1. Introduction
Thyroid carcinoma (TC) is the most prevalent
malignant tumor of the endocrine system, as well as the head and
neck. It is the most common type of tumor among adolescents and
youths, and its incidence growth rate is highest among females
(1-3). Statistically, approximately 70% of
thyroid nodules are diagnosed as benign prior to the surgical
procedure, 5-10% as malignant (4),
and approximately 10-30% remain uncategorized (5). Among these uncategorized nodules,
only 20-25% are eventually identified as TC following surgery,
accounting for approximately 75-80% of unnecessary thyroidectomies
in patients (6,7). Thus, the detection of specific
diagnostic markers for goiter that do not implicate fine needle
aspiration may mitigate unnecessary thyroidectomies (8). Additionally, apart from papillary TC
(PTC) and follicular TC (FTC), approximately 3% of TC cases are
categorized as myeloid TC (MTC) and anaplastic TC (ATC). However,
MTC and ATC are poorly differentiated, which hamper their
treatment. Thus, an in-depth analysis of MTC and ATC may improve
the overall level of treatment in TC (9).
Extracellular vesicles (EVs) are small membrane
vesicles with lipid bilayer membrane structure (10). EVs were isolated from serum,
saliva, urine, semen, breast milk, amniotic fluid, ascites,
cerebrospinal fluid, bile and a variety of body fluids (11). Liquid biopsy plays a crucial role
in the primary screening of cancer, the identification of malignant
nodules, the prediction of local TC metastasis, the assessment of
prognosis and the treatment response in cancer. In addition, liquid
biopsy is comparatively less invasive than traditional diagnostic
tests. Moreover, EVs may also regulate a myriad of intracellular
life processes by exerting autocrine and paracrine functions
(12). EVs play vital
physiological roles in immune regulation, tumor growth
infiltration, coagulation, tissue homeostasis and neurodegenerative
diseases (13). Additionally, EVs
are considered promising natural drug carriers due to their
relatively stable membrane structure and better biocompatibility
(14,15). The contents and transportation
routes of EVs can be engineered, rendering EVs an efficient tool
for targeted tumor therapy (16)
and for counteracting drug resistance (17). The present review systematically
expounds the recent research investigations on EVs in TC and
discusses the new opportunities and challenges as regards the
application of EVs in thyroid tumors.
2. Characteristics of EVs
Generally, EVs are categorized as exosomes (30-200
nm in diameter), microvesicles (200-1,000 nm in diameter) and
apoptotic bodies (50-5,000 nm in diameter) (18,19).
Early endosomes are formed by non-clathrin or clathrin-mediated
endocytic processes. Early endosomes wrap part of the cytosol by
invaginating inward to form intraluminal vesicles (ILVs). At this
time, early endosomes mature to form multivesicular bodies (MVBs).
Exosomes are formed when ILVs stored in MVBs are released into the
extracellular space, post-MVB and cell membrane fusion. Unlike
exosomes, microvesicles are released directly in the extracellular
space via exocytosis (20).
Moreover, when the cell initiates the apoptotic program, the fusion
of MVBs and lysosomes forms apoptotic bodies. EVs mediate
intercellular communication by secreting proteins (21) and nucleic acids (22). Intercellular communication is
crucial in multicellular organisms, and it occurs either by the
uptake or by the secretion of the signaling factors. All eukaryotic
(such as amoebae, Caenorhabditis elegans and mammalian
parasites) and prokaryotic cells release vesicles into the
extracellular environment (23)
(Fig. 1). It should be noted that
the fusion of MVBs with lysosomes often results in the degradation
of MVBs and the recycling of their raw materials. Thus, apoptotic
bodies are not secreted in the extracellular environment, so they
do not participate in intercellular communication. Exosomes and
microvesicles have become major concerns as regards cancer
progression (12). Microvesicles
are usually larger than exosomes, and their composition is
identical to that of parent cells (24). Exosomes carrying specific signaling
substances can participate in a variety of life processes through
intercellular communication (Table
I). However, the inability of the commonly used commercial
exosome purification kits and protocols to segregate the
microvesicles and exosomes needs to be emphasized (10). Furthermore, published articles have
not fully succeeded in specifying the subtypes of their vesicular
samples. Therefore, as per the recommendation of the International
EVs Society, microvesicles and exosomes should be collectively
addressed as EVs. However, the term subtype should be used with
caution in the absence of reliable subcellular origin markers
(25).
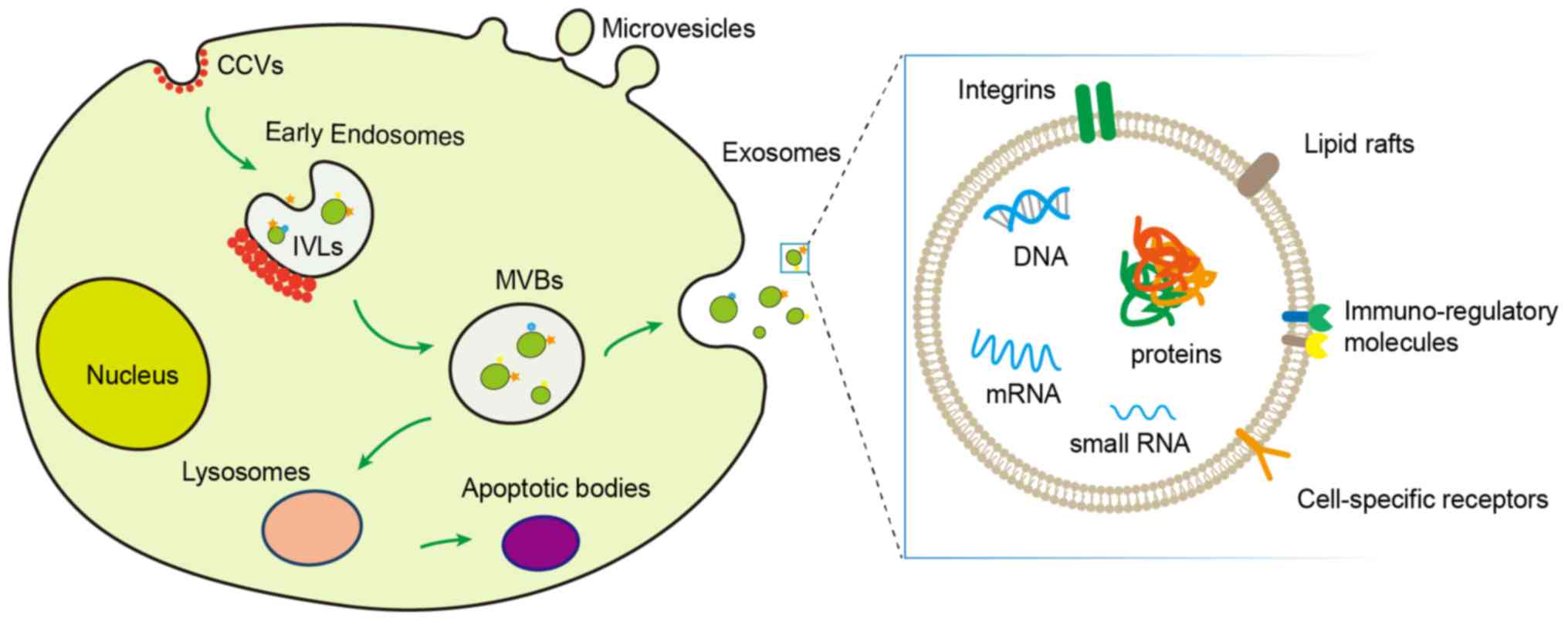 | Figure 1Biogenesis and structural composition
of EVs. EVs include exosomes, microvesicles and apoptotic bodies.
After the fusion of MVBs with the cell membrane, the IVLs in the
MVBs are released outside the cell and become exosomes. The
microvesicles bud directly from the cell surface, and apoptotic
bodies are formed by the fusion of MVBs and lysosomes. EVs contain
proteins, lipids, and nucleic acids (DNA, mRNA and small RNA),
which are crucial signaling molecules for intercellular
communication functions. Red dots represent CCVs. Triangles,
rectangles and pentagons represent membrane-associated and
transmembrane proteins on the vesicles. Arrows indicate the
transport direction of proteins and lipids between organelles, MVBs
and plasma membrane during exosome secretion. EVs, extracellular
vesicles; MVBs, multivesicular bodies; IVLs, intraluminal vesicles;
CCVs, clathrin-coated vesicles. |
 | Table IGeneral characterizations of the
extracellular vesicles. |
Table I
General characterizations of the
extracellular vesicles.
| Markers | Size (nm) | Origin | Intercellular
communication |
|---|
| Exosomes | Proteins:
Tetraspanins (CD9, CD82, CD81, CD63), cytoplasmic proteins (Rab,
annexins), co-stimulatory molecules (CD86), adhesion molecules
(CD54 and CD11b), membrane proteins (CD55, CD59), heat shock
proteins (Hsp70, Hsp90).
Immunomodulators: MHC-I; MHC-I.
Nucleic acids: DNAs, miRNAs, mRNAs, non-coding RNAs.
Lipids | 30-200 | Fusion of MVBs with
plasma membrane | Yes |
| Microvesicles | Selectins;
integrins; tissues factor and cell-specific markers | 200-1,000 | Exocytosis of cell
plasma membrane | Yes |
| Apoptotic
bodies | Histones;
organelles | 50-5,000 | Fusion of MVBs with
lysosomes | No |
Initially, vesicles 40-1,000 nm in diameter released
by different types of cells were termed as exosomes (26). Subsequently, Harding et al
reported that MVBs and plasma membrane fusion initiates the release
of exosomes in extracellular space. Their study demonstrated that
MVBs were involved in the process of exosomal release (27). Based on this finding, exosomes were
considered to be vesicles 40-100 nm in diameter released by the
fusion of the MVBs with the plasma membrane (28). Previous studies confined the
cellular functions of vesicles, primarily to the waste secretion,
and thus EVs failed to attract ample attention. Subsequently, EVs
re-entered the researcher's vision as an essential intercellular
communication medium. In the 1990s, Raposo et al and
Zitvogel et al found that the EVs released by B lymphocytes
and dendritic cells had the antigen-presenting ability, which
stimulated the T-cell-dependent anti-tumor effects (29,30).
In 2007, Valadi et al, for the first time, reported the
exosome-mediated intercellular exchange of genetic materials.
Exosomes from tumorigenic cells promoted the tumor progression and
metastasis, and exosomes secreted by adjacent normal cells had
tumor-killing capacity (31).
These findings shifted the focus of researchers to EVs and
encouraged a plethora of studies, exploring the potential of
clinical translation of EVs. EVs secreted by Gram-negative bacteria
can be utilized as vectors to deliver lipopolysaccharide (LPS) into
the host cytoplasm, triggering an intracellular
caspase-11-dependent immune response (32). In addition, EVs carrying suicide
mRNA and protein following genetic engineering can play a role in
the treatment of schwannoma (33).
These studies have paved the way for the advancement of clinical
application of EVs. Recently, a series of discoveries have tapped
the role of EVs in cancer and the immune system. Several new
studies on TC are currently focusing on the development, diagnosis,
and treatment of TC. In view of this, it is necessary to summarize
the role of EVs in TC.
3. Role of EVs in TC progression
EVs persist in the body fluid and tumor
microenvironment of cancer patients. Within 2 h of secretion by
tumor cells, EVs directly interact with other tumor cells and
become engulfed within 24 h by these cells (34). These internalized signaling
molecules regulate the pathophysiological state of the recipient
cells. Therefore, functional characterization of the TC-derived
exosomal content can provide the clues for unraveling the
underlying mechanism for the TC occurrence and development
(Fig. 2).
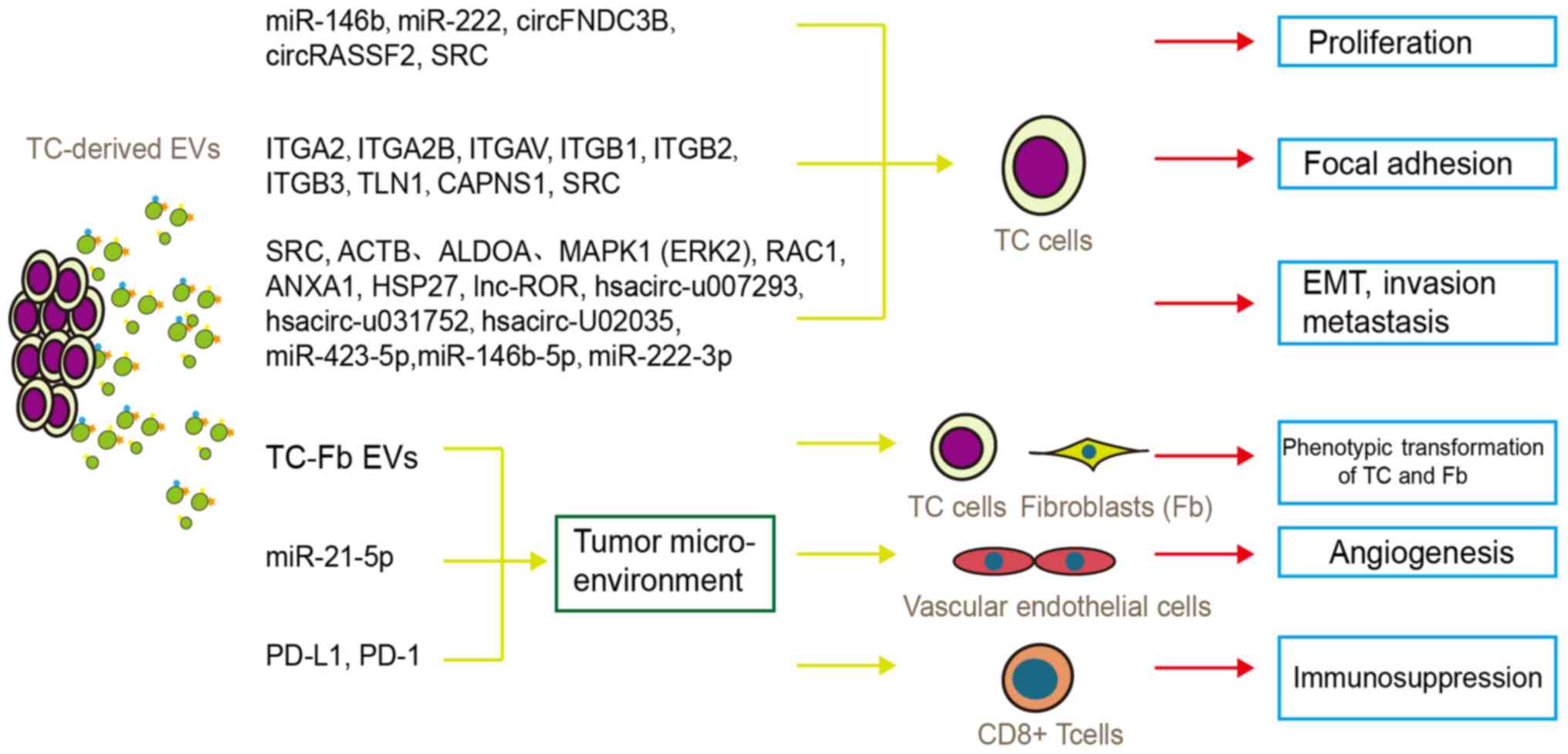 | Figure 2Function of tumor-derived EVs in TC
progression and tumor microenvironment regulation. TC-derived EVs
participate in tumor cell proliferation, invasion, metastasis, EMT,
and microenvironmental regulation (phenotypic transformation of TC
and Fbs, angiogenesis, and immunosuppression) via multiple proteins
and RNAs. EVs, extracellular vesicles; EMT, epithelial-mesenchymal
transition; Fb, fibroblast; TC, thyroid carcinoma. |
EVs affect the growth of TC
The content of tumor-derived EVs is also involved in
the regulation of TC proliferation. The miR-146b and miR-222 levels
in the TPC-1 derived exosomes (PTC cell line) were higher than
those of NTHY cells (normal thyroid cells). The T-EXO (TPC-1
derived exosomes) inhibited the proliferation of TPC-1 and NTHY
cells (35). However, some studies
have demonstrated that these two miRNAs increased the proliferation
of normal thyroid and TC cells (36,37).
The inconsistency in these studies may be as the latter did not
involve EV-mediated miRNA transfer; in addition, miRNAs may
regulate receptor cells through different targets in different
processes. Moreover, circFNDC3B and circRASSF2 were significantly
upregulated in serum purified EVs (SPEs) derived from patients with
PTC, and their downregulation inhibited the proliferation and
promoted the apoptosis of PTC cells. Further research studies found
that both of these circRNAs act as a sponge of miR-1178 and
regulate the miR-1178-TLR4 axis in promoting the PTC progression
(38,39).
EVs promote the invasion and metastasis
of TC
The contents of tumor-derived EVs regulate the
invasion and metastasis of recipient cells during the process of
intercellular exchange (40). A
previous study reported that the typical proteins associated with
the integrin-mediated cell adhesion pathway, i.e., ITGA2, ITGA2B,
ITGAV, ITGB1, ITGB2 and ITGB3, along with the proteins located in
the upstream of the integrin pathway, i.e., TLN1, ITGB2, CAPNS1 and
SRC, were overexpressed in SPEs of patients with PTCs with lymph
node metastasis (LNM) (41). As
per the previous classic hypothesis on metastasis, tumor cells
secreted EVs prior to the onset of metastasis, and these EVs
reached the pre-metastatic site via the circulatory system. The
components in EVs can affect the recipient cell environment and
form a microenvironment suitable for tumor cell growth, the
'pre-metastatic niche' (42-44).
Therefore, the abnormal expression of integrin in SPEs of patients
with PTC with LNM may be involved in the formation of a
pre-metastatic niche. SRC inhibitors have also been shown to
inhibit the proliferation and invasion of PTC cells (45). Furthermore, ACTB, ALDOA, MAPK1
(ERK2), RAC1, ANXA1 and HSP27 have been shown to also be
overexpressed in SPEs from patients with PTC with LNM, which
promoted the invasion and metastasis of TC. Among these, RAC1 gene
knockdown was shown to alleviate the migration and invasion of
TPCB1 and HTH83TC cells (46).
The underlying mechanism for the EV-mediated
regulation of TC metastasis is not limited to proteins only, but it
includes a number of non-coding RNAs, such as lncRNAs, cricRNAs and
miRNAs. According to a previous study, TC stem cell (TCSC)-derived
EVs induce epithelial-mesenchymal transition (EMT) in normal
thyroid cells by transferring lnc-ROR (47). EMT leads to a loss of polarity,
close intercellular connection and adhesion (loss of e-cadherin,
and so on) in epithelial cells through a series of reactions and
facilitates invasion and migration in these cells. lnc-ROR
overexpression in TCSCs significantly enhances the proliferation
and invasion of target cells. This indicates that the intervention
of stem cells, stem cell-derived EVs or lncRNAs has the potential
to inhibit tumor metastasis.
A recent study reported the expression levels of
circRNAs in SPEs of PTC and benign goiter by using high-throughput
sequencing. In that study, SPEs from patients with PTC exhibited 3
upregulated (hsacirc-u007293, hsacirc-u031752 and hsacirc-u020135)
and 19 downregulated circRNAs. It was also found that these
differentially expressed circRNAs regulated 16 signaling pathways,
among which the PI3K-Akt and AMPK signaling pathways participated
in the invasion and metastasis of TC (48). Apart from circRNAs, some studies
have reported that miRNAs in EVs derived from patients with PTC
also play a role in metastasis. The augmented migration and
invasion of PTC cells have been observed in patients with PTC with
significantly escalated miR-423-5p levels in SPEs (49). Also, increased levels of
miR-146b-5p and miR-222-3p were observed in SPEs from PTC patients
with LNM as compared to PTC patients without LNM. Wound healing
experiments and Transwell assays have proven that the upregulation
of miR-146b-5p and miR-222-3p in PTC cell lines (K1 cells and BCPAP
cells) significantly enhances the invasion and migration of PTC
cells and their downregulation results in opposite effects
(50).
EVs affect the microenvironment of
TC
The tumor microenvironment promotes the onset of the
tumor and sustains its development, in a similar manner to that of
soil with seeds. Immune cells, nascent tumor cells, tumor
extracellular matrix and tumor blood vessels in the tumor
microenvironment co-evolve with tumor cells (51). Multiple mechanisms have been
proposed to explain the TC microenvironment, with EVs being an
integral part of these.
Effects of TC-derived EVs on
fibroblasts
Recently, a new mode of EVs involved in TC
metastasis has been proposed, TC and fibroblast (Fb) cell
crosstalk. Herein, it was previously demonstrated that Fb or Fb and
TC cell co-culture-derived conditioned medium (CM) induces the
migratory phenotype of TC cells, such as MMP2 activation and the
formation of filopodia and lamellipodia in TC. Moreover, EVs
extracted from the CM of Fb-TC exhibited CD147 overexpression and
MMP2 secretion. The CD147 interaction with interstitial tumor cells
promoted MMP2 secretion and activation. The Fb and TC cell
co-cultured CM and EVs promoted metastasis and invasive phenotypic
alterations in TC and Fb cells (52). The TC and Fb crosstalk promoted TC
cells and Fb EMT and tumor progression in general.
Effects of TC-derived EVs on
angiogenesis
It was previously reported that the miR-21-5p
expression level in PTC cell (BCPAP and KTC-1 cell lines)-derived
EVs under hypoxic conditions was significantly higher than that of
normal thyroid follicular cell line (Nthy-ori-3-1) and normoxic PTC
cells. In addition, the miR-21-5p expression level in SPEs of
patients with PTC was higher than that of healthy individuals. PTC
cells derived exosomes under hypoxic conditions promoted the
vascular endothelial cells formation, and conversely, miR-21-5p
downregulation or normoxic conditions inhibited it. The angiogenic
ability of SPEs from patients with PTC was also confirmed at the
tissue level. It was found that miR-21-5p promoted this process by
directly targeting and inhibiting TGFBI and COL4A1 (53).
Effects of TC-derived EVs on immune
cells
The status of immune cells in the tumor
microenvironment is also closely related to tumor progression.
Immune cells, such as B cells, tumor-associated macrophages (TAM)
and Tregs suppress cytotoxic T cells and CD8+ T cells
via EVs, and promote immune escape and drug resistance in tumor
cells (54). Tumor cells also
secrete EVs to mitigate the immune cell activation and cytokine
secretion. A previous study reported that the PD-1 and PD-L1 levels
were significantly higher in children with PTC than in healthy
children. When EVs with high PD-L1 expression were co-incubated
with CD8+ T cells, the PD-1 level remained unaffected,
although the levels of inflammatory cytokines (CD69, IFN-γ and
TNF-α) were significantly depleted. This suggests that PD-L1 can
bind to PD-1 expressed on the surface of CD8+ T cells
and interfere with the effector T cell activation (55).
4. Role of EVs in TC diagnosis
The diagnostic accuracy of tumors can be enhanced by
employing liquid biopsy as an alternative and complementary
diagnostic method in cases where the cytological diagnosis is
uncertain. It can also reduce the rate of invasive puncture, easing
the overtreatment of patients with benign nodules. EVs secreted
into body fluids stably retain the vesicle's content and partial
characteristics of secretory cells (56). The signaling biomolecules of EVs
can be categorized as non-specific and specific. Non-specific
biomolecules are primarily related to the common biological
characteristics of EVs, such as vesicle formation, binding with
receptor cells and so on. Therefore, these molecules are employed
for the isolation and identification of EVs. However, the specific
biomolecules precisely reflect the parent cell condition, such as
carcinogenesis or metastasis (57). Thus, summarizing the TC-specific
molecules in the EVs could assist the diagnosis and prognosis of TC
along with the recurrence and metastasis prediction.
Previous studies have reported that miR-146b,
miR-222 (35), miR-5189-3p
(58), circFNDC3B (circ_0006156)
(38), circRASSF2 (circ_0059354)
(39), miR-31-5p, miR-21-5p
(59), miR-346, miR-10a-5p,
miR-34a-5p (60), hsacirc_007293,
hsacirc_031752, hsacirc_020135 (48) and miR-4433a-5p (61), which were overexpressed in EVs,
serve as diagnostic biomarkers for PTC and FTC, to identify TC and
distinguish it from healthy or benign thyroid nodules (Table II) (62). In addition, miR-485-3p functions as
a prognostic biomarker for TC. The overexpression of miR-485-3p in
the SPEs from patients with PTC is associated with a tumor size ≥1
cm, extrathyroid metastasis, lymph node metastasis and BRAF
mutation. Thus, miR-485-3p can be used as a biomarker to
distinguish high-risk from low-risk PTC (61). Concurrently, EV markers suggesting
the LNM of TC have also been discovered. The overexpression of
miR-146b-5p and miR-222-3p in SPEs indicates LNM in patients with
PTC, and these biomarkers have demonstrated 81.1 and 83.4% area
under the curve (AUC) values, respectively. Notably, the AUC values
increased to 89.5% when expression levels of these two miRNAs were
collectively analyzed (50).
Previous studies have stated the diagnostic advantages of using
multiple biomarkers. The combined analysis of miR-21-5p and
miR-181a expression in SPEs can distinguish PTC and FTC with 100%
sensitivity and 87% specificity (59). The combined analysis of
miR-16-2-3p, miR-223-5p, miR-101-3p and miR-34c-5p expression in
SPEs can differentiate PTC from benign goiter (BG) with 74% AUCs,
100% sensitivity and 87% specificity (63), yielding higher values than those
estimated using individual biomarkers.
 | Table IIThyroid carcinoma biomarkers in
extracellular vesicles (62). |
Table II
Thyroid carcinoma biomarkers in
extracellular vesicles (62).
| Biomarkers | Expression level in
TC | Research
objects | Predictive
indicator | Sample sources | Sensitivity,
specificity or AUC values | (Refs.) |
|---|
miR-146b
miR-222 | Up | PTC vs. HC | Differential
diagnosis | Cell lines | Not mentioned | (35) |
| miR-5189-3p | Up | PTC vs. BG | Differential
diagnosis | SPEs | 95.1% AUC | (58) |
| circFNDC3B
(circ_0006156) | Up | PTC vs. HC | Differential
diagnosis | SPEs | Not mentioned | (38) |
| circRASSF2
(circ_0059354) | Up | PTC vs. HC | Differential
diagnosis | SPEs | Not mentioned | (39) |
| miR-31-5p | Up | PTC vs. BG | Differential
diagnosis | SPEs | Not mentioned | (59) |
| miR-21-5p | Up | FTC vs. BG | Differential
diagnosis | SPEs | Not mentioned | (59) |
| miR-346,
miR-10a-5p, miR-34a-5p | Up | PTC vs. HC | Differential
diagnosis | SPEs | Not mentioned | (60) |
| hsacirc_007293,
hsacirc_031752 and hsacirc_020135 | Up | PTC vs. BG | Differential
diagnosis | SPEs | Not mentioned | (48) |
miR-485-3p
miR-4433a-5P | Up | PTC vs. HC vs.
BG | Differential
diagnosis, Prognosis (just for miR-485-3p) | SPEs | Not mentioned with
89.5% AUCs for two miRNAs used together | (61) |
| miR-146b-5p,
miR-222-3p | Up | PTC with vs.
without LNM | LNM | SPEs | with 89.5% AUCs for
two miRNAs used together | (50) |
miR-21-5p
miR-181a | Up | PTC vs. FTC | Differential
diagnosis | SPEs | With 100%
sensitivity and 87% specificity for two miRNAs used together | (59) |
| miR-16-2-3p,
miR-223-5p, miR-101-3p, and miR-34c-5p | Only up in the
first two miRNAs | PTC vs. BG PTC and
FTC before vs. after the surgery | Differential
diagnosis | SPEs | with 74% AUCs,
71.43% sensitivity and 73.33% specificity for four miRNAs used
together | (63) |
| Thyroglobulin | Up | PTC and FTC before
vs. after the surgery | Recurrence,
Prognosis | UEs | Not mentioned | (64) |
| Hsp27, Hsp60, and
Hsp90 | Up | PTC vs. BG vs. PT,
PTC before vs. after the surgery | Differential
diagnosis, recurrence | SPEs | Not mentioned | (65) |
| PD-1, PD-L1 | Up | PTC children vs. HC
children | Differential
diagnosis | SPEs | 81.4% AUCs for
PD-L1 | (55) |
In addition to non-coding RNAs, EV proteins can also
serve as diagnostic biomarkers for TC. A previous study included
patients with differentiated thyroid carcinoma (DTC) at different
stages and analyzed the levels of serum thyroglobulin and urinary
exosomal thyroglobulin (U-Ex-Tg). The outcomes indicated that the
U-Ex-Tg levels were higher in TC patients with T3 stage or LNM than
in those with T1 and T2 stages. Notably, increased serum
thyroglobulin levels, post-surgery, were not detected in TC
patients with a high risk of recurrence. However, the U-Ex-Tg
levels were significantly elevated. This suggests that U-Ex-Tg can
act as a more sensitive molecular marker for prognosis and the
post-operative recurrence prediction of DTC (64). It was also found that HSP27, HSP60
and HSP90 in SPEs from patients with PTC were higher than those in
peritumoral tissues (PT) and BG. These SPEs markers from
preoperative PTC were higher than in PTC, post-surgery. Moreover,
these markers were located in normal sites, such as the cytoplasm
in BG samples, but closer to the plasma membrane in PTC tissues;
thus, it is likely that EVs enclosed these markers, which later
participated in the intercellular communication of TC (65). Moreover, the PD-L1 and PD-1 levels
in SPEs from children with PTC were found to be significantly
higher than in healthy children, and PD-L1 had an 81.4% AUC value.
Moreover, the PD-L1 level in SPEs prior to PTC surgery was higher
than that post-surgery, and PD-L1 overexpression was associated
with T staging (55).
5. Potential applications of EVs in the
treatment of TC
TC-derived EVs can target tumor
regions
Due to the low immunogenicity and toxicity, stable
membrane structure, high biocompatibility and biological
permeability of EVs (15,66,67),
several studies have explored the applicability of EVs in tumor
therapy. The receptor present on the tumor-derived EVs binds to the
ligand present on the tumor cells and mediates the fusion of tumor
cells with the tumor-derived EVs (68). Previous studies have demonstrated
that tumor-derived EVs were more easily internalized by tumor cells
compared with EVs derived from epithelial cells (69). The specific expression of
tetraspanins (a receptor protein) in EVs enables its preferential
binding to specific ligands for the selection of receptor cells
(70). Therefore, engineered EVs
can target a specific tumor type and can function as a therapeutic
agent. It can effectively mitigate the side-effects caused by other
modes of cancer treatment. EVs with hepatocyte growth factor (HGF)
siRNAs that are transported to gastric cancer (GC) cells have been
shown to potentiate the therapeutic effect of tumor growth and
angiogenesis inhibition (71).
This provides a valuable reference for EVs in the treatment of TC.
In the study conducted by Gangadaran et al, CAL62 (ATC cell
line)-derived EVs labeled with Renilla luciferase (Rluc)
were injected into tumor-bearing mice and the targeting accuracy of
EVs for thyroid tumors was monitored (72). Rluc is an efficient monitoring tool
due to its real-time monitoring and high sensitivity. This method
prevented the inclusion of non-specific signal interference and EV
damage caused by the lipophilic dyes, the high signal-to-noise
ratio of bioluminescent imaging (BLI), and the low sensitivity of
MRI. The authors of that study noted that the CAL62-cells derived
EVs were explicitly targeted and internalized into the mouse CAL62
tumor model after 30 min of systemic injection. The targeting
characteristics of TC-derived EVs have been successfully validated,
which indicates that EVs can act as an effective therapeutic agent
by carrying tumor-killing factors.
The killing effect of NK cell-derived EVs
on TC
As a key subpopulation of natural immune
lymphocytes, natural killer (NK) cells can carry out their
anti-tumor functions through cytotoxic effects. The tumor-killing
ability of NK cells, coupled with the targeting ability of EVs, can
expedite the progression of immunotherapeutic clinical
applications. Zhu et al reported that the NK
cell-derived-EVs, which were co-incubated with IL-15, exerted
significant cell lytic and tumor growth inhibitory activity in
human cancer cell lines (glioblastoma, breast cancer and TC), which
was not significantly toxic to normal cells or mice (73). Furthermore, previous studies have
illustrated that although the production of EVs exists in both
physiological and pathological processes (74), the production of naturally secreted
EVs is low (75). Therefore, to
advance the clinical application of EVs, significant issues, such
as EV production, need to be addressed. Incubation with IL-15
increases the NK cell production of EVs (73). Moreover, for the first time, Yang
et al reported the continuous extrusion of NK cells with
filters of different pore sizes (76). This method obtained exosome
mimetics (EMs) with high yields and controllable size. The amount
of EMs thus obtained, was higher in concentration than the purified
EVs, and EMs had a better-targeted killing effect on a variety of
tumor cells, including ATC. These EMs were not toxic to normal
cells and tissues. Zhu et al used this method to embed siRNA
into EMs through electroporation packages and verified the
targeting and tumor-killing effect of NK cell-derived EMs once
again (77). These methods have
greatly enhanced the therapeutic prospects of anti-tumor
cell-derived EVs in a variety of tumors, including TC.
6. Conclusions and future perspectives
Recent advances in microscopic and EV separation
technology have led to an advancement in the research of EVs in TC.
Their rich content and functions render EVs a complex cellular
component. Differentially expressed proteins and RNAs in TC-derived
EVs play a significant role in cancer progression. Over time, a
plethora of potential diagnostic markers have been identified,
which can facilitate the accurate diagnosis of TC in the future,
reducing unnecessary thyroidectomies. TC-derived EVs can target
tumor regions, and NK cell-derived EVs can inhibit thyroid
progression, which opens the door to the use of EVs in TC therapy.
EVs have a targeted therapeutic effect on ATC, which provides a
breakthrough for its poor prognosis and limited treatment. However,
quite a few issues in this field still remain unresolved. The
research samples included by TC-derived EVs are generally
insufficient, and more significant conclusions can be drawn by
increasing the sample size and eliminating individual differences.
In addition, there is still no direct evidence that EVs obtained
from serum or urine are derived from TC cells, which remains a
challenging question in exosomal research. Concisely, in-depth
research investigations are required to unravel the unexplored
areas of EV research, which may also unravel the precise mechanisms
for the EV-mediated onset and progression of TC along with the
clinical diagnosis and treatment.
Acknowledgments
Not applicable.
Funding
The present study was supported by the Science
Foundation of Gansu Province (no. 18JR3RA343) and Science and
Technology Bureau 2018 Fund of the Chengguan District (no.
2018KJGG0037).
Availability of data and materials
Not applicable.
Authors' contributions
YL, YZ and WY were involved in the conception of the
study subject. NL, XN and SP were involved in the study design and
the acquisition of data for the purposes of the present review. YL,
NL and WY were involved in the writing of the article and in
revising the manuscript. All authors have read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2020. CA Cancer J Clin. 70:7–30. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang L, Zheng RS, Wang N, Zeng HM, Yuan
YN, Zhang SW, Li HC, Liu S, Chen WQ and He J: Analysis of incidence
and mortality of thyroid cancer in China, 2013. Zhonghua Zhong Liu
Za Zhi. 39:862–867. 2017.In Chinese. PubMed/NCBI
|
|
3
|
Cronin KA, Lake AJ, Scott S, Sherman RL,
Noone AM, Howlader N, Henley SJ, Anderson RN, Firth AU, Ma J, et
al: Annual report to the nation on the status of cancer, part I:
National cancer statistics. Cancer. 124:2785–2800. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yassa L, Cibas ES, Benson CB, Frates MC,
Doubilet PM, Gawande AA, Moore FD Jr, Kim BW, Nose V, Marqusee E,
et al: Long-term assessment of a multidisciplinary approach to
thyroid nodule diagnostic evaluation. Cancer. 111:508–516. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cooper DS, Doherty GM, Haugen BR, Kloos
RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Pacini F,
Schlumberger M, et al: Revised American Thyroid Association
management guidelines for patients with thyroid nodules and
differentiated thyroid cancer. Thyroid. 19:1167–1214. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sahin M, Gursoy A, Tutuncu NB and Guvener
DN: Prevalence and prediction of malignancy in cytologically
indeterminate thyroid nodules. Clin Endocrinol (Oxf). 65:514–518.
2006. View Article : Google Scholar
|
|
7
|
Gomez Saez JM: Diagnostic and prognostic
markers in differentiated thyroid cancer. Curr Genomics.
12:597–608. 2011. View Article : Google Scholar
|
|
8
|
Jegerlehner S, Bulliard JL, Aujesky D,
Rodondi N, Germann S, Konzelmann I and Chiolero A: Overdiagnosis
and overtreatment of thyroid cancer: A population-based temporal
trend study. PLoS One. 12:e01793872017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Miller KD, Nogueira L, Mariotto AB,
Rowland JH, Yabroff KR, Alfano CM, Jemal A, Kramer JL and Siegel
RL: Cancer treatment and survivorship statistics, 2019. CA Cancer J
Clin. 69:363–385. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tkach M and Thery C: Communication by
extracellular vesicles: Where we are and where we need to go. Cell.
164:1226–1232. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Raposo G and Stoorvogel W: Extracellular
vesicles: Exosomes, microvesicles, and friends. J Cell Biol.
200:373–383. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Maas SLN, Breakefield XO and Weaver AM:
Extracellular vesicles: Unique intercellular delivery vehicles.
Trends Cell Biol. 27:172–188. 2017. View Article : Google Scholar
|
|
13
|
Gallo A, Tandon M, Alevizos I and Illei
GG: The majority of microRNAs detectable in serum and saliva is
concentrated in exosomes. PLoS One. 7:e306792012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ren J, He W, Zheng L and Duan H: From
structures to functions: Insights into exosomes as promising drug
delivery vehicles. Biomater Sci. 4:910–921. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
EL Andaloussi S, Mäger I, Breakefield XO
and Wood MJ: Extracellular vesicles: Biology and emerging
therapeutic opportunities. Nat Rev Drug Discov. 12:347–357. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gilligan KE and Dwyer RM: Engineering
exosomes for cancer therapy. Int J Mol Sci. 18:11222017. View Article : Google Scholar
|
|
17
|
Namee NM and O'Driscoll L: Extracellular
vesicles and anti-cancer drug resistance. Biochim Biophys Acta Rev
Cancer. 1870:123–136. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mori MA, Ludwig RG, Garcia-Martin R,
Brandão BB and Kahn CR: Extracellular miRNAs: From biomarkers to
mediators of physiology and disease. Cell Metab. 30:656–673. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Suchorska WM and Lach MS: The role of
exosomes in tumor progression and metastasis (Review). Oncol Rep.
35:1237–1244. 2016. View Article : Google Scholar
|
|
20
|
Margolis L and Sadovsky Y: The biology of
extracellular vesicles: The known unknowns. PLoS Biol.
17:e30003632019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vella LJ, Hill AF and Cheng L: Focus on
extracellular vesicles: Exosomes and their role in protein
trafficking and biomarker potential in alzheimer's and parkinson's
disease. Int J Mol Sci. 17:1732016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang Y, Xi H, Nie X and Yuan W, Zhang P,
Lan N, Lu Y, Liu J and Yuan W: Assessment of miR-212 and other
biomarkers in the diagnosis and treatment of HBV-infection-related
liver diseases. Curr Drug Metab. 20:785–798. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Colombo M, Raposo G and Thery C:
Biogenesis, secretion, and intercellular interactions of exosomes
and other extracellular vesicles. Annu Rev Cell Dev Biol.
30:255–289. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pegtel DM and Gould SJ: Exosomes Annu Rev
Biochem. 88:487–514. 2019. View Article : Google Scholar
|
|
25
|
Théry C, Witwer KW, Aikawa E, Alcaraz MJ,
Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F,
Atkin-Smith GK, et al: Minimal information for studies of
extra-cellular vesicles 2018 (MISEV2018): A position statement of
the International Society for Extracellular Vesicles and update of
the MISEV2014 guidelines. J Extracell Vesicles. 7:15357502018.
View Article : Google Scholar
|
|
26
|
Trams EG, Lauter CJ, Salem N Jr and Heine
U: Exfoliation of membrane ectoenzymes in the form of
micro-vesicles. Biochim Biophys Acta. 645:63–70. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Harding C, Heuser J and Stahl P:
Endocytosis and intracellular processing of transferrin and
colloidal gold-transferrin in rat reticulocytes: Demonstration of a
pathway for receptor shedding. Eur J Cell Biol. 35:256–263.
1984.PubMed/NCBI
|
|
28
|
Johnstone RM, Adam M, Hammond JR, Orr L
and Turbide C: Vesicle formation during reticulocyte maturation.
Association of plasma membrane activities with released vesicles
(exosomes). J Biol Chem. 262:9412–9420. 1987.PubMed/NCBI
|
|
29
|
Raposo G, Nijman HW, Stoorvogel W,
Liejendekker R, Harding CV, Melief CJ and Geuze HJ: B lymphocytes
secrete antigen-presenting vesicles. J Exp Med. 183:1161–1172.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zitvogel L, Regnault A, Lozier A, Wolfers
J, Flament C, Tenza D, Ricciardi-Castagnoli P, Raposo G and
Amigorena S: Eradication of established murine tumors using a novel
cell-free vaccine: Dendritic cell-derived exosomes. Nat Med.
4:594–600. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Valadi H, Ekström K, Bossios A, Sjöstrand
M, Lee JJ and Lötvall JO: Exosome-mediated transfer of mRNAs and
microRNAs is a novel mechanism of genetic exchange between cells.
Nat Cell Biol. 9:654–659. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vanaja SK, Russo AJ, Behl B, Banerjee I,
Yankova M, Deshmukh SD and Rathinam VAK: Bacterial outer membrane
vesicles mediate cytosolic localization of LPS and caspase-11
activation. Cell. 165:1106–1119. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mizrak A, Bolukbasi MF, Ozdener GB,
Brenner GJ, Madlener S, Erkan EP, Ströbel T, Breakefield XO and
Saydam O: Genetically engineered microvesicles carrying suicide
mRNA/protein inhibit schwannoma tumor growth. Mol Ther. 21:101–108.
2013. View Article : Google Scholar
|
|
34
|
Maacha S, Bhat AA, Jimenez L, Raza A,
Haris M, Uddin S and Grivel JC: Extracellular vesicles-mediated
intercellular commu-nication: Roles in the tumor microenvironment
and anti-cancer drug resistance. Mol Cancer. 18:552019. View Article : Google Scholar
|
|
35
|
Lee JC, Zhao JT, Gundara J, Serpell J,
Bach LA and Sidhu S: Papillary thyroid cancer-derived exosomes
contain miRNA-146b and miRNA-222. J Surg Res. 196:39–48. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Geraldo MV, Yamashita AS and Kimura ET:
MicroRNA miR-146b-5p regulates signal transduction of TGF-β by
repressing SMAD4 in thyroid cancer. Oncogene. 31:1910–1922. 2012.
View Article : Google Scholar
|
|
37
|
Visone R, Russo L, Pallante P, De Martino
I, Ferraro A, Leone V, Borbone E, Petrocca F, Alder H, Croce CM and
Fusco A: MicroRNAs (miR)-221 and miR-222, both overexpressed in
human thyroid papillary carcinomas, regulate p27Kip1 protein levels
and cell cycle. Endocr Relat Cancer. 14:791–798. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wu G, Zhou W, Pan X, Sun Z, Sun Y, Xu H,
Shi P, Li J, Gao L and Tian X: Circular RNA profiling reveals
exosomal circ_0006156 as a novel biomarker in papillary thyroid
cancer. Mol Ther Nucleic Acids. 19:1134–1344. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wu G, Zhou W, Lin X, Sun Y, Li J, Xu H,
Shi P, Gao L and Tian X: circRASSF2 Acts as ceRNA and promotes
papillary thyroid carcinoma progression through miR-1178/TLR4
signaling pathway. Mol Ther Nucleic Acids. 19:1153–1163. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Greening DW, Gopal SK, Mathias RA, Liu L,
Sheng J, Zhu HJ and Simpson RJ: Emerging roles of exosomes during
epithelial-mesenchymal transition and cancer progression. Semin
Cell Dev Biol. 40:60–71. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Luo D, Zhan S, Xia W, Huang L, Ge W and
Wang T: Proteomics study of serum exosomes from papillary thyroid
cancer patients. Endocr Relat Cancer. 25:879–891. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Peinado H, Alečković M, Lavotshkin S,
Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M,
Williams C, García-Santos G, Ghajar C, et al: Melanoma exosomes
educate bone marrow progenitor cells toward a pro-metastatic
phenotype through MET. Nat Med. 18:883–891. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Melo SA, Luecke LB, Kahlert C, Fernandez
AF, Gammon ST, Kaye J, LeBleu VS, Mittendorf EA, Weitz J, Rahbari
N, et al: Glypican-1 identifies cancer exosomes and detects early
pancreatic cancer. Nature. 523:177–182. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Costa-Silva B, Aiello NM, Ocean AJ, Singh
S, Zhang H, Thakur BK, Becker A, Hoshino A, Mark MT, Molina H, et
al: Pancreatic cancer exosomes initiate pre-metastatic niche
formation in the liver. Nat Cell Biol. 17:816–826. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Henderson YC, Toro-Serra R, Chen Y, Ryu J,
Frederick MJ, Zhou G, Gallick GE, Lai SY and Clayman GL: Src
inhibitors in suppression of papillary thyroid carcinoma growth.
Head Neck. 36:375–384. 2014. View Article : Google Scholar
|
|
46
|
Wang C, Yan G, Zhang Y, Jia X and Bu P:
Long non-coding RNA MEG3 suppresses migration and invasion of
thyroid carcinoma by targeting of Rac1. Neoplasma. 62:541–549.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hardin H, Helein H, Meyer K, Robertson S,
Zhang R, Zhong W and Lloyd R V: Thyroid cancer stem-like cell
exosomes: Regulation of EMT via transfer of lncRNAs. Lab Invest.
98:1133–1142. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yang C, Wei Y, Yu L and Xiao Y:
Identification of altered circular RNA expression in serum exosomes
from patients with papillary thyroid carcinoma by high-throughput
sequencing. Med Sci Moni. 25:2785–2791. 2019. View Article : Google Scholar
|
|
49
|
Ye W, Deng X and Fan Y: Exosomal
miRNA423-5p mediated oncogene activity in papillary thyroid
carcinoma: A potential diagnostic and biological target for cancer
therapy. Neoplasma. 66:516–523. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Jiang K, Li G, Chen W, Song L, Wei T, Li
Z, Gong R, Lei J, Shi H and Zhu J: Plasma Exosomal miR-146b-5p and
miR-222-3p are potential biomarkers for lymph node metastasis in
papillary thyroid carcinomas. Onco Targets Ther. 13:1311–1319.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Thery C, Zitvogel L and Amigorena S:
Exosomes: Composition, biogenesis and function. Nat Rev Immunol.
2:569–579. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
52
|
Bravo-Miana RDC, Della Vedova AB, De Paul
AL, Remedi MM, Guantay ML, Gilardoni MB, Pellizas CG and Donadio
AC: Thyroid tumor cells-fibroblasts crosstalk: Role of
extracellular vesicles. Endocr Connect. 9:506–518. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wu F, Li F, Lin X, Xu F, Cui RR, Zhong JY,
Zhu T, Shan SK, Liao XB, Yuan LQ and Mo ZH: Exosomes increased
angiogenesis in papillary thyroid cancer microenvironment. Endocr
Relat Cancer. 26:525–538. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
van Niel G, D'Angelo G and Raposo G:
Shedding light on the cell biology of extracellular vesicles. Nat
Rev Mol Cell Biol. 19:213–228. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wang G, Wang S, Zhang M, Li Y, Liu Q, Sun
N, Zhang X, Liu Y, Zhang J, He L, et al: EV PD-L1 is correlated
with clinical features and contributes to t cell suppression in
pediatric thyroid cancer. J Clin Endocrinol Metab. 105:dgaa3092020.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Cheng L, Sharples RA, Scicluna BJ and Hill
AF: Exosomes provide a protective and enriched source of miRNA for
biomarker profiling compared to intracellular and cell-free blood.
J Extracell Vesicles. 3:2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Qiao F, Pan P, Yan J, Sun J, Zong Y, Wu Z,
Lu X, Chen N, Mi R, Ma Y and Ji Y: Role of tumor-derived
extracellular vesicles in cancer progression and their clinical
applications (Review). Int J Oncol. 54:1525–1533. 2019.PubMed/NCBI
|
|
58
|
Pan Q, Zhao J, Li M, Liu X, Xu Y, Li W, Wu
S and Su Z: Exosomal miRNAs are potential diagnostic biomarkers
between malignant and benign thyroid nodules based on
next-generation sequencing. Carcinogenesis. 41:18–24. 2020.
|
|
59
|
Samsonov R, Burdakov V, Shtam T,
Radzhabovsmall Z, Vasilyev D, Tsyrlina E, Titov S, Ivanov M,
Berstein L, et al: Plasma exosomal miR-21 and miR-181a
differentiates follicular from papillary thyroid cancer. Tumour
Biol. 37:12011–12021. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Wang Z, Lv J, Zou X, Huang Z, Zhang H, Liu
Q, Jiang L, Zhou X and Zhu W: A three plasma microRNA signature for
papillary thyroid carcinoma diagnosis in Chinese patients. Gene.
693:37–45. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Dai D, Tan Y, Guo L, Tang A and Zhao Y:
Identification of exosomal miRNA biomarkers for diagnosis of
papillary thyroid cancer by small RNA sequencing. Eur J Endocrinol.
182:111–121. 2020. View Article : Google Scholar
|
|
62
|
Rappa G, Puglisi C, Santos MF, Forte S,
Memeo L and Lorico A: Extracellular vesicles from thyroid
carcinoma: The new frontier of liquid biopsy. Int J Mol Sci.
20:11142019. View Article : Google Scholar
|
|
63
|
Liang M, Yu S, Tang S, Bai L, Cheng J, Gu
Y, Li S, Zheng X, Duan L, Wang L, et al: A Panel of Plasma exosomal
miRNAs as potential biomarkers for differential diagnosis of
thyroid nodules. Front Genet. 11:4492020. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Huang TY, Wang CY, Chen KY and Huang LT:
Urinary exosomal thyroglobulin in thyroid cancer patients with
post-ablative therapy: A new biomarker in thyroid cancer. Front
Endocrinol (Lausanne). 11:3822020. View Article : Google Scholar
|
|
65
|
Caruso Bavisotto C, Cipolla C, Graceffa G,
Barone R, Bucchieri F, Bulone D, Cabibi D, Campanella C, Marino
Gammazza A, Pitruzzella A, et al: Immunomorphological pattern of
molecular chaperones in normal and pathological thyroid tissues and
circulating exosomes: Potential use in Clinics. Int J Mol Sci.
20:44962019. View Article : Google Scholar
|
|
66
|
Zhuang X, Xiang X, Grizzle W, Sun D, Zhang
S, Axtell RC, Ju S, Mu J, Zhang L, Steinman L, et al: Treatment of
brain inflammatory diseases by delivering exosome encapsulated
anti-inflammatory drugs from the nasal region to the brain. Mol
Ther. 19:1769–1779. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Yang T, Martin P, Fogarty B, Brown A,
Schurman K, Phipps R, Yin VP, Lockman P and Bai S: Exosome
delivered anticancer drugs across the blood-brain barrier for brain
cancer therapy in Danio rerio. Pharm Res. 32:2003–2014. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Smyth T, Kullberg M, Malik N, Smith-Jones
P, Graner MW and Anchordoquy TJ: Biodistribution and delivery
efficiency of unmodified tumor-derived exosomes. J Control Release.
199:145–155. 2015. View Article : Google Scholar
|
|
69
|
Kim SM, Yang Y, Oh SJ, Hong Y, Seo M and
Jang M: Cancer-derived exosomes as a delivery platform of
CRISPR/Cas9 confer cancer cell tropism-dependent targeting. J
Control Release. 266:8–16. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Rana S, Yue S, Stadel D and Zöller M:
Toward tailored exosomes: The exosomal tetraspanin web contributes
to target cell selection. Int J Biochem Cell Biol. 44:1574–1584.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Zhang H, Wang Y, Bai M, Wang J, Zhu K, Liu
R, Ge S, Li J, Ning T, Deng T, et al: Exosomes serve as
nanoparticles to suppress tumor growth and angiogenesis in gastric
cancer by delivering hepatocyte growth factor siRNA. Cancer Sci.
109:629–641. 2018. View Article : Google Scholar
|
|
72
|
Gangadaran P, Li XJ, Kalimuthu SK, Min OJ,
Hong CM, Rajendran RL, Lee HW, Zhu L, Baek SH and Jeong SY: New
optical imaging reporter-labeled anaplastic thyroid cancer-derived
extracellular vesicles as a platform for in vivo tumor targeting in
a mouse model. Sci Rep. 8:135092018. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Zhu L, Kalimuthu S, Oh JM, Gangadaran P,
Baek SH, Jeong SY, Lee SW, Lee J and Ahn BC: Enhancement of
antitumor potency of extracellular vesicles derived from natural
killer cells by IL-15 priming. Biomaterials. 190-191:38–50. 2019.
View Article : Google Scholar
|
|
74
|
Lugini L, Cecchetti S, Huber V, Luciani F,
Macchia G, Spadaro F, Paris L, Abalsamo L, Colone M, Molinari A, et
al: Immune surveillance properties of human NK cell-derived
exosomes. J Immunol. 189:2833–2842. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Kim OY, Choi SJ, Jang SC, Park KS, Kim SR,
Choi JP, Lim JH, Lee SW, Park J, Di Vizio D, et al: Bacterial
protoplast-derived nanovesicles as vaccine delivery system against
bacterial infection. Nano Lett. 15:266–274. 2015. View Article : Google Scholar
|
|
76
|
Yang Z, Xie J, Zhu J, Kang C, Chiang C,
Wang X, Wang X, Kuang T, Chen F, Chen Z, et al: Functional
exosome-mimic for delivery of siRNA to cancer: In vitro and in vivo
evaluation. J Control Release. 243:160–171. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Zhu L, Gangadaran P, Kalimuthu S, Oh JM,
Baek SH, Jeong SY, Lee SW, Lee J and Ahn BC: Novel alternatives to
extracellular vesicle-based immunotherapy-exosome mimetics derived
from natural killer cells. Artif Cells Nanomed Biotechnol.
46(sup3): S166–S179. 2018. View Article : Google Scholar
|
















