Breast cancer is characterized by heterogeneity at
the molecular and clinical levels. Several biomarkers including
estrogen receptor α (ERα), progesterone receptor (PR), and human
epidermal growth factor receptor-2 (ERBB2/HER2) have been
established, and the main breast cancer subtypes are classified
according to their molecular profile (1,2).
Traditional staging of breast cancer is based on tumor size, lymph
node involvement, and presence of metastasis, and recently biologic
markers have been incorporated in the 8th edition of the American
Joint Committee on Cancer (AJCC), improving the prognostic
discrimination over anatomic staging alone (3).
Triple-negative breast cancer (TNBC) is
characterized as having ≤1% cellular expression of ER and PR as
determined by immunohistochemistry (IHC), and having HER2
expression of 0 to 1+ by IHC, or 2+ by IHC and fluorescence in
situ hybridization (FISH) negative (i.e. not an amplified gene
copy number), according to American Society of Clinical
Oncology/College of American Pathologists (ASCO/CAP) guidelines
(4,5). TNBCs are comprised of at least four
distinct transcriptional subtypes: Two basal subtypes, BL1 and BL2;
a mesenchymal subtype M, which is devoid of immune cells; and a
luminal androgen receptor (AR) subtype LAR (1,2).
TNBC is also subdivided into 6 different subgroups based on
molecular heterogeneity: Basal-like; mesenchymal-like; mesenchymal
stem-like; luminal AR expression; immunomodulatory; and unstable
type (6). TNBC represents
approximately 15-20% of all newly diagnosed breast cancers and is
generally a more aggressive disease with a poorer prognosis and
higher grade than other types of breast cancer, accounting for 5%
of all cancer-related deaths annually. The median overall survival
(OS) for the disease is 10.2 months with current therapies, with a
5-year survival rate of ~65% for regional tumors and 11% for those
that have spread to distant organs (7,8).
In this review, we discuss current TNBC treatments
and key examples of improved clinical benefit, as well as new
therapeutic strategies with which to treat the disease.
TNBC is chemotherapy sensitive, and this treatment
remains the standard of care (SOC). Common chemotherapies include
anthracycline (e.g., DNA intercalating agent and topoisomerase II
blocker doxorubicin), alkylating agents (e.g., cyclophosamide), an
anti-microtubule agent taxane, and an anti-metabolite fluorouracil
(5-FU). The current SOC for newly diagnosed early TNBC consists of
neoadjuvant chemotherapy, followed by surgery. For patients with
relapsed/refractory TNBC, there is no standard chemotherapy
regimen. Responses to treatment are usually short in duration and
followed by rapid relapse, and visceral and brain metastases are
common. Available therapies for patients with advanced TNBC include
anti-metabolites capecitabine and gemcitabine, non-taxane
microtubule inhibitor eribulin, and DNA cross-linker platinums. The
median progression-free survival (PFS) with chemotherapy ranges
from 1.7 to 3.7 months; the median OS from the onset of metastasis
is 10 to 13 months. In clinical trials, patients with advanced TNBC
treated with single-agent taxane- or platinum-based chemotherapy
had a median PFS of 4 to 6 months and a median OS of 11 to 17
months (9-11).
New treatment options for patients with advanced
TNBC have recently emerged, especially in cases where surgery is
not an option.
TNBC is more immunogenic than other breast cancer
subtypes with tumor-infiltrating lymphocytes (TILs) in its
microenvironment. However, TNBC also displays a high level of
programmed cell death-ligand 1 (PD-L1) expression (12,13).
Thus, immunotherapies targeting the programmed cell death-1 (PD-1)
receptor/PD-L1 pathway that maintains immunosuppression in the
tumor environment in TNBC have been explored and atezolizumab
(anti-PD-L1 antibody) in combination with nanoparticle
albumin-bound (nab)-paclitaxel was approved as a first-line therapy
by the US Food and Drug Administration (FDA) based on the
IMpassion130 trial (NCT02425891) in 2019. This immunochemotherapy
became SOC for patients with PD-L1+, unresectable,
locally advanced or metastatic TNBC. Note that the survival benefit
was exclusively in PD-L1+ TNBC patients. The threshold
is 1% PD-L1 expression on infiltrating immune cells by an approved
companion diagnostic SP142 IHC assay and 41% of enrolled patients
showed PD-L1-positive expression in the IMpassion130 trial. This is
in contrast to studies in other types of cancer which showed
benefit for checkpoint inhibitor therapy even in patients with
negative PD-L1 expression. In the first interim analysis of
IMpassion130, the median PFS was 7.5 vs. 5.0 months with
chemotherapy and the median OS was 25.0 vs. 15.5 months with
chemotherapy among patients with PD-L1+ tumors (14). In the pre-specified second interim
analysis (data cutoff January 2, 2019), the median OS was 25.0 vs.
18.0 months with chemotherapy. Overall, the combination was
well-tolerated and immune-related adverse events (AEs) included
rash, hypothyroidism, and pneumonitis (15). Another immunotherapy, pembrolizumab
(anti-PD-1 antibody), was approved in 2017 as a histology agnostic
immunotherapy in all microsatellite instability-high (MSI-H) and/or
mismatch repair deficient (dMMR) tumors. This is the first
FDA-approved cancer treatment based on a tumor biomarker without
regard to the original location of the tumor. However, MSI-H is
rare in breast cancer (<2%) (16-18).
BRCA1 and BRCA2-deficient tumors exhibit impaired
homologous recombination repair (HRR) and synthetic lethality with
poly(ADP-ribose) polymerase (PARP) inhibitors (19,20).
The FDA approved olaparib and talazoparib in 2018 to treat
advanced-stage HER2-negative breast cancer in individuals with a
Brca1 or Brca2 mutation. The FDA also approved the
companion diagnostic test to identify germline Brca-mutated
(gBRCAm) breast cancer patients. Approximately 5% of patients with
breast cancer carry a gBRCAm. Olaparib approval was based on data
from the OlympiAD Phase III (NCT02000622) trial comparing olaparib
to physician's choice of chemotherapy (capecitabine, vinorelbine or
eribulin). Olaparib was associated with a 42% increase in median
PFS as compared to the control group (7 vs. 4 months) in gBRCAm
HER2-negative meta-static breast cancer patients with previous
chemotherapy (21). There was no
statistically significant improvement in OS with olaparib compared
to the control group (19.3 vs. 17.1 months), but there was
potential OS benefit among patients with no prior chemotherapy for
metastatic breast cancer (HR 0.51, 95% CI 0.29-0.90) (22). Olaparib was generally
well-tolerated, with no evidence of cumulative toxicity including
the risk of developing anemia during extended exposure. Talazoparib
approval was based on data from the EMBRACA Phase III (NCT01945775)
trial comparing talazoparib to gemcitabine or to the same physician
choice of standard therapy as the OlympiAD trial. Talazoparib
increased median PFS by 46% (8.6 vs. 5.6 months) in gBRCAm
HER2-negative locally advanced or metastatic breast cancer patients
with previous chemotherapy including an anthracycline and/or
taxane. Talazoparib presented with hematologic grade 3-4 AEs
(primarily anemia), which occurred in 55 vs. 38% of the patients
with standard therapy, and an improved side-effect profile in
patient-reported outcomes (23).
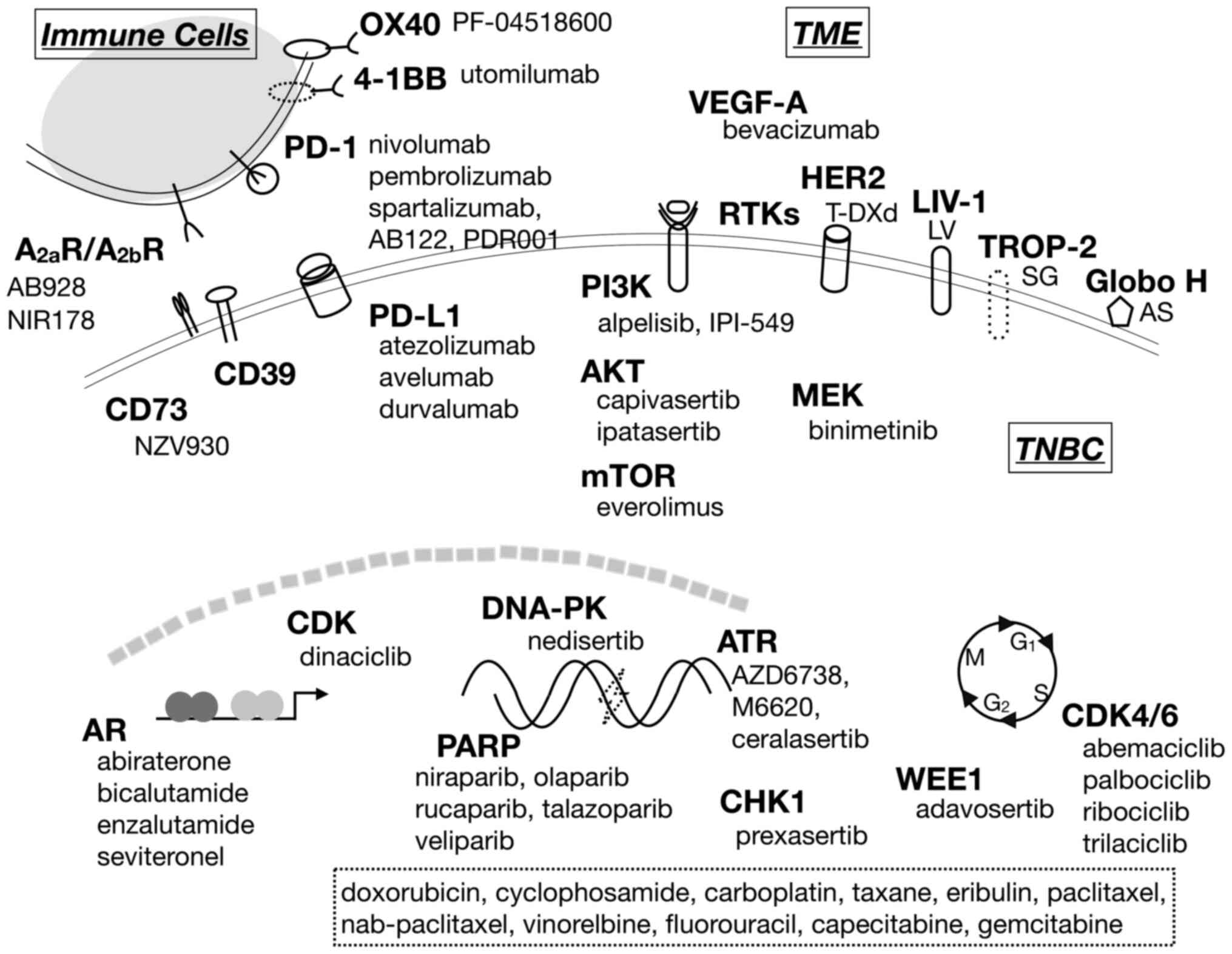
To improve therapeutic benefit in TNBC treatment,
various agents have been explored in clinical studies. They include
immuno- and targeted-therapies in the networks of tumor-stroma, DNA
damage response (DDR), cell surface or intracellular receptors, and
signaling pathways as well as cell surface markers for selective
drug delivery, and antibody-drug conjugates (ADCs) (Fig. 1). As of March 2020, 399 ongoing
studies for TNBC have been listed on ClinicalTrials.gov and select
Phase III studies are listed in Table
I.
TILs are frequent in TNBC, correlate with increased
pathologic complete response (pCR) to neoadjuvant chemotherapy, and
are predictive of disease-free survival (DFS) and OS in early-stage
TNBC (24-26). Expression of immune regulatory
checkpoints is an adaptive method of tumor resistance to
infiltrating lymphocytes within the tumor microenvironment.
Multiple strategies have been used to enhance the response to
PD-1/PD-L1 blockade in pre-clinical and early clinical studies,
including several intratumoral immune modulators and targeted
agents (27). The activity of
immunotherapy, such as immune checkpoint inhibitors, can be
enhanced by chemotherapeutic agents through the stimulation/release
of antigens, thus leading to promotion of immunogenic cell death.
Currently, clinical trials investigating the use of immune
checkpoint inhibitors are ongoing either as a single agent or in
various combinations with other agents beyond the metastatic
setting and even in the first-line setting (28).
Studies determining benefit from neoadjuvant
checkpoint inhibitor therapy have yielded mixed outcomes.
Neoadjuvant chemotherapy with pembrolizumab have demonstrated
manageable safety and promising anti-tumor activity for patients
with early-stage TNBC in the KEYNOTE-173 Phase 1b (NCT02622074)
(29) and I-SPY2 Phase II
(NCT01042379) trials (30). The
KEYNOTE-522 Phase III trial (NCT03036488) further explored
neoadjuvant chemotherapy with or without pembrolizumab followed by
surgery and pembrolizumab or placebo adjuvantly. The neoad-juvant
combination showed a significantly higher pCR rate than the
placebo-chemotherapy group (65 vs. 51%). Note that a similar pCR
benefit (~15%) in both the PD-L-positive and -negative subgroups
was observed, suggesting that neoadjuvant pembrolizumab may benefit
patients regardless of PD-L1 levels. This is different from the
advanced setting where only the PD-L1-positive patients benefit
from atezolizumab. The toxicity profiles were as expected for each
treatment, with similar rates (78 vs. 73%) of grade ≥3
treatment-related AEs (TRAEs) (31).
NeoTRIPaPDL1 Phase III (NCT02620280) trial also
explored neoadjuvant chemotherapy with or without atezolizumab
followed by surgery and four cycles of an anthracycline regimen.
However, in this trial for patients with early-stage high-risk or
locally advanced unilateral breast cancer there was no improvement
in pCR with the combination therapy (44 vs. 41% with the control
arm) (32). Note that the
neoadjuvant chemo-regimen was different from KEYNOTE-522 which
included another round of chemotherapy following carboplatin and
nab-paclitaxel. The difference in the targets, PD-1 for
pembrolizumab vs. PD-L1 for atezolizumab, may also have contributed
to the different outcomes. Another Phase III (NCT03197935) trial,
IMpassion031 study also explored atezolizumab in combination with
chemotherapy (nab-paclitaxel followed by doxorubicin and
cyclophosphamide) in comparison to placebo plus chemotherapy in the
neoadjuvant setting. Treatment with atezolizumab continued
adjuvantly for those in the combination arm of the study (33). The primary endpoint was pCR.
As a first-line treatment option for patients with
locally recurrent, inoperable or metastatic TNBC, pembrolizumab was
evaluated in combination with investigator's choice of chemotherapy
(i.e. nab-paclitaxel, paclitaxel or
gemcitabine/carboplatin), compared to placebo plus chemo-therapy
(KEYNOTE-355 Phase III trial, NCT02819518). A significant PFS
benefit with the pembrolizumab-chemo combination in patients whose
tumors expressed PD-L1 (CPS ≥10) was reported (9.7 vs. 5.6 months
for chemotherapy alone) (34). The
study is currently in progress to evaluate OS, the other primary
endpoint of the trial.
In contrast to other studies of immunotherapy
combined with SOC chemotherapy, the Tonic trial (NCT02499367) in
metastatic TNBC was based on an adaptive trial design that explores
a sequential treatment with anti-PD-1 antibody nivolumab after 2
weeks of chemotherapy or radiotherapy. The hypothesis is that
short-term treatment induces a more favorable tumor
microenvironment that would enhance sensitivity to immune
checkpoint blockade in TNBC. The highest overall response rate
(ORR) was observed with doxorubicin induction (35%) followed by
nivolumab/doxorubicin. Doxorubicin induction also upregulated
immune-related genes as well as inflammation, JAK-STAT, and TNF-α
signaling-related genes, suggesting a more favorable tumor
microenvironment induced by these chemotherapies (35). The InCITe Phase II trial
(NCT03971409) also includes a two-week induction of binimetinib
(MEK inhibitor), utomilumab (4-1BB agonist), or PF-04518600
(anti-OX40 antibody) which may help activate the immune system. The
trial explores how well anti-PD-L1 antibody avelumab might work
with one of those agents after induction in stage IV or
unresectable and recurrent TNBC.
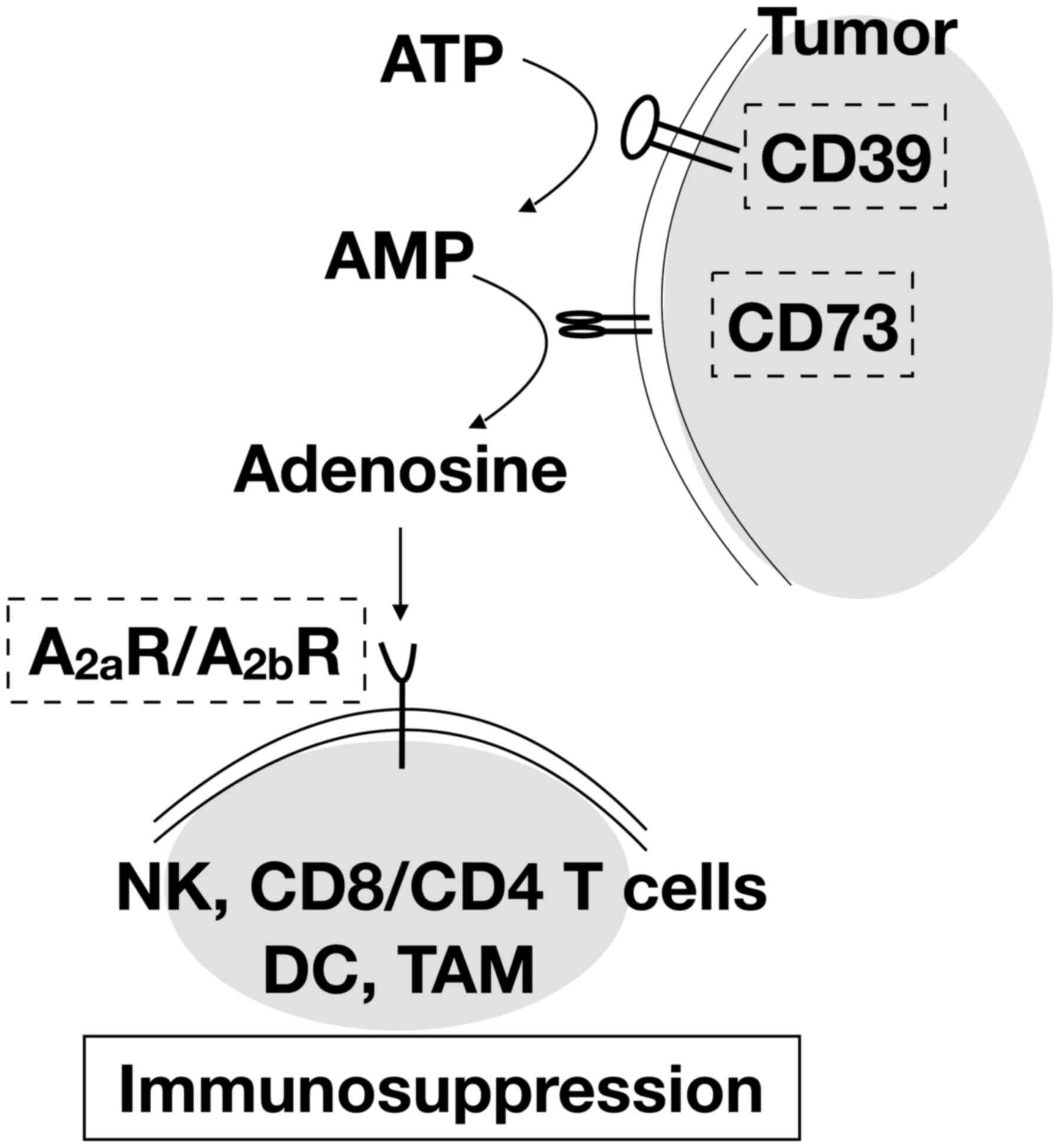
Adenosine is catabolized from ATP and often
overproduced and released by tumor cells. It is also converted from
extracellular nucleotides by the plasma membrane protein, cluster
of differentiation 73 (CD73), which is upregulated in many cancer
types (36,37). The excess adenosine in the tumor
microenvironment activates the adenosine 2A receptor
(A2aR) and 2B receptor (A2bR) (38,39)
which are highly expressed on the cell surfaces of lymphocytes and
myeloid cells, respectively, leading to immunosuppressive effects
(Fig. 2). Targeting these
receptors and enzymes could lead to reactivation of antitumor
immunity by abrogating the inhibitory effect on the immune system
and enhancing the cytotoxic T lymphocyte (CTL)-mediated immune
response (40,41).
Combinations of adenosine pathway inhibitors and
immune checkpoint inhibitors have been explored in clinical trials.
NZV930 (SRF373) is an anti-CD73 monoclonal antibody that binds to
CD73 on tumor cells, leading to internalization of CD73, thereby
preventing CD73-mediated conversion of extracellular AMP to
adenosine. A Phase I/Ib study (NCT03549000) is underway to evaluate
NZV930 alone and in combination with PD-1 inhibitor PDR001 and/or
A2aR antagonist NIR178 in patients with advanced
malignancies including TNBC. NIR178 is an antagonist of
A2aR, blocking adenosine/A2aR-mediated
inhibition of T lymphocytes. A Phase II study (NCT03207867) is
underway for NIR178 in combination with PD-1 inhibitor
spartalizumab in multiple solid tumors and diffuse large B-cell
lymphoma (DLBCL) to assess if the addition of the adenosine
antagonist improves the efficacy of PD-1 inhibition. A dual
adenosine A2aR/A2bR receptor antagonist,
AB928, is currently being evaluated in a Phase I study
(NCT03629756) in combination with the PD-1 inhibitor AB122 in
patients with advanced malignancies. Early results show a favorable
safety profile of AB928 combination therapy and predictable PK/PD
correlation (42).
A PARP inhibitor appears to have efficacy for
neoadjuvant treatment of patients with gBRCAm TNBC. Talazoparib
achieved encouraging pCR in patients with gBRCAm breast cancer,
including TNBC, and HR+ breast cancer, as a neoadjuvant
single-agent without the addition of chemotherapy (46). Currently a larger, multi-center,
neoadjuvant Phase II trial (NCT03499353) is ongoing. However, the
addition of a PARP inhibitor to standard neoadjuvant chemotherapy
was found to be not beneficial. In the BrighTNess Phase III trial
(NCT02032277) the addition of PARP inhibitor veliparib to
carboplatin and paclitaxel followed by doxorubicin and
cyclophosphamide did not improve pCR whereas the addition of
veliparib and carboplatin to paclitaxel did. Therefore, the
addition of carboplatin but not veliparib to paclitaxel was
proposed as a potential component of neoadjuvant chemotherapy for
patients with high-risk TNBC (47).
PARP inhibitors have also been studied as an
adjuvant single-agent therapy. The OlympiA Phase III trial
(NCT02032823) was designed to assess olaparib in patients with
gBRCAm and high-risk HER2-negative breast cancer who completed
definitive local treatment and neoadjuvant or adjuvant
chemotherapy. The primary outcome measure will be invasive DFS with
a time frame of up to 10 years.
A crosstalk exists between PARP inhibition and the
PD-L1/PD-1 immune checkpoint axis. PARP inhibitors upregulate PD-L1
expression on tumor cells by inhibiting glycogen synthase kinase 3
beta (GSK3β) and activating the cGAS-STING pathway (48). Thus, primary/acquired resistance to
PARP inhibitors seems to be associated with the development of
immune evasion mechanisms. Multiple clinical studies are underway
to assess synergy between therapeutic strategies of PARP inhibition
and immune checkpoint blockers.
In platinum-resistant, advanced, or metastatic TNBC,
niraparib combined with pembrolizumab (TOPACIO/KEYNOTE-162 Phase II
trial, NCT02657889) showed higher response rates in patients with
tumor Brca mutations (tBRCAm): ORR of 28% in all
(biomarker-unselected) patients vs. 60% for tBRCAm patients. The
combination therapy was safe with a tolerable safety profile
(49).
In MEDIOLA Phase I/II trial (NCT02734004) the
combi-nation of olaparib and durvalumab showed ORR of 63% in a
cohort of patients with gBRCAm metastatic breast cancer (50). In the I-SPY 2 Phase II study
(NCT01042379), adding the same combination to neoadjuvant
paclitaxel led to improved pCR rates in patients with high-risk,
HER2-negative stage II/III breast cancer compared with single-agent
paclitaxel. In those with TNBC, the pCR rate was 47 vs. 27% with
paclitaxel alone. AEs were consistent with the known safety
profiles of each agent alone (51). In metastatic TNBC, the efficacy of
induction treatment of olaparib followed by the combination
treatment of olaparib and durvalumab is being assessed in a Phase
II study (NCT03801369) (52).
Patients with ≤2 prior chemotherapy regimens for metastatic breast
cancer are eligible, but patients with gBRCAm TNBC are excluded.
The primary end point is ORR.
The DORA Phase II trial (NCT03167619) is evaluating
olaparib as a maintenance therapy with or without durvalumab in
patients with advanced TNBC who achieve at least stable disease
after 3 cycles of platinum-based chemo-therapy. Another study of a
PARP inhibitor as a maintenance therapy, KEYLYNK-009 Phase II/III
trial (NCT04191135), is underway in metastatic TNBC to assess the
efficacy of olaparib plus pembrolizumab vs. chemotherapy plus
pembrolizumab after induction with first-line chemotherapy plus
pembrolizumab (53).
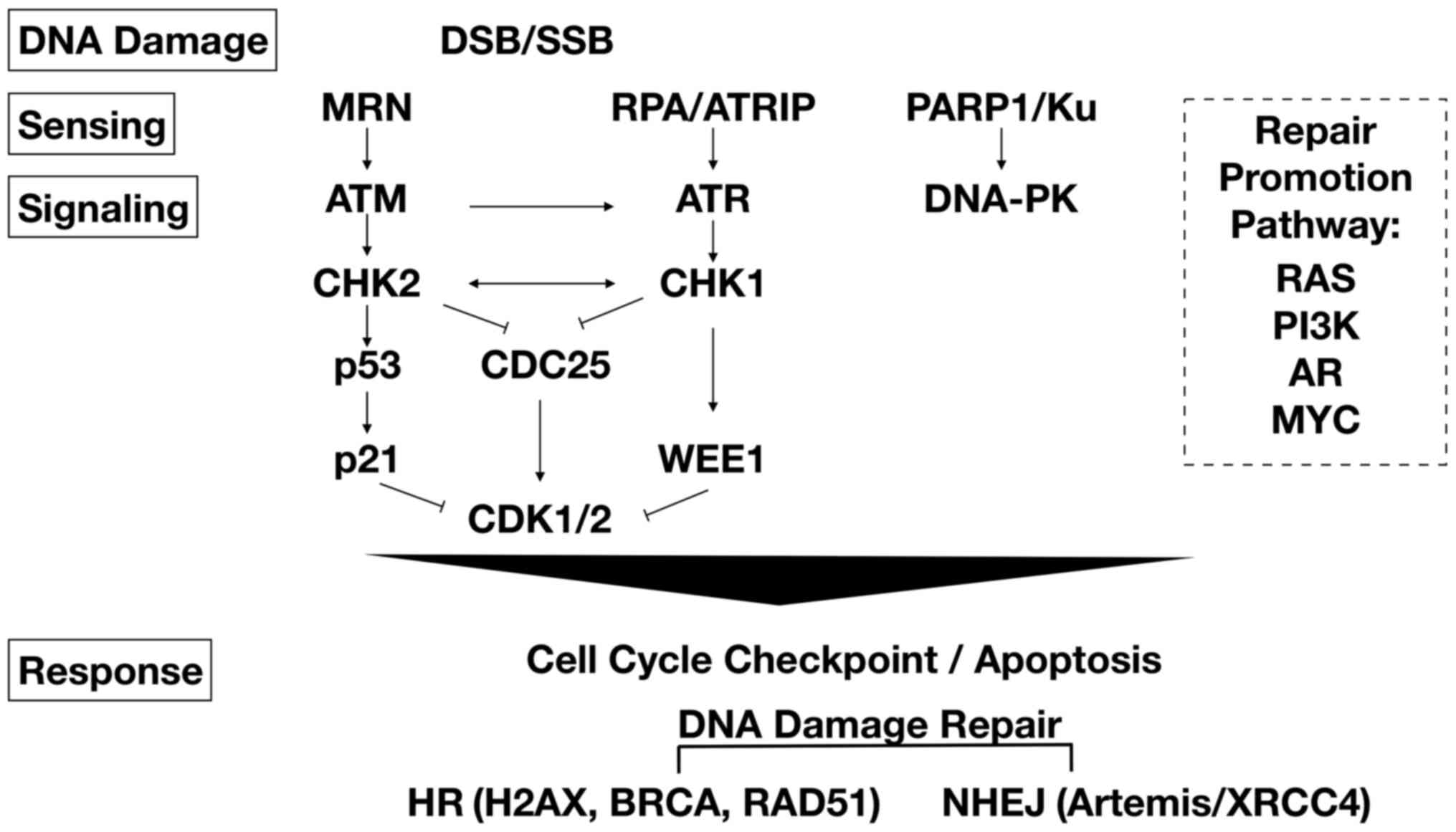
Resistance to PARP inhibitors can occur in certain
cancer contexts by various mechanisms, including increased HRR
capacity and decreased cell cycle progression and DNA replication
stress. RAD51 overexpression has been observed in a wide range of
human cancers, particularly TNBCs and serous ovarian cancers
(54,55). Upregulation of RAD51 in
BRCA1-defective cells is also associated with resistance to PARP
inhibitor (56,57). Inhibitors of key mediators of DNA
repair and replication, such as ataxia telangiectasia mutated
kinase (ATM), ataxia telangiectasia and Rad3-related kinase (ATR),
checkpoint kinase 1 (CHK1) and checkpoint kinase 2 (CHK2),
DNA-dependent protein kinase (DNA-PK), and WEE1 kinase (Fig. 3) have been assessed to determine if
they can sensitize tumor cells to treatment with PARP inhibitors,
as these inhibitors were found to prevent the accumulation of RAD51
in TNBC (58).
The VIOLETTE Phase II study (NCT03330847) was set up
to assess the combinatory inhibition of PARP and a component of the
ATR-CHK1-WEE1 axis. Olaparib with DDR kinase ATR inhibitor AZD6738
was compared to olaparib monotherapy in the second- or third-line
setting of metastatic TNBC. Patients were stratified by Brca
and HRR gene mutation status and the primary endpoint was PFS
(59). The study also included a
combination arm of olaparib with the first-in-class WEE1 inhibitor
adavosertib. WEE1 inhibitor was found to potentiate the activity of
DNA-damaging agents in preclinical TNBC models (60,61)
and its potential clinical value was observed in a Phase I study in
patients with Brca mutations (62). However, the combination treatment
arm of olaparib and adavosertib was discontinued in the VIOLETTE
study and patients were offered the opportunity to continue
treatment on olaparib monotherapy. The CHK1 inhibitor prexasertib
in combination with olaparib was also explored in early clinical
trials (63), but development of
prexasertib was discontinued by the sponsor in 2019.
A wide range of malignancies including TNBC show
dysregulated phosphatase and tensin homolog (PTEN)/phosphoinositide
3-kinases (PI3K)/protein kinase B (AKT)/mammalian target of
rapamycin (mTOR) signaling due to mutations in multiple signaling
components. Loss of PTEN, a negative regulator of AKT, was found to
be correlated with decreased T-cell infiltration at tumor sites in
patients, and inhibition of the PI3K-AKT pathway re-sensitized to
T-cell-mediated immunotherapy (64). As the PI3K/AKT pathway has emerged
as a potential mechanism of resistance to immunotherapy and
chemotherapy, multiple clinical trials have assessed inhibitors of
the various pathway components.
Alpelisib is an oral PI3K inhibitor that selectively
inhibits p110α. It showed efficacy in targeting
Pik3ca-mutated breast cancer (65) and was FDA approved in 2019 in
combination with fulvestrant for postmenopausal women and men, with
HR+, HER2-negative, Pik3ca-mutated, advanced or
metastatic breast cancer following progression on or after an
endocrine-based regimen. For patients with advanced TNBC, the
EPIK-B3 Phase III trial (NCT04251533) is planned with study start
date of April 2020 to assess alpelisib in combination with
nab-paclitaxel. Patients have Pik3ca mutations or PTEN loss
with ≤1 prior line of therapy for metastatic disease.
IPI-549 is a selective PI3K-gamma inhibitor
targeting immune-suppressive tumor-associated myeloid cells. The
MARIO-3 Phase II study (NCT03961698) was designed to explore the
addition of IPI-549 to the FDA approved regimen
atezolizumab/nab-paclitaxel in front-line TNBC. Cohort A will be
composed of patients with locally advanced, metastatic TNBC, which
will include two sub-cohorts based on PD-L1 IHC status. The primary
objective is CR rate.
Ipatasertib and capivasertib are pan-AKT inhibitors
that bind to all three isoforms of AKT. Both are now in Phase III
trials evaluating the efficacy of combination with paclitaxel as
first-line therapy for locally advanced or metastatic TNBC. In the
LOTUS Phase II trial, adding ipatasertib to first-line paclitaxel
improved PFS, particularly in patients with PTEN/PI3K/AKT-altered
tumors (HR, 0.44) (66). In this
subgroup of patients, median OS was 23.1 vs. 16.2 months with
placebo (HR, 0.65) (67). To
confirm the findings from LOTUS, the IPATunity130 Phase III trial
(NCT03337724) is evaluating ipatasertib + paclitaxel for
PTEN/PI3K/AKT-altered advanced TNBC or HR+,
HER2-negative breast cancers. The primary endpoint is PFS (68). An independent trial also supported
the potential benefit for addition of AKT inhibitor to
chemotherapy. In the PAKT Phase II study (NCT02423603), addition of
the oral AKT inhibitor capivasertib to first-line paclitaxel
resulted in significantly longer PFS and OS in patients with
advanced TNBC, especially in patients with PTEN/PI3K/AKT-altered
tumors. The median PFS duration was 5.9 vs. 4.2 months with
placebo, meeting the predefined significance level, and better
benefit in patients with PTEN/PI3K/AKT-altered tumors with median
PFS of 9.3 months (HR, 0.30). The median OS was prolonged by 6.5
months with capivasertib (69).
The most common AEs of grade ≥3 were diarrhea, infection, rash, and
fatigue, similar to those observed with ipatasertib in the LOTUS
trial. The CAPItello-290 Phase III trial (NCT03997123) is underway
and the primary endpoints are PFS and OS (70).
Efficacy of immunotherapy was also found to be
enhanced by AKT inhibitors as a first-line therapy for locally
advanced/metastatic TNBC. Phase Ib study (NCT03800836) was designed
to evaluate the triplet combination of ipatasertib (I),
atezolizumab (A), and paclitaxel or nab-paclitaxel (P). Preliminary
efficacy and safety data up to January 5, 2019 showed that the
triplet regimen had promising antitumor activity (73% confirmed
ORR), irrespective of biomarker PD-L1 status or PTEN/PI3K/AKT
alteration status, and manageable toxicity (71). In Phase III trial (NCT04177108),
patients were enrolled in two cohorts according to PD-L1 status:
Cohort 1 for PD-L1-negative tumors and cohort 2 for PD-L1-positive
tumors. Three arms, P + I + A vs. P + I vs. P, will be evaluated in
cohort 1 and 2 arms, P + I + A vs. P + A, will be evaluated in
cohort 2.
Preclinical combination studies of CDK4/6 inhibitors
with chemotherapy suggest that the timing and sequence of drug
exposure/drug delivery schedule might play a critical role in drug
activity, and the evaluation of different schedules of treatment
may represent a new approach (73,74).
The hypothesis was that reversible G1 arrest of
palbociclib could synchronize tumor cells in the cell cycle and
following their re-entry later would ensure a higher fraction in
mitosis (M) phase when exposed to paclitaxel. In the first
combination trial for palbociclib and paclitaxel (NCT01320592) an
alternative dosing schedule was feasible and safe, without evidence
of additive toxicity in Rb+ breast cancer regardless of
subtype (75). Phase I follow-up
trial (NCT02599363) of ribocilcib and weekly paclitaxel is in
progress in patients with Rb+ advanced breast cancer. In
this study, pharmacodynamic, histologic, and imaging biomarkers
will be utilized to confirm synchronization and schedule and
identify a patient population that benefits from this treatment
approach.
The standard chemotherapy regimen causes
treatment-limiting cumulative myelosuppression that may compromise
antitumor efficacy in TNBC. CDK4/6 inhibitors induce transient
G1 arrest in immune cells and hematopoietic stem and
progenitor cells, potentially helping to preserve T-cell function
and bone marrow. To test this hypothesis, an investigational CDK4/6
inhibitor trilaciclib in combination with gemcitabine and
carboplatin was explored to evaluate benefit for patients with ≤2
prior chemotherapy regimens in metastatic TNBC. Phase II trial
(NCT02978716) was negative for a safety-related primary endpoint
(i.e. no difference in the frequency or duration of severe grade 4
neutropenia). However, the median OS was improved by more than 60%,
which was likely due to increased chemotherapy duration and
exposure. Trilaciclib-treated patients also had a higher number of
activated CD8+ T cells over the first 5 cycles of
chemotherapy, which potentially enhanced antitumor immunity
(76).
Transcription factor c-MYC triggers selective gene
expression to promote cell growth and proliferation. It is
amplified in several different cancer types including TNBC,
functioning as a proto-oncogene (77). c-MYC compensates for BRCA loss by
upregulating HRR through increased RAD51 expression (55,78).
TNBC patients with high c-MYC and RAD51 expression exhibit poor
prognosis and less favorable response to chemotherapy and PARP
inhibitors (55,57,79).
c-MYC blockade in TNBC was found to be synthetic lethal with PARP
inhibitors, independent of BRCA status (80). c-MYC pathway activation in TNBC is
also synthetic lethal with CDK inhibition (81). Dinaciclib is a pan-CDK (CDK1/2/5/9)
inhibitor and the combination with PARP1 inhibitor veliparib is
currently being pursued in patients with advanced solid tumors for
which no curative therapy exists (Phase I trial, NCT01434316).
Dinaciclib induced immunogenic cell death (ICD) but also increased
expression of PD1 on tumor-infiltrating T cells and expression of
PD-L1 on tumor cells, thus limiting its antitumor effect in
preclinical studies. However, dinaciclib inhibits tumor growth in
combination with anti-PD-1 (82).
Phase Ib trial (NCT01676753) was designed to evaluate the efficacy
of combined dinaciclib and pembrolizumab in patients with
metastatic or locally advanced and unresectable TNBC. Its clinical
benefit rate was 47% in preliminary efficacy analysis and high
c-MYC expression correlated significantly with clinical response,
warranting further validation of c-MYC as a predicative biomarker
of response to CDK/checkpoint inhibitors (83).
The androgen receptor (AR) is an intracellular
steroid receptor that dimerizes and translocates to the nucleus
after binding androgen ligands. In the nucleus, AR binds to
androgen response elements to promote target gene transcription in
a tissue-specific manner. AR can also be activated in a
ligand-independent manner through crosstalk with key signaling
pathways, including PI3K/AKT and ERK (84). AR is involved in cell cycle
regulation and the epithelial-to-mesenchymal transition (EMT)
(85,86). AR has emerged as a new biomarker
and a potential therapeutic target in TNBC. AR is expressed in ≥40%
of TNBCs and its expression level varies considerably among TNBC
molecular subtypes. It has been associated with favorable
prognosis, with better DFS and higher OS in the LAR subtype
(87,88). However, patients with
AR+ TNBCs have a decreased chance of achieving pCR to
neoadjuvant chemotherapy and the LAR subtype has been linked to
poorer response to chemotherapy compared to other TNBC patients
(89-91). Multiple selective AR inhibitors
have been approved by the FDA for the treatment of prostate cancer
and are currently part of standard care (92). The role of the AR in signaling
pathways in TNBC is still not clear and clinical studies are
underway to provide more insight into the role of the AR as well as
to assess whether AR targeting is a valuable therapeutic strategy
in TNBC.
The first proof-of-concept trial of AR-targeted
treatment established activity of the first-generation AR
antagonist bicalutamide in patients with advanced AR+
TNBC. The TBCRC 011 Phase II trial (NCT00468715) showed a modest
clinical benefit rate (CBR) of 19% at 6 months and a median PFS
duration of 12 weeks (93).
As one of the second-generation anti-androgen
therapies, abiraterone is a steroidal CYP17 inhibitor with potent
hydroxylase activity, targeting androgen biosynthesis. The French
Breast Cancer Intergroup (UCBG) 12-1 Phase II trial (NCT01842321)
was designed to evaluate abiraterone acetate (AA) with its
requisite concomitant medication prednisone in AR+
advanced or metastatic TNBC. Androgen deprivation by AA resulted in
20% of the 6-month CBR. This treatment appeared to be beneficial
for some patients with molecular apocrine tumors, a subtype that
expresses AR but not ERα (96).
Considering that prednisone stimulates the glucocorticoid receptor
(GR), which is expressed in approximately 25% of TNBCs, GR activity
might limit the efficacy of AA.
Seviteronel is an investigational lyase-selective
non-steroidal CYP17 inhibitor that targets androgen and estrogen
production. The CLARITY-01 Phase I/II trial (NCT02580448) was set
up to evaluate seviteronel in locally advanced or metastatic TNBC
or ER+ breast cancer. It revealed that seviteronel was
generally well-tolerated and provided clinical benefit. A total of
26 and 11% of patients reached at least a CBR at 4 and 6 months,
respectively. Levels of circu-lating tumor cells (CTCs) also
decreased (97,98).
A second-generation AR antagonist enzalutamide not
only competitively binds to the AR ligand-binding domain, but also
inhibits nuclear translocation of AR, DNA binding, and coactivator
recruitment. Phase II single arm study (NCT01889238) assessed the
efficacy of enzalutamide in patients with locally advanced or
metastatic, AR+ TNBC. The primary endpoint was CBR at 16
weeks, which was 25% in the intention-to-treat (ITT) population and
33% in the evaluable subgroup whose tumors expressed ≥10% nuclear
AR. The only treatment-related grade 3 or greater AE occurring in
≥2% of patients was fatigue (3.4%) (99). The randomized ENDEAR Phase III
study (NCT02929576) comparing enzalutamide and paclitaxel to
placebo and paclitaxel in advanced TNBC was in place (100) but withdrawn in 2018, citing that
further understanding about the role of androgen signaling in TNBC
was required. The TBCRC 032 Phase Ib/II trial (NCT02457910)
investigated the safety and efficacy of enzalutamide alone or in
combination with PI3K inhibitor taselisib in patients with
metastatic AR+ TNBC. Primary endpoint of CBR at 16 weeks
was 36% and median PFS was 3.4 months. The trial was not completed
due to termination of the development of taselisib. Although this
study was exploratory due to sample size limitation, it revealed
subtype-specific treatment response (favorable trend for luminal
over non-luminal) and identified novel Fgfr2 gene fusions
that likely activate the PI3K pathway and AR splice variants that
may contribute to enzalu-tamide resistance. Therefore, an AR IHC
score of ≥10% alone may not identify patients with AR-dependent
tumors, and LAR subtype and AR splice variants may help identify
patients likely to benefit from AR antagonists (101).
The Globo H antigen is a hexasaccharyl sphingolipid
expressed on the surface of various cancer types and has been
explored as a potential target for vaccine therapy. Adagloxad
simolenin (AS) is an immune stimulant comprising the Globo H
hexasaccharide epitope linked to the carrier protein keyhole limpet
hemocyanin (KLH). KLH facilitates a more vigorous immune response
given the weak antigen, Globo H. As a first-in-class active
immunotherapy in development for metastatic breast cancer, AS with
the saponin-based adjuvant OBI-821 induced antibodies reactive with
Globo H+ tumor cells that mediate antibody-dependent
cell-mediated cytotoxicity (ADCC) and complement-dependent
cytotoxicity (CDC) (102). Phase
II trial (NCT01516307) assessed low-dose cyclophosphamide with or
without active immunotherapy (AS + adjuvant) in post-treated
metastatic breast cancer subjects with stable disease or response
to treatment. Although it did not meet its primary efficacy
endpoint of PFS, patients who developed an immune response to the
vaccine showed significantly improved PFS and OS (103). Based on these subgroup data,
Phase III study (NCT03562637) of AS with adjuvant vs. placebo
treatment is in progress for high-risk early-stage TNBC patients
following neoadjuvant or adjuvant chemotherapy. Patients will be
screened for Globo H expression (IHC H-score ≥15) and the primary
objective is improvement of invasive disease-free survival (IDFS)
in the time frame of 5 years.
An ADC is designed to be stable in plasma, target a
tumor cell surface antigen with a high affinity and specificity,
and is internalized, cleaved, and releases a payload drug which
drives antitumor activity through direct cytotoxic cell killing and
induces ICD.
Sacituzumab govitecan-hziy (SG) targets a
glycoprotein, the human trophoblast cell-surface antigen 2
(TROP-2), that is expressed in more than 90% of TNBCs. Its payload
is the active metabolite of irinotecan (SN-38), which is conjugated
to the anti-TROP-2 antibody by a cleavable linker. Phase I/II
single group study (NCT01631552) included 108 patients with TNBC
and 80% of patients had visceral metastases. The median number of
prior regimens was 3 (range, 2-10), which included chemotherapies
and checkpoint inhibitors. Although it did not include biomarker
selection of patients, 57 patients had moderate (2+) to strong (3+)
and 5 patients had weak or absent TROP-2 expression by IHC
according to available data. The ORR was 33% and the median
duration of response (DOR) was 7.7 months. The median PFS was 5.5
months and the median OS was 13.0 months. Myelotoxic effects were
the main adverse reactions and grade 3 or 4 AEs included anemia and
neutropenia (104). The
confirmatory ASCENT Phase III study (NCT02574455) of SG in
comparison with treatment of physician's choice for patients with
metastatic TNBC was stopped due to compelling evidence of efficacy
across multiple endpoints and SG was granted accelerated approval
by the FDA based on the results of the IMMU-132-01 Phase II
clinical trial for the treatment of adult patients with metastatic
TNBC who have received ≥2 prior therapies for metastatic disease.
It is the first ADC approved by the FDA specifically for relapsed
or refractory metastatic TNBC as well as the first FDA-approved
anti-TROP-2 ADC.
Ladiratuzumab vedotin (LV) targets LIV-1, which is
expressed in >90% of breast tumors with limited expression in
normal tissues. LIV-1 is a transmembrane protein with zinc
transporter and metalloproteinase activity. The payload of LV is
the microtubule disrupting agent monomethyl auristatin E (MMAE).
Phase I study (NCT01969643) in patients with heavily pretreated
metastatic TNBC showed 25% ORR and medium PFS of 11 weeks.
Treatment was generally well-tolerated and related AEs were
neutropenia, anemia, and neuropathy (105). LV was further explored in
combination studies and in earlier lines of treatment. The
SGNLVA-002 Phase Ib/II trial (NCT03310957) was designed to assess
whether combining LV and pembrolizumab results in synergistic
activity through LV-induced ICD that creates a microenvironment
favorable for enhanced anti-PD-L1 activity. It was for first-line
treatment of patients with unresectable locally advanced or
metastatic TNBC. Initial dose-finding studies revealed ORR of 35%
with responses independent of PD-L1 status and manageable toxicity
(106).
ADC has also been explored for HER2-low or negative
breast cancer. The rationale is based on the bystander effect, that
is, the cleaved drug from an ADC may leak from the targeted tumor
cell and affect cells in close proximity regardless of their target
antigen expression status. Thus, an ADC having a high
drug-to-antibody ratio and high-potency payload would increase the
killing of tumor cells even with low HER2 expression. Trastuzumab
deruxtecan (T-DXd) is the first HER2-targeted agent to demonstrate
promising clinical antitumor activity with a manageable safety
profile in patients considered to be HER2-negative. T-DXd delivers
a potent topoisomerase I inhibitor payload (an exatecan derivative)
which is linked to a humanized anti-HER2 anti-body. In Phase Ib
(NCT02564900) trial of T-DXd for heavily pretreated patients with
advanced HER2-low breast cancer, ORR was 37% with the median DOR
being 10.4 months. Most toxicities were gastrointestinal or
hematologic-related, and interstitial lung disease (ILD) was an
important identified risk (107).
The DESTINY-Breast04 Phase III (NCT03734029) was initiated to
compare the efficacy and safety of T-DXd to physician's choice
(capecitabine, eribulin, gemcitabine, paclitaxel, or
nab-paclitaxel) in patients with HER2-low, unresectable, and/or
metastatic breast cancer (108).
Gene expression analysis and functional studies have
revealed a high degree of plasticity and heterogeneity in luminal
and basal-like tumors. Expression of ERα, FOXA1 or GATA3 can result
in transition from basal-like breast cancer to luminal type whereas
epigenetic reprogramming can result in a reverse transition
(109-111). The CDK2-EZH2 axis in tumors with
TNBC phenotype (i.e. basal-like breast cancer) has been explored
for conversion to the ERα+ subtype. Epigenetic enzyme
EZH2, a histone-lysine N-methyltransferase that promotes histone H3
lysine 27 mono-, di- and tri-methylation (H3K27me1/2/3), drives
transcriptional repression (112,113). EZH2 can be phosphorylated at T416
(pT416-EZH2) by cyclin E/CDK2 and >80% of TNBC patient specimens
exhibit high pT416-EZH2 levels, which correlate with poorer
survival (114). In preclinical
studies, transgenic expression of a phospho-mimicking mutant
EZH2(T416D) in the mammary glands of mice reprogramed the committed
luminal breast cancer cells into the basal-like TNBC phenotype. In
this setting inhibition of the CDK2-EZH2 axis by EZH2 inhibitors
reactivated ERα expression and thus combination with tamoxifen
suppressed tumor growth and improved the survival of mice bearing
tumors with the TNBC phenotype (115). Therefore, inhibitors of CDK2 or
EZH2 combined with hormonal therapy may be a novel therapeutic
strategy in TNBC with especially high pT416-EZH2 levels.
Another mechanism-based therapy exploits the lack of
ER expression due to hypermethylation of the ERα promoter. A
combination epigenetic therapy of a DNA methyltransferase (DNMT)
inhibitor and a histone deacetylase (HDAC) inhibitor led to
re-expression of genes including ERα and restored tamoxifen
sensitivity in ER-negative breast cancer models (116,117). However, Phase II study
(NCT01349959) in patients with advanced hormone-resistant breast
cancer or TNBC revealed that combination of DNMT inhibitor
5-azacitidine and HDAC inhibitor entinostat did not induce ERα
expression and primary endpoint ORR was not met (118). ERα re-expression induced by
DNMT/HDAC inhibition might be attenuated by an active CDK2-EZH2
axis, which affected outcomes in this study.
Under the master protocol framework, basket trials,
where a targeted therapy is evaluated for multiple diseases that
share common molecular alterations, and umbrella trials, where
multiple targeted therapies are evaluated for a single disease that
is stratified into multiple subgroups based on different molecular
factors, have been developed (121). Recently there have been more
adaptive, signal-finding clinical trial designs coupled with
correlative studies to investigate mechanisms of action. They also
facilitate identifying active drug combinations as well as novel
tumor indications. Patients are enrolled based on molecular markers
from genetic profiling performed on their tumors. Some examples are
listed below.
In the OLAPCO Phase II trial (NCT02576444), PARP
inhibitor olaparib was assessed in combination with various agents
according to identified tumor mutations. It included AKT inhibitor
capivasertib for tumors with mutations in the PI3K-AKT pathway,
WEE1 inhibitor adavosertib for tumors with tp53 or/and
Kras mutations, and ATR inhibitor ceralasertib for tumors
with mutations in HRR genes. Primary outcome measure was ORR, and
the trial also identified genetic determinants of response and
resistance. Another Phase II trial (NCT03718091) evaluated ATR
inhibitor M6620 in selected solid tumors. Patients were enrolled in
different cohorts based on tumor mutation status, including
truncating Atm mutations, germline Brca mutations,
somatic Brca mutations or other HRR gene mutations, c-MYC
amplification, Fbxw7 mutations, cyclin E amplification, and
Arid1a mutations. Primary outcome measures included disease
control rate (DCR) and changes in pCHK1 and γH2AX levels. The I-SPY
2 Phase II trial (NCT01042379) was a neoadjuvant breast cancer
trial using response-adaptive randomization. It had multiple
concurrent experimental arms with shared controls. Each biomarker
signature was established at trial entry. A new regimen of
combination with standard chemotherapy will be moved up to Phase
III trial if it shows a high probability of improved pCR over
standard chemotherapy.
Developing novel treatments in both early and
advanced TNBC settings remains a significant unmet need. Recent
advances with novel agents have been made for specific subgroups
with PD-L1+ tumors or gBRCAm tumors. However, only a
fraction of those patients respond to immune check-point or PARP
inhibitors, and even those who do respond often develop resistance
and relapse. In diverse tumor microenvironments, a given
therapeutic agent shows variable responses, thus compromising the
survival endpoints especially in an unselected TNBC population.
Therefore, developing novel predictive biomarkers are crucial for
selecting patients that will benefit the most from a given therapy.
Single cell technologies will provide additional insight on
tumor-stroma interactions and facilitate compel-ling rationale for
new treatments based on novel biomarkers. A non-invasive testing of
plasma circulating tumor DNA (ctDNA) and CTCs can potentially
provide real-time disease monitoring and even early therapy
modification. However, their prognostic value needs further
evaluation. With recent advances in multiomic analyses of cancers,
there appears to be genomic and molecular similarities between TNBC
and high-grade serous ovarian carcinoma (HGSOC), suggesting that
similar biological mechanisms drive some aspects of both cancer
types. Therefore, treatment strategies for HGSOC can be explored in
TNBC as well. The recent increase in the number of clinical trials
investigating various new agents and combination strategies
reflects further efforts to under-stand molecular and immunological
aspects of TNBC. This may lead to more meaningful clinical
benefits, including event-free and overall survival.
The authors would like to thank Professor Ian
Collins of the Institute of Cancer Research, UK for valuable
discussions on the DNA damage response pathways and checkpoint
kinases.
No funding was declared.
All information provided in this review is
documented with relevant and current references.
KAW was responsible for conceptualization, design,
interpretation and visualization. KAW and CS were responsible for
writing, reviewing and editing. Both authors approved the final
manuscript.
Not applicable.
Not applicable.
No competing interests are declared.
|
1
|
Lehmann BD, Bauer JA, Chen X, Sanders ME,
Chakravarthy AB, Shyr Y and Pietenpol JA: Identification of human
triple-negative breast cancer subtypes and preclinical models for
selection of targeted therapies. J Clin Invest. 121:2750–2767.
2011.PubMed/NCBI
|
|
2
|
Lehmann BD, Jovanović B, Chen X, Estrada
MV, Johnson KN, Shyr Y, Moses HL, Sanders ME and Pietenpol JA:
Refinement of triple-negative breast cancer molecular subtypes:
Implications for neoadjuvant chemotherapy selection. PLoS One.
11:e01573682016.PubMed/NCBI
|
|
3
|
Giuliano AE, Connolly JL, Edge SB,
Mittendorf EA, Rugo HS, Solin LJ, Weaver DL, Winchester DJ and
Hortobagyi GN: Breast cancer-major changes in the American Joint
Committee on cancer eighth edition cancer staging manual. CA Cancer
J Clin. 67:290–303. 2017.PubMed/NCBI
|
|
4
|
Wolff AC, Hammond MEH, Allison KH, Harvey
BE, Mangu PB, Bartlett JMS, Bilous M, Ellis IO, Fitzgibbons P,
Hanna W, et al: Human epidermal growth factor receptor 2 testing in
breast cancer: American Society of Clinical Oncology/College of
American pathologists clinical practice guideline focused update. J
Clin Oncol. 36:2105–2122. 2018.PubMed/NCBI
|
|
5
|
Allison KH, Hammond MEH, Dowsett M,
McKernin SE, Carey LA, Fitzgibbons PL, Hayes DF, Lakhani SR,
Chavez-MacGregor M, Perlmutter J, et al: Estrogen and progesterone
receptor testing in breast cancer: ASCO/CAP guideline update. J
Clin Oncol. 38:1346–1366. 2020.PubMed/NCBI
|
|
6
|
Yam C, Mani SA and Moulder SL: Targeting
the molecular subtypes of triple negative breast cancer:
Understanding the diversity to progress the field. Oncologist.
22:1086–1093. 2017.PubMed/NCBI
|
|
7
|
Bonotto M, Gerratana L, Poletto E, Driol
P, Giangreco M, Russo S, Minisini AM, Andreetta C, Mansutti M, Pisa
FE, et al: Measures of outcome in metastatic breast cancer:
Insights from a real-world scenario. Oncologist. 19:608–615.
2014.PubMed/NCBI
|
|
8
|
Kohler BA, Sherman RL, Howlader N, Jemal
A, Ryerson AB, Henry KA, Boscoe FP, Cronin KA, Lake A, Noone AM, et
al: Annual report to the nation on the status of cancer, 1975-2011
featuring incidence of breast cancer subtypes by race/ethnicity,
poverty, and state. J Natl Cancer Inst. 107:djv0482015.
|
|
9
|
O'Shaughnessy J, Schwartzberg L, Danso MA,
Miller KD, Rugo HS, Neubauer M, Robert N, Hellerstedt B, Saleh M,
Richards P, et al: Phase III study of iniparib plus gemcitabine and
carboplatin versus gemcitabine and carboplatin in patients with
metastatic triple-negative breast cancer. J Clin Oncol.
32:3840–3847. 2014.PubMed/NCBI
|
|
10
|
Caswell-Jin JL, Plevritis SK, Tian L,
Cadham CJ, Xu C, Stout NK, Sledge GW, Mandelblatt JS and Kurian AW:
Change in survival in metastatic breast cancer with treatment
advances: Meta-analysis and systematic review. JNCI Cancer Spectr.
2:pky0622018.
|
|
11
|
Plevritis SK, Munoz D, Kurian AW, Stout
NK, Alagoz O, Near AM, Lee SJ, van den Broek JJ, Huang X, Schechter
CB, et al: Association of screening and treatment with breast
cancer mortality by molecular subtype in US women, 2000-2012. JAMA.
319:154–164. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Stanton SE, Adams S and Disis ML:
Variation in the incidence and magnitude of tumor-infiltrating
lymphocytes in breast cancer subtypes: A systematic review. JAMA
Oncol. 2:1354–1360. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Safonov A, Jiang T, Bianchini G, Győrffy
B, Karn T, Hatzis C and Pusztai L: Immune gene expression is
associated with genomic aberrations in breast cancer. Cancer Res.
77:3317–3324. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schmid P, Adams S, Rugo HS, Schneeweiss A,
Barrios CH, Iwata H, Diéras V, Hegg R, Im SA, Shaw Wright G, et al:
Atezolizumab and Nab-Paclitaxel in advanced triple-negative breast
cancer. N Engl J Med. 379:2108–2121. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schmid P, Rugo HS, Adams S, Schneeweiss A,
Barrios CH, Iwata H, Diéras V, Henschel V, Molinero L, Chui SY, et
al: Atezolizumab plus nab-paclitaxel as first-line treatment for
unresectable, locally advanced or metastatic triple-negative breast
cancer (IMpassion130): Updated efficacy results from a randomised,
double-blind, placebo-controlled, phase 3 trial. Lancet Oncol.
21:44–59. 2020. View Article : Google Scholar
|
|
16
|
Dudley JC, Lin MT, Le DT and Eshleman JR:
Microsatellite instability as a biomarker for PD-1 blockade. Clin
Cancer Res. 22:813–820. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bonneville R, Krook MA, Kautto EA, Miya J,
Wing MR, Chen HZ, Reeser JW, Yu L and Roychowdhury S: Landscape of
microsatellite instability across 39 cancer types. JCO Precis
Oncol. 2017:PO.17.00073. 2017.PubMed/NCBI
|
|
18
|
Kurata K, Kubo M, Mori H, Kawaji H,
Motoyama Y, Kuroki L, Yamada M, Kaneshiro K, Kai M and Nakamura M:
Microsatellite instability in triple negative breast cancers. In:
Proceedings of the 2018 San Antonio Breast Cancer Symposium. Cancer
Res. 79(Suppl 4): Abstract nr P1-06-11. 2019.
|
|
19
|
Bryant HE, Schultz N, Thomas HD, Parker
KM, Flower D, Lopez E, Kyle S, Meuth M, Curtin NJ and Helleday T:
Specific killing of BRCA2-deficient tumours with inhibitors of
poly(ADP-ribose) polymerase. Nature. 434:913–917. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Farmer H, McCabe N, Lord CJ, Tutt AN,
Johnson DA, Richardson TB, Santarosa M, Dillon KJ, Hickson I,
Knights C, et al: Targeting the DNA repair defect in BRCA mutant
cells as a therapeutic strategy. Nature. 434:917–921. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Robson M, Im SA, Senkus E, Xu B, Domchek
SM, Masuda N, Delaloge S, Li W, Tung N, Armstrong A, et al:
Olaparib for meta-static breast cancer in patients with a germline
BRCA mutation. N Engl J Med. 377:523–533. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Robson ME, Tung N, Conte P, Im SA, Senkus
E, Xu B, Masuda N, Delaloge S, Li W, Armstrong A, et al: OlympiAD
final overall survival and tolerability results: Olaparib versus
chemotherapy treatment of physician's choice in patients with a
germline BRCA mutation and HER2-negative metastatic breast cancer.
Ann Oncol. 30:558–566. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Litton JK, Rugo HS, Ettl J, Hurvitz SA,
Gonçalves A, Lee KH, Fehrenbacher L, Yerushalmi R, Mina LA, Martin
M, et al: Talazoparib in patients with advanced breast cancer and a
germ-line BRCA mutation. N Engl J Med. 379:753–763. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Denkert C, von Minckwitz G, Darb-Esfahani
S, Lederer B, Heppner BI, Weber KE, Budczies J, Huober J, Klauschen
F, Furlanetto J, et al: Tumour-infiltrating lymphocytes and
prog-nosis in different subtypes of breast cancer: A pooled
analysis of 3771 patients treated with neoadjuvant therapy. Lancet
Oncol. 19:40–50. 2018. View Article : Google Scholar
|
|
25
|
Hida AI, Watanabe T, Sagara Y, Kashiwaba
M, Sagara Y, Aogi K, Ohi Y and Tanimoto A: Diffuse distribution of
tumor-infiltrating lymphocytes is a marker for better prognosis and
chemo-therapeutic effect in triple-negative breast cancer. Breast
Cancer Res Treat. 178:283–294. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Loi S, Drubay D, Adams S, Pruneri G,
Francis PA, Lacroix-Triki M, Joensuu H, Dieci MV, Badve S, Demaria
S, et al: Tumor-infiltrating lymphocytes and prognosis: A pooled
individual patient analysis of early-stage triple-negative breast
cancers. J Clin Oncol. 37:559–569. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Galon J and Bruni D: Approaches to treat
immune hot, altered and cold tumours with combination
immunotherapies. Nat Rev Drug Discov. 18:197–218. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Adams S, Gatti-Mays ME, Kalinsky K, Korde
LA, Sharon E, Amiri-Kordestani L, Bear H, McArthur HL, Frank E,
Perlmutter J, et al: Current landscape of immunotherapy in breast
cancer: A review. JAMA Oncol. Apr 11–2019.Epub ahead of print.
View Article : Google Scholar
|
|
29
|
Schmid P, Salgado R, Park YH,
Muñoz-Couselo E, Kim SB, Sohn J, Im S-A, Foukakis T, Kuemmel S,
Dent R, et al: Pembrolizumab plus chemotherapy as neoadjuvant
treatment of high-risk, early-stage triple-negative breast cancer:
Results from the phase 1b open-label, multicohort KEYNOTE-173
study. Ann Oncol. 31:569–581. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nanda R, Liu MC, Yau C, Shatsky R, Pusztai
L, Wallace A, Chien AJ, Forero-Torres A, Ellis E, Han H, et al:
Effect of pembrolizumab plus neoadjuvant chemotherapy on Pathologic
complete response in women with early-stage breast cancer: An
analysis of the ongoing phase 2 adaptively randomized I-SPY2 trial.
JAMA Oncol. 6:1–9. 2020. View Article : Google Scholar
|
|
31
|
Schmid P, Cortes J, Pusztai L, McArthur H,
Kümmel S, Bergh J, Denkert C, Park YH, Hui R, Harbeck N, et al:
Pembrolizumab for early triple-negative breast cancer. N Engl J
Med. 382:810–821. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gianni L, Huang CS, Egle D, Bermejo B,
Zamagni C, Thill M, Anton A, Zambelli S, Bianchini G, Russo S and
Ciruelos E: Pathologic complete response (pCR) to neoadjvaunt
treatment with or without atezolizumab in triple negative, early
high-risk and locally advanced breast cancer. NeoTRIPaPDL1
Michelangelo randomized study. In: Proceedings of the 2019 San
Antonio Breast Cancer Symposium. Cancer Res. 80(Suppl 4): Abstract
nr GS3-04. 2020.
|
|
33
|
Mittendorf E, Barrios CH, Harbeck N, Miles
D, Saji S, Zhang H, Duc AN, Rafii S and Lai C: IMpassion031: A
phase III study comparing neoadjuvant atezolizumab vs placebo in
combination with nab-paclitaxel-based chemotherapy in early
triple-negative breast cancer (TNBC). In: Proceedings of the 2017
San Antonio Breast Cancer Symposium. Cancer Res. 78(Suppl 4):
Abstract nr OT2-07-03. 2018.
|
|
34
|
Cortes J, Cescon DW, Rugo HS, Nowecki Z,
Im SA, Yusof MM, Gallardo C, Lipatov O, Barrios CH, Holgado E, et
al: KEYNOTE-355: Randomized, double-blind, phase III study of
pembrolizumab + chemotherapy versus placebo + chemotherapy for
previously untreated locally recurrent inoperable or meta-static
triple-negative breast cancer. J Clin Oncol. 38(Suppl 15): S1000.
2020. View Article : Google Scholar
|
|
35
|
Voorwerk L, Slagter M, Horlings HM,
Sikorska K, van de Vijver KK, de Maaker M, Nederlof I, Kluin RJC,
Warren S, Ong S, et al: Immune induction strategies in meta-static
triple-negative breast cancer to enhance the sensitivity to PD-1
blockade: The TONIC trial. Nat Med. 25:920–928. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Allard B, Longhi MS, Robson SC and Stagg
J: The ectonucleotidases CD39 and CD73: Novel checkpoint inhibitor
targets. Immunol Rev. 276:121–144. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ghalamfarsa G, Kazemi MH, Raoofi Mohseni
S, Masjedi A, Hojjat-Farsangi M, Azizi G, Yousefi M and
Jadidi-Niaragh F: CD73 as a potential opportunity for cancer
immunotherapy. Expert Opin Ther Targets. 23:127–142. 2019.
View Article : Google Scholar
|
|
38
|
Duhant X, Schandené L, Bruyns C, Gonzalez
NS, Goldman M, Boeynaems JM and Communi D: Extracellular adenine
nucleotides inhibit the activation of human CD4+ T lymphocytes. J
Immunol. 169:15–21. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Allard B, Beavis PA, Darcy PK and Stagg J:
Immunosuppressive activities of adenosine in cancer. Curr Opin
Pharmacol. 29:7–16. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ohta A: A metabolic immune checkpoint:
Adenosine in tumor microenvironment. Front Immunol. 7:1092016.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Buisseret L, Pommey S, Allard B, Garaud S,
Bergeron M, Cousineau I, Ameye L, Bareche Y, Paesmans M, Crown JPA,
et al: Clinical significance of CD73 in triple-negative breast
cancer: Multiplex analysis of a phase III clinical trial. Ann
Oncol. 29:1056–1062. 2018.
|
|
42
|
Powderly J, Spira A, Gutierrez R, DiRenzo
D, Udyavar A, Karakunnel JJ, Rieger A, Colabella J, Lai DW and de
Souza P: Phase 1 evaluation of AB928, a novel dual adenosine
receptor antagonist, combined with chemotherapy or AB122
(anti-PD-1) in patients with advanced malignancies. Ann Oncol.
30(Suppl 5): v475–v532. 2019.
|
|
43
|
Hartman AR, Kaldate RR, Sailer LM, Painter
L, Grier CE, Endsley RR, Griffin M, Hamilton SA, Frye CA, Silberman
MA, et al: Prevalence of BRCA mutations in an unselected population
of triple-negative breast cancer. Cancer. 118:2787–2795.
2012.PubMed/NCBI
|
|
44
|
Okuma HS and Yonemori K: BRCA gene
mutations and poly(ADP-Ribose) polymerase inhibitors in
triple-negative breast cancer. Adv Exp Med Biol. 1026:271–286.
2017.PubMed/NCBI
|
|
45
|
Lord CJ and Ashworth A: BRCAness
revisited. Nat Rev Cancer. 16:110–120. 2016.PubMed/NCBI
|
|
46
|
Litton JK, Scoggins ME, Hess KR, Adrada
BE, Murthy RK, Damodaran S, DeSnyder SM, Brewster AM, Barcenas CH,
Valero V, et al: Neoadjuvant talazoparib for patients with operable
breast cancer with a germline BRCA pathogenic variant. J Clin
Oncol. 38:388–394. 2020.
|
|
47
|
Loibl S, O'Shaughnessy J, Untch M, Sikov
WM, Rugo HS, McKee MD, Huober J, Golshan M, von Minckwitz G, Maag
D, et al: Addition of the PARP inhibitor veliparib plus carboplatin
or carboplatin alone to standard neoadjuvant chemotherapy in
triple-negative breast cancer (BrighTNess): A randomised, phase 3
trial. Lancet Oncol. 19:497–509. 2018.PubMed/NCBI
|
|
48
|
Jiao S, Xia W, Yamaguchi H, Wei Y, Chen
MK, Hsu JM, Hsu JL, Yu WH, Du Y, Lee HH, et al: PARP inhibitor
upregulates PD-L1 expression and enhances cancer-associated
immunosuppression. Clin Cancer Res. 23:3711–3720. 2017.PubMed/NCBI
|
|
49
|
Vinayak S, Tolaney SM, Schwartzberg L,
Mita M, McCann G, Tan AR, Wahner-Hendrickson AE, Forero A, Anders
C, Wulf GM, et al: Open-label clinical trial of niraparib combined
with pembrolizumab for treatment of advanced or metastatic
triple-negative breast cancer. JAMA Oncol. 5:1132–1140. 2019.
|
|
50
|
Domchek S, Postel-Vinay S, Im S, Park YH,
Delord J, Italiano A, Alexandre J, You B, Bastian S, Krebs MG, et
al: Phase II study of olaparib (o) and durvalumab (d) (MEDIOLA):
Updated results in patients (pts) with germline BRCA-mutated
(gBRCAm) meta-static breast cancer (mbc). Ann Oncol. 30(Suppl 5):
v475–v532. 2019.
|
|
51
|
Pusztai L, Han HS, Yau C, Wolf D, Wallace
AM, Shatsky R, Helsten T, Boughey JC, Haddad T, Stringer-Reasor E,
et al: Durvalumab in combination with olaparib and paclitaxel in
high-risk HER2 negative stage II/III breast cancer: Results from
the I-SPY 2 trial. In: Proceedings of the Annual Meeting of the
American Association for Cancer Research 2020. Cancer Res. 80(Suppl
16): Abstract nr CT011. 2020.
|
|
52
|
Mitri ZI, Vuky J, Kemmer KA, Savin MA,
Parmar S, Kolodzie AK, Johnson B, Williams-Belizaire R, Gray JW and
Mills GB: A phase II trial of olaparib and durvalumab in metastatic
BRCA wild type triple-negative breast cancer. J Clin Oncol.
37:TPS11112019.
|
|
53
|
Rugo HS, Llombart-Cussac A, Andre F,
Robson ME, Saji S, Harbeck N, Schmid P, Cescon DW, Ahn JS, Nanda R,
et al: KEYLYNK-009: A phase II/III, open-label, randomized study of
pembrolizumab (pembro) plus olaparib vs pembro plus chemotherapy
after induction with first-line pembro plus chemo-therapy in
patients with locally recurrent inoperable or metastatic
triple-negative breast cancer (TNBC). J Clin Oncol.
38:TPS5962020.
|
|
54
|
Maacke H, Opitz S, Jost K, Hamdorf W,
Henning W, Krüger S, Feller AC, Lopens A, Diedrich K, Schwinger E
and Stürzbecher HW: Over-expression of wild-type Rad51 correlates
with histological grading of invasive ductal breast cancer. Int J
Cancer. 88:907–913. 2000.PubMed/NCBI
|
|
55
|
Martin RW, Orelli BJ, Yamazoe M, Minn AJ,
Takeda S and Bishop DK: RAD51 up-regulation bypasses BRCA1 function
and is a common feature of BRCA1-deficient breast tumors. Cancer
Res. 67:9658–9665. 2007.PubMed/NCBI
|
|
56
|
Wiegmans AP, Yap PY, Ward A, Lim YC and
Khanna KK: Differences in expression of key DNA damage repair genes
after epigenetic-induced BRCAness dictate synthetic lethality with
PARP1 inhibition. Mol Cancer Ther. 14:2321–2331. 2015.PubMed/NCBI
|
|
57
|
Liu Y, Burness ML, Martin-Trevino R, Guy
J, Bai S, Harouaka R, Brooks MD, Shang L, Fox A, Luther TK, et al:
RAD51 mediates resistance of cancer stem cells to PARP inhibition
in triple-negative breast cancer. Clin Cancer Res. 23:514–522.
2017.
|
|
58
|
Marzio A, Puccini J, Kwon Y, Maverakis NK,
Arbini A, Sung P, Bar-Sagi D and Pagano M: The F-Box
domain-dependent activity of EMI1 regulates PARPi sensitivity in
triple-negative breast cancers. Mol Cell. 73:224–237.e6. 2019.
|
|
59
|
Tutt A, Stephens C, Frewer P, Pierce A,
Rhee J, So K, Ottesen L, Dean E and Hollingsworth SJ: VIOLETTE: A
randomized phase II study to assess DNA damage response inhibitors
in combination with olaparib (Ola) vs. Ola monotherapy in patients
(pts) with metastatic, triple-negative breast cancer (TNBC)
stratified by alterations in homologous recombination repair
(HRR)-related genes. J Clin Oncol. 36(Suppl 15): TPS11122018.
|
|
60
|
Hirai H, Arai T, Okada M, Nishibata T,
Kobayashi M, Sakai N, Imagaki K, Ohtani J, Sakai T, Yoshizumi T, et
al: MK-1775, a small molecule Wee1 inhibitor, enhances anti-tumor
efficacy of various DNA-damaging agents, including 5-fluorouracil.
Cancer Biol Ther. 9:514–522. 2010.PubMed/NCBI
|
|
61
|
Pitts TM, Simmons DM, Bagby SM, Hartman
SJ, Yacob BW, Gittleman B, Tentler JJ, Cittelly D, Ormond DR,
Messersmith WA, et al: Wee1 inhibition enhances the anti-tumor
effects of capecitabine in preclinical models of triple-negative
breast cancer. Cancers (Basel). 12:7192020. View Article : Google Scholar
|
|
62
|
Do K, Wilsker D, Ji J, Zlott J, Freshwater
T, Kinders RJ, Collins J, Chen AP, Doroshow JH and Kummar S: Phase
I study of single-agent AZD1775 (MK-1775), a Wee1 kinase inhibitor,
in patients with refractory solid tumors. J Clin Oncol.
33:3409–3415. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Do KT, Hill SJ, Kochupurakkal B, Supko JG,
Gannon C, Anderson A, Muzikansky A, Wolanski A, Hedglin J, Parmar
K, et al: Abstract CT232: Phase I combination study of the CHK1
inhibitor prexasertib (LY2606368) and olaparib in patients with
high-grade serous ovarian cancer and other advanced solid tumors.
In: Proceedings of the American Association for Cancer Research
Annual Meeting 2019. Cancer Res. 79(Suppl 13): Abstract nr CT232.
2019.
|
|
64
|
Peng W, Chen JQ, Liu C, Malu S, Creasy C,
Tetzlaff MT, Xu C, McKenzie JA, Zhang C, Liang X, et al: Loss of
PTEN promotes resistance to T cell-mediated immunotherapy. Cancer
Discov. 6:202–216. 2016. View Article : Google Scholar :
|
|
65
|
André F, Ciruelos E, Rubovszky G, Campone
M, Loibl S, Rugo HS, Iwata H, Conte P, Mayer IA, Kaufman B, et al:
Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced
breast cancer. N Engl J Med. 380:1929–1940. 2019. View Article : Google Scholar
|
|
66
|
Kim SB, Dent R, Im SA, Espié M, Blau S,
Tan AR, Isakoff SJ, Oliveira M, Saura C, Wongchenko MJ, et al:
Ipatasertib plus paclitaxel versus placebo plus paclitaxel as
first-line therapy for metastatic triple-negative breast cancer
(LOTUS): A multicentre, randomised, double-blind,
placebo-controlled, phase 2 trial. Lancet Oncol. 18:1360–1372.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Dent R, Im SA, Espie M, Blau S, Tan AR,
Isakoff SJ, Oliveira M, Saura C, Wongchenko M, Kapp AV, et al:
Overall survival (OS) update of the double-blind placebo
(PBO)-controlled random-ized phase 2 LOTUS trial of first-line
ipatasertib (IPAT) + paclitaxel (PAC) for locally
advanced/metastatic triple-negative breast cancer (mTNBC). J Clin
Oncol. 36:10082018. View Article : Google Scholar
|
|
68
|
Dent R, Kim SB, Oliveira M, Isakoff SJ,
Barrios CH, O'Shaughnessy J, Lu X, Wongchenko M, Bradley D, Mani A,
et al: IPATunity130: A pivotal randomized phase III trial
evaluating ipatasertib (IPAT) + paclitaxel (PAC) for
PIK3CA/AKT1/PTEN-altered advanced triple-negative (TN) or hormone
receptor-positive HER2-negative (HR+/HER2-) breast cancer (BC). J
Clin Oncol. 36(Suppl 15): TPS11172018. View Article : Google Scholar
|
|
69
|
Schmid P, Abraham J, Chan S, Wheatley D,
Brunt AM, Nemsadze G, Baird RD, Park YH, Hall PS, Perren T, et al:
Capivasertib plus paclitaxel versus placebo plus paclitaxel as
first-line therapy for metastatic triple-negative breast cancer:
The PAKT trial. J Clin Oncol. 38:423–433. 2020. View Article : Google Scholar
|
|
70
|
Schmid P, Cortes J, Robson M, Iwata H,
Hegg R, Verma S, Nechaeva M, Xu B, Haddad V, Imedio RE, et al:
Abstract OT2-08-02: Capivasertib and paclitaxel in first-line
treatment of patients with metastatic triple-negative breast
cancer: A phase III trial (CAPItello-290). In: Proceedings of the
2019 San Antonio Breast Cancer Symposium. Cancer Res. 80(Suppl 4):
Abstract nr OT2-08-02. 2020.
|
|
71
|
Schmid P, Loirat D, Savas P, Espinosa E,
Boni V, Italiano A, White S, Singel MS, Withana N, Mani A, et al:
Phase Ib study evaluating a triplet combination of ipatasertib
(IPAT), atezoli-zumab (atezo), and paclitaxel (PAC) or nab-PAC as
first-line (1L) therapy for locally advanced/metastatic
triple-negative breast cancer (TNBC). In: Proceedings of the
American Association for Cancer Research Annual Meeting 2019.
Cancer Res. 79(Suppl 13): Abstract nr CT049. 2019.
|
|
72
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Dean JL, McClendon AK and Knudsen ES:
Modification of the DNA damage response by therapeutic CDK4/6
inhibition. J Biol Chem. 287:29075–29087. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Cretella D, Fumarola C, Bonelli M, Alfieri
R, La Monica S, Digiacomo G, Cavazzoni A, Galetti M, Generali D and
Petronini PG: Pre-treatment with the CDK4/6 inhibitor palbociclib
improves the efficacy of paclitaxel in TNBC cells. Sci Rep.
9:130142019. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Clark AS, McAndrew NP, Troxel A, Feldman
M, Lal P, Rosen M, Burrell J, Redlinger C, Gallagher M, Bradbury
AR, et al: Combination paclitaxel and palbociclib: Results of a
phase I trial in advanced breast cancer. Clin Cancer Res.
25:2072–2079. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Tan AR, Wright GS, Thummala AR, Danso MA,
Popovic L, Pluard TJ, Han HS, Vojnović Ž, Vasev N, Ma L, et al:
Trilaciclib plus chemotherapy versus chemotherapy alone in patients
with metastatic triple-negative breast cancer: A multicentre,
randomised, open-label, phase 2 trial. Lancet Oncol. 20:1587–1601.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Stine ZE, Walton ZE, Altman BJ, Hsieh AL
and Dang CV: MYC, metabolism, and cancer. Cancer Discov.
5:1024–1039. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Ambrosio S, Amente S, Napolitano G, Di
Palo G, Lania L and Majello B: MYC impairs resolution of
site-specific DNA double-strand breaks repair. Mutat Res. 774:6–13.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Wiegmans AP, Al-Ejeh F, Chee N, Yap PY,
Gorski JJ, Da Silva L, Bolderson E, Chenevix-Trench G, Anderson R,
Simpson PT, et al: Rad51 supports triple negative breast cancer
metastasis. Oncotarget. 5:3261–3272. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Carey JPW, Karakas C, Bui T, Chen X,
Vijayaraghavan S, Zhao Y, Wang J, Mikule K, Litton JK, Hunt KK and
Keyomarsi K: Synthetic lethality of PARP inhibitors in combination
with MYC blockade is independent of BRCA status in triple negative
breast cancer. Cancer Res. 78:742–757. 2018. View Article : Google Scholar :
|
|
81
|
Horiuchi D, Kusdra L, Huskey NE,
Chandriani S, Lenburg ME, Gonzalez-Angulo AM, Creasman KJ, Bazarov
AV, Smyth JW, Davis SE, et al: MYC pathway activation in
triple-negative breast cancer is synthetic lethal with CDK
inhibition. J Exp Med. 209:679–696. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Hossain DMS, Javaid S, Cai M, Zhang C,
Sawant A, Hinton M, Sathe M, Grein J, Blumenschein W, Pinheiro EM
and Chackerian A: Dinaciclib induces immunogenic cell death and
enhances anti-PD1-mediated tumor suppression. J Clin Invest.
128:644–654. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Chien AJ, Gliwa AS, Rahmaputri S, Dittrich
HF, Majure MC, Rugo HS, Melisko ME, Munster PN, Park JW, Moasser
MM, et al: A phase Ib trial of the cyclin-dependent kinase
inhibitor dinaci-clib (dina) in combination with pembrolizumab (P)
in patients with advanced triple-negative breast cancer (TNBC) and
response correlation with MYC-overexpression. J Clin Oncol.
38(1076)2020. View Article : Google Scholar
|
|
84
|
Kono M, Fujii T, Lim B, Karuturi MS,
Tripathy D and Ueno NT: Androgen receptor function and androgen
receptor-targeted therapies in breast cancer: A Review. JAMA Oncol.
3:1266–1273. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Gerratana L, Basile D, Buono G, De Placido
S, Giuliano M, Minichillo S, Coinu A, Martorana F, De Santo I, Del
Mastro L, et al: Androgen receptor in triple negative breast
cancer: A potential target for the targetless subtype. Cancer Treat
Rev. 68:102–110. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Anestis A, Zoi I, Papavassiliou AG and
Karamouzis MV: Androgen receptor in breast cancer-clinical and
preclinical research insights. Molecules. 25(358)2020. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Masuda H, Baggerly KA, Wang Y, Zhang Y,
Gonzalez-Angulo AM, Meric-Bernstam F, Valero V, Lehmann BD,
Pietenpol JA, Hortobagyi GN, et al: Differential response to
neoadjuvant chemotherapy among 7 triple-negative breast cancer
molecular subtypes. Clin Cancer Res. 19:5533–5540. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Thike AA, Yong-Zheng Chong L, Cheok PY, Li
HH, Wai-Cheong Yip G, Huat Bay B, Tse GM, Iqbal J and Tan PH: Loss
of androgen receptor expression predicts early recurrence in
triple-negative and basal-like breast cancer. Mod Pathol.
27:352–360. 2014. View Article : Google Scholar
|
|
89
|
Echavarria I, Lopez-Tarruella S, Picornell
A, García-Saenz JA, Jerez Y, Hoadley K, Gómez HL, Moreno F,
Monte-Millan MD, Márquez-Rodas I, et al: Pathological response in a
triple-negative breast cancer cohort treated with neoadjuvant
carboplatin and docetaxel according to Lehmann's refined
classification. Clin Cancer Res. 24:1845–1852. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Santonja A, Sánchez-Muñoz A, Lluch A,
Chica-Parrado MR, Albanell J, Chacón JI, Antolín S, Jerez JM, de la
Haba J, de Luque V, et al: Triple negative breast cancer subtypes
and pathologic complete response rate to neoadjuvant chemotherapy.
Oncotarget. 9:26406–26416. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Venema CM, Bense RD, Steenbruggen TG,
Nienhuis HH, Qiu SQ, van Kruchten M, Brown M, Tamimi RM, Hospers
GAP, Schröder CP, et al: Consideration of breast cancer subtype in
targeting the androgen receptor. Pharmacol Ther. 200:135–147. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Rice MA, Malhotra SV and Stoyanova T:
Second-generation antiandrogens: From discovery to standard of care
in castration resistant prostate cancer. Front Oncol. 9:8012019.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Gucalp A, Tolaney S, Isakoff SJ, Ingle JN,
Liu MC, Carey LA, Blackwell K, Rugo H, Nabell L, Forero A, et al:
Phase II trial of bicalutamide in patients with androgen
receptor-positive, estrogen receptor-negative metastatic breast
cancer. Clin Cancer Res. 19:5505–5512. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Gucalp A, Edelweiss M, Patil S, Gounder
MM, Feigin KN, Corben A, Arumov A and Traina TA: Abstract P3-11-04:
Phase I/II trial of palbociclib in combination with bicalutamide
for the treatment of androgen receptor (AR)+ metastatic breast
cancer (MBC). In: Proceedings of the 2017 San Antonio Breast Cancer
Symposium. Cancer Res 2018. 78(Suppl 4): Abstract nr P3-11-04.
2018.
|
|
95
|
Gucalp A, Boyle LA, Alano T, Arumov A,
Gounder MM, Patil S, Feigin K, Edelweiss M, D'Andrea G, Bromberg J,
et al: Phase II trial of bicalutamide in combination with
palbociclib for the treatment of androgen receptor (+) metastatic
breast cancer. J Clin Oncol. 38:2020. View Article : Google Scholar
|
|
96
|
Bonnefoi H, Grellety T, Tredan O,
Saghatchian M, Dalenc F, Mailliez A, L'Haridon T, Cottu P,
Abadie-Lacourtoisie S, You B, et al: A phase II trial of
abiraterone acetate plus prednisone in patients with
triple-negative androgen receptor positive locally advanced or
metastatic breast cancer (UCBG 12-1). Ann Oncol. 27:812–818. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Gucalp A, Danso MA, Elias AD, Bardia A,
Ali HY, Potter D, Gabrail NY, Haley BB, Khong HT, Riley EC, et al:
Phase (Ph) 2 stage 1 clinical activity of seviteronel, a selective
CYP17-lyase and androgen receptor (AR) inhibitor, in women with
advanced AR+ triple-negative breast cancer (TNBC) or estrogen
receptor (ER)+ BC: CLARITY-01. J Clin Oncol. 35:11022017.
View Article : Google Scholar
|
|
98
|
Bardia A, Gucalp A, DaCosta N, Gabrail N,
Danso M, Ali H, Blackwell KL, Carey LA, Eisner JR, Baskin-Bey ES
and Traina TA: Phase 1 study of seviteronel, a selective CYP17
lyase and androgen receptor inhibitor, in women with estrogen
receptor-positive or triple-negative breast cancer. Breast Cancer
Res Treat. 171:111–120. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Traina TA, Miller K, Yardley DA, Eakle J,
Schwartzberg LS, O'Shaughnessy J, Gradishar W, Schmid P, Winer E,
Kelly C, et al: Enzalutamide for the treatment of androgen
receptor-expressing triple-negative breast cancer. J Clin Oncol.
36:884–890. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Dent R, Schmid P, Cortes J, Kim SB, Andre
F, Abramson V, Cardoso F, Colleoni M, Morris P, Steinberg J, et al:
Abstract OT3-02-02: ENDEAR: A randomized international phase 3
study comparing the efficacy and safety of enzalutamide in
combination with paclitaxel chemotherapy or as mono-therapy vs
placebo with paclitaxel in patients with advanced
diagnostic-positive triple-negative breast cancer. Cancer Res.
77:Abstract OT3-02-02. 2017.
|
|
101
|
Lehmann BD, Abramson VG, Sanders ME, Mayer
EL, Haddad TC, Nanda R, Van Poznak C, Storniolo AM, Nangia JR,
Gonzalez-Ericsson PI, et al: TBCRC 032 IB/II multicenter study:
Molecular insights to AR antagonist and PI3K inhibitor efficacy in
patients with AR+ metastatic triple-negative breast
cancer. Clin Cancer Res. 26:2111–2123. 2020. View Article : Google Scholar
|
|
102
|
Gilewski T, Ragupathi G, Bhuta S, Williams
LJ, Musselli C, Zhang XF, Bornmann WG, Spassova M, Bencsath KP,
Panageas KS, et al: Immunization of metastatic breast cancer
patients with a fully synthetic globo H conjugate: A phase I trial.
Proc Natl Acad Sci USA. 98:3270–3275. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Huang CS, Yu AL, Tseng LM, Chow LWC, Hou
MF, Hurvitz SA, Schwab RB, Wong CH, Murray JL, Chang SC, et al:
Randomized phase II/III trial of active immunotherapy with
OPT-822/OPT-821 in patients with metastatic breast cancer. J Clin
Oncol. 34(Suppl 15): S10032016. View Article : Google Scholar
|
|
104
|
Bardia A, Mayer IA, Vahdat LT, Tolaney SM,
Isakoff SJ, Diamond JR, O'Shaughnessy J, Moroose RL, Santin AD,
Abramson VG, et al: Sacituzumab govitecan-hziy in refractory
metastatic triple-negative breast cancer. N Eng J Med. 380:741–751.
2019. View Article : Google Scholar
|
|
105
|
Modi S, Pusztai L, Forero A, Mita M,
Miller KD, Weise A, Burris H III, Kalinsky K, Tsai M, Liu MC, et
al: Abstract PD3-14: Phase 1 study of the antibody-drug conjugate
SGN-LIV1A in patients with heavily pretreated triple-negative
metastatic breast cancer. Cancer Res. 78:2018.
|
|
106
|
Han HS, Alemany CA, Brown-Glaberman UA,
Pluard TJ, Sinha R, Sterrenberg D, Albain KS, Basho RK, Biggs D,
Boni V, et al: SGNLVA-002: Single-arm, open label phase Ib/II study
of ladiratuzumab vedotin (LV) in combination with pembrolizumab for
first-line treatment of patients with unresectable locally advanced
or metastatic triple-negative breast cancer. J Clin Oncol. 37(Suppl
15): TPS11102019. View Article : Google Scholar
|
|
107
|
Modi S, Park H, Murthy RK, Iwata H, Tamura
K, Tsurutani J, Moreno-Aspitia A, Doi T, Sagara Y, Redfern C, et
al: Antitumor activity and safety of trastuzumab Deruxtecan in
patients with HER2-low-expressing advanced breast cancer: Results
from a phase Ib study. J Clin Oncol. 38:1887–1896. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Modi S, Ohtani S, Lee CC, Wang K, Saxena K
and Cameron DA: A phase III, multicenter, randomized, open label
trial of [fam-] trastuzumab deruxtecan (DS-8201a) versus
investigator's choice in HER2-low breast cancer. J Clin Oncol.
37(Suppl 15): TPS11022019. View Article : Google Scholar
|
|
109
|
Molyneux G, Geyer FC, Magnay FA, McCarthy
A, Kendrick H, Natrajan R, Mackay A, Grigoriadis A, Tutt A,
Ashworth A, et al: BRCA1 basal-like breast cancers originate from
luminal epithelial progenitors and not from basal stem cells. Cell
Stem Cell. 7:403–417. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Bernardo GM, Bebek G, Ginther CL, Sizemore
ST, Lozada KL, Miedler JD, Anderson LA, Godwin AK, Abdul-Karim FW,
Slamon DJ and Keri RA: FOXA1 represses the molecular pheno-type of
basal breast cancer cells. Oncogene. 32:554–563. 2013. View Article : Google Scholar
|
|
111
|
Su Y, Subedee A, Bloushtain-Qimron N,
Savova V, Krzystanek M, Li L, Marusyk A, Tabassum DP, Zak A,
Flacker MJ, et al: Somatic cell fusions reveal extensive
heterogeneity in basal-like breast cancer. Cell Rep. 11:1549–1563.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Cao R, Wang L, Wang H, Xia L,
Erdjument-Bromage H, Tempst P, Jones RS and Zhang Y: Role of
histone H3 lysine 27 methylation in Polycomb-group silencing.
Science. 298:1039–1043. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Yamagishi M and Uchimaru K: Targeting EZH2
in Cancer Therapy. Curr Opin Oncol. 29:375–381. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Yang CC, LaBaff A, Wei Y, Nie L, Xia W,
Huo L, Yamaguchi H, Hsu YH, Hsu JL, Liu D, et al: Phosphorylation
of EZH2 at T416 by CDK2 contributes to the malignancy of triple
negative breast cancers. Am J Transl Res. 7:1009–1020.
2015.PubMed/NCBI
|
|
115
|
Nie L, Wei Y, Zhang F, Hsu YH, Chan LC,
Xia W, Ke B, Zhu C, Deng R, Tang J, et al: CDK2-mediated
site-specific phosphorylation of EZH2 drives and maintains
triple-negative breast cancer. Nat Commun. 10:51142019. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Yang X, Phillips DL, Ferguson AT, Nelson
WG, Herman JG and Davidson NE: Synergistic activation of functional
estrogen receptor (ER)-alpha by DNA methyltransferase and histone
deacetylase inhibition in human ER-alpha-negative breast cancer
cells. Cancer Res. 61:7025–7029. 2001.PubMed/NCBI
|
|
117
|
Sharma D, Saxena NK, Davidson NE and
Vertino PM: Restoration of tamoxifen sensitivity in estrogen
receptor-negative breast cancer cells: Tamoxifen-bound reactivated
ER recruits distinctive corepressor complexes. Cancer Res.
66:6370–6378. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Connolly RM, Li H, Jankowitz RC, Zhang Z,
Rudek MA, Jeter SC, Slater SA, Powers P, Wolff AC, Fetting JH, et
al: Combination epigenetic therapy in advanced breast cancer with
5-azacitidine and entinostat: A phase II National Cancer
Institute/Stand up to cancer study. Clin Cancer Res. 23:2691–2701.
2017. View Article : Google Scholar :
|
|
119
|
Anderberg C, Li H, Fredriksson L, Andrae
J, Betsholtz C, Li X, Eriksson U and Pietras K: Paracrine signaling
by platelet-derived growth factor-CC promotes tumor growth by
recruitment of cancer-associated fibroblasts. Cancer Res.
69:369–378. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Roswall P, Bocci M, Bartoschek M, Li H,
Kristiansen G, Jansson S, Lehn S, Sjölund J, Reid S, Larsson C, et
al: Microenvironmental control of breast cancer subtype elicited
through paracrine platelet-derived growth factor-CC signaling. Nat
Med. 24:463–473. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Park JJH, Hsu G, Siden EG, Thorlund K and
Mills EJ: An overview of precision oncology basket and umbrella
trials for clinicians. CA Cancer J Clin. 70:125–137. 2020.
View Article : Google Scholar : PubMed/NCBI
|