Han JY: Claudin 10 is a glandular epithelial marker
in the chicken model as human epithelial ovarian cancer. Int J
Gynecol Cancer. 20:1465–1473. 2010.
|
|
28
|
Huang GW, Ding X, Chen SL and Zeng L:
Expression of claudin 10 protein in hepatocellular carcinoma:
Impact on survival. J Cancer Res Clin Oncol. 137:1213–1218. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhou Y, Xiang J, Bhandari A, Guan Y, Xia
E, Zhou X, Wang Y and Wang O: CLDN10 is Associated with Papillary
Thyroid Cancer Progression. J Cancer. 9:4712–4717. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bulut G, Fallen S, Beauchamp EM, Drebing
LE, Sun J, Berry DL, Kallakury B, Crum CP, Toretsky JA, Schlegel R,
et al: Beta-catenin accelerates human papilloma virus type-16
mediated cervical carcinogenesis in transgenic mice. PLoS One.
6:e272432011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
González-Mariscal L, Garay E and Quirós M:
Identification of claudins by western blot and immunofluorescence
in different cell lines and tissues. Methods Mol Biol. 762:213–231.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee C and Laimins LA: The
differentiation-dependent life cycle of human papillomaviruses in
keratinocytes. The Papillomaviruses. Garcea RL and DiMaio D:
Springer; US, Boston, MA: pp. 45–67. 2007, View Article : Google Scholar
|
|
33
|
Günzel D, Stuiver M, Kausalya PJ, Haisch
L, Krug SM, Rosenthal R, Meij IC, Hunziker W, Fromm M and Müller D:
Claudin-10 exists in six alternatively spliced isoforms that
exhibit distinct localization and function. J Cell Sci.
122:1507–1517. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Miranda J, Martín-Tapia D,
Valdespino-Vázquez Y, Alarcón L, Espejel-Nuñez A, Guzmán-Huerta M,
Muñoz-Medina JE, Shibayama M, Chávez-Munguía B, Estrada-Gutiérrez
G, et al: Syncytiotrophoblast of Placentae from Women with Zika
Virus Infection Has Altered Tight Junction Protein Expression and
Increased Paracellular Permeability. Cells. 8:82019. View Article : Google Scholar
|
|
35
|
Ortega-Olvera JM, Winkler R,
Quintanilla-Vega B, Shibayama M, Chávez-Munguía B, Martín-Tapia D,
Alarcón L and González-Mariscal L: The organophosphate pesticide
methami-dophos opens the blood-testis barrier and covalently binds
to ZO-2 in mice. Toxicol Appl Pharmacol. 360:257–272. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gutiérrez J, García-Villa E,
Ocadiz-Delgado R, Cortés-Malagón EM, Vázquez J, Roman-Rosales A,
Alvarez-Rios E, Celik H, Romano MC, Üren A, et al: Human
papillomavirus type 16 E7 oncoprotein upregulates the retinoic acid
receptor-beta expression in cervical cancer cell lines and K14E7
transgenic mice. Mol Cell Biochem. 408:261–272. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
McFarland DC: Preparation of pure cell
cultures by cloning. Methods Cell Sci. 22:63–66. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gonzalez-Mariscal L, Chávez de Ramírez B
and Cereijido M: Tight junction formation in cultured epithelial
cells (MDCK). J Membr Biol. 86:113–125. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Her nández-Monge J, Ga ray E, Raya-Sandino
A, Vargas-Sierra O, Díaz-Chávez J, Popoca-Cuaya M, Lambert PF,
González-Mariscal L and Gariglio P: Papillomavirus E6 oncoprotein
up-regulates occludin and ZO-2 expression in ovariectomized mice
epidermis. Exp Cell Res. 319:2588–2603. 2013. View Article : Google Scholar
|
|
40
|
Latorre IJ, Roh MH, Frese KK, Weiss RS,
Margolis B and Javier RT: Viral oncoprotein-induced mislocalization
of select PDZ proteins disrupts tight junctions and causes polarity
defects in epithelial cells. J Cell Sci. 118:4283–4293. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ramirez L, Betanzos A, Raya-Sandino A,
González-Mariscal L and Del Angel RM: Dengue virus enters and exits
epithelial cells through both apical and basolateral surfaces and
perturbs the apical junctional complex. Virus Res. 258:39–49. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Nava P, López S, Arias CF, Islas S and
González-Mariscal L: The rotavirus surface protein VP8 modulates
the gate and fence function of tight junctions in epithelial cells.
J Cell Sci. 117:5509–5519. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Svensson L, Finlay BB, Bass D, von
Bonsdorff CH and Greenberg HB: Symmetric infection of rotavirus on
polarized human intestinal epithelial (Caco-2) cells. J Virol.
65:4190–4197. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Amasheh S, Meiri N, Gitter AH, Schöneberg
T, Mankertz J, Schulzke JD and Fromm M: Claudin-2 expression
induces cation-selective channels in tight junctions of epithelial
cells. J Cell Sci. 115:4969–4976. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yu AS, Cheng MH, Angelow S, Günzel D,
Kanzawa SA, Schneeberger EE, Fromm M and Coalson RD: Molecular
basis for cation selectivity in claudin-2-based paracellular pores:
Identification of an electrostatic interaction site. J Gen Physiol.
133:111–127. 2009. View Article : Google Scholar :
|
|
46
|
Van Itallie CM, Fanning AS and Anderson
JM: Reversal of charge selectivity in cation or anion-selective
epithelial lines by expression of different claudins. Am J Physiol
Renal Physiol. 285:F1078–F1084. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Furuse M, Furuse K, Sasaki H and Tsukita
S: Conversion of zonulae occludentes from tight to leaky strand
type by introducing claudin-2 into Madin-Darby canine kidney I
cells. J Cell Biol. 153:263–272. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Van Itallie C, Rahner C and Anderson JM:
Regulated expression of claudin-4 decreases paracellular
conductance through a selective decrease in sodium permeability. J
Clin Invest. 107:1319–1327. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hou J, Renigunta A, Yang J and Waldegger
S: Claudin-4 forms paracellular chloride channel in the kidney and
requires claudin-8 for tight junction localization. Proc Natl Acad
Sci USA. 107:18010–18015. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chaicharoenaudomrung N, Kunhorm P and
Noisa P: Three-dimensional cell culture systems as an in vitro
platform for cancer and stem cell modeling. World J Stem Cells.
11:1065–1083. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Tavares S, Vieira AF, Taubenberger AV,
Araújo M, Martins NP, Brás-Pereira C, Polónia A, Herbig M, Barreto
C, Otto O, et al: Actin stress fiber organization promotes cell
stiffening and proliferation of pre-invasive breast cancer cells.
Nat Commun. 8:152372017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Herber R, Liem A, Pitot H and Lambert PF:
Squamous epithelial hyperplasia and carcinoma in mice transgenic
for the human papillomavirus type 16 E7 oncogene. J Virol.
70:1873–1881. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Sobel G, Szabó I, Páska C, Kiss A,
Kovalszky I, Kádár A, Paulin F and Schaff Z: Changes of cell
adhesion and extracellular matrix (ECM) components in cervical
intraepithelial neoplasia. Pathol Oncol Res. 11:26–31. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Chen Y, Miller C, Mosher R, Zhao X, Deeds
J, Morrissey M, Bryant B, Yang D, Meyer R, Cronin F, et al:
Identification of cervical cancer markers by cDNA and tissue
microarrays. Cancer Res. 63:1927–1935. 2003.PubMed/NCBI
|
|
55
|
Zinner B, Gyöngyösi B, Babarczi E, Kiss A
and Sobel G: Claudin 1 expression characterizes human uterine
cervical reserve cells. J Histochem Cytochem. 61:880–888. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Sobel G, Páska C, Szabó I, Kiss A, Kádár A
and Schaff Z: Increased expression of claudins in cervical squamous
intraepithelial neoplasia and invasive carcinoma. Hum Pathol.
36:162–169. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Ersoz S, Mungan S, Cobanoglu U, Turgutalp
H and Ozoran Y: Prognostic importance of Claudin-1 and Claudin-4
expression in colon carcinomas. Pathol Res Pract. 207:285–289.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Süren D, Yıldırım M, Kaya V, Alikanoğlu
AS, Bülbüller N, Yıldız M and Sezer C: Loss of tight junction
proteins (Claudin 1, 4, and 7) correlates with aggressive behavior
in colorectal carcinoma. Med Sci Monit. 20:1255–1262. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Jung H, Jun KH, Jung JH, Chin HM and Park
WB: The expression of claudin-1, claudin-2, claudin-3, and
claudin-4 in gastric cancer tissue. J Surg Res. 167:e185–e191.
2011. View Article : Google Scholar
|
|
60
|
Wang H and Yang X: The expression patterns
of tight junction protein claudin-1, -3, and -4 in human gastric
neoplasms and adjacent non-neoplastic tissues. Int J Clin Exp
Pathol. 8:881–887. 2015.PubMed/NCBI
|
|
61
|
Tokuhara Y, Morinishi T, Matsunaga T,
Ohsaki H, Kushida Y, Haba R and Hirakawa E: Claudin-1, but not
claudin-4, exhibits differential expression patterns between well-
to moderately-differentiated and poorly-differentiated gastric
adenocarcinoma. Oncol Lett. 10:93–98. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Lourenço SV, Coutinho-Camillo CM, Buim ME,
Pereira CM, Carvalho AL, Kowalski LP and Soares FA: Oral squamous
cell carcinoma: Status of tight junction claudins in the different
histopathological patterns and relationship with clinical
parameters. A tissue-microarray-based study of 136 cases. J Clin
Pathol. 63:609–614. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Tzelepi VN, Tsamandas AC, Vlotinou HD,
Vagianos CE and Scopa CD: Tight junctions in thyroid
carcinogenesis: Diverse expression of claudin-1, claudin-4,
claudin-7 and occludin in thyroid neoplasms. Mod Pathol. 21:22–30.
2008. View Article : Google Scholar
|
|
64
|
Székely E, Törzsök P, Riesz P, Korompay A,
Fintha A, Székely T, Lotz G, Nyirády P, Romics I, Tímár J, et al:
Expression of claudins and their prognostic significance in
noninvasive urothelial neoplasms of the human urinary bladder. J
Histochem Cytochem. 59:932–941. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Resnick MB, Konkin T, Routhier J, Sabo E
and Pricolo VE: Claudin-1 is a strong prognostic indicator in stage
II colonic cancer: A tissue microarray study. Mod Pathol.
18:511–518. 2005. View Article : Google Scholar
|
|
66
|
Shibutani M, Noda E, Maeda K, Nagahara H,
Ohtani H and Hirakawa K: Low expression of claudin-1 and presence
of poorly-differentiated tumor clusters correlate with poor
prognosis in colorectal cancer. Anticancer Res. 33:3301–3306.
2013.PubMed/NCBI
|
|
67
|
Jiang L, Yang L, Huang H, Liu BY and Zu G:
Prognostic and clinical significance of claudin-1 in colorectal
cancer: A systemic review and meta-analysis. Int J Surg.
39:214–220. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Higashi Y, Suzuki S, Sakaguchi T, Nakamura
T, Baba S, Reinecker HC, Nakamura S and Konno H: Loss of claudin-1
expression correlates with malignancy of hepatocellular carcinoma.
J Surg Res. 139:68–76. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Bouchagier KA, Assimakopoulos SF, Karavias
DD, Maroulis I, Tzelepi V, Kalofonos H, Karavias DD, Kardamakis D,
Scopa CD and Tsamandas AC: Expression of claudins-1, -4, -5, -7 and
occludin in hepatocellular carcinoma and their relation with
classic clinicopathological features and patients' survival. In
Vivo. 28:315–326. 2014.PubMed/NCBI
|
|
70
|
Sheehan GM, Kallakury BV, Sheehan CE,
Fisher HA, Kaufman RP Jr and Ross JS: Loss of claudins-1 and -7 and
expression of claudins-3 and -4 correlate with prognostic variables
in prostatic adenocarcinomas. Hum Pathol. 38:564–569. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Chao YC, Pan SH, Yang SC, Yu SL, Che TF,
Lin CW, Tsai MS, Chang GC, Wu CH, Wu YY, et al: Claudin-1 is a
metastasis suppressor and correlates with clinical outcome in lung
adeno-carcinoma. Am J Respir Crit Care Med. 179:123–133. 2009.
View Article : Google Scholar
|
|
72
|
Moldvay J, Fábián K, Jäckel M, Németh Z,
Bogos K, Furák J, Tiszlavicz L, Fillinger J, Döme B and Schaff Z:
Claudin-1 Protein Expression Is a Good Prognostic Factor in
Non-Small Cell Lung Cancer, but only in Squamous Cell Carcinoma
Cases. Pathol Oncol Res. 23:151–156. 2017. View Article : Google Scholar
|
|
73
|
Blanchard AA, Skliris GP, Watson PH,
Murphy LC, Penner C, Tomes L, Young TL, Leygue E and Myal Y:
Claudins 1, 3 and 4 protein expression in ER negative breast cancer
correlates with markers of the basal phenotype. Virchows Arch.
454:647–656. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Kulka J, Szász AM, Németh Z, Madaras L,
Schaff Z, Molnár IA and Tokés AM: Expression of tight junction
protein claudin-4 in basal-like breast carcinomas. Pathol Oncol
Res. 15:59–64. 2009. View Article : Google Scholar
|
|
75
|
Ma F, Ding X, Fan Y, Ying J, Zheng S, Lu N
and Xu B: A CLDN1-negative phenotype predicts poor prognosis in
triple-negative breast cancer. PLoS One. 9:e1127652014. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Morohashi S, Kusumi T, Sato F, Odagiri H,
Chiba H, Yoshihara S, Hakamada K, Sasaki M and Kijima H: Decreased
expression of claudin-1 correlates with recurrence status in breast
cancer. Int J Mol Med. 20:139–143. 2007.PubMed/NCBI
|
|
77
|
Szasz AM, Tokes AM, Micsinai M, Krenacs T,
Jakab C, Lukacs L, Nemeth Z, Baranyai Z, Dede K, Madaras L, et al:
Prognostic significance of claudin expression changes in breast
cancer with regional lymph node metastasis. Clin Exp Metastasis.
28:55–63. 2011. View Article : Google Scholar
|
|
78
|
Steinau M, Rajeevan MS, Lee DR, Ruffin MT,
Horowitz IR, Flowers LC, Tadros T, Birdsong G, Husain M, Kmak DC,
et al: Evaluation of RNA markers for early detection of cervical
neoplasia in exfoliated cervical cells. Cancer Epidemiol Biomarkers
Prev. 16:295–301. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Vázquez-Ortíz G, Ciudad CJ, Piña P,
Vazquez K, Hidalgo A, Alatorre B, Garcia JA, Salamanca F,
Peralta-Rodriguez R, Rangel A, et al: Gene identification by cDNA
arrays in HPV-positive cervical cancer. Arch Med Res. 36:448–458.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Benczik M, Galamb Á, Koiss R, Kovács A,
Járay B, Székely T, Szekerczés T, Schaff Z, Sobel G and Jeney C:
Claudin-1 as a Biomarker of Cervical Cytology and Histology. Pathol
Oncol Res. 22:179–188. 2016. View Article : Google Scholar
|
|
81
|
Hoellen F, Waldmann A, Banz-Jansen C,
Holtrich U, Karn T, Oberländer M, Habermann JK, Hörmann M, Köster
F, Ribbat-Idel J, et al: Claudin-1 expression in cervical cancer.
Mol Clin Oncol. 7:880–884. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Gröne J, Weber B, Staub E, Heinze M,
Klaman I, Pilarsky C, Hermann K, Castanos-Velez E, Röpcke S, Mann
B, et al: Differential expression of genes encoding tight junction
proteins in colorectal cancer: Frequent dysregulation of claudin-1,
-8 and -12. Int J Colorectal Dis. 22:651–659. 2007. View Article : Google Scholar
|
|
83
|
Kinugasa T, Akagi Y, Yoshida T, Ryu Y,
Shiratuchi I, Ishibashi N and Shirouzu K: Increased claudin-1
protein expression contributes to tumorigenesis in ulcerative
colitis-associated colorectal cancer. Anticancer Res. 30:3181–3186.
2010.PubMed/NCBI
|
|
84
|
Dhawan P, Singh AB, Deane NG, No Y, Shiou
SR, Schmidt C, Neff J, Washington MK and Beauchamp RD: Claudin-1
regulates cellular transformation and metastatic behavior in colon
cancer. J Clin Invest. 115:1765–1776. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Aro K, Rosa LE, Bello IO, Soini Y, Mäkitie
AA, Salo T and Leivo I: Expression pattern of claudins 1 and 3-an
auxiliary tool in predicting behavior of mucoepidermoid carcinoma
of salivary gland origin. Virchows Arch. 458:341–348. 2011.
View Article : Google Scholar
|
|
86
|
Németh J, Németh Z, Tátrai P, Péter I,
Somorácz A, Szász AM, Kiss A and Schaff Z: High expression of
claudin-1 protein in papillary thyroid tumor and its regional lymph
node metastasis. Pathol Oncol Res. 16:19–27. 2010. View Article : Google Scholar
|
|
87
|
Gyorffy H: Study of claudins and
prognostic factors in some gastrointestinal diseases. Magy Onkol.
53:377–383. 2009.In Hungarian.
|
|
88
|
Montgomery E, Mamelak AJ, Gibson M, Maitra
A, Sheikh S, Amr SS, Yang S, Brock M, Forastiere A, Zhang S, et al:
Overexpression of claudin proteins in esophageal adenocar-cinoma
and its precursor lesions. Appl Immunohistochem Mol Morphol.
14:24–30. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Li W, Dong Q, Li L, Zhang Z, Cai X and Pan
X: Prognostic significance of claudin-1 and cyclin B1 protein
expression in patients with hypopharyngeal squamous cell carcinoma.
Oncol Lett. 11:2995–3002. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Li WJ, Zhang ZL, Yu XM, Cai XL, Pan XL and
Yang XY: Expression of claudin-1 and its relationship with
lymphatic microvessel generation in hypopharyngeal squamous cell
carcinoma. Genet Mol Res. 14:11814–11826. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Bello IO, Vilen ST, Niinimaa A, Kantola S,
Soini Y and Salo T: Expression of claudins 1, 4, 5, and 7 and
occludin, and relationship with prognosis in squamous cell
carcinoma of the tongue. Hum Pathol. 39:1212–1220. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Babkair H, Yamazaki M, Uddin MS, Maruyama
S, Abé T, Essa A, Sumita Y, Ahsan MS, Swelam W, Cheng J, et al:
Aberrant expression of the tight junction molecules claudin-1 and
zonula occludens-1 mediates cell growth and invasion in oral
squamous cell carcinoma. Hum Pathol. 57:51–60. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Sappayatosok K and Phattarataratip E:
Overexpression of Claudin-1 is Associated with Advanced Clinical
Stage and Invasive Pathologic Characteristics of Oral Squamous Cell
Carcinoma. Head Neck Pathol. 9:173–180. 2015. View Article : Google Scholar :
|
|
94
|
Weeraratna AT, Becker D, Carr KM, Duray
PH, Rosenblatt KP, Yang S, Chen Y, Bittner M, Strausberg RL,
Riggins GJ, et al: Generation and analysis of melanoma SAGE
libraries: SAGE advice on the melanoma transcriptome. Oncogene.
23:2264–2274. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Leotlela PD, Wade MS, Duray PH, Rhode MJ,
Brown HF, Rosenthal DT, Dissanayake SK, Earley R, Indig FE,
Nickoloff BJ, et al: Claudin-1 overexpression in melanoma is
regulated by PKC and contributes to melanoma cell motility.
Oncogene. 26:3846–3856. 2007. View Article : Google Scholar
|
|
96
|
Shiozaki A, Shimizu H, Ichikawa D, Konishi
H, Komatsu S, Kubota T, Fujiwara H, Okamoto K, Iitaka D, Nakashima
S, et al: Claudin 1 mediates tumor necrosis factor alpha-induced
cell migration in human gastric cancer cells. World J
Gastroenterol. 20:17863–17876. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Fritzsche FR, Oelrich B, Johannsen M,
Kristiansen I, Moch H, Jung K and Kristiansen G: Claudin-1 protein
expression is a prognostic marker of patient survival in renal cell
carcinomas. Clin Cancer Res. 14:7035–7042. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Huang J, Li J, Qu Y, Zhang J, Zhang L,
Chen X, Liu B and Zhu Z: The expression of claudin 1 correlates
with β-catenin and is a prognostic factor of poor outcome in
gastric cancer. Int J Oncol. 44:1293–1301. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Sun BS, Yao YQ, Pei BX, Zhang ZF and Wang
CL: Claudin-1 correlates with poor prognosis in lung
adenocarcinoma. Thorac Cancer. 7:556–563. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Zhang ZF, Pei BX, Wang AL, Zhang LM, Sun
BS, Jiang RC and Wang CL: Expressions of CLDN1 and insulin-like
growth factor 2 are associated with poor prognosis in stage N2
non-small cell lung cancer. Chin Med J (Engl). 126:3668–3674.
2013.
|
|
101
|
Hahn-Strömberg V, Askari S, Ahmad A,
Befekadu R and Nilsson TK: Expression of claudin 1, claudin 4, and
claudin 7 in colorectal cancer and its relation with CLDN DNA
methylation patterns. Tumour Biol. 39:10104283176975692017.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Zwanziger D, Badziong J, Ting S, Moeller
LC, Schmid KW, Siebolts U, Wickenhauser C, Dralle H and Fuehrer D:
The impact of CLAUDIN-1 on follicular thyroid carcinoma
aggressiveness. Endocr Relat Cancer. 22:819–830. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Ma H, Yan J, Zhang C, Qin S, Qin L, Liu L,
Wang X and Li N: Expression of papillary thyroid
carcinoma-associated molecular markers and their significance in
follicular epithelial dysplasia with papillary thyroid
carcinoma-like nuclear alterations in Hashimoto's thyroiditis. Int
J Clin Exp Pathol. 7:7999–8007. 2014.
|
|
104
|
Abd El AttiRM and Shash LS: Potential
diagnostic utility of CD56 and claudin-1 in papillary thyroid
carcinoma and solitary follicular thyroid nodules. J Egypt Natl
Canc Inst. 24:175–184. 2012. View Article : Google Scholar
|
|
105
|
Furuse M, Hata M, Furuse K, Yoshida Y,
Haratake A, Sugitani Y, Noda T, Kubo A and Tsukita S: Claudin-based
tight junctions are crucial for the mammalian epidermal barrier: A
lesson from claudin-1-deficient mice. J Cell Biol. 156:1099–1111.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Reyes JL, Lamas M, Martin D, del Carmen
Namorado M, Islas S, Luna J, Tauc M and González-Mariscal L: The
renal segmental distribution of claudins changes with development.
Kidney Int. 62:476–487. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Lu Z, Ding L, Lu Q and Chen YH: Claudins
in intestines: Distribution and functional significance in health
and diseases. Tissue Barriers. 1:e249782013. View Article : Google Scholar
|
|
108
|
Garcia-Hernandez V, Quiros M and Nusrat A:
Intestinal epithelial claudins: Expression and regulation in
homeostasis and inflammation. Ann N Y Acad Sci. 1397:66–79. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Tőkés AM, Szász AM, Juhász E, Schaff Z,
Harsányi L, Molnár IA, Baranyai Z, Besznyák I Jr, Zaránd A, Salamon
F, et al: Expression of tight junction molecules in breast
carcinomas analysed by array PCR and immunohistochemistry. Pathol
Oncol Res. 18:593–606. 2012. View Article : Google Scholar
|
|
110
|
Németh Z, Szász AM, Tátrai P, Németh J,
Gyorffy H, Somorácz A, Szíjártó A, Kupcsulik P, Kiss A and Schaff
Z: Claudin-1, -2, -3, -4, -7, -8, and -10 protein expression in
biliary tract cancers. J Histochem Cytochem. 57:113–121. 2009.
View Article : Google Scholar :
|
|
111
|
Zhang Z, Wang A, Sun B, Zhan Z, Chen K and
Wang C: Expression of CLDN1 and CLDN10 in lung adenocarcinoma in
situ and invasive lepidic predominant adenocarcinoma. J
Cardiothorac Surg. 8:952013. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Cheung ST, Leung KL, Ip YC, Chen X, Fong
DY, Ng IO, Fan ST and So S: Claudin-10 expression level is
associated with recurrence of primary hepatocellular carcinoma.
Clin Cancer Res. 11:551–556. 2005.PubMed/NCBI
|
|
113
|
Sanada Y, Yoshida K and Itoh H: Comparison
of CT enhancement patterns and histologic features in
hepatocellular carcinoma up to 2 cm: Assessment of malignant
potential with claudin-10 immunohistochemistry. Oncol Rep.
17:1177–1182. 2007.PubMed/NCBI
|
|
114
|
Aldred MA, Huang Y, Liyanarachchi S,
Pellegata NS, Gimm O, Jhiang S, Davuluri RV, de la Chapelle A and
Eng C: Papillary and follicular thyroid carcinomas show distinctly
different microarray expression profiles and can be distinguished
by a minimum of five genes. J Clin Oncol. 22:3531–3539. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Barros-Filho MC, Marchi FA, Pinto CA,
Rogatto SR and Kowalski LP: High Diagnostic Accuracy Based on
CLDN10, HMGA2, and LAMB3 Transcripts in Papillary Thyroid
Carcinoma. J Clin Endocrinol Metab. 100:E890–E899. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Blaskewicz CD, Pudney J and Anderson DJ:
Structure and function of intercellular junctions in human cervical
and vaginal mucosal epithelia. Biol Reprod. 85:97–104. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Cereijido M, Robbins ES, Dolan WJ, Rotunno
CA and Sabatini DD: Polarized monolayers formed by epithelial cells
on a permeable and translucent support. J Cell Biol. 77:853–880.
1978. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Martinez-Palomo A, Meza I, Beaty G and
Cereijido M: Experimental modulation of occluding junctions in a
cultured transporting epithelium. J Cell Biol. 87:736–745. 1980.
View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Meza I, Ibarra G, Sabanero M,
Martínez-Palomo A and Cereijido M: Occluding junctions and
cytoskeletal components in a cultured transporting epithelium. J
Cell Biol. 87:746–754. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Meza I, Sabanero M, Stefani E and
Cereijido M: Occluding junctions in MDCK cells: Modulation of
transepithelial permeability by the cytoskeleton. J Cell Biochem.
18:407–421. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Cereijido M, Stefani E and Palomo AM:
Occluding junctions in a cultured transporting epithelium:
Structural and functional heterogeneity. J Membr Biol. 53:19–32.
1980. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Balda MS, Whitney JA, Flores C, González
S, Cereijido M and Matter K: Functional dissociation of
paracellular perme-ability and transepithelial electrical
resistance and disruption of the apical-basolateral intramembrane
diffusion barrier by expression of a mutant tight junction membrane
protein. J Cell Biol. 134:1031–1049. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Fanning AS, Little BP, Rahner C,
Utepbergenov D, Walther Z and Anderson JM: The unique-5 and -6
motifs of ZO-1 regulate tight junction strand localization and
scaffolding properties. Mol Biol Cell. 18:721–731. 2007. View Article : Google Scholar :
|
|
124
|
Van Itallie CM, Gambling TM, Carson JL and
Anderson JM: Palmitoylation of claudins is required for efficient
tight-junction localization. J Cell Sci. 118:1427–1436. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Colegio OR, Van Itallie C, Rahner C and
Anderson JM: Claudin extracellular domains determine paracellular
charge selectivity and resistance but not tight junction fibril
architecture. Am J Physiol Cell Physiol. 284:C1346–C1354. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Colegio OR, Van Itallie CM, McCrea HJ,
Rahner C and Anderson JM: Claudins create charge-selective channels
in the paracellular pathway between epithelial cells. Am J Physiol
Cell Physiol. 283:C142–C147. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
González-Mariscal L, Chávez de Ramírez B
and Cereijido M: Effect of temperature on the occluding junctions
of mono-layers of epithelioid cells (MDCK). J Membr Biol.
79:175–184. 1984. View Article : Google Scholar
|
|
128
|
Gonzalez-Mariscal L, Contreras RG, Bolívar
JJ, Ponce A, Chávez De Ramirez B and Cereijido M: Role of calcium
in tight junction formation between epithelial cells. Am J Physiol.
259:C978–C986. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Contreras RG, Miller JH, Zamora M,
González-Mariscal L and Cereijido M: Interaction of calcium with
plasma membrane of epithelial (MDCK) cells during junction
formation. Am J Physiol. 263:C313–C318. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Amaya E, Alarcón L, Martín-Tapia D,
Cuellar-Pérez F, Cano-Cortina M, Ortega-Olvera JM, Cisneros B,
Rodriguez AJ, Gamba G and González-Mariscal L: Activation of the
Ca2+ sensing receptor and the PKC/WNK4 downstream
signaling cascade induces incorporation of ZO-2 to tight junctions
and its separation from 14-3-3. Mol Biol Cell. 30:2377–2398. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Balda MS, Gonzalez-Mariscal L, Matter K,
Cereijido M and Anderson JM: Assembly of the tight junction: The
role of diacylglycerol. J Cell Biol. 123:293–302. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Balda MS, González-Mariscal L, Contreras
RG, Macias-Silva M, Torres-Marquez ME, García-Sáinz JA and
Cereijido M: Assembly and sealing of tight junctions: Possible
participation of G-proteins, phospholipase C, protein kinase C and
calmodulin. J Membr Biol. 122:193–202. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Sonoda N, Furuse M, Sasaki H, Yonemura S,
Katahira J, Horiguchi Y and Tsukita S: Clostridium perfringens
enterotoxin fragment removes specific claudins from tight junction
strands: Evidence for direct involvement of claudins in tight
junction barrier. J Cell Biol. 147:195–204. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Feldman G, Kiely B, Martin N, Ryan G,
McMorrow T and Ryan MP: Role for TGF-beta in cyclosporine-induced
modulation of renal epithelial barrier function. J Am Soc Nephrol.
18:1662–1671. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
García-Hernández V, Flores-Maldonado C,
Rincon-Heredia R, Verdejo-Torres O, Bonilla-Delgado J,
Meneses-Morales I, Gariglio P and Contreras RG: EGF regulates
claudin-2 and -4 expression through Src and STAT3 in MDCK cells. J
Cell Physiol. 230:105–115. 2015. View Article : Google Scholar
|
|
136
|
Inai T, Kobayashi J and Shibata Y:
Claudin-1 contributes to the epithelial barrier function in MDCK
cells. Eur J Cell Biol. 78:849–855. 1999. View Article : Google Scholar
|
|
137
|
McCarthy KM, Francis SA, McCormack JM, Lai
J, Rogers RA, Skare IB, Lynch RD and Schneeberger EE: Inducible
expression of claudin-1-myc but not occludin-VSV-G results in
aberrant tight junction strand formation in MDCK cells. J Cell Sci.
113:3387–3398. 2000.PubMed/NCBI
|
|
138
|
Van Itallie CM, Mitic LL and Anderson JM:
Claudin-2 forms homodimers and is a component of a high molecular
weight protein complex. J Biol Chem. 286:3442–3450. 2011.
View Article : Google Scholar :
|
|
139
|
Milatz S, Piontek J, Hempel C, Meoli L,
Grohe C, Fromm A, Lee IM, El-Athman R and Günzel D: Tight junction
strand formation by claudin-10 isoforms and claudin-10a/-10b
chimeras. Ann N Y Acad Sci. 1405:102–115. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Nakagawa S and Huibregtse JM: Human
scribble (Vartul) is targeted for ubiquitin-mediated degradation by
the high-risk papillomavirus E6 proteins and the E6AP
ubiquitin-protein ligase. Mol Cell Biol. 20:8244–8253. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Golebiewski L, Liu H, Javier RT and Rice
AP: The avian influenza virus NS1 ESEV PDZ binding motif associates
with Dlg1 and Scribble to disrupt cellular tight junctions. J
Virol. 85:10639–10648. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Johnson C, Sanders K and Fan H: Jaagsiekte
sheep retrovirus transformation in Madin-Darby canine kidney
epithelial cell three-dimensional culture. J Virol. 84:5379–5390.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Töyli M, Rosberg-Kulha L, Capra J,
Vuoristo J and Eskelinen S: Different responses in transformation
of MDCK cells in 2D and 3D culture by v-Src as revealed by
microarray techniques, RT-PCR and functional assays. Lab Invest.
90:915–928. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Mettlen M, Platek A, Van Der Smissen P,
Carpentier S, Amyere M, Lanzetti L, de Diesbach P, Tyteca D and
Courtoy PJ: Src triggers circular ruffling and macropinocytosis at
the apical surface of polarized MDCK cells. Traffic. 7:589–603.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Rahikkala M, Sormunen R and Eskelinen S:
Effects of src kinase and TGFbeta1 on the differentiation and
morphogenesis of MDCK cells grown in three-dimensional collagen and
Matrigel environments. J Pathol. 195:391–400. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Tsukamoto T and Nigam SK: Cell-cell
dissociation upon epithelial cell scattering requires a step
mediated by the proteasome. J Biol Chem. 274:24579–24584. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Takeda H and Tsukita S: Effects of
tyrosine phosphorylation on tight junctions in
temperature-sensitive v-src-transfected MDCK cells. Cell Struct
Funct. 20:387–393. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Connolly-Andersen AM, Magnusson KE and
Mirazimi A: Basolateral entry and release of Crimean-Congo
hemorrhagic fever virus in polarized MDCK-1 cells. J Virol.
81:2158–2164. 2007. View Article : Google Scholar :
|
|
149
|
Nunbhakdi-Craig V, Craig L, Machleidt T
and Sontag E: Simian virus 40 small tumor antigen induces
deregulation of the actin cytoskeleton and tight junctions in
kidney epithelial cells. J Virol. 77:2807–2818. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Tafazoli F, Zeng CQ, Estes MK, Magnusson
KE and Svensson L: NSP4 enterotoxin of rotavirus induces
paracellular leakage in polarized epithelial cells. J Virol.
75:1540–1546. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Mo C, Schneeberger EE and Arvin AM:
Glycoprotein E of varicella-zoster virus enhances cell-cell contact
in polarized epithelial cells. J Virol. 74:11377–11387. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Rajasekaran AK, Hojo M, Huima T and
Rodriguez-Boulan E: Catenins and zonula occludens-1 form a complex
during early stages in the assembly of tight junctions. J Cell
Biol. 132:451–463. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Gottlieb TA, Ivanov IE, Adesnik M and
Sabatini DD: Actin microfilaments play a critical role in
endocytosis at the apical but not the basolateral surface of
polarized epithelial cells. J Cell Biol. 120:695–710. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Stephens EB, Compans RW, Earl P and Moss
B: Surface expression of viral glycoproteins is polarized in
epithelial cells infected with recombinant vaccinia viral vectors.
EMBO J. 5:237–245. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Schoenenberger CA, Zuk A, Kendall D and
Matlin KS: Multilayering and loss of apical polarity in MDCK cells
trans-formed with viral K-ras. J Cell Biol. 112:873–889. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Gravotta D, Adesnik M and Sabatini DD:
Transport of influenza HA from the trans-Golgi network to the
apical surface of MDCK cells permeabilized in their basolateral
plasma membranes: Energy dependence and involvement of GTP-binding
proteins. J Cell Biol. 111:2893–2908. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
van Meer G and Simons K: The function of
tight junctions in maintaining differences in lipid composition
between the apical and the basolateral cell surface domains of MDCK
cells. EMBO J. 5:1455–1464. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Rindler MJ, Ivanov IE, Plesken H,
Rodriguez-Boulan E and Sabatini DD: Viral glycoproteins destined
for apical or basolateral plasma membrane domains traverse the same
Golgi apparatus during their intracellular transport in doubly
infected Madin-Darby canine kidney cells. J Cell Biol.
98:1304–1319. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Roth MG and Compans RW: Delayed appearance
of pseudotypes between vesicular stomatitis virus influenza virus
during mixed infection of MDCK cells. J Virol. 40:848–860. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Noyce RS, Delpeut S and Richardson CD: Dog
nectin-4 is an epithelial cell receptor for canine distemper virus
that facilitates virus entry and syncytia formation. Virology.
436:210–220. 2013. View Article : Google Scholar
|
|
161
|
Hernandez S, Chavez Munguia B and
Gonzalez-Mariscal L: ZO-2 silencing in epithelial cells perturbs
the gate and fence function of tight junctions and leads to an
atypical monolayer architecture. Exp Cell Res. 313:1533–1547. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Raya-Sandino A, Castillo-Kauil A,
Domínguez-Calderón A, Alarcón L, Flores-Benitez D, Cuellar-Perez F,
López-Bayghen B, Chávez-Munguía B, Vázquez-Prado J and
González-Mariscal L: Zonula occludens-2 regulates Rho proteins
activity and the development of epithelial cytoarchitecture and
barrier function. Biochim Biophys Acta Mol Cell Res.
1864:1714–1733. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Günzel D and Yu AS: Claudins and the
modulation of tight junction permeability. Physiol Rev. 93:525–569.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Enck AH, Berger UV and Yu AS: Claudin-2 is
selectively expressed in proximal nephron in mouse kidney. Am J
Physiol Renal Physiol. 281:F966–F974. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Rahner C, Mitic LL and Anderson JM:
Heterogeneity in expression and subcellular localization of
claudins 2, 3, 4, and 5 in the rat liver, pancreas, and gut.
Gastroenterology. 120:411–422. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Van Itallie CM, Rogan S, Yu A, Vidal LS,
Holmes J and Anderson JM: Two splice variants of claudin-10 in the
kidney create paracellular pores with different ion selectivities.
Am J Physiol Renal Physiol. 291:F1288–F1299. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Ikari A, Sato T, Takiguchi A, Atomi K,
Yamazaki Y and Sugatani J: Claudin-2 knockdown decreases matrix
metallopro-teinase-9 activity and cell migration via suppression of
nuclear Sp1 in A549 cells. Life Sci. 88:628–633. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Hicks DA, Galimanis CE, Webb PG, Spillman
MA, Behbakht K, Neville MC and Baumgartner HK: Claudin-4 activity
in ovarian tumor cell apoptosis resistance and migration. BMC
Cancer. 16:7882016. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Dahiya N, Becker KG, Wood WH III, Zhang Y
and Morin PJ: Claudin-7 is frequently overexpressed in ovarian
cancer and promotes invasion. PLoS One. 6:e221192011. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Ashikari D, Takayama KI, Obinata D,
Takahashi S and Inoue S: CLDN8, an androgen-regulated gene,
promotes prostate cancer cell proliferation and migration. Cancer
Sci. 108:1386–1393. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Ip YC, Cheung ST, Lee YT, Ho JC and Fan
ST: Inhibition of hepatocellular carcinoma invasion by suppression
of claudin-10 in HLE cells. Mol Cancer Ther. 6:2858–2867. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Sun L, Feng L and Cui J: Increased
expression of claudin-17 promotes a malignant phenotype in
hepatocyte via Tyk2/Stat3 signaling and is associated with poor
prognosis in patients with hepatocellular carcinoma. Diagn Pathol.
13:722018. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Shang X, Lin X, Alvarez E, Manorek G and
Howell SB: Tight junction proteins claudin-3 and claudin-4 control
tumor growth and metastases. Neoplasia. 14:974–985. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Ren Y, Wu Q, Liu Y, Xu X and Quan C: Gene
silencing of claudin-6 enhances cell proliferation and migration
accompanied with increased MMP-2 activity via p38 MAPK signaling
pathway in human breast epithelium cell line HBL-100. Mol Med Rep.
8:1505–1510. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Li Y, Gong Y, Ning X, Peng D, Liu L, He S,
Gong K, Zhang C, Li X and Zhou L: Downregulation of CLDN7 due to
promoter hypermethylation is associated with human clear cell renal
cell carcinoma progression and poor prognosis. J Exp Clin Cancer
Res. 37:2762018. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Li HP, Peng CC, Wu CC, Chen CH, Shih MJ,
Huang MY, Lai YR, Chen YL, Chen TW, Tang P, et al: Inactivation of
the tight junction gene CLDN11 by aberrant hypermethylation
modulates tubulins polymerization and promotes cell migration in
nasopharyngeal carcinoma. J Exp Clin Cancer Res. 37:1022018.
View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Webb PG, Spillman MA and Baumgartner HK:
Claudins play a role in normal and tumor cell motility. BMC Cell
Biol. 14:192013. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Ikari A, Takiguchi A, Atomi K, Sato T and
Sugatani J: Decrease in claudin-2 expression enhances cell
migration in renal epithelial Madin-Darby canine kidney cells. J
Cell Physiol. 226:1471–1478. 2011. View Article : Google Scholar
|
|
179
|
Handorf AM, Zhou Y, Halanski MA and Li WJ:
Tissue stiffness dictates development, homeostasis, and disease
progression. Organogenesis. 11:1–15. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Indra I and Beningo KA: An in vitro
correlation of metastatic capacity, substrate rigidity, and ECM
composition. J Cell Biochem. 112:3151–3158. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Cartagena-Rivera AX, Van Itallie CM,
Anderson JM and Chadwick RS: Apical surface supracellular
mechanical properties in polarized epithelium using noninvasive
acoustic force spectroscopy. Nat Commun. 8:10302017. View Article : Google Scholar : PubMed/NCBI
|