To adapt to the severe nutrient deficient tumor
microenvironment, cancer cells have been indicated to adjust their
metabolic processes, including the abnormal function of signaling
path-ways and cellular metabolism (1). Cancer cells can express certain key
glycolysis enzymes that act on the mammalian target of rapamycin
(mTOR) signaling pathway to maintain clonality and promote
migration (2,3). In recent years, increasing evidence
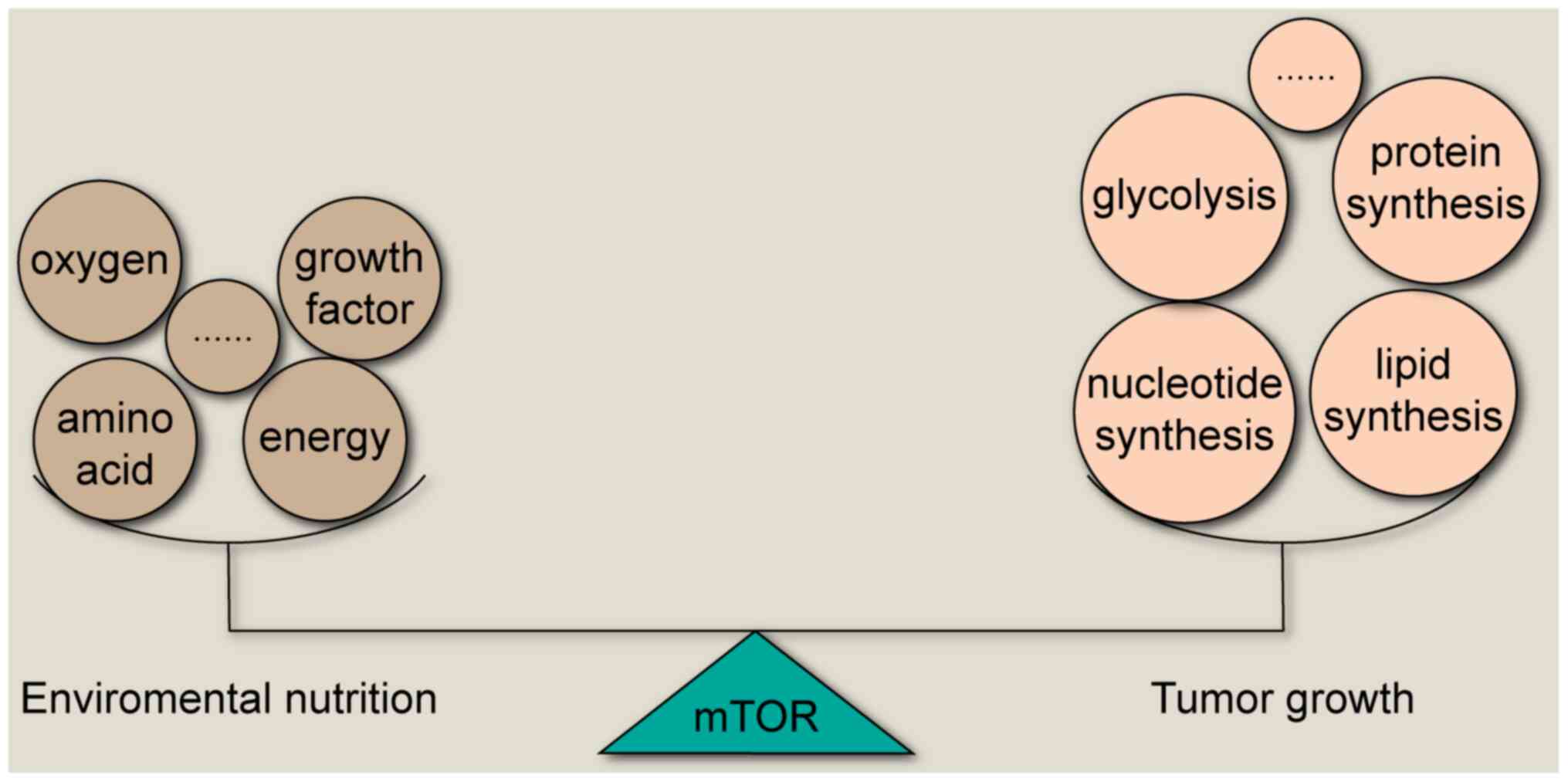
has suggested that mTOR is a core network in cancer cells,
regulating several key enzymes to maintain the balance between
tumor growth and nutrition outside the cancer cells (4) (Fig.
1). The activation of mTOR has been indicated to promote mRNA
transcription, protein synthesis, glucose metabolism and lipid
synthesis that is necessary for cell growth (5,6).
mTOR is an essential serine/threonine protein kinase that belongs
to the PI3K family, and it includes two different catalytic subunit
protein complexes, mTOR complex 1 (mTORC1) and mTOR complex 2
(mTORC2) (7-9). Both complexes have been revealed to
serve important roles in regulating the metabolism of cancer cells
(9).
Carcinomas are often accompanied by metabolic
alterations that are recognized as hallmarks of cancer during tumor
development, and mTOR has been indicated to serve a vital role in
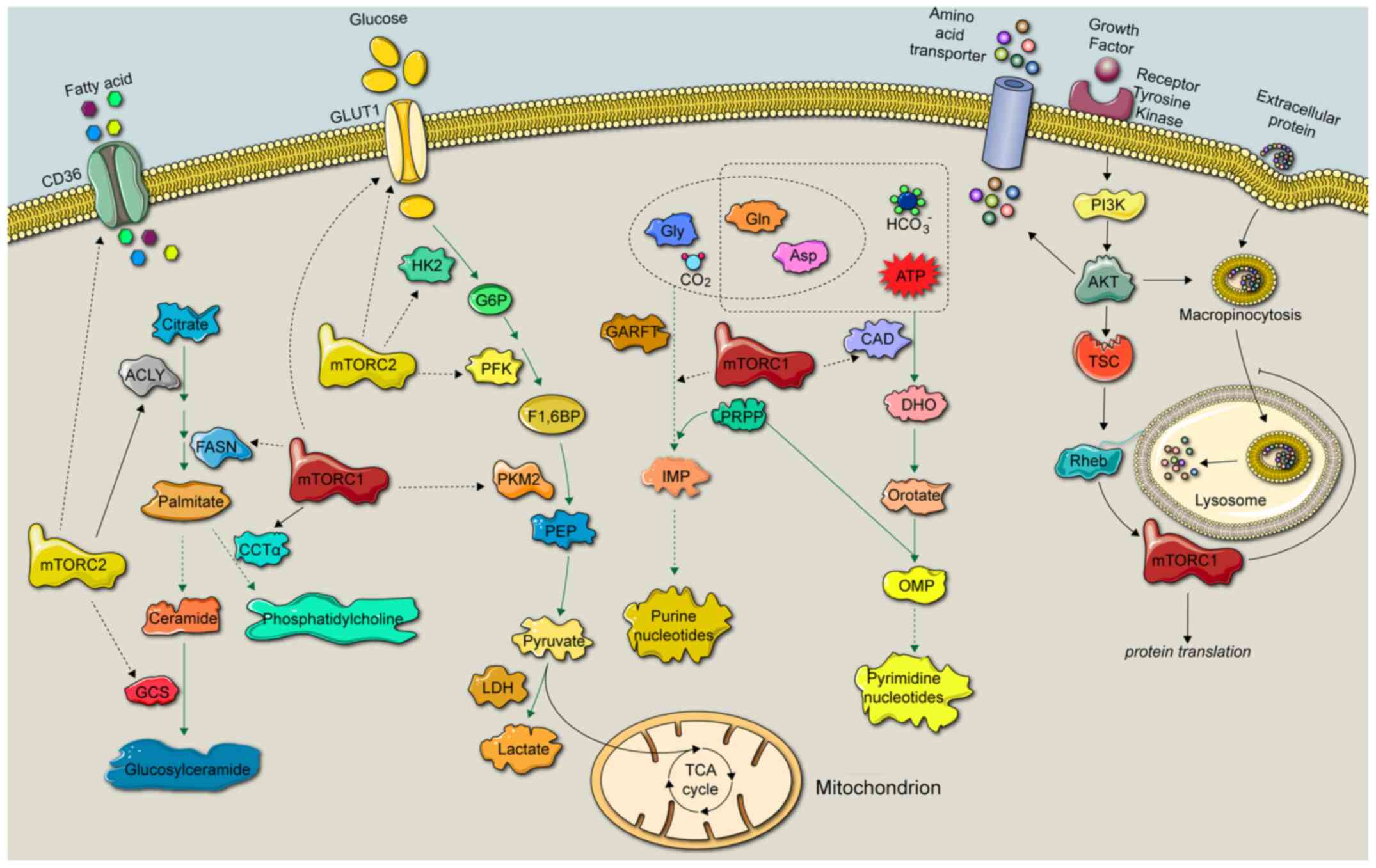
cancer metabolism (4). There are
several types of metabolism in cancer cells, including amino acid,
glucose, nucleotide and lipid metabolism (10) (Fig.
2).
Amino acids are vital nutrient substrates for
protein synthesis, and this process has been indicated to be
closely regulated by mTOR in cancer cells. Both essential and
nonessential amino acid metabolism has been associated with the
mTOR signaling pathway (4). L-type
amino acid transporter (LAT) 1, which transports the essential
amino acid leucine, has been associated with the activation of mTOR
pathway in colorectal cancer (11). Glutamine, a nonessential amino
acid, contributes to the synthesis of other amino acids, lipids and
nucleotides (4), and
glutaminolysis has been indicated to be promoted by the mTORC1 and
mTORC2 pathways (12,13).
Cancer cells can synthesize fatty acids
intracellularly or incorporate extracellular fatty acids, and mTOR
has been indicated to regulate fatty acid transporters, such as
CD36 and synthesis enzymes, including ATP citrate lyase and fatty
acid synthase (4). The
transmembrane glycoprotein CD147 has been reported to reprogram
fatty acid metabolism via the AKT/mTOR/sterol regulatory element
binding protein-1c and p38/peroxisome proliferator-activated
receptor α pathways in hepatocellular carcinoma cells (15).
Glucose, which is the main cellular energy source,
has been demonstrated to promote growth, proliferation and
metastasis of cancer cells; therefore, the sense, uptake and
utilization of glucose are essential for maintaining life, and the
mTOR signaling pathway has been indicated to participate in these
processes (16). Otto Warburg has
revealed that cancer cells often rely on glycolysis even in the
presence of oxygen, and this phenomenon is also called aerobic
glycolysis or the Warburg effect (17).
Given the significance and complexity of aerobic
glycolysis and mTOR in cancer cells, it is necessary to review the
relationship between mTOR and aerobic glycolysis to suggest a
potential novel strategy for clinical cancer therapy that depends
on both these factors.
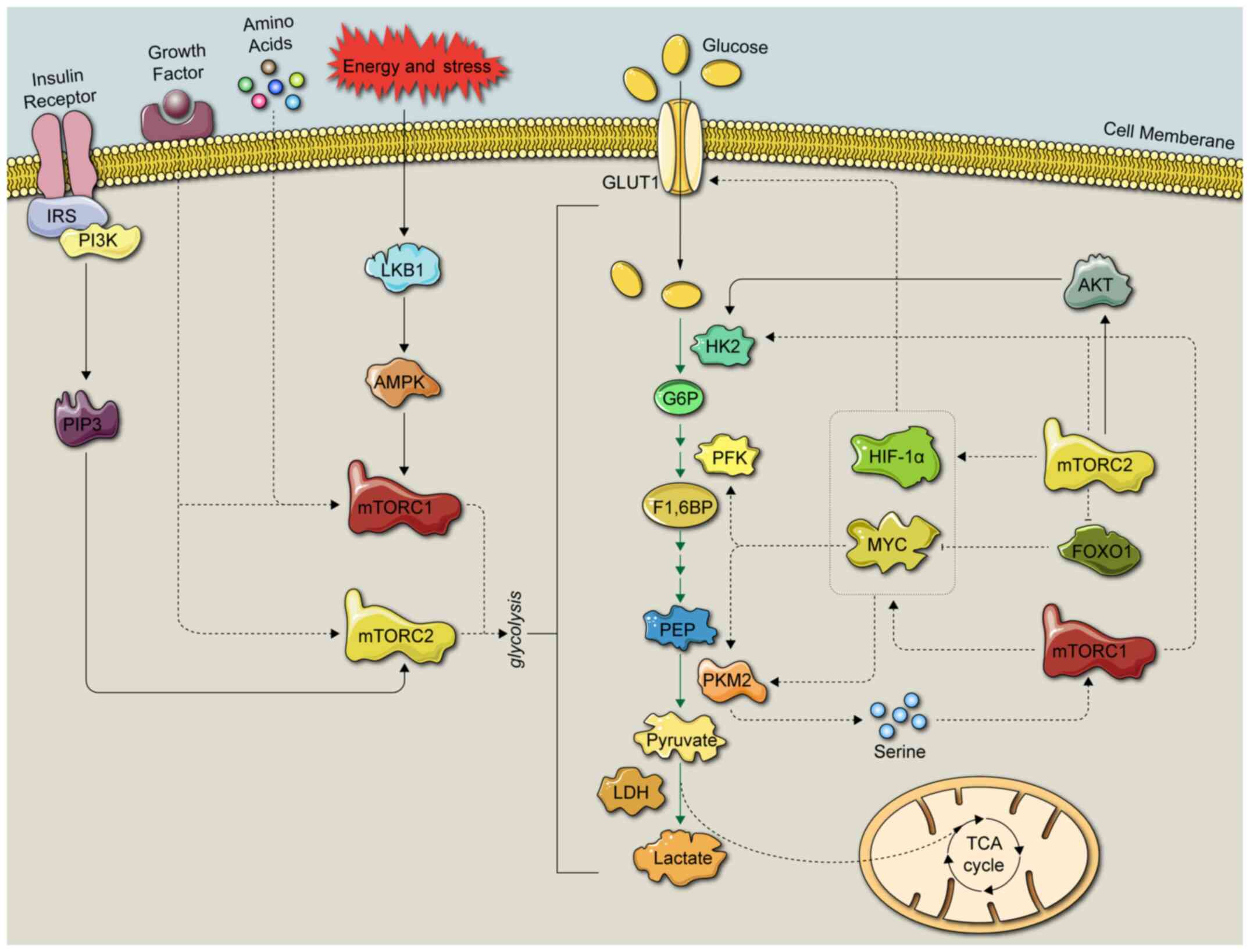
Glycolysis is a process in which glucose is
metabolized into several products, including pyruvate, lactate and
hydrogen ions, via multistep enzymatic reactions in the cytoplasm
(18). The detailed glycolytic
process is depicted in Figs. 2 and
3. Glycolytic enzymes have been
indicated to be closely associated with the mTOR signaling pathway
(4,19). In a first step, cancer cells
overexpress glucose transporters, such as glucose transporter
(GLUT)1 and sodium-glucose linked trans-porter 1, transporting
glucose into the cytoplasm to maintain high levels of glucose
consumption during glycolysis (20,21).
Subsequently, hexokinase (HK) phosphorylates intracellular glucose
to form glucose 6-phosphate (22,23).
In a second step, glucose 6-phosphate isomerase catalyzes the
production of fructose-6-phosphate (24). In a third step,
fructose-6-phos-phate is phosphorylated by phosphofructokinase
(PFK)-1 and PFK-2 into the unstable fructose-1,6-biphosphate and
the relatively stable fructose-2,6-biphosphate (19), respectively. Glucose 6-phosphate is
also converted into glyceraldehyde-3-phosphate (25), which is subsequently converted into
glycerate-1,3-biphosphate, and glycerate-1,3-biphosphate is
converted into 3-phosphoglycerate by phosphoglycerate kinase
(19). Subsequently,
phosphoglyceromutase drives the isomerization of 3-phosphoglycerate
to phosphoglycerate 2-phosphate, followed by the formation of
phosphoenolpyruvate (PEP). In the last step of glycolysis, PEP is
converted into pyruvate by pyruvate kinase (PK) (26).
As aforementioned, mTOR has been associated with
several fundamental cellular processes (4), such as protein synthesis (27), autophagy (28) and cancer (29). Moreover, glycolysis is considered a
principal driver of carcinoma metastasis (30) due to the energy support provided by
glycolysis (30). Ka et al
(28) revealed that
pharmacological inhibition of
phos-phofructokinase-2/fructose-2,6-bisphosphatase 3 suppressed
tumor growth and alleviated metastasis in head and neck squamous
cell carcinoma. In vitro and in vivo experiments have
demonstrated that glycolysis promoted breast cancer growth and
metastasis via the regulation of miR-30a-5p (31). These results suggested that
targeting glycolysis may be a promising strategy to inhibit cancer
growth. The present review subsequently focused on the effects of
the mTOR signaling pathway on energy metabolism, especially cancer
cell glycolysis (Fig. 3).
mTORC1 integrates four major regulatory inputs:
Nutrients, growth factors, energy and stress (32). mTORC1 has been indicated to be
frequently overactivated in glycolytic cancer cells, suggesting
that the inhibition of factors upstream of mTORC1 may be more
effective compared with the inhibition of downstream factors to
inhibit glycolysis in cancer cells (4).
Previous evidence has suggested that amino acids are
critical to cancer cell growth by stimulating the mTORC1 signaling
pathway, and among these, leucine and glutamine have been revealed
to be the most important amino acids (33). Therefore, targeting transporters
may be a reasonable strategy to inhibit tumor growth. LAT2, which
mainly imports neutral amino acids, including leucine and
glutamine, has been indicated to bind to phosphorylated
(p)-mTORSer2448 and regulate the
glutamine/p-mTORSer2448/glutamine synthetase feedback
loop, maintaining mTORC1 activation, which activates glycolysis
(34).
Previous studies have revealed that mTORC1 is a
downstream mediator of growth factors (9,35).
Insulin, one of these growth factors, has been indicated to
upregulate pyruvate kinase M (PKM)2 expression via the
PI3K/mTOR-mediated hypoxia inducible factor-1α (HIF-1α) induction,
but reduce PKM2 activity independent of this pathway (36,37).
Insulin-induced PKM2 upregulation has been revealed to enhance
aerobic glycolysis, but the reduction in PKM2 activity may result
in a characteristic pooling of glycolytic intermediates and the
accumulation of NADPH (37).
A previous study has indicated that mTORC2 can be
activated by growth factors, such as insulin (32). Recent studies have revealed that
mTORC2 can also be stimulated by glucose (44), glutamine and mTORC1 (9,45).
The mTORC2 pathway is essential for normal glucose homeostasis and
metabolic regulation in the body, and the overactivation of mTORC2
may be linked to tumor growth (46). The activation of insulin-like
growth factor 1-TORC2 has been indicated to drive the metastasis of
nasopharyngeal carcinoma with reprogrammed glucose metabolism
(47).
AMPK serves as an energy sensitive receptor in the
context of energy stress and can be viewed as a negative regulator
of the Warburg effect in human cancers, suggesting that a high
level of AMPK indicates a better prognosis (48). Given that both AMPK and mTOR serve
important roles in regulating glucose metabolism, it has been
demonstrated that AMPK is indeed a regulator of mTOR and
phosphorylates regulatory-associated protein of mTOR, which is a
component of mTORC1, inhibiting its activity (49). The levels of aerobic glycolysis and
tumor growth were indicated to be downregulated by AMPK in
vivo, and Myc-induced lymphomagenesis was accelerated when the
α1 catalytic subunit of AMPK was ablated (50). Moreover, a metabolic shift to
aerobic glycolysis has been observed in both transformed and
untransformed cells when AMPK was inactivated, which increased the
allocation of glucose-derived carbon into lipids and biomass
accumulation (50). However, the
role of AMPK in regulating glycolysis may be different in other
contexts. For example, tamoxifen has been indicated to inhibit
oxygen consumption via inhibiting mitochondrial complex I,
increasing the AMP/ATP ratio and activating the AMPK signaling
pathway, thereby promoting glycolysis (51).
Moreover, glucose promotes glycolysis by regulating
the AMPK/AKT/mTOR/S6 kinase and MAPK pathways (52). The small polyphenol resveratrol has
been indicated to inhibit glycolysis in female ovarian cancer cells
via activating the AMPK/mTOR signaling pathway (53). However, AMPK has also been
demonstrated to regulate glycolysis via phosphorylating and
activating 6-phosphofructo-2-kinase/fruc-tose-2,6-bisphosphatase
(PFKFB)3, thereby increasing the level of fructose-2,6-bisphosphate
(54).
The upstream and downstream pathways of the
PI3K/AKT/mTOR signaling pathway have been revealed to regulate
cancer cell glycolysis. For example, knockdown of Forkhead box
protein O (FOXO)6, a FOXO family member, has been indicated to
inhibit PI3K/AKT/mTOR pathway activation and alter cellular
metabolism via inhibiting glycolysis and promoting mitochondrial
respiration in colorectal cancer (CRC), indicating that FOXO6 may
serve as a potential mTOR-dependent target for CRC therapy
(61). Krüppel-like factor 5
(KLF5) silencing has been reported to suppress HIF-1α-dependent
glycolysis in non-small cell lung cancer (NSCLC) via inactivating
the PI3K/AKT/mTOR pathway (62).
Knockdown of glutamate receptor, iono-tropic, N-methyl
D-aspartate-associated protein 1, which is also known as GRINA, has
been demonstrated to inhibit PI3K/AKT/mTOR signaling and glycolytic
metabolism in gastric cancer cells (63). CD147 has been indicated to promote
glycolytic metabolism in hepatocellular carcinoma cells via the
PI3K/AKT/mTOR signaling pathway (64). CD276 has been reported to enhance
the Warburg effect via promoting the PI3K/AKT/mTOR/HIF-1α pathway,
as well as its downstream targets GLUT1 and PFKFB3, in oral
squamous carcinoma (65).
Tripartite motif-containing protein 59 may enhance glycolysis via
the PI3K/AKT/mTOR signaling pathway, ultimately contributing to
pancreatic cancer progression (66). Type Iγ phosphatidylinositol
phosphate kinase has been indicated to upregulate c-Myc and HIF-1α
levels by stimulating the PI3K/AKT/mTOR signaling pathway, thereby
regulating the expression of glycolytic enzymes to enhance
glycolysis in CRC (67).
The PI3K/AKT/mTOR signaling pathway is one of the
most commonly activated signaling pathways associated with drug
resistance in various cancers, such as breast cancer, ovarian
cancer and NSCLC (62,68,69).
A recent study on the drug resistance of breast cancer has revealed
that the PI3K/AKT/mTOR signaling pathway serves an important role
in endocrine resistance, since it was indicated to be activated in
response to CC-chemokine ligand 2 secreted by tumor-associated
macrophages, which promoted an endocrine resistance feedback loop
in the tumor microenvironment (68). In addition to breast cancer, the
PI3K/AKT/mTOR signaling pathway has also been revealed to
facilitate the chemoresistance of ovarian cancer. For example, this
pathway has been indicated to trigger the expression of cancer stem
cell markers, including CD44v6, CD117, aldehyde dehydrogenase 1
family member A1 and Snail, resulting in cisplatin resistance in
epithelial ovarian cancer (70).
Hypoxia-induced cisplatin resistance in NSCLC has been demonstrated
to be suppressed after knockdown of KLF5, which may occur following
inhibition of HIF-1α-dependent glycolysis via PI3K/AKT/mTOR
inactivation (62).
There are various types of glucose transporters and
three key rate-limiting enzymes in glycolysis, HK, PFK and PK
(71), and all of these
transporters and enzymes have been reported to be associated with
the mTOR signaling pathway (23,72).
Glucose transporters act as gatekeepers of
glycolysis and have been reported to be elevated to facilitate
glucose uptake due to the low efficiency of glycolysis in various
types of cancers, such as NSCLC (73), hepatocellular carcinoma and breast
cancer (74). A previous study has
revealed that glucose uptake and GLUT1 expression were increased in
multiple cell types due to the absence of the downstream target
protein of mTOR, tuberous sclerosis (TSC)2 protein (75). Moreover, mTORC1 has been indicated
to be activated in renal angiomyolipomas as a result of the loss of
TSC1/2 function, and TSC2 has been reported to regulate the
membrane localization of various glucose transporter proteins, such
as GLUT1, GLUT2 and GLUT4, ultimately affecting glucose uptake
(76). In addition, the
proliferation of CRC has been indicated to be inhibited following
GLUT1 gene silencing, which was mediated by the TGF-β/PI3K/AKT/mTOR
signaling pathway, providing a novel basis for targeted therapy and
the development of novel drugs to treat CRC (77). It has been demonstrated that GLUT1
translocation may be regulated by the PI3K/AKT/mTOR-mediated
activation of the RhoA/Rho-associated protein kinase 1 pathway or
by stimulating glycolysis, but this remains to be elucidated in the
future (59). Certain researchers
have focused on the effects of oncogenes that are downstream of the
mTOR signaling pathway, including c-Myc and HIF-1α, on the
expression of glucose transporters (78,79).
In addition to GLUT1, the effects of other members of the glucose
family, such as GLUT2 and GLUT5, have also been examined in the
context of cancer (74). In
studying the role of microRNA (miRNA/miR)-21 in glycolysis and
tumorigenesis in T24 bladder cancer cells, it has been revealed
that GLUT3 was downregulated when miRNA-21 was sponged and that the
associated mechanism may implicate the PI3K/AKT/mTOR signaling
pathway (56). Similarly, the
expression of GLUT3 has been indicated to be positively regulated
by mTORC1 via the activation of IKK/NF-κB signaling, and reduction
in aerobic glycolysis, inhibition of cell proliferation,
suppression of colony formation and delay in tumor growth have been
revealed to occur following GLUT3 knockdown (80).
Glucose phosphorylation, which is the first step in
glucose metabolism, is under the control of HK (81). There are five different types of
hexokinase isoforms that have been discovered in mammalian cells
(82). HK1 is widely expressed in
human cells and is considered to be a housekeeping isoform, while
HK2 is only expressed in certain tissues, including skeletal
muscle, cardiac muscle and adipose tissues (83,84),
as well as in cancer cells (85).
HK3 is less characterized compared with the other isoforms due to
its low levels, and it is considered to be inhibited by glucose
under physiological conditions (86). Moreover, recent research has
indicated that the upregulation of HK3 is closely associated with
the occurrence of EMT in CRC and may be of importance in metabolic
adaptation for the rapid proliferation, survival and metastasis of
CRC cells (87). HK4, which is
also called glucokinase, is expressed in the liver and pancreas
(88) and is involved in the
migration of regulatory T cells via a PI3K-mTORC2-mediated pathway
(89). The importance of HK2 in
cancer cells and its relationship with mTOR is highlighted below.
HK2 has also been indicated to bind to mitochondrial porins and
catalyze the first step of glycolysis (90). AKT can activate HK2 associated with
mitochondria, thereby promoting glycolysis (91). In addition, HK2 expression has been
revealed to be enhanced by HIF-1α (92,93),
which is regulated by the PI3K/AKT/mTORC1 pathway (4,94).
To investigate the contributions of c-Src, a proto-oncogene closely
associated with mTOR (95,96), to the metabolic reprogramming of
cancer cells, Conde et al (92) demonstrated that c-Src
phosphorylated HK1 at Tyr732 and HK2 at Tyr686, strengthening their
catalytic activity and enhancing glycolysis. Cell glycolysis,
proliferation and metastasis were diminished when the c-Src
phosphorylation site of either HK1 or HK2 was mutated (97). In addition, HK may be affected by
miRNAs. miR-214 downregulation has been revealed to inhibit
glycolysis by decreasing the expression of both HK2 and PKM2 via
the PTEN/AKT/mTOR pathway in NSCLC cells, revealing the
significance of miR-214 and the importance of mTOR-mediated
HK-regulated glycolysis in NSCLC treatment (98).
HIF-1 and c-Myc, which are both downstream mediators
of the mTOR signaling pathway, are two major regulators of
glycolytic enzymes, including HK2, PFK and LDHA (96,99).
Fructose 6-phos-phate is converted to fructose 1,6-bisphosphate by
PFK-1, which is a well-known 'gatekeeper' of glycolysis, and this
constitutes the commitment step of glycolysis (100). PFK-1 is considered to be a key
regulator of metabolic repro-gramming in various types of cancers
(101,102), including glioblastoma (103), lung cancer (104) and breast cancer (105). It has been indicated that c-Myc
directly transactivated genes encoding PFK and increased glucose
uptake in Rat-1 fibroblasts (106). A previous study has revealed that
PFK-1 platelet isoform (PFKP), which is the main PFK-1 isoform, was
overexpressed in human glioblastoma due to the loss of PTEN- and
EGFR-mediated PI3K activation, contributing to glycolysis and
tumorigenesis (103). These
results highlighted the potential role and regulation of PFKP and
mTOR-related AKT signaling in human glioblastoma development
(103). It has been revealed that
PFKFB is a key glycolysis regulator that modulates fructose
2,6-bisphosphate levels and glucose uptake (107). PFKFB3 has been reported to
contribute to the development, metastasis and chemotherapy
resistance of cancer cells (108-111). Previous studies have indicated
that mTORC2 activated AKT by phosphorylating S473, leading to the
allosteric activation of PFK-1 (108,110). Moreover, PFKFB3 has been
demonstrated to be upregulated by mTORC1, which was dependent on
HIF-1α in acute myeloid leukemia (112), and has been indicated to be
activated by AKT in another study, which was downstream of mTORC2,
thereby contributing to tumor angiogenesis (113). In addition to PFKFB3, PFKFB4 has
also been reported to be associated with certain malignancies
(114,115). PFKFB4 expression in human bladder
cancer has been demonstrated to be associated with hypoxia via
HIF-1α (116). The aforementioned
results have indicated that mTOR-mediated PFK signaling is tightly
associated with carcinogenesis.
There are two genes encoding four PK isoforms, where
two PK isoforms correspond to each gene (117). The PKLR gene encodes PKL and PKR,
while PKM1 and PKM2 are encoded by the PKM gene (118). It has been indicated that there
is a potential relationship between PK and mTOR. The final step of
glycolysis is catalyzed by PKM2, which converts PEP to pyruvate via
the transfer of a phosphate group to ADP (4). PKM2 has been widely studied in past
decades, and both inhibitors and activators have been developed for
anticancer purposes in different contexts due to the multifaceted
characteristics of cancers (119,120). Certain PKM2 activators have been
developed to induce the tetramerization of PKM2, causing PKM2 to
function as PKM1, which is the dominant glyco-lytic enzyme in
healthy adult tissues (121,122). A number of scientists believe
that the inhibition of PKM2 occurs as a result of its high
expression in various types of cancers, including lung (123), breast (124) and gastric cancer (125). The PI3K/AKT/mTOR pathway has been
implicated in regulating PKM2 expression levels, and evidence has
indicated that rapamycin decreased PKM2 expression, highlighting
the role of mTOR in regulating PKM2 (126,127). HIF-1α and c-Myc have also both
been closely associated with the activity of PKM2 (128,129). It has been demonstrated that when
mTOR was activated or inhibited, the expression levels of PKM2 and
HIF-1α, as well as glycolysis, were altered in esophageal squamous
cell carcinoma, indicating that mTOR promoted aerobic glycolysis in
esophageal squamous cell carcinoma by upregulating PKM2 expression
(130). Yes-associated protein is
a powerful regulator that has been indicated to be overexpressed in
various types of cancers, such as hepatocellular carcinoma
(131), binds to HIF-1α in the
nucleus to maintain the stability of HIF-1α protein and also binds
to PKM2 gene and directly activates PKM2 transcription to
accelerate glycolysis, providing a novel therapeutic target based
on HIF-1α (131). Moreover, the
interaction of PKM2 with the HIF-1α subunit stimulates PKM2
transactivation via a feedback loop (132). Following ligand stimulation and
post-translational modification, PKM2 is transferred into the
nucleus activating HIF-1α, thereby promoting the glycolytic pathway
(122). The correlation between
c-Myc expression and increased PKM2 synthesis in various cancers,
such as gastric (133), head and
neck (129), liver (134) and breast cancer (135), highlights the possible role of
c-Myc in PKM2 expression (134).
A recent study has revealed that c-Myc expression was upregulated
by p21-activated kinase 2 (PAK2) and that c-Myc bound to the PKM
promoter to induce PKM2 expression, which provided a potential
framework for head and neck cancer therapy by targeting the
PAK2-cMyc-PKM2 axis (129).
Furthermore, mTOR has been indicated to regulate TSC1/2 to control
the expression of PAK2, but the regulation of glycolysis via PKM2
remains to be explored (136).
After the bidirectional enzyme LDH converts pyruvate
to lactate, certain cancer cells have been indicated to retain
lactate as a metabolic substrate, although others secrete it
(4,137,138). Glycolytic cancer cells have been
revealed to overexpress MCT-4 to export lactate, maintaining a
proper pH for tumor growth, while MCT-1 has been indicated to be
highly expressed in normoxic cancer cells in close proximity to
blood vessels to import lactate for the synthesis of amino acids
(139,140). The phenomenon of lactate re-use
in two different types of cells is called metabolic symbiosis, and
it is regarded as a potent therapeutic target for cancer (139,141,142). Notably, MCT-1 and MCT-4 have been
demonstrated to be critical in metabolic symbiosis (143,144). In arsenite-induced
carcinogenesis, it has been reported that arsenite caused high
HIF-1α-mediated expression of MCT-4 in liver cells, enhancing
glycolysis and resulting in hepatoxicity (145).
Cancer cell metabolism shifts from oxidative
phosphorylation to glycolysis even in the presence of oxygen, which
is also called the Warburg effect, to accommodate the rapid
increase in the energy requirements of cancer cells (4). Cancer cells often overexpress
catalytic glycolysis enzymes to promote energy production, which is
often associated with the abnormal activation of the mTOR signaling
pathway (146). All molecules of
the entire glycolytic pathway from GLUT to PK are indispensable for
glycolysis, and disruption of any factor can lead to glycolysis
disorders (4). More importantly,
the molecules involved in glycolysis do not function alone, but are
regulated by other molecules (147). mTOR-mediated pathways have been
revealed to act as central regulators to control the entire
glycolytic process (4). Currently,
several drugs have been indicated to affect glycolytic proteins,
and certain of these drugs will undergo clinical testing (148). Although the Warburg effect has
been identified in the past century, its application in cancer has
been investigated until recent decades (149). mTOR inhibitors that have been
used in the clinic to treat cancer have also been revealed to exert
unsatisfactory effects (148).
Therefore, in reviewing the importance of aerobic glycolysis and
mTOR in carcino-genesis and the limitations of a single application
in cancer treatment, we hypothesize that simultaneously blocking
glycolysis-associated proteins and the mTOR pathway may be a better
alternative for cancer therapy compared with the single-agent
inhibition of glycolysis.
The present study was funded by National Natural
Science Foundation of China (grant nos. 81673648, 81673725 and
81673795) and the Natural Science Foundation of Higher School of
Jiangsu Province (grant no. 17KJA360003).
Data sharing is not applicable to this article, as
no datasets were generated or analyzed during the current
study.
WXC, YL and SJW conceived and supervised the study;
HF and YYW wrote the original draft; HF, SYY, XML and AYW wrote and
edited the review; HF prepared the figures; WXC and YL acquired
funding. All the authors have read and approved the final
manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
|
1
|
Vander Heiden MG and DeBerardinis RJ:
Understanding the intersections between metabolism and cancer
biology. Cell. 168:657–669. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vijayakrishnapillai LMK, Desmarais JS,
Groeschen MN and Perlin MH: Deletion of ptn1, a PTEN/TEP1
orthologue, in ustilago maydis reduces pathogenicity and teliospore
development. J Fungi (Basel). 5:12018. View Article : Google Scholar
|
|
3
|
Huang S, Yang C, Li M, Wang B, Chen H, Fu
D and Chong T: Effect of dual mTOR inhibitor on TGFβ1-induced
fibrosis in primary human urethral scar fibroblasts. Biomed
Pharmacother. 106:1182–1187. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mossmann D, Park S and Hall MN: mTOR
signalling and cellular metabolism are mutual determinants in
cancer. Nat Rev Cancer. 18:744–757. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kenerson HL, Aicher LD, True LD and Yeung
RS: Activated mammalian target of rapamycin pathway in the
pathogenesis of tuberous sclerosis complex renal tumors. Cancer
Res. 62:5645–5650. 2002.PubMed/NCBI
|
|
6
|
Dowling RJ, Topisirovic I, Fonseca BD and
Sonenberg N: Dissecting the role of mTOR: Lessons from mTOR
inhibitors. Biochim Biophys Acta. 1804:433–439. 2010. View Article : Google Scholar
|
|
7
|
Kim DH, Sarbassov DD, Ali SM, King JE,
Latek RR, Erdjument-Bromage H, Tempst P and Sabatini DM: mTOR
inter-acts with raptor to form a nutrient-sensitive complex that
signals to the cell growth machinery. Cell. 110:163–175. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hara K, Maruki Y, Long X, Yoshino KI,
Oshiro N, Hidayat S, Tokunaga C, Avruch J and Yonezawa K: Raptor, a
binding partner of target of rapamycin (TOR), mediates TOR action.
Cell. 110:177–189. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Saxton RA and Sabatini DM: mTOR signaling
in growth, metabolism, and disease. Cell. 169:361–371. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu GY and Sabatini DM: mTOR at the nexus
of nutrition, growth, ageing and disease. Nat Rev Mol Cell Biol.
21:183–203. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hayase S, Kumamoto K, Saito K, Kofunato Y,
Sato Y, Okayama H, Miyamoto K, Ohki S and Takenoshita S: L-type
amino acid transporter 1 expression is upregulated and associated
with cellular proliferation in colorectal cancer. Oncol Lett.
14:7410–7416. 2017.
|
|
12
|
Villar VH, Nguyen TL, Delcroix V, Terés S,
Bouchecareilh M, Salin B, Bodineau C, Vacher P, Priault M,
Soubeyran P and Durán RV: mTORC1 inhibition in cancer cells
protects from glutaminolysis-mediated apoptosis during nutrient
limitation. Nat Commun. 8:141242017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
van der Vos KE, Eliasson P,
Proikas-Cezanne T, Vervoort SJ, van Boxtel R, Putker M, van Zutphen
IJ, Mauthe M, Zellmer S, Pals C, et al: Modulation of glutamine
metabolism by the PI(3) K-PKB-FOXO network regulates autophagy. Nat
Cell Biol. 14:829–837. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wise DR and Thompson CB: Glutamine
addiction: A new therapeutic target in cancer. Trends Biochem Sci.
35:427–433. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li J, Huang Q, Long X, Zhang J, Huang X,
Aa J, Yang H, Chen Z and Xing J: CD147 reprograms fatty acid
metabolism in hepatocellular carcinoma cells through
Akt/mTOR/SREBP1c and P38/PPARα pathways. J Hepatol. 63:1378–1389.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Harachi M, Masui K, Okamura Y, Tsukui R,
Mischel PS and Shibata N: mTOR complexes as a nutrient sensor for
driving cancer progression. Int J Mol Sci. 19:32672018. View Article : Google Scholar :
|
|
17
|
Vander Heiden MG, Cantley LC and Thompson
CB: Understanding the Warburg effect: The metabolic requirements of
cell proliferation. Science. 324:1029–1033. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ganapathy-Kanniappan S: Molecular
intricacies of aerobic glycolysis in cancer: Current insights into
the classic metabolic phenotype. Crit Rev Biochem Mol Biol.
53:667–682. 2018. View Article : Google Scholar
|
|
19
|
Chen XS, Li LY, Guan YD, Yang JM and Cheng
Y: Anticancer strategies based on the metabolic profile of tumor
cells: Therapeutic targeting of the Warburg effect. Acta Pharmacol
Sin. 37:1013–1019. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lei S, Yang J, Chen C, Sun J, Yang L, Tang
H, Yang T, Chen A, Zhao H, Li Y and Du X: FLIP(L) is critical for
aerobic glycolysis in hepatocellular carcinoma. J Exp Clin Cancer
Res. 35:792016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shi Y, Liu S, Ahmad S and Gao Q: Targeting
key transporters in tumor glycolysis as a novel anticancer
strategy. Curr Top Med Chem. 18:454–466. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu B and Yu S: Amentoflavone suppresses
hepatocellular carcinoma by repressing hexokinase 2 expression
through inhibiting JAK2/STAT3 signaling. Biomed Pharmacother.
107:243–253. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gao X and Han H: Jolkinolide B inhibits
glycolysis by down-regulating hexokinase 2 expression through
inactivating the Akt/mTOR pathway in non-small cell lung cancer
cells. J Cell Biochem. 119:4967–4974. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu B, Huang ZB, Chen X, See YX, Chen ZK
and Yao HK: Mammalian target of rapamycin 2 (MTOR2) and C-MYC
modulate glucosamine-6-phosphate synthesis in glioblastoma (GBM)
cells through glutamine: Fructose-6-phosphate aminotransferase 1
(GFAT1). Cell Mol Neurobiol. 39:415–434. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ghashghaeinia M, Koberle M, Mrowietz U and
Bernhardt I: Proliferating tumor cells mimick glucose metabolism of
mature human erythrocytes. Cell Cycle. 18:1316–1334. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Prakasam G, Singh RK, Iqbal MA, Saini SK,
Tiku AB and Bamezai RNK: Pyruvate kinase M knockdown-induced
signaling via AMP-activated protein kinase promotes mitochondrial
biogenesis, autophagy, and cancer cell survival. J Biol Chem.
292:15561–15576. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang R, Jiao H, Zhao J, Wang X and Lin H:
L-arginine enhances protein synthesis by phosphorylating mTOR (Thr
2446) in a nitric oxide-dependent manner in C2C12 cells. Oxid Med
Cell Longev. 2018:75691272018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ka M, Smith AL and Kim WY: MTOR controls
genesis and autophagy of GABAergic interneurons during brain
development. Autophagy. 13:1348–1363. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Caron A, Briscoe DM, Richard D and
Laplante M: DEPTOR at the nexus of cancer, metabolism, and
immunity. Physiol Rev. 98:1765–1803. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Payen VL, Porporato PE, Baselet B and
Sonveaux P: Metabolic changes associated with tumor metastasis,
part 1: Tumor pH, glycolysis and the pentose phosphate pathway.
Cell Mol Life Sci. 73:1333–1348. 2016. View Article : Google Scholar
|
|
31
|
Li L, Kang L, Zhao W, Feng Y, Liu W, Wang
T, Mai H, Huang J, Chen S, Liang Y, et al: miR-30a-5p suppresses
breast tumor growth and metastasis through inhibition of
LDHA-mediated Warburg effect. Cancer Lett. 400:89–98. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zoncu R, Efeyan A and Sabatini DM: mTOR:
From growth signal integration to cancer, diabetes and ageing. Nat
Rev Mol Cell Biol. 12:21–35. 2011. View Article : Google Scholar
|
|
33
|
Wyant GA, Abu-Remaileh M, Wolfson RL, Chen
WW, Freinkman E, Danai LV, Vander Heiden MG and Sabatini DM: mTORC1
activator SLC38A9 is required to efflux essential amino acids from
lysosomes and use protein as a nutrient. Cell. 171:642–654 e612.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Feng M, Xiong G, Cao Z, Yang G, Zheng S,
Qiu J, You L, Zheng L, Zhang T and Zhao Y: LAT2 regulates
glutamine-dependent mTOR activation to promote glycolysis and
chemoresistance in pancreatic cancer. J Exp Clin Cancer Res.
37:2742018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yoshida S, Pacitto R, Yao Y, Inoki K and
Swanson JA: Growth factor signaling to mTORC1 by amino acid-laden
macropinosomes. J Cell Biol. 211:159–172. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Alessi DR, James SR, Downes CP, Holmes AB,
Gaffney PR, Reese CB and Cohen P: Characterization of a
3-phos-phoinositide-dependent protein kinase which phosphorylates
and activates protein kinase Balpha. Curr Biol. 7:261–269. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Iqbal MA, Siddiqui FA, Gupta V,
Chattopadhyay S, Gopinath P, Kumar B, Manvati S, Chaman N and
Bamezai RNK: Insulin enhances metabolic capacities of cancer cells
by dual regulation of glycolytic enzyme pyruvate kinase M2. Mol
Cancer. 12:722013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Neil J, Shannon C, Mohan A, Laurent D,
Murali R and Jhanwar-Uniyal M: ATP-site binding inhibitor
effectively targets mTORC1 and mTORC2 complexes in glioblastoma.
Int J Oncol. 48:1045–1052. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yang H, Jiang X, Li B, Yang HJ, Miller M,
Yang A, Dhar A and Pavletich NP: Mechanisms of mTORC1 activation by
RHEB and inhibition by PRAS40. Nature. 552:368–373. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ma Y, Vassetzky Y and Dokudovskaya S:
mTORC1 pathway in DNA damage response. Biochim Biophys Acta Mol
Cell Res. 1865:1293–1311. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Dewar JM and Walter JC: Mechanisms of DNA
replication termination. Nat Rev Mol Cell Biol. 18:507–516. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hsieh HJ, Zhang W, Lin SH, Yang WH, Wang
JZ, Shen J, Zhang Y, Lu Y, Wang H, Yu J, et al: Systems biology
approach reveals a link between mTORC1 and G2/M DNA damage
check-point recovery. Nat Commun. 9:39822018. View Article : Google Scholar
|
|
43
|
Silvera D, Ernlund A, Arju R, Connolly E,
Volta V, Wang J and Schneider RJ: mTORC1 and -2 coordinate
transcriptional and translational reprogramming in resistance to
DNA damage and replicative stress in breast cancer cells. Mol Cell
Biol. 37:e005772017. View Article : Google Scholar :
|
|
44
|
Javary J, Allain-Courtois N, Saucisse N,
Costet P, Heraud C, Benhamed F, Pierre R, Bure C, Pallares-Lupon N,
Do Cruzeiro M, et al: Liver reptin/RUVBL2 controls glucose and
lipid metabolism with opposite actions on mTORC1 and mTORC2
signalling. Gut. 67:2192–2203. 2018. View Article : Google Scholar
|
|
45
|
Byun JK, Choi YK, Kim JH, Jeong JY, Jeon
HJ, Kim MK, Hwang I, Lee SY, Lee YM, Lee IK and Park KG: A positive
feedback loop between sestrin2 and mTORC2 is required for the
survival of glutamine-depleted lung cancer cells. Cell Rep.
20:586–599. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yuan T, Lupse B, Maedler K and Ardestani
A: mTORC2 signaling: A path for pancreatic β cell's growth and
function. J Mol Biol. 430:904–918. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhang J, Jia L, Liu T, Yip YL, Tang WC,
Lin W, Deng W, Lo KW, You C, Lung ML, et al: mTORC2-mediated PDHE1α
nuclear translocation links EBV-LMP1 reprogrammed glucose
metabolism to cancer metastasis in nasopharyngeal carcinoma.
Oncogene. 38:4669–4684. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Li W, Wong CC, Zhang X, Kang W, Nakatsu G,
Zhao Q, Chen H, Go MYY, Chiu PWY, Wang X, et al: CAB39L elicited an
anti-Warburg effect via a LKB1-AMPK-PGC1α axis to inhibit gastric
tumorigenesis. Oncogene. 37:6383–6398. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Varshney R, Gupta S and Roy P:
Cytoprotective effect of kaempferol against palmitic acid-induced
pancreatic β-cell death through modulation of autophagy via
AMPK/mTOR signaling pathway. Mol Cell Endocrinol. 448:1–20. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Faubert B, Boily G, Izreig S, Griss T,
Samborska B, Dong Z, Dupuy F, Chambers C, Fuerth BJ, Viollet B, et
al: AMPK is a negative regulator of the Warburg effect and
suppresses tumor growth in vivo. Cell Metab. 17:113–124. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Daurio NA, Tuttle SW, Worth AJ, Song EY,
Davis JM, Snyder NW, Blair IA and Koumenis C: AMPK activation and
metabolic repro-gramming by tamoxifen through estrogen
receptor-independent mechanisms suggests new uses for this
therapeutic modality in cancer treatment. Cancer Res. 76:3295–3306.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Han J, Zhang L, Guo H, Wysham WZ, Roque
DR, Willson AK, Sheng X, Zhou C and Bae-Jump VL: Glucose promotes
cell proliferation, glucose uptake and invasion in endometrial
cancer cells via AMPK/mTOR/S6 and MAPK signaling. Gynecol Oncol.
138:668–675. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Liu Y, Tong L, Luo Y, Li X, Chen G and
Wang Y: Resveratrol inhibits the proliferation and induces the
apoptosis in ovarian cancer cells via inhibiting glycolysis and
targeting AMPK/mTOR signaling pathway. J Cell Biochem.
119:6162–6172. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Liang J and Mills GB: AMPK: A contextual
oncogene or tumor suppressor? Cancer Res. 73:2929–2935. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Hauge M, Bruserud O and Hatfield KJ:
Targeting of cell metabolism in human acute myeloid leukemia-more
than targeting of isocitrate dehydrogenase mutations and
PI3K/AKT/mTOR signaling? Eur J Haematol. 96:211–221. 2016.
View Article : Google Scholar
|
|
56
|
Yang X, Cheng Y, Li P, Tao J, Deng X,
Zhang X, Gu M, Lu Q and Yin C: A lentiviral sponge for miRNA-21
dimin-ishes aerobic glycolysis in bladder cancer T24 cells via the
PTEN/PI3K/AKT/mTOR axis. Tumour Biol. 36:383–391. 2015. View Article : Google Scholar
|
|
57
|
Wang P, Guan Q, Zhou D, Yu Z, Song Y and
Qiu W: miR-21 inhibitors modulate biological functions of gastric
cancer cells via PTEN/PI3K/mTOR pathway. DNA Cell Biol. 37:38–45.
2018. View Article : Google Scholar
|
|
58
|
Wang WJ, Yang W, Ouyang ZH, Xue JB, Li XL,
Zhang J, He WS, Chen WK, Yan YG and Wang C: MiR-21 promotes ECM
degradation through inhibiting autophagy via the PTEN/akt/mTOR
signaling pathway in human degenerated NP cells. Biomed
Pharmacother. 99:725–734. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Makinoshima H, Takita M, Saruwatari K,
Umemura S, Obata Y, Ishii G, Matsumoto S, Sugiyama E, Ochiai A, Abe
R, et al: Signaling through the phosphatidylinositol 3-kinase
(PI3K)/mammalian target of rapamycin (mTOR) axis is responsible for
aerobic glycolysis mediated by glucose transporter in epidermal
growth factor receptor (EGFR)-mutated lung adeno-carcinoma. J Biol
Chem. 290:17495–17504. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Fruman DA and Rommel C: PI3K and cancer:
Lessons, challenges and opportunities. Nat Rev Drug Discov.
13:140–156. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Li Q, Tang H, Hu F and Qin C: Silencing of
FOXO6 inhibits the proliferation, invasion, and glycolysis in
colorectal cancer cells. J Cell Biochem. 120:3853–3860. 2019.
View Article : Google Scholar
|
|
62
|
Gong T, Cui L, Wang H, Wang H and Han N:
Knockdown of KLF5 suppresses hypoxia-induced resistance to
cisplatin in NSCLC cells by regulating HIF-1α-dependent glycolysis
through inactivation of the PI3K/Akt/mTOR pathway. J Transl Med.
16:1642018. View Article : Google Scholar
|
|
63
|
Xu DH, Li Q, Hu H, Ni B, Liu X, Huang C,
Zhang ZZ and Zhao G: Transmembrane protein GRINA modulates aerobic
glycolysis and promotes tumor progression in gastric cancer. J Exp
Clin Cancer Res. 37:3082018. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Li X, Zhang Y, Ma W, Fu Q, Liu J, Yin G,
Chen P, Dai D, Chen W, Qi L, et al: Enhanced glucose metabolism
mediated by CD147 contributes to immunosuppression in
hepatocellular carcinoma. Cancer Immunol Immunother. 69:535–548.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Li Z, Liu J, Que L and Tang X: The
immunoregulatory protein B7-H3 promotes aerobic glycolysis in oral
squamous carcinoma via PI3K/Akt/mTOR pathway. J Cancer.
10:5770–5784. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Li R, Weng L, Liu B, Zhu L, Zhang X, Tian
G, Hu L, Li Q, Jiang S and Shang M: TRIM59 predicts poor prognosis
and promotes pancreatic cancer progression via the
PI3K/AKT/mTOR-glycolysis signaling axis. J Cell Biochem.
121:1986–1997. 2020. View Article : Google Scholar
|
|
67
|
Peng W, Huang W, Ge X, Xue L, Zhao W and
Xue J: Type Ig phosphatidylinositol phosphate kinase promotes tumor
growth by facilitating Warburg effect in colorectal cancer.
EBioMedicine. 44:375–386. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Li D, Ji H, Niu X, Yin L, Wang Y, Gu Y,
Wang J, Zhou X, Zhang H and Zhang Q: Tumor-associated macrophages
secrete CC-chemokine ligand 2 and induce tamoxifen resistance by
activating PI3K/Akt/mTOR in breast cancer. Cancer Sci. 111:47–58.
2020. View Article : Google Scholar
|
|
69
|
Gasparri ML, Besharat ZM, Farooqi AA,
Khalid S, Taghavi K, Besharat RA, Sabato C, Papadia A, Panici PB,
Mueller MD and Ferretti E: MiRNAs and their interplay with
PI3K/AKT/mTOR pathway in ovarian cancer cells: A potential role in
platinum resistance. J Cancer Res Clin Oncol. 144:2313–2318. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Deng J, Bai X, Feng X, Ni J, Beretov J,
Graham P and Li Y: Inhibition of PI3K/Akt/mTOR signaling pathway
alleviates ovarian cancer chemoresistance through reversing
epithelial-mesenchymal transition and decreasing cancer stem cell
marker expression. BMC Cancer. 19:6182019. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Massari F, Ciccarese C, Santoni M,
Iacovelli R, Mazzucchelli R, Piva F, Scarpelli M, Berardi R,
Tortora G, Lopez-Beltran A, et al: Metabolic phenotype of bladder
cancer. Cancer Treat Rev. 45:46–57. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Wang Y, Han X, Fu M, Wang J, Song Y, Liu
Y, Zhang J, Zhou J and Ge J: Qiliqiangxin attenuates
hypoxia-induced injury in primary rat cardiac microvascular
endothelial cells via promoting HIF-1α-dependent glycolysis. J Cell
Mol Med. 22:2791–2803. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Koh YW, Lee SJ and Park SY: Differential
expression and prognostic significance of GLUT1 according to
histologic type of non-small-cell lung cancer and its association
with volume-dependent parameters. Lung Cancer. 104:31–37. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Hamann I, Krys D, Glubrecht D, Bouvet V,
Marshall A, Vos L, Mackey JR, Wuest M and Wuest F: Expression and
function of hexose transporters GLUT1, GLUT2, and GLUT5 in breast
cancer-effects of hypoxia. FASEB J. 32:5104–5118. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Buller CL, Loberg RD, Fan MH, Zhu Q, Park
JL, Vesely E, Inoki K, Guan KL and Brosius FC III: A
GSK-3/TSC2/mTOR pathway regulates glucose uptake and GLUT1 glucose
trans-porter expression. Am J Physiol Cell Physiol. 295:C836–C843.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Jiang X, Kenerson H, Aicher L, Miyaoka R,
Eary J, Bissler J and Yeung RS: The tuberous sclerosis complex
regulates trafficking of glucose transporters and glucose uptake.
Am J Pathol. 172:1748–1756. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Wu XL, Wang LK, Yang DD, Qu M, Yang YJ,
Guo F, Han L and Xue J: Effects of Glut1 gene silencing on
proliferation, differentiation, and apoptosis of colorectal cancer
cells by targeting the TGF-β/PI3K-AKT-mTOR signaling pathway. J
Cell Biochem. 119:2356–2367. 2018. View Article : Google Scholar
|
|
78
|
Barron CC, Bilan PJ, Tsakiridis T and
Tsiani E: Facilitative glucose transporters: Implications for
cancer detection, prognosis and treatment. Metabolism. 65:124–139.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Do SK, Jeong JY, Lee SY, Choi JE, Hong MJ,
Kang HG, Lee WK, Seok Y, Lee EB, Shin KM, et al: Glucose
transporter 1 gene variants predict the prognosis of patients with
early-stage non-small cell lung cancer. Ann Surg Oncol.
25:3396–3403. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Zha X, Hu Z, Ji S, Jin F, Jiang K, Li C,
Zhao P, Tu Z, Chen X, Di L, et al: NFκB up-regulation of glucose
transporter 3 is essential for hyperactive mammalian target of
rapamycin-induced aerobic glycolysis and tumor growth. Cancer Lett.
359:97–106. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
DeWaal D, Nogueira V, Terry AR, Patra KC,
Jeon SM, Guzman G, Au J, Long CP, Antoniewicz MR and Hay N:
Hexokinase-2 depletion inhibits glycolysis and induces oxidative
phosphorylation in hepatocellular carcinoma and sensitizes to
metformin. Nat Commun. 9:4462018. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Hay N: Reprogramming glucose metabolism in
cancer: Can it be exploited for cancer therapy? Nat Rev Cancer.
16:635–649. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Esteves JV, Yonamine CY, Pinto-Junior DC,
Gerlinger-Romero F, Enguita FJ and Machado UF: Diabetes modulates
MicroRNAs 29b-3p, 29c-3p, 199a-5p and 532-3p expression in muscle:
Possible role in GLUT4 and HK2 repression. Front Endocrinol
(Lausanne). 9:5362018. View Article : Google Scholar
|
|
84
|
Marampon F, Antinozzi C, Corinaldesi C,
Vannelli GB, Sarchielli E, Migliaccio S, Di Luigi L, Lenzi A and
Crescioli C: The phosphodiesterase 5 inhibitor tadalafil regulates
lipidic homeostasis in human skeletal muscle cell metabolism.
Endocrine. 59:602–613. 2018. View Article : Google Scholar
|
|
85
|
DeWaal D, Nogueira V, Terry AR, Patra KC,
Jeon SM, Guzman G, Au J, Long CP, Antoniewicz MR and Hay N: Author
correction: Hexokinase-2 depletion inhibits glycolysis and induces
oxidative phosphorylation in hepatocellular carcinoma and
sensitizes to metformin. Nat Commun. 9:25392018. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Kudryavtseva AV, Fedorova MS, Zhavoronkov
A, Moskalev AA, Zasedatelev AS, Dmitriev AA, Sadritdinova AF,
Karpova IY, Nyushko KM, Kalinin DV, et al: Effect of
lentivirus-mediated shRNA inactivation of HK1, HK2, and HK3 genes
in colorectal cancer and melanoma cells. BMC Genet. 17(Suppl 3):
S1562016. View Article : Google Scholar
|
|
87
|
Pudova EA, Kudryavtseva AV, Fedorova MS,
Zaretsky AR, Shcherbo DS, Lukyanova EN, Popov AY, Sadritdinova AF,
Abramov IS, Kharitonov SL, et al: HK3 overexpression associated
with epithelial-mesenchymal transition in colorectal cancer. BMC
Genomics. 19(Suppl 3): S1132018. View Article : Google Scholar
|
|
88
|
Fujieda H, Kogami M, Sakairi M, Kato N,
Makino M, Takahashi N, Miyazawa T, Harada S and Yamashita T:
Discovery of a potent glucokinase activator with a favorable liver
and pancreas distribution pattern for the treatment of type 2
diabetes mellitus. Eur J Med Chem. 156:269–294. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Kishore M, Cheung KCP, Fu H, Bonacina F,
Wang G, Coe D, Ward EJ, Colamatteo A, Jangani M, Baragetti A, et
al: Regulatory T cell migration is dependent on
glucokinase-mediated glycolysis. Immunity. 47:875–889 e10. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Yeung SJ, Pan J and Lee MH: Roles of p53,
MYC and HIF-1 in regulating glycolysis - the seventh hallmark of
cancer. Cell Mol Life Sci. 65:3981–3999. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Roberts DJ and Miyamoto S: Hexokinase II
integrates energy metabolism and cellular protection: Akting on
mitochondria and TORCing to autophagy. Cell Death Differ.
22:3642015. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Conde E, Giménez-Moyano S, Martín-Gómez L,
Rodríguez M, Ramos ME, Aguado-Fraile E, Blanco-Sanchez I, Saiz A
and García-Bermejo ML: HIF-1α induction during reperfusion avoids
maladaptive repair after renal ischemia/reperfusion involving
miR127-3p. Sci Rep. 7:410992017. View Article : Google Scholar
|
|
93
|
Zhang T, Zhu X, Wu H, Jiang K, Zhao G,
Shaukat A, Deng G and Qiu C: Targeting the ROS/PI3K/AKT/HIF-1α/HK2
axis of breast cancer cells: Combined administration of polydatin
and 2-deoxy-d-glucose. J Cell Mol Med. 23:3711–3723. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Miyazaki M, Miyazaki K, Chen S, Chandra V,
Wagatsuma K, Agata Y, Rodewald HR, Saito R, Chang AN, Varki N, et
al: The E-Id protein axis modulates the activities of the
PI3K-AKT-mTORC1-Hif1a and c-myc/p19Arf pathways to suppress innate
variant TFH cell development, thymocyte expansion, and
lymphomagenesis. Genes Dev. 29:409–425. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Sudhagar S, Sathya S and Lakshmi BS: Rapid
non-genomic signalling by 17β-oestradiol through c-Src involves
mTOR-dependent expression of HIF-1α in breast cancer cells. Br J
Cancer. 105:953–960. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Huang C, Bruggeman LA, Hydo LM and Miller
RT: Shear stress induces cell apoptosis via a c-Src-phospholipase
D-mTOR signaling pathway in cultured podocytes. Exp Cell Res.
318:1075–1085. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Zhang J, Wang S, Jiang B, Huang L, Ji Z,
Li X, Zhou H, Han A, Chen A, Wu Y, et al: c-Src phosphorylation and
activation of hexokinase promotes tumorigenesis and metastasis. Nat
Commun. 8:137322017. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Zhang K, Zhang M, Jiang H, Liu F, Liu H
and Li Y: Down-regulation of miR-214 inhibits proliferation and
glycolysis in non-small-cell lung cancer cells via down-regulating
the expression of hexokinase 2 and pyruvate kinase isozyme M2.
Biomed Pharmacother. 105:545–552. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Singh D, Arora R, Kaur P, Singh B, Mannan
R and Arora S: Overexpression of hypoxia-inducible factor and
metabolic path-ways: Possible targets of cancer. Cell Biosci.
7:622017. View Article : Google Scholar
|
|
100
|
Webb BA, Forouhar F, Szu FE, Seetharaman
J, Tong L and Barber DL: Structures of human phosphofructokinase-1
and atomic basis of cancer-associated mutations. Nature.
523:111–114. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Yi W, Clark PM, Mason DE, Keenan MC, Hill
C, Goddard WA III, Peters EC, Driggers EM and Hsieh-Wilson LC:
Phosphofructokinase 1 glycosylation regulates cell growth and
metabolism. Science. 337:975–980. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Moreno-Sánchez R, Marin-Hernández A,
Gallardo-Pérez JC, Quezada H, Encalada R, Rodríguez-Enríquez S and
Saavedra E: Phosphofructokinase type 1 kinetics, isoform
expression, and gene polymorphisms in cancer cells. J Cell Biochem.
113:1692–1703. 2012.PubMed/NCBI
|
|
103
|
Lee JH, Liu R, Li J, Zhang C, Wang Y, Cai
Q, Qian X, Xia Y, Zheng Y, Piao Y, et al: Stabilization of
phosphofructokinase 1 platelet isoform by AKT promotes
tumorigenesis. Nat Commun. 8:9492017. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Tang H, Lee M, Sharpe O, Salamone L,
Noonan EJ, Hoang CD, Levine S, Robinson WH and Shrager JB:
Oxidative stress-responsive microRNA-320 regulates glycolysis in
diverse biological systems. FASEB J. 26:4710–4721. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Gomez LS, Zancan P, Marcondes MC,
Ramos-Santos L, Meyer-Fernandes JR, Sola-Penna M and Da Silva D:
Resveratrol decreases breast cancer cell viability and glucose
metabolism by inhibiting 6-phosphofructo-1-kinase. Biochimie.
95:1336–1343. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Holmes B, Lee J, Landon KA,
Benavides-Serrato A, Bashir T, Jung ME, Lichtenstein A and Gera J:
Mechanistic target of rapamycin (mTOR) inhibition synergizes with
reduced internal ribosome entry site (IRES)-mediated translation of
cyclin D1 and c-MYC mRNAs to treat glioblastoma. J Biol Chem.
291:14146–14159. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Bartrons R, Simon-Molas H,
Rodríguez-Garcia A, Castaño E, Navarro-Sabaté À, Manzano A and
Martinez-Outschoorn UE: Fructose 2,6-bisphosphate in cancer cell
metabolism. Front Oncol. 8:3312018. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Wang C, Qu J, Yan S, Gao Q, Hao S and Zhou
D: PFK15, a PFKFB3 antagonist, inhibits autophagy and proliferation
in rhabdomyosarcoma cells. Int J Mol Med. 42:359–367.
2018.PubMed/NCBI
|
|
109
|
Ros S and Schulze A: Balancing glycolytic
flux: The role of 6-phosphofructo-2-kinase/fructose
2,6-bisphosphatases in cancer metabolism. Cancer Metab. 1:82013.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Cantelmo AR, Conradi LC, Brajic A, Goveia
J, Kalucka J, Pircher A, Chaturvedi P, Hol J, Thienpont B, Teuwen
LA, et al: Inhibition of the glycolytic activator PFKFB3 in
endothelium induces tumor vessel normalization, impairs metastasis,
and improves chemotherapy. Cancer Cell. 30:968–985. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Atsumi T, Chesney J, Metz C, Leng L,
Donnelly S, Makita Z, Mitchell R and Bucala R: High expression of
inducible 6-phos-phofructo-2-kinase/fructose-2,6-bisphosphatase
(iPFK-2; PFKFB3) in human cancers. Cancer Res. 62:5881–5887.
2002.PubMed/NCBI
|
|
112
|
Feng Y and Wu L: mTOR up-regulation of
PFKFB3 is essential for acute myeloid leukemia cell survival.
Biochem Biophys Res Commun. 483:897–903. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Ziegler ME, Hatch MM, Wu N, Muawad SA and
Hughes CC: mTORC2 mediates CXCL12-induced angiogenesis.
Angiogenesis. 19:359–371. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Shi L, Pan H, Liu Z, Xie J and Han W:
Roles of PFKFB3 in cancer. Signal Transduct Target Ther.
2:170442017. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Dasgupta S, Rajapakshe K, Zhu B, Nikolai
BC, Yi P, Putluri N, Choi JM, Jung SY, Coarfa C, Westbrook TF, et
al: Metabolic enzyme PFKFB4 activates transcriptional coactivator
SRC-3 to drive breast cancer. Nature. 556:249–254. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Zhang H, Lu C, Fang M, Yan W, Chen M, Ji
Y, He S, Liu T, Chen T and Xiao J: HIF-1α activates hypoxia-induced
PFKFB4 expression in human bladder cancer cells. Biochem Biophys
Res Commun. 476:146–152. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Zhang Z, Deng X, Liu Y, Liu Y, Sun L and
Chen F: PKM2, function and expression and regulation. Cell Biosci.
9:522019. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Nguyen A, Loo JM, Mital R, Weinberg EM,
Man FY, Zeng Z, Paty PB, Saltz L, Janjigian YY, de Stanchina E and
Tavazoie SF: PKLR promotes colorectal cancer liver colonization
through induction of glutathione synthesis. J Clin Invest.
126:681–694. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Adem S, Comakli V and Uzun N: Pyruvate
kinase activators as a therapy target: A patent review 2011-2017.
Expert Opin Ther Pat. 28:61–68. 2018. View Article : Google Scholar
|
|
120
|
Liu VM and Vander Heiden MG: The role of
pyruvate kinase M2 in cancer metabolism. Brain Pathol. 25:781–783.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Warner SL, Carpenter KJ and Bearss DJ:
Activators of PKM2 in cancer metabolism. Future Med Chem.
6:1167–1178. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Wang HJ, Hsieh YJ, Cheng WC, Lin CP, Lin
YS, Yang SF, Chen CC, Izumiya Y, Yu JS, Kung HJ and Wang WC: JMJD5
regulates PKM2 nuclear translocation and reprograms HIF-1α-mediated
glucose metabolism. Proc Natl Acad Sci USA. 111:279–284. 2014.
View Article : Google Scholar
|
|
123
|
Kim DJ, Park YS, Kim ND, Min SH, You YM,
Jung Y, Koo H, Noh H, Kim JA, Park KC and Yeom YI: A novel pyruvate
kinase M2 activator compound that suppresses lung cancer cell
viability under hypoxia. Mol Cells. 38:373–379. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Huang L, Yu Z, Zhang Z, Ma W, Song S and
Huang G: Interaction with pyruvate kinase M2 destabilizes
tristetraprolin by proteasome degradation and regulates cell
proliferation in breast cancer. Sci Rep. 6:224492016. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Wang C, Jiang J, Ji J, Cai Q, Chen X, Yu
Y, Zhu Z and Zhang J: PKM2 promotes cell migration and inhibits
autophagy by mediating PI3K/AKT activation and contributes to the
malignant development of gastric cancer. Sci Rep. 7:28862017.
View Article : Google Scholar : PubMed/NCBI
|
|
126
|
van Niekerk G and Engelbrecht AM: Role of
PKM2 in directing the metabolic fate of glucose in cancer: A
potential therapeutic target. Cell Oncol (Dordr). 41:343–351. 2018.
View Article : Google Scholar
|
|
127
|
Nemazanyy I, Espeillac C, Pende M and
Panasyuk G: Role of PI3K, mTOR and Akt2 signalling in hepatic
tumorigenesis via the control of PKM2 expression. Biochem Soc
Trans. 41:917–922. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Moloughney JG, Kim PK, Vega-Cotto NM, Wu
CC, Zhang S, Adlam M, Lynch T, Chou PC, Rabinowitz JD, Werlen G and
Jacinto E: mTORC2 responds to glutamine catabolite levels to
modulate the hexosamine biosynthesis enzyme GFAT1. Mol Cell.
63:811–826. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Gupta A, Ajith A, Singh S, Panday RK,
Samaiya A and Shukla S: PAK2-c-Myc-PKM2 axis plays an essential
role in head and neck oncogenesis via regulating Warburg effect.
Cell Death Dis. 9:8252018. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Xiaoyu H, Yiru Y, Shuisheng S, Keyan C,
Zixing Y, Shanglin C, Yuan W, Dongming C, Wangliang Z, Xudong B and
Jie M: The mTOR pathway regulates PKM2 to affect glycolysis in
esophageal squamous cell carcinoma. Technol Cancer Res Treat.
17:15330338187800632018. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Zhang X, Li Y, Ma Y, Yang L, Wang T, Meng
X, Zong Z, Sun X, Hua X and Li H: Yes-associated protein (YAP)
binds to HIF-1 α and sustains HIF-1α protein stability to promote
hepatocellular carcinoma cell glycolysis under hypoxic stress. J
Exp Clin Cancer Res. 37:2162018. View Article : Google Scholar
|
|
132
|
Demaria M and Poli V: PKM2, STAT3 and
HIF-1α: The Warburg's vicious circle. JAKSTAT. 1:194–196.
2012.PubMed/NCBI
|
|
133
|
Gao S, Chen M, Wei W, Zhang X, Zhang M,
Yao Y, Lv Y, Ling T, Wang L and Zou X: Crosstalk of mTOR/PKM2 and
STAT3/c-Myc signaling pathways regulate the energy metabolism and
acidic microenvironment of gastric cancer. J Cell Biochem.
2018.Epub ahead of print.
|
|
134
|
Mendez-Lucas A, Li X, Hu J, Che L, Song X,
Jia J, Wang J, Xie C, Driscoll PC, Tschaharganeh DF, et al: Glucose
catabolism in liver tumors induced by c-MYC can be sustained by
various PKM1/PKM2 ratios and pyruvate kinase activities. Cancer
Res. 77:4355–4364. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Yu P, Li AX, Chen XS, Tian M, Wang HY,
Wang XL, Zhang Y, Wang KS and Cheng Y: PKM2-c-Myc-survivin cascade
regulates the cell proliferation, migration, and tamoxifen
resistance in breast cancer. Front Pharmacol. 11:5504692020.
View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Alves MM, Fuhler GM, Queiroz KC, Scholma
J, Goorden S, Anink J, Spek CA, Hoogeveen-Westerveld M, Bruno MJ,
Nellist M, et al: PAK2 is an effector of TSC1/2 signaling
independent of mTOR and a potential therapeutic target for tuberous
sclerosis complex. Sci Rep. 5:145342015. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Hui S, Ghergurovich JM, Morscher RJ, Jang
C, Teng X, Lu W, Esparza LA, Reya T, Zhan L, Guo JY, et al: Glucose
feeds the TCA cycle via circulating lactate. Nature. 551:115–118.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Faubert B, Li KY, Cai L, Hensley CT, Kim
J, Zacharias LG, Yang C, Do QN, Doucette S, Burguete D, et al:
Lactate metabolism in human lung tumors. Cell. 171:358–371 e359.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Allen E, Mieville P, Warren CM, Saghafinia
S, Li L, Peng MW and Hanahan D: Metabolic symbiosis enables
adaptive resistance to anti-angiogenic therapy that is dependent on
mTOR signaling. Cell Rep. 15:1144–1160. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Kim HK, Lee I, Bang H, Kim HC, Lee WY, Yun
SH, Lee J, Lee SJ, Park YS, Kim KM and Kang WK: MCT4 expression is
a potential therapeutic target in colorectal cancer with peritoneal
carcinomatosis. Mol Cancer Ther. 17:838–848. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Pisarsky L, Bill R, Fagiani E, Dimeloe S,
Goosen RW, Hagmann J, Hess C and Christofori G: Targeting metabolic
symbiosis to overcome resistance to anti-angiogenic therapy. Cell
Rep. 15:1161–1174. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Morrot A, da Fonseca LM, Salustiano EJ,
Gentile LB, Conde L, Filardy AA, Franklim TN, da Costa KM,
Freire-de-Lima CG and Freire-de-Lima L: Metabolic symbiosis and
immunomodulation: How tumor cell-derived lactate may disturb innate
and adaptive immune responses. Front Oncol. 8:812018. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Pavlova NN and Thompson CB: The emerging
hallmarks of cancer metabolism. Cell Metab. 23:27–47. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Curry JM, Tuluc M, Whitaker-Menezes D,
Ames JA, Anantharaman A, Butera A, Leiby B, Cognetti DM, Sotgia F,
Lisanti MP and Martinez-Outschoorn UE: Cancer metabolism, stemness
and tumor recurrence: MCT1 and MCT4 are functional biomarkers of
metabolic symbiosis in head and neck cancer. Cell Cycle.
12:1371–1384. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Luo F, Zou Z, Liu X, Ling M, Wang Q, Wang
Q, Lu L, Shi L, Liu Y, Liu Q and Zhang A: Enhanced glycolysis,
regulated by HIF-1α via MCT-4, promotes inflammation in
arsenite-induced carcinogenesis. Carcinogenesis. 38:615–626. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Tan FH, Bai Y, Saintigny P and Darido C:
mTOR signalling in head and neck cancer: Heads up. Cells.
8:3332019. View Article : Google Scholar :
|
|
147
|
Jewell JL and Guan KL: Nutrient signaling
to mTOR and cell growth. Trends Biochem Sci. 38:233–242. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Martelli AM, Buontempo F and McCubrey JA:
Drug discovery targeting the mTOR pathway. Clin Sci (Lond).
132:543–568. 2018. View Article : Google Scholar
|
|
149
|
Liberti MV and Locasale JW: The Warburg
effect: How does it benefit cancer cells? Trends Biochem Sci.
41:211–218. 2016. View Article : Google Scholar : PubMed/NCBI
|