1. Introduction
There has been substantial improvement during
previous decades concerning understanding of the genetics and
molecular pathways involved in the development of breast cancer
(BC). However, BC remains the most commonly occurring cancer and
primary cause of cancer-related death in women worldwide (1). For example, data reported by the
World Health Organization estimate that there will be >20
million new cases of BC in the world by 2025 (2). The most frequent cause of mortality
in cases of BC is due to tumor metastasis and disease recurrence,
reflected by a <22% 5-year survival rate in patients with
metastasis or recurrence (3).
BC tumors exhibit a high degree of complexity
resulting from interactions between various cell types, including
proliferating tumor cells and non-cancerous cells in the stroma,
such as cancer-associated fibroblasts, endothelial cells,
infiltrating inflammatory cells, adipocytes and immune cells
(4). Another important component
is the extracellular matrix (ECM) (5). In breast tumors, the ECM is primarily
comprised of collagens, as well as fibronectin, laminins,
glycoproteins, polysaccharides and lipids from adipose tissue
(6,7). Of note, the ECM progressively changes
during the course of BC, becoming denser and richer in collagen;
along with the appearance of new blood vessels, both of these are
events are associated with the onset of metastasis (8-10).
In the tumor microenvironment, there are frequently progressive
changes in the expression of certain receptors in tumor cells,
including estrogen receptor (ER), progesterone receptor and/or
human epidermal growth factor receptor 2 (HER2), as well as the
gene expression patterns of specific genes, some of which are used
to perform molecular categorization of breast tumors (11). The sum of all of these properties
results in a highly heterogeneous environment that influences tumor
proliferation and cellular metastasis. Due the high incidence of BC
and its importance as a public health problem, understanding the
role of each element in BC tumors comprises a great challenge for
research groups to develop new and more effective treatments.
In order to study the progression of BC, in
vitro and in vivo models have been utilized to
recapitulate the main aspects of human tumors. The cultivation of
tumor cell lines in two-dimensional models (2D) is the most widely
employed in vitro model used to study tumor physiology
(12,13). This technique has the advantage of
providing an inexpensive and standardized high-throughput system.
Additionally, characterization of a specific cell response under a
specific condition is possible. However, in this model, it is not
possible to replicate all aspects observed physiologically in
tumors, such as the diversity in cellular population, ECM compounds
and interactions in a three-dimensional (3D) environment (14). Therefore, information obtained from
these 2D models should be interpreted with caution and extensively
confirmed. In order to overcome these limitations, animal models
have been used (14). In these,
certain deficiencies that arise from 2D culture of cell lines are
resolved; however, other points must be considered, such as the low
or absent expression of specific biomarkers observed in human BC,
that mean that they are cannot fully recapitulate the human
disease.
Although the tumor niche is highly complex, its
recapitulation in new models may improve understanding of the roles
that its components serve in leading to tumor progression and
metastasis. In vitro 3D models are proposed to address some
of these issues, such as the 3D conditions and interactions with
ECM, thus developing cellular conditions closer to those observed
in vivo (15). In addition,
under 3D conditions, stromal cells can be added in physiologically
relevant proportions to the culture to mimic the cellular
composition observed in vivo (15). The advantages of these approaches
for displaying tumor characteristics have been demonstrated. For
example, tumor cells in 3D models exhibit greater resistance to
drugs and invasive ability compared with those cultured under 2D
conditions (16,17). The present review explores the
application of these 3D models to study BC, discussing spheroid
models, organ-on-a-chip models, hydrogel models and bio-printed
models in particular. To easily consult the applications of each
model, a brief summary of them and their advantages and
disadvantages is presented in Table
I.
 | Table IApplication of 3D models to study
BC. |
Table I
Application of 3D models to study
BC.
| Model type | Description | Advantages | Disadvantages | Applications | (Refs.)a |
|---|
| Spheroid | Micro-scale 3D
model that reproduces early tumor stages. They have a spheroidal
shape, with a central necrotic core, surrounded by quiescent cells
and an outer layer with proliferating cells | Gradients of pH,
oxygen, and metabolic compounds, as in in vivo
conditions | Their production is
expensive and time-consuming, and some methods require a rigorous
standardization process | Reproduction of
tumor features: Spheroids can be generated using single cell lines
or via co-culture of several cell lines, with well-regulated
communication between cells to enable successful formation,
differentiation, and invasion | (19,28,33,40,41) |
| Chemosensitivity:
Spheroids are less sensitive to cisplatin, docetaxel and epirubicin
compared with 2D cell cultures, potentially due to an increase in
BC stem cell number in 3D conditions | (25,31) |
| Metastasis and
invasion: Spheroids embedded in high-stiffness scaffolds exhibit
tumor cells with reduced invasiveness compensated by actin
protrusions that remodel the fibronectin matrix and promote cell
invasion; additionally, it has been demonstrated that tumor cells
are able to breach basement membranes | (29,30) |
| High-throughput
screening: Novel methodologies have been developed to produce
organoids of a standardized size to be used in the high-throughput
screening of chemotherapeutics and molecular inhibitors | (49,50,58-60) |
| Organ-on-
a-chip | Micropatterned
surfaces capable of supporting the correct spatial arrangement of
cells. Microfluidic applications can mimic the specific stages of
tumor cell distribution, as well as the gradients of
biomolecules | Due to their small
size, smaller sample sizes and reagent volumes are employed | Qualified personnel
are required for its design and use | Study of tumor
microenvironment: Close communication between BC cells,
macrophages, stromal cells and fibroblasts has been investigated,
as well as the effects of microenvironmental signals on BC
progression | |
| Their manufacturing
characteristics enable high reproducibility and large-scale
production | PDMS, the material
commonly used for their construction, absorbs small hydrophobic
molecules, interfering with some drugs-screening studies | Metastasis and
invasion: The culture of spheroids in microfluidic devices enables
the study of early invasion processes, as well as the important
role of cell adhesion molecules on motility and the importance of
cell interactions between tumoral, endothelial and immunological
cells in invasion; additionally intravasation processes can be
studied | (57,63,65,67,
68,70) |
| Hydrogel | Scaffolds that
reproduce a simplified tumor microenvironment to replicate specific
stages of tumor behavior | Low toxicity and
good biocompatibility | Mechanical weakness
under some culture conditions | Study of tumor
microenvironment: The use of synthetic hydrogels has supported
their application in 3D models due their capability to reproduce
natural matrixes; those models have been studied to investigate the
effects of hypoxic conditions on gene expression and the impact of
3D conditions on cancer stem cell populations | (77,78,94-96) |
| Promoting cell
attachment and proliferation, and the presentation of tumor cues
for cell migration | | Study of
interactions between BC cells and stroma: Scaffolds models have
been used to study interactions between tumor cells, fibroblasts
and osteoblasts, highlighting the participation of soluble factors
in promoting tumoral progression | (79-81) |
| Metastasis and
invasion: With this approach, the importance of the ECM for cell
migration has been demonstrated | (82) |
| Bio- printing | Bio-printing
employs printable hydrogels encapsulating living cells in order to
sequentially form a 3D scaffold | Good performance
for recreating the complex tumor environment in high-resolution
under 3D conditions | Challenges include
improvements in cell viability, resolution and print fidelity | Study of the
interactions between BC cells and stroma: Successful co-culture of
BC cells and mesenchymal stem cells using a bioink comprised of
alginate and highly hydrated cellulose has been reported | (119,120) |
| The type and
concentration of the selected bioink can greatly affect the
scaffold properties | Implementation of
bio-printing to develop organoids: Using bio-printing principles,
it has been possible to obtain organoids with a standardized size
on a large scale | |
2. Spheroid models
Spheroids or mammospheres, depending on whether they
derive from BC tumor cells or BC cell lines, are micro-scale 3D
models characterized by their ability to form spherical aggregates
of cells by auto-assembly (radii, 100-600 µm) (18-20).
Spheroids display several features observed in tumors, such as a
central necrotic core surrounded by quiescent viable cells and, in
the outer layer, a coat of actively proliferating cells (Fig. 1A) (18). In these models, other tumoral
features have been observed, such as gradients of pH, oxygen and
metabolic compounds (Fig. 1A)
(21), and, in some cases,
micrometastases (22).
Nevertheless, the major inconvenience that these models involve is
that they are expensive, and their production is
time-consuming.
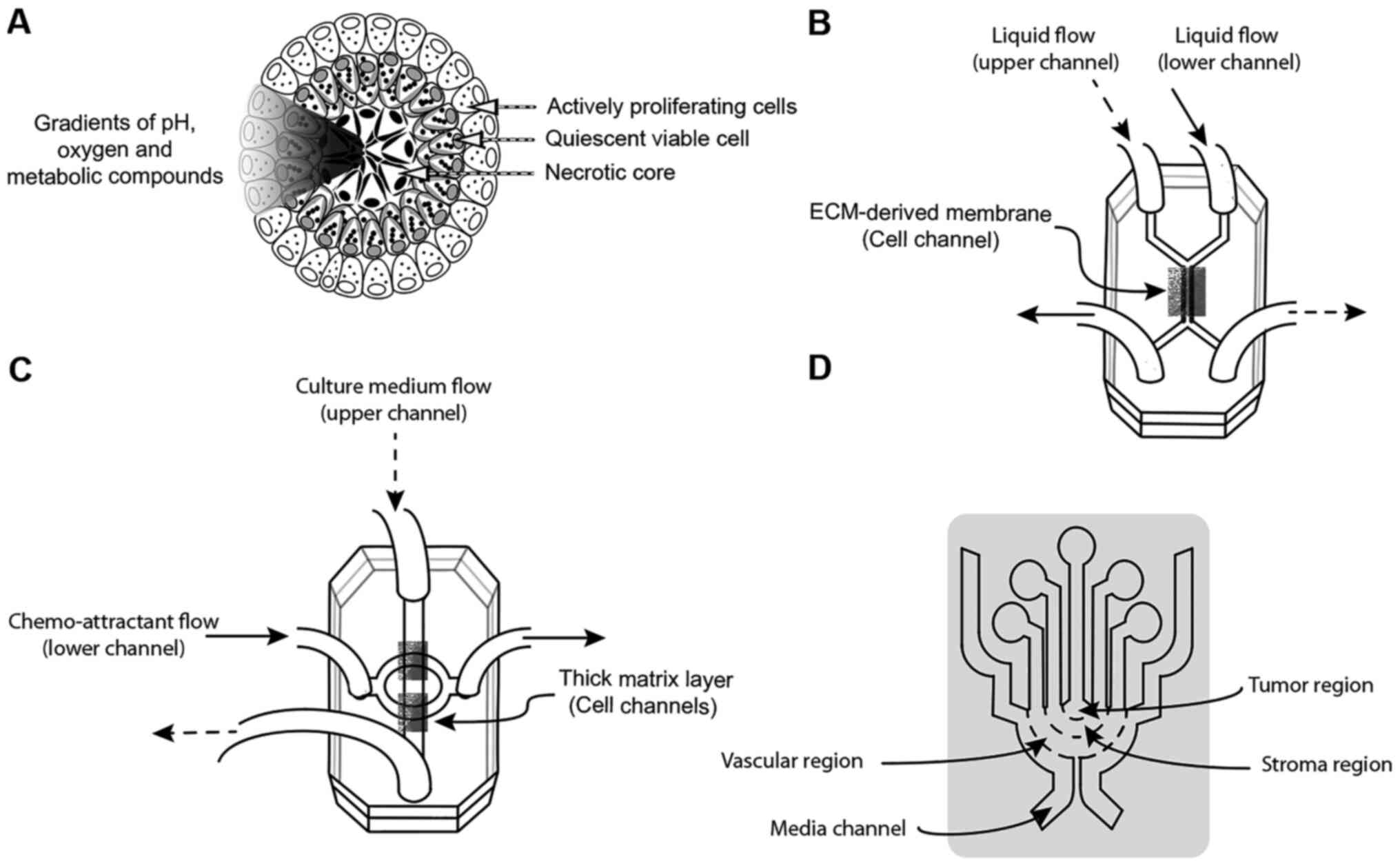 | Figure 1Schematic representation of 3D models
employed to study BC. (A) Schematic of spheroid models, spherical
aggregates of cells with tumor features, such as a central necrotic
core surrounded by quiescent viable cells and a coat of actively
proliferating cells. Additionally, as in tumors, gradients of pH,
oxygen and metabolic compounds are observed. These models are used
to study early-stage breast cancer. (B) Schematic of an
organ-on-a-chip model: A 3D compart-mentalized microfluidic device
with an upper channel and a lower channel, separated by an
ECM-derived membrane. Above this membrane, mammary epithelial cells
and spheroids are cultivated. A stromal layer and mammary
fibroblasts are added in the lower channel. This chip is able to
recapitulate the general aspects of mammary ducts and ductal
carcinoma. (C) 3D microfluidic device compartmentalized into two
microfluidic channels separated by two thick matrix layers. In the
upper channel, BC cells such as MDA-MB-231 or MCF-7 cells are
seeded. In the lower channel, chemo-attractants are added. This
model is employed to study epithelial-mesenchymal transition. (D)
Microfluidic system consisting of concentric layers containing
tumor cells, a stromal region and vascular cells, used to study the
intravasation of BC cells into the blood capillaries. Liquid flow
into the upper channel is represented by dashed arrowheads. Liquid
flow into the lower channel is represented by solid arrowheads. BC,
breast cancer; ECM, extracellular matrix; 3D,
three-dimensional. |
At present, there are several methods for producing
mammospheres in order to allow for cellular growth in suspension or
embedded in a 3D matrix, including the hanging drop method
(23). In this method, drops of
media containing cells are seeded on the lids of culture plate. To
increase the thickness of the media, a viscous component is usually
added. Then, the cells are cultured in an upside-down position to
promote cell aggregation and the formation of spheroids by gravity
(21). Although this method is
simple and inexpensive, a rigorous standardization process is
necessary (24). Using this
method, it was demonstrated that, in spheroids developed from
MCF-7, BT474 and trastuzumab-resistant BT474 cells, hypoxia
regulates resistance to trastuzumab through an increase in BC stem
cells and the expression of HER2 (25). This approach has also been adapted
for high-throughput screening using 3D printing methodology
(26). An alternative method for
producing mammospheres involves cell culture in spinner flasks. In
this method, a cell suspension is cultured under continuous
agitation to prevent adhesion to the flask, allowing cell-cell
attachment and the formation of spheroids (19,20).
The advantage of this method lies in the production of mammospheres
on a large scale, but specialized equipment is required, and the
experimental conditions need to be standardized to avoid cell
damage, and variation in spheroid size and shape (21). Another approach is the liquid
overlay technique (23). In this
method, cells are seeded in round-bottomed well plates pre-coated
with agarose or polyethylene glycol (PEG) to create a non-adherent
substrate. These conditions encourage cellular aggregation,
avoiding their attachment to the surface of the plate (21,23,27).
Currently, this is one of the most widely used methods to develop
mammospheres. Using this approach, the important role of cellular
communication during sphere growth, differentiation and collective
invasion was demonstrated (28).
Additionally, the impact of tumor stiffness on metastasis was
recently demonstrated (29); in
spheroids from MDA-MB-231 cells seeded in high-stiffness scaffolds,
a low invasive capability was observed, but cells with high amounts
of actin-enriched protrusions and high levels of Mena protein were
also observed. These elevated levels of Mena were in turn
associated with remodeling of the fibronectin matrix to promote
cell migration (29).
Different stages of BC invasion have been studied
using spheroid models. The process of breaching the basement
membrane by BC cells and subsequent invasion of the ECM were
studied in spheroids from non-tumor MCF10A cells and malignant
MCF10A-HRas cells surrounded with basement membrane proteins,
seeded within a collagen matrix intended to replicate the ECM
(30). Under these conditions, it
was shown that the basement membrane prevents the invasion of
non-tumor cells; conversely, tumor cells were able to breach this
membrane and invade the surrounding matrix. Furthermore, in
malignant cells, although invasive abilities were independent of
matrix-degrading enzymes, the breaching of the basement membrane
was a completely enzyme-dependent process (30). Additionally, the responses to
chemotherapeutic agents under 3D conditions have been evaluated. In
non-proliferative SUM1315 spheroids and in proliferative MDA-MB-231
spheroids, a less sensitive response was observed to cisplatin,
docetaxel and epirubicin compared with 2D cell cultures (31). An important point to be considered
during spheroid implementation is that sphere size affects the
nutrient distribution in the model core, consequently diminishing
cellular growth and response (32). In order to address this, several
studies have been conducted. For example, comparison of several
procedures to develop spheroids revealed that the liquid overlay
technique is an efficient tool to generate uniform mammospheres
from the commonly used BC cell lines, MCF-7 and MDA-MB-213
(19); additionally, this
methodology permitted the development of co-cultures with human
fibroblasts (33). The major
disadvantages with this approach concern the implementation of the
proper parameters required during cultivation and the acquisition
of a sufficient skill level to ensure a regular size and shape in
the spheroids (34).
As previously mentioned, the main obstacle in the
implementation of spheroid technology lies in the difficulty to
make the size and shape of the spheres consistently reproducible.
Previous studies showed that small spheroids (diameter, 100-300
µm) lose oxygen, nutrient and biochemical compound
gradients; as a result, they also lose the hypoxic core (35,36).
Conversely, large spheroids (diameter, 500-1,000 µm)
frequently exhibits extensive necrotic cores due to the lack of
nutrients and stimuli, rendering their biological relevance
questionable (33,37). Additionally, the analysis of these
large spheroids using conventional microscopy tools is complicated
(37). Spheroids of medium size
(diameter, 300-500 µm) exhibit an equilibrium between the
biological signatures of tumors, and their study using conventional
tools is also viable (38).
Therefore, methods have been developed that attempt to generate
spheroids of optimal size in a more consistent, efficient and
scalable manner. Among these methods, the magnetic levitation
method inserts magnetic nanoparticles into the cells for assembling
3D models (39). These models
allow the formation of spheroids from single-tumor BC cell lines
such as MDA-MB-231, Hs785bst and Hs371.t, or their co-cultivation
with human pulmonary fibroblast cell lines such as SUM159 (40) and, cells from patient-derived
xenografts (PDXs) (41). In all
these models, preservation of tissue architecture and the
expression profile of key biomarkers from the original tissue was
observed (40,41). A major advantage of this approach
that it is important to emphasize is its capability to develop
spheroids in a short period of time; however, in terms of
challenges concerning its implementation, it is important to
mention that specialized instrumentation and particular laboratory
skills are required (42).
3D printing is another approach used to develop
mammo-spheres (43), involving the
incorporation of living cells within biomaterials via the use of
robotic manufacturing to provide control over cell distribution
(44). For example, using the
laser-direct writing bio-printing approach, spheroids were
constructed of MDA-MB-231 cells (45); this approach enabled control of the
size, spatial placement and overall geometry of the aggregate.
Another example is the use of 96-well magnetic bio-printed plates
(41). In this method, PDX-derived
cells were coated with a nanoparticle assembly of iron oxide and
iron nanoparticles cross-linked with poly-L-lysine; then, the
formation of spheroids was conducted via cell seeding under a
magnetic field in the 96-well magnetic bio-printed plates, a method
that has been successfully employed as preclinical platform for
high-throughput drug screening (41). Finally, another methodology to
develop mammospheres involves the use of an aqueous two-phase
system in which two hydrophilic solutions are mixed to entrap the
cells into drops of a more hydrophilic phase. The usefulness of
this method to develop spheroids was showed in MDA-MB-157 cells
(46). Likewise, to form
spheroids, MDA-MB-231 cells were absorbed into drops of aqueous
dextran; subsequently, these drops were immersed in PEG and culture
medium to ensure free diffusion of nutrients and the removal of
waste between both phases. These spheroids displayed a standardized
size and the typical features of solid tumors, such as compact
morphology, hypoxia and ECM deposition (47,48).
In addition, they were also successfully used as models in the
high-throughput screening of chemotherapeutics and molecular
inhibitors (49,50).
In conclusion, the features observed in organoids,
such as a necrotic core surrounded by quiescent cells and a coat of
actively proliferating cells, enable study of the early stages of
tumor progression. The presence of gradients of pH, oxygen and
metabolic compounds, and micrometastases provide the possibility
for the high-throughput screening of chemo-therapeutics and
molecular inhibitors. However, for this, it is necessary to resolve
technical challenges, such as the development of approaches to
produce organoids on a large scale and consistently. At present,
there are several systems to produce these and the application of
novel techniques could allow, within a few years, the production of
these models on a large scale.
3. Organ-on-a-chip models
A combination of microfabrication approaches, such
as soft lithography, molding and micromachining techniques, has
been biologically adapted to develop microfluidic 3D devices
(51,52). These models are constructed
primarily with optically clear plastic, glass or flexible polymers
such as polydimethylsiloxane (PDMS) (53). As a result of developing
micropatterned surfaces, tumor configuration and microenvironmental
cues are reproduced in the 3D conditions, supporting the accurate
arrangement of cells. The major advantage of these chips over
static models is through the manipulation of microfluidic amounts
of fluids and living cells via channels with dimensions of hundreds
of µm that mimic tumor cell distributions, as well as the
gradients of nutrients and factors (54,55).
Despite the great advantages shown by microfluidic chips, there are
certain limitations that should be considered in terms of their
implementation. First, their standardization requires qualified
personnel for their design and use. Second, PDMS, which is the
material most commonly utilized for chip construction, is able to
absorb small hydrophobic molecules, resulting in interference in
certain studies on drug screening (56). Finally, the use of
spheroids-on-chips results in difficulties in providing long-term
cultures (24).
At present, different aspects of the behavior of BC
tumors have been evaluated using organ-on-a-chip models. For
example, the early stages of BC tumors have been explored. Using
these chips, co-culture of breast tumor spheroids with human
mammary ductal epithelial cells and mammary fibroblasts in a
compartmentalized 3D microfluidic device was successfully
implemented (Fig. 1B) (57). This design consists of upper and
lower microchannels separated by a thin ECM-derived membrane
(Fig. 1B). On the upper side of
the membrane, mammary epithelial cells and spheroids were seeded.
On the lower side of the membrane, a stromal layer was added that
contained mammary fibroblasts. Both chambers were permanently
infused with culture medium (Fig.
1B). This chip was able to recapitulate the general aspects of
the mammary duct and of ductal mammary carcinoma, and also allowed
the study of anticancer drugs and their effects on proliferation
and invasion (57).
Additionally, the impact of the microenvironment has
been studied in terms of growth and progression in other models.
For example, the co-culture of mammary fibroblasts and T47D human
BC cells with different ECM proteins (collagen, fibronectin and
laminin) showed that fibroblasts and high amounts of fibronectin
stimulate the growth of tumor cells (58). In addition, in a cell panel of BC
cells, it was determined that microenvironmental signals promote
the ligand-independent activation of ERα (59). Furthermore, by means of a
multi-culture device, BC cells, macrophages and stromal cells were
co-cultured, and it was determined that cellular interactions
altered gene expression in all of the evaluated cells (60). Another important aspect studied
using micro-chip models is the epithelial-mesenchymal transition of
BC cells. This is a cellular process normally observed during
embryogenesis; however, during tumor progression, this process
leads to the loss of cell-cell and cell-ECM interactions following
the activation of specific genes, promoting a mesenchymal phenotype
with high metastatic potential and resistance to therapeutic drugs
(61). To investigate this
process, a chip with two microfluidic channels separated by a thick
matrix layer was employed (Fig.
1C) (62,63). In this device, MDA-MB-231 were
seeded into the upper channel. Then, they were attracted toward the
matrix layer to the lower channel (Fig. 1C). By using this microfluidic
system, it was determined that cells not expressing E-cadherin
(MDA-MB-231) invaded mostly as single cells, whilst cells with
normal E-cadherin expression (MCF-7) collectively invaded (63).
In addition, migration and invasion processes have
also been studied using organ-on-a-chip tools (64). Through the use of a multifluidic
platform, a physical interaction was demonstrated between
metastatic BC cells and an endothelial monolayer that facilitated
tumor invasion through a collagen type I gel (65). In two parallel microfluidic
channels, a co-culture was evaluated comprising human umbilical
vein endothelial cells (HUVECs) as an endothelial layer and
MCF-10A, MCF-7 or MDA-MB-231 cells as epithelial cells (66). In this system, a higher migration
profile was observed for MDA-MB-231 cells compared with MCF-10A and
MCF-7 cells, with a lack of movement from HUVEC cells. The system
also was proposed as a tool to study the interaction between
endothelial and epithelial layers and their effects during cell
migration and invasion. Additionally, the interaction between tumor
cells and immunological cells was evaluated using a microfluidic 3D
migration assay (67), which
reported that macrophages promoted an increase in the speed and
persistence of cancer cell migration.
The establishment of models to study the
intravasation of BC cells into blood capillaries has been
challenging. To address this, microfluidic approaches have been
used. For example, in a microfluidic system consisting of
concentric layers containing BC cells and vascular networks formed
by HUVECs, it was demonstrated that only the highly metastatic
MDA-MB-231 cell line, and not the less invasive MCF-7 cell line,
was able to either enhance invasion or intravasation, or to
increase vascular permeability (Fig.
1D) (68). Similar results
were observed in the co-culture of primary human BC cells and the
vascular network; the permeability of the vessels was significantly
increased in response to tumor cells or tumor cell-conditioned
medium (69). Furthermore, these
models have been utilized to study BC cell responses against
anticancer therapies (70).
In brief, the greatest advantage of these models is
their small size, allowing the use of smaller sample sizes and
reagent volumes. As a result, they have high reproducibility and
capacity for large-scale production. Additionally, their design
permits the study of specific stages of BC development, employing
single cells or the co-culture of cell lines under controlled
conditions. By the use of these models, the study of BC cell
interactions with vascular networks, endothelial and epithelial
cells has been possible, along with the study of ECM components
during tumor metastasis, invasion or angiogenesis.
4. Hydrogel models
Hydrogel models are scaffolds that reproduce a
simplified tumor microenvironment to understand tumor behavior
(71). These platforms are
fabricated with a naturally derived hydrogel, such as collagen
(72,73), fibrin (74) or reconstituted basement membrane
(Matrigel) (75,76). The advantages of hydrogel models
include low toxicity, biocompatibility, the promotion of cell
attachment and proliferation, and the display of tumor cues for
cell migration (71). However, as
in all of the models, they demonstrate limitations, such as
mechanical weakness under certain culture conditions.
The biological relevance of 3D-based scaffold models
made with natural hydrogels has been investigated. Comparisons
between 2D and 3D conditions reveal that, although ERα protein was
expressed in a 3D collagen-scaffold model maintained in hypoxia,
these molecules were downregulated in 2D models under the same
hypoxic conditions (77).
Additionally, scaffolds comprised of polycaprolactone (PCL), a
biocompatible polymer, exhibit an increase in cancer stem cell
populations under 3D conditions (78). These data demonstrate the effects
of culture environment on cellular response and indicate that 3D
models mimic in vivo conditions. Naturally-derived hydrogels
have been employed to evaluate novel behaviors of BC cells. For
example, in order to study the complex interactions between BC
cells and stroma, human fibroblasts were seeded on a Petri dish
pre-coated with collagen. Above this layer, a mix of Matrigel +
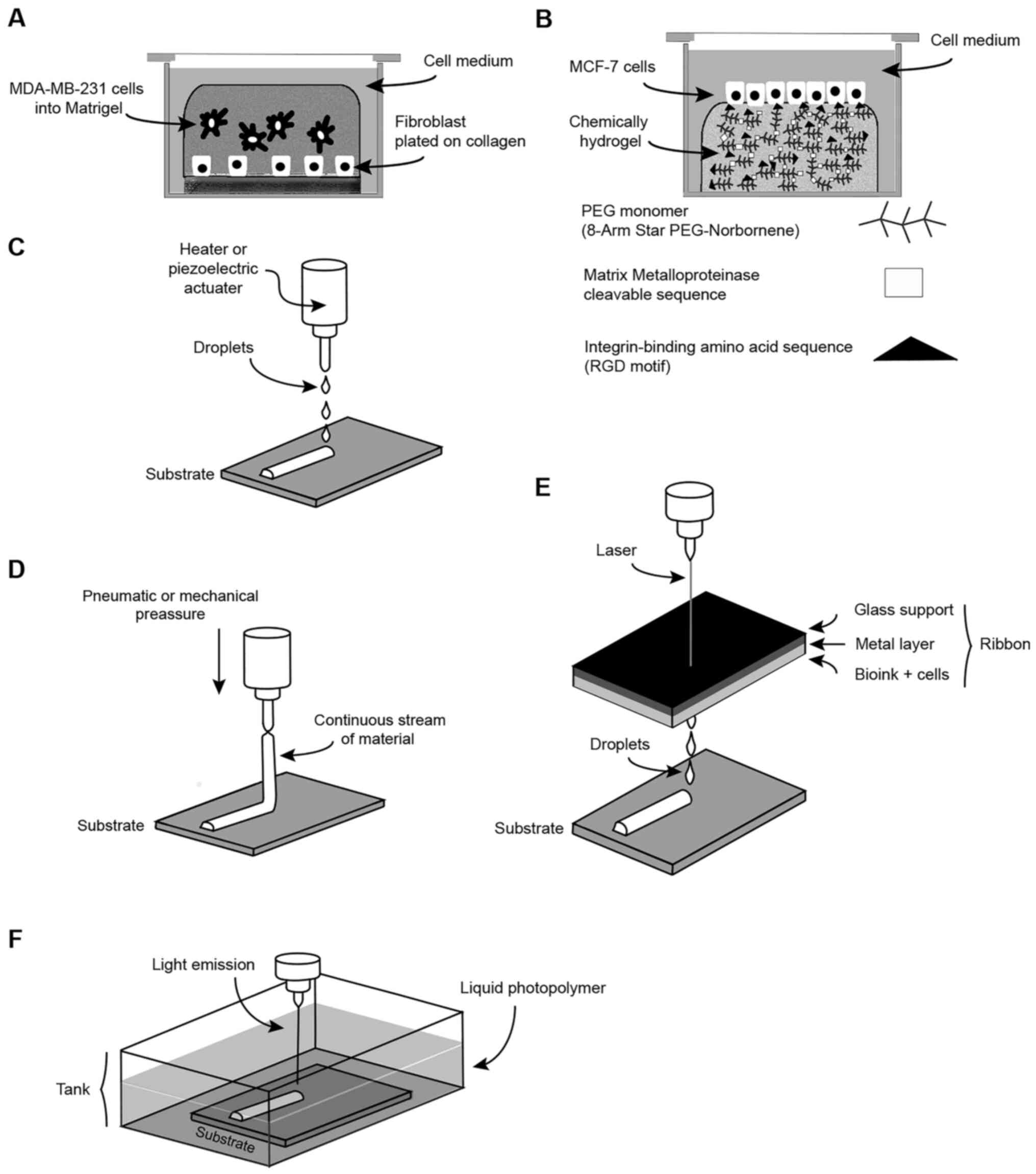
MDA-MB-231 cells was added (Fig.
2A) (79). This
collagen-Matrigel scaffold demonstrated that MDA-MB-231 cells and
human fibroblasts interact by the release of soluble factors
secreted by tumor cells (79),
which promotes alterations in fibroblast shape, motility and gene
expression. In a reciprocal manner, fibroblasts release soluble
factors that accelerate BC cell aggregation (Fig. 2A) (79). These data emphasize the important
role of the stroma on tumor growth.
Another study evaluated the interaction between
MC3T3-E1 pre-osteoblasts and MDA-MB-231 tumor cells in a dense 3D
collagenous environment (cellularized scaffold from rat tail
collagen) (80). Under these
co-culture conditions, BC cells promote MC3T3-E1 differentiation
into osteoblasts, reducing the mineralization of the
osteoblast-mediated media, which is hypothesized to result in
promoted bone resorption. Similarly, in a 3D scaffold model
developed by a collagen-glycosaminoglycan combination, it was
demonstrated that the murine mammary adenocarcinoma 4T1 cell line
was able to mineralize under appropriate conditions (80). These findings support the
hypothesis that BC cells possess the ability to osteomimitize in
order to promote metastasis into the bone microenvironment
(81) and alter the bone
microenvironment for survival. Additionally, the importance of
collagen fibers, independent of matrix stiffness during tumor cell
invasion, was revealed using collagen scaffolds and MDA-MB-231
cells (82). Although this
observation was previously postulated following observation of
human samples (83,84), it was verified in a 3D model.
Furthermore, the importance of substrate fibers in promoting cell
migration was shown. This was studied in 3D scaffolds comprising
Matrigel, collagen type I and porcine ECM-derived tissue matrix gel
(TMG). Comparison of these hydrogels revealed that in collagen and
TMG matrix, in which there were high amounts of fibers, MDA-MB-231
cells exhibited more cellular protrusions, which could be
associated with their invasive properties (82).
As mentioned above, the use of natural hydrogels has
certain limitations. To counteract this, chemical hydrogels have
been developed (85). Among them,
the most used in tumor research are PEG, poly(lactic acid),
poly(glycolic acid) and poly(lactic-co-glycolic acid) (PLGA)
(86). One important
characteristic displayed by these models is their ability to
repro-duce 3D tumor environment properties, such as adhesiveness,
degradable components and the diffusion of chemoattractant
molecules. Thus, they can be designed as modular settings
containing peptide sequences or protein domains in the hydrogel
backbone; for example, the PEG monomer, (8-arm star
PEG-norbornene), incorporates a cross-linker sequence and
cell-adhesion domains (Fig. 2B)
(87). Adhesion domains utilize
biological molecules such as the integrin-binding amino acid
sequence (RGD motif), which more effectively reproduce tumor
aspects such as cellular proliferation, adhesion and migration
(Fig. 2B) (88,89).
Other cell adhesion molecules that are utilized include
fibronectin/vitronectin (RGDS motif), collagen (GFOGER motif) and
laminin (IKVAV motif) combined with PEG to develop scaffolds with
different matrix densities (90).
The successful proliferation of the MDA-MB-231 and T47D cell lines
was observed using these molecules; the cell lines responded
distinctly according to the synthetic matrix employed (90). Finally, cross-linker sequences such
as the matrix metalloproteinase-cleavable sequences
(KCGGPQGIAGQGCK-NH2) can be added to permit cell invasion (Fig. 2B) (87). Recently, the biological relevance
of chemical hydrogels such as PEG in terms of reproducing the
effects of natural hydrogels such as collagen and Matrigel was
evaluated (87). It was shown
using the MCF-7 cell line that PEG hydrogels exhibit similar
performance compared with collagen and Matrigel scaffolds,
rendering them suitable for use in cell culture experiments
(87). Additionally, MDA-MB-231
and MCF-7 cells recently exhibited their ability to grow on both
natural hydrogels, such as a 1% alginate scaffold, and synthetic
matrices, such as a thiol-modified hyaluronan (HA-SH cross-linked
with PEGDA) device (91).
Additionally, 3D porous scaffolds from synthetic
polymers have demonstrated their utility for mimicking tumor
conditions (92,93). For example, in a 3D porous PCL
scaffold model, it was demonstrated that MDA-MB-231 cells exhibit
enhanced proliferation and a significant increase in the expression
of genes associated with BC metastasis, tissue remodeling and
cancer inflammation, indicating the recreation of in vivo
conditions in the 3D model (94).
Similarly, in 3D scaffolds made from PCL, it has been observed that
the 3D microenvironment promotes the cell dormancy of
chemoresistant BC cells compared with cells cultured under 2D
conditions (95). Finally, it was
reported that MDA-MB-231 cells seeded in PLGA and PCL porous
scaffolds exhibited increased expression of ECM receptors and
reduced sensitivity to 4-hydroxytamoxifen treatment compared with
cells cultured under 2D conditions (96).
In conclusion, these hydrogel models have opened the
possibility to study the cellular response of single tumor cells
and stroma cells to specific environments. Additionally, they have
been used to evaluate how this cellular communication promotes
tumor growth, invasion, metastasis and changes in the
microenvironment in order for them to colonize and survive in new
tissues.
5. Bio-printing models
In bio-printing models, 3D bio-printing technologies
are used to create complex structures (97). In them, 2D layers of biomaterials
or bioinks, which are printable hydrogels with living cell
encapsulation, are sequentially printed to form a 3D scaffold
(98). Currently, the most common
methods of bio-printing employed in Biology include inkjet printing
(99,100), micro-extrusion printing (101), laser-assisted printing (102) and stereolithographic printing
(103). For all of these, their
principal advantage is their ability to reproduce high-resolution
3D structures that recreate the complex tumor environment. However,
at present, there are specific challenges that should be resolved
for each method, such as cell viability, resolution and print
fidelity; in addition, the bioink selected and its concentration
are also important points that impact the printing characteristics.
The specific properties, advantages and disadvantages of each
method are covered below.
In the inkjet printing method, the biomaterial that
contains the cells is vaporized into microbubbles via a thermal
process (a heated element is used to form droplets) or a
piezoelectric process (acoustic waves are used to eject droplets),
and they are deposited to print the desired pattern (Fig. 2C) (97,104,105). This method has several
advantages, such as high resolution (~50 µm), the capacity
to replicate complex biological structures and high cell viability
(97). Its principal disadvantage
is its inability to print large-scale scaffolds; in addition, the
use of viscous bioinks represents a challenge (106).
Micro-extrusion printing is a pressure-driven
technology that is connected with nozzles or needles to cartridges
loaded with bioink (Fig. 2D). In
this method, the biomaterial is driven by pneumatic or mechanical
pressure to produce a continuous stream of material that is
directly deposited onto the substrate (Fig. 2D) (107). Some advantages include the
ability of this method to print high cell densities in viscous
biomaterials and the possibility of using multiple cartridges to
print heterogenous structures with several types of cells (101,107). Notable drawbacks include a
reduced resolution in comparison with inkjet printing (~100
µm) (97), the possible
distortion of cell structures and the loss of cellular viability
due to the force used to expel the biomaterial (107,108).
In the laser-assisted approach, laser energy is used
to vaporize a thin layer of metal and eject the biomaterial in
droplets. In brief, the procedure consists of three steps. In the
first of these, a laser source is focused on a laser-absorbing
support called the ribbon. In this ribbon, there are three layers:
A transparent glass support, a thin layer of metal and the layer of
bioink containing cells (Fig. 2E)
(97). In the second step, the
metal layer is vaporized by laser pulses to release bioink
droplets. In the third step, the free droplets containing the cells
are printed on the receiving slide (Fig. 2E) (106). The major advantages shown for
this methodology are a high cellular viability (97) and a high resolution for printing 3D
models (10-50 µm) (109).
However, there are some limitations to consider for its
implementation. For example, during the fabrication process,
rigorous standardization is necessary to minimize the possibility
of over-drying of the sample by the laser power, and to adjust the
distance between the ribbon and receiving slide (102). Another important point to bear in
mind is the high cost of the required equipment (104).
Stereolithographic printing is the oldest technique
of bio-printing that exists (110). In this approach, a liquid
photo-polymer or photoinitiator is irradiated with ultraviolet
(UV), infrared or visible light to promote its photopolymerization
(Fig. 2F). The 3D scaffold is
formed by stacking all of the solidified layers via a layer-by
layer process (layer heights ranging from 25-200 µm)
(Fig. 2F) (106,111) The resolution for this method
ranges from 5-300 µm and is dictated by the light source
(112). In terms of advantages,
the limitations observed using other printing methods resulting
from the bioink viscosity are avoided. Additionally, the
fabrication speed of 3D scaffolds using this method is fast, which
can provide high-resolution molds, and eliminate the distortion of
cell structures and the loss of cellular viability observed using
micro-extrusion printing. However, there are some disadvantages,
such as the difficulty in fabricating using multimaterial
structures (112). Another point
to consider is the illumination source; when the modeling of the 3D
structures employs UV or laser light, it can affect the cells and
introduce mutations in them. To eliminate the harmful effects of
this radiation, it is possible to employ this method using visible
light, allowing the manufacturing of 3D scaffolds containing cells
throughout the polymer resin (113). Also, the selection of the
concentration of the photoinitiator is a critical point, as the
amount of this element defines the stiffness and matrix density of
the scaffold; however, a high concentration may induce cytotoxic
effects (97).
Biomaterials or bioinks employed in bio-printing are
hydro-gels that protect the cells during the printing process and
also reproduce the ECM environment to support cellular functions
such as cell viability, proliferation and morphology. The type of
bioink selected will exert an important effect on the 3D scaffold
structure, and certain properties, such as viscosity, gelation and
cross-linking capabilities, must be considered prior to its
selection (97). In general, two
types of biomaterials are utilized to produce 3D platforms: Natural
polymers and synthetic polymers (110). Among the natural substrates used
are alginate (114), collagen
(115), fibrin (116), gelatin and hyaluronic acid
(117). Some examples of
synthetic polymers include PCL, PEG, PLGA and Poloxamer 407
(110). Of note, natural polymers
are the most frequently used bioink due to their biological
compatibility (118).
The implementation of micro-extrusion printing has
allowed for the successful co-culture of mesenchymal stem cells and
MDA-MB-231 cells in bioinks comprised of alginate and highly
hydrated cellulose in order to explore the communication between
these cells (119). Additionally,
through the implementation of bio-printing, organoids from MCF-7
and MDA-MB-468 tumor cells, as well as MCF-12A non-tumor cells,
have been generated using a novel human-derived breast hydrogel
(120).
These systems also permit the study of
microenvironmental effects on tumor progression; however, to our
knowledge, there are few works at present that employ this method
to study BC. Nevertheless, it is predicted that their
implementation in upcoming years will provide new data concerning
the progression of BC, facilitating the development of new and more
effective therapies.
6. Conclusions
Present understanding of the development of BC
derives from studies conducted in patients and animal models, or
from the culture of cell lines under 2D conditions. Although the
information obtained from these studies has been very important,
each method and model exhibits limitations. To generate solutions
for these challenges, novel 3D models have been developed. In
these, various aspects observed under in vivo conditions
have been recapitulated, such as interactions between tumor cells
and the stroma, as well as the presence of the ECM and 3D
environment. These particularities have rendered possible the study
of novel aspects of BC progression under standardized conditions.
However, their regular implementation requires the resolution of
some important points. For example, in spheroid models, the lack of
vascularization limits their use as a model of the genesis of
tumors, although not the later stages of tumor development.
Concerning organ-on-a-chip and hydrogel models, these have a
well-organized spatial distribution of tumor cells and ECM
components, but their implementation reproduces only one specific
point in tumor progression. Finally, the imple-mentation of
bio-printing methods depends upon resolving several technical
challenges, such as improvements in bioink materials and
cell-seeding conditions.
Advances concerning present knowledge of BC
progression require the use of 3D models and improved laboratory
skills. Additionally, it is necessary to improve these models to
allow their regular use, as well as to improve their ability to
reflect the complexity of the tumor microenvironment and increase
knowledge of cancer biology. To resolve these limitations,
interdisciplinary work between various areas of science will be
necessary. These improvements without doubt will be an important
step for the development of more efficient therapeutic strategies.
Based on current understanding of 3D models, it is predicted that
in the near future, it will be possible, for example, to combine
two or more conventional 3D tumor models, such as organoids
contained in microfluidic devices or bio-printed scaffolds, raising
the possibility of even more complex models that will be able to
recapitulate complex cell-ECM interactions and tumor
compartmentalization and further understanding BC.
Funding
This study was supported by the Health Research Fund
of the Mexican Institute of Social Security (IMSS; grant nos.
FIS/IMSS/PROT/EMER18/1848 and FIS/IMSS/PROT/G15/1472). Funding was
also received courtesy of Research Career Development Awards from
the IMSS Foundation.
Availability of data and materials
Not applicable.
Authors' contributions
MHR and AAR conceived and drafted the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Ahmad A: Breast cancer statistics: Recent
trends. Adv Exp Med Biol. 1152:1–7. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rivera-Franco MM and Leon-Rodriguez E:
Delays in breast cancer detection and treatment in developing
countries. Breast Cancer (Auckl). 12:11782234177526772018.
|
|
3
|
Global Burden of Disease Cancer
Collaboration; Fitzmaurice C, Dicker D, Pain A, Hamavid H,
Moradi-Lakeh M, MacIntyre MF, Allen C, Hansen G, Woodbrook R, et
al: The global burden of cancer 2013. JAMA Oncol. 1:505–527. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hanahan D and Coussens LM: Accessories to
the crime: Functions of cells recruited to the tumor
microenvironment. Cancer Cell. 21:309–322. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mittal S, Brown NJ and Holen I: The breast
tumor microenvironment: Role in cancer development, progression and
response to therapy. Expert Rev Mol Diagn. 18:227–243. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Conklin MW and Keely PJ: Why the stroma
matters in breast cancer: Insights into breast cancer patient
outcomes through the examination of stromal biomarkers. Cell Adh
Migr. 6:249–260. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Insua-Rodríguez J and Oskarsson T: The
extracellular matrix in breast cancer. Adv Drug Deliv Rev.
97:41–55. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Friedl P and Alexander S: Cancer invasion
and the microenvironment: Plasticity and reciprocity. Cell.
147:992–1009. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kaushik S, Pickup MW and Weaver VM: From
transformation to metastasis: Deconstructing the extracellular
matrix in breast cancer. Cancer Metastasis Rev. 35:655–667. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Natal RA, Paiva GR, Pelegati VB, Marenco
L, Alvarenga CA, Vargas RF, Derchain SF, Sarian LO, Franchet C,
Cesar CL, et al: Exploring collagen parameters in pure special
types of invasive breast cancer. Sci Rep. 9:77152019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vuong D, Simpson PT, Green B, Cummings MC
and Lakhani SR: Molecular classification of breast cancer. Virchows
Arch. 465:1–14. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Comşa Ş, Cimpean AM and Raica M: The story
of MCF-7 breast cancer cell line: 40 years of experience in
research. Anticancer Res. 35:3147–3154. 2015.PubMed/NCBI
|
|
13
|
Ravi M, Sneka MK and Joshipura A: The
culture conditions and outputs from breast cancer cell line in
vitro experiments. Exp Cell Res. 383:1115482019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim JB, O'Hare MJ and Stein R: Models of
breast cancer: Is merging human and animal models the future?
Breast Cancer Res. 6:22–30. 2004. View
Article : Google Scholar :
|
|
15
|
Bersini S, Jeon JS, Dubini G, Arrigoni C,
Chung S, Charest JL, Moretti M and Kamm RD: A microfluidic 3D in
vitro model for specificity of breast cancer metastasis to bone.
Biomaterials. 35:2454–2461. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
dit Faute MA, Laurent L, Ploton D, Poupon
MF, Jardillier JC and Bobichon H: Distinctive alterations of
invasiveness, drug resistance and cell-cell organization in
3D-cultures of MCF-7, a human breast cancer cell line, and its
multidrug resistant variant. Clin Exp Metastasis. 19:161–168. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lovitt CJ, Shelper TB and Avery VM:
Evaluation of chemotherapeutics in a three-dimensional breast
cancer model. J Cancer Res Clin Oncol. 141:951–959. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mehta G, Hsiao AY, Ingram M, Luker GD and
Takayama S: Opportunities and challenges for use of tumor spheroids
as models to test drug delivery and efficacy. J Control Release.
164:192–204. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Froehlich K, Haeger JD, Heger J,
Pastuschek J, Photini SM, Yan Y, Lupp A, Pfarrer C, Mrowka R,
Schleußner E, et al: Generation of multicellular breast cancer
tumor spheroids: Comparison of different protocols. J Mammary Gland
Biol Neoplasia. 21:89–98. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bahcecioglu G, Basara G, Ellis BW, Ren X
and Zorlutuna P: Breast cancer models: Engineering the tumor
microenvironment. Acta Biomater. 106:1–21. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nagelkerke A, Bussink J, Sweep FC and Span
PN: Generation of multicellular tumor spheroids of breast cancer
cells: How to go three-dimensional. Anal Biochem. 437:17–19. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Singh M, Mukundan S, Jaramillo M,
Oesterreich S and Sant S: Three-dimensional breast cancer models
mimic hallmarks of size-induced tumor progression. Cancer Res.
76:3732–3743. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang W, Li C, Baguley BC, Zhou F, Zhou W,
Shaw JP, Wang Z, Wu Z and Liu J: Optimization of the formation of
embedded multicellular spheroids of MCF-7 cells: How to reliably
produce a biomimetic 3D model. Anal Biochem. 515:47–54. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Asghar W, El Assal R, Shafiee H, Pitteri
S, Paulmurugan R and Demirci U: Engineering cancer
microenvironments for in vitro 3-D tumor models. Mater Today
(Kidlington). 18:539–553. 2015. View Article : Google Scholar
|
|
25
|
Rodríguez CE, Berardi DE, Abrigo M, Todaro
LB, Bal de Kier Joffé ED and Fiszman GL: Breast cancer stem cells
are involved in trastuzumab resistance through the HER2 modulation
in 3D culture. J Cell Biochem. 119:1381–1391. 2018. View Article : Google Scholar
|
|
26
|
Zhao L, Xiu J, Liu Y, Zhang T, Pan W,
Zheng X and Zhang X: A 3D printed hanging drop dripper for tumor
spheroids analysis without recovery. Sci Rep. 9:197172019.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Metzger W, Sossong D, Bächle A, Pütz N,
Wennemuth G, Pohlemann T and Oberringer M: The liquid overlay
technique is the key to formation of co-culture spheroids
consisting of primary osteoblasts, fibroblasts and endothelial
cells. Cytotherapy. 13:1000–1012. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang S, Yuan N, Wang G, Wu F, Feng L, Luo
M, Li M, Luo A, Zhao X and Zhang L: Cellular communication promotes
mammosphere growth and collective invasion through microtubule-like
structures and angiogenesis. Oncol Rep. 40:3297–3312.
2018.PubMed/NCBI
|
|
29
|
Berger AJ, Renner CM, Hale I, Yang X,
Ponik SM, Weisman PS, Masters KS and Kreeger PK: Scaffold stiffness
influences breast cancer cell invasion via EGFR-linked Mena
upregulation and matrix remodeling. Matrix Biol. 85:80–93. 2020.
View Article : Google Scholar
|
|
30
|
Guzman A, Sánchez Alemany V, Nguyen Y,
Zhang CR and Kaufman LJ: A novel 3D in vitro metastasis model
elucidates differential invasive strategies during and after
breaching basement membrane. Biomaterials. 115:19–29. 2017.
View Article : Google Scholar
|
|
31
|
Dubois C, Dufour R, Daumar P, Aubel C,
Szczepaniak C, Blavignac C, Mounetou E, Penault-Llorca F and Bamdad
M: Development and cytotoxic response of two proliferative
MDA-MB-231 and non-proliferative SUM1315 three-dimensional cell
culture models of triple-negative basal-like breast cancer cell
lines. Oncotarget. 8:953162017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Smalley KS, Lioni M, Noma K, Haass NK and
Herlyn M: In vitro three-dimensional tumor microenvironment models
for anti-cancer drug discovery. Expert Opin Drug Discov. 3:1–10.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Costa EC, Gaspar VM, Coutinho P and
Correia IJ: Optimization of liquid overlay technique to formulate
heterogenic 3D co-cultures models. Biotechnol Bioeng.
111:1672–1685. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Costa EC, de Melo-Diogo D, Moreira AF,
Carvalho MP and Correia IJ: Spheroids formation on non-adhesive
surfaces by liquid overlay technique: Considerations and practical
approaches. Biotechnol J. 13:2018. View Article : Google Scholar
|
|
35
|
Kosaka T, Tsuboi S, Fukaya K, Pu H, Ohno
T, Tsuji T, Miyazaki M and Namba M: Spheroid cultures of human
hepatoblastoma cells (HuH-6 line) and their application for
cytotoxicity assay of alcohols. Acta Medica Okayama. 50:61–66.
1996.PubMed/NCBI
|
|
36
|
Kelm JM, Timmins NE, Brown CJ, Fussenegger
M and Nielsen LK: Method for generation of homogeneous
multicel-lular tumor spheroids applicable to a wide variety of cell
types. Biotechnol Bioeng. 83:173–180. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Robertson FM, Ogasawara MA, Ye Z, Chu K,
Pickei R, Debeb BG, Woodward WA, Hittelman WN, Cristofanilli M and
Barsky SH: Imaging and analysis of 3D tumor spheroids enriched for
a cancer stem cell phenotype. J Biomol Screen. 15:820–829. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lorenzo C, Frongia C, Jorand R, Fehrenbach
J, Weiss P, Maandhui A, Gay G, Ducommun B and Lobjois V: Live cell
division dynamics monitoring in 3D large spheroid tumor models
using light sheet microscopy. Cell Div. 6:222011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Leonard F and Godin B: 3D in vitro model
for breast cancer research using magnetic levitation and
bioprinting method. Methods Mol Biol. 1406:239–251. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jaganathan H, Gage J, Leonard F,
Srinivasan S, Souza GR, Dave B and Godin B: Three-dimensional in
vitro co-culture model of breast tumor using magnetic levitation.
Sci Rep. 4:64682014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Eckhardt BL, Gagliardi M, Iles L, Evans K,
Ivan C, Liu X, Liu CG, Souza G, Rao A, Meric-Bernstam F, et al:
Clinically relevant inflammatory breast cancer patient-derived
xenograft-derived ex vivo model for evaluation of tumor-specific
therapies. PLoS One. 13:e01959322018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lin RZ and Chang HY: Recent advances in
three-dimensional multicellular spheroid culture for biomedical
research. Biotechnol J. 3:1172–1184. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Swaminathan S, Hamid Q, Sun W and Clyne
AM: Bioprinting of 3D breast epithelial spheroids for human cancer
models. Biofabrication. 11:0250032019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ong CS, Yesantharao P, Huang CY, Mattson
G, Boktor J, Fukunishi T, Zhang H and Hibino N: 3D bioprinting
using stem cells. Pediatr Res. 83:223–231. 2018. View Article : Google Scholar
|
|
45
|
Kingsley DM, Roberge CL, Rudkouskaya A,
Faulkner DE, Barroso M, Intes X and Corr DT: Laser-based 3D
bioprinting for spatial and size control of tumor spheroids and
embryoid bodies. Acta Biomater. 95:357–370. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ham SL, Joshi R, Luker GD and Tavana H:
Engineered breast cancer cell spheroids reproduce biologic
properties of solid tumors. Adv Healthc Mater. 5:2788–2798. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ham SL, Thakuri PS, Plaster M, Li J, Luker
KE, Luker GD and Tavana H: Three-dimensional tumor model mimics
stromal-breast cancer cells signaling. Oncotarget. 9:249–267. 2017.
View Article : Google Scholar
|
|
48
|
Atefi E, Lemmo S, Fyffe D, Luker GD and
Tavana H: High throughput, polymeric aqueous two-phase printing of
tumor spheroids. Adv Funct Mater. 24:6509–6515. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lemmo S, Atefi E, Luker GD and Tavana H:
Optimization of aqueous biphasic tumor spheroid microtechnology for
anti-cancer drug testing in 3D culture. Cell Mol Bioeng. 7:344–354.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Shahi Thakuri P, Ham SL, Luker GD and
Tavana H: Multiparametric analysis of oncology drug screening with
aqueous two-phase tumor spheroids. Mol Pharm. 13:3724–3735. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Sackmann EK, Fulton AL and Beebe DJ: The
present and future role of microfluidics in biomedical research.
Nature. 507:181–189. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sen AK, Raj A, Banerjee U and Iqbal SR:
Soft lithography, molding, and micromachining techniques for
polymer micro devices. Microfluidic Electrophoresis. Methods in
Molecular Biology. Dutta D: 1906. Humana Press Springer; New York,
NY: pp. 13–54. 2019, View Article : Google Scholar
|
|
53
|
Halldorsson S, Lucumi E, Gómez-Sjöberg R
and Fleming RMT: Advantages and challenges of microfluidic cell
culture in polydimethylsiloxane devices. Biosens Bioelectron.
63:218–231. 2015. View Article : Google Scholar
|
|
54
|
Sontheimer-Phelps A, Hassell BA and Ingber
DE: Modelling cancer in microfluidic human organs-on-chips. Nat Rev
Cancer. 19:65–81. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yum K, Hong SG, Healy KE and Lee LP:
Physiologically relevant organs on chips. Biotechnol J. 9:16–27.
2014. View Article : Google Scholar
|
|
56
|
Wang JD, Douville NJ, Takayama S and
ElSayed M: Quantitative analysis of molecular absorption into PDMS
microfluidic channels. Ann Biomed Eng. 40:1862–1873. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Choi Y, Hyun E, Seo J, Blundell C, Kim HC,
Lee E, Lee SH, Moon A, Moon WK and Huh D: A microengineered
patho-physiological model of early-stage breast cancer. Lab Chip.
15:3350–3357. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Montanez-Sauri SI, Sung KE, Berthier E and
Beebe DJ: Enabling screening in 3D microenvironments: Probing
matrix and stromal effects on the morphology and proliferation of
T47D breast carcinoma cells. Integr Biol (Camb). 5:631–640. 2013.
View Article : Google Scholar
|
|
59
|
Lang JD, Berry SM, Powers GL, Beebe DJ and
Alarid ET: Hormonally responsive breast cancer cells in a
microfluidic co-culture model as a sensor of microenvironmental
activity. Integr Biol (Camb). 5:807–816. 2013. View Article : Google Scholar
|
|
60
|
Regier MC, Maccoux LJ, Weinberger EM,
Regehr KJ, Berry SM, Beebe DJ and Alarid ET: Transitions from
mono-to co-to tri-culture uniquely affect gene expression in breast
cancer, stromal, and immune compartments. Biomed Microdevices.
18:702016. View Article : Google Scholar
|
|
61
|
Dongre A and Weinberg RA: New insights
into the mechanisms of epithelial-mesenchymal transition and
implications for cancer. Nat Rev Mol Cell Biol. 20:69–84. 2019.
View Article : Google Scholar
|
|
62
|
Eslami Amirabadi H, SahebAli S, Frimat JP,
Luttge R and den Toonder JMJ: A novel method to understand tumor
cell invasion: Integrating extracellular matrix mimicking layers in
microfluidic chips by 'selective curing'. Biomed Microdevices.
19:922017. View Article : Google Scholar
|
|
63
|
Eslami Amirabadi H, Tuerlings M,
Hollestelle A, SahebAli S, Luttge R, van Donkelaar CC, Martens JWM
and den Toonder JMJ: Characterizing the invasion of different
breast cancer cell lines with distinct E-cadherin status in 3D
using a microfluidic system. Biomed Microdevices. 21:1012019.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Toh YC, Raja A, Yu H and van Noort D: A 3D
microfluidic model to recapitulate cancer cell migration and
invasion. Bioengineering (Basel). 5:292018. View Article : Google Scholar
|
|
65
|
Blaha L, Zhang C, Cabodi M and Wong JY: A
microfluidic platform for modeling metastatic cancer cell matrix
invasion. Biofabrication. 9:0450012017. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Devadas D, Moore TA, Walji N and Young
EWK: A microfluidic mammary gland coculture model using parallel 3D
lumens for studying epithelial-endothelial migration in breast
cancer. Biomicrofluidics. 13:0641222019. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Li R, Hebert JD, Lee TA, Xing H,
Boussommier-Calleja A, Hynes RO, Lauffenburger DA and Kamm RD:
Macrophage-secreted TNFα and TGFβ1 influence migration speed and
persistence of cancer cells in 3D tissue culture via independent
pathways. Cancer Res. 77:279–290. 2017. View Article : Google Scholar
|
|
68
|
Nagaraju S, Truong D, Mouneimne G and
Nikkhah M: Microfluidic tumor-vascular model to study breast cancer
cell invasion and intravasation. Adv Healthc Mater. 7:17012572018.
View Article : Google Scholar
|
|
69
|
Tang Y, Soroush F, Sheffield JB, Wang B,
Prabhakarpandian B and Kiani MF: A biomimetic microfluidic tumor
microenvironment platform mimicking the EPR effect for rapid
screening of drug delivery systems. Sci Rep. 7:93592017. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Lanz HL, Saleh A, Kramer B, Cairns J, Ng
CP, Yu J, Trietsch SJ, Hankemeier T, Joore J, Vulto P, et al:
Therapy response testing of breast cancer in a 3D high-throughput
perfused microfluidic platform. BMC Cancer. 17:7092017. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Bahlmann LC, Smith LJ and Shoichet MS:
Designer biomaterials to model cancer cell invasion in vitro:
Predictive tools or just pretty pictures. Adv Funct Mater.
30:19090322020. View Article : Google Scholar
|
|
72
|
Chen L, Xiao Z, Meng Y, Zhao Y, Han J, Su
G, Chen B and Dai J: The enhancement of cancer stem cell properties
of MCF-7 cells in 3D collagen scaffolds for modeling of cancer and
anti-cancer drugs. Biomaterials. 33:1437–1444. 2012. View Article : Google Scholar
|
|
73
|
Antoine EE, Vlachos PP and Rylander MN:
Review of collagen I hydrogels for bioengineered tissue
microenvironments: Characterization of mechanics, structure, and
transport. Tissue Eng Part B Rev. 20:683–696. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Liu J, Tan Y, Zhang H, Zhang Y, Xu P, Chen
J, Poh YC, Tang K, Wang N and Huang B: Soft fibrin gels promote
selection and growth of tumorigenic cells. Nat Mater. 11:734–741.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Kleinman HK and Martin GR: Matrigel:
Basement membrane matrix with biological activity. Semin Cancer
Biol. 15:378–386. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Krause S, Maffini MV, Soto AM and
Sonnenschein C: The microenvironment determines the breast cancer
cells' phenotype: Organization of MCF7 cells in 3D cultures. BMC
Cancer. 10:2632010. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Whitman NA, Lin ZW, Kenney RM, Albertini L
and Lockett MR: Hypoxia differentially regulates estrogen receptor
alpha in 2D and 3D culture formats. Arch Biochem Biophys. 671:8–17.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Palomeras S, Rabionet M, Ferrer I, Sarrats
A, Garcia-Romeu ML, Puig T and Ciurana J: Breast cancer stem cell
culture and enrichment using poly(ε-Caprolactone) scaffolds.
Molecules. 21:5372016. View Article : Google Scholar
|
|
79
|
Wessels DJ, Pradhan N, Park YN, Klepitsch
MA, Lusche DF, Daniels KJ, Conway KD, Voss ER, Hegde SV, Conway TP
and Soll DR: Reciprocal signaling and direct physical interactions
between fibroblasts and breast cancer cells in a 3D environment.
PLoS One. 14:e02188542019. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
James-Bhasin M, Siegel PM and Nazhat SN: A
three-dimensional dense collagen hydrogel to model cancer
cell/osteoblast interactions. J Funct Biomater. 9:722018.
View Article : Google Scholar
|
|
81
|
Cox RF, Jenkinson A, Pohl K, O'Brien FJ
and Morgan MP: Osteomimicry of mammary adenocarcinoma cells in
vitro; increased expression of bone matrix proteins and
proliferation within a 3D collagen environment. PLoS One.
7:e416792012. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Berger AJ, Linsmeier KM, Kreeger PK and
Masters KS: Decoupling the effects of stiffness and fiber density
on cellular behaviors via an interpenetrating network of
gelatin-methacrylate and collagen. Biomaterials. 141:125–135. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Conklin MW, Eickhoff JC, Riching KM,
Pehlke CA, Eliceiri KW, Provenzano PP, Friedl A and Keely PJ:
Aligned collagen is a prognostic signature for survival in human
breast carcinoma. Am J Pathol. 178:1221–1232. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Bredfeldt JS, Liu Y, Conklin MW, Keely PJ,
Mackie TR and Eliceiri KW: Automated quantification of aligned
collagen for human breast carcinoma prognosis. J Pathol Inform.
5:282014. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Wang Y, Mirza S, Wu S, Zeng J, Shi W, Band
H, Band V and Duan B: 3D hydrogel breast cancer models for studying
the effects of hypoxia on epithelial to mesenchymal transition.
Oncotarget. 9:321912018. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Peppas NA, Hilt JZ, Khademhosseini A and
Langer R: Hydrogels in biology and medicine: From molecular
principles to bionanotechnology. Adv Mater. 18:1345–1360. 2006.
View Article : Google Scholar
|
|
87
|
Livingston MK, Morgan MM, Daly WT, Murphy
WL, Johnson BP, Beebe DJ and Virumbrales-Muñoz M: Evaluation of
PEG-based hydrogel influence on estrogen receptor driven responses
in MCF7 breast cancer cells. ACS Biomater Sci Eng. 5:6089–6098.
2019. View Article : Google Scholar
|
|
88
|
Bellis SL: Advantages of RGD peptides for
directing cell association with biomaterials. Biomaterials.
32:4205–4210. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Wang F, Li Y, Shen Y, Wang A, Wang S and
Xie T: The functions and applications of RGD in tumor therapy and
tissue engineering. Int J Mol Sci. 14:13447–13462. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Sawicki LA, Ovadia EM, Pradhan L, Cowart
JE, Ross KE, Wu CH and Kloxin AM: Tunable synthetic extracellular
matrices to investigate breast cancer response to biophysical and
biochemical cues. APL Bioeng. 3:0161012019. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Schmid R, Schmidt SK, Hazur J, Detsch R,
Maurer E, Boccaccini AR, Hauptstein J, Teßmar J, Blunk T, Schrüfer
S, et al: Comparison of hydrogels for the development of
well-defined 3D cancer models of breast cancer and melanoma.
Cancers (Basel). 12:23202020. View Article : Google Scholar :
|
|
92
|
Feng S, Duan X, Lo PK, Liu S, Liu X, Chen
H and Wang Q: Expansion of breast cancer stem cells with fibrous
scaffolds. Integr Biol (Camb). 5:768–777. 2013. View Article : Google Scholar
|
|
93
|
Zhang YS, Duchamp M, Oklu R, Ellisen LW,
Langer R and Khademhosseini A: Bioprinting the cancer
microenvironment. ACS Biomater Sci Eng. 2:1710–1721. 2016.
View Article : Google Scholar
|
|
94
|
Balachander GM, Balaji SA, Rangarajan A
and Chatterjee K: Enhanced metastatic potential in a 3D tissue
scaffold toward a comprehensive in vitro model for breast cancer
metastasis. ACS Appl Mater Interfaces. 7:27810–27822. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Guiro K, Patel SA, Greco SJ, Rameshwar P
and Arinzeh TL: Investigating breast cancer cell behavior using
tissue engineering scaffolds. PLoS One. 10:e01187242015. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Rijal G, Bathula C and Li W: Application
of synthetic polymeric scaffolds in breast cancer 3D tissue
cultures and animal tumor models. Int J Biomater. 2017:80748902017.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Kačarević ŽP, Rider PM, Alkildani S,
Retnasingh S, Smeets R, Jung O, Ivanišević Z and Barbeck M: An
introduction to 3D bioprinting: Possibilities, challenges and
future aspects. Materials (Basel). 11:21992018. View Article : Google Scholar
|
|
98
|
Cha rbe N, McCa r ron PA and Tambuwala MM:
Three-dimensional bio-printing: A new frontier in oncology
research. World J Clin Oncol. 8:21–36. 2017. View Article : Google Scholar
|
|
99
|
Derby B: Bioprinting: Inkjet printing
proteins and hybrid cell-containing materials and structures. J
Mater Chem. 18:5717–5721. 2008. View Article : Google Scholar
|
|
100
|
Negro A, Cherbuin T and Lutolf MP: 3D
inkjet printing of complex, cell-laden hydrogel structures. Sci
Rep. 8:170992018. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Panwar A and Tan LP: Current status of
bioinks for micro-extrusion-based 3D bioprinting. Molecules.
21:6852016. View Article : Google Scholar
|
|
102
|
Koch L, Gruene M, Unger C and Chichkov B:
Laser assisted cell printing. Curr Pharm Biotechnol. 14:91–97.
2013.PubMed/NCBI
|
|
103
|
Mondschein RJ, Kanitkar A, Williams CB,
Verbridge SS and Long TE: Polymer structure-property requirements
for stereo-lithographic 3D printing of soft tissue engineering
scaffolds. Biomaterials. 140:170–188. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Cui X, Boland T, D'Lima DD and Lotz MK:
Thermal inkjet printing in tissue engineering and regenerative
medicine. Recent Pat Drug Deliv Formul. 6:149–155. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Cheng E, Yu H, Ahmadi A and Cheung KC:
Investigation of the hydrodynamic response of cells in drop on
demand piezoelectric inkjet nozzles. Biofabrication. 8:0150082016.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Yi HG, Lee H and Cho DW: 3D printing of
organs-on-chips. Bioengineering (Basel). 4:102017. View Article : Google Scholar
|
|
107
|
Bishop ES, Mostafa S, Pakvasa M, Luu HH,
Lee MJ, Wolf JM, Ameer GA, He TC and Reid RR: 3-D bioprinting
technologies in tissue engineering and regenerative medicine:
Current and future trends. Genes Dis. 4:185–195. 2017. View Article : Google Scholar
|
|
108
|
Chang R, Nam J and Sun W: Effects of
dispensing pressure and nozzle diameter on cell survival from solid
freeform fabrication-based direct cell writing. Tissue Eng Part A.
14:41–48. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Murphy SV and Atala A: 3D bioprinting of
tissues and organs. Nat Biotechnol. 32:773–785. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Park JH, Jang J, Lee JS and Cho DW:
Three-dimensional printing of tissue/organ analogues containing
living cells. Ann Biomed Eng. 45:180–194. 2017. View Article : Google Scholar
|
|
111
|
Sears NA and Seshadri DR: A review of
three-dimensional printing in tissue engineering. Tissue Eng Part B
Rev. 22:298–310. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Raman R, Bhaduri B, Mir M, Shkumatov A,
Lee MK, Popescu G, Kong H and Bashir R: High-resolution projection
microstereolithography for patterning of neovasculature. Adv
Healthc Mater. 5:610–619. 2016. View Article : Google Scholar
|
|
113
|
Lin H, Zhang D, Alexander PG, Yang G, Tan
J, Cheng AW and Tuan RS: Application of visible light-based
projection stereo-lithography for live cell-scaffold fabrication
with designed architecture. Biomaterials. 34:331–339. 2013.
View Article : Google Scholar
|
|
114
|
Axpe E and Oyen ML: Applications of
alginate-based bioinks in 3D bioprinting. Int J Mol Sci.
17:19762016. View Article : Google Scholar
|
|
115
|
Nerger BA, Brun PT and Nelson CM:
Microextrusion printing cell-laden networks of type I collagen with
patterned fiber alignment and geometry. Soft Matter. 15:5728–5738.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Włodarczyk-Biegun MK and del Campo A: 3D
bioprinting of structural proteins. Biomaterials. 134:180–201.
2017. View Article : Google Scholar
|
|
117
|
Albritton JL and Miller JS: 3D
bioprinting: Improving in vitro models of metastasis with
heterogeneous tumor microenvironments. Dis Model Mech. 10:3–14.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Mancha Sánchez E, Gómez-Blanco JC, López
Nieto E, Casado JG, Macías-García A, Díaz Díez MA, Carrasco-Amador
JP, Torrejón Martín D, Sánchez-Margallo FM and Pagador JB:
Hydrogels for bioprinting: A systematic review of hydrogels
synthesis, bioprinting parameters, and bioprinted structures
behavior. Front Bioeng Biotechnol. 8:7762020. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Moore CA, Shah NN, Smith CP and Rameshwar
P: 3D bioprinting and stem cells. Methods Mol Biol. 1842:93–103.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Mollica PA, Booth-Creech EN, Reid JA,
Zamponi M, Sullivan SM, Palmer XL, Sachs PC and Bruno RD: 3D
bioprinted mammary organoids and tumoroids in human mammary derived
ECM hydrogels. Acta Biomater. 95:201–213. 2019. View Article : Google Scholar : PubMed/NCBI
|