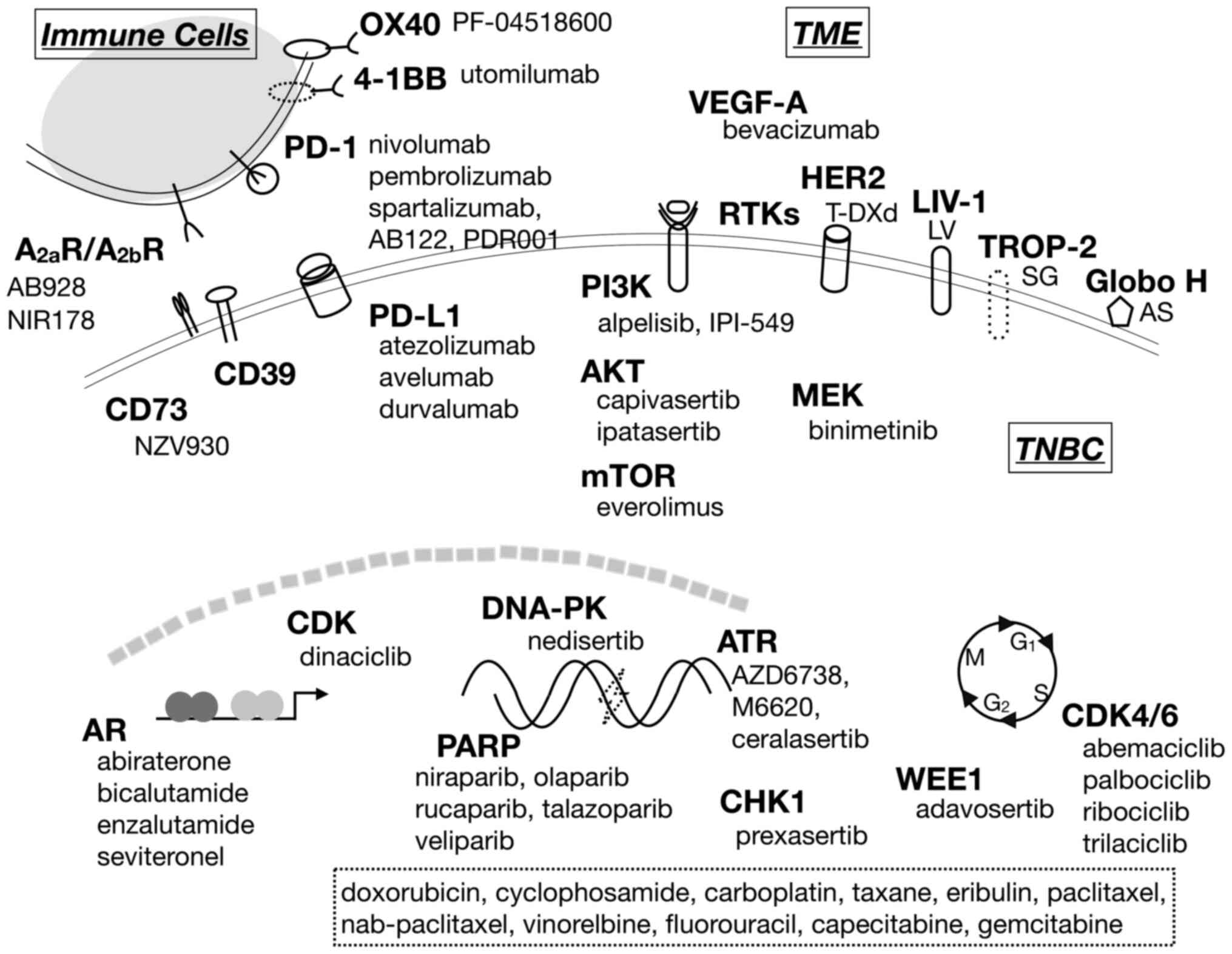
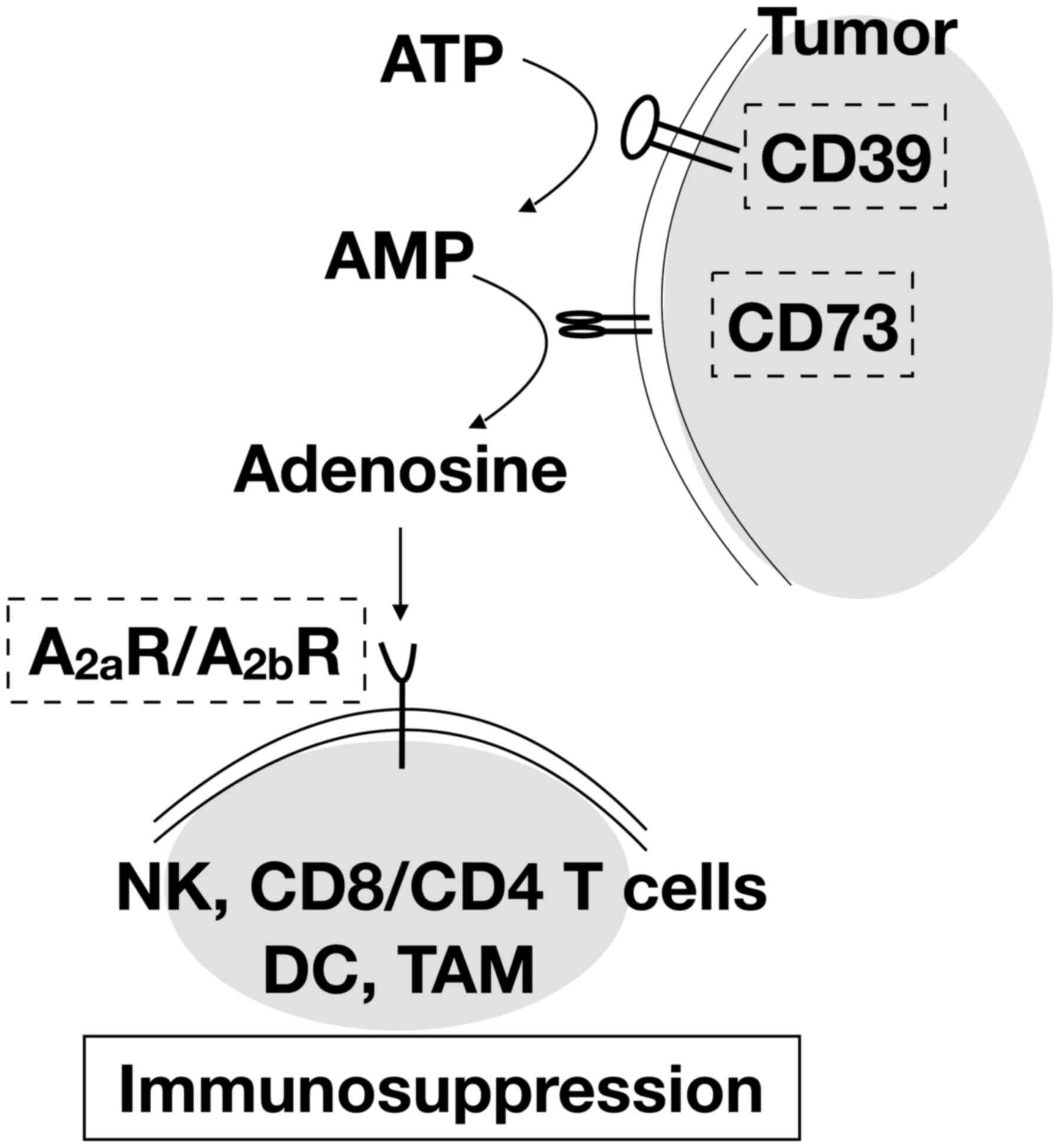
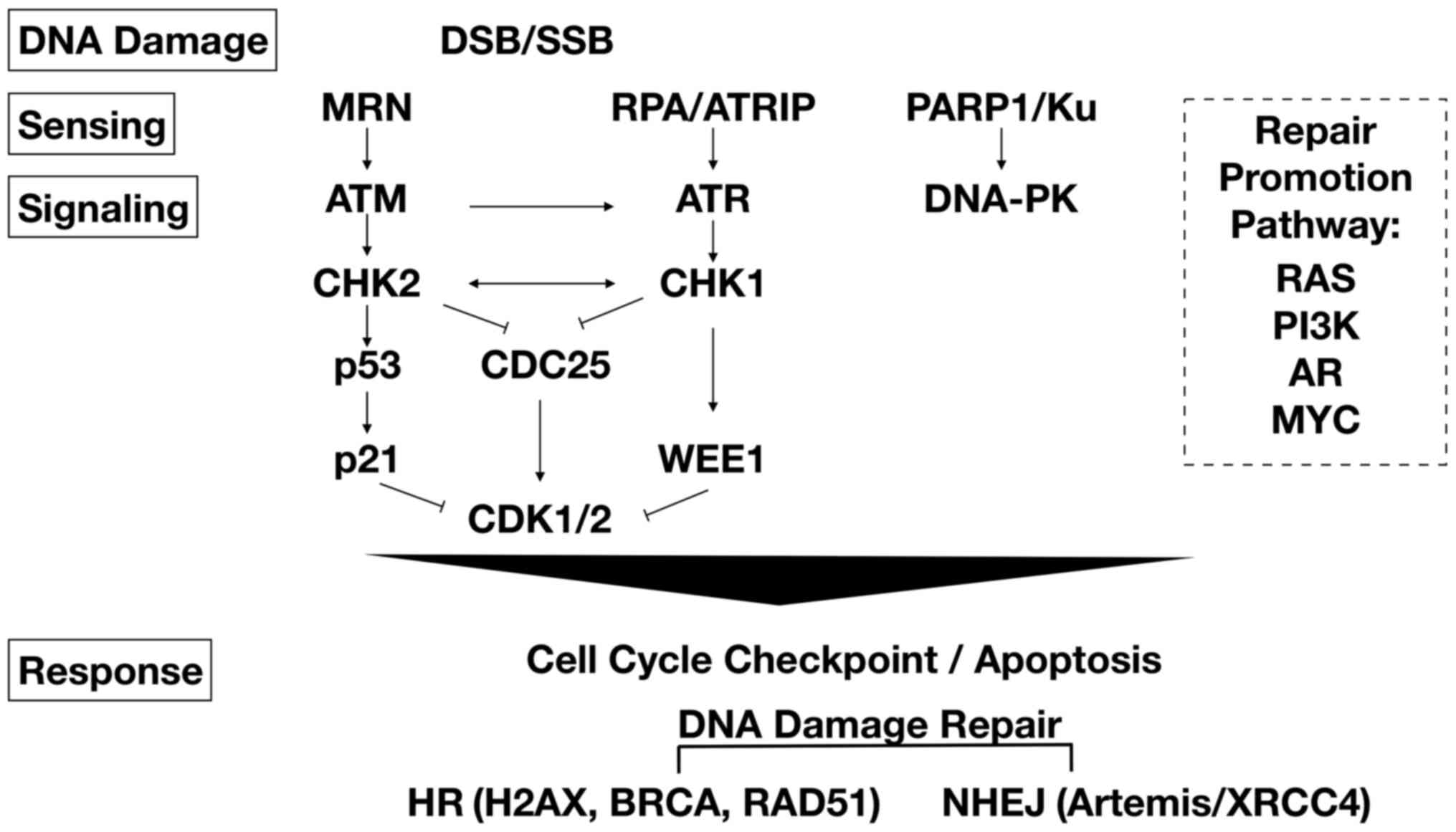
|
1
|
Lehmann BD, Bauer JA, Chen X, Sanders ME,
Chakravarthy AB, Shyr Y and Pietenpol JA: Identification of human
triple-negative breast cancer subtypes and preclinical models for
selection of targeted therapies. J Clin Invest. 121:2750–2767.
2011.PubMed/NCBI
|
|
2
|
Lehmann BD, Jovanović B, Chen X, Estrada
MV, Johnson KN, Shyr Y, Moses HL, Sanders ME and Pietenpol JA:
Refinement of triple-negative breast cancer molecular subtypes:
Implications for neoadjuvant chemotherapy selection. PLoS One.
11:e01573682016.PubMed/NCBI
|
|
3
|
Giuliano AE, Connolly JL, Edge SB,
Mittendorf EA, Rugo HS, Solin LJ, Weaver DL, Winchester DJ and
Hortobagyi GN: Breast cancer-major changes in the American Joint
Committee on cancer eighth edition cancer staging manual. CA Cancer
J Clin. 67:290–303. 2017.PubMed/NCBI
|
|
4
|
Wolff AC, Hammond MEH, Allison KH, Harvey
BE, Mangu PB, Bartlett JMS, Bilous M, Ellis IO, Fitzgibbons P,
Hanna W, et al: Human epidermal growth factor receptor 2 testing in
breast cancer: American Society of Clinical Oncology/College of
American pathologists clinical practice guideline focused update. J
Clin Oncol. 36:2105–2122. 2018.PubMed/NCBI
|
|
5
|
Allison KH, Hammond MEH, Dowsett M,
McKernin SE, Carey LA, Fitzgibbons PL, Hayes DF, Lakhani SR,
Chavez-MacGregor M, Perlmutter J, et al: Estrogen and progesterone
receptor testing in breast cancer: ASCO/CAP guideline update. J
Clin Oncol. 38:1346–1366. 2020.PubMed/NCBI
|
|
6
|
Yam C, Mani SA and Moulder SL: Targeting
the molecular subtypes of triple negative breast cancer:
Understanding the diversity to progress the field. Oncologist.
22:1086–1093. 2017.PubMed/NCBI
|
|
7
|
Bonotto M, Gerratana L, Poletto E, Driol
P, Giangreco M, Russo S, Minisini AM, Andreetta C, Mansutti M, Pisa
FE, et al: Measures of outcome in metastatic breast cancer:
Insights from a real-world scenario. Oncologist. 19:608–615.
2014.PubMed/NCBI
|
|
8
|
Kohler BA, Sherman RL, Howlader N, Jemal
A, Ryerson AB, Henry KA, Boscoe FP, Cronin KA, Lake A, Noone AM, et
al: Annual report to the nation on the status of cancer, 1975-2011
featuring incidence of breast cancer subtypes by race/ethnicity,
poverty, and state. J Natl Cancer Inst. 107:djv0482015.
|
|
9
|
O'Shaughnessy J, Schwartzberg L, Danso MA,
Miller KD, Rugo HS, Neubauer M, Robert N, Hellerstedt B, Saleh M,
Richards P, et al: Phase III study of iniparib plus gemcitabine and
carboplatin versus gemcitabine and carboplatin in patients with
metastatic triple-negative breast cancer. J Clin Oncol.
32:3840–3847. 2014.PubMed/NCBI
|
|
10
|
Caswell-Jin JL, Plevritis SK, Tian L,
Cadham CJ, Xu C, Stout NK, Sledge GW, Mandelblatt JS and Kurian AW:
Change in survival in metastatic breast cancer with treatment
advances: Meta-analysis and systematic review. JNCI Cancer Spectr.
2:pky0622018.
|
|
11
|
Plevritis SK, Munoz D, Kurian AW, Stout
NK, Alagoz O, Near AM, Lee SJ, van den Broek JJ, Huang X, Schechter
CB, et al: Association of screening and treatment with breast
cancer mortality by molecular subtype in US women, 2000-2012. JAMA.
319:154–164. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Stanton SE, Adams S and Disis ML:
Variation in the incidence and magnitude of tumor-infiltrating
lymphocytes in breast cancer subtypes: A systematic review. JAMA
Oncol. 2:1354–1360. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Safonov A, Jiang T, Bianchini G, Győrffy
B, Karn T, Hatzis C and Pusztai L: Immune gene expression is
associated with genomic aberrations in breast cancer. Cancer Res.
77:3317–3324. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schmid P, Adams S, Rugo HS, Schneeweiss A,
Barrios CH, Iwata H, Diéras V, Hegg R, Im SA, Shaw Wright G, et al:
Atezolizumab and Nab-Paclitaxel in advanced triple-negative breast
cancer. N Engl J Med. 379:2108–2121. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schmid P, Rugo HS, Adams S, Schneeweiss A,
Barrios CH, Iwata H, Diéras V, Henschel V, Molinero L, Chui SY, et
al: Atezolizumab plus nab-paclitaxel as first-line treatment for
unresectable, locally advanced or metastatic triple-negative breast
cancer (IMpassion130): Updated efficacy results from a randomised,
double-blind, placebo-controlled, phase 3 trial. Lancet Oncol.
21:44–59. 2020. View Article : Google Scholar
|
|
16
|
Dudley JC, Lin MT, Le DT and Eshleman JR:
Microsatellite instability as a biomarker for PD-1 blockade. Clin
Cancer Res. 22:813–820. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bonneville R, Krook MA, Kautto EA, Miya J,
Wing MR, Chen HZ, Reeser JW, Yu L and Roychowdhury S: Landscape of
microsatellite instability across 39 cancer types. JCO Precis
Oncol. 2017:PO.17.00073. 2017.PubMed/NCBI
|
|
18
|
Kurata K, Kubo M, Mori H, Kawaji H,
Motoyama Y, Kuroki L, Yamada M, Kaneshiro K, Kai M and Nakamura M:
Microsatellite instability in triple negative breast cancers. In:
Proceedings of the 2018 San Antonio Breast Cancer Symposium. Cancer
Res. 79(Suppl 4): Abstract nr P1-06-11. 2019.
|
|
19
|
Bryant HE, Schultz N, Thomas HD, Parker
KM, Flower D, Lopez E, Kyle S, Meuth M, Curtin NJ and Helleday T:
Specific killing of BRCA2-deficient tumours with inhibitors of
poly(ADP-ribose) polymerase. Nature. 434:913–917. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Farmer H, McCabe N, Lord CJ, Tutt AN,
Johnson DA, Richardson TB, Santarosa M, Dillon KJ, Hickson I,
Knights C, et al: Targeting the DNA repair defect in BRCA mutant
cells as a therapeutic strategy. Nature. 434:917–921. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Robson M, Im SA, Senkus E, Xu B, Domchek
SM, Masuda N, Delaloge S, Li W, Tung N, Armstrong A, et al:
Olaparib for meta-static breast cancer in patients with a germline
BRCA mutation. N Engl J Med. 377:523–533. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Robson ME, Tung N, Conte P, Im SA, Senkus
E, Xu B, Masuda N, Delaloge S, Li W, Armstrong A, et al: OlympiAD
final overall survival and tolerability results: Olaparib versus
chemotherapy treatment of physician's choice in patients with a
germline BRCA mutation and HER2-negative metastatic breast cancer.
Ann Oncol. 30:558–566. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Litton JK, Rugo HS, Ettl J, Hurvitz SA,
Gonçalves A, Lee KH, Fehrenbacher L, Yerushalmi R, Mina LA, Martin
M, et al: Talazoparib in patients with advanced breast cancer and a
germ-line BRCA mutation. N Engl J Med. 379:753–763. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Denkert C, von Minckwitz G, Darb-Esfahani
S, Lederer B, Heppner BI, Weber KE, Budczies J, Huober J, Klauschen
F, Furlanetto J, et al: Tumour-infiltrating lymphocytes and
prog-nosis in different subtypes of breast cancer: A pooled
analysis of 3771 patients treated with neoadjuvant therapy. Lancet
Oncol. 19:40–50. 2018. View Article : Google Scholar
|
|
25
|
Hida AI, Watanabe T, Sagara Y, Kashiwaba
M, Sagara Y, Aogi K, Ohi Y and Tanimoto A: Diffuse distribution of
tumor-infiltrating lymphocytes is a marker for better prognosis and
chemo-therapeutic effect in triple-negative breast cancer. Breast
Cancer Res Treat. 178:283–294. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Loi S, Drubay D, Adams S, Pruneri G,
Francis PA, Lacroix-Triki M, Joensuu H, Dieci MV, Badve S, Demaria
S, et al: Tumor-infiltrating lymphocytes and prognosis: A pooled
individual patient analysis of early-stage triple-negative breast
cancers. J Clin Oncol. 37:559–569. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Galon J and Bruni D: Approaches to treat
immune hot, altered and cold tumours with combination
immunotherapies. Nat Rev Drug Discov. 18:197–218. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Adams S, Gatti-Mays ME, Kalinsky K, Korde
LA, Sharon E, Amiri-Kordestani L, Bear H, McArthur HL, Frank E,
Perlmutter J, et al: Current landscape of immunotherapy in breast
cancer: A review. JAMA Oncol. Apr 11–2019.Epub ahead of print.
View Article : Google Scholar
|
|
29
|
Schmid P, Salgado R, Park YH,
Muñoz-Couselo E, Kim SB, Sohn J, Im S-A, Foukakis T, Kuemmel S,
Dent R, et al: Pembrolizumab plus chemotherapy as neoadjuvant
treatment of high-risk, early-stage triple-negative breast cancer:
Results from the phase 1b open-label, multicohort KEYNOTE-173
study. Ann Oncol. 31:569–581. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nanda R, Liu MC, Yau C, Shatsky R, Pusztai
L, Wallace A, Chien AJ, Forero-Torres A, Ellis E, Han H, et al:
Effect of pembrolizumab plus neoadjuvant chemotherapy on Pathologic
complete response in women with early-stage breast cancer: An
analysis of the ongoing phase 2 adaptively randomized I-SPY2 trial.
JAMA Oncol. 6:1–9. 2020. View Article : Google Scholar
|
|
31
|
Schmid P, Cortes J, Pusztai L, McArthur H,
Kümmel S, Bergh J, Denkert C, Park YH, Hui R, Harbeck N, et al:
Pembrolizumab for early triple-negative breast cancer. N Engl J
Med. 382:810–821. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gianni L, Huang CS, Egle D, Bermejo B,
Zamagni C, Thill M, Anton A, Zambelli S, Bianchini G, Russo S and
Ciruelos E: Pathologic complete response (pCR) to neoadjvaunt
treatment with or without atezolizumab in triple negative, early
high-risk and locally advanced breast cancer. NeoTRIPaPDL1
Michelangelo randomized study. In: Proceedings of the 2019 San
Antonio Breast Cancer Symposium. Cancer Res. 80(Suppl 4): Abstract
nr GS3-04. 2020.
|
|
33
|
Mittendorf E, Barrios CH, Harbeck N, Miles
D, Saji S, Zhang H, Duc AN, Rafii S and Lai C: IMpassion031: A
phase III study comparing neoadjuvant atezolizumab vs placebo in
combination with nab-paclitaxel-based chemotherapy in early
triple-negative breast cancer (TNBC). In: Proceedings of the 2017
San Antonio Breast Cancer Symposium. Cancer Res. 78(Suppl 4):
Abstract nr OT2-07-03. 2018.
|
|
34
|
Cortes J, Cescon DW, Rugo HS, Nowecki Z,
Im SA, Yusof MM, Gallardo C, Lipatov O, Barrios CH, Holgado E, et
al: KEYNOTE-355: Randomized, double-blind, phase III study of
pembrolizumab + chemotherapy versus placebo + chemotherapy for
previously untreated locally recurrent inoperable or meta-static
triple-negative breast cancer. J Clin Oncol. 38(Suppl 15): S1000.
2020. View Article : Google Scholar
|
|
35
|
Voorwerk L, Slagter M, Horlings HM,
Sikorska K, van de Vijver KK, de Maaker M, Nederlof I, Kluin RJC,
Warren S, Ong S, et al: Immune induction strategies in meta-static
triple-negative breast cancer to enhance the sensitivity to PD-1
blockade: The TONIC trial. Nat Med. 25:920–928. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Allard B, Longhi MS, Robson SC and Stagg
J: The ectonucleotidases CD39 and CD73: Novel checkpoint inhibitor
targets. Immunol Rev. 276:121–144. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ghalamfarsa G, Kazemi MH, Raoofi Mohseni
S, Masjedi A, Hojjat-Farsangi M, Azizi G, Yousefi M and
Jadidi-Niaragh F: CD73 as a potential opportunity for cancer
immunotherapy. Expert Opin Ther Targets. 23:127–142. 2019.
View Article : Google Scholar
|
|
38
|
Duhant X, Schandené L, Bruyns C, Gonzalez
NS, Goldman M, Boeynaems JM and Communi D: Extracellular adenine
nucleotides inhibit the activation of human CD4+ T lymphocytes. J
Immunol. 169:15–21. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Allard B, Beavis PA, Darcy PK and Stagg J:
Immunosuppressive activities of adenosine in cancer. Curr Opin
Pharmacol. 29:7–16. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ohta A: A metabolic immune checkpoint:
Adenosine in tumor microenvironment. Front Immunol. 7:1092016.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Buisseret L, Pommey S, Allard B, Garaud S,
Bergeron M, Cousineau I, Ameye L, Bareche Y, Paesmans M, Crown JPA,
et al: Clinical significance of CD73 in triple-negative breast
cancer: Multiplex analysis of a phase III clinical trial. Ann
Oncol. 29:1056–1062. 2018.
|
|
42
|
Powderly J, Spira A, Gutierrez R, DiRenzo
D, Udyavar A, Karakunnel JJ, Rieger A, Colabella J, Lai DW and de
Souza P: Phase 1 evaluation of AB928, a novel dual adenosine
receptor antagonist, combined with chemotherapy or AB122
(anti-PD-1) in patients with advanced malignancies. Ann Oncol.
30(Suppl 5): v475–v532. 2019.
|
|
43
|
Hartman AR, Kaldate RR, Sailer LM, Painter
L, Grier CE, Endsley RR, Griffin M, Hamilton SA, Frye CA, Silberman
MA, et al: Prevalence of BRCA mutations in an unselected population
of triple-negative breast cancer. Cancer. 118:2787–2795.
2012.PubMed/NCBI
|
|
44
|
Okuma HS and Yonemori K: BRCA gene
mutations and poly(ADP-Ribose) polymerase inhibitors in
triple-negative breast cancer. Adv Exp Med Biol. 1026:271–286.
2017.PubMed/NCBI
|
|
45
|
Lord CJ and Ashworth A: BRCAness
revisited. Nat Rev Cancer. 16:110–120. 2016.PubMed/NCBI
|
|
46
|
Litton JK, Scoggins ME, Hess KR, Adrada
BE, Murthy RK, Damodaran S, DeSnyder SM, Brewster AM, Barcenas CH,
Valero V, et al: Neoadjuvant talazoparib for patients with operable
breast cancer with a germline BRCA pathogenic variant. J Clin
Oncol. 38:388–394. 2020.
|
|
47
|
Loibl S, O'Shaughnessy J, Untch M, Sikov
WM, Rugo HS, McKee MD, Huober J, Golshan M, von Minckwitz G, Maag
D, et al: Addition of the PARP inhibitor veliparib plus carboplatin
or carboplatin alone to standard neoadjuvant chemotherapy in
triple-negative breast cancer (BrighTNess): A randomised, phase 3
trial. Lancet Oncol. 19:497–509. 2018.PubMed/NCBI
|
|
48
|
Jiao S, Xia W, Yamaguchi H, Wei Y, Chen
MK, Hsu JM, Hsu JL, Yu WH, Du Y, Lee HH, et al: PARP inhibitor
upregulates PD-L1 expression and enhances cancer-associated
immunosuppression. Clin Cancer Res. 23:3711–3720. 2017.PubMed/NCBI
|
|
49
|
Vinayak S, Tolaney SM, Schwartzberg L,
Mita M, McCann G, Tan AR, Wahner-Hendrickson AE, Forero A, Anders
C, Wulf GM, et al: Open-label clinical trial of niraparib combined
with pembrolizumab for treatment of advanced or metastatic
triple-negative breast cancer. JAMA Oncol. 5:1132–1140. 2019.
|
|
50
|
Domchek S, Postel-Vinay S, Im S, Park YH,
Delord J, Italiano A, Alexandre J, You B, Bastian S, Krebs MG, et
al: Phase II study of olaparib (o) and durvalumab (d) (MEDIOLA):
Updated results in patients (pts) with germline BRCA-mutated
(gBRCAm) meta-static breast cancer (mbc). Ann Oncol. 30(Suppl 5):
v475–v532. 2019.
|
|
51
|
Pusztai L, Han HS, Yau C, Wolf D, Wallace
AM, Shatsky R, Helsten T, Boughey JC, Haddad T, Stringer-Reasor E,
et al: Durvalumab in combination with olaparib and paclitaxel in
high-risk HER2 negative stage II/III breast cancer: Results from
the I-SPY 2 trial. In: Proceedings of the Annual Meeting of the
American Association for Cancer Research 2020. Cancer Res. 80(Suppl
16): Abstract nr CT011. 2020.
|
|
52
|
Mitri ZI, Vuky J, Kemmer KA, Savin MA,
Parmar S, Kolodzie AK, Johnson B, Williams-Belizaire R, Gray JW and
Mills GB: A phase II trial of olaparib and durvalumab in metastatic
BRCA wild type triple-negative breast cancer. J Clin Oncol.
37:TPS11112019.
|
|
53
|
Rugo HS, Llombart-Cussac A, Andre F,
Robson ME, Saji S, Harbeck N, Schmid P, Cescon DW, Ahn JS, Nanda R,
et al: KEYLYNK-009: A phase II/III, open-label, randomized study of
pembrolizumab (pembro) plus olaparib vs pembro plus chemotherapy
after induction with first-line pembro plus chemo-therapy in
patients with locally recurrent inoperable or metastatic
triple-negative breast cancer (TNBC). J Clin Oncol.
38:TPS5962020.
|
|
54
|
Maacke H, Opitz S, Jost K, Hamdorf W,
Henning W, Krüger S, Feller AC, Lopens A, Diedrich K, Schwinger E
and Stürzbecher HW: Over-expression of wild-type Rad51 correlates
with histological grading of invasive ductal breast cancer. Int J
Cancer. 88:907–913. 2000.PubMed/NCBI
|
|
55
|
Martin RW, Orelli BJ, Yamazoe M, Minn AJ,
Takeda S and Bishop DK: RAD51 up-regulation bypasses BRCA1 function
and is a common feature of BRCA1-deficient breast tumors. Cancer
Res. 67:9658–9665. 2007.PubMed/NCBI
|
|
56
|
Wiegmans AP, Yap PY, Ward A, Lim YC and
Khanna KK: Differences in expression of key DNA damage repair genes
after epigenetic-induced BRCAness dictate synthetic lethality with
PARP1 inhibition. Mol Cancer Ther. 14:2321–2331. 2015.PubMed/NCBI
|
|
57
|
Liu Y, Burness ML, Martin-Trevino R, Guy
J, Bai S, Harouaka R, Brooks MD, Shang L, Fox A, Luther TK, et al:
RAD51 mediates resistance of cancer stem cells to PARP inhibition
in triple-negative breast cancer. Clin Cancer Res. 23:514–522.
2017.
|
|
58
|
Marzio A, Puccini J, Kwon Y, Maverakis NK,
Arbini A, Sung P, Bar-Sagi D and Pagano M: The F-Box
domain-dependent activity of EMI1 regulates PARPi sensitivity in
triple-negative breast cancers. Mol Cell. 73:224–237.e6. 2019.
|
|
59
|
Tutt A, Stephens C, Frewer P, Pierce A,
Rhee J, So K, Ottesen L, Dean E and Hollingsworth SJ: VIOLETTE: A
randomized phase II study to assess DNA damage response inhibitors
in combination with olaparib (Ola) vs. Ola monotherapy in patients
(pts) with metastatic, triple-negative breast cancer (TNBC)
stratified by alterations in homologous recombination repair
(HRR)-related genes. J Clin Oncol. 36(Suppl 15): TPS11122018.
|
|
60
|
Hirai H, Arai T, Okada M, Nishibata T,
Kobayashi M, Sakai N, Imagaki K, Ohtani J, Sakai T, Yoshizumi T, et
al: MK-1775, a small molecule Wee1 inhibitor, enhances anti-tumor
efficacy of various DNA-damaging agents, including 5-fluorouracil.
Cancer Biol Ther. 9:514–522. 2010.PubMed/NCBI
|
|
61
|
Pitts TM, Simmons DM, Bagby SM, Hartman
SJ, Yacob BW, Gittleman B, Tentler JJ, Cittelly D, Ormond DR,
Messersmith WA, et al: Wee1 inhibition enhances the anti-tumor
effects of capecitabine in preclinical models of triple-negative
breast cancer. Cancers (Basel). 12:7192020. View Article : Google Scholar
|
|
62
|
Do K, Wilsker D, Ji J, Zlott J, Freshwater
T, Kinders RJ, Collins J, Chen AP, Doroshow JH and Kummar S: Phase
I study of single-agent AZD1775 (MK-1775), a Wee1 kinase inhibitor,
in patients with refractory solid tumors. J Clin Oncol.
33:3409–3415. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Do KT, Hill SJ, Kochupurakkal B, Supko JG,
Gannon C, Anderson A, Muzikansky A, Wolanski A, Hedglin J, Parmar
K, et al: Abstract CT232: Phase I combination study of the CHK1
inhibitor prexasertib (LY2606368) and olaparib in patients with
high-grade serous ovarian cancer and other advanced solid tumors.
In: Proceedings of the American Association for Cancer Research
Annual Meeting 2019. Cancer Res. 79(Suppl 13): Abstract nr CT232.
2019.
|
|
64
|
Peng W, Chen JQ, Liu C, Malu S, Creasy C,
Tetzlaff MT, Xu C, McKenzie JA, Zhang C, Liang X, et al: Loss of
PTEN promotes resistance to T cell-mediated immunotherapy. Cancer
Discov. 6:202–216. 2016. View Article : Google Scholar :
|
|
65
|
André F, Ciruelos E, Rubovszky G, Campone
M, Loibl S, Rugo HS, Iwata H, Conte P, Mayer IA, Kaufman B, et al:
Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced
breast cancer. N Engl J Med. 380:1929–1940. 2019. View Article : Google Scholar
|
|
66
|
Kim SB, Dent R, Im SA, Espié M, Blau S,
Tan AR, Isakoff SJ, Oliveira M, Saura C, Wongchenko MJ, et al:
Ipatasertib plus paclitaxel versus placebo plus paclitaxel as
first-line therapy for metastatic triple-negative breast cancer
(LOTUS): A multicentre, randomised, double-blind,
placebo-controlled, phase 2 trial. Lancet Oncol. 18:1360–1372.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Dent R, Im SA, Espie M, Blau S, Tan AR,
Isakoff SJ, Oliveira M, Saura C, Wongchenko M, Kapp AV, et al:
Overall survival (OS) update of the double-blind placebo
(PBO)-controlled random-ized phase 2 LOTUS trial of first-line
ipatasertib (IPAT) + paclitaxel (PAC) for locally
advanced/metastatic triple-negative breast cancer (mTNBC). J Clin
Oncol. 36:10082018. View Article : Google Scholar
|
|
68
|
Dent R, Kim SB, Oliveira M, Isakoff SJ,
Barrios CH, O'Shaughnessy J, Lu X, Wongchenko M, Bradley D, Mani A,
et al: IPATunity130: A pivotal randomized phase III trial
evaluating ipatasertib (IPAT) + paclitaxel (PAC) for
PIK3CA/AKT1/PTEN-altered advanced triple-negative (TN) or hormone
receptor-positive HER2-negative (HR+/HER2-) breast cancer (BC). J
Clin Oncol. 36(Suppl 15): TPS11172018. View Article : Google Scholar
|
|
69
|
Schmid P, Abraham J, Chan S, Wheatley D,
Brunt AM, Nemsadze G, Baird RD, Park YH, Hall PS, Perren T, et al:
Capivasertib plus paclitaxel versus placebo plus paclitaxel as
first-line therapy for metastatic triple-negative breast cancer:
The PAKT trial. J Clin Oncol. 38:423–433. 2020. View Article : Google Scholar
|
|
70
|
Schmid P, Cortes J, Robson M, Iwata H,
Hegg R, Verma S, Nechaeva M, Xu B, Haddad V, Imedio RE, et al:
Abstract OT2-08-02: Capivasertib and paclitaxel in first-line
treatment of patients with metastatic triple-negative breast
cancer: A phase III trial (CAPItello-290). In: Proceedings of the
2019 San Antonio Breast Cancer Symposium. Cancer Res. 80(Suppl 4):
Abstract nr OT2-08-02. 2020.
|
|
71
|
Schmid P, Loirat D, Savas P, Espinosa E,
Boni V, Italiano A, White S, Singel MS, Withana N, Mani A, et al:
Phase Ib study evaluating a triplet combination of ipatasertib
(IPAT), atezoli-zumab (atezo), and paclitaxel (PAC) or nab-PAC as
first-line (1L) therapy for locally advanced/metastatic
triple-negative breast cancer (TNBC). In: Proceedings of the
American Association for Cancer Research Annual Meeting 2019.
Cancer Res. 79(Suppl 13): Abstract nr CT049. 2019.
|
|
72
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Dean JL, McClendon AK and Knudsen ES:
Modification of the DNA damage response by therapeutic CDK4/6
inhibition. J Biol Chem. 287:29075–29087. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Cretella D, Fumarola C, Bonelli M, Alfieri
R, La Monica S, Digiacomo G, Cavazzoni A, Galetti M, Generali D and
Petronini PG: Pre-treatment with the CDK4/6 inhibitor palbociclib
improves the efficacy of paclitaxel in TNBC cells. Sci Rep.
9:130142019. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Clark AS, McAndrew NP, Troxel A, Feldman
M, Lal P, Rosen M, Burrell J, Redlinger C, Gallagher M, Bradbury
AR, et al: Combination paclitaxel and palbociclib: Results of a
phase I trial in advanced breast cancer. Clin Cancer Res.
25:2072–2079. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Tan AR, Wright GS, Thummala AR, Danso MA,
Popovic L, Pluard TJ, Han HS, Vojnović Ž, Vasev N, Ma L, et al:
Trilaciclib plus chemotherapy versus chemotherapy alone in patients
with metastatic triple-negative breast cancer: A multicentre,
randomised, open-label, phase 2 trial. Lancet Oncol. 20:1587–1601.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Stine ZE, Walton ZE, Altman BJ, Hsieh AL
and Dang CV: MYC, metabolism, and cancer. Cancer Discov.
5:1024–1039. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Ambrosio S, Amente S, Napolitano G, Di
Palo G, Lania L and Majello B: MYC impairs resolution of
site-specific DNA double-strand breaks repair. Mutat Res. 774:6–13.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Wiegmans AP, Al-Ejeh F, Chee N, Yap PY,
Gorski JJ, Da Silva L, Bolderson E, Chenevix-Trench G, Anderson R,
Simpson PT, et al: Rad51 supports triple negative breast cancer
metastasis. Oncotarget. 5:3261–3272. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Carey JPW, Karakas C, Bui T, Chen X,
Vijayaraghavan S, Zhao Y, Wang J, Mikule K, Litton JK, Hunt KK and
Keyomarsi K: Synthetic lethality of PARP inhibitors in combination
with MYC blockade is independent of BRCA status in triple negative
breast cancer. Cancer Res. 78:742–757. 2018. View Article : Google Scholar :
|
|
81
|
Horiuchi D, Kusdra L, Huskey NE,
Chandriani S, Lenburg ME, Gonzalez-Angulo AM, Creasman KJ, Bazarov
AV, Smyth JW, Davis SE, et al: MYC pathway activation in
triple-negative breast cancer is synthetic lethal with CDK
inhibition. J Exp Med. 209:679–696. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Hossain DMS, Javaid S, Cai M, Zhang C,
Sawant A, Hinton M, Sathe M, Grein J, Blumenschein W, Pinheiro EM
and Chackerian A: Dinaciclib induces immunogenic cell death and
enhances anti-PD1-mediated tumor suppression. J Clin Invest.
128:644–654. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Chien AJ, Gliwa AS, Rahmaputri S, Dittrich
HF, Majure MC, Rugo HS, Melisko ME, Munster PN, Park JW, Moasser
MM, et al: A phase Ib trial of the cyclin-dependent kinase
inhibitor dinaci-clib (dina) in combination with pembrolizumab (P)
in patients with advanced triple-negative breast cancer (TNBC) and
response correlation with MYC-overexpression. J Clin Oncol.
38(1076)2020. View Article : Google Scholar
|
|
84
|
Kono M, Fujii T, Lim B, Karuturi MS,
Tripathy D and Ueno NT: Androgen receptor function and androgen
receptor-targeted therapies in breast cancer: A Review. JAMA Oncol.
3:1266–1273. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Gerratana L, Basile D, Buono G, De Placido
S, Giuliano M, Minichillo S, Coinu A, Martorana F, De Santo I, Del
Mastro L, et al: Androgen receptor in triple negative breast
cancer: A potential target for the targetless subtype. Cancer Treat
Rev. 68:102–110. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Anestis A, Zoi I, Papavassiliou AG and
Karamouzis MV: Androgen receptor in breast cancer-clinical and
preclinical research insights. Molecules. 25(358)2020. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Masuda H, Baggerly KA, Wang Y, Zhang Y,
Gonzalez-Angulo AM, Meric-Bernstam F, Valero V, Lehmann BD,
Pietenpol JA, Hortobagyi GN, et al: Differential response to
neoadjuvant chemotherapy among 7 triple-negative breast cancer
molecular subtypes. Clin Cancer Res. 19:5533–5540. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Thike AA, Yong-Zheng Chong L, Cheok PY, Li
HH, Wai-Cheong Yip G, Huat Bay B, Tse GM, Iqbal J and Tan PH: Loss
of androgen receptor expression predicts early recurrence in
triple-negative and basal-like breast cancer. Mod Pathol.
27:352–360. 2014. View Article : Google Scholar
|
|
89
|
Echavarria I, Lopez-Tarruella S, Picornell
A, García-Saenz JA, Jerez Y, Hoadley K, Gómez HL, Moreno F,
Monte-Millan MD, Márquez-Rodas I, et al: Pathological response in a
triple-negative breast cancer cohort treated with neoadjuvant
carboplatin and docetaxel according to Lehmann's refined
classification. Clin Cancer Res. 24:1845–1852. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Santonja A, Sánchez-Muñoz A, Lluch A,
Chica-Parrado MR, Albanell J, Chacón JI, Antolín S, Jerez JM, de la
Haba J, de Luque V, et al: Triple negative breast cancer subtypes
and pathologic complete response rate to neoadjuvant chemotherapy.
Oncotarget. 9:26406–26416. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Venema CM, Bense RD, Steenbruggen TG,
Nienhuis HH, Qiu SQ, van Kruchten M, Brown M, Tamimi RM, Hospers
GAP, Schröder CP, et al: Consideration of breast cancer subtype in
targeting the androgen receptor. Pharmacol Ther. 200:135–147. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Rice MA, Malhotra SV and Stoyanova T:
Second-generation antiandrogens: From discovery to standard of care
in castration resistant prostate cancer. Front Oncol. 9:8012019.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Gucalp A, Tolaney S, Isakoff SJ, Ingle JN,
Liu MC, Carey LA, Blackwell K, Rugo H, Nabell L, Forero A, et al:
Phase II trial of bicalutamide in patients with androgen
receptor-positive, estrogen receptor-negative metastatic breast
cancer. Clin Cancer Res. 19:5505–5512. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Gucalp A, Edelweiss M, Patil S, Gounder
MM, Feigin KN, Corben A, Arumov A and Traina TA: Abstract P3-11-04:
Phase I/II trial of palbociclib in combination with bicalutamide
for the treatment of androgen receptor (AR)+ metastatic breast
cancer (MBC). In: Proceedings of the 2017 San Antonio Breast Cancer
Symposium. Cancer Res 2018. 78(Suppl 4): Abstract nr P3-11-04.
2018.
|
|
95
|
Gucalp A, Boyle LA, Alano T, Arumov A,
Gounder MM, Patil S, Feigin K, Edelweiss M, D'Andrea G, Bromberg J,
et al: Phase II trial of bicalutamide in combination with
palbociclib for the treatment of androgen receptor (+) metastatic
breast cancer. J Clin Oncol. 38:2020. View Article : Google Scholar
|
|
96
|
Bonnefoi H, Grellety T, Tredan O,
Saghatchian M, Dalenc F, Mailliez A, L'Haridon T, Cottu P,
Abadie-Lacourtoisie S, You B, et al: A phase II trial of
abiraterone acetate plus prednisone in patients with
triple-negative androgen receptor positive locally advanced or
metastatic breast cancer (UCBG 12-1). Ann Oncol. 27:812–818. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Gucalp A, Danso MA, Elias AD, Bardia A,
Ali HY, Potter D, Gabrail NY, Haley BB, Khong HT, Riley EC, et al:
Phase (Ph) 2 stage 1 clinical activity of seviteronel, a selective
CYP17-lyase and androgen receptor (AR) inhibitor, in women with
advanced AR+ triple-negative breast cancer (TNBC) or estrogen
receptor (ER)+ BC: CLARITY-01. J Clin Oncol. 35:11022017.
View Article : Google Scholar
|
|
98
|
Bardia A, Gucalp A, DaCosta N, Gabrail N,
Danso M, Ali H, Blackwell KL, Carey LA, Eisner JR, Baskin-Bey ES
and Traina TA: Phase 1 study of seviteronel, a selective CYP17
lyase and androgen receptor inhibitor, in women with estrogen
receptor-positive or triple-negative breast cancer. Breast Cancer
Res Treat. 171:111–120. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Traina TA, Miller K, Yardley DA, Eakle J,
Schwartzberg LS, O'Shaughnessy J, Gradishar W, Schmid P, Winer E,
Kelly C, et al: Enzalutamide for the treatment of androgen
receptor-expressing triple-negative breast cancer. J Clin Oncol.
36:884–890. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Dent R, Schmid P, Cortes J, Kim SB, Andre
F, Abramson V, Cardoso F, Colleoni M, Morris P, Steinberg J, et al:
Abstract OT3-02-02: ENDEAR: A randomized international phase 3
study comparing the efficacy and safety of enzalutamide in
combination with paclitaxel chemotherapy or as mono-therapy vs
placebo with paclitaxel in patients with advanced
diagnostic-positive triple-negative breast cancer. Cancer Res.
77:Abstract OT3-02-02. 2017.
|
|
101
|
Lehmann BD, Abramson VG, Sanders ME, Mayer
EL, Haddad TC, Nanda R, Van Poznak C, Storniolo AM, Nangia JR,
Gonzalez-Ericsson PI, et al: TBCRC 032 IB/II multicenter study:
Molecular insights to AR antagonist and PI3K inhibitor efficacy in
patients with AR+ metastatic triple-negative breast
cancer. Clin Cancer Res. 26:2111–2123. 2020. View Article : Google Scholar
|
|
102
|
Gilewski T, Ragupathi G, Bhuta S, Williams
LJ, Musselli C, Zhang XF, Bornmann WG, Spassova M, Bencsath KP,
Panageas KS, et al: Immunization of metastatic breast cancer
patients with a fully synthetic globo H conjugate: A phase I trial.
Proc Natl Acad Sci USA. 98:3270–3275. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Huang CS, Yu AL, Tseng LM, Chow LWC, Hou
MF, Hurvitz SA, Schwab RB, Wong CH, Murray JL, Chang SC, et al:
Randomized phase II/III trial of active immunotherapy with
OPT-822/OPT-821 in patients with metastatic breast cancer. J Clin
Oncol. 34(Suppl 15): S10032016. View Article : Google Scholar
|
|
104
|
Bardia A, Mayer IA, Vahdat LT, Tolaney SM,
Isakoff SJ, Diamond JR, O'Shaughnessy J, Moroose RL, Santin AD,
Abramson VG, et al: Sacituzumab govitecan-hziy in refractory
metastatic triple-negative breast cancer. N Eng J Med. 380:741–751.
2019. View Article : Google Scholar
|
|
105
|
Modi S, Pusztai L, Forero A, Mita M,
Miller KD, Weise A, Burris H III, Kalinsky K, Tsai M, Liu MC, et
al: Abstract PD3-14: Phase 1 study of the antibody-drug conjugate
SGN-LIV1A in patients with heavily pretreated triple-negative
metastatic breast cancer. Cancer Res. 78:2018.
|
|
106
|
Han HS, Alemany CA, Brown-Glaberman UA,
Pluard TJ, Sinha R, Sterrenberg D, Albain KS, Basho RK, Biggs D,
Boni V, et al: SGNLVA-002: Single-arm, open label phase Ib/II study
of ladiratuzumab vedotin (LV) in combination with pembrolizumab for
first-line treatment of patients with unresectable locally advanced
or metastatic triple-negative breast cancer. J Clin Oncol. 37(Suppl
15): TPS11102019. View Article : Google Scholar
|
|
107
|
Modi S, Park H, Murthy RK, Iwata H, Tamura
K, Tsurutani J, Moreno-Aspitia A, Doi T, Sagara Y, Redfern C, et
al: Antitumor activity and safety of trastuzumab Deruxtecan in
patients with HER2-low-expressing advanced breast cancer: Results
from a phase Ib study. J Clin Oncol. 38:1887–1896. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Modi S, Ohtani S, Lee CC, Wang K, Saxena K
and Cameron DA: A phase III, multicenter, randomized, open label
trial of [fam-] trastuzumab deruxtecan (DS-8201a) versus
investigator's choice in HER2-low breast cancer. J Clin Oncol.
37(Suppl 15): TPS11022019. View Article : Google Scholar
|
|
109
|
Molyneux G, Geyer FC, Magnay FA, McCarthy
A, Kendrick H, Natrajan R, Mackay A, Grigoriadis A, Tutt A,
Ashworth A, et al: BRCA1 basal-like breast cancers originate from
luminal epithelial progenitors and not from basal stem cells. Cell
Stem Cell. 7:403–417. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Bernardo GM, Bebek G, Ginther CL, Sizemore
ST, Lozada KL, Miedler JD, Anderson LA, Godwin AK, Abdul-Karim FW,
Slamon DJ and Keri RA: FOXA1 represses the molecular pheno-type of
basal breast cancer cells. Oncogene. 32:554–563. 2013. View Article : Google Scholar
|
|
111
|
Su Y, Subedee A, Bloushtain-Qimron N,
Savova V, Krzystanek M, Li L, Marusyk A, Tabassum DP, Zak A,
Flacker MJ, et al: Somatic cell fusions reveal extensive
heterogeneity in basal-like breast cancer. Cell Rep. 11:1549–1563.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Cao R, Wang L, Wang H, Xia L,
Erdjument-Bromage H, Tempst P, Jones RS and Zhang Y: Role of
histone H3 lysine 27 methylation in Polycomb-group silencing.
Science. 298:1039–1043. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Yamagishi M and Uchimaru K: Targeting EZH2
in Cancer Therapy. Curr Opin Oncol. 29:375–381. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Yang CC, LaBaff A, Wei Y, Nie L, Xia W,
Huo L, Yamaguchi H, Hsu YH, Hsu JL, Liu D, et al: Phosphorylation
of EZH2 at T416 by CDK2 contributes to the malignancy of triple
negative breast cancers. Am J Transl Res. 7:1009–1020.
2015.PubMed/NCBI
|
|
115
|
Nie L, Wei Y, Zhang F, Hsu YH, Chan LC,
Xia W, Ke B, Zhu C, Deng R, Tang J, et al: CDK2-mediated
site-specific phosphorylation of EZH2 drives and maintains
triple-negative breast cancer. Nat Commun. 10:51142019. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Yang X, Phillips DL, Ferguson AT, Nelson
WG, Herman JG and Davidson NE: Synergistic activation of functional
estrogen receptor (ER)-alpha by DNA methyltransferase and histone
deacetylase inhibition in human ER-alpha-negative breast cancer
cells. Cancer Res. 61:7025–7029. 2001.PubMed/NCBI
|
|
117
|
Sharma D, Saxena NK, Davidson NE and
Vertino PM: Restoration of tamoxifen sensitivity in estrogen
receptor-negative breast cancer cells: Tamoxifen-bound reactivated
ER recruits distinctive corepressor complexes. Cancer Res.
66:6370–6378. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Connolly RM, Li H, Jankowitz RC, Zhang Z,
Rudek MA, Jeter SC, Slater SA, Powers P, Wolff AC, Fetting JH, et
al: Combination epigenetic therapy in advanced breast cancer with
5-azacitidine and entinostat: A phase II National Cancer
Institute/Stand up to cancer study. Clin Cancer Res. 23:2691–2701.
2017. View Article : Google Scholar :
|
|
119
|
Anderberg C, Li H, Fredriksson L, Andrae
J, Betsholtz C, Li X, Eriksson U and Pietras K: Paracrine signaling
by platelet-derived growth factor-CC promotes tumor growth by
recruitment of cancer-associated fibroblasts. Cancer Res.
69:369–378. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Roswall P, Bocci M, Bartoschek M, Li H,
Kristiansen G, Jansson S, Lehn S, Sjölund J, Reid S, Larsson C, et
al: Microenvironmental control of breast cancer subtype elicited
through paracrine platelet-derived growth factor-CC signaling. Nat
Med. 24:463–473. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Park JJH, Hsu G, Siden EG, Thorlund K and
Mills EJ: An overview of precision oncology basket and umbrella
trials for clinicians. CA Cancer J Clin. 70:125–137. 2020.
View Article : Google Scholar : PubMed/NCBI
|